ABSTRACT
Diet quality indicators (DQIns) are tools that aim to assess an individual's overall diet quality. Previous reviews focused mainly on health-related outcomes but did not provide detailed information about components, assessment variables, or important methodological issues for the development and application of DQIns in the pediatric age. The current mapping review aims to provide comprehensive guidance regarding DQIns developed through a priori methodology in children aged ≤14 y that have been applied worldwide. A mapping review was conducted, whereby 1665 original articles describing the development, modifications, and updates of DQIns, published up to June 26, 2020, in English and Spanish, were retrieved. A total of 139 articles were identified and classified into 13 subgroups. There were 10 overall DQIns: Healthy Eating Indexes (n = 25), Dietary Diversity Scores (n = 20), Diet Quality Indexes (n = 16), Food Variety Scores (n = 11), Healthy and Unhealthy Scores (n = 11), Feeding and Eating Indexes (n = 10), Diet Quality Scores (n = 5), Nutritional Adequacy and Micronutrients Indexes (n = 5), Dietary Guidelines Indexes (n = 5), and Other Healthy Diet Indexes (n = 13). Three additional subgroups of dietary and lifestyle indicators found were Mediterranean Diet Indexes (n = 10), Diet–Lifestyle Indexes (n = 5), and Breakfast Quality Indexes (n = 3). This compilation of DQIns will help researchers select the most appropriate tool for future epidemiological studies by considering a careful selection of information about the assessment components, scoring methods, and key methodological issues. The main limitations of this review are that, due to its nature, a risk-of-bias assessment was not performed and the article screening was completed in 2 databases (PubMed/MEDLINE and Scopus). More research is needed to identify health-related outcomes associated with DQIns in the pediatric population, using clearer and more standardized methodological criteria.
Keywords: dietary indicators, diet quality, diet index, dietary assessment, child, adolescent, pediatrics, methodological criteria, index specification, mapping review
Introduction
Nutrition is an essential factor for growth and development during childhood and adolescence (1). In the first years of life, infant feeding practices and dietary patterns transition from breast milk or formula to semisolid food intake and, later, to the adult diet (2). Feeding skills, dietary habits, food preferences, and nutritional knowledge are learned during pediatric age and carried through into adulthood (3).
Recent systematic reviews (SRs), which included dietary patterns, have examined the timing, types, and amounts of foods and beverages during infancy and toddlerhood in relation to growth and developmental outcomes into adulthood (4, 5), but only moderate or limited conclusions were drawn primarily from a lack of available evidence on diet quality (DQ) early in life. Dietary patterns represent a broader picture of food and nutrient consumption, and they may thus be more predictive of disease risk compared with individual foods or nutrients (6).
There can be concerning changes in the dietary pattern and lifestyle during childhood and adolescence, leading to overweight and obesity. Indeed, the risk factors that contribute to the gaining of body weight are the reduced consumption of fruits, vegetables, and legumes; the increased consumption of ultra-processed foods, unhealthy fats, added sugars, and refined carbohydrates; and sedentary habits (7). According to WHO (8), >340 million children and adolescents aged 5–19 y were overweight or obese in 2016, and 38 million children aged <5 y were overweight or obese in 2019. The prevention and treatment of obesity in the pediatric population can reduce long-term comorbidities in adulthood (e.g., arterial hypertension, dyslipidemia, diabetes, and cardiovascular diseases) (7).
An overall dietary assessment is necessary to identify consumption patterns in the pediatric population. In epidemiological research, diet assessment includes valid dietary methods such as diet history, FFQs, 24-h dietary recalls (24h-DRs), and food records. These methods are associated with a high respondent burden; they are time-consuming and need additional analysis (9). However, there are blunt instruments, a priori diet quality indicators (DQIns), that describe an overall dietary pattern based on current nutritional knowledge (10). These tools can represent the combined effects of all foods and evaluate dietary guidelines and recommendations (10).
DQIns use mathematical algorithms to quantify the degree of food and nutrient adequacy (11). DQIns are designed for a specific purpose and population, and they are used to evaluate the overall DQ and to categorize chronic disease risks according to eating behaviors (12). DQIns measure nutrient adequacy, food variety or food diversity, and the moderation of food consumption and nutrients (13). The advantages of DQIns include the possibility of extended use in epidemiological studies because of their rapid assessment and simple interpretation (14).
Although single-nutrient analyses are valuable to nutrition epidemiology, a more recent and informative approach is dietary pattern analysis, which addresses the combined effects that multiple nutrients may have when consumed as foods in the overall diet over time (15). Dietary patterns have also become a foundational core component of the Dietary Guidelines for Americans (DGAs) (16, 17).
Nutritional recommendations refer to the nutrient amount expressed as dietary reference intake—for instance, the RDA, Adequate Intake, or estimated mean requirement (18). Regarding food intake, national nutritional guidelines encourage healthy dietary patterns in the population (19). DQIns have been assessed using those approaches, initially at the population level (20). Later, DQIns were developed or adapted to evaluate consumption patterns in the pediatric population. Some of these instruments were short tools considered according to their reliability and validity for later use as dietary indexes (21).
Compared with the pediatric population, DQIn research is widely extended in the adult population (22). Hence, DQIns play an essential role in identifying modifiable dietary factors that can improve health and nutrition by preventing chronic diseases.
Several SRs on DQIns in the pediatric population have been published since various indexes have been developed. In 2011, an SR that compiled 40 DQIns by identifying a priori methods to assess dietary patterns in children aged 1–5 y and their association with sociodemographic characteristics in high-income countries was published (23). Another SR in low-income countries grouped 90 DQIns by their intended use, such as assessing dietary quality, dietary habits, and association with health-related outcomes (24). Moreover, an SR made an inventory of 119 DQIns and identified applications in low- and high-income countries (25). An update of this SR has been published describing 128 DQIns from 33 countries; the SR focused on the associations between DQIns and health-related outcomes (IQ, quality of life, blood pressure, body composition, and metabolic syndrome) and on the evaluation of validity and reliability of these tools (26).
Previous reviews of DQIns focused essentially on health-related outcomes. Despite their completeness, insufficient detail has been reported about the components, the scoring methods, and the main methodological issues that have been taken into consideration for the development and application of DQIns. Considering that the differences in these methodological features (types of components and scoring methods) are fundamental, it has been necessary to perform a review emphasizing these issues. The current mapping review approach provides the methodological criteria on which a priori DQIns have been developed in children. This compilation aims to be a comprehensive reference to future epidemiological studies conducted in children aged ≤14 y by facilitating the selection of indicators (previously developed, updated, or modified) that assess DQ.
Methods
We conducted a mapping review of DQIns including observational (mainly cross-sectional and cohort studies) and intervention studies (randomized controlled trials) in childhood. The protocol was registered on PROSPERO (International Prospective Register of Systematic Reviews) as CRD42020201571. The review processes were documented following a pre-established protocol for reviews about the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (except the assessment of the risk of bias) (27). It was decided not to assess the risk of bias of selected articles because of the methodological approach of the review that is describing the DQIns main features as instruments of the evaluation of the DQ. The steps are described in more detail later.
Literature search strategy
The research was performed on 2 electronic databases of MEDLINE (PubMed) and Scopus. The literature search included documents published up to June 26, 2020. The years covered by this review were not limited. The search was conducted in articles published in English and Spanish.
We selected keywords that are similar and related to DQIns. We included the following in the research statement: (diet OR dietary OR food OR meal) and (quality OR diversity OR variety) and (index OR score OR indices). Medical subject heading (MeSH) terms were also identified and included in the search query: (diet OR diet healthy OR diet survey OR nutrition surveys). The search was limited to human studies on infants, preschoolers, children, or adolescents. Excluded health conditions were food hypersensitivity, celiac disease, cognitive dysfunction, diabetes mellitus type 1, depression, severity of illness, irritable bowel, mental disorder, neoplasm, and pregnancy. These were excluded using the appropriate MeSH terms. The search strategy utilized (a combination of MeSH terms, keywords, and Boolean operators) is shown in Supplemental Table 1. In addition to the literature search strategy, previous systematic and narrative reviews about DQIns were collected, and their references were checked in order to include them in our mapping review if they did not appear in our original search.
Study eligibility criteria and data synthesis
The inclusion and exclusion criteria were established by the PICOS criteria (population/patient, intervention exposure, comparison, outcome, and setting) (28) and are summarized in Supplemental Appendix 1. Inclusion criteria for participants were children and adolescents aged ≤14 y from throughout the world. Publications that evaluated the population level were also included. The outcome of interest included articles that described the development, update, or modification of a DQIn. The selected articles described the scoring system according to the food groups or foods, nutrients, or lifestyle-related factors that were part of the tools. The DQIns had to evaluate ≥1 d of food consumption.
Exclusion criteria were studies that included a health condition, such as a primary disability, or behavioral or learning difficulties. Studies using an existing DQIn without any modification, articles that analyzed mealtimes (except breakfast), or studies that scored for single nutrients such as protein quality index or glycemic index were excluded. Articles with no full text available and no response after contacting the authors were also excluded.
Data collection
The screening process included the review of titles and abstracts first to identify eligible articles. Duplicates were removed. Then, full-text articles were screened to confirm eligibility. Studies were first screened by title and abstract by a first reviewer (LAD-J); the screening was supervised by a second reviewer (ÁH-R). A reverse search was performed by manually checking the references of previously published reviews (ÁH-R). The final study selection was performed based on a full-text review. Any discrepancies were resolved by consulting a third reviewer (MJS-M).
For data extraction, if several tools were developed in the same article, only the most complete DQIn was selected. If 2 developed DQIns were similarly complex (components and scoring systems), data from both were extracted. If several indicators were applied in a publication, the novel DQIn developed in the manuscript or the indicator with greater modifications was selected. Hence, articles were classified only in a specific subgroup. The initial references of the rest of the instruments used in those articles were retrieved and used in their original version.
Data extraction and management
Data extraction was conducted by 2 reviewers (LAD-J and ÁH-R) through a predefined standardized form to collect the information required: 1) author, publication year (reference), indicator (abbreviation), indicator subgroup, country, population, year of recruitment, age, sex, study name, study design, main objective, and results; 2)food and food group, nutrient, and lifestyle-related factor components included in each DQIn; 3) component information (n, and type of component), and global scoring method; 4) cutoff values, overall score, and methodological issues. If the publication was a population-based study, only the results for children and adolescents were extracted. At this stage, articles that failed to provide all the necessary information to make them applicable were excluded. The references were managed by Mendeley software (29) and checked by data extraction to avoid citation mistakes.
Results
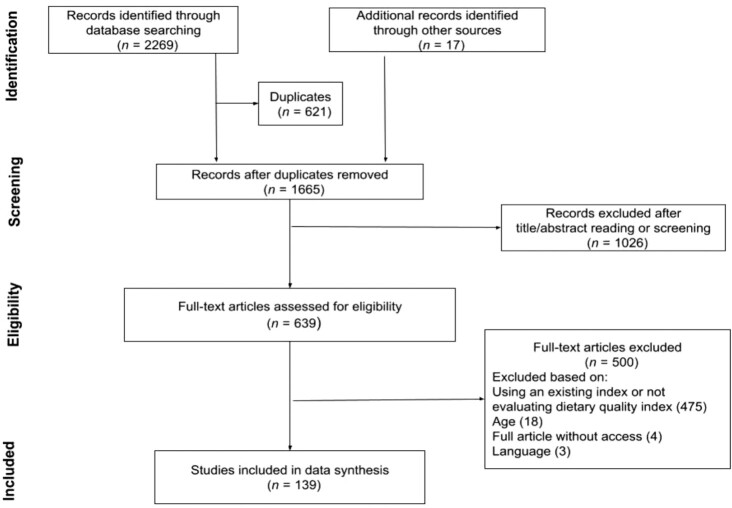
The research strategy resulted in 2269 articles. An additional 17 articles were included by screening the references of previous reviews and eligible articles. After the removal of 621 duplicates, 1665 references were left. The initial title–abstract screening resulted in 639 eligible articles. After reading the methodology, we proceeded to verify whether they were the identical versions of the original articles, modified versions excluding any of the original components, or modified versions adding new information. Five hundred additional articles were excluded, resulting in 139 articles. Figure 1 shows the PRISMA flow diagram for the selection of studies.
FIGURE 1.
PRISMA flow diagram for the selection of studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Presentation of the main results
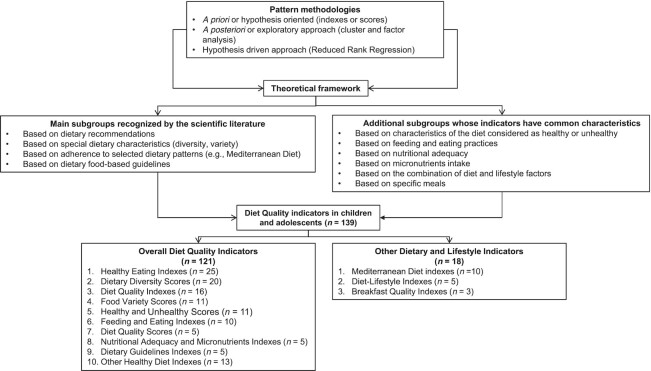
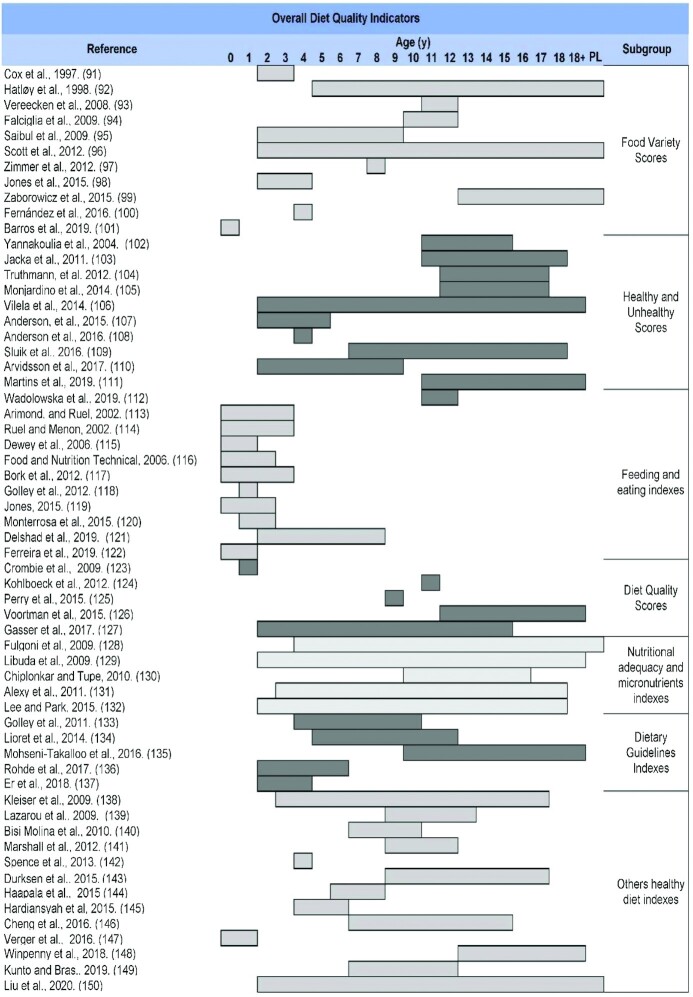
Because all indicators assess the DQ, but from different approaches, a classification of these tools was proposed to improve the understanding of these diverse perspectives. This classification is based on the main subgroups recognized by the scientific literature and additional subgroups whose indicators have common characteristics. In addition, several subgroups of Other Dietary and Lifestyle Indicators have been included that, although not assessing overall DQ in the strict sense, evaluate dietary characteristics associated with a better dietary profile and improved health. Figure 2 shows this proposed classification of the DQIns or their modifications that were retrieved in this mapping review. The results of all studies included in this mapping review are presented by types of components, scoring methods, and other relevant methodological issues applied to the DQIns. Subsequently, a brief description is given in narrative and tabular forms by the subgroup of key indicators of the most noteworthy aspects.
FIGURE 2.
Proposed classification of the indicator approaches for assessing diet quality indicators in children and adolescents.
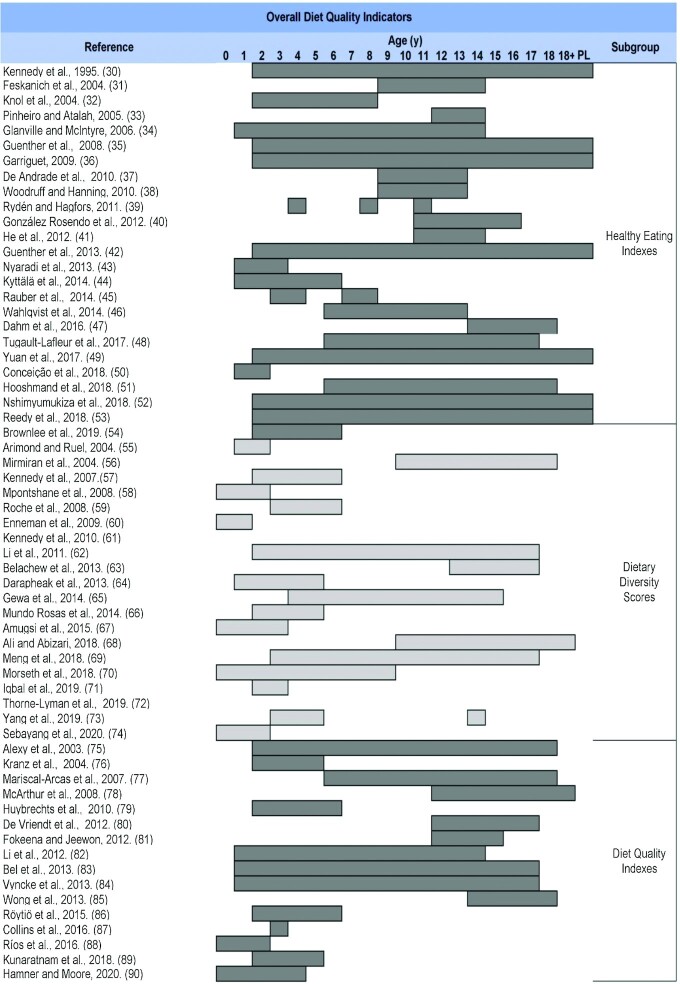
Supplemental Table 2 shows an overview of all relevant DQIns, including the main indicators’ subgroups, ordered according to the number of articles found in each category. The original version and the first modified version were included and placed in chronological order in each DQIns subgroup. A total of 139 original DQIns or their modifications were retrieved; they are mainly classified in the following 10 subgroups: Healthy Eating Indexes (n = 25) (30–54); Dietary Diversity Scores (n = 20) (55–74); Diet Quality Indexes (n = 16) (75–90); Food Variety Scores (n = 11) (91–101); Healthy and Unhealthy Scores (n = 11) (102–112); Feeding and Eating Indexes (n = 10) (113–122); Diet Quality Scores (n = 5) (123–127); Nutritional Adequacy and Micronutrients Indexes (n = 5) (128–132); Dietary Guidelines Indexes (n = 5) (133–137); and Other Healthy Diet Indexes (n = 13) (138–150). Indicators classified as Other Dietary and Lifestyle Indicators were Mediterranean Diet Indexes (n = 10) (151–160), Diet–Lifestyle Indexes (n = 5) (161–165), and Breakfast Quality Indexes (n = 3) (166–168).
Table 1 shows a summary of the main features of the studies retrieved in this mapping review, in which a DQIn was developed or updated through modifications (to components or to scoring methods), chronologically organized according to previously defined subgroups.
TABLE 1.
Main characteristics of the first study in children involving each diet quality indicator (n = 139)1
| Authors, publication year (Ref) | Indicator | Country, population, year of recruitment, age, sex | Study name, study design | Main objective and results |
|---|---|---|---|---|
| Overall diet quality indicators | ||||
| Healthy Eating Indexes | ||||
| Kennedy et al., 1995 (30) | Healthy Eating Index (HEI) | United Statesn = 7463 1989–1990 ≥2 y Female percentage not found | Cross-sectional study | Nutrient adequacyCorrelation coefficient of the HEI and the intake of nutrients 0.06—0.42 |
| Feskanich et al., 2004 (31) | Youth Healthy Eating Index (YHEI) | United States n = 16,540 1996 9–14 y Female (54%) | Growing Up Today StudyCohort study | Assessed healthful and unhealthful foods and eating behaviorsThere was no strong correlation with total energy intake (r = 0.12) and an inverse association with time spent in inactive pursuits (r = –0.27) |
| Knol et al., 2004 (32) | Healthy Eating Index Variety Score (HEI-variety) | United Statesn = 27481994–1996, 19982–8 yFemale, percentage not found | Cross-sectional study | Relation between food sufficiency status and dietary varietyVariety scores were low for all children but did not differ by food sufficiency status |
| Pinheiro and Atalah, 2005 (33) | Healthy Eating Index Chile (HEI-CHL) | Chilen = 536Date not found13.4 yFemale, percentage not found | Exposure to environmental arsenic in children from ChileCross-sectional study | Characteristics of the nutrition intakeThe scores revealed that most of the study population needed changes in their diet |
| Glanville and McIntyre, 2006 (34) | Healthy Eating Index Canada 2006 (HEI-C06) | Canadan = 3031999–20001–14 yFemale (50%) | Cross-sectional study | Nutritional characteristics of food-insecure householdsYounger children scored ∼22% higher than mothers and 12% higher than their older siblings (P < 0.05) |
| Guenther et al., 2008 (35) | Healthy Eating Index 2005 (HEI-2005) | United Statesn = 86502001–2002≥2 yFemale, percentage not found | Cross-sectional study | Measure of nutrient densityNutrient density was calculated for 1000–3200 kcal |
| Garriguet, 2009 (36) | Healthy Eating Index Canada modified (HEI-C-mod) | Canadan = 33,6642004≥2 yFemale, percentage not found | Cross-sectional study | Characteristics of the nutrition intake and dietary, socioeconomic, and lifestyle factorsChildren had a mean score of 65 and adolescents 55 |
| De Andrade et al., 2010 (37) | Healthy Eating Index Brazil (HEI-BRA14) | Braziln = 15842001–200212–20 yFemale (51%) | ISA-SPCross-sectional study | Characteristics of the nutrition intake according to age, sex, and lifestyle factorsThere was a significant difference in the mean scores by sex, age, physical activity, and type of housing (r2 = 0.027, P < 0.001) |
| Woodruff and Hanning, 2010 (38) | Healthy Eating Index Canada (HEIC-2009) | Canadan = 40520019–13 yFemale (52%) | Cross-sectional study | Characteristics of the nutrition intake and comparison with the Healthy Eating Index Canada tool75% of the participants were classified as needing improvements in their diet compared with 71% of the HEI-C |
| Rydén and Hagfors, 2011 (39) | Healthy Eating Index 2005 (HEI-2005) | Swedenn = 216020034–11 yFemale (49%) | Cross-sectional study | Assessment of dietary intake by food group, BMI, and sociodemographic factorsChildren with more educated parents had higher scores. The highest cost quintile consumed a better diet |
| González Rosendo et al., 2012 (40) | Healthy Eating Index Mexico (HEI-MEX) | Mexicon = 5140Date not found11–16 yFemale | Cross-sectional study | Characteristics of the nutrition intakeOnly 1% had a healthy nutrient intake, 75% needed modifications in their diet, and 24% had an unhealthy diet |
| He et al., 2012 (41) | Healthy Eating Index 2005 Canadian Modification (HEIC-2005) | Canadan = 8102006–200711–14 yFemale (52%) | Cross-sectional study | Relation between the neighborhood food environment and dietary intakeClose proximity to convenience stores was associated with low scores (P = 0.03) |
| Guenther et al., 2013 (42) | Healthy Eating Index 2010 (HEI-2010) | United States2001–2002≥2yFemale, percentage not found | Update of the HEI | Capture the recommendations of the 2010 Dietary Guidelines for American populationNew components were added |
| Nyaradi et al., 2013 (43) | Youth Healthy Eating Index Australia (YHEI-AUS) | Australian = 28681991–19951–3 yFemale (49%) | RaineCohort study | Association between diet during the first 3 y of life and cognitive outcomes at 10 yA better DQ during the early years may have a positive effect on cognitive ability later in life (ß: 0.12; 95% CI: 0.05, 0.19) |
| Kyttälä et al., 2014 (44) | Finnish Children Healthy Eating Index (FCHEI) | Finlandn = 16392003–20051–6 yFemale, percentage not found | The type 1 diabetes prediction and prevention nutrition studyCross-sectional study | Nutritional recommendations and relation with family characteristicsLowest scores were associated with semi-urban areas of residence and low maternal education and smoking |
| Rauber et al., 2014 (45) | Healthy Eating Index Brazil (HEI-BRA14) | Braziln = 6523–4 y, 7–8 y2001, 2002Female (57%) | Healthy feeding infants: São LeopoldoCohort study | Changes in children's dietary patternScores changed from 65.7 ± 11.2 at 3–4 y to 65.0 ± 8.8 at 7–8 years. The score correlates positively with the dietary variety |
| Wahlqvist et al., 2014 (46) | Youth Healthy Eating Index Taiwan (YHEI-TW) | Taiwann = 23892001–20026–13 yFemale (46.3%) | Cross-sectional study | Intergenerational association of DQ |
| Dahm et al., 2016 (47) | High School Alternative Healthy Eating Index (HS-AHEI) | United Statesn = 42,112199814–18 yFemale | Nurses’ Health StudyCohort study | Association of diet and risk of developing cardiovascular disease risk factorsHigher scores were associated with lower risk of developing ≥1 risk factor. HR comparing the highest with the lowest quintile: 0.82 (0.77—0.87) |
| Tugault-Lafleur et al., 2017 (48) | School Healthy Eating Index (School-HEI) | Canadan = 35,10720046–17 yFemale (49%) | Cross-sectional study | Differences between school-hours and non-school-hours dietary intakes and demographic and socioeconomic characteristicsThe mean score was 53.4. There was a difference between school-hours and non-school-hours dietary intakes. Demographic and socioeconomic characteristics show a weak correlation with school-hours DQ |
| Yuan et al., 2017 (49) | Healthy Eating Index China (HEI-CHN) | Chinan = 14,5842011≥2 yFemale (52%) | Cross-sectional study | Assess DQ and its association with sociodemographic factorsThere is an association between education (P < 0.001) and urbanization levels (P < 0.001) |
| Conceição et al., 2018 (50) | Healthy Eating Index Brazil Infants (HEI-BRA-I) | Braziln = 11851–2 y2001, 2002Female (49%) | BRISACohort study | Food intake dimension, energy intake, and componentsThe mean score was 74.8 ± 13. Components presented low correlations with total energy intake (r = 0.29), and the correlation of individual food types was moderate (r = 0.53) |
| Hooshmand et al., 2018 (51) | Healthy Eating Index Iran (HEI-IRN) | Irann = 4246–18 y2006–2011Female (57%) | Tehran Lipid and Glucose StudyCohort study | Assess the relation between the tool and the development of metabolic syndromeMetabolic syndrome decreased in the highest score quartile compared with the lowest quartile (OR: 0.35; 95% CI: 0.13, 0.98; P for trend = 0.025) |
| Nshimyumukiza et al., 2018 (52) | Healthy Eating Index Canada (HEI-C-2010) | Canadan = 18,4092004,2015≥2 yFemale, percentage not found | Cross-sectional study | Assess the changes in DQ and the associated economic burdenOn average, there were temporal improvements on DQ |
| Reedy et al., 2018 (53) | Healthy Eating Index 2015 (HEI-2015) | United Statesn = 7935; n = 422,9281994–2012≥2 yFemale, percentage not found | Update of the HEI | Capture the recommendations of the 2015–2020 Dietary Guidelines for American population. Components showed construct validity, reliability, and criterion validity |
| Brownlee et al., 2019 (54) | Healthy Eating Index Singapur (HEI-SGP) | Singaporen = 5612014–20152–6 yFemale (53%) | Cross-sectional study | Overall DQ and association with demographic factors and BMIThe median score was 65.4. There is a statistical difference in the DQ and demographic factors/BMI category (P > 0.05) |
| Dietary Diversity Scores | ||||
| Arimond and Ruel, 2004 (55) | Dietary Diversity Score (DDS-04) | 11 low- and middle-income countriesn = 958; n = 36621999–20016–23 moFemale, percentage not found | Cross-sectional study | Assessed DD and anthropometric parametersAssociation between DD and height-for-age z score was significant as a main effect in 7 countries |
| Mirmiran et al., 2004 (56) | Dietary Diversity Score Iran (DDS-IRN) | Irann = 3041999–200110–18 yFemale (53%) | Cross-sectional study | Nutrient adequacyMean of DDS 6.25 ± 1.08. Pearson correlation and MAR was 0.42 |
| Kennedy et al., 2007 (57) | Dietary Diversity Score Filipinas (DDS-PHI) | Philippinesn = 3164199324–71 moFemale (49%) | Cross-sectional study | Nutrient adequacyThe Pearson's correlation coefficient between DDS and MAR was 0.36 (P < 0.001) and between DDS 10 g and MAR was 0.44 (P < 0.001) |
| Mpontshane et al., 2008 (58) | Dietary Diversity Score South Africa (DDS-ZAF) | South African = 381Date not found6–28 moFemale, percentage not found | Randomized controlled trial | Assessed dietary intake in an area with a high prevalence of HIVHIV-infected children's diet was significantly less diverse than the diet of uninfected children |
| Roche et al., 2008 (59) | Traditional Food Diversity Score (TFDS) | Perun = 3620043–6 yFemale, percentage not found | Cross-sectional study | Assessed the strengths of the traditional food systemThe TFDS ranged from 2 to 17, with a median of 8. Higher scores were correlated with higher nutrient intakes |
| Enneman et al., 2009 (60) | Dietary Diversity Score Guatemala–USAID (DDS-GT-US-CP-INCAP) | Guatemalan = 1282007, 20086–12 moFemale (39%) | Cross-sectional study | Difference in the distributions according to food characteristics and between 1-d and 3-d intakeMedian score was 18 for the 3-d DR |
| Kennedy et al., 2010 (61) | Individual Dietary Diversity Score (IDDS) | Guideline to measure DDS in developing countries | Cross-sectional study | Nutrient adequacy |
| Li et al., 2011 (62) | Dietary Diversity Score China (DDS-CHN10) | Chinan = 13,77020022–17 yFemale (46%) | Cross-sectional study | Associations between dietary food/nutrient intake and plasma lipid profiles related to stunting and overweight statusThe ORs for prevalent dyslipidemia were as follows: OR: 1.32; 95% CI: 1.13, 1.53 (stunted); OR: 1.76; 95% CI: 1.48, 2.09 (overweight); and OR: 2.59; 95% CI: 1.65, 4.07 (stunted–overweight) |
| Belachew et al., 2013 (63) | Dietary Diversity Score Ethiopia (DDS-ETH) | Ethiopian =20842005–200613–17 yFemale (49%) | Adolescents in Jimma ZoneCohort study | Association between food insecurity and dietary practicesFood insecurity was negatively associated with the likelihood of having a diversified diet (P < 0.001) |
| Darapheak et al., 2013 (64) | Dietary Diversity Score WHO modification (DDS-WHOm) | Cambodian = 620912–59 mo2005Female, percentage not found | Cross-sectional study | Association between DD and stunting, underweight, wasting, and diarrheaStunting was negatively associated with DD (OR: 0.95; 95% CI: 0.91, 0.99). The consumption of animal source foods was associated with reduced risk of stunting (OR: 0.69; 95% CI: 0.54, 0.89) and underweight (OR: 0.74; 95% CI: 0.57, 0.96; P = 0.03) |
| Gewa et al., 2014 (65) | Dietary Diversity Score Kenya (DDS-KEN) | Kenyan = 5291998–20004–15 yFemale, percentage not found | Cross-sectional study | Nutrient adequacyA 1-unit increase in the DDS was associated with a significant increase in the mean probability of adequacy |
| Mundo-Rosas et al., 2014 (66) | Dietary Diversity Score Mexico (DDS-MX) | Mexicon = 955201224–59 moFemale (49%) | Cross-sectional study | Nutrient adequacy and food insecurity levelThe DDS is associated with food insecurity |
| Amugsi et al., 2015 (67) | Dietary Diversity Score Ghana 2015 (DDS-GHA15) | Ghanan = 118720086–36 moFemale (49%) | Cross-sectional study | Association between maternal and child DDA difference of 1 food group in mother's consumption was associated with a difference of 0.72 food groups in the child's food consumption (95% CI: 0.63, 0.82) |
| Ali and Abizari, 2018 (68) | Dietary Diversity Score Ghana 2018 (DDS-GHA18) | Ghanan = 366201710–19 yFemale (50%) | Cohort study | Characterization of dietary patterns and body weight changes during RamadanThe mean DD was statistically significant across the assessment stages (F = 7.152, P < 0.001) |
| Meng et al., 2018 (69) | Dietary Diversity Score China 2018 (DDS-CHN18) | Chinan = 201220113–17 yFemale (47.8%) | Cross-sectional study | Nutrient adequacyDDS was positively associated with dietary micronutrient intake and negatively associated with micronutrient inadequacy |
| Morseth et al., 2018 (70) | Dietary Diversity Score WHO modification (DDS-WHOm) | Nepaln = 2292010–20129–24 moFemale (46%) | MAL-EDCohort study | Assessed the DDS and the sociodemographic factors associated with tracking complementary feeding practicesLow SES significantly increased the odds of tracking of low vs. high DDS (OR: 3.31; 95% CI: 1.44, 7.60) and meal frequency (OR: 3.46; 95% CI: 1.54, 7.76) |
| Iqbal et al., 2019 (71) | Dietary Diversity Score Bangladesh (DDS-BGD) | Bangladeshn = 3242015–20172–3 yFemale (50%) | Cross-sectional study | Association between dietary intakes and DNA methylationLower dietary intakes increased global methylation (OR: 3.05; 95% CI: 0.24, 38.7) |
| Thorne-Lyman et al., 2019 (72) | Individual Dietary Diversity Score modification (mIDDS) | Nepaln = 30514 y2013–2014Female (48%) | Cohort study | Association between diet and child development (OR: 4.6; 95% CI: –2.0, 11.2) |
| Yang et al., 2019 (73) | Dietary Diversity Score China 2019 (DDS-CHN19) | Chinan = 55320153–5 yFemale (45%) | Cluster randomized trial | Relation between food insecurity and undernutritionFood-insecure households presented stunting 6.5 times more compared with food-secure households (OR: 6.5; 95% CI: 1.60, 7.54) |
| Sebayang et al., 2020 (74) | Dietary Diversity Score Indonesia (DDS-IDN) | Indonesian = 11,6872012 and 20170–23 moFemale (49%) | Cross-sectional study | Assessed the determinants of DDAge and antenatal care were associated with DD consumption |
| Diet Quality Indexes | ||||
| Alexy et al., 2003 (75) | Diet Quality Index Germany (DQI-DEU) | Germanyn = 84219852–18 yFemale (51%) | Dortmund Nutritional and Anthropometric Longitudinal Designed (DONALD) studyCohort study | Characterized sugar intake and DQ13% of boys and 21% of girls had very low DQ |
| Kranz et al., 2004 (76) | Diet Quality Index for Children (C-DQI) | United Statesn = 86281997–19982–5 yFemale (∼50%) | Cross-sectional study | Determined the DQ trends between 1977 and 1998The mean total scores were 43.7, 45.0, and 45.7 in the 3 national surveys |
| Mariscal-Arcas et al., 2007 (77) | Diet Quality Index International adaptation (DQI-Ia) | Spainn = 2882002–20056–18 yFemale (44%) | Cross-sectional study | Evaluated the diet of young Mediterranean populationThe DQI was significantly associated with the breakfast duration (P = 0.029) |
| McArthur et al., 2008 (78) | Rapid Assessment Diet Quality Index (RADQI) | Latin American = 1279No date found12–19 yFemale (54%) | Cross-sectional study | Characterized dietary and physical activity patterns and the association with socioeconomic characteristics and BMIThe RADQI scores ranged from 53.0 points (±10.5) in Panama City to 44.7 points (±8.3) in Santiago |
| Huybrechts et al., 2010 (79) | Diet Quality Index for Flemish Preschoolers (DQI-FP) | Belgiumn = 5682002–20132.5–6.5 yFemale, percentage not found | Cross-sectional study | Validity and reproducibility of the DQIThe reproducibility correlation was 0.82. Actual values for surrogate FFQ tertiles showed a progressive increase in DQI score (P < 0.001) |
| De Vriendt et al., 2012 (80) | Diet Quality Index for Adolescents 2012 (DQI-A-12) | Europen = 7042006–200712.5–17.5 yFemale (62%) | Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA)Cross-sectional part | Relation of level of perceived stress and the overall DQStress influences dietary behavior (ß: – 0.02; P = 0.040) |
| Fokeena and Jeewon, 2012 (81) | Diet Quality Index Mauritius (DQI-MUS) | Mauritiusn = 200No date mentioned12–15 yFemale (52%) | Cross-sectional study | Association between SES and BMI mediated by physical activity and DQMean score in higher SES 6.48 ± 1.86 and in lower SES 5.87 ± 1.95 were significantly different (P = 0.02) |
| Li et al., 2012 (82) | Diet Quality Index Australian Children and Adolescents (DQI-AUS-CA) | Australian = 16292003–20061–14 yFemale (49%) | Western Australian PregnancyCohort study | Examined the impact of parental work hours from age 1 y to age 14 y on adolescent DQ. The mean working hours of mothers who worked full-time were negatively associated with adolescent DQ |
| Bel et al., 2013 (83) | Diet Quality Index for Adolescents with Meal Index (DQI-AM) | Europen = 15222006–200712.5–17.5 yFemale (47%) | Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA)Cross-sectional study | Association between self-reported sleep duration and DQThere is a significant negative association between the mean sleep duration and the DQI-AM (ß: 0.027; 95% CI: 0.018, 0.037) |
| Vyncke et al., 2013 (84) | Diet Quality Index for Adolescents 2013 (DQI-A-13) | Europen = 18042006–200712.5–17.5 yFemale (53%) | Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA)Cross-sectional study | Association between the DQI scores and nutritional biomarkersA positive association was found with 25-hydroxyvitamin D (ß: 0.30; 95% CI: 0.16, 0.44), holotranscobalamin (ß: 1.01; 95% CI: 1.00, 1.01), and n–3 fatty acid serum levels (ß: 0.38; 95% CI: 0.11, 0.65) |
| Wong et al., 2013 (85) | Diet Quality Index for New Zealand Adolescents (NZDQI-A) | New Zealandn = 412010–201114–18 yFemale (61%) | Cross-sectional study | Assessed a DQI and examined the relative validityThe score showed good reliability (r = 0.65) and a reasonable agreement in ranking participants by scores (r = 0.39) |
| Röytiö et al., 2015 (86) | Diet Quality Index for Finland Children (FINDQI-C) | Finlandn = 3742–6 y2009–2010Female (52%) | Cross-sectional study | Assessed diet in young childrenThe diet was good in 29.8%, moderate in 43.7%, and poor in 26.5% |
| Collins et al., 2016 (87) | Revised Children's Diet Quality Index Australia (RC-DQI-AUS) | Australian = 2442008–20133.6 yFemale (50%) | Melbourne Infant Feeding, Activity, and Nutrition TrialTrial study | Assessed diet and its predictorsBreastfeeding status (OR: 3.09; 95% CI: 1.63, 5.85) and modeling (OR: 2.01; 95% CI: 1.04, 3.88) |
| Ríos et al., 2016 (88) | Diet Quality Index Score for Puerto Rico Infants (DQIS-PRIn) | Puerto Ricon = 2962014–20158–24 moFemale, percentage not found | Cross-sectional study | Assessed diet of infantsThere was a trend for higher odds of excessive weight in those with poor diets compared with those with excellent diets (OR: 2.01; 95% CI: 0.85, 5.18) |
| Kunaratnam et al., 2018 (89) | Diet Quality Index for Australian Preschoolers (DQI-AusP) | Australian = 622007–20082–5 yFemale (55%) | Cross-sectional study | Assessed the association between DQI scores and nutrientsThe analysis indicated that increases in the DQI scores were related to increases in carbohydrate, folate, β-carotene, magnesium, calcium, protein, and total fat and to decreases in sugar, starch, niacin, vitamin C, phosphorus, polyunsaturated fat, and monounsaturated fat (adjusted r2 = 0.426, P < 0.05) |
| Hamner and Moore, 2020 (90) | Diet Quality Index Score for American Infants (DQIS-USIn) | United Statesn = 26752011–20166 mo–4 yFemale, percentage not found | Cross-sectional study | Assessed diet of childrenThe mean score was 22.4. There were significant differences between races |
| Food Variety Scores | ||||
| Cox et al., 1997 (91) | Variety Index for Toddlers (VIT) | United Statesn = 12419972–3 yFemale (44%) | Cross-sectional study | Dietary adequacyVIT scores were strongly correlated to the MAR score of nutrient adequacy (r = 0.74, P < 0.01) |
| Hatløy et al., 1998 (92) | Food Variety Score (FVS) | Malin = 771995<5 yFemale (55%) | Cross-sectional study | Nutritional adequacyA positive correlation was found between FVS and MAR (r = 0.33, P < 0.001). With cutoff points for FVS at 23, the indexes had a high ability to identify those with a nutritionally inadequate diet. MAR increased with increasing FVS |
| Vereecken et al., 2008 (93) | Variety Index | Belgium, Italyn = 226200811–12 yFemale (56%) | Cross-sectional study | Food group intakes and dietary indexesThe Variety Index was significantly correlated with fiber intake (0.26) |
| Falciglia et al., 2009 (94) | Three types of dietary variety | United Statesn = 7220059–12 yFemale (53%) | Prospective study | Predicted 15-d cumulative dietary variety score from 3 consecutive days and 3 interval days of dietary dataThree days of dietary data accurately estimated dietary variety over time using the predictive equation. Three interval days predicted 15-d food variety more precisely than did 3 consecutive days |
| Saibul et al., 2009 (95) | Food Variety Score (FVS) | Malaysian = 2842002–20052–9 yFemale (47%) | Cross-sectional study | Association between burden households and weightDual-burden households were associated with women's employment status (OR: 3.18) and FVS of children (OR: 0.71). The FVS of children (OR: 0.49) remained significant even when dual-burden households were compared with only households with normal-weight mother/normal-weight child |
| Scott et al., 2012 (96) | Core Food Variety Score (CFVS); Fruit and Vegetable Variety Score (FVVS) | Australian = 28681989–1992<2 yFemale (48%) | Western Australian Pregnancy Cohort (Raine) studyLongitudinal study | Duration of breastfeedingBreastfeeding duration was independently and directly associated with the CFVS (P < 0.001). Maternal age was independently and directly associated with the CFVS (P < 0.001). The presence of older siblings was independently and inversely associated with the CFVS (P = 0.003) |
| Zimmer et al., 2012 (97) | Food Variety (FV) score | United Statesn = 4419998 yFemale (32%) | Case–control study | Nutritional statusChildren with autism had a higher mean intake of magnesium and lower mean intake of protein, calcium, vitamin B-12, and vitamin D |
| Jones et al., 2015 (98) | Healthy Plate Variety Score (HPVS) | United Kingdom, France, Portugal, Greecen = 14,7631991–20072–4 yFemale (47%) | ALSPAC, EDEN, Generation XXI Birth Cohort, Greek EuroPrevallCohort studies | Maternal diet and early infant feeding experiencesMost children were not eating a full variety of healthy foods. There was no consistent association between the timing of complementary feeding and HPVS |
| Zaborowicz et al., 2015 (99) | Food Intake Variety Questionnaire (FIVeQ) | Polandn = 1862011–201213–21 yFemale | Gebahealth projectPilot project | AttitudesGirls from the upper tertile with favorable attitudes on food health benefits had an OR for adequate fat intake of 3.1 (P < 0.05) compared with those from the middle-neutral attitudes tertile (OR 1.05, not significant) |
| Fernández et al., 2016 (100) | Overall Variety Score | United Statesn = 340 Date not found 4.2 y Female (51%) | Follow-up study | Association between variety of diet and BMI z score. Overall variety and annual increases in BMI z score (β: 0.007; P = 0.02) |
| Barros et al., 2019 (101) | Healthy Dietary Variety Index (DVI) | Portugaln = 84954 y2006Female (49%) | Generation XXICohort studies | Dietary variety and adequacy and screen time, participation in sports, breastfeeding duration, siblings, family structureScreen time (≥120 vs. <120 min/d: β̂: −0.012), no regular participation in sports (β̂: −0.022), underweight status (β̂: −0.081), shorter breastfeeding duration (<4 vs. ≥6 mo: β̂: −0.012), no siblings (0 vs. ≥2: β̂: −0.023), and a 2-parent family structure (vs. single-parent; β̂: −0.010) |
| Healthy and Unhealthy Scores | ||||
| Yannakoulia et al., 2004 (102) | Unhealthy Food Choices Score (UFCS) | Greecen = 42111997–199811–15 yFemale (52%) | Health Behavior in School Aged ChildrenCross-sectional study | Characterization of nutrition-related habitsGirls were found to have lower score values compared with boys (24.7 ± 4.6 vs. 24.1 ± 4.5, respectively; P < 0.001) |
| Jacka et al., 2011 (103) | Healthy (HDS-A) and unHealthy Diet Scores Australia (uHDS-A) | Australian = 30402005–200811–18 yFemale (44%) | Prospective study | Association between DQ and adolescent mental healthThere was a significant association between both scores and adolescent mental health. HDS-A is a stronger predictor: Healthy (β: 0.14; 95% CI: 0.10, 0.18; P < 0.001); unhealthy (β: –0.009; 95% CI: –0.13, –0.6; P < 0.001) |
| Truthmann et al., 2012 (104) | Indicator Food Index (IFI) | Germanyn = 51982003–200612–17 yFemale (49%) | Cross-sectional study | Association of dietary indexes with biomarkers of dietary exposure and cardiovascular statusFruit/vegetable intake was associated with the IFI for boys. Among girls, positive associations were seen between vitamin B-12 and the IFI and between diastolic blood pressure and the IFI as well as fruit/vegetable intake. A negative association was found between homocysteine and the IFI |
| Monjardino et al., 2014 (105) | Oslo Health Study Dietary Index (OHS) | Portugaln = 12642003–200812–17 yFemale (54%) | The EPITeen cohortCohort study | Association between dietary patterns defined a priori and bone mineral densityThere is a lack of clear association between a priori dietary patterns and bone mineral density (girls OR: –0.29; 95% CI: –0.93, 0.34; boys OR: –0.59; 95% CI: 1.28, 0.084) |
| Vilela et al., 2014 (106) | Healthy Eating Index Score Portugal (HEIS-PRT) | Portugaln = 70520062–24 yFemale (49%) | Generation XXI prospectiveCohort study | Association between the consumption of less-healthy foods at age 2 y and the consumption of foods at age 4 yConsumption of energy-dense foods at age 2 y was associated with lower healthy eating score at age 4 y (IRR: 0.75; 95% CI: 0.58, 0.96; IRR: 0.56; 95% CI: 0.41, 0.77) |
| Anderson et al., 2015 (107) | Healthy (HDS-USA15) and unHealthy Diet Scores USA 2015 (uHDS-USA15) | United Statesn = 3572012–20132–5 yFemale (49%) | Cross-sectional study | Association between healthy and unhealthy foods and differences in younger and older children.The number of healthy diet behaviors was not related to the number of unhealthy diet behaviors or the unhealthy diet score (P trend values ranged from 0.26 to 0.90) |
| Anderson et al., 2016 (108) | Healthy (HDS-USA16) and unHealthy Diet Scores USA 2016 (uHDS-USA16) | United Statesn = 8900200152.5 moFemale (49%) | Early Childhood Longitudinal Study–Birth cohortCohort study | Relation between frequency of healthy foods intake and intake of unhealthy foodsThe rank correlation between healthy and unhealthy diet scores was positive (r = 0.09). The relation between healthy and unhealthy scores differed by household income-to-poverty ratio (P-interaction = 0.01), child racial–ethnic group (P = 0.005), and maternal education (P < 0.001) |
| Sluik et al., 2016 (109) | Dutch Healthy Diet index (DHD-index) | Netherlandsn = 17132007–20107–18 yFemale (50%) | Cross-sectional study | Assessed overall DQ and estimated the intake of total sugarsTotal sugar consumption was 136–153 g in boys and 125–138 g in girls |
| Arvidsson et al., 2017 (110) | Healthy Dietary Adherence Score (HDAS) | Europen = 76752007–20082–9 yFemale (49%) | Prevention of dietary-lifestyle-induced health effect in children and infants. Cohort study | Association between children's adherence to healthy dietary guidelines and their well-being. A higher HDAS at baseline was associated with better self-esteem (OR: 1.2; 95% CI: 1.0, 1.4) and fewer emotional problems (OR: 1.2; 95% CI:1.1, 1.3) and peer problems (OR: 1.3; 95% CI: 1.2, 1.4) |
| Martins et al., 2019 (111) | Healthy (HDS-BRA) and unHealthy (uHDS-BRA) Diet Scores Brazil | Braziln = 102,072201511–19 yFemale, percentage not found | Cross-sectional study | Association between eating meals with parents and DQEating meals with parents was positively associated with healthy diet scores (β: 1.86; P < 0.001) and inversely associated with unhealthy diet scores (β: –0.62; P < 0.001) |
| Wadolowska et al., 2019 (112) | Pro-Healthy Diet Index (pHDI); non-Healthy Diet Index (nHDI) | Polandn = 4642015–201611–12 yFemale (54%) | Intervention study | Sustainability of education on a sedentary and active lifestyle, DQ, and body composition. The chance of adherence to the nHDI was significantly lower (35%; P < 0.05) after 9-mo intervention |
| Feeding and Eating Indexes | ||||
| Arimond and Ruel, 2002 (113) | Infant and Child Feeding Index (ICFI) | Ethiopian = 462420026–36 moFemale, percentage not found | Cross-sectional study | Association between infant and child feeding practices with nutritional statusDD and frequency of feeding component were positively associated with height-for-age z score |
| Ruel and Menon, 2002 (114) | Child Feeding Index (CFI) | Latin American = 23,0161994–19996–36 moFemale, percentage not found | Cross-sectional study | Association between child feeding practices and nutritional status according to characteristics of the child, mother, or householdChild feeding practices were associated with better nutritional status, especially for children with lower SES |
| Dewey et al., 2006 (115) | Food Group Indicator–8 (FGI-8) | Peru, Bangladesh, Ghana, Hondurasn = 18666–9 moFemale, percentage not found | Cross-sectional study | Mean micronutrient density adequacyMean nutrient density adequacy increased with increasing DD, although the relation was not always linear. It ranged from 0.37 to 0.74 |
| Food and Nutrition Technical Assistance Project, 2006 (116) | Food Group Indicator (FGI) | Africa, Asian, Latin American = 10,0141994–20046–23 moFemale, percentage not found | Cross-sectional study | Mean micronutrient density adequacy. For practical purposes, 1 g restriction is better than 10 g restriction.The association between mean micronutrient density adequacy and DD was significant |
| Bork et al., 2012 (117) | Infant and Child Feeding Index Senegal (ICFI-Sen) | Senegaln = 106020096–36 moFemale, percentage not found | Cross-sectional study | Association between height-for-age z score and feeding indicatorHeight‐for‐age z score was positively associated with the ICFI-Sen (P < 0.05) |
| Golley et al., 2012 (118) | Complementary Feeding Utility Index (CFUI) | United Kingdomn = 606519916 mo, 8 mo, and 3 yFemale, percentage not found | The Avon Longitudinal Study of Parents and Children (ALSPAC)Cohort study | Association between index scores, sociodemographic factors, food and nutrient intakes, and dietary patternsThe index score was associated with “processed” (β: –0.234; 95% CI: –0.260, –0.209) and “healthy” (β: 1.185; 95% CI: 0.155. 0.215) dietary patterns |
| Jones, 2015 (119) | Infant and Child Feeding Index modification (ICFIm) | Bolivian = 25220096–23 moFemale (∼50%) | Cross-sectional study | Association between infant and child feeding index and factors related to agricultural productionThere was a positive association with score and the length-for-age z score, the energy intake, and the micronutrient adequacy |
| Monterrosa et al., 2015 (120) | Infant and child Feeding Index Mexico | Mexicon = 3701999–20011–2 yFemale (43%) | Cross-sectional study | Association between maternal pre-pregnancy BMI and feeding practices.Maternal pre-pregnancy BMI was not associated with children feeding practices and breastfeeding |
| Delshad et al., 2019 (121) | Diet Index for a Child's Eating (DICE) | New Zealandn = 652007–20082–8 yFemale (55%) | Cross-sectional study | DICE and assessment of adherence to nutritional guidelinesCorrelation coefficients showed significance (P < 0.001) between 4-d food record and DICE. Analysis showed that increases of vitamin C, vitamin A, vitamin D, and calcium were associated with increasing tertiles of DICE |
| Ferreira et al., 2019 (122) | Child Feeding Index modification (CFIm) | Braziln = 6172008–20106–15 moFemale (47%) | Randomized controlled trial | Assessed feeding practices in intervention compared with control groupChildren from the intervention group showed a higher score index in comparison to the control group (mean difference: 0.22; 95% CI: 0.24, 1.11) at 6 mo (mean difference: 0.23; 95% CI: 0.35, 0.56) |
| Diet Quality Scores | ||||
| Crombie et al., 2009 (123) | Scottish Diet Quality Score (SDQS) | Scotlandn = 3002005–20062 yFemale, percentage not found | Cross-sectional study | Assessed maternal factors associated with poor diet among disadvantaged children85% of the children had a poor DQ: lack of limitation of sweets (OR: 21.63; 95% CI: 2.70, 173.30) and difficulties for providing fruits (OR: 2.94; 95% CI: 1.0, 7.95) |
| Kohlboeck et al., 2012 (124) | German Optimized Mixed Diet Quality Score (GOMDQS) | Germanyn = 33611995–1999∼11 yFemale (49%) | The German Infant Nutritional Intervention, GINI-plus; and Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood, LISA-plus | Association between food intake, DQ, and behavioral problemsA higher DQS was associated with a lower likelihood of emotional symptoms (OR: 0.89; 95% CI: 0.80, 0.98) |
| Perry et al., 2015 (125) | Diet Quality Score Ireland (DQS-IRL) | Irelandn = 81362007–20089 yFemale (48.7%) | Growing Up in IrelandCross-sectional study | Association between DQ and childhood overweight or obesityChildhood obesity was significantly associated with poor DQ (OR: 1.56; 95% CI: 1.02, 2.38) |
| Voortman et al., 2015 (126) | Diet Quality Score The Netherlands (DQS-NL) | Netherlandsn = 36292001–200512–19 moFemale (51%) | Generation R Cohort study | Identified the sociodemographic and lifestyle determinants of the DQA higher score was associated with maternal folic acid supplement during pregnancy, no smoking during pregnancy, and children watching less television |
| Gasser et al., 2017 (127) | Dietary Quality Score Australia 2017 (DQS-AUS17) | Australian = 977420042–15 yFemale (49%) | Baby (B) cohort study and Kindergarten (K) cohort study | Derived and compared dietary score and pattern trajectories from a parallel population-based cohortSimilar trajectories of dietary scores emerged for B and K cohorts, respectively: never healthy (8.8% and 11.9%), moderately healthy (24% and 20.7%), becoming less healthy (16.6% and 27.3%), and always healthy (50.7% and 40.2%) |
| Nutritional Adequacy and Micronutrients Indexes | ||||
| Fulgoni et al., 2009 (128) | Nutrient-Rich Foods Index | United Statesn = 15,5371999–2002≥4 yFemale (50%) | NHANESCross-sectional study | Validation against the HEIMaximum variance in HEI was explained using 6 or 9 nutrients to encourage; index performance declined with the inclusion of additional vitamins and minerals |
| Libuda et al., 2009 (129) | Nutrient-based Nutritional Quality Score (NQI) | Germanyn = 106919852–19 yFemale (51%) | Dortmund Nutritional and Anthropometric Longitudinally Study Designed (DONALD) studyOpen cohort study | Association between SSB consumption and nutrient intakeSSB consumption was positively associated with percentage total energy from carbohydrates and added sugars and negatively with percentage total energy from protein and fat. SSB consumption was negatively associated with folate and calcium intake, for which mean intake levels were inadequate in girls |
| Chiplonkar and Tupe, 2010 (130) | Adolescent Micronutrient Quality Index (AMQI) | Indian = 6302006–200710–16 yFemale | Cross-sectional study | Micronutrient adequacyThe AMQI was correlated with nutrient intakes and the ratio of observed intake to reference intake (P < 0.01). Higher AMQI scores were associated with higher concentrations of plasma vitamin C (r = 0.26), β-carotene (r = 0.34), and zinc (r = 0.12) |
| Alexy et al., 2011 (131) | Nutrient Quality Index (NQI) | Germanyn = 5852004–20083–18 yFemale (49%) | Dortmund Nutritional and Anthropometric Longitudinally Study Designed (DONALD) studyOpen cohort study | Body weight statusThe NQI showed a significant negative trend with increased consumption of convenience foods (P = 0.0013) |
| Lee and Park, 2015 (132) | Index of Nutritional Quality (INQ) | Korean = 19691998–20122–18 yFemale (42%) | Korean National Health and Nutrition Examination Survey (KNHANES)Cross-sectional study | Poor micronutrition and dietCalcium was the most commonly underconsumed micronutrient. More than half of the sons and daughters showed insufficient vitamin A, vitamin C, and iron intake. The correlation between a poor diet in parents and that in offspring was 0.17 (P < 0.001). Eating breakfast provided a significant protective effect against the risk of poor nutrition in offspring |
| Dietary Guidelines Indexes | ||||
| Golley et al., 2011 (133) | Dietary Guideline Index for Children and Adolescents (DGI-CA) | Australian = 341620064–10 yFemale, percentage not found | Australian National Children's Nutrition and Physical Activity SurveyCross-sectional study | Index scores, food and nutrient intake, sociodemographic characteristics, and measures of adiposityDGI-CA was associated with socioeconomic characteristics and measures of family circumstance. Weak positive associations were observed between DGI-CA and BMI or waist circumference z scores in the 4- to 10-y and 12- to 16-y age groups only |
| Lioret et al., 2014 (134) | Dietary Guideline Index (DGI) | Australian = 21620075–12 yFemale (56%) | Resilience for Eating and Activity Despite Inequality (READI) studyCohort study | Relation between diet quality and BMI z scoreChange in DQ was found to be inversely associated with a change in BMI z score only in children who were overweight at baseline (P = 0.035) |
| Mohseni-Takalloo et al., 2016 (135) | Dietary Guidelines for Americans Adherence Index (DGAI) | Irann = 7222008–201110–19 yFemale (54%) | Tehran Lipid and Glucose StudyCross-sectional study | Obesity-associated phenotypesNo association was observed between different types of obesity and DGAI |
| Rohde et al., 2017 (136) | Diet Quality Index based on the Danish national guidelines (DQI-Danish national guidelines) | Denmarkn = 6352009–20112–6 yFemale (42%) | Healthy Start StudyRandomized controlled trial | Macronutrient profileChildren in the intervention group had a lower energy intake after the 15-mo intervention (P = 0.02) compared with the control group. There were lower intakes of carbohydrates and added sugar in the intervention group compared with the control group after the intervention (P = 0.002 and P = 0.01, respectively) |
| Er et al., 2018 (137) | Children's Food Trust guidelines (CFT guidelines) and NAP SACC UK Nutrition Best Practice Standards score | Englandn = 1502015–20162–4 yFemale (52%) | NAP SACC UK studyCross-sectional study | Association between PA parameters and BMI z scoresMinutes spent in light PA (β: 0.08; 95% CI: 0.0, 0.15) and active time (β: 0.07; 95% CI: 0.01, 0.12) were positively associated with BMI z scores |
| Other Healthy Diet Indexes | ||||
| Kleiser et al., 2009 (138) | Healthy Nutrition Score (HuSKY) | Germanyn = 14,1052003–20063–17 yFemale (49%) | German Health Interview and Examinations Study for Children and Adolescents (KiGGS) | Association between DQ and nutritional recommendationsThe score was significantly associated with age, sex, lower socioeconomic status R2 |
| Lazarou et al., 2009 (139) | Foods E-KINDEX score (E-KINDEX) | Cyprusn = 6222004–20059–13 yFemale (52%) | Cross-sectional study | Association between DQ and children's obesity status and blood pressure levelsCompared with children with a low diet score, those with at least an average Foods E-KINDEX score were 57% (OR: 0.43; 95% CI: 0.19, 0.98) less likely to have elevated systolic blood pressure levels |
| Bisi Molina et al., 2010 (140) | School Child Diet Index (ALES) | Braziln = 128220077–10 yFemale (58%) | Cross-sectional study | Association between socioeconomic factors and low DQLow maternal level of education (OR: 3.93; 95% CI: 2.58, 5.99), father not present in the household (OR: 2.03; 95% CI: 1.68, 2.99), and not having lunch at the table (OR: 1.47; 95% CI: 1.1, 1.93) were associated with low DQ |
| Marshall et al., 2012 (141) | Australian Child and Adolescent Recommended Food Score (ACARFS) | Australian = 69120059–12 yFemale (56%) | Cross-sectional study | Evaluation of the DQ and nutrient intakes. Statistically significant correlations between scores and all vitamins, minerals and energy intake, were moderate to strong and positive (r = 0.42–0.70) |
| Spence et al., 2013 (142) | Obesity Protective Dietary Index (OPDI) | Australian = 3982008–2010∼4 yFemale (47%) | The Melbourne InFANT program clusterRandomized controlled trial | Effect of healthy eating and PA intervention on dietary patternsEnergy-adjusted model regression coefficient (β: 1.33; 95% CI: 0.28, 2.39; P = 0.01) |
| Durksen et al., 2015 (143) | Unhealthy Eating Index Canada (UEI-Ca15) | Canadan = 33020089–17 yFemale, percentage not found | Cross-sectional study | Association between unhealthy diet and light, moderate, or vigorous PAThere was no statistical difference in the consumption of unhealthy food between levels of PA (OR: 1.01; 95% CI: 0.41, 2.46; P = 0.8) |
| Haapala et al., 2015 (144) | Baltic Sea Diet Score (BSDS) | Finlandn = 4282007–20096–8 yFemale (50%) | Physical Activity and Nutrition in Children (PANIC) intervention study | Association between diet and cognitionDQ was directly associated with cognition test (ß: 0.122; P = 0.012) |
| Hardiansyah et al., 2015 (145) | Balanced Diet Index (BDI) | Indonesian = 10,22120104–6 yFemale, percentage not found | Cross-sectional study | Assessed nutritional adequacyPearson correlation coefficient, r = 0.42 |
| Cheng et al., 2016 (146) | Chinese Children Dietary Index (CCDI) | Chinan = 171920137–15 yFemale (49%) | Cross-sectional study | Characterization of DQ and associated factorsMean score 88.1. Age, paternal education level, and family size were associated with DQ |
| Verger et al., 2016 (147) | Diet Quality Index Based on the Probability of Adequate Nutrient Intake (PANDiet) | United Kingdomn = 115220112–18 moFemale (49%) | Cross-sectional study | Nutritional adequacy according to the consumption of formula and infant foodsA lower score was associated with lower intakes of formula, infant foods, vegetables, and fruits |
| Winpenny et al., 2018 (148) | Dietary Approaches to Stop Hypertension index (DASH index) | United Kingdomn = 29572008–201613–30 yFemale (51%) | Cross-sectional study | Association between DQ and ageThe regression showed no significant change in DQ. There was a slight improvement among older females (ß: 2.39; 95% CI: 0.53, 4.26) |
| Kunto and Bras, 2019 (149) | Berry Index (BI) | Indonesian = 64782000–20157–12 yFemale, percentage not found | Cross-sectional study | DQ and sociodemographic characteristicsSex and household SES were associated with the DQ |
| Liu et al., 2020 (150) | American Heart Association Score (AHAS) | United Statesn = 31,4201999–20162–19 yFemale (49%) | Cross-sectional study | Trends in DQ in youthYouth with poor diets declined from 76.8% to 56.1% and with intermediate diets increased from 23.2% to 43.7% (P < 0.001 for each trend) |
| Other Dietary and Lifestyle Indicators | ||||
| Mediterranean Diet Indexes | ||||
| Serra-Majem et al., 2004 (151) | Mediterranean Diet Quality Index for Children and Adolescents (KIDMED) | Spainn = 385020002–24 yFemale, percentage not found | EnKid studyCross-sectional study | Levels of adherenceLower percentages of high DQ were observed in low socioeconomic groups compared with middle and upper income cohorts (42.8%, 47.6%, and 54.9%, respectively) |
| Jennings et al., 2011 (152) | Modified Mediterranean Diet Score (mMDS) | United Kingdomn = 170020079–10 yFemale (56%) | Sport, Physical Activity, and Eating Behaviour: Environmental Determinants in Young People (SPEEDY) studyCross-sectional study | Adherence to the Med diet and anthropometric parametersNo significant associations were observed with the MDS and weight status, waist circumference, and body fat |
| Schroder et al., 2011 (153) | Brief Mediterranean Diet Screener (bMDSC): 2 subscores—Antioxidant Food Score (ANTOX-S) and Modified Mediterranean Diet Score (mMDS) | Spainn = 63522004–20063–80 yFemale (52%) | Cross-sectional study | Valid assessments of DQ for use in time-limited settingsCorrelated (P < 0.001) with the corresponding 24h-DR (r = 0.45). Dietary intakes of fiber, vitamin C, vitamin E, magnesium, and potassium reported on the 24h-DR were positively associated (P = 0.04) with ANTOX-S and mMDS |
| Lazarou and Matalas, 2015 (154) | Modified Mediterranean Diet Quality Index for Children and Adolescents (mKIDMED) | Cyprusn = 832006–20079.2 yFemale (37%) | Cross-sectional study | Med diet adherence, PA, obesity, selected cardiovascular risk markers, and iron statusBreakfast skippers vs. regular breakfast eaters were 40% more likely to have a Modified Mediterranean DQI score higher by 1 point (OR: 1.41; 95% CI: 1.05, 1.84).Combining breastfeeding percentage and waist circumference into a composite variable (OR: 0.20; 95% CI: 0.06, 0.69) |
| Kastorini et al., 2016 (155) | Modified Mediterranean Diet Quality Index for Children and Adolescents (mKIDMED) | Greecen = 39412012–20133–18 yFemale (52%) | DIATROFI studyCohort study | Evaluation of the effects of healthy nutrition program. Modified Mediterranean DQI increased statistically significantly in adolescent girls (P = 0.042), whereas the consumption frequency of all foods promoted by the intervention—milk, fruits, vegetables, and whole-grain products—increased for children and adolescents, boys and girls (P = 0.002) |
| Rivas et al., 2016 (156) | Mediterranean Diet Score (MDS) | Spainn = 13220146–8 yFemale (43%) | Cross-sectional study | Bisphenol A migration and exposureSignificant association between the MDS and low bisphenol A exposure. Bisphenol A exposure below the median level was significantly associated with a higher score (P < 0.05) |
| Haapala et al., 2017 (157) | Mediterranean Diet Score (MDS) | Finlandn = 4712007–20096–8 yFemale (46%) | Physical Activity and Nutrition in Children (PANIC) studyIntervention study | Academic achievementMDS was positively associated with reading comprehension in a group of children (β: 0.167; P = 0.032) |
| Montero et al., 2017 (158) | Mediterranean Adequacy Index (MAI) | Morocco, Spainn = 327; n = 772007–201012–19 yFemale (59%); female (51%) | Cross-sectional study | Eating patterns of teenagers from Morocco living in MadridTeenagers from Morocco living in Madrid consumed more calories, proteins, saturated fats, and simple sugars (P < 0.001) than those living in Morocco. Their diet was of lower quality than that of their peers in Morocco |
| Carvalho et al., 2018 (159) | Adolescent Mediterranean Diet Score (aMDS) | European countriesn = 242200512.5–17.5 yFemale (57%) | Healthy Lifestyle in Europe by Nutrition in Adolescence Cross-Sectional Study (HELENA-CSS)Cross-sectional study | Association between cortisol levels, inflammation, and Med diet. Cortisol levels were inversely associated with adherence to the Med diet (β: –1.023; P = 0.002). Adolescents with higher adherence to the Med diet had lower levels of IL-1, IL-2, IL-6, and TNF-α compared with those who did not adhere |
| Aparicio-Ugarriza et al., 2019 (160) | Adapted Mediterranean Diet Score for Adolescents (MDS_A)Adapted Mediterranean Diet Quality Index for Children and Adolescents (KIDMED_A) | European countriesn = 2330200514.7 yFemale (56%) | Healthy Lifestyle in Europe (HELENA) studyCross-sectional study | Better food/nutrient intakes and nutritional biomarkersPositive associations for KIDMED_A with serum levels of vitamin D, vitamin C, plasma folate, holotranscobalamin, β-carotene, and n–3 fatty acids, whereas there was a negative association with trans-fatty acid serum levels. KIDMED_A positive associations with vegetables and fruits intake and negative associations with energy-dense and low-nutritious foods |
| Diet–Lifestyle Indexes | ||||
| Kosti et al., 2009 (161) | Diet–Lifestyle Index | Greecen = 20082004–200812–17 yFemale (49%) | Cross-sectional study | Prevalence of overweight/obesityThe index was inversely associated with the odds of being obese/overweight (OR: 0.93; 95% CI: 0.90, 0.96). An 11/57–unit increase of the index was associated with a 6% and 9% decrease in the odds of being overweight/obese in boys and girls (P < 0.001) |
| Manios et al., 2010 (162) | Preschoolers Diet–Lifestyle Index (PDL-Index) | Greecen = 22872003–20042–5 yFemale (48%) | Exercise and Nutrition Epidemiological Study in preSchoolers (GENESIS) studyCross-sectional study | ObesityIt was observed that a 1/44-unit increase in the score of the PDL-Index was associated with ∼5% and ∼3% lower odds of being obese and overweight/obese, respectively |
| Manios et al., 2010 (b) (163) | Healthy Lifestyle–Diet Index (HLD-Index) | Greecen = 729200710–12 yFemale, percentage not found | Healthy Growth StudyCross-sectional study | IR20.9% of participants were found to be IR. A 1-unit increase in the score is associated with ∼8% lower odds for being IR |
| Manios et al., 2015 (164) | Revised Healthy Lifestyle–Diet Index (R-HLD-Index) | Greecen = 266020079–13 yFemale (49%) | Healthy Growth StudyCross-sectional study | Obesity and iron deficiencyAn increase in the R-HLD-Index score by 1 unit was associated with a 6% lower odds for obesity. No significant association was observed between the R-HLD-Index score and iron deficiency |
| Ertaş Öztürk et al., 2018 (165) | Healthy Lifestyle–Diet for Turkey (HLD-TR) | Turkeyn = 16420159–13 yFemale (42%) | Cross-sectional study | IRA 1-unit increase in the index score decreased the IR risk (OR: 0.91; 95% CI: 0.85, 0.97) |
| Breakfast Quality Indexes | ||||
| Radcliffe et al., 2004 (166) | Five food groups in Australian Guide to Healthy Eating (AGHE) Food Group Score (FGS) | Australian = 832200211–12 yFemale (50%) | Queensland School Breakfast Project (QSBP) studyCross-sectional study | Breakfast characteristicsThe most common breakfast item reported was breakfast cereal (55%), and 22% of children consumed energy-dense, micronutrient-poor foods or beverages |
| Herrero Lozano and Fillat Ballesteros, 2006 (167) | enKID study criteria | Spainn = 141200612–13 yFemale (50%) | Cross-sectional study | Intellectual performanceThe mean mark systematically increased as breakfast quality increased from a mean score of 5.63 in the group with poor-quality breakfast to a mean score of 7.73 in the group with a good-quality breakfast |
| Hallström et al., 2012 (168) | Breakfast Quality Index (BQI) | European citiesn = 26722006–200712–17 yFemale (53%) | Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) studyCross-sectional study | Breakfast habits and sociodemographic factorsMost of the adolescents reported a breakfast that scored poorly on the BQI. Older adolescents, adolescents from the southern area of Europe, and adolescents from families with low SES were more likely to consume a low-quality breakfast |
DD, dietary diversity; DDS, Dietary Diversity Score; DQ, diet quality; DQI, diet quality index; DR, dietary recall; FVS, Food Variety Score; HEI, Healthy Eating Index; IR, insulin resistance; MAR, mean adequacy ratio; Med diet, Mediterranean diet; PA, physical activity; SES, socioeconomic status; SSB, sugar-sweetened beverage.
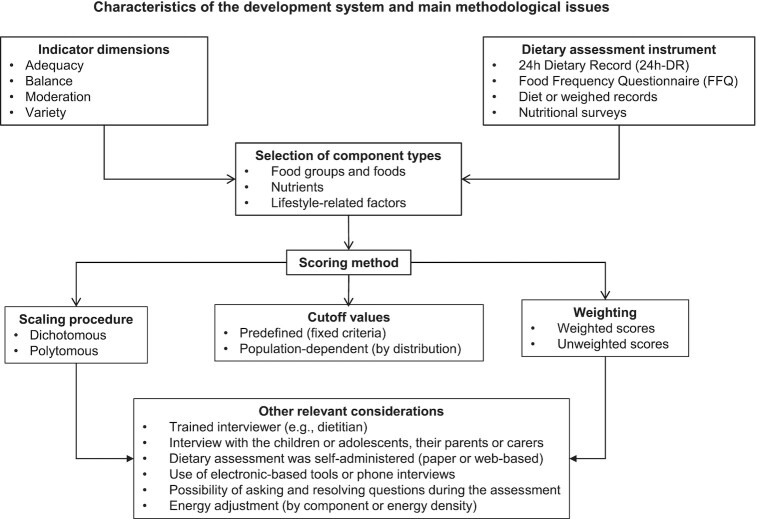
Key methodological issues: components and scoring method of the DQIns
Tables 2a – j and 3a–g describe the components found in the DQIns reviewed. All the components were classified into 3 types: food groups and food, nutrients, and lifestyle-related factors (Supplemental Table 2).
TABLE 2a.
Food group components included in the Healthy Eating Indexes diet quality indicators1
| Authors, publication year (Ref) | Indicator | Vegetables | Fruits | Cereals | Legumes | Nuts | Fats | Dairy | Eggs | Fish | Meat | Snacks | SSBs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kennedy et al., 1995 (30) | Healthy Eating Index (HEI) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)2 | ✓ (+)2 | ✓ (+)3 | ✓ (+)2 | |||||
| Feskanich et al., 2004 (31) | Youth Healthy Eating Index (YHEI) | ✓ (+) | ✓ (+) | ✓ (+)4 | ✓ (+)2 | ✓ (+)2 | ✓ (–)5 | ✓ (.)6 | ✓ (+)2 | ✓ (+)2 | ✓ (.)2, 7 | ✓ (–)8 | ✓ (–) |
| Knol et al., 2004 (32) | Healthy Eating Index Variety Score (HEI-variety) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)2 | ✓ (+)2 | ✓ (+)3 | ✓ (+)2 | |||||
| Pinheiro and Atalah, 2005 (33) | Healthy Eating Index Chile (HEI-CHL) | ✓ (+) | ✓ (+)9 | ✓ (+)10 | ✓ (+)2 | ✓ (+)2 | ✓ (+)11 | ✓ (+)2 | ✓ (+)2 | ✓ (.)2 | |||
| Glanville and McIntyre, 2006 (34) | Healthy Eating Index Canada 2006 (HEI-C06) | ✓ (+)12 | ✓ (+)9 | ✓ (+) | ✓ (+)2 | ✓ (+)2 | ✓ (+) | ✓ (+) | ✓ (+)2 | ✓ (+)2 | ✓ (–)8 | ✓ (–) | |
| Guenther et al., 2008 (35) | Healthy Eating Index 2005 (HEI-2005) | ✓ (+)13 | ✓ (+)9 | ✓ (+)14 | ✓ (+)2 | ✓ (+)15 | ✓ (+)11 | ✓ (+)2 | |||||
| Garriguet, 2009 (36) | Healthy Eating Index Canada modification (HEI-C-mod) | ✓ (+)13 | ✓ (+) | ✓ (+)14 | ✓ (+)2 | ✓ (+)15 | ✓ (+)11 | ✓ (+)2 | |||||
| De Andrade et al., 2010 (37) | Healthy Eating Index Brazil (HEI-BRA14) | ✓ (+) | ✓ (+) | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+)2 | ✓ (+)2 | |||||
| Woodruff et al., 2010 (38) | Healthy Eating Index Canada (HEIC-2009) | ✓ (+)13 | ✓ (+) | ✓ (+) | ✓ (+)16 | ✓ (+)2 | ✓ (+)2, 17 | ||||||
| Rydén and Hagfors, 2011 (39) | Healthy Eating Index 2005 (HEI-2005) | ✓ (+)13 | ✓ (+)9 | ✓ (+)14 | ✓ (+)2 | ✓ (+)15 | ✓ (+)11 | ✓ (+)2 | |||||
| González Rosendo et al., 2012 (40) | Healthy Eating Index Mexico (HEI-MEX) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (.) | ||||||
| He et al., 2012 (41) | Healthy Eating Index 2005 Canadian modification (HEIC-2005) | ✓ (+) | ✓ (+)9 | ✓ (+)14 | ✓ (+)2 | ✓ (+)2 | ✓ (+)2 | ✓ (+)2 | |||||
| Guenther et al., 2013 (42) | Healthy Eating Index 2010 (HEI-2010) | ✓ (+) | ✓ (+) | ✓ (.)14 | ✓ (+)2 | ✓ (+)18 | ✓ (+)11 | ✓ (+)2 | ✓ (+)18 | ✓ (+)2 | |||
| Nyaradi et al., 2013 (43) | Youth Healthy Eating Index Australia (YHEI-AUS) | ✓ (+) | ✓ (+) | ✓ (+)4 | ✓ (+)2 | ✓ (+)2 | ✓ (+)11 | ✓ (+)2 | ✓ (+)2, 7 | ✓ (–)8 | ✓ (–) | ||
| Kyttälä et al., 2014 (44) | Finnish Children Healthy Eating Index (FCHEI) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)16 | ✓ (+) | ✓ (–) | ||||||
| Rauber et al., 2014 (45) | Healthy Eating Index Brazil (HEI-BRA14) | ✓ (+) | ✓ (+)9 | ✓ (+)10 | ✓ (+)2 | ✓ (+)2 | ✓ (+)11 | ✓ (+)2 | ✓ (+)2 | ||||
| Wahlqvist et al., 2014 (46) | Youth Healthy Eating Index Taiwan (YHEI-TW) | ✓ (+) | ✓ (+) | ✓ (+)7 | ✓ (+)2 | ✓ (+)2 | ✓ (+)2 | ✓ (+)2, 7 | ✓ (–)8 | ✓ (–) | |||
| Dahm et al., 2016 (47) | High School Alternative Healthy Eating Index (HS-AHEI) | ✓ (+) | ✓ (+) | ✓ (+)4 | ✓ (+)18 | ✓ (+)18 | ✓ (+) | ✓(-)19 | ✓ (–) | ||||
| Tugault-Lafleur et al., 2017 (48) | School Healthy Eating Index (School-HEI) | ✓ (+)13 | ✓ (+) | ✓ (+)14 | ✓ (+) | ✓ (.) | ✓ (.) | ✓(–)21 | ✓(–)21 | ||||
| Yuan et al., 2017 (49) | Healthy Eating Index China (HEI-CHN) | ✓ (+)13 | ✓ (+) | ✓(+)12, 14 | ✓ (+) | ✓ (+)15 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (.)20 | |||
| Conceição et al., 2018 (50) | Healthy Eating Index Brazil Infants (HEI-BRA-I) | ✓ (+) | ✓ (+) | ✓ (+)10 | ✓ (+)2 | ✓ (+) | ✓ (+)2 | ✓ (+)2 | |||||
| Hooshmand et al., 2018 ( 51) | Healthy Eating Index Iran (HEI-IRN) | ✓ (+) | ✓ (+) | ✓ (+)10 | ✓ (+)5 | ✓ (+) | ✓ (+)7 | ✓ (–)8 | ✓ (–) | ||||
| Nshimyumukiza et al., 2018 (52) | Healthy Eating Index Canada (HEI-C-2010) | ✓ (+) | ✓ (+) | ✓ (+)14 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (.) | |||||
| Reedy et al., 2018 (53) | Healthy Eating Index 2015 (HEI-2015) | ✓ (+) | ✓ (+) | ✓ (.)14 | ✓ (+) | ✓ (+) | ✓ (+)2 | ✓ (+) | ✓ (+)2 | ||||
| Brownlee et al., 2019 (54) | Healthy Eating Index Singapur (HEI-SGP) | ✓ (+) | ✓ (+) | ✓ (+)14 | ✓ (.) | ✓ (.) | |||||||
| No. of studies using the particular component in this indicators subgroup | 25 | 25 | 24 | 20 | 9 | 9 | 23 | 12 | 10 | 24 | 6 | 8 | |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets a higher score when it fits within a specific range or the score is unspecified. DQ, diet quality; SSBs, sugar-sweetened beverages.
Included in the meat component.
Milk.
Whole grains only.
Margarine and butter.
Includes ice cream and whole-fat milk.
Ratio from the consumption of meat and other protein sources, where the denominator included dark meat, including beef, pork, lamb, liver, and cold cuts; and the numerator included poultry, fish, dried beans, tofu, eggs, nuts, and seeds.
Salty snacks (e.g., potato chips, corn chips, nachos, popcorn, pretzels, and crackers) and snacks with added sugar (e.g., cake, snack cake, toaster pastry, sweet roll/Danish/pastry, doughnut, brownie, cookies, pie, chocolate, candy bar with chocolate, candy without chocolate, fruit rollup, popsicle, and flavored gelatin).
Includes fruit juices.
Cereal, tubers, and roots.
Cow milk, goat milk, and soy drinks.
Includes potatoes.
Includes dark green and orange vegetables.
Whole grains as separate component.
Nonhydrogenated vegetable oils and oils in fish, nuts, and seeds.
Skimmed milk and alternatives.
Lean meat and alternatives.
Plant proteins.
Red processed meats.
Red meat and poultry.
As other food component.
TABLE 2j.
Food group components included in the Other Healthy Diet Indexes diet quality indicator1
| Authors, publication, year (Ref) | Indicator | Water | Vegetables | Fruits | Cereals | Legumes | Nuts | Fats | Dairy | Eggs | Fish | Meat | Snacks | FF | Sweets | SSBs | Miscellaneous |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kleiser et al., 2009 (138) | Healthy Nutrition Score (HuSKY) | ✓ (+)2 | ✓ (+) | ✓ (+) | ✓ (+)3, 4 | ✓ (.)5 | ✓ (.) | ✓ (+) | ✓ (+) | ✓ (+)6 | ✓ (–)7 | ✓ (–)7 | ✓ (–)7 | ✓ (–)2 | |||
| Lazarou et al., 2009 (139) | Foods E-KINDEX score (E-KINDEX) | ✓ (+) | ✓ (+)8 | ✓ (+)9 | ✓ (+) | ✓ (.) | ✓ (.)10 | ✓ (-)7 | ✓ (-)7 | ✓ (-) | |||||||
| Bisi Molina et al., 2010 (140) | School Child Diet Index (ALES) | ✓ (+) | ✓ (+)8 | ✓ (+) | ✓ (–)11 | ✓ (+)12 | ✓ (+) | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–) | ||||||
| Marshall et al., 2012 (141) | Australian Child and Adolescent Recommended Food Score (ACARFS) | ✓ (+) | ✓ (+) | ✓ (+)13 | ✓ (+)14 | ✓ (+)15 | ✓ (+)15 | ✓ (.)16 | ✓ (+)17 | ✓ (+)17 | ✓ (+)17 | ✓ (.) | |||||
| Spence et al., 2013 (142) | Obesity Protective Dietary Index (OPDI) | ✓ (+)4 | ✓ (+) | ✓ (–)7 | ✓ (–)7 | ✓ (–)7 | ✓ (–)7 | ||||||||||
| Durksen et al., 2015 (143) | Unhealthy Eating Index Canada (UEI-Ca15) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–)7 | ✓ (–)7 | ✓ (–)7 | ✓ (–)7 | ||||||||
| Haapala et al., 2015 (144) | Baltic Sea Diet Score (BSDS) | ✓ (+)7 | ✓ (+)7 | ✓ (+)3 | ✓ (+)7 | ✓ (+) | ✓ (–)17 | ||||||||||
| Hardiansyah et al, 2015 (145) | Balanced Diet Index (BDI) | ✓ (+) | ✓ (+) | ✓ (+)15 | ✓ (+) | ✓ (+)7 | ✓ (+)7 | ✓ (+)7 | |||||||||
| Cheng et al., 2016 (146 ) | Chinese Children Dietary Index (CCDI) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (.) | ✓ (+) | ✓ (.) | ✓ (.) | ✓ (+) | ✓ (.) | ✓ (–) | ||||||
| Winpenny et al., 2018 (148) | Dietary Approaches to Stop Hypertension index (DASH index) | ✓ (+) | ✓ (+) | ✓ (.)3 | ✓ (+)15 | ✓ (+)15 | ✓ (–) | ✓ (.)16 | ✓ (.)7 | ✓ (.)7 | ✓ (.)7 | ✓ (–) | |||||
| Kunto and Bras, 2019 (149) | Berry Index (BI) | ✓ (+) | ✓ (+) | ✓ (.) | ✓ (.) | ✓ (.) | ✓ (.) | ||||||||||
| Liu et al., 2020 (150) | American Heart Association Score (AHAS) | ✓ (+) | ✓ (+) | ✓ (+)18 | ✓ (+)15 | ✓ (+)15 | ✓ (+) | ✓ (–)19 | ✓ (–) | ||||||||
| No. of studies using this component in this indicators subgroup | 3 | 12 | 12 | 8 | 8 | 3 | 3 | 9 | 6 | 9 | 9 | 5 | 5 | 6 | 7 | 1 | |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets the higher score when it fits within a specific range or the score is unspecified. DQ, diet quality; FF, fast food; SSBs, sugar-sweetened beverages.
Beverages components.
Whole grains as single component.
Includes potatoes.
Butter and margarine.
Meat, poultry, and sausage.
In the same component.
Includes juices.
Bread as single component.
Salted and smoked meat as single component.
Mayonnaise.
Milk.
Includes fresh, canned, and dried fruit.
Includes whole grain.
Plant proteins.
Includes whole-fat and skim milk and soy drinks.
Includes sausage.
Only tubers.
Processed meat.
TABLE 3a.
Nutrient components included in the Healthy Eating Indexes diet quality indicator1
| Macronutrients | Mineral | |||||||
|---|---|---|---|---|---|---|---|---|
| Authors, publication year (Ref) | Indicator | Sugar | Alcohol | Total fat | Saturated fat | Cholesterol | Trans fat | Sodium |
| Kennedy et al., 1995 (30) | Healthy Eating Index (HEI) | ✓ | ✓ | ✓ | ✓ | |||
| Pinheiro and Atalah, 2005 (33) | Healthy Eating Index Chile (HEI-CHL) | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Glanville and McIntyre, 2006 (34) | Healthy Eating Index Canada 2006 (HEI-C06) | ✓ | ✓ | ✓ | ✓ | |||
| Guenther et al., 2008 (35) | Healthy Eating Index 2005 (HEI-2005) | ✓ | ✓ | ✓ | ✓ | |||
| Garriguet, 2009 (36) | Healthy Eating Index Canada modification (HEI-C-mod) | ✓ | ✓ | ✓ | ✓ | |||
| De Andrade et al., 2010 (37) | Healthy Eating Index Brazil (HEI-BRA14) | ✓ | ✓ | ✓ | ||||
| Woodruff et al., 2010 (38) | Healthy Eating Index Canada (HEIC-2009) | ✓ | ✓ | ✓ | ||||
| Rydén and Hagfors, 2011 (39) | Healthy Eating Index 2005 (HEI-2005) | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| González Rosendo et al., 2012 (40) | Healthy Eating Index Mexico (HEI-MEX) | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| He et al., 2012 (41) | Healthy Eating Index 2005 Canadian modification (HEIC-2005) | ✓ | ✓ | ✓ | ||||
| Guenther et al., 2013 (42) | Healthy Eating Index 2010 (HEI-2010) | ✓ | ✓ | ✓ | ||||
| Kyttälä et al., 2014 (44) | Finnish Children Healthy Eating Index (FCHEI) | ✓ | ||||||
| Rauber et al., 2014 (45) | Healthy Eating Index Brazil (HEI-BRA14) | ✓ | ✓ | ✓ | ✓ | |||
| Dahm et al., 2016 (47) | High School Alternative Healthy Eating Index (HS-AHEI) | ✓ | ✓ | |||||
| Tugault-Lafleur et al., 2017 (48) | School Healthy Eating Index (School-HEI) | ✓ | ✓ | ✓ | ||||
| Yuan et al., 2017 (49 ) | Healthy Eating Index China (HEI-CHN) | ✓ | ✓ | ✓ | ||||
| Conceição et al., 2018 (50) | Healthy Eating Index Brazil Infants (HEI-BRA-I) | ✓ | ✓ | ✓ | ✓ | |||
| Nshimyumukiz et al., 2018 ( 52) | Healthy Eating Index Canada (HEI-C-2010) | ✓ | ✓ | ✓ | ||||
| Reedy et al., 2018 (53) | Healthy Eating Index 2015 (HEI-2015) | ✓ | ✓ | ✓ | ||||
| Brownlee et al., 2019 (54) | Healthy Eating Index Singapur (HEI-SGP) | ✓ | ✓ | ✓ | ✓ | |||
| No. of studies using this component in this indicators subgroup | 13 | 4 | 11 | 16 | 7 | 1 | 18 | |
Check symbol denotes that a component is included in the indicator.
TABLE 3g.
Nutrient components included in the Other Healthy Diet Indexes diet quality indicator1
| Authors, publication year (Ref) | Macronutrients | Vitamins | Minerals | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indicator | Protein | Carbohydrates | Fiber | Sugar | Total fat | Unsaturated fat | SFA | PUFA:SFA | A | C | B2 | D | Folate | Calcium | Zi nc | Sodium | Potassium | Iron | Selenium | Phosphorus | Copper | Iodine | |
| Haapala et al., 2015 (144) | Baltic Sea Diet Score (BSDS) | ✓ | ✓ | ✓ | |||||||||||||||||||
| Hardiansyah et al., 2015 (145) | Balanced Diet Index (BDI) | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Cheng et al., 2016 (146 ) | Chinese Children Dietary Index (CCDI) | ✓ | ✓ | ✓ | ✓ | ||||||||||||||||||
| Verger et al., 2016 (147 ) | Diet Quality Index Based on the Probability of Adequate Nutrient Intake (PANDiet) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Liu et al., 2020 (150) | American Heart Association Score (AHAS) | ✓ | ✓ | ||||||||||||||||||||
| No. of studies using this component in this indicators subgroup | 1 | 1 | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | |
SFA, Saturated Fatty Acids; PUFA, Polyunsaturated Fatty Acids.
Check symbol denotes that a component is included in the indicator.
Vitamin B: thiamin, riboflavin, niacin, B6, and B12.
TABLE 2b.
Food group components included in the Dietary Diversity Scores diet quality indicator1
| Authors, publication year (Ref) | Indicator | Vegetables | Fruits | Cereals | Legumes | Nuts | Fats | Dairy | Eggs | Fish | Meat | Snacks | Sweets | SSBs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arimond and Ruel, 2004 (55) | Dietary Diversity Score (DDS04) | ✓ (+)2 | ✓(+)3, 4 | ✓ (+)5 | ✓ (+) | ✓ (+)6 | ✓ (+)7 | ✓ (+)8 | ✓ (+)8 | ✓ (+)8, 9 | ||||
| Mirmiran et al., 2004 (56) | Dietary Diversity Score Iran (DDS-IRN) | ✓ (+)3 | ✓ (+)3, 4 | ✓ (+) | ✓ (+) | ✓ (+)8 | ✓ (+)8 | ✓ (+)8, 9 | ||||||
| Kennedy et al., 2007 (57) | Dietary Diversity Score Filipinas (DDS-PHI) | ✓ (+)3 | ✓ (+)3 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)8 | ✓ (+)8, 9 | |||
| Mpontshane et al., 2008 (58 ) | Dietary Diversity Score South Africa (DDS-ZAF) | ✓ (+)10 | ✓ (+)10 | ✓ (+)11 | ✓ (+) | ✓ (+)12 | ✓ (+)8 | ✓ (+)8 | ✓ (+)8 | ✓ (–)13 | ✓ (–)13 | ✓ (–)13 | ||
| Enneman et al., 2009 (60) | Dietary Diversity Score Guatemala USAID (DDS-GT-US-CP-INCAP) | ✓ (+)2, 3 | ✓ (+)3 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–) | ✓ (–) | ✓ (–) |
| Kennedy et al., 2010 (61) | Individual Dietary Diversity Score (IDDS) | ✓ (+)2, 3 | ✓ (+)3 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+)8 | ✓ (+)8, 14 | ||||
| Li et al., 2011 (62) | Dietary Diversity Score China (DDS-CHN10) | ✓ (+)2 | ✓ (+) | ✓ (+)5 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | |||||
| Belachew et al., 2013 (63) | Dietary Diversity Score Ethiopia (DDS-ETH) | ✓ (+)2, 3 | ✓ (+)3 | ✓ (+) | ✓ (+)8 | ✓ (+)8 | ✓ (+)6 | ✓ (+)16 | ✓ (+)8 | ✓ (+)8 | ✓ (+)8, 9 | ✓ (–) | ||
| Darapheak et al., 2013 (64) | Dietary Diversity Score WHO modification (DDS-WHOm) | ✓ (+)2, 3 | ✓ (+)3 | ✓ (+)5 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)8 | ✓ (+)8, 14 | ||||
| Gewa et al., 2014 (65) | Dietary Diversity Score Kenya (DDS-KEN) | ✓ (+)2, 3, 15 | ✓ (+)3 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+)8 | ✓ (+)8 | ✓ (+) 8, 9 | ||||
| Mundo-Rosas et al., 2014 (66) | Dietary Diversity Score Mexico (DDS-MX) | ✓ (+)3 | ✓ (+)3 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)3 | ✓ (+)8 | ✓ (+)8, 9 | ✓ (–) | ✓ (–) | ||
| Amugsi et al., 2015 (67) | Dietary Diversity Score Ghana (DDS-GHA) | ✓ (+)2, 3 | ✓ (+)3 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–) | ||
| Ali and Abizari, 2018 (68) | Dietary Diversity Score Ghana 2018 (DDS-GHA18) | ✓ (+) 2, 3 | ✓ (+)3 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)14 | |||
| Meng et al., 2018 (69 ) | Dietary Diversity Score China (DDS-CHN18) | ✓ (+)17 | ✓ (+)4 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–) | ✓ (–) | ✓ (–) | |
| Morseth et al., 2018 (70) | Dietary Diversity Score (DDS-NPL17) | ✓ (+)3 | ✓ (+)3 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ||||||
| Iqbal et al., 2019 (71) | Dietary Diversity Score Bangladesh (DDS-BGD) | ✓ (+)3 | ✓ (+)3 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–) | ✓ (–) | ✓ (–) | ||
| Thorne-Lyman et al., 2019 (72) | Individual Dietary Diversity Score (IDDS) | ✓ (+)3 | ✓ (+)3 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | |||
| Yang et al., 2019 (73) | Dietary Diversity Score China 2019 (DDS-CHN19) | ✓ (+)2 | ✓ (+) | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)9 | ||||
| Sebayang et al., 2020 (74) | Dietary Diversity Score Indonesia (DDS-IDN) | ✓ (+)3 | ✓ (+)3 | ✓ (+)5 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (+) | |||||
| No. of studies using each component in this indicator subgroup | 19 | 19 | 19 | 16 | 12 | 9 | 17 | 19 | 16 | 19 | 4 | 6 | 6 | |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets the higher score when it fits within a specific range or the score is unspecified. DQ, diet quality; SSBs, sugar-sweetened beverages.
Green leafy vegetables as separate component.
Vitamin A–rich group as separate component.
Fruit juices.
Cereal, tubers, and roots.
Oil and butter or fat.
Dairy other than breast milk, cheese, or yogurt.
In the same component as meat or protein.
Including meat and poultry.
In the same component.
Bread, cereal porridge, and maize meal.
Breast milk and formula milk are included as a separate component.
In the same component as snacks.
Including organ meat.
Vitamin C–rich group as a separate component.
Milk and cheese.
Vegetable juices.
TABLE 2c.
Food group components included in the Diet Quality Indexes diet quality indicator1
| Authors, publication year (Ref) | Indicator | Water | Vegetables | Fruits | Juice | Cereals | Legumes | Nuts | Fats | Dairy | Eggs | Fish | Meat | Snacks | FF | Sweets | SSBs | Alcohol |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alexy et al., 2003 (75) | Diet Quality Index Germany (DQI-DEU) | ✓ (+) | ||||||||||||||||
| Kranz et al., 2004 (76) | Diet Quality Index for Children (C-DQI) | ✓ (+)2 | ✓ (+)2 | ✓ (+) | ✓ (+) | ✓ (.) | ||||||||||||
| Mariscal-Arcas et al., 2007 (77) | Diet Quality Index International adaptation (DQI-Ia) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)3 | ✓ (+)4 | ✓ (.)3 | ✓ (+)3 | ✓ (+)3 | ✓ (+)3, 5 | ✓ (–)4 | ✓ (–)4 | ✓ (–)4 | |||||
| McArthur et al., 2008 (78) | Rapid Assessment Diet Quality Index (RADQI) | ✓ (+)6 | ✓ (+)7 | ✓ (+)8 | ✓ (+)9 | ✓ (.)10 | ✓ (+)2 | ✓ (+)2, 11 | ✓ (–) | ✓ (–) | ✓ (–)8 | |||||||
| Huybrechts et al., 2010 (79) | Diet Quality Tool for Flemish Preschoolers (DQT-FP) | ✓ (+)12 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)13 | ✓ (.)14 | ✓ (+)2 | ✓ (+)2, 15 | ✓ (–) | ✓ (–) | ✓ (–) | ||||||
| De Vriendt et al., 2012 (80) | Diet Quality Index for Adolescents (DQI-A) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–)8 | ✓ (+)13 | ✓ (+) | ✓ (.)16 | ✓ (+)2 | ✓ (+)2 | ✓ (+)17 | ✓ (–)4 | ✓ (–)4 | ✓ (–)8 | ✓ (–)8 | |||
| Fokeena and Jeewon, 2012 (81) | Diet Quality Index Mauritius (DQI-MUS) | ✓ (+)18 | ✓ (+) | ✓ (+)19 | ✓ (+) | ✓ (.)10 | ✓ (+)3 | ✓ (+)3 | ✓ (–)2 | ✓ (–)2 | ||||||||
| Li et al., 2012 (82) | Diet Quality Index Australian Children and Adolescents (DQI-AUS-CA) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)3 | ✓ (+)3 | ✓ (+)3 | ✓ (+)3 | ✓ (+)3 | ✓ (+)3, 11 | ✓ (–)2 | ✓ (–)2 | ✓ (–)2 | ✓ (–)2 | ||||
| Bel et al., 2013 (83) | Diet Quality Index for Adolescents with Meal Index (DQI-AM) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)13 | ✓ (+)16 | ✓ (+)3 | ✓ (+)3, 17 | ✓ (–)2 | ✓ (–)2 | ✓ (–)2 | |||||||
| Vyncke et al., 2013 (84) | Diet Quality Index for Adolescents 2013 (DQI-A-13) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)13 | ✓ (+) | ✓ (+)16 | ✓ (+)3 | ✓ (+)3 | ✓ (+)3 | ||||||||
| Wong et al., 2013 (85) | Diet Quality Index for New Zealand Adolescents (NZDQI-A) | ✓ (+)20, 21 | ✓ (+)20 | ✓ (+) | ✓ (+) | ✓ (+)3 | ✓ (+)3 | ✓ (+)3, 11, 17 | ||||||||||
| Röytiö et al., 2015 (86) | Diet Quality Index for Finland Children (FINDQI-C) | ✓ (+) | ✓ (+) | ✓ (+)22 | ||||||||||||||
| Collins et al., 2016 (87) | Revised Children's Diet Quality Index Australia (RC-DQI-AUS) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)19 | ✓ (+) | ||||||||||||
| Kunaratnam et al., 2018 (89) | Diet Quality Index for Australian Preschoolers (DQI-AusP) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)23 | ✓ (–)24 | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–) | |||||||
| Hamner and Moore, 2020 (90) | Diet Quality Index Score for American Infants (DQIS-USIn) | ✓ (+)2 | ✓ (+) | ✓ (+) | ✓ (+)19 | ✓ (+)2 | ✓ (+)23, 25 | ✓ (.)3 | ✓ (.)3 | ✓ (.)3 | ✓ (–) | ✓ (–) | ✓ (–) | |||||
| No. of studies using each component in this indicator subgroup | 6 | 14 | 14 | 7 | 13 | 3 | 1 | 4 | 13 | 6 | 10 | 11 | 8 | 2 | 9 | 8 | 2 | |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets the higher score when it fits within a specific range or the score is unspecified. DQ, diet quality; FF, fast food; SSBs, sugar-sweetened beverages.
Classified in the same component.
As protein component.
As empty calorie food component or low-nutrient energy dense.
Cold meats and pate considered as empty-calorie food.
Including canned vegetables and starchy vegetables such as potatoes.
Including canned fruits.
As low-nutrient energy-dense drinks.
Including breakfast cereal.
Whole milk and low-fat milk.
Meat and poultry.
Including all drinks except drinks from other mentioned groups.
Bread and cereals as one component and potatoes and grains as another component.
Milk, yogurt, milk desserts, and soy drinks.
Including meat, poultry, and game.
Milk, yogurt, and milk desserts as one component, and cheese as another component.
Including vegetarian substitutes.
Raw and cooked vegetables as separate components.
Whole grains and refined cereals as separate components.
Fresh, frozen, and canned.
Including potatoes.
Whole grains.
Milk.
Processed meat.
Includes breastfeeding or formula feeding.
TABLE 2d.
Food group components included in the Food Variety Scores diet quality indicator1
| Authors, publication year (Ref) | Indicator | Cereals | Vegetables | Fruits | Dairy | Meat | Fish | Eggs |
|---|---|---|---|---|---|---|---|---|
| Cox et al., 1997 (91) | Variety Index for Toddlers (VIT) | ✓ (.)2 | ✓ (.)3 | ✓ (.)4 | ✓ (.)5 | ✓ (.)6 | ✓ (.)6 | |
| Vereecken et al., 2008 (93) | Variety Index | ✓ (.)7 | ✓ (.) | ✓ (.) | ✓ (.)8 | ✓ (.)6 | ||
| Saibul et al., 2009 (95) | Food Variety Score (FVS) | ✓ (.)9 | ✓ (.) | ✓ (.) | ✓ (.)10 | ✓ (.)11 | ✓ (.) | ✓ (.)11 |
| Scott et al., 2012 (96) | Core Food Variety Score (CFVS) | ✓ (.)12 | ✓ (.)13 | ✓ (.) | ✓ (.) | ✓ (.)14 | ✓ (.)14 | ✓ (.)14 |
| Jones et al., 2015 (98) | Healthy Plate Variety Score (HPVS)15 | ✓ (.)2 | ✓ (.)15 | ✓ (.)4 | ✓ (.)5 | ✓ (.)6 | ✓ (.)6 | |
| Barros et al., 2019 (101) | Dietary Variety Index (DVI) | ✓ (.)16 | ✓ (.) | ✓ (.) | ✓ (.) | ✓ (.)17 | ✓ (.)17 | ✓ (.)17 |
| No. of studies using each component in this indicator subgroup | 6 | 6 | 6 | 6 | 5 | 3 | 6 | |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets the higher score when it fits within a specific range or the score is unspecified. DQ, diet quality.
Bread, cereal, pasta, rice, crackers, and sweets.
Vegetables, processed or cooked; potatoes; vegetables, raw leafy and whole raw.
Fruit, large raw (apple, banana); fruit, small raw (apricot, plum); and fruit, processed (applesauce).
Milk, yogurt, pudding, cheese, natural, and ice cream.
Meat, eggs, peanut butter, beans, and nuts.
Brown bread.
Whole-fat milk, semi-skimmed milk, cheese, and other milk products.
Rice/rice porridge, wet noodles, rice vermicelli, instant noodles, traditional pancake, white bread, filled bun, unleavened bread, corn, crackers/biscuits, tapioca, sweet potato, yam, potato, traditional cakes.
Chocolate milk (ultra-high-temperature), powdered full-cream milk, soy milk, and sweet condensed milk.
Beef, chicken, egg, pork.
Breakfast cereals, breads and rolls, rice and pasta, crackers, pretzels, rice cakes, cereal or granola bars, pizza/savory pastry/pies, and other grains and grain products.
Vegetables, potatoes, and French fries/hot chips.
Meat and other nondairy protein sources (eggs, nuts and seeds, dried beans and peas, vegetarian meat substitutes, red meat, chicken or turkey, fish and shellfish, hot dogs, sausages, cold cuts, offal, and other unspecified meats).
Cakes, biscuits/cookies, puddings, and fried potatoes were excluded from the VIT.
Bread, cookies, rice, pasta, and potatoes.
Eggs, white meats, meat, pork products, and fish.
TABLE 2e.
Food group components included in the Healthy and Unhealthy Scores diet quality indicator1
| Authors, publication year (Ref) | Indicator | Vegetables | Fruits | Cereals | Legumes | Dairy | Eggs | Fish | Meat | Snacks | FF | Sweets | SSBs | Coffee |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jacka et al., 2011 (103) | Unhealthy Food Choices Score (UFCS) | ✓ (+)2 | ✓ (+) | ✓ (.)3 | ✓ (+)4 | ✓ (–) | ✓ (–) | ✓ (-) | ✓ (–) | ✓ (.) | ||||
| Truthmann et al., 2012 (104) | Healthy (HDS-A) and unHealthy Diet Scores Australia (uHDS-A) | ✓ (+) | ✓ (+) | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–) | |||||||
| Monjardino et al., 2014 (105) | Healthy Eating Index Score Portugal (HEIS-PRT) | ✓ (+)2 | ✓ (+) | ✓ (+)6 | ✓ (+)7 | ✓ (+)7 | ✓ (.)7, 8 | ✓ (–) | ✓ (–)8 | ✓ (–) | ✓ (–)9 | |||
| Vilela et al., 2014 (106) | Indicator Food Index (IFI) | ✓ (+) | ✓ (+) | ✓ (+)5 | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–) | ||||||
| Anderson et al., 2015 (107) | Pro-Healthy Diet Index (pHDI) and Non-Healthy Diet Index (nHDI) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–) | ✓ (–) | ✓ (–)11 | ||||||
| Anderson et al., 2016 ( 108) | Healthy (HDS-USA15) and unHealthy Diet Scores USA 2015 (uHDS-USA15) | ✓ (+) | ✓ (+)12 | ✓ (.) | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–) | ||||||
| Sluik et al., 2016 (109 ) | Healthy (HDS-USA16) and unHealthy Diet Scores USA 2016 (uHDS-USA16) | ✓ (+) | ✓ (+) | ✓ (+)13 | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–) | ||||||
| Arvidsson et al., 2017 (110) | Dutch Healthy Diet index (DHD-index) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–) | |||||||||
| Arvidsson et al., 2017 (110) | Healthy Dietary Adherence Score (HDAS) | ✓ (+) | ✓ (+) | ✓ (+)14 | ✓ (+) | |||||||||
| Martins et al., 2019 (111) | Healthy (HDS-BRA) and unHealthy (uHDS-BRA) Diet Scores Brazil | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–) | ||||||
| Wadolowska et al., 2019 (112) | Oslo Health Study Dietary Index (OHS) | ✓ (+)10 | ✓ (+)10 | ✓ (–) | ||||||||||
| No. of studies using each component in this indicators subgroup | 11 | 11 | 3 | 1 | 5 | 1 | 4 | 1 | 7 | 7 | 8 | 10 | 1 | |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets the higher score when it fits within a specific range or the score is unspecified. DQ, diet quality; FF, fast food; SSBs, sugar-sweetened beverages.
Includes raw and cooked vegetables.
Whole and white bread.
Whole-fat and low-fat milk.
Whole bread.
Semi-skimmed milk, skimmed milk, cheese, and yogurt.
Part of the meat component.
Red meat and white meat.
Part of the snack component.
Fresh, canned, or juice.
Includes carbonated drinks and energy drinks.
Juice.
Milk.
Whole meal.
TABLE 2f.
Food group components included in the Feeding and Eating Indexes diet quality indicator1
| Authors, publication year (Ref) | Indicator | Water | Vegetables | Fruits | Cereals | Legumes | Nuts | Fats | Dairy | Eggs | Fish | Meat | Snacks | Sweets | SSBs | Coffee/tea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arimond and Ruel, 2002 (113) | Infant and Child Feeding Index (ICFI) | ✓ (+)2 | ✓ (+)2 | ✓ (+)3 | ✓ (+) | ✓ (.) | ✓ (+)4, 5 | ✓ (+)5 | ✓ (+)5 | ✓ (.)5, 6 | ||||||
| Ruel and Menon, 2002 (114) | Child Feeding Index (CFI) | ✓ (+) | ✓ (+)7 | ✓ (+)8 | ✓ (+)8 | ✓ (.) | ||||||||||
| Dewey et al., 2006 (115) | Food Group Indicator–8 (FGI-8) | ✓ (+)9 | ✓ (+)9 | ✓ (+)3 | ✓ (+)10 | ✓ (+)10 | ✓ (.) | ✓ (.) | ✓ (+) | ✓ (+)5 | ✓ (.)5, 6 | ✓ (–) | ||||
| Food and Nutrition Technical Assistance Project, 2006 (116) | Food Group Indicator (FGI-7) | ✓ (+)9 | ✓ (+)9 | ✓ (+)3 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+)5 | ✓ (+)5 | ✓ (.)5, 6 | ||||||
| Bork et al., 2012 (117) | Infant and Child Feeding Index Senegal (ICFI-Sen) | ✓ (+)9 | ✓ (+)9 | ✓ (+)3 | ✓ (+)10 | ✓ (+)10 | ✓ (.) | ✓ (+)7 | ✓ (+)5 | ✓ (+)5 | ✓ (.)5, 6 | |||||
| Golley et al., 2012 (118) | Complementary Feeding Utility Index (CFUI) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)5 | ✓ (+)5 | ✓ (.)5 | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–) | ||||
| Jones, 2015 (119) | Infant and Child Feeding Index modification (ICFIm) | ✓ (+)9 | ✓ (+)9 | ✓ (+)3 | ✓ (+)10 | ✓ (+)10 | ✓ (+) | ✓ (+)5 | ✓ (+)5 | ✓ (.)5,6 | ||||||
| Monterrosa et al., 2015 (120) | Infant and Child Feeding Index Mexico | ✓ (+) | ✓ (+) | ✓ (+)3 | ✓ (+)10 | ✓ (+)10 | ✓ (.)5 | ✓ (+) | ✓ (+)5 | ✓ (.)5 | ||||||
| Delshad et al., 2019 (121) | Diet Index for a Child's Eating (DICE) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)11 | ✓ (+)10 | ✓ (+)10 | ✓ (+)10 | ✓ (+)10 | ✓ (+)10 | ✓ (.)10 | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–) | |
| Ferreira et al., 2019 (122) | Child Feeding Index modification (CFIm) | ✓ (+) | ✓ (+) | ✓ (+)3 | ✓ (+) | ✓ (+)10 | ✓ (+)10 | ✓ (.)10 | ||||||||
| No. of studies using this component in this indicators subgroup | 1 | 9 | 9 | 10 | 8 | 6 | 3 | 9 | 10 | 10 | 10 | 2 | 2 | 3 | 2 | |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets the higher score when it fits within a specific range or the score is unspecified. DQ, diet quality; SSBs, sugar-sweetened beverages.
Includes juices.
Grains, roots, and tubers.
Yogurt.
Animal protein component.
Meat and poultry.
Milk.
Foods in the same component; includes poultry.
Vitamin A–rich food.
In the same component.
Whole-grain products as different component.
TABLE 2g.
Food group components included in the Diet Quality Scores diet quality indicator1
| Authors, publication year (Ref) | Indicator | Water | Vegetables | Fruits | Cereals | Legumes | Fats | Dairy | Eggs | Fish | Meat | Snacks | FF | Sweets | SSBs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crombie et al., 2009 (123) | Scottish Diet Quality Score (SDQS) | ✓ (+)2 | ✓ (+)2 | ✓ (+)3 | ✓ (+)4 | ✓ (+)5 | ✓ (+)5, 6 | ✓ (–)7 | ✓ (–)7 | ||||||
| Kohlboeck et al., 2012 (124) | German Optimized Mixed Diet Quality Score (GOMDQS) | ✓ (+) | ✓ (+) | ✓ (+)8 | ✓ (.) | ✓ (.) | ✓ (.) | ✓ (+) | ✓ (.)6 | ✓ (–) | ✓ (–) | ✓ (–) | |||
| Perry et al., 2015 (125) | Diet Quality Score Ireland (DQS-IRL) | ✓ (+)9 | ✓ (+)10 | ✓ (+) | ✓ (+) | ✓ (.)11 | ✓ (.) | ✓ (+) | ✓ (.)12 | ✓ (–) | ✓ (–) | ✓ (–) | ✓ (–)13 | ||
| Voortman et al., 2015 (126) | Diet Quality Score The Netherlands (DQS-NL) | ✓ (+) | ✓ (+) | ✓ (+)2, 8 | ✓ (+)2 | ✓ (.) | ✓ (.) | ✓ (.)5 | ✓ (+) | ✓ (.)5, 14 | ✓ (–) | ✓ (–) | ✓ (–) | ||
| Gasser et al., 2017 (127) | Dietary Quality Score Australia 2017 (DQS-AUS17) | ✓ (+) | ✓ (+)10 | ✓ (+) | ✓ (.)15 | ✓ (–)2 | ✓ (–)2 | ✓ (–) | ✓ (–) | ||||||
| No. of studies using this component in this indicators subgroup | 2 | 5 | 5 | 4 | 1 | 2 | 5 | 3 | 4 | 4 | 5 | 2 | 5 | 4 | |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets the higher score when it fits within a specific range or the score is unspecified. DQ, diet quality; FF, fast food; SSBs, sugar-sweetened beverages.
In the same component.
Includes breakfast cereal and potatoes.
All milk was included irrespective of fat content or type.
In the meat component.
Includes meat and poultry, processed and nonprocessed.
In the same component as snacks.
Bread and cereals in one component; potatoes, pasta, and rice in another component.
Includes juice.
Cooked and raw vegetables.
Different components for full-cream milk, skimmed milk, cheese and yogurt, and low-fat cheese and yogurt.
Meat and poultry in one component; meat pie sausage in another component.
Includes diet soft drinks.
Includes meat substitutes.
Full-cream milk and products, skimmed milk and products, and soya drink and products.
TABLE 2h.
Food group components included in the Nutritional Adequacy and Micronutrients Indexes diet quality indicator1
| Authors, publication year (Ref) | Indicator | Vegetables | Fruits | Cereals | 50% whole grains | Legumes | 50% legumes, micronutrient dense | Dairy | Fats | Fried foods | Variety | Fermented foods/salads | Sugar | Tea/coffee with meals |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chiplonkar and Tupe, 2010 (130) | Adolescent Micronutrient Quality Index (AMQI) | ✓ (+)2 | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+)3 | ✓ (+) | ✓ (.) | ✓ (–) | ✓ (+)4 | ✓ (+) | ✓ (.) | ✓ (–) |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets the higher score when it fits within a specific range or the score is unspecified. DQ, diet quality.
Green leafy, roots and tubers, and other vegetables.
Micronutrient-dense legumes (e.g., chick peas, moth beans, or lentils).
Food variety based on all subgroups and weekly variety in vegetables and fruits.
TABLE 2i.
Food group components included in the Dietary Guidelines Indexes diet quality indicator1
| Authors, publication year (Ref) | Indicator | Fruits | Vegetables | Cereals | Whole-grain cereals | Meat, alternatives | Dairy | Fish | Reduced-fat dairy | Beverages | Extra foods | Healthy fats | Legumes | Eggs | Dietary variety | SFI | Oil | Dessert | Snacks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Golley et al., 2011 (133) | Dietary Guideline Index for Children and Adolescents (DGI-CA) | ✓ (.)2 | ✓ (.)3 | ✓ (.)4 | ✓ (.)4 | ✓ (.)5 | ✓ (.) | ✓ (.)5 | ✓ (.) | ✓ (.) | ✓ (.)6 | ✓ (.)7 | ✓ (.)3 | ✓ (.)8 | |||||
| Lioret et al., 2014 (134) | Dietary Guideline Index (DGI) | ✓ (+) | ✓ (+)3 | ✓ (+)4 | ✓ (+)4 | ✓ (+)5 | ✓ (+)9 | ✓ (+)9 | ✓ (+)9 | ✓ (–)10 | ✓ (+)11 | ||||||||
| Mohseni-Takalloo et al., 2016 (135) | Dietary Guidelines for Americans Adherence Index (DGAI) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (+) | ✓ (–)12 | ✓ (–)13 | ✓ (–)12 | ✓ (-) | ||||||||||
| Rohde et al., 2017 (136) | Diet Quality Index based on the Danish national guidelines (DQI-Danish national guidelines) | ✓ (+)14 | ✓ (+)14 | ✓ (+)15 | ✓ (+) | ||||||||||||||
| Er et al., 2018 (137) | Children's Food Trust guidelines (CFT guidelines) and NAP SACC UK Nutrition Best Practice Standards score | ✓ (+)14 | ✓ (+)14 | ✓ (+)16 | ✓ (+)17 | ✓ (+)17 | ✓ (-)18 | ✓ (+)17 | ✓ (+)17 | ✓ (–)19 | ✓ (–)20 | ||||||||
| No. of studies using this component in this indicators subgroup | 5 | 5 | 5 | 3 | 4 | 3 | 3 | 2 | 3 | 2 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | |
Check symbol denotes that a component is included in the indicator. +, the component is scored positively or as improving the DQ; –, the component is scored negatively or as decreasing the DQ; dot, the component gets the higher score when it fits within a specific range or the score is unspecified. DQ, diet quality; SFI, saturated fat intake.
Maximum 125 mL 100% fruit juice.
Including nonfried potatoes and legumes.
Eat plenty of cereals (including breads, rice, pasta, and noodles), preferably whole grain.
Meat alternatives (excluding processed meats).
Choose foods low in salt; consume only moderate amounts of foods containing sugar; moderate total fat.
Healthy fats, oils, nuts, and seeds: kilojoules from healthy fats as a proportion of total fats and oils.
Sum of food types within core food groups over 2 d (0.5 minimum serving).
Preference for low-fat dairy products.
Potatoes cooked in fat, crisps, confectioneries, cakes, and sweet biscuits, savory pastries, fast foods, pizzas, meat products, flavored milks, soft drinks, and fruit juices.
Saturated fat intake: trimming of fat from meat.
Includes in the same component meats and legumes.
Milk.
Fruit and vegetables in the same component.
Potatoes, rice, or pasta (whole-grain bread not included).
Starchy foods (bread, crackers, pizza, rice, pasta, high-fiber cereals, and other cereals; exclude, croissant, cakes, chips, and sugar-coated cereal).
Meat, fish, eggs, beans, and other nondairy sources of protein.
Drinking chocolate, fizzy drink, squash, fruit drink diet, and low-calorie drink.
Yogurt or fromage frais, fruit salad (tinned or fresh), mousse, milk puddings (e.g., rice pudding), ice cream, frozen dessert, cream, custard, cakes, buns, sponge pudding, sweet pies, tarts, and crumbles.
High-sugar or high-fat snacks (crisps, savory snacks, cereal bars, biscuits, cakes, ice cream, frozen dessert, cream, custard mousse, puddings, buns, sponge pudding, sweets, toffees, mints, and chocolate bars).
TABLE 3b.
Nutrient components included in the Diet Quality Indexes diet quality indicator1
| Macronutrients | Vitamins | Minerals | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, publication year (Ref) | Indicator | Pro | Fiber | Sugar | TF | UF | SF | Cholesterol | Fatty acid ratio | Empty calories | Macro-nutrient ratio | C | E | A | B1 | Folate | Calcium | Iron | Sodium |
| Alexy et al., 2003 (75) | Diet Quality Index Germany (DQI-DEU) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Kranz et al., 2004 (76) | Diet Quality Index for Children (C-DQI) | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Mariscal-Arcas et al., 2007 (77) | Diet Quality Index International adaptation (DQI-Ia) | ✓ | ✓ | ✓ | ✓2 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Li et al., 2012 (82) | Diet Quality Index Australian Children and Adolescents (DQI-AUS-CA) | ✓ | ✓ | ✓ | ✓ | ✓3 | ✓ | ✓ | ✓ | ✓ | |||||||||
| Röytiö et al., 2015 (86) | Diet Quality Index for Finland Children (FINDQI-C) | ✓ | ✓4 | ✓ | ✓ | ||||||||||||||
| Collins et al., 2016 (87) | Revised Children's Diet Quality Index Australia (RC-DQI-AUS) | ✓ | ✓2 | ||||||||||||||||
| No. of studies using this component in this indicators subgroup | 2 | 3 | 2 | 5 | 3 | 4 | 2 | 2 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 4 | 4 | 1 | |
Pro, protein; TF, Total Fat, UF, Unsaturated Fat; SF, Saturated Fat.
Check symbol denotes that a component is included in the indicator.
Linoleic acid, linolenic acid, DHA + EPA.
Omega-6: omega-3.
MUFAs and PUFAs.
TABLE 3c.
Nutrient components included in the Healthy and Unhealthy Scores diet quality indicator1
| Macronutrients | Minerals | |||||||
|---|---|---|---|---|---|---|---|---|
| Authors, publication year (Ref) | Indicator | Fiber | Sugar | Alcohol | Total fat | Saturated fat | Trans fat | Sodium |
| Sluik et al., 2016 ( 109) | Dutch Healthy Diet index (DHD-index) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Arvidsson et al., 2017 (110) | Healthy Dietary Adherence Score (HDAS) | ✓ | ✓ | |||||
| No. of studies using this component in this indicators subgroup | 1 | 2 | 1 | 1 | 1 | 1 | 1 | |
Check symbol denotes that a component is included in the indicator.
TABLE 3d.
Nutrient components included in the Feeding and Eating Indexes diet quality indicator1
| Authors, publication year (Ref) | Indicator | Protein |
|---|---|---|
| Golley et al., 2012 (118 ) | Complementary Feeding Utility Index (CFUI) | ✓ |
Check symbol denotes that a component is included in the indicator.
TABLE 3e.
Nutrient components included in the Nutritional Adequacy and Micronutrients Indexes diet quality indicator1
| Authors, publication year (Ref) | Macronutrients | Vitamins | Minerals | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indicator | Protein | Fiber | Added sugar | MUFA | SFA | C | D | E | K | B1 | B2 | B3 | B5 | B6 | B9 | B12 | Calcium | Iron | Zinc | Potassium | Magnesium | Phosphorus | Sodium | |
| Fulgoni et al., 2009 (128) | Nutrient-Rich Foods Index | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Libuda et al., 2009 (129) | Nutrient-based Nutritional Quality Score (NQI) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Alexy et al., 2011 (131) | Nutrient Quality Index (NQI) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| Lee and Park, 2015 (132) | Index of Nutritional Quality (INQ) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||||
| No. of studies using this component in this indicators subgroup | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 4 | 3 | 2 | 1 | 1 | 3 | 2 | 4 | 4 | 3 | 3 | 2 | 2 | 1 | |
MUFA, Monounsaturated Fatty Acids; SFA, Saturated Fatty Acids.
Check symbol denotes that a component is included in the indicator.
TABLE 3f.
Nutrient components included in the Dietary Guidelines Indexes diet quality indicator1
| Macronutrients | Mineral | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Authors, publication year (Ref) | Indicator | Fiber | Added sugar | Fat | Low-fat choices | SFA | Trans fatty acids | Cholesterol | Sodium |
| Mohseni-Takalloo et al., 2016 (135) | Dietary Guidelines for Americans Adherence Index (DGAI) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Rohde et al., 2017 (136) | Diet Quality Index based on the Danish national guidelines (DQI-Danish national guidelines) | ✓ | ✓ | ✓ | |||||
| No. of studies using this component in this indicators subgroup | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
SFA, Saturated Fatty Acids.
Check symbol denotes that a component is included in the indicator.
The number of components in each DQIns varied from 3 to 20. Healthy Eating Indexes, Diet Quality Indexes, Healthy and Unhealthy Indexes, and Dietary Guidelines Indexes included all 3 types of components. Feeding and Eating Indexes, Diet–Lifestyle Indexes, and Nutritional Adequacy and Micronutrients used 2 types of components, where the food groups were present in the possible combinations with the other 2 types of components. Last, Dietary Diversity Scores, Food Variety Scores, and Breakfast Quality Indexes evaluated only food group components.
The scoring system included 3 major issues that need to be considered. First is the scaling procedure between dichotomous (2 categories) or polytomous (>2 categories). Second is the appraisal of cutoff values, which can be calculated through fixed criteria (predefined) or by distribution (population-dependent). Finally, the weighting system is an issue that is not usually considered because in the majority of the indicators, all components contribute the same weight to the overall score. The sum of all the scores of each component of DQIns provided the overall score, which is usually interpreted as the higher the score, the better the DQ.
Types of components included in each DQIn
The food groups, foods, nutrients, and lifestyle-related factor components are shown in Tables 2a–j and 3a–g and Supplemental Tables 3–5. These components were part of the countries’ dietary national guidelines or public health recommendations.
Food groups and food components
The most prevalent food components found were fruits, milk and dairy products, and vegetables; they were mentioned in 116, 104, and 100 DQIns, respectively (Tables 2a–j, Supplemental Table 3). In order of frequency, additional food components found included the following: legumes, fish, eggs, water, and nuts.
Regarding the fruit component, some DQIns included fruit and fruit juices or consider fruit and vegetables in the same component. In the vegetable component, it is noteworthy that most of the indicators also included potatoes; however, many other DQIns included potatoes in the cereal component.
Concerning the dairy component, it is noteworthy that most of the DQIns valued total consumption, without distinction by product type or fat content. Regarding the dairy component, it is notable that in children, it is considered positive in the majority of the DQIns without distinction by product type or fat content. In reference to the components concerning protein-rich foods (meat, fish, eggs, and legumes), some authors have considered all these foods in the same component. However, most of the DQIns considered them individually due to their different effects on health. Most indicators contemplated total meat consumption (including all types of meat and processed meat) as a negative component. Nevertheless, other DQIns included red and white meat or only white meat and value them as positive or negative.
Moreover, food components that required moderation in their consumption were identified; the most prevalent were meat, sweets and confectionery items, and sugar-sweetened beverages. These components were mentioned in 91, 49, and 46 indicators, respectively. Other food components that required moderation, in order of frequency, were fats and oils, snacks, juices, fast food, coffee, and alcoholic drinks.
The sweets component sometimes also included bakery products, which at other times are classified in the cereals component. It is noteworthy that in some DQIns, the sweets and snacks components are combined. In numerous cases, the snacks component refers to salty snack products. In other cases, it also includes sweet products.
Nutrient components
Tables 3a–g and Supplemental Table 4 show the nutrient components. This type of component was less frequently used in comparison to the food groups component. The nutrient components were selected by their health-related outcomes. In order of frequency of appearance, the nutrient components found were calcium, iron, fiber, vitamin C, vitamin A, protein, thiamin, folate, zinc, and niacin. Moreover, the most frequently identified nutrients that required moderation to prevent chronic diseases were sodium, total fat, saturated fat, sugar, cholesterol, and trans fats. Fat-related components warrant special note because they are the most frequently negatively scored.
One issue to be noted is that when DQIns use food components, the majority of food groups or items included are those to be scored as positive (e.g., fruits, vegetables); on the contrary, when DQIns considered nutrients as components, the majority listed are those to be scored as negative.
Finally, Supplemental Table 5 shows lifestyle-related factor components. These were less frequently used in comparison with the other types of components (food groups and nutrients).
Scoring methods applied to the DQIns
Supplemental Table 6 shows the essential methodological issues identified in each indicator. Scoring methods for each component regarding the type of cutoff values, the interpretation of the overall score, and other relevant methodological considerations (e.g., description of the dietary assessment instrument used to evaluate the indicator) are described in-depth.
Cutoff values
The cutoff values were classified as predefined (fixed criteria) or population-dependent (by distribution) (169). The cutoff values were established beforehand according to fixed amounts of dietary intake described by several criteria. For food groups and foods, these cutoff points are defined by servings per day (or week) or grams (or milliliters) per day (or week), by the percentage of the frequency of consumption, or by the score according to adherence to dietary guidelines. For nutrients, the percentage of the energy intake per day or the nutritional recommendations—milligrams (nutrient) per day or nutrient density (per 1000 kcal)—were used. Some DQIns considered cutoff values fixed through a variety of components per day or the number of different food items eaten during the registration period.
The population-dependent cutoff values vary based on the distribution of the dietary intake in the studied population, using quantiles. The cutoff points most frequently applied in this approach were medians (or sex- and age-specific medians), tertiles, quartiles, quintiles, or deciles. Other indicator components were calculated as algorithms based on multiple criteria or as the individual harmonic mean of the individual intake.
Dietary assessment methods
The dietary assessment instrument most frequently used was the 24h-DR, followed by the FFQ, the diet record, the weight record, and nutritional surveys. The 24h-DR was used in more than half of the articles reviewed and assessed a single day or multiple days. The FFQ considered different periods (more frequently past month or year), the more frequent are those of a qualitative or semiquantitative approach with between 120 and 170 items. The diet records evaluated at least 3–4 d of dietary intake. Moreover, only 2 articles used a nutritional survey, and 1 article used a weighed dietary record. In addition, in some studies, researchers used 2 dietary assessment methods, usually the 24h-DR and the FFQ.
Other relevant considerations found in the methodology were as follows: the energy adjustment, a trained interviewer or the dietitian's participation in the face-to-face interview or by phone, if the dietary assessment was self-administered, the use of paper or electronic-based tools (computer or smartphone), and the possibility of asking and resolving questions during the evaluation.
A synthesis of the main issues analyzed in this mapping review in terms of components, scoring and statistical methods, and other relevant considerations is shown in Figure 3.
FIGURE 3.
Synthesis of the main issues analyzed in terms of components and scoring methods.
Main features of the subgroups of the DQIns
Overall DQIns
Indicator subgroup: Healthy Eating Indexes
A total of 25 DQIns based on the concept of Healthy Eating Indexes were classified in this subgroup with different modifications (30–54). For this subgroup, 6 population-based studies were found. In general, this group of DQIns was developed based on the most important recommendations of the DGAs (170). These guidelines and the indexes account for the population older >2 y and have been updated every 5 y. Originally, the components included were food groups, nutrients, and variety (30). The score was based on adequacy, moderation, and frequency of consumption. For the food group, higher scores represented higher adequacy. The score for the moderation component is reversed: lower consumption accounted for a higher score. Since 2005, the adequacy and moderation components have been expressed based on energy density, allowing the evaluation of mixed dishes (35). Modifications and updates in the food components have been made from the original Healthy Eating Index to evaluate food and dietary intakes of children and adolescents. This process included evaluating whole cereals, snack foods, sugar-sweetened drinks (31, 43), and fish (47). Moreover, adjustments in food components to assess diet in different settings were found. For instance, in Brazil, the researchers included the beans food group component (45 ).
Indicator subgroup: Dietary Diversity Scores
Twenty-one indicators were collected to assess DQ from a dietary diversity approach (55–74). Diversity is one of the dimensions of DQ and is the desirable consumption of foods or food groups; these indicators are usually used in low-income countries (13). The components of these indicators were the food groups. The scoring system is dichotomous, and it accounted for each item consumed with or without the number of servings or cutoff limits during a given time (171). The food groups selected in each index considered the nutritional aspects and the local food culture (60). For instance, the group of fruits and vegetables were classified as sources of vitamin A, or the meat group included other food sources of protein such as fish or eggs. The primary reference for Dietary Diversity Scores is that proposed by WHO, the minimum dietary diversity with 7 food groups: 1) grains, roots, and tubers; 2) legumes and nuts; 3) dairy (milk, yogurt, and cheese); 4) flesh foods (meat, fish, poultry, and liver/organ meats); 5) eggs; 6) vitamin A–rich fruits and vegetables; and 7) other fruits and vegetables (172).
Indicator subgroup: Diet Quality Indexes
The Diet Quality Indexes subgroup had the third most prevalent indicators found in this mapping review. We found 16 indexes (75–90). All evaluated food group components, excluding 1 publication that evaluated only the nutrient components (75). Different from previous subgroups, Diet Quality Indexes included the assessment of water, fruit juices, and alcohol. Five publications also considered the nutrient components in addition to food (77, 82, 86, 87, 173), and only 3 evaluated the lifestyles component (77, 87, 173). The scoring method varied per component, and each component was weighted. In 4 publications, the score per component considered diversity, quality, equilibrium, and adequacy for each food group (79, 80, 83, 84).
Indicator subgroup: Food Variety Scores
In this mapping review, 11 studies were found in which different indicators were developed or updated to assess DQ by Food Variety Scores (91–101). Dietary variety has been considered a desirable feature of most dietary patterns because a greater variety of foods means a greater likelihood of meeting nutritional requirements (1). For this reason, the development of indicators from the perspective of variety is a fundamental issue for children and adolescents. Variety can be defined from several approaches; one of the most frequent is the number of different food items eaten during the registration period, most often between 60 and 75 items (92, 95, 99). The main foods to assess the DQ according to variety belong to cereals, vegetables, fruits, milk and dairy products, meat, and fish.
Indicator subgroup: Healthy and Unhealthy Scores
A total of 11 indicators were classified in this group, assessing different aspects considered healthy or unhealthy (102–112). The Healthy and Unhealthy Scores are presented independently of each other or in combination. Independent scores are compared to each other by showing their percentage of consumption. In combination, higher scores can indicate better DQ or adherence to dietary guidelines. The components were food groups. The healthy index included food groups such as fruits and vegetables that are considered beneficial for health. The Unhealthy Scores included food groups such as sugar-sweetened drinks that are considered risky for human health. The scores were based on the frequency of consumption of the food groups. DQIns were used to evaluate health-related outcomes such as blood pressure (104), mineral density (105), and mental health (103), or they were used to compare food intake patterns in populations (111).
Indicator subgroup: Feeding and Eating Indexes
In this indicator subgroup, 10 tools were classified that assessed different aspects related to feeding and eating practices (113–122). The Feeding and Eating Indexes assess the DQ through a combination of indicators composed by independent elements. These combinations included food groups that assess diet diversity; diet variety; frequency of consumption; and lifestyle feeding practices such as breastfeeding, baby-bottle use, and food consistency (solid food). Therefore, the scoring system was more complicated. First, each component was scored separately according to its criteria and cutoff points. Then, the score was added together or transformed into a probability function to derive a unit-free component (118 ). Moreover, the score for each component was dependent on the infants’ age. For instance, breastfeeding had a higher score at 6–12 mo, and food frequency of consumption evaluated the consumption of fruits and vegetables separately after 12 mo (122).
Indicator subgroup: Diet Quality Scores
In this mapping review, 5 DQIns were recovered and classified in this subgroup (123–127). The main components of the Diet Quality Scores were the food groups, which were assessed by an FFQ. All the Diet Quality Scores represented different national nutritional guidelines; consequently, the scores had cutoff points dependent on each population under study. The scores account for a positive total. Three indicators had a dichotomous score (123, 124, 126). One indicator scored according to the consumption level of food groups, and if the food group required moderation, a higher score was given with less consumption (127). In the last indicator, the food groups were added or subtracted from the score if they were considered healthy or unhealthy, respectively (125).
Indicator subgroup: Nutritional Adequacy and Micronutrients Indexes
To evaluate DQ from the perspective of nutritional adequacy, 5 indicators were found (128–132). Intake of certain nutrients, especially some micronutrients, is critical in some periods of childhood and adolescence for adequate growth and development. Therefore, one way of assessing the quality of the diet is to consider dietary components in the form of nutrients rather than foods, as it is more common in most indicator subgroups. The indicators that assess nutritional adequacy are characterized by micronutrient components, with the most common being vitamins A, C, E, thiamine, and riboflavin, and the most common minerals being calcium, iron, zinc, and potassium. The least frequent vitamins were vitamins D, K, pantothenic acid, and B6. Among the minerals, sodium and phosphorus were the least frequent.
Indicator subgroup: Dietary Guidelines Indexes
In this subgroup, 5 publications were collected (133–137). These indicators are based on dietary guidelines from different countries. For instance, the Australian guidelines and the DGA Adherence Index (174) are remarkable references for many countries and institutions globally. Regarding the food or nutrient components, those related to the lipid profile are highlighted. This subgroup excludes indicators based on the recommendations of DGA within the concept of Healthy Eating Indexes, which, in this mapping review, constitute an independent subgroup.
Indicator subgroup: Other Healthy Diet Indexes
In this subgroup, indicators that evaluate certain aspects of healthy patterns or characteristics of the diet and that have not been studied much in the scientific literature in this population group have been compiled, obtaining 13 tools (138–150). In this classification, the indexes were developed or modified according to the studies' needs by adapting characteristics of the previous described indexes. The components were food groups, nutrients, or lifestyles. The Baltic Sea Diet Score (175), American Heart Association Score (176), the PAN diet (177), and the Dietary Approaches to Stop Hypertension-DASH score (178) were developed initially for adults with the purpose of measuring the adherence to dietary guidelines or the associations with health conditions.
Other dietary and lifestyle indicators
A detailed description of the types of components of other dietary and lifestyle indicators (Mediterranean Diet Indexes, Diet–Lifestyle Indexes, and Breakfast Quality Indexes) is shown in Supplemental Tables 3–5.
Indicator subgroup: Mediterranean Diet Indexes
In this mapping review, 11 studies were retrieved in which Mediterranean Diet Indexes were developed or applied in children (151–160). The indexes based on the adherence to the Mediterranean diet pattern have been developed with the concept of assessing adherence to an established healthy dietary pattern and with scientific evidence of its association with the prevention of chronic diseases, especially in the adult population (169). Numerous indexes of this subgroup have been developed, presenting concepts such as those applied to the adult population. However, it is necessary to highlight the main controversies and differences in these indexes when applied to children and adolescents. Many authors have eliminated the alcohol component or have included it as negative because its consumption is not recommended in children and adolescents. Regarding dairy products, although many authors do not consider it a traditional characteristic component of the Mediterranean diet pattern, and therefore it is usually included as a negative component in the indexes, other authors have modified their assessment, considering it a positive component because of its richness in nutrients, especially in critical nutrients in the pediatric population (179).
Indicator subgroup: Diet–Lifestyle Indexes
Five studies assessing DQ in conjunction with lifestyle characteristics have been included in the current mapping review (161–165). Lifestyle characteristics have been included less as components in the DQIns. This subgroup is characterized by the inclusion of components related to lifestyles in conjunction with dietary components. The beneficial one is the practice of moderate to vigorous physical activity, and the harmful one is watching television. Most of these indicators were characterized by the inclusion of food and food groups (vegetables, fruits, and cereals most frequently) and the importance of valuing sweets, added sugars, and soft drinks.
Discussion
The current mapping review provides a practical compilation of 139 overall DQIns developed through a priori methodology in the pediatric population and classified into 13 subgroups: Healthy Eating Indexes, Dietary Diversity Scores, Diet Quality Indexes, Food Variety Scores, Healthy and Unhealthy Scores, Mediterranean Diet Indexes, Feeding and Eating Indexes, Diet Quality Scores, Nutritional Adequacy and Micronutrients Indexes, Dietary Guideline Indexes, Diet–Lifestyle Indexes, Breakfast Quality Indexes, and Other Healthy Diet Indexes. We have also included a description of the DQIns according to the components information, scoring method, and key methodological issues. This compilation of DQIns will facilitate the researcher in selecting the most appropriate tool for future epidemiological studies, considering, in a special way, the main methodological aspects collected in this mapping review.
The DQIns in the current mapping review represent a great variety of tools, including those that measure adherence to dietary consumption patterns (13, 23, 180, 181). Several DQIns only contain minor adjustments of the original ones; this is especially the case for the indicator subgroups belonging to Healthy Eating Indexes (50), Dietary Diversity Scores (72), Diet Quality Indexes (77), Food Variety Scores (101), and Healthy and Unhealthy Scores (104). The modifications from the DQIns’ original versions included new food components such as fruit juices or lifestyle factor components related to pediatric age; exclusion of specific food components (i.e., alcohol); cutoff values according to the pediatric age recommendations; and other alternatives concerning the scoring method, such as giving more weight to specific components according to the study age (i.e., breastfeeding). These issues hinder comparisons across DQIns regarding their impact on the primary endpoints of interest in child and adolescent growth and development. These tools for assessing eating habits and lifestyles take a holistic perspective, considering both positive and negative interactions (synergism and antagonism) of foods, food groups, nutrients, and lifestyle-related factors (12).
In the current research, the most practical use of DQIns is to associate the DQ with health-related outcomes (chronic diseases or specific-cause mortality). The main objective in these DQIns was to assess the diet globally by categorizing individuals in a population according to their adherence to the pre-established recommendations or dietary patterns. These DQIns measure and quantify the intake of foods, food groups, nutrients, and lifestyle-related factors concerning specific diet recommendations or characteristics considered beneficial or detrimental to health (182). All DQIns included components that expressed a dimension within the indicator and had a specific weight within the overall score. However, the development of a weighting system dependent on the association of each of the components with selected aspects of the diet or with diseases is currently one of the most controversial issues. The global computation of all the components allowed for assessing dietary habits and other lifestyle aspects related to food intake—for instance, physical activity or anthropometric parameters.
Several studies have found significant associations between the DQIns and health-related outcomes: adiposity and anthropometric parameters (133, 135, 154, 161, 162, 164), lifestyle-related factors (101), insulin resistance (163, 165), growth patterns (100, 134, 137), cognition (43, 144), academic achievement (157, 167), mental health (103), overweight and obesity (125), metabolic syndrome (51), cardiovascular risk factors (47), and levels of biomarkers of inflammation (159). These findings support that higher adherence to predefined dietary patterns with more nutrient-dense foods/components is typically associated with a healthier diet. Therefore, this approach could provide higher protection from diet-related chronic diseases in adulthood, mainly cardiovascular diseases, cancer, diabetes, or metabolic syndrome (183).
Publications were in agreement regarding specific components, despite several disagreements concerning children's DQIns. Among the foods and food groups, the most frequently used were fruits, milk and dairy products, vegetables, cereals, legumes, and fish. Most of these components are considered potentially beneficial to health. Regarding the cereal component, it is notable that few DQIns emphasize the importance of consuming preferably whole-grain cereals because, in general, this component considers all types of cereals and derived products, without considering their fiber content. It is also necessary to emphasize that some researchers name this group starchy foods, including the cereals group, together with bakery and pastry products and some ready-to-eat products (pizza, quiche).
Regarding the dairy component, it generates more controversy because it is considered as positive or negative depending on the indicator. This is perhaps one of the main discrepancies regarding the adult population's indicators; in children, it is considered positive in many more indicators. In the protein-rich foods components, meat is undoubtedly the one that generates more discrepancies, and it must be noted that most of the DQIns consider total consumption and their evaluation is negative. Agreement is more often found when referring to the harmful components to health frequently included in the DQIns: processed meat, sweets, and confectionery items, snacks, and sugar-sweetened beverages.
Nutrient components scored as negative required moderate or limited intake. Those that appeared frequently were sodium, total fat, saturated fat, sugar, cholesterol, and trans fats. Nevertheless, it is noteworthy that in childhood, fats are not only a prime source of energy but also provide essential fatty acids and PUFAs, which are critical for appropriate growth, cognitive development, and prevention of chronic disease in adulthood.
Nutrients considered as positive that appeared frequently were calcium, iron, fiber, vitamin C, vitamin A, protein, thiamin, folate, zinc, and niacin. Some of these are antioxidant components that can halt oxidative stress and the subsequent development of oxidative stress-related chronic diseases in adulthood. Moreover, some researchers have provided evidence for a potential beneficial effect of diet quality against the development of certain high-risk micronutrient deficiencies in children (184).
Compared to DQIns developed for the adult population, specific dietary components such as red wine (153) and alcohol intake (35, 39, 49, 52, 80, 109, 160) are considered in a few indicators. The reason is that the benefit that certain types of alcoholic beverages could have for some segments of the population (185) is inapplicable to children and young people for many reasons, and their consumption is discouraged in this population group.
The remarkable components that generate controversy in children's DQIns are dairy products, meat, and fats. Dairy products are not part of specific patterns of consumption, such as the Mediterranean diet pattern. Other authors have described the positive components of dairy consumption, including those related to bone growth (186). In a critical review, the authors summarized studies that evaluate dairy products and the development of obesity. In 9 publications, a positive association was found between milk and other dairy products and body fatness (187).
Regarding meat consumption, high-quality dietary protein and micronutrients can improve the diet, especially in low-income countries (188). In contrast, their consumption is associated with increased risk of chronic diseases, such as cardiovascular disease and cancer (189). The consumption of oils and fats, or specific types of fats (saturated fats, monounsaturated fats, and polyunsaturated fats), can be considered harmful, but olive oil consumption was considered positive in the Mediterranean Diet Indexes (190).
The relation between DQ and certain health-related outcomes in the pediatric population was controversial—for instance, blood pressure levels and the risk of developing high blood pressure (139), bone mineral density and changes in bone mineralization (105), and the association between DQ and metabolic risk factors (191). Other variables that would be of particular interest to study because of their increased prevalence in young people in recent years are alterations in lipid profiles, insulin resistance, and diabetes (192).
Elements that have not been estimated in the developed tools are components related to food safety level, noncaloric sweeteners, salt added at the table, total antioxidant capacity, smoking, sleep, and rest. These components have additional difficulties to be assessed in the child population. Another way to measure the overall DQ or dietary patterns is by estimating the dietary–lifestyle total oxidative capacity with oxidative balance scores. To date, no oxidative balance score has been designed specifically for the youth population (193).
Regarding the scoring methods, some discrepancies and issues to consider are noteworthy. The differences between the cutoff point values hinder comparability, even within each of the 2 approaches (predefined or population-dependent). The fixed criteria are very different between the DQIns (servings per day or week, grams or milliliters per day or week, or the percentage of the frequency of consumption). Concerning the distribution approach, differences have also been observed in relation to the quantile applied (mainly medians, tertiles, and quintiles), even when age- and sex-specific. The establishment of cutoff values based on the distribution of intake of the sample population limits the comparability between studies. In this aspect, the majority of the articles retrieved in this review presented predefined cutoff point values. Consequently, the use of fixed criteria cutoff points could be a successful strategy for comparability between studies.
Therefore, the methodological study (components and scoring systems) of the DQIns is a fundamental issue due to the great heterogeneity with respect to their development, which hinders their comparison, even when studies are performed in similar populations and for a common outcome variable.
Chronic diet-related diseases are a major public health concern; their causes are the interactions between different nutrient and nonnutrient components. Nevertheless, there are gaps in the knowledge concerning the mechanistic effect of nonnutritive components and their impact on health (194).
The dietary patterns of populations can be studied from 3 different and complementary approaches: a priori (indexes or scores), a posteriori (exploratory analyses), and hypothesis-driven (reduced rank regression) approaches (Figure 2) (195). In this mapping review, the focus was the a priori indicators because they are the most studied in the literature (182). The a posteriori approach has been applied in other studies to develop and evaluate dietary patterns in epidemiological research on children (196, 197). This approach has been used less often because of its poor reproducibility, the limitations concerning its interpretation, and its application in public health.
Limitations
This mapping review presents some significant limitations that need to be considered. Only 2 electronic databases were used (MEDLINE-PubMed and Scopus). However, it is necessary to emphasize that the 2 databases selected are the most relevant to the biomedical literature search. It is important to mention that most of the articles identified in this review are observational, predominantly cross-sectional studies.
The global pediatric DQIns research is limited. Despite the relatively high number of indicators found, Figure 4 shows the distribution of the ages covered by DQIns. This figure shows the gaps in ages 4–8 y, the age group with the fewest indicators. Few DQIns have been validated with different relevant health-related outcomes, such as overweight and obesity (125), metabolic syndrome (51), or cardiovascular risk factors (47). However, studies with an empirical validation have shown more or less the same predictive capacity for the risk of chronic diseases (180).
FIGURE 4.
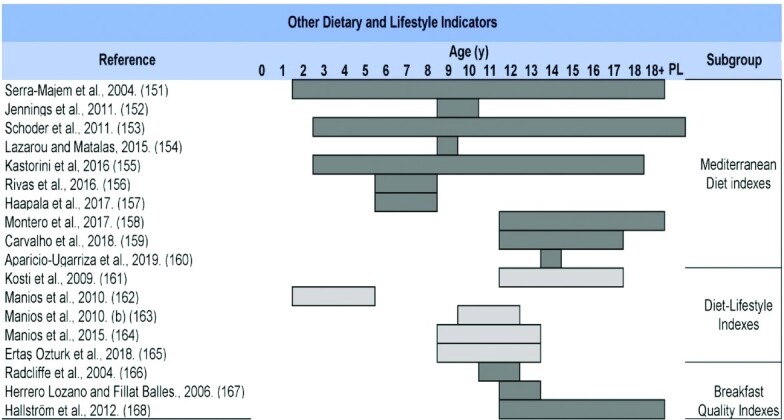
Overall distribution of diet quality indicators and other dietary and lifestyle indicators according to covered ages. Dark and light gray bars show the ages covered in each publication. PL, population level.
The conclusions of DQIn studies must be interpreted with great caution for several reasons. One of the most remarkable is that some DQIns have not been specifically developed for children and adolescents because they encompassed all ages of the population (30, 32, 35, 42, 49, 52, 53, 96, 150). The lack of validation studies in some DQIns might yield the wrong results. It is advised to choose DQIns with validation or reproducibility assessment. This assessment is carried out with other instruments widely validated in the scientific literature (associations with nutrient biomarkers and chronic diseases). In addition, the methodological criteria for the development and validation of DQIns were usually based on dietary guidelines (173, 198) and predefined healthy dietary patterns (113, 151). These dietary guidelines are limited by the current knowledge of diet, health, and disease.
Conclusions
We have provided a practical compilation of 139 DQIns developed through a priori methodology in the pediatric population and classified into 13 subgroups. The subgroup differences should be considered because the DQIns include multiple dimensions to evaluate DQ. Some subgroups have greater insight with regard to validity because of their extensive scientific research and scientific literature availability. Therefore, a previous selection of validated DQIns should be taken into account for future epidemiological studies. If the intention is to develop a new tool, the first step should be justifying the need for such an instrument because there are many previously validated DQIns. In addition, careful selection of the indicator components, the scoring system, the applied methodology for its development, a comparison with internationally recognized DQIns, and potential advantages should be reported in detail.
Future research
Development of DQIns is one of the fastest-growing areas in nutritional epidemiology; additional and more specific research is needed in children and adolescents. More studies in which numerous indicators of DQ are developed and validated against instruments widely validated in the literature through the application of robust methodologies are needed. Because of the increasing prevalence of some chronic diseases in the child population, it would be interesting to study the associations between DQIns and the risk of developing risk factors related to blood pressure levels, alterations in lipid profiles or anthropometric parameters, and diseases such as diabetes and obesity.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ÁH-R and LAD-J: designed the study, conducted the literature search and data extraction, and performed the interpretation of the data; ÁH-R, LAD-J, CM, MJS-M, AK, and AG: drafted and revised the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–6 and Supplemental Appendix 1 are available from the “Supplementary data” link in the online posting of the article and the same link in the online table of contents at https://academic.oup.com/advances.
ÁH-R and LAD-J contributed equally to this work.
Abbreviations used: DGA, Dietary Guidelines for Americans; DQ, diet quality; DQIn, diet quality indicator; MeSH, medical subject heading; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SR, systematic review; 24h-DR, 24-h dietary recall.
Contributor Information
Ángela Hernández-Ruiz, Iberoamerican Nutrition Foundation (FINUT), Granada, Spain.
Liza Alejandra Díaz-Jereda, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
Casandra Madrigal, Iberoamerican Nutrition Foundation (FINUT), Granada, Spain; Department of Nutrition and Food Science, Faculty of Pharmacy, University of Granada, Granada, Spain.
María José Soto-Méndez, Iberoamerican Nutrition Foundation (FINUT), Granada, Spain.
Anneleen Kuijsten, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
Ángel Gil, Iberoamerican Nutrition Foundation (FINUT), Granada, Spain; Department of Biochemistry and Molecular Biology II, University of Granada, Granada, Spain; Institute of Nutrition and Food Technology “José Mataix,” Biomedical Research Center, University of Granada, Granada, Spain; Biosanitary Research Institute ibs.GRANADA, Granada University Hospital Complex, Granada, Spain; CIBEROBN (Physiopathology of Obesity and Nutrition), Instituto de Salud Carlos III, Madrid, Spain.
References
- 1. Corkins MR, Daniels SR, de Ferranti SD, Golden NH, Kim JH, Magge SN, Schwarzenberg SJ. Nutrition in children and adolescents. Med Clin North Am. 2016;100:1217–35. [DOI] [PubMed] [Google Scholar]
- 2. Stewart L, Thompson J. Early years nutrition and healthy weight. Chichester (UK): Wiley-Blackwell; 2015. [Google Scholar]
- 3. Haines J, Haycraft E, Lytle L, Nicklaus S, Kok FJ, Merdji M, Fisberg M, Moreno LA, Goulet O, Hughes SO. Nurturing children's healthy eating: position statement. Appetite. 2019;137:124–33. [DOI] [PubMed] [Google Scholar]
- 4. English LK, Obbagy JE, Wong YP, Butte NF, Dewey KG, Fox MK, Greer FR, Krebs NF, Scanlon KS, Stoody EE. Types and amounts of complementary foods and beverages consumed and growth, size, and body composition: a systematic review. Am J Clin Nutr. 2019;109:956S–77S. [DOI] [PubMed] [Google Scholar]
- 5. English LK, Obbagy JE, Wong YP, Butte NF, Dewey KG, Fox MK, Greer FR, Krebs NF, Scanlon KS, Stoody EE. Complementary feeding and developmental milestones: a systematic review. Am J Clin Nutr. 2019;109:879S–89S. [DOI] [PubMed] [Google Scholar]
- 6. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 7. Pandita A, Sharma D, Pandita D, Pawar S, Tariq M, Kaul A. Childhood obesity: prevention is better than cure. Diabetes Metab Syndr Obes Targets Ther. 2016;9:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO . Obesity and overweight. Geneva (Switzerland): WHO; 2020. [Google Scholar]
- 9. Shim J-S, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Willett W. Nutritional epidemiology. 3rd ed. Oxford University Press. New York: Oxford University Press; 2013. [Google Scholar]
- 11. Guerrero MLP, Pérez-Rodríguez F. Diet quality indices for nutrition assessment: types and applications. In: Functional food—improve health through adequate food. London:InTech; 2017. [Google Scholar]
- 12. Gil Á, de Victoria EM, Olza J. Indicadores de evaluación de la calidad de la dieta. Nutr Hosp. 2015;31:128–44.25719781 [Google Scholar]
- 13. Trijsburg L, Talsma EF, De Vries JHM, Kennedy G, Kuijsten A, Brouwer ID. Diet quality indices for research in low- and middle-income countries: a systematic review. Nutr Rev. 2019;77:515–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flood VM, Webb K, Rangan A. Recommendations for short questions to assess food consumption in children for the NSW Health Surveys. Sydney (Australia):NSW Centre for Public Health Nutrition; 2005. [Google Scholar]
- 15. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11790957%5Cnhttp://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 16. USDA and US Department of Health and Human Services . 2020–2025 dietary guidelines for Americans. Alexandria (VA):USDA; 2020; [Internet]. Available from: https://www.dietaryguidelines.gov. [Google Scholar]
- 17. US Department of Health and Human Services . 2015–2020 dietary guidelines for Americans. Washington (DC):USDA;2015; [Internet]. Available from: http://health.gov/dietaryguidelines/2015/guidelines. [Google Scholar]
- 18. Trumbo PR, Barr SI, Murphy SP, Yates AA. Dietary reference intakes: cases of appropriate and inappropriate uses. Nutr Rev. 2013;71:657–64. [DOI] [PubMed] [Google Scholar]
- 19. Herforth A, Arimond M, Álvarez-Sánchez C, Coates J, Christianson K, Muehlhoff E. A global review of food-based dietary guidelines. Adv Nutr. 2019;10:590–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104:615–35. [DOI] [PubMed] [Google Scholar]
- 21. Bell LK, Golley RK, Magarey AM. Review article: short tools to assess young children's dietary intake: a systematic review focusing on application to dietary index research. J Obesity. 2013;2013:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Asghari G, Mirmiran P, Yuzbashian E, Azizi F. A systematic review of diet quality indices in relation to obesity. Br J Nutr. 2017;117:1055–65. [DOI] [PubMed] [Google Scholar]
- 23. Smithers LG, Golley RK, Brazionis L, Lynch JW. Characterizing whole diets of young children from developed countries and the association between diet and health: a systematic review. Nutr Rev. 2011;69:449–67. [DOI] [PubMed] [Google Scholar]
- 24. Lazarou C, Newby PK. Use of dietary indexes among children in developed countries. Adv Nutr. 2011;2:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marshall S, Burrows T, Collins CE. Systematic review of diet quality indices and their associations with health-related outcomes in children and adolescents. J Hum Nutr Diet. 2014;27(6):577–98. [DOI] [PubMed] [Google Scholar]
- 26. Dalwood P, Marshall S, Burrows TL, McIntosh A, Collins CE. Diet quality indices and their associations with health-related outcomes in children and adolescents: An updated systematic review. Nutr J. 2020;19:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG; the PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fineout-Overholt E, Johnston L. Teaching EBP: asking searchable, answerable clinical questions. Worldviews Evidence-Based Nurs. 2005;2:157–60. [DOI] [PubMed] [Google Scholar]
- 29. Glyph & Cog . Mendeley software. 2008. [Google Scholar]
- 30. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95:1103–8. [DOI] [PubMed] [Google Scholar]
- 31. Feskanich D, Rockett HRH, Colditz GA. Modifying the Healthy Eating Index to assess diet quality in children and adolescents. J Am Diet Assoc. 2004;104:1375–83. [DOI] [PubMed] [Google Scholar]
- 32. Knol LL, Haughton B, Fitzhugh EC. Food insufficiency is not related to the overall variety of foods consumed by young children in low-income families. J Am Diet Assoc. 2004;104:640–4. [DOI] [PubMed] [Google Scholar]
- 33. Pinheiro AC, Atalah E. Propuesta de una metodología de análisis de la calidad global de la alimentación. Rev Med Chil. 2005;133:175–82. [DOI] [PubMed] [Google Scholar]
- 34. Glanville NT, McIntyre L. Diet quality of Atlantic families headed by single mothers. Can J Diet Pract Res. 2006;67:28–35. [DOI] [PubMed] [Google Scholar]
- 35. Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index–2005. J Am Diet Assoc. 2008;108:1896–901. [DOI] [PubMed] [Google Scholar]
- 36. Garriguet D. Diet quality in Canada. Health Rep. 2009;20:41–52. [PubMed] [Google Scholar]
- 37. de Andrade SC, de Azevedo Barros MB, Carandina L, Goldbaum M, Cesar CLG, Fisberg RM. Dietary quality index and associated factors among adolescents of the state of Sao Paulo, Brazil. J Pediatr. 2010;156:456–60. [DOI] [PubMed] [Google Scholar]
- 38. Woodruff SJ, Hanning RM. Development and implications of a revised Canadian Healthy Eating Index (HEIC-2009). Public Health Nutr. 2010;13:820–5. [DOI] [PubMed] [Google Scholar]
- 39. Rydén PJ, Hagfors L. Diet cost, diet quality and socio-economic position: how are they related and what contributes to differences in diet costs?. Public Health Nutr. 2011;14:1680–92. [DOI] [PubMed] [Google Scholar]
- 40. González Rosendo G, Puga Díaz R, Quintero Gutiérrez AG. Índice de alimentación saludable en mujeres adolescentes de Morelos, México. Rev Esp Nutr Comunitaria. 2012;18:12–18. [Google Scholar]
- 41. He M, Tucker P, Irwin JD, Gilliland J, Larsen K, Hess P. Obesogenic neighbourhoods: the impact of neighbourhood restaurants and convenience stores on adolescents’ food consumption behaviours. Public Health Nutr. 2012;15:2331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HAB, Kuczynski KJ, Kahle LL, Krebs-Smith SM. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113:569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nyaradi A, Li J, Hickling S, Whitehouse AJO, Foster JK, Oddy WH. Diet in the early years of life influences cognitive outcomes at 10 years: a prospective cohort study. Acta Paediatr. 2013;102:1165–73. [DOI] [PubMed] [Google Scholar]
- 44. Kyttälä P, Erkkola M, Lehtinen-Jacks S, Ovaskainen ML, Uusitalo L, Veijola R, Simell O, Knip M, Virtanen SM. Finnish Children Healthy Eating Index (FCHEI) and its associations with family and child characteristics in pre-school children. Public Health Nutr. 2014;17:2519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rauber F, da Costa Louzada ML, Vitolo MR. Healthy Eating Index measures diet quality of Brazilian children of low socioeconomic status. J Am Coll Nutr. 2014;33:26–31. [DOI] [PubMed] [Google Scholar]
- 46. Wahlqvist ML, Huang LY, Lee MS, Chiang PH, Chang YH, Tsao AP. Dietary quality of elders and children is interdependent in Taiwanese communities: a NAHSIT mapping study. Ecol Food Nutr. 2014;53:81–97. [DOI] [PubMed] [Google Scholar]
- 47. Dahm CC, Chomistek AK, Jakobsen MU, Mukamal KJ, Eliassen AH, Sesso HD, Overvad K, Willett WC, Rimm EB, Chiuve SE. Adolescent diet quality and cardiovascular disease risk factors and incident cardiovascular disease in middle-aged women. J Am Heart Assoc. 2016;5:e003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tugault-Lafleur CN, Black JL, Barr SI. Examining school-day dietary intakes among Canadian children. Appl Physiol Nutr Metab. 2017;42:1064–72. [DOI] [PubMed] [Google Scholar]
- 49. Yuan Y-Q, Li F, Dong R-HR-H, Chen J-SJ-S, He G-SG-S, Li S-GS-G, Chen BB. The development of a Chinese healthy eating index and its application in the general population. Nutrients. 2017;9:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. da Conceição SIO, de Oliveira BR, Rizzin M, da Silva AAM. Healthy Eating Index: adaptation for children aged 1 to 2 years. Cienc Saude Coletiva. 2018;23:4095–106. [DOI] [PubMed] [Google Scholar]
- 51. Hooshmand F, Asghari G, Yuzbashian E, Mahdavi M, Mirmiran P, Azizi F. Modified healthy eating index and incidence of metabolic syndrome in children and adolescents: Tehran Lipid and Glucose Study. J Pediatr. 2018;197:134–139.e2. [DOI] [PubMed] [Google Scholar]
- 52. Nshimyumukiza L, Lieffers J, Ekwaru JP, Ohinmaa A, Veugelers PJ. Temporal changes in diet quality and the associated economic burden in Canada. PLoS One. 2018;13:e0206877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reedy J, Lerman JL, Krebs-Smith SM, Kirkpatrick SI, Pannucci TE, Wilson MM, Subar AF, Kahle LL, Tooze JA. Evaluation of the Healthy Eating Index–2015. J Acad Nutr Diet. 2018;118:1622–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brownlee IA, Low J, Duriraju N, Chun M, Ong JXY, Tay ME, Hendrie GA, Santos-Merx L. Evaluation of the proximity of Singaporean children's dietary habits to food-based dietary guidelines. Nutrients. 2019;11:2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arimond M, Ruel MT. Dietary diversity is associated with child nutritional status: evidence from 11 demographic and health surveys. J Nutr. 2004;134:2579–85. [DOI] [PubMed] [Google Scholar]
- 56. Mirmiran P, Azadbakht L, Esmaillzadeh A, Azizi F. Dietary diversity score in adolescents—a good indicator of the nutritional adequacy of diets: Tehran Lipid and Glucose Study. Asia Pac J Clin Nutr. 2004;13:56–60. [PubMed] [Google Scholar]
- 57. Kennedy GL, Pedro MR, Seghieri C, Nantel G, Brouwer I. Dietary diversity score is a useful indicator of micronutrient intake in non-breast-feeding Filipino children. J Nutr. 2007;137:472–7. [DOI] [PubMed] [Google Scholar]
- 58. Mpontshane N, Van Den Broeck J, Chhagan M, Luabeya KKA, Johnson A, Bennish ML. HIV infection is associated with decreased dietary diversity in South African children. J Nutr. 2008;138:1705–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roche ML, Creed-Kanashiro HM, Tuesta I, Kuhnlein HV. Traditional food diversity predicts dietary quality for the Awajún in the Peruvian Amazon. Public Health Nutr. 2008;11:457–65. [DOI] [PubMed] [Google Scholar]
- 60. Enneman A, Hernández L, Campos R, Vossenaar M, Solomons NW. Dietary characteristics of complementary foods offered to Guatemalan infants vary between urban and rural settings. Nutr Res. 2009;29:470–9. [DOI] [PubMed] [Google Scholar]
- 61. Kennedy G, Ballard T, Dop M. Guidelines for measuring household and individual dietary diversity. New York: FAO; 2010. [Google Scholar]
- 62. Li Y, Lai J, He Y, Hu X, Liu A, Du S, Zhang J, Yang X, Chen C, Hu FBet al. Lack of dietary diversity and dyslipidaemia among stunted overweight children: the 2002 China National Nutrition and Health Survey. Public Health Nutr. 2011;14:896–903. [DOI] [PubMed] [Google Scholar]
- 63. Belachew T, Lindstrom D, Gebremariam A, Hogan D, Lachat C, Huybregts L, Kolsteren P. Food insecurity, food based coping strategies and suboptimal dietary practices of adolescents in Jimma Zone southwest Ethiopia. PLoS One. 2013;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Darapheak C, Takano T, Kizuki M, Nakamura K, Seino K. Consumption of animal source foods and dietary diversity reduce stunting in children in Cambodia. Int Arch Med. 2013;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gewa CA, Murphy SP, Weiss RE, Neumann CG. Determining minimum food intake amounts for diet diversity scores to maximize associations with nutrient adequacy: an analysis of schoolchildren's diets in rural Kenya. Public Health Nutr. 2014;17:2667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mundo-Rosas V, de la Cruz-Góngora V, Jiménez-Aguilar A, Shamah-Levy T. Diversidad de la dieta y consumo de nutrimentos en niños de 24 a 59 meses de edad y su asociación con inseguridad alimentaria. Salud Publica Mex. 2014;56:39–46. [PubMed] [Google Scholar]
- 67. Amugsi DA, Mittelmark MB, Oduro A. Association between maternal and child dietary diversity: an analysis of the Ghana Demographic and Health Survey. PLoS One. 2015;10(8):e0136748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ali Z, Abizari A-R. Ramadan fasting alters food patterns, dietary diversity and body weight among Ghanaian adolescents. Nutr J. 2018;17:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meng L, Wang Y, Li T, van Loo-Bouwman CA, Zhang Y, Szeto IM-Y. Dietary diversity and food variety in Chinese children aged 3–17 years: are they negatively associated with dietary micronutrient inadequacy?. Nutrients. 2018;10:1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Morseth MS, Torheim LE, Gebremariam MK, Chandyo RK, Ulak M, Shrestha SK, Shrestha B, Henjum S. Tracking of infant and young child feeding practices among 9- to 24-month-old children in Nepal: the MAL-ED Birth Cohort Study. Public Health Nutr. 2018;21:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Iqbal MS, Rahman S, Haque MA, Bhuyan MJ, Faruque ASG, Ahmed T. Lower intakes of protein, carbohydrate, and energy are associated with increased global DNA methylation in 2- to 3-year-old urban slum children in Bangladesh. Matern Child Nutr. 2019;15:e12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thorne-Lyman AL, Shrestha M, Fawzi WW, Pasqualino M, Strand TA, Kvestad I, Hysing M, Joshi N, Lohani M, Miller LC. Dietary diversity and child development in the far west of Nepal: a cohort study. Nutrients. 2019;11:1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang Q, Yuan T, Yang L, Zou J, Ji M, Zhang Y, Deng J, Lin Q. Household food insecurity, dietary diversity, stunting, and anaemia among left-behind children in poor rural areas of China. Int J Environ Res Public Health. 2019;16:4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sebayang SK, Dibley MJ, Astutik E, Efendi F, Kelly PJ, Li M. Determinants of age-appropriate breastfeeding, dietary diversity, and consumption of animal source foods among Indonesian children. Matern Child Nutr. 2020;16:e12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alexy U, Kersting M, Schultze-Pawlitschko V. Two approaches to derive a proposal for added sugars intake for German children and adolescents. Public Health Nutr. 2003;6:697–702. [DOI] [PubMed] [Google Scholar]
- 76. Kranz S, Siega-Riz AM, Herring AH. Changes in diet quality of American preschoolers between 1977 and 1998. Am J Public Health. 2004;94:1525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mariscal-Arcas M, Romaguera D, Rivas A, Feriche B, Pons A, Tur JA, Olea-Serrano F. Diet quality of young people in southern Spain evaluated by a Mediterranean adaptation of the Diet Quality Index–International (DQI-I). Br J Nutr. 2007;98:1267–73. [DOI] [PubMed] [Google Scholar]
- 78. McArthur LH, Holbert D, Peña M. Development and application of rapid assessment diet and physical activity indexes, which suggest high consumption of energy-dense foods and inadequate exercise among adolescents from 6 Latin American cities: a pilot study. Nutr Res. 2008;28:590–9. [DOI] [PubMed] [Google Scholar]
- 79. Huybrechts I, Vereecken C, De Bacquer D, Vandevijvere S, Van Oyen H, Maes L, Vanhauwaert E, Temme L, De Backer G, De Henauw S. Reproducibility and validity of a diet quality index for children assessed using a FFQ. Br J Nutr. 2010;104:135–44. [DOI] [PubMed] [Google Scholar]
- 80. De Vriendt T, Clays E, Huybrechts I, De Bourdeaudhuij I, Moreno LA, Patterson E, Molnár D, Mesana MI, Beghin L, Widhalm Ket al. European adolescents’ level of perceived stress is inversely related to their diet quality: the Healthy Lifestyle in Europe by Nutrition in Adolescence study. Br J Nutr. 2012;108:371–80. [DOI] [PubMed] [Google Scholar]
- 81. Fokeena WB, Jeewon R. Is there an association between socioeconomic status and body mass index among adolescents in Mauritius?. Sci World J. 2012;2012:750659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li J, O'Sullivan T, Johnson S, Stanley F, Oddy W. Maternal work hours in early to middle childhood link to later adolescent diet quality. Public Health Nutr. 2012;15:1861–70. [DOI] [PubMed] [Google Scholar]
- 83. Bel S, Michels N, De Vriendt T, Patterson E, Cuenca-García M, Diethelm K, Gutin B, Grammatikaki E, Manios Y, Leclercq Cet al. Association between self-reported sleep duration and dietary quality in European adolescents. Br J Nutr. 2013;110:949–59. [DOI] [PubMed] [Google Scholar]
- 84. Vyncke K, Cruz Fernandez E, Fajó-Pascual M, Cuenca-García M, De Keyzer W, Gonzalez-Gross M, Moreno LA, Beghin L, Breidenassel C, Kersting Met al. Validation of the Diet Quality Index for Adolescents by comparison with biomarkers, nutrient and food intakes: The HELENA study. Br J Nutr. 2013;109:2067–78. [DOI] [PubMed] [Google Scholar]
- 85. Wong JE, Parnell WR, Howe AS, Black KE, Skidmore PML. Development and validation of a food-based diet quality index for New Zealand adolescents. BMC Public Health. 2013;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Röytiö H, Jaakkola J, Hoppu U, Poussa T, Laitinen K. Development and evaluation of a stand-alone index for the assessment of small children's diet quality. Public Health Nutr. 2015;18:1941–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Collins LJ, Lacy KE, Campbell KJ, McNaughton SA. The predictors of diet quality among Australian children aged 3.5 years. J Acad Nutr Diet. 2016;116:1114–26..e2. [DOI] [PubMed] [Google Scholar]
- 88. Ríos EM, Sinigaglia O, Diaz B, Campos M, Palacios C. Development of a diet quality score for infants and toddlers and its association with weight. J Nutr Heal Food Sci. 2016;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kunaratnam K, Halaki M, Wen LM, Baur LA, Flood VM. Reliability and comparative validity of a diet quality index for assessing dietary patterns of preschool-aged children in Sydney, Australia. Eur J Clin Nutr. 2018;72:464–8. [DOI] [PubMed] [Google Scholar]
- 90. Hamner HC, Moore LV. Dietary quality among children from 6 months to 4 years, NHANES 2011–2016. Am J Clin Nutr. 2020;111:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cox DR, Skinner JD, Carruth BR, Moran J, Houck KS. A food Variety Index for Toddlers (VIT): development and application. J Am Diet Assoc. 1997;97:1382–6. [DOI] [PubMed] [Google Scholar]
- 92. Hatløy A, Torheim LE, Oshaug A. Food variety—a good indicator of nutritional adequacy of the diet? A case study from an urban area in Mali, West Africa. Eur J Clin Nutr. 1998;52:891–8. [DOI] [PubMed] [Google Scholar]
- 93. Vereecken CA, Rossi S, Giacchi MV, Maes L. Comparison of a short food-frequency questionnaire and derived indices with a seven-day diet record in Belgian and Italian children. Int J Public Health. 2008;53:297–305. [DOI] [PubMed] [Google Scholar]
- 94. Falciglia GA, Horner SL, Liang J, Couch SC, Levin LS. Assessing dietary variety in children: development and validation of a predictive equation. J Am Diet Assoc. 2009;109:641–7. [DOI] [PubMed] [Google Scholar]
- 95. Saibul N, Shariff ZM, Lin KG, Kandiah M, Ghani NA, Rahman HA. Food variety score is associated with dual burden of malnutrition in Orang Asli (Malaysian indigenous peoples) households: implications for health promotion. Asia Pac J Clin Nutr. 2009;18:412–22. [PubMed] [Google Scholar]
- 96. Scott JA, Chih TY, Oddy WH. Food variety at 2 years of age is related to duration of breastfeeding. Nutrients. 2012;4:1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zimmer MH, Hart LC, Manning-Courtney P, Murray DS, Bing NM, Summer S. Food variety as a predictor of nutritional status among children with autism. J Autism Dev Disord. 2012;42:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Jones L, Moschonis G, Oliveira A, De Lauzon-Guillain B, Manios Y, Xepapadaki P, Lopes C, Moreira P, Charles MA, Emmett P. The influence of early feeding practices on healthy diet variety score among pre-school children in four European birth cohorts. Public Health Nutr. 2015;18:1774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zaborowicz K, Czarnocińska J, Wądołowska L, Kowalkowska J, Jeżewska-Zychowicz M, Babicz-Zielińska E, Sobaś K, Kozirok W. The effect of girls attitudes towards the health benefits of food on selected dietary characteristics: The GEBahealth Project. Rocz Państwowego Zakładu Hig. 2015;66:69–75. [PubMed] [Google Scholar]
- 100. Fernández C, Kasper NM, Miller AL, Lumeng JC, Peterson KE. Association of dietary variety and diversity with body mass index in US preschool children. Pediatrics. 2016;137:e20152307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Barros L, Lopes C, Oliveira A. Child and family characteristics are associated with a dietary variety index in 4-year-old children from the Generation XXI cohort. Nutr Res. 2019;63:76–85. [DOI] [PubMed] [Google Scholar]
- 102. Yannakoulia M, Karayiannis D, Terzidou M, Kokkevi A, Sidossis LS. Nutrition-related habits of Greek adolescents. Eur J Clin Nutr. 2004;58:580–6. [DOI] [PubMed] [Google Scholar]
- 103. Jacka FN, Kremer PJ, Berk M, de Silva-Sanigorski AM, Moodie M, Leslie ER, Pasco JA, Swinburn BA. A prospective study of diet quality and mental health in adolescents. PLoS One. 2011;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Truthmann J, Richter A, Thiele S, Drescher L, Roosen J, Mensink GB. Associations of dietary indices with biomarkers of dietary exposure and cardiovascular status among adolescents in Germany. Nutr Metab. 2012;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Monjardino T, Lucas R, Ramos E, Barros H. Associations between a priori–defined dietary patterns and longitudinal changes in bone mineral density in adolescents. Public Health Nutr. 2014;17:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Vilela S, Oliveira A, Ramos E, Moreira P, Barros H, Lopes C. Association between energy-dense food consumption at 2 years of age and diet quality at 4 years of age. Br J Nutr. 2014;111:1275–82. [DOI] [PubMed] [Google Scholar]
- 107. Anderson SE, Kaye G, Andridge R, Smathers C, Peng J, Pirie P. Interrelationships of more healthful and less healthful aspects of diet quality in a low-income community sample of preschool-aged children. Matern Child Health J. 2015;19:2663–72. [DOI] [PubMed] [Google Scholar]
- 108. Anderson SE, Ramsden M, Kaye G. Diet qualities: healthy and unhealthy aspects of diet quality in preschool children. Am J Clin Nutr. 2016;103:1507–13. [DOI] [PubMed] [Google Scholar]
- 109. Sluik D, van Lee L, Engelen AI, Feskens EJM. Total, free, and added sugar consumption and adherence to guidelines: The Dutch National Food Consumption Survey 2007–2010. Nutrients. 2016;8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Arvidsson L, Eiben G, Hunsberger M, De Bourdeaudhuij I, Molnar D, Jilani H, Thumann B, Veidebaum T, Russo P, Tornatitis Met al. Bidirectional associations between psychosocial well-being and adherence to healthy dietary guidelines in European children: prospective findings from the IDEFICS study. BMC Public Health. 2017;17:926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Martins BG, Ricardo CZ, Machado PP, Rauber F, Azeredo CM, Levy RB. Eating meals with parents is associated with better quality of diet for Brazilian adolescents [Fazer refeições com os pais está associado à maior qualidade da alimentação de adolescentes brasileiros]. Cad Saude Publica. 2019;35:e00153918. [DOI] [PubMed] [Google Scholar]
- 112. Wadolowska L, Hamulka J, Kowalkowska J, Ulewicz N, Hoffmann M, Gornicka M, Bronkowska M, Leszczynska T, Glibowski P, Korzeniowska-Ginter R. Changes in sedentary and active lifestyle, diet quality and body composition nine months after an education program in Polish students aged 11–12 years: report from the ABC of Healthy Eating Study. Nutrients. 2019;11:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Arimond M, Ruel M. Progress in developing an infant and child feeding index. Washington (DC): International Food Policy Research Institute; 2002. [Google Scholar]
- 114. Ruel MT, Menon P. Child feeding practices are associated with child nutritional status in Latin America: innovative uses of the Demographic and Health Surveys. J Nutr. 2002;132:1180–7. [DOI] [PubMed] [Google Scholar]
- 115. Dewey KG, Cohen RJ, Arimond M, Ruel MT, Ainy E, Mirmiran P, Azizi F, Gogoi G, Ahmed FU, Nahar S. Developing and validating simple indicators of complementary food intake and nutrient density for breastfed children in developing countries. Washington (DC): Food and Nutrition Technical Assistance Project, Academy for Education Development;2006. [Google Scholar]
- 116. Food and Nutrition Technical Assistance Project . Developing and validating simple indicators of dietary quality and energy intake of infants and young children in developing countries: summary of findings from analysis of 10 data sets. Washington (DC): Food and Nutrition Technical Assistance Project; 2006. [Google Scholar]
- 117. Bork K, Cames C, Barigou S, Cournil A, Diallo A. A summary index of feeding practices is positively associated with height-for-age, but only marginally with linear growth, in rural Senegalese infants and toddlers. J Nutr. 2012;142:1116–22. [DOI] [PubMed] [Google Scholar]
- 118. Golley RK, Smithers LG, Mittinty MN, Brazionis L, Emmett P, Northstone K, Campbell K, McNaughton SA, Lynch JW. An index measuring adherence to complementary feeding guidelines has convergent validity as a measure of infant diet quality. J Nutr. 2012;142:901–8. [DOI] [PubMed] [Google Scholar]
- 119. Jones AD. The production diversity of subsistence farms in the Bolivian Andes is associated with the quality of child feeding practices as measured by a validated summary feeding index. Public Health Nutr. 2015;18:329–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Monterrosa EC, Frongillo EA, Neufeld LM, Egan KA, Ramakrishnan U, Rasmussen KM. Maternal pre-pregnancy body mass index is not associated with infant and young child feeding in low-income Mexican children 1–24 months old. Matern Child Nutr. 2015;11:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Delshad M, Beck KL, von Hurst PR, Mugridge O, Conlon CA. The validity and reliability of the Dietary Index for a Child's Eating in 2–8-year old children living in New Zealand. Matern Child Nutr. 2019;15:e12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ferreira VR, Sangalli CN, Leffa PS, Rauber F, Vitolo MR. The impact of a primary health care intervention on infant feeding practices: a cluster randomised controlled trial in Brazil. J Hum Nutr Diet. 2019;32:21–30. [DOI] [PubMed] [Google Scholar]
- 123. Crombie IK, Kiezebrink K, Irvine L, Wrieden WL, Swanson V, Power K, Slane PW. What maternal factors influence the diet of 2-year-old children living in deprived areas? A cross-sectional survey. Public Health Nutr. 2009;12:1254–60. [DOI] [PubMed] [Google Scholar]
- 124. Kohlboeck G, Sausenthaler S, Standl M, Koletzko S, Bauer CP, Von Berg A, Berdel D, Kärmer U, Schaaf B, Lehmann Iet al. Food intake, diet quality and behavioral problems in children: results from the GINI-plus/LISA-plus studies. Ann Nutr Metab. 2012;60:247–56. [DOI] [PubMed] [Google Scholar]
- 125. Perry CP, Keane E, Layte R, Fitzgerald AP, Perry IJ, Harrington JM. The use of a dietary quality score as a predictor of childhood overweight and obesity. BMC Public Health. 2015;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Voortman T, Kiefte-de Jong JC, Geelen A, Villamor E, Moll HA, de Jongste JC, Raat H, Hofman A, Jaddoe VWV, Franco OHet al. The development of a diet quality score for preschool children and its validation and determinants in the Generation R study. J Nutr. 2015;145:306–14. [DOI] [PubMed] [Google Scholar]
- 127. Gasser C, Kerr J, Mensah F, Wake M. Stability and change in dietary scores and patterns across six waves of the Longitudinal Study of Australian Children. Br J Nutr. 2017;117:1137–50. [DOI] [PubMed] [Google Scholar]
- 128. Fulgoni VL, Keast DR, Drewnowski A. Development and validation of the nutrient-rich foods index: a tool to measure nutritional quality of foods. J Nutr. 2009;139:1549–54. [DOI] [PubMed] [Google Scholar]
- 129. Libuda L, Alexy U, Buyken AE, Sichert-Hellert W, Stehle P, Kersting M. Consumption of sugar-sweetened beverages and its association with nutrient intakes and diet quality in German children and adolescents. Br J Nutr. 2009;101:1549–57. [DOI] [PubMed] [Google Scholar]
- 130. Chiplonkar SA, Tupe R. Development of a diet quality index with special reference to micronutrient adequacy for adolescent girls consuming a lacto-vegetarian diet. J Am Diet Assoc. 2010;110:926–31. [DOI] [PubMed] [Google Scholar]
- 131. Alexy U, Libuda L, Mersmann S, Kersting M. Convenience foods in children's diet and association with dietary quality and body weight status. Eur J Clin Nutr. 2011;65:160–6. [DOI] [PubMed] [Google Scholar]
- 132. Lee HA, Park H. Correlations between poor micronutrition in family members and potential risk factors for poor diet in children and adolescents using Korean National Health and Nutrition Examination Survey data. Nutrients. 2015;7:6346–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Golley RK, Hendrie GA, Mcnaughton SA. Scores on the Dietary Guideline Index for Children and Adolescents are associated with nutrient intake and socio-economic position but not adiposity. J Nutr. 2011;141:1340–7. [DOI] [PubMed] [Google Scholar]
- 134. Lioret S, McNaughton SA, Cameron AJ, Crawford D, Campbell KJ, Cleland VJ, Ball K. Three-year change in diet quality and associated changes in BMI among schoolchildren living in socio-economically disadvantaged neighbourhoods. Br J Nutr. 2014;112:260–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Mohseni-Takalloo S, Hosseini-Esfahani F, Mirmiran P, Azizi F. Associations of pre-defined dietary patterns with obesity associated phenotypes in Tehranian adolescents. Nutrients. 2016;8:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Rohde JF, Larsen SC, Ängquist L, Olsen NJ, Stougaard M, Mortensen EL, Heitmann BL. Effects of the Healthy Start randomized intervention on dietary intake among obesity-prone normal-weight children. Public Health Nutr. 2017;20:2988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Er V, Dias KI, Papadaki A, White J, Wells S, Ward DS, Metcalfe C, Jago R, Kipping R. Association of diet in nurseries and physical activity with zBMI in 2–4-year olds in England: a cross-sectional study. BMC Public Health. 2018;18:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kleiser C, Mensink GBM, Scheidt-Nave C, Kurth BM. HuSKY: A healthy nutrition score based on food intake of children and adolescents in Germany. Br J Nutr. 2009;102:610–18. [DOI] [PubMed] [Google Scholar]
- 139. Lazarou C, Panagiotakos DB, Matalas AL. Foods E-KINDEX: a dietary index associated with reduced blood pressure levels among young children: The CYKIDS Study. J Am Diet Assoc. 2009;109:1070–5. [DOI] [PubMed] [Google Scholar]
- 140. Bisi Molina MC, Lopéz PM, de Faria CP, Cade NV, Zandonade E. Socioeconomic predictors of child diet quality. Rev Saude Publica. 2010;44:785–92. [DOI] [PubMed] [Google Scholar]
- 141. Marshall S, Watson J, Burrows T, Guest M, Collins CE. The development and evaluation of the Australian Child and Adolescent Recommended Food Score: a cross-sectional study. Nutr J. 2012;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Spence AC, McNaughton SA, Lioret S, Hesketh KD, Crawford DA, Campbell KJ. A health promotion intervention can affect diet quality in early childhood. J Nutr. 2013;143:1672–8. [DOI] [PubMed] [Google Scholar]
- 143. Durksen A, Downs S, Mollard R, Forbes L, Ball GDC, McGavock J. The association between time spent in vigorous physical activity and dietary patterns in adolescents: a cross-sectional study. J Phys Act Heal. 2015;12:208–15. [DOI] [PubMed] [Google Scholar]
- 144. Haapala EA, Eloranta AM, Venäläinen T, Schwab U, Lindi V, Lakka TA. Associations of diet quality with cognition in children: the Physical Activity and Nutrition in Children Study. Br J Nutr. 2015;114:1080–7. [DOI] [PubMed] [Google Scholar]
- 145. Hardiansyah A, Hardinsyah, Sukandar D. Alternative indices for the assessment of nutritional quality of balanced diet of Indonesian children 4–6 years old. Pakistan J Nutr. 2015;14:716–20. [Google Scholar]
- 146. Cheng G, Duan R, Kranz S, Libuda L, Zhang L. Development of a dietary index to assess overall diet quality for Chinese school-aged children: the Chinese Children Dietary Index. J Acad Nutr Diet. 2016;116:608–17. [DOI] [PubMed] [Google Scholar]
- 147. Verger EO, Eussen S, Holmes BA. Evaluation of a nutrient-based diet quality index in UK young children and investigation into the diet quality of consumers of formula and infant foods. Public Health Nutr. 2016;19:1785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Winpenny E, Greenslade S, Corder K, van Sluijs E. Diet quality through adolescence and early adulthood: cross-sectional associations of the Dietary Approaches to Stop Hypertension diet index and component food groups with age. Nutrients. 2018;10:1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kunto YS, Bras H. Ethnic group differences in dietary diversity of school-aged children in Indonesia: the roles of gender and household SES. Food Nutr Bull. 2019;40:182–201. [DOI] [PubMed] [Google Scholar]
- 150. Liu J, Rehm C, Onopa J, Mozaffarian D. Trends in diet quality among youth in the United States, 1999–2016. JAMA. 2020;323:1161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Serra-Majem L, Ribas L, Ngo J, Ortega RM, García A, Pérez-Rodrigo C, Aranceta J. Food, youth and the Mediterranean diet in Spain. Development of KIDMED, Mediterranean diet quality index in children and adolescents. Public Health Nutr. 2004;7:931–5. [DOI] [PubMed] [Google Scholar]
- 152. Jennings A, Welch A, Sluijs EMF V, Griffin SJ. Diet quality is independently associated with weight status in children aged 9–10 years. J Nutr. 2011;141:453–9. [DOI] [PubMed] [Google Scholar]
- 153. Schroder H, Corella D, Salas-Salvado J, Lamuela-Ravento R, Ros E, Salaverrı I, Vinyoles E, Go E. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141:1140–5. [DOI] [PubMed] [Google Scholar]
- 154. Lazarou C, Matalas AL. Breakfast intake is associated with nutritional status, Mediterranean diet adherence, serum iron and fasting glucose: the CYFamilies study. Public Health Nutr. 2015;18:1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kastorini CM, Lykou A, Yannakoulia M, Petralias A, Riza E, Linos A, Katerina B, Archontoula D, Dafni G, Natalia Get al. The influence of a school-based intervention programme regarding adherence to a healthy diet in children and adolescents from disadvantaged areas in Greece: the DIATROFI study. J Epidemiol Community Health. 2016;70:671–7. [DOI] [PubMed] [Google Scholar]
- 156. Rivas A, Monteagudo C, Heras-Gonzalez L, Mariscal-Arcas M, Lorenzo-Tovar ML, Olea-Serrano F. Association of bisphenol A exposure with dietary quality indices in Spanish schoolchildren. Food Chem Toxicol. 2016;94:25–30. [DOI] [PubMed] [Google Scholar]
- 157. Haapala EA, Eloranta A-MM, Venäläinen T, Jalkanen H, Poikkeus A-MM, Ahonen T, Lindi V, Lakka TA. Diet quality and academic achievement: a prospective study among primary school children. Eur J Nutr. 2017;56:2299–308. [DOI] [PubMed] [Google Scholar]
- 158. Montero MDP, Mora-Urda AI, Anzid K, Cherkaoui M, Marrodan MD. Diet quality of Moroccan adolescents living in Morocco and in Spain. J Biosoc Sci. 2017;49:173–86. [DOI] [PubMed] [Google Scholar]
- 159. Carvalho KMB, Ronca DB, Michels N, Huybrechts I, Cuenca-Garcia M, Marcos A, Molnár D, Dallongeville J, Manios Y, Schaan BDet al. Does the Mediterranean diet protect against stress-induced inflammatory activation in European adolescents? The HELENA study. Nutrients. 2018;10:1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Aparicio-Ugarriza R, Cuenca-García M, Gonzalez-Gross M, Julián C, Bel-Serrat S, Moreno LA, Breidenassel C, Kersting M, Arouca AB, Michels Net al. Relative validation of the adapted Mediterranean Diet Score for Adolescents by comparison with nutritional biomarkers and nutrient and food intakes: the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Public Health Nutr. 2019;22:2381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kosti RI, Panagiotakos DB, Mariolis A, Zampelas A, Athanasopoulos P, Tountas Y. The diet–lifestyle index evaluating the quality of eating and lifestyle behaviours in relation to the prevalence of overweight/obesity in adolescents. Int J Food Sci Nutr. 2009;60:34–47. [DOI] [PubMed] [Google Scholar]
- 162. Manios Y, Kourlaba G, Grammatikaki E, Androutsos O, Moschonis G, Roma-Giannikou E. Development of a diet–lifestyle quality index for young children and its relation to obesity: the Preschoolers Diet–Lifestyle Index. Public Health Nutr. 2010;13:2000–9. [DOI] [PubMed] [Google Scholar]
- 163. Manios Y, Kourlaba G, Grammatikaki E, Koubitski A, Siatitsa PE, Vandorou A, Kyriakou K, Dede V, Moschonis G. Development of a lifestyle–diet quality index for primary schoolchildren and its relation to insulin resistance: the Healthy Lifestyle–Diet Index. Eur J Clin Nutr. 2010;64:1399–406. [DOI] [PubMed] [Google Scholar]
- 164. Manios Y, Moschonis G, Papandreou C, Politidou E, Naoumi A, Peppas D, Mavrogianni C, Lionis C, Chrousos GP. Revised healthy lifestyle–diet index and associations with obesity and iron deficiency in schoolchildren: the Healthy Growth Study. J Hum Nutr Diet. 2015;28:50–8. [DOI] [PubMed] [Google Scholar]
- 165. Ertaş Öztürk Y, Bozbulut R, Döǧer E, Bideci A, Köksal E. The relationship between diet quality and insulin resistance in obese children: adaptation of the Healthy Lifestyle–Diet Index in Turkey. J Pediatr Endocrinol Metab. 2018;31:391–8. [DOI] [PubMed] [Google Scholar]
- 166. Radcliffe BC, Ogden C, Coyne T, Craig P. Breakfast consumption patterns of upper primary school students in 14 Queensland schools. Nutr Diet. 2004;61:151–8. [Google Scholar]
- 167. Herrero Lozano R, Fillat Ballesteros JC. Estudio sobre el desayuno y el rendimiento escolar en un grupo de adolescentes. Nutr Hosp. 2006;21:346–52. [PubMed] [Google Scholar]
- 168. Hallström L, Vereecken CA, Labayen I, Ruiz JR, Le Donne C, García MC, Gilbert CC, Martínez SG, Grammatikaki E, Huybrechts Iet al. Breakfast habits among European adolescents and their association with sociodemographic factors: The HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) study. Public Health Nutr. 2012;15:1879–89. [DOI] [PubMed] [Google Scholar]
- 169. Hernández Ruiz A, García-Villanova B, Guerra Hernández EJ, Amiano P, Azpiri M, Molina Montes E. Description of indexes based on the adherence to the Mediterranean dietary pattern: a review. Nutr Hosp. 2015;32:1872–84. [DOI] [PubMed] [Google Scholar]
- 170. Kirkpatrick SI, Reedy J, Krebs-Smith SM, Pannucci TE, Subar AF, Wilson MM, Lerman JL, Tooze JA. Applications of the Healthy Eating Index for surveillance, epidemiology, and intervention research: considerations and caveats. J Acad Nutr Diet. 2018;118:1603–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Ruel MT. Is dietary diversity an indicator of food security or dietary quality? A review of measurement issues and research needs. Food Nutr Bull. 2003;24(2):231–2. [DOI] [PubMed] [Google Scholar]
- 172. Daelmans B, Dewey K, Arimond M. New and updated indicators for assessing infant and young child feeding. Food Nutr Bull. 2009;30:256–62. [DOI] [PubMed] [Google Scholar]
- 173. Kranz S, Hartman R T, Anna R, Siega-Riz M, Herring AH. A diet quality index for American preschoolers based on current dietary intake recommendations and an indicator of energy balance. J Am Diet Assoc. 2006;106:1594–604. [DOI] [PubMed] [Google Scholar]
- 174. Fogli-Cawley JJ, Dwyer JT, Saltzman E, McCullough ML, Troy LM, Jacques PF. The 2005 Dietary Guidelines for Americans Adherence Index: development and application. J Nutr. 2006;136:2908–15. [DOI] [PubMed] [Google Scholar]
- 175. Kanerva N, Kaartinen NE, Schwab U, Lahti-Koski M, Männistö S. The Baltic Sea Diet Score: a tool for assessing healthy eating in Nordic countries. Public Health Nutr. 2014;17:1697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GFet al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 177. Verger EO, Holmes BA, Huneau JF, Mariotti F. Simple changes within dietary subgroups can rapidly improve the nutrient adequacy of the diet of French adults. J Nutr. 2014;144:929–36. [DOI] [PubMed] [Google Scholar]
- 178. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–20. [DOI] [PubMed] [Google Scholar]
- 179. De Lamas C, De Castro MJ, Gil-Campos M, Gil Á, Couce ML, Leis R. Effects of dairy product consumption on height and bone mineral content in children: a systematic review of controlled trials. Adv Nutr. 2019;10:S88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Burggraf C, Teuber R, Brosig S, Meier T. Review of a priori dietary quality indices in relation to their construction criteria. Nutr Rev. 2018;76:747–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Wu XY, Zhuang LH, Li W, Guo HW, Zhang JH, Zhao YK, Hu JW, Gao QQ, Luo S, Ohinmaa Aet al. The influence of diet quality and dietary behavior on health-related quality of life in the general population of children and adolescents: a systematic review and meta-analysis. Qual Life Res. 2019;28:1989–2015. [DOI] [PubMed] [Google Scholar]
- 182. Waijers P, Feskens EJM, Ocké MC. A critical review of predefined diet quality scores. Br J Nutr. 2007;97:219–31. [DOI] [PubMed] [Google Scholar]
- 183. Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118:74–100.e11. [DOI] [PubMed] [Google Scholar]
- 184. Bruins MJ, Bird JK, Aebischer CP, Eggersdorfer M. Considerations for secondary prevention of nutritional deficiencies in high-risk groups in high-income countries. Nutrients. 2018;10(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ Res. 2019;124:779–98. [DOI] [PubMed] [Google Scholar]
- 186. Tunick MH, Van Hekken DL. Dairy products and health: recent insights. J Agric Food Chem. 2015;63:9381–8. [DOI] [PubMed] [Google Scholar]
- 187. Dougkas A, Barr S, Reddy S, Summerbell CD. A critical review of the role of milk and other dairy products in the development of obesity in children and adolescents. Nutr Res Rev. 2019;32:106–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. De Smet S, Vossen E. Meat: the balance between nutrition and health—a review. Meat Sci. 2016;120:145–56. [DOI] [PubMed] [Google Scholar]
- 189. Vernooij RWM, Zeraatkar D, Han MA, El Dib R, Zworth M, Milio K, Sit D, Lee Y, Gomaa H, Valli Cet al. Patterns of red and processed meat consumption and risk for cardiometabolic and cancer outcomes: a systematic review and meta-analysis of cohort studies. Ann Intern Med. 2019;171:732–41. [DOI] [PubMed] [Google Scholar]
- 190. Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health: a critical review. Circ Res. 2019;124:779–98. [DOI] [PubMed] [Google Scholar]
- 191. Winpenny EM, van Sluijs EMF, Forouhi NG. How do short-term associations between diet quality and metabolic risk vary with age?. Eur J Nutr. 2021;60(1):517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Barbetti F, De Filippo G, Hospital B, Zucchini FS, Chiarelli F, Tagi VM, Giannini C. Insulin resistance in children. Front Endocrinol. 2019;1:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Hernández-Ruiz Á, García-Villanova B, Guerra-Hernández E, Amiano P, Ruiz-Canela M, Molina-Montes E. A review of a priori defined oxidative balance scores relative to their components and impact on health outcomes. Nutrients. 2019;11:1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Lohner S, Toews I, Meerpohl JJ. Health outcomes of non-nutritive sweeteners: analysis of the research landscape. Nutr J. 2017;16(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ocké MC. Evaluation of methodologies for assessing the overall diet: dietary quality scores and dietary pattern analysis. Proc Nutr Soc. 2013;72:191–9. [DOI] [PubMed] [Google Scholar]
- 196. Haszard JJ, Diana A, Daniels L, Houghton LA, Gibson RS. Development of a nutrient quality score for the complementary diets of Indonesian infants and relationships with linear growth and stunting: a longitudinal analysis. Br J Nutr. 2019;122:71–7. [DOI] [PubMed] [Google Scholar]
- 197. Plaza-Díaz J, Molina-Montes E, Soto-Méndez MJ, Madrigal C, Hernández-Ruiz Á, Valero T, Villoslada FL, Leis R, de Victoria EM, Moreno JMet al. Clustering of dietary patterns and lifestyles among Spanish children in the ESNUPI study. Nutrients. 2020;12:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Krebs-Smith SM, Pannucci TRE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118:1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.