Abstract
Breast cancer (BC) has high incidence and mortality rates, making it a major global health issue. BC treatment has been challenging due to the presence of drug resistance and the limited availability of therapeutic options for triple-negative and metastatic BC, thereby urging the exploration of more effective anti-cancer agents. Hesperidin and its aglycone hesperetin, two flavonoids from citrus species, have been extensively evaluated for their anti-cancer potentials. In this review, available literatures on the chemotherapeutic and chemosensitising activities of hesperidin and hesperetin in preclinical BC models are reported. The safety and bioavailability of hesperidin and hesperetin as well as the strategies to enhance their bioavailability are also discussed. Overall, hesperidin and hesperetin can inhibit cell proliferation, migration and BC stem cells as well as induce apoptosis and cell cycle arrest in vitro. They can also inhibit tumour growth, metastasis and neoplastic changes in tissue architecture in vivo. Moreover, the co-administration of hesperidin or hesperetin with doxorubicin, letrozole or tamoxifen can enhance the efficacies of these clinically available agents. These chemotherapeutic and chemosensitising activities of hesperidin and hesperetin have been linked to several mechanisms, including the modulation of signalling pathways, glucose uptake, enzymes, miRNA expression, oxidative status, cell cycle regulatory proteins, tumour suppressor p53, plasma and liver lipid profiles as well as DNA repair mechanisms. However, poor water solubility, extensive phase II metabolism and apical efflux have posed limitations to the bioavailability of hesperidin and hesperetin. Various strategies for bioavailability enhancement have been studied, including the utilisation of nano-based drug delivery systems and the co-administration of hesperetin with other flavonoids. In particular, nanoformulated hesperidin and hesperetin possess greater chemotherapeutic and chemosensitising activities than free compounds. Despite promising preclinical results, further safety and efficacy evaluation of hesperidin and hesperetin as well as their nanoformulations in clinical trials is required to ascertain their potentials to be developed as clinically useful agents for BC treatment.
Keyword: Bioavailability, Biosafety, Breast cancer, Hesperetin, Hesperidin, Nanoformulation
1. Introduction
Breast cancer (BC) remains a major public health issue worldwide (Tao et al., 2015), where female BC recorded approximately 2.26 million cases and 684,996 deaths globally in 2020 (Sung et al., 2021). Global BC incidence has been predicted to rise to around 3.2 million cases per year by 2050 (Momenimovahed and Salehiniya, 2019). There is thus an urgent need for the discovery of more effective preventive and treatment options for BC. It has been widely known that BC is a heterogenous disease, with the presence of a high degree of molecular and phenotypic variations both within (i.e., intratumoural heterogeneity) and between (i.e., intertumoural heterogeneity) tumours (Lüönd et al., 2021). These variations serve as the basis for the classification of breast tumours into three major subtypes, including 1) luminal A/B (Oestrogen receptor [ER]-positive and/or progesterone receptor [PR]-positive), 2) human epidermal growth factor receptor 2 (HER2)-enriched and 3) triple-negative (ER-negative, PR-negative and HER2-negative) subtypes (Dai et al., 2015). As different BC subtypes demonstrate variabilities in their prognosis and treatment response, subtype classification is critical when making treatment decisions (Tong et al., 2018, Waks and Winer, 2019).
At present, BC treatment relies mainly on surgery, radiotherapy, chemotherapy, endocrine therapy and targeted therapy (Nounou et al., 2015). Breast conserving surgery has become the trending treatment approach for patients with low-grade BC (Tailby and Boyages Am, 2017). Surgery is often followed by adjuvant treatment in order to inhibit or kill micro-metastases that could occur after surgery (Chew, 2001). One example of adjuvant treatment is radiotherapy (Nounou et al., 2015). The decision on systemic adjuvant treatment (i.e., chemotherapy, endocrine therapy and targeted therapy), on the other hand, is usually made based on the subtypes of BC (Table 1) (Mehanna et al., 2019, Tong et al., 2018, Waks and Winer, 2019).
Table 1.
Typical systemic adjuvant therapeutic options for the treatment of luminal A/B, HER2-enriched and triple-negative BC subtypes.
| Chemotherapy | Endocrine Therapy | HER2-targeted Therapy | References | |
|---|---|---|---|---|
| Luminal A/B (70%) | Certain patients only | All patients | Not applicable | Tong et al., 2018, Waks and Winer, 2019 |
| HER2-enriched (15–20%) | All patients | Patients with HR-positive tumours only | All patients | Tong et al., 2018, Waks and Winer, 2019 |
| Triple-negative (15%) | All patients | Not applicable | Not applicable | Mehanna et al. (2019) |
Abbreviations: HER2, Human epidermal growth factor receptor 2; HR, Hormone receptor.
Importantly, there have been reports of resistance to chemotherapy, endocrine therapy and HER2-targeted therapy in the clinics (Haque and Desai, 2019, Ji et al., 2019, Rexer and Arteaga, 2012). Triple-negative BC, the subtype with the poorest prognosis, has limited therapeutic options (i.e., chemotherapy) (Mehanna et al., 2019). However, chemotherapy is non-specific, which can cause damage to normal tissues and result in significant adverse effects (Agarwal, 2016). Moreover, early-stage BC in around 20–30% of patients may progress to become metastatic, which is largely incurable (Pulido et al., 2017). Collectively, it is crucial to discover and develop more effective and less toxic anti-cancer agents to address these challenges associated with BC treatment.
Natural products have been an important source of anti-cancer drugs, where greater than 60% of the currently available anti-cancer drugs are natural product-derived (Cragg and Pezzuto, 2016). Some successful examples of natural product-derived anti-cancer drugs include paclitaxel, docetaxel, vinblastine, vincristine, etoposide and irinotecan (Cragg and Pezzuto, 2016). In the 1990s, there was a shift in the focus of the pharmaceutical industry from natural product-based drug discovery to synthetic compounds due to issues such as their low availability, incompatibility with high-throughput screening and the needs for identifying bioactive compound(s) of interest (Dutta et al., 2019). However, low US Food and Drug Administration (FDA) approval rates of these newly introduced synthetic drugs as well as recent scientific and technological advancements that could address the issues associated with natural products have reignited the interest of the industry in natural product-based drug discovery (Atanasov et al., 2021, Dutta et al., 2019).
Phytochemicals, the bioactive non-nutritive plant chemicals, have attracted tremendous research interest for their anti-cancer potentials (Israel et al., 2018, Kapinova et al., 2018). Notably, phytochemicals may offer several advantages over conventional anti-cancer drugs, which include their capability to target multiple pathways, chemosensitising properties and favourable safety profiles (Magura et al., 2020). A number of phytochemicals, including anthocyanins, β-elemene, carotenoids, curcumin, daidzein, epigallocatechin gallate, γ-tocotrienol, genistein, hesperetin, hesperidin, kaempferol, lignans, quercetin, resveratrol and tangeritin, have demonstrated anti-cancer activities against BC (Abotaleb et al., 2019, Israel et al., 2018, Kapinova et al., 2018, Rahman et al., 2021). The anti-cancer activities of these phytochemicals are believed to be related to their impacts on BC stem cells (BCSCs), angiogenesis, apoptosis, cell proliferation, inflammation, metastasis and/or non-coding ribonucleic acids (Kapinova et al., 2018).
Hesperidin (hesperetin 7-O-β-rutinoside) and its aglycone hesperetin (4′-methoxy-3′,5,7-trihydroxyflavanone) are flavonoids that can be richly found in citrus species such as grapefruits, lemons and oranges (Parhiz et al., 2015). They have been reported to possess various pharmacological activities, including cardioprotective (Agrawal et al., 2014, Trivedi et al., 2011), hepatoprotective (He et al., 2019, Tabeshpour et al., 2020), neuroprotective (Roohbakhsh et al., 2014), hypolipidemic (Akiyama et al., 2009, Wilcox et al., 2001), anti-cancer (Ferreira de Oliveira et al., 2020), anti-diabetic (Dhanya and Jayamurthy, 2020), antimicrobial (Abuelsaad et al., 2013, Moon et al., 2013), anti-inflammatory and antioxidant (Parhiz et al., 2015) activities. These pharmacological activities have directly reflected the therapeutic potentials of hesperidin and hesperetin in the treatment of multiple medical conditions, including cancer, cardiovascular diseases, diabetes mellitus, diabetic cardiomyopathy, endothelial dysfunction, atherosclerosis and neurodegenerative disorders (Mahmoud et al., 2019).
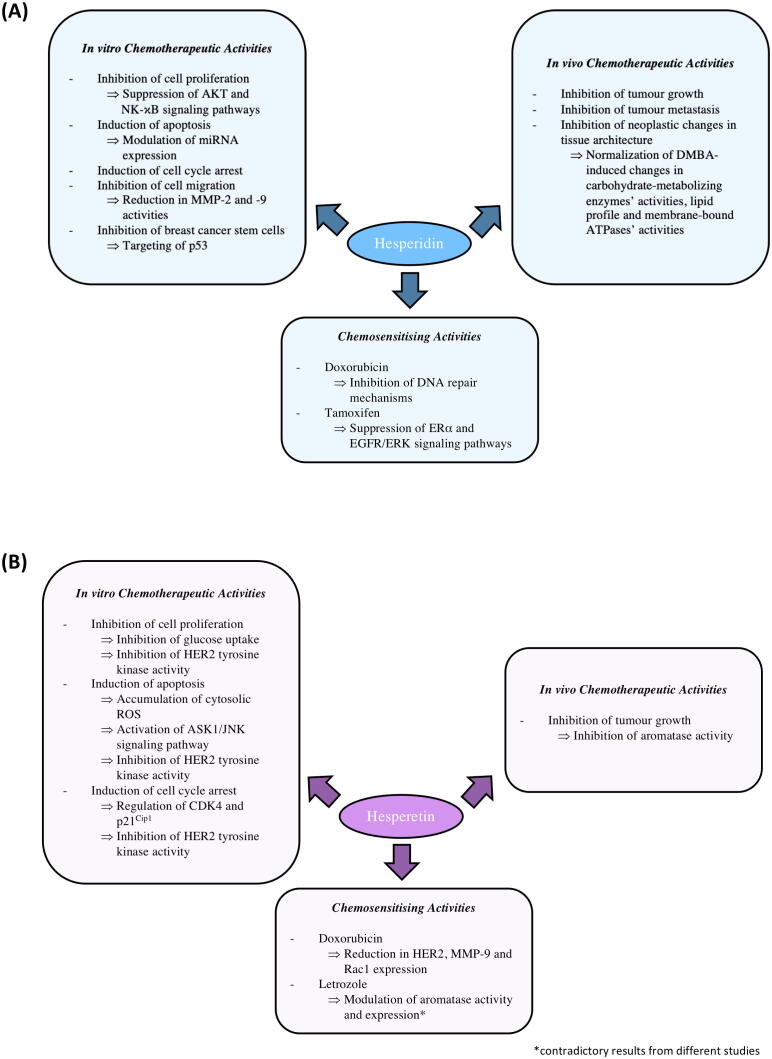
This review aims to provide a comprehensive overview on the anti-BC potentials of hesperidin and hesperetin by describing particularly their chemotherapeutic and chemosensitising activities in preclinical BC models. Besides, their safety and bioavailability as well as the strategies to improve their bioavailability are also discussed. Fig. 1 summarises the reported preclinical chemotherapeutic and chemosensitising activities of hesperidin and hesperetin as well as the associated molecular mechanisms that have been proposed for these activities. However, hesperidin and hesperetin have not been evaluated in clinical trials for BC treatment, thus representing an important future research direction.
Fig. 1.
Anti-cancer (chemotherapeutic and chemosensitising) activities and associated molecular mechanisms of hesperidin (A) and hesperetin (B) in BC. AKT, Protein kinase B; ASK1/JNK, Apoptosis signal-regulating kinase 1/c-Jun N-terminal kinase; ATPases, adenosine triphosphatases; CDK4, cyclin dependent kinase 4; DMBA, 7,12-dimethylbenz(a)anthracene; DNA, Deoxyribonucleic acid; EGFR/ERK, Epidermal growth factor receptor/extracellular signal-regulated kinase; ERα, Oestrogen receptor-alpha; HER2, Human epidermal growth factor receptor 2; MMP-2 and −9, Matrix metalloproteinase-2 and −9; miRNAs, Micro-ribonucleic acids; NF-κB, Nuclear factor kappa B; Rac1, Ras-related C3 botulinum toxin substrate 1; ROS, Reactive oxygen species.
2. Chemotherapeutic activities of hesperidin and hesperetin
2.1. Chemotherapeutic activities in in vitro breast cancer models
2.1.1. Inhibition of cell proliferation
Hesperidin has demonstrated anti-proliferative activity in both luminal and triple-negative BC cell lines (Fig. 2). In a study, Lee et al. (2010) observed that hesperidin treatment (100 µM) induced a significant inhibition in the proliferation of MCF-7 cells transfected with green fluorescent protein/α-tubulin (MCF-7-GFP-Tubulin; luminal A) but had insignificant impact on the number of mitotic cells, suggesting that the observed anti-proliferative effect of hesperidin is unlikely a result of mitotic inhibition. Other studies similarly revealed the ability of hesperidin (20–300 µM or 20–140 µg/mL) to dose- and time-dependently inhibit MCF-7 cell proliferation (Al-Rikabi et al., 2020, Magura et al., 2020).
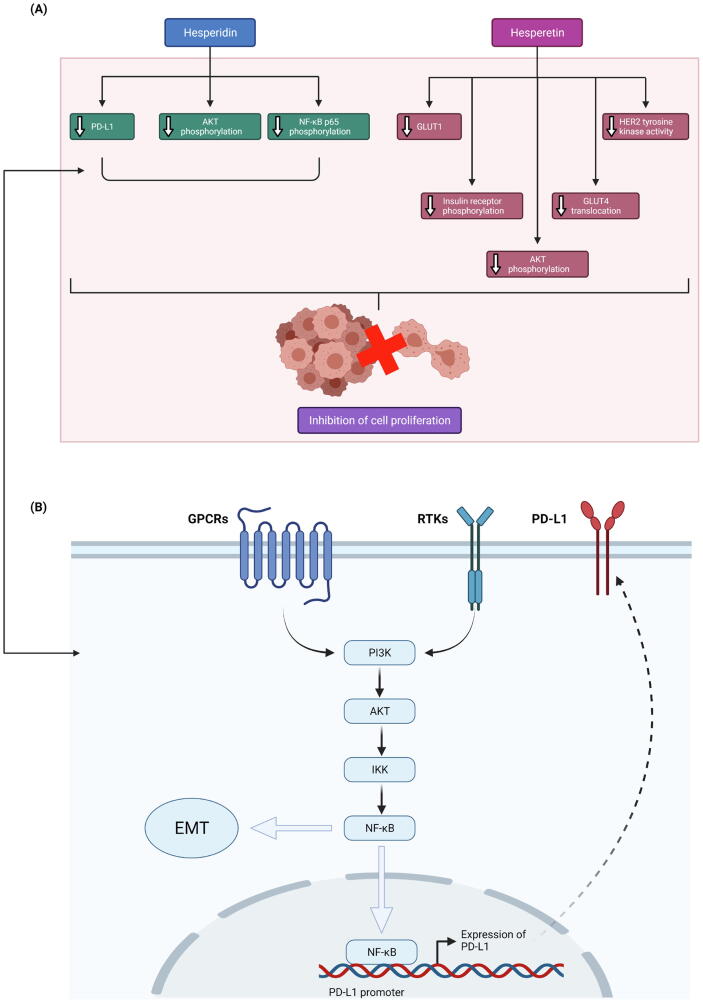
Fig. 2.
Anti-proliferative activity of hesperidin and hesperetin in BC cells. A) Effects of hesperidin and hesperetin on associated molecular targets. B) Involvement of AKT and NF-κB signalling pathways in regulation of PD-L1 expression. AKT, Protein kinase B; EMT, Epithelial-mesenchymal transition; GLUT1/4, Glucose transporter type 1/4; GPCRs, G-protein-coupled receptors; HER2, Human epidermal growth factor receptor 2; IKK, IκB kinase; NF-κB, Nuclear factor kappa B; PD-L1, Programmed death-ligand 1; PI3K, Phosphoinositide 3-kinase; RTKs, Receptor tyrosine kinases.
Kabała-Dzik et al. (2018) reported that hesperidin (5–100 µM) can dose- and time-dependently inhibit the proliferation of not only MCF-7 cells but also MDA-MB-231 (triple-negative) cells, with IC50 at 9.39 µM and 50.83 µM, respectively. Another study conducted by Kongtawelert et al. (2020) also reported the dose- and time-dependent anti-proliferative effect of hesperidin treatment (50–200 µM) on MDA-MB-231 cells. In the same study, mRNA and protein expression analyses revealed significantly downregulated expression of programmed death-ligand 1 (PD-L1; an immune checkpoint protein), the overexpression of which could contribute to cancer cell evasion of the T-cell immunity (Cha et al., 2019), in MDA-MB-231 cells treated with hesperidin. A reduction in the phosphorylation levels of protein kinase B (AKT) and p65 was also observed, which is suggestive of a suppression of the AKT and nuclear factor kappa B (NF-κB) signalling pathways, respectively (Kongtawelert et al., 2020). Both phosphoinositide 3-kinase (PI3K)/AKT and NF-κB signalling pathways have been reported to play a role in the induction of epithelial-mesenchymal transition (EMT) process (Xu et al., 2015), through which PD-L1 can be upregulated in BC cells (Noman et al., 2017). Therefore, these findings collectively suggest that the anti-proliferative activity of hesperidin in MDA-MB-231 cells is likely linked to a downregulation of PD-L1 via the suppression of AKT and NF-κB signalling pathways (Kongtawelert et al., 2020).
In contrast to hesperidin, the anti-proliferative activity of hesperetin has been reported in not only luminal and triple-negative BC cell lines but also HER2-enriched BC cell lines (Fig. 2). For instance, Choi (2007) showed that hesperetin (1–100 µM) can induce a significant reduction in MCF-7 cell proliferation in both a dose- and time- dependent manner. Similarly, in another study, hesperetin (20–200 µM) was capable of dose- and time-dependently inhibit the proliferation of both MCF-7 and MDA-MB-231 cells (Palit et al., 2015). The same study also revealed the cancer cell selectivity of hesperetin, as evidenced by its inability to significantly influence the proliferation of normal (HMEC) and immortalised normal mammary epithelial cells (MCF-10A).
The Warburg effect refers to the metabolic switch in cancer cells from oxidative phosphorylation to aerobic glycolysis (Yu et al., 2017). This glycolytic switch leads to dramatic increases in glucose uptake and lactate production, and is important in providing substrates for cancer cells to sustain a high proliferation rate (Lin et al., 2020). In a study, Yang et al. (2013) reported that hesperetin (100 µM) can significantly attenuate both basal and insulin-stimulated proliferation of MDA-MB-231 cells by inhibiting the basal (~45%) and insulin-stimulated (~40%) uptake of glucose in these cells. The authors suggested that the inhibition of basal glucose uptake may be attributed to a dose-dependent reduction in the mRNA and protein levels of glucose transporter type 1 (GLUT1), the predominant transporter that is overexpressed in breast tumours under basal conditions (Wellberg et al., 2016). On the other hand, the inhibition of insulin-stimulated glucose uptake was suggested to be linked to a reduction in the phosphorylation of insulin receptor (an initial step of insulin signalling) and AKT (a downstream substrate of insulin signalling that triggers GLUT4 translocation) as well as the insulin-stimulated cell surface translocation of glucose transporter type 4 (GLUT4) (Yang et al., 2013).
In another study, hesperetin was identified as a potential inhibitor of HER2 tyrosine kinase activity via computational modelling, whereby it was found to exhibit a strong and stable association at the ATP-binding site of HER2 tysosine kinase domain that can potentially prevent ATP binding (Chandrika et al., 2016). The inhibitory effect of hesperetin against HER2 tyrosine kinase activity (IC50 20 µM) was further validated via a luminescence-based HER2 kinase assay (Chandrika et al., 2016). As HER2 signalling is highly associated with cell survival and growth and its overexpression is correlated with a more aggressive disease and a worse prognosis in BC, HER2 represents a promising anti-BC target (Browne et al., 2009). As expected, hesperetin (150–500 µM) was observed to dose-dependently inhibit SKBR3 (HER2-enriched) cell proliferation, with IC50 at 500 µM (Chandrika et al., 2016). Interestingly, hesperetin was found to exhibit a significantly greater anti-proliferative activity against HER2-positive SKBR3 cells than HER2-negative MDA-MB-231 cells, suggesting the action of hesperetin may be HER2-dependent (Chandrika et al., 2016).
2.1.2. Induction of apoptosis
The pro-apoptotic effect of hesperidin has been reported in luminal and triple-negative BC cell lines (Fig. 3). In a study by Magura et al. (2020), flow cytometric analysis of annexin V- and propidium iodide (PI)-stained MCF-7 cells showed that hesperidin (100 µg/mL) can induce a significant increase in the population of apoptotic cells. The pro-apoptotic effect of hesperidin on MCF-7 was further validated via Hoechst staining, whereby microscopic images of hesperidin-treated cells revealed apoptotic morphological changes (i.e., chromatin condensation and nuclear fragmentation) (Magura et al., 2020). In the same study, subsequent gene expression analysis linked the pro-apoptotic activity of hesperidin to the altered expression of various key apoptotic regulatory genes and apoptosis-related micro-ribonucleic acids (miRNAs). The results revealed an upregulation of caspase-3 (CASP3; an effector caspase), caspase-9 (CASP9; an initiator caspase of intrinsic apoptotic pathway) and Bcl-2-associated X protein (Bax; a pro-apoptotic protein) together with a downregulation of B-cell lymphoma 2 (Bcl-2; an anti-apoptotic protein), suggesting the induction of intrinsic apoptotic pathway (Magura et al., 2020). Furthermore, hesperidin-treated MCF-7 cells also showed an upregulated expression of miR-16 and miR-34a and a downregulated expression of miR-21 (Magura et al., 2020). It has been reported that while miR-16 and miR-34a can induce apoptosis by negatively regulating Bcl-2 expression, miR-21 can exert an anti-apoptotic effect by promoting Bcl-2 expression (Gu et al., 2018, Lin et al., 2014, Pekarsky et al., 2018). In another study, Kabała-Dzik et al. (2018) reported that hesperidin (50–100 µM) can promote apoptotic cell death in both MCF-7 and MDA-MB-231 cells. Interestingly, a greater effect was observed in MCF-7 cells in comparison to MDA-MB-231 cells (Kabała-Dzik et al., 2018).
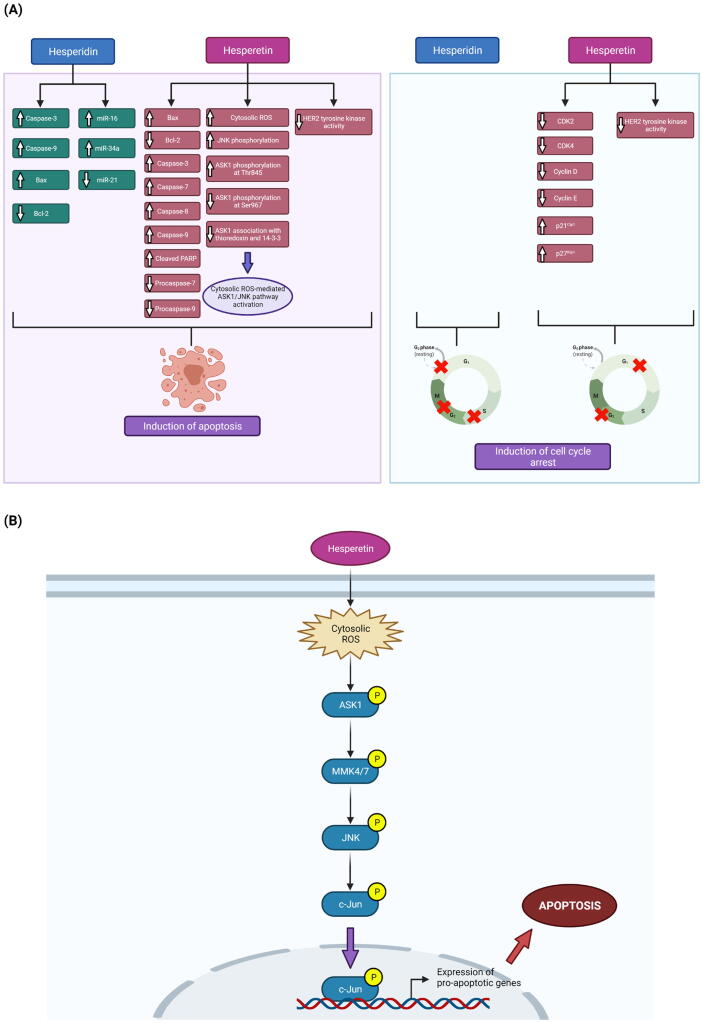
Fig. 3.
Pro-apoptotic and cell cycle arrest-inducing activities of hesperidin and hesperetin in BC cells. A) Effects of hesperidin and hesperetin on associated molecular targets. B) Cytosolic ROS-mediated activation of ASK1/JNK pathway by hesperetin. ASK1, Apoptosis signal-regulating kinase 1; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; CDK2/4, Cyclin dependent kinase 2/4; HER2, Human epidermal growth factor receptor 2; JNK, c-Jun N-terminal kinase; PARP, Poly (ADP-ribose) polymerase; ROS, Reactive oxygen species.
Similarly, hesperetin has also shown pro-apoptotic activity in luminal and triple-negative BC cell lines (Fig. 3). For instance, Choi (2007) reported that hesperetin (1–100 µM) can trigger a reduction in anti-apoptotic Bcl-2 level and an increase in pro-apoptotic Bax level in both a dose- and time-dependent manner, both of which could promote apoptosis. In another study, Palit et al. (2015) reported the time-dependent pro-apoptotic effect of hesperetin (150 or 120 µM) on both MCF-7 and MDA-MB-231 cells. In hesperetin-treated MCF-7 cells, increased Bax/Bcl-2 ratio, mitochondrial membrane potential (ΔΨm) loss (an early stage in intrinsic apoptotic signalling), mitochondrial cytochrome c release, increased caspase-7 (CASP7; an effector caspase), CASP9 and cleaved poly (ADP-ribose) polymerase (PARP) protein levels, decreased procaspase-7 and procaspase-9 protein levels as well as unchanged procaspase-8 and caspase-8 (CASP8; an initiator caspase of extrinsic apoptotic pathway) protein levels were observed (Palit et al., 2015). In hesperetin-treated MDA-MB-231 cells, there was an increase in the Bax/Bcl-2 ratio, an increase in the protein levels of CASP3, CASP7 and CASP9 as well as an insignificant change in the protein level of CASP8 (Palit et al., 2015). These findings collectively suggest the ability of hesperetin to induce apoptosis in MCF-7 and MDA-MB-231 cells by triggering the intrinsic but not the extrinsic apoptotic pathway (Palit et al., 2015). Further mechanistic studies revealed an increase in intracellular reactive oxygen species (ROS) level, the inability of a mitochondrial uncoupler (i.e., carbonyl cyanide m-chlorophenylhydrazone [CCCP]) to disrupt hesperetin-induced ROS generation, the inability of hesperetin to increase the number of dihydrorhodamine 123 (DHR123; a dye used for mitochondrial ROS detection)-positive cells and the attenuation of hesperetin-induced apoptosis by antioxidants (i.e., N-acetylcysteine [NAC] and glutathione [GSH]), all of which suggested the involvement of high cytosolic ROS levels in hesperetin-induced apoptosis in MCF-7 cells (Palit et al., 2015). It was also observed that NAC can suppress hesperetin-induced c-Jun N-terminal kinase (JNK) phosphorylation, and a JNK inhibitor (i.e., SP600125) can reverse hesperetin-induced phosphorylation of Bcl-2 and disruption of Bax/Bcl-2 association (Palit et al., 2015). Moreover, hesperetin also activated apoptosis signal-regulating kinase 1 (ASK1), the silencing of which attenuated hesperetin-induced JNK phosphoprylation and apoptosis (Palit et al., 2015). Taken together, these findings indicate that hesperetin treatment increases the generation of cytoslic ROS that can in turn activate the ASK1/JNK signalling pathway, whereby JNK phosphorylates Bcl-2 to reduce Bax/Bcl-2 association and induce apoptosis in MCF-7 cells (Palit et al., 2015).
In contrast to hesperidin, hesperetin has also been reported to be capable of inducing apoptosis in a HER2-enriched BC cell line, namely the SKBR3 cell line (Chandrika et al., 2016) (Fig. 3). The study revealed ΔΨm loss, chromatin condensation and increased protein levels of CASP3 and CASP8 in SKBR3 cells treated with hespetretin (500 µM), suggesting the involvement of both intrinsic and extrinsic apoptotic pathways. Interestingly, hesperetin was observed to exert a higher pro-apoptotic activity in HER2-positive SKBR3 cells than HER2-negative MCF-7 and MDA-MB-231 cells (Chandrika et al., 2016). This exhilarated apoptotic cell death seen in HER2-positive BC cells may be attributed to the HER2 tyrosine kinase-inhibitory activity of hesperetin (Chandrika et al., 2016).
2.1.3. Induction of cell cycle arrest
There have been reports of the cell cycle arrest-inducing ability of hesperidin in luminal and triple-negative BC cell lines (Fig. 3). In a study, Kabała-Dzik et al. (2018) reported that hesperidin (50–100 µM; 24–48 h) can induce significant changes in the progression of cell cycle in both MCF-7 and MDA-MB-231 cells. In MCF-7 cells, 24 h of treatment with 50 µM and 100 µM hesperidin significantly increased the cell population in G0/G1 phase and G2/M phase, respectively. On the other hand, 48 h of treatment with 50 µM and 100 µM hesperidin significantly increased the cell population in G2/M phase. In MDA-MB-231 cells, 24 h of treatment with 100 µM hesperidin induced a significant increase in the S phase and G2/M phase cell populations. After 48 h of treatment with 50 µM and 100 µM hesperidin, there was a significant increase in the G2/M phase and G0/G1 phase cell populations, respectively. Collectively, these findings suggest that while hesperidin can induce only G0/G1- and G2/M−phase arrest in MCF-7 cells, it is capable of inducing S-, G2/M and G0/G1-phase arrest in MDA-MB-231 cells. Similarly, the cell cycle arrest-inducing ability of hesperidin was also reported in another study (Magura et al., 2020). The study showed that treatment with 100 µg/mL hesperidin for 48 h induced a high accumulation of MCF-7 cells in G0/G1 phase, reflecting a G0/G1 phase arrest (Magura et al., 2020).
On ther other hand, hesperetin has demonstrated cell cycle arrest-inducing ability in luminal and HER2-enriched BC cell lines (Fig. 3). For instance, Choi (2007) reported that hesperetin (1–100 µM; 48–72 h) can induce a dose-dependent G1 phase arrest in MCF-7 cells, and linked this arrest to a reduction in the protein levels of cyclin dependent kinase (CDK)2, CDK4, cyclin D and cyclin E as well as an increase in the protein levels of p21Cip1 and p27Kip1. As p21Cip1, p27Kip1 and p57Kip2 are members of the CIP/KIP family of CDK inhibitors (CKIs) that can preferentially interfere with the activities of CDK2, CDK4 and CDK6 implicated in G1-to-S-phase transition (Hume et al., 2020), the upregulation of these CKIs can thus mediate a G1 phase arrest (Pfeuty, 2012). In the same study, increased formation of CDK4-p21Cip1 but not that of CDK4-p27Kip1 and CDK4-p57Kip2 was also observed, suggesting that hesperetin-induced G1 phase arrest in MCF-7 cells may be mediated predominantly via its regulation of CDK4 and p21Cip1 (Choi, 2007). In another study conducted by Chandrika et al. (2016), flow cytometric analysis of PI-stained cells showed that hesperetin treatment (500 µM; 24 h) promoted a significant G2 phase arrest in HER2-positive SKBR3 cells but not in HER2-negative MDA-MB-231 cells. This differential inhibitory effect of hesperetin on cell cycle progression may reflect that its action is HER2-dependent.
2.1.4. Inhibition of cell migration
Thus far, only one study has evaluated the inhibitory effect of hesperidin on BC cell migration (Kongtawelert et al., 2020). The study reported that hesperidin (10–50 µM) can inhibit MDA-MB-231 cell migration by significantly reducing the activities of matrix metalloproteinase (MMP)-2 and MMP-9. MMP-2 and MMP-9, members of a family of zinc-dependent endopeptidases, possess enzymatic activity against basement membrane type IV collagen and have thus been implicated in the promotion of cancer metastasis (Li et al., 2017a).
2.1.5. Inhibition of breast cancer stem cells
BCSCs are a small and dynamic subpopulation of cells found in the breast tumour bulk (Raman et al., 2020). They exhibit stem cell properties and have been found to play an important role in tumour initiation and maintenance as well as chemoresistance (Economopoulou et al., 2012). Following conventional chemotherapy treatment, inherently chemoresistant-BCSCs survive and re-establish the tumour, thereby causing a cancer relapse (Raman et al., 2020). As such, the discovery and development of BCSC-targeting agents would be highly needed to improve BC treatment.
Hermawan et al. (2021) investigated the effect of hesperidin on MCF-7 mammospheres (3D) that exhibit enriched BCSC properties, as confirmed via the observation of significantly upregulated cancer stem cell markers (i.e., nanog homeobox [NANOG] and aldehyde dehydrogenase 1 [ALDH1]) in the mammospheres. A series of in vitro experiments revealed that hesperidin inhibited mammosphere and colony formation as well as exerted cytotoxic and anti-migratory effects against mammosphere-derived MCF-7 cells (3D). Moreover, hesperidin also induced G0/G1 phase arrest and apoptosis in both 2D and 3D MCF-7 cells. These in vitro findings collectively indicate the ability of hesperidin to inhibit BCSCs.
In the same study, both in vitro and in silico approaches were employed to elucidate the underlying molecular mechanisms of the inhibitory effect of hesperidin on BCSCs (Hermawan et al., 2021). Effects of heperidin on the gene expression of the markers for EMT (i.e., MMP-9), stemness (i.e., ALDH1 and p53), cell cycle (i.e., p21 and cyclin D1) and apoptosis (i.e., Bcl-2) were evaluated in vitro. In 2D MCF-7 cells, there was a significant decrease in MMP-9 and p21 mRNA levels and a significant increase in p53 and cyclin D1 mRNA levels. On the other hand, 3D MCF-7 cells showed a significant downregulation in the mRNA levels of MMP-9, ALDH1, p21 and Bcl-2 as well as a significant upregulation in the mRNA levels of p53 and cyclin D1. Apart from these, a series of in silico work was also performed. Bioinformatics analysis led to the identification of 11 direct target proteins (DTPs) for hesperidin, including aurora kinase B (AURKB), B-cell lymphoma 6 (BCL6), CASP3, CD80, ghrelin and obestatin prepropeptide (GHRL), glucose-6-phosphate dehydrogenase (G6PD), heme oxygenase 1 (HMOX1), interferon regulatory factor 7 (IRF-7), MMP9, tumour protein 53 (TP53) and specificity protein 1 (SP1). Besides, a total of 195 indirect target proteins (ITPs) were found for these DTPs. Subsequently, a Venn diagram was prepared using DTPs, ITPs and BCSC-related genes from PubMed search to identify the potential therapeutic targets (THs) upon which hesperidin acts on in BCSCs. The generation of a protein–protein interaction (PPI) network for the 75 THs identified revealed that TP53 was the only DTP with a high degree score among the selected hub genes, suggesting that p53 is strongly correlated with the BCSC-inhibitory activity of hesperidin. Furthermore, among the five THs (i.e., AKT1, ataxia-telangiectasia mutated kinase [ATM], CDK1, beta-catenin [CTNNB1] and TP53) chosen for genetic alteration analysis, TP53 genetic alterations could particularly be found in 39% of BC patients. Overall, the in vitro and in silico findings suggest that hesperidin is likely to inhibit BCSCs by targeting p53 (Hermawan et al., 2021), whose mutations have been associated with a higher aggressiveness and a lower overall survival in BC (Gasco et al., 2002).
2.2. Chemotherapeutic activities in in vivo breast cancer models
2.2.1. Inhibition of tumour growth
In a study, gavage administration of hesperidin (30 mg/kg/day) was discovered to significantly reduce primary tumour volume and tumour weight in mice bearing mouse 4T1 mammary gland (triple-negative) cancer xenografts while having an insignificant impact on their body weight, suggesting the lack of adverse effects (Pang et al., 2021).
On the other hand, Ye et al. (2012) reported the growth-inhibitory activity of hesperetin in a post-menopausal and ER-positive BC model (i.e., ovariectomised athymic mice bearing aromatase-overexpressing MCF-7 xenografts), and suggested that this activity is linked to the inhibition of aromatase (CYP19A1), which is a rate-limiting enzyme that catalyses testosterone-to-oestrogen conversion in oestrogen biosynthesis (Chumsri, 2015). In the study, mice were subjected to both hesperetin treatment mixed in diet (500–5000 ppm) and androstenedione (AD) injection every other day (Ye et al., 2012). The results demonstrated a significant reduction in tumour volume and tumour weight as compared to AD controls. No significant changes were found in the body and liver weights of hesperetin-treated mice, suggesting the absence of side effects. Furthermore, it was observed that hesperetin inhibited aromatase activity (IC50 = 5 µM) and significantly suppressed AD-induced increase in serum oestradiol (E2) concentration and pS2 (an oestrogen-responsive gene) expression. In addition, hesperetin also attenuated AD-induced expression of CYP1A1, which is an enzyme involved in oestrogen metabolism; reflecting that hesperetin is unlikely to reduce serum E2 level via elimination. Subsequent protein expression analysis of cell cycle- and apoptosis-related proteins revealed that hesperetin can suppress AD-induced increase in the protein levels of cyclin D1, CDK4 and anti-apoptotic Bcl-xL as well as revert AD-induced decrease in the protein level of p57kip2, all of which are likely to promote cell cycle arrest and apoptosis and thereby contribute to the observed reduction in tumour growth.
2.2.2. Inhibition of tumour metastasis
The inhibitory effect of hesperidin on tumour metastasis has been evaluated in a study using mice bearing 4T1 xenografts (Pang et al., 2021). It was found that gavage administration of hesperidin (30 mg/kg/day) significantly reduced the number of lung metastasis nodules relative to untreated controls. A modified in vivo circulating tumour cell (CTC) capture technology was also utilised to determine the number of CTC cells in 4T1 xenograft mice. CTCs have been identified to be representative of an intermediate step in cancer metastasis, thus making CTC enumeration useful in monitoring cancer progression and metastasis (Castro-Giner and Aceto, 2020, Micalizzi et al., 2017). It was observed that hesperidin-treated mice had a significantly lower number of CTCs than untreated controls, which is suggestive of a significant inhibition in metastasis and consistent with the finding from counting lung metastasis nodules (Pang et al., 2021).
2.2.3. Inhibition of neoplastic changes in tissue architecture
In a study, Nandakumar et al. (2014) induced breast carcinogenesis in female Sprague-Dawley rats using 7,12-dimethylbenz(a)anthracene (DMBA), and noted significant changes in the activities of carbohydrate-metabolising enzymes, the plasma and liver lipid profiles and the activities of membrane-bound adenosine triphosphatases (ATPases). Specifically, there was a significant increase in the activities of glycolytic enzymes (i.e., hexokinase, phosphoglucoisomerase and aldolase) as well as a significant decrease in the activties of gluconeogenic enzymes (i.e., glucose-6-phosphatase and fructose-1,6-diphosphatase) (Nandakumar et al., 2014), which is consistent with a high rate of glycolysis and a low rate of gluconeogenesis that are frequently observed in cancers (Lin et al., 2020, Wang and Dong, 2019). DMBA-induced BC-bearing rats also showed significantly increased plasma levels of total cholesterol, free cholesterol, phospholipids, triglycerides and free fatty acids as well as significantly decreased plasma level of ester cholesterol (Nandakumar et al., 2014). In liver, on the other hand, there was a significant increase in the levels of total cholesterol, free cholesterol, ester cholesterol, phospholipids, triglycerides and free fatty acids. These abnormalities in lipid metabolism may promote cancer development and metastasis via certain signalling pathways (Long et al., 2018). Moreover, the activities of Na+/K+-, Ca2+- and Mg2+-ATPases were also found to be significantly suppressed in both erythrocyte membrane and liver of DMBA-induced BC-bearing rats (Nandakumar et al., 2014). This may be a result of DMBA-induced oxidative stress, as DMBA metabolism can generate ROS and cause lipid peroxidation that has been reported to inhibit the activities of membrane-bound ATPases (Amin, 2008, Pizzimenti et al., 2010, Racay et al., 1997, Rauchová et al., 1995).
In the same study, Nandakumar et al. (2014) observed the protective effect of the intragastric adminstration of hesperidin (30 mg/kg/day; 45 days) against DMBA-induced BC in rats, as evidenced by the maintenance of almost normal breast tissue architecture and normal fibro fatty tissue structure. Furthermore, hesperidin treatment also significantly ameliorated DMBA-induced biochemical parameters mentioned above, leading to the proposal that the observed protective effect of hesperidin against DMBA-induced breast carcinogenesis is associated with the suppression of glycolysis, the normalisation of gluconeogenesis and lipid metabolism as well as the stabilisation of cell membranes.
3. Chemosensitising activities of hesperidin and hesperetin
3.1. Doxorubicin
Doxorubicin belongs to the anthracycline class of compounds that share similarities in their structures (Yang et al., 2014). It is a type of cytotoxic chemotherapeutic agent that is commonly used in the treatment of BC patients whose tumours have demonstrated resistance to endocrine therapy or are metastatic (Xiang et al., 2015). Despite its extensive clinical usage, the exact anti-cancer molecular mechanisms of doxorubicin have not been fully elucidated (Yang et al., 2014). Various mechanisms have been proposed to explain how doxorubicin induces cancer cell death, including topoisomerase II poisoning (Atwal et al., 2019), DNA adduct formation (Coldwell et al., 2008), oxygen-derived free radical formation (Cervantes et al., 1988), increased ceramide synthesis (Rath et al., 2009) and histone eviction promotion (Pang et al., 2013). However, the use of doxorubicin has been challenged by drug resistance and cardiotoxicity (Cabeza et al., 2015, Kalyanaraman, 2020). Thus, further research efforts are required to reduce the anti-cancer effective dose and side effects of doxorubicin as well as its resistance. Interestingly, hesperidin and hesperetin have demonstrated chemosensitising activities when used in combination with doxorubicin in BC, suggesting their potentials to be developed as co-chemotherapeutic agents.
A study by Korga-Plewko et al. (2020) reported that doxorubicin (1 µM) reduced cell viability, induced significant morphological changes (i.e., round, shrunk and with poor adhesion), increased both sub-G1 phase (corresponding to dead cells) and early apoptotic cell populations and raised phosphorylated histone H2AX (a marker of DNA double-strand breaks) level in MCF-7 cells. These effects induced by doxorubicin were intensified when co-administered with hesperidin (50 µM), which may reflect the presence of synergism. Gene expression analysis revealed that the co-administration of doxorubicin and hesperidin downregulated the mRNA levels of various DNA repair-related genes, including PARP1, excision repair cross-complementing group 1 (ERCC1), mutS homolog 2 (MSH2), 8-oxoguanine-DNA glycosylase (OGG1), O(6)-methylguanine-DNA methyltransferase (MGMT) and mutL homolog 1 (MLH1), in comparison to individual treatments. Moreover, the co-administration of doxorubicin and hesperidin was also observed to diminish doxorubicin-induced DNA oxidative damage, as indicated by a reduction in the level of apurinic/apyrimidinic (AP) sites and the reversal of doxorubicin-induced reduction in GSH level. These findings collectively suggest that the observed synergistic effect between hesperidin and doxorubicin can potentially be explained by the inhibition of DNA repair mechanisms but not the induction of oxidative stress.
The chemosensitising activity of hesperetin has also been reported by Nurhayati et al. (2020). In the study, the co-adminstration of doxorubicin and hesperetin exerted greater cytotoxic, G2/M phase arrest-inducing and pro-apoptotic effects against a HER2-overexpressing BC cell line (i.e., MCF-7/HER2) in comparison to either treatment alone. Specifically, the combination of 0.2 µM doxorubicin and 95 µM hesperetin yielded a combination index (CI) of 0.63 (<1), signifying a synergistic effect. Furthermore, it was observed that the co-administration of doxorubicin with hesperetin could reduce the extent of lamellipodia elongation and cell migration that would otherwise be induced by doxorubicin alone. Subsequent protein expression analysis revealed the ability of doxorubicin and hesperetin co-treatment to downregulate the protein expression of HER2, MMP-9 and Ras-related C3 botulinum toxin substrate 1 (Rac1; a Rho GTPase implicated in lamellipodia formation), of which can likely explain the observed cytotoxic, G2/M phase arrest-inducing, pro-apoptotic and anti-migratory activities of the co-treatment.
3.2. Letrozole
Around 70% of human BC cases are ER-positive, in which oestrogen signalling plays a key role in promoting disease progression (Lumachi et al., 2013, Saha Roy and Vadlamudi, 2012). Endocrine therapy, including selective oestrogen-receptor modulators (SERMs; i.e., tamoxifen) and aromatase inhibitors (AIs; i.e., anastrozole, letrozole and exemestane), has shown beneficial effects in the management of ER-positive BC patients (Saha Roy and Vadlamudi, 2012, Waks and Winer, 2019). AIs have been classified into two major classes based on their chemical structures (Chumsri, 2015). While type 1 steroidal inhibitors (i.e., exemestane) mediate their anti-oestrogenic activities by competitively and irreversibly binding to and inhibiting aromatase, type 2 non-steroidal inhibitors (i.e., anastrozole and letrozole) bind to aromatase in a reversible manner; both of which reduce oestrogen biosynthesis (Miller, 2003). Letrozole has been used extensively in the management of post-menopausal women with advanced, recurrent or metastatic BCs, as it has a high selectivity of action against aromatase-producing peripheral tissues that represent the major source of oestrogen in these patients (He and Ma, 2016). However, the development of acquired resistance to letrozole has been reported, and it is associated with a more aggressive disease phenotype (Tilghman et al., 2013). In addition, the use of AIs (including letrozole) has been associated with oestrogen deficiency-related conditions such as bone loss (Rachner et al., 2020).
In the recent decade, only the chemosensitising activity of hesperetin with regards to letrozole has been evaluated. In a study, ovariectomised athymic mice bearing aromatase-overexpressing MCF-7 xenografts (a mouse model of post-menopausal BC) were subjected to 84 days of treatment with hesperetin (5000 ppm) mixed in diet as well as subcutaneous injection of AD (0.1 mg/day) and letrozole (0.04, 0.2 and 2 µg/day) (Li et al., 2013). Notably, the co-administration of letrozole and hesperetin suppressed AD-induced tumour growth to a greater extent than either treatment alone. This growth-inhibitory effect of letrozole, hesperetin and their combination can likely be attributed to their suppression of AD-induced increase in plasma E2 level and pS2 expression. Importantly, higher bone volume fraction (BV/TV) and trabecular thickness were observed in the co-treatment groups as compared to the letrozole treatment group, indicating that hesperetin can ameliorate letrozole-induced bone loss.
A more recent study by Rahideh et al. (2016) reported that while letrozole (0.01–200 µM) had insignificant impact on MCF-7 cell viability, hesperetin (200 µM) induced a significant time-dependent reduction in MCF-7 cell viability. However, the same study revealed that while letrozole (0.1–10 µM) expectedly exhibited a significant aromatase-inhibitory activity in MCF-7 cells, hesperetin (1–25 µM) and its co-administration with letrozole only demonstrated a slight but insignificant aromatase-inhibitory activity. Additionally, analysis of aromatase gene expression in MCF-7 cells treated with letrozole, hesperetin or their combination showed that only low dose hesperetin (1 µM) was capable of inducing a significant downregulation of aromatase expression. Overall, these findings reflect the absence of synergism between letrozole and hesperetin in inhibiting aromatase activity and expression. Similarly, another study conducted by the same research group reported that the co-administration of letrozole (1 µM) and hesperetin (5 µM) induced no significant changes in both aromatase activity and expression in MCF-7 cells (Rahideh et al., 2017).
On the other hand, Li et al. (2011) observed that hesperetin can significantly inhibit recombinant aromatase activity at 5–20 µM, as well as significantly upregulate aromatase activity and expression in MCF-7 cells at 20 µM and 10–25 µM, respectively. These observations are contradictory to those of Rahideh et al. (2016), thereby warranting further investigation to ascertain the effects of hesperetin on aromatase activity and expression as well as to re-evaluate the usefulness of hesperetin as a co-therapeutic agent for letrozole. In the same study, Li et al. (2011) also found that hesperetin (20 µM) can significantly enhance the aromatase transactivity driven by promoters I.3 and PII as well as the binding of CCAAT-enhancer-binding proteins (C/EBPs; a family of transcription factors) to the (-56/0) region of promoters, both of which can potentially explain the observed increase in aromatase mRNA level following hesperetin treatment. Subsequent protein expression analysis linked the abovementioned effects of hesperetin to an enhanced extracellular signal-regulated kinase (ERK) signalling (Li et al., 2011), whereby hesperetin (0.5–20 µM) increased the phosphorylated levels of ERK1/2 that has been implicated in C/EBP phosphorylation and activation (Borland et al., 2009).
3.3. Tamoxifen
Tamoxifen, a SERM, exerts its anti-oestrogenic effect by competitively binding to ER and preventing oestrogen from binding (Criscitiello et al., 2011). Its therapeutic effects against ER-positive BC include a reduction in cell proliferation, migration and invasion as well as an induction of apoptosis (Li et al., 2017b). It has been reported that the long-term (i.e., 10 years) use of tamoxifen provides a better therapeutic outcome than a shorter-term (i.e., 5 years) use (Heery et al., 2018). However, continuous usage of tamoxifen has been associated with adverse effects (e.g., hot flashes, arthralgia hypertension, liver abnormalities, lymphedema, nausea, ocular toxicity, sexual dysfunction, vomiting, weight loss/gain and increased risk of venous thromboembolic events, fractures, endometrial and uterine cancers) and drug resistance (Ali et al., 2016, Heery et al., 2018). As such, there have been multiple studies focusing on the approaches to improve both efficacy and side effect profiles of tamoxifen.
In a study by Khamis et al. (2018), the co-administration of tamoxifen and hesperidin (at a non-constant ratio from IC50 fractions of the two compounds) demonstrated a greater anti-proliferative effect against ER-positive MCF-7 and T47D (luminal A) cells than either treatment alone. The co-treatment groups yielded CI values of less than one, reflecting a synergism between hesperentin and tamoxifen. Subsequent gene expression analysis revealed that tamoxifen, hesperidin and their combination resulted in a significant upregulation of BAX and a significant downregulation of BCL2, ERα and EGFR, implying the induction of apoptosis (by a high Bax/Bcl-2 ratio) and the blockage of ERα and EGFR/ERK signalling pathways. Moreover, a significant increase in G0/G1 phase cell population was also observed, suggesting the induction of a G0/G1 phase arrest. These findings collectively suggest that hesperidin may potentiate the anti-cancer activity of tamoxifen via both cytostatic (G0/G1 phase arrest induction) and cytotoxic (apoptosis induction) approaches, which could allow for a reduction in tamoxifen concentration and its associated adverse effects.
4. Safety profiles of hesperidin and hesperetin
Numerous studies have reported on the in vitro and in vivo anti-cancer efficacies of hesperidin and hesperetin, thus making them promising compounds for BC treatment. However, it is of the utmost importance to ensure that their safety profiles are established before translating to clinical settings. Various studies have, thus, attempted to evaluate the preclinical safety of hesperidin and hesperetin, as detailed below.
Hardigree and Epler (1978) evaluated the in vitro mutagenic potentials of plant flavonoids, including hesperidin and hesperetin, using a Salmonella typhimurium reverse mutation assay termed the Ames test. The results showed that both hesperidin and hesperetin showed no mutagenic effect against frameshift strain TA98. In another study, Ortiz-Andrade et al. (2020) assessed the preclinical safety of hesperidin via a combination of in vitro, in vivo and in silico approaches. The MTT assay revealed the lack of cytotoxicity of hesperidin (125–1000 µg/mL) against VERO and MDCK kidney cell lines. When the acute oral toxicity of hesperidin (300 or 2000 mg/kg; single dose) was evaluated in female Wistar rats, no deaths, signs of toxicity or significant abnormalities in gross pathology and serum biochemical parameters was observed at the end of the study period (14 days). However, a considerable weight gain difference was observed in the low dose group on the 7th and 10th days, and a markedly lower neutrophil count was observed in both low and high dose groups. Furthermore, the ACD/Tox Suite® platform was employed to predict the toxicity of hesperidin in terms of its acute toxicity (rodent LD50) as well as its capabilities to block human Ether-à-go-go-Related Gene (hERG) channel or cytochrome P450 (CYP450). The software gave rise to predicted LD50 values of 1600 mg/kg (intraperitoneal injection) and 3000 mg/kg (oral administration) (Ortiz-Andrade et al., 2020), placing hesperidin in the acute toxicity category IV (i.e., non-toxic) according to the OECD guide 423 (Organisation for Economic Co-operation and Development, 2001). Additionally, therapeutic concentrations of hesperidin were predicted to have 0% probability of inducing hERG blockage that would otherwise cause long QT syndrome and sudden cardiac death, together with very low probabilities of inhibiting CYP450 activities (Ortiz-Andrade et al., 2020).
On the other hand, Li et al. (2019) evaluated the acute and sub-chronic oral toxicities of hesperidin in both male and female Sprague-Dawley rats. In the acute toxicity study, lower doses of hesperidin (55, 175, 550 or 1750 mg/kg; single dose) did not cause any deaths or significant abnormalities in food/water intake, body weight, organ weights and gross pathology at the end of the study period (14 days). However, a higher dose of hesperidin (5000 mg/kg; single dose) resulted in a 10% mortality rate, hypoactivity, asthenia and a significant increase in absolute liver and spleen weights. In the sub-chronic toxicity study, rats treated with lower doses of hesperidin (250 or 500 mg/kg/day; 13 weeks) did not demonstrate any significant abnormalities in food/water intake, body weight, ophthalmoscopic examination, functional observation tests, haematological parameters, serum biochemical parameters, urine analysis, organ weights and gross pathology. However, a higher dose of hesperidin (1000 mg/kg/day; 13 weeks) induced significant changes in body and organ weights, haematological parameters, serum biochemical parameters and tissue histopathology of rats. As the observed changes in haematological and serum biochemical parameters are within the normal or laboratory ranges and not dose-dependent, they are considered to be of limited toxicological relevance. Overall, hesperidin has a LD50 at 4837.5 mg/kg and a low observed adverse effect level (LOAEL) at 1000 mg/kg. Similarly, another study also showed that hesperidin (5% or 10%) caused no significant changes in the food intake, food efficiency ratio and body weight of Sprague-Dawley rats (Kawaguchi et al., 1997).
Collectively, the abovementioned findings suggest that hesperidin is considered to be a low-risk and safe compound for further drug development. In contrast, there have not been any reports of animal toxicity studies for hesperetin to date, thereby warranting further investigation into its safety profile. Importantly, the extrapolation of findings from animal toxicity studies to humans often gives a poor prediction of drug toxicity in humans (Bracken, 2009), thus emphasising the importance of clinical evaluation in determining the safe dose range for humans.
5. Pharmacokinetics and bioavailability of hesperidin and hesperetin
The in vivo efficacy of phytochemicals is highly dependent on their bioavailability, which is formally defined by the FDA as ‘the rate and extent to which the active ingredient or active moiety from the drug product is absorbed and becomes available at the site of drug action’ (Chow, 2014). Thus, bioavailability covers the process of absorption, distribution, metabolism and excretion that collectively define a compound’s pharmacokinetics. However, due to the difficulties in assessing in vivo drug target organs in humans, absolute bioavailability of a compound is often evaluated instead. Absolute bioavailability, defined as the fraction of an administered compound that reaches the systemic circulation, is calculated as a ratio of the area under the curve (AUC) of plasma concentration–time curves between oral ingestion and intravenous injection (Jiang et al., 2016).
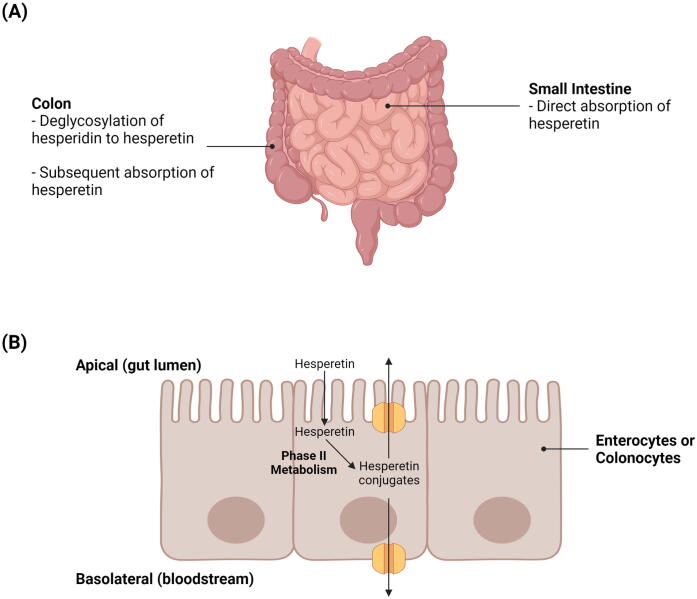
Hesperidin has demonstrated limited bioavailability in studies owing to its poor water solubility that can greatly limit its absorption (Jiao et al., 2020, Li and Schluesener, 2017, Li et al., 2008). It has been discovered that while hesperetin can be directly absorbed in the small intestine by enterocytes, the absorption of hesperidin relies on its deglycosylation by colonic microbiota to a form that can be more readily absorbed (e.g., hesperetin) (Roohbakhsh et al., 2014). Deglycosylation of hesperidin to hesperetin may occur in either a one-step or a two-step process (Mas-Capdevila et al., 2020). The one-step deglycosylation process is catalysed by α-rhamnosyl-β-glucosidase, whereas the two-step process is catalysed by two monoglycosidases (i.e., α-rhamnosidase and β-glucosidase) and involves hesperetin-7-O-glucoside as an intermediate product (Mas-Capdevila et al., 2020). In the colon, the absorption of hesperetin by colonocytes can occur via proton-coupled active transport and/or transcellular passive diffusion (Shen et al., 2016). These suggest that the removal of rutinoside in hesperidin to form hesperetin results in an improvement in bioavailability. For instance, Li et al. (2008) found that the absolute bioavailability of hesperetin is 2-fold higher than that of hesperidin (i.e., 61.5 versus 30.1 nmol . h/mL).
Following absorption, hesperetin is subjected to extensive phase II metabolism by uridine diphosphate (UDP)-glucuronosyltransferases and sulfotransferases to yield glucuronide and sulfate metabolites, respectively (Bredsdorff et al., 2010). This extensive metabolism of hesperetin in intestinal cells, in combination with the subsequent efflux of hesperetin conjugates back into the gut lumen by apical adenosine triphosphate (ATP)-binding cassette (ABC) transporters (e.g., ABC transporter G2 [ABCG2]), may ultimately lower the bioavailability of hesperetin (Brand et al., 2010, Brand et al., 2008). Fig. 4 illustrates the pharmacokinetics of hesperidin and hesperetin in the small intestine and colon
Fig. 4.
Pharmacokinetics of hesperidin and hesperetin. A) Direct absorption of hesperetin in the small intestine and deglycosylation of hesperidin to hesperetin in the colon. B) Phase II metabolism of hesperetin and subsequent apical ABC transporter-mediated efflux of hesperetin conjugates in enterocytes or colonocytes.
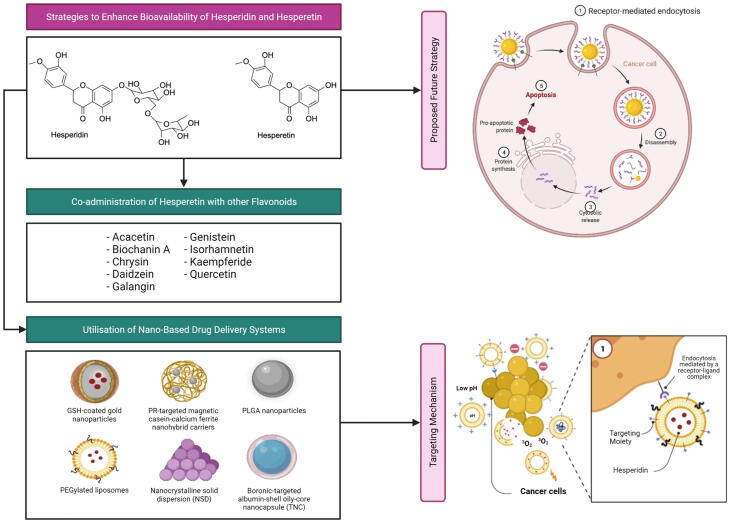
6. Strategies to enhance bioavailability of hesperidin and hesperetin
6.1. Nano-based drug delivery systems
Nano-based drug delivery systems are a rapidly developing technology that utilises nanocarriers to rationally deliver drugs to their sites of action (Patra et al., 2018). This technology has been very promising in cancer treatment, as it allows for the targeted delivery of chemotherapeutic agents to tumour cells, thus avoiding damages to normal healthy cells (Liyanage et al., 2019). In addition, they also possess various properties that make their utilisation in cancer treatment advantageous, including their non-toxicity, high drug loading capacity and capability to resolve poor drug solubility and bioavailability problems (Liyanage et al., 2019). Some examples of nanomedicines that have been approved by the FDA for BC treatment include PEGylated liposomal doxorubicin (Doxil®) that has demonstrated an improvement in the delivery and systemic toxicity profile of doxorubicin; as well as albumin-bound paclitaxel nanoparticles (Abraxane®) that has demonstrated an improvement in the solubility and delivery of paclitaxel (Wu et al., 2017, Yan et al., 2020).
Several classes of nanocarriers (i.e., lipid-based nanoparticles, metallic nanoparticles, nanocrystals, polymeric nanoparticles and protein-based nanoparticles) or their hybrids have been employed to deliver hesperidin or hesperetin in preclinical BC models, with the aim of overcoming their poor bioavailability. Each of these nanocarrier classes is associated with a distinct set of advantages and limitations (Table 2) (Colone et al., 2020, García-Pinel et al., 2019, Gigliobianco et al., 2018, Kianfar, 2021, Mitchell et al., 2021, Sharma et al., 2018), thus emphasising the importance of selecting the most appropriate nanocarrier system for a particular compound. In a study, Sulaiman et al. (2020) loaded hesperidin onto GSH-coated gold nanoparticles (Hsp-AuNPs) via electrostatic attraction between the amine group of hesperidin and the carboxyl group of GSH, and assessed this nanoformulation in both in vitro and in vivo BC models. It was found that Hsp-AuNPs (25–125 µg/mL) exhibited a greater cytotoxicity against MDA-MB-231 cells than hesperidin (25–125 µg/mL), as evidenced by the observations of a greater reduction in cell viability and a greater extent of morphological changes (i.e., formation of cell clusters with less extensions) in Hsp-AuNPs-treated cells. Additionally, Hsp-AuNPs also demonstrated a greater pro-apoptotic activity than free hesperidin, where Hsp-AuNPs-treated cells showed a greater extent of membrane integrity disruption and chromatin condensation. Importantly, ultraviolet–visible spectrum analysis revealed a more rapid release of hesperidin from AuNPs in acidic (pH 5.04) than neutral (pH 7.4) environments, reflecting the potential of Hsp-AuNPs to preferentially deliver hesperidin to tumours whose microenvironment is usually acidic due to acidic metabolic waste product accumulation. Moreover, Hsp-AuNPs also demonstrated a less significant cytotoxicity against human normal breast epithelial cells (HBL-100) than MDA-MB-231 cells, further implying a preferential targeting of cancer cells by this nanoformulation. When evaluated in vivo, it was observed that Hsp-AuNP treatment (20 mg/kg; 3 days in a week) improved the functional activity of macrophages in mice bearing Ehrlich ascites carcinoma cells (Sulaiman et al., 2020), which may help to slow down tumour growth (Klimp et al., 2002). Moreover, Hsp-AuNPs were found to be generally safe in these mice, as reflected by the lack of significant abnormalities in the body weight, the liver and kidney functionality biomarkers as well as the histology of kidneys, liver, lungs and spleen of mice (Sulaiman et al., 2020).
Table 2.
Advantages and limitations associated with the major classes of nanocarriers that have been employed for the delivery of hesperidin or hesperetin in preclinical BC models.
| Advantages | Limitations | References | |
|---|---|---|---|
| Lipid-based Nanoparticles |
|
|
Colone et al., 2020, García-Pinel et al., 2019 |
| Metallic Nanoparticles |
|
|
Colone et al., 2020, Sharma et al., 2018 |
| Nanocrystals |
|
|
Gigliobianco et al. (2018) |
| Polymeric Nanoparticles |
|
|
Mitchell et al., 2020 |
| Protein-based Nanoparticles |
|
|
Kianfar (2021) |
In another study, Purushothaman et al. (2020) encapsulated citrus peel extracted hesperidin drug (CHD) and hesperidin into PR-targeted magnetic casein-calcium ferrite (CaFe2O4) nanohybrid carriers, and found that this nanoformulation can similarly improve the anti-cancer efficacy of CHD and hesperidin. For instance, it was observed that nano-encapsulated CHD and hesperidin respectively induced a greater reduction in MDA-MB-231 cell viability than CHD (i.e., IC50 at 0.92 versus 31.88 µg/mL pure CHD) and hesperidin (i.e., IC50 at 2.01 versus 32.86 µg/mL pure hesperidin) treatment alone. Moreover, this nanoformulation also demonstrated preferential release of hesperidin under acidic conditions (pH 5.4) and enabled sustained release of hesperidin for up to 12 h, which can enhance the anti-cancer efficacy and reduce the systemic toxicity of hesperidin as well as prolong its anti-cancer effect. Interestingly, it was noted that the application of an external magnetic field can trigger hesperidin release from the nanocarriers. In addition, this nanocarrier system was found to be biocompatible (i.e., IC50 greater than 500 µg/mL in L929 mouse fibroblasts), thus suggesting its potential applicability in magnetic-controlled targeting and release of drugs at their sites of action.
On the other hand, El‐Sisi et al. (2020) evaluated the in vivo anti-cancer efficacy and toxicity of poly (lactide-co-glycolide) (PLGA)-encapsulated imatinib mesylate and/or hesperidin in Swiss albino mice bearing solid Ehrlich carcinoma (SEC). The study showed that the intraperitoneal injection of nano-encapsulated imatinib mesylate (Nano-IM; 3.75 or 6.25 mg/mL) or nano-encapsulated hesperidin (Nano-HES; 6.25 mg/mL) induced a more significant reduction in both tumour volume and tumour weight than their respective treatment (50 mg/kg). This growth-inhibitory effect was intensified when Nano-IM (3.75 mg/mL) was co-admimistered with Nano-HES (6.25 mg/mL), potentially reflecting a synergistic anti-cancer effect between these nanoformulations. The presence of synergism was validated in MCF-7 cells with a CI value of lower than 1. Similarly, protein expression analysis revealed that while Nano-IM or Nano-HES induced a greater extent of reduction in vascular endothelial growth factor (VEGF; an angiogenesis marker) and Ki-67 (a proliferation marker) levels as well as increase in CASP3 (an apoptotic marker) level than their free counterparts, the greatest extent of changes were observed with the co-administration of Nano-IM and Nano-HES. Interestingly, gene expression analysis suggested that the nano-encapsulation of imatinib can prevent it from inducing the expression of multidrug resistance protein 1 (MDR1) (El‐Sisi et al., 2020), a drug efflux pump that may confer resistance to imatinib (Mahon et al., 2003). Additionally, hesperidin, Nano-HES as well as the co-admimistration of Nano-IM and Nano-HES can further downregulate MDR1 expression to a level below that of untreated mice, thereby reducing the efflux of imatinib and overcoming its resistance (El‐Sisi et al., 2020). While imatinib-treated mice had significantly lower levels of hematological markers, significantly higher levels of cardiac markers and altered cardiac tissue histology (which pointed to anemia, reduced immunity and cardiotoxicity), these phenomena were not observed in mice treated with Nano-IM or Nano-IM and Nano-HES combination. Overall, the study findings suggest that the nano-encapsulation of imatinib and hesperidin as well as the co-adminstration of their nanoformulations can improve the anti-cancer efficacy (i.e., tumour growth-inhibitory, anti-angiogenic, anti-proliferative and pro-apoptotic effects) of both compounds and protect against imatinib-induced hematotoxicity and cardiotoxicity.
The first liposome-based formulation of hesperetin has been developed and reported by Wolfram et al. (2016) in a study, whereby the incorporation of polyethylene glycol (PEG) into liposomes helped to enhance the stability of the nanoformulation in serum and during storage. This nanoformulation was observed to enable a gradual release of hesperetin, starting from the 1st h and reaching approximately 86% after 72 h. Moreover, fluorescent microscopic analysis confirmed the successful cellular internalisation of liposomal hesperetin within 24 h, the time period during which the majority (~75%) of hesperetin release occurred. Notably, void liposomal carriers had no significant effect on MDA-MB-231 cell viability while liposomal hesperetin attenuated MDA-MB-231 cell viability to a greater extent than DMSO-dissolved hesperetin of an equivalent dose (100 µM hesperetin), reflecting the biocompatibility of this nanocarrier system and its ability to enhance the anti-cancer efficacy of hesperetin. It was also suggested that hesperetin is unlikely to be a MDR1 substrate, as both liposomal and DMSO-dissolved hesperetin exhibited a similar level of anti-cancer activity against both non-transfected and MDR1-transfected MDA-MB-231 cells.
In the following years, various studies have also attempted to improve the bioavailability of hesperetin via the development of various nanoformulations. For instance, Sheokand et al. (2019) developed a nanocrystalline solid dispersion (NSD) of hesperetin and evaluated its oral bioavailability and chemopreventive potential against DMBA-induced breast carcinogenesis in female Sprague-Dawley rats. In comparison to physical mixtures of hesperetin and excipients (PM), NSD (20 mg/kg; single dose) demonstrated significantly improved absorption rate and extent, as indicated by a higher Cmax (182.5 ± 31.3 versus 604.7 ± 60.7 ng/mL) and a greater AUC of plasma concentration–time curve (2800.7 ± 457.4 versus 6081.0 ± 911.8 ng . h/mL), respectively. Additionally, NSD also had a lower Tmax of 0.5 ± 0.1 h, which may be indicative of a faster onset of hesperetin action. Moreover, it was observed that DMBA-treated rats subjected to PM or NSD treatment (20 mg/kg/day; days 8–112) had lower tumour indicence and tumour growth as well as higher tumour latency in comparison to the DMBA control group. However, an intensified effect was reported in the NSD-treated group. Histologically, both NSD and PM protected against significant DMBA-induced malignancy-associated changes in mammary tissues, whereby only benign form of breast tumours were observed in these treatment groups. Importantly, both NSD and PM also suppressed DMBA-induced increase in liver functionality markers. However, a greater extent of suppression was observed in the NSD- than the PM-treated groups. These findings collectively suggest that although hesperetin exhibits protective effect against DMBA-induced breast carcinogenesis and hepatotoxicity regardless of its formultation type, the NSD formulation tends to offer a better protection than the PM formulation.
In another study, Gaber et al. (2019) observed a synergistic anti-cancer effect between exemestane and hesperetin both in vitro and in vivo, in MCF-7 cells and BALB/C mice bearing Ehrlich ascites tumours, thus justifying the co-administration of these compounds in BC treatment. In the same study, the authors loaded the hydrophobic exemestane and hesperetin combination into a boronic-targeted albumin-shell oily-core nanocapsule (TNC) formulation, where albumin-mediated binding to albondin receptors and secreted protein, acidic and rich in cysteine (SPARC) as well as 3-aminophenylboronic acid-mediated binding to sialic acid residues can allow for the targeted delivery of compounds to tumours. The MTT assay revealed that TNCs (IC50 = 1.96 µM) exhibited a greater cytotoxicity against MCF-7 cells than its non-targeted counterparts, including exemestane- and hesperetin-loaded albumin nanocapsules (NCs; IC50 = 2.09 µM) as well as nanoemulsions of exemestane and hesperetin (NEs; IC50 = 2.35 µM), and the free drug combination (IC50 = 2.69 µM). Similarly, the lowest CI value was obtained for TNCs (0.662), followed by NCs (0.705), NEs (0.794) and the free drug combination (0.909). Consistent with the in vitro trend, mice subjected to intravenous injections of TNCs (30 mg/kg exemestane and 7.5 mg/kg hesperetin; biweekly for 3 weeks) had the greatest suppression in tumour growth, the highest necrosis score and the lowest Ki-67 protein expression, followed by those subjected to treatment with NCs and the free drug combination. Collectively, the study findings suggest that the combined use of exemestane and hesperetin as well as the utilisation of nanocarrier systems and targeting ligands can enhance the in vitro and in vivo anti-cancer efficacies of exemestane and hesperetin. Importantly, TNC formulation also demonstrated a dose-reducing capability in which a dose reduction index (DRI) of 8.22 and 1.84 could be achieved for exemestane and hesperetin, respectively.
6.2. Co-administration with other flavonoids
As detailed in Section 5, the low bioavailability of hesperetin can be accounted for by extensive phase II metabolism and ABCG2-mediated apical efflux of hesperetin metabolites (i.e., glucuronide and sulfate metabolites) back into the gut lumen. In a study by Brand et al. (2010), it was observed that the co-administration of hesperetin with certain flavonoids can potentially suppress hesperetin metabolism and hesperetin metabolite efflux, thereby improving hesperetin bioavailability. The study was based on the two-compartment transwell Caco-2 (a human colorectal adenocarcinoma cell line) monolayer system, which is an in vitro intestinal barrier model whereby the apical (AP) compartment simulated the gut lumen side and the basolateral (BL) compartment simulated the bloodstream side. When 10 µM hesperetin was co-incubated with 10 µM of a selected list of flavonoids [i.e., acacetin, biochanin A, (+)-catechin, chrysin, daidzein, (-)-epigallocatechin gallate, (-)-epicatechin, galangin, genistein, hesperidin, isorhamnetin, kaempferide, phloretin, quercetin and rutin], acacetin, biochanin A, chrysin, daidzein, galangin, genistein, isorhamnetin, kaempferide and quercetin markedly decreased the AP efflux and increased the BL transport of hesperetin metabolites. In terms of the BL/AP transport ratio of hesperetin metabolites, chrysin, isorhamnetin and quercetin (highest ratio of 1.7) yielded a ratio of greater than 1, which was indicative of the predominance of BL transport over AP efflux. This observed improvement in the BL/AP ratio is potentially linked to the direct modulation of ABCG2 activity by these flavonoids or the competitive inhibition of ABCG2-mediated AP efflux by flavonoids that are also substrates for ABCG2. Moreover, the co-administration of hesperetin and quercetin also resulted in a significant decrease (27%) in the total amount (both extracellularly [AP and BL compartments] and intracellularly) of hesperetin metabolites that could be detected. In combination with the observation of a significantly higher (70%) hesperetin level with quercetin co-administration, these findings suggest that quercetin is likely to be capable of suppressing the phase II metabolism of hesperetin. As quercetin is also subjected to phase II metabolism (i.e., glucuronidation, sulfation and methylation) in Caco-2 cells (Del Mar Contreras et al., 2016), the observed quercetin-mediated inhibition of hesperetin phase II metabolism is likely of a competitive nature (Brand et al., 2010).
7. Conclusion and future perspectives
In summary, hesperidin and its aglycone hesperetin have good in vitro anti-cancer activities in BC models based on their inhibition of cell proliferation, migration (hesperidin only) and BCSCs (hesperidin only) as well as their induction of apoptosis and cell cycle arrest. In in vivo BC models, they exert their anti-cancer effects by inhibiting tumour growth, metastasis (hesperidin only) and neoplastic changes in tissue architecture (hesperidin only). The co-administration of hesperidin with doxorubicin or tamoxifen as well as the co-administration of hesperetin with doxorubicin or letrozole can enhance the anti-BC effects of the clinically available anti-cancer drugs. These chemotherapeutic and chemosensitising activities of hesperidin and hesperetin have been associated with various mechanisms, including the suppression of AKT, NF-κB, ERα and EGFR/ERK signalling pathways, the inhibition of glucose uptake, the inhibition of HER2 tyrosine kinase activity, the modulation of miRNA (i.e., miR-16, −21 and miR-34a) expression, the accumulation of cytosolic ROS, the activation of ASK1/JNK signalling pathway, the regulation of cell cycle regulatory proteins (i.e., CDK4 and p21Cip1), the reduction of MMP (i.e., MMP-2 and −9) activities, the targeting of p53, the modulation of aromatase activity and expression, the normalisation of DMBA-induced changes in carbohydrate-metabolising enzyme activities, lipid profile and membrane-bound ATPases activities, the inhibition of DNA repair mechanisms and the downregulation of HER2, MMP-9 and Rac1 expression.
Although several toxicity studies have suggested the low-risk and safe nature of hesperidin, little is known about the safety profile of hesperetin. Thus, extensive preclinical toxicity studies are required before these compounds can be considered for further drug development and clinical use. In addition, poor oral bioavailability has been another important limitation for both hesperidin and hesperetin. Attempts such as the use of nano-based drug delivery systems or the co-administration of hesperetin with other flavonoids seem to be promising in improving the oral bioavailability of hesperidin and hesperetin to facilitate their future clinical use. In particular, several nanoformulations of hesperidin and hesperetin, including GSH-coated gold nanoparticles, PR-targeted magnetic casein-calcium ferrite nanohybrid carriers, PLGA nanoparticles, PEGylated liposomes, nanocrystalline solid dispersions and boronic-targeted albumin-shell oily-core nanocapsules, have been developed. The said nanoformulations can improve the chemotherapeutic activity and the chemosensitising activity (to imatinib mesylate and exemestane) of hesperidin and hesperetin against BC. Another important flavonoid, quercetin, can suppress hesperetin metabolism and hesperetin metabolite efflux, thereby improving the bioavailability of hesperetin when co-adminstered. Taken together, hesperidin and hesperetin are highly promising candidates to be developed as chemotherapeutic and chemosensitising agents for BC treatment, although much work remains to be done with regards to confirming their efficacy, safety and bioavailability in humans via clinical studies.
For future work, one approach that can be delved into for improving the anti-cancer efficacy of hesperdin/hesperetin includes the nanocarrier-mediated co-delivery of hesperidin/hesperetin and a plasmid carrying a gene that encodes for a pro-apoptotic protein (Fig. 5). By further conjugating the nanocarrier surface with targeting ligands, targeted delivery of hesperidin/hesperetin and plasmid can be achieved, thereby resulting in an effective induction of apoptosis in cancer cells with minimal systemic toxicity.
Fig. 5.
Current and proposed future strategies for bioavailability and anti-cancer efficacy enhancement of hesperidin and hesperetin.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors appreciate Universiti Kuala Lumpur Royal College of Medicine Perak, Ipoh, Perak, Malaysia for providing the facilities and services required to complete the study. Figures were created with the support of https://biorender.com under the paid subscription.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abotaleb M., Samuel S.M., Varghese E., Varghese S., Kubatka P., Liskova A., Büsselberg D. Flavonoids in cancer and apoptosis. Cancers (Basel). 2019;11:28. doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuelsaad A.S.A., Mohamed I., Allam G., Al-Solumani A.A. Antimicrobial and immunomodulating activities of hesperidin and ellagic acid against diarrheic Aeromonas hydrophila in a murine model. Life Sci. 2013;93(20):714–722. doi: 10.1016/j.lfs.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Agarwal M.B. Is cancer chemotherapy dying? Asian. J. Transfus. Sci. 2016;10:S1–S7. doi: 10.4103/0973-6247.182735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal Y.O., Sharma P.K., Shrivastava B., Ojha S., Upadhya H.M., Arya D.S., Goyal S.N., Das A. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PLoS ONE. 2014;9(11):e111212. doi: 10.1371/journal.pone.0111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama S., Katsumata S.-I., Suzuki K., Ishimi Y., Wu J., Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2009;46(1):87–92. doi: 10.3164/jcbn.09-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rikabi R., Al-Shmgani H., Dewir Y.H., El-Hendawy S. In vivo and in vitro evaluation of the protective effects of hesperidin in lipopolysaccharide-induced inflammation and cytotoxicity of cell. Molecules. 2020;25:478. doi: 10.3390/molecules25030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Rasool M., Chaoudhry H., Pushparaj P.N., Jha P., Hafiz A., Mahfooz M., Sami G.A., Kamal M.A., Bashir S., Ali A., Jamal M.S. Molecular mechanisms and mode of tamoxifen resistance in breast cancer. Bioinformation. 2016;12(3):135–139. doi: 10.6026/bioinformation10.6026/012.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin A. Chemopreventive effect of chlorella on the antioxidant system in 7, 12-dimethylbenz[a]anthracene-induced oxidative stress in liver. Int. J. Pharmacol. 2008;4(3):169–176. doi: 10.3923/ijp.2008.169.176. [DOI] [Google Scholar]
- Atanasov A.G., Zotchev S.B., Dirsch V.M., Taskforce I.N.P.S., Supuran C.T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 2021;20:200–216. doi: 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal M., Swan R.L., Rowe C., Lee K.C., Lee D.C., Armstrong L., Cowell I.G., Austin C.A. Intercalating TOP2 poisons attenuate topoisomerase action at higher concentrations. Mol. Pharmacol. 2019;96(4):475–484. doi: 10.1124/mol.119.117259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland G., Bird R.J., Palmer T.M., Yarwood S.J. Activation of protein kinase Calpha by EPAC1 is required for the ERK- and CCAAT/enhancer-binding protein beta-dependent induction of the SOCS-3 gene by cyclic AMP in COS1 cells. J. Biol. Chem. 2009;284:17391–17403. doi: 10.1074/jbc.M109.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009;102(3):120–122. doi: 10.1258/jrsm.2008.08k033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand W., Padilla B., van Bladeren P.J., Williamson G., Rietjens I.M.C.M. The effect of co-administered flavonoids on the metabolism of hesperetin and the disposition of its metabolites in Caco-2 cell monolayers. Mol. Nutr. Food Res. 2010;54(6):851–860. doi: 10.1002/mnfr.200900183. [DOI] [PubMed] [Google Scholar]
- Brand W., van der Wel P.A.I., Rein M.J., Barron D., Williamson G., van Bladeren P.J., Rietjens I.M.C.M. Metabolism and transport of the citrus flavonoid hesperetin in Caco-2 cell monolayers. Drug Metab. Dispos. 2008;36(9):1794–1802. doi: 10.1124/dmd.107.019943. [DOI] [PubMed] [Google Scholar]
- Bredsdorff L., Nielsen I.L., Rasmussen S.E., Cornett C., Barron D., Bouisset F., Offord E., Williamson G. Absorption, conjugation and excretion of the flavanones, naringenin and hesperetin from alpha-rhamnosidase-treated orange juice in human subjects. Br. J. Nutr. 2010;103:1602–1609. doi: 10.1017/S0007114509993679. [DOI] [PubMed] [Google Scholar]
- Browne B.C., O’Brien N., Duffy M.J., Crown J., O’Donovan N. HER-2 signaling and inhibition in breast cancer. Curr. Cancer Drug Targets. 2009;9:419–438. doi: 10.2174/156800909788166484. [DOI] [PubMed] [Google Scholar]
- Cabeza L., Ortiz R., Arias J.L., Prados J., Ruiz Martínez M.A., Entrena J.M., Luque R., Melguizo C. Enhanced antitumor activity of doxorubicin in breast cancer through the use of poly(butylcyanoacrylate) nanoparticles. Int. J. Nanomedicine. 2015;10:1291–1306. doi: 10.2147/IJN.S74378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Giner F., Aceto N. Tracking cancer progression: from circulating tumor cells to metastasis. Genome Med. 2020;12:31. doi: 10.1186/s13073-020-00728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes A., Pinedo H.M., Lankelma J., Schuurhuis G.J. The role of oxygen-derived free radicals in the cytotoxicity of doxorubicin in multidrug resistant and sensitive human ovarian cancer cells. Cancer Lett. 1988;41(2):169–177. doi: 10.1016/0304-3835(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Cha J.-H., Chan L.-C., Li C.-W., Hsu J.L., Hung M.-C. Mechanisms controlling PD-L1 expression in cancer. Mol. Cell. 2019;76(3):359–370. doi: 10.1016/j.molcel.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrika B.B., Steephan M., Kumar T.R.S., Sabu A., Haridas M. Hesperetin and naringenin sensitize HER2 positive cancer cells to death by serving as HER2 tyrosine kinase inhibitors. Life Sci. 2016;160:47–56. doi: 10.1016/j.lfs.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Chew H.K. Adjuvant therapy for breast cancer: who should get what? West. J. Med. 2001;174:284–287. doi: 10.1136/ewjm.174.4.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.J. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: involvement of CDK4 and p21. Nutr. Cancer. 2007;59(1):115–119. doi: 10.1080/01635580701419030. [DOI] [PubMed] [Google Scholar]
- Chow S.-C. Bioavailability and bioequivalence in drug development. Wiley Interdiscip. Rev. Comput. Stat. 2014;6(4):304–312. doi: 10.1002/wics.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumsri S. Clinical utilities of aromatase inhibitors in breast cancer. Int. J. Womens Health. 2015;7:493–499. doi: 10.2147/IJWH.S69907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldwell K.E., Cutts S.M., Ognibene T.J., Henderson P.T., Phillips D.R. Detection of Adriamycin–DNA adducts by accelerator mass spectrometry at clinically relevant Adriamycin concentrations. Nucleic Acids Res. 2008;36 doi: 10.1093/nar/gkn439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colone M., Calcabrini A., Stringaro A. Drug delivery systems of natural products in oncology. Molecules. 2020;25:4560. doi: 10.3390/molecules25194560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G.M., Pezzuto J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016;25:41–59. doi: 10.1159/000443404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscitiello C., Fumagalli D., Saini K.S., Loi S. Tamoxifen in early-stage estrogen receptor-positive breast cancer: overview of clinical use and molecular biomarkers for patient selection. Onco. Targets. Ther. 2011;4:1–11. doi: 10.2147/OTT.S10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Li T., Bai Z., Yang Y., Liu X., Zhan J., Shi B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015;5:2929–2943. [PMC free article] [PubMed] [Google Scholar]
- Del Mar Contreras M., Borrás-Linares I., Herranz-López M., Micol V., Segura-Carretero A. Further exploring the absorption and enterocyte metabolism of quercetin forms in the Caco-2 model using nano-LC-TOF-MS. Electrophoresis. 2016;37:998–1006. doi: 10.1002/elps.201500375. [DOI] [PubMed] [Google Scholar]
- Dhanya R., Jayamurthy P. In vitro evaluation of antidiabetic potential of hesperidin and its aglycone hesperetin under oxidative stress in skeletal muscle cell line. Cell Biochem. Funct. 2020;38(4):419–427. doi: 10.1002/cbf.v38.410.1002/cbf.3478. [DOI] [PubMed] [Google Scholar]
- Dutta S., Mahalanobish S., Saha S., Ghosh S., Sil P.C. Natural products: An upcoming therapeutic approach to cancer. Food Chem. Toxicol. 2019;128:240–255. doi: 10.1016/j.fct.2019.04.012. [DOI] [PubMed] [Google Scholar]
- Economopoulou P., Kaklamani V.G., Siziopikou K. The role of cancer stem cells in breast cancer initiation and progression: potential cancer stem cell-directed therapies. Oncologist. 2012;17(11):1394–1401. doi: 10.1634/theoncologist.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Sisi A.E., Sokkar S.S., Ibrahim H.A., Hamed M.F., Abu‐Risha S.E. Targeting MDR-1 gene expression, BAX/BCL2, caspase-3, and Ki-67 by nanoencapsulated imatinib and hesperidin to enhance anticancer activity and ameliorate cardiotoxicity. Fundam. Clin. Pharmacol. 2020;34(4):458–475. doi: 10.1111/fcp.v34.410.1111/fcp.12549. [DOI] [PubMed] [Google Scholar]
- Ferreira de Oliveira J.M.P., Santos C., Fernandes E. Therapeutic potential of hesperidin and its aglycone hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine. 2020;73:152887. doi: 10.1016/j.phymed.2019.152887. [DOI] [PubMed] [Google Scholar]
- Gaber M., Hany M., Mokhtar S., Helmy M.W., Elkodairy K.A., Elzoghby A.O. Boronic-targeted albumin-shell oily-core nanocapsules for synergistic aromatase inhibitor/herbal breast cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;105:110099. doi: 10.1016/j.msec.2019.110099. [DOI] [PubMed] [Google Scholar]
- García-Pinel B., Porras-Alcalá C., Ortega-Rodríguez A., Sarabia F., Prados J., Melguizo C., López-Romero J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials. 2019;9:638. doi: 10.3390/nano9040638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasco M., Shami S., Crook T. The p53 pathway in breast cancer. Breast Cancer Res. 2002;4:70–76. doi: 10.1186/bcr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliobianco M.R., Casadidio C., Censi R., Di Martino P. Nanocrystals of poorly soluble drugs: Drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10:134. doi: 10.3390/pharmaceutics10030134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J.B., Bao X.B., Ma Z. Effects of miR-21 on proliferation and apoptosis in human gastric adenocarcinoma cells. Oncol. Lett. 2018;15:618–622. doi: 10.3892/ol.2017.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M.M., Desai K.V. Pathways to endocrine therapy resistance in breast cancer. Front. Endocrinol. Lausanne. 2019;10:573. doi: 10.3389/fendo.2019.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardigree A.A., Epler J.L. Comparative mutagenesis of plant flavonoids in microbial systems. Mutat. Res. 1978;58(2-3):231–239. doi: 10.1016/0165-1218(78)90014-9. [DOI] [PubMed] [Google Scholar]
- He D.X., Ma X. Clinical utility of letrozole in the treatment of breast cancer: a Chinese perspective. Onco. Targets Ther. 2016;9:1077–1084. doi: 10.2147/OTT.S81087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Xia Z., Yu D., Wang J., Jin L., Huang D., Ye X., Li X., Zhang B. Hepatoprotective effects and structure-activity relationship of five flavonoids against lipopolysaccharide/d-galactosamine induced acute liver failure in mice. Int. Immunopharmacol. 2019;68:171–178. doi: 10.1016/j.intimp.2018.12.059. [DOI] [PubMed] [Google Scholar]
- Heery M., et al. Precautions for patients taking tamoxifen. J. Adv. Pract. Oncol. 2018;9(1) doi: 10.6004/jadpro10.6004/jadpro.2018.9.110.6004/jadpro.2018.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermawan A., Khumaira A., Ikawati M., Putri H., Jenie R.I., Angraini S.M., Muflikhasari H.A. Identification of key genes of hesperidin in inhibition of breast cancer stem cells by functional network analysis. Comput. Biol. Chem. 2021;90:107427. doi: 10.1016/j.compbiolchem.2020.107427. [DOI] [PubMed] [Google Scholar]
- Hume S., Dianov G.L., Ramadan K. A unified model for the G1/S cell cycle transition. Nucleic Acids Res. 2020;48:12483–12501. doi: 10.1093/nar/gkaa1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel B.B., Tilghman S.L., Parker-Lemieux K., Payton-Stewart F. Phytochemicals: current strategies for treating breast cancer. Oncol. Lett. 2018;15:7471–7478. doi: 10.3892/ol.2018.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Lu Y., Tian H., Meng X., Wei M., Cho W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019;114:108800. doi: 10.1016/j.biopha.2019.108800. [DOI] [PubMed] [Google Scholar]
- Jiang W., Yang J.J., Cao L., Xiao X., Shi X.L., Cao Y.X. Modifications of the method for calculating absolute drug bioavailability. J. Pharm. Pharm. Sci. 2016;19:181–187. doi: 10.18433/J3RG78. [DOI] [PubMed] [Google Scholar]
- Jiao Q., Xu L., Jiang L., Jiang Y., Zhang J., Liu B. Metabolism study of hesperetin and hesperidin in rats by UHPLC-LTQ-Orbitrap MSn. Xenobiotica. 2020;50(11):1311–1322. doi: 10.1080/00498254.2019.1567956. [DOI] [PubMed] [Google Scholar]
- Kabała-Dzik A., Rzepecka-Stojko A., Kubina R., Iriti M., Wojtyczka R.D., Buszman E., Stojko J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7 - a comparative study. Cell. Mol. Biol. 2018;64(8):1–10. doi: 10.14715/cmb/2018.64.8.1. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree? Redox Biol. 2020;29:101394. doi: 10.1016/j.redox.2019.101394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinova A., Kubatka P., Golubnitschaja O., Kello M., Zubor P., Solar P., Pec M. Dietary phytochemicals in breast cancer research: anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018;23:36. doi: 10.1186/s12199-018-0724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K., Mizuno T., Aida K., Uchino K. Hesperidin as an inhibitor of lipases from porcine pancreas and Pseudomonas. Biosci. Biotechnol. Biochem. 1997;61(1):102–104. doi: 10.1271/bbb.61.102. [DOI] [PubMed] [Google Scholar]
- Khamis A.A.A., Ali E.M.M., El-Moneim M.A.A., Abd-Alhaseeb M.M., El-Magd M.A., Salim E.I. Hesperidin, piperine and bee venom synergistically potentiate the anticancer effect of tamoxifen against breast cancer cells. Biomed. Pharmacother. 2018;105:1335–1343. doi: 10.1016/j.biopha.2018.06.105. [DOI] [PubMed] [Google Scholar]
- Kianfar E. Protein nanoparticles in drug delivery: animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnology. 2021;19:159. doi: 10.1186/s12951-021-00896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimp A.H., de Vries E.G.E., Scherphof G.L., Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit. Rev. Oncol. Hematol. 2002;44(2):143–161. doi: 10.1016/S1040-8428(01)00203-7. [DOI] [PubMed] [Google Scholar]
- Kongtawelert P., Wudtiwai B., Shwe T.H., Pothacharoen P., Phitak T. Inhibitory effect of hesperidin on the expression of programmed death ligand (PD-L1) in breast cancer. Molecules. 2020;25:252. doi: 10.3390/molecules25020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korga-Plewko A., Michalczyk M., Adamczuk G., Humeniuk E., Ostrowska-Lesko M., Jozefczyk A., Iwan M., Wojcik M., Dudka J. Apigenin and hesperidin downregulate dna repair genes in MCF-7 breast cancer cells and augment doxorubicin toxicity. Molecules. 2020;25:4421. doi: 10.3390/molecules25194421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.J., Wilson L., Jordan M.A., Nguyen V.y., Tang J., Smiyun G. Hesperidin suppressed proliferations of both human breast cancer and androgen-dependent prostate cancer cells. Phyther. Res. 2010;24(S1):S15–S19. doi: 10.1002/ptr.2856. [DOI] [PubMed] [Google Scholar]
- Li C., Schluesener H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017;57(3):613–631. doi: 10.1080/10408398.2014.906382. [DOI] [PubMed] [Google Scholar]
- Li F., Chow S., Cheung W.-H., Chan F.L., Chen S., Leung L.K. The citrus flavonone hesperetin prevents letrozole-induced bone loss in a mouse model of breast cancer. J. Nutr. Biochem. 2013;24(6):1112–1116. doi: 10.1016/j.jnutbio.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Li F., Ye L., Lin S.-M., Leung L.K. Dietary flavones and flavonones display differential effects on aromatase (CYP19) transcription in the breast cancer cells MCF-7. Mol. Cell. Endocrinol. 2011;344(1-2):51–58. doi: 10.1016/j.mce:2011.06.024. [DOI] [PubMed] [Google Scholar]
- Li H., Qiu Z., Li F., Wang C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 2017;14:5865–5870. doi: 10.3892/ol.2017.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Shi X., Xu Y., Wan J., Wei S., Zhu R. Tamoxifen promotes apoptosis and inhibits invasion in estrogen-positive breast cancer MCF-7 cells. Mol. Med. Rep. 2017;16:478–484. doi: 10.3892/mmr.2017.6603. [DOI] [PubMed] [Google Scholar]
- Li Y., Kandhare A.D., Mukherjee A.A., Bodhankar S.L. Acute and sub-chronic oral toxicity studies of hesperidin isolated from orange peel extract in Sprague Dawley rats. Regul. Toxicol. Pharm. 2019;105:77–85. doi: 10.1016/j.yrtph.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Li Y.-M., Li X.-M., Li G.-M., Du W.-C., Zhang J., Li W.-X., Xu J., Hu M., Zhu Z.e. In vivo pharmacokinetics of hesperidin are affected by treatment with glucosidase-like BglA protein isolated from yeasts. J. Agric. Food Chem. 2008;56(14):5550–5557. doi: 10.1021/jf800105c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Guan H., Huang Z., Liu J., Li H., Wei G., Cao X., Li Y. Downregulation of Bcl-2 expression by miR-34a mediates palmitate-induced Min6 cells apoptosis. J. Diabetes Res. 2014;2014:1–7. doi: 10.1155/2014/258695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Xiao Z., Chen T., Liang S.H., Guo H. Glucose metabolism on tumor plasticity, diagnosis, and treatment. Front. Oncol. 2020;10:317. doi: 10.3389/fonc.2020.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage P.Y., Hettiarachchi S.D., Zhou Y., Ouhtit A., Seven E.S., Oztan C.Y., Celik E., Leblanc R.M. Nanoparticle-mediated targeted drug delivery for breast cancer treatment. Biochim. Biophys. Acta - Rev. Cancer. 2019;1871(2):419–433. doi: 10.1016/j.bbcan.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J., Zhang C.J., Zhu N., Du K., Yin Y.F., Tan X., Liao D.F., Qin L. Lipid metabolism and carcinogenesis, cancer development. Am. J. Cancer Res. 2018;8:778–791. [PMC free article] [PubMed] [Google Scholar]
- Lumachi F., Brunello A., Maruzzo M., Basso U., Basso S.M. Treatment of estrogen receptor-positive breast cancer. Curr. Med. Chem. 2013;20:596–604. doi: 10.2174/092986713804999303. [DOI] [PubMed] [Google Scholar]
- Lüönd F., Tiede S., Christofori G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer. 2021;125(2):164–175. doi: 10.1038/s41416-021-01328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magura J., Moodley R., Mackraj I. The effect of hesperidin and luteolin isolated from Eriocephalus africanus on apoptosis, cell cycle and miRNA expression in MCF-7. J. Biomol. Struct. Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1833757. [DOI] [PubMed] [Google Scholar]
- Mahmoud A.M., Hernández Bautista R.J., Sandhu M.A., Hussein O.E. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid. Med. Cell. Longev. 2019;2019:1–19. doi: 10.1155/2019/5484138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon F.X., Belloc F., Lagarde V., Chollet C., Moreau-Gaudry F., Reiffers J., Goldman J.M., Melo J.V. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101:2368–2373. doi: 10.1182/blood.V101.6.2368. [DOI] [PubMed] [Google Scholar]
- Mas-Capdevila A., Teichenne J., Domenech-Coca C., Caimari A., Del Bas J.M., Escoté X., Crescenti A. Effect of hesperidin on cardiovascular disease risk factors: the role of intestinal microbiota on hesperidin bioavailability. Nutrients. 2020;12:1488. doi: 10.3390/nu12051488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehanna J., Haddad F.G., Eid R., Lambertini M., Kourie H.R. Triple-negative breast cancer: current perspective on the evolving therapeutic landscape. Int. J. Womens Health. 2019;11:431–437. doi: 10.2147/IJWH.S178349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micalizzi D.S., Maheswaran S., Haber D.A. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 2017;31(18):1827–1840. doi: 10.1101/gad.305805.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W.R. Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin. Oncol. 2003;30:3–11. doi: 10.1016/s0093-7754(03)00302-6. [DOI] [PubMed] [Google Scholar]
- Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momenimovahed Z., Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019;11:151–164. doi: 10.2147/BCTT.S176070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon S.H., Lee J.H., Kim K.T., Park Y.S., Nah S.Y., Ahn D.U., Paik H.D. Antimicrobial effect of 7-O-butylnaringenin, a novel flavonoid, and various natural flavonoids against Helicobacter pylori strains. Int. J. Environ. Res. Public Health. 2013;10:5459–5469. doi: 10.3390/ijerph10115459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar N., Rengarajan T., Balamurugan A., Balasubramanian M.P. Modulating effects of hesperidin on key carbohydrate-metabolizing enzymes, lipid profile, and membrane-bound adenosine triphosphatases against 7,12-dimethylbenz(a)anthracene-induced breast carcinogenesis. Hum. Exp. Toxicol. 2014;33(5):504–516. doi: 10.1177/0960327113485252. [DOI] [PubMed] [Google Scholar]
- Noman M.Z., Janji B., Abdou A., Hasmim M., Terry S., Tan T.Z., Mami-Chouaib F., Thiery J.P., Chouaib S. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology. 2017;6(1):e1263412. doi: 10.1080/2162402X.2016.1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nounou M.I., ElAmrawy F., Ahmed N., Abdelraouf K., Goda S., Syed-Sha-Qhattal H. Breast cancer: conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer Targets Ther. 2015;9:17–34. doi: 10.4137/BCBCR.S29420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurhayati I.P., Khumaira A., Ilmawati G.P.N., Meiyanto E., Hermawan A. Cytotoxic and antimetastatic activity of hesperetin and doxorubicin combination toward Her2 expressing breast cancer cells. Asian Pacific J. Cancer Prev. 2020;21:1259–1267. doi: 10.31557/APJCP.2020.21.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development, 2001. OECD Guideline for Testing of Chemicals [WWW Document]. URL https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd_gl423.pdf (accessed 4.17.21).
- Ortiz-Andrade R., Araujo-León J.A., Sánchez-Recillas A., Navarrete-Vazquez G., González-Sánchez A.A., Hidalgo-Figueroa S., Alonso-Castro Á.J., Aranda-González I., Hernández-Núñez E., Coral-Martínez T.I., Sánchez-Salgado J.C., Yáñez-Pérez V., Lucio-Garcia M.A. Toxicological screening of four bioactive citroflavonoids: in vitro, in vivo, and in silico approaches. Molecules. 2020;25:5959. doi: 10.3390/molecules25245959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palit S., Kar S., Sharma G., Das P.K. Hesperetin induces apoptosis in breast carcinoma by triggering accumulation of ROS and activation of ASK1/JNK pathway. J. Cell. Physiol. 2015;230(8):1729–1739. doi: 10.1002/jcp.24818. [DOI] [PubMed] [Google Scholar]
- Pang B., Qiao X., Janssen L., Velds A., Groothuis T., Kerkhoven R., Nieuwland M., Ovaa H., Rottenberg S., van Tellingen O., Janssen J., Huijgens P., Zwart W., Neefjes J. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat. Commun. 2013;4:1908. doi: 10.1038/ncomms2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S., Jia M., Gao J., Liu X., Guo W., Zhang H. Effects of dietary patterns combined with dietary phytochemicals on breast cancer metastasis. Life Sci. 2021;264:118720. doi: 10.1016/j.lfs.2020.118720. [DOI] [PubMed] [Google Scholar]
- Parhiz H., Roohbakhsh A., Soltani F., Rezaee R., Iranshahi M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phyther. Res. 2015;29(3):323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- Patra J.K., Das G., Fraceto L.F., Campos E.V.R., Rodriguez-Torres M.D.P., Acosta-Torres L.S., Diaz-Torres L.A., Grillo R., Swamy M.K., Sharma S., Habtemariam S., Shin H.S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnology. 2018;16:71. doi: 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y., Balatti V., Croce C.M. BCL2 and miR-15/16: from gene discovery to treatment. Cell Death Differ. 2018;25(1):21–26. doi: 10.1038/cdd.2017.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuty B. Strategic cell-cycle regulatory features that provide mammalian cells with tunable G1 length and reversible G1 arrest. PLoS ONE. 2012;7(4):e35291. doi: 10.1371/journal.pone.0035291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzimenti S., Toaldo C., Pettazzoni P., Dianzani M.U., Barrera G. The “two-faced” effects of reactive oxygen species and the lipid peroxidation product 4-hydroxynonenal in the hallmarks of cancer. Cancers (Basel). 2010;2:338–363. doi: 10.3390/cancers2020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido C., Vendrell I., Ferreira A.R., Casimiro S., Mansinho A., Alho I., Costa L. Bone metastasis risk factors in breast cancer. Ecancermedicalscience. 2017;11:715. doi: 10.3332/ecancer.2017.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman B.K., Maheswari P.U., Begum K.M.M.S. Magnetic casein-CaFe2O4 nanohybrid carrier conjugated with progesterone for enhanced cytotoxicity of citrus peel derived hesperidin drug towards breast and ovarian cancer. Int. J. Biol. Macromol. 2020;151:293–304. doi: 10.1016/j.ijbiomac.2020.02.172. [DOI] [PubMed] [Google Scholar]
- Racay P., Kaplán P., Mézesová V., Lehotský J. Lipid peroxidation both inhibits Ca(2+)-ATPase and increases Ca2+ permeability of endoplasmic reticulum membrane. IUBMB Life. 1997;41(4):647–655. doi: 10.1080/15216549700201691. [DOI] [PubMed] [Google Scholar]
- Rachner T.D., Göbel A., Jaschke N.P., Hofbauer L.C. Challenges in preventing bone loss induced by aromatase inhibitors. J. Clin. Endocrinol. Metab. 2020;105:3122–3133. doi: 10.1210/clinem/dgaa463. [DOI] [PubMed] [Google Scholar]
- Rahideh S.T., Keramatipour M., Nourbakhsh M., Koohdani F., Hoseini M., Shidfar F. The effects of Nobiletin, Hesperetin, and Letrozole in a combination on the activity and expression of aromatase in breast cancer cells. Cell. Mol. Biol. 2017;63:9–13. doi: 10.14715/cmb/2017.63.2.2. [DOI] [PubMed] [Google Scholar]
- Rahideh S.T., Shidfar F., Nourbakhsh M., Hoseini M., Koohdani F., Entezam M., Keramatipour M. The individual or combinational effects of Hesperetin and Letrozole on the activity and expression of aromatase in MCF-7 cells. Cell. Mol. Biol. 2016;62(6):38–43. doi: 10.14715/cmb/2016.62.6.7. [DOI] [PubMed] [Google Scholar]
- Rahman M.A., Hannan M.A., Dash R., Rahman M.H., Islam R., Uddin M.J., Sohag A.A.M., Rahman M.H., Rhim H. Phytochemicals as a complement to cancer chemotherapy: Pharmacological modulation of the autophagy-apoptosis pathway. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.639628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman D., Tiwari A.K., Tiriveedhi V., Rhoades Sterling J.A. Editorial: the role of breast cancer stem cells in clinical outcomes. Front. Oncol. 2020;10:299. doi: 10.3389/fonc.2020.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath G., Schneider C., Langlois B., Sartelet H., Morjani H., Btaouri H.E.L., Dedieu S., Martiny L. De novo ceramide synthesis is responsible for the anti-tumor properties of camptothecin and doxorubicin in follicular thyroid carcinoma. Int. J. Biochem. Cell Biol. 2009;41(5):1165–1172. doi: 10.1016/j.biocel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Rauchová H., Ledvinková J., Kalous M., Drahota Z. The effect of lipid peroxidation on the activity of various membrane-bound ATPases in rat kidney. Int. J. Biochem. Cell Biol. 1995;27(3):251–255. doi: 10.1016/1357-2725(94)00083-N. [DOI] [PubMed] [Google Scholar]
- Rexer B.N., Arteaga C.L. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit. Rev. Oncog. 2012;17(1):1–16. doi: 10.1615/CritRevOncog.v17.i110.1615/CritRevOncog.v17.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohbakhsh A., Parhiz H., Soltani F., Rezaee R., Iranshahi M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—a mini-review. Life Sci. 2014;113(1-2):1–6. doi: 10.1016/j.lfs.2014.07.029. [DOI] [PubMed] [Google Scholar]
- Saha Roy S., Vadlamudi R.K. Role of estrogen receptor signaling in breast cancer metastasis. Int. J. Breast Cancer. 2012;2012:1–8. doi: 10.1155/2012/654698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Goyal A.K., Rath G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2018;26(8):617–632. doi: 10.1080/1061186X.2017.1400553. [DOI] [PubMed] [Google Scholar]
- Shen C., Qian Z., Chen R., Meng X., Hu T., Chen Z., Li Y., Huang C., Hu C., Li J. Single dose oral and intravenous pharmacokinetics and tissue distribution of a novel hesperetin derivative MTBH in rats. Eur. J. Drug Metab. Pharmacokinet. 2016;41(6):675–688. doi: 10.1007/s13318-015-0293-2. [DOI] [PubMed] [Google Scholar]
- Sheokand S., Navik U., Bansal A.K. Nanocrystalline solid dispersions (NSD) of hesperetin (HRN) for prevention of 7, 12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in Sprague-Dawley (SD) rats. Eur. J. Pharm. Sci. 2019;128:240–249. doi: 10.1016/j.ejps.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Sulaiman G.M., Waheeb H.M., Jabir M.S., Khazaal S.H., Dewir Y.H., Naidoo Y. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci. Rep. 2020;10:9362. doi: 10.1038/s41598-020-66419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tabeshpour J., Hosseinzadeh H., Hashemzaei M., Karimi G. A review of the hepatoprotective effects of hesperidin, a flavanon glycoside in citrus fruits, against natural and chemical toxicities. Daru. 2020;28(1):305–317. doi: 10.1007/s40199-020-00344-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailby E., Boyages Am J. Conservation surgery and radiation therapy in early breast cancer - An update. Aust. Fam. Physician. 2017;46:214–219. [PubMed] [Google Scholar]
- Tao Z., Shi A., Lu C., Song T., Zhang Z., Zhao J. Breast cancer: epidemiology and etiology. Cell Biochem. Biophys. 2015;72(2):333–338. doi: 10.1007/s12013-014-0459-6. [DOI] [PubMed] [Google Scholar]
- Tilghman S.L., Townley I., Zhong Q., Carriere P.P., Zou J., Llopis S.D., Preyan L.C., Williams C.C., Skripnikova E., Bratton M.R., Zhang Q., Wang G. Proteomic signatures of acquired letrozole resistance in breast cancer: suppressed estrogen signaling and increased cell motility and invasiveness. Mol. Cell. Proteomics. 2013;12(9):2440–2455. doi: 10.1074/mcp.M112.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C.W.S., Wu M., Cho W.C.S., To K.K.W. Recent advances in the treatment of breast cancer. Front. Oncol. 2018;8:227. doi: 10.3389/fonc.2018.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P.P., Kushwaha S., Tripathi D.N., Jena G.B. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovasc. Toxicol. 2011;11(3):215–225. doi: 10.1007/s12012-011-9114-2. [DOI] [PubMed] [Google Scholar]
- Waks A.G., Winer E.P. Breast cancer treatment: a review. J. Am. Med. Assoc. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- Wang Z., Dong C. Gluconeogenesis in cancer: function and regulation of PEPCK, FBPase, and G6Pase. Trends Cancer. 2019;5(1):30–45. doi: 10.1016/j.trecan.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Wellberg E.A., Johnson S., Finlay-Schultz J., Lewis A.S., Terrell K.L., Sartorius C.A., Abel E.D., Muller W.J., Anderson S.M. The glucose transporter GLUT1 is required for ErbB2-induced mammary tumorigenesis. Breast Cancer Res. 2016;18:131. doi: 10.1186/s13058-016-0795-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox L.J., Borradaile N.M., de Dreu L.E., Huff M.W. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J. Lipid Res. 2001;42(5):725–734. doi: 10.1016/S0022-2275(20)31634-5. [DOI] [PubMed] [Google Scholar]
- Wolfram J., Scott B., Boom K., Shen J., Borsoi C., Suri K., Grande R., Fresta M., Celia C., Zhao Y., Shen H., Ferrari M. Hesperetin liposomes for cancer therapy. Curr. Drug Deliv. 2016;13(5):711–719. doi: 10.2174/1567201812666151027142412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Si M., Xue H.Y., Wong H.L. Nanomedicine applications in the treatment of breast cancer: current state of the art. Int. J. Nanomedicine. 2017;12:5879–5892. doi: 10.2147/IJN.S123437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang S., Dauchy R.T., Hauch A., Mao L., Yuan L., Wren M.A., Belancio V.P., Mondal D., Frasch T., Blask D.E., Hill S.M. Doxorubicin resistance in breast cancer is driven by light at night induced disruption of the circadian melatonin signal. J. Pineal Res. 2015;59(1):60–69. doi: 10.1111/jpi.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Yang Z., Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015;9(4):317–324. doi: 10.1080/19336918.2015.1016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Shen J., Wang J., Yang X., Dong S., Lu S. Nanoparticle-based drug delivery system: a patient-friendly chemotherapy for oncology. Dose Response. 2020;18(3) doi: 10.1177/1559325820936161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F., Teves S.S., Kemp C.J., Henikoff S. Doxorubicin, DNA torsion, and chromatin dynamics. BBA. 2014;1845(1):84–89. doi: 10.1016/j.bbcan.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wolfram J., Boom K., Fang X., Shen H., Ferrari M. Hesperetin impairs glucose uptake and inhibits proliferation of breast cancer cells. Cell Biochem. Funct. 2013;31(5):374–379. doi: 10.1002/cbf.v31.510.1002/cbf.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L., Chan F.L., Chen S., Leung L.K. The citrus flavonone hesperetin inhibits growth of aromatase-expressing MCF-7 tumor in ovariectomized athymic mice. J. Nutr. Biochem. 2012;23(10):1230–1237. doi: 10.1016/j.jnutbio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Yu L.i., Chen X., Sun X., Wang L., Chen S. The glycolytic switch in tumors: how many players are involved? J. Cancer. 2017;8(17):3430–3440. doi: 10.7150/jca.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]