Abstract
Zijian Feng and colleagues argue that sustained elimination of SARS-CoV-2 in China offers flexibility in covid-19 vaccination policy and discuss the anticipated challenges and systematic monitoring necessary to keep the immunisation component of the response on track
The epidemiology of covid-19 in China is unusual but not unique. SARS-CoV-2 was contained by April 2020 through a population response comprising non-pharmaceutical interventions.1 The overarching policy adopted from the start of the epidemic was (and remains) “zero tolerance for local transmission.” Although successful and able to be sustained for 18 months with only two post-containment covid-19 deaths (fig 1), zero tolerance has major implications for the maintenance of elimination and for China’s covid-19 vaccination strategies.
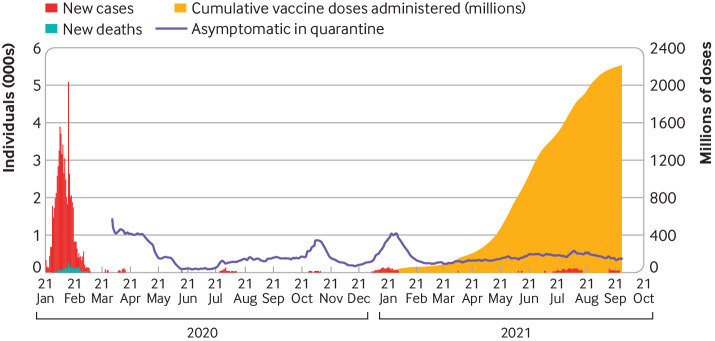
Fig 1.
Daily reports of covid-19 cases, deaths, asymptomatic cases, asymptomatic cases in quarantine (most of whom were diagnosed on PCR testing on entering China after negative testing abroad), and vaccine doses administered in China from January 2020 to September 2021. Source: Chinese Center for Disease Control and Prevention Weekly, National Health Commission, and weekly.chinacdc.cn/news/TrackingtheEpidemic.htm
The goal of China’s covid-19 response has always been to minimise morbidity, mortality, and socioeconomic damage—reflecting the values of human health, unimpeded societal functioning, and economic health. Carefully monitored use of the new covid-19 vaccines to build population immunity and protect people is integral to China’s response. We describe analyses of the epidemiological situation in China that are important for vaccination strategy and discuss the challenges and systematic monitoring necessary to keep the immunisation component of the response on track.
Analysis
Three characteristics of the post-containment epidemiology of SARS-CoV-2 inform vaccination strategy: total population susceptibility; effective but imperfect protection from imported virus through an international border quarantine programme; and a SARS-CoV-2 effective reproduction number (Rt) greater than 1, despite routine use of individual, environmental, and population non-pharmaceutical interventions.
Representative serological surveys taken one month after containment showed that 4.4% of Wuhan’s population, 0.4% of Hubei’s population outside of Wuhan; and less than 0.1% of the rest of China’s population had been infected, indicating that over 99.9% of the population was susceptible to SARS-CoV-2.2
Every month, border quarantine stops over 700 infected people from entering China, but the process is imperfect—more than 20 outbreaks were caused by imported virus in 2020.3 Although stopped while small (6 to 1064 cases), each outbreak required enormous effort to contain, including test and trace, smartphone identification of close contacts, quarantine of contacts, and isolated management of infected people; some outbreaks also required total population PCR screening to identify asymptomatic infections.3
This huge effort shows that routinely used non-pharmaceutical interventions such as masks, physical distancing, travel restrictions, and hand hygiene are inadequate to stop community transmission, so Rt remained >1 in China in the period after containment but before vaccination.
Strategy
Experience with other diseases that are preventable by vaccines tells us that only a major vaccination effort can make it safe to lift the zero tolerance policy without substantial loss of life and societal damage. Emerging variants may be able to break through border protection more easily, transmit more efficiently, cause more severe illness, or even escape vaccine induced immunity.4 Containment bought China precious time to strengthen its non-pharmaceutical interventions and develop vaccines, but it needed to be consolidated with vaccination as soon as possible.
In July 2020, after three vaccines (two inactivated and one adenovirus) were approved for overseas phase III clinical trials, China’s regulatory authority allowed their emergency use in mainland China (fig 2). Only people in occupations at risk of infection were vaccinated, such as healthcare workers in direct contact with patients and people working overseas. After successful phase III trials, conditional market authorisation was granted at the end of 2020, starting a large, carefully sequenced vaccination effort.5 As global public goods, the approved vaccines are also exported to meet overseas commitments as well as being used domestically.
Fig 2.
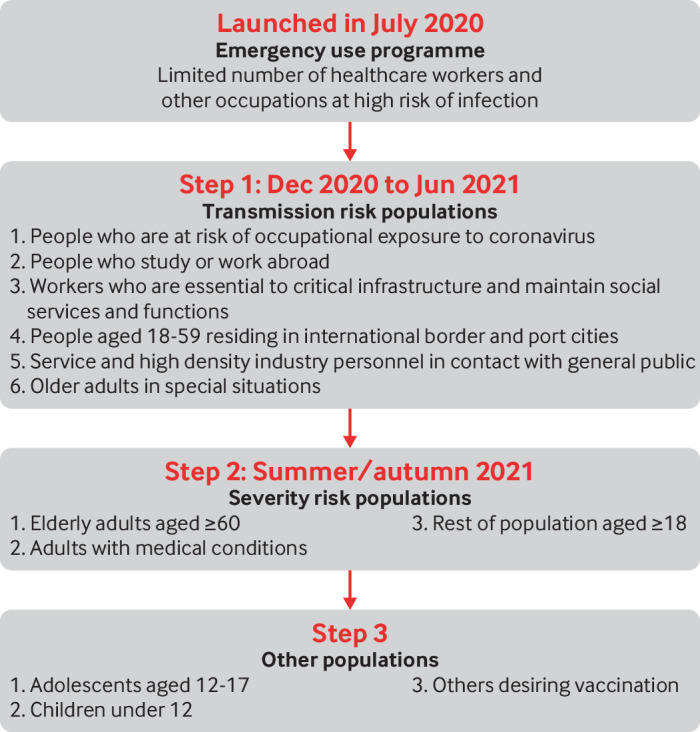
Covid-19 vaccination strategy in China. Shown are target populations and stages of vaccination starting with the emergency programme and expanding to large populations after covid-19 vaccines were granted conditional approval
There are three epidemiologically distinct but partially overlapping populations to consider in the vaccination strategy: people at occupational or residential risk of SARS-CoV-2 infection, people at increased risk of serious illness if infected, and people at risk of transmitting SARS-CoV-2 once infected.
People at occupational risk of infection and workers essential to maintaining social functions were vaccinated in the first quarter of 2021. The first large population to be vaccinated consisted of working age adults in broadly defined border communities—cities with international airports or ports and provinces with international borders, workers in service and high density industries, and students and staff in universities. This group is the most vulnerable to importation outbreaks and is at the highest risk of transmission to others if infected; people in this group were vaccinated by June 2021 in a time of increasing vaccine supply. The remaining working age adults and older adults rounded out the second stage of the campaign, which was completed in September 2021. Children aged 18 years and under complete the third stage, starting from adolescents aged 12-17 (who have been vaccinated) and working down in age (currently under way) (fig 2).
This sequencing is different from that recommended by the World Health Organization for countries with community transmission, in that people at the greatest risk of severe illness were not targeted immediately after healthcare workers.6 Having zero tolerance for local transmission effectively protects older adults, people with comorbidities, and children in the first, second, and third stages of the campaign. Vaccinating working age adults protects those who make society function and those who can more readily transmit the virus. After adults are vaccinated, children will be the only wholly susceptible population, and emerging SARS-CoV-2 variants could cause severe illness in children. Three covid-19 inactivated vaccines are approved for children aged 3 years and older. Although a decision for children under 12 has not been made yet, they are certain to follow adults in China’s vaccination campaign.
Strategy evaluation
The vaccination campaign is concurrent with the zero tolerance policy—how can policy makers determine whether the vaccination strategy is working in a country with little local transmission?
China relies on widespread, routine PCR testing in medical facilities, workplaces, environmental surfaces, and other accessible places to identify infections.3 To ensure that no infections or outbreaks are missed, the Protocol for Prevention and Control of Covid-19,7 has been frequently updated to incorporate increasingly sensitive population and environmental testing strategies.
If the sequencing strategy is working, deaths and hospital admissions from covid-19 should remain at or near zero. Vaccination of those at occupational risk should strengthen border quarantine protection, making importations less frequent. In contrast to the 20 importations in the last eight months of 2020, there have been four in the first eight months of 2021 (in Yunnan, Guangdong, Jiangsu, and Yunnan again). Measured Rt should fall as population immunity is built,8 and when we have identified laboratory correlates of protection, serological surveys will be useful for determining population protection. After the zero tolerance policy is lifted, importation and transmission will occur, but hospital admissions, severe illnesses, and deaths should remain low.
Vaccine evaluation
Vaccine performance and the vaccination campaign must be monitored closely. Phase III clinical trials showed vaccine efficacies in WHO’s acceptable range, but too few participants had comorbidities or were older. Given ethical problems and the impracticalities of conducting placebo controlled trials when vaccines are widely available, real world evaluation is essential to fill evidence gaps.
Vaccine effectiveness against current and emerging SARS-CoV-2 variants can be assessed overseas9 and in China. Vaccine effectiveness against imported variants that escape border quarantine can be assessed in close contacts, 10 as was done in Guangdong, showing 70% effectiveness against the delta variant.11 We don’t yet know how well China’s covid-19 vaccines prevent transmission of SARS-CoV-2. As with routine childhood vaccines, some indirect protection will likely be seen with many covid-19 vaccines; evidence is emerging.12 Optimal dosing is yet to be determined—specifically whether a third dose will be needed for effective and longer term protection.14
Vaccine safety is monitored by the Chinese Center for Disease Control and Prevention and the National Center for Adverse Drug Reaction Monitoring using a passive adverse events system that was established 15 years ago.15 A characteristic of the system is that all serious adverse events are evaluated for causality. For covid-19 vaccines, the system was modified to identify adverse events of special interest, and active vaccine safety is being pilot tested.16 To date, no serious safety problem has been identified beyond severe allergic reactions that are associated with all vaccines.
Lifting “zero tolerance”
China currently has no deadline for lifting the zero tolerance policy. One goal of building population immunity is to create an epidemiological environment in which it is safe to relax or retire some non-pharmaceutical interventions—especially international border quarantine. Before we do that, we need to know the conditions under which unknowingly infected people can enter communities without causing loss of epidemic control and what levels of coverage immunity (and in which populations) are needed so that SARS-CoV-2 transmission can occur without causing significant morbidity and mortality. Trial and error is one way to find out, but a better way is mathematical transmission models that use vaccine performance characteristics, local vaccine coverage, and demographic data.8 Models can be refined with real world experience. Vaccination coverage is known and monitored in detail in China, making it feasible to construct high resolution maps of Rt and the consequences of spread to help policy makers safely retire non-pharmaceutical interventions.
Building population immunity with vaccines requires a high degree of compliance with vaccination recommendations, which is challenged by vaccine hesitancy or refusal due to complacency about low risk of infection or concerns about vaccines. China does not have vaccine mandates, but vaccination is considered by law to be a shared duty between the public and the government. Before any covid-19 vaccines were approved, China had the highest rate of vaccine demand among 19 large countries with covid-19 epidemics.17 But confidence is volatile, potentially changing with external events.18 Articulating clearly why it is important to get vaccinated and that vaccine safety and effectiveness are monitored carefully will help sustain confidence. Vaccination is enabled by a strong local public health workforce,19 making vaccines readily and freely available for all. Incentives, such as vaccine passports, are not currently national policy, but requiring proof of vaccination is becoming more common and may help attain and sustain immunity.
A corollary to Walt Orenstein’s admonition that “vaccines don’t save lives; vaccinations save lives”20 is that vaccines don’t build population immunity; vaccination does. As at 26 September 2021, over 2.2 billion doses of covid-19 vaccine had been administered in China (fig 1), fully vaccinating over 70% of the population. The challenges of this campaign are many (box 1), but consolidating containment with vaccine induced population immunity and sustaining protective immunity using emerging scientific evidence to adjust vaccines, vaccination schedules, policy, and the vaccination programme is a key part of China’s path out of the pandemic.
Box 1. Challenges of China’s vaccination campaign.
Inability to conduct large vaccine effectiveness studies in China owing to little local transmission
Deciding when it is safe to stop the international border quarantine programme
Ensuring that emerging SARS-CoV-2 variants do not escape vaccines or non-pharmaceutical interventions
Sustaining confidence in a lengthy vaccination campaign
Key messages.
Elimination of SARS-CoV-2 from China is providing a window of time to consolidate containment by building population immunity through vaccination
China’s population was virtually entirely susceptible after containment, making this window of protection fragile and dependent on continued strong use of non-pharmaceutical interventions while building immunity
Premature lifting of non-pharmaceutical interventions invites loss of epidemic control and morbidity and mortality from covid-19, especially among elderly people and those with comorbidities
Sustained containment allows flexibility to logically sequence target populations for building immunity and to ultimately lift the policy of “zero tolerance for local transmission” with minimal covid-19 morbidity and mortality
Assessing real world vaccine performance for updating policy and vaccines is reliant, in part, on overseas studies owing to the paucity of domestic infections
Acknowledgments
We thank Li-Ming Li and Jin-Ling Tang for their insightful comments and suggestions and Yuanqiu Li for figure 2.
Contributors and sources: ZJF is deputy director general of the China Center for Disease Control and Prevention over emergency response and immunisation and is the lead of its technical working group on covid-19 vaccines for China’s National Immunization Advisory Committee. ZDY is deputy working group lead; ZJA, FZW, and LR are working group members. ZJF conceived the idea for this manuscript and led development of strategies, policies, and plans for vaccination described in the manuscript. Epidemiologist and research scientist AP contributed strategies for people with comorbidities. LR, ZJA, and AP wrote the first draft of the manuscript; all authors contributed and approved the submitted manuscript. ZJF is the guarantor for the manuscript.
Patient involvement: No patients were involved in the work presented in this manuscript.
Competing interests: We have read and understood BMJ policy on declaration of interests and have no interests to declare.
This article is part of a collection proposed by the Peking University Centre for Public Health and Epidemic Preparedness and Response. Open access fees were funded by individual institutions. The BMJ commissioned, peer reviewed, edited, and made the decision to publish these articles. Li-Ming Li advised on commissioning for this collection. Jin-Lign Tang, Di Wang, and Kamran Abbasi were the lead editors for The BMJ.
References
- 1. Zhou L, Wu Z, Li Z, et al. One hundred days of coronavirus disease 2019 prevention and control in China. Clin Infect Dis 2021;72:332-9. 10.1093/cid/ciaa725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Z, Guan X, Mao N, et al. Antibody seroprevalence in the epicenter Wuhan, Hubei, and six selected provinces after containment of the first epidemic wave of covid-19 in China. Lancet Reg Health West Pac 2021;8:100094. 10.1016/j.lanwpc.2021.100094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Z, Liu F, Cui J, et al. Comprehensive large-scale nucleic acid-testing strategies support China’s sustained containment of covid-19. Nat Med 2021;27:740-2. 10.1038/s41591-021-01308-7 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
- 5.Covid-19 Vaccines Technical Working Group. Technical vaccination recommendations for covid-19 vaccines in China (first edition). China CDC Weekly 2021;3:459-461. 10.46234/ccdcw2021.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO SAGE roadmap for prioritizing uses of covid-19 vaccines in the context of limited supply. https://www.who.int/publications/i/item/who-sage-roadmap-for-prioritizing-uses-of-covid-19-vaccines-in-the-context-of-limited-supply.
- 7. Liu FF, Zheng CJ, Wang LP, et al. Interpretation of the protocol for prevention and control of covid-19 in China (edition 8). China CDC Weekly 2021;3:527-530. 10.46234/ccdcw2021.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan A, Liu L, Wang C, et al. Association of public health interventions with the epidemiology of the covid-19 outbreak in Wuhan, China. JAMA 2020;323:1915-23. 10.1001/jama.2020.6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jara A, Undurraga EA, González C, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med 2021;385:875-84 . 10.1056/NEJMoa2107715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Liu J, Yang Z, et al. Transmission of severe acute respiratory syndrome coronavirus 2 to close contacts, China, January-February 2020. Emerg Infect Dis 2021;27:2288-93. 10.3201/eid2709.202035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kang M, Yi Y, Li Y, et al. Effectiveness of inactivated covid-19 vaccines against covid-19 pneumonia and severe illness caused by the B.1.617.2 (delta) variant: evidence from an outbreak in Guangdong, China. SSRN 2021:3895639 [Preprint]. 10.2139/ssrn.3895639. [DOI]
- 12. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021;384:1412-23. 10.1056/NEJMoa2101765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Strategic advisory group of experts on immunization. Covid-19 vaccines technical documents. https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization/covid-19-materials.
- 14. Li MJ, Yang J, Wang L, et al. A booster dose is immunogenic and will be needed for older adults who have completed two doses vaccination with CoronaVac: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. medRxiv 2021; 10.1101/2021.08.03.21261544. [DOI]
- 15. Liu D, Wu W, Li K, et al. Surveillance of adverse events following immunization in China: Past, present, and future. Vaccine 2015;33:4041-6. 10.1016/j.vaccine.2015.04.060 [DOI] [PubMed] [Google Scholar]
- 16. Liu Z, Meng R, Yang Y, et al. Progress of active surveillance for vaccine safety in China. China CDC Wkly 2021;3:581-3. 10.46234/ccdcw2021.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med 2021;27:225-8. 10.1038/s41591-020-1124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Larson HJ, Broniatowski DA. Volatility of vaccine confidence. Science 2021;371:1289. 10.1126/science.abi6488 [DOI] [PubMed] [Google Scholar]
- 19. Li Z, Gao GF. Strengthening public health at the community-level in China. Lancet Public Health 2020;5:e629-30. . 10.1016/S2468-2667(20)30266-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orenstein W. Vaccines don’t save lives. Vaccinations save lives. Hum Vaccin Immunother 2019;15:2786-9. 10.1080/21645515.2019.1682360 [DOI] [PMC free article] [PubMed] [Google Scholar]