Abstract
Advancement in both bioengineering and cell biology of the liver led to the establishment of the first-generation humanized liver chimeric mouse (HLCM) model in 2001. The HLCM system was initially developed to satisfy the necessity for a convenient and physiologically representative small animal model for studies of hepatitis B virus and hepatitis C virus infection. Over the last two decades, the HLCM system has substantially evolved in quality, production capacity, and utility, thereby growing its versatility beyond the study of viral hepatitis. Hence, it has been increasingly employed for a variety of applications including, but not limited to, the investigation of drug metabolism and pharmacokinetics and stem cell biology. To date, more than a dozen distinctive HLCM systems have been established, and each model system has similarities as well as unique characteristics, which are often perplexing for end-users. Thus, this review aims to summarize the history, evolution, advantages, and pitfalls of each model system with the goal of providing comprehensive information that is necessary for researchers to implement the ideal HLCM system for their purposes. Furthermore, this review article summarizes the contribution of HLCM and its derivatives to our mechanistic understanding of various human liver diseases, its potential for novel applications, and its current limitations.
Keywords: humanized liver chimeric mouse, human hepatocyte, liver diseases, liver biology, bioengineering
The liver consists of six different cell types with hepatocytes as the predominant and sole parenchymal cell type, occupying up to 80% of the organ volume and 60% of the total liver cell count.1 The hepatocyte plays a central role in regulating a broad spectrum of critical functions required for the maintenance of homeostatic body conditions. Hepatocytes govern the catabolism, anabolism, storage, and systemic distribution of nutrients.2,3 Moreover, hepatocytes facilitate toxin detoxification, and xenobiotics metabolism. In addition, products biosynthesized in hepatocytes participate in the regulation of cardiovascular, digestive, endocrinological, hematological, immune, neurological, respiratory, and skeletal muscle systems. For example, proteins synthesized in hepatocytes, such as albumin and complement, are required for the maintenance of optimal plasma oncotic pressure and the immune system’s innate ability to defend against bacterial infections. Furthermore, lipoprotein particles secreted by hepatocytes not only distribute critical energy sources to peripheral organs, but also play a role in the antibacterial defense program.4 Similarly, cholesterol metabolism in hepatocytes results in the production of bile acids, important products for exogenous lipophilic nutrient absorption.
Given the essential roles of hepatocytes in the maintenance of systemic homeostasis, the functional impairment of or substantial reduction in number of hepatocytes results in the manifestation of pathological changes across multiple functional systems. For instance, the insufficient production of albumin, angiotensinogen, coagulation factors, and deficiency in ammonia metabolism lead to the development of anasarca, hypotension, bleeding disorders, and hepatic encephalopathy, respectively. Therefore, furthering our understanding of hepatocyte biology and pathology is essential for learning how the liver contributes to the maintenance of homeostasis in multiple organs and functional systems and systemic manifestations of liver diseases.
Historically, rodents and their derivatives, such as freshly isolated primary hepatocytes, had been considered the gold standard research tool of human liver biology. Alternatively, hepatoma or immortalized hepatocyte cell lines had satisfied the demands from researchers to a certain extent. However, reliance on these tools is coupled with numerous limitations and pitfalls due to interspecies difference between humans and rodents or the lack of genuine hepatocytic function in cancerous cells. Consequently, demand for a research tool that facilitates the study of bona fide human hepatocyte function arose, leading to the establishment of the humanized liver chimeric mouse (HLCM) system by two independent investigators in 2001.5,6 Since then, the HLCM system has greatly expanded its utility due to evolutions in its stability, quality, and producibility. To date, more than a dozen distinct HLCM systems have been developed5-20 (►Table 1). Each HLCM system has numerous similarities with and differences from each other that obscure researchers in determining which model system to employ.
Table 1.
The establishment and evolution of HLCM systems
| Year | Mechanism of liver toxicity |
Type of immunodeficiency |
Evolution and unique features | Ref. | ||
|---|---|---|---|---|---|---|
| Transgene promoter |
Responsible gene |
|||||
| 1 | 2001 | Alb | uPA tg | Rag2−/− | One of the two original HLCM models. | 5 |
| 2 | 2001 | Alb | uPA tg | SCID-beige | One of the two original HLCM models. | 6 |
| 3 | 2004 | Alb | uPA tg | SCID | Another uPA-based HLCM with improved RI compared with models #1 and 2. | 7 |
| 4a | 2007 | – | Fah−/− | Rag2−/−/IL2rg−/− | The original model of FAH−/− based model, which had several advantages over earlier uPA-based models, such as the lack of extrahepatic organ injury and inducible liver toxicity. However, the overall RI remained comparable to previous models. This system is one of the current major HLCM systems. | 8,9 |
| 5 | 2008 | Alb | uPA tg | NOG | The 2nd generation uPA-based HLCM. A modification in the transgene expression mechanism from 5× minigene (used in earlier models) to 1 copy of cDNA alleviated the risk of extrahepatic organ injury, female infertility, and also prevented the reversion of transgene. | 10 |
| 6 | 2010 | Alb | uPA tg | Rag2−/−/IL2rg−/− | uPA-based model with higher degree of immunodeficiency, which demonstrated an improvement of overall RI compared with model #2. | 11 |
| 7 | 2011 | Alb | FC8 tg | Rag2−/−/IL2rg−/− | Alb-FC8 transgenic system induces liver toxicity by the activation of caspase 8 pathway upon administration of AP20187. Notably, this system was used for the first time for the reconstitution of human adaptive immune system in HLCM, referred to as dual humanized mouse. | 12 |
| 8a | 2011 | Alb | HSV-TK tg | NOG | One of the three current mainstay HLCM systems, comprised of inducible liver toxicity and the greatest degree of immunodeficiency. The overall RI demonstrated an incremental improvement compared with earlier models of uPA-based HLCM. | 13 |
| 9 | 2011 | – | Fah−/− | NOD/SCID | This version of Fah−/− based system improved the longevity of host mice in comparison with model #4. | 14 |
| 10 | 2013 | MUP | uPA tg | SCID-beige | MUP promoter driven gene expression partially alleviated extrahepatic organ injuries of uPA tg. | 15 |
| 11 | 2014 | – | Fah−/− | NOD/Rag2−/−/IL2rg−/− | A modified version of model #4 with a higher order of immunodeficiency, which was employed as a platform for the establishment of dual humanized mice. | 299 |
| 12 | 2014 | MUP | uPA tg | Rag2−/−/IL2rg−/− | MUP-uPA system with a much higher order of immunodeficiency without notable improvement in the RI. | 16 |
| 13 | 2015 | Alb | uPA tg | Rag2−/−/IL2rg−/−NOD.sirpa | HLCM with the greatest degree of immunodeficiency was utilized for the establishment of dual humanization system. | 300 |
| 14 | 2015 | Alb | uPA tg | SCID | uPA-SCID HCLM system established with a host mouse lacking an entire CYP3A locus, can be used for certain types of DMPK studies. | 17 |
| 15 a | 2015 | Alb | uPA tg | SCID | One of the three major HLCM systems, which offers consistently high RI and absence of extrahepatic organ damage. | 18 |
| 16 | 2017 | – | Fah−/− | Rag2−/−/IL2rg−/− | Fah−/− based system with host mice lacking NADPH-P450 oxidoreductase, which eliminates the involvement of murine CYPs in DMPK. | 20 |
Abbreviations: Alb, albumin enhancer promoter; CYP, cytochrome P450; DMPK, drug metabolism and pharmacokinetics; Fah, fumarylacetoacetate hydrolase; FC8, FK506-Caspase8; HLCM, humanized liver chimeric mouse; HSV-TK, Herpes simplex virus type 1 thymidine kinase; IL2rg, interleukin 2 receptor subunit gamma; MUP, major urinary protein promoter; NADPH, nicotinamide adenine dinucleotide phosphate; NOD, nonobese diabetic; NOG, NOD/Shi-SCID/IL2rg−/−; RI, replacement index; Rag2, recombination activating 2; SCID, severe combined immunodeficiency; sirpa, signal regulatory protein α; uPA, urokinase type plasminogen activator.
Three major HCLM systems described in ►Table 2 in detail.
Therefore, this comprehensive review is designed to aid end users by providing necessary information that is required for the proper implementation and utilization of this powerful research tool for their respective research activities. To achieve this goal, the first half of this article summarizes the fundamental basis of HLCM by covering the history of its evolution, the similarities, and unique characteristics of each system, and factors influencing the outcome and interpretation of research outcomes. Furthermore, this article will discuss its tremendous versatility in the study of homeostatic liver function and pathophysiology of liver diseases along with our perspectives on its potential utility in health care.
The Prologue: Historical Background of the HCLM
The fundamental concept of HLCM involves the proliferation of xenotransplanted human hepatocytes in the liver of host mice to replace endogenous hepatocytes, which is facilitated by a combination of an immunodeficiency and a mechanism of induced toxicity to endogenous hepatocytes. This concept potentially originated from the well-documented proliferative potential of rodent hepatocytes in vitro,21 which led to the successful allo- and xenotransplantation of rodent hepatocytes into immunodeficient mice harboring toxic gene expression in endogenous hepatocyte.22,23
In contrast, the poor colony-forming potential of primary human hepatocytes (PHH) in vitro led to perceived challenges in inducing their proliferation in the liver of HLCM.24 Surprisingly, the unique in vivo environment of HLCM was capable of triggering robust proliferation of xenotransplanted PHH, resulting in the establishment of the first generation HLCM in 20015,6 and catapulting it to its current state as an indispensable research tool for the study of liver biology and diseases.
Required Components for the Establishment of HLCM
In principle, the establishment of HLCM requires a high-quality xenograft, such as highly viable cryopreserved or freshly isolated PHH. The quality of PHH can be assessed by its viability, morphology, culture dish platability, and synthetic/metabolic capacity, along with the age/disease status of the donor.25 In general, PHH with at least 70% viability, and preferably from a younger donor, is recommended for the production of a high-quality HLCM based on our experience and observation by others.26
A host mouse of HLCM requires to have both: (1) a lack of immunity, and (2) a mechanism of induced cytotoxicity in endogenous hepatocytes to create the space for the proliferation of transplanted PHH within the liver of the host mouse (►Tables 1 and 2). Of note, the first report of successful engraftment of rat hepatocytes was performed using nude mice harboring hepatocyte-specific expression of urokinase-type plasminogen activator (uPA) transgene.23 The lack of FOXN1 gene in nude mouse impairs the development of thymus and thus predominantly abrogates T-cell immunity.27 Though the degree of immunodeficiency in nude mice appears adequate to accommodate the xenograft, it has been considered suboptimal due to extrathymic T-cell development with age, and the preservation of B-cell immunity. Therefore, mouse models with a mutation in Prkdc (protein kinase, DNA-activated, catalytic subunit), referred to as severe combined immunodeficiency (SCID: PrkdcSCID), or higher-order, multigenic immunodeficiency such as Rag2−/−/IL2rg−/−, Rag2−/−/IL2rg−/−/nonobese diabetic (NOD), and NOG (NOD/Shi-SCID/IL2rg−/−) have been employed as platforms for the establishment of HLCM (►Tables 1 and 2).
Table 2.
Comparison of three major HLCM models
| Models | ||||
|---|---|---|---|---|
| uPA-SCID18 | FRG8,9 | TK-NOG13 | ||
| Immune deficiency77 | Responsible gene | SCID (Prkdc gene mutation) | Rag2−/−/IL2rg−/− | NOG (IL2rg−/−/NOD/Shi-SCID) |
| Innate immunity | Intact | Lack of NK cells | Lack of NK cells, dysfunctional dendric cell and macrophage, reduced complement activity. | |
| Adaptive immunity | Impaired T/B cell maturation | Complete inhibition of T/B cell maturation. | Complete inhibition of T/B cell maturation. | |
| Pros and cons |
|
|
|
|
| Hepatotoxicity | Responsible gene | uPA tg | Fah−/− | HSV-TK tg |
| Induction required | No | Yes (NTBC withdrawal)8,9 | Yes (ganciclovir administration)13 | |
| Maintenance required | No | Yes (Frequent adjustment of NTBC treatment based on the animal condition is required until it achieves a high RI)100 | No | |
| Pros and cons |
|
|
||
Abbreviations: FAH, fumarylacetoacetate hydrolase; HGF, hepatocyte growth factor; HLCM, humanized liver chimeric mouse; HSV-TK, herpes simplex virus thymidine kinase; Il2rg, common gamma chain of the interleukin 2 receptor; NK, natural killer; NOD, nonobese diabetic; NOG, nonobese diabetic/Shi-SCID Il2rg(KO); NTBC, 2-(2-nitro-4-trifluoro-methylbenzoyl)-1,3-cyclohexanedione; Rag2, recombination activating gene 2; RI, replacement index; SCID, severe combined immunodeficiency; uPA, urokinase type plasminogen activator.
In brief, both PrkdcSCID and Rag are involved in V(D)J recombination, which is required for the rearrangement of the antigen-binding region of antibodies and T-cell receptors during B- and T-cell development, respectively.28 Thus, the loss-of-function mutation or genetic deletion of these genes leads to impairment of T- and B-cell immunity while preserving innate immunity governed by complement, macrophage, dendritic cells, and natural killer (NK) cells. The deletion of interleukin-2 receptor subunit gamma (IL-2rg) further augments the degree of immunodeficiency as IL-2 plays a critical role in the activation of T, B, and NK cells.29 The NOD background abrogates NK cell activity, complement system, and antigen-presenting cells such as macrophages and dendritic cells.30-32 To date, SCID and other higher-order, multigenic immunodeficiency models serve as mainstay host mice for the production of HLCM (►Table 2).
Regarding the induction of cytotoxicity in endogenous hepatocytes, a few distinct mechanisms have been utilized such as transgenic uPA, herpes simplex virus type 1 thymidine kinase (HSV-TK) overexpression, and the genetic deletion of fumarylacetoacetate (FAA) hydrolase (Fah)13,33-35 (►Table 2). The overexpression of uPA, a type of serine protease, under albumin-promoter constitutively causes the hepatotoxicity, presumably by nonspecific proteolytic effects. In contrast, HSV-TK transgene itself does not autonomously lead to cytotoxicity. Upon administration of ganciclovir, HSV-TK promotes the production of its triphosphate metabolite, an acyclic deoxyguanosine analog, which induces the cell toxicity by inhibition of deoxyribonucleic acid (DNA) synthesis.36 Lastly, the genetic deletion of Fah, a gene responsible for the development of hypertyrosinemia, induces the death of hepatocytes by accumulating toxic tyrosine metabolite intermediates, such as maleylacetoacetate and FAA.35 With this mechanism, it constantly requires administering nitisi-none(NTBC; 2-[2-nitro-4-trifluoro-methylbenzoyl]-1,3-cyclohexanedione), an inhibitor of the tyrosine metabolic pathway, to prevent embryonic lethality and to maintain animal viability. There might be unique advantages and disadvantages associated with each mechanism, which will be discussed in the following sections.
The Quality Control of HLCM
In general, the quality of HLCM is determined by the degree of endogenous hepatocyte replacement with xenotransplanted PHH within the liver of the host mouse. Conventionally, the degree of replacement can be estimated by the area occupied by proliferating PHH over the total area of host mouse liver using representative histology sections (►Fig. 1). Notably, the median cell volume of mouse hepatocytes is approximately 8,000 μm3, which is twice as large as that of human hepatocytes; therefore, the actual cell number-based replacement is substantially higher than the dimensional replacement.37,38 Hereafter, the dimension-based estimation of the replacement rate is referred to as the replacement index (RI), the most critical determinant of overall quality of HLCM. In addition, human albumin (hALB) and α1-antitrypsin (hAAT) levels in the blood or serum of HLCM have strong positive correlations to RI regardless of the model systems as evidenced by the correlation coefficient (r) of 0.88 to 0.95 between hALB and RI.13,18,37 Thus, hALB and hAAT level are well-accepted surrogates for determining RI, thereby serving as alternative indicators for HLCM quality (►Fig. 2).
Fig. 1.
Immunohistology of the liver of a high-RI HLCM. A representative liver section of a high-RI HLCM (RI: 91.2%) was subjected to immunohistochemical staining using STEM 121 antibody for the detection of engrafted human hepatocytes. The area stained in brown and unstained foci show xenografted human hepatocytes (H) and residual murine hepatocytes (M), respectively. The RI was determined by the proportion of area occupied by xenograft to the total area of the liver section. CV, central vein; HLCM, humanized liver chimeric mouse; RI, replacement index; PV, portal vein.
Fig. 2.
Correlation of RI and blood human albumin level. The scatter plot demonstrates the correlation between the detention-based RI and the blood human albumin (hALB) level of each uPA-SCID HLCM (n = 91) at 70 to 105 days after cell transplant surgery. The dots represent individual animals and the thick black line represents the approximation curve. The black lines indicate RI of 70% and its corresponding blood hALB (Approximately 7 mg/mL). The data shown is a reanalysis of the data used in our published work.18 HLCM, humanized liver chimeric mouse; RI, replacement index; SCID, severe combined immunodeficiency; uPA, urokinase-type plasminogen activator.
Factors Contributing to the Quality of HLCM
As discussed in the previous section, the most critical determinant of overall HLCM quality is the degree of RI. To date, more than a dozen HLCM systems have been developed by numerous investigators (►Table 1). Of those, uPA-SCID, FAH−/−Rag2−/−IL2rg−/− (hereafter FRG), and HSV-TK-NOG (hereafter TK-NOG) are the current mainstay HLCM systems (►Table 2). These three model systems are commercially available, and by far the most commonly referenced in publications. Consequently, there is copious publicly available information that can be used to assess the overall quality of HLCM based on the type of liver toxicity and immunodeficiency. A comparison of the RI in the three major HLCM models demonstrated a substantial variability depending on the model system. The average and median RI ranges from 42% in FRG and TK-NOG to 80% in uPA-SCID.13,18,37 This variability might be attributed to the mechanism and degree of immunodeficiency, hepatotoxicity, or the combination of both.
Of important note, the comparison of two distinct uPA-SCID–based HLCM systems suggests that the degree of hepatotoxicity, but not the mechanism, might be the crucial determinant of RI.18 The intoxicated hepatocytes of the host mice are required to preserve enough functionality to sustain life until the host mice mature enough to tolerate surgical manipulation for xenograft transplantation. However, the hepatocytes of the host mice should still surrender in the competition over space, allowing the xenograft to robustly proliferate and achieve a high RI. For example, the first generation uPA transgenic mouse comprises five-copies of uPA mini-genes.34 This animal suffers from extrahepatic organ impairment due to the overwhelming degree of uPA activity as well as the re-growth of murine hepatocytes as a result of the reversion of transgene via homologous recombination.33 In contrast, the current version of uPA-transgenic mouse, carrying one copy of cDNA, exhibits a stable and appropriate degree of hepatotoxicity suitable to achieve a high RI.18 These observations strongly indicate that the copy number of the transgene and the loci of the toxic-gene insertion likely dictate the aforementioned fine-tuned balance between life sustainability and competition for space.18,34
There might be some advantages and disadvantages unique to each type of hepatotoxicity that contribute to the differential degree of RI. For example, the overexpression of uPA not only exhibits cytotoxicity in endogenous hepatocytes, but also enhances the growth of PHH through the activation of hepatocyte growth factor (HGF) signaling pathway. This effect is mediated by its proteolytic conversion of plasminogen to plasmin. Plasmin then digests promatrix metalloproteinases (Pro-MMP) to MMP, which mobilizes pro-HGF trapped on extracellular matrix.39 Furthermore, the high homology of the cleavage sites of pro-HGF and plasminogen allows uPA to facilitate the conversion of pro-HGF to its active form, HGF.40,41 This is demonstrated by the significantly compromised regenerative capability of hepatocytes in uPA knockout (KO) mice.42 Of important note, murine HGF is capable of activating human HGF-receptor, mesenchymal-epithelial transition receptor (c-MET),43 indicating that the uPA-HGF axis is viable and likely contributes to the robust proliferation of PHH in the liver of HLCM.
In contrast, two other mechanisms of hepatotoxicity might be coupled with some disadvantages. In the case of FRG-based HLCM, the tyrosine toxicity in the Fah−/− hepatocyte would be alleviated by the xenotransplanted PHH as it exhibits normal FAH activity.8 Consequently, the endogenous hepatocytes likely regain their viability, thereby potentially competing with the xenograft over space. To alleviate this fundamental disadvantage, an additional mechanism of hepatotoxicity, such as adeno virus-mediated overexpression of uPA gene, might be required to achieve a high RI.8
Similarly, the two-time administration of ganciclovir in TK-NOG mice system likely allows the residual mouse hepatocytes to regain their proliferation potential over time.13 Theoretically, the potential competition between the HSV-TK expressing endogenous hepatocytes and proliferating PHH can be remediated by the repetitive administration of ganciclovir. However, it may lead to the development of drug-induced toxicity to the PHH.44
Regarding the significance of the type of immunodeficiency in determining the RI, the higher-order, multigenic immunodeficiency models might be advantageous over SCID due to the absence of NK cells activity (►Table 2). NK cells play a significant role in both allo- and xenograft rejection via direct cytotoxicity or antibody-mediated rejection.45,46 This notion holds true in the case of HLCM as the intrahepatic activation of NK cells prohibits efficient engraftment and proliferation of human hepatocytes.8,11 Thus, immunodeficiency systems lacking NK cell activity, such as Rag2−/−/IL2rg−/− and NOG, could be advantageous as a platform for the establishment of HLCM. However, this particular disadvantage is remedied by the one-time injection of anti-NK cell antibodies when utilizing a system with residual NK cell activity such as SCID.7,11 Although the rationale behind the requirement of only a temporal depletion remains elusive, it is likely that its constitutive activation renders the induction of NK cell tolerance.47,48
Overall, evidence suggests that the type of immunodeficiency and hepatotoxicity would both influence the degree of RI; however, cross-sectional, single-variable comparison of these factors is required to define the predominant factors influencing and the combination most suitable for the production of HLCM.
Recreation of Human Liver Physiology at the Organ System Level in HLCM
The value of HLCM lies in its ability to represent the normal physiology of the human liver, requiring a xenograft to re-establish an appropriate histological structure, even at the ultrastructural level. A proper arrangement of hepatic cords, containing two rows of hepatocytes that are aligned along the line connecting between the portal triad and central vein allows the xenograft to emulate the histological structure of the hepatic lobules. The importance of this histological rearrangement is coupled with the concept of hepatic zonation. The clusters of hepatocyte that belong to each zonation have distinct functionalities, which lead to the functional compartmentalization of hepatocytes to support the multifaceted function of the liver.49
To date, assessment of HLCM in the context of the proper formation of hepatic zonation has largely remained elusive and controversial. For example, a study with the FRG-based system suggested that the liver of HLCM failed to form the hepatic zonation based on their mathematical assessment of the pharmacokinetics.50 In contrast, our previous study and unpublished work with uPA-SCID demonstrated that the liver of HLCM with particularly high RI has appropriate patterns of zone-dependent expression of representative cytochrome P450 (CYP) enzymes and arginase I, respectively51(►Fig. 3). The properly aligned cord of hepatocytes in the liver of high RI HLCM also allowed appropriate distribution of nonparenchymal cell types of the liver, such as Kupffer cells, liver sinusoidal endothelial cells (LSEC), and hepatic stellate cells (HSC).52 These observations provide grounds for the assumption that the liver of high RI HLCM re-establishes the proper structure of the hepatic lobule.
Fig. 3.
Re-establishment of hepatic zonation in the liver of HLCM. Immunohistochemical analysis of the liver of an extremely high RI (approximately 96%) HLCM for the detection of human Arginase I expression (A) low magnification and (B) magnified image of the corresponding boxed areas of the lower magnification. CV, central vein; HLCM, humanized liver chimeric mouse; RI, replacement index; PV, portal vein; scale bars = 200 μm.
The formation of proper ultrastructure is also essential to recapitulate the physiology of the liver. In the normal human liver tissue, two adjacent polarized hepatocytes form intercellular structures such as tight junction, gap junction, and bile canaliculi.53 Reassuringly, a high-quality transmission electron microscope (TEM) analysis of HLCM revealed that implanted human hepatocytes re-establish the intercellular structures among proliferated xenograft as well as with hepatocytes of the host mouse.52,54,55 The xenograft also establishes a physical contact with HSC via processes, namely stellate cell projection, which facilitates the synaptic interaction between these cell types.52,56,57 Furthermore, the liver of HLCM re-establishes the space of Disse, a buffering area that separates hepatocytes from the lining of LSEC where nutrients and toxins translocate from circulation through a structure called fenestra.58
Lastly, the liver of HLCM re-establishes the bile secretion machinery. As mentioned above, TEM analyses reassured the formation of bile canaliculi in between xenotransplanted hepatocyte, which then networks to the intrahepatic bile ductules, namely Canals of Herring, followed by further connections to the intrahepatic bile duct.52,59,60 Consequently, the composition of human hepatocytes-derived bile acid in the gall bladder of HLCM shows a positive correlation to the degree of RI.61 This structural and functional re-establishment of the bile excretion machinery is particularly important for the in vivo study of xenobiotic metabolism, as the physiological biliary excretion system is an essential route for the elimination of drug metabolites. Also, this mechanism allows the establishment of enterohepatic circulation of human bile salts as well as the exposure of the gut microbiome to bile secreted from human hepatocytes. The recent growth of interest in the gut microbiome and its association with the liver–intestine axis makes this aspect of the HLCM system enticing not only for the investigation of metabolic liver diseases but also for a variety of systemic diseases beyond the liver.62-64
Current Limitations and Progress in Overcoming Them
It is extremely important to recognize the limitations that are uniquely associated with the HLCM system and how these factors restrict its utility. There are two fundamental limitations: (1) the lack of an adaptive immune system, and (2) the impairment of intercell type crosstalk due to interspecies difference.
The study of viral hepatitis exemplifies the former short-coming, as the adaptive immune response of virally infected cells play a central role in the development of hepatic necroinflammation.65,66 The lack of adaptive immunity in HLCM may also pose questions about its reliability for the study of metabolic liver disease and drug-induced liver injury (DILI) in certain aspects.67-70 Fortunately, recent seminal studies have shown a framework that would alleviate this fundamental constraint by reconstituting the humanized adaptive immune system.12,71-76 This approach requires the transplantation of hematopoietic stem cells, preferably with the concurrent transplantation of bone marrow, spleen, and thymus from the same donor to re-establish the human adaptive immune system in HLCM. Then, the reconstituted adaptive immune cells undergo both positive and negative selection, thereby preventing human hepatocytes from suffering immune rejection while maintaining their capability of eliciting proper immune responses upon recognition of exogenous products such as viral pathogens. For production of dual-humanized HLCM, it is necessary to use higher order immunodeficiency models without functional NK cells and macrophages such as NOG- or NOD/Rag2−/−/IL2rg−/−-based HLCM systems (►Tables 1 and 2). Unless these immunodeficiency mechanisms are employed, murine NK cells prevent the engraftment of xenotransplanted human hematopoietic cells due to the absence of inhibitory signals that result from major histocompatibility complex (MHC) mismatch.77-82 Similarly, signal regulatory protein α expressed on the cell surface of murine macrophages is incapable of recognizing human CD47, the “don’t eat me” signal, resulting in xenograft rejection by enhancing phagocytosis.83,84 This mechanism plays a particularly significant role in the immune rejection of blood cells such as hematopoietic stem cells.85,86 Accordingly, SCID- and NOD-based HLCM systems are not suitable for the establishment of dual humanized HLCM as residual NK cells and macrophages elicit innate immune rejection of xenografted human hematopoietic cells.87,88 Although the dual-humanization is a resource and labor-intensive approach, this modification has greatly improved the versatility of the HLCM system. Accordingly, several groups have already applied the immune-reconstituted HLCM system for the study of HBV and HCV infection,12,72,73,89 which exhibited a broad spectrum of immunopathogenic phenotypes.
Another major obstacle of HLCM is paucity of intercell type crosstalk between xenotransplanted hepatocytes and nonparenchymal cells of the liver, or perhaps cells in distant organs. For example, the transplanted human hepatocytes lack response to murine FGF15 (homolog to hFGF19).90 In a physiological setting, the reabsorbed bile acid in enterocytes at the terminal ileum promotes the secretion of FGF15. Then secreted FGF15, FGF19 in the case of humans, suppresses the expression of CYP7A1 in hepatocytes, thereby reducing the biogenesis of bile acid.91 Thus, the disruption of this critical regulator of enterohepatic circulation in HLCM renders an abnormally high serum bile acid level.90,92,93 Accordingly, the impairment of bile acid homeostasis was alleviated in HLCM by expression of transgenic hFGF19.92 However, this remedy is case-specific, and other examples that emphasize this obstacle have been documented, such as the irresponsiveness of xenografted human hepatocytes to murine growth hormone, resulting in the deregulation of lipid homeostasis.94,95 As such, users of the HLCM system should be aware of potential undiscovered miscommunications of humoral factors.
Selection of Appropriate HLCM System for In Vivo Studies; the RI Matters
The decision for the employment of HLCM for any research activity should be determined based on considerations of the balance between the advantages and disadvantages, including the aforementioned fundamental limitations. Then, it is extremely important to cautiously select the most suitable HLCM system for each experimental application. Although the selection of an appropriate system could be case-by-case depending on the specific scientific question addressed, the general recommendation is to employ a HLCM with as high RI as possible to avoid misinterpretation of experimental results due to compensation of liver function by residual mouse hepatocytes. The benefits of utilizing a high RI HLCM are several fold.
For example, HLCM with extremely high RI is particularly essential for the study of drug metabolism and pharmacokinetics (DMPK). Given the multifaceted, complex nature of the drug metabolic pathway, which will be discussed in the following DMPK section in great detail, the presence of residual mouse hepatocyte in low RI animal will have a confounding impact on the xenobiotics metabolism due to interspecies difference.96 Indeed, the administration of Debrisoquine, which is a specific substrate of human CYP2D6, resulted in the generation of its metabolite, 4′-hydroxydebrisoquin (4-OH DB), in a RI-dependent manner.97
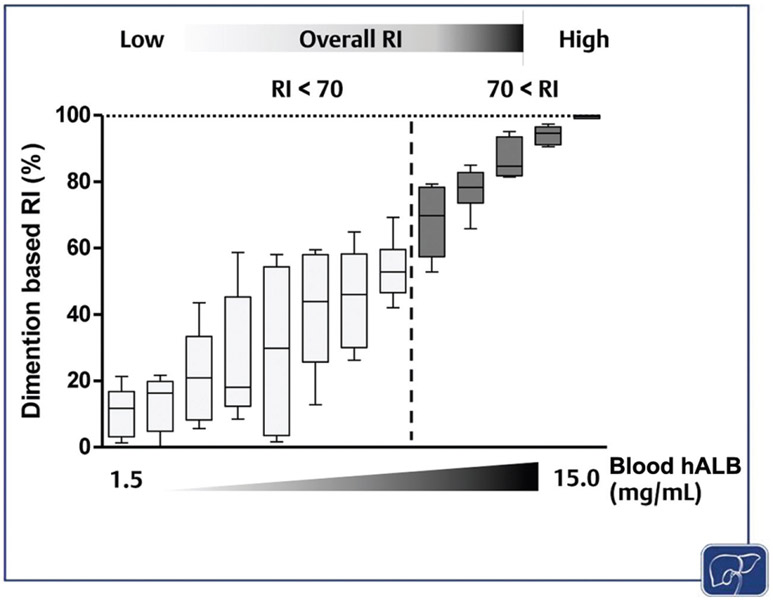
In addition, human hepatocytes in high RI HLCM demonstrate characteristics of the terminally-differentiated hepatocyte, thereby recapitulating the homeostatic cell biology of hepatocytes in the human liver; however, proliferating human hepatocyte colonies in the liver of low-RI mice exhibits an inadequate degree of maturation.98,99 Furthermore, our unpublished study revealed that HLCM with higher RI (RI over 70%) would have much less variability in the rate of replacement within and among the hepatic lobes (►Fig. 4). Thus, experiments using low-RI HLCM will have a great deal of sampling error due to compositional heterogeneity in both quality and quantity of transplanted human hepatocytes, and thus a higher risk of data misrepresentation. Therefore, a high-RI, at least over 70%, is recommended for any applications.
Fig. 4.
The correlation between the overall RI and the RI variability among seven hepatic lobes of HLCM. The dimension-based RI of each of the seven hepatic lobes were individually determined to calculate the RI variability between lobes for each uPA-SCID-based HLCM (n = 13). Then, the RI of each lobe was normalized by the volume of the corresponding lobe followed by the determination of overall RI of each HLCM (top). Lines at the extremes represent the lowest and the highest RI and the box represents interquartile range of RI among lobes in each animal. The black horizontal line in each box indicates median RI among seven hepatic lobes. The range of blood hALB level is shown in x-axis. hALB, human albumin; HLCM, humanized liver chimeric mouse; RI, replacement index; SCID, severe combined immunodeficiency; uPA, urokinase-type plasminogen activator.
There are a few additional important criteria that users should take into consideration. These include the postsurgical survival rate, high-precision production capacity of high-RI HLCM, and ease of handling. These factors are critical to optimally utilize HLCM, especially when a large number of high-quality animals are necessary. Notably, it takes at least 8 weeks, perhaps much longer, for the xenograft to complete the proliferation phase and reach the plateau.18,100 Thus, the long-term survival rate after cell transplant surgery serves as one of the determinants of the capacity of a stable supply of HLCM. For example, the 6-week posttransplantation survival rates of uPA-SCID-based HLCM span a wide range, from 54 to 100%,18,101 indicating that the mass production capacity varies significantly, even among the same model system depending on the manufacturer. This disparity is most likely due to the difference in technical expertise and the quality of the mouse maintenance facilities. Currently, there are no cross-sectional studies that compare the long-term postsurgery survival rates of high RI animals of each HLCM production facility.
The Spinoff of the HLCM System; the HLCM-Derived Human Hepatocytes
The liver has a great regenerative capacity in response to surgical resection or liver injury for the maintenance of organ volume and function.102 The substantial regenerative capability of the liver is largely due to the self-renewal and proliferation potential of hepatocytes. In the case of HLCM production, implanted human hepatocytes duplicate at least ten times. Of note, this process is not associated with the expression of alfa-fetoprotein, a marker gene of hepatocyte progenitors.103 This indicates that the liver of HLCM leads terminally-differentiated human hepatocytes to enter the cell cycle without undergoing dedifferentiation into liver progenitor cells.
Utilizing the liver of HLCM as organ scaffold for the induction of robust proliferation of hepatocytes has enabled mass-production of high-quality human hepatocytes without compromising the degree of differentiation.8 With this approach, a single tube containing 5 × 106 of commercially-available cryopreserved human hepatocytes would support the production of approximately 600 HLCM after two rounds of serial expansion. In our experience, one HLCM with RI of over 90% usually yields approximately 2 × 108 human hepatocytes with an average viability of 85%. This means a vial of cryopreserved hepatocytes could be expanded to approximately 1 × 1011 viable cells upon two rounds of serial expansion. Of important note, the proliferation of somatic cells, such as matured hepatocytes, is associated with a shortening of telomere that eventually leads to the induction of cellular senescence.104,105 Accordingly, our internal data indicated that hepatocytes extracted from juvenile donors appear much superior as they are equipped with longer telomeres, thus having a higher Hayflick limit. Nevertheless, this technical breakthrough enables the mass amplification of genetically-identical human hepatocytes and permits the use of HLCM as a source of human hepatocytes for in vitro applications.8
Freshly isolated HLCM-derived human hepatocytes generally offer superior cell viability, platability, and preservation of hepatocyte function when compared with commercially available cryopreserved PHH. We defined platability as the rate of viable cell attachment to the culture dish. It is important to note that the viability, which is usually determined by trypan blue staining, does not serve as an indicator of the “platability.” The viability and platability of HLCM-derived human hepatocytes are consistently over 80%, which allow users to plate the cells at desired confluency. This is extremely important because hepatocytes cultured at optimal confluency appropriately polarize through the formation of tight junctions and bile canaliculi with neighboring cells, thereby preventing the cells from undergoing epithelial to mesenchymal transition (EMT) and thus sustaining precise morphology and functionality over a long-term culture.106-108 In contrast, these factors, especially the platability and the degree of hepatocyte function preservation of commercially available cryopreserved PHH, are highly variable.
Of another important note, the stable, long-term culture of freshly isolated primary rodent hepatocytes has been conceived impossible as it undertakes rapid EMT soon after plating into culture dishes. Although the reason why only human hepatocytes, especially HLCM-derived human hepatocytes, tolerate long-term culture without undergoing EMT remains entirely elusive, this is another advantage of the HLCM system as it enables a variety of in vitro studies with bona fide characteristics of primary hepatocytes.
Accordingly, HLCM serves as the source to provide consistently high-quality human hepatocytes at much lower cost compared with commercially available cryopreserved PHH. HLCM-derived human hepatocyte has been heavily utilized in a broad spectrum of in vitro experimental applications such as, but not limited to the studies of hepatitis virus infection, DMPK, and regenerative biology.106,107,109-111 In addition, HLCM-derived human hepatocyte could be applied to the development of microfabricated in vitro culture systems that mimic the cellular environment of tissue and organ. These include co-culture nonparenchymal cells of the liver, 3D organoid culture, and organ-on a-chip system in conjunction with microfluid circulatory system. These culture methods that miniaturize the environment of liver tissue have been shown to greatly enhance the functionality of hepatocytes when compared with that of the conventional 2D culture condition.112,113 However, the success rate in the establishment of organ-mimicking culture system with cryopreserved fleshly isolated PHH is unsatisfactory as up to 60% of attempts fail, largely owing to the inconsistent viability and platability.112 In contrast, our internal unpublished observations as well as by others with freshly isolated HLCM-derived human hepatocytes exhibited its promising potential in the establishment of microfabricated culture systems along with the significant utilities for the study of metabolic liver diseases and DMPK.108
Taken together, emerging shreds of evidence indicate the substantial advantage of HLCM-derived human hepatocytes over conventional PHH. Numerous investigators have already utilized it for the study of a broad spectrum of liver biology and diseases, which will be further discussed in the following sections.
Application of HLCM System for the Study of Human Liver Biology and Diseases
The HLCM system has tremendously contributed to furthering our understanding of the pathophysiology underlying human liver diseases, especially in the field of viral hepatitis. Moreover, this system has been a gold standard for preclinical studies of DMPK. In contrast, it has not established a position for the study of metabolic liver diseases. This section will discuss the current utility, advantages, pitfalls, and future perspectives of the HLCM system for the study of each liver disease. Also, the application of HLCM derivatives, such as human hepatocytes extracted from the liver of HLCM as well as the host mice of HLCM, as experimental tools will be discussed in each clinical and scientific discipline.
Infectious Diseases
In theory, any studies of human pathogens that target hepatocytes can benefit from the use of HLCM systems. Among such pathogens, viral hepatitides such as hepatitis A, B, C, D, E viruses, have been the most well-studied pathogens. These pathogens belong to different viral families and have unique lifecycles, thereby causing diseases with a variety of pathophysiologic mechanisms. Furthermore, chronic viral hepatitis poses a significant threat to public health, as it is the leading cause of end-stage liver diseases worldwide.
Since the discoveries of these devastating pathogens in the 1970s to 1990s, the demand for a stable and physiological small animal model became the driving force for the development of HLCM. Indeed, the conceptual and methodological framework of HLCM was developed by investigators who specialized in the virology of HBV.114 First, these investigators developed a chimeric liver mouse model using woodchuck hepatocytes as the xenograft and then utilized them for the study of woodchuck hepatitis virus, an orthologue of HBV. Subsequently, these works led to the successful establishment of the first HLCM system in 2001.5,6 Since then, HLCM has been extensively utilized for the study of HBV and HCV infection; however, recent studies demonstrated its potential utility for the study of other pathogenic microorganisms beyond its application for the study of viral hepatitis.
Hepatitis A Virus
The annual worldwide burden of hepatitis A virus (HAV) infection is 8.8 million, which results in approximately 11,000 deaths.115 HAV is primarily transmitted through the oral/fecal route and thus is considered a disease associated with poor hygiene. As such, the incidence of acute HAV infection has remained extremely low in developed countries, and thus its significance is considered relatively low, especially after the development of a highly effective vaccine in 1995. However, the recent outbreaks of HAV infection in the United States showed an unexpectedly high severity and mortality, suggesting the resurgence of this infectious disease.116
To date, there has not been a study that employed the HLCM system for the in vivo infection study of HAV. However, HLCM has a potential advantage compared with the conventional murine model as HAV has a restricted host range only to human and nonhuman primates. The narrow host range of HAV is attributed to the capacity of the viral protein, 3ABC, to antagonize the protein involved in the type I interferon (IFN) production pathway, in infected hepatocytes.117,118 Accordingly, both PHH and HLCM-derived human hepatocytes are capable of supporting the viral lifecycle of HAV in vitro.119,120 This evidence strongly encourages us to consider employing HLCM as an in vivo study model of HAV infection.
Hepatitis B Virus
HBV infection is one of the most prevalent chronic viral infectious diseases and estimated that over 240 million people are currently infected worldwide.121 Over the course of chronic infection, up to 40% of the affected individuals will develop end-stage liver diseases, if untreated.122 Therefore, the clinical and economic burden associated with HBV infection has been one of the most significant threats to public health.
HBV has a narrow host range, and only infects humans, nonhuman primates, and northern treeshrew.123,124 The narrow host range is largely due to the species differences in the amino acid sequence of sodium taurocholate cotransporting polypeptide (NTCP), which is one of the bile acid transporters expressed on the cell surface of hepatocytes.125-127 NTCP interaction with the HBV envelope glycoprotein, Pre-S1, facilitates viral entry into hepatocytes.126 Accordingly, the overexpression of NTCP renders HepG2 cells, a hepatoblastoma cell line, susceptible to HBV infection.128,129 Accordingly, a transgenic mouse expressing human NTCP (hNTCP) also allows HBV entry into murine hepatocytes; however, it does not support the formation of a covalently closed circular DNA (cccDNA), which is the episomal viral genome required for the establishment of a stable viral lifecycle.130 Thus, the expression of hNTCP and intracellular environment of a human hepatocyte are both required for the establishment of HBV lifecycle.
Hence, HLCM is highly susceptible to HBV, to the extent that only one copy of viral inoculum is required to establish stable infection and support all stages of the HBV life cycle, including the formation of cccDNA.131-133 More importantly, the HBV infection of HLCM is representative of infection in the human liver in terms of susceptibility, the abundance of cccDNA, replication efficiency, virion stability in the systemic circulation, and response to antiviral therapy.134,135
HLCM-derived human hepatocytes also have a substantial superiority over other in vitro tools such as NTCP-expressing HepG2 cells or HepaRG cells. They retain a comparable susceptibility to HBV to that of in vivo infection of HLCM, thus only requiring an inoculum containing physiological titers whereas the aforementioned cell lines require an approximately 1,000 times higher titer to achieve the infection.128,136 Moreover, the viral replication efficiency in HLCM-derived human hepatocytes appears equivalent to that of the liver of HBV-infected HLCM or chronically infected patients.105
The complete cure for chronic HBV infection requires eradication of cccDNA. The current mainstay of anti-HBV drugs, such as tenofovir disoproxil fumarate, has a negligible effect on the elimination of cccDNA regardless of the length of antiviral therapy.137,138 Thus, a research tool that allows the mechanistic investigation of the path to cccDNA eradication plays a critical role in mitigating the burdens of HBV infection. To this end, HLCM is advantageous as the abundance of cccDNA in the liver of HBV-infected HLCM is comparable to that of the liver of an HBV-infected patient.134,139 Furthermore, the abundance of cccDNA heavily fluctuates during cell division.140 Therefore, studies addressing the regulatory mechanisms of cccDNA should be conducted with tools with absent or a minimal degree of cell division. For this reason, HLCM with extremely high RI or in vitro cultured HLCM-derived hepatocytes serve as ideal platforms when compared with other study tools.
Hepatitis C Virus
Currently, over 70 million people are living with chronic HCV infection worldwide.115 Nearly 50% of the affected individuals eventually develop end-stage liver diseases, thus chronic HCV infection is the leading indication for liver transplantation. The recent introduction of direct-acting antivirals (DAAs) has revolutionized the management of HCV infection as it enabled sustained virological response in nearly all patients.141 However, it is expected that only a fraction of patients would benefit from DAA therapy because the affected population is usually unaware of their infection status due to the insidious nature of the disease.142 Moreover, there is a sharp rise in the number of new HCV infection cases due to the opioid pandemic.143 Therefore, HCV infection likely continues to be a serious public health problem.
HCV also has an extremely narrow host range and can primarily infect primates such as chimpanzees and humans.144,145 Accordingly, the demand from HCV-virologists was also one of the driving forces for the development of HLCM6; it has since been widely employed for in vivo studies of HCV infection.146,147 HLCM efficiently supports the establishment of chronic infection of multiple HCV genotypes of clinical isolates.148-151 This is one of the major advantages of the HLCM system as other experimental tools are limited to the use of a few genotypes. For example, Huh7 cells, a hepatoma cell line established from well-differentiated hepatocellular carcinoma, is only susceptible to cell culture-adapted, but not clinical isolate, strains.152-154
There are currently a few other small animal models available for the study of HCV infection, such as transgenic mice with hepatocytes that either harbor the HCV genome or express human factors necessary for the entry of reporter virus.155,156 Though these models exhibit the signatures of viral infection and certain pathologic features the efficiency of viral replication appears to be insufficient for in vivo biological studies. For these reasons, HLCM serves as the mainstay platform for in vivo HCV infection studies.
Lastly, it is important to recognize the substantial contribution of HLCM to the development of DAAs, especially at the stage of the preclinical trial. HLCM has been employed as a reliable preclinical in vivo evaluation tool for nearly all clinically available anti-HCV agents including IFNs, NS3–4A protease inhibitors, NS5B polymerase inhibitors, and NS5A inhibitors.147 More importantly, the potency of anti-HCV drug evaluated in studies with HLCM appears to well predict the clinical efficacy and outcomes of antiviral therapy.157 Collectively, the HLCM system has enormously contributed to our understanding of HCV virology in vivo and bridged the gap between in vitro studies and human clinical trials.
Hepatitis D Virus
Hepatitis D virus (HDV) is a satellite virus, and thus the lifecycle is dependent on co-infection with HBV as the formation of the viral particle requires an outer lipoprotein envelope made of the HBV surface antigen HBsAg. The superinfection of HDV represents a severe form of chronic viral hepatitis and is thus associated with an accelerated liver disease progression compared with that of HBV monoinfection.158 Currently, WHO estimates that 5% of the chronically HBV-infected population, approximately 15 to 30 million people, is chronically co-infected with HDV.158
At present, the molecular mechanism through which superinfection by HDVexhibits pronounced disease progression remains elusive. Moreover, there has not been a definitive therapeutic strategy for the eradication of HDV. Thus, using physiological study models, such as HLCM, would play a critical role in furthering our understanding of HDV virology and pathogenesis. The host range, in terms of intracellular replication of HDV but not its entry, seems promiscuous as it is capable of establishing stable replication in the hepatocytes of various species including, but not limited to, murine, woodchuck, swine, and humans.127,159,160 Furthermore, even a nonhepatocyte cell line, HEK293 cells, is capable of supporting the replication of HDV.161 Nevertheless, the reliance on HBV for the entry determines the host range of HDV infection. Accordingly, various investigators have employed HLCM systems for the in vivo study of molecular virology of HDV.162-167 In these studies, HLCM supported stable coinfection and replication of HDV and HBV, and also recapitulated the viral interferes of the HBV lifecycle by HDV replication observed in the clinical setting.165,166,168 Additionally, HLCM demonstrated its value by serving as a model system for the preclinical evaluation of novel antiviral compounds.162,165,167
Regarding in vitro studies of HDV infection, freshly isolated human hepatocytes and human hepatoma cell lines, such as Huh7 and HepG2 cells expressing NTCP, have been shown to support the replication of HDV.169-171 Among these, PHH, and to a lesser extent, HepG2 cells with NTCP overexpression deem advantageous as they comprehensively support the HDV lifecycle via an establishment of HBV-HDV superinfection.160,171,172 Although not yet demonstrated, an extrapolation of this anticipates that HLCM-derived human hepatocytes will serve as an in vitro study tool for the study of HDV.
Hepatitis E Virus
Similar to HAV, hepatitis E virus (HEV) is transmitted fecalorally and thus is associated with poor hygiene.173 HEV infection is the most common cause of acute viral hepatitis worldwide, infecting over 20 million people annually.174 In general, HEV infection is a self-limited disease, and the mortality rate of HEV infection is low. Moreover, a recent study reported the successful development of a definitive prevention strategy.175 Therefore, HEV infection is not deemed a significant threat to public health if compared with other hepatitis viruses.
However, HEV infection is of importance in certain high-risk populations, such as pregnant women and immunosuppressed individuals. HEV infection in pregnant women is associated with an increased mortality.176 In addition, HEV infection in immunosuppressed populations, such as post-organ transplant population and HIV infected patients, are known to have higher risk for development of chronic infection with resultant rapid progression of liver fibrosis.177,178 These notions emphasize the importance of further investigation into HEV infection.
HEV establishes infection in a variety of species, such as swine, rabbit, rat, chimpanzees, and masques179,180; therefore the utility of HLCM as an in vivo study model is limited. However, it has a few advantages. First, it could still prove relevant in the study of human diseases. Another advantage is for the study of chronic HEV infection. As mentioned above, an immunocompromised host is prone to developing chronic HEV infection. Accordingly, the immune deficient nature of HLCM led to the establishment of chronic HEV infection.173 The inoculation of HLCM with serum or fecal extract of infected patients supported persistent HEV infection for at least 6 months.181
Currently, only two antiviral therapies, pegylated interferon (PEG-IFN) and ribavirin have shown clinical efficacy against HEV infection.182 Accordingly, the administration of PEG-IFN and ribavirin to HEV-infected HLCM markedly suppressed the serum HEV-RNA titer.181,183 These observations indicate that the HLCM system has a certain value as a human disease-relevant experimental model system of HEV infection.
Other Infectious Diseases
Theoretically, the HLCM system can be employed for the study of any pathogenic microorganisms with tropism for human hepatocytes. One prominent example is Plasmodium falciparum,184-186 the pathogen that causes malaria in humans. In vivo studies of malaria have been hampered by the strict specificity of the pathogen for particular host cells. However, injection of Plasmodium sporozoites into HLCM enabled the initiation of hepatic stage lifecycle, facilitating the production site of schizonts. Moreover, transfusion of malaria-infected human red blood cells into HLCM supported the P. falciparum lifecycle in both the liver stage and blood stages.187
Another pathogen capable of initiating its lifecycle in HLCM is cytomegalovirus. HLCM has been used to test the antiviral efficacy of ganciclovir and human NK cells.188 The utility of HLCM in the study of microorganisms is not limited to their pathogenicity. Adeno-associated virus (AAV), a virus, known to exhibit a broad range of host and tissue tropism depending on the serotypes with serotypes 2, 5, and 8 having strong hepatotropism in humans, has been heavily utilized for gene therapy.189 The HLCM system has proved to be a powerful preclinical trial study model of AAV-based gene therapy.190-192 A preclinical trial with HLCM demonstrated an encouraging result in which AAV mediated introduction of human low-density lipoprotein receptor (LDLR) substantially improved the clinical presentation in HLCM harboring human hepatocytes of familial hypercholesterolemia.193 These examples well justify the potential utility of the HLCM system for infectious diseases beyond the study of viral hepatitis.
Metabolic Liver Diseases
Nonalcoholic fatty liver diseases (NAFLD) and alcoholic liver diseases (ALD) are the two major metabolic liver diseases. Currently, ALD is the most prevalent cause of chronic liver disease in the United States, and NAFLD is the most rapidly growing cause of cirrhosis in the United States and worldwide.194,195 Definitive therapeutic and preventative strategies for these conditions have not been established, resulting in a significant demand for basic research to further develop our understanding of their pathophysiology.
To the best of our knowledge, the HLCM system has not yet been employed for the study of ALD and NAFLD. This is partly because there are numerous convenient models with conventional rodent systems currently available. Therefore, the cost and labor-intensive nature of HLCM make it less attractive to investigators studying metabolic liver diseases. However, the insight that HLCM would offer by eliminating interspecies differences between rodent and human hepatocytes in their capacity to and mechanisms of handling metabolic insults may be undervalued. It is plausible that HLCM can be a unique and prevailing in vivo experiment model as it enables the study of alcohol toxicity, lipotoxicity, and glucotoxicity occurring in bona fide human hepatocytes. This section describes our perspectives on the potential use of HLCM for the study of metabolic liver diseases.
Alcoholic Liver Diseases
Chronic alcohol abuse leads to the development of a broad spectrum of ALD, including fatty liver, foamy fatty degeneration, intrahepatic cholestasis, and alcoholic hepatitis.196-200 Unless achieving long-term abstinence, ALD eventually progresses to the end-stage liver diseases, and these conditions are often ineligible for liver transplantation due to the uncertainty of lifelong abstinence.201 Thus, it is crucial to establish a medical intervention that effectively prevents disease progression or promotes the regression of ALD, which requires a further understanding of the underlying molecular pathophysiology.
Conventionally, in vivo studies of ALD have been relying on rodent models with an ethanol containing Lieber-DeCarli liquid diet feeding protocol.202,203 These model systems certainly demonstrate signatures of liver injury along with the development of liver steatosis.203 While these models somewhat imitate the disease presentation of alcoholic fatty liver disease, the mildest form of ALD, it hardly recapitulates severe forms of ALD, such as alcoholic hepatitis and cirrhosis.203 The severe forms of ALD are the researcher’s major focus, as there are no definitive therapeutic strategies for these extremely high mortality diseases. Therefore, there remains a significant demand for the establishment of a physiological small animal model for the investigation of severe forms of ALD.
The relatively modest disease presentations of the mouse ALD models are likely attributed to the interspecies difference in alcohol metabolization and cellular response to toxic metabolites. Though both human and mouse hepatocytes are known to express the two major alcohol metabolizing enzymes alcohol dehydrogenase (ADHs) and CYP2E1,204 the enzymatic activity and extent of ethanol-induced oxidative stress could differ between species. For example, differences in the enzymatic activity of CYP2E1, the major inducer of oxidative stress in cells metabolizing alcohol, determine the severity of the liver injury.205 Thus, the inferior inductive potential of oxidative stress of murine CYP2E1 when compared with human CYP2E1 exemplifies the interspecies differences in the enzymatic activity, one that would hamper our precise understanding of ALD when employing the conventional rodent models.
Excessive EtOH-metabolism also reduces the abundance of nicotinamide adenine dinucleotide (NAD), a co-factor of ADH.206 Thus, chronic alcohol abuse alters the NAD/NADH (nicotinamide adenine dinucleotide) ratio, which likely disturbs numerous homeostatic redox reactions, such as glycolysis, citric acid cycle, and retinoid metabolism.204,207 In humans, the hepatocyte expresses ADH1A, 1B, 1C, 4, 6, and ADHFE1.204 These ADHs are expressed at a differential abundance and also equipped with highly variable enzymatic activities on ethanol, NAD, and NADH.208 In mouse, ADH1, 4, and 5 are expressed in the liver. To date, their relative abundance and enzymatic characterization between species remain entirely elusive, leading to significant uncertainties on the conventional murine model as a surrogate of human alcohol metabolism.
In contrast, HLCM is likely advantageous as it allows the study of the cellular response to EtOH metabolism that is occurring in human hepatocytes. Furthermore, cumulative evidence suggests that innate immunity, which is fully equipped in uPA-SCID HLCM system, plays a critical role in the development of a severe form of ALD209,210 (►Table 2). For example, the histopathology of alcoholic hepatitis demonstrated a massive infiltration of neutrophils and to a much lesser extent, macrophages.211-213 In general, these cells are the major source of proinflammatory cytokines and reactive oxygen species (ROS), thus considered the major aggravating factors.67,214
Altogether, the HLCM system is equipped with EtOH metabolism machinery of human hepatocytes and necessary immune factors, thereby having tremendous potential as a study tool of ALD that compensates the weaknesses associated with the conventional rodent model system.
Nonalcoholic Fatty Liver Diseases
NAFLD consists of two distinct forms: (1) steatosis without histological signatures of necroinflammation nor liver fibrosis, and (2) steatosis with varying degrees of inflammation with or without liver fibrosis. The former case is called simple steatosis and occurs mainly as a part of metabolic syndrome. The latter case is classified as nonalcoholic steatohepatitis (NASH). Simple steatosis is considered the pre-requisite to but not sufficient for the development of NASH as only 30% of the affected population progress to NASH, which eventually progresses to end-stage liver diseases.215 Currently, the molecular mechanism by which only a small fraction of people with simple steatosis progress to NASH remains largely unclear. Given the exponential growth of the affected population, NAFLD is now the mainstay of both clinical and basic research in the field of hepatology.
There exists a significant concern on conventional mouse models, such as ob/ob, choline/methionine-deficient diet feeding, and high-fat diet feeding models,216 for in vivo studies of NAFLD. This is because there are substantial interspecies differences between rodents and human in nutrient metabolism.193,217,218 Nevertheless, these “conventional” rodent models serve as the mainstay due to their immediate availability and status as convenient and well-accepted systems.
Consequently, there have not been any publications utilizing the HLCM system for the study of NAFLD. The under-utilization could be due to the technical hurdle for animal propagation, inconvenience of maintenance, and high-cost nature. Moreover, there are numerous additional reservations that might hinder investigators from utilizing HLCM. For example, there are significant uncertainties in establishing physiological crosstalk between xenotransplanted human hepatocytes and murine cells of the host mouse over humoral factors. In particular, visceral adiposity and insulin resistance, two major risk factors for the development of NAFLD, increase the abundance of lipid droplets in hepatocytes via deregulations of endocrinological messengers such as adipokines or insulin/glucagon.219-222 In brief, central obesity promotes the transformation of white adipose tissue into a central hub for systemic and chronic inflammation via the recruitment of proinflammatory macrophages.223-226 This event results in the alteration of multiorgan cross-talks that are mediated by numerous humoral factors such as insulin, inflammatory adipokines, and hepatokines, which further promote the elevation of plasma glucose and free fatty acids. This event leads to the accumulation of lipid droplets in the hepatocytes along with sensitizing hepatocytes to lipo- and glucotoxicity, resulting in necroinflammation of the liver.227,228 To date, it remains entirely uncertain whether these critical cross-talks of humoral factors take place in between murine cells of the host mouse and the xenotransplanted human hepatocytes. The relevance of this concern is well-demonstrated in our previous work demonstrating the interspecies mismatch in the growth hormone (GH) signaling pathway.94,95 Because of the “glitch” in GH signaling, HLCM naturally develops a moderate degree of steatosis even under standard chow diet maintenance, which was alleviated by the administration of human growth factor (hGH). This case well exemplifies the criticality of having physiological, endocrinological cross-talks between hepatocytes and cells in distant organs.
The substantial involvement of adaptive immunity in the disease process could be another pitfall of utilizing HLCM for the study of NAFLD. In NASH, both innate and adaptive immune systems significantly contribute to the development and progression of NAFLD. In general, hepatic macrophages, Kupffer cells, and NK cells are considered as aggravating factors while dendritic cells play a protective role in NASH, although there are some controversies.210,229-235 Certainly, these innate immune cells are equipped in uPA-SCID HLCM and thus, might not hamper the investigator’s enthusiasm (►Table 2). However, the potential contribution of adaptive immunity cannot be overlooked in NAFLD, especially for NASH. The inflammatory cells seen in the liver tissue of NASH are predominantly activated T lymphocytes.230,236 While this could be a nonspecific response, the T lymphocytes could still exhibit cytokine-dependent bystander hepatic injury.237 Alternatively, the excessive lipid peroxidation in hepatocytes may result in the generation of immunogenic adducts, which might be targeted by antigen-specific T cells.238,239 It is also important to note that the liver of NASH has altered ratio of CD4(+) and CD8(+) T cells in both human and conventional mouse models.240 The dysregulated lipid metabolism induces the mitochondrial toxicity, resulting in the selective loss of CD4(+) T cells. Then, the altered CD4(+)/CD8(+) ratio impairs antitumor immunity, which leads to the development of liver cancer. This emerging evidence collectively indicates the significant involvement of adaptive immunity in the disease processes, which might be an additional disadvantage of utilizing HLCM for the study of NAFLD.
Drug Metabolism, Pharmacokinetics, and Drug-Induced Liver Injury
The development of clinical drugs is a long and cost-intensive journey starting from the target discovery in basic research and progressing to compound synthesis, and finally preclinical trial followed by phase I–III clinical trials. The average cost required for the commercialization of a new drug keeps escalating and is currently 2.6 billion dollars per compound.241 Also, nearly 90% of the compounds drop out at some point during clinical trials, in which hepatotoxicity is one of the major causes of the attrition.242,243 Moreover, hepatotoxicity is also one of the most common causes of postmarketing drug withdrawals.244,245 Thus, it is crucial to precisely assess the DMPK of a candidate compound as well as the potential occurrence of DILI to maximize the chance of success in drug development. This section will focus on summarizing the considerable contribution of HLCM for the study of DMPK and DILI.
Drug Metabolism and Pharmacokinetics
The hepatocyte serves as the principal site of drug metabolism (DM) due to the predominant expression of xenobiotics-metabolizing enzymes.246 In general, DM involves three distinct steps: phase I: modification, phase II: conjugation, and phase III: secretion. In phase I, xenobiotics undergo oxidative modifications mainly facilitated by multiple CYP enzymes.247 In phase II, xenobiotics receive further detoxifications facilitated by conjugation enzymes such as uridine diphosphate glucuronosyltransferases (UGTs), N-acetyltransferase 2 (NAT2), and glutathione S-transferases (GSTs). Phase I and II metabolic processes, and phase III secretion machinery cooperatively determine the rate of DMPK.
The precise understanding of DMPK of a candidate compound is one of the critical milestones as it provides necessary information to determine appropriate dosing and administration schedule. However, the accurate prediction of DMPK is enormously complicated because numerous phase I and II enzymes, and phase III transporters simultaneously and reciprocally participate in the processes,248 thereby requiring a highly physiological experimental tool. Historically, rodents, dogs, and primates had been employed for in vivo studies of DMPK. However, these are considered suboptimal given the substantial interspecies differences in the expression and capacity of xenobiotic metabolizing enzymes.249-251 Alternatively, cryopreserved human hepatocytes, purified microsomes, immortalized hepatocytes, and hepatoma cell lines have served as convenient study tools252,253; however, these tools hardly recapitulate the in vivo DMPK and thus should only be suitable for limited purposes.
In contrast, HLCM is immensely advantageous as it preserves the expression profiles of drug-metabolizing enzymes and xenobiotic uptake/efflux transporters of the human liver.98,99,254,255 The assessment of DMPK requires additional information, such as the rate of hepatic extraction, referred to as hepatic clearance (CLhepatic), and the volume of distribution.256 The hepatic blood flow (Qh) determined with uPA-SCID-based HLCM now allows estimation of the rate of hepatic extraction using the formula: CLhepatic = Qh(Cin – Cout)/Cin.52 In addition, DMPK studies with two independent mathematical modeling methods, a single-species allometric scaling and physiologically-based pharmacokinetics, showed that the total clearance (CLt) and volume of distribution at steady state (Vdss) of clinically available compounds are comparable between humans and HLCM.96,257 Other studies with a similar approach revealed that HLCM well mimics the CLt and Vdss of the human, compared with that of macaques and rattus.258,259 Lastly, the drug–drug interactions—which are caused by competition over CYP enzymes, non-CYP enzymes, and transporters—occurring in the liver of HLCM well resemble that of the human liver.97,260-262 Collectively, HLCM most accurately reiterates the DMPK of the human liver and justifies its superiority over other in vitro and in vivo study tools.96,263,264
Drug-Induced Liver Injury
The incidence of DILI continues to increase largely due to the exponential growth in the cumulative number of approved clinical medications in recent years.265 Moreover, the increase in the average life expectancy worldwide is coupled with the growth in the number of individuals with multiple chronic illnesses, who concomitantly use multiple medications.266,267 This sets up another layer of complexity as the occurrence of DILI is hardly predictable in the presence of multiple xenobiotics given the potential competitive and/or inhibitory effect of each compound on drug-metabolizing enzymes.247 Although the exact mechanism remains largely elusive, the DM by CYPs, UGTs, NAT2, and GSTs partially underlie the occurrence of DILI via the formation of reactive metabolites.268-271 As HLCM is equipped with the physiological machinery of DM, it serves as an ideal platform for the in vivo study of DILI. Accordingly, numerous studies revealed the administration of troglitazone, amiodarone, and bosentan, which are indicated for type 2 diabetes, arrhythmia, and pulmonary hypertension, respectively, resulted in the generation of human-specific reactive metabolites in the liver of HLCM, which led to the development of liver injury.60,272-275
Of important note, emerging evidence revealed that for most, but not all, the occurrence of DILI results from the activation of the adaptive immune response.69 Genome-wide association studies demonstrated a strong association between HLA typing and the occurrence of DILI.70 The potential explanation for this phenomenon is the creation of neoantigens via adduct formation of reactive drug metabolite on cellular components, which leads to the activation of the adaptive immune response against drug-metabolizing hepatocytes. One compelling example is the development of autoantibodies against CYPs seen in isoniazid (INH)-induced hepatotoxicity.276 This study showed that INH covalently bound to CYP2E1, CYP3A4, and CYP2C9, resulting in the development of autoantibodies against the INH-CYPs adducts. Thus, the adaptive immunodeficiency of the conventional HLCM is surely a disadvantage if the mode of toxicity of the testing compound is mediated by adaptive immunity.
In recent years, there is an exponential growth in the development of new classes of therapeutic interventions such as immunotherapy with monoclonal antibodies (mAbs) for various disease conditions. The metabolism/catabolism of therapeutic-mAbs is mediated through proteolytic degradation, which is independent of the conventional DM pathways of the hepatocytes. Thus, it is expected that the therapeutic-mAbs less likely causes DILI. However, there are numerous incidences where therapeutic mAbs, such as immune checkpoint inhibitors, antitumor necrosis factor (anti-TNF) therapy, anti-TNF–related apoptosis-inducing ligand receptor (anti-TRAIL-R) exhibited a significant degree of hepatoxicity.277-279 As the mode of DILI associated with therapeutic-mAbs is likely immune medicated, HLCM would not be helpful in understanding how it exhibits hepatotoxicity. However, a few pieces of evidence suggest that such preconceived ideation might not be the case. The administration of anti-hTRAIL-R2 mAbs triggered a marked cell death of xenotransplanted human hepatocytes in HLCM.280 Moreover, the toxicity of anti-hTRAIL-R mAbs was not observed in rodents and monkeys,279 indicating that there might exist an unrecognized mode of species-specific antibody-mediated hepatotoxicity. Taken together, this emerging evidence suggests that HLCM may serve as a tool to seek out the mechanisms of mAbs-mediated hepatotoxicity.
Regenerative Biology and Genetic Liver Diseases
Regenerative Biology
The substantial regenerative capability of the liver in response to various types of insults is largely due to the proliferation potential of hepatocytes. Studies with partial hepatectomy have indicated that terminally differentiated hepatocytes have great self-renewal and proliferation potential without compromising the degree of differentiation.102
Since the demonstration that the liver of HLCM supports robust proliferation of human hepatocytes without involving their dedifferentiation, the HLCM has received interest from researchers of stem cell biology.
After the discovery of pluripotency in embryonic stem (ES) and induced pluripotent stem (iPS) cells,281-283 stem cell biologists have made significant efforts to establish methods to differentiate ES/iPS cells into a variety of cell types. Accordingly, protocols to differentiate ES/iPS cells into hepatocytes, hereafter iHep, has also evolved substantially over time; however, the degree of maturity is still far from that of freshly isolated PHH.284-287 For example, transcriptome analysis of iHep demonstrated a highly distinctive gene expression profile compared with that of PHH.284 These important observations collectively indicate that there remains a significant gap in terms of the degree of differentiation/maturation between genuine hepatocytes and iHeps. Of great interest, it has been shown that the implantation of iHep into the liver of HLCM prompted engraftment, proliferation, and further maturation.288,289 Similarly, the liver of HLCM supports the terminal differentiation of chemically induced hepatic progenitor cells.290,291 These breakthrough discoveries indicate that the liver of HLCM is equipped with a unique organ-specific microenvironment that is required for the terminal differentiation of hepatocyte progenitors, thereby shedding light upon a path to overcoming the widely-recognized obstacles to achieving propagation of fully matured iHep. Taken together, these observations well exemplify the substantial potential of HLCM system in the field of developmental and stem cell biology of the liver.
Genetic Liver Diseases
The proliferation potential of hepatocytes in the liver of HLCM is highly exploitable for the development of disease models and therapeutic strategies of genetic liver diseases. A few studies demonstrated the utility of HLCM for the study of genetic diseases of the liver, in which a variety of disease model systems using hepatocytes extracted from livers of individuals with congenital metabolic disorders, including α-1-antitrypsin deficiency, ornithine transcarbamylase deficiency, and Crigler–Najjar syndrome, were successfully established.292,293 These congenital metabolic diseases are caused by impaired metabolic function of hepatocytes, but do not lead to the development of hepatotoxicity. Thus, hepatocytes extracted from livers out of these conditions would not have compromised proliferation potential, and could be successfully engrafted into the livers of HLCM.
Another example is the establishment of a model system for the study of homozygous familial hypercholesterolemia (HFHC), a relatively rare multifactorial inheritance disorder.294 In the most common form of HFHC, mutations in LDLR lead to the development of consistently high plasma cholesterol level, resulting in the development of atherosclerosis early in life that eventually leads to the onset of ischemic heart disease and stroke. To date, a few in vivo models developed with conventional gene manipulation approaches, such as LDLR-KO mice, have been established. However, they hardly mimic the disease presentation in humans given the substantial interspecies differences in the expression of other key proteins involved in the maintenance of lipid homeostasis.193,295 In contrast, an HLCM comprised of hepatocytes of HFHC well recapitulated the disease presentation.193
To date, liver transplantation is the only definitive therapeutic intervention for HFHC296; however, reservation exists for a variety of reasons. First, the hepatocytes of HFHC remain normal lifelong and thus maintain normal liver function except for LDL uptake. Numerous other concerns include the invasive nature of liver transplantation, posttransplant management with a lifelong immunosuppressant, and significant shortage of donor livers. As a result, there is a substantial degree of demand for the development of a novel therapeutic approach. To this end, a study mentioned above has already paved the way for the development of gene therapy for HFHC where the injection of AAV encoding wild-type LDLR into HLCM-based HFHC achieved complete normalization of apolipoprotein profiles in plasma.193 These compelling pieces of evidence well support the superiority of HLCM-based genetic disease model system along with its usefulness for the therapeutic investigations.
These cumulating pieces of evidence encourage the utilization of HLCM system for the establishment of a highly physiological disease model system of genetic liver diseases. The only caveat is it might not be feasible for genetic deficiencies that compromise the proliferation of xenotransplanted hepatocytes. Nevertheless, stem cell biology and regenerative medicine are particularly exciting fields for the application of HLCM. Given the availability of genome editing approaches, it is anticipated that patient-derived iPS cells could be used to repair genetic mutations where the HLCM system serves the purpose of propagation of mutation-repaired hepatocytes toward cell transplant therapy as a replacement for liver transplantation.
Conclusion
Since its establishment in 2001, HLCM has exponentially expanded in its application to variety aspects of human liver biology and diseases. Accordingly, the HLCM system has established a strong position as an experimental platform for the study of hepatotropic infectious diseases, DMPK, and regenerative medicine. It is expected that this exciting research tool will continue to evolve in its quality and expand in its utility by achieving milestones to overcome its weaknesses and shortfalls. A significant accomplishment in this regard is the successful establishment of the “immune reconstituted” HLCM system, which allows investigators to employ HLCM for the study of immune-mediated disease mechanisms.
Emerging shreds of evidence indicate that intercell type crosstalk between hepatocytes and other cell types plays a critical role in both homeostatic and disease condition.297 Thus, the potential uncertainty in the establishment of intercell type communication over humoral factors due to the interspecies chimerism is another fundamental weakness. However, this aspect of weakness might be alleviated relatively soon as the growth in stem cell research has already made solid stride toward the establishment of dual-humanization of both parenchymal and nonparenchymal cells of the liver.298
Taken altogether, the HLCM system will continue to be an attractive model system not only for the investigation of “human liver biology and disease” but also, and more importantly, for the study of therapeutic interventions.
Financial Support
This work was supported by funds from NIH NIAAA (R21AA022751: TS), NIH NIAID (R21AI139954: TS), NIH NIDDK (RO1DK101773: TS), and research funding from PhoenixBio.
Footnotes
Conflicts of Interest
T.S. reports grants from NIH and PhoenixBio, during the conduct of the study.
References
- 1.Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol 1977;72(02): 441–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng X, Kim SY, Okamoto H, et al. Glucagon contributes to liver zonation. Proc Natl Acad Sci U S A 2018;115(17):E4111–E4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dancygier H Clinical Hepatology Principles and Practice of Hepatobiliary Diseases. Berlin, Heidelberg: Springer; 2010:75102 [Google Scholar]
- 4.Kaji H High-density lipoproteins and the immune system. J Lipids 2013;2013:684903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandri M, Burda MR, Török E, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology 2001;33(04):981–988 [DOI] [PubMed] [Google Scholar]
- 6.Mercer DF, Schiller DE, Elliott JF, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med 2001;7(08): 927–933 [DOI] [PubMed] [Google Scholar]
- 7.Tateno C, Yoshizane Y, Saito N, et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol 2004;165(03):901–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azuma H, Paulk N, Ranade A, et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat Biotechnol 2007;25(08):903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bissig KD, Le TT, Woods NB, Verma IM Repopulation of adult and neonatal mice with human hepatocytes: a chimeric animal model. Proc Natl Acad Sci U S A 2007;104(51):20507–20511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suemizu H, Hasegawa M, Kawai K, et al. Establishment of a humanized model of liver using NOD/Shi-SCID IL2Rgnull mice. Biochem Biophys Res Commun 2008;377(01):248–252 [DOI] [PubMed] [Google Scholar]
- 11.Kawahara T, Douglas DN, Lewis J, et al. Critical role of natural killer cells in the rejection of human hepatocytes after xenotransplantation into immunodeficient mice. Transpl Int 2010;23 (09):934–943 [DOI] [PubMed] [Google Scholar]
- 12.Washburn ML, Bility MT, Zhang L, et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology 2011;140(04):1334–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa M, Kawai K, Mitsui T, et al. The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochem Biophys Res Commun 2011;405(03):405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su B, Liu C, Xiang D, et al. Xeno-repopulation of Fah −/− Nod/Scid mice livers by human hepatocytes. Sci China Life Sci 2011;54 (03):227–234 [DOI] [PubMed] [Google Scholar]
- 15.Tesfaye A, Stift J, Maric D, Cui Q, Dienes HP, Feinstone SM. Chimeric mouse model for the infection of hepatitis B and C viruses. PLoS One 2013;8(10):e77298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komori J, DeWard AD, Gramignoli R, Strom SC, Fontes P, Lagasse E. Potential barriers to human hepatocyte transplantation in MUP-uPAtg(+/+)Rag2−/−γC−/− mice. Cell Transplant 2014;23 (12):1537–1544 [DOI] [PubMed] [Google Scholar]
- 17.Kato K, Ohbuchi M, Hamamura S, et al. Development of murine Cyp3a knockout chimeric mice with humanized liver. Drug Metab Dispos 2015;43(08):1208–1217 [DOI] [PubMed] [Google Scholar]
- 18.Tateno C, Kawase Y, Tobita Y, et al. Generation of novel chimeric mice with humanized livers by using hemizygous cDNA-uPA/SCID mice. PLoS One 2015;10(11):e0142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Jong YP, Dorner M, Mommersteeg MC, et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med 2014;6(254):254ra129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barzi M, Pankowicz FP, Zorman B, et al. A novel humanized mouse lacking murine P450 oxidoreductase for studying human drug metabolism. Nat Commun 2017;8(01):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tateno C, Yoshizato K. Growth and differentiation in culture of clonogenic hepatocytes that express both phenotypes of hepatocytes and biliary epithelial cells. Am J Pathol 1996;149(05): 1593–1605 [PMC free article] [PubMed] [Google Scholar]
- 22.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science 1994;263(5150):1149–1152 [DOI] [PubMed] [Google Scholar]
- 23.Rhim JA, Sandgren EP, Palmiter RD, Brinster RL. Complete reconstitution of mouse liver with xenogeneic hepatocytes. Proc Natl Acad Sci U S A 1995;92(11):4942–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamasaki C, Tateno C, Aratani A, et al. Growth and differentiation of colony-forming human hepatocytes in vitro. J Hepatol 2006; 44(04):749–757 [DOI] [PubMed] [Google Scholar]
- 25.Knobeloch D, Ehnert S, Schyschka L, et al. Human hepatocytes: isolation, culture, and quality procedures. Methods Mol Biol 2012;806:99–120 [DOI] [PubMed] [Google Scholar]
- 26.Kawahara T, Toso C, Douglas DN, et al. Factors affecting hepatocyte isolation, engraftment, and replication in an in vivo model. Liver Transpl 2010;16(08):974–982 [DOI] [PubMed] [Google Scholar]
- 27.Kelland LR. Of mice and men: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur J Cancer 2004;40(06):827–836 [DOI] [PubMed] [Google Scholar]
- 28.Jung D, Alt FW, Unraveling V. Unraveling V(D)J recombination; insights into gene regulation. Cell 2004;116(02):299–311 [DOI] [PubMed] [Google Scholar]
- 29.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity 1995;2(03):223–238 [DOI] [PubMed] [Google Scholar]
- 30.Ogasawara K, Hamerman JA, Hsin H, et al. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity 2003;18(01):41–51 [DOI] [PubMed] [Google Scholar]
- 31.Kataoka S, Satoh J, Fujiya H, et al. Immunologic aspects of the nonobese diabetic (NOD) mouse. Abnormalities of cellular immunity. Diabetes 1983;32(03):247–253 [DOI] [PubMed] [Google Scholar]
- 32.Pearson T, Markees TG, Serreze DV, et al. Genetic dissociation of autoimmunity and resistance to costimulation blockade-induced transplantation tolerance in nonobese diabetic mice. J Immunol 2003;171(01):185–195 [DOI] [PubMed] [Google Scholar]
- 33.Sandgren EP, Palmiter RD, Heckel JL, Daugherty CC, Brinster RL, Degen JL. Complete hepatic regeneration after somatic deletion of an albumin-plasminogen activator transgene. Cell 1991;66(02):245–256 [DOI] [PubMed] [Google Scholar]
- 34.Heckel JL, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Neonatal bleeding in transgenic mice expressing urokinase-type plasminogen activator. Cell 1990;62(03):447–456 [DOI] [PubMed] [Google Scholar]
- 35.Grompe M, al-Dhalimy M, Finegold M, et al. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev 1993;7 (12A):2298–2307 [DOI] [PubMed] [Google Scholar]
- 36.Rubsam LZ, Davidson BL, Shewach DS. Superior cytotoxicity with ganciclovir compared with acyclovir and 1-beta-D-arabinofur-anosylthymine in herpes simplex virus-thymidine kinase-expressing cells: a novel paradigm for cell killing. Cancer Res 1998;58(17):3873–3882 [PubMed] [Google Scholar]
- 37.Bissig KD, Wieland SF, Tran P, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment.J Clin Invest 2010;120(03):924–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th ed. New York: W. H. Freeman; 2000 [Google Scholar]
- 39.Eddy AA, Fogo AB. Plasminogen activator inhibitor-1 in chronic kidney disease: evidence and mechanisms of action. J Am Soc Nephrol 2006;17(11):2999–3012 [DOI] [PubMed] [Google Scholar]
- 40.Miyazawa K Hepatocyte growth factor activator (HGFA): a serine protease that links tissue injury to activation of hepatocyte growth factor. FEBS J 2010;277(10):2208–2214 [DOI] [PubMed] [Google Scholar]
- 41.Mizuno K, Tanoue Y, Okano I, Harano T, Takada K, Nakamura T. Purification and characterization of hepatocyte growth factor (HGF)-converting enzyme: activation of pro-HGF. Biochem Biophys Res Commun 1994;198(03):1161–1169 [DOI] [PubMed] [Google Scholar]
- 42.Shimizu M, Hara A, Okuno M, et al. Mechanism of retarded liver regeneration in plasminogen activator-deficient mice: impaired activation of hepatocyte growth factor after Fas-mediated massive hepatic apoptosis. Hepatology 2001;33(03):569–576 [DOI] [PubMed] [Google Scholar]
- 43.Hill KS, Gaziova I, Harrigal L, et al. Met receptor tyrosine kinase signaling induces secretion of the angiogenic chemokine interleukin-8/CXCL8 in pancreatic cancer. PLoS One 2012;7(07):e40420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplowitz N Drug-Induced Liver Disease. 3rd ed. London: Elsevier; 2013 [Google Scholar]
- 45.Castro-Dopico T, Clatworthy MR. Fcγ receptors in solid organ transplantation. Curr Transplant Rep 2016;3(04):284–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griesemer A, Yamada K, Sykes M. Xenotransplantation: immunological hurdles and progress toward tolerance. Immunol Rev 2014;258(01):241–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawahara T, Rodriguez-Barbosa JI, Zhao Y, Zhao G, Sykes M. Global unresponsiveness as a mechanism of natural killer cell tolerance in mixed xenogeneic chimeras. Am J Transplant 2007;7 (09):2090–2097 [DOI] [PubMed] [Google Scholar]
- 48.Orr MT, Lanier LL. Natural killer cell education and tolerance. Cell 2010;142(06):847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEnerney L, Duncan K, Bang BR, et al. Dual modulation of human hepatic zonation via canonical and non-canonical Wnt pathways. Exp Mol Med 2017;49(12):e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chow EC, Wang JZ, Quach HP, et al. Functional integrity of the chimeric (humanized) mouse liver: enzyme zonation, physiologic spaces, and hepatic enzymes and transporters. Drug Metab Dispos 2016;44(09):1524–1535 [DOI] [PubMed] [Google Scholar]
- 51.Kakuni M, Yamasaki C, Tachibana A, Yoshizane Y, Ishida Y, Tateno C. Chimeric mice with humanized livers: a unique tool for in vivo and in vitro enzyme induction studies. Int J Mol Sci 2013;15(01):58–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tateno C, Miya F, Wake K, et al. Morphological and microarray analyses of human hepatocytes from xenogeneic host livers. Lab Invest 2013;93(01):54–71 [DOI] [PubMed] [Google Scholar]
- 53.Vinken M, Henkens T, De Rop E, Fraczek J, Vanhaecke T, Rogiers V. Biology and pathobiology of gap junctional channels in hepatocytes. Hepatology 2008;47(03):1077–1088 [DOI] [PubMed] [Google Scholar]
- 54.Peterson RA, Krull DL, Brown HR, de Serres M. Morphologic characterization of PhoenixBio (uPA+/+/SCID) humanized liver chimeric mouse model. Drug Metab Lett 2010;4(03):180–184 [DOI] [PubMed] [Google Scholar]
- 55.Meuleman P, Libbrecht L, De Vos R, et al. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology 2005;41(04):847–856 [DOI] [PubMed] [Google Scholar]
- 56.Wake K Hepatic stellate cells: three-dimensional structure, localization, heterogeneity and development. Proc Jpn Acad, Ser B, Phys Biol Sci 2006;82(04):155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atzori L, Poli G, Perra A. Hepatic stellate cell: a star cell in the liver. Int J Biochem Cell Biol 2009;41(8-9):1639–1642 [DOI] [PubMed] [Google Scholar]
- 58.Phillips MJ, Poucell S, Patterson J, Valencia P. The Liver: An Atlas and Text of Ultrastructural Pathology. New York, NY: Raven Press; 1987 [Google Scholar]
- 59.Ekdahl A, Weidolf L, Baginski M, Morikawa Y, Thompson RA, Wilson ID. The metabolic fate of fenclozic acid in chimeric mice with a humanized liver. Arch Toxicol 2018;92(09):2819–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu D, Wu M, Nishimura S, et al. Chimeric TK-NOG mice: a predictive model for cholestatic human liver toxicity. J Pharmacol Exp Ther 2015;352(02):274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujino C, Sanoh S, Tamura Y, et al. Changes in bile acid concentrations in chimeric mice transplanted with different replacement indexes of human hepatocytes. BPB Rep 2019;2:29–34 [Google Scholar]
- 62.Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 2017;152(07):1679–1694.e3 [DOI] [PubMed] [Google Scholar]
- 63.Cani PD. Microbiota and metabolites in metabolic diseases. Nat Rev Endocrinol 2019;15(02):69–70 [DOI] [PubMed] [Google Scholar]
- 64.Du Toit A The gut microbiome and mental health. Nat Rev Microbiol 2019;17(04):196. [DOI] [PubMed] [Google Scholar]
- 65.Chisari FV, Isogawa M, Wieland SF. Pathogenesis of hepatitis B virus infection. Pathol Biol (Paris) 2010;58(04):258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koziel MJ. Cellular immune responses against hepatitis C virus. Clin Infect Dis 2005;41(Suppl 1):S25–S31 [DOI] [PubMed] [Google Scholar]
- 67.Gao B, Ahmad MF, Nagy LE, Tsukamoto H. Inflammatory pathways in alcoholic steatohepatitis. J Hepatol 2019;70(02): 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol 2020;17 (02):81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel: Chair; Panel members; EASL Governing Board representative. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol 2019;70(06):1222–1261 [DOI] [PubMed] [Google Scholar]
- 70.Urban TJ, Daly AK, Aithal GP. Genetic basis of drug-induced liver injury: present and future. Semin Liver Dis 2014;34(02): 123–133 [DOI] [PubMed] [Google Scholar]
- 71.Bility MT, Li F, Cheng L, Su L. Liver immune-pathogenesis and therapy of human liver tropic virus infection in humanized mouse models. J Gastroenterol Hepatol 2013;28(Suppl 1): 120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bility MT, Cheng L, Zhang Z, et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog 2014;10(03):e1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dusséaux M, Masse-Ranson G, Darche S, et al. Viral load affects the immune response to HBV in mice with humanized immune system and liver. Gastroenterology 2017;153(06):1647–1661.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Washburn K Model for end-stage liver disease exceptions: proceed with caution. Liver Transpl 2011;17(10):1123–1124 [DOI] [PubMed] [Google Scholar]
- 75.Billerbeck E, Mommersteeg MC, Shlomai A, et al. Humanized mice efficiently engrafted with fetal hepatoblasts and syngeneic immune cells develop human monocytes and NK cells. J Hepatol 2016;65(02):334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keng CT, Sze CW, Zheng D, et al. Characterisation of liver pathogenesis, human immune responses and drug testing in a humanised mouse model of HCV infection. Gut 2016;65(10): 1744–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol 2007;7(02): 118–130 [DOI] [PubMed] [Google Scholar]
- 78.Christianson SW, Greiner DL, Hesselton RA, et al. Enhanced human CD4+ T cell engraftment in beta2-microglobulin-deficient NOD-SCID mice. J Immunol 1997;158(08):3578–3586 [PubMed] [Google Scholar]
- 79.McKenzie JL, Gan OI, Doedens M, Dick JE. Human short-term repopulating stem cells are efficiently detected following intra-femoral transplantation into NOD/SCID recipients depleted of CD122+ cells. Blood 2005;106(04):1259–1261 [DOI] [PubMed] [Google Scholar]
- 80.Forte P, Baumann BC, Weiss EH, Seebach JD. HLA-E expression on porcine cells: protection from human NK cytotoxicity depends on peptide loading. Am J Transplant 2005;5(09):2085–2093 [DOI] [PubMed] [Google Scholar]
- 81.Shultz LD, Banuelos S, Lyons B, et al. NOD/LtSz-Rag1nullPfpnull mice: a new model system with increased levels of human peripheral leukocyte and hematopoietic stem-cell engraftment. Transplantation 2003;76(07):1036–1042 [DOI] [PubMed] [Google Scholar]
- 82.Tian X, Woll PS, Morris JK, Linehan JL, Kaufman DS. Hematopoietic engraftment of human embryonic stem cell-derived cells is regulated by recipient innate immunity. Stem Cells 2006;24(05):1370–1380 [DOI] [PubMed] [Google Scholar]
- 83.Navarro-Alvarez N, Yang YG. CD47: a new player in phagocytosis and xenograft rejection. Cell Mol Immunol 2011;8(04):285–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang YG. CD47 in xenograft rejection and tolerance induction. Xenotransplantation 2010;17(04):267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamauchi T, Takenaka K, Urata S, et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood 2013;121(08):1316–1325 [DOI] [PubMed] [Google Scholar]
- 86.Takenaka K, Prasolava TK, Wang JC, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 2007;8(12):1313–1323 [DOI] [PubMed] [Google Scholar]
- 87.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c) (null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 2002;100(09):3175–3182 [DOI] [PubMed] [Google Scholar]
- 88.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol 2005;174(10):6477–6489 [DOI] [PubMed] [Google Scholar]
- 89.Uchida T, Hiraga N, Imamura M, et al. Human cytotoxic T lymphocyte-mediated acute liver failure and rescue by immunoglobulin in human hepatocyte transplant TK-NOG mice. J Virol 2015;89(19):10087–10096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ellis EC, Naugler WE, Parini P, et al. Mice with chimeric livers are an improved model for human lipoprotein metabolism. PLoS One 2013;8(11):e78550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chiang JY. Bile acid metabolism and signaling. Compr Physiol 2013;3(03):1191–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Naugler WE, Tarlow BD, Fedorov LM, et al. Fibroblast growth factor signaling controls liver size in mice with humanized livers. Gastroenterology 2015;149(03):728–40.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chow EC, Quach HP, Zhang Y, et al. Disrupted murine gut-to-human liver signaling alters bile acid homeostasis in humanized mouse liver models. J Pharmacol Exp Ther 2017;360(01):174–191 [DOI] [PubMed] [Google Scholar]
- 94.Tateno C, Kataoka M, Utoh R, et al. Growth hormone-dependent pathogenesis of human hepatic steatosis in a novel mouse model bearing a human hepatocyte-repopulated liver. Endocrinology 2011;152(04):1479–1491 [DOI] [PubMed] [Google Scholar]
- 95.Masumoto N, Tateno C, Tachibana A, et al. GH enhances proliferation of human hepatocytes grafted into immunodeficient mice with damaged liver. J Endocrinol 2007;194(03):529–537 [DOI] [PubMed] [Google Scholar]
- 96.Sanoh S, Naritomi Y, Fujimoto M, et al. Predictability of plasma concentration-time curves in humans using single-species allometric scaling of chimeric mice with humanized liver. Xenobiotica 2015;45(07):605–614 [DOI] [PubMed] [Google Scholar]
- 97.Katoh M, Sawada T, Soeno Y, et al. In vivo drug metabolism model for human cytochrome P450 enzyme using chimeric mice with humanized liver. J Pharm Sci 2007;96(02):428–437 [DOI] [PubMed] [Google Scholar]
- 98.Katoh M, Matsui T, Nakajima M, et al. Expression of human cytochromes P450 in chimeric mice with humanized liver. Drug Metab Dispos 2004;32(12):1402–1410 [DOI] [PubMed] [Google Scholar]
- 99.Katoh M, Matsui T, Okumura H, et al. Expression of human phase II enzymes in chimeric mice with humanized liver. Drug Metab Dispos 2005;33(09):1333–1340 [DOI] [PubMed] [Google Scholar]
- 100.Foquet L, Wilson EM, Verhoye L, et al. Successful engraftment of human hepatocytes in uPA-SCID and FRG® KO mice. Methods Mol Biol 2017;1506:117–130 [DOI] [PubMed] [Google Scholar]
- 101.Vanwolleghem T, Libbrecht L, Hansen BE et al. Factors determining successful engraftment of hepatocytes and susceptibility to hepatitis B and C virus infection in uPA-SCID mice. J Hepatol 2010;53:468–476 [DOI] [PubMed] [Google Scholar]
- 102.Itoh T, Miyajima A. Liver regeneration by stem/progenitor cells. Hepatology 2014;59(04):1617–1626 [DOI] [PubMed] [Google Scholar]
- 103.Yin Y, Lim YK, Salto-Tellez M, Ng SC, Lin CS, Lim SK. AFP(+), ESC-derived cells engraft and differentiate into hepatocytes in vivo. Stem Cells 2002;20(04):338–346 [DOI] [PubMed] [Google Scholar]
- 104.Greider CW. Telomeres and senescence: the history, the experiment, the future. Curr Biol 1998;8(05):R178–R181 [DOI] [PubMed] [Google Scholar]
- 105.Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 2015;350(6265):1193–1198 [DOI] [PubMed] [Google Scholar]
- 106.Watari R, Kakiki M, Oshikata A, et al. A long-term culture system based on a collagen vitrigel membrane chamber that supports liver-specific functions of hepatocytes isolated from mice with humanized livers. J Toxicol Sci 2018;43(08):521–529 [DOI] [PubMed] [Google Scholar]
- 107.Ishida Y, Yamasaki C, Yanagi A, et al. Novel robust in vitro hepatitis B virus infection model using fresh human hepatocytes isolated from humanized mice. Am J Pathol 2015;185(05): 1275–1285 [DOI] [PubMed] [Google Scholar]
- 108.Maher SP, Crouse RB, Conway AJ, et al. Microphysical space of a liver sinusoid device enables simplified long-term maintenance of chimeric mouse-expanded human hepatocytes. Biomed Microdevices 2014;16(05):727–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kanamori T, Togawa-Iwata Y, Segawa H, et al. Use of hepatocytes isolated from a liver-humanized mouse for studies on the metabolism of drugs: application to the metabolism of fentanyl and acetylfentanyl. Forensic Toxicol 2018;36(02):467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Watari R, Kakiki M, Yamasaki C, et al. Prediction of human hepatic clearance for cytochrome P450 substrates via a new culture method using the collagen vitrigel membrane chamber and fresh hepatocytes isolated from liver humanized mice. Biol Pharm Bull 2019;42(03):348–353 [DOI] [PubMed] [Google Scholar]
- 111.Kanamori T, Iwata YT, Segawa H, et al. Metabolism of butyrylfentanyl in fresh human hepatocytes: chemical synthesis of authentic metabolite standards for definitive identification. Biol Pharm Bull 2019;42(04):623–630 [DOI] [PubMed] [Google Scholar]
- 112.Vorrink SU, Ullah S, Schmidt S, et al. Endogenous and xenobiotic metabolic stability of primary human hepatocytes in long-term 3D spheroid cultures revealed by a combination of targeted and untargeted metabolomics. FASEB J 2017;31(06): 2696–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lauschke VM, Shafagh RZ, Hendriks DFG, Ingelman-Sundberg M. 3D primary hepatocyte culture systems for analyses of liver diseases, drug metabolism, and toxicity: emerging culture paradigms and applications. Biotechnol J 2019;14(07):e1800347. [DOI] [PubMed] [Google Scholar]
- 114.Petersen J, Dandri M, Gupta S, Rogler CE. Liver repopulation with xenogenic hepatocytes in B and T cell-deficient mice leads to chronic hepadnavirus infection and clonal growth of hepatocellular carcinoma. Proc Natl Acad Sci U S A 1998;95(01):310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.WHO. Global Hepatitis Report, 2017. Available at: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. Accessed January 27, 2020 [Google Scholar]
- 116.Moyer MW. American epidemic. Sci Am 2018;318(05):44–57 [DOI] [PubMed] [Google Scholar]
- 117.Hirai-Yuki A, Hensley L, McGivern DR, et al. MAVS-dependent host species range and pathogenicity of human hepatitis A virus. Science 2016;353(6307):1541–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang Y, Liang Y, Qu L, et al. Disruption of innate immunity due to mitochondrial targeting of a picornaviral protease precursor. Proc Natl Acad Sci U S A 2007;104(17):7253–7258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Win NN, Kanda T, Nakamoto S, et al. Inhibitory effect of Japanese rice-koji miso extracts on hepatitis A virus replication in association with the elevation of glucose-regulated protein 78 expression. Int J Med Sci 2018;15(11):1153–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dotzauer A, Gebhardt U, Bieback K, et al. Hepatitis A virus-specific immunoglobulin A mediates infection of hepatocytes with hepatitis A virus via the Asialoglycoprotein receptor. J Virol 2000;74(23):10950–10957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet 2015;386(10003):1546–1555 [DOI] [PubMed] [Google Scholar]
- 122.Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA 2018;319(17):1802–1813 [DOI] [PubMed] [Google Scholar]
- 123.Sanada T, Tsukiyama-Kohara K, Yamamoto N, et al. Property of hepatitis B virus replication in Tupaia belangeri hepatocytes. Biochem Biophys Res Commun 2016;469(02):229–235 [DOI] [PubMed] [Google Scholar]
- 124.Tsukiyama-Kohara K, Kohara M. Tupaia belangeri as an experimental animal model for viral infection. Exp Anim 2014;63(04): 367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stieger B The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol 2011;5(201):205–259 [DOI] [PubMed] [Google Scholar]
- 126.Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012;1:e00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yan H, Peng B, He W, et al. Molecular determinants of hepatitis B and D virus entry restriction in mouse sodium taurocholate cotransporting polypeptide. J Virol 2013;87(14):7977–7991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Iwamoto M, Watashi K, Tsukuda S, et al. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem Biophys Res Commun 2014;443(03):808–813 [DOI] [PubMed] [Google Scholar]
- 129.Lempp FA, Wiedtke E, Qu B, et al. Sodium taurocholate cotransporting polypeptide is the limiting host factor of hepatitis B virus infection in macaque and pig hepatocytes. Hepatology 2017;66(3):703–716 [DOI] [PubMed] [Google Scholar]
- 130.Li H, Zhuang Q, Wang Y, et al. HBV life cycle is restricted in mouse hepatocytes expressing human NTCP. Cell Mol Immunol 2014;11(02):175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tabuchi A, Tanaka J, Katayama K, et al. Titration of hepatitis B virus infectivity in the sera of pre-acute and late acute phases of HBV infection: transmission experiments to chimeric mice with human liver repopulated hepatocytes. J Med Virol 2008;80(12): 2064–2068 [DOI] [PubMed] [Google Scholar]
- 132.Allweiss L, Dandri M. Experimental in vitro and in vivo models for the study of human hepatitis B virus infection. J Hepatol 2016;64 (1, Suppl):S17–S31 [DOI] [PubMed] [Google Scholar]
- 133.Hayes CN, Chayama K. HBV culture and infectious systems. Hepatol Int 2016;10(04):559–566 [DOI] [PubMed] [Google Scholar]
- 134.Ishida Y, Chung TL, Imamura M, et al. Acute hepatitis B virus infection in humanized chimeric mice has multiphasic viral kinetics. Hepatology 2018;68(02):473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tsuge M, Hiraga N, Takaishi H, et al. Infection of human hepatocyte chimeric mouse with genetically engineered hepatitis B virus. Hepatology 2005;42(05):1046–1054 [DOI] [PubMed] [Google Scholar]
- 136.Schulze A, Mills K, Weiss TS, Urban S. Hepatocyte polarization is essential for the productive entry of the hepatitis B virus. Hepatology 2012;55(02):373–383 [DOI] [PubMed] [Google Scholar]
- 137.Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004;126(07):1750–1758 [DOI] [PubMed] [Google Scholar]
- 138.Wong DK, Yuen MF, Ngai VW, Fung J, Lai CL. One-year entecavir or lamivudine therapy results in reduction of hepatitis B virus intrahepatic covalently closed circular DNA levels. Antivir Ther 2006;11(07):909–916 [PubMed] [Google Scholar]
- 139.Volz T, Lutgehetmann M, Wachtler P, et al. Impaired intrahepatic hepatitis B virus productivity contributes to low viremia in most HBeAg-negative patients. Gastroenterology 2007;133(03):843–852 [DOI] [PubMed] [Google Scholar]
- 140.Allweiss L, Volz T, Giersch K, et al. Proliferation of primary human hepatocytes and prevention of hepatitis B virus reinfection efficiently deplete nuclear cccDNA in vivo. Gut 2018;67(03): 542–552 [DOI] [PubMed] [Google Scholar]
- 141.Vermehren J, Park JS, Jacobson IM, Zeuzem S. Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection. J Hepatol 2018;69(05):1178–1187 [DOI] [PubMed] [Google Scholar]
- 142.Chhatwal J, Wang X, Ayer T, et al. Hepatitis C disease burden in the United States in the era of oral direct-acting antivirals. Hepatology 2016;64(05):1442–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liang TJ, Ward JW. Hepatitis C in injection-drug users—a hidden danger of the opioid epidemic. N Engl J Med 2018;378(13): 1169–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lanford RE, Bigger C, Bassett S, Klimpel G. The chimpanzee model of hepatitis C virus infections. ILAR J 2001;42(02):117–126 [DOI] [PubMed] [Google Scholar]
- 145.Scull MA, Shi C, de Jong YP, et al. Hepatitis C virus infects rhesus macaque hepatocytes and simianized mice. Hepatology 2015;62(01):57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chayama K, Hayes CN, Hiraga N, Abe H, Tsuge M, Imamura M. Animal model for study of human hepatitis viruses. J Gastroenterol Hepatol 2011;26(01):13–18 [DOI] [PubMed] [Google Scholar]
- 147.Mesalam AA, Vercauteren K, Meuleman P. Mouse systems to model hepatitis C virus treatment and associated resistance. Viruses 2016;8(06):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Umehara T, Sudoh M, Yasui F, et al. Serine palmitoyltransferase inhibitor suppresses HCV replication in a mouse model. Biochem Biophys Res Commun 2006;346(01):67–73 [DOI] [PubMed] [Google Scholar]
- 149.Hiraga N, Imamura M, Tsuge M, et al. Infection of human hepatocyte chimeric mouse with genetically engineered hepatitis C virus and its susceptibility to interferon. FEBS Lett 2007; 581 (10):1983–1987 [DOI] [PubMed] [Google Scholar]
- 150.Shi N, Hiraga N, Imamura M, et al. Combination therapies with NS5A, NS3 and NS5B inhibitors on different genotypes of hepatitis C virus in human hepatocyte chimeric mice. Gut 2013;62 (07):1055–1061 [DOI] [PubMed] [Google Scholar]
- 151.Katsume A, Tokunaga Y, Hirata Y, et al. A serine palmitoyltransferase inhibitor blocks hepatitis C virus replication in human hepatocytes. Gastroenterology 2013;145(04):865–873 [DOI] [PubMed] [Google Scholar]
- 152.Kim S, Date T, Yokokawa H, et al. Development of hepatitis C virus genotype 3a cell culture system. Hepatology 2014;60(06): 1838–1850 [DOI] [PubMed] [Google Scholar]
- 153.Wakita T, Pietschmann T, Kato T, et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med 2005;11(07):791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc Natl Acad Sci U S A 2006;103(07):2310–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lerat H, Honda M, Beard MR, et al. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology 2002;122(02): 352–365 [DOI] [PubMed] [Google Scholar]
- 156.Dorner M, Horwitz JA, Donovan BM, et al. Completion of the entire hepatitis C virus life cycle in genetically humanized mice. Nature 2013;501(7466):237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kneteman NM, Weiner AJ, O’Connell J, et al. Anti-HCV therapies in chimeric scid-Alb/uPA mice parallel outcomes in human clinical application. Hepatology 2006;43(06):1346–1353 [DOI] [PubMed] [Google Scholar]
- 158.Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet 2011;378(9785):73–85 [DOI] [PubMed] [Google Scholar]
- 159.Taylor J, Mason W, Summers J, et al. Replication of human hepatitis delta virus in primary cultures of woodchuck hepatocytes. J Virol 1987;61(09):2891–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gudima S, He Y, Chai N, et al. Primary human hepatocytes are susceptible to infection by hepatitis delta virus assembled with envelope proteins of woodchuck hepatitis virus. J Virol 2008;82 (15):7276–7283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chang J, Gudima SO, Tarn C, Nie X, Taylor JM. Development of a novel system to study hepatitis delta virus genome replication. J Virol 2005;79(13):8182–8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lütgehetmann M, Mancke LV, Volz T, et al. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology 2012; 55(03):685–694 [DOI] [PubMed] [Google Scholar]
- 163.Giersch K, Helbig M, Volz T, et al. Persistent hepatitis D virus mono-infection in humanized mice is efficiently converted by hepatitis B virus to a productive co-infection. J Hepatol 2014;60 (03):538–544 [DOI] [PubMed] [Google Scholar]
- 164.Tsuge M, Uchida T, Walsh K, et al. Early multiphasic HBV infection initiation kinetics is not clone-specific and is not affected by hepatitis D virus (HDV) infection. Viruses 2019;11(03):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Giersch K, Homs M, Volz T, et al. Both interferon alpha and lambda can reduce all intrahepatic HDV infection markers in HBV/HDV infected humanized mice. Sci Rep 2017;7(01):3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Giersch K, Allweiss L, Volz T, et al. Hepatitis delta co-infection in humanized mice leads to pronounced induction of innate immune responses in comparison to HBV mono-infection. J Hepatol 2015;63(02):346–353 [DOI] [PubMed] [Google Scholar]
- 167.Ye X, Tateno C, Thi EP, et al. Hepatitis B virus therapeutic agent ARB-1740 has inhibitory effect on hepatitis delta virus in a new dually-infected humanized mouse model. ACS Infect Dis 2019;5 (05):738–749 [DOI] [PubMed] [Google Scholar]
- 168.Jardi R, Rodriguez F, Buti M, et al. Role of hepatitis B, C, and D viruses in dual and triple infection: influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology 2001;34(02):404–410 [DOI] [PubMed] [Google Scholar]
- 169.Wu JC, Chen PJ, Kuo MY, Lee SD, Chen DS, Ting LP. Production of hepatitis delta virus and suppression of helper hepatitis B virus in a human hepatoma cell line. J Virol 1991;65(03):1099–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Blanchet M, Sureau C, Labonté P. Use of FDA approved therapeutics with hNTCP metabolic inhibitory properties to impair the HDV lifecycle. Antiviral Res 2014;106:111–115 [DOI] [PubMed] [Google Scholar]
- 171.Kaneko M, Watashi K, Kamisuki S, et al. A novel tricyclic polyketide, vanitaracin A, specifically inhibits the entry of hepatitis B and D viruses by targeting sodium taurocholate cotransporting polypeptide. J Virol 2015;89(23):11945–11953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gudima S, He Y, Meier A, et al. Assembly of hepatitis delta virus: particle characterization, including the ability to infect primary human hepatocytes. J Virol 2007;81(07):3608–3617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gouttenoire J, Moradpour D. A mouse model for hepatitis E virus infection. J Hepatol 2016;64(05):1003–1005 [DOI] [PubMed] [Google Scholar]
- 174.Hoofnagle JH, Nelson KE, Purcell RH, Hepatitis E Hepatitis E N Engl J Med 2012;367(13):1237–1244 [DOI] [PubMed] [Google Scholar]
- 175.Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010;376(9744):895–902 [DOI] [PubMed] [Google Scholar]
- 176.Patra S, Kumar A, Trivedi SS, Puri M, Sarin SK. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med 2007;147(01):28–33 [DOI] [PubMed] [Google Scholar]
- 177.Dalton HR, Keane FE, Bendall R, Mathew J, Ijaz S. Treatment of chronic hepatitis E in a patient with HIV infection. Ann Intern Med 2011;155(07):479–480 [DOI] [PubMed] [Google Scholar]
- 178.Lenggenhager D, Weber A, Hepatitis E. Hepatitis E virus and the liver: clinical settings and liver pathology. Gastroenterol Clin North Am 2017;46(02):393–407 [DOI] [PubMed] [Google Scholar]
- 179.Meng XJ. Expanding host range and cross-species infection of hepatitis E virus. PLoS Pathog 2016;12(08):e1005695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Longer CF, Denny SL, Caudill JD, et al. Experimental hepatitis E: pathogenesis in cynomolgus macaques (Macaca fascicularis). J Infect Dis 1993;168(03):602–609 [DOI] [PubMed] [Google Scholar]
- 181.Allweiss L, Gass S, Giersch K, et al. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. J Hepatol 2016;64(05):1033–1040 [DOI] [PubMed] [Google Scholar]
- 182.Peters van Ton AM, Gevers TJ, Drenth JP. Antiviral therapy in chronic hepatitis E: a systematic review. J Viral Hepat 2015;22 (12):965–973 [DOI] [PubMed] [Google Scholar]
- 183.van de Garde MDB, Pas SD, van Oord GW, et al. Interferon-alpha treatment rapidly clears hepatitis E virus infection in humanized mice. Sci Rep 2017;7(01):8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Morosan S, Hez-Deroubaix S, Lunel F, et al. Liver-stage development of Plasmodium falciparum, in a humanized mouse model. J Infect Dis 2006;193(07):996–1004 [DOI] [PubMed] [Google Scholar]
- 185.Sacci JB Jr, Alam U, Douglas D, et al. Plasmodium falciparum infection and exoerythrocytic development in mice with chimeric human livers. Int J Parasitol 2006;36(03):353–360 [DOI] [PubMed] [Google Scholar]
- 186.Vaughan AM, Mikolajczak SA, Wilson EM, et al. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J Clin Invest 2012;122(10):3618–3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Soulard V, Bosson-Vanga H, Lorthiois A, et al. Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat Commun 2015;6:7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kawahara T, Lisboa LF, Cader S, et al. Human cytomegalovirus infection in humanized liver chimeric mice. Hepatol Res 2013;43(06):679–684 [DOI] [PubMed] [Google Scholar]
- 189.Srivastava A In vivo tissue-tropism of adeno-associated viral vectors. Curr Opin Virol 2016;21:75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lisowski L, Dane AP, Chu K, et al. Selection and evaluation of clinically relevant AAV variants in a xenograft liver model. Nature 2014;506(7488):382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vercauteren K, Hoffman BE, Zolotukhin I, et al. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther 2016;24(06):1042–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Paulk NK, Pekrun K, Zhu E, et al. Bioengineered AAV capsids with combined high human liver transduction in vivo and unique humoral seroreactivity. Mol Ther 2018;26(01):289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bissig-Choisat B, Wang L, Legras X, et al. Development and rescue of human familial hypercholesterolaemia in a xenograft mouse model. Nat Commun 2015;6:7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Manos MM, Leyden WA, Murphy RC, Terrault NA, Bell BP. Limitations of conventionally derived chronic liver disease mortality rates: results of a comprehensive assessment. Hepatology 2008;47(04):1150–1157 [DOI] [PubMed] [Google Scholar]
- 195.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(01):73–84 [DOI] [PubMed] [Google Scholar]
- 196.Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 2015;12(04):231–242 [DOI] [PubMed] [Google Scholar]
- 197.Osna NA, Donohue TM Jr, Kharbanda KK. Alcoholic liver disease: pathogenesis and current management. Alcohol Res 2017;38 (02):147–161 [PMC free article] [PubMed] [Google Scholar]
- 198.Glover SC, McPhie JL, Brunt PW. Cholestasis in acute alcoholic liver disease. Lancet 1977;2(8052-8053):1305–1307 [DOI] [PubMed] [Google Scholar]
- 199.Uchida T, Kao H, Quispe-Sjogren M, Peters RL. Alcoholic foamy degeneration—a pattern of acute alcoholic injury of the liver. Gastroenterology 1983;84(04):683–692 [PubMed] [Google Scholar]
- 200.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141(05):1572–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation 2016;100(05):981–987 [DOI] [PubMed] [Google Scholar]
- 202.Tsukamoto H, Towner SJ, Ciofalo LM, French SW. Ethanol-induced liver fibrosis in rats fed high fat diet. Hepatology 1986;6 (05):814–822 [DOI] [PubMed] [Google Scholar]
- 203.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 2013;8(03):627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cho NE, Bang BR, Gurung P, et al. Retinoid regulation of antiviral innate immunity in hepatocytes. Hepatology 2016;63(06): 1783–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lu Y, Wu D, Wang X, Ward SC, Cederbaum AI. Chronic alcohol-induced liver injury and oxidant stress are decreased in cytochrome P4502E1 knockout mice and restored in humanized cytochrome P4502E1 knock-in mice. Free Radic Biol Med 2010;49(09):1406–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zakhari S, Li TK. Determinants of alcohol use and abuse: impact of quantity and frequency patterns on liver disease. Hepatology 2007;46(06):2032–2039 [DOI] [PubMed] [Google Scholar]
- 207.Cantó C, Menzies KJ, Auwerx J. NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab 2015;22(01):31–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chase JR, Poolman MG, Fell DA. Contribution of NADH increases to ethanol’s inhibition of retinol oxidation by human ADH isoforms. Alcohol Clin Exp Res 2009;33(04):571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nagy LE. The role of innate immunity in alcoholic liver disease. Alcohol Res 2015;37(02):237–250 [PMC free article] [PubMed] [Google Scholar]
- 210.Kazankov K, Jørgensen SMD, Thomsen KL, et al. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol 2019;16(03): 145–159 [DOI] [PubMed] [Google Scholar]
- 211.Jaeschke H Neutrophil-mediated tissue injury in alcoholic hepatitis. Alcohol 2002;27(01):23–27 [DOI] [PubMed] [Google Scholar]
- 212.Bautista AP. Neutrophilic infiltration in alcoholic hepatitis. Alcohol 2002;27(01):17–21 [DOI] [PubMed] [Google Scholar]
- 213.Sheron N, Bird G, Koskinas J, et al. Circulating and tissue levels of the neutrophil chemotaxin interleukin-8 are elevated in severe acute alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology 1993;18(01):41–46 [PubMed] [Google Scholar]
- 214.Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology 2014;146(05):1231–9.e1, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.DeWeerdt S Disease progression: divergent paths. Nature 2017; 551(7681):551. [DOI] [PubMed] [Google Scholar]
- 216.Lau JK, Zhang X, Yu J. Animal models of non-alcoholic fatty liver disease: current perspectives and recent advances. J Pathol 2017; 241(01):36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr 2005;135(11):2499–2502 [DOI] [PubMed] [Google Scholar]
- 218.Zhang Y, Qin C, Yang L, Lu R, Zhao X, Nie G. A comparative genomics study of carbohydrate/glucose metabolic genes: from fish to mammals. BMC Genomics 2018;19(01):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am 2008;37(04):841–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 2006;131(03):934–945 [DOI] [PubMed] [Google Scholar]
- 221.Junker AE, Gluud LL, van Hall G, Holst JJ, Knop FK, Vilsbøll T. Effects of glucagon-like peptide-1 on glucagon secretion in patients with non-alcoholic fatty liver disease. J Hepatol 2016; 64(04):908–915 [DOI] [PubMed] [Google Scholar]
- 222.Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res 2009;50(Suppl): S74–S79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Shimobayashi M, Albert V, Woelnerhanssen B, et al. Insulin resistance causes inflammation in adipose tissue. J Clin Invest 2018;128(04):1538–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012;18(03):363–374 [DOI] [PubMed] [Google Scholar]
- 225.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett 2008;582(01):97–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117(01):175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci 2015;36(07):461–470 [DOI] [PubMed] [Google Scholar]
- 228.Stefan N, Häring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol 2013;9(03):144–152 [DOI] [PubMed] [Google Scholar]
- 229.Yu Y, Liu Y, An W, et al. STING-mediated inflammation in Kupffer cells contributes to progression of nonalcoholic steatohepatitis. J Clin Invest 2019;129(02):546–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lefkowitch JH, Haythe JH, Regent N Kupffer cell aggregation and perivenular distribution in steatohepatitis. Mod Pathol 2002;15 (07):699–704 [DOI] [PubMed] [Google Scholar]
- 231.Kahraman A, Schlattjan M, Kocabayoglu P, et al. Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. Hepatology 2010;51(01):92–102 [DOI] [PubMed] [Google Scholar]
- 232.Tosello-Trampont AC, Krueger P, Narayanan S, Landes SG, Leitinger N, Hahn YS. NKp46(+) natural killer cells attenuate metabolism-induced hepatic fibrosis by regulating macrophage activation in mice. Hepatology 2016;63(03):799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Heier EC, Meier A, Julich-Haertel H, et al. Murine CD103+ dendritic cells protect against steatosis progression towards steatohepatitis. J Hepatol 2017;66(06):1241–1250 [DOI] [PubMed] [Google Scholar]
- 234.Henning JR, Graffeo CS, Rehman A, et al. Dendritic cells limit fibroinflammatory injury in nonalcoholic steatohepatitis in mice. Hepatology 2013;58(02):589–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Handa P, Kowdley KV. Dendritic cells in NASH: friend or foe? Ann Hepatol 2013;12(03):508–509 [PubMed] [Google Scholar]
- 236.Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2010;7(04):195–203 [DOI] [PubMed] [Google Scholar]
- 237.Bowen DG, Warren A, Davis T, et al. Cytokine-dependent bystander hepatitis due to intrahepatic murine CD8 T-cell activation by bone marrow-derived cells. Gastroenterology 2002;123(04):1252–1264 [DOI] [PubMed] [Google Scholar]
- 238.Sutti S,Jindal A, Bruzzì S, Locatelli I, Bozzola C, Albano E Is there a role for adaptive immunity in nonalcoholic steatohepatitis? World J Hepatol 2015;7(13):1725–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sutti S, Jindal A, Locatelli I, et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology 2014;59(03):886–897 [DOI] [PubMed] [Google Scholar]
- 240.Ma C, Kesarwala AH, Eggert T, et al. NAFLD causes selective CD4 (+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature 2016;531(7593):253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Avorn J The $2.6 billion pill—methodologic and policy considerations. N Engl J Med 2015;372(20):1877–1879 [DOI] [PubMed] [Google Scholar]
- 242.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 2004;3(08):711–715 [DOI] [PubMed] [Google Scholar]
- 243.Regev A Drug-induced liver injury and drug development: industry perspective. Semin Liver Dis 2014;34(02):227–239 [DOI] [PubMed] [Google Scholar]
- 244.Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC Med 2016;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA 2002;287(17):2215–2220 [DOI] [PubMed] [Google Scholar]
- 246.Issa NT, Wathieu H, Ojo A, Byers SW, Dakshanamurthy S. Drug metabolism in preclinical drug development: a survey of the discovery process, toxicology, and computational tools. Curr Drug Metab 2017;18(06):556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Guengerich FP. Cytochromes P450, drugs, and diseases. Mol Interv 2003;3(04):194–204 [DOI] [PubMed] [Google Scholar]
- 248.Zhang Z, Tang W. Drug metabolism in drug discovery and development. Acta Pharm Sin B 2018;8(05):721–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2006;2(06):875–894 [DOI] [PubMed] [Google Scholar]
- 250.Anderson S, Luffer-Atlas D, Knadler MP. Predicting circulating human metabolites: how good are we? Chem Res Toxicol 2009; 22(02):243–256 [DOI] [PubMed] [Google Scholar]
- 251.Sanoh S, Tayama Y, Sugihara K, Kitamura S, Ohta S. Significance of aldehyde oxidase during drug development: effects on drug metabolism, pharmacokinetics, toxicity, and efficacy. Drug Metab Pharmacokinet 2015;30(01):52–63 [DOI] [PubMed] [Google Scholar]
- 252.Bale SS, Moore L, Yarmush M, Jindal R. Emerging in vitro liver technologies for drug metabolism and inter-organ interactions. Tissue Eng Part B Rev 2016;22(05):383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Bale SS, Vernetti L, Senutovitch N, et al. In vitro platforms for evaluating liver toxicity. Exp Biol Med (Maywood) 2014;239 (09):1180–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Nishimura M, Yoshitsugu H, Yokoi T, et al. Evaluation of mRNA expression of human drug-metabolizing enzymes and transporters in chimeric mouse with humanized liver. Xenobiotica 2005;35(09):877–890 [DOI] [PubMed] [Google Scholar]
- 255.Ohtsuki S, Kawakami H, Inoue T, et al. Validation of uPA/SCID mouse with humanized liver as a human liver model: protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases by LC-MS/MS. Drug Metab Dispos 2014;42(06):1039–1043 [DOI] [PubMed] [Google Scholar]
- 256.Wilkinson GR, Shand DG. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther 1975; 18(04):377–390 [DOI] [PubMed] [Google Scholar]
- 257.Nakayama K, Ito S, Suzuki M, Takubo H, Yamazaki H, Nomura Y. Prediction of human pharmacokinetics of typical compounds by a physiologically based method using chimeric mice with humanized liver. Xenobiotica 2019;49(04):404–414 [DOI] [PubMed] [Google Scholar]
- 258.Miyamoto M, Iwasaki S, Chisaki I, et al. Prediction of human pharmacokinetics of long half-life compounds using chimeric mice with humanised liver. Xenobiotica 2019;49(12): 1379–1387 [DOI] [PubMed] [Google Scholar]
- 259.Miyamoto M, Iwasaki S, Chisaki I, Nakagawa S, Amano N, Hirabayashi H. Comparison of predictability for human pharmacokinetics parameters among monkeys, rats, and chimeric mice with humanised liver. Xenobiotica 2017;47(12):1052–1063 [DOI] [PubMed] [Google Scholar]
- 260.Katoh M, Matsui T, Nakajima M, et al. In vivo induction of human cytochrome P450 enzymes expressed in chimeric mice with humanized liver. Drug Metab Dispos 2005;33(06):754–763 [DOI] [PubMed] [Google Scholar]
- 261.Suzuki E, Koyama K, Nakai D, Goda R, Kuga H, Chiba K. Observation of clinically relevant drug interaction in chimeric mice with humanized livers: the case of valproic acid and carbapenem antibiotics. Eur J Drug Metab Pharmacokinet 2017;42(06): 965–972 [DOI] [PubMed] [Google Scholar]
- 262.Uchida M, Tajima Y, Kakuni M, et al. Organic anion-transporting polypeptide (OATP)-mediated drug-drug interaction study between rosuvastatin and cyclosporine A in chimeric mice with humanized liver. Drug Metab Dispos 2018;46(01):11–19 [DOI] [PubMed] [Google Scholar]
- 263.Foster JR, Lund G, Sapelnikova S, Tyrrell DL, Kneteman NM. Chimeric rodents with humanized liver: bridging the preclinical/clinical trial gap in ADME/toxicity studies. Xenobiotica 2014; 44(02):109–122 [DOI] [PubMed] [Google Scholar]
- 264.Naritomi Y, Sanoh S, Ohta S. Chimeric mice with humanized liver: application in drug metabolism and pharmacokinetics studies for drug discovery. Drug Metab Pharmacokinet 2018; 33(01):31–39 [DOI] [PubMed] [Google Scholar]
- 265.Hayashi PH, Chalasani NP. Increasing impact of drug-induced liver injury. Clin Liver Dis (Hoboken) 2015;5(06):136–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tragni E, Casula M, Pieri V, et al. Prevalence of the prescription of potentially interacting drugs. PLoS One 2013;8(10):e78827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gu Q, Dillon CF, Burt VL. Prescription drug use continues to increase: U.S. prescription drug data for 2007–2008. NCHS Data Brief 2010;(42):1–8 [PubMed] [Google Scholar]
- 268.Lammert C, Bjornsson E, Niklasson A, Chalasani N. Oral medications with significant hepatic metabolism at higher risk for hepatic adverse events. Hepatology 2010;51(02):615–620 [DOI] [PubMed] [Google Scholar]
- 269.Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res 2001;482(1-2):21–26 [DOI] [PubMed] [Google Scholar]
- 270.Zhou S, Chan E, Duan W, Huang M, Chen YZ. Drug bioactivation, covalent binding to target proteins and toxicity relevance. Drug Metab Rev 2005;37(01):41–213 [DOI] [PubMed] [Google Scholar]
- 271.Hinson JA, Forkert PG. Phase II enzymes and bioactivation. Can J Physiol Pharmacol 1995;73(10):1407–1413 [DOI] [PubMed] [Google Scholar]
- 272.Sanoh S, Yamachika Y, Tamura Y, et al. Assessment of amiodarone-induced phospholipidosis in chimeric mice with a humanized liver. J Toxicol Sci 2017;42(05):589–596 [DOI] [PubMed] [Google Scholar]
- 273.Barnes AJ, Baker DR, Hobby K, et al. Endogenous and xenobiotic metabolite profiling of liver extracts from SCID and chimeric humanized mice following repeated oral administration of troglitazone. Xenobiotica 2014;44(02):174–185 [DOI] [PubMed] [Google Scholar]
- 274.Foster JR, Jacobsen M, Kenna G, et al. Differential effect of troglitazone on the human bile acid transporters, MRP2 and BSEP, in the PXB hepatic chimeric mouse. Toxicol Pathol 2012;40 (08):1106–1116 [DOI] [PubMed] [Google Scholar]
- 275.Kakuni M, Morita M, Matsuo K, et al. Chimeric mice with a humanized liver as an animal model of troglitazone-induced liver injury. Toxicol Lett 2012;214(01):9–18 [DOI] [PubMed] [Google Scholar]
- 276.Metushi IG, Sanders C, Lee WM, Uetrecht J; Acute Liver Study Group. Detection of anti-isoniazid and anti-cytochrome P450 antibodies in patients with isoniazid-induced liver failure. Hepatology 2014;59(03):1084–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jennings JJ, Mandaliya R, Nakshabandi A, Lewis JH. Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol 2019;15(03):231–244 [DOI] [PubMed] [Google Scholar]
- 278.García Aparicio AM, Rey JR, Sanz AH, Alvarez JS. Successful treatment with etanercept in a patient with hepatotoxicity closely related to infliximab. Clin Rheumatol 2007;26(05):811–813 [DOI] [PubMed] [Google Scholar]
- 279.von Karstedt S, Montinaro A, Walczak H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat Rev Cancer 2017;17(06):352–366 [DOI] [PubMed] [Google Scholar]
- 280.Nihira K, Nan-Ya KI, Kakuni M, et al. Chimeric mice with humanized livers demonstrate human-specific hepatotoxicity caused by a therapeutic antibody against TRAIL-receptor 2/death receptor 5. Toxicol Sci 2019;167(01):190–201 [DOI] [PubMed] [Google Scholar]
- 281.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 1981;78(12):7634–7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126(04):663–676 [DOI] [PubMed] [Google Scholar]
- 283.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981;292(5819):154–156 [DOI] [PubMed] [Google Scholar]
- 284.Gao Y, Zhang X, Zhang L, et al. Distinct gene expression and epigenetic signatures in hepatocyte-like cells produced by different strategies from the same donor. Stem Cell Reports 2017;9 (06):1813–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Song W, Lu YC, Frankel AS, An D, Schwartz RE, Ma M. Engraftment of human induced pluripotent stem cell-derived hepatocytes in immunocompetent mice via 3D co-aggregation and encapsulation. Sci Rep 2015;5:16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Sakurai F, Mitani S, Yamamoto T, et al. Human induced-pluripotent stem cell-derived hepatocyte-like cells as an in vitro model of human hepatitis B virus infection. Sci Rep 2017;7:45698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kondo Y, Iwao T, Nakamura K, et al. An efficient method for differentiation of human induced pluripotent stem cells into hepatocyte-like cells retaining drug metabolizing activity. Drug Metab Pharmacokinet 2014;29(03):237–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Nagamoto Y, Takayama K, Tashiro K, et al. Efficient engraftment of human induced pluripotent stem cell-derived hepatocyte-like cells in uPA/SCID mice by overexpression of FNK, a Bcl-xL mutant gene. Cell Transplant 2015;24(06):1127–1138 [DOI] [PubMed] [Google Scholar]
- 289.Carpentier A, Tesfaye A, Chu V, et al. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. J Clin Invest 2014;124(11):4953–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Katsuda T, Kawamata M, Hagiwara K, et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell 2017;20 (01):41–55 [DOI] [PubMed] [Google Scholar]
- 291.Katsuda T, Matsuzaki J, Yamaguchi T, et al. Generation of human hepatic progenitor cells with regenerative and metabolic capacities from primary hepatocytes. eLife 2019;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Gramignoli R, Tahan V, Dorko K, et al. New potential cell source for hepatocyte transplantation: discarded livers from metabolic disease liver transplants. Stem Cell Res (Amst) 2013;11(01): 563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Srinivasan RC, Zabulica M, Hammarstedt C, et al. A liver-humanized mouse model of carbamoyl phosphate synthetase 1-deficiency. J Inherit Metab Dis 2019;42(06):1054–1063 [DOI] [PubMed] [Google Scholar]
- 294.Varghese MJ. Familial hypercholesterolemia: a review. Ann Pediatr Cardiol 2014;7(02):107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 1993;92(02):883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mansoorian M, Kazemi K, Nikeghbalian S, et al. Liver transplantation as a definitive treatment for familial hypercholesterolemia: a series of 36 cases. Pediatr Transplant 2015;19(06): 605–611 [DOI] [PubMed] [Google Scholar]
- 297.Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol 2016;65(03): 608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Koui Y, Kido T, Ito T, et al. An in vitro human liver model by iPSC-derived parenchymal and non-parenchymal cells. Stem Cell Reports 2017;9(02):490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wilson EM, Bial J, Tarlow B, et al. Extensive double humanization of both liver and hematopoiesis in FRGN mice. Stem Cell Res (Amst) 2014;13(3 Pt A):404–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Strick-Marchand H, Dusséaux M, Darche S, et al. A novel mouse model for stable engraftment of a human immune system and human hepatocytes. PLoS One 2015;10(03):e0119820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Brezillon NM, DaSilva L, L’Hôte D, et al. Rescue of fertility in homozygous mice for the urokinase plasminogen activator transgene by the transplantation of mouse hepatocytes. Cell Transplant 2008;17(07):803–812 [DOI] [PubMed] [Google Scholar]
- 302.Bissig KD, Grompe M. Response to “Can ‘humanized’ mice improve drug development in the 21st century?” Trends Pharmacol Sci 2013;34(08):425. [DOI] [PubMed] [Google Scholar]
- 303.Braun RE, Lo D, Pinkert CA, et al. Infertility in male transgenic mice: disruption of sperm development by HSV-tk expression in postmeiotic germ cells. Biol Reprod 1990;43(04):684–693 [DOI] [PubMed] [Google Scholar]