Abstract
We present a systematic review and pooled analysis of clinical studies to date that (1) specifically compare the protection of natural immunity in the COVID-recovered versus the efficacy of complete vaccination in the COVID-naive, and (2) the added benefit of vaccination in the COVID-recovered, for prevention of subsequent SARS-CoV-2 infection. Using the PRISMA 2020 guidance, we first conducted a systematic review of available literature on PubMed, MedRxIV and FDA briefings to identify clinical studies either comparing COVID vaccination to natural immunity or delineating the benefit of vaccination in recovered individuals. After assessing eligibility, studies were qualitatively appraised and formally graded using the NOS system for observational, case-control and RCTs. Incidence rates were tabulated for the following groups: never infected (NI) and unvaccinated (UV), NI and vaccinated (V), previously infected (PI) and UV, PI and V. Pooling were performed by grouping the RCTs and observational studies separately, and then all studies in total. Risk ratios and differences are reported for individual studies and pooled groups, in 1) NPI/V vs PI/UV and 2) PI/UV vs PI/V analysis. In addition, the number needed to treat (NNT) analysis was performed for vaccination in naïve and previously infected cohorts. Nine clinical studies were identified, including three randomized controlled studies, four retrospective observational cohorts, one prospective observational cohort, and a case-control study. The NOS quality appraisals of these articles ranged from four to nine (out of nine stars). All of the included studies found at least statistical equivalence between the protection of full vaccination and natural immunity; and, three studies found superiority of natural immunity. Four observational studies found a statistically significant incremental benefit to vaccination in the COVID-recovered individuals. In a total pooled analysis, the incidence in NPI/V trended higher than PI/UV groups (RR=1.86 [95%CI 0.77-4.51], P=0.17). Vaccination in COVID-recovered individuals provided modest protection from reinfection (RR=1.82 [95%CI 1.21-2.73], P=0.004), but the absolute risk difference was extremely small (AR= 0.004 person-years [95% CI 0.001-0.007], P=0.02). The NNT to prevent one annual case of infection in COVID-recovered patients was 218, compared to 6.5 in COVID-naïve patients, representing a 33.5-fold difference in benefit between the two populations. COVID-recovered individuals represent a distinctly different benefit-risk calculus. While vaccinations are highly effective at protecting against infection and severe COVID-19 disease, our review demonstrates that natural immunity in COVID-recovered individuals is, at least, equivalent to the protection afforded by complete vaccination of COVID-naïve populations. There is a modest and incremental relative benefit to vaccination in COVID-recovered individuals; however, the net benefit is marginal on an absolute basis. Therefore, vaccination of COVID-recovered individuals should be subject to clinical equipoise and individual preference.
Keywords: sars-cov-2 reinfection, sars-cov-2, a systematic review, covid-19 vaccines, natural immunity, covid-19
Introduction and background
With the emergence of the COVID-19 pandemic beginning in early 2020, the rapid development and release of effective COVID-19 vaccinations represent a crowning achievement of the pharmaceutical and medical establishment. In the United States, Pfizer/NBiotech and Moderna achieved Emergency Use Authorization ("EUA") for the use of novel mRNA vaccines in general populations. Later, Johnson and Johnson (J&J) was awarded a EUA for a one-dose viral vector vaccination. The efficacy of these vaccines are excellent, with the Pfizer and Moderna vaccinations reported to achieve 90.3-97.6% [1] and 89.3-96.8% [2] efficacy, and the J&J viral vector vaccine be in the range of 55-74% [3]. The overall risk of severe adverse effects is generally considered to be extremely low [1-3].
While COVID-19 vaccinations are generally recommended for all persons 12 years of age and older without contraindications [4], the risk/benefit calculus may differ for individuals who may not expect the same benefit or may be at higher risk of adverse effects. One major subpopulation in this category are those individuals who were previously infected with SARS-CoV-2 and recovered (i.e., "COVID-Recovered"), which now exceed 180 million persons worldwide [5]. Significant public debate is now occurring as to whether recovered COVID-19 patients possess sufficient natural immunity and any substantial incremental benefit to COVID vaccination. In the United States, the Centres for Disease Control (CDC) currently recommends vaccinations in previously infected individuals without exception.
From early in the pandemic's start through November 2020, a growing number of case reports and small series were published demonstrating the possibility of reinfection in previously infected individuals [6]. However, the incidence and risk factors were only hypothesized. In December of 2020, Abu Raddad et al. published a study of a Qatari population of 133,266 previously infected individuals and found positivity and symptomatic incidence of reinfection of 0.18% and 0.04%, respectively, over six months [7]. Lawandi et al. reported a retrospective cohort study spanning 238 US hospitals and 131,773 patients and found suspected reinfection in 0.2% of patients [8]. A systematic review of the literature in May 2021 found that SARS-CoV-2 reinfection was an uncommon event, ranging from 0-1.1%, with no study reporting an increase in infection risk over time [9].
Similarly, several investigators have reported longitudinal and observational studies that directly compare the relative incidence of reinfection in COVID-recovered persons to COVID-naïve populations and found at significant risk reduction via natural immunity. In January 2021, Hanrath et al. reported a series of 17,126 health care workers (HCW) in the UK. They found a 0% risk of reinfection, compared to a 2.9% positivity rate, resulting in a 100% risk reduction due to prior infection (P<0.0001) [10]. In February of 2021, Lumley et al. published an observational cohort study of 12,541 HCWs, with known seropositive or seronegative status and found an adjusted risk reduction of 89%, with no symptomatic infections in seropositive individuals [11]. Hall et al. published the results of a prospective study of 30,625 participants and reported an 84% risk reduction, with a minimum median protective effect of at least seven months after primary infection [12]. Vitale et al. published a report on 15,075 individuals in the general Italian population and found a 94% risk reduction lasting at least one year. Leidi et al. studied an observational cohort of 10,547 essential Swiss workers and found a 94% risk reduction lasting at least eight months [13]. Few studies reported more modest risk reduction but greater than 74% [14-16].
The phenomena of reinfection in the COVID-recovered are considered being relatively low. With the protective effect of the previous infection on par with the primary available COVID-19 vaccinations, the next important question is the comparative benefit of vaccination in COVID-recovered individuals? As this is an evolving yet substantially important question, we consider published and pre-published studies and independently appraise the strength of findings. In this systematic review, we focus on the existing clinical studies that comparatively delineate the efficacy of natural immunity in COVID-recovered persons and the incremental benefit of vaccination in this same population. We also perform a pooled analysis of the eligible literature to aggregate and add power to the findings.
This review has important policy implications, as vaccine mandates are emerging in the public and private sectors. With few exceptions, these mandates do not generally grant natural immunity persons the same status as COVID-naïve individuals who have been "fully vaccinated". If, however, the evidence objectively shows equivalence in protection, then these civic vaccination policies in the COVID-recovered should be seriously questioned based on medical necessity, ethical principles and legal precepts governing the maintenance of bodily integrity.
Review
METHODS
Search strategy and data extraction
We performed a qualitative and inclusive systematic review guided by the principles outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines published in 2020. We used PubMed as our primary database for published and peer-reviewed articles and MedRxIV for pre-published studies. We also included publically available briefings or communications available on the FDA and CDC websites, given their central position in the dynamics of the pandemic. These briefings included significant data on subgroups that were not presented in the academic literature. The search period was defined as December 1, 2020, to August 31, 2021. This period was selected to include vaccination efficacy trials that occurred before the large-scale administration of vaccinations. Search words included "COVID-19", "SARS-CoV-2", "Coronavirus", in combination with terms, "previously infected", "reinfection", "recovered", "convalescent", "natural immunity", "recurrence", "antibody", "seropositive", intersecting with searches on "vaccination" or "immunization".
Articles included were screened independently by the authors, by title, or if necessary, the abstract or available manuscript. Our review was limited to original articles of clinical outcome studies published in English, without limitations on age, gender, the geography of the study population, or vaccine manufacturer. Selected articles included direct comparisons of unvaccinated COVID-recovered individuals (determined either by RT-PCR, antigen or serology) to other populations (either vaccinated or unvaccinated). We excluded case reports, other systemic or scoping reviews, opinion publications, guidelines, comments, editorials, animal studies, in vitro studies (typically testing for immune response outcomes), duplicate studies, or studies not electronically accessible. We also excluded studies with less than 500 total participants in the previously infected or baseline seropositive cohorts. All listings were reviewed and either included or excluded by the authors.
Quality appraisal of identified studies
Of the identified studies, each study was appraised. Observational, case-control and randomized controlled studies were assessed according to the respective Newcastle-Ottawa Scales (NOS), segmented by selection, comparability and outcome quality. Additionally, each study was qualitatively assessed regarding study design, patient/case definitions, methodology, and outcome measurement. Criteria on these scales were applied consistently across studies.
Incidence analysis and study pooling
Four groups were constructed: never previously infected and unvaccinated (NPI/UV), never previously infected and vaccinated (NPI/V), previously infected and unvaccinated (PI/UV), and previously infected and vaccinated (PI/V). In each of the identified studies, these cohorts were identified from the primary data, and event (infection) numbers, total cohort size, and time at risk (in person-years) was tabulated. In contrast, most data was available in the manuscripts and briefings, and some required personal communication with authors. If we did not get a reply from the authors, we made conservative assumptions (details in table). Studies were excluded if they did not contain absolute event counts or if the study's design did not conform to a pool. All data were then tabulated to include the unadjusted infection events, total participants, and the person-time follow-up (converted to standardized person-years) for each study. We then conducted two basic comparisons. First, we analyzed the NPI/V versus PI/UV groups to delineate the relative and absolute differences in protection between vaccination and natural immunity. Second, we compared the P/UV versus PI/V groups to define the absolute and relative incidence differences due to vaccination within the PI group.
The risk ratio (RR) and absolute risk (AR) differences were calculated for each study and comparison, along with 95% confidence intervals. A pooling method was performed combining the RCTs, the observational studies, and all studies, using Mantel-Haenszel (M-H) methods to arrive at pooled risk ratios and differences, along with confidence intervals and P values. M-H methods were utilized to provide pooled risk ratios across designated strata, in this case, randomized studies versus observational studies. Weighting was also conducted per M-H methods. When applicable, a discontinuity correction was performed. All pooling and statistical analyses were performed in RevMan Version 5.4. A p-value of 0.05 was used as the threshold for significance. For the PI/UV vs PI/V analysis, the risk differences (1/AR) were used to generate a number needed to treat (NNT) per annum for the NPI and PI pooled groups.
RESULTS
Search outcome
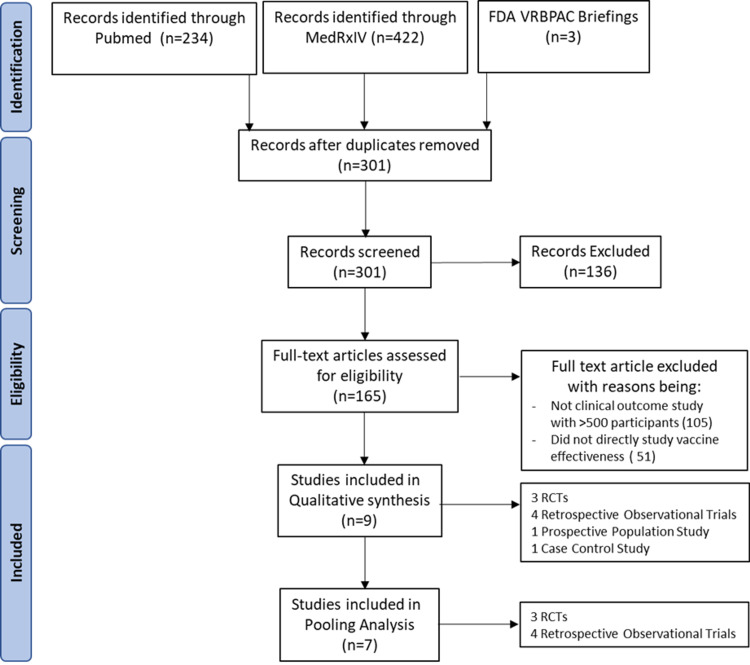
We began with the known FDA Vaccine Related and Biological Product Advisory Committee (VRBPAC) briefings for Pfizer, Moderna, and J&J [17-19]. These VRBPAC briefings contained significant subgroup data not included in the academic publications [1-3]. From the initial database searches in PubMed and MEDRxiv, we identified 234 and 422 results, of which six met criteria [20-25]. Therefore, we identified nine clinical studies reporting infection rates in COVID-recovered, COVID-naïve, vaccinated and unvaccinated individuals. Three of these studies were randomized controlled studies (RCTs) [17-19] with subgroup analysis. Four of these studies were classified as retrospective observational cohort studies [21,22,24,25]. One study was a prospective observational cohort [20], and another study was case-control [23]. Figure 1 depicts the PRISMA flow diagram. Table 1 summarizes the identified studies, listed in chronological order of publication.
Table 1. Description of identified clinical studies, type, purpose and result/conclusion.
VRBPAC- Vaccine Related and Biological Product Advisory Committee
| Author | Date of Publication | Study Type | Study Purpose | Result/Conclusion |
| Pfizer FDA VRBPAC [17] | 12/10/2020 | Subgroup Analysis of a Randomized Controlled Study | Pivotal trial to estimate Pfizer vaccine safety and efficacy overall, and within various subgroups. Subgroup analysis of previously infected provided. | Overall, 3% of all participants had prior infection with SARS-CoV-2. There were 1/567 (0.2%) and 1/526 (0.2%) reinfections in placebo and vaccination arms respectively. The overall incidence of unvaccinated and vaccinated incidence was 0.93% and 0.05%, respectively. |
| Moderna FDA VRBPAC [18] | 12/17/2020 | Subgroup Analysis of a Randomized Control Study | Pivotal trial to estimate Moderna vaccine safety and efficacy overall, and within various subgroups. Subgroup analysis of previously infected provided. | Overall, 0.15% of participants had baseline SARS-CoV2 seropositivity. There was 1/334 (0.3%) and 0/341 (0.0%) reinfections in the placebo and vaccination arms respectively. The overall incidence of infection in vaccinated seronegative participants was (<0.1%) |
| J&J FDA VRBPAC [19] | 2/26/2021 | Subgroup Analysis of a Randomized Control Study | Pivotal trial to estimate Johnson and Johnson vaccine safety and efficacy overall, and within various subgroups. Subgroup analysis of previously infected provided. | Overall, 9.6% of all participants had baseline SARS-CoV-2 seropositivity. There were 4/2030 (0.2%) and 3/2122 (0.14%) reinfection in the placebo and vaccination arms respectively. The incidence of primary infection in vaccinated seronegative participants was 0.8%. |
| Goldberg et. al. [20] | 4/24/2021 | Prospective Observational Analysis | To estimate vaccine efficacy vs. natural immunity, to varying severities of disease, stratified amongst age groups. | Study found excellent overall vaccine efficacy against infection, hospitalization and death (92.8%, 94.2%, 94.4%, 93.7%) . Previously infected individuals also had excellent protection to infection, hospitalization and illness (94.8%, 94.1%, 96.4%). Superior efficacy held up in every age group, for each severity. |
| Shrestha et. al. [21] | 6/5/2021 | Retrospective Observational Cohort | To assess for reinfection in previously infected (with/without vaccination), vaccinated (Pz, Md) and unvaccinated HCWs between 12/16/20-5/15/2021. | No reinfections (0.0%) in any HCW with previous infection, regardless of vaccination status. Vaccination in COVID naïve HCWS was (0.7%). No statistically significant benefit in vaccinating previously infected individuals (HR 0.313 [95% CI 0 to Inf]) |
| Lumley et al. [22] | 7/3/2021 | Retrospective Observational Cohort | To study the incidence SARS-COV-2 infection (Alpha strain) in HCWS, according to antibody and vaccination (PZ, AZ) status. | When compared to unvaccinated seronegative individuals, aRR for vaccinated immunity and previous infection were 90% [62-98%] and 85% [74-92%], respectively . |
| Cavanaugh et. al. [23] | 8/13/2021 | Case-control study | To determine the relative odds of non-vaccination (PZ, Md, JnJ) in newly reinfected and matched uninfected/recovered controls, in May/June 2021 in Kentucky. | Reinfection in previously infected individuals was associated with a 2.34x [1.58-3.57] odds of non-vaccinated status. |
| Satwik et. al. [24] | 8/15/2021 | Retrospective Observational Cohort | To assess vaccination efficacy (AZ) and previous infection in preventing symptomatic, severe disease, and death in one tertiary hospital in India, where the Delta variant is suspected to be the dominant strain. | Previous infection was significantly protective against symptomatic and moderate/severe disease with 93% [87-96%] and 89% [57-97%] effectiveness, respectively. Two doses of AZ vaccine led to reduction of symptomatic and moderate/severe disease by 24% [6-38%] and 65% [42-79%]. |
| Gazit et. al. [25] | 8/24/2021 | Retrospective Observational Analysis | To compare reinfection in previously infected individuals with and without vaccination, to a naïve vaccinated control. | Found prior infection afforded 13x protection from reinfection, compared to never infected but vaccinated individuals – when prior infection and vaccination were matched for time. Prior infection afforded 5.97x protection when unmatched for time. Vaccination resulted in 0.53x odds of reinfection, in previously infected group. |
Figure 1. PRISMA flowchart.
Description and appraisal of identified studies
Each study was meticulously reviewed qualitatively and objectively appraised based upon the respective NOS rating scales and other characteristics. Table 2 depicts the strengths and weaknesses of each study and the NOS score.
Table 2. Strengths, weaknesses and appraisals of identified studies.
VRBPAC- Vaccine Related and Biological Product Advisory Committee
| Author | Strengths | Weaknesses | NOS | Assessment |
| Pfizer FDA VRBPAC [17] | Randomized controlled, pivotal study - Methodologically robust - 112-day follow-up follow-up | Subgroup analysis of previously infected relatively small (3% of overall cohort). - Industry-sponsored - 2x withdrawal by a participant in the placebo group. Only 2/19 cases were included in subgroup analysis. | Selection: ★★★ Comparability: ★★ Outcome: ★★★ | (8/9) |
| Moderna FDA VRBPAC [18] | Randomized controlled, pivotal study -Methodologically robust - 104-day follow-up | Subgroup analysis of previously infected relatively small (0.15% of overall cohort). - Industry-sponsored | Selection: ★★★★ Comparability: ★★ Outcome: ★★★ | (9/9) |
| J&J FDA VRBPAC [19] | Randomized controlled, pivotal study -Methodologically robust - 125-day follow-up | Subgroup analysis of previously infected relatively small (3% of overall cohort). - Timing of reinfections not reported related to vaccination dosing. - Industry-sponsored | Selection: ★★★★ Comparability: ★★ Outcome: ★★★ | (9/9) |
| Goldberg et al. [20] | A large whole population study conducted in Israel - Statistically robust, adjusting for individual cohort dynamics. - Reported outcomes by age and severity of illness. - Study period during Israeli B.1.17 surge. | 3 month follow-up period - Did not compare vaccination efficacy in previously infected -Limited to PZ vaccination only. | Selection: ★★★ Comparability: ★★ Outcome: ★★★ | (8/9) |
| Shrestha et al. [21] | Large observational study of HCWs - a 5-month observational period - Specifically attempts to answer the research question of vaccine efficacy in previously infected. - Included both PZ, Moderna vaccines. - Adjusted for a phase of an epidemic -Rigorous testing protocol | Based on HCWs in 1 large U.S. health system - Despite a large overall sample size, the previously infected cohort was underpowered. -Studied only symptomatic infection. | Selection: ★★ Comparability: ★★ Outcome: ★★★ | (7/9) |
| Lumley et al. [22] | High-risk exposure group (HCWs) - Serology used to confirm exposure and control groups. - Adjusted for days at risk, demographics, staff occupational role and patient contact -Considered variants of concern (B.1.1.7), symptomatic illness | Limited to HCWs, so may not be generalizable, particularly in children or elderly populations. -Underpowered to resolve a difference between vaccinated/ seronegative and seropositive groups. | Selection: ★★ Comparability: ★★ Outcome: ★★★ | (7/9) |
| Cavanaugh et al. [23] | Specifically attempts to answer the research question of vaccine efficacy in previously infected. - Focus on the general population | Limited to two months (May/June) in one state. - Not a test-negative design, underestimating possible infection. - Not controlled for serological status, only history of the prior test. - Not powered to elucidate subgroup trends. - Case-control methodology disallows for calculation of absolute risk reduction. | Selection: ★ Comparability: ★★ Exposure: ★ | (4/9) |
| Satwik et al. [24] | An observational study, performed during the Indian Delta variant surge. - Stratified results by the severity of disease, and a number of vaccine doses. - 5-month observational period | Based on HCWs in 1 tertiary Indian hospital; a small sample size. - Limited to AZ vaccine use only. - Studied only symptomatic infection - | Selection: ★★ Comparability: ★★ Outcome: ★★★ | (7/9) |
| Gazit et al. [25] | 1:1 matched cohort study with a high volume of subjects from a national database -Adjusted for age, gender, geographic area, socioeconomic status - Designed to answer questions pertinent to policy ( protection of the previous infection vs. vaccination, the durability of protection, and risk reduction of vaccination in previously infected. - Timed during Delta surge in Israel | Retrospective in nature. - Results may specifically reflect Delta strain phenomena - Assessed only Pfizer vaccine | Selection: ★★★ Comparability: ★★ Outcome: ★★★ | (8/9) |
The pivotal vaccine trials (Pfizer, Moderna, and J&J) each contained subgroups of participants that were previously infected, either by medical history or serological status. These studies were extensive and methodically regulated randomized controlled trials (RCTs), specifically designed to assess vaccine efficacy in a general population. None of these three studies could statistically conclude that vaccination in the previously infected or serologically positive population would benefit from the vaccine, due mainly to the small overall reinfection incidence. In the Pfizer trial, when observing all participants from enrollment (without exclusion), there were a total of 19 reinfections (9 in the placebo arm and 10 in the vaccination arm). However, the exact timings of the second dose are not reported in the briefing. Overall, these are well-conducted trials, with the limitations of power for this subgroup analysis, and relatively short follow-up (3 to 4 months), with differing follow-up periods within various phases of the pandemic. Critically, the Moderna and J&J trials achieve a full score (9/9) for NOS evaluation. The Pfizer trial received an 8/9 score, with one star deducted due to a lack of confirming the presence or absence of current or prior infection before intervention.
Goldberg et al. [20] released a study set in the unique situation of Israel, which undertook a massive vaccination campaign. However, during the study period, previously infected individuals were explicitly excluded from vaccination. This policy allowed for a large direct volume and prospective comparison of COVID-naive vaccinated individuals to COVID-recovered unvaccinated individuals. The overall study population included 6.3 million individuals 18 years and older and utilized a dynamic cohort model that accounted for individuals progress through the first dose to full vaccination status. The statistical methodology was robust, executing a Poisson regression, and adjusting for age, gender, prior PCR results, and municipal risk. Overall, the results found excellent vaccine efficacy in the NPI/V group of 92.8%, 94.2%, 94.4% and 93.7% against infection, hospitalization, severe illness and death, respectively. However, protection in the PI/UV cohort was superior with 94.8%, 94.1%, 96.4% against infection, hospitalization and severe illness. There were so few deaths in the PI/UV cohort that it could not be statistically calculated. The trend of superior protection from natural immunity held up in every age demographic for all severities of illness.
Additionally, this study was conducted during the Israeli surge of the B.1.1.7 (Alpha) variant, suggesting robust natural immunity to variants of concern. Limitations of this study include a bias towards symptomatic testing and the lesser likelihood of vaccinated or previously infected individuals getting symptomatic testing. As such, incidence rates may be underestimated, but not for more severe illnesses. In terms of appraisal, the study achieves 8/9 stars, with only a one-star deduction for the inability to confirm current infection before vaccination.
Shrestha et al. [21] performed an observational study in the context of occupational health, set at the Cleveland Clinic, OH, USA. A total of 52,238 employees were enrolled, of which 2,579 had a history of previous SARS-CoV-2 infection. Of these previously infected individuals, 53% remained unvaccinated during the observation period. Throughout the entire study, not a single previously infected individual (0%) presented with reinfection, regardless of vaccination status (PI/V and PI/UV). Consequently, the practical risk reduction by the previous infection was effectively 100%.
Conversely, the NPI/V cohort had a breakthrough of 0.7%. As expected, the vast majority of study positives were in the NPI/UV cohort. Using a Cox proportional hazard methodology, the authors found a significantly lower risk by vaccination in COVID-naïve individuals but no significant difference in COVID-recovered persons attributable to vaccination. Limitations of this study include its definition of the previous infection, which may neglect asymptomatic infections. Additionally, constraining the study to HCWs limits the study's generalizability to demographics not represented in this particular workforce. Regarding NOS scoring, the study achieved 7/9 stars, with two-star deductions for its limited focus on HCWs, and the absence of a demonstration that infection was present at the start of the study.
Lumley et al. [22] represent a high-quality observational cohort study, performed at Oxford University Hospitals, that evaluated the incidence of SARS-CoV-2 reinfection in 13,109 HCWs, stratified by serological and vaccination (one and two doses) status. Of note, this study coincided with the B.1.1.7 surge (Alpha ) in the United Kingdom. There were 327 infections in the study group, with 326 in the seronegative unvaccinated or partially vaccinated group and only one reinfection in the seropositive group. There were no infections in the vaccinated, seronegative group. The authors calculated a 90% and 85% risk reduction for vaccination in seronegative and seropositive, respectively, without statistical difference [P=0.96]).
Additionally, the authors conducted a study on viral loads in symptomatic infection. They found that the pre-vaccination seropositive cohort had the lowest viral loads in infected persons across the study. The authors concluded that "Natural immunity resulting in detectable anti-spike antibodies and the two-dose vaccine does both provide robust protection against SARS-CoV-2 infection, including the B.1.1.7 variant".
The strengths of Lumley et al. are its robust methodology that utilized a Poisson regression model to estimate the incidence per day-at-risk, adjusted for a month, age, gender, ethnicity, staff role and direct patient contact. The cohort was drawn from a well-defined population of a serologically tested individual with either a positive or negative status before the study period. While the study pertains to a narrow population of HCWs, that are underrepresent certain age and ethnicity demographics in general populations, the high-risk setting contributes to the confidence of the result. The study was somewhat limited in its uncontrolled approach to testing, as some cohorts may have different tendencies to get tested. Finally, while the study was powered to resolve differences between unvaccinated seronegative and the other cohorts, it was underpowered to determine superiority between fully-vaccinated and unvaccinated seropositive individuals due to low rates in both groups. The study informs on comparability in protection but non-superiority of either vaccinated or seropositive status. Per our appraisal, the study rated 7/9 stars, with the only deductions being for lack of demonstration that infection was not present at the start of the study and its focus on HCWs.
Cavanaugh et al. [23] presented a case-control study in the state of Kentucky, United States. The study used linked state infection and vaccination databases, reconciled by name and date of birth. The authors identified 246 total "case" reinfections in May and June 2021, drawn from all Kentucky residents aged ≥18 years, with a positive SARS-CoV-2 test in 2020. Case patients were then matched 1:2 to control (492 individuals) non-reinfected patients, based on sex, age, and date of an initial positive test. Unvaccinated individuals accounted for 72.8% of case patients, whereas only 57.7% of the controls were unvaccinated. This calculates an adjusted odds ratio (OR) of 2.34 (95% CI 1.58-3.47). The authors suggest that "among persons with previous SARS-CoV-2 infection, full vaccination provides additional protection against reinfection."
While Cavanaugh et al. was specifically designed to assess for the the superiority of vaccination versus non-vaccination in previously infected individuals, the study had several limitations to consider. First, the study represents a single-state experience drawing only 246 reinfected patients in May and June of 2021 (out of potentially 275,000 eligible [26]), based upon a database matching algorithm, by which inefficient matching (duplicate names, incomplete records) could lead to disproportionate selection bias in this small sample. Second, the control group was not confirmed "test-negative", and vaccinated individuals (symptomatic or asymptomatic) may be less inclined to get tested. Consequently, the case and control groups are not matched according to their likelihood of getting tested, which is a critical confounder. Third, case matching was only performed based on age, gender, and month of the previous infection; however, several other salient parameters should have been addressed. For example, race, socioeconomics, and geography are all variables that could impact getting vaccinated or getting tested. Fourth, only reinfections reported in May and June of 2021 were used to identify case subjects, even though vaccinations were made available beginning December 2020. NOS assessment for case-control studies was rated 4 of 9 stars, with three-star deductions in the subject selection and two-star deductions in assessment and follow-up of the outcome.
Satwik et al. [24] reported a small observational study performed on HCWs at one tertiary hospital in New Dehli, India, where primarily the Astra-Zeneca (ChAdOx1 Nov-19) vaccination was available for 4,296 employees. The authors report the effectiveness of 93% [95% CI 87-96%] versus two-dose vaccination efficacy of 24% [95% CI 6-38%] for all symptomatic infections. For moderate to severe disease, the effectiveness of the previous infection was 89% [95% CI 57 to 97] versus 65% [95% CI 42-79%] for two-dose vaccination. There were no deaths in the previous infection or two-dose cohort. This study is notable for setting during the B.1.617.2 (Delta) variant surge experienced in India during this time. A separate study performed at this institution also noted approximately a 50% penetration of the Delta variant [27]. The underwhelming vaccine efficacy observed in this study was in line with other studies about the Delta variant during the same observation period [28]. The limitations of this study are its relatively small size within a group of HCWs, lack of adjustments for basic demographics, testing of symptomatic individuals only, and primary use of the ChAdOx1 Nov-19 vaccine, which differs from other studies in this review. The study also occurred during a difficult Delta strain emergence period, leading to shortened average follow-up in vaccinated individuals. Nevertheless, the authors conclude that "[the previous infection offered] higher protection than that offered by single or double dose vaccine." NOS assessment attributed 7 of 9 stars to this study due to lack of confirming presence or absence of infection at the start of the study, and the short duration of follow-up, particularly in vaccinated cohorts.
Gazit et al. [25] recently presented a retrospective observational study in Israel during the Delta surge with a matched cohort analysis. The authors defined three groups: never infected and two doses of vaccination (Pfizer), previously infected and never vaccinated, and previously infected, and one dose of vaccination (Pfizer). These groups then underwent a matched cohort comparison, controlling for age, gender, geographic area, and socioeconomic status. When comparing the vaccinated COVID-naive group with the unvaccinated COVID-recovered in a matched timing analysis, they found a 13.06 (95% CI 8.08-21.11, P<0.001) increased risk of infection in the vaccinated cohort. For symptomatic infections only, the risk increased to 27.02-fold [95%CI12.7-57.5]). When time matching was removed, there still was a 5.96 [95% CI 4.85-7.33, P<0.001] increased risk of infection in the vaccinated no prior infection group. Finally, they compared vaccination to non-vaccination in previously infected individuals and found a 0.53-fold risk reduction (95%CI 0.3-0.92, P<0.05). However, the absolute risk reduction was only 0.1% (17 cases/14,029 subjects). Similarly, the risk was reduced 0.68-fold (95%CI 0.38-1.21) for symptomatic individuals with an absolute risk reduction of 0.04%, without reaching statistical significance. The authors bluntly conclude, "This study demonstrated that natural immunity confers longer-lasting and stronger protection against infection, symptomatic disease and hospitalization caused by the Delta variant of SARS-CoV-2, compared to the BNT162b2 two-dose vaccine-induced immunity [the previously infected] given a single dose of the vaccine gained additional protection against the Delta variant."
The Gazit et al. study was designed to answer pertinent clinical questions with robust methodology and adjustments precisely. The study's strength is the size of the cohorts and its matched design, which allowed for multivariable adjustments. The limitations of the study include its applicability primarily to the Delta variant and Pfizer vaccine only. As the authors only reported total events without respect to time, time-varying complicating factors could alter the result. In terms of NOS rating, it achieved 8/9 stars, with its only deduction being the lack of demonstration of infection at the start of the study, due to its population database methodology.
Risk analysis: Vaccination vs Natural Immunity ( NPI/V vs PI/UV)
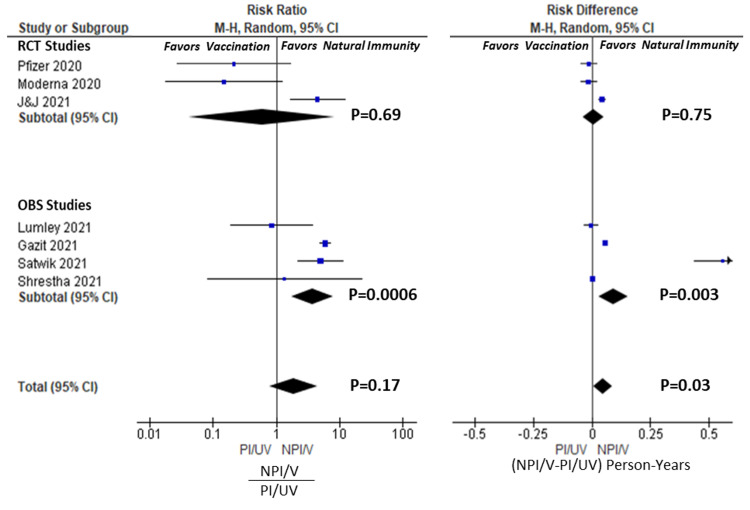
The infection events per person were tabulated for each study and are presented in Table 3, along with the risk difference and risk ratios between NPI/V and PI/UV groups. Figure 2 depicts a forest plot of these results graphically for individual studies, RCTs, observational, and total pooled groups. Four studies [19,21,24,25] favoured an increased risk ratio in the vaccinated NPI/V group (three statistically significant) versus three studies suggesting increased risk in the natural immunity PI/UV group [17,18,22] (none statistically significant). This observation was similar in the absolute risk difference analysis. The pooled RCT studies led to an overall RR of 0.59 [95% CI 0.04-8.28, P=0.69], whereas the RR for pooled observational studies was 3.71 [95%CI 1.75-7.86; P=0.0006]. Overall, the total pooled RR was 1.86 [95% CI 0.77-4.51, P=0.17]. Goldberg et al. [20] were excluded from this analysis as numeric data for events and cohort sizes were not reported; however, it supports the pooled findings in finding superiority of natural immunity over-vaccination. Cavanaugh et al. [23] were also excluded as they only included analysis of previously infected individuals. Consequently, no study could conclude the superiority of vaccination protection over natural immunity with statistical confidence, but observational studies endorsed an advantage for protection by natural immunity.
Table 3. Summary of Reported Infection/Reinfection Incidences (per person, person-years).
1 Lumley et al. included 1- and 2-dose vaccination groups. For this analysis, only the 2-dose vaccination group was counted. 2 Satwik et al. data was not entirely provided in the publication. Data obtained via personal communication. 3 Gazit et al. described three models of study, with a matching comparison. For this analysis, Model 2 was used for NPI/V, and PI/UV analysis and Model 3 was used for the PI/V group. An NPI/UV group was not reported in this study and therefore omitted from the analysis. 4 Risk ratios and differences calculated from Events/Person-Year figures 5 Pooled RR and risk differences adjusted and determined by M-H methods. 6 Time-at-risk not published in Shrestha et. al. manuscript. Estimated by taking a number of people in the group, multiplied by the length of study (in years) 7 Noted that there is no difference in incidence between NPI/UV and NPI/V groups. The authors of this study were contacted, and relayed at vaccine efficacy of 28%, after adjustment by a Cox proportion regression. Additionally, the author notes the implementation of vaccination during the peak phase of Delta and the shorter follow-up in the vaccinated cohort.
| Study | NPI/UV | NPI/V | PI/UV | PI/V | NPI/V vs. PI/UV | PI/UV vs PI/V |
| Cases (N) | Cases (N) | Cases (N) | Cases (N) | RR [95% CI]4 | RR [95% CI]4 | |
| % | % | % | % | AR [95%CI]4 | AR [95%CI]4 | |
| Person-Years | Person-Years | Person-Years | Person-Years | |||
| Events/P-Y | Events/P-Y | Events/P-Y | Events/P-Y | |||
| Pfizer [17] | 164 (17,720) | 8 (17,637) | 1 (567) | 1 (526) | 0.21 [0.03-1.69] | 0.93 [0.06-14.57] |
| 0.93% | 0.05% | 0.18% | 0.19% | -0.013 [-0.046-0.019] | -0.001 [-0.049-0.046] | |
| 2,242 | 2,237 | 60 | 56 | |||
| 0.073 | 0.004 | 0.017 | 0.018 | |||
| Moderna [18] | 90 (14,730) | 6 (14,312) | 1 (334) | 0 (341) | 0.15 [0.02-1.21] | 3.05 [0.13-73.39] |
| 0.63% | 0.04% | 0.30% | 0.0% | -0.014 [-0.47-0.019] | 0.017 [-0.029-0.063] | |
| 2,787 | 2,387 | 59 | 60 | |||
| 0.032 | 0.002 | 0.017 | 0.0 | |||
| J&J [19] | 509 (19,544) | 173 (19,514) | 4 (2030) | 3(2122) | 4.46 [1.67-11.93] | 1.40 [0.31-6.19] |
| 2.60% | 0.89% | 0.20% | 0.14% | 0.043 [0.029-0.058] | 0.004 [-0.012-0.019] | |
| 3,089 | 3,114 | 321 | 336 | |||
| 0.164 | 0.056 | 0.012 | 0.009 | |||
| RCT Pooled | 763 (51,634) | 187 (52463) | 6 (2931) | 4 (2989) | 0.59 [0.04-8.28]5 | 1.45 [0.43-4.85] |
| 1.48% | 0.36% | 0.20% | 0.13% | 0.007 [-0.037--.051]5 | 0.004 [-0.010-0.019] | |
| 8,118 | 7,738 | 439 | 453 | |||
| 0.094 | 0.024 | 0.014 | 0.009 | |||
| Shrestha et.al [21] | 2139 (15,317) | 15 (28,836) | 0 (1265) | 0 (1265) | 1.36 [0.08-22.71] | Not estimable |
| 14.0% | 0.05% | 0.0% | 0.0% | 0.0026 [-0.003-0.0081] | 0.000 [-0.008-0.008] | |
| 3,5116 | 5,8466 | 2566 | 2476 | |||
| 0.609 | 0.002 | 0.0 | 0.0 | |||
| Lumley et al. [22]1 | 635 (10,513) | 2 (940) | 12 (1273) | 1 (974 | 0.85 [0.19-3.73] | 2.03 [0.27-15.42] |
| 6.0% | 0.21% | 0.94% | 0.10% | -0.003 [-0.032-0.025] | 0.011 [-0.013-0.036] | |
| 6,231 | 107 | 543 | 92 | |||
| 0.102 | 0.019 | 0.022 | 0.011 | |||
| Satwik et. al. [24]2 | 128 (790) | 323 (2120) | 5 (147) | 4 (596) | 5.01 [2.21-11.32] | 4.48 [1.27-15.82] |
| 16.2% | 15.2% | 3.4% | 0.67% | 0.55 [0.4354-0.6775] | 0.108 [-0.0090-0.225] | |
| 182 | 430 | 36 | 129 | |||
| 0.6857 | 0.6967 | 0.138 | 0.031 | |||
| Gazit et. al. [25]3 | - | 640 (46,035) | 108 (46,035) | 20 (14,029) | 5.93 [4.84-7.25] | 1.65 [1.02-2.65] |
| 1.39% | 0.23% | 0.14% | 0.056 [0.051-0.062] | 0.005 [0.001-0.008] | ||
| 9,459 | 9,459 | 2,882 | ||||
| 0.068 | 0.011 | 0.007 | ||||
| OBS Pooled | 2902(26,620) | 980(77,931) | 125(48,720) | 25 (16,819) | 3.71 [1.75-7.86]5 | 1.94 [1.17-3.21]5 |
| 10.9% | 1.26% | 0.26% | 0.15% | 0.092 [0.031-0.153]5 | 0.0038 [-0.0036-0.011]5 | |
| 9,925 | 15,842 | 10,296 | 3,352 | |||
| 0.292 | 0.062 | 0.012 | 0.007 | |||
| Total Pooled | 3665(78,254) | 1167 (129,394) | 131(51,651) | 29(19,808) | 1.86 [0.77-4.51]5 | 1.82 [1.21-2.73]5 |
| 4.68% | 0.90% | 0.25% | 0.15% | 0.049 [0.0084-0.0893]5 | 0.0039 [0.001-0.007]5 | |
| 18,042 | 23,580 | 10,735 | 3,804 |
Figure 2. Forest plots for NPI/V vs. PI/UV Analysis. Risk ratio and risk differences calculated for individual studies, and RCT, OBS, and Total pools. Risk ratio calculated as incidence ratio of (NPI/V)/PI/UV). Risk difference calculated as incidence difference of NPI/V-PI/UV.
RCT= Randomized Controlled Studies; OBS=Observational Studies; NPI/V= not previously infected, vaccinated ; PI/UV=previously infected, unvaccinated; M-H= Mantel-Haenszel methods. CI = confidence interval. Weights (Risk Ratio): Pfizer 10.1%, Moderna 9.9%, J&J 17.7%, Lumley 13.9%, Gazit 22.4%, Satwik 19.0%, Shrestha 6.9%. Weights (Risk Difference): Pfizer 14.8%, Moderna 14.8%, J&J 16.0%, Lumley 15.1%, Gazit 16.3%, Satwik 6.6%, Shrestha 16.3%.
Citations: Pfizer [17], Moderna [18], J&J [19], Shrestha [21], Lumley [22], Satwik [24], Gazit [25].
Risk analysis: Vaccination vs Non-vaccination in the Previously Infected (PI/V vs. PI/UV)
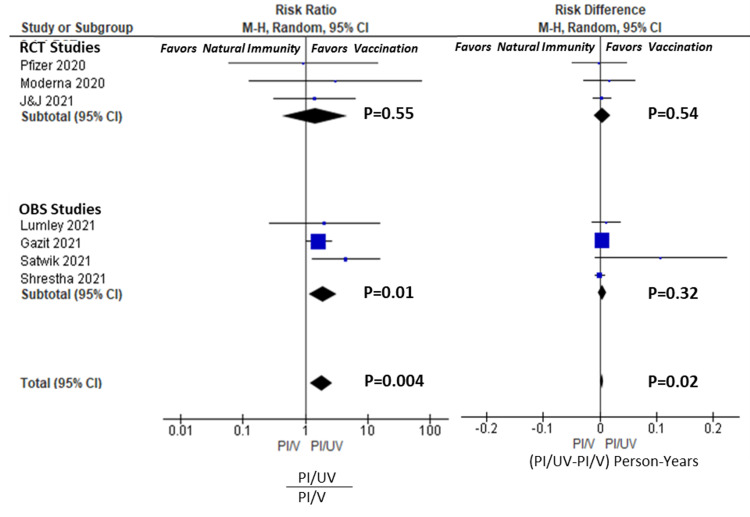
Table 3 also summarizes the incidence rates, the RR and AR for reinfection in the PI/V vs. PI/UV groups. Forest plots are given in Figure 3. None of the individual RCTs found a statistically significant advantage to vaccination in preventing reinfection. The RCT pooled results resulted in a RR of 1.45 [95%CI 0.43-4.85, P=0.55], and the AR of 0.004 person-years [95%CI -0.010-0.019, P=0.54 ]. However, three of the four observational trials did find an advantage to vaccination in the previously infected with an RR of 1.94 [1.17-3.21, P=0.01], but the AR was only 0.004 person-years [95%CI -0.004-0.011, P=0.32 ]. Overall, there was significant protection to vaccination in COVID-recovered persons in the total pooled group, with an RR of 1.82 [1.21-2.73, P=.004], but this effect was modest on an absolute scale, 0.004 [95%CI 0.001-0.007, P=0.02]. Table 4 displays the applicable NNTs for each RCT, observational and total pool. The NNT by vaccination in COVID-recovered individuals in the 218, 207, and 214 in Total, RCT, and Observational pools, respectively. Comparatively, the NNTs in COVID-naïve individuals were 6.5, 14.3, and 4.3 persons, respectively. This represents a 33.5-fold larger population that needs to be vaccinated to prevent one case of COVID per year in the total pool.
Table 4. Summary of Vaccination Risk Difference and NNT in Pooled Populations.
RCT= Randomized Controlled Studies; OBS=Observational Studies; NNT- Number needed to treat; PI/UV=previously infected, unvaccinated; PI/V=previously infected, vaccinated
| Study | NPI/UV - NPI/V (person-years) | PI/UV-PI/V (person-years) | NNT (NPI) | NNT (PI) | Ratio (PI/NPI) |
| RCTs | 0.070 | 0.005 | 14.3 | 207 | 14.5 |
| OBS | 0.231 | 0.005 | 4.33 | 214 | 49.2 |
| Total | 0.154 | 0.005 | 6.5 | 218 | 33.5 |
Figure 3. Forest plots for PI/UV vs. PI/V Analysis. Risk ratio and differences for individual studies, RCT, OBS and Total pools. Risk ratio calculated as incidence ratio of (PI/UV)/(PI/V). Risk difference calculated as incidence differences of PI/UV-PI/V.
RCT= Randomized Controlled Studies; OBS=Observational Studies; PI/V= previously infected, vaccinated ; PI/UV=previously infected, unvaccinated; M-H= Mantel-Haenszel methods. CI = confidence interval. Weights (Risk Ratio): Pfizer 2.2%, Moderna 1.7%, J&J 7.5%, Lumley 4.1%, Gazit 74.0%, Satwik 10.5%. Weights (Risk Difference): Pfizer 0.5%, Moderna 0.5%,, J&J 4.2%, Lumley 1.7%, Gazit 75.7%, Satwik 0.1%, Shrestha 17.3%. Citations: Pfizer [17], Moderna [18], J&J [19], Shrestha [21], Lumley [22], Satwik [24], Gazit [25].
Discussion
Despite the massive investigational attention on SARS-CoV-2 infection, COVID illnesses and vaccine efficacy, our systematic review identified only a relatively few outcome studies that investigated the comparative benefit of vaccination in COVID-recovered individuals. Two relevant but separate questions need to be considered. First, does previous infection protect individuals to the same degree as what we currently consider "full vaccination" in COVID-naïve individuals? Second, is there an incremental benefit of vaccination to previously infected individuals? Table 5 summarizes the significance of our findings, and each question is considered below. In summary, our analysis demonstrates that natural immunity in the COVID-recovered performs better than full-vaccination alone in COVID-naïve persons. However, there is a small absolute benefit to vaccination in COVID-recovered persons.
Table 5. Summary of Pooled Analysis Results and Statistical Significance.
RCT= Randomized Controlled Studies; OBS=Observational Studies; NNT- Number needed to treat; PI/UV=previously infected, unvaccinated; PI/V=previously infected, vaccinated; RR-risk ratio; AR- absolute risk
| NPI/V vs. PI/UV | PI/UV vs. PI/V | |
| RR | RR | |
| Pool | AR | AR |
| RCTs | P>0.05 | P>0.05 |
| P>0.05 | v P>0.05 | |
| OBS | Favors Natural Immunity | Favors Vaccination |
| Favors Natural Immunity | P>0.05 | |
| Total | P>0.05 | Favors Vaccination |
| Favors Natural Immunity | Favors Vaccination |
Does natural immunity provide at least equivalent protection from infection to that afforded by vaccination in the COVID-naïve persons?
The CDC currently recommends vaccination in all individuals 12 and older, regardless of a history of the previous infection, assuming that recovered individuals are still at risk for reinfection and transmission. However, as discussed earlier, the data indicate that this reinfection rate is low. From a policy perspective, it is relevant to understand if natural immunity in COVID-recovered individuals provides similar protection from reinfection than vaccination in COVID-naïve persons, given the newfound social status of being "fully vaccinated". We emphasize that COVID-naïve persons should not seek infection as a means to avoid vaccination, and the risk of COVID illness (serious or otherwise) far exceeds the risk of vaccination. However, if natural immunity is at least equivalent to some brands of vaccination (i.e., adenoviral vs mRNA COVID-19 vaccines), then any rigid mandate to vaccinate COVID-recovered individuals would be questionable legal and ethical standing, based on suspect medical necessity and even a potential for harm.
Our systematic review showed no clear evidence that vaccinated COVID-naïve individuals enjoyed greater protection than unvaccinated COVID-recovered counterparts. Four observational studies [20,24,25] and one RCT [19] found that natural immunity provided superior protection to vaccination in the COVID-naive. None of the identified studies, or pooled groups, found a statistically significant advantage to vaccination in the COVID-naïve population, although the RCTs favoured vaccination. There was solid internal consistency between the conclusions of our pooled analysis and the conclusions of the studies' authors, taken individually.
While RCTs are more methodologically sound, observational studies yield a more practically realizable result. In our study, pooled RCTs favoured vaccination, while the pooled observational studies more strongly supported natural immunity. This disparity can be partially explained by the difference in timing of the studies, as the RCTs were performed in an earlier phase of the pandemic compared to the observational trials which occurred during the emergence of variants. For example, Satwik et al. (India) and Gazit et al. (Israel), which occurred during the mid-2021 Delta phases of the pandemic, found stronger and statistically significant natural immunity benefits than vaccination alone. Gazit et al. found 13-fold increased odds of all infection in the vaccinated COVID-naive compared to unvaccinated COVID-recovered individuals and a 27-fold increase in symptomatic infection. These more recent and stronger findings of stronger natural immunity highlight the time-dependent sensitivity of this analysis due to variants of concern and potential waning vaccination efficacy. This observation is in line with other recent studies by Keehner et al. [29] and Goldberg et al. [30], demonstrating a time-dependent decline in vaccine efficacy during the pandemic's Delta phase of the pandemic.
In total, the evidence points quite convincingly to at least the equivalency between the protection of natural versus vaccinated immunity, with the possibility of enhanced durability of protection from natural immunity in non-controlled settings and later phases of the pandemic.
Does vaccination in the previously infected provide a reduction in risk of reinfection?
Regarding this question, the vaccination RCTs did not find a significant benefit to vaccination in the previously infected, either individually or in pooled RCT analysis. This conclusion is reflected in the official briefing narratives, which explicitly stated an absence of observed benefit of vaccination in recovered individuals, due primarily to the limitation of study power. However, in the pooled observational trials, the stronger relative effect was seen favouring vaccination, but the absolute effect was still small. Overall, the total pooled results demonstrated a statistically significant 1.86x enhanced protection by vaccination in COVID-recovered persons, which generally agrees with Gazit et al. (1/.53=1.89x) and Cavanaugh et al. (2.34x), the latter of which was not included in the pooled analysis. Generally, we can conclude that vaccination in the COVID-recovered roughly halves the risk of reinfection, based on our pooled results and individual studies. These studies were relatively short in their follow-up period (<3 months), and therefore longer-term efficacy (>6 months) remains to be seen.
However, on an absolute basis, the risk reduction is relatively modest. This is most tangibly seen in our pooled NNT analysis, where 218 recovered individuals would need to be vaccinated to prevent one case of COVID annually, compared to only 6.5 COVID-naïve individuals. This represents a 33.5-fold difference in the absolute effect size between COVID-naïve and COVID-recovered individuals.
This disparity in NNT highlights the muted absolute benefit of vaccination to COVID-recovered individuals, compared to that enjoyed by COVID-naïve individuals. While our systematic review did not specifically cover the risk of vaccination, recent studies have shown that vaccinations have a small but excess risk of adverse events appears in the range of 2-80 events per 100,000 [31]. There are also some reports, though no consensus, that previously infected individuals may have an increased risk of local and systemic adverse effects [32]. Therefore, while vaccination is overwhelmingly safe for the general population, and even for most COVID-recovered individuals, higher-risk subgroups are subject to a distinctly different risk/benefit calculus and narrower therapeutic window, suggesting that individual factors with clinical equipoise should be utilized. Further evaluation of adverse events specifically within COVID-recovered individuals is warranted, as is a formal evaluation of the risk/benefit calculus. Civil policies, including vaccine mandates, should strongly consider automatic exemption from vaccination based on a history of prior infection or serological evidence of immunity until the risk/benefit is better delineated.
Strengths and Limitations
This systematic review has several strengths: It uses the available literature to address timely questions about vaccination policy. Our systematic review standardizes the metrics of vaccine and natural immunity by reporting both relative and absolute measures to provide a broader perspective on policy implications. Using inclusive criteria, including FDA briefings and the MedRxIv pre-print server, it aggregated all possible evidence on the subject current to the time in a rapidly evolving setting. Our strategy of stratifying the pooling of RCTs, observational studies, and then in total pools via M-H methods also allows for assessing the internal consistency of our conclusions. Each study is also presented individually to provide some internal validation to the overall pooled results.
However, this systematic review has its limitations. First and foremost, the question of vaccine efficacy in recovered COVID individuals is rapidly evolving, with new data and studies continuously being published. Indeed, several of the studies included in this review are still in peer review [20,21,25]. However, we have objectively attempted to appraise the quality of each of these studies with the use of the established NOS rating scale. Moreover, after the defined search dates of this study, several newer studies (including updates to the RCTs) have been released with updated data to be included in a follow-on study. Second, we included results from PubMed, MedRxiv, and FDA/CDC briefings while excluding briefings from other international regulatory organizations and other public databases. This could lead to a selection bias in identified studies. Third, the comparability of these studies is not precise, with some variation in the case and endpoint definitions, follow-up periods, the pandemic phase, and other methodological variations (incidence calculation, statistical tests etc.). This can confound the direct comparison of results between studies and assumptions for pooling.
The use of M-H methods for pooling leads to an automated weighting mechanism that depends partly on the quality of the study effect and other factors. In the NPI/V vs PI/UV analysis, the RCTs were weighted 37.8% and 45.6% in risk ratio and risk difference analyses, respectively. However, in analysis PI/UV vs PI/V, the RCTs were weighted only 11.4% and 5.2%, respectively. These lower weights reflect that the RCTs had relatively small populations of COVID-recovered individuals getting placebo or vaccination, and therefore, the variances were considerably higher. As expected at six months, follow-up data from these RCTs may alter the variances and weighting. This is subject to further updated publication.
Finally, when considering pooling analyses, the question of study homogeneity needs to be considered. While the RCTs were relatively homogenous in their study design and methodology, the other observational and case-control studies had significant differences that warrant consideration. For example, Shrestha et al., Lumley et al., and Satwik et al. considered only HCWs in single health systems, whereas Goldberg et al. and Gazit et al. considered entire national populations. Satwik et al. appear to be somewhat of an outlier in the magnitude of its results (but not the trend of its conclusion) due to the rapid rise of Delta during the implementation of vaccination and the short follow-up in this group. This is a different scenario from Gazit et al., where a significant portion of the Israeli population was already fully vaccinated during the study period. Studies also differed in the difference in vaccination brand utilization, vaccination cadence (one versus two doses and interval), definitions of endpoints, inclusion and exclusion criteria, timing within the pandemic phases, and variants of concern. Despite these disparities, the observations we have made are remarkably consistent across the included studies and pools: that protection of vaccinated and natural immunity is at least roughly equivalent; and, while there may be some incremental protection to vaccination in COVID-recovered individuals, the absolute magnitude of that protection is dramatically lower compared to that experienced by COVID-naïve individuals.
Conclusions
Overall, our comprehensive systematic review identified nine clinical studies of various designs, of which seven could be included in a pooled analysis. From a review of these studies, we conclude that there is currently no statistical advantage to vaccination in the COVID-naive compared to natural immunity in the COVID-recovered. Vaccination in the COVID-recovered may provide some incremental protective benefit, but the total size of this benefit is marginal. Explicitly, COVID-naïve individuals should not seek infection to bypass vaccination, as the risks of infection far exceed the low risks associated with vaccination. However, until further data is available, unvaccinated COVID-recovered individuals should be considered to have at least equal protection to their vaccinated COVID-naïve counterparts. The COVID-recovered represent a unique population segment with distinct risk/benefit considerations and a narrower therapeutic window than their COVID-naïve counterparts. National policy should reflect the need for clinical equipoise and restraint in vaccinating these individuals by mandate.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. Polack FP, Thomas SJ, Kitchin N, et al. N Engl J Med. 2020;31:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. Baden LR, El Sahly HM, Essink B, et al. N Engl J Med. 2021;4:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. Sadoff J, Gray G, Vandebosch A, et al. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Your Vaccination. [ Sep; 2021 ];https://www.cdc.gov/coronavirus/2019-ncov/vaccines/your-vaccination.html 2021
- 5.COVID-19 false dichotomies and a comprehensive review of the evidence regarding public health, COVID-19 symptomatology, SARS-CoV-2 transmission, mask wearing, and reinfection. Escandón K, Rasmussen AL, Bogoch II, et al. BMC Infect Dis. 2021;27:710. doi: 10.1186/s12879-021-06357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Hall VJ, Foulkes S, Charlett A, et al. Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Abu-Raddad LJ, Chemaitelly H, Malek JA, et al. Clin Infect Dis. 2020;14:1846. doi: 10.1093/cid/ciaa1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suspected SARS-CoV-2 reinfections: incidence, predictors, and healthcare use among patients at 238 US Healthcare Facilities, 1 June 2020 to 28 February 2021 . Lawandi A, Warner S, Sun J, et al. Clin Infect Dis. 2021:671. doi: 10.1093/cid/ciab671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quantifying the risk of SARS-CoV-2 reinfection over time. O Murchu E, Byrne P, Carty PG, et al. Rev Med Virol. 2021;27:2260. doi: 10.1002/rmv.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. Hanrath AT, Payne BAI, Duncan CJA. J Infect. 2021;82:29–30. doi: 10.1016/j.jinf.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antibody status and incidence of SARS-CoV-2 infection in health care workers. Lumley SF, O'Donnell D, Stoesser NE, et al. N Engl J Med. 2021;11:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assessment of SARS-CoV-2 reinfection 1 year after primary infection in a population in Lombardy, Italy. Vitale J, Mumoli N, Clerici P, et al. JAMA Intern Med. 2021;28:212959. doi: 10.1001/jamainternmed.2021.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SEROCoV-POP study group. risk of reinfection after seroconversion to SARS-CoV- 2: a population-based propensity-score matched cohort study. Leidi A, Koegler F, Dumont R, et al. Clin Infect Dis. 2021;27:495. doi: 10.1093/cid/ciab495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Hansen CH, Michlmayr D, Gubbels SM, et al. Lancet. 2021;27:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinfection rates among patients who previously tested positive for COVID- 19: a retrospective cohort study. Sheehan MM, Reddy AJ, Rothberg MB. Clin Infect Dis. 2021;15:234. doi: 10.1093/cid/ciab234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serologic status and SARS-CoV-2 infection over 6 months of follow up in healthcare workers in Chicago: a cohort study. Wilkins JT, Hirschhorn LR, Gray EL, et al. Infect Control Hosp Epidemiol. 2021:1–9. doi: 10.1017/ice.2021.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaccines and related biological products advisory committee meeting, December 10, 2020; FDA briefing document; Pfizer-BioNTech COVID-19 vaccine. [ Sep; 2021 ];https://www.fda.gov/media/144245/download 2020
- 18.Vaccines and Related Biological Products Advisory Committee Meeting, December 17, 2020; FDA Briefing Document; Moderna COVID-19 Vaccine. [ Sep; 2021 ];https://www.fda.gov/media/144434/download 2020
- 19.Vaccines and Related Biological Products Advisory Committee Meeting, February 26, 2021 FDA Briefing Document; Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. [ Sep; 2021 ];https://www.fda.gov/media/146217/download 2021
- 20.Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: a three-month nationwide experience from Israel [Pre-Print] Goldberg Y, Mandel M, Woodbridge Y, et al. medRxiv. 2021 doi: 10.1093/aje/kwac060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Necessity of COVID-19 vaccination in previously infected individuals [Pre-Print] Shrestha NK, Burke PC, Nowacki AS, et al. medRxiv. 2021 [Google Scholar]
- 22.An observational cohort study on the incidence of SARS-CoV-2 infection and B.1.1.7 variant infection in healthcare workers by antibody and vaccination status. Lumley SF, Rodger G, Constantinides B, et al. Clin Infect Dis. 2021;3:608. doi: 10.1093/cid/ciab608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reduced Risk of Reinfection with SARS-CoV-2 After COVID-19 Vaccination — Kentucky, May-June 2021. Cavanaugh AM, Spicer KB, Thoroughman D, et al. MMWR. 2021;13:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ChAdOx1 nCoV-19 effectiveness during an unprecedented surge in SARS COV-2 infections. Satwik R, Satwik A, Katoch S, Saluja S. Eur J Intern Med. 2021 doi: 10.1016/j.ejim.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections [pre-Print] Gazit S, Shlezinger R, Perez G, et al. medRxiv. 2021 [Google Scholar]
- 26.Total Coronavirus Cases in Kentucky. [ Sep; 2021 ];https://www.worldometers.info/coronavirus/usa/Kentucky 2020
- 27.Genomic characterization and Epidemiology of an emerging SARS-CoV-2 variant in Delhi, India [Pre-Print] Dhar MS, Marwal R, Radhakrishnan VS, Ponnusamy K, Jolly B, Bhoyar RC, Fatihi S, et al. https://www.medrxiv.org/content/10.1101/2021.06.02.21258076v1. Medrxiv. 2021 doi: 10.1126/science.abj9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Sheikh A, McMenamin J, Taylor B, Robertson C. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. Keehner J, Horton LE, Binkin NJ, et al. N Engl J Med. 2021;385:1330–1332. doi: 10.1056/NEJMc2112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waning immunity of the BNT162b2 vaccine: a nationwide study from Israel [Pre-Print] Goldberg Y, Mandel M, Bar-On YM, et al. MedRxiv. 2021 doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. Barda N, Dagan N, Ben-Shlomo Y, et al. N Engl J Med. 2021;385:1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Menni C, Klaser K, May A, et al. Lancet Infect Dis. 2021;21:939–949. doi: 10.1016/S1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]