Abstract
Type 2 diabetes mellitus (T2DM) is a noteworthy worldwide public health problem. It represents a complex metabolic disorder, mainly characterized as hyperglycemia and lipid dysfunction. The gut microbiota dysbiosis has been proposed to play a role in the development of diabetes. Recently, there has been considerable interest in the use of medicine food homology (MFH) and functional food herbs (FF) to ameliorate diabetes and lead to a natural and healthy life. Hence, this review compiles some reports and findings to demonstrate that the practical use of the MFH/FF can modulate the homoeostasis of gut microbiota, thereby ameliorating the development of T2DM. The results provided useful data to support further investigation of the functional basis and application of MFH/FF to treat T2DM through maintaining intestinal homeostasis.
Keywords: medicine food homology, functional food herbs, type 2 diabetes mellitus, gut microbiota
1. Introduction
Diabetes mellitus is one of the major public health problems and has become a health global burden. Based on the data of IDF, there were approximately 451 million diabetic patients aged 18 to 99 in the world in 2017. By 2045, this figure is expected to increase to 693 million [1]. Diabetes mainly includes Type-1 (T1DM) and Type-2 Diabetes Mellitus (T2DM), of which T2DM accounts for roughly 90–95% [2]. T2DM is a complex metabolic disorder, which is chiefly characterized by hyperglycemia, with glycolipid dysfunction, progressive loss and dysfunction of islet β-cell, and insulin resistance. T2DM is usually accompanied by oxidative stress and inflammation, and long-term hyperglycemia may lead to diverse diabetic complications [3].
One of the main reasons for the sharp increase in the incidence of T2DM is the significant changes in human behavior and lifestyle. Through diet modification, regular exercise, weight control, and patient education, T2DM can be managed and medications can be avoided. Diabetes is characterized by “leaky gut” syndrome, where bacterial cell wall components enter the blood circulation of the animal host in a large amount, which may cause metabolic endotoxemia and systemic low-grade inflammation [4]. Gut microbiota acts an important role in modulating the systemic and intestinal immunity and metabolic homeostasis [5]. Studies have shown that the consortium of gut microbiota is closely related to host genetics and other diverse conditions, such as food habits, stresses, exposure to drugs or toxins [5]. It was reported that the gut microbiota in healthy people is diversified, achieving more short chain fatty acids (SCFA) and producing more branched amino acids, while the intestinal flora of diabetes is more likely to produce compounds that affects glucose metabolism. The intestinal microbiota can digest diverse dietary fibers that cannot be digested by the host, and produce SCFAs as its metabolites, such as acetate, butyrate and propionate [6]. Propionate can maintain gluconeogenesis in the intestinal tract, thereby making better use of energy, while butyrate, with anti-inflammatory activity, can reduce the permeability of the intestine [6]. In T2DM patients, butyrate producing microbiota are significantly reduced, specifically the Clostridiales order, including the genera Ruminococcus and Subdoligranulum, and species such as Roseburia intestinalis and Roseburia inulinivorans [7,8].
In ancient China, diabetes was called ‘Xiao Ke’, manifested as persistent thirst and hunger, excessive urination, and weight loss. For thousands of years, Chinese herbal prescriptions and traditional Chinese medicine (TCM) medicinal materials have been commonly used to intervene in ‘Xiaoke’ disease. Historically, many of the formulations and medicinal herbs have been used as food for safe and effective long-term consumption [9]. Natural plants are essential for the management of many human diseases, such as diabetes [10]. Numerous herbal medicinal plants are natural sources of antioxidants, which can reduce the oxidative stress generated by STZ in β-cells. World Health Organization (WHO) has recommended the evaluation and application of traditional botanical treatments for diabetes because they are effective and non-toxic, have fewer side effects or have no side effects, and are considered excellent candidates for oral therapy [11]. In recent years, more and more researchers have been paying attention to natural products from traditional herbs and foods for their safety, efficacy, and potency in treating diabetes [12].
The concept of ‘medicine and food homology’ was proposed in the Huang Di Nei Jing Su Wen: ‘Eating on an empty stomach as food, and administering to the patient as medication’ embodies the theory of medicine food homology (MFH); that is, some food classes can also be used as drugs. Functional food (FF), also known as health food, refers to a specific type of food that is not aimed at curing diseases but can modulate human body functions. “Notice on Further Regulating the Management of Raw Materials for Health Foods” was issued in 2012 by the Ministry of Health, which covers both foods and medicines [9]. In addition, 110 MHF and 114 FF are currently included in this promulgated management method. More and more clinical evidence clarifies that the occurrence and development of T2DM can be prevented or delayed by regular intake of foods that are believed to be functional and affect glycemic control, antioxidant enzymes activity and intestinal flora, while also inhibiting the excessive production of pro-inflammatory cytokines during diabetes [13].
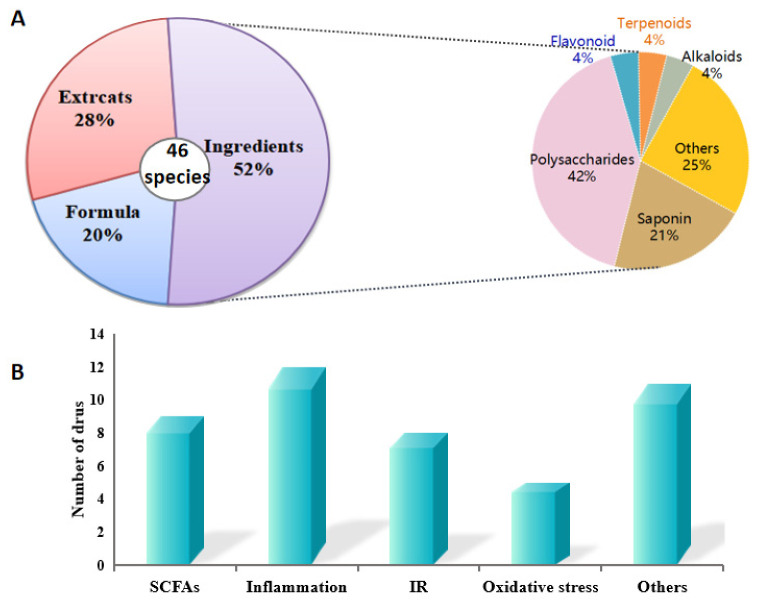
Therefore, in this review, we searched various online databases (PubMed, ScienceDirect, CNKI) and scientific publications from the library using qualitative systematic reviews. The review was based on MHF and FF application for possessing medicinal value against T2DM by modulating the gut microbiota (Figure 1A).
Figure 1.
Analysis of MFH and FF showing anti-diabetic effects by modulating gut microbiota in this review. (A) Classification about the active MFH and FF species. (B) the relational mechanism of MFH and FF on T2DM associated with gut micorobiota.
2. Association between Gut Microbiota and T2DM
2.1. Alteration of Gut Microbiota Composition with T2DM
Although T2DM is caused due to various factors, the human gut microbiota plays a vital role in the progression of T2DM [4]. The impact of gut microbiota on T2DM has attracted widespread attention; studies have been done over the past few years to research the relationship between the two [12]. ‘Human microbiome’ was firstly defined by Joshua Lederberg in 2001 as an ecological community of symbiotic and pathogenic microorganisms that share our body space. An adult is colonized by almost 100 trillion microbes, which mainly exist in the gastrointestinal tract, with the largest group living in the colon. Human health is strongly affected by the microbiota that coexist with our body [14]. The diversity of the intestinal flora of T2DM patients is significantly decreased compared to that of healthy controls [15,16]. As Larson et al. reported, the abundance of Firmicutes phylum in diabetic patients was reduced when compared to non-diabetic patients, and the ratio of Bacteroides to Firmicutes was positively correlated with blood glucose levels [17].
2.2. Mechanism of Gut Microbiota Alteration Causing T2DM
T2DM, characterized by “leaky gut” syndrome, is known to have markedly enhanced intestinal permeability, allowing bacteria to translocate across the intestinal epithelium, resulting in host metabolic endotoxemia and triggering low-grade inflammation. In T2DM, the abundance and diversity of the gut microbiota both decreased, accompanied by an increase in the abundance of pathogenic microorganisms and a decrease in the abundance of symbiotic microorganisms [18]. For example, the generas Faecalibacterium, Roseburia and Bifdobacterium, with noticeable abilities to reduce intestinal permeability, have been shown to be exhausted in T2DM [19]. The changes in the above-mentioned microbiota would lead to low-grade inflammation, resulting in a decrease in mucus layer and disintegration of the epithelial membrane. This is followed by an increase in intestinal permeability, allowing lipopolysaccharides (LPS) to enter the blood circulation. Bacterial fragments and LPS can be recognized by innate toll-like receptors (TLRs), particularly TLR-4, which subsequently stimulates the activation of transcription factor κB (NF-κB) and the release of pro-inflammatory mediators in intracellular signaling pathways [20]. The release of pro-inflammatory cytokines would further result in the destruction of glucose metabolism and insulin signaling pathways [21]. Metabolic endotoxemia and low-grade inflammation occurs, subsequently. The systemic low-grade inflammation affects all vital organs or tissues, such as the pancreas, liver, and kidney [22]. For example, the level of TNF-α in T2DM is significantly increased, which is closely related to islet dysfunction [16,23]. Under this circumstance, the homeostasis of glucose metabolism no longer exists and thus results in type 2 diabetes. In this condition, the steady state of glucose metabolism collapses and T2DM is developed [24].
3. Bioactive Ingredients of MFH and FF Target for Microbiota in T2DM
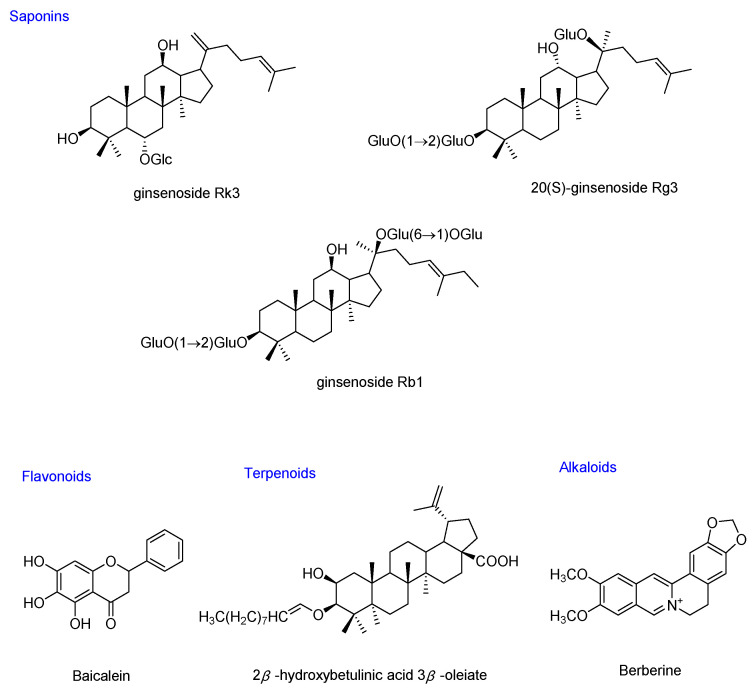
The diet and its metabolites have a major physiological impact on the composition of gut microbiota and the health of the host [22]. In China, MFH and FF refers to a group of foods that can also be used as medicines, many of which possessed anti-hyperglycemic activities. Regular consumption of MFH, which is considered to affect glycemic control, activation of antioxidant enzymes and gut microbiota, and to inhibit the excessive production of pro-inflammatory cytokines, to prevent or treat T2DM [25]. MFH and FF have been widely sought recently, and research into their use for T2DM has evoked considerable interest. Herein, the bioactive ingredients of MFH and MHF were divided into: saponins, polysaccharides, flavonoids, terpenoids, alkaloids, and others, and their anti-diabetic effects via gut miocrobiota regulation were listed in Table 1, and the chemical structures of the representative compounds are shown in Figure 2.
Table 1.
Effects of ingredients from MFH and FF on T2DM via gut microbiota.
| Ingredients | Source | Microbiota Findings | Mechanism | Study Types and Sequencing Method | Animals | Dose and Duration | Refs. | |
|---|---|---|---|---|---|---|---|---|
| Saponins | Ginsenoside Rk3 | Panax notoginseng | ↑ Lactobacillaceae, Helicobacteraceae, Neococcaceae, Bifidobacteriaceae ↓ Ratio of Firmicute to Bacteroidete; |
Inhibit the inflammatory cascade by suppressing the TLR4/NF-κB pathway | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 Mice | 60 mg/kg/day; 8 weeks | [26] |
| 20(S)-ginsenoside Rg3 | Panax ginseng C. A. Meyer | ↑ Bacterial diversity | Improve bacterial diversity | In vivo; principal component analysis |
Male Wistar rats | 20 mg/kg/day; 2 weeks | [27] | |
| Ginsenoside Rb1 | Panax ginseng C. A. Meyer | Unclear | Inhibit deglycosylation in the diabetic rats | In vivo; 16S rRNA Sequencing Analysis | Male Sprague-Dawley rats | 100 mg/kg/day; 72 h | [28] | |
| Saponin-containing Korean red ginseng extracts | Korean red ginseng (Panax ginseng Meyer) | ↑ Parabacteroides, Allistipes, Lactobacillus ↓ Barnesiella, Mucispirillum, Lactococcus, Oscillibacter, Helicobacter |
Improve IR and glucose intolerance | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 | 235 mg/kg/day; 4 weeks | [29] | |
| Saponin extract of Polygonatum sibiricum | Polygonatum sibiricum (Liliaceae) | ↑ Bifidobacteria, Lactobacillus; ↓ Enterobacteriaceae, Enterococcus, C. perfringens |
Improve IR | In vivo; Bacteria plate count | ICR male mice | 1.0, 1.5, or 2.0 g/kg/day; 5 weeks | [30] | |
| Polysaccharides | Polysaccharides (MDG-1) from Ophiopogonis Radix |
Ophiopogon japonicus (Thunb.) Ker-Gawl. (Liliaceae) | ↑ Lactobacillus, Bifidobacterium; ↓ Escherichia coli, Streptococcus |
Improve SCFAs metabolism | In vivo; 16S rRNA Sequencing Analysis | KKay mice | 300 mg/kg/day; 8 weeks | [31,32] |
| Homogeneous polysaccharides from crude Lycium barbarum polysaccharides | Lycium barbarum L. | ↑ Firmicutes/Bacteroides, SCFAs | Regulate SCFAs levels | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 | 50 mg/kg/day; 12 weeks | [33] | |
| Polygonatum sibiricum polysaccharide | Polygonatum sibiricum (Liliaceae) | ↑ Firmicutes, Veillonella, Escherichia-Shigella, Klebsiella; ↓ Proteobacteria, Bacteroides |
Regulate bacterial diversity | In vitro; 16S rRNA Sequencing Analysis | / | / | [34] | |
| polysaccharide-rich extracts of A. venetum | Apocynum venetum | ↑ Odoribacter, Anaeroplasma, Parasutterella, Muribaculum; ↓ Enterococcus, Klebsiella, Aerococcus. |
Attenuate oxidative stress and SCFAs levels | In vivo; 16S rRNA Sequencing Analysis | Male C57BL/6 J mice | 400 mg/kg/day; 4 weeks | [35] | |
| Maydis stigma polysaccharides | Zea mays subsp. mays | ↑ Lactobacillus and Bacteroides | Restore the intestinal microflora balance | In vivo; 16S rRNA Sequencing Analysis | Male KM mice | 400, 600, 800 mg/kg/day; 5 weeks | [36] | |
| Plantago asiatica L. polysaccharides | Plantago asiatica L. | ↑ Colon bacterial diversity, Bacteroides vulgatus, Lactobacillus fermentum, Prevotella loescheii, Bacteroides vulgates | Increase the levels of SCFAs | In vivo; 16S rRNA Sequencing Analysis | Wistar rats | 100, 200 or 400 mg/kg/day; 5 weeks | [37] | |
| Pseudostellariae Radix | Pseudostellaria heterophylla (Miq.) Pax ex Paxet Hoffm. | ↑ Lactobacillus, Bifidobacterium | Attenuate oxidative stress; suppress inflammatory response | In vivo; 16S rRNA Sequencing Analysis | Male C57BL/6 J | 500 mg/kg/day; 4 weeks | [38] | |
| Polysaccharides of Lactobacillus plantarum-fermented Momordica charantia | Momordica charantia L. | ↑ Lactococcus laudensis, Prevotella loescheii, diversity of gut microbiota, SCFAs
↓ pH value |
Attenuate oxidative stress | In vivo; 16S rRNA Sequencing Analysis | Male Wistar rats | 50, 100 mg/kg/day; 4 weeks | [39] | |
| mulberry fruit polysaccharide | Morus alba L. | ↑ Lactobacillus, Allobaculum, Bacteroides, Akkermansia, SCFA (butyrate, propionate). ↓ Firmicutes, Bacillus, Lactobacillus |
Attenuate oxidative stress | In vivo; 16S rRNA Sequencing Analysis | Male db/db mice | 500, 800 mg/kg/day; 8 weeks | [40] | |
| Pumpkin polysaccharide | Cucurbita moschata (Duch. ex Lam.) | ↑ Bacteroidetes, Prevotella, Deltaproteobacteria, Oscillospira, Veillonellaceae, Phascolarctobacterium, Sutterella, Bilophila | Increase SCFAs production | In vivo; 16S rRNA Sequencing Analysis | Male Wistar rats | 1000 mg/kg/day; 4 weeks | [41] | |
| Flavonoids | Baicalein | Oroxylum indicum, Scutellaria baicalensis | ↑ Bacteroides, Bacteroidales S24-7 | Alleviate inflammation and IR | In vivo; 16S rRNA Sequencing Analysis | Male Wistar rats | 50, 150 mg/kg/day; 4 weeks | [42] |
| Terpenoids | 2β-hydroxybetulinic acid 3β-oleiate | Euryale ferox salisb. | Unclear | Reduce blood glucose, regulate dyslipidemia and antioxidant enzymes, protect pancreatic β-cell | In vivo | Male Wistar rats | 60 mg/kg/day; 45 days | [43] |
| Alkaloids | Berberine | Coptidis rhizoma and Berberis vulgaris | ↑ Bacteroidetes, Lactobacillaceae; diversity of the gut microbiota ↓ Proteobacteria, Verrucomicrobia |
Alleviate inflammation via NF-κB signaling pathways | In vivo; Real-Time PCR Assay | Male Sprague-Dawley rats | 200 mg/kg/day; 6 weeks | [44,45] |
| Others | total glycoside from R. glutinosa leaves | Rehmannia glutinosa | ↑ Firmicutes, norank_f_Bacteroidales_S24-7_group | Regulate glycolipid, inhibit the expression of α-SMA, TGF-β1, Smad3 and Smad4 in the kidney tissues | In vivo; 16S rRNA Sequencing Analysis | db/db mice | 520 mg/kg/day; 6 weeks | [46] |
| low-polar S. grosvenorii glycosides | Siraitia grosvenorii (Swingle) C. | ↑ Elusimicrobium, Lachnospiraceae_UCG-004 | Increase SCFAs production (acetate, butyrate, and 1β-hydroxycholic acid) | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley rats | 20 mg/kg/day; 14 days | [47] | |
| sea buckthorn protein | Hippophae rhamnoides L. | ↑ Bifidobacterium, Lactobacillus, Bacteroides
↓ Clostridium coccoides, PH value; |
Increase intestinal microorganism diversity and SCFAs levels | In vivo; 16S rRNA Sequencing Analysis | ICR mice | 50, 100 and 200 mg/kg/day; 30 days | [48] | |
| Long chain of inulin-type fructans | inulin | ↑ Firmicutes/Bacteroidetes ratio; Ruminococcaceae, Lactobacilli | Regulate SCFAs levels | In vivo; 16S rRNA Sequencing Analysis | Female NOD/LtJ mice | 5% diet; 24 weeks | [49] | |
| cinnamon oil | Cortex Cinnamomi | ↑ Bacteroides ↓ Clostridia flora IV |
Improve IR | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley rats | 0.384 g/kg/day; 30 days | [50,51] | |
Abbreviations: SCFAs, short-chain fatty acid. IR, insulin resistance. ↑, Increase. ↓, Decrease.
Figure 2.
Chemical structures of the representative hypoglycemic compounds from MFH and FF that can modulate gut microbiota in T2DM.
3.1. Saponins
Saponins are a class of glycosides composed of triterpenes or spirostanes, which are widely present in nature [52]. Saponins have been reported to have a wide range of hypoglycemic targets and pathways, which can directly repair damaged islet cells and increase insulin levels to maintain normal blood sugar. Saponins can also regulate blood lipids and improve glucose tolerance. This suggests that they have broad research and development prospects as anti-diabetic drugs [53]. In this review, saponins from Panax ginseng, Panax notoginseng, Korean red ginseng and Polygonatum sibiricum were researched. It was observed that these saponins can intervene on T2DM and are associated with their modulating the imbalance of gut microbiota and inhibiting the low-grade inflammation and insulin resistance.
Ginseng, a perennial herb of the genus Panax, has been used widely as a TCM herbs in China and Asia for thousands of years. About 4000 years ago, “Shen Nong Ben Cao Jing” is the earliest surviving TCM monograph in China. It records the use of ginseng as a health medicine to delay aging and nourish the body without side effects [54]. According to Zhang Zhongjing’s Shang Han Za Bing Lun in the Han Dynasty, ginseng was used to cure thirst, which is the main symptom of “Xiaoke” (diabetes). Additionally, the “Tai Ping Hui Min He Ji Ju Fang”, an official traditional Chinese medicine book in Song Dynasty, recorded the use of ginseng to treat “Xiaoke disease”. Many of the Chinese patent medicines approved by the government for the treatment of diabetes contain ginsenosides, such as Tianqi capsules [55], Jinlida Granule [56], and ShenMai Injection [28,57]. Ginseng has several therapeutic functions, such as anti-stress, maintaining and strengthening the central and immune system, preventing certain chronic diseases, and delaying aging. While American ginseng is more effective in treating cardiovascular disease [58]. Ginsenosides are extracted from the roots and rhizomes of Panax ginseng C. A. Meyer. Research has shown that ginsenosides show noticeable anti-diabetic activities and have been used as adjuvants for diabetes treatment in China. It was reported that saponins isolated from ginseng, such as Ginsenoside Rk3, and 20(S)-ginsenoside Rg3 showed potential anti-diabetic activities by regulation of gut microbiota.
It was observed that Ginsenoside Rk3 at the dose of 30 and 60 mg/kg/day could alleviate the abundance imbalance of gut microbiota and inhibit the expression of pro-inflammatory cytokines by reducing intestinal permeability and LPS levels, thereby preventing low-grade colon inflammation caused by a high-fat diet in mice. An 8-week intervention of Rk3 could significantly decrease the ratio of Firmicute to Bacteroidete, and restore the abundance of Lactobacillaceae, Helicobacteraceae, Neococcaceae, and Bifidobacteriaceae at the dose of 60 mg/kg/day. Ginsenoside Rk3 can effectively improve the C57BL/6 Mice metabolic disorder of gut microbiota by decreasing the ratio of Firmicute/Bacteroidete, and inhibit the inflammatory cascade by suppressing the TLR4/NF-κB pathway [26]. It was found that 20(S)-ginsenoside Rg3 at a dose of 20 mg/kg can reduce the blood glucose by regulating the metabolism of gut flora in T2DM rats [27].
Polygonatum sibiricum, a perennial herb of Liliaceae family, has diverse activities, such as hypoglycemic effect, regulating blood lipids, delaying aging, and strengthening immunity. A saponin was isolated from P. sibiricum (PSS) and administered to diabetic mice at the dose of 1.0, 1.5, or 2.0 g/kg/day. It was found that PSS could alleviate the symptoms of polyphagia and polydipsia and regulate the gut microbiota in the diabetic mice. PPS increased the abundance of probiotics (including Bifidobacteria and Lactobacillus), and down-regulated the harmful bacteria (such as Enterobacteriaceae, Enterococcus, and C. perfringens) [30].
3.2. Polysaccharides
Polysaccharides are formed by the polymerization of monosaccharide molecules through glycosidic bonds, which are generally composed of hundreds or even thousands of monosaccharides molecules with a relatively high molecular weight. Polysaccharides, as a kind of abundant natural product, are found in organisms such as fungi and plant roots [59]. As prebiotics, polysaccharides have been found to affect the populations and metabolism of the gut microbiota, and attracted widespread attention in biochemical and medical research [60]. The polysaccharides from MFH have been studied to show potential impact on T2DM, which is associated with the regulation of gut microbiota.
Ophiopogonis Radixa, the Chinese name Maidong, is the tuberous roots of Ophiopogon japonicus (Thunb.) Ker-Gawl (Liliaceae), which is a popular TCM. Maidong is widely used as a functional food in China. Maidong has been used to relieve diabetes and cardiovascular diseases for years [31]. The polysaccharide is one of the main active ingredients of Maidong. A homogeneous polysaccharide fraction was isolated and characterized from Ophiopogonis Radix collected from Sichuan. Ophiopogonis Radix and was analyzed for anti-diabetic effects in targeting β-cell dysfunction, insulin enhancement and inhibiting α-amylase and α-glucosidase [61]. The anti-diabetic effects of polysaccharides, isolated from Maidong, have been reported [62,63]. More than 15 kinds of polysaccharides have been isolated from Maidong, which show a good anti-diabetic effect, and the main mechanism is associated with improving β-cell dysfunction, enhancing insulin and inhibiting α-glucosidase and α-amylase [64]. For example, MDG-1, a kind of polysaccharide isolated from Maidong, possessed anti-diabetic effects in diabetic mice and regulated intestinal flora in obese mice [31,64]. In KKay mice, the abundance of Escherichia coli and Streptococcus increased, while the abundance of Lactobacillus and Bifidobacterium decreased. However, oral administration of 300 mg/kg MDG-1 can reduce the number of pathogenic E. coli and Streptococcus, and increase the number of Lactobacillus (p < 0.05). It has been proven that oral MDG-1 can improve the glucose tolerance of diabetic mice and is related to its regulating effect on the intestinal microecological balance [32].
Lycium barbarum L. and its mature fruits have been used as a TCM and functional food in China for about 2000 years. The leaves of L. barbarum, also named Tianjing grass, are widely used as tea, food and medicine in China due to its activities of reinforcing deficiency and benefiting essence, as well as anti-thermic and eye-clearing effects [65,66]. An HFD/STZ-induced T2DM rat model was established to study the anti-diabetic effects of the water extract of L. barbarum leaf (LLB). It was found that LLB can improve T2DM, which is mainly associated with the reversal of gut microbiota imbalance, and regulation of nicotinate/nicotinamide, arachidonic acid/purine metabolism. Administration of LLB at 2.08 g/kg T2DM rats significantly reduced excessive abundance of Parasutterella, Marvinbryantia, Blautia, Ruminococcus_1, and Prevotellaceae_NK3B31_group, and reversed the ratio of Firmicutes to Bacteroidetes in the gut microbiota of diabetic rats [66]. The homogeneous polysaccharide (LBP-W) was purified from crude Lycium barbarum polysaccharides (LBPs), and administration of 50 mg/kg LBP-W could improve obesity by modulating the composition of gut microbiota and the metabolism of SCFAs in C57BL/6 mice on a high fat diet. LBP-W intervention reversed the HFD-induced changes in Firmicutes and Bacteroides, and the ratio of Firmicutes/Bacteroides was noticeably reduced (p < 0.01) [33].
Plantago asiatica L. is a kind of TCM and has been used as a folk medicine worldwide [67,68]. A high-fat diet and STZ induced T2DM rat model has been established, and the anti-diabetic effect of Plantago asiatica L. polysaccharide (PLP) was studied. It was observed that administration of PLP (at dose of 100, 200 or 400 mg/kg) significantly decreased the level of blood glucose, insulin, serum lipids, non-esterifified fatty acid and maleic dialdehyde, and noticeably increased the activities of antioxidant enzymes in T2DM rats after 4 weeks of PLP intervention. The concentrations of SCFA were noticeably higher in the feces of diabetic rats after treating with PLP. Moreover, colon bacterial diversity and abundance of bacteria, including Bacteroides vulgatus, Lactobacillus fermentum, Prevotella loescheii and Bacteroides vulgates were markedly increased by PLP intervention. It indicated that the anti-diabetic effect of PLP inT2DM rats was related to the regulation of gut microbiota and increased levels of SCFAs production [37].
Apocynum venetum is a perennial herbaceous or half-shrub plant, and its leaves have been traditionally consumed as a tea beverage in China. A. venetum is widely distributed in saline-alkali land, riverbanks, fluvial plains and sandy soils of Asia and North America [69]. Hypoglycemic and hypolipidemic effects of polysaccharide-rich extracts from A. venetum leaves on T2DM mice has been studied. Treatment of alkaline extracted polysaccharide-rich products markedly decreased the levels of fasting blood glucose, serum insulin, and serum lipids. Meanwhile, the reduced glycogen contents in liver were prominently improved, and the oxidative damage was markedly ameliorated by alkaline extracted polysaccharide products in diabetic mice. Furthermore, the polysaccharide-rich extracts could reverse the gut microbiota dysbiosis in T2DM mice by increasing the abundance of genera Odoribacter, Anaeroplasma, Parasutterella, and Muribaculum, while decreasing the abundance of genera Enterococcus, Klebsiella, and Aerococcus. Thus, polysaccharide-rich extracts of A. venetum showed good anti-diabetic effects for treating T2DM, which was associated with the intervention of gut microbiota [35].
In this review, polysaccharides from MFH and FF were summarized and their impacts on T2DM by regulating gut microbiota were listed in Table 1. It was found that the polysaccharides play an important role in maintaining intestinal flora steady state, which was associated with the promotion of short-chain fatty acids (SCFAs). SCFA mainly include acetate, butyrate and propionate at the ratio of 3:1:1 in human gut microbiota, which are usually present in the human intestine at a ratio of 3:1:1 and are in a steady state [70]. Butyrate possesses anti-inflammatory effects and can reduce intestinal permeability, and propionate also maintains gluconeogenesis in the intestines, thereby making better use of energy [24]. Individuals with T2DM have reduced butyrate-producing gut microbiota, which promotes low-grade inflammation [70].
3.3. Flavonoids
Flavonoids are meaningful natural compounds that exist widely in the plant kingdom and have a basic 2-phenyl-chromone structure. They are a class of secondary plant compound with noticeable physiological effects and various health benefits [9]. Flavonoids possess extensive pharmacological effects, among which are antioxidant and free radical scavenging activities, which are of particular interest to the pharmaceutical industry [71]. Flavonoids are widely reported to prevent and treat T2DM by affecting the function of islet β-cells and anti-lipid peroxidation [72]. However, not so many flavonoids from natural herbs were found to intervene T2DM by the regulation of gut microbiota.
Baicalein is a dietary flavonoid and is a main component of Oroxylum indicum and Scutellaria baicalensis. It is used as a dietary supplement or as tea in Asia, Europe and the Americas. Based on Zhang’s study in 2018, four weeks intervention of baicalein (50, 150 mg/kg·d) significantly decreased the blood glucose and LPS and improved insulin resistance, inflammation, and lipid profile in T2DM rat dose-developmentally. These anti-diabetic effects are owing to the increase in SCFAs content and the thickness of the intestinal mucus layer, which is closely related to the regulation of the intestinal microbiota, especially the abundance of Bacteroides and Bacteroides S24-7. They had the highest relative abundance in rats receiving 150 mg/kg baicalein, and they were positively correlated with improving T2DM-related phenotypes [42]. As reported, Bacteroidales S24-7, Prevotella, Blautia, and Butyricoccus are the key SCFA-producing bacteria, which may relieve inflammation and insulin resistance, by reducing the intestinal endotoxins entering the circulation, thereby alleviating T2DM [73,74].
Plumula nelumbinis, also named “Lian-Zi-Xin” in Chinese, is the dried embryo of the ripe seeds of Nelumbo nucifera Gaertn (Nelumbonaceae). It is a traditional Chinese medicine (TCM), and also an ordinary health food. It is commonly used in several counties around the world. In TCM, Lian-Zi-Xin has been used to clear heart heat, calm the mind, and treat high fever, promote astringent essence and hemostasis [75,76]. As Qiuzhe Li reported in 2015, the total flavonoids from Lotus plumule showed noticeable anti-diabetic effects by reducing the blood glucose level, regulating blood lipid levels and improving the glucose tolerance in the T2DM mice.
3.4. Terpenoids
Terpenes are natural hydrocarbons and can be linked in diverse ways through isoprene or isopentane. It mainly includes monoterpenes, sesquiterpenes, diterpenes and triterpenes, which play a vital role in organisms. Studies have shown that some terpenoids possess a preventive effect on T2DM; the mechanism may be mediated by protecting islet β-cells and increasing glucose tolerance and hepatic glycogen synthesis [71]. However, there are few studies on the effects of terpenoids treating T2DM by regulating gut microbiota.
A pentacyclic triterpene, 2β-hydroxybetulinic acid 3β-oleiate (HBAO), was isolated from the seeds of Euryale ferox salisb. Oral administration of 60 mg/kg/d HBAO could ameliorate glycemic homeostasis and alleviate oxidative stress in the streptozocin (STZ)-induced diabetic rats. It was observed that HBAO normalized the blood glucose, glycosylated hemoglobin (HbA1c), hepatic hexokinase and plasma insulin, improved damaged pancreatic β-cell, regulated dyslipidemia and antioxidant enzymes (such as superoxide dismutase, catalase and glutathione peroxidase) in the diabetic rats (p < 0.05) [43]. STZ-induced diabetic mice were administrated by the triterpenoid-rich extracts of Euryale ferox shell (ES) orally at doses of 200, 300, 400, 500 ± 2 mg/L for 4 weeks. It was found that the triterpenoid-rich extracts of ES could regulate glucose metabolism (p < 0.01), normalize the body weight of the diabetic mice (p < 0.01), reduce the expression of the negative regulation protein PTP1B gene and increase insulin receptor IRS-1 protein expression (p < 0.05) [77].
3.5. Alkaloids
Alkaloids are a class of nitrogen-containing organic compounds derived in nature, mainly in the plant kingdom. Most alkaloids are alkaline and have significant biological activity and are a kind of important bioactive ingredient in MFH and FF [71]. It has been found that the hypoglycemic activities of alkaloids are mainly mediated by inhibition of gluconeogenesis, regulation of gut microbiota structure, promotion of glycolysis and anti-glucagon activities, promotion of the secretion of pancreatic β-cells, and scavenging of oxygen free radicals [78]. For example, neferine could reduce the levels of blood glucose, improve insulin resistance and regulate the disorder of lipid metabolism in T2DM rats [75]. Isoliensinine was found to attenuate T2DM with hyperlipidemia in a KK-Ay mouse model by regulating GLUT4, SREBP-1c, PPARγ, AMPK and ACC phosphorylation [76]. However, there are few studies on the effects of alkaloids treating T2DM through gut microbiota regulation.
Berberine is an isoquinoline quaternary alkaloid that is widely found in Coptidis rhizoma and Berberis vulgaris [79]. Berberine has a long history in Chinese and Western medicine treatment [80]. In China, Berberine has been used to treat diarrhea caused by bacteria as an over-the-counter drug for years [45,81]. Berberine was administrated to T2DM rats, and it was found that the anti-diabetic effects of Berberine is related to its regulation of gut microbiota. The community richness and diversity of the gut microbiota were noticeably increased by Berberine, and the abundance of Bacteroidetes was increased, while the number of Proteobacteria and Verrucomicrobia were decreased. At the family level, a probiotic Lactobacillaceae was markedly increased after Berberine intervention, which was negatively related to the risk of T2DM [44]. It suggested that Berberine can alleviate T2DM in rats by modulating gut microbiota composition.
3.6. Others
Some other kinds of compounds in medical herbs, such as proteins, fibers, essential oil and glycosides, also show significant hypoglycemic activities. We also researched and summarized those kinds of ingredients from MFH and FF to find the potential anti-diabetic compounds.
Rehmannia glutinosa is a kind of perennial herbaceous plant of the Scrophulariaceae family. The R. glutinosa leaves’ total glycoside (DHY) is mainly composed of iridoid glycosides and phenylethanoid glycosides extracted from R. glutinosa leaves. Studies have shown that DHY has been used in the clinical treatment of various kidney diseases, due to its protection on kidneys by improving glomerular permeability and reducing proteinuria. DHY was also found to improve STZ-induced gut microbiota imbalance in diabetic nephropathy rats [46]. DHY was observed to significantly decrease the levels of blood glucose, serum lipid (such as total cholesterol and triglyceride) and improve kidney damage, and inhibit the expression of α-SMA, TGF- β1, Smad3 and Smad4 in the kidney tissues of db/db mice. DHY had noticeable up-regulation effect on Firmicutes in db/db mice. At the genus level, DHY were dominant for the recovery of norank_f_Bacteroidales_S24_7_group in db/db mice. Therefore, DHY may restore the dysfunctional intestinal flora to normal and regulate glycolipid level of db/db mice [46].
Salvia miltiorrhiza Bge., a TCM for promoting blood circulation and removing blood stasis, has been used as a health-care food recently. The aerial parts of S. miltiorrhiza Bge. (DJ) are rich in phenolic acids similar to the rhizome [82]. The 60% ethanol extracts of DJ were found to strengthen the intestinal barrier of diabetic mice by up-regulating the tight junction proteins expressions in ileum and colon, but not in duodenum. DJ could modulate the diabetes-induced gut microbiota imbalance. At phylum level, that the number of Proteobacteria was significantly increased while Tenericutes was significantly decreased in DJ group compared to the control group [82].
Dietary fibers can modify the gut barrier and microbiota homeostasis, thereby impacting the progression of diabetes. Inulin-type fructans (ITFs) are natural soluble dietary fibers with different fermentation degrees in chicory root, which can regulate the occurrence and development of diabetes. Female nonobese diabetic mice were weaned to long-and short-chain ITFs, ITF(l) and ITF(s) supplemented diet up to 24 weeks. Expression of barrier reinforcing tight junction proteins occludin and claudin-2, antimicrobial peptides-defensin-1, and cathelicidin-related antimicrobial peptide as well as short-chain fatty acid production were enhanced by ITF(l). It was found that ITF(l) enhanced Firmicutes/Bacteroidetes ratio to an antidiabetogenic balance and enriched modulatory Ruminococcaceae and Lactobacilli [49]. The inulin was found to alleviate different stages of T2DM in diabetic mice by modulating gut microbiota. It increased the relative abundance of Cyanobacteria and Bacteroides, and reduced the relative abundance of Deferribacteres and Tenericutes. Dietary inulin can ameliorate diverse stages of T2DM by suppressing inflammation and modulating gut microbiota, especially in pre-diabetic and early diabetic stages, thus it potentially serves as an inexpensive intervention for the prevention and treatment of T2DM patients [15].
4. Herb Extracts of MFH and FF Target for Microbiota in T2DM
4.1. Single Herb Extracts of MFH and FF Target for Microbiota in T2DM
Generally, MFH and FF are usually taken in the form of decoction or direct consumption. Therefore, the anti-diabetic effect of the water extracts or total extracts is worthy of attention. We investigated the anti-diabetic effects of the extracts of MFH and FF by regulating the imbalance of the intestinal microbiota, such as Fructus Aurantii Immaturus, Atractylodis macrocephalae Rhizoma, Radix Puerariae, sea buckthorn, Anemarrhena asphodeloides, Dendrobium officinale, listed in Table 2.
Table 2.
The role and mechanism of extracts in MFH and FF on T2DM through modulating gut microbiota.
| MFH/FF | Source | Microbiota Findings | Mechanism | Test Sections | Study Type and Sequencing Method | Animals | Dose and Duration | Refs. |
|---|---|---|---|---|---|---|---|---|
| Fructus Aurantii Immaturus | Citrus aurantium L. | ↓ Lachnospiraceae NK4A136, Prevo tellaceae UCG-003, Prevotellaceae NK3B31, Lachnospiraceae UCG-008, Ruminiclostridium 9, Ruminococcaceae UCG-014; ↑ Lactobacillus, Alloprevotella, Treponema 2 |
Restore the intestinal microflora balance | Water extracts of fried Fructus Aurantii Immaturus with wheat bran decoction | In vivo; 16S rRNA Sequencing Analysis | Male Sprague-Dawley rats | 9 g/kg/day; 14 d | [83] |
| Atractylodes macrocephala Koidz | Atractylodes macrocephala Koidz (Compositae) | ↑ Bacteroides thetaiotaomicron, Methanobrevibacter smithii | Upregulate GLP-1R, PI3K, PDX-1 expressions, and suppress inflammation (decrease FOXO1, NF-κB p65) | Water extracts of Atractylodis macrocephalae Rhizoma (AMK) | In vivo; 16S rRNA Sequencing Analysis | db/db mice | 100 mg/kg/day; 3 weeks | [84] |
| Anemarrhena asphodeloides | Anemarrhena asphodeloides Bge. | ↑ Blautia coccoides (in vitro) ↓ Proteobacteria, Facklamia, Oligella, and Klebsiella |
Suppress the increased oxidative stress and inflammatory activation. | Water extract of A. asphodeloides | In vivo; 16S rRNA Sequencing Analysis | Male SPF Wistar rats | 20, 60, 180 mg/kg/day; 4 weeks. | [85] |
| Lycium barbarum | Lycium barbarum L. | ↑ the ratio of Firmicutes to Bacteroidetes; ↓ Parasutterella, Marvinbryantia, Blautia, Ruminococcus_1, Prevotellaceae_NK3B31_group |
Improve liver, kidney, and pancreas injury and regulate metabolic profiles | Water extract of L. barbarum leaf | In vivo; 16S rRNA Sequencing Analysis | (SPF)-grade rat | 1.04, 2.08 g/kg/day; 4 weeks | [66] |
| Alpinia oxyphylla Miq. | Alpinia oxyphylla Miq. (Zingiberaceae) | ↑ Akkermansia; ↓ Helicobacter |
Modulate gut microbiota composition | Water extract of Alpinia oxyphylla Miq. | In vivo; 16S rRNA Sequencing Analysis | db/db mice | 100, 300, 500 mg/kg/day; 8 weeks | [86] |
| Chinese propolis | Chinese propolis | ↑ Roseburia, Intestinimonas, Parabacteroides goldsteinii, Parabacteroides distasonis; ↓ Faecalibacterium, Prevotella, Bacteroides vulgatus |
Reduce inflammation | Ethanol extract of propolis | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 | 200, 300 mg/kg/day; 12 weeks |
[87,88] |
| Puerariae Radix | Pueraria lobata | ↑ Lactococcus, Ruminococcus | Inhibit obesity and inflammatory-related parameters | 30% ethanol extracts of dried root of P. lobata | In vivo; 16S rRNA Sequencing Analysis | Female C57BL/6 J mice | 400 mg/kg/day; 10 weeks | [89] |
| Mulberry leaf | Morus alba L. | ↑ Bacteroidetes, Proteobacteria; Clostridia | Improve IR | mulberry leaf powder | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley male rats | 20% (w/w) in diet; 13 weeks |
[90] |
| Coicis Semen | Coix lacryma-jobi L. var. ma-yuen (Roman.) Stapf | ↑ Lactobacillus, Coprococcus, Akkermansia, Akkermansia muciniphila, Lactobacillus agilis | Improve glucose homeostasis | Coicis Semen power included in diet | In vivo; 16S rRNA Sequencing Analysis | C57BL/6 mice | 0.5 g/100 g; 5 weeks | [91] |
| Astragali Radix |
Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) |
↑ ratio of Firmicutes/Bacteroidota; Lactobacillales | Regulate gut microbiota | Astragali Radix decoction vesicle-like nanoparticles extracted by ltracentrifugation; | In vivo; 16S rRNA Sequencing Analysis | db/db mice | 5.3, 10.6, 21.1 g/kg/day; 3 weeks | [92] |
| Dendrobium candidum | Dendrobium candidum Wall Ex Lindl | ↑ Akkermansia, Parabacteroides | Improve glucose intolerance and IR | Dendrobium officinale extract | In vivo; 16S rRNA Sequencing Analysis | T2D mice | 1.0 g/kg/day; 30 days | [93] |
| hemp seed | Cannabis sativa L. | ↑ Bacteroidetes; ↓ Firmicutes |
Modulate gut microbiota | hemp seed oil-water mixture | In vivo; 16S rRNA Sequencing Analysis | Female KM mice | 0.2, 0.4 mL; 10 days | [94] |
| Dioscoreae Rhizoma | Dioscorea opposita Thunb. | ↑ Bifidobacterium, Adolescentis, Bifidobacterium infantis | Modulate gut microbiota | yam gruel | In vivo; 16S rRNA Sequencing Analysis | Human patients | 150 g/day; 3 months | [95] |
Abbreviations: IR, insulin resistance. ↑, Increase. ↓, Decrease.
Atractylodis macrocephalae Rhizoma is widely used as a functional food in Asia. The water extracts of A. macrocephalae (AMK) at a dose of 100 mg/kg noticeably increased the relative abundance of Bacteroides thetaiotaomicron and Methanobrevibacter smithii in gut microbiota of the diabetic mice. It was found that AMK could significantly decrease the blood glucose and serum lipids, and improve the insulin resistance, which was associated with its inhibitory effects on inflammation and its regulation of gut miocrobiota imbalance.
Chinese propolis, is a resinous substance collected by bees from plants exudates that is mixed with wax and mandibular gland secretions [96]. Propolis has long been recognized as a natural nutraceutical has shown a beneficial effect on alleviating by exerting good anti-inflammatory, anti-oxidant effects [96]. Studies have reported that propolis extract could boost lipid metabolism, alleviate insulin resistance, and delay obesity in high-fat diet-fed mice and rats with T2DM [87]. Propolis were abserved to reverse the elevation of Firmicutes and inflammatory biomarkers expression induced by HFD in the obese mice [88]. Propolis intervention can regulate gut microbiota by decreasing Alistipes, and increasing Lactobacillus in male mice, which are playing an important role in the preventive effect on obesity and T2DM.
4.2. Herb Formula Consisted of MFH for T2DM by Regulating Microbiota
Chinese herbal formulas with anti-diabetic effects have been well studied, and many of them have commonly been used in “Xiaoke” patients since ancient times. In the traditional Chinese medicine system, the relationship between the gut microbiota and disease is actually the relationship between the intestine and disease, which was early mentioned in the “Huang Di Nei Jing”. Therefore, we summarized the herb formulas consisted of MFH, which act anti-diabetic effects by regulating the imbalance of gut microbiota, and listed in Table 3.
Table 3.
The role and mechanism of formula extracts in MFH and FF on T2DM through modulating gut microbiota.
| MFH and FF | Microbiota Findings | Mechanism | Test Sections | Study Type and Sequencing Method | Animals | Dose and Duration | Ref. |
|---|---|---|---|---|---|---|---|
| Wumeiwan | ↓ Bacteroidetes, Actinobacteria, Bacteroides, Clostridium; ↑ Firmicutes, DeltaProteobacteria, Lactobacillus |
Improve SCFA, inhibit inflammatory mediums (TNF-α, IL-10) | Decoction concentrate | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley rats | 5, 10, 20 g/kg/day; 4 weeks | [97] |
| Daesiho-Tang | ↑ Bacteroidetes, Bacteroidetes/Firmicutes ratio, Akkermansia Bifidobacterium, Lactobacillus; ↓ Firmicutes |
Modulate intestinal microbiota | Water extracts | In vivo; 16S rRNA Sequencing Analysis | Male C57BL/6 mice | 700 mg/kg/day; 12 weeks | [98] |
| Gegen Qinlian Decoction | ↑ Lactobacillus johnsonii, Stomatobaculum longum strain ACC2, Bacteroides vulgatus | Suppress inflammation: reduce the levels of LPS, TNF-α, IL-6 | Crude drugs | In vivo; 16S rRNA Sequencing Analysis | KK-Ay mice | 4.44, 13.30, 40.00 g/kg/day; 4 weeks | [99] |
| A mixture of D. officinale and American ginseng | ↑ ratio of Bacteroidetes to Firmicutes, Prevotella, Akkermansia; and SCFA-producing bacteria; ↓ S24-7/Rikenella/Escherichia coli. |
Decrease inflammation (IL-6 and TNF-α) and oxidative stress; improve intestinal flora balance | Mixture of D. officinale and American ginseng | In vivo; 16S rRNA Sequencing Analysis | Dogs | 160 mg/kg/day; 60 days | [100] |
|
Chinese Herbal Formula Shenzhu Tiaopi
Granule |
↑ Lactobacillus; ↓ Firmicutes/Bacteroidetes ratio, Bacteroidetes, Allobaculum, Desulfovibrionaceae |
Inhibit inflammation, ameliorate IR | Shenzhu Tiaopi Granule |
In vivo; 16S rRNA Sequencing Analysis | Male Goto-Kakizaki (GK) | 1000 mg/kg/day; 8 weeks | [101] |
| Qijian Mixture | ↑ Bacteroidetes | Inhibit inflammation and oxidative stress | Qijian Mixture | In vivo; 16S rRNA Sequencing Analysis | Male KKay mice | 1.795, 5.385 g/kg/day; 5 weeks | [102] |
| Anemarrhena asphodeloides Bge.and Phellodendron chinense Schneid | ↓ Bacteroidetes; Bacilli, Lactobacillus ↑ Firmicutes, Proteobacteria; Clostridia, Romboutsia, Bacteroides |
Improve intestinal microbiota | Decoction concentrate | In vivo; 16S rRNA Sequencing Analysis | Sprague-Dawley rats | 6.48 g/kg/day; 30 days | [103] |
| Combination of Aronia, Red Ginseng, Shiitake Mushroom and Nattokinase | ↓ Clostridales; ↑ Bacterioidales |
Improve IR | Water extracts of the combination | In vivo; 16S rRNA Sequencing Analysis | Sprague Dawley rats | 0.5, 1.0 g/kg/day; 12 weeks | [104] |
| Scutellaria baicalensis Georgi, SR and Coptis chinensis Franch, CR | ↑ SCFAs-producing bacteria: Bacteroidales S24-7 group_norank, Eubacterium nodatum group, Parasutterella, Prevotellaceae UCG-001, Ruminiclostridium, Ruminiclostridium
↓ Secondary bile acid-producing bacteria Escherichia Shigella; |
Increase microbially derived SCFAs | Water extracts | In vivo; 16S rRNA Sequencing Analysis | Male Sprague-Dawley rats | 6.3 g/kg/day; 1 month | [105] |
Abbreviations: IR, insulin resistance. ↑, Increase. ↓, Decrease.
Wumei Wan was first recorded in Zhang Zhongjing’s “Shanghan lun”, and is the main prescription for the treatment of Jueyin disease. “Xiaoke” disease was considered to be one of Jueyin diseases as recorded in ancient China. It was found that Wumei Wan (at the dose of 20, 10, 5 g/kg/d) could significantly enrich the functional bacteria, such as Firmicutes, DeltaProteobacteria, and Lactobacillus, and decrease the abundance of Bacteroidetes, Actinobacteria, Bacteroides, Clostridium in the T2DM rats [97]. It has been proven that Wumei Pill can regulate the balance of intestinal flora in T2DM model rats, increase the content of short-chain fatty acids (including acetic acid, propionic acid, butyric acid), thereby lowering blood glucose and ameliorating T2DM. Daesiho-Tang is another important formulation in TCM, known for its anti-diabetic and anti-hepatotoxic effects. It has been found that Daesiho-Tang treatment noticeably increased the relative abundance of Bacteroidetes, Bacteroidetes/Firmicutes ratio, Akkermansia Bifidobacterium, Lactobacillus, and decreased the level of Firmicutes [98].
5. Conclusions and Perspective
T2DM, as one of the major public health problems worldwide, is currently prevailing and seems likely to continue for some time. Therefore, there is an urgent need for new methods to prevent and treat this disease. However, most of the treatments currently in use, especially drugs with proven effects, generally focus on agents designed to directly affect signaling pathways that directly modulate the blood glucose, which usually show some side effects. However, better underlying causes of T2DM indicates that regulating the gut microbiota may be a potential way to treat this disorder.
Natural plants, especially medicine food homology and functional foods, are considered to be an ideal candidate for oral treatment because of their effective, non-toxic, few side effects, and have received widespread attention in the of management of T2DM. As described in this review, research, especially in animal models, supports this view. Additionally, studies on MFH and FF suggests that their beneficial effects on T2DM may be partly mediated by their influences on gut microbiota. In fact, approaches such as inhibiting low-grade inflammation to prevent T2DM through regulating gut microbiota have existed, but recent studies on impacts of gut microbiota suggest it may be a possible medium for preventing this disorder. In this regard, further studies on the impacts of MFH and FF on the gut microbiota are worthy of in-depth attention, in humans, paving the way for better treatment and prevention of T2DM.
Author Contributions
X.X. and J.X. designed and revised the manuscript; J.X. analyzed the data. X.X. wrote the paper, and both authors critically reviewed and approved the final form of the manuscript. All data were generated in-house, and no paper mill was used. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Natural Science Foundation of China (Grant No. 82104061); LiaoNing Revitalization Talents Program (XLYC1802037).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi J.H., Jin S.W., Choi C.Y., Kim H.G., Lee G.H., Kim Y.A., Chung Y.C., Jeong H.G. Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-beta1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J. Agric. Food Chem. 2017;65:317–326. doi: 10.1021/acs.jafc.6b04805. [DOI] [PubMed] [Google Scholar]
- 2.Da Rocha Fernandes J., Ogurtsova K., Linnenkamp U., Guariguata L., Seuring T., Zhang P., Cavan D., Makaroff L.E. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res. Clin. Pract. 2016;117:48–54. doi: 10.1016/j.diabres.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Danda R.S., Habiba N.M., Rincon-Choles H., Bhandari B.K., Barnes J.L., Abboud H.E., Pergola P.E. Kidney involvement in a nongenetic rat model of type 2 diabetes. Kidney Int. 2005;68:2562–2571. doi: 10.1111/j.1523-1755.2005.00727.x. [DOI] [PubMed] [Google Scholar]
- 4.Al-Jameel S.S. Association of diabetes and microbiota: An update. Saudi J. Biol. Sci. 2021;28:4446–4454. doi: 10.1016/j.sjbs.2021.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Needell J.C., Zipris D. The Role of the Intestinal Microbiome in Type 1 Diabetes Pathogenesis. Curr. Diabetes Rep. 2016;16:89. doi: 10.1007/s11892-016-0781-z. [DOI] [PubMed] [Google Scholar]
- 6.Koh A., De Vadder F., Kovatcheva-Datchary P., Backhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 7.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F., Wang M., Yang J., Xu Q., Liang C., Chen B., Zhang J., Yang Y., Wang H., Shang Y., et al. Response of gut microbiota in type 2 diabetes to hypoglycemic agents. Endocrine. 2019;66:485–493. doi: 10.1007/s12020-019-02041-5. [DOI] [PubMed] [Google Scholar]
- 9.Gong X., Ji M., Xu J., Zhang C., Li M. Hypoglycemic effects of bioactive ingredients from medicine food homology and medicinal health food species used in China. Crit. Rev. Food Sci. Nutr. 2020;60:2303–2326. doi: 10.1080/10408398.2019.1634517. [DOI] [PubMed] [Google Scholar]
- 10.Kankanala J., Kirby K.A., Huber A.D., Casey M.C., Wilson D.J., Sarafianos S.G., Wang Z. Design, synthesis and biological evaluations of N-Hydroxy thienopyrimidine-2,4-diones as inhibitors of HIV reverse transcriptase-associated RNase H. Eur. J. Med. Chem. 2017;141:149–161. doi: 10.1016/j.ejmech.2017.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Zhao Z., Fan H., Li Z., He Y., Liu C. Safety and therapeutic effects of anti-fibrotic Traditional Chinese Medicine Fuzheng Huayu on persistent advanced stage fibrosis following 2 years entecavir treatment: Study protocol for a single arm clinical objective performance criteria trial. Contemp. Clin. Trials Commun. 2020;19:100601. doi: 10.1016/j.conctc.2020.100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou M., Hong Y., Lin X., Shen L., Feng Y. Recent pharmaceutical evidence on the compatibility rationality of traditional Chinese medicine. J. Ethnopharmacol. 2017;206:363–375. doi: 10.1016/j.jep.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Alkhatib A., Tsang C., Tiss A., Bahorun T., Arefanian H., Barake R., Khadir A., Tuomilehto J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients. 2017;9:1310. doi: 10.3390/nu9121310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Relman D.A., Falkow S. The meaning and impact of the human genome sequence for microbiology. Trends Microbiol. 2001;9:206–214. doi: 10.1016/S0966-842X(01)02041-8. [DOI] [PubMed] [Google Scholar]
- 15.Li B.Y., Xu X.Y., Gan R.Y., Sun Q.C., Meng J.M., Shang A., Mao Q.Q., Li H.B. Targeting Gut Microbiota for the Prevention and Management of Diabetes Mellitus by Dietary Natural Products. Foods. 2019;8:440. doi: 10.3390/foods8100440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham A.L., Stephens J.W., Harris D.A. Gut microbiota influence in type 2 diabetes mellitus (T2DM) Gut Pathog. 2021;13:50. doi: 10.1186/s13099-021-00446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen N., Vogensen F.K., van den Berg F.W., Nielsen D.S., Andreasen A.S., Pedersen B.K., Al-Soud W.A., Sorensen S.J., Hansen L.H., Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilg H., Moschen A.R. Microbiota and diabetes: An evolving relationship. Gut. 2014;63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 19.Cani P.D., Possemiers S., Van de Wiele T., Guiot Y., Everard A., Rottier O., Geurts L., Naslain D., Neyrinck A., Lambert D.M., et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 21.Sedighi M., Razavi S., Navab-Moghadam F., Khamseh M.E., Alaei-Shahmiri F., Mehrtash A., Amirmozafari N. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. 2017;111:362–369. doi: 10.1016/j.micpath.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 22.Dabke K., Hendrick G., Devkota S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019;129:4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Yuan G., Pan Y., Wang C., Chen H. Network Pharmacology Studies on the Bioactive Compounds and Action Mechanisms of Natural Products for the Treatment of Diabetes Mellitus: A Review. Front. Pharmacol. 2017;8:74–84. doi: 10.3389/fphar.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hung W.-W., Hung W.-C. How gut microbiota relate to the oral antidiabetic treatment of type 2 diabetes. Med. Microecol. 2020;3:100007. doi: 10.1016/j.medmic.2020.100007. [DOI] [Google Scholar]
- 25.Li L.J., Wu Z.W., Xiao D.S., Sheng J.F. Changes of gut flora and endotoxin in rats with D-galactosamine-induced acute liver failure. World J. Gastroenterol. 2004;10:2087–2090. doi: 10.3748/wjg.v10.i14.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen H., Yang H., Deng J., Fan D. Ginsenoside Rk3 Ameliorates Obesity-Induced Colitis by Regulating of Intestinal Flora and the TLR4/NF-kappaB Signaling Pathway in C57BL/6 Mice. J. Agric. Food Chem. 2021;69:3082–3093. doi: 10.1021/acs.jafc.0c07805. [DOI] [PubMed] [Google Scholar]
- 27.Niu J., Pi Z.F., Yue H., Yang H., Wang Y., Yu Q., Liu S.Y. Effect of 20(S)-ginsenoside Rg3 on streptozotocin-induced experimental type 2 diabetic rats: A urinary metabonomics study by rapid-resolution liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2012;26:2683–2689. doi: 10.1002/rcm.6392. [DOI] [PubMed] [Google Scholar]
- 28.Bai L., Gao J., Wei F., Zhao J., Wang D., Wei J. Therapeutic Potential of Ginsenosides as an Adjuvant Treatment for Diabetes. Front. Pharmacol. 2018;9:423. doi: 10.3389/fphar.2018.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S.Y., Yuk H.G., Ko S.G., Cho S.G., Moon G.S. Gut Microbiome Prolongs an Inhibitory Effect of Korean Red Ginseng on High-Fat-Diet-Induced Mouse Obesity. Nutrients. 2021;13:926. doi: 10.3390/nu13030926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo J., Chai Y., Zhao M., Guo Q., Bao Y. Hypoglycemic effects and modulation of gut microbiota of diabetic mice by saponin from Polygonatum sibiricum. Food Funct. 2020;11:4327–4338. doi: 10.1039/D0FO00428F. [DOI] [PubMed] [Google Scholar]
- 31.Wang H.-Y., Guo L.-X., Hu W.-H., Peng Z.-T., Wang C., Chen Z.-C., Liu E.Y.L., Dong T.T.X., Wang T.-J., Tsim K.W.K. Polysaccharide from tuberous roots of Ophiopogon japonicus regulates gut microbiota and its metabolites during alleviation of high-fat diet-induced type-2 diabetes in mice. J. Funct. Foods. 2019;63:103593–103603. doi: 10.1016/j.jff.2019.103593. [DOI] [Google Scholar]
- 32.Wang L., Wang S., Wang Y., Ruan K.F., Feng Y. Effect of MDG-1 on oral glucose tolerance and intestinal microecological balance in diabetic mice. World Chin. J. Dig. 2011;19:2058–2062. doi: 10.11569/wcjd.v19.i19.2058. [DOI] [Google Scholar]
- 33.Yang Y., Chang Y., Wu Y., Liu H., Liu Q., Kang Z., Wu M., Yin H., Duan J. A homogeneous polysaccharide from Lycium barbarum: Structural characterizations, anti-obesity effects and impacts on gut microbiota. Int. J. Biol. Macromol. 2021;183:2074–2087. doi: 10.1016/j.ijbiomac.2021.05.209. [DOI] [PubMed] [Google Scholar]
- 34.Yang M.C., Yuan M.X., Lu W., Bao Y.H., Chai Y.Y. In Vitro Digestion Properties of Polygonatum sibiricum Polysaccharide and Its Regulatory Action on the Gut Microbiota in T2DM Mice. Mod. Food Sci. Technol. 2021;37:8–22. [Google Scholar]
- 35.Yuan Y., Zhou J., Zheng Y., Xu Z., Li Y., Zhou S., Zhang C. Beneficial effects of polysaccharide-rich extracts from Apocynum venetum leaves on hypoglycemic and gut microbiota in type 2 diabetic mice. Biomed. Pharmacother. 2020;127:110182. doi: 10.1016/j.biopha.2020.110182. [DOI] [PubMed] [Google Scholar]
- 36.Wang C., Yin Y., Cao X., Li X. Effects of Maydis stigma polysaccharide on the intestinal microflora in type-2 diabetes. Pharm. Biol. 2016;54:3086–3092. doi: 10.1080/13880209.2016.1211153. [DOI] [PubMed] [Google Scholar]
- 37.Nie Q., Hu J., Gao H., Fan L., Chen H., Nie S. Polysaccharide from Plantago asiatica L. attenuates hyperglycemia, hyperlipidemia and affects colon microbiota in type 2 diabetic rats. Food Hydrocol. 2019;86:34–42. doi: 10.1016/j.foodhyd.2017.12.026. [DOI] [Google Scholar]
- 38.Wang Q., Chai D.D., Wu X.H., Ren L.W., Liu Y.N., Yu Z.W. Radix Pseudostellariae polysaccharide attenuates high fat diet induced hepatic insulin resistance in mice. Chin. J. Pathophysiol. 2015;31:5–10. [Google Scholar]
- 39.Gao H., Wen J.J., Hu J.L., Nie Q.X., Chen H.H., Xiong T., Nie S.P., Xie M.Y. Polysaccharide from fermented Momordica charantia L. with Lactobacillus plantarum NCU116 ameliorates type 2 diabetes in rats. Carbohydr. Polym. 2018;201:624–633. doi: 10.1016/j.carbpol.2018.08.075. [DOI] [PubMed] [Google Scholar]
- 40.Chen C., You L.J., Huang Q., Fu X., Zhang B., Liu R.H., Li C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018;9:3732–3742. doi: 10.1039/C7FO01346A. [DOI] [PubMed] [Google Scholar]
- 41.Liu G., Liang L., Yu G., Li Q. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018;115:711–717. doi: 10.1016/j.ijbiomac.2018.04.127. [DOI] [PubMed] [Google Scholar]
- 42.Zhang B., Sun W., Yu N., Sun J., Yu X., Li X., Xing Y., Yan D., Ding Q., Xiu Z., et al. Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats. J. Funct. Foods. 2018;46:256–267. doi: 10.1016/j.jff.2018.04.070. [DOI] [Google Scholar]
- 43.Ahmed D., Khan M.I., Sharma M., Khan M.F. Novel pentacyclic triterpene isolated from seeds of Euryale Ferox Salisb. ameliorates diabetes in streptozotocin induced diabetic rats. Interdiscip. Toxicol. 2018;11:275–288. doi: 10.2478/intox-2018-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong J., Hu M., Huang Z., Fang K., Wang D., Chen Q., Li J., Yang D., Zou X., Xu L., et al. Berberine Attenuates Intestinal Mucosal Barrier Dysfunction in Type 2 Diabetic Rats. Front. Pharmacol. 2017;8:42–54. doi: 10.3389/fphar.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu D., Zhang Y., Liu Y., Hou L., Li S., Tian H., Zhao T. Berberine Modulates Gut Microbiota and Reduces Insulin Resistance via the TLR4 Signaling Pathway. Exp. Clin. Endocrinol. Diabetes. 2018;126:513–520. doi: 10.1055/s-0043-125066. [DOI] [PubMed] [Google Scholar]
- 46.Xu Z., Dai X.X., Zhang Q.Y., Su S.L., Yan H., Zhu Y., Shang E.X., Qian D.W., Duan J.A. Protective effects and mechanisms of Rehmannia glutinosa leaves total glycoside on early kidney injury in db/db mice. Biomed. Pharmacother. 2020;125:109926. doi: 10.1016/j.biopha.2020.109926. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y., Peng Y., Zhao L., Zhou G., Li X. Regulating the gut microbiota and SCFAs in the faeces of T2DM rats should be one of antidiabetic mechanisms of mogrosides in the fruits of Siraitia grosvenorii. J. Ethnopharmacol. 2021;274:114033–114044. doi: 10.1016/j.jep.2021.114033. [DOI] [PubMed] [Google Scholar]
- 48.Yuan H., Shi F., Meng L., Wang W. Effect of sea buckthorn protein on the intestinal microbial community in streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2018;107:1168–1174. doi: 10.1016/j.ijbiomac.2017.09.090. [DOI] [PubMed] [Google Scholar]
- 49.Chen K., Chen H., Faas M.M., de Haan B.J., Li J., Xiao P., Zhang H., Diana J., de Vos P., Sun J. Specific inulin-type fructan fibers protect against autoimmune diabetes by modulating gut immunity, barrier function, and microbiota homeostasis. Mol. Nutr. Food Res. 2017;61:1601006. doi: 10.1002/mnfr.201601006. [DOI] [PubMed] [Google Scholar]
- 50.Lira Neto J.C.G., Damasceno M.M.C., Ciol M.A., de Freitas R., de Araujo M.F.M., Teixeira C.R.S., Carvalho G.C.N., Lisboa K., Marques R.L.L., Alencar A., et al. Efficacy of Cinnamon as an Adjuvant in Reducing the Glycemic Biomarkers of Type 2 Diabetes Mellitus: A Three-Month, Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. J. Am. Coll. Nutr. 2021:1–9. doi: 10.1080/07315724.2021.1878967. (online ahead of print) [DOI] [PubMed] [Google Scholar]
- 51.Peng X.C., Huang L.Z., Zhan H.L., Wang C., Zhang N., Liu L. Effects of essential oil from Cinnamomum Cassia on Clostridia flora IV and Bacteroides in gut of rats. Chin. Tradit. Herb. Drugs. 2013;44:437–443. [Google Scholar]
- 52.Liu J., Henkel T. Traditional Chinese medicine (TCM): Are polyphenols and saponins the key ingredients triggering biological activities? Curr. Med. Chem. 2002;9:1483–1488. doi: 10.2174/0929867023369709. [DOI] [PubMed] [Google Scholar]
- 53.Adeshirlarijaney A., Gewirtz A.T. Considering gut microbiota in treatment of type 2 diabetes mellitus. Gut Microbes. 2020;11:253–264. doi: 10.1080/19490976.2020.1717719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Y., Guo M., Feng Y., Zheng H., Lei P., Ma X., Han X., Guan H., Hou D. Effect of ginseng polysaccharides on NK cell cytotoxicity in immunosuppressed mice. Exp. Ther. Med. 2016;12:3773–3777. doi: 10.3892/etm.2016.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pang B., Zhang Y., Liu J., He L.S., Zheng Y.J., Lian F.M. Correction to: Prevention of Type 2 diabetes with the Chinese herbal medicine tianqi capsule: A systematic review and meta-analysis. Diabetes Ther. 2017;8:1243–1245. doi: 10.1007/s13300-017-0331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tian J., Lian F., Yang L., Tong X. Evaluation of the Chinese Herbal medicine jinlida in Type 2 diabetes patients based on stratification: Results of subgroup analysis from a 12-week trial. J. Diabetes. 2018;10:112–120. doi: 10.1111/1753-0407.12559. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y.C., Lu B.J., Zhao M.H., Rong Y.Z., Chen R.M. Effect of shengmai injection on vascular endothelial and heart functions in patients with coronary heart disease complicated with diabetes mellitus. Chin. J. Integr. Med. 2008;14:281–286. doi: 10.1007/s11655-008-0281-3. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y., Choi H.K., Brinckmann J.A., Jiang X., Huang L. Chemical analysis of Panax quinquefolius (North American ginseng): A review. J. Chromatogr. A. 2015;14:1426. doi: 10.1016/j.chroma.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Gan L., Zhang S.H., Yang X.L., Xu H.B. Immunomodulation and antitumor activity by a polysaccharide protein complex from Lycium barbarum. Int. Immunopharmacol. 2004;4:563–569. doi: 10.1016/j.intimp.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 60.Parnell J.A., Reimer R.A. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes. 2012;3:29–34. doi: 10.4161/gmic.19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He J., Ye L., Fang C., Li J., Liu L., Zhang W. Identification of changes in volatile organic compounds in Ophiopogonis Radix containing spoiled products in different proportions by headspace-gas chromatography-ion mobility spectrometry. J. Food Biochem. 2021:e13802. doi: 10.1111/jfbc.13802. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y., Zhou Y. Shashen-Maidong Decoction-Mediated IFN-gamma and IL-4 on the Regulation of Th1/Th2 Imbalance in RP Rats. BioMed Res. Int. 2019;2019:6012473. doi: 10.1155/2019/6012473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He Q., Zhang T., Jin B., Wu Y., Wu J., Gao P., Wu S. Exploring the Regulatory Mechanism of Modified Huanglian Maidong Decoction on Type 2 Diabetes Mellitus Biological Network Based on Systematic Pharmacology. Evid. Based Complement. Altern. Med. 2021;2021:1768720. doi: 10.1155/2021/1768720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mao D., Tian X.Y., Mao D., Hung S.W., Wang C.C., Lau C.B.S., Lee H.M., Wong C.K., Chow E., Ming X., et al. A polysaccharide extract from the medicinal plant Maidong inhibits the IKK-NF-kappaB pathway and IL-1beta-induced islet inflammation and increases insulin secretion. J. Biol. Chem. 2020;295:12573–12587. doi: 10.1074/jbc.RA120.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L., Lao W., Ji Q.S., Yang Z.H., Yu G.C., Zhong J.X. Lycium barbarum polysaccharides protected human retinal pigment epithelial cells against oxidative stress-induced apoptosis. Int. J. Ophthalmol. 2015;8:11–16. doi: 10.3980/j.issn.2222-3959.2015.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao X.Q., Guo S., Lu Y.Y., Hua Y., Zhang F., Yan H., Shang E.X., Wang H.Q., Zhang W.H., Duan J.A. Lycium barbarum L. leaves ameliorate type 2 diabetes in rats by modulating metabolic profiles and gut microbiota composition. Biomed. Pharmacother. 2020;121:109559. doi: 10.1016/j.biopha.2019.109559. [DOI] [PubMed] [Google Scholar]
- 67.Samuelsen A.B. The traditional uses, chemical constituents and biological activities of Plantago major L. A review. J. Ethnopharmacol. 2000;71:1–21. doi: 10.1016/S0378-8741(00)00212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michaelsen T.E., Gilje A., Samuelsen A.B., Hogasen K., Paulsen B.S. Interaction between human complement and a pectin type polysaccharide fraction, PMII, from the leaves of Plantago major L. Scand. J. Immunol. 2000;52:483–490. doi: 10.1046/j.1365-3083.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 69.Ren H., Cao J., Chen Y., Li G. Current research state and exploitation of Apocynum venetum L. North. Hortic. 2008;7:4. [Google Scholar]
- 70.Everard A., Cani P.D. Diabetes, obesity and gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2013;27:73–83. doi: 10.1016/j.bpg.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Jin D.L., Chen X.B. Progress in research on hypoglycemic effect of traditional Chinese medicine. Zhejiang J. Integr. Tradit. Chin. West. Med. 2015;25:1–3. [Google Scholar]
- 72.Chen F., Liu D.B. Advances in anti-diabetes mechanism of active components in Traditional Chinese Medicine. Acta Chin. Med. Pharmacol. 2012;40:1–5. [Google Scholar]
- 73.Zhang B., Yue R., Chen Y., Yang M., Huang X., Shui J., Peng Y., Chin J. Gut Microbiota, a Potential New Target for Chinese Herbal Medicines in Treating Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2019;2019:2634898. doi: 10.1155/2019/2634898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou S.S., Xu J., Zhu H., Wu J., Xu J.D., Yan R., Li X.Y., Liu H.H., Duan S.M., Wang Z., et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci. Rep. 2016;6:22474. doi: 10.1038/srep22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng J., Tian W., Yang C., Shi W., Cao P., Long J., Xiao L., Wu Y., Liang J., Li X., et al. Identification of flavonoids in Plumula nelumbinis and evaluation of their antioxidant properties from different habitats. Ind. Crop. Prod. 2019;127:36–45. doi: 10.1016/j.indcrop.2018.08.020. [DOI] [Google Scholar]
- 76.Chen S., Li X., Wu J., Li J., Xiao M., Yang Y., Liu Z., Cheng Y. Plumula Nelumbinis: A review of traditional uses, phytochemistry, pharmacology, pharmacokinetics and safety. J. Ethnopharmacol. 2021;266:113429. doi: 10.1016/j.jep.2020.113429. [DOI] [PubMed] [Google Scholar]
- 77.Yuan H., Meng S., Wang G., Gong Z., Sun W., He G. Hypoglycemic effect of triterpenoid-rich extracts from Euryale ferox shell on normal and streptozotocin-diabetic mice. Pak. J. Pharm. Sci. 2014;27:859–864. [PubMed] [Google Scholar]
- 78.Zhang T.T., Jiang J.G. Active ingredients of traditional Chinese medicine in the treatment of diabetes and diabetic complications. Expert Opin. Investig. Drugs. 2012;21:1625–1642. doi: 10.1517/13543784.2012.713937. [DOI] [PubMed] [Google Scholar]
- 79.Wang N., Tan H.Y., Li L., Yuen M.F., Feng Y. Berberine and Coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015;176:35–48. doi: 10.1016/j.jep.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 80.Schiffelers M. Liposome encapsulated berberine treatment attenuates cardiac dysfunction after myocardial infarction. J. Control. Release. 2017;247:7. doi: 10.1016/j.jconrel.2016.12.042. [DOI] [PubMed] [Google Scholar]
- 81.Lan J., Zhao Y., Dong F., Yan Z., Zheng W., Fan J., Sun G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 82.Gu J.-F., Su S.-L., Guo J.-M., Zhu Y., Zhao M., Duan J.-A. The aerial parts of Salvia miltiorrhiza Bge. strengthen intestinal barrier and modulate gut microbiota imbalance in streptozocin-induced diabetic mice. J. Funct. Foods. 2017;36:362–374. doi: 10.1016/j.jff.2017.06.010. [DOI] [Google Scholar]
- 83.Wang T., Sun J., Liu C., He Q., Zhou X., Wan J. Effects of Fried Fructus Aurantii Immaturus with Wheat Bran Decoction on Intestinal Flora in rats with Functional Dyspepsia. Chin. Pharm. J. 2021;56:1068–1075. [Google Scholar]
- 84.Zhang W.-Y., Zhang H.-H., Yu C.-H., Fang J., Ying H.-Z. Ethanol extract of Atractylodis macrocephalae Rhizoma ameliorates insulin resistance and gut microbiota in type 2 diabetic db/db mice. J. Funct. Foods. 2017;39:139–151. doi: 10.1016/j.jff.2017.10.020. [DOI] [Google Scholar]
- 85.Yan H., Lu J., Wang Y., Gu W., Yang X., Yu J. Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine. 2017;26:45–54. doi: 10.1016/j.phymed.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 86.Xie Y., Xiao M., Ni Y., Jiang S., Feng G., Sang S., Du G. Alpinia oxyphylla Miq. Extract Prevents Diabetes in Mice by Modulating Gut Microbiota. J. Diabetes Res. 2018;2018:4230590. doi: 10.1155/2018/4230590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xue M., Liu Y., Xu H., Zhou Z., Ma Y., Sun T., Liu M., Zhang H., Liang H. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed. Pharmacother. 2019;118:109393. doi: 10.1016/j.biopha.2019.109393. [DOI] [PubMed] [Google Scholar]
- 88.Zheng Y., Wu Y., Tao L., Chen X., Jones T.J., Wang K., Hu F. Chinese Propolis Prevents Obesity and Metabolism Syndromes Induced by a High Fat Diet and Accompanied by an Altered Gut Microbiota Structure in Mice. Nutrients. 2020;12:959. doi: 10.3390/nu12040959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi Y., Bose S., Shin N.R., Song E.J., Nam Y.D., Kim H. Lactate-Fortified Puerariae Radix Fermented by Bifidobacterium breve Improved Diet-Induced Metabolic Dysregulation via Alteration of Gut Microbial Communities. Nutrients. 2020;12:276. doi: 10.3390/nu12020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sheng Y., Zheng S., Ma T., Zhang C., Ou X., He X., Xu W., Huang K. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci. Rep. 2017;7:12041. doi: 10.1038/s41598-017-12245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu S., Li F., Zhang X. Structural modulation of gut microbiota reveals Coix seed contributes to weight loss in mice. Appl. Microbiol. Biotechnol. 2019;103:5311–5321. doi: 10.1007/s00253-019-09786-z. [DOI] [PubMed] [Google Scholar]
- 92.Gao W., Hou M., Chen X., Wang P., Ren J., Liu J. Mechanism of Astragali Radix Vesicle-like Nanoparticles for Reducing Blood Glucose in db/db Diabetic Mice by Regulating Gut Microbiota. Chin. J. Exp. Tradit. Med. Formulae. 2021;27:111–118. [Google Scholar]
- 93.Wang S., Li X.Y., Shen L. Modulation effects of Dendrobium officinale on gut microbiota of type 2 diabetes model mice. FEMS Microbiol. Lett. 2021;368:1–5. doi: 10.1093/femsle/fnab020. [DOI] [PubMed] [Google Scholar]
- 94.Wang H. Master’s Thesis. Guangdong Pharmaceutical University; Guangzhou, China: 2016. Effects of Hemp Seed Oil-Water Mixture on Intestinal Microbial and Intestinal Immunity in Mice. [Google Scholar]
- 95.Xin H. Master’s Thesis. Fujian University of Traditional Chinese Medicine; Fuzhou, China: 2016. Effect Study of Yam Gruel on Bifidobacterium in the Gut with Diabetic Patients of Type 2. [Google Scholar]
- 96.Sforcin J.M., Bankova V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011;133:253–260. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 97.Zhou G., Wu F., Zhu J., Gao Y., Wan H. Effect of Wumeiwan on Intestinal Microflora, Inflammatory Factor and Short Chain Fatty Acids in Type 2 Diabetic Rat. Chin. J. Exp. Tradit. Med. Formulae. 2020;26:8–15. [Google Scholar]
- 98.Hussain A., Yadav M.K., Bose S., Wang J.H., Lim D., Song Y.K., Ko S.G., Kim H. Daesiho-Tang Is an Effective Herbal Formulation in Attenuation of Obesity in Mice through Alteration of Gene Expression and Modulation of Intestinal Microbiota. PLoS ONE. 2016;11:e0165483. doi: 10.1371/journal.pone.0165483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang C., Ma G., Deng Y., Wang X., Chen Y.C., Tu X.Y., Yu M., Sheng J. Effect of Gegen Qinlian Decoction on LPS, TNF-α, IL-6, and intestinal flora in diabetic KK-Ay mice. Chin. Tradit. Herb. Drugs. 2017;48:1611–1616. [Google Scholar]
- 100.Liu C.-Z., Chen W., Wang M.-X., Wang Y., Chen L.-Q., Zhao F., Shi Y., Liu H.-J., Dou X.-B., Liu C., et al. Dendrobium officinale Kimura et Migo and American ginseng mixture: A Chinese herbal formulation for gut microbiota modulation. Chin. J. Nat. Med. 2020;18:446–459. doi: 10.1016/S1875-5364(20)30052-2. [DOI] [PubMed] [Google Scholar]
- 101.Zhao J., Li Y., Sun M., Xin L., Wang T., Wei L., Yu C., Liu M., Ni Y., Lu R., et al. The Chinese Herbal Formula Shenzhu Tiaopi Granule Results in Metabolic Improvement in Type 2 Diabetic Rats by Modulating the Gut Microbiota. Evid. Based Complement. Altern. Med. 2019;2019:6976394. doi: 10.1155/2019/6976394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao K., Yang R., Zhang J., Wang Z., Jia C., Zhang F., Li S., Wang J., Murtaza G., Xie H., et al. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol. Res. 2018;130:93–109. doi: 10.1016/j.phrs.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 103.Fan S., Zhang C., Li X., Ao M., Yu L. Effect of Raw and Salt-processed Herb Pair Anemarrhenae Rhizoma-Phellodendri Chinensis Cortex on Gut Microbiota of Type 2 Diabetic Rats Based on 16S T Sequencing Technique. Pharmacol. Clin. Chin. Med. 2020;36:150–156. [Google Scholar]
- 104.Yang H.J., Kim M.J., Kwon D.Y., Kim D.S., Zhang T., Ha C., Park S. Combination of Aronia, Red Ginseng, Shiitake Mushroom and Nattokinase Potentiated Insulin Secretion and Reduced Insulin Resistance with Improving Gut Microbiome Dysbiosis in Insulin Deficient Type 2 Diabetic Rats. Nutrients. 2018;10:948. doi: 10.3390/nu10070948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiao S., Liu C., Chen M., Zou J., Zhang Z., Cui X., Jiang S., Shang E., Qian D., Duan J. Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites. Appl. Microbiol. Biotechnol. 2020;104:303–317. doi: 10.1007/s00253-019-10174-w. [DOI] [PubMed] [Google Scholar]