Abstract
Background
Comparative effectiveness of 7 glucagon-like peptide 1 (GLP-1) agents on weight loss (WL) in obesity remains unknown.
Methods
We performed a systematic review, network meta-analysis (NMA) utilizing the following data sources: MEDLINE, EMBASE, Scopus, Cochrane Central and clinical trial registries, from inception to March 2, 2021. The prespecified criteria for study inclusion were randomized clinical trials (RCTs) of ≥12 weeks’ duration. The data appraisal and extraction were performed by two investigators independently, using the published reports. The main outcomes and statistical methods were weight loss over placebo (WLOP) and adverse events (AEs) among GLP-1 agents using random-effects NMA (frequentist approach); relative ranking using surface under the cumulative ranking (SUCRA) method and certainty of evidence using grading of recommendations, assessment, development and evaluations (GRADE).
Findings
64 RCTs (from 2004 to 2021) included 27018 patients (median of age, 55.1 years old; 57.4% women; baseline weight 94.8kg and BMI 33.0kg/m2; trial duration 26 weeks). Direct meta-analysis showed significant WLOP with: -1.44kg (95% CI, -2.14 to -0.74) with dulaglutide ≥1.5 mg; -1.82kg (-2.42 to -1.23) with exenatide immediate release (IR); -2.20kg (-4.31 to -0.08) with exenatide extended release (ER); -3.20kg (-6.53 to 0.15) with efpeglenatide; -2.72kg (-3.35 to -2.09) with liraglutide ≤1.8mg; -4.49kg (-5.26 to -3.72) with liraglutide >1.8mg; -0.62kg (-1.22 to -0.02) with lixisenatide; -4.33kg (-5.71 to -3.00) with semaglutide SQ <2.4mg; -9.88kg (-13.17 to -6.59) with semaglutide SQ 2.4mg; -2.73kg (-4.81 to -0.65) with semaglutide oral; and -1.71kg (-2.64 to -0.78) with taspoglutide. Highest WLOP were with semaglutide SQ 2.4mg and <2.4mg, and liraglutide >1.8mg (SUCRAs 100, 86.1, 82.8 respectively). Highest SUCRAs for discontinuation due to AEs were with taspoglutide and liraglutide >1.8mg. Risk of bias was high or unclear for random sequence generation (29.7%), allocation concealment (26.6%), and incomplete outcome data (26.6%). Heterogeneity (I2 >50%) in WL and AEs reflected magnitude, not direction of effect.
Research in context.
Evidence before this study
Prior systematic reviews and meta-analyses have documented the effects of diverse pharmacological treatments for obesity on weight loss, cardiometabolic risk factors, and adverse effects. The most efficacious class of pharmacological treatments are the GLP-1 agonists or analogs. However, the relative efficacy of the latter agents remains unclear.
Added value of this study
The current study, based on 64 randomized, controlled trials (2004-2021) and 27018 patients, provides an estimate of the relative efficacy of the available GLP-1 agonists or analogs, as well as the risk of adverse effects. The network meta-analysis suggests that the greatest efficacy is observed with liraglutide and semaglutide.
Implications of all the available evidence
This analysis, as well as future studies replicating these findings, may inform clinical practice as well as guidelines and policies with regards to pharmacological treatment of obesity.
Alt-text: Unlabelled box
1. Introduction
It is estimated that about 1.9 billion adults are overweight and 600 million have obesity worldwide [1]. Obesity increases the risk of type 2 diabetes and cardiovascular disease, in part related to genetic and lifestyle-related causes [2] leading to high health-care costs attributable to obesity-related diseases [3]. Prior systematic reviews have documented the relative efficacy of different classes of obesity medications, including glucagon-like peptide-1 (GLP-1) analogs and agonists, on weight loss and cardiometabolic health.[4,5] The odds of discontinuation of therapy due to medication-related adverse events were not different for phentermine-topiramate, liraglutide, and naltrexone-bupropion [4]. The magnitude of weight loss relative to placebo treatment achieved with the GLP-1 analog, liraglutide, was greater than that observed with lorcaserin and orlistat, similar to naltrexone-bupropion, and slightly lower than that observed in two trials with phentermine-topiramate [4]. This observation and the multiple GLP-1 agents tested or available for prescription provided the rationale for the current study of the relative efficacy of the diverse GLP-1 agents.
The metabolic effects of GLP-1 agonists and analogs reflect the effects described for endogenous GLP-1 on peripheral and central mechanisms involved in weight control such as appetite, food intake, and glycemic control. The peripheral actions include activation of the ileal brake, delay in gastric emptying, increase in glucose-dependent insulin release, decrease in glucagon secretion, and increase in pancreatic β cell growth. [6,7] GLP-1 and GLP-1 agonists or analogs also reduce appetite through effects on several regions of the human central nervous system that express GLP-1 receptors including the parietal and orbitofrontal cortex, hypothalamus, [8] and medulla (such as the nucleus of solitary tract); for example, the GLP-1 analog, liraglutide, alters brain activity related to highly desirable food cues [9], [10], [11], [12], [13]. Retardation of gastric emptying is partly impacted by tachyphylaxis, but upper gastrointestinal symptoms such as nausea or vomiting may lead to discontinuation, sometimes affecting 6 to 16% of participants in randomized clinical trials (RCTs) of liraglutide [14], [15], [16], [17]. In an earlier analysis, discontinuations with GLP-1 agents were reported as exenatide and liraglutide 1.2mg (both 19%), and liraglutide 1.8mg (16%) [18]. The US Food and Drug Administration (FDA) has approved liraglutide 3.0mg and subcutaneous (SQ) semaglutide 2.4mg for weight loss, and several GLP-1 analogs or agonists in diverse formulations or doses for the treatment of type 2 diabetes mellitus: dulaglutide, exenatide [immediate release (IR) and extended release (ER)], efpeglenatide, liraglutide, lixisenatide, semaglutide (SQ and oral), and taspoglutide.
However, there is a paucity of RCT evidence comparing the efficacy and adverse effects of the different GLP-1 agonist or analog treatments with each other. It is relevant that data regarding relative efficacy and adverse effects of each drug can inform patients, health care practitioners, and policymakers regarding the optimal GLP-1 medication prescription to treat obesity and overweight. The objective of this systematic review and network meta-analysis was to compare the associations of each GLP-1 agonist or analog with weight loss and adverse effects using a direct meta-analysis and random-effects network meta-analysis using a frequentist approach.
2. Methods
We conducted a systematic review and network meta-analysis. The meta-analysis was conducted following principles described in the Cochrane Handbook for Systematic Reviews of Interventions [19]. Pairwise random-effect meta-analysis was performed by synthesizing data from trials comparing at least one GLP-1 agonist or analog drug with placebo. Network meta-analysis was conducted to compare different drugs and doses. Results were reported according to PRISMA Extension Statement for Reporting of Systematic Incorporating Network Meta-analyses of Health Care Interventions [20]. Certainty of evidence was assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for network meta-analysis [21].
3. Criteria for study inclusion
We included parallel, randomized clinical trials which studied one or more GLP-1 agonist or analog drug compared to placebo treatment for at least 12 weeks (a recognized minimum duration in the application of pharmacotherapy for obesity for achieving meaningful and, for many medications, near-maximal weight loss), and at least 12 weeks of follow-up. The included studies were conducted in adults (>18 years) with obesity or overweight [body mass index (BMI) >25 kg/m2) in White, Hispanic, and Black individuals, and BMI >23kg/m2 in Asian populations] and documented the measured change in mean weight.
Patients with or without diabetes mellitus or nonalcoholic fatty liver disease (NAFLD) were included. We excluded open-labeled, cross-over design, or non-placebo-controlled trials (e.g., trials with active or no intervention comparators), as well as trials in specific populations such as patients with polycystic ovary syndrome. There was no language limitation.
4. Data sources and searches
A medical librarian (LJP) developed and conducted the search strategy (see eMethods in Supplemental Material) using inputs from the study investigators. The search included Ovid MEDLINE, Cochrane Central Register of Controlled Trials, EMBASE, clinical trial registries and Scopus from inception to March 2, 2021. A repeat literature search conducted on October 10, 2021 revealed no relevant additional clinical trials pertaining exclusively to this class of GLP-1 agents. Published review articles and content experts were used to identify relevant articles missed by the electronic search. The data sought included individual patient data. We did not seek data from any unpublished studies. Details of the electronic search strategy are presented in eMethods in Supplemental Material.
5. Study selection
Two investigators (KV and KK) independently screened the titles and abstracts to select potentially eligible studies based on prespecified eligibility criteria. Discrepancies were discussed until a consensus was reached. Any potentially relevant citation was then retrieved in full-text and reviewed by the same two investigators to evaluate the inclusion/exclusion criteria and to determine inclusion. Discrepancies were resolved through discussion with a third investigator (MC). Excluded studies at this step were listed with the reason for exclusion.
6. Data extraction and quality assessment
Two investigators (JA and LK) independently reviewed the main reports and supplementary materials, including data reported in the ClinicalTrials.gov portal, and extracted trial and patient characteristics, duration of treatment strategies, and outcomes using a predesigned form in order to prepare the data for synthesis. Discrepancies were resolved by referring to the original article in consultation with a third investigator (MC).
Details of the extracted variables are presented in eTable 1 in the Supplemental Material. The data extraction form is included in eFigure 1 in the Supplemental Material. Active durations of treatments of each trial were classified into one of the following drug-dosage combinations: dulaglutide <1.5mg, dulaglutide ≥1.5mg, exenatide IR, exenatide ER, efpeglenatide, liraglutide ≤1.8mg, liraglutide >1.8mg, lixisenatide, semaglutide SQ <2.4mg, semaglutide SQ 2.4mg, semaglutide oral, and taspoglutide. In trials where participants were randomized to different dosages of a GLP-1 agonist or analog, the intervention arms with different dosages were pooled and classified into the pertinent drug-dosage category.
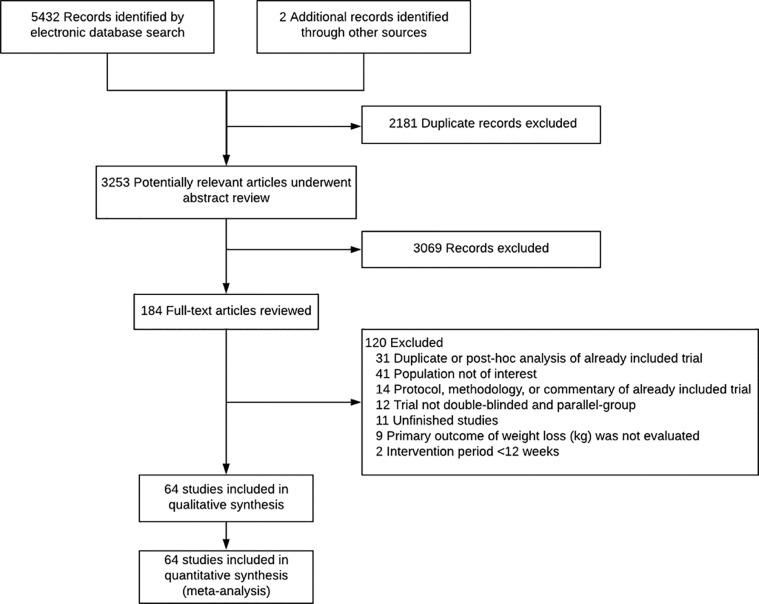
Figure 1.
Flow chart summarizing study retrieval and identification
The primary outcome was to assess the effect of GLP-1 agonists or analogs on change in weight compared to placebo in adults with obesity or overweight. The primary safety outcome was the rate of individuals with adverse events leading to discontinuation of trial treatment. Secondary safety outcomes were rates of participants with nausea, vomiting, and serious adverse events.
Two reviewers (JA and LK) assessed the study risk of bias using the Cochrane Risk of Bias assessment tool [22]. Certainty in the pooled estimates was assessed using the GRADE approach for network meta-analysis [21]. In this approach, investigators rate the certainty of evidence separately for direct, indirect, and NMA (combined direct + indirect) estimates. Certainty in direct estimates obtained from head-to-head comparisons starts as high and can be downgraded to moderate, low, and very low based on risk of bias, indirectness, imprecision, inconsistency (or heterogeneity), and publication bias. This was followed by rating of the indirect estimates, which starts at the lowest rating of the two pairwise estimates that contribute as first-order loops to the indirect estimate without considering imprecision. Intransitivity (significant differences between the characteristics of the studies in the first-order loop) was considered a reason to lower certainty in the indirect estimate. Finally, certainty of NMA estimate was defined as the highest rating between direct and indirect evidence of a given comparison, if the direct and indirect estimates were similar (i.e., coherent), and after applying the rules of imprecision to the NMA estimate. If the direct and indirect estimates were incoherent, we rated down the NMA estimate for incoherence. Alternatively, the direct estimates were used as the best evidence.
7. Outcomes, data synthesis and analysis
The primary outcomes extracted were weight loss and adverse effects, in particular discontinuation due to adverse effects. The analysis performed to assess discontinuations did not adjust for differences in dose escalation regimens across the RCTs which ranged from inflexible (subjects exit if unable to tolerate the medication) to flexible dose escalation protocols with ability to decrease the dose or maintain a given dose for a longer period of time before escalating to the next dose level to enable retention in the study on treatment.
Treatment effects and 95% confidence intervals were estimated by pairwise meta-analysis using DerSimonian and Laird random-effects model [23]. The effects of treatment compared to placebo were estimated using mean difference (MD) for weight loss and relative risk (RR) for adverse events leading to discontinuation, nausea, vomiting, and serious adverse events (together with 95% confidence interval).
I[2] statistics was used to quantify the degree of heterogeneity (variability within studies of each of the GLP-1 agents) with values over 50%, indicating substantial heterogeneity [24]. Publication bias was assessed by examining funnel plot asymmetry using the linear regression method of Egger's test [25].
To integrate indirect and direct estimates, random-effects network meta-analysis was performed using a frequentist approach. Relative ranking of agents was assessed using surface under the cumulative ranking (SUCRA) method. Higher SUCRA scores reflect higher probability of being most effective in terms of weight loss and lower likelihood of adverse events. The analysis was conducted using the network suite[26,27] in STATA (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.).
8. Role of the funding source
There was no funding used to conduct this study other than support of research time for the PI's research program on a GLP-1 agent in obesity from NIH R01-DK67071. The funding source had no access to data and was not involved in the decision to submit for publication. All of the authors had access to the data and decided to submit the manuscript for publication.
9. Results
The search strategy retrieved 3253 unique references from RCTs. Of these references, 64 RCTs fulfilled the inclusion criteria (Figure 1). The data extracted from the included randomized clinical trials are shown in eTable 1 in Supplemental Material; the characteristics of included randomized clinical trial including route, dose, and frequency of medications, duration of trial, study population as well as the total number of participants randomized, included in the weight or BMI analysis, completers, and attrition rate are summarized in eTable 2 in Supplemental Material. The baseline patient characteristics in the included randomized controlled trials with each GLP-1 agent are summarized in eTable 3 in Supplemental Material.
Table 2.
Weight loss vs. adverse events leading to medication discontinuation comparison of GLP-1 agonist/analog drug-dosage groups by network meta-analysis
| Mean difference (95% CrI) of weight loss | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relative risk (95% CrI) of discontinuation due to adverse events | Placebo | -0.41 (-1.89 to 1.06) | -1.43 (-2.70 to -0.15) | -1.77 (-2.75 to -0.79) | -2.19 (-5.02 to 0.65) | -3.15 (-4.99 to -1.31) | -2.61 (-3.38 to -1.84) | -4.19 (-5.26 to -3.13) | -0.72 (-2.89 to 1.46) | -4.49 (-5.55 to -3.43) | -9.72 (-11.34 to -8.11) | -2.79 (-4.42 to -1.17) | -1.71 (-4.80 to 1.39) |
| 0.81 (0.26 to 2.46) | Dulaglu-tide <1.5mg | -1.01 (-2.49 to 0.46) | -1.36 (-3.14 to 0.41) | -1.78 (-4.97 to 1.42) | -2.74 (-5.10 to -0.38) | -2.20 (-3.86 to -0.53) | -3.78 (-5.60 to -1.96) | -0.31 (-2.93 to 2.32) | -4.08 (-5.89 to -2.26) | -9.31 (-11.50 to -7.13) | -2.38 (-4.58 to -0.19) | -1.29 (-4.72 to 2.13) | |
| 2.07 (0.84 to 5.11) | 2.57 (0.98 to 6.71) | Dulaglu-tide ≥1.5 mg | -0.35 (-1.95 to 1.26) | -0.76 (-3.87 to 2.35) | -1.72 (-3.96 to 0.51) | -1.18 (-2.67 to 0.30) | -2.77 (-4.42 to -1.11) | 0.71 (-1.81 to 3.23) | -3.06 (-4.72 to -1.41) | -8.30 (-10.35 to -6.24) | -1.37 (-3.43 to 0.69) | -0.28 (-3.63 to 3.07) | |
| 2.05 (1.21 to 3.48) | 2.54 (0.74 to 8.75) | 0.99 (0.35 to 2.82) | Exana-tide IR | -0.41 (-3.41 to 2.59) | -1.37 (-3.46 to 0.71) | -0.83 (-2.08 to 0.41) | -2.42 (-3.87 to -0.97) | 1.06 (-1.32 to 3.44) | -2.72 (-4.16 to -1.27) | -7.95 (-9.84 to -6.06) | -1.02 (-2.92 to 0.87) | 0.07 (-3.18 to 3.31) | |
| 0.29 (0.02 to 3.58) | 0.36 (0.02 to 5.63) | 0.14 (0.01 to 2.02) | 0.14 (0.01 to 1.85) | Exana-tide ER | -0.96 (-4.34 to 2.42) | -0.42 (-3.36 to 2.51) | -2.01 (-5.04 to 1.02) | 1.47 (-2.10 to 5.04) | -2.30 (-5.33 to 0.72) | -7.54 (-10.80 to -4.27) | -0.61 (-3.87 to 2.66) | 0.48 (-3.72 to 4.68) | |
| 1.91 (0.80 to 4.53) | 2.36 (0.57 to 9.73) | 0.92 (0.26 to 3.22) | 0.93 (0.34 to 2.57) | 6.60 (0.46 to 94.62) | Efpegla-natide | 0.54 (-1.45 to 2.53) | -1.05 (-3.17 to 1.08) | 2.43 (-0.41 to 5.28) | -1.34 (-3.46 to 0.78) | -6.57 (-9.02 to -4.13) | 0.35 (-2.10 to 2.81) | 1.44 (-2.16 to 5.04) | |
| 1.93 (1.29 to 2.89) | 2.40 (0.73 to 7.89) | 0.93 (0.35 to 2.50) | 0.94 (0.49 to 1.84) | 6.70 (0.52 to 85.79) | 1.02 (0.39 to 2.64) | Liraglu-tide ≤ 1.8mg | -1.59 (-2.82 to -0.35) | 1.89 (-0.41 to 4.19) | -1.88 (-3.14 to -0.63) | -7.11 (-8.89 to -5.33) | -0.19 (-1.91 to 1.54) | 0.90 (-2.29 to 4.09) | |
| 2.31 (1.53 to 3.49) | 2.87 (0.87 to 9.44) | 1.12 (0.41 to 3.01) | 1.13 (0.58 to 2.21) | 8.00 (0.62 to 102.67) | 1.21 (0.46 to 3.17) | 1.19 (0.72 to 1.99) | Liraglu -tide >1.8mg | 3.48 (1.06 to 5.90) | -0.30 (-1.74 to 1.15) | -5.53 (-7.45 to -3.60) | 1.40 (-0.52 to 3.32) | 2.49 (-0.78 to 5.76) | |
| 1.80 (0.73 to 4.44) | 2.23 (0.53 to 9.40) | 0.87 (0.24 to 3.11) | 0.88 (0.31 to 2.51) | 6.22 (0.43 to 90.35) | 0.94 (0.27 to 3.30) | 0.93 (0.34 to 2.50) | 0.78 (0.29 to 2.10) | Lixise-natide | -3.77 (-6.19 to -1.36) | -9.01 (-11.71 to -6.30) | -2.08 (-4.79 to 0.63) | -0.99 (-4.77 to 2.79) | |
| 2.12 (1.32 to 3.41) | 2.63 (0.78 to 8.88) | 1.02 (0.37 to 2.83) | 1.04 (0.51 to 2.11) | 7.34 (0.57 to 95.22) | 1.11 (0.41 to 2.99) | 1.10 (0.62 to 1.93) | 0.92 (0.51 to 1.64) | 1.18 (0.43 to 3.28) | Sema-glutide SQ <2.4mg | -5.23 (-7.06 to -3.41) | 1.69 (-0.13 to 3.52) | 2.78 (-0.49 to 6.05) | |
| 2.09 (1.07 to 4.09) | 2.60 (0.71 to 9.55) | 1.01 (0.33 to 3.12) | 1.02 (0.44 to 2.40) | 7.25 (0.54 to 98.14) | 1.10 (0.37 to 3.28) | 1.08 (0.50 to 2.35) | 0.91 (0.42 to 1.97) | 1.17 (0.38 to 3.60) | 0.99 (0.47 to 2.09) | Sema-glutide SQ 2.4mg | 6.93 (4.66 to 9.20) | 8.02 (4.53 to 11.51) | |
| 1.45 (0.72 to 2.95) | 1.80 (0.48 to 6.78) | 0.70 (0.22 to 2.21) | 0.71 (0.29 to 1.72) | 5.04 (0.37 to 68.92) | 0.76 (0.25 to 2.34) | 0.75 (0.35 to 1.62) | 0.63 (0.28 to 1.40) | 0.81 (0.26 to 2.56) | 0.69 (0.33 to 1.45) | 0.70 (0.27 to 1.80) | Sema-glutide Oral | 1.09 (-2.40 to 4.58) | |
| 3.86 (1.02 to 14.68) | 4.79 (0.84 to 27.35) | 1.87 (0.37 to 9.36) | 1.89 (0.45 to 7.94) | 13.38 (0.77 to 231.40) | 2.03 (0.41 to 9.96) | 2.00 (0.50 to 8.06) | 1.67 (0.41 to 6.76) | 2.15 (0.43 to 10.81) | 1.82 (0.44 to 7.52) | 1.85 (0.41 to 8.22) | 2.66 (0.59 to 12.04) | Taspo-glutide | |
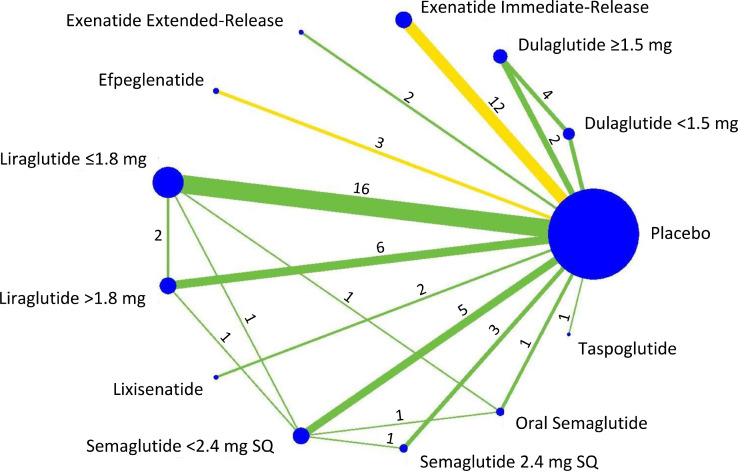
After classification of the active durations of treatments based on the predefined drug-dosage combinations, 53 of these RCTs provided two-group comparisons consisting of a placebo group and one drug-dosage group (dulaglutide ≥1.5mg, 2 trials; [28,29] exenatide IR, 12 trials; [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41] exenatide ER, 2 trials; [42,43] efpeglenatide, 3 trials;[44], [45], [46] liraglutide ≤1.8mg, 16 trials; [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62] liraglutide >1.8mg, 6 trials;[17,[63], [64], [65], [66], [67]] lixisenatide, 2 trials; [68,69] semaglutide SQ <2.4mg, 5 trials; [70], [71], [72], [73], [74] semaglutide SQ 2.4mg, 3 trials; [75], [76], [77] semaglutide oral, 1 trial; [78] and taspoglutide, 1 trial [79]). In addition, 11 studies provided three-group comparisons consisting of one placebo group and two drug-dosage groups (dulaglutide <1.5mg and dulaglutide ≥1.5mg, 4 trials; [80], [81], [82], [83] liraglutide ≤1.8mg and liraglutide >1.8mg, 2 trials; [84,85] liraglutide ≤1.8mg and semaglutide SQ <2.4mg, 1 trial; [86] semaglutide SQ <2.4mg and semaglutide oral, 1 trial; [87] liraglutide >1.8mg and semaglutide SQ <2.4mg, 1 trial;[88] semaglutide SQ <2.4mg and semaglutide SQ 2.4mg, 1 trial; [89] and liraglutide ≤1.8mg and semaglutide oral, 1 trial [90]). A network of direct comparisons is presented in Figure 2.
Figure 2.
Network of included trials and available direct comparisons for weight loss. Color of the comparison lines shows the average estimated risk of bias for each direct comparison: green reflects low risk and yellow reflects unclear risk of bias. Numbers above lines indicate numbers of trials for each comparison.
10. Trial and participant characteristics and risk of bias
Overall, 64 RCTs reported between 2004 and 2021 and involving 27018 participants were included. In three of these RCTs (2629 participants), two different GLP-1 agonists or analogs were tested vs. placebo. The number of participants randomized to active treatments ranged from 1140 to 248766 [median, 196 (IQR, 35-322)]. The attrition rate was generally comparable between the active and placebo treatment groups, and ranged from 0% to 47% [median, 11% (IQR, 5.5-17%)] in active treatment groups and from 0% to 56% [median, 11% (IQR, 6-18.5%)] in placebo groups.
The duration of treatments ranged from 12 to 160 weeks, with a median of 26 weeks (IQR, 20-30 weeks). Four studies administered a glucose lowering drug as a co-treatment to all participants in the placebo and active treatment groups (metformin, 3 studies; [53,54,82] and dapaglifozin, 1 trial [43]). Two RCTs did not allow any glucose lowering medications alongside the trial treatment, including one trial which recruited only treatment-naïve participants [35] and one trial which excluded any patients who received anti-diabetes medications within 90 days before screening [78]. The rest of the trials allowed at least one glucose or weight lowering agent alongside the trial treatment. Details of the RCTs included in this meta-analysis are summarized in eTable 2 in the Supplemental Material.
Overall, 57.4% of the participants were female (range, 19.8%-81.5%) and 74.9% were of white race (range 36.6%-100%). The median of the mean age at randomization was 55.1 years (range, 37-65.8 years). At randomization, the median of mean weight was 94.3kg (IQR, 90.5-100kg) in placebo groups and 95.3kg (IQR, 92.1-100.5kg) in active treatment groups, and the median of mean BMI was 32.9kg/m2 (IQR, 31.9-35kg/m2) in placebo groups and 33.1kg/m2 (IQR, 32-34.4kg/m2) in active treatment groups. eTable 3 in the Supplemental Material presents details of patient characteristics in the included RCTs.
Overall, the risk of bias was high or unclear for random sequence generation in 19 trials (29.7%), allocation concealment in 17 trials (26.6%), and incomplete outcome data in 17 trials (26.6%). Blinding of participants and personnel, blinding of outcome assessment, and possibility of selective reporting were adequate in all trials. Study level and overall risk of bias assessments are summarized in eFigure 2 in the Supplemental Material.
11. Primary assessments
11.1. Pairwise direct meta-analysis
The results of treatment effects and adverse events estimates of GLP-1 agonists or analogs compared to placebo in pairwise meta-analyses are shown in Table 1. Overall, all drug-dosage groups combined, and GLP-1 agonists or analogs showed excess weight loss of 3.11kg (95% CI, -3.64 to -2.57) compared to placebo. All drug-dosage categories except dulaglutide <1.5mg and efpeglenatide (all doses) were associated with significant excess weight loss compared to placebo. The excess weight losses compared to placebo (see Forest plot in eFigure 3 in the Supplemental Material) are detailed in Table 1.
Table 1.
Estimates of pairwise meta-analyses for excess weight loss and adverse events vs. placebo (I=immediate; E= extended; AE= adverse event).
| GLP-1 agents and dosage | Weight loss vs. placebo |
Discontinuation of GLP-1 agonist/analog due to AE |
No. of patients with nausea |
No. of patients with vomiting |
||||
|---|---|---|---|---|---|---|---|---|
| No. of Trials | Weighted mean difference, kg (95% CI) | No. of trials | Relative risk (95% CI) | No. of trials | Relative risk (95% CI) | No. of trials | Relative risk (95% CI) | |
| Dulaglutide <1.5 mg | 4 | -0.48 (-1.24 to 0.28) | 4 | 0.50 (0.16 to 1.52) | 4 | 1.42 (0.72 to 2.78) | 4 | 0.99 (0.27 to 3.72) |
| Dulaglutide ≥1.5 mg | 6 | -1.44 (-2.14 to -0.74) | 6 | 4.14 (1.66 to 10.33) | 6 | 2.71 (1.47 to 5.00) | 5 | 2.76 (1.42 to 5.38) |
| Exenatide I Release | 12 | -1.82 (-2.42 to -1.23) | 12 | 2.01 (1.36 to 2.96) | 9 | 2.58 (1.70 to 3.91) | 9 | 3.73 (2.65 to 5.26) |
| Exenatide E Release | 2 | -2.20 (-4.31 to -0.08) | 2 | 0.29 (0.03 to 3.25) | 1 | 1.36 (0.33 to 5.62) | 1 | 0.41 (0.01 to 19.4) |
| Efpeglenatide | 3 | -3.20 (-6.53 to 0.15) | 3 | 2.49 (1.06 to 5.86) | 3 | 3.02 (1.06 to 8.64) | 3 | 3.91 (1.81 to 8.45) |
| Liraglutide ≤1.8mg | 20 | -2.72 (-3.35 to -2.09) | 20 | 2.12 (1.44 to 3.12) | 13 | 3.07 (1.99 to 4.72) | 11 | 2.33 (1.59 to 3.41) |
| Liraglutide >1.8mg | 9 | -4.49 (-5.26 to -3.72) | 9 | 2.32 (1.49 to 3.63) | 7 | 2.62 (2.37 to 2.90) | 6 | 3.62 (2.88 to 4.55) |
| Lixisenatide | 2 | -0.62 (-1.22 to -0.02) | 2 | 1.74 (0.86 to 3.51) | 2 | 3.82 (1.24 to 11.75) | 2 | 4.35 (0.07 to 256.65) |
| Semaglutide <2.4mg | 9 | -4.33 (-5.71 to -3.00) | 9 | 2.56 (1.44 to 4.58) | 9 | 3.47 (2.73 to 4.41) | 9 | 5.08 (3.30 to 7.81) |
| Semaglutide 2.4mg | 4 | -9.88 (-13.17 to -6.59) | 4 | 1.98 (1.32 to 2.98) | 4 | 2.65 (2.30 to 3.04) | 4 | 3.41 (2.59 to 4.49) |
| Semaglutide oral | 3 | -2.73 (-4.81 to -0.65) | 3 | 2.55 (1.24 to 5.24) | 3 | 5.41 (2.14 to 13.65) | 3 | 2.72 (1.07 to 6.93) |
| Taspoglutide | 1 | -1.71 (-2.64 to -0.78) | 1 | 3.87 (1.44 to 10.35) | 0 | N/A | 0 | N/A |
| Total # of trials (# of comparisons) | 64 (75) | -3.11 (-3.64 to -2.57) | 64 (75) | 2.17 (1.85 to 2.56) | 48 (61) | 2.75 (2.44 to 3.09) | 46 (57) | 3.22 (2.74 to 3.78) |
All drug-dosage groups were associated with a significantly higher rate of medication discontinuation due to adverse events except dulaglutide <1.5mg with a relative risk (RR) of 0.5 (95% CI, 0.16 to 1.52); exenatide ER with an RR of 0.29 (95% CI, 0.03 to 3.25); and lixisenatide with an RR of 1.74 (95% CI, 0.86 to 3.51) (see Forest plot in eFigure 4 in the Supplemental Material). Overall, GLP-1 agonists or analogs (all drug-dosages combined) increased the number of patients with nausea [RR, 2.75 (95% CI, 2.44 to 3.09)] and vomiting [RR, 3.22 (95% CI, 2.74 to 3.78)] compared to placebo.
Substantial heterogeneity (I2 >50%) was observed for most of the weight loss and adverse events comparisons. Variations, however, were mostly in the magnitude of effect, not in the direction of effect.
11.2. Comparison of efficacy of GLP-1 agents in only diabetic or only non-diabetic participants
eTable 4 in the Supplemental Material shows the pairwise analysis for excess weight loss over placebo in all studies, and separately for studies performed only in diabetic or non-diabetic participants. Two of the 67 studies were excluded from this analysis because they enrolled both diabetic and non-diabetic participants. Both groups of participants (weighted mean difference of -3.11 [95% CI -3.64 to -2.57] kg overall, -2.19 [95% CI -2.56 to -1.83] kg in diabetic and -6.09 [95% CI -7.62 to -4.57] kg in non-diabetic participants) had significant overall effects on excess weight loss over placebo. In addition, meta-regression model showed that, after adjustment for drug/dosage group, being non-diabetic was associated with excess weight loss with a coefficient of -2.62 (95% CI, -3.82 to -1.42) kg (p<0.001).
Forest plots of the excess weight losses compared to placebo for the studies focused on diabetic participants and non-diabetic participants are included in Supplemental Material eFigures 5 and 6.
11.3. Network meta-analysis
11.3.1. Excess weight loss compared with placebo
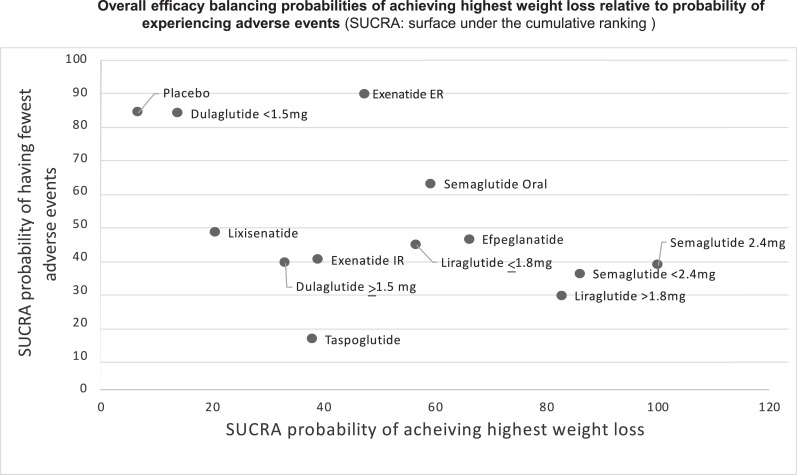
In network meta-analysis compared with placebo, the following were associated with significant excess weight loss: dulaglutide ≥1.5mg, exenatide IR;, liraglutide ≤1.8mg, liraglutide >1.8mg, semaglutide SQ <2.4mg,semaglutide SQ 2.4mg, and semaglutide oral. The following did not show a statistically significant excess weight loss in comparison with placebo: dulaglutide <1.5mg, exenatide ER, lixisenatide; and taspoglutide. Network meta-analysis showed that semaglutide SQ 2.4mg was associated with excess weight loss compared to all other active agents (Table 2). SUCRA-based treatment ranking suggested that semaglutide SQ 2.4mg (SUCRA, 100) was most likely to result in the highest excess weight loss, followed by semaglutide <2.4mg (SUCRA, 86.1), liraglutide >1.8mg (SUCRA, 82.8), efpeglenatide (SUCRA, 66.2), semaglutide oral (SUCRA, 59.4), liraglutide ≤1.8mg (SUCRA, 58), exenatide ER (SUCRA, 56.7), exenatide IR (SUCRA, 39.1), taspoglutide (SUCRA, 38), dulaglutide ≥1.5mg (SUCRA, 33.2), lixisenatide (SUCRA, 20.6), and dulaglutide <1.5mg (SUCRA, 13.9) (Figure 3 and see Forest plots in eFigure 7 for SUCRA curves for weight loss and eFigure 8 for SUCRA curves for adverse events leading to discontinuation in the Supplemental Material).
Figure 3.
Overall efficacy balancing probabilities of achieving highest weight loss relative to probability of experiencing adverse events
The network meta-analysis maps and SUCRA-based treatment rankings for diabetic and non-diabetic participants analyzed separately are shown in Supplemental Material eFigure 9, eFigure 10, and eFigure 11. These additional analyses were generally consistent with the analyses performed for all participants in the studies reviewed to assess overall efficacy of weight loss over placebo for the diabetic and non-diabetic participants together.
11.3.2. Adverse events
In the network meta-analysis, the following showed higher risk of being associated with discontinuation due to adverse events compared to placebo (Table 2): exenatide IR liraglutide ≤1.8mg liraglutide >1.8mg semaglutide SQ <2.4mgsemaglutide SQ 2.4mg; and taspoglutide . The SUCRA ranking suggested that taspoglutide (SUCRA, 15.1) and liraglutide >1.8mg (SUCRA, 28.3) were associated with the highest probability of being discontinued because of adverse events, whereas exenatide ER (SUCRA, 89.6) and dulaglutide <1.5mg (SUCRA, 83.6) were associated with the lowest probability of being discontinued because of adverse events (Figure 3 and see Forest plots respectively for nausea and vomiting in eFigure 12 and eFigure 13 in Supplemental Material).
12. Publication bias and network coherence
Visual evaluation of funnel plot symmetry (eFigure 14 in Supplemental Material) and quantitative analysis of small study effects (Egger's regression test, P >0.05) suggested no evidence of publication bias.
13. Certainty of evidence
Details of certainty assessment are presented in eTables 5 and 6 in the Supplemental Material. Overall, the certainty of the evidence was judged to be high or moderate for the NMA estimates that were derived from head-to-head direct comparisons. The trials were well done with low risk of bias, and the point estimates were mostly consistent. Some pooled estimates were imprecise due to wide confidence intervals that overlapped clinically important benefit with lack of benefit. Direct meta-analysis estimate of efpeglenatide was not statistically significant, but the network estimate had higher precision and was statistically significant. Comparisons of exenatide ER, lixisenatide, and taspoglutide had wider confidence intervals in the network than those derived from direct estimates, suggesting that the direct estimates may be more reliable and should be used for decision-making [91].
In this systematic review and network meta-analysis, direct and indirect evidence from 64 RCTs in 27,018 patients with obesity or overweight was appraised and synthesized to compare the associations of each GLP-1 agonist or analog with relative weight loss and adverse events.
14. Discussion
Our study suggests that dulaglutide ≥1.5mg, exenatide IR and exenatide ER, efpeglenatide, liraglutide ≤1.8mg, liraglutide >1.8mg, lixisenatide and semaglutide <2.4mg or 2.4mg SQ, semaglutide oral, and taspoglutide were all associated with significant weight loss compared with placebo. On the other hand, dulaglutide <1.5mg did not significantly affect body weight relative to placebo in both direct meta-analysis and NMA. Based on NMA, semaglutide SQ and oral, and liraglutide were the most efficacious GLP-1 agents to induce weight loss over at least 12 weeks’ treatment.
All GLP-1 agonists or analogs were associated with higher odds of discontinuation of treatment due to adverse effects during the conduct of the RCTs compared to placebo, with the highest odds ratios for taspoglutide and liraglutide >1.8mg. Frequently encountered symptoms were nausea and vomiting. By SUCRA probabilities, the GLP-1 agonists or analogs least likely to be associated with discontinuation because of adverse events were exenatide ER and dulaglutide <1.5 mg.
Our study has suggested the relative efficacy of all available GLP 1-agents for weight loss. This is the first systematic review and network meta-analysis of the 7 different agents in this category, and it enhances the previously published systematic reviews and meta analyses of pharmacological treatments for obesity and their effects on weight loss as well as cardio- metabolic factors [4,5].
In recommendations on prevention of obesity-related morbidity and mortality in adults, [92] the US Preventive Services Task Force (USPSTF) recommended multi-component treatments including pharmacological therapies and surgical weight loss procedures. The current study expands on the insights included in the USPSTF document of 2018 [93] which summarized pharmacotherapy trials that evaluated liraglutide (4 trials), lorcaserin (4 trials), naltrexone and bupropion (3 trials), orlistat (21 trials), and phentermine-topiramate (3 trials) in combination with behavioral counseling. In addition, [4] a systematic review and meta-analysis of the broader spectrum of drugs included in the USPSTF recommendation found moderate-quality evidence for phentermine-topiramate being associated with higher odds of achieving predefined thresholds of clinically meaningful weight loss compared with other currently approved agents.
In the future, comparison of selective GLP-1 agonists or analogs with dual glucose-stimulated insulinotropic peptide agonist, tirzepatide, will be of significant interest, given a recent report [94] of greater weight loss with 10 and 15 mg SQ tirzepatide weekly in comparison to 1mg SQ semaglutide weekly in patients with type 2 diabetes mellitus. In addition, comparisons with SQ semaglutide doses >2.4mg weekly would be of great interest, given the results showing this was the most effective GLP-1 agent in the current NMA.
There is paucity of direct comparative trials which are needed to confirm indirect comparisons obtained through NMA. On the other hand, the risk of bias in the available trials was judged to be low. Most trials attempted to control for concomitant interventions such as diet, exercise, and behavioral modification, although these were not necessarily measured as potential confounders in appraising the relative efficacy of each of the GLP-1 agents assessed. The fact that the attrition rates in the active treatment arms and placebo groups were similar (median 11%) is nevertheless reassuring that dropout is less likely to lead to important bias in the assessment of efficacy or adverse events. Risk of bias was high or unclear for random sequence generation (29.7%), allocation concealment (26.6%), and incomplete outcome data (26.6%). Heterogeneity (I2 >50%) in weight loss and adverse events reflected magnitude, not direction of effect.
The strengths of this systematic review and meta-analysis relate to selecting studies by independent pairs and following an a priori established analysis plan. Limitations include that analysis did not correct for quantity of data (i.e., numbers of studies, numbers of enrollees) for different medications, analysis performed to assess discontinuations did not adjust for differences in dose escalation regimens across the RCTs, and racial diversity was not sufficient to ensure that the results and conclusions are generalizable to races other than Caucasians. In addition, there is uncertainty in the strength of conclusions due to the presence of substantial heterogeneity, and some of the medications evaluated are either not yet marketed (efpeglenatide) or are no longer in development (taspoglutide). Future research needs to include direct comparisons between the different agents, particularly semaglutide and liraglutide which appear to demonstrate the greatest efficacy in the current network meta-analysis.
In summary, in our analysis among adults with obesity or overweight, semaglutide (SQ and oral) and liraglutide were the most efficacious GLP-1 agents to induce weight loss over at least 12 weeks of treatment. This analysis, as well as future studies replicating these findings, may inform clinical practice as well as guidelines and policies with regards to pharmacological treatment of obesity.
Contributors
K. Vosoughi and J. Atieh: co-first authors of the paper, fellow investigators, literature search
L. Khanna and K. Khoshbin: fellow investigators, literature search; co-authors
L.J. Prokop: librarian, literature searches; co-author
P. Davitkov: GRADE methodologist and risk of bias assessment; co-author
M.H. Murad: supervisor of all analyses (including SRMA), conduct of network meta-analysis, co-author
M. Camilleri: principal investigator, content expertise, data analysis and principal authorship
Interpretation
According to our analysis, semaglutide (SQ, oral) and liraglutide (SQ) were the most efficacious GLP-1 agents to induce WL during ≥12 weeks’ treatment.
Funding
There was no funding used to conduct this study other than support of research time for the PI's research program on a GLP-1 agent in obesity from NIH R01-DK67071.
Data sharing
There are no new original data in this article, as this is a review of the literature.
Funding
There was no extramural funding used to conduct this study other than support of research time for the PI's research program on a GLP-1 agent in obesity from NIH R01-DK67071.
Declaration of Competing Interest
Dr. Camilleri is supported by grant R01-DK67071 from National Institutes of Health for mechanistic studies of obesity including GLP-1 agonists/analogs. Dr. Camilleri serves on an advisory board for Phenomix Sciences regarding the company's development of a test to predict weight loss response to obesity therapy, and he is a stockholder in the company. Phenomix Sciences has obtained an exclusive license to Dr. Camilleri's and a Mayo Clinic co-investigator's biomarker technology, submitted patent, and know-how to develop a biomarker to predict response to obesity pharmacotherapy. Dr. Camilleri also serves as a consultant to Kallyope for the company's development program regarding obesity (the consulting fee is paid to his employer, Mayo Clinic). Dr. Camilleri has a patent application pending regarding obesity metabolomics to identify different phenotypes with the United States Patent and Trademark Office. The other authors have no conflicts of interest.
Acknowledgements
The authors thank Mrs. Cindy Stanislav for secretarial support.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2021.101213.
Appendix. Supplementary materials
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diab Endocrinol. 2020;8:616–627. doi: 10.1016/S2213-8587(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- 4.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of pharmacologic treatments for obesity with weight loss and adverse events: a systematic review and network meta-analysis. JAMA. 2016;315:2424–2434. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khera R, Pandey A, Chandar AK, Murad MH, Prokop LJ, Neeland IJ, et al. Effects of weight-loss medications on cardiometabolic risk profiles: a systematic review and network meta-analysis. Gastroenterology. 2018;154:1309–1319. doi: 10.1053/j.gastro.2017.12.024. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cifuentes L, Camilleri M, Acosta A. Gastric sensory and motor functions and energy intake in health and obesity - therapeutic implications. Nutrients. 2021 Apr 1;13:1158. doi: 10.3390/nu13041158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maselli DB, Camilleri M. Effects of GLP-1 and its analogs on gastric physiology in diabetes mellitus and obesity. Adv Exp Med Biol. 2021;1307:171–192. doi: 10.1007/5584_2020_496. [DOI] [PubMed] [Google Scholar]
- 8.Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124:4473–4488. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coveleskie K, Kilpatrick LA, Gupta A, Stains J, Connolly L, Labus JS, et al. The effect of the GLP-1 analogue Exenatide on functional connectivity within an NTS-based network in women with and without obesity. Obes Sci Pract. 2017;3:434–445. doi: 10.1002/osp4.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Bloemendaal L, Veltman DJ, Ten Kulve JS, Groot PFC, Ruhé HG, Barkhof F, et al. Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes Metab. 2015;17:878–886. doi: 10.1111/dom.12506. [DOI] [PubMed] [Google Scholar]
- 11.Farr OM, Sofopoulos M, Tsoukas MA, Dincer F, Thakkar B, Sahin-Efe A, et al. GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;59:954–965. doi: 10.1007/s00125-016-3874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ten Kulve JS, Veltman DJ, van Bloemendaal L, Groot PFC, Ruhé HG, Barkhof F, et al. Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. J Endocrinol. 2016;229:1–12. doi: 10.1530/JOE-15-0461. [DOI] [PubMed] [Google Scholar]
- 13.Farr OM, Upadhyay J, Rutagengwa C, DiPrisco B, Ranta Z, Adra A, et al. Longer-term liraglutide administration at the highest dose approved for obesity increases reward-related orbitofrontal cortex activation in response to food cues: Implications for plateauing weight loss in response to anti-obesity therapies. Diabetes Obes Metab. 2019;21:2459–2464. doi: 10.1111/dom.13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lean ME, Carraro R, Finer N, Hartvig H, Lindegaard ML, Rössner S, et al. Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond) 2014;38:689–697. doi: 10.1038/ijo.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382:2117–2128. doi: 10.1056/NEJMoa1916038. [DOI] [PubMed] [Google Scholar]
- 16.Capehorn MS, Catarig A-M, Furberg JK, Janez A, Price HC, Tadayon S, et al. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10) Diabetes Metab. 2020;46:100–109. doi: 10.1016/j.diabet.2019.101117. [DOI] [PubMed] [Google Scholar]
- 17.le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, et al. Three years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389:1399–1409. doi: 10.1016/S0140-6736(17)30069-7. [DOI] [PubMed] [Google Scholar]
- 18.Niswender K, Pi-Sunyer X, Buse J, Jensen KH, Toft AD, Russell-Jones D, et al. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab. 2013;15:42–54. doi: 10.1111/j.1463-1326.2012.01673.x. [DOI] [PubMed] [Google Scholar]
- 19.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. 2011. www.cochrane.org/training/cochrane-handbook
- 20.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 21.Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JPT, Jackson D, Barrett JL, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Research Synthesis Methods. 2012;3:98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White IR, Barrett JK, Jackson D, et al. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. 2012;3:111–125. doi: 10.1002/jrsm.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frias JP, Wynne AG, Matyjaszek-Matuszek B, Bartaskova D, Cox DA, Woodward B, et al. Efficacy and safety of an expanded dulaglutide dose range: A phase 2, placebo-controlled trial in patients with type 2 diabetes using metformin. Diabetes Obes Metab. 2019;21:2048–2057. doi: 10.1111/dom.13764. [DOI] [PubMed] [Google Scholar]
- 29.Dungan KM, Weitgasser R, Perez Manghi F, Pintilei E, Fahrbach JL, Jiang HH, et al. A 24-week study to evaluate the efficacy and safety of once-weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8) Diabetes Obes Metab. 2016;18:47–82. doi: 10.1111/dom.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenstock J, Buse JB, Azeem R, Prabhakar P, Kiems L, Huang H, et al. Efficacy and safety of ITCA 650, a novel drug-device GLP-1 receptor agonist, in type 2 diabetes uncontrolled with oral antidiabetes drugs: The FREEDOM-1 Trial. Diabetes Care. 2018;41:333–340. doi: 10.2337/dc17-1306. [DOI] [PubMed] [Google Scholar]
- 31.Derosa G, Cicero AFG, Franzetti IG, Querci F, Carbone A, Ciccarelli L, et al. Effects of exenatide and metformin in combination on some adipocytokine levels: a comparison with metformin monotherapy. Can J Physiol Pharmacol. 2013;91:724–732. doi: 10.1139/cjpp-2012-0300. [DOI] [PubMed] [Google Scholar]
- 32.Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AYM, et al. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154:103–112. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 33.Apovian CM, Bergenstal RM, Cuddihy RM, Qu Y, Lenox S, Lewis MS, et al. Effects of exenatide combined with lifestyle modification in patients with type 2 diabetes. Am J Med. 2010;123:468. doi: 10.1016/j.amjmed.2009.11.019. e9–17. [DOI] [PubMed] [Google Scholar]
- 34.Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, et al. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010 Jan 28;9:6. doi: 10.1186/1475-2840-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448–1460. doi: 10.1016/j.clinthera.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 36.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 37.Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 38.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 39.Liutkus J, Rosas Guzman J, Norwood P, Pop L, Northrup J, Cao D, et al. A placebo-controlled trial of exenatide twice-daily added to thiazolidinediones alone or in combination with metformin. Diabetes Obes Metab. 2010;12:1058–1065. doi: 10.1111/j.1463-1326.2010.01251.x. [DOI] [PubMed] [Google Scholar]
- 40.Scalzo RL, Moreau KL, Ozemek C, Herlache L, McMillin S, Gilligan S, et al. Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes. J Diabetes Complications. 2017;31:449–455. doi: 10.1016/j.jdiacomp.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joubert M, Opigez V, Pavlikova B, Peyro Saint Paul L, Jeandidier N, Briant AR, et al. Efficacy and safety of exenatide as add-on therapy for patients with type 2 diabetes with an intensive insulin regimen: A randomized double-blind trial. Diabetes Obes Metab. 2021;23:374–381. doi: 10.1111/dom.14225. [DOI] [PubMed] [Google Scholar]
- 42.Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 43.Harreiter J, Just I, Leutner M, Bastian M, Brath H, Schelkshorn C, et al. Combined exenatide and dapagliflozin has no additive effects on reduction of hepatocellular lipids despite better glycaemic control in patients with type 2 diabetes mellitus treated with metformin: EXENDA, a 24-week, prospective, randomized, placebo-controlled pilot trial. Diabetes Obes Metab. 2021;23:1129–1139. doi: 10.1111/dom.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pratley RE, Kang J, Trautmann ME, Hompesch M, Han OP, Stewart J, et al. Body weight management and safety with efpeglenatide in adults without diabetes: A phase II randomized study. Diabetes Obes Metab. 2019;21:2429–2439. doi: 10.1111/dom.13824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenstock J, Sorli CH, Trautmann ME, Morales C, Wendisch U, Dailey G, et al. Once-weekly efpeglenatide dose-range effects on glycemic control and body weight in patients with type 2 diabetes on metformin or drug naive, referenced to liraglutide. Diabetes Care. 2019;42:1733–1741. doi: 10.2337/dc18-2648. [DOI] [PubMed] [Google Scholar]
- 46.Del Prato S, Kang J, Trautmann ME, Stewart J, Sorli CH, Derwahl M, et al. Efficacy and safety of once-monthly efpeglenatide in patients with type 2 diabetes: Results of a phase 2 placebo-controlled, 16-week randomized dose-finding study. Diabetes Obes Metab. 2020;22:1176–1186. doi: 10.1111/dom.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mensberg P, Nyby S, Jorgensen PG, Storgaard H, Jensen MT, Sivertsen J, et al. Near-normalization of glycaemic control with glucagon-like peptide-1 receptor agonist treatment combined with exercise in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:172–180. doi: 10.1111/dom.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smits MM, Tonneijck L, Muskiet MHA, Kramer MHH, Pouwels PJW, Pieters-van den Bos IC, et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia. 2016;59:2588–2593. doi: 10.1007/s00125-016-4100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fremming Dejgaard T, Seerup Frandsen C, Stenbaek Hansen T, Almdal T, Urhammer S, Pedersen-Bjergaard U, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2016;4:221–232. doi: 10.1016/S2213-8587(15)00436-2. [DOI] [PubMed] [Google Scholar]
- 50.Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 51.Nandy D, Johnson C, Basu R, Joyner M, Brett J, Svendsen CB, et al. The effect of liraglutide on endothelial function in patients with type 2 diabetes. Diab Vasc Dis Res. 2014;11:419–430. doi: 10.1177/1479164114547358. [DOI] [PubMed] [Google Scholar]
- 52.Kim SH, Abbasi F, Lamendola C, Liu A, Ariel D, Schaaf P, et al. Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes Care. 2013;36:3276–3282. doi: 10.2337/dc13-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner AM, Miranda-Calderin G, Ugarte-Lopetegui MA, Marrero-Santiago H, Suárez-Castellano L, López-Madrazo MJ, et al. Effect of liraglutide on physical performance in type 2 diabetes: Results of a randomized, double-blind, controlled trial (LIPER2) Diabetes Metab. 2019;45:268–275. doi: 10.1016/j.diabet.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Vanderheiden A, Harrison L, Warshauer J, Li X, Adams-Huet B, Lingvay I. Effect of adding liraglutide vs placebo to a high-dose insulin regimen in patients with type 2 diabetes: a randomized clinical trial. JAMA Intern Med. 2016;176:939–947. doi: 10.1001/jamainternmed.2016.1540. [DOI] [PubMed] [Google Scholar]
- 58.Lind M, Hirsch IB, Tuomilehto J, Dahlqvist S, Ahrén B, Torffvit O, et al. Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: randomised clinical trial (MDI Liraglutide trial) BMJ. 2015;351:h5364. doi: 10.1136/bmj.h5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dejgaard TF, Schmidt S, Frandsen CS, Vistisen D, Madsbad S, Andersen HU, et al. Liraglutide reduces hyperglycaemia and body weight in overweight, dysregulated insulin-pump-treated patients with type 1 diabetes: The Lira Pump trial-a randomized, double-blinded, placebo-controlled trial. Diabetes Obes Metab. 2020;22:492–500. doi: 10.1111/dom.13911. [DOI] [PubMed] [Google Scholar]
- 60.Ghanim H, Batra M, Green K, Abuaysheh S, Heina J, Makdissi A, et al. Liraglutide treatment in overweight and obese patients with type 1 diabetes: A 26-week randomized controlled trial; mechanisms of weight loss. Diabetes Obes Metab. 2020;22:1742–1752. doi: 10.1111/dom.14090. [DOI] [PubMed] [Google Scholar]
- 61.Hygum K, Harsløf T, Rye Jørgensen N, Rungby J, Pedersen SB, Langdahl BL. Bone resorption is unchanged by liraglutide in type 2 diabetes patients: A randomised controlled trial. Bone. 2020;132 doi: 10.1016/j.bone.2019.115197. [DOI] [PubMed] [Google Scholar]
- 62.van Eyk HJ, Paiman EHM, Bizino MB, IJzermans SL, Kleiburg F, Boers TGW, et al. Liraglutide decreases energy expenditure and does not affect the fat fraction of supraclavicular brown adipose tissue in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2020;30:616–624. doi: 10.1016/j.numecd.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Nexøe-Larsen CC, Sørensen PH, Hausner H, Agersnap M, Baekdal M, Brønden A, et al. Effects of liraglutide on gallbladder emptying: A randomized, placebo-controlled trial in adults with overweight or obesity. Diabetes Obes Metab. 2018;20:2557–2564. doi: 10.1111/dom.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Halawi H, Khemani D, Eckert D, O'Neill J, Kadouh H, Grothe K, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2:890–899. doi: 10.1016/S2468-1253(17)30285-6. [DOI] [PubMed] [Google Scholar]
- 65.Wadden TA, Hollander P, Klein S, Niswender K, Woo V, Hale PM, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond) 2013;37:1443–1451. doi: 10.1038/ijo.2013.120. [DOI] [PubMed] [Google Scholar]
- 66.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. doi: 10.1056/NEJMoa1411892. [DOI] [PubMed] [Google Scholar]
- 67.Gudbergsen H, Overgaard A, Henriksen M, Ejlersen Waehrens E, Bliddal H, Christensen R, et al. Liraglutide after diet-induced weight loss for pain and weight control in knee osteoarthritis: a randomized controlled trial. Am J Clin Nutr. 2021;113:314–323. doi: 10.1093/ajcn/nqaa328. [DOI] [PubMed] [Google Scholar]
- 68.Pinget M, Goldenberg R, Niemoeller E, Muehlen-Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P) Diabetes Obes Metab. 2013;15:1000–1007. doi: 10.1111/dom.12121. [DOI] [PubMed] [Google Scholar]
- 69.Bolli GB, Munteanu M, Dotsenko S, Niemoeller E, Boka G, Wu Y, et al. Efficacy and safety of lixisenatide once daily vs. placebo in people with Type 2 diabetes insufficiently controlled on metformin (GetGoal-F1) Diabet Med. 2014;31:176–184. doi: 10.1111/dme.12328. [DOI] [PubMed] [Google Scholar]
- 70.Sorli C, Harashima S-I, Tsoukas GM, Unger J, Derving Karsbøl J, Hansen T, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:251–260. doi: 10.1016/S2213-8587(17)30013-X. [DOI] [PubMed] [Google Scholar]
- 71.Nauck MA, Petrie JR, Sesti G, Mannucci E, Courrèges J-P, Lindegaard ML, et al. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39:231–241. doi: 10.2337/dc15-0165. [DOI] [PubMed] [Google Scholar]
- 72.Zinman B, Bhosekar V, Busch R, Holst I, Ludvik B, Thielke D, et al. Semaglutide once weekly as add-on to SGLT-2 inhibitor therapy in type 2 diabetes (SUSTAIN 9): a randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019;7:356–367. doi: 10.1016/S2213-8587(19)30066-X. [DOI] [PubMed] [Google Scholar]
- 73.Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291–2301. doi: 10.1210/jc.2018-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 75.Friedrichsen M, Breitschaft A, Tadayon S, Wizert A, Skovgaard D. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754–762. doi: 10.1111/dom.14280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: The STEP 4 Randomized Clinical Trial. JAMA. 2021;325:1414–1425. doi: 10.1001/jama.2021.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 78.Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. 2019;42:1724–1732. doi: 10.2337/dc19-0749. [DOI] [PubMed] [Google Scholar]
- 79.Bergenstal RM, Forti A, Chiasson J-L, Woloschak M, Boldrin M, Balena R. Efficacy and safety of taspoglutide versus sitagliptin for type 2 diabetes mellitus (T-emerge 4 trial) Diabetes Ther. 2012 Dec;3(1):13. doi: 10.1007/s13300-012-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ, 3rd, et al. The effects of LY2189265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab. 2011;13:418–425. doi: 10.1111/j.1463-1326.2011.01366.x. [DOI] [PubMed] [Google Scholar]
- 81.Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabet Med. 2012;29:1260–1267. doi: 10.1111/j.1464-5491.2012.03745.x. [DOI] [PubMed] [Google Scholar]
- 82.Skrivanek Z, Gaydos BL, Chien JY, Geiger MJ, Heathman MA, Berry S, et al. Dose-finding results in an adaptive, seamless, randomized trial of once-weekly dulaglutide combined with metformin in type 2 diabetes patients (AWARD-5) Diabetes Obes Metab. 2014;16:748–756. doi: 10.1111/dom.12305. [DOI] [PubMed] [Google Scholar]
- 83.Ludvik B, Frias JP, Tinahones FJ, Wainstein J, Jiang H, Robertson KE, et al. Dulaglutide as add-on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD-10): a 24-week, randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6:370–381. doi: 10.1016/S2213-8587(18)30023-8. [DOI] [PubMed] [Google Scholar]
- 84.Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 85.Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Vang Skiøth T, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: The SCALE Diabetes Randomized Clinical Trial. JAMA. 2015;314:687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 86.Lingvay I, Desouza CV, Lalie KS, Rose L, Hansen T, Zacho J, et al. A 26-week randomized controlled trial of semaglutide once daily versus liraglutide and placebo in patients with type 2 diabetes suboptimally controlled on diet and exercise with or without metformin. Diabetes Care. 2018;41:1926–1937. doi: 10.2337/dc17-2381. [DOI] [PubMed] [Google Scholar]
- 87.Davies M, Pieber TR, Hartoft-Nielsen M-L, Hansen OKH, Jabbour S, Rosenstock J. Effect of oral semaglutide compared with placebo and subcutaneous semaglutide on glycemic control in patients with type 2 diabetes: a randomized clinical trial. JAMA. 2017;318:1460–1470. doi: 10.1001/jama.2017.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O'Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392:637–649. doi: 10.1016/S0140-6736(18)31773-2. [DOI] [PubMed] [Google Scholar]
- 89.Davies M, Faerch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2•4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 90.Pratley R, Amod A, Tetens Hoff S, Kadowaki T, Lingvay I, Nauck M, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394:39–50. doi: 10.1016/S0140-6736(19)31271-1. [DOI] [PubMed] [Google Scholar]
- 91.Brignardello-Petersen R, Murad MH, Walter SD, McLeod S, Carrasco-Labra A, Rochwerg B, et al. GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol. 2019;105:60–67. doi: 10.1016/j.jclinepi.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 92.U.S. Preventive Services. Final Recommendation Statement: Weight Loss to Prevent Obesity-Related Morbidity and Mortality in Adults: Behavioral Interventions. September 18, 2018. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/obesity-in-adults-interventionsLast accessed 8/23/2021
- 93.LeBlanc EL, Patnode CD, Webber EM, Redmond N, Rushkin M, O'Connor EA. Agency for Healthcare Research and Quality (US); Rockville, MD: 2018 Sep. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: an updated systematic review for the US Preventive Services Task Force [Internet] Report No. 18-05239-EF-1. [PubMed] [Google Scholar]
- 94.Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503–515. doi: 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.