Abstract
Objective
To evaluate the efficacy of financial incentives dependent on continuous smoking abstinence on smoking cessation and birth outcomes among pregnant smokers.
Design
Single blind, randomised controlled trial.
Setting
Financial Incentive for Smoking Cessation in Pregnancy (FISCP) trial in 18 maternity wards in France.
Participants
460 pregnant smokers aged at least 18 years who smoked ≥5 cigarettes/day or ≥3 roll-your-own cigarettes/day and had a pregnancy gestation of <18 weeks were randomised to a financial incentives group (n=231) or a control group (n=229).
Interventions
Participants in the financial incentives group received a voucher equivalent to €20 (£17; $23), and further progressively increasing vouchers at each study visit if they remained abstinent. Participants in the control group received no financial incentive for abstinence. All participants received a €20 show-up fee at each of six visits.
Main outcome measures
The main outcome measure was continuous smoking abstinence from the first post-quit date visit to visit 6, before delivery. Secondary outcomes in the mothers were point prevalence abstinence, time to smoking relapse, withdrawal symptoms, blood pressure, and alcohol and cannabis use in past 30 days. Secondary outcomes in the babies were gestational age at birth, birth characteristics (birth weight, length, head circumference, Apgar score), and a poor neonatal outcome—a composite measure of transfer to the neonatal unit, congenital malformation, convulsions, or perinatal death.
Results
Mean age was 29 years. In the financial incentives and control groups, respectively, 137 (59%) and 148 (65%) were employed, 163 (71%) and 171 (75%) were in a relationship, and 41 (18%) and 31 (13%) were married. The participants had smoked a median of 60 cigarettes in the past seven days. The continuous abstinence rate was significantly higher in the financial incentives group (16%, 38/231) than control group (7%, 17/229): odds ratio 2.45 (95% confidence interval 1.34 to 4.49), P=0.004). The point prevalence abstinence rate was higher (4.61, 1.41 to 15.01, P=0.011), the median time to relapse was longer (visit 5 (interquartile range 3-6) and visit 4 (3-6), P<0.001)), and craving for tobacco was lower (β=−1.81, 95% confidence interval −3.55 to −0.08, P=0.04) in the financial incentives group than control group. Financial incentives were associated with a 7% reduction in the risk of a poor neonatal outcome: 4 babies (2%) in the financial incentives group and 18 babies (9%) in the control group: mean difference 14 (95% confidence interval 5 to 23), P=0.003. Post hoc analyses suggested that more babies in the financial incentives group had birth weights ≥2500 g than in the control group: unadjusted odds ratio 1.95 (95% confidence interval 0.99 to 3.85), P=0.055; sex adjusted odds ratio 2.05 (1.03 to 4.10), P=0.041; and sex and prematurity adjusted odds ratio 2.06 (0.90 to 4.71), P=0.086. As these are post hoc analyses, the results should be interpreted with caution.
Conclusions
Financial incentives to reward smoking abstinence compared with no financial incentives were associated with an increased abstinence rate in pregnant smokers. Financial incentives dependent on smoking abstinence could be implemented as a safe and effective intervention to help pregnant smokers quit smoking.
Trial registration
ClinicalTrials.gov NCT02606227.
Introduction
Maternal smoking during pregnancy is an avoidable risk factor for negative pregnancy and birth outcomes and could have negative health effects on children who are exposed in utero to tobacco smoking.1 2 3
Smoking cessation is critical in preventing smoking associated risks in pregnancy and negative birth outcomes.4 The US Preventive Services Task Force concluded that the benefit of behavioural interventions for smoking cessation is substantial but that the evidence of the benefit and harm of pharmacotherapy interventions is insufficient.5 In a recent Cochrane review, nicotine replacement therapy was found to increase smoking cessation rates in late pregnancy, but this evidence is of low certainty as the effect was not found when non-placebo controlled randomised controlled trials were excluded from the analysis.6 Evidence that nicotine replacement therapy has an impact on birth outcomes, particularly on birth weight,6 is lacking. New treatment options should be researched to help pregnant smokers quit.4
A new research area is using financial incentives to treat addictions based on the probability that providing incentives or reinforcers increase the chance of drug avoiding behaviour. Previous trials have suggested that financial incentives might be a promising intervention to help pregnant smokers quit smoking7 8 9 10 11 12 13; however, this therapeutic approach has not been implemented in clinical practice probably because of the large variability of settings, types of interventions, and lack of conclusive, pivotal studies.
We assessed the efficacy of progressively higher financial incentives dependent on smoking cessation on continuous smoking abstinence among pregnant smokers. Secondary aims were to assess the efficacy of financial incentives on point prevalence smoking abstinence, maternal craving for tobacco, tobacco withdrawal symptoms, weight, blood pressure, and birth outcomes.
Methods
Trial design and participants
The Financial Incentive for Smoking Cessation in Pregnancy (FISCP) trial was a randomised, two parallel groups, trial conducted in 18 maternity wards in France.14 Pregnant smokers were included if they were 18 years or older, smoked ≥5 cigarettes/day or ≥3 roll-your-own cigarettes/day, had a gestation of <18 weeks, were motivated to quit smoking (scored >5 on a visual analogue scale ranging from 0 for not at all to 10 for extremely motivated), were affiliated to the social health insurance system as required by the French law on biomedical research, and had signed the informed written consent and agreed to the collection of the birth characteristics of their offspring.
Exclusion criteria included current treatment for a chronic psychiatric disorder using neuroleptics, antidepressants, or anxiolytics, use of tobacco products other than cigarettes, use of either bupropion or varenicline, which is contraindicated in pregnancy, and already participating in a biomedical research project. We excluded electronic cigarette users as little is known about the benefit:risk ratio of these devices. Multiple pregnancy was not an exclusion criterion.
Randomisation
A statistician independent of the study prepared a computer generated randomisation list in blocks of 4. The individual randomisation list by centre was incorporated into the electronic case report form. After inclusion and exclusion criteria had been checked and written informed consent obtained, a randomisation number was allocated to the participant at the first visit. Participants and investigators were blinded to assignment group at randomisation but not at subsequent visits.
Interventions
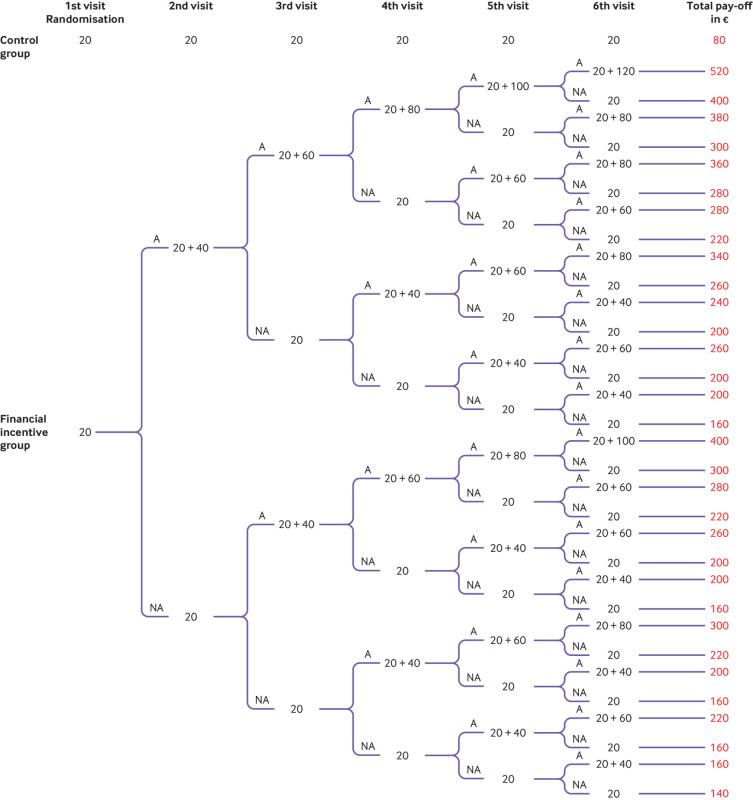
Fig 1 depicts the pay-off tree (€) showing what participants could earn according to their assignment group and abstinence, under the assumption that they completed six visits.
Fig 1.
Pay-off tree showing what participants could earn according to group assignment and smoking abstinence status under the assumption that they completed six visits. At the first visit, participants were randomised to either the financial incentives group or the control group if they agreed to take part in the study. All participants received a €20 (£17; $23) voucher at the end of the randomisation visit. A=abstinent; NA=not abstinent
Control group—Participants randomised to the control group received a €20 (£17; $23) voucher at the end of each visit as a show-up fee, but abstinence was not rewarded. The total pay-off depended on the total number of visits attended. The maximum amount a participant could earn was €120 after six visits.
Financial incentives group—Participants could earn additional vouchers dependent on abstinence. The pay-offs were based on two principles: a reward for abstinence today and a reward for continuous abstinence. Hence, the pay-off increased with the number of visits at which abstinence was biochemically confirmed and with the length of continuous abstinence. For example, if participants were abstinent during six consecutive visits, they could earn up to €520 in vouchers (see supplementary material).
Study outline and follow-up
A quit date was set at randomisation (visit 1)—determined in collaboration with the participants, who could choose a quit date between randomisation and day 15 post-randomisation in both the financial incentives group and the control group. The start date for abstinence was the quit date. Monthly face-to-face visits (visits 2-6) were planned up to the expected delivery date. If participants missed visits, they were contacted by telephone at least twice, and if they did not respond they were sent a letter to encourage them to attend the next visit. Participants received follow-up telephone calls six months after delivery.
At each visit, all participants received a minimum 10 minute intervention for smoking cessation according to national guidelines. The intervention included motivational counselling, support, relapse prevention, and skills training elements for behavioural modifications.15 16
Outcome measures
Primary outcome
The primary outcome measure was continuous smoking abstinence from the predefined quit date until the sixth visit. Abstinence was defined as a self-report of no smoking in the past seven days and expired air carbon monoxide (eCO) ≤8 ppm measured by a Bedfont Smokelyzer piCO (Kent, UK).17 Although the point prevalence abstinence rate might be useful to show the efficacy of an intervention, continuous abstinence from smoking might be associated with greater efficacy in preventing negative fetal growth and birth outcomes (see supplementary material).
Secondary outcomes
At each visit we recorded the maternal secondary outcome measures of point prevalence abstinence, defined as self-report of no smoking in the past seven days and eCO level ≤8 ppm, time (days) to the first cigarette after quit date, lapse (a few puffs) or relapse (smoking a cigarette), total number of cigarettes smoked/day, craving for tobacco (12 item French Tobacco Craving Questionnaire, FTCQ-12),18 tobacco withdrawal symptoms (Minnesota nicotine withdrawal scale),19 weight, sitting systolic and diastolic blood pressure, and cannabis and alcohol use in the past 30 days.
Secondary outcomes in newborns were recorded from medical charts: gestational age at birth (in weeks), birth weight, head circumference, length, Apgar score at five minutes, and poor neonatal outcomes (a composite measure of transfer to the neonatal unit, congenital malformation, convulsions, or perinatal death). Serious adverse events were collected by the sponsor’s pharmacovigilance system.
Statistical analyses
The primary outcome was analysed on an intention-to-treat basis, defined as all randomised pregnant smokers.
We hypothesised a 10% continuous abstinence rate, double that of the previously observed continuous abstinence rate14 in the control group, presuming the show-up fee might increase the abstinence rate by itself. Assuming a 20% continuous abstinence rate in the financial incentives group, with an α=0.05 and 1−β=0.80, we planned to randomise at least 199 women to each group.8 The targeted sample size was 420; the randomisation of 460 to 480 participants was planned, hypothesising a dropout rate of 9% to 12% (40 to 60 participants).
The log rank test was used to compare the time to relapse. We also compared point prevalence abstinence rate, use of nicotine replacement therapy in the past 30 days with mixed effects logistic models, and craving for tobacco (12 item French Tobacco Craving Questionnaire) with a mixed effects linear model.
Several sensitivity analyses were performed to assess the robustness of the results using alternative definitions of continuous abstinence and the smoking status of participants who did not attend appointments. The supplementary files provide further details.
We considered participants who did not show up for an appointment to be non-abstinent. The end of follow-up was defined as the end of pregnancy: delivery or other end of pregnancy (eg, miscarriage). Participants were considered to have been continuously abstinent if they showed up at each visit after the quit date, self-reported abstinence, and had a negative eCO test result.
Because of the variable birth weight of twins compared with singleton pregnancies as well as the unbalanced proportion of twins between groups, we excluded twins from the data analysis of newborns.20 As a post hoc analysis we defined low birth weight as <2500 g.21
The author (LG) who performed most of the data analyses was blinded when she ran the first main analyses. The code for grouping was opened on 22 July 2020 when she reported the results for the first time.
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. No patients were asked to advice on interpretation or writing up of results. In 2014, when the research protocol was written and approved by health authorities, patient and public involvement was not a requirement or suggestion.
Results
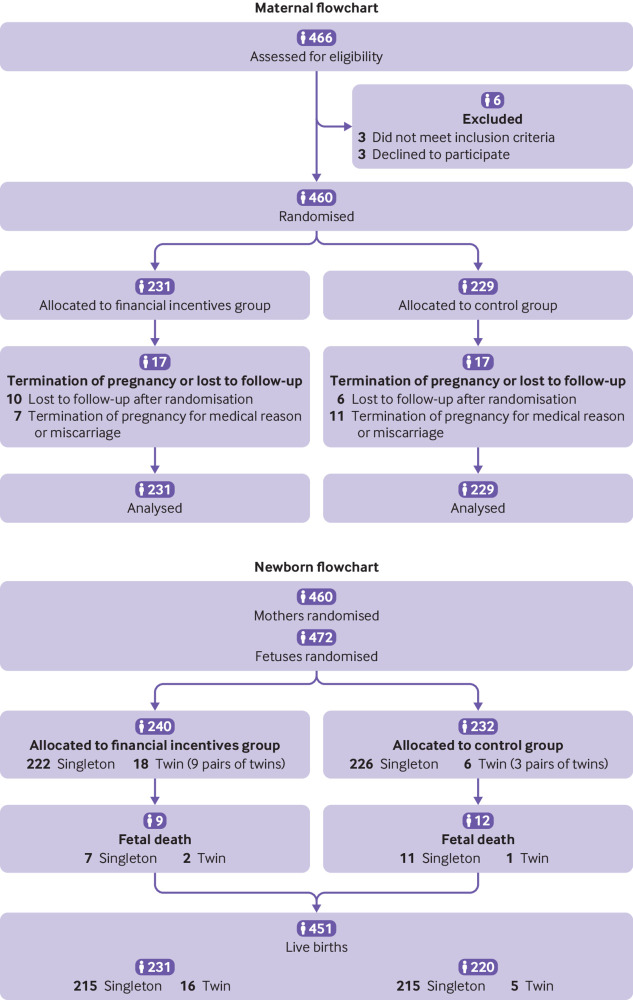
Four hundred and sixty six pregnant smokers were assessed for eligibility and 460 were randomised: 231 to the financial incentives group and 229 to the control group (fig 2). Baseline characteristics (table 1) and number of visits (median=4, P=0.499) were similar between the two groups.
Fig 2.
Flow of pregnant smokers and their offspring through the study
Table 1.
Sociodemographic characteristics, tobacco and alcohol use, and medico-obstetrical characteristics of pregnant smokers at randomisation. Values are numbers (percentages) unless stated otherwise
| Characteristics | Financial incentives (n=231) | Control (n=229) |
|---|---|---|
| Sociodemographic | ||
| Mean (95% CI) age (years); min-max | 29 (28 to 30); 18-42 | 29 (29 to 30); 18-42 |
| Yearly income (€): | ||
| <10 000 | 42 (18) | 40 (17) |
| 10 000 to 18 000 | 51 (22) | 52 (23) |
| 18 000 to <30 000 | 67 (29) | 72 (31) |
| 30 000 to <54 000 | 56 (24) | 50 (22) |
| 54 000 to 100 000 | 6 (3) | 8 (3) |
| Refused to answer or no answer | 9 (4) | 7 (3) |
| Self-reported ethnic origin: | ||
| African | 10 (4) | 10 (4) |
| Asian | 1 (0.4) | 2 (1) |
| European | 217 (94) | 214 (93) |
| Other | 3 (1) | 3 (1) |
| Marital status: | ||
| Single | 21 (9) | 20 (9) |
| Divorced | 1 (0.4) | 2 (1) |
| In a relationship | 163 (71) | 171 (75) |
| Married | 41 (18) | 31 (13) |
| Separated | 5 (2) | 5 (2) |
| Employment status: | ||
| Employed | 137 (59) | 148 (65) |
| Unemployed | 41 (18) | 40 (17) |
| Homemaker | 26 (11) | 16 (7) |
| Other inactive | 20 (9) | 23 (10) |
| Student | 7 (3) | 2 (1) |
| Tobacco and alcohol use | ||
| Mean (95% CI) motivation to quit smoking; min-max (scale 0-10) | 8.3 (8.1 to 8.5); 6-10 | 8.4 (8.2 to 8.6); 6-10 |
| Mean (95% CI) age of first cigarette (years); min-max | 14.9 (14.5 to 15.3); 5-34 | 14.8 (14.4 to 15.1); 8-32 |
| Mean (95% CI) age of daily smoking (years); min-max | 16.6 (16.1 to 17); 10-37 | 16.4 (16 to 16.7); 8-32 |
| Median (IQR) No of smoking cessation attempts | 1 (0-1) | 1 (0-1) |
| Median (IQR) No of cigarettes smoked in past 7 days; min-max | 56 (35-80); 3-210 | 60 (42-77); 1-210 |
| Median (IQR) expired air carbon monoxide (ppm); min=max | 14 (8-19); 0-49 | 15 (10-21); 0-50 |
| Partner smokes: | ||
| No partner | 13 (6) | 11 (5) |
| No | 51 (22) | 51 (22) |
| Yes | 167 (72) | 167 (73) |
| Other smoker at home: | ||
| No | 206 (89) | 200 (87) |
| Yes | 25 (11) | 29 (13) |
| Exposure to secondhand smoke at work or during leisure (other than by partner): | ||
| No | 93 (40) | 106 (46) |
| Yes | 138 (60) | 123 (54) |
| Mean (95% CI) FTCD total score | 4.6 (4.4 to 4.9) | 4.6 (4.3 to 4.9) |
| Mean (95% CI) FTCQ-12 total score; min-max | 42.2 (40.8 to 43.6); 12-82 | 40.7 (39.2 to 42.2); 12- 81 |
| CAGE questionnaire for alcohol problems (No of positive answers): | ||
| 0 | 179 (77) | 187 (82) |
| 1 | 22 (10) | 17 (7) |
| 2 | 16 (7) | 17 (7) |
| 3 | 13 (6) | 7 (3) |
| 4 | 1 (0.4) | 1 (0.4) |
| Medical and obstetrical | ||
| Medical or psychiatric history: | ||
| No | 118 (51) | 125 (55) |
| Yes | 112 (48) | 104 (45) |
| Not available | 1 (0.4) | 0 |
| Obstetrical history: | ||
| No | 80 (35) | 90 (39) |
| Yes | 151 (65) | 139 (61) |
| Median (IQR) No of previous pregnancies; min-max | 1 (2-0); 0-12 | 1 (2-0); 0-13 |
| Median (IQR) No of children; min-max | 0 (1-0); 0-5 | 0 (1-0); 0-7 |
| Median (IQR) No of preterm births; min-max | 0 (0-0); 0-2 | 0 (0-0); 0-2 |
| Median (IQR) small for gestational age at birth; min-max | 0 (0-0); 0-2 | 0 (0-0); 0-2 |
| Median (IQR) BMI before pregnancy; min-max | 22.5 (27-20); 16-43 | 23 (27-20); 16-40 |
| Mean (95% CI) maternal weight before pregnancy (kg); min-max | 65 (63-67); 36-120 | 65 (63-67); 40-116 |
| Median (IQR) BMI at randomisation; min-max | 23 (21-27); 17-50 | 24 (21-28); 16-40 |
| Mean (95% CI) gestational age (weeks); min-max | 13.6 (13.2 to 14.0); 4-17 | 13.7 (13.3 to 14.1); 3-17 |
| Maternal health disorder reported since start of pregnancy: | ||
| No | 211 (91) | 207 (90) |
| Yes | 20 (9) | 22 (10) |
| No fetal disorder diagnosed since start of pregnancy | 231 (100) | 229 (100) |
| Mean (95% CI) systolic blood pressure (mm Hg); min-max | 113 (111.5 to 114.5); 85-146 | 112 (110.6 to 113.5); 80-140 |
| Mean (95% CI) diastolic blood pressure (mm Hg); min-max | 65.8 (64.6 to 67); 50-90 | 66.3 (65.1 to 67.5); 50-90 |
| Twins (pairs): | ||
| No | 222 (96) | 226 (99) |
| Yes | 9 (4) | 3 (1) |
Primary outcome
The continuous abstinence rate was significantly higher in the financial incentives group (16%, 38/231) than control group (7%, 17/229): odds ratio 2.45 (95% confidence interval 1.34 to 4.49), P=0.004). Sensitivity analyses confirmed the robustness of this result (see supplementary table 2).
Secondary outcomes
The point prevalence abstinence rate was consistently higher in the financial incentives group than control group (mixed effects logistic model, odds ratio 4.61, 95% confidence interval 1.41 to 15.01, P=0.011; fig 3).
Fig 3.
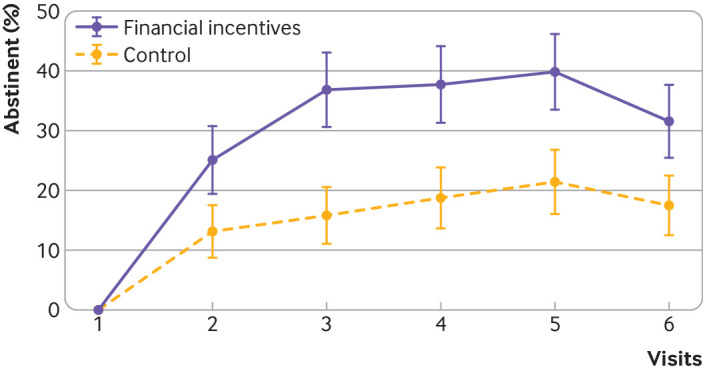
Point prevalence smoking abstinence rate by visit. Mixed effects logistic model: odds ratio 4.61 (95% confidence interval 1.41 to 15.01), P=0.011. Whiskers represent 95% confidence intervals
Time to relapse to the first cigarette occurred significantly later in the financial incentives group than control group (fig 4). The median relapse occurred at visit 5 (interquartile range 3-6) in the financial incentives group and at visit 4 (3-6) in the control group (log rank test, P<0.001).
Fig 4.
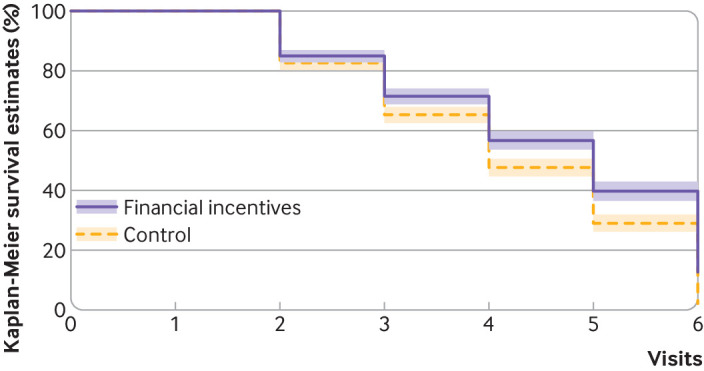
Time to lapse or relapse to first cigarette (log rank test, P<0.001) by percentage abstinent. Financial incentives group: median 5 (interquartile range 3-6), control group: median 4 (3-6). Shaded areas represent 95% confidence intervals
Craving for tobacco was lower in the financial incentives group than control group throughout pregnancy: β=−1.81 (95% confidence interval −3.55 to −0.08), P=0.04; fig 5). Participants in the financial incentives group smoked fewer cigarettes than those in the control group (mean difference −163, 95% confidence interval −302 to−23, P=0.022). No difference was found in the Minnesota nicotine withdrawal scale total score, systolic and diastolic blood pressure, weight change, or cannabis or alcohol use in the past 30 days.
Fig 5.
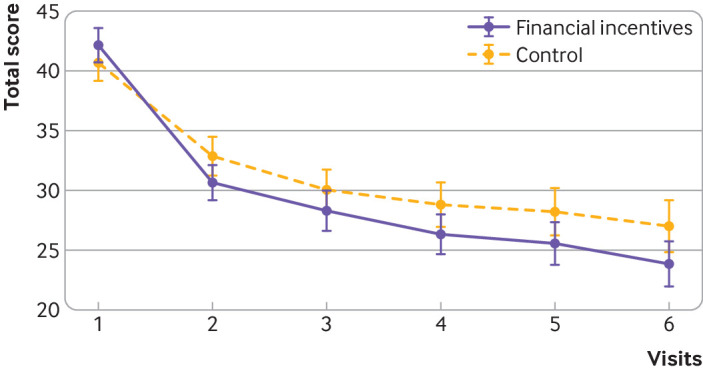
12 item French Tobacco Craving Questionnaire. Mixed effects linear model: β= −1.81 (95% confidence interval −3.55 to −0.08), P=0.040. Whiskers represent 95% confidence intervals
Concomitant use of nicotine replacement therapy
The mixed effects logistic model showed similar past 30 day use of nicotine replacement therapy (odds ratio 0.88, 95% confidence interval 0.62 to 1.24, P=0.462); the time by group comparisons suggested that use of nicotine replacement therapy was less likely in the financial incentives group than control group at visits 4 and 5 (supplementary table 4a and 4b). No interaction with use of nicotine replacement therapy was observed (supplementary table 3).
Newborns
The 460 randomised mothers had 472 fetuses, 240 (51%, nine pairs of twins) in the financial incentives group and 232 (49%, three pairs of twins) in the control group. Nine fetal deaths (4%) occurred in the financial incentives group and 12 (5%) in the control group (fig 2). Birth weight data were available for 202 (84%) and 205 (88%) singleton live newborns. The mean difference in birth weight of 47g was not statistically significant. Significantly fewer newborns in the financial incentives group (n=4) than in the control group (n=18) had poor neonatal outcomes (a composite measure of transfer to the neonatal unit, congenital malformation, convulsions, or perinatal death), this difference represents a 7% points reduction (table 2). Supplementary table 6 shows the number of poor neonatal outcomes.
Table 2.
Univariate analyses of birth characteristics (ordinary least squares and logistic models). Values are means (95% confidence intervals) unless stated otherwise
| Characteristics | Financial incentives | Control | Difference | |||||
|---|---|---|---|---|---|---|---|---|
| No* | Estimate (95% CI) | No* | Estimate (95% CI) | Estimate (95% CI) | P value | |||
| Birth weight (g) | 202 | 3176 (3107 to 3245) | 205 | 3130 (3053 to 3207) | 47 (−56 to 150) | 0.374 | ||
| Length (cm) | 191 | 49 (48 to 49) | 187 | 49 (48 to 49) | −0.03 (−0.6 to 0.5) | 0.925 | ||
| Head circumference (cm) | 186 | 34 (34 to 34) | 185 | 34 (34 to 34) | 0.00 (−0.4 to 0.4) | 1.000 | ||
| Apgar score at 5 min (range 0-10) | 199 | 9.9 (9.8 to 9.9) | 201 | 9.8 (9.7 to 9.9) | 0.05 (−0.1 to 0.2) | 0.497 | ||
| Gestational age (weeks) | 208 | 37.6 (37.3 to 37.8) | 209 | 37.3 (37 to 37.6) | 0.24 (−0.14 to 0.63) | 0.217 | ||
| Poor neonatal outcome† | 202 | 4 (0 to 8) | 209 | 18 (10 to 26) | 14 (5 to 23) | 0.0028 | ||
Data are from singleton live newborns.
Composite measure including transfer to neonatal unit, congenital malformation, convulsions, or perinatal deaths. Numbers are number of babies with poor neonatal outcome.
Post hoc analysis of dichotomised birth weight
Analysis of dichotomised birth weight ≥2500 g versus <2500 g was not included in the research protocol14; it was added as a post hoc analysis, as a recommended approach for birth weight.21 Therefore the following results are only hypothesis generating and should be interpreted with caution. The effect of financial incentives on delivering a baby of birth weight ≥2500 g versus <2500 g without adjustment closely approached but did not reach statistical significance (odds ratio 1.95, 95% confidence interval 0.99 to 3.85, P=0.055). Overall, girls comprised 53% of the births in the financial incentives group and 38% in the control group. Because birth weight is associated with the sex of babies, when birth weight was adjusted for sex in the post hoc analysis, a significant effect was shown of an increased likelihood of having a birth weight ≥2500 g in the financial incentives group compared with the control group (odds ratio 2.05, 95% confidence interval 1.03 to 4.10, P=0.041). After further adjustment for prematurity22 the statistical significance of the association showed some reduction (2.06, 0.90 to 4.71, P=0.086; see supplementary table 7).
Serious adverse event rates yielded similar results (supplementary table 8).
Costs
The vouchers totalled €49 040 in the financial incentives group and €19 520 in the control group (difference €29 520; see supplementary material).
Discussion
In this multicentre randomised controlled trial, financial incentives rewarding smoking abstinence compared with no financial incentives were associated with significantly increased continuous and point prevalence abstinence rates, a prolongation in the time to relapse, and a reduction in craving for tobacco. Financial incentives were also associated with a decrease in the probability of having a low birthweight baby.
Strengths of this study
A notable feature of this trial is the rigorous design and sufficient statistical power to determine the validity of the observed outcomes. The use of continuous abstinence as the main outcome measure rather than intermittent point prevalence abstinence might have contributed to the observed benefit on birth weight.
Post hoc analysis of birth weight suggested that the number of babies with a birth weight ≥2500 g was higher in the financial incentives group than control group after adjustment for the babies’ sex. When the groups were compared for birth weight (g) as a continuous variable, there was no difference but a difference was found when the number of babies with birth weights ≥2500 g was compared (see table 2). This discrepancy suggests that the effect of the financial incentives on birth weight might not be linear. When we adjusted for prematurity, the statistical significance of the effect of financial incentive on birth weights ≥2500 g became attenuated, suggesting the effect might differ according to prematurity. Further studies should include as a primary outcome the analysis of dichotomised birth weight according to recommendations21 and investigate whether the effect varies with prematurity.
That both groups received similar show-up fees enabled us to compare the net effect of progressively higher financial incentives on smoking abstinence.
Similar with the study by Tappin et al,13 use of nicotine replacement therapy was permitted. As use of nicotine replacement therapy is a principal confounding factor, controlling for it suggests that the effect of financial incentives was independent of use.
The study lasted three years and three months from the time that participants were first randomised to the time of the last assessment. Because participants were included during the first trimester and because the last assessment was at six months’ follow-up, participants remained in the study for 12 months. Recruitment occurred between April 2016 and July 2018—that is, two years and three months. This seems to be an acceptable recruitment period considering the treatment approach was unusual, only treatment seeking pregnant smokers were candidates for participation, and investigators conducted the study voluntarily and without payment.
This trial was conducted in 18 maternity wards throughout France, which used different organisational processes and healthcare professionals, lending representativeness to the data.
This national representativeness could allow the trial to be considered as an effectiveness study. Furthermore, by the nature of the intervention, financial incentives compared with drugs are not ostensibly associated with adverse events.
Limitations of this study
Limitations of this study include the lack of systematic follow-up of mothers and their offspring after delivery. It also might not be possible to generalise the findings to other countries and cultures; replications are needed. We did not include users of electronic cigarettes. We cannot, however, be sure that electronic cigarettes were not used because information was obtained only by participant self-report at each visit. A pragmatic limitation is the lack of information on the participants’ as well as healthcare professionals’ acceptability of financial incentives, and assessment of cost effectiveness in the real world. This is a sensitive issue and might vary according to national, cultural, and socioeconomic influences.23 Also, real world cost effectiveness of financial incentives to encourage smoking cessation during pregnancy have yet to be obtained.
To specifically control for tobacco use, in our study protocol we included urinary anabasine and anatabine, both biochemical measures of tobacco uptake.14 Because the sensitivity and specificity of these measures were below 55%, these measures could not be used as an objective control of tobacco intake. Furthermore, sufficiently powered studies should assess the usefulness of these non-nicotinic alkaloids as markers of exposure to tobacco.
Comparison with other studies
A Cochrane review on incentives for smoking cessation partly addressed prenatal and postnatal incentives for pregnant smokers.24 Nine randomised trials were included that comprised various populations, different settings, and diverse interventions (cash, vouchers, gift card, social support). Without distinctions between the prenatal and postnatal interventions, financial incentives showed a significant advantage over no incentives control (relative risk 2.38, 95% confidence interval 1.54 to 3.69, n=2273).
Four previous studies with a design similar to ours also found an association between financial incentives and increased abstinence.7 8 9 10 Our design differs by the magnitude of incentives increments, the absence of reset of vouchers value back to the initial value (€20) if the participant was not abstinent or did not show up, and sample size (supplementary material, discussion).
Policy implications, unanswered questions, and future research
Smoking prevalence is higher among people on a low income. Forty per cent of our participants’ households reported an annual income of <€18 000; further analyses by our group will investigate the efficacy of financial incentives in this subgroup. Further research should investigate who among pregnant smokers could benefit even more from being rewarded for smoking abstinence. Conception, planning, recruitment, and design of future trials should involve pregnant smokers, healthcare professionals, and policy makers in the assessment of the cost effectiveness and acceptability of this treatment approach. Finally, future studies should compare the efficacy of incentives or contingency management with that of nicotine replacement therapy.
The smoking rate of partners was as high as 72% in the financial incentives group and 73% in the control group. Exposure to secondhand tobacco smoke during pregnancy regardless of whether the mother smokes is associated with an increased risk of smoking related negative health outcomes. We suggest that future studies should assess the efficacy of financial incentives in helping the partners of pregnant smokers to quit smoking.
Financial incentives dependent on smoking abstinence could be implemented as a safe and effective intervention to help pregnant smokers quit smoking.
Conclusions
Among pregnant smokers motivated to quit smoking, financial incentives to reward abstinence in a progressive manner were associated with increased continuous and point prevalence abstinence rates and, in post hoc analyses, seemed to increase the number of newborns with birth weights ≥2500 g. Financial incentives progressively rewarding smoking abstinence could be implemented in the routine healthcare of pregnant smokers. Future studies should assess the long term effectiveness of financial incentives on smoking abstinence after delivery.
What is already known on this topic
Smoking cessation is crucial to prevent smoking associated risks in pregnancy and negative birth outcomes
The benefit:risk ratio of drug treatments for smoking cessation in pregnancy is unclear
Previous studies with various financial incentives schedules showed promising results for point prevalence abstinence rates but no effect on birth weight
What this study adds
This multicentre randomised controlled trial implemented progressive financial incentives as vouchers and found that these were associated with an increase in continuous abstinence rate throughout pregnancy (16% v 7%)
Vouchers for abstinence were also associated with a more than doubled point prevalence abstinence rate compared with no vouchers
Financial incentives were associated with a 7% reduction in the risk of poor neonatal outcomes, a composite measure of transfer to the neonatal unit, congenital malformation, convulsions, or perinatal death
Acknowledgments
We thank the investigators for their interest, time, and enthusiasm in running this trial: F Aubourg, Paris; F Bottet, Caen; M Breton, Brest; L Galmiche, Marmande; C Denis-Vatant, Saint-Etienne; A Gentil, Angers; G Grangé, Paris; I Granger, Parthenay and Thouars; N Hochedé, Divion; B Lecollaire, Dieppe; D Levisse, F Lhenry, Valenciennes; M Malecot, Lyon; C Marçais-Espiand, Montpellier; C Meier, Pau; C Monard, Nantes; D Levisse, Valenciennes; B Mollet-Guiliani, Angers; A Noblet, Grenoble; P Riffat, Caen; R Targhetta, Nimes; C Vannimenus, and Lille; P Villemonteix, Bressuire. We thank members of the Unité de Recherche Clinique, Pitié-Salpêtrière Hospital who contributed to the conduct of this trial: Jessica Palmyre (coordinator), France Boyaud, Alexis Caloc, Damien Gauthier, and Ramdane Slimi (research assistants), and Shoreh Azimi (project manager at the Délégation à la Recherche Clinique et Innovation, Assistance Publique-Hôpitaux de Paris). We thank Lirio S Covey who reviewed the English in the manuscript and Matthieu Cassou for statistical advice; the postdoctoral financial support to LG from the health chair—a joint initiative between Paris Sciences et Lettres (PSL), Université Paris-Dauphine, l’École nationale de la statistique et de l’administration économique (ENSAE), Mutuelle générale de l’Éducation nationale (MGEN), Université Paris-Dauphine, and ISTYA Collectives under the aegis of the Fondation du Risque and from this grant.
Web extra.
Extra material supplied by authors
Supplementary material: Additional information
Contributors: IB, NB, LG, and FJ conceived and planned the study. IB drafted the manuscript with regular major input from the coauthors. LG performed the data management, and did the statistical analyses with regular major input from IB, NB, and FJ. All authors contributed to interpretation of the data, revision of the manuscript, and approved the final manuscript. IB is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no other meeting the criteria have been omitted.
Funding: This research was funded by the French National Cancer Institute (INCa) Recherche en Prévention Primaire (grant No 2014-100). Logistic support was provided by Assistance Publique-Hôpitaux de Paris, Direction à la Recherche Clinique et Innovation, Paris, France. The present paper represents the opinions of the authors and does not necessarily reflect the position of their employers.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from the French National Cancer Institute (INCa); no support from any organisation for the submitted work with the exception of LG who was supported by the study’s grant and by a grant from the health chair—a joint initiative between Paris Sciences et Lettres (PSL), Université Paris-Dauphine, l’École nationale de la statistique et de l’administration économique (ENSAE), Mutuelle générale de l’Éducation nationale (MGEN), Université Paris-Dauphine, and ISTYA Collectives under the aegis of the Fondation du Risque as a postdoctoral fellow. All authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
The lead author (IB) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We plan to disseminate the findings to pregnant smokers, healthcare professionals involved in smoking cessation treatments, health authorities, and policy makers. The sponsor, Assistance publique-Hôpitaux de Paris, will launch a press release and summarise the results on its website. Investigators will communicate the results in their respective maternity wards to healthcare professionals and pregnant smokers. The Ministry of health, France plans to formally inform the regional health services about the results.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The research protocol was approved by the ethics committee (Comité de Protection des Personnes) Ile de France VI on 17 April 2015. Conduct of the trial was approved by the Agence nationale de sécurité du médicament et des produits de santé on 22 April 2015.
Data availability statement
Requests for data sharing should be submitted to the data owner Assistance Publique-Hôpitaux de Paris, France. Contact project manager Shoreh Azimi (shoreh.azimi@aphp.fr).
References
- 1. National Centre for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention, 2014. [PubMed] [Google Scholar]
- 2.US Centres for Disease Control and Prevention, U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. CDC, 2006. www.ncbi.nlm.nih.gov/books/NBK44324/
- 3. Grangé G, Berlin I, Bretelle F, et al. Smoking and smoking cessation in pregnancy. Synthesis of a systematic review. J Gynecol Obstet Hum Reprod 2020;49:101847. 10.1016/j.jogoh.2020.101847. [DOI] [PubMed] [Google Scholar]
- 4. Tobacco and Nicotine Cessation During Pregnancy: ACOG Committee Opinion. Number 807. Obstet Gynecol 2020;135:e221-9. 10.1097/AOG.0000000000003822. [DOI] [PubMed] [Google Scholar]
- 5. US Preventive Services Task Force . Interventions for Tobacco Smoking Cessation in Adults, Including Pregnant Persons: US Preventive Services Task Force Recommendation Statement. JAMA 2021;19:265-79. 10.1001/jama.2020.25019. [DOI] [PubMed] [Google Scholar]
- 6. Claire R, Chamberlain C, Davey MA, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2020;3:CD010078. 10.1002/14651858.CD010078.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Higgins ST, Heil SH, Solomon LJ, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 2004;6:1015-20. 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- 8. Heil SH, Higgins ST, Bernstein IM, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction 2008;103:1009-18. 10.1111/j.1360-0443.2008.02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins ST, Bernstein IM, Washio Y, et al. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction 2010;105:2023-30. 10.1111/j.1360-0443.2010.03073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Higgins ST, Washio Y, Heil SH, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med 2012;55(Suppl):S33-40. 10.1016/j.ypmed.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins ST, Washio Y, Lopez AA, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med 2014;68:51-7. 10.1016/j.ypmed.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy. Nicotine Tob Res 2012;14:351-60. 10.1093/ntr/ntr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tappin D, Bauld L, Purves D, et al. Cessation in Pregnancy Incentives Trial Team . Financial incentives for smoking cessation in pregnancy: randomised controlled trial. BMJ 2015;350:h134. 10.1136/bmj.h134. [DOI] [PubMed] [Google Scholar]
- 14. Berlin N, Goldzahl L, Jusot F, Berlin I. Protocol for study of financial incentives for smoking cessation in pregnancy (FISCP): randomised, multicentre study. BMJ Open 2016;6:e011669. 10.1136/bmjopen-2016-011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conférence de consensus . “grossesse et tabac” : texte des recommandations. Prof Sage-Femme 2005;(112):19-34. [Google Scholar]
- 16.Grossesse et arrêt du tabac : Accompagner par l’écoute et le dialogue - Les essentiels de l’Inpes - Essentiel_GrossesseArretTabac.pdf. Accessed February 15, 2016. http://www.inpes.sante.fr/30000/pdf/2014/Essentiel_GrossesseArretTabac.pdf
- 17. SRNT Subcommittee on Biochemical Verification . Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4:149-59. 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 18. Berlin I, Singleton EG, Heishman SJ. Validity of the 12-item French version of the Tobacco Craving Questionnaire in treatment-seeking smokers. Nicotine Tob Res 2010;12:500-7. 10.1093/ntr/ntq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 1986;43:289-94. 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 20. Taylor L, Claire R, Campbell K, et al. Fetal safety of nicotine replacement therapy in pregnancy: systematic review and meta-analysis. Addiction 2021;116:239-77. 10.1111/add.15185. [DOI] [PubMed] [Google Scholar]
- 21.Weise A. WHA Global Nutrition Targets 2025: Low Birth Weight Policy Brief. WHO Publ. Published online 2012:1-7. www.who.int/nutrition/topics/globaltargets_stunting_policybrief.pdf
- 22.Born too soon: the global action report on preterm birth. 2012. www.who.int/pmnch/media/news/2012/201204_borntoosoon-execsum-eng.pdf
- 23. Berlin N, Goldzahl L, Bauld L, Hoddinott P, Berlin I. Public acceptability of financial incentives to reward pregnant smokers who quit smoking: a United Kingdom-France comparison. Eur J Health Econ 2018;19:697-708. 10.1007/s10198-017-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Notley C, Gentry S, Livingstone-Banks J, Bauld L, Perera R, Hartmann-Boyce J. Incentives for smoking cessation. Cochrane Database Syst Rev 2019;7:CD004307. 10.1002/14651858.CD004307.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob Res 2012;14:75-8. 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- 26. Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA 1984;252:1905-7. 10.1001/jama.1984.03350140051025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Additional information
Data Availability Statement
Requests for data sharing should be submitted to the data owner Assistance Publique-Hôpitaux de Paris, France. Contact project manager Shoreh Azimi (shoreh.azimi@aphp.fr).