Graphical abstract
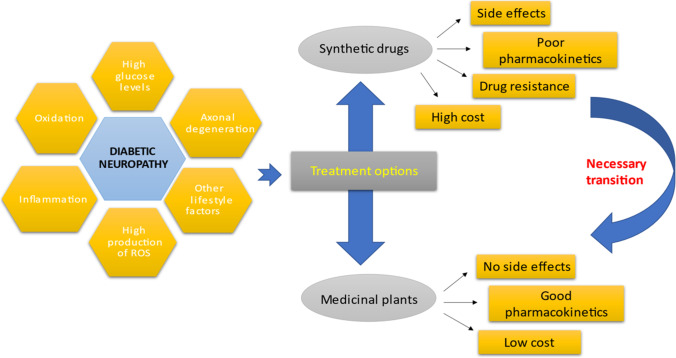
The global pandemic of prediabetes and diabetes has led to a severe corresponding complication of these disorders. Neuropathy is one of the most prevalent complication of diabetes is, affecting blood supply of the peripheral nervous system that may eventually results into loss of sensations, injuries, diabetic foot and death. The utmost identified risk of diabetic neuropathy is uncontrolled high blood glucose levels. However, aging, body mass index (BMI), oxidative stress, inflammation, increased HbA1c levels and blood pressure are among the other key factors involved in the upsurge of this disease. The so far treatment to deal with diabetic neuropathy is controlling metabolic glucose levels. Apart from this, drugs like reactive oxygen species (ROS) inhibitors, aldose reductase inhibitors, PKC inhibitors, Serotonin-norepinephrine reuptake inhibitors (SNRIs), anticonvulsants, N-methyl-D-aspartate receptor (NMDAR) antagonists, are the other prescribed medications. However, the related side-effects (hallucinations, drowsiness, memory deficits), cost, poor pharmacokinetics and drug resistance brought the trust of patients down and thus herbal renaissance is occurring all over the word as the people have shifted their intentions from synthetic drugs to herbal remedies. Medicinal plants have widely been utilized as herbal remedies against number of ailments in Indian medicinal history. Their bioactive components are very much potent to handle different chronic disorders and complications with lesser-known side effects. Therefore, the current article mainly concludes the etiology and pathophysiology of diabetic neuropathy. Furthermore, it also highlights the important roles of medicinal plants and their naturally occurring bioactive compounds in addressing this disease.
Keywords: Diabetes, Diabetic neuropathy, Peripheral nervous system, Herbal therapy, Traditional medicine, Medicinal plants, Bioactive compounds
Introduction
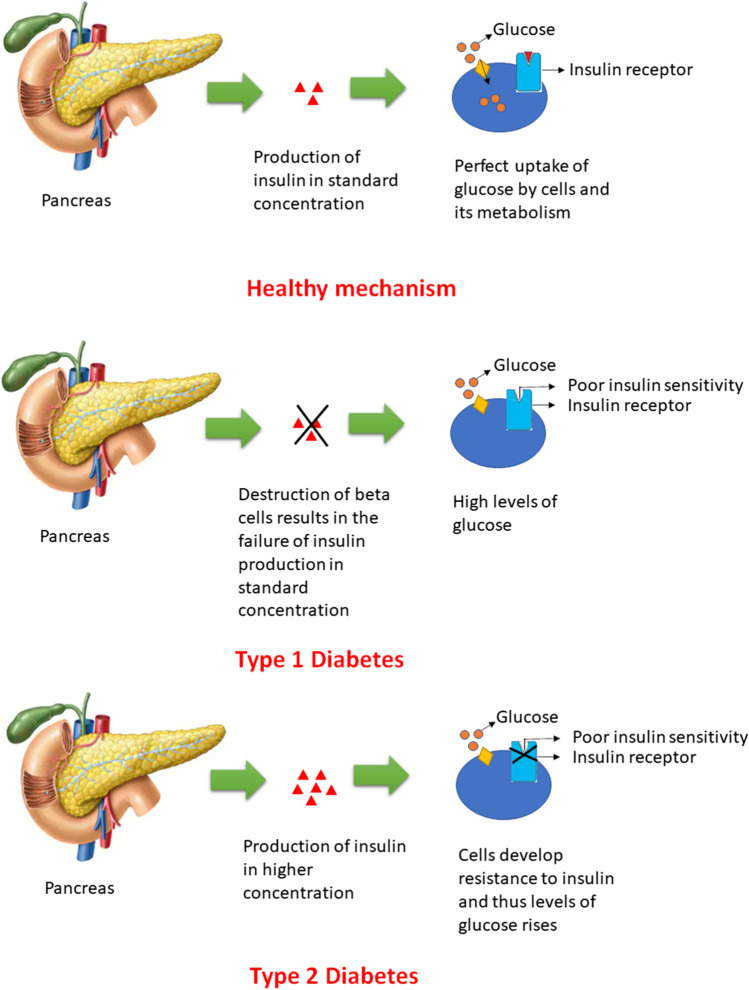
Diabetic neuropathy, a multifaceted disorder is characterized by the presence of signs and indications that may led to the impairment of peripheral nerves mainly due to the altered levels of insulin [1]. Insulin is a polypeptide hormone that primarily maintains the required glucose levels in body and any alteration in levels of insulin led to the progression of diabetes with or without accompanying complications [2, 3]. Diabetic neuropathy is an example of accompanying distress resulted from diabetes mellitus and thus understanding the types, mechanisms and associated risk factors of diabetes mellitus is much more important. There are two main classes of diabetes mellitus (type 1 and type 2). Type 1 is an insulin dependent diabetes mellitus, in which the body’s immune system specifically targets the insulin secreting β-cells of pancreas. However, in type 2 diabetes (insulin independent diabetes), cells may either become unresponsive to the insulin or develop insulin resistance [4] (Fig. 1). The most common mechanism that mainly gets affected in the diabetic patients is Glucose-Insulin-Glucagon mechanism. The antagonistic effects of insulin and glucagon chiefly helps to maintain the optimized blood glucose levels [5–7]. Number of risk factors associated with diabetes mellitus includes sedentary lifestyle (smoking, lack of physical activity, alcohol consumption), obesity, age, diet, environmental factors, gut microflora, disturbed circadian rhythms and many more [8–17].
Fig. 1.
Classification of diabetes mellitus
Etiology and risk factors
The diabetic neuropathy mainly elicits due to the high levels of glucose in plasma (diabetes mellitus) that usually disrupts the ability of neurons to communicate, which results into a weakness of blood capillaries supplying nutrients and oxygen to the nerves [18]. In addition to this, insulin resistance, hyperglycemia and dyslipidemia are the other contributing factors resulting a disturbed biochemical pathway which ultimately ends up in high ROS production due to disturbed mitochondrial redox status [19], and thereby leading to loss of axonal vitality. Thus, oxidative stress and inflammation can surely lead to nerve dysfunction and eventual cell death [20]. Other risk factors include age, weight, body mass index, HbA1c levels, diabetic retinopathy, 2-h glucose C peptide, duration (time span) of diabetes, smoking, high blood pressure, fasting plasma glucose, levels of blood urea nitrogen, etc. [21–29].
In diabetic neuropathy, the early modifications that occurs in type ‘C’ unmyelinated fibers results into the loss of sensation in distal parts of body (toe and foot), pain, physical sensitivity and allodynia mainly due to the axonal degeneration [30, 31]. The four main classes of diabetic neuropathy based on the part that got affected includes peripheral neuropathy, autonomic neuropathy, focal neuropathy and proximal neuropathy [32]. Peripheral neuropathy is a most common and broadly distressing about 50% of the diabetes population, affecting the motor and sensory nerves around the periphery of brain [33]. In Autonomic neuropathy the entire autonomic nervous system got affected; focal neuropathy leads to isolated mononeuropathies, radiculopathy or polyradiculopathywhileproximal neuropathy results in severe unilateral anterior thigh pain followed by weakness of the quadriceps muscle with a loss of the knee jerk [34, 35].
Pathophysiology
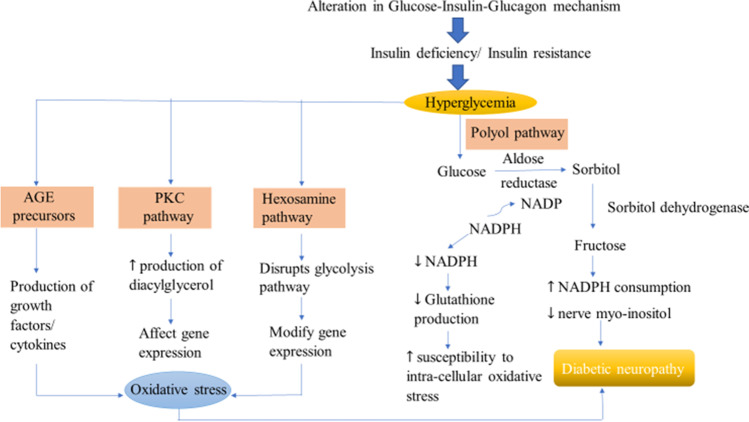
The pathogenesis of diabetic peripheral neuropathy is complex and is defined by different metabolic and vascular factors. The normal physiological mechanisms of our body maintain optimum glucose levels in blood plasma. However, any alteration in these mechanisms causes diabetes and its related complications. There are four different significant pathways that are ascribed to the development and progression of diabetic neuropathy. These pathways depict the metabolic abnormalities of nerve physiology and are named as Polyol pathway, Intracellular production of Advance Glycation end products (AGE) precursors, Protein Kinase C (PKC) activation and Hexosamine pathway. The high levels of glucose hyperactivates the polyol pathway in which the enzymes aldose reductase (converts glucose to sorbitol) and sorbitol dehydrogenase (converts sorbitol to fructose) begins the process of conversion of glucose to fructose. During the conversion of glucose to sorbitol, aldose reductase consumes NADPH which lessens the production of glutathione and thereby leading to increased susceptibility to intra-cellular oxidative stress. The enhanced action of polyol pathway also reduces myo-inositol that ultimately results in diabetic neuropathy [36–39]. AGE precursors respond by three mechanisms where the alteration in intracellular proteins disrupts gene transcription; diffusion of AGE precursors leading to cellular dysfunction and finally modification of circulating proteins in blood after diffusion activate the AGE receptors that results in the production of growth factors and cytokines and thus causes vascular pathology [40]. The activation of PKC requires phosphorylation after which it binds to its activating cofactor diacylglycerol whose production rate is enhanced by high glucose levels. Activated PKC affects gene expression by either upregulating (eg. endothelin-1) or downregulating (eg. endothelial Nitric Oxide Synthase) the expression ultimately disrupts axonal transport [41]. Hexosamine pathway involves the redirection of fructose-6 phosphate to the other signaling pathways (Glycolysis: original pathway) in which enzyme glutamine: fructose-6 phosphate amido transferase (GFAT) converts the fructose-6 phosphate to glucosamine-6 phosphate and finally to UDP (uridine diphosphate) N-acetyl glucosamine which then leads to the modification in gene expression after binding to serine and threonine (Fig. 2) [42].
Fig. 2.
Pathophysiological mechanism of diabetic neuropathy
Treatment
Despite the extensive research, the definitive cure of diabetic neuropathy is not found yet. However, taking some measures like maintaining optimum levels of blood glucose, physical fitness, HgbA1C (glycosylated hemoglobin) levels below 7, avoiding drinking-smoking, controlling cholesterol levels, taking care of foot minimizes the risk for its development.
Metabolic control
Controlling the levels of blood glucose is one of the principal treatments that can prevent diabetic neuropathy. As per the data published by Diabetes Control and Complications Center (DCCT), neuropathy has been reduced by 57% in the patients of type 1 diabetes mellitus who are on tight glycemic control. Likewise, the Epidemiology of Diabetes Interventions and Complications (EDIC) study illustrated the less prevalence of diabetic neuropathy in type 2 diabetic mellitus patients undergoing insulin treatment [43].
ROS inhibitors
Alpha lipoic acid is an anti-oxidant, authorized in Germany to cure diabetic polyneuropathy [44]. Improved symptoms were observed when patients were administered intravenously with 600 mg/day of alpha lipoic acid for more than 3 weeks in a meta-examination of four placebo-controlled trials [45]. However, the randomized, placebo-controlled and double-blind experimental trials showed no improvements in the symptoms after four years of treatment with alpha lipoic acid [46].
Aldose reductase inhibitors
Aldose reductase is a key enzyme involved in polyol pathway responsible for blood glucose metabolism. Aldose reductase inhibitors such as sorbinil, tolrestat, ponalrestat, fidarestat, epalrestat halt the progression of diabetic neuropathy either by preventing sorbitol accumulation, increasing nerve conduction velocity, maturing nerve fibers or by regulating polyol pathway [47]. However, various tested inhibitors showed notable side effects with low effectiveness [48].
Protein kinase-C inhibitors
Ruboxistaurin is one of the inhibitors of PKC-β that binds to its active site and inhibits the substrate phosphorylation by interfering with the process of ATP binding [49]. It helps in improving the functions of nerve fiber and microvascular blood flow. It also enhances the quality of life in patients having diabetic neuropathy [50].
Tricyclic anti-depressants
They hinder the reuptake process of serotonin and non-adrenaline from the synaptic clefts. Previous reports showed 61%, 74% and 41% pain reduction upon intake of doses of desipramine, amitriptyline and placebo respectively by the patients suffering from diabetic neuropathy in a randomized, double-blind and place-controlled trial. However, administering overdoses (more than 100 mg) increased the risk of sudden death [51]. Also, various side effects associated with TCAs restricted their use in diabetic patients [52].
Serotonin-norepinephrine reuptake inhibitors (SNRIs)
Duloxetine was approved as the first drug in 2014 by US Food and Drug Administration (FDA). The drug was formulated based on the results obtained from the randomized controlled trials and showed remarkable improvement in pain when patients were administered with daily doses of 60 mg and 120 mg duloxetine [53, 54]. However, doses lower than these were not efficacious and higher than these resulted in minor side effects such as constipation and drowsiness [54, 55]. Also, doses of Venlafaxine (another SNRI) above 150 mg/per day exhibited improved response in managing neuropathic pain. However, the dose less than 150 mg/day alter the process of transmission of serotonin and does not act as an SNRI [56].
Anticonvulsants
The first discovered carboxamide that blocks the Na channels in order to reduce the excitability of the peripheral nerve, ultimately help in managing diabetic neuropathy was Carbamazepine. Though, the studies suggested the role of carbamazepine in regulating neuropathic pain but due to its unfavourable outcomes such as osteoporosis and suppression of bone marrow, the other agents have taken its place [44, 57, 58]. Gabapentin is an analogue of neurotransmitter GABA and helps in reducing the neuropathic pain by inhibiting the α2δ unit of the calcium channel [59, 60]. Topiramate, during a double-blind and randomised placebo- controlled trial showed symptomatic relief in neuropathic pain [61]. However, no significant change was found in pain relief with topiramate in the investigation of three small studies [62].
N-methyl-D-aspartate receptor (NMDAR) antagonists
These drugs are commonly used to manage the pain that occurs during a period following surgery. The increased activity of NMDAR causes the condition of central sensitization that helps in the maintenance and management of chronic neuropathic pain [63]. The most clinically and commonly used NMDAR antagonists are dextromethorphan and ketamine. Dextromethorphan in high doses have been reported to exhibit tolerable results in case of managing neuropathic pain. Also, studies revealed the reduction in pain scores in the patients suffering from diabetic neuropathy upon administering dextromethorphan orally [64]. The administration of ketamine in low doses via intranasal route showed pain scores reduction in patients of diabetic neuropathy [65]. However, detrimental effects like restlessness, hallucinations, drowsiness, memory deficits, vivid dreams and dissociation have been observed and documented when NMDAR antagonists administered systemically [63].
Natural therapies
Though numerous treatments and medications are available in market for the cure of diabetic neuropathy, but the downsides of these medications such as, poor pharmacokinetics, cost and drug resistance urge the need to have a transition from these chemical medications towards the natural traditional medications in a form of herbal treatment. Plants are utilized as an essential source for various drugs in Indian medicinal history. The complete description of all the curative properties of the medicinal plants has been listed in Rigveda. In the era of medieval Persia, herbal therapies were utilized for therapeutic purposes in order to relieve pain originated from nerve cell. Herbal ointments derived from topical oil with hot temperaments such as thyme, pepper, colocynth, flax and chamomile were used for the pain management. The scholars of medieval Persian suggested lavender syrup as the most accepted cure for controlling neuropathic pain. They also used Takmid (warm compress) as non-pharmacological treatment [66]. After a while, various synthetic drugs developed and commenced which due to their drawbacks failed. Recently, the trend of using plants is again rising and is being utilized in a confident manner. The herbal drugs are prescribed widely because of their effectiveness, fewer side effects and relatively low cost and the traditional plant medicines are used throughout the world for a range of diabetic complications. Preclinical studies have crossed the doorstep of laboratories and reached to the bed-side of the patients. Clinically, various studies have been done on humans and reported the anti-diabetic potential of plants such as Curcumin longa, Cinnamomum cassia, Portulaca oleracea L. seeds, Scoparia dulcis, Ficus racemosa bark. The research on their herbal preparations in lab has reached to diabetic patients by the brand name of Glyoherb®, Diabeta Plus® and Diabecon® [67]. For instance, Diabeta plus is made of polyherbal composition Vijayasar (Pterocarpus marsupium), Gurmar (Gymnemasylves), Jamun (Syzygiumcumini), Karela (Momordica charantia), Shilajit (Asphaltum), Madagascar periwinkle (Catharanthus roseus) [68]. Further, the frontline anti-diabetic drug, Metformin constitute Galega officinalis as a bioactive compound and proves to be successful and efficacious glucose-lowering agent. Other examples of branded drugs formulated with medicinal plants include diasulin, pancreatic tonic 180 cp, dia-care, diabetes-daily care, diabecure (Table 1) [69].
Table 1.
Pharmacokinetic profile of bioactive compounds of plants used in diabetic neuropathy
| Parameters | Curcumin | Ginkgolide B | Cucurbitacin B | Saponins | Methyl eugenol | Thymoquinone | |
|---|---|---|---|---|---|---|---|
| Formula | C21H20O6 | C20H24O10 | C32H46O8 | C58H94O27 | C11H14O2 | C10H12O2 | |
| Molecular weight (g/mol) | 368.38 | 424.40 | 558.70 | 1223.35 | 178.23 | 164.20 | |
| Water solubility | Log S | -3.94 | -2.17 | -4.57 | -4.82 | -2.61 | -2.18 |
| Solubility | 4.22e−02 mg/ml | 2.90 e+00 | 1.50e−02 mg/ml | 1.86e−02 mg/ml | 4.37e−01 mg/ml | 1.09e+00 mg/ml | |
| Class | Soluble | Soluble | Moderately soluble | Moderately soluble | Soluble | Soluble | |
| Gastrointestinal absorption | High | Low | Low | Low | High | High | |
| p-glycoprotein substrate | No | Yes | Yes | Yes | No | No | |
| Lipophilicity (Log Po/w) | 3.03 | -0.12 | 3.19 | -2.77 | 2.58 | 1.85 | |
| CYP1A2 inhibitor | No | No | No | No | Yes | No | |
| CYP2C19 inhibitor | No | No | No | No | No | No | |
| CYP2C9 inhibitor | Yes | No | No | No | No | No | |
| CYP2D6 inhibitor | No | No | No | No | No | Yes | |
| CYP3A4 inhibitor | No | No | Yes | No | No | No | |
| Bioavailability score | 0.55 | 0.55 | 0.55 | 0.17 | 0.55 | 0.55 | |
| Any violation to Lipinski’s rule | No | No | Yes (1 violation) MW > 350 | Yes (3 violations) MW > 500, N or O > 10, NH or OH > 5 | No | No | |
Role of Curcuma longa
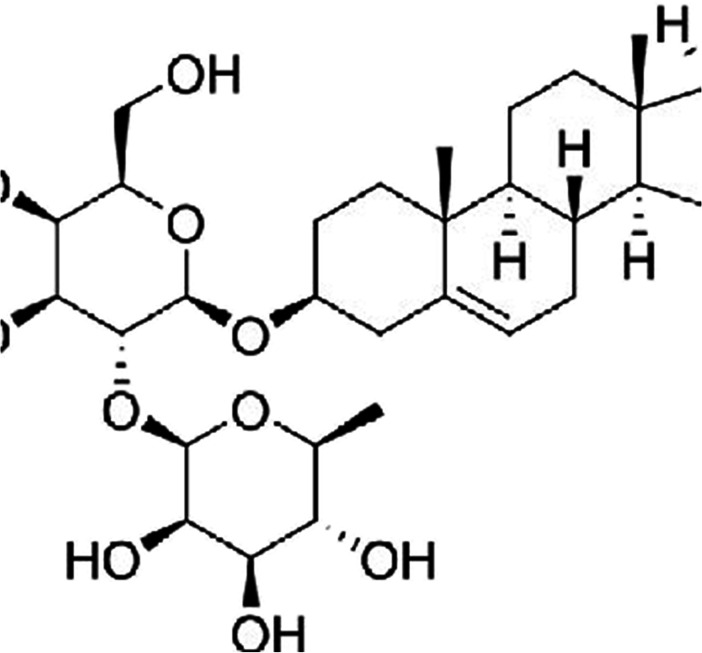
Curcuma longa is one of the demanding spices of Indian cooking/cuisine. The active component found in Curcuma longa is curcumin. However, other active constituents are demethoxycurcumin, bisdemethoxycurcumin, volatile oils (tumerone, zingiberene and atlantone) [70]. It is known by its astonishing activities to cure several diseases. It possesses anti-inflammatory, anti-oxidant, anti-diabetic, anti-infectious, healing, anti-viral, hypolipidemic activities [71, 72]. Specifically, for diabetic neuropathy curcumin have been reported to slow down the progression of diabetes mellitus (type 2), ameliorates the functions of beta cells, prohibits the death of beta cells, scavenges reactive oxygen species, lowers the syndrome of insulin resistance [73, 74]. The proposed mechanisms by which curcumin prevents the development of diabetic neuropathy includes obstructing the process of production of NO (nitric oxide), TNF-alpha, IL-Iβ (interleukin-lβ), IL-8 (interleukin- 8) and by reducing levels of nitrite in the brain [75, 76]. However, no decline in inflammatory markers (IL-1β, IL-6, TNF-α) in patients having chronic inflammatory diseases was observed by White et al. in their meta-analysis of randomized controlled trials [77]. Further, significant decrease in triglyceride levels, body weight, lypoprotein cholesterol total cholesterol and body mass index (as compared to standard) was observed in a double blind, randomized study of hyperlipidemic patients having type 2 diabetes upon turmeric supplementation [78]. The administration of curcumin at a dose of 1 g per day in diabetic patients decrease skin flux at foot’s surface and improves microangiography [72]. Poolsup et al. done a meta-analysis and found the decreased levels of HbA1c and fasting blood glucose when 1.5 g of turmeric was administered to prediabetic individuals for the period of 9 months (Fig. 3) [79, 80].
Fig. 3.
Chemical structure of curcumin illustrating anti-diabetic and anti-neuropathic activity
Role of Ginkgo biloba
Gingko biloba is the single representative of the family Ginkgoaceae and thus known as a living fossil. The bioactive constituents responsible for the pharmacological actions include flavonoids glycosides, ginkgolide B and terpenes trilactones [81]. The vital and sparkling potential of the plant led to explore its applications in numerous fields like health care, food supplements. The extract of Gingko leaves is used as a remedy for memory impairment, concentration difficulties, and the disorders related to dementia. It also possesses neuroprotective, anti-oxidant, wound healing, anti-asthmatic properties. In addition to this, various studies have been done and reported indicating its potential role in the management of neuropathic pain. Previous studies showed significant reduction in pain upon administering standardized extract of Ginkgo biloba in different doses to the rats with neuropathic pain. The different objective tests were conducted in order to evaluate the effect of gingko on rats expose to cold and pressure and for both the stimuli (cold and pressure), positive results were shown. The observation of the study was that higher the dose of Ginkgo biloba extract, the more prominent the alleviating impact on pain. The possible mechanisms behind the pain-relieving effect including anti-inflammatory, neuroprotective or anti-oxidant effects. Other studies included the treatment of diabetic neuropathy via administering extract of EGb 761 (120 mg/day) or placebo on 156 patients for 6 months. The after results of the treatment were found to be good as within the period of 6 months remarkable reduction in intensity of pain was observed along with improved sense of touch [82]. Also, combinatorial studies have been done indicating the positive results in the management of diabetic neuropathy. Gingko and methylcobalamin have been proposed by a group of Chinese researchers. The treatment was done in three groups on 144 patients for the period of 12 weeks. Out of the three groups i.e., tablets of Ginkgo biloba and methylcobalmin; epalrestat and methylcobalmin; placebo, the treatment of ginkgo tablets and methylcobalmin showed effective results (minimized physical indications and improved functions of nerve) as compared to placebo (Fig. 4).
Fig. 4.
Chemical structure of ginkgolide B illustrating anti-diabetic and anti-neuropathic activity
Role of Citrullus colocynthis
Citrullus colocynthis is a member of family Cucurbitaceae. It is a very popular traditional fruit that is involved in diverse applications, known by bitter apple, tumba, bitter cucumber, vine-of-sodom, wild gourd, and colosynth [83]. It has been reported to have anti-oxidative, anti-cancerous, anti-inflammatory, analgesic, gastrointestinal, hypolipidemic effects [84, 85]. Also, the plant is well-known for its anti-diabetic property and medication. Some of the bioactive compounds due to which it has potential to cure diseases include phenolics, flavonoids, α-aelaterin, cucurbitacins (A, B, C, D) [86]. The presence of anti-oxidative and anaesthetic effects in Citrullus colcynthis makes it as a therapeutic agent for the treatment of diabetic neuropathy. In one of the pilot interventional study, formulation of Citrullus colocynthis was applied on eight adults (diagnosed with diabetic neuropathy) topically for 3 months twice a day and were evaluated in terms of pain scales. Significant reduction in mean pain scores was observed with no reported local or systemic effects [87]. Oral administration of this extract is practically not recommended due to very little research evidence (Fig. 5).
Fig. 5.
Chemical structure of cucurbutacin A illustrating anti-diabetic and anti-neuropathic activity
Role of Ocimum sanctum
The common name of Ocimum sanctum is Tulsi belongs to the Lamiaceae family [88]. It is a traditional plant that is rich in various medicinal bioactive compounds like saponin, eugenol, oleanolic acid, caryophyllene, rosmarinic acid, linalool, ursolic acid, linalool [89]. The tremendous capabilities of plant to cure abundant diseases makes it an incredible shrub in the field of medicine. Tulsi, the elixir of life, is worth and worshipped for its both medicinal and spiritual properties, and thus known as “Mother Medicine of Nature”, “The Queen of Herbs,” and “The Incomparable One, in the ancient system of Indian medicine (Ayurveda) [90]. It possesses biological activities like anti-bacterial, anti-oxidant, anti-inflammatory, anti-pyretic, anti-diabetic, anti-allergic, memory enhancing and many more [91]. It’s role in alleviating neuropathic pain in diabetic neuropathy patients is also well-defined. The studies reported the beneficial/ remedial effect of Ocimum sanctum in reducing neuropathic pain upon its administration along with saponins (bioactive compound) for 14 days in the doses of 100 and 200 mg/kg p.o. The treatment also reduces the oxidative stress and levels of calcium [92]. Other study investigated the combinatorial impact of Ocimum sanctum (methanolic leaf extract) with metformin in diminishing and managing the neuropathic pain where they got significant anti-nociceptive effects as compared to the group treated alone (Fig. 6) [93].
Fig. 6.
Chemical structure of saponin illustrating anti-diabetic and anti-neuropathic activity
Role of Artemisia dracunculus
The Artemisia genus incorporates more than 1500 species and is a member of family Asteraceae. Studies reported the potential of plant in treating and managing various disorders. The plant is investigated for pharmacological activities and found to have anti-inflammatory, anti-bacterial, hepato and gastroprotective, anti-fungal, anti-convulsant and anti-diabetic properties. The active and key biological compounds are essential oils, phenolcarbonic acids, flavonoids and coumarins. The components of essential oil include methyl eugenol, estragole and other monoterpenoids where methyl eugenol hampers TRP (Transient Receptor Potential) channels and acts as a pain reliever [94, 95]. In a study, the neuropathy induced by high fat diet was found to be reduced upon intake of ethanolic extract of Artemisia dracunculus, PMI-5011. The induction of neuropathy was done by feeding the C57Bl6/J mice with high fat diets for 16 weeks and due to the high fat diet, mice developed obesity, hyperglycaemia, hypoalgesia, allodynia and nerve conduction deficits. Also, they showed the over expression of 12/15-lipoxygenase, accumulation of 12(S)-hydroxyeicosatetraenoic acid and nitrosative stress in peripheral nerves and spinal cord. The administration of 500 mg/kg of PMI-5011 per day for 7 weeks normalized the levels of blood glucose, alleviated sensory pain, reduced the over expression of 12/15-lipoxygenase and the levels of nitrated protein in PNS. Thus, the study concluded and proved that PMI-5011 is safe and non-toxic extract of to be used in the management of neuropathic pain (Fig. 7) [96].
Fig. 7.
Chemical structure of methyl eugenol illustrating anti-diabetic and anti-neuropathic activity
Role of Nigella sativa
Nigella sativa, a representative of Ranunculaceae family, famous by black seed in India. Fixed oil and volatile oils are the active components of the plant where monoterpenes (α-piene and p-cymene) and thymoquinone are the constituents of volatile oil. The plant has been reported to possess various therapeutic activities like anti-bacterial, hepatoprotective, immunomodulatory, hypotensive and anti-diabetic activity [97]. The curative effects of black seeds and its active component thymoquinone have been investigated by Kanter on the histopathological changes of sciatic nerves in streptozotocin (STZ)-induced diabetic rats. The sharp decline in the levels of serum glucose and enhanced concentration of serum insulin were observed when streptozotocin (STZ)-induced diabetic rats in treated groups for black seeds and thymoquinone were administered orally with 400 mg/kg of back seeds and 50 mg/kg of thymoquinone once a day [95]. Also, less alterations in morphology found when the nerve tissues of treated rats were evaluated histologically. The breakdown of myelin reduced appreciably following the treatment with black seeds and thymoquinone. Significant improvement was noticed in the ultra-structural characteristics of axons. Thus, the treatments (black seeds and thymoquinone) if taken ahead to pre-clinical studies can prove to be potential therapeutics for diabetic neuropathy (Fig. 8) [97].
Fig. 8.
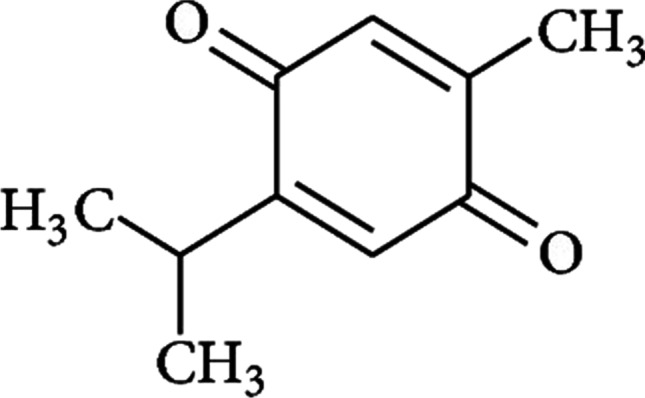
Chemical structure of thymoquinone illustrating anti-diabetic and anti-neuropathic activity
Discussion and future directions
Since diabetes mellitus is a silent-killer that slowly progress to neuropathy and leads to severe pain, it is important to take measures to manage neuropathic pain [98]. Herbal medicines possessing anti-diabetic, anti-oxidant and anti-inflammatory properties should be the primary concern for reducing glucose levels and managing neuropathic pain as numerous side effects and poor pharmacokinetics reported against synthetic agents [99]. The medicinal herbs can thus be utilized as an alternative to available synthetic drugs with few/no side effects [100–102]. Sustained attempts must be done to continue the utilization of therapeutic plants. They ought to follow the Primary Healthcare Strategy (PHC) with the aim that worth and advancement of market worldwide stays centred.
Efforts must be taken to sustain the usage of medicinal plants. In order to ensure the accurate information on the presence of plants in specific domains, data should be maintained regional wise in a tabular form [103]. The sharing of R and D should be done on a regular basis to circumvent duplication. Safety must also be considered, and standardization of medicinal plants is essential for the further studies on them [104]. The dosage quantity should also be taken care for the formulation of herbal medicine as below appropriate quantity, the drug would not be effective and above which the drug becomes too toxic. Also, it is important to determine the pharmacokinetic profile of bioactive compounds for minimum herb-drug interactions as some drugs are considered to interact with chemical drugs. For instance, Ginkgo biloba, a herb approved for managing neuropathic pain but it also possess blood thinning properties and thus not recommended for the ones who are consuming either any kind of anti-coagulants, non-steroidal anti-inflammatory drugs or aspirin [82, 105]. The pharmacokinetic profile of plants used in the treatment of diabetic neuropathy is listed in Table 1. Moreover, efficient and reliable bioassays should be developed to identify the necessary bioactive compounds. Most anti diabetic agents from medicinal plants are prophylactic in nature rather than therapeutic, and clinical trials have not yet been undertaken. If such studies are encouraged and performed, more herbal drugs for human use may soon be available (Table 2).
Table 2.
Clinical application of bioactive compounds involve in diabetic neuropathy
| Plant | Bioactive component | Clinical application/ potential | Potential benefits | References |
|---|---|---|---|---|
| Curcuma longa | Curcumin | Anti-inflammatory, anti-oxidant, anti-diabetic, anti-infectious, healing, anti-viral, hypolipidemic | Slow down the progression of diabetes mellitus, ameliorates the functions of beta cells, prohibits the death of beta cells, scavenges reactive oxygen species, lowers the syndrome of insulin resistance, decrease skin flux at foot’s surface, improves microangiography | [71–74] |
| Ginkgo biloba | Ginkgolide B | Neuroprotective, anti-oxidant, wound healing, anti-asthmatic, anti-inflammatory, | Reduce intensity of pain, improves sense of touch, minimize physical indications and improved functions of nerve | [82] |
| Citrullus colocynthis | Cucurbutacin A | Anti-oxidative, anti-cancerous, anti-inflammatory, analgesic, gastrointestinal, hypolipidemic effects, anti-diabetic | Significant reduction in mean pain scores | [84–86] |
| Ocimum sanctum | Saponin | Anti-bacterial, anti-oxidant, anti-inflammatory, anti-pyretic, anti-diabetic, anti-allergic, memory enhancer | Alleviate neuropathic pain, reduces oxidative stress, | [91–93] |
| Artemisia dracunculus | Methyl eugenol | Anti-inflammatory, anti-bacterial, hepato and gastroprotective, anti-fungal, anti-convulsant and anti-diabetic properties | Normalize the levels of blood glucose, alleviate sensory pain | [94–96] |
| Nigella sativa | Thymoquinone | Anti-bacterial, hepatoprotective, immunomodulatory, hypotensive and anti-diabetic activity | Decline levels of serum glucose, enhance concentration of serum insulin, reduce myelin breakdown, improve ultra-structural characteristics of axons | [97] |
Conclusion
Diabetic neuropathy is a complex and serious lifestyle associated disorder that keeps on increasing continuously and can soon become as one of the health epidemics. The consumption of sugar in excess amount, obesity, physical inactivity, environmental factors are causing increased levels of glucose which in turn affects the peripheral nerves of the brain leading to diabetic neuropathy. Other lifestyle factors and inadequate knowledge of nutritional habits also have an important role in its development. The available medications are not much in use due to their poor pharmacokinetics and side effects. Herbal medicinal plants and their bioactive compounds can be a solution for diabetic neuropathy with least/no side effects if sustained attempts to use therapeutic plants continues as they are involved in the analgesic effects and pathways including anti-inflammatory, neuroprotective, anti-oxidant, anti-apoptotic and calcium inhibitory actions. The beneficial effects of plants in the management of diabetic neuropathy have been reported, however not considered for clinical trials yet. Thus, herbal formulation, as discussed, should be uplifted and encouraged so that the issues associated with synthetic drugs can be avoided.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
The authors agreed to participate in writing this manuscript.
Consent for publication
The manuscript is approved by all the authors for publication.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bansal V, Kalita J, Misra UK. Diabetic neuropathy. Postgrad Med J. 2006;82(964):95–100. doi: 10.1136/pgmj.2005.036137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad K. Insulin sources and types: a review of insulin in terms of its mode on diabetes mellitus. J Tradit Chin Med. 2014;34(2):234–237. doi: 10.1016/s0254-6272(14)60084-4. [DOI] [PubMed] [Google Scholar]
- 3.Villegas-Valverde CC, Kokuina E, Breff-Fonseca MC. Strengthening National Health Priorities for Diabetes Prevention and Management. MEDICC Rev. 2018;20(4):5. doi: 10.37757/MR2018.V20.N4.2. [DOI] [PubMed] [Google Scholar]
- 4.Adapa D, Sarangi TK. A review on diabetes mellitus: complications, management and treatment modalities. J Med Health Sci. 2015; 4(3).
- 5.Rottschäfer V. Analysis of glucose-insulin-glucagon interaction models. Bachelor thesis. Leiden University Mathematical Institute. 2017; 1–57.
- 6.Röder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48(3):e219. doi: 10.1038/emm.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284(4):E671–E678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- 8.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Wilett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among U.S Male Physicians. JAMA. 1992;268:63–67. doi: 10.1001/jama.1992.03490010065031. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon GC, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 10.Cullmann M, Hilding A, Östenson CG. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a Swedish population. Diabet Med. 2012;29(4):441–452. doi: 10.1111/j.1464-5491.2011.03450.x. [DOI] [PubMed] [Google Scholar]
- 11.Belkina CA, Denis VG. Obesity genes and insulin resistance. Curr Opin Endocrinol Diabetes Obes. 2010;17(5):472–477. doi: 10.1097/MED.0b013e32833c5c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Manson JE, Stampfer MJ, Hu FB, Giovannucci E, Colditz GA, Hennekens CH, Willett WC. A prospective study of whole-grain intake and risk of type 2 diabetes mellitus in US women. Am J Public Health. 2000;90(9):1409–1415. doi: 10.2105/ajph.90.9.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dam MB, Rimm BE, Willett CW, Stampfer JM, Hu BF. Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med. 2002;136(3):201–9. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 14.Schulze BM, Manson EJ, Ludwig SD, Colditz AG, Stampfer JM, Willett CW, Hu BF. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 15.Meneilly SG, Elliott T. Metabolic alterations in middle-aged and elderly obese patients with type 2 diabetes. Diabetes Care. 1999;22(1):112–118. doi: 10.2337/diacare.22.1.112. [DOI] [PubMed] [Google Scholar]
- 16.Raman GP. Environmental factors in causation of diabetes mellitus. 2016. 10.5772/62543. (Book chapter).
- 17.Gan Y, Chen Y, Tong X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2015;72(1):72–78. doi: 10.1136/oemed-2014-102150. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Xu Y, An M, Zeng Q. The risk factors for diabetic peripheral neuropathy: a meta-analysis. PLoS ONE. 2019;14(2):e0212574. doi: 10.1371/journal.pone.0212574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajic M. Mitochondrial dynamics in peripheral neuropathies. Antioxid Redox Signal. 2014;21(4):601–620. doi: 10.1089/ars.2013.5822. [DOI] [PubMed] [Google Scholar]
- 20.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93(6):1296–1313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiani J, Moghimbeigi A, Azizkhani H, Kosarifard S. The prevalence and associated risk factors of peripheral diabetic neuropathy in Hamedan, Iran. Arch Iran Med. 2013;16(1):17–19. [PubMed] [Google Scholar]
- 22.Tesfaye S, Stevens LK, Stephenson JM, Fuller JH, Plater M, Ionescu-Tirgoviste C, et al. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39(11):1377–1384. doi: 10.1007/s001250050586. [DOI] [PubMed] [Google Scholar]
- 23.Nie C, Bao HP. Analysis of the related risk factors of diabetic peripheral neuropathy. Chin J Exp Clin Virol. 2012;26(6):467–9. [PubMed] [Google Scholar]
- 24.Mahroos AF, Roomi AK. Diabetic neuropathy, foot ulceration, peripheral vascular disease and potential risk factors among patients with diabetes in Bahrain: a nationwide primary care diabetes clinic-based study. Ann Saudi Med. 2007;27(1):25–31. doi: 10.5144/0256-4947.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maser RE, Steenkiste AR, Dorman JS, Nielsen VK, Bass EB, Manjoo Q, et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh epidemiology of diabetes complications study. Diabetes. 1989;38(11):1456–61. doi: 10.2337/diab.38.11.1456. [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Bi Y, Hu Y, Tong G, Zhu D. Prevalence and risk factors of diabetic peripheral neuropathy. J Med Postgrad. 2011;24(10):1035–1038. doi: 10.3969/j.issn.1008-8199.2011.10.007. [DOI] [Google Scholar]
- 27.Wei W, Wang Z, Zhou L, Zhao S, Mao H. Risk factors analysis of diabetic peripheral neuropathy in patients with type 2 diabetes. Hainan Med J. 2017;28(20):3379–81. doi: 10.3969/j.issn.1003-6350.2017.20.035. [DOI] [Google Scholar]
- 28.Chen L, Zheng F, Li H. A change of serum cystatin C in diabetic peripheral neuropathy in type 2 diabetic patients and its clinical significance. Chin J Diabetes. 2014;22(8):700–703. doi: 10.3969/j.issn.1006-6187.2014.08.007. [DOI] [Google Scholar]
- 29.Xu F, Zhao LH, Su JB, Chen T, Wang XQ, Chen JF, et al. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol Metab Syndr. 2014;6(1):139. doi: 10.1186/1758-5996-6-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green AQ, Krishnan S, Finucane FM, Rayman G. Altered C-fiber function as an indicator of early peripheral neuropathy in individuals with impaired glucose tolerance. Diabetes Care. 2010;33(1):174–176. doi: 10.2337/dc09-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik RA, Veves A, Walker D, Siddique I, Lye RH, Schady W, et al. Sural nerve fibre pathology in diabetic patients with mild neuropathy: relationship to pain, quantitative sensory testing and peripheral nerve electrophysiology. Acta Neuropathol. 2001;101(4):367–374. doi: 10.1007/s004010000287. [DOI] [PubMed] [Google Scholar]
- 32.Llewelyn GJ. The diabetic neuropathies: types, diagnosis and management. J Neurol Neurosurg Psychiatry. 2003;74(2):15–19. doi: 10.1136/jnnp.74.suppl_2.ii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vinik A, Casellini C, Nevoret LM. Diabetic Neuropathies. 2018, Endotext [Internet]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. South Dartmouth (MA): MDText.com, Inc.
- 34.Feldman LE, Callaghan CB, Pop-Busui R, Zochodne WD, Wright ED, Bennett LD, Bril V, Russell WJ and Viswanathan V. Diabetic neuropathy. Nature Reviews | DiSeASePrimerS | Article citation ID: 2019 5:41. 1–18. [DOI] [PubMed]
- 35.Koca TT. Concomitance of diabetic neuropathic amyotrophy and cachexia: a case report with review of the literature. North Clin Istanb. 2015;2(2):165–170. doi: 10.14744/nci.2015.52523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robert J, Tanenbergpeter D, Donofrio. Chapter 3 - Neuropathic problems of the lower limbs in diabetic patients. In Levin and O’Neal’s, The Diabetic Foot (Seventh Edition), 2008 pp. 33–74.
- 37.Lee YA, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13(1):23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 38.Brownlee M. The pathobiology of diabetic complications. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 39.Llewelyn JG, Tomlinsonp DR, Thomas K. Diabetic neuropathies. Chapter no. 85. In peripheral neuropathy (Fourth Edition), 2005, 2, pp. 1951–1991. 10.1016/B978-0-7216-9491-7.50088-0
- 40.Brownlee M, Aiello PL, Cooper EM, Vinik IA, Plutzky J, Boulton MJA. Complications of diabetes mellitus. Chapter no. 33. In Williams textbook of endocrinology (Thirteenth Edition), 2016; pp. 1484–1581. 10.1016/B978-0-323-29738-7.00033-2
- 41.Cancelliere P. A review of the pathophysiology and clinical sequelae of diabetic polyneuropathy in the feet. J Diabetes Metab Disord Control. 2016;3(2):21–24. doi: 10.15406/jdmdc.2016.03.00062. [DOI] [Google Scholar]
- 42.Feldman EN, Vincent EM. The prevalence, impact, and multifactorial pathogenesis of diabetic peripheral neuropathy. Adv Stud Med. 2004;4(8A):S642–S649. [Google Scholar]
- 43.Albers J, Herman W, Pop-Busui R, Feldman E, Martin C, Cleary P, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chong M, Hester J. Diabetic painful neuropathy: current and future treatment options. Drugs. 2007;67:569–585. doi: 10.2165/00003495-200767040-00006. [DOI] [PubMed] [Google Scholar]
- 45.Ziegler D, Nowak H, Kempler P, Vargha P, Low P. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004;21(2):114–121. doi: 10.1111/j.1464-5491.2004.01109.x. [DOI] [PubMed] [Google Scholar]
- 46.Dyck P, Norell J, Tritschler H, Schuette K, Samigullin R, Ziegler D, et al. Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care. 2007;30(10):2619–2625. doi: 10.2337/dc06-2479. [DOI] [PubMed] [Google Scholar]
- 47.Sing R, Kishore L, Kaur N. Diabetic peripheral neuropathy: current perspective and future directions. Pharmacolog Res. 2014;80:21–35. doi: 10.1016/j.phrs.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Boulton A, Kempler P, Ametov A, Ziegler D. Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev. 2013;29:327–333. doi: 10.1002/dmrr.2397. [DOI] [PubMed] [Google Scholar]
- 49.Bansal D, Badhan Y, Gudala K, Schifano F. Ruboxistaurin for the treatment of diabetic peripheral neuropathy: a systematic review of randomized clinical trials. Diabetes Metab J. 2013;37(5):375–384. doi: 10.4093/dmj.2013.37.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh KA, Kumar A, Karmakar D, Jha KR. Association of B12 deficiency and clinical neuropathy with metformin use in type 2 diabetes patients. J Postgrad Med. 2013;59(4):253–257. doi: 10.4103/0022-3859.123143. [DOI] [PubMed] [Google Scholar]
- 51.Tahrani AA, Aswith T, Stevens MJ. Emerging drugs for diabetic neuropathy. Expert Opin Emerg Drugs. 2010;15(4):661–683. doi: 10.1517/14728214.2010.512610. [DOI] [PubMed] [Google Scholar]
- 52.Javed S, Petropoulos NI, Alam U, Malik AR. Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis. 2015;6(1):15–28. doi: 10.1177/2040622314552071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavakoli M, Mojaddidi M, Fadavi H, Malik R. Pathophysiology and treatment of painful diabetic neuropathy. Curr Pain Headache Rep. 2008;12(3):192–197. doi: 10.1007/s11916-008-0034-1. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein D, Lu Y, Detke M, Lee T, Iyengar S. Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1–2):109–118. doi: 10.1016/j.pain.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 55.Lunn M, Hughes R, Wiffen P. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rowbotham M, Goli V, Kunz N, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697–706. doi: 10.1016/j.pain.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 57.Rull J, Quibrera R, Gonzalez-Millan H, Lozano Castaneda O. Symptomatic treatment of peripheral diabetic neuropathy with carbamazepine (Tegretol): double blind crossover trial. Diabetologia. 1969;5(4):215–218. doi: 10.1007/BF01212087. [DOI] [PubMed] [Google Scholar]
- 58.Wilton T. Tegretol in the treatment of diabetic neuropathy. S Afr Med J. 1974;48(20):869–872. [PubMed] [Google Scholar]
- 59.Mellegers M, Furlan A, Mailis A. Gabapentin for neuropathic pain: systematic review of controlled and uncontrolled literature. Clin J Pain. 2001;17(4):284–295. doi: 10.1097/00002508-200112000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Rudroju N, Bansal D, Talakokkula S, Gudala K, Hota D, Bhansali A, et al. Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician. 2013;16:E705–E714. [PubMed] [Google Scholar]
- 61.Raskin P, Donofrio DP, Rosenthal RN, et al. Topiramate vs placebo in painful diabetic neuropathy: analgesic and metabolic effects. Neurology. 2004;63(5):865–873. doi: 10.1212/01.wnl.0000137341.89781.14. [DOI] [PubMed] [Google Scholar]
- 62.Thienel U, Neto W, et al. Topiramate in painful diabetic polyneuropathy: findings from three double-blind placebo-controlled trials. Acta Neurol Scand. 2004;110:221–231. doi: 10.1111/j.1600-0404.2004.00338.x. [DOI] [PubMed] [Google Scholar]
- 63.Zhou YH, Chen RS, Pan LH. Targeting N-methyl-D-aspartate receptors for treatment of neuropathic pain. Expert Rev Clin Pharmacol. 2011;4(3):379–388. doi: 10.1586/ecp.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson KA, Park KM, Robinovitz E, Tsigos C, Max MB. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48:1212–1218. doi: 10.1212/wnl.48.5.1212. [DOI] [PubMed] [Google Scholar]
- 65.Huge V, Lauchart M, Magerl W, Schelling G, Beyer A, Thieme D, Azad CS. Effects of low-dose intranasal (S)-ketamine in patients with neuropathic pain. Eur J Pain. 2010;14(4):387–394. doi: 10.1016/j.ejpain.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Heydari M, Shams M, Hashempur MH, Zargaran A, Dalfardi B, Borhani-Haghighi A. The origin of the concept of neuropathic pain in early Medieval Persia (9TH-12TH CENTURY CE) Acta Med Hist Adriat. 2015;13(Supl. 2):9–22. [PubMed] [Google Scholar]
- 67.Choudhurya H, Pandeya M, Huaa CK, Muna CS, Jinga JK, Konga L, Erna LY, Ashraf NA, Kit SW, Yee TS, Pichika MR, Gorain B, Kesharwani P. An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J Tradit Complement Med. 2018;8(3):361–376. doi: 10.1016/j.jtcme.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diabeta plus – maintain good blood sugar level – natural herbal supplements. AyurvedicCure.com.
- 69.Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TP. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40(3):163–173. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dasgupta A. Chapter 4 - antiinflammatory herbal supplements. Translational inflammation. Perspectives in translational cell biology. 2019, pp. 69–91. 10.1016/B978-0-12-813832-8.00004-2.
- 71.Krup V, Hedge Prakash L, Harini A. Pharmacological activities of turmeric (Curcuma longalinn): a review. J Homeop Ayurv Med. 2013;2(4):1000133. doi: 10.4172/2167-1206.1000133. [DOI] [Google Scholar]
- 72.Ahmad RS, et al. Biochemistry, safety, pharmacological activities, and clinical applications of turmeric: a mechanistic review. Evid Based Complement Alternat Med. 2020;2020:7656919. doi: 10.1155/2020/7656919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seo KI, Choi MS, Jung UJ, et al. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. 2008;52:995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 74.Jang EM, Choi MS, Jung UJ, et al. Beneficial effect of curcumin on hyperlipidaemia and insulin resistance in high-fat-fed hamsters. Metabolism. 2008;57:1576–1583. doi: 10.1016/j.metabol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 75.Sharma S, Kulkarni SK, Agrewala JN, Chopra K. Curcumin attenuates thermal hyperalgesia in a diabetic mouse model of neuropathic pain. Eur J of Pharmacol. 2006;536(3):256–261. doi: 10.1016/j.ejphar.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 76.Kulkarni SK, Dhir A. An overview of curcumin in neurological disorders. Indian J Pharm Sci. 2010;72(2):149–154. doi: 10.4103/0250-474X.65012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.White MC, Pasupuleti V, Roman MY, Li Y, Hernandez VA. Oral turmeric/curcumin effects on inflammatory markers in chronic inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2019;146:104280. doi: 10.1016/j.phrs.2019.104280. [DOI] [PubMed] [Google Scholar]
- 78.Adab Z, et al. Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytother Res. 2019;33(4):1173–1181. doi: 10.1002/ptr.6312. [DOI] [PubMed] [Google Scholar]
- 79.Poolsup N, Suksomboon N, Kurnianta P, Deawjaroen K. Effects of curcumin on glycemic control and lipid profile in prediabetes and type 2 diabetes mellitus: a systematic review and meta-analysis. PLoS ONE. 2019;14:e0215840. doi: 10.1371/journal.pone.0215840. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Singletary K. Turmeric: potential health benefits. Nutr Today. 2020;55(1):45–56. doi: 10.1097/NT.0000000000000392. [DOI] [Google Scholar]
- 81.Sasaki K, Wada K, Haga M. Chemistry and biological activities of Ginkgo Biloba. Stud Nat Prod Chem. 2003; 28(Part I): 165–198. 10.1016/S1572-5995(03)80141-2.
- 82.Numan A, Masud F, Khawaja IK, Khan FF, Qureshi BA, Burney S, Ashraf K, Ahmad N, Yousaf SM, Rabbani I, Zaneb H, Rehman H. Clinical and electrophysiological efficacy of leaf extract of Gingko biloba L (Ginkgoaceae) in subjects with diabetic sensorimotor polyneuropathy. Trop J Pharm Res. 2016;15(10):2137–2145. doi: 10.4314/tjpr.v15i10.12. [DOI] [Google Scholar]
- 83.Sebbagh N, Cruciani-Guglielmacci C, Ouali F, Berthault MF, Rouch C, Sari DC, et al. Comparative effects of Citrullus colocynthis, sunflower and olive oil-enriched diet in streptozotocin-induced diabetes in rats. Diabetes Metab. 2009;35(3):178–184. doi: 10.1016/j.diabet.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 84.Al-Snafi EA. Chemical constituents and phar macological effects of Citrullus. IOSR J Pharm. 2016;6(3):57–67. [Google Scholar]
- 85.Bansal D, Badhan Y, Gudala K, Schifano F. Ruboxistaurin for the treatment of diabetic peripheral neuropathy: a systematic review of randomized clinical trials. Diabetes Metab J. 2013;37(5):375–384. doi: 10.4093/dmj.2013.37.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oryan A, Hashemnia M, Hamidi RA, Mohammadalipour A. Effects of hydro-ethanol extract of Citrullus colocynthis on blood glucose levels and pathology of organs in alloxan-induced diabetic rats. Asian Pac J Trop Dis. 2014;4(2):125–130. doi: 10.1016/S2222-1808(14)60328-5. [DOI] [Google Scholar]
- 87.Mojtaba H, Kaynoosh H, Hashem MH, Mesbah S. Topical Citrullus colocynthis (bitter apple) extract oil in painful diabetic neuropathy: a double-blind randomized placebo-controlled clinical trial†. J Diabetes. 2016;8(2):246–252. doi: 10.1111/1753-0407.12287. [DOI] [PubMed] [Google Scholar]
- 88.Cohen MM. Tulsi - Ocimum sanctum: A herb for all reasons. J Ayurveda Integr Med. 2014;5(4):251–259. doi: 10.4103/0975-9476.146554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Upadhyay KR. Tulsi: a holy plant with high medicinal and therapeutic value. Int J Green Pharm. 2017;11(1):S1–S12. [Google Scholar]
- 90.Singh N, Hoette Y, Miller R. Tulsi: the mother medicine of nature. 2. Lucknow: International Institute of Herbal Medicine; 2010. pp. 28–47. [Google Scholar]
- 91.Pandey G, Sharma M. Pharmacological activities of Ocimum sanctum (Tulsi): a review. Int J Pharm Sci Rev Res. 2010;5(1):61–66. [Google Scholar]
- 92.Kaur G, Bali A, Singh N, Jaggi SA. Ameliorative potential of Ocimum sanctum in chronic constriction injury-induced neuropathic pain in rats. An Acad Bras Cienc. 2015;87(1):417–29. doi: 10.1590/0001-3765201520130008. [DOI] [PubMed] [Google Scholar]
- 93.Kumar S, Manjunath, Prem Kumar N. Preventive and curative effect of METHANOLIC leaves extract of Ocimum sanctum with metformin in diabetes induced neuropathy in rats. 3rd World Congress on Diabetes & Metabolism; 2012.
- 94.Obolskiy D, Pischel I, Feistel B, Glotov N, Heinrich M. Artemisia dracunculus L. (tarragon): a critical review of its traditional use, chemical composition, pharmacology and safety. J Agric Food Chem. 2011;59(21):11367–11384. doi: 10.1021/jf202277w. [DOI] [PubMed] [Google Scholar]
- 95.Watcho P, Stavniichuk R, Ribnicky DM, Ilya R, Obrosova IG. High-fat diet induced neuropathy of prediabetes and obesity: effect of pmi-5011, an ethanolic extract of Artemiesiadracunculus L. Mediators Inflamm. 2010;2010:268547. doi: 10.1155/2010/268547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Watcho P, Stavniichuk R, Tane P, Shevalye H, Maksimchyk Y, Pacher P, Obrosova IG. Evaluation of PMI-5011, an ethanolic extract of Artemisia dracunculus L., on peripheral neuropathy in streptozotocin-diabetic mice. Int J Mol Med. 2011;27(3):299–307. doi: 10.3892/ijmm.2011.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kanter M. Effects of Nigella sativa and its major constituent 21. thymoquinone on sciatic nerves in experimental diabetic neuropath. Neurochem Res. 2008;33:87–96. doi: 10.1007/s11064-007-9419-5. [DOI] [PubMed] [Google Scholar]
- 98.Kaur S, Pandhi P, Dutta P. Painful diabetic neuropathy: an update. Ann Neurosci. 2011;18(4):168–175. doi: 10.5214/ans.0972-7531.1118409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salehi B, Ata A, V Anil Kumar N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, Abdulmajid Ayatollahi S, Valere Tsouh Fokou P, Kobarfard F, Amiruddin Zakaria Z, Iriti M. Antidiabetic potential of medicinal plants and their active components. Biomolecules. 2019;9(10):551. [DOI] [PMC free article] [PubMed]
- 100.Karimi A, Majlesi M, Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol. 2015;4(1):27–30. [PMC free article] [PubMed] [Google Scholar]
- 101.Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res Int. 2015;2015:515042. doi: 10.1155/2015/515042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choudhury H, Pandey M, Hua CK, Mun CS, Jing JK, Kong L, Ern LY, Ashraf NA, Kit SW, Yee TS, Pichika MR. An update on natural compounds in the remedy of diabetes mellitus: a systematic review. J Tradit Complement Med. 2018;8(3):361–376. doi: 10.1016/j.jtcme.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen LS, Yu H, Luo MH, Wu Q, Li FC, Steinmetz A. Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin Med. 2016;11:37. doi: 10.1186/s13020-016-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eldin BIA. Quality control of herbal medicines and related areas. 2011 Edn: Rijeka: InTechJanezaTrdine; 2011.
- 105.AltCareDex® System [intranet database]. Version 5.1. Greenwood Village: Thomson Healthcare.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.