Abstract
There are 150 million people with diabetes worldwide who require insulin replacement therapy and the prevalence of diabetes is rising fastest in middle and low-income countries. Current formulations require costly refrigerated transport and storage to prevent loss of insulin integrity. This study shows the development of simple “drop-in” amphiphilic copolymer excipients to maintain formulation integrity, bioactivity, pharmacokinetics and pharmacodynamics for over 6 months when subjected to severe stressed aging conditions that cause current commercial formulation to fail in under 2 weeks. Further, when these copolymers are added to Humulin R (Eli Lilly) in original commercial packaging they prevent insulin aggregation for up to 4 days at 50 °C compared to less than 1 day for Humulin R alone. These copolymers demonstrate promise as simple formulation additives to increase the cold chain resilience of commercial insulin formulations, thereby expanding global access to these critical drugs for treatment of diabetes.
Keywords: Diabetes, Insulin, Global Access, Drug Development
INTRODUCTION
There are over 150 million people with diabetes requiring insulin replacement therapy worldwide.1 The worldwide population of people with insulin-deficient diabetes is increasing at a rate of 3–5% annually, with the highest increases in prevalence occurring in warm regions such as Africa, the Western Pacific, and the Middle East.1–2 Unfortunately, insulin is prone to irreversible aggregation when exposed to high temperatures and/or agitation and requires careful storage and refrigerated transport (the cold chain) to retain activity over its shelf life. Maintaining insulin integrity presents a challenge for the pharmaceutical industry, health care providers, and people with diabetes worldwide.3–4 Indeed, annual global costs for the refrigerated transport of all biopharmaceuticals globally exceed $15 billion (USD), and losses due to interruptions in the cold chain reach $35 billion (USD) annually.4
A primary driver of the loss of formulation integrity is the propensity of proteins to aggregate at hydrophobic interfaces when exposed to elevated temperatures.5 In the case of insulin formulations, agitation and elevated temperatures – conditions common to worldwide transport and cold chain interruptions – increase interactions between partially unfolded insulin monomers adsorbed to interfaces, leading to nucleation of insulin amyloid fibrils.6–9
Recent studies in insulin stabilization have relied on covalent or non-covalent attachment of hydrophilic polymers directly to insulin or through encapsulation of insulin or insulin crystals in hydrogels.10–15 While these strategies have successfully shielded insulin from interfacial adsorption or insulin-insulin interactions and increased insulin stability, they can also lead to increased absorption times and longer circulation times in vivo. A more translatable stabilization method would utilize an inactive excipient that could be incorporated into existing formulations without altering the drug pharmacokinetics.
Amphiphilic copolymers present an alternative to polymer-protein conjugation, exploiting their propensity to gather at the air-water interface to hinder insulin-interface interactions.6, 16–18 Poloxamers have been successfully used to improve insulin stability and have been employed in commercial formulations (Insuman U400, Sanofi-Aventis). Yet, poloxamers have not been widely adopted and have not succeeded in reducing reliance on the cold chain for insulin transport.
Previously we have shown that biocompatible and non-toxic acrylamide carrier/dopant copolymers (AC/DC), a class of amphiphilic copolymers comprising water-soluble “carrier” and hydrophobic “dopant” monomers, can enable the development of ultra-fast insulin formulations.19 We hypothesized that these excipients could be applied more broadly to improve the stability of current commercial insulin formulations in the context of reducing cold chain reliance and improving formulation resilience. In this work, we aim to understand the stabilization mechanism of AC/DC copolymer excipients and to test the limits of their stabilizing capacity long-term and under extreme environmental conditions. We report the ability of select copolymers to act as simple “drop-in” excipients to stabilize commercial insulin formulations (Humulin R, Eli Lilly) without altering their bioactivity, pharmacokinetics or pharmacodynamics, constituting an important step toward improving global access to these critical drugs.
EXPERIMENTAL METHODS
Materials
Humulin R (Eli Lilly) was purchased and used as received. Solvents N,N-dimethylformamide (DMF; HPLC Grade, Alfa Aeser, >99.7%), hexanes (Fisher, Certified ACS, >99.9%), ether (Sigma, Certified ACS, Anhydrous,>99%) and CDCl3 (Acros, >99.8%) were used as received. Monomers N-(3-methoxypropyl)acrylamide (MPAM; Sigma, 95%), 4-acryloylmorpholine (MORPH; Sigma, >97%) were filtered with basic alumina prior to use. Monomers N-phenylacrylamide (PHE; Sigma, 99%) and N-isopropylacrylamide (NIPAM; Sigma, >99%) were used as received. RAFT chain transfer agents 2-cyano-2-propyl dodecyl trithiocarbonate (2-CPDT; Strem Chemicals, >97%) and 4-((((2-carboxyethyl)thio)carbonothioyl)thio)-4-cyanopentanoic acid (BM1433; Boron Molecular, >95%) were used as received. Initiator 2,2’-azobis(2-methyl-propionitrile) (AIBN; Sigma, >98%) was recrystallized from methanol (MeOH; Fisher, HPLC Grade, >99.9%) and dried under vacuum before use. Z-group removing agents lauroyl peroxide (LPO; Sigma, 97%) and hydrogen peroxide (H2O2; Sigma, 30%) were used as received. Streptozotocin (99.58%) was purchased from MedChem Express. All other reagents were purchased from Sigma-Aldrich unless otherwise specified.
Surface Tension
Time resolved surface tension of the air-solution interface was measured with a Platinum/Iridium Wilhelmy plate connected to an electrobalance (KSV Nima, Finland). The Wilhelmy plate was partially immersed in the aqueous solution in a Petri dish, and the surface tension of the interface was recorded for 50 minutes from the formation of a fresh interface. Equilibrium surface tension values (t = 50 min) were reported as these values more closely describe the environment in a stored vial before agitation. Two replicates were taken and averaged.
Interfacial Rheology
Interfacial shear rheology was measured using the Discovery HR-3 rheometer (TA Instruments) with an interfacial geometry comprising of a Du Noüy ring made of Platinum/Iridium wire (CSC Scientific, Fairfax, VA, catalog No. 70542000). Before each experiment, the Du Noüy ring was rinsed with ethanol and water and flame treated to remove organic contaminants. The solution chamber consisted of a double-wall Couette flow cell with an internal Teflon cylinder and an external glass beaker. A time-sweep was performed with a strain of 1% (within the linear regime) and a frequency of 0.05 Hz (low enough for instrument inertia to not be significant). Interfacial complex shear viscosity was measured for 30 minutes. The experiment was repeated in triplicate.
Polymer Synthesis
Polymers were synthesized via reversible addition fragmentation transfer as described in previous literature. Detailed methods can be found in the supplementary information. Resulting composition and molecular weights were determined via1H NMR spectroscopy and SEC with poly(ethylene glycol) standards (Table S1).
In Vitro Insulin Stability Assay (Accelerated Aging)
50 μL of AC/DC excipient (MoNi23%, MpPhe8%, MoPhe6%) in milli-Q water (2.1, 21, or 105 mg/mL) or 50 μL of milli-Q water was added to 1 mL of Humulin R (Eli Lilly 100U) in a glass autosampler vial (J.G. Finneran, 2.0mL Clear R.A.M.™ Large Opening Vial, 12 × 32mm, 9mm Thread) and capped, yielding 95U Humulin either as a control or formulated with 0.01, 0.1, or 0.5 wt.% AC/DC excipient. These vials were incubated at 37 °C and agitated at 150 RPM for 2, 4, and 6 months (in addition, Humulin only control was agitated at 2 weeks and 1 month). The preparation of formulations was staggered so that all samples reached their endpoint age at the same time. Vials were refrigerated until testing upon reaching selected aging timepoint. Following initial transmittance experiments, all further experiments were done with formulations with 0.01 wt.% AC/DC excipient to minimize copolymer concentration. In addition, 500 μL of 2.1 mg/mL MoNi23% or milli-Q water were added to 10 mL of unadulterated Humulin (Eli Lilly 100U) in its commercial vial to generate 95U Humulin control and formulation with 0.01 wt.% AC/DC excipient. These vials were placed in the original individual packaging boxes with the instruction papers. These packages were incubated at either 37 °C or 50 °C until significant opacity change. 300–400 μL aliquots were removed every 24 hours for the first 7 days and refrigerated. Following that, intermittent aliquots were taken to conserve volume. Every 24 hours, the bottoms of the vials were photographed to track the change in opacity. Methods for aggregation assays for recombinant human insulin were adapted from Webber et al.11 Formulation samples were plated at 150 μL per well in a clear 96-well plate and an absorbance reading was taken at 540 nm (BioTek Synergy H1 microplate reader). The aggregation of insulin leads to light scattering, which results in an increase in the measured absorbance. The time-to-aggregation (tA) was defined as the timepoint when a 10% increase in transmittance from time zero was observed.
Circular dichroism
Circular dichroism was used to validate that aging with AC/DC excipients does not result in changes to the secondary structure of insulin. Aged Humulin (0.5, 1, 2, 4, and 6 months) or Humulin aged with 0.01 wt.% AC/DC excipients (2, 4, and 6 months) were evaluated against an unaged Humulin control or unaged Humulin with 0.01 wt.% AC/DC excipient. Formulation samples were diluted to 0.2 mg/mL in PBS (pH=7.4). Samples were left to equilibrate for 15 minutes at room temperature before measurement. Near-UV circular dichroism spectroscopy was performed at 20 °C with a J-815 CD Spectropolarimeter (Jasco Corporation) over a wavelength range of 200–260 nm using a 0.1 cm pathlength cell.
In vitro insulin cellular activity assay
In vitro insulin activity was tested using the AKT phosphorylation pathway using AlphaLISA SureFire Ultra (Perkin-Elmer) kits for detection of phosphorylated AKT 1/2/3 (pS473) compared to total Akt1. Humulin, Aged Humulin (t = 6 months), Humulin + MoNi23%, and Aged Humulin + MoNi23% (t = 6 months) formulations were tested. Methods have been previously described elsewhere.13–14 Detailed methods can be found in the supplemental information. Results were plotted as a ratio of [pAKT]/[AKT] for each sample (n=3 cellular replicates) and an EC50 regression (log(agonist) vs. response (three parameters)) was plotted using GraphPad Prism 8.
In vitro cytotoxicity of AC/DC excipients
NIH 3T3 culture: NIH 3T3s were cultured according to ATCC’s recommendations. Media consisted of Dulbecco’s Modified Eagle Medium, 10% FBS and 1% Penicillin-Streptomycin. Cells were split at an approximate ratio of 1:4 every 3–4 days. Promega CellTiter-Glo 3D Cell Viability Assay was used to characterize the short-term cell viability in different formulation conditions. Cells were seeded at 10,000 cells per well in an opaque 96 well plate in 100 uL of media containing polymers of interest. MoNi23%, MPPhe8% and MoPhe6% polymers were added to media at concentrations of 10, 5, 2.5, 1, 0.5, and 0.1 mg/mL. Four wells were used as replicates for each polymer concentration. Relative viability was measured after 1 day in culture by adding 100 uL per well of the CellTiter-Glo reagent, mixing for 5 minutes, allowing the plate to sit for 25 minutes, and then reading the luminescent signal with a 1 second integration time. LC50 values were determined for each polymer by plotting the absolute signal for each of the four replicates in GraphPad Prism 9 and using the [Agonist] vs. Response – Find ECanything nonlinear fit. Fit parameter F was constrained to 50, Bottom was constrained to 4 (the negative control for the assay) and the Top was constrained to be the same for all data sets (cell viability should be equal for all data sets as polymer concentration approaches 0).
Streptozotocin (STZ) induced model of diabetes in rats
Male Sprague Dawley rats (Charles River) were used for experiments. Animal studies were performed in accordance with the guidelines for the care and use of laboratory animals; all protocols were approved by the Stanford Institutional Animal Care and Use Committee. The protocol used for STZ induction adapted from the protocol by Kenneth K. Wu and Youming Huan,20 and have been previously described.13, 21 Detailed methods are included in the supplemental information. Diabetes was defined as having 3 consecutive blood glucose measurements >300 mg/dL in non-fasted rats.
In vivo pharmacodynamics in diabetic rats
Diabetic rats were fasted for 4–6 hours. For initial blood glucose studies rats were injected subcutaneously (1.5U/kg) with the following formulations: (i) Humulin, or (ii) Humulin with 0.01 wt.%AC/DC excipient (MoNi23%, MpPhe8%, MoPhe6%). Humulin formulations were tested at 6 aging time points of 0, 0.5, 1, 2, 4, and 6 months, and Humulin with AC/DC excipient was tested at 0, 2, 4, and 6 months of aging. The preparation of formulations was staggered so that all samples reached their endpoint age at the same time and all aging timepoints could be compared in the same cohort of rats. 32 rats with fasting glucose levels >300 mg/dL were randomized to a formulation group (8 rats/group) and each rat received that formulation at all levels of aging (the order of the aging timepoints rats received was also randomized). For blood glucose studies after formulation aging at 50 °C, rats were injected subcutaneously (1.5U/kg) with the following formulations: (i) Humulin, (ii) Aged Humulin (t = 1 day), (iii) Humulin + MoNi23%, or (iv) Aged Humulin + MoNi23% (t = 4 days). 16 Rats with fasting glucose levels >300 mg/dL were randomized to either the Humulin control group or the MoNi23% group. Within both groups, the order that the aged formulations were given was also randomized and formulations were administered on separate experimental days. Before injection, baseline blood glucose was measured. After injection, blood was sampled every 30 minutes for 5 hours. Blood glucose was measured using a handheld blood glucose monitor. The maximum change in blood glucose measured from baseline was used as a metric of bioactivity of each formulation to assess in vivo bioactivity after aging.
In vivo pharmacokinetics in diabetic rats
Diabetic rats were fasted for 4–6 hours. For pharmacokinetic studies rats were injected subcutaneously (1.5U/kg) with the following formulations: (i) Humulin, (ii) Aged Humulin (t = 6 months), (iii) Humulin + MoNi23%, or (iv) Aged Humulin + MoNi23% (t = 6 months). 16 diabetic rats were randomized to a formulation group: Humulin or Humulin + MoNi23% (8 rats/group). Within each group, rats received both the fresh (t = 0 months) or aged (t = 6 months) formulations in a randomized order. After subcutaneous injection, blood was sampled every 15 minutes for 2 hours and blood was collected in serum tubes (Sarstedt) for analysis with ELISA. Serum insulin concentrations were quantified using a Human Insulin ELISA kit (Mercodia).
Statistics
All data is shown as mean ± standard error unless specified. For the in vitro activity assay (AKT) EC50 regression (log(agonist) vs. response (three parameters)) was plotted using GraphPad Prism 8. GraphPad Prism 8 Extra sum-of-squares F-test was used to test if Log(EC50) differed between datasets. Data sets were compared in pairs, and Bonferroni post-hoc tests were used to adjust for multiple comparisons (alpha=0.008). For blood glucose measurements, a REML repeated measures mixed model was used to test for differences at different aging timepoints within a formulation (JMP Pro 14). Rat was included as a random effect and the age of the formulation as a within-subject fixed effect. A post-hoc Tukey HSD test was used on Humulin formulations to determine statistical significance between aging timepoints.
RESULTS AND DISCUSSION
AC/DC excipient insulin stabilizing mechanism
We selected our three top performing candidates from a previous screen of amphiphilic AC/DC excipients for their ability to stabilize monomeric insulin.19 These excipients were composed of either acryloylmorpholine (Mo) or methoxypropylacrylamide (Mp) as a hydrophilic carrier monomer copolymerized with either N-Isopropylacrylamide (Ni) or phenylacrylamide (Phe) as a hydrophobic dopant monomer. This excipient design is hypothesized to preferentially occupy the air-water interface and consequently inhibit insulin-insulin interactions occurring at these interfaces (Figure 1). We sought to evaluate this hypothesis through time-resolved surface tension and interfacial rheology experiments with a model AC/DC excipient, poly(acryloylmorpholine77%-co-N-Isopropylacrylamide23%) (MoNi23%), co-formulated with commercial Humulin R (Eli Lilly) (Figure 2).
Figure 1. Scheme of the cold chain and insulin aggregation mechanism.
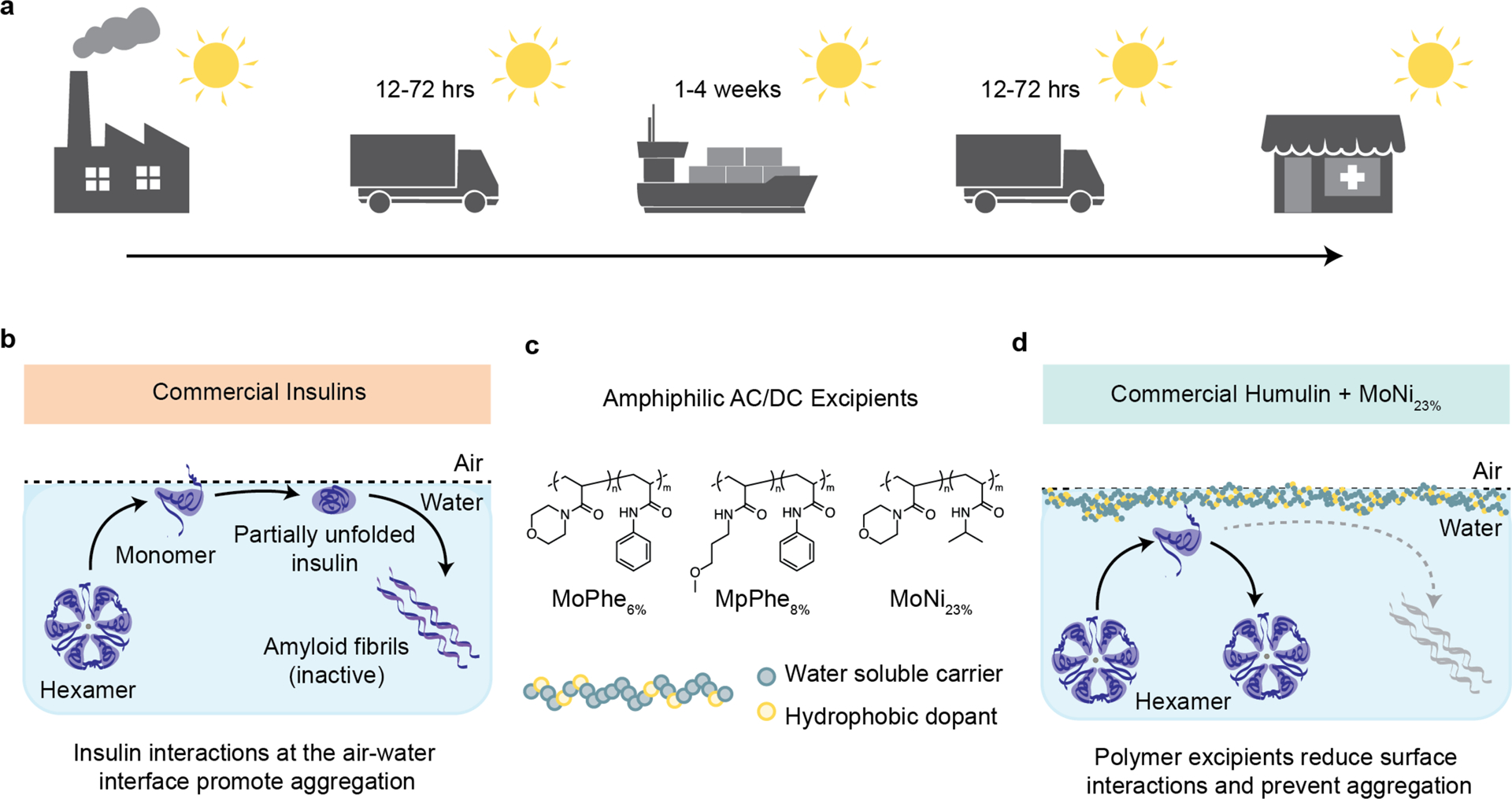
a, To maintain integrity, commercial insulin formulations must currently be transported and stored in refrigerated containers for the weeks-long duration of worldwide distribution. b, Aggregation mechanism of commercial insulin formulations. The insulin hexamer is at equilibrium with monomers in formulation. These monomers interact at the interface, where the exposure of hydrophobic domains during insulin-insulin interaction nucleate amyloid fiber formation. c, Chemical structure of AC/DC excipients, poly(acryloylmorpholine77%-co-N-isopropylacrylamide23%) (MoNi23%). AC/DC excipients have a molecular weight between 2–5 kDa (See Table S1). d, AC/DC excipients are amphiphilic copolymers that adsorb to interfaces, reducing insulin-insulin interactions and delaying the nucleation of insulin amyloidosis.
Figure 2: Experimental insight into the mechanism of AC/DC excipient stabilization.
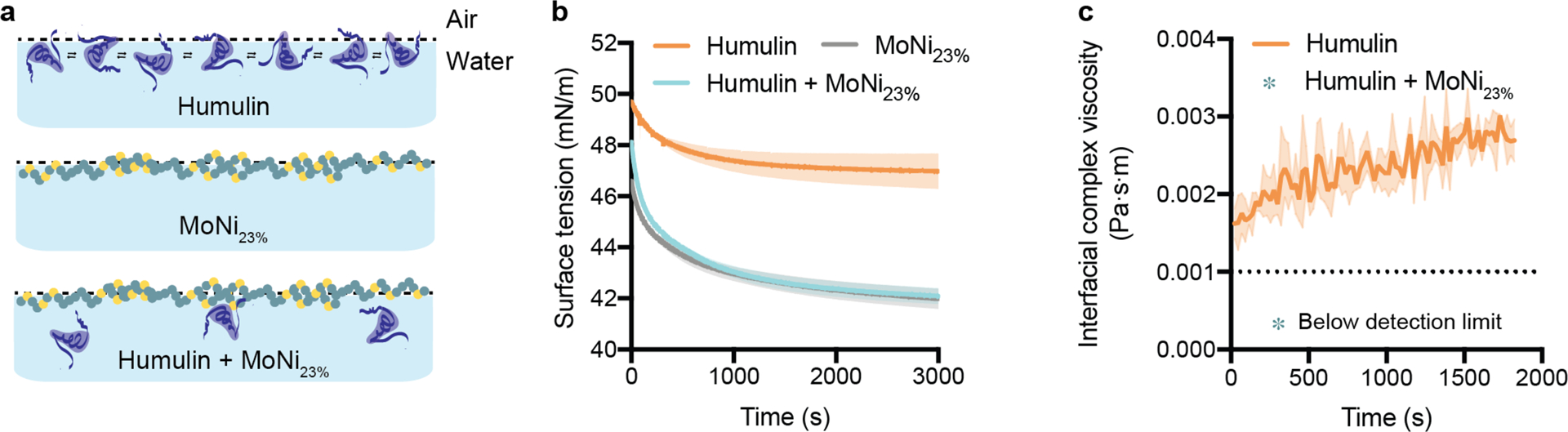
a, Illustration of proposed stabilization mechanism: (i) In commercial Humulin, monomers at the interface have associative interactions. (ii) Alone, MoNi23% occupies the interface without the presence of insulin. (iii) In combination with Humulin formulations, MoNi23% disrupts insulin-insulin surface interactions, providing a mechanism for inhibiting aggregation. b, Surface tension measurements of Humulin, MoNi23% (0.01 wt.%) formulated with formulation excipients, and Humulin formulated with MoNi23% (0.01 wt.%) (n=2). c, Interfacial rheology measurements of Humulin. Measurements for Humulin formulated with MoNi23% (0.01 wt.%) fell below the resolution of the instrument, indicating that there is no protein aggregation at the interface (n=3).
Equilibrium surface tension measurements of Humulin R, Humulin R containing MoNi23% (0.01 wt.%), and a solution of MoNi23% (0.01 wt.%) containing the same formulation excipients (i.e., Humulin R without the insulin) revealed that the presence of MoNi23% resulted in surface tension values well below Humulin R (approximately 42 vs. 47 mN/m, Figure 2b). Moreover, a ten-fold increase in the MoNi23% concentration (0.1 wt.%) further reduced the surface tension of the formulation (Supplementary Figure 1A). The decrease in surface tension upon addition of MoNi23% to Humulin indicates that there are more species at the interface when MoNi23% and Humulin are formulated together, compared to Humulin alone. The decreased surface tension concomitant with the increased concentration of MoNi23% in the absence of Humulin indicates that the surface is not saturated at 0.01 wt.% MoNi23%. However, the surface tension is identical for formulations of Humulin and MoNi23% and MoNi23% with formulation excipients, indicating that there are similar number of molecular species at the interface regardless of the presence of Humulin. Further, the addition of completely hydrophilic poly(acryloylmorpholine) (Mo) to Humulin did not lower the surface tension, indicating that the amphiphilic copolymer is required to displace insulin (Supplementary Figure 1B). Together, these surface tension experiments help demonstrate that MoNi23% preferentially adsorbs and dominates the air-water interface.22
Interfacial shear rheology measurements demonstrated that addition of MoNi23% (0.01 wt.%) to Humulin R reduced interfacial complex viscosity to below the detection limit of the instrument compared to Humulin R, which exhibited values between 0.002 – 0.003 Pa·s·m (Figure 2c). The complex viscosity of Humulin is indicative of associative insulin-insulin interactions that can dissipate viscous energy at the interface. While not quantitative, the lowering of the interfacial complex viscosity below instrument detection limits is indicative that the addition of MoNi23% disrupts insulin-insulin interactions at the interface.
When this complex interface is subjected to interfacial stresses and agitation, it is likely that these insulin-insulin associations can nucleate amyloid fibril formation and lead to aggregation. Together, the surface tension and interfacial rheology experiments suggest a mechanism of AC/DC enhanced insulin stabilization where preferential adsorption of the AC/DC excipient to the air-water interface disrupts insulin-insulin interactions.
AC/DC excipients for long-term stability of insulin
We then sought to evaluate the capacity of our three previous top performing AC/DC excipients, MoNi23%, poly(acryloylmorpholine94%-co-phenylacrylamide6%) (MoPhe6%) and poly(methoxypropylacrylamide92%-co-phenylacrylamide8%) (MpPhe8%) to act as simple “drop-in” excipients to stabilize Humulin R through stressed aging. Formulations of Humulin alone or Humulin with an AC/DC excipient added were prepared and aged for 0, 2, 4, or 6 months at 37 °C with constant agitation (150 rpm on an orbital shaker plate). The preparation of formulations was staggered so that all samples reached their endpoint age at the same time. Both visual inspection and a transmittance assays were used to determine if the insulin had aggregated (Figure 3).11, 13 Insulin aggregates scatter light, and thus aggregation can be defined as a change in transmittance greater than 10%.11, 13 Humulin alone began to aggregate after 2 weeks of stressed aging. In contrast, all insulin formulations containing AC/DC excipients MoPhe6%, MpPhe8%, and MoNi23% at concentrations of 0.01, 0.1 or 0.5 wt.% did not show any signs of insulin aggregation over the course of the 6-month study, with the exception of MpPhe8% at 0.5 wt.% (Figure 3b, Supplementary Figure 2). Thus, to minimize the amount of copolymer excipient in formulation, only the 0.01 wt.% formulations were used for the rest of the studies reported here.
Figure 3: Formulation with AC/DC copolymers stabilizes insulin.
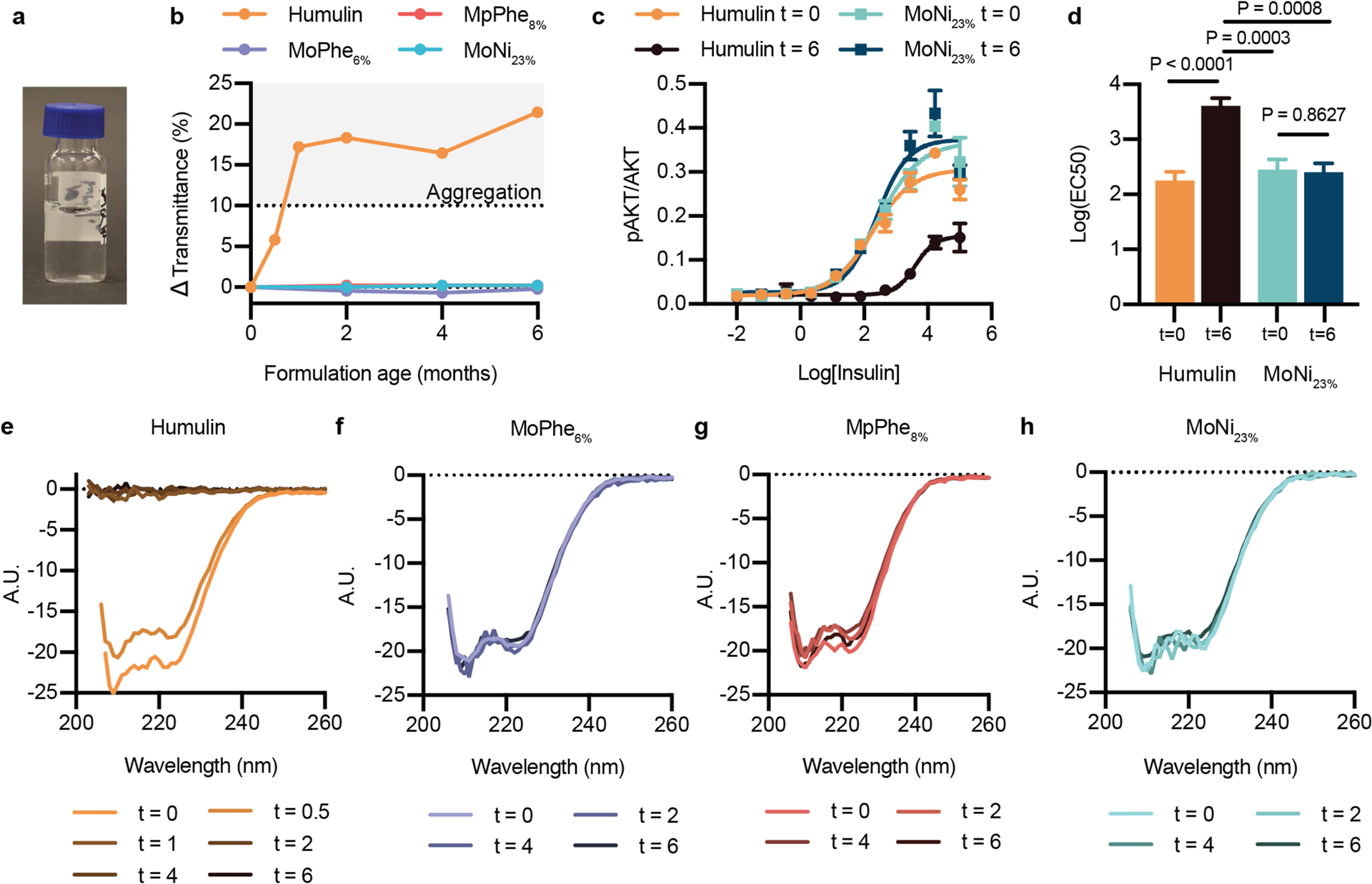
a, 1mL of Commercial Humulin or Humulin with the addition of AC/DC excipients (i) MoPhe6%, (ii) MpPhe8%, (iii) MoNi23% were aliquoted into 2mL glass vials and aged at 37°C with constant agitation (150 rpm) for 0, 2, 4, and 6 months. Additional 2 week and 1 month timepoints were added for the Humulin control. All formulations were at a concentration of 95U/mL (diluted so that copolymers could be added to commercial Humulin). b, Transmittance assay to assess the aggregation of proteins in formulation over time by monitoring changes in transmittance at 540nm (n=1 per formulation timepoint). c, In vitro activity by assaying for phosphorylation of Ser473 on AKT after stimulation with either Humulin or MoNi23% at 0 month and 6 month timepoints. Insulin concentrations are shown as Log(ng/mL). d, Log(EC50) values for each formulation. Statistical significance was assessed using the Extra sum-of-squares F-test to determine if Log(EC50) differed between datasets. Data sets were compared in pairs, and Bonferroni post-hoc tests were used to adjust for multiple comparisons (alpha=0.008). e-h, Circular dichroism spectra from 200–260nm for each formulation (diluted to 0.2 mg/mL in PBS) at each time point. c, Results shown are mean ± s.e plotted as a ratio of [pAKT]/[AKT] for each sample (n=3 cellular replicates) and an EC50 regression (log(agonist) vs. response (three parameters)) was plotted using GraphPad Prism 8. d, Statistical significance was assessed using the Extra sum-of-squares F-test to determine if Log(EC50) differed between datasets. Data sets were compared in pairs, and Bonferroni post-hoc tests were used to adjust for multiple comparisons (alpha=0.008).
To further validate the transmittance results, which only assess insulin aggregation, we evaluated in vitro activity by assaying for phosphorylation of Ser473 on protein kinase B (AKT) after stimulating C2C12 cells with either Humulin or Humulin containing MoNi23% (0.01 wt.%) at both the 0 month and 6 month timepoints (Figure 3c–d). Fresh formulations and the aged Humulin+MoNi23% formulation showed equivalent bioactivity (Humulint=0 Log(EC50) = 2.252 ± 0.158; MoNi23%,t=0 Log(EC50) = 2.448 ± 0.186; MoNi23%,t=6 Log(EC50) = 2.405 ± 0.158), whereas aged Humulin R exhibited almost complete loss of bioactivity (Humulint=6 Log(EC50) = 3.606 ± 0.139) (Figure 3c–d).
While these in vitro AKT assay results supported the transmittance data, we sought to further confirm insulin formulation integrity by using circular dichroism to observe insulin secondary structure for each formulation timepoint (Figure 3e–h). Formulations stabilized with the AC/DC excipients exhibited no changes in secondary structure after stressed aging, whereas Humulin alone had lost all structural features by 1 month. These data corroborate both the transmittance and in vitro activity data.
Bioactivity of aged insulin in diabetic rats
To evaluate the integrity of the aged insulin formulations in a functional setting in vivo, we assessed formulation activity in diabetic rats. Administration of streptozotocin was used to induce insulin-dependent diabetes in a cohort of 32 male rats. These rats were randomly assigned to one of four formulation groups: (i) Humulin, or Humulin comprising either (ii) MoPhe6%, (iii) MpPhe8%, or (iv) MoNi23% at 0.01 wt.%, and each rat received that formulation at each aging timepoint (0, 2, 4, 6 months). The preparation of formulations was staggered so that all samples reached their endpoint age at the same time and all aging timepoints could be compared in the same cohort of rats. Insulin was administered subcutaneously in fasted rats (1.5U/kg) and blood glucose levels were measured every 30 minutes. Active formulations resulted in a distinct initial drop in blood glucose from extreme hyperglycemia that reached a minimum in the range of normoglycemia between 60–100 minutes after administration (Figure 4, Supplementary Figure 3). After this phase, blood glucose levels began to rise as insulin was cleared. In contrast, formulations that appeared aggregated in the in vitro transmittance assays following aging did not show this distinct reduction in glucose reminiscent of insulin action and instead resulted in a gradual decrease in glucose levels. The gradual decrease in glucose may suggest that some of the insulin is initially trapped in reversible aggregates, and over time these aggregates dissociate and result in a slow-acting insulin effect. The maximum difference in blood glucose from baseline to the minimum glucose levels was plotted for each formulation as a measure of formulation potency. All copolymer-stabilized formulations showed no difference in activity between aging timepoints, but Humulin alone demonstrated a large difference between aging timepoints (F3,21=23.83, P<0.0001), where a post-hoc Tukey HSD test revealed that Humulin after 2, 4, and 6 months of aging had decreased activity compared to fresh Humulin (t=0 months). These observations were corroborated by evaluation of insulin pharmacokinetics, where no differences were observed between fresh Humulin R (t=0 months) and Humulin+MoNi23% initially (t=0 months) and after 6 months of aging, but a decrease in exposure was observed for the aged Humulin (t=6 months) (Figure 4f, Supplementary Figure 4). These data suggest that AC/DC excipients function as stabilizing ingredients for commercial formulations such as Humulin R without altering the insulin pharmacokinetics or pharmacodynamics.
Figure 4: Insulin activity after aging in diabetic rats.
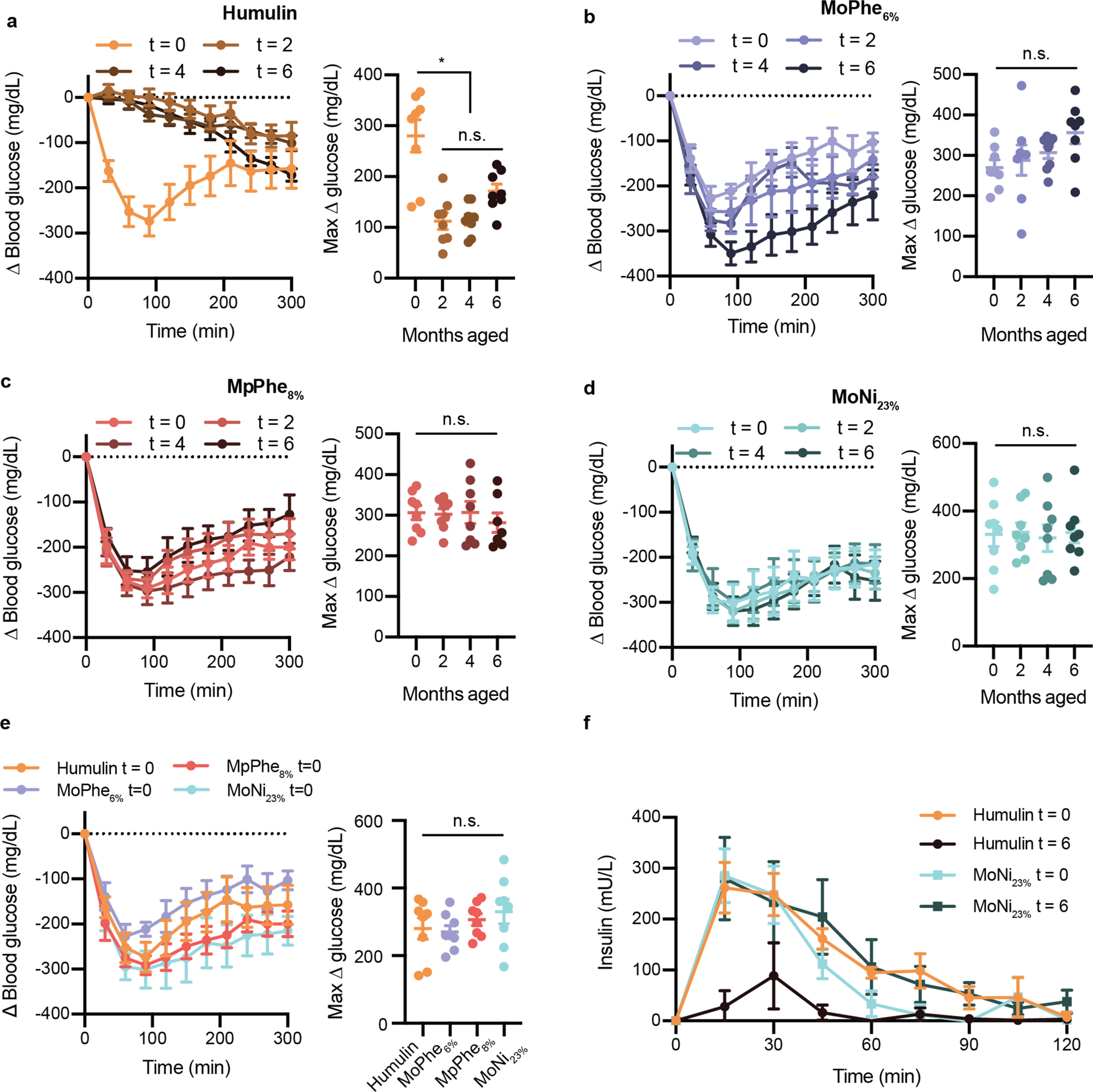
Fasted diabetic male rats received subcutaneous administration (1.5U/kg) of each insulin formulation a, Humulin b, Humulin with MoPhe6%, c, Humulin with MpPhe8%, or d, Humulin with MoNi23% at each aging timepoint (0, 2, 4, 6 months). e, Comparison of each formulation a t=0 months. In these assays, 32 rats were randomly assigned to one of the four formulation groups (n=8) and each rat received one dose of the formulation at each aging timepoint in a random order. Blood glucose levels were measured every 30 minutes using a handheld glucose monitor and the change in blood glucose relative to baseline glucose measurements were plotted. Baseline glucose measurements ranged from 300–600 mg/dL (See Supplementary Figure 5 for raw glucose curves). The maximum difference in glucose from baseline (Δ glucose) was also plotted for each formulation as a measure of formulation potency. f, Pharmacokinetics of Humulin and Humulin with MoNi23% at t=0 and t=6 months. All data is shown as mean ± s.e. Statistical significance between max Δ glucose was assessed using a REML repeated measures mixed model with rat as a random effect and the age of the formulation as a within-subject fixed effect. A post-hoc Tukey HSD test was used on Humulin formulations to determine statistical significance between aging timepoints.
High temperature aging of insulin formulations
To determine the capacity of AC/DC excipients to improve insulin cold-chain resilience, we evaluated the extent of stability imbued by one of our excipients, MoNi23%, under extreme manufacturing and distribution conditions (37 °C and 50 °C with constant agitation) (Figure 5). MoNi23% was selected for further testing following cytotoxicity tests that revealed MoNi23% and MoPhe6% had more favorable LC50 values compared to MPPhe8% (Supplementary Figure 6). These results, combined with previous success using MoNi23% to stabilize monomeric insulin,19 led us to choose to advance MoNi23% through the extreme aging tests. Temperatures were selected to represent the temperature on a hot summer day (37 °C), and the upper temperature range that a shipping container or truck without refrigeration or insulation could reach during the peak of summer (50 °C).23 Humulin R can be purchased in 10mL glass vials that are packaged and shipped in cardboard boxes (Figure 5a, Supplementary Figure 7). MoNi23 (0.01 wt.%) was added to new vials of Humulin R using a syringe (dilution from 100 U/mL to 95U/mL to allow addition of copolymer; control vial was diluted with water) and the vials were then replaced in the original cardboard packaging with the package insert (Supplementary Figure 7a). The cardboard packaging was affixed to a rotary shaker inside a temperature-controlled incubator and agitated at 150 RPM (Supplementary Figure 7b).
Figure 5: Stressed aging in commercial packaging.
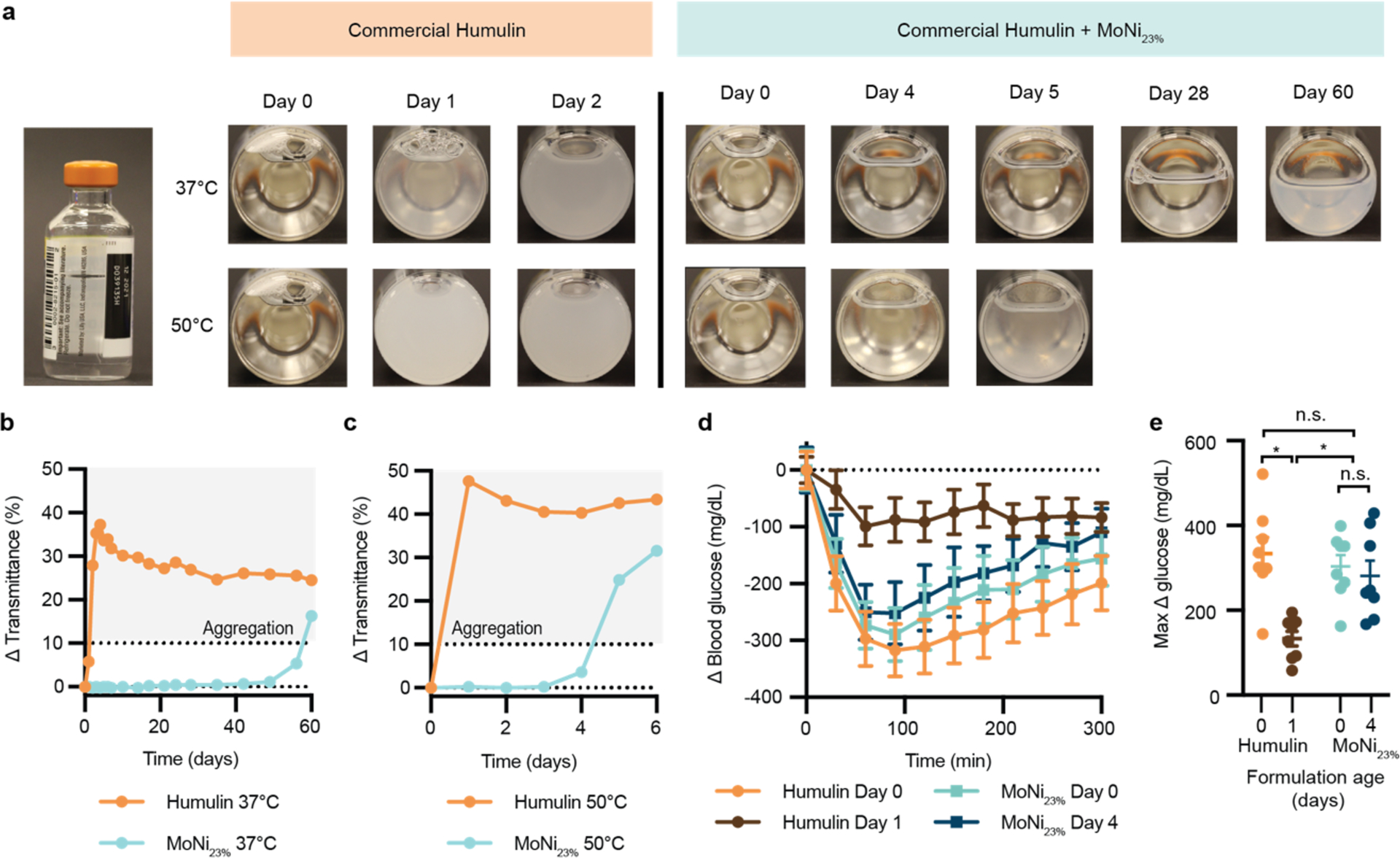
a, Humulin is often sold in standardized 10mL glass vials and packaged in cardboard boxes. Here we tested the stabilizing capacity of AC/DC copolymers in commercial packaging conditions under stressed conditions. 10mL vials of U100 Humulin R were diluted to 95 U/mL with the addition of 50 uL of a MoNi23% stock solution (to a final concentration of 0.01 wt.% copolymer) or water (control). Dilution was necessary to add copolymer to the formulation. These vials were replaced in their original boxes and taped to a shaker plate (150 rpm) in a 37 °C (n=1 per formulation) or 50 °C (n=1 per formulation) incubator. Samples were observed and imaged daily. b,c, Transmittance assays for Humulin or Humulin comprising MoNi23% after aging at (b) 37 °C and (c) 50 °C. Single samples (n=1) were tested for each transmittance curve. d, Blood glucose curves and e, maximum change in blood glucose (Δ glucose) in fasted diabetic rats for samples aged at 50 °C. Data is shown as mean ± s.e. Statistical significance between max Δ glucose was assessed using a REML repeated measures mixed model with rat as a random effect and the age of the formulation as a within-subject fixed effect. A post-hoc Tukey HSD test was used to determine statistical significance between aging timepoints and groups.
Visual inspection combined with a transmittance assay were used as the primary measures of insulin integrity (Figure 5a). These assays are consistent with our earlier experiments that demonstrated that the transmittance readings correlate well with both in vitro and in vivo functional activity assays. At 37 °C, Humulin alone begins to show visual changes in opacity at day 1 and becomes fully opaque by day 2. In contrast, when formulated with MoNi23%, the insulin formulation shows no visual changes in opacity until day 56 and remains below a 10% change in transmittance for 56 days. At 50 °C, commercial Humulin became fully opaque within one day. In contrast, formulation with MoNi23% extends stability under these extreme conditions to past 4 days before the formulation becomes cloudy on day 5. These qualitative observations were consistent with quantitative transmittance readings (Figure 5b–c).
To verify functional insulin activity after aging at 50 °C in vivo, these formulations were evaluated in diabetic rats. The ability of (i) Humulin (t = 0 day), (ii) aged Humulin (t = 1 day), (iii) Humulin with MoNi23% (t = 0 day), or (iv) aged Humulin with MoNi23% (t = 4 days) to decrease glucose levels was measured in fasted diabetic rats. After subcutaneous administration of formulations (1.5U/kg), blood glucose levels were measured every 30 minutes (Figure 5d). Humulin (t = 0 day), Humulin with MoNi23% (t = 0 day), and aged Humulin with MoNi23% (t = 4 day) demonstrated an initial blood glucose drop that reached a minimum between 60–100 minutes after injection. These results were consistent with active formulations in earlier experiments. This characteristic glucose drop was absent in rats who received aged Humulin (t = 1 day), consistent with inactive formulations in earlier experiments. The maximum difference in glucose from baseline was also plotted for each formulation as a metric of formulation potency (Figure 5e).
Statistical analysis identified a difference between the potency of these formulations (F3,18.18=10.71, P=0.0003), whereby a post-hoc Tukey HSD test revealed that aged Humulin alone had significantly decreased activity compared to the other formulations. In contrast, there was no statistical difference between unaged Humulin, unaged Humulin with MoNi23%, as well as aged Humulin with MoNi23% after stressed aging at 50 °C for 4 days.
CONCLUSIONS
In this study, we report on the application of AC/DC excipients to imbue long term stability and cold chain resilience to commercial insulin formulations. While commercial insulin formulations have good shelf lives when stored properly, interruptions in the cold chain can decrease insulin bioactivity and formulation integrity. The primary mechanism of insulin destabilization is through insulin adsorption at the air water interface, where insulin-insulin interactions promote amyloid fibril nucleation.6–7, 9 We hypothesized that amphiphilic copolymer excipients could potentially displace insulin from the interfacial region, and thus improve stability by reducing the probability of nucleation of insulin-insulin aggregation events at the interface. Surface tension and interfacial rheology data on our top-performing excipient MoNi23% support this hypothesis by showing that these copolymers preferentially adsorb to the air-water interface and disrupt insulin-insulin interactions. No thermo-responsive behavior was observed by the MoNi23% excipient at the temperatures tested in this study (Figure S8), thus disruption of surface interactions remains the most likely mechanism for stability.
When used as simple, low concentration (0.01 wt.%) formulation additives, AC/DC excipients preserve insulin activity through 6 months of stressed aging without modifying formulation pharmacokinetics, protein secondary structure, formulation clarity, or in vivo bioactivity. When further subjected to harsh stressed aging tests in standard packaging, Humulin R formulated with our MoNi23% excipient did not aggregate for over 56 days of constant agitation at 37 °C, and four days of constant agitation at 50 °C, whereas Humulin R alone aggregated within two days at 37 °C and within one day at 50 °C. While agitation experienced by pharmaceuticals during typical storage and transportation can be highly complex, these processes can be mimicked in vitro using elevated temperatures and continuous agitation. Stressed aging, through incubation at elevated temperatures with continuous agitation, has been previously used to test insulin stability.11, 13–14, 19, 21 Even in hot climates with limited cold chain infrastructure, it is unlikely shipping containers would remain at 50 °C with continuous agitation with no reprieve for over four days and nights. Further, in our harsh stressed aging studies we use 10 mL vials, which resulted in a higher rate of interfacial exchange compared to initial stability studies in autosampler vials due to a larger diameter. The increased stability observed for Humulin R formulated with our MoNi23% excipient compared to Humulin R alone suggests that our MoNi23% excipient has utility in stabilizing insulin under various agitation conditions where interfacial turnover may be higher (i.e., horizontal agitation). The conditions evaluated in this study represent extreme exposure conditions during shipping in uninsulated containers or trucks in the hottest climates in the world where transport can take weeks before reaching patients.18 In addition to refrigerated transport in the early stages of the cold chain, maintaining proper transport and storage conditions during local distribution and once in patients’ hands presents a challenge in many parts of the world.24 This work suggest that the addition of AC/DC excipients can preserve insulin formulation integrity during even severe cold chain interruptions, enabling a reduction in cold chain requirements for insulin transportation and storage that are difficult to maintain in under-resourced environments.3, 24–29
These AC/DC excipients are synthesized at molecular weights below the glomerular filtration threshold to ensure they are eliminated without tissue accumulation, and they are prepared with facile and scalable controlled radical polymerization techniques from inexpensive starting materials. Previous exploration of this class of amphiphilic copolymer showed that they do not alter blood chemistry markers for liver and kidney function following repeated administration in rats, and exhibit cytotoxicity levels comparable to the phenolic preservatives used in commercial insulin formulations.19
Taken together, the results reported herein demonstrate the potential of AC/DC excipients as insulin formulation additives to improve cold chain resilience, thereby expanding global access to these critical drugs for treatment of diabetes. Further, based on the identified stabilization mechanism, AC/DC excipients are promising candidates to stabilize other biopharmaceuticals, such as monoclonal antibodies, that lose bioactivity as a result of aggregation at interfaces.22, 30 Future studies will require continued evaluation of the limits of AC/DC stabilized insulin and explore the application of these excipients to other protein therapeutics.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the Veterinary Service Centre staff for their technical assistance. The authors also acknowledge the High Throughput Bioscience Center (HTBC) at Stanford Medicine.
Funding Sources
This work was funded in part by NIDDK R01 (NIH grant #R01DK119254) and a Pilot and Feasibility funding from the Stanford Diabetes Research Center (NIH grant #P30DK116074), as well as the American Diabetes Association Grant (1-18-JDF-011). Support is also provided by the Stanford Maternal and Child Health Research Institute through the SPARK Translational Research Program. C.L.M. was supported by the NSERC Postgraduate Scholarship and the Stanford BioX Bowes Graduate Student Fellowship. J.L.M was supported Department of Defense NDSEG Fellowship and by a Stanford Graduate Fellowship. C.M.M. was supported by a Stanford Graduate Fellowship. A.A.A.S. was funded by grant NNF18OC0030896 from the Novo Nordisk Foundation and the Stanford Bio-X Program.
Footnotes
Declarations of interest: E.A.A., J.L.M., and C.L.M. are listed as inventors on a provisional patent application (63/011,928) filed by Stanford University describing the technology reported in this manuscript.
REFERENCES
- 1.Garg SK; Rewers AH; Akturk HK, Ever-Increasing Insulin-Requiring Patients Globally. Diabetes Technol. Ther 2018, 20 (S2), S21–S24. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation IDF Diabetes Atlas, 9th edn.; https://www.diabetesatlas.org., 2019. [Google Scholar]
- 3.Ogle GD; Abdullah M; Mason D; Januszewski AS; Besançon S, Insulin storage in hot climates without refrigeration: temperature reduction efficacy of clay pots and other techniques. Diabetic Med. 2016, 33 (11), 1544–1553. [DOI] [PubMed] [Google Scholar]
- 4.Basta N; Lipowicz M 2019 Biopharma Cold Chain Sourcebook Pharmaceutical Commerce: 2019. [Google Scholar]
- 5.Brange J; Andersen L; Laursen ED; Meyn G; Rasmussen E, Toward understanding insulin fibrillation. J. Pharm. Sci 1997, 86 (5), 517–525. [DOI] [PubMed] [Google Scholar]
- 6.Sluzky V; Klibanov AM; Langer R, Mechanism of insulin aggregation and stabilization in agitated aqueous solutions. Biotechnol. Bioeng 1992, 40 (8), 895–903. [DOI] [PubMed] [Google Scholar]
- 7.Sluzky V; Tamada JA; Klibanov AM; Langer R, Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc. Natl. Acad. Sci. U. S. A 1991, 88 (21), 9377–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharp JS; Forrest JA; Jones RAL, Surface Denaturation and Amyloid Fibril Formation of Insulin at Model Lipid−Water Interfaces. Biochemistry 2002, 41 (52), 15810–15819. [DOI] [PubMed] [Google Scholar]
- 9.Nault L; Guo P; Jain B; Bréchet Y; Bruckert F; Weidenhaupt M, Human insulin adsorption kinetics, conformational changes and amyloidal aggregate formation on hydrophobic surfaces. Acta Biomater. 2013, 9 (2), 5070–5079. [DOI] [PubMed] [Google Scholar]
- 10.Hinds K; Koh JJ; Joss L; Liu F; Baudys M; Kim SW, Synthesis and characterization of poly(ethylene glycol)-insulin conjugates. Bioconjug. Chem 2000, 11 (2), 195–201. [DOI] [PubMed] [Google Scholar]
- 11.Webber MJ; Appel EA; Vinciguerra B; Cortinas AB; Thapa LS; Jhunjhunwala S; Isaacs L; Langer R; Anderson DG, Supramolecular PEGylation of biopharmaceuticals. Proc. Natl. Acad. Sci. U. S. A 2016, 113 (50), 14189–14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y; Lee J; Mansfield KM; Ko JH; Sallam S; Wesdemiotis C; Maynard HD, Trehalose Glycopolymer Enhances Both Solution Stability and Pharmacokinetics of a Therapeutic Protein. Bioconjugate Chem. 2017, 28 (3), 836–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maikawa CL; Smith AAA; Zou L; Roth GA; Gale EC; Stapleton LM; Baker SW; Mann JL; Yu AC; Correa S; Grosskopf AK; Liong CS; Meis CM; Chan D; Troxell MD; Maahs DM; Buckingham BA; Webber MJ; Appel EA, A co-formulation of supramolecularly stabilized insulin and pramlintide enhances mealtime glucagon suppression in diabetic pigs. Nat. Biomed. Eng 2020, 4, 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meis CM; Salzman EE; Maikawa CL; Smith AAA; Mann JL; Grosskopf AK; Appel EA, Self-Assembled, Dilution-Responsive Hydrogels for Enhanced Thermal Stability of Insulin Biopharmaceuticals. ACS Biomater. Sci. Eng 2020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contreras-Montoya R; Arredondo-Amador M; Escolano-Casado G; Mañas-Torres MC; González M; Conejero-Muriel M; Bhatia V; Díaz-Mochón JJ; Martínez-Augustin O; de Medina FS; Lopez-Lopez MT; Conejero-Lara F; Gavira JA; de Cienfuegos LÁ, Insulin Crystals Grown in Short-Peptide Supramolecular Hydrogels Show Enhanced Thermal Stability and Slower Release Profile. ACS Appl. Mater. Interfaces 2021, 13 (10), 11672–11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rayaprolu BM; Strawser JJ; Anyarambhatla G, Excipients in parenteral formulations: selection considerations and effective utilization with small molecules and biologics. Drug Dev. Ind. Pharm 2018, 44 (10), 1565–1571. [DOI] [PubMed] [Google Scholar]
- 17.Akers MJ, Excipient-drug interactions in parenteral formulations. J. Pharm. Sci 2002, 91 (11), 2283–2300. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ; McAuley A; Schilke KF; McGuire J, Molecular origins of surfactant-mediated stabilization of protein drugs. Adv. Drug Delivery Rev 2011, 63 (13), 1160–1171. [DOI] [PubMed] [Google Scholar]
- 19.Mann JL; Maikawa CL; Smith AAA; Grosskopf AK; Baker SW; Roth GA; Meis CM; Gale EC; Liong CS; Correa S; Chan D; Stapleton LM; Yu AC; Muir B; Howard S; Postma A; Appel EA, An Ultra-fast Insulin Formulation Enabled by High Throughput Screening of Polymeric Excipients. Sci. Transl. Med 2020, 12, eaba6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu KK; Huan Y, Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol 2008, 5.47.1–5.47.14. [DOI] [PubMed] [Google Scholar]
- 21.Maikawa CL; Smith AAA; Zou L; Meis CM; Mann JL; Webber MJ; Appel EA, Stable Monomeric Insulin Formulations Enabled by Supramolecular PEGylation of Insulin Analogues. Adv. Ther 2019, 75, 1900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannan A; Shieh IC; Fuller GG, Linking aggregation and interfacial properties in monoclonal antibody-surfactant formulations. J. Colloid Interface Sci 2019, 550, 128–138. [DOI] [PubMed] [Google Scholar]
- 23.Schafer HC Field-Measured Temperature Profiles of Truck-Carried Materiel in Desert, Tropic, Mountainous Temperate, and Arctic Locations; Naval Weapons Center: 1983, 1983; pp 1–75. [Google Scholar]
- 24.Sykes C, Time- and Temperature-Controlled Transport: Supply Chain Challenges and Solutions. Pharm. Ther 2018, 43 (3), 154–170. [PMC free article] [PubMed] [Google Scholar]
- 25.Gill G; Price C; English P; Eriksson-Lee J, Traditional clay pots as storage containers for insulin in hot climates. Trop. Doct 2002, 32 (4), 237–238. [DOI] [PubMed] [Google Scholar]
- 26.Vimalavathini R; Gitanjali B, Effect of temperature on the potency & pharmacological action of insulin. Indian J. Med. Res 2009, 130 (0971–5916 (Print)), 166–169. [PubMed] [Google Scholar]
- 27.Subzwari M; Nasir SZ; Others, Preserving Efficacy of Temperature Sensitive Medicines--Logistics Management in Pharmaceutical Supply Chain. South Asian Journal of Management 2015, 9 (1), 1–9. [Google Scholar]
- 28.Bahendeka S; Kaushik R; Swai AB; Otieno F; Bajaj S; Kalra S; Bavuma CM; Karigire C, EADSG Guidelines: Insulin Storage and Optimisation of Injection Technique in Diabetes Management. Diabetes Ther. 2019, 10 (2), 341–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khurana G; Gupta V, Effect on Insulin upon Storage in Extreme Climatic Conditions (Temperature and Pressure) and Their Preventive Measures. J. Soc. Health Diabetes 2019, 07 (01), 006–010. [Google Scholar]
- 30.Kannan A; Shieh IC; Leiske DL; Fuller GG, Monoclonal Antibody Interfaces: Dilatation Mechanics and Bubble Coalescence. Langmuir 2018, 34 (2), 630–638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


