Abstract
Background:
Measuring recent HIV infections from routine surveillance systems could allow timely and granular monitoring of HIV incidence patterns. We evaluated the relationship of two recent infection indicators with alternative denominators to true incidence patterns.
Methods:
We used a mathematical model of HIV testing behaviours, calibrated to population-based surveys and HIV testing services programme data, to estimate the number of recent infections diagnosed annually from 2010 to 2019 in Côte d’Ivoire, Malawi, and Mozambique. We compared two different denominators to interpret recency data: those at risk of HIV acquisition (HIV-negative tests and recent infections) and all people testing HIV positive. Sex and age-specific longitudinal trends in both interpretations were then compared with modelled trends in HIV incidence, testing efforts and HIV positivity among HIV testing services clients.
Results:
Over 2010–2019, the annual proportion of the eligible population tested increased in all countries, while positivity decreased. The proportion of recent infections among those at risk of HIV acquisition decreased, similar to declines in HIV incidence among adults (≥15 years old). Conversely, the proportion of recent infections among HIV-positive tests increased. The female-to-male ratio of the proportion testing recent among those at risk was closer to 1 than the true incidence sex ratio.
Conclusion:
The proportion of recent infections among those at risk of HIV acquisition is more indicative of HIV incidence than the proportion among HIV-positive tests. However, interpreting the observed patterns as surrogate measures for incidence patterns may still be confounded by different HIV testing rates between population groups or over time.
Keywords: epidemiologic surveillance, HIV infection diagnosis, HIV infections, incidence, routine diagnostic tests
Introduction
Monitoring HIV incidence allows identifying priority populations at high risk of HIV acquisition, essential information for planning interventions and evaluating public health programmes [1]. Biomarker-based assays that detect recent HIV seroconversions, defined as those that have occurred within 4–6 months of HIV infection, are increasingly used to identify ongoing transmission, monitor incidence changes, and identify population groups and geographic areas with higher relative infection levels [1–7]. These assays are often employed in recent infection testing algorithms (RITAs) that reclassify false-recent infections based on additional diagnostics, such as viral load, antiretroviral metabolites or other epidemiological information [1,8–11]. RITAs are now commonly applied to estimate HIV incidence in national household surveys in sub-Saharan Africa [12–15].
Tests for recent infection among people diagnosed with HIV have been implemented as part of routine surveillance systems in several resource-rich countries [16–20]. In sub-Saharan Africa, they have recently been implemented in countries with support from the President's Emergency Plan for AIDS Relief (PEPFAR), wherein HIV testing is more widespread and uniquely identifying new diagnoses is a major challenge [12,15]. In such contexts, detecting recent infections supports contact tracing activities to curtail onward community transmission [10,21]. RITAs implemented in routine surveillance systems could additionally contribute to HIV incidence estimation and identifying populations with high risk of acquiring HIV [12,22,23]. There are multiple options to interpret recency data as a surrogate measure for HIV incidence, such as the proportion of recent infections among new diagnoses (HIV-positive tests) or the proportion of recent infections among people at risk of acquiring HIV (HIV-negative tests and recent infections). Although measurable, interpretation of recent infections among persons testing for HIV is challenging because levels of recency among new diagnoses may depend on HIV testing efforts and healthcare seeking patterns across population groups [16,17].
We aimed to characterize the relationship between recent infection among HIV testing services (HTS) clients and population-level incidence, and consider the interpretation of two different indicators for recent infections in routine surveillance systems. We used a mathematical model of HIV testing behaviors and examined trends in the proportion of recent infections among the population at risk of HIV acquisition and the proportion of recent infections among HIV-positive tests (new diagnoses). These were compared with modeled trends in HIV incidence, the proportion tested among the adult population (testing effort), and positivity among HTS clients in Côte d’Ivoire, Malawi and Mozambique over 2010–2019.
Materials and methods
We used Shiny90, a mathematical model of HIV testing behaviors developed to estimate HIV status awareness and the number of people being diagnosed through routine HTS with recent HIV infection. Details on model structure, parameterization and calibration have been presented elsewhere [24,25]. Briefly, the model uses as inputs UNAIDS estimates of HIV incidence, prevalence, mortality and ART coverage produced by the Spectrum/EPP model (2019 projections files were used) [26,27]. The model estimates rates of HIV testing over time using the proportion of adults ever tested for HIV measured in population-based surveys, and the total annual numbers of tests and of HIV positive tests obtained from HTS programme data.
Shiny90 produces annual estimates of HIV testing rates among adults that vary by age, sex, previous HIV testing history, awareness and treatment status, and CD4+ cell count category. Using these rates, we calculated the probability of an incident infection being diagnosed within 6 months of HIV acquisition [25]. This period was chosen as it roughly corresponds to the setting-specific mean duration of recent infection (MDRI) value used to classify infections as recent or long-standing. To account for the imperfect sensitivity of the HIV testing algorithm during the window period of detection, we assumed that only people with HIV tested 4 weeks after HIV acquisition would be detected [2].
The steps outlined above enabled us to calculate the annual number of recent infections, the number of adults tested for HIV by serostatus and the number tested that are classified as recent infections. We then compared 2010–2019 trends in the proportion of recent infections among persons at risk of HIV acquisition (HIV-negative tests and recent infections) and the proportion of recent infections diagnosed among HIV-positive tests in Côte d’Ivoire, Malawi and Mozambique; three countries with several high-quality population-based surveys and HTS programme data. We examined time trends for these two metrics stratified by sex (male and female) and by broad age groups (15–24 and 25+). We compared these trends to the modelled trends in HIV incidence, the proportion tested among the adult population (testing effort) and the proportion HIV-positive among HTS clients (positivity). Total adult population annual estimates were obtained from Spectrum/EPP.
Ethics
Ethics approval was granted by the McGill Institutional Review Board (A10–E72–17A).
Results
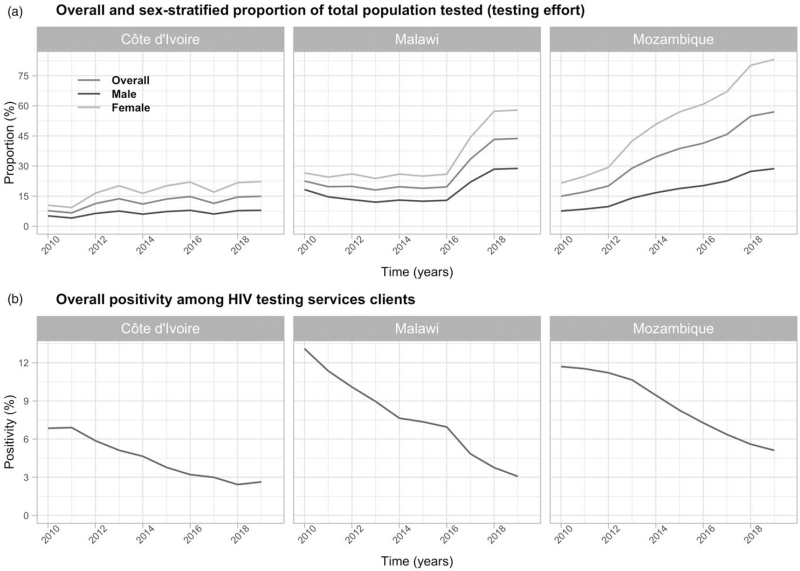
There was good agreement between the model and empirical data in all three countries (Figure S1). Testing rates increased in all three countries between 2010 and 2019. The annual proportion of adults tested for HIV (number of tests/total population) increased twofold in Malawi and fourfold in Mozambique (Fig. 1a). Increases were larger among women than men over the same period (Fig. 1a). Meanwhile, HIV positivity among HTS clients declined from 6.8 to 2.6% in Côte d’Ivoire, 13.1 to 3.0% in Malawi and 11.7 to 5.1% in Mozambique (Fig. 1b).
Fig. 1.
Modelled trends in HIV testing effort and positivity.
(a) Overall and sex-stratified modelled trends in testing effort (defined as the annual number of individual tested for HIV/total population) among people aged ≥15 years from 2010 to 2019 in Côte d’Ivoire, Malawi and Mozambique. (b) Overall modeled trends in positivity among HIV testing services clients aged ≥15 years from 2010 to 2019 in Côte d’Ivoire, Malawi and Mozambique.
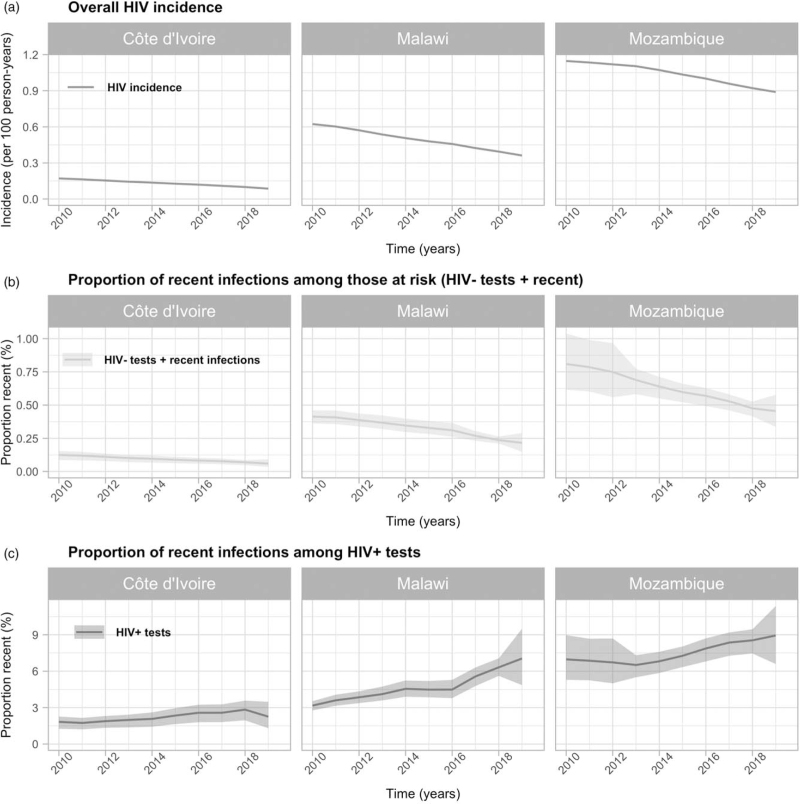
Modelled annual HIV incidence was higher than the proportion of recent infections among persons at risk of HIV acquisition in all countries, but both measures declined similarly over the period (Fig. 2a). The proportion of recent infections among those at risk of HIV acquisition declined from 0.12 to 0.06% in Côte d’Ivoire, from 0.41 to 0.22% in Malawi and from 0.81 to 0.45% in Mozambique over 2010–2019. Meanwhile, HIV incidence declined from 0.17 per 100 person-years (PY) to 0.09 per 100 PY in Côte d’Ivoire, 0.62 per 100 PY to 0.36 per 100 PY in Malawi, and 1.14 per 100 PY to 0.89 per 100 PY in Mozambique. In contrast, the proportion of recent infections among HIV-positive tests increased from 1.8 to 2.3% in Côte d’Ivoire, 3.2 to 7.0% in Malawi and 7.0 to 8.9% in Mozambique (Fig. 2b).
Fig. 2.
HIV incidence and recent infections expressed as proportions of HIV tests.
(a) HIV incidence (estimated from Spectrum/EPP); (b) proportion of recent infections among those at risk of HIV acquisition (HIV-negative tests and recent infections); and (c) proportion of recent infection among HIV-positive (HIV+) tests in Côte d’Ivoire, Malawi and Mozambique among people aged ≥15 years from 2010 to 2019.
The proportion of recent infections among those at risk of HIV acquisition declined for both men and women, concomitant with reductions in sex-stratified HIV incidence (Figure S2). The proportion of recent infections among those at risk of HIV acquisition declined more among women than men: 55 versus 49% in Côte d’Ivoire, 50 versus 44% in Malawi, and 47 versus 33% in Mozambique. In contrast, modeled reductions in HIV incidence were the same for both sexes – 49% in Côte d’Ivoire, 42% in Malawi and 22% in Mozambique (Figure S2) – but rates of HIV testing increased more for women than men (Fig. 1a) resulting in disproportionately larger reductions in the proportion recent among those testing positive.
In Côte d’Ivoire and Malawi, the relative change in the proportion of recent infections among those at risk of HIV acquisition by age groups 15–24 years and 25 and older mirrored trends in HIV incidence. (Figure S2). The difference was somewhat larger in Mozambique, where the proportion of recent infections among people at risk of HIV aquisition decreased 11% more among those aged 15–24 years than among those aged at least 25 years. In the three countries, younger people had a greater proportion of recent infection among HIV-positive tests, which increased faster than among people aged at least 25 years (Figure S2).
Discussion
The use of recency assays among people newly diagnosed with HIV has been promoted to enhance real-time HIV surveillance [7,12,23]. Implementing these assays in routine surveillance systems could also improve the timeliness and granularity of efforts to monitor HIV incidence trends and identifying population groups with higher infection risk. However, the interpretation of recency assays from routine surveillance systems should consider both denominators and testing efforts.
We compared two interpretations of data on recent infections among persons testing for HIV in Côte d’Ivoire, Malawi and Mozambique over the 2010–2019 period. Our modelling results strongly suggest that the proportion of recent infections diagnosed among people at risk of HIV acquisition provides a better indication of incidence trends and relative levels than the proportion recently infected among those testing HIV-positive. The proportion of recent infections among those diagnosed with HIV, which mostly increased in all countries, did not mirror trends in incidence. This was likely due to increases in testing effort and diagnosis coverage (Fig. 1a). Further, sex-specific trends in this metric were inconsistent in some countries, which could be attributed to reductions in the proportion of undiagnosed infections and changes in testing efforts or test-seeking behaviours, rather than shifts in active transmission.
However, we also found that sex-stratified trends in the proportion of recently infected among those at risk of HIV acquistion was not reflective of the true sex-stratified trends for HIV incidence. This was due to differences in testing rates by sex. Women tend to seek testing earlier in the course of their infection and test more frequently due to their engagement in antenatal care and other targeted testing activities [10,28]. This conclusion also implies caution when using the proportion of recent infections among those at risk of HIV acquisition to characterize other risk groups or relative incidence patterns where apparent differences may be confounded by different testing patterns. Interpreting programmatic recency data within a framework that explicitly represents differences in testing behaviors, such as the model analysed here, may help to address these limitations.
Our results must be interpreted in light of some limitations. First, we assumed perfect sensitivity and specifity of HIV infection detection, such that there are no false-positive or false-negative. The accuracy and precision of RITA in routine programmatic settings is a distinct and important area of ongoing research that could further compound interpretation of trends [3]. Second, modelled rates of testing were identical for people with recent infection and those with long-standing infections in the model at high CD4+ cell counts (≥350 CD4+). This assumption implies that our results could slightly underestimate the proportions of recent infections, as people with recent infection might engage in at-risk behaviours or experience acute HIV symptoms that motivate seeking healthcare and HIV testing [29,30]. Last, our results should be carefully interpreted in the context of large-scale routine surveillance systems rather than at local levels.
This work is an effort to examine under which circumstances recency data represent an accurate indicator of active HIV transmission and incidence trends. Strengths of our work include, firstly, the integration of routinely collected HTS programme data, population-based surveys and standardized statistical estimates of the HIV epidemic in a coherent modeling framework. Second, we considered sex and age-specific patterns of HIV testing and stratified our results by age and sex. Third, we systematically compared and contrasted our different metrics to incidence estimates, testing efforts and positivity.
Conclusions
Our analysis showed that the proportion of adults recently infected among those at risk of HIV acquisition will more accurately identify areas or groups of individuals at high risk of HIV acquisition and trends in HIV incidence. This denominator should be preferred over HIV-positive tests. However, patterns in this proportion may be a biased reflection of relative HIV incidence patterns in populations with different HIV testing behaviours, such as among men versus women or geographically targeted testing campaigns. Data from routine recent infection testing must be interpreted in the context of patterns and changes in HIV testing.
Acknowledgements
We acknowledge funding from the Steinberg Fund for Interdisciplinary Global Health Research (McGill University), the Canadian Institutes of Health Research, and the Bill and Melinda Gates Foundation. M.M.G. is supported by a Canada Research Chair (Tier 2) in Population Health Modeling. K.G. was supported by Postdoctoral Fellowships from the Fonds the recherche du Québec – Santé and the Canadian Institutes of Health Research. J.W.E. was supported by UNAIDS, the Bill and Melinda Gates Foundation, and National Institute of Allergy and Infectious Disease of the National Institutes of Health (award number R01AI136664).
A.G., K.G., J.W.E. and M.M.G. conceptualized the study. J.W.E., K.M. and M.M.-G. conceived and designed the model. A.G., K.G., A.J., F.M., E.E., J.W.E., K.M. and M.M.-G. obtained, administered and processed the different databases. A.G., K.G., A.J., J.W.E., K.M. and M.M.-G. contributed to model development and/or revisions. A.G., K.G., J.W.E. and M.M.-G. performed the analyses and all authors contributed to results interpretation. A.G. drafted the manuscript and all authors critically reviewed it for important intellectual content. All authors approved the final version.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.UNAIDS/WHO Working Group on Global HIV/AIDS/STI Surveillance, WHO Technical Working Group on HIV Incidence Assays, World Health Organization, Department of HIV/AIDS. When and how to use assays for recent infection to estimate HIV incidence at a population level. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Grebe E, Facente SN, Hampton D, Cheng C, Owen R, Keating SM, et al. Consortium for the Evaluation and Performance of HIV Incidence Assays (2019, October 11). Asanté™ HIV-1 rapid recency® assay evaluation report (version 1.0). Zenodo 2019; 10.5281/zenodo.3509834 [DOI] [Google Scholar]
- 3.Galiwango RM, Ssuuna C, Kaleebu P, Kigozi G, Kagaayi J, Nakigozi G, et al. Validation of the Asante HIV-1 rapid recency assay for detection of recent HIV-1 infections in Uganda. AIDS Res Hum Retroviruses 2021; doi: 10.1089/AID.2020.0279. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Laeyendecker O, Wang R. Cross-sectional human immunodeficiency virus incidence estimation accounting for heterogeneity across communities. Biometrics 2019; 75:1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassanjee R, Pilcher CD, Busch MP, Murphy G, Facente SN, Keating SM, et al. Viral load criteria and threshold optimization to improve HIV incidence assay characteristics. AIDS Lond Engl 2016; 30:2361–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Centre for Disease Prevention and Control. Monitoring recently acquired HIV infections in the European context. Stockholm: ECDC; 2013. [Google Scholar]
- 7.World Health Organization. WHO working group on HIV incidence measurement and data use; 3–4 March 2018; Boston, MA, USA: meeting report. Geneva: World Health Organization; 2018. https://apps.who.int/iris/handle/10665/272940. [Google Scholar]
- 8.Mastro TD, Kim AA, Hallett T, Rehle T, Welte A, Laeyendecker O, et al. Estimating HIV incidence in populations using tests for recent infection: issues, challenges and the way forward. J HIV AIDS Surveill Epidemiol 2010; 2:1–14. [PMC free article] [PubMed] [Google Scholar]
- 9.Hallett TB. Estimating the HIV incidence rate – recent and future developments. Curr Opin HIV AIDS 2011; 6:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice BD, Wit M, Welty S, Risher K, Cowan FM, Murphy G, et al. Can HIV recent infection surveillance help us better understand where primary prevention efforts should be targeted? Results of three pilots integrating a recent infection testing algorithm into routine programme activities in Kenya and Zimbabwe. J Int AIDS Soc 2020; 23:e25513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim AA, Rehle T. Short communication: assessing estimates of HIV incidence with a recent infection testing algorithm that includes viral load testing and exposure to antiretroviral therapy. AIDS Res Hum Retroviruses 2018; 34:863–866. [DOI] [PubMed] [Google Scholar]
- 12.Kim AA, Behel S, Northbrook S, Parekh BS. Tracking with recency assays to control the epidemic: real-time HIV surveillance and public health response. AIDS Lond Engl 2019; 33:1527–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maman D, Chilima B, Masiku C, Ayouba A, Masson S, Szumilin E, et al. Closer to 90–90–90. The cascade of care after 10 years of ART scale-up in rural Malawi: a population study. J Int AIDS Soc 2016; 19:20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehle T, Johnson L, Hallett T, Mahy M, Kim A, Odido H, et al. A ccmparison of South African National HIV incidence estimates: a critical appraisal of different methods. PLoS One 2015; 10:e0133255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hines JZ, Sachathep K, Pals S, Davis SM, Toledo C, Bronson M, et al. HIV incidence by male circumcision status from the population-based HIV impact assessment (PHIA) surveys: eight sub-Saharan African countries, 2015–2017. JAIDS J Acquir Immune Defic Syndr 2021; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aghaizu A, Murphy G, Tosswill J, DeAngelis D, Charlett A, Gill O, et al. Recent infection testing algorithm (RITA) applied to new HIV diagnoses in England, Wales and Northern Ireland, 2009 to 2011. Euro Surveill 2014; 19:20673. [DOI] [PubMed] [Google Scholar]
- 17.Robinson E, Moran J, O’Donnell K, Hassan J, Tuite H, Ennis O, et al. Integration of a recent infection testing algorithm into HIV surveillance in Ireland: improving HIV knowledge to target prevention. Epidemiol Infect 2019; 147:e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV incidence in the United States, 2006–2009. PloS One 2011; 6:e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Vu S, Le Strat Y, Barin F, Pillonel J, Cazein F, Bousquet V, et al. Population-based HIV-1 incidence in France, 2003–08: a modelling analysis. Lancet Infect Dis 2010; 10:682–687. [DOI] [PubMed] [Google Scholar]
- 20.Fisher M, Pao D, Murphy G, Dean G, McElborough D, Homer G, et al. Serological testing algorithm shows rising HIV incidence in a UK cohort of men who have sex with men: 10 years application. AIDS Lond Engl 2007; 21:2309–2314. [DOI] [PubMed] [Google Scholar]
- 21.Oster AM, France AM, Panneer N, Ocfemia MCB, Campbell E, Dasgupta S, et al. Identifying clusters of recent and rapid HIV transmission through analysis of molecular surveillance data. J Acquir Immune Defic Syndr 1999 2018; 79:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welty S, Motoku J, Muriithi C, Rice B, de Wit M, Ashanda B, et al. Brief report: recent HIV infection surveillance in routine HIV testing in Nairobi, Kenya: a feasibility study. JAIDS J Acquir Immune Defic Syndr 2020; 84:5–9. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Health, Malawi. Estimating HIV incidence and detecting recent infection among pregnant adolescent girls and young women in Malawi, 2017–2018: final report. Lilongwe: Ministry of Health; 2019. [Google Scholar]
- 24.Maheu-Giroux M, Marsh K, Doyle CM, Godin A, Lanièce Delaunay C, Johnson LF, et al. National HIV testing and diagnosis coverage in sub-Saharan Africa: a new modeling tool for estimating the ‘first 90’ from program and survey data. AIDS 2019; 33:S255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giguère K, Eaton JW, Marsh K, Johnson LF, Johnson CC, Ehui E, et al. Trends in knowledge of HIV status and efficiency of HIV testing services in sub-Saharan Africa, 2000–20: a modelling study using survey and HIV testing programme data. Lancet HIV 2021; 8:e284–e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stover J, Glaubius R, Mofenson L, Dugdale CM, Davies M-A, Patten G, et al. Updates to the Spectrum/AIM model for estimating key HIV indicators at national and subnational levels. AIDS 2019; 33:S227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.UNAIDS. UNAIDS data 2020. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2020. [Google Scholar]
- 28.Staveteig S, Croft TN, Kampa KT, Head SK. Reaching the ‘first 90’: gaps in coverage of HIV testing among people living with HIV in 16 African countries. PloS One 2017; 12:e0186316–e1186316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders EJ, Chirro O, Oduor C, Mangi J, Wahome E, Price MA, et al. Point-of-care HIV RNA testing and immediate antiretroviral therapy initiation in young adults seeking out-patient care in Kenya. AIDS Lond Engl 2019; 33:923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders EJ, Wahome E, Mwangome M, Thiong’o AN, Okuku HS, Price MA, et al. Most adults seek urgent healthcare when acquiring HIV-1 and are frequently treated for malaria in coastal Kenya. AIDS Lond Engl 2011; 25:1219–1224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.