Abstract
Eating disorders are life-interrupting psychiatric conditions with high morbidity and mortality, yet the basic mechanisms underlying these conditions are understudied compared to other psychiatric disorders. In this Opinion piece, we suggest that recent knowledge gleaned from genomic and neuroimaging investigations of eating disorders in humans presents a rich opportunity to sharpen animal models of eating disorders and to identify neural mechanisms that contribute to risk and maintenance of the conditions. Our review reflects the state of the science, with a primary focus on anorexia nervosa and binge-eating behavior, and encourages further study of all conditions categorized under feeding and eating disorders.
Keywords: anorexia nervosa, animal models, GWAS, neuroimaging, neural circuits, genomics
The Challenge of Studying Eating Disorders
Basic research on eating disorders, especially food-restricting disorders such as anorexia nervosa (AN), has lagged behind other psychiatric illnesses. AN is characterized by extreme low weight coupled with fears of and behaviors to prevent weight gain [1]. Current conceptualizations implicate both genetic and environmental etiological factors [2]. Mortality is high and impact on other aspects of health (e.g., osteoporosis, amenorrhea, cardiac function, anemia) is extensive [3]. Bulimia nervosa (BN) and binge-eating disorder (BED) are primarily characterized by binge eating (eating an unusually large amount of food coupled with a sense of loss of control). They are differentiated by the presence of recurrent compensatory behaviors (e.g., self-induced vomiting, laxative abuse), which is seen in BN but not BED [1]. The high co-occurrence of all eating disorders with other psychiatric illnesses such as anxiety disorders, major depressive disorder (MDD), and obsessive-compulsive disorder (OCD) (between 40% and 90%) [4], and their intersection with weight and appetite regulation mechanisms tells us that building the eating disorders knowledge base has the potential to illuminate factors that also influence other related psychiatric and somatic conditions and traits.
Research into the etiology and pathogenesis of eating disorders is in search of refined paths forward. Discovery has been slow due to the lack of tractable models and genetic leads. Importantly, moderate to high twin-based heritability estimates [2]—on par with other major psychiatric traits [5]—imply that strong biological mechanisms are implicated that have yet to be discovered. We discuss how recent research provides insights into the mechanisms underlying restrictive eating disorders, which have the potential to direct basic science researchers toward testable science.
AN is perplexing, with affected individuals defying, failing to respond to, or not experiencing biological drives central to survival (i.e., hunger) and claiming to feel better while doing so. Coined a paradoxical response to negative energy balance [6] (see Glossary), this core feature of AN directly opposes a global trend toward positive energy balance and progressive weight gain [7]. The core features of AN are packaged within cultural or psychological epiphenomena—such as religiosity of the Middle Ages [8] or the contemporary societal drive for thinness [9], which can obscure the central features of restricting food intake and/or increasing energy output to achieve and maintain negative energy balance. We acknowledge the importance of cultural and psychological factors, and suggest that a more concerted focus on the core biology could be key to unlocking mechanisms underlying eating disorders.
Individuals with BN or BED display dysregulated eating in the opposite direction by overriding or failing to experience typical satiety signals during binge episodes [10]. Individuals with BN tend to be in the normal to overweight range, whereas individuals with BED, often, but not always maintain positive energy balance and fall within the overweight or obese range [11].
Animal models are an important research tool for many psychiatric disorders. Although animal behaviors cannot fully recapitulate human diseases, the core behavioral features of many diseases can be modeled in animals to gain insight into underlying neural circuits and mechanisms. Examples include fear conditioning in the study of anxiety [12] and post-traumatic stress disorder [13], social defeat for depression [14], and grooming or reversal learning for obsessive-compulsive behaviors [15]. The intention is clearly not to perfectly recapitulate the target disorder, but rather to model foundational behavioral aspects of the disease that isolate implicated neural underpinnings [16]. A comparison of AN to OCD is informative, given their strong shared genetic basis [17]. Animal models of OCD never claim to study obsessive cognitions, which are impossible to assess in rodents, but rather compulsive behaviors, which can be observed and quantified [15]. Similarly, it is impossible (and even humorous) to propose to observe obsessions with thinness in rodents, but it is feasible to observe and quantify voluntary food restriction.
A number of animal models have been advanced to study AN [18, 19] and binge-eating behavior (see Box 1). Early models of AN tended not to recapitulate core features of the disease (e.g., experimenter-imposed food restriction has little to do with the psychiatric condition of AN) and may have fueled skepticism about the utility of animal models. Several animal models of binge eating behavior exist, but cannot fully capture the syndromes of BN or BED. A complete model of BN, for example, would include both binge eating and compensatory behaviors. Animal models can capture excessive consumption, but cannot model the psychological criterion of “feeling out of control.” Moreover, mice and rats, commonly used for behavioral neuroscience studies, are physiologically unable to vomit, rendering animal models of BN only partially attainable. Similarly, BED often co-occurs with obesity and shares numerous features, complicating the ability of animal models to differentiate between the two. In addition to these practical limitations, the historical focus on sociocultural aspects of eating disorders [20] may have contributed to a systemic bias against animal models. As the science of eating disorders matures, however, we anticipate a greater acceptance of the relevance of animal models for studying both restrictive eating disorders and other patterns of dysregulated eating.
Text Box 1: Animal Models Relevant for Eating Disorders.
Numerous animal models have been proposed to study AN and binge eating, and these models are variably useful depending on which aspects of the disease one is trying to model. Early models that used simple food restriction or deprivation paradigms [18] to lower body weight have been mostly discarded as they do not involve voluntary food restriction [18, 19, 66] and appear to mainly reflect energy balance changes. Similarly, sham-feeding (i.e., draining liquid food before entering the intestinal tract) serves as a model for binge eating in which rodents get positive orosensory feedback from consumption with negative intestinal feedback, although the behavior is not self-initiated [76]. Binge-like behavior of palatable foods can be induced under limited access conditions, but this access schedule is also experimenter-initiated.
Genetic mutations that lead to catastrophic weight loss in which mice die soon after birth, such as the anx/anx mutation [77], are intriguing and informative, although significant phenotypic differences including timing of neurodevelopmental onset differ from that seen in anorexia nervosa. Similarly, ob/ob mice engage in severe hyperphagia, but this is likely due to a perceived sensation of starvation due to the lack of circulating leptin which is not characteristic of binge-like behavior and the mice exhibit other unrelated phenotypes such as hypothermia, reduced fertility, and decreased bone mass [78].
Simple stress models, such as restraint stress or social isolation stress can induce reduction in body weight and/or food intake in the absence of imposed food restriction, but other chronic stress models, including after footshock, can induce hyperphagia [79], highlighting the importance of dissecting out these competing mechanisms. However, these models may preferentially reflect energy balance changes over food intake changes and may also more closely model the type of food restriction or increase that occurs during simple HPA axis activation.
More sophisticated animal models of eating disorders incorporate multiple parameters. For binge eating, cue-mediated overconsumption is a model that incorporates learning about food cues with restriction/refeeding, which induces overconsumption in the sated state when prompted by the cue [80]. The most popular animal model of AN currently in use is activity-based anorexia (ABA) [18], which combines moderate food restriction (usually 1 hour a day of food access) in combination with access to a running wheel. This model leads to severe food restriction compared to control groups that are also food restricted but do not have access to a running wheel. The ABA model also successfully models hyperactivity—a pernicious and often intractable symptom of AN. Despite this, the model requires a food restriction paradigm for the duration of the experiment in order to maintain voluntary food restriction, which contrasts with the voluntary food restriction seen in patients with AN despite all efforts to encourage consumption. Another recent gene x environment x diet (GED) model incorporates moderate food restriction for 11 days, genetic predisposition to anxiety (BDNFVal66Met gene variant), and social isolation stress in adolescent mice [19]. Compared to ABA, the GED model imposes food restriction for only a short period of time, and may therefore more accurately model the type of voluntary food restriction seen in patients with AN following initial dieting. It is notably the only model proposed to date that incorporates gene, environment, and diet changes to produce anorexia-like behaviors.
Here, we outline research developments into the biological mechanisms underlying eating disorders and discuss how they will enable an updated framework for future research in this area (Figure 1).
Figure 1.
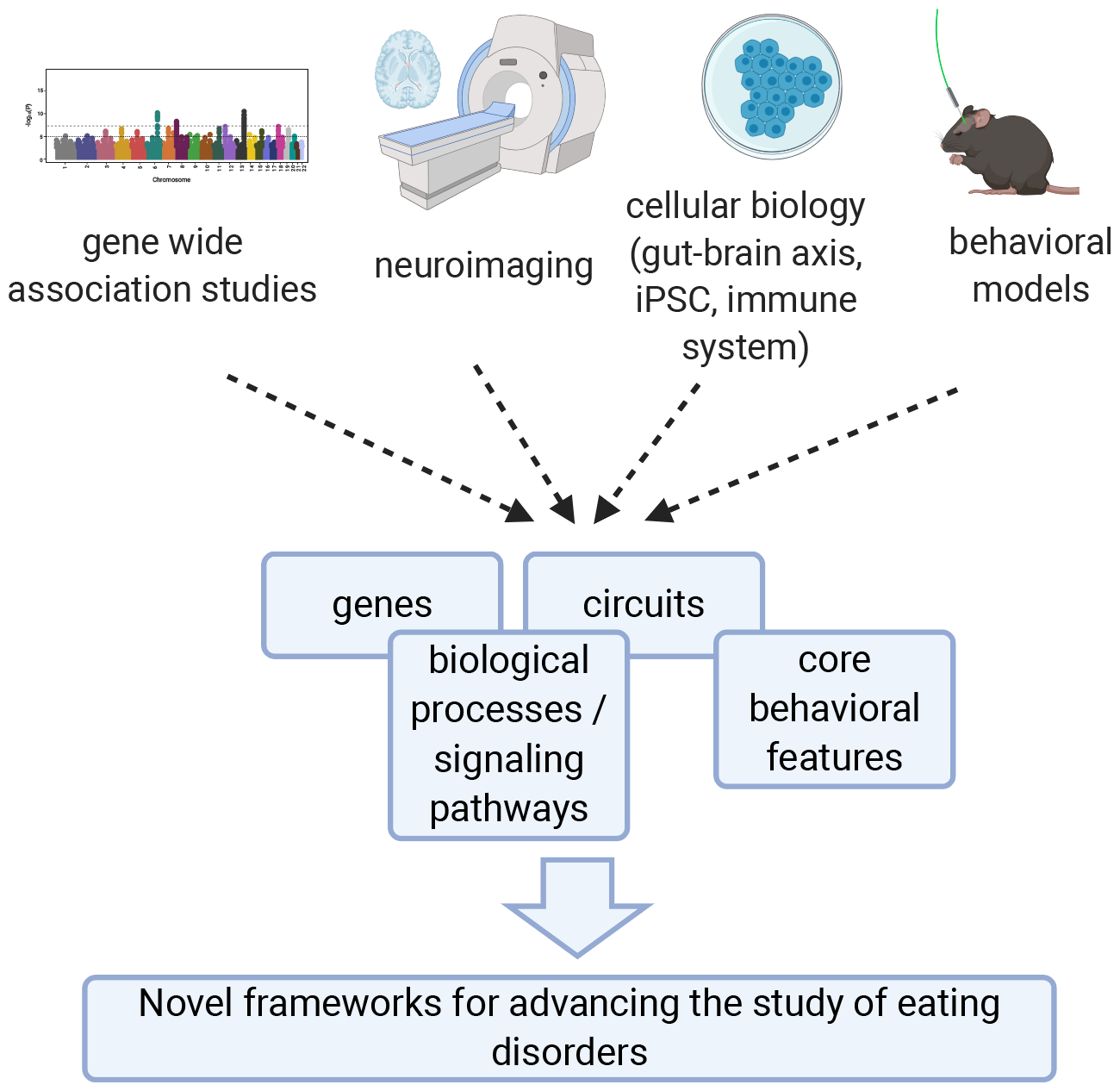
Schematic for proposed refined framework for incorporating and integrating emerging knowledge from genomics, neuroimaging, cellular biology, and animal models to identify implicated genes, circuits, and underlying biological processes associated with both core behavioral features and threshold eating disorder syndromes. Created with BioRender.com.
What can be learned from GWAS?
Encouraging science related to restrictive eating disorders has emerged from genome-wide association studies (GWAS). The largest published GWAS (~17K cases and 55K controls), yielded 8 significant loci with clearest evidence for the single gene loci that intersected with CADM1, MGMT, FOXP1 and PTBP2, suggesting that these genes may have a role in the AN etiology. Moreover, we observed expected positive SNP-based genetic correlations with other psychiatric illnesses (such as OCD, MDD, anxiety, schizophrenia) and intriguing negative genetic correlations with metabolic and anthropometric traits fueling a reconceptualization of AN as a metabo-psychiatric illness [21]. Moreover, positive genetic correlations with educational attainment and measured physical activity suggested that features previously believed to be driven by family pressure (academic achievement) or treatment resistance (driven physical activity) may have their roots in underlying shared genetic factors.
One significant biological pathway emerged, positive regulation of embryonic development, tentatively pointing to a role of developmental processes in AN etiology. In addition, genes associated with AN were enriched for expression in most brain regions and, at finer resolution in 24 brain cell types, significant enrichment was found for striatal medium spiny neurons and pyramidal neurons from hippocampal CA1. Notably, striatum and hippocampus have been associated with feeding behaviors including food motivation and reward [22, 23]. The genetic data were used to estimate gene expression levels (using PrediXcan), and 36 genes were predicted to be differentially expressed most notably for the DNA methyltransferase MGMT to be downregulated in the caudate. The sample sizes analyzed to date are still insufficient but, nonetheless, these are the first indications of specific pathways, tissues, and cell types that may mediate genetic risk for AN [21]. No GWAS have yet been published for other eating disorders, but global efforts are underway.
As the genetic research evolves and sample sizes increase, we anticipate that more robust results will yield insight into underlying biological pathways that will inform animal models and guide drug repositioning or development toward compounds that target dysregulated biology.
What can be learned from neuroimaging?
Neuroimaging provides insight into the neuropsychological processes that underlie certain aspects of eating disorders [24]. A full review of the eating disorders imaging literature is beyond our scope, so we focus on how recent studies have pinpointed central mechanisms underlying behavioral changes in AN and binge eating, and how these findings may translate into focusing basic research on core systems.
Initial findings of altered gray matter volume and cortical thickness in AN were intriguing, although most studies indicate that brain structure is modified strongly by food intake (or lack thereof), and that these abnormalities resolve when eating is restored [25]. Functional connectivity studies have been more consistent, pointing to dysfunction in the “salience network” (anterior cingulate, insula, and orbitofrontal cortex), as well as altered connectivity between ventral striatum and the prefrontal cortex [26].
A third approach correlates specific task performances (e.g., attention, reward, perception, and decision-making) with alterations in brain activity using functional magnetic resonance imaging (fMRI) in patients with AN vs healthy controls. Using food images as cues, studies of AN consistently report alterations in pleasure derived from (e.g., liking) and motivation to consume (e.g., wanting) high-calorie foods, as well as implicit liking of neutral stimuli following food images [27]. Neuroimaging studies utilizing these tasks also support a role of the brain reward system in AN [25]. In contrast, there is no clear consensus as to whether individuals with AN display attentional biases toward or against food images, and no perceptual deficits have been identified. Imaging studies also support the role of both trait and state anxiety during food-related tasks and consumption in patients with AN [27].
Neuroimaging studies have also examined regional activation during passive viewing of food, interoception [28], conditioned reward learning [29], and inhibitory control [30], all of which may provide important insights into brain mechanisms underlying aspects of AN. Many of these studies have reported alterations in amygdala and insula activation, although the directionality is inconsistent [25, 31]—a pattern not unlike that seen in OCD [15]. Nonetheless, consensus implicates alterations in the striatal reward and habit systems, along with the insula interoceptive network and other frontal and limbic regions. Limitations in task structures and sample sizes hinder the ability to pinpoint whether these alterations are causes or consequences of disease, as well as the directionality of effects.
The neuroimaging literature on BN and BED is somewhat less mature than AN, but a recent systematic review highlighted hypoactivity in the frontostriatal circuits; and aberrant responses in the insula, amygdala, middle frontal gyrus and occipital cortex to a range of different stimuli (including food and body image stimuli) [32]. A consistent observation relates greater neural changes with higher binge frequency [32].
Overall, neuroimaging studies provide important insights into brain alterations underlying AN and other eating disorders. Moreover, they point to numerous behavioral modalities on which to focus animal models, as well as brain regions that are candidates for basic mechanistic research. Neuroimaging studies can sharpen animal models by improving behavioral models that focus on the core findings of reward and salience and their corresponding brain region networks. Several innovative teams are advancing neuroimaging research in eating disorders, which has the potential to yield more consistent predictions and informative findings [25].
What can be learned from neural circuits and rodent models?
Study of neural circuits underlying feeding behavior provides important insights into mechanisms underlying eating disorders. Similar to the manner in which basic researchers have translated findings from fMRI studies on OCD, which converge on the cortico-striato-thalamo-cortical (CSTC) circuits to inform optogenetics and circuit-based animal model research [15], eating disorders may also benefit from an interrogation into conserved brain circuits involved in feeding—particularly those that mediate environmental influences on feeding. As mentioned above, neuroimaging studies converge on cortico-limbic circuits, including the insular cortex, and striatal circuits, whereas animal model research has primarily focused on the amygdala and hypothalamus. In mice, stimulation of the BNST (bed nucleus of stria terminalis) → LH (lateral hypothalamus) → [33], amygdala prepronociceptin neurons mediate palatable food consumption [34], and similarly, unpublished work reported an insular cortex → central amygdala pathway mediates cue-induced overconsumption [35]. Limbic circuits that specifically mediate suppression of feeding include a lateral septum (LS) → LH circuit that is specifically activated by stress [36] and a PVH (paraventricular hypothalamus) → LS circuit that mediates feeding and anxiety-like behavior [37]. The reasons for the discrepancy between neuroimaging and animal model research is not obvious, but may be due to methodological factors as well as historical predispositions. Nonetheless, it will be important for future animal model research to incorporate findings from neuroimaging and vice versa. For example, a number of neuroimaging studies have converged around the idea of harm avoidance as a personality trait common in AN patients [38, 39]. In rodents, harm avoidance may be akin to what is known as risk aversion, therefore, studying this behavioral domain may reveal important insights [40, 41], especially as they relate to effects on feeding. In particular, further examination of these circuits in the context of behavioral models (see Box 1) may reap important insights. In that context, it is critical to differentiate between homeostatic circuits that orchestrate normal hunger and satiety responses to those that may be involved in non-homeostatic feeding changes that underlie eating disorders.
What are other entry points into eating disorders research?
Immune and autoimmune function.
Several influential studies point to an association between the immune system, inflammatory processes, and eating disorders [42-44]. One study found that patients who had been previously hospitalized for infection were 22% more likely to develop AN and 39% more likely to develop a non-specified eating disorder [45]. Another reported that those with autoimmune or autoinflammatory diseases had a 36% higher hazard for AN, 73% for BN, and 72% for an eating disorder not otherwise specified [44]—these are far from subtle associations.
Further evidence supporting an autoimmune connection comes from the study of PANDAS—a rare set of pediatric conditions that emerge post-Group A Streptococcal (GAS) infections marked by tics, obsessive compulsive, or eating disorder symptoms [46]. Given the high twin- and SNP-based genetic correlations between OCD and AN [17, 47], this connection is particularly intriguing. PANDAS animal models have relied on GAS immunization or passive transfer of GAS antigens to naïve animals reporting behavioral disturbances, including increased grooming and rearing, anxiety like behaviors, motor changes, and impaired food manipulation [48, 49]. Given that severe food aversions occur in a subset of PANDAS patients [50, 51], future work could assess differences in food seeking or intake after GAS infection.
One mechanism whereby infections or their treatment could influence eating disorders risk is via the gut microbiota, which can influence behavior through the gut-brain axis [52]. The gut-brain axis has been implicated in numerous diseases, including psychiatric disorders [53], and a general rule of thumb is that a diverse microbiota is a healthy microbiota. The microbial richness (diversity within a community-alpha diversity) is reported to be lower in individuals with AN than controls in most studies and diversity between communities—beta diversity indices—reveal differences between individuals with AN and controls [54-57]. The reduced gut microbial richness only partially normalizes with recovery [57] and specific alterations vary widely across studies. Designs that track the community of microorganisms in the gut across clinical course may be used to benchmark recovery [58, 59].
Although the gut microbiota is influenced strongly by the host diet, its composition also directly influences metabolism and host behavior, suggesting a feedback cycle that influences the maintenance of restrictive feeding behaviors [60]. One study transplanted stool from patients with AN into germ-free (GF) mice and found reduced body weight compared to mice colonized with healthy control microbiota [61]. The observed weight reduction was not only due to decreased food intake, indicating an impairment in the maintenance of energy balance. Furthermore, AN-transplanted mice exhibited behavioral abnormalities compared to control mice in the open field and in marble burying, indicating that the microbiota may also be relevant to understanding comorbidities in eating disorders. Unpublished results from our group, however, are inconsistent with the aforementioned findings, and the efficacy of transfer from humans to GF mice has been questioned [62]. This branch of eating disorders science is emerging and requires harmonized procedures, longitudinal sampling, and larger sample sizes.
A related consideration is that patients with AN report high stress and anxiety [63], and the documented association between stress and an altered neuroimmune environment increases the risk for numerous psychiatric disorders [64, 65]. Stress has been incorporated into several animal models of eating disorders in the form of restraint stress (stress-induced anorexia), exercise (activity-based anorexia), and social isolation stress [18, 19, 66], which lead to food restriction and chronic stress, and in turn to binge-like behavior (see Box 1). Fear conditioning or impaired fear extinction have also been posited to underlie AN [63, 67], but to our knowledge, this has not yet been examined in animal models. One study showed that deletions of MC4R, which leads to obesity, ameliorates the effects of grooming from deletion of SAPAP3, providing further supporting the relationship between anxiety and feeding [68]. Thus, studying the neurobiological and immunological mechanisms underlying whereby stress alters eating behavior is a fruitful future direction for basic science.
Pluripotent Stem Cells
Yet another inroad into the study of AN is via pluripotent stem cells (IPSCs) [69]. In this approach, IPSCs derived from patients with AN and healthy controls are induced to differentiate into a particular cell type, for example, neurons. This methodology affords the study of differentiated cell types from distinct populations, enabling precise experimental designs that are not available using human tissues or other genetic methods [69]. In particular, one study showed that neuronal IPSCs derived from patients with AN showed dysregulation in the TACR1 gene [70], which is implicated in reward processing, small body size, increased fat mass, and hyperactivity [71]. Inhibition of Tacr1 leads to decreased food intake in mice [72, 73], further implicating this system in the regulation of feeding, although no studies on animal models of restrictive eating have been performed. Pluripotent stem cells may also be useful for building organoids, which although in their infancy, can used to study the gut-brain axis [74, 75] and could potentially hold insights into eating disorders as the field matures. Studies using these and other approaches that discriminate across different stages of the disease will provide important insights into the biological mechanisms underlying AN risk and course.
Concluding Remarks
Recent scientific advances, particularly in GWAS, functional neuroimaging, and the study of the intestinal microbiota have opened new directions to advance animal models of eating disorders. Although AN has been the primarily focus, intriguing advances are emerging in animal models of binge eating, which we have highlighted. We encourage future research to focus on circuit-based studies of restrictive feeding and other dysregulated eating behaviors (see Outstanding Questions) in order to identify underlying mechanisms, along with generating new transgenic models based on newly identified genes and gene pathways.
Supplementary Material
Acknowledgements:
Dr. Bulik acknowledges funding from the National Institute of Mental Health (R01MH120170, R01MH119084, R01MH118278, R21MH115397, R01MH105684, U01MH109528), the Swedish Research Council (Vetenskapsrådet, award: 538-2013-8864), and Lundbeckfonden. Dr. Stern acknowledges funding from a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation, the National Institute on Drug Abuse (K99DA048749) and the Klarman Family Foundation.
Declaration of Interest:
Dr. Bulik declares the following interests: Shire (Takeda) (grant recipient, Scientific Advisory Board member); Idorsia (consultant); Pearson (author, royalty recipient). Dr. Stern reports no conflicts.
Glossary
- Alpha diversity
in the study of the intestinal microbiota, alpha diversity refers to the diversity within a sample. It can measure richness of species or how evenly distributed the microbes are
- Beta diversity
refers to diversity between samples.
- Food restriction/deprivation
Restriction is a behavioral paradigm wherein the investigator limits either the amount of food given on any given day or restricts the time of day during which eating is permitted. Deprivation refers experimenter-induced fasting for a specified period of time
- Functional magnetic resonance imaging (fMRI)
a technique for indirectly measuring and mapping brain activity by detecting changes associated with cerebral blood flow, which is coupled with neuronal activation
- Genome-wide association study
scanning markers across the complete sets of DNA, or genomes, of large samples of people with and without a particular disease to identify genetic variations associated with the target disease
- Genetic mutations
an alteration in the DNA sequence that makes up a gene. In rodents, mutations can be spontaneous (as in the case of the anx/anx mouse), or engineered (as in the case of the BDNFVal66Met mouse)
- Germ free mice
Germ-free mice (or gnotobiotic) mice are bred in isolators to keep them completely free of detectable microorganisms, including those that are typically found in the gut
- Negative energy balance
the state of expending more energy than you are consuming
- Optogenetics
a technique wherein a cell type of interest is genetically modified to express a light-gated ion channel, thereby allowing artificial activation or inhibition of those cells
- Organoids
a multicellular in vitro tissue construct, usually grown from embryonic or induced pluripotent stem cells, which represents a simplified version of the modelled organ, while displaying realistic micro-anatomy
- PANDAS
is short for Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. PANDAS can be diagnosed when obsessive-compulsive disorder (OCD), tic disorder, or both suddenly appear following a streptococcal (strep) infection, such as strep throat or scarlet fever
- Restraint stress
a behavioral paradigm to induce stress by restricting the rodent’s movement, usually by placing it into a small, inescapable container with a breathing hole
- Salience network
A group of intrinsically connected brain regions identified through fMRI studies, anchored by the anterior insular cortex and the dorsal anterior cingulate cortex, thought to be involved in detecting, and responding to, salient stimuli in the environment
- Sham feeding
A feeding technique that allows liquid food to be drained before it reaches the intestine to preserve the consummatory component and eliminate the intestinal feedback
- Social isolation stress
a behavioral paradigm in rodents to induce stress by housing subjects individually rather than with cagemates
References
- 1.American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition. American Psychiatric Association [Google Scholar]
- 2.Yilmaz Z, et al. (2015) Genetics and epigenetics of eating disorders. Adv Genom Genet 5, 131–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treasure J, et al. (2015) Anorexia nervosa. Nat Rev Dis Primers 1, 15074. [DOI] [PubMed] [Google Scholar]
- 4.Herpertz-Dahlmann B (2015) Adolescent eating disorders: update on definitions, symptomatology, epidemiology, and comorbidity. Child Adolesc Psychiatr Clin N Am 24, 177–196 [DOI] [PubMed] [Google Scholar]
- 5.Polderman TJC, et al. (2015) Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet 47, 702–709 [DOI] [PubMed] [Google Scholar]
- 6.Bulik CM (2016) Towards a science of eating disorders: Replacing myths with realities: The fourth Birgit Olsson lecture. Nord J Psychiatry, 224–230 [DOI] [PubMed] [Google Scholar]
- 7.NCD Risk Factor Collaboration (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387, 1377–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espi Forcen F and Espi Forcen C (2015) The practice of holy fasting in the late middle ages: A psychiatric approach. J Nerv Ment Dis 203, 650–653 [DOI] [PubMed] [Google Scholar]
- 9.Striegel-Moore R, et al. (1986) Toward an understanding of risk factors for bulimia. Am Psychologist 41, 246–263 [DOI] [PubMed] [Google Scholar]
- 10.Sysko R, et al. (2018) Rigor and reproducibility via laboratory studies of eating behavior: A focused update and conceptual review. Int J Eat Disord 51, 608–616 [DOI] [PubMed] [Google Scholar]
- 11.Schaumberg K, et al. (2017) The science behind the Academy for Eating Disorders’ Nine Truths About Eating Disorders. Eur Eat Disord Rev 25, 432–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jovanovic T, et al. (2013) Translational neuroscience measures of fear conditioning across development: applications to high-risk children and adolescents. Biol Mood Anxiety Disord 3, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Careaga MBL, et al. (2016) Understanding posttraumatic stress disorder through fear conditioning, extinction and reconsolidation. Neurosci Biobehav Rev 71, 48–57 [DOI] [PubMed] [Google Scholar]
- 14.Hollis F and Kabbaj M (2014) Social defeat as an animal model for depression. ILAR J 55, 221–232 [DOI] [PubMed] [Google Scholar]
- 15.Ahmari SE (2016) Using mice to model obsessive compulsive disorder: From genes to circuits. Neurosci 321, 121–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteggia LM, et al. (2018) Meeting Report: Can we make animal models of human mental illness? Biol Psychiatry 84, 542–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yilmaz Z, et al. (2018) Examination of the shared genetic basis of anorexia nervosa and obsessive–compulsive disorder. Mol Psychiatry, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mequinion M, et al. (2015) The use of animal models to decipher physiological and neurobiological alterations of anorexia nervosa patients. Front Endocrinol (Lausanne) 6, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeltser LM and Madra M (2018) A Framework for Elucidating Causes and Consequences of Malnutrition in Anorexia Nervosa. In Neurobiology of Abnormal Emotion and Motivated Behaviors (Sangha S and Foti D, eds), pp. 92–118, Elsevier [Google Scholar]
- 20.Culbert KM, et al. (2015) Research Review: What we have learned about the causes of eating disorders - a synthesis of sociocultural, psychological, and biological research. J Child Psychol Psychiatry 56, 1141–1164 [DOI] [PubMed] [Google Scholar]
- 21.Watson H, et al. (2019) Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet 51, 1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, et al. (2017) Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93, 1464–1479 e1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Connor EC, et al. (2015) Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88, 553–564 [DOI] [PubMed] [Google Scholar]
- 24.McGuire PK and Matsumoto K (2004) Functional neuroimaging in mental disorders. World Psychiatry 3, 6–11 [PMC free article] [PubMed] [Google Scholar]
- 25.King JA, et al. (2018) Structural neuroimaging of anorexia nervosa: future directions in the quest for mechanisms underlying dynamic alterations. Biol Psychiatry 83, 224–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank GKW, et al. (2019) The neurobiology of eating disorders. Child Adolesc Psychiatr Clin N Am 28, 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd EC and Steinglass JE (2018) What can food-image tasks teach us about anorexia nervosa? A systematic review. J Eat Disord 6, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacquemot AMMC and Park R (2020) The role of interoception in the pathogenesis and treatment of anorexia nervosa: A narrative review. Front Psychiatry 11, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank GK, et al. (2016) Prediction error and somatosensory insula activation in women recovered from anorexia nervosa. J Psychiatry Neurosci 41, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullmann S, et al. (2014) Impaired inhibitory control in anorexia nervosa elicited by physical activity stimuli. Soc Cogn Affect Neurosci 9, 917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuglset TS, et al. (2016) Functional brain alterations in anorexia nervosa: a scoping review. J Eat Disord 4, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donnelly B, et al. (2018) Neuroimaging in bulimia nervosa and binge eating disorder: a systematic review. J Eat Disord 6, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jennings JH, et al. (2013) The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 341, 1517–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardaway JA, et al. (2019) Central amygdala prepronociceptin-expressing neurons mediate palatable food consumption and reward. Neuron 102, 1037–1052 e1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stern SA, et al. (2019) A molecularly defined insular cortex --> central amygdala circuit mediates conditioned overconsumption of food. bioRxiv, 684498 [Google Scholar]
- 36.Azevedo EP, et al. (2020) A limbic circuit selectively linking active escape to food suppression. Elife 9, e58894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Y, et al. (2019) Identification of a neurocircuit underlying regulation of feeding by stress-related emotional responses. Nat Commun 10, 3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frank GKW, et al. (2018) Association of Brain Reward Learning Response With Harm Avoidance, Weight Gain, and Hypothalamic Effective Connectivity in Adolescent Anorexia Nervosa. JAMA Psychiatry 75, 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bailer UF, et al. (2004) Altered 5-HT(2A) receptor binding after recovery from bulimia-type anorexia nervosa: relationships to harm avoidance and drive for thinness. Neuropsychopharmacol 29, 1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon NW, et al. (2011) Dopaminergic modulation of risky decision-making. J Neurosci 31, 17460–17470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zalocusky KA, et al. (2016) Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature 531, 642–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hedman A, et al. (2018) Bidirectional relationship between eating disorders and autoimmune diseases. J Child Psychol Psychiatry 60, 803–812 [DOI] [PubMed] [Google Scholar]
- 43.Raevuori A, et al. (2014) The increased risk for autoimmune diseases in patients with eating disorders. PLoS One 9, e104845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zerwas S, et al. (2017) Eating disorders, autoimmune, and autoinflammatory disease. Pediatrics 140, e20162089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breithaupt L, et al. (2019) Nationwide study of exposure to infections and anti-infective agents in adolescents and the risk of eating disorders. JAMA Psychiatry 76, 800–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leonard HL and Swedo SE (2001) Paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS). Int J Neuropsychopharmacol 4, 191–198 [DOI] [PubMed] [Google Scholar]
- 47.Cederlöf M, et al. (2015) Etiological overlap between obsessive-compulsive disorder and anorexia nervosa: A longitudinal cohort, family and twin study. World Psychiatry 14, 333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brimberg L, et al. (2012) Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacol 37, 2076–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yaddanapudi K, et al. (2010) Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry 15, 712–726 [DOI] [PubMed] [Google Scholar]
- 50.Calkin CV and Carandang CG (2007) Certain eating disorders may be a neuropsychiatric manifestation of PANDAS: case report. J Can Acad Child Adolesc Psychiatry 16, 132–135 [PMC free article] [PubMed] [Google Scholar]
- 51.Vincenzi B, et al. (2010) PANDAS and anorexia nervosa--a spotters’ guide: suggestions for medical assessment. Eur Eat Disord Rev 18, 116–123 [DOI] [PubMed] [Google Scholar]
- 52.Cryan J and Dinan T (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 13, 701–712 [DOI] [PubMed] [Google Scholar]
- 53.Dinan TG and Cryan JF (2017) The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am 46, 77–89 [DOI] [PubMed] [Google Scholar]
- 54.Hanachi M, et al. (2019) Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: An explicative factor of functional intestinal disorders? Clin Nutr 38, 2304–2310 [DOI] [PubMed] [Google Scholar]
- 55.Mack I, et al. (2016) Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep 6, 26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morita C, et al. (2015) Gut dysbiosis in patients with anorexia nervosa. PLoS One 10, e0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kleiman SC, et al. (2015) The intestinal microbiota in acute anorexia nervosa and during renourishment: Relationship to depression, anxiety, and eating disorder psychopathology. Psychosom Med 77, 969–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruusunen A, et al. (2019) The gut microbiome in anorexia nervosa: relevance for nutritional rehabilitation. Psychopharmacol (Berl) 236, 1545–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glenny EM, et al. (2017) Eating disorders and the intestinal microbiota: Mechanisms of energy homeostasis and behavioral influence. Curr Psychiatry Rep 19, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carr J, et al. (2016) Can attention to the intestinal microbiota improve understanding and treatment of anorexia nervosa? Expert Rev Gastroenterol Hepatol 10, 565–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hata T, et al. (2019) The gut microbiome derived from anorexia nervosa patients impairs weight gain and behavioral performance in female mice. Endocrinol 160, 2441–2452 [DOI] [PubMed] [Google Scholar]
- 62.Fouladi F, et al. (2020) Sequence variant analysis reveals poor correlations in microbial taxonomic abundance between humans and mice after gnotobiotic transfer. ISME J 14, 1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guarda AS, et al. (2015) Anorexia nervosa as a motivated behavior: Relevance of anxiety, stress, fear and learning. Physiol Behav 152, 466–472 [DOI] [PubMed] [Google Scholar]
- 64.Frank MG, et al. (2016) Stress-induced neuroinflammatory priming: A liability factor in the etiology of psychiatric disorders. Neurobiol Stress 4, 62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cryan JF (2016) Stress and the microbiota-gut-brain axis: An evolving concept in psychiatry. Can J Psychiatry 61, 201–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Donohoe TP (1984) Stress-induced anorexia: implications for anorexia nervosa. Life Sci 34, 203–218 [DOI] [PubMed] [Google Scholar]
- 67.Murray SB, et al. (2018) Fear as a translational mechanism in the psychopathology of anorexia nervosa. Neurosci Biobehav Rev 95, 383–395 [DOI] [PubMed] [Google Scholar]
- 68.Xu P, et al. (2013) Double deletion of melanocortin 4 receptors and SAPAP3 corrects compulsive behavior and obesity in mice. Proc Natl Acad Sci U S A 110, 10759–10764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maussion G, et al. (2019) Induced pluripotent stem cells: New tools for investigating molecular mechanisms in anorexia nervosa. Front Nutr 6, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Negraes PD, et al. (2017) Modeling anorexia nervosa: transcriptional insights from human iPSC-derived neurons. Transl Psychiatry 7, e1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pillidge K, et al. (2016) The NK1R−/− mouse phenotype suggests that small body size, with a sex- and diet-dependent excess in body mass and fat, are physical biomarkers for a human endophenotype with vulnerability to attention deficit hyperactivity disorder. J Psychopharmacol 30, 848–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karagiannides I, et al. (2008) Substance P as a novel anti-obesity target. Gastroenterol 134, 747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barbier E, et al. (2013) The NK1 receptor antagonist L822429 reduces heroin reinforcement. Neuropsychopharmacol 38, 976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chukwurah E, et al. (2019) All together now: modeling the interaction of neural with non-neural systems using organoid models. Front Neurosci 13, 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaelberer MM, et al. (2018) A gut-brain neural circuit for nutrient sensory transduction. Science 361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corwin RL and Buda-Levin A (2004) Behavioral models of binge-type eating. Physiol Behav 82, 123–130 [DOI] [PubMed] [Google Scholar]
- 77.Nilsson IA (2019) The anx/anx mouse–a valuable resource in anorexia nervosa research. Front Neurosci 13, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Friedman JM (2019) Leptin and the endocrine control of energy balance. Nat Metab 1, 754–764 [DOI] [PubMed] [Google Scholar]
- 79.Hagan MM, et al. (2002) A new animal model of binge eating: key synergistic role of past caloric restriction and stress. Physiol Behav 77, 45–54 [DOI] [PubMed] [Google Scholar]
- 80.Petrovich GD (2013) Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav 121, 10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


