Abstract
Objective
This non-inferiority study aimed to determine the burden of obesity in a hospital outpatient setting of a developing country, using three commonly employed metrics as predictors of hypertension (HTN).
Design
A cross-sectional study design was adopted.
Setting
This study was conducted in Health Promotion and Risk Factor Screening Services of a tertiary hospital for eye and ear, nose, throat in a semiurban area of Nepal.
Participants
2256 randomly selected outpatients between 40 and 69 years old.
Outcome measures
The three obesity metrics and HTN were analysed for association using correlation, the area under the receiver operating characteristic (ROC) curve and ORs.
Results
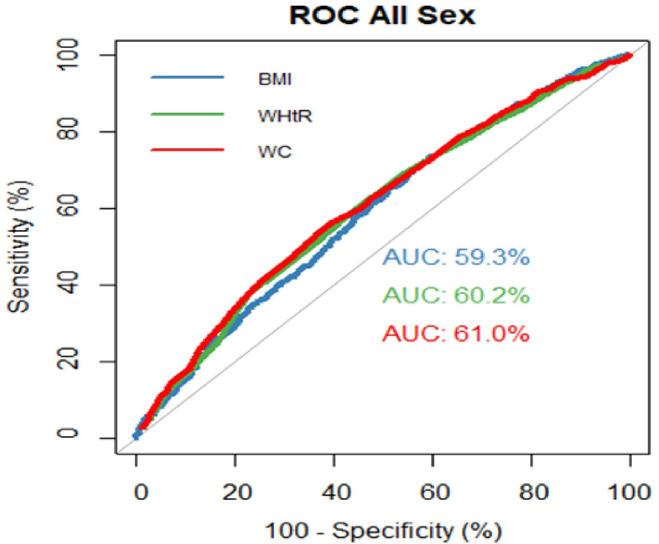
The prevalence of obesity or overweight by body mass index (BMI) was 58.29%; by waist-to-height ratio (WHtR) was 85.95%, high waist circumference (WC) was observed among 66.76% of participants. Female participants had a greater prevalence of high WC (77.46%) than males (53.73%) (p<0.001). Prevalence of HTN and pre-HTN were 40.67% and 36.77%, respectively. The areas under the ROC curve were significantly higher than 0.5 for BMI (0.593), WHtR (0.602) and WC (0.610).
Conclusion
This study showed that WHtR and WC measured were not inferior to BMI as a metric for obesity detection and HTN prediction. Because of its low cost, simplicity of measurement and better ability to predict HTN, it may become a more usable metric in health facilities of low-income and middle-income countries.
Keywords: hypertension, public health, other metabolic, e.g. iron, porphyria
Strengths and limitations of this study.
This is the first study done in large sample size among hospital outpatients in Nepal.
Waist to height ratio and waist circumference were also included for picking up obesity with higher cardiovascular risk despite normal body mass index.
This study could have some selection bias as it was conducted at tertiary eye hospital and underlying causes and co-morbidities are not included in this analysis, which are being reported in a separate paper.
This observational, cross-sectional study cannot infer a causal relationship between overweight or obesity and raised blood pressure.
This is a single institution-based study which requires further studies in general and multispecialty hospitals.
Introduction
The world is rapidly becoming obese.1 2 According to WHO, obesity rates have tripled since 1975. In 2016, more than 1.9 billion people above the age of 17 were overweight, and of those, over 650 million were obese.3 The trends in adult body mass index (BMI) in 200 countries showed that the age-standardised prevalence of obesity increased from 3.2% to 10.8% in men, from 6.4% to 14.9% in women from 1975 to 2014.4 Obesity is known to be strongly associated with hypertension (HTN), heart disease and diabetes.5 6 Worldwide, 23% of Ischaemic heart disease and 44% of diabetes has been reported to be associated with being overweight or obese.7 Moreover, obesity and HTN are significant causes of premature death worldwide.8 9 WHO has estimated that 1.13 billion people worldwide have HTN; among them, two-thirds live in low-income and middle-income countries (LMICs). The rising burden of non-communicable diseases (NCDs)—major killers of the world’s population, closely parallel with the rise in the burden of obesity.10 11 Most NCDs have obesity as an important risk factor.12 13 Recently, obesity has also been reported to be a high risk for morbidity and mortality from COVID-19.14 COVID-19 has also been reported to have resulted in a higher incidence of obesity.15
Additionally, the emerging epidemic has put patients with HTN at greater risk of death due to their inability to access treatment. HTN is the most common cause of cardiovascular diseases (CVDs), and it is a leading cause of CVD deaths—45% due to heart disease and 51% due to stroke.16 The estimated cost of obesity and NCDs to the countries has been estimated to be up to 9.3% of gross domestic product.17
NCDs country profiles 2018 estimated NCDs to account for 66% of all deaths, while CVD caused 30% of all deaths.18 The prevalence of HTN in Nepal tripled to 18.5% in 25 years (1981 and 2006) and took only 15 years (2003 to 2019) to double to 24.5%.19–21 and projected to be 44.7% in men in 2025.22 A similar trend has been seen in the prevalence of overweight or obesity, which increased from 7.20% to 24.30% between 2008 and 2019.20 23
Like many LMICs, Nepal is battling a triple burden of diseases: communicable, NCDs and injuries, with CVDs being the most common.24 Research done in the last decade shows that the conventional risk factors for CVDs are present in a high proportion of the Nepalese population.23 In the absence of a routine surveillance or registry system, the actual burden and trend of CVDs in Nepal remain uncertain. Early detection of individuals at high CVD risk is the cornerstone of primary prevention. Simple routine screening methods such as measuring waist to height ratio (WHtR), waist circumference (WC), blood pressure (BP), which help detect CVDs early, are not routinely practiced in LMICs because of heavy patient loads and staff shortages.
The Nepal government has been implementing the WHO Package for essential NCDs since 2016. Piloted in 3 districts, this has now been proposed to be implemented in all 77 districts beginning during the 2021–2022 fiscal year.25 The government has also constituted the Nepal Non-Communicable Disease and Injury Poverty Commission, which has provided recommendations to address NCD in the larger context of poverty.26
Most reported studies on obesity, HTN and their association had been conducted in community settings. There is a paucity of hospital data on the burden of obesity, HTN and an association between them in outpatient hospital settings of LMICs. Therefore, this study was designed to find the burden of obesity and HTN among patients attending a hospital and compare the ability of three different currently available anthropometric measurements to predict HTN in hospital outpatients in a low-income setting.
Methods
Study design and study population
This hospital-based cross-sectional descriptive study was conducted from June to December 2019 at the Hospital for Children, Eye, ENT and Rehabilitation Services (CHEERS), located in a semiurban setting of the Bhaktapur district, Nepal. We used systematic random sampling to select the participants. During the study period, every third participant aged 40–69 years attending the Health Promotion and Risk Factor Screening (HP-RFS) service of CHEERS constituted the study population. The sample size was calculated based on the HTN prevalence of 46.7% (P).27 The margin of error is considered as 5% (D), using a 95% confidence level (Z=1.96) and with an 80% response rate. The formulae used for sample size calculation was N=(Z2*P(1−P))/(D2). The calculated sample size was multiplied by the number of domains to obtain the final sample size. The number of domains was decided to be four; two age groups and two genders. Based on these calculations, the minimum sample size required was 1913. All participants were informed about the purpose of the screening service, and informed consent was obtained before proceeding with the anthropometric measurements.
Inclusion and exclusion criteria
All outpatients between 40 and 69 years of age who provided written consent during the study period were included. Pregnant women and people unable to stand correctly were excluded from data analysis for this study. Participants with any abnormal body composition which did not allow measuring height, weight and WC were excluded from the analysis.
Anthropometric measurements
A standardised protocol for anthropometric measurements was followed at the hospital. A community medicine auxiliary staff member was trained on an existing protocol for obtaining anthropometric measurements for height, weight, WC and BP. Height, weight and WC were measured using a portable digital weighing scale (Equinox Weighing Scale), stadiometer (Prestige stadiometer) and constant tension tape. Participants were asked to remove bulky clothes, shoes and cap before taking measurements. The WC in cm was measured at the midpoint between the lower edge of the rib cage and the iliac crest after a full expiration. BMI was calculated as weight (kg) divided by height in metres squared (m2). The WHtR was calculated as WC in cm divided by height in cm. Besides anthropometric measurements, sociodemographic information (age and sex) and history of HTN (anti-HTN medication) were also enquired.
The standard value for WHtR was considered as ‘no increased risk’ (WHtR <0.5); ‘increased risk’ (WHtR ≥0.5 to <0.6) and ‘very high-risk’ (WHtR ≥0.6). The cut-off value for WHtR was considered as 0.5. Similarly, WC ≥90 cm for males and ≥80 cm for females were considered ‘cut-off values’.28 The standard weight BMI classifications for normal was 18.5–24.9 kg/m2, whereas BMI values of 25.0 - 29.9 kg/m2 and ≥30 kg/m2 were considered overweight and obese, respectively, according to WHO classifications.29 The cut-off value for BMI was therefore set at 25 kg/m2.
The BP of the participants was measured by aneroid sphygmomanometer using an adult cuff size. Participants were asked to sit quietly for 15 min, legs uncrossed in a comfortable position with back supported. The BP was measured from the left arm, placing the artery mark of the cuff over the brachial artery of the arm at heart level. BP in participants with an initial high BP reading was remeasured after 15 min of rest.
Statistical analysis
Participants were classified as hypertensive if their systolic BP (SBP) was 140 mm Hg or higher, and/or diastolic BP (DBP) was 90 mm Hg or higher and prehypertensive (pre-HTN) if SBP levels were between 120 and 139 mm Hg and/or DBP levels were between 80 and 89 mm Hg.30 The participants were also considered hypertensive if they took antihypertensive medication, even if their BP was normal at the time of measurement. The participants who did not fit in all of the above categories were considered normotensive.
Collected data were instantly entered into an Excel file (MS Office 2010). Data analysis was done using R language, V.4.0.0. Continuous variables are shown as the mean, SD and categorical variables as frequency and percentage. An independent sample t-test was applied to compare mean values of continuous variable between different groups. We used logistic regression analysis separately to find the effect of socio-demographic variables and different obesity metrics. An adjusted OR (AOR) for hypertensive compared with the non-hypertensive group was analysed by entering age and sex in a model with different obesity metrics in separate analysis models. ORs were reported within a 95% CI. We also did correlation analysis and calculated the Spearman’s product-moment correlation coefficient. The area under the receiver operating characteristic (ROC) curve (including 95% CI) of BMI, WHtR and WC was calculated for predicting HTN. The CI, which did not include 0.5, was considered to indicate significant results. A p<0.05 was considered significant for all tests.
Patient and public involvement
Patients or the public were not involved in the design, conduct or reporting of this research.
Results
This study included 2256 randomly selected participants from 6769 persons, between 40 and 69 years of age, visiting the HP-RFS service at CHEERS from June to December 2019. The non-response rate was 5%. The non-participation was mainly due to lack of time and unwillingness. The participants were outpatients visiting the hospital for eye and ear, nose, throat (ENT) consultations. The mean (SD) age of the participants was 51.75 (8.47) years. More than half of the participants were female (56.0%) than male (44.0%). About two-thirds of the participants (62.8%) were 40–54 years old, and about one-third (37.2%) were 55–69 years old.
The mean (SD) BMI was 25.29 (3.81) kg/m2 and 26.72 (4.44) kg/m2 for male and female participants respectively (p<0.01). The mean BMI was observed to gradually decrease with advancing age in both male and female participants. The mean (SD) WHtR was 0.56 (0.06) and 0.58 (0.08) for male and female (p<0.01), respectively. Similarly, the mean (SD) WC was 90.96 (10.32) cm and 88.24 (10.94) cm for males and females (p<0.01), respectively (table 1).
Table 1.
Mean (SD) of different anthropometric measurements according to age and gender
| Age group (years) | Gender | |||||||||
| 40–54 | 55–69 | P value | Male | Female | P value* | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| SBP (mm Hg) | 118.46 | 14.96 | 122.77 | 16.49 | <0.01 | 121.47 | 15.50 | 118.90 | 15.74 | <0.01 |
| DBP (mm Hg) | 79.21 | 10.74 | 79.39 | 10.25 | 0.69 | 79.98 | 10.67 | 78.69 | 10.43 | <0.01 |
| Weight (KG) | 65.55 | 10.81 | 61.58 | 11.41 | <0.01 | 67.41 | 11.15 | 61.32 | 10.48 | <0.01 |
| Height (CM) | 157.60 | 8.84 | 155.47 | 9.15 | <0.01 | 163.19 | 7.23 | 151.54 | 6.62 | <0.01 |
| WC (CM) | 89.51 | 10.38 | 89.40 | 11.36 | 0.83 | 90.96 | 10.32 | 88.24 | 10.94 | <0.01 |
| WHtR | 0.57 | 0.07 | 0.58 | 0.08 | 0.02 | 0.56 | 0.06 | 0.58 | 0.08 | <0.01 |
| BMI | 26.41 | 4.08 | 25.50 | 4.41 | <0.01 | 25.29 | 3.81 | 26.72 | 4.44 | <0.01 |
*Independent sample t-test.
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; WC, waist circumference; WHtR, waist-to-height ratio.
Prevalence of obesity and overweight
The prevalence of obesity and overweight using BMI was 16.09% and 42.20%, respectively. Female participants had higher prevalence of obesity (21.40%) than males (9.60%) (p<0.001). The observed prevalence of overweight using BMI among males and females was 42.80% and 41.70%, respectively, and this difference was not statistically significant (p =0.612). Participants of younger age groups (40–54 years) had a significantly higher prevalence (p<0.001) of overweight and obesity in both genders.
Using WHtR, female participants had a significantly higher (p<0.001) prevalence (40.10%) of obesity than male participants (23.80%). Among females, the younger age group of 40–54 years had a higher prevalence of abdominal obesity than the older group (37.59% vs 44.52%, p=0.0195). However, there was no significant difference between age groups among male participants (23.96% vs 23.66%, p=0.974).
Higher than cut-off WC values were observed among 66.76% of all participants, with the greatest prevalence in female participants (77.46%) compared with males (53.73%) (p<0.001). The prevalence of WHtR higher than cut-off values among all participants was 85.95%, which exceeded the prevalence exceeding cut-off values for both WC (66.76%) and BMI (58.29%). Similar patterns were found among gender and age groups as well (table 2).
Table 2.
BMI, WHtR and WC classification according to age and sex
| Characteristic | N | BMI | WHtR | WC* (CM) | ||||||
| Age group (years) | ≥30.0 kg/m2 | 25.0–29.9 kg/m2 | <25.0 kg/m2 | >0.60 | 0.50–0.59 | <0.50 | ≥Cut-off | <Cut-off | ||
| All | 2256 | 16.09 | 42.20 | 41.71 | 32.76 | 53.19 | 14.05 | 66.76 | 33.24 | |
| Male | All age | 1048 | 9.60 | 42.80 | 47.50 | 23.80 | 60.00 | 16.20 | 53.73 | 46.27 |
| 40–54 | 634 | 10.88 | 47.48 | 41.64 | 23.65 | 60.25 | 16.09 | 55.68 | 44.32 | |
| 55–69 | 384 | 7.55 | 35.16 | 57.29 | 23.96 | 59.63 | 16.41 | 50.52 | 49.48 | |
| Female | All age | 1238 | 21.40 | 41.70 | 36.90 | 40.10 | 47.60 | 12.30 | 77.46 | 22.54 |
| 40–54 | 782 | 21.74 | 44.37 | 33.89 | 37.59 | 50.00 | 12.40 | 77.75 | 22.25 | |
| 55–69 | 456 | 20.83 | 37.06 | 42.10 | 44.52 | 43.42 | 12.06 | 76.97 | 23.06 | |
*Cut-off value of WC for male is 90 cm and for female is 80 cm.
BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio.
Prevalence of HTN
The overall prevalence of HTN and pre-HTN was 40.67% and 36.77%, respectively. Though not statistically significant, male participants had a slightly higher prevalence of HTN (42.72%) than female participants (39.00%). The prevalence of HTN for both genders was found to increase with age. The prevalence of pre-HTN was found to be 38.78% and 35.11% among male and female participants. A staggering 81.50% of male and 74.11% of female participants had either established HTN or pre-HTN. A concerning finding was that among 916 participants with HTN, 57.4% did not know they had raised BP before this study.
Age AOR for being hypertensive for males compared with females was 0.86 (95% CI 0.72 to 1.02), and sex AOR for being hypertensive was 1.61 (95% CI 1.35 to 1.91) for the age group 55–69 compared with age group 40–54 years (table 3).
Table 3.
HTN, sociodemographic variables, and risk factors
| Pre HTN n (%) |
HTN n (%) |
Unadjusted OR for HTN (95% CI) | Adjusted OR (AOR)* for HTN (95% CI) | ||
| All | 828 (36.77) | 916 (40.67) | |||
| Sex | Female | 434 (35.11) | 482 (39.00) | 1 | 1 |
| Male | 394 (38.78) | 434 (42.72) | 0.86 (0.72 to 1.01) | 0.86 (0.72 to 1.02) | |
| Age group (years) | 40–54 | 544 (38.50) | 514 (36.38) | 1 | 1 |
| 55–69 | 284 (33.85) | 402 (47.91) | 1.61 (1.35 to 1.91) | 1.61 (1.35 to 1.91) | |
| BMI | <25 kg/m2 | 337 (35.93) | 312 (33.26) | 1 | 1 |
| ≥25 kg/m2 | 491 (37.37) | 604 (45.97) | 1.74 (1.43 to 2.03) | 1.89 (1.58 to 2.26) | |
| WHtR | <0.5 | 93 (31.74) | 83 (28.33) | 1 | 1 |
| ≥0.5 | 735 (37.52) | 833 (42.52) | 1.87 (1.43 to 2.45) | 1.92 (1.46 to 2.52) | |
| WC† (CM) | <cut-off | 281 (37.57) | 235 (31.42) | 1 | 1 |
| ≥cut-off | 547 (36.37) | 681 (45.28) | 1.81 (1.50 to 2.17) | 2.02 (1.66 to 2.45) |
*AOR were adjusted for age and sex variables.
†Cut-off value of WC for male is 90 cm and for female is 80 cm.
BMI, body mass index; HTN, hypertension; WC, waist circumference; WHtR, waist-to-height ratio.
Obesity and HTN
The prevalence of HTN among the participants with BMI, WHtR, and WC more than or equal to cut-off value was 45.97%, 42.52% and 45.28%, respectively. Not surprisingly, a higher prevalence of HTN was found among participants with any obesity metrics higher than cut-off value when compared with participants with normal value (p<0.001). Age and sex AOR for being hypertensive among higher BMI, WHtR and WC than the cut-off was 1.89 (95% CI 1.58 to 2.26), 1.92 (95% CI 1.46 to 2.52) and 2.02 (95% CI 1.66 to 2.45), respectively, indicating that WHtR and WC’s were better able to predict HTN than BMI.
Table 4 shows the correlation between BMI, WC, WHtR, BP and Age, including the significance level. A strong positive correlation (r=0.682, p<0.01) was found between WC and BMI. Moreover, WC had a very weak positive correlation (r=0.188, p<0.01) with SBP, though higher than the other two metrics. However, BMI had a weak positive correlation (r=0.214, p<0.01) with HTN, higher than other metrics. The relationship between all three anthropometric metrics with SBP was lower than with DBP.
Table 4.
Correlation between BMI, WHtR, WC, SBP, DBP and age
| BMI | WC (CM) | WHtR | SBP (mm Hg) | DBP (mm Hg) | Age (years) | |
| BMI | 1 | 0.682* | 0.770* | 0.154* | 0.214* | −0.120* |
| WC (CM) | 0.682* | 1 | 0.884* | 0.188* | 0.192* | −0.004 |
| WHtR | 0.770* | 0.884* | 1 | 0.168* | 0.183* | 0.054† |
| SBP (mm Hg) | 0.154* | 0.188* | 0.168* | 1 | 0.726* | 0.137* |
| DBP (mm Hg) | 0.214* | 0.192* | 0.183* | 0.726* | 1 | −0.007 |
| Age (years) | −0.120* | −0.004 | 0.054† | 0.137* | −0.007 | 1 |
*Correlation is significant at the 0.01 level.
†Correlation is significant at the 0.05 level.
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; WC, waist circumference; WHtR, waist-to-height ratio.
ROC analyses were used to determine the relative ability of the three obesity metrics to predict HTN, as depicted in figure 1 and table 5. The areas under the curve (AUC) were significantly higher than 0.5 for BMI (0.593, 95% CI 0.569 to 0.616), WC (0.610, 95% CI 0.586 to 0.633) and WHtR (0.602, 95% CI 0.578 to 0.625). In both genders and all age groups, AUC is significantly higher than 0.5 (p<0.01)
Figure 1.
Comparison of the ROC curves of WC, WHtR and BMI in total participants. AUC, areas under the curve; BMI, body mass index; ROC, receiver operating characteristic; WC, waist circumference; WHtR, waist-to-height ratio.
Table 5.
Sex-specific and age-specific comparisons of the area under ROC curves of BMI, WC and WHtR
| Area (95% CI) under the curve | ||||
| BMI | WC (CM) | WHtR | ||
| All | 0.593 (0.569 to 0.616)* | 0.610 (0.586 to 0.633)* | 0.602 (0.578 to 0.625)* | |
| Sex | Male | 0.620 (0.585 to 0.654)* | 0.620 (0.586 to 0.655)* | 0.629 (0.595 to 0.664)* |
| Female | 0.581 (0.549 to 0.614)* | 0.598 (0.566 to 0.630)* | 0.590 (0.558 to 0.623)* | |
| Age (years) | 40–54 | 0.600 (0.570 to 0.631)* | 0.606 (0.563 to 0.625)* | 0.594 (0.563 to 0.625)* |
| 55–69 | 0.610 (0.572 to 0.648)* | 0.620 (0.583 to 0.658)* | 0.609 (0.572 to 0.647)* | |
*Correlation is significant at the 0.01 level.
BMI, body mass index; ROC, receiver operating characteristic; WC, waist circumference; WHtR, waist-to-height ratio.
Discussion
This descriptive study is the outcome of an opportunistic RFS for NCDs, obesity and HTN at the Health Promotion Unit of a tertiary eye and ENT hospital in Bhaktapur, Nepal.
The overall prevalence of obesity using BMI in this study was 16.09%, higher than the national obesity prevalence reported in Nepal’s 2019 NCD Risk Factors STEPS survey, where only 5.42% of the same age group were reported to be obese.20 This might be due to the different settings of the two studies, as this study was done in a semiurban area of Kathmandu valley, whereas STEPS survey covered both urban and rural areas. Additionally, using BMI, we found that more than 2 in 10 (21.40%) women and nearly 1 in 10 (9.60%) men coming to the hospital were obese, which is nearly double the previously reported values in the Nepal Demographic and Health Survey (DHS) of 2016, for both genders (women: 9.5% and men: 5.1%).31 Again, these are not comparable because of the different settings in which these studies were done, and the different parameters used but may indicate an increasing trend.
Using the International Diabetes Federation recommended WC cut-off points (male 90 cm and female 80 cm) for South Asians,28 two-thirds (66.76%) of the study participants were obese as opposed to only 16.09% when measured with BMI. In our study, more than three-quarters (77.46%) of women and over half (53.73%) of men were obese.
The overall prevalence of abdominal obesity by WHtR was 32.76%, with a higher prevalence among women (40.1%) than men (23.8%). This indicates that obesity, as measured by WHtR, missed a significant proportion of the most important CVD risk factor. Also, WC could detect more obesity cases than either BMI or WHtR proving itself superior to the other two metrics.
The observed higher prevalence of overweight/obesity in women than men, using all three obesity metrics in our study, is supported by other studies.27 32 33 The reported increase in abdominal obesity with each pregnancy independent of total body fat34 may explain higher abdominal obesity observed among women in this study.
Regardless of the metrics used, this study shows a higher prevalence of obesity among Hospital OPD patients, indicating that screening for obesity in this setting has a higher potential to detect a larger number of people with obesity than in community settings. However, the latter is essential for population-based data.
The higher prevalence of obesity in this study may be due to study design, a selection bias as people reporting to hospitals may also have some or other conditions which could have obesity as a possible background of their illness. For example, this is an eye hospital, and the number of patients reporting retinopathy associated with NCDs is a potential source of bias.
Examined within the larger population context of the non-utilisation of health services, subjects in this study may be the ones with better health-seeking behaviours, therefore, not truly representative of the wider community. However, hospitals draw visitors from their local community confirms a high prevalence of this risk factor in the local community. This would need to be confirmed through multicentric studies in different regions. To the best of our knowledge, this is the first hospital OPD based data on obesity prevalence from Nepal.
The prevalence of HTN in our study was 40.67%, and men had a slightly higher prevalence of HTN (42.72%) than women (39.00%). Our findings are comparable to the 2019 STEPS survey Nepal and 2016 Nepal DHS, where the prevalence of HTN was 40.91% and 32.6%, respectively.31 In this study and the other two nationwide surveys, the prevalence of HTN increased with increasing age. One in three participants with pre-HTN detected in this study would be an important finding indicating the potential of progressing to HTN and the need to consider possible interventions to prevent HTN. A disturbing finding of this study was that 57.6% of hypertensive patients, who presumably had better health-seeking behaviour, did not know that they had HTN before this study. This should alert hospital leaders to launch health promotion programmes to raise awareness about HTN in hospitals and their surrounding communities.
At this hospital, persons found to be overweight or obese were referred to a counsellor in the next room who advises them for appropriate lifestyle modifications and, if necessary, refers them to an in-house exercise unit and an in-house general practitioner for other associated disease conditions.
Obesity and HTN
In this study, using all metrics, a significantly higher prevalence of HTN was found among participants with either overweight or obesity than participants with normal weight. Also, participants with WC measures greater than the cut-off value were twice as likely to be hypertensive (2.02; 95% CI 1.66 to 2.45) than people with normal WC. This is supported by several studies in different countries (Italy, USA, India).35–38
This study also found a statistically significant positive correlation between all three anthropometric metrics and SBP and DBP. These findings agree with other studies in different populations that support a strong relationship between different obesity metrics and BP across developed and low/middle-income countries. The Olivetti Heart Study also showed a weak, but significant and positive correlation between WC and SBP (r=0.191, p<0.001), and DBP (r=0.166, p<0.001) as well.39
An important finding of this study is that while BMI, WC and WHtR were all predictors of HTN, the WC and WHtR metrics were better predictors than BMI. Other studies have also shown that both WC or WHtR are better predictors for HTN than BMI. A Brazilian cohort study also showed that WC and WHtR were better predictors of HTN in adults over 18, with AUC values of 0.66 and 0.64, respectively, while BMI was 0.62.38 A study in India showed AUC values as 0.694, 0.667 and 0.634 for WHtR, WC and BMI, respectively.40 The study done in eastern India also showed AUC values of BMI, WC and WHtR that were 0.654, 0.676 and 0.693, respectively, indicating WC and WHtR as a better predictor for HTN than BMI.41
The greater value of this study lies in its ability to single out a very high prevalence of obesity and HTN in people coming to a tertiary care hospital, which is being missed in the routine clinical settings of busy hospitals in LMICs faced with inadequate health resources.
A simple anthropometric measurement could be used to determine the risk of having HTN Several studies show that the different anthropometric measurements of obesity can predict CVDs risk, such as HTN. However, the best anthropometric measurement as a predictor of HTN remains contentious and controversial. BMI, WC and WHtR are commonly used anthropometric screening tools to predict HTN and other CVDs.42 Some studies have suggested that WC and WHtR may be better predictors for HTN and CVD risk,43–48 while other studies suggest that BMI and WC as better predictors of HTN.49–51 A meta-analysis suggested that WC is a better predictor for CVDs risks such as HTN and recommended that it should be used in clinics and research.52
Although prospective, this single-centre, observational, cross-sectional study design cannot establish a causal relationship between increased weight over the optimal level and raised BP. The findings of this hospital-based study could not be generalised to the whole population of the country. The study was performed with a limited objective of finding an inexpensive, easily used measure of obesity that could be conducted by even non-medical personnel and could be used to predict risk for HTN.
WHtR had higher predictability than WC and BMI to predict HTN. WHtR thus proved to be non-inferior to BMI and WC as a screening measurement. This inexpensive and simple non-tension tape measurement may play an important role in the future detection of obesity and the prediction of HTN in resource-constrained settings of low/middle-income countries.
Conclusions
This study showed that WHtR and WC measured with an inexpensive non-tension tape was not inferior to BMI as a metric for obesity detection and HTN prediction. Because of its low cost, simplicity of measurement and better ability to predict HTN, it may become a more valuable tool in health facilities.
However, validation through larger studies in different settings (multicentre studies) is required for further confirmation before becoming a universal tool for routine and research use at the national level. Regardless of the anthropometric metrics used to measure obesity, the hospital setting is an opportune venue to screen overweight, obesity, and HTN, major NCDs risk factors. This is not standard practice as yet in many LMICs. Therefore, our group recommends that WC and BP measurement be introduced in all healthcare settings.
Finally, this study is an outcome of the evolving model of CHEERS’ proactive practice of person-centred care, which is an approach to reorienting the health system by incorporating holistic health promotion activities in contrast to the mainstream health system’s current practice of disease-focused, organ-centred, fragmented care.
Supplementary Material
Acknowledgments
We are grateful to Ms Jeera Budha, who was involved in data collection. We would like to express our sincere thanks to CHEERS hospital administration for allowing us to conduct this study and giving permission to submit it for publication.
Footnotes
Collaborators: no any collaborator.
Contributors: RS and MPU designed the study. RS, BK and JRB were involved in proposal writing. RS and BK involve in data analysis. RS, BK and SKU were involved in drafting the manuscript. RS, BK, MPU, JRB, SKU and MK are involved in the critical analysis and manuscript review. All authors have read the manuscript carefully and approved its submission. RS is responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. Data will be available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by Nepal Health Research Council (207/2019). Informed consent was taken from every participant before the interview and anthropometric measurement. Participation in this study was voluntary.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet 2014;384:766–81. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popkin BM. The world is fat. Sci Am 2007;297:88–95. 10.1038/scientificamerican0907-88 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Obesity and overweight 2020. Available: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [Accessed 1 Apr 2020].
- 4.NCD Risk Factor Collaboration (NCD-RisC) . Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377–96. 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall JE, Kuo JJ, da Silva AA, et al. Obesity-associated hypertension and kidney disease. Curr Opin Nephrol Hypertens 2003;12:195–200. 10.1097/00041552-200303000-00011 [DOI] [PubMed] [Google Scholar]
- 6.Rahmouni K, Correia MLG, Haynes WG, et al. Obesity-associated hypertension: new insights into mechanisms. Hypertension 2005;45:9–14. 10.1161/01.HYP.0000151325.83008.b4 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . Global health risks: mortality and burden of disease attributable to selected major risks, 2009. [Google Scholar]
- 8.Prospective Studies Collaboration, Whitlock G, Lewington S, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083–96. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . Hypertension 2019. Available: https://www.who.int/news-room/fact-sheets/detail/hypertension [Accessed 1 Sep 2020].
- 10.Rithie H, Roser M. Obesity and BMI: our world in data, 2019. Available: https://ourworldindata.org/obesity [Accessed 15 Apr 2020].
- 11.Roser M, Ritchie H. Burden of disease: our world in data, 2019. Available: https://ourworldindata.org/burden-of-disease [Accessed 15 Apr 2020].
- 12.Banjare JB, Bhalerao S. Obesity associated non-communicable disease burden. Int J Health Allied Sci 2016;5:81. [Google Scholar]
- 13.World Health Organization . Burden: mortality, morbidity and risk factors. Global status report on non-communicable diseases 2011;2010. [Google Scholar]
- 14.Yadav R, Aggarwal S, Singh A. SARS-CoV-2-host dynamics: increased risk of adverse outcomes of COVID-19 in obesity. Diabetes Metab Syndr 2020;14:1355–60. 10.1016/j.dsx.2020.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbas AM, Fathy SK, Fawzy AT, et al. The mutual effects of COVID-19 and obesity. Obes Med 2020;19:100250. 10.1016/j.obmed.2020.100250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waters H, Graf M. America’s obesity crisis, 2018. [Google Scholar]
- 18.World Health Organization . Non-communicable diseases country profiles 2018, 2018. [Google Scholar]
- 19.Vaidya A, Pathak RP, Pandey MR. Prevalence of hypertension in Nepalese community triples in 25 years: a repeat cross-sectional study in rural Kathmandu. Indian Heart J 2012;64:128–31. 10.1016/S0019-4832(12)60045-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhimal MBB, Bhattarai S, Dixit LP, et al. Report of non communicable disease risk factors: steps survey Nepal 2019. Kathmandu, Nepal: Nepal Health Research Council (NHRC), 2020. [Google Scholar]
- 21.WHO . Research report on NCD risk factors surveillance in Nepal, 2003. [Google Scholar]
- 22.Dhungana RR, Pandey AR, Shrestha N. Trends in the prevalence, awareness, treatment, and control of hypertension in Nepal between 2000 and 2025: a systematic review and meta-analysis. Int J Hypertens 2021;2021:6610649 10.1155/2021/6610649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nepal Health Research Council . Non communicable diseases risk factors survey 2007/08, 2008. [Google Scholar]
- 24.Thienemann F, Ntusi NAB, Battegay E, et al. Multimorbidity and cardiovascular disease: a perspective on low- and middle-income countries. Cardiovasc Diagn Ther 2020;10:376–85. 10.21037/cdt.2019.09.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Department of Health Services MOHP . Annual report 2018/19, 2020. [Google Scholar]
- 26.The Nepal NCDI Poverty Commission . The Nepal NCDI poverty commission national report - 2018, 2018. [Google Scholar]
- 27.Nepal Health Research Council . Non communicable diseases risk factors: steps survey Nepal 2013, 2013. [Google Scholar]
- 28.International Diabetes Federation . The IDF consensus worldwide definition of the metabolic syndrome, 2006. [Google Scholar]
- 29.Center for Disease Control . About adult BMI 2017. Available: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html [Accessed 20 Aug 2020].
- 30.Armstrong C, Joint National Committee . JNC8 guidelines for the management of hypertension in adults. Am Fam Physician 2014;90:503–4. [PubMed] [Google Scholar]
- 31.Ministry of Health, New ERA, ICF . Nepal demographic and health survey 2016. Kathmandu, Nepal: MOH/Nepal, New ERA, and ICF, 2017. [Google Scholar]
- 32.Vaidya A, Shakya S, Krettek A. Obesity prevalence in Nepal: public health challenges in a low-income nation during an alarming worldwide trend. Int J Environ Res Public Health 2010;7:2726–44. 10.3390/ijerph7062726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford ND, Patel SA, Narayan KMV. Obesity in low- and middle-income countries: burden, drivers, and emerging challenges. Annu Rev Public Health 2017;38:145–64. 10.1146/annurev-publhealth-031816-044604 [DOI] [PubMed] [Google Scholar]
- 34.Gunderson EP, Sternfeld B, Wellons MF, et al. Childbearing may increase visceral adipose tissue independent of overall increase in body fat. Obesity 2008;16:1078–84. 10.1038/oby.2008.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guagnano MT, Ballone E, Colagrande V, et al. Large waist circumference and risk of hypertension. Int J Obes Relat Metab Disord 2001;25:1360–4. 10.1038/sj.ijo.0801722 [DOI] [PubMed] [Google Scholar]
- 36.Levine DA, Calhoun DA, Prineas RJ, et al. Moderate waist circumference and hypertension prevalence: the REGARDS study. Am J Hypertens 2011;24:482–8. 10.1038/ajh.2010.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouf A, Rasool M, SK SM, et al. Prevalence of hypertension and its association with waist circumference in adult population of block Hazratbal, Srinagar, India. Ann Med Health Sci Res 2018. [Google Scholar]
- 38.Rezende AC, Souza LG, Jardim TV, et al. Is waist-to-height ratio the best predictive indicator of hypertension incidence? a cohort study. BMC Public Health 2018;18:281. 10.1186/s12889-018-5177-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siani A, Cappuccio FP, Barba G, et al. The relationship of waist circumference to blood pressure: the Olivetti heart study. Am J Hypertens 2002;15:780–6. 10.1016/S0895-7061(02)02976-X [DOI] [PubMed] [Google Scholar]
- 40.Vikram NK, Latifi AN, Misra A, et al. Waist-to-height ratio compared to standard obesity measures as predictor of cardiometabolic risk factors in Asian Indians in North India. Metab Syndr Relat Disord 2016;14:492–9. 10.1089/met.2016.0041 [DOI] [PubMed] [Google Scholar]
- 41.Prasad DS, Kabir Z, Suganthy JP, et al. Appropriate anthropometric indices to identify cardiometabolic risk in South Asians. WHO South East Asia J Public Health 2013;2:142–8. 10.4103/2224-3151.206760 [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization . Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008.
- 43.Park S-H, Choi S-J, Lee K-S, et al. Waist circumference and waist-to-height ratio as predictors of cardiovascular disease risk in Korean adults. Circ J 2009;73:1643–50. 10.1253/circj.CJ-09-0161 [DOI] [PubMed] [Google Scholar]
- 44.Zeng Q, He Y, Dong S, et al. Optimal cut-off values of BMI, waist circumference and waist:height ratio for defining obesity in Chinese adults. Br J Nutr 2014;112:1735–44. 10.1017/S0007114514002657 [DOI] [PubMed] [Google Scholar]
- 45.Lee CMY, Huxley RR, Wildman RP, et al. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol 2008;61:646–53. 10.1016/j.jclinepi.2007.08.012 [DOI] [PubMed] [Google Scholar]
- 46.Caminha TCS, Ferreira HS, Costa NS, et al. Waist-to-height ratio is the best anthropometric predictor of hypertension: a population-based study with women from a state of northeast of Brazil. Medicine 2017;96:e5874. 10.1097/MD.0000000000005874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W-C, Chen I-C, Chang Y-C, Loke S-S, et al. Waist-to-height ratio, waist circumference, and body mass index as indices of cardiometabolic risk among 36,642 Taiwanese adults. Eur J Nutr 2013;52:57–65. 10.1007/s00394-011-0286-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayeed MA, Mahtab H, Latif ZA, et al. Waist-to-height ratio is a better obesity index than body mass index and waist-to-hip ratio for predicting diabetes, hypertension and lipidemia. Bangladesh Med Res Counc Bull 2003;29:1–10. [PubMed] [Google Scholar]
- 49.Li N, Yang T, Yu W-Q, et al. Is waist-to-height ratio superior to body mass index and waist circumference in predicting the incidence of hypertension? Ann Nutr Metab 2019;74:215–23. 10.1159/000499073 [DOI] [PubMed] [Google Scholar]
- 50.Sakurai M, Miura K, Takamura T, et al. Gender differences in the association between anthropometric indices of obesity and blood pressure in Japanese. Hypertens Res 2006;29:75–80. 10.1291/hypres.29.75 [DOI] [PubMed] [Google Scholar]
- 51.Bennasar-Veny M, Lopez-Gonzalez AA, Tauler P, et al. Body adiposity index and cardiovascular health risk factors in Caucasians: a comparison with the body mass index and others. PLoS One 2013;8:e63999. 10.1371/journal.pone.0063999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Dijk SB, Takken T, Prinsen EC, et al. Different anthropometric adiposity measures and their association with cardiovascular disease risk factors: a meta-analysis. Neth Heart J 2012;20:208–18. 10.1007/s12471-011-0237-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request. Data will be available upon reasonable request.