ABSTRACT
Radiotherapy is one of the curative mainstays of prostate cancer; however, its efficacy is often diminished by tumor radioresistance. Depending on the stage of disease, tumors can relapse in approximately 50% of patients with prostate cancer after radiotherapy. Nevertheless, the mechanisms that drive tumor cell survival are not fully characterized, and reliable molecular prognostic markers of prostate cancer radioresistance are missing. Similar to other tumor entities, prostate cancer cells are heterogeneous in their capability to maintain tumor growth. The populations of cancer stem cells (CSCs) with self-renewal and differentiation properties are responsible for tumor development and recurrence after treatment. Eradication of these CSC populations is of utmost importance for efficient tumor cure. In a recently published study, we showed that prostate cancer cells could be radiosensitized by glutamine deprivation, resulting in DNA damage, oxidative stress, epigenetic modifications, and depletion of CSCs. Conversely, prostate cancer cells with resistance to glutamine depletion show an activation of ATG-mediated macroautophagy/autophagy as a survival strategy to withstand radiation-induced damage. Thus, a combination of targeting glutamine metabolism and autophagy blockade lead to more efficient prostate cancer radiosensitization.
Abbreviations: ATG5: autophagy related 5; CSCs: cancer stem cells; GLS: glutaminase; TCA cycle: tricarboxylic acid cycle
KEYWORDS: ATG5, autophagy, cancer stem cells, GLS1, glutamine, MYC, prostate cancer, radioresistance
Radiotherapy is one of the main treatment options for prostate cancer. The curative potential of radiotherapy is mediated by irradiation-induced oxidative stress and DNA damage in tumor cells. However, treatment efficacy is often compromised by intrinsic or acquired tumor radioresistance. Recurrence of tumors after radiotherapy results from the survival of a cancer stem cell (CSC) population. CSCs possess inherent resistance to chemo- and radiotherapy and the ability to self-renew and give rise to new populations of tumor cells. In addition, recent studies showed that CSCs have unique metabolic demands, relying on the utilization of specific amino acids and other nutrients. Targeting these metabolic pathways and associated signaling can be a promising approach to render radioresistant properties of CSCs and, therefore, increase the radiocurability of prostate tumors.
Our recent study demonstrated that prostate cancer cells and CSCs, in particular, are addicted to glutamine [1]. Glutamine is a non-essential amino acid that becomes conditionally essential for fast-proliferating tissues, including cancer. Glutamine is utilized by cells via transforming into glutamate by the enzyme GLS (glutaminase); glutamate can be further metabolized into alpha-ketoglutarate, which undergoes incorporation into the tricarboxylic acid (TCA) cycle, and serves as a building block for glutathione and as a co-factor for DNA and histone demethylases. We combined global gene expression profiling and the targeted metabolomics approach to study metabolic processes, which differed between parental prostate cancer cell lines (DU145, LNCaP, and PC3) and their radioresistant derivatives created by applying fractionated X-ray irradiation. This approach demonstrated that radioresistant prostate cancer cells have deregulated glutamine metabolism compared to parental counterparts. Further experiments showed that glutamine withdrawal leads to radiosensitization of prostate cancer cells by increasing DNA damage, shifting redox balance, and decreasing CSC properties, such as in vivo tumorigenicity and resistance to anoikis (Figure 1). Moreover, depletion of glutamine attenuates the histone methylation profile of prostate cancer cells. Targeting glutamine metabolism of patient-derived primary prostate cell cultures by chemical inhibition of GLS and MYC leads to a significant radiosensitization of cancer cells. In contrast, this treatment does not cause toxic effects and even protects the benign prostate hyperplasia cells. Furthermore, we correlated expression levels of the GLS and MYC genes in tumor tissues and amino acid concentrations in blood plasma to progression-free survival in prostate cancer patients, therefore proposing GLS and MYC as potential prognostic biomarkers for prostate cancer patients treated with radiotherapy.
Figure 1.
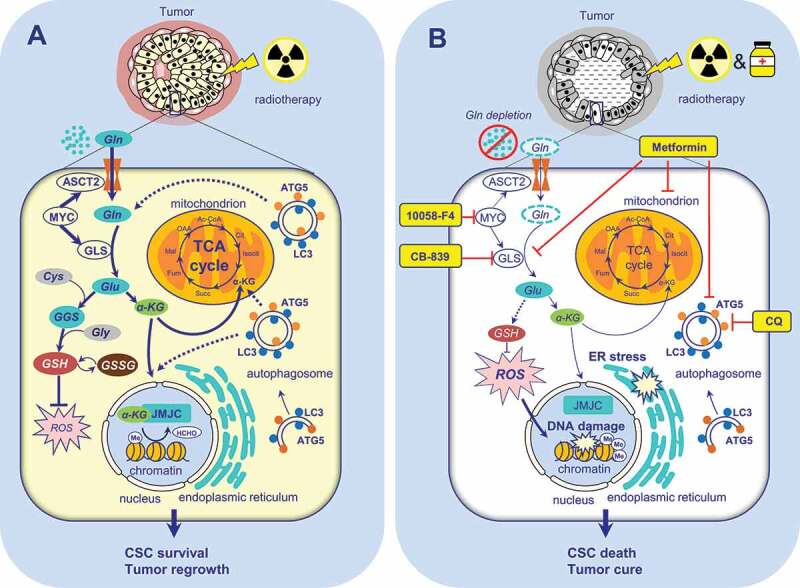
The role of Gln metabolism and autophagy in the regulation of prostate cancer radioresistance. (A) Gln serves for energy production in the TCA cycle, maintaining the redox state, epigenetic regulation, and the maintenance of CSCs. The activation of autophagy improves cell survival in a low glutamine microenvironment. (B) Targeting glutamine metabolism and autophagy results in tumor radiosensitization. αKG – alpha ketoglutarate; ATG5 – autophagy related 5; CSC – cancer stem cell; CQ – chloroquine; Cys – cysteine; ER - endoplasmic reticulum; GGC - γ-glutamylcysteine;GLS – glutaminase; Gln – glutamine; Glu – glutamate; Gly – glycine; GSH - glutathione (reduced form); GSSG - glutathione disulfide (oxidized form of GSH); HCHO – formaldehyde, a product of histone demethylation; JMJC – Jumonji C (JmjC)-domain-containing histone demethylases; LC3 – microtubule-associated protein 1 light chain 3; Me – histone methylation; ROS – reactive oxygen species; RS – radiosensitizers; SLC1A5/ASCT2 – solute carrier family 1 member 5; TCA – tricarboxylic acid cycle
Autophagy is one of the critical mechanisms involved in cancer cell survival upon nutrient deficiency. Our work demonstrated that, in contrast to the glutamine-dependent DU145 cells, LNCaP cells do not exhibit significant radiosensitization upon glutamine withdrawal. To elaborate on this phenomenon more, we compared gene expression profiling of DU145 and LNCaP cells. Our findings showed that DU145 cells do not express the canonical isoform of ATG5. The ATG5 gene encodes one of the key components in autophagosome formation. Presumably, cells lacking ATG5 may not be able to have a fully functional autophagic pathway. Indeed, in contrast to LNCaP cells, DU145 does not show LC3 lipidation and autophagosome formation upon glutamine starvation. By inhibiting the maturation of autolysosomes with chloroquine or by siRNA-mediated knockdown of ATG5, we are able to radiosensitize LNCaP cells upon glutamine deprivation. These findings suggest that a combination of nutrient deprivation with inhibition of autophagy, which serves as a backup source of energy and biosynthesis, may be a promising approach for radiosensitization of prostate cancer cells, which are otherwise resistant to nutrient depletion (Figure 1).
Although the role of autophagy as a regulator of tumor radiosensitivity is yet a matter of debate and targeting autophagy is not yet clinically approved as an anticancer treatment, recent studies showed that the diabetes drug metformin at micromolar concentrations inhibits both glutamine metabolism and autophagy in tumor cells. Our study confirmed the radiosensitizing effect of low doses of metformin in prostate cancer cells resistant to glutamine deprivation. Furthermore, early-stage clinical trials are currently exploiting metabolic vulnerabilities of solid tumors with mutations in IDH1 (isocitrate dehydrogenase (NADP(+)) 1) or IDH2 by a combination of metformin and chloroquine (NCT02496741).
To conclude, our work demonstrated that glutamine metabolism could be considered as a promising therapeutic target for prostate cancer radiosensitization. Moreover, targeting autophagy in nutrient deprivation-insensitive cells can be an additional approach to re-sensitize resistant cells to radiotherapy. Further experiments in the patient-derived xenograft models and clinical studies are warranted to ascertain the potential therapeutic utility of targeting glutamine metabolism and autophagy for prostate cancer radiosensitization and the implication of autophagy-related biomarkers for the prediction of prostate cancer recurrence after radiotherapy.
Funding Statement
Work in AD lab was partially supported by grants from Deutsche Forschungsgemeinschaft (DFG): SPP 2084 µBONE (project number 401326337) and project number 416001651.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Mukha A, Kahya U, Linge A, et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics. 2021;11(16):7844–7868. [DOI] [PMC free article] [PubMed] [Google Scholar]


