Abstract
Background
As concerns about the prevalence of infections that are resistant to available antibiotics increase, attention has turned toward the use of these medicines both within and outside of formal healthcare settings. Much of what is known about use beyond formal settings is informed by survey-based research. Few studies to date have used comparative, mixed-methods approaches to render visible patterns of use within and between settings as well as wider points of context shaping these patterns.
Design
This article analyses findings from mixed-methods anthropological studies of antibiotic use in a range of rural and urban settings in Zimbabwe, Malawi and Uganda between 2018 and 2020. All used a ‘drug bag’ survey tool to capture the frequency and types of antibiotics used among 1811 households. We then undertook observations and interviews in residential settings, with health providers and key stakeholders to better understand the stories behind the most-used antibiotics.
Results
The most self-reported ‘frequently used’ antibiotics across settings were amoxicillin, cotrimoxazole and metronidazole. The stories behind their use varied between settings, reflecting differences in the configuration of health systems and antibiotic supplies. At the same time, these stories reveal cross-cutting features and omissions of contemporary global health programming that shape the contours of antibiotic (over)use at national and local levels.
Conclusions
Our findings challenge the predominant focus of stewardship frameworks on the practices of antibiotic end users. We suggest future interventions could consider systems—rather than individuals—as stewards of antibiotics, reducing the need to rely on these medicines to fix other issues of inequity, productivity and security.
Keywords: qualitative study, public health
What is already known?
Antimicrobial resistance is a key health challenge of our time, its rise attributed to widespread use of antibiotics.
Capturing the volume, types and stories of antibiotics used both within and outside formal prescriber settings is challenging but important to guide efforts to optimise antibiotic use.
Existing survey research in low- and middle-income countries contributes to a behaviour-oriented agenda by pinpointing demographic and educational associations with antibiotic use.
What are the new findings?
The most frequently used antibiotics among selected households in Malawi, Zimbabwe and Uganda were amoxicillin, cotrimoxazole and metronidazole.
The reasons for varying antibiotic use profiles between and within countries reflected particular histories, systems and relations.
Cross-cutting drivers of antibiotic use included gaps in public sector healthcare, the prominence of donor programmes, the lack of attention to disease prevention and deeply entrenched inequalities.
What do the new findings imply?
Research foregrounding individual characteristics and behaviour risks overlooking structural and systemic reasons for antibiotic use.
There is a need to consider systems - rather than individuals - as stewards of antibiotics ifsafe, sustainable reductions in antibiotic use are to be achieved within and beyond prescriber settings.
Introduction
To mitigate against the predicted health and economic impacts of antimicrobial resistance (AMR), the WHO’s global action plan has called for improved surveillance of antibiotic use to inform antimicrobial stewardship strategies.1 Many low-income and middle-income countries (LMICs) have experienced steep rises in antibiotic use in recent decades2–6 and been identified as particular targets for interventions. Surveillance at national level has been challenging to implement, and has generally prioritised the collection of aggregated consumption data from sales and imports, accompanied by limited hospital data, and even more limited information outside of hospitals on how antibiotics are actually used by different provider types and patients.7 A particular area of concern is antibiotic use beyond formal prescriber settings (retail pharmacies, drug shops, markets, households and farms), an understanding of which is crucial for producing a comprehensive picture of antibiotic use in LMICs and for developing context-sensitive interventions, particularly in settings with large informal pharmaceutical markets.8 9 Such data, however, are more resource intensive to collect systematically than consumption and prescribing data and are made especially challenging by the fact that ‘antibiotic’ is a complex category with numerous classes and uses and does not translate well either linguistically or conceptually in many settings.10 11
A growing number of surveys, systematic reviews and meta-analyses have nonetheless begun to elucidate patterns and predictors of antibiotic use at the community level. Analyses of demographic and health surveys and multi-indicator cluster surveys suggest that the greatest rises in antibiotic use in LMICs have been among rural, the poorest and least educated populations, particularly in low-income countries and South-East Asia, although the greatest overall rates are seen in urban centres and among the most affluent and most educated demographics.3 12 Studies have further sought to capture the extent of the misuse of antibiotics, undergirded by benchmarking ‘(ir)rational’ practice against indicators of expected clinical need. Findings indicate high rates of inappropriate use across LMICs,13–16 and that non-prescription use or ‘self-medication’ can make up as much as 100% in some populations.16 A recent study identified a greater prevalence of and reliance on informal providers in Asian countries than in African countries, with self-medication generally considered a cheaper, more convenient option than attending healthcare facilities,17 although this and other studies show many factors affect if/where treatment is sought, including policy and health system context, symptoms, disease severity, distance to travel, age of patient and trust in medicines and providers.3 13 14 18 Some studies have also broken down use profiles by antibiotic type and, increasingly, have mapped these onto the WHO’s Access, Watch, Reserve (AWaRe) classification system.19 The majority of antibiotic use in LMICs appears to be predominated by substances in the Access group,13 17 20–23 although use profiles vary considerably by setting and Watch group antibiotics appear to be more widely used in Asia than in Africa.17
While quantitative survey data are important for targeting interventions to particular populations, qualitative methods are vital for explaining antibiotic use in context.24 25 A strength of social research, including the observational techniques of ethnography, is the ability to capture, analyse and articulate the often taken-for-granted backdrops that shape—and are reproduced by—the ways people live in the world. An expanding body of such research has demonstrated that many of the antibiotic use practices that would be considered ‘irrational’ from a biomedical perspective (eg, storing antibiotics for later, sharing them with others and using informal providers) are highly rational within the material and moral worlds inhabited by people living in contexts of scarcity and precarity.26–29 Anthropologists have shown that expectations for medicinal care and increasing reliance on antibiotics are tied to processes of pharmaceuticalisation that have disproportionately affected people living in LMICs, in which care has been progressively stripped to little more than the giving and receiving of medicines.26 30 31 The work of social scientists has contributed to a growing recognition of the wider structural processes shaping antibiotic use practices in LMICs and that these should be considered in stewardship strategies moving forward.32 33
In-depth qualitative approaches necessarily require a focus on particular sites and contexts. Few studies using such methods, however, have attempted the kind of multi-sited, comparative analyses more common in survey research to compare patterns of, and underlying reasons for, antibiotic use across different settings.17 18 Such research that can build in the power of comparative approaches—that render visible what might otherwise be taken for granted in a given setting—can enhance the ability of social research to contribute to interventions at multiple levels of scale. This article analyses findings from anthropological studies of household antibiotic use in Malawi, Uganda and Zimbabwe, which used mixed-methods research designs to capture, situate and compare the stories of antibiotics and their use in different settings. Our aim is both to increase understanding of patterns and reasons behind antibiotic use at community level and to contribute to ongoing debates around who or what should be considered antibiotic ‘stewards’ beyond the better-known architectures of formal prescriber settings.
METHODS
Studies and settings
The data on which this article is based are drawn from anthropological research on antibiotic use in Malawi, Zimbabwe and Uganda, which took place within two projects—Febrile Illness Evaluation in a Broad Range of Endemicities (FIEBRE) and Antimicrobials in Society (AMIS)—between April 2018 and December 2020. The concept for this cross-site paper emerged during the analysis phase of both projects in late 2019. The multidisciplinary FIEBRE and AMIS teams were comprised of social scientists (mostly anthropologists), epidemiologists, clinical and laboratory scientists, pharmacists, postgraduate students, research assistants, project managers and administrators.
Malawi, Zimbabwe and Uganda are postcolonial states in sub-Saharan Africa with different historical relationships with biomedicine and antibiotics,34 but all have high rates of poverty, inequality and donor-dependent health systems. FIEBRE sites (Chikwawa District, Malawi, and suburbs of Harare, Zimbabwe) were selected to evaluate febrile illness in malaria-endemic and non-endemic settings, while AMIS sites (Kampala and Tororo District, Uganda) were selected purposively to compare urban and rural antibiotic use (figure 1). FIEBRE and AMIS focused on low-income residential areas, but there was also considerable heterogeneity between sites that enables comparison between a range of different urban and rural environments, health system contexts and degrees of poverty (table 1).
Figure 1.
Map showing study settings and population density in Malawi, Uganda and Zimbabwe. Maps were generated using ESRI ArcMap 10.8.1 (ESRI 2020; Redlands, California, USA). Population data were sourced from www.worldpop.org and consists of 2020 population estimates for each country at the scale of 100 m2 grid cells.
Table 1.
Overview of study settings and healthcare context
| Malawi | Zimbabwe | Uganda |
| Malawi, a country with a population of 17.6 million, is among the poorest countries in the world.59 It ranks 174 out of 189 countries in the human development index and had a per capita gross national income (GNI) in 2019 of $550.60 Malawi gained independence from British colonial rule in 1964, with Hastings Kamuzu Banda governing the country under autocratic rule until 1994. He used international donor money for large-scale infrastructure projects,61 often in urban centres leaving rural areas underdeveloped. Severe debt, limited public sector funds, corruption and HIV have undermined modest development gains.62–64 Today, the majority of funds for public services, including health, come from international donors.65 Chikwawa is a rural district in the country’s southern region, where subsistence farming is the predominant livelihood. Malaria is endemic and, annually, Chikwawa records one of the highest rates in Malawi.66 As is the case nationally, government health services are free, and residents are highly reliant on primary facilities.67 However, there are geographical inequities in healthcare coverage, with rural areas facing persistent staff shortages and regular medicine stockouts.62 67 Private and informal providers exist but are less prominent in Chikwawa than in cities, partially due to the lack of cash available to most residents. | Zimbabwe has a population of 14.6 million, of which 1.5 million live in the capital of Harare. Zimbabwe ranks 150 of 189 countries in the human development index and in 2019 had a per capita GNI of $1200.68 Zimbabwe boasted one of Africa’s strongest postcolonial healthcare systems following independence in 1980, with robust primary healthcare infrastructure and a strong essential drugs programme.69 However, by the late 1990s, these achievements were undone by political repression, structural adjustment, hyperinflation, increasing privatisation and the HIV pandemic.70 Life expectancy was less than 50 years during the 2000s, and the neglect of water and sanitation infrastructure led to a cholera outbreak in 2008–2009 that killed over 4000 people, many in high-density suburbs of Harare.39 Since then, frequent and increasingly drug-resistant outbreaks of cholera and typhoid have occurred.40 Mbare and Budiriro, where we conducted research, are among such suburbs. Most residents live within walking distance of a public clinic, yet healthcare is not readily accessible: residents must pay a user fee and, furthermore, must often purchase prescribed medicines at a retail pharmacy.50 While Zimbabwe has historically had a strong regulatory environment, since economic collapse, informal markets have grown substantially, with Mbare’s expansive marketplace a hotspot of medicine vending. | Uganda has a large population of 44.2 million and ranks 159 of 189 in the human development index, with a per capita GNI in 2019 of $780.71 Before independence in 1962, the Ugandan health system was entirely funded by the government but, following political unrest and economic decline in the 1970s and 80s, during the 1990s health sector reforms were implemented, including decentralisation, forming a multilayered public healthcare system.72 Healthcare in the public sector in Uganda is free but suffers severe resources shortages, including frequent drug stockouts and a lack of equipment and supplies.73 The country’s large private sector includes hospitals, clinics, pharmacies and drug shops, the latter of which are licensed and regulated but generally extend beyond their formal roles. This includes the unlicensed sale of antibiotics.74 Namuwongo is a large informal settlement in Kampala, where many people who work in the city centre and the surrounding affluent suburbs reside. The settlement has notoriously poor water and sanitation infrastructure.75 Nagongera is a subcounty in the rural district of Tororo, where over 70% of the population survive on subsistence farming and where most live hand to mouth, with over 50% of the population living on less than $1 per day.76 |
Household surveys
Data collection commenced with six household surveys, including two in each country (see table 2). In the FIEBRE settings (Zimbabwe and Malawi), the selection of households in the two larger surveys (ZU1 and MR1) followed the eligibility criteria for study’s clinical protocol. Specifically, community ‘controls’, matched by age, sex and residential proximity to patient cases attending outpatient facilities with febrile illness, were identified and invited to take part in the study. A survey of antibiotic use practices was conducted during the same visit as for the clinical activities among consenting households. The smaller, purposively sampled surveys (ZU2 and MR2) were conducted to include households in geographical areas of relevance to antibiotic use not captured in the larger surveys, which in ZU2 included a suburb with known informal markets for antibiotics (Mbare) and in MR2 included villages further from public healthcare facilities. The inclusion of households in these areas did not follow predefined demographic criteria, but we sought to iteratively capture a range of livelihoods and points of access to medicines and care in these settings. The AMIS surveys (UU and UR) were not attached to a clinical study and used the same sampling strategy as the smaller FIEBRE surveys within defined study areas in Uganda.
Table 2.
Overview of surveys
| Country | Survey name* | Setting | Survey description | Sampled households (n) | Sample method | Number of antibiotics in drug bag† |
| Malawi | MR1 | Chikwawa district (rural) |
Survey conducted with households of community controls who had been randomly matched with participating febrile patient cases presenting at FIEBRE study facilities | 825 | Semi-random | 27 |
| MR2 | Chikwawa district (rural) |
Targeted survey that included households both in same areas as MR1 and in more remote areas of Chikwawa, further from public healthcare facilities. Selected households followed up ethnographically | 100 | Purposive | ||
| Zimbabwe | ZU1 | Harare–Budiriro, Glenview and Glen Norah (urban) | Survey conducted with households of community controls who had been randomly matched with participating febrile patient cases presenting at FIEBRE study facilities | 336 | Semi-random | 30 |
| ZU2 | Harare–Mbare and Budiriro (urban) |
Targeted survey that included households both in same areas as ZU1 and in the dense township of Mbare with large marketplace. Selected households followed up ethnographically | 100 | Purposive | ||
| Uganda | UU | Kampala–Namuwongo (urban) |
Survey in an informal settlement in Kampala, conducted as part of AMIS study. Selected households followed up ethnographically | 350 | Purposive | 37 |
| UR | Tororo district–Nagongera (rural) |
Survey in a rural town in Tororo district, conducted as part of AMIS study. Selected households followed up ethnographically | 100 | Purposive | 16 |
*M, Malawi, Z, Zimbabwe, U, Uganda; 1larger, semi-random survey, 2smaller, purposively sampled survey; R, rural, U, urban.
†The number indicates how many different antibiotic substances were included. Where possible, we also included different modes of administration for the same substances to maximise the chances of recognition by respondents; however, these fine-grained differences are not explicitly represented in our quantitative analyses to avoid overcomplication. See online supplemental material for breakdown of drug bag contents by antibiotic substance and mode of administration.
AMIS, Antimicrobials in Society; FIEBRE, Febrile Illness Evaluation in a Broad Range of Endemicities.
bmjgh-2021-006920supp001.pdf (85.7KB, pdf)
Surveys were conducted by postgraduate researchers and/or experienced research assistants. The questions asked of respondents were tailored towards each project’s aims, with AMIS seeking to understand the roles of antibiotics in lives, livelihoods and society more broadly, and FIEBRE more specifically focused on the relationship between febrile illness and antibiotic use. These differences aside, surveys commonly included questions about household context and demographics, acute illnesses frequently experienced by household members, medicines (including antibiotics) used to treat these common illnesses and challenges associated with accessing medicines and care. These core domains form the basis of the analysis we present here.
In asking households about antibiotics specifically, all surveys used a ‘drug bag’ method that we developed, methodological reflections of which are published elsewhere.11 This involved showing respondents physical samples of antibiotics to establish common referents with them without assuming that they use biomedical categories, both to stimulate deeper conversation within the interview itself and to quantitatively identify patterns of antibiotic use to be followed up on through further ethnographic fieldwork. Drug bags specific to each site were assembled by visiting all spaces where antibiotics could be acquired locally, including clinics and hospitals, retail pharmacies, drug shops, iterant drug sellers and market vendors. We included different modes of administration of the same drug where these existed, but this analysis only includes systemic formulations (ie, tablets, suspensions and injections, not topical creams or eye ointments). All drugs were clearly labelled with packaging included, so that respondents were able to recognise the drug in a number of different ways, including appearance, generic name and branding. A full breakdown of drug bag contents is provided as online supplemental material. During the surveys, a series of ‘pile sorting’ exercises were conducted to identify which antibiotics respondents recognised, had used before, used in the month preceding the survey, used ‘frequently’ (as they interpreted ‘frequently’) and were unable to access when needed (as they interpreted ‘need’). In designing these exercises, we avoided asking participants about non-prescription use unless they raised the topic themselves, to avoid imposing a normative biomedical framework that could have made respondents feel judged and thus feel reluctant to open up about the many other dimensions of antibiotic use. Quantitative data were produced by capturing the contents of the piles, while narrative qualitative data were produced through the conversations stimulated by the process.
Ethnographic fieldwork
The household surveys took place within a broader ethnographic methodology that included fieldwork in clinics, hospitals, pharmacies, markets and with residents, as well as with key stakeholders in antimicrobial use and resistance. Fieldwork, conducted by anthropologists, postgraduate researchers and research assistants, aimed to gain a deeper understanding of the social, economic and health system context of antibiotic use profiles identified during the surveys. Following Whyte et al’s observation that medicines have social lives analytically separable from those of human actors,35 our aim was to draw out the stories of antibiotics by following them as they circulated within and beyond formal healthcare settings in different countries. Households were purposively sampled for ethnogaphic research from among those who expressed interest during surveys. At each site, 15–25 households were purposively selected to capture a range of household contexts and environments. Healthcare providers accessible to these households were eligible for inclusion, including public, private and informal providers. Observation of antibiotic prescribing and dispensing took place with a total of 23 providers (4 in Malawi, 7 in Zimbabwe and 12 in Uganda). Key informants and stakeholders, including individuals with relevant knowledge and influence at local, district and national levels, were interviewed, totalling 113 over the study period (57 in Malawi, 34 in Zimbabwe and 22 in Uganda). Ethnographic observation entailed taking part in everyday routines, including management of ill health. Interviews followed tailored and regularly updated topic guides which drew on findings from other concurrent fieldwork activities. The studies in the three countries were designed collaboratively between the researchers in all three teams, who remained in frequent contact for the duration of the fieldwork and analysis to share insights, compare findings across sites and add further lines of inquiry stimulated by interim analyses.
Patient and public involvement
Participants and stakeholders were engaged throughout the research process across all settings. While protocols were being developed, key stakeholders were identified to help to refine research questions and to address ethical and logistical challenges; these same individuals, some of whom became participants in the research themselves, were regularly updated throughout the research on findings as they emerged. Preliminary findings were also shared with residents and health providers where possible, discussions which were iteratively fed back into data collection. On completion of data collection, findings were disseminated to all participants and stakeholders, a process which in turn fed back into analysis and writing of outputs, including the present article.
Data management and analysis
All data were captured and stored on encrypted devices. Data were collected using ODK and transferred to secure servers at the London School of Hygiene and Tropical Medicine (FIEBRE data) and the Infectious Diseases Research Collaboration, Kampala (AMIS data).
Quantitative data, which included quantitative survey data and basic prescribing data abstracted from field notes with antibiotic providers, was analysed using R.36 Data were analysed separately according to the protocols of each project, and when the concept for this article was developed, comparable variables were merged into unified dataframes, and descriptive statistics were reported for each variable. During the analysis, individual antibiotic substances were reclassified into antibiotic classes and WHO’s AWaRe categories to enable greater comparison between sites and to draw out implications for stewardship. Qualitative data, which included qualitative survey data as well as field notes and interview data from the ethnographic phase, were analysed using NVivo 12. Themes were generated by each country team on an iterative basis during which codes of progressively higher orders of abstraction were used to explain and theorise the data. Comparative analysis was achieved through frequent cross-site sharing of findings and discussions of themes emerging, with inter-project meetings held at least every 2 weeks for the duration of the 3.5-year projects.
Results
Demographics and common illnesses
Across all sites, a total of 1811 households participated in the household surveys, of which 925 (51.0%) were in Malawi, 436 (24.0%) were in Zimbabwe and 450 (24.8%) were in Uganda. Demographic characteristics of households are summarised in table 3. The most common occupations across study settings were labouring (eg, building, making, fixing, cleaning or cooking), informal vending and subsistence farming, the latter the primary occupation of most rural households. Median household size varied but was greater in urban areas, with the largest households in ZU2 (median: 4). Overcrowding was especially common in high-rise flats in Mbare, where several households often occupied a single room separated by a divider. The primary respondents were predominantly female, ranging from 56.0% (UR) to 79.0% (ZU2), for various reasons, including surveys being conducted during weekday working hours, and men often referring our field team to women, who were seen to know more about care and medicines, especially for children. The median age of respondents ranged from 27 years (MR1) to 47 years (UR). The most common self-reported acute illnesses were colds, flus and coughs, but beyond this there were notable differences between settings, especially between urban and rural study sites. In urban settings, stomach pain and diarrhoea were a more regular occurrence likely due to contaminated water sources, while in rural areas, where malaria was endemic, malaria and fevers were self-reported as the most frequent causes of sickness. Households were asked how often they were able to get the medicines needed for these illnesses, the most common answer to which was ‘sometimes’ (MR1: 31.2%, ZU1: 58.9%, UU: 13.1%).
Table 3.
Household context, common illnesses and medicines availability
| Variable | Malawi | Zimbabwe | Uganda | ||||
| MR1 | MR2 | ZU1 | ZU2 | UU | UR | ||
| Total households (n) | 825 | 100 | 336 | 100 | 350 | 100 | |
| Setting | Urban/rural | Rural | Rural | Urban | Urban | Urban | Rural |
| Primary respondent | Age (median, range) (years) | 27 (18–77) | 42 (18–78) | 29 (18–71) | – | – | 47 (18–87) |
| Sex (n, % female) | 645 (78.1) | 58 (58.0) | 243 (72.3) | 79 (79.0) | 256 (73.1) | 56 (56.0) | |
| Household | Occupants (median, range) | 3 (1–13) | – | 2 (1–13) | 4 (1–12) | – | – |
| Primary occupation | Farmer (n, %) | – | 82 (82.0) | – | 2 (2.0) | 10 (2.9) | 89 (89.0) |
| Merchant/vendor (n, %) | – | 9 (9.0) | – | 57 (57.0) | 127 (36.3) | 1 (1.0) | |
| Labourer (n, %) | – | 7 (7.0) | – | 15 (15.0) | 30 (8.6) | 1 (1.0) | |
| Other (n, %) | – | 2 (2.0) | – | 26 (26.0) | 183 (52.3) | 9 (9.0) | |
| Common self-reported acute illnesses (top 5) |
Cold/flu (n, %) | 67 (8.1) | 5 (5.0) | 252 (75.0) | 65 (65.0) | 270 (77.1)† | 41 (41.0)† |
| Malaria (n, %) | 609 (73.8) | 80 (80.0) | 39 (11.6) | 2 (2.0) | 160 (45.7) | 77 (77.0) | |
| Cough (n, %) | 410 (49.7) | 37 (37.0) | 177 (52.7) | 26 (26.0) | –† | –† | |
| Stomach pain (n, %) | 128 (15.5) | 23 (23.0) | 220 (65.4) | 40 (40.0) | 12 (3.4) | 19 (19.0) | |
| Diarrhoea (n, %) | 175 (21.2) | 16 (16.0) | 142 (42.3) | 30 (30.0) | 39 (11.1) | 11 (19.0) | |
| Medicines are available for common illnesses | Always (n, %) | 126 (15.2) | Respondents were asked qualitatively about medicine availability* | 38 (11.3) | Respondents were asked qualitatively about medicine availability* | 293 (83.7) | Respondents were asked qualitatively about medicine availability* |
| Usually (n, %) | 297 (36.0) | 39 (11.6) | 0 (0.0) | ||||
| Sometimes (n, %) | 257 (31.2) | 198 (58.9) | 46 (13.1) | ||||
| Rarely (n, %) | 125 (15.2) | 57 (17.0) | 10 (2.9) | ||||
| Never (n, %) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
M, Malawi, Z, Zimbabwe, U, Uganda; 1larger, semi-random survey, 2smaller, purposively sampled survey; R, rural, U, urban.
*Respondents across all three countries generally indicated that medicines were challenging to access for reasons including affordability, stockouts and inability to obtain a prescription. Even where medicines were available, this was often at considerable expense to the household.
†In the Ugandan surveys, 'cold/flu' and 'cough' are grouped together because participants used language that combined these categories of illness.
Patterns of antibiotic use
The drug bags were a source of considerable curiosity wherever we went. With word travelling quickly, surveys were usually conducted with several onlookers gathered around, attentively watching the pile sorting exercises. The main findings regarding patterns of antibiotic use are summarised in table 4. Antibiotics were widely recognised by respondents, but this varied considerably within and between settings (ranging from a median pile size of 4 in UR to 7.5 in ZU2). Some antibiotics were especially well recognised, the most familiar of which were amoxicillin, doxycycline, cotrimoxazole, metronidazole, ciprofloxacin, erythromycin and an ampicillin/cloxacillin combination drug (the latter only in Uganda). These were referred to by short-hand or brand name terms such as ‘amoxyl’ (amoxicillin), ‘cotri’ or ‘bactrim’ (cotrimoxazole), ‘doxy’ (doxycline) and ‘flagyl’ or ‘metro’ (metronidazole). These same antibiotics were most likely to have been used before, with a considerable proportion of households having used an antibiotic in the last month (range: 31.0% in ZU1–71.0% in UR). In Zimbabwe and Malawi, most households’ last antibiotic was prescribed at a public facility; in Uganda, private providers were more commonly used, particularly in Namuwongo (UU).
Table 4.
Household antibiotic use
| Variable | Malawi | Zimbabwe | Uganda | ||||
| MR1 | MR2 | ZU1 | ZU2 | UU | UR | ||
| Denominator: antibiotics in drug bags | 27 | 27 | 30 | 30 | 37 | 16 | |
| Pile sorting exercises—median drugs selected (range) | Recognised | 6 (0–27) | 5.5 (0–19) | 5 (0–23) | 7.5 (3–25) | 7 (1–17) | 4 (0–11) |
| Used | 3 (0–15) | 5 (0–15) | 2 (0–12) | 5 (0–24) | 5 (1–14) | – | |
| Frequently used | 1 (0–6) | 2 (0–9) | 1 (0–7) | 2 (0–13) | 2 (0–10) | 2 (0–7) | |
| Needed but inaccessible | 0 (0–7) | 0 (0–1) | 0 (0–3) | 1 (0–20) | 1 (0–12) | 2 (0–6) | |
| Denominator: total households | 825 | 100 | 336 | 100 | 350 | 100 | |
| Last antibiotic used, n (%) |
≤1 month | 467 (56.7) | 53 (53.0) | 104 (31.0) | – | 144 (41.1) | 71 (71.0) |
| >1 month | 352 (42.7) | 38 (38.0) | 224 (66.7) | 206 (58.9) | 24 (24.0) | ||
| Top 3 most frequently used antibiotics—antibiotic, n (%) | Amoxicillin, 395 (47.9) | Cotrimoxazole, 68 (68.0) | Amoxicillin, 192 (57.1) | Amoxicillin, 64 (64.0) | Metronidazole, 208 (59.4) | Amoxicillin, 58 (58.0) | |
| Cotrimoxazole, 311 (37.7) | Amoxicillin, 39 (39.0) | Cotrimoxazole, 88 (26.2) | Cotrimoxazole, 51 (51.0) | Ampicillin/cloxacillin, 106 (30.3) | Cotrimoxazole, 42 (42.0) | ||
| Metronidazole, 62 (7.5) | Erythromycin, 19 (19.0) | Metronidazole, 37 (11.0) | Metronidazole, 36 (36.0) | Amoxicillin, 97 (27.7) | Metronidazole, 40 (40.0) | ||
| Frequent use of ≥1 antibiotic in AWaRe classification, n (%): | Access | 608 (73.7) | 80 (80.0) | 231 (68.8) | 74 (74.0) | 291 (83.1) | 94 (94.0) |
| Watch | 82 (9.9) | 21 (21.0) | 28 (8.3) | 25 (25.0) | 85 (24.3) | 15 (15.0) | |
| Denominator: subset of households* | 173 | 100 | 101 | 100 | 350 | 100 | |
| Source of antibiotics, n (%) | Public clinic | 5 (2.9) | 3 (3.0) | 38 (37.6) | Respondents asked qualitatively about where antibiotics were generally obtained and why† | 55 (15.7)‡ | 84 (84.0)‡ |
| Public hospital | 111 (64.2) | 63 (63.0) | 8 (7.9) | ||||
| Private facility | 8 (4.6) | 16 (16.0) | 7 (6.9) | 165 (47.1) | 79 (79.0) | ||
| Research clinic/NGO | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (2.3) | 1 (1.0) | ||
| Retail pharmacy | 7 (4.0) | 1 (1.0) | 37 (36.6) | 107 (30.6) | 2 (2.0) | ||
| Other shop | 7 (4.0) | 1 (1.0) | 0 (0.0) | 8 (2.3) | 14 (14.0) | ||
| Informal vendor | 6 (3.5) | 2 (2.0) | 2 (2.0) | 0 (0.0) | 0 (0.0) | ||
| Someone else | 29 (16.8) | 4 (4.0) | 9 (8.9) | 0 (0.0) | 0 (0.0) | ||
| Other | 0.0 | 10 (0.0) | 4 (4.0) | 4 (1.1) | 2 (2.0) | ||
M, Malawi, Z, Zimbabwe, U, Uganda; 1larger, semi-random survey, 2smaller, purposively sampled survey; R, rural, U, urban.
*In MR1 and ZU1, a smaller subset of households who reported using an antibiotic during a prior fever episode were asked where the antibiotic was obtained from. In MR2, all respondents were asked about where the last antibiotic used in the household was obtained from. In UU and UR, respondents were asked to list the sources from which they generally obtain medicines, including antibiotics.
†A common theme among respondents was that the clinic shelves were perceived as often being empty, and so they felt pushed towards retail pharmacies and the informal sector to obtain antibiotics—discussed in section ‘Amoxicillin and the gaps in public sector healthcare’.
‡In Uganda, both public clinics and public hospitals were grouped together under public facilities.
AWaRe, Access, Watch, Reserve; NGO, non-governmental organisation.
Aiming to determine which antibiotics featured most prominently in everyday life, we were especially interested in the category of self-reported ‘frequent use’. Most households reported frequently using at least one antibiotic, with a median of one antibiotic being frequently used in ZU1 and MR1 and two in all other surveys. Frequent use was heavily concentrated on three drugs—amoxicillin, cotrimoxazole and metronidazole—all of which belong to the WHO Access group. These drugs were the three most frequently used antibiotics in 4/6 surveys (MR1, ZU1, ZU2 and UR) and the remaining two surveys (MR2 and UU) had two of these drugs in their top three (see table 4). Expressed as a proportion of total frequent use (visualised in figure 2), amoxicillin made up the greatest proportion (range: 13.5% in UU–53.0% in ZU1), followed by cotrimoxazole (range: 8.1% in UU–37.9% in MR2) and then metronidazole (range: 6.1% in MR2–28.5% in UU). Across all our surveys, a greater proportion of households were frequently using one or more antibiotics from the Access group (range: 68.8% in ZU1–94% in UR) than from the Watch group (range: 8.3% in ZU1–25.0% in ZU2) (see table 4). The most frequently used drugs from the Watch group were ciprofloxacin, which also belongs to the Access group, and erythromycin (figure 2). We observed, however, that while frequent use of erythromycin was commonly reported in Malawi and this was the third most frequently used of all antibiotics in MR2 (table 4), its use seemed likely over-reported due to its very similar appearance and applications for pain and inflammation to ibuprofen.
Figure 2.
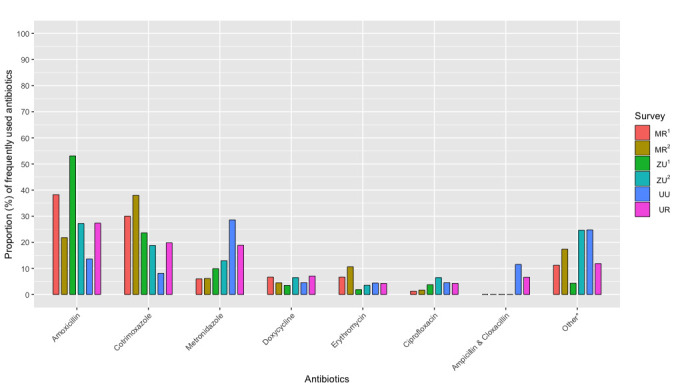
Proportion (%) of total ‘frequently used’ antibiotics among households in Malawi (MR1, n=825; MR2, n=100), Zimbabwe (ZU1, n=336; ZU2, n=100) and Uganda (UU, n=350; UR, n=100). For a full breakdown, see online supplemental material. *Includes all other antibiotic substances in the drug bags. M, Malawi, Z, Zimbabwe, U, Uganda; 1larger, semi-random survey, 2smaller, purposively sampled survey; R, rural, U, urban.
With amoxicillin, cotrimoxazole and metronidazole the most frequently used antibiotics across all settings, three noteworthy features of their patterning prompted particular ethnographic attention as our research progressed: (1) amoxicillin was the most frequently used antibiotic in 4/6 surveys and in the top 3 in all (table 4), with the antibiotic making up the greatest proportion of frequent use in ZU1 (53.0%) relative to other surveys (figure 2); (2) cotrimoxazole made up a greater proportion of frequent use in the Malawian surveys than in other countries (MR1: 30.0%, MR2: 37.9%) (figure 2); and (3) metronidazole made up a greater proportion of frequent use in Uganda than in other countries, particularly Namuwongo (UU), where it was the most used antibiotic, making up 28.5% of all frequent use (figure 2). In the following sections, we home in on the biographies35 of these three antibiotics in different settings: amoxicillin in Zimbabwe, cotrimoxazole in Malawi and metronidazole in Uganda. Our aim is not to offer a representative analysis of antibiotic use in these countries, but to highlight both particular and cross-cutting features of health systems and supply chains that shape the use of these antibiotics at household level and that are pertinent for informing antimicrobial stewardship interventions.
Amoxicillin and the gaps in public sector healthcare
During ethnographic fieldwork in primary care clinics in Harare, Zimbabwe, nurses often referred to amoxicillin as the ‘drug of choice’. This is because of the drug’s centrality to the country’s national treatment guideline (EDLIZ), which specified which essential medicines should be available on the clinic shelves and how they should be used. A broad-spectrum penicillin, amoxicillin is crucial to the syndromic management approach taken by EDLIZ and is a first-line treatment for a wide range of conditions, including pneumonia, sinusitis, otitis media and urinary tract infections. In the two clinics in which we worked in Mbare and in Buduriro, amoxicillin was the most prescribed antibiotic, making up 107/253 (42.3%) and 88/258 (33.7%) of observed antibiotic prescriptions, respectively. Additionally, we found that consultations tended to gravitate around the norm and expectation of an antibiotic being given, which we similarly found in healthcare facilities in Malawi and Uganda. Because of its pervasive use in outpatient settings, amoxicillin was perhaps expectedly the most widely recognised and used across our household surveys.
However, stockouts of first-line antibiotics, including amoxicillin, were a regular occurrence in all of our study settings in Zimbabwe, Malawi and Uganda. In Zimbabwe, the situation analysis on AMR attributed stockouts to ‘a lack of finances at the national level and fragmented funding from donors, which are often interested in funding drugs for specific programmes rather than the workhorse antimicrobials’.37 As box 1 illustrates, residents in Zimbabwe like Sisi Betty were accustomed to a situation in which, more often than not, all they had to show for paying a steep user fee and queuing for hours at the clinic outpatient department was a ‘piece of paper’ with which to seek an antibiotic themselves. Purchasing from a retail pharmacy was, however, often not an option due to high medicine costs, driving people towards the informal marketplace for medicines. Indeed, with experience dictating that the clinic was unlikely to have medicines in stock, respondents reported that they were increasingly being forced to ‘bypass’ the clinic altogether to get the medicines they needed. In this process, residents used the information and experience available to them to source the correct medicine:
Box 1. Amoxicillin use in Harare, Zimbabwe.
Sisi Betty smiled as she quickly recognised and picked up a packet of amoxicillin as the first medicine that she recognised from a pile of antibiotics from the drug bag. This 35-year-old mother of 3 stayed just a street away from the busy marketplace of Mbare where medicines were a common sight in the hands of drug vendors. ‘This one, I bought very cheap at the market, I got two packets with 20 amoxicillin tablets for just a dollar. These days I never go to the clinic, it is a waste of time and money. The clinic shelves are empty. You only go there to pay for a card to see a nurse who only gives you a paper to go to the pharmacy. At the pharmacy, you will find everything but the prices there will tell you to go away. To get something, you are forced to buy very cheap medicine at the market like what I do. The marketplace is now my clinic’. (SM fieldnotes, Mbare, Zimbabwe, June 2018)
At times when a neighbour's child goes to the clinic and is given a prescription for a similar disease like flu you can just opt to go to the market and buy say amoxyl [sic]. You see what the medication the neighbour was told to buy, and you go to the market to buy the medication—you avoid paying consultation fee at the clinic if you check what someone else with the same condition was told to buy. The market has almost all the drugs; I buy there. (Grandmother, 49, Mbare, respondent in ZU2)
It must be stressed that the informal markets in Harare were widely viewed as a last resort, with some having realised that the cost of using a potentially substandard drug at the marketplace could eventually be even higher than the pharmacy-sourced medicines: ‘$1 for 10 pills when you buy from the street as compared with those that cost $3 from pharmacy the cost may be low but you end up going there a lot of times with no real change’ (female, informal food vendor selling mealies, Mbare). Additionally, our surveys suggest that residents reported their last antibiotic often to have been received at a clinic (37.6% in ZU1) or from a retail pharmacy (36.6% in ZU1). However, despite respondents’ observed attempts to be responsible medicines users, there was often simply no alternative than to use the informal sector given the gaps in the public healthcare system.
Cotrimoxazole and the programme landscape of ‘global health’
Unlike amoxicillin, an antibiotic that is comparatively well funded by international donors is cotrimoxazole. In Malawi, as in many LMICs, cotrimoxazole is primarily used in the management of HIV, having previously been more widely used in outpatient care (much like amoxicillin). Following WHO guidelines,38 cotrimoxazole is prescribed as part of antiretroviral therapy initiation and prescribed indefinitely afterwards as prophylaxis against opportunistic infections. In Chikwawa, such was the scarcity of essential medicines in government healthcare facilities, particularly those further away from the district hospital, that the only medicines consistently available were those funded by donor programmes. This included, most notably, antimalarials, TB medicines and HIV-related medicines, including cotrimoxazole. This meant that cotrimoxazole was routinely prescribed for acute illnesses, regardless of patients’ HIV status, simply because it was consistently in stock. Even though in Malawi, unlike in Zimbabwe, residents did not have to pay for public sector healthcare (table 1), with households surviving through subsistence farming and with almost no access to cash they were more heavily reliant on these few donor-funded medicines.
Surveys in Chikwawa revealed that cotrimoxazole accounted for a high proportion of household use, particularly MR2 which included households in more remote villages further from the district hospital. As we found, residents knew that febrile illness—an extremely common occurrence in this malaria-endemic region—would mean the prescription of either antimalarials or, if the rapid test was negative, cotrimoxazole:
My daughter had fever and general body pain, but she was not diagnosed malaria, so she was given cotrimoxazole and Panadol (paracetamol). (Female, 32, subsistence farmer, MR2)
We always get prescribed [cotrimoxazole] from the hospital when we go for treatment. (Male, 49, subsistence farmer, MR1)
However, given the considerable geographical inequalities in access to government health facilities (table 1), accessing healthcare and medications could be expensive for many. As the vignette in box 2 shows, central to household coping and care strategies was a moral economy of reciprocity that included the storing and sharing of medicines.28 Catastrophic healthcare costs could be mitigated through borrowing medicine to treat acute illness and reciprocating at a later date when one had leftover medicine. Securing access to cotrimoxazole could be further improved through having a household member or a neighbour living with HIV and who thus had a consistent supply of the medicine, a practice we also observed during ethnographic fieldwork in Uganda and Zimbabwe. The centrality of cotrimoxazole to prescribing in Malawi and, in turn, to household coping and care strategies stems from systemic prioritisation of HIV and malaria at the expense of more ‘mundane’ acute illnesses.
Box 2. Cotrimoxazole use in Chikwawa, Malawi.
At 21:00 one night, one of the neighbours called by the compound I was staying in. Her child had developed a fever, cough and fast breathing. The family I was staying with did not have any medicine stored that day. But one of the women in the nearby house gave them cotrimoxazole. I called by the next day to understand what had happened. The child’s mother narrated that all the local shops were closed. She had no money to hire a taxi to take the child to the hospital (approximately 8 km away on mostly dirt roads). The child’s father was away, and they couldn’t reach him because he could not afford a phone. She said her neighbour had given her 10 cotrimoxazole tablets. She was giving it to the child in the same dose they give you at the hospital—two in the morning, afternoon and evening. When I called by again, the child was better, and the mother decided not to take her to the clinic. She was relieved that the child had got better, because paying for transport had an impact on the household. They would not be able to afford food for two meals that day so they would have to go to bed on empty stomachs. When I probed around whether she would need to return the medicine, she explained that because it was cotrimoxazole it would be easy to replace. She said it was the one drug that the clinic always gives. In the household, once the patient is feeling better, she will be able to share the leftover dose. (AK fieldnotes, Chikwawa, Malawi, December 2019)
Metronidazole and the neglect of disease prevention
One consequence of the positioning of high-priority pathogens to be addressed through narrow pharmaceutical solutions is the systematic neglect of disease prevention measures, including water and sanitation infrastructure. In Namuwongo, Uganda (UU), such infrastructure was almost completely absent bar a few fee-for-use public latrines that were unaffordable to most and in any case were too dirty to use. Residents were forced to use buckets or plastic bags for toileting, disposed of in open drainage channels or community rubbish piles. Like many urban informal settlements globally, Namuwongo was portrayed in the media as an ‘illegal slum’ that was criminally inhabited. Thus, there was little more than sporadic political will to tackle these issues and, as a result, diarrhoea and the associated crippling stomach cramps were an almost daily occurrence for households. Similar struggles with persistent diarrhoea were faced by households in Harare. While many occupied legal structures in legitimate areas of the city, Harare has long been beset with dilapidated water infrastructure, which infamously caused a cholera outbreak in 2008–2009 that caused the deaths of thousands.39 With very little investment in water infrastructure since, Harare has experienced recurring and increasingly drug-resistant cholera and typhoid outbreaks to the present day.40
Metronidazole is an antibiotic and antiprotozoal drug commonly used in the treatment of anaerobic bacterial infections. In clinics in Harare, metronidazole was prescribed second only to amoxicillin. While Zimbabwe’s national treatment guideline recommends its use only for bloody diarrhoea, such was the anxiety of sending patients home without antibiotics given the cholera and typhoid situation that metronidazole was prescribed for almost all gastrointestinal symptoms. Metronidazole was widely recognised across our surveys, particularly in urban Uganda and Zimbabwe (UU and ZU1), but what surprised us was the extent of metronidazole use in Namuwongo, where three quarters of households regularly used the drug, in most cases the week preceding the survey. As box 3 illustrates, for the residents served by health providers like Maria and Martha, metronidazole was an essential antibiotic and one that could be purchased relatively cheaply and stored at home. Metronidazole made the symptoms, if not completely better, at least tolerable, and enabled the residents of Namuwongo to continue to get by. Metronidazole can, thus, be seen as a ‘quick fix’ for hygiene and productivity, enabling the everyday life to go on amid gaping structural inequalities.
Box 3. Metronidazole use in Kampala, Uganda.
I sat with Maria in her clinic for hours with no patients in sight. She told me she had not served any patients since she opened that morning. It had been raining all morning and according to Maria it was not surprising that she has not received any patients, ‘they do not come while it is raining.’ We talk about the flooding further down in the settlement during the rainy season. She tells me that when she first started living in Namuwongo close to 25 years ago, most of the area further downstream was a swamp with papyrus vegetation, explaining the flooding that happens when it rains. A few weeks back, I had spent some time with Martha, a long-term resident and community health worker who told me there had been a lot of diarrhoea in the area recently. She knew this because she was selling a lot of zinc and ORS that she buys at a subsidised price from an NGO that does health promotion and livelihoods improvement work within some communities in the country. I wondered if the increased diarrhoea was linked to infections from the aftermath of heavy rains and flooding that happens in the rainy season. Maria tells me she has plans to stock a lot of flagyl (metronidazole) as she expects many patients with diarrhoea now that the rains have started. (CN fieldnotes, Namuwongo, Uganda, December 2018)
Discussion
Recognition of significant increases in antibiotic use in LMICs2–6 and its implications for rising AMR has led to urgent calls to understand reasons for antibiotic (mis)use, especially beyond prescriber settings.8 9 This article presents a mixed-methods, multi-country analysis of the patterns of and reasons behind household antibiotic use practices in a range of rural and urban settings in Malawi, Uganda and Zimbabwe. Embedding the use of a ‘drug bag’ survey tool11 within an overarching ethnographic methodology, we highlighted antibiotics being frequently used—most notably, amoxicillin, cotrimoxazole and metronidazole—before explaining their use in terms of systemic processes within and between settings that often elude attempts at quantification. In this discussion, we draw out the significance of our findings in relation to existing quantitative and qualitative scholarship before using our wide-angle lens to propose a decentring of individual behaviour in LMIC antimicrobial stewardship frameworks.
Quantitative data produced using the drug bag survey tool add to a growing understanding of the local contours of antibiotic use within and between study settings. Our findings are broadly consistent with Do et al’s multi-country analysis of antibiotic use in Africa and Asia, which found that access to antibiotics in African locations was generally restricted, particularly in Southern Africa.17 As the authors similarly observed in South Africa and Mozambique,17 most antibiotic use in Zimbabwe and Malawi stemmed from public sector prescribing, with use of private pharmacies and informal providers often forced by clinic stockouts. In Uganda, antibiotics were obtained from a wider range of sources, reflecting the country’s large private sector (table 1), yet even in this setting most antibiotics were oral antibiotics from the WHO’s Access group rather than classes of medicines with higher resistance potential that are to be used Watchfully.19 Our finding that antibiotic use was heavily concentrated on a limited number of Access group medicines across all our surveys is well supported in the literature. Amoxicillin, cotrimoxazole and/or metronidazole have been found to be the most widely used antibiotics by several multi-country surveys in African settings, including that by Vialle-Valentin et al in The Gambia, Ghana, Kenya, Nigeria and Uganda,13 Padget et al in Senegal and Madagascar,41 and Fink et al in Malawi, Namibia, Senegal, Tanzania and Uganda.14 In short, our quantitative data form part of a broader emerging picture in Africa of generally restricted antibiotic use with a public sector bias, predominated by use of a small number of first-line antibiotics.
In addition to the quantitative data produced during surveys, our analysis extends to in-depth ethnographic data that enable us to tell the stories of the most widely used antibiotics.35 Our findings add to a small but growing body of work that locates antibiotic use as emergent of political and economic structures and systems, thereby decentring behaviour as the focal point of antibiotic use trends.42 The amoxicillin stories showed both the centrality of antibiotics to outpatient care and their persistent under-funding in LMICs not only by national governments but also the northern donors on whom public sector care in many LMICs has been rendered dependent. Thus, residents like Sisi Betty in Harare, Zimbabwe, were forced toward the informal sector in the frequent occurrence that this drug was out of stock. If amoxicillin is the story of under-funded public sector healthcare, cotrimoxazole is the story of that which has come to fill the gap: an antibiotic funded simply by virtue of its relationship to a ‘big’ disease like HIV.43 That public sector healthcare and household coping strategies alike in rural Malawi gravitate around this old antibiotic known to have limited efficacy44 can be read as a residual consequence of the more dramatic and widely documented ‘therapeutic citizenships’45 enacted by northern-funded donor programmes in Africa. Finally, the heavy reliance on metronidazole among residents of Namuwongo, Uganda, represents the story of what has been lost in the turn towards increasingly pharmaceuticalised forms of care: the older public health emphasis on disease prevention in the form of improved hygiene and sanitation infrastructure. While some aspects of these stories are particular to each setting, they also point toward deep-seated, transnational issues that many are aware of but that tend to get back-grounded in the practice of global health.46–48 With much at stake in the allocation of risk and responsibility for rising rates of AMR globally,25 49 making explicit connections between the use of antibiotics at household level and their entanglement within wider national and transnational systems has arguably never been more important.
Our data prompt questions about how antibiotic use data can inform AMR policy. Commentators have warned of a vicious cycle of surveillance and decision-making that results in the imposition of interventions to restrict and correct antibiotic use that may not be appropriate or a high priority in LMIC settings, including education and awareness campaigns, prescribing audits and formulary restrictions.50–52 These risks may be mitigated with more situated research and interpretation of data. There has been an increase in surveillance capacity in many LMICs to understand local use and resistance profiles, some of which is sensitive to socioeconomic and political factors driving high and rising antibiotic use and resistance.3 14 53 However, the epidemiological technologies and rationalities underpinning the majority of research and surveillance on which interventions are based—even those acknowledging wider structural factors—has tended to centre the behaviour of end users, aiming to factorialise and predict ‘misuse’ by different demographic groups. Our research demonstrates the need to decentre human behaviour and bring to the fore the systemic reasons for antibiotic use. This may be challenging, given the comparatively greater availability of expertise and templates for understanding and responding to antibiotic use through a knowledge–attitudes–practices lens.54 55 This challenge is compounded by a lack of resources and support in LMICs to develop and implement national action plans.51 This underscores the need for embedded social research expertise in AMR policy and programme implementation roles.
How might antibiotic stewardship decentre human behaviour? Following a growing number of commentators advocating for stewardship frameworks that extend beyond behaviour,51 54–56 we propose that it is systems, not individuals, that should be considered stewards of antibiotics. This is not a new or especially radical proposition; successful stewardship programmes in high-income countries rely on multiple resource and organisational components.57 However, these do not easily travel, and systems for supporting stewardship in LMICs will need to be constructed in response to local scenarios. As a first step, more sophisticated methods and metrics for knowing antibiotic use in context are needed. Our mixed-methods approach grounded in the drug bag survey tool and following antibiotics beyond the moment of use represents one among a growing number of research and surveillance protocols that produce both granular user-level data and connect use profiles to the deployment of antibiotics by the systems that are entrusted to deliver medicines and care.8 11 51 Such a wide-angle lens in turn expands possible pathways for interventions to include those that are ‘structural’ or ‘AMR sensitive’—for example, health system strengthening, infrastructural improvements and poverty alleviation strategies—which may be expensive in the short term but more cost-effective in the longer term.32 33 We further contend the importance of attending to transnational dynamics between global north and south as part of stewardship frameworks. Taking systems as stewards seriously means stepping outside the entrenched ‘grooves’ within which we collectively have come to operate and to design-out our reliance on antibiotics (and narrower techniques for evaluating their use) from the architecture of global healthcare.42 50 This is a tremendous challenge and will require bold interdisciplinary collaborations, including the emerging field of implementation science58 to develop stewardship strategies for particular LMICs. However, this is far more likely than narrow behavioural approaches to result in sustainable reductions in use both within and beyond formal prescriber settings.
The approach taken in this analysis had several limitations. First, our household surveys used purposive and semi-random sampling techniques which limit the generalisability of findings to wider populations. The quantitative findings, however, are consistent with other research in sub-Saharan Africa,13 14 17 and this approach enabled us to follow a more in-depth analysis of antibiotic stories. The drug bags were time and resource intensive to assemble and implement and, particularly as part of an overarching ethnographic methodology, may not be feasible to implement in the context of routine surveillance, although elsewhere we have considered ways in which the drug bag tool could be used to generate representative data with fewer resources.11 Finally, working with different protocols in each country meant that some data, for instance, regarding household demographics, were collected differently or not at all for each dataset, limiting some of the comparability across different studies. At the same time, however, the combined analysis did not happen retrospectively; conducted within the same anthropology of AMR research group, social scientists in the FIEBRE and AMIS teams met fortnightly to compare, contrast and collectively learn from experiences in different settings. This both strengthened the immersive, single-site ethnographic studies of each country team and became the cornerstone of our cross-country analysis here. It further generated opportunities for forging not only north–south but also south–south connection and collaboration.
Conclusion
This article presented a mixed-methods, multi-country analysis of household antibiotic use. Drawing on survey and ethnographic data from Zimbabwe, Malawi and Uganda, the stories presented of the three most widely used antibiotics—amoxicillin, cotrimoxazole and metronidazole—illustrate cross-cutting characteristics and omissions of the national and transnational systems that are entrusted to deliver medicines and care. Our findings challenge the predominant focus of current stewardship frameworks on restrictive and corrective interventions aimed at antibiotic end users. We suggest future strategies could consider systems—not individuals—as stewards of antibiotics, reducing the need to rely on these medicines to fix other issues of inequity, productivity and security.
Acknowledgments
We are grateful to all participants who took part in this study and to the Febrile Illness Evaluation in a Broad Range of Endemicities and Antimicrobials in Society teams. Special thanks go to Heidi Hopkins, David Mabey, Yuzana Khine Zaw, Katharina Kranzer, Ioana Olaru, Clever Mazhanga, Phillip Tinaapi, Nicholas Feasey, Mabvuto Chimenya, John Mankhomwa, Frank Mbalume, Fletcher Nangupeta, Freddy Kitutu, David Waiswa and Ceaser Scott Barole. We thank Simon P Kigozi for assistance with image design for figure 1. We finally thank two anonymous reviewers and the BMJ Global Health editorial team for their generous insights and suggestions.
Footnotes
Handling editor: Seye Abimbola
Twitter: @JustinD_902, @eleanormacp, @susannaiga, @salomemaku1, @ChristineNabir1, @MiriamKayendeke, @Alexand26085762, @kenny_sithole, @rashida_abbferr, @sham_l4l, @chrissyhroberts, @ldenyerwillis, @staedke, @AnthroAMR
Contributors: Data were collected by SM, JD, PM, KS, SN, CN, MK, EEM, AN and ES. Quantitative analysis was carried out by JD, EEM, CIRC, SN, CN, MK, CR, SL, JB, SGS, SY, RAF and EG. Qualitative data were analysed by SM, EEM, SN, CN, MK, JD, CdLH, LDW and CIRC. The manuscript was drafted by JD and CIRC and revised and approved by all other authors. JD and CIRC are the guarantors responsible for the overall content.
Funding: The surveys in Zimbabwe and Malawi were funded by the the Foreign, Commonwealth and Development Office, UK (Febrile Illness Evaluation in a Broad Range of Endemicities project number: PO7856). Surveys in Uganda were funded by the Antimicrobial Resistance Cross Council Initiative through the Economic and Social Research Council (Antimicrobials in Society project number: ES/P008100/1).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are available upon request from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The studies on which this article are based were approved by the London School of Hygiene and Tropical Medicine ethics board (reference numbers: 41616 (Febrile Illness Evaluation in a Broad Range of Endemicities (FIEBRE), Zimbabwe), 41617 (FIEBRE, Malawi) and 15244 (Antimicrobials in Society, Uganda)). Additionally, ethical approval was granted by the Biomedical Research and Training Institute, Zimbabwe (reference number: AP146/2018), the Medical Research Council of Zimbabwe (reference number: MRCZ/A/2288), the College of Medicine Research Ethics Committee, Malawi (reference number: P06/182429) and the School of Biomedical Sciences Research and Ethics Committee, Makerere University College of Health Sciences, Uganda (reference number: 562). All research participants provided written informed consent to take part in the research; all participant names that appear in this article are pseudonyms.
References
- 1.World Heath Organization . Global action plan on antimicrobial resistance. Geneva, Switzerland, 2015. Available: https://www.who.int/antimicrobial-resistance/global-action-plan/en/ [Accessed 19 Jul 2016].
- 2.Shankar PR. Medicines use in primary care in developing and transitional countries: fact book summarizing results from studies reported between 1990 and 2006. Bull World Health Organ 2009;87:804. 10.2471/BLT.09.070417 [DOI] [Google Scholar]
- 3.Allwell-Brown G, Hussain-Alkhateeb L, Sewe MO, et al. Determinants of trends in reported antibiotic use among sick children under five years of age across low-income and middle-income countries in 2005-17: a systematic analysis of user characteristics based on 132 national surveys from 73 countries. Int J Infect Dis 2021;108:473–82. 10.1016/j.ijid.2021.05.058 [DOI] [PubMed] [Google Scholar]
- 4.Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A 2018;115:E3463–70. 10.1073/pnas.1717295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014;14:742–50. 10.1016/S1473-3099(14)70780-7 [DOI] [PubMed] [Google Scholar]
- 6.Hsia Y, Sharland M, Jackson C, et al. Consumption of oral antibiotic formulations for young children according to the WHO access, watch, reserve (AWaRe) antibiotic groups: an analysis of sales data from 70 middle-income and high-income countries. Lancet Infect Dis 2019;19:1–9. 10.1016/S1473-3099(18)30547-4 [DOI] [PubMed] [Google Scholar]
- 7.Frost I, Kapoor G, Craig J, et al. Status, challenges and gaps in antimicrobial resistance surveillance around the world. J Glob Antimicrob Resist 2021;25:222–6. 10.1016/j.jgar.2021.03.016 [DOI] [PubMed] [Google Scholar]
- 8.Queenan K, Chandler C, Goodman C. Meeting report: metrics and methods for assessing antibiotic use at the granular level in humans and livestock in LMICs, 2017. Available: https://researchonline.lshtm.ac.uk/id/eprint/4650709/ [Accessed 3 Apr 2019].
- 9.Padget M, Guillemot D, Delarocque-Astagneau E. Measuring antibiotic consumption in low-income countries: a systematic review and integrative approach. Int J Antimicrob Agents 2016;48:27–32. 10.1016/j.ijantimicag.2016.04.024 [DOI] [PubMed] [Google Scholar]
- 10.Haenssgen MJ, Charoenboon N, Khine Zaw Y. It is time to give social research a voice to tackle antimicrobial resistance? J Antimicrob Chemother 2018;73:1112–3. 10.1093/jac/dkx533 [DOI] [PubMed] [Google Scholar]
- 11.Dixon J, MacPherson E, Manyau S, et al. The ‘drug bag’ method: lessons from anthropological studies of antibiotic use in Africa and South-East Asia. Glob Health Action 2019;12:1639388. 10.1080/16549716.2019.1639388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allwell-Brown G, Hussain-Alkhateeb L, Kitutu FE, et al. Trends in reported antibiotic use among children under 5 years of age with fever, diarrhoea, or cough with fast or difficult breathing across low-income and middle-income countries in 2005–17: a systematic analysis of 132 national surveys from 73 countries. Lancet Glob Health 2020;8:e799–807. 10.1016/S2214-109X(20)30079-6 [DOI] [PubMed] [Google Scholar]
- 13.Vialle-Valentin CE, Lecates RF, Zhang F, et al. Predictors of antibiotic use in African communities: evidence from medicines household surveys in five countries. Trop Med Int Health 2012;17:211–22. 10.1111/j.1365-3156.2011.02895.x [DOI] [PubMed] [Google Scholar]
- 14.Fink G, D'Acremont V, Leslie HH, et al. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: a cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect Dis 2020;20:1–9. 10.1016/S1473-3099(19)30572-9 [DOI] [PubMed] [Google Scholar]
- 15.Auta A, Hadi MA, Oga E, et al. Global access to antibiotics without prescription in community pharmacies: a systematic review and meta-analysis. J Infect 2019;78:8–18. 10.1016/j.jinf.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 16.Morgan DJ, Okeke IN, Laxminarayan R, et al. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis 2011;11:692–701. 10.1016/S1473-3099(11)70054-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Do NTT, Vu HTL, Nguyen CTK, et al. Community-based antibiotic access and use in six low-income and middle-income countries: a mixed-method approach. Lancet Glob Health 2021;9:1–10. 10.1016/S2214-109X(21)00024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wertheim HFL, Chuc NTK, Punpuing S, et al. Community-level antibiotic access and use (ABACUS) in low- and middle-income countries: Finding targets for social interventions to improve appropriate antimicrobial use - an observational multi-centre study. Wellcome Open Res 2017;2:58. 10.12688/wellcomeopenres.11985.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Heath Organization . WHO list of essential medicines (EML). 20th edn, 2017. http://www.who.int/medicines/publications/essentialmedicines/en/ [Google Scholar]
- 20.Horumpende PG, Said SH, Mazuguni FS, et al. Prevalence, determinants and knowledge of antibacterial self-medication: a cross sectional study in North-eastern Tanzania. PLoS One 2018;13:1–13. 10.1371/journal.pone.0206623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erku DA, Mekuria AB, Belachew SA. Inappropriate use of antibiotics among communities of Gondar town, Ethiopia: a threat to the development of antimicrobial resistance. Antimicrob Resist Infect Control 2017;6:1–7. 10.1186/s13756-017-0272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkaff RN, Kamigaki T, Saito M. Use of antibiotics for common illnesses among children aged under 5 years in a rural community in Indonesia: a cross-sectional study. Trop Med Health 2019;47:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Togoobaatar G, Ikeda N, Ali M, et al. A survey of non-prescribed use of antibiotics for children in an urban community in Mongolia. Bull World Health Organ 2010;88:930–6. 10.2471/BLT.10.079004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ESRC Working Group . Anti-Microbial resistance: setting the social science agenda, 2014. Available: https://esrc.ukri.org/files/funding/funding-opportunities/amr/anti-microbial-resistance-setting-the-social-science-agenda/ [Accessed 10 Jun 21].
- 25.Chandler CIR, Hutchinson E, Hutchison C. Addressing Antimicrobial Resistance Through Social Theory: An Anthropologically Oriented Report. London School of Hygiene & Tropical Medicine, 2016. Available: http://www.lshtm.ac.uk/php/ghd/research/app/anthropologyofantimicrobialresistance.html [Accessed 12 Jul 2018].
- 26.Denyer Willis L, Chandler C. Quick fix for care, productivity, hygiene and inequality: reframing the entrenched problem of antibiotic overuse. BMJ Glob Health 2019;4:e001590. 10.1136/bmjgh-2019-001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigues CF. Self-medication with antibiotics in Maputo, Mozambique: practices, rationales and relationships. Palgrave Commun 2020;6:1–12. [Google Scholar]
- 28.MacPherson E, Reynolds J, Sanudi E. Understanding antimicrobial use in Subsistence farmers in Chikwawa district Malawi, implications for public awareness campaigns. SocArXiv 2021. 10.31235/osf.io/e7b6n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haenssgen MJ, Charoenboon N, Zanello G, et al. Antibiotics and activity spaces: protocol of an exploratory study of behaviour, marginalisation and knowledge diffusion. BMJ Glob Health 2018;3:e000621. 10.1136/bmjgh-2017-000621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon J, Chandler C. Opening up ‘fever’, closing down medicines: algorithms as blueprints for global health in an era of antimicrobial resistance. Med Anthropol Theory 2019;6:53–79. [Google Scholar]
- 31.Biehl JG. Pharmaceuticalization: AIDS treatment and global health politics. Anthropol Q 2007;80:1083–126. [Google Scholar]
- 32.Interagency Core Group on Antimicrobial Resistance . Reduce unintentional exposure and the need for antimicrobials, and optimize their use, 2018. Available: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_Optimize_use_of_antimicrobials_120718.pdf [Accessed 23 Feb 2020].
- 33.World Bank Group . Pulling together to beat superbugs: knowledge and implementation gaps in addressing antimicrobial resistance. Washington, DC, 2019. Available: https://documents1.worldbank.org/curated/en/430051570735014540/pdf/Pulling-Together-to-Beat-Superbugs-Knowledge-and-Implementation-Gaps-in-Addressing-Antimicrobial-Resistance.pdf [Accessed 5 Nov 2020].
- 34.Palanco P, Chandler CIR. Histories of antibiotics: a one health account of the arrival of antimicrobial drugs to Zimbabwe, Malawi and Uganda. Report for the improving human health Flagship initiative, agriculture for nutrition and health research programme, CGIAR, 2020. Available: https://researchonline.lshtm.ac.uk/id/eprint/4658867/ [Accessed 23 Aug 2021].
- 35.Whyte SR, Van der Geest S, Hardon A. Social lives of medicines. Cambridge: Cambridge University Press, 2002. [Google Scholar]
- 36.R Core Team . R: A language and environment for statistical computing [online]. Vienna: R foundation for statistical computing, 2020. Available: https://www.r-project.org/
- 37.Zimbabwe Antimicrobial Resistance Core Group . Situation analysis on antimicrobial resistance in humans and animals in Zimbabwe, 2017. Available: https://cddep.org/wp-content/uploads/2017/10/SITUATION-ANALYSIS-OF-ANTIMICROBIAL-USE-AND-RESISTANCE-IN-HUMANS-AND-ANIMALS-IN-ZIMBABWE-1.pdf [Accessed 17 May 2018].
- 38.World Health Organization . Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and children: recommendations for a public health approach. Geneva, Switzerland, 2014. https://apps.who.int/iris/handle/10665/145719 [PubMed] [Google Scholar]
- 39.Chigudu S. The political life of an epidemic: cholera, crisis and citizenship in Zimbabwe. Cambridge: Cambridge University Press, 2020. [Google Scholar]
- 40.Olaru ID, Mtapuri-Zinyowera S, Feasey N, et al. Typhoid Vi-conjugate vaccine for outbreak control in Zimbabwe. Lancet Infect Dis 2019;19:930. 10.1016/S1473-3099(19)30425-6 [DOI] [PubMed] [Google Scholar]
- 41.Padget M, Tamarelle J, Herindrainy P, et al. A community survey of antibiotic consumption among children in Madagascar and Senegal: the importance of healthcare access and care quality. J Antimicrob Chemother 2017;72:564–73. 10.1093/jac/dkw446 [DOI] [PubMed] [Google Scholar]
- 42.Tompson AC, Manderson L, Chandler CIR. Understanding antibiotic use: practices, structures and networks. JAC Antimicrob Resist 2021;3:dlab150. 10.1093/jacamr/dlab150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGoey L, Reiss J, Wahlberg A. Editors’ introduction: the global health complex. Biosocieties 2011;6:106–18. [Google Scholar]
- 44.World Health Organization . Who report on surveillance of antibiotic consumption: 2016-2018 early implementation. Geneva, Switzerland, 2018. https://www.who.int/medicines/areas/rational_use/oms-amr-amc-report-2016-2018/en/ [Google Scholar]
- 45.Nguyen V-K. The republic of therapy : triage and sovereignty in West Africa’s time of AIDS. Durham: Duke University Press, 2010. [Google Scholar]
- 46.Biehl JG, Petryna A, eds. When People Come First: Critical Studies in Global Health. New Jersey: Princeton University Press, 2013. [Google Scholar]
- 47.Prince RJ, Marsland R, eds. Making and Unmaking Public Health in Africa: Ethnographic and Historical Perspectives. Ohio: Ohio University Press, 2014. [Google Scholar]
- 48.Packard RM. A history of global health: interventions into the lives of other peoples. Baltimore: Johns Hopkins University Press, 2016. [Google Scholar]
- 49.Chandler CIR. Current accounts of antimicrobial resistance: stabilisation, individualisation and antibiotics as infrastructure. Palgrave Commun 2019;5:1–13. 10.1057/s41599-019-0263-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dixon J, Manyau S, Kandiye F, et al. Antibiotics, rational drug use and the architecture of global health in Zimbabwe. Soc Sci Med 2021;272:113594. 10.1016/j.socscimed.2020.113594 [DOI] [PubMed] [Google Scholar]
- 51.Kirchhelle C, Atkinson P, Broom A, et al. Setting the standard: multidisciplinary hallmarks for structural, equitable and tracked antibiotic policy. BMJ Glob Health 2020;5:e003091. 10.1136/bmjgh-2020-003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broom A, Kenny K, Kirby E. Improvisation, therapeutic brokerage and antibiotic (mis)use in India: a qualitative interview study of Hyderabadi physicians and pharmacists. Crit Public Health 2020;30:16–27. [Google Scholar]
- 53.Collignon P, Beggs JJ, Walsh TR, et al. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health 2018;2:e398–405. 10.1016/S2542-5196(18)30186-4 [DOI] [PubMed] [Google Scholar]
- 54.Will CM. The problem and the productivity of ignorance: public health campaigns on antibiotic stewardship. Sociol Rev 2020;68:55–76. 10.1177/0038026119887330 [DOI] [Google Scholar]
- 55.Broom J, Broom A, Kirby E. The drivers of antimicrobial use across institutions, stakeholders and economic settings: a paradigm shift is required for effective optimization. J Antimicrob Chemother 2019;74:2803–9. 10.1093/jac/dkz233 [DOI] [PubMed] [Google Scholar]
- 56.Kakkar AK, Shafiq N, Singh G, et al. Antimicrobial stewardship programs in resource constrained environments: understanding and addressing the need of the systems. Front Public Health 2020;8:1–10. 10.3389/fpubh.2020.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dyar OJ, Huttner B, Schouten J, et al. What is antimicrobial stewardship? Clin Microbiol Infect 2017;23:793–8. 10.1016/j.cmi.2017.08.026 [DOI] [PubMed] [Google Scholar]
- 58.Rogers Van Katwyk S, Hoffman SJ, Mendelson M, et al. Strengthening the science of addressing antimicrobial resistance: a framework for planning, conducting and disseminating antimicrobial resistance intervention research. Health Res Policy Syst 2020;18:1–13. 10.1186/s12961-020-00549-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malawi Population and Housing National Statistical Office . Malawi population and housing census report, 2019. [Google Scholar]
- 60.World Bank . Malawi. world development indicators, 2021. Available: https://data.worldbank.org/country/malawi [Accessed 15 Sep 2021].
- 61.Messac L. Birthing a nation: political legitimacy and health policy in hastings kamuzu Banda’s Malawi, 1962–1980. J South Afr Stud 2020;46:209–28. 10.1080/03057070.2020.1689008 [DOI] [Google Scholar]
- 62.Abiiro GA, Mbera GB, De Allegri M. Gaps in universal health coverage in Malawi: a qualitative study in rural communities. BMC Health Serv Res 2014;14:1–10. 10.1186/1472-6963-14-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalipeni E. Structural adjustment and the health-care crisis in Malawi. Proteus-Shippensburg 2004;21:23–30. [Google Scholar]
- 64.Mwale B. HIV/AIDS in Malawi. Malawi Med J 2002;14:2–3. [PMC free article] [PubMed] [Google Scholar]
- 65.Page S. The development aid situation in Malawi. In: Development, sexual cultural practices and HIV/AIDS in Africa. London: Palgrave Macmillan, 2019: 43–60. [Google Scholar]
- 66.Ewing VL, Tolhurst R, Kapinda A, et al. Understanding interpretations of and responses to childhood fever in the Chikhwawa district of Malawi. PLoS One 2015;10:1–18. 10.1371/journal.pone.0125439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Makwero MT. Delivery of primary health care in Malawi. African J Prim Heal Care Fam Med 2018;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.World Bank . Zimbabwe. World development indicators, 2021. Available: https://data.worldbank.org/country/zimbabwe?view=chart [Accessed 10 Sep 2021].
- 69.Woelk GB. Primary health care in Zimbabwe: can it survive? Soc Sci Med 1994;39:1027–35. 10.1016/0277-9536(94)90374-3 [DOI] [PubMed] [Google Scholar]
- 70.Green A. Zimbabwe post-Mugabe era: reconstructing a health system. Lancet 2018;391:17–18. 10.1016/S0140-6736(18)30007-2 [DOI] [PubMed] [Google Scholar]
- 71.World Bank . Uganda. world development indicators, 2021. Available: https://data.worldbank.org/country/uganda [Accessed 14 Sep 2021].
- 72.Tashobya CK, Ssengooba F, Cruz VO. Health systems reforms in Uganda: processes and outputs. Health Systems Development Programme 2006. [Google Scholar]
- 73.Batwala V, Magnussen P, Nuwaha F. Challenges to implementation of artemisinin combination therapy policy in Uganda. Int Health 2010;2:262–8. 10.1016/j.inhe.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 74.Mbonye AK, Buregyeya E, Rutebemberwa E, et al. Prescription for antibiotics at drug shops and strategies to improve quality of care and patient safety: a cross-sectional survey in the private sector in Uganda. BMJ Open 2016;6:1–6. 10.1136/bmjopen-2015-010632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.London School of Economics and Political Science Blog. Namuwongo: Key to Kampala’s present and future development, 2015. Available: https://blogs.lse.ac.uk/africaatlse/2015/05/28/namuwongo-key-to-kampalas-present-and-future-development/ [Accessed 15 Sep 2021].
- 76.Tororo District Local Government . Tororo district development plan 2015-2020, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2021-006920supp001.pdf (85.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are available upon request from the corresponding author.