Abstract
The physical–chemical, structural, hydrodynamic, and biological properties of hyaluronic acid within tendons are still poorly investigated. Medical history and clinical applications of hyaluronic acid for tendinopathies are still debated. In general, the properties of hyaluronic acid depend on several factors including molecular weight. Several preclinical and clinical experiences show a good efficacy and safety profile of hyaluronic acid, despite the absence of consensus in the literature regarding the classification according to molecular weight. In in vitro and preclinical studies, hyaluronic acid has shown physical–chemical properties, such as biocompatibility, mucoadhesivity, hygroscopicity, and viscoelasticity, useful to contribute to tendon healing. Additionally, in clinical studies, hyaluronic acid has been used with promising results in different tendinopathies. In this narrative review, findings encourage the clinical application of HA in tendinopathies such as rotator cuff, epicondylitis, Achilles, and patellar tendinopathy.
Keywords: hyaluronic acid, receptor, biology, structure, tendon, tendinopathy, degeneration, inflammation, viscoelastic, hygroscopic, effect
1. Introduction
Hyaluronic acid (HA) is a non-sulphated glycosaminoglycan formed by repetitive units of glucuronic acid and N-acetyl glucosamine. HA is widely present in the extracellular matrix in vertebrates and invertebrates to confer mechanical support, viscoelastic and hygroscopic properties, and anti-inflammatory effects to cells and tissue. HA is one of the fundamental components of cartilage and tendon tissue, contributing to their viscoelastic properties [1,2,3,4,5]. HA enhances the cellular activities of fibroblasts, including their adhesivity, extracellular matrix (ECM) synthesis, and proliferation, but, despite its influence on cells and tendon structure, its role on the biomechanical function is still not clarified [6,7]. The ECM of tendons is predominantly composed of type I collagen (Figure 1) and proteoglycans, with a small amount of the other types of collagen (type II, III, V, VI, IX, XI) (Table 1). The predominant cells type within the tendon are tenoblasts or tenocytes, accounting for 90–95% of cells present in tendon tissue between collagen fibres [8]. Tenoblasts and tenocytes show different metabolic features: while tenoblasts have high activity, tenocytes are metabolically less active. Both types of cells produce collagen, elastin, ECM, and proteins [9]. Other types of cells present in lesser quantities include chondrocytes at the bone attachment and insertion sites, synovial cells in the tendon sheath, and vascular cells, capillary endothelial cells, and arterioles’ smooth muscle cells. All cells that produce the ECM are involved in endogenous HA production as well. HA injections are widely used to treat osteoarthritis (OA), but their efficacy in the management of tendinopathies is still debated [10]. Tendinopathies are characterised by tendon structure disruption, ineffective neovascularisation, decreased collagen I, and enhanced collagen II production [10]. Recently, the anti-inflammatory and viscoelastic effects of HA on connective tissue have been suggested to warrant the use of HA for the treatment of tendinopathies [11]. Some studies proved and support its use to improve function and reduce pain in tendinopathies [12,13], avoiding the complications of corticosteroids [14]. This review outlines the role of HA in reducing inflammation and increasing regeneration in tendinopathies in order to better understand its clinical applications.
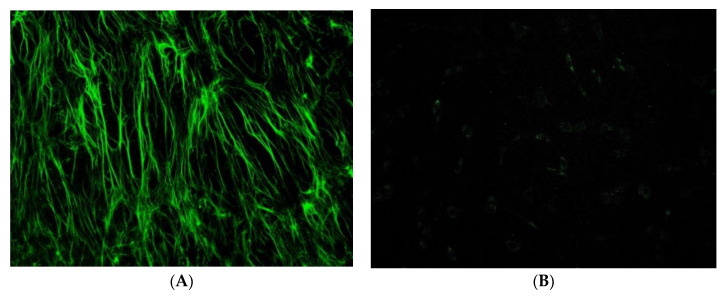
Figure 1.
(A) Type I collagen in tenocytes, harvested from degenerated human supraspinatus tendon, stimulated for 14 days with 1000 μg/mL (>500 KDa) of HA; (B) untreated cells.
Table 1.
ECM components of tendons.
| ECM Components | % |
|---|---|
| Collagen | 86% (type I: 98%) |
| Proteoglycan | 1–5% |
| Elastin | 2% |
| Decorin | <1% |
| Aggrecan | <1% |
| Other proteins | <1% |
2. HA Synthesis, Properties, and Degradation
HA is synthesised by a class of three integral membrane proteins named HA synthases (HAS1, HAS2 and HAS3). They synthetise HA by repeated addition of glucuronic acid and N-acetyl-D-glucosamine groups, and HA molecules are exported through transporters through the cell wall into the cells [15]. HAS1 and HAS2 proteins are moderately active and responsible for the synthesis of high molecular weight HA (HMW-HA) (>500 kDa), whereas HAS3, with high activity, synthetises low molecular weight HA (LMW-HA) ranging from 20 to 450 kDa (Table 2).
Table 2.
HA synthesis enzymes; HMW-HA, high molecular weight HA; MMW-HA, medium molecular weight HA; LMW-HA, low molecular weight HA; HAS, HA synthases.
| HA SYNTHASES | |
|---|---|
| HMW-HA and LMW-HA | HAS1 |
| HAS2 | |
| LMW-HA | HAS3 |
HA synthesis is reversed by a 4-methylumbelliferone (hymecromone, heparvit), a 7-hydroxy-4-methylcoumarin derivate [16]. The degradation of tendon collagen fibrils is mainly regulated by a complex system of metalloproteinases (MMP), which self-regulates by means of specific inhibitors (TIMP). The activity of this system is in relation to the levels of the mechanical structure of the tendon. Historically, in some in vitro experimental animal models, HA does not directly block the production of MMPs or increase the synthesis of its inhibitors by itself [17]. However, HA is likely able to regulate the activity of some MMPs through a downregulation mechanism [18]. In experimental studies in humans, HA is also able to block the action of fibronectin fragments (a molecule synthesised during tendon repair capable of activating MMP), inhibiting degradative processes and promoting repair and biosynthesis of the molecular constituents of the tendon fascicles [19]. HA acts on the ECM through specific and non-specific interactions with several receptors (Table 3).
Table 3.
HA interactions. RHAMM, receptor for hyaluronan-mediated motility; LYVE-1, lymphatic vessel endothelial hyaluronan receptor 1; TLR-4, Toll-like receptors 4; TSG6, TNF-stimulated gene 6; GHAP, glial hyaluronate-binding protein; LMW-HA, low molecular weight HA.
| HA and ECM Receptors Interactions | |
|---|---|
| Function | |
| CD44 Receptor for hyaluronan-mediated motility (RHAMM) |
Binding HA: anti-inflammatory, wound healing, antiangiogenic, immunosuppressive |
| Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) | Lymphatic transport of leukocytes |
| Toll-like receptor 4 (TLR-4) | Binding LMW-HA: pro-inflammatory |
| TNF-stimulated gene 6 (TSG-6) | Tissue protective and anti-inflammatory |
| Glial hyaluronate-binding protein (GHAP) | Reduces the spread of inflammatory cells in nerve tissue |
| Neurocan Versican Aggrecan |
Development, cell migration, maturation and differentiation, cell survival, and tissue homeostasis |
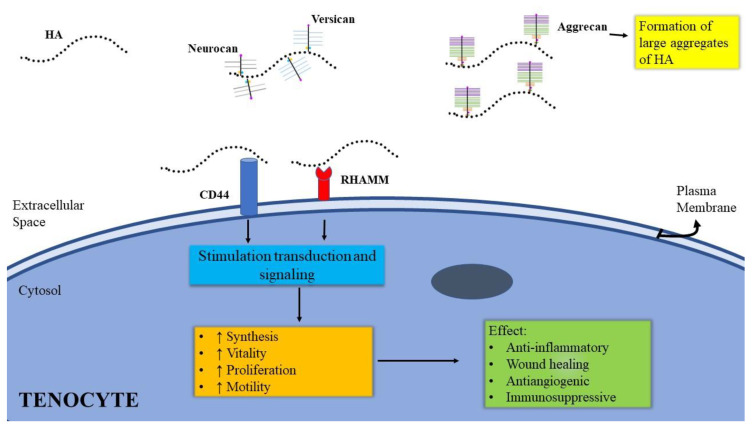
Some examples of receptors and molecules that show an interaction with HA are CD44, the receptor for hyaluronan-mediated motility (RHAMM), lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), Toll-like receptors 2-4 (TLR2-4), TNF-stimulated gene 6 (TSG6), glial hyaluronate-binding protein (GHAP), versican, aggrecan, and neurocan [20]. Versican, aggrecan, and neurocan are HA-bound chondroitin sulphate and keratan sulphate proteoglycans that can be found in the ECM of different tissues. Neurocan is mainly located in the nervous tissue; it is involved in cell adhesion and plays an important role in local axonal growth [21]. Aggrecan and versican also regulate cell growth and are involved in cell migration and haemostasis [22,23]. Aggrecan is mainly located in the cartilage, and it plays a key role in joint cartilage function and in the development of OA [23]. Versican is involved in skeletal development, cardiovascular, and nervous morphogenesis during embryogenesis [24]. Aggrecan and versican, linked to HA, allow the tendon to acquire a high resistance to compression and traction forces due to loading and mobilisation [25]. RHAMM is a protein located in the cytoplasm, nucleus, and plasma membrane [26]. The nuclear receptor for hyaluronan mediated motility (Nuclear-RHAMM) regulates cell motility and inflammation [27]. In the cytoplasm, RHAMM interacts directly or indirectly via binding proteins with actin filaments and microtubules, regulating cell polarity and cell migration [27,28,29]. Extracellular RHAMM acts on cell transformation and migration during the HA-dependent tissue damage and repair process [2]. Extracellularly, it acts with CD44 [27]. RHAMM is expressed in several healthy and neoplastic human tissues. Greater expression of the RHAMM protein in tumour tissues is associated with a higher degree of malignancy [30,31,32,33,34]. In healthy adult tissues, the RHAMM protein shows limited expression. RHAMM has been found in the thymus, lymph nodes, small intestine, colon, skin, and bone marrow [35]. LYVE-1 is a receptor found on endothelial cells of lymphatic tissue that binds to HA. This protein is involved in the lymphatic transport of leukocytes [36]. Toll-like receptors 2 and 4 (TLR-2 and TLR-4) are receptors expressed on chondrocytes and peritendinous cells [37,38]. It has been observed that the expression of TLR-4 is increased on the cell surface of chondrocytes in patients with rheumatoid arthritis, osteoarthritis, and tendinopathies [38,39]. LMW-HA binds to TLR-4 producing an inflammatory response in the joint, while HMW-HA reduces this stimulation hiding the active site of TLR-4 [40]. TSG-6 is a secreted protein with immunomodulating action for mesenchymal/stromal stem cells. The connection among HA and TSG-6 leads to anti-inflammatory processes and protective action on the tissues [41]. Glial hyaluronate-binding protein (GHAP) is an ECM protein of nervous tissues that reduces the spread of the inflammatory process [42]. CD44 regulation plays a fundamental role in lymphocyte cell stimulation and contributes to cell adhesion interaction necessary for cell growth [43]. There is also a marked increase in the expression of HAS2 at the onset of tendon growth, with expression falling substantially by 28 days. As reported by Schwartz et al., in the original tendon, HA was located immediately adjacent to tenocytes, while HA was found throughout the neotendon ECM. By 7 days, the majority of the neotendon matrix stained positive for collagen, and at 28 days, nearly the entire neotendon area was occupied by collagen. While it is difficult to relate differences in abundance of a specific MMP with changes within the ECM, the stark upregulation in MMP13, and to a lesser extent in MMP2, MMP3, TIMP1, and TIMP2, suggests changes in proteolytic activity, which, along with increases in collagen expression and hydroxyproline content, suggest an active-matrix remodelling process [44]. Mitsui et al. reported the effect of HA on the expression of mRNAs for proinflammatory cytokines (IL-6, IL-1β, and TNF-α), and COX-2/PGE2 production in IL-1 stimulated subacromial synovial fibroblasts from patients with rotator cuff disease [45]. Aggrecan monomers form large aggregates consequent to their HA binding in the presence of Hyaluronan and proteoglycan link protein 1 (HAPLN1), resulting in considerable water absorption, making HA responsible for flexibility in animal tissues. While the quality in terms of size of HA in animal tissue decreases with age, its quantity increases [46]. HA is also a significant component of skin [47]: exposure of skin to ultraviolet rays causes reddening, and the derma stops HA production and increases its degradation [48]. HA is additionally helpful for the growth of epithelial tissue cells, eosinophils, and macrophages, and is also essential in healing and scar formation [49]. HA is degraded by a group of several enzymes named hyaluronidases. HA degradation products such as oligosaccharides and LMW-HA (<500 KDa) show proangiogenic properties [50]. HA is degraded under different conditions such as pH, temperature, mechanical, free radical, ultrasonic, and enzymatic stresses [51]. HA can also be degraded with non-enzymatic reactions such as acidic, alkaline hydrolysis, and oxidant decomposition [52]. ICAM-1 is considered a metabolic cell surface receptor of HA. It is involved in the clearance of HA from body fluid and plasma. Binding to this receptor triggers a coordinated cascade of events that induces endocytic vesicles fusion with primary lysosomes and catalyses its digestion to monosaccharides, transmembrane transport, phosphorylation, and catalyst deacetylation [53]. ICAM-1 can also act as a cell adhesion molecule, and the binding with HA might contribute to regulate ICAM-1-mediated inflammatory activation [54]. Edsfeldt et al. reported the role of PXL01, a synthetic peptide derived from lactoferrin, which exhibits an inhibitory effect on adhesion by reducing secretion of inflammatory cytokines, promoting fibrinolysis and reducing infections [55]. Tendon healing develops in three stages: haemorrhage–inflammation, proliferation–scar formation, and remodelling. Sodium hyaluronate (NaHA) has a strong effect on angiogenesis, as shown by VEGF and type IV collagen expression. Type IV collagen gradually accumulates in the subendothelial space, appearing in the early stage of angiogenesis and in the late stages of the healing process. Repetitive administration of NaHA demonstrated endothelial cell proliferation with strongly increased expression of VEGF [56].
3. Effects on Tendons
Overuse and traumatic events are capable of inducing remodelling of the tendon matrix through a mechanism that involves the degradation of collagen, the phagocytosis of the fragments obtained by the tenocytes, and a subsequent compensatory fibrillogenesis that restores the integrity of the tendon structure [57]. To our knowledge, the first experimental observation regarding the effects of HA on biomechanics and tendon repair dates back to 1980 and was performed on monkeys. St Onge et al. showed an improvement in the range of motion of the treated tendon, suggesting a possible role of HA in primary tendon repair [58]. Since then, the effect of HA application has been evaluated in numerous animal models, from rodents to primates, both in vitro and in vivo, and, more recently, in clinical trials with a limited number of participants [59]. To date, multiple mechanisms have been identified in the pathophysiological process of tendinopathies. HA had a beneficial effect on both the repair site and synovial sheath by decreasing the peripheral inflammatory response and promoting contact healing via involvement of epitenon and endotenon cells in the repair process [60]. Cell damage at epitenon level is evident—namely, necrosis, cells engaged in a phagocytic activity, massive destruction, and involution of large areas of the matrix [61]. HA 1% and 0.5% are more effective than triamcinolone in the prevention of adhesion and do not interfere with tendon healing [62]. Rapid change or an abrupt increase in the loading forces acting on the tendons can cause repeated microtraumas [63], capable of weakening the tendon structure in the long term. On the contrary, tendon disuse produces pathological variations of the collagen pattern similar to those of overuse [64]. Recently, Chisari et al. highlighted that the prolonged state of low-grade inflammation usually found in chronic tendinopathy can be considered as a risk factor for a “failed healing response” following an acute tendon insult [65]. In both cases, the tendon loses its functional and structural integrity with disruption of the fibres and reduction in resistance to mechanical stress. The first therapeutic approach consists of conservative treatment through the intake of anti-inflammatory drugs, the application of topical agents, injection of corticosteroids, and functional rehabilitation exercises of the affected joint. Recently, some clinical trials highlighted the beneficial effect of HA on tendon viscoelasticity [66]. The superiority of HA injections over other conservative treatments has been reported [67]. Several studies investigated the link between the inhibition of fibroblastic proliferation induced by HA, the stabilisation of type II collagen, and the reduction in type III collagen concentration in the tendon [68,69]. Currently, the ability of HA to stimulate new indirect synthesis of type I collagen is still being discussed, while its ability to increase cell viability and proliferation has been proven (Figure 2) [20]. Furthermore, HA seems to inhibit the expression of intermediate factors that play a key role in inflammatory pathways (NF-kB) in a dose-dependent manner [70]. This anti-inflammatory action is added to the ability of exogenous HA to reduce fragmentation of endogenous HA, stimulating new synthesis [71]. Regarding the therapeutic protocol, one or two high molecular weight HA injections are described in isolated tendinopathies for suitable short-term outcome [11,63].
Figure 2.
Effects of HA on tenocytes.
4. Tissue Engineering and Tendon Healing
Regenerative medicine procedures are possible disease-modifying therapies for tendinopathies [72]. Several bioengineered systems have been developed using HA. Cells and growth factors alone cannot achieve optimal results in stimulating tenocytes differentiation without appropriate mechanical stimulation [73]. HA has been proposed as hydrogel or a 3D scaffold in combination with orthobiologics, such as tenoblasts, biomaterials, and growth factors to develop and support implantable tenocytes [74]. Scaffolds using HA are biodegradable, biocompatible, and bioabsorbable; HA plays a crucial role in cell signalling and cell growth, which can be attuned to the scaffold through its functional groups and functional domains [75,76]. HA hydrogel can be used as a carrier of drugs or biomaterials, combining it with epigallocatechin gallate (EGCG), a natural polyphenol with antioxidative and anti-inflammatory properties. This combination led to the mitigation of ischaemic and oxidative injury, and a suppression of the increased collagen III/I ratio in the tendinopathy group [72]. Recently, an esterified HA matrix (eHAM) has been used as a 3D scaffold to treat complicated lower extremity wounds with bone and tendon exposure with successful coverage and healing of exposed bone and tendons, because of the bridge role of eHAM providing a scaffold for fibroblast and endothelial cells and stimulating angiogenesis [77]. The use of a 3D scaffold has a double function: first, HA scaffold can carry biomaterials and growth factors into the tendinopathic tendon; then, it provides the mechanical stimulation necessary to tenocytic differentiation [78,79]. Ciardulli et al. investigated the effects of the HY-FIB 3D HA scaffold on human bone marrow mesenchymal stem cells (hBM-MSCs) in static and dynamic scenarios, showing a significant increase in tenogenic differentiation and anti-inflammatory cytokines production in dynamic conditions [80]. HA has been used also as a resorbable, suturable, and biocompatible mesh in the treatment of neglected Achilles tendon rupture. In 2014, Esenyel et al. applied Hyalonect as a reinforcement of a turndown gastrocnemius–soleus fascial flap, reporting an excellent or good return of function after surgery [81].
5. Tendinopathies and HA Clinical Applications
Tendinopathies are increasingly common, negatively impacting patients’ quality of life [82]. Several intrinsic and extrinsic risk factors can contribute to the pathogenesis of tendinopathies, including mechanical overload, poor vascularity, age, gender, and genetic, endocrine, and metabolic factors [83,84,85]. Tendinopathic tendons show diffuse structural changes: increased tenocyte apoptosis, disruption of collagen fibres with decreased collagen type I production, disorderly increased type III collagen production, and ineffective neoangiogenesis [83]. Patients report pain at the tendon site which increases with exercise and everyday life activities and limits sports performances [86]. Other clinical signs are local tendon tenderness, limited range of motion, and swelling [87]. The management of tendinopathy is debated, and there is still no consensus about a gold standard treatment. However, HA could have several disease-modifying effects, leading to increased tenocytes regeneration, restoration of collagen type I/type III ratio, reduced apoptosis, and angiogenetic changes [18,80,81].
6. Rotator Cuff Tendinopathy and HA
Rotator cuff tendinopathy is the most common cause of shoulder pain, with an increasing frequency that varies from 5% to 40%, especially affecting the supraspinatus tendon [88]. Recently, several authors compared HA injections with different management options. Meloni et al. evaluated the effects of ultrasound-guided HA injections in supraspinatus tendinopathy HA induced symptoms and disability improvement, compared with placebo, up to 9 months follow-up [89]. Merolla et al. compared ultrasound-guided subacromial injections of HA with physical therapy. Both treatments lead to pain relief and clinical improvement in the short term, but the HA group maintained a significant improvement at 12 weeks of follow-up. Frizziero et al. in a prospective study investigated the effects of low molecular weight HA injections in the subacromial space compared with extracorporeal shockwaves therapy in patients with non-calcific supraspinatus tendinopathy. This study confirmed Merolla et al.’s findings, showing that one low molecular weight HA injection a week for three weeks produced clinical relief at the end of the treatment and for up to 3 months. No significant difference was found between the HA injections and extracorporeal shockwave therapy in terms of safety and efficacy [67]. Comparing physical therapy alone or combined with HA injections in the management of supraspinatus tendinopathy, Flores et al. found that patients treated with physical therapy and HA returned significantly earlier to work and needed fewer rehabilitation sessions [90].
7. Patellar Tendinopathy and HA
Patellar tendinopathy is common in sports in which athletes jump, as in volleyball, basketball, or triple jump [91]. Kumai et al. evaluated the effects of a single high molecular weight HA injection in patients with patellar tendinopathy, finding improvement in pain and visual analogue scale (VAS) values at short-term follow-up (one week) [13]. Muneta et al. treated 50 young athletes with two high molecular weight HA injections without ultrasound (US) guidance. This was effective, safe, and repeatable [92]. Fogli et al. showed that a cycle of one HA injection a week for three weeks was effective and safe in patellar tendinopathy, with a significant decrease in pain, mean VAS values, and improvement of US appearance [93]. Kaux et al. compared US-guided injections of platelet-rich plasma (PRP) versus two HA injections, reporting that both treatments could be effective. The PRP group showed significant improvement in quadriceps strength, while HA had a greater impact on the improvement of symptoms [94]. Recently, Frizziero et al. showed good results after three medium molecular weight US-guided HA injections. This study reported pain relief and improvement of the Victorian Institute of Sport Assessment for the patellar tendon (VISA-P) values at 90 days follow-up, with a decrease in vascularisation and tendon thickness at US and Power Doppler analysis [10].
8. Achilles Tendinopathy and HA
Achilles tendinopathy affects mainly athletes, especially runners, but also non-athletes [95]. The therapeutic use of HA in Achilles tendinopathy has been recently described (Figure 3A,B). Lynen et al. compared the effects of two HA peritendinous injections with shockwave therapy in patients with mid-portion Achilles tendinopathy. At 6 months, patients treated with HA injections reported better outcomes, with greater symptom improvement and restored function [14]. Similarly, Fogli et al. and Frizziero et al. showed that three US-guided medium molecular weight HA injections induce a clinically relevant increase in VISA-A values, pain relief, and US parameters improvement [10,93]. Recently, Gervasi et al. investigated the clinical, viscoelastometric, and biochemical effects of three US-guided medium molecular weight HA injections in runners with unilateral Achilles tendinopathy. Indeed, patients reported improvement in clinical assessment, decreased pain and stiffness of the tendon, and reduction in the viscoelastometric and functional asymmetry between the affected tendon and the healthy limb [96,97].
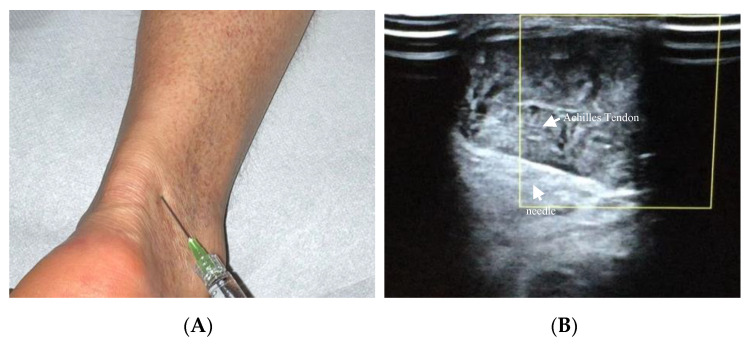
Figure 3.
(A) Injection of HA in a dorsolateral approach of Achilles tendon; (B) US visualisation (5–12 MHz linear probe and PRF set at 0.5 kHz) of the needle (22-gauge) introduced at a 30-degree angle in the mesotendon, with the probe in a transverse plane.
9. Epicondylitis and HA
Lateral epicondylitis is a common cause of chronic elbow pain and affects 1% to 3% of the general population per year [98]. The tendon structures most affected are the insertions of the extensor of the forearm, located on the lateral side of the elbow [98]. Petrella et al. compared outcomes in patients who received HA injections versus a control group, who received an injection of 1.2 mL of a saline placebo, finding significantly greater improvement in VAS pain at rest and after grip testing up to 1-year follow-up [99]. Khan et al. reported the efficacy of a single HA injection in the management of moderate epicondylitis (VAS pain score < 7), while it was not effective in severe lateral epicondylitis [100]. In a prospective randomised trial, Tosun et al. compared a combined HA-chondroitin sulphate injection versus a corticosteroid injection, showing an equal reduction in pain and improvement of function in the short term, while HA resulted in better outcomes at long-term follow-up [101].
10. Conclusions
Anti-inflammatory, wound healing, antiangiogenic, and immunosuppressive effects of HA have been reported in in vitro and in vivo studies. These findings encourage the clinical application of HA in tendinopathies such as rotator cuff, epicondylitis, Achilles, and patellar tendinopathy. However, there are still questions to be answered and issues to be addressed. First of all, many aspects of HA metabolism, receptor clustering, and affinity still need to be explored to understand the different biological actions that hyaluronan has in the inflammatory process also in relation to molecular weight. Understanding these mechanisms could provide opportunities to extend and improve hyaluronan pharmaceutical, biomedical, cosmetics, and food supplements applications, obtaining more targeted effects.
Author Contributions
Conceptualisation, F.O. and A.F.; methodology, F.O. and A.C.B.; investigation, E.M. and G.A.; data curation, E.M. and G.A.; writing—original draft preparation, E.M. and G.A.; writing—review and editing, N.M. and F.O.; visualisation, E.M. and G.A.; supervision, N.M. and F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balazs E.A., Laurent T.C., Jeanloz R.W. Nomenclature of hyaluronic acid. Biochem. J. 1986;235:903. doi: 10.1042/bj2350903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turley E.A., Noble P.W., Bourguignon L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 3.Toole B.P., Wight T.N., Tammi M.I. Hyaluronan-cell interactions in cancer and vascular disease. J. Biol. Chem. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- 4.Hascall V.C., Majors A.K., De La Motte C.A., Evanko S.P., Wang A., Drazba J.A., Strong S.A., Wight T.N. Intracellular hyaluronan: A new frontier for inflammation? Biochim. Biophys. Acta. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Kogan G., Soltes L., Stern R., Gemeiner P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007;29:17–25. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 6.Sawaguchi N., Majima T., Iwasaki N., Funakoshi T., Shimode K., Onodera T., Minami A. Extracellular matrix modulates expression of cell-surface proteoglycan genes in fibroblasts. Connect Tissue Res. 2006;47:141–148. doi: 10.1080/03008200600685459. [DOI] [PubMed] [Google Scholar]
- 7.Sikes K.J., Renner K., Li J., Grande-Allen K.J., Connell J.P., Cali V., Midura R.J., Sandy J.D., Plaas A., Wang V.M. Knockout of hyaluronan synthase 1, but not 3, impairs formation of the retrocalcaneal bursa. J. Orthop. Res. 2018;36:2622–2632. doi: 10.1002/jor.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess G.P., Cappiello W.L., Poole R.M., Hunter S.C. Prevention and Treatment of Overuse Tendon Injuries. Sports Med. 1989;8:371–384. doi: 10.2165/00007256-198908060-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bolon B. Mini-Review: Toxic Tendinopathy. Toxicol. Pathol. 2017;45:834–837. doi: 10.1177/0192623317711614. [DOI] [PubMed] [Google Scholar]
- 10.Frizziero A., Oliva F., Vittadini F., Vetrano M., Bernetti A., Giordan N., Vulpiani M., Santilli V., Masiero S., Maffulli N., et al. Efficacy of ultrasound-guided hyaluronic acid injections in achilles and patellar tendinopathies:a prospective multicentric clinical trial. Muscles Ligaments Tendon J. 2019;09:305. doi: 10.32098/mltj.03.2019.01. [DOI] [Google Scholar]
- 11.Kaux J.F., Samson A., Crielaard J.M. Hyaluronic acid and tendon lesions. Muscles Ligaments Tendons J. 2015;5:264–269. doi: 10.32098/mltj.04.2015.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Merolla G., Bianchi P., Porcellini G. Ultrasound-guided subacromial injections of sodium hyaluronate for the management of rotator cuff tendinopathy: A prospective comparative study with rehabilitation therapy. Musculoskelet. Surg. 2013;97((Suppl. S1)):49–56. doi: 10.1007/s12306-013-0259-y. [DOI] [PubMed] [Google Scholar]
- 13.Kumai T., Muneta T., Tsuchiya A., Shiraishi M., Ishizaki Y., Sugimoto K., Samoto N., Isomoto S., Tanaka Y., Takakura Y. The short-term effect after a single injection of high-molecular-weight hyaluronic acid in patients with enthesopathies (lateral epicondylitis, patellar tendinopathy, insertional Achilles tendinopathy, and plantar fasciitis): A preliminary study. J. Orthop. Sci. 2014;19:603–611. doi: 10.1007/s00776-014-0579-2. [DOI] [PubMed] [Google Scholar]
- 14.Lynen N., De Vroey T., Spiegel I., Van Ongeval F., Hendrickx N.J., Stassijns G. Comparison of Peritendinous Hyaluronan Injections Versus Extracorporeal Shock Wave Therapy in the Treatment of Painful Achilles’ Tendinopathy: A Randomized Clinical Efficacy and Safety Study. Arch. Phys. Med. Rehabil. 2017;98:64–71. doi: 10.1016/j.apmr.2016.08.470. [DOI] [PubMed] [Google Scholar]
- 15.Schulz T., Schumacher U., Prehm P. Hyaluronan export by the ABC transporter MRP5 and its modulation by intracellular cGMP. J. Biol. Chem. 2007;282:20999–21004. doi: 10.1074/jbc.M700915200. [DOI] [PubMed] [Google Scholar]
- 16.Vasvani S., Kulkarni P., Rawtani D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020;151:1012–1029. doi: 10.1016/j.ijbiomac.2019.11.066. [DOI] [PubMed] [Google Scholar]
- 17.Han F., Ishiguro N., Ito T., Sakai T., Iwata H. Effects of sodium hyaluronate on experimental osteoarthritis in rabbit knee joints. Nagoya J. Med. Sci. 1999;62:115–126. [PubMed] [Google Scholar]
- 18.Takahashi K., Goomer R.S., Harwood F., Kubo T., Hirasawa Y., Amiel D. The effects of hyaluronan on matrix metalloproteinase-3 (MMP-3), interleukin-1beta(IL-1beta), and tissue inhibitor of metalloproteinase-1 (TIMP-1) gene expression during the development of osteoarthritis. Osteoarthr. Cartil. 1999;7:182–190. doi: 10.1053/joca.1998.0207. [DOI] [PubMed] [Google Scholar]
- 19.Kang Y., Eger W., Koepp H., Williams J.M., Kuettner K.E., Homandberg G.A. Hyaluronan suppresses fibronectin fragment-mediated damage to human cartilage explant cultures by enhancing proteoglycan synthesis. J. Orthop. Res. 1999;17:858–869. doi: 10.1002/jor.1100170611. [DOI] [PubMed] [Google Scholar]
- 20.Osti L., Berardocco M., di Giacomo V., Di Bernardo G., Oliva F., Berardi A.C. Hyaluronic acid increases tendon derived cell viability and collagen type I expression in vitro: Comparative study of four different Hyaluronic acid preparations by molecular weight. BMC Musculoskelet. Disord. 2015;16:284. doi: 10.1186/s12891-015-0735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grumet M., Friedlander D.R., Sakurai T. Functions of brain chondroitin sulfate proteoglycans during developments: Interactions with adhesion molecules. Perspect. Dev. Neurobiol. 1996;3:319–330. [PubMed] [Google Scholar]
- 22.Wight T.N. Provisional matrix: A role for versican and hyaluronan. Matrix Biol. J. Int. Soc. Matrix Biol. 2017;60-61:38–56. doi: 10.1016/j.matbio.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roughley P.J., Mort J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014;1:8. doi: 10.1186/s40634-014-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadas A., Asimakopoulos F. Versican in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020;1272:55–72. doi: 10.1007/978-3-030-48457-6_4. [DOI] [PubMed] [Google Scholar]
- 25.Yoon J.H., Halper J. Tendon proteoglycans: Biochemistry and function. J. Musculoskelet. Neuronal Interact. 2005;5:22–34. [PubMed] [Google Scholar]
- 26.Maxwell C.A., McCarthy J., Turley E. Cell-surface and mitotic-spindle RHAMM: Moonlighting or dual oncogenic functions? J. Cell Sci. 2008;121:925–932. doi: 10.1242/jcs.022038. [DOI] [PubMed] [Google Scholar]
- 27.Tolg C., McCarthy J.B., Yazdani A., Turley E.A. Hyaluronan and RHAMM in wound repair and the “cancerization” of stromal tissues. BioMed Res. Int. 2014;2014:103923. doi: 10.1155/2014/103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Assmann V., Jenkinson D., Marshall J.F., Hart I.R. The intracellular hyaluronan receptor RHAMM/IHABP interacts with microtubules and actin filaments. Pt 22J. Cell Sci. 1999;112:3943–3954. doi: 10.1242/jcs.112.22.3943. [DOI] [PubMed] [Google Scholar]
- 29.Silverman-Gavrila R., Silverman-Gavrila L., Hou G., Zhang M., Charlton M., Bendeck M.P. Rear polarization of the microtubule-organizing center in neointimal smooth muscle cells depends on PKCα, ARPC5, and RHAMM. Am. J. Pathol. 2011;178:895–910. doi: 10.1016/j.ajpath.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du Y.C., Chou C.K., Klimstra D.S., Varmus H. Receptor for hyaluronan-mediated motility isoform B promotes liver metastasis in a mouse model of multistep tumorigenesis and a tail vein assay for metastasis. Proc. Natl. Acad. Sci. USA. 2011;108:16753–16758. doi: 10.1073/pnas.1114022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D., Narula N., Azzopardi S., Smith R.S., Nasar A., Altorki N.K., Mittal V., Somwar R., Stiles B.M., Du Y.N. Expression of the receptor for hyaluronic acid mediated motility (RHAMM) is associated with poor prognosis and metastasis in non-small cell lung carcinoma. Oncotarget. 2016;7:39957–39969. doi: 10.18632/oncotarget.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Augustin F., Fiegl M., Schmid T., Pomme G., Sterlacci W., Tzankov A. Receptor for hyaluronic acid-mediated motility (RHAMM, CD168) expression is prognostically important in both nodal negative and nodal positive large cell lung cancer. J. Clin. Pathol. 2015;68:368–373. doi: 10.1136/jclinpath-2014-202819. [DOI] [PubMed] [Google Scholar]
- 33.Ishigami S., Ueno S., Nishizono Y., Matsumoto M., Kurahara H., Arigami T., Uchikado Y., Setoyama T., Arima H., Yoshiaki K., et al. Prognostic impact of CD168 expression in gastric cancer. BMC cancer. 2011;11:106. doi: 10.1186/1471-2407-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koelzer V.H., Huber B., Mele V., Iezzi G., Trippel M., Karamitopoulou E., Zlobec I., Lugli A. Expression of the hyaluronan-mediated motility receptor RHAMM in tumor budding cells identifies aggressive colorectal cancers. Hum. Pathol. 2015;46:1573–1581. doi: 10.1016/j.humpath.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y.T., Chen Z., Du Y.N. Immunohistochemical analysis of RHAMM expression in normal and neoplastic human tissues: A cell cycle protein with distinctive expression in mitotic cells and testicular germ cells. Oncotarget. 2018;9:20941–20952. doi: 10.18632/oncotarget.24939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson D.G. Leucocyte Trafficking via the Lymphatic Vasculature- Mechanisms and Consequences. Front. Immunol. 2019;10:471. doi: 10.3389/fimmu.2019.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Mos M., Joosten L.A., Oppers-Walgreen B., van Schie J.T., Jahr H., van Osch G.J., Verhaar J.A. Tendon degeneration is not mediated by regulation of Toll-like receptors 2 and 4 in human tenocytes. J. Orthop. Res. 2009;27:1043–1047. doi: 10.1002/jor.20834. [DOI] [PubMed] [Google Scholar]
- 38.Su S.L., Tsai C.D., Lee C.H., Salter D.M., Lee H.S. Expression and regulation of Toll-like receptor 2 by IL-1beta and fibronectin fragments in human articular chondrocytes. Osteoarthr. Cartil. 2005;13:879–886. doi: 10.1016/j.joca.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Kim H.A., Cho M.L., Choi H.Y., Yoon C.S., Jhun J.Y., Oh H.J., Kim H.Y. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:2152–2163. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- 40.Campo G.M., Avenoso A., D’Ascola A., Prestipino V., Scuruchi M., Nastasi G., Calatroni A., Campo S. Hyaluronan differently modulates TLR-4 and the inflammatory response in mouse chondrocytes. BioFactors. 2012;38:69–76. doi: 10.1002/biof.202. [DOI] [PubMed] [Google Scholar]
- 41.Day A.J., Milner C.M. TSG-6: A multifunctional protein with anti-inflammatory and tissue-protective properties. Matrix Biol. J. Int. Soc. Matrix Biol. 2019;78-79:60–83. doi: 10.1016/j.matbio.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Bignami A., Hosley M., Dahl D. Hyaluronic acid and hyaluronic acid-binding proteins in brain extracellular matrix. Anat. Embryol. 1993;188:419–433. doi: 10.1007/BF00190136. [DOI] [PubMed] [Google Scholar]
- 43.Chang E.J., Kim H.J., Ha J., Kim H.J., Ryu J., Park K.H., Kim U.H., Lee Z.H., Kim H.M., Fisher D.E., et al. Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J. Cell Sci. 2007;120:166–176. doi: 10.1242/jcs.03310. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz A.J., Sarver D.C., Sugg K.B., Dzierzawski J.T., Gumucio J.P., Mendias C.L. p38 MAPK signaling in postnatal tendon growth and remodeling. PLoS ONE. 2015;10:e0120044. doi: 10.1371/journal.pone.0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitsui Y., Gotoh M., Nakama K., Yamada T., Higuchi F., Nagata K. Hyaluronic acid inhibits mRNA expression of proinflammatory cytokines and cyclooxygenase-2/prostaglandin E(2) production via CD44 in interleukin-1-stimulated subacromial synovial fibroblasts from patients with rotator cuff disease. J. Orthop. Res. 2008;26:1032–1037. doi: 10.1002/jor.20558. [DOI] [PubMed] [Google Scholar]
- 46.Holmes M.W., Bayliss M.T., Muir H. Hyaluronic acid in human articular cartilage. Age-related changes in content and size. Biochem. J. 1988;250:435–441. doi: 10.1042/bj2500435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stecco C., Stern R., Porzionato A., Macchi V., Masiero S., Stecco A., De Caro R. Hyaluronan within fascia in the etiology of myofascial pain. Surg. Radiol. Anat. 2011;33:891–896. doi: 10.1007/s00276-011-0876-9. [DOI] [PubMed] [Google Scholar]
- 48.Huskisson E.C., Donnelly S. Hyaluronic acid in the treatment of osteoarthritis of the knee. Rheumatology. 1999;38:602–607. doi: 10.1093/rheumatology/38.7.602. [DOI] [PubMed] [Google Scholar]
- 49.McKee C.M., Penno M.B., Cowman M., Burdick M.D., Strieter R.M., Bao C., Noble P.W. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Investig. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tesar B.M., Jiang D., Liang J., Palmer S.M., Noble P.W., Goldstein D.R. The role of hyaluronan degradation products as innate alloimmune agonists. Am. J. Transpl. 2006;6:2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 51.Stern R., Kogan G., Jedrzejas M., Soltés L. The many ways to cleave hyaluronan. Biotechnol. Adv. 2007;25:537–557. doi: 10.1016/j.biotechadv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Aruffo A., Stamenkovic I., Melnick M., Underhill C.B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-A. [DOI] [PubMed] [Google Scholar]
- 53.Stern R., Maibach H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin. Derm. 2008;26:106–122. doi: 10.1016/j.clindermatol.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Hubbard A.K., Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol. Med. 2000;28:1379–1386. doi: 10.1016/S0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- 55.Edsfeldt S., Holm B., Mahlapuu M., Reno C., Hart D.A., Wiig M. PXL01 in sodium hyaluronate results in increased PRG4 expression: A potential mechanism for anti-adhesion. Ups J. Med. Sci. 2017;122:28–34. doi: 10.1080/03009734.2016.1230157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halici M., Karaoglu S., Canoz O., Kabak S., Baktir A. Sodium hyaluronate regulating angiogenesis during Achilles tendon healing. Knee Surg. Sports Traumatol. Arthrosc. 2004;12:562–567. doi: 10.1007/s00167-004-0536-2. [DOI] [PubMed] [Google Scholar]
- 57.Slack C., Bradley G., Beaumont B., Poole A., Flint M. Changes in the morphology and synthetic activity of cultured rat tail tendon. Cell Tissue Res. 1986;245:359–368. doi: 10.1007/BF00213943. [DOI] [PubMed] [Google Scholar]
- 58.St Onge R., Weiss C., Denlinger J.L., Balazs E.A. A preliminary assessment of Na-hyaluronate injection into “no man’s land” for primary flexor tendon repair. Clin. Orthop. Relat. Res. 1980;146:269–275. [PubMed] [Google Scholar]
- 59.Gopinath C., Nathar T.J., Ghosh A., Hickstein D.D., Nelson E.J.R. Contemporary Animal Models For Human Gene Therapy Applications. Curr. Gene Ther. 2015;15:531–540. doi: 10.2174/1566523215666150929110424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amiel D., Ishizue K., Billings E., Jr., Wiig M., Vande Berg J., Akeson W.H., Gelberman R. Hyaluronan in flexor tendon repair. J. Hand Surg. Am. 1989;14:837–843. doi: 10.1016/S0363-5023(89)80085-1. [DOI] [PubMed] [Google Scholar]
- 61.Sakata T., Scudamore R.A., Cooke T.D. Antigen-induced tenosynovitis in hypersensitized rabbits: A model for rheumatoid tenosynovitis. Rheumatol. Int. 1988;8:47–53. doi: 10.1007/BF00271834. [DOI] [PubMed] [Google Scholar]
- 62.Yuzawa K. Experimental studies on the healing and restoration of gliding function of the injured digital flexor tendon. Part 9: The use of drugs to prevent adhesion formation of the injured tendon. Nihon Seikeigeka Gakkai Zasshi. 1985;59:1107–1118. [PubMed] [Google Scholar]
- 63.Tallon C., Maffulli N., Ewen S.W. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med. Sci. Sports Exerc. 2001;33:1983–1990. doi: 10.1097/00005768-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Maganaris C.N., Narici M.V., Almekinders L.C., Maffulli N. Biomechanics and pathophysiology of overuse tendon injuries: Ideas on insertional tendinopathy. Sports Med. 2004;34:1005–1017. doi: 10.2165/00007256-200434140-00005. [DOI] [PubMed] [Google Scholar]
- 65.Chisari E., Rehak L., Khan W.S., Maffulli N. Tendon healing in presence of chronic low-level inflammation: A systematic review. Br. Med. Bull. 2019;132:97–116. doi: 10.1093/bmb/ldz035. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura H., Gotoh M., Kanazawa T., Ohta K., Nakamura K., Honda H., Ohzono H., Shimokobe H., Mitsui Y., Shirachi I., et al. Effects of corticosteroids and hyaluronic acid on torn rotator cuff tendons in vitro and in rats. J. Orthop. Res. 2015;33:1523–1530. doi: 10.1002/jor.22921. [DOI] [PubMed] [Google Scholar]
- 67.Frizziero A., Vittadini F., Barazzuol M., Gasparre G., Finotti P., Meneghini A., Maffulli N., Masiero S. Extracorporeal shockwaves therapy versus hyaluronic acid injection for the treatment of painful non-calcific rotator cuff tendinopathies: Preliminary results. J. Sports Med. Phys. Fit. 2017;57:1162–1168. doi: 10.23736/S0022-4707.16.06408-2. [DOI] [PubMed] [Google Scholar]
- 68.Wiig M., Abrahamsson S.O., Lundborg G. Effects of hyaluronan on cell proliferation and collagen synthesis: A study of rabbit flexor tendons in vitro. J. Hand Surg. Am. 1996;21:599–604. doi: 10.1016/S0363-5023(96)80010-4. [DOI] [PubMed] [Google Scholar]
- 69.Dean B.J., Franklin S.L., Carr A.J. A systematic review of the histological and molecular changes in rotator cuff disease. Bone Jt. Res. 2012;1:158–166. doi: 10.1302/2046-3758.17.2000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Neumann A., Schinzel R., Palm D., Riederer P., Munch G. High molecular weight hyaluronic acid inhibits advanced glycation endproduct-induced NF-kappaB activation and cytokine expression. FEBS Lett. 1999;453:283–287. doi: 10.1016/S0014-5793(99)00731-0. [DOI] [PubMed] [Google Scholar]
- 71.Ozgenel G.Y., Etoz A. Effects of repetitive injections of hyaluronic acid on peritendinous adhesions after flexor tendon repair: A preliminary randomized, placebo-controlled clinical trial. Ulus Travma Acil. Cerrahi Derg. 2012;18:11–17. doi: 10.5505/tjtes.2012.95530. [DOI] [PubMed] [Google Scholar]
- 72.Hsiao M.Y., Lin A.C., Liao W.H., Wang T.G., Hsu C.H., Chen W.S., Lin F.H. Drug-loaded hyaluronic acid hydrogel as a sustained-release regimen with dual effects in early intervention of tendinopathy. Sci. Rep. 2019;9:4784. doi: 10.1038/s41598-019-41410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doroski D.M., Levenston M.E., Temenoff J.S. Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels. Tissue Eng. Part A. 2010;16:3457–3466. doi: 10.1089/ten.tea.2010.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pereira H., Sousa D.A., Cunha A., Andrade R., Espregueira-Mendes J., Oliveira J.M., Reis R.L. Hyaluronic Acid. Adv. Exp. Med. Biol. 2018;1059:137–153. doi: 10.1007/978-3-319-76735-2_6. [DOI] [PubMed] [Google Scholar]
- 75.Baier Leach J., Bivens K.A., Patrick C.W., Jr., Schmidt C.E. Photocrosslinked hyaluronic acid hydrogels: Natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng. 2003;82:578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- 76.Tiwari S., Bahadur P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019;121:556–571. doi: 10.1016/j.ijbiomac.2018.10.049. [DOI] [PubMed] [Google Scholar]
- 77.Kozusko S.D., Hassouba M., Hill D.M., Liu X., Dadireddy K., Velamuri S.R. Esterified Hyaluronic Acid Matrix in Lower Extremity Reconstruction With Exposed Tendon and Bone: A Retrospective Review. J. Burn. Care Res. 2020;41:828–834. doi: 10.1093/jbcr/iraa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cipollaro L., Ciardulli M.C., Della Porta G., Peretti G.M., Maffulli N. Biomechanical issues of tissue-engineered constructs for articular cartilage regeneration: In vitro and in vivo approaches. Br. Med. Bull. 2019;132:53–80. doi: 10.1093/bmb/ldz034. [DOI] [PubMed] [Google Scholar]
- 79.d’Angelo M., Benedetti E., Tupone M.G., Catanesi M., Castelli V., Antonosante A., Cimini A. The Role of Stiffness in Cell Reprogramming: A Potential Role for Biomaterials in Inducing Tissue Regeneration. Cells. 2019;8:1036. doi: 10.3390/cells8091036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ciardulli M.C., Marino L., Lovecchio J., Giordano E., Forsyth N.R., Selleri C., Maffulli N., Porta G.D. Tendon and Cytokine Marker Expression by Human Bone Marrow Mesenchymal Stem Cells in a Hyaluronate/Poly-Lactic-Co-Glycolic Acid (PLGA)/Fibrin Three-Dimensional (3D) Scaffold. Cells. 2020;9:1268. doi: 10.3390/cells9051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Esenyel C.Z., Tekin C., Cakar M., Bayraktar K., Saygili S., Esenyel M., Tekin Z.N. Surgical treatment of the neglected achilles tendon rupture with Hyalonect. J. Am. Podiatr. Med. Assoc. 2014;104:434–443. doi: 10.7547/0003-0538-104.5.434. [DOI] [PubMed] [Google Scholar]
- 82.Maffulli N., Longo U.G., Loppini M., Denaro V. Current treatment options for tendinopathy. Expert Opin. Pharm. 2010;11:2177–2186. doi: 10.1517/14656566.2010.495715. [DOI] [PubMed] [Google Scholar]
- 83.Del Buono A., Battery L., Denaro V., Maccauro G., Maffulli N. Tendinopathy and inflammation: Some truths. Int. J. Immunopathol. Pharm. 2011;24:45–50. doi: 10.1177/03946320110241S209. [DOI] [PubMed] [Google Scholar]
- 84.Frizziero A., Salamanna F., Della Bella E., Vittadini F., Gasparre G., Nicoli Aldini N., Masiero S., Fini M. The Role of Detraining in Tendon Mechanobiology. Front. Aging Neurosci. 2016;8:43. doi: 10.3389/fnagi.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oliva F., Piccirilli E., Berardi A.C., Frizziero A., Tarantino U., Maffulli N. Hormones and tendinopathies: The current evidence. Br. Med. Bull. 2016;117:39–58. doi: 10.1093/bmb/ldv054. [DOI] [PubMed] [Google Scholar]
- 86.Ackermann P.W., Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193–201. doi: 10.1177/1941738112440957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abate M., Silbernagel K.G., Siljeholm C., Di Iorio A., De Amicis D., Salini V., Werner S., Paganelli R. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res. Ther. 2009;11:235. doi: 10.1186/ar2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamamoto A., Takagishi K., Osawa T., Yanagawa T., Nakajima D., Shitara H., Kobayashi T. Prevalence and risk factors of a rotator cuff tear in the general population. J. Shoulder. Elb. Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 89.Meloni F., Milia F., Cavazzuti M., Doria C., Lisai P., Profili S., Meloni G.B. Clinical evaluation of sodium hyaluronate in the treatment of patients with sopraspinatus tendinosis under echographic guide: Experimental study of periarticular injections. Eur. J. Radiol. 2008;68:170–173. doi: 10.1016/j.ejrad.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 90.Flores C., Balius R., Alvarez G., Buil M.A., Varela L., Cano C., Casariego J. Efficacy and Tolerability of Peritendinous Hyaluronic Acid in Patients with Supraspinatus Tendinopathy: A Multicenter, Randomized, Controlled Trial. Sports Med. Open. 2017;3:22. doi: 10.1186/s40798-017-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaux J.F., Forthomme B., Goff C.L., Crielaard J.M., Croisier J.L. Current opinions on tendinopathy. J. Sports Sci. Med. 2011;10:238–253. [PMC free article] [PubMed] [Google Scholar]
- 92.Muneta T., Koga H., Ju Y.J., Mochizuki T., Sekiya I. Hyaluronan injection therapy for athletic patients with patellar tendinopathy. J. Orthop. Sci. 2012;17:425–431. doi: 10.1007/s00776-012-0225-9. [DOI] [PubMed] [Google Scholar]
- 93.Fogli M., Giordan N., Mazzoni G. Efficacy and safety of hyaluronic acid (500–730 kDa) Ultrasound-guided injections on painful tendinopathies: A prospective, open label, clinical study. Muscles Ligaments Tendons J. 2017;7:388–395. doi: 10.32098/mltj.02.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaux J., Bornheim S., Dardenne N., Deroisy R., Samson A.J., Roberjot M., Croisier J.L. Comparison between platelet-rich plasma injections and hyaluronic acid injections in the treatment of patellar tendinopathies: A randomized trial. Muscles Ligaments Tendons J. 2019;09:156. doi: 10.32098/mltj.03.2019.03. [DOI] [Google Scholar]
- 95.Vora A.M., Myerson M.S., Oliva F., Maffulli N. Tendinopathy of the main body of the Achilles tendon. Foot Ankle Clin. 2005;10:293–308. doi: 10.1016/j.fcl.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 96.Gervasi M., Barbieri E., Capparucci I., Annibalini G., Sisti D., Amatori S., Carrabs V., Valli G., Donati Zeppa S., Rocchi M.B.L., et al. Treatment of Achilles Tendinopathy in Recreational Runners with Peritendinous Hyaluronic Acid Injections: A Viscoelastometric, Functional, and Biochemical Pilot Study. J. Clin. Med. 2021;10:1397. doi: 10.3390/jcm10071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coari G.P.F., Tajana G. Fisiopatologia tendinea & Acido Ialuronico. Med. Sport. 2001;1:79. [Google Scholar]
- 98.Verhaar J.A. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int. Orthop. 1994;18:263–267. doi: 10.1007/BF00180221. [DOI] [PubMed] [Google Scholar]
- 99.Petrella R.J., Cogliano A., Decaria J., Mohamed N., Lee R. Management of Tennis Elbow with sodium hyaluronate periarticular injections. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2010;2:4. doi: 10.1186/1758-2555-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan I.U., Awan A.S., Khan A.S., Marwat I., Meraj M. Efficacy Of A Single-Injection Sodium Hyaluronate Treatment In Lateral Epicondylitis. J. Ayub Med. Coll. Abbottabad. 2018;30:85–89. [PubMed] [Google Scholar]
- 101.Tosun H.B., Gumustas S., Agir I., Uludag A., Serbest S., Pepele D., Ertem K. Comparison of the effects of sodium hyaluronate-chondroitin sulphate and corticosteroid in the treatment of lateral epicondylitis: A prospective randomized trial. J. Orthop. Sci. 2015;20:837–843. doi: 10.1007/s00776-015-0747-z. [DOI] [PubMed] [Google Scholar]