Abstract
The current international crisis situation caused by the COVID-19 pandemic is having a strong psychological impact on our subjectivities. We are constantly threatened by the danger of i) being infected, ii) infecting other people, and (iii) by the loss of social relation. Departing from these premises, we here aim to investigate the psychological and neurodynamics of this complex phenomenon. First, we discuss about recent psychological and neuronal findings on fear and its disorders, related to an unbalanced intero-exteroceptive processing and emotional regulation. Secondly we move to the psychological and neuronal dynamics of self and others characterized by a temporo-spatial alignment with the world. Due to the neural overlap of emotion and self and the deep-reaching neuro-ecological layers of self, emotional feelings like fear and anxiety cannot be detached and dissociated from the world; they signify the world–brain relation, and, more specifically, our self-other relation. The deepest neuro-ecological and neuro-social layers of self are threatened by the loss of subjectivity, which is manifest in our loss of body and thus the fear of dying, and the loss of intersubjectivity that surfaces in our fear of infecting others, which reflect the intimate anchorage of the self with the world. In our opinion the pandemic of COVID-19 deeply affect our sense of self and its spatio-temporal neuronal dynamics providing the prerequisites for the manifestation of fear and existential anxiety, thus disrupting the brain-world relation with significant repercussions on our psyche and on our daily lives.
Keywords: self, others, world, interoception, neuroecological, spatio-temporal psychopathology, existential fear, COVID-19
The self is like a crowd... being oneself, one is also like many. One cannot individuate without being with other human beings... Being an individual is always a link in a chain... how little you can exist... without responsibilities and duties and the relation of other people to yourself... The Self... plants us in otherness – of other people, and of the transcendent. Jung, 1988, v. 1, p. 102
1. Introduction
What is happening nowadays with the pandemic spread of COVID-19 is an existential threat for us as individual selves related with others, both belonging to a shared world.
Our sense of self and others is threatened by the danger of i) being infected, ii) infecting other people, and (iii) the loss of social relation. This abnormal situation has an impact on us as subjectivities being intrinsically related with others and the world, leading to different neuronal and psychological responses on the basis of our basic emotional feelings, as is the case of fear. Our self is existentially threatened at its most deepest level, that is, its relationship to others and, more generally, the world – this kind of existential fear of losing ourselves through losing our relation to the world corresponds well to the kind of deep anxieties existential philosophers such as Kierkegaard, Heidegger, and Sartre, among others, described. In order to shed some light on such existential fear in the midst of the current Coronavirus crisis, we first need to understand the self and its different layers.
Specifically, to better deepen the psychological and neurodynamics concerning the sense of self and its relationship to others, we proceed in two steps. We first discuss recent psychological and neuronal findings on fear and related disorders. This serves as stepping stone to show the relevance of reaching deeper beyond the emotion of fear itself to the underlying self who experiences that very same emotion, i.e., fear. The nature of self and its different layers will be discussed in a second part. We suppose that it is the intimate linkage of the emotion of fear to the self and the latter’s deeper neuro-social and neuro-ecological layers that first and foremost renders the emotion of fear as threat to our existence, i.e., existential fear. Only by considering the self, including its deep neuro-ecological anchorage in the world, we can fully comprehend the far-reaching nature of our emotions like fear in a situation where events in the world threaten our existence as in the corona virus crisis.
2. Fear – neurobiological and psychological characterization
2.1 The FEAR/Anxiety System – fear is relational and neuro-ecological
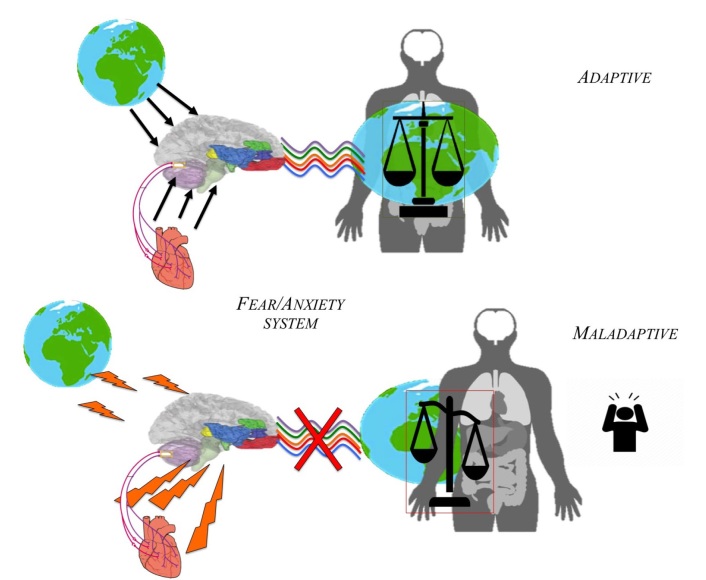
The FEAR/Anxiety System (Panksepp, 1990, pp. 3–58; Panksepp, Fuchs, & Iacobucci, 2011; Davis, Walker, Miles, & Grillon, 2009) was designed during brain evolution to help animals escape and avoid the many dangers of the world. In humans higher levels of fear destroys the sense of security in the world producing higher level of stress and anxiety. In these extreme cases the world is not perceived as a “secure base”. The FEAR/Anxiety System can be activated by various external events as well as by internal ones. These are usually triggered by specific external events that have been paired with pain or other threatening stimuli, but feelings of fear can also emerge simply from the internal dynamics of the brain, what has been called free-floating anxieties. Indeed, when humans are stimulated intensely, primary-process FEAR circuits motivate to freeze and flee in apparent frights. In these conditions people report being engulfed by intense anxiety and fear (e.g. “scared to death”) with no necessary environmental cause (Nashold, Wilson, & Slaughter, 1969).
Neuroscientists have tended to focus on information that enters the FEAR system via so called ‘highroads’ (more cognitive-perceptual inputs), and via ‘low-roads’ (the more primitive sensory inputs). Jaak Panksepp focused mainly on what he described as the ‘Royal Road’, which is the evolved FEAR system itself, a circuit that course between central amygdala and periaqueductal gray of midbrain, that governs the instinctual action apparatus, which intrinsically helps animals avoid danger (Panksepp, 2004). In external or internal threaten circumstances, the arousal of anxieties systems lead to mental tensions that characterize various anxiety disorders (Panksepp, 1990). In order to better understand the emotional feeling of fear, we must investigate the neurodynamics of the brain and its spatio-temporal properties that might lead to different disorders.
We can see that even in animals, there is a most basic subcortical fear-anxiety system. That very same system receives plenty of input from especially the own body – we are thus intimately connected with our body and that relationship can transform into fear and anxiety if discrepancies in the inputs, i.e, between intero- and extero-ceptive stimuli, occur. Fear and anxiety can then be seen as strategies of the brain to re-balance and ultimately ‘normalize’ its intero- and extero-ceptive input – the brain establishes relationship to the body, which is manifest in fear/anxiety. More deeply, it tells us that even an emotion as basic as fear is not isolated and locked-up in the brain or the organism. Instead, the data tell us that fear is intrinsically relational, something constituted neuro-vegetatively as based on the interoceptive input from the body. That, in turn, establishes relationship to the environment and the world – taken in a more general sense, emotions like fear/anxiety are thus about the balance between world, body, and brain and are thus genuinely relational and neuro-ecological.
2.2. Mechanisms of fear – involvement of the body with interoception and prediction
Anxiety may be defined as a state of worry due to anticipation of uncertain or undesirable outcomes (Grupe & Nitschke, 2013). Anxiety can be useful and adaptive in imagining and avoiding negative events. However, extreme responses, such persistent worry due to perceiving innocuous stimuli or events perceived as threatening, might result in a psychopathological disorder. Recently, it has been proposed that abnormal interoceptive processing might represent a basis for anxiety (Barrett & Simmons, 2015; Paulus & Stein, 2010). In this view, behavioural and autonomic symptoms are attributed to top-down modulation from brain regions that monitor interoceptive signals of the body.
Predictive coding theories suggest that the brain prospectively models the anticipated or expected bodily input (Seth, Suzuki, & Critchley, 2012; Seth, 2013). When this expected input diverges from the received input, error signals are generated to ‘correct’ the received input (i.e. interoceptive afferent), i.e., prediction error. For example, worrying about a threatening condition is expected to be associated with increased heart rate. While in the absence of expected increase in the heart rate, top down signals increase the heart rate to match the prediction (Barrett & Simmons, 2015). In the brain, this predictive interoception is attributed particularly to the anterior insula. Anxiety featured by persistent uncertainty and associated worry is suggested to produce strong expectations of arousal (hyper-precise errors), which are not responsive or changed in the face of contrary information (such as absence of increased heart rate) (Paulus, Feinstein, & Khalsa, 2019). As such, if either a strong expectation of abnormality or an imprecise input produces an error, the somatic signal may be amplified to match the belief – this results in anxiety and fear.
How exactly does the brain integrate information into its own neuronal activity that it receives from the body’s interoceptive inputs? The above-mentioned theories can be complemented by the recently suggested spatiotemporal approach, which lays emphasis upon the temporal structure of neural activity and its spatial patterns in the brain (Northoff, 2016a). The temporal structure of ongoing neural activity would thus represent the a priori predictions that remain largely robust to external inputs. In this approach, interoceptive inputs represent a special case. These inputs continuously provide critical information of survival value. Consequently, and unlike external inputs, interoceptive inputs must also be continuously integrated with the ongoing (resting state) spontaneous activity (Northoff, 2014). Thus, a noisy interoceptive input or afferent may affect the ongoing neural activity, and regions that receive this input may function abnormally. That, in turn, leads to the somato-cardiac symptoms and abnormal interoception in anxiety disorders that may thus be intimately related to the brain’s abnormal interoceptive processing.
2.3. Disorders of fear and anxiety I – prefrontal cortex with convergence of emotion and self
Anxiety disorders are the most common psychiatric condition, with a lifetime prevalence between 5%-30% (Kessler et al., 2005) and are mainly divided into generalized anxiety disorder (GAD), panic disorder (PD), specific phobia (SP), and social phobia (or social anxiety disorder, SAD); high rates of comorbidity across these diagnoses suggest common vulnerabilities (Hamm et al., 2014). This class of disorders is characterized by significant feelings of anxiety and fear (American Psychiatric Association, 2013).
The latter are related, but different phenomena that can be distinguished on the neurophysiological level (e.g. Davis, 1998; Grillon et al., 2006). At the same time, there is evidence suggesting that both fear and anxiety are related to all anxiety disorders to varying degrees (Barlow, 2001; Etkin & Wager, 2007).
As said in the previous sections, a constant state of worry, i.e., being afraid of something bad, which is about to happen, and a hyper vigilance for external and internal inputs are the hallmark of anxiety disorders (Bishop, 2007; Bijsterbosch, Smith, Forster, John, & Bishop, 2014). Hypersensitivity to emotional stimuli has been broadly shown in individuals with GAD (Weinberg & Hajcak, 2010), SAD (Voegler et al., 2018), and PD (Ludewig, 2003). Attentional biases, subserving such hypervigilant reactions, engage our attention into detecting threat-related stimuli from the environment and within our own body feeding worry symptoms (Clark & Watson, 1991). This leads to a hyperactivity of the amygdala, taking control over the brain and affecting the prefrontal cortices activity (Schienle, Schäfer, Walter, Stark, & Vaitl, 2005; Rauch, Shin, & Wright, 2006; Hermann et al., 2007). Indeed in healthy individuals, the activity of amygdala, in response to threat-related stimuli, is mitigated from prefrontal cortices, which are involved in cognitive regulation of emotions. In this stage of cognitive processing, the organism chooses coping strategies, and then finally applies emotion regulation strategies to cope with the fearful situation. This emotion regulation capacity is impaired in anxiety disorders, reflecting an imbalance of amygdala-prefrontal cortices connectivity (Mennin, 2006; Amstadter, 2008; Aldao, Nolen-Hoeksema, & Schweizer, 2010; Cisler, Olatunji, Feldner, & Forsyth, 2009). Hence, the relationship between prefrontal cortices hypo-activation and functional impairment suggests that the failure to engage prefrontal cortices during emotion regulation may be part of the critical transition from dispositional high anxiety to an acute manifestation of anxiety disorder (e.g., Ball, Ramsawh, Campbell-Sill, Paulus, & Stein, 2013; Straube, Mentzel, & Miltner, 2005; Mucci, Scalabrini, & Northoff, 2018).
On the neural level, neuroimaging studies have provided evidence of alterations in functional connectivity (FC) within and/or between several brain networks in anxiety and anxiety disorders in response to fear-related stimuli (see Xu et al., 2019 for a review). In particular, the affective network (AN), which includes amygdala, orbitofrontal cortex (OFC), temporal cortex, pallidum, and insular cortex, results to have hypo-connectivity with default mode network (DMN) and cortical midline structures (CMS-Northoff & Bermpohl, 2004). The involvement of the DMN provides connection of emotion/anxiety to the self as the DMN is well known to be responsible for processing self-related content (Northoff et al., 2006; Qin & Northoff, 2011). The hypo-activation in anxiety disorder occurs especially with the vmPFC, which has been shown to be critical to emotion regulation via modulating amygdala activity (Motzkin, Philippi, Wolf, Baskaya, & Koenigs, 2015). How that very same abnormal emotion regulation of vmPFC affects and modulates the self that is also processed in the same region remains to be investigated.
The vmPFC also plays an important role in value-based decision-making and social interaction, underpinning the avoidance responses, subserving the self-defense mechanism from physical and psychological threats typical of anxiety disorders (Hiser & Koenigs, 2018). Anxious individuals tend to generate emotionally charged and overgeneralized threat-related future events, consistent with previous findings in anxious younger adults and asymptomatic older adults (Brown et al., 2013, Brown, Addis et al., 2014; Kleim, Graham, Fihosy, Stott, & Ehlers, 2014; Schacter, Gaesser, & Addis, 2013). They report impairments in imagining themself in the future (Schacter et al., 2013), contributing to poor decision-making and problem-solving capacities (Gupta et al., 2009; Lyons, Henry, Rendell, Corballis, & Suddendorf, 2014). Together, these data support close relationship between the neural correlates of anxiety/fear on the one hand and those implicated in self-related processing and our sense of self on the other.
2.4. Disorders of fear and anxiety II – abnormal intero-exteroceptive balance as world-body-brain relation
In addition, many studies reported an abnormal interoception across anxiety disorders (Andor, Gerlach, & Rist, 2008; Chan et al., 2015; Chan, von Leupoldt, Liu, & Hsu, 2014; Ehlers & Breuer, 1992; Grossi et al., 2017; Hoehn-Saric, McLeod, & Zimmerli, 1989), thus suggesting how altered interoception might play a key role in the pathogenesis of these disorders.
An increased anterior insular activity was indeed associated with interoceptive awareness in phobic patients (Caseras et al., 2011). Furthermore, Cui and colleagues (2016) reported an increased functional connectivity (FC) from thalamus to somatosensory cortex in panic disorder (PD) patients causing abnormal high interoceptive sensitivity and somatosensory stimulus processing, which underlies the typical symptoms of PD, such as the extreme feeling of heartbeat. Finally, in generalized anxiety disorder (GAD) abnormal interoceptive awareness was correlated with altered neural activity (resting and task-related) in the left anterior insular cortex (AI) (Cui et al., 2020).
Why is there such abnormally heightened interoceptive awareness in anxiety and fear disorders? Usually, regions like the insula process both intero-and exteroceptive stimuli and integrate them (Craig, 2009; Wiebking et al., 2014; Wiebking & Northoff, 2014). There is thus a certain intero-exteroceptive processing balance in the neural activity of the insula. If now, for some reason, the exteroceptive input recedes or is traumatic for the respective subject, the intero-exteroceptive balance is shifted towards its interoceptive pole. This shifts the awareness. While the awareness is usually divided and balanced between intero- and exteroceptive contents, it is now abnormally strongly dominated by the predominating interoceptive input. Neural activity is thus strongly shaped by the relatively stronger interoceptive input which obviously enhances the subject’s perception of the interoceptive inputs resulting in increased interoceptive awareness. In contrast, exteroceptive awareness is decreased and recedes into the background.
Figure 1.
Adaptive vs. maladaptive conceptual model of Fear/anxiety system
If one now perceives and is more aware of the one’s own interoceptive processing, the individual certainly becomes anxious when, for instance, continuously perceives the one’s own heartbeat and especially its otherwise ‘normal’ irregularities as related to the “healthy” heart rate variability (Wiebking et al. 2014; deGreck et al., 2011). The anxious and fearful reaction to such abnormally heightened interoceptive awareness of one’s heartbeat’s irregularities is then nothing but a very healthy and normal reaction – except that the psychiatrists would define it as “anxiety disorder”.
Thus, having reported how pathological anxiety consumes mental resources impairing daily functioning and our quality of life (Mathews & MacLeod, 2005; Rodriguez, Bruce, Pagano, & Keller, 2005), it is possible to understand how the current international pandemic crisis situation due to COVID-19 has a strong impact on our fear/anxiety levels. Just as the virus’ global spread accelerated, the perception and the attention towards our inner state have increased sharply. So while externally, media and social networks are gathering attention over the threatened worldwide situation, internally, we experience increased interoceptive awareness, in an attempt to early detect the symptoms of the virus. The prospect of being infected or of being able to infect our loved significant others makes us experience the constant state of worry lived daily by those suffering from an anxiety disorder. The more these possibilities become concrete, i.e., by becoming aware of virus-positive friends and acquaintances, the more our own self-integrity and its relationship with the world are threatened which, in turn, induces anxiety/ fear. We will now see how that anxiety/fear becomes literally existential by shedding some light on the deep relational and neuro-ecological layers of self (as based on what conceptually has been described as ‘world-brain relation’; Northoff, 2018).
3. The Self and its fears
3.1. The Self, the other and the spontaneous activity of the brain – temporo-spatial alignment to the world
The sense of self is the term we use to profoundly investigate what concerns our subjectivity. It is my self who feels pain when I am hurt; others can see the manifestation of my self, my behaviour, feelings and cognition, they can mirror/empathize with my self to get closer and aligned to what is my self experiences but they do not feel my same subjective pain. However self and other are intrinsically connected to each other, being mutually dependent in a continuous dynamic dialectical exchange of similarities and differences.
From a neuronal perspective, self-processing has been operationalized in many experimental studies in terms of self-relatedness (SR, Northoff, 2016a) and has been shown to modulate behavioral responses related to reward (de Greck et al., 2008, 2010, 2012; Yankouskaya, Bührle, Lugt, Stolte, & Sui, 2018), attention (Sui, Chechlacz, & Humphreys, 2012; Sui, Rotshtein, & Humphreys, 2013; Sui, Sun, Peng, & Humphreys, 2014; Sui & Humphreys, 2015), perception (Sui et al., 2012, 2013), action (Frings & Wentura, 2014), emotion (Phan et al., 2004; Northoff et al., 2007; Yankouskaya et al., 2020), and decision making (e.g. Nakao, Ohira, & Northoff, 2012; Nakao et al., 2013, 2019). Most interestingly, in addition to task evoked paradigms, the investigation of the brain’s spontaneous activity (or resting state) and its elaborate spatio-temporal structure have been gained more and more attention during the years (Logothethis et al., 2009; Northoff 2014a,b; Raichle 2015a,b). The brain’s spontaneous activity can spatially be characterized by various networks, like the Default Mode Network (DMN) that include mainly the cortical midline structures (CMS) and show strong low frequency fluctuations (Raichle 2001, 2009; Buckner, Andrews-Hanna, & Schacter, 2008), and other networks such as sensorimotor, salience, central executive networks, etc. (see Menon, 2011 for a review). Intriguingly, CMS have been associated with SR not only during the stimulus-induced states but also during the resting state characterized by spontaneous thought (Gusnard & Raichle, 2001; Zhu, 2004; D’Argembeau et al., 2005; Moran, Macrae, Heatherton, Wyland, & Kelley, 2006; Schneider et al., 2008; Enzi, De Greck, Pröesch, Tempelmann, & Northoff, 2009; Northoff, Qin, & Nakao, 2010; Whitfield-Gabrieli et al., 2011; Hu et al., 2016). Therefore, the ‘‘rest-self overlap’’ concept (Bai et al., 2016; Northoff, 2016b) has been introduced to describe the convergence in anterior and posterior CMS (Qin & Northoff, 2011; Murray, Debbane, Fox, Bzdok, & Eickhoff, 2014; Davey, Pujol, & Harrison, 2016) between the self and the brain’s spontaneous (or resting state) activity.
Raichle (2015a) proposed that the “default mode functionality” of the brain maintains an intrinsic state of preparedness for anticipating or predisposing the demands placed continuously over time. Based on “rest-self overlap” findings and the “default mode functionality” properties, one may hypothesize that the spontaneous activity of the brain may contain some specific information related to the self, serving to process and assign contents to the subsequent internal or external stimuli. Therefore, one may conceptualize a ‘‘rest-self overlap/containment’’ (Huang, Obara, Davis, Pokorny, & Northoff, 2016; Huang et al., 2017; Northoff, 2016b; Scalabrini, Mucci, & Northoff 2018), where the self-specific information not only overlaps with the resting state but, much stronger, is contained in the spontaneous activity itself. That self-specific information encoded and contained in the spontaneous activity itself may, in turn, provide the basis and thus the “default mode functionality” for the assignment of contents as processed in affective, cognitive, social and sensorimotor functions.
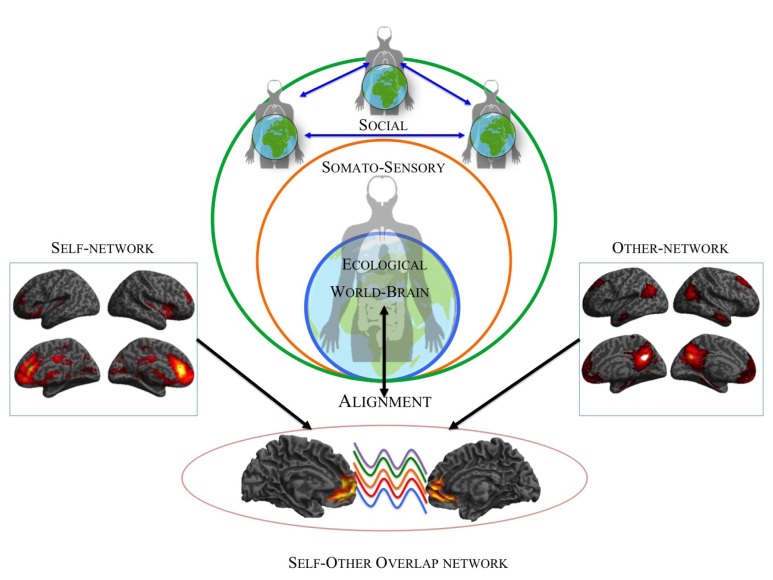
If this holds, one would expect that the spontaneous activity’s degree of self-relatedness should modulate task-related activity and its self-specificity. That is indeed supported by recent empirical data. For instance a recent paper (Scalabrini et al., 2019) showed how the scale-free properties (i.e., the shape of the power spectrum with its long-range temporal correlation) of resting state activity in the anterior cingulate cortex predict the subsequent task evoked neuronal activity related to the animate environment i.e. social. These findings expand the notion of rest-self overlap/ containment to the notion of self-other overlap and distinction. These and others findings (see Northoff, 2016b; Wolff et al., 2019; Huang et al., 2016) strongly suggest that the spontaneous activity’s temporo-spatial dynamic (like its scale-free properties) provide the link between the environmental and social context on the one hand and our sense of self on the other. Our self is thus intrinsically relational, i.e., related to others and the socio-environmental context, which is mediated by temporo-spatial features – one can thus speak of temporo-spatial alignment of the self to the environment (Northoff, 2018; Scalabrini et al., 2018).
Such temporo-spatial alignment of the self to others, both at a neuronal and at a psychological level, is put into doubt by the Coronavirus crisis. As long as the threat of fear affects our relationships we might no longer feel the other as closer in space and time – the self-other relation might be disrupted, which basically means that the self has to cut some of the most fundamental conditions on the basis of which it constitutes itself. That, combined with the threat of death for the one’s own self and for the others results in the existential fear of literally losing the self through losing the other and the world – anxiety/fear thus become existential as manifest in existential fear of the loss of both self and world as so well described by existential philosophers. We here provide empirical evidence that these early existential philosophers were indeed right, as their description of existential fear and loss of self can nowadays be supported by the neuroscience of self as described above.
3.2. The intrinsic relation between self and other – Inter-subjectivity and attachment
Supporting the notion of self-other overlap and distinction, Murray and colleagues (Murray et al., 2014; Murray, Schaer, & Debbané, 2012), based on functional connectivity analysis of a large resting state data set, demonstrated anterior midline regions like perigenual anterior cingulate cortex (PACC) and ventro medial (vm)PFC, as well as the anterior insula, to form a ‘self network’ in the resting state (see also Huang et al., 2016; Lou, Changeux, & Rosenstand, 2017). Intriguingly they also described a ‘other network’ that includes posterior midline regions like posterior cingulate cortex (PCC) and the temporal parietal junction (TPJ), while a ‘self–other overlap network’ in resting state revealed a shared connectivity within the anterior cortical midline structures, i.e. the vmPFC bordering into the ACC (Murray et al., 2014).
These findings imply that in the anterior portion of CMS there is an overlap between self and others while regions like PCC and TPJ are specific for the so called ‘other network’ and more higher order functions like theory of mind (particularly for TPJ), while the ‘self network’ is mainly characterized by the anterior CMS and the insula. The right anterior insula, in particular, has been related to the interoceptive processing to give rise to mental image of one’s physical state, that in turn provide the basis for subjective awareness of emotional feeling and one’s self as “material me” (Craig, 2002, 2003, 2004, 2009, 2010, 2011).
Figure 2.
Self-Other overlap and temporo-spatial alignment to the world through body and social relatedness
From a psychological perspective, recent investigations in psychodynamic psychology and neurobiology suggest how the brain and the related construction of the self depends from the interaction with the animate environment (e.g. significant others) that plays a crucial role in development of the sense of self and relatedness with others (Trevarthen & Aitken, 2001; Schore, 2000; Mucci, 2017, 2018; Mucci, Scalabrini & Northoff, 2018; Scalabrini et al., 2017). The early growth and maturation of brain regions involved in self and social development (Pfeifer & Peake, 2012) is experience-dependent and requires nurturing self-other interactions in the context of attachment for developing the different characteristics related to the construct of self, e.g., continuity, constancy, regulation of cognitive and emotional states. This process of relational internalization is enabled by the human capacity for intersubjectivity, attunement, empathy and the predisposition to joint meaning-making and companionship, which are present from birth (Trevarthen, 2001; Stern, 2000; Tronick, 2007; Lyons-Ruth, 2008). Both attachment and SR processing (Northoff, 2011) enable the constitution and differentiation between self and others: “Attachment begins before any sense of self and before any sense of object to attach to” (Brockman, 2002, p.90).
Recently, several studies investigating the neurobiology of attachment in animals (Insel & Young, 2001) and humans using functional magnetic resonance imaging (fMRI; Lorberbaum et al., 2002; Bartels & Zeki, 2004; Swain, Lorberbaum, Kose, & Strathearn, 2007; Strathearn, Li, Fonagy, & Montague, 2008; Laurita, Hazan, & Spreng, 2017, 2018) showed how the regions prevalently located in the CMS and limbic areas (e.g. amygdala and hippocampus) are fundamentally involved in the context of attachment and have an impact on SR functions. Therefore we might assume that the spontaneous activity of the brain and the SR are experience-dependent, i.e., influenced by the individual experience of the first encounter with the environment (particularly the animate one, e. g. caregivers).
Supporting the connection between the relation between the rest/self – other overlap and the role of attachment we might consider the impact on brain and mind of adverse childhood experiences (ACE). A recent study by Nakao and colleagues (2013) show how ACEs are associated neuronally with decreased functional connectivity within the default mode network (DMN) in the resting state. Moreover, they found a greater deactivation of medial PFC during a self-oriented task. Extending these findings Duncan and colleagues (2015) showed how ACEs have long-term effects on the structure and function of the brain. Alterations have been noted in grey and white matter, in the brain’s resting state, on the glutamatergic system, and on neural and behavioural responses to aversive stimuli. These findings highlight the impact of ACEs on multiple inter-related brain systems. In particular, they highlight the role of a prefrontal-insular-motor cortical network in the processing and response to aversive stimuli and its potential adaptability by ACEs. The enduring effect of early traumatization does not allow the connection between the limbic areas and superior orbito-frontal areas creating the dysfunctions typical of personality and psychiatric pathologies, characterized by long-term abuse and dysfunctional families (Felitti et al., 1998; Mucci, 2013; Schore, 2012; Schimmenti & Caretti, 2016; Liotti, 2017; Scalabrini Cavicchioli, Fossati, & Maffei, 2017).
What do these data tell us about existential fear? On the deepest level of our existence, we are intrinsically connected with the other – we constitute our self through our intrinsic connection with the other. That very same intrinsic connection to the other shapes our self through what psychology describes as attachment. The data show that early life events like traumatic childhood events in our relation with others strongly shape our self, leading to malfunctioning attachment and major changes or deficits in the structure of self.
Even more interesting, early traumatic childhood events leave their traces in the brain that can be followed up even during adulthood. Those traces are left in form of altered temporo-spatial dynamic in the brain’s spontaneous activity like abnormal entropy (disorder) or scale-free activity. Hence, though seemingly isolated within the skull, our brain’s spontaneous activity is intrinsically social and ecological, i.e., neuro-social and neuro-ecological – that is the lesson brain imaging studies on self and other as well as psychological attachment studies can teach us. Most importantly, it makes clear that the social disruption caused by the Coronavirus-emergency touches us so deeply upon the basis and fundament of our self and its existence – no wonder that our self cannot react otherwise than with existential fear as who would not be afraid if one’s ground one stands on is pulled away? And who can avoid the re-activation of earlier childhood trauma that, remaining completely unconscious, was hidden deep in the dark basements of the self?
3.3 The Self-Other Relationship – the role of Emotional Feeling and the body
We showed how self and others are related with each other and how the development of the self is experience dependent since the early stages of life from the “animate” environment. How do we experience the intrinsic relation between self and other being part of the world. One of the main human characteristics is that we experience emotional feelings, which are the subjective experiences of emotions. For instance, the neural activity in various subcortical regions of the brain (e.g., the amygdala) has been associated with specifically negative emotions such as sadness and fear—and that very same region has also been observed to be hyperactive in depression and anxiety. Positive emotions such as happiness have been linked to other subcortical regions (e.g., the striatum) that are closely linked to reward.
Some authors (e.g., James, 1890; see Schachter & Singer, 1962, for details) argue that emotions are simply a class of feelings that must be distinguished from mere sensation and proprioception by their experience. Emotions can also be defined by their objects, rather than by the feelings of the emotions. It does not matter whether the object is real or virtual as long as it is associated with specific emotions. In this sense, the objects that elicit them define emotions. However, in our perspective emotional feelings are then at the very core of emotions, with emotions depending on subjective feelings associated with them.
Thus, do emotional feelings result from our brain’s neuronal processes, the perception of the body’s interoceptive stimuli, or from the exteroceptive stimuli from the environment? Following Jaak Panksepp, the father of Affective neuroscience (Panksepp, 1998a, 1998b, 2007a, 2007b, 2011a, 2011b), he assumes that the neural activity in the first-order structures is already, by itself, associated with emotional feelings. As soon as the affective regions become active by, for instance, processing the sensory input from the body and the motor output to the body, emotional feelings are generated. Subcortical regions such as the periaqueductal gray are central here. They process the linkage between sensory input from body and environment and at the same time link it to motor functions. Emotional feelings are assumed to result from such linkage between bodily and environmental sensory input to the body’s motor functions. This represents a system that was conceptualized as “the core self” (Northoff & Panksepp, 2008), which is a central integrative neural system made up of subcortical–cortical midline structures (SCMSs).
For Panksepp (1998a), any input from body and world that is processed into the brain directly leads to emotional feelings. Metaphorically we would say that emotional feelings are the manifestation of our temporospatial alignment and its underlying world–brain relation (Northoff, 2018) in our consciousness. Due to the neural overlap of emotion and self (see first part on fear) and the deep-reaching neuro-ecological layers of self, emotional feelings like fear and anxiety cannot avoid being existential: they are experienced, i.e., felt by the self and therefore signify our existence within the world, our “Dasein” as the German philosopher Martin Heidegger said at the beginning of the 20th century. In short, emotional feelings cannot be detached and dissociated from the world. They are not located in the cognitive elaborations of our brain. Instead, they signify the world–brain relation, and, more specifically, our (or our self’s) relation to other persons (and their selves).
3.4 Self and world – Collapse of their relationship
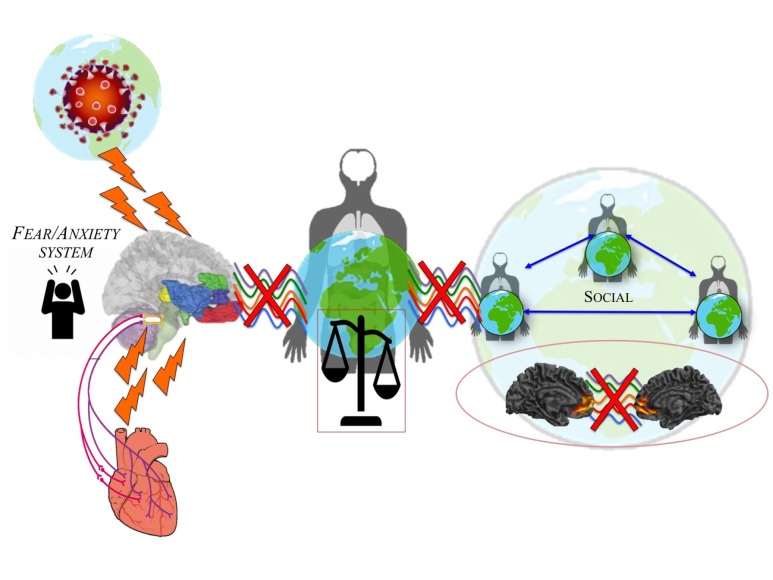
When the basis of our self in its underlying the world-brain relation is disrupted or even lost, non-integration of internal and external stimuli might occur in order to avoid the painful experience of existential fear – this process might result in dissociation at different levels (see Scalabrini et al., 2020). More specifically, the desaggreation of intero-exteroceptive function on the basis of impaired spatiotemporal integration leads to alterations in embodiment and, even further, the disruption of our self’s relationship with the world and its environmental context. This failure of integration can thus be seen as a disconnection that induces an instant collapse of both subjectivity and intersubjectivity (Scalabrini et al. 2020; Schimmenti & Sar, 2019).
Let us start with the collapse of self and its subjectivity. Such disruptions of embodiment and first-person perspective are well reflected in trauma related disorders. Stressful stimuli, especially those associated with painful emotional effects, are thus not experienced in consciousness, and they are associated with what Bromberg (2014) terms “not-me” self-states. At the same time, intero-exteroceptive desaggregation leads to disruption of the self’s temporo-spatial alignment to the world. If the world’s exteroceptive stimuli can no longer be integrated with the body’s interoceptive stimuli, the self becomes decupled and thus dissociated from the world. That ultimately leads to the collapse of inter-subjectivity and the self’s neuro-ecological relation to its world. This can be well observed in dissociative states where either parts of the own body, indexing the collapse of the subjectivity, and/or parts of the environmental context, signifying the collapse of inter-subjectivity, can be observed.
Figure 3.
Existential anxiety leading disruption of subjectivity and intersubjectivity. The threats of COVID-19 trigger an existential anxiety feeling resulting in a collapse of the relation between world, body and brain.
Conclusions
The threat of the coronavirus presents us with a double threat – collapse of our subjectivity as due to the risk of our body being infected and collapse of our inter-subjectivity as by social isolation of our self from others. The double threat of both subjectivity and inter-subjectivity provides a depth dimension to our emotions including our fears and anxieties resulting in existential fear. Such intimate connection of fear, self, and existence, is due to the way our brain and its spontaneous activity are organized, that is, it stabilizes itself by aligning to body and world in very much the same way we stabilize our movements during dancing by aligning to the rhythm of the music. That very same alignment of our brain to body and world, i.e., temporo-spatial alignment is threatened if not disrupted by the corona virus crisis – in the same, one dances erratically when being detached from the music’s rhythms, we become existentially threatened and erratic when the brain’s and ultimately our self’s temporo-spatial neuro-ecological alignment to the world are threatened by both the virus and the defense measures of physical and social distancing.
Nowadays reading life on the basis of daily confrontation with the death and connected existential fears is at the same time the threat and the value of this time. Loss of subjectivity is manifest in our loss of body and thus the fear of dying, while the loss of intersubjectivity surfaces in our fear of infecting others and thereby losing a key component of our relation and alignment to the world, that is, ultimately, the others and thereby our self. In those instances, one would wish that our brain would operate differently. However, that is a hope doomed to fail as we, through our existential fears, pay the price for the virtues of our brain as it lets us experience our own self in synchrony with and being aligned to others and the wider world.
References
- Aldao, A., Nolen-Hoeksema, S., & Schweizer, S. (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30(2), 217–237. 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders. Washington, DC, United States of America: Amer Psychiatric Pub Incorporated. [Google Scholar]
- Amstadter, A. (2008). Emotion regulation and anxiety disorders. Journal of Anxiety Disorders, 22(2), 211–221. 10.1016/j.janxdis.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andor, T., Gerlach, A. L., & Rist, F. (2008). Superior perception of phasic physiological arousal and the detrimental consequences of the conviction to be aroused on worrying and metacognitions in GAD. Journal of Abnormal Psychology, 117(1), 193–205. 10.1037/0021-843x.117.1.193 [DOI] [PubMed] [Google Scholar]
- Bai, Y., Nakao, T., Xu, J., Qin, P., Chaves, P., Heinzel, A., … Northoff, G. (2015). Resting state glutamate predicts elevated pre-stimulus alpha during self-relatedness: A combined EG-MRS study on “rest-self overlap.” Social Neuroscience, 11(3), 249–263. 10.1080/17470919.2015.1072582 [DOI] [PubMed] [Google Scholar]
- Ball, T. M., Ramsawh, H. J., Campbell-Sills, L., Paulus, M. P., & Stein, M. B. (2012). Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychological Medicine, 43(7), 1475–1486. 10.1017/s0033291712002383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow, D. H. (2001). Anxiety and Its Disorders. New York, NY, United States of America: Guilford Publications. [Google Scholar]
- Barrett, L. F., & Simmons, W. K. (2015). Interoceptive predictions in the brain. Nature Reviews Neuroscience, 16(7), 419–429. 10.1038/nrn3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels, A., & Zeki, S. (2004). The neural correlates of maternal and romantic love. NeuroImage, 21(3), 1155–1166. 10.1016/j.neuroimage.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Bijsterbosch, J., Smith, S., Forster, S., John, O. P., & Bishop, S. J. (2014). Resting State Correlates of Subdimensions of Anxious Affect. Journal of Cognitive Neuroscience, 26(4), 914–926. 10.1162/jocn_a_00512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, S. J. (2007). Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences, 11(7), 307–316. 10.1016/j.tics.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Brockman, R. (2002). Self, Object, Neurobiology. Neuropsychoanalysis, 4(1), 89–101. 10.1080/15294145.2002.10773382 [DOI] [Google Scholar]
- Bromberg, P. M. (2014). Standing in the spaces: Essays on clinical process trauma and dissociation. Routledge. [Google Scholar]
- Brown, A. D., Addis, D. R., Romano, T. A., Marmar, C. R., Bryant, R. A., Hirst, W., & Schacter, D. L. (2013). Episodic and semantic components of autobiographical memories and imagined future events in post-traumatic stress disorder. Memory, 22(6), 595–604. 10.1080/09658211.2013.807842 [DOI] [PubMed] [Google Scholar]
- Brown, A. D., Root, J. C., Romano, T. A., Chang, L. J., Bryant, R. A., & Hirst, W. (2013). Overgeneralized autobiographical memory and future thinking in combat veterans with posttraumatic stress disorder. Journal of Behavior Therapy and Experimental Psychiatry, 44(1), 129–134. 10.1016/j.jbtep.2011.11.004 [DOI] [PubMed] [Google Scholar]
- Buckner, R. L., Andrews-Hanna, J. R., & Schacter, D. L. (2008). The Brain’s Default Network. Annals of the New York Academy of Sciences, 1124(1), 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Caseras, X., Murphy, K., Mataix-Cols, D., López-Solà, M., Soriano-Mas, C., Ortriz, H., … Torrubia, R. (2011). Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Human Brain Mapping, 34(5), 1220–1229. 10.1002/hbm.21503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, P.-Y. S., Cheng, C.-H., Hsu, S.-C., Liu, C.-Y., Davenport, P. W., & Leupoldt, A. von. (2015). Respiratory sensory gating measured by respiratory-related evoked potentials in generalized anxiety disorder. Frontiers in Psychology, 6. 10.3389/fpsyg.2015.00957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, P.-Y. S., von Leupoldt, A., Liu, C.-Y., & Hsu, S.-C. (2014). Respiratory perception measured by cortical neural activations in individuals with generalized anxiety disorder. Respiratory Physiology & Neurobiology, 204, 36–40. 10.1016/j.resp.2014.09.009 [DOI] [PubMed] [Google Scholar]
- Cisler, J. M., Olatunji, B. O., Feldner, M. T., & Forsyth, J. P. (2009). Emotion Regulation and the Anxiety Disorders: An Integrative Review. Journal of Psychopathology and Behavioral Assessment, 32(1), 68–82. 10.1007/s10862-009-9161-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, L. A., & Watson, D. (1991). Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100(3), 316–336. 10.1037/0021-843x.100.3.316 [DOI] [PubMed] [Google Scholar]
- Craig, A.D. (Bud). (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–666. 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Craig, A.D. (Bud). (2003). Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology, 13(4), 500–505. 10.1016/s0959-4388(03)00090-4 [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (Bud). (2004). Human feelings: why are some more aware than others? Trends in Cognitive Sciences, 8(6), 239–241. 10.1016/j.tics.2004.04.004 [DOI] [PubMed] [Google Scholar]
- Craig, A. D. (Bud). (2009). Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1525), 1933–1942. 10.1098/rstb.2009.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. D. (Bud). (2010). The sentient self. Brain Structure and Function, 214(5–6), 563–577. 10.1007/s00429-010-0248-y [DOI] [PubMed] [Google Scholar]
- Craig, A.D. (Bud). (2011). Significance of the insula for the evolution of human awareness of feelings from the body. Annals of the New York Academy of Sciences, 1225(1), 72–82. 10.1111/j.1749-6632.2011.05990.x [DOI] [PubMed] [Google Scholar]
- Cui, H., Zhang, B., Li, W., Li, H., Pang, J., Hu, Q., … Northoff, G. (2020). Insula shows abnormal task-evoked and resting-state activity in first-episode drug-naïve generalized anxiety disorder. Depression and Anxiety. 10.1002/da.23009 [DOI] [PubMed]
- Cui, H., Zhang, J., Liu, Y., Li, Q., Li, H., Zhang, L., … Northoff, G. (2016). Diferential alterations of resting-state functional connectivity in generalized anxiety disorder and panic disorder. Human Brain Mapping, 37(4), 1459–1473. 10.1002/hbm.23113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau, A., Collette, F., Van der Linden, M., Laureys, S., Del Fiore, G., Degueldre, C., … Salmon, E. (2005). Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage, 25(2), 616–624. 10.1016/j.neuroimage.2004.11.048 [DOI] [PubMed] [Google Scholar]
- Davey, C. G., Pujol, J., & Harrison, B. J. (2016). Mapping the self in the brain’s default mode network. NeuroImage, 132, 390–397. 10.1016/j.neuroimage.2016.02.022 [DOI] [PubMed] [Google Scholar]
- Davis, M. (1998). Are different parts of the extended amygdala involved in fear versus anxiety? Biological Psychiatry, 44(12), 1239–1247. 10.1016/s0006-3223(98)00288-1 [DOI] [PubMed] [Google Scholar]
- Davis, M., Walker, D. L., Miles, L., & Grillon, C. (2009). Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology, 35(1), 105–135. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Greck, M., Rotte, M., Paus, R., Moritz, D., Thiemann, R., Proesch, U., … Northoff, G. (2008). Is our self based on reward? Self-relatedness recruits neural activity in the reward system. NeuroImage, 39(4), 2066–2075. 10.1016/j.neuroimage.2007.11.006 [DOI] [PubMed] [Google Scholar]
- de Greck, M., Enzi, B., Prösch, U., Gantman, A., Tempelmann, C., & Northoff, G. (2010). Decreased neuronal activity in reward circuitry of pathological gamblers during processing of personal relevant stimuli. Human Brain Mapping, NA. 10.1002/hbm.20981 [DOI] [PMC free article] [PubMed]
- de Greck, M., Wang, G., Yang, X., Wang, X., Northoff, G., & Han, S. (2011). Neural substrates underlying intentional empathy. Social Cognitive and Afective Neuroscience, 7(2), 135–144. 10.1093/scan/nsq093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan, N. W., Hayes, D. J., Wiebking, C., Tiret, B., Pietruska, K., Chen, D. Q., … Northoff, G. (2015). Negative childhood experiences alter a prefrontal-insular-motor cortical network in healthy adults: A preliminary multimodal rsfMRI-fMRI-MRS-dMRI study. Human Brain Mapping, 36(11), 4622–4637. 10.1002/hbm.22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers, A., & Breuer, P. (1992). Increased cardiac awareness in panic disorder. Journal of Abnormal Psychology, 101(3), 371–382. 10.1037/0021-843x.101.3.371 [DOI] [PubMed] [Google Scholar]
- Enzi, B., de Greck, M., Prösch, U., Tempelmann, C., & Northoff, G. (2009). Is Our Self Nothing but Reward? Neuronal Overlap and Distinction between Reward and Personal Relevance and Its Relation to Human Personality. PLoS ONE, 4(12), e8429. 10.1371/journal.pone.0008429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin, A., & Wager, T. D. (2007). Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. American Journal of Psychiatry, 164(10), 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., BA, … Marks, J. S. (1998). Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. American Journal of Preventive Medicine, 14(4), 245–258. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed] [Google Scholar]
- Frings, C., & Wentura, D. (2014). Self-priorization processes in action and perception. Journal of Experimental Psychology: Human Perception and Performance, 40(5), 1737–1740. 10.1037/a0037376 [DOI] [PubMed] [Google Scholar]
- Grillon, C., Baas, J. M. P., Pine, D. S., Lissek, S., Lawley, M., Ellis, V., & Levine, J. (2006). The Benzodiazepine Alprazolam Dissociates Contextual Fear from Cued Fear in Humans as Assessed by Fear-potentiated Startle. Biological Psychiatry, 60(7), 760–766. 10.1016/j.biopsych.2005.11.027 [DOI] [PubMed] [Google Scholar]
- Grossi, D., Longarzo, M., Quarantelli, M., Salvatore, E., Cavaliere, C., De Luca, P., … Aiello, M. (2017). Altered functional connectivity of interoception in illness anxiety disorder. Cortex, 86, 22–32. 10.1016/j.cortex.2016.10.018 [DOI] [PubMed] [Google Scholar]
- Grupe, D. W., & Nitschke, J. B. (2013). Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501. 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, R., Duff, M. C., Denburg, N. L., Cohen, N. J., Bechara, A., & Tranel, D. (2009). Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia, 47(7), 1686–1693. 10.1016/j.neuropsychologia.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard, D. A., & Raichle, M. E. (2001). Searching for a baseline: Functional imaging and the resting human brain. Nature Reviews Neuroscience, 2(10), 685–694. 10.1038/35094500 [DOI] [PubMed] [Google Scholar]
- Hamm, L. L., Jacobs, R. H., Johnson, M. W., Fitzgerald, D. A., Fitzgerald, K. D., Langenecker, S. A., … Phan, K. L. (2014). Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biology of Mood & Anxiety Disorders, 4(1). 10.1186/s13587-014-0015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, A., Schäfer, A., Walter, B., Stark, R., Vaitl, D., & Schienle, A. (2007). Diminished medial prefrontal cortex activity in blood-injection-injury phobia. Biological Psychology, 75(2), 124–130. 10.1016/j.biopsycho.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Hiser, J., & Koenigs, M. (2018). The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biological Psychiatry, 83(8), 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric, R. (1989). Somatic Manifestations in Women With Generalized Anxiety Disorder. Archives of General Psychiatry, 46(12), 1113. 10.1001/archpsyc.1989.01810120055009 [DOI] [PubMed] [Google Scholar]
- Hu, C., Di, X., Eickhoff, S. B., Zhang, M., Peng, K., Guo, H., & Sui, J. (2016). Distinct and common aspects of physical and psychological self-representation in the brain: A meta-analysis of self-bias in facial and self-referential judgements. Neuroscience & Biobehavioral Reviews, 61, 197–207. 10.1016/j.neubiorev.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Huang, Z., Obara, N., Davis, H. (Hap), IV, Pokorny, J., & Northoff, G. (2016). The temporal structure of resting-state brain activity in the medial prefrontal cortex predicts self-consciousness. Neuropsychologia, 82, 161–170. 10.1016/j.neuropsychologia.2016.01.025 [DOI] [PubMed] [Google Scholar]
- Huang, Z., Zhang, J., Longtin, A., Dumont, G., Duncan, N. W., Pokorny, J., … Northoff, G. (2017). Is There a Nonadditive Interaction Between Spontaneous and Evoked Activity? Phase-Dependence and Its Relation to the Temporal Structure of Scale-Free Brain Activity. Cerebral Cortex, bhv288. 10.1093/cercor/bhv288 [DOI] [PubMed]
- Insel, T. R., & Young, L. J. (2001). The neurobiology of attachment. Nature Reviews Neuroscience, 2(2), 129–136. 10.1038/35053579 [DOI] [PubMed] [Google Scholar]
- James, W. (1890). The Principles of Psychology. Cambridge, MA, United States of America: Harvard University Press. [Google Scholar]
- Jung, C.,G. (1988). Nietzsche’s “Zarathustra”, 2 vols. Princeton, NJ: Princeton University Press. [Google Scholar]
- Kessler, R. C., Berglund, P., Demler, O., Jin, R., Merikangas, K. R., & Walters, E. E. (2005). Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kleim, B., Graham, B., Fihosy, S., Stott, R., & Ehlers, A. (2013). Reduced Specificity in Episodic Future Thinking in Posttraumatic Stress Disorder. Clinical Psychological Science, 2(2), 165–173. 10.1177/2167702613495199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurita, A. C., Hazan, C., & Spreng, R. N. (2017). Dissociable patterns of brain activity for mentalizing about known others: a role for attachment. Social Cognitive and Affective Neuroscience, 12(7), 1072–1082. 10.1093/scan/nsx040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurita, A. C., Hazan, C., & Spreng, R. N. (2018). Neural signatures of chronic accessibility in parent – adult child attachment bonds. Social Neuroscience, 14(4), 462–469. 10.1080/17470919.2018.1494037 [DOI] [PubMed] [Google Scholar]
- Liotti, G. (2017). Conflicts between motivational systems related to attachment trauma: Key to understanding the intra-family relationship between abused children and their abusers. Journal of Trauma & Dissociation, 18(3), 304–318. 10.1080/15299732.2017.1295392 [DOI] [PubMed] [Google Scholar]
- Logothetis, N. K., Murayama, Y., Augath, M., Steffen, T., Werner, J., & Oeltermann, A. (2009). How not to study spontaneous activity. NeuroImage, 45(4), 1080–1089. 10.1016/j.neuroimage.2009.01.010 [DOI] [PubMed] [Google Scholar]
- Lorberbaum, J. P., Newman, J. D., Horwitz, A. R., Dubno, J. R., Lydiard, R. B., Hamner, M. B., … George, M. S. (2002). A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry, 51(6), 431–445. 10.1016/s0006-3223(01)01284-7 [DOI] [PubMed] [Google Scholar]
- Lou, H. C., Changeux, J. P., & Rosenstand, A. (2017). Towards a cognitive neuroscience of self-awareness. Neuroscience & Biobehavioral Reviews, 83, 765–773. 10.1016/j.neubiorev.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Ludewig, S. (2003). Decision-making strategies by panic disorder subjects are more sensitive to errors. Journal of Afective Disorders, 76(1–3), 183–189. 10.1016/s0165-0327(02)00089-7 [DOI] [PubMed] [Google Scholar]
- Lyons, A. D., Henry, J. D., Rendell, P. G., Corballis, M. C., & Suddendorf, T. (2014). Episodic foresight and aging. Psychology and Aging, 29(4), 873–884. 10.1037/a0038130 [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth, K. (2008). Contributions of the mother–infant relationship to dissociative, borderline, and conduct symptoms in young adulthood. Infant Mental Health Journal, 29(3), 203–218. 10.1002/imhj.20173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews, A., & MacLeod, C. (2005). Cognitive Vulnerability to Emotional Disorders. Annual Review of Clinical Psychology, 1(1), 167–195. 10.1146/annurev.clinpsy.1.102803.143916 [DOI] [PubMed] [Google Scholar]
- Mennin, D. S. (2006). Emotion Regulation Therapy: An Integrative Approach to Treatment-Resistant Anxiety Disorders. Journal of Contemporary Psychotherapy, 36(2), 95–105. 10.1007/s10879-006-9012-2 [DOI] [Google Scholar]
- Menon, V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences, 15(10), 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Moran, J. M., Macrae, C. N., Heatherton, T. F., Wyland, C. L., & Kelley, W. M. (2006). Neuroanatomical Evidence for Distinct Cognitive and Affective Components of Self. Journal of Cognitive Neuroscience, 18(9), 1586–1594. 10.1162/jocn.2006.18.9.1586 [DOI] [PubMed] [Google Scholar]
- Motzkin, J. C., Philippi, C. L., Wolf, R. C., Baskaya, M. K., & Koenigs, M. (2015). Ventromedial Prefrontal Cortex Is Critical for the Regulation of Amygdala Activity in Humans. Biological Psychiatry, 77(3), 276–284. 10.1016/j.biopsych.2014.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci, C. (2013). Beyond Individual and Collective Trauma. Amsterdam, Netherlands: Adfo Books. [Google Scholar]
- Mucci, C. (2017). Ferenczi’s Revolutionary Therapeutic Approach*. The American Journal of Psychoanalysis, 77(3), 239–254. 10.1057/s11231-017-9104-7 [DOI] [PubMed] [Google Scholar]
- Mucci, C. (2018). Borderline Bodies: Affect Regulation Therapy for Personality Disorders (Norton Series on Interpersonal Neurobiology). W Norton & Comp [Google Scholar]
- Mucci, C., Scalabrini, A., & Northoff, G. (2018). Traumatogenic Disturbances: PTSD, Complex PTSD and Trauma-Related Disorders. Neuropsychodynamic Psychiatry, 351–376. 10.1007/978-3-319-75112-2_17 [DOI]
- Murray, R. J., Debbané, M., Fox, P. T., Bzdok, D., & Eickhoff, S. B. (2014). Functional connectivity mapping of regions associated with self- and other-processing. Human Brain Mapping, 36(4), 1304–1324. 10.1002/hbm.22703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, R. J., Schaer, M., & Debbané, M. (2012). Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self- and other-reflection. Neuroscience & Biobehavioral Reviews, 36(3), 1043–1059. 10.1016/j.neubiorev.2011.12.013 [DOI] [PubMed] [Google Scholar]
- Nakao, T., Matsumoto, T., Morita, M., Shimizu, D., Yoshimura, S., Northoff, G., … Yamawaki, S. (2013). The Degree of Early Life Stress Predicts Decreased Medial Prefrontal Activations and the Shift from Internally to Externally Guided Decision Making: An Exploratory NIRS Study during Resting State and Self-Oriented Task. Frontiers in Human Neuroscience, 7. 10.3389/fnhum.2013.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao, T., Miyagi, M., Hiramoto, R., Wolff, A., Gomez-Pilar, J., Miyatani, M., & Northoff, G. (2019). From neuronal to psychological noise – Long-range temporal correlations in EG intrinsic activity reduce noise in internally-guided decision making. NeuroImage, 201, 116015. 10.1016/j.neuroimage.2019.116015 [DOI] [PubMed] [Google Scholar]
- Nakao, T., Ohira, H., & Northoff, G. (2012). Distinction between Externally vs. Internally Guided Decision-Making: Operational Differences, Meta-Analytical Comparisons and Their Theoretical Implications. Frontiers in Neuroscience, 6. 10.3389/fnins.2012.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashold, B. S., Wilson, W. P., & Slaughter, D. G. (1969). Sensations Evoked by Stimulation in the Midbrain of Man. Journal of Neurosurgery, 30(1), 14–24. 10.3171/jns.1969.30.1.0014 [DOI] [PubMed] [Google Scholar]
- Northoff, G. (2011). Neuropsychoanalysis in Practice. Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- Northoff, G. (2014). How Is Our Self Altered in Psychiatric Disorders? A Neurophenomenal Approach to Psychopathological Symptoms. Psychopathology, 47(6), 365–376. 10.1159/000363351 [DOI] [PubMed] [Google Scholar]
- Northoff, G. (2016a). Is the self a higher-order or fundamental function of the brain? The “basis model of self-specificity” and its encoding by the brain’s spontaneous activity. Cognitive Neuroscience, 7(1–4), 203–222. 10.1080/17588928.2015.1111868 [DOI] [PubMed] [Google Scholar]
- Northoff, G. (2016b). Spatiotemporal psychopathology I: No rest for the brain’s resting state activity in depression? Spatiotemporal psychopathology of depressive symptoms. Journal of Afective Disorders, 190, 854–866. 10.1016/j.jad.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Northof, G. (2018). The brain’s spontaneous activity and its psychopathological symptoms – “Spatiotemporal binding and integration.” Progress in Neuro-Psychopharmacology and Biological Psychiatry, 80, 81–90. 10.1016/j.pnpbp.2017.03.019 [DOI] [PubMed] [Google Scholar]
- Northoff, G., & Bermpohl, F. (2004). Cortical midline structures and the self. Trends in Cognitive Sciences, 8(3), 102–107. 10.1016/j.tics.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Northoff, G., Heinzel, A., de Greck, M., Bermpohl, F., Dobrowolny, H., & Panksepp, J. (2006). Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage, 31(1), 440–457. 10.1016/j.neuroimage.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Northoff, G., & Panksepp, J. (2008). The trans-species concept of self and the subcortical–cortical midline system. Trends in Cognitive Sciences, 12(7), 259–264. 10.1016/j.tics.2008.04.007 [DOI] [PubMed] [Google Scholar]
- Northof, G., Qin, P., & Nakao, T. (2010). Rest-stimulus interaction in the brain: a review. Trends in Neurosciences, 33(6), 277–284. 10.1016/j.tins.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Northof, G., Schneider, F., Rotte, M., Matthiae, C., Tempelmann, C., Wiebking, C., … Panksepp, J. (2007). Differential parametric modulation of self-relatedness and emotions in diferent brain regions. Human Brain Mapping, 30(2), 369–382. 10.1002/hbm.20510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp, J. (1990). The psychoneurology of fear: Evolutionary perspectives and the role of animal models in understanding human anxiety. In Burrows G. D., Roth M., & Noyes R. Jr. (Eds.) Handbook of Anxiety (pp. 3–58). Amsterdam: Elsevier ISBN-10: 0444812369 [Google Scholar]
- Panksepp, J. (1998a). The periconscious substrates of consciousness: affective states and the evolutionary origins of the SELF. J. Conscious. Stud. 5, 566–582. [Google Scholar]
- Panksepp, J. (1998b). Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford, United Kingdom: Oxford University Press
- Panksepp, J. (2004). Textbook of Biological Psychiatry. Hoboken, NJ, United States: Wiley. [Google Scholar]
- Panksepp, J. (2007a). Affective Consciousness. The Blackwell Companion to Consciousness, 114–129. 10.1002/9780470751466.ch9 [DOI]
- Panksepp, J. (2007b). The neuroevolutionary and neuroaffective psychobiology of the prosocial brain. Oxford Handbooks Online. 10.1093/oxfordhb/9780198568308.013.0012 [DOI]
- Panksepp, J. (2011a). Cross-Species Affective Neuroscience Decoding of the Primal Affective Experiences of Humans and Related Animals. PLoS ONE, 6(9), e21236. 10.1371/journal.pone.0021236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp, J. (2011b). The basic emotional circuits of mammalian brains: Do animals have affective lives? Neuroscience & Biobehavioral Reviews, 35(9), 1791–1804. 10.1016/j.neubiorev.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Panksepp, J., Fuchs, T., & Iacobucci, P. (2011). The basic neuroscience of emotional experiences in mammals: The case of subcortical FEAR circuitry and implications for clinical anxiety. Applied Animal Behaviour Science, 129(1), 1–17. 10.1016/j.applanim.2010.09.014 [DOI] [Google Scholar]
- Paulus, M. P., Feinstein, J. S., & Khalsa, S. S. (2019). An Active Inference Approach to Interoceptive Psychopathology. Annual Review of Clinical Psychology, 15(1), 97–122. 10.1146/annurev-clinpsy-050718-095617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus, M. P., & Stein, M. B. (2010). Interoception in anxiety and depression. Brain Structure and Function, 214(5–6), 451–463. 10.1007/s00429-010-0258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer, J. H., & Peake, S. J. (2012). Self-development: Integrating cognitive, socioemotional, and neuroimaging perspectives. Developmental Cognitive Neuroscience, 2(1), 55–69. 10.1016/j.dcn.2011.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan, K. L., Taylor, S. F., Welsh, R. C., Ho, S.-H., Britton, J. C., & Liberzon, I. (2004). Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage, 21(2), 768–780. 10.1016/j.neuroimage.2003.09.072 [DOI] [PubMed] [Google Scholar]
- Qin, P., & Northoff, G. (2011). How is our self related to midline regions and the default-mode network? NeuroImage, 57(3), 1221–1233. 10.1016/j.neuroimage.2011.05.028 [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. (2009). A Paradigm Shift in Functional Brain Imaging. Journal of Neuroscience, 29(41), 12729–12734. 10.1523/jneurosci.4366-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E., MacLeod, A. M., Snyder, A. Z., Powers, W. J., Gusnard, D. A., & Shulman, G. L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle, M. E. (2015a). The Brain’s Default Mode Network. Annual Review of Neuroscience, 38(1), 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Raichle, M. E. (2015b). The restless brain: how intrinsic activity organizes brain function. Philosophical Transactions of the Royal Society B: Biological Sciences, 370(1668), 20140172. 10.1098/rstb.2014.0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch, S. L., Shin, L. M., & Wright, C. I. (2006). Neuroimaging Studies of Amygdala Function in Anxiety Disorders. Annals of the New York Academy of Sciences, 985(1), 389–410. 10.1111/j.1749-6632.2003.tb07096.x [DOI] [PubMed] [Google Scholar]
- Rodriguez, B. F., Bruce, S. E., Pagano, M. E., & Keller, M. B. (2005). Relationships among psychosocial functioning, diagnostic comorbidity, and the recurrence of generalized anxiety disorder, panic disorder, and major depression. Journal of Anxiety Disorders, 19(7), 752–766. 10.1016/j.janxdis.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Scalabrini, A., Cavicchioli, M., Fossati, A., & Maffei, C. (2017). The extent of dissociation in borderline personality disorder: A meta-analytic review. Journal of Trauma & Dissociation, 1–22. 10.1080/15299732.2016.1240738 [DOI] [PubMed]
- Scalabrini, A., Huang, Z., Mucci, C., Perrucci, M. G., Ferretti, A., Fossati, A., Romani, G.L., Northoff, G. & Ebisch, S. J. (2017). How spontaneous brain activity and narcissistic features shape social interaction. Scientific reports, 7(1), 1-12. 10.1038/s41598-017-10389-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalabrini, A., Mucci, C., & Northoff, G. (2018). Is Our Self Related to Personality? A Neuropsychodynamic Model. Frontiers in Human Neuroscience, 12. 10.3389/fnhum.2018.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalabrini, A., Ebisch, S. J. H., Huang, Z., Di Plinio, S., Perrucci, M. G., Romani, G. L., Mucci, C., Northoff, G. (2019). Spontaneous Brain Activity Predicts Task-Evoked Activity During Animate Versus Inanimate Touch. Cerebral Cortex, 29(11), 4628–4645. 10.1093/cercor/bhy340 [DOI] [PubMed] [Google Scholar]
- Scalabrini, A., Mucci, C., Esposito, R., Damiani, S., & Northoff, G. (2020). Dissociation as a disorder of integration – On the footsteps of Pierre Janet. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 101, 109928. 10.1016/j.pnpbp.2020.109928 [DOI] [PubMed] [Google Scholar]
- Schachter, S., & Singer, J. (1962). Cognitive, social, and physiological determinants of emotional state. Psychological Review, 69(5), 379–399. 10.1037/h0046234 [DOI] [PubMed] [Google Scholar]
- Schacter, D. L., Gaesser, B., & Addis, D. R. (2013). Remembering the Past and Imagining the Future in the Elderly. Gerontology, 59(2), 143–151. 10.1159/000342198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle, A., Schäfer, A., Walter, B., Stark, R., & Vaitl, D. (2005). Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neuroscience Letters, 388(1), 1–6. 10.1016/j.neulet.2005.06.025 [DOI] [PubMed] [Google Scholar]
- Schimmenti, A., & Caretti, V. (2016). Linking the overwhelming with the unbearable: Developmental trauma, dissociation, and the disconnected self. Psychoanalytic Psychology, 33(1), 106–128. 10.1037/a0038019 [DOI] [Google Scholar]
- Schimmenti, A., & Sar, V. (2019). A correlation network analysis of dissociative experiences. Journal of Trauma & Dissociation, 20(4), 402-419. 10.1080/15299732.2019.1572045 [DOI] [PubMed] [Google Scholar]
- Schneider, F., Bermpohl, F., Heinzel, A., Rotte, M., Walter, M., Tempelmann, C., … Northoff, G. (2008). The resting brain and our self: Self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience, 157(1), 120–131. 10.1016/j.neuroscience.2008.08.014 [DOI] [PubMed] [Google Scholar]
- Schore, A. N. (2000). Attachment and the regulation of the right brain. Attachment & Human Development, 2(1), 23–47. 10.1080/146167300361309 [DOI] [PubMed] [Google Scholar]
- Schore, A.N. (2012). The Science of the Art of Psychotherapy (Norton Series on Interpersonal Neurobiology). New York, NY, United States of America: W. W. Norton. [Google Scholar]
- Seth, A. K., Suzuki, K., & Critchley, H. D. (2012). An Interoceptive Predictive Coding Model of Conscious Presence. Frontiers in Psychology, 2. 10.3389/fpsyg.2011.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth, A. K. (2013). Interoceptive inference, emotion, and the embodied self. Trends in Cognitive Sciences, 17(11), 565–573. 10.1016/j.tics.2013.09.007 [DOI] [PubMed] [Google Scholar]
- Stern, D. N. (2000). The Interpersonal World Of The Infant. Amsterdam, Netherlands: Adfo Books. [Google Scholar]
- Strathearn, L., Li, J., Fonagy, P., & Montague, P. R. (2008). What’s in a Smile? Maternal Brain Responses to Infant Facial Cues. PEDIATRICS, 122(1), 40–51. 10.1542/peds.2007-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube, T., Mentzel, H.-J., & Miltner, W. H. R. (2005). Common and Distinct Brain Activation to Threat and Safety Signals in Social Phobia. Neuropsychobiology, 52(3), 163–168. 10.1159/000087987 [DOI] [PubMed] [Google Scholar]
- Sui, J., Rotshtein, P., & Humphreys, G. W. (2013). Coupling social attention to the self forms a network for personal significance. Proceedings of the National Academy of Sciences, 110(19), 7607–7612. 10.1073/pnas.1221862110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, J., Chechlacz, M., & Humphreys, G. W. (2012). Dividing the self: Distinct neural substrates of task-based and automatic self-prioritization after brain damage. Cognition, 122(2), 150–162. 10.1016/j.cognition.2011.10.008 [DOI] [PubMed] [Google Scholar]
- Sui, J., & Humphreys, G. W. (2015). The Integrative Self: How Self-Reference Integrates Perception and Memory. Trends in Cognitive Sciences, 19(12), 719–728. 10.1016/j.tics.2015.08.015 [DOI] [PubMed] [Google Scholar]
- Sui, J., Sun, Y., Peng, K., & Humphreys, G. W. (2014). The automatic and the expected self: separating self-and familiarity biases effects by manipulating stimulus probability. Attention, Perception, & Psychophysics, 76(4), 1176–1184. 10.3758/s13414-014-0631-5 [DOI] [PubMed] [Google Scholar]
- Swain, J. E., Lorberbaum, J. P., Kose, S., & Strathearn, L. (2007). Brain basis of early parent?infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry, 48(3–4), 262–287. 10.1111/j.1469-7610.2007.01731.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarthen, C. (2001). Intrinsic motives for companionship in understanding: their origin, development, and significance for infant mental health. Infant Ment. Health J. 22, 95–131. 10.1002/1097-0355(200101/04)22:1<95::aid-imhj4>3.0.co;2-6 [Google Scholar]
- Trevarthen, C., & Aitken, K. J. (2001). Infant intersubjectivity: research, theory, and clinical applications. J. Child Psychol. Psychiatry 42, 3–48. 10.1017/s0021963001006552 [PubMed] [Google Scholar]
- Tronick, E. (2007). The Neurobehavioral and Social-emotional Development of Infants and Children. New York, NY, United States of America: W. W. Norton & Company. [Google Scholar]
- Voegler, R., Peterburs, J., Lemke, H., Ocklenburg, S., Liepelt, R., & Straube, T. (2018). Electrophysiological correlates of performance monitoring under social observation in patients with social anxiety disorder and healthy controls. Biological Psychology, 132, 71–80. 10.1016/j.biopsycho.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Weinberg, A., & Hajcak, G. (2010). Electrocortical evidence for vigilance-avoidance in Generalized Anxiety Disorder. Psychophysiology, 48(6), 842–851. 10.1111/j.1469-8986.2010.01149.x [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli, S., Moran, J. M., Nieto-Castañón, A., Triantafyllou, C., Saxe, R., & Gabrieli, J. D. E. (2011). Associations and dissociations between default and self-reference networks in the human brain. NeuroImage, 55(1), 225–232. 10.1016/j.neuroimage.2010.11.048 [DOI] [PubMed] [Google Scholar]
- Wiebking, C., Duncan, N. W., Tiret, B., Hayes, D. J., Marjaǹska, M., Doyon, J., … Northoff, G. (2014). GABA in the insula — a predictor of the neural response to interoceptive awareness. NeuroImage, 86, 10–18. 10.1016/j.neuroimage.2013.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebking, C., & Northoff, G. (2014). Interoceptive Awareness and the Insula – Application of Neuroimaging Techniques in Psychotherapy. GSTF Journal of Psychology, 1(1). 10.5176/2345-7872_1.1.8 [DOI] [Google Scholar]
- Wolff, A., Yao, L., Gomez-Pilar, J., Shoaran, M., Jiang, N., & Northoff, G. (2019). Neural variability quenching during decision-making: Neural individuality and its prestimulus complexity. NeuroImage, 192, 1–14. 10.1016/j.neuroimage.2019.02.070 [DOI] [PubMed] [Google Scholar]
- Xu, J., Van Dam, N. T., Feng, C., Luo, Y., Ai, H., Gu, R., & Xu, P. (2019). Anxious brain networks: A coordinate-based activation likelihood estimation meta-analysis of resting-state functional connectivity studies in anxiety. Neuroscience & Biobehavioral Reviews, 96, 21–30. 10.1016/j.neubiorev.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Yankouskaya, A., Bührle, R., Lugt, E., Stolte, M., & Sui, J. (2018). Intertwining personal and reward relevance: evidence from the drift-diffusion model. Psychological Research, 84(1), 32–50. 10.1007/s00426-018-0979-6 [DOI] [PubMed] [Google Scholar]
- Zhu, Y. (2004). Neuroimaging studies of self-reflection. Progress in Natural Science, 14(4), 296–302. 10.1080/10020070412331343511 [DOI] [Google Scholar]