Abstract
Aim: Seaweed contains soluble dietary fibers, potassium, and flavonoids and was recently reported to be inversely associated with the risk of coronary heart disease and mortality from stroke. However, epidemiological evidence on this issue has remained scarce.
Methods: At the baseline survey of four Japanese communities between 1984 and 2000, we enrolled 6,169 men and women aged 40–79 years who had no history of cardiovascular disease. We assessed their seaweed intake using the data from a 24 h dietary recall survey and categorized the intake into four groups (0, 1–5.5, 5.5–15, and ≥ 15 g/day). We used sex-specific Cox proportional hazards models to examine the association between seaweed intake and risk of cardiovascular disease (stroke, stroke subtypes, and coronary heart disease).
Results: During the 130,248 person-year follow-up, 523 cases of cardiovascular disease occurred: 369 cases of stroke and 154 cases of coronary heart disease. Seaweed intake levels were inversely associated with the risk of total stroke and cerebral infarction among men but not among women. Adjustment for cardiovascular risk factors did not change the associations: the hazard ratios (95% confidence intervals; P for trend) for the highest versus lowest categories of seaweed intake were 0.63 (0.42–0.94; 0.01) for total stroke and 0.59 (0.36–0.97; 0.03) for cerebral infarction. No associations were observed between seaweed intake and risks of intraparenchymal hemorrhage, subarachnoid hemorrhage, or coronary heart disease among men or women.
Conclusions: We found an inverse association between seaweed intake and risk of total stroke, especially that from cerebral infarction, among Japanese men.
Keywords: Epidemiology, Stroke, Risk factor, Cohort study, Japan
Introduction
Seaweed is not commonly eaten in most Western countries but is often consumed in East Asian countries 1) , where it is a traditional food and has been believed to be beneficial for health. A cohort study in Japan reported that Japanese dietary patterns characterized by a high contribution of soybeans, fish, vegetables, seaweed, mushrooms, and fruits 2) were inversely associated with risk of death from cardiovascular disease 3 - 6) .
Seaweed is rich in dietary fiber, vitamins, and minerals 7 , 8) . These components affect blood pressure 9 - 13) , serum lipids 14 - 16) , fatty acid 17) , blood glucose 14 , 18 , 19) , and body weight 20 , 21) , which are known risk factors for cardiovascular disease. As such, seaweeds have been suggested to have various beneficial effects on cardiovascular disease. However, only two cohort studies have examined the association between seaweed intake and risks of mortality 22) and of incidence 23) from cardiovascular disease. Both studies concluded that seaweed intake may be beneficial for preventing cardiovascular disease; however, a part of the findings on sex and outcome differed between them 22 , 23) . Thus, we sought to examine the association between seaweed intake and risk of incidence of cardiovascular disease in different populations from those in the previous two studies, under the Circulatory Risk in Communities Study (CIRCS), a large community-based cohort study in Japan.
Methods
Study Cohort
CIRCS is an ongoing community-based epidemiological study using dynamic prospective cohorts involving approximately 12,000 persons every year in five Japanese municipalities; the details of the study have been described elsewhere 24 , 25) . The present study includes 6,169 residents aged 40– 79 years who participated in at least one dietary survey between 1984 and 2000 in four CIRCS communities (Ikawa, Yao, Noichi, and Kyowa) and who have no history of coronary heart disease and/or stroke at baseline. Fig.1 shows the study flowchart for patients’ selection.
Fig.1.
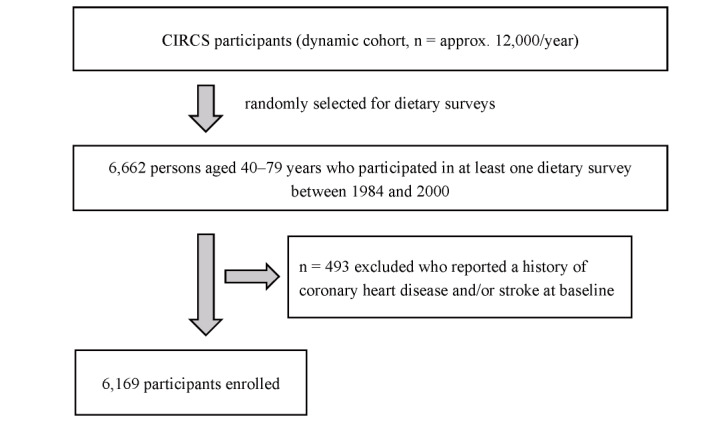
Flowchart of study participants of the present study
Baseline Examination and Assessment of Nutrient Intake
Trained study physicians or nurses measured blood pressure using standard mercury sphygmomanometers with standardized methods 25) . Height without shoes and weight in light clothing were measured. Face-to-face interviews were conducted to determine drinking and smoking status and use of antihypertensive, cholesterol-lowering, or antidiabetic medication. Blood samples were collected without fasting requirement from the participants; approximately 15% of the participants were in a fasting state. Serum total cholesterol, triglyceride, and glucose were measured. The details of the methods in baseline examination were described elsewhere 26) . Hypertension was defined as systolic blood pressure of ≥ 140 mmHg or diastolic blood pressure of ≥ 90 mmHg or as the use of antihypertensive medication. Diabetes mellitus was defined as a fasting serum glucose of ≥ 126 mg/dL or a nonfasting serum glucose of ≥ 200 mg/dL or as the use of antidiabetic medication.
We adopted the 24 h dietary recall method to collect the dietary data. In this method, the participants were interviewed by trained dietitians or nutritionists about everything they had eaten within 24 h. Actual-sized food models, pictures of food materials and dishes, and/or real foods and dishes were used to help the recall and estimate the amount of the foods. Nutrient intakes were calculated based on the estimated amount using the Standard Tables of Food Composition in Japan, 2015 (seventh revised edition) 27) .
We examined the reproducibility of seaweed intake in the 24 h dietary recall survey among the 1,866 participants who had responded to two 24 h dietary recall surveys. The surveys were undertaken 4.3 years apart on average. We categorized seaweed intake into four groups for the first and second surveys and compared the two surveys for group concordance. The quadratic weighted kappa coefficient of the seaweed intake of the two groups in the two surveys was 0.24. In addition, Spearman’s rank correlation coefficient between the groups of seaweed intake was 0.14.
Follow-up and Determination of Ischemic Cardiovascular Disease
Follow-up lasted until the end of 2012 for Noichi, until the end of 2015 for Kyowa, and until the end of 2016 for Ikawa and Minami-Takayasu and was terminated at the first incident of coronary heart disease or stroke, moving out of the original community, or death. The median follow-up was 22 years for stroke or coronary heart disease.
The details of the endpoint determination have been described in previous CIRCS reports 26 , 28) . For all the residents, cardiovascular disease endpoints were ascertained from the death certificates, national insurance claims, reports by local physicians, reports by public health nurses and health volunteers, and annual cardiovascular risk surveys. To confirm the diagnosis, all living patients were telephoned, visited, or invited to take part in risk factor surveys, or a medical history was obtained from their families. In addition, the medical records in the local clinics and hospitals were reviewed. In the cases of death, the histories were obtained from the patients’ families and/or attending physicians, and the medical records were reviewed.
The criteria for coronary heart disease, i.e., definite and probable myocardial infarctions, definite angina pectoris, and sudden cardiac death within 1 h of onset, were modified from those of the World Health Organization Expert Committee 29) . The criterion for incident stroke was a focal neurologic disorder with rapid onset and persisting for at least 24 h or until death. Stroke cases were further classified as intraparenchymal hemorrhage, subarachnoid hemorrhage, ischemic stroke (lacunar, large-artery occlusive, or embolic stroke), or stroke of undetermined type primarily using computed tomography (CT)/magnetic resonance imaging (MRI) findings 30) . CT and/or MRI imaging findings were available for 97% of the stroke cases. The final diagnosis of coronary heart disease or stroke was performed by a panel of two to four physicians participating in this study who were blinded to the data from the risk factor survey.
Statistical Analyses
For the analyses, we categorized seaweed intake into four groups. We sorted a group whose seaweed intake was zero from the participants who had consumed seaweed, and then, we divided those into tertiles. As a result, the four groups of seaweed intake were as follows: intake of 0 g ( n =2,478), between 1 and 5.5 g ( n =1,244), between 5.5 and 15 g ( n =1,214), and more than 15 g ( n =1,233) per day. The analysis of covariance was used to test for differences in age-adjusted means and prevalence of baseline characteristics in terms of seaweed intake. Person-years were calculated as the sum of the individual follow-up times until the occurrence of incident coronary heart disease, stroke, death, or emigration, or the end of follow-up. We used Cox proportional hazards models to calculate the sex-specific and age-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for total stroke, cerebral infarction, intraparenchymal hemorrhage, subarachnoid hemorrhage, and coronary heart disease using the risk for persons, with the group of no seaweed intake as the reference (model 1). A test for the trend of association between seaweed intake and cardiovascular disease was also conducted using the median value of seaweed intake for each category. We further adjusted for age; body mass index (quartiles); smoking status (never, past, or current smoker); drinking status (never, past, or current drinker); total energy intake; and dietary intakes of vegetables, fruits, fish, meat, soy, and sodium (continuous) (model 2). To test the potential mediating effects, we further adjusted for hypertension (dichotomous), serum total cholesterol (quartiles), cholesterol-lowering medication use (dichotomous), and diabetes mellitus (dichotomous). Nutrient residual models were not applied in any foods/nutrients since seaweed contains little energy. The nutrient residual models did not alter the main results.
We used SAS version 9.4 software (SAS Institute) for all statistical analyses. All probability values for statistical tests were two-tailed, and P values below 0.05 were considered significant.
Ethical Considerations
The CIRCS protocol was approved by the ethics committees of the Osaka Center for Cancer and Cardiovascular Disease Prevention (R2-Rinri-4), Osaka University (14285-7), and of the University of Tsukuba (66-8). Informed consent was obtained verbally before the dietary surveys, and an opt-out option was provided to all the participants.
Results
Seaweed intake was positively associated with total energy intake and dietary intakes of soy, total fiber, and sodium for both sexes ( Table 1 ) . Seaweed intake was positively associated with age, cholesterol-lowering medication use, and dietary intake of fish only among men; conversely, it was associated with dietary intakes of vegetables and fruits only among women. Seaweed intake was not associated with body mass index, smoking status, and drinking status in either sex.
Table 1. Age-adjusted baseline characteristics according to the categories of seaweed intake among 6,169 Japanese men and women aged 40 to 79 years who participated in CIRCS between 1984 and 2000.
| None | Tertiles of seaweed intake, g/day | P for overall difference | |||
|---|---|---|---|---|---|
| T1 (0-5.5) | T2 (5.5-15) | T3 (≥ 15) | |||
| Men ( n = 2,792) | |||||
| Number at risk | 1,166 | 514 | 544 | 568 | |
| Age at baseline * , years | 52.5 | 51.9 | 52.9 | 54.0 | <0.001 |
| Current smoker, % | 55.1 | 52.7 | 55.6 | 54.1 | 0.78 |
| Current drinker, % | 73.9 | 74.7 | 77.3 | 73.6 | 0.42 |
| Body mass index, kg/m 2 | 23.4 | 23.5 | 23.3 | 23.5 | 0.52 |
| Systolic blood pressure, mmHg | 135 | 134 | 134 | 134 | 0.38 |
| Diastolic blood pressure, mmHg | 83 | 82 | 82 | 83 | 0.44 |
| Antihypertensive medication use, % | 14.4 | 16.6 | 16.6 | 16.1 | 0.49 |
| Hypertension, % | 48.1 | 46.4 | 45.1 | 47.0 | 0.69 |
| Serum total cholesterol, mg/dL | 192 | 192 | 193 | 193 | 0.85 |
| Casual serum triglycerides, mg/dL | 156 | 160 | 151 | 148 | 0.30 |
| Cholesterol-lowering medication use, % | 9.9 | 12.3 | 13.3 | 15.0 | 0.01 |
| Diabetes mellitus, % | 11.3 | 14.8 | 13.3 | 11.7 | 0.18 |
| Dietary intakes | |||||
| Total energy, kcal/day | 2,251 | 2,266 | 2,278 | 2,349 | 0.02 |
| Vegetables, g/day | 268 | 249 | 254 | 262 | 0.12 |
| Fruits, g/day | 137 | 128 | 118 | 122 | 0.15 |
| Seaweed, g/day | 0 | 3 | 10 | 35 | <0.001 |
| Fish, g/day | 114 | 111 | 121 | 127 | 0.01 |
| Meat, g/day | 57 | 54 | 51 | 51 | 0.20 |
| Soy, g/day | 62 | 59 | 67 | 73 | 0.01 |
| Total fiber, g/day | 14.0 | 14.3 | 14.4 | 15.9 | <0.001 |
| Sodium, g/day | 11.3 | 11.8 | 11.8 | 12.9 | <0.001 |
| Women ( n = 3,377) | |||||
| Number at risk | 1,312 | 730 | 670 | 665 | |
| Age at baseline * , years | 53.4 | 53.6 | 54.2 | 54.4 | 0.06 |
| Current smoker, % | 5.5 | 3.9 | 4.2 | 4.6 | 0.36 |
| Current drinker, % | 10.9 | 9.8 | 10.9 | 10.0 | 0.85 |
| Body mass index, kg/m 2 | 23.7 | 23.6 | 23.6 | 23.8 | 0.50 |
| Systolic blood pressure, mmHg | 133 | 134 | 134 | 133 | 0.31 |
| Diastolic blood pressure, mmHg | 80 | 80 | 80 | 80 | 0.90 |
| Antihypertensive medication use, % | 18.8 | 15.5 | 18.9 | 16.0 | 0.11 |
| Hypertension, % | 45.3 | 40.1 | 44.7 | 41.2 | 0.11 |
| Serum total cholesterol, mg/dL | 205 | 205 | 207 | 202 | 0.13 |
| Casual serum triglycerides, mg/dL | 122 | 126 | 119 | 118 | 0.29 |
| Cholesterol-lowering medication use, % | 13.8 | 10.8 | 15.4 | 14.6 | 0.05 |
| Diabetes mellitus, % | 10.6 | 9.8 | 11.7 | 11.8 | 0.50 |
| Dietary intakes | |||||
| Total energy, kcal/day | 1,683 | 1,724 | 1,693 | 1,758 | 0.007 |
| Vegetables, g/day | 281 | 265 | 270 | 290 | 0.008 |
| Fruits, g/day | 168 | 192 | 173 | 181 | 0.02 |
| Seaweed, g/day | 0 | 3 | 10 | 34 | <0.001 |
| Fish, g/day | 86 | 89 | 94 | 94 | 0.05 |
| Meat, g/day | 41 | 37 | 39 | 41 | 0.11 |
| Soy, g/day | 50 | 55 | 63 | 63 | <0.001 |
| Total fiber, g/day | 14.6 | 15.1 | 15.5 | 16.9 | <0.001 |
| Sodium, g/day | 9.6 | 10.0 | 10.2 | 11.3 | <0.001 |
Abbreviations: dL, deciliter; g, gram; kcal, kilocalorie; kg, kilogram; m 2 , square meter; mg, milligram; mmHg, millimeter hydrargyrum; n , number * Unadjusted
During a median 22 year follow-up totaling 130,248 person-years, we confirmed a total of 523 cases of total cardiovascular diseases (330 men) including 369 total strokes (205 men), 234 cerebral infarctions (145 men), 85 intraparenchymal hemorrhages (45 men), 45 subarachnoid hemorrhages (12 men), and 154 coronary heart disease events (105 men). As shown in Table 2 , seaweed intake levels were inversely associated with the risk of total stroke, especially cerebral infarction, among men. Adjustment for cardiovascular risk factors did not change the association: the HRs (95% CIs; P for trend) for the highest versus lowest category of seaweed intake were 0.63 (0.42–0.94; 0.01) for total stroke and 0.59 (0.36–0.97; 0.03) for cerebral infarction. No significant associations with seaweed intake were observed for coronary heart disease among men and women: the HRs were 1.10 (0.63–1.92; 0.56) among men and 0.57 (0.25–1.32; 0.19) among women (model 2). Furthermore, no significant associations were observed for intraparenchymal and subarachnoid hemorrhages in either sex. After further adjustment for potential mediators, these associations were not materially altered for any outcomes for both men and women; for example, the HRs were 0.63 (0.42–0.95; 0.02) for total stroke and 0.59 (0.36–0.98; 0.04) for cerebral infarction (data not shown in the Tables).
Table 2. Multivariable adjusted HRs and 95% CIs of ischemic cardiovascular disease (stroke and coronary heart disease) according to the four categories of seaweed intake, CIRCS.
| None | Tertiles of seaweed intake, g/day | P for trend | |||
|---|---|---|---|---|---|
| T1 (0-5.5) | T2 (5.5-15) | T3 (≥ 15) | |||
| Men | |||||
| Person-years | 24,083 | 10,674 | 11,359 | 11,947 | |
| Number at risk | 1166 | 514 | 544 | 568 | |
| Total stroke | |||||
| Number of cases | 98 | 38 | 33 | 36 | |
| Model 1 | 1.00 | 0.92 (0.63-1.35) | 0.65 (0.44-0.97) | 0.62 (0.41-0.92) | 0.01 |
| Model 2 | 1.00 | 0.93 (0.63-1.36) | 0.64 (0.43-0.95) | 0.63 (0.42-0.94) | 0.01 |
| Intraparenchymal hemorrhage | |||||
| Number of cases | 21 | 10 | 6 | 8 | |
| Model 1 | 1.00 | 1.18 (0.55-2.52) | 0.57 (0.23-1.41) | 0.71 (0.30-1.66) | 0.29 |
| Model 2 | 1.00 | 1.10 (0.51-2.36) | 0.53 (0.21-1.33) | 0.63 (0.26-1.49) | 0.19 |
| Subarachnoid hemorrhage | |||||
| Number of cases | 4 | 4 | 1 | 3 | |
| Model 1 | 1.00 | 2.48 (0.61-10.1) | 0.56 (0.06-5.03) | 1.06 (0.23-4.96) | 0.76 |
| Model 2 | 1.00 | 3.40 (0.77-15.0) | 0.64 (0.07-5.90) | 1.32 (0.27-6.44) | 0.90 |
| Cerebral infarction | |||||
| Number of cases | 71 | 24 | 26 | 24 | |
| Model 1 | 1.00 | 0.80 (0.50-1.27) | 0.70 (0.44-1.11) | 0.56 (0.34-0.91) | 0.02 |
| Model 2 | 1.00 | 0.80 (0.50-1.28) | 0.68 (0.43-1.08) | 0.59 (0.36-0.97) | 0.03 |
| Coronary heart disease | |||||
| Number of cases | 47 | 16 | 22 | 20 | |
| Model 1 | 1.00 | 0.75 (0.42-1.32) | 1.03 (0.62-1.71) | 1.07 (0.62-1.84) | 0.63 |
| Model 2 | 1.00 | 0.77 (0.43-1.36) | 1.05 (0.63-1.76) | 1.10 (0.63-1.92) | 0.56 |
| Women | |||||
| Person-years | 28,173 | 15,515 | 13,955 | 14,542 | |
| Number at risk | 1312 | 730 | 670 | 665 | |
| Total strokes | |||||
| Number of cases | 69 | 32 | 29 | 34 | |
| Model 1 | 1.00 | 0.89 (0.58-1.36) | 0.85 (0.55-1.31) | 0.83 (0.54-1.26) | 0.40 |
| Model 2 | 1.00 | 0.89 (0.58-1.37) | 0.86 (0.55-1.33) | 0.85 (0.55-1.31) | 0.49 |
| Intraparenchymal hemorrhage | |||||
| Number of cases | 19 | 8 | 5 | 8 | |
| Model 1 | 1.00 | 0.82 (0.36-1.88) | 0.54 (0.20-1.45) | 0.73 (0.31-1.71) | 0.43 |
| Model 2 | 1.00 | 0.78 (0.34-1.83) | 0.54 (0.20-1.46) | 0.65 (0.27-1.55) | 0.32 |
| Subarachnoid hemorrhage | |||||
| Number of cases | 14 | 7 | 6 | 6 | |
| Model 1 | 1.00 | 0.91 (0.36-2.26) | 0.87 (0.33-2.27) | 0.72 (0.27-1.90) | 0.50 |
| Model 2 | 1.00 | 0.97 (0.39-2.45) | 0.93 (0.35-2.46) | 0.70 (0.26-1.91) | 0.48 |
| Cerebral infarction | |||||
| Number of cases | 34 | 17 | 18 | 20 | |
| Model 1 | 1.00 | 0.99 (0.55-1.79) | 1.04 (0.59-1.86) | 0.96 (0.54-1.69) | 0.90 |
| Model 2 | 1.00 | 1.00 (0.55-1.80) | 1.02 (0.57-1.83) | 1.05 (0.59-1.88) | 0.86 |
| Coronary heart disease | |||||
| Number of cases | 23 | 10 | 8 | 8 | |
| Model 1 | 1.00 | 0.81 (0.38-1.71) | 0.71 (0.32-1.59) | 0.58 (0.26-1.32) | 0.20 |
| Model 2 | 1.00 | 0.81 (0.38-1.72) | 0.70 (0.31-1.57) | 0.57 (0.25-1.32) | 0.19 |
Abbreviations: HR, hazard ratio; CIs, confidence intervals
Model 1 was adjusted for age. Model 2 was adjusted for age; body mass index (quartiles); smoking status (never, current, or past smoker); drinking status (never, current, or past drinker); total energy intake; and dietary intakes of vegetables, fruits, fish, meat, soy, and sodium (continuous).
Discussion
In this large, long-term prospective cohort study of middle-aged Japanese individuals, we observed that seaweed intake was inversely associated with the risk of total stroke, especially cerebral infarction, among men but not among women. This is the first study to find an inverse association of seaweed intake with risk of incident total stroke and cerebral infarction.
Recently, two large Japanese cohort studies reported an association between seaweed intake and risk of or mortality from cardiovascular disease. The Japan Public Health Center (JPHC) Study, involving 86,113 men and women aged 40– 69 years during the baseline period (1990–1994), showed that the frequency of seaweed intake was inversely associated with the risk of incident coronary heart disease among men and women but not of stroke 23) . By contrast, another recent report from the Japan Collaborative Cohort (JACC) Study, involving 96,215 men and women aged 40– 79 years during the baseline period (1988–1990), showed that the frequency of seaweed intake based on a food frequency questionnaire was inversely associated with mortality from total stroke, cerebral infarction, and total cardiovascular disease among women but not among men 22) . In that study, seaweed intake was not associated with mortality from coronary heart disease in either sex.
Our results are partly concordant with those of the JACC Study, although we did not find any associations among women. The reasons for the discrepancies between our study and these previous ones are unknown. However, possible reasons are differences in the methods in the dietary survey (food frequency questionnaire in the JPHC and the JACC vs 24 h dietary records in the CIRCS); categories of seaweed intake (frequency in a week: four categories in the JPHC and five categories in the JACC vs the amount in a day in the CIRCS); modes of outcome (incidence in the JPHC and the CIRCS vs mortality in the JACC); the era of the baseline (the 1990s in the JPHC and the JACC vs the 1980-90s in the CIRCS); follow-up periods; and study populations. Notwithstanding these differences, our study and the two previous studies showed that seaweed intake was inversely associated with the risk of coronary heart disease or stroke.
No intervention trials proved the preventive effect of seaweed on cardiovascular diseases. However, the beneficial impacts of seaweed on blood pressure, lipids, diabetes mellitus, weight reduction, and related factors in humans have been shown in the previous trials 1) , which support our and previous observational findings.
As for the bioactive components, alginates and fucoidan (both are polysaccharides), fucosterols (lipids), and fucoxanthin (carotenoids) are contained specifically in seaweed 31) . Alginates, the main polysaccharides of seaweed, contribute to lower cholesterol levels 32) , improved postprandial blood glucose 32) , and lower blood pressure 10 , 12) . Another polysaccharide, fucoidan, contained only in brown algae (which is the most commonly consumed seaweeds 33) ) 31) , has antioxidant, anticoagulant, antithrombotic, and anti-inflammatory effects 34) , as well as cholesterol-lowering 35) and blood pressure-lowering 36) effects. As for an anticoagulant effect, an in vivo experiment of Wistar rats demonstrated that the application of fucoidan (ointment containing dry Fucus extracts) for 7 days increased the activated partial thromboplastin time (21.3% vs 2.7%, P <0.05) and prothrombin time (42.0% vs 6.0%, P <0.05) in comparison with the control group (no manipulation). In addition, the prothrombin time after the application of the ointment of dry Fucus extracts was similar to that of heparin application (42.0% vs 41.3%) 37) .
The other nutrients, fucosterols, contained as lipids especially in brown and red algae 38) , lower blood glucose and blood pressure levels 39) . Fucoxanthin, an algal carotenoid, also has a stronger antioxidant effect than other carotenoids such as α-tocopherol 40) . In addition, although they are not specifically contained in seaweeds, potassium 41) and calcium 42) lower blood pressure levels. Seaweed is also rich in vitamins A, B (including vitamins B 1 , B 2 , B 6 , and B 12 ), C, and E, which have antioxidant effects 43) . The concentration of vitamin C is comparable with that in common vegetables such as tomatoes and lettuce 43 , 44) . Taken together, several mechanisms may be involved in the pathophysiology of seaweed–stroke associations. Our observation that the adjustment for mediating factors such as hypertension, dyslipidemia, and diabetes mellitus did not alter the associations suggested that pathways other than blood pressure, cholesterol, and glucose may be relatively essential roles for the prevention of stroke.
The major strengths of our study are its large sample size, prospective design, long follow-up duration, and high follow-up rate. Unlike most Western populations, the Japanese are unique in that they frequently eat seaweeds, which allowed us to examine the association between seaweed intake and the risk of incident ischemic cardiovascular disease. In addition, by adopting the 24 h dietary recall survey, we could estimate the absolute amount of commonly consumed seaweed intake.
Several limitations of our study must be mentioned. First, since we used a single data from the 24 h dietary recall survey to determine the exposure of seaweed intake, we did not take any subsequent dietary changes into account; thus, the participants might have been categorized incorrectly by the single data source for seaweed intake. In the present study, the reproducibility of seaweed intake in the 24 h dietary recall survey was not high (quadratic weighted kappa coefficient=0.24, Spearman’s correlation coefficients=0.14) compared with the reproducibility estimated by using repeated food frequency questionnaires in the other study 23) (Spearman’s correlation coefficients=0.35). In previous studies, the frequency of seaweed intake was usually one to two times or three to four times a week 22 , 23) ; therefore, the participants may not necessarily have consumed seaweed the day before the survey. Since such measurement errors would occur randomly across the exposure groups, they likely diluted the association between seaweed intake and risk of cardiovascular disease. Second, we cannot negate the possibility of residual confounding by unmeasured variables such as socioeconomic status. Seaweed intake might reflect healthy dietary patterns 3) , but the adjustment for vegetable, fruit, fish, soy, salt, and meat intake did not change the results materially. Finally, no significant results were obtained in women, the reasons for which are unknown to us. The sex difference in the association of seaweed and cardiovascular disease was also found in previous studies 22) . It might be due to the difference in hormones, underlying risk factors of cardiovascular disease (such as smoking and hypertension), and the amount of food intake.
Nevertheless, our findings suggest the usefulness of seaweeds in preventing ischemic cardiovascular disease. Since seaweed can be easily adapted into dietary habits, it could attract worldwide attention as a health food. Although active pathways to metabolic processes of various nutrients in seaweed have been reported, most of them are from animal experiments. In addition, the absorption rates of those nutrients from the digestive tract in humans have not been revealed.
Conclusion
The present study revealed that seaweed intake was inversely associated with the risk of incident total stroke, especially cerebral infarction, among Japanese men.
Acknowledgements
The authors thank the health care staff of the Ikawa, Yao, and Kyowa communities and of the Osaka Center for Cancer and Cardiovascular Disease Prevention. We also thank Flaminia Miyamasu, Medical English Communications Center, University of Tsukuba, for editorial assistance. The full list of CIRCS investigators is presented in reference number 25.
Notice of Grand Support
This study was partly supported by JSPS Kakenhi [grant numbers 17H04121, 18K10097, 19H03901]; and the Ministry of Health, Labour and Welfare, Japan (Research on Health Services: H17–Kenkou–007; Comprehensive Research on Cardiovascular Disease and Life–Related Disease: H18–Junkankitou [Seishuu]–Ippan–012; H19–Junkankitou [Seishuu]–Ippan–012; H20–Junkankitou [Seishuu]–Ippan–013; H23–Junkankitou [Seishuu]–Ippan–005; H26-Junkankitou [Seisaku]-Ippan-001; H29–Junkankitou [Seishuu]–Ippan–003 and 20FA1002; H30-Junkankitou-Ippan-005)
COI
No conflicts of interest to declare. The funding agencies for this study made no decisions regarding its study design, data collection, data analysis, manuscript preparation, or decision to publish.
References
- 1).Murai U, Yamagishi K, Kishida R, Iso H: Impact of seaweed intake on health. Eur J Clin Nutr, 2020; https: //doi.org/10.1038/s41430-020-00739-8 [Google Scholar]
- 2).Nanri A, Shimazu T, Ishihara J, Takachi R, Mizoue T, Inoue M and Tsugane S: Reproducibility and validity of dietary patterns assessed by a food frequency questionnaire used in the 5-year follow-up survey of the Japan Public Health Center-Based Prospective Study. J Epidemiol, 2012; 22: 205-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Maruyama K, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Inaba Y and Tamakoshi A: Dietary patterns and risk of cardiovascular deaths among middle-aged Japanese: JACC Study. Nutr Metab Cardiovasc Dis, 2013; 23: 519-527 [DOI] [PubMed] [Google Scholar]
- 4).Okada C, Imano H, Yamagishi K, Cui R, Umesawa M, Maruyama K, Muraki I, Hayama-Terada M, Shimizu Y, Sankai T, Okada T, Kiyama M, Kitamura A and Iso H: Dietary Intake of Energy and Nutrients from Breakfast and Risk of Stroke in The Japanese Population: The Circulatory Risk in Communities Study (CIRCS). J Atheroscler Thromb, 2019; 26: 145-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Shimazu T, Kuriyama S, Hozawa A, Ohmori K, Sato Y, Nakaya N, Nishino Y, Tsubono Y and Tsuji I: Dietary patterns and cardiovascular disease mortality in Japan: a prospective cohort study. Int J Epidemiol, 2007; 36: 600-609 [DOI] [PubMed] [Google Scholar]
- 6).Yamori Y, Miura A and Taira K: Implications from and for food cultures for cardiovascular diseases: Japanese food, particularly Okinawan diets. Asia Pac J Clin Nutr, 2001; 10: 144-145 [DOI] [PubMed] [Google Scholar]
- 7).MacArtain P, Gill CI, Brooks M, Campbell R and Rowland IR: Nutritional value of edible seaweeds. Nutr Rev, 2007; 65: 535-543 [DOI] [PubMed] [Google Scholar]
- 8).Cardoso SM, Pereira OR, Seca AM, Pinto DC and Silva AM: Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar Drugs, 2015; 13: 6838-6865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Sato M, Oba T, Yamaguchi T, Nakano T, Kahara T, Funayama K, Kobayashi A and Nakano T: Antihypertensive effects of hydrolysates of wakame (Undaria pinnatifida) and their angiotensin-I-converting enzyme inhibitory activity. Ann Nutr Metab, 2002; 46: 259-267 [DOI] [PubMed] [Google Scholar]
- 10).Sato M, Hosokawa T, Yamaguchi T, Nakano T, Muramoto K, Kahara T, Funayama K, Kobayashi A and Nakano T: Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J Agric Food Chem, 2002; 50: 6245-6252 [DOI] [PubMed] [Google Scholar]
- 11).Ikeda K, Kitamura A, Machida H, Watanabe M, Negishi H, Hiraoka J and Nakano T: Effect of Undaria pinnatifida (Wakame) on the development of cerebrovascular diseases in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol, 2003; 30: 44-48 [DOI] [PubMed] [Google Scholar]
- 12).Yamori Y, Nara Y, Tsubouchi T, Sogawa Y, Ikeda K and Horie R: Dietary prevention of stroke and its mechanisms in stroke-prone spontaneously hypertensive rats--preventive effect of dietary fibre and palmitoleic acid. J Hypertens Suppl, 1986; 4: S449-452 [PubMed] [Google Scholar]
- 13).Suetsuna K, Maekawa K and Chen JR: Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J Nutr Biochem, 2004; 15: 267-272 [DOI] [PubMed] [Google Scholar]
- 14).Yoshinaga K, Nakai Y, Izumi H, Nagaosa K, Ishijima T, Nakano T and Abe K: Oral Administration of Edible Seaweed Undaria Pinnatifida (Wakame) Modifies Glucose and Lipid Metabolism in Rats: A DNA Microarray Analysis. Mol Nutr Food Res, 2018; 62: e1700828 [DOI] [PubMed] [Google Scholar]
- 15).Yokota T, Nomura K, Nagashima M and Kamimura N: Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoE(shl) mice deficient in apolipoprotein E expression. J Nutr Biochem, 2016; 32: 46-54 [DOI] [PubMed] [Google Scholar]
- 16).Maeda H, Yamamoto R, Hirao K and Tochikubo O: Effects of agar (kanten) diet on obese patients with impaired glucose tolerance and type 2 diabetes. Diabetes Obes Metab, 2005; 7: 40-46 [DOI] [PubMed] [Google Scholar]
- 17).Shijo Y, Maruyama C, Nakamura E, Nakano R, Shima M, Mae A, Okabe Y, Park S, Kameyama N and Hirai S: Japan Diet Intake Changes Serum Phospholipid Fatty Acid Compositions in Middle-Aged Men: A Pilot Study. J Atheroscler Thromb, 2019; 26: 3-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Yoshinaga K and Mitamura R: Effects of Undaria pinnatifida (Wakame) on Postprandial Glycemia and Insulin Levels in Humans: a Randomized Crossover Trial. Plant Foods Hum Nutr, 2019; 74: 461-467 [DOI] [PubMed] [Google Scholar]
- 19).Maeda H, Hosokawa M, Sashima T and Miyashita K: Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J Agric Food Chem, 2007; 55: 7701-7706 [DOI] [PubMed] [Google Scholar]
- 20).Paxman JR, Richardson JC, Dettmar PW and Corfe BM: Daily ingestion of alginate reduces energy intake in free-living subjects. Appetite, 2008; 51: 713-719 [DOI] [PubMed] [Google Scholar]
- 21).Hosokawa M, Miyashita T, Nishikawa S, Emi S, Tsukui T, Beppu F, Okada T and Miyashita K: Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch Biochem Biophys, 2010; 504: 17-25 [DOI] [PubMed] [Google Scholar]
- 22).Kishida R, Yamagishi K, Muraki I, Sata M, Tamakoshi A and Iso H: Frequency of Seaweed Intake and Its Association with Cardiovascular Disease Mortality: The JACC Study. J Atheroscler Thromb, 2020; 27: 1340-1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Murai U, Yamagishi K, Sata M, Kokubo Y, Saito I, Yatsuya H, Ishihara J, Inoue M, Sawada N, Iso H and Tsugane S: Seaweed intake and risk of cardiovascular disease: the Japan Public Health Center-based Prospective (JPHC) Study. Am J Clin Nutr, 2019; 110: 1449-1455 [DOI] [PubMed] [Google Scholar]
- 24).Imano H, Iso H, Kiyama M, Yamagishi K, Ohira T, Sato S, Noda H, Maeda K, Okada T, Tanigawa T and Kitamura A: Non-fasting blood glucose and risk of incident coronary heart disease in middle-aged general population: the Circulatory Risk in Communities Study (CIRCS). Prev Med, 2012; 55: 603-607 [DOI] [PubMed] [Google Scholar]
- 25).Yamagishi K, Muraki I, Kubota Y, Hayama-Terada M, Imano H, Cui R, Umesawa M, Shimizu Y, Sankai T, Okada T, Sato S, Kitamura A, Kiyama M and Iso H: The Circulatory Risk in Communities Study (CIRCS): A Long-Term Epidemiological Study for Lifestyle-Related Disease Among Japanese Men and Women Living in Communities. J Epidemiol, 2019; 29: 83-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Iso H, Imano H, Yamagishi K, Ohira T, Cui R, Noda H, Sato S, Kiyama M, Okada T, Hitsumoto S, Tanigawa T, Kitamura A and Investigators C: Fasting and non-fasting triglycerides and risk of ischemic cardiovascular disease in Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Atherosclerosis, 2014; 237: 361-368 [DOI] [PubMed] [Google Scholar]
- 27).Ministry of Education Culture, Sports, Science and Technology. Japan standard tables of food composition in Japan, 2015 [Google Scholar]
- 28).Kitamura A, Noda H, Nakamura M, Kiyama M, Okada T, Imano H, Ohira T, Sato S, Yamagishi K and Iso H: Association between non-high-density lipoprotein cholesterol levels and the incidence of coronary heart disease among Japanese: the Circulatory Risk in Communities Study (CIRCS). J Atheroscler Thromb, 2011; 18: 454-463 [DOI] [PubMed] [Google Scholar]
- 29).WHO Expert Committee on Prevention of Coronary Heart Disease. Prevention of coronary heart disease. World Health Organization - Technical Report Series. 1982: 1-53 [PubMed] [Google Scholar]
- 30).Iso H, Rexrode K, Hennekens CH and Manson JE: Application of computer tomography-oriented criteria for stroke subtype classification in a prospective study. Ann Epidemiol, 2000; 10: 81-87 [DOI] [PubMed] [Google Scholar]
- 31).Wells ML, Potin P, Craigie JS, Raven JA, Merchant SS, Helliwell KE, Smith AG, Camire ME and Brawley SH: Algae as nutritional and functional food sources: revisiting our understanding. J Appl Phycol, 2017; 29: 949-982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Paxman JR, Richardson JC, Dettmar PW and Corfe BM: Alginate reduces the increased uptake of cholesterol and glucose in overweight male subjects: a pilot study. Nutr Res, 2008; 28: 501-505 [DOI] [PubMed] [Google Scholar]
- 33).Nisizawa K, Noda H, Kikuchi R and Watanabe T: The main seaweed foods in Japan. 1987; 5-29 [Google Scholar]
- 34).Luthuli S, Wu S, Cheng Y, Zheng X, Wu M and Tong H: Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar Drugs, 2019; 17: 487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Park MK, Jung U and Roh C: Fucoidan from marine brown algae inhibits lipid accumulation. Mar Drugs, 2011; 9: 1359-1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Li X, Li J, Li Z, Sang Y, Niu Y, Zhang Q, Ding H and Yin S: Fucoidan from Undaria pinnatifida prevents vascular dysfunction through PI3K/Akt/eNOS-dependent mechanisms in the l-NAME-induced hypertensive rat model. Food Funct, 2016; 7: 2398-2408 [DOI] [PubMed] [Google Scholar]
- 37).Obluchinsksya ED, Makarova MN, Pozharitskaya ON and Shikov AN: Effects of Ultrasound Treatment on the Chemical Composition and Anticoagulant Properties of Dry Fucus Extract. Pharmaceutical Chemistry Journal, 2015; 49: 183-186 [Google Scholar]
- 38).Pereira CMP, Nunes CFP, Zambotti-Villela L, Streit NM, Dias D, Pinto E, Gomes CB and Colepicolo P: Extraction of sterols in brown macroalgae from Antarctica and their identification by liquid chromatography coupled with tandem mass spectrometry. Journal of Applied Phycology, 2017; 29: 751-757 [Google Scholar]
- 39).Abdul QA, Choi RJ, Jung HA and Choi JS: Health benefit of fucosterol from marine algae: a review. J Sci Food Agric, 2016; 96: 1856-1866 [DOI] [PubMed] [Google Scholar]
- 40).Sachindra NM, Sato E, Maeda H, Hosokawa M, Niwano Y, Kohno M and Miyashita K: Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Agric Food Chem, 2007; 55: 8516-8522 [DOI] [PubMed] [Google Scholar]
- 41).MacGregor GA, Smith SJ, Markandu ND, Banks RA and Sagnella GA: Moderate potassium supplementation in essential hypertension. Lancet, 1982; 2: 567-570 [DOI] [PubMed] [Google Scholar]
- 42).McCarron DA and Morris CD: Blood pressure response to oral calcium in persons with mild to moderate hypertension. A randomized, double-blind, placebo-controlled, crossover trial. Ann Intern Med, 1985; 103: 825-831 [DOI] [PubMed] [Google Scholar]
- 43).Fabregas J and Herrero C: Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J Ind Microbiol, 1990; 5: 259-263 [Google Scholar]
- 44).Hani N and Ching C: Nutritional composition of edible seaweed Gracilaria changgi. Food Chem, 2000; 68: 69-76 [Google Scholar]


