Abstract
Introduction
The effect of the natural sources of fructose such as high fructose fruits and honey on the risk of fatty liver is still challenging. This study aimed to compare the effect of fructose, high fructose fruits, and honey on the metabolic factors and non-alcoholic fatty liver disease (NAFLD).
Methods
Forty-four rats were divided into four groups including normal diet group, high fructose group (HF), high fructose fruits group (HFF), and honey group (HO). After 120 days of intervention, the levels of insulin resistance, hepatic enzyme, and lipid profile were measured. Also, the expression levels of the acetyl-coA carboxylase (ACC), sterol regulatory element-binding protein 1c (SREBP-1c), Interleukin 6 (IL-6), and transforming growth factor-beta (TGF-β) genes were assessed. In addition, a histopathologic assessment was performed on liver tissues.
Results
Insulin resistance (IR) increased significantly in the HF, HFF, and HO groups (All P < 0.05). The levels of liver enzymes was significantly increased only in the group receiving the HF regimen (P < 0.01). A significant decrease in total cholesterol and HDL-C (high density lipoprotein cholesterol) levels was found in HO group compared to the control group (P < 0.05). The expression levels of ACC and SREBP-1c genes in HF, HFF, and HO groups were significantly higher than the control group (All P < 0.05). The HF group had a greater increase in the level of gene expression of IL-6 and TGF-β (All P < 0.05). Histopathological assessment did not find any changes in fatty liver formation and inflammatory damage.
Conclusion
Consumption of fructose-rich honey and fruits improved the status of inflammatory markers and liver enzymes compared with the industrial fructose-rich products.
Keywords: Fructose, Non-alcoholic fatty liver, Inflammatory markers
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the result of lipid accumulation in the liver [1] in which, more than 5 to 10 percent of the liver’s weight is from visible intracellular triglycerides [2]. The prevalence of fatty liver is estimated to be between 20 to 30% worldwide [3]. Of these, about 20% of the patients progress to the disease in the form of non-alcoholic steatohepatitis (NASH) and may even end up in end-stage liver disease (ESLD) [4]. In Iran, it is estimated that 7% of children and 35% of adults suffer from NAFLD [5]. NAFLD is more common in men than in women and its prevalence is higher in premenopausal women [6].
Unhealthy diet, obesity, overweight, and type 2 diabetes are considered as the underlying causes of NAFLD development and progression [7–10]. Fructose and fructose-rich sweeteners are reported to be important factors in the development of non-alcoholic fatty liver since an increase in the prevalence of NAFLD was associated with higher fructose consumptions [11, 12]. Fructose is a highly lipogenic sugar as a precursor of fatty acid production in the cytosol through the phosphofructokinase pathway [13]. Moreover, fructose interferes with insulin signaling, which can lead to insulin resistance [14]. Fructose promotes fat accumulation in the liver not only by increasing the fatty acids synthesis but also by decreasing fatty acids oxidation [15]. Fructose also plays an important role in oxidative and inflammatory damage. Studies have shown that administration of fructose in human [16] and animal models [17] increases the active oxygen species, which is a main factor in the development of steatosis towards NASH [18]. Furthermore, despite the liver damage caused by the NAFLD, cardiovascular diseases (CVDs) are now considered the most important cause of death in patients who suffers from NAFLD [19]. Recent studies identified a positive relationship between high fructose intake and developing CVDs [20, 21].
Restriction of food sources of dietary fructose includes high fructose beverages, sweets, and desserts can be crucial strategies against NAFLD development. However, some previous studies claimed that higher consumption of fruits is protective against NAFLD [22] and the effect of fructose-rich fruits on fatty liver indices is not yet clear. Similarly, this question arises for honey which is a significant source of polyphenolic compounds and antioxidant enzymes but also rich in fructose [23]. The possible effects of fructose in fruits or honey compared to fructose in artificial foods have not been well assessed. So, this study aimed to investigate the effect of high fructose fruits, honey, and pure fructose on the metabolic factors and NAFLD. The purpose of this study was to examine that whether other mediators in fruits and honey including vitamins, minerals, fiber and phenolic compounds could change the health consequences of intake of high fructose foods.
Material and methods
A total of 44 male Sprague Dawley rats aged 10–12 weeks and weighing approximately 200–300 g were transferred to the experimental laboratory and kept in cages at 23- 20° C, under 12 h of light/12 h of the dark cycle.
Animals were divided into 4 groups (11 rats in each group) using random assignment as follow: the control group had free access to a standard diet and water, the high fructose group (HF) had free access to water and a diet containing 15% artificial fructose and 15% artificial glucose, the high fructose fruits group (HFF) had free access to water and diet containing a 15% fructose and 15% glucose from a mixture of high fructose fruits including apples, dates and grapes which were mixed with equal proportions, and honey group (HO) which had free access to water and diet containing natural honey with 15.5% fructose and 14% glucose. All diets were prepared in the animal pellet form.
Preparation of pellets
To prepare the HF diet, artificial fructose and glucose were added to the standard diet so that the final HF pellet contains 15 g fructose and 15 g glucose per 100 g. HFF pellet was made up of a combination of three high fructose fruits (apples, grapes and dates) with standard pellet. The fruits were thoroughly washed and dried. The non-edible parts of the fruit were separated and then were mixed in a blender with an equal proportion. The fructose and glucose content of each fruit was derived from the standard values published by the United States Department of Agriculture (USDA) [24]. The gala apple contains 6.1 g of fructose and 3.0 g of glucose per 100 g, white and red grapes contain 8.1 g of fructose and 7.2 g of glucose per 100 g, and the Medjool dates contain 32 g of fructose and 33.6 g of glucose per 100 g. Finally, the mixture of fruits was blended with the standard pellet powder, and after drying, its moisture content dropped to 12%. At this stage, every 100 g of the final product contained 15.4 g of fructose and 14 g of glucose. To prepare the honey group diet, natural honey was added to standard pellets powder so that the new pellets had 15 g of fructose and 13.5 g of glucose per 100 g.
Biochemical, pathological, and genetics assays
Animal weights were measured at baseline and monthly throughout the study. Moreover, a digital scale with 0.1 kg accuracy was used to measure the weight of rats and consumed foods on a weekly basis. The duration of the intervention was 120 days, which was determined based on previous studies [25, 26]. At the end of the intervention, the rats were kept fasted for 12 h followed by ketamine injection with the dose of 45–60 mg/kg bw and diazepam 2.5 mg/kg bw for anesthetic induction. Blood samples were collected from the heart and centrifuged at 3000 rpm for 15 min at 4° C and serums were kept at -80° C. Serum samples were used to determine the level of glucose, insulin, total cholesterol, triglyceride, HDL-C (High-density lipoprotein cholesterol), LDL-C (Low-density lipoprotein cholesterol), ALT (Alanine transaminase), AST (Aspartate transaminase) and albumin by the International Federation of Clinical Chemistry (IFCC) methods using the BT1500 auto analyzer (Cobas-Fara, Italy).
The serum level of insulin was measured by the ELISA (Enzyme-Linked Immunosorbent Assay) method using the IBL kit (IBL International, Germany) using Microplate reader stat fax 2200 (Awareness Technology Inc, USA) to read the plates. The stat fax 2200 shaker incubator was used to incubate the plates and the stat fax 2600 washer (Awareness Technology Inc, USA) was used to wash the plates.
After the end of the intervention period, the animals were euthanized, and the liver tissue was removed, immediately weighed and then fixed. The blocking steps of the slides were then performed by staining Hematoxylin and Eosin (H&E) to assess the extent of fat accumulation and Azan staining method was used for the evaluation of inflammation and fibrosis.
After weighing the liver tissue, a portion of that was kept in the liquid nitrogen tank to determine the changes in genes expression. The expression level of ACACA, SREBP-1c, IL-6, and TGF-β genes was assessed. RNA (Ribonucleic acid) extraction was done by standard kits (Cinnagen, Iran). The cDNA (complementary Deoxyribonucleic acid) was constructed by reverse transcriptase and using the extracted RNAs. The dilution of cDNAs was performed at a concentration of approximately 300 ng/μl using DNase free distilled water CinnnaGen kit (CinnaGen Inc., Tehran, Iran). The gene expression was measured by Real-Time PCR and CinnnaGen kit was used for gene duplication. The primers and probes were designed by AlleleID software version 7.5 (Premier Biosoft, USA) (Primer sequence is listed in Table 1). The relative quantity in real-time PCR was assessed by measuring the increase in fluorescence radiation due to the binding of the Cybergreen (Cybergreen TaKaRa SYBR Premix Ex Taq II) using the ABI 7300 machine.
Table 1.
Sequences of primers used to assess the level of genes expression
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | TTCAACGGCACAGTCAAGG | CTCAGCACCAGCATCACC |
| IL6 | AAGTCAACTCCATCTGCC | TATCCTCTGTGAAGTCTCCT |
| TGFB1 | ATTCCTGGCGTTACCTTG | CTGTATTCCGTCTCCTTGG |
| SREBP-1c | ATCAACAACCAAGACAGT | GAAGCAGGAGAAGAGAAG |
| ACACA | TGGTGCTTATATTGTGGATGG | CCGAGGATTGATGGTTGG |
The real-time PCR (polymerase chain reaction) components in the final volume of 20 μl are shown in Table 2. The rPCR was performed using the TaKaRa SYBR Premix Ex Taq II kit (TaKaRa, Japan) and Rotor-Gene Q (Qiagen, Hilden, Germany). The rPCR was started with the activation of the single-polymerase enzyme at 95° C for 30 s. Then, 45-times three-stages reactions (denaturation at 95° C for 5 s, annealing at 57° C for 20 s, and extending for 30 s at 72° C) were performed for replication. By the end of the quantitative real-time PCR reaction, Rotor-Gene Q Series version 2.0.2 software was used for data analysis.
Table 2.
Content of the vials of the PCR reaction
| Reagent | Volume | Final Concentration |
|---|---|---|
| SYBER Master Mix (2X) | 10 μl | 1x |
| Forward Primer (10 μl) | 0.6 μl | 0.3 μM |
| Reverse Primer (10 μl) | 0.6 μl | 0.3 μM |
| Rox Reference Dye (50X) | 0.4 μl for ABI Step One/Plus | 1x |
| Template | 2 μl | < 100 ng |
| DDW | 7.2 μl | |
| Total | 20 μl |
Statistical analysis
The normality of the data was examined with Shapiro–Wilk test. The data were normally distributed and presented as mean ± standard deviation. One-way analysis of variance (ANOVA) test was used to perform a comparison between groups and if there was a significant difference between groups, Tukey's post hoc test was applied. All the statistical analyses were performed by SPSS version 25 and P < 0.05 was considered significant.
Results
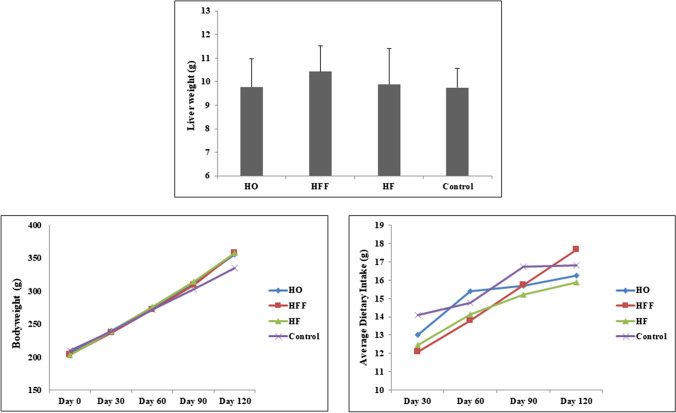
The amounts of food intake and weight gain were significantly increased during study period (all P < 0.05), but there was no significant difference between the groups (Fig. 1). At the end of the study, fasting blood glucose levels were significantly higher in the HF (208.33 ± 50.25) and HFF groups (214.12 ± 47.86) than in the control group (145.00 ± 17.57) (P < 0.05). There was no significant difference between the fasting blood glucose levels of HO group (163.00 ± 37.40) and the control group. Fasting insulin levels in HF and HO groups significantly increased compared with the control group. In addition, calculating HOMA-IR (Homeostatic Model Assessment for Insulin Resistance) showed that all intervention diets increased insulin resistance compared to the standard diet. Changes in levels of liver enzymes indicated a significant increase in the level of these enzymes only in the group receiving the HF regimen (P < 0.01). There were no significant changes in lipid indices in the groups, and only the HO group showed a significant decrease in total cholesterol and HDL-C levels compared to the control group (P < 0.05) (Table 3).
Fig. 1.
Change in bodyweight and dietary intake of pellets during study period and liver weight at the end of study
Table 3.
Biochemical indices in experimental groups
| Control (Mean ± SD) | HF (Mean ± SD) | HFF (Mean ± SD) | HO (Mean ± SD) | |
|---|---|---|---|---|
| Bodyweight change (g) | 121.33 ± 22.92 | 151.64 ± 34.70 | 157.75 ± 22.42 | 148.01 ± 43.21 |
| FBS (mg/dl) | 145.00 ± 17.57 | 208.33 ± 50.25 ** | 214.12 ** ± 47.86 | 163.00 ± 37.40 |
| Triglyceride (mg/dl) | 91.88 ± 30.53 | 110.88 ± 12.76 | 122.37 ± 31.30 | 101.55 ± 12.08 |
| Total cholesterol (mg/dl) | 62.66 ± 7.33 | 62.00 ± 5.26 | 46.87 ± 15.42 | 56.88* ± 6.52 |
| LDL-C (mg/dl) | 12.00 ± 1.22 | 11.66 ± 2.34 | 12.37 ± 2.06 | 12.11 ± 2.42 |
| HDL-C (mg/dl) | 35.33 ± 4.76 | 32.44 ± 4.90 | 31.62 ± 5.31 | 27.77* ± 7.29 |
| AST (mg/dl) | 120.33 ± 25.91 | 170.76 ± 47.91** | 118.12 ± 17.29 | 159.34 ± 76.49 |
| ALT (mg/dl) | 44.65 ± 13.21 | 70.44 ± 9.12** | 45.12 ± 8.69 | 41.62 ± 16.74 |
| Insulin (µU/ml) | 5.50 ± 1.11 | 8.74 ± 0.99** | 7.15 ± 1.49 | 7.89** ± 0.76 |
| HOMA-IR | 1.95 ± 0.39 | 4.511.31** | 3.79** ± 1.15 | 3.20* ± 0.92 |
Kruskal–Wallis test was used for alterations between the groups
LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, AST Aspartate transaminase, ALT Alanine transaminase, HOMA-IR Homeostatic Model Assessment for Insulin Resistance
P Values < 0.05 * and < 0.01 ** compared to the control group
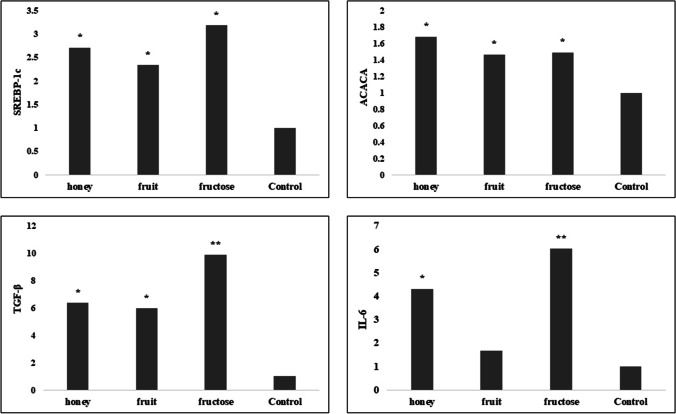
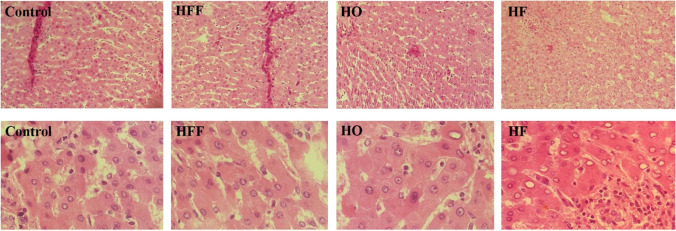
The expression level of genes encoding inflammatory factors increased in rats fed with high fructose diets compared with the control group. The group receiving HF and HO regimens had significantly increased expression of Interleukin 6 (IL-6) gene and HF, HFF and HO groups had significantly increased expression of transforming growth factor-beta (TGF-β) gene compared to the control group. The increased gene expression of inflammatory markers in the HF group was higher compared to the other groups. The examination of acetyl-coA carboxylase (ACC) and sterol regulatory element-binding protein 1c (SREBP-1c) genes showed a significant increase in their gene expression in all high fructose diets (Fig. 2). The histopathological evaluations showed no association between high fructose diets and fatty liver induction as shown in Fig. 3. Based on histopathologic assessments on liver tissue, high fructose diets did not promote necrosis and hepatitis compared to the control group (P > 0.05). However, mild inflammation was observed in the HF group.
Fig. 2.
Difference in gene expression between the groups at the end of study. Kruskal–Wallis test was used for alterations between the groups. P Values < 0.05 * and < 0.01 ** compared to the control group
Fig. 3.
Histopathology charechtristic of study groups. First row is with 100 magnification, second row is with 400 magnification. Micrograph shows normal portal triad in the control, HFF, and HO groups. HF group displays mild inflamation and congestion
Discussion
The results of this study indicated that high fructose intake through the synthetic HF regimen, high fructose fruits, or honey has a similar adverse effect on insulin resistance and ACC and SREBP-1c genes expression. However, HF group had a greater increase in the expression levels of IL-6 and TGF-β genes compared to the control group at the end of the intervention. Increasing the fasting blood sugar or fasting insulin in high fructose groups resulted in a significant increase in insulin resistance. Higher levels of blood sugar and especially insulin resistance in the HF group was predictable, as many studies showed increased insulin resistance in people who consumed high amounts of fructose [27, 28]. Based on animal and molecular studies, decreasing insulin sensitivity following increased fructose consumption may be due to changes in the insulin signaling pathway or increased oxidative stress and inflammation [29, 30]. In addition, declining of adiponectin production and secretion as a result of consuming a high fructose diet can also exacerbate insulin resistance [31]. The changes in insulin resistance in HO and HFF groups were similar to HF group. However, several studies have investigated the effect of honey consumption in controlling blood glucose and the results were inconsistent. Studies that have reported positive effects of honey on blood glucose control mainly focused on the antioxidant potential of honey, which can vary among different types of honey [32–34]. In the present study, increased fasting insulin and insulin resistance was consistent with the results of Mohammadimansesh et al. study, which observed increased fasting insulin after 75 days of intervention with honey in diabetic rats [35]. Also, in a study conducted by Bahrami et al. on patients with type 2 diabetes, honey consumption had positive effects on weight control and lipid indices. Nevertheless, the glycosylated hemoglobin increased in these patients [36]. These differences in the results of studies can be related to the dual effect of fructose in controlling blood glucose. In the short term, honey can lower or stabilize blood glucose, but in the long term, due to changes in plasma levels of hormones and liver metabolism, insulin resistance is exacerbated [37]. Increasing insulin resistance in the HFF group also suggests that despite higher amounts of fiber and polyphenolic and antioxidant compounds in HFF diet, the amount of carbohydrates in the diet has an important effect on its metabolic consequences and this diet did not prevent insulin resistance. Meta-analysis of prospective studies emphasized on the preventive effects of higher fruit consumption on insulin resistance, which is not consistent with the results of the present study [38, 39]. On the other hand, some studies found no relationship between the consumption of fruits and diabetes [40, 41]. Although, the differences in fructose, fiber, and polyphenolic compounds content of different fruits can be considered as the reasons for differences in the results of the studies. Wang et al. reported that using more Berry family fruits due to higher polyphenolic content have stronger effects in reducing the risk of diabetes [42].
The acyl CoA carboxylase enzyme is one of the most sensitive lipid synthesis pathway enzymes which is affected by high carbohydrate diets [24]. High fructose diets may increase the gene expression of this enzyme, which can result in increased lipid synthesis [43]. In the present study, there was a significant increase in the expression level of acyl-CoA carboxylase gene in high fructose diets. In addition, expression of the SREBP-1c, which plays a role in the gene expression of enzymes involved in lipogenesis, increased in the intervention groups compared with the control group. Previous studies have shown that fructose can independently activate the expression of SREBP-1c gene, which subsequently increases the expression of genes that plays a role in lipid synthesis [44, 45]. However, the results of the present study indicated that these changes in gene expression could not affect pathological parameters such as fat accumulation in the liver or lipid profile.
Increased expression of TGF-β and IL-6 genes was observed HF, HFF, and HO groups in comparison with the control group. These results indicated the role of fructose in stimulating inflammatory activity. This stimulation can be mainly due to the role of fructose in increasing the production of reactive oxygen species compared to glucose [46]. However, the lower levels of inflammatory indices in HFF and HO groups compared to the HF group can be due to antioxidant and the phenolic contents of fruits and honey, which decreases the severity of the damage caused by reactive oxygen species [46]. This protective effect can reduce the damage to the cell, especially the destruction of hepatocytes. As the present study identified, elevations of liver enzymes in the HF group, which reflects the liver damage, were not observed in HO and HFF groups. The effects of fructose on liver tissue may occur in a longer duration compared to the duration of the present study.
Conclusion
Consumption of fructose-rich honey and fruits, compared to an industrial fructose-rich product, partially improved the status of inflammatory markers and lipid profiles and liver enzymes. However, all three groups had higher insulin resistance and higher levels of expression of genes related to lipid synthesis than the control group. There was no histopathological difference in liver tissue between the groups. Future human studies are warranted to understand the effects of different types of fructose on biochemical, pathological, and inflammatory biomarkers.
Acknowledgements
We would like to thank all the staff from Comparative and Experimental Medicine center, Shiraz, Iran for their contribution in this study. We also appreciate the unlimited cooperation and guidance of Prof. A Behzad-Behbahani from Diagnostic Laboratory Sciences and Technology Research Center. This work was supported by a research grant from the research deputy of Shiraz University of Medical Sciences (grant no. 93-8704).
Declarations
Ethical approval
The experimental protocol of this research was approved by ethics committee of Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS.REC.1393.8704).
Conflict of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8(3):521–533. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Brunt EM. Nonalcoholic steatohepatitis. In Seminars in liver disease. 2004. Copyright© 2004 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New ….
- 3.Bedogni G, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42(1):44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 4.Fassio E, et al. Natural history of nonalcoholic steathepatitis: a longitudinal study of repeat liver biopsies. Hepatology. 2004;40(4):820–826. doi: 10.1002/hep.20410. [DOI] [PubMed] [Google Scholar]
- 5.Alavian SM, et al. Non-alcoholic fatty liver disease prevalence among school-aged children and adolescents in Iran and its association with biochemical and anthropometric measures. Liver Int. 2009;29(2):159–163. doi: 10.1111/j.1478-3231.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, et al. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology. 2008;47(2):746–754. doi: 10.1002/hep.22009. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani NP. Goldman's Cecil Medicine. Elsevier; 2012. Alcoholic and nonalcoholic steatohepatitis; pp. 996–999. [Google Scholar]
- 8.Chalasani N, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 9.Mehrdad M, et al. Association of FTO rs9939609 polymorphism with serum leptin, insulin, adiponectin, and lipid profile in overweight adults. Adipocyte. 2020;9(1):51–56. doi: 10.1080/21623945.2020.1722550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezaei S, et al. Hydroalcoholic extract of Achillea millefolium improved blood glucose, liver enzymes and lipid profile compared to metformin in streptozotocin-induced diabetic rats. Lipids Health Dis. 2020;19(1):1–7. doi: 10.1186/s12944-019-1182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vos MB, et al. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10(7):160. [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr. 2013;162(3):496–500. e1. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park O-J, et al. Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem J. 1992;282(3):753–757. doi: 10.1042/bj2820753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Y, Pagliassotti MJ. Hepatospecific effects of fructose on c-jun NH2-terminal kinase: implications for hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2004;287(5):E926–E933. doi: 10.1152/ajpendo.00185.2004. [DOI] [PubMed] [Google Scholar]
- 15.Jegatheesan P, De Bandt JP. Fructose and NAFLD: the multifaceted aspects of fructose metabolism. Nutrients. 2017;9(3):230. doi: 10.3390/nu9030230. [DOI] [Google Scholar]
- 16.Bernardes N, et al. Excessive consumption of fructose causes cardiometabolic dysfunctions through oxidative stress and inflammation. Can J Physiol Pharmacol. 2017;95(10):1078–1090. doi: 10.1139/cjpp-2016-0663. [DOI] [PubMed] [Google Scholar]
- 17.García-Berumen CI, et al. The severity of rat liver injury by fructose and high fat depends on the degree of respiratory dysfunction and oxidative stress induced in mitochondria. Lipids Health Dis. 2019;18(1):1–11. doi: 10.1186/s12944-019-1024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierantonelli I, Svegliati-Baroni G. Nonalcoholic fatty liver disease: basic pathogenetic mechanisms in the progression from NAFLD to NASH. Transplantation. 2019;103(1):e1–e13. doi: 10.1097/TP.0000000000002480. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Lu H-Y. Nonalcoholic fatty liver disease and cardiovascular disease. World J Gastroenterol: WJG. 2014;20(26):8407. doi: 10.3748/wjg.v20.i26.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuyumcu A, Kuyumcu MS. High fructose consumption may have part in the pathophysiology of coronary artery ectasia. Ann Med Res. 2020;27(6):1536–1541. doi: 10.5455/annalsmedres.2019.12.865. [DOI] [Google Scholar]
- 21.Rippe JM, Angelopoulos TJ. Fructose-containing sugars and cardiovascular disease. Adv Nutr. 2015;6(4):430–439. doi: 10.3945/an.114.008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S-A, Shin S. Fruit and vegetable consumption and non-alcoholic fatty liver disease among Korean adults: a prospective cohort study. J Epidemiol Community Health. 2020;74(12):1035–1042. doi: 10.1136/jech-2020-214568. [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Suarez JM, et al. The composition and biological activity of honey: a focus on Manuka honey. Foods. 2014;3(3):420–432. doi: 10.3390/foods3030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsurada A, et al. Effects of nutrients and hormones on transcriptional and post-transcriptional regulation of acetyl-CoA carboxylase in rat liver. Eur J Biochem. 1990;190(2):435–441. doi: 10.1111/j.1432-1033.1990.tb15593.x. [DOI] [PubMed] [Google Scholar]
- 25.Lennox RR, et al. Effects of glucose-dependent insulinotropic polypeptide receptor knockout and a high-fat diet on cognitive function and hippocampal gene expression in mice. Mol Med Rep. 2015;12(1): 1544–8. Erratum in10.3892/mmr.2015.4078. [DOI] [PubMed]
- 26.Jensen VS, et al. Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague-Dawley rats. Diabetol Metab Syndr. 2018;10(1):1–13. doi: 10.1186/s13098-018-0307-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Softic S, et al. Fructose and hepatic insulin resistance. Crit Rev Clin Lab Sci. 2020;57(5):308–322. doi: 10.1080/10408363.2019.1711360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolderup A, Svihus B. Fructose metabolism and relation to atherosclerosis, type 2 diabetes, and obesity. J Nutr Metab. 2015;2015:823081. doi: 10.1155/2015/823081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibitoye OB, Ajiboye TO. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch Physiol Biochem. 2018;124(5):410–417. doi: 10.1080/13813455.2017.1415938. [DOI] [PubMed] [Google Scholar]
- 30.Sangüesa G et al. Liquid fructose and liver insulin signaling: molecular mechanisms controlling hepatic steatosis. Molecular Nutrition: Carbohydrates, 2019: p. 149–72.
- 31.Marek G, et al. Adiponectin resistance and proinflammatory changes in the visceral adipose tissue induced by fructose consumption via ketohexokinase-dependent pathway. Diabetes. 2015;64(2):508–518. doi: 10.2337/db14-0411. [DOI] [PubMed] [Google Scholar]
- 32.Omotayo EO, et al. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int J Vitam Nutr Res. 2010;80(1):74. doi: 10.1024/0300-9831/a000008. [DOI] [PubMed] [Google Scholar]
- 33.Adesoji F, Oluwakemi A. Differential effect of honey on selected variables in alloxan-induced and fructose-induced diabetic rats. Afr J Biomed Res. 2008;11(2).
- 34.Pasupuleti VR, et al. A review on oxidative stress, diabetic complications, and the roles of honey polyphenols. Oxid Med Cell Longev. 2020;2020. [DOI] [PMC free article] [PubMed]
- 35.Mohammadimanesh A, et al. The effect of different types of honey on levels of glucose, fructosamine and insulin in diabetes: an animal study. Journal of North Khorasan University of Medical Sciences. 2016;7(4):905–916. doi: 10.29252/jnkums.7.4.905. [DOI] [Google Scholar]
- 36.Bahrami M, et al. Effects of natural honey consumption in diabetic patients: an 8-week randomized clinical trial. Int J Food Sci Nutr. 2009;60(7):618–626. doi: 10.3109/09637480801990389. [DOI] [PubMed] [Google Scholar]
- 37.Bobiş O, Dezmirean DS, Moise AR. Honey and diabetes: the importance of natural simple sugars in diet for preventing and treating different type of diabetes. Oxid Med Cell Longev. 2018;2018. [DOI] [PMC free article] [PubMed]
- 38.Cooper AJ, et al. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr. 2012;66(10):1082–1092. doi: 10.1038/ejcn.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muraki I, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. Bmj. 2013;347. [DOI] [PMC free article] [PubMed]
- 40.Mamluk L, et al. Fruit and vegetable intake and risk of incident of type 2 diabetes: results from the consortium on health and ageing network of cohorts in Europe and the United States (CHANCES) Eur J Clin Nutr. 2017;71(1):83–91. doi: 10.1038/ejcn.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auerbach BJ, et al. Associations of 100% fruit juice versus whole fruit with hypertension and diabetes risk in postmenopausal women: results from the Women's Health Initiative. Prev Med. 2017;105:212–218. doi: 10.1016/j.ypmed.2017.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang PY, et al. Higher intake of fruits, vegetables or their fiber reduces the risk of type 2 diabetes: A meta-analysis. Journal of diabetes investigation. 2016;7(1):56–69. doi: 10.1111/jdi.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imai N, Cohen DE. Trimming the fat: acetyl-CoA carboxylase inhibition for the management of NAFLD. Hepatology (Baltimore, Md.) 2018;68(6):2062. doi: 10.1002/hep.30206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C, et al. Endoplasmic reticulum stress is involved in hepatic SREBP-1c activation and lipid accumulation in fructose-fed mice. Toxicol Lett. 2012;212(3):229–240. doi: 10.1016/j.toxlet.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Herman MA, Samuel VT. The sweet path to metabolic demise: fructose and lipid synthesis. Trends Endocrinol Metab. 2016;27(10):719–730. doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bose T, Chakraborti AS. Fructose-induced structural and functional modifications of hemoglobin: implication for oxidative stress in diabetes mellitus. Biochim Biophys Acta Gen Subj. 2008;1780(5):800–808. doi: 10.1016/j.bbagen.2008.02.001. [DOI] [PubMed] [Google Scholar]