Abstract
Water-related opportunistic pathogens (OPs) are a leading cause of drinking-water-related disease outbreaks, especially in developed countries such as the United States (US). Physicochemical water quality parameters, especially disinfectant residuals, control the (re)growth, presence, colonization, and concentrations of OPs in drinking water distribution systems (DWDSs), while the relationship between OPs and those parameters remain unclear. This study aimed to quantify how physicochemical parameters, mainly monochloramine residual concentration, hydraulic residence time (HRT), and seasonality, affected the occurrence and concentrations of four common OPs (Legionella, Mycobacterium, Pseudomonas, and Vermamoeba vermiformis) in four full-scale DWDSs in the US. Legionella as a dominant OP occurred in 93.8% of the 64 sampling events and had a mean density of 4.27 × 105 genome copies per liter. Legionella positively correlated with Mycobacterium, Pseudomonas, and total bacteria. Multiple regression with data from the four DWDSs showed that Legionella had significant correlations with total chlorine residual level, free ammonia concentration, and trihalomethane concentration. Therefore, Legionella is a promising indicator of water-related OPs, reflecting microbial water quality in chloraminated DWDSs. The OP concentrations had strong seasonal variations and peaked in winter and/or spring possibly because of reduced water usage (i.e., increased water stagnation or HRT) during cold seasons. The OP concentrations generally increased with HRT presumably because of disinfectant residual decay, indicating the importance of well-maintaining disinfectant residuals in DWDSs for OP control. The concentrations of Mycobacterium, Pseudomonas, and V. vermiformis were significantly associated with total chlorine residual concentration, free ammonia concentration, and pH and trihalomethane concentration, respectively. Overall, this study demonstrates how the significant spatiotemporal variations of OP concentrations in chloraminated DWDSs correlated with physicochemical water quality parameters such as disinfectant residual levels. This work also indicates that Legionella is a promising indicator of OPs and microbial water quality in chloraminated DWDSs.
Keywords: Mycobacterium, Pseudomonas, Spatiotemporal variations, Disinfectant residuals, Monochloramine, Indicator
Graphical Abstract
1. Introduction
Water utilities in the United States (US) are required to maintain “detectable” levels of disinfectant residuals throughout drinking water distribution systems (DWDSs) as a critical barrier to suppress microbial (re)growth (Clark et al., 1995; McGuire, 2006; US EPA, 2019b; Waak et al., 2018; Zhang and Lu, 2021b; Zhang and Liu, 2019). However, this barrier is often ineffective or insufficient to ensure microbial drinking water quality and protect the health of end-consumers (Payment, 1999; Propato and Uber, 2004; Silvestry-Rodriguez et al., 2008; Zhang et al., 2019). For instance, opportunistic pathogens (OPs) (re)grow and frequently occur at high concentrations in municipal water systems despite the presence of disinfectant residuals (Lu et al., 2016; Ma et al., 2020a; Wang et al., 2012b; Whiley et al., 2014a; Zhang and Lu, 2021b). The (re)growth is due to favorable conditions in water systems (i.e., disinfectant residual decay, low levels of organic matter, water stagnation, and protection resulting from biofilms and amoebae) and high disinfectant resistance of OPs (Falkinham et al., 2015a; Jjemba et al., 2010; Liu et al., 2013).
OPs, mainly Legionella (e.g., L. pneumophila) and Mycobacterium (e.g., non-tuberculosis mycobacteria or NTM), are natural or normal inhabitants rather than contaminants in municipal water systems (Falkinham, 2020). The frequent occurrence of those OPs in water threatens the health of immunocompromised and immunocompetent people (Falkinham, 2015; Lu et al., 2017; Perrin et al., 2019; Wang et al., 2012b). For instance, Legionella, especially L. pneumophila, is the leading cause of drinking-water-related disease (e.g., Legionnaires’ disease or legionellosis) outbreaks, especially in developed countries such as the US (Baron et al., 2020; Donohue et al., 2019a; Falkinham et al., 2015b; Smith et al., 2019). In the US, Legionella annually caused at least ten waterborne disease outbreaks, 50 illnesses, 45 hospitalizations, and six fatalities from 2011 to 2014 (Beer et al., 2015; Benedict et al., 2017). In addition, the annual crude incidence rate of Legionnaires’ disease per million residences in the US increased dramatically from 4.2 in 2000 to 18.9 in 2015 (Shah et al., 2018). Therefore, closely monitoring the dynamics of OPs such as Legionella and controlling their (re)growth in DWDSs are critical to ensuring drinking water quality and protecting public health.
Previous studies have well documented the occurrence and spatiotemporal variations of OPs in building premise plumbing systems (PPSs) (Donohue et al., 2019a; Haig et al., 2020; Huo et al., 2021; Isaac and Sherchan, 2020; Leoni et al., 2005; Lu et al., 2017; Serrano-Suárez et al., 2013; Sharaby et al., 2019; Tang et al., 2020; Wang et al., 2012a; Wolf-Baca and Siedlecka, 2020; Zhang et al., 2021). Although PPSs and DWDSs are directly connected and are both indispensable components of municipal water systems, microbial growth conditions are distinct in those two components. For instance, PPSs have smaller pipe diameters and greater surface-to-volume ratios than the water mains of DWDSs. DWDSs and PPSs also have different water ages, water temperatures, water velocities, disinfectant residual concentrations, and biofilm conditions. Therefore, OP dynamics in PPSs and DWDSs are distinct, and the spatiotemporal variations of OPs in PPSs do not represent those in DWDSs. To distinguish the contributions of DWDSs and PSSs to OP prevalence in drinking water, one must also closely monitor OP dynamics in DWDSs. Understanding the complete “life-course” of OPs in municipal water systems (i.e., from distribution system entry points to end taps/showerheads) allows the development of more effective OP control strategies to better protect public health.
Studies on OPs in DWDSs in Australia (Kaestli et al., 2019; Thomson et al., 2013; Whiley et al., 2014a), China (Tang et al., 2021), France (Perrin et al., 2019), Leeward Antilles (Valster et al., 2011), and the US (LeChevallier, 2019a; b; Lu et al., 2016; Lu et al., 2015; Pryor et al., 2004; Qin et al., 2017) are available but limited. Because accessing drinking water in full-scale DWDSs is restricted (especially in the US for privacy and regulation reasons), the profiles of OPs and how OPs correlate with physicochemical water quality parameters in distribution systems are unclear. For instance, Pryor et al. (2004) assessed the presence/absence and speciation but not the concentrations of Legionella and Mycobacterium in a US DWDS. Two recent studies reported the concentrations of L. pneumophila in several US DWDSs but did not quantify other important OPs (LeChevallier, 2019a; b). Other studies determined the concentrations of multiple OPs in DWDSs with a low sampling frequency or without measuring physicochemical water quality parameters. For example, in two studies assessing the occurrence of OPs, single-time grab samples were collected from storage tanks in DWDSs throughout the US (i.e., one-time sampling from each tank during its cleaning), and the spatiotemporal variations of OP concentrations were unable to be measured (Lu et al., 2015; Qin et al., 2017). A parallel study monitored dominant OPs in US DWDSs but did not determine the relationship between OPs and physicochemical water quality parameters (Lu et al., 2016).
More comprehensive studies on OP dynamics in DWDSs and the correlations between OPs and physicochemical water quality parameters, such as disinfectant residuals, hydraulic residence time (HRT), disinfection by-products (DBPs), and seasonality, are highly needed. Understanding the correlations between OPs and such physicochemical parameters in DWDSs is essential to evaluating the efficacies of disinfectant residuals and developing effective OP control strategies to ensure drinking water quality and protect public health. For instance, “detectable” levels of disinfectant residuals in DWDSs are a critical barrier preventing OP (re)growth but are often ineffective partially because of the strong disinfectant resistance of OPs (Falkinham, 2020; Zhang and Lu, 2021b). With more information on the correlation between OP concentrations and disinfectant residual levels, we may update disinfectant residual maintenance guidance to more effectively prevent OP (re)growth. Such information could better guide water utilities to boost disinfectant residual levels at points/regions in DWDSs where OP concentrations exceed certain levels or during seasons/periods when OP concentrations peak (i.e., OP episodes), thereby enhancing the disinfection barrier and ensuring microbial drinking water quality (Cope et al., 2019; LeChevallier, 1990; Lytle et al., 2021).
This study aimed to determine the spatiotemporal variations of OPs in four representative full-scale chloraminated DWDSs (CDWDSs) in the US. Our parallel study determined the spatiotemporal variations of multiple physicochemical water quality parameters in those systems (Abulikemu et al., 2021). We focused on assessing the correlations between OP concentrations and critical physicochemical water quality parameters, especially disinfectant residual concentrations, HRT, and seasonality. The main hypothesis was that disinfectant residual concentrations would correlate with OP concentrations so that disinfectant residual decay would promote OP (re)growth. A second hypothesis was that OP concentrations were greater in warm seasons. To test these hypotheses, we sampled water in four consecutive seasons in 2017 (winter) and 2018 (spring, summer, and fall) from four sites in each CDWDS. We then assessed how the concentrations of dominant OPs (Legionella, Mycobacterium, Pseudomonas, and Vermamoeba vermiformis) correlated with critical physicochemical water quality parameters, focusing on the correlation between regulated levels of disinfectant residuals and OP concentrations.
This study is novel for three reasons. First, this study comprehensively assessed the spatiotemporal variations of OPs in four full-scale CDWDSs. A study determining and comparing the dynamics of OPs in multiple full-scale distribution systems does not exist. To the best of our knowledge, this is the first large-scale, multi-DWDS study conducted in the US that assessed the dynamics of multiple OPs. Second, this study correlated the dynamics of OPs with multiple physicochemical water quality parameters, which was missing in previous studies. This study also assessed the correlations among OPs. Third, on the basis of the correlations among OPs and between OPs and physicochemical water quality parameters, this work proved that Legionella is a promising indicator of OPs and microbial water quality in CDWDSs (Zhang and Lu, 2021a). This is a pioneering work proposing and validating a non-conventional indicator (i.e., Legionella) that is suitable to indicate or infer microbial drinking water quality in municipal engineered water systems.
2. Materials and methods
2.1. Drinking water sampling and processing
Abulikemu et al. (2021) presents detailed information on the four water utilities and the corresponding CDWDSs. Briefly, the source water for utility GW is groundwater, while the source water for the other utilities (SW1, SW2, and SW3) is surface water. Table S1A shows the treatment processes, water production capacities, and populations served for the four water utilities. Each utility doses monochloramine at the Entry Point (EP) of its distribution system for secondary disinfection. In each CDWDS, four sampling sites were selected, covering different HRTs (Table S1B). For SW1, SW2, or SW3, the four sites are an EP representing the minimum HRT (plant effluent or finished water); a disinfectant residual monitoring site representing the average HRT (ART); a drinking water storage tank (Tank); and an end site representing the maximum HRT (MRT). Because GW has no water storage tank in its distribution system, samples were taken from EP, two sites representing the average HRT (ART1 and ART2), and MRT.
Drinking water was sampled aseptically from each sampling site (three minutes flushing before sampling) in December 2017, March 2018, June 2018, and September 2018 (64 sampling events in total). Abulikemu et al. (2021) describes the measurements of physicochemical water quality parameters [pH, water temperature, and concentrations of total chlorine residual, monochloramine residual, free ammonia, nitrite, four trihalomethanes (THMs), and nine haloacetic acids (HAAs)]. For each sampling event, two biological replicates (1 L for each replicate; 128 samples in total) were separately collected in high-density polyethylene bottles and shipped overnight on ice to our laboratory for biological analysis. We stored the samples at 4 °C immediately after receiving them and then processed them for DNA isolation. The total holding time of the water samples under cooling conditions was less than 24 h (US EPA, 2016).
2.2. DNA isolation and quantitative polymerase chain reactions (qPCRs)
We isolated total genomic DNA from each water sample (i.e., each biological replicate) (Text S1) and subsequently quantified four OPs (Legionella, Mycobacterium, Pseudomonas, and V. vermiformis) and total bacteria in each sample via qPCRs following established protocols (Lu et al., 2017; Lu et al., 2016) (Text S2). Tables S2A and S3 list the details of the qPCRs. The four individual OPs are collectively denoted by “total OPs” hereinafter. We used both original (i.e., undiluted) and ten-fold diluted DNA extracts of each water sample as qPCR templates to detect potential qPCR inhibition and manual operational errors. The OP concentrations in the drinking water samples are expressed in GCN·L−1 (GCN: gene copy number or genome copy number). In addition, we quantified L. pneumophila in all water samples and Naegleria fowleri in the samples of December 2017 and March 2018 using qPCRs. The concentrations of L. pneumophila and N. fowleri in all tested samples were below the limits of detection (LOD), suggesting that they could be absent from the four CDWDSs. The occurrence of N. fowleri in the CDWDSs is not discussed hereinafter.
2.3. PCR amplicon sequencing
We identified the speciation of Legionella for 20 sampling events following an established protocol (Lu et al., 2017; Lu et al., 2008; Lu et al., 2015) (Table S2B). Those 20 sampling events included the four sampling events for GW in December 2017 and all sampling events for the four CDWDSs in March 2018. For each sampling event, we mixed the two DNA extracts from the two biological replicates (i.e., the two drinking water samples) and then used the mixture as the template in the PCR to amplify a fraction of the 16S rRNA gene of Legionella (Table S2C). The PCR amplicons were inserted into Invitrogen™ pCR™4 TOPO® TA Vectors (Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA). We then randomly selected 12 individual clones for each sampling event and Sanger-sequenced them using a BigDye™ Terminator Cycle Sequencing Kit on a 3730xl DNA Analyzer [both from Applied Biosystems™ (Thermo Fisher Scientific Inc.), Waltham, Massachusetts, USA]. We processed the raw sequences using a Sequencher® 5.2.4 DNA Sequence Analysis Software (Gene Codes Corporation, Ann Arbor, Michigan, USA) for homology searching and comparison analysis. We further identified and removed chimeric sequences using Bellerophon (Huber et al., 2004). Most clone sequences were Legionella-positive sequences. We aligned the positive sequences using Clustal W (Larkin et al., 2007; Thompson et al., 2003; Thompson et al., 1994). We finally generated unrooted neighbor-joining dendrograms using MEGA X (Molecular Evolutionary Genetics Analysis) (Adzhubei et al., 2010; Kumar et al., 2018) to compare those PCR-amplified sequences with reference Legionella species or sequences. Representative partial 16S rRNA gene sequences (PCR amplicons) from the Legionella clone libraries are available in GenBank (MW524842 to MW524859).
2.4. Data filtering, analysis, and reporting
The qPCR datapoints with significant PCR inhibition or other issues were removed (data filtering) (Text S3). The filtered qPCR data have multiple datapoints that are below the LOD or undetected. We used a reasonable strategy to estimate those datapoints when plotting data and performing statistical analysis (Table S4). Unless specified otherwise, we used another reasonable strategy to estimate the physicochemical water quality datapoints (i.e., monochloramine residual concentration) that are below the LOD for data plotting and statistical analysis (Table S4). The arithmetic mean (AM), arithmetic sample standard deviation (SD), median, geometric mean (GM), and geometric standard deviation (GSD) for each qPCR target at each sampling event were calculated and reported (Bohidar, 1991; Dodge, 2008; Habib, 2012) (Table S5).
We used Microsoft Office 365’s ProPlus Excel® (version 1908, Microsoft Corporation, Redmond, Washington, USA) to manage data and plot figures. We used SPSS® Statistics (version 26.0, The International Business Machines Corporation, Armonk, New York, USA) for statistical analysis (significance level 5%). We used an Excel® add-on XLSTAT (version 2020.2.3) (Addinsoft, 2020) and PAleontological STatistics (PAST, version 4.02) (Hammer et al., 2001) in parallel but independently to conduct a canonical correspondence analysis (CCA).
3. Results and Discussion
3.1. The occurrence of OPs in the CDWDSs
We quantified four common OPs (Legionella, Mycobacterium, Pseudomonas, and V. vermiformis) in the four CDWDSs using qPCRs. The major OPs in the bacterial genera Legionella, Mycobacterium, and Pseudomonas are L. pneumophila, NTM, and P. aeruginosa, respectively (Falkinham, 2015; Wang et al., 2017). However, we view the whole genera Legionella, Mycobacterium, and Pseudomonas to be OPs for three reasons. First, other than L. pneumophila and P. aeruginosa, other species in Legionella and Pseudomonas (such as L. micdadei, L. bozemanii, L. londiniensis, L. longbeachae, L. anisa, P. fluorescens, and P. stutzer) could be OPs and infect humans (Donnarumma et al., 2010; Lachant and Prasad, 2015; Lau and Ashbolt, 2009; Madi et al., 2010a; Madi et al., 2010b; Muder and Victor, 2002; Picot et al., 2001; Scales et al., 2014; Stallworth et al., 2012; Vaz-Moreira et al., 2012; Yu et al., 2002). Those additional species also appear in drinking-water-related settings such as distribution systems and may cause disease outbreaks (Brunkard et al., 2011; Craun et al., 2010; Dennis et al., 1993; Dilger et al., 2018; Papapetropoulou et al., 1994; Penna et al., 2002; Salinas et al., 2021; Thomas et al., 2008; Vaz-Moreira et al., 2012; Wullings and Van Der Kooij, 2006). On the other hand, our current understanding of the pathogenicity and speciation of Legionella and Pseudomonas is incomplete. Both the total number of species and the number of (opportunistically) pathogenic species in Legionella are increasing over time (De Giglio et al., 2019; Dennis et al., 1993; Fields, 1996; Gomes et al., 2018; Lesnik et al., 2016; Mercante and Winchell, 2015; Muder and Victor, 2002; Pascale et al., 2020; Pearce et al., 2012; Wu et al., 2019). New Legionella species are been constantly isolated and identified (De Giglio et al., 2019). In addition, new (opportunistically) pathogenic species in Legionella continue to be identified, and certain Legionella species that are not considered to be (opportunistically) pathogenic could be determined to be so in newer studies. For instance, L. londiniensis had been believed to have no implications in human infections since its first isolation/identification in the 1980s (Dennis et al., 1993). However, a study later on found that L. londiniensis is an OP and can cause Legionnaires’ disease in humans (Stallworth et al., 2012). L. londiniensis was isolated from municipal water systems (Dilger et al., 2018). Another example is P. fluorescens. P. fluorescens was historically considered to be nonpathogenic to humans, while modern studies reveal that this species can cause opportunistic infections in humans and acts as an OP (Donnarumma et al., 2010; Madi et al., 2010a; Madi et al., 2010b; Picot et al., 2001; Scales et al., 2014). Therefore, assessing microbial drinking water quality by detecting only the dominant opportunistically pathogenic species in Legionella and Pseudomonas (i.e., L. pneumophila and P. aeruginosa) could underestimate the health risks of drinking water. To be more inclusive and assess all Legionella and Pseudomonas species that may infect humans, we view Legionella and Pseudomonas to be OPs.
Second, Legionella is the key focus and the most important genus in the current study. L. pneumophila is the leading disease-causing species in Legionella which causes over 80% of Legionella infections (Amemura-Maekawa et al., 2018; Hornei et al., 2007; Reingold et al., 1984; Stout and Yu, 1997; Yu et al., 2002). However, most or even all species in Legionella are potential OPs to humans (Borella et al., 2005; Fields, 1996; Lesnik et al., 2016; Mercante and Winchell, 2015; Muder and Victor, 2002). Therefore, considering the genus Legionella to be an OP in drinking water is valid.
Third, in the current literature, it is common to view the whole genera Legionella, Pseudomonas, and Mycobacterium to be OPs (Allen et al., 2004; Buse et al., 2020; Cullom et al., 2020; Edrees and Al-Awar, 2020; Falkinham et al., 2015a; Keenum et al., 2021; Lytle et al., 2021; Ma et al., 2020b; Pryor et al., 2004; Scaturro et al., 2020; Senbadejo, 2017; Shen et al., 2019; Whiley, 2017; Whiley et al., 2014b; Wilson, 2019). As a result, we define Legionella, Pseudomonas, and Mycobacterium to be OPs and used genus-specific primers to quantify them in the current study (Table S2A).
V. vermiformis (née Hartmanella vermiformis; a protist) is a free-living amoeba inhabiting both natural and engineered aquatic environments including DWDSs (Delafont et al., 2019; Delafont et al., 2018; Lu et al., 2017; Lu et al., 2016; Qin et al., 2017). V. vermiformis is an important host and/or vehicle for bacteria including OPs and itself could be pathogenic and an etiological agent of human infections (Abdul Majid et al., 2017; Buse et al., 2020; Delafont et al., 2018; Kennedy et al., 1995; Molmeret et al., 2005; Park, 2016; Scheid et al., 2019; Siddiqui et al., 2021; van der Loo et al., 2021; Wang et al., 2019).
The high frequencies of detection (FOD) of Legionella (93.8%) and Mycobacterium (89.1%) indicate their persistence in the CDWDSs (Table 1A). By contrast, the much lower FOD of Pseudomonas (57.8%) and V. vermiformis (31.3%) suggest their sporadic occurrence (Donohue et al., 2019a). Previous studies similarly found that Legionella (or L. pneumophila) and Mycobacterium occurred more frequently than P. aeruginosa and V. vermiformis in DWDSs in the US (Lu et al., 2016; Qin et al., 2017) and France (Perrin et al., 2019).
Table 1.
Table 1A. Occurrence of Legionella, Mycobacterium, Pseudomonas, and V. vermiformis in the four CDWDSs.
Table 1B. Occurrence of Legionella, Mycobacterium, Pseudomonas, and V. vermiformis in different seasons
Table 1C. Occurrence of Legionella, Mycobacterium, Pseudomonas, and V. vermiformis at different sampling sites
| OP | SW11 | SW21 | SW31 | GW1 | All CDWDSs2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| F (%) | n | F (%) | n | F (%) | n | F (%) | n | F (%) | n | |
| Legionella | 87.5 | 14 | 100.0 | 16 | 87.5 | 14 | 100.0 | 16 | 93.8 | 60 |
| Mycobacterium | 100.0 | 16 | 100.0 | 16 | 81.3 | 13 | 75.0 | 12 | 89.1 | 57 |
| Pseudomonas | 6.3 | 1 | 75.0 | 12 | 62.5 | 10 | 87.5 | 14 | 57.8 | 37 |
| V. vermiformis | 31.3 | 5 | 43.8 | 7 | 37.5 | 6 | 12.5 | 2 | 31.3 | 20 |
| OP | December 20171 | March 20181 | June 20181 | September 20181 | ||||
|---|---|---|---|---|---|---|---|---|
| F (%) | n | F (%) | n | F (%) | n | F (%) | n | |
| Legionella | 100.0 | 16 | 100.0 | 16 | 87.5 | 14 | 87.5 | 14 |
| Mycobacterium | 87.5 | 14 | 93.8 | 15 | 87.5 | 14 | 87.5 | 14 |
| Pseudomonas | 43.8 | 7 | 56.3 | 9 | 50.0 | 8 | 81.3 | 13 |
| V. vermiformis | 75.0 | 12 | 0.0 | 0 | 18.8 | 3 | 31.3 | 5 |
| OP | EP1 | ART3 | Tank4 | MRT1 | ||||
|---|---|---|---|---|---|---|---|---|
| F (%) | n | F (%) | n | F (%) | n | F (%) | n | |
| Legionella | 75.0 | 12 | 100.0 | 20 | 100.0 | 12 | 100.0 | 16 |
| Mycobacterium | 56.3 | 9 | 100.0 | 20 | 100.0 | 12 | 100.0 | 16 |
| Pseudomonas | 37.5 | 6 | 75.0 | 15 | 50.0 | 6 | 62.5 | 10 |
| V. vermiformis | 12.5 | 2 | 35.0 | 7 | 50.0 | 6 | 31.3 | 5 |
F: Frequency of detection. n: Number of sampling events where an OP was detected (i.e., number of positive sampling events). A sampling event was positive for a target OP if the concentration of that OP was above the LOD in at least one of the four DNA samples (Tables S4 and S6).
Number of sampling events: 16.
Number of sampling events: 64.
Number of sampling events: 20.
Number of sampling events: 12.
Although Pseudomonas occurred less frequently than Legionella and Mycobacterium, its maximum concentration among individual sampling events (8.75 × 106 GCN·L−1) was comparable to that of Legionella (9.01 × 106 GCN·L−1) and greater than that of Mycobacterium (1.55 × 105 GCN·L−1) (Table S6 and Figure 1). In addition, the overall AM of Pseudomonas concentrations for the four CDWDSs (2.69 × 105 GCN·L−1) was comparable to that for Legionella (4.27 × 105 GCN·L−1) and greater than that for Mycobacterium (1.67 × 104 GCN·L−1) (Table S7A). Therefore, the occurrence of Pseudomonas had a unique pattern: Pseudomonas occurred in only approximately 60% of the sampling events (FOD 57.8%), but when it appeared, its concentration was relatively high, indicating possible release from pipe biofilms. Similar to previous studies (Lu et al., 2016; Lu et al., 2015; Qin et al., 2017), V. vermiformis occurred much less frequently and had a much lower concentration than the three bacterial OPs. The FOD for V. vermiformis was only approximately 30% (Table 1A), and its overall AM (5.66 × 102 GCN·L−1) (Table S7A) and overall GM (3.66 × 101 GCN·L−1) (Table S8A) for the four CDWDSs were much lower than those of the three bacterial OPs.
Figure 1.
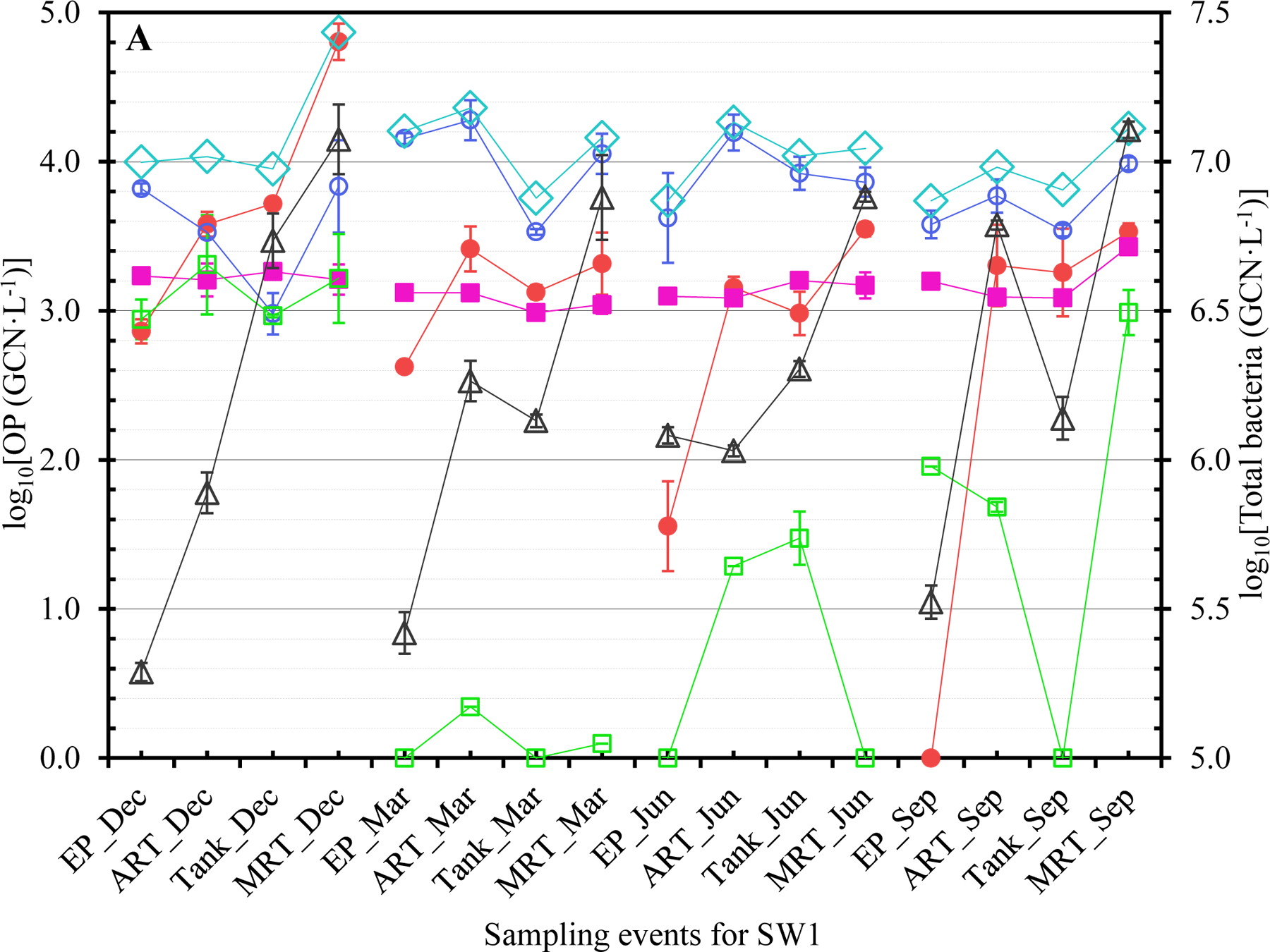
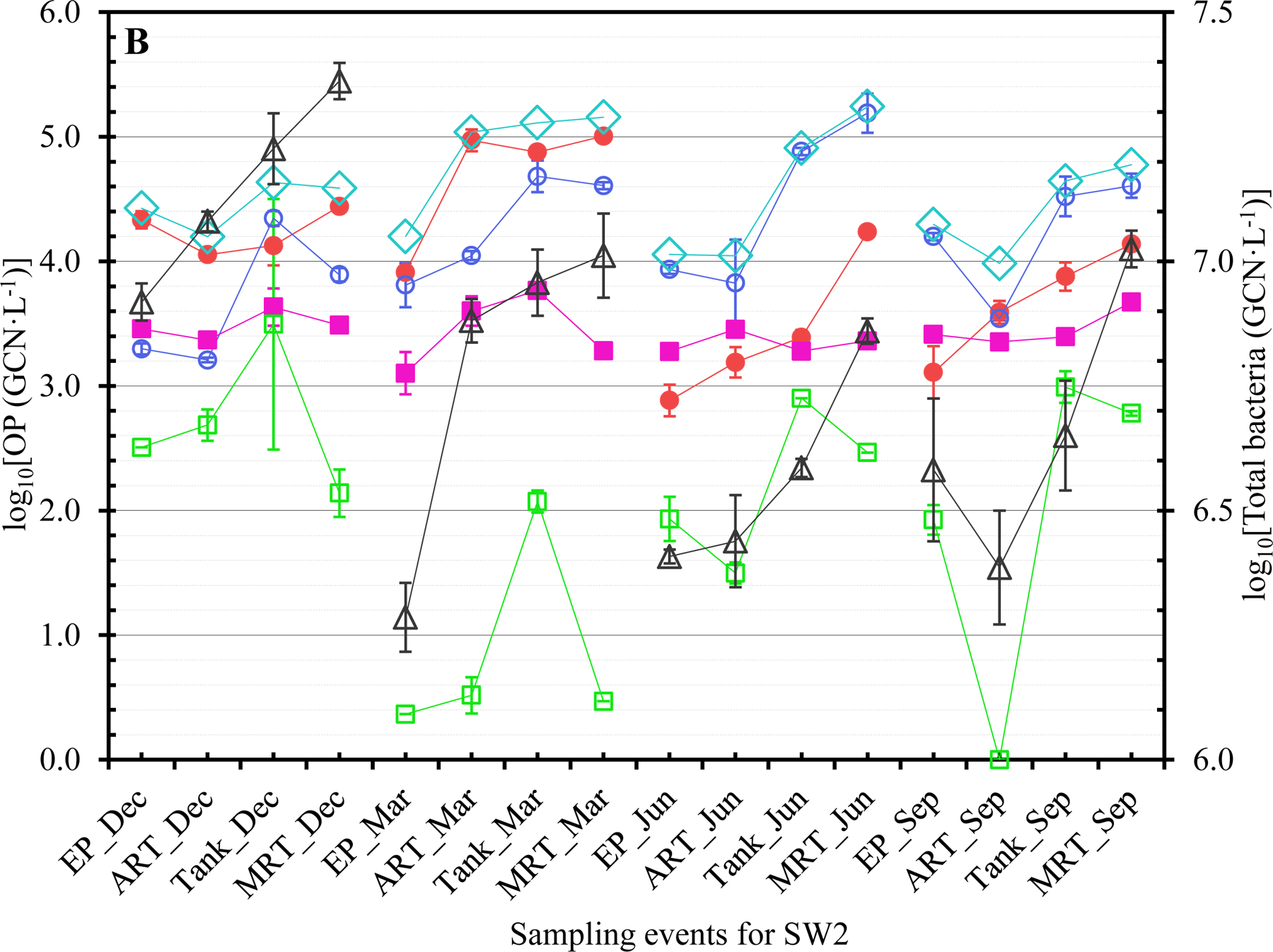
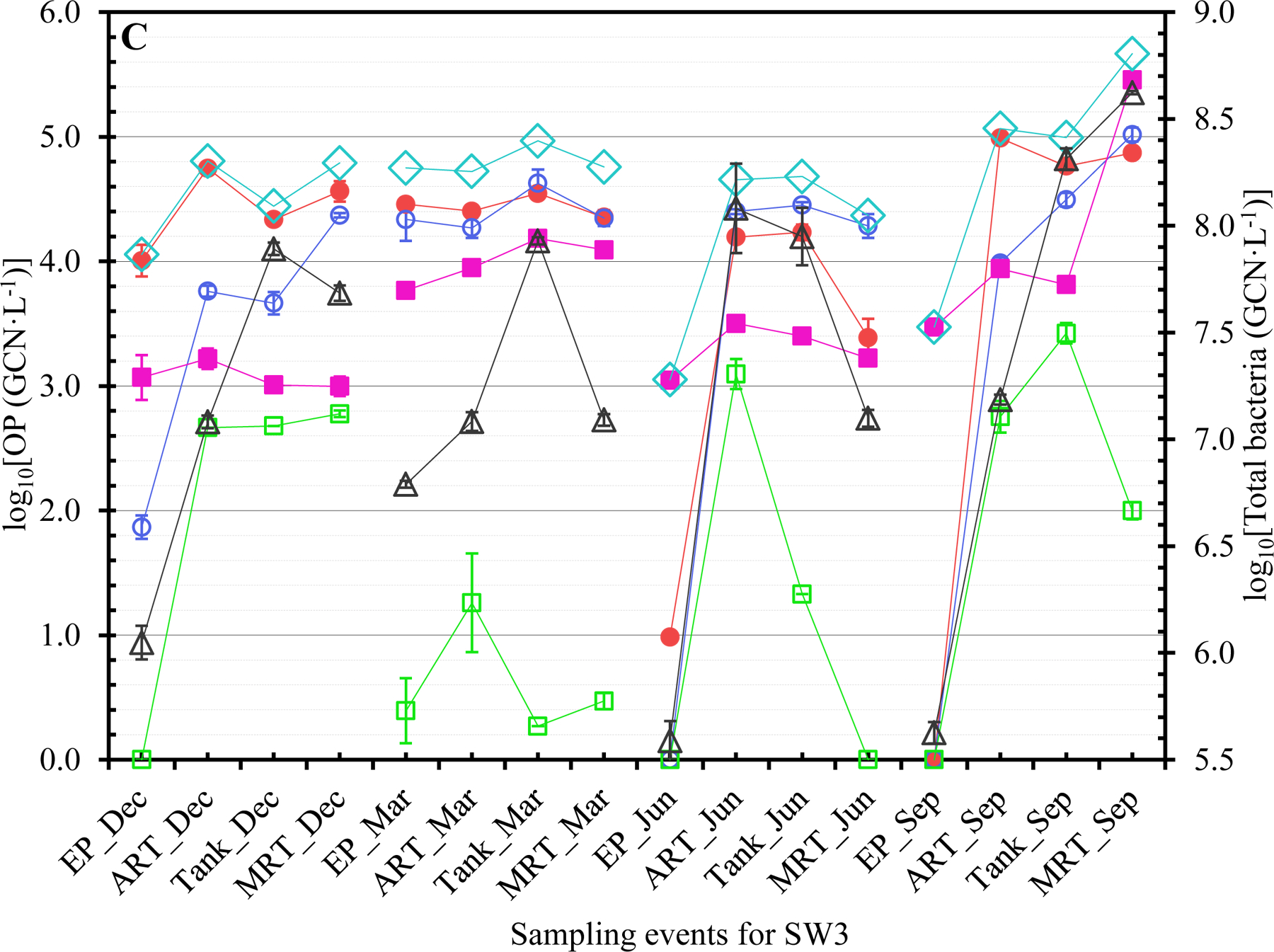
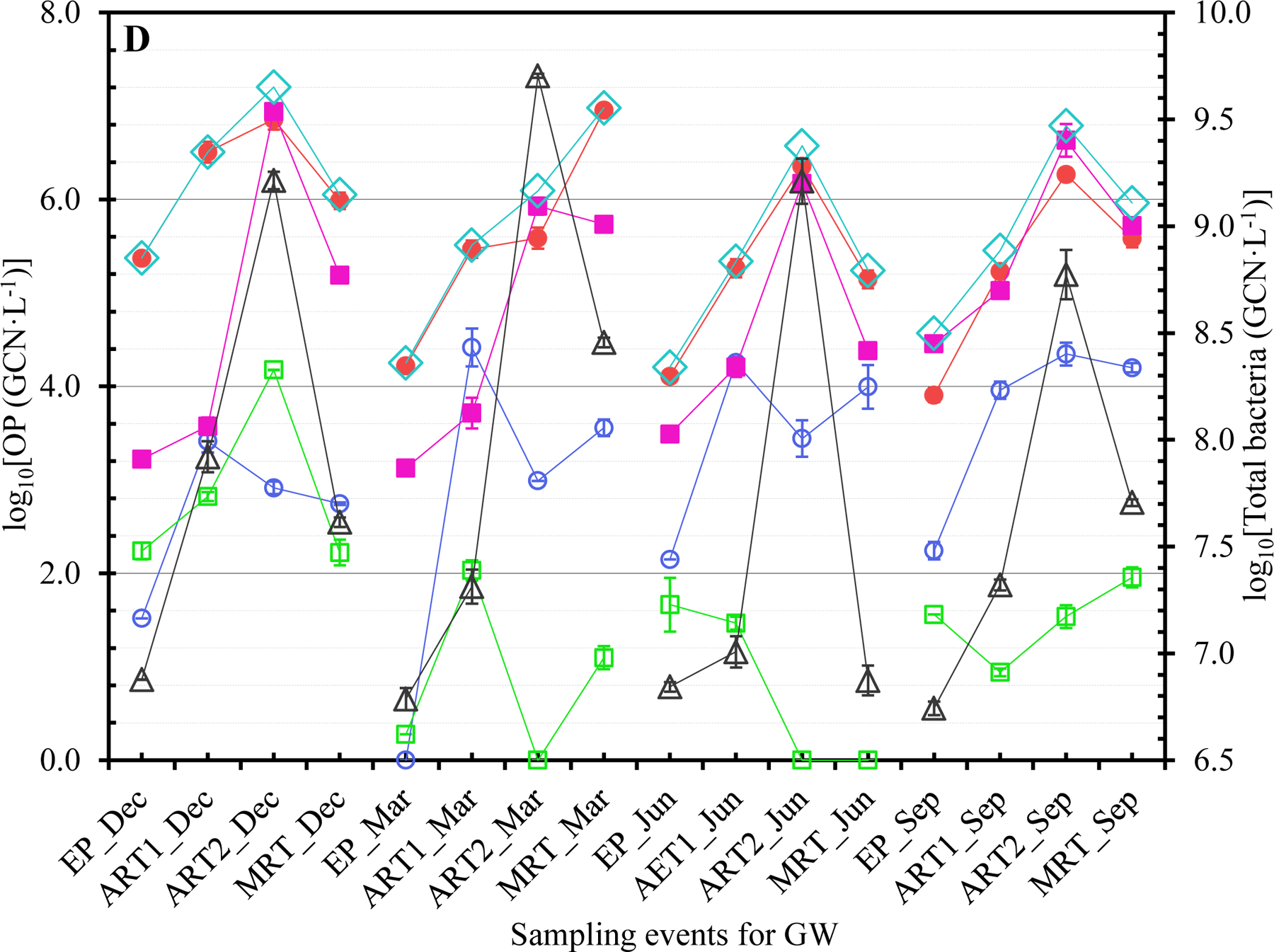
The concentrations of Legionella (closed red circles ●), Mycobacterium (open blue circles ○), Pseudomonas (closed pink squares ■), V. vermiformis (open green squares □), total OPs (open blue diamonds ◇), and total bacteria (open black triangles △) at each sampling event for (A) SW1, (B) SW2, (C) SW3, and (D) GW. Dec: December 2017. Mar: March 2018. Jun: June 2018. Sep: September 2018. The error bars indicate the standard errors of the means.
The OPs generally occurred more frequently in winter and/or spring (Tables 1B and S6). The greatest FOD for Legionella, Mycobacterium, Pseudomonas, and V. vermiformis occurred in December 2017 and March 2018 (both 100%), March 2018 (93.8%), September 2018 (81.3%), and December 2017 (75.0%), respectively. During cold seasons, municipal water demand is typically (much) lower or reduced (Franklin and Maidment, 1986; Hansen and Narayanan, 1981; Maidment and Parzen, 1984; Miaou, 1990; Opalinski et al., 2020). The greater FOD of OPs in winter and/or spring could be due to reduced water usage (i.e., pronounced stagnation or increased HRT) with cold weather. Presumably, the effect of water stagnation on the occurrence of OPs was stronger than that of water temperature so that the FOD of OPs were generally greater in winter and/or spring even though the water temperature was lower in winter and spring (Abulikemu et al., 2021). A previous study similarly showed that the FOD of L. pneumophila and P. aeruginosa in unchlorinated DWDSs had substantial seasonal variations (universal seasonal effects did not exist) (van der Wielen and van der Kooij, 2013). Future studies should explore the exact underlying mechanisms for the greater FOD of OPs during cold seasons in the CDWDSs. The greater FOD of each OP at ART, Tank, and MRT than those at EP (Table 1C) suggest that the OPs occurred more frequently downstream of EP because of disinfectant residual decay and OP (re)growth.
GW had a distinct OP concentration profile from the other CDWDSs (Figures 1, 2, and S1). Specifically, even though the disinfectant residual concentrations in GW were greater than the other CDWDSs (Abulikemu et al., 2021), the concentrations of Legionella, Pseudomonas, total OPs, and total bacteria in GW were generally greater (Figures 2A, 2B, and 2C). By contrast, Mycobacterium concentrations in GW were much lower than those in SW1, SW2, and SW3 where Mycobacterium concentrations were comparable (Figure 2A). Presumably, GW had a unique OP profile because its source water (groundwater) was different from the other water utilities (surface water as source water). The underlying reasons for the unique OP concentration profile in GW require further exploration.
Figure 2.
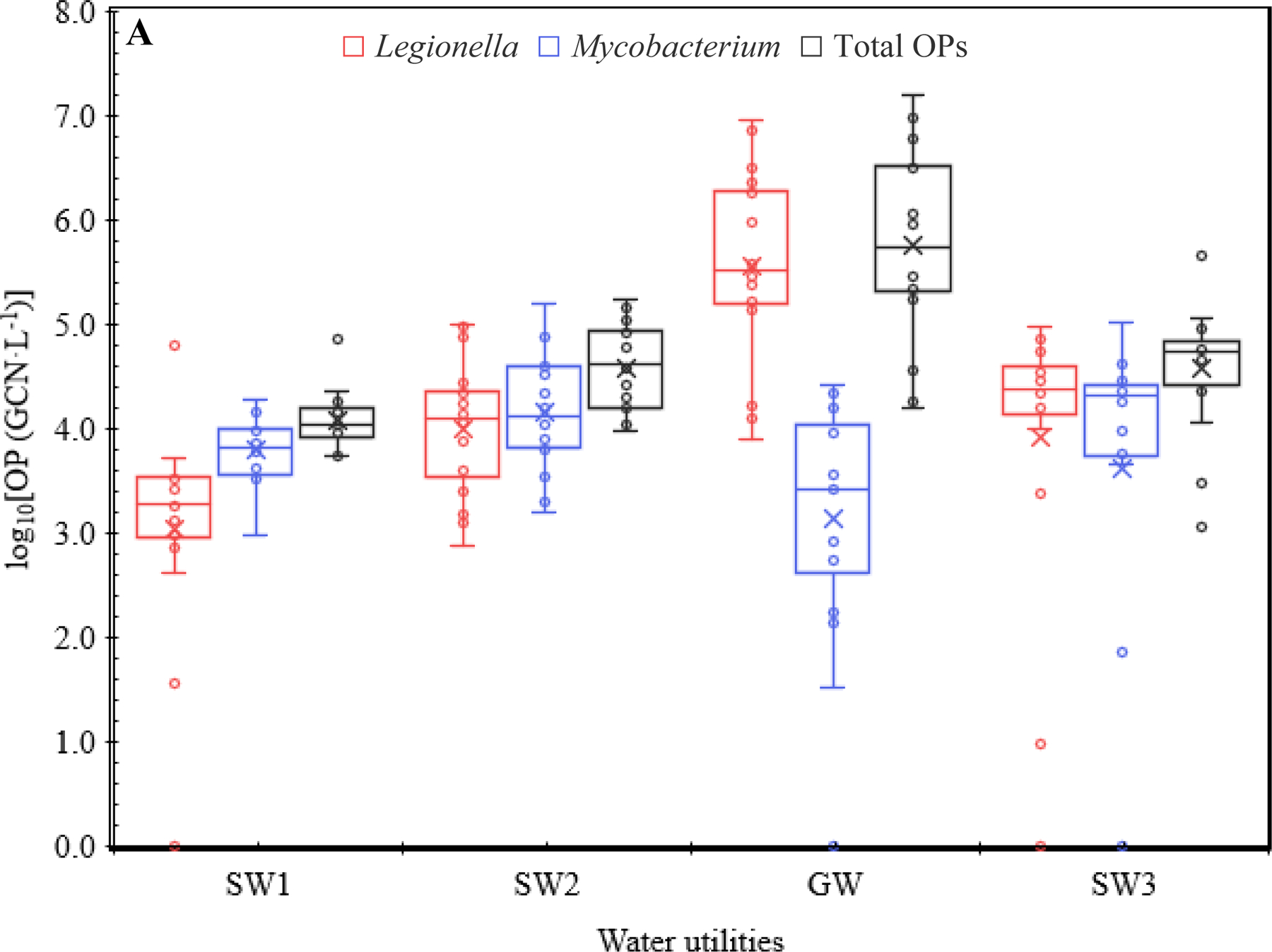
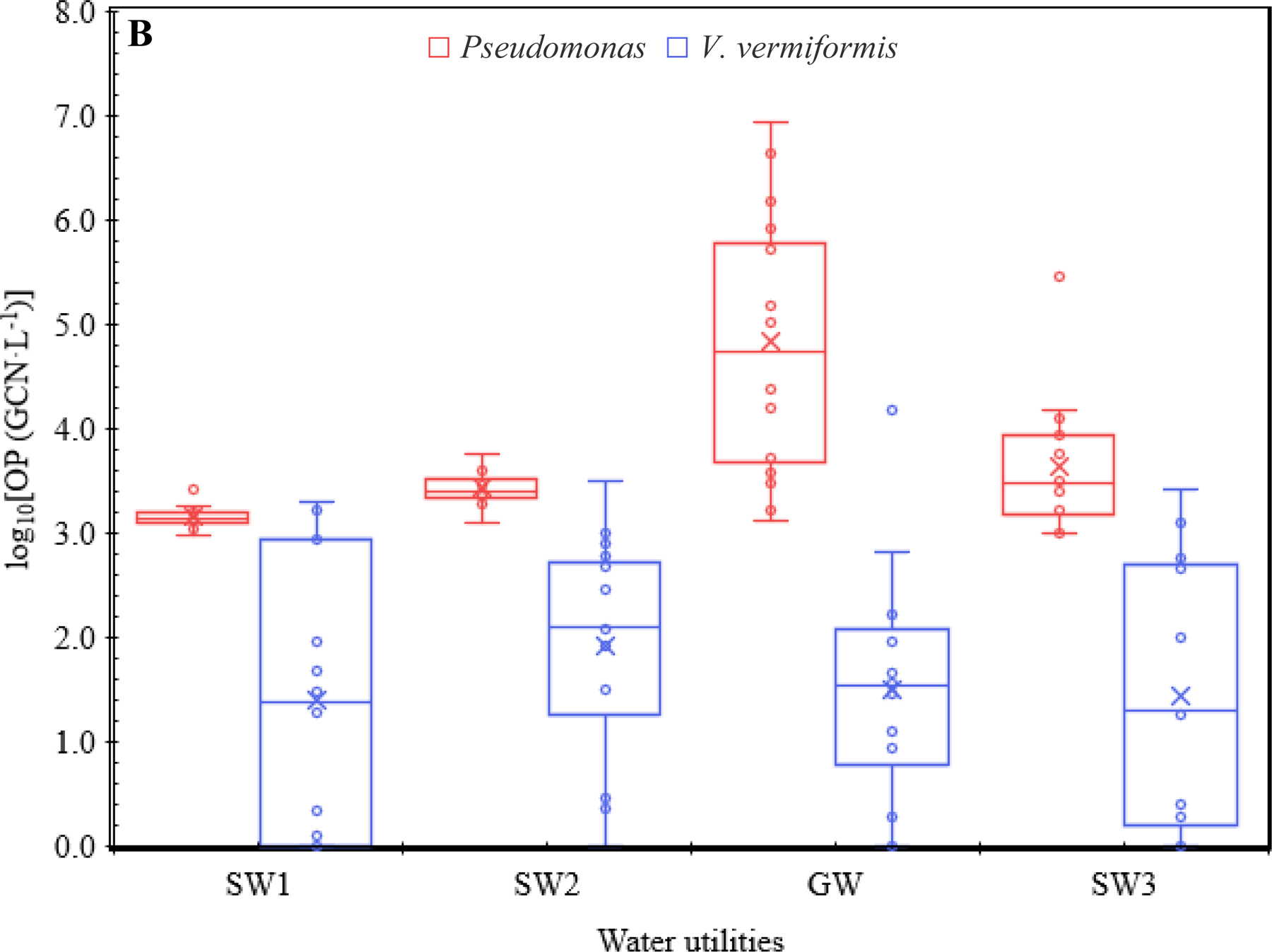
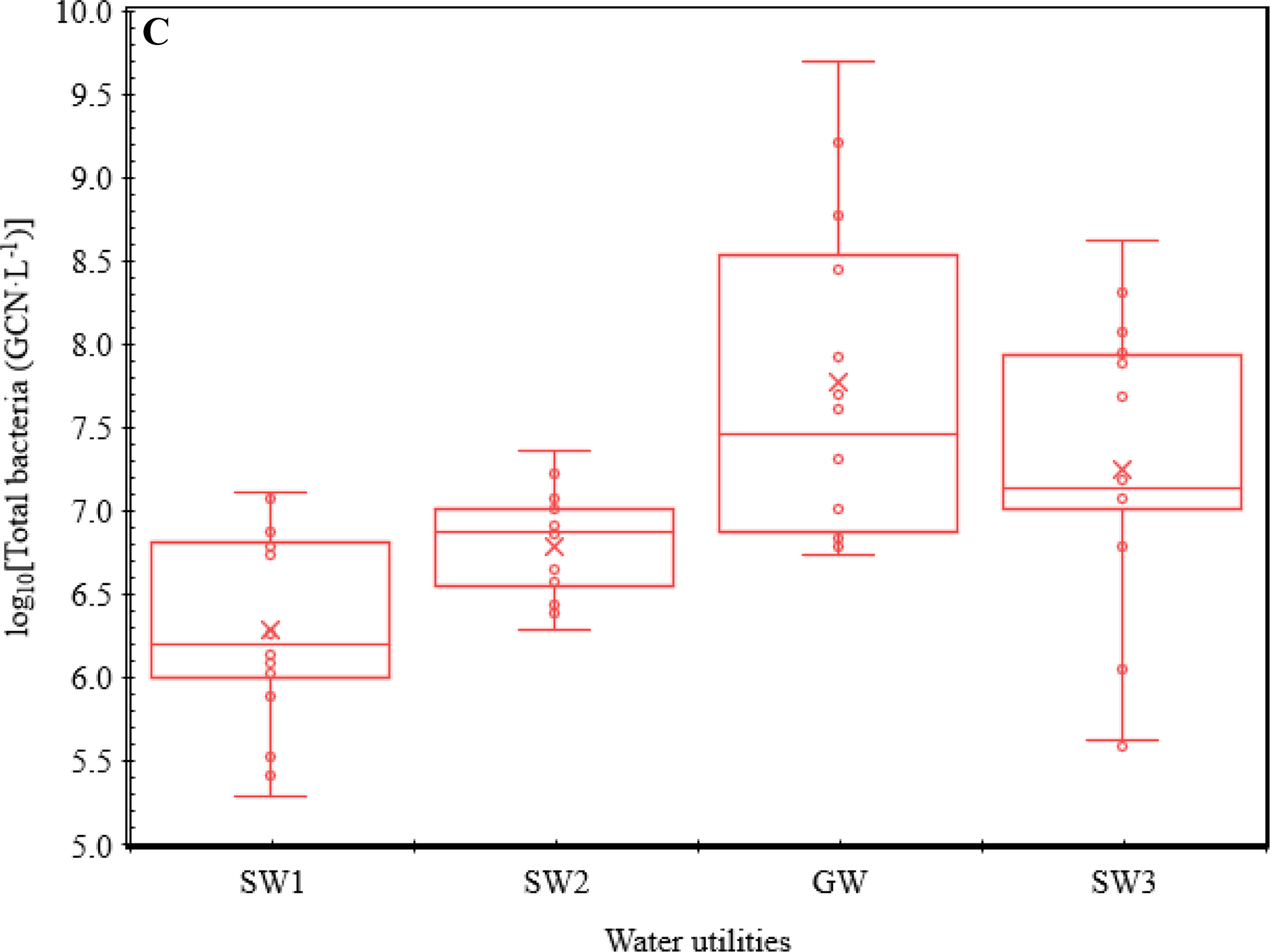
Box-and-whisker plots for the log10-transformed concentrations of (A) Legionella, (A) Mycobacterium, (B) Pseudomonas, (B) V. vermiformis, (A) total OPs, and (C) total bacteria in the four CDWDSs. The lower and upper box boundaries represent the first and third quartiles, respectively. The line inside a box: Median. The cross sign inside a box: Arithmetic mean. The lower and upper error lines: The lowest and highest observations, respectively. Both inner and outlier points (the open circles) are shown. The calculation of the quartiles included the median.
3.2. The speciation and phylogeny of Legionella in the CDWDSs
The speciation and phylogeny of Legionella for 20 sampling events (i.e., the four sampling events for GW in December 2017 and all sampling events for the four CDWDSs in March 2018) were analyzed (Table S2B). The exact Legionella species in the four CDWDSs are unknown. However, the PCR-amplified Legionella sequences are highly similar (identity ≥ 97%), indicating that they belong to closely related species or even one species. The high similarity among those sequences was presumably because the four CDWDSs are geographically close and all four water utilities use monochloramine for secondary disinfection (Table S1A). The PCR-amplified sequences are also similar (identity ≥ 97%) to Legionella or Legionella-like species from municipal drinking water storage tank sediments (Lu et al., 2015), DWDS bulk water (Lu et al., 2016), cold tap and shower water from a building PPS (Lu et al., 2017), a drinking water biofilm (Williams et al., 2005), cooling tower water (Campocasso et al., 2012; Inoue et al., 2015), and water utility water (Corsaro et al., 2010; Edagawa et al., 2019) (Figures 3A, 3B, 3C, and 3D). Therefore, Legionella in various water systems might share a core community with limited diversity. In addition, the PCR-amplified Legionella sequences are also similar to some clinically related Legionella species or isolates (identity > 96%) (Cordes et al., 1979; Garrity et al., 1980; Han et al., 2015; Hookey et al., 1996; Kozak-Muiznieks et al., 2018; Slow et al., 2018; Thacker et al., 1991). Future studies need to explore the virulence and pathogenicity of the Legionella species in the four full-scale CDWDSs.
Figure 3.
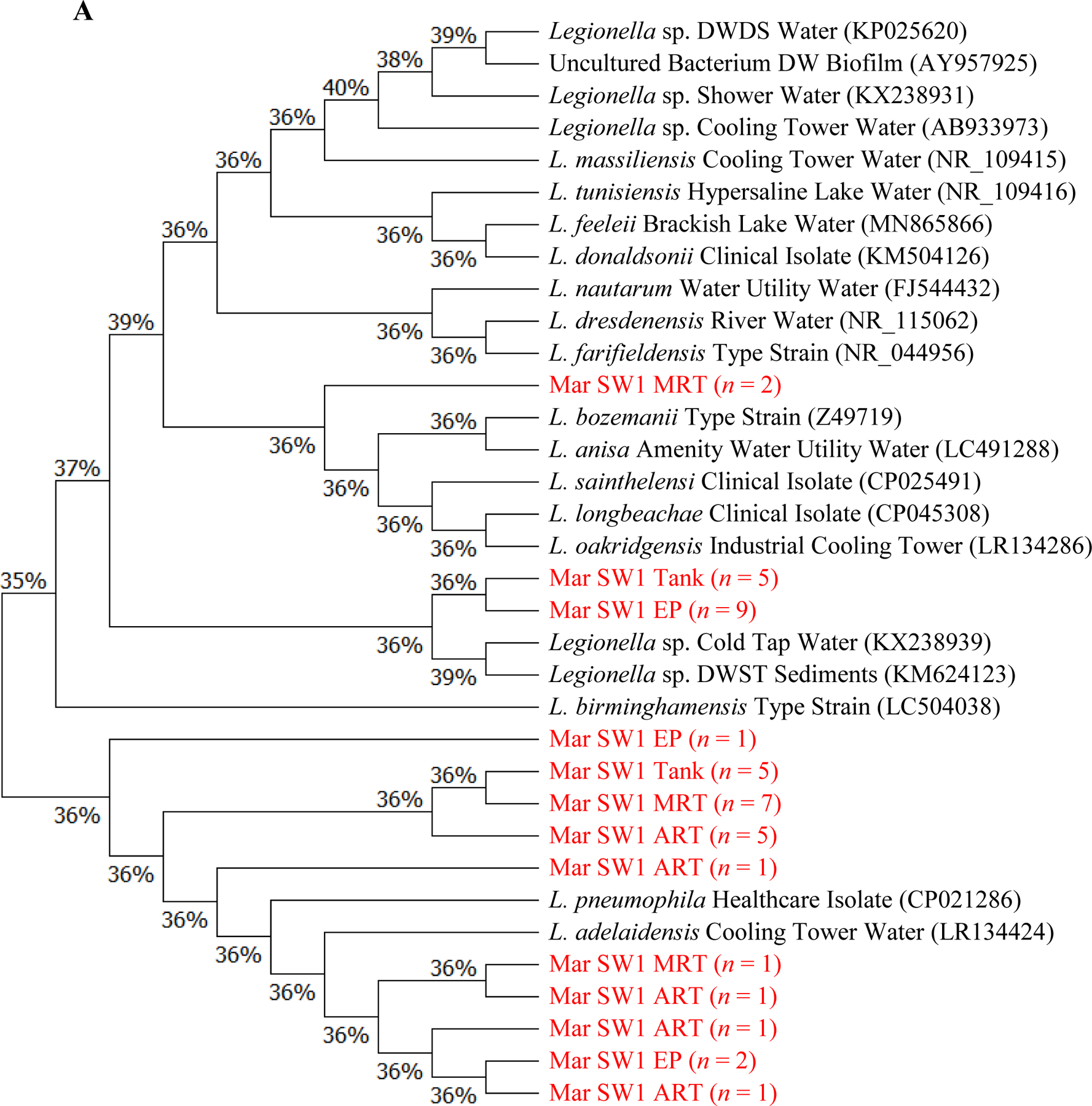
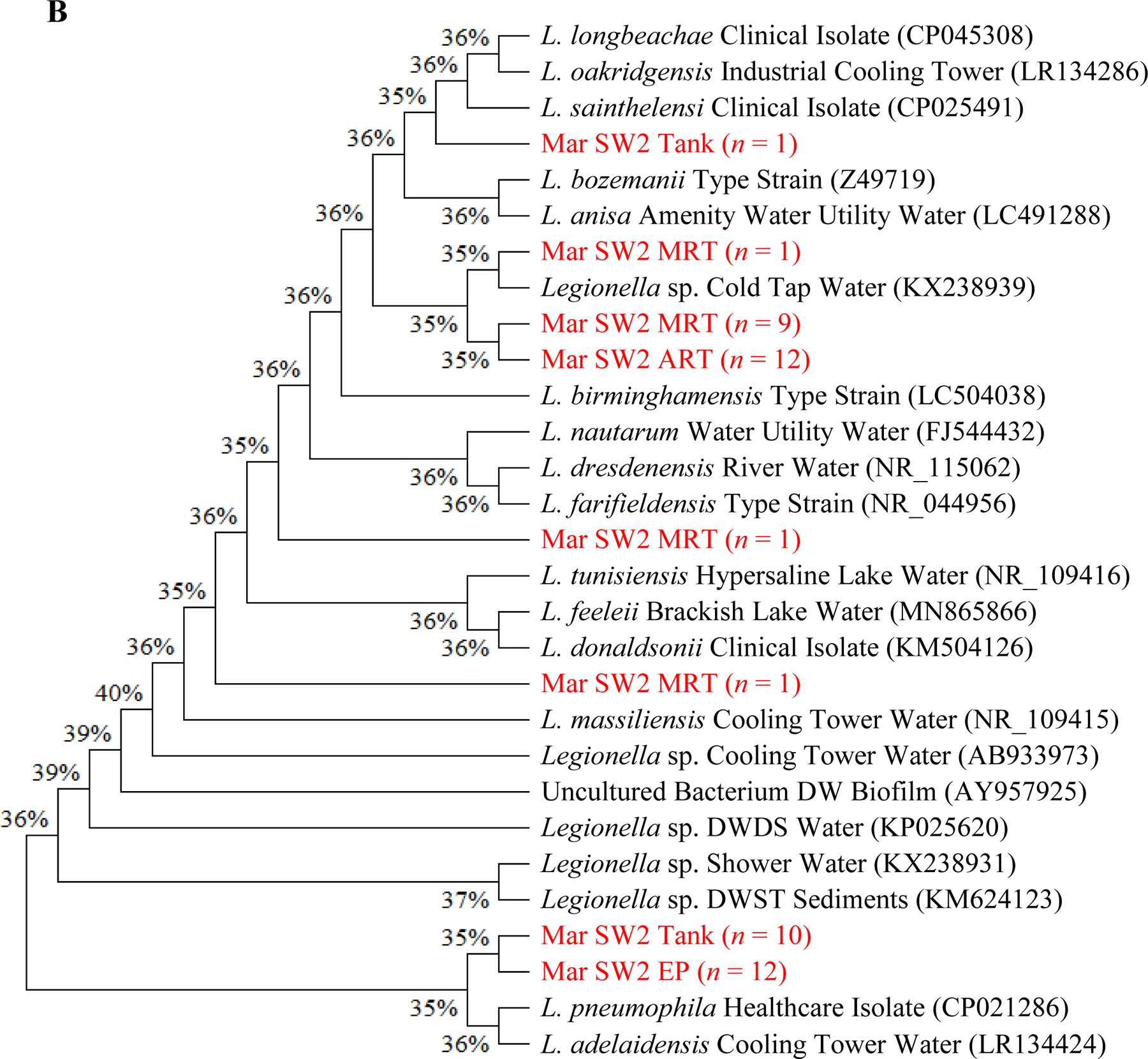
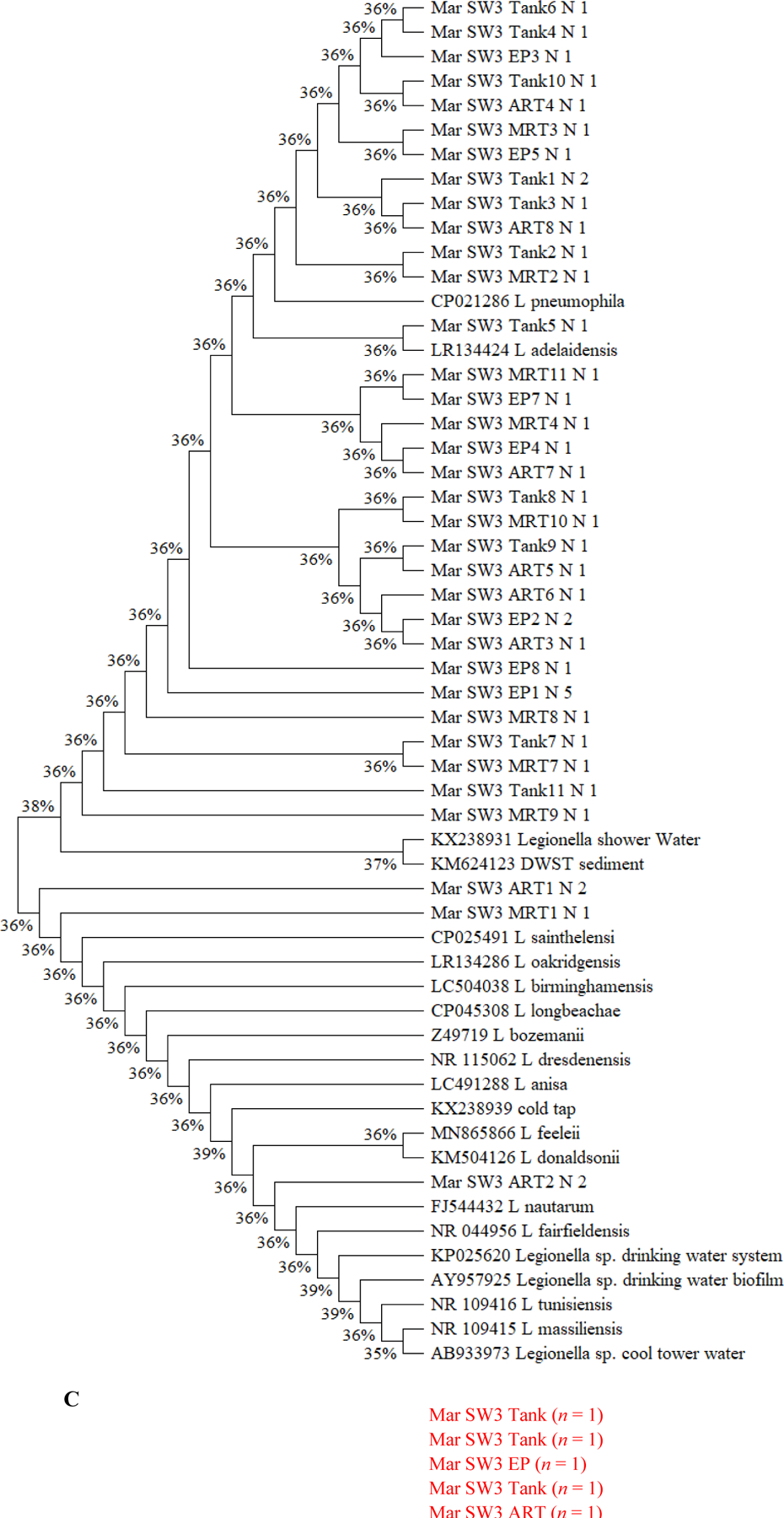
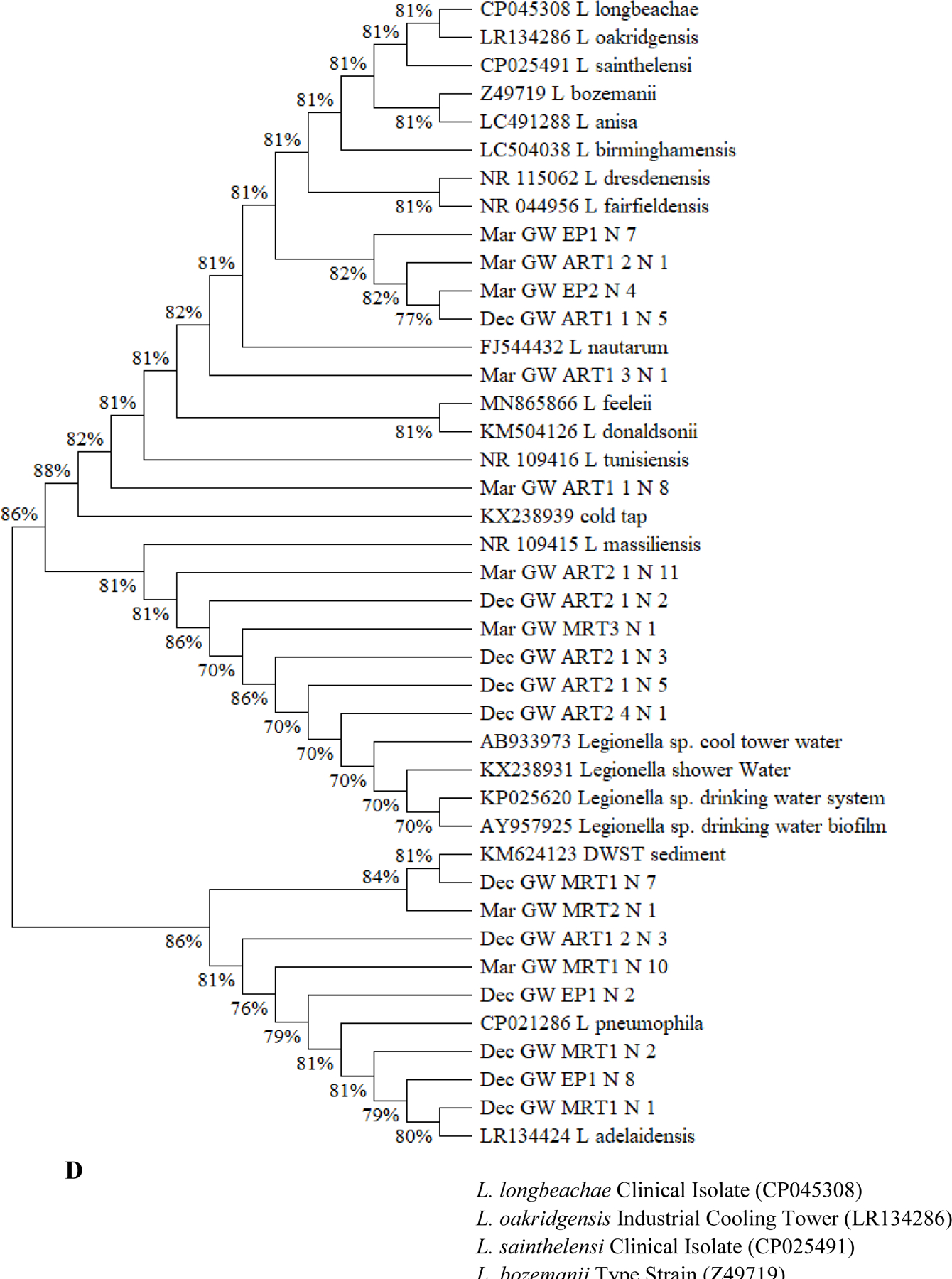
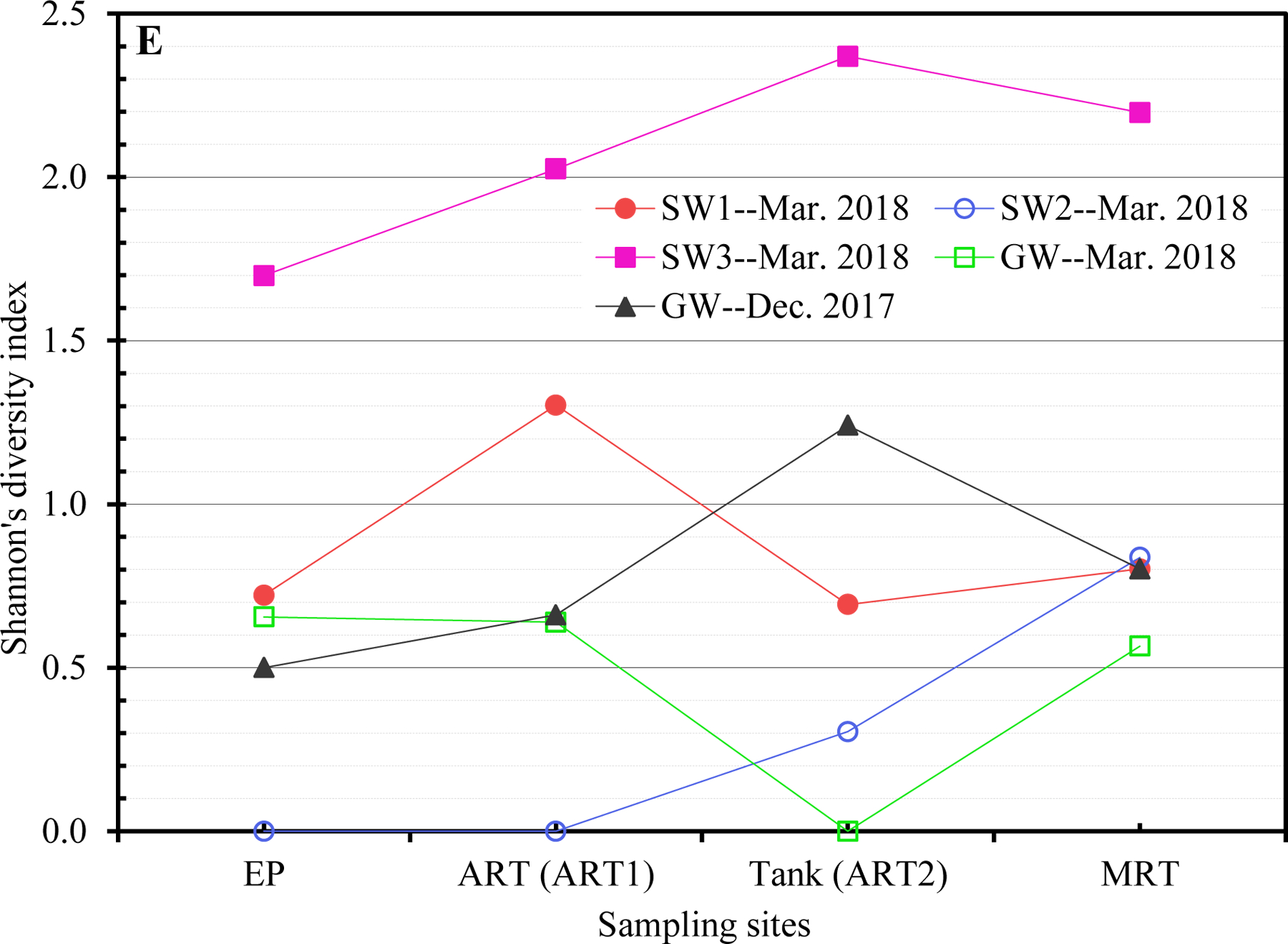
(A to D) Unrooted neighbor-joining dendrograms of PCR-amplified 16S rRNA gene sequences (obtained from clone libraries) of Legionella for (A) SW1, (B) SW2, (C) SW3, and (D) GW. Test scores based on bootstrap values (1,000 replicates) are shown at the nodes. For the samples from the current study, the name of each branch provides the following information (in this order): Sampling date [December 2017 (i.e., Dec) or March 2018 (i.e., Mar)]; Water utility name (i.e., SW1, SW2, SW3, or GW); Sampling site; and number (n) of Legionella-positive clones/sequences (1 to 12; sequences with a similarity ≥ 99% were clustered into one branch). For the reference species or sequences, the name of each branch provides the following information (in this order): Name of the species or sequence; Place/sample where the species or species was isolated or identified from; and NCBI accession number (in the round brackets). DW: Drinking water. DWDS: Drinking water distribution system. DWST: Drinking water storage tank. NCBI: National Center for Biotechnology Information (ncbi.nlm.nih.gov). AY957925: Closet match in GenBank is Legionella/Amoeba proteus symbiont. CP021286: Originally isolated from human lung tissue. CP025491 and CP045308: Isolated from patients with Legionnaires’ disease. FJ544432: Isolated from ozonated water. KM504126: Isolated from bronchoscopy specimens of cancer patients (bronchoalveolar lavage). LC504038: Originally isolated from lung biopsy. NR_109415: Identical to JF779685. NR_109416: Identical to JF779686. NR_115062: Identical to AM747393. NR_044956: Identical to Z49722 (originally isolated from cooling tower water). Z49719 (current name: Fluoribacter bozemanae): Originally isolated from necropsy lung tissue of a patient with pneumonia. References: AB933973: Inoue et al. (2015). AY957925: Williams et al. (2005). CP021286: Kozak-Muiznieks et al. (2018). CP025491: Slow et al. (2018). FJ544432: Corsaro et al. (2010). KM624123: Lu et al. (2015). KM504126: Han et al. (2015). KP025620: Lu et al. (2016). KX238931 and KX238939: Lu et al. (2017). LC491288: Edagawa et al. (2019). MN865866: Ibrahim et al. (2020). NR_109415 and NR_109416: Campocasso et al. (2012). NR_115062: Lück et al. (2010). NR_044956: Hookey et al. (1996) and Thacker et al. (1991). Z49719: Hookey et al. (1996), Garrity et al. (1980), and Cordes et al. (1979). (E) Shannon’s diversity index values for the 20 sampling events (the four sampling events for GW in December 2017 and all sampling events for the four CDWDSs in March 2018). For each sampling event, the two DNA extracts from the two biological replicates (i.e., the two drinking water samples) were mixed and then used as the template in the PCR.
Although the PCR-amplified Legionella sequences across the four CDWDSs are similar (identity ≥ 97%) (Figures 3A, 3B, 3C, and 3D), Legionella at the genotype or strain level was diverse among different sampling sites (Table S5 and Figure 3E). Shannon’s diversity index values fluctuated among the four sampling sites for each CDWDS. For SW2 (March 2018), SW3 (March 2018), and GW (December 2017), Shannon’s diversity index values and disinfectant residual concentrations (Abulikemu et al., 2021) had a clear negative correlation. For SW1 (March 2018) or GW (March 2018), disinfectant residuals and Shannon’s diversity index lacked such a clear correlation; however, Shannon’s diversity index increased in at least one section of the CDWDS (i.e., between two consecutive sampling sites) where disinfectant residual concentrations decreased. Therefore, disinfectant residuals strongly affected the diversity of Legionella, and a greater Legionella community diversity tended to occur where the residual concentrations were lower. The strong effect of disinfectant residuals on Legionella community diversity confirms the importance of well-maintaining disinfectant residuals in distribution systems for ensuring microbial drinking water quality (Zhang and Lu, 2021b).
Shannon’s diversity index values for SW1, SW2, and GW are comparable and much lower than those for SW3 (Figure 3E). Figure 3C similarly shows that Legionella genotype diversity for SW3 was high, especially at Tank and MRT where disinfectant residual concentrations were relatively low (Abulikemu et al., 2021). The relatively high and unique Legionella community diversity in SW3 warrants future research.
3.3. Spatial variations of OP concentrations in the CDWDSs
Both the genotypic diversity (Figure 3) and concentration (Tables S6 to S8 and Figures 1 and S1A) of Legionella generally increased with HRT. For instance, except for SW3 in March 2018, Legionella concentration at MRT was greater than that at EP for each CDWDS in each season (Figure 1). In addition, the concentration of Legionella often increased in multiple sections along the CDWDSs. Mycobacterium concentration also generally increased with HRT. At MRT, Mycobacterium concentration was comparable to or greater than that at EP for each CDWDS in each season (Figure 1). Pseudomonas concentration was either relatively stable along the CDWDSs or increased with HRT (Figure 1). V. vermiformis concentration was generally much lower than those of the three bacterial OPs and changed along the CDWDSs without an apparent universal pattern (Figures 1 and S1B). The concentration of total OPs (i.e., the summed concentration of the four monitored OPs) was relatively stable or increased with HRT (Figures 1 and S1A). Therefore, the OP concentrations generally increased with HRT presumably responding to disinfectant residual decay and water stagnation, which would be more significant downstream of the CDWDSs (Abulikemu et al., 2021).
The general increase in OP concentrations with HRT (Tables S6 to S8 and Figures 1, S1A, and S1B) suggests that OPs are natural inhabitants and (re)grow well in municipal water systems (Falkinham, 2020; Zhang and Lu, 2021b). The (re)growth correlated with disinfectant residual decay (i.e., loss of efficiency) especially because the weak disinfectant monochloramine was used for secondary disinfection in these CDWDSs (Zhang et al., 2018). Presumable significant water stagnation downstream of the CDWDSs could also contribute to the increase in OP concentrations with HRT (Ley et al., 2020; Nisar et al., 2020). Previous studies similarly found that the concentrations of dominant OPs downstream of DWDSs were generally greater (Lu et al., 2016; van der Wielen and van der Kooij, 2013; Whiley et al., 2014a). Similar to OP concentrations, total bacterial concentration generally increased with HRT and was always greater at MRT than at EP for each CDWDS in each season (Figures 1 and S1C). The general increase in the concentrations of OPs and total bacteria with HRT suggests that drinking water downstream of the CDWDSs has a higher risk of causing disease outbreaks and threatening public health. Therefore, OP monitoring and distribution system maintenance (i.e., booster disinfection) should be more frequent or even routine downstream of the CDWDSs.
This study used the distances of the sampling sites from the water utilities as a surrogate for HRT. This use has limitations. For instance, HRT increases from EP to ART (or ART1 and ART2) and from ART (or ART1 and ART2) to MRT, while HRT for Tank could be shorter than or equal to that for ART even though Tank is physically farther from EP. Moreover, all water samples of the same season for each CDWDS were taken on the same day and therefore not from the same water packet moving along the CDWDS (e.g., the MRT sample might have left EP days before sampling). Future studies should determine actual HRTs of water samples to accurately evaluate how HRT correlates with OP concentrations.
3.4. Seasonal variations of OP concentrations in the CDWDSs
Even though total bacterial concentrations were comparable in the four monitored seasons for each CDWDS, OP concentrations had strong seasonal variations (Figures 1, S1D, S1E, and S1F). Previous studies also revealed significant seasonal variations of OP concentrations in water systems (Perrin et al., 2019; van der Wielen and van der Kooij, 2013; Whiley et al., 2014a). The seasonal variations of Legionella concentration were CDWDS-specific (Tables S6 to S8 and Figures 1 and S1D). For SW1 and GW, Legionella concentration generally decreased from December 2017 to September 2018 (Figure S1D). For SW2, Legionella concentration in December 2017 and March 2018 was greater than that in June 2018 and September 2018 (concentration peaked in March 2018). For SW3, Legionella concentration in December 2017 and March 2018 was comparable and greater than that in June 2018 and September 2018. Therefore, Legionella concentration generally peaked in December 2017 and/or March 2018 possibly because of low or reduced water usage (i.e., enhanced water stagnation) during cold seasons (Franklin and Maidment, 1986; Hansen and Narayanan, 1981; Ley et al., 2020; Maidment and Parzen, 1984; Miaou, 1990; Nisar et al., 2020; Opalinski et al., 2020).
Mycobacterium concentration for SW1, SW2, and SW3 initially increased and then decreased from December 2017 to September 2018 (Figures 1 and S1D). For SW1 and SW3, Mycobacterium concentration peaked in March 2018. For SW2, Mycobacterium concentration peaked in June 2018. For GW, Mycobacterium concentration increased from December 2017 to September 2018. Pseudomonas concentration for SW1 or SW2 was comparable in the four monitored seasons (Figures 1 and S1E). For SW3, Pseudomonas concentration in March 2018 and September 2018 was comparable and greater than that in December 2017 and June 2018. For GW, Pseudomonas concentration peaked in September 2018 and was comparable in December 2017, March 2018, and June 2018. From December 2017 to September 2018, V. vermiformis concentration for each CDWDS initially decreased and then increased (Figures 1 and S1E). For SW1, SW2, and GW, V. vermiformis concentration peaked in December 2017. For SW3, V. vermiformis concentration was comparable in December 2017 and September 2018 and greater than that in March 2018 and June 2018. Total OP concentration also had strong seasonal variations (Figures 1 and S1D). For SW1, SW2, and GW, total OP concentration peaked in December 2017 or March 2018. For SW3, total OP concentration in March 2018 and September 2018 was comparable and greater than that in December 2017 and June 2018.
We hypothesized that OP concentrations should be greater during summer (June 2018) or fall (September 2018) because the water temperature was generally greater in these seasons (Abulikemu et al., 2021). Indeed, mycobacterial (i.e., NTM) concentration in eight unchlorinated DWDSs in the Netherlands was greater during summer than winter because of the much greater summer water temperature (van der Wielen and van der Kooij, 2013). Unexpectedly, the OP concentrations in the current study tended to peak during winter (December 2017) and/or spring (March 2018). Legionella, Mycobacterium, and V. vermiformis also occurred more frequently (i.e., greater FOD) during winter and/or spring (Table 1B). The underlying mechanisms for the greater OP concentrations and FOD during winter and/or spring need further exploration. Possibly, other environmental parameters than water temperature had a stronger effect on OP concentrations and FOD (Whiley et al., 2014a). For instance, water usage is typically lower during cold seasons (Franklin and Maidment, 1986; Hansen and Narayanan, 1981; Maidment and Parzen, 1984; Miaou, 1990; Opalinski et al., 2020), increasing HRT and promoting stagnation (i.e., prolonged water age) and therefore favoring OP (re)growth and colonization (Hozalski et al., 2020; Lautenschlager et al., 2010; Li et al., 2019; Lipphaus et al., 2014; Manuel et al., 2009; Zhang et al., 2015; Zlatanović et al., 2017). A recent study found that reduced water usage in a building premise plumbing increased water stagnation, which, in turn, had a significant positive correlation with the occurrence of Legionella spp. (p < 0.001) and Mycobacterium spp. (p < 0.001) (Ley et al., 2020). In addition, a review of 24 studies shows that water stagnation and Legionella colonization were positively correlated in premise plumbing in 22 studies (Nisar et al., 2020). In summary, the OP concentrations had significant seasonal variations and tended to peak during cold seasons (i.e., December 2017 and/or March 2018) presumably because of significant water stagnation and prolonged HRT due to reduced water usage with cold weather.
All water samples in the current study were grab samples. The relatively low sampling frequency (i.e., four times over 10 months for each sampling site) might have biased the results. Future studies should rely on more frequent and prolonged sampling to more accurately assess the seasonal variations of OP concentrations and explore the underlying mechanisms for the greater OP concentrations and FOD during cold seasons.
3.5. The correlations between OP concentrations and physicochemical water quality parameters
The main objective of this study was to assess the correlations between OP concentrations and physicochemical water parameters in the four full-scale CDWDSs. OP concentrations generally increased with HRT in the four CDWDSs (Figures 1, S1A, and S1B). For SW1 and SW2, the log10-transformed Legionella concentration (GM of all seasons) had a strong positive correlation with HRT (R2 0.777 for SW1 and 0.986 for SW2) (Figure S2A). For SW3 and GW, Legionella concentration at MRT was less than that at Tank (for SW3 in March 2018 and June 2018) and ART2 (for GW in December 2017, June 2018, and September 2018) (Figures 1C and 1D). The lower Legionella concentration at MRT was possibly because disinfectant residual concentrations at MRT in those seasons were greater than those at Tank (for SW3) and ART2 (for GW) (Abulikemu et al., 2021). After excluding MRT, we found that the log10-transformed Legionella concentration (GM of all seasons) for SW3 (R2 0.823) and GW (R2 0.935) also had strong positive correlations with HRT (Figure S2A). The concentrations of the other three OPs (Mycobacterium, Pseudomonas, and V. vermiformis) and total OPs were stable or increased with HRT (Figures 1, S1A, and S1B).
Similar to OP concentrations, total bacterial concentration generally increased with HRT and was greater at MRT than at EP for each CDWDS in each season (Figures 1 and S1C). For SW1 and SW2, the log10-transformed total bacterial concentration (GM of all seasons) had strong positive linear correlations with HRT (R2 0.842 for SW1 and 0.935 for SW2) (Figure S2B). After excluding MRT, we found that the log10-transformed total bacterial concentration (GM of all seasons) for SW3 (R2 0.997) and GW (R2 0.562) also positively correlated with HRT. We excluded MRT in the linear regression because of the lower total bacterial concentration at MRT due to greater disinfectant residual concentrations at MRT in some seasons (Abulikemu et al., 2021).
The general increase in the concentrations of OPs and total bacteria with HRT (Figures 1, S1A, S1B, S1C, and S2) could be due to disinfectant residual decay (Abulikemu et al., 2021). Multiple factors such as the reactions between disinfectant residuals and inorganic/organic substances (DBPs produced), disinfectant consumption by microbes (e.g., the OPs), and off-gassing in Tank (i.e., water storage tanks) could cause the decay. The concentrations of total chlorine and monochloramine residuals for the four CDWDSs had a significant positive linear correlation (R2 0.926, p < 0.001) (Figure S3). Therefore, we used total chlorine residual to represent disinfectant residuals hereinafter even though monochloramine was the actual disinfectant used for secondary disinfection for the four CDWDSs. For SW1 and SW2, total chlorine residual concentration generally decreased with increasing HRT (R2 0.806 for SW1 and 0.990 for SW2) (Figure S4).
For SW3 (March 2018 and June 2018) or GW (December 2017 and June 2018), the total chlorine residual concentration at MRT was much greater than that at Tank (for SW3) or ART2 (for GW) (Abulikemu et al., 2021). After excluding MRT, we found that total chlorine residual concentration for SW3 (R2 0.933) and GW (R2 0.864) also had strong negative linear correlations with HRT (Figure S4). Similarly, in a chlorinated DWDS and a CDWDS in South Australia, disinfectant residual concentrations generally decreased with increasing HRT (Whiley et al., 2014a). In the current study, the increase in OP concentrations (Figures 1, S1A, S1B, and S2A) and the decrease in disinfectant residual concentrations (Figure S4) cooccurred when HRT increased (Figure S5). Therefore, we speculate that disinfectant residual decay promoted OP (re)growth and contributed to greater OP concentrations and FOD downstream of the CDWDSs.
We used the CCA to assess the linear relationship between two multivariate sets of variables: I) eight physicochemical water quality parameters (distance of each sampling site from the corresponding EP as a surrogate of HRT, water temperature, pH, total chlorine residual concentration, free ammonia concentration, nitrite concentration, total THM concentration, and total HAA concentration) and II) OP concentrations. The CCA conducted with XLSTAT indicates that these water quality parameters linearly correlated with OP concentrations (p < 0.0001). However, the constrained CCA (XLSTAT) explained only 29% of the total inertia, indicating that some environmental parameters not determined in the current study such as organic carbon concentration, biofilm detachment, flow velocity, and possible water main breaks or leaks significantly affected OP concentrations (Perrin et al., 2019). The CCA plots from XLSTAT (Figure 4) and PAST (Figure S6) are distinct but commonly show that Legionella concentration was associated with greater free ammonia concentration and lower water temperature. The association between Legionella concentration and low water temperature might be because Legionella concentration generally peaked in December 2017 and/or March 2018 (Tables S6 to S8 and Figures 1 and S1D). As discussed above, the greater Legionella concentration in winter and/or spring was possibly due to reduced water usage and pronounced water stagnation during cold seasons. Mycobacterium concentration was sensitive to total THM concentration and water temperature. Pseudomonas concentration was associated with greater pH, greater total chlorine residual concentration, and greater free ammonia concentration. The close associations between Legionella/Pseudomonas concentrations and free ammonia concentration could be because free ammonia is the preferred nitrogen source for these two OPs (Rittmann and McCarty, 2020). Because monochloramine residual decay releases free ammonia (Woolschlager et al., 2001; Zhang and Edwards, 2009), the positive correlations between Legionella/Pseudomonas concentrations and free ammonia concentration could partially explain why OP concentrations generally increased with decreasing disinfectant residual concentrations (or increasing HRT). V. vermiformis concentration was associated with lower pH.
Figure 4.
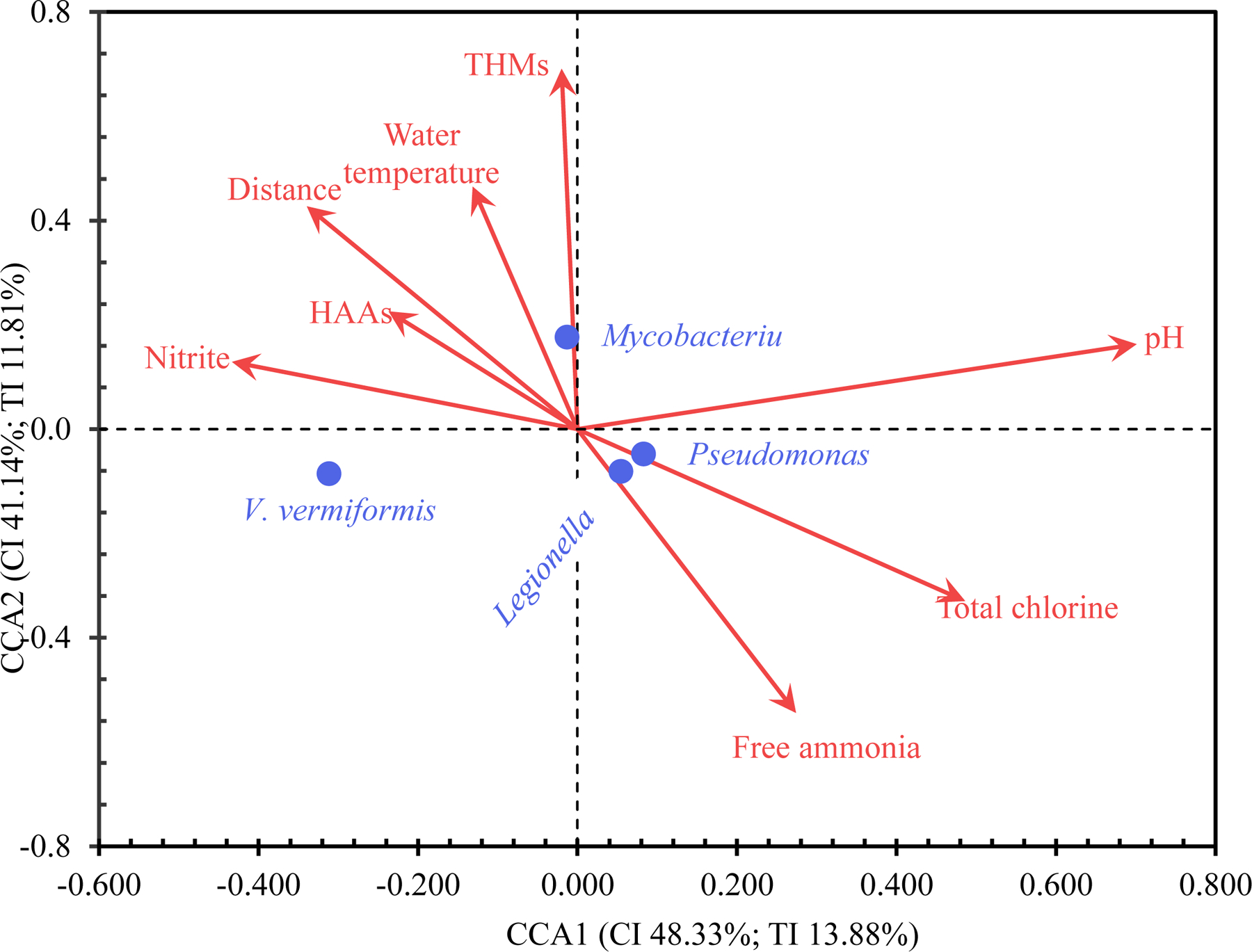
A CCA plot (conducted with XLSTAT) for the relationship between eight physicochemical water quality parameters and log10-transformed OP concentrations (unit: GCN·L−1). Missing values were replaced with arithmetic means before the analysis. CI: Constrained inertia. TI: Total inertia. CCA1, CCA2, and CCA3 explained 48.33%, 41.14%, and 10.52% of the CI, respectively. CCA1, CCA2, and CCA3 explained 13.88%, 11.81%, and 3.02% of the TI, respectively.
In addition to the CCA, we performed a multiple linear regression to simulate the concentrations of Legionella, Mycobacterium, Pseudomonas, V. vermiformis, and total OPs with seven physicochemical water quality parameters as independent variables (Table 2). Those parameters are water temperature, pH, total chlorine residual concentration, free ammonia concentration, nitrite concentration, total THM concentration, and total HAA concentration. Legionella had significant linear correlations with water temperature (p 0.002), total chlorine residual concentration (p 0.012), free ammonia concentration (p 0.037), and total THM concentration (p 0.019) (negative correlations except for with free ammonia concentration). Other studies similarly found that Legionella closely correlated with multiple physicochemical water quality parameters in municipal water systems (Bargellini et al., 2011; Isaac and Sherchan, 2020; Leoni et al., 2005; Qin et al., 2017; Serrano-Suárez et al., 2013; Zacheus and Martikainen, 1994). Mycobacterium had significant negative linear correlations with total chlorine residual concentration (p < 0.001) and total HAA concentration (p 0.018). Pseudomonas had a significant positive linear correlation with free ammonia concentration (p 0.010). V. vermiformis had significant negative linear correlations with pH (p 0.024) and total THM concentration (p 0.048).
Table 2.
A multiple linear regression to predict OP concentrations
| Legionella | Mycobacterium | Pseudomonas | V. vermiformis | Total OPs | |
|---|---|---|---|---|---|
| Overall regression model |
R2 0.551; p-value < 0.001* |
R2 0.422; p-value 0.002* |
R2 0.436; p-value 0.001* |
R2 0.290; p-value 0.048* |
R2 0.542; p-value < 0.001* |
| Water temperature (°C) |
B −0.102; p-value 0.002* |
B 0.019; p-value 0.499 |
B −0.014; p-value 0.559 |
B 0.002; p-value 0.949 |
B −0.038; p-value 0.063 |
| pH |
B 0.664; p-value 0.265 |
B 0.085; p-value 0.873 |
B 0.659; p-value 0.157 |
B −1.530; p-value 0.024* |
B 0.588; p-value 0.126 |
| Total chlorine residual (mg Cl2·L−1) |
B −0.338; p-value 0.012* |
B −0.488; p-value < 0.001* |
B −0.201; p-value 0.051 |
B −0.206; p-value 0.158 |
B −0.201; p-value 0.019* |
| Free ammonia (mg N·L−1) |
B 1.425; p-value 0.037* |
B −0.879; p-value 0.147 |
B 1.385; p-value 0.010* |
B 0.151; p-value 0.838 |
B 1.276; p-value 0.004* |
| Nitrite (mg N·L−1) |
B 5.272; p-value 0.383 |
B −3.346; p-value 0.538 |
B 0.201; p-value 0.966 |
B 8.089; p-value 0.231 |
B 2.671; p-value 0.489 |
| THMs (μg·L−1) |
B −0.015; p-value 0.019* |
B 0.003; p-value 0.572 |
B −0.009; p-value 0.053 |
B −0.014; p-value 0.048* |
B −0.010; p-value 0.014* |
| HAAs (μg·L−1) |
B −0.020; p-value 0.139 |
B −0.029; p-value 0.018* |
B −0.014; p-value 0.184 |
B 0.016; p-value 0.271 |
B −0.012; p-value 0.148 |
Statistical analysis: SPSS (missing values: pairwise deletion). The seven physicochemical water quality parameters were independent variables in the regression. Unit for OP concentrations: log10(GCN·L−1). GCN: Gene copy number or genome copy number. B: Unstandardized coefficient.
Significant correlation (i.e., p < 0.05).
Total chlorine residual concentration negatively correlated with the concentrations of Legionella, Mycobacterium, Pseudomonas, V. vermiformis, and total OPs although the correlations were not significant for Pseudomonas and V. vermiformis (Table 2). Indeed, OP concentrations increased, while total chlorine residual concentration decreased with increasing HRT (Figures 1, S1A, S1B, S2A, S4, and S5). In addition, the concentrations of Legionella, Pseudomonas, V. vermiformis, and total OPs positively correlated with free ammonia concentration (Table 2). Therefore, the general increase in the concentrations of OPs (i.e., regrowth) with HRT had at least two underlying mechanisms. First, total chlorine residual decayed with increasing HRT (Figure S4). The disinfectant residuals with lower concentrations downstream of the CDWDSs had a lower efficiency in inhibiting OP (re)growth (i.e., compromised disinfection power) (Zhang and Edwards, 2009). Second, monochloramine residual decay would release free ammonia (Abulikemu et al., 2021; Huang et al., 2019; Roy et al., 2020; Woolschlager et al., 2001; Yang et al., 2008), while free ammonia concentration positively correlated with the concentrations of multiple OPs, especially Legionella (Table 2 and Figures 4 and S6). The positive correlations between the concentrations of OPs and free ammonia might be because ammonia as a nitrogen source promoted OP (re)growth (Krishna and Sathasivan, 2010; Rittmann and McCarty, 2020; Woolschlager et al., 2001; Zhang et al., 2009). The release of free ammonia from monochloramine might also promote nitrification, which could further reduce the disinfection efficacy of monochloramine residual (Li et al., 2019; Pressman et al., 2012; Wilczak et al., 1996).
In addition to disinfectant residual decay and free ammonia release, biofilms in the CDWDSs might contribute to the increase in OP concentrations with HRT. Biofilms might provide resources (i.e., nutrients and organic matter) to OPs in bulk water. Biofilms on pipe walls might also shed OPs to bulk water through detachment, which could be due to water pressure differentials, water hammers, and other disturbances in the distribution systems (i.e., main breaks and line scouring).
In conclusion, physicochemical water quality parameters (especially the concentrations of disinfectant residuals and free ammonia) closely correlated with and might have significantly affected the dynamics of OPs in the four CDWDSs. A deep understanding of the correlations between OP concentrations and physicochemical water quality parameters would help us to develop more effective OP control strategies. For instance, the negative correlation between the concentrations of OPs and disinfectant residuals suggests that maintaining an appropriate level of residuals (i.e., through booster disinfection and other approaches) downstream of the CDWDSs is necessary. However, as indicated by the CCA, the eight physicochemical water quality parameters tested in the current study explain only a small portion of the variations of OP concentrations. Therefore, further studies should comprehensively assess the roles and impacts of these and other parameters (such as water usage and stagnation, water quality in the source water, pipe materials, biofilms, natural organic matter, and nutrients) on OP dynamics in the four CDWDSs.
3.6. The correlations between OP concentrations and total chlorine residual levels
Water utilities in the US and many other countries maintain disinfectant residuals in DWDSs to control microbial or OP (re)growth and prevent distribution system upsets (Zhang and Lu, 2021b; Zhang and Liu, 2019). The multiple regression shows that the concentrations of Legionella (p 0.012), Mycobacterium (p < 0.001), and total OPs (p 0.019) significantly and negatively correlated with total chlorine residual concentration (Table 2). The concentrations of Pseudomonas (p 0.051) and V. vermiformis (p 0.158) also negatively correlated with total chlorine residual concentration although the correlations were not significant (for Pseudomonas, the correlation was nearly significant). In addition, we assessed the Pearson product-moment correlations between OP concentrations and total chlorine residual concentration in each CDWDS (Table 3). The correlation assessment reveals that the concentration of each OP or total OPs and total chlorine residual concentration were negatively correlated in each CDWDS (Pearson correlation coefficients from −0.809 to −0.169) except for Mycobacterium in SW1 (Pearson correlation coefficient 0.009). In addition, the concentration of Legionella had significant negative correlations with total chlorine residual concentration in SW1 (p 0.003), SW3 (p 0.005), and GW (p 0.004). For SW2, the negative correlation between Legionella concentration and total chlorine residual concentration was nearly significant (p 0.054). Moreover, the concentrations of Mycobacterium and total OPs had significant negative correlations with total chlorine residual concentration in SW2, SW3, and GW (Pearson correlation coefficients from −0.768 to −0.541, p ≤ 0.031) (Table 3). The negative correlation between total chlorine residual concentration and Pseudomonas in GW (p < 0.001) or V. vermiformis in SW3 (p < 0.001) was also significant. Therefore, OP concentrations and disinfectant residual levels in the four CDWDSs generally had (significant) negative correlations, and disinfectant residual decay could promote the (re)growth of OPs and contribute to greater OP concentrations and FOD downstream of the CDWDSs. Thus, maintaining sufficient disinfectant residuals in CDWDSs, especially downstream of the systems where disinfectant decay is more pronounced (Figure S4) (Abulikemu et al., 2021), is necessary to controlling OPs and protecting public health.
Table 3.
Pearson product-moment correlation coefficients between OP concentrations and total chlorine residual concentration for each CDWDS
| OP | SW1 | SW2 | SW3 | GW |
|---|---|---|---|---|
| Legionella |
ρ −0.685; p-value 0.003* |
ρ −0.490; p-value 0.054 |
ρ −0.665; p-value 0.005* |
ρ −0.677; p-value 0.004* |
| Mycobacterium |
ρ 0.009; p-value 0.975 |
ρ −0.561; p-value 0.024* |
ρ −0.768; p-value 0.001* |
ρ −0.541; p-value 0.031* |
| Pseudomonas |
ρ −0.177; p-value 0.513 |
ρ −0.268; p-value 0.316 |
ρ −0.372; p-value 0.156 |
ρ −0.809; p-value < 0.001* |
| V. vermiformis |
ρ −0.169; p-value 0.532 |
ρ −0.224; p-value 0.404 |
ρ −0.778; p-value < 0.001* |
ρ −0.203; p-value 0.451 |
| Total OPs |
ρ −0.407; p-value 0.118 |
ρ −0.637; p-value 0.008* |
ρ −0.765; p-value 0.001* |
ρ −0.752; p-value 0.001* |
Statistical analysis: SPSS. Sample size for each pairwise comparison: 16 (i.e., 16 sampling events for each CDWDS). Unit for total chlorine residual concentration: mg Cl2·L−1. Unit for OP concentrations: log10(GCN·L−1). GCN: Gene copy number or genome copy number. ρ: Pearson (product-moment) correlation coefficient (i.e., Pearson’s r). p-value: Two-tailed.
Significant correlation (i.e., p < 0.05).
Total OP concentration increased with decreasing total chlorine residual concentration in each CDWDS (Table 3 and Figure S5). Different water utilities may optimize total chlorine residual levels in their CDWDSs to minimize OP (re)growth. For SW1 (Figure S7A) and GW (Figure S7D), total chlorine residual concentration of 2 to 3 mg Cl2·L−1 and 3 to 4 mg Cl2·L−1 (i.e., the middles of the ranges of the total chlorine residual levels detected in the CDWDSs), respectively, could well control OPs. For SW2 (Figure S7B) and SW3 (Figure S7C), total chlorine residual concentration of slightly less than 4 mg Cl2·L−1 (i.e., the highest level detected in the CDWDSs) could minimize OP (re)growth while preventing potential issues associated with high disinfectant residual levels (i.e., odor of tap water).
3.7. Legionella as an indicator of OPs and microbial drinking water quality
Water-related OPs are natural inhabitants in DWDSs, have high concentrations and FOD, and cause drinking-water-related disease (such as legionellosis) outbreaks (Falkinham, 2015; Lu et al., 2016; Wang et al., 2012a; Zhang and Lu, 2021b). Therefore, monitoring OP dynamics in municipal DWDSs and understanding how physicochemical water quality parameters affect the dynamics are key to developing effective OP control strategies and protecting public health. Detecting and quantifying all (opportunistic) pathogens in waterbodies to fully assess microbial water quality is time-consuming, impractical, and often unnecessary. Examining the dynamics of indicator microorganisms is the predominant means to assess microbial water quality, and conventional indicator microorganisms (e.g., total coliforms, fecal coliforms, enterococci, and Escherichia coli) are the main or even exclusive indicators of fecal/bacterial contamination and enteric pathogens (Ashbolt et al., 2001; Gerba, 2015; Lu et al., 2016; McLellan and Eren, 2014; Tallon et al., 2005). However, OPs are not contaminants in DWDSs and do not correlate with those conventional indicators (Falkinham, 2015; 2020; Lu et al., 2016; Wang et al., 2017). For instance, OPs generally (re)grow, whereas those conventional indicators decay with HRT in distribution systems. In the current study, the FOD (Table 1) and concentrations (Tables S6 to S8) of OPs were high and generally increased with HRT (Figures 1, S1A, S1B, S2A, and S5), while coliforms were generally absent from the four CDWDSs (data not shown). In addition, the purpose of using conventional indicators to monitor drinking water quality is detecting treatment (i.e., disinfection) failure, infiltration (i.e., main breaks), and aquifer contamination (for utilities using groundwater as source water) rather than assessing OP prevalence within pipelines.
Previous studies proposed that Mycobacterium and Legionella can be potential indicators of OPs or even microbial drinking water quality in municipal water systems (Lu et al., 2017; Lu et al., 2016). Our recent literature review systematically describes the reasons why Legionella is an appropriate indicator of OPs and microbial water quality in municipal water systems (Zhang and Lu, 2021a). The current work confirms that Legionella is a promising indicator of OPs and microbial drinking water quality in CDWDSs or even general municipal water systems. For instance, Legionella represented the other three OPs in the four CDWDSs: The concentration of Legionella significantly and positively correlated with those of Mycobacterium (p 0.042), Pseudomonas (p < 0.001), and total OPs (p < 0.001) (Table 4). Legionella also had a nearly significant positive linear correlation with V. vermiformis (p 0.054). Other studies similarly found that Legionella in municipal water systems closely correlated with multiple dominant OPs (Lu et al., 2016; Valster et al., 2011; Wang et al., 2012a). The natural association between Legionella and total bacteria (which contain OPs) could be the underlying mechanism for the strong, positive correlations between Legionella and the other three OPs. The concentrations of Legionella and total bacteria in the four distributions systems had a significant positive correlation (Pearson correlation coefficient 0.779; p < 0.001) (Table 4). OPs are not contaminants but natural or normal inhabitants in drinking water systems (such as DWDSs) and thus are a critical component of total bacteria (Falkinham, 2015; 2020; Zhang and Lu, 2021b). Because OPs are natural inhabitants in drinking water, they share many features with total bacteria (i.e., comparable growth patterns/rates, comparable responses to physicochemical water quality parameters, and comparable spatiotemporal variations in concentrations). Among the target OPs, Legionella is a typical, representative, and dominant genus (Table 4) (Zhang and Lu, 2021a). Therefore, Legionella significantly correlated with total bacteria and the other three OPs (Mycobacterium, Pseudomonas, and V. vermiformis). However, the strong correlations between Legionella and the other three OPs as well as total bacteria do not mean that they have causative or causal relationships (i.e., correlations do not imply causations). Most likely, the strong correlations between Legionella and the other three OPs as well as total bacteria are simply because they all are natural inhabitants in drinking water and share similar growth features and comparable responses to ever-changing physicochemical water quality parameters.
Table 4.
Pearson product-moment correlation coefficients between pairwise groups of target microorganisms (OPs and total bacteria)
| Legionella | Mycobacterium | Pseudomonas | V. vermiformis | Total OPs | Total bacteria | |
|---|---|---|---|---|---|---|
| Legionella | ρ 1.000 |
ρ 0.255; p-value 0.042* |
ρ 0.643; p-value < 0.001* |
ρ 0.242; p-value 0.054 |
ρ 0.881; p-value < 0.001* |
ρ 0.779; p-value < 0.001* |
| Mycobacterium |
ρ 0.255; p-value 0.042* |
ρ 1.000 |
ρ 0.059; p-value 0.642 |
ρ 0.234; p-value 0.063 |
ρ 0.242; p-value 0.054 |
ρ 0.222; p-value 0.078 |
| Pseudomonas |
ρ 0.643; p-value < 0.001* |
ρ 0.059; p-value 0.642 |
ρ 1.000 |
ρ 0.048; p-value 0.709 |
ρ 0.829; p-value < 0.001* |
ρ 0.742; p-value < 0.001* |
| V. vermiformis |
ρ 0.242; p-value 0.054 |
ρ 0.234; p-value 0.063 |
ρ 0.048; p-value 0.709 |
ρ 1.000 |
ρ 0.224; p-value 0.075 |
ρ 0.217; p-value 0.086 |
| Total OPs |
ρ 0.881; p-value < 0.001* |
ρ 0.242; p-value 0.054 |
ρ 0.829; p-value < 0.001* |
ρ 0.224; p-value 0.075 |
ρ 1.000 |
ρ 0.809; p-value < 0.001* |
| Total bacteria |
ρ 0.779; p-value < 0.001* |
ρ 0.222; p-value 0.078 |
ρ 0.742; p-value < 0.001* |
ρ 0.217; p-value 0.086 |
ρ 0.809; p-value < 0.001* |
ρ 1.000 |
Statistical analysis: SPSS. Sample size for each pairwise comparison: 64 (i.e., 64 sampling events for the four CDWDSs). ρ: Pearson (product-moment) correlation coefficient (i.e., Pearson’s r). p-value: Two-tailed.
Significant correlation (p < 0.05). Unit for the concentrations of OPs and total bacteria: log10(GCN·L−1). GCN: Gene copy number or genome copy number.
Among the other three OPs, Pseudomonas had a significant positive linear correlation with Legionella (p < 0.001), total OPs (p < 0.001), and total bacteria (p < 0.001) (Table 4). Mycobacterium had a significant positive linear correlation with only Legionella (p 0.042), while V. vermiformis was not significantly correlated with any other target microorganisms.
Overall, Legionella had significant linear correlations with more physicochemical water quality parameters (Table 2) and more OPs (Table 4) than the other three OPs (Mycobacterium, Pseudomonas, and V. vermiformis). Indeed, Legionella concentration closely correlated with total chlorine residual concentration, free ammonia concentration, and water temperature (Tables 2 and 3 and Figures 4, S5, and S6). Furthermore, Legionella had high FOD (e.g., its overall FOD in the four CDWDSs of 93.8% was greater than those of the other three OPs) (Table 1A) and concentration (e.g., the overall GM and median of its concentration for the four CDWDSs were greater than those of the other three OPs) (Table S8A). The high Legionella FOD and concentration make its quantification feasible through molecular techniques such as qPCRs (Lu et al., 2017; Wang et al., 2017). Therefore, Legionella is a promising indicator of microbial drinking water quality, especially the occurrence and concentrations of OPs, in CDWDSs. Water utilities may use a simple and cost-effective presence/absence test of Legionella as an alternative or supplementary approach to routinely monitor water quality in CDWDSs.
Currently, using Legionella as an indicator of microbial drinking water quality has certain difficulties. First, no current water policies or laws require a routine detection of Legionella in drinking water systems. The concept of using Legionella to indicate OPs and drinking water quality is not widely accepted and practiced. We need to conduct more research on the indication role of Legionella in municipal water systems. Second, detecting Legionella in drinking water using the “gold standard” culture-based assays requires special culture media, is complicated, and is time-consuming (Lee et al., 2011; Wang et al., 2017). In addition, Legionella in (chloraminated/chlorinated) drinking water systems is often non-culturable or in a viable-but-non-culturable (VBNC) state (Alleron et al., 2008; Cullom et al., 2020; Fu et al., 2021; Kirschner, 2016; Sciuto et al., 2021). VBNC Legionella is alive and infective, producing virulence proteins (Alleron et al., 2013; Dietersdorfer et al., 2018). However, a culture-based approach cannot detect or recover VBNC Legionella (Whiley, 2017). To resolve this issue, we need to develop and upgrade fast, cost-effective, and easy-to-handle advanced molecular techniques (especially qPCRs) for better Legionella detection and quantification (Buse et al., 2012; Fields, 2007; Kirschner, 2016; Marre et al., 2002; US EPA, 2019a; Wang et al., 2017; Whiley and Taylor, 2016). Third, Legionella as a single indicator cannot reflect all aspects of microbial drinking water quality. For instance, Legionella concentration in chloraminated water systems could be low, while the concentration of Mycobacterium or NTM could be high (Donohue et al., 2019b; Moore et al., 2006; Pryor et al., 2004). In addition, Legionella cannot indicate drinking water treatment system failure, water main breaks, and fecal contamination (Falkinham, 2015). Therefore, using Legionella as the sole indicator would generate biased results. We need to use Legionella in conjugation with other indicator microorganisms (such as E. coli, fecal and total coliforms, and even Mycobacterium) to comprehensively reflect microbial drinking water quality in municipal water systems (Zhang and Lu, 2021a).
5. Conclusions
In four full-scale CDWDSs, correlations of four common OPs (Legionella, Mycobacterium, Pseudomonas, and V. vermiformis) with critical physicochemical water quality parameters (especially HRT, seasonality, and disinfectant residual concentrations) were closely assessed. Legionella (overall FOD 93.8%; overall GM 1.36 × 104 GCN·L−1) and Mycobacterium (overall FOD 89.1%; overall GM 4.74 × 103 GCN·L−1) occurred more frequently and had greater concentrations than Pseudomonas (overall FOD 57.8%; overall GM 5.85 × 103 GCN·L−1) and V. vermiformis (FOD 31.3%; overall GM 3.66 × 101 GCN·L−1). The OP concentrations generally increased with HRT. This increase might be due to the decrease in disinfectant (mainly monochloramine) residual concentrations with increasing HRT (i.e., residual decay). The OP concentrations had significant seasonal variations and tended to peak during winter and/or spring possibly because of lower water usage in cold seasons (i.e., increased HRT and enhanced water stagnation). Legionella significantly correlated with more physicochemical water quality parameters and more OPs than the other three studied OPs (Mycobacterium, Pseudomonas, and V. vermiformis). Therefore, Legionella could be a promising indicator for microbial drinking water quality, especially the occurrence and concentrations of OPs, in municipal water systems. This study sheds light on OP profiles in full-scale CDWDSs and provides meaningful information to water engineers for better and enhanced OP control and public health protection.
Supplementary Material
Highlights.
Spatiotemporal dynamics of opportunistic pathogens (OPs) occurred in four full-scale chloraminated DWDSs.
Increasing OP concentrations along DWDSs correlated with disinfectant residual decay.
Monochloramine residual dictated OP concentrations and (re)growth in the DWDSs.
OP concentrations peaked in winter and/or spring because of reduced water usage during cold seasons.
Legionella was a dominant OP and could be a promising indicator of OPs in DWDSs.
Acknowledgments
The United States Environmental Protection Agency (US EPA) through its Office of Research and Development funded and collaborated in the research described here [Regional Applied Research Effort Program (US EPA Region 6 RARE) and Safe and Sustainable Water Resources (SSWR 7.2.1)]. This article has been subjected to US EPA’s peer review and has been approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation by the US EPA for use. We thank Stephanie Brown, Christy Muhlen, Colleen Platten, and Ian Raffenberg for laboratory support.
Footnotes
Declaration of Competing Interest
No potential or actual conflict of interest exists in this work.
References
- Abdul Majid MA, Mahboob T, Mong BG, Jaturas N, Richard RL, Tian-Chye T, Phimphila A, Mahaphonh P, Aye KN, Aung WL (2017) Pathogenic waterborne free-living amoebae: an update from selected Southeast Asian countries. PLOS ONE 12 (2), e0169448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulikemu G, Mistry JH, Wahman DG, Alexander MT, Kennicutt AR, Bollman JD, Pressman JG (2021) Investigation of chloramines, DBPs, and nitrification in chloraminated drinking water distribution systems (In review). AWWA Water Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addinsoft (2020) (Accessed 2020) XLSTAT Statistical and Data Analysis Solution. New York, USA: https://www.xlstat.com. [Google Scholar]
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nature Methods 7 (4), 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MJ, Edberg SC, Reasoner DJ (2004) Heterotrophic plate count bacteria—what is their significance in drinking water? International Journal of Food Microbiology 92 (3), 265–274. [DOI] [PubMed] [Google Scholar]
- Alleron L, Khemiri A, Koubar M, Lacombe C, Coquet L, Cosette P, Jouenne T, Frere J (2013) VBNC Legionella pneumophila cells are still able to produce virulence proteins. Water Research 47 (17), 6606–6617. [DOI] [PubMed] [Google Scholar]
- Alleron L, Merlet N, Lacombe C, Frère J (2008) Long-term survival of Legionella pneumophila in the viable but nonculturable state after monochloramine treatment. Current Microbiology 57 (5), 497–502. [DOI] [PubMed] [Google Scholar]
- Amemura-Maekawa J, Kura F, Chida K, Ohya H, Kanatani J. i., Isobe J, Tanaka S, Nakajima H, Hiratsuka T, Yoshino S (2018) Legionella pneumophila and other Legionella species isolated from legionellosis patients in Japan between 2008 and 2016. Applied and Environmental Microbiology 84 (18), e00721–00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbolt NJ, Grabow WO, Snozzi M (2001) Indicators of microbial water quality. In: Water Quality: Guidelines, Standards and Health. Fewtrell L and Bartram J (eds), pp. 289–316, IWA Publishing, London, UK. [Google Scholar]
- Bargellini A, Marchesi I, Righi E, Ferrari A, Cencetti S, Borella P, Rovesti S (2011) Parameters predictive of Legionella contamination in hot water systems: association with trace elements and heterotrophic plate counts. Water Research 45 (6), 2315–2321. [DOI] [PubMed] [Google Scholar]
- Baron J, Morris L, Stout J (2020) Control of Legionella in hospital potable water systems. In: Decontamination in Hospitals and Healthcare. Cambridge M, USA: (ed), pp. 71–100, Elsevier Ltd. (Woodhead Publishing Limited). [Google Scholar]
- Beer KD, Gargano JW, Roberts VA, Hill VR, Garrison LE, Kutty PK, Hilborn ED, Wade TJ, Fullerton KE, Yoder JS (2015) Surveillance for waterborne disease outbreaks associated with drinking water-United States, 2011–2012. Morbidity and Mortality Weekly Report 64 (31), 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict KM, Reses H, Vigar M, Roth DM, Roberts VA, Mattioli M, Cooley LA, Hilborn ED, Wade TJ, Fullerton KE (2017) Surveillance for waterborne disease outbreaks associated with drinking water-United States, 2013–2014. Morbidity and Mortality Weekly Report 66 (44), 1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohidar N (1991) Determination of geometric standard deviation for dissolution. Drug Development and Industrial Pharmacy 17 (10), 1381–1387. [Google Scholar]
- Borella P, Guerrieri E, Marchesi I, Bondi M, Messi P (2005) Water ecology of Legionella and protozoan: environmental and public health perspectives. Biotechnology Annual Review 11, 355–380. [DOI] [PubMed] [Google Scholar]
- Brunkard JM, Ailes E, Roberts VA, Hill V, Hilborn ED, Craun GF, Rajasingham A, Kahler A, Garrison L, Hicks L (2011) Surveillance for waterborne disease outbreaks associated with drinking water—United States, 2007–2008. Morbidity and Mortality Weekly Report 60 (SS12), 38–68. [PubMed] [Google Scholar]
- Buse HY, Morris BJ, Gomez-Alvarez V, Szabo JG, Hall JS (2020) Legionella diversity and spatiotemporal variation in the occurrence of opportunistic pathogens within a large building water system. Pathogens 9 (7), 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse HY, Schoen ME, Ashbolt NJ (2012) Legionellae in engineered systems and use of quantitative microbial risk assessment to predict exposure. Water Research 46 (4), 921–933. [DOI] [PubMed] [Google Scholar]
- Campocasso A, Boughalmi M, Fournous G, Raoult D, La Scola B (2012) Legionella tunisiensis sp. nov. and Legionella massiliensis sp. nov., isolated from environmental water samples. International Journal of Systematic and Evolutionary Microbiology 62 (Pt_12), 3003–3006. [DOI] [PubMed] [Google Scholar]
- Clark RM, Rossman LA, Wymer LJ (1995) Modeling distribution system water quality: regulatory implications. Journal of Water Resources Planning and Management 121 (6), 423–428. [Google Scholar]
- Cope JR, Kahler AM, Causey J, Williams JG, Kihlken J, Benjamin C, Ames AP, Forsman J, Zhu Y, Yoder JS (2019) Response and remediation actions following the detection of Naegleria fowleri in two treated drinking water distribution systems, Louisiana, 2013–2014. Journal of Water & Health 17 (5), 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes L, Gorman G, Wilkinson H, Fikes B, Fraser D (1979) Atypical Legionella-like organisms: fastidious water-associated bacteria pathogenic for man. The Lancet 314 (8149), 927–930. [DOI] [PubMed] [Google Scholar]
- Corsaro D, Pages GS, Catalan V, Loret J-F, Greub G (2010) Biodiversity of amoebae and amoeba-associated bacteria in water treatment plants. International Journal of Hygiene and Environmental Health 213 (3), 158–166. [DOI] [PubMed] [Google Scholar]
- Craun GF, Brunkard JM, Yoder JS, Roberts VA, Carpenter J, Wade T, Calderon RL, Roberts JM, Beach MJ, Roy SL (2010) Causes of outbreaks associated with drinking water in the United States from 1971 to 2006. Clinical Microbiology Reviews 23 (3), 507–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullom AC, Martin RL, Song Y, Williams K, Williams A, Pruden A, Edwards MA (2020) Critical review: propensity of premise plumbing pipe materials to enhance or diminish growth of Legionella and other opportunistic pathogens. Pathogens 9 (11), 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giglio O, Fasano F, Diella G, Lopuzzo M, Napoli C, Apollonio F, Brigida S, Calia C, Campanale C, Marzella A (2019) Legionella and legionellosis in touristic-recreational facilities: Influence of climate factors and geostatistical analysis in Southern Italy (2001–2017). Environmental Research 178, 108721. [DOI] [PubMed] [Google Scholar]
- Delafont V, Perraud E, Brunet K, Maisonneuve E, Kaaki S, Rodier M-H (2019) Vermamoeba vermiformis in hospital network: a benefit for Aeromonas hydrophila. Parasitology Research 118 (11), 3191–3194. [DOI] [PubMed] [Google Scholar]
- Delafont V, Rodier M-H, Maisonneuve E, Cateau E (2018) Vermamoeba vermiformis: a free-living amoeba of interest. Microbial Ecology 76 (4), 991–1001. [DOI] [PubMed] [Google Scholar]
- Dennis PJ, Brenner DJ, Thacker WL, Wait R, Vesey G, Steigerwalt AG, Benson RF (1993) Five new Legionella species isolated from water. International Journal of Systematic and Evolutionary Microbiology 43 (2), 329–337. [DOI] [PubMed] [Google Scholar]
- Dietersdorfer E, Kirschner A, Schrammel B, Ohradanova-Repic A, Stockinger H, Sommer R, Walochnik J, Cervero-Aragó S (2018) Starved viable but non-culturable (VBNC) Legionella strains can infect and replicate in amoebae and human macrophages. Water Research 141, 428–438. [DOI] [PubMed] [Google Scholar]
- Dilger T, Melzl H, Gessner A (2018) Legionella contamination in warm water systems: a species-level survey. International Journal of Hygiene and Environmental Health 221 (2), 199–210. [DOI] [PubMed] [Google Scholar]
- Dodge Y (2008) The Concise Encyclopedia of Statistics. Springer-Verlag (Springer Science + Business Media, LLC), New York, USA. [Google Scholar]
- Donnarumma G, Buommino E, Fusco A, Paoletti I, Auricchio L, Tufano M (2010) Effect of temperature on the shift of Pseudomonas fluorescens from an environmental microorganism to a potential human pathogen. International Journal of Immunopathology and Pharmacology 23 (1), 227–234. [DOI] [PubMed] [Google Scholar]
- Donohue M, King D, Pfaller S, Mistry J (2019a) The sporadic nature of Legionella pneumophila, Legionella pneumophila Sg1 and Mycobacterium avium occurrence within residences and office buildings across 36 states in the United States. Journal of Applied Microbiology 126 (5), 1568–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MJ, Vesper S, Mistry J, Donohue JM (2019b) Impact of chlorine and chloramine on the detection and quantification of Legionella pneumophila and Mycobacterium species. Applied and Environmental Microbiology 85 (24), e01942–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edagawa A, Kimura A, Miyamoto H (2019) Investigations on contamination of environmental water samples by Legionella using real-time quantitative PCR combined with amoebic co-culturing. Biocontrol Science 24 (4), 213–220. [DOI] [PubMed] [Google Scholar]
- Edrees WH, Al-Awar MS (2020) Bacterial contamination of mobile phones of medical laboratory workers at Sana’a city, Yemen and their antimicrobial susceptibility. Journal of Pharmacy & Pharmacognosy Research 8 (6), 591–599. [Google Scholar]
- Falkinham J III, Pruden A, Edwards M (2015a) Opportunistic premise plumbing pathogens: increasingly important pathogens in drinking water. Pathogens 4 (2), 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham JO (2015) Common features of opportunistic premise plumbing pathogens. International Journal of Environmental Research and Public Health 12 (5), 4533–4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham JO (2020) Living with Legionella and other waterborne pathogens. Microorganisms 8 (12), 2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham JO III, Hilborn ED, Arduino MJ, Pruden A, Edwards MA (2015b) Epidemiology and ecology of opportunistic premise plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environmental Health Perspectives 123 (8), 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields BS (1996) The molecular ecology of legionellae. Trends in Microbiology 4 (7), 286–290. [DOI] [PubMed] [Google Scholar]
- Fields BS (2007) Legionellae and Legionnaires’ disease. In: Manual of Environmental Microbiology. Hurst CJ, Crawford RL, Garland JL, Lipson DA, Mills AL and Stetzenbach LD (eds), pp. 1005–1015, ASM Press, Washington, D.C., USA. [Google Scholar]
- Franklin SL, Maidment DR (1986) An evaluation of weekly and monthly time series forecasts of municipal water use. Journal of the American Water Resources Association 22 (4), 611–621. [Google Scholar]
- Fu Y, Peng H, Liu J, Nguyen TH, Hashmi MZ, Shen C (2021) Occurrence and quantification of culturable and viable but non-culturable (VBNC) pathogens in biofilm on different pipes from a metropolitan drinking water distribution system. Science of the Total Environment 764, 142851. [DOI] [PubMed] [Google Scholar]
- Garrity G, Brown A, Vickers R (1980) Tatlockia and Fluoribacter: two new genera of organisms resembling Legionella pneumophila. International Journal of Systematic Bacteriology 30 (4), 609–614. [Google Scholar]
- Gerba CP (2015) Indicator microorganisms. In: Environmental Microbiology. Gerba CP, Pepper IL and Gentry TJ (eds), pp. 419–434, Elsevier (Academic Press), San Diego, California, USA. [Google Scholar]
- Gomes TS, Gjiknuri J, Magnet A, Vaccaro L, Ollero D, Izquierdo F, Fenoy S, Hurtado C, Del Águila C (2018) The influence of Acanthamoeba–Legionella interaction in the virulence of two different legionella species. Frontiers in Microbiology 9, 2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib EA (2012) Geometric mean for negative and zero values. International Journal of Research and Reviews in Applied Sciences 11 (3), 419–432. [Google Scholar]
- Haig S-J, Kotlarz N, Kalikin LM, Chen T, Guikema S, LiPuma JJ, Raskin L (2020) Emerging investigator series: bacterial opportunistic pathogen gene markers in municipal drinking water are associated with distribution system and household plumbing characteristics. Environmental Science: Water Research & Technology 6 (11), 3032–3043. [Google Scholar]
- Hammer Ø, Harper DA, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4 (1), 4. [Google Scholar]
- Han XY, Ihegword A, Evans SE, Zhang J, Li L, Cao H, Tarrand JJ, El-Kweifi O (2015) Microbiological and clinical studies of legionellosis in 33 patients with cancer. Journal of Clinical Microbiology 53 (7), 2180–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen RD, Narayanan R (1981) A monthly time series model of municipal water demand. Journal of the American Water Resources Association 17 (4), 578–585. [Google Scholar]
- Hookey J, Saunders N, Fry N, Birtles R, Harrison T (1996) Phylogeny of legionellaceae based on small-subunit ribosomal DNA sequences and proposal of Legionella lytica comb. nov. for Legionella-like amoebal pathogens. International Journal of Systematic Bacteriology 46 (2), 526–531. [Google Scholar]
- Hornei B, Ewig S, Exner M, Tartakovsky I, Lajoie L, Dangendorf F, Surman-Lee S, Fields B (2007) Legionellosis. In: Legionella and the Prevention of Legionellosis. Bartram J, Chartier Y, Lee JV, Pond K and Surman-Lee S (eds), pp. 1–28, World Health Organization, India. [Google Scholar]
- Hozalski RM, LaPara TM, Zhao X, Kim T, Waak MB, Burch T, McCarty M (2020) Flushing of stagnant premise water systems after the COVID-19 shutdown can reduce infection risk by Legionella and Mycobacterium spp. Environmental Science & Technology 54 (24), 15914–15924. [DOI] [PubMed] [Google Scholar]
- Huang J, Chow CW, Kuntke P, Cruveiller L, Gnos G, Davey DE, Teasdale PT (2019) The development and evaluation of a microstill with conductance detection for low level ammonia monitoring in chloraminated water. Talanta 200, 256–262. [DOI] [PubMed] [Google Scholar]
- Huber T, Faulkner G, Hugenholtz P (2004) Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20 (14), 2317–2319. [DOI] [PubMed] [Google Scholar]
- Huo L, Pan L, Chen R, Shi B, Wang H, He S (2021) Effects of disinfectants and particles on the occurrence of different microorganisms in drinking water distribution systems. Environmental Science: Water Research & Technology 7 (5), 983–992. [Google Scholar]
- Inoue H, Fujimura R, Agata K, Ohta H (2015) Molecular characterization of viable Legionella spp. in cooling tower water samples by combined use of ethidium monoazide and PCR. Microbes and Environments 30 (1), 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac TS, Sherchan SP (2020) Molecular detection of opportunistic premise plumbing pathogens in rural Louisiana’s drinking water distribution system. Environmental Research 181, 108847. [DOI] [PubMed] [Google Scholar]
- Jjemba PK, Weinrich LA, Cheng W, Giraldo E, LeChevallier MW (2010) Regrowth of potential opportunistic pathogens and algae in reclaimed-water distribution systems. Applied and Environmental Microbiology 76 (13), 4169–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestli M, O’Donnell M, Rose A, Webb JR, Mayo M, Currie BJ, Gibb K (2019) Opportunistic pathogens and large microbial diversity detected in source-to-distribution drinking water of three remote communities in Northern Australia. PLOS Neglected Tropical Diseases 13 (9), e0007672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenum I, Medina MC, Garner E, Pieper KJ, Blair MF, Milligan E, Pruden A, Ramirez-Toro G, Rhoads WJ (2021) Source-to-tap assessment of microbiological water quality in small rural drinking water systems in puerto rico six months after hurricane maria. Environmental Science & Technology 55 (6), 3775–3785. [DOI] [PubMed] [Google Scholar]
- Kennedy S, Devine P, Hurley C, Ooi Y-S, Collum L (1995) Corneal infection associated with Hartmannella vermiformis in contact-lens wearer. The Lancet 8975 (346), 637–638. [PubMed] [Google Scholar]
- Kirschner AK (2016) Determination of viable legionellae in engineered water systems: Do we find what we are looking for? Water Research 93, 276–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak-Muiznieks NA, Morrison SS, Mercante JW, Ishaq MK, Johnson T, Caravas J, Lucas CE, Brown E, Raphael BH, Winchell JM (2018) Comparative genome analysis reveals a complex population structure of Legionella pneumophila subspecies. Infection, Genetics and Evolution 59, 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna KB, Sathasivan A (2010) Does an unknown mechanism accelerate chemical chloramine decay in nitrifying waters? Journal AWWA 102 (10), 82–90. [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35 (6), 1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachant D, Prasad P (2015) Legionella micdadei: a forgotten etiology of growing cavitary nodules: a case report and literature review. Case Reports in Pulmonology 2015, 535012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23 (21), 2947–2948. [DOI] [PubMed] [Google Scholar]
- Lau H, Ashbolt N (2009) The role of biofilms and protozoa in Legionella pathogenesis: implications for drinking water. Journal of Applied Microbiology 107 (2), 368–378. [DOI] [PubMed] [Google Scholar]
- Lautenschlager K, Boon N, Wang Y, Egli T, Hammes F (2010) Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition. Water Research 44 (17), 4868–4877. [DOI] [PubMed] [Google Scholar]
- LeChevallier MW (1990) Coliform regrowth in drinking water: a review. Journal AWWA 82 (11), 74–86. [Google Scholar]
- LeChevallier MW (2019a) Monitoring distribution systems for Legionella pneumophila using Legiolert. AWWA Water Science 1 (1), e1122. [Google Scholar]
- LeChevallier MW (2019b) Occurrence of culturable Legionella pneumophila in drinking water distribution systems. AWWA Water Science 1 (3), e1139. [Google Scholar]
- Lee J, Lai S, Exner M, Lenz J, Gaia V, Casati S, Hartemann P, Lück C, Pangon B, Ricci M (2011) An international trial of quantitative PCR for monitoring Legionella in artificial water systems. Journal of Applied Microbiology 110 (4), 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoni E, De Luca G, Legnani P, Sacchetti R, Stampi S, Zanetti F (2005) Legionella waterline colonization: detection of Legionella species in domestic, hotel and hospital hot water systems. Journal of Applied Microbiology 98 (2), 373–379. [DOI] [PubMed] [Google Scholar]
- Lesnik R, Brettar I, Höfle MG (2016) Legionella species diversity and dynamics from surface reservoir to tap water: from cold adaptation to thermophily. The ISME Journal 10 (5), 1064–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley CJ, Proctor CR, Singh G, Ra K, Noh Y, Odimayomi T, Salehi M, Julien R, Mitchell J, Nejadhashemi AP (2020) Drinking water microbiology in a water-efficient building: stagnation, seasonality, and physicochemical effects on opportunistic pathogen and total bacteria proliferation. Environmental Science: Water Research & Technology 6 (10), 2902–2913. [Google Scholar]
- Li RA, McDonald JA, Sathasivan A, Khan SJ (2019) Disinfectant residual stability leading to disinfectant decay and by-product formation in drinking water distribution systems: a systematic review. Water Research 153, 335–348. [DOI] [PubMed] [Google Scholar]
- Lipphaus P, Hammes F, Kötzsch S, Green J, Gillespie S, Nocker A (2014) Microbiological tap water profile of a medium-sized building and effect of water stagnation. Environmental Technology 35 (5), 620–628. [DOI] [PubMed] [Google Scholar]
- Liu G, Verberk J, Van Dijk J (2013) Bacteriology of drinking water distribution systems: an integral and multidimensional review. Applied Microbiology and Biotechnology 97 (21), 9265–9276. [DOI] [PubMed] [Google Scholar]
- Lu J, Buse H, Struewing I, Zhao A, Lytle D, Ashbolt N (2017) Annual variations and effects of temperature on Legionella spp. and other potential opportunistic pathogens in a bathroom. Environmental Science and Pollution Research 24 (3), 2326–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Santo Domingo JW, Lamendella R, Edge T, Hill S (2008) Phylogenetic diversity and molecular detection of bacteria in gull feces. Applied and Environmental Microbiology 74 (13), 3969–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Struewing I, Vereen E, Kirby A, Levy K, Moe C, Ashbolt N (2016) Molecular detection of Legionella spp. and their associations with Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in a drinking water distribution system. Journal of Applied Microbiology 120 (2), 509–521. [DOI] [PubMed] [Google Scholar]
- Lu J, Struewing I, Yelton S, Ashbolt N (2015) Molecular survey of occurrence and quantity of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa and amoeba hosts in municipal drinking water storage tank sediments. Journal of Applied Microbiology 119 (1), 278–288. [DOI] [PubMed] [Google Scholar]
- Lytle DA, Pfaller S, Muhlen C, Struewing I, Triantafyllidou S, White C, Hayes S, King D, Lu J (2021) A comprehensive evaluation of monochloramine disinfection on water quality, Legionella and other important microorganisms in a hospital. Water Research 189, 116656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Li G, Chen R, Yu Y, Tao H, Zhang G, Shi B (2020a) Revealing the changes of bacterial community from water source to consumers tap: a full-scale investigation in eastern city of China. Journal of Environmental Sciences 87, 331–340. [DOI] [PubMed] [Google Scholar]
- Ma X, Pierre D, Bibby K, Stout JE (2020b) Bacterial community structure correlates with Legionella pneumophila colonization of New York City high rise building premises plumbing systems. Environmental Science: Water Research & Technology 6 (5), 1324–1335. [Google Scholar]
- Madi A, Lakhdari O, Blottière HM, Guyard-Nicodème M, Le Roux K, Groboillot A, Svinareff P, Doré J, Orange N, Feuilloley MG (2010a) The clinical Pseudomonas fluorescens MFN1032 strain exerts a cytotoxic effect on epithelial intestinal cells and induces Interleukin-8 via the AP-1 signaling pathway. BMC Microbiology 10, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madi A, Svinareff P, Orange N, Feuilloley MG, Connil N (2010b) Pseudomonas fluorescens alters epithelial permeability and translocates across Caco-2/TC7 intestinal cells. Gut Pathogens 2, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidment DR, Parzen E (1984) Cascade model of monthly municipal water use. Water Resources Research 20 (1), 15–23. [Google Scholar]
- Manuel C, Nunes O, Melo L (2009) Unsteady state flow and stagnation in distribution systems affect the biological stability of drinking water. Biofouling 26 (2), 129–139. [DOI] [PubMed] [Google Scholar]
- Marre R, Abu Kwaik Y, , Bartlett C, Cianciotto N, Fields B, Frosch M, Hacker J, Lück P (2002) Legionella. ASM Press, Washington, D.C., USA. [Google Scholar]
- McGuire MJ (2006) Eight revolutions in the history of US drinking water disinfection. Journal AWWA 98 (3), 123–149. [Google Scholar]
- McLellan SL, Eren AM (2014) Discovering new indicators of fecal pollution. Trends in Microbiology 22 (12), 697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercante JW, Winchell JM (2015) Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clinical Microbiology Reviews 28 (1), 95–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaou SP (1990) A class of time series urban water demand models with nonlinear climatic effects. Water Resources Research 26 (2), 169–178. [Google Scholar]
- Molmeret M, Horn M, Wagner M, Santic M, Abu Kwaik Y (2005) Amoebae as training grounds for intracellular bacterial pathogens. Applied and Environmental Microbiology 71 (1), 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MR, Pryor M, Fields B, Lucas C, Phelan M, Besser RE (2006) Introduction of monochloramine into a municipal water system: impact on colonization of buildings by Legionella spp. Applied and Environmental Microbiology 72 (1), 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muder RR, Victor LY (2002) Infection due to Legionella species other than L. pneumophila. Clinical Infectious Diseases 35 (8), 990–998. [DOI] [PubMed] [Google Scholar]
- Nisar MA, Ross KE, Brown MH, Bentham R, Whiley H (2020) Water stagnation and flow obstruction reduces the quality of potable water and increases the risk of legionelloses. Frontiers in Environmental Science 8, 611611. [Google Scholar]
- Opalinski NF, Bhaskar AS, Manning DT (2020) Spatial and seasonal response of municipal water use to weather across the contiguous us. Journal of the American Water Resources Association 56 (1), 68–81. [Google Scholar]
- Papapetropoulou M, Iliopoulou J, Rodopoulou G, Detorakis J, Paniara O (1994) Occurrence and antibiotic-resistance of Pseudomonas species isolated from drinking water in southern Greece. Journal of Chemotherapy 6 (2), 111–116. [DOI] [PubMed] [Google Scholar]
- Park JS (2016) First record of potentially pathogenic amoeba Vermamoeba vermiformis (Lobosea: Gymnamoebia) isolated from a freshwater of Dokdo Island in the East Sea, Korea. Animal Systematics, Evolution and Diversity 32 (1), 1–8. [Google Scholar]
- Pascale MR, Mazzotta M, Salaris S, Girolamini L, Grottola A, Simone ML, Cordovana M, Bisognin F, Dal Monte P, Sabattini MAB (2020) Evaluation of MALDI–TOF mass spectrometry in diagnostic and environmental surveillance of Legionella species: a comparison wth culture and Mip-gene sequencing technique. Frontiers in Microbiology 11, 589369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payment P (1999) Poor efficacy of residual chlorine disinfectant in drinking water to inactivate waterborne pathogens in distribution systems. Canadian Journal of Microbiology 45 (8), 709–715. [PubMed] [Google Scholar]
- Pearce MM, Theodoropoulos N, Mandel MJ, Brown E, Reed KD, Cianciotto NP (2012) Legionella cardiaca sp. nov., isolated from a case of native valve endocarditis in a human heart. International Journal of Systematic and Evolutionary Microbiology 62 (Pt_12), 2946–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna VTC, Martins SAM, Mazzola PG (2002) Identification of bacteria in drinking and purified water during the monitoring of a typical water purification system. BMC Public Health 2, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin Y, Bouchon D, Héchard Y, Moulin L (2019) Spatio-temporal survey of opportunistic premise plumbing pathogens in the Paris drinking water distribution system. International Journal of Hygiene and Environmental Health 222 (4), 687–694. [DOI] [PubMed] [Google Scholar]
- Picot L, Abdelmoula SM, Merieau A, Leroux P, Cazin L, Orange N, Feuilloley MG (2001) Pseudomonas fluorescens as a potential pathogen: adherence to nerve cells. Microbes and Infection 3 (12), 985–995. [DOI] [PubMed] [Google Scholar]
- Pressman JG, Lee WH, Bishop PL, Wahman DG (2012) Effect of free ammonia concentration on monochloramine penetration within a nitrifying biofilm and its effect on activity, viability, and recovery. Water Research 46 (3), 882–894. [DOI] [PubMed] [Google Scholar]
- Propato M, Uber JG (2004) Vulnerability of water distribution systems to pathogen intrusion: How effective is a disinfectant residual? Environmental Science & Technology 38 (13), 3713–3722. [DOI] [PubMed] [Google Scholar]
- Pryor M, Springthorpe S, Riffard S, Brooks T, Huo Y, Davis G, Sattar S (2004) Investigation of opportunistic pathogens in municipal drinking water under different supply and treatment regimes. Water Science & Technology 50 (1), 83–90. [PubMed] [Google Scholar]
- Qin K, Struewing I, Domingo J, Lytle D, Lu J (2017) Opportunistic pathogens and microbial communities and their associations with sediment physical parameters in drinking water storage tank sediments. Pathogens 6 (4), 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reingold AL, Thomason BM, Brake BJ, Thacker L, Wilkinson HW, Kuritsky JN (1984) Legionella pneumonia in the United States: the distribution of serogroups and species causing human illness. Journal of Infectious Diseases 149 (5), 819–819. [DOI] [PubMed] [Google Scholar]
- Rittmann BE, McCarty PL (2020) Environmental Biotechnology: Principles and Applications. McGraw-Hill Education, 2nd Edition. New York, USA. [Google Scholar]
- Roy D, McEvoy J, Khan E (2020) Abundance and activity of ammonia oxidizing archaea and bacteria in bulk water and biofilm in water supply systems practicing chlorination and chloramination: Full and laboratory scale investigations. Science of the Total Environment 715, 137043. [DOI] [PubMed] [Google Scholar]
- Salinas MB, Fenoy S, Magnet A, Vaccaro L, Gomes TD, Hurtado C, Ollero D, Valdivieso E, Del Águila C, Pozuelo MJ (2021) Are pathogenic Legionella non-pneumophila a common bacteria in water distribution networks? Water Research 196, 117013. [DOI] [PubMed] [Google Scholar]
- Scales BS, Dickson RP, LiPuma JJ, Huffnagle GB (2014) Microbiology, genomics, and clinical significance of the Pseudomonas fluorescens species complex, an unappreciated colonizer of humans. Clinical Microbiology Reviews 27 (4), 927–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaturro M, Poznanski E, Mupo M, Blasior P, Seeber M, Prast A-M, Romanin E, Girolamo A, Rota MC, Bella A (2020) Evaluation of GVPC and BCYE media for Legionella detection and enumeration in water samples by ISO 11731: does plating on bcye medium really improve yield? Pathogens 9 (9), 757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid PL, Lâm T-T, Sinsch U, Balczun C (2019) Vermamoeba vermiformis as etiological agent of a painful ulcer close to the eye. Parasitology Research 118 (6), 1999–2004. [DOI] [PubMed] [Google Scholar]
- Sciuto EL, Laganà P, Filice S, Scalese S, Libertino S, Corso D, Faro G, Coniglio MA (2021) Environmental management of Legionella in domestic water systems: consolidated and innovative approaches for disinfection methods and risk assessment. Microorganisms 9 (3), 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senbadejo TY (2017) Antibiotic susceptibility patterns of Pseudomonas species isolated from clinical and environmental samples in Awba Dam, Ibadan. Fountain Journal of Natural and Applied Sciences 6 (2), 9–14. [Google Scholar]
- Serrano-Suárez A, Dellundé J, Salvadó H, Cervero-Aragó S, Méndez J, Canals O, Blanco S, Arcas A, Araujo R (2013) Microbial and physicochemical parameters associated with Legionella contamination in hot water recirculation systems. Environmental Science and Pollution Research 20 (8), 5534–5544. [DOI] [PubMed] [Google Scholar]
- Shah PP, Barskey AE, Binder AM, Edens C, Lee S, Smith JC, Schrag S, Whitney CG, Cooley LA (2018) Legionnaires’ Disease Surveillance Summary Report, United States: 2014–1015 Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, USA. [Google Scholar]
- Sharaby Y, Rodríguez-Martínez S, Höfle M, Brettar I, Halpern M (2019) Quantitative microbial risk assessment of Legionella pneumophila in a drinking water supply system in Israel. Science of the Total Environment 671, 404–410. [DOI] [PubMed] [Google Scholar]
- Shen B, Hu J, Song H, Wang Z, Fan J, Sun Y, Wang Q (2019) Antibiotics exacerbated colitis by affecting the microbiota, Treg cells and SCFAs in IL10-deficient mice. Biomedicine & Pharmacotherapy 114, 108849. [DOI] [PubMed] [Google Scholar]
- Siddiqui R, Makhlouf Z, Khan NA (2021) The Increasing Importance of Vermamoeba vermiformis (In press). Journal of Eukaryotic Microbiology, e12857. [DOI] [PubMed] [Google Scholar]
- Silvestry-Rodriguez N, Bright KR, Slack DC, Uhlmann DR, Gerba CP (2008) Silver as a residual disinfectant to prevent biofilm formation in water distribution systems. Applied and Environmental Microbiology 74 (5), 1639–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slow S, Anderson T, Biggs P, Kennedy M, Murdoch D, Cree S (2018) Complete genome sequence of Legionella sainthelensi isolated from a patient with legionnaires’ disease. Genome Announcements 6 (5), e01588–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AF, Huss A, Dorevitch S, Heijnen L, Arntzen VH, Davies M, Robert-Du Ry van Beest Holle M, Fujita Y, Verschoor AM, Raterman B (2019) Multiple sources of the outbreak of Legionnaires’ disease in Genesee County, Michigan, in 2014 and 2015. Environmental Health Perspectives 127 (12), 127001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallworth C, Steed L, Fisher MA, Nolte FS (2012) Legionnaires’ disease caused by Legionella londiniensis. Journal of Clinical Microbiology 50 (12), 4178–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout JE, Yu VL (1997) Legionellosis. New England Journal of Medicine 337 (10), 682–687. [DOI] [PubMed] [Google Scholar]
- Tallon P, Magajna B, Lofranco C, Leung KT (2005) Microbial indicators of faecal contamination in water: a current perspective. Water, Air, & Soil Pollution 166 (1–4), 139–166. [Google Scholar]
- Tang W, Li Q, Chen L, Zhang W. x., Wang H (2021) Biofilm community structures and opportunistic pathogen gene markers in drinking water mains and the role of pipe materials. ACS ES&T Water 1 (3), 630–640. [Google Scholar]
- Tang W, Mao Y, Li Q, Meng D, Chen L, Wang H, Zhu R, Zhang W (2020) Prevalence of opportunistic pathogens and diversity of microbial communities in the water system of a pulmonary hospital. Biomedical and Environmental Sciences 33 (4), 248–259. [DOI] [PubMed] [Google Scholar]
- Thacker WL, Benson R, Hawes L, Gidding H, Dwyer B, Mayberry W, Brenner D (1991) Legionella fairfieldensis sp. nov. isolated from cooling tower waters in Australia. Journal of Clinical Microbiology 29 (3), 475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V, Loret JF, Jousset M, Greub G (2008) Biodiversity of amoebae and amoebae - resisting bacteria in a drinking water treatment plant. Environmental Microbiology 10 (10), 2728–2745. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Higgins DG (2003) Multiple sequence alignment using ClustalW and ClustalX. Current Protocols in Bioinformatics Chapter 2: Unit 2.3. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22 (22), 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RM, Carter R, Tolson C, Coulter C, Huygens F, Hargreaves M (2013) Factors associated with the isolation of Nontuberculous mycobacteria (NTM) from a large municipal water system in Brisbane, Australia. BMC Microbiology 13, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA (2016) Quick Guide to Drinking Water Sample Collection (Second Edition, Update) US EPA, Golden, Colorado, USA. [Google Scholar]
- US EPA (2019a) (Accessed 2021) Clean Water Act Analytical Methods--Other Clean Water Act Test Methods: Microbiological--Not Approved Under 40 CFR Part 136 https://www.epa.gov/cwa-methods/other-clean-water-act-test-methods-microbiological.
- US EPA (2019b) How to Conduct a Sanitary Survey of Drinking Water Systems: A Learner’s Guide (EPA 816-R-17–001) US EPA, Cincinnati, Ohio, USA. [Google Scholar]
- Valster RM, Wullings BA, van den Berg R, van der Kooij D (2011) Relationships between free-living protozoa, cultivable Legionella spp., and water quality characteristics in three drinking water supplies in the Caribbean. Applied and Environmental Microbiology 77 (20), 7321–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo C, Bartie C, Barnard TG, Potgieter N (2021) Detection of free-living amoebae and their intracellular bacteria in borehole water before and after a ceramic pot filter point-of-use intervention in rural communities in South Africa. International Journal of Environmental Research and Public Health 18 (8), 3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wielen PW, van der Kooij D (2013) Nontuberculous mycobacteria, fungi, and opportunistic pathogens in unchlorinated drinking water in The Netherlands. Applied and Environmental Microbiology 79 (3), 825–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz-Moreira I, Nunes OC, Manaia CM (2012) Diversity and antibiotic resistance in Pseudomonas spp. from drinking water. Science of the Total Environment 426, 366–374. [DOI] [PubMed] [Google Scholar]
- Waak MB, LaPara TM, Hall C, Hozalski RM (2018) Occurrence of Legionella spp. in water-main biofilms from two drinking water distribution systems. Environmental Science & Technology 52 (14), 7630–7639. [DOI] [PubMed] [Google Scholar]
- Wang H, Bedard E, Prevost M, Camper AK, Hill VR, Pruden A (2017) Methodological approaches for monitoring opportunistic pathogens in premise plumbing: a review. Water Research 117, 68–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Edwards M, Falkinham JO, Pruden A (2012a) Molecular survey of the occurrence of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa, and amoeba hosts in two chloraminated drinking water distribution systems. Applied and Environmental Microbiology 78 (17), 6285–6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Masters S, Hong Y, Stallings J, Falkinham III JO, Edwards MA, Pruden A (2012b) Effect of disinfectant, water age, and pipe material on occurrence and persistence of Legionella, mycobacteria, Pseudomonas aeruginosa, and two amoebas. Environmental Science & Technology 46 (21), 11566–11574. [DOI] [PubMed] [Google Scholar]
- Wang H, Xu J, Tang W, Li H, Xia S, Zhao J, Zhang W, Yang Y (2019) Removal efficacy of opportunistic pathogens and bacterial community dynamics in two drinking water treatment trains. Small 15 (2), 1804436. [DOI] [PubMed] [Google Scholar]
- Whiley H (2017) Legionella risk management and control in potable water systems: argument for the abolishment of routine testing. International Journal of Environmental Research and Public Health 14 (1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley H, Keegan A, Fallowfield H, Bentham R (2014a) Detection of Legionella, L. pneumophila and Mycobacterium avium [sic] complex (MAC) along potable water distribution pipelines. International Journal of Environmental Research and Public Health 11 (7), 7393–7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley H, Keegan A, Fallowfield H, Ross K (2014b) Uncertainties associated with assessing the public health risk from Legionella. Frontiers in Microbiology 5, 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley H, Taylor M (2016) Legionella detection by culture and qPCR: comparing apples and oranges. Critical Reviews in Microbiology 42 (1), 65–74. [DOI] [PubMed] [Google Scholar]
- Wilczak A, Jacangelo JG, Marcinko JP, Odell LH, Kirmeyer GJ (1996) Occurrence of nitrification in chloraminated distribution systems. Journal AWWA 88 (7), 74–85. [Google Scholar]
- Williams MM, Santo Domingo JW, Meckes MC (2005) Population diversity in model potable water biofilms receiving chlorine or chloramine residual. Biofouling 21 (5–6), 279–288. [DOI] [PubMed] [Google Scholar]
- Wilson N (2019) A microbial hitchhiker’s guide to the galaxy: researchers race to understand effects of deep space on microbiome. Bioscience 69 (1), 5–11. [Google Scholar]
- Wolf-Baca M, Siedlecka A (2020) Prevalence of Legionella spp. and Escherichia coli in the drinking water distribution system of Wrocław (Poland). Water Supply 20 (3), 1083–1090. [Google Scholar]
- Woolschlager J, Rittmann B, Piriou P, Kiene L, Schwartz B (2001) Using a comprehensive model to identify the major mechanisms of chloramine decay in distribution systems. Water Science and Technology: Water Supply 1 (4), 103–110. [Google Scholar]
- Wu H-Y, Yan H, Zheng M-L, Sun M-M, Wang Q, Hu C-M, Zhan X-Y, Yuan M-G, Qu P-H, Hu C-H (2019) Legionella qingyii sp. nov., isolated from water samples in China. International Journal of Systematic and Evolutionary Microbiology 69 (7), 2017–2022. [DOI] [PubMed] [Google Scholar]
- Wullings BA, Van Der Kooij D (2006) Occurrence and genetic diversity of uncultured Legionella spp. in drinking water treated at temperatures below 15 °C. Applied and Environmental Microbiology 72 (1), 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Harrington GW, Noguera DR (2008) Nitrification modeling in pilot-scale chloraminated drinking water distribution systems. Journal of Environmental Engineering 134 (9), 731–742. [Google Scholar]
- Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL (2002) Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. The Journal of infectious diseases 186 (1), 127–128. [DOI] [PubMed] [Google Scholar]
- Zacheus OM, Martikainen PJ (1994) Occurrence of legionellae in hot water distribution systems of Finnish apartment buildings. Canadian Journal of Microbiology 40 (12), 993–999. [DOI] [PubMed] [Google Scholar]
- Zhang C, Brown PJ, Hu Z (2018) Thermodynamic properties of an emerging chemical disinfectant, peracetic acid. Science of the Total Environment 621, 948–959. [DOI] [PubMed] [Google Scholar]
- Zhang C, Brown PJ, Miles RJ, White TA, Grant DG, Stalla D, Hu Z (2019) Inhibition of regrowth of planktonic and biofilm bacteria after peracetic acid disinfection. Water Research 149, 640–649. [DOI] [PubMed] [Google Scholar]
- Zhang C, Lu J (2021a) Legionella: a supplementary indicator of microbial water quality in municipal engineered water systems (In revision). Frontiers in Environmental Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Lu J (2021b) Optimizing disinfectant residual dosage in engineered water systems to minimize the overall health risks of opportunistic pathogens and disinfection by-products. Science of the Total Environment 770, 145356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Qin K, Struewing I, Buse H, Santo Domingo J, Lytle D, Lu J (2021) The bacterial community diversity of bathroom hot tap water was significantly lower than that of cold tap and shower water. Frontiers in Microbiology 12, 625324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-H, Chen S-N, Huang T-L, Shang P-L, Yang X, Ma W-X (2015) Indoor heating drives water bacterial growth and community metabolic profile changes in building tap pipes during the winter season. International Journal of Environmental Research and Public Health 12 (10), 13649–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Edwards M (2009) Accelerated chloramine decay and microbial growth by nitrification in premise plumbing. Journal AWWA 101 (11), 51–62. [Google Scholar]
- Zhang Y, Liu W-T (2019) The application of molecular tools to study the drinking water microbiome–current understanding and future needs. Critical Reviews in Environmental Science and Technology 49 (13), 1188–1235. [Google Scholar]
- Zhang Y, Love N, Edwards M (2009) Nitrification in drinking water systems. Critical Reviews in Environmental Science and Technology 39 (3), 153–208. [Google Scholar]
- Zlatanović L, Van Der Hoek J, Vreeburg J (2017) An experimental study on the influence of water stagnation and temperature change on water quality in a full-scale domestic drinking water system. Water Research 123, 761–772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


