Abstract
We sought to evaluate the effetc of metformin on body mass index (BMI) and metabolic parameters in HIV-positive patients. We performed a comprehensive search through five major indexing databases, using keywords (“metformin” OR “dimethylguanylguanidine” OR “biguanide” OR “Glucophage”) AND (“HIV” OR “human immunodeficiency virus” OR “AIDS” OR “Acquired immunodeficiency syndrome”), and all possible combinations until January 15, 2021. We measured standardized mean differences (SMD) and 95% confidence intervals (CI) for each outcome. We finally included 12 RCTs (577 participants, 274 in the metformin group and 303 in the comparators). Metformin did not significantly change BMI index compared to various comparators. Metformin generally improve LDL levels (SMD = 0.29, 95% CI: − 1.00 1.57, P = 0.01), HDL levels (SMD = − 0.15, 95% CI: − 0.72 0.41, P = 0.001), triglycerides values (SMD = 0.46, 95% CI: − 0.36 1.27, P < 0.00001), fasting glucose (SMD = − 0.82, 95% CI: − 1.80 0.15, P < 0.00001), insulin 120 min (SMD = − 0.82, 95% CI: − 1.59–0.04, P = 0.02), and glucose 120 min (SMD = − 1.24, 95% CI: − 2.57 0.10, P < 0.0001), but worsened total cholesterol values (SMD = 1.24, 95% CI: − 0.98 3.46, P = 0.0001). Metformin is safe for weight loss in obese people; however, this drug may not be suitable for everyone, especially those who are not overweight. Nevertheless the body of evidences may suggest that metformin had promising impacts on metabolic parameters in patients with both HIV, it is still unknown that such surrogate changes will translate to long-standing clinical advantages.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-021-00869-1.
Keywords: HIV-positive patients, Metabolic syndrome, Meta-analysis, Anthropometric indices, Metabolic parameters, Insulin sensitizing-agents
Introduction
Acquired immunodeficiency syndrome (AIDS) is an infectious chronic condiction that refers to a set of symptoms caused by the human immunodeficiency virus (HIV), in which the human immune system is severely becomes weaker [1]. HIV directly attacks and abolishes the immune system and makes the body vulnerable to many forms of infections and cancer [2]. AIDS is a global pandemic and has been reported in nearly every country, and it was estimated that 38 million people across the globe living with the disease [3]. Clinical manifestations of HIV infection are of wide spectrum depending on the stage of infection [4]. HIV infection further reduces food intake and leads to weight loss due to sore throat, anorexia nervosa, depression, and disturbances of consciousness, as well as adverse events of gastrointestinal medications and poverty [5].
Patients with AIDS are recommended to use a balanced diet containing four main food groups to maintain body weight and prevent body weakness [6]. Nutritional disorders and weight loss due to decreased in dietary intake are one of the most common complications in people with HIV is a [6]. Weight loss, depletion of cell stores, reduction of prephiral and central fats, hypoalbuminemia, and loss of iron stores have been reported frequently in patients with HIV [7, 8]. So far, numerous reearches about the effect of nutritional interventions on anthropometric indices of patients with HIV have been conducted glolbally [9–13]. Thus, several studies highly recommended measuring anthropometric condition in patients with HIV, which may help a lot in follow the disease process and treatment. Besides, studies show that some medications in patients with HIV may cause changes in body fat composition [14, 15]. One suggested mechanism is that endocrine function may directly affect by viral proteins, through the production of systemic and local cytokines and the inflammatory response through glandular involvement with HIV infection [16].
Preceding study has shown that weight gain is asscoiated with starting antiretroviral therapy (ART) among treatment-naive patients with HIV [17]. Recent studies showed that male patients with HIV who are on antiretroviral medications are four times more likely at risk of developing diabetes than healthy individuals [18, 19]. Metformin is safe blood glucose lowering agent that use for weight loss in obese people; however, this drug may not be suitable for people who are not severely overweight [20]. Metformin only reduces calorie intake and has a beneficial effect on body weight; however, little is known about the effects of this drug on body fat percentage [21]. Metformin is the drug of choice for improving insulin sensitivity and lowering blood sugar levels by reducing hepatic gluconeogenesis through the LKB1/AMPK mechanism and intestinal glucose uptake [22]. This Insulin sensitizier lowers blood glucose by reducing hepatic glucose production as well as improving insulin function in muscle and adipose tissue [22]. This drug also reduces glucose uptake from the gastrointestinal lumen, increases glycolysis, and decreases gluconeogenesis, as well as reduces plasma triglyceride levels by 15 to 20% [23]. Other uses of metformin include the treatment of polycystic ovary syndrome (PCOS) and HIV-induced lipodystrophy syndrome [24].
Considering the effective solution in the treatment and prevention of changes in anthropometric indices of patients with HIV, and the contradictory findings in various studies, we sought to conduct a systematic review and meta-analysis study to assess the efficacy and safety of metformin on body mass index (BMI) and metabolic parameters in HIV-positive patients.
Methods
We used standard meta-analysis methods in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [25].
Search strategy
A comprehensive search in Medline, Embase, ISI web of science, Scopus, Cochrane central and CINAHL databases, using keywords (“metformin” OR “dimethylguanylguanidine” OR “biguanide” OR “Glucophage”) AND (“HIV” OR “human immunodeficiency virus” OR “AIDS” OR “Acquired immunodeficiency syndrome”), and all possible combinations until January 15, 2021. Grey literatures were also searched from Ahvaz Jundishapur University of Medical Sciences (AJUMS) and Iranian Ministry of Health research repository online library. Besides, a hand searching through the reference lists of included articles was performed.
Inclusion criteria
Studies were included if they met the following inclusion criteria, including randomized clinical/control trials (RCTs), studies included patients with HIV and no history of diabetes with metabolic syndrome, patients in the intervention group had been treated by metformin, patient received no medication, life style modification, placebo or drugs other than metformin were used as the comparators, and (5) studies reported weight or BMI change, as well as anthropometric changes.
Exclusion criteria
Studies without the outcome of interest, studies with only abstracts, case studies, qualitative studies and citations without full-text were excluded.
Data extraction
Data extraction was performed individually by FR and MZ. All information, including number of patients, type of intervention, type of comparators, outcomes, duration of follow-up, the completeness of follow-up, and evidence level, were retrived. The level of evidence was determined using GRADE [26]. Any disagreements were resolved by a third researcher (GM).
Quality assessment
Quality assessment was performed individually by FR and MZ.The methodological quality assessment was performed considering factors that may increase the risk of bias. We used RoB 2 (A revised cochrane risk-of-bias tool for randomized trials), including random sequence generation (selection bias), allocation concellement (selection bias), blinding participants or personnel (performance bias), blinding outcome assessors (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias [27].
Data analysis
We used RevMan 5.3 software for data analysis, as well as used standardized mean diefference as effect size. Heterogenity was discrubied as the total variability (I2). The significant heterogeneity was tested by χ2 test. Low heterogentiy was indicated as I2 < 40%. In case the heterogeneity was significant (I2 > 75%), the source of heterogentity was detected before meta-analysis. We conducted sub-group analyses based on various comaprators. To assess publication bias we used funnel plots.
Results
Characteristics of included studies
A total of 779 publications were retrieved, of which 542 selected after title and abstract screening. After excluding 531 literatures, we finially included 12 publications (Fig. 1).
Fig. 1.
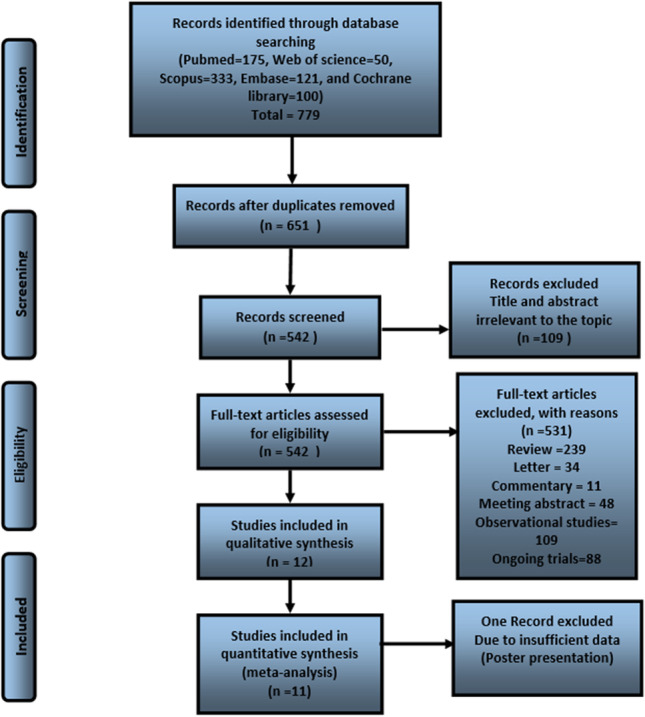
The follow diagram of study selection
We included 12 RCTs (577 participants, 274 in the metformin group and 303 in the comparators) [28–39]. Six trials compared metformin with placebo [29–32, 34, 36], four trials compared metformin with rosiglitazone [32, 33, 38, 39], three trilas compared metformin with life style modification (LSM) [28, 30, 35], and two RCTs compared metformin with no treatment [33, 37]. The treatment duration ranged from 14 to 60 days. Participants were aged 18–65 years old, following the course of treatment was 2–12 months (Table 1).
Table 1.
Baseline characteristics of randomized controlled trials for HIV-positive patients with metabolic diseases
| References | Country | Number | Mean age (years) | Gender male) | Mean CD4 (cells/mm3) | Current NRTI* use (%) | Current PI** use (%) | Intervention (dose) | Comparison (dose) | Duration of follow up | Completeness of follow up (%) | Level of evidencea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metformin versus Placebo, LSM, or no treatment | ||||||||||||
| Jiriyasin [27] | Thailand | 54 | 49.6 | 68 | 570 | 73 | 85 | Metformin (1500 mg daily) | LSM | 6 months | 100 | Very low |
| Srinivasa [28] | USA | 50 | 47 | 76 | 576 | 86 | 56 | Metformin (850 mg bid) | Placebo | 12 months | 100 | High |
| Fitch [29] | USA | 50 | 49 | 70 | 583 | 64 | 100 | Metformin (500 mg bid) | LSM | 6 months | 68 | High |
| Fitch [29] | USA | 50 | 47 | 72 | 691 | 55 | 82 | Metformin (500 mg bid) | Placebo | 6 months | 65 | High |
| Kohli [30] | USA | 48 | 42 | 56 | 394 | 67 | 73 | Metformin (1500 mg daily) | Placebo | 24 weeks | 81 | High |
| Mulligan [31] | USA | 53 | 44 | 64 | 609 | 95 | 61 | Metformin (1000 mg bid) | Placebo | 16 weeks | 91 | High |
| Silic [32] | Slovenia | 60 | 42.8 | 100 | 340 | 100 | 100 | Metformin (500 mg bid) | No Treatment | 48 weeks | 100 | Moderate |
| Martinez [33] | Spain | 71 | 41.5 | 70 | NR | 100 | 100 | Metformin (850 mg bid) | Placebo | 12 months | 96 | High |
| Driscoll [34] | USA | 25 | 42 | 80 | 429 | 92 | 67 | Metformin (850 mg bid) | LSM | 12 months | 100 | Moderate |
| Hadigan [35] | USA | 26 | 45 | 76 | 508 | 100 | 88 | Metformin (500 g bid) | Placebo | 3 months | 96 | High |
| St-Marc [36] | France | 29 | 40 | 74 | 377 | NR | 100 | Metformin (850 mg tid) | No Treatment | 2 months | 93i | low |
| Metformin vs. rosiglitazone | ||||||||||||
| van Wijk [37] | The Netherlands | 37 | 47 | 78 | 697 | 39 | 68 | Metformin (1000 mg bid) | Rosiglitazone (8 mg daily) | 4 months | 100 | Moderate |
| Mulligan [38]*** | USA | 53 | 45 | 66 | 562 | 98 | 71 | Metformin (1000 mg bid) | Rosiglitazone (4 mg daily) | 16 weeks | 89 | High |
| Coll [38] | The Netherlands | 39 | 48 | 100 | 637 | 100 | 64 | Metformin (1000 mg bid) | Rosiglitazone (8 mg daily) | 6 months | 95 | Very low |
| Silic [32] | Slovenia | 60 | 41.8 | 100 | 372 | 100 | 100 | Metformin (500 mg bid) | Rosiglitazone (4 mg daily) | 48 weeks | 90 | Moderate |
aGRADE: an emerging consensus on rating quality of evidence and strength of recommendations: High quality— Further research is very unlikely to change our confidence in the estimate of effect; Moderate quality— Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality— Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality— Any estimate of effect is very uncertain
*NRTI = nucleoside reverse transcriptase inhibitor, **PI = protease inhibitor; ***Mulligan study had double placebos; NR = not reported, mg = milligrams, bid = twice a day, LSM, Life style modification, DM, diabetes
Risk of bias in included trials
All 12 RCTs reported the method to randomize participants and was considered as low risk of bias due to using either dynamic block randomization or random number table. Only seven studies were considered as low-risk of bias by reporting adequate allocation concealment [30–34, 36, 38]. Only three of the studies reported blinding participants or outcome assessors, which were considered low risk of bias [31, 32, 34]. A total seven RCTs reported none of participants and outcome assessors were blinded, which were considered as high risk of bias [28–30, 33, 35, 36, 38], and two studies were not mentioned whether participants and outcome assessors were blinded that were considered as unclear risk of bias [37, 39]. The informations of the assessment of risk of bias of selected RCTs is shown in Fig. 2.
Fig. 2.

Risk of bias of randomized clinical trials of metformin for patient with HIV and metabolic syndrome
Meta-analyses
Body mass index (BMI)
Metformin did not significantly change BMI index (SMD = 0.15, 95% CI: − 0.25—0.55, P = 0.02) compared to placebo (Fig. 3). Metformin has no significant impact on BMI index compared to no treatment (SMD = − 0.22, 95% CI: − 0.64—0.21, P = 0.56) (Fig. 3). As shown in figure, there were no statistically significant differences between the metformin and rosiglitazone in terms of BMI values (SMD = 0.96, 95% CI: − 0.32–2.25, P < 0.001) (Fig. 3). There were no statistically significant differences between meformine and LSM with regard to BMI value (SMD = − 0.54, 95% CI: − 1.09–0.02, P = 0.38) (Fig. 3).
Fig. 3.
Effects of metformin versus placebo (Placebo /LSM/no treatment/Rosiglitazone) on body mass index in patients with HIV affected suffer from metabolic syndrome
Low-density lipoprotein (LDL)
Our results indicated that there were no statistically significant differences between metformin and placebo groups in terms of LDL levels (SMD = − 0.10, 95% CI: − 0.43 0.24, P = 0.54). However, we found a significant difference between the metformin and rosiglitazone groups in terms of LDL levels (SMD = 0.29, 95% CI: − 1.00 1.57, P = 0.01) (Fig. 4).
Fig. 4.
Effects of Metformin versus Placebo and Rosiglitazone on LDL in patients with HIV
High-density lipoprotein (HDL)
There were a statistically significant differences between the metformin and placebo groups in terms of HDL levels (SMD = − 0.15, 95% CI: − 0.72 0.41, P = 0.001). However, we found no statistically differences between the metformin and rosiglitazone groups in terms of HDL levels (SMD = 0.49, 95% CI: − 0.42 1.41, P = 0.06) (Fig. 5).
Fig. 5.
Effects of Metformin versus Placebo and Rosiglitazone on HDL in patients with HIV
Triglycerides (TG)
Five studies compared the metformin and placebo effects on triglycerides parameter in 262 subjects, which showed a statistically significant differences between the two groups in term of these values (SMD = 0.46, 95% CI: − 0.36 1.27, P < 0.00001) (Figure S1).
Fasting insulin (FI)
Only two unique trials compared Metformin and placebo effects on "fasting insulin" parameter in 67 subjects, and showed no statistical significant differences between the two groups with regard to "fasting insulin" value (SMD = 0.22, 95% CI: − 0.54 0.99, P = 0.15) (Figure S2).
Fasting glucose (FG)
Five studies compared the effects of metformin and placebo on fasting glucose (FG, 244 patients), which demonstrated that FG values in metformin group were significantly lower than the control group (SMD = -0.82, 95% CI: -1.80 0.15, P < 0.00001) (Figure S3).
Total cholesterol
Only two studies compared metformin and rosiglitazone in terms of total cholesterol (69 patients), and showed that total cholesterol values in metformin group were significantly higher than the rosiglitazone group (SMD = 1.24, 95% CI: − 0.98 3.46, P = 0.0001) (Figure S4).
Insulin 120 minute
Three studies compared the metformin and placebo effects for insulin 120 min, and showed that mean values of insulin 120 min were significantly reduced in metformin group than the placebo group (SMD = − 0.82, 95% CI: − 1.59–0.04, P = 0.02) (Figure S5).
Glucose 120 minute
Three studies compared metformin in terms of glucose 120 min parameter (165 patients), and showed that, glucose 120 min values were significantly higher in comaprators than the metformin group (SMD = − 1.24, 95% CI: − 2.57 0.10, P < 0.0001) (Figure S5).
Adverse events
Our analyses show that metformin was well tolerated. All studies reported 9 different adverse events and included in meta-analysis. The result showed that no significant difference in many of adverse events between metformin and the comparators. However, adverse events such as diarrhea (OR = 0.23 95%CI (0.08, 0.68)) and nausea (OR = 23.38 95%CI (2.25, 242.64)) were significantly higher in metformin group comparing to both rosiglitazone and placebo groups (Table 2). But, other adverse events, including headache, muscle strain, increased lactate, and elevated liver function tests were not significantly higher in metformin group.
Table 2.
All studies reported 9 different adverse events and included in meta-analysis
| Adverse events | Group | n | OR (95% CI) | I2 (%) | Chi-square (P-value) | P-value* |
|---|---|---|---|---|---|---|
| Headache | Metformin vs. Rosiglitazone | 1 | 10.36 [0.53, 201.45] | – | – | 0.12 |
| Muscle Strain | Metformin vs. LSM | 1 | 5.00 [0.22, 114.22] | – | – | 0.31 |
| Increased lactate | Metformin vs. Placebo | 1 | 4.83 [0.45, 89.14] | – | – | 0.58 |
| Elevated LFT | Metformin vs. Placebo | 1 | 3.12 [0.37, 94.14] | – | – | 0.28 |
| Gasterointestinal | Metformin vs. Rosiglitazone | 2 | 0.23 [0.08, 0.68] | 0 | 0.93 (P = 0.33) | 0.008 |
| Metformin vs. Placebo | 3 | 23.38 [2.25, 242.64] | 42 | 3.46 (P = 0.18) | 0.008 | |
| Total | 5 | 2.53 [0.15, 43.99] | 85 | 26.08 (P < 0.0001) | 0.52 |
Publication bias
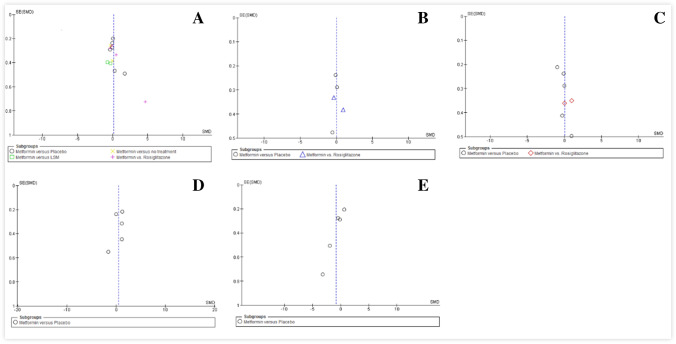
The funnel plots give us a visual observation of the publication bias of five comparisons, including SMD of BMI (Fig. 6A), SMD of LDL (Fig. 6B), SMD of HDL (Fig. 6C), SMD of TG (Fig. 6D), and SMD of FG (Fig. 6E). Obviously, five symmetrical figures showed that the publication bias isn’t large. Funnel plot was useless for other comparisons, including fasting insulin, total cholesterol, insulin 120 min, and glucose 120 min because of less than 5 studies included.
Fig. 6.
Funnel plot of the effect of metformin on body mass index (BMI) (A), low-density lipoprotein (LDL) (B), high-density lipoprotein (HDL) (C), triglycerides (TG) (D), and fasting glucose (FG) (E) in HIV-Positive patients
Discussion
This systematic review and meta-analysis summarized and assessed 12 RCTs that focused on optimizing the use of metformin in patients with HIV and metabolic syndrome. Overall methodological quality of included studies were of moderate to high, and the effectiveness of the interventions reviewed on different outcomes varied.
Our pooled analyses have suggested no significant effect of metformin on body weight versus placebo. In this context, Golay in a meta-analysis summarized the effects of metformin on body weight in both diabetes and obesity with special focous on longer treatment duration (more than 6 months), and reported no significant effect of metformin versus placebo [40]. In contrast, Desilets et al., in a systematic review assessed the efficacy and safety of metformin on body weight in overweight or obese adults without type 2 diabetes, and suggested that the weight loss associated with metformin is promising in these poepole [41]. The difference between last meta-analysis with our finndings and Golay, may be due to this fact that they included RCTs limited by small sample size and weak design [42].
In line with recent literature on the same topic our pooled analyses also showed that metformin may also impose a affirmative effect on metabolic parameters such as LDL, HDl, fasting glucose levels, total cholesterol and triglycerides [41, 43, 44]. This is may be due to favorable effects of metformin glucose homeostasis and metabolism in obese insulin-resistant people,especially children [45]. Other possible reasons are that metformin has a potential mechanism by which affect lipid metabolism [46],as well as metformin is able to decrease intestine-derived TG-rich lipoproteins measured in plasma, especially in patient with type 2 diabetes [47]. In contrast, Solymár et al. perform a meta-analysis of RCTs investigating the body weight, total cholesterol and LDL levels changes upon metformin treatment in the elderly48]. The difference in findings may be due to the age category of included patients [49].
Our analyses show that metformin was well tolerated and induce no serious adverse events; thus, metformin is not just an antihyperglycaemic drug but also has protective effects. In this context, Feng and Yang performed a susyematic review and meta-analysis on metformin use in the management of gestational diabetes mellitus (GDM), and sugge that this drug is an effective and safe alternative to insulin in such patients [50]. Besides, Schlender et al. counducted a meta-anlysis on the safety and efficacy of metformin in older adults with type 2 diabetes, and metformin has a better effcicay and surperior safety than other treatments [51].
HIV associated lipodystrophy syndrome is associated with central fat accumulation and peripheral fat loss which cannot accurately assessed by BMI. While the most consistently reported morphology-related variables, including BMI, waist-to-hip ratio (WHR) and VAT–capture elements of central adiposity, they do not specifically address peripheral wasting, which may be particularly important in the decision to use metformin in patients with lipoatrophy. In this context, sevral meta-analyses assessed the efficacy of pharmacotherapyin HIV-associated lipodystrophy syndrome [52–56]. Sheth and Larson in a meta-anlysis assessed the safety and efficacy of Insulin sensitizing-agents (rosiglitazone, pioglitazone or metformin) for the treatment of HIV associated lipodystrophy syndrome [55]. They reported that metformin significantly reduced WHR and BMI in patients with HIV-associated lipodystrophy syndrome; thus, metformin is the only Insulin sensitizing-agent that has beneficial effects on all morphology-related variables.
According to research, metformin helps some people lose weight; however, it is not clear exactly why metformin causes weight loss. Waist and hip circumference show how fat is distributed in the body and are influenced by lifestyle factors (smoking, alcohol consumption and physical activity), and today the most common indicator used to identify how fat is distributed in the body is the WHR. Most studies have suggested WHR as a more accurate non-invasive indicator than BMI to identify abdominal obesity and risk factors for cardiovascular disease [57, 58], especially in case of using Insulin sensitizing-agents such as metformin [59, 60].
The exact molecular mechanism of action of metformin in such conditions has not been elucidated to date, but its possible mechanisms, include first metformin was reported to alter the composition of the gut microbiota which may improve inflammation [61]. Various pathogens are colonized in the intestinal mucosa, leading to a strong inflammatory response, followed by the transmission of intestinal bacteria. Infection is one of the most common diseases caused by microbial imbalance, as well as treatment of infectious diseases affects the composition of the intestinal microbiota. Changes in the microbiota are reported in diseases such as HIV and hepatitis B [62]. The effect of microbiota in HIV infection may not be direct but they are indirectly effective through the microbiota-gut-brain axis [63]. Second this insulin sensitizer also could be a promising addition to ART in HIV patients, because of its ability to improve immune functions. In addition, metformin induces immune system activation and apoptosis and reduces growth factor signaling. The immunomodulatory effect and aniviral activity of metformin can also be due to a reduction in the production of proinflammatory cytokines using macrophages and causes the formation of neutrophil extracellular traps (NETs), as well as inhibiting the cytokine production of pathogenic Th1 and Th17 cells [64].
Limitations
This meta-analysis was limited mainly by only screening peer‐reviewed published English studies and did not access unpublished data, which may leads to language and publication biases. The metodlogical quality of specific studies also varied broadly. Anopther limitation is that the heterogeneity of treatments, placebos, and ‘no treatments’ used in the meta-analysis may call into question the justification for pooling results. Thus, using placebos, or ‘no treatments’ have been shown to have different effects. Moreover, such unblinded trials favored the intervention group more strongly than comparable blinded trials for the outcome evaluated in these trials, thereby possibly inflating pooling estimates and could have been susceptible to performance bias. The overall moderate quality and existing relatively poor methodologies could lead to biases that can results in over‐ or underestimates of the effectiveness of intervention of interest.
Conclusion
Continuing attempts to recognize and compare possible interventions for patients with HIV would be beneficial. Metformin is safe for weight loss in obese people; however, this drug may not be suitable for everyone, especially those who are not overweight. One theory is that metformin may reduce appetite or alter how fat is stored and used. Besides, regarding the comparison of metformin with rosiglitazone, there is a consensus and satisfactory evidences suggesting that rosiglitazone should not be given to patients with HIV-associated metabolic syndrome [55]. Finally, though the body of evidences may suggest that metformin had promising effects on metabolic parameters in patients with both HIV and metabolic syndromes, it is still unknown that such surrogate changes will translate to long-standing clinical advantages.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Varadé J, Magadán S, González-Fernández Á. Human immunology and immunotherapy: main achievements and challenges. Cellular Mol Immunol. 2021:18; 805–828. [DOI] [PMC free article] [PubMed]
- 2.Boasso A, Shearer GM, Chougnet C. Immune dysregulation in human immunodeficiency virus infection: know it, fix it, prevent it? J Intern Med. 2009;265(1):78–96. doi: 10.1111/j.1365-2796.2008.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Challacombe SJ. Global inequalities in HIV infection. Oral Dis. 2020;26(S1):16–21. doi: 10.1111/odi.13386. [DOI] [PubMed] [Google Scholar]
- 4.Mindel A, Tenant-Flowers M. ABC of AIDS: Natural history and management of early HIV infection. BMJ (Clinical Research Ed) 2001;322(7297):1290–1293. doi: 10.1136/bmj.322.7297.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duggal S, Chugh TD, Duggal AK. HIV and malnutrition: effects on immune system. Clin Dev Immunol. 2012;2012:784740. doi: 10.1155/2012/784740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendricks K, Gorbach S. Nutrition issues in chronic drug users living with HIV infection. Addict Sci Clin Pract. 2009;5(1):16–23. doi: 10.1151/ascp095116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira da Silva R, Santos Borges de Araújo IL, Coelho Cabral P, Pessoa de Araújo Burgos MG. Effects of oral nutritional support in hospitalized patients with AIDS. Nutr Hosp. 2013;28(2):400–404. doi: 10.3305/nh.2013.28.2.6276. [DOI] [PubMed] [Google Scholar]
- 8.Forrester J, Spiegelman D, Woods M, Knox T, Fauntleroy J, Gorbach S. Weight and body composition in a cohort of HIV-positive men and women. Public Health Nutr. 2001;4:743–747. doi: 10.1079/phn200099. [DOI] [PubMed] [Google Scholar]
- 9.Wig N, Bhatt SP, Sakhuja A, Srivastava S, Agarwal S. Dietary adequacy in Asian Indians with HIV. AIDS Care. 2008;20(3):370–375. doi: 10.1080/09540120701583753. [DOI] [PubMed] [Google Scholar]
- 10.Uthman OA. Prevalence and pattern of HIV-related malnutrition among women in sub-Saharan Africa: a meta-analysis of demographic health surveys. BMC Public Health. 2008;8:226. doi: 10.1186/1471-2458-8-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger MR, Fields-Gardner C, Wagle A, Hollenbeck CB. Prevalence of malnutrition in human immunodeficiency virus/acquired immunodeficiency syndrome orphans in the Nyanza province of Kenya: a comparison of conventional indexes with a composite index of anthropometric failure. J Am Diet Assoc. 2008;108(6):1014–1017. doi: 10.1016/j.jada.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Ludy MJ, Hendricks K, Houser R, et al. Body composition in adults infected with human immunodeficiency virus in Khon Kaen, Thailand. Am J Trop Med Hyg. 2005;73(4):815–819. [PubMed] [Google Scholar]
- 13.Forrester JE, Tucker KL, Gorbach SL. Dietary intake and body mass index in HIV-positive and HIV-negative drug abusers of Hispanic ethnicity. Public Health Nutr. 2004;7(7):863–870. doi: 10.1079/phn2004617. [DOI] [PubMed] [Google Scholar]
- 14.Koethe JR, Lagathu C, Lake JE, et al. HIV and antiretroviral therapy-related fat alterations. Nat Rev Dis Primers. 2020;6(1):48. doi: 10.1038/s41572-020-0181-1. [DOI] [PubMed] [Google Scholar]
- 15.dos Santos AP, Navarro AM, Schwingel A, et al. Lipodystrophy diagnosis in people living with HIV/AIDS: prediction and validation of sex-specific anthropometric models. BMC Public Health. 2018;18(1):806. doi: 10.1186/s12889-018-5707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirza FS, Luthra P, Chirch L. Endocrinological aspects of HIV infection. J Endocrinol Invest. 2018;41(8):881–899. doi: 10.1007/s40618-017-0812-x. [DOI] [PubMed] [Google Scholar]
- 17.Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4):e25484. doi: 10.1002/jia2.25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan AD, Goff LM, Peters BS. Type 2 diabetes prevalence and its risk factors in HIV: a cross-sectional study. PLoS ONE. 2018;13(3):e0194199. doi: 10.1371/journal.pone.0194199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl 1):S27–36 [DOI] [PMC free article] [PubMed]
- 20.Abdelgadir E, Ali R, Rashid F, Bashier A. Effect of metformin on different non-diabetes related conditions, a special focus on malignant conditions: review of literature. J Clin Med Res. 2017;9(5):388–395. doi: 10.14740/jocmr2922e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tokubuchi I, Tajiri Y, Iwata S, et al. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS ONE. 2017;12(2):e293. doi: 10.1371/journal.pone.0171293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horakova O, Kroupova P, Bardova K, et al. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci Rep. 2019;9(1):6156–6156. doi: 10.1038/s41598-019-42531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu T, Xie C, Wu H, Jones K, Horowitz M, Rayner C. Metformin reduces the rate of small intestinal glucose absorption in type 2 diabetes: metformin and glucose absorption. Diab Obes Metab. 2016;19 [DOI] [PubMed]
- 24.Mahmood K, Naeem M, Rahimnajjad N. Metformin: The hidden chronicles of a magic drug. Eur J Int Med. 2012;24 [DOI] [PubMed]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical Research Ed). 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical Research Ed) 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research Ed). 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 28.Jiriyasin S, Nimitphong H, Sungkanuparph S. 2250. Metformin for Preventing Diabetes Mellitus in HIV-Infected Patients with Prediabetes: A Randomized Controlled Trial. Open Forum Infect Dis. 2018 Nov 26;5(Suppl 1):S665. 10.1093/ofid/ofy210.1903 (eCollection 2018 Nov)
- 29.Srinivasa S, Wong K, Fitch KV, et al. Effects of lifestyle modification and metformin on irisin and FGF21 among HIV-infected subjects with the metabolic syndrome. Clin Endocrinol. 2015;82(5):678–685. doi: 10.1111/cen.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fitch K, Abbara S, Lee H, et al. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV-infected patients with the metabolic syndrome. AIDS (London, England) 2012;26(5):587–597. doi: 10.1097/QAD.0b013e32834f33cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med. 2007;8(7):420–426. doi: 10.1111/j.1468-1293.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 32.Mulligan K, Yang Y, Wininger DA, et al. Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio. AIDS (London, England) 2007;21(1):47–57. doi: 10.1097/QAD.0b013e328011220e. [DOI] [PubMed] [Google Scholar]
- 33.Silic A, Janez A, Tomazic J, et al. Effect of rosiglitazone and metformin on insulin resistance in patients infected with human immunodeficiency virus receiving highly active antiretroviral therapy containing protease inhibitor: randomized prospective controlled clinical trial. Croat Med J. 2007;48(6):791–799. doi: 10.3325/cmj.2007.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez E, Domingo P, Ribera E, et al. Effects of metformin or gemfibrozil on the lipodystrophy of HIV-infected patients receiving protease inhibitors. Antivir Ther. 2003;8(5):403–410. [PubMed] [Google Scholar]
- 35.Driscoll SD, Meininger GE, Ljungquist K, et al. Differential effects of metformin and exercise on muscle adiposity and metabolic indices in human immunodeficiency virus-infected patients. J Clin Endocrinol Metab. 2004;89(5):2171–2178. doi: 10.1210/jc.2003-031858. [DOI] [PubMed] [Google Scholar]
- 36.Hadigan C, Rabe J, Grinspoon S. Sustained benefits of metformin therapy on markers of cardiovascular risk in human immunodeficiency virus-infected patients with fat redistribution and insulin resistance. J Clin Endocrinol Metab. 2002;87(10):4611–4615. doi: 10.1210/jc.2002-020709. [DOI] [PubMed] [Google Scholar]
- 37.Saint-Marc T, Touraine J-L. Effects of metformin on insulin resistance and central adiposity in patients receiving effective protease inhibitor therapy. AIDS (London, England) 1999;13:1000–1002. doi: 10.1097/00002030-199905280-00023. [DOI] [PubMed] [Google Scholar]
- 38.van Wijk JP, Hoepelman AI, de Koning EJ, Dallinga-Thie G, Rabelink TJ, Cabezas MC. Differential effects of rosiglitazone and metformin on postprandial lipemia in patients with HIV-lipodystrophy. Arterioscler Thromb Vasc Biol. 2011;31(1):228–233. doi: 10.1161/ATVBAHA.110.216192. [DOI] [PubMed] [Google Scholar]
- 39.Coll B, van Wijk JP, Parra S, et al. Effects of rosiglitazone and metformin on postprandial paraoxonase-1 and monocyte chemoattractant protein-1 in human immunodeficiency virus-infected patients with lipodystrophy. Eur J Pharmacol. 2006;544(1–3):104–110. doi: 10.1016/j.ejphar.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Golay A. Metformin and body weight. Int J Obes. 2008;32(1):61–72. doi: 10.1038/sj.ijo.0803695. [DOI] [PubMed] [Google Scholar]
- 41.Desilets AR, Dhakal-Karki S, Dunican KC. Role of metformin for weight management in patients without type 2 diabetes. Ann Pharmacother. 2008;42(6):817–826. doi: 10.1345/aph.1K656. [DOI] [PubMed] [Google Scholar]
- 42.Lin L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS ONE. 2018;13(9):e0204056. doi: 10.1371/journal.pone.0204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pradas I, Rovira-Llopis S, Naudí A, et al. Metformin induces lipid changes on sphingolipid species and oxidized lipids in polycystic ovary syndrome women. Sci Rep. 2019;9(1):16033. doi: 10.1038/s41598-019-52263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lord J, Thomas R, Fox B, Acharya U, Wilkin T. The effect of metformin on fat distribution and the metabolic syndrome in women with polycystic ovary syndrome—a randomised, double-blind, placebo-controlled trial. BJOG Int J Obstetr Gynaecol. 2006;113(7):817–824 [DOI] [PubMed]
- 45.Yanovski JA, Krakoff J, Salaita CG, et al. Effects of metformin on body weight and body composition in obese insulin-resistant children: a randomized clinical trial. Diabetes. 2011;60(2):477–485. doi: 10.2337/db10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Stee MF, de Graaf AA, Groen AK. Actions of metformin and statins on lipid and glucose metabolism and possible benefit of combination therapy. Cardiovasc Diabetol. 2018;17(1):94. doi: 10.1186/s12933-018-0738-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jeppesen J, Zhou MY, Chen YD, Reaven GM. Effect of metformin on postprandial lipemia in patients with fairly to poorly controlled NIDDM. Diabetes Care. 1994;17(10):1093–1099. doi: 10.2337/diacare.17.10.1093. [DOI] [PubMed] [Google Scholar]
- 48.Solymár M, Ivic I, Pótó L, et al. Metformin induces significant reduction of body weight, total cholesterol and LDL levels in the elderly—a meta-analysis. PLoS ONE. 2018;13(11):e0207947. doi: 10.1371/journal.pone.0207947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glossmann HH, Lutz OMD. Metformin and aging: a review. Gerontology. 2019;65(6):581–590. doi: 10.1159/000502257. [DOI] [PubMed] [Google Scholar]
- 50.Feng Y, Yang H. Metformin - a potentially effective drug for gestational diabetes mellitus: a systematic review and meta-analysis. J Maternal-Fetal Neonatal Med Off J Eu Assoc Perin Med Feder Asia Oceania Perin Soc Int Soc Perin Obstet. 2017;30(15):1874–1881. doi: 10.1080/14767058.2016.1228061. [DOI] [PubMed] [Google Scholar]
- 51.Schlender L, Martinez YV, Adeniji C, et al. Efficacy and safety of metformin in the management of type 2 diabetes mellitus in older adults: a systematic review for the development of recommendations to reduce potentially inappropriate prescribing. BMC Geriatr. 2017;17(Suppl 1):227. doi: 10.1186/s12877-017-0574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batterham MJ. Investigating heterogeneity in studies of resting energy expenditure in persons with HIV/AIDS: a meta-analysis. Am J Clin Nutr. 2005;81(3):702–713. doi: 10.1093/ajcn/81.3.702. [DOI] [PubMed] [Google Scholar]
- 53.Clumeck N, Hill A, Moecklinghoff C. Effects of switching to protease inhibitor monotherapy on nucleoside analogue-related adverse events. AIDS Rev. 2014;16(4):236–245. [PubMed] [Google Scholar]
- 54.Falutz J, Mamputu JC, Potvin D, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010;95(9):4291–4304. doi: 10.1210/jc.2010-0490. [DOI] [PubMed] [Google Scholar]
- 55.Sheth SH, Larson RJ. The efficacy and safety of insulin-sensitizing drugs in HIV-associated lipodystrophy syndrome: a meta-analysis of randomized trials. BMC Infect Dis. 2010;10:183. doi: 10.1186/1471-2334-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veloso S, Olona M, Peraire J, et al. No relationship between TNF-α genetic variants and combination antiretroviral therapy-related lipodystrophy syndrome in HIV type 1-infected patients: a case-control study and a meta-analysis. AIDS Res Hum Retroviruses. 2011;27(2):143–152. doi: 10.1089/aid.2009.0312. [DOI] [PubMed] [Google Scholar]
- 57.Ravensbergen HRJC, Lear SA, Claydon VE. Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury. J Neurotrauma. 2014;31(3):292–300. doi: 10.1089/neu.2013.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmad N, Adam SIM, Nawi AM, Hassan MR, Ghazi HF. Abdominal obesity indicators: waist circumference or waist-to-hip ratio in Malaysian adults population. Int J Prev Med. 2016;7:82–82. doi: 10.4103/2008-7802.183654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiao Q, Nyamdorj R. Is the association of type II diabetes with waist circumference or waist-to-hip ratio stronger than that with body mass index? Eur J Clin Nutr. 2010;64(1):30–34. doi: 10.1038/ejcn.2009.93. [DOI] [PubMed] [Google Scholar]
- 60.Papaetis GS, Papakyriakou P, Panagiotou TN. Central obesity, type 2 diabetes and insulin: exploring a pathway full of thorns. Arch Med Sci. 2015;11(3):463–482. doi: 10.5114/aoms.2015.52350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouyang J, Isnard S, Lin J, et al. Metformin effect on gut microbiota: insights for HIV-related inflammation. AIDS Res Ther. 2020;17(1):10. doi: 10.1186/s12981-020-00267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mathew S, Smatti MK, Al Ansari K, Nasrallah GK, Al Thani AA, Yassine HM. Mixed viral-bacterial infections and their effects on gut microbiota and clinical illnesses in children. Sci Rep. 2019;9(1):865–865. doi: 10.1038/s41598-018-37162-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenhill C. Effects of metformin mediated by gut microbiota. Nat Rev Endocrinol. 2018;15(1):2. doi: 10.1038/s41574-018-0133-y. [DOI] [PubMed] [Google Scholar]
- 64.Chen X, Guo H, Qiu L, Zhang C, Deng Q, Leng Q. Immunomodulatory and antiviral activity of metformin and its potential implications in treating coronavirus disease 2019 and lung injury. Front Immunol. 2020;11:2056. doi: 10.3389/fimmu.2020.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.