ABSTRACT
The athlete's goal is to optimize their performance. Towards this end, nutrition has been used to improve the health of athletes’ brains, bones, muscles, and cardiovascular system. However, recent research suggests that the gut and its resident microbiota may also play a role in athlete health and performance. Therefore, athletes should consider dietary strategies in the context of their potential effects on the gut microbiota, including the impact of sports-centric dietary strategies (e.g., protein supplements, carbohydrate loading) on the gut microbiota as well as the effects of gut-centric dietary strategies (e.g., probiotics, prebiotics) on performance. This review provides an overview of the interaction between diet, exercise, and the gut microbiota, focusing on dietary strategies that may impact both the gut microbiota and athletic performance. Current evidence suggests that the gut microbiota could, in theory, contribute to the effects of dietary intake on athletic performance by influencing microbial metabolite production, gastrointestinal physiology, and immune modulation. Common dietary strategies such as high protein and simple carbohydrate intake, low fiber intake, and food avoidance may adversely impact the gut microbiota and, in turn, performance. Conversely, intake of adequate dietary fiber, a variety of protein sources, and emphasis on unsaturated fats, especially omega-3 (ɷ-3) fatty acids, in addition to consumption of prebiotics, probiotics, and synbiotics, have shown promising results in optimizing athlete health and performance. Ultimately, while this is an emerging and promising area of research, more studies are needed that incorporate, control, and manipulate all 3 of these elements (i.e., diet, exercise, and gut microbiome) to provide recommendations for athletes on how to “fuel their microbes.”
Keywords: microbiome, athletic performance, gastrointestinal health, protein, carbohydrates, prebiotics, probiotics
Statement of Significance: This review provides a comprehensive evaluation of the current evidence for the effects of diet, as it pertains to athletic performance, on the gut microbiota, and the potential for the gut microbiota to impact athletic performance as a result of diet-induced modifications.
Introduction
The human body integrates thousands of biochemical processes to manifest the various aspects of its metabolic phenotype. The athlete's goal is to optimize this complex system to enhance performance. Nutrition has long been used as a tool by athletes to feed their brains, bones, muscles, and cardiovascular system to foster peak performance (1). However, recent scientific advances suggest that nutrition may also influence athletic performance via the gut and the trillions of microorganisms that inhabit this ecosystem (Text Box 1) (2–4). Importantly, diet affects the microbial community within the gut (5–7). As a result, the gut microbiota mediates and modulates many of the effects of diet and nutrition and health, such as the risk of chronic diseases including obesity, type 2 diabetes, and cardiovascular disease (8, 9). However, athletes are interested not only in preventing disease but also in optimizing health and performance.
Text Box 1.
Definitions
Gut microbiota: the collection of microorganisms, including bacteria, archaea, fungi, and viruses, in the gut.
Gut microbiome: the collection of genetic information contained within the microbiota that provides information about what microorganisms are present as well as the functional capacity of the ecosystem.
Physical activity: any body movement produced by skeletal muscles that results in energy expenditure.
Exercise: a subcategory of physical activity that encompasses planned, structure, and repetitive movement and has as a final or an intermediate objective the improvement in or maintenance of physical fitness.
References: 3, 4.
Given the gut microbiota's potential to influence athletic performance and its responsiveness to diet, “fueling your microbes” should be seen as a strategy for athletes attempting to optimize performance. Therefore, this report aims to provide a comprehensive review of research on 1) common dietary strategies utilized by athletes and their effects on the gut microbiota and 2) dietary strategies utilized to improve gastrointestinal health and effects on athletic performance (Figure 1). This review summarizes clinical research investigating connections between the gut microbiota/microbiome and exercise since 2008. However, research on the gut microbiota/microbiome or exercise from any time was included when necessary to provide context, including mechanisms of action. This review aims to synthesize nutrition, exercise, and gut microbiota research to highlight what is known, gaps in the literature, and future directions for research to optimize the interaction between diet, sports, and the gut microbiota for health and athletic performance.
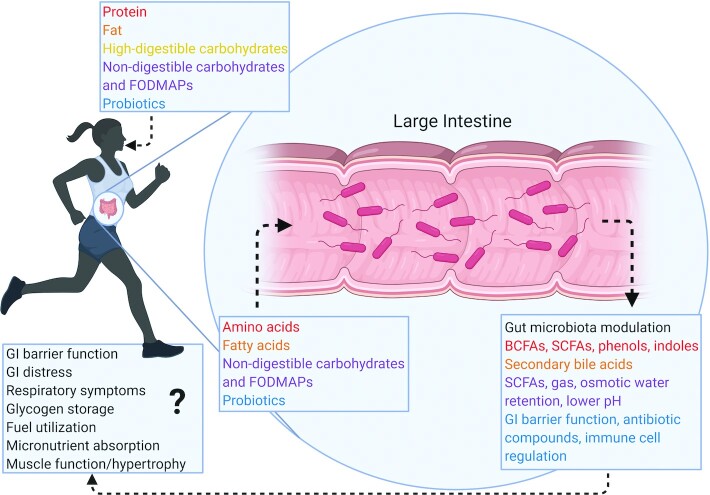
FIGURE 1.
Fueling your microbes for athletic performance. Sport-centric and gut-centric dietary strategies both modulate the composition and function of the gut microbiota, which may then mediate or modulate the effects of these dietary strategies on athletic performance. Human digestive processes produce amino acids and fatty acids from ingested protein and fat, respectively, while nondigestible carbohydrates make it down to the large intestine intact. These components as well as ingested supplements such as probiotics then interact with the gut microbiota, which produces metabolites that influence local gastrointestinal barrier function as well as systemic functions such as glycogen storage, fuel utilization, and muscle function that have the potential to affect athletic performance. BCFA, branched-chain fatty acid; GI, gastrointestinal; FODMAP, fermentable oligo-, di-, monosaccharides and polyols.
The Effects of Diet and Sport on the Gut Microbiome
Diet affects the gut microbiota composition and fluctuations (7, 10) over both short (5) and long (6) time frames. In addition to well-studied nutrients like fiber (11), the effects of specific foods (e.g., nuts, avocados) (12, 13) and dietary patterns (e.g., Mediterranean diet pattern) (14) are also recognized. Recent reviews have detailed how exercise influences the gut microbiota, depending on the type, intensity, and exercise duration (2, 10, 15–17). Indeed, evidence suggests that exercise increases α-diversity and microbial metabolites such as SCFAs (2). Effects of exercise on individual taxa are variable but typically reveal increases in commensal taxa such as Bifidobacterium, Lactobacilli, and Akkermansia (2). The gut microbiota may also influence exercise performance by producing metabolites such as SCFAs, which are utilized as fuel by colonocytes or absorbed into systemic circulation (acetate, 36%; propionate, 9%; butyrate 2%) (18). In skeletal muscle, SCFAs can be oxidized, incorporated into glucose via gluconeogenesis, or increase the bioavailability of glucose, glycogen, and fatty acids during exercise (2, 18–23). SCFAs also contribute to increased blood flow, insulin sensitivity, skeletal muscle mass preservation, and an oxidative phenotype (21). The multiple mechanisms by which SCFAs influence athletic performance via modulation of skeletal muscle function is an area of growing research.
Population-based cohort studies have documented the correlation between the gut microbiota and both physical activity, particularly vigorous physical activity, and exercise (24–27). It is important to distinguish between “physical activity” and “exercise” (3) when considering the interaction between sport and the gut microbiota (Text Box 1). When assessing the effect of sport on the gut microbiota or the role of the gut microbiota in athlete health and performance, exercise is the more accurate classification. However, exercise has different modes and varying degrees of intensity, which may differentially impact the gut microbiota (23, 28–32). For instance, cardiorespiratory exercise induced immediate changes in the gut microbiota composition, while resistance exercise had no effect (23). This may be due to differences in the metabolic pathways involved in and activated by different exercise modalities (23).
Additionally, factors such as dietary intake, colonic transit time, training status, shared training environment, health or disease status, age, or gender may present confounding factors in assessing the bidirectional relation between sport and the gut microbiota (2, 33–35). Recent reviews have discussed dietary intake (2, 15) and supplements (17) on the gut microbiota of athletes. Numerous cross-sectional studies have reported the relation between exercise, athletes’ habitual diets, and the gut microbiota (36). Additional studies have investigated the impact of dietary and exercise interventions on the gut microbiota in rodents or in sedentary or otherwise unhealthy human populations (2). Importantly, there is research on the effects of combined exercise and dietary interventions in athletes (Table 1) (37–44).
TABLE 1.
Combined diet, exercise, and gut microbiota interventions in athletes1
| Study (reference) | Year | Diet/supplement | Exercise/test | Duration | Participants | Microbiota effects | Metabolite effects | Metabolic effects | Performance effects |
|---|---|---|---|---|---|---|---|---|---|
| Moreno-Pérez et al. (37) | 2018 | Protein supplement (10 g whey isolate + 10 g beef hydrosylate) vs. control (maltodextrin) | Habitual training (≥5× per week) | 10 wk | 24 males, ages 18–45 y, cross-country runners | ↑ Bacteroidetes in protein group; ↓ Roseburia, Blautia, Bif. longum in protein group | ↔ Fecal pH, water content, ammonia, SCFA concentration, plasma or urine malondialdehyde in protein group | — | — |
| Murtaza et al. (38) | 2019 | HCHO vs. PCHO vs. LCHF | Habitual training test: VO2peak, walking economy, 10-km race time, 25-km long walk time, respiratory exchange ratio, fuel oxidation rate | 3 wk | 21 males, ages 20–35 y, race walkers | ↔ Enterotype, diversity (Shannon, Simpson, weighted/unweighted UniFrac) in diet groups; ↓ Faecalibacterium spp., Bifidobacterium, Veillonella, Streptococcus, Succinivibrio, Odoribacter, Lachnospira spp. in LCHF group; ↑ Dorea spp., Bacteroides spp., Enterobacteriaceae, Peptostreptococcaceae, Barnesiellaceae, Akkermansia in LCHF group; LCHF × enterotype interaction (↓ Bif., ↑ Sutterella in Bacteroides enterotype; ↑ Clostridiales in Prevotella enterotype); ↑ Clostridiales, Ruminococcaceae, Coprococcus spp., Akkermansia muciniphila, Bifidobacterium, Streptococcus in PCHO group; ↑ Clostridiaceae, Lachnospiraceae, Ruminococcaceae, Streptophyta in HCHO group | — | ↑ Fat oxidation in LCHF group Bacteroides ∼ fat oxidation (–) in LCHF group | ↓ Exercise economy, 10-km race performance in LCHF group; ↑ exercise economy, 10-km race performance in HCHO and PCHO groups; Dorea ∼ exercise economy (–) in LCHF group |
| Karl et al. (39) | 2017 | Rations (control) vs. rations + protein supplement (whey) vs. rations + carbohydrate supplement | 4-d cross-country ski-march (STRESS) | 4 d | 73 soldiers, age >18 y, 71 men, 2 women | ↑ α-Diversity (Shannon) post STRESS (no difference between groups); ↔ richness (Chao1, total observed OTUs); ↑ Firmicutes/Bacteroidetes ratio post STRESS (no difference between groups) | ↑ p-Cresol post STRESS (no difference between groups) | ↑ Sucralose and mannitol excretion post STRESS (no difference between groups); ↔ LPS | — |
| Son et al. (40) | 2020 | Probiotic supplement (1012 CFU each Lactobacillus acidophilus, L. casei, L. helveticus, Bifidobacterium bifidum) vs. placebo; divided into groups based on dietary intake: group 1 (high-protein, reduced fiber), group 2 (high-protein, adequate fiber), group 3 (adequate protein, restricted fiber), group 4 (sedentary control) | Habitual training | 60 d | 15 males, bodybuilders | ↔ α-Diversity (Shannon, Simpson), probiotic bacteria (Lactobacilli, Bifidobacterium) in probiotic vs. placebo group; ↑ Paraprevotella in probiotic group; ↑ Megamonas, Anaerostipes, Dorea in placebo group; ↔ α-diversity in group 1 vs. group 4; ↑ no. of species, Chao1 richness, ACE, Jacknife in groups 2 and 3 vs. group 4; ↑ Haemophilus, Streptococcus in group 1; ↑ Bifidobacterium in group 2; ↑ Faecalibacterium in group 3 | ↔ SCFAs | — | — |
| Huang et al. (41) | 2020 | Probiotic supplement (3× 1010 CFU/d L. plantarum 128) vs. placebo | Habitual training test: 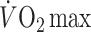 , endurance performance , endurance performance |
4 wk | 20 males, triathletes | ↑ Akkermansia, Bifidobacterium, Butyricimonas, Lactobacilli in Probiotic group; ↓ α-diversity (Shannon), Anaerotruncus, Caproiciproducens, Coprobacillus, Desulfovibrio, Dielma, Family_XIII, Holdemania, Oxalobacter in Probiotic group; ↔ Firmicutes/Bacteroidetes ratio | ↑ SCFAs (acetate, propionate, butyrate) in probiotic group | ↔ Body composition (bone, fat, lean %), blood biochemistry (glucose, lipids, creatinine, liver enzymes, blood cell counts) | ↔ 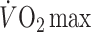 ; ↑ endurance performance ; ↑ endurance performance |
| Martarelli et al. (42) | 2011 | Probiotic supplement (109 CFU/d, 1:1 of L. rhamnosus IMC 501, L. paracasei IMC 502) vs. control (no supplement); all athletes on controlled diet developed based on athlete's basal metabolism, body composition, and energy expenditure (methods not described) | Controlled training developed based on athlete's basal metabolism, body composition and energy expenditure (methods not described) | 4 wk | 24 males, cyclists | ↑ Lactobacilli in Probiotic group | — | ↑ Reactive oxygen metabolites after physical activity in control group (not in probiotic group); ↑ plasma antioxidants in probiotic group | — |
| West et al. (43) | 2011 | Probiotic supplement (109 CFU/d L. fermentum PCC) vs. placebo | Habitual training test: 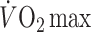 , peak power output, postexercise cytokine response , peak power output, postexercise cytokine response |
11 wk | 64 males, 35 females, cyclists | ↑ Lactobacilli in males in probiotic group (not in females)—obtained from subset of 10 males and 10 females from each group | — | ↓ Severity of GI and lower respiratory illness in males in probiotic vs. placebo; ↑ number and duration (↓ severity) of lower respiratory illness in females in probiotic vs. placebo; ↓ cytokine response to acute exercise in probiotic group; ↔ upper respiratory tract infection, mucosal immunity (lactoferrin, lysozyme, SIgA) | ↔ 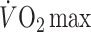
|
| Axelrod et al. (44) | 2020 | Probiotic supplement (2× 108 CFU/d L. salivarius UCC118) vs. placebo | Test 2-h treadmill at 60% 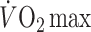
|
4 wk | 7 trained endurance athletes, ages 18–45 y | ↓ Verrumicrobia, Verrumicrobiae, Verrumicrobiales, Verrumicrobiaceae, Prosthecobacter fusiformis in Probiotic group; ↔ diversity/richness (Chao1, ACE, Shannon, Simpson) | — | ↓ GI permeability of sucrose in probiotic group; ↔ lactulose and rhamnose excretion, fecal zonulin, core temperature, IL-6 | — |
ACE, Abundance-based Coverage Estimator;Bif., Bifidobacterium; GI, gastrointestinal; HCHO, high carbohydrate; LCHF, ketogenic low-carbohydrate, high-fat; OTU, operational taxonomic unit; PCHO, periodized carbohydrate; SIgA, secretory immunoglobulin A; STRESS, 4-d cross-country ski march, 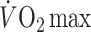 , maximal oxygen uptake; VO2peak, peak oxygen uptake; ↑, significant increase; ↓, significant decrease; ↔, no significant difference.
, maximal oxygen uptake; VO2peak, peak oxygen uptake; ↑, significant increase; ↓, significant decrease; ↔, no significant difference.
The Effect of Sport on the Gut
In addition to affecting the gut microbiota, exercise also impacts gastrointestinal physiology. Although exercise typically acts as a beneficial, or “hormetic,” stress, it can become detrimental if increased duration and intensity are not supported by adequate training, rest, nutrition, and antioxidant status (17). Exercise activates the autonomic nervous system, increasing circulating concentrations of cortisol and catecholamines, epinephrine, and norepinephrine, in peripheral tissues and the gastrointestinal tract (45). This results in reduced blood flow to the gastrointestinal tract, causing hypoxia, ATP depletion, and oxidative stress (46). These effects damage the gut barrier, increasing intestinal permeability, endotoxemia, nutrient depletion, and inflammation (46). The gastrointestinal tract responds to stress activation by releasing neurotransmitters such as γ-aminobutyric acid (GABA), neuropeptide Y, and dopamine, which are associated with gastrointestinal disturbances (45). These physiological effects are proportional to the intensity, duration, and frequency of exercise (45, 46).
While low- to moderate-intensity exercise promotes gastrointestinal motility and transit time, intense [>60% maximal oxygen uptake (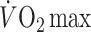 )] or prolonged (≥2 h) exercise may have the opposite effect, as well as create acute gastrointestinal disturbances (45, 47, 48). Regular exercise promotes adaptations to maintain intestinal blood flow and reduce inflammation, although recovery must also be adequate (46). Gastrointestinal issues are common, particularly among endurance athletes, with 30–50% of athletes experiencing gastrointestinal distress symptoms (48). These symptoms may be caused by physiological, mechanical, psychological, and nutritional factors, including reduced blood flow, increased gut permeability, increased production of stress hormones and inflammatory cytokines, and inadequate gastric emptying (45, 47, 48). However, outside of endurance running, gastrointestinal symptoms are rarely assessed (49). For instance, a study in soldiers participating in a 4-d rigorous cross-country ski march revealed increased intestinal permeability but did not report gastrointestinal symptoms, making the implications on subjective experience and the impact on exercise performance unclear (39).
)] or prolonged (≥2 h) exercise may have the opposite effect, as well as create acute gastrointestinal disturbances (45, 47, 48). Regular exercise promotes adaptations to maintain intestinal blood flow and reduce inflammation, although recovery must also be adequate (46). Gastrointestinal issues are common, particularly among endurance athletes, with 30–50% of athletes experiencing gastrointestinal distress symptoms (48). These symptoms may be caused by physiological, mechanical, psychological, and nutritional factors, including reduced blood flow, increased gut permeability, increased production of stress hormones and inflammatory cytokines, and inadequate gastric emptying (45, 47, 48). However, outside of endurance running, gastrointestinal symptoms are rarely assessed (49). For instance, a study in soldiers participating in a 4-d rigorous cross-country ski march revealed increased intestinal permeability but did not report gastrointestinal symptoms, making the implications on subjective experience and the impact on exercise performance unclear (39).
The gut epithelium has a high turnover rate (3–5 d) and requires large amounts of energy and nutrients (50). Athletes training at high intensities for long periods without adequate fueling are at risk for disturbances in gut integrity and function and gastrointestinal symptoms. In particular, inadequate habitual carbohydrate intake increases the proinflammatory stress response to prolonged, continuous strenuous exercise (47, 51). However, research has primarily focused on the effects of acute intake (before and during) on gastrointestinal symptoms during exercise rather than habitual diet, although an increasing number of athletes and researchers focus on food-avoidance strategies, such as a low fermentable oligo-, di-, monosaccharides and polyols (FODMAP) diet or gluten-free diet, as discussed below (47, 48).
The increased oxidative stress and disturbances to the gut barrier function that cause gastrointestinal symptoms also influence the gut microbiota (22, 45). Translocation of LPS, components of gram-negative bacteria resulting from increased gut permeability, causes endotoxemia and triggers proinflammatory cytokine secretion into the gastrointestinal tract that may influence the gut microbiota and further exacerbate the condition (22). Conversely, the microbial metabolites butyrate and propionate serve as energy sources for colonocytes, reducing mucosal degradation, gastrointestinal permeability, and inflammatory cytokines (22, 45). As alterations in microbial composition and diversity have been associated with gastrointestinal distress prevalence in athletes, the gut microbiota composition may be used as a biomarker for metabolic and systemic stress after exercise (22). For instance, a study investigating the acute effects of an exercise bout on the serum and fecal metabolome and the gut microbiota demonstrated that a single bout of exercise upregulated metabolic pathways of skeletal muscle substrate utilization and carbohydrate metabolites in serum, increased fecal ammonia and amino acid metabolites, and increased the abundance of Clostridia (52). Thus, acute changes in microbial and metabolite profiles may provide information regarding the effects of exercise on the gastrointestinal tract and metabolism. Furthermore, gastrointestinal symptom assessments could complement information on gut microbiota composition when considering the impact of exercise on the gut microbiota and the need for gut-centric dietary strategies.
Dietary Strategies for Sport and the Gut
Diet is 1 tool that athletes use to optimize their fitness, performance, and recovery (1). Dietary strategies for sport seek to optimize training, performance, and recovery via supplementation of specific nutrients (e.g., protein, carbohydrate loading, iron), restriction of energy or certain food categories (e.g., low-FODMAP diet, gluten-free), and adequate hydration; however, the effects of these dietary strategies on the gut microbiota are not well understood (17, 53, 54). Alternatively, increasing research indicates that dietary strategies for improving gastrointestinal health (e.g., probiotics, prebiotics, and synbiotics) represent promising opportunities to optimize the interaction between the gut and sport, with the potential to enhance athletes’ health and performance. The following sections discuss the effects of dietary strategies on the gut microbiota and athletic performance.
Protein
Protein is the main component of skeletal muscle. However, specific amino acids differ in their uptake and catabolism by the liver and skeletal muscle and their ability to regulate the muscle protein synthetic response (55). Essential amino acids, particularly branched-chain amino acids (BCAAs), are crucial for muscle protein synthesis and result in a greater muscle protein synthetic response than nonessential amino acids (55, 56). Therefore, dietary protein influences protein utilization and the anabolic response of skeletal muscle to exercise (55).
Although recommendations vary, athletes may need upwards of twice as much protein as the general population (1.2–1.7 vs. 0.8 g · kg–1 · d–1) to maintain protein synthesis, energy production, immune function, and gut integrity as a result of exercise-induced stress (54). This is true for endurance and resistance-trained athletes. Indeed, endurance athletes may need to ingest a higher amount of protein in the postexercise recovery period (∼0.5 vs. ∼0.3 g · kg–1 within 3–5 h of exercise), particularly if endurance exercise is performed in a fasted state, as this may increase myofilament proteolysis (57–59). Although variable based on dietary and physiological factors such as digestibility, quantity and composition of amino acids, the food matrix, and presence of other nutrients (60, 61), ∼10% of protein is not digested and may undergo proteolytic fermentation by bacterial proteases in the colon (62–64).
Concerning gut microbiota metabolism, amino acids can be classified by their fermentation metabolites: sulfur-containing amino acids, aromatic amino acids, and tryptophan (60). These metabolites include branched-chain fatty acids and SCFAs, ammonia, sulfides, indolic, and phenolic compounds (61, 64). While some of these metabolites (e.g., SCFAs and indole) may have beneficial effects like improving gut integrity, other metabolites (e.g., ammonia and p-cresol) decrease gut epithelium integrity (64, 65). Excess protein intake may lead to levels of proteolytic metabolite production that overwhelm the hosts’ ability to assimilate, transform, or detoxify harmful metabolites (61), contributing to adverse effects on intestinal barrier function, inflammation, and colonic health (60, 61, 63–66).
Protein supplements, including BCAAs and taurine added to energy drinks, are commonly used by athletes to enhance the anabolic and adaptive effects of exercise on skeletal muscle and improve recovery (67–69). Excess taurine leads to elevated taurocholic acid (TCA), deoxycholic acid (DCA), and hydrogen sulfide (H2S) concentrations, which are associated with increased risk of colorectal cancer (70); however, the effects of these supplements on the athletic gut microbiota are unclear (17, 54). To our knowledge, there is only one intervention study that has investigated the effects of protein supplements on the gut microbiota in athletes (37). In this study, male cross-country runners consumed a protein supplement (10 g whey isolate and 10 g beef hydrosylate) or a placebo (maltodextrin) for 10 wk. Protein powder consumption was associated with a decrease in Lachnospiraceae, Roseburia, Blautia, Synergistales, Coprococcus, Lactobacillales, Bacilli, and Bifidobacterium longum, as well as a higher abundance of Bacteroidetes and lower abundance of Firmicutes relative to the placebo. There were no differences between groups at baseline or after the intervention in α-diversity (Chao1, equitability, phylogenetic tree, number of observed species, Shannon index, Simpson index), β-diversity (unweighted UniFrac), or microbial metabolites (i.e., SCFAs, ammonia). Thus, protein supplementation influenced the abundance of specific taxa with limited effects on the community's diversity and function (i.e., metabolites).
Additional studies have investigated the effects of protein supplementation or high-protein diets in sedentary adults with overweight and obesity. McKenna et al. (71) investigated the effects of moderate (0.8–1.0 g · kg–1 · d–1) and high (1.6–1.8 g · kg–1 · d–1) beef protein consumption combined with resistance training in a cohort of healthy, overweight, middle-aged adults. In this study, participants in the high-protein group had decreased abundance of Veillonellaceae, Akkermansia, uncultured Eggerthellaceae, and Ruminococcaceae UCG-010 following 1 wk of dietary habituation relative to baseline (71). However, there were no differences between the moderate- and high-protein groups in strength gains in response to resistance training (71). Cronin et al. (72) investigated the effect of whey protein supplementation (24-g blend of whey concentrate, isolate, and hydrolysate) in sedentary adults with overweight and obesity with or without exercise on the gut microbiome and reported no effects of the protein supplement or exercise on microbiota composition or metabolic pathways. The lack of effect of the supplement on gut microbial composition may have been due to lack of dietary control or the relatively short duration of the intervention (8 wk) compared with habitual exercise and supplementation undertaken by athletes (65). However, the authors did report a shift in the gut virome in the protein-supplemented groups with and without exercise due to virus particles present in the supplement and increases in trimethylamine-N-oxide (TMAO) and phenylacetylglycine (PAG) in the protein-supplemented group without exercise.
TMAO, produced from carnitine, choline, and phosphatidylcholine, is metabolized by the gut microbiota to trimethylamine (TMA), which is then converted to TMAO in the liver (14). TMAO and PAG are associated with increased risk of cardiovascular disease and adverse outcomes in cardiovascular disease patients (73, 74) and are elevated in athletes versus sedentary controls, potentially resulting from increased protein intake (75). In contrast, the addition of exercise decreases TMAO (72). The associations between TMAO and disease may be confounded by or dependent on kidney function, the gut microbiota, and the flavin-containing monooxygenase 3 (FMO3) genotype (4, 76–78). Fish is rich in preformed TMAO and has the greatest impact on circulating TMAO concentrations; however, fish intake is associated with decreased risk of cardiovascular disease (76, 77). Additionally, habitual intake of red meat, containing the TMAO precursor carnitine, and acute feeding of phosphatidylcholine, the predominant form of choline in foods such as eggs, are not associated with increased circulating TMAO (76, 4, 78, 79). Ultimately, the connections between TMAO, lifestyle factors (diet and exercise), and disease are complex, and it is difficult to draw conclusions based on the current state of the science.
Beaumont et al. (80) investigated the effects of a high-protein diet (∼30% energy intake) using either casein or soy (both providing 15% of energy intake) in overweight individuals. They reported no shift in the gastrointestinal microbiota, perhaps due to variability in the protein sources consumed by participants outside of the supplements or other aspects of dietary intake. However, this study reported a shift in bacterial metabolism and metabolite profiles toward products of amino acid degradation, including a decrease in butyrate and increases in 2-methylbutyrate, phenylacetylglutamine, and indoxyl sulfate.
The results of these protein-supplementation studies indicate that, while protein supplements may impact the gut microbiota composition, they have a greater impact on microbial metabolites (81, 82). The decrease in butyrate, a key SCFA, and increase in proteolytic metabolites could be detrimental to gastrointestinal health. Therefore, protein supplementation in athletes should be further assessed to determine whether this population experiences the same metabolic effects and whether these changes are associated with increased gastrointestinal distress or inflammation and performance.
Cross-sectional studies examining the relation between dietary intake and the microbiota in athletes have reported inconsistent results. For instance, Clarke et al. (83) reported that protein intake was positively correlated with microbial diversity, while Jang et al. (84) reported a negative association between protein intake and microbial diversity. These contradictory findings may be due to the athletes’ fiber intake, as those in the study by Clarke et al. met recommended fiber intake requirements, while those in the study by Jang et al. did not (15). A follow-up study to Clarke et al. investigating the metabolomic and metagenomic signatures of athletes and sedentary controls reported an increase in microbial genes related to amino acid biosynthesis and carbohydrate metabolism, as well as an increase in amino acid metabolites (e.g., TMAO and PAG) and SCFAs, suggesting that fiber intake was sufficient to balance the increased protein intake (75). Indeed, it has been suggested that the fiber, calorie, and fat content of the diet may have significant impacts on the effects of protein amount and type on the gut microbiota and health (65, 85, 86).
Animal studies investigating the effects of different protein types, focusing primarily on the comparison between animal- versus plant-based proteins, have reported differential effects such as a higher abundance of Lactobacilli (∼5-fold) and the ratio of Firmicutes to Bacteroidetes and lower butyrate (−1.4-fold), SCFA-producing bacteria (e.g., Bacteroides and Prevotella), LPS-binding protein (∼ −2-fold), and transcription factor CD14 receptor (∼ −0.4-fold) with meat versus nonmeat proteins (87–90). LPS-binding protein binds to CD14 to mediate the activation of macrophages to produce inflammatory cytokines, serving as a proxy for inflammation (89). Thus, these results suggest that soy, the plant-based protein used in these studies, elicited a greater inflammatory response than animal proteins (88, 89). Dairy proteins appear to have an intermediate effect between meat and nonmeat proteins (88), although results may differ between whey and casein components (91). However, these studies of protein type have been conducted almost exclusively in rodents, primarily use isolated protein sources, and often use protein intakes above the recommended daily guidelines. Effects of protein sources such as beef on the gut microbiota have more consistent findings in animal models than humans, in which limited to no impact of protein type has been reported, highlighting the need for more studies in humans (90). To our knowledge, only 1 study has investigated the effects of different protein types on the gut microbiota in humans; however, this study also added a high– or low–saturated fat component to the study design and reported that saturated fat consumption masked the effects of protein type (85). This again demonstrates the need to account for the intake of other dietary components (e.g., fat) in addition to protein.
Evidence suggests that the gut microbiota contributes to protein absorption and utilization (92) as well as skeletal muscle anabolism and functionality (gut–muscle axis) via fuel availability and storage and modulation of inflammation (19, 20, 93, 94). For example, probiotic supplementation (Lactobacillus paracasei) enhanced the bioavailability of plant proteins, elevating essential amino acid and BCAA concentrations to comparable concentrations of animal proteins (95). Additionally, when co-administered with protein, the probiotic Bacillus coagulans (GBI-30,6086) decreased epithelial cell inflammation, improved nutrient absorption, and produced proteases that increase amino acid absorption in humans (92). These effects may reduce muscle damage and boost muscle recovery, thereby enhancing adaptation and performance (92). Increasing the bioavailability and absorption of dietary protein and increasing muscle protein synthesis is 1 mechanism by which the gut microbiota may influence muscle mass and function. These effects may be partially regulated by SCFA production, affecting insulin sensitivity, inflammation, and release of insulin-like growth factor I (IGF-I) that modulate the balance between anabolic and catabolic processes (93, 96). Therefore, the gut–muscle axis may mediate the positive effects of exercise and diet on muscle anabolism and play a role in the age-related decline in muscle mass (i.e., sarcopenia) and disease-related muscle wasting (19, 20, 93, 94, 96, 97). For instance, increased abundances of Oscillospira and Ruminoccocus and decreased abundances of Barnesiellaceae and Christenellaceae helped accurately predict individuals with physical frailty and sarcopenia (97). However, due to the small sample size, it is unclear if differences in body composition, diet, and physical activity contributed to these differences in muscle function independently of the gut microbiota. However, alterations in the gut microbiota have been associated with phenomena including “anabolic resistance” that contribute to the development of sarcopenia (96). Therefore, growing research suggests that the gut microbiota plays a role in muscle function and anabolism via modulation of protein metabolism.
An additional area of interest is the effects of whole-food protein versus protein supplements as whole foods have been shown to have equal or superior ergogenic effects (1, 98–100). For instance, ingestion of whole eggs versus egg whites and whole milk versus fat-free milk result in greater amino acid uptake and postexercise myofibrillar protein synthesis (100, 101), suggesting that nonprotein components (e.g., lipids, carbohydrates, micronutrients, and other bioactive compounds) contribute to the postexercise protein synthetic response. The food matrix may also contribute to differential effects of whole-food protein sources on the gut microbiota, as the same quantity of protein in supplement form and the change in the amino acid profile as a result of protein isolation impact the protein digestion and absorption (60, 61, 98). For instance, purified proteins are digested more efficiently than protein-rich foods consumed in a mixed meal, which may decrease the amount of protein delivered to the large intestine, although the amount of purified protein ingested likely also influences colonic availability (60). It is unclear to what extent these differences in digestibility of protein types, and potential modulating effects of gut transit time, affect the athlete gut microbiota, health, and performance.
In summary, high-protein diets and protein supplements appear to have limited effects on the gut microbiota composition but shift the metabolite profile to greater production of proteolytic metabolites. This may lead to detrimental effects on gastrointestinal health and exacerbate exercise stress–induced symptoms of gastrointestinal distress in athletes, which may impair training and performance. However, these effects may be specific to the protein supplement type and depend on concomitant carbohydrate or fiber intake. Furthermore, the gut microbiota may also contribute to muscle protein anabolism and function throughout the lifespan via modulation of protein absorption and utilization.
Fat
Intramuscular triglycerides and adipose tissue provide important fuel substrates for athletes during exercise (102, 103). Additionally, dietary fat modulates the gut microbiota composition and subsequently impacts metabolic health (104). The amount and type of dietary fat are important aspects of dietary quality and are important considerations for both athletic performance (102, 103, 105) and the health of the gut microbiota (104, 106–108).
Dietary fat intake is variable based on sport modality, training level, and body-composition goals (84, 102). Pre-exercise meals or snacks are generally low in fat to facilitate gastric emptying and minimize gastrointestinal distress during exercise (109). Conversely, there is interest in high-fat, low-carbohydrate ketogenic diets for athletes for performance enhancement or weight control (110, 111). However, while a high-fat, low-carbohydrate diet does enhance fat oxidation, there is no evidence to support the notion that it increases performance; instead, there is evidence that it may decrease exercise performance at higher intensities (102, 103, 110, 112). Alternatively, supplementation of omega-3 (ɷ-3) essential fatty acids may positively affect exercise performance via improved endurance capacity, recovery, and immune modulation (105). However, most studies have been conducted in untrained, amateur populations, and few focus on performance as an outcome, limiting the ability to determine their ergogenic effects in athletes (105).
Concerning the gut microbiota, research on fat intake has primarily centered on the effects of a high-fat, particularly high-saturated-fat, Western-style diet (104, 107, 113, 114). These studies reveal that the Western-style dietary pattern is associated with an increased Firmicutes to Bacteroidetes ratio and increased abundance of Proteobacteria, Mollicutes, and Bilophila wadsworthia, as well as a decrease in Akkermansia muciniphila, Bifidobacterium spp., and butyrate-producing taxa (62, 104, 113).
Additionally, a high-fat diet with concomitant restriction of carbohydrates, as in a ketogenic diet, may have differential effects on the gut microbiota and inflammation compared with a high-fat diet without carbohydrate restriction due to ketone body production (115). However, there are conflicting results regarding the effects of the ketogenic diet on gut microbiota composition, although evidence suggests that the gut microbiota mediates some of the beneficial effects of the ketogenic diet on neurological outcomes (116). In men with overweight and obesity, consumption of a ketogenic diet decreased Bifidobacterium and Lactobacilli and increased Fusobacteria and Escherichia (115). To our knowledge, only 1 study has investigated the effects of a ketogenic diet, compared with either a high-carbohydrate or periodized carbohydrate diet, on the gut microbiota of athletes (elite race walkers). The authors reported an increase in Bacteroides and Dorea and a reduction in Faecalibacterium, a known butyrate producer (38). Additionally, the abundance of Bacteroides and Dorea following the intervention was negatively associated with fat oxidation and exercise economy, respectively, suggesting a negative correlation of these taxa with exercise performance (38). Furthermore, recent reviews indicate that ketone supplementation does not benefit athletic performance, cognition, or muscle recovery in athletes and may induce gastrointestinal symptoms (117, 118).
In addition to the amount, the type of fat modulates the gut microbiota and downstream inflammatory signaling, which may have implications for athletic performance. While acute inflammation in response to exercise is necessary for the adaptive response and functional recovery of muscle, chronic or excessive inflammation can lead to detrimental effects such as reduced muscle strength and mass (93, 119). Different types of fat are associated with varying effects on the gut microbiota and consequential effects on inflammation (107, 114, 120). Saturated fat intake is associated with decreased microbiota diversity and richness in humans and increased availability and transport of LPS, leading to proinflammatory Toll-like receptor (TLR) activation in preclinical models (107, 121). Monounsaturated fat intake is also associated with decreased total bacterial numbers in humans and increased LPS in preclinical models but still leads to lower inflammation than saturated fat (121). However, polyunsaturated fat has no effect on diversity or richness in humans and increases the abundance of Bifidobacterium, Lactobacilli, and Akkermansia muciniphila, which are also increased by exercise (2, 107). ɷ-3 PUFAs increase SCFAs, improve gastrointestinal integrity and inflammation, and potentially affect communication along the gut–brain axis (108). Therefore, beneficial effects of ɷ-3 fatty acids on the gut microbiota may mimic the effects of exercise and contribute to health and performance benefits by promoting an anti-inflammatory bacterial profile and production of SCFAs. Conversely, the proinflammatory effects of high saturated fat intake on the gut microbiota may impair exercise-induced performance benefits on muscle anabolism.
Bile acids may also mediate some of the disparate effects of different dietary fats on lipid and carbohydrate metabolism, energy expenditure, and inflammation via the farnesoid X receptor (FXR) and G protein–coupled membrane receptor 5 (TGR5) (106, 121–123). Interactions of bile acids with these receptors also increase energy expenditure in skeletal muscle and decrease muscle fat deposition, suggesting that microbiota-mediated changes in the bile acid pool may influence skeletal muscle function (94, 124). Increased intramuscular triglycerides (IMTGs) have been reported in individuals with obesity and associated with insulin resistance, although athletes exhibit similarly high concentrations of IMTGs that can be used as fuel during exercise (125). It is now thought that the association with insulin resistance is due to increased intramuscular lipid metabolite concentrations, not IMTGs, and that accumulation of these metabolites is prevented by high IMTG turnover with exercise (125). Secondary bile acids produced by the gut microbiota also interact with FXR and TGR5 receptors and increase mitochondrial oxidative phosphorylation and fatty acid β-oxidation, which may have performance benefits such as better oxygen uptake, energy availability, and fatigue resistance (126). It is unclear whether bile acid modulation of IMTG content or mitochondrial function influence exercise capacity in athletes and, if so, how to optimize the concentration and composition of bile acids and secondary bile acids via type and amount of fat intake.
In summary, high fat, particularly high saturated fat, intake is linked to a proinflammatory microbiota composition with a reduced capacity to produce SCFAs and may induce gastrointestinal permeability, both of which can adversely impact performance. Conversely, ɷ-3 fatty acids may promote a beneficial microbiota profile, increased SCFAs, and reduced gastrointestinal permeability. However, current research on the ergogenic effects of ɷ-3 fatty acids is inconclusive (127).
Carbohydrate and fiber
Highly digestible and readily absorbed carbohydrates are of great interest for sport. However, nondigestible carbohydrates (i.e., fibers and resistant starches) are of greater interest when considering the gut microbiota.
Carbohydrates function as one of the primary fuel sources during exercise (128). Dietary recommendations for athletes suggest high intakes of simple carbohydrates to maintain glucose homeostasis and low fiber intake before exercise to reduce gastrointestinal distress, also adding that plant-based high-fiber diets may reduce energy availability (17, 109). Ingestion of simple carbohydrates (e.g., glucose, fructose, sucrose, dextrose) before and during exercise can reduce fatigue, improve performance, and promote water reabsorption and maintenance of euhydration (45, 129, 130). However, glucose and fructose load and the fructose-to-glucose ratio affect gut microbial fermentation and gastrointestinal distress (131). Ingesting fructose and glucose in equal quantities optimizes fructose absorption (132, 133) and reduces microbial fermentation, potentially reducing gastrointestinal distress symptoms. Lactose may also serve as a good fuel source before, during, and after exercise for increased performance and recovery while also potentially promoting beneficial effects on the gut microbiota, such as increases in Bifidobacteria and Lactobacilli (134).
Carbohydrate loading is also a common strategy used by endurance athletes to maximize glycogen concentrations before a competition (135). The goal of carbohydrate loading is to maximize carbohydrate absorption and glycogen storage. Thus, carbohydrates that will not be digested and absorbed in the small intestine, like fiber and resistant starch, are generally avoided. Interestingly, ingestion of potatoes during cycling is as effective as carbohydrate gels to support performance, despite having a much higher fiber content (11.2 vs. 2.3 g) (136). However, gastrointestinal symptoms (abdominal pain, bloating, and discomfort) were higher in the potato group, limiting the use of such practices among athletes.
Athletes focused on maximizing glycogen storage may ingest high amounts of carbohydrates but avoid nondigestible carbohydrates (45, 137). Evidence suggests that a high-carbohydrate, low-fiber dietary pattern has detrimental effects on intestinal health and microbes, including altered intestinal transit times, loss of bacterial diversity, and reduced SCFA production (11, 138, 139). There is a positive association between total dietary fiber per kilocalorie energy and the abundance of Bifidobacterium (140). Furthermore, adequate intake of nondigestible carbohydrates may also negate the potentially adverse effects of microbial proteolytic fermentation and its metabolites as nondigestible carbohydrates are preferentially metabolized by the gastrointestinal microbiota (60, 64, 65). Indeed, bodybuilders with high protein and restricted dietary fiber intake had greater microbiota similarity to sedentary controls (i.e., reduced α-diversity) compared with bodybuilders with adequate fiber intake (40). These microbiota characteristics may adversely affect long-term health and induce short-term gastrointestinal distress in athletes. This makes it even more important for athletes consuming high-protein diets to ensure adequate intake of nondigestible carbohydrates to prevent gastrointestinal distress and inflammation (45). Since athletes typically have increased energy intake relative to sedentary individuals (83), fiber intake should be scaled appropriately. Ultimately, athletes should strive for adequate fiber intake (14 g/1000 kcal) to promote gastrointestinal health and athletic performance, although avoidance directly before or after exercise may be warranted due to the potential for gastrointestinal distress.
SCFAs are linked to muscle function and glycogen accretion in skeletal muscle (19, 20). Therefore, reduced SCFAs due to a low-fiber diet may affect exercise capacity and performance. Studies in mice by Donatto et al. (141) (oat bran containing β-glucan, 300 g/kg chow) and Okamoto et al. (142) (hemicellulose and lignin, 14.6% neutral detergent fiber) revealed that nondigestible carbohydrate supplementation with exercise, either swimming or treadmill running, respectively, increases muscle glycogen concentration, SCFA production, and time to exhaustion while decreasing the postexercise inflammatory response. While muscle glycogen content is well correlated with endurance performance (143), the effect of increased SCFA production and systemic availability (18) on athletic performance in humans is unclear. Okamoto et al. (142) reported that infusion with acetate improved endurance exercise capacity in antibiotic-treated mice while Scheiman et al. (144) reported increased performance with propionate and Veillonella atypica, which converts lactate to propionate, inoculation in mice. The mechanism(s) of these ergogenic effects may involve increased glycogen or glucose fuel availability (19), increased water reabsorption (145), or direct utilization of metabolites (e.g., propionate) (144). Fiber intake and SCFAs may also decrease gastrointestinal permeability (146) and influence the immune response and inflammation via interaction with the gut-associated lymphoid tissue (GALT) (147). A study on the effects of butyrylated high-amylose maize starch in healthy adult cyclists increased butyrate and propionate concentrations, increased Parabacteroides distasonis and Faecalibacterium prausnitzii, and maintained IL-10 concentrations (an anti-inflammatory cytokine) (148). Another study on the effects of a low-dose (6 g/d), partially hydrolyzed guar gum fiber on the gut microbiota and recovery in athletes revealed increased Actinobacteria, decreased Bacteroidetes and Clostridium cluster XI, fecal defecation characteristic improvements, and reduced diarrhea (149), thus having a potential indirect effect on performance.
Prebiotics
A prebiotic is “a substrate that is selectively utilized by host microorganisms conferring a health benefit” (150). While many fibers have prebiotic effects and are considered candidate prebiotics (e.g., resistant starch; polydextrose; β-glucans; pectin; soy-, xylo-, arabinoxylo-, and malto-oligosaccharides) (150–152), only fructo-oligosaccharides (present in artichokes, asparagus, bananas, chicory root, garlic, onions, leeks, wheat) (11) and galacto-oligosaccharides (derived from lactose) (153) are readily accepted as prebiotics (150). The health benefits of prebiotics include gastrointestinal health (e.g., pathogen inhibition), mental health (e.g., energy and cognition), and bone health (e.g., mineral absorption), all of which play important roles in the health and performance of athletes (150, 154).
While increasing prebiotic intake may decrease effective carbohydrate intake and glycogen storage, it has been postulated that microbial production of SCFAs from prebiotic fermentation may improve glycogen storage and metabolism (19, 155). To our knowledge, no studies have investigated the effects of prebiotic supplementation alone on exercise performance in athletes (156) (Table 2) (141, 142, 157–161). However, a study in asthmatic adults with hyperpnea-induced bronchoconstriction, a surrogate for exercise-induced bronchoconstriction, demonstrated that galacto-oligosaccharide supplementation (5.5 g/d) improved exercise-induced bronchoconstriction and reduced inflammation (157). Another study investigated the effect of exercise training in combination with inulin-propionate ester (IPE) supplementation in women with overweight and reported that IPE increased fat oxidation compared with a placebo (158). However, IPE has distinct effects compared with inulin alone on the gut microbiota and metabolome (162), making it difficult to determine whether the observed effects were due to inulin's prebiotic capacity or the esterified propionate.
TABLE 2.
Prebiotic or synbiotic supplementation with exercise1
| Study (reference) | Year | Prebiotic/synbiotic | Exercise/test | Duration | Subjects | Results |
|---|---|---|---|---|---|---|
| Prebiotics | ||||||
| Donatto et al. (141) | 2010 | Oat bran (300 g/kg chow) | Swimming | 8 wk | Rats (Wistar, male, 2 mo old) | ↑ Time to exhaustion, hepatic glycogen in oat bran group; ↓ IL-6, IL-10, corticosterone in oat bran group |
| Okamoto et al. (142) | 2019 | LMC (cellulose) vs. HMC (cellulose, hemicellulose, lignin) vs. antibiotics vs. control | Forced-treadmill running | 6 wk | Mice (C57BL/6J, male, 10 wk old) | ↓ Time to exhaustion, muscle mass in LMC and antibiotic groups; ↑ time to exhaustion in antibiotic mice with acetate infusion (not with butyrate); ↔ body mass, muscle mass in antibiotic mice with acetate infusion and in LMC mice with HMC fecal transplant + inulin; ↑ time to exhaustion, SCFAs in LMC mice with HMC fecal transplant + inulin; ↑ white adipose tissue mass in LMC group; ↔ body mass gain, dietary intake; ↓ SCFAs (fecal and plasma) in LMC and antibiotic groups; ↑ Firmicutes/Bacteroidetes, Actinobacteria, Lactococcus, Allobaculum in LMC group; ↓ Shannon diversity, Prevotella in LMC group |
| Williams et al. (157) | 2016 | B-GOS (5.5 g/d) vs. placebo | EVH | 3 wk | 10 adults (with asthma and HIB, 5 males, 5 females) and 8 adult controls (5 males, 3 females) | ↓ Peak post-EVH fall in pulmonary function following B-GOS; ↓ airway inflammation (chemokine CC ligand 17, TNF-α) following B-GOS in HIB group; ↓ CRP following B-GOS in HIB and control groups |
| Malkova et al. (158) | 2020 | IPE (10 g/d) vs. placebo | Supervised endurance exercise, submaximal VO2 treadmill test | 4 wk | 20 adults (overweight women) | ↑ Fat oxidation in IPE group |
| Synbiotics | ||||||
| West et al. (159) | 2012 | Gut Balance (synbiotic; Lactobacillus paracasei 431, Bifidobacterium animalis ssp. lactis BB-12, L. acidophilus LA-5, L. rhamnosus LGG, raftiline, raftilose, lactoferrin, immunoglobulins, acacia gum) vs. acacia gum (prebiotic) | Habitual exercise (cycling) | 3 wk | 22 adults (healthy males) | ↑ L. paracasei in synbiotic vs. prebiotic group; ↓ increase in IL-16 in synbiotic vs. prebiotic group; ↔ fecal SCFAs, immunity, GI permeability in both groups |
| Coman et al. (160) | 2017 | Fermented milk (synbiotic; L. rhamnosus IMC 501, L. paracasei IMC 502, oat bran fiber) vs. control (fermented milk) | Habitual exercise (intense gym-training program) | 4 wk | 10 adults (healthy, 3 males, 7 females) | ↑ Lactobacilli spp., Bifidobacterium spp., secretory IgA, improvement in intestinal regularity, ease of defecation, and improved upper respiratory symptoms in synbiotic group; ↓ Lipid hydroperoxide in synbiotic group |
| Roberts et al. (161) | 2016 | Pro/prebiotic/antioxidant (L. acidophilus CUL-60, L. acidophilus CUL-21, Bif. bifidum CUL-20, Bif. animalus ssp. lactis CUL-34, fructo-oligosaccharides, α-lipoic acid, N-acetyl-carnitine) vs. pro/prebiotic vs. placebo | Long-distance triathlon | 12 wk pre-race, 6 d post-race | 30 adults (healthy, recreational athletes, 25 males, 5 females) | ↓ Endotoxin pre- and post-race with pro/prebiotic/antioxidant, post-race with pro/prebiotic; ↑ gastrointestinal permeability with placebo; ↔ mean race time |
B-GOS, bimuno-galacto-oligosaccharide; Bif.,Bifidobacterium; CRP, C-reactive protein; EVH, eucapnic voluntary hyperpnea; GI, gastrointestinal; HIB, hyperpnea-induced bronchoconstriction; HMC, high microbiome-accessible carbohydrate; IPE, inulin-propionate ester; LMC, low microbiome-accessible carbohydrate; ↑, significant increase; ↓, significant decrease; ↔, no significant difference.
Probiotics
Probiotic supplementation is a topic of interest among athletes to increase health and performance (36, 156, 163–170). Probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” (171). Conventional probiotics include Bifidobacterium spp. and Lactobacilli, although other bacteria investigated in athletes include Bacillus spp., Enterococcus spp., Streptococcus spp., Veillonella, or the yeast Saccharomyces boulardii, which have been reviewed elsewhere (2, 36, 156, 163–170, 172).
Briefly, probiotics reduce infection, inflammation, muscle soreness, and gastrointestinal permeability or distress. Thus far, the most substantive evidence for probiotic benefits is improvements in the incidence, duration, and severity of upper respiratory tract infections (2, 163), which may indirectly improve athletic performance (156). The studies reporting improvement in respiratory symptoms include organisms from the Lactobacilli family (172, 173). L. salivarius, may also reduce gastrointestinal permeability via increases in butyrate-producing taxa Roseburia and Lachnospiraceae and decreases in Verrumicrobia (44). While there is evidence of shared mechanisms of probiotic functions, the benefits of probiotics are often dependent on the strain and dose of the probiotic (163, 164). The majority of studies reporting positive effects on gastrointestinal barrier function use multi-strain formulations (172). Probiotics may attenuate the effects of intense exercise on gastrointestinal distress and muscle soreness in athletes by improving intestinal permeability and antioxidant status and reducing inflammation (36, 156, 163, 171, 174, 175), potentially via interaction with GALT (176). For example, a daily multi-strain probiotic (Ultrabiotic 60TM, Bioceuticals, Australia AustL# 259813) containing 10 different strains from the genera Lactobacillus, Bifidobacterium, and Streptococcus and SBFloractiv™ (Bioceuticals, Australia AustL# 285024) containing the yeast Saccharomyces boulardii decreased muscle soreness in elite rugby athletes (175). While there was no main effect of treatment on inflammation, muscle soreness was positively correlated with salivary C-reactive protein (CRP) and negatively correlated with motivation and sleep quantity and quality (175). A combination of L. rhamnosus and L. paracasei increased plasma antioxidants and mitigated the exercise-induced rise in reactive oxygen species (ROS) while also increasing Lactobacillus in participants (42). Of interest to athletes while traveling, Saccharomyces boulardii and a combination of L. acidophilus and B. bifidum help prevent traveler's diarrhea (177).
Probiotics may also improve nutrient absorption and utilization, glycogen storage, body composition, energy harvest, hormone production, and cognition and mood via mechanisms such as bioactive metabolite production (e.g., SCFAs, neurotransmitters), modulation of gut pH, and alterations in the gut microbiota activities (36, 92, 163, 164, 169, 178, 179). For instance, L. plantarum increased endurance performance in triathletes concurrent with an increase in fecal SCFAs (41). A study in mice revealed that a bacterial strain isolated from an Olympic weightlifting athlete [L. salivarius subsp. salicinius (SA-03)] improved endurance performance and muscle strength via increased hepatic and muscular glycogen and decreased lactate, blood urea nitrogen, ammonia, and creatine kinase after exercise (178). However, more studies show ergogenic effects of multi-strain probiotics than single-strain probiotics (163), suggesting that multiple strains may act in a complementary way to provide performance benefits. Probiotics may, therefore, benefit athletic performance via both direct and indirect mechanisms, although the evidence of ergogenic effects remains scarce (156, 163, 166).
Differences in strains and doses of probiotics and individuals’ baseline diet, immune status, and microbiota composition may contribute to variability in findings between studies, making comparisons and conclusions difficult (2, 36, 40, 168). Most probiotic supplementation studies in athletes do not assess the gut microbiota, making it difficult to determine whether efficacy depends on baseline or changes in the participants’ gut microbiota composition (2, 166). Concurrent dietary intake, particularly intake of fiber and prebiotic substrates, may also impact the probiotic effects and should therefore be accounted for in analyses (180). This is important as consumers should be aware that probiotic supplementation alone may not have the intended effects if not supported by a diet with adequate nutrition. Additionally, probiotic supplementation studies in athletes typically have small sample sizes (i.e., 10 to 30 participants) and often include only or predominantly male participants (167), often the case in sports and exercise research (181), but which is problematic because there may be gender-specific effects (43). For example, in West et al. (43), probiotic supplementation with Lactobacillus fermentum (PCC®, Probiomics Ltd, Sydney, Australia) decreased gastrointestinal symptoms in males but increased the incidence and duration of symptoms in females.
There is increasing interest in the effect of live cultures in fermented foods (171, 182), and their effects or association with the gut microbiota (183). However, few studies have investigated the effects of fermented foods, including yogurt, kefir, sauerkraut, on exercise (184–187). Three studies using kefir or fermented milk reported decreased exercise-induced CRP or creatine phosphokinase and muscle soreness, indicating a positive effect of these fermented foods on reducing inflammation (185–187). One study in mice reported an ergogenic effect of kefir on strength and endurance (184). Therefore, fermented foods containing live microorganisms may confer beneficial effects on inflammation and exercise performance.
Synbiotics
A synbiotic is “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host” (180). A synbiotic may be a combination of a probiotic and a prebiotic (complementary synbiotic), although the individual components do not necessarily need to meet the criteria for pro- and prebiotics as long as they act synergistically when co-administered (synergistic synbiotic) (180). Thus, the prebiotic component may enhance the functionality of the probiotic (synergistic synbiotic), or the 2 components may provide independent beneficial functions upon introduction to the gut and its resident microbes (complementary synbiotic) (180). This combination of microorganisms and selectively utilized substrates (159–161) may have different effects than either prebiotic or probiotic supplementation alone (Table 2). However, to our knowledge, only 1 study has investigated the synergistic and independent effects of these components in physically active humans (159). West et al. (159) reported that synbiotic supplementation (Lactobacillus paracasei 431, Bifidobacterium animalis subsp.lactis BB-12, L. acidophilus LA-5, L. rhamnosus LGG, raftiline, raftilose, lactoferrin, immunoglobulins, acacia gum) was associated with a smaller increase in serum IL-16 concentrations compared with prebiotic (acacia gum) supplementation alone, but neither synbiotic supplementation nor acacia gum alone influenced SCFA concentrations, immunity, or gastrointestinal permeability. Therefore, synbiotics may have different or additional effects on athlete health and performance than prebiotic or probiotic supplementation alone.
Micronutrients
Micronutrients contribute to immune function, inflammation, energy metabolism, and bone health, impacting athletic performance (51, 188–190). Adequate intakes of iron, zinc and vitamins A, E, C, B-6, and B-12 are essential for proper immune function, which may be compromised under conditions of heavy training and competition in athletes (51). Furthermore, dietary requirements for some micronutrients may be increased in athletes due to losses in sweat and urine and increased oxidative stress (51, 188). Additionally, female athletes are at higher risk of iron deficiency, compromising health and performance (191).
Micronutrient deficiencies can also impact the gut microbiota (192). Lack of antioxidant micronutrients (e.g., vitamins C and E and selenium) decrease the abundance of commensal gut bacteria while promoting an increase in Escherichia coli (192). In animals under increased stress conditions, an antioxidant blend of vitamin C, vitamin E, polyphenols, lipoic acid, and microbial antioxidants restored intestinal redox status, which was correlated with increased Bifidobacterium and Lactobacilli and decreased E. coli (193). However, excessive intake of some micronutrients may also increase infection susceptibility (51). For example, excessive iron supplementation in infants increases pathogenic microbes, including E. coli, and contributes to intestinal inflammation (194, 195). Thus, micronutrient supplementation under conditions of increased stress or micronutrient deficiency may have microbiota-mediated benefits on immunity and inflammation.
Calcium and vitamin D support bone health. Additionally, vitamin D may impact skeletal muscle mass and strength via regulation of calcium-dependent contraction, protein-dependent skeletal muscle anabolism, mitochondrial function, and insulin sensitivity (196, 197). Increases in Bifidobacterium, Lachnospiraceae, and Bacteroides in response to fiber intake are positively correlated with increased calcium absorption (195, 198). This may be due to SCFA production, which increases calcium absorption by lowered colonic pH or regulation of signaling pathways or gene expression (199). Vitamin D intake also impacts the gut microbiota, although variability in results precludes the ability to determine the effect of supplementation on specific taxa (200). The bidirectional relation between intake calcium and vitamin D and the gut microbiota has important implications for bone health (201) in athletes of all ages, whether for growth or maintenance of bone density, to reduce the risk of fractures.
Food avoidance
Gastrointestinal issues are common among athletes. To alleviate symptoms, athletes may avoid or restrict certain foods that trigger symptoms. Athletes may also adopt nutritional strategies to increase gastric emptying and improve absorption of water and nutrients, including avoidance of high-FODMAP foods and gluten-containing foods (202).
FODMAP are nondigestible short-chain carbohydrates that increase the osmotic load within the gastrointestinal tract. Intestinal microbes can ferment these dietary components to form gas, which results in bloating and gastrointestinal distress in certain individuals (203). A recent study investigating FODMAP intake in endurance athletes reported high intake, both habitually and surrounding exercise, contributing to gastrointestinal symptoms (204). Preliminary results indicate that a low-FODMAP diet alleviates gastrointestinal symptoms in athletes (203, 205, 206). However, FODMAP also act as fuel for the gut microbiota, and their restriction may impact the composition and function of the community (207).
It has been postulated that it is the reduction in FODMAP foods on the gluten-free diet that may be affecting improvement in gastrointestinal symptoms rather than gluten itself (208–210). To our knowledge, only 1 study has investigated the effects of a gluten-free diet in nonceliac endurance athletes (211), which reported no effect of the gluten-free diet on performance, gastrointestinal symptoms, well-being, intestinal injury, or inflammatory markers relative to a gluten-containing diet. However, this was a small study (n = 13) with a short duration (7 d) and did not assess effects on the gut microbiota, limiting its ability to draw conclusions on effects for the general athlete population or assess potential long-term effects on health or the gut microbiota.
Energy intake
Food avoidance may also be applied more generally to energy restriction to achieve a particular physique or weight for sport. This is prevalent among female athletes and may result in inadequate energy availability, menstrual dysfunction, and decreased bone mineral density termed the “female athlete triad” (212). Energy deficiency contributes to gastrointestinal distress in athletes (213). At the extreme, anorexia decreases gut microbiota diversity and richness and increases Methanobrevibacter smithii, Proteobacteria, and the ratio of Bacteroidetes to Firmicutes (214). Anorexia is also associated with an altered metabolomic profile, including reduced SCFAs (215). These differences in the microbiota and metabolomic profiles may contribute to the clinical manifestations of inadequate energy availability, including gastrointestinal symptoms and compromised bone density (214). Prebiotic and probiotic supplementation and SCFAs have shown promising effects in the maintenance of and improvement in bone density and bone resorption, potentially via increased calcium absorption or IGF-I (214, 216). Therefore, microbiota-targeted therapies may complement dietary and psychiatric treatments for athletes with inadequate energy intake and/or disordered eating.
On the other side of the spectrum, many athletes have increased energy intake relative to sedentary controls (83). Much of this energy is utilized to support the energy demands of exercise, muscle remodeling and repair, and the health of the brain and immune system. However, greater quantities of food intake result in greater amounts of substrates being delivered to the large intestine due to the general efficiency of digestion and absorption. Each day, ∼15% of carbohydrates, 10% of protein, and 7% of fat escape digestion and are available for microbial fermentation (217). Maldigestion and malabsorption of nutrients may also be exacerbated by decreased blood flow and oxygen delivery (i.e., hypoxia) to the gut during exercise, causing changes in absorption, gut motility, and transit time (218). Intestinal hypoxia may also alter the mucosal-associated gut microbiota composition and disturb the balance of metabolic functions within this niche, potentially compounding the effects of these changes in gastrointestinal physiology on maldigestion and malabsorption. Increased caloric intake, independent of macronutrient composition changes, increases Firmicutes and decreases Bacteroidetes and microbiota diversity (219). Total calorie intake is positively correlated with the abundance of circulating serum zonulin, a marker of gastrointestinal permeability, in a large cohort of women, including athletes, anorexia nervosa patients, and normal-weight, overweight, and individuals with obesity (220). Zonulin was also negatively correlated with Ruminococcaceae and Faecalibacterium, both of which are butyrate-producing taxa, suggesting alterations in the gut microbiota composition (220). There were no differences in zonulin or gut microbiota composition detected between athletes and nonathletes, but differences in dietary intakes between the groups were not discussed, and therefore it is unclear whether disparities in dietary intake, or lack thereof, may have contributed to this homogeneity. Thus, while higher energy intake may contribute to differences in gastrointestinal function and the microbiota, athletes should obtain adequate dietary intake to support increased energy demands.
Hydration
Hydration status is crucial for athlete health and performance and is supported by water and electrolyte transport across the gastrointestinal barrier. There is limited information on the effects of hydration status on the gut microbiota. However, lubiprostone, a clinical agent that is used to stimulate Cl– secretion and thus cause water and electrolyte secretion in the gut, alters in the intestinal mucus layer and increases Lactobacilli in mice (221, 222). Additionally, dehydration can lead to constipation (223). Constipation has been associated with decreased Bacteroides, Roseburia, and Coprococcusand increased abundances of genes involved in gas production (224). Furthermore, stool consistency and transit time are linked to the diversity and composition of the gut microbiota (225). Dehydration also increases gastrointestinal distress symptoms (218), suggesting that insufficient fluid replacement affects gut function and may impact the gut microbiota.
The gut microbiota may also influence hydration status via cellular transport of solutes through the gastrointestinal mucosa (22). Hydration status biomarkers, including copeptin, urine volume, and urine nitrogen concentration, are associated with substrate utilization and energy expenditure (226) as well as long-term health outcomes such as metabolic syndrome, diabetes, obesity, kidney disease, and heart disease (227) and may therefore be useful measurements to assess the relation of the gut microbiota with hydration and health outcomes. These associations would help assess the effects of the gut microbiota on hydration status, or vice versa, and subsequent effects on substrate utilization and gastrointestinal distress in athletes during competition, both of which could potentially impact performance.
Additionally, carbohydrate, electrolyte, and energy beverages are commonly used by endurance athletes (228) but, to our knowledge, no studies have investigated the effects of carbohydrate or concentrated sports drinks on the gut microbiota (17). However, intake of both caloric and low/noncaloric sweeteners and food emulsifiers commonly contained in these beverages may have harmful, proinflammatory effects (229). Both sucralose and emulsifiers such as carrageenan have been shown to trigger proinflammatory responses, including upregulation of TNF-α as well as increased gastrointestinal permeability in both humans and animal models (146, 229). Ultimately, the effects of low/noncaloric sweeteners on the gut microbiota of athletes remain unclear (138, 230–233).
Sport supplements
To support and enhance athletic performance, athletes frequently consume nutritional supplements that may also have additional, unintended, impacts on the gut microbiota (17). Some of these, such as protein supplements, BCAAs, taurine, ɷ-3 fatty acids, vitamin D, and probiotics, have already been discussed. However, other commonly used supplements include antioxidants, nitrates, sodium bicarbonate, creatine, B-alanine, and caffeine (17, 234).
While some degree of exercise-induced oxidative stress is necessary for muscle adaptation, excessive ROS concentrations may compromise health, immunity, and recovery (17). Polyphenols are plant-derived compounds commonly used for their antioxidant properties to mitigate excessive oxidative stress in athletes (235). However, the bioavailability, absorption, and effects of polyphenols often depend on their conversion by the gut microbiota into more bioavailable, bioactive metabolites (236, 237). Additionally, polyphenols exert prebiotic-like effects on the gut microbiota composition by increasing the abundances of commensal bacteria, including Bifidobacterium, Lactobacilli, Akkermansia muciniphila, Faecalibacterium prausnitzii, and Roseburia spp. (237). Therefore, in addition to direct effects on reducing excess ROS, polyphenols may improve recovery and performance via their effects on the gut microbiota and the production of microbial metabolites.
Nitrates, mainly in the form of beetroot juice, improve athletic performance via increased oxygen uptake efficiency by skeletal muscle (238). Conversion of dietary nitrate to nitrite may also influence the gut microbiota composition via antimicrobial properties and modulation of intestinal permeability (238). However, it is difficult to isolate the role of nitrates from other compounds, such as polyphenols provided by vegetable intake (238). Certain bacteria can also utilize nitrate as a nutrient, which may increase bioavailability in skeletal muscle and contribute to its ergogenic effect (238).
Sodium bicarbonate is used to enhance buffering capacity, thus mitigating the increase in intracellular acidosis during intense exercise (239). Bicarbonate-rich mineral water consumption increases Christenellaceae, Bacteroidaceae, and Erysipelotrichaceae and decreases Bifidobacteriaceae (240). While higher abundance of Christenellaceae has been reported in lean individuals relative to individuals with obesity (241), it is unclear whether changes in the gut microbiota resulting from sodium bicarbonate supplementation may contribute to its ergogenic effects during exercise.
Creatine increases the muscle phosphocreatine reservoir, enhancing rapid ATP regeneration during high-intensity exercise (234). To our knowledge, there are no studies of the effects of creatine supplementation on the gut microbiota. Higher doses of creatine (≥10g) increase gastrointestinal distress and the risk of diarrhea (242). However, lower doses of creatine do not affect gastrointestinal symptoms (242) and research in mice suggests that glycine amidinotransferase (GATM), the enzyme that catalyzes the rate-limiting step of creatine biosynthesis, has a beneficial effect on gastrointestinal barrier integrity (243).
B-alanine is the rate-limiting precursor for carnosine synthesis, and supplementation is used to elevate muscle carnosine concentration, providing a benefit for high-intensity exercise (234). The effects of B-alanine supplementation on the gut microbiota or the effects of the gut microbiota on B-alanine supplementation efficacy have not been investigated. However, certain bacteria, including Lactobacilli and Streptococcus thermophilus, have functional genes capable of B-alanine metabolism (244). Furthermore, animal models using antibiotic treatment and stress induced changes in microbial metabolism of B-alanine (245, 246). Therefore, it is plausible that the gut microbiota may influence the ergogenic effects of B-alanine supplementation.
Caffeine is widely used to reduce perceived effort, fatigue, or pain during exercise (234). Caffeine can be consumed in coffee, tea, energy drinks, pills, or foods. While some research has shown modest effects of coffee on the gut microbiota, such as increases in Bifidobacterium and Bacteroides (247, 248), coffee and tea contain complex mixtures of other compounds, such as polyphenols and chlorogenic acid, that may also impact the gut microbiota. A study in mice investigated the effects of coffee or coffee components (i.e., caffeine or chlorogenic acid) on the gut microbiota and demonstrated that caffeine increased butyrate and propionate (249). However, chlorogenic acid induced greater increases in acetate, propionate, and butyrate, while coffee had no significant effect, although another study in rats revealed an increase in SCFAs in response to coffee intake (250). Therefore, it is difficult to determine the potential for the gut microbiota and SCFAs to mediate the ergogenic effects of caffeine or coffee intake.
Overall, there is evidence to suggest that supplements commonly used by athletes may also affect the gut microbiota and the production of metabolites such as SCFAs. The implications of these changes in the gut microbiota on the ergogenic effects of these supplements are unclear but could involve mediation of effects via the gut–muscle axis.
Future Directions
There is currently a lack of research in humans on the interaction between the gut microbiota and exercise, particularly in combination with a controlled diet, which is a significant confounding factor. Researchers should implement validated approaches to assess acute (Automated Self-Administered 24-hour [ASA-24] dietary recall) and habitual dietary intake (Food-Frequency Questionnaire [FFQ]), which also allow for the calculation of standardized values such as the Healthy Eating Index (HEI). Dietary quality, commonly measured using the HEI, is associated with better physical performance (251), although it has been proposed that an Athlete Diet Index targeted specifically at assessing dietary quality for athletes may be more relevant (252). In terms of nutrient intake, higher protein intake in athletes compared with sedentary controls has been documented (83). However, an emphasis on protein, carbohydrate, and fat intake differs by sport modality (84, 253), gender (254), and as a result of fluctuations in training (255). Therefore, accurate measurement of both nutrient content and diet quality will help separate the effects of sport on the gut microbiota from other confounding factors. Studies should also record fluid intake or measure hydration biomarkers (e.g., copeptin) to determine whether hydration status affects the gut microbiota or vice versa. Additionally, the effects of diet and exercise on the gut microbiota are often transient and do not persist after completion of the intervention (23, 256, 257). This suggests that long-term lifestyle habits are necessary to induce stable shifts in the gut microbiota. Alternatively, certain interventions or interventions during critical development windows may have more lasting effects on the gut microbiota, although this requires further investigation.
Although animal models are useful because factors such as diet can be stringently controlled and tissue samples are available to study mechanisms, differences in gastrointestinal physiology, microbiota compositions, effects of genetic background in mice, coprophagy, housing conditions, and feeding, as well as insufficient numbers of “donor microbiomes” in the case of human microbiota transplants in rodents all limit the translation of rodent research (258, 259). Future research should focus on using a tiered approach in which human clinical trials are used to identify target bacteria that may benefit athletic performance and animal and in vitro studies are used to determine causality and mechanisms. Human trials may then be used again to determine whether supplementation with the identified bacteria or implementation of dietary practices (e.g., prebiotics/nondigestible carbohydrates, ɷ-3 fatty acid supplements, type/amount of protein intake) that enhance bacterial abundance and/or functionality are beneficial for athletic performance.
Clinical studies investigating the effects of high-protein diets, whole-food protein sources, and protein supplements in the context of a controlled diet are needed to determine the impact of these dietary patterns and components on the gut microbiota in athletes. Additionally, more research is needed to clarify the effects of amounts and types of dietary fat on the gut microbiota and subsequent microbiota-mediated (e.g., via bile acids) effects on exercise performance. This research should consider differences among athletes practicing sports of different durations and intensities and effects in female athletes, as fat oxidation during exercise is higher in women versus men (260). Research is also needed to establish the potential detrimental effects of a diet low in nondigestible carbohydrates on the athletic gut microbiota as well as the potential beneficial effects of different types, doses, and timing of fiber intake and other candidate prebiotics, in whole foods or as isolated supplements, on athlete health and performance while addressing issues of tolerability and gastrointestinal distress. To assess the role of microbial metabolites, future studies should consider the use of intrinsically labeled SCFAs to assess systemic availability and their incorporation into biologically relevant molecules (18). While some research suggests a potential ergogenic effect of probiotic strains in athletes (2, 36, 156, 261), confirmatory trials to replicate findings are rare. Therefore, correlations of single strains or multi-strain formulations with certain performance or health outcomes are primarily based on a single study (172). More evidence is needed to clarify the potential for ingestion of probiotics or fermented foods to enhance athletic performance. Additionally, more research is needed with larger and more diverse sample populations to determine the specific strains or combinations of strains that may induce specific, desired responses in athletes and potential modification of effects by individual factors, such as gender, as this has been reported to impact gastrointestinal structure, function, and microbiota at rest and during exercise (35). Similarly, response to prebiotics, probiotics, and dietary strategies such as a low-FODMAP or gluten-free diet may differ based on an individual's baseline microbiota composition (8, 262), indicating that researchers must take a precision nutrition approach to account for interindividual differences that may influence the efficacy of avoidance of certain dietary components. This may also be true of the microbial and ergogenic response to dietary supplements, although more research is needed to understand the interaction between sports supplements and the gut microbiota.
It is increasingly recognized that the responses of an individual's gut microbiota to diet are personalized depending on characteristics such as the presence or abundance of keystone species (e.g., Ruminococcus bromii or Prevotella copri) (155, 263) or metabotypes (264). Interindividual variability in microbial responses then contributes to variability in metabolic responses (e.g., glycemic response) and health outcomes (e.g., weight loss) (7, 8). Therefore, dietary strategies require a nuanced approach to optimize health via the gut microbiota. To capture this complexity, future research should also integrate other “omics” data to determine potential metabolites, genes, and epigenetic modifications that may cause, contribute to, mediate, or modulate the effects of diet and exercise on the gut microbiota (265–267). The use of “omics” data coupled with machine-learning methods has the potential to uncover novel associations between the gut microbiota and its metabolites, diet, and athletic performance, as well as predict personalized responses to dietary strategies (16). The impacts of these findings include the potential for enhanced performance in athletes and improved health, particularly gastrointestinal and respiratory health. Additionally, the research will lead to a greater understanding of the interaction between the gut microbiota, diet, and human health that may have implications and applications that extend beyond the athletic population to benefit the health of all.
Conclusions
To achieve optimum performance, athletes must fuel, train, and utilize their entire supraorganism, including their gut microbiota, by implementing gut-centric dietary strategies (Text Box 2). There is a growing body of research on the role of the gut microbiota in sport and performance. Current evidence suggests that the gut microbiota may contribute to the effects of dietary intake on athletic performance via production of metabolites (e.g., SCFAs, secondary bile acids), influence on gastrointestinal physiology (e.g., nutrient absorption, barrier integrity, motility, gas production), and immune modulation (e.g., pathogen inhibition, GALT). Common dietary strategies in athletes, such as high protein and simple carbohydrate intake, low intake of nondigestible carbohydrates, and food avoidance, may adversely impact the gut microbiota and predispose athletes to gastrointestinal distress and thus impair performance. Conversely, intake of adequate dietary fiber, a variety of protein sources, and emphasis on unsaturated fats, especially ɷ-3 fatty acids, as well as supplementation with pre-, pro-, and synbiotics, have shown promising results in optimizing the health of the athlete and their gut microbiota with potential beneficial effects on performance.
Text Box 2.
Summary
• Diet and exercise affect the composition and function of the gut microbiome via substrate availability and physiological changes to the gastrointestinal environment.
• Sport-centric dietary strategies such as high protein, carbohydrate loading, and FODMAP restriction as well as gut-centric dietary strategies such as pro-, pre-, and synbiotics all represent opportunities to impact both the gut microbiota and athletic performance.
• High-protein diets and use of protein supplements show a greater effect on microbial metabolites than on the gut microbiota composition. The gut microbiota may contribute to muscle protein anabolism and function by modulating protein absorption and utilization.
• High-fat and saturated fat intake are associated with a proinflammatory gut microbiota composition, although ɷ-3 fatty acids promote SCFA production. Effects of these changes on athletic performance is inconclusive.
• Intake of highly digestible carbohydrates at the expense of fiber has detrimental effects on the gut microbiota, whereas SCFAs produced by the gut microbiota from dietary fiber are positively associated with muscle function.
• Pro-, pre-, and synbiotics can alter the gut microbiota and positively affect athletic performance and recovery. Variability in strains, doses, and other individual factors makes it difficult to identify the ergogenic effects of these gut-centric dietary strategies.
• The gut microbiota influences the absorption of certain micronutrients, including calcium, that are important for aspects of athlete health and performance, such as bone health.
• Short-term or pre-exercise avoidance of certain foods or food groups, such as FODMAPs or gluten, may be warranted for some individuals but the long-term effects of these strategies on the athletic gut microbiota and performance are unclear.
• Energy deficiency or excess both influence the gut microbiota. The gut microbiota and microbiota-based therapies may help alleviate detrimental effects of both extremes including gastrointestinal symptoms and compromised bone density.
• There is limited evidence on the effect of hydration status or sports drinks on the gut microbiota, although dehydration is associated with constipation and gastrointestinal symptoms that affect or indicate effects on the gut microbiota.
• Sports supplements are used for their ergogenic effects but their effects on the gut microbiota are unclear and warrant further research.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—RLH: performed the literature review and composed the manuscript; HDH: provided content and formatting advice and edited the final manuscript; and both authors: read and approved the final manuscript.
Notes
This work was partially supported by Danone Research.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: BCAA, branched-chain amino acid; FODMAP, fermentable oligo-, di-, monosaccharides and polyols; FXR, farnesoid X receptor; GALT, gut-associated lymphoid tissue; HEI, Healthy Eating Index; IGF-I, insulin-like growth factor I; IMTG, intramuscular triglyceride; IPE, inulin-propionate ester; PAG, phenylacetylglycine; ROS, reactive oxygen species; TGR5, G protein–coupled receptor 5; TMAO, trimethylamine-N-oxide; 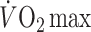 , maximal oxygen uptake.
, maximal oxygen uptake.
Contributor Information
Riley L Hughes, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana, IL, USA.
Hannah D Holscher, Department of Food Science and Human Nutrition, University of Illinois at Urbana-Champaign, Urbana, IL, USA; Division of Nutrition Sciences, University of Illinois at Urbana-Champaign, Urbana, IL, USA.
References
- 1. Costa MS, Toscano LT, Tavares Toscano LdL, Luna VR, Torres RA, Silva JA, Silva AS. Ergogenic potential of foods for performance and recovery: a new alternative in sports supplementation? A systematic review. Crit Rev Food Sci Nutr. 23 Nov 2020:1–22.. 10.1080/10408398.2020.1844137. [DOI] [PubMed] [Google Scholar]
- 2. Hughes RL. A review of the role of the gut microbiome in personalized sports nutrition. Front Nutr. 2020;6(191):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31. [PMC free article] [PubMed] [Google Scholar]
- 4. Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. [DOI] [PubMed] [Google Scholar]
- 5. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MAet al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight Ret al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. [DOI] [PubMed] [Google Scholar]
- 8. Hughes RL, Kable ME, Marco M, Keim NL. The role of the gut microbiome in predicting response to diet and the development of precision nutrition models. Part II: results. Adv Nutr. 2019;10(6):979–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, Abrouk M, Farahnik B, Nakamura M, Zhu THet al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mailing LJ, Allen JM, Buford TW, Fields CJ, Woods JA. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc Sport Sci Rev. 2019;47(2):75–85. [DOI] [PubMed] [Google Scholar]
- 11. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Creedon AC, Hung ES, Berry SE, Whelan K. Nuts and their effect on gut microbiota, gut function and symptoms in adults: a systematic review and meta-analysis of randomised controlled trials. Nutrients. 2020;12(8):2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shinn LM, Li Y, Mansharamani A, Auvil LS, Welge ME, Bushell C, Khan NA, Charron CS, Novotny JA, Baer DJet al. Fecal bacteria as biomarkers for predicting food intake in healthy adults. J Nutr. 2021;151(2):423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merra G, Noce A, Marrone G, Cintoni M, Tarsitano MG, Capacci A, De Lorenzo A. Influence of Mediterranean diet on human gut microbiota. Nutrients. 2021;13(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mohr AE, Jäger R, Carpenter KC, Kerksick CM, Purpura M, Townsend JR, West NP, Black K, Gleeson M, Pyne DBet al. The athletic gut microbiota. J Int Soc Sports Nutr. 2020;17(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mancin L, Rollo I, Mota JF, Piccini F, Carletti M, Susto GA, Valle G, Paoli A. Optimizing microbiota profiles for athletes. Exerc Sport Sci Rev. 2021;49(1):42–9. [DOI] [PubMed] [Google Scholar]
- 17. Donati Zeppa S, Agostini D, Gervasi M, Annibalini G, Amatori S, Ferrini F, Sisti D, Piccoli G, Barbieri E, Sestili P. Mutual interactions among exercise, sport supplements and microbiota. Nutrients. 2020;12(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boets E, Gomand SV, Deroover L, Preston T, Vermeulen K, De Preter V, Hamer HM, Van den Mooter G, De Vuyst L, Courtin CM. Systemic availability and metabolism of colonic-derived short-chain fatty acids in healthy subjects: a stable isotope study. J Physiol. 2017;595(2):541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hawley JA. Microbiota and muscle highway—two way traffic. Nat Rev Endocrinol. 2019;16(2):1–2. [DOI] [PubMed] [Google Scholar]
- 20. Nay K, Jollet M, Goustard B, Baati N, Vernus B, Pontones M, Lefeuvre-Orfila L, Bendavid C, Rué O, Mariadassou M. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. Am J Physiol Endocrinol Metab. 2019;317(1):E158–71. [DOI] [PubMed] [Google Scholar]
- 21. Frampton J, Murphy KG, Frost G, Chambers ES. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat Metab. 2020;2(9):1–9. [DOI] [PubMed] [Google Scholar]
- 22. Mach N, Fuster-Botella D. Endurance exercise and gut microbiota: a review. J Sport Health Sci. 2017;6(2):179–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bycura D, Santos AC, Shiffer A, Kyman S, Winfree K, Sutliffe J, Pearson T, Sonderegger D, Cope E, Caporaso JG. Impact of different exercise modalities on the human gut microbiome. Sports. 2021;9(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manor O, Dai CL, Kornilov SA, Smith B, Price ND, Lovejoy JC, Gibbons SM, Magis AT. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat Commun. 2020;11(1):5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu Q, Jiang S, Du G. Effects of exercise frequency on the gut microbiota in elderly individuals. Microbiology Open. 2020;9(8):e1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bai J, Hu Y, Bruner DW. Composition of gut microbiota and its association with body mass index and lifestyle factors in a cohort of 7–18 years old children from the American Gut Project. Pediatr Obesity. 2019;14(4):e12480. [DOI] [PubMed] [Google Scholar]
- 27. Kern T, Blond MB, Hansen TH, Rosenkilde M, Quist JS, Gram AS, Ekstrøm CT, Hansen T, Stallknecht B. Structured exercise alters the gut microbiota in humans with overweight and obesity—a randomized controlled trial. Int J Obes. 2020;44(1):125–35. [DOI] [PubMed] [Google Scholar]
- 28. O'Donovan CM, Madigan SM, Garcia-Perez I, Rankin A, O’ Sullivan O, Cotter PD. Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. J Sci Med Sport. 2020;23(1):63–8. [DOI] [PubMed] [Google Scholar]
- 29. Morita E, Yokoyama H, Imai D, Takeda R, Ota A, Kawai E, Hisada T, Emoto M, Suzuki Y, Okazaki K. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients. 2019;11(4):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allen JM, Berg Miller ME, Pence BD, Whitlock K, Nehra V, Gaskins HR, White BA, Fryer JD, Woods JA. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol. 2015;118(8):1059–66. [DOI] [PubMed] [Google Scholar]
- 31. Lamoureux EV, Grandy SA, Langille MGI. Moderate exercise has limited but distinguishable effects on the mouse microbiome. mSystems. 2017;2(4):e00006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Batacan R, Fenning A, Dalbo V, Scanlan A, Duncan M, Moore R, Stanley D. A gut reaction: the combined influence of exercise and diet on gastrointestinal microbiota in rats. J Appl Microbiol. 2017;122(6):1627–38. [DOI] [PubMed] [Google Scholar]
- 33. Roager HM, Hansen LB, Bahl MI, Frandsen HL, Carvalho V, Gøbel RJ, Dalgaard MD, Plichta DR, Sparholt MH, Vestergaard H. Colonic transit time is related to bacterial metabolism and mucosal turnover in the gut. Nat Microbiol. 2016;1(9):1–9. [DOI] [PubMed] [Google Scholar]
- 34. Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S. Cohabiting family members share microbiota with one another and with their dogs. elife. 2013;2:e00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pugh JN, Lydon K, O'Donovan CM, O'Sullivan O, Madigan S. More than a gut feeling: what is the role of the gastrointestinal tract in female athlete health?. Eur J Sport Sci. May 20 2021:1–10.. doi: 10.1080/17461391.2021.1921853. [DOI] [PubMed] [Google Scholar]
- 36. Marttinen M, Ala-Jaakkola R, Laitila A, Lehtinen MJ. Gut microbiota, probiotics and physical performance in athletes and physically active individuals. Nutrients. 2020;12(10):2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moreno-Pérez D, Bressa C, Bailén M, Hamed-Bousdar S, Naclerio F, Carmona M, Pérez M, González-Soltero R, Montalvo-Lominchar MG, Carabaña C. Effect of a protein supplement on the gut microbiota of endurance athletes: a randomized, controlled, double-blind pilot study. Nutrients. 2018;10(3):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murtaza N, Burke LM, Vlahovich N, Charlesson B, O’ Neill H, Ross ML, Campbell KL, Krause L, Morrison M. The effects of dietary pattern during intensified training on stool microbiota of elite race walkers. Nutrients. 2019;11(2):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Karl JP, Margolis LM, Madslien EH, Murphy NE, Castellani JW, Gundersen Y, Hoke AV, Levangie MW, Kumar R, Chakraborty Net al. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol. 2017;312(6):G559–71. [DOI] [PubMed] [Google Scholar]
- 40. Son J, Jang L-G, Kim B-Y, Lee S, Park H. The effect of athletes’ probiotic intake may depend on protein and dietary fiber intake. Nutrients. 2020;12(10):2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang W-C, Pan C-H, Wei C-C, Huang H-Y. Lactobacillus plantarum PS128 improves physiological adaptation and performance in triathletes through gut microbiota modulation. Nutrients. 2020;12(8):2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martarelli D, Verdenelli MC, Scuri S, Cocchioni M, Silvi S, Cecchini C, Pompei P. Effect of a probiotic intake on oxidant and antioxidant parameters in plasma of athletes during intense exercise training. Curr Microbiol. 2011;62(6):1689–96. [DOI] [PubMed] [Google Scholar]
- 43. West NP, Pyne DB, Cripps AW, Hopkins WG, Eskesen DC, Jairath A, Christophersen CT, Conlon MA, Fricker PA. Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutr J. 2011;10(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Axelrod CL, Brennan CJ, Cresci G, Paul D, Hull M, Fealy CE, Kirwan JP. UCC118 supplementation reduces exercise-induced gastrointestinal permeability and remodels the gut microbiome in healthy humans. Physiol Rep. 2019;7(22):e14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clark A, Mach N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: a systematic review for athletes. J Int Soc Sports Nutr. 2016;13(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty LA, Soares JW. Effects of psychological, environmental and physical stressors on the gut microbiota. Front Microbiol. 2018;9(2013). doi: 10.3389/fmicb.2018.02013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Costa R, Snipe R, Kitic C, Gibson P. Systematic review: exercise-induced gastrointestinal syndrome—implications for health and intestinal disease. Aliment Pharmacol Ther. 2017;46(3):246–65. [DOI] [PubMed] [Google Scholar]
- 48. de Oliveira EP, Burini RC, Jeukendrup A. Gastrointestinal complaints during exercise: prevalence, etiology, and nutritional recommendations. Sports Med. 2014;44(S1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pugh JN, Fearn R, Morton JP, Close GL. Gastrointestinal symptoms in elite athletes: time to recognise the problem?. Br J Sports Med. 2018;52(8):487–8. [DOI] [PubMed] [Google Scholar]
- 50. Arike L, Seiman A, van der Post S, Rodriguez Piñeiro AM, Ermund A, Schütte A, Bäckhed F, Johansson MEV, Hansson GC. Protein turnover in epithelial cells and mucus along the gastrointestinal tract is coordinated by the spatial location and microbiota. Cell Rep. 2020;30(4):1077–87, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gleeson M, Nieman DC, Pedersen BK. Exercise, nutrition and immune function. J Sports Sci. 2004;22(1):115–25. [DOI] [PubMed] [Google Scholar]
- 52. Tabone M, Bressa C, García-Merino JA, Moreno-Pérez D, Van EC, Castelli FA, Fenaille F, Larrosa M. The effect of acute moderate-intensity exercise on the serum and fecal metabolomes and the gut microbiota of cross-country endurance athletes. Sci Rep. 2021;11(1):3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cronin O, O'Sullivan O, Barton W, Cotter PD, Molloy MG, Shanahan F. Gut microbiota: implications for sports and exercise medicine. Br J Sports Med. 2017;51(9):700–1. [DOI] [PubMed] [Google Scholar]
- 54. Kårlund A, Gómez-Gallego C, Turpeinen AM, Palo-oja O-M, El-Nezami H, Kolehmainen M. Protein supplements and their relation with nutrition, microbiota composition and health: is more protein always better for sportspeople?. Nutrients. 2019;11(4):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tipton KD, Wolfe RR. Protein and amino acids for athletes. J Sports Sci. 2004;22(1):65–79. [DOI] [PubMed] [Google Scholar]
- 56. Phillips SM, Van Loon LJC. Dietary protein for athletes: from requirements to optimum adaptation. J Sports Sci. 2011;29(Suppl 1):S29–38. [DOI] [PubMed] [Google Scholar]
- 57. Moore DR. One size doesn't fit all: postexercise protein requirements for the endurance athlete. Am J Clin Nutr. 2020;112(2):249–50. [DOI] [PubMed] [Google Scholar]
- 58. Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Betz MW, Senden JM, Goessens JPB, Gijsen AP, Rollo I, Verdijk LB, van Loon LJC. Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: a double-blind randomized trial. Am J Clin Nutr. 2020;112(2):303–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moore DR. Maximizing post-exercise anabolism: the case for relative protein intakes. Front Nutr. 2019;6(147):e00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yao CK, Muir JG, Gibson PR. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther. 2016;43(2):181–96. [DOI] [PubMed] [Google Scholar]
- 61. Oliphant K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shen Q, Chen YA, Tuohy KM. A comparative in vitro investigation into the effects of cooked meats on the human faecal microbiota. Anaerobe. 2010;16(6):572–7. [DOI] [PubMed] [Google Scholar]
- 63. Diether NE, Willing BP. Microbial fermentation of dietary protein: an important factor in diet–microbe–host interaction. Microorganisms. 2019;7(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Portune KJ, Beaumont M, Davila A-M, Tomé D, Blachier F, Sanz Y. Gut microbiota role in dietary protein metabolism and health-related outcomes: the two sides of the coin. Trends Food Sci Technol. 2016;57:213–32. [Google Scholar]
- 65. Blachier F, Beaumont M, Portune KJ, Steuer N, Lan A, Audebert M, Khodorova N, Andriamihaja M, Airinei G, Benamouzig Ret al. High-protein diets for weight management: interactions with the intestinal microbiota and consequences for gut health. A position paper by the My New Gut Study Group. Clin Nutr. 2019;38(3):1012–22. [DOI] [PubMed] [Google Scholar]
- 66. Madsen L, Myrmel LS, Fjære E, Liaset B, Kristiansen K. Links between dietary protein sources, the gut microbiota, and obesity. Front Physiol. 2017;8:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–64. [DOI] [PubMed] [Google Scholar]
- 68. Rahimi MH, Shab-Bidar S, Mollahosseini M, Djafarian K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: a meta-analysis of randomized clinical trials. Nutrition. 2017;42:30–6. [DOI] [PubMed] [Google Scholar]
- 69. Caine JJ, Geracioti TD. Taurine, energy drinks, and neuroendocrine effects. Cleve Clin J Med. 2016;83(12):895–904. [DOI] [PubMed] [Google Scholar]
- 70. Wolf PG, Gaskins HR, Ridlon JM, Freels S, Hamm A, Goldberg S, Petrilli P, Schering T, Vergis S, Gomez-Perez Set al. Effects of taurocholic acid metabolism by gut bacteria: a controlled feeding trial in adult African American subjects at elevated risk for colorectal cancer. Contemp Clin Trials Comm. 2020;19:100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McKenna CF, Salvador AF, Hughes RL, Scaroni SE, Alamilla RA, Askow AT, Paluska SA, Dilger AC, Holscher HD, Lisio MDet al. Higher protein intake during resistance training does not potentiate strength, but modulates gut microbiota, in middle-aged adults: a randomized control trial. Am J Physiol Endocrinol Metab. 2021;320(5):E900–13. [DOI] [PubMed] [Google Scholar]
- 72. Cronin O, Barton W, Skuse P, Penney NC, Garcia-Perez I, Murphy EF, Woods T, Nugent H, Fanning A, Melgar S. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. MSystems. 2018;3(3):e00044–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Israr MZ, Bernieh D, Salzano A, Cassambai S, Yazaki Y, Heaney LM, Jones DJL, Ng LL, Suzuki T. Association of gut-related metabolites with outcome in acute heart failure. Am Heart J. 2021;234:71–80. [DOI] [PubMed] [Google Scholar]
- 75. Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E, Shanahan F, Cotter PD, O'Sullivan O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut. 2018;67(4):625–33. [DOI] [PubMed] [Google Scholar]
- 76. Cho CE, Caudill MA. Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire?. Trends Endocrinol Metab. 2017;28(2):121–30. [DOI] [PubMed] [Google Scholar]
- 77. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, Sutter JL, Caudill MA. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res. 2017;61(1). doi: 10.1002/mnfr.201600324. [DOI] [PubMed] [Google Scholar]
- 78. Hamaya R, Ivey KL, Lee DH, Wang M, Li J, Franke A, Sun Q, Rimm EB. Association of diet with circulating trimethylamine-N-oxide concentration. Am J Clin Nutr. 2020;112(6):1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cho CE, Aardema NDJ, Bunnell ML, Larson DP, Aguilar SS, Bergeson JR, Malysheva OV, Caudill MA, Lefevre M. Effect of choline forms and gut microbiota composition on trimethylamine-N-oxide response in healthy men. Nutrients. 2020;12(8):2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Beaumont M, Portune KJ, Steuer N, Lan A, Cerrudo V, Audebert M, Dumont F, Mancano G, Khodorova N, Andriamihaja Met al. Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am J Clin Nutr. 2017;106(4):1005–19. [DOI] [PubMed] [Google Scholar]
- 81. Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut. 2016;65(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, Gonzalez A, Kosciolek T, McCall L-I, McDonald Det al. Best practices for analysing microbiomes. Nat Rev Microbiol. 2018;16(7):410–22. [DOI] [PubMed] [Google Scholar]
- 83. Clarke SF, Murphy EF, O'Sullivan O, Lucey AJ, Humphreys M, Hogan A, Hayes P, O'Reilly M, Jeffery IB, Wood-Martin Ret al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut. 2014;63(12):1913–20. [DOI] [PubMed] [Google Scholar]
- 84. Jang L-G, Choi G, Kim S-W, Kim B-Y, Lee S, Park H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: an observational study. J Int Soc Sports Nutr. 2019;16(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lang JM, Pan C, Cantor RM, Tang WHW, Garcia-Garcia JC, Kurtz I, Hazen SL, Bergeron N, Krauss RM, Lusis AJ. Impact of individual traits, saturated fat, and protein source on the gut microbiome. mBio. 2018;9(6):e01604–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Toden S, Bird AR, Topping DL, Conlon MA. Resistant starch prevents colonic DNA damage induced by high dietary cooked red meat or casein in rats. Cancer Biol Ther. 2006;5(3):267–72. [DOI] [PubMed] [Google Scholar]
- 87. Zhu Y, Lin X, Li H, Li Y, Shi X, Zhao F, Xu X, Li C, Zhou G. Intake of meat proteins substantially increased the relative abundance of genus lactobacillus in rat feces. PLoS One. 2016;11(4):e0152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhu Y, Lin X, Zhao F, Shi X, Li H, Li Y, Zhu W, Xu X, Li C, Zhou G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci Rep. 2015;5:15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhu Y, Shi X, Lin X, Ye K, Xu X, Li C, Zhou G. Beef, chicken, and soy proteins in diets induce different gut microbiota and metabolites in rats. Front Microbiol. 2017;8:1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Albracht-Schulte K, Islam T, Johnson P, Moustaid-Moussa N. Systematic review of beef protein effects on gut microbiota: implications for health. Adv Nutr. 2021;12(1):102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sprong RC, Schonewille AJ, van der Meer R. Dietary cheese whey protein protects rats against mild dextran sulfate sodium–induced colitis: role of mucin and microbiota. J Dairy Sci. 2010;93(4):1364–71. [DOI] [PubMed] [Google Scholar]
- 92. Jäger R, Purpura M, Farmer S, Cash HA, Keller D. Probiotic Bacillus coagulans GBI-30, 6086 improves protein absorption and utilization. Probiotics Antimicrob Proteins. 2018;10(4):611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ticinesi A, Lauretani F, Tana C, Nouvenne A, Ridolo E, Meschi T. Exercise and immune system as modulators of intestinal microbiome: implications for the gut-muscle axis hypothesis. Exerc Immunol Rev. 2019;25:84–95. [PubMed] [Google Scholar]
- 94. Bindels LB, Delzenne NM. Muscle wasting: the gut microbiota as a new therapeutic target?. Int J Biochem Cell Biol. 2013;45(10):2186–90. [DOI] [PubMed] [Google Scholar]
- 95. Jäger R, Zaragoza J, Purpura M, Iametti S, Marengo M, Tinsley GM, Anzalone AJ, Oliver JM, Fiore W, Biffi A. Probiotic administration increases amino acid absorption from plant protein: a placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob Proteins. 2020;12(4):1330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Casati M, Ferri E, Azzolino D, Cesari M, Arosio B. Gut microbiota and physical frailty through the mediation of sarcopenia. Exp Gerontol. 2019;124:110639. [DOI] [PubMed] [Google Scholar]
- 97. Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho-Júnior HJ, Gervasoni J, Primiano A, Putignani L, Del Chierico Fet al. Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the BIOSPHERE study. Nutrients. 2020;12(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Burd NA, Beals JW, Martinez IG, Salvador AF, Skinner SK. Food-first approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Sports Med. 2019;49(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. van Vliet S, Beals JW, Martinez IG, Skinner SK, Burd NA. Achieving optimal post-exercise muscle protein remodeling in physically active adults through whole food consumption. Nutrients. 2018;10(2):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. van Vliet S, Shy EL, Abou Sawan S, Beals JW, West DW, Skinner SK, Ulanov AV, Li Z, Paluska SA, Parsons CMet al. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am J Clin Nutr. 2017;106(6):1401–12. [DOI] [PubMed] [Google Scholar]
- 101. Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc. 2006;38(4):667–74. [DOI] [PubMed] [Google Scholar]
- 102. Thomas DT, Erdman KA, Burke LM. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: nutrition and athletic performance. J Acad Nutr Diet. 2016;116(3):501–28. [DOI] [PubMed] [Google Scholar]
- 103. Fritzen AM, Lundsgaard A-M, Kiens B. Dietary fuels in athletic performance. Annu Rev Nutr. 2019;39:1, 45. [DOI] [PubMed] [Google Scholar]
- 104. Shen W, Gaskins HR, McIntosh MK. Influence of dietary fat on intestinal microbes, inflammation, barrier function and metabolic outcomes. J Nutr Biochem. 2014;25(3):270–80. [DOI] [PubMed] [Google Scholar]
- 105. Thielecke F, Blannin A. Omega-3 fatty acids for sport performance—are they equally beneficial for athletes and amateurs? a narrative review. Nutrients. 2020;12(12):3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kübeck R, Bonet-Ripoll C, Hoffmann C, Walker A, Müller VM, Schüppel VL, Lagkouvardos I, Scholz B, Engel K-H, Daniel Het al. Dietary fat and gut microbiota interactions determine diet-induced obesity in mice. Mol Metab. 2016;5(12):1162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wolters M, Ahrens J, Romani-Perez M, Watkins C, Sanz Y, Benitez-Paez A, Stanton C, Gunther K. Dietary fat, the gut microbiota, and metabolic health—a systematic review conducted within the MyNewGut project. Clin Nutr. 2019;38(6):2504–20. [DOI] [PubMed] [Google Scholar]
- 108. Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18(12):2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rodriguez NR, Di Marco NM, Langley S. American College of Sports Medicine position stand. Nutrition and athletic performance. Med Sci Sports Exerc. 2009;41(3):709–31. [DOI] [PubMed] [Google Scholar]
- 110. Bailey CP, Hennessy E. A review of the ketogenic diet for endurance athletes: performance enhancer or placebo effect?. J Int Soc Sports Nutr. 2020;17(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Paoli A, Bianco A, Grimaldi KA. The ketogenic diet and sport: a possible marriage?. Exerc Sport Sci Rev. 2015;43(3):153–62. [DOI] [PubMed] [Google Scholar]
- 112. Murphy NE, Carrigan CT, Margolis LM. High-fat ketogenic diets and physical performance: a systematic review. Adv Nutr. 2021;12(1):223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci. 2010;107(33):14691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Backhed F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab. 2015;22(4):658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, Turnbaugh JA, Verdin E, Hall KD, Leibel RLet al. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell. 2020;181(6):1263–1275.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Paoli A, Mancin L, Bianco A, Thomas E, Mota JF, Piccini F. Ketogenic diet and microbiota: friends or enemies?. Genes. 2019;10(7):534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Margolis LM, O'Fallon KS. Utility of ketone supplementation to enhance physical performance: a systematic review. Adv Nutr. 2019;11(2):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Valenzuela PL, Castillo-García A, Morales JS, Lucia A. Perspective: ketone supplementation in sports—does it work?. Adv Nutr. 2021;12(2):305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Peake JM, Neubauer O, Gatta PAD, Nosaka K. Muscle damage and inflammation during recovery from exercise. J Appl Physiol. 2017;122(3):559–70. [DOI] [PubMed] [Google Scholar]
- 120. Huang EY, Leone VA, Devkota S, Wang Y, Brady MJ, Chang EB. Composition of dietary fat source shapes gut microbiota architecture and alters host inflammatory mediators in mouse adipose tissue. JPEN J Parenter Enteral Nutr. 2013;37(6):746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bailey MA, Holscher HD. Microbiome-Mediated effects of the Mediterranean diet on inflammation. Adv Nutr. 2018;9(3):193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The role of the gut microbiota in bile acid metabolism. Ann Hepatol. 2018;16(1):21–6. [DOI] [PubMed] [Google Scholar]
- 123. Gérard P. Metabolism of cholesterol and bile acids by the gut microbiota. Pathogens. 2014;3(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cerdá B, Pérez M, Pérez-Santiago JD, Tornero-Aguilera JF, González-Soltero R, Larrosa M. Gut microbiota modification: another piece in the puzzle of the benefits of physical exercise in health?. Front Physiol. 2016;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shaw CS, Clark J, Wagenmakers AJM. The effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu Rev Nutr. 2010;30(1):13–34. [DOI] [PubMed] [Google Scholar]
- 126. Clark A, Mach N. The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol. 2017;8:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mickleborough TD. Omega-3 polyunsaturated fatty acids in physical performance optimization. Int J Sport Nutr Exerc Metab. 2013;23(1):83. [DOI] [PubMed] [Google Scholar]
- 128. Hargreaves M. Skeletal muscle metabolism during exercise in humans. Clin Exp Pharmacol Physiol. 2000;27(3):225–8. [DOI] [PubMed] [Google Scholar]
- 129. Jeukendrup A. A step towards personalized sports nutrition: carbohydrate intake during exercise. Sports Medicine. 2014;44(S1):S25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jeukendrup AE. Carbohydrate intake during exercise and performance. Nutrition. 2004;20(7-8):669–77. [DOI] [PubMed] [Google Scholar]
- 131. Erickson J, Wang Q, Slavin J. White grape juice elicits a lower breath hydrogen response compared with apple juice in healthy human subjects: a randomized controlled trial. J Acad Nutr Diet. 2017;117(6):908–13. [DOI] [PubMed] [Google Scholar]
- 132. Corpe CP, Burant CF, Hoekstra JH. Intestinal fructose absorption: clinical and molecular aspects. J Pediatr Gastroenterol Nutr. 1999;28(4):364–74. [DOI] [PubMed] [Google Scholar]
- 133. Truswell AS, Seach JM, Thorburn A. Incomplete absorption of pure fructose in healthy subjects and the facilitating effect of glucose. Am J Clin Nutr. 1988;48(6):1424–30. [DOI] [PubMed] [Google Scholar]
- 134. Odell OJ, Wallis GA. The application of lactose in sports nutrition. Int Dairy J. 2021;116:104970. [Google Scholar]
- 135. Burke LM, van Loon LJC, Hawley JA. Postexercise muscle glycogen resynthesis in humans. J Appl Physiol. 2017;122(5):1055–67. [DOI] [PubMed] [Google Scholar]
- 136. Salvador AF, McKenna CF, Alamilla RA, Cloud RM, Keeble AR, Miltko A, Scaroni SE, Beals JW, Ulanov AV, Dilger RN. Potato ingestion is as effective as carbohydrate gels to support prolonged cycling performance. J Appl Physiol. 2019;127(6):1651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Heaney S, O'Connor H, Gifford J, Naughton G. Comparison of strategies for assessing nutritional adequacy in elite female athletes’ dietary intake. Int J Sport Nutr Exerc Metab. 2010;20(3):245. [DOI] [PubMed] [Google Scholar]
- 138. Di Rienzi SC, Britton RA. Adaptation of the gut microbiota to modern dietary sugars and sweeteners. Adv Nutr. 2020;11(3):616–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Payne AN, Chassard C, Lacroix C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: implications for host–microbe interactions contributing to obesity. Obes Rev. 2012;13(9):799–809. [DOI] [PubMed] [Google Scholar]
- 140. Holscher HD, Bauer LL, Gourineni V, Pelkman CL, Fahey GC Jr, Swanson KS. Agave inulin supplementation affects the fecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145(9):2025–32. [DOI] [PubMed] [Google Scholar]
- 141. Donatto FF, Prestes J, Frollini AB, Palanch AC, Verlengia R, Cavaglieri CR. Effect of oat bran on time to exhaustion, glycogen content and serum cytokine profile following exhaustive exercise. J Int Soc Sports Nutr. 2010;7(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Okamoto T, Morino K, Ugi S, Nakagawa F, Lemecha M, Ida S, Ohashi N, Sato D, Fujita Y, Maegawa H. Microbiome potentiates endurance exercise through intestinal acetate production. Am J Physiol Endocrinol Metab. 2019;316(5):E956–66. [DOI] [PubMed] [Google Scholar]
- 143. Bergström J, Hermansen L, Hultman E, Saltin B. Diet, muscle glycogen and physical performance. Acta Physiol Scand. 1967;71(2–3):140–50. [DOI] [PubMed] [Google Scholar]
- 144. Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham L-D, Wibowo MC, Wurth RC, Punthambaker S, Tierney BTet al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med. 2019;25(7):1104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Cook S. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 1998;12(6):499–507. [DOI] [PubMed] [Google Scholar]
- 146. Khoshbin K, Camilleri M. Effects of dietary components on intestinal permeability in health and disease. Am J Physiol Gastrointest Liver Physiol. 2020;319(5):G589–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Qi X, Tester RF. Gut associated lymphoid tissue: carbohydrate interactions within the intestine. Bioact Carbohydr Diet Fibre. 2020;21:100210. [Google Scholar]
- 148. West NP, Christophersen CT, Pyne DB, Cripps AW, Conlon MA, Topping DL, Kang S, McSweeney CS, Fricker PA, Aguirre D. Butyrylated starch increases colonic butyrate concentration but has limited effects on immunity in healthy physically active individuals. Exerc Immunol Rev. 2013;19:102–19. [PubMed] [Google Scholar]
- 149. Kapoor MP, Koido M, Kawaguchi M, Timm D, Ozeki M, Yamada M, Mitsuya T, Okubo T. Lifestyle related changes with partially hydrolyzed guar gum dietary fiber in healthy athlete individuals—a randomized, double-blind, crossover, placebo-controlled gut microbiome clinical study. J Funct Foods. 2020;72:104067. [Google Scholar]
- 150. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491. [DOI] [PubMed] [Google Scholar]
- 151. Delcour JA, Aman P, Courtin CM, Hamaker BR, Verbeke K. Prebiotics, fermentable dietary fiber, and health claims. Adv Nutr. 2016;7(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Scott KP, Grimaldi R, Cunningham M, Sarbini SR, Wijeyesekera A, Tang MLK, Lee JC-Y, Yau YF, Ansell J, Theis Set al. Developments in understanding and applying prebiotics in research and practice—an ISAPP conference paper. J Appl Microbiol. 2020;128(4):934–49. [DOI] [PubMed] [Google Scholar]
- 153. Macfarlane G, Steed H, Macfarlane S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J Appl Microbiol. 2008;104(2):305–44. [DOI] [PubMed] [Google Scholar]
- 154. Scholz-Ahrens KE, Ade P, Marten B, Weber P, Timm W, Aςil Y, Glüer C-C, Schrezenmeir J. Prebiotics, probiotics, and synbiotics affect mineral absorption, bone mineral content, and bone structure. J Nutr. 2007;137(3):838S–46S. [DOI] [PubMed] [Google Scholar]
- 155. Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Bjorck I, Backhed F. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of prevotella. Cell Metab. 2015;22(6):971–82. [DOI] [PubMed] [Google Scholar]
- 156. Calero CQ, Rincón EO, Marqueta PM. Probiotics, prebiotics and synbiotics: useful for athletes and active individuals? A systematic review. Beneficial Microbes. 2020;11(2):135–49. [DOI] [PubMed] [Google Scholar]
- 157. Williams NC, Johnson MA, Shaw DE, Spendlove I, Vulevic J, Sharpe GR, Hunter KA. A prebiotic galactooligosaccharide mixture reduces severity of hyperpnoea-induced bronchoconstriction and markers of airway inflammation. Br J Nutr. 2016;116(5):798–804. [DOI] [PubMed] [Google Scholar]
- 158. Malkova D, Polyviou T, Rizou E, Gerasimidis K, Chambers ES, Preston T, Tedford MC, Frost G, Morrison DJ. Moderate intensity exercise training combined with inulin-propionate ester supplementation increases whole body resting fat oxidation in overweight women. Metabolism. 2020;104:154043. [DOI] [PubMed] [Google Scholar]
- 159. West NP, Pyne DB, Cripps A, Christophersen CT, Conlon MA, Fricker PA. Gut Balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes. 2012;3(3):221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Coman MM, Verdenelli MC, Silvi S, Cecchini C, Gabbianelli R, Amadio E, Orpianesi C, Cresci A. Knowledge and acceptance of functional foods: a preliminary study on influence of a synbiotic fermented milk on athlete health. Int J Probiotics Prebiotics. 2017;12(1):33–42. [Google Scholar]
- 161. Roberts JD, Suckling CA, Peedle GY, Murphy JA, Dawkins TG, Roberts MG. An exploratory investigation of endotoxin levels in novice long distance triathletes, and the effects of a multi-strain probiotic/prebiotic, antioxidant intervention. Nutrients. 2016;8(11):733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chambers ES, Byrne CS, Morrison DJ, Murphy KG, Preston T, Tedford C, Garcia-Perez I, Fountana S, Serrano-Contreras JI, Holmes Eet al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut. 2019;68(8):1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Jäger R, Mohr AE, Carpenter KC, Kerksick CM, Purpura M, Moussa A, Townsend JR, Lamprecht M, West NP, Black K. International Society of Sports Nutrition position stand: probiotics. J Int Soc Sports Nutr. 2019;16(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jäger R, Mohr AE, Pugh JN. Recent advances in clinical probiotic research for sport. Curr Opin Clin Nutr Metab Care. 2020;23(6):428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Miles MP. Probiotics and gut health in athletes. Curr Nutr Rep. 2020;9(3):129–36. [DOI] [PubMed] [Google Scholar]
- 166. Möller GB, da Cunha Goulart MJV, Nicoletto BB, Alves FD, Schneider CD. Supplementation of probiotics and its effects on physically active individuals and athletes: systematic review. Int J Sport Nutr Exerc Metab. 2019;29(5):481–92. [DOI] [PubMed] [Google Scholar]
- 167. Wosinska L, Cotter PD, O'Sullivan O, Guinane C. The potential impact of probiotics on the gut microbiome of athletes. Nutrients. 2019;11(10):2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. West N, Pyne D, Peake J, Cripps A. Probiotics, immunity and exercise: a review. Exerc Immunol Rev. 2009;15(107):e26. [PubMed] [Google Scholar]
- 169. Nichols AW. Probiotics and athletic performance: a systematic review. Curr Sport Med Rep. 2007;6(4):269–73. [PubMed] [Google Scholar]
- 170. Pyne DB, West NP, Cox AJ, Cripps AW. Probiotics supplementation for athletes—clinical and physiological effects. Eur J Sport Sci. 2015;15(1):63–72. [DOI] [PubMed] [Google Scholar]
- 171. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen Set al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 172. Agans RT, Giles GE, Goodson MS, Karl JP, Leyh S, Mumy KL, Racicot K, Soares JW. Evaluation of probiotics for warfighter health and performance. Front Nutr. 2020;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zheng J, Wittouck S, Salvetti E, Franz C, Harris HMB, Mattarelli P, O'Toole PW, Pot B, Vandamme P, Walter Jet al. A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol. 2020;70(4):2782–858. [DOI] [PubMed] [Google Scholar]
- 174. Jäger R, Purpura M, Stone JD, Turner SM, Anzalone AJ, Eimerbrink MJ, Pane M, Amoruso A, Rowlands DS, Oliver JM. Probiotic Streptococcus thermophilus FP4 and Bifidobacterium breve BR03 supplementation attenuates performance and range-of-motion decrements following muscle damaging exercise. Nutrients. 2016;8(10):642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Harnett JE, Pyne DB, McKune AJ, Penm J, Pumpa KL. Probiotic supplementation elicits favourable changes in muscle soreness and sleep quality in rugby players. J Sci Med Sport. 2021;24(2):195–9. [DOI] [PubMed] [Google Scholar]
- 176. Sanz Y, De Palma G. Gut microbiota and probiotics in modulation of epithelium and gut-associated lymphoid tissue function. Int Rev Immunol. 2009;28(6):397–413. [DOI] [PubMed] [Google Scholar]
- 177. McFarland LV. Meta-analysis of probiotics for the prevention of traveler's diarrhea. Travel Med Infect Dis. 2007;5(2):97–105. [DOI] [PubMed] [Google Scholar]
- 178. Lee M-C, Hsu Y-J, Ho H-H, Hsieh S-H, Kuo Y-W, Sung H-C, Huang C-C. Lactobacillus salivarius subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue. Microorganisms. 2020;8(4):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Axling U, Önning G, Combs MA, Bogale A, Högström M, Svensson M. The effect of Lactobacillus plantarum 299v on iron status and physical performance in female iron-deficient athletes: a randomized controlled trial. Nutrients. 2020;12(5):1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NMet al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Costello JT, Bieuzen F, Bleakley CM. Where are all the female participants in Sports and Exercise Medicine research?. Eur J Sport Sci. 2014;14(8):847–51. [DOI] [PubMed] [Google Scholar]
- 182. Sanders M, Merenstein D, Merrifield C, Hutkins R. Probiotics for human use. Nutr Bull. 2018;43(3):212–25. [Google Scholar]
- 183. Taylor BC, Lejzerowicz F, Poirel M, Shaffer JP, Jiang L, Aksenov A, Litwin N, Humphrey G, Martino C, Miller-Montgomery S. Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. Msystems. 2020;5(2):e00901–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Hsu Y-J, Huang W-C, Lin J-S, Chen Y-M, Ho S-T, Huang C-C, Tung Y-T. Kefir supplementation modifies gut microbiota composition, reduces physical fatigue, and improves exercise performance in mice. Nutrients. 2018;10(7):862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Iwasa M, Aoi W, Mune K, Yamauchi H, Furuta K, Sasaki S, Takeda K, Harada K, Wada S, Nakamura Yet al. Fermented milk improves glucose metabolism in exercise-induced muscle damage in young healthy men. Nutr J. 2013;12(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Salehzadeh K. The effects of probiotic yogurt drink on lipid profile, CRP and record changes in aerobic athletes. Int J Life Sci. 2015;9(4):32–7. [Google Scholar]
- 187. O'Brien KV, Stewart L, Forney L, Aryana K, Prinyawiwatkul W, Boeneke C. The effects of postexercise consumption of a kefir beverage on performance and recovery during intensive endurance training. J Dairy Sci. 2015;98(11):7446–9. [DOI] [PubMed] [Google Scholar]
- 188. Clarkson PM. Micronutrients and exercise: anti-oxidants and minerals. J Sports Sci. 1995;13(sup1):S11–24. [DOI] [PubMed] [Google Scholar]
- 189. Heffernan SM, Horner K, De Vito G, Conway GE. The role of mineral and trace element supplementation in exercise and athletic performance: a systematic review. Nutrients. 2019;11(3):696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Maughan RJ. Role of micronutrients in sport and physical activity. Br Med Bull. 1999;55(3):683–90. [DOI] [PubMed] [Google Scholar]
- 191. DellaValle DM. Iron supplementation for female athletes: effects on iron status and performance outcomes. Curr Sports Med Rep. 2013;12(4):234. [DOI] [PubMed] [Google Scholar]
- 192. Mach N, Clark A. Micronutrient deficiencies and the human gut microbiota. Trends Microbiol. 2017;25(8):607–10. [DOI] [PubMed] [Google Scholar]
- 193. Xu J, Xu C, Chen X, Cai X, Yang S, Sheng Y, Wang T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;30(5):584–9. [DOI] [PubMed] [Google Scholar]
- 194. Rusu IG, Suharoschi R, Vodnar DC, Pop CR, Socaci SA, Vulturar R, Istrati M, Moroșan I, Fărcaș AC, Kerezsi ADet al. Iron supplementation influence on the gut microbiota and probiotic intake effect in iron deficiency—a literature-based review. Nutrients. 2020;12(7):1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Skrypnik K, Suliburska J. Association between the gut microbiota and mineral metabolism. J Sci Food Agric. 2018;98(7):2449–60. [DOI] [PubMed] [Google Scholar]
- 196. Agostini D, Donati Zeppa S, Lucertini F, Annibalini G, Gervasi M, Ferri Marini C, Piccoli G, Stocchi V, Barbieri E, Sestili P. Muscle and bone health in postmenopausal women: role of protein and vitamin D supplementation combined with exercise training. Nutrients. 2018;10(8):1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Rizzoli R, Stevenson JC, Bauer JM, van Loon LJC, Walrand S, Kanis JA, Cooper C, Brandi M-L, Diez-Perez A, Reginster J-Y. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas. 2014;79(1):122–32. [DOI] [PubMed] [Google Scholar]
- 198. Wallace TC, Marzorati M, Spence L, Weaver CM, Williamson PS. New frontiers in fibers: innovative and emerging research on the gut microbiome and bone health. J Am Coll Nutr. 2017;36(3):218–22. [DOI] [PubMed] [Google Scholar]
- 199. Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13(2):125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Waterhouse M, Hope B, Krause L, Morrison M, Protani MM, Zakrzewski M, Neale RE. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur J Nutr. 2019;58(7):2895–910. [DOI] [PubMed] [Google Scholar]
- 201. Rizzoli R. Nutritional influence on bone: role of gut microbiota. Aging Clin Exp Res. 2019;31(6):743–51. [DOI] [PubMed] [Google Scholar]
- 202. Lis DM, Kings D, Larson-Meyer DE. Dietary practices adopted by track-and-field athletes: gluten-free, low FODMAP, vegetarian, and fasting. Int J Sport Nutr Exerc Metab. 2019;29(2):236–45. [DOI] [PubMed] [Google Scholar]
- 203. Lis DM, Stellingwerff T, Kitic CM, Fell JW, Ahuja KD. Low FODMAP: a preliminary strategy to reduce gastrointestinal distress in athletes. Med Sci Sports Exerc. 2018;50(1):116–23. [DOI] [PubMed] [Google Scholar]
- 204. Killian LA, Muir JG, Barrett JS, Burd NA, Lee S-Y. High fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) consumption among endurance athletes and relationship to gastrointestinal symptoms. Front Nutr. 2021;8:637160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lis D, Ahuja KDK, Stellingwerff T, Kitic CM, Fell J. Case study: utilizing a low FODMAP diet to combat exercise-induced gastrointestinal symptoms. Int J Sport Nutr Exerc Metab. 2016;26(5):481. [DOI] [PubMed] [Google Scholar]
- 206. Gaskell SK, Costa RJS. Applying a low-FODMAP dietary intervention to a female ultraendurance runner with irritable bowel syndrome during a multistage ultramarathon. Int J Sport Nutr Exerc Metab. 2019;29(1):61. [DOI] [PubMed] [Google Scholar]
- 207. Gibson PR, Halmos EP, Muir JG. Review article: FODMAPS, prebiotics and gut health—the FODMAP hypothesis revisited. Aliment Pharmacol Ther. 2020;52(2):233–46. [DOI] [PubMed] [Google Scholar]
- 208. Lis DM. Exit gluten-free and enter low FODMAPs: a novel dietary strategy to reduce gastrointestinal symptoms in athletes. Sports Med. 2019;49(S1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Skodje GI, Sarna VK, Minelle IH, Rolfsen KL, Muir JG, Gibson PR, Veierød MB, Henriksen C, Lundin KEA. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018;154(3):529–539.e2. [DOI] [PubMed] [Google Scholar]
- 210. Lis DM, Fell JW, Ahuja KDK, Kitic CM, Stellingwerff T. Commercial hype versus reality: our current scientific understanding of gluten and athletic performance. Curr Sports Med Rep. 2016;15(4):262–8. [DOI] [PubMed] [Google Scholar]
- 211. Lis DM, Stellingwerff T, Kitic CM, Ahuja KDK, Fell J. No effects of a short-term gluten-free diet on performance in nonceliac athletes. Med Sci Sports Exerc. 2015;47(12):2563–70. [DOI] [PubMed] [Google Scholar]
- 212. American College of Sports Medicine . The female athlete triad. Med Sci Sports Exerc. 2007;39(10):1867–82. [DOI] [PubMed] [Google Scholar]
- 213. Rankin A, O'Donovan C, Madigan SM, O'Sullivan O, Cotter PD. “Microbes in sport”—the potential role of the gut microbiota in athlete health and performance. Br J Sports Med. 2017;51(9):698–9. [DOI] [PubMed] [Google Scholar]
- 214. Aurigemma NC, Koltun KJ, VanEvery H, Rogers CJ, De Souza MJ. Linking the gut microbiota to bone health in anorexia nervosa. Curr Osteoporos Rep. 2018;16(1):65–75. [DOI] [PubMed] [Google Scholar]
- 215. Monteleone AM, Troisi J, Fasano A, Dalle Grave R, Marciello F, Serena G, Calugi S, Scala G, Corrivetti G, Cascino Get al. Multi-omics data integration in anorexia nervosa patients before and after weight regain: a microbiome-metabolomics investigation. Clin Nutr. 2021;40(3):1137–46. [DOI] [PubMed] [Google Scholar]
- 216. Tatsuya I, Eri T, Suguru T, Motoko T. Prebiotic food intake may improve bone resorption in Japanese female athletes. J Int Soc Sports Nutr. 2021;9(6):82. doi: 10.21203/rs.3.rs-115305/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res. 2013;69(1):52–60. [DOI] [PubMed] [Google Scholar]
- 218. Brouns F, Beckers E. Is the gut an athletic organ?. Sports Med. 1993;15(4):242–57. [DOI] [PubMed] [Google Scholar]
- 219. Frame LA, Costa E, Jackson SA. Current explorations of nutrition and the gut microbiome: a comprehensive evaluation of the review literature. Nutr Rev. 2020;78(10):798–812. [DOI] [PubMed] [Google Scholar]
- 220. Mörkl S, Lackner S, Meinitzer A, Mangge H, Lehofer M, Halwachs B, Gorkiewicz G, Kashofer K, Painold A, Holl AKet al. Gut microbiota, dietary intakes and intestinal permeability reflected by serum zonulin in women. Eur J Nutr. 2018;57(8):2985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Colgan SP. Swimming through the gut: implications of fluid transport on the microbiome. Dig Dis Sci. 2013;58(3):602–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Musch MW, Wang Y, Claud EC, Chang EB. Lubiprostone decreases mouse colonic inner mucus layer thickness and alters intestinal microbiota. Digestive diseases and sciences. 2013;58(3):668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Arnaud MJ. Mild dehydration: a risk factor of constipation?. Eur J Clin Nutr. 2003;57(S2):S88–95. [DOI] [PubMed] [Google Scholar]
- 224. Mancabelli L, Milani C, Lugli GA, Turroni F, Mangifesta M, Viappiani A, Ticinesi A, Nouvenne A, Meschi T, van Sinderen Det al. Unveiling the gut microbiota composition and functionality associated with constipation through metagenomic analyses. Sci Rep. 2017;7(1):9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016;65(1):57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Chang DC, Basolo A, Piaggi P, Votruba SB, Krakoff J. Hydration biomarkers and copeptin: relationship with ad libitum energy intake, energy expenditure, and metabolic fuel selection. Eur J Clin Nutr. 2020;74(1):158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Perrier ET. Shifting focus: from hydration for performance to hydration for health. Ann Nutr Metab. 2017;70(Suppl 1):4–12. [DOI] [PubMed] [Google Scholar]
- 228. Jeukendrup AE. Nutrition for endurance sports: marathon, triathlon, and road cycling. J Sports Sci. 2011;29(Suppl 1):S91–9. [DOI] [PubMed] [Google Scholar]
- 229. Crowson MM, McClave SA. Does the intestinal microbiome impact athletic performance?. Curr Gastroenterol Rep. 2020;22(11):53. [DOI] [PubMed] [Google Scholar]
- 230. Lobach AR, Roberts A, Rowland IR. Assessing the in vivo data on low/no-calorie sweeteners and the gut microbiota. Food Chem Toxicol. 2019;124:385–99. [DOI] [PubMed] [Google Scholar]
- 231. Ruiz-Ojeda FJ, Plaza-Díaz J, Sáez-Lara MJ, Gil A. Effects of sweeteners on the gut microbiota: a review of experimental studies and clinical trials. Adv Nutr. 2019;10(Suppl 1):S31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Turner A, Veysey M, Keely S, Scarlett CJ, Lucock M, Beckett EL. Intense sweeteners, taste receptors and the gut microbiome: a metabolic health perspective. Int J Environ Res Public Health. 2020;17(11):4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Khan TA, Ayoub-Charette S, Sievenpiper JL, Comelli EM. Non-nutritive sweeteners and their effects on human health and the gut microbiome. Encyclopedia of Gastroenterology (Second Edition). 2020:676–84.. doi: 10.1016/B978-0-12-801238-3.62162-1. [Google Scholar]
- 234. Close GL, Hamilton DL, Philp A, Burke LM, Morton JP. New strategies in sport nutrition to increase exercise performance. Free Radic Biol Med. 2016;98:144–58. [DOI] [PubMed] [Google Scholar]
- 235. D'Angelo S. Polyphenols: potential beneficial effects of these phytochemicals in athletes. Curr Sports Med Rep. 2020;19(7):260–5. [DOI] [PubMed] [Google Scholar]
- 236. Laparra JM, Sanz Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol Res. 2010;61(3):219–25. [DOI] [PubMed] [Google Scholar]
- 237. Espín JC, González-Sarrías A, Tomás-Barberán FA. The gut microbiota: a key factor in the therapeutic effects of (poly)phenols. Biochem Pharmacol. 2017;139:82–93. [DOI] [PubMed] [Google Scholar]
- 238. González-Soltero R, Bailén M, de Lucas B, Ramírez-Goercke MI, Pareja-Galeano H, Larrosa M. Role of oral and gut microbiota in dietary nitrate metabolism and its impact on sports performance. Nutrients. 2020;12(12):3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Burke LM. Practical considerations for bicarbonate loading and sports performance. Nutritional Coaching Strategy to Modulate Training Efficiency. 75:2013;15–26. Switzerland: Nestec Ltd & S. Karger AG; [DOI] [PubMed] [Google Scholar]
- 240. Murakami S, Goto Y, Ito K, Hayasaka S, Kurihara S, Soga T, Tomita M, Fukuda S. The consumption of bicarbonate-rich mineral water improves glycemic control. Evidence-Based Complement Altern Med. 2015;2015:824395. doi: 10.1155/2015/824395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, Beaumont M, Van Treuren W, Knight R, Bell JTet al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Ostojic SM, Ahmetovic Z. Gastrointestinal distress after creatine supplementation in athletes: are side effects dose dependent?. Res Sports Med. 2008;16(1):15–22. [DOI] [PubMed] [Google Scholar]
- 243. Turer E, McAlpine W, Wang K-w, Lu T, Li X, Tang M, Zhan X, Wang T, Zhan X, Bu C-Het al. Creatine maintains intestinal homeostasis and protects against colitis. Proc Natl Acad Sci. 2017;114(7):E1273–E1281.. doi: 10.1073/pnas.1621400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Luo G, Li B, Yang C, Wang Y, Bian X, Li W, Liu F, Huo G. Major traditional probiotics: comparative genomic analyses and roles in gut microbiome of eight cohorts. Front Microbiol. 2019;10(712):e00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Sun L, Zhang X, Zhang Y, Zheng K, Xiang Q, Chen N, Chen Z, Zhang N, Zhu J, He Q. Antibiotic-induced disruption of gut microbiota alters local metabolomes and immune responses. Front Cell Infect Microbiol. 2019;9(99):e00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Li Y, Guo Y, Wen Z, Jiang X, Ma X, Han X. Weaning stress perturbs gut microbiome and its metabolic profile in piglets. Sci Rep. 2018;8(1):18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. González S, Salazar N, Ruiz-Saavedra S, Gómez-Martín M, de Los Reyes-Gavilán CG, Gueimonde M. Long-term coffee consumption is associated with fecal microbial composition in humans. Nutrients. 2020;12(5):1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Jaquet M, Rochat I, Moulin J, Cavin C, BibiloniR. Impact of coffee consumption on the gut microbiota: a human volunteer study. Int J Food Microbiol. 2009;130(2):117–21. [DOI] [PubMed] [Google Scholar]
- 249. Nishitsuji K, Watanabe S, Xiao J, Nagatomo R, Ogawa H, Tsunematsu T, Umemoto H, Morimoto Y, Akatsu H, Inoue Ket al. Effect of coffee or coffee components on gut microbiome and short-chain fatty acids in a mouse model of metabolic syndrome. Sci Rep. 2018;8(1):16173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Cowan TE, Palmnäs MSA, Yang J, Bomhof MR, Ardell KL, Reimer RA, Vogel HJ, Shearer J. Chronic coffee consumption in the diet-induced obese rat: impact on gut microbiota and serum metabolomics. J Nutr Biochem. 2014;25(4):489–95. [DOI] [PubMed] [Google Scholar]
- 251. Farina EK, Thompson LA, Knapik JJ, Pasiakos SM, Lieberman HR, Mcclung JP. Diet quality is associated with physical performance and Special Forces selection. Med Sci Sports Exerc. 2020;52(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Capling L, Gifford JA, Beck KL, Flood VM, Slater GJ, Denyer GS, O'Connor HT. Development of an athlete diet index for rapid dietary assessment of athletes. Int J Sport Nutr Exerc Metab. 2019;29(6):643. [DOI] [PubMed] [Google Scholar]
- 253. Burke LM, Slater G, Broad EM, Haukka J, Modulon S, Hopkins WG. Eating patterns and meal frequency of elite Australian athletes. Int J Sport Nutr Exerc Metab. 2003;13(4):521. [DOI] [PubMed] [Google Scholar]
- 254. Burke LM, Cox GR, Cummings NK, Desbrow B. Guidelines for daily carbohydrate intake. Sports Med. 2001;31(4):267–99. [DOI] [PubMed] [Google Scholar]
- 255. Erdman KA, Tunnicliffe J, Lun VM, Reimer RA. Eating patterns and composition of meals and snacks in elite Canadian athletes. Int J Sport Nutr Exerc Metab. 2013;23(3):210. [DOI] [PubMed] [Google Scholar]
- 256. Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA, Holscher HD, Woods JA. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc. 2018;50(4):747–57. [DOI] [PubMed] [Google Scholar]
- 257. Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. 2019;11(12):2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Nguyen TL, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research?. Dis Models Mechanisms. 2015;8(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Walter J, Armet AM, Finlay BB, Shanahan F. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell. 2020;180(2):221–32. [DOI] [PubMed] [Google Scholar]
- 260. Purdom T, Kravitz L, Dokladny K, Mermier C. Understanding the factors that effect maximal fat oxidation. J Int Soc Sports Nutr. 2018;15(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Smarkusz-Zarzecka J, Ostrowska L, Leszczyńska J, Orywal K, Cwalina U, Pogodziński D. Analysis of the impact of a multi-strain probiotic on body composition and cardiorespiratory fitness in long-distance runners. Nutrients. 2020;12(12):3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137(3):830S–7S. [DOI] [PubMed] [Google Scholar]
- 263. Ze X, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6(8):1535–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Bolca S, Van de Wiele T, Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol. 2013;24(2):220–5. [DOI] [PubMed] [Google Scholar]
- 265. Antoni C, Noemí B, Núria C, Pol H, Jordi M-P, Lluís A, Puiggròs F. Chapter Nineteen—Metabolomics and proteomics as tools to advance the understanding of exercise responses: the emerging role of gut microbiota in athlete health and performance. In: Barh D, Ahmetov II, editors. Sports, exercise, and nutritional genomics. London: Academic Press; 2019. p. 433–59. [Google Scholar]
- 266. Sorrenti V, Fortinguerra S, Caudullo G, Buriani A. Deciphering the role of polyphenols in sports performance: from nutritional genomics to the gut microbiota toward phytonutritional epigenomics. Nutrients. 2020;12(5):1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Guest NS, Horne J, Vanderhout SM, El-Sohemy A. Sport nutrigenomics: personalized nutrition for athletic performance. Front Nutr. 2019;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]