Abstract
SSeCKS, first isolated as a G1→S inhibitor that is downregulated in src- and ras-transformed cells, is a major cytoskeleton-associated PKC substrate with tumor suppressor and kinase-scaffolding activities. Previous attempts at constitutive expression resulted in cell variants with truncated ectopic SSeCKS products. Here, we show that tetracycline-regulated SSeCKS expression in NIH 3T3 cells induces G1 arrest marked by extracellular signal-regulated kinase 2-dependent decreases in cyclin D1 expression and pRb phosphorylation. Unexpectedly, the forced reexpression of cyclin D1 failed to rescue SSeCKS-induced G1 arrest. Confocal microscopy analysis revealed cytoplasmic colocalization of cyclin D1 with SSeCKS. Because the SSeCKS gene encodes two potential cyclin-binding motifs (CY) flanking major in vivo protein kinase C (PKC) phosphorylation sites (Ser507/515), we addressed whether SSeCKS encodes a phosphorylation-dependent cyclin scaffolding function. Bacterially expressed SSeCKS-CY bound cyclins D1 and E, whereas K→S mutations within either CY motif ablated binding. Activation of PKC in vivo caused a rapid translocation of cyclin D1 to the nucleus. Cell permeable, penetratin-linked peptides encoding wild-type SSeCKS-CY, but not K→S or phospho-Ser507/515 variants, released cyclin D1 from its cytoplasmic sequestration and induced higher saturation density in cyclin D1-overexpressor cells or rat embryo fibroblasts. Our data suggest that SSeCKS controls G1→S progression by regulating the expression and localization of cyclin D1. These data suggest that downregulation of SSeCKS in tumor cells removes gating checkpoints for saturation density, an effect that may promote contact independence.
Cell cycle progression from the first gap (G1) to the DNA synthetic (S) phase is mainly controlled by the activity of complexes containing cyclin D–cyclin-dependent kinase 4 (CDK4) or CDK6 and cyclin E-CDK2 (53). In contrast to cycling cells in which regulation of these activities has been well studied, little is known regarding the control of cyclin-dependent complexes when cells become contact inhibited or how these controls are subverted in cancer or diseases marked by cell hyperplasia.
Cyclin protein levels are largely controlled at the transcriptional level and by ubiquitin-mediated degradation. Except for cyclin D, whose expression increases rapidly in G1 and stays high until the end of G2/M, the remaining cyclins are expressed in discrete phases (20, 49). In contrast, the levels of CDKs are stable throughout the cell cycle; however, their phosphorylation status is cell cycle regulated (48). Current models suggest that cyclin D-CDK4 or -CDK6 complexes are responsible for G1 progression whereas cyclin E-CDK2 complexes are responsible for G1→S transition. The primary role of cyclin D in cell cycle regulation is most likely to phosphorylate pRb, because microinjection of antibodies to cyclin D1 has no effect in Rb−/− cells (54). Phosphorylation of pRb by cyclin D-CDK4 or -CDK6, which occurs in mid-G1, leads to the release of pRb-bound E2F transcription factors, which in turn are free to transcribe genes required for S-phase entry (16).
Accumulation of cyclin D1 mRNA is dependent on both mitogen- and anchorage-induced signals. Induction of cyclin D1 in early G1 requires the sustained activation of the extracellular signal-regulated kinase (ERK) subfamily of mitogen-activated protein kinases (1, 37). Thus, addition of growth factors in the absence of adhesion, or vice versa, only results in a transient ERK activation, insufficient to induce cyclin D1 expression. Degradation of cyclin D1 is dependent on phosphorylation-triggered, ubiquitin-mediated proteolysis (21), which is also a mitogen-dependent process involving a Ras-PI3K-Akt-GSK3β-mediated pathway (19). Cyclin D1 is increasingly located in the nucleus of human fibroblasts as the G1 phase progresses. However, the intensity of cyclin D1 nuclear staining is highly heterogeneous in asynchronized cultures, with the strongest staining in late G1 phase. Nuclear cyclin D1 levels decrease rapidly once cells enter S phase (2). Recent evidence indicates that mutation of cyclin D residues required for correct protein folding (21) causes retention of cyclin D in the cytoplasm and growth arrest in G1 (20).
One enigma in this field is how untransformed cells growth arrest due to contact inhibition even in the presence of growth factors and cell adhesion signals. This phenomenon implies the existence of at least one negative regulatory pathway that is dominant over the growth factor- and integrin-mediated effects on cyclin D expression. Contact inhibition is defined in reality by a saturation density set point, because untransformed cells do not undergo growth arrest at the precise point of cell-cell contact. One view is that mechanical forces defined by actin-based cytoskeletal architecture control mitogenic signaling, cell cycle progression and cell motility (33). Another is that cytoskeletal networks control the recruitment of signaling proteins such as mitogen-activated protein kinases and Rho family GTPases to sites of activation such as focal adhesion complexes during mitogenesis (60). Thus, saturation density set points are likely controlled by cytoskeletal proteins that influence cell shape in response to both growth factors and integrin-mediated adhesion.
Few genes have been identified whose expression is induced during contact inhibition and, presumably, whose products might participate in G1→S control. Unlike genes such as sdr, whose expression increases following serum starvation but not contact inhibition (30), and genes such as GAS2, whose expression is induced by either serum starvation or contact inhibition (3), genes such as those encoding contactinhibin, phosphatidylinositol-3 kinase, and candidate tumor suppressors p27 and neurofibromin are induced in contact-inhibited cells only (15, 29, 35, 45, 59).
Our laboratory isolated a novel protein kinase C (PKC) substrate, SSeCKS, whose transcription is increased in contact-inhibited cultures but downregulated in src- and ras-transformed fibroblasts and epithelial cells or in response to mitogenic activation (39, 43). In contrast, SSeCKS protein, which is long-lived (half-life, >24 h), is phosphorylated rapidly in late G1 phase (43), simultaneous with its translocation from cytoskeletal and plasma membrane sites to the perinucleus (25, 40). Moreover, in ras-transformed Rat-6 cells, the remaining SSeCKS protein is hyperphosphorylated, suggesting that SSeCKS function is lost by either downregulation or hyperphosphorylation (40).
Multiple attempts to express SSeCKS constitutively indicated that high levels inhibit cell proliferation, resulting in the selection of cell lines expressing deletion variants of SSeCKS (39). Recently, we demonstrated that the tetracycline (TET)-regulated expression of SSeCKS induces G1 arrest in NIH 3T3 marked by cell flattening, a transient loss of focal adhesion complexes and stress fibers, and the formation of filopodium- and lamellipodium-like projections (25).
Ectopic expression of SSeCKS is able to suppress morphological transformation and tumorigenicity in src-transformed rodent fibroblasts and in ras-activated rat prostate cancer cell lines, indicating a potential role as a tumor suppressor (38; W. Xia, J. Nelson, and I. H. Gelman, submitted for publication). A putative human orthologue, gravin, maps to 6q24-25.2, a hot spot for chromosomal deletion in advanced prostate, breast, and ovarian cancer (I. H. Gelman, A. Bulua, and A. Wang, submitted for publication). Indeed, SSeCKS or Gravin expression is severely downregulated in human prostate and breast cancer cell lines and in well-differentiated prostate cancers in situ (Gelman et al., submitted for publication; Xia et al., submitted for publication). Gravin, first identified as an autoantigen in some myasthenia gravis patients, is identical to the A kinase anchoring protein, AKAP250, and based on its PKA-binding activity and its ability to bind PKC in a phosphatidylserine-dependent manner has been postulated to function as a kinase scaffolding protein (42). The fact that SSeCKS shares these functions, as well as the ability to bind calmodulin, led us to postulate that the scaffolding function of SSeCKS or Gravin is directly related to their putative roles as negative mitogenic regulators and/or tumor suppressors. Phosphorylation of SSeCKS or Gravin by PKC (and presumably other kinases) decreases their scaffolding activities, suggesting that their regulatory functions are lost in late G1.
In this report, we determine that SSeCKS-induced G1 arrest in NIH 3T3 cells correlates with a lack of cyclin D1, possibly due to a decrease in the level of serum-inducible ERK2 activation. Unexpectedly, the forced expression of cyclin D1 failed to rescue growth arrest because it was sequestered in the cytoplasm, most likely mediated by tandem cyclin-binding motifs (CY) in SSeCKS. We conclude that SSeCKS controls cell cycle progression by inhibiting cyclin D1 expression and/or by sequestering cyclin D1 in the cytoplasm. This scaffolding function of SSeCKS is likely to influence the saturation density set point in untransformed cells.
MATERIALS AND METHODS
Cells.
S2-6 cells (gift of David Schatz, Yale School of Medicine), NIH 3T3 cells expressing a TET-regulated version of the TET transactivator, tTA (55), were grown in histidine-deficient Dulbecco's modified Eagle's medium (DMEM) (Irvine Scientific) supplemented with 0.5 μM l-histidinol (Sigma, St. Louis, Mo.), 10% calf serum, penicillin-streptomycin-amphotericin B (GIBCO, Gaithersburg, Md.), and TET (0.5 μg/ml; Sigma). φNX cells, an ecotropic packaging line (a gift of Gary Nolan, Stanford University), were grown in DMEM supplemented with 10% calf serum.
TET-regulated SSeCKS-overexpressing cell lines.
An EcoRI fragment encoding the full-length SSeCKS cDNA was spliced into pUHD10-3, a plasmid containing a tTA-dependent promoter (gift of Hermann Bujard [28]). Ten micrograms of pUHD10-3[SSeCKS] or pUHD10-3 DNA was cotransfected into S2-6 cells with 1 μg of pBabepuro using CaPO4 precipitates. Stable cell lines were selected in S2-6 medium supplemented with puromycin (2 μg/ml; Sigma) and TET (5 μg/ml). S2-6/S24, an SSeCKS-expressing cell clone, and S2-6/V3, transfected with vector alone, were used to produce cyclin D1 overexpressors.
Cyclin D1-overexpressing cell lines.
Stocks of ecotropic viruses encoding pLJ/cyclin D1 (gift of Robert Krauss, Mount Sinai School of Medicine) or pLJ retrovirus vector were produced by transient transfection of φNX cells (24) and then filtering medium through 0.2-μm-pore-size low protein-binding filters (Gelman Sciences). Stably infected cell lines were selected in S2-6 medium supplemented with G418 (400 μg/ml; GIBCO) and TET (0.5 μg/ml).
Western blot analysis.
Cells were washed thrice with ice-cold phosphate-buffered saline (PBS), scraped into microtubes, and lysed with RIPA buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 5 mM EDTA, 8% glycerol, 1% Triton X-100, 0.1% sodium dodecyl sulfate [SDS], 0.5% sodium deoxycholate, 1 mM sodium vanadate, 10 mM sodium fluoride, 1 mM phenylmethylsulfonyl fluoride [PMSF], and 2 μg (each) of aprotinin, leupeptin, antipain, and pepstatin per ml). Protein content was normalized using protein assay kits (Bio-Rad Laboratories). Equal amounts of protein were separated by SDS–polyacrylamide gel electrophoresis (PAGE) (5% polyacrylamide), electrophoretically transferred to PolyScreen polyvinylidene difluoride membrane (NEN, Boston, Mass.), and immunoblotted as described before (25). Primary polyclonal and monoclonal antibodies (PAbs and MAbs, respectively) included SSeCKS (PAb) (40), ERK2 (PAb), cyclin D1 (PAb), cyclin A or E (MAb), CDK2, -4, or -6 (MAb), CKIs p21 and p27 (MAb) (Santa Cruz Biotechnology), CKIs p18 and p19 (MAb) (gifts of Selina Chen-Kiang, Weill Medical School of Cornell University), pRb or cyclin D1 (MAb) (PharMingen), or CKI p16 (MAb) (Clontech). Following three PBS washes, the filter was incubated with either horseradish peroxidase (Chemicon)- or alkaline phosphatase (Boehringer Mannheim)-conjugated secondary antibody for 1 h. After extensive washing, the secondary antibodies were visualized using ECL (Amersham) or Western Blue (Promega) substrates, respectively. For detection of pRb phosphorylation, cells were lysed in NETN buffer (1% NP-40, 2 mM EDTA, 50 mM Tris-HCl [pH 8.0], 250 mM NaCl, 1 mM dithiothreitol [DTT], 1 mM Na3VO4, 10 mM NaF, 1 mM PMSF, and 2 μg (each) of aprotinin, leupeptin, antipain, and pepstatin per ml) followed by SDS-PAGE and immunoblotting. In some cases, blots were stripped of antibody probes by incubating in 500 ml of preheated (50°C) 62.7 mM Tris-HCl, pH 6.7, containing 2% SDS and 0.1 M β-mercaptoethanol, followed by extensive washes in PBS.
Proliferation assay.
A total of 104 cells were seeded onto 24-well plates, and the next day an aliquot of cells was trypsinized and counted to establish a baseline plating efficiency. The remaining cells were grown in medium in the presence or absence of tetracycline. Duplicate wells were trypsinized and counted every two days using a hemacytometer (Fisher Scientific).
ERK2 kinase assay.
Cells were serum starved overnight and then stimulated with 10% calf serum-containing medium for various periods. Following lysis in RIPA buffer, the lysates were incubated with rabbit anti-ERK2 antibody prebound to Affi-Prep protein A beads (Bio-Rad). The immunocomplex was washed twice with RIPA buffer and twice with kinase buffer (10 mM HEPES [pH 7.5], 10 mM magnesium acetate). A 20-μl aliquot of the bead-antibody-antigen complex was resuspended in 40 μl containing a 1:1 dilution of myelin basic protein (MBP) (2 mg/ml; Sigma) and 3× hot mix (30 mM HEPES [pH 7.5], 30 mM magnesium acetate, 150 μM ATP, 10 μCi of [γ-32P]ATP) and incubated for 30 min at 30°C. The reaction was stopped by adding 60 μl of 2× protein loading dye. This mixture was boiled and electrophoresed through an SDS–15% polyacrylamide gel, and this was followed by autoradiography.
CDK2 kinase assay.
RIPA lysates were incubated with anti-cyclin E antibodies prebound to protein A beads. The immunocomplexes were washed three times with RIPA buffer and two times with histone H1 assay buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 10 mM MgCl2, 2 mM EGTA, 1 mM DTT) and then resuspended in 25 μl of assay buffer supplemented with 20 μM ATP and 4 μg of histone H1 (GIBCO). The kinase assay was initiated by adding 10 μCi of [γ-32P]ATP. After 10 min of incubation at 30°C, the supernatants were collected and electrophoresed through an SDS–10% polyacrylamide gel, and this was followed by autoradiography.
Cell cycle analysis.
The percentage of cells in different phases of the cell cycle was quantified using flow cytometry as described (34, 62). Synchronized cells were harvested by trypsinization, washed in PBS, and fixed in ice-cold 70% ethanol (106 cells/ml) for at least 2 h at −20°C. Before flow cytometric analysis, the pelleted cells were washed in PBS and stained for 2 h at room temperature with propidium iodide (20 μg/ml; Sigma) containing 1 μg of RNase A per ml. Analysis was performed on a FACScan machine (Becton Dickinson) using the CellFIT analysis software.
Construction and expression of GST-SSeCKS-CY fusion proteins.
SSeCKS fragments (amino acid [aa] residues 389 to 552) were generated by PCR amplification and cloned in pBluescript II KS (Stratagene) (40). Mutations in two potential CY were generated using a Transformer site-directed mutagenesis kit (Clontech). A unique restriction site in pBluescript, Sca I, was chosen as a selection marker (ScaI to StuI). Trans and switch selection primers were 5′GTGACTGGTGAGGCCTCAACCAAGTC (Sca I to Stu I) and 5′GTGACTGGTGAGTACTCAACCAAGTC (Stu I to Sca I), respectively (restriction sites are underlined, and changed residues are in boldface). Trans-mutagenic primers were as follows: 5′GGAAGTCCCTTGTCGAGCCTCTTCAGTAGC (first KK to SS), 5′GCTCAGGCTTAAGCTCGCTGTCTGGG (second KK to SS), 5′CCCTTGAAGAAAAGCTTCAGTAGC (first L to S), and 5′GGCTTAAAGAAGTCGTCTGGGAAG (second L to S). Switch-mutagenic primers were 5′CCCTTGTCGAGCAGCTTCAGTAGC (first L to S) and 5′GGCTTAAGCTCGTCGTCTGGGAAG (second L to S). After denaturation, the target SSeCKS plasmid was annealed with primers, and this was followed by synthesis of the mutant strand DNA. Primary selection was carried out by restriction digestion. The mutated plasmid was amplified and then was subjected to a second round of restriction enzyme digestion. All mutations were confirmed by sequencing using Sequenase 2.0 kits (U.S. Biochemicals). The resulting SSeCKS variants were spliced back to pGEX 5x-1 for fusion protein expression. BL21(DE3) pLysS bacteria (Novagen) were transformed with these constructs, grown in Luria-Bertani–ampicillin medium containing 20 mM glucose at 37°C, and glutathione-S-transferase (GST) fusion protein induced and purified as described previously (40, 52).
In vitro cyclin pull-down assay.
Cyclin D1 pull-down assays were performed as described previously (11). Briefly, cells were lysed in binding buffer (20 mM Tris-HCl [pH 7.4]; 1 mM EDTA; 25 mM NaCl; 10% glycerol; 0.01% NP-40; a 1 mM concentration [each] of DTT, Na3VO4, and PMSF; and 2 μg [each] of aprotinin, leupeptin, antipain, and pepstatin A per ml). A total of 500 μg of the lysates was incubated with 15 μg of GST-SSeCKS or GST prebound to glutathione-Sepharose beads for 3 h at 4°C on a rotating wheel. After four washes in binding buffer, the beads were boiled in protein loading dye, and the proteins were analyzed by immunoblotting using anti-cyclin D1 antibody.
Immunofluorescence analysis.
Cells grown on 22-mm2 coverslips were fixed in 60% acetone–2% formaldehyde at −20°C for 20 min, then incubated with either immunoaffinity-purified anti-SSeCKS (40) and/or anti-cyclin D1, and then stained with fluorescein isothiocyanate (FITC)- or tetramethyl rhodamine isothiocyanate (TRITC)-labeled secondary antibodies as described previously (25). Slides were visualized on either a Leica confocal laser scanning microscope or an Olympus IX-70 fluorescence microscope fitted with a Sony Catseye digital camera, and digital images were processed using Photoshop 4.01 and NIH Image software on a Macintosh Power PC 8100/100AV.
Peptide treatment.
Peptides were synthesized by BioWorld 2000 or the Mount Sinai Peptide Core Facility and were >85% pure as determined by ion-spray mass spectroscopy. The following peptides were produced, either linked to penetratin peptide (RQIKIWFQNRRMKWKK) or as the sequences shown (changed residues are in boldface, and Pi indicates phosphate): wild-type SSeCKS CY (LKKLFSSSGLKKLSGK), mutated CY (LSSSFSSSGLSSSSGK) or phosphoserine CY (LKKLFSPiSSGLKKLSPiGK). N-terminal biotinylation was performed on half the penetratin-linked and half the non-penetratin-linked peptide product. Peptides were resuspended in DMEM and then incubated with cells at a final concentration of 100 μg/ml for 2 to 4 h. Peptide entry into cells was monitored by fixation of cells in ice-cold ethanol-acetone (9:1) for 5 min at −20°C, washing with DMEM–10% CS, and incubation with PAb anti-cyclin D1 and then with TRITC-labeled anti-rabbit immunoglobulin (Chemicon) and FITC-labeled avidin (Molecular Probes). Coverslips were mounted and photographed as described above.
Cell fractionation.
Cytosolic and nuclear fractions were prepared according to the technique of Hochholdinger et al. (32) with the following modifications. After centrifuging the homogenate at 500 × g for 10 min at 4°C, the supernatant (typically 1 ml) was collected and SDS and Triton X-100 were added to final concentrations of 0.2 and 1%, respectively; this was followed by vortexing and storage at −70°C. The pellets (nuclear fractions) were resuspended in 100 to 200 μl of hypotonic lysis buffer (1 mM EDTA, 1 mM EGTA, 10 mM β-glycerophosphate, 1 mM Na3 VO4, 2 mM MgCl2, 10 mM KCl, 1 mM DTT, PMSF (40 μg/ml), aprotinin (10 μg/ml), and leupeptin (10 μg/ml). This fraction was loaded atop 1 ml of 1 M sucrose in hypotonic lysis buffer and centrifuged at 1600 × g for 20 min at 4°C. The pellets were resuspended in 100 μl of buffer, brought to final concentrations of 0.2% SDS and 1% Triton X-100, vortexed, and stored at −70°C.
RESULTS
Overexpression of SSeCKS results in G1 arrest.
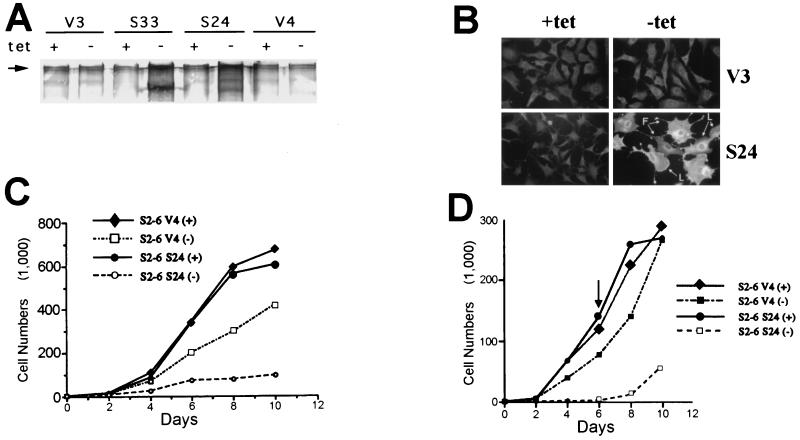
Previous attempts to produce stable constitutive expression of SSeCKS resulted in the selection of variants with their transduced SSeCKS cDNA copies deleted (39). Using S6 cells, we produced cells lines which express full-length rat SSeCKS following the removal of TET (25). A number of resulting cell lines, e.g., S24 and S33, showed background levels of SSeCKS in the presence of TET and >25-fold induction of the 290-kDa SSeCKS isoform following TET removal (Fig. 1A). At least 40% of the cells lines selected showed a similar profile of inducibility of apparently full-length protein (data not shown). As we described previously (25), overexpression of SSeCKS caused severe cell flattening and the production of exaggerated cell projections (Fig. 1B).
FIG. 1.
SSeCKS overexpression results in growth arrest. (A) Western blot showing expression of SSeCKS in TET-regulated clones. Cell lysates of TET-SSeCKS clones (S24 and S33) and vector control clones (V3 and V4) grown in the presence (+) or absence (−) of TET were used for Western blotting analysis normalized for 35 μg of protein per lane. In the absence of TET, ectopic expression of the 290-kDa SSeCKS isoform was induced in S24 and S33 cells (Fig. 8A shows better resolution of the 280-kDa–290-kDa doublet). (B) Morphological changes after SSeCKS expression. S24 and V3 cells grown in the presence or absence of TET were immunostained for SSeCKS expression. SSeCKS overexpression results in cell flattening and production of filopodium (F)- and lamellipodium (L)-like projections. Removal of TET did not affect the morphology of control V3 cells. Magnification, ×220. (C) SSeCKS induces growth arrest. The proliferation rate of S24 cells is dramatically reduced when SSeCKS is overexpressed. The reduced proliferation of V4 cells is most likely due to squelching effects of the tTA transactivator (27). (D) SSeCKS-induced growth arrest is reversible. TET (0.5 μg/ml) was added back to cell cultures at day 6 after its removal. Two days later, coincident with the degradation of ectopic SSeCKS (data not shown), S24 cells were proliferating exponentially, indicating that SSeCKS overexpression does not induce cellular senescence.
We tested the effect of SSeCKS overexpression on proliferation rates in the presence of serum growth factors. In vector control cells, removal of TET decreased proliferation rates 20 to 40% (Fig. 1C). This phenomenon, described previously by others (27), is most likely due to squelching of endogenous transcription factors by the VP-16 moiety of the tTA. In contrast, S24 cells expressing full-length SSeCKS underwent growth arrest following TET removal, showing only a marginal increase in cell number after 10 days of incubation in the presence of serum growth factors (Fig. 1C). The finding of SSeCKS-induced growth arrest in a number of independently derived clones (Table 1) indicates that this phenomenon is not due to the idiosyncrasies of a particular cell line.
TABLE 1.
Correlation between G1 phase growth arrest, cell flattening, and loss of cyclin D1
| Observation | Cell Line
|
||||||
|---|---|---|---|---|---|---|---|
| V3 | V9 | S24 | S26 | S33 | S23a | S38a | |
| Flattening | − | − | + | + | + | − | − |
| Growth arrest | − | − | + | + | + | − | − |
| G1 arrest | − | − | + | + | + | − | − |
| Suppression of cyclin D1 | − | − | + | + | + | − | − |
Cell lines expressing slightly truncated SSeCKS product.
Figure 1D shows that unlike p53 overexpression, which induces cell senescence (56), readdition of TET after 6 days resulted in the full recovery of proliferative ability. On the possibility that the proliferating cells represented a “breakout” population, we isolated 20 S24 subclones and showed that each could recover equally from growth arrest by the readdition of TET (data not shown). The proliferating cells could be rearrested by readding TET, indicating that these cells were still SSeCKS responsive (data not shown). However, after more than four rounds of arrest and release, it became more difficult to induce full arrest, likely due to the selective advantage of proliferating variants.
To determine where in the cell cycle SSeCKS arrests cell proliferation, S24 or control cells were put into G0 phase by serum starvation and then induced with serum in the presence or absence of TET, and this was followed by propidium iodide staining and fluorescence-activated cell sorter analysis. Table 2 shows a two- to threefold reduction in the percentage of S phase following expression of ectopic SSeCKS. Several independently derived TET-SSeCKS clones (S26 and S33) showed similar S-phase decreases concomitant with increases in G1 phase (Table 2), indicating an overall G1 phase arrest. Interestingly, a small number of TET-SSeCKS clones (<15% of all clones derived) typified by S23 and S38, showed neither G1 arrest nor cell flattening (Table 1). Although these clones express apparently full-length protein, a more careful analysis (long-run SDS-PAGE analysis using 5% gels) showed that these clones contain small truncations (not shown).
TABLE 2.
Percent of control (V) or SSeCKS (S) cells in various cell cycle phases
| Clone | % of cells in phaseb:
|
|||||
|---|---|---|---|---|---|---|
| G0/G1
|
S
|
G2/M
|
||||
| +TET | −TET | +TET | −TET | +TET | −TET | |
| V3 | 54.6 | 58.9 | 20.0 | 19.2 | 25.2 | 21.8 |
| V9 | 59.9 | 63.2 | 23.2 | 23.0 | 16.9 | 13.8 |
| S24 | 54.5 | 72.5 | 23.9 | 11.7 | 21.6 | 15.8 |
| S26 | 49.6 | 70.2 | 26.9 | 14.2 | 23.5 | 15.5 |
| S33 | 54.3 | 72.1 | 23.9 | 10.2 | 21.8 | 17.7 |
| S23a | 60.8 | 63.7 | 21.1 | 18.4 | 18.1 | 17.9 |
| S38a | 60.9 | 56.3 | 21.7 | 21.8 | 17.4 | 21.8 |
Cell lines expressing slightly truncated SSeCKS product.
Cells were grown and labeled with propidium iodide as described in Materials and Methods. Percentages are based on a single experiment in which all clones were grown under the same condition (with or without TET [+TET and −TET, respectively]). Two repeats of the experiment showed less than a 10% variation for individual clones.
Overexpression of SSeCKS suppresses cyclin D1 expression and serum-inducible ERK2 activation.
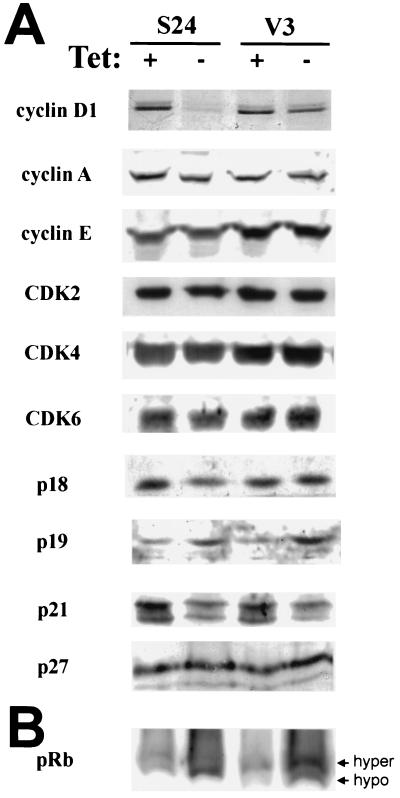
To investigate which cell cycle components are affected by SSeCKS overexpression, cell lysates from S24 and V3 cells were analyzed by Western blotting. Among all the components examined—including cyclins D1, E, and A; CDK2, -4, and -6; and CKIs (p16, p18, p19, p21, and p27)—only the expression of cyclin D1 was dramatically reduced in S24 cells grown in the absence of TET in comparison with V3 cells (Fig. 2A). The expression of p16 was undetectable in these NIH 3T3-derived cell lines. p21 expression was decreased in both S24 and V3 cells after removal of TET, probably due to the nonspecific effect of tTA. Similarly, the level of p19 was increased in both S24 and V3 cells in the absence of TET. However, the absence of pRb hyperphosphorylation in S24 cells after TET removal (Fig. 2B) correlates with G1 arrest due to cyclin D1 deficiency.
FIG. 2.
SSeCKS overexpression results in the loss of cyclin D1 expression and pRb hypophosphorylation. S24 and V3 cells were grown in the presence (+) or absence (−) of TET for 4 days before collection of cell lysates. (A) Western blot analysis showing the steady-state levels of cyclins, CDKs, and CKIs. Among the examined cell cycle regulators, only the level of cyclin D1 is specifically reduced in response to SSeCKS overexpression. The relative protein levels between lanes were normalized by loading equal aliquots of protein and by stripping and reprobing the same blot. These results were confirmed at least twice more. (B) Western blot showing the phosphorylation status of pRb. The relative abundance of hypophosphorylated (hypo) (faster migrating band) pRb is greatly increased when SSeCKS expression is induced. hyper, hyperphosphorylated.
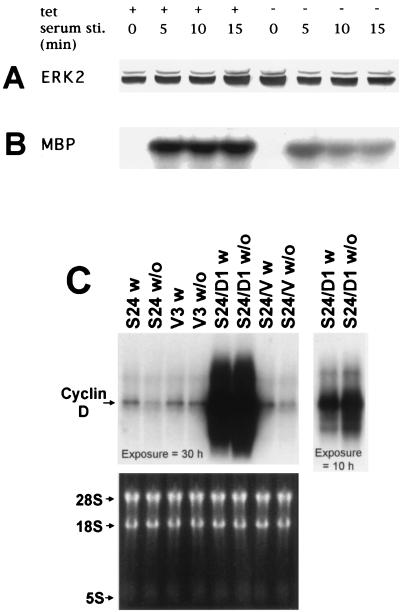
Because the expression of cyclin D1 is dependent on sustained ERK activation (1), we examined whether SSeCKS affects serum-inducible ERK activation. We previously showed that SSeCKS's ability to suppress src-induced oncogenesis correlated with a growth factor-independent superinduction of ERK2 activity (38), indicating that SSeCKS could modulate ERK-activating mechanisms. Figure 3B shows that serum-inducible ERK2 activation, as measured by the ability of ERK2 immunoprecipitates to phosphorylate MBP, was depressed fivefold by SSeCKS overexpression. Moreover, SSeCKS attenuated the length of ERK2 activation, presumably to levels insufficient for cyclin D induction. SSeCKS expression had no effect on ERK2 protein levels (Fig. 3A). Finally, Fig. 3C shows that TET removal resulted in a loss in steady-state cyclin D message levels in the S24 cells but not in the V3 controls. Thus, SSeCKS may inhibit cyclin D transcription by down-modulating ERK2 activation.
FIG. 3.
SSeCKS overexpression inhibits cyclin D transcription and serum-inducible ERK2 activity. S24 cells grown in the presence (+) or absence (−) of TET were starved of serum overnight and then stimulated (sti.) with medium supplemented with 10% calf serum for various periods. (A) ERK2 levels detected by Western blotting analysis. (B) ERK2 kinase activity measured by in vitro phosphorylation of MBP substrate. SSeCKS overexpression results in a >5-fold decrease in serum-inducible ERK2 kinase activity and a decrease in the length of ERK2 activation. (C) Northern blot analysis of total RNA (25 μg/lane) from S24, V3, S24/D1, and S24/V cell lines grown in the presence or absence of TET and probed with 32P-labeled cyclin D1 cDNA. Note the decreased accumulation of cyclin D1 RNA in S24 and S24/V cells when SSeCKS is reexpressed, in contrast to V3 and S24/D1 cells in which cyclin D1 levels do not decrease in the absence of TET. The S24/D1 lanes are shown at right with a shorter exposure to facilitate comparison. RNA levels were normalized to rRNA levels shown at bottom.
Overexpression of SSeCKS correlates with G1 arrest, suppression of cyclin D1, and cell flattening.
We showed previously that SSeCKS overexpression caused dramatic morphological changes, including cell flattening, a transient loss of F-actin stress fibers and vinculin-associated adhesion plaques, and the formation of filopodium- and lamellipodium-like projections (25). Table 2 shows a correlation between these effects and SSeCKS-induced G1 arrest in several independent TET SSeCKS clones. In contrast, the variant clones such as S23 neither flattened nor arrested in G1. Additionally, G1 arrest always correlated with a loss of cyclin D1, whereas the nonarresting clones expressed cyclin D1 in the absence of TET. These data demonstrate a strong correlation between SSeCKS-induced G1 arrest, loss of cyclin D1, and SSeCKS-induced cytoskeletal reorganization.
Ectopic expression of cyclin D1 fails to rescue SSeCKS-induced growth arrest.
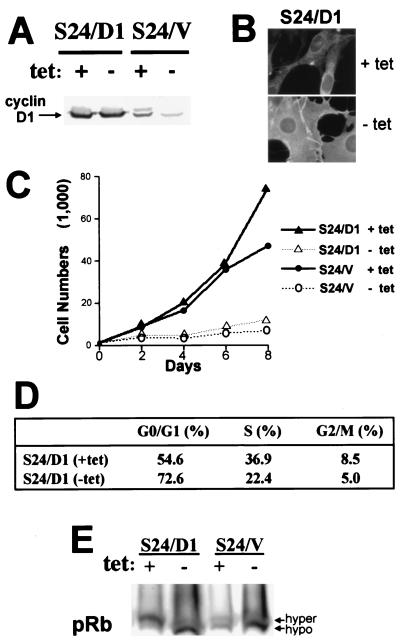
If loss of cyclin D1 was sufficient to induce the G1 arrest, we assumed that the forced expression of exogenous cyclin D1 should rescue the SSeCKS-induced arrest. Figure 4A shows that following retroviral transduction of cyclin D1 into S24 cells, cyclin D1 levels were unaffected by SSeCKS overexpression. Surprisingly, the forced expression of cyclin D1 failed to rescue the G1 arrest, as shown by a lack of proliferation (Fig. 4C), by the continued flattened cell morphology of S24/D1 cells grown without TET (Fig. 4B), and by a loss of S-phase staining in fluorescence-activated cell sorter analysis (Fig. 4D). Most significantly, pRb remains hypophosphorylated in S24/D1 cells grown in the absence of TET (Fig. 4E). However, there was no change relative to S24/V controls in the steady-state levels of cyclins E and A; CDK2, -4, and -6; CKIs p15, p18, p19, p21, or p27; or in the total cell CDK4-, -6-, or -2-associated kinase activities (data not shown).
FIG. 4.
Ectopic expression of cyclin D1 fails to rescue SSeCKS-induced G1 arrest. (A) Western blot analysis (150 μg of total protein/lane) showing ectopic expression of cyclin D1 in S24 cells (S24/D1) achieved by retroviral transduction, followed by G418 selection for stable clones. S24/V are vector-infected S24 cells. +, presence; −, absence. (B) Morphology of S24/D1 cells. S24/D1 cells grown in the presence or absence of TET were immunostained for SSeCKS expression. Ectopic expression of D1 fails to revert SSeCKS-induced cell flattening. Magnification, ×850. (C) SSeCKS overexpression inhibits the proliferation of S24/D1 cells to a similar extent as with S24/V cells. (D) Cell cycle analysis as described in Materials and Methods. SSeCKS overexpression results in an increased abundance of S24/D1 cells in G1 phase. (E) Western blot analysis showing the mobility status of pRb on SDS-PAGE. The majority of pRb in S24/D1 and S24/V cells is hypophosphorylated (hypo) in response to SSeCKS expression. hyper, hyperphosphorylated.
Recent data indicate that binding of c-Abl protein to the C terminus of pRb is required along with pRb hyperphosphorylation to insure G1→S transition (36). Because SSeCKS (aa 468 to 496) shows similarity with the C-terminal domain of pRb involved in c-Abl binding (the Rb “C pocket” [aa 780 to 860]) (Fig. 5), we hypothesized that overexpressed SSeCKS might compete directly for c-Abl binding and thus inhibit Rb-mediated G1→S transition. However, overexpression of SSeCKS did not affect the levels of pRb-bound c-Abl (data not shown).
FIG. 5.
Sequence similarity between SSeCKS and the Abl-binding domain in pRb. Identical amino acid residues (vertical lines) or similarly charged residues (colons) are shown for the SSeCKS and newt Rb (GenBank accession no. Y09226) proteins.
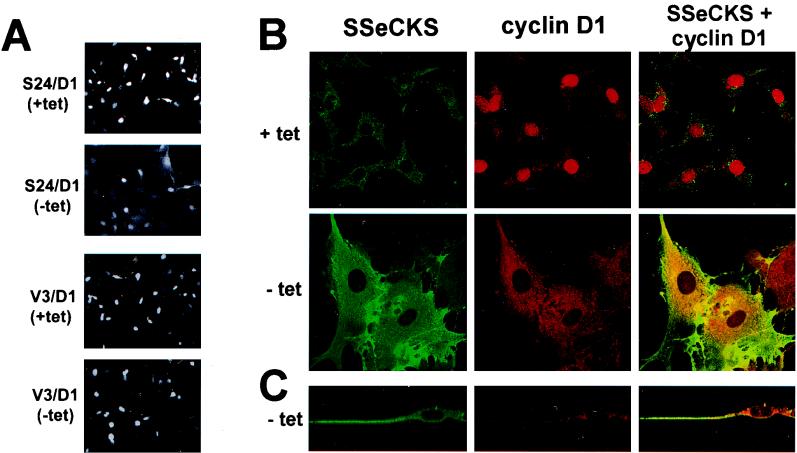
We entertained the possibility that active cyclin D1-CDK4 complexes were not accessible to their downstream target, pRb. Figure 6 shows that the majority of cyclin D1 in S24/D1 cells grown without TET is cytoplasmic in contrast to cells grown with TET, in which most of the staining is nuclear. Additionally, confocal microscopy shows that cyclin D1 colocalizes with SSeCKS in the cytoplasm (Fig. 6B). Although some S24/D1 cells grown without TET exhibited nuclear staining, there was a 70% reduction in the amount of nuclear cyclin D1 and the number of cells with nuclear staining compared to controls (Table 3). These data indicate that SSeCKS induces the cytoplasmic sequestration of ectopic cyclin D1.
FIG. 6.
SSeCKS overexpression redirects cyclin D1 to the cytoplasm. (A) Confocal analysis of cyclin D1 immunostaining. S24/D1 and V3/D1 (V3 cells overexpressing D1) cells grown in the presence (+) or absence (−) of TET were fixed and immunostained using PAb anti-cyclin D1. The nuclear staining of cyclin D1 is diminished and cytoplasmic staining is increased in response to SSeCKS overexpression. Note that the total cyclin D1 protein level is unchanged by TET in S24/D1 as shown in Fig. 4A. Magnification, ×88 (B) Confocal images of SSeCKS and cyclin D1 coimmunostaining. Cyclin D1 colocalizes in the cytoplasm with SSeCKS following SSeCKS overexpression. (C) Confocal images of SSeCKS and cyclin D1 double immunostaining viewed from the plane of X and Z axes. Magnification, ×550.
TABLE 3.
Percentage of cells showing nuclear or cytoplasmic staining of cyclin D1a
| Cell type | TET | % of cells showing staining of:
|
|
|---|---|---|---|
| Cytoplasm | Nucleus | ||
| V3/D1 | + | 3 ± 3 | 97 ± 4 |
| V3/D1 | − | 3 ± 2 | 93 ± 4 |
| S24/D1 | + | 4 ± 2 | 94 ± 4 |
| S24/D1 | − | 98 ± 4 | 24 ± 5 |
| S24/D1 + PMAc | + | NAd | 95 ± 4 |
| S24/D1 + PMA | − | NA | 78 ± 5 |
| 293T+D1-pBABEhygro (1:2.5)b | 22 ± 6 | 86 ± 4 | |
| 293T+D1-SSeCKS (1:2.5) | 78 ± 4 | 27 ± 4 | |
| 293T+D1-pBABEhygro (1:10) | 35 ± 4 | 80 ± 2 | |
| 293T+D1-SSeCKS (1:10) | 73 ± 6 | 18 ± 4 | |
Cells grown on 22-mm2 coverslips under various TET conditions (+, with; −, without) for 2 days were fixed and stained for cyclin D1 using PAb, as described in Materials and Methods. Independent fields of cells were counted (total of 250 to 300 cells for each analysis).
293T cells were transfected transiently with Lipofectamine (GIBCO-BRL) containing pEGFP-1, pRcCyclin D1 (gift of A. Dutta) plus pBABEhyg, or pBABEhyg/SSeCKS at either a 1:2.5 or 1:10 ratio, fixed after 40 h, and then stained for cyclin D as described above.
Cells grown with PMA (200 nM final concentration) for 30 min.
NA, not applicable because PMA treatment decreased the cytoplasmic, but not nuclear area.
On the possibility that the SSeCKS-induced sequestration of cyclin D was an artifact of our particular cell lines (i.e., due to the tTa transactivator, for example), we transiently transfected 293T cells with a cyclin D1 expressor plasmid with excess molar ratios of either an SSeCKS expressor plasmid or vector alone. Table 3 shows that SSeCKS induced a three- to fourfold increase in cytoplasmic cyclin D1 compared to vector alone. This clearly shows that SSeCKS can direct the cytoplasmic sequestration of cyclin D1 in several cell types, under conditions of both transient and stable expression and in the absence of the TET-regulated system.
SSeCKS binds G1 phase cyclins in vitro via tandem CY.
Work from Dutta's group identified a so-called CY which facilitates the binding of cyclins to several cell cycle components such as p21 (10). The SSeCKS gene encodes two closely spaced potential CY, KKLFSXXXXKKLSG [(K/R)(K/R) followed by two nonpolar residues, with the first usually Leu]. This domain also contains two major in vivo PKC sites, Ser507 and Ser515 (9, 40). We tested whether a GST fusion protein containing the SSeCKS CY (SSeCKS2) could bind G1-phase cyclins in an in vitro pull-down assay. Indeed, GST-SSeCKS2, but not GST alone, bound endogenous and ectopic cyclin D1 from lysates prepared from S24, S24/D1, S24/V, and V3 cells grown in the presence or absence of TET (data not shown). Stripping of the blot and reprobing with cyclin E-specific antibody showed that GST-SSeCKS2 also bound cellular cyclin E. The levels of cyclins D1 or E bound by GST-SSeCKS2 corresponded to their relative stoichiometry in the cells tested, indicating saturation in the binding kinetics. Thus, higher amounts of cyclin D1 were bound in the S24/D1 cell lysates irrespective of TET conditions, whereas in S24 cells, where SSeCKS overexpression suppresses D1 levels, less cyclin D1 was bound in the condition lacking TET compared to the condition with TET. In contrast, the binding to cyclin E was relatively constant throughout the cells lines, reflecting the similar levels of cyclin E in these cells, whether in conditions with or without TET (not shown). Additionally, prephosphorylation of GST-SSeCKS2 with rabbit brain PKC (Upstate Biotechnology) ablated cyclin D and E binding (data not shown).
Mutation of either the up- or downstream KK residues reduced cyclin D1 binding roughly 70%; the KK→SS mutation in both motifs reduced binding >95%. In contrast, the L→S mutations had little effect on cyclin D1 binding (data not shown). Importantly, none of the mutations affected the expression level or stability of the bacterially expressed GST-SSeCKS fusion products. These data show a dependence on the charged residues in the CY motifs for cyclin binding and further indicate that the CY motifs function both independently and in tandem.
SSeCKS-induced sequestration of cyclin D1 is PKC regulated.
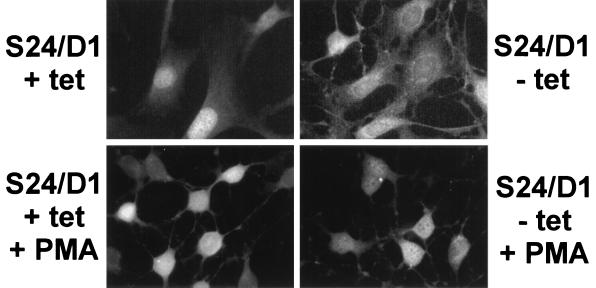
Our working hypothesis is that SSeCKS's scaffolding functions are down-modulated by kinases that are activated during G1→S progression. Nascent SSeCKS protein synthesized during early G1 or in confluent cultures is underphosphorylated and following mitogenic stimulation, becomes rapidly serine phosphorylated (43). Additionally, prephosphorylation of SSeCKS by PKC severely decreases its ability to bind phosphatidylserine and calmodulin (40; X. Lin and I. H. Gelman, unpublished data). Based on our finding that PKC-induced phosphorylation of GST-SSeCKS2 ablates in vitro binding activity to cyclins (above), we determined whether the in vivo activation of PKC affects the putative binding of SSeCKS to cyclin D1. Figure 7 and Table 3 show that the short-term addition of phorbol 12-myristate 13-acetate (PMA) to S24/D1 cells grown in the absence of TET caused a rapid translocation of cyclin D1 from cytoplasmic sites to the nucleus. Additionally, PMA induced SSeCKS translocation to perinuclear sites, as we and others showed previously (8, 25, 40). These data suggest that some of SSeCKS's in vivo scaffolding functions, possibly including binding to cyclins, are regulated by G1-phase phosphorylation.
FIG. 7.
Short-term activation of PKC induces the nuclear translocation of cyclin D1 sequestered in the cytoplasm. S24/D1 cells grown on coverslips in the presence (+) or absence (−) of TET were serum starved overnight and treated for 30 min with 200 nM PMA or solvent (dimethyl sulfoxide). The cells were fixed and stained for cyclin D1 as described in Materials and Methods. Note the increase in nuclear cyclin D1 staining after PKC activation (typified by the exclusion of nucleolar compartments) in the absence of TET.
Decreasing the levels of ectopic SSeCKS rescues G1 arrest and the nuclear translocation of cyclin D1.
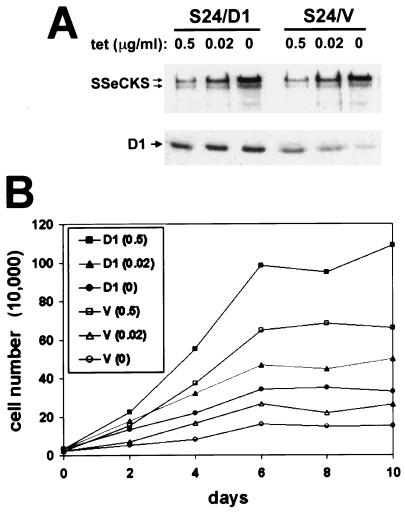
In S24/D1 cells grown in the absence of TET, ectopic SSeCKS levels are more abundant than the levels of ectopic cyclin D1 (Fig. 8A). We assumed that decreasing the levels of SSeCKS should increase the ability of ectopic D1 to translocate to the nucleus and, thus, rescue G1 arrest. To examine this possibility, we took advantage of the fact that the levels of ectopic SSeCKS could be modulated by varying the concentration of TET added. Figure 8A shows the effect of varying TET levels, such that at 0.02 μg/ml, the level of ectopic SSeCKS in both S24/V and S24/D1 cells was roughly equal to that in parental S6 cells. Most importantly, increasing the concentration of TET had no effect on the levels of ectopic D1 in S24/D1 cells but clearly caused an increase in the levels of endogenous D1 in S24/V cells due to a decrease in the ectopic levels of SSeCKS. Figure 8B shows that at a TET concentration of 0.02 μg/ml, S24/D1 cells proliferated at a significantly greater rate than S24/V cells, which were still partially growth arrested in G1. Moreover, there is a direct correlation between the increasing levels of ectopic SSeCKS and increasing levels of cytoplasmic cyclin D1 in the S24/D1 cells (Table 4). (Note: S24/D1 cells could not be compared directly to parental NIH 3T3/D1 cells because the latter lack the tTA-mediated effects on proliferation described in Fig. 1.) These data support the notion that SSeCKS-induced growth arrest and cyclin D1 translocation are dependent on the expression level of SSeCKS.
FIG. 8.
SSeCKS-induced growth arrest is not simply due to its overexpression at high levels. (A) Western blot showing the expression levels of SSeCKS and cyclin D1. S24/D1 and S24/V cells grown in the presence of TET (0.5, 0.02, or 0 μg/ml) were lysed for Western blotting analysis. A 0.02-μg/ml concentration of TET induces ectopic SSeCKS expression levels only two- to threefold above those of endogenous SSeCKS as determined by densitometric comparison. (B) S24/D1 cells are less growth arrested than S24/V cells as the level of ectopic SSeCKS is decreased (compare proliferation rates of S24/D1 at TET concentrations 0.5 and 0.02 μg/ml).
TABLE 4.
Increased levels of induced ectopic SSeCKS correlates with increased cytoplasmic cyclin D1 sequestrationa
| Cell type | TET concn (μg/ml) | % of cells showing staining of:
|
|
|---|---|---|---|
| Cytoplasm | Nucleus | ||
| S24/V | 0 | 3 ± 3 | 15 ± 4 |
| S24/V | 0.02 | 8 ± 4 | 34 ± 5 |
| S24/V | 0.5 | 7 ± 4 | 70 ± 4 |
| S24/D1 | 0 | 85 ± 6 | 32 ± 4 |
| S24/D1 | 0.02 | 29 ± 4 | 59 ± 4 |
| S24/D1 | 0.5 | 8 ± 6 | 96 ± 3 |
Cells grown on 22-mm2 coverslips in medium containing various concentrations of TET for 2 days were fixed and stained for cyclin D1 using PAb, as described in Materials and Methods. Independent fields of cells were counted (total of 250 to 300 cells for each analysis). Note that the decrease in nuclear staining in the S24/V cells correlating with decreasing TET concentration relates to the SSeCKS-induced downregulation of cyclin D1 (Fig. 8A).
Penetratin-linked CY encoding peptides compete for in vivo SSeCKS-cyclin D binding.
We were confounded in our attempts to show coimmunoprecipitation from cellular lysates due to SSeCKS association with the cytoskeleton: mild lysis conditions (e.g., 0.5% NP-40) resulted in the nonspecific coprecipitation of proteins with SSeCKS, whereas stronger detergent conditions (e.g., RIPA) stripped off all interacting proteins. Moreover, coprecipitating overexpressed proteins does not reflect physiological conditions nor do they rule out the involvement of intermediary or adapter proteins.
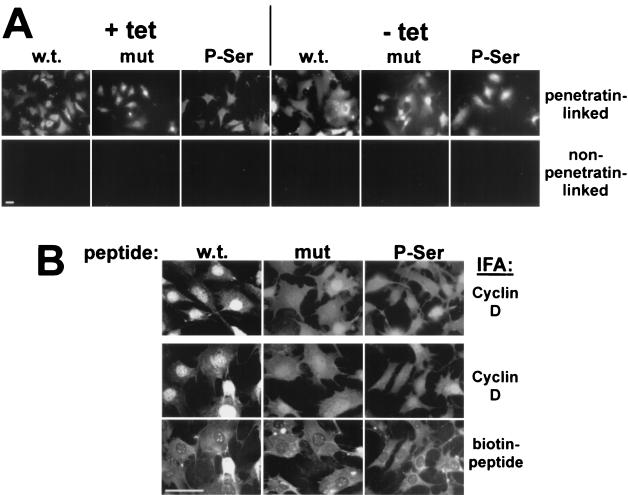
As an alternative approach to studying the in vivo interaction between SSeCKS and cyclin D1, we treated S24/D1 cells grown in the absence of TET with penetratin-linked peptides encoding either the wild-type (wt) SSeCKS CY domains, K→S mutants peptides, or peptides with phospho-Ser507/515. Penetratin corresponds to a homeodomain of the Drosophila Antennapedia transcription factor that facilitates the internalization of peptides, even phosphorylated versions (47), and oligonucleotides into many cell types (18), possibly by the formation of inverted micelles (reviewed in reference 17). Figure 9A shows that biotin-labeled versions of the penetratin-linked CY peptides (wt, K→S mutation, or phospho-Ser507/515) entered S24/D1 cells with equal efficiency, indicating that the CY motif variations had no effect on penetratin-mediated transduction. In contrast, biotin-labeled CY peptides lacking the penetratin motif did not enter at all, indicating that transduction is penetratin-mediated. Figure 9B and Table 5 show that incubation of S24/D1 cells for 4 h with the penetratin-wt CY peptide caused a roughly twofold increase in cyclin D translocation to nuclei, whereas the penetratin-K→S or -phospho-Ser507/515 peptides or the peptides lacking the penetratin motif did not significantly decrease the cytoplasmic sequestration of D1 (Table 5). These data indicate that the wt CY peptide competes with the in vivo binding between SSeCKS and cyclin D but that mutation of the basic KK residues or addition of phosphoserines in the CY domains ablates the competing activity.
FIG. 9.
Penetratin-linked peptides encoding the SSeCKS CY motifs induce nuclear translocation of cyclin D. (A) Entry of penetratin-linked peptides. S24/D1 cells grown on coverslips in the absence (−) or presence (+) of TET were incubated for 2 h in DMEM containing biotinylated peptides (100 μg/ml), washed, fixed, and incubated with FITC-labeled avidin as described in Materials and Methods. In contrast to the penetratin-linked peptides which entered cells, the non-penetratin-linked biotinylated peptides failed to enter cells. wt CY, w.t.; K→S mutant, mut; phospho-Ser507/515, P-Ser. (B) S24/D1 cells grown on 22-mm2 coverslips in the absence of TET were incubated for 4 h with either unlabeled (top panel) or biotinylated (middle and bottom panels) penetratin-linked wt CY, K→S, or phospho-Ser507/515 peptides; washed; fixed; and then stained for peptide with FITC-avidin (bottom panel) or for cyclin D1 with PAb and TRITC-labeled anti-rabbit immunoglobulin (top and middle panels). Note that the unlabeled penetratin peptides show similar effects on cyclin D compartmentalization as the biotinylated forms. Also note that although the phospho-Ser507/515 peptide fails to chase cyclin D into nuclei, it caused partial reduction in cell flattening. Size bars = 1 nm.
TABLE 5.
Percentage of cells showing nuclear staining of cyclin D1 following treatment with penetratin-linked peptidesa
| Cell type | Peptide added | TET | % of cells showing nuclear staining |
|---|---|---|---|
| S24/D1 | -- | + | 95 ± 3 |
| S24/D1 | -- | − | 28 ± 4 |
| S24/D1 | Penetratin-wt CY | − | 82 ± 5 |
| S24/D1 | Penetratin-K→S | − | 30 ± 5 |
| S24/D1 | Penetratin-phospho-Ser507/515 | − | 39 ± 4 |
| S24/D1 | wt CY | − | 95 ± 4 |
| S24/D1 | K→S | − | 95 ± 5 |
| S24/D1 | Phospho-Ser507/515 | − | 91 ± 5 |
Cells grown on 22-mm2 coverslips with (+) or without (−) TET for 3 days and then with or without peptide (100 μg/ml) for 4 h were fixed and stained for cyclin D1 using PAb, as described in Materials and Methods. Three independent fields of cells were counted (total of 250 to 300 cells for each analysis).
Cytoplasmic sequestration of cyclin D and E correlates with contact inhibition and induction of SSeCKS expression.
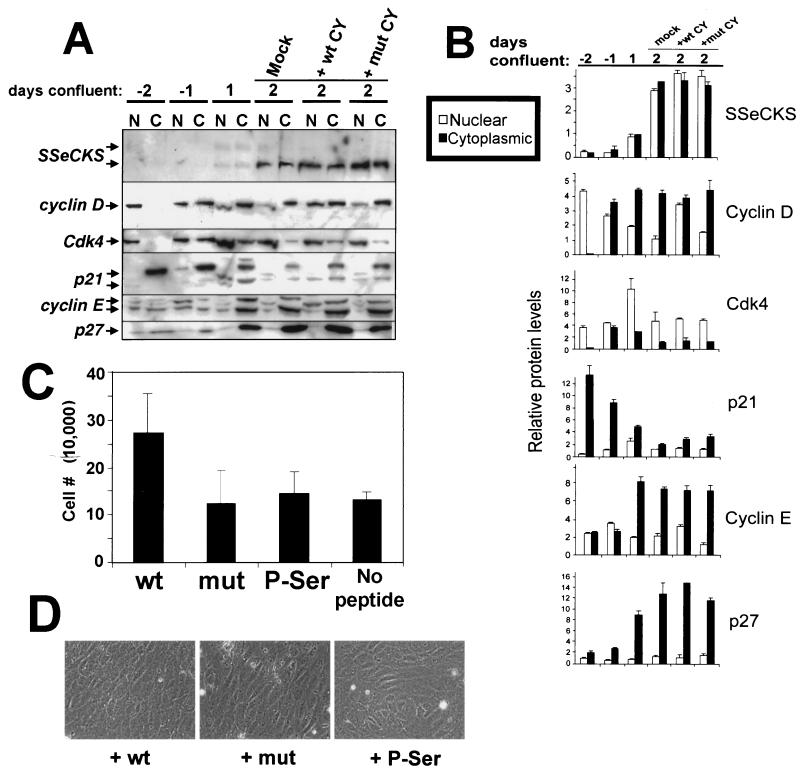
It is possible that the SSeCKS-induced sequestration of cyclins may be an artifact of SSeCKS overexpression. Thus, we investigated the level and compartmentalization of G1→S cyclins, CKIs and CDK4 as cycling untransformed rat embryo fibroblasts transition to contact inhibition. Figure 10A confirms our previous findings (39, 43) that the level of SSeCKS protein is induced by confluency. Although some nuclear SSeCKS has been identified by confocal immunofluorescence microscopy (25), we attribute much of the nuclear component here as perinuclear and/or cytoskeletal SSeCKS. Also, as described previously by others (61), contact inhibition results in increased p27 and decreased p21 levels relative to cycling cells. Much of the sustained levels of cyclin D in contact-inhibited populations is likely to be preexisting protein because the level of cyclin D transcription in confluent cells drops precipitously (data not shown). This agrees with the finding above that increased SSeCKS levels, whether ectopically induced by TET or endogenously induced by confluency, leads to inhibition of cyclin D transcription.
FIG. 10.
In vivo cytoplasmic sequestration of cyclin D by SSeCKS via CY domains correlates with contact inhibition. (A) Immunoblots of cell lysates from untransformed rat embryo fibroblasts grown pre- and postconfluency, probed for SSeCKS (arrows show the 290- and 280-kDa isoforms), cyclins D and E, CDK4, and CKIs p21 and p27. Actively dividing, subconfluent cultures (−2 days) were allowed to achieve saturation density (day 1 of confluence), and the following day they were either mock treated or supplemented twice daily for 2.5 more days with 100 μg of SSeCKS-wt-CY or mutant K→S-CY peptide per ml as described in the legend to Fig. 9. (B) The relative level of each protein band in panel A was determined by densitometric scanning and is represented at right as nuclear and cytoplasmic fractions. Relative protein levels were controlled by stripping the cyclin D blot and then reprobing with the other antibodies shown. The error bars reflect the composite of duplicate experiments. (C) Proliferation index of 2-day confluent cultures mock treated or treated with wt, K→S mutant, or P-Ser507/515 CY peptides. After peptide treatment, cells were trypsinized, stained with trypan blue, and counted using a hemacytometer. Error bars represent standard deviation based on analysis of triplicate samples for each treatment regimen. (D) Phase-contrast microscopy of rat embryo fibroblasts treated as in panel A, showing increased saturation density of the cells treated with the wt CY peptide yet no increase in cell refractility. Magnification, ×340.
The data in Fig. 10A clearly indicate a cytoplasmic shift for p27 and cyclins D and E correlating with confluency. In contrast, the earliest stages of cell confluence (days −1 and 1) are marked by increases in CDK4 level and an equilibrium between nuclear and cytoplasmic components with the majority of protein being nuclear. Lastly, cytoplasmic p21 levels, which are high in cycling cells, transiently shift to the nucleus at the onset of cell confluence.
To determine if SSeCKS plays a role in cytoplasmic sequestration during contact inhibition, rat embryo fibroblasts kept confluent for 2 days were treated for 2.5 additional days with repeated doses of either the wt or mutant penetratin-CY peptides. Figures 10A and B show that the wt CY peptide induced a three- to fivefold increase in nuclear cyclin D compared the mutant-CY peptide or mock treatment. This correlated with a twofold increase in cell density (Fig. 10C) in the absence of any increase in cell refractility (Fig. 10D). A marginal increase in nuclear cyclin E was also detected after wt CY peptide treatment. In contrast, the CY peptides had no effect on the localization of CDK4, p21, or p27. These data clearly indicate that antagonism of a putative SSeCKS-cyclin D cytoplasmic complex leads to cyclin D nuclear translocation and an increase in saturation density.
DISCUSSION
SSeCKS-induced growth arrest correlates with the downregulation of cyclin D1.
We present evidence that the inducible overexpression of SSeCKS in untransformed NIH 3T3 results in growth arrest in G1 accompanied by cell flattening and the loss of cyclin D1 expression. This result is predicted by our previous data showing that expression and phosphorylation of SSeCKS is cell cycle regulated during G1→S and that attempts to produce stable SSeCKS-expressing cell lines resulted in the selection of SSeCKS deletion mutants. SSeCKS-induced growth arrest cannot be viewed as simple toxicity since we showed elsewhere that v-src-transformed NIH 3T3 cells overexpressing SSeCKS (S24/ts72v-src cells) lose oncogenic growth characteristics but do not undergo growth arrest (38).
Our data also indicate that SSeCKS most likely prevents induction of cyclin D1 expression by preventing sustained activation of ERK2. However, unlike S24 cells, where the overexpression of SSeCKS inhibits serum-induced ERK2 activation, the proliferative ability of S24/ts72v-src cells correlates with an enigmatic superinduction of ERK2 (38). SSeCKS may inhibit the induction of cyclin D transcription by inducing cytoskeletal reorganization and, thus, integrin-mediated signals. We showed previously that SSeCKS induces an integrin-independent activation of the focal adhesion kinase (FAK) (25), which prevents apoptosis of S24 cells kept in suspension. However, unlike other systems in which integrin-mediated FAK activation has been tied to ERK2 activation during mitogenesis (6, 7), in S24 cells SSeCKS prevents FAK degradation and also disengages FAK from ERK2 activation (K. Moissoglu and I. H. Gelman, unpublished data). The fact that v-src superinduces ERK2 activity during SSeCKS overexpression indicates the existence of some crosstalk between SSeCKS-mediated pathways and v-src-induced, SSeCKS-independent pathways.
The SSeCKS-induced cell flattening phenomenon associated with G1-phase arrest is reminiscent of the effects of several other G1-phase regulators, such as p53 and pRb, which induce cell flattening when overexpressed (31, 56). However, in contrast to the effects of p53 and Rb, the SSeCKS-arrested cells are not senescent, because readdition of TET induces proliferative activity.
Our data show consistently that overexpression of full-length SSeCKS product results in similar G1 arrest patterns that correlate with cell flattening and loss of cyclin D1. In contrast, several cell lines containing SSeCKS products with small deletions, such as S23, did not induce any of these effects. Preliminary mapping indicates that the ectopic SSeCKS in S23 cells contains an ∼75-aa deletion covering the CY domains and other important scaffolding regions (data not shown). Thus, it is unclear whether the loss of cyclin binding sites per se is responsible for the loss of cellular effects and G1 arrest in S23. We speculate that the failure of mitogenically stimulated cells to transition to S phase causes a “backslide” to an early G1 or G0 state similar to that in differentiated cells. This might include the elaboration of an extensive cytoskeletal infrastructure (i.e., flattening) that maximizes rigidity and minimizes motility. Indeed, SSeCKS-induced cell flattening is linked with a cytoskeletal reorganization, specifically, the splaying of microfilament and microtubule networks, and the translocation of ERK2 away from focal adhesion complexes (25), possibly explaining how SSeCKS inhibits ERK2 activation.
The inverse relationship between the suppression of cyclin D1 transcription and the induction of endogenous SSeCKS expression in either early G1 phase or in confluent cultures strongly suggests that SSeCKS plays a direct role in negatively modulating G1→S progression. There has been an enigma as to why untransformed cells shut off cyclin D transcription when they reach a specific saturation density (read: contact inhibition) even in the presence of growth factors and clustered integrins. We speculate that SSeCKS serves as a gating protein for saturation density which functions by inhibiting cyclin D expression, and thus, G1→S progression. Indeed, knockout of SSeCKS expression by retrovirus transduction of antisense cDNAs causes increased spindle-like morphologies in stellate glomerular mesangial cells (44) and growth to higher saturation densities (Moissoglu and Gelman, unpublished data), suggesting that loss of SSeCKS decreases the cytoskeletal and signaling constraints for G1→S progression.
The finding that SSeCKS possibly sequesters cyclin D to the cytoplasm by direct binding is not surprising in light of the scaffolding functions attributed to the SSeCKS/Gravin protein family (23, 42, 46). Our group and others have speculated that the scaffolding functions for SSeCKS/Gravin in vivo are modulated by kinases activated in G1, specifically that phosphorylation of SSeCKS/Gravin decreases their binding affinity for partners and causes translocation of SSeCKS/Gravin from plasma membrane and cytoskeletal sites to the perinucleus (8, 25, 40). Nauert et al. (42) showed that prephosphorylation of Gravin decreased its phosphatidylserine-dependent binding of PKC, and we showed that prephosphorylation of SSeCKS by PKC decreased its binding of calmodulin (Lin and Gelman, unpublished data). Our data here indicate that the activation of PKC causes a rapid translocation of ectopic cyclin D1 to the nucleus even in the presence of overexpressed SSeCKS, presumably due to the direct PKC-mediated phosphorylation of Ser507/515 near the SSeCKS CY domains. This conclusion is strengthened by the inability of the penetratin–SSeCKS-CY-Ser507/515 peptide to inhibit the cytoplasmic sequestration of cyclin D1 by ectopic SSeCKS.
Little is known regarding the regulation of cyclin D1 protein during G1→S progression. Whereas cyclin D1 can be found in the cell nucleus during G1 and then rapidly translocates out of the nucleus during S, the total level of cyclin D as determined by immunoblotting analysis declines just slightly during S, suggesting that cyclin D1 function may be regulated by cell compartmentalization (54). Diehl and Sherr (20) showed that a T156A substitution in cyclin D1 caused retention of kinase-inactive D-CDK4 complexes in the cytoplasm, and furthermore, that the CDK4 in these complexes cannot be phosphorylated in vitro by the CDK-activating kinase. Because the cyclin DT156A-CDK4 complexes could be forced into the nucleus by the simultaneous overexpression of the CDK inhibitor, p21CIP1, these data suggest that something other than p21 can sequester cyclin D-CDK4 complexes in the cytoplasm. Also, because p21 knockout mice have no apparent phenotype (5), it is likely that other cyclin-binding proteins can also facilitate cyclin D-CDK4 complex formation and nuclear translocation. Indeed, p27 is the most likely candidate because mouse embryo fibroblasts deficient in both p21 and p27 have reduced levels of cyclin D, lack kinase-active cyclin D-CDK complexes, and are unable to transport cyclin D to the nucleus (12). One problem is that the p21-p27 knockout fibroblasts proliferate normally, suggesting that they were selected in vitro to be independent of cyclin D-CDK complexes for cell cycle progression. However, others have shown that cyclin E-CDK complexes can partially rescue G1→S progression in the absence of cyclin D (26), and indeed, the p21-p27 knockout cells have increased cyclin E levels and cyclin E-CDK activity (12).
These data are complicated by an even greater lack of knowledge of cyclin, CDK, and CKI function and expression during the process of contact inhibition. Kato et al. (35) showed that in response to contact-inhibited growth arrest, CDK4 remains complexed to cyclin D yet is enzymatically inactive. Interestingly, inhibition of calmodulin activity using N-(4-aminobutyl)-5-chloro-2-naphthalensulfonamide caused G1 phase arrest in normal rabbit kidney fibroblasts marked by the retention of cyclin D-CDK4 complexes in the cytoplasm (57). However, Weiser et al. (58) show opposite results, namely, that an increase in p16INK4 in confluent cultures of untransformed cells leads to the dissociation of CDK4 and cyclin D1. Our current data showing an inverse relationship in the nuclear and cytoplasmic levels between CDK4 and cyclin D during confluency agrees with the latter study.
A major role for cyclin D in cell cycle regulation is to complex with and activate CDK4, ultimately leading to the hyperphosphorylation of pRb and the release of S-phase inducing transcription factors such as E2F. Consistent with this, pRb remained hypophosphorylated when cyclin D1 expression was inhibited in our SSeCKS overexpressor cells. However, little is known regarding the regulation of cyclin D activity by cell compartmentalization. Our results indicate that cyclin D is sequestered in the cytoplasm during contact inhibition. We speculate that when cells are released from contact inhibition (i.e., replated at a lower density in the presence of growth factors), cytoplasmic cyclin D begins to form active complexes with CDK4 which translocate to the nucleus, ultimately leading to growth factor independence and G1→S progression. In support of this mechanism, Dietrich et al. (22) showed that platelet-derived growth factor-induced proliferation of contact-inhibited FH109 untransformed fibroblasts correlated with a nuclear translocation of kinase-active cyclin E-CDK2 complexes.
SSeCKS is not a direct in vitro substrate of baculovirus-produced, enzymatically active cyclin D-CDK4 complexes (data not shown), ruling out the possibility that overexpressed SSeCKS merely acts as a sink for CDK activity. Also, addition of GST-SSeCKS failed to block the in vitro phosphorylation of C-terminal sites in pRb by active cyclin D-CDK4 (not shown). This agrees with our in vivo data that overexpression of SSeCKS, even in the presence of overexpressed cyclin D1, does not alter cyclin D-associated CDK4 activity on a pRb substrate, although these lysates are prepared from total cells and not nuclei alone. Thus, we cannot rule out that SSeCKS-sequestered cytoplasmic D1 may be complexed with CDKs.
SSeCKS binds G1-phase cyclins in vitro via CY in a phosphorylation-dependent manner.
CY were originally identified as sequences in the N terminus of p21 required for formation of complexes between p21 and cyclin-CDKs and for the inhibitory effects of p21 on CDK activity. A second CY at the C terminus of p21, which also contributes to p21-cyclin interaction, seems less involved with modulating CDK activity (10). The CY is also present in other CDK inhibitors such as p27 and p57, activator CDC25, and substrates p107, p130, and E2F1, all of which form stable complexes with cyclin-CDKs (51). Although not yet demonstrated formally, the ability of p21 and/or p27 to facilitate the formation of kinase-active cytoplasmic cyclin D-CDK complexes (above) may be mediated by the interaction of p21-p27 CY domains with cyclin D.
Our results demonstrate that SSeCKS sequesters cyclin D in vivo and that an SSeCKS protein fragment encoding CY binds cyclins D and E in vitro. Interestingly, the SSeCKS CY domains (a.a. 503 to 507 and a.a. 512 to 515) are coincident with two major PKC phosphorylation sites on SSeCKS, and activation of PKC in vivo results in translocation of cyclin D1 to the nucleus, suggesting that SSeCKS's binding activity to cyclins is decreased by PKC-mediated phosphorylation and possibly by other mitogen-activated kinases. Mutagenesis analysis revealed that mutation of the charged residues (KK) in either CY greatly reduced binding, and that mutation of KK in both motifs resulted in even greater loss of binding. This indicates that both CY are functional individually but that they also function cooperatively. Interestingly, L→S mutation in either or both motifs had little effect on cyclin D binding. The fact that the KK→SS mutations in both CY were sufficient to abolish cyclin D binding suggests that a putative leucine zipper motif (LAPEEKTLPKHPEGIVSEVEML) just upstream in the GST-SSeCKS-2 product is not involved in cyclin binding.
The binding of a bacterially expressed SSeCKS protein fragment to cellular cyclin D1 and cyclin E suggests that SSeCKS and cyclins may form direct complexes in vivo. However, we were not able to detect association between SSeCKS and cyclins inside cells by coimmunoprecipitation although we could by confocal microscopy. Several possibilities may explain the failure: (i) polyclonal antibodies against SSeCKS or cyclin D1 used for immunoprecipitation may disrupt the complex, (ii) the detergents used may dissociate the interaction between SSeCKS and cyclins, and (iii) the association of the SSeCKS-cyclin complex with the cortical cytoskeleton may prevent their accessibility to the soluble cell fraction after lysis. In defense of the latter notion, several groups including our own have been unable to demonstrate in vivo binding between the SSeCKS/Gravin protein family and their in vitro binding partners such as PKC, PKA, or calmodulin (9, 40, 42). However, our demonstration that penetratin-linked CY peptides cause SSeCKS-sequestered cyclin D1 to translocate to the nucleus strongly suggests an in vivo interaction between SSeCKS and cyclin D facilitated by CY sequences. Most importantly, CY have been shown by others to facilitate in vitro protein-protein binding in the absence of adaptor proteins, strongly suggesting that the interaction between SSeCKS and cyclin in vivo is direct.
In untransformed rat embryo fibroblasts, the cytoplasmic sequestration of cyclin D correlates with an increase in both SSeCKS and p27 levels. However, fibroblasts from p27−/− mice are normal for contact inhibition and saturation density (41). This argues that p27 is not responsible for the cyclin D sequestration to the cytoplasm although it may play a role in the formation of kinase-active complexes. Additionally, because cytoplasmic p21 levels decrease with contact inhibition and fibroblasts from Rb-p21 double-knockout mice are contact inhibited (4), a role for p21 in cyclin D sequestration is unlikely.
The biological significance of the putative SSeCKS-cyclin D interaction in the context of contact inhibition is underlined by our findings that (i) the cytoplasmic sequestration of cyclin D in confluent cells correlates with the onset of SSeCKS expression and (ii) the addition of wt but not mutant CY peptides induces the nuclear translocation of cyclin D and an increase in saturation density. These data further our thesis that SSeCKS controls both G1→S progression and saturation density by sequestering cyclin D in the cytoplasm. Thus, the loss of the SSeCKS-cyclin D interaction in oncogene-transformed cells or primary tumors is most probably involved with the decontrol of cell-cell interactions that trigger contact-dependent saturation density. The finding that high endogenous levels of SSeCKS (in confluent cultures) correlate with high levels of cytoplasmic cyclin D yet decreased levels of nascent cyclin D RNA strongly suggests that the SSeCKS protein domain responsible for cyclin D transcriptional repression differs from the cyclin D-binding domains. Therefore, we would predict that an SSeCKS CY domain mutant would induce only higher saturation density but would not lose its ability to cause growth arrest when overexpressed.
Model for G1→S control by SSeCKS.
The expression and phosphorylation pattern of SSeCKS within G1→S transition suggests that it functions as a negative mitogenic regulator. Specifically, nascent SSeCKS protein induced by mitogens in nonconfluent cultures is underphosphorylated, and our results from in vitro assays indicate that this form encodes the greatest scaffolding activity. Continued treatment with mitogens causes a severe decrease in SSeCKS transcript levels and a concomitant increase in the relative level of SSeCKS serine phosphorylation and as we presume, a decrease in scaffolding activity in vivo. Interestingly, in confluent cultures, SSeCKS RNA and protein levels are superinduced in a serum-independent manner, and the addition of mitogens to these cultures fails to induce the serine phosphorylation of SSeCKS (43). Confluent cultures of untransformed Rat-6 or NIH 3T3 cells often express levels of SSeCKS similar to that induced in subconfluent cultures overexpressing SSeCKS using the TET system. Therefore, growth arrest in either confluent cultures or in the TET-regulated subconfluent cells may simply reflect the stoichiometric relationship between SSeCKS and mitogen-activated kinases: in confluent or overexpressor cultures, SSeCKS levels are in excess and thus, most SSeCKS is in the underphosphorylated, scaffolding-competent form, whereas in actively dividing cells, where SSeCKS levels are much lower, mitogen-activated kinases can sufficiently phosphorylate SSeCKS and inactivate scaffolding activity.
We postulate that high SSeCKS levels in untransformed cells facilitates both cytoskeletal organization and signaling control that typify the quiescent state. Most importantly, this includes preventing cyclin D transcription even in the presence of mitogens and engaged integrins. As predicted from this assumption, SSeCKS levels are severely downregulated in many oncogene-induced transformed cells and cancer cell lines, and knockout of SSeCKS expression leads to increased saturation densities and proliferation rates (above). Our data also suggest that SSeCKS ensures contact-inhibited growth through a secondary, fail-safe function, that being an ability to scaffold residual cyclin D protein in the cytoplasm. In agreement with this, several studies have shown that continuous plating of untransformed chick embryo fibroblasts at high densities increases the frequencies of spontaneous oncogenic transformation compared to cells plated at low densities (13, 14, 50).
ACKNOWLEDGMENTS
We thank Jean Wang for providing Abl-specific reagents, David Schatz for providing S2-6 cells, Gary Nolan for providing φNX ecotropic packaging cells, J. W. Harper for providing baculovirus-synthesized cyclin D1-CDK4 enzyme, Robert Krauss for stocks of ecotropic viruses encoding pLJ/cyclin D1, and Hermann Bujard for components of the TET-regulated expression system. We thank Lily Ossowski and Ed Johnson for critical review of the manuscript and Scott Henderson for help with confocal microscopy.
This work was supported by NCI grant R29-CA65787 to I.H.G.
REFERENCES
- 1.Assoian R K. Anchorage-dependent cell cycle progression. J Cell Biol. 1997;136:1–4. doi: 10.1083/jcb.136.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 3.Brancolini C, Schneider C. Phosphorylation of the growth arrest-specific protein Gas2 is coupled to actin rearrangements during G0→G1 transition in NIH 3T3 cells. J Cell Biol. 1994;124:743–756. doi: 10.1083/jcb.124.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brugarolas J, Bronson R T, Jacks T. p21 is a critical CDK2 regulator essential for proliferation control in Rb-deficient cells. J Cell Biol. 1998;141:503–514. doi: 10.1083/jcb.141.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon G J. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 6.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility, and signaling. Annu Rev Cell Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- 7.Burridge K. Signal transduction—crosstalk between Rac and Rho. Science. 1999;283:2028–2029. doi: 10.1126/science.283.5410.2028. [DOI] [PubMed] [Google Scholar]
- 8.Chapline C, Cottom J, Tobin H, Hulmes J, Crabb J, Jaken S. A major, transformation-sensitive PKC-binding protein is also a PKC substrate involved in cytoskeletal remodeling. J Biol Chem. 1998;273:19482–19489. doi: 10.1074/jbc.273.31.19482. [DOI] [PubMed] [Google Scholar]
- 9.Chapline C, Mousseau B, Ramsay K, Duddy S, Li Y, Kiley S C, Jaken S. Identification of a major protein kinase C-binding protein and substrate in rat embryo fibroblasts—decreased expression in transformed cells. J Biol Chem. 1996;271:6417–6422. doi: 10.1074/jbc.271.11.6417. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Saha P, Kornbluth S, Dynlacht B D, Dutta A. Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Farmer A A, Chen C F, Jones D C, Chen P L, Lee W H. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 1996;56:3168–3172. . (Erratum, 56:4074.) [PubMed] [Google Scholar]
- 12.Cheng M, Olivier P, Diehl J A, Fero M, Roussel M F, Roberts J M, Sherr C J. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow M, Rubin H. Evidence for cellular aging in long-term confluent cultures: heritable impairment of proliferation, accumulation of age pigments and their loss in neoplastic transformation. Mech Ageing Dev. 1996;89:165–183. doi: 10.1016/0047-6374(96)01744-7. [DOI] [PubMed] [Google Scholar]
- 14.Chow M, Rubin H. Irreversibility of cellular aging and neoplastic transformation: a clonal analysis. Proc Natl Acad Sci USA. 1996;93:9793–9798. doi: 10.1073/pnas.93.18.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daduang S, Kimura K, Nagata S, Fukui Y. Density dependent elevation of phosphatidylinositol-3 kinase level in rat 3Y1 cells. Biochim Biophys Acta. 1998;1401:113–120. doi: 10.1016/s0167-4889(97)00108-0. [DOI] [PubMed] [Google Scholar]
- 16.DelSal G, Loda M, Pagano M. Cell cycle and cancer: critical events at the G1 restriction point. Crit Rev Oncol. 1996;7:127–142. doi: 10.1615/critrevoncog.v7.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 17.Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- 18.Derossi D, Joliot A H, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J Biol Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 19.Diehl J A, Cheng M, Roussel M F, Sherr C J. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diehl J A, Sherr C J. A dominant-negative cyclin D1 mutant prevents nuclear import of cyclin-dependent kinase 4 (CDK4) and its phosphorylation by CDK-activating kinase. Mol Cell Biol. 1997;17:7362–7374. doi: 10.1128/mcb.17.12.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diehl J A, Zindy F, Sherr C J. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 1997;11:957–972. doi: 10.1101/gad.11.8.957. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich C, Wallenfang K, Oesch F, Wieser R. Translocation of cdk2 to the nucleus during G1-phase in PDGF-stimulated human fibroblasts. Exp Cell Res. 1997;232:72–78. doi: 10.1006/excr.1997.3507. [DOI] [PubMed] [Google Scholar]
- 23.Faux M C, Scott J D. Molecular glue: kinase anchoring and scaffold proteins. Cell. 1996;85:9–12. doi: 10.1016/s0092-8674(00)81075-2. [DOI] [PubMed] [Google Scholar]
- 24.Gelman I, Khan S, Hanafusa H. Morphological transformation, tumorigenicity and src-specific cytotoxic T lymphocyte-mediated tumor immunity induced by murine 3T3 cells expressing src oncogenes encoding novel non-myristylated N-terminal domains. Oncogene. 1993;8:2995–3004. [PubMed] [Google Scholar]
- 25.Gelman I H, Lee K, Tombler E, Gordon R, Lin X. Control of cytoskeletal architecture by the src-suppressed C kinase substrate, SSeCKS. Cell Motil Cytoskeleton. 1998;41:1–17. doi: 10.1002/(SICI)1097-0169(1998)41:1<1::AID-CM1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Geng Y, Whoriskey W, Park M Y, Bronson R T, Medema R H, Li T, Weinberg R A, Sicinski P. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell. 1999;97:767–777. doi: 10.1016/s0092-8674(00)80788-6. [DOI] [PubMed] [Google Scholar]
- 27.Gossen M, Bonin A L, Bujard H. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem Sci. 1993;18:471–475. doi: 10.1016/0968-0004(93)90009-c. [DOI] [PubMed] [Google Scholar]
- 28.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gradl G, Faust D, Oesch F, Wieser R J. Density-dependent regulation of cell growth by contactinhibin and the contactinhibin receptor. Curr Biol. 1995;5:526–535. doi: 10.1016/s0960-9822(95)00105-9. [DOI] [PubMed] [Google Scholar]
- 30.Gustincich S, Schneider C. Serum deprivation response gene is induced by serum starvation but not by contact inhibition. Cell Growth Differ. 1993;4:753–760. [PubMed] [Google Scholar]
- 31.Hinds P W, Mittnacht S, Dulic V, Arnold A, Reed S I, Weinberg R A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- 32.Hochholdinger F, Baier G, Nogalo A, Bauer B, Grunicke H H, Uberall F. Novel membrane-targeted ERK1 and ERK2 chimeras which act as dominant negative, isotype-specific mitogen-activated protein kinase inhibitors of Ras-Raf-mediated transcriptional activation of c-fos in NIH 3T3 cells. Mol Cell Biol. 1999;19:8052–8065. doi: 10.1128/mcb.19.12.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ingber D E. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan M H, Daniel C, Schindler U, Grusby M J. Stat proteins control lymphocyte proliferation by regulating p27Kip1 expression. Mol Cell Biol. 1998;18:1996–2003. doi: 10.1128/mcb.18.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato A, Takahashi H, Takahashi Y, Matsushime H. Inactivation of the cyclin D-dependent kinase in the rat fibroblast cell line, 3Y1, induced by contact inhibition. J Biol Chem. 1997;272:8065–8070. doi: 10.1074/jbc.272.12.8065. [DOI] [PubMed] [Google Scholar]
- 36.Knudsen E S, Wang J Y. Dual mechanisms for the inhibition of E2F binding to RB by cyclin-dependent kinase-mediated RB phosphorylation. Mol Cell Biol. 1997;17:5771–5783. doi: 10.1128/mcb.17.10.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavoie J N, Rivard N, L'Allemain G, Pouyssegur J. A temporal and biochemical link between growth factor-activated MAP kinases, cyclin D1 induction and cell cycle entry. Prog Cell Cycle Res. 1996;2:49–58. doi: 10.1007/978-1-4615-5873-6_5. [DOI] [PubMed] [Google Scholar]
- 38.Lin X, Gelman I H. Re-expression of the major protein kinase C substrate, SSeCKS, suppresses v-src-induced morphological transformation and tumorigenesis. Cancer Res. 1997;57:2304–2312. [PubMed] [Google Scholar]
- 39.Lin X, Nelson P J, Frankfort B, Tombler E, Johnson R, Gelman I H. Isolation and characterization of a novel mitogenic regulatory gene, 322, which is transcriptionally suppressed in cells transformed by src and ras. Mol Cell Biol. 1995;15:2754–2762. doi: 10.1128/mcb.15.5.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin X, Tombler E, Nelson P J, Ross M, Gelman I H. A novel src- and ras-suppressed protein kinase C substrate associated with cytoskeletal architecture. J Biol Chem. 1996;271:28430–28438. doi: 10.1074/jbc.271.45.28430. [DOI] [PubMed] [Google Scholar]
- 41.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D Y. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 42.Nauert J, Klauck T, Langeberg L K, Scott J D. Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffolding protein. Curr Biol. 1997;7:52–62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- 43.Nelson P, Gelman I H. Cell-cycle regulated expression and serine phosphorylation of the myristylated protein kinase C substrate, SSeCKS: correlation with cell confluency, G0 phase and serum response. Mol Cell Biochem. 1997;175:233–241. doi: 10.1023/a:1006836003758. [DOI] [PubMed] [Google Scholar]
- 44.Nelson P J, Moissoglu K, Vargas J J, Klotman P E, Gelman I H. Involvement of the protein kinase C substrate, SSeCKS, in the actin-based stellate morphology of mesangial cells. J Cell Sci. 1999;112:361–370. doi: 10.1242/jcs.112.3.361. [DOI] [PubMed] [Google Scholar]
- 45.Norton K K, Mahadeo D K, Geist R T, Gutmann D H. Expression of the neurofibromatosis 1 (NF1) gene during growth arrest. Neuroreport. 1996;7:601–604. doi: 10.1097/00001756-199601310-00054. [DOI] [PubMed] [Google Scholar]
- 46.Pawson T, Scott J D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 47.Peck D, Isacke C M. Hyaluronan-dependent cell migration can be blocked by a CD44 cytoplasmic domain peptide containing a phosphoserine at position 325. J Cell Sci. 1998;111:1595–1601. doi: 10.1242/jcs.111.11.1595. [DOI] [PubMed] [Google Scholar]
- 48.Pines J. Cell cycle. Conformational change. Nature. 1995;376:294–295. doi: 10.1038/376294a0. [DOI] [PubMed] [Google Scholar]
- 49.Reed S I. G1/S regulatory mechanisms from yeast to man. Prog Cell Cycle Res. 1996;2:15–27. doi: 10.1007/978-1-4615-5873-6_2. [DOI] [PubMed] [Google Scholar]
- 50.Rubin H, Yao A, Chow M. Neoplastic development: paradoxical relation between impaired cell growth at low population density and excessive growth at high density. Proc Natl Acad Sci USA. 1995;92:7734–7738. doi: 10.1073/pnas.92.17.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saha P, Eichbaum Q, Silberman E D, Mayer B J, Dutta A. p21CIP1 and Cdc25A: competition between an inhibitor and an activator of cyclin-dependent kinases. Mol Cell Biol. 1997;17:4338–4345. doi: 10.1128/mcb.17.8.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory Manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 53.Sherr C J. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 54.Sherr C J, Roberts J M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 55.Shockett P, Difilippantonio M, Hellman N, Schatz D G. A modified tetracycline-regulated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci USA. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugrue M M, Shin D Y, Lee S W, Aaronson S A. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci USA. 1997;94:9648–9653. doi: 10.1073/pnas.94.18.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taules M, Rius E, Talaya D, Lopez-Girona A, Bachs O, Agell N. Calmodulin is essential for cyclin-dependent kinase 4 (Cdk4) activity and nuclear accumulation of cyclin D1-cdk4 during G1. J Biol Chem. 1998;273:33279–33286. doi: 10.1074/jbc.273.50.33279. [DOI] [PubMed] [Google Scholar]
- 58.Wieser R J, Faust D, Dietrich C, Oesch F. p16INK4 mediates contact-inhibition of growth. Oncogene. 1999;18:277–281. doi: 10.1038/sj.onc.1202270. [DOI] [PubMed] [Google Scholar]
- 59.Wieser R J, Schutz S, Tschank G, Thomas H, Dienes H P, Oesch F. Isolation and characterization of a 60–70-kD plasma membrane glycoprotein involved in the contact-dependent inhibition of growth. J Cell Biol. 1990;111:2681–2692. doi: 10.1083/jcb.111.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamada K M. Integrin signaling. Matrix Biol. 1997;16:137–141. doi: 10.1016/s0945-053x(97)90001-9. [DOI] [PubMed] [Google Scholar]
- 61.Yanagisawa K, Kosaka A, Iwahana H, Nakanishi M, Tominaga S. Opposite regulation of the expression of cyclin-dependent kinase inhibitors during contact inhibition. J Biochem (Tokyo) 1999;125:36–40. doi: 10.1093/oxfordjournals.jbchem.a022265. [DOI] [PubMed] [Google Scholar]
- 62.Zhu L, van-den-Heuvel S, Helin K, Fattaey A, Ewen M, Livingston D, Dyson N, Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993;7:1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]