Abstract
In recent years, and particularly associated with the increase of cancer patients’ life expectancy, the occurrence of cancer treatment sequelae, including cognitive impairments, has received considerable attention. Chemotherapy-induced cognitive impairments (CICI) can be observed not only during pharmacological treatment of the disease but also long after cessation of this therapy. The lack of effective tools for its diagnosis together with the limited treatments currently available for alleviation of the side-effects induced by chemotherapeutic agents, demonstrates the need of a better understanding of the mechanisms underlying the pathology. This review focuses on the comprehensive appraisal of two main processes associated with the development of CICI: neuroinflammation and oxidative stress, and proposes the endogenous cannabinoid system (ECS) as a new therapeutic target against CICI. The neuroprotective role of the ECS, well described in other cognitive-related neuropathologies, seems to be able to reduce the activation of pro-inflammatory cytokines involved in the neuroinflammatory supraspinal processes underlying CICI. This review also provides evidence supporting the role of cannabinoid-based drugs in the modulation of oxidative stress processes that underpin cognitive impairments, and warrant the investigation of endocannabinoid components, still unknown, that may mediate the molecular mechanism behind this neuroprotective activity. Finally, this review points forward the urgent need of research focused on the understanding of CICI and the investigation of new therapeutic targets.
Keywords: chemotherapy-induced cognitive impairment, cannabinoid drugs, endocannabinoid system, neuroinflammation, oxidative stress
Introduction
The occurrence of sequelae after chemotherapeutic treatment has recently attracted increasing interest, particularly given the higher life expectancy of those with a lived experience of cancer. The cognitive alterations described following cancer experience normally occur during pharmacological treatment of the disease, however, it can prevail long after the cessation of therapy. This phenomenon is known as chemotherapy-induced cognitive impairment (CICI), chemofog or chemobrain. Preclinical research has shown that chemotherapeutic agents such as oxaliplatin, paclitaxel, cyclophosphamide, methotrexate, 5-fluorouracil or doxorubicin can induce short- and long-term deleterious effects in working memory and fear and spatial learning in a wide variety of rodent models (Table 1). Moreover, neuroimaging studies have collected data from patients following chemotherapeutic regime supporting chemotherapy induced alterations on brain structure and plasticity. These studies showed the presence of cognitive alterations independently on the tumour location; suggesting that chronic chemotherapy treatment may induce alterations on cognitive functionality (Wefel and Schagen, 2012; Conroy et al., 2013; McDonald et al., 2013; Sleurs et al., 2016).
TABLE 1.
Summary of cognitive deficits induced by chemotherapeutic drugs in preclinical animal models of chemotherapy-induced cognitive impairment (CICI).
| Chemotherapeutic drug | Animal model | Regime | Cognitive impairments observed | References |
|---|---|---|---|---|
| Cyclophosphamide (CPA) | Young adult male ICR mice | One i.p. administration (40 mg/kg) | • CPA induced deficits in memory retention in the PAT and the NOR 12 h after administration | Yang et al. (2010) |
| • These CPA-related effects on cognition were not observed 10 days after drug administration | ||||
| Young adult male ICR mice | Weekly i.p. administration for 4 consecutive weeks (80 mg/kg per administration) | • Learning deficiencies in the PAT. | Hou et al. (2013) | |
| • Impairment of spatial memory in the Y-maze | ||||
| Young adult male athymic nude rats | Weekly i.p. administration for 5 consecutive weeks (50 mg/kg per administration) | • CPA administration caused an impairment of spatial memory in the NLR. | Christie et al. (2012) | |
| • In the FC paradigm, CPA caused a decrease of freezing upon re-exposure to the context, but not to the cue | ||||
| Oxaliplatin (OXA) | Male and female hooded Wistar rats | One i.p. administration (6 mg/kg) | • Male and female animals treated with OXA exhibited a deficit of working memory in the NOR. | Johnston et al. (2017) |
| • OXA induced a significant impairment of spatial memory in the NLR. | ||||
| • In the FC paradigm, OXA impaired the renewal of extinguished fear conditioning for up to 19 days after administration | ||||
| Male Sprague-Dawley rats | One i.p. administration (12 mg/kg) | • OXA administration induced an impairment in the renewal of extinguished fear in the FC paradigm | Sharpe et al. (2012) | |
| Male hooded Wistar rats | Weekly i.p. administration for 3 consecutive weeks (0.6, 2 and 6 mg/kg per administration) | • Only the highest dose of OXA (6 mg/kg) induced a gradual deterioration of the recognition memory in the NOR. This impairment became appreciable 4 months after and lasted up to 11 months | Fardell et al. (2015) | |
| • In the NLR the lower doses of OXA (0.6 and 2 mg/kg) induced a deficit of spatial memory 15 and 30 days after treatment, although this deleterious effect was not observed 4 and 11 months after OXA administration | ||||
| • The highest dose (6 mg/kg) induced a long lasting (up to 11 months after administration) deficit of spatial memory in the NLR. | ||||
| Cisplatin | Infant and adolescent male Sprague-Dawley rats | Weekly i.p. administration for 5 consecutive weeksd (2 mg/kg per administration) | • Cisplatin induced in infant and adolescent animals an impairment of the recognition memory in the NOR. | John et al. (2017) |
| • Only adolescent animals exhibited an impairment of spatial memory in the NLR. | ||||
| • In the FC paradigm, cisplatin impaired contextual memory, but not cued memory, of infant and adolescent animals | ||||
| 5-Fluorouracil (5-FU) | Young adult male C57BL/6 J mice | One i.p. administration (75 mg/kg) | • 5-FU caused short-term (2–12 weeks) impairments of spatial memory in the NLR and the Barnes maze. Likewise, 5-FU impaired recognition memory in the NOR. | Seigers et al. (2015) |
| • In the long term (15–25 weeks) only the spatial memory impairment in the NLR persisted | ||||
| Methotrexate (MTX) | Male Sprague-Dawley rats | One i.p. administration (20 mg/kg) | • Animals treated with MTX exhibited in the short-term deficits of memory retention in the PAT and an impairment of spatial memory in the Y-maze | Shalaby et al. (2019) |
| Infant female C57BL/6 J mice | One i.p. administration (20 mg/kg) | • Administration of MTX during infancy induced in the adulthood an impairment of spatial memory in the Morris water maze | Elens et al. (2019) | |
| Young adult male Long Evans rats | - One i.t. administration (0.5 mg/kg) | • Both administration schedules of MTX induced a deficit in recognition and spatial memory measured by the NOR and the NLR respectively | Vijayanathan et al. (2011) | |
| - Four i.t. administrations over 10 days (0.5 mg/kg per administration) | • Repeated MTX administration induced a longer deleterious effect on cognition than the single administration protocol | |||
| Infant male and female Swiss-Webster mice | Daily i.p. administration for 3 consecutive days (2 mg/kg per administration) | • Infant administration of MTX induced in the adolescence an impairment of recognition memory in the NOR. | Bisen-Hersh et al. (2013) | |
| Paclitaxel | Young adult male Sprague-Dawley rats | Four i.p. administrations every 2 days (2 mg/kg per administration) | • Impairment of spatial memory in the Morris water test | (Li et al., 2017), (Li et al., 2018) |
| Young adult male C57BL/6 J mice | One i.p. administration (33 mg/kg) | • Paclitaxel induced in the short (2–12 weeks) and the long term (15–25 weeks) an impairment of spatial memory in the NLR. | Seigers et al. (2015) | |
| Doxorubicin (DOX) | Young adult male C57BL/6 J mice | One i.v. administration (5 or 10 mg/kg) | • The lowest dose (5 mg/kg) impaired spatial memory in the NLR. | Seigers et al. (2015) |
| • The highest dose (10 mg/kg) induced an impairment of the recognition memory in the NOR and the spatial memory in the NLR and the Barnes maze | ||||
| Young adult male Wistar rats | Four i.p. administrations every 2 days (2 mg/kg per administration) | • DOX caused an impairment of spatial memory in the Morris water maze and memory retention in the PAT. | Park et al. (2018) | |
| Young adult male Wistar rats | One administration every 5 days over 50 days (2.5 mg/kg) | • DOX impaired recognition memory in the NOR. | Verma et al. (2017) |
- Chemotherapeutic agents: 5-FU, 5-Fluorouracil; CPA, cyclophosphamide; DOX, doxorubicin; MTX, methotrexate; OXA, oxaliplatin.
- Type of administration: i.p., intraperitoneal; i.t., intrathecal; i.v., intravenous.
- Behavioural test: FC, fear conditioning; NLR, novel location recognition test; NOR, novel object recognition test; PAT, passive avoidance test.
Despite the great number of antineoplastic drugs available in the market, only a few of them have been tested on preclinical and clinical studies of CICI, emphasizing the lack of clinical evaluation of cognition-related side effects. In addition, the majority of models investigating CICI have limited their attention on non-CNS cancer types (Wefel and Schagen, 2012), especially on breast cancer, biasing thus the investigation of CICI into one sex population and type of cancer disease.
Among the most common cognitive deficiencies reported, are those of short-term working and visuospatial memories, verbal ability, executive functions and attention span (Conroy et al., 2013; Du et al., 2013; McDonald et al., 2013; Sleurs et al., 2016). These deficiencies are difficult to detect since the cognitive levels observed in CICI patients are often placed at the lower end of the normal range of the population. In addition, the lack of approved tests for CICI diagnosis complicates medical evaluation (Horowitz et al., 2018; Nguyen and Ehrlich, 2020). Similar limitations are observed in the cognitive rehabilitation of CICI patients. The current, palliative, therapies available involves physical activity and cognitive-behavioural therapy (Kesler et al., 2013; Ferguson et al., 2014; Fernandes et al., 2019). Even though these therapies seem to improve the life quality of the patients, they require a lot of time, effort and economical aids. Therefore, the ongoing investigation of CICI leads the attention to develop new pharmacotherapies attending to the neurobiological alterations associated with this disease.
There are a great number of biological mechanisms that seem to be implicated in the cognitive deficits induced by chemotherapy agents, including: direct neurotoxic effects, impaired neurogenesis or increased death of nervous cells, white matter abnormalities, inflammatory responses, oxidative stress and even alterations in the levels of sex and stress hormones (Sleurs et al., 2016; El-Agamy et al., 2019; Mounier et al., 2020; Nguyen and Ehrlich, 2020).
The endogenous cannabinoid system (ECS) is a complex signalling system comprised of cannabinoid type 1 (CB1) and cannabinoid type 2 (CB2) receptors; endocannabinoid ligands: anandamide (AEA) and 2-arachidonoylglycerol (2-AG); and catabolizing enzymes: fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) (Viveros et al., 2012; Kaur et al., 2016; Woodhams et al., 2017; Fraguas-Sánchez et al., 2018; Gorzkiewicz and Szemraj, 2018; Micale and Drago, 2018; Zou and Kumar, 2018). Other related biogenic lipids such as oleoylethanolamine (OEA) and palmitoylethanolamine (PEA) are also included within the ECS as endocannabinoid-related compounds (Di Marzo, 2018). Interestingly, pharmacological modulation of the ECS has been shown to reduce cancer-induced side effects such as nausea, vomiting (Taylor et al., 2020) and peripheral neuropathy (Lynch et al., 2014; Masocha, 2018; Blanton et al., 2019). Several studies in animal models have evaluated the role of the ECS in the modulation of cognitive functions (Schreiner and Dunn, 2012; Cohen and Weinstein, 2018) indicating for example the anxiolytic effects of low doses of cannabinoids. However, only few trials with cannabinoids have evaluated the mood state of cancer patients. ∆-9-tetrahydrocannabinol (THC) and nabilone have been proposed as alleviators for cancer-related psychological disorders, including depression and anxiety (Guzmán, 2003), however they need to be further evaluated though clinical trials. As a matter of fact, to the best of our knowledge no study has ever analysed the potential therapeutic value of cannabinoid drugs in CICI (Kleckner et al., 2019).
In this review we aim to describe the role of the ECS in two well-known CICI-associated processes: neuroinflammation and oxidative stress. In lack of specific studies on the topic, we will review the involvement of the ECS in cancer disease and other pathologies exhibiting similar cognitive phenotype to CICI.
Cannabinoids and Cancer
From a preclinical perspective, several studies have reported the involvement of the ECS in cancer disease. Increased expression of endocannabinoid receptors and ligand levels have been classically associated with carcinogenesis processes and a higher aggressiveness of cancer (Zhu et al., 2000; Hart et al., 2004; McKallip et al., 2005). Additionally, CB2 receptors have been demonstrated to regulate HER2 (human epidermal growth factor receptor 2) oncogene expression, whose upregulation increases vulnerability to leukemia induced by viral infection (Pérez-Gómez et al., 2015).
Regarding the ECS as a therapeutic target against cancer activity, it has been observed its implication in the inhibition of cell proliferation and/or angiogenesis in different tumour types (Śledziński et al., 2020). Attending to cancer cell type and substance, the anti-tumorigenic effects of cannabinoids have been shown to be mediated via CB1, CB2 and TRPV1 receptors. Cell activation of CB2 receptors led to a reduced cell motility in bladder cancer, decreasing proliferation rates (Bettiga et al., 2017). The phytocannabinoids THC and Cannabidiol (CBD) have been also reported to exert anti-tumour effects on U-87 MG cell-derived tumour xenografts by decreasing cancer growth via cell apoptosis (Torres et al., 2011). THC was shown to induce apoptosis of primary brain tumour cells (Carracedo et al., 2006) and to inhibit tumour growth and survival in a murine Lewis lung adenocarcinoma model (Ramer and Hinz, 2017). Interestingly, knockout mice for CB1/CB2 receptors exhibited a lower incidence to develop skin cancer after treatment with ultraviolet radioation (Surh et al., 2008). In vivo investigations have revealed cannabinoid-inhibition of tumour angiogenesis by inhibition of vascular endothelial cell migration and survival; as well as suppression of proangiogenic factor and matrix metalloprotease (MMP) expression in tumours (Blázquez e al., 2003). Cannabinoid administration has also been associated with a significant decrease in the expression of proangiogenic factors VEGF and Ang2, which result essential for the vascularization of different types of tumours (Carmeliet and Jain, 2000; Casanova et al., 2002). Altogether, the anti-tumour activity, including cancer cell death induction and angiogenesis inhibition, of cannabinoid drugs remark their potential as emergent and effective pharmacological targets in cancer.
Despite the potential anti tumorigenic effects demonstrated in numerous preclinical evaluations only one clinical study tested THC phytocannabinoid as systemically anticancer agent in glioblastoma multiforme (Guzmán et al., 2006). THC was injected intracranially into patients with an early diagnosed glioblastoma. However, the experiment failed to provide strong data supporting THC’s efficacy at that cancer stage. Recent clinical investigations have tested the administration of exocannabinoid compounds, such as Sativex, CBD or dexanabidiol, in different modalities of cancer (e.g. glioblastoma, advanced solid tumours, brain cancer, and neck squamous cell carcinoma); showing reductions in circulating tumour cells, reductions in tumour size, improved survival rate or reduced risk of head and neck squamous cell carcinoma (Moreno et al., 2019). Another possible approach could combine the use of chemotherapeutic agents and cannabinoid drugs to establish whether cannabinoids can enhance the current drug treatments. The few experiments that have investigated this hypothesis have shown controversial results. One study, using γ-radiation combined with a cannabinoid-based treatment demonstrated increased leukemic cell death than single administration of γ-radiation (Jacobsson et al., 2000). However, synergism was not observed when cannabinoids and tamoxifen were combined to induce glioma cell death (Radin, 2003).
It is important to remark that cannabinoids are currently used in palliative medicine for treatment of nausea and vomiting in cancer patients undergoing chemotherapy (Besner et al., 1992; Hall et al., 2001; Walsh et al., 2002). In addition, several preclinical studies have shown beneficial effects of cannabinoid drugs in chemotherapy-induced neuropathy, which is a common side effect of several chemotherapeutic agents, especially platinum-based compounds and taxanes (Abrams and Guzman, 2015; Blanton et al., 2019). Even though the anticancer effectiveness of cannabinoid drugs still remains unclear, its clinical use for the alleviation of cancer side effects such as pain, vomiting, nausea or anorexia is well stablished (Abrams and Guzman, 2015; Dariš et al., 2019; Vecera et al., 2020).
Taking this context into account, the following sections aim to clarify the involvement of the ECS in the two main processes underlying chemotherapy-induced cognitive impairment: neuroinflammation and oxidative stress.
Cannabinoids and Neuroinflammation
The presence of a tumour and/or the pharmacological management of cancer provokes the activation of the immune system. This mechanism of defence promotes the release of pro-inflammatory mediators responsible for an inflammatory response (Mounier et al., 2020). The pro-inflammatory factors reach the central nervous system (CNS) enhancing the inflammatory response through the activation of glial cells such as microglia and astroglia (Nguyen and Ehrlich, 2020) and promote the release of proinflammatory cytokines such as: tumour necrosis factor alpha (TNFα), interleukin 1 (IL-1) and interleukin 6 (IL-6). A persistent neuroinflammatory response provokes, among others, alterations in neurogenesis and changes in the myelination processes (Mounier et al., 2020), which are responsible for the emergence of cognitive impairments (Fourrier et al., 2019).
The ECS plays a key role in the homeostasis of the immune system. The ECS modulation of the immune system can promote neurogenesis or neurodegeneration (Molina-Holgado and Molina-Holgado, 2010; Tanasescu et al., 2013). Cannabinoid drugs have been used as therapeutic tools in a great number of neuroinflammatory and ageing animal models that involve cognitive dysregulation (Bisogno and di Marzo, 2008; Bilkei-Gorzo, 2012; Chiurchiù et al., 2018; Estrada and Contreras, 2020). As reported below, several studies have analysed the neuroprotective actions of cannabinoid drugs in pathologies that combine neuroinflammatory responses and cognitive impairments, but present different aetiologies, such as Parkinson’s disease (PD), Alzheimer’s disease (AD) or traumatic brain injury (TBI) (Schurman and Lichtman, 2017; Rodrigues et al., 2019; Uddin et al., 2020). In this section, we propose to analyse the modulatory effect of cannabinoids in these neuropathologies to envision the potential beneficial role over CICI.
PD is a progressive and chronic neurodegenerative disorder characterized by the death of dopaminergic neurons in the substantia nigra pars compacta and the presence of intraneuronal inclusions of the protein a-synuclein, generally known as Lewy bodies (Braak et al., 2004; Concannon et al., 2015a; Rodrigues et al., 2019). In PD patients and animal models of PD, the ECS is highly dysregulated (Concannon et al., 2015a; Concannon et al., 2015b), suggesting an implication of this system in the pathology and progression of the disease. In addition, it has also been observed that pharmacological modulation of the ECS can induce neuroprotective actions in PD (Aymerich et al., 2018). For instance, the CB1 receptor agonist HU-210 exhibited neuroprotective properties to 6-hydroxydopamine (6-OHDA) neurotoxicity in vitro. This neuroprotective effect was greater in the presence of glial cells, suggesting that HU-210 neuroprotection depends on its ability to modify this type of cells (Lastres-Becker et al., 2005). Similar results were observed in two animal models of PD-induced neuroinflammation; PD induced by lipopolysaccharide (LPS) and PD induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Both animal models exhibited a reduction of microglial activation, thus pro-inflammatory cytokines expression, following HU-210 and WIN55,212-2, CB1 and CB1/CB2 agonists respectively, administration (Chung et al., 2011; Chung et al., 2012). In addition to WIN55,212–2, the CB2 receptor agonist JWH155 induced a similar effect against MPTP neurotoxicity, while CB2 receptor genetic ablation exacerbated MPTP neurotoxicity (Price et al., 2009). Likewise, CB2 receptor knockout mice are more sensitive to the neuroinflammatory effects induced by LPS compared to their wild littermates (García et al., 2011). Additionally, an increase in CB2 receptor expression has been positively correlated with an increase of microglial activation (Concannon et al., 2015b) in animal models of neuroinflammation and neutoxocity. Moreover, recent post-mortem studies have shown that there is an increase in the expression of CB2 receptors in microglia of the substantia nigra and a decreased expression of this cannabinoid receptor in tyrosine hydroxylase-positive cells in patients suffering from PD (García et al., 2015; Gómez-Gálvez et al., 2016). Additionally, it was detected, in neurotoxic and inflammation-driven animal models of PD, an increase in CB2 receptor expression that correlated with an increase of microglial activation (Concannon et al., 2015b), attributing clinical relevance to the involvement of CB2 receptors in neuroinflammatory processes associated with PD.
AD is a neuropsychiatric and neurodegenerative disorder with an important neuroinflammatory component. In fact, chronic inflammation contributes to the pathophysiology of AD and is closely associated to the neuropathological and cognitive syndromes of AD (Marchalant et al., 2008; Bonnet and Marchalant, 2015). Several studies have observed that the activation of CB2 receptors decrease neuroinflammation in animal models of AD (Uddin et al., 2020). In a recent study the administration of the CB2 receptor agonist JWH-015 induced a significant reduction of the gene expression of pro-inflammatory cytokines in the prefrontal cortex of the APP/PS1 double transgenic mice linked to a decrease of the microglial biomarker Iba-1. Yet, CB2 activation did not reduce neuroinflammation in the hippocampus or decreased the β-amyloid plaque deposition (Li et al., 2019). In addition, administration of JWH-015 in these transgenic mice improved their working memory in the novel object recognition test, but not their spatial memory measured in the Morris water maze (Li et al., 2019). In the same animal model, the administration of the CB1 receptor agonist ACEA decreased astroglial response in the vicinity of β-amyloid plaques and decreased the expression of the pro-inflammatory cytokine interferon-γ in astrocytes (Aso et al., 2012). ACEA also improved the working memory and decreased the activity of Akt and ERK in the hippocampus of another AD animal model consisting in intracerebroventricular administration of streptozotocin (STZ) (Crunfli et al., 2019). CBD is one of the main pharmacologically active phytocannabinoids of the plant Cannabis sativa L. (Atalay et al., 2020), but, unlike THC, it does not produce psychotropic effects and presents no affinity to CB1 and CB2 receptors. In vitro studies have described the anti-inflammatory effects of CBD (Esposito et al., 2006a; Esposito et al., 2006b; Martín-Moreno et al., 2011), however, recent in vivo studies have failed to relate these effects with a reversion of cognitive impairments in animal models of AD (Cheng et al., 2014a; Cheng et al., 2014b; Watt et al., 2020) which may indicate that CB1 and CB2 receptors play a crucial role in the cognitive impairments induced by the inflammatory response and they are potential therapeutic targets to take into account in future experiments.
Traumatic Brain injury (TBI) is a non-degenerative disease induced by a mechanical neuronal damage. This type of damage triggers a cascade of neuroinflammatory events usually followed by an increase of endocannabinoid ligand levels: AEA and 2-AG. This effect is thought to be an immediate response to maintain brain-related homeostasis since binding of these ligands to CB1 and CB2 receptors generate an anti-inflammatory response in an attempt to counteract the injury-related inflammation (Vázquez et al., 2015; Schurman and Lichtman, 2017). AEA levels have been shown to be increased in the brain ipsilateral side of the lesion in different TBI animal models, a compensatory effect that is thought to prevent cell degeneration. Administration of the FAAH inhibitor PF-3845 prevented dendritic loss and restored the levels of synaptophysin, a synaptic transmission precursor, in the ipsilateral dentate gyrus. Furthermore, the administration of PF-3845 (5 mg/kg) reversed TBI-induced impairment of hippocampal-dependent memory. However, since PF3845 not only induced an increase on AEA levels but also 2-AG levels (Tchantchou et al., 2014), both endocannabinoid ligands could be involved in this neuroprotective activity observed in the ipsilateral brain. In addition, CB1 receptor antagonists reverted 2-AG anti-inflammatory effects suggesting 2-AG-mediated activation of CB1 receptors induce neuroprotection (Panikashvili et al., 2001; Panikashvili et al., 2005; Panikashvili et al., 2006). TBI also induces a significant increase of CB2 receptors expression in the injured cortex. Activation of CB2 receptors by GP1a (a CB2 receptor agonist) induced a significant decrease in the levels of pro-inflammatory cytokines as well as an increase in the number of M2 macrophages in a TBI animal model (Braun et al., 2018). Since CB1 and CB2 activation plays such an important role counteracting the neuroinflammatory response after TBI it is not surprising that two well-known neuroprotective compounds with no direct relation with the ECS, such as the antibiotic minocycline and the hormone leptin, had their anti-inflammatory properties blocked when CB1 and CB2 receptor antagonists were administered (Lopez-Rodriguez et al., 2015; Lopez-Rodriguez et al., 2016). Although the use of cannabinoid drugs following TBI has been linked to decreased inflammatory cell activation and decreases in pro-inflammatory cytokine production (Price et al., 2009), little is known about the prevention or reversion of the development of cognitive impairments after TBI.
Cannabinoids and Oxidative Stress
Chemotherapeutic drugs induce an increase of the mitochondrial production and accumulation of reactive oxygen and nitrogen species (ROS/RNS), a phenomenon known as oxidative stress (Lipina and Hundal, 2016; Atalay et al., 2020; Mounier et al., 2020). Intracellular accumulation of ROS and RNS results in cell damage and subsequent death (Lipina and Hundal, 2016; Umeno et al., 2017; Gallelli et al., 2018; Atalay et al., 2020). Oxidative stress is especially toxic in cancer cells due to their high metabolic rate, however, healthy cells in the CNS can also be damaged by the oxidative stress-related toxicity generated by chemotherapeutic agents (Kawai et al., 2006; Rajamani et al., 2006; Joshi et al., 2007; Tangpong et al., 2007; Liu et al., 2009).
In the past few years, it has been observed a correlation between the ECS and the synthesis of ROS/RNS. For instance, the ECS has been demonstrated to modulate the activity and expression of key enzymes involved in the synthesis of oxygen reactive species in the CNS, such as NOX2 and NOX4 (Lipina and Hundal, 2016; Gallelli et al., 2018). Moreover, AEA is able to partially reverse oxidative stress induced by exposure to hydrogen peroxide in a culture of hippocampal neural HT22 cells. In particular, AEA increased the cellular metabolic rate and decreased the number of apoptotic cells. AEA also increased the expression of the antioxidant enzyme superoxide dismutase (SOD) and decreased mRNA expression of NOX2 provoking a significant reduction of the intracellular levels of ROS. These AEA-related antioxidant effects were attributed to the activation of CB1 receptors, since their pharmacological and genetic blockade reversed those effects (Jia et al., 2014). The ECS can also regulate oxidative stress and lipid peroxidation by conveying beneficial free radical scavenging effects or through directly targeting CB1 and CB2 receptors (Lipina and Hundal, 2016; Gallelli et al., 2018). Interestingly, the beneficial or detrimental effects induced by the activation of cannabinoid receptors on ROS/RNS synthesis, seems to depend on the cell type and the aetiology and stage of the disease, and CB1 and CB2 receptors seem to have opposite effects in ROS formation. In the murine macrophage cell line RAW264.7, CB1 receptor activation promoted ROS formation via phosphorylation of p38-mitogen-activated protein kinase, whereas CB2 receptors suppressed this CB1 receptor-mediated effect (Han et al., 2009). Interestingly, this opposite action of CB1 and CB2 receptors has been documented in studies in which the oxidative stress was caused by a chemotherapeutic agent. For instance, acute and chronic administration of doxorubicin increased markers of oxidative/nitrosative stress in the myocardium of CB1 +/+ mice. This effect was attenuated in CB1 −/− mice, suggesting the implication of CB1 receptors in the oxidative stress induced by doxorubicin (Mukhopadhyay et al., 2010a). In addition, CB1 receptor agonists, such as AEA and HU-210, increased ROS generation in human cardiomyocytes, and this effect was attenuated by the concomitant application of the CB1 receptor antagonists SR1 and AM281 (Mukhopadhyay et al., 2010b). Similarly, cisplatin administration induced a significant increased expression of renal ROS/RNS synthesising enzymes, such as NOX2 and NOX4, and cell death. These deleterious effects were attenuated by the blockade of CB1 receptor or activation of CB2 receptors thus protecting against tubular damage (Mukhopadhyay et al., 2010a; Mukhopadhyay et al., 2010c; Horváth et al., 2012).
There is a great number of neuropathologies that cause an increment of the oxidative stress, including neurodegenerative diseases that are commonly associated with the development of cognitive deficits such as AD and PD. In fact, the antioxidant properties of cannabinoid drugs and their effect on cognition have been extensively studied in neurodegenerative animal models. In the STZ animal model of AD, a chronic treatment with the CB1 receptor agonist ACEA induced a reduction of nitric oxide (NO) accompanied by an improvement of the short- and long-term working memory measured by novel object recognition test (Crunfli et al., 2019). In a neurotoxic animal model of AD, the injection of β-amyloid peptide in the frontal cortex induced an important neural loss in the CA1, CA2 and CA3 hippocampal regions accompanied with the increased expression of biomarkers for apoptosis and gliosis, only 12 days following β-amyloid peptide administration. It was also observed an increase of the pro-oxidative enzyme inducible nitric oxide synthase (iNOS). Acute administration of VDM11, an inhibitor of AEA cellular reuptake, ameliorated the amnesia induced by β-amyloid peptide administration in the passive avoidance task. Interestingly, the significant increase in the hippocampal levels of AEA induced by the repeated administration of VDM11 reduced the neuronal loss and also the expression of iNOS (van der Stelt et al., 2006). A similar effect was observed when CB1 receptors were pharmacologically activated by administration of HU-210 or WIN55,212–2 in the MPTP-induced animal model of PD. Treated animals showed enhanced survival of nigrostriatal dopaminergic neurons, suppressed NOX enzymes and decreased ROS production (Chung et al., 2011).
Other cannabinoid-related compounds have recently attracted attention for their neuroprotective and antioxidant properties. One of these compounds is the endogenous lipid mediator PEA. In the 3xTg genetic mouse model, which contains three well established mutations for the development of AD, a chronic treatment for 90 days with ultramicronized PEA resulted in the rescue of the memory deficits typically observed in this phenotype of mice (Scuderi et al., 2018). Interestingly, this treatment also reversed astrogliosis and neuroinflammation, incremented the expression levels of BDNF in the hippocampus and decreased iNOS levels (Bronzuoli et al., 2018). Another different cannabinoid compound that has been extensively studied for its antioxidant properties is CBD. CBD, like other antioxidants, can modify the level and activity of oxidants and antioxidants and interrupt free radical chain reactions (Atalay et al., 2020). CBD administration also reduces the oxidant effects of chemotherapy drugs. For instance, CBD reduced iNOS levels in cardiac tissue and decreased serum levels of NO in mice treated with doxorubicin (Fouad et al., 2013). In addition, in the mouse model of cisplatin-induced nephropathy, CBD markedly attenuated cisplatin-induced oxidative/nitrosative stress, inflammation and cell death, improving renal function (Pan et al., 2009). As previously mentioned, these CBD antioxidant effects are similar to that provoked by the blockade of CB1 or the activation of CB2 receptors in this same animal model of nephropathy (Mukhopadhyay et al., 2010a; Mukhopadhyay et al., 2010c; Horváth et al., 2012). In the neurotoxic animal model of AD induced by the intracerebroventricular administration of β-amyloid peptide in mice, chronic administration of CBD reduced the hippocampal expression of iNOS and the subsequent NO release (Esposito et al., 2007) and prevented the spatial memory deficits usually observed in this animal model (Martín-Moreno et al., 2011). CBD was also able to recover 6-OHDA-induced dopamine depletion in this animal model of PD, but only when it was administered immediately after the lesion. This effect was accompanied by an increase in the levels of SOD (García-Arencibia et al., 2006).
Clinical evidence for the use of cannabinoid drugs in cognitive-related diseases
The preclinical findings on the antioxidant and anti-inflamatory effects induced by the pharmacological modulation of the ECS during PD or AD has also been translated into the clinical field. Despite the vast evidence of cannabinoids-induced neuroprotection in TBI, there are still no studies translating those finding into humans. Elevated endocannabinoid levels have been found in the cerebrospinal fluid of PD patients, together with decreased CB1 receptors in the basal ganglia (Hurley et al., 2003; Pisani et al., 2005). A small human trial performed in patients suffering from PD revealed that cannabinoid-related drugs such as CBD, nabilone or even cannabis improved motor symptoms an attenuated levodopa-induced dyskinesia. Moreover, resting tremor, rigidity, bradykinesia, and posture were corrected, followed by a decrease on pain sensitivity and amelioriated sleep quality (Carroll et al., 2004; Mesnage et al., 2004; Lotan et al., 2014). CBD has also been associated with diminished REM sleep behavior disorder in PD patiens (Chagas et al., 2014).
A post-mortem study in human brain samples of AD patients showed an increased expression of CB2 receptors in microglia associated with β-amyloid-enriched neuritic plaques. This effect was not detected in CB1 receptors expression, suggesting the involvement of CB2 dependent mechanisms in this disorder (Benito et al., 2003). Several clincial studies have investigated the effects of dronabinol, which is a synthetic version of THC, in advanced stages of AD. Dronabinol improved side effects associated with late stages of AD such as food intake, sleep duration and circadian rhythm; decreasing also agitation (Volicer et al., 1997; Woodward et al., 2014; Suryadevara et al., 2017).
These reports demonstrate the potential therapeutic activity of cannabinoid drugs to relieve PD and AD symthomatology. However, to the best of our knowledge, there is no clinical evidence of improvement in the cognitive alterations associated with these neurodegenerative disorders yet. Further clinical and preclinical research is required to assess the cognitive-related therapeutic effects that cannabinoid drugs may exert.
Discussion
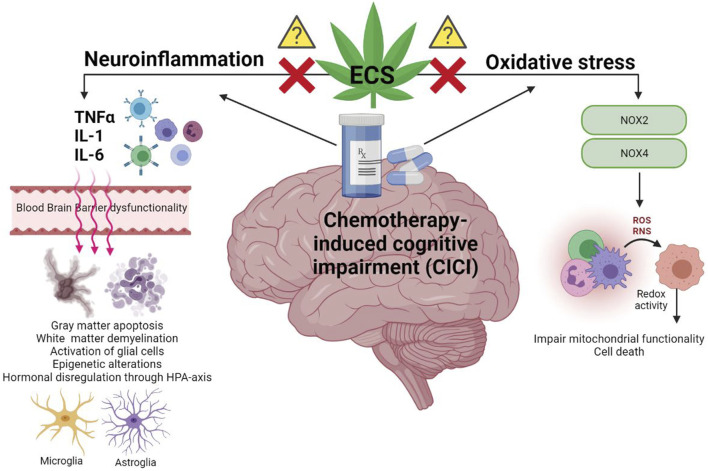
Due to the increased survival of cancer patients, there is an urgent need to address the possible sequelae that the current treatments may provoke. Amongst these adverse effects, those affecting cognition and other brain functionality are particularly worrying. The occurrence of chemotherapy-induced cognitive impairment (CICI) has been demonstrated in animal models and human patients. Different biological mechanisms seem to be involved, however there is a big gap in the understanding of those yet. The ECS is implicated in neuroinflammation and oxidative stress (Figure 1). This review comprehends evidence on the use of cannabinoid-related drugs for the modulation of neuroinflammation and oxidative stress in different pathologies with similar cognitive phenotype to CICI, as well as their anti-tumour activity.
FIGURE 1.
Graphic representation of the neuroinflammatory processes and oxidative stress underlying chemotherapy-induced cognitive impairment (CICI). The activation of the pro-inflammatory cascade, following chemotherapeutic drug administration, is characterized by the central/peripheral release of pro-inflammatory cytokines such as TNFα, IL-1 and IL-6. Transport of these cytokines through the blood brain barrier (BBB) affects its functionality, facilitating, thus, the access of further cytokines and chemotherapeutic drugs into the supraspinal central nervous system (CNS). Therefore, persistent neuroinflammation in the CNS alters brain’s plasticity and functionality mediating the development of cognitive alterations. The commencement of oxidative stress cascade, following chemotherapeutic drug administration, is characterised by the activation of NOX2/NOX4 enzymes which synthesize oxygen and nitrogen reactive species (ROS and RNS, respectively). Increased redox activity due to intracellular accumulation of ROS/RNS induces protein and DNA isoforms alterations that lead to cell death. The endocannabinoid system (ECS), as previously described in similar cognitive-related alterations, proposes a new target for the inhibition of neurotoxicity, providing thus neuroprotection. However, the mechanisms through which the ECS may mediate this process are still unknown in CICI pathology.
The data collected elucidate the positive outcomes of cannabinoid-based drugs in the relief of PD- and AD-side effects in human patients. These results highlight the possible therapeutic potential of cannabinoid drugs in the treatment of CICI. The lack of clinical evidence supporting the anti-cancer role described of the ECS in animal and in vitro models, emphasizes the importance of translating the preclinical findings into humans. This fact points forward the urgent need of clinical assays where the preclinical effectiveness of cannabinoid drugs in the recovery of chemotherapy-induced cognitive alterations can be also investigated.
Author Contributions
AL-B wrote the first draft of the manuscript and the table. LB prepared the figure. LB and RA reviewed and edited the manuscript.
Funding
LB and AL-B are supported by the Irish Research Council (IRCL/2017/78). RA is supported by Ministerio de Ciencia, Innovación y Universidades (PID2019-111510RB-I00) and Universidad Rey Juan Carlos.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abrams D. I., Guzman M. (2015). Cannabis in cancer care. Clin. Pharmacol. Ther. 97, 575–86. 10.1002/cpt.108 [DOI] [PubMed] [Google Scholar]
- Aso E., Palomer E., Juvés S., Maldonado R., Muñoz F. J., Ferrer I. (2012). CB1 Agonist ACEA Protects Neurons and Reduces the Cognitive Impairment of AβPP/PS1 Mice. J. Alzheimers Dis. 30, 439–459. 10.3233/JAD-2012-111862 [DOI] [PubMed] [Google Scholar]
- Atalay S., Jarocka K. I., Skrzydlewska E. (2020). Antioxidative and Anti-inflammatory Properties of Cannabidiol. Antioxidants (Basel) 9, 21. 10.3390/antiox9010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymerich M. S., Aso E., Abellanas M. A., Tolon R. M., Ramos J. A., Ferrer I., et al. (2018). Cannabinoid Pharmacology/therapeutics in Chronic Degenerative Disorders Affecting the central Nervous System. Biochem. Pharmacol. 157, 67–84. 10.1016/j.bcp.2018.08.016 [DOI] [PubMed] [Google Scholar]
- Benito C., Núñez E., Tolón R. M., Carrier E. J., Rábano A., Hillard C. J., Romero J. (2003). Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J. Neurosci. 23 (35), 11136–11141. 10.1523/jneurosci.23-35-11136.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besner G. E., Whelton D., Crissman-Combs M. A., Steffen C. L., Kim G. Y., Brigstock D. R. (1992). Interaction of Heparin-Binding EGF-like Growth Factor (HB-EGF) with the Epidermal Growth Factor Receptor: Modulation by Heparin, Heparinase, or Synthetic Heparin-Binding HB-EGF Fragments. Growth Factors 7, 289–296. 10.3109/08977199209046411 [DOI] [PubMed] [Google Scholar]
- Bettiga A., Aureli M., Colciago G., Murdica V., Moschini M., Lucianò R., et al. (2017). Bladder Cancer Cell Growth and Motility Implicate Cannabinoid 2 Receptor-Mediated Modifications of Sphingolipids Metabolism. Sci. Rep. 7, 42157. 10.1038/srep42157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilkei G. A. (2012). The Endocannabinoid System in normal and Pathological Brain Ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 3326–3341. 10.1098/rstb.2011.0388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisen H. E. B., Hineline P. N., Walker E. A. (2013). Effects of Early Chemotherapeutic Treatment on Learning in Adolescent Mice: Implications for Cognitive Impairment and Remediation in Childhood Cancer Survivors. Clin. Cancer Res. 19, 3008–3018. 10.1158/1078-0432.CCR-12-3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T., di Marzo V. (2008). The Role of the Endocannabinoid System in Alzheimer's Disease: Facts and Hypotheses. Curr. Pharm. Des. 14, 2299–3305. 10.2174/138161208785740027 [DOI] [PubMed] [Google Scholar]
- Blanton H. L., Brelsfoard J., DeTurk N., Pruitt K., Narasimhan M., Morgan D. J., et al. (2019). Cannabinoids: Current and Future Options to Treat Chronic and Chemotherapy-Induced Neuropathic Pain. Drugs 79, 969–995. 10.1007/s40265-019-01132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez C., Casanova M. L., Planas A., Gómez Del Pulgar T., Villanueva C., Fernández-Aceñero M. J., et al. (2003). Inhibition of Tumor Angiogenesis by Cannabinoids. FASEB J. 17, 529–531. 10.1096/fj.02-0795fje [DOI] [PubMed] [Google Scholar]
- Bonnet A. E., Marchalant Y. (2015). Potential Therapeutical Contributions of the Endocannabinoid System towards Aging and Alzheimer's Disease. Aging Dis. 6, 400–405. 10.14336/AD.2015.0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Ghebremedhin E., Rüb U., Bratzke H., Del Tredici K. (2004). Stages in the Development of Parkinson's Disease-Related Pathology. Cell Tissue Res 318, 121–134. 10.1007/s00441-004-0956-9 [DOI] [PubMed] [Google Scholar]
- Braun M., Khan Z. T., Khan M. B., Kumar M., Ward A., Achyut B. R., et al. (2018). Selective Activation of Cannabinoid Receptor-2 Reduces Neuroinflammation after Traumatic Brain Injury via Alternative Macrophage Polarization. Brain Behav. Immun. 68, 224–237. 10.1016/j.bbi.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronzuoli M. R., Facchinetti R., Steardo L., Romano A., Stecca C., Passarella S., et al. (2018). Palmitoylethanolamide Dampens Reactive Astrogliosis and Improves Neuronal Trophic Support in a Triple Transgenic Model of Alzheimer's Disease: In Vitro and In Vivo Evidence. Oxid. Med. Cel. Longev. 2018, 4720532. 10.1155/2018/4720532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Jain R. K. (2000). Angiogenesis in Cancer and Other Diseases. Nature 407, 249–257. 10.1038/35025220.16 [DOI] [PubMed] [Google Scholar]
- Carracedo A., Lorente M., Egia A., Blázquez C., García S., Giroux V., et al. (2006). The Stress-Regulated Protein P8 Mediates Cannabinoid-Induced Apoptosis of Tumor Cells. Cancer Cell 9, 301–312. 10.1016/j.ccr.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Carroll C. B., Bain P. G., Teare L., Liu X., Joint C., Wroath C., et al. (2004). Cannabis for Dyskinesia in Parkinson Disease: a Randomized Double-Blind Crossover Study. Neurology 63 (7), 1245–1250. 10.1212/01.wnl.0000140288.48796.8e [DOI] [PubMed] [Google Scholar]
- Casanova M. L., Larcher F., Casanova B., Murillas R., Fernández-Aceñero M. J., Villanueva C., et al. (2002). A Critical Role for Ras-Mediated, Epidermal Growth Factor Receptor-dependent Angiogenesis in Mouse Skin Carcinogenesis. Cancer Res. 62, 3402–3407. [PubMed] [Google Scholar]
- Chagas M. H., Eckeli A. L., Zuardi A. W., Pena-Pereira M. A., Sobreira-Neto M. A., Sobreira E. T., et al. (2014). Cannabidiol Can Improve Complex Sleep-Related Behaviours Associated with Rapid Eye Movement Sleep Behaviour Disorder in Parkinson's Disease Patients: a Case Series. J. Clin. Pharm. Ther. 39 (5), 564–566. 10.1111/jcpt.12179 [DOI] [PubMed] [Google Scholar]
- Cheng D., Low J. K., Logge W., Garner B., Karl T. (2014). Chronic Cannabidiol Treatment Improves Social and Object Recognition in Double Transgenic APPswe/PS1∆E9 Mice. Psychopharmacology (Berl) 231, 3009–3017. 10.1007/s00213-014-3478-5 [DOI] [PubMed] [Google Scholar]
- Cheng D., Spiro A. S., Jenner A. M., Garner B., Karl T. (2014). Long-term Cannabidiol Treatment Prevents the Development of Social Recognition Memory Deficits in Alzheimer's Disease Transgenic Mice. J. Alzheimers Dis. 42, 1383–1396. 10.3233/JAD-140921 [DOI] [PubMed] [Google Scholar]
- Chiurchiù V., van der Stelt M., Centonze D., Maccarrone M. (2018). The Endocannabinoid System and its Therapeutic Exploitation in Multiple Sclerosis: Clues for Other Neuroinflammatory Diseases. Prog. Neurobiol. 160, 82–100. 10.1016/j.pneurobio.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Christie L. A., Acharya M. M., Parihar V. K., Nguyen A., Martirosian V., Limoli C. L. (2012). Impaired Cognitive Function and Hippocampal Neurogenesis Following Cancer Chemotherapy. Clin. Cancer Res. 18, 1954–1965. 10.1158/1078-0432.CCR-11-2000 [DOI] [PubMed] [Google Scholar]
- Chung E. S., Bok E., Chung Y. C., Baik H. H., Jin B. K. (2012). Cannabinoids Prevent Lipopolysaccharide-Induced Neurodegeneration in the Rat Substantia Nigra In Vivo through Inhibition of Microglial Activation and NADPH Oxidase. Brain Res. 1451, 110–116. 10.1016/j.brainres.2012.02.058 [DOI] [PubMed] [Google Scholar]
- Chung Y. C., Bok E., Huh S. H., Park J. Y., Yoon S. H., Kim S. R., et al. (2011). Cannabinoid Receptor Type 1 Protects Nigrostriatal Dopaminergic Neurons against MPTP Neurotoxicity by Inhibiting Microglial Activation. J. Immunol. 187, 6508–6517. 10.4049/jimmunol.1102435 [DOI] [PubMed] [Google Scholar]
- Cohen K., Weinstein A. (2018). The Effects of Cannabinoids on Executive Functions: Evidence from Cannabis and Synthetic Cannabinoids-A Systematic Review. Brain Sci. 8, 40. 10.3390/brainsci8030040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon R. M., Okine B. N., Finn D. P., Dowd E. (2015). Differential Upregulation of the Cannabinoid CB2 Receptor in Neurotoxic and Inflammation-Driven Rat Models of Parkinson's Disease. Exp. Neurol. 269, 133–141. 10.1016/j.expneurol.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Concannon R. M., Finn D. P., Dowd E. (2015). “Cannabinoids in Neurologic and Mental Disease,” in Cannabinoids in Neurologic and Mental Disease (Liana Fattore Elsevier Inc; ), Amsterdam, Netherlands. 10.1016/C2013-0-00592-0 [DOI] [Google Scholar]
- Conroy S. K., McDonald B. C., Smith D. J., Moser L. R., West J. D., Kamendulis L. M., et al. (2013). Alterations in Brain Structure and Function in Breast Cancer Survivors: Effect of post-chemotherapy Interval and Relation to Oxidative DNA Damage. Breast Cancer Res. Treat. 137, 493–502. 10.1007/s10549-012-2385-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunfli F., Vrechi T. A., Costa A. P., Torrão A. S. (2019). Cannabinoid Receptor Type 1 Agonist ACEA Improves Cognitive Deficit on STZ-Induced Neurotoxicity through Apoptosis Pathway and NO Modulation. Neurotox. Res. 35, 516–529. 10.1007/s12640-018-9991-2 [DOI] [PubMed] [Google Scholar]
- Dariš B., Tancer Verboten M., Knez Ž., Ferk P. (2019). Cannabinoids in Cancer Treatment: Therapeutic Potential and Legislation. Bosn. J. Basic Med. Sci. 19, 14–23. 10.17305/bjbms.2018.3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V. (2018). New Approaches and Challenges to Targeting the Endocannabinoid System. Nat. Rev. Drug Discov. 17, 623–639. 10.1038/nrd.2018.115 [DOI] [PubMed] [Google Scholar]
- Du X. L., Cai Y., Symanski E. (2013). Association between Chemotherapy and Cognitive Impairments in a Large Cohort of Patients with Colorectal Cancer. Int. J. Oncol. 42, 2123–2133. 10.3892/ijo.2013.1882 [DOI] [PubMed] [Google Scholar]
- El-Agamy S. E., Abdel- A. A. K., Esmat A., Azab S. S. (2019). Chemotherapy and Cognition: Comprehensive Review on Doxorubicin-Induced Chemobrain. Cancer Chemother. Pharmacol. 84, 1–14. 10.1007/s00280-019-03827-0 [DOI] [PubMed] [Google Scholar]
- Elens I., Dekeyster E., Moons L., D'Hooge R. (2019). Methotrexate Affects Cerebrospinal Fluid Folate and Tau Levels and Induces Late Cognitive Deficits in Mice. Neuroscience 404, 62–70. 10.1016/j.neuroscience.2019.01.024 [DOI] [PubMed] [Google Scholar]
- Esposito G., de Filippis D., Maiuri M. C., de Stefano D., Carnuccio R., Iuvone T. (2006). Cannabidiol Inhibits Inducible Nitric Oxide Synthase Protein Expression and Nitric Oxide Production in Beta-Amyloid Stimulated PC12 Neurons through P38 MAP Kinase and NF-kappaB Involvement. Neurosci. Lett. 399, 91–95. 10.1016/j.neulet.2006.01.047 [DOI] [PubMed] [Google Scholar]
- Esposito G., Scuderi C., Savani C., Steardo L., Jr, De Filippis D., Cottone P., et al. (2007). Cannabidiol In Vivo Blunts Beta-Amyloid Induced Neuroinflammation by Suppressing IL-1beta and iNOS Expression. Br. J. Pharmacol. 151, 1272–1279. 10.1038/sj.bjp.0707337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G., de Filippis D., Carnuccio R., Izzo A. A., Iuvone T. (2006). The Marijuana Component Cannabidiol Inhibits β-amyloid-induced Tau Protein Hyperphosphorylation through Wnt/β-Catenin Pathway rescue in PC12 Cells. J. Mol. Med. 84, 253–258. 10.1007/s00109-005-0025-1 [DOI] [PubMed] [Google Scholar]
- Estrada J. A., Contreras I. (2020). Endocannabinoid Receptors in the CNS: Potential Drug Targets for the Prevention and Treatment of Neurologic and Psychiatric Disorders. Curr. Neuropharmacol. 18, 769–787. 10.2174/1570159x18666200217140255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardell J. E., Vardy J., Monds L. A., Johnston I. N. (2015). The Long-Term Impact of Oxaliplatin Chemotherapy on Rodent Cognition and Peripheral Neuropathy. Behav. Brain Res. 291, 80–88. 10.1016/j.bbr.2015.04.038 [DOI] [PubMed] [Google Scholar]
- Ferguson R. J., Mcdonald B. C., Rocque M. A., Furstenberg C. T., Horrigan S., Ahles T. A., et al. (2014). Development of CBT for Chemotherapy-Related Cognitive Change: Results of a Waitlist Control Trial. Psychooncology 21, 176–186. 10.1002/pon.1878.Development [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes H. A., Richard N. M., Edelstein K. (2019). Cognitive Rehabilitation for Cancer-Related Cognitive Dysfunction: a Systematic Review. Support. Care Cancer 27, 3253–3279. 10.1007/s00520-019-04866-2 [DOI] [PubMed] [Google Scholar]
- Fouad A. A., Albuali W. H., Al-mulhim A. S., Jresat I. (2013). Cardioprotective Effect of Cannabidiol in Rats Exposed to Doxorubicin Toxicity. Environ. Toxicol. Pharmacol. 36, 347–357. 10.1016/j.etap.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Fourrier C., Singhal G., Baune B. T. (2019). Neuroinflammation and Cognition across Psychiatric Conditions. CNS Spectr. 24, 4–15. 10.1017/S1092852918001499 [DOI] [PubMed] [Google Scholar]
- Fraguas S. A. I., Martín S. C., Torres S. A. I. (2018). Insights into the Effects of the Endocannabinoid System in Cancer: a Review. Br. J. Pharmacol. 175, 2566–2580. 10.1111/bph.14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallelli C. A., Calcagnini S., Romano A., Koczwara J. B., de Ceglia M., Dante D., et al. (2018). Modulation of the Oxidative Stress and Lipid Peroxidation by Endocannabinoids and Their Lipid Analogues. Antioxidants (Basel) 7, 93. 10.3390/antiox7070093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García C., Palomo- G. C., García- A. M., Ramos J., Pertwee R., Fernández- R. J. (2011). Symptom-relieving and Neuroprotective Effects of the Phytocannabinoid Δ9-THCV in Animal Models of Parkinson's Disease. Br. J. Pharmacol. 163, 1495–1506. 10.1111/j.1476-5381.2011.01278.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- García M. C., Cinquina V., Palomo-Garo C., Rábano A., Fernández-Ruiz J. (2015). Identification of CB2 Receptors in Human Nigral Neurons that Degenerate in Parkinson's Disease. Neurosci. Lett. 587, 1–4. 10.1016/j.neulet.2014.12.003 [DOI] [PubMed] [Google Scholar]
- García-Arencibia M., González S., de Lago E., Ramos J. A., Mechoulam R., Fernández-Ruiz J. (2006). Evaluation of the Neuroprotective Effect of Cannabinoids in a Rat Model of Parkinson's Disease: Importance of Antioxidant and Cannabinoid Receptor-independent Properties. Brain Res. 1134, 162–170. 10.1016/j.brainres.2006.11.063 [DOI] [PubMed] [Google Scholar]
- Gómez-Gálvez Y., Palomo-Garo C., Fernández-Ruiz J., García C. (2016). Potential of the Cannabinoid CB(2) Receptor as a Pharmacological Target against Inflammation in Parkinson's Disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 64, 200–208. 10.1016/j.pnpbp.2015.03.017 [DOI] [PubMed] [Google Scholar]
- Gorzkiewicz A., Szemraj J. (2018). Brain Endocannabinoid Signaling Exhibits Remarkable Complexity. Brain Res. Bull. 142, 33–46. 10.1016/J.BRAINRESBULL.2018.06.012 [DOI] [PubMed] [Google Scholar]
- Guzmán M. (2003). Cannabinoids: Potential Anticancer Agents. Nat. Rev. Cancer 3, 745–755. 10.1038/nrc1188 [DOI] [PubMed] [Google Scholar]
- Guzmán M., Duarte M. J., Blázquez C., Ravina J., Rosa M. C., Galve-Roperh I., et al. (2006). A Pilot Clinical Study of Delta9-tetrahydrocannabinol in Patients with Recurrent Glioblastoma Multiforme. Br. J. Cancer 95, 197–203. 10.1038/sj.bjc.6603236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W. D., Degenhardt L. J., Currow D. (2001). Allowing the Medical Use of Cannabis. Med. J. Aust. 175, 39–40. 10.5694/j.1326-5377.2001.tb143512.x [DOI] [PubMed] [Google Scholar]
- Han K. H., Lim S., Ryu J., Lee C. W., Kim Y., Kang J. H., et al. (2009). CB1 and CB2 Cannabinoid Receptors Differentially Regulate the Production of Reactive Oxygen Species by Macrophages. Cardiovasc. Res. 84, 378–386. 10.1093/cvr/cvp240 [DOI] [PubMed] [Google Scholar]
- Hart S., Fischer O. M., Ullrich A. (2004). Cannabinoids Induce Cancer Cell Proliferation via Tumor Necrosis Factor Alpha-Converting Enzyme (TACE/ADAM17)-mediated Transactivation of the Epidermal Growth Factor Receptor. Cancer Res. 64, 1943–1950. 10.1158/0008-5472.can-03-3720 [DOI] [PubMed] [Google Scholar]
- Horowitz T. S., Suls J., Treviño M. (2018). A Call for a Neuroscience Approach to Cancer-Related Cognitive Impairment. Trends Neurosci. 41, 493–496. 10.1016/j.tins.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Horváth B., Mukhopadhyay P., Kechrid M., Patel V., Tanchian G., Wink D. A., et al. (2012). β-Caryophyllene Ameliorates Cisplatin-Induced Nephrotoxicity in a Cannabinoid 2 Receptor-dependent Manner. Free Radic. Biol. Med. 52, 1325–1333. 10.1016/j.freeradbiomed.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J. G., Xue J. J., Lee M. R., Sun M. Q., Zhao X. H., Zheng Y. N., et al. (2013). Compound K Is Able to Ameliorate the Impaired Cognitive Function and Hippocampal Neurogenesis Following Chemotherapy Treatment. Biochem. Biophys. Res. Commun. 436, 104–109. 10.1016/j.bbrc.2013.05.087 [DOI] [PubMed] [Google Scholar]
- Hurley M. J., Mash D. C., Jenner P. (2003). Expression of Cannabinoid CB1 Receptor mRNA in Basal Ganglia of normal and Parkinsonian Human Brain. J. Neural Transm. (Vienna) 110 (11), 1279–1288. 10.1007/s00702-003-0033-7 [DOI] [PubMed] [Google Scholar]
- Jacobsson S. O., Rongård E., Stridh M., Tiger G., Fowler C. J. (2000). Serum-dependent Effects of Tamoxifen and Cannabinoids upon C6 Glioma Cell Viability. Biochem. Pharmacol. 60, 1807–1813. 10.1016/s0006-2952(00)00492-5 [DOI] [PubMed] [Google Scholar]
- Jia J., Ma L., Wu M., Zhang L., Zhang X., Zhai Q., et al. (2014). Anandamide Protects HT22 Cells Exposed to Hydrogen Peroxide by Inhibiting CB1 Receptor-Mediated Type 2 NADPH Oxidase. Oxid. Med. Cel. Longev. 2014, 893516. 10.1155/2014/893516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John T., Lomeli N., Bota D. A. (2017). Systemic Cisplatin Exposure during Infancy and Adolescence Causes Impaired Cognitive Function in Adulthood. Behav. Brain Res. 319, 200–206. 10.1016/j.bbr.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston I. N., Tan M., Cao J., Matsos A., Forrest D. R. L., Si E., et al. (2017). Ibudilast Reduces Oxaliplatin-Induced Tactile Allodynia and Cognitive Impairments in Rats. Behav. Brain Res. 334, 109–118. 10.1016/j.bbr.2017.07.021 [DOI] [PubMed] [Google Scholar]
- Joshi G., Hardas S., Sultana R., St Clair D. K., Vore M., Butterfield D. A. (2007). Glutathione Elevation by Gamma-Glutamyl Cysteine Ethyl Ester as a Potential Therapeutic Strategy for Preventing Oxidative Stress in Brain Mediated by In Vivo Administration of Adriamycin: Implication for Chemobrain. J. Neurosci. Res. 85, 497–503. 10.1002/jnr.21158 [DOI] [PubMed] [Google Scholar]
- Kaur R., Ambwani S. R., Singh S. (2016). Endocannabinoid System: A Multi-Facet Therapeutic Target. Curr. Clin. Pharmacol. 11, 110–117. 10.2174/1574884711666160418105339 [DOI] [PubMed] [Google Scholar]
- Kawai Y., Nakao T., Kunimura N., Kohda Y., Gemba M. (2006). Relationship of Intracellular Calcium and Oxygen Radicals to Cisplatin-Related Renal Cell Injury. J. Pharmacol. Sci. 100, 65–72. 10.1254/jphs.fp0050661 [DOI] [PubMed] [Google Scholar]
- Kesler S., Hadi Hosseini S. M., Heckler C., Janelsins M., Palesh O., Mustian K., et al. (2013). Cognitive Training for Improving Executive Function in Chemotherapy-Treated Breast Cancer Survivors. Clin. Breast Cancer 13, 299–306. 10.1016/j.clbc.2013.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner A. S., Kleckner I. R., Kamen C. S., Tejani M. A., Janelsins M. C., Morrow G. R., et al. (2019). Opportunities for Cannabis in Supportive Care in Cancer. Ther. Adv. Med. Oncol. 11, 1758835919866362. 10.1177/1758835919866362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres-Becker I., Molina-Holgado F., Ramos J. A., Mechoulam R., Fernández-Ruiz J. (2005). Cannabinoids Provide Neuroprotection Against 6-hydroxydopamine Toxicity In Vivo and In Vitro: Relevance to Parkinson's Disease. Neurobiol. Dis. 19, 96–107. 10.1016/j.nbd.2004.11.009 [DOI] [PubMed] [Google Scholar]
- Li C., Shi J., Wang B., Li J., Jia H. (2019). CB2 Cannabinoid Receptor Agonist Ameliorates Novel Object Recognition but Not Spatial Memory in Transgenic APP/PS1 Mice. Neurosci. Lett. 707, 134286. 10.1016/j.neulet.2019.134286 [DOI] [PubMed] [Google Scholar]
- Li Z., Liu P., Zhang H., Zhao S., Jin Z., Li R., et al. (2017). Role of GABAB Receptors and p38MAPK/NF-Κb Pathway in Paclitaxel-Induced Apoptosis of Hippocampal Neurons. Pharm. Biol. 55, 2188–2195. 10.1080/13880209.2017.1392987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhao S., Zhang H. L., Liu P., Liu F. F., Guo Y. X., et al. (2018). Proinflammatory Factors Mediate Paclitaxel-Induced Impairment of Learning and Memory. Mediators Inflamm. 2018, 3941840. 10.1155/2018/3941840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina C., Hundal H. S. (2016). Modulation of Cellular Redox Homeostasis by the Endocannabinoid System. Open Biol. 6, 150276. 10.1098/rsob.150276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. J., Jamieson S. M., Subramaniam J., Ip V., Jong N. N., Mercer J. F., et al. (2009). Neuronal Expression of Copper Transporter 1 in Rat Dorsal Root Ganglia: Association with Platinum Neurotoxicity. Cancer Chemother. Pharmacol. 64, 847–856. 10.1007/s00280-009-1017-6 [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez A. B., Mela V., Acaz-Fonseca E., Garcia-Segura L. M., Viveros M. P. (2016). CB2 Cannabinoid Receptor Is Involved in the Anti-inflammatory Effects of Leptin in a Model of Traumatic Brain Injury. Exp. Neurol. 279, 274–282. 10.1016/j.expneurol.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez A. B., Siopi E., Finn D. P., Marchand-Leroux C., Garcia-Segura L. M., Jafarian-Tehrani M., et al. (2015). CB1 and CB2 Cannabinoid Receptor Antagonists Prevent Minocycline-Induced Neuroprotection Following Traumatic Brain Injury in Mice. Cereb. Cortex 25, 35–45. 10.1093/cercor/bht202 [DOI] [PubMed] [Google Scholar]
- Lotan I., Treves T. A., Roditi Y., Djaldetti R. (2014). Cannabis (Medical Marijuana) Treatment for Motor and Non-motor Symptoms of Parkinson Disease: an Open-Label Observational Study. Clin. Neuropharmacol. 37 (2), 41–44. 10.1097/WNF.0000000000000016 [DOI] [PubMed] [Google Scholar]
- Lynch M. E., Cesar-Rittenberg P., Hohmann A. G. (2014). A Double-Blind, Placebo-Controlled, Crossover Pilot Trial with Extension Using an Oral Mucosal Cannabinoid Extract for Treatment of Chemotherapy-Induced Neuropathic Pain. J. Pain Symptom Manage. 47, 166–173. 10.1016/j.jpainsymman.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Marchalant Y., Brothers H. M., Wenk G. L. (2008). Inflammation and Aging: Can Endocannabinoids Help? Biomed. Pharmacother. 62, 212–217. 10.1016/j.biopha.2008.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Moreno A. M., Reigada D., Ramírez B. G., Mechoulam R., Innamorato N., Cuadrado A., et al. (2011). Cannabidiol and Other Cannabinoids Reduce Microglial Activation In Vitro and In Vivo: Relevance to Alzheimer's Disease. Mol. Pharmacol. 79, 964–973. 10.1124/mol.111.071290.Alzheimer [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masocha W. (2018). Targeting the Endocannabinoid System for Prevention or Treatment of Chemotherapy-Induced Neuropathic Pain: Studies in Animal Models. Pain Res. Manage. 2018, 1–9. 10.1155/2018/5234943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B. C., Conroy S. K., Smith D. J., West J. D., Saykin A. J. (2013). Frontal gray Matter Reduction after Breast Cancer Chemotherapy and Association with Executive Symptoms: A Replication and Extension Study. Brain Behav. Immun. 30 Suppl, S117–S125. 10.1016/j.bbi.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKallip R. J., Nagarkatti M., Nagarkatti P. S. (2005). Delta-9-tetrahydrocannabinol Enhances Breast Cancer Growth and Metastasis by Suppression of the Antitumor Immune Response. J. Immunol. 174, 3281–3289. 10.4049/jimmunol.174.6.3281 [DOI] [PubMed] [Google Scholar]
- Mesnage V., Houeto J. L., Bonnet A. M., Clavier I., Arnulf I., Cattelin F., et al. (2004). Neurokinin B, Neurotensin, and Cannabinoid Receptor Antagonists and Parkinson Disease. Clin. Neuropharmacol. 27 (3), 108–110. 10.1097/00002826-200405000-00003 [DOI] [PubMed] [Google Scholar]
- Micale V., Drago F. (2018). Endocannabinoid System, Stress and HPA axis. Eur. J. Pharmacol. 834, 230–239. 10.1016/J.EJPHAR.2018.07.039 [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E., Molina-Holgado F. (2010). Mending the Broken Brain: Neuroimmune Interactions in Neurogenesis. J. Neurochem. 114, 1277–1290. 10.1111/j.1471-4159.2010.06849.x [DOI] [PubMed] [Google Scholar]
- Moreno E., Cavic M., Krivokuca A., Casadó V., Canela E. (2019). The Endocannabinoid System as a Target in Cancer Diseases: Are We There yet?. Front. Pharmacol. 10, 339. 10.3389/fphar.2019.00339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounier N. M., Abdel-Maged A. E., Wahdan S. A., Gad A. M., Azab S. S. (2020). Chemotherapy-Induced Cognitive Impairment (CICI): An Overview of Etiology and Pathogenesis. Life Sci. 258, 118071. 10.1016/j.lfs.2020.118071 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P., Pan H., Rajesh M., Bátkai S., Patel V., Harvey-White J., et al. (2010). CB1 Cannabinoid Receptors Promote Oxidative/nitrosative Stress, Inflammation and Cell Death in a Murine Nephropathy Model. Br. J. Pharmacol. 160, 657–668. 10.1111/j.1476-5381.2010.00769.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P., Rajesh M., Bátkai S., Patel V., Kashiwaya Y., Liaudet L., et al. (2010). CB1 Cannabinoid Receptors Promote Oxidative Stress and Cell Death in Murine Models of Doxorubicin-Induced Cardiomyopathy and in Human Cardiomyocytes. Cardiovasc. Res. 85, 773–784. 10.1093/cvr/cvp369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P., Rajesh M., Pan H., Patel V., Mukhopadhyay B., Bátkai S., et al. (2010). Cannabinoid-2 Receptor Limits Inflammation, Oxidative/nitrosative Stress, and Cell Death in Nephropathy. Free Radic. Biol. Med. 48, 457–467. 10.1016/j.freeradbiomed.2009.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L. D., Ehrlich B. E. (2020). Cellular Mechanisms and Treatments for Chemobrain: Insight from Aging and Neurodegenerative Diseases. EMBO Mol. Med. 12, e12075. 10.15252/emmm.202012075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H., Mukhopadhyay P., Rajesh M., Patel V., Mukhopadhyay B., Gao B., et al. (2009). Cannabidiol Attenuates Cisplatin-Induced Nephrotoxicity by Decreasing Oxidative/Nitrosative Stress, Inflammation, and Cell Death. J. Pharmacol. Exp. Ther. 328, 708–714. 10.1124/jpet.108.147181.cells [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D., Mechoulam R., Beni S. M., Alexandrovich A., Shohami E. (2005). CB1 Cannabinoid Receptors Are Involved in Neuroprotection via NF-Kappa B Inhibition. J. Cereb. Blood Flow Metab. 25, 477–484. 10.1038/sj.jcbfm.9600047 [DOI] [PubMed] [Google Scholar]
- Panikashvili D., Shein N. A., Mechoulam R., Trembovler V., Kohen R., Alexandrovich A., et al. (2006). The Endocannabinoid 2-AG Protects the Blood-Brain Barrier after Closed Head Injury and Inhibits mRNA Expression of Proinflammatory Cytokines. Neurobiol. Dis. 22, 257–264. 10.1016/j.nbd.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Panikashvili D., Simeonidou C., Ben-shabat S., Hanus L., Breuer A., Mechoulam R., et al. (2001). An Endogenous Cannabinoid (2-AG) Is Neuroprotective after Brain Injury. Nature 413, 527–531. 10.1038/35097089 [DOI] [PubMed] [Google Scholar]
- Pisani A., Fezza F., Galati S., Battista N., Napolitano S., Finazzi-Agrò A., et al. (2005). High Endogenous Cannabinoid Levels in the Cerebrospinal Fluid of Untreated Parkinson's Disease Patients. Ann. Neurol. 57 (5), 777–779. 10.1002/ana.20462 [DOI] [PubMed] [Google Scholar]
- Park H. S., Kim C. J., Kwak H. B., No M. H., Heo J. W., Kim T. W. (2018). Physical Exercise Prevents Cognitive Impairment by Enhancing Hippocampal Neuroplasticity and Mitochondrial Function in Doxorubicin-Induced Chemobrain. Neuropharmacology 133, 451–461. 10.1016/j.neuropharm.2018.02.013 [DOI] [PubMed] [Google Scholar]
- Pérez-Gómez E., Andradas C., Blasco-Benito S., Caffarel M. M., García-Taboada E., Villa-Morales M., et al. (2015). Role of Cannabinoid Receptor CB2 in HER2 Pro-oncogenic Signaling in Breast Cancer. J. Natl. Cancer Inst. 107, djv077. 10.1093/jnci/djv077 [DOI] [PubMed] [Google Scholar]
- Price D. A., Martinez A. A., Seillier A., Koek W., Acosta Y., Fernandez E., et al. (2009). WIN55,212-2, a Cannabinoid Receptor Agonist, Protects against Nigrostriatal Cell Loss in the 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine Mouse Model of Parkinson's Disease. Eur. J. Neurosci. 29, 2177–2186. 10.1111/j.1460-9568.2009.06764.x.WIN55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin N. S. (2003). Killing Tumours by Ceramide-Induced Apoptosis: a Critique of Available Drugs. Biochem. J. 371, 243–256. 10.1042/BJ20021878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamani R., Muthuvel A., Senthilvelan M., Sheeladevi R. (2006). Oxidative Stress Induced by Methotrexate Alone and in the Presence of Methanol in Discrete Regions of the Rodent Brain, Retina and Optic Nerve. Toxicol. Lett. 165, 265–273. 10.1016/j.toxlet.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Ramer R., Hinz B. (2017). New Insights into Antimetastatic and Antiangiogenic Effects of Cannabinoids. Int. Rev. Cel Mol. Biol. 314, 43–116. 10.1016/bs.ircmb.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Rodrigues L. S., Fagotti J., D S Targa A., D Noseda A. C., L Ilkiwa J., Chuproski A. P., et al. (2019). Potential New Therapies against a Toxic Relationship: Neuroinflammation and Parkinson's Disease. Behav. Pharmacol. 30, 676–688. 10.1097/FBP.0000000000000512 [DOI] [PubMed] [Google Scholar]
- Schreiner A. M., Dunn M. E. (2012). Residual Effects of Cannabis Use on Neurocognitive Performance after Prolonged Abstinence: A Meta-Analysis. Exp. Clin. Psychopharmacol. 20, 420–429. 10.1037/a0029117 [DOI] [PubMed] [Google Scholar]
- Schurman L. D., Lichtman A. H. (2017). Endocannabinoids: A Promising Impact for Traumatic Brain Injury. Front. Pharmacol. 8, 69. 10.3389/fphar.2017.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi C., Bronzuoli M. R., Facchinetti R., Pace L., Ferraro L., Broad K. D., et al. (2018). Ultramicronized Palmitoylethanolamide Rescues Learning and Memory Impairments in a Triple Transgenic Mouse Model of Alzheimer's Disease by Exerting Anti-inflammatory and Neuroprotective Effects. Transl. Psychiatry 8, 32. 10.1038/s41398-017-0076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigers R., Loos M., Van Tellingen O., Boogerd W., Smit A. B., Schagen S. B. (2015). Cognitive Impact of Cytotoxic Agents in Mice. Psychopharmacology (Berl) 232, 17–37. 10.1007/s00213-014-3636-9 [DOI] [PubMed] [Google Scholar]
- Shalaby Y. M., Menze E. T., Azab S. S., Awad A. S. (2019). Involvement of Nrf2/HO-1 Antioxidant Signaling and NF-Κb Inflammatory Response in the Potential Protective Effects of Vincamine against Methotrexate-Induced Nephrotoxicity in Rats: Cross Talk between Nephrotoxicity and Neurotoxicity. Arch. Toxicol. 93, 1417–1431. 10.1007/s00204-019-02429-2 [DOI] [PubMed] [Google Scholar]
- Sharpe M. J., Fardell J. E., Vardy J., Johnston I. N. (2012). The Chemotherapy Agent Oxaliplatin Impairs the Renewal of Fear to an Extinguished Conditioned Stimulus in Rats. Behav. Brain Res. 227, 295–299. 10.1016/j.bbr.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Śledziński P., Nowak-Terpiłowska A., Zeyland J. (2020). Cannabinoids in Medicine: Cancer, Immunity, and Microbial Diseases. Ijms 22, 263. 10.3390/ijms22010263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadevara U., Bruijnzeel D. M., Nuthi M., Jagnarine D. A., Tandon R., Bruijnzeel A. W. (2017). Pros and Cons of Medical Cannabis Use by People with Chronic Brain Disorders. Curr. Neuropharmacol 15 (6), 800–814. 10.2174/1570159X14666161101095325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleurs C., Deprez S., Emsell L., Lemiere J., Uyttebroeck A. (2016). Chemotherapy-induced Neurotoxicity in Pediatric Solid Non-CNS Tumor Patients: An Update on Current State of Research and Recommended Future Directions. Crit. Rev. Oncol. Hematol. 103, 37–48. 10.1016/j.critrevonc.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Surh Y. J., Bode A. M., Zhao Q., Cho Y. Y., Zhu F., Ma W. Y., et al. (2008). The Cannabinoid Receptors Are Required for Ultraviolet-Induced Inflammation and Skin Cancer Development. Cancer Res. 68, 3992–3998. 10.1158/0008-5472.CAN-07-6594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasescu R., Gran B., Constantinescu C. S. (2013). The Endocannabinoid System: A Revolving Plate in Neuro-Immune Interaction in Health and Disease. Amino Acids 45, 95–112. 10.1007/s00726-012-1252-8 [DOI] [PubMed] [Google Scholar]
- Tangpong J., Cole M. P., Sultana R., Estus S., Vore M., St Clair W., et al. (2007). Adriamycin-mediated Nitration of Manganese Superoxide Dismutase in the central Nervous System: Insight into the Mechanism of Chemobrain. J. Neurochem. 100, 191–201. 10.1111/j.1471-4159.2006.04179.x [DOI] [PubMed] [Google Scholar]
- Taylor B., Mueller M., Sauls R. (2020). “Cannabinoid Antiemetic Therapy,” in StatPearls [Internet] (Treasure Island (FL): StatPearls Publishing; ). [PubMed] [Google Scholar]
- Tchantchou F., Tucker L. B., Fu A. H., Bluett R. J., Mccabe J. T., Patel S., et al. (2014). The Fatty Acid Amide Hydrolase Inhibitor PF-3845 Promotes Neuronal Survival, Attenuates Inflammation and Improves Functional Recovery in Mice with Traumatic Brain Injury. Neuropharmacology 85, 427–439. 10.1016/j.neuropharm.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres S., Lorente M., Rodríguez-Fornés F., Hernández-Tiedra S., Salazar M., García-Taboada E., et al. (2011). A Combined Preclinical Therapy of Cannabinoids and Temozolomide against Glioma. Mol. Cancer Ther. 10, 90–103. 10.1158/1535-7163.MCT-10-0688 [DOI] [PubMed] [Google Scholar]
- Uddin M. S., Mamun A. A., Sumsuzzman D. M., Ashraf G. M., Perveen A., Bungau S. G., et al. (2020). Emerging Promise of Cannabinoids for the Management of Pain and Associated Neuropathological Alterations in Alzheimer's Disease. Front. Pharmacol. 11, 1097. 10.3389/fphar.2020.01097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeno A., Biju V., Yoshida Y. (2017). In Vivo ROS Production and Use of Oxidative Stress-Derived Biomarkers to Detect the Onset of Diseases Such as Alzheimer's Disease, Parkinson's Disease, and Diabetes. Free Radic. Res. 51, 413–427. 10.1080/10715762.2017.1315114 [DOI] [PubMed] [Google Scholar]
- van der Stelt M., Mazzola C., Esposito G., Matias I., Petrosino S., de Filippis D., et al. (2006). Endocannabinoids and Beta-Amyloid-Induced Neurotoxicity In Vivo: Effect of Pharmacological Elevation of Endocannabinoid Levels. Cell. Mol. Life Sci. 63, 1410–1424. 10.1007/s00018-006-6037-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez C., Tolón R. M., Pazos M. R., Moreno M., Koester E. C., Cravatt B. F., et al. (2015). Endocannabinoids Regulate the Activity of Astrocytic Hemichannels and the Microglial Response against an Injury: In Vivo Studies. Neurobiol. Dis. 79, 41–50. 10.1016/j.nbd.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Vecera L., Gabrhelik T., Prasil P., Stourac P. (2020). The Role of Cannabinoids in the Treatment of Cancer. Bratisl. Lek. Listy. 121, 79–95. 10.4149/BLL_2020_012 [DOI] [PubMed] [Google Scholar]
- Verma T., Mallik S. B., Ramalingayya G. V., Nayak P. G., Kishore A., Pai K. S. R., et al. (2017). Sodium Valproate Enhances Doxorubicin-Induced Cognitive Dysfunction in Wistar Rats. Biomed. Pharmacother. 96, 736–741. 10.1016/j.biopha.2017.09.150 [DOI] [PubMed] [Google Scholar]
- Vijayanathan V., Gulinello M., Ali N., Cole P. D. (2011). Persistent Cognitive Deficits, Induced by Intrathecal Methotrexate, Are Associated with Elevated CSF Concentrations of Excitotoxic Glutamate Analogs and Can Be Reversed by an NMDA Antagonist. Behav. Brain Res. 225, 491–497. 10.1016/j.bbr.2011.08.006 [DOI] [PubMed] [Google Scholar]
- Viveros M. P., Llorente R., Suarez J., Llorente-Berzal A., López-Gallardo M., de Fonseca F. R. (2012). The Endocannabinoid System in Critical Neurodevelopmental Periods: Sex Differences and Neuropsychiatric Implications. J. Psychopharmacol. 26, 164–176. 10.1177/0269881111408956 [DOI] [PubMed] [Google Scholar]
- Volicer L., Stelly M., Morris J., McLaughlin J., Volicer B. J. (1997). Effects of Dronabinol on Anorexia and Disturbed Behavior in Patients with Alzheimer's Disease. Int. J. Geriatr. Psychiatry 12 (9), 913–919. [DOI] [PubMed] [Google Scholar]
- Walsh D., Nelson K. A., Mahmoud F. A. (2002). Established and Potential Therapeutic Applications of Cannabinoids in Oncology. Support Care Cancer 11, 137–143. 10.1007/s00520-002-0387-7 [DOI] [PubMed] [Google Scholar]
- Watt G., Shang K., Zieba J., Olaya J., Li H., Garner B., et al. (2020). Chronic Treatment with 50 mg/kg Cannabidiol Improves Cognition and Moderately Reduces Aβ40 Levels in 12-Month-Old Male AβPPswe/PS1ΔE9 Transgenic Mice. J. Alzheimers Dis. 74, 937–950. 10.3233/JAD-191242 [DOI] [PubMed] [Google Scholar]
- Wefel J. S., Schagen S. B. (2012). Chemotherapy-Related Cognitive Dysfunction. Curr. Neurol. Neurosci. Rep. 12, 267–275. 10.1007/s11910-012-0264-9 [DOI] [PubMed] [Google Scholar]
- Woodhams S. G., Chapman V., Finn D. P., Hohmann A. G., Neugebauer V. (2017). The Cannabinoid System and Pain. Neuropharmacology 124, 105–120. 10.1016/J.NEUROPHARM.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward M. R., Harper D. G., Stolyar A., Forester B. P., Ellison J. M. (2014). Dronabinol for the Treatment of Agitation and Aggressive Behavior in Acutely Hospitalized Severely Demented Patients with Noncognitive Behavioral Symptoms. Am. J. Geriatr. Psychiatry 22 (4), 415–419. 10.1016/j.jagp.2012.11.022 [DOI] [PubMed] [Google Scholar]
- Yang M., Kim J. S., Song M. S., Kim S. H., Kang S. S., Bae C. S., et al. (2010). Cyclophosphamide Impairs Hippocampus-dependent Learning and Memory in Adult Mice: Possible Involvement of Hippocampal Neurogenesis in Chemotherapy-Induced Memory Deficits. Neurobiol. Learn. Mem. 93, 487–494. 10.1016/j.nlm.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Zhu L. X., Sharma S., Stolina M., Gardner B., Roth M. D., Tashkin D. P., et al. (2000). Delta-9-tetrahydrocannabinol Inhibits Antitumor Immunity by a CB2 Receptor-Mediated, Cytokine-dependent Pathway. J. Immunol. 165, 373–380. 10.4049/jimmunol.165.1.373 [DOI] [PubMed] [Google Scholar]
- Zou S., Kumar U. (2018). Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the central Nervous System. Int. J. Mol. Sci. 19, 833. 10.3390/ijms19030833 [DOI] [PMC free article] [PubMed] [Google Scholar]