ABSTRACT
The search for new antiobesogenic agents is increasing because of the current obesity pandemic. Capsaicin (Caps), an exogenous agonist of the vanilloid receptor of transient potential type 1 (TRPV1), has shown promising results in the treatment of obesity. This scoping review aims to verify the pathways mediating the effects of Caps in obesity and the different methods adopted to identify these pathways. The search was carried out using data from the EMBASE, MEDLINE (PubMed), Web of Science, and SCOPUS databases. Studies considered eligible evaluated the mechanisms of action of Caps in obesity models or cell types involved in obesity. Nine studies were included and 100% (n = 6) of the in vivo studies showed a high risk of bias. Of the 9 studies, 66.6% (n = 6) administered Caps orally in the diet and 55.5% (n = 5) used a concentration of Caps of 0.01% in the diet. In vitro, the most tested concentration was 1 μM (88.9%; n = 8). Capsazepine was the antagonist chosen by 66.6% (n = 6) of the studies. Seven studies (77.8%) linked the antiobesogenic effects of Caps to TRPV1 activation and 3 (33.3%) indicated peroxisome proliferator-activated receptor (PPAR) involvement as an upstream connection to TRPV1, rather than a direct metabolic target of Caps. The main secondary effects of Caps were lower weight gain (33.3%; n = 3) or loss (22.2%; n = 2), greater improvement in lipid profile (33.3%; n = 3), lower white adipocyte adipogenesis (33.3%; n = 3), browning process activation (44.4%; n = 4), and higher brown adipocyte activity (33.3%; n = 3) compared with those of the control treatment. Some studies have shown that PPAR agonists modulate TRPV1 activity, and no study has evaluated the simultaneous antagonism of these 2 receptors. Consequently, further studies are necessary to elucidate the role of each of these signaling molecules in the antiobesogenic effects of Caps.
Keywords: obesity, capsaicin, TRPV1, PPARγ, thermogenesis, adipogenesis, browning
Statement of Significance: Some narrative reviews have addressed the antiobesity effects of capsaicin. However, no study has systematically reviewed the literature on the mechanism and pathways of such antiobesogenic actions.
Introduction
Obesity is a chronic metabolic disorder associated with excessive adiposity (1–3). The global prevalence of obesity is >13% of the adult population and ∼40 million children <5 y of age were overweight or obese in 2018 (4). Currently, overweight and obesity lead to more deaths than low weight and undernutrition (3). Obesity is a multifactorial disease that includes genetic, dietary, and environmental factors (5). Obesity is associated with comorbidities (6), including type 2 diabetes (7, 8), dyslipidemia (9), systemic arterial hypertension (10, 11), other cardiovascular diseases (12, 13) and cancer (14, 15). Moreover, obesity is associated with an increase in deaths from all causes (16–18), mainly cancer and cardiovascular diseases (19–21).
Therapeutic strategies for obesity are based on lifestyle changes such as avoiding a sedentary lifestyle and adopting balanced diets. Nonetheless, approaches that induce thermogenesis and satiety or reduce food absorption could be used as adjuvant tools in the treatment of obesity (22). Thus, active compounds of natural products have been tested (23–25), including 8-methyl-N-vanilil-6-nonenamide [capsaicin (Caps)], the main capsaicinoid of Capsicum peppers, which has shown positive antiobesogenic effects in clinical, experimental, and cell lineage studies (26–28). Caps is an exogenous agonist of the transient receptor potential cation channel subfamily V member 1 (TRPV1) that acts on obesity by controlling the appetite (29, 30), increasing fat oxidation, reducing adipogenesis (31–33), and inducing thermogenesis (34–36). Moreover, Caps has also been related to improvement in the intestinal microbiota profile and SCFA production (37, 38).
Although the effects of Caps on weight loss and adiposity have already been established and published in the literature, the pathways mediating these actions are still controversial. Some studies have reported that Caps acts by activating TRPV1 (39–41), which modulates fatty acid and glucose metabolism and high-fat-diet (HFD)–induced metabolic stress (42–46). These results suggest that TRPV1 activity may attenuate obesity and its complications (41, 47). However, the results of studies in TRPV1−/− mice are conflicting, with some demonstrating protective (48, 49) or neutral (50) effects against the development of obesity compared with wild-type mice.
Nonetheless, some studies have shown a TRPV1-independent effect of Caps related to its action as an agonist of peroxisome proliferator-activated receptors (PPARs) (45, 51–54), especially PPARγ (31, 53–56). The role of PPARγ in the transcriptional regulation of adipogenesis could contribute to the antiobesity action of Caps. Indeed, Caps was demonstrated to induce the expression of adiponectin and reduce IL-6 and chemokine (C-C motif) ligand 2 (CCL2)/monocyte chemoattractant protein-1 (MCP1) (31, 53–56) in adipocytes of mice fed an HFD. These effects were associated with the activation of PPARγ and inactivation of NF-κB (51).
Although several narrative reviews have addressed the antiobesity effects of Caps (57–59), there is no systematic review and consensus in the literature regarding the mechanism and pathways of such actions. Systematic reviews generally seek to answer different clinical questions regarding the efficacy, adequacy, or viability of a specific intervention (60). The main objective of this study was not to evaluate the efficacy of Caps as an antiobesogenic agent but to provide an overview of the evidence regarding the mechanisms of action of this compound in obesity, and to clarify the nature and diversity of available evidence. Consequently, we believed that conducting a scoping review rather than a systematic review would be more appropriate. Thus, this scoping review aims to verify Caps-mediated pathways related to obesity and the different methods used for their identification.
Methods
Protocol and checklist
This scoping review was performed to understand the concepts underpinning a research area and explain work definitions or theoretical limits of a topic. This review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) (61) and the review protocol was registered in the Open Science Framework (https://osf.io/8svn7/).
Databases and search strategy
The following databases were searched for relevant studies in June 2019: EMBASE, MEDLINE (PubMed), Web of Science, and SCOPUS. The search strategy included using terms related to population, concept, and context according to the Joanna Briggs Institute (62): obesity, Caps, and data divergence regarding the mechanism of action (Supplemental Table 1). To complement the electronic search, gray literature was searched at the Digital Library of the Federal University of Minas Gerais, Federal University of São Paulo, State University of Campinas, the bank of theses and dissertations from the Coordination for the Improvement of Higher Education Personnel, and the Brazilian Digital Library of Theses and Dissertations. In addition, further searches of the British Journal of Pharmacology, Biophysical Journal, and articles included in the last phase 3 of the scoping review were conducted. To avoid missing any crucial publications, we used sensitive search strategies in the search platforms and contacted the authors of unavailable studies, as well as other systematic reviews (63–66).
Selection of studies and eligibility criteria
Initially, the studies retrieved from the search platform were unified on a single basis to exclude duplicates using EndNote software, version 7x (Clarivate Analytics; https://www.endnote.com). The unified database was implemented in Rayyan, a web application developed for this stage of the systematic review (identification, screening, eligibility, and inclusion) (67). Next, 2 independent reviewers (DLA and NAMN) evaluated the titles (phase 1), abstracts (phase 2), and full texts (phase 3). Any disagreements were resolved by reaching a consensus between the 2 reviewers or, if necessary, a third reviewer (PHRFA) was involved.
Data collection and analysis
The characteristics of the various studies (year of publication, type of study, experimental model, types of animals or cell cultures used, Caps intervention time used in vivo, Caps/antagonist concentration, identification of the Caps pathway of action, among other variables) were extracted by 2 independent reviewers (DLA and NAMN). Any disagreements were resolved by a consensus being reached between the 2 reviewers or, if necessary, a third reviewer (PHRFA) was involved. For this step, an online form prepared and tested on the Google Forms platform was used.
The included studies were submitted to a descriptive synthesis, which involved summarizing the data collected from the included studies. The studies were grouped according to the method design type, along with outcome measures and publication characteristics. Tables and figures were used to represent the results of the included studies. The program used to create the drawings was Adobe Illustrator CC, version 2014.0.0 (Adobe; http://www.adobe.com/products/illustrator.html).
The risk-of-bias (RoB) tool for animal intervention studies [SYstematic Review Center for Laboratory Animal Experimentation's (SYRCLE's) RoB tool] was used to assess the bias of the studies included in the scoping review (68). SYRCLE's RoB tool contains 10 entries, which are related to selection, performance, detection, attrition, and reporting biases, as well as other biases. However, according to SYRCLE's RoB tool guidelines, entry 9 (reporting bias) was not used, as most animal studies did not have a previous research protocol specifying the experimental design and statistical analysis.
In addition, only the secondary outcomes of the included studies were evaluated, because they were the only ones that used animals (in vivo). After the analysis, 1 of the following bias classifications was designated for each type of bias analyzed: low risk, high risk, or unclear risk of bias. Two authors (DLA and NAMN) independently assessed the RoB of the studies and any disagreements were resolved by a consensus being reached between them or, where necessary, a third reviewer (PHRFA) was involved. Similar to other previously published scoping reviews (69, 70), this study strictly followed the recommendations of PRISMA-ScR (61) and the Joanna Briggs Institute (62).
Results
Search
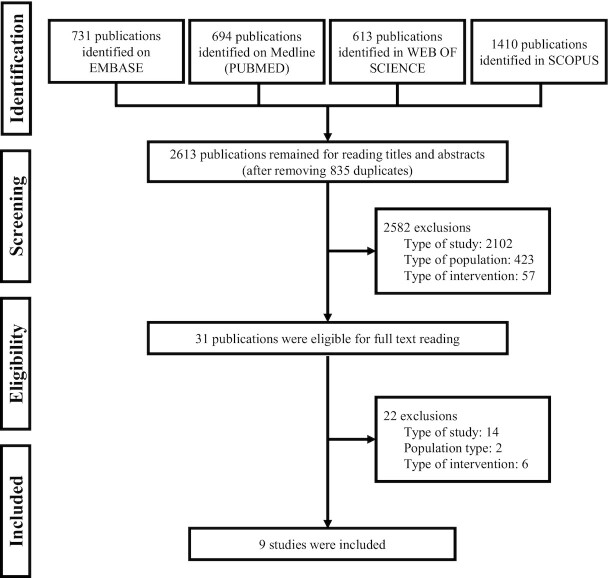
The search platforms returned 5501 reports (Figure 1), and after removing duplicates and reading titles and abstracts (phase 1 and phase 2), 31 reports were selected for the full reading (phase 3). Most full-text reports were excluded because they did not present the appropriate study design (Supplemental Table 2). The complementary search did not identify any other publications. Finally, 9 studies were included (71–79).
FIGURE 1.
Overview of the flowchart of the selection of included studies.
General characteristics of included studies
Among the included studies, 77.8% (n = 7) were published in the last 4 y (2015–2019) (72–77, 79), 88.9% (n = 8) had public funding for their execution (71–74, 76–79), and 33.3% (n = 3) were performed in the United States (73, 74, 79). Experimental designs using in vivo and in vitro models were adopted by 77.8% of studies (n = 7) (71–75, 78, 79) (Table 1).
TABLE 1.
General characteristics of in vivo and in vitro studies included in the scoping review1
| Country | Type of study | Experimental model: in vivo | Experimental model: in vitro | Type of Caps intervention | Caps intervention time: in vivo | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | USA | China | Japan | India | In vivo | In vitro | C57BL/6–WT mice | C57BL/6 -TRPV1−/− mice | db/db Mice | ob/ob Mice | Wistar rats | LACA | HEK293 | 3T3-L1 | Mouse brown adipocyte (primary culture) | Mouse white adipocyte (primary culture) | Human white adipocyte (primary culture) | Oral–via diet | Oral–via gavage | Incubation (cell-cell culture) | Up to 12 wk | 16–20 wk | 32–38 wk |
| Baboota et al. (78) | – | – | – | X | X | X | – | – | – | – | X | X | X | – | – | – | – | X | X | X2 | – | – | |
| Baskaran et al. (73) | X | – | – | – | X | X | X | X | – | – | – | – | X | – | – | X | – | X | – | X | – | – | X |
| Baskaran et al. (79) | X | – | – | – | X | X | X | X | – | – | – | – | X | – | X | – | – | X | – | X | – | X | X |
| Chen et al. (72) | – | X | – | – | X | X | X | X | X | – | – | – | – | X | – | – | X | X | – | X | – | X | – |
| Fan et al. (77) | – | X | – | – | – | X | – | – | – | – | – | – | – | X | – | – | – | – | – | X | – | – | – |
| Kida et al. (75) | – | – | X | – | X | X | X | – | – | – | – | – | – | – | X | – | – | – | – | X | – | – | – |
| Kida et al. (76) | – | – | X | – | – | X | – | – | – | – | – | – | – | – | X | – | – | – | – | X | – | – | – |
| Krishnan et al. (74) | X | – | – | – | X | X | – | – | X | – | – | – | X | X | – | X | – | X | – | X | – | – | X |
| Zhang et al. (71) | – | X | – | – | X | X | X | X | X | X | – | – | – | X | – | – | – | X | – | X | X | X | – |
1The included studies were published between the years 2007 and 2019 (n = 9). The “X” symbol represents the presence of the characteristic in the study. The “-” symbol represents the absence. Caps, capsaicin; TRPV1, transient receptor potential vanilloid 1; WT, wild-type.
2Administration on alternate days.
Risk of bias
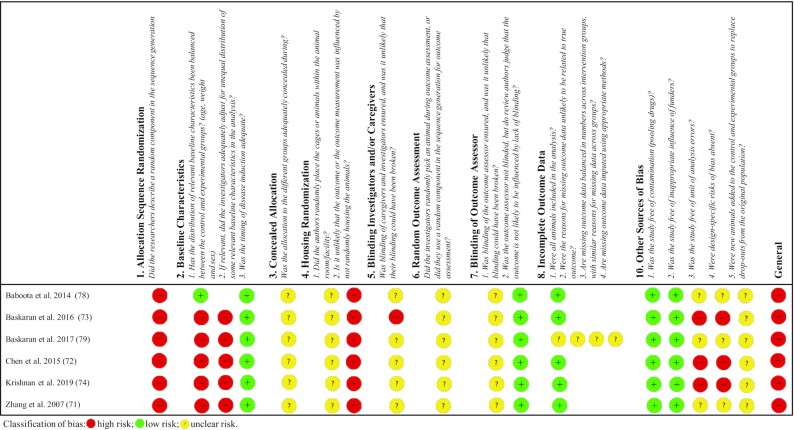
The analysis showed that 100% (n = 6) of the studies in vivo evaluated (71–74, 78, 79) had a high RoB, as shown in Figure 2. All studies (100%, n = 6) (71–74, 78, 79) showed a high RoB in sequence generation (selection bias), and although all the authors reported that the allocation sequences were randomly generated, none described the random component used. In addition, 83.3% (n = 5) (71–74, 79) also showed a high RoB for the balanced distribution of relevant baseline characteristics (sex, age, and weight) between groups (selection bias), and 100% (n = 6) (71–74, 78, 79) showed a high RoB for housing randomization (performance bias).
FIGURE 2.
Bias risk assessment of studies included in the scoping review by the RoB tool for animal intervention studies: SYRCLE's RoB tool. RoB, risk-of-bias; SYRCLE, SYstematic Review Centre for Laboratory animal Experimentation.
An unclear RoB was found with some domains: concealed allocation (selection bias; 100%, n = 6) (71–74, 78, 79); blinding of investigators, caregivers, or both (performance bias; 83.3%, n = 5) (71, 72, 74, 78, 79); random outcome assessment (detection bias; 100%, n = 6) (71–74, 78, 79); and blinding of outcome assessor (detection bias; 100%, n = 6) (71–74, 78, 79). With regard to other sources of bias, 50% (n = 3) of the studies showed an unclear RoB (71, 78, 79), whereas the remaining 50% (n = 3) (72–74) revealed a high RoB. It is noteworthy that 83.3% (n = 5) (71–74, 78) of the 6 studies evaluated demonstrated a low RoB for the incomplete outcome data domain (attrition bias). The full details for determining the bias risk can be found in Supplemental Table 3.
Characterization of “in vivo” models
Among the 7 studies that performed in vivo experiments (71–75, 78, 79), 71.4% (n = 5) used TRPV1−/− C57Bl/6 mice to investigate the involvement of the TRPV1 pathway in the Caps-mediated effects (71–74, 79) (Table 1). In 85.7% of them (n = 6), obesity was induced using an HFD (71–74,78, 79).
Caps intervention (in vivo)
Of the 7 studies that adopted in vivo models (71–75, 78, 79), in 71.4% (n = 5) Caps was added to the diet (orally administered) (71–74, 79) (Table 1). In 71.4% (n = 5) of the studies, the Caps concentration in the diet was 0.01% (71–74, 79) (Table 2). Although 1 study (14.3%) used an in vivo model (Table 1), the C57BL/6 mice were not treated with Caps and were only used to obtain brown adipose tissue (BAT) (75).
TABLE 2.
Capsaicin, antagonist, and agonist concentrations used in the in vivo and in vitro experiments of the included studies1
| Caps concentration | Antagonist concentration, μM | ||||
|---|---|---|---|---|---|
| Reference | In vivo | In vitro, μM | Antagonist | Other agonists | |
| Baboota et al. (78) | 2 mg/kg body weight | 0.1, 0.5, 1, 10, 50, and 100 | Capsazepine | 1, 10 and 20 | Resiniferatoxin |
| Baskaran et al. (73) | 0.01% in the diet | 1 | Capsazepine | 10 | — |
| Baskaran et al. (79) | 0.003%, 0.01% and 0.03% in the diet | 1 | Capsazepine | 10 | — |
| Chen et al. (72) | 0.01% in the diet | 1 | Capsazepine | 1 | — |
| GSK0660 | 10 | ||||
| Fan et al. (77) | — | 25, 50, and 100 | T0070907 | 10 | Capsiate |
| SR59230A | 10 | ||||
| Kida et al. (75) | — | 0.1, 1, and 10 | 5'-Iodoresiniferatoxin | 200 | — |
| Kida et al. (76) | — | 0.1, 1, 10, 30, and 100 | 5'-Iodoresiniferatoxin | 1 | — |
| Krishnan et al. (74) | 0.01% in the diet | 1 | Capsazepine | 10 | Troglitazone |
| Zhang et al. (71) | 0.01% in the diet | 0.01, 0.1, and 1 | Capsazepine | 1 | — |
1The table describes the concentrations of Caps used in vivo and in vitro for each of the included studies, as well as which antagonists were used and their respective concentrations. The last column also describes the use of other agonists. Caps, capsaicin.
Although the in vivo administration route was similar in most studies, the intervention time varied among the studies. Caps was administered for up to 12 wk in 22.2% of the studies (n = 2) (71, 78), 16–20 wk in 33.3% (n = 3) (71, 72, 79), and 32–38 wk in 33.3% of studies (n = 3) (73, 74, 79) (Table 1).
In vitro model characterization
Despite the importance of using TRPV1−/− mice to evaluate the in vivo dependence of TRPV1 on the Caps-mediated effects, all studies (100%, n = 9) included in the analysis used in vitro models to investigate the signaling pathways involved in the Caps action (71–79). The white adipocyte 3T3-L1 cell line was used in 55.5% (n = 5) of the studies (71, 72, 74, 77, 78), whereas the others (33.3%, n = 3) used murine primary brown adipocytes (75, 76, 79) to evaluate the Caps signaling pathways (Table 1).
Caps intervention (in vitro)
Most in vitro studies (88.9%, n = 8) used a Caps concentration of 1 μM (71–76, 78, 79), and 44.4% (n = 4) used >1 concentration (75–78) or tested other components in addition to Caps (33.3%, n = 3) (74, 77, 78) (Table 2). Antagonists were used in 100% (n = 9) of the studies (71–79) and, particularly, capsazepine, a classic TRPV1 antagonist, which was used in 66.7% of studies (n = 6) (71–74, 78, 79) and at a concentration of 10 μM in 44.4% of studies (n = 4) (73, 74, 78, 79) (Table 2).
Action pathways
All studies (100%, n = 9) investigated the involvement of the TRPV1 ion channel in the Caps-mediated effects (71–79). Among these studies, 6 (66.7%, n = 6) found a TRPV1-dependent action (71–74, 78, 79), whereas 44.4% (n = 4) of the reviewed studies reported that the effects of Caps were mediated by the activation of alternative signaling pathways (74–78). One study (11.1%, n = 1) evaluating low and high concentrations of Caps found concentration-dependent TRPV1-dependent and TRPV1-independent effects (78) (Figure 3).
FIGURE 3.
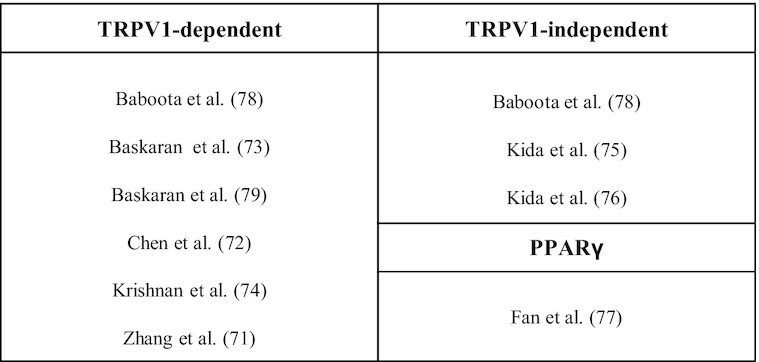
Capsaicin pathways of action found in the studies reviewed from experiments conducted in vivo and in vitro. The studies included in this scoping review were divided into 3 main outcomes: TRPV1-dependent, TRPV1-independent, and PPARγ. PPARγ, peroxisome proliferator-activated receptor γ; TRPV1, transient receptor potential vanilloid 1.
Almost all studies investigated the action of Caps on 2 metabolic axes related to obesity: 1) fat storage in white adipose tissue (WAT) (adipogenesis and lipogenesis) was investigated in 3 studies (33.3%, n = 3) (71, 72, 78) and 2) thermogenesis induction directly in brown adipocytes (33.3%, n = 3) (75, 76, 79) or indirectly in white adipocyte browning (44.4%, n = 4) (73, 74, 77, 78). All 3 studies (100%, n = 3) that evaluated the action of Caps in adipogenesis found a TRPV1-dependent action when cells were exposed to a low concentration (71, 72, 78). One of those studies (33.3%) demonstrated that, at higher concentrations, Caps stimulated adipogenesis in a TRPV1-independent manner (78).
Among the 4 studies that examined Caps-induced browning (73, 74, 77, 78), 75% (n = 3) demonstrated its dependence on TRPV1 activity (73, 74, 78), whereas 1 study (25%) found a PPARγ-related action (77). Considering only the 3 studies exploring brown adipocyte activity (75, 76, 79), 66.7% (n = 2) found TRPV1-independent (75, 76) Caps activity, whereas 33.3% (n = 1) found TRPV1-dependent activity (79) (Supplemental Table 4).
Secondary effects
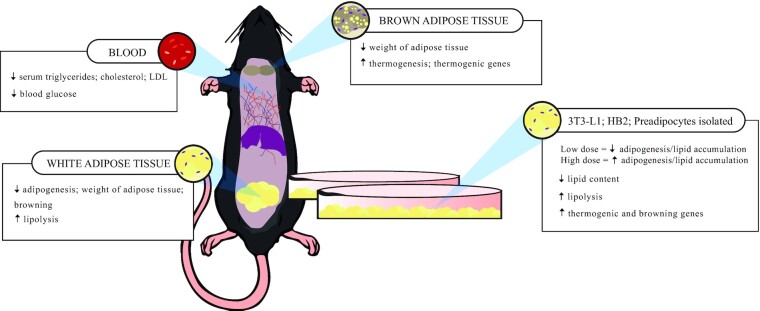
The main in vivo effects of Caps found in the reviewed studies were a lower weight gain (33.3%, n = 3) (71–73) or loss (22.2%, n = 2) (74, 79); a reduction in triglyceridemia, cholesterolemia (33.3%, n = 3) (71–73), and fasting glycemia (11.1%, n = 1) (79); and higher expression of thermogenic genes or browning inducers (33.3%, n = 3) compared with that induced by control treatments (73–75) (Figure 4). In the in vitro studies, 55.5% (n = 5) observed reduced adipogenesis, increased lipolysis, and/or lower intracellular lipid content in white adipocytes (71, 73, 74, 78, 79). In brown adipocytes, 2 studies (22.2%) found increased adipogenesis, as well as a higher expression of adipogenic genes (75, 76) (Figure 4).
FIGURE 4.
Main secondary capsaicin effects found in vivo and in vitro in the included studies. The in vivo findings were divided by tissue or organ in which they were analyzed. All in vitro findings were grouped in a single box.
Discussion
Although the assessment of the bias risk of included studies is less common in scoping reviews than it is in other reviews, we opted to use this tool because some of the studies reviewed here investigated, in addition to the Caps action pathway, its efficacy as an antiobesogenic agent in animal models of obesity. All evaluated studies presented a high RoB. This was an expected result, because previous research suggests that animal studies show a certain commitment in internal validity, which is related to methodological biases (80–82). In addition, it should be noted that many entries in SYRCLE's RoB tool were determined to exhibit an unclear risk of bias, as a consequence of the dearth of more accurate information on the methodological parameters adopted.
This is not a surprising finding. One study evaluating the quality of research conducted in animals, including 271 studies, revealed that the description of experimental details of the materials and methods used is rather weak (83). In addition, Ioannidis (84) highlighted a series of initiatives where researchers collaborated for the efficient execution of systematic reviews and meta-analyses of animal studies, such as the Collaborative Approach to Meta-Analysis and Review of Animal Experimental Studies (CAMARADES), which demonstrated that this type of research has low reliability (84). These studies suggest that the low study reliability is not because animal models are not appropriate for the study of human diseases, but is likely because of quality deficits, selective reports, and other biases related to basic research (82, 85–88).
These results demonstrate that it is necessary to improve the quality of experimental designs and to record essential experimental details in animal studies. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) is an important tool for assessing the quality of evidence in systematic reviews of randomized controlled trials (RCTs) and cohort studies (prospective and retrospective) that seek to analyze the efficacy, effectiveness, and safety of a health technology (89–93). Wei et al. (94) adapted the GRADE tool for animal studies (94). Considering this scenario, we recommend the use of the CAMARADES initiative in primary studies and the RoB and GRADE tools in systematic reviews of efficacy analyses using animal models. Thus, it is expected that the lack of reproducibility of results frequently observed between animal models and clinical tests can be partially minimized.
Studies suggest that TRPV1 activity is associated with metabolic homeostasis (40, 95). The mechanisms may include appetite control, improved pancreatic function, thermogenesis, and lipogenesis regulation (40, 96). All these factors are related to the development of obesity. However, studies with TRPV−/− mice reported conflicting results of a reduction (48), gain (41), or no effect (50, 97) on body weight. In addition, some studies of different clinical conditions, including obesity, have shown that the actions of Caps may also be mediated through activation of receptors, such as PPARs, independent of TRPV1 activity (31, 45, 51, 53, 56, 98–100).
The duration of Caps treatment varied considerably among the studies, which hindered a definitive conclusion. Kang et al. (45) induced obesity in C57BL/6 mice by feeding them an HFD for 10 wk and then supplemented the diet with Caps for another 10 wk. Caps-supplemented mice lost weight in the initial 5 wk but subsequently regained weight. Similarly, Lee et al. (101) induced obesity in C57BL/6 mice by feeding them an HFD for 8 wk before initiating topical treatment with Caps, which prevented body-weight gain compared with the nonsupplemented controls. These data reflect the lack of standardization of intervention time with Caps, as revealed by the studies included in this review. However, no adverse effects were observed in the in vivo studies.
The use of different in vitro concentrations makes it possible to verify the different Caps-induced effects. Lee et al. (102) investigated the effect of different concentrations of Caps (0.1, 1, and 10 μM) on lipid catabolism in 3T3-L1 adipocytes and found that Caps exerts lipolytic action by increasing triacylglycerol hydrolysis only at 10 μM. Moreover, when analyzing the regulation of genes related to lipid metabolism, the effects were found at 1 and 10 μM. The studies included in this review used concentrations ranging from 0.01 to 100 μM, which caused different effects (71–79). The action of Caps in reducing white adipocyte adipogenesis was observed at concentrations up to 1 μM (71, 72, 78). At higher concentrations, the opposite effect was observed in both white and brown adipocytes (75, 76, 78). The studies included in this review support the idea that Caps modulates adipogenesis and regulates genes related to lipid metabolism, thereby reducing body adiposity (71–79).
Previous studies have shown that Caps and its nonpungent analogs reduce the lipid content in murine white adipocytes in a TRPV1-dependent manner (103, 104). In our review, all studies evaluating the effect of Caps in reducing lipid storage in white adipocytes found a TRPV1-dependent action (71, 72, 78) at concentrations up to 1 μM. Nonetheless, 1 study showed that, at higher concentrations (50 and 100 μM), Caps increased adipogenesis independently of TRPV1 activation (78). Studies have shown that calcium ions are involved in the prevention of adipogenesis (105–107), thus supporting the results showing that a low Caps concentration reduces adipogenesis because TRPV1 activation induces calcium intracellular influx.
With regard to the browning process, our review found that most studies investigating the potential of Caps to induce the browning phenotype showed a TRPV1-dependent action (73, 74, 78). All of these studies demonstrated that the calcium influx triggered by Caps activation of TRPV1 stimulated the transcriptional activity of PPARγ, culminating in browning induction. These results suggest that PPARγ functions as a downstream target of Caps-triggered TRPV1 signaling. One study reported that the combined effect of Caps and capsiate on the browning process was triggered by PPARγ and β3-adrenergic receptors (77). Because PPARγ may be activated via TRPV1 signaling, further studies using cells with these receptors knocked out or TRPV1 antagonists are necessary to confirm that the effects of Caps are independent of the TRPV1 pathway.
Among the 3 studies evaluating the action of Caps directly in BAT, 2 studies suggested that the thermogenic stimulus was associated with alternative pathways (75, 76). One study demonstrated that these effects were associated with increased intracellular calcium induction by endoplasmic reticulum stress rather than TRPV1 activation (76). However, a third study found a dependency on TRPV1 (79).
Different studies have demonstrated that capsaicinoids and capsioids stimulate brown adipocyte activity or promote browning (28, 34–36, 57, 108–110). Some studies have shown thermogenic responses to the sympathetic nervous system (SNS) activated through β-adrenergic signaling (111–113). In addition, consistent evidence links the activation of PPARγ to brown adipocyte thermogenesis and browning program induction (114–118). The ability of Caps to stimulate thermogenesis may be related to 1 or even both of those mechanisms, as demonstrated by the studies we reviewed. Nonetheless, it is unclear whether the induction of browning or brown cell activity by Caps is exclusively dependent on upstream TRPV1 signaling or whether different metabolic targets of Caps (and TRPV1 independent) are also involved. Studies suggest that Caps acts in various pathological conditions independently of TRPV1 activation (31, 53, 55).
Nevertheless, several studies have associated the effect of Caps on the browning process and brown adipocytes with TRPV1 activation (28, 35, 39, 40), which supports the evidence presented by some studies reported in this review. Data from studies published in the literature suggest that this action is generally linked to stimulation of the TRPV1–SNS axis via β-adrenergic signaling (119–121). Most of the studies reviewed here associated the thermogenic ability of Caps with the regulation of the transcriptional activity of PPARγ via TRPV1 activation, which induces the expression of genes related to the browning process (73, 74, 78) and adipogenesis in brown adipocytes (79).
Some studies have suggested that cross-talk occurs between PPARγ and TRPV1 (122–124). Lieder et al. (123) demonstrated that the modulation of TRPV1 activity by an alkamide reduced the lipid accumulation in 3T3-L1 adipocytes by reducing PPARγ expression. Alsalem et al. (122) showed that the dual PPARα/γ agonist tesaglitazar caused analgesic effects via TRPV1 and subsequently desensitized nociceptive cells. Moreover, Ambrosino et al. (124) showed that different PPARα agonists stimulated TRPV1-induced ionic currents. It should be noted that most studies exploring the link between these receptors focused on analgesia or lipid metabolism in white cells.
Thus, the studies included in this review are pioneers in the investigation of these thermogenesis pathways. Nonetheless, further studies are required to better understand the possible cross-talk between TRPV1 and PPARs in BAT and WAT metabolism. Since PPAR agonists can stimulate TRPV1 activity and consequently calcium influx, future investigations are required to examine whether Caps acts on adipose tissue exclusively via direct TRPV1 activation or if it generates calcium currents by alternative mechanisms.
Inflammation of adipokines, dysregulation of lipids, and glucose homeostasis are characteristics of obesity, contributing to the development or worsening of several metabolic disorders (6, 125, 126). Caps has been shown to minimize the effect of obesity in those disorders (37, 45) and improve glucose homeostasis by reducing hyperglycemia (45, 101, 127–129). Among the studies included and reviewed in this analysis, only 1 analyzed glucose homeostasis (79), whereas others found lower serum triglyceride and cholesterol concentrations associated with the dietary use of Caps, minimizing complications related to excess body adiposity (71–73). Moreover, all studies included in this review showed a positive effect of Caps in controlling obesity through lowering weight gain (71–73) or favoring weight loss (74, 79).
Although the results favored the intervention of Caps in both the primary weight-loss outcome and the other secondary outcomes, they presented a high RoB. However, RCTs also demonstrated positive results, but they notably commonly evaluated secondary outcomes such as appetite, energy expenditure (EE), respiratory quotient (RQ), and fat oxidation (30, 32, 130). We did not find any RCT that effectively evaluated the effects of Caps on weight-loss parameters. In an RCT meta-analysis exploring the effects of Caps and capsiate on EE and RQ, Zsiborás et al. (27) demonstrated that Caps effectively increased EE and reduced RQ in individuals with a BMI (in kg/m2) >25 (27).
Although other reviews have reported positive effects (57, 109, 131), the clinical significance of these results cannot be confirmed because, in addition to the lack of systematic reviews demonstrating the effect of Caps directly on weight loss, no RCT reviews have explored the RoB or the quality of the evidence. Another limitation of the existing literature is the lack of studies evaluating the potential sustainability of the effects of Caps, considering that most had a short follow-up period. Because obesity is a chronic disease, we considered this to be a relevant deficiency. Finally, studies evaluating the effectiveness and safety of Caps are necessary to validate their effect in uncontrolled scenarios (real-world data).
Finally, although 9 studies were included in this review, some were conducted by the same group of researchers. Consequently, 2 studies (75, 76) used the same method of evaluating the thermogenesis of BAT, and subsequently concluded that the underlying mechanism was TRPV1-independent. A TRPV1-independent action of Caps was also found in another study, from another group, although the process was not evaluated in thermogenesis but rather in adipogenesis (78). Other studies from the same research group (73, 74, 79) investigated browning and found that the effects of Caps were TRPV1-dependent. Although the effects and observed outcomes were the same, the methods and techniques used by the researchers were diverse. However, another independent study that also evaluated the browning process (77) reported an action of Caps that was mediated via PPARγ. Other studies have reported the same outcome, linking the action of Caps to TRPV1, but not by evaluating the browning process (71, 72, 78). It is important to emphasize that to reach a conclusion about the target of the action of Caps under each evaluated condition, further studies with experimental strategies that can be reproduced by other groups are necessary to corroborate the existing findings (84).
Limitations
The limitations of this scoping review include the lack of standardization of the Caps intervention time in the in vivo models, the scarcity of studies on human adipocyte culture, and the lack of studies that simultaneously evaluated the possible effects of antagonism or overexpression of PPARγ and TRPV1 in the activity of Caps. Future studies are necessary to fill these gaps and clarify the signaling pathways involved in the action of Caps. Nevertheless, the present scoping review included studies with diverse methods, which allowed us to collate information from in vitro and in vivo studies evaluating Caps under different conditions and, thus, identified aspects to be improved in future studies.
Conclusions
Most of the studies reviewed related the obesity-reducing activity of Caps to the activation of TRPV1 and had a high RoB. Some studies showed PPARγ to be a downstream target of the signaling cascade triggered by Caps-induced TRPV1 activation. Further studies would be necessary to evaluate the effects of antagonism or overexpression of PPARs in the presence or absence of TRPV1 activity. The complete analysis of these pathways will contribute to the elucidation of the role of these receptors in Caps-mediated antiobesogenic effects.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—DLA, NAMN, and PHRFA: conceptualization and data curation, formal analysis, methodology, and writing of original draft; PHRFA, COBR, JASG, and JIA-L: writing—review and editing; and all authors: read and approved the final manuscript.
Notes
This project is funded by the following research funding institutions: National Council for Scientific and Technological Development [Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)] and Coordination for the Improvement of Higher Education Personnel [Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)], through doctoral and postdoctoral fellowships.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BAT, brown adipose tissue; Caps, capsaicin; CAMARADES, Collaborative Approach to Meta-Analysis and Review of Animal Experimental Studies; EE, energy expenditure; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HFD, high-fat diet; PPAR, peroxisome proliferator-activated receptor; PRISMA-ScR, Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews; RCT, randomized controlled trial; RoB, risk-of-bias; RQ, respiratory quotient; SNS, sympathetic nervous system; SYRCLE, SYstematic Review Centre for Laboratory animal Experimentation; TRPV1, transient receptor potential vanilloid 1; WAT, white adipose tissue.
Contributor Information
Danielle L Ávila, Instituto de Ciências Biológicas, Departamento de Bioquímica e Imunologia, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
Núbia A M Nunes, Instituto de Ciências Biológicas, Departamento de Bioquímica e Imunologia, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
Paulo H R F Almeida, Programa de Pós-Graduação em Medicamentos e Assistência Farmacêutica, Departamento de Farmácia Social, Faculdade de Farmácia, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
Juliana A S Gomes, Instituto de Ciências Biológicas, Departamento de Morfologia, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
Carla O B Rosa, Faculdade de Nutrição, Departamento de Nutrição e Saúde, Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil.
Jacqueline I Alvarez-Leite, Instituto de Ciências Biológicas, Departamento de Bioquímica e Imunologia, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
References
- 1. Elagizi A, Kachur S, Lavie CJ, Carbone S, Pandey A, Ortega FB, Milani RV. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–50. [DOI] [PubMed] [Google Scholar]
- 2. Zhang F, Ye J, Zhu X, Wang L, Gao P, Shu G, Jiang Q, Wang S. Anti-obesity effects of dietary calcium: the evidence and possible mechanisms. Int J Mol Sci. 2019;20:3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . Health topics. Obesity. [Internet]. WHO; 2020; [cited 2020 Apr 28]. Available from: https://www.who.int/health-topics/obesity. [Google Scholar]
- 4. Organisation for Economic Co-operation and Development (OECD) . International regulatory co-operation: the role of international organisations in fostering better rules of globalisation. [Internet]. World Health Organization;2016; [cited 2021 Feb 8]. Available from: 10.1787/9789264244047-en. [DOI] [Google Scholar]
- 5. Wan-Loy C, Siew-Moi P. Marine algae as a potential source for anti-obesity agents. Mar Drugs. 2016;14:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C. Obesity as a disease. Med Clin North Am. 2018;102:13–33. [DOI] [PubMed] [Google Scholar]
- 7. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23:804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. [DOI] [PubMed] [Google Scholar]
- 9. Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5:1218–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10:364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. 2017;122:1–7. [DOI] [PubMed] [Google Scholar]
- 12. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118:1752–70. [DOI] [PubMed] [Google Scholar]
- 13. Mandviwala T, Khalid U, Deswal A. Obesity and cardiovascular disease: a risk factor or a risk marker?. Curr Atheroscler Rep. 2016;18:21. [DOI] [PubMed] [Google Scholar]
- 14. Ackerman SE, Blackburn OA, Marchildon F, Cohen P. Insights into the link between obesity and cancer. Curr Obes Rep. 2017;6:195–203. [DOI] [PubMed] [Google Scholar]
- 15. Tahergorabi Z, Khazaei M, Moodi M, Chamani E. From obesity to cancer: a review on proposed mechanisms. Cell Biochem Funct. 2016;34:533–45. [DOI] [PubMed] [Google Scholar]
- 16. GBD 2015 Obesity Collaborators; Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh Met al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu H, Cupples LA, Stokes A, Liu C-T. Association of obesity with mortality over 24 years of weight history: findings from the Framingham Heart Study. JAMA Netw Open. 2018;1:e184587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Global BMI Mortality Collaboration; Angelantonio ED, Bhupathiraju S, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns B, Huxley R, Jackson Cet al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, Willett WC, Hu FB. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, Ju W, Huo C, Zhang S, Wang X, Huang K. Overweight and obesity as independent factors for increased risk of hepatocellular cancer-related mortality: a meta-analysis. J Am Coll Nutr. 2021;40:287–93. [DOI] [PubMed] [Google Scholar]
- 21. Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3.6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6:944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, Loomba R, Camilleri M, Singh S. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA. 2016;315:2424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Horvath C, Wolfrum C. Feeding brown fat: dietary phytochemicals targeting non-shivering thermogenesis to control body weight. Proc Nutr Soc. 2020;79:338–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amiot MJ, Riva C, Vinet A. Effects of dietary polyphenols on metabolic syndrome features in humans: a systematic review. Obes Rev. 2016;17:573–86. [DOI] [PubMed] [Google Scholar]
- 25. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leung FW. Capsaicin as an anti-obesity drug. Prog Drug Res. 2014;68:171–9. [DOI] [PubMed] [Google Scholar]
- 27. Zsiborás C, Mátics R, Hegyi P, Balaskó M, Pétervári E, Szabó I, Sarlós P, Mikó A, Tenk J, Rostás Iet al. Capsaicin and capsiate could be appropriate agents for treatment of obesity: a meta-analysis of human studies. Crit Rev Food Sci Nutr. 2018;58:1419–27. [DOI] [PubMed] [Google Scholar]
- 28. Montanari T, Boschi F, Colitti M. Comparison of the effects of browning-inducing capsaicin on two murine adipocyte models. Front Physiol. 2019;10:1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whiting S, Derbyshire EJ, Tiwari B. Could capsaicinoids help to support weight management? A systematic review and meta-analysis of energy intake data. Appetite. 2014;73:183–8. [DOI] [PubMed] [Google Scholar]
- 30. Yoshioka M, St-Pierre S, Drapeau V, Dionne I, Doucet E, Suzuki M, Tremblay A. Effects of red pepper on appetite and energy intake. Br J Nutr. 1999; 82:2, 115–23. [PubMed] [Google Scholar]
- 31. Bort A, Sánchez BG, Mateos-Gómez PA, Díaz-Laviada I, Rodríguez-Henche N. Capsaicin targets lipogenesis in HepG2 cells through AMPK activation, AKT inhibition and PPARs regulation. Int J Mol Sci. 2019;20:1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lejeune M, Kovacs EMR, Westerterp-Plantenga MS. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br J Nutr. 2003;90:651–9. [DOI] [PubMed] [Google Scholar]
- 33. Payab M, Hasani-Ranjbar S, Baeeri M, Rahimifard M, Arjmand B, Haghi-Aminjan H, Abdollahi M, Larijani B. Development of a novel anti-obesity compound with inhibiting properties on the lipid accumulation in 3T3-L1 adipocytes. Iran Biomed J. 2020;24:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saito M, Yoneshiro T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr Opin Lipidol. 2013;24:71–7. [DOI] [PubMed] [Google Scholar]
- 35. Saito M. Capsaicin and related food ingredients reducing body fat through the activation of TRP and brown fat thermogenesis. Adv Food Nutr Res. 2015;76:1–28. [DOI] [PubMed] [Google Scholar]
- 36. Okla M, Kim J, Koehler K, Chung S. Dietary factors promoting brown and beige fat development and thermogenesis. Adv Nutr. 2017;8:473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen W, Shen M, Zhao X, Zhu H, Yang Y, Lu S, Tan Y, Li G, Li M, Wang Jet al. Anti-obesity effect of capsaicin in mice fed with high-fat diet is associated with an increase in population of the gut bacterium. Front Microbiol. 2017;8:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Y, Tang C, Tang Y, Yin H, Liu X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short-chain fatty acid concentrations. Food Nutr Res. 2020;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Panchal SK, Bliss E, Brown L. Capsaicin in metabolic syndrome. Nutrients. 2018;10:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Christie S, Wittert GA, Li H, Page AJ. Involvement of TRPV1 channels in energy homeostasis. Front Endocrinol. 2018;9:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee E, Jung DY, Kim JH, Patel PR, Hu X, Lee Y, Azuma Y, Wang H-F, Tsitsilianos N, Shafiq Uet al. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015;29:3182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma L, Zhong J, Zhao Z, Luo Z, Ma S, Sun J, He H, Zhu T, Liu D, Zhu Zet al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc Res. 2011;92:504–13. [DOI] [PubMed] [Google Scholar]
- 43. Sun J, Pu Y, Wang P, Chen S, Zhao Y, Liu C, Shang Q, Zhu Z, Liu D.. TRPV1-mediated UCP2 upregulation ameliorates hyperglycemia-induced endothelial dysfunction. Cardiovasc Diabetol. 2013;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang P, Yan Z, Zhong J, Chen J, Ni Y, Li L, Ma L, Zhao Z, Liu D, Zhu Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes. 2012;61:2155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang J-H, Goto T, Han I-S, Kawada T, Kim YM, Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity. 2010;18:780–7. [DOI] [PubMed] [Google Scholar]
- 46. Baskaran P, Markert L, Bennis J, Zimmerman L, Fox J, Thyagarajan B.. Assessment of pharmacology, safety, and metabolic activity of capsaicin feeding in mice.Sci Rep. 2019;9:8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li Q, Li L, Wang F, Chen J, Zhao Y, Wang P, Nilius B, Liu D, Zhu Z. Dietary capsaicin prevents nonalcoholic fatty liver disease through transient receptor potential vanilloid 1-mediated peroxisome proliferator-activated receptor δ activation. Pflugers Arch. 2013;465:1303–16. [DOI] [PubMed] [Google Scholar]
- 48. Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51(2):159–212. [PubMed] [Google Scholar]
- 50. Marshall NJ, Liang L, Bodkin J, Dessapt-Baradez C, Nandi M, Collot-Teixeira S, Smillie S-J, Lalgi K, Fernandes ES, Gnudi Let al. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension. 2013;61:246–52. [DOI] [PubMed] [Google Scholar]
- 51. Kang J-H, Kim C-S, Han I-S, Kawada T, Yu R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007;581:4389–96. [DOI] [PubMed] [Google Scholar]
- 52. Tan S, Gao B, Tao Y, Guo J, Su Z-Q. Antiobese effects of capsaicin-chitosan microsphere (CCMS) in obese rats induced by high fat diet. J Agric Food Chem. 2014;62:1866–74. [DOI] [PubMed] [Google Scholar]
- 53. Kim C-S, Park W-H, Park J-Y, Kang J-H, Kim M-O, Kawada T, Yoo H, Han I-S, Yu R. Capsaicin, a spicy component of hot pepper, induces apoptosis by activation of the peroxisome proliferator-activated receptor gamma in HT-29 human colon cancer cells. J Med Food. 2004;7:267–73. [DOI] [PubMed] [Google Scholar]
- 54. Bitencourt S, de Mesquita FC, Caberlon E, da Silva GV, Basso BS, Ferreira GA, de Oliveira JR. Capsaicin induces de-differentiation of activated hepatic stellate cell. Biochem Cell Biol. 2012;90:683–90. [DOI] [PubMed] [Google Scholar]
- 55. Choi JH, Jin SW, Choi CY, Kim HG, Lee GH, Kim YA, Chung YC, Jeong HG.. Capsaicin inhibits dimethylnitrosamine-induced hepatic fibrosis by inhibiting the TGF-β1/Smad pathway via peroxisome proliferator-activated receptor gamma activation. J Agric Food Chem. 2017;65:317–26. [DOI] [PubMed] [Google Scholar]
- 56. Park J-Y, Kawada T, Han I-S, Kim B-S, Goto T, Takahashi N, Fushiki T, Kurata T, Yu R. Capsaicin inhibits the production of tumor necrosis factor alpha by LPS-stimulated murine macrophages, RAW 264.7: a PPARgamma ligand-like action as a novel mechanism. FEBS Lett. 2004;572:266–70. [DOI] [PubMed] [Google Scholar]
- 57. Zheng J, Zheng S, Feng Q, Zhang Q, Xiao X. Dietary capsaicin and its anti-obesity potency: from mechanism to clinical implications. Biosci Rep. 2017;37:BSR20170286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li R, Lan Y, Chen C, Cao Y, Huang Q, Ho C-T, Lu M. Anti-obesity effects of capsaicin and the underlying mechanisms: a review. Food Funct. 2020;11:7356–70. [DOI] [PubMed] [Google Scholar]
- 59. Rosca AE, Iesanu MI, Zahiu CDM, Voiculescu SE, Paslaru AC, Zagrean A-M. Capsaicin and gut microbiota in health and disease. Molecules. 2020;25:5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks Let al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73. [DOI] [PubMed] [Google Scholar]
- 62. Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. Chapter 11: scoping reviews (2020 version). In: Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer's Manual. Joanna Briggs Institute; 2020. [Google Scholar]
- 63. Almeida PHRF, Silva TBC, de Assis Acurcio F, Guerra Júnior AA, Araújo VE, Diniz LM, Godman B, Almeida AM, Alvares J. Quality of life of patients with type 1 diabetes mellitus using insulin analog glargine compared with NPH insulin: a systematic review and policy implications. Patient. 2018;11:377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Silva TBC, Almeida PHRF, Araújo VE, Acurcio F de A, Guerra Júnior AA, Godman B, Alvares J. Effectiveness and safety of insulin glargine detemir analysis in patients with type 1 diabetes: systematic review and meta-analysis. Ther Adv Endocrinol Metab. 2018;9:241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Silva WC, de Araujo VE, Lima E, Dos Santos JBR, da Silva MRR, Almeida PHRF, de Assis Acurcio F, Godman B, Kurdi A, Cherchiglia MLet al. Comparative effectiveness and safety of monoclonal antibodies (bevacizumab, cetuximab, and panitumumab) in combination with chemotherapy for metastatic colorectal cancer: a systematic review and meta-analysis. BioDrugs. 2018;32:585–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lemos LLP, Carvalho de Souza M, Pena Moreira D, Almeida PHRF, Godman B, Verguet S, Guerra Júnior AA, Leal Cherchiglia M. Stage at diagnosis and stage-specific survival of breast cancer in Latin America and the Caribbean: a systematic review and meta-analysis. PLoS One. 2019;14:e0224012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hooijmans CR, Rovers MM, de Vries RBM, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Method BioMed Central. 2014;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fernandes BD, Almeida PHRF, Foppa AA, Sousa CT, Ayres LR, Chemello C. Pharmacist-led medication reconciliation at patient discharge: a scoping review. Res Social Adm Pharm. 2020;16:605–13. [DOI] [PubMed] [Google Scholar]
- 70. Sousa MDCVB, Fernandes BD, Foppa AA, Almeida PHRF, Mendonça SAM, Chemello C. Tools to prioritize outpatients for pharmaceutical service: a scoping review. Res Social Adm Pharm. 2020;16:1645–57. [DOI] [PubMed] [Google Scholar]
- 71. Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJet al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–70. [DOI] [PubMed] [Google Scholar]
- 72. Chen J, Li L, Li Y, Liang X, Sun Q, Yu H, Zhong J, Ni Y, Chen J, Zhao Zet al. Activation of TRPV1 channel by dietary capsaicin improves visceral fat remodeling through connexin43-mediated Ca2+ influx. Cardiovasc Diabetol. 2015;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173:2369–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Krishnan V, Baskaran P, Thyagarajan B. Troglitazone activates TRPV1 and causes deacetylation of PPARγ in 3T3-L1 cells. Biochim Biophys Acta Mol Basis Dis. 2019;1865:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kida R, Yoshida H, Murakami M, Shirai M, Hashimoto O, Kawada T, Matsui T, Funaba M. Direct action of capsaicin in brown adipogenesis and activation of brown adipocytes. Cell Biochem Funct. 2016;34:34–41. [DOI] [PubMed] [Google Scholar]
- 76. Kida R, Noguchi T, Murakami M, Hashimoto O, Kawada T, Matsui T, Funaba M. Supra-pharmacological concentration of capsaicin stimulates brown adipogenesis through induction of endoplasmic reticulum stress. Sci Rep. 2018;8:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fan L, Xu H, Yang R, Zang Y, Chen J, Qin H. Combination of capsaicin and capsiate induces browning in 3T3-L1 white adipocytes via activation of the peroxisome proliferator-activated receptor γ/β-adrenergic receptor signaling pathways. J Agric Food Chem. 2019;67:6232–40. [DOI] [PubMed] [Google Scholar]
- 78. Baboota RK, Singh DP, Sarma SM, Kaur J, Sandhir R, Boparai RK, Kondepudi KK, Bishnoi M. Capsaicin induces “brite” phenotype in differentiating 3T3-L1 preadipocytes. PLoS One. 2014;9:e103093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Baskaran P, Krishnan V, Fettel K, Gao P, Zhu Z, Ren J, Thyagarajan B. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int J Obes. 2017;41:739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Begley CG, Ellis LM. Drug development: raise standards for preclinical cancer research. Nature. 2012;483:531–3. [DOI] [PubMed] [Google Scholar]
- 81. Faggion CM Jr, Diaz KT, Aranda L, Gabel F, Listl S, Alarcón MA. The risk of bias of animal experiments in implant dentistry: a methodological study. Clin Oral Implants Res. 2017;28:e39–45. [DOI] [PubMed] [Google Scholar]
- 82. Tsilidis KK, Panagiotou OA, Sena ES, Aretouli E, Evangelou E, Howells DW, Al-Shahi Salman R, Macleod MR, Ioannidis JPA. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol. 2013;11:e1001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kilkenny C, Parsons N, Kadyszewski E, Festing MFW, Cuthill IC, Fry D, Hutton J, Altman DG. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS One. 2009;4:e7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ioannidis JPA. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 2016;94:485–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC.. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA. 1992;268(2):240–8. [PubMed] [Google Scholar]
- 86. Iqbal SA, Wallach JD, Khoury MJ, Schully SD, Ioannidis JPA. Reproducible research practices and transparency across the biomedical literature. PLoS Biol. 2016;14:e1002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sena ES, van der Worp HB, Bath PMW, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, Bath PMW, Macleod M, Dirnagl U. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke. 2008;39:929–34. [DOI] [PubMed] [Google Scholar]
- 89. Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Yet al. GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277–82. [DOI] [PubMed] [Google Scholar]
- 90. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist Get al. GRADE guidelines: 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–93. [DOI] [PubMed] [Google Scholar]
- 91. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EAet al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–302. [DOI] [PubMed] [Google Scholar]
- 92. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist Get al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64:1303–10. [DOI] [PubMed] [Google Scholar]
- 93. Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, Atkins D, Kunz R, Brozek J, Montori Vet al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–6. [DOI] [PubMed] [Google Scholar]
- 94. Wei D, Tang K, Wang Q, Estill J, Yao L, Wang X, Chen Y, Yang K. The use of GRADE approach in systematic reviews of animal studies. J Evid Based Med. 2016;9:98–104. [DOI] [PubMed] [Google Scholar]
- 95. Zsombok A. Vanilloid receptors—do they have a role in whole body metabolism? Evidence from TRPV1. J Diabetes Complications. 2013;27:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sun F, Xiong S, Zhu Z. Dietary capsaicin protects cardiometabolic organs from dysfunction. Nutrients. 2016;8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kentish SJ, Frisby CL, Kritas S, Li H, Hatzinikolas G, O'Donnell TA, Wittert GA, Page AJ. TRPV1 channels and gastric vagal afferent signalling in lean and high fat diet induced obese mice. PLoS One. 2015;10:e0135892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tang J, Luo K, Li Y, Chen Q, Tang D, Wang D, Xiao J. Capsaicin attenuates LPS-induced inflammatory cytokine production by upregulation of LXRα. Int Immunopharmacol. 2015;28:264–9. [DOI] [PubMed] [Google Scholar]
- 99. Hirai S, Takahashi N, Goto T, Lin S, Uemura T, Yu R, Kawada T. Functional food targeting the regulation of obesity-induced inflammatory responses and pathologies. Mediators Inflamm. 2010;2010:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rigano D, Sirignano C, Taglialatela-Scafati O. The potential of natural products for targeting PPAR. Acta Pharm Sin B. 2017;7:427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lee G-R, Shin MK, Yoon D-J, Kim A-R, Yu R, Park N-H, Han I-S. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity. 2013;21:115–22. [DOI] [PubMed] [Google Scholar]
- 102. Lee M-S, Kim C-T, Kim I-H, Kim Y. Effects of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytother Res. 2011;25:935–9. [DOI] [PubMed] [Google Scholar]
- 103. Hsu C-L, Yen G-C. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J Agric Food Chem. 2007;55:1730–6. [DOI] [PubMed] [Google Scholar]
- 104. Hong Q, Xia C, Xiangying H, Quan Y. Capsinoids suppress fat accumulation via lipid metabolism. Mol Med Rep. 2015;11:1669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ntambi JM, Takova T. Role of Ca2+ in the early stages of murine adipocyte differentiation as evidenced by calcium mobilizing agents. Differentiation. 1996;60:151–8. [DOI] [PubMed] [Google Scholar]
- 106. Shi H, Halvorsen YD, Ellis PN, Wilkison WO, Zemel MB. Role of intracellular calcium in human adipocyte differentiation. Physiol Genomics. 2000;3:75–82. [DOI] [PubMed] [Google Scholar]
- 107. Zhai M, Yang D, Yi W, Sun W. Involvement of calcium channels in the regulation of adipogenesis. Adipocyte. 2020;9:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ohyama K, Nogusa Y, Shinoda K, Suzuki K, Bannai M, Kajimura S. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes. 2016;65:1410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ludy M-J, Moore GE, Mattes RD. The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chem Senses. 2012;37:103–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Osuna-Prieto FJ, Martinez-Tellez B, Sanchez-Delgado G, Aguilera CM, Lozano-Sánchez J, Arráez-Román D, Segura-Carretero A, Ruiz JR. Activation of human brown adipose tissue by capsinoids, catechins, ephedrine, and other dietary components: a systematic review. Adv Nutr. 2019;10:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria Aet al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21:33–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Peng X-R, Gennemark P, O'Mahony G, Bartesaghi S. Unlock the thermogenic potential of adipose tissue: pharmacological modulation and implications for treatment of diabetes and obesity. Front Endocrinol. 2015;6:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Song N-J, Chang S-H, Li DY, Villanueva CJ, Park KW. Induction of thermogenic adipocytes: molecular targets and thermogenic small molecules. Exp Mol Med. 2017;49:e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tai TA, Jennermann C, Brown KK, Oliver BB, MacGinnitie MA, Wilkison WO, Brown HR, Lehmann JM, Kliewer SA, Morris DCet al. Activation of the nuclear receptor peroxisome proliferator-activated receptor gamma promotes brown adipocyte differentiation. J Biol Chem. 1996;271:29909–14. [DOI] [PubMed] [Google Scholar]
- 117. Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, Wang H, Scherer PE, Farmer SR. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29:4714–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lasar D, Rosenwald M, Kiehlmann E, Balaz M, Tall B, Opitz L, Lidell ME, Zamboni N, Krznar P, Sun Wet al. Peroxisome proliferator activated receptor gamma controls mature brown adipocyte inducibility through glycerol kinase. Cell Rep. 2018;22:760–73. [DOI] [PubMed] [Google Scholar]
- 119. Whiting S, Derbyshire E, Tiwari BK. Capsaicinoids and capsinoids: a potential role for weight management? A systematic review of the evidence. Appetite. 2012;59:341–8. [DOI] [PubMed] [Google Scholar]
- 120. Liu J, Wang Y, Lin L. Small molecules for fat combustion: targeting obesity. Acta Pharm Sin B. 2019;9:220–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Yoneshiro T, Saito M. Transient receptor potential activated brown fat thermogenesis as a target of food ingredients for obesity management. Curr Opin Clin Nutr Metab Care. 2013;16:625–31. [DOI] [PubMed] [Google Scholar]
- 122. Alsalem M, Haddad M, Aldossary SA, Kalbouneh H, Azab B, Dweik A, Imraish A, El-Salem K. Effects of dual peroxisome proliferator-activated receptors and activation in two rat models of neuropathic pain. PPAR Res. 2019;2019:2630232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lieder B, Zaunschirm M, Holik A-K, Ley JP, Hans J, Krammer GE, Somoza V. The alkamide -pellitorine targets PPARγ via TRPV1 and TRPA1 to reduce lipid accumulation in developing 3T3-L1 adipocytes. Front Pharmacol. 2017;8:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ambrosino P, Soldovieri MV, De Maria M, Russo C, Taglialatela M. Functional and biochemical interaction between PPARα receptors and TRPV1 channels: potential role in PPARα agonists-mediated analgesia. Pharmacol Res. 2014;87:113–22. [DOI] [PubMed] [Google Scholar]
- 125. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jung UJ, Choi M-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Okumura T, Tsukui T, Hosokawa M, Miyashita K. Effect of caffeine and capsaicin on the blood glucose levels of obese/diabetic KK-A(y) mice. J Oleo Sci. 2012;61:515–23. [DOI] [PubMed] [Google Scholar]
- 128. Kang J-H, Tsuyoshi G, Le Ngoc H, Kim H-M, Tu TH, Noh H-J, Kim C-S, Choe S-Y, Kawada T, Yoo Het al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J Med Food. 2011;14:310–15. [DOI] [PubMed] [Google Scholar]
- 129. Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med. 1986;183:250–6. [DOI] [PubMed] [Google Scholar]
- 130. Rigamonti AE, Casnici C, Marelli O, De Col A, Tamini S, Luchetti E, Tringali G, Di Micheli R, Abbruzzese L, Bortolotti Met al. Acute administration of capsaicin increases resting energy expenditure in young obese subjects without affecting energy intake, appetite, and circulating levels of orexigenic/anorexigenic peptides. Nutr Res. 2018; 52:71–9. [DOI] [PubMed] [Google Scholar]
- 131. Fattori V, Hohmann MSN, Rossaneis AC, Pinho-Ribeiro FA, Verri WA. Capsaicin: current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules. 2016;21:844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.