Abstract
Background
Around 30% world population affected by acute and chronic pain due to inflammation and accidental injuries. Pain is a uncomfortable sensation and it reduce the patients’ life quality.
Objective
The present exploration focuses to explore the beneficial effects of butein on the different chemical and thermal-provoked nociceptive and inflammatory mice models.
Methodology
The nociception was induced to the Swiss mice using different chemical (formalin, acetic acid, glutamate, and capsaicin) and thermal (hot plate and tail immersion) methods. the mice were supplemented with 10, 15, and 20 mg/kg of butein and respective standard drugs like morphine, diclofenac sodium, and dexamethasone. The anti-inflammatory effects of butein was studied using carrageenan-provoked inflammation in mice.
Results
The present findings clearly demonstrated that the butein was substantially lessened the different thermal and chemical provoked nociception in mice. The carrageenan-triggered paw edema and inflammatory cell infiltrations were appreciably suppressed by the butein treatment. The TNF-α, IL-1β, and IL-6 levels in the carrageenan-induced mice were effectively depleted by the butein.
Conclusion
Altogether, the present findings evidenced the potent antinociceptive and anti-inflammatory properties of the butein in different nociceptive mice models.
Keywords: Nociception, Butein, Inflammation, Tail immersion, Capsaicin
1. Introduction
Pain is a uncomfortable sensation due to the sensory and tissue injuries and it severely affects all the human functions (Puebla, 2005). Pain may arise in any part of the body for instance, muscles, joints, limbs, stomach, and head and is escorted by insomnia, anxiety, fatigue, less appetite, and even limb dysfunctions (Chou et al., 2016). Pain is instigated by nociceptors, a dedicated peripheral sensory neurons that was stimulated by deleterious stimuli like chemical, thermal, and mechanical stimuli. These nociceptors was primarily occurs in the muscle, tissues, vessels, and bone (Zakaria et al., 2019). Several mechanisms are actively participated in the regulation of pain for instance, certain receptors, regulators, and neurotransmitters concerned in a central and peripheral sensitization (Garimella and Cellini, 2013). The protection and warning are the primary roles of the pain in order to prevent the further damages. It cautions about the tissue injuries caused by damage or disease and provokes reflexion and behavioral response to lessen the effects of this injury. The significance of such behaviors is clearly demonstrated in the pathological incidences of innate insensitivity to painful stimuli (Sneddon, 2018). If the tissue injury is inevitable, the changes takes place in both central and peripheral nervous systems to express the hypersensitivity and pain as a result of inflammation in the tissues adjacent nerve systems.
It was reported that nearly 30% of the peoples around the world affected from acute and chronic pain, and these victims needs five times more health service than the others. As a result, pain is regarded as a global public health problem (Vasconcelos and de Araujo, 2018). Inflammation is a multifaceted process provoked by numerous stimuli for instance, mechanical damages, tissue ischemia, infectious, and toxic agents and distinguished by tissue changes which permits the intense relocation of immune cells to the inflammatory sites (Chen et al., 2018, Gupta et al., 2018). Inflammation is distinguished by numerous mechanisms relating augmented blood flow, vascular permeability and penetration of inflammatory cells into the injury site (Serhan et al., 2015). Pain is a general indicator of inflammation, and it provides as a guarding mechanism provoked by the hostile sensory experiences connected with tissue injury (Raja et al., 2020). Additionally, pain is a representative of inflammation due to the tissue damages (Oliveira et al., 2016).
The extreme release of inflammatory regulators could directs to the triggering of inflammatory pain (Sekiguchi et al., 2018, Ji et al., 2016). For instance, the inflammatory regulators like TNF-α, IL-1β, and IL-6, which is generated by macrophages and other immune cells interrelate with nociceptive neurons and regulated central sensitization and pain (Pinho-Ribeiro et al., 2017, Cook et al., 2018). Additionally, other mediators generated by immune cells like prostaglandins and histamines also performs a significant functions in pain mediation (Obara et al., 2020). In this case, inflammation and pain has the closely regulated connections, since the nociceptive neurons also mediate the inflammatory reactions (Matsuda et al., 2019).
The primary goal of pain treatment is to lessen or eradicate the pain and uneasiness with none adverse effects (Pogatzki-Zahn et al., 2017). The anti-inflammatory and analgesic medications are generally administered to lessen the pain (Moraes et al., 2020, Goucke and Chaudakshetrin, 2018). Currently existing medications for the pain management are opiates like morphine and pentazocine or non– steroidal anti-inflammatory drugs (NSAIDs) like diclofenac and indomethacin (Kumar and Shankar, 2009). Both NSAIDs and opiates are often experienced with many side effects for instance, depletion of respiration, cardiovascular complications, gastric injury, nephron and hepatotoxicities (Lacy et al., 2008). The novel drugs to alleviate the pain without possessing any adverse effects are greatly required around the world as a potential alternatives to conventional synthetic drugs. Recently, a great interests has been paid to screen the novel natural agents with antinociceptive potentials to lessen the pain without side effects.
The herbal plants were extensively utilized in the form of medications to alleviate the pain since history with less or no toxicities for instance, morphine and acetyl salicylic acid are the most imperative analgesics primarily obtained from plant sources (Almeida et al., 2001, Khan et al., 2011). Butein (3,4,2′,4′‐Tetrahydroxychalcone) is a chalcone compound occurs in a several medicinal plants like Toxicodendron vernicifluum (Yang et al., 2012, Padmavathi et al., 2015). Butein was already reported to hold multitude pharmacological activities like anticancer, anti-inflammatory, antimicrobial, and antioxidant property (Padmavathi et al., 2017). Butein also demonstrated the anti-depressive (Guan and Liu, 2016), nueroprotective (Cho et al., 2012), anti-adipogenic, antidiabetic, and antifibrogenic properties (Semwal et al., 2015). Though, the anti-inflammatory role of butein was well reported, the antinociceptive actions of butein was remain unexplored. The current study focuses to explore the beneficial actions of butein against the different chemical and thermal stimuli provoked nociceptive and inflammatory mice models.
2. Materials and methods
2.1. Chemicals
Butein, naloxone, morphine, diclofenac sodium, dexamethasone, formalin, acetic acid, capsaicin, glutamate, and other chemicals were procured from the Sigma-Aldrich, USA. The assay kits inflammatory markers were purchased from Biocompare, USA.
2.2. Experimental animals
The Swiss albino mice from both genders, 6–8 weeks aged, weighing 25–35 g were purchased from the institutional animal facility and preserved in a organized infection free environment (temperature 23 ± 2 °C, 12 h light/dark series) in throughout the experiments with free access to rodent-specific diet and water. All animals were fasted for 2 h of food in prior to the investigations. All the experiments were done as per the guidelines suggested by institutional ethics committee. Animals were adapted for seven days in the laboratory before the experiments initiation.
2.3. Hot plate test
Hot plate test was executed in order to assess the nociceptive responses of experimental animals (Carino et al., 2010)). For this, animals were located into the acrylic cylinder on the heated surface (55 ± 0.2 °C). The time gap between the placement of animals onto the platform and hind paws licking or shaking or jumping was noted carefully as a latency of response. Animals were supplemented with the diverse doses of butein (10, 15, and 20 mg/kg) on 30 min before the thermal stimulus in a hot plate. Morphine (5 mg/kg) was administered as a positive control. the opioid antagonist naloxone (2 mg/kg) was also administered along with the butein and morphine to detect the reversal effects. All animals were carefully monitored before and at 0, 30, 60, 90, and 120 min after the respective sample drug supplementation. The cut-off of 30 s were fixed and this exposition period were sufficient to notice any mice responses without provoking any tissue injuries.
2.4. Tail immersion test
The central anti-nociceptive action of butein was detected as per the procedure described by Shwetha et al. (2014). The water bath was adjusted at a preferred temperature (55 ± 0.5 °C) for this assay. For this assay, mice were treated with the sample and standard drugs as specified in the hot plate test. Roughly 3 cm of the mice’s tail end was waterlogged in hot water. The responses of pain was noticed if a speedy removal of tail from the hot water. The time of tail immersion and withdrawal from hot water were noticed on 30 min before and after the 30, 60, 90, and 120 min of treatment were noted carefully. As the over tail immersion in hot water may cause tissue damages, 15 s time period were fixed as maximal immersion time and an increased immersion time denotes the analgesic effect of butein.
2.5. Acetic acid-induced nociceptive assay
The antinociceptive potential of butein was scrutinized by acetic acid-provoked writhing test as described by Koster et al. (1959). For this, animals were supplemented with the butein (10, 15, and 20 mg/kg) and diclofenac (5 mg/kg) as a positive control 30 min before the acetic acid challenge (0.1 ml/10 g b.wt 0.6% v/v). The total incidences of abdominal writhing during 25 min after the acetic acid challenge were noted and tabulated.
2.6. Capsaicin and glutamate-induced nociceptive assays
The antinociceptive actions of butein was assessed by capsaicin and glutamate-provoked nociceptive models as recommended by Giorno et al. (2015). All mice were supplemented with the sample and standard drugs as specified in the acetic acid test on one hour before the intraplantar administration of capsaicin (20 µl, 1.6 µg/paw). All animals were located separately onto the transparent glass monitoring cabins to assess the nociceptive responsive and the paw licking numbers were noted and tabulated. For the glutamate-provoked licking assay, animals were treated respectively as specified in the acetic acid test on 60 min before the intraplantar administration of glutamate (20 µl, 3.7 ng/paw). After the administration, all mice were located onto the transparent glass monitoring cabins and the total licking numbers were tabulated.
2.7. Formalin-induced nociceptive assay
The formalin provoked nociceptive assay was performed as described earlier by Ortiz and Castaneda, (2008). For this, 1% formalin in 0.9% saline, were administered (50 μl/paw) onto the hind paw surface of animals (i.p), and mice were located on a transparent monitoring cabins. Animal were supplemented with the butein (10, 15, and 20 mg/kg) and morphine (5 mg/kg) as a positive control 30 min before the formalin administration. To determine the local antinociceptive effects, the relative time period of animals spent for flinching the formalin administered paw was noticed and tabulated.
2.8. Carrageenan-induced paw edema
The anti-inflammatory potentials of butein was assessed against the carrageenan-triggered paw edema as described by Ponce-Monter et al. (2010). The paw edema of animals were assessed using plethysmometer, the basal right hind paw volume was detected before any treatments. After that, mice were supplemented with the butein (10, 15, and 20 mg/kg) and indomethacin (5 mg/kg) as a positive control orally on 30 min before the carrageenan (100 μl/paw i.p.) administration. The paw volume of each animals were assessed 4 h after the inflammatory provocation.
2.9. Determination of leukocyte infiltration on the peritoneal cavity
The anti-inflammatory property of butein were scrutinized by measuring the leukocyte penetrations on the peritoneal cavity. For this, animals were treated with the sample and standard drugs as specified in the formalin test. After the 1 h, animals were challenged with 500 µg of carrageenan (1% i.p.). Then the peritoneal fluid was gathered after the 6 h of carrageenan challenge and the leukocytes content in the peritoneal fluid were determined in order to detect the inflammatory responses in the control and treated mice.
2.10. Determination of inflammatory markers in the carrageenan induced air pouch model
The decreasing effect of butein on the inflammatory markers in the carrageenan-provoked air pouch test was performed. In prior to the experiment initiation, animals were anesthetized with ether and back of the animals were shaved properly using clean blades. The air pouch was created through administering clean air into the mice’s back twice at a period of three days. Animals with pouches were challenged with the 0.5 ml carrageenan to stimulate the inflammatory response then supplemented with the butein (10, 15, and 20 mg/kg) and dexamethasone (5 mg/kg) as a positive control. After an hour, animals were sacrificed and air pouches of animals injected with 2 ml of saline to gather the inflammatory cells. The levels of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 were detected using respective assay kits (Biocompare, USA).
2.11. Determination of behavioural changes by open field test
The open field test was employed to detect the sedative effects of butein on the animals. For this, animals were supplemented For this, animals were treated with the sample and standard drugs as specified in the formalin test. After 1 h of treatments, all mice was placed onto the open field setup measuring 50 cm × 50 cm × 50 cm and separated into 25 squares. The behavioral changes in the animals were detected.
2.12. Statistical analysis
Results were represented as mean ± SD of the data achieved from triplicate evaluations. One-way ANOVA successively Dunnett's test was performed for statistical study of outcomes engaging SPSS version 22.0. For significance between groups, p < 0.05 was fixed.
3. Results
3.1. Butein reduced the hot plate-induced nociception in mice
The inhibitory effects of butein on the hot plate provoked nociception in the mice was evaluated and the outcomes were presented in the Table 1. The response time of the control animals were relatively lesser than the butein administered animals. The butein (10, 15, and 20 mg/kg) supplemented animals demonstrated the response time in the hot plate when related with the control, which demonstrates the antinociceptive action. The standard drug morphine (5 mg/kg) also improved the response time of mice on the hot plate, which resembles the 20 mg/kg of butein treatment (Table 1). The butein treatment also improved the response time of mice on the hot plate when administered along with the naloxone (2 mg/kg).
Table 1.
Effect of butein on the hot plate-induced nociception in mice.
| Treatment (mg/kg) |
pre treatment | Response time(s)(%MPE) |
|||
|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | ||
| Control | 10.23 ± 0.28 | 10.38 ± 0.26 | 10.72 ± 0.29 | 10.9 ± 0.31 | 12.44 ± 0.68 |
| Butein (5 mg) | 10.24 ± 0.47 | 12.27 ± 0.61 | 14.32 ± 0.83 | 15.23 ± 0.94 | 15.61 ± 0.99 |
| Butein (10 mg) | 9.53 ± 0.37 | 12.15 ± 0.68 | 14.45 ± 0.73 | 15.27 ± 0.91 | 15.64 ± 0.96 |
| Butein (15 mg) | 10.05 ± 0.48 | 13.20 ± 0.77 | 14.66 ± 0.81 | 15.37 ± 0.93 | 16.83 ± 1.12 |
| Morphine(5 mg) | 9.42 ± 0.34 | 15.44 ± 0.93 | 17.14 ± 1.15 | 18.23 ± 1.22 | 19.73 ± 1.27 |
| NLX(2 mg) + Control | 9.75 ± 0.37 | 9.95 ± 0.39 | 10.34 ± 0.42 | 10.84 ± 0.45 | 11.13 ± 0.53 |
| NLX(2 mg) + Butein (5 mg) | 9.94 ± 0.39 | 10.44 ± 0.43 | 11.16 ± 0.54 | 11.56 ± 0.57 | 13.28 ± 0.74 |
| NLX(2 mg) + Butein (10 mg) | 9.80 ± 0.36 | 10.14 ± 0.42 | 11.53 ± 0.54 | 12.36 ± 0.67 | 13.35 ± 0.71 |
| NLX(2 mg) + Butein (15 mg) | 9.26 ± 0.33 | 10.08 ± 0.41 | 10.77 ± 0.48 | 12.56 ± 0.69 | 14.34 ± 0.82 |
| NLX(2 mg) + Morphine(5 mg) | 9.98 ± 0.39 | 10.27 ± 0.46 | 11.59 ± 0.53 | 13.33 ± 0.74 | 16.83 ± 1.28 |
Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
3.2. Butein lessened the tail immersion induced nociception in mice
The antinociceptive actions of butein was assessed by tail immersion test and the outcomes were represented in the Table 2. The response time of the butein (10, 15, and 20 mg/kg) administered animals were relatively improved in the tail immersion on hot water when evaluated with control. The untreated control animals exhibited the reduced response time than the butein treated animals. The morphine treatment also improved the tail immersion response time, which is similar with butein treatment. Even with the naloxone, the butein and morphine treatments improved the response time of tail immersion, which indicates the antinocicpetive activity of butein (Table 1).
Table 2.
Effect of butein on the tail immersion induced nociception in mice.
| Treatment (mg/kg) |
pre treatment | Response time(s)(%MPE) |
|||
|---|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | ||
| Control | 5.29 ± 0.32 | 6.15 ± 0.43 | 6.27 ± 0.47 | 6.88 ± 0.49 | 5.48 ± 0.37 |
| Butein (5 mg) | 5.12 ± 0.36 | 5.83 ± 0.34 | 6.07 ± 0.42 | 7.07 ± 0.51 | 7.21 ± 0.53 |
| Butein (10 mg) | 4.68 ± 0.23 | 5.97 ± 0.35 | 6.69 ± 0.48 | 7.56 ± 0.53 | 8.28 ± 0.64 |
| Butein (15 mg) | 4.74 ± 0.27 | 4.95 ± 0.29 | 7.00 ± 0.53 | 7.75 ± 0.58 | 8.16 ± 0.67 |
| Morphine(5 mg) | 4.93 ± 0.29 | 6.85 ± 0.43 | 7.20 ± 0.56 | 7.75 ± 0.59 | 8.35 ± 0.64 |
| NLX(2 mg) + Control | 4.85 ± 0.27 | 5.04 ± 0.32 | 5.37 ± 0.37 | 5.84 ± 0.39 | 6.2 ± 0.43 |
| NLX(2 mg) + Butein (5 mg) | 5.03 ± 0.36 | 5.82 ± 0.39 | 6.05 ± 0.41 | 6.35 ± 0.43 | 6.65 ± 0.47 |
| NLX(2 mg) + Butein (10 mg) | 4.85 ± 0.24 | 7.68 ± 0.53 | 8.05 ± 0.61 | 8.31 ± 0.67 | 8.64 ± 0.69 |
| NLX(2 mg) + Butein (15 mg) | 5.27 ± 0.34 | 5.96 ± 0.37 | 6.14 ± 0.43 | 6.56 ± 0.46 | 6.72 ± 0.48 |
| NLX(2 mg) + Morphine(5 mg) | 3.77 ± 0.13 | 4.68 ± 0.27 | 4.95 ± 0.29 | 5.15 ± 0.34 | 6.19 ± 0.47 |
Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
3.3. Butein decreased the carrageenan-induced paw edema in mice
The inhibitory effects of butein against the provoked paw edema was investigated and the outcomes were depicted in the Table 3. The treatment with the 10, 15, and 20 mg/kg of butein was remarkably decreased the carrageenan-provoked paw edema in mice, when related with control. The 20 mg/kg of butein demonstrated the better action than the other doses and the outcomes were closely related with the indomethacin (10 mg/kg) treatment.
Table 3.
Effect of butein on the carrageenan-induced paw edema in mice.
| Treatment(mg/kg) | Response time(s)(%MPE) |
||||
|---|---|---|---|---|---|
| Basal | 1st h | 2nd h | 3rd h | 4th h | |
| Control | 49.37 ± 16.37 | 173.97 ± 87.13 | 164.88 ± 80.64 | 153.74 ± 63.79 | 125.53 ± 40.32 |
| Butein (5 mg) | 46.14 ± 14.43 | 115.64 ± 33.39 | 112.82 ± 31.77 | 108.23 ± 28.22 | 98.52 ± 25.67 |
| Butein (10 mg) | 45.81 ± 16.39 | 108.28 ± 28.74 | 104.36 ± 24.13 | 91.37 ± 21.73 | 87.88 ± 19.83 |
| Butein (15 mg) | 48.28 ± 18.56 | 110.91 ± 30.47 | 98.90 ± 25.23 | 90.23 ± 20.49 | 86.71 ± 18.91 |
| Indomethacin(10 mg) | 47.27 ± 17.93 | 92.71 ± 22.78 | 88.83 ± 20.37 | 84.50 ± 17.83 | 76.02 ± 16.47 |
Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
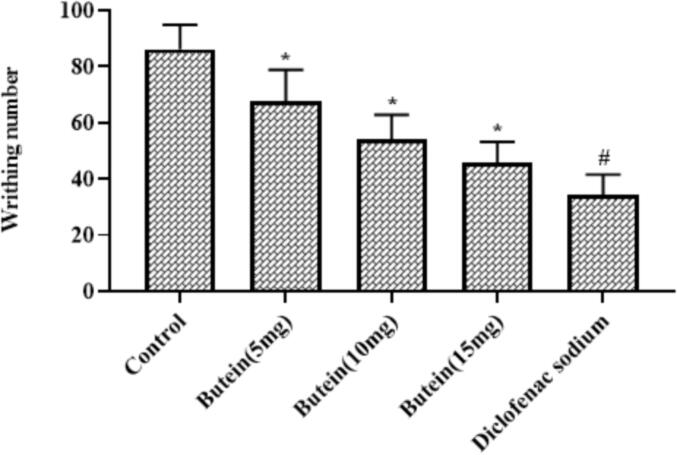
3.4. Butein reduced the acetic acid-induced nociception in mice
As demonstrated in the Fig. 1, the acetic acid-induced animals displayed the improved writing numbers. Interestingly, the treatment with the 10, 15, and 20 mg/kg of butein substantially decreased the writhing number in the acetic acid-provoked animals. The standard drug diclofenac sodium also decreased the writhing number of acetic acid-provoked animals. The outcomes of 20 mg/kg of butein and diclofenac sodium treatments were found similar with each other, which demonstrates the antinocicetive actions of butein (Fig. 1).
Fig. 1.
Effect of butein on the acetic acid-induced nociception in mice Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
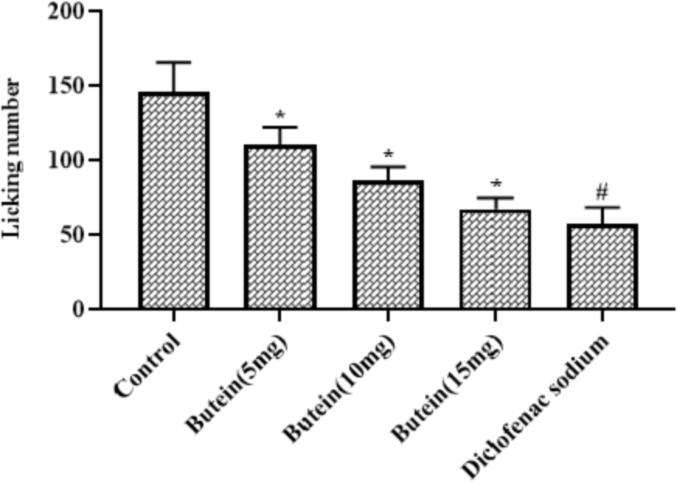
3.5. Butein reduced the glutamate-induced nociception in mice
Fig. 2 demonstrates the inhibitory effects of butein on the glutamate-provoked nociception in mice. The glutamate-provoked animals displayed the increased licking numbers that shows the pain sensation. Conversely, the butein (10, 15, and 20 mg/kg) supplementation demonstrates the substantial reduction in the writhing number of glutamate-challenged animals (Fig. 2). Both diclofenac sodium and 20 mg/kg of butein treatment effectively reduced the writhing numbers of glutamate-provoked animals.
Fig. 2.
Effect of butein on the glutamate-induced nociception in mice Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
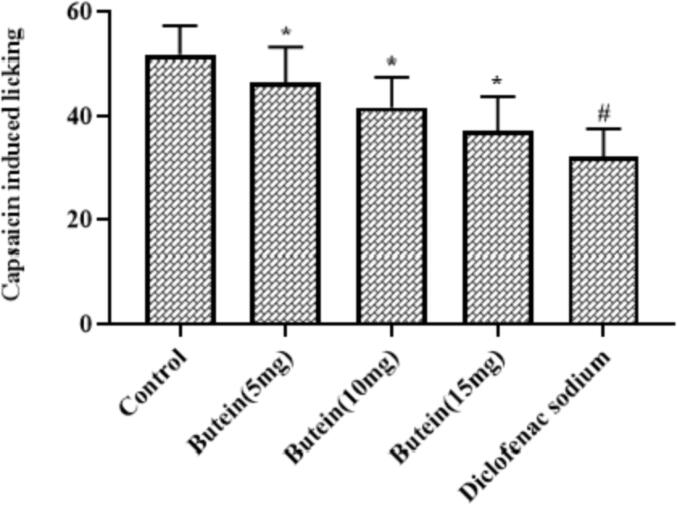
3.6. Butein lessened the capsaicin induced nociception in mice
As represented in the Fig. 3, the capsaicin challenge remarkably improved the licking numbers of mice. Interestingly, the 10, 15, and 20 mg/kg of butein treatment markedly suppressed the capsaicin-triggered licking numbers in the mice, which shows its antinociceptive property. The diclofenac sodium treatment also decreased the capsaicin-provoked licking numbers in the mice, which is similar to the 20 mg/kg of butein treatment.
Fig. 3.
Effect of butein on the capsaicin induced nociception in mice Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
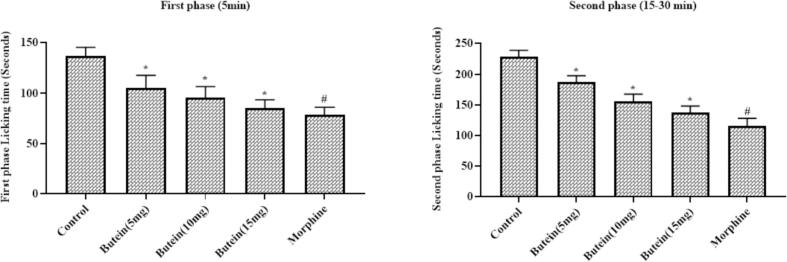
3.7. Butein decreased the formalin-induced nociception in mice
Fig. 4 represents the inhibitory effects of butein on the formalin-provoked biphasic nociception in mice. The formalin alone administered animals displayed the increased licking numbers, which indicates the nociception. Interestingly, the treatment with the 10, 15, and 20 mg/kg of butein was substantially lessened the formalin-triggered licking numbers in both phases. The morphine treatment also reduced the formalin-provoked licking numbers in both phases. The outcomes of 20 mg/kg of butein and morphine treatments were found similar with each other (Fig. 4).
Fig. 4.
Effect of butein on the formalin induced nociception in mice Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
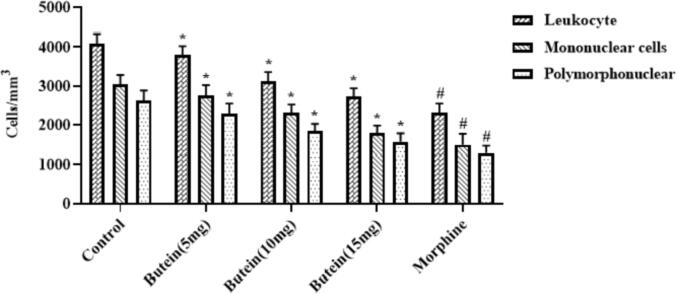
3.8. Butein reduced the carrageenan induced peritoneal leukocyte infiltration in mice
Fig. 5 displays the inhibitory effect of butein on the carrageenan-triggered peritoneal inflammatory cell infiltrations in the mice. It revealed that the carrageenan-provoked animals demonstrated the increased peritoneal infiltrations of leukocytes, mononuclear, and polymorphonuclear cells. These infiltrations were appreciably decreased by the butein treatment. The supplementation of 10, 15, and 20 mg/kg of butein was remarkably suppressed the peritoneal leukocytes, mononuclear, and polymorphonuclear cell infiltrations (Fig. 5). The morphine treatment also decreased the carrageenan-triggered peritoneal inflammatory cell infiltrations.
Fig. 5.
Effect of butein on the carrageenan-induced peritoneal leukocyte infiltration in mice Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
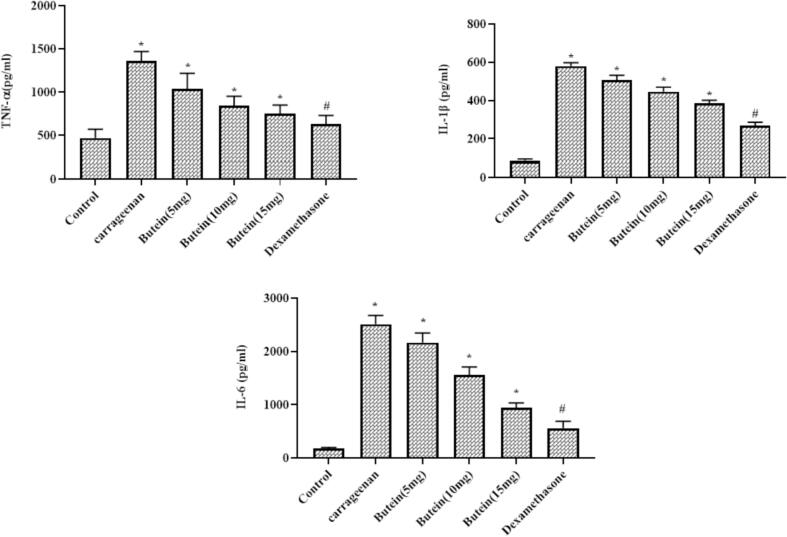
3.9. Butein reduced the pro-inflammatory cytokines in the carrageenan induced air pouch mice model
As represented in the Fig. 6, the TNF-α, IL-1β, and IL-6 levels were markedly increased in the carrageenan-provoked air pouch animals. Conversely, these elevations were appreciably suppressed by the butein treatment. The administration of 10, 15, and 20 mg/kg of butein markedly depleted the TNF-α, IL-1β, and IL-6 levels in the carrageenan-challenged mice air pouch. The dexamethasone treatment also lessened the TNF-α, IL-1β, and IL-6 status that is similar to the 20 mg/kg of butein, which demonstrate its anti-inflammatory property (Fig. 6).
Fig. 6.
Effect of butein on the pro-inflammatory cytokines in the carrageenan induced air pouch mice model Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
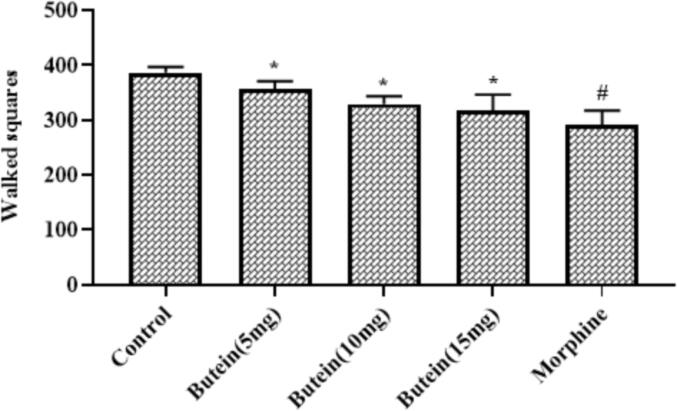
3.10. Butein reduced the behavioural changes in the mice assessed by open field test
The sedative effects of butein was evaluated by open field test and the outcomes were illustrated in the Fig. 7. The 10, 15, and 20 mg/kg of butein supplemented animals displayed the relatively decreased walking squares than the control, which indicates the mild sedative effects. The morphine treatment also reduced the walked squares due to sedative effects, which is found similar to the 20 mg/kg of butein treatment.
Fig. 7.
Effect of butein on the behavioural changes in the mice assessed by open field test Results were represented as mean ± SD obtained from three separate measurements. Outcomes were scrutinized by one-way ANOVA and Dunnett's test using SPSS. ‘*’ p < 0.05 compared with control and ‘#’ p < 0.01 compared with butein treated animals.
4. Discussion
Pain is triggered by various mechanisms, and it can be categorised as neuropathic, inflammatory and/or physiological pain (Campbell and Meyer, 2006). The more number of deleterious endogenous and exogenous pathological events like damages, autoimmune reactions, infections, tissue dysfunctions can stimulate the several biochemical cascades that could directs to the development of inflammatory reactions and pain initiation. The poorly treated pain could directs to the several prolonged complications. The unrestrained acute pain is related with the chronic pain development, with the patients life quality reduction. The activation of inflammatory cells and penetration with proinflammatory regulators release participate in the nociception (Leuti et al., 2020).
The severe inflammatory reaction is distinguished by enhanced vascular permeability and inflammatory cell penetration that directs to the edema development, as a consequence of several protein and fluid extravasation and leukocyte accretion at the inflammatory sites (Posadas et al, 2004). IL-1β are the vital player in the commencement and elevation of inflammatory reactions participated in numerous cellular functions. TNF-α actively participates in the inflammatory reactions that involved in initiating its own accretion and generation of other inflammatory mediators (Chu, 2013, Lin et al., 2014). There are plentiful reports that highlights several proinflammatory mediators for instance, IL-1B, IL-6, and TNF-α are the vital players of pain (Zhang and An, 2007). Our findings from this study unveiled that the butein treatment substantially alleviated the IL-1B, IL-6, and TNF-α status in mice that demonstrates its strong anti-inflammatory properties.
In carrageenan-provoked paw edema assay, the inflammatory reaction provoked by carrageenan is distinguished by the biphasic response, a marked edema resulting from the over accumulation of numerous inflammatory regulators like histamines and bradykinins were observed during phase I. The phase II is distinguished by the prostaglandin release acquiring a peak at 3 h (Seibert et al., 1994). Our findings of this assay markedly proved that the butein administration remarkably suppressed the carrageenan-provoked edema in mice through its strong anti-inflammatory property.
The opioid analgesics are known as a antinociceptive in both phases, though the first phase is highly sensitive to these analgesics. Contrastingly, NSAIDs like indomethacin are know to lessen only second phase, although the analgesic property of diclofenac involves its anti-inflammatory actions (Ortiz et al., 2012). The systemic and local effects of butein in second denote the probable anti-inflammatory property, hindering the inflammatory regulators release that further sensitize and stimulate the peripheral nociceptors. The nociceptive effects provoked by acetic acid is also reliant on the deliverance of inflammatory regulators like IL-6 and TNF-α via regulating the macrophage and mast cell responses situated in the peritoneal cavity (Couture et al., 2001). We found that the butein supplementation effectively depleted the inflammatory cells like leukocytes, monocytes, and polymorphonuclear cells in the peritoneal cavity of mice.
Glutamate is a primary regulator of excitatory synaptic transmission in the central nervous system and stimulates numerous intracellular mechanisms, for instance changes in the intracellular calcium status, stimulation of cellular regulators and ion channel openings (Zhuo, 2017). Glutamate also triggers the discharge of prostaglandins, kinins, and excitatory amino acids and enhances the sensitive fibers stimulation that trigger the discharge of several mediators (Beirith et al., 2002). Capsaicin is an well known agonist of pain receptors and can stimulate the nociceptive fibers. These provocations is also regulated by the discharge of many neurotransmitters, which actively participates in the initiation of nociception (Medvedeva et al., 2008). Our data clearly proved that the butein supplementation demonstrated a marked reduction in the licking events of mice, which is administered with capsaicin and glutamate. Hence it was clear that the butein can alleviate the glutamate and capsaicin provoked nociception in mice.
The tail immersion assay is well established model to study the antinociceptive properties of sample drugs, in which the latency time was determined. Based on the intensity of heat activation, the tail immersion assay encompasses supraspinal and spinal systems (Negus et al., 2006). It is remarkable that related to other options of heat stimulation, the tail immersion assay does not provoke perceptible stimulus because the heat beaming establishes a reasonably selective stimulus for nociceptors. The latency time extension in the tail immersion assay is connected to the central analgesic outcome of managed drugs (Hutchinson et al., 2004). In this exploration, we found that the butein administered mice demonstrated the enhanced latency time in the tail immersion, which proves the antinociceptive actions of butein.
The formalin assay is another well known model generally employed for detecting the antinociceptive actions of sample drug. The formalin-provoked assay encompasses central, inflammatory, and neurogenic mechanisms of nociception, which makes it a most suitable model for antinociceptive study (Mogil et al., 2010). To extricate the peripheral and central antinociceptive actions of butein, the formalin-provoked nociceptive assay was executed. The subcutaneous administration of formalin to the hind paw demonstrates the biphasic nociceptive response. The early phase is a neurogenic pain which is provoked by direct stimulation of nociceptive fibers and neuropeptides discharge. The late phase is a inflammatory pain aroused due to the inflammatory responses provoked by tissue damage with successive discharge of inflammatory regulators like prostaglandins and sensitization in the spinal cord (Le Bars et al., 2001). The outcomes of the formalin-provoked nociceptive assay exhibited that the butein markedly lessened the licking incidences of mice in both phases, which proves the antinociceptive actions of butein.
The acetic acid-provoked writhing test were extensively employed to scrutinize the antinociceptive actions of sample drugs. It is known as a sensitive technique to evaluate the peripheral analgesics on visceral and inflammatory nociception. The nociception in the acetic acid-provoked writhing is because of the local inflammatory reactions mediate by the discharge of endogenous regulators, which trigger the peripheral pain nerves. In animals, it enhances the prostaglandin, serotonin, histamine, bradykinin, and other inflammatory regulators (Begnami et al., 2018). In this exploration, we noted that the butein supplementation substantially lessened the abdominal writhing incidences in the acetic acid provoked mice, which demonstrates its antinocicpetive action.
5. Conclusion
In conclusion, the present findings revealed the antinociceptive and anti-inflammatory properties on the different thermal and chemical induced pain and inflammatory models. Butein remarkably suppressed the chemical and thermal-provoked nociception and carrageenan-provoked inflammation mice. These findings recommend the additional studies in the future to better understanding of antinociceptive and anti-inflammatory properties of butein in order to develop a novel analgesic agent.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This project was supported by Researchers Supporting Project number (RSP-2021/230) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Almeida R.N., Navarro D.S., Barbosa J.M. Plants with central analgesic activity. Phytomedicine. 2001;8:310e322. doi: 10.1078/0944-7113-00050. [DOI] [PubMed] [Google Scholar]
- Begnami A.F., Spindola H.M., Ruiz A.L.T.G., de Carvalho J.E., Groppo F.C., Rehder V.L.G. Antinociceptive and anti-edema properties of the ethyl acetate fraction obtained from extracts of Coriandrum sativum Linn. Leaves. Biomed. Pharmacother. 2018;103:1617–1622. doi: 10.1016/j.biopha.2018.04.196. [DOI] [PubMed] [Google Scholar]
- Beirith A., Santos A.R.S., Calixto J.B. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002;924:219–228. doi: 10.1016/s0006-8993(01)03240-1. [DOI] [PubMed] [Google Scholar]
- Campbell J.N., Meyer R.A. Mechanisms of neuropathic pain. Neuron. 2006;52(1):77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariño-Cortés R., Gayosso-De-Lucio J.A., Ortiz M.I., Sánchez-Gutiérrez M., García-Reyna P.B., Cilia-López V.G., Pérez-Hernández N., Moreno E., Ponce-Monter H. Antinociceptive, genotoxic and histopathological study of Heliopsis longipes S.F. Blake in mice. J. Ethnopharmacol. 2010;130(2):216–221. doi: 10.1016/j.jep.2010.04.037. [DOI] [PubMed] [Google Scholar]
- Chen L., Deng H., Cui H., et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N., Choi J.H., Yang H., Jeong E.J., Lee K.Y., Kim Y.C., Sung S.H. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem. Toxicol. 2012;50(6):1940–1945. doi: 10.1016/j.fct.2012.03.052. [DOI] [PubMed] [Google Scholar]
- Chou R., Gordon D.B., de Leon-Casasola O.A., Rosenberg J.M., Bickler S., Brennan T., Carter T., Cassidy C.L., Chittenden E.H., Degenhardt E., Griffith S., Manworren R., McCarberg B., Montgomery R., Murphy J., Perkal M.F., Suresh S., Sluka K., Strassels S., Thirlby R., Viscusi E., Walco G.A., Warner L., Weisman S.J., Wu C.L. Management of postoperative pain: a clinical practice guideline from the American pain society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J. Pain. 2016;17(2):131–157. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Chu W.-M. Tumor necrosis factor. Cancer Lett. 2013;328(2):222–225. doi: 10.1016/j.canlet.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A.D., Christensen A.D., Tewari D., McMahon S.B., Hamilton J.A. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39(3):240–255. doi: 10.1016/j.it.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Couture R., Harrisson M., Vianna R.M., Cloutier F. Kinin receptors in pain inflammation. Eur. J. Pharmacol. 2001;429:161–176. doi: 10.1016/s0014-2999(01)01318-8. [DOI] [PubMed] [Google Scholar]
- De Moraes, S.Z.; da Silva, J.P.; Melo, M.A.O.; Shan, A.Y.K.V.; de Araújo, B.S.; Quintans, J.d.S.S.; Passos, F.R.; Júnior, L.J.Q.; de Oliveira Barreto, E.; Brandão, G.C. Antinociceptive and anti-inflammatory effect of Poincianella pyramidalis (Tul). LP Queiroz. J. Ethnopharmacol. 2020, 254, 112563. [DOI] [PubMed]
- Garimella Veerabhadram, Cellini Christina. Postoperative pain control. Clin. Colon. Rectal. Surg. 2013;26(03):191–196. doi: 10.1055/s-0033-1351138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorno Thais Biondino Sardella, Ballard Y.L.L., Cordeiro Millena Santos, Silva B.V., Pinto A.C., Fernandes Patricia Dias. Central and peripheral antinociceptive activity of 3-(2-oxopropyl)-3-hydroxy-2-oxindoles. Pharmacol. Biochem. Behav. 2015;135:13–19. doi: 10.1016/j.pbb.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Goucke C.R., Chaudakshetrin P. Pain: A neglected problem in the low-resource setting. Anesth. Analg. 2018;126:1283–1286. doi: 10.1213/ANE.0000000000002736. [DOI] [PubMed] [Google Scholar]
- Guan Li-Ping, Liu Bing-Yu. Antidepressant-like effects and mechanisms of flavonoids and related analogues. Eur. J. Med. Chem. 2016;121:47–57. doi: 10.1016/j.ejmech.2016.05.026. [DOI] [PubMed] [Google Scholar]
- Gupta S.C., Kunnumakkara A.B., Aggarwal S., Aggarwal B.B. Inflammation, a double-edge sword for cancer and other age-related diseases. Front. Immunol. 2018;9:2160. doi: 10.3389/fimmu.2018.02160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson K.J., Gómez-Pinilla F., Crowe M.J., Ying Z., Basso D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Ji R.R., Chamessian A., Zhang Y.Q. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.J., Ajazuddin V.A., Singh M., Singh D. Acute and chronic effect of Hibiscus rosa sinensis flower extract on anxiety induced exploratory and locomotor activity in mice. J Plant Sci. 2011;6(2):102–107. [Google Scholar]
- Koster R., Anderson M., DeBeer E.J. Acetic acid for analgesic screening. J. Fed. Proc. 1959;18:412–417. [Google Scholar]
- Kumar J.P., Shankar N.B. Analgesic activity of Mollugo pentaphylla Linn. by tail immersion method, Asian J. Pharmaceut. Clin. Res. 2009;2:61–63. [Google Scholar]
- Lacy F., Armstrong L., Goldman M., Lance L. fifth ed. Lexi-Comp; USA: 2008. Drug Information Handbook. [Google Scholar]
- Le Bars D., Gozariu M., Cadden S. Animal Models of Nociception. Pharmacol. Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Leuti A., Fazio D., Fava M., Piccoli A., Oddi S., Maccarrone M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020;159:133–169. doi: 10.1016/j.addr.2020.06.028. [DOI] [PubMed] [Google Scholar]
- Lin T.-H., Tamaki Y., Pajarinen J., Waters H.A., Woo D.K., Yao Z., Goodman S.B. Chronic inflammation in biomaterial-induced periprosthetic osteolysis: NF-κB as a therapeutic target. Acta Biomater. 2014;10(1):1–10. doi: 10.1016/j.actbio.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Huh Y., Ji R.-R. Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J. Anesthesia. 2019;33(1):131–139. doi: 10.1007/s00540-018-2579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedeva Y.V., Kim M.S., Usachev Y.M. Mechanisms of prolonged presynaptic Ca2þ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J. Neurosci. 2008;28:5295–5311. doi: 10.1523/JNEUROSCI.4810-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil J.S., Davis K.D., Derbyshire S.W. The necessity of animal models in pain research. Pain. 2010;151:12–17. doi: 10.1016/j.pain.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Negus S.S., Vanderah T.W., Brandt M.R., Bilsky E.J., Becerra L., Borsook D. Preclinical assessment of candidate analgesic drugs: Recent advances and future challenges. J. Pharmacol. Exp. Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Obara Ilona, Telezhkin Vsevolod, Alrashdi Ibrahim, Chazot P.L. Histamine, histamine receptors, and neuropathic pain relief. British J. Pharmacol. 2020;177(3):580–599. doi: 10.1111/bph.14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira J.F., Nonato F.R., Zafred R.R.T., Leite N.M.S., Ruiz A.L.T.G., Carvalho J.E., et al. Evaluation of anti-inflammatory effect of derivative (E)-N-(4-bromophenyl)-2-(thiophen-2-ylmethylene)-thiosemicarbazone. Biomed. Pharmacother. 2016;80:388–392. doi: 10.1016/j.biopha.2016.03.047. [DOI] [PubMed] [Google Scholar]
- Ortiz Mario I., Castañeda-Hernández Gilberto, Izquierdo-Vega Jeannett A., Sánchez-Gutiérrez Manuel, Ponce-Monter Héctor A., Granados-Soto Vinicio. Role of ATP-sensitive K+ channels in the antinociception induced by non-steroidal anti-inflammatory drugs in streptozotocin-diabetic and non-diabetic rats. Pharmacol. Biochem. Behav. 2012;102(1):163–169. doi: 10.1016/j.pbb.2012.03.032. [DOI] [PubMed] [Google Scholar]
- Ortiz M.I., Castaneda H.G. Examination of the interaction between peripheral lumiracoxib and opioids on the 1% formalin test in rats. Eur. J. Pain. 2008;12:233–241. doi: 10.1016/j.ejpain.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Padmavathi Ganesan, Rathnakaram Sivakumar Raju, Monisha Javadi, Bordoloi Devivasha, Roy Nand Kishor, Kunnumakkara Ajaikumar B. Potential of butein, a tetrahydroxychalcone to obliterate cancer. Phytomedicine. 2015;22(13):1163–1171. doi: 10.1016/j.phymed.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Padmavathi G., Roy N.K., Bordoloi D., et al. Butein in health and disease: A comprehensive review. Phytomedicine. 2017;25:118–127. doi: 10.1016/j.phymed.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Pinho-Ribeiro F.A., Verri W.A., Jr, Chiu I.M. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 2017;38(1):5–19. doi: 10.1016/j.it.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki-Zahn E.M., Segelcke D., Schug S.A. Postoperative pain—from mechanisms to treatment. Pain Rep. 2017;2(2):e588. doi: 10.1097/PR9.0000000000000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce M.H., Fernandez M.E., Ortiz M.I., Ramirez M.M.L., Cruz E.D., Perez H.N., Carino C.R. Spasmolytic and anti-inflammatory effects of Aloysia triphylla and citral, in vitro and in vivo studies. J. Smooth Muscle Res. 2010;46(6):309–319. doi: 10.1540/jsmr.46.309. [DOI] [PubMed] [Google Scholar]
- Posadas I, Bucci M, Roviezzo F, Rossi A, Parente L, Sautebin L, et al. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. Br J Pharmacol 2004; 142: 331–338. [DOI] [PMC free article] [PubMed]
- Puebla Diaz F. Types of pain and WHO therapeutic scale Iatrogenic pain. Oncol. (Barc.) 2005;28(3):33–37. [Google Scholar]
- Raja S.N., Carr D.B., Cohen M., Finnerup N.B., Flor H., Gibson S., Keefe F.J., Mogil J.S., Ringkamp M., Sluka K.A., et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert K., Zhang Y., Leahy K., Hauser S., Masferrer J., Perkins W., Lee L., Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. USA. 1994;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi F., Tsubota M., Kawabata A. Involvement of voltage-gated calcium channels in inflammation and inflammatory pain. Biolog. Pharm. Bullet. 2018;41(8):1127–1134. doi: 10.1248/bpb.b18-00054. [DOI] [PubMed] [Google Scholar]
- Semwal R.B., Semwal D.K., Combrinck S., Viljoen A. Butein: from ancient traditional remedy to modern nutraceutical. Phytochem. Lett. 2015;11:188–201. [Google Scholar]
- Serhan C.N., Chiang N., Dalli J. The resolution code of acute inflammation: Novel pro–resolving lipid mediators in resolution. Semin. Immunol. 2015;27(3):200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shwetha C., Latha K.P., Asha K.A. Study on analgesic activity of Holarrhena antidysenterica leaves. J. Herb. Med. 2014;2:14–16. [Google Scholar]
- Sneddon Lynne U. Comparative physiology of nociception and pain. Physiology. 2018;33(1):63–73. doi: 10.1152/physiol.00022.2017. [DOI] [PubMed] [Google Scholar]
- Vasconcelos F.H., de Araujo G.C. Prevalence of chronic pain in Brazil: a descriptive study. Brazilian J. Pain. 2018;1:176–179. [Google Scholar]
- Yang L.H., Ho Y.J., Lin J.F., Yeh C.W., Kao S.H., Hsu L.S. Butein inhibits the proliferation of breast cancer cells through generation of reactive oxygen species and modulation of ERK and p38 activities. Mol. Med. Rep. 2012;6:1126–1132. doi: 10.3892/mmr.2012.1023. [DOI] [PubMed] [Google Scholar]
- Zakaria Z.A., Rahim M.H.A., Sani M.H.M., Omar M.H., Ching S.M., Kadir A.A., Ahmed Q.U. Antinociceptive activity of petroleum ether fraction obtained from methanolic extract of Clinacanthus nutans leaves involves the activation of opioid receptors and NO-mediated/cGMP-independent pathway. BMC Complement. Altern. Med. 2019;19:79. doi: 10.1186/s12906-019-2486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo, M. Ionotropic glutamate e receptors contribute to pain transmission and chronic pain. Neuropharmacology 2017, 112, 228–234. [DOI] [PubMed]