Abstract
Background/Objective:
Smoking prevalence in patients with chronic pancreatitis [CP] is high. We aimed to understand lifetime history of smoking and cohort trends in CP patients to inform effective strategies for smoking cessation.
Method:
Data on 317 CP patients from the North American Pancreatitis Study 2 [NAPS2] Continuation and Validation Study and the NAPS2 Ancillary Study were analyzed. Smoking history was assessed for each phase of life from the onset of smoking to study enrollment. Data on second-hand smoke and drinking history were also collected. We compared demographic factors, drinking history, pain level and pancreas morphology by smoking status at age 25 (non-smoking, <1 pack per day [PPD], ≥1 PPD). We compared smoking prevalence by birth cohorts: 1930-1949, 1950-1969, 1970-1989.
Result:
Fifty-one percent of CP patients reported smoking at the time of enrollment. Those who smoked ≥1 PPD at age 25 smoked a cumulative total of 30.3 pack-years of cigarettes over a lifetime. Smoking at age 25 was associated with greater lifetime drinking and greater exposure to second-hand smoke at home and at workplace. Pancreatic atrophy and pseudocysts were more common among smokers. Pancreatic pain was more severe among smokers, and 12-13% of smokers reported smoking to alleviate pain. Male CP patients bom in 1950-1969 reported the highest peak prevalence of smoking, and female CP patients bom in 1970-1989 reported highest peak prevalence of smoking.
Conclusion:
CP patients exhibit intense and sustained smoking behavior once established in the 20s. Regardless, cohort analyses demonstrate that the behaviors could potentially be altered by policy changes.
Introduction
Smoking is an established risk factor of chronic pancreatitis (CP)1, 2 impacting every pathophysiologic stage of CP.3 Cumulative smoking of 20 pack-years or more is associated with a 2-fold increased risk of acute pancreatitis.4 Daily smoking increases the risk of CP independent of alcohol consumption,5-7 and accelerates the progression of CP.8, 9 In animal model studies, nicotine and the tobacco products have been shown to induce morphological changes in the pancreas consistent with pancreatitis, cause abnormal pancreatic secretion and zymogen activation, and promote inflammation leading to acinar cell damage.10
A majority of patients report continued smoking after diagnosis of CP, despite abstaining from alcohol.11 Some patients even report smoking to alleviate pain.11 While physicians have historically frequently failed to identify smoking as a contributing factor among CP patients who smoke,3 more recent data demonstrates that physician’s recognition of smoking as a contributing cause of pancreatitis has improved among current smokers.12 While cumulative and lifetime changes in drinking in CP patients have been described in the literature,11, 13, 14 lifetime smoking history is not well-understood in this population. Moreover, the landscape of smoking behavior has changed over the years in the U.S. with anti-tobacco campaigns, smoking bans, and cigarette taxes.15,16 It is unknown how the change in tobacco policies has impacted the manifestation and incidence of CP. Also unexplored is the influence of secondhand smoking at home and in the workplace on CP risk. Given this, we aimed to describe the lifetime smoking patterns of CP and examine the association of smoking with clinical manifestation of CP and comorbidity. In addition, we also aimed to describe cohort effects on prevalence of smoking and explore exposures to second-hand smoke.
Methods
Study population
We analyzed data from study participants enrolled in the North American Pancreatitis Study 2 Continuation and Validation Study (NAPS2-CV) and North American Pancreatitis Study 2– Ancillary Study (NAPS2-AS).17, 18 which prospectively enrolled subjects with a diagnosis of CP from 2008 to 2014. Among 424 patients with CP in whom lifetime drinking was evaluated, we included data on 317 participants who provided complete information on lifetime smoking history and who had not developed CP prior to turning age 25. We purposely excluded those with CP onset prior to age 25, to capture a population that would have been had an opportunity to start smoking legally.
Smoking and drinking data
We assessed lifetime smoking patterns by interviewing the patients about average number of cigarettes smoked on a smoking day, the frequency of smoking per month (or per week), and the duration of smoking for each phase of life starting from the time the participant began smoking until the diagnosis of CP. Current smoking was assessed at the time of enrollment. Any major changes in cigarette smoking frequency or intensity were noted, including periods of cessation. For the purpose of comparing heavy smokers to moderate smokers and non-smokers, we categorized patients according to intensity of smoking at age 25, to be able to assess the influence of early smoking on age of CP onset. Heavy smoking was defined as smoking 1 PPD or more on a smoking day, moderate smoking as <1 PPD on a smoking day and never smoking as less than 100 cigarettes in a lifetime. Cumulative smoking was estimated in pack-years by summing the products of cigarettes smoked on smoking day X number of smoking days per month X 12 months/year for each phase and dividing by 20 cigarettes/pack. In addition to personal history of smoking, we inquired about the duration of second hand and environmental smoke exposure at home as a child and separately as an adult. We also included a question about duration of working in an environment where co-workers smoked cigarettes. We applied a validated 5-item alcohol disorder instrument called TWEAK19 to evaluate alcohol consumption prior to diagnosis of pancreatitis. TWEAK is scored from a scale of 0-7, with ≥3 indicating problematic drinking. We assessed lifetime drinking history following the approach by Skinner et al.20 from the time of onset of drinking to diagnosis of CP. Detailed methods for lifetime drinking has been presented previously.11 Briefly, patients were asked to provide the age at the beginning of each drinking phase, drinks consumed on a drinking day, frequency of drinking per month, number of days per month when drinking exceeded the usual levels. Periods of abstinence were also noted. Current drinking status was assessed relative to the time of enrollment.
Covariates
The morphology of CP was determined by standard of care pancreatic imaging, including abdominal computed tomography, magnetic resonance imaging, magnetic resonance cholangiopancreatography, or endoscopic ultrasound, as reported by the enrolling physician in a structured case report form. Features of interest included presence of calcifications, pseudocyst, atrophy, pancreatic ductal irregularity and/or dilation. Similarly, the enrolling physicians noted the presence of diabetes and exocrine insufficiency. The severity of pain due to CP was assessed by the patients as mild, moderate or severe and the patterns of pain due to CP was described as none, intermittent or constant. We assessed any self-reported comorbidity present at the time of enrollment. For the purposes of the current analyses, we summarized data on smoking-related comorbid conditions including chronic obstructive pulmonary disease, hypertension, heart disease, stroke, liver disease, and cancer.
Statistical analysis
Descriptive characteristics are reported as median (interquartile range) for continuous variables and n (%) for categorical variables for the entire population as well as by smoking category at age 25 (never smoked, smoked <1PPD, smoked>=1PPD as defined previously). Differences between groups are tested using Kruskal-Wallis tests for continuous variables and chi-squared tests for categorical variables; additionally, Jonckheere-Terpstra, Cochran-Armitage and linear regression trend tests, are presented for tests of trend across the three smoking categories where relevant. We also present color component bar diagrams to illustrate individual-level data on smoking intensity across the lifespan. Statistical analyses were performed in SAS 9.4.
Birth Cohort
To identify potential temporal changes in smoking prevalence over time, we conducted a cohort analysis of smoking prevalence by birth decade and by age of smoking. We grouped patients by decades starting from the earliest birth year represented in the participants to the latest and combined decades with sparse data (1930-1949, 1950-1969, 1970-1989). Within each birth cohort, we computed the prevalence of smoking at each age to evaluate the age of smoking initiation and the age at peak prevalence of smoking. Data points based on fewer than 10 were not included.
Results
A total of 317 individuals with a CP diagnosis were included in the analysis. At the time of enrollment 51% of the study participants reported smoking. At the age of 25, 105 (33%) were not smoking, 85 (27%) smoked less than 1 pack per day and 127 (40%) were smoking 1 PPD or more. Table 1 presents the distribution of demographic factors, drinking history by the smoking status at age 25. Smokers were more likely to be male than non-smokers, and heavy smokers were more likely to be of White race. While age of CP diagnosis was lowest among heavy smokers (47 years), this was not statistically different from that of non-smokers (49 years) and moderate smokers (51 years). Smokers were leaner at enrollment and at their peak weight as compared to non-smokers. Current drinking status was similar between the three groups (20-25%), but moderate and heavy smokers were more likely to have a higher consumption of alcohol in the past than non-smokers. Moderate and heavy smokers reported higher number of drinks on a drinking day (9.7-10.0 drinks/day) at their maximum drinking phase as compared to non-smokers (4.7 drinks/day) and higher frequency of drinking (30 days/month) as compared to non-smokers (8 days/month). (Table 1)
Table 1.
Distribution of demographic and alcohol-related variables by smoking status at age 25 in persons diagnosed with chronic pancreatitis
| Variable | All (N = 317) |
Did not smoke [A] (N = 105) |
Smoked <1 PPD [B] (N = 85) |
Smoked ≥1 PPD [C] (N = 127) |
Trend P-Value |
A&B vs C P-Value |
A vs B&C P-Value |
|---|---|---|---|---|---|---|---|
| n (%) or median (interquartile range) | n (%) or median (interquartile range) | n (%) or median (interquartile range) | n (%) or median (interquartile range) | ||||
| Male sex | 187 (59.0) | 52 (49.5) | 50 (58.8) | 85 (66.9) | 0.0073a | 0.0188 | 0.0159 |
| Black Race | 128 (40.4) | 34 (32.4) | 50 (58.8) | 44 (34.6) | 0.8808a | 0.089 | 0.0411 |
| Age at enrollment (years) | 53.0 (45.0-59.0) | 54.0 (44.0-61.0) | 53.0 (47.0-59.0) | 51.0 (45.0-57.0) | 0.1982b | 0.1165 | 0.5643 |
| Age at CP Diagnosis (years) | 48.0 (40.0-57.0) | 49.0 (39.0-58.0) | 51.0 (41.0-56.0) | 47.0 (41.0-54.0) | 0.5182b | 0.316 | 0.9771 |
| Body Mass Index at enrollment (kg/m2) | 24.2 (21.0-28.3) | 25.8 (22.5-29.9) | 23.5 (20.4-26.3) | 23.6 (20.6-27.4) | 0.0121b | 0.1956 | 0.0009 |
| Highest Body Mass Index ever (kg/m2) | 29.2 (25.6-33.9) | 30.4 (26.9-34.3) | 28.4 (24.8-33.4) | 28.4 (25.2-34.9) | 0.1538b | 0.4745 | 0.0622 |
| Drinking at enrollment | 71 (22.4) | 21 (20.0) | 18 (21.2) | 32 (25.2) | 0.9722a | 0.7522 | 0.7772 |
| TWEAK score of ≥ 3 prior to CP diagnosis | 172 (54.3) | 33 (31.4) | 54 (63.5) | 85 (66.9) | <.0001a | 0.0002 | <.0001 |
| Average # of drinks per day during period of drinking | 2.0 (0.1- 5.5) | 0.2 (0.0- 2.8) | 2.7 (1.0- 6.2) | 4.0 (0.7- 7.9) | <.0001b | <.0001 | <.0001 |
| Average # of drinks on a drinking day during periods of drinking | 4.8 (1.8- 8.1) | 2.0 (0.0- 5.0) | 5.6 (3.0- 8.5) | 6.1 (3.0-10.3) | <.0001b | <.0001 | <.0001 |
| # of drinks on a drinking day during maximum drinking period | 8.0 (4.0-12.6) | 4.7 (2.0- 9.0) | 9.7 (6.0-13.6) | 10.0 (5.6-16.3) | <.0001b | 0.0006 | <.0001 |
| History of heavy drinking based on maximum lifetime drinking period | 202 (63.7) | 36 (34.3) | 67 (78.8) | 99 (78.0) | <.0001a | <.0001 | <.0001 |
| The # of drinking days | 25.0 (8.0-30.0) | 8.0 (3.0-30.0) | 30.0 (13.0-30.0) | 30.0 (10.0-30.0) | 0.0002b | 0.0178 | <.0001 |
| per month during the maximum drinking period | |||||||
| Drinking duration (years) | 27.0 (18.0-36.0) | 25.0 (16.0-35.0) | 30.5 (22.0-37.0) | 27.0 (18.0-36.0) | 0.7054b | 0.8059 | 0.2351 |
| Duration spent at maximum drinking level in years | 8.0 (4.0-18.0) | 9.0 (3.0-20.0) | 8.0 (4.0-15.0) | 8.0 (4.0-17.0) | 0.9851b | 0.9595 | 0.9768 |
| Cumulative Lifetime Alcohol Consumption in Kilograms of alcohol | 352 (65.7- 766) | 72.9 (13.2- 480) | 414 (192- 698) | 414 (112-1014) | <.0001b | 0.0034 | <.0001 |
PPD = Packs per day,
= Cochran-Armitage,
= Jonckheere-Terpstra
Smoking onset, lifetime smoking history are presented in Table 2 for CP patients who smoked at age 25. Age at smoking initiation was younger (16 years) in heavy smokers as compared to moderate smokers (18 years). (Table 2) Thirty-four % of patients who were not smoking at age 25 reported ever smoking over a lifetime, with 13% continuing to smoke at the time of enrollment. In contrast, 63.5% and 74% of CP patient who were smoking moderately or heavily at age 25 were continuing to smoke at the time of enrollment, past CP diagnosis. A small, but non-trivial (12-13%) proportion of smokers reported smoking to alleviate pain. During periods of most intense smoking, heavy smokers smoked on average of 1 pack per day (19.9 cigarettes per smoking day), moderate smokers reported smoking on average of half of a pack (10 cigarettes/day). Those who smoked heavily at age 25 smoked 10,440 packs over an average of 30 years until the CP diagnosis, equivalent to 30.3 pack-years of smoking. Those who smoked moderately at age 25 smoked on average a cumulative total of 4,540 packs over an average duration of 30 years until CP diagnosis, equivalent to 13.9 packs-years of smoking.
Table 2.
Distribution of lifetime smoking variables by intensity of smoking at age 25 in persons diagnosed with chronic pancreatitis
| Variable | Category | Smoked <1 PPD (N = 85) | Smoked ≥1 PPD (N = 127) | P-value |
|---|---|---|---|---|
| n (%) or median (interquartile range) | n (%) or median (interquartile range) | |||
| Age when smoking started (years) | 18.0 (15.0-20.0) | 16.0 (14.0-18.0) | 0.0015 | |
| Smoking status at enrollment | Current smoker |
54 (63.5) | 94 (74.0) | 0.1031 |
| Former smoker |
31 (36.5) | 33 (26.0) | ||
| Never smoker | 0 (0.0) | 0 (0.0) | ||
| Smoking to alleviate pain | 11 (12.9) | 15 (11.8) | 0.7726 | |
| Average # of cigarettes per day during periods of smoking | 9.0 (5.0-12.2) | 19.3 (16.4-24.7) | <0.0001 | |
| Average # of cigarettes per day on a smoking day during periods of smoking | 10.0 (8.0-12.9) | 19.9 (17.7-26.5) | <0.0001 | |
| Smoking duration (years) | 30.0 (22.0-37.0) | 30.0 (23.0-36.0) | 0.9290 | |
| Cumulative # of packs smoked until CP diagnosis | 4540 (2104-6976) | 10440 (6480-14040) | <0.0001 | |
| Smoking pack-years until CP diagnosis | 13.9 (7.3-20.5) | 30.3 (21.0-41.1) | <0.0001 |
PPD = packs per day
Second-hand smoke exposure history is presented in Table 3. The distribution of childhood exposure to smoke for 10 or more years were similar between the three smoking groups (61-70%), while both moderate (55%) and heavy smokers (59%) more likely to be exposed also to 10 or more years of second-hand smoke at home as an adult as compared to non-smokers (33%). (Table 3) There was also greater exposure to second-hand smoke at workplace for at least 10 years among heavy smokers (61%), as compared to moderate smokers (49%) and non-smokers (37%). (Table 3)
Table 3.
Distribution of environmental smoke exposure by smoking intensity at age 25 in persons diagnosed with chronic pancreatitis
| Variable | Category | All (N = 317) | Did not smoke [A] (N = 105) | Smoked <1 PPD [B] (N = 85) | Smoked ≥1 PPD [C] (N = 127) | Trend P-Value | A&B vs C P-Value | A vs B&C P-Value | B vs C P-Value |
|---|---|---|---|---|---|---|---|---|---|
| n (%) or median (interquartile range) | n (%) or median (interquartile range) | n (%) or median (interquartile range) | n (%) or median (interquartile range) | ||||||
| Childhood household smoke exposure | <1 year | 75 (23.7) | 26 (24.8) | 22 (25.9) | 27 (21.3) | 0.3768c | 0.6077 | 0.6671 | 0.6574 |
| 1-9 years | 20 (6.3) | 5 (4.8) | 7 (8.2) | 8 (6.3) | . | . | . | ||
| 10-18 years | 209 (65.9) | 64 (61.0) | 56 (65.9) | 89 (70.1) | . | . | . | ||
| Household passive smoke exposure as adult | <1 year | 86 (27.1) | 40 (38.1) | 18 (21.2) | 28 (22.0) | 0.0003c | 0.0148 | 0.0024 | 0.2833 |
| 1-9 years | 60 (18.9) | 20 (19.0) | 18 (21.2) | 22 (17.3) | . | . | . | ||
| 10-19 years | 56 (17.7) | 16 (15.2) | 17 (20.0) | 23 (18.1) | . | . | . | ||
| 20-29 years | 48 (15.1) | 7 (6.7) | 12 (14.1) | 29 (22.8) | . | . | . | ||
| 30-39 years | 30 (9.5) | 7 (6.7) | 13 (15.3) | 10 (7.9) | . | . | . | ||
| 40+ years | 22 (6.9) | 4 (3.8) | 5 (5.9) | 13 (10.2) | . | . | . | ||
| Years worked in place where people smoked | <1 year | 74 (23.3) | 29 (27.6) | 20 (23.5) | 25 (19.7) | 0.0008c | 0.0083 | 0.0453 | 0.1675 |
| 1-9 years | 72 (22.7) | 28 (26.7) | 22 (25.9) | 22 (17.3) | . | . | . | ||
| 10-19 years | 60 (18.9) | 19 (18.1) | 17 (20.0) | 24 (18.9) | . | . | . | ||
| 20-29 years | 48 (15.1) | 10 (9.5) | 9 (10.6) | 29 (22.8) | . | . | . | ||
| 30+ years | 51 (16.1) | 10 (9.5) | 16 (18.8) | 25 (19.7) | . | . | . |
PPD = packs per day,
= Linear Regression
Comorbid conditions
Comparison of morphologic findings of pancreatitis and comorbid conditions by smoking status at age 25 are presented in Table 4. Heavy smokers (45%) were less likely than non-smokers (53%) and moderate smokers (61%) to show calcifications, although the trend was not significant. Moderate (35%) and heavy smokers (39%) were more likely to show pseudocysts than non-smokers (26%). Heavy smokers were more likely to show atrophy (66%) as compared to non-smokers (51%) and moderate smokers (49%). While the prevalence of severe pain was largely prevalent across the smoking categories, moderate (71%) and heavy smokers (76%) were also more likely to report severe pain as compared to non-smokers (59%). As anticipated, the prevalence of COPD was higher among heavy smokers (10%) as compared to moderate (7.1%) and non-smoker (3.8%), but the difference did not reach statistical significance. There was no difference in other major comorbidity including heart disease, liver disease, or cancer.
Table 4.
Distribution of morphologic findings and comorbidities by smoking intensity at age 25 in persons with chronic pancreatitis
| Variable | All (N = 317) | Did not Smoke [A] (N = 105) | Smoked <1 PPD [B] (N = 85) | Smoked ≥1 PPD [C] (N = 127) | Trend P-value | A&B vs C P-Value | A vs B&C P-Value | B vs C P-Value |
|---|---|---|---|---|---|---|---|---|
| Morphologic severity of CP - n (%) | ||||||||
| Calcifications | 165 (52.1) | 56 (53.3) | 52 (61.2) | 57 (44.9) | 0.1682a | 0.0367 | 0.7476 | 0.02 |
| Pseudo cysts | 107 (33.8) | 27 (25.7) | 30 (35.3) | 50 (39.4) | 0.0301a | 0.0838 | 0.0331 | 0.5485 |
| Atrophy | 180 (56.8) | 54 (51.4) | 42 (49.4) | 84 (66.1) | 0.0199a | 0.006 | 0.1757 | 0.015 |
| PD irregularity | 183 (57.7) | 64 (61.0) | 47 (55.3) | 72 (56.7) | 0.5308a | 0.7602 | 0.4135 | 0.8406 |
| PD dilation | 210 (66.2) | 72 (68.6) | 59 (69.4) | 79 (62.2) | 0.2730a | 0.245 | 0.4404 | 0.3848 |
| Diabetes | 115 (36.3) | 41 (39.0) | 29 (34.1) | 45 (35.4) | 0.5855a | 0.7982 | 0.4704 | 0.8439 |
| Exocrine Insufficiency | 134 (42.3) | 46 (43.8) | 37 (43.5) | 51 (40.2) | 0.5664a | 0.5334 | 0.6964 | 0.6253 |
| Pain Severity — n (%) | ||||||||
| No pain | 46 (14.5) | 20 (19.0) | 11 (12.9) | 15 (11.8) | 0.015c | 0.0978 | 0.0316 | 0.6819 |
| Mild/Moderate | 53 (16.7) | 23 (21.9) | 14 (16.5) | 16 (12.6) | . | . | . | |
| Severe | 218 (68.8) | 62 (59.0) | 60 (70.6) | 96 (75.6) | . | . | . | |
| Pain Temporal — n (%) | ||||||||
| No pain | 46 (14.5) | 20 (19.0) | 11 (12.9) | 15 (11.8) | 0.0604c | 0.3588 | 0.1581 | 0.9089 |
| Intermittent | 93 (29.3) | 33 (31.4) | 25 (29.4) | 35 (27.6) | . | . | . | |
| Constant | 178 (56.2) | 52 (49.5) | 49 (57.6) | 77 (60.6) | . | . | . | |
| Smoking-related comorbidity at enrollment — n (%) | ||||||||
| COPD | 23 (7.3) | 4 (3.8) | 6 (7.1) | 13 (10.2) | 0.0601a | 0.0944 | 0.096 | 0.4273 |
| Hypertension | 150 (47.3) | 43 (41.0) | 47 (55.3) | 60 (47.2) | 0.3846a | 0.9827 | 0.1101 | 0.2506 |
| Heart Disease | 30 (9.5) | 7 (6.7) | 11 (12.9) | 12 (9.4) | 0.5114a | 0.9941 | 0.2312 | 0.4229 |
| Stroke | 16 (5.0) | 3 (2.9) | 7 (8.2) | 6 (4.7) | 0.5697a | 0.83 | 0.21 | 0.2964 |
| Any Liver Disease | 21 (6.6) | 5 (4.8) | 6 (7.1) | 10 (7.9) | 0.3481a | 0.4646 | 0.348 | 0.8257 |
| Cirrhosis | 18 (5.7) | 5 (4.8) | 6 (7.1) | 7 (5.5) | 0.8308a | 0.9166 | 0.6198 | 0.6454 |
| Heart Disease or Stroke | 42 (13.2) | 10 (9.5) | 17 (20.0) | 15 (11.8) | 0.6854a | 0.5369 | 0.1685 | 0.1026 |
| Any Liver Disease or Cirrhosis | 29 (9.1) | 7 (6.7) | 7 (8.2) | 15 (11.8) | 0.1713a | 0.1788 | 0.2808 | 0.4028 |
| History of Cancers — n (%) | ||||||||
| Colon | 4 (1.3) | 1 (1.0) | 1 (1.2) | 2 (1.6) | 0.6699a | 0.6832 | 0.7283 | 0.8098 |
| Liver | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.8) | 0.2743a | 0.2205 | 0.4809 | 0.4122 |
| Breast | 6 (1.9) | 2 (1.9) | 3 (3.5) | 1 (0.8) | 0.4936a | 0.2377 | 0.9912 | 0.1504 |
| Ovarian | 1 (0.3) | 0 (0.0) | 0 (0.0) | 1 (0.8) | 0.2006a | 0.1462 | 0.4049 | 0.3582 |
| Endometrial | 3 (0.9) | 0 (0.0) | 0 (0.0) | 3 (2.4) | 0.0255a | 0.0112 | 0.146 | 0.1068 |
| Other (free text) | 18 (5.7) | 6 (5.7) | 5 (5.9) | 7 (5.5) | 0.9435a | 0.9166 | 0.9844 | 0.9089 |
| Any Cancer | 32 (10.1%) | 9 (8.6%) | 9 (10.6%) | 14 (11.0%) | 0.5434a | 0.6535 | 0.5264 | 0.9204 |
PPD = packs per day,
= Cochran-Armitage,
= Linear Regression
Smoking component bar diagrams
Figures 1 illustrates individual-level data on smoking intensity over a lifetime from birth to CP diagnosis, stratified by sex and by alcohol etiology of CP (Figure la for males and Figure lb for females). Each bar represents smoking trajectory in each participant with the length of the colored bars indicating the duration of smoking at that colored intensity. Gray bars indicate periods of non-smoking, green bars indicate periods of smoking ≤5 cigarettes/day, yellow bars indicate smoking 6-10 cigarettes/day, orange bars indicate smoking 11-20 cigarettes/day and red bars indicate smoking more than 20 cigarettes/day. Several observations are noteworthy: (i) the prevalence of smoking is greater among CP patients of alcohol etiology than in CP patients of non-alcohol etiology in both males and females, (ii) smoking prevalence and intensity is higher among males as compared to females, (iii) smokers generally begin at a level of ≤5 cigarettes/day (green bars) regardless of how intense they ultimately smoke, (iv) smoking prevalence and intensity changes dramatically in the teenage years with most male patients establishing peak intensity by age 25 and most female patients establishing peak intensity by age 30 (changing colored bars prior to age 30), (v) smoking intensity is highly variable between patients at each age, while smoking intensity remains relatively stable throughout the lifetime once established (long colored bars), and (vi) smoking status does not change prior to CP diagnosis, indicating no change in behavior with antecedent symptoms.
Figure 1. Component bar diagram of individual smoking trajectory in chronic pancreatitis patients by alcohol etiology, Male (A) and Female (B).
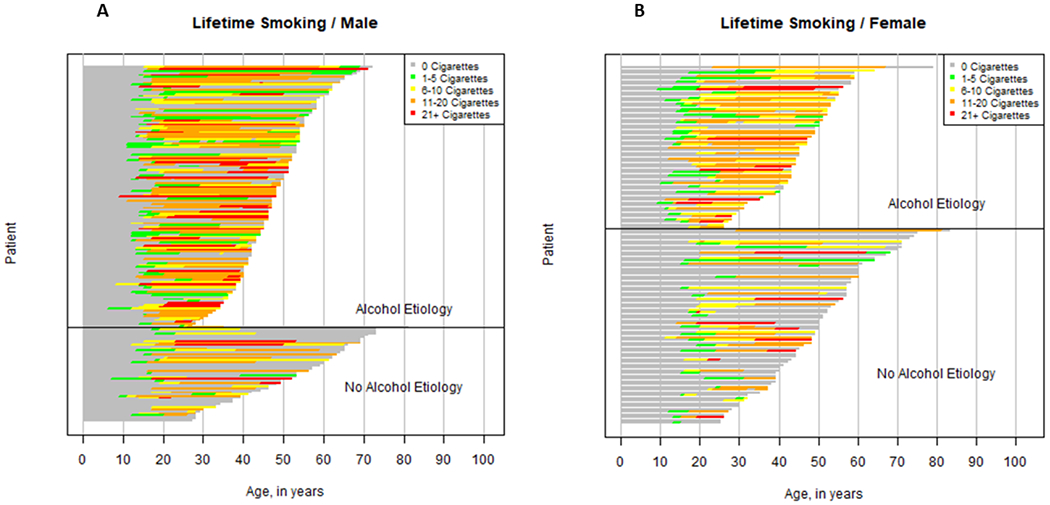
Each bar represents smoking trajectory in each participant with the length of the colored bars indicating the duration of smoking at that colored intensity. Gray bars indicate periods of non-smoking, green bars indicate periods of smoking ≤5 cigarettes/day, yellow bars indicate smoking 6-10 cigarettes/day, orange bars indicate smoking 11-20 cigarettes/day and red bars indicate smoking more than 20 cigarettes/day. Bars end at age of CP diagnosis.
Cohort analysis
Cohort-specific smoking prevalence by age are presented in Figure 2a (Male) and Figure 2b (Female). Sufficient data were available for birth cohorts 1930-1949, 1950-69, and 1970-1989 for assessing age-specific prevalence. The three birth cohorts show similar rate of increase in prevalence of smoking by age between 10 and 20 years of age among male CP patients. Peak prevalence of smoking is reached between 20 and 30 years of age for each cohort. The peak prevalence is highest for the birth cohort of 1950-1969, and lowest for the birth cohort of 1970-1989. The prevalence of smoking decreases gradually with age in birth cohorts 1930-1949 and 1950-1969. Data on male CP patients bom in 1970-1989 was not sufficient to assess age-specific change in smoking prevalence beyond the age of 35.
Figure 2.
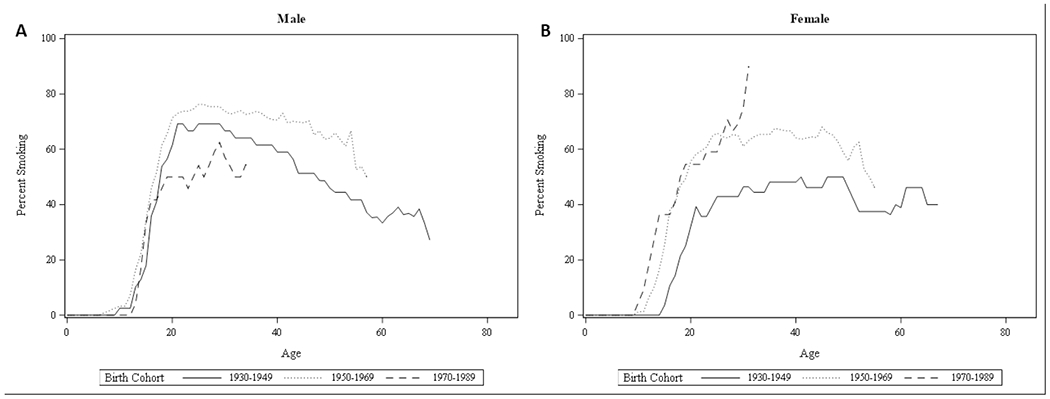
Prevalence of smoking by age in three birth cohorts of chronic pancreatitis patients from 1930 to 1989, Male (A) and Female (B)
In contrast to male CP patients, female CP patients showed differences in age of initiation by birth cohorts. Female CP patients born in 1950-1969 and in 1970-1989 showed earlier rise in prevalence of smoking between 10 and 20 years of age, reaching 50% smoking prevalence by age 20. By contrast, those born in 1930-1949 showed later rise in prevalence of smoking, well into the teenage years, with prevalence reaching 40% by age 20. Peak prevalence was highest for the birth cohort born in 1970-1989, but were determined with smaller sample size than for older patients. Data on female CP patients born in 1970-1989 was not sufficient to assess age-specific change in smoking prevalence beyond the age of 30. In contrast to male CP patients, prevalence of smoking did not decrease after 30 years and remained stable at around 60% until mid 40s for female CP patients born in 1950-1969, and even increased from 40% up to 50% by late 40s among those born in 1930-1949.
Discussion
In this analysis of 317 CP patients, we found that smoking behavior typically began in teenage years and that smoking continues for a lifetime once established in 20s even after a diagnosis of CP. CP patients who smoked at least 1 pack of cigarettes per day in their 20s cumulatively were exposed to an average of 30 pack-years of smoking over their lifetime. The extensive cumulative history of smoking and the fact that 40% of deaths among CP patients are attributable to cancer21 warrant active surveillance for cancer risk in CP patients, especially for lung cancer.22
Smoking continues after a diagnosis of CP even as drinking discontinues, as evident in the high prevalence of smoking (64-74%) and lower prevalence of drinking (21-25%) at the time of enrollment among those with a smoking history at age 25. Smoking with the intention to alleviate pain may contribute to continued smoking, but it does not explain a large proportion of smoking behavior. Adults with co-use of alcohol and smoking report that it is as difficult or more difficult to give up cigarettes than alcohol or other substance, demonstrating the challenges of nicotine dependence.23 Physicians by and large do recognize smoking as an etiologic factor in CP patients who smoke.12 Whether this translates to referral for smoking cessation program is unknown. Literature points to suboptimal uptake of nicotine replacement therapy in smokers, especially in minorities, addressing the barriers to which could help improve smoking cessation in this population.24 Intervention studies of substance use suggest that substance-using patients who smoke have a desire to quit smoking and that in some cases smoking cessation can enhance the intervention for substance use.25 In a single-arm intervention study of QuitWorks, in which 27 CP patients set quit dates and were offered nicotine patches and one-on-one counseling, none of the patients were able to quit smoking completely after 18 months of follow-up as compared to 19% quit rate among smokers without CP, demonstrating the challenges of behavior change in pancreatitis populations.26 Innovative and sustainable interventions are needed to help reduce tobacco exposure in CP patients.
In addition to personal history of smoking; moderate and heavy smokers were more likely to be exposed to second-hand smoke at home and at the workplace for 10 or more years as well as have greater consumption of alcohol over a lifetime. Due to small sample size of patients with passive smoke exposure but without personal smoking history, we were not able to examine the independent association of environmental smoke exposure and clinical features of CP. While the question of whether secondhand smoke could influence one’s risk of CP independent of first-hand smoke is beyond the scope of this study, we hypothesize the second-hand smoke could have contributed to the pathogenesis of CP and further studies are warranted.
In contrast to what has been reported about the reduced risk of calcifications with cessation of smoking in CP patients,27 we found that history of heavy smoking is associated a with lower prevalence of calcification. Because heavy smoking leads to increased risk of other diseases with fatal consequences, such as heart disease, our cross-sectional analyses could have been influenced by survivor’s bias, whereby CP patients with calcifications could have been excluded due to afflictions with other smoking-related diseases. Moreover, smoking may be associated with development of pancreatic cancer, which may manifest as CP.28 It is also possible that non-smokers in our study population were more likely to have late-onset idiopathic CP, in whom pancreatic calcifications are the hallmark of diagnosing the disease, often in the setting of no or mild symptoms. Pseudocysts were more common among smokers, especially heavy smokers, a finding that confirms prior observations of the association between smoking and pseudocysts in CP patients.29, 30
Birth cohort analyses of age-specific smoking prevalence in CP patients is consistent with national trends in smoking prevalence in that (i) smoking behavior peaked earlier in males than in females, (ii) smoking prevalence in males has substantially decreased over time and with age, and (iii) age of initiation of smoking has decreased in females over time.31 Smoking trends in CP population differs, however, in that smoking prevalence peaked later in CP population than in national population, and that peak prevalence (>70% in males, >60% in females) was higher among CP patients than nationwide peak prevalence. Our results suggest that patients who develop CP have more common smoking history than the general population and that cessation campaigns to influence behavior change had a delayed effect in the CP population than in the general population. Given that smoking prevalence is decreasing with younger male cohorts, we expect smoking-related CP incidence to decrease. CP incidence has reportedly fallen from 2007 to 2014 in the U.S.32 Whether smoking cessation is responsible for these trends merits investigation. The trends for females tell a contrasting story. Age of onset of smoking has decreased in female CP patients and prevalence has increased. Could this have led to greater CP incidence and offset the decrease in males? Future epidemiologic investigations of CP should provide sex-specific statistics to examine population-level influences of smoking behavior on pancreatitis trends.
Our study brings several novel insights on smoking trends in CP patients, but has some limitations. First, we did not ask about the use of electronic nicotine delivery systems (ENDS) or the use of other tobacco products, such as cigars. Future studies that include data on a more diverse array of smoking or vaping products would help to assess their independent influence on pancreatitis pathogenesis in lieu of the changing smoking landscape in the U.S. Secondly, we lacked information on intensity of second-hand smoke, thus we are not able to assess whether the exposure was appreciable to the level of promoting pancreatic injury. Also, there may be some misclassification of smoking and lifetime drinking due to inaccurate recall. The use of a calendar and milestone-aided instrument helped to improve participant memory, but the extent of drinking and smoking may have been over- or under-estimated for exposure in distant past. Finally, association analyses between CP morphology and smoking could have been confounded by other correlated factors, especially heavy alcohol consumption, which has been associated with acute pancreatic pseudocysts.33, 34
In sum, our study sheds light on the extensive and sustained smoking behaviors in CP patients and related clinical factors. We also bring to light birth cohort differences in prevalence of smoking in CP patients, which indicate that smoking policy changes can have an impact on long-term epidemiologic trends of CP. Of note, female CP patients have shown increased prevalence and earlier initiation of smoking over time and warrants further studies on how this portends future epidemiologic trends of CP in females. Studies on interventions for smoking cessation and surveillance for cancer are necessary to develop novel prevention strategies of cancer and other diseases in patients with CP.
Acknowledgements:
This research was partly supported by the NIH DK061451 (D.C.W.), DK077906 (D.Y.), U01 DK108306 (D.C.W.,D.Y.), U01DK108314 (C.Y.J.) and Department of Defense (DoD) WX81XWH-19-10888 (D.Y., C. Y. J.). This publication was made possible in part by Grant Number UL1 RR024153 and UL1TR000005 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research (University of Pittsburgh. PI, Steven E. Reis, MD). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR, NIH or DoD. The authors report no potential conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cote GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2011;9:266–73; quiz e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aune D, Mahamat-Saleh Y, Norat T, et al. Tobacco smoking and the risk of pancreatitis: A systematic review and meta-analysis of prospective studies. Pancreatology 2019;19:1009–1022. [DOI] [PubMed] [Google Scholar]
- 3.Yadav D, Slivka A, Sherman S, et al. Smoking is underrecognized as a risk factor for chronic pancreatitis. Pancreatology 2010;10:713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadr-Azodi O, Andren-Sandberg A, Orsini N, et al. Cigarette smoking, smoking cessation and acute pancreatitis: a prospective population-based study. Gut 2012;61:262–7. [DOI] [PubMed] [Google Scholar]
- 5.Talamini G, Bassi C, Falconi M, et al. Cigarette smoking: an independent risk factor in alcoholic pancreatitis. Pancreas 1996;12:131–7. [PubMed] [Google Scholar]
- 6.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 2009;169:1035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav D, Whitcomb DC. The role of alcohol and smoking in pancreatitis. Nat Rev Gastroenterol Hepatol 2010;7:131–45. [DOI] [PubMed] [Google Scholar]
- 8.Maisonneuve P, Lowenfels AB, Mullhaupt B, et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut 2005;54:510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masamune A, Nabeshima T, Kikuta K, et al. Prospective study of early chronic pancreatitis diagnosed based on the Japanese diagnostic criteria. J Gastroenterol 2019;54:928–935. [DOI] [PubMed] [Google Scholar]
- 10.Greer JB, Thrower E, Yadav D. Epidemiologic and Mechanistic Associations Between Smoking and Pancreatitis. Curr Treat Options Gastroenterol 2015;13:332–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon CY, Whitcomb DC, Slivka A, et al. Lifetime Drinking History of Persons With Chronic Pancreatitis. Alcohol Alcohol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muniraj T, Yadav D, Abberbock JN, et al. Increased awareness enhances physician recognition of the role of smoking in chronic pancreatitis. Pancreatology 2019;19:500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juliusson SJ, Nielsen JK, Runarsdottir V, et al. Lifetime alcohol intake and pattern of alcohol consumption in patients with alcohol-induced pancreatitis in comparison with patients with alcohol use disorder. Scand J Gastroenterol 2018:1–7. [DOI] [PubMed] [Google Scholar]
- 14.Masamune A, Kume K, Shimosegawa T. Sex and age differences in alcoholic pancreatitis in Japan: a multicenter nationwide survey. Pancreas 2013;42:578–83. [DOI] [PubMed] [Google Scholar]
- 15.Agaku IT, Odani S, Okuyemi KS, et al. Disparities in current cigarette smoking among US adults, 2002-2016. Tob Control 2020;29:269–276. [DOI] [PubMed] [Google Scholar]
- 16.Marynak KL, Gammon DG, King BA, et al. National and State Trends in Sales of Cigarettes and E-Cigarettes, U.S., 2011-2015. Am J Prev Med 2017;53:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox CM, Yadav D, Ye T, et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol 2015;13:552–60; quiz e28-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcox CM, Sandhu BS, Singh V, et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. Am J Gastroenterol 2016;111:1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan AW, Pristach EA, Welte JW, et al. Use of the TWEAK test in screening for alcoholism/heavy drinking in three populations. Alcohol Clin Exp Res 1993;17:1188–92. [DOI] [PubMed] [Google Scholar]
- 20.Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol 1982;43:1157–70. [DOI] [PubMed] [Google Scholar]
- 21.de la Iglesia-Garcia D, Vallejo-Senra N, Iglesias-Garcia J, et al. Increased Risk of Mortality Associated With Pancreatic Exocrine Insufficiency in Patients With Chronic Pancreatitis. J Clin Gastroenterol 2018;52:e63–e72. [DOI] [PubMed] [Google Scholar]
- 22.Wood DE, Kazerooni EA, Baum SL, et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:412–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozlowski LT, Skinner W, Kent C, et al. Prospects for smoking treatment in individuals seeking treatment for alcohol and other drug problems. Addict Behav 1989;14:273–8. [DOI] [PubMed] [Google Scholar]
- 24.Trinidad DR, Perez-Stable EJ, White MM, et al. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am J Public Health 2011;101:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat 2009;36:205–19. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Kheder J, Bocelli L, et al. Smoking Cessation in a Chronic Pancreatitis Population. Pancreas 2016;45:1303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talamini G, Bassi C, Falconi M, et al. Smoking cessation at the clinical onset of chronic pancreatitis and risk of pancreatic calcifications. Pancreas 2007;35:320–6. [DOI] [PubMed] [Google Scholar]
- 28.Jeon CY, Chen Q, Yu W, et al. Identification of Individuals at Increased Risk for Pancreatic Cancer in a Community-Based Cohort of Patients With Suspected Chronic Pancreatitis. Clin Transl Gastroenterol 2020;11:e00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hao L, Pan J, Wang D, et al. Risk factors and nomogram for pancreatic pseudocysts in chronic pancreatitis: A cohort of 1998 patients. J Gastroenterol Hepatol 2017;32:1403–1411. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Wang D, Hao L, et al. Risk Factors Analysis and Nomogram Development for Pancreatic Pseudocyst in Idiopathic Chronic Pancreatitis. Pancreas 2020;49:967–974. [DOI] [PubMed] [Google Scholar]
- 31.Anderson CM, Burns DM, Dodd KW, et al. Chapter 2: Birth-cohort-specific estimates of smoking behaviors for the U.S. population. Risk Anal 2012;32 Suppl 1:S14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sellers ZM, MacIsaac D, Yu H, et al. Nationwide Trends in Acute and Chronic Pancreatitis Among Privately Insured Children and Non-Elderly Adults in the United States, 2007-2014. Gastroenterology 2018;155:469–478 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui ML, Kim KH, Kim HG, et al. Incidence, risk factors and clinical course of pancreatic fluid collections in acute pancreatitis. Dig Dis Sci 2014;59:1055–62. [DOI] [PubMed] [Google Scholar]
- 34.Kim KO, Kim TN. Acute pancreatic pseudocyst: incidence, risk factors, and clinical outcomes. Pancreas 2012;41:577–81. [DOI] [PubMed] [Google Scholar]


