Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer‐related mortality worldwide. Early detection of HCC enables patients to avail curative therapies that can improve patient survival. Current international guidelines advocate for the enrollment of patients at high risk for HCC, like those with cirrhosis, in surveillance programs that perform ultrasound every 6 months. In recent years, many studies have further characterized the utility of established screening strategies and have introduced new promising tools for HCC surveillance. In this review, we provide an overview of the most promising new imaging modalities and biomarkers for the detection of HCC. We discuss the role of imaging tools like ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) in the early detection of HCC, and describe recent innovations which can potentially enhance their applicability, including contrast enhanced ultrasound, low‐dose CT scans, and abbreviated MRI. Next, we outline the data supporting the use of three circulating biomarkers (i.e., alpha‐fetoprotein [AFP], AFP lens culinaris agglutinin‐reactive fraction, and des‐gamma‐carboxy prothrombin) in HCC surveillance, and expand on multiple emerging liquid biopsy biomarkers, including methylated cell‐free DNA (cfDNA), cfDNA mutations, extracellular vesicles, and circulating tumor cells. These promising new imaging modalities and biomarkers have the potential to improve early detection, and thus improve survival, in patients with HCC.
HCC is a challenging disease to detect in the early stages due to poor adherence to screening guidelines, and more importantly, a paucity of highly sensitive and specific screening tools. Recent advances in ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) techniques have improved upon the sensitivity and specificity for detection, but they are in large part hindered by cost, safety and availability. The future of HCC surveillance likely lies in circulating liquid biopsy biomarkers which have the potential to be cost‐effectively and non‐invasively implemented in populations worldwide.

Abbreviations
- AASLD
Association for the Study of Liver Diseases
- AFP
alpha‐fetoprotein
- AFP‐L3
AFP lens culinaris agglutinin‐reactive fraction
- aMRI
abbreviated MRI
- AUC
area under the curve
- cfDNA
cell‐free DNA
- CI
confidence interval
- CT
computed tomography
- CTC
circulating tumor cell
- ctDNA
circulating tumor DNA
- DCP
des‐gamma‐carboxy prothrombin
- EV
extracellular vesicle
- FDA
Food and Drug Administration
- GPC3
glypican‐3
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- LDCT
low‐dose CT
- LI‐RADS
Liver Imaging Reporting and Data System
- MDM
methylated DNA marker
- MRI
magnetic resonance imaging
- miRNA
microRNA
- ncRNA
noncoding RNA
- RCT
randomized controlled trial
- TP53
tumor protein P53
- US
ultrasound
Liver cancer is the sixth most common cancer worldwide and is now the third‐leading cause of cancer‐related death, behind lung cancer and colorectal cancer.( 1 ) In 2020, more than 900,000 cases of liver cancer were diagnosed globally, with more than 830,000 liver cancer–related deaths, underscoring the high mortality index of this cancer.( 1 ) Hepatocellular carcinoma (HCC) accounts for 75%‐85% of primary liver cancers. The global incidence of HCC has increased by more than 75% in the last 30 years, especially in Western countries,( 2 , 3 , 4 ) and is expected to continue to grow in the near future. Unfortunately, survival rates for patients with HCC in the United States remain dismally low and essentially unchanged over the past 30 years,( 5 ) with only 3%‐34% of patients with HCC surviving 5 years after diagnosis.( 6 ) The high HCC mortality rates can be attributed to several factors, with delayed diagnosis of cancer at more advanced stages of disease being an important reason.( 2 , 7 , 8 )
HCC typically arises in the background of the cirrhotic liver, where chronic inflammation and fibrosis induce genomic alterations that render the hepatocytes vulnerable to malignant transformation.( 9 , 10 , 11 ) The presence of cirrhosis is the strongest risk factor for HCC, with 90% of HCCs arising in cirrhotic livers. The common chronic liver diseases with increased risk for HCC include hepatitis B (HBV), hepatitis C (HCV), alcohol‐associated liver disease (ALD), or nonalcoholic steatohepatitis (NASH). Early detection of HCC enables patients to avail curative therapies like resection or liver transplantation, leading to an improvement in overall survival.( 12 , 13 , 14 , 15 ) In this review, we discuss the rationale behind current surveillance guidelines and highlight promising imaging and biomarker tools for the early detection of HCC in patients with cirrhosis.
What Are the Benefits and Risks of HCC Surveillance?
The goal of an effective cancer surveillance program is to decrease cancer‐related mortality. In a randomized controlled trial (RCT) in 2004, Zhang et al. showed that biannual alpha‐fetoprotein (AFP) serum measurements and abdominal ultrasounds in more than 9,000 randomized HBV‐infected patients led to a higher frequency of HCC detection at early stages (60.5% vs. 0%), and a 37% reduction in HCC‐related mortality compared with an unscreened control group.( 16 ) Although these results provide level I evidence for the effectiveness of HCC surveillance, there were concerns about both the randomization strategy and the lack of adherence to intention‐to‐treat principles in this trial. More importantly, the benefit of this surveillance strategy for the detection of non‐HBV HCCs and HCCs arising in cirrhotic livers remains unclear. Unfortunately, additional attempts to replicate these RCTs are hindered by feasibility challenges and ethical concerns associated with randomizing patients to a control group. Thus, later RCT studies mostly aimed instead to optimize the frequency or imaging method of surveillance.( 17 , 18 , 19 ) Several meta‐analyses and observational studies have shown higher rates of HCC detection at early stages and improved 3‐year survival rates in those patients who were actively under surveillance compared with those who were not.( 14 , 20 , 21 , 22 ) Furthermore, a large study reported that even a single mass ultrasound (US) screening of 11,114 high‐risk individuals in Taiwan was associated with a 31% reduction in HCC‐related mortality.( 23 ) Overall, the survival benefits and cost effectiveness of HCC surveillance are comparable with that of other cancer surveillance programs,( 24 , 25 , 26 ) thus providing staying power for the current HCC surveillance program.
Surveillance programs are not without risk (Fig. 1). For HCC, US or AFP measurements themselves may be safe procedures with minimal inherent risk. However, follow‐up biopsies and computed tomography (CT)/magnetic resonance imaging (MRI) scans have their own risks, and remaining on surveillance can be associated with significant financial and psychological harm. In a study of a large cohort of patients with cirrhosis at a safety‐net system with optimal surveillance compliance, over one‐fourth of the patients experienced surveillance‐related “harm” due to repeated cross‐sectional imaging or invasive evaluation, to further investigate indeterminate nodules found on US.( 27 ) Similar results have been recently demonstrated from another structured US‐based HCC surveillance program at an academic tertiary care center.( 28 ) Hence, there remains a need to improve upon HCC surveillance to better estimate its impact on disease‐related mortality, and also quantify its risks.
FIG. 1.
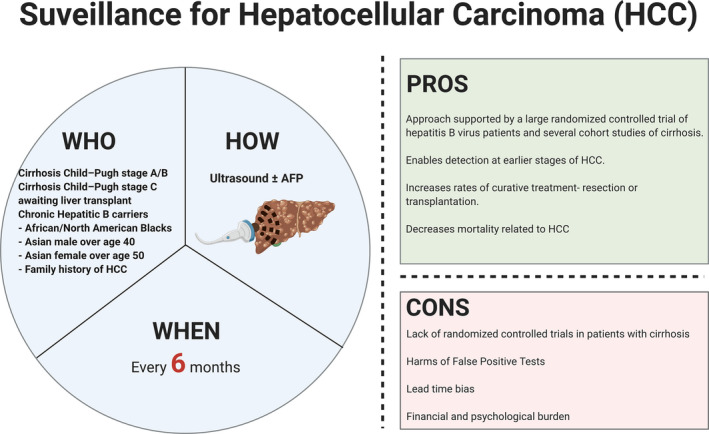
Overview of HCC surveillance. This schematic detail at‐risk populations that would most benefit from HCC surveillance, in addition to the modality and recommended frequency of surveillance. At‐risk populations are identified based on demographic and clinicopathologic factors that determine the yearly risk of HCC. Currently, according to AASLD guidelines, at‐risk individuals should be screened by US, with or without AFP, every 6 months. The pros and cons of HCC surveillance are also highlighted.
Which High‐Risk Group of Patients Should Undergo HCC Surveillance?
Ideally, HCC surveillance should be offered to patients in whom early detection of HCC can facilitate the receipt of curative therapies. The Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) have recommended that surveillance with US, with or without complementary AFP testing, be performed biannually (with an optimum range of 4‐8 months) for any patient with risk for HCC above 1.5% per year.( 29 , 30 ) HCC incidence rates vary based on multiple factors, including etiology of liver disease, and range from 0.2% to 8% per year.( 29 , 30 ) These recommendations are largely based on the cost effectiveness of these specific methods, with data suggesting that surveillance for an HCC risk of 1.5%/year results in a 3‐month increase in survival, roughly approximating the 100‐day longevity target above which an intervention is considered effective.( 31 , 32 , 33 ) Moreover, the biannual frequency of surveillance is based on an estimated tumor doubling time of 120 days for untreated HCC, although this surveillance frequency also benefits patients with a less‐conservative doubling time of 60 days.( 34 , 35 )
HCC surveillance is recommended for all patients with cirrhosis secondary to either viral hepatitis (hepatitis B or hepatitis C) or nonviral causes (e.g., ALD, nonalcoholic fatty liver disease [NAFLD], hemochromatosis)( 29 ) (Fig. 1). Special groups of patients with noncirrhotic hepatitis B (Asian males ≥ 40 years, Asian females ≥ 50 years, African persons ≥ 20 years, and those with family history of HCC) are also recommended to undergo HCC surveillance (Fig. 1). The role for HCC surveillance in patients with noncirrhotic NAFLD or patients with F3 fibrosis after HCV viral eradication is not clear, and current guidelines do not recommend routine surveillance in these patients. The use of validated multivariable HCC risk calculators is a promising strategy to estimate HCC risk, and can hopefully be used to personalize decision making in these patients.( 36 )
The necessity for HCC surveillance is determined not only by the individual risk for HCC but also other important factors like age, comorbidities, performance status, liver function, ability to receive curative therapies, and patient willingness. For instance, surveillance is not recommended for those with Child‐Turcotte‐Pugh class C cirrhosis who are not on the transplant waiting list, as liver‐related mortality is already high with this degree of liver dysfunction, and these patients are generally not candidates for other forms of HCC therapy, thus making early detection of HCC unlikely to offer a survival benefit.
What Are the Current and Emerging Imaging Tools for HCC Surveillance?
Ultrasound
US imaging is recommended as the main modality for HCC surveillance by many international guidelines and is the definite workhorse for screening patients with cirrhosis.( 29 , 30 , 37 , 38 , 39 ) Overall, the benefits of US as an HCC screening modality are many: It is cost‐effective, widely available, and well‐tolerated. Additionally, there is a strong body of evidence supporting the use of the US for HCC surveillance.( 40 , 41 ) A systematic review of 14 US studies showed that the pooled estimates of sensitivity of US for HCC detection was 60% (95% confidence interval [CI] 44‐76), while the specificity was 97% (95% CI 95‐98).( 40 ) In another large meta‐analysis of 32 studies comprising 13,367 patients, US was shown to have an overall sensitivity of 84% (95% CI 76%‐92%) for HCC of any stage and a sensitivity of 47% (95% CI 33%‐61%) for early‐stage HCC.( 41 ) Real‐world data from a retrospective cohort multicenter study of 374 patients found that 42% of the HCCs had been diagnosed on screening, and US detection was associated with a higher rate of early tumor detection (63.1 vs. 36.4%; P < 0.001), a higher rate of curative treatment (31% vs. 13%; P = 0.02), and improved survival (hazard ratio 0.41; 95% CI 0.26‐0.65), compared with HCC not detected by screening.( 42 ) Collectively, these studies show that US remains an effective tool for HCC screening.
One of the main shortcomings of US in the detection of HCC is the heterogeneity in its interpretation, reporting, and management recommendations across health care sites. This issue was partially addressed by the adoption of the US Liver Imaging Reporting and Data System (LI‐RADS; American College of Radiology, Reston, VA) criteria, introduced in 2011 by the American College of Radiology.( 43 ) The LI‐RADS detection score designations (US‐1, obviously benign observations or no observations; US‐2, non‐definitely benign observations <10 mm in diameter; and US‐3, non‐definitely benign observations ≥10 mm or new portal vein thrombosis) offer guidance on the need for additional imaging or more frequent surveillance, whereas the visualization score provides granularity on the imaging limitations (e.g., body habitus, bowel gas) that may change the sensitivity of detection of focal lesions. Although there are currently no recommendations for management based on LI‐RADS visualization scores, early multicenter studies have validated the overall approach of the LI‐RADS criteria,( 44 , 45 , 46 ) and prospective studies are ongoing.
There are a few other notable shortcomings of US as a screening tool for HCC. It performs relatively poorly in the detection of early lesions, with a pooled sensitivity of 45% in a meta‐analysis of 32 studies( 41 , 47 ) and 63% in another meta‐analysis of 13 studies( 22 ) for the early detection of HCC. Another challenge is the poor sensitivity of US in distinguishing early HCCs from dysplastic nodules. It appears that contrast‐enhanced US may be able to aid in this problem by providing information about vascularity, arterial hyperenhancement patterns, and contrast distribution patterns in these lesions, in addition to improving the overall sensitivity of the assay.( 48 , 49 , 50 , 51 , 52 ) Finally, US exams do suffer from uneven quality and interobserver variability, with a blinded review of 941 USs showing that up to 20% of the studies were of inadequate quality for surveillance.( 53 ) Thus, US does suffer from several limitations as a screening tool, but additional improvements in US technology, standardization of reporting, and selective use of contrast agents can help address its current limitations.
MRI and CT
The limitations of US imaging can theoretically be addressed by the technical superiority of MRI and CT, which have both been shown to have a high sensitivity and specificity for the diagnosis of HCC.( 54 , 55 , 56 , 57 ) As a diagnostic tool, MRI has a sensitivity and specificity over 90% for HCC lesions greater than 2 cm in diameter, and its per‐patient sensitivity and specificity for all size lesions is still relatively high (88% and 94%, respectively).( 58 , 59 ) Here again, the likelihood of a lesion being designated as an HCC is determined by LI‐RADS criteria specific to MRI or CT. HCC lesions should have one or more of these radiological features for diagnosis: arterial phase hyperenhancement (sensitivity 65%‐98%),( 60 ) washout (sensitivity 50%‐79%), and an encompassing pseudocapsule (sensitivity 42%‐64%).( 60 ) However, the number of features required for diagnosis depends on the lesion size, non‐rim arterial phase hyperenhancement, and other ancillary features.( 61 ) A meta‐analysis of 17 studies involving 2,760 patients confirmed the usefulness of the LI‐RADS classification; it showed that the percentage of HCCs was higher in lesions of higher LI‐RADS category.( 55 )
Despite this excellent diagnostic capability, MRI is currently not a first‐line screening modality for HCC. Its general tolerability, high safety profile, and diagnostic superiority do not make up for its restricted availability, potential contraindications, and suboptimal cost‐effectiveness. Furthermore, MRI has low specificity for the detection of HCC nodules in the 10‐20‐mm range.( 62 ) MRI likely is a great first‐line screening tool in a selected subset of patients. For instance, MRI does appear to be cost‐effective in patients at high or intermediate risk for HCC, particularly those with viral hepatitis–induced compensated cirrhosis.( 26 , 63 , 64 ) MRI is, however, universally recommended as a second‐line modality for patients with a positive screening test.( 29 , 30 , 37 , 38 , 39 ) Recently, several abbreviated MRI (aMRI) protocols have been developed for HCC screening to overcome the barrier created by traditionally long imaging times. These aMRIs use specific sequences that maintain MRI diagnostic accuracy and include noncontrast protocols with T2 and diffusion‐weighted imaging, or dynamic protocols with either extracellular gadolinium contrast agent or gadoxetate‐enhanced T1 and T2‐weighted imaging.( 64 , 65 , 66 , 67 ) A recent comparison of aMRI with complete MRI in 86 patients with cirrhosis found comparable sensitivities and specificities between the two (0.92 vs. 0.94 and 0.87 vs. 0.87, respectively) in the detection of HCC.( 66 ) Another study with 330 patients who underwent aMRI revealed a sensitivity and specificity of 0.92 and 0.91, respectively,( 68 ) whereas a smaller study of 19 patients revealed 90% sensitivity and 89% specificity for the detection of early‐stage HCCs.( 65 ) Overall, with its shorter examination times of 5‐15 minutes and its ability to detect and concurrently characterize HCC lesions, aMRI may gain acceptance as a suitable alternative to US for HCC screening in the near future, especially in transplant centers.
Multiphase CT is the other second‐line imaging modality for HCC screening. Even though its diagnostic accuracy is lower than that of extracellular contrast‐enhanced MRI, it remains a viable alternative for HCC diagnosis because of its lower cost, faster imaging times, and lower technical complexity.( 58 , 69 ) Studies have also assessed the utility of CT as a first‐line imaging modality.( 19 , 62 , 70 ) In an RCT of 163 patients, multiphase CT was shown to have a sensitivity similar to that of US (66.% vs. 71.4%, respectively) for the detection of HCC in patients with cirrhosis.( 19 ) However, as expected, CT was less cost‐effective than US. Furthermore, the sensitivity (62.5%) and specificity (76.5%) of CT for the early‐stage detection of HCC remains low,( 19 , 62 ) which, despite still being comparable to US, makes CT a less‐palatable surveillance tool because of the additional risk of radiation exposure and contrast‐induced nephrotoxicity. Low‐dose CT scans (LDCTs), however, aim for a targeted reduction of 30% in both radiation and contrast media. A recent study compared the sensitivities of biannual US and two‐phase LDCT in patients with a >5% risk (≥2.33 risk index) of developing HCC.( 71 ) In 27 of 137 high‐risk patients who received both biannual US and 1‐3 LDCTs per year, LDCTs showed significantly higher sensitivity (83.3% vs. 29.2%) and specificity (95.6% vs. 87.7%), as well as lower false‐positive rates for the detection of HCC. Finally, when the cost‐effectiveness of seven strategies for HCC surveillance was compared, surveillance and diagnosis with CT (with MRI follow‐up for inadequate surveillance) were determined to be the most cost‐effective strategies when optimal compliance was assumed.( 72 ) When conservative compliance was assumed, surveillance with aMRI with CT follow‐up for inadequate diagnosis was the most cost‐effective strategy. Thus, both MRI and CT have the potential to replace US as the primary screening tool, especially in high‐risk patients, with further innovations and improvements in techniques, access, and cost. In Fig. 2 we have summarized the results from meta‐analyses that have reported the sensitivities of the various imaging modalities for detection of HCC.( 22 , 40 , 41 , 52 , 57 , 58 , 69 , 70 )
FIG. 2.
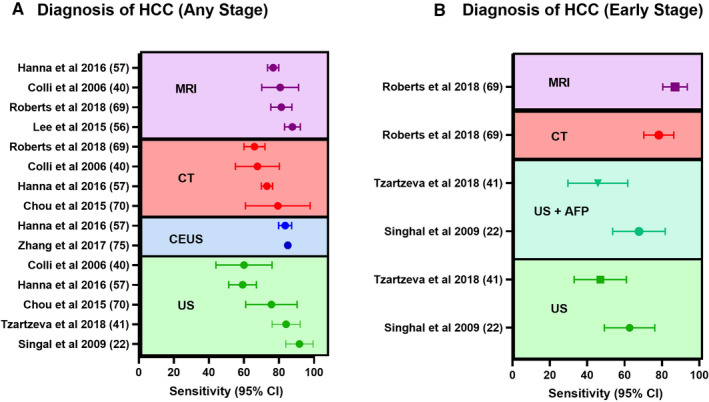
Sensitivity of imaging tools for the HCC surveillance. Forest plots comparing meta‐analyses that describe the sensitivity of the three imaging modalities (US, CT, and MRI) in the detection of any stage (A) and early‐stage (B) HCC. Abbreviation: CEUS, contrast‐enhanced ultrasound.
What Are the Current and Emerging Circulating Biomarkers for HCC Surveillance?
Alpha‐Fetoprotein
AFP, a member of the albumin family of proteins, is the main serological marker used in the detection of HCC.( 73 ) Elevated AFP can be detected in 60%‐80% of HCCs when using 20 ng/mL plasma concentration as a pathological threshold for diagnosis.( 74 ) In a recent meta‐analysis of 59 studies, AFP thresholds of 400 ng/mL, 200 ng/mL, and 20 ng/mL were associated with pooled sensitivities of 32%, 49% and 61%, respectively.( 75 ) Several studies have suggested that these metrics can be improved with longitudinal AFP testing and cirrhosis etiology‐based changes in the AFP threshold.( 76 , 77 , 78 , 79 , 80 , 81 ) Importantly, AFP measurements are usually performed in conjunction with US imaging, and a meta‐analysis confirmed that the US alone has lower sensitivity rates for any‐stage HCC (relative risk [RR] 0.88; 95% CI 0.83‐0.93) and early‐stage HCC (RR 0.81; 95% CI 0.71‐0.93) compared to US with AFP.( 41 )
Despite its diagnostic utility in HCC, AFP measurements are not currently recommended by the AASLD or EASL as a solitary screening test for HCC due to several limitations. First, AFP is expressed in other cancers and conditions, such as chronic hepatitis, liver cirrhosis, neurodegenerative diseases, and non‐seminomatous testicular cancer.( 82 , 83 ) Additionally, it has a relatively inadequate sensitivity and specificity for surveillance, low positive predictive value, and inadequate diagnostic accuracy for early‐stage HCCs.( 84 ) Despite these limitations, AFP continues to be the most widely used biomarker for HCC, given its low cost, wide availability, easy measurement, and a strong body of work showing its association with HCC. However, AFP should not be used in isolation as a tool for HCC surveillance.
AFP‐L3
As mentioned previously, the specificity of AFP is relatively low, as it can be elevated in non‐HCC malignancies and other chronic inflammatory conditions of the liver. This limitation has partially been overcome by the identification of the biomarker AFP‐L3, lens culinaris agglutinin‐reactive fraction of AFP, which was shown to be more specifically associated with HCC and to have a superior diagnostic efficacy when combined with AFP measurements.( 80 , 85 , 86 ) Furthermore, a study by Kumada et al. revealed elevated AFP‐L3 levels to be predictive of early HCC even in the absence of elevated AFP.( 87 ) However, despite having a higher specificity than AFP, AFP‐L3 measurements still suffer from poor sensitivity.( 88 ) AFP‐L3 is currently approved by the Food and Drug Administration FDA for the assessment of liver cancer risk as a part of the GALAD score.( 89 )
Des‐Gamma‐Carboxy Prothrombin
Des‐gamma‐carboxy prothrombin (DCP), or protein‐induced by vitamin K absence‐II, is another serum biomarker for HCC. It is a prothrombin precursor that has differentially undergone abnormal post‐translational carboxylation in malignant cells. DCP has been found to be elevated in patients with HCC. The body of evidence supporting DCP as a potential biomarker for HCC continues to grow. Not only do DCP levels correlate with HCC stage and survival, but DCP may be more sensitive than AFP for the detection of HCC.( 85 , 90 , 91 , 92 , 93 ) In a recent study of 90 patients with cirrhosis with US evidence of liver nodules, 40 were diagnosed to have HCC at very early/early stages. In these patients, DCP was found to be significantly associated with HCC at a cutoff level of 60 mAU/mL, whereas AFP at a cutoff level of 6.5 ng/mL was not.( 94 ) DCP was found to have a sensitivity of 60%, specificity of 80%, positive predictive value (PPV) of 80%, and negative predictive value of 73%, compared with 67%, 68%, 63% and 72%, respectively, for AFP.( 94 ) Importantly, in accordance with the findings of other published studies,( 95 , 96 , 97 ) the combination of both DCP and AFP improved the diagnostic accuracy of the assay, with a higher sensitivity (70%), specificity (94%), and area under the curve (AUC; 0.76). Some studies, however, have produced contradictory results suggesting that the addition of DCP to AFP does not improve the sensitivity of HCC detection.( 80 ) Given its potential promise, DCP is currently approved by the FDA for the determination of HCC risk, and commercial immunoassays like Roche’s Elecsys DCP (Basel, Switzerland) have been developed for quantitative measurement of the protein. However, the overall lack of consensus on the use of DCP as a biomarker has resulted in variations in international recommendations regarding its use. Japan, for instance, recommends using both DCP and AFP for HCC surveillance, whereas DCP use is not explicitly recommended for surveillance and diagnosis by the AASLD.( 29 , 38 )
Heterogeneity in biomarker testing frequency and cutoff levels have made it difficult to compare them against each other. We have summarized the sensitivity and specificity of AFP, AFP‐L3, and DCP reported in various meta‐analyses in Table 1.( 75 , 88 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 ) Moreover, several studies have suggested that a combination of biomarkers is likely the best tool for detection of HCC. In fact, in March 2020, the FDA approved breakthrough device designation for Roche’s Elecsys GALAD score,( 106 ) which noninvasively uses an algorithmic score that combines gender, age, AFP, AFP‐L3, and DCP biomarker levels to detect early HCC.( 107 ) In multiple international studies, the GALAD score has been shown to perform better than its individual components for the early detection of HCC, with area under the receiver operating characteristic curve (AUROC) crossing 0.9.( 108 , 109 , 110 , 111 )
TABLE 1.
Summary of Meta‐analyses Evaluating the Performance of Three Common Serum Biomarkers for the Diagnosis of HCC
| Biomarker | Meta‐analysis | Sensitivity | Specificity | AUC | Reference |
|---|---|---|---|---|---|
| AFP | 60 studies; 11,731 patients | 61% (60%‐62%) | 86% (86%‐87%) | 0.83 | Zhang et al.( 75 ) |
| 326 studies, 144,570 patients | 60% (58%‐62%) | 84% (82%‐86%) | ‐ | Colli et al.( 98 ) | |
| 20 studies; 12,906 patients | 89% (88%‐90%) | 82% (81%‐83%) | 0.75 | Chen et al.( 99 ) | |
| 16 studies; 4,573 patients | 59% (57%‐61%) | 83% (81%‐85%) | 0.73 | Chen et al.( 100 ) | |
| 12 studies; 2,426 patients | 64% (54%‐73%) | 96% (91%‐98%) | 0.88 | Sun et al.( 101 ) | |
| 11 studies; 1,838 patients | 54% (51%‐57%) | 83% (80%‐85%) | 0.79 | Xu et al.( 102 ) | |
| AFP‐L3 | 12 studies; 2,245 patients | 48% (46%‐51%) | 93% (92%‐94%) | 0.75 | Yi et al.( 88 ) |
| 16 studies; 4,573 patients | 56% (54%‐58%) | 90% (88%‐91%) | 0.84 | Chen et al.( 100 ) | |
| DCP | 20 studies; 5,911 patients | 67% (58%‐74%) | 92% (88%‐94%) | 0.89 | Gao et al.( 103 ) |
| 38 studies; 11,124 patients | 66% (65%‐68%) | 88% (87%‐90%) | 0.90 | De et al.( 104 ) | |
| 12 studies; 3,058 patients | 71% (68%–73%) | 84% (83%‐86%) | 0.89 | Zhu et al.( 105 ) | |
| 20 studies; 12,906 patients | 69% (68%‐70%) | 88% (87%‐89%) | 0.88 | Chen et al.( 99 ) |
Other Circulating Biomarkers for HCC
Glypican‐3 (GPC3), a heparan‐sulfate proteoglycan, is known to be elevated in HCC tissues. Although serum GPC3, both the full length and N‐terminal form, has repeatedly been shown to be elevated in patients with HCC,( 112 , 113 , 114 ) it does not appear to perform better than AFP in detecting early HCC.( 102 ) Golgi protein‐73 (GP73) has been reported to be a potential biomarker for early detection of HCC, with ranges of AUROC (0.79‐0.94), sensitivities (69%‐95%), and specificities (83.9%‐92.9%) rivaling those of AFP.( 115 , 116 ) However, the clinical utility of GP73 is limited by the lack of an FDA‐approved immunoassay that detects the specific glycosylated isoform associated with HCC. Other promising circulating biomarkers for the early diagnosis of HCC include osteopontin,( 101 , 117 , 118 , 119 ) midkine,( 120 , 121 , 122 , 123 , 124 ) Dickkopf‐1,( 125 , 126 ) squamous cell carcinoma antigen,( 127 , 128 ) and fibronectin.( 129 , 130 ) These biomarkers need further validation in large, multicenter studies that include patients with HCCs arising from different etiologies.
Circulating Noncoding RNA as Noninvasive Diagnostic Tools of HCC
Noncoding RNAs (ncRNAs), like microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), are regulatory molecules. Unlike messenger RNA, ncRNAs are relatively stable in circulation, making them well‐suited for quantitative assays. MiRNAs have shown to be promising biomarkers for the early detection of HCC in multiple early phase studies.( 131 , 132 , 133 ) Zhang et al. reported that a three‐miRNA panel (miR‐92‐3p, miR‐107, and miR‐3126‐5p) had an AUC of 0.98 for detecting early‐stage HCC and a promising AUC of 0.97 for detecting HCC in patients with low AFP levels.( 132 ) Yamamoto et al. recently reported promising diagnostic values for a circulating miRNA panel, with a high sensitivity (97.7%), specificity (94.7%), and AUC (0.99) in the detection of early HCC.( 133 ) Several lncRNAs, including highly up‐regulated in liver cancer,( 134 ) long intergenic non‐coding RNA 152,( 134 , 135 ) metastasis‐associated lung adenocarcinoma transcript 1( 134 , 135 , 136 ) and urothelial cancer associated 1,( 137 ) have also been shown to be promising biomarkers in diagnosing HCC. Variations in assays and nonstandardized nomenclature make it difficult to compare across studies. However, ncRNAs remain promising tools for the surveillance of HCC.
What Is the Role of Liquid Biopsy in HCC Surveillance?
Even though there have been significant improvements in HCC surveillance imaging modalities, the most exciting developments in this field lie in the diagnostic promise of “liquid biopsy” biomarkers. Liquid biopsy refers to the molecular analysis of tumor‐derived nucleic acids or extracellular vesicles (EVs) in the bloodstream. Here, we discuss advances in the use of methylation and mutation profiling of circulating tumor DNA (ctDNA) and EV profiling in the detection of HCC.
Methylation Profiling of ctDNA
Hypermethylated or hypomethylated cell‐free DNA (cfDNA) markers have been scrutinized as tools for early cancer detection because of growing evidence suggesting that epigenetic changes play an important role in carcinogenesis.( 138 , 139 ) More importantly, it is much more convenient to detect, amplify, and quantitate aberrant DNA hypermethylation of ctDNA compared with genomic mutations. Efforts to identify methylated DNA markers (MDMs) that predict the development of HCC have been years in the making.( 140 , 141 , 142 , 143 , 144 , 145 ) However, the technical improvements in the ability to isolate circulating cfDNA in recent years have led to significant progress in the development of methylated cfDNA as biomarkers for HCC.
In a large study, Xu et al. identified an HCC‐specific methylation panel that showed high correlation between methylation profiles of 401 markers in HCC tumors and matched plasma ctDNA samples.( 146 ) They went on to construct a highly sensitive and specific diagnostic prediction model using these profiles on 1,098 patients with HCC and 835 healthy controls.( 146 ) In a different study, Kiesel et al. used a reduced‐representation bisulfite method of cfDNA sequencing to identify 302 candidate MDMs with AUCs above 0.75, 16 of which were then tested in phase 1 and phase 2 plasma validation cohorts (21 HCC cases, 30 controls; and 95 HCC cases, 51 controls; respectively).( 147 ) Ultimately, a six‐marker MDM panel consisting of homeobox A1 (HOXA1), empty spiracles homeobox 1 (EMX1), AK055957, ECE1 (endothelin converting enzyme 1), PFKP (phosphofructokinase, platelet), and CLEC11A (C‐type lectin domain containing 11A) genes was shown to have a 95% sensitivity and a 92% specificity in the detection of HCC from plasma samples.( 147 ) In another recent multicenter case‐control study of 135 HCC cases and 302 controls, three MDMs (HOXA1, EMX1, and TSPYL5 [testis‐specific Y‐encoded‐like protein 5]) and two protein markers (AFP and AFP‐L3) with high sensitivities and specificities for the detection of early‐stage HCC were identified, with an AUC for the multitarget panel of 0.92 for HCC at any stage, regardless of liver disease etiology, presence of cirrhosis, or sex.( 148 ) Finally, a recent study in 80 healthy volunteers, 45 patients with chronic liver disease, and 136 patients with HCC revealed methylated SEPT9 to be a promising marker for HCC detection, with an assay sensitivity and specificity of 63.2% and 90.0% for the detection of HCC.( 149 ) Overall, given substantial improvements in the diagnostic capabilities of methylation markers in recent years, these markers will hopefully soon be widely adopted into clinical practice, if phase 3 trials confirm the promising results.
Mutation Profiling of cfDNA
Recent studies on comprehensive genomic profiling have enabled us to identify key driver mutations in common genes like tumor protein P53 (TP53), TERT promoter, and catenin beta 1 (CTNNB1) in HCCs.( 150 , 151 , 152 ) cfDNA are also likely to harbor these mutations, and their detection can serve as potential biomarkers for the early detection of HCC.( 153 , 154 ) Recently, Kaseb et al. evaluated blood samples from 206 patients with HCC using comprehensive genomic testing of cfDNA. They found that 88% of the patients had at least one detectable genetic alteration, with TP53, EGFR (epidermal growth factor receptor), MET, and ARID1A (AT‐rich interaction domain 1A) being some of the common ones.( 155 ) In another large Chinese study of over 10,000 patients with different kinds of cancer, ctDNA was detected in 77% of the 571 patients with HCC, with TP53 and TERT promoter mutations being common,( 153 ) further demonstrating the feasibility of this approach in the early detection of HCC. A study of 331 hepatitis B surface antigen–positive at‐risk patients recently showed that an assay designed to detect highly prevalent mutations in hepatitis B core antigen–HCC cfDNA (i.e., TP53, CTNNB1, AXIN1 [axin 1], or TERT promoter) positively identified four early‐stage HCC cases that were negative by AFP and US, resulting in a 100% sensitivity, 94% specificity, and 17% PPV for the assay.( 133 , 156 ) Finally, Wang et al. showed that genome scale profiling of cfDNA can be used to develop a diagnostic model (including genomic features such as nucleosome footprint, fragmentation, and base mismatch) to accurately differentiate HCCs from liver cirrhosis, with a sensitivity of 95.4%, specificity of 97.7%, and an AUC of 0.997.( 157 ) Early study results such as these highlight the exciting promise of cfDNA as noninvasive diagnostic tools for the early detection of HCC.
Extracellular Vesicles
EVs are lipid bilayer‐delimited particles that are secreted by cells that contain biologically active cargo like proteins, DNA, or miRNAs.( 158 ) They are classified based on their size and properties as either apoptotic bodies (50‐5,000 nm), microvesicles (100‐1,000 nm), or exosomes (30‐150 nm). There is an expanding understanding of the biological significance of EVs, and their potential to serve as biomarkers for HCC surveillance is being explored.( 159 ) A few studies have shown the promise of EVs as prognostic biomarkers.( 160 , 161 ) However, their role in the diagnosis of early‐stage HCC is not yet clear. Sun et al. used an EV purification technology to isolate EVs from 158 patients with HCC and showed that an HCC 10‐gene molecular signature had a high sensitivity of 94.4% and specificity of 88.5% in detecting early HCC.( 162 ) Even though small early studies have shown promise,( 163 , 164 ) further standardization of EV capture and quantification is needed.
Circulating Tumor Cells
There has been a longstanding interest in detecting circulating tumor cells (CTCs) to diagnose HCC.( 165 ) A few studies have shown the value of epithelial cell adhesion molecule (EpCAM)–positive CTCs in the diagnosis and prognosis of HCC.( 166 , 167 , 168 ) However, the detection of CTCs in HCC has a low sensitivity, as most HCCs do not express EpCAM and the number of CTCs in early‐stage tumors is low, making detection of early‐stage tumors challenging. CTCs positive for other surface markers like AFP, GPC3, DNA‐dependent protein kinase, vimentin, and TWIST1 (twist family BHLH transcription factor 1) have also been reported to be useful to diagnose HCC.( 166 , 169 , 170 ) A recent meta‐analysis of 20 studies including 1,191 patients showed that CTC testing has a sensitivity of 95% and a specificity of 60% for diagnosing HCC, but there was significant heterogeneity in the kind of assay used and in the clinical features of the study population.( 171 ) Thus, these challenges in assay standardization need to be overcome in additional large‐scale prospective validation studies before CTCs can be useful in the surveillance of HCC.
Future Directions
Several critical areas need urgent research to increase the rate of early detection of HCC and make an impact on patient outcomes. A few areas of unmet need for biomarker development for HCC include the need to (1) more precisely determine the risk for HCC in specific cohorts among patients with liver diseases, like those with hepatitis C following sustained virological response or NASH with F3 stage fibrosis; (2) collaboratively perform large‐scale validation of promising biomarkers in prospective phase 3 clinical trials; (3) standardize technology and optimize sample processing to maximize sensitivity and enable cross‐validation; and (4) use new technological advances to develop targeted contrast agents for imaging and noninvasive biomarkers for HCC. All of these efforts need to be simultaneously paired with strategies to implement care pathways in primary care for HCC surveillance, remove barriers for access to surveillance testing, and improve patient adherence to surveillance.
Conclusions
HCC is a challenging disease to detect in the early stages, and we need highly sensitive and specific screening tools to achieve this. Recent advances in imaging techniques have improved upon their sensitivity and specificity of detection. Moreover, novel circulating noninvasive biomarkers, which have the potential to be widely implemented, have recently been introduced (Fig. 3). Future prospective studies with these promising markers will help to better define their utility as diagnostic tools. Improvements in screening and surveillance will hopefully usher in a new era of earlier tumor detection, higher rates of curative treatment, and ultimately improved survival rates for patients with HCC.
FIG. 3.
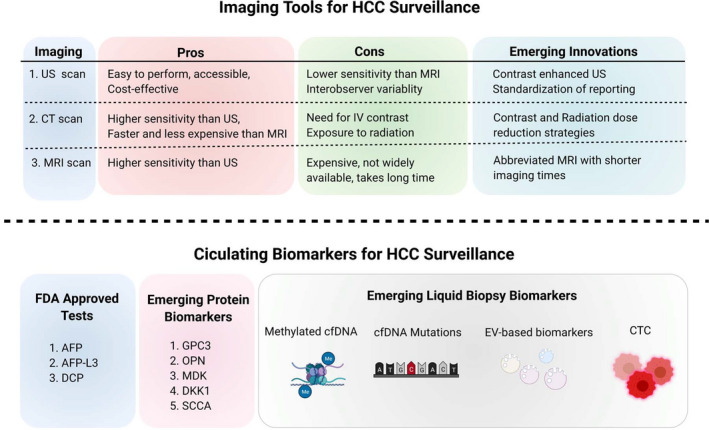
Existing and emerging biomarkers for HCC surveillance. US, CT, and MRI imaging modalities all have their pros and cons when used in the surveillance of at‐risk patients. Emerging innovations in these modalities, mostly by way of adjustments to imaging protocols and addition of agents that allow for more accurate detection, will improve upon existing limitations. AFP, AFP‐L3, and DCP are known FDA‐approved circulating biomarkers for HCC surveillance, but several emerging protein biomarkers (GPC3, OPN, MDK, DKK1, and SCCA) are proving to be clinically promising. The newest wave of innovation in tools for HCC surveillance lies in liquid biopsy biomarkers, which include methylated cfDNA, cfDNA mutations, EV‐based biomarkers, and CTC detection. Abbreviations: DKK1, Dickkopf‐1; IV, intravenous; MDK, midkine; OPN, osteopontin; SCCA, squamous cell carcinoma antigen.
Supported by the National Cancer Institute (CA222676).
Potential conflict of interest: Nothing to report.
References
- 1. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I & Bray F Cancer Today 2020. Global Cancer Observatory: International Agency for Research on Cancer. https://gco.iarc.fr/today/home. [Accessed July 15, 2021].
- 2. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol 2020;72:250‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Z, Suo C, Mao X, Jiang Y, Jin L, Zhang T, et al. Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990‐2017. Cancer 2020;126:2267‐2278. [DOI] [PubMed] [Google Scholar]
- 4. Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD. Global trends in incidence rates of primary adult liver cancers: a systematic review and meta‐analysis. Front Oncol 2020;10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertuccio P, Turati F, Carioli G, Rodriguez T, La Vecchia C, Malvezzi M, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67:302‐309. [DOI] [PubMed] [Google Scholar]
- 6. Liver Cancer Survival Rates . American Cancer Society. https://www.cancer.org/cancer/liver‐cancer/detection‐diagnosis‐staging/survival‐rates.html. [Accessed May 21, 2020].
- 7. Robinson A, Tavakoli H, Liu B, Bhuket T, Wong RJ. Advanced hepatocellular carcinoma tumor stage at diagnosis in the 1945‐1965 birth cohort reflects poor use of hepatocellular carcinoma screening. Hepatol Commun 2018;2:1147‐1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park J, Chen M, Colombo M, Roberts LR, Schwartz M, Chen P, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155‐2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adeniji N, Dhanasekaran R. Genomic Landscape of HCC. Curr Hepatol Rep 2020;19:448‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 2006;6:674‐687. [DOI] [PubMed] [Google Scholar]
- 11. Llovett JM, Zucman‐Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 12. Zhang X, El‐Serag HB, Thrift AP. Predictors of five‐year survival among patients with hepatocellular carcinoma in the United States: an analysis of SEER‐Medicare. Cancer Causes Control 2021. April. 10.1007/s10552-020-01386-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13. Ulahannan SV, Duffy AG, McNeel TS, Kish JK, Dickie LA, Rahma OE, et al. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatology 2014;60:1637‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta‐analysis. PLoS Med 2014;11:e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pandya P, Kanwal F. Adding to the evidence base: effectiveness of hepatocellular carcinoma surveillance in clinical practice. Hepatol Commun 2017;1:723‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang B‐H, Yang B‐H, Tang Z‐Y. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417‐422. [DOI] [PubMed] [Google Scholar]
- 17. Trinchet J‐C, Chaffaut C, Bourcier V, Degos F, Henrion J, Fontaine H, et al. Ultrasonographic surveillance of hepatocellular carcinoma in cirrhosis: a randomized trial comparing 3‐ and 6‐month periodicities. Hepatology 2011;54:1987‐1997. [DOI] [PubMed] [Google Scholar]
- 18. Wang J‐H, Chang K‐C, Kee K‐M, Chen P‐F, Yen Y‐H, Tseng P‐L, et al. Hepatocellular carcinoma surveillance at 4‐ vs. 12‐month intervals for patients with chronic viral hepatitis: a randomized study in community. Am J Gastroenterol 2013;108:416‐424. [DOI] [PubMed] [Google Scholar]
- 19. Pocha C, Dieperink E, McMaken KA, Knott A, Thuras P, Ho SB. Surveillance for hepatocellular cancer with ultrasonography vs. computed tomography—a randomised study. Aliment Pharmacol Ther 2013;38:303‐312. [DOI] [PubMed] [Google Scholar]
- 20. van Meer S, de Man RA, Coenraad MJ, Sprengers D, van Nieuwkerk KMJ, Klümpen H‐J, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: results from a large cohort in the Netherlands. J Hepatol 2015;63:1156‐1163. [DOI] [PubMed] [Google Scholar]
- 21. Costentin CE, Layese R, Bourcier V, Cagnot C, Marcellin P, Guyader D, et al. Compliance with hepatocellular carcinoma surveillance guidelines associated with increased lead‐time adjusted survival of patients with compensated viral cirrhosis: a multi‐center cohort study. Gastroenterology 2018;155:431‐442.e10. [DOI] [PubMed] [Google Scholar]
- 22. Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MAM, et al. Meta‐analysis: surveillance with ultrasound for early‐stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30:37‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeh Y‐P, Hu T‐H, Cho P‐Y, Chen H‐H, Yen AM‐F, Chen SL‐S, et al. Evaluation of abdominal ultrasonography mass screening for hepatocellular carcinoma in Taiwan. Hepatology 2014;59:1840‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tina Shih Y‐C, Dong W, Xu Y, Shen Y. Assessing the cost‐effectiveness of updated breast cancer screening guidelines for average‐risk women. Value Health 2019;22:185‐193. [DOI] [PubMed] [Google Scholar]
- 25. Khalili F, Najafi B, Mansour‐Ghanaei F, Yousefi M, Abdollahzad H, Motlagh A. Cost‐effectiveness analysis of colorectal cancer screening: a systematic review. Risk Manag Healthc Policy 2020;13:1499‐1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goossens N, Singal AG, King LY, Andersson KL, Fuchs BC, Besa C, et al. Cost‐effectiveness of risk score–stratified hepatocellular carcinoma screening in patients with cirrhosis. Clin Transl Gastroenterol 2017;8:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Atiq O, Tiro J, Yopp AC, Muffler A, Marrero JA, Parikh ND, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konerman MA, Verma A, Zhao B, Singal AG, Lok AS, Parikh ND. Frequency and outcomes of abnormal imaging in patients with cirrhosis enrolled in a hepatocellular carcinoma surveillance program. Liver Transpl 2019;25:369‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Clin Liver Dis 2019;13:1. [DOI] [PubMed] [Google Scholar]
- 30. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182‐236. [DOI] [PubMed] [Google Scholar]
- 31. Sarasin FP, Giostra E, Hadengue A. Cost‐effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child‐Pugh class A cirrhosis. Am J Med 1996;101:422‐434. [DOI] [PubMed] [Google Scholar]
- 32. Lin OS, Keeffe EB, Sanders GD, Owens DK. Cost‐effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther 2004;19:1159‐1172. [DOI] [PubMed] [Google Scholar]
- 33. Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473‐481. [PMC free article] [PubMed] [Google Scholar]
- 34. Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, et al. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology 1985;89:259‐266. [DOI] [PubMed] [Google Scholar]
- 35. Han K‐H, Do Young K, Park JY, Ahn SH, Kim J, Kim SU, et al. Survival of hepatocellular carcinoma patients may be improved in surveillance interval not more than 6 months compared with more than 6 months. J Clin Gastroenterol 2013;47:538‐544. [DOI] [PubMed] [Google Scholar]
- 36. Ioannou GN. HCC surveillance after SVR in patients with F3/F4 fibrosis. J Hepatol 2021;74:458‐465. [DOI] [PubMed] [Google Scholar]
- 37. National Comprehensive Cancer Network . NCCN guidelines for patients: liver cancer. 2020. https://www.nccn.org/professionals/physician_gls/default.aspx. [Accessed July 15, 2021].
- 38. Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, et al. Management of hepatocellular carcinoma in Japan: consensus‐based clinical practice guidelines proposed by the Japan Society of Hepatology. Dig Dis 2011;29:339‐364. [DOI] [PubMed] [Google Scholar]
- 39. Omata M, Cheng A‐L, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia‐Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha‐fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol 2006;101:513‐523. [DOI] [PubMed] [Google Scholar]
- 41. Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta‐analysis. Gastroenterology 2018;154:1706‐1718.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Singal AG, Mittal S, Yerokun OA, Ahn C, Marrero JA, Yopp AC, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med 2017;130:1099‐1106.e1. [DOI] [PubMed] [Google Scholar]
- 43. Morgan TA, Maturen KE, Dahiya N, Sun MRM, Kamaya A. American College of Radiology Ultrasound Liver Imaging and Reporting Data System (US LI‐RADS) Working Group. US LI‐RADS: ultrasound liver imaging reporting and data system for screening and surveillance of hepatocellular carcinoma. Abdom Radiol 2018;43:41‐55. [DOI] [PubMed] [Google Scholar]
- 44. Son JH, Choi SH, Kim SY, Jang HY, Byun JH, Won HJ, et al. Validation of US Liver Imaging Reporting and Data System Version 2017 in patients at high risk for hepatocellular carcinoma. Radiology 2019;292:390‐397. [DOI] [PubMed] [Google Scholar]
- 45. Millet JD, Kamaya A, Choi HH, Dahiya N, Murphy PM, Naveed MZ, et al. ACR Ultrasound Liver Reporting and Data System: multicenter assessment of clinical performance at one year. J Am Coll Radiol 2019;16:1656‐1662. [DOI] [PubMed] [Google Scholar]
- 46. da Silva PH, Gomes MM, de Matos CAL, de Souza E, Silva IS, Gonzalez AM, et al. HCC detection on surveillance US: comparing focused liver protocol using US LI‐RADS technical guidelines to a general complete abdominal US protocol. J Ultrasound Med 2021. Jan 19. 10.1002/jum.15637. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47. Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:161‐167. [DOI] [PubMed] [Google Scholar]
- 48. Park HJ, Choi BI, Lee ES, Park SB, Lee JB. How to differentiate borderline hepatic nodules in hepatocarcinogenesis: emphasis on imaging diagnosis. Liver Cancer 2017;6:189‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duan Y, Xie X, Li Q, Mercaldo N, Samir AE, Kuang M, et al. Differentiation of regenerative nodule, dysplastic nodule, and small hepatocellular carcinoma in cirrhotic patients: a contrast‐enhanced ultrasound–based multivariable model analysis. Euro Radiol 2020;30:4741‐4751. [DOI] [PubMed] [Google Scholar]
- 50. Fan P, Xia H, Ding H, Dong Y, Chen L, Wang W. Characterization of early hepatocellular carcinoma and high‐grade dysplastic nodules on contrast‐enhanced ultrasound. J Ultrasound Med 2020;39:1799‐1808. [DOI] [PubMed] [Google Scholar]
- 51. Goshima S. Use of imaging techniques to screen hepatocellular carcinoma. Hepatocellular Carcinoma 2016;355‐365. [Google Scholar]
- 52. Zhang J, Yu Y, Li Y, Wei L. Diagnostic value of contrast‐enhanced ultrasound in hepatocellular carcinoma: a meta‐analysis with evidence from 1998 to 2016. Oncotarget 2017;8:75418‐75426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther 2017;45:169‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liang Y, Xu F, Guo Y, Lai L, Jiang X, Wei X, et al. Diagnostic performance of LI‐RADS for MRI and CT detection of HCC: a systematic review and diagnostic meta‐analysis. Eur J Radiol 2021;134:109404. [DOI] [PubMed] [Google Scholar]
- 55. van der Pol CB, Lim CS, Sirlin CB, McGrath TA, Salameh J‐P, Bashir MR, et al. Accuracy of the Liver Imaging Reporting and Data System in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy‐a systematic review. Gastroenterology 2019;156:976‐986. [DOI] [PubMed] [Google Scholar]
- 56. Lee S, Kim S‐S, Roh YH, Choi J‐Y, Park M‐S, Kim M‐J. Diagnostic performance of CT/MRI Liver Imaging Reporting and Data System v2017 for hepatocellular carcinoma: a systematic review and meta‐analysis. Liver Int 2020;40:1488‐1497. [DOI] [PubMed] [Google Scholar]
- 57. Hanna RF, Miloushev VZ, Tang A, Finklestone LA, Brejt SZ, Sandhu RS, et al. Comparative 13‐year meta‐analysis of the sensitivity and positive predictive value of ultrasound, CT, and MRI for detecting hepatocellular carcinoma. Abdom Radiol 2016;41:71‐90. [DOI] [PubMed] [Google Scholar]
- 58. Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging—a systematic review and meta‐analysis. Radiology 2015;275:97‐109. [DOI] [PubMed] [Google Scholar]
- 59. Becker‐Weidman DJS, Kalb B, Sharma P, Kitajima HD, Lurie CR, Chen Z, et al. Hepatocellular carcinoma lesion characterization: single‐institution clinical performance review of multiphase gadolinium‐enhanced MR imaging—comparison to prior same‐center results after MR systems improvements. Radiology 2011;261:824‐833. [DOI] [PubMed] [Google Scholar]
- 60. Tang A, Bashir MR, Corwin MT, Cruite I, Dietrich CF, Do RKG, et al. Evidence supporting LI‐RADS major features for CT‐ and MR imaging–based diagnosis of hepatocellular carcinoma: a systematic review. Radiology 2018;286:29‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moreno CC, Hang T‐VP. MRI screening for hepatocellular carcinoma. Appl Radiol 2020;49:9‐15. [Google Scholar]
- 62. Aubé C, Oberti F, Lonjon J, Pageaux G, Seror O, N’Kontchou G, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int 2017;37:1515‐1525. [DOI] [PubMed] [Google Scholar]
- 63. Kim H, An J, Park J, Park S, Lim Y, Lee E. Magnetic resonance imaging is cost‐effective for hepatocellular carcinoma surveillance in high‐risk patients with cirrhosis. Hepatology 2019;69:1599‐1613. [DOI] [PubMed] [Google Scholar]
- 64. Park HJ, Jang HY, Kim SY, Lee SJ, Won HJ, Byun JH, et al. Non‐enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: comparison with ultrasound. J Hepatol 2020;72:718‐724. [DOI] [PubMed] [Google Scholar]
- 65. Besa C, Lewis S, Pandharipande PV, Chhatwal J, Kamath A, Cooper N, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion‐weighted and T1‐weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol 2017;42:179‐190. [DOI] [PubMed] [Google Scholar]
- 66. Khatri G, Pedrosa I, Ananthakrishnan L, de Leon AD, Fetzer DT, Leyendecker J, et al. Abbreviated‐protocol screening MRI vs. complete‐protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: an equivalence study using LI‐RADS v2018. J Magn Reson Imaging 2020;51:415‐425. [DOI] [PubMed] [Google Scholar]
- 67. Vietti Violi N, Taouli B. Abbreviated MRI for HCC surveillance: is it ready for clinical use? Eur Radiol 2020;30:4147‐4149. [DOI] [PubMed] [Google Scholar]
- 68. Brunsing RL, Chen DH, Schlein A, Wolfson T, Gamst A, Mamidipalli A, et al. Gadoxetate‐enhanced abbreviated MRI for hepatocellular carcinoma surveillance: preliminary experience. Radiol Imaging Cancer 2019;1:e190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta‐analysis Hepatology 2018;67:401‐421. [DOI] [PubMed] [Google Scholar]
- 70. Chou R, Cuevas C, Fu R, Devine B, Wasson N, Ginsburg A, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systematic review and meta‐analysis. Ann Intern Med 2015;162:697‐711. [DOI] [PubMed] [Google Scholar]
- 71. Yoon JH, Lee JM, Lee DH, Joo I, Jeon JH, Ahn SJ, et al. A comparison of biannual two‐phase low‐dose liver CT and US for HCC surveillance in a group at high risk of HCC development. Liver Cancer 2020;9:503‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lima PH, Fan B, Bérubé J, Cerny M, Olivié D, Giard J‐M, et al. Cost‐utility analysis of imaging for surveillance and diagnosis of hepatocellular carcinoma. AJR Am J Roentgenol 2019;213:17‐25. [DOI] [PubMed] [Google Scholar]
- 73. Sauzay C, Petit A, Bourgeois A‐M, Barbare J‐C, Chauffert B, Galmiche A, et al. Alpha‐foetoprotein (AFP): a multi‐purpose marker in hepatocellular carcinoma. Clin Chim Acta 2016;463:39‐44. [DOI] [PubMed] [Google Scholar]
- 74. Zhao Y‐J, Ju Q, Li G‐C. Tumor markers for hepatocellular carcinoma. Mol Clin Oncol 2013;1:593‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhang J, Chen G, Zhang P, Zhang J, Li X, Gan Da’nan, et al. The threshold of alpha‐fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: a systematic review and meta‐analysis. PLoS One 2020;15:e0228857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Frenette C. Hepatocellular carcinoma: moving into the 21st Century. In: Clinics Liver Disease, Volume 24‐4. Amsterdam, Netherlands: Elsevier; 2020. [Google Scholar]
- 77. Tayob N, Lok ASF, Do K‐A, Feng Z. Improved detection of hepatocellular carcinoma by using a longitudinal alpha‐fetoprotein screening algorithm. Clin Gastroenterol Hepatol 2016;14:469‐475.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. White DL, Richardson P, Tayoub N, Davila JA, Kanwal F, El‐Serag HB. The updated model: an adjusted serum alpha‐fetoprotein–based algorithm for hepatocellular carcinoma detection with hepatitis C virus‐related cirrhosis. Gastroenterology 2015;149:1986‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hu J, Wang N, Yang Y, Ma L, Han R, Zhang W, et al. Diagnostic value of alpha‐fetoprotein combined with neutrophil‐to‐lymphocyte ratio for hepatocellular carcinoma. BMC Gastroenterol 2018;18:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Choi J, Kim G, Han S, Lee W, Chun S, Lim Y. Longitudinal assessment of three serum biomarkers to detect very early‐stage hepatocellular carcinoma. Hepatology 2019;69:1983‐1994. [DOI] [PubMed] [Google Scholar]
- 81. Hughes DM, Berhane S, Emily de Groot CA, Toyoda H, Tada T, Kumada T, et al. Serum levels of α‐fetoprotein increased more than 10 years before detection of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2021;19;162‐170.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nicholson BD, Jones NR, Protheroe A, Joseph J, Roberts NW, Van den Bruel A, et al. The diagnostic performance of current tumour markers in surveillance for recurrent testicular cancer: a diagnostic test accuracy systematic review. Cancer Epidemiol 2019;59:15‐21. [DOI] [PubMed] [Google Scholar]
- 83. Schieving JH, de Vries M, van Vugt JMG, Weemaes C, van Deuren M, Nicolai J, et al. Alpha‐fetoprotein, a fascinating protein and biomarker in neurology. Eur J Paediatr Neurol 2014;18:243‐248. [DOI] [PubMed] [Google Scholar]
- 84. Chen J‐G, Parkin DM, Chen Q‐G, Lu J‐H, Shen Q‐J, Zhang B‐C, et al. Screening for liver cancer: results of a randomised controlled trial in Qidong, China. J Med Screen 2003;10:204‐209. [DOI] [PubMed] [Google Scholar]
- 85. Caviglia GP, Abate ML, Petrini E, Gaia S, Rizzetto M, Smedile A. Highly sensitive alpha‐fetoprotein, Lens culinaris agglutinin‐reactive fraction of alpha‐fetoprotein and des‐gamma‐carboxyprothrombin for hepatocellular carcinoma detection. Hepatol Res 2016;46:e130‐e135. [DOI] [PubMed] [Google Scholar]
- 86. Tateishi R, Yoshida H, Matsuyama Y, Mine N, Kondo Y, Omata M. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int 2008;2:17‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, et al. High‐sensitivity Lens culinaris agglutinin‐reactive alpha‐fetoprotein assay predicts early detection of hepatocellular carcinoma. J Gastroenterol 2014;49:555‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yi X, Yu S, Bao Y. Alpha‐fetoprotein‐L3 in hepatocellular carcinoma: a meta‐analysis. Clin Chim Acta 2013;425:212‐220. [DOI] [PubMed] [Google Scholar]
- 89. Roscoe D. AFP‐L3 Immunological Test Systems—Class II Special Controls Guidance Document for Industry and FDA Staff. Food and Drug Administration. 2005. https://www.fda.gov/medical‐devices/guidance‐documents‐medical‐devices‐and‐radiation‐emitting‐products/afp‐l3‐immunological‐test‐systems‐class‐ii‐special‐controls‐guidance‐document‐industry‐and‐fda‐staff. [Accessed February 1, 2021] [Google Scholar]
- 90. Inagaki Y, Tang W, Makuuchi M, Hasegawa K, Sugawara Y, Kokudo N. Clinical and molecular insights into the hepatocellular carcinoma tumour marker des‐γ‐carboxyprothrombin. Liver Int 2011;31:22‐35. [DOI] [PubMed] [Google Scholar]
- 91. Seo SI, Kim HS, Kim WJ, Shin WG, Kim DJ, Kim KH, et al. Diagnostic value of PIVKA‐II and alpha‐fetoprotein in hepatitis B virus‐associated hepatocellular carcinoma. World J Gastroenterol 2015;21:3928‐3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, et al. Evidence‐based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH‐HCC Guidelines). Hepatol Res 2015;45. [DOI] [PubMed] [Google Scholar]
- 93. Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, et al. Performance of PIVKA‐II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol 2015;62:848‐854. [DOI] [PubMed] [Google Scholar]
- 94. Saitta C, Raffa G, Alibrandi A, Brancatelli S, Lombardo D, Tripodi G, et al. PIVKA‐II is a useful tool for diagnostic characterization of ultrasound‐detected liver nodules in cirrhotic patients. Medicine 2017;96:e7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, et al. A combination of α‐fetoprotein and des‐γ‐carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion 2013;87:121‐131. [DOI] [PubMed] [Google Scholar]
- 96. Loglio A, Iavarone M, Facchetti F, Di Paolo D, Perbellini R, Lunghi G, et al. The combination of PIVKA‐II and AFP improves the detection accuracy for HCC in HBV caucasian cirrhotics on long‐term oral therapy. Liver Int 2020;40:1987‐1996. [DOI] [PubMed] [Google Scholar]
- 97. Tarao K, Nozaki A, Komatsu H, Komatsu T, Taguri M, Tanaka K, et al. Real impact of tumor marker AFP and PIVKA‐II in detecting very small hepatocellular carcinoma (≤2 cm, Barcelona stage 0)—assessment with large number of cases. World J Hepatol 2020;12:1046‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Colli A, Nadarevic T, Miletic D, Giljaca V, Fraquelli M, Štimac D, et al. Abdominal ultrasound and alpha‐foetoprotein for the diagnosis of hepatocellular carcinoma in adults with chronic liver disease. Cochrane Database Syst Rev 2021;4:CD013346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chen H, Chen S, Li S, Chen Z, Zhu X, Dai M, et al. Combining des‐gamma‐carboxyprothrombin and alpha‐fetoprotein for hepatocellular carcinoma diagnosing: an update meta‐analysis and validation study. Oncotarget 2017;8:90390‐90401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen S, Li J, Tan X, Xu Q, Mo Y, Qin H, et al. Clinical role of combining alpha‐fetoprotein and lens culinaris agglutinin‐reactive fraction of alpha‐fetoprotein for hepatocellular carcinoma: evidence from literature and an original study. J Clin Lab Anal 2020;34:e23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sun T, Tang Y, Sun D, Bu Q, Li P. Osteopontin versus alpha‐fetoprotein as a diagnostic marker for hepatocellular carcinoma: a meta‐analysis. Onco Targets Ther 2018;11:8925‐8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xu D, Su C, Sun L, Gao Y, Li Y. Performance of serum glypican 3 in diagnosis of hepatocellular carcinoma: a meta‐analysis. Ann Hepatol 2019;18:58‐67. [DOI] [PubMed] [Google Scholar]
- 103. Gao P, Li M, Tian QB, Liu D‐W. Diagnostic performance of des‐γ‐carboxy prothrombin (DCP) for hepatocellular carcinoma: a bivariate meta‐analysis. Neoplasma 2012;59:150‐159. [DOI] [PubMed] [Google Scholar]
- 104. De J, Shen Y, Qin J, Feng L, Wang Y, Yang L. A systematic review of des‐γ‐carboxy prothrombin for the diagnosis of primary hepatocellular carcinoma. Medicine 2016;95:e3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhu R, Yang J, Xu L, Dai W, Wang F, Shen M, et al. Diagnostic performance of des‐γ‐carboxy prothrombin for hepatocellular carcinoma: a meta‐analysis. Gastroenterol Res Pract 2014;2014:529314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev 2014;23:144‐153. [DOI] [PubMed] [Google Scholar]
- 107. Roche . FDA grants Breakthrough Device Designation for Roche’s Elecsys GALAD score to support earlier diagnosis of hepatocellular carcinoma. 2020. https://www.roche.com/dam/jcr:2b18da09‐fd2c‐43d3‐a776‐857a649555de/en/roche‐mediarelease‐04032020‐en.pdf. [Accessed December 16, 2020]
- 108. Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, et al. Role of the GALAD and BALAD‐2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol 2016;14:875‐886.e6. [DOI] [PubMed] [Google Scholar]
- 109. Best J, Bilgi H, Heider D, Schotten C, Manka P, Bedreli S, et al. The GALAD scoring algorithm based on AFP, AFP‐L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z Gastroenterol 2016;54:1296‐1305. [DOI] [PubMed] [Google Scholar]
- 110. Best J, Bechmann LP, Sowa J‐P, Sydor S, Dechêne A, Pflanz K, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2020;18:728‐735.e4. [DOI] [PubMed] [Google Scholar]
- 111. Liu M, Wu R, Liu X, Xu H, Chi X, Wang X, et al. Validation of the GALAD model and establishment of GAAP model for diagnosis of hepatocellular carcinoma in Chinese patients. J Hepatocell Carcinoma 2020;7:219‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hsu HC, Cheng W, Lai PL. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res 1997;57:5179‐5184. [PubMed] [Google Scholar]
- 113. Zhu ZW, Friess H, Wang L, Abou‐Shady M, Zimmermann A, Lander AD, et al. Enhanced glypican‐3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut 2001;48:558‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, et al. Glypican‐3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003;125:89‐97. [DOI] [PubMed] [Google Scholar]
- 115. Ba M‐C, Long H, Tang Y‐Q, Cui S‐Z. GP73 expression and its significance in the diagnosis of hepatocellular carcinoma: a review. Int J Clin Exp Pathol 2012;5:874‐881. [PMC free article] [PubMed] [Google Scholar]
- 116. Mao Y, Yang H, Xu H, Lu X, Sang X, Du S, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010;59:1687‐1693. [DOI] [PubMed] [Google Scholar]
- 117. Abdel‐Hafiz SM, Hamdy HEM, Khorshed FM, Aboushousha TS, Safwat G, Saber MA, et al. Evaluation of osteopontin as a biomarker in hepatocellular carcinomas in Egyptian patients with chronic HCV cirrhosis. Asian Pac J Cancer Prev 2018;19:1021‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology 2012;55:483‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wan H‐G, Xu H, Gu Y‐M, Wang H, Xu W, Zu M‐H. Comparison osteopontin vs AFP for the diagnosis of HCC: a meta‐analysis. Clinic Res Hepatol Gastroenterol 2014;38:706‐714. [DOI] [PubMed] [Google Scholar]
- 120. Hung Y‐J, Lin ZHY, Cheng T‐I, Liang C‐T, Kuo T‐M, Kao K‐J. Serum midkine as a prognostic biomarker for patients with hepatocellular carcinoma. Am J Clin Pathol 2011;136:594‐603. [DOI] [PubMed] [Google Scholar]
- 121. Zhu W‐W, Guo J‐J, Guo L, Jia H‐L, Zhu M, Zhang J‐B, et al. Evaluation of midkine as a diagnostic serum biomarker in hepatocellular carcinoma. Clin Cancer Res 2013;19:3944‐3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yin Z, Luo X, Kang X, Wu Z, Qian H, Wu M. Correlation between midkine protein overexpression and intrahepatic metastasis in hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 2002;24:27‐29. [PubMed] [Google Scholar]
- 123. Filippou PS, Karagiannis GS, Constantinidou A. Midkine (MDK) growth factor: a key player in cancer progression and a promising therapeutic target. Oncogene 2020;39:2040‐2054. [DOI] [PubMed] [Google Scholar]
- 124. Lu Q, Li J, Cao H, Lv C, Wang X, Cao S. Comparison of diagnostic accuracy of midkine and AFP for detecting hepatocellular carcinoma: a systematic review and meta‐analysis. Biosci Rep 2020;40:BSR20192424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shen Q, Fan J, Yang X‐R, Tan Y, Zhao W, Xu Y, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large‐scale, multicentre study. Lancet Oncol 2012;13:817‐826. [DOI] [PubMed] [Google Scholar]
- 126. Jiang X, Hui F, Qin X, Wu Y, Liu H, Gao J, et al. Diagnosis accuracy and prognostic significance of the Dickkopf‐1 protein in gastrointestinal carcinomas: systematic review and network meta‐analysis. J Cancer 2020;11:7091‐7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pontisso P, Quarta S, Caberlotto C, Beneduce L, Marino M, Bernardinello E, et al. Progressive increase of SCCA‐IgM immune complexes in cirrhotic patients is associated with development of hepatocellular carcinoma. Int J Cancer 2006;119:735‐740. [DOI] [PubMed] [Google Scholar]
- 128. Biasiolo A, Trotta E, Fasolato S, Ruvoletto M, Martini A, Gallotta A, et al. Squamous cell carcinoma antigen‐IgM is associated with hepatocellular carcinoma in patients with cirrhosis: a prospective study. Dig Liver Dis 2016;48:197‐202. [DOI] [PubMed] [Google Scholar]
- 129. Kim H, Park J, Kim Y, Sohn A, Yeo I, Jong YuS, et al. Serum fibronectin distinguishes the early stages of hepatocellular carcinoma. Sci Rep 2017;7:9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Krishnan MS, Rajan KDA, Park J, Arjunan V, Garcia Marques FJ, Bermudez A, et al. Genomic analysis of vascular invasion in hepatocellular carcinoma (HCC) reveals molecular drivers and predictive biomarkers. Hepatology 2021;73:2342‐2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Jin Y, Wong YS, Goh BKP, Chan CY, Cheow PC, Chow PKH, et al. Circulating microRNAs as potential diagnostic and prognostic biomarkers in hepatocellular carcinoma. Sci Rep 2019;9:10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhang Y, Li T, Qiu Y, Zhang T, Guo P, Ma X, et al. Serum microRNA panel for early diagnosis of the onset of hepatocellular carcinoma. Medicine 2017;96:e5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yamamoto Y, Kondo S, Matsuzaki J, Esaki M, Okusaka T, Shimada K, et al. Highly sensitive circulating microRNA panel for accurate detection of hepatocellular carcinoma in patients with liver disease. Hepatol Commun 2020;4:284‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Huang J, Zheng Y, Xiao X, Liu C, Lin J, Zheng S, et al. A Circulating long noncoding RNA panel serves as a diagnostic marker for hepatocellular carcinoma. Dis Markers 2020;2020:5417598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yuan W, Sun Y, Liu L, Zhou B, Wang S, Gu D. Circulating lncRNAs serve as diagnostic markers for hepatocellular carcinoma. Cell Physiol Biochemistry 2017;44:125‐132. [DOI] [PubMed] [Google Scholar]
- 136. Konishi H, Ichikawa D, Yamamoto Y, Arita T, Shoda K, Hiramoto H, et al. Plasma level of metastasis‐associated lung adenocarcinoma transcript 1 is associated with liver damage and predicts development of hepatocellular carcinoma. Cancer Sci 2016;107:149‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zheng Z‐K, Pang C, Yang Y, Duan Q, Zhang J, Liu W‐C. Serum long noncoding RNA urothelial carcinoma‐associated 1: a novel biomarker for diagnosis and prognosis of hepatocellular carcinoma. J Int Med Res 2018;46:348‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Liu MC, Oxnard GR, Klein EA, Swanton C, Seiden MV, Liu MC, et al. Sensitive and specific multi‐cancer detection and localization using methylation signatures in cell‐free DNA. Ann Oncol 2020;31:745‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mah W‐C, Lee CGL. DNA methylation: potential biomarker in Hepatocellular Carcinoma. Biomarker Res 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Katoh H, Shibata T, Kokubu A, Ojima H, Fukayama M, Kanai Y, et al. Epigenetic instability and chromosomal instability in hepatocellular carcinoma. Am J Pathol 2006;168:1375‐1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ko E, Kim Y, Kim S‐J, Joh J‐W, Song S, Park C‐K, et al. Promoter hypermethylation of the p16 gene is associated with poor prognosis in recurrent early‐stage hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 2008;17:2260‐2267. [DOI] [PubMed] [Google Scholar]
- 142. Saelee P, Chuensumran U, Wongkham S, Chariyalertsak S, Tiwawech D, Petmitr S. Hypermethylation of suppressor of cytokine signaling 1 in hepatocellular carcinoma patients. Asian Pac J Cancer Prev 2012;13:3489‐3493. [DOI] [PubMed] [Google Scholar]
- 143. Lee S, Lee HJ, Kim J‐H, Lee H‐S, Jang JJ, Kang GH. Aberrant CpG island hypermethylation along multistep hepatocarcinogenesis. Am J Pathol 2003;163:1371‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kwon GY, Yoo BC, Koh KC, Cho JW, Park WS, Park CK. Promoter methylation of E‐cadherin in hepatocellular carcinomas and dysplastic nodules. J Korean Med Sci 2005;20:242‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Yang B, Guo M, Herman JG, Clark DP. Aberrant promoter methylation profiles of tumor suppressor genes in hepatocellular carcinoma. Am J Pathol 2003;163:1101‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Xu R‐H, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater 2017;16:1155‐1161. [DOI] [PubMed] [Google Scholar]
- 147. Kisiel JB, Dukek BA, V.S.R. Kanipakam R, Ghoz HM, Yab TC, Berger CK, et al. Hepatocellular carcinoma detection by plasma methylated DNA: discovery, phase I pilot, and phase II clinical validation. Hepatology 2019;69:1180‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Chalasani NP, Ramasubramanian TS, Bhattacharya A, Olson MC, EdwardsV DK, Roberts LR, et al. A novel blood‐based panel of methylated DNA and protein markers for detection of early‐stage hepatocellular carcinoma. Clin Gastroenterol Hepatol 2020. Sep 2;S1542‐3565(20)31224‐6. [DOI] [PubMed] [Google Scholar]
- 149. Kotoh Y, Suehiro Y, Saeki I, Hoshida T, Maeda M, Iwamoto T, et al. Novel liquid biopsy test based on a sensitive methylated SEPT9 assay for diagnosing hepatocellular carcinoma. Hepatol Commun 2020;4:461‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell 2017;169:1327–1341.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, et al. Trans‐ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet 2014;46:1267‐1273. [DOI] [PubMed] [Google Scholar]
- 152. Dhanasekaran R, Nault J‐C, Roberts LR, Zucman‐Rossi J. Genomic medicine and implications for hepatocellular carcinoma prevention and therapy. Gastroenterology 2019;156:492‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhang Y, Yao Y, Xu Y, Li L, Gong Y, Zhang K, et al. Pan‐cancer circulating tumor DNA detection in over 10,000 Chinese patients. Nat Commun 2021;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Mody K, Kasi PM, Yang JD, Surapaneni PK, Ritter A, Roberts A, et al. Feasibility of circulating tumor DNA testing in hepatocellular carcinoma. J Gastrointest Oncol 2019;10:745‐750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kaseb AO, Sánchez NS, Sen S, Kelley RK, Tan B, Bocobo AG, et al. Molecular profiling of hepatocellular carcinoma using circulating cell‐free DNA. Clin Cancer Res 2019;25:6107‐6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Qu C, Wang Y, Wang P, Chen K, Wang M, Zeng H, et al. Detection of early‐stage hepatocellular carcinoma in asymptomatic HBsAg‐seropositive individuals by liquid biopsy. Proc Natl Acad Sci U S A 2019;116:6308‐6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Chen L, Abou‐Alfa GK, Zheng B, Liu J‐F, Bai J, Du L‐T, et al. Genome‐scale profiling of circulating cell‐free DNA signatures for early detection of hepatocellular carcinoma in cirrhotic patients. Cell Res 2021;31:589‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Margolis L, Sadovsky Y. The biology of extracellular vesicles: the known unknowns. PLoS Biol 2019;17:e3000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Sukowati CHC, Cabral LKD, Tiribelli C, Pascut D. Circulating long and circular noncoding RNA as non‐invasive diagnostic tools of hepatocellular carcinoma. Biomedicines 2021;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lu Y, Duan Y, Xu Q, Zhang L, Chen W, Qu Z, et al. Circulating exosome‐derived bona fide long non‐coding RNAs predicting the occurrence and metastasis of hepatocellular carcinoma. J Cell Mol Med 2020;24:1311‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lee YR, Kim G, Tak WY, Jang SY, Kweon YO, Park JG, et al. Circulating exosomal noncoding RNAs as prognostic biomarkers in human hepatocellular carcinoma. Int J Cancer 2019;144:1444‐1452. [DOI] [PubMed] [Google Scholar]
- 162. Sun N, Lee Y‐T, Zhang RY, Kao R, Teng P‐C, Yang Y, et al. Purification of HCC‐specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat Commun 2020;11:4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Cho HJ, Eun JW, Baek GO, Seo CW, Ahn HR, Kim SS, et al. Serum exosomal microRNA, miR‐10b‐5p, as a potential diagnostic biomarker for early‐stage hepatocellular carcinoma. J Clin Med Res 2020;9:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wang Y, Zhang C, Zhang P, Guo G, Jiang T, Zhao X, et al. Serum exosomal microRNAs combined with alpha‐fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med 2018;7:1670‐1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ahn JC, Teng P‐C, Chen P‐J, Posadas E, Tseng H‐R, Lu SC, et al. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology 2021;73:422‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Qi L‐N, Xiang B‐D, Wu F‐X, Ye J‐Z, Zhong J‐H, Wang Y‐Y, et al. Circulating tumor cells undergoing EMT provide a metric for diagnosis and prognosis of patients with hepatocellular carcinoma. Cancer Res 2018;78:4731‐4744. [DOI] [PubMed] [Google Scholar]
- 167. Guo W, Yang X‐R, Sun Y‐F, Shen M‐N, Ma X‐L, Wu J, et al. Clinical significance of EpCAM mRNA‐positive circulating tumor cells in hepatocellular carcinoma by an optimized negative enrichment and qRT‐PCR‐based platform. Clin Cancer Res 2014;20:4794‐4805. [DOI] [PubMed] [Google Scholar]
- 168. Sun Y‐F, Xu Y, Yang X‐R, Guo W, Zhang X, Qiu S‐J, et al. Circulating stem cell‐like epithelial cell adhesion molecule‐positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology 2013;57:1458‐1468. [DOI] [PubMed] [Google Scholar]
- 169. Ogle LF, Orr JG, Willoughby CE, Hutton C, McPherson S, Plummer R, et al. Imagestream detection and characterisation of circulating tumour cells—a liquid biopsy for hepatocellular carcinoma? J Hepatol 2016;65:305‐313. [DOI] [PubMed] [Google Scholar]
- 170. Hamaoka M, Kobayashi T, Tanaka Y, Mashima H, Ohdan H. Clinical significance of glypican‐3‐positive circulating tumor cells of hepatocellular carcinoma patients: a prospective study. PLoS One 2019;14:e0217586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Cui K, Ou Y, Shen Y, Li S, Sun Z. Clinical value of circulating tumor cells for the diagnosis and prognosis of hepatocellular carcinoma (HCC): a systematic review and meta‐analysis. Medicine 2020;99:e22242. [DOI] [PMC free article] [PubMed] [Google Scholar]


