Abstract
Among those many individuals who experience a reduced odor sensitivity (hyposmia/anosmia), some individuals also have disorders that lead to odor distortion, such as parosmia (i.e. distorted odor with a known source), or odor phantoms (i.e. odor sensation without an odor source). We surveyed a large population with at least one olfactory disorder (N = 2031) and found that odor distortions were common (46%), with respondents reporting either parosmia (19%), phantosmia (11%), or both (16%). In comparison to respondents with hyposmia or anosmia, respondents with parosmia were more likely to be female, young, and suffering from post-viral olfactory loss (P < 0.001), while respondents with phantosmia were more likely to be middle-aged (P < 0.01) and experiencing symptoms caused by head trauma (P < 0.01). In addition, parosmia, compared to phantosmia or anosmia/hyposmia, was most prevalent 3 months to a year after olfactory symptom onset (P < 0.001), which coincides with the timeline of physiological recovery. Finally, we observed that the frequency and duration of distortions negatively affects the quality of life, with parosmia showing a higher range of severity than phantosmia (P < 0.001). Previous research often grouped these distortions together, but our results show that they have distinct patterns of demographics, medical history, and loss in quality of life.
Keywords: olfactory, impairment, anosmia, distortion, smell
Introduction
Olfactory dysfunction affects a quarter of the population, and with the advent of COVID-19, this number is likely to rise (Pellegrino et al. 2020a). In addition to reduced odor sensitivity, some individuals also experience odor distortion (Leopold 2002; Keller and Malaspina 2013; Burges Watson et al. 2020). Reduced sensitivity has been well described in the literature leading to better diagnosis and treatment (Hummel et al. 2017; Oleszkiewicz et al. 2019). Still, despite the differences between parosmia (i.e. distorted odor with a known source) and phantosmia (i.e. odor sensation without an odor source) (Hummel et al. 2017), most studies do not separate them. This is partly due to the large variance in their clinical presentation (Frasnelli et al. 2004) and because many patients report having both symptoms (Sjölund et al. 2017).
In general, when patients with parosmia inhale odorants their perception does not match their memory from before the distortion. In most cases of parosmia, the distorted odors are usually perceived as unpleasant (“cacosmia”), but there have been cases in which the distortions were pleasant (“euosmia,” (Landis et al. 2006)). Additionally, recent evidence suggests that specific odors, such as coffee, meat, onion, and toothpaste, are more likely to trigger parosmia than others (Parker et al. 2021b). Phantosmia, on the other hand, describes the perception of an odor in the absence of a source—there is only the illusion of a smell. Parosmia has been reported among 10%–60% of olfactory dysfunction patients (Nordin et al. 1996; Reden et al. 2007; Parma et al. 2020) while the range is much smaller (3%–16%) for phantosmia (Nordin et al. 1996; Ohayon 2000; Reden et al. 2007; Rawal et al. 2016; Sjölund et al. 2017; Bainbridge et al. 2018). These numbers indicate that incidences of parosmias and phantosmias are not rare, but the variance indicates that the reported frequency depends on the definition of parosmia or phantosmia.
Most parosmia appears to co-occur with olfactory loss due to viral infection, with the majority of cases resolving within a year (Nordin et al. 1996; Quint et al. 2001; Reden et al. 2007; Liu et al. 2020a). Patients suffering from parosmia also had smaller olfactory bulbs compared to those with reduced sensitivity and no distortion (Mueller et al. 2005; Rombaux et al. 2009). In addition, parosmia was eliminated by preventing odors from entering the olfactory cleft in a case study (Liu et al. 2020b). This supports a peripheral etiology and is consistent with the theory that parosmia results from mistargeting that occurs when olfactory sensory neurons regrow axons to the olfactory bulb during recovery (Holbrook et al. 2005; Hong et al. 2012).
With phantosmia, peripheral origins of distortion may be maintained through abnormally active olfactory sensory neurons, loss of inhibitory neurons, or microbial infection creating a malodor (Leopold 2002). The removal of the olfactory epithelium or even briefly occluding a nostril (irrelevant of side) has been shown to eliminate the olfactory illusions for some patients (Leopold et al. 1991, 2002). Many phantosmia patients have a history of head trauma (Leopold 2002; Sjölund et al. 2017), psychiatric disorders (Frasnelli et al. 2004; Croy et al. 2013), temporal lobe epilepsy, and phantosmic episodes in the form of auras (Leopold 2002; Aiello and Hirsch 2013), suggesting a central etiology from overactive neurons.
Patients with symptoms of olfactory distortion may suffer to a larger extent than those with a reduced sensitivity, as they are continually reminded of their problem. In fact, individuals with reduced perception of odors are often not even aware of their disorder (Oleszkiewicz and Hummel 2019; Oleszkiewicz et al. 2020). However, most reports on odor distortions have not used a quantitative approach to compare them with anosmia and hyposmia—instead reporting anecdotal patient experiences. Here, we compared them directly using a survey designed to gather information about parosmia and phantosmia. This quantitative approach allowed us to provide diagnostic criteria and reveal patterns of the disorder. Using this method, we saw several distinct differences among the disorders and created a severity metric for clinical use.
Materials and methods
Participants
A total of 2246 individuals filled out an online questionnaire survey that was distributed globally in English with English-speaking countries (the United Kingdom and the United States) representing the largest proportion of respondents. The survey was launched in parallel with a new informational website about smell loss (www.abscent.org) which had two parts: an area with information that could be accessed by anyone, and a “member area” with a closed forum, access to the Snif Smell Training app, and other more premium features. Access to the member area was given to anyone who completed the survey. Primary areas of recruitment were the AbScent website and social media posts to AbScent’s Facebook and Twitter accounts. Survey data were collected between May 2019 and October 2020. This procedure was conducted according to the Declaration of Helsinki for studies on human subjects and approved by the University of Tennessee IRB review for research involving human subjects (IRB # 19-05253-XM).
Procedure
The Sense of Smell Questionnaire was created from prior research surveys (Frasnelli et al. 2004; Landis et al. 2010; Keller and Malaspina 2013) and patient observations by the authors. It was designed to specifically address features of odor distortion (Supplementary Appendix I). Two binary response (yes or no) questions accompanied by a descriptive caption were used to create four groups of smell impairment:
Parosmia—the experience of distorted smells which have an obvious source:
Do you have parosmia (distorted sense of smell)?
Phantosmia—the experience of smells that have no obvious origin:
Do you experience smells that are not present (phantosmia)?
Participants who only chose A or B were classified as Parosmic and Phantosmic, respectively, while those choosing both were considered Parosmic/Phantosmic. All other smell impaired participants were considered Anosmic/Hyposmic. The questionnaire used a branching design such that questions specific to each disorder were only presented to those who responded with “Yes” to the quality disorder.
Statistical analysis
We used a unimodal analysis to look at differences across groups. We used chi-square analysis for categorical responses and analysis of variance (ANOVA) for continuous responses. Responses were bootstrapped to provide confidence intervals using the boot package (Davison and Hinkley 1997). Here, we resampled (with replacement) the responses 1000 times to estimate error in all comparisons and visualizations.
To determine the degree of severity, three questions were considered—each were asked within each odor distortion question block (answering “Yes” to Parosmia or Phantosmia) and had the same options. Below is an example of the questions for Phantosmia/Parosmia.
How often do you experience smells that are not present (for phantosmia) or how often do you experience parosmia (distorted sense of smell)? (daily, once every week, once every month)
How long does a Phantosmia/Parosmia episode last? (seconds, minutes, hours, days)
How would you describe your Phantosmia/Parosmia? (mild, strong)
These questions together had a low intercorrelation coefficient (0.55). Questions A and C loaded onto the same principal component with question C explaining less variance (89% compared to 77%). Therefore, question C was dropped and A and B were summed to create a severity score of the disorder. Analysis was done with the psych package in R (Revelle 2017).
Two open-ended text questions describing distortions of parosmia or phantosmia underwent text analysis. Sentences were cleaned and words were spell checked with hunspell using a large English dictionary (Ooms 2020). Sentiment analysis, using the sentimentr package (Rinker 2019), was done at the sentence level across participants that provided sentences longer than the first quartile length of all sentences (>7 words), and density plots were used to provide a visual representation. Average sentiment and negative emotion count of each sentence were then used as predictors for degree of severity. Furthermore, sentences were broken down into one-word nouns with SpacyR (Benoit and Matsuo 2018). Summary tables of counts were constructed and visually represented in wordclouds with the size representing the frequency using ggwordcloud (Pennec and Slowikowski 2018).
All analyses were performed using R (version 4.3), and the code along with data can be found here: https://osf.io/5ebjt/
Results
Only participants reporting an olfactory disorder, 18 years of age or over, and not born with the smell problem (congenital) were considered in the analysis (N = 2031). From this large population with an olfactory disorder, we report that odor distortions are common to smell impairment (46%, N = 1106) with individuals reporting either parosmia (19%, N = 376), phantosmia (11%, N = 224), or both (16%, N = 325). Exploratory analysis revealed individuals reporting “both” types of odor distortion did not represent parosmia and phantosmia evenly (Supplementary Fig. 1). Due to this heterogeneity, we excluded this population from the rest of the analysis leaving three groups—Anosmic/Hyposmic, Parosmic, and Phantosmic. Parosmia and phantosmia showed distinct patterns, both from each other as well as from those with reduced sensitivity, in demographics, medical history, and impacts to quality of life. Using two questions, we were able to derive a severity score that influences many of these patterns.
Demographics and medical history
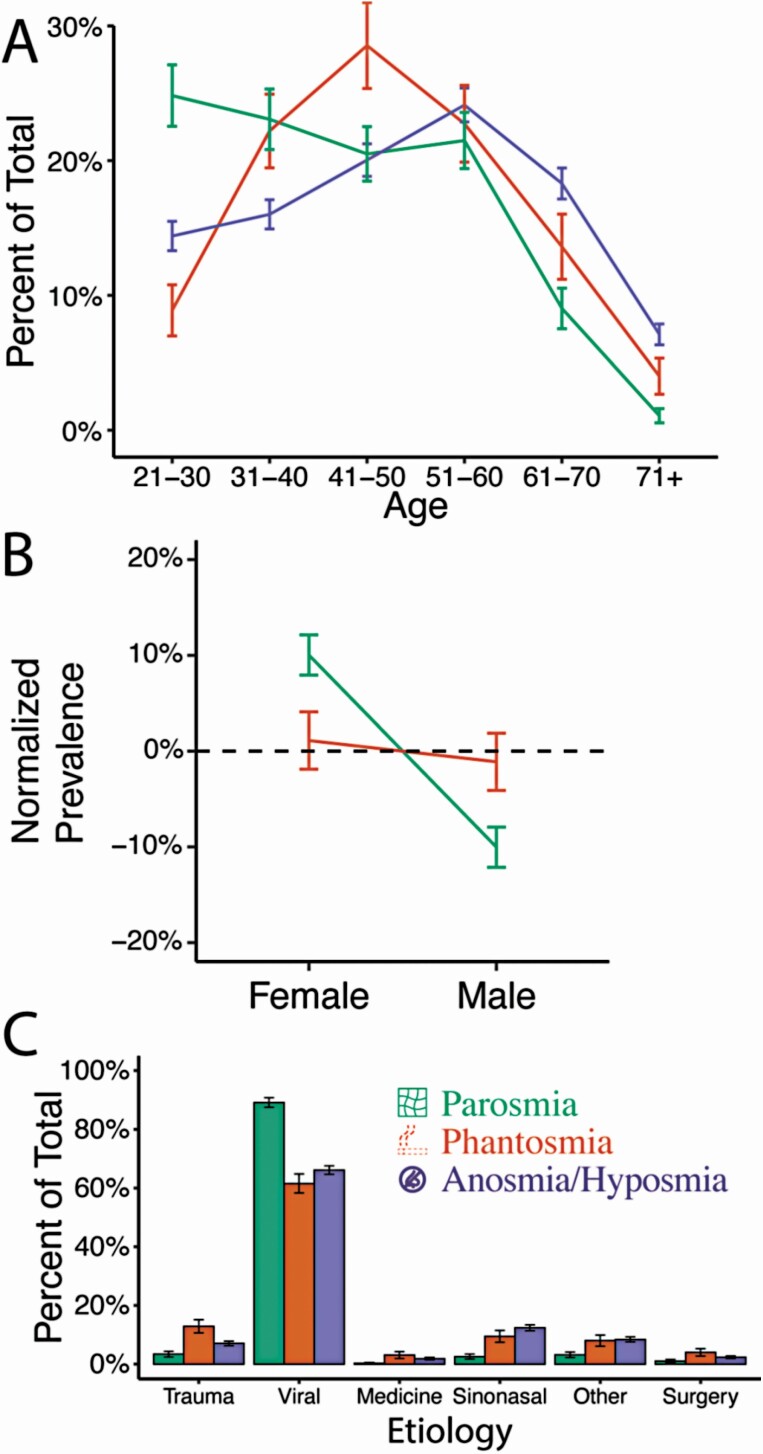
Our sample was predominantly female (72%) with an age range from 21 to over 71 (see Supplementary Table 1). Respondents with parosmia were more likely to be female and younger than phantosmic (χ 2 = 5.84, P = 0.047 and χ 2 = 4.79, P < 0.001, respectively) or anosmic/hyposmic individuals (χ 2 = 14.12, P < 0.001 and χ 2 = 4.62, P < 0.001, respectively) (Fig. 1A and B). In contrast, phantosmia prevalence peaked for 41–50 years old (χ 2 = 2.82, P = 0.01) and anosmia/hyposmia was more prominent in older individuals (61 and over; χ 2 = 5.18, P < 0.001). There were no differences in gender between phantosmic vs. anosmic/hyposmic populations (χ 2 = 0.08, P = 0.78).
Fig 1.
Parosmia and phantosmia are distinct disorders in demographics and etiology. The demographics of respondents were distinct by age (A), sex (B), and etiology (C). Colors and icons represent olfactory disorders: green with a distorted grid icon represents individuals with parosmia, orange with an outlined cigarette icon represents individuals with phantosmia, as cigarette smell was a common phantom smell reported in our sample pool (14% of terms), and purple with a nose deny icon represents individuals with neither parosmia nor phantosmia, but who reported an issue with smell (hyposmia/anosmia). Normalized prevalence represents the frequency difference between anosmia/hyposmia (baseline) and the other two olfactory disorders (parosmia or phantosmia). Error bars represent bootstrapped standard errors.
The three most common etiologies resulting in an olfactory disorder are viral (70%), sinonasal disease (10%), and traumatic impact (8%) (Fig. 1C). Among those with post-viral disorders, parosmia was the most common disorder (χ 2 = 8.58, P < 0.001), and among those who suffered the traumatic impact, phantosmia was the most common disorder (χ 2 = 3.69, P = 0.006).
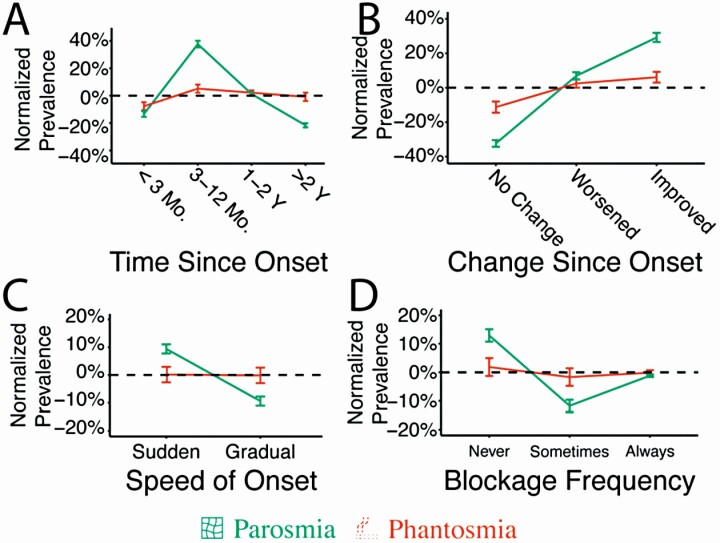
Parosmia, compared to other olfactory conditions, was less likely to last more than 2 years (χ 2 = 8.36, P < 0.001) and more likely to appear during recovery from the initial olfactory impairment (3–12 months) (χ 2 = 13.35, P < 0.001) (Fig. 2A). Similarly, parosmic individuals were more likely to say their condition was improving (χ 2 = 10.02, P < 0.001) and less likely to report their condition as unchanged (χ 2 = 2.68, P = 0.02). Phantosmia, on the other hand, was more stable, with no change in improvement across time in comparison to the anosmic/hyposmic group (χ 2 = 1.59, P = 0.33) (Fig. 2B). Compared to phantosmic and anosmic/hyposmic individuals, parosmia occurred suddenly (χ 2 = 3.61, P < 0.001) with less nasal blockage (χ 2 = 4.56, P < 0.001) (Fig. 2C and D). Overall, parosmic individuals showed the most deviation from the other olfactory disorders (phantosmia and anosmia/hyposmia).
Fig. 2.
Parosmia and phantosmia are distinct disorders by medical history. (A) The two disorders were distinct in the time course of disease (A–C), and amount of nasal congestion (D). Normalized prevalence represents the frequency difference between anosmia/hyposmia (baseline) and the other two olfactory disorders (parosmia or phantosmia). Error bars represent bootstrapped standard errors.
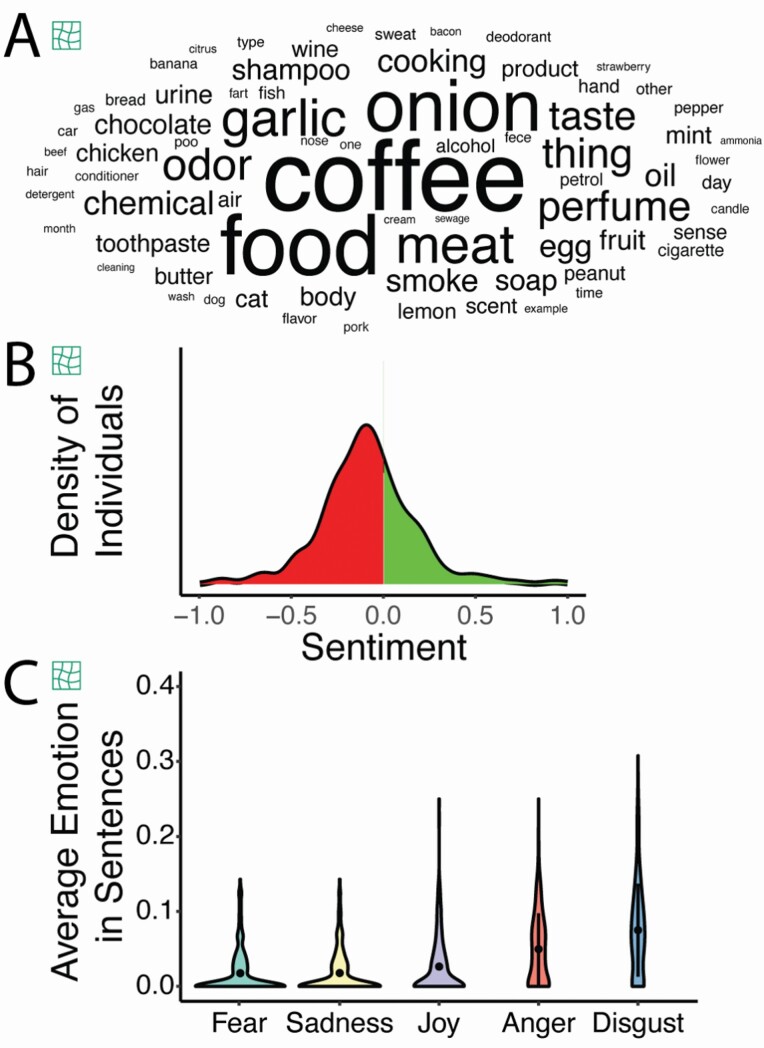
Parosmia is defined as distortion with an odor source, but the triggers for phantosmia are unknown. We report that all but one parosmic patient had specific sources that were distorted (99.7%, Fig. 3A) while only a few phantosmic individuals had situations that triggered a distorted episode (17.0%). Sentences (N = 547) used to describe distortions for parosmia mostly had a negative sentiment, but there were positively described distortions (e.g. “my smell disorders are actually pleasant, flatulence smell like extra virgin olive oil and sometimes bubble gum”) (Fig. 3B). Disgust was the highest emotion (Fig. 3C, F(3) = 107.63, P < 0.001). Compiling words that trigger a distorted episode, parosmic individuals frequently reported foods that are roasted (coffee, meat) or contain sulfur (onion, egg, garlic). Phantosmic individuals instead reported places (room, house) or temporal events (e.g. time, week) while some referred to specific sensory (loud tv, cigarette smoke) or cognitive events (stress, memory).
Fig. 3.
Text analysis of descriptions of parosmic episodes by individuals with parosmia. (A) Word cloud of nouns used to describe triggers of parosmia with size representing word frequency across 375 parosmics. (B) Distribution of sentences having a negative (in red) or positive (in green) sentiment. (C) Average emotions in sentences describing parosmia episodes.
Quality of life
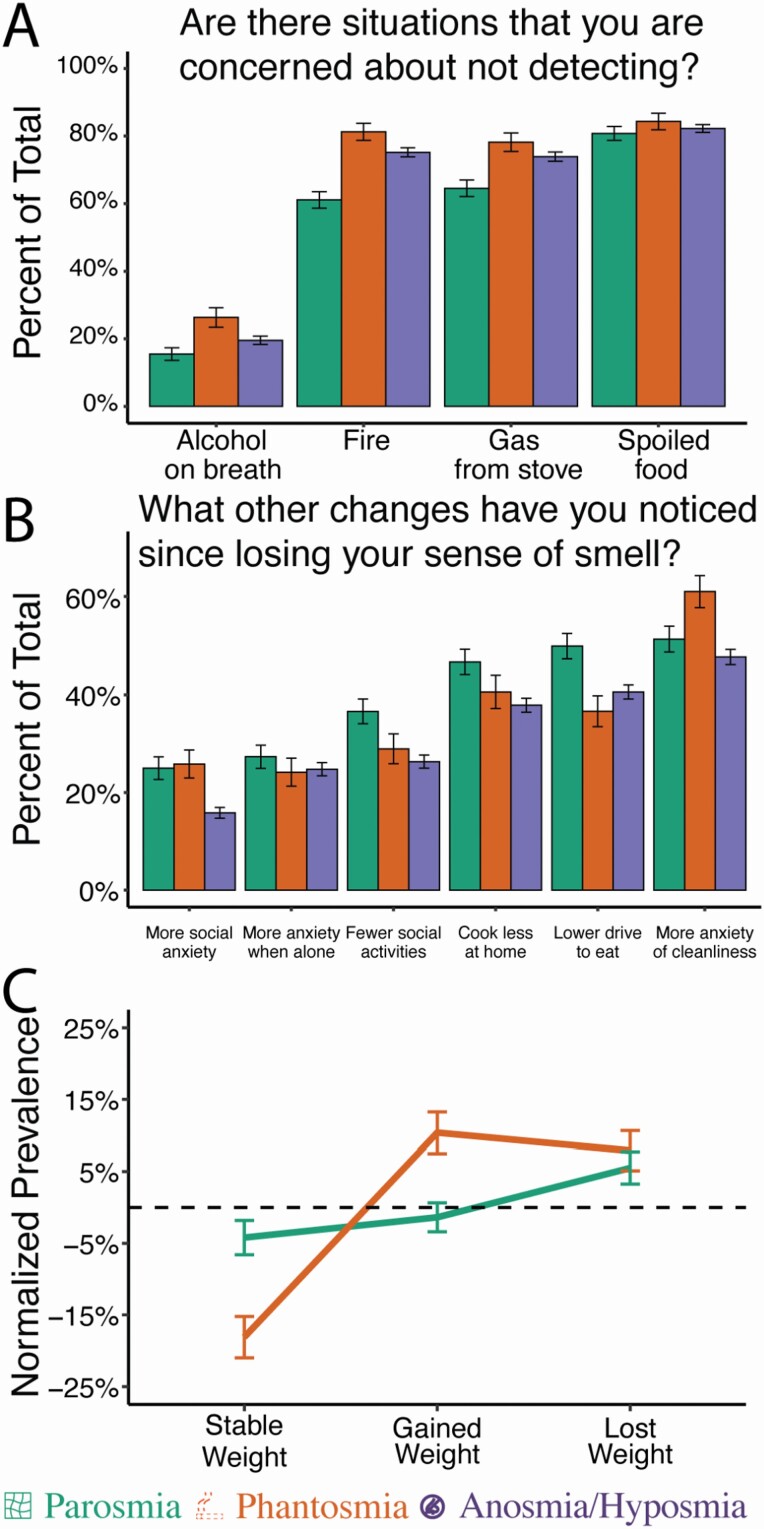
All olfactory disorders affect the overall quality of life, but each in different ways. Smell impaired individuals are concerned with failing to detect a hazard (>50%) such as spoiled food (82.2%) followed by fire (72.8%) and gas (72.3%) (Fig. 4A). Phantosmic and anosmic/hyposmic individuals showed a higher concern for failing to detect fire and gas than parosmic individuals.
Fig. 4.
Impacts on quality of life. Percentage of respondents (A) concerned about failing to detect common hazards and (B) reporting changes in common behaviors. (C) Differences in reports of weight fluctuation compared to the anosmia/hyposmia baseline. Error bars represent bootstrapped standard errors.
Other changes to quality of life include increased anxiety about being alone (25.2%), being in social settings (19.1%), cleanliness (50.4%), and cooking (40.2%) followed by a reported decrease in socializing (29.0%) and motivation to eat (42.1%) (Fig. 4B). Among olfactory disorders, there was a higher anxiety for cleanliness among those with phantosmia, whereas those with parosmia had a lower motivation to eat, cook, and socialize. Both olfactory disorders had higher reports of social anxiety than anosmia/hyposmia. Parosmic individuals also found it difficult to adjust to their disorder (χ 2 = 3.76, P < 0.001) which might be a result of its acute nature during recovery. Phantosmics reported changes in their weight, with some gaining and others losing weight since the onset of the disorder (χ 2 = 5.27, P < 0.001) (Fig. 4C). Intimacy was altered among 24% of respondents, but there were no differences across olfactory disorders (χ 2 = 5.40, P = 0.24).
Developing a severity score
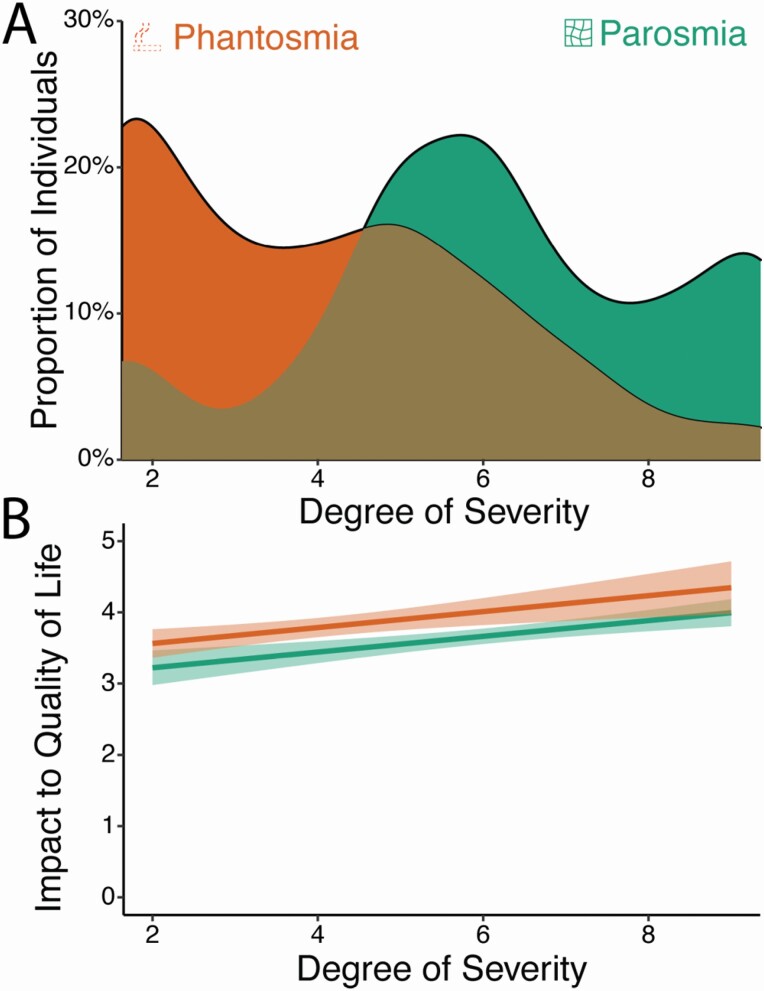
A single scale of severity from structured questions has proven to be a clinically useful measure for parosmia, and here we extend this idea to phantosmia (Landis et al. 2010). We combined the frequency and duration of distortion episodes to develop a severity score for both phantosmia and parosmia. Those with parosmia had higher severity scores than those with phantosmia (B = 1.96, t = 11.66, P < 0.001); Fig. 5A), and an increased severity score was inversely correlated with overall quality of life for both disorders (B = −0.39, t = 4.16, P < 0.001; Fig. 5B). More specifically, BMI trended toward a significant correlation with severity score for those with phantosmia (B = 0.05, t = 1.79, P = 0.07). As determined by the sentiment analysis, there was no relationship between severity score and negative emotions (B = −1.35, SE = 2.45, t = 0.55, P = 0.58), the type of emotion (F(3) = 0.02, P = 0.99), or overall sentiment (B = −0.53, SE = 0.49, t = 1.09, P = 0.28).
Fig. 5.
Degree of severity for parosmia and phantosmia. (A) Distribution of severity scores among parosmic and phantosmic groups. Higher scores represent more frequent and/or longer episodes. (B) The severity score correlates with the reported impact of the olfactory disorder on their quality of life. Error bands represent 95% confidence intervals. Error bars represent bootstrapped standard errors.
Discussion
To date, little attention has been given to parosmia and phantosmia—with studies often combining them rather than studying them separately. Our study reveals some distinct differences between parosmia and phantosmia, as well as from hyposmia/anosmia. They are common olfactory impairments, with half of the participants with smell dysfunction reporting these disorders. Both parosmia and phantosmia vary in severity and are distinct in terms of demographics, medical history, and quality of life issues. Our survey also suggests that parosmia and phantosmia have distinct underlying mechanisms.
Parosmia
Parosmia represents a distortion of smell when an odorous source is present. Instead of smells becoming weaker, as described in hyposmia/anosmia, they change in quality such that perceived smells are not the same as patients remember from before the onset of parosmia. In our survey, there is a distinct demographic that more commonly experiences parosmia—individuals who are younger, female, and recovering from a virus.
In general, there is a negative correlation between age and recovery from smell loss, such that losing smell at an older age results in slower recovery. One possibility is that parosmia is a symptom of recovery, and those who are older have a smaller chance of developing parosmia (Reden et al. 2006; London et al. 2008; Hummel and Lötsch 2010; Cavazzana et al. 2018; Ogawa et al. 2020). Supporting this idea, individuals in the early stages of recovery from smell loss who report parosmia also reported more improvement over time than those with either phantosmia or a simple reduction in smell. Others have reported this co-occurrence of parosmia through times of recovery (Nordin et al. 1996; Quint et al. 2001; Reden et al. 2007; Liu et al. 2020a). The presence of parosmia has indicated faster return to the sense of smell in some studies (Reden et al. 2007; Liu et al. 2020a), but not others (Hummel and Lötsch 2010). This discrepancy may be due to patient age, since older patients have reduced olfactory regenerative capacity (Mobley et al. 2014).
Past research has reported parosmia commonly occurs with olfactory loss due to viral infection and frequently resolves within a year of the incident, with only 26% of an initial parosmic patient sample (N = 112) having parosmia after 14 months (Nordin et al. 1996; Quint et al. 2001; Reden et al. 2007; Liu et al. 2020a). Similarly, in a study by Damm et al. (2014), 26% from a group of 47 initially parosmic patients reported no parosmia after an observation period of 4 months (Damm et al. 2014). Parosmia was the most prevalent outcome among post-viral disorders in our sample (nearly 90% of parosmics) while parosmia had the lowest prevalence among those suffering from head trauma or conductive loss etiologies (e.g. polyps). As mentioned, patients with parosmia also showed higher prevalence of the disorder after the initial incident (>3 months to 1 year), not during, and did not show issues with nasal patency.
Leading theories for parosmia suggest a peripheral origin of the disorder. Although these patients do show differences in neural activation (Iannilli et al. 2019), this might be a downstream effect. In fact, in hyposmic patients with parosmia, olfactory bulb volumes have been shown to be smaller compared to hyposmic patients without parosmia (Mueller et al. 2005; Rombaux et al. 2009). In neurogenesis, the axons of newly born sensory neurons must find the correct targets in the olfactory bulb. Abnormalities may occur during the process (Murai et al. 2016; Schwob et al. 2017), such that a sensory neuron tuned to one odor mistakenly stimulates an area of the bulb that signals the presence of a different odor. Axons reach the bulb approximately 1–3 months after injury, which matches the timing of parosmia in this survey. Taken together, our data support a peripheral cause of distortion that may result from a variety of mechanisms related to recovery such as differences across olfactory sensory neurons in time to recover or a mismatch in rewiring in the olfactory bulb. This is supported by animal models where olfactory maps significantly change after regeneration of ablated neurons, leading animals to have to relearn the correct odor match (Yee and Costanzo 1998), and this is most likely due to mistargeting by a receptor-defined subset of peripheral neurons (Christensen et al. 2001; Holbrook et al. 2005).
Parosmic patients showed higher disturbances to their social life, leading to an avoidance of social and eating activities. In comparison to hyposmia/anosmia, this did not lead to any associated behavioral outcomes that we measured, such as weight fluctuation, but more rigorous assessments are warranted (Mattes and Cowart 1994). For instance, we clearly show that individuals with parosmia are reminded of their disorder regularly, which has been hypothesized as a reason for greater disruption in daily life (Frasnelli and Hummel 2005; Hong et al. 2012; Croy et al. 2013). These patients also report more difficulty adjusting to their disorder, which may explain a recent report showing higher depression and anxiety symptoms in this patient group (Lecuyer Giguere et al. 2020).
The distortions experienced describe a common thread of sources (e.g. coffee) that has been reported in the literature, and there is little doubt that the terms used to describe these distortions generally have a negative valence associated with them (dirty, sewage, unpleasant, rotting, disgusting, sickly sweet, and vomit-inducing) (Keller and Malaspina 2013; Burges Watson et al. 2020; Parker et al. 2021b). Importantly, many culprit compounds causing negative distortions have a low detection threshold (Parker et al. 2021b). Another explanation for this negative valence toward distorted odors may be the low familiarity to odors activating unlearned neuronal mapping which may be similar to nonspecific electrical stimulation of the olfactory epithelium/olfactory bulb (Aronsohn 1884; Straschill et al. 1983; Kumar et al. 2012). Similarly, disordered input during recovery, either through lack of myelination causing cross-activation or disordered inhibitory wiring, are often perceived as unpleasant. Support for this theory comes from epilepsy research in which sporadic activation of the orbital frontal cortex leads to phantom smells that are mostly unpleasant, but also pleasant (Fox et al. 2018; Bérard et al. 2021). Although our question about distortions had a negative phrasing, “Which odors do you find particularly unpleasant and distorted? (Describe in as much detail as possible),” individuals still reported some positive changes. Looking at the positive and negative sentimental sentences, there seems to be a valence shift in which odors commonly perceived as positive are described negatively, but a few, usually related to body odors, shift from negative to positive. For instance, fecal smells may turn pleasant whereas coffee becomes unpleasant. One explanation for this shift from negative to positive is that some of the key aroma compounds responsible for the strong and usually repulsive smell of feces were not perceived at all by those with parosmia (Parker et al. 2021a). In the absence of these potent odors, other pleasant compounds may dominate the perception of the mixture. Additionally, as referenced before, some epilepsy patients receiving direct stimulation of the cortex experience pleasant phantom odors.
Phantosmia
Phantosmia is an olfactory experience when there is no odor source present. These phantom odors may be high or low in intensity and may be familiar or unfamiliar odors and cannot be perceived by others nearby. Unlike previous reports done at a population level (Sjölund et al. 2017; Bainbridge et al. 2018), in our sample females were not more prone to phantosmia (P = 0.78). This difference in findings may be due to previous studies categorizing parosmia and phantosmia together. For instance, a population-level study found females to be almost twice as likely to have phantosmia than men, but the group under study also reported they were 6 times more likely to have parosmia thus representing a heterogeneous group (Sjölund et al. 2017). However, our results do agree with previous findings regarding age, in which individuals between 40 and 60 years of age were more likely to have phantosmia than older individuals (>60 years) (Bainbridge et al. 2018).
Phantosmia was the most common olfactory disorder among those who suffered a head trauma. Phantosmic patients have previously been reported to have a history of head trauma (Leopold 2002; Sjölund et al. 2017), as well as psychiatric disorders (Frasnelli et al. 2004; Croy et al. 2013), temporal lobe epilepsy, and phantosmic episodes commonly preceding seizures, and migraines in the form of auras (Leopold 2002; Aiello and Hirsch 2013). Additionally, we show that phantosmic patients had more sinonasal diseases (e.g. polyps) and more blockage than those suffering from parosmia. This suggests that at least some of these phantom odors do not come from an odorous source, as airflow is needed to carry volatiles.
The mechanisms of phantosmia are largely unknown. Hallucinations in other senses can be due to overactive neurons, either peripheral or in the brain. Olfactory sensations can also result from temporal lobe seizure or direct stimulation of the olfactory bulb (R.N.DeJ 1954; Kumar et al. 2012; Bérard et al. 2021). Debilitating cases of phantosmia have been treated by the removal of the olfactory bulb (Kaufman et al. 1988; Markert et al. 1993), removal of the olfactory epithelium (Leopold et al. 1991, 2002), or unilateral blockage of the olfactory cleft (Liu et al. 2020b). The central or peripheral origin of phantosmia is unclear and may be heterogeneous across cases (Leopold 2002).
Phantosmic patients, compared to parosmics, reported worries about not being able to detect hazards (fire, gas) that might be noticed through smell. As previously discussed, phantosmics showed increased blockage and sinonasal diseases and this might decrease odor sensitivity to all odors, including hazards. Only a few (~20%) had recurring situations that triggered a phantom episode, describing these triggers as places, temporal, or cognitive events. Additionally, phantosmic patients reported more changes in weight, with individuals experiencing more severe phantoms having an increase in weight (measured by BMI). Fluctuation in appetite with olfactory dysfunction occurs due to its involvement in metabolic status (Guzmán-Ruiz et al. 2021), which could lead to changes in food preference (Pellegrino et al. 2020b) and weight (Kershaw and Mattes 2018). However, it is difficult to say whether phantoms are causal. For example, insulin-dependent diabetics, who often have a comorbidity of being overweight, were twice as likely to experience phantom odors (Chan et al. 2018). This may warrant additional studies to replicate our findings and delve into specific dietary changes and whether adiposity is related to a higher rate of smell phantoms.
Study limitations
Our study is based on cross-sectional data from a survey, therefore, the direction of associations among variables with time cannot be established and this may undermine our causal inference in recovery for parosmia patients. Longitudinal studies with this patient group should be done to confirm our results. In addition, respondents self-reported their olfactory disorder types. We provided clear definitions of each type of distorted disorder, but it is difficult to exclude the possibility that some participants did not understand the meaning of parosmia and phantosmia. Indeed, we have a large category of respondents reporting both parosmia and phantosmia that were not included in the analysis as these respondents did not fall into a separate group and were difficult to interpret. The group who reported both parosmia and phantosmia could be either those who experience distortions from both known and unknown sources, or those with a scenario where they recognize the source of their distortions, but for whom the distorted smell persists for hours or days after the stimulus has disappeared. Patients have reported this before as a “smell lock” and clinicians have referred to it as olfactory perseveration (Parker et al. 2021a). Lastly, we sampled from a smell loss group that has interest in their disorder (actively joining an interest group/charity) that might have prioritized severe cases relative to mild ones. There was also an overlap in our sampling times with the COVID-19 pandemic. Post-viral loss was the most prominent etiology in our sample—this might be due to our sampling times overlapping with the COVID-19 pandemic, where smell loss is a prominent symptom of the disease. This overshadowed other important etiologies from our analysis including smell disorders induced by chemotherapy, neurotoxicity, and neurodegenerative diseases. Thus, our results may not represent the typical patient population at large. We report that half of smell loss patients experience odor distortion—this should be considered a liberal estimate.
Conclusion
Two common symptoms of olfactory dysfunction, parosmia, and phantosmia, represent distinct conditions that, along with hyposmia and anosmia, have characteristic patterns of medical history, demographics, and impact on quality of life. They are not rare, with almost half our sample reporting symptoms, and cause additional distress typically after an initial olfactory dysfunction starts to resolve. The mechanisms for distinct features of these smell distortions should undergo consideration in the clinic and research setting. If parosmia relates to neurogenesis, what does the character of distortion tell us about the underlying population of recovered neurons? Similarly, if phantosmia is centrally caused what does this tell us about our perception of reality? Distortions among olfactory disorders may provide insight into the organization and function of the olfactory system.
Supplementary Material
Funding
Research conducted by (RP) and reported in this publication was supported by the National Institute on Deafness and other communication disorders of the National Institutes of Health under award number T32DC000014.
Conflict of interest
The authors declare no conflict of interest.
References
- Aiello SR, Hirsch AR. 2013. Phantosmia as a meteorological forecaster. Int J Biometeorol. 57(5):813–815. [DOI] [PubMed] [Google Scholar]
- Aronsohn E. 1884. Über elektrische Geruchsempfindung. Arch. f. Anat. u. Physiol./Phys. Abteil, b. 460. [Google Scholar]
- Bainbridge KE, Byrd-Clark D, Leopold D. 2018. Factors associated with phantom odor perception among US adults: findings from the national health and nutrition examination survey. JAMA Otolaryngol Head Neck Surg. 144(9):807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit K, Matsuo A. 2018. Package ‘spacyr’. R Package version 1.2.1. https://cran.r-project.org/web/packages/spacyr/index.html, 27 May 2021. [Google Scholar]
- Bérard N, Landis BN, Legrand L, Tyrand R, Grouiller F, Vulliémoz S, Momjian S, Boëx C. 2021. Electrical stimulation of the medial orbitofrontal cortex in humans elicits pleasant olfactory perceptions. Epilepsy Behav. 114(Pt A):107559. [DOI] [PubMed] [Google Scholar]
- Burges Watson DL, Campbell M, Hopkins C, Smith B, Kelly C, Deary V. 2020. Altered smell and taste: anosmia, parosmia and the impact of long covid-19. MedRxiv 20239152. 10.1101/2020.11.26.20239152, 30 November 2020, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzana A, Larsson M, Münch M, Hähner A, Hummel T. 2018. Postinfectious olfactory loss: a retrospective study on 791 patients. Laryngoscope. 128(1):10–15. [DOI] [PubMed] [Google Scholar]
- Chan JYK, García-Esquinas E, Ko OH, Tong MCF, Lin SY. 2018. The association between diabetes and olfactory function in adults. Chem Senses. 43(1):59–64. [DOI] [PubMed] [Google Scholar]
- Christensen MD, Holbrook EH, Costanzo RM, Schwob JE. 2001. Rhinotopy is disrupted during the re-innervation of the olfactory bulb that follows transection of the olfactory nerve. Chem Senses. 26(4):359–369. [DOI] [PubMed] [Google Scholar]
- Croy I, Yarina S, Hummel, T. 2013. Research letter enhanced parosmia and phantosmia in patients with severe depression. Psychol Med. 43(11):2460–2464. [DOI] [PubMed] [Google Scholar]
- Damm M, Pikart LK, Reimann H, Burkert S, Göktas Ö, Haxel B, Frey S, Charalampakis I, Beule A, Renner B, et al. 2014. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 124(4):826–831. [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV. 1997. Bootstrap methods and their application. In: Cambridge series of statistical and probabilistic mathematics. Vol. 1. Cambridge: Cambridge University Press. [Google Scholar]
- Fox KCR, Yih J, Raccah O, Pendekanti SL, Limbach LE, Maydan DD, Parvizi J. 2018. Changes in subjective experience elicited by direct stimulation of the human orbitofrontal cortex. Neurology. 91(16):e1519–e1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasnelli J, Hummel T. 2005. Olfactory dysfunction and daily life. Eur Arch Otorhinolaryngol. 262(3):231–235. [DOI] [PubMed] [Google Scholar]
- Frasnelli J, Landis BN, Heilmann S, Hauswald B, Hüttenbrink KB, Lacroix JS, Leopold DA, Hummel T. 2004. Clinical presentation of qualitative olfactory dysfunction. Eur Arch Otorhinolaryngol. 261(7):411–415. [DOI] [PubMed] [Google Scholar]
- Guzmán-Ruiz MA, Jiménez A, Cárdenas-Rivera A, Guerrero-Vargas NN, Organista-Juárez D, Guevara-Guzmán R. 2021. Regulation of metabolic health by an “olfactory-hypothalamic axis” and its possible implications for the development of therapeutic approaches for obesity and T2D. Cell Mol Neurobiol: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook EH, Leopold DA, Schwob JE. 2005. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope. 115(12):2144–2154. [DOI] [PubMed] [Google Scholar]
- Hong S-C, Holbrook EH, Leopold DA, Hummel T. 2012. Distorted olfactory perception: a systematic review. Acta Otolaryngol. 132(sup1), S27–S31. [DOI] [PubMed] [Google Scholar]
- Hummel T, Lötsch J. 2010. Prognostic factors of olfactory dysfunction. Arch Otolaryngol Head Neck Surg. 136(4):347–351. [DOI] [PubMed] [Google Scholar]
- Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM, Damm M, Frasnelli J, Gudziol H, Gupta N, et al. 2017. Position paper on olfactory dysfunction. Rhinol Suppl. 54(26):1–30. [DOI] [PubMed] [Google Scholar]
- Iannilli E, Leopold DA, Hornung DE, Hummel T. 2019. Advances in understanding parosmia: an fMRI study. ORL J Otorhinolaryngol Relat Spec. 81(4):185–192. [DOI] [PubMed] [Google Scholar]
- Kaufman MD, Lassiter KR, Shenoy BV. 1988. Paroxysmal unilateral dysosmia: a cured patient. Ann Neurol. 24(3):450–451. [DOI] [PubMed] [Google Scholar]
- Keller A, Malaspina D. 2013. Hidden consequences of olfactory dysfunction: a patient report series. BMC Ear Nose Throat Disord. 13(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw JC, Mattes RD. 2018. Nutrition and taste and smell dysfunction. World J Otorhinolaryngol Head Neck Surg. 4(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Juhász C, Sood S, Asano E. 2012. Olfactory hallucinations elicited by electrical stimulation via subdural electrodes: effects of direct stimulation of olfactory bulb and tract. Epilepsy Behav. 24(2):264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis BN, Frasnelli J, Croy I, Hummel T. 2010. Evaluating the clinical usefulness of structured questions in parosmia assessment. Laryngoscope. 120(8):1707–1713. [DOI] [PubMed] [Google Scholar]
- Landis BN, Frasnelli J, Hummel T. 2006. Euosmia: a rare form of parosmia. Acta Otolaryngol. 126(1):101–103. [DOI] [PubMed] [Google Scholar]
- Lecuyer Giguere F, Jobin B, Robert J, Bastien L, Giguère J-F, De Beaumont L, de Guise E, Frasnelli J. 2020. Early parosmia signs and affective states predict depression and anxiety symptoms 6 months after a mild traumatic brain injury. Chem Senses. 45(6):483–490. [DOI] [PubMed] [Google Scholar]
- Leopold D. 2002. Distortion of olfactory perception: diagnosis and treatment. Chem Senses. 27(7):611–615. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Loehrl TA, Schwob JE. 2002. Long-term follow-up of surgically treated phantosmia. Arch Otolaryngol Head Neck Surg. 128(6):642–647. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Schwob JE, Youngentob SL, Hornung DE, Wright HN, Mozell MM. 1991. Successful treatment of phantosmia with preservation of olfaction. Arch Otolaryngol Head Neck Surg. 117(12):1402–1406. [DOI] [PubMed] [Google Scholar]
- Liu DT, Sabha M, Damm M, Philpott C, Oleszkiewicz A, Hähner A, Hummel T. 2020a. Parosmia is associated with re levant olfactory recovery after olfactory training. Laryngoscope. 131(3):618–623. [DOI] [PubMed] [Google Scholar]
- Liu J, Pinheiro-Neto CD, Zhao J, Chen Z, Wang Y. 2020b. A novel surgical treatment for long lasting unilateral peripheral parosmia: olfactory cleft blocking technique. Auris Nasus Larynx. 48(6):1209–1213. [DOI] [PubMed] [Google Scholar]
- London B, Nabet B, Fisher AR, White B, Sammel MD, Doty RL. 2008. Predictors of prognosis in patients with olfactory disturbance. Ann Neurol. 63(2):159–166. [DOI] [PubMed] [Google Scholar]
- Markert JM, Hartshorn DO, Farhat SM. 1993. Paroxysmal bilateral dysosmia treated by resection of the olfactory bulbs. Surg Neurol. 40(2):160–163. [DOI] [PubMed] [Google Scholar]
- Mattes RD, Cowart BJ. 1994. Dietary assessment of patients with chemosensory disorders. J Am Diet Assoc. 94(1):50–56. [DOI] [PubMed] [Google Scholar]
- Mobley AS, Rodriguez-Gil DJ, Imamura F, Greer CA. 2014. Aging in the olfactory system. Trends Neurosci. 37(2):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A, Rodewald A, Reden J, Gerber J, von Kummer R, Hummel T. 2005. Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. Neuroreport. 16(5):475–478. [DOI] [PubMed] [Google Scholar]
- Murai A, Iwata R, Fujimoto S, Aihara S, Tsuboi A, Muroyama Y, Saito T, Nishizaki K, Imai T. 2016. Distorted coarse axon targeting and reduced dendrite connectivity underlie dysosmia after olfactory axon injury. eNeuro. 3(5):ENEURO.0242-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin S, Murphy C, Davidson TM, Quiñonez C, Jalowayski AA, Ellison DW. 1996. Prevalence and assessment of qualitative olfactory dysfunction in different age groups. Laryngoscope. 106(6):739–744. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Nakamura K, Yamamoto S, Tojima I, Shimizu T. 2020. Recovery over time and prognostic factors in treated patients with post-infectious olfactory dysfunction: a retrospective study. Ann Otol Rhinol Laryngol. 129(10):977–982. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. 2000. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 97(2–3):153–164. [DOI] [PubMed] [Google Scholar]
- Oleszkiewicz A, Hummel T. 2019. Whose nose does not know? Demographical characterization of people unaware of anosmia. Eur Arch Otorhinolaryngol. 276(6):1849–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszkiewicz A, Kunkel F, Larsson M, Hummel T. 2020. Consequences of undetected olfactory loss for human chemosensory communication and well-being. Philos Trans R Soc Lond B Biol Sci. 375(1800):20190265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. 2019. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 276(3):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms J. 2020. hunspell: high-performance stemmer, tokenizer, and spell checker (3.0.1) [Computer software]. https://cran.r-project.org/web/packages/hunspell/index.html, 15 June 2021. [Google Scholar]
- Parker JK, Kelly CE, Gane SB. 2021a. Molecular mechanism of parosmia. MedRxiv 21251085. 10.1101/2021.02.05.21251085, 8 February 2021, preprint: not peer reviewed. [DOI] [Google Scholar]
- Parker JK, Kelly CE, Smith B, Hopkins C, Gane SB. 2021b. An analysis of patients’ perspectives on qualitative olfactory dysfunction using social media. MedRxiv 20249029. 10.1101/2020.12.30.20249029, 4 January 2021, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, et al. ; GCCR Group Author . 2020. More than smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 45(7):609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino R, Cooper KW, Di Pizio A, Joseph PV, Bhutani S, Parma V. 2020a. Coronaviruses and the chemical senses: past, present, and future. Chem Senses. 45(6):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino R, Hummel T, Emrich R, Chandra R, Turner J, Trone T, Dorminy C, Luckett CR. 2020b. Cultural determinants of food attitudes in anosmic patients. Appetite. 147(1):104563. [DOI] [PubMed] [Google Scholar]
- Pennec E, Slowikowski K. 2018. ggwordcloud: a word cloud geom for ‘ggplot2’. R Package Version 0.3.0 [Computer software] [accessed 2020 Sep 3]. https://cran.r-project.org/package=ggwordcloud. [Google Scholar]
- Quint C, Temmel AF, Schickinger B, Pabinger S, Ramberger P, Hummel T. 2001. Patterns of non-conductive olfactory disorders in eastern Austria: a study of 120 patients from the Department of Otorhinolaryngology at the University of Vienna. Wien Klin Wochenschr. 113(1–2):52–57. [PubMed] [Google Scholar]
- Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB. 2016. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses. 41(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reden J, Maroldt H, Fritz A, Zahnert T, Hummel T. 2007. A study on the prognostic significance of qualitative olfactory dysfunction. Eur Arch Otorhinolaryngol. 264(2):139–144. [DOI] [PubMed] [Google Scholar]
- Reden J, Mueller A, Mueller C, Konstantinidis I, Frasnelli J, Landis BN, Hummel T. 2006. Recovery of olfactory function following closed head injury or infections of the upper respiratory tract. Arch Otolaryngol Head Neck Surg. 132(3):265–269. [DOI] [PubMed] [Google Scholar]
- Revelle WR. 2017. psych: procedures for personality and psychological research.https://www.scholars.northwestern.edu/en/publications/psych-procedures-for-personality-and-psychological-research, 25 May 2021.
- Rinker TW. 2019. sentimentr: calculate text polarity sentiment. R Package Version 2.7.1. https://cran.r-project.org/web/packages/sentimentr/sentimentr.pdf, 27 May 2021. [Google Scholar]
- R.N.DeJ . 1954. Epilepsy and the functional anatomy of the human brain. Neurology. 4(6):483–483. [Google Scholar]
- Rombaux P, Duprez T, Hummel T. 2009. Olfactory bulb volume in the clinical assessment of olfactory dysfunction. Rhinology. 47(1):3–9. [PubMed] [Google Scholar]
- Schwob JE, Jang W, Holbrook EH, Lin B, Herrick DB, Peterson JN, Hewitt Coleman J. 2017. Stem and progenitor cells of the mammalian olfactory epithelium: taking poietic license. J Comp Neurol. 525(4):1034–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund S, Larsson M, Olofsson JK, Seubert J, Laukka EJ. 2017. Phantom smells: prevalence and correlates in a population-based sample of older adults. Chem Senses. 42(4):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straschill M, Stahl H, Gorkisch K. 1983. Effects of electrical stimulation of the human olfactory mucosa. Appl Neurophysiol. 46(5–6):286–289. [DOI] [PubMed] [Google Scholar]
- Yee KK, Costanzo RM. 1998. Changes in odor quality discrimination following recovery from olfactory nerve transection. Chem Senses. 23(5):513–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.