Abstract
We previously showed that exposure to a high-sugar and moderate-fat diet (i.e., Western diet, WD) in mice induces appreciable skin inflammation and enhances the susceptibility to imiquimod-induced psoriasiform dermatitis (PsD), suggesting that dietary components may render the skin susceptible to psoriatic inflammation. Herein, utilizing an IL-23 minicircle (MC)-based model with features of both PsD and psoriatic arthritis (PsA), we showed that intake of WD for 10 weeks predisposed mice not only to skin but also joint inflammation. Both WD-induced skin and joint injuries were associated with an expansion of IL-17A-producing γδ T cells and increased expression of Th17 cytokines. After IL-23 MC delivery, WD-fed mice had reduced microbial diversity and pronounced dysbiosis. Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in WD-fed mice. Strikingly, reduced skin and joint inflammation with a partial reversion of the gut microbiota were noted when mice switched from a WD to a standard diet after IL-23 MC delivery. These findings reveal that short-term WD intake-induced dysbiosis is accompanied by enhanced psoriasis-like skin and joint inflammation. Modifications toward a healthier dietary pattern should be considered in patients with psoriatic skin and/or joint disease.
Keywords: Western diet, psoriasis, psoriatic arthritis, gut microbiota
INTRODUCTION
Psoriasis is a chronic autoimmune skin disease that is associated with multiple comorbidities. One of the most prevalent is psoriatic arthritis (PsA), which can develop in up to 30% of psoriasis patients and lead to a significant decrease in quality of life (Ritchlin et al., 2017). Emerging data suggest unhealthy diet pattern as an important and independent risk factor for psoriasis. For example, an increase in dietary saturated fatty acids (SFAs) in healthy, lean mice alone was sufficient to induce an exacerbation of psoriasiform inflammation , and conversely, a reduction in dietary SFAs diminished the psoriatic phenotype in non-obese mice (Herbert et al., 2018). Our recently published work further suggested a critical role for dietary composition in the pathogenesis of psoriasis by demonstrating that exposure to a high-sugar, moderate-fat diet (i.e. Western diet, WD) alone was enough to induce appreciable skin inflammation without inducing obesity (Shi et al., 2020b).
The mechanisms behind dietary regulation of inflammation in psoriasiform dermatitis (PsD) are only beginning to be elucidated in experimental models. Several studies suggest that gut microbiota may potentially play key roles in shaping inflammation (Stehlikova et al., 2019, Zakostelska et al., 2016). The mammalian intestinal tract is colonized by a large number of microorganisms, including trillions of bacteria referred to collectively as the gut microbiota. Perturbation of the gut microbiome by environmental factors such as diet promotes the overgrowth of harmful pathobionts, disrupts immune homeostasis, and leads to the development of inflammatory diseases (Jena et al., 2020, Sheng et al., 2017b).
Among the numerous factors known to influence gut microbiota composition, dietary composition has repeatedly been shown to be one of the most critical modifiable factors regulating the gut microbiota (Martinez et al., 2017). The Western diet, in particular, can lead to a rapid and detrimental impact on microbial community and function, contributing to systemic inflammation and other metabolically compromised phenotypes (Jena et al., 2018, Jena et al., 2017, Sheng et al., 2017a). We previously have shown that feeding a diet analogous to the WD in mice induces spontaneous skin inflammation (Shi et al., 2020b) and enhances susceptibility to imiquimod-induced PsD (Yu et al., 2018). Herein, we used an IL-23 minicircle DNA (IL-23 MC)-based murine model with features of PsD and PsA (Shi et al., 2020a) to study the effect of WD on both psoriasis-like skin and joint disease and tested the hypothesis that intestinal dysbiosis is implicated in skin and joint inflammation.
RESULTS
WD intake enhances the susceptibility to IL-23-mediated skin inflammation
We first investigated whether or not relatively short-term exposure to WD predisposes mice to IL-23-mediated skin inflammation. When injected with 10 ug of IL-23 MC DNA to induce systemic IL-23 over-expression, chow diet (CD)-fed mice of C57BL/6 background developed psoriatic inflammation as previously described (Shi et al., 2020a), but otherwise their general health was fine. Contrarily, WD-fed mice developed severe gut symptoms including loose stool and diarrhea, and most of them did not survive 2 weeks post-delivery of MC (Supplementary figure 1). Therefore, we reduced the dose of MC by half so that the WD-fed mice would be tolerant to the IL-23 MC treatment. After 6 weeks of feeding with WD or CD, mice were injected with IL-23 MC or control GFP MC and maintained on their respective diets for additional 4 weeks (Figure 1a). IL-23 MC injected mice revealed a significant elevation of serum IL-23 whereas GFP MC injected mice did not have detectable levels of IL-23 in either CD or WD-fed groups (Figure 1b). 6 weeks before MC delivery, WD-fed mice gained more weight than CD-fed mice. At 10 weeks, WD intake led to higher body weight in GFP-MC treated mice, but failed to induce significant weight gain in IL-23 MC-treated mice (Figure 1c). Mice from the WD+IL-23 MC group developed skin changes consisting of erythema and scaling, which were remarkably milder in CD+IL-23 MC groups and absent in GFP MC groups (Figure 1d). Ear thickness followed a similar pattern with GFP MC mice exhibiting the least amount of ear swelling (Figure 1e). Histological analysis showed increased epidermal thickness in ears from WD+GFP MC mice compared to CD+GFP MC mice. IL-23 MC promoted epidermal hyperplasia in both diet groups but had a greater effect on mice fed with WD (Figure 1f, g). WD-fed mice had increased nuclear staining of Ki-67 in the epidermis and higher gene expression of Keratin 16, consistent with enhanced keratinocyte proliferation (Figure 1h, i). In summary, short-term WD intake appears sufficient to enhance susceptibility to IL-23-mediated psoriasis-like skin inflammation.
Figure 1. Short-term exposure to Western diet (WD) enhances the susceptibility to IL-23-mediated skin inflammation.
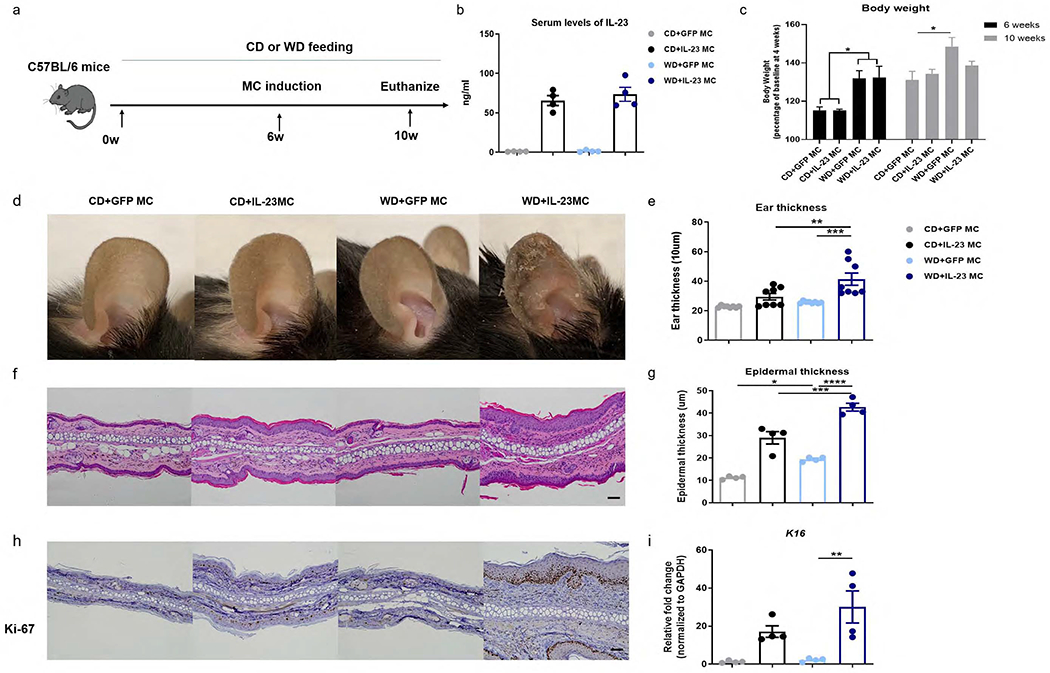
(a) C57BL/6 mice were fed with a WD or a standard chow diet (CD) for 6 weeks and then injected with IL-23 minicircle DNA (MC) or control GFP MC. After MC delivery, mice were maintained on their respective diets for an additional 4 weeks. (b) Serum levels of IL-23 by ELISA of each group at 24 hours after MC delivery. (c) Body weight, (d) clinical photographs showing erythema and scales, (e) ear thickness, (f) image of H&E section (scale bars, 50 μm), (g) histological analysis of epidermal thickness, (h) representative images of immunohistochemical Ki-67 (scale bars, 50 μm) and (i) gene expression of K16 in ear skin. All of the data are presented as mean± SEM. 4 mice per group. Data are representative of two independent experiments. * p <0.05, ** p <0.01,*** p <0.001,ns, not significant, by using one-way ANOVA with Bonferroni’s test.
WD promotes IL-23-mediated skin inflammation accompanied by infiltration of γδ T cells and enhanced expression of Th17 cytokines
We next sought to characterize the immunological features of IL-23-mediated skin inflammation. As we previously described (Shi et al., 2020b), WD-fed mice had greater numbers of γδ-low T cells in ear skin than CD-fed mice (Figure 2a). Following the delivery of IL-23 MC, this difference was amplified as WD-fed mice responded with more infiltration of γδ-low T cells than CD mice. In a previous study, we showed that a subset of CC chemokine receptor-6 (CCR6) positive , γδ-low T cells expressing Th17 cytokines in mouse skin participates in IL-23-induced PsD (Mabuchi et al., 2013). As expected, WD+ IL-23 MC mice had upregulated CCR6 expression on γδ-low T cells (Figure 2b) and a greater potential to produce IL-17A compared to CD+IL-23 MC mice (Figure 2c). Keratinocytes from WD-fed, IL-23 MC-injected mice demonstrated stronger nuclear staining of phospho-Stat3, a transcription factor involved in Th17-mediated inflammation and cell differentiation (Figure 2d) (Sano et al., 2005). There was also a trend towards higher numbers of neutrophils in WD-fed mice versus CD-fed mice (Figure 2e). IL-23 MC delivery also resulted in increased expression of psoriasis-related cytokines, including Th17 cytokines, neutrophil chemoattractants, and antimicrobial peptides (AMPs) in both CD- and WD-fed groups (Figure 2f). Notably, WD-fed mice showed higher levels of Il17a and Il17f and some, albeit insignificant, increase in the expression of Cxcl2, S100a8 and S100a9 compared to CD-fed mice. Together, our data suggest that WD results in exacerbated IL-23-mediated, Th17-predominant skin inflammation.
Figure 2. WD intake promotes IL-23-mediated skin inflammation accompanied by infiltration of γδ T cells and an enhanced Th17 response.
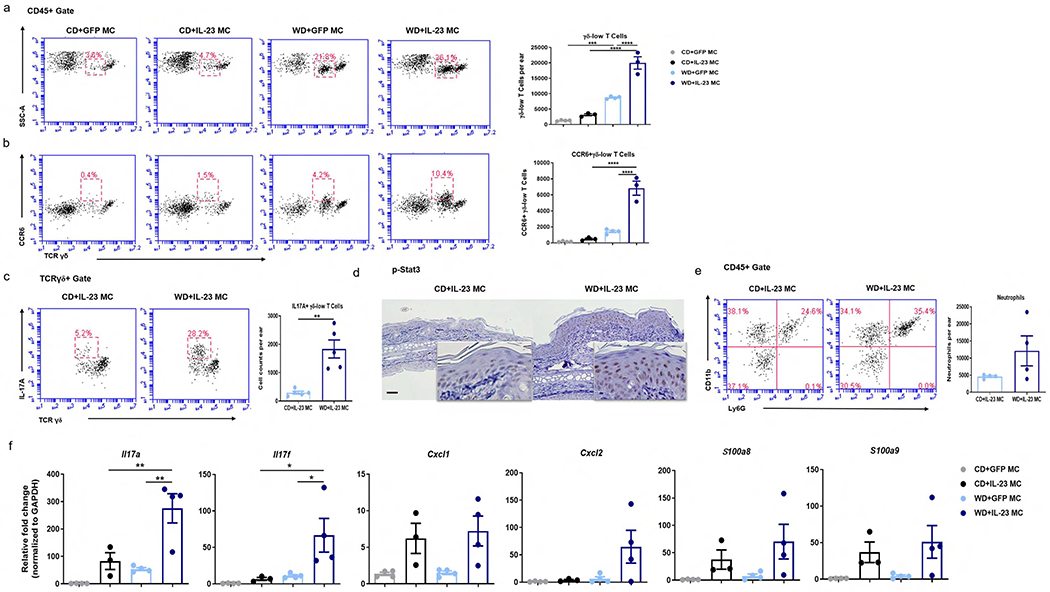
(a, b) Representative flow cytometry plots and absolute numbers of total (a) and CCR6-positive γδ-low T cells (b) in ear skin from mice with indicated treatment. (c) Representative flow cytometry plots and absolute numbers of IL-17A-producing γδ-low T cells, (d) representative images of immunohistochemical staining of p-stat3 (scale bars, 50 μm), (e) representative flow cytometry plots and absolute numbers of neutrophils in ear skin from CD-fed and WD-fed after IL-23 MC delivery. (f) Gene expression of psoriasis-related cytokines in ear skin from mice with indicated treatment. All of the data are presented as mean ± SEM. 3-4 mice per group. Data are representative of two independent experiments. * p <0.05, ** p <0.01. *** p <0.001, **** p <0.0001, by using one-way ANOVA with Bonferroni’s test in (a, b, f) and Student’s t-test in (c, e).
WD intake enhances susceptibility to IL-23-mediated joint inflammation
C57BL/6 mice are known to be a strain that is relatively resistant to experimental arthritis (Pan et al., 2004). Previously, we showed that around 80% mice of C57BL/6 background developed digital swelling after IL-23 MC delivery at a dose of 10 μg (Shi et al., 2020a). When we injected half dose to improve the survival of the WD group, however, we failed to observe clinical signs of joint inflammation in CD-fed mice. With the 5 ug dose, WD-fed mice developed gross dactylitis 4 weeks following IL-23 gene transfer (Figure 3a). Histologically, the “sausage” digit in WD+IL-23 MC was characterized by subcutaneous cellular infiltrate and edema. Cellular infiltrate surrounding the tendon and its bone insertion was also observed in distal interphalangeal joints from WD-fed but not from CD-fed mice. Moderate hypertrophy of the synovial lining and increased cellular infiltrates in the synovium were noted in metatarsophalangeal joints from WD-fed mice. Apparent signs of bone destruction or inflammation in Achilles tendon were found in neither CD-fed mice nor WD-fed mice (Figure 3b). The proportion and absolute number of IL-17A-producing CD3+ T cells (Figure 3c) and γδ T cells (Figure 3d) were increased in the popliteal lymph nodes (pLN) from WD+IL-23 MC mice compared to those from CD+IL-23 MC mice. WD exposure resulted in elevation of all measured pro-inflammatory markers in paw tissue, including Il17a, Il17f, Il6, Il1b and Tnf (Figure 3e). In addition, the expression of Tnfsf11 (RANKL, a gene involved in bone remodeling) (Walsh and Choi, 2014) was also increased in WD+IL-23 MC mice compared to CD+IL-23 MC mice. However, no difference was observed in the expression of Tnfrsf11b (OPG, the natural inhibitor of RANKL) (Walsh and Choi, 2014) between the two groups.
Figure 3. Exposure to WD exacerbates IL-23-mediated joint inflammation in C57BL/6 mice.
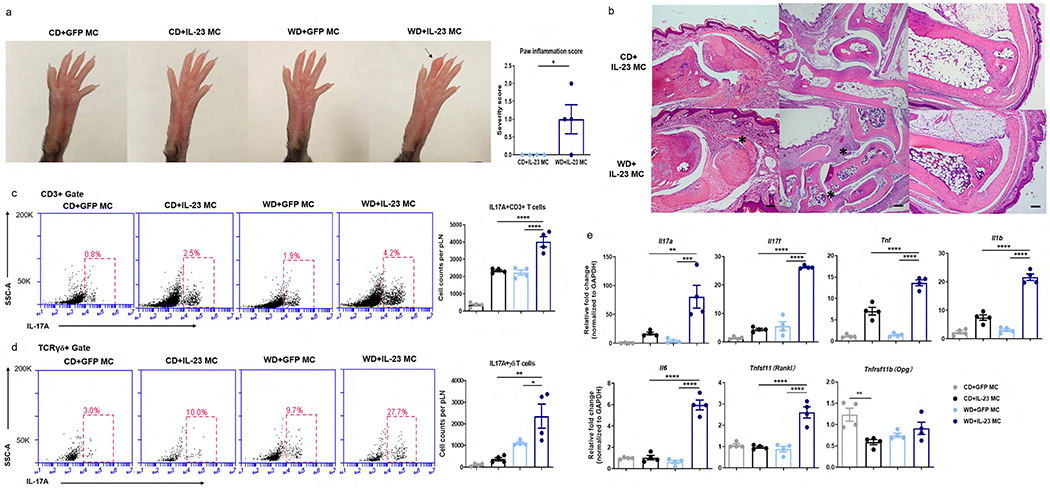
(a) Clinical photographs showing dactylitis and paw inflammation score. Arrow indicates dactylitis.(b) Images of H&E sections from distal interphalangeal joint (left panel, scale bars, 50 μm, asterisk indicates tendon inflammation), ankle joints (middle panel, scale bars, 200 μm, asterisk indicates synovial inflammation ) and Achilles tendon (right panel, scale bars, 200 μm) in WD-fed, IL-23 MC injected mice. (c, d) Representative flow cytometry plots and absolute numbers of IL-17A-producing CD3+ T cells (c) and γδ T cells (d) in popliteal LN. (e) Gene expression of pro-inflammatory cytokines and osteoclastogenesis-related markers in paw tissue from mice with indicated treatment. All of the data are presented as mean± SEM. 4 mice per group. Data are representative of two independent experiments. * p <0.05, ** p <0.01. *** p <0.001, **** p <0.0001, by using Student’s T test in (c, d) and one-way ANOVA with Bonferroni’s test in (e).
To further confirm the impact of diet on IL-23-mediated joint inflammation, we subjected B10.RIII mice, an autoimmune-prone mouse strain (Nandakumar and Holmdahl, 2005), to a similar feeding and hydrodynamic injection protocol, but adjusted the dose of IL-23 MC to 3 μg, as this strain experienced even more severe gut symptoms with a dose of 10 μg (data not shown). WD-fed B10.RIII mice developed remarkably swollen wrist and ankle joints 4 weeks after gene delivery, whilst CD-fed mice only developed dactylitis (Supplementary figure 2a). Consistently, the paw swelling score was significantly higher in WD-fed versus CD-fed mice (Supplementary figure 2b). Histologically, WD-fed mice had remarkable pannus formation with destruction of the articular surface, while CD-fed mice only exhibited moderate synovial inflammatory cell infiltration (Supplementary figure 2c). IL-23 MC delivery also resulted in enthesitis, as evidenced by leukocytic infiltration near the Achilles tendon and synovio–entheseal complex in mice fed with WD, which was absent in mice on CD (Supplementary figure 2d, e). The enhanced inflammation in WD-fed mice was corroborated by increased infiltration of Gr-1+ neutrophils and F4/80+ macrophages into ankle joints and entheses (Supplementary figure 2f, g). Collectively, in addition to skin inflammation, WD consumption also predisposes mice to IL-23-mediated joint inflammation.
The effect of WD intake and IL-23 overexpression on the gut microbiota
Because recent studies suggest gut microbiota is dysbiotic in patients with psoriasis (Benhadou et al., 2018), we next investigated the effect of WD intake on gut microbiota in the IL-23-mediated psoriasis mouse models. Alpha diversity analysis (Observed OTUs measure) showed that WD-fed had a lower diversity compared to CD-fed mice ,and that overexpression of IL-23 led to loss of microbial diversity only in WD-fed mice (Figure 4a). Beta diversity analysis using unweighted UniFrac distance PCoA (Principal Coordinates Analysis) plots showed a clear separation between CD and WD-fed mice and that IL-23 overexpression had a more pronounced effect in WD-fed than on CD-fed mice (Figure 4b). These results show that WD had a striking effect in the microbiome community structure and that WD-induced changes are exacerbated with IL-23 overexpression.
Figure 4. Diet and over-expression of IL-23 influence gut microbiota in a synergistic manner.
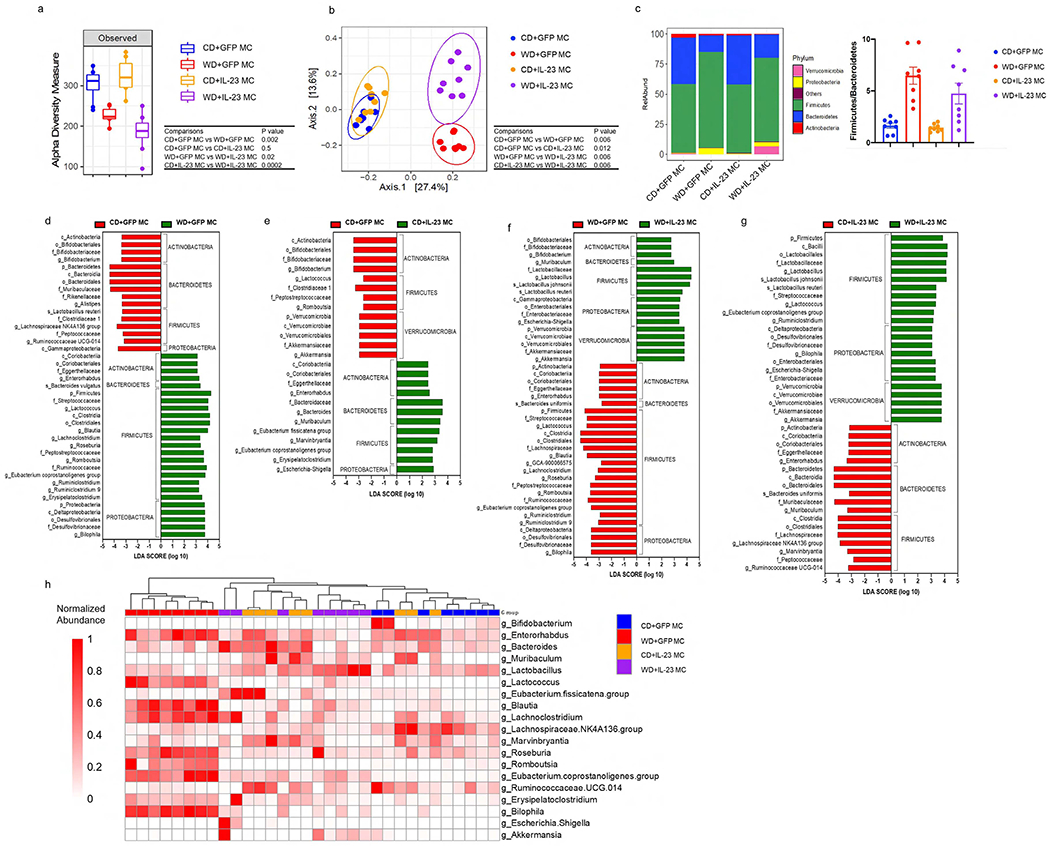
(a) Observed index of bacterial diversity, (b) PCoA of UniFrac distances, (c) bacterial classification at the phylum level and (d-g) LEfSe analysis showing significant differences in bacterial abundance between indicated groups. The threshold of logarithmic LDA score was 2. (h) Heatmap showing the most discriminative genus among the groups. n = 8 per group. The significance was assessed by Mann–Whitney U test in (a) and by Paired-Permanova test with Bonferroni correction in (b).
The most abundant phyla were Firmicutes and Bacteroidetes in all four groups of mice. Increased Firmicutes/Bacteroidetes (F/B) ratio has been associated with obesity and WD consumption (Turnbaugh et al., 2008). In mice injected with GFP MC, WD led to a 4-fold increase in the F/B ratio, thus confirming previously results (Turnbaugh et al., 2008). However, IL-23 overexpression did not affect the F/B ratio in CD-fed and WD-fed mice (Figure 4c).
We applied LEfSe (Linear discriminant analysis Effect Size) analysis to identify bacterial taxa differentially associated with each treatment by performing pairwise comparisons between groups (CD+GFP MC vs. WD+GFP MC; CD+GFP MC vs. CD+IL-23 MC; WD+GFP MC vs. WD+IL-23 MC; CD+IL-23MC vs. WD+IL-23MC) (Figure 4d–g). Within the Firmicutes phylum, in animals injected with GFP MC, WD led to increased abundance of several members of Rumminococaccea and Lachnospiracea family, including Blautia, Lachnoclostridium and Roseburia. By contrast, Bacteroidales (Muribaculaceae, Rikenellaceae and Alistipes) were decreased in WD-fed mice relative to CD-fed mice (Figure 4d).
The effects of IL-23 overexpression were markedly more pronounced in WD-fed than in CD-fed mice. A total of 25 and 43 differentially abundant taxa were found in CD-fed and WD-fed mice, respectively, following IL-23 MC injection (Figure 4e, 4f). CD+IL-23 MC mice had increased abundance of Bacteroidetes associated with depletion of Verrucomicrobia (Akkermansia). Escherichia-Shigella (Gammaproteobacteria) and Eubacterium were also enriched whereas Bifidobacterium was depleted (Figure 4e). WD+IL-23 MC mice showed higher levels of Bifidobacterium, Lactobacillus, Escherichia-Shigella and Akkermansia levels. This increase was associated with depletion of several Firmicutes (Blautia, Roseburia, Ruminoclostridum, etc) (Figure 4f). Interestingly, some changes induced by IL-23 delivery showed an opposite pattern in CD-fed and WD-fed mice. For instance, Akkermansia and Bifidobacterium were depleted in CD+IL23 MC compared to CD+GFP MC mice but enriched in WD+IL23 MC compared to WD+GFP MC group (Figures 4e, 4f). These results indicate that IL-23-induced changes are dependent of the baseline composition of the gut microbiota. Compared with CD+IL-23 MC group, WD+IL-23 MC group had enriched abundance of Lactobacillales (Lactobacillus and Lactococcus), Proteobacteria and Akkermansia whereas Lachnospiraceae, Bacteroidetes and Actinobacteria members were predominantly depleted (Figures 4g). The most discriminative bacteria genera among the groups are represented in the Heatmap (Figure 4h). All the bacteria taxa discriminated between the groups are provided in Supplemental Table 1–4. Together, our data suggest that WD and over-expression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner.
The pro-inflammatory effect of WD intake is abrogated by antibiotic treatment
We next investigated whether or not gut dysbiosis is a potential mechanism by which WD predisposes mice to IL-23-mediated inflammation. WD-fed mice were treated with broad-spectrum coverage antibiotics (ATB) 6 weeks prior to IL-23 MC induction until the end of the experiment. Strikingly, compared to vehicle-treated mice, ATB-treated mice had reduced erythema and scaling (Figure 5a) along with diminished ear swelling (Figure 5b). H&E staining further revealed that ATB-treated mice had reduced celluar infiltrates and a 40% reduction in epidermal hyperplasia (Figure 5c, d). Additionally, treatment with ATB suppressed nuclear staining of Ki-67 and phospho-Stat3 (Figure 5e). Consistently, infiltration of IL-17A-producing γδ-low T cells and neutrophils was profoundly impaired (Figure 5f), and Cxcl2 expression was reduced in the skin of ATB-treated mice (Figure 5g). Compared to vehicle-treated mice, pLN from ATB-treated mice exhibited a reduction in IL-17A-producing CD3+ T cell and γδ T cell infiltration (Figure 5h). ATB treatment also suppressed Il6 and RANKL expression in paw tissue (Figure 5i). Of note, the effects of ATB treatment did not solely affect WD-fed mice. CD-fed mice also demonstrated moderate attenuations in skin inflammation following ATB treatment as evidenced by reduced ear thickness, epidermal thickness, and infiltration of IL-17A-producing γδ-low T cells and neutrophils (Supplementary figure 3). Interestingly, when naïve mice were co-housed with MC-injected mice to achieve transmission of gut microbes via coprophagy, the numbers of IL-17A-producing T cells in cervical lymph nodes (cLN) and pLNs were significantly higher in naïve mice co-housed with IL-23 MC-injected mice than those with GFP-injected mice (Supplementary figure 4). These data suggest that systemic IL-23 expression leads to gut dysbiosis, which may in return exacerbate the IL-23-mediated inflammation.
Figure 5. The pro-inflammatory effect of WD is abrogated by antibiotic treatment.
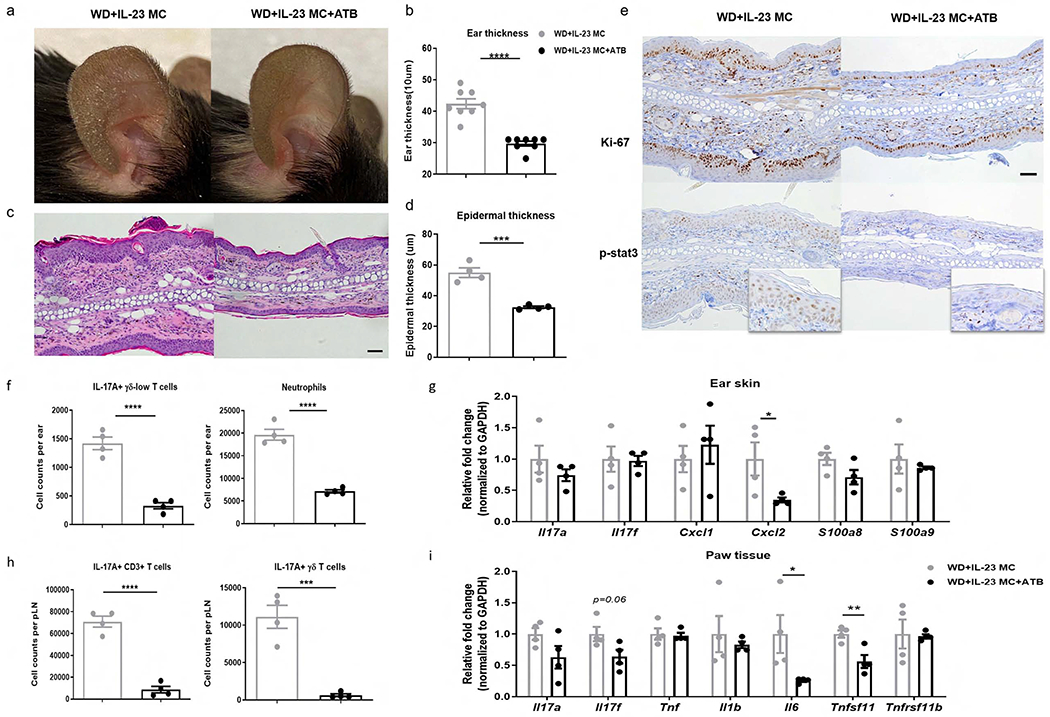
WD-fed C57BL/6 mice were treated with broad-spectrum combination antibiotics (ATB) or vehicle daily for 6 weeks by oral gavage and then injected with IL-23 MC to initiate disease. (a) Representative photographs, (b) ear thickness, (c) image of H&E section (scale bars, 50 μm), (d) histological analysis of epidermal thickness, (e) representative images of immunohistochemical Ki-67 and p-stat3 (scale bars, 50 μm), (f) absolute numbers of IL-17A-producing γδ-low T cells and neutrophils, and (g) gene expression of proinflammatory markers in ear skin. (h) Absolute numbers of IL-17A-producing CD3+ T cells and γδ T cells in popliteal LN. (i) Gene expression of proinflammatory cytokines and osteoclastogenesis-related markers in paw tissue. All of the data are presented as mean± SEM. 4 mice per group. Data are representative of two independent experiments. * p <0.05, ** p <0.01. *** p <0.001, by using Student’s t-test.
To further establish a cause-effect relationship between gut microbiota and diet-induced alterations in immunity, we recolonized ATB-treated mice reared in SPF conditions by fecal microbiota transplantation (FMT) using stool from mice fed with WD or CD for 6 weeks followed by IL-23 MC injection (Supplementary figure 5a). Although there was no obvious difference in ear thickness between two groups of mice (data not shown), FMT from WD-fed mice resulted in significantly increased infiltration of CD45+leukocytes, neutrophils and γδ-low T cells in the ear skin after IL-23 MC delivery (Supplementary figure 5b). Moreover, mice received FMT from WD-fed mice had higher numbers of IL-17A-producing CD3+ T cells in both cLNs and mesenteric lymph nodes (mLN) as compared to those received FMT from CD-fed mice (Supplementary figure 5c). Collectively, these findings demonstrate that gut microbiota contributes to maximal WD-promoted IL-23-mediated inflammation.
Switching from a WD to CD attenuates IL-23-mediated inflammation
An important question that arises is whether or not augmentation of skin and joint inflammation caused by WD is reversible by changing diets. To test this possibility, mice were fed a WD for 6 weeks and then injected with IL-23 MC to initiate the disease. Mice were then randomly divided into two groups: (1) continued on the WD for another 4 weeks, (2) switched to a CD for the same length of time (Figure 6a). Mice that were switched to a CD had less scaling and reduced ear thickness compared to those maintained on a WD (Figure 6b, c). Similarly, histological analysis revealed diminished epidermal thickness and cellular infiltrates in mice switched from a WD to a CD (Figure 6d, e). This change in diet profoundly diminished Ki-67 nuclear staining but only slightly reduced phospho-Stat3 staining (Figure 6f). Flow cytometric analysis demonstrated a remarkable reduction in the absolute number of IL-17A+ producing γδ-low T as well as a notable, but statistically insignificant, decline in neutrophil infiltration in the ear skin from mice switched to CD (Figure 6g). Additionally, diet change resulted in significantly decreased levels of Cxcl2 in ear skin but had no effect on other markers (Figure 6h). Similar to what was observed in skin, there were less IL-17A producing CD3+ T cells and γδ T cells in pLN in diet-switched mice (Figure 6i). Moreover, diet-switched mice had reduced Il17a, Il6, and RANKL in the paw tissue compared to those maintained on a WD (Figure 6j).
Figure 6. Switching from a WD to CD attenuates IL-23-mediated inflammation.
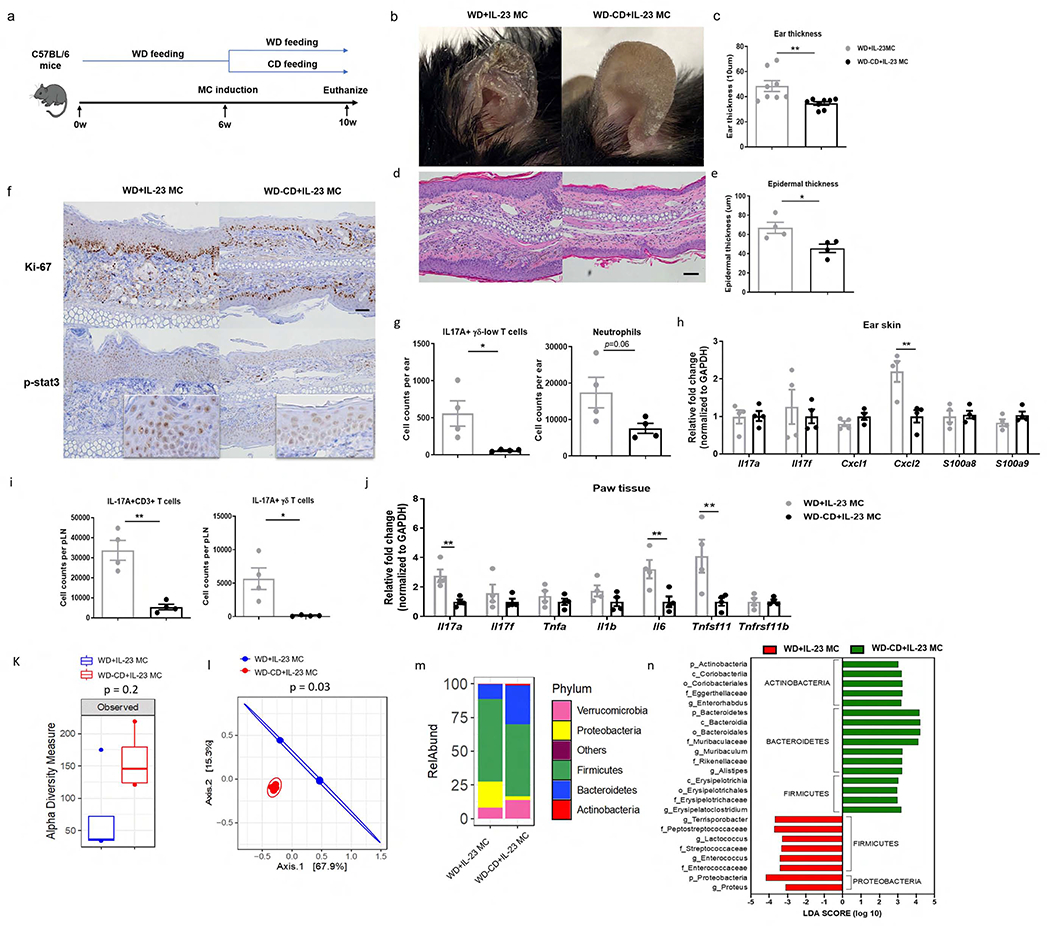
(a) C57BL/6 mice first received WD for 6 weeks and injected with IL-23 MC, which was then changed to CD for an additional 4 weeks. (b) Clinical photographs showing erythema and scales, (c) ear thickness, (d) image of H&E section (scale bars, 50 μm), (e) histological analysis of epidermal thickness, (f) representative images of immunohistochemical Ki-67 and p-stat3 (scale bars, 50 μm), (g) absolute numbers of IL-17A-producing γδ-low T cells and neutrophils, and (h) gene expression of proinflammatory markers in ear skin. (i) Absolute numbers of IL-17A-producing CD3+ T cells and γδ T cells in popliteal LN. (j) Gene expression of pro-inflammatory cytokines and osteoclastogenesis-related markers in paw tissue. (k) Observed index of bacterial diversity, (l) PCoA of UniFrac distances, (m) bacterial classification at the phylum level and (n) LEfSe analysis showing significant differences in bacterial abundance between two groups. The threshold logarithmic LDA score was 2. All of the data are presented as mean± SEM. 4 mice per group. Data are representative of two independent experiments. * p <0.05, ** p <0.01. *** p <0.001, by using Student’s T test in (c,e,g,h,i,j), by Mann–Whitney U test in (k) and by Paired-Permanova test with Bonferroni correction in (l).
Notably, diet switch also altered the gut microbiota composition. Compared to mice remaining on a WD, mice switched to a CD had increased α-diversity (Figure 6k). PCoA plots showed a high degree of divergence between mice maintained on a WD and mice switched to a CD (Figure 6l). Switching to CD led to an increase in the abundance of Bacteroidetes and a decrease in Proteobacteria and a subset of Firmicutes (Figure 6m,n,Supplemental Table5). In sum, these data suggest that the IL-23-mediated proinflammatory effects and alteration of gut microbiota by WD could be partially reversed by changes in diet.
DISCUSSION
Recently, reports revealed that gut dysbiosis is implicated in the development of psoriasis and psoriatic arthritis (Benhadou et al., 2018, Eppinga et al., 2014). Animal studies further showed that broad spectrum antibiotic treatment and germ-free environment lead to resistance to IMQ-induced PsD in mice (Stehlikova et al., 2019, Zakostelska et al., 2016), emphasizing the importance of gut microbiota in the pathogenesis of psoriasis. Interestingly, we found that overexpression of IL-23 in CD-fed mice increases the abundance of Lachnospiraceae and Ruminococcaceae but decreases that of Akkermansia and Bifidobacterium. Ruminococcaceae and Lachnospiraceae have been linked to several immune-mediated inflammatory diseases including inflammatory bowel disease (IBD) (Forbes et al., 2016) whilst Akkermansia and Bifidobacterium have been proposed as anti-inflammatory probiotics (Allen et al., 2017, de Vos, 2017). Moreover, we showed that co-housing with IL-23 MC-injected mice to achieve transmission of gut flora via coprophagy was enough to promote immune response in naïve mice (Supplementary figure 4). Thus, our data suggest for the first time that systemic IL-23 expression alters the microbiota composition in mice and possibly leads to dysbiosis that is associated with the development of exacerbated psoriatic skin and joint inflammation.
Consuming excess fat and carbohydrates as part of a WD negatively impacts the gut microbiota (Jena et al., 2017, Statovci et al., 2017). We and others showed WD exposure leads to dysregulated gut microbiota profiles in mice, including increased Firmicutes to Bacteroides ratio (Jena et al., 2018, Liu et al., 2018). Interestingly, several studies suggested that patients with psoriasis and/or PsA had similar signatures of dysbiosis to those of healthy controls (Benhadou et al., 2018, Scher et al., 2015), potentially making intestinal microbiota a pathogenic link between diet and the clinical manifestations of psoriatic inflammation. Indeed, our data showed that in mice switching from WD to CD, the ratio of Firmicutes to Bacteroidetes was decreased with ameliorated inflammation.
Of interest, WD intake with IL-23 MC treatment decreased the mRNA expression of barrier-forming tight junction (TJ) proteins (data not shown). This may be relevant since increased intestinal permeability, which is termed “leaky gut” has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis and enhanced immune response (Mu et al., 2017). A leaky gut has been observed in a number of autoimmune diseases, including psoriasis (Sikora et al., 2018). Furthermore, depletion of gut microbiota by antibiotic treatment restored the expression of TJ proteins in WD-fed, IL-23 MC-injected mice (Data not shown). We also found that the absolute numbers of IL-17A-producing CD3+ T cells and γδ T cells were increased in the mesenteric lymph nodes (mLN) from WD+IL-23 MC mice compared to those from CD+IL-23 MC mice and reversed by a switch to CD and through antibiotic treatment (supplementary figure 6). Thus, modulating gut microbiota may serve as a potential method for regulating intestinal permeability and inflammation.
While FMT from WD-fed mice promoted infiltration of immune cells in skin and draining LNs after IL-23 delivery, it failed to recapitulate clinical skin and joint phenotype, which might be due to suboptimal engraftment. Further studies using germ-free facilities with examination of engraft efficacy are warranted to demonstrate microbial sufficiency in promoting psoriatic phenotypes.
In conclusion, our study highlights the causative role of dietary components in the exacerbation of IL-23-mediated skin and joint inflammation, possibly through modulation of gut microbiota. Potentially, direct modulation of the gut microbiome may be a novel method for controlling psoriatic inflammation.
MATERIALS AND METHODS
Mice and diets
C57BL/6 mice or B10.RIII-H2r H2-T18b/(71NS)SnJ (B10.RIII) were purchased from The Jackson Laboratory (Bar Harbor, ME) and used in institutionally approved animal protocols (University of California, Davis). WD (21.2% fat, 17.3% protein, 46.7% carbohydrate, 34.1% sucrose, % by weight) and CD was purchased from Envigo Teklad (Madison, WI; WD: TD.140414, CD: Teklad global 18% protein).
IL-23 MC DNA production and hydrodynamic delivery
Minicircle-RSV.Flag.mIL23.elasti.bpA or RSV.eGFP.bpA was produced as described (Chen et al., 2005). Hydrodynamic delivery of IL-23 or GFP MC DNA via tail vein injection was performed as previously described (Adamopoulos et al., 2011). C57BL/6 mice was injected with 5 μg of IL-23 MC and B10.RIII mice was injected with 3 μg of IL-23 MC.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a National Psoriasis Foundation Discovery and Translational Grants to STH, a NIH/NIAMS R01 grant (1R01AR063091-01A1) to STH, and a NCI/NIM grant (1R01CA222490) to YJW. We thank Dr. Sebastian Yu for helpful comments and Drs. Iannis E. Adamopoulos and Cuong Thach Nguyen for providing IL-23 MC plasmid.
Funding sources: This study was supported by a National Psoriasis Foundation Discovery and Translational Grants and NIH/NIAMS R01 grant (1R01AR063091-01Al) to STH, a NIH/NCI grant (1R01CA222490 ) to YJW and the Department of Dermatology, University of California, Davis.
Abbreviations:
- AMPs
antimicrobial peptides
- ATB
broad-spectrum antibiotics
- CD
chow diet
- IL
interleukin
- H&E
hematoxylin and eosin
- HD
Hydrodynamic delivery
- MC
minicircle DNA
- PsA
psoriatic arthritis
- PsD
psoriasiform dermatitis
- γδ-low
TCR γδ-low expressing
- Th
T helper
- TNF-α
tumor necrosis factor-α
- WD
Western diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Detailed experimental procedures are described in supplementary methods.
DATA AVAILABILITY STATEMENT
Datasets related to this article can be found in the supplementary Data file or at https://trace.ncbi.nlm.nih.gov/Traces/sra/?study=SRP294311, hosted at Sequence Read Archive (SRA) data.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
REFERENCES
- Adamopoulos IE, Tessmer M, Chao CC, Adda S, Gorman D, Petro M, et al. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol 2011;187(2):951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AP, Clarke G, Cryan JF, Quigley EMM, Dinan TG. Bifidobacterium infantis 35624 and other probiotics in the management of irritable bowel syndrome. Strain specificity, symptoms, and mechanisms. Curr Med Res Opin 2017;33(7):1349–51. [DOI] [PubMed] [Google Scholar]
- Benhadou F, Mintoff D, Schnebert B, Thio HB. Psoriasis and Microbiota: A Systematic Review. Diseases 2018;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, He CY, Kay MA. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum Gene Ther 2005;16(1):126–31. [DOI] [PubMed] [Google Scholar]
- de Vos WM. Microbe Profile: Akkermansia muciniphila: a conserved intestinal symbiont that acts as the gatekeeper of our mucosa. Microbiology 2017;163(5):646–8. [DOI] [PubMed] [Google Scholar]
- Eppinga H, Konstantinov SR, Peppelenbosch MP, Thio HB. The microbiome and psoriatic arthritis. Curr Rheumatol Rep 2014;16(3):407. [DOI] [PubMed] [Google Scholar]
- Forbes JD, Van Domselaar G, Bernstein CN. The Gut Microbiota in Immune-Mediated Inflammatory Diseases. Front Microbiol 2016;7:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D, Franz S, Popkova Y, Anderegg U, Schiller J, Schwede K, et al. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J Invest Dermatol 2018;138(9):1999–2009. [DOI] [PubMed] [Google Scholar]
- Jena PK, Sheng L, Di Lucente J, Jin LW, Maezawa I, Wan YY. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity. FASEB J 2018;32(5):2866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena PK, Sheng L, Li Y, Wan YY. Probiotics VSL#3 are effective in reversing non-alcoholic steatohepatitis in a mouse model. Hepatobiliary Surg Nutr 2020;9(2):170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena PK, Sheng L, Liu HX, Kalanetra KM, Mirsoian A, Murphy WJ, et al. Western Diet-Induced Dysbiosis in Farnesoid X Receptor Knockout Mice Causes Persistent Hepatic Inflammation after Antibiotic Treatment. Am J Pathol 2017;187(8):1800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Zhang Y, Wang R, An Y, Gao W, Bai L, et al. Western diet feeding influences gut microbiota profiles in apoE knockout mice. Lipids Health Dis 2018;17(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Singh TP, Takekoshi T, Jia GF, Wu X, Kao MC, et al. CCR6 is required for epidermal trafficking of gammadelta-T cells in an IL-23-induced model of psoriasiform dermatitis. J Invest Dermatol 2013;133(1):164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez KB, Leone V, Chang EB. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 2017;8(2):130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Q, Kirby J, Reilly CM, Luo XM. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol 2017;8:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar KS, Holmdahl R. A genetic contamination in MHC-congenic mouse strains reveals a locus on chromosome 10 that determines autoimmunity and arthritis susceptibility. Eur J Immunol 2005;35(4):1275–82. [DOI] [PubMed] [Google Scholar]
- Pan M, Kang I, Craft J, Yin Z. Resistance to development of collagen-induced arthritis in C57BL/6 mice is due to a defect in secondary, but not in primary, immune response. J Clin Immunol 2004;24(5):481–91. [DOI] [PubMed] [Google Scholar]
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic Arthritis. N Engl J Med 2017;376(10):957–70. [DOI] [PubMed] [Google Scholar]
- Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med 2005;11(1):43–9. [DOI] [PubMed] [Google Scholar]
- Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol 2015;67(1):128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L, Jena PK, Hu Y, Liu HX, Nagar N, Kalanetra KM, et al. Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation. J Pathol 2017a;243(4):431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng L, Jena PK, Liu HX, Kalanetra KM, Gonzalez FJ, French SW, et al. Gender Differences in Bile Acids and Microbiota in Relationship with Gender Dissimilarity in Steatosis Induced by Diet and FXR Inactivation. Sci Rep 2017b;7(1):1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Garcia-Melchor E, Wu X, Yu S, Nguyen M, Rowland DJ, et al. Differential Requirement for CCR6 in IL-23-Mediated Skin and Joint Inflammation. J Invest Dermatol 2020a. [DOI] [PubMed] [Google Scholar]
- Shi Z, Wu X, Yu S, Huynh M, Jena PK, Nguyen M, et al. Short-Term Exposure to a Western Diet Induces Psoriasiform Dermatitis by Promoting Accumulation of IL-17A-Producing gammadelta T Cells. J Invest Dermatol 2020b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora M, Chrabaszcz M, Maciejewski C, Zaremba M, Waskiel A, Olszewska M, et al. Intestinal barrier integrity in patients with plaque psoriasis. J Dermatol 2018;45(12):1468–70. [DOI] [PubMed] [Google Scholar]
- Statovci D, Aguilera M, MacSharry J, Melgar S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front Immunol 2017;8:838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlikova Z, Kostovcikova K, Kverka M, Rossmann P, Dvorak J, Novosadova I, et al. Crucial Role of Microbiota in Experimental Psoriasis Revealed by a Gnotobiotic Mouse Model. Front Microbiol 2019;10:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 2008;3(4):213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front Immunol 2014;5:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Wu X, Zhou Y, Sheng L, Jena PK, Han D, et al. A Western diet, but not high fat and low sugar diet, predisposes mice to enhanced susceptibility to imiquimod-induced psoriasiform dermatitis. J Invest Dermatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakostelska Z, Malkova J, Klimesova K, Rossmann P, Hornova M, Novosadova I, et al. Intestinal Microbiota Promotes Psoriasis-Like Skin Inflammation by Enhancing Th17 Response. PLoS One 2016;11(7):e0159539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


