Abstract
Polycyclic aromatic hydrocarbons like benzo[a]pyrene (BaP) are generated during incomplete combustion of organic materials. Prior research has demonstrated that BaP is a prenatal ovarian toxicant and carcinogen. However, the metabolic pathways active in the embryo and its developing gonads and the mechanisms by which prenatal exposure to BaP predisposes to ovarian tumors later in life remain to be fully elucidated. To address these data gaps, we orally dosed pregnant female mice with BaP from embryonic day (E) 6.5 to E11.5 (0, 0.2, or 2 mg/kg/day) for metabolite measurement or E9.5 to E11.5 (0 or 3.33 mg/kg/day) for embryonic gonad RNA sequencing. Embryos were harvested at E13.5 for both experiments. The sum of BaP metabolite concentrations increased significantly with dose in the embryos and placentas, and concentrations were significantly higher in female than male embryos and in embryos than placentas. RNA sequencing revealed that enzymes involved in metabolic activation of BaP are expressed at moderate to high levels in embryonic gonads and that greater transcriptomic changes occurred in the ovaries in response to BaP than in the testes. We identified 490 differentially expressed genes (DEGs) with false discovery rate P-values < 0.05 when comparing BaP-exposed to control ovaries but no statistically significant DEGs between BaP-exposed and control testes. Genes related to monocyte/macrophage recruitment and activity, prolactin family genes, and several keratin genes were among the most upregulated genes in the BaP-exposed ovaries. Results show that developing ovaries are more sensitive than testes to prenatal BaP exposure, which may be related to higher concentrations of BaP metabolites in female embryos.
Keywords: polycyclic aromatic hydrocarbon, benzo[a]pyrene, ovary, testis, developmental origins of health and disease (DOHAD)
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental contaminants to which essentially all people are exposed (1). PAHs are formed during incomplete combustion of organic materials, and common sources of exposure are ingestion of grilled or smoked foods, inhalation of tobacco, wood, and wildfire smoke and particulate matter air pollution. Benzo[a]pyrene (BaP) is a common PAH, which we and others have shown to be a transplacental ovarian toxicant and tumorigen that induces apoptosis of germ cells in the prenatal mouse ovary (2-4). The developing testis is less sensitive than the developing ovary in that higher in vivo doses during prenatal development are required to elicit decreased testicular and epidydimal sperm counts postnatally compared to follicle depletion in females (4,5). Consistent with these in vivo findings, concentrations that result in germ cell apoptosis in cultured embryonic ovaries do not induce apoptosis in cultured embryonic testes (3). We previously estimated that the average daily intake of BaP from all exposure routes in a highly environmentally exposed, occupationally unexposed woman is 0.08 μg/kg/day (6), while occupational exposures alone may result in more than 15-fold higher daily exposures (7,8).
BaP and other PAHs generally require metabolism to reactive metabolites to exert toxicity. There are several main metabolic pathways. Several begin with oxidation by cytochrome P450 enzymes (CYPs), primarily CYP1A1 and CYP1B1, to epoxides, followed by hydrolysis of the epoxide by epoxide hydrolases (EPHX1 and EPHX2) (9). The resultant dihydrodiols can undergo further oxidation by CYPs to diol epoxides, such as BaP-7,8-diol epoxide, which are highly reactive and form DNA adducts that can result in mutations if not repaired (10). Alternatively, the dihydrodiols can be metabolized by aldoketoreductases (AKR) to ketols, which can tautomerize to catechols, which then undergo 1- and 2-electron oxidation to o-semiquinones and o-quinones, producing reactive oxygen species in the process (9,11,12). Yet another pathway involves BaP acting as a reducing factor for lipid peroxides formed by the action of prostaglandin endoperoxide synthase (PTGS1 and 2, also known as COX1 and COX2), forming BaP radical cation, another DNA reactive and mutagenic metabolite (13). Cyp1a1 is constitutively expressed in the liver, while Cyp1b1 is expressed in target tissues. Cyp1a1 and Cyp1b1 expression are induced by arylhydrocarbon receptor (AHR) activation in a tissue-specific manner (14). Several PAH metabolites, including ortho quinones and dihydrodiols, are AHR activators (12,15), which results in upregulation of their metabolism. AHR activation has been shown to play a role in destruction of germ cells in the embryonic ovary by the PAH 7,12-dimethylbenz[a]anthracene (16). Diol epoxides, o-quinones, reactive oxygen species, and PAH radical cations can react with DNA to form adducts, which if not repaired can result in mutations. Detection of BaP-7,8-diol epoxide DNA adducts in human fetal tissue demonstrates that maternal exposure results in exposure of the developing embryo to reactive metabolites of BaP (10,17). Consistent with exposure of the developing embryo to reactive metabolites of BaP, in utero exposure to BaP has been shown to cause dose-dependent increases in mutations in the ovaries, sperm, and bone marrow of the offspring (18,19).
While the tissue distribution of expression of PAH metabolizing enzyme genes has been well-characterized in adults, relatively little is known about their expression in the developing gonads. The adult mouse ovary is known to express Cyp1b1, Ephx1 and Ephx2, Ptgs1 and Ptgs2, and various AKRs, while less is known about the expression of these genes in the prenatal ovaries (14,20-22). Similarly, the adult mouse testis expresses these genes, but little is known about metabolism in the developing testis (14,23). Prior studies suggest that basal and inducible CYP expression is low prior to birth and increases around PND5 in mice, but these studies analyzed messenger RNA (mRNA) and protein expression at the whole embryo level or in the embryonic liver (24,25). Based on these prior findings, it was postulated that metabolism via PTGS is the main metabolic pathway for BaP during prenatal development (26,27).
We hypothesized that BaP is metabolized to reactive metabolites by the developing mouse gonads and that these metabolites alter the transcriptomes of the embryonic ovaries and testes. We measured BaP metabolites in placentas and whole embryos and performed transcriptomic analyses of embryonic ovaries and testes.
Materials and Methods
Chemicals and Other Supplies
Methanol, ethanol, chloroform, and isopropanol used in this study were of chromatographic grade; BaP, sesame oil, sodium dodecyl sulfate, tris, EDTA, and high-performance liquid chromatography (HPLC) water were of analytical/reagent grade and purchased from Sigma-Aldrich Chemical Co (St. Louis, MO, USA). The Corning® syringe filters were also purchased from Sigma-Aldrich. The HPLC mobile phase filtration assembly and accessories were purchased from Phenomenex (Torrance, CA, USA). Autosampler vials (amber color) and vial inserts were bought from the DWK Lifesciences, LLC (Millville, NJ, USA). BaP metabolite standards were obtained from the National Cancer Institute Chemical Carcinogen Repository within the Midwest Research Institute (Kansas City, MO, USA). Other reagents and chemicals were purchase from Fisher Scientific or Sigma-Aldrich unless otherwise noted.
Animals
All animal procedures were reviewed and approved by the University of California Irvine Institutional Animal Care and Use Committee and followed the recommendations of the Guide for the Care and Use of Laboratory Animals (28). Female and male C57BL/6J mice were purchased from Jackson Labs at 8 weeks of age and were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International–accredited vivarium on 14-h/10-h light/dark cycles with temperature maintained at 70°F to 72°F and humidity maintained at 40% to 60%. The mice were allowed to acclimate to the vivarium for at least 1 week prior to experimentation. Females underwent vaginal cytology to monitor estrous cycles (29). On the evening of proestrus determined by vaginal cytology, females were mated with a proven breeder male. The next morning females were checked for vaginal plugs. The day a vaginal plug was found was designated embryonic day (E) 0.5.
Experimental Protocols
For BaP metabolite measurements and immunostaining, pregnant female mice were orally dosed via pipet inserted into the animal’s mouth with 0, 0.2, or 2 mg/kg/day BaP dissolved in sesame oil vehicle from E6.5 to E11.5 (total doses of 1.2 or 12 mg/kg). The animals avidly consume the oil, and this method is less stressful than oral gavage. The doses were chosen because we previously published that 2 mg/kg/day from E6.5 to E15.5 resulted in greater than 60% depletion of the primordial follicle pool in 5- to 6-week-old offspring (4,6). With the shorter dosing window of E6.5 to E11.5 used in the present study, we observed a 50% depletion of primordial follicles using the 2 mg/kg/day dose, while the 0.2 mg/kg/day dose does not significantly deplete germ cell numbers (unpublished). On E13.5, pregnant females were euthanized by CO2 asphyxiation, and embryos were removed and dissected with the aid of a dissecting microscope. Embryo sex was identified by the appearance of the gonads, with E13.5 testes having clearly visible testicular cords while ovaries present a more homogeneous appearance (3,30). Ovaries and testes with attached mesonephros were harvested for immunostaining and immediately fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in phosphate buffered saline at 4°C for 2 to 3 h and then cryoprotected in 30% sucrose for 2 h and embedded in optimal cutting temperature compound (Sakura Finetek) and stored at −80°C. Remaining embryos (minus gonads) and placentas were snap frozen for BaP metabolite extraction and measurement and stored at −80°C until use. Since BaP and its metabolite standards are potential carcinogens and mutagens, they were handled in accordance with National Institutes of Health guidelines (31).
For E13.5 gonad RNA sequencing (RNA-seq), pregnant female mice were orally dosed as previously mentioned with 0 or 3.33 mg/kg/day from E9.5 to E11.5 (total dose of 10 mg/kg). On E13.5, pregnant females were euthanized by CO2 asphyxiation for isolation of the embryonic gonads with attached mesonephros as previously described (3). The mesonephros was then trimmed away from the gonads. Paired gonads from 1 or 2 male embryos and 1 or 2 female embryos per each of 3 or 4 litters were snap frozen and stored at −80°C for RNA-seq.
A daily dose of 3.33 mg/kg is about 3.5 orders of magnitude higher than the mouse-adjusted estimated high daily intake of BaP in a nonoccupationally exposed woman using allometric interspecies dose adjustment (6,32). The 3.33 mg/kg/day dose is similar to the lowest dose of 5 mg/kg/day and 2 orders of magnitude lower than the highest oral doses of BaP used in previously published papers that examined transcriptome-wide effects of BaP in livers, lungs, and forestomachs of adult mice (33,34).
Measurement of BaP Metabolites
For metabolite measurements, 3 embryos of the same sex or 3 placentas from embryos of the same sex were pooled per replicate. Whenever possible, all 3 embryos or placentas in a pool were from the same litter. While it would have been interesting to examine the effect of intrauterine position on BaP metabolites, the limited technical sensitivity for quantitative detection of the BaP metabolites required pooling of embryos and placentas.
Sample Preparation for BaP Metabolite Quantification
The frozen tissues were thawed, and each tissue sample was cut into small pieces using a Technocut scalpel. Then, the samples of cut tissues were minced with Supercut Iris Scissors and thoroughly mixed to obtain a homogeneous mixture of minced individual tissue. From this mixture, 450 to 750 mg of tissue was weighed and transferred using Dumont tweezers into a sample holding tube. The extraction and analyses of samples for BaP metabolites followed the method of Ramesh et al (35) with modifications as detailed in the following discussion.
Liquid-Liquid Extraction
The previously mentioned tissue preparation was suspended in 2 volumes of Tris sucrose-EDTA buffer (Tris 0.10 M; sucrose 0.25 M; EDTA 0.10 M; pH 7.4) and processed using an Ultra-Turrax® benchtop Tube Drive system (IKA-Werke, Staufen, Germany) for 2 min. This system facilitates grinding, stirring, and homogenizing the sample. Ten microliters of sodium dodecyl sulfate (0.1%, w/v) were added to the homogenate and vortexed for 30 sec 3 times. Subsequently, the homogenate was subjected to a 2-step extraction procedure designed to separate organic from aqueous metabolites. The first extraction employed methanol + chloroform + water (30:15:10, v/v/v). The sample-solvent mixture was transferred to a 10 mL screwcap vial and was shaken vigorously at 5-min intervals for 15 min. After separation, the top aqueous layers were pipetted into a Corning tube. The bottom organic layer was transferred into another Corning tube. The lipid interface was re-extracted with the previously mentioned solvents and centrifuged at 2000 × g for 20 min to not miss the analytes of interest that may be present in the emulsion. For each sample, the organic phases from all extractions were pooled. The extracts were passed through disposable glass columns that were packed with activated Florisil on the top and a layer of anhydrous sodium sulfate below (to remove lipid and moisture) and a glass wool placed underneath the sodium sulfate (to prevent the adsorbent coming off the column along with the sample or eluent.
The sample extracts were blown off under a gentle stream of N2 using a Meyer Analytical Evaporator (Organomation Associates Inc., Berlin, MA, USA). The dried organic phase was resuspended in 500 μL of methanol. Particulates from the sample suspension were removed by filtration through Corning® syringe filters (0.45 µm; 25 mm diameter). The final extracts were transferred to amber-colored screw top vials to prevent photodegradation of BaP metabolites and stored at 4°C until analyzed by HPLC.
BaP Metabolite Analysis by HPLC
The metabolites were analyzed by HPLC (Model 1100; Agilent, Wilmington, DE, USA) equipped with a variable wavelength and fluorescence detectors. The instrument was operated using ChemStation software (Agilent) for instrument control and data acquisition. Using an automatic sampler, 50 µL of sample were injected onto a C18 reverse phase column (ODS Hypersil, 5 mm, 250 × 4.6 mm; Thermo Fisher Scientific, Waltham, MA, USA). The column (temperature 25°C) was eluted for 45 min at a flow rate of 1.0 mL/min with a ternary elution: isocratic, water-methanol-ethanol (40:40:20, v/v/v) from 0 to 20 min; again water-methanol-ethanol (30:46:24, v/v/v) from 20 to 30 min; 100% methanol from 30 to 40 min; and returning to the initial conditions from 40 to 45 min. The excitation and emission wavelengths for the fluorescence detector were 244 and 410 nm, respectively. The wavelength for ultraviolet detection was set at 254 nm. After 10 sample runs, the column was flushed with 100% isopropanol to remove any impurities or interfering substances that may have retained on the column. The resolved BaP metabolites were identified by comparison of retention times and peak areas of the samples with that of authentic standards, injected prior to sample analyses.
RNA Sequencing
Gene expression profiles were determined by RNA-seq as described (36,37). Dissected and frozen testicular and ovarian tissue specimens were subjected to complimentary DNA synthesis using the direct cell lysis protocol of Takara SMART-Seq v4 Ultra Low Input RNA Kit for Sequencing (Takara Bio USA). Of particular note, this protocol, which involves oligo(dT) priming of reverse transcription, permits mRNA-enriched RNA-seq library synthesis without poly(A)+ mRNA purification or ribosomal RNA depletion. The resulting complimentary DNA was fragmented by sonication using Covaris S2 sonicator to 200 to 300 bp and subjected to synthesis of single-indexed Illumina deep sequencing libraries using Takara Low Input Library Prep Kit. Libraries were subjected to size distribution measurement using High Sensitivity D1000 DNA Screen Tape (Agilent) and quantitated using a quantitative polymerase chain reaction kit (Kapa Biosystems). Successfully synthesized libraries with size peaking at ~300 bp were subjected to deep sequencing using the Illumina NextSeq500 deep sequencer with High Output Kit v2 (Illumina) for 75 nt + 75 nt paired-end multiplexed sequencing. The RNA-seq data discussed in this publication have been deposited in National Center for Biotechnology Information’s Gene Expression Omnibus (38).
Bioinformatics
The fastq-format raw sequence reads were generated from the bcl NextSeq output files in a local Linux server using the bcl2fastq software (Illumina) and subjected to quality control analysis using the fastQC tool (Babraham Institute, Cambridge, UK). Adaptor sequences and low-quality reads (<30) were trimmed from fastq reads using the Trim Galore! Tool (Babraham Institute) with its default configurations. Quality-control–filtered, paired-end reads were then aligned to the GRCm38/mm10 mouse genome reference sequence using the STAR aligner (39). Duplicated reads were removed from the resulting bam format alignment files using the sambamba tool package (40), yielding 37 to 60 million per library uniquely mapped reads. Aligned reads assigned to exons of the mm10 gene model were counted using the Bioconductor package “Rsubread,” (41) and the counts were normalized using the negative binominal, trimmed mean of M-values method implemented by the Bioconductor package edgeR (42). The general linear model likelihood ratio test was performed using edgeR to identify differentially expressed genes (DEGs) where the mean expression value of a gene in any group is significantly different from other sample groups. The obtained P-values were corrected by the Benjamini-Hochberg method to obtain false discovery rate (FDR).
DAVID and Gene Ontology Analysis
Functional annotation clustering in lists of DEGs (BaP-exposed vs control ovaries and BaP-exposed vs control testes) was examined using the DAVID server with default annotation categories (GOTERM_CC_Direct, GOTERM_MF_Direct, GOTERM_BP_Direct, KEGG Pathway, INTERPRO, UP_Keywords, UP_Seq_Feature, and SMART) with initial and final group membership of at least 3 and similarity threshold of 0.50 (43). The same gene lists were entered into the GeneOntology Panther tool (44,45), again using a cutoff of at least 3 genes per group. Common Gene Ontology (GO) terms between the DAVID functional annotation clusters and the GeneOntology GO analyses were found, and P-values and numbers of genes per term were compared. DAVID and Panther analyses were performed on subsets of the DEGs that differed by more than 1.5-fold and had unadjusted P-values < 0.05.
Immunofluorescence and Quantification
Optimal cutting temperature compound–embedded fixed ovaries were cryosectioned at 7-μm thickness. Sections were pretreated in 10-mM citric acid for 15 min at 95°C, blocked in phosphate-buffered saline–Triton X-100 solution with 5% normal goat serum and 1% bovine serum albumin for 1 h and incubated with primary antibodies, rabbit anti-KRT18 (1:200; Abcam cat no. ab133263, RRID:AB_11155892; http://antibodyregistry.org/AB_11155892), rabbit anti-KRT19 (1:200; Abcam cat no. ab52625, RRID:AB_2281020; http://antibodyregistry.org/AB_2281020), rabbit anti-CCL2 (1:100; ThermoFisher cat no. PA5-115555, RRID:AB_2893318; http://antibodyregistry.org/AB_2893318), rabbit anti-SPP1 (also called osteopontin, 1:300; Millipore cat no. AB10910, RRID:AB_1587339; http://antibodyregistry.org/AB_1587339), and rat anti-TRA98 (commonly referred to as GCNA; 1:200; Abcam cat no. ab82527, RRID:AB_1659152; http://antibodyregistry.org/AB_1659152), at 4°C overnight. After washing, the primary antibodies were detected with corresponding secondary antibodies, goat anti-rabbit Alexa Fluor 633 (ThermoFisher, A21070, RRID:AB_2535731; http://antibodyregistry.org/AB_2535731) and goat anti-rat Alexa Fluor 488 (ThermoFisher, A11006, RRID:AB_2534074; http://antibodyregistry.org/AB_2534074), for 1 h at room temperature. Slides were mounted in Prolong glass antifade mountant with Hoechst 33342 (NucBlue, ThermoFisher, P36981). Images of co-immunofluorescence with TRA98 (germ cells) plus KRT18, KRT19, CCL2, or SPP1 were generated using a Leica TCS SP8 confocal microscope and processed using Bitplane Imaris imaging software (v.9.7.0). Image gain settings (laser power and gain) and image processing settings (background subtraction) from each channel were adjusted for optimal signal and identically applied to all the images acquired from the same experiment. Image J was used to quantify mean immunofluorescence intensity of KRT18-, KRT19-, CCL2-, or SPP1-stained cells in the area of an ovarian section. Specifically, images were converted to 16-bit grayscale by selecting “Conversions” from the Edit menu, and the whole ovary was selected by the “Polygon” tool from the Image menu. Then, the mean intensity of fluorescence signal was measured by selecting “Measurement” from the Analyze menu.
Statistical Analyses
Data are presented as means ± SE of the mean unless otherwise noted. General linear models with BaP dose as covariate and sex as fixed factor were used to analyze total BaP metabolite concentrations in placentas and embryos. Intergroup comparisons between dose groups were done using Kruskal-Wallis test, followed by Mann-Whitney tests. For immunofluorescence quantification, t-tests and Mann-Whitney tests were used to compare the means of 2 groups, adjusted for unequal variances if necessary. Statistical significance was set at P < 0.05. Statistical analyses were carried out using SPSS 25 for Mac.
Results
BaP Metabolites in Whole Embryos and Placentas
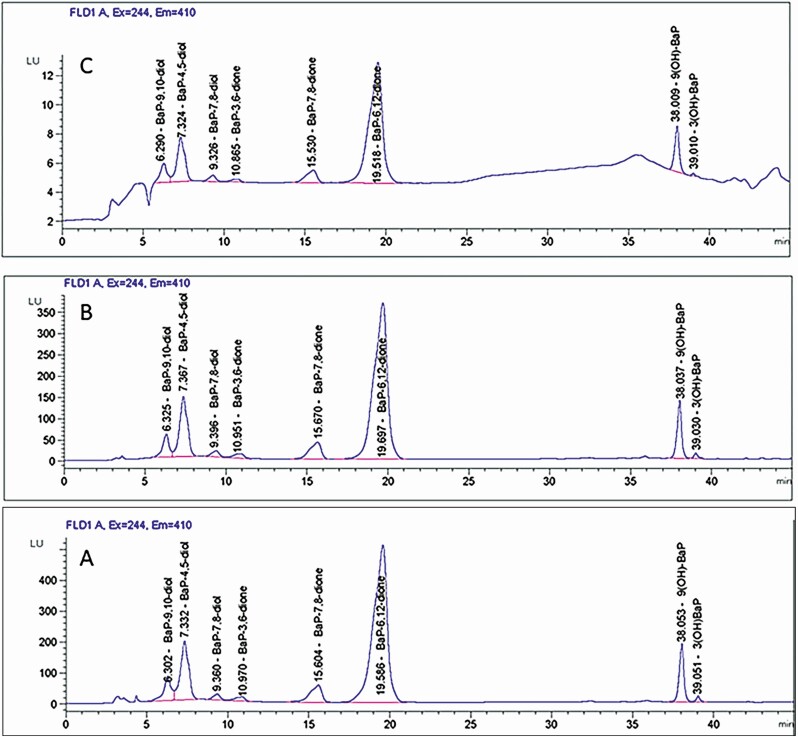
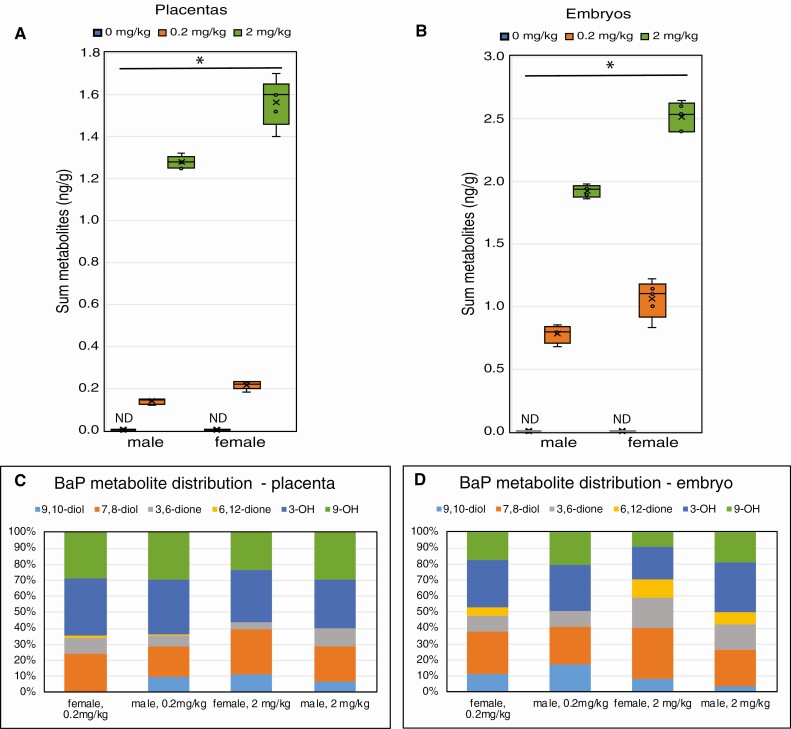
Representative chromatograms are shown in Figure 1 for standards (Fig. 1A) and for embryos (Fig. 1B) and placentas (Fig. 1C) from 0.2 mg/kg/day-dosed mothers. The sum of BaP metabolite concentrations increased with dose in the embryos and placentas (P < 0.001 effect of dose) (Fig. 2A and 2B), and concentrations were higher in female than male embryos (P < 0.001, effect of sex) (Fig. 2A and 2B). Total BaP metabolite concentrations were highly correlated between embryos and placentas (Pearson correlation = 0.947, P < 0.0001). BaP metabolites were undetectable in control embryos and placentas. The most abundant BaP metabolites detected in placentas from treated male and female embryos were 3(OH)BaP and 9(OH)BaP, while in both female and male embryos, BaP-7,8-diol and 3(OH)BaP were the most abundant metabolites (Fig. 2C and 2D). Embryos had greater proportions of BaP-6,12 quinone and BaP-3,6-quinone than placentas. Female embryos had higher proportions of these quinones than male embryos, and the proportions increased with increased dose of BaP.
Figure 1.
Representative high-performance liquid chromatography with fluorescence detection chromatograms of benzo[a]pyrene (BaP) metabolites extracted by the liquid-liquid extraction method using methanol + chloroform + water (15:30:10, v/v/v). (A) BaP metabolite standards (1000 ppb concentration). (B) Metabolites detected in embryos. (C) Metabolites detected in the bodies of mouse placentas exposed in utero to 0.2 mg BaP/kg/bw/day. Identification of metabolites was accomplished by comparison of retention times and peak areas of the samples (computed using the Agilent ChemStation software) with that of the BaP metabolite standards obtained from the National Cancer Institute Chemical Carcinogen Repository within the Midwest Research Institute (Kansas City, MO, USA).
Figure 2.
Benzo[a]pyrene (BaP) metabolite concentrations in placentas and embryos 48 h after the last of 6 daily doses to the mother. Pregnant female mice were orally administered 0.2 or 2 mg/kg BaP dissolved in sesame oil or sesame oil alone daily from E6.5 to E11.5. Mice were euthanized and embryos and placentas were harvested on E13.5 for quantification of BaP metabolites by high-performance liquid chromatography with fluorescence detection. Box-and-whisker plots show the mean (x), median (line between 2 boxes), and individual data points in the middle quartiles (°) of the sum of BaP metabolites in placentas (A) and embryos (B). The effects of BaP dose and sex on BaP metabolite concentrations were statistically significant for both placentas and embryos (*P < 0.001). Relative abundance of BaP metabolites in placentas (C) and embryos (D) averaged for all samples per group and sex. n = 4-5 pools of 3 embryos of the same sex each per dose group.
Effects of Maternal BaP Exposure on Embryonic Gonadal Gene Expression
RNA-seq was performed on gonads of embryos exposed to 0 (females: n = 6; males: n = 6) or 3.33 (females: n = 6; males: n = 5) mg/kg/day BaP from E9.5 to E11.5 via oral dosing to the pregnant mother and harvested on E13.5, 48 h after the last dose. As a part of routine quality control analyses, unsupervised hierarchical clustering of normalized RNA-seq data was performed, and it was noted that 6 samples had high expression of hemoglobin-related genes (1 control female, 3 BaP females, 2 control males). Out of concern that these samples were contaminated with blood during dissection, we analyzed the RNA-seq data, including all samples (hereafter referred to as “full data set”) and again with those 6 samples removed (hereafter referred to as “blood-free data set”). A total of 15 754 expressed genes were identified in the full data set and 15 289 expressed genes were identified in the blood-free data set.
Using the full data set, there were more than 1000 DEGs with unadjusted P-values < 0.05 and 490 of the top 1000 DEGs had FDR P-values < 0.05 when comparing their expression in BaP-exposed to control ovaries [Supplemental Data 1 (46)]. All of the top 1000 DEGs differed by ≥1.5-fold in BaP-exposed compared to control ovaries. There were more than 1000 genes that differed by ≥1.5-fold with unadjusted P-values < 0.05, but no genes with FDR P-values < 0.05 when comparing BaP-exposed to control testes [Supplemental Data 2 (46)].
Using the blood-free data set, there were more than 1000 genes with unadjusted P-values < 0.05, and 32 genes with FDR P-values < 0.05 when comparing their expression in BaP-exposed to control ovaries. Of the top 1000 DEGs, 783 differed by ≥1.5-fold in BaP-exposed compared to control ovaries. There were 687 DEGs that differed by ≥1.5-fold when comparing expression in the BaP-exposed to control testes, but only 440 of these had unadjusted P-values < 0.05, and only 4 (Ngp, Rhox10, Slc15a2, 2210417A02Rik) had FDR P-values < 0.05. The results with the full and the blood-free data sets demonstrate that BaP exposure from E6.5 to E11.5 affects gene expression in the developing ovary to a much greater extent than in the developing testis. Because no genes were significantly differentially expressed in the full testis data set, we focused our subsequent analyses on the ovarian data set.
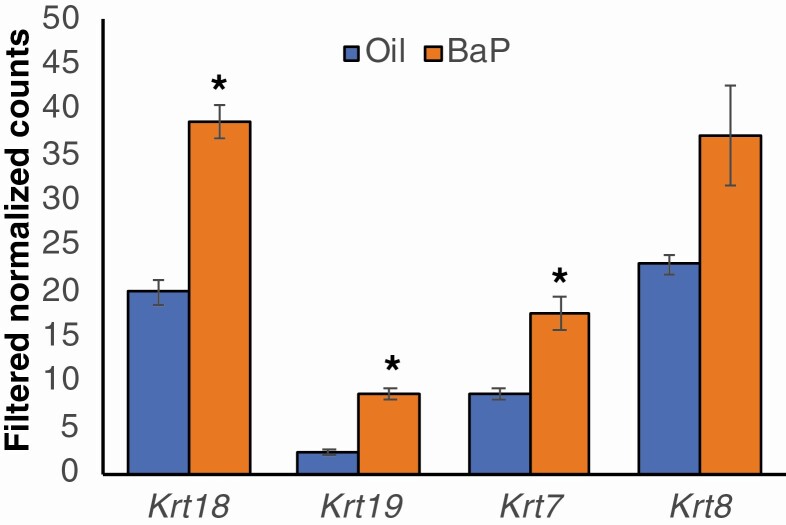
The 32 most statistically significant DEGs between BaP and sesame oil-exposed ovaries for the full and the blood-free data sets are shown in Tables 1 and 2, respectively. Notable among the top 32 DEGs in both data sets are 4 prolactin (PRL) family genes in both (Prl2a1, Prl2c2, Prl2c5, and Prl8a9 in the blood-free data set and Prl2c2, Prl2c4, Prl2c3, and Prl7a1 in the full data set). Chemokine (C-C motif) ligand 2 (Ccl2), Ccl7, and secreted phosphoprotein 1 (Spp1) are also among the 32 most significantly upregulated genes in both data sets. Keratin (Krt) 19 is the most statistically significant DEG, and it and Krt7 are both among the 32 most significantly upregulated genes in the blood-free data set, while Krt17 is 1 of the 32 most significantly upregulated genes in the full data set. In total, 16 Krt genes were significantly expressed in both data sets. Expression of Krt17, Krt18, Krt19, Krt7, and Krt8 was increased in BaP-exposed compared to control ovaries in both data sets; the increase was statistically significant for Krt18, Krt19, and Krt7 by t-test in the blood-free data set (Fig. 3).
Table 1.
Top 32 differentially expressed genes (benzo[a]pyrene vs oil ovaries), full data set
| Gene symbol | logFC | FDR P-value |
|---|---|---|
| Pitx2 | 4.31 | 0.007 |
| Prl2c2 | 5.58 | 0.007 |
| Cxcr7 | 2.55 | 0.007 |
| Gjb2 | 4.82 | 0.007 |
| Prl2c4 | 5.19 | 0.007 |
| Prl2c3 | 5.17 | 0.007 |
| Hsd3b6 | 5.21 | 0.007 |
| Slc25a37 | 2.74 | 0.007 |
| Prl7a1 | 6.44 | 0.007 |
| Spp1 | 3.67 | 0.007 |
| Myoz2 | 5.13 | 0.007 |
| Tbx18 | 3.77 | 0.007 |
| S100g | 5.28 | 0.007 |
| Rhd | 4.19 | 0.007 |
| Gm648 | 6.22 | 0.007 |
| Fam46c | 3.00 | 0.007 |
| Ttn | 3.34 | 0.007 |
| Ccl2 | 4.61 | 0.007 |
| Foxa3 | 3.68 | 0.007 |
| Alas2 | 4.88 | 0.007 |
| Hsd3b2 | 4.14 | 0.007 |
| Kng1 | 6.23 | 0.008 |
| Arhgef6 | 2.52 | 0.008 |
| Hbb-b1 | 4.64 | 0.008 |
| Hbb-b2 | 4.64 | 0.008 |
| Hba-a1 | 4.46 | 0.008 |
| Hba-a2 | 4.46 | 0.008 |
| Grap2 | 4.76 | 0.008 |
| Krt17 | 3.68 | 0.008 |
| Lgi4 | 4.32 | 0.008 |
| Ccl7 | 4.93 | 0.008 |
| Beta-s | 4.34 | 0.007 |
Abbreviation: FDR, false discovery rate.
Table 2.
Top 32 differentially expressed genes (benzo[a]pyrene vs oil ovaries), blood-free data set
| Gene symbol | logFC | FDR P-value |
|---|---|---|
| Krt19 | 1.88 | <0.0001 |
| Lgals3 | 5.10 | 0.004 |
| Ctxn1 | 1.00 | 0.006 |
| Ccl2 | 5.37 | 0.006 |
| Ccl7 | 5.79 | 0.006 |
| Cyp1b1 | -0.91 | 0.007 |
| Ier3 | 1.74 | 0.014 |
| Clec1b | -1.57 | 0.014 |
| Lgi4 | 3.21 | 0.016 |
| Prl2a1 | 6.02 | 0.017 |
| Clec4d | 3.42 | 0.018 |
| 1700019A02Rik | -1.30 | 0.018 |
| Efcab10 | -1.52 | 0.018 |
| Prl2c5 | 5.88 | 0.018 |
| Tsc22d3 | 0.86 | 0.020 |
| 4930524B15Rik | 1.61 | 0.020 |
| 4930513N10Rik | -1.16 | 0.020 |
| Spp1 | 2.79 | 0.023 |
| Fxyd3 | 3.02 | 0.026 |
| Dmrtc2 | 2.18 | 0.028 |
| Ube2s | 1.11 | 0.028 |
| H2-Aa | 2.99 | 0.028 |
| Spdya | 1.00 | 0.028 |
| Mmp10 | 3.51 | 0.028 |
| Gm1653 | -1.66 | 0.031 |
| Ppbp | -2.73 | 0.038 |
| Krt7 | 1.04 | 0.038 |
| Prl2c2 | 3.38 | 0.043 |
| Gm4312 | -4.89 | 0.043 |
| Gm4301 | -4.89 | 0.043 |
| Prl8a9 | 6.51 | 0.043 |
| F3 | 1.71 | 0.046 |
Abbreviation: FDR, false discovery rate.
Figure 3.
Increased keratin gene expression in benzo[a]pyrene (BaP)-exposed ovaries. Pregnant female mice were orally administered 3.33 mg/kg BaP dissolved in sesame oil or sesame oil alone daily from E9.5 to E11.5. Mice were euthanized and embryonic ovaries were harvested for RNA sequencing. The graph shows means ± SE of the mean of filtered normalized counts for the keratin genes, Krt18, Krt19, Krt7, and Krt8 in oil and BaP-exposed E13.5 ovaries. *P < 0.05, t-test.
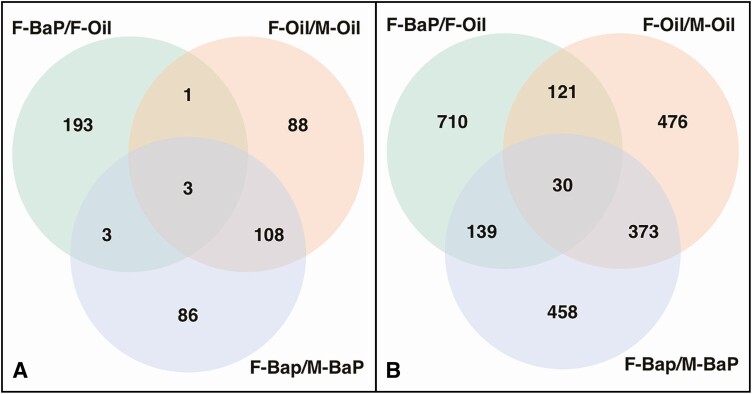
As expected, there were more than 1000 genes that differed significantly by unadjusted and FDR P-values between control E13.5 ovaries and testes in both data sets [Supplemental Data 3 (46)]. Figure 4A shows that more than half of the top 200 DEGs between BaP-exposed ovaries compared to BaP-exposed testes were shared in common with the top 200 DEGs between control ovaries and control testes, while less than 10% of the top 200 DEGs between BaP-exposed ovaries and control ovaries were shared in common with the top 200 DEGs between BaP-exposed ovaries and BaP-exposed testes. Similar results are shown in Figure 4B for the top 1000 DEGs for each of the 3 comparisons.
Figure 4.
Pregnant mice were treated and embryonic ovaries were processed for RNA sequencing as in Figure 3. Venn diagrams of top 1000 differentially expressed genes (DEGs) for the indicated comparisons in the full (A) and truncated (B) data sets. Abbreviations: F, female. M: male; Oil, vehicle control.
DAVID Functional Annotation Clusters
DAVID was used for functional annotation of the DEG lists. Only DEGs that differed by at least 1.5-fold and had unadjusted P-values < 0.05 were subjected to DAVID analysis. Top results of the functional annotation cluster analyses for the DEGs between BaP-exposed ovaries and control ovaries are shown in Tables 3 and 4 for the full and blood-free data sets, respectively. DEGs associated with these GO terms are shown in Supplemental Tables 1 and 2 (47), respectively. Both Tables 3 and 4 show all annotation clusters with enrichment scores > 3. The most enriched cluster for the full data set and the second most enriched cluster in the blood-free data set had annotation terms related to secreted glycoprotein/extracellular/disulfide and included the GO terms “extracellular region” and “extracellular space.” DEGs associated with these GO terms included “cytokines,” “chemokines,” “prolactin family genes,” “serine protease inhibitors,” “growth factors,” and “collagen genes,” among others. The most enriched annotation cluster in the blood-free data set and the third most enriched cluster in the full data set had annotation terms related to somatotropin activity/hormone activity and included the GO term “hormone activity.” DEGs associated with this GO term included PRL family members, neuropeptide Y, and secretin. The third most enriched annotation cluster in the blood-free data set and the sixth most enriched term in the full data set had annotation terms related to proteinase/protease inhibitor. The GO terms associated with these annotation clusters differed between the 2 data sets; however, they shared the common DEG cystatin 11, a cysteine protease inhibitor. The second most enriched annotation cluster in the full data set had annotation terms related to oxygen transport/globin/hemoglobin complex/erythrocyte/metal ion binding and included the GO terms “erythrocyte development,” “oxygen transporter activity,” “oxygen binding,” “iron ion binding,” “heme binding,” and “hemoglobin complex.” This is consistent with blood contamination of 3 of 6 BaP-exposed ovary samples in the full data set. However, despite this, the other most highly enriched annotation clusters were similar between the full and blood-free data sets. For both full and blood-free ovarian DEG sets, the Panther analysis identified nearly all of the GO terms in the DAVID annotation clusters (Tables 3 and 4), and the P-values were similar.
Table 3.
DAVID annotation clusters (differentially expressed genes ≥1.5-fold different and unadjusted P-value < 0.05 from full ovary data set)
| DAVID annotation cluster | Enrichment score | GO terms in cluster | DAVID number of genes | DAVID FDR P-value | Panther FDR P-value |
|---|---|---|---|---|---|
| Secreted glycoprotein, extracellular, disulfide | 25.85 | Extracellular region (CC) | 177 | 2.0E-25 | 8.8E-8 |
| Extracellular space (CC) | 158 | 2.3E-24 | 9.7E-5 | ||
| Oxygen transport, globin, hemoglobin complex, erythrocyte, metal ion binding | 5.86 | Erythrocyte development (BP) | 9 | 4.1E-3 | 1.5E-4 |
| Oxygen transport (BP) | 6 | 6.3E-3 | 4.2E-3 | ||
| Oxygen transporter activity (MF) | 11 | 1.5E-7 | 5.6E-6a | ||
| oxygen binding (MF) | 12 | 2.1E-6 | 1.8E-5 | ||
| Iron ion binding (MF) | 21 | 5.4E-2 | |||
| Heme binding (MF) | 17 | 1.6E-1 | |||
| Hemoglobin complex (CC) | 10 | 2.1E-8 | 8.1E-3 | ||
| Somatotropin hormone, hormone activity | 5.4 | Hormone activity (MF) | 16 | 1.1E-2 | 3E-3 |
| Immunity, immune system | 4.83 | Immune system process (BP) | 38 | 3.1E-3 | 9.5E-3 |
| Innate immune response (BP) | 29 | 2.9E-1 | |||
| Lysosome | 3.84 | Lysosome (CC) | 32 | 1.2E-3 | 7.2E-3 |
| Lysosomal membrane (CC) | 19 | 1.5E-1 | |||
| Serpin, protease inhibitor | 3.82 | Negative regulation of peptidase activity (BP) | 14 | 9.1E-2 | 2.2E-4 |
| Serine-type endopeptidase inhibitor activity (MF) | 17 | 1.1E-2 | 2.6E-3 | ||
| Peptidase inhibitor activity (MF) | 14 | 1.1E-1 | 3.2E-4 | ||
| Development, differentiation | 3.47 | Multicellular organism development (BP) | 71 | 1.5E-2 | 5.7E-21 |
| Cell differentiation (BP) | 49 | 2.9E-1 | 1.6E-10 | ||
| Responses to peptide hormone and cytokine | 3.28 | Response to peptide hormone (BP) | 13 | 9.2E-3 | 1.7E-3 |
| Response to cytokine (BP) | 12 | 4.8E-2 |
Abbreviations: BP, biological process; CC, cellular component; FDR, false discovery rate; MF, molecular function.
aOxygen carrier activity.
Table 4.
DAVID annotation clusters (differentially expressed genes ≥1.5-fold different and unadjusted P-value < 0.05 from blood-free ovary data set)
| DAVID annotation cluster | Enrichment score | GO terms in cluster | DAVIDa genes | DAVID FDR P-value | Panther FDR P-value |
|---|---|---|---|---|---|
| Somatotropin hormone, hormone activity | 7.16 | Hormone activity (MF) | 15 | 7.6E-3 | 3.2E-4 |
| Secreted glycoprotein, extracellular, disulfide | 4.2 | Extracellular region (CC) | 93 | 7.3E-5 | 3.2E-4 |
| proteinase inhibitor | 3.49 | Cysteine-type endopeptidase inhibitor activity (MF) | 9 | 2.5E-2 | 3.2E-2a |
Abbreviations: CC, cellular component; MF, molecular function.
aEndopeptidase inhibitor activity (MF).
Expression of Genes Involved in BaP Metabolism in Embryonic Gonads
We were a priori interested in the expression in embryonic testes and ovaries of key genes known to be involved in metabolism of BaP to reactive metabolites. Expression levels of Phase I and Phase II BaP metabolism genes are shown in Table 5. Cyp1b1 was expressed at moderately high levels in both embryonic ovaries and testes and was statistically significantly decreased by 50% in ovaries after BaP treatment (FDR P-value = 0.007 in the blood-free data set; P = 0.047, t-test in full data set), while its expression was unchanged by BaP exposure in testes. Cyp1a1 was not expressed in control or BaP-exposed ovaries or testes. Akr1a1 was highly expressed in both ovaries and testes and was not affected by BaP exposure. Statistically significant sex differences in expression were noted for Ephx1, Ephx2, Gstm6, Gstm7, Gstp1, Gstp2, Gsta2, Gsta3, and Gsta4; none was significantly affected by BaP exposure in ovaries or testes.
Table 5.
Expression of genes related to benzo[a]pyrene metabolism
| Gene symbol | Ovaries | Testes | ||
|---|---|---|---|---|
| Oil | BaP | Oil | BaP | |
| Ahr | 15.0 | 12.8 | 20.2 | 29.0 |
| Cyp1a1 | ND | ND | ND | ND |
| Cyp1b1 | 69a | 33 | 88 | 125 |
| Ephx1 | 0.8 | 1.7 | 12.2b | 13.3b |
| Ephx2 | 13.7 | 11.6 | 12.5 | 11.8 |
| Akr1a1 | 248.1 | 343.2 | 290.9 | 191.0 |
| Ptgs1 | 5.9 | 10.6 | 5.7 | 7.8 |
| Ptgs2 | 0.3 | 0.6 | 0.2 | 1.0 |
| Gstm1 | 217.3 | 251.6 | 589.0 | 348.0 |
| Gstm2 | 310.1 | 240.9 | 534.0 | 381.4 |
| Gstm5 | 153.5 | 153.2 | 164.0 | 171.4 |
| Gstm6 | 100.9 | 79.6 | 229.4b | 204.4 |
| Gstm7 | 22.7 | 24.1 | 60.6b | 40.1 |
| Gstp1 | 219.1 | 425.0 | 342.0 | 120.0b |
| Gstp2 | 171.2 | 348.3 | 270.6 | 97.3b |
| Gsta2 | 0.3 | 0.2 | 14.5b | 16.3b |
| Gsta3 | 4.1 | 8.6 | 22.9b | 27.7b |
| Gsta4 | 52.6 | 60.6 | 150.3b | 141.2b |
| Gclc | 40.9 | 25.2 | 28.2 | 29.7 |
| Gclm | 29.1 | 47.3 | 35.6 | 19.2 |
| Gsr | 67.4 | 65.2 | 72.8 | 59.6 |
| Gpx1 | 314.8 | 1352.3 | 935.0 | 172.5 |
| Gpx4 | 155.1 | 323.3 | 235.7 | 51.6 |
| Gpx8 | 185.2 | 124.7 | 232.8 | 386.9 |
| Ugt1a1,10,2,5,6a,6b,7c,9a | <3.3 | <3.3 | <3.3 | <3.3 |
| Ugt2b34,37,38,5 | <4.2 | <4.2 | <4.2 | <4.2 |
| Ugt8a | 8.6 | 6.0 | 4.7b | 3.2 |
Abbreviations: BaP, benzo[a]pyrene; ND, not detected.
aSignificantly different from BaP of same sex.
bSignificantly different from ovary of same treatment group, P < 0.05.
Protein Expression of Krt19, Krt18, Ccl2, and Spp1 in BaP-exposed Embryonic Ovaries
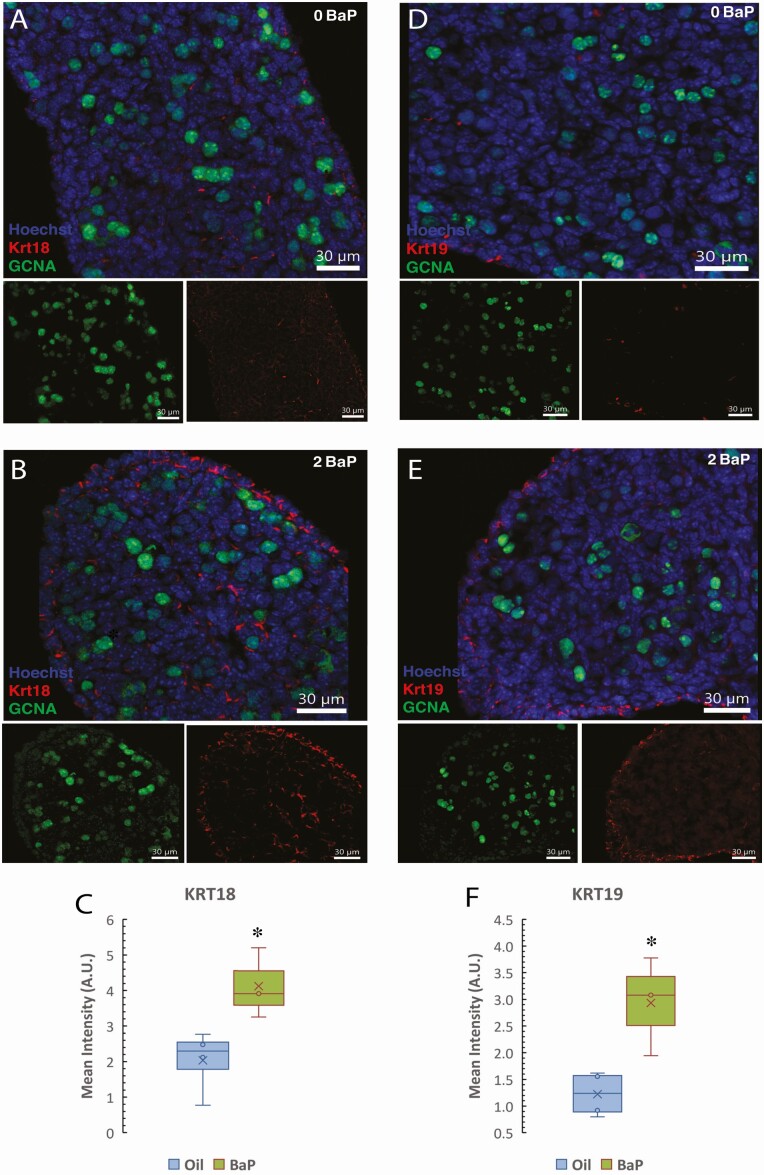
Several of the most DEGs between control and BaP-exposed ovaries were keratins. KRT18 and KRT19 are acidic (type I) intermediate filament proteins, which modulate cytokine receptor trafficking, and several cytokine/chemokine genes were also highly upregulated. Therefore, we further investigated the effect of prenatal exposure to BaP on Krt18 and Krt19 protein levels in the E13.5 ovaries exposed in utero to 0 or 2 mg/kg-day BaP using immunofluorescence (Fig. 5). These results show that Krt18 protein is weakly expressed in somatic cells on the surface and in the ovigerous cords of the control ovaries, and its expression increased in BaP-exposed mice (Fig. 5A and 5B). Quantification of immunofluorescence intensity per unit area revealed that KRT18 (P = 0.042, t-test, P = 0.034, Mann-Whitney test) (Fig. 5C) protein expression was significantly increased in ovaries from E13.5 embryos of mothers exposed from E6.5 to 11.5 to 2 mg/kg/day BaP compared to oil-exposed controls. Similarly, KRT19 protein was found in rare somatic cells within ovigerous cords and on the surface of control ovaries and increased significantly in BaP-exposed compared to oil-exposed controls (P = 0.021, t-test, P = 0.034, Mann-Whitney test) (Fig. 5D-5F).
Figure 5.
Increased KRT18 and KRT19 protein expression in embryonic day (E)13.5 ovaries exposed to BaP E6.5 to 11.5. Immunofluorescence images show increased KRT18 (A and B) and KRT19 (D and E) immunofluorescence (red) in somatic cells of E13.5 ovaries in BaP-exposed (B and E) compared to controls (A and D). Germ cells are stained with GCNA antibody (green, also called TRA98) and nuclei with Hoechst. Scale bars 30 μm. (C) Quantification of KRT18 and (F) KRT19 fluorescence (oil control: n = 4; 2-mg/kg/day BaP: n = 3). Box-and-whisker plots show the mean (x), median (line between 2 boxes), and individual data points in the middle quartiles (°). *P < 0.05, t-test BaP vs oil.
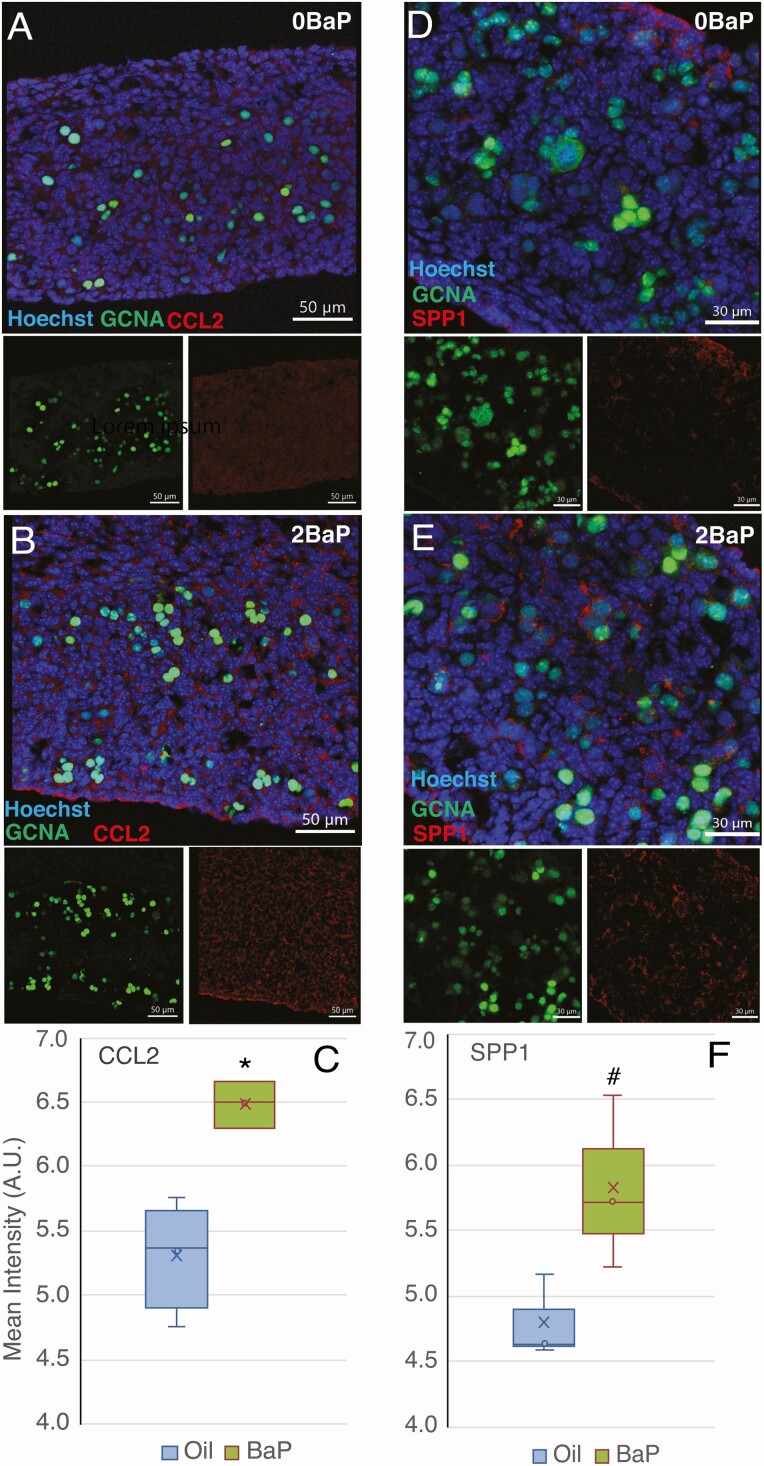
Ccl2, Ccl7, and Spp1 mRNA expression were highly upregulated in BaP-exposed compared to control ovaries (Tables 1 and 2). CCL2 and CCL7 are monocyte chemoattractants, and SPP1 is a macrophage-secreted factor. All 3 of these genes have been associated with ovarian cancer (48-50). Therefore, we investigated the effects of prenatal exposure to 2 mg/kg/day BaP from E6.5 to E11.5 on ovarian CCL2 and SPP1 proteins using immunofluorescence. CCL2 protein expression was significantly increased in somatic cells of E13.5 ovaries from BaP-exposed mice (P = 0.006, t-test; P = 0.034, Mann-Whitney test) (Fig. 6A-6C); it was not expressed in germ cells. Increased SPP1 protein expression, which approached statistical significance, was observed in somatic cells of E13.5 ovaries (P = 0.050, Mann-Whitney test; P = 0.073, t-test) (Fig. 6D-6F).
Figure 6.
Increased CCL2 and SPP1 protein expression in E13.5 ovaries exposed to benzo[a]pyrene (BaP) embryonic day (E) 6.5-11.5. Immunofluorescence images show increased CCL2 (A and B; scale bars 50 μm) and SPP1 (E and D; scale bars 30 μm) immunofluorescence (red) in somatic cells of E13.5 ovaries in BaP-exposed compared to controls. Germ cells are stained with GCNA antibody (green, also called TRA98) and nuclei with Hoechst. (C) Quantification of CCL2 (oil control: n = 4; 2-mg/kg/day BaP: n = 3) and (D) SPP1 immunofluorescence (oil control: n = 3; 2-mg/kg/day BaP: n = 3). Box-and-whisker plots show the mean (x), median (line between 2 boxes), and individual data points in the middle quartiles (°). *P < 0.05, BaP vs oil. #P = 0.050, BaP vs oil.
Discussion
Our results show that BaP metabolites are present in mouse embryos and placentas after oral dosing to the mother and that the concentrations are higher in female than male embryos and are higher in embryos than placentas. We further found that embryonic gonadal gene expression is altered to a greater extent by transplacental BaP exposure in the embryonic ovary than testis. Genes related to monocyte/macrophage recruitment and activity, keratin genes, and PRL family genes were among the most highly upregulated genes in the BaP-exposed ovaries. Our transcriptomic analyses also demonstrated that genes involved in BaP metabolism are expressed in both embryonic ovaries and testes at E13.5. However, expression of most of these metabolic genes was not affected by BaP exposure.
The BaP metabolites we detected in mouse placenta and embryo tissues were BaP 4,5-diol, BaP 7,8-diol, BaP 9,10-diol, BaP 3,6-dione, BaP 6,12-dione, 3-hydroxy BaP, and 9-hydroxy BaP, and their concentrations increased in a BaP dose-dependent manner. These BaP metabolites have also been detected in the uterine myometrium from Long Evans rats gestationally exposed to BaP (51). BaP metabolite concentrations measured in the hearts of postnatal rat offspring exposed via a single dose to the mother on E14 also revealed the presence of diol and quinone metabolites (52). The presence of these metabolites in embryonic tissues in our study and prior studies supports that the CYP, EPHX, and AKR pathway is active in mouse and rat embryos, and our transcriptomic data show that Cyp1b1, Akr1a1, Ephx1, and Ephx2 are expressed in embryonic ovaries and testes. Our findings are supported by publicly available RNA-seq data of E13.5 ovaries and testes, which similarly reported expression of Cyp1b1, Akr1a1, Ephx1, and Ephx2 in E13.5 ovaries and testes (53,54). Several BaP metabolites, including BaP diones measured in our study, are AHR ligands (12,55), and Ahr was expressed in both embryonic ovaries and testes in our transcriptomic data. In addition, we detected BaP-7,8-diol, the precursor to BaP 7,8-dione, a potent AHR activator (12,55), in both embryos and placentas. However, our transcriptomic analysis did not demonstrate increased expression of Ahr or genes known to be upregulated by AHR activation, such as Cyp1b1 and Cyp1a1, in the BaP-exposed embryonic gonads. Unlike our observation in prenatally BaP-exposed gonads, prenatal exposure of mice to 2,3,7,8-tetrachlorodibenzodioxin, the most potent AHR receptor ligand, on gestational day 12.5 significantly upregulated Cyp1b1 in whole perinatal brains (gestational days 15.5 and 18.5 and postnatal day 1) of males and females (56).
The lower concentrations of BaP metabolites in placenta relative to embryos suggest efficient transfer of BaP metabolites through placenta. Additionally, the elevated concentrations of BaP metabolites in embryos compared to placenta are strongly supportive of embryonic metabolism of BaP. Crowell et al (57) made similar observations in mice orally administered with dibenzo(d,e,f,p)chrysene, another PAH compound. BaP metabolites have also been detected in placenta and embryos of rats orally administered BaP, suggesting that uterine tissues are capable of metabolizing BaP (51), which may have been transported to the fetus through the placenta (58), in addition to the fetal metabolism of BaP. Activity-based protein profiling studies by Crowell et al (57) revealed a 2- to 10-fold reduction in activities of PAH biotransformation enzymes in pregnant-compared to naïve mice. Given this scenario, the possibility of BaP parent compound that was not metabolized in the pregnant mother’s body or placenta being subjected to further metabolism in the embryonic tissues cannot be ruled out. Transplacental exposure of mice to the PAH compound dibenzo(a.l)pyrene showed bioactivation of CYP1B1 in fetal tissues (59).
We are not aware of any studies measuring tissue concentrations of BaP metabolites in human fetal tissues or cord blood. BaP concentrations measured in human lung tissue obtained at autopsy ranged from 0.1 to 0.8 ng/g dry weight (60), which roughly translates to 0.02 to 0.16 ng/g wet weight (61). For comparison, total BaP metabolite concentrations in placentas from mice exposed to 0.2 mg/kg/day BaP in our study ranged from 0.14 to 0.23 ng/g wet weight. BaP-7,8-diol epoxide DNA adducts have been measured in human umbilical cord arteries and veins, as well as cord blood, consistent with fetal exposure (10,17). Humans are exposed to dozens of mutagenic PAHs, which undergo similar metabolic activation and have similar mechanisms of action as BaP, in tobacco smoke, air pollution, and foods (7,62-64), and multiple of these PAHs have been shown to be ovotoxicants in rodents (65-68). We chose to study BaP as a prototypical PAH. We estimated the total daily intake of mutagenic PAHs in a highly environmentally exposed, nonoccupationally exposed 60 kg woman at 0.5 μg/kg/day and daily intake of BaP at 0.08 μg/kg/day (6). After allometric interspecies correction (32), these translate to 3.7 and 0.6 μg/kg/day, respectively, in a mouse weighing 20 g. Therefore, the lowest dose of 0.2 mg/kg/day is about 2 orders of magnitude higher, and the high dose of 3.33 mg/kg/day is about 3.5 orders of magnitude higher than the estimated daily BaP dose in a highly environmentally exposed woman. By contrast, occupational exposures can be much higher, with measured air concentrations of BaP in coke production facilities reported to range up to 161 μg/m3 (8). Exposure to this concentration over an 8 h working day would result in inhalation of 1.23 μg/kg-day (mouse equivalent dose 9.1 μg/kg/day), assuming a doubling of resting minute ventilation to 10 L/min during work in a 60 kg woman. We acknowledge that the doses used in the present study are much higher than human nonoccupational and occupational doses and are unlikely to occur in pregnant women.
Some of the most highly upregulated genes in the ovaries from embryos exposed to BaP in utero included multiple cytokeratins associated with ovarian cancer in humans (Krt19, Krt18, and Krt8) (69-73), as well as Ccl2, Ccl7, Lgals3 (lectin galactose binding soluble 3, which codes for the protein galectin-3), and osteopontin (Spp1). Consistent with these RNA-seq results, immunofluorescence quantification revealed significantly increased protein levels of KRT18, KRT19, CCL2, and SPP1. We previously discovered that keratin-positive, epithelial ovarian tumors arise in mice exposed prenatally to BaP (4). KRT18 and KRT19 are acidic (type I) intermediate filament proteins, which modulate cytokine receptor trafficking and are associated with resistance to apoptosis and poor prognosis in human ovarian cancers (74-76). Knockdown of KRT18 and its basic (type II) partner KRT8 enhanced FAS expression and increased FAS antibody-induced apoptosis of KGN human ovarian tumor cells (74). High expression of KRT19 in human ovarian cancer stem cells is associated with chemoresistance (75). Ccl2, Ccl7, Lgals3, and Spp1 have been linked with the tumor microenvironments of several tumor types, including ovarian cancer. CCL2 and CCL7 are potent chemo-attractants of macrophages, which are found in ascites fluid of human ovarian carcinoma patients (48). CCL2 was demonstrated to recruit macrophages to the tumor microenvironment in a mouse model of human pancreatic cancer (77). Localized immunomodulation with interleukin 12 of the tumor microenvironment in a mouse model of metastatic ovarian carcinoma resulted in decreased expression of CCL2 and CCL7, decreased macrophage infiltration, and decreased tumor burden (50). SPP1 (osteopontin) is highly overexpressed in over 90% of human ovarian cancers (49). SPP1 is secreted by macrophages in the tumor microenvironment in a mouse model of glioblastoma, thereby supporting tumor survival (78). Galectins are soluble proteins, which bind to β-galactoside-containing glycans and are involved in regulating inflammation and apoptosis (79). Tumoral and peritumoral expression of galectin 3 is associated with lower progression-free survival in ovarian cancer (80). Thus, our RNA-seq and immunostaining findings suggest that prenatal exposure to BaP induces an ovarian inflammatory response, with increased expression of genes related to chemokine/cytokine signaling and monocyte/macrophage recruitment.
Four different PRL family genes were expressed at low or undetectable levels in controls and were significantly upregulated in BaP-exposed ovaries, with FDR P-values < 0.05 in E13.5 ovaries in the blood-free data set (Prl2a1, Prl2c2, Prl2c5, and Prl8a9) and in the full data set (Prl2c2, Prl2c4, Prl2c3, and Prl7a1). These genes contributed to 2 significant DAVID functional annotation clusters, which included the GO terms “extracellular region/extracellular space” and “hormone activity,” respectively. PRL and growth hormone genes are derived from a single ancestral gene (81). In humans and dogs, the growth hormone gene has expanded, while in mice and rats, the PRL locus has expanded, to 23 genes in mice located on chromosome 13 (81). These genes are important in immune, hematopoietic, placental, vascular, and metabolic adaptations to physiological stressors during pregnancy (81,82). The PRL family genes are expressed at the maternal-fetal interface, particularly in the trophoblast lineage cells (83). Among the differentially expressed PRL family members in our data, Prl2c genes, called Proliferins, have angiogenic actions, and Prl2c2 is considered a marker of trophoblast giant cells (83,84). Prl2a1 is considered a trophoblast invasion marker, and Prl7a1 promotes hematopoiesis (83,84). Consistent with our findings in control ovaries, Prl2c3, Prl2c5, and Prl2a1 were reported to be expressed at low levels, while expression of Prl2c2 and Prl7a1 were reported as below the cutoff, in the E13.5 mouse ovary (53,54). The function of Prl8a9 is unknown; consistent with our data, it has been reported to be expressed at low levels in the E13.5 mouse ovary (53,54). Functions of these PRL family members in the developing ovary have not been investigated to our knowledge.
Conclusion
In conclusion, the much greater effect of prenatal BaP on the embryonic ovarian transcriptome than on the testicular transcriptome in the present study is consistent with our prior work showing that doses of BaP that destroy germ cells in the developing ovary have minimal effects on germ cells in the developing testis (3-5). The finding that prenatal exposure to BaP increased mRNA and protein expression of genes related to chemokine/cytokine signaling and monocyte/macrophage recruitment suggests that BaP exposure creates a proinflammatory ovarian microenvironment. Future studies should investigate whether these ovarian changes are persistent and, if so, whether they contribute to ovarian tumor development.
Acknowledgments
Financial Support: National Institute of Environmental Health Sciences of the National Institutes of Health (NIH) R01ES020454 to U.L. and T.S. Center for Occupational and Environmental Health, University of California Irvine. UC Irvine Chao Family Comprehensive Cancer Center, supported by the National Cancer Institute of the NIH under award number P30CA062203.
Additional Information
Disclosure Statement: The authors have nothing to disclose.
Data Availability
Some data generated or analyzed during this study are included in this published article or in the data repositories listed in the references (applies to raw RNAseq data, supplemental tables, and supplemental data). Links to these data are provided. Other data generated or analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. National Health and Nutrition Examination Survey. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019. Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 2. MacKenzie KM, Angevine DM. Infertility in mice exposed in utero to benzo(a)pyrene. Biol Reprod. 1981;24(1):183-191. [DOI] [PubMed] [Google Scholar]
- 3. Lim J, Kong W, Lu M, Luderer U. The mouse fetal ovary has greater sensitivity than the fetal testis to benzo[a]pyrene-induced germ cell death. Toxicol Sci. 2016;152(2):372-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim J, Lawson GW, Nakamura BN, et al. Glutathione-deficient mice have increased sensitivity to transplacental benzo[a]pyrene-induced premature ovarian failure and ovarian tumorigenesis. Cancer Res. 2013;73(2):908-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nakamura BN, Mohar I, Lawson GW, et al. Increased sensitivity to testicular toxicity of transplacental benzo[a]pyrene exposure in male glutamate cysteine ligase modifier subunit knockout (Gclm−/−) mice. Toxicol Sci. 2012;126(1):227-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luderer U, Myers MB, Banda M, McKim KL, Ortiz L, Parsons BL. Ovarian effects of prenatal exposure to benzo[a]pyrene: Roles of embryonic and maternal glutathione status. Reprod Toxicol. 2017;69:187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry; 1995. [PubMed] [Google Scholar]
- 8. Wallingford KM. Occupational exposure to benzo[a]pyrene. In: Cooke MP, Dennis AJ, eds. Polynuclear Aromatic Hydrocarbons: Mechanisms, Methods and Metabolism. NIOSHTIC no. 00159018. NIOSH; 1985. [Google Scholar]
- 9. Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005;206(1):73-93. [DOI] [PubMed] [Google Scholar]
- 10. Hansen C, Asmussen I, Autrup H. Detection of carcinogen-DNA adducts in human fetal tissues by the 32P-postlabeling procedure. Environ Health Perspect. 1993;99:229-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Penning TM. Aldo-keto reductases and formation of polycyclic aromatic hydrocarbon o-quinones. Methods Enzymol. 2004;378:31-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burczynski ME, Penning TM. Genotoxic polycyclic aromatic hydrocarbon ortho-quinones generated by aldo-keto reductases induce CYP1A1 via nuclear translocation of the aryl hydrocarbon receptor. Cancer Res. 2000;60(4):908-915. [PubMed] [Google Scholar]
- 13. Cavalieri EL, Rogan EG. Central role of radical cations in metabolic activation of polycyclic aromatic hydrocarbons. Xenobiotica. 1995;25(7):677-688. [DOI] [PubMed] [Google Scholar]
- 14. Shimada T, Sugie A, Shindo M, et al. Tissue-specific induction of cytochromes P450 1A1 and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in engineered C57BL/6J mice of arylhydrocarbon receptor gene. Toxicol Appl Pharmacol. 2003;187(1):1-10. [DOI] [PubMed] [Google Scholar]
- 15. Anderson RA, McIlwain L, Coutts S, Kinnell HL, Fowler PA, Childs AJ. Activation of the aryl hydrocarbon receptor by a component of cigarette smoke reduces germ cell proliferation in the human fetal ovary. Mol Hum Reprod. 2014;20(1):42-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matikainen TM, Moriyama T, Morita Y, et al. Ligand activation of the aromatic hydrocarbon receptor transcription factor drives Bax-dependent apoptosis in developing fetal ovarian germ cells. Endocrinology. 2002;143(2):615-620. [DOI] [PubMed] [Google Scholar]
- 17. Autrup H, Vestergaard AB. Transplacental transfer of environmental genotoxins–polycyclic aromatic hydrocarbon-albumin in nonsmoking women. Environ Health Perspect. 1996;104(suppl 3):625-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Luderer U, Meier MJ, Lawson GW, Beal MA, Yauk CL, Marchetti F. In utero exposure to benzo[a]pyrene induces ovarian mutations at doses that deplete ovarian follicles in mice. Environ Mol Mutagen. 2019;60(5):410-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meier MJ, O’Brien JM, Beal MA, Allan B, Yauk CL, Marchetti F. In utero exposure to benzo[a]pyrene increases mutation burden in the soma and sperm of adult mice. Environ Health Perspect. 2017;125(1):82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Madden JA, Hoyer PB, Devine PJ, Keating AF. Acute 7,12-dimethylbenz[a]anthracene exposure causes differential concentration-dependent follicle depletion and gene expression in neonatal rat ovaries. Toxicol Appl Pharmacol. 2014;276(3):179-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang WL, Cai KQ, Smedberg JL, et al. A reduction of cyclooxygenase 2 gene dosage counters the ovarian morphological aging and tumor phenotype in Wv mice. Am J Pathol. 2007;170(4):1325-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nio-Kobayashi J, Kudo M, Sakuragi N, Iwanaga T, Duncan WC. Loss of luteotropic prostaglandin E plays an important role in the regulation of luteolysis in women. Mol Hum Reprod. 2017;23(5):271-281. [DOI] [PubMed] [Google Scholar]
- 23. Griffin DK, Ellis PJ, Dunmore B, Bauer J, Abel MH, Affara NA. Transcriptional profiling of luteinizing hormone receptor-deficient mice before and after testosterone treatment provides insight into the hormonal control of postnatal testicular development and Leydig cell differentiation. Biol Reprod. 2010;82(6):1139-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rich KJ, Boobis AR. Expression and inducibility of P450 enzymes during liver ontogeny. Microsc Res Tech. 1997;39(5):424-435. [DOI] [PubMed] [Google Scholar]
- 25. McCarver DG, Hines RN. The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. J Pharmacol Exp Ther. 2002;300(2):361-366. [DOI] [PubMed] [Google Scholar]
- 26. Parman T, Wells PG. Embryonic prostaglandin H synthase-2 (PHS-2) expression and benzo[a]pyrene teratogenicity in PHS-2 knockout mice. Faseb J. 2002;16(9):1001-1009. [DOI] [PubMed] [Google Scholar]
- 27. Wells PG, McCallum GP, Chen CS, et al. Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci. 2009;108(1):4-18. [DOI] [PubMed] [Google Scholar]
- 28. National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. National Research Council, National Academy of Sciences, National Academies Press; 2011. [Google Scholar]
- 29. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84-97. [DOI] [PubMed] [Google Scholar]
- 30. Livera G, Delbes G, Pairault C, Rouiller-Fabre V, Habert R. Organotypic culture, a powerful model for studying rat and mouse fetal testis development. Cell Tissue Res. 2006;324(3):507-521. [DOI] [PubMed] [Google Scholar]
- 31. National Institutes of Health. NIH Guidelines for the Laboratory Use of Chemical Carcinogens. NIH publication no. 81-2385. U.S. Government Printing Office; 1981. [Google Scholar]
- 32. Environmental Protection Agency. Recommended Use of Body Weight3/4as the Default Method in Derivation of the Oral Reference Dose. Environmental Protection Agency; 2011. [Google Scholar]
- 33. Moffat I, Chepelev N, Labib S, et al. Comparison of toxicogenomics and traditional approaches to inform mode of action and points of departure in human health risk assessment of benzo[a]pyrene in drinking water. Crit Rev Toxicol. 2015;45(1):1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Halappanavar S, Wu D, Williams A, et al. Pulmonary gene and microRNA expression changes in mice exposed to benzo(a)pyrene by oral gavage. Toxicology. 2011;285(3):1879-3185. [DOI] [PubMed] [Google Scholar]
- 35. Ramesh A, Inyang F, Hood DB, Archibong A, Knuckles ME, Nyanda AM. Metabolism, bioavailability, and toxicokinetics of Benzo(a) pyrene in F-344 rats following oral administration. Exp Toxic Pathol. 2001;53:275-290. [DOI] [PubMed] [Google Scholar]
- 36. Hu WY, Hu DP, Xie L, et al. Isolation and functional interrogation of adult human prostate epithelial stem cells at single cell resolution. Stem Cell Res. 2017;23:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitsunaga S, Odajima J, Yawata S, et al. Relevance of iPSC-derived human PGC-like cells at the surface of embryoid bodies to prechemotaxis migrating PGCs. Proc Natl Acad Sci U S A. 2017;114(46):E9913-E9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gene Expression Omnibus Repository. National Center for Biotechnology Information. 2021. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE184417.
- 39. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tarasov A, Vilella AJ, Cuppen E, Nijman IJ, Prins P. Sambamba: fast processing of NGS alignment formats. Bioinformatics. 2015;31(12):2032-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liao Y, Smyth GK, Shi W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads. Nucleic Acids Res. 2019;47(8):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44-57. [DOI] [PubMed] [Google Scholar]
- 44. Mi H, Ebert D, Muruganujan A, et al. PANTHER version 16: a revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2020;49(D1):D394-D403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mi H, Muruganujan A, Huang X, et al. Protocol Update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat Protoc. 2019;14(3):703-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lim J, Ramesh A, Shioda T, Leon Parada K, Luderer U. DEGs in embryonic gonads after prenatal benzo[a]pyrene v oil vehicle and in vehicle embryonic testes v ovaries. Figshare.2021. doi: 10.6084/m9.figshare.16677724 [DOI] [PMC free article] [PubMed]
- 47. Lim J, Ramesh A, Shioda T, Leon Parada K, Luderer U. Genes driving DAVID functional annotation clusters and PANTHER GO terms for DEGs in Benzopyrene v vehicle exposed embryonic ovaries. Figshare.2021. doi: 10.6084/m9.figshare.16678042 [DOI] [PMC free article] [PubMed]
- 48. Schutyser E, Struyf S, Proost P, et al. Identification of biologically active chemokine isoforms from ascitic fluid and elevated levels of CCL18/pulmonary and activation-regulated chemokine in ovarian carcinoma. J Biol Chem. 2002;277(27):24584-24593. [DOI] [PubMed] [Google Scholar]
- 49. Atai NA, Bansal M, Lo C, et al. Osteopontin is up-regulated and associated with neutrophil and macrophage infiltration in glioblastoma. Immunology. 2011;132(1):39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cohen CA, Shea AA, Heffron CL, Schmelz EM, Roberts PC. Interleukin-12 immunomodulation delays the onset of lethal peritoneal disease of ovarian cancer. J Interferon Cytokine Res. 2016;36(1):62-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Laknaur A, Foster TL, Bobb LE, et al. Altered expression of histone deacetylases, inflammatory cytokines and contractile-associated factors in uterine myometrium of Long Evans rats gestationally exposed to benzo[a]pyrene. J Appl Toxicol. 2016;36(6):827-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jules GE, Pratap S, Ramesh A, Hood DB. In utero exposure to benzo(a)pyrene predisposes offspring to cardiovascular dysfunction in later-life. Toxicology. 2012;295(1-3):56-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. GXD. Gene expression database (GXD), mouse genome informatics web site. World wide web. http://www.informatics.jax.org. Accessed May 2021, 2021.
- 54. Smith CM, Hayamizu TF, Finger JH, et al. The mouse gene expression database (GXD): 2019 update. Nucleic Acids Res. 2019;47(D1):D774-D779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park JH, Mangal D, Frey AJ, Harvey RG, Blair IA, Penning TM. Aryl hydrocarbon receptor facilitates DNA strand breaks and 8-oxo-2’-deoxyguanosine formation by the aldo-keto reductase product benzo[a]pyrene-7,8-dione. J Biol Chem. 2009;284(43):29725-29734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mitsui T, Taniguchi N, Kawasaki N, Kagami Y, Arita J. Fetal exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin induces expression of the chemokine genes Cxcl4 and Cxcl7 in the perinatal mouse brain. J Appl Toxicol. 2011;31(3):279-284. [DOI] [PubMed] [Google Scholar]
- 57. Crowell SR, Sharma AK, Amin S, et al. Impact of pregnancy on the pharmacokinetics of dibenzo[def,p]chrysene in mice. Toxicol Sci. 2013;135(1):48-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dong X, Wang Q, Peng J, Wu M, Pan B, Xing B. Transfer of polycyclic aromatic hydrocarbons from mother to fetus in relation to pregnancy complications. Sci Total Environ. 2018;636:61-68. [DOI] [PubMed] [Google Scholar]
- 59. Castro DJ, Baird WM, Pereira CB, et al. Fetal Mouse Cyp1b1 and transplacental carcinogenesis from maternal exposure to Dibenzo[a,l]pyrene. Cancer Prev Res. 2008;1:128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lodovici M, Akpan V, Giovannini L, Migliani F, Dolara P. Benzo[a]pyrene diol-epoxide DNA adducts and levels of polycyclic aromatic hydrocarbons in autoptic samples from human lungs. Chem Biol Interact. 1998;116(3):199-212. [DOI] [PubMed] [Google Scholar]
- 61. Matsuyama H, Amaya F, Hashimoto S, et al. Acute lung inflammation and ventilator-induced lung injury caused by ATP via the P2Y receptors: an experimental study. Respir Res. 2008;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lodovici M, Akpan V, Evangelisti C, Dolara P. Sidestream tobacco smoke as the main predictor of exposure to polycyclic aromatic hydrocarbons. J Appl Toxicol. 2004;24(4):277-281. [DOI] [PubMed] [Google Scholar]
- 63. Menzie CA, Potocki BB, Santodonato J. Ambient concentrations and exposure to carcinogenic pahs in the environment. Environ Sci Technol. 1992;26(7):1278-1284. [Google Scholar]
- 64. Shopland DR, Burns DM, Benowitz NL, Amacher RH, eds. Risks Associated with Smoking Cigarettes with Low Machine-Measured Yields of Tar and Nicotine. Smoking and Tobacco Control Monographs no. 13. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 2001. [Google Scholar]
- 65. Mattison DR. Difference in sensitivity of rat and mouse primordial oocytes to destruction by polycyclic aromatic hydrocarbons. Chem Biol Interact. 1979;28(1):133-137. [DOI] [PubMed] [Google Scholar]
- 66. Mattison DR, Nightingale MS. Oocyte destruction by polycyclic aromatic hydrocarbons is not linked to the inducibility of ovarian aryl hydrocarbon (benzo(a)pyrene) hydroxylase activity in (DBA/2N X C57BL/6N) F1 X DBA/2N backcross mice. Pediatr Pharmacol (New York). 1982;2(1):11-21. [PubMed] [Google Scholar]
- 67. Mattison DR, Singh H, Takizawa K, Thomford PJ. Ovarian toxicity of benzo(a)pyrene and metabolites in mice. Reprod Toxicol. 1989;3(2):115-125. [DOI] [PubMed] [Google Scholar]
- 68. Borman SM, Christian PJ, Sipes IG, Hoyer PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol. 2000;167(3):191-198. [DOI] [PubMed] [Google Scholar]
- 69. Chen X, Aravindakshan J, Yang Y, Sairam MR. Early alterations in ovarian surface epithelial cells and induction of ovarian epithelial tumors triggered by loss of FSH receptor. Neoplasia. 2007;9(6):521-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495(7440):241-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ng A, Tan S, Singh G, et al. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat Cell Biol. 2014;16(8):745-757. [DOI] [PubMed] [Google Scholar]
- 72. Kolostova K, Pinkas M, Jakabova A, et al. Molecular characterization of circulating tumor cells in ovarian cancer. Am J Cancer Res. 2016;6(5):973-980. [PMC free article] [PubMed] [Google Scholar]
- 73. Jin C, Yang M, Han X, et al. Evaluation of the value of preoperative CYFRA21-1 in the diagnosis and prognosis of epithelial ovarian cancer in conjunction with CA125. J Ovarian Res. 2019;12(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Trisdale SK, Schwab NM, Hou X, Davis JS, Townson DH. Molecular manipulation of keratin 8/18 intermediate filaments: modulators of FAS-mediated death signaling in human ovarian granulosa tumor cells. J Ovarian Res. 2016;9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu M, Mor G, Cheng H, et al. High frequency of putative ovarian cancer stem cells with CD44/CK19 coexpression is associated with decreased progression-free intervals in patients with recurrent epithelial ovarian cancer. Reprod Sci. 2013;20(5):605-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim S, Coulombe PA. Intermediate filament scaffolds fulfill mechanical, organizational, and signaling functions in the cytoplasm. Genes Dev. 2007;21(13):1581-1597. [DOI] [PubMed] [Google Scholar]
- 77. Hou P, Kapoor A, Zhang Q, et al. Tumor microenvironment remodeling enables bypass of oncogenic KRAS dependency in pancreatic cancer. Cancer Discov. 2020;10(7):1058-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen P, Zhao D, Li J, et al. Symbiotic macrophage-glioma cell interactions reveal synthetic lethality in PTEN-null glioma. Cancer Cell. 2019;35(6):868-884.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hisrich BV, Young RB, Sansone AM, et al. Role of human galectins in inflammation and cancers associated with endometriosis. Biomolecules. 2020;10(2):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pergialiotis V, Papoutsi E, Androutsou A, et al. Galectins-1, -3, -7, -8 and -9 as prognostic markers for survival in epithelial ovarian cancer: A systematic review and meta-analysis. Int J Gynaecol Obstet. 2021;152(3):299-307. [DOI] [PubMed] [Google Scholar]
- 81. Soares MJ, Konno T, Alam SM. The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol Metab. 2007;18(3):114-121. [DOI] [PubMed] [Google Scholar]
- 82. Ain R, Canham LN, Soares MJ. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: novel endocrine phenotype and regulation. Dev Biol. 2003;260(1):176-190. [DOI] [PubMed] [Google Scholar]
- 83. Simmons DG, Rawn S, Davies A, Hughes M, Cross JC. Spatial and temporal expression of the 23 murine prolactin/placental lactogen-related genes is not associated with their position in the locus. BMC Genomics. 2008;9:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chakraborty S, Ain R. NOSTRIN: a novel modulator of trophoblast giant cell differentiation. Stem Cell Res. 2018;31:135-146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some data generated or analyzed during this study are included in this published article or in the data repositories listed in the references (applies to raw RNAseq data, supplemental tables, and supplemental data). Links to these data are provided. Other data generated or analyzed during this study are not publicly available but are available from the corresponding author on reasonable request.