Abstract
The gut-brain axis plays an important role in maintaining homeostasis. Many intrinsic and extrinsic factors influence signaling along this axis, modulating the function of both the enteric and central nervous systems. More recently the role of the microbiome as an important factor in modulating gut-brain signaling has emerged and the concept of a microbiota-gut-brain axis has been established. In this review, we highlight the role of this axis in modulating enteric and central nervous system function and how this may impact disorders such as Irritable Bowel Syndrome and disorders of mood and affect. We examine the overlapping biological constructs that underpin these disorders with a special emphasis on the neurotransmitter serotonin, which plays a key role in both the gastrointestinal tract and in the brain. Overall, it is clear that although animal studies have shown much promise, more progress is necessary before these findings can be translated for diagnostic and therapeutic benefit in patient populations.
Lay Summary:
25–30 words and very briefly summarize the article’s fundamental components: In this comprehensive review, the authors have united the known and most recent evidence of how the microbiota-gut-brain axis modulates enteric and central nervous system development and function to impact disorders such as Irritable Bowel Syndrome and those affecting mood and affect
Introduction: Microbiota-gut-brain axis
The understanding that the brain and gut participate in continuous, bidirectional communication was recognized as remotely as in Ancient Greece where philosophers such as Hippocrates, Plato and Aristotle postulated that the brain and the rest of the body are intrinsically connected. This notion led to the understanding that in order to study disease processes, the whole person must be considered rather than an isolated organ system1. It wasn’t until the 1840s, however, that William Beaumont experimentally showed that emotional status affected the rate of digestion and, thus, that the brain affects the gut and that there is a brain-gut axis. Although this concept was subsequently recognized by the greats of modern biology including Darwin, Pavlov, James, Bernard and Cannon2, it took until the early to mid 20th century for the first scientifically recorded observations to be made that correlated gut physiology changes with changes in emotion. These studies were limited, however, by simple techniques and the lack of study of the reciprocal effects of changes in gut physiology on mental function1. Emerging data has confirmed connections between brain and gut health and has further suggested several mechanistic underpinnings. Alterations in gastrointestinal (GI) function and GI symptoms have been reported to accompany an increasing number of central nervous system (CNS) disorders and, as in the case of Parkinson’s disease, GI dysfunction might occur even before central neurological symptoms become evident3. Similarly, GI symptoms are an important component of disorders of brain-gut interactions such as IBS, which are commonly associated with psychological symptoms and psychiatric diagnoses. Moreover, with the advent of brain imaging, the reciprocal interactions can be visualized for the first time, demonstrating that gut stimuli can activate key brain regions involved in emotion regulation2.
Most aspects of GI physiology are under neural control, which is exerted via a vast network of intrinsic enteric neurons and glia that span throughout the enteric nervous system (ENS), GI smooth muscle and the lamina propria of the mucosa, as well as extrinsic innervation from primary afferent and autonomic fibers that connect the intestine to the spinal cord and the brain4, 5. Although the ENS can regulate GI peristalsis largely independent of CNS input, GI motility is also modulated by factors extrinsic to the ENS, including the brain6 and other divisions of the autonomic nervous system (ANS), the gut associated immune system and the gut microbiome. The influence on the gut is not unidirectional, as the gut also sends information to these various systems through complex pathways that function as bidirectional conduits for homeostasis, and alterations in this communication are associated with disease. Adequate gut function is thus critical for not only long-term survival but also for brain-gut homeostasis. Precisely how gut-brain communication occurs in health and disease in humans, however, remains an active area of investigation.
More recently, the microbiome (the trillions of microrganisms that reside in the gut) has emerged as an integral player in gut-brain communication and a microbiome-gut-brain axis has been proposed7–10. Although mechanistic studies on how this expansive community of microorganisms influences human ENS and CNS development11, GI motility12, mood10, cognition and learning13 are still in their infancy, it offers itself as a potentially important site for future therapeutic interventions (Figure 1). Gut microbes communicate to the CNS through neuronal, endocrine, and immune signaling channels. Conversely, the CNS can affect the gut microbiota directly via stress mediator-induced virulence gene expression and indirectly through ANS-mediated control of gut function (e.g., motility, immune modulation and secretion)14 (Figure 1). In addition, the ENS can directly modulate microbial composition through changes in secretion, motility, permeability and immunological defense. These parallel and interacting pathways are thus emerging to investigators as a complex communication matrix which also has been referred to as the gut connectome15.
Figure 1: Pathways of communication between microbiota & brain.
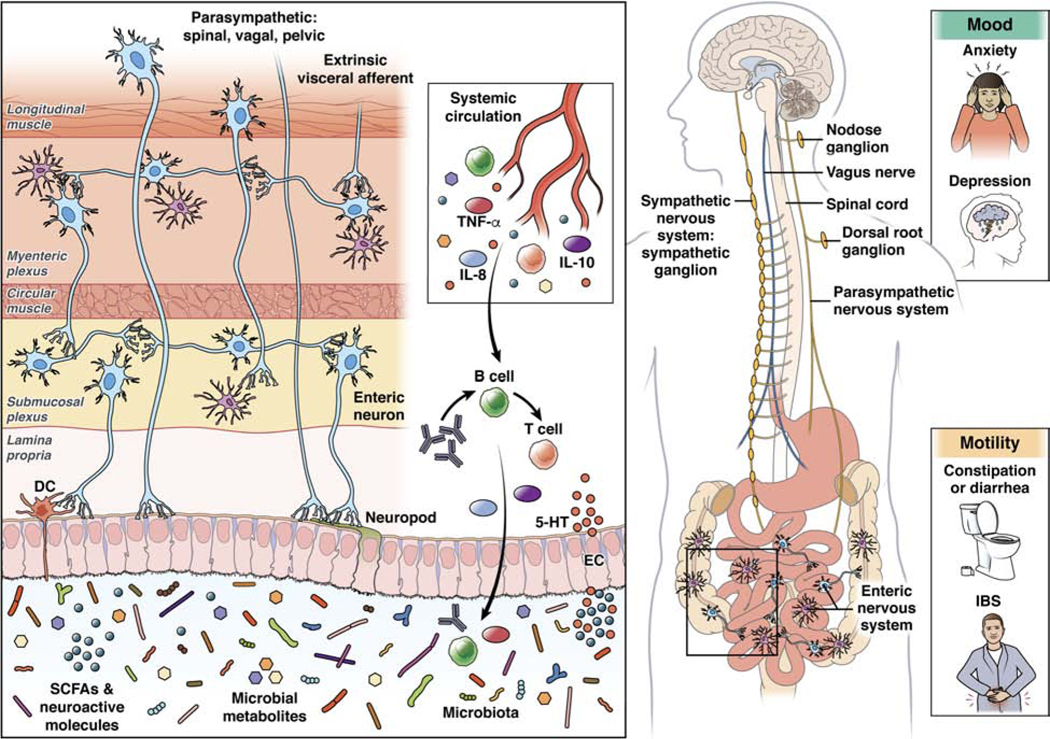
A growing body of research is implicating different pathways of communication between the microbiome and brain in disorders of both mood and motility. Multiple direct and indirect (via systemic circulation) pathways exist through which the gut microbiota can modulate the gut-brain axis. They include endocrine (cortisol), immune (cytokines) and neural (vagus, enteric nervous system and spinal nerves) pathways. Several gut microbes are capable of synthesizing neurotransmitters (i.e., γ-amino butyric acid (GABA), noradrenaline, and dopamine) locally, which can act on target cells in the gut and act as an important avenue of communication. Neuroactive microbial metabolites can modulate brain and behavior through a number of ways that are still being elucidated. These include affecting epithelial cells to impact gut barrier function and enteroendocrine cells (EECs) to release GI hormones, as well as dendritic cells (DCs) to modulate immune function. Specialized structures on EECs and ECCs, known as neuropods have been shown to transduce sensory signals from the intestinal milieu to the brain through forming synapse-like connections to afferent nerves, including the vagus nerve. The enteric nervous system is perfectly poised to be an integral hub for microbial signals and can communicate with the brain via vagal and spinal pathways. However, the exact molecular signaling pathways of all these pathways involved remain to be defined.
In addition to the contributions of the microbiome, studies in animal models have provided evidence that some GI dysfunction in neurological conditions may also be due to genetic defects and/or environmental influences that can simultaneously impact gut and brain development and/or function. Supportive of this notion are the demonstrations that the ENS, often described as a “second brain,” shares many likenesses with the CNS. Their shared structure, developmental patterns and neurochemistry have formed the basis for research in understanding how pathogenic mechanisms that give rise to CNS disorders might also lead to ENS dysfunction and vice versa. For example, one of the key transmitters in the CNS and the intestine, serotonin (5-HT), can act in neuroendocrine, endocrine and/or paracrine fashions to impact the development and long-term functions of both the ENS and CNS.
Given the critical involvement of the ENS, CNS and the microbiome in brain and gut development and function, a greater understanding of the relationships between these systems is likely to enable the development of novel therapeutic targets for some of the most common yet poorly understood medical conditions. The current state of research, while impressive, leaves many vital unanswered questions that need to be addressed in order to facilitate novel, effective therapeutic development. In this review, we address current evidence supporting the ways in which the brain, the gut and the gut microbiota interact and the emerging data supporting its contribution to human disease.
The Microbiota-Gut-Brain (MGB) Axis
Driven by the development of next-generation sequencing technologies in tandem with large cohort studies, the past decade has seen a dramatic increase in our understanding of the microbiome in many aspects of health and disease16. In humans, the greatest abundance of microbes is found in the distal gut that hosts approximately 3·1013 microbes from more than 60 genera17. Although bacteria are the most abundant and best-studied gut microorganisms, the multitude of archaea, yeasts, single-celled eukaryotes, helminth parasites and viruses are also more recently being considered, though the role that these other microorganisms play in MGB interactions is currently unknown. There are large inter-individual differences in the microbial composition and we are only beginning to understand the factors that influence this in health and disease 16. Moreover, there is a growing appreciation from cross sectional human studies that changes in the diversity and relative abundances of the microbiota and microbial metabolites are associated with a wide array of neurological and psychiatric disorders, including Parkinson’s disease, Alzheimer’s disease, Autism spectrum disorders and depression18. Results from these studies, however, have been inconsistent and without evidence establishing causality for the gut microbiome. Further, examination of the MGB axis in clinical populations has been mostly restricted to cross-sectional studies demonstrating associations of gut microbes with brain architecture in healthy subjects or disease states 19. There is thus a large gap in our understanding of the underlying mechanisms involved in MGB dialogue. Based largely on results obtained in preclinical animal models, the more well-studied routes of communication thus far include the immune system20, metabolites and neurotransmitters and vagal nerve activation 21, 22.
Microbiome-Gut-Brain Axis in early Life
Whether microbial colonization occurs in utero is not yet fully understood23. Maternal diet 24 and maternal stress exposure during pregnancy25, 26, however, have been shown to influence the infant microbiome, and it is clear that the maternal microbiome can play a key role in shaping infant host development and physiology 27, 28,29, 30. Intriguingly, the periods of major change in the developing microbiota overlap partially with the time-frames for development of other bodily systems and particularly the brain31 and the enteric nervous system11. This parallel development is likely to be biologically relevant, and these periods may correspond to sensitive periods in the development of the MGB axis that will be critical for establishing appropriate communication along the axis throughout the lifespan.
The postnatal microbiota is relatively volatile, gaining stability and diversity across maturation32. Most colonization of the infant gut starts at birth when delivery exposes the infant to a complex microbiota that are dependent on many elements, including mode of delivery, breastfeeding, prematurity, the environment, host genetics, antibiotic exposure, and maternal factors such as infection status, stress and/or obesity.
After the first several days of life, there is a shift towards a microbiota population focused on extracting nutrients in order to support the rapid development of the brain and body of the host33. A key component of gut microbiota differences may be dependent on whether an infant is breast or formula fed. While heterogeneity exists among the population characteristics and study techniques, most studies show that both diversity and richness of the microbiome are lower in breastfed than formula fed infants, with higher levels of Proteobacteria and Bifidobacteria, and lower levels Bacteroidetes and Firmicutes found in breast fed infants compared to those who are formula fed34, 35. However, these differences do not appear to be linked to infant behavioral distinctions such as those associated with colic35.
The last major change takes place at weaning, as the infant shifts from breastmilk or formula to a solid diet with a pattern observed across species, including humans and rodents36, 37. Although there are continuous changes well into adolescence 32, these alterations are more gradual and geared towards an adult-like profile 38. In the adult, diet has the greatest life-long influence on microbiota composition39 although antibiotic usage is also a key factor in disrupting the microbiota across the lifespan40.
Microbiota and CNS Development
Overall, fundamental central neural processes including development, myelination, neurogenesis and microglia activation have shown to be dependent on the composition of the microbiota 41,42−48,49, 50. The strongest evidence for a role of the microbiota in neurodevelopment comes from research in germ-free (GF) mice 41, 51. Studies where GF rodents have been recolonized with “normal” microbiota (i.e., from specific pathogen-free animals) at different ages have shown that post-weaning re-colonization is more effective at restoring GF deficits than re-colonization later in life, at least for specific aspects of brain or immune function and behavior52–56. Still other functions in GF animals, such as those affecting CNS serotonergic neurotransmission, cannot be restored by re-colonization by the age of weaning, suggesting that the window for microbial influence on these functions is already closed56.
Although these studies in GF mice have been important in providing evidence supporting the concept that the microbiome is involved in brain processes involved in stress hormone signaling, neural function and neuroprotection 51, there are significant limitations to human translation of these findings including, but not limited to, the presence of defects in the immune system development, ENS formation and CNS maturation of GF animals 57,41,42–48,49, 50. The mechanistic underpinnings of these relationships are also little understood.
The human studies that have sought to evaluate the relationship between the microbiota and CNS development remain limited; most have been conducted in infants and are largely cross-sectional. Studies that have extended follow-up to two years of age, however, have continued to show connections. Antibiotic exposure in infancy has been reported to have a negative impact on cognitive development 58. Another study linked cognitive function at two years of age with microbiota composition assessed one year earlier 59. More recently, the same research group demonstrated that microbiota alpha diversity was also related to cognitive outcomes at two years of age and, further, was associated with functional connectivity between the supplementary motor area and the inferior parietal lobule in infancy. Importantly, this connectivity was also related to cognitive outcomes at two years of age 60.
Enteric Nervous System Development
In the early postnatal period, enteric neuro- and glio-genesis is accompanied by a functional maturation of intestinal neural circuits. 33, 61. This evolution has been shown to continue beyond the postnatal period in preclinical models; enteric gliogenesis is maintained at low levels throughout life 62–64, enteric neuronal turnover may occur even more rapidly than that of glial cells 65 and changes in synaptic contacts within the enteric circuitry are seen in mice through adolescence 66.
To date, ENS development has been examined primarily from its molecular and genetic origins 67. An increase in the understanding of the importance of postnatal ENS development, however, has led to an emergence of literature focusing on factors that are contained within the postnatal gut microenvironment, including the presence of a complex gut microbiota and immune system11, 68–70.
Gut microbiota-driven effects on ENS development and function have been exemplified in studies on GF mice. These mice harbor reduced numbers and subtype distributions of enteric neurons that are associated with deficits in gut motility68–70 as well as attenuated excitability of intrinsic primary afferent neurons, a key component of gut-brain neural pathways71, 72. Conventionalization of adult GF mice reduces the deficit in intestinal transit time69, 73, restores neuronal excitability71, alters the chemical coding of enteric neurons and normalizes enteric glial cell density and gut physiology64, 69 demonstrating an important role for the microbiota in ENS plasticity. Similar effects have been noted after bacterial exposure through probiotics or specific bacterial strains74–76. Moreover, studies have also provided insight as to which microbial mechanisms may affect enteric nerve activity77, 78,78, 79. These include G-protein-coupled receptor-mediated signaling pathways 80, 5-HT, short chain fatty acids (SCFAs) 78, microbial-epithelial interactions 79 and the transcription factor, aryl hydrocarbon receptor (Ahr).
Ahr is a recognized biosensor for intestinal epithelial- and immune-cell homeostasis in the gut. As such, enteric neuron specific Ahr may serve as a node that integrates signals from the luminal microbiota environment with the physiological output of intestinal neural circuits to maintain gut homeostasis81. Interestingly, in a recent study it was shown that neuron-specific deletion of Ahr, or constitutive overexpression of its negative feedback regulator, CYP1A1, results in reduced peristaltic activity of the colon, similar to that observed in microbiota-depleted mice 76. Moreover, expression of Ahr in the enteric neurons of mice treated with antibiotics partially restores intestinal motility. These studies suggest that the ENS possesses an ability to monitor the luminal microbial environment and adjust neuronal activity and motility accordingly and thus provides a further basis for studies that examine the microbial detection mechanisms used to alter GI and or gut-brain physiology. Overall, more research is needed to understand the mechanisms by which the microenvironment of the gut lumen influences ENS plasticity.
Finally, reverse regulation in which the ENS contributes to the shaping of the microbiome is also possible and has been addressed by several preclinical studies, including several in which alterations in the composition of colonic and/or fecal microbiota were observed in murine or zebrafish models of congenital aganglionosis82,83. It still needs to be determined, however, whether these abnormalities represent direct effects of ENS circuits on microbiota or are merely the consequences of abnormal peristalsis.
Mechanisms of Microbiota to Gut-Brain Signaling
Vagus Nerve
As a major bidirectional highway of brain-gut connection, the afferent branch of the vagus nerve has been the focus of multiple studies examining its effects on brain-gut communication in health and disease 14, 84. While the sensory vagus nerve and ENS are intrinsically linked, the mechanisms underpinning this interaction and the role of vagal signalling from the ENS to the brain remain incompletely understood.
The afferent branch of the vagus nerve is the main neural conduit connecting the gastrointestinal tract to the nucleus of the solitary tract and higher emotion-regulating networks in the mammalian brain 85. Although it does not appear to interact with the gut microbiota directly, evidence suggests that the vagus nerve can sense microbial signals in the form of bacterial metabolites, or be influenced via microbiota-mediated modulation of enteroendocrine (EECs) and enterochromaffin (ECCs) cells in the gut epithelium 86 (Figure 2). For example, gut bacteria produce SCFA metabolites (e.g., butyrate, propionate, acetate and valerate) that regulate physiological intestinal functions, including those involving motility, secretion and inflammation (see below), through their cognate free fatty acid receptors (FFARs)87. Further, other receptors on vagal nerve fibers such as those for serotonin (5-HT3, 5-HT4) and other gut peptide receptors may also facilitate these messenger pathways86, 88. Vagotomy studies in mice also highlight possible roles for the vagus nerve in CNS-microbiota communication which may translate to human mood and neurobehavioral disorders. For example, in mice, vagotomy has been shown to block central signaling of Lactobacillus and Bifidobacterium species, resulting in the besiegement of their mood-modifying effects55, 89, 90.
Figure 2: Serotonin (5-HT) as a critical regulator of gut-brain-microbiome axis signaling.
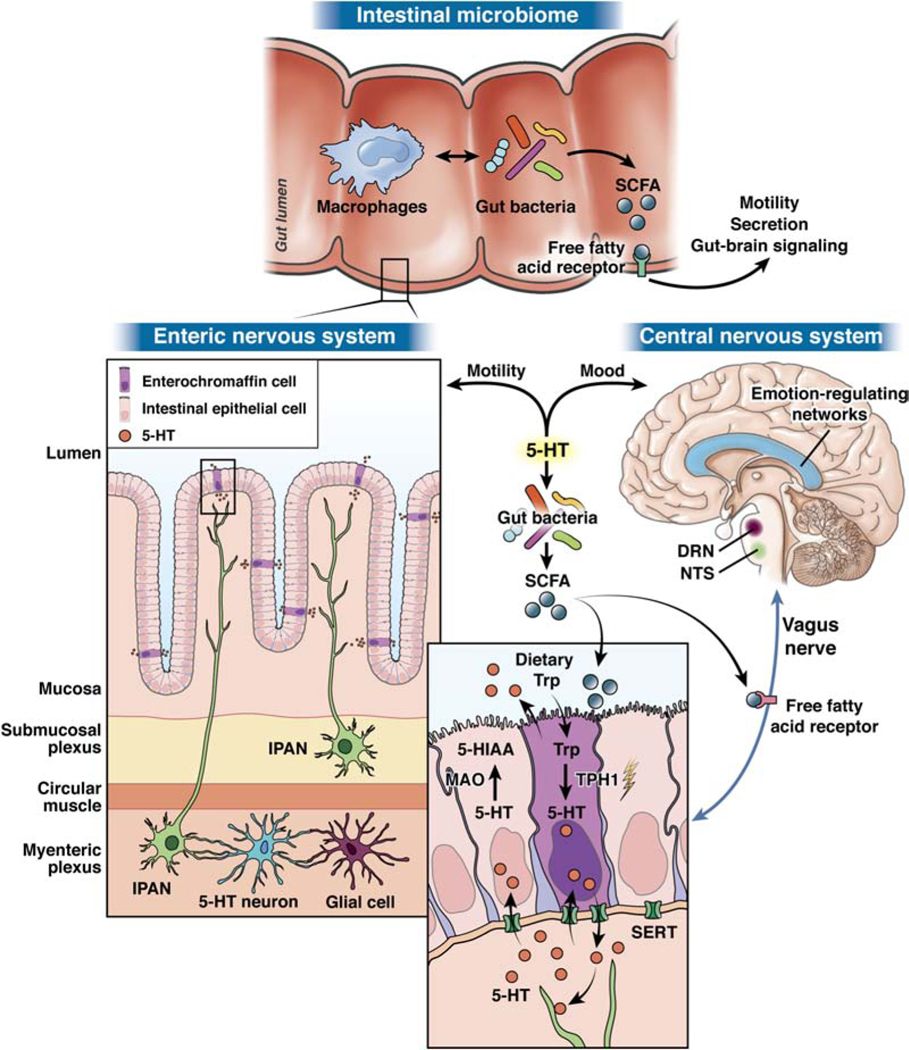
Gut bacteria in the intestinal microbiome produce short-chain fatty acids (SCFAs) that directly stimulate tryptophan hydroxylase 1 (TPH1), resulting in 5-HT synthesis in and secretion from intestinal enterochromaffin (ECC) cells. 5-HT released from the basal membrane of intestinal EC cells then interacts with receptors from neurons in the enteric nervous system to modulate motility and, during development, neuronal development and differentiation. Vagal afferents signal to the nucleus of the solitary tract (NTS) and the dorsal raphe nucleus (DRN), the latter of which houses the majority of the brain’s 5-HT neurons. These areas then interact with emotion-regulating brain networks that influence mood. Of note, SCFAs produced by gut bacteria can also directly stimulate free fatty acid receptors on multiple cell types, including epithelial cells, ECCs, immune cells, and nerve cells, including the vagus nerve and primary afferent neurons. This signaling can also modulate downstream regulation of motility, secretion, and gut-brain signaling. Abbreviations: Trp: tryptophan; SERT: serotonin reuptake transporter; MAO: monoamine oxidase.
A bidirectional communication system between diet, the gut microbiome, ECCs and the vagus nerve has recently been reported. ECCs contain more than 90% of the body’s serotonin (5-HT) and 5-HT synthesis and release in ECCs is modulated by SCFAs and 2BAs produced by spore-forming Clostridiales12, 91. These microbes increase their stimulatory actions on ECCs with increased dietary tryptophan availability92. ECCs also communicate with afferent nerve fibers through synaptic connections of neuropod-like extensions of ECCs93. On the other hand, the ANS can activate ECCs to release 5-HT into the gut lumen, where it can both be taken up by serotonin transporter-like mechanisms and influence gut microbial function 94.
Immune Mechanisms for microbiota to gut-brain signaling
In the gut, an intact immune system is critical for maintaining the careful balance between homeostatic tolerance of commensal organisms and the simultaneous protection of the body against pathogenic microbial invasion. In addition,immunity also serves a critical role in mediating communication between the gut microbiota, the ENS and the brain. Toll-like receptors (TLRs) and peptidoglycans (PGNs) mediate the immune response to microbes by acting as sensors of microbial components95, 96.An intact gut barrier also prevents the inappropriate activation of immune cells and the development of systemic immune activation.
Bacteria can release immune agonists, such as lipopolysaccharide (LPS) and PGN, into the circulation where they can gain access to the brain. TLRs have been found in the brain of mouse disease models, and especially the microglia, where they have been studied in the development of Alzheimer’s Disease97, Parkinson’s disease 98, visceral pain99 and depression100. GF and antibiotic-treated mice also both display a reduction in the expression of several of the receptors that detect PGN in the striatum, which suggests that gene expression in the brain is sensitive to microbiota manipulation. 101. Moreover, knockdown of a PGN sensing receptor, PGN-recognition protein 2, resulted in an increase in sociability in mice, indicating that loss of the ability to sense peptidoglycan results in host behavioral changes101. More research is needed, however, to unravel the functional consequences of such immune signaling across the lifespan in health and disease
Diet-induced changes in the gut microbiome can lead to a compromised mucus layer, allowing access of luminal microbes to extensions of dendritic cells, resulting in activation of these cells by both pathogens and commensals. This local immune activation can lead to increased permeability of the epithelial tight junctions that further compromises the intestinal barrier. The diet-induced release of immune mediators into the systemic circulation is referred to as metabolic endotoxemia, which can lead to immune activation in different organs, including the brain102. This low grade immune activation has been implicated in the pathophysiology of some forms of depression and neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease.
Immune Signaling and the ENS
TLRs and other components of the innate immune system (e.g., macrophages) may serve as sensors of gut microbial presence and deliver messages to the ENS that result in changes in gut nervous system development and function. Enteric neurons and glia have the machinery necessary to detect the gut microbiota; they both express TLR2 and TLR468, 103. Further, antibiotic depletion of the microbiota alters TLR expression in mice and also results in concomitant alterations in GI motility and sensitivity to acetycholine 104.These effects may, at least in part, may be mediated by TLR4 and/or TLR2. TLR4 deficient mice have decreased numbers of nitrergic neurons and reduced motility, a similar phenotype to that observed in GF and antibiotic-treated mice68. Mice deficient in TLR2 signaling exhibit abnormalities in the neurochemical coding of the ENS that is accompanied by gut dysmotility and attenuated chloride production in intestinal explants 103. Further, antibiotic treatment of wild-type mice leads to ENS abnormalities that can be reversed after supplementation with a TLR2 agonist, further confirming the idea that the gut microbiota-TLR2 axis is important for ENS morphology and function103.
These data suggest that the ENS has the capacity to respond to stimuli from distinct types of microbes affecting its physiology. Precisely how microbe-TLR communication affects ENS structure and function, and how these changes relate to gut-brain signaling, have yet to be determined. For example, it would be important to determine how alterations in TLR activation during early-life, including those induced by infections and/or antibiotics, can affect ENS development and long-term function of brain-gut interactions. The plasticity of the ENS demonstrated in these studies and others make this likely to be high-yielding area of therapeutic investigation.
Macrophages are present throughout the gut where they play an essential role in the reparative response to intestinal injury 105, 106. Monocyte-derived and tissue-resident macrophages are decreased in quantity in GF mice or in mice that that are microbiota-depleted with antibiotics, implying a role for the microbiota in intestinal macrophage recruitment and differentiation107. Further, muscularis macrophages (MMs) engage in a bidirectional relationship with enteric neurons that appears to be regulated by the gut microbiota; MM activation by the cytokine, bone morphogenetic protein 2 (BMP-2) results in alterations in GI motility and production of macrophage colony-stimulating factor 1 (CSF1), a critical mediator of MM development, and both CSF1 and BMP2 production are decreased after antibiotic treatment 107. Microbiota-neuron-macrophage interactions have also been exemplified in studies showing that sympathetic ganglia activation elicited by S. typhimurium may influence macrophages to protect the ENS by stimulating processes that both limit enteric neuronal damage and enhance gut motility108. The ENS may also protect itself from invasive S. typhimurium infection by producing IL-18, a cytokine that both drives goblet cell antimicrobial peptide production and maintains the mucosal barrier 109. It has most recently been demonstrated that the gut microbiota may influence gut-extrinsic sympathetic activation through a gut-brain circuit110.
Microbial Metabolites
The gut microbiota generate metabolites that are implicated in the modulation of both CNS and ENS physiology and behavior. Although in vitro studies have shown that specific bacteria can produce neurotransmitters (e.g., noradrenaline, dopamine and GABA)111, 112, whether these neurotransmitters are capable of reaching specific targets within the CNS, and/or in sufficient concentrations, is unknow. The short half-lives of most neurotransmitters and their limited ability to cross the blood brain barrier, however, make this possibility unlikely. The way this gut-brain communication may happen has been studied most extensively for serotonin and other tryptophan metabolites (see below) which have been shown to exert major influences on ENS and CNS development and functions including GI motility, mood and behavior113–118.
Short Chain Fatty Acids
Gut bacteria produce SCFA metabolites that can regulate motility, secretion, and gut-brain signaling by acting through FFARs on epithelial cells, EECs, ECCs, immune cells, and intrinsic and extrinsic neurons12, 91 (Figure 2) 119. Centrally, administration of acetate, propionate and butyrate is capable of restoring morphological deficits of microglia in GF mice 120 and reversing the behavioral and physiological effects of chronic stress 121. Moreover, SCFAs may influence the production of neurotransmitters in the brain through regulating the expression of enzymes involved in their biosynthesis; administration of propionate and butyrate to PC12 cells (neuroblastic cells which differentiate to neuron-like cells) in vitro increased the expression of tyrosine hydroxylase, the rate-limiting enzyme involved in noradrenaline and dopamine synthesis 122. Whether these microbial metabolites are capable of regulating neurotransmission in vivo, however, is not clear. More evidence is also needed to determine the extent to which physiologically relevant concentrations of SCFAs are capable of reaching the brain given their relatively short half-life (25 minutes to three hours). To date, studies investigating the effect of exogenous SCFA administration upon brain physiology and behavior have typically used concentrations that far exceed what is microbially derived 123.
Serotonin: A key regulator of the MGB Axis in the Gut and Brain
There is growing appreciation that the neurotransmitter 5-HT is one of the key players in MGB axis signaling in both the brain, the gut and in gut to brain communication. Indeed, recent studies have shown that specific spore-forming bacteria from both humans and mice increase colonic and serum 5-HT levels in GF mice and ameliorate GF-associated gut dysmotility by producing SCFAs124, which increase 5-HT production by up-regulating Tph1 expression in ECCs12, 91 (Figure 2). Recent evidence suggests that 5-HT released from ECCs communicates with the gut microbiota, Turicibacter sanguinis, that possesses serotonin uptake mechanisms which are involved in its colonization and host physiology125. This may account for some of the bidirectional influences that occur between some psychotropic drugs, including SSRIs, and the intestinal microbiota126. Gut microbiota has also been shown to induce maturation of the adult ENS via activation of 5-HT4 receptors (5-HT4R); GF mice retain a higher degree of nestin-expressing neuronal stem cells and exhibit slower intestinal transit and both factors normalize following bacterial colonization that is dependent on 5-HT4R signaling73. Finally, the gut microbiota may also act through neurotransmitter precursors; it can influence serotonergic neurotransmission through regulating the availability of the 5-HT precursor, tryptophan. Circulating tryptophan concentrations are significantly higher in male GF mice relative to conventional controls 56 and these altered tryptophan levels correspond with an increase in hippocampal serotonin, and its metabolite, 5-hydroxy-indole acetic acid 56. Whether this has any bearing on the social deficits observed in these animals, however, requires further investigation.
Tryptophan Metabolism: Beyond Serotonin
The main physiological pathway for tryptophan metabolism is along the kynurenine pathway. GF mouse studies have shown an increased availability of tryptophan in GF animals as a consequence of a reduction in peripheral kynurenine pathway activation56. Moreover, in a rodent model of chronic variable stress, a stress induced reduction of Lactobacilli reduced hydrogen peroxide (H202)-mediated inhibition of Indoleamine 2, 3-Dioxygenase 1 (IDO1). This inhibition resulted in an increase in the conversion of tryptophan to kynurenine, a feature that was linked to depression-like behavioral alterations in mice exposed to chronic stress127. In contrast to serotonin, kynurenine can traverse the blood brain barrier and negatively impact brain health by inducing neuroinflammation and neurodegeneration128.
Indole production which is limited to the gut microbiota, is catalyzed by the microbial enzyme, tryptophan hydroxylase and indole has been detected in blood, brain and the GI tract 92. There is a growing literature supporting the concept that the microbial processing of tryptophan to indole affects gut-brain axis function129. Indoles exert many beneficial actions on intestinal and systemic homeostasis130.Yet, adverse effects on the gut-brain axis are also evident following absorption from the gut and host processing, where some indole derivatives, have been shown to exert neurodepressive-like affects on behavior, at least in preclinical studies131.
Tryptamine
Bacteria express tryptophan decarboxylase and are capable of producing tryptamine from dietary tryptophan. It has recently been demonstrated that bacterial-derived tryptamine can act via the 5-HT4R to impact gastrointestinal motility88. The capacity of the gut microbiota to produce metabolites like tryptamine, that can influence host physiology by activation of G protein-coupled receptors, is an important facet of host-microbe interactions that warrants increased attention132,133. It is currently unclear, however, if microbially derived tryptamine reaches the CNS and whether it acts there to control behavior.
The Microbiota-Gut-Brain axis in Healthy subjects
There are a number of studies that demonstrate the effects of microbiota-targeted interventions in healthy individuals 19. Four weeks of probiotic consumption by healthy females led to changes in the functional connectivity of an emotion recognition network in the brain134. In a different cohort of female healthy control subjects, white and gray matter imaging parameters were associated with two bacterial genus-based clusters that each differed in the structures associated with emotional, attentional, and sensory processing and also in fMRI-measured emotional reactivity 135. Several trials involving the administration of a Lactobacillus rhamnosus (JB-1) supplement to healthy males showed inconsistent outcomes in stress-related behaviors with either a positive136 or no137 difference in those receiving the supplement. In another examination of healthy subjects, however, probiotics improved emotional decision-making and affect relative to placebo with concomitant changes in specific taxa138. In contrast to preclinical models, there is currently no solid evidence that probiotic ingestion in healthy individuals can modulate behavior, as reported studies on probiotic consumption in small clinical studies have produced contradictory outcomes139–143.
Disorders of brain-gut interactions: IBS
IBS is the most common disorder of brain-gut interaction, occurring in up to 4.8 % of the population worldwide144. Based on the current symptom criteria,145 IBS is defined by chronically recurring abdominal pain associated with altered bowel habits in the absence of detectable organic disease. This gut-restricted definition overlooks the findings that up to 50% of individuals who meet diagnostic criteria for an anxiety disorder have IBS, and that individuals with IBS have a greater than three-fold risk of meeting diagnostic criteria for an anxiety disorder146. While in the majority of IBS patients, CNS-related precipitants in early and adult life (e.g., psychological trauma, stress, abuse, maternal neglect) 147 have been identified, about half of IBS patients present after an intestinal trigger 148. The bidirectional nature of brain-gut involvement in IBS was illustrated in a one-year population-based prospective study that evaluated individuals with anxiety +/− depression and IBS as well as control individuals with neither condition. At the conclusion of the study, it was found that individuals with higher baseline levels of anxiety and depression were significantly more likely to develop IBS and, conversely, that those subjects with baseline IBS reported significantly higher levels of anxiety or depression. Interestingly, in 2/3 of these co-morbid cases, an IBS diagnosis preceded the mood disorder, implying that in some patients primary gut dysfunction might serve as a driver for mood disorders149.
Alterations in fMRI-detected brain activity are linked to abdominal pain150. Connections have been shown between brain networks that mediate anxiety and ANS output, like the amygdala, with mechanisms that modulate colonic sensitivity and gut motility. Increased and decreased activation of endogenous pain excitatory and inhibitory pathways, respectively, have also been observed in CNS locations that have been associated with visceral afferent processing and emotional arousal151–153. Interestingly, these pathways share significant homology to a stress circuit in rodents that implicates the involvement of corticotropin releasing factor in the central and peripheral regulation of brain-gut interactions in IBS154.
IBS and the Microbiome
A causal role for altered gut microbiota in IBS symptoms remains to be determined, even though a number of cross sectional studies have reported alterations in fecal microbial community composition in IBS subjects, based on disease subtype (IBS-D, IBS-C, IBS-M), age (pediatric vs. adult), and/or compartment (mucosa vs. stool)155. Recent evidence suggests the presence of IBS subgroups based on gut microbial community structure, with groups not differing from healthy controls despite GI symptoms156, 157. In one study, a dysbiotic IBS subgroup differed in regional brain volumes from a group with normal gut microbiota157, suggesting a relationship between microbial community composition and brain structure. However, as both microbiota-defined subgroups met the IBS diagnostic criteria and did not differ in any clinical parameters, these findings put into question a causative role for dysbiosis in IBS symptoms. The absence of group differences in the microbial composition between healthy controls and individuals with IBS has been reproduced elsewhere, though IBS symptom severity was found to be correlated with dysbiosis158. A more recent cross-sectional study revealed significant differences among IBS subtypes in the distribution of Clostridiales. Relative Clostridiales abundance was correlated with significant differences in the level of fecal SCFAs, which together were associated with altered fecal cytokine levels159. Even though this study aimed to identify mechanistic pathways in gut microbe-host interactions, the findings need to be confirmed in a study with a control population and a larger sample size.
The diverse findings from the IBS microbiota studies have been attributed to the extensive range of technologies employed for microbiota study, sample source, differences in IBS subtype, differential effects of the ANS on other aspects of physiology (e.g., mucus secretion, intestinal permeability and mucosal immunity; reviewed in160) as well as the many other influences that affect microbial composition and function (e.g., age, diet, antibiotic exposure, geography, probiotic intake, medication exposure)155, 161. Further complicating this dynamic is the need to factor in the CNS-mediated aspects of motility and gut physiology, including sleep quality and stress. The examination of much larger cohorts in longitudinal studies that also integrate clinical phenotypes and diet are thus needed for a more comprehensive understanding of these populations.
IBS and serotonin
Serotonin is one of most highly studied neurotransmitters in IBS physiology. As a major determinant of ENS and CNS development and a modulator of IBS-related symptoms (e.g., motility, secretion and visceral hypersensitivity) as well as mood 113, serotonin may thus be an important developmental modulator of the comorbid mood and IBS diagnoses made in some affected patients. Alterations in enteric mucosal and blood serotonin signaling have also been demonstrated in adults and children with IBS, potentially indicative of GI-initiated serotonergic dysregulation162, 163. Although serotonin can activate more than 15 receptors/receptor subtypes in the brain and intestine, the majority of IBS research has focused on the 5-HT3 and 5-HT4 receptors, as both have been shown to have effects on mood, motility and abdominal pain (reviewed previously113, 164–167).
Altogether, these data suggest a possible role of the gut microbiome in altered brain gut interactions in IBS. They also provide the basis for larger, longitudinal interventional studies, both clinical and functional, to identify the roles of specific microbiota in behavioral and gut dysfunction in IBS and also the utility of serotonin-based modulators as potential therapeutic targets (Figure 2).
Gut Microbiome in Depression
In recent years, there have been an increasing number of studies showing that patients with Major Depressive disorder (MDD) have an altered gut microbiome composition when compared to healthy controls, although the nature of the alterations in each study are diverse168–170. This variation in outcomes is likely due to similar reasons as those noted for IBS. It is worth noting that studies have also shown that transferring the microbiome of a depressed individual into a healthy rodent can induce depressive-like behaviors in the murine recipient suggesting the possibility of a causal role for the microbiota in pathophysiology of depression and opening up the concept of targeting the microbiome for mental health benefit19, 169.
Mood and IBS: Targeting the Microbiota-Gut-Brain Axis
The effects of enteric microbial manipulations in controlled clinical trials in patients with depression and/or IBS have been evaluated with probiotics, antibiotics and the low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) diet. Several studies have demonstrated the effectiveness of a low FODMAP diet in the short-term treatment of IBS symptoms, and diet induced changes in the gut microbiome have been implicated as an underlying mechanism. The low FODMAP diet results in a decreased production of gas and osmotically active metabolites, as a result of decreased microbial fermentation, that is thought to lead to improvements in bloating, flatulence, and pain171. In line with this theory, adult RCTs demonstrate that intake of a low FODMAP diet improves IBS symptoms, regardless of subtype, as well as health-related quality of life (QOL), anxiety, and activity impairment in adults with IBS-D171. However, even though these study results indirectly support a role for the gut microbiome in some IBS symptoms and may be useful for short term treatment of some IBS symptoms, the value of a low FODMAP diet for long term treatment for IBS has been questioned171. This reduction efficacy may be because of the reduction of oligosaccharides important for the diversity and abundance of the gut microbiota, and/or because of the low compliance rate associated with long-term adherence171.
A different dietary approach has been taken in the treatment of depression which comes under the umbrella of Nutritional Psychiatry. There is clinical evidence from both epidemiological and several interventional studies that a largely plant based diet, such as the traditional Mediterranean diet, has benefits in the adjuvant treatment of depression172, 173. There are many open questions that remain, especially in regard to the mechanisms of how diet may impact mood and which components are the most beneficial.
Supplementing the Gut Microbiome to improve MGB Axis
Apart from dietary intervention, probiotics have also been tried as a treatment meant to target the microbiome. Although numerous preclinical and some clinical studies have reported beneficial effects of specific probiotics on mood and emotional behaviors, clinically significant effects of probiotics in the treatment of psychiatric disorders have not been demonstrated174. There is thus a need for high quality randomized controlled clinical studies in human subjects to demonstrate that the beneficial effects observed in preclinical models can be translated and confirmed in human settings.
Studies on probiotics, including strains of Bifidobacterium and/or Lactobacillus, as well as VSL#3, have been shown to improve symptom severity in adults and children with IBS175–177. Due to the insufficient quality of these studies, however, the recently published AGA Guidelines on probiotics do not recommend their use in IBS, other than in controlled studies176, 177.
Interestingly, although some probiotic supplementation in humans changes the gut microbiota composition, as judged by 16S rRNA sequencing, others show no or only transient modification of the collective microbiome transcriptional state. These findings suggest that measurement of probiotic intervention on gut microbial profiles must be accompanied by technologies assessing microbial function, such as metagenomics or metabolomics.
Fecal microbiome transplantation as a therapy for IBS & mood disturbances
Clinical studies on fecal microbiota transplantation (FMT) remain limited and systematic review fails to show any overall benefit178, 179. Two recent RCT studies showed alterations in gut microbial composition in the group receiving a FMT. However, while one study showed a significant IBS symptom reduction three months after FMT, the other study demonstrated greater symptom improvement in the placebo-treated group178–180. High quality FMT studies in patients with depression are in the pipeline.
Conclusions and Future Directions
Considerable progress has been made in the understanding of the MGB axis in preclinical models of human brain disorders and in the potential translation of these findings to humans. A growing body of research has confirmed that disorders of brain gut interactions like IBS have a strong brain component and that many brain disorders have a GI facet to their manifestations or even their origins. A causal role of the gut microbiome in these interactions remains to be determined. This valuable knowledge will inform the development of cross-disciplinary therapeutic approaches well into the future.
Key areas that have not yet been examined extensively are the roles that sex and race play in microbiome-gut-brain-axis development and disease. Accumulating evidence has shown, however, that both sex and race may exert important influences over the gut microbiota. The microbiome may alter brain development in a sex-specific manner and females are significantly more likely to suffer from stress-related and functional GI- disorders56, 181, 182. On the other hand, at least in animal models, the male brain appears to be more susceptible to microbial disturbances in early life than the female brain183. These sex-specific effects have given rise to justified calls for more intensive study of the “microgenderome”181, 184. Although racial diversity of the gut microbiome has been explored somewhat in cancer and other medical conditions, there has been no in-depth exploration of how race impacts microbial diversity and its relationship with brain-gut axis conditions185. The current knowledge, however, emphasizes the need to understand how the sexual dimorphism and racial diversity impact the microbiome and contribute to brain-gut axis disorders.
It is important to reiterate that translation of preclinical findings into more effective therapies for human brain disorders has largely been unsuccessful to date (Figure 3). Moving forward, the development of live biotherapeutics or substances whose beneficial effects on the brain are bacteria-mediated (i.e., psychobiotics) are currently being investigated as direct and/or adjunctive therapies for brain disorders, but this field is very much in its infancy19. Until identification of gut microbiome-related patient subtypes becomes possible and new pharmacologic and specific microbiome targeted approaches emerge, the most effective treatments for IBS and other disorders of MGB interactions remain a combination of personalized diet approaches, behavioral therapies and a limited number of pharmacologic treatments aimed at improvement of bowel function186.
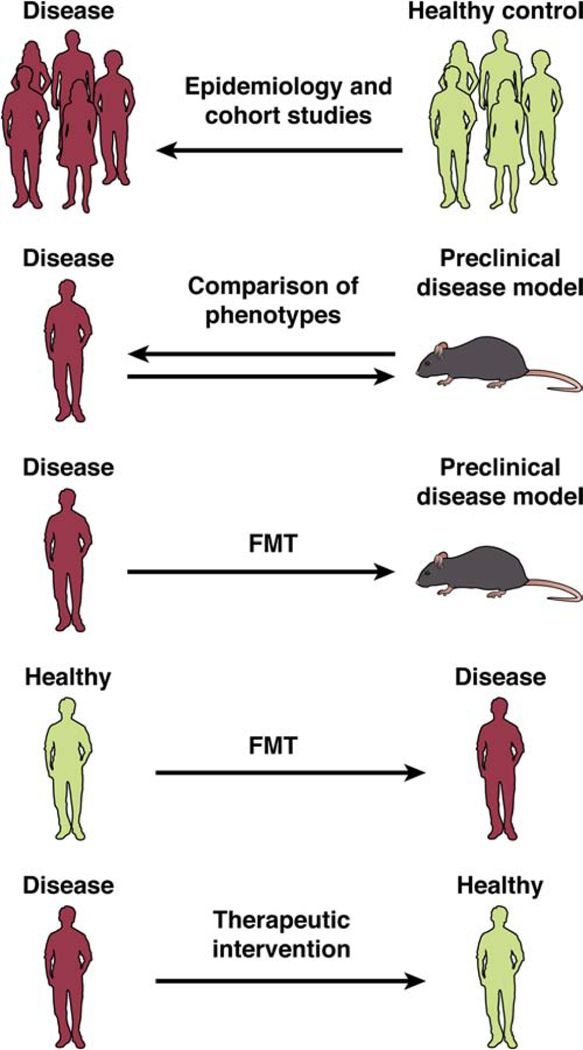
Figure 3: Challenges in Translational Research.
Schematic representation of research approaches aimed to identify a causal role of the gut microbiome in human brain and brain gut disorders. There is extensive evidence for cross sectional differences in the gut microbial composition between defined disease populations and healthy control populations (top row). A number of rodent models of human brain diseases have been developed that mimic certain disease aspects (second row from top). More recently, studies have been reported in which fecal microbial transplants from patients with certain brain diseases into germ free mice have resulted in altered mouse behaviors, mimicking some aspects of the human phenotype (middle row). Fecal microbial transplants from healthy human subjects into individuals with brain disorders have not resulted in consistent improvement in respective symptoms to date (second row from bottom). To date, there is limited evidence for the effectiveness of therapeutic interventions targeted at the microbiome (bottom row).
What You Need to Know.
BACKGROUND AND CONTEXT:
A growing body of research has confirmed that disorders of brain gut interactions (e.g., IBS) have a strong brain component and that many brain disorders have a GI facet to their manifestations or even their origins. The gut-brain axis plays an important role in maintaining homeostasis. Hence, when this system goes awry, disease can develop. More recently, the role of the microbiome as an important factor in modulating gut-brain signaling has emerged and the concept of a microbiota-gut-brain (MGB) axis has been established.
NEW FINDINGS:
In this review, we highlight the role of the MGB axis in modulating enteric and central nervous system function and how this modulation may impact disorders such as Irritable Bowel Syndrome and disorders of mood and affect. We also examine the overlapping biological constructs that underpin these disorders with a special emphasis on the neurotransmitter serotonin, which plays a key role in both the gastrointestinal tract and in the brain.
LIMITATIONS:
Although considerable progress has been made in the understanding of the MGB axis in preclinical models of disease and in the potential translation of these findings to humans, several limitations remain. These limitations include a lack of understanding of whether there is a causal role of the gut microbiome in these interactions and the identification of gut microbiome-related patient subtypes.
IMPACT:
Overall, it is clear that although animal studies have shown much promise, more progress is necessary before these findings can be translated for diagnostic and therapeutic benefit. Although in its infancy, the development of live biotherapeutics or substances whose beneficial effects on the brain or gut are bacteria-mediated may ultimately serve as novel, effective therapies for brain-gut disorders.
Acknowledgements:
We would like to thank Narek Israelyan (Columbia University), Dr. Kenneth O’Riordan (University College Cork) and Cathy Liu (UCLA) for their contributions to the figures.
GRANT SUPPORT: This research was supported by grants from the National Institutes of Health: RO1 NS015547 (KGM), R01 DK048351 (EAM); the Department of Defense: PR160365 (KGM), Science Foundation Ireland SFI/12/RC/ 2273_P2 (JFC), the Saks Kavanaugh Foundation (JFC) and EU H2020 project DLV-848228 DISCOvERIE (development, diagnosis and prevention of gender-related somatic and mental comorbidities in irritable bowel syndrome in Europe) (JFC).
Footnotes
DISCLOSURES: KGM has been a consultant for Takeda. JFC has been an invited speaker at meetings organized by Yakult, Alkermes, Ordesa & Alimentary Health, has been a consultant for Alkermes & Nestle and has received research funding from Mead Johnson, Cremo, Nutricia, Dupont, Pharmavite & 4D Pharma. EAM is a scientific advisory board member of Danone, Axial Biotherapeutics, Viome, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, APC Microbiome Ireland.
Study concept and design: KGM, JC, EAM
Acquisition of data: KGM, JC, EAM
Drafting of the manuscript: KGM, JC, EAM
Critical revision of the manuscript for important intellectual content: KGM, JC, EAM
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology 2016. [DOI] [PubMed] [Google Scholar]
- 2.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci 2011;12:453–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bove C, Travagli RA. Neurophysiology of the brain stem in Parkinson’s disease. J Neurophysiol 2019;121:1856–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margolis KG, Gershon MD, Bogunovic M. Cellular Organization of Neuroimmune Interactions in the Gastrointestinal Tract. Trends Immunol 2016;37:487–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao M, Gershon MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol 2016;13:517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browning KN, Travagli RA. Central control of gastrointestinal motility. Curr Opin Endocrinol Diabetes Obes 2019;26:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012;10:735–42. [DOI] [PubMed] [Google Scholar]
- 9.Cryan JF, O’Riordan KJ, Cowan CSM, et al. The Microbiota-Gut-Brain Axis. Physiol Rev 2019;99:1877–2013. [DOI] [PubMed] [Google Scholar]
- 10.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. [DOI] [PubMed] [Google Scholar]
- 11.Obata Y, Pachnis V. The Effect of Microbiota and the Immune System on the Development and Organization of the Enteric Nervous System. Gastroenterology 2016;151:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reigstad CS, Salmonson CE, Rainey JF 3rd, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 2015;29:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gareau MG. Microbiota-gut-brain axis and cognitive function. Adv Exp Med Biol 2014;817:357–71. [DOI] [PubMed] [Google Scholar]
- 14.Osadchiy V, Martin CR, Mayer EA. Gut Microbiome and Modulation of CNS Function. Compr Physiol 2019;10:57–72. [DOI] [PubMed] [Google Scholar]
- 15.Ye L, Liddle RA. Gastrointestinal hormones and the gut connectome. Curr Opin Endocrinol Diabetes Obes 2017;24:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert JA, Blaser MJ, Caporaso JG, et al. Current understanding of the human microbiome. Nat Med 2018;24:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biology 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastiaanssen TFS, Cowan CSM, Claesson MJ, et al. Making Sense of … the Microbiome in Psychiatry. Int J Neuropsychopharmacol 2019;22:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long-Smith C, O’Riordan KJ, Clarke G, et al. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu Rev Pharmacol Toxicol 2020;60:477–502. [DOI] [PubMed] [Google Scholar]
- 20.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009;9:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease: Springer, 2014:115–133. [DOI] [PubMed] [Google Scholar]
- 22.Fülling C, Dinan TG, Cryan JF. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron 2019;101:998–1002. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Munoz ME, Arrieta MC, Ramer-Tait AE, et al. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 2017;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu DM, Antony KM, Ma J, et al. The early infant gut microbiome varies in association with a maternal high-fat diet. Genome Med 2016;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jasarevic E, Howard CD, Misic AM, et al. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep 2017;7:44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golubeva AV, Crampton S, Desbonnet L, et al. Prenatal stress-induced alterations in major physiological systems correlate with gut microbiota composition in adulthood. Psychoneuroendocrinology 2015;60:58–74. [DOI] [PubMed] [Google Scholar]
- 27.Ganal-Vonarburg SC, Hornef MW, Macpherson AJ. Microbial-host molecular exchange and its functional consequences in early mammalian life. Science 2020;368:604–607. [DOI] [PubMed] [Google Scholar]
- 28.Vuong HE, Pronovost GN, Williams DW, et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura I, Miyamoto J, Ohue-Kitano R, et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020;367. [DOI] [PubMed] [Google Scholar]
- 30.McDonald B, McCoy KD. Maternal microbiota in pregnancy and early life. Science 2019;365:984–985. [DOI] [PubMed] [Google Scholar]
- 31.Cowan CSM, Dinan TG, Cryan JF. Annual Research Review: Critical windows - the microbiota-gut-brain axis in neurocognitive development. J Child Psychol Psychiatry 2020;61:353–371. [DOI] [PubMed] [Google Scholar]
- 32.Stewart CJ, Ajami NJ, O’Brien JL, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018;562:583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 2011;108 Suppl 1:4578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis EC, Dinsmoor AM, Wang M, et al. Microbiome Composition in Pediatric Populations from Birth to Adolescence: Impact of Diet and Prebiotic and Probiotic Interventions. Dig Dis Sci 2020;65:706–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouald Chaib A, Levy EI, Ouald Chaib M, et al. The influence of the gastrointestinal microbiome on infant colic. Expert Rev Gastroenterol Hepatol 2020:1–13. [DOI] [PubMed] [Google Scholar]
- 36.Al Nabhani Z, Dulauroy S, Marques R, et al. A Weaning Reaction to Microbiota Is Required for Resistance to Immunopathologies in the Adult. Immunity 2019;50:1276–1288 e5. [DOI] [PubMed] [Google Scholar]
- 37.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature 2012;486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agans R, Rigsbee L, Kenche H, et al. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiology Ecology 2011;77:404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandhu KV, Sherwin E, Schellekens H, et al. Feeding the microbiota-gut-brain axis: diet, microbiome, and neuropsychiatry. Transl Res 2017;179:223–244. [DOI] [PubMed] [Google Scholar]
- 40.Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol 2011;9:233–43. [DOI] [PubMed] [Google Scholar]
- 41.Spichak S, Guzzetta KE, O’Leary OF, et al. Without a bug’s life: Germ-free rodents to interrogate microbiota-gut-neuroimmune interactions. Drug Discovery Today: Disease Models 2018;28:79–83. [Google Scholar]
- 42.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Science Translational Medicine 2014;6:art. no. 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chu C, Murdock MH, Jing D, et al. The microbiota regulate neuronal function and fear extinction learning. Nature 2019;574:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gacias M, Gaspari S, Santos PM, et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoban AE, Stilling RM, Moloney G, et al. The microbiome regulates amygdala-dependent fear recall. Mol Psychiatry 2018;23:1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoban AE, Stilling RM, Ryan FJ, et al. Regulation of prefrontal cortex myelination by the microbiota. Transl Psychiatry 2016;6:e774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogbonnaya ES, Clarke G, Shanahan F, et al. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol Psychiatry 2015;78:e7–9. [DOI] [PubMed] [Google Scholar]
- 49.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 2011;108:3047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neufeld KA, Kang N, Bienenstock J, et al. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol 2011;4:492–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luczynski P, McVey Neufeld KA, Oriach CS, et al. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int J Neuropsychopharmacol 2016;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olszak T, An D, Zeissig S, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012;336:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diaz Heijtz R, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences of the United States of America 2011;108:3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of Physiology 2004;558:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buffington SA, Di Prisco GV, Auchtung TA, et al. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016;165:1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clarke G, Grenham S, Scully P, et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 2013;18:666–73. [DOI] [PubMed] [Google Scholar]
- 57.Walter J, Armet AM, Finlay BB, et al. Establishing or Exaggerating Causality for the Gut Microbiome: Lessons from Human Microbiota-Associated Rodents. Cell 2020;180:221–232. [DOI] [PubMed] [Google Scholar]
- 58.Slykerman RF, Hood F, Wickens K, et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017;24:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlson AL, Xia K, Azcarate-Peril MA, et al. Infant Gut Microbiome Associated With Cognitive Development. Biol Psychiatry 2018;83:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao W, Salzwedel AP, Carlson AL, et al. Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology (Berl) 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts RR, Murphy JF, Young HM, et al. Development of colonic motility in the neonatal mouse-studies using spatiotemporal maps. Am J Physiol Gastrointest Liver Physiol 2007;292:G930–8. [DOI] [PubMed] [Google Scholar]
- 62.Joseph NM, He S, Quintana E, et al. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest 2011;121:3398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laranjeira C, Sandgren K, Kessaris N, et al. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest 2011;121:3412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kabouridis PS, Lasrado R, McCallum S, et al. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 2015;85:289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulkarni S, Micci MA, Leser J, et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A 2017;114:E3709–E3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parathan P, Wang Y, Leembruggen AJ, et al. The enteric nervous system undergoes significant chemical and synaptic maturation during adolescence in mice. Dev Biol 2020;458:75–87. [DOI] [PubMed] [Google Scholar]
- 67.Lake JI, Heuckeroth RO. Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol 2013;305:G1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anitha M, Vijay-Kumar M, Sitaraman SV, et al. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 2012;143:1006–16 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kashyap PC, Marcobal A, Ursell LK, et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 2013;144:967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hyland NP, Cryan JF. Microbe-host interactions: Influence of the gut microbiota on the enteric nervous system. Dev Biol 2016;417:182–7. [DOI] [PubMed] [Google Scholar]
- 71.McVey Neufeld KA, Mao YK, Bienenstock J, et al. The microbiome is essential for normal gut intrinsic primary afferent neuron excitability in the mouse. Neurogastroenterol Motil 2013;25:183–e88. [DOI] [PubMed] [Google Scholar]
- 72.Perez-Burgos A, Mao YK, Bienenstock J, et al. The gut-brain axis rewired: adding a functional vagal nicotinic “sensory synapse”. FASEB J 2014;28:3064–74. [DOI] [PubMed] [Google Scholar]
- 73.De Vadder F, Grasset E, Manneras Holm L, et al. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A 2018;115:6458–6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamm K, Hoppe S, Breves G, et al. Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of myenteric neurones in pig jejunum. Neurogastroenterol Motil 2004;16:53–60. [DOI] [PubMed] [Google Scholar]
- 75.di Giancamillo A, Vitari F, Bosi G, et al. The chemical code of porcine enteric neurons and the number of enteric glial cells are altered by dietary probiotics. Neurogastroenterol Motil 2010;22:e271–8. [DOI] [PubMed] [Google Scholar]
- 76.Obata Y, Castano A, Boeing S, et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020;578:284–289. [DOI] [PubMed] [Google Scholar]
- 77.Khoshdel A, Verdu EF, Kunze W, et al. Bifidobacterium longum NCC3001 inhibits AH neuron excitability. Neurogastroenterol Motil 2013;25:e478–84. [DOI] [PubMed] [Google Scholar]
- 78.Kunze WA, Mao YK, Wang B, et al. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med 2009;13:2261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al-Nedawi K, Mian MF, Hossain N, et al. Gut commensal microvesicles reproduce parent bacterial signals to host immune and enteric nervous systems. FASEB J 2015;29:684–95. [DOI] [PubMed] [Google Scholar]
- 80.Mao YK, Kasper DL, Wang B, et al. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat Commun 2013;4:1465. [DOI] [PubMed] [Google Scholar]
- 81.Schiering C, Wincent E, Metidji A, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 2017;542:242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ward NL, Pieretti A, Dowd SE, et al. Intestinal aganglionosis is associated with early and sustained disruption of the colonic microbiome. Neurogastroenterol Motil 2012;24:874–e400. [DOI] [PubMed] [Google Scholar]
- 83.Rolig AS, Mittge EK, Ganz J, et al. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol 2017;15:e2000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fulling C, Dinan TG, Cryan JF. Gut Microbe to Brain Signaling: What Happens in Vagus. Neuron 2019;101:998–1002. [DOI] [PubMed] [Google Scholar]
- 85.Han W, Tellez LA, Perkins MH, et al. A Neural Circuit for Gut-Induced Reward. Cell 2018;175:665–678.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci 2018;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016;165:1332–1345. [DOI] [PubMed] [Google Scholar]
- 88.Bhattarai Y, Williams BB, Battaglioli EJ, et al. Gut Microbiota-Produced Tryptamine Activates an Epithelial G-Protein-Coupled Receptor to Increase Colonic Secretion. Cell Host Microbe 2018;23:775–785 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011;108:16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bercik P, Park AJ, Sinclair D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil 2011;23:1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Agus A, Planchais J, Sokol H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018;23:716–724. [DOI] [PubMed] [Google Scholar]
- 93.Bohorquez DV, Shahid RA, Erdmann A, et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest 2015;125:782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sgritta M, Dooling SW, Buffington SA, et al. Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019;101:246–259 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004;118:229–41. [DOI] [PubMed] [Google Scholar]
- 96.Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol 2013;14:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin C, Zhao S, Zhu Y, et al. Microbiota-gut-brain axis and toll-like receptors in Alzheimer’s disease. Comput Struct Biotechnol J 2019;17:1309–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perez-Pardo P, Dodiya HB, Engen PA, et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 2019;68:829–843. [DOI] [PubMed] [Google Scholar]
- 99.Tramullas M, Finger BC, Moloney RD, et al. Toll-like receptor 4 regulates chronic stress-induced visceral pain in mice. Biol Psychiatry 2014;76:340–8. [DOI] [PubMed] [Google Scholar]
- 100.Kelly JR, Clarke G, Cryan JF, et al. Brain-gut-microbiota axis: challenges for translation in psychiatry. Ann Epidemiol 2016;26:366–72. [DOI] [PubMed] [Google Scholar]
- 101.Arentsen T, Qian Y, Gkotzis S, et al. The bacterial peptidoglycan-sensing molecule Pglyrp2 modulates brain development and behavior. Mol Psychiatry 2017;22:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Andre P, Laugerette F, Feart C. Metabolic Endotoxemia: A Potential Underlying Mechanism of the Relationship between Dietary Fat Intake and Risk for Cognitive Impairments in Humans? Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brun P, Giron MC, Qesari M, et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology 2013;145:132–333. [DOI] [PubMed] [Google Scholar]
- 104.Grasa L, Abecia L, Forcen R, et al. Antibiotic-Induced Depletion of Murine Microbiota Induces Mild Inflammation and Changes in Toll-Like Receptor Patterns and Intestinal Motility. Microb Ecol 2015;70:835–48. [DOI] [PubMed] [Google Scholar]
- 105.Farache J, Zigmond E, Shakhar G, et al. Contributions of dendritic cells and macrophages to intestinal homeostasis and immune defense. Immunol Cell Biol 2013;91:232–9. [DOI] [PubMed] [Google Scholar]
- 106.Mikkelsen HB, Rumessen JJ. Characterization of macrophage-like cells in the external layers of human small and large intestine. Cell Tissue Res 1992;270:273–9. [DOI] [PubMed] [Google Scholar]
- 107.Muller PA, Koscso B, Rajani GM, et al. Crosstalk between Muscularis Macrophages and Enteric Neurons Regulates Gastrointestinal Motility. Cell 2014;158:1210. [DOI] [PubMed] [Google Scholar]
- 108.Gabanyi I, Muller PA, Feighery L, et al. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 2016;164:378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jarret A, Jackson R, Duizer C, et al. Enteric Nervous System-Derived IL-18 Orchestrates Mucosal Barrier Immunity. Cell 2020;180:813–814. [DOI] [PubMed] [Google Scholar]
- 110.Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 2020;583:441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taj A, Jamil N. Bioconversion of Tyrosine and Tryptophan Derived Biogenic Amines by Neuropathogenic Bacteria. Biomolecules 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roshchina VV. New Trends and Perspectives in the Evolution of Neurotransmitters in Microbial, Plant, and Animal Cells. Adv Exp Med Biol 2016;874:25–77. [DOI] [PubMed] [Google Scholar]
- 113.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013;10:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gross ER, Gershon MD, Margolis KG, et al. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 2012;143:408–17 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 2011;31:8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Margolis KG. A role for the serotonin reuptake transporter in the brain and intestinal features of autism spectrum disorders and developmental antidepressant exposure. J Chem Neuroanat 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Margolis KG, Li Z, Stevanovic K, et al. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest 2016;126:2221–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brummelte S, Mc Glanaghy E, Bonnin A, et al. Developmental changes in serotonin signaling: Implications for early brain function, behavior and adaptation. Neuroscience 2017;342:212–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Del Colle A, Israelyan N, Gross Margolis KG. Novel Aspects of Enteric Serotonergic Signaling in Health and Brain-Gut Disease. Am J Physiol Gastrointest Liver Physiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci 2015;18:965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van de Wouw M, Boehme M, Lyte JM, et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J Physiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nankova BB, Agarwal R, MacFabe DF, et al. Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells--possible relevance to autism spectrum disorders. PLoS One 2014;9:e103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.MacFabe DF, Cain NE, Boon F, et al. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: Relevance to autism spectrum disorder. Behav Brain Res 2011;217:47–54. [DOI] [PubMed] [Google Scholar]
- 124.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504:446–50. [DOI] [PubMed] [Google Scholar]
- 125.Fung TC, Vuong HE, Luna CDG, et al. Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cussotto S, Clarke G, Dinan TG, et al. Psychotropics and the Microbiome: a Chamber of Secrets. Psychopharmacology (Berl) 2019;236:1411–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marin IA, Goertz JE, Ren TT, et al. Microbiota alteration is associated with the development of stress-induced despair behavior. Scientific Reports 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kennedy PJ, Cryan JF, Dinan TG, et al. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017;112:399–412. [DOI] [PubMed] [Google Scholar]
- 129.Osadchiy V, Labus JS, Gupta A, et al. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS One 2018;13:e0201772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galligan JJ. Beneficial actions of microbiota-derived tryptophan metabolites. Neurogastroenterol Motil 2018;30. [DOI] [PubMed] [Google Scholar]
- 131.Gheorghe CE, Martin JA, Manriquez FV, et al. Focus on the essentials: tryptophan metabolism and the microbiome-gut-brain axis. Curr Opin Pharmacol 2019;48:137–145. [DOI] [PubMed] [Google Scholar]
- 132.Cohen LJ, Esterhazy D, Kim SH, et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 2017;549:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Colosimo DA, Kohn JA, Luo PM, et al. Mapping Interactions of Microbial Metabolites with Human G-Protein-Coupled Receptors. Cell Host Microbe 2019;26:273–282 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 2013;144:1394–401, 1401 e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tillisch K, Mayer EA, Gupta A, et al. Brain Structure and Response to Emotional Stimuli as Related to Gut Microbial Profiles in Healthy Women. Psychosom Med 2017;79:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gawronska A, Dziechciarz P, Horvath A, et al. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther 2007;25:177–84. [DOI] [PubMed] [Google Scholar]
- 137.Francavilla R, Miniello V, Magista AM, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 2010;126:e1445–52. [DOI] [PubMed] [Google Scholar]
- 138.Bagga D, Reichert JL, Koschutnig K, et al. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018;9:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr 2011;105:755–64. [DOI] [PubMed] [Google Scholar]
- 140.Messaoudi M, Violle N, Bisson JF, et al. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011;2:256–61. [DOI] [PubMed] [Google Scholar]
- 141.Steenbergen L, Sellaro R, van Hemert S, et al. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun 2015;48:258–64. [DOI] [PubMed] [Google Scholar]
- 142.McKean J, Naug H, Nikbakht E, et al. Probiotics and Subclinical Psychological Symptoms in Healthy Participants: A Systematic Review and Meta-Analysis. J Altern Complement Med 2017;23:249–258. [DOI] [PubMed] [Google Scholar]
- 143.Ng QX, Peters C, Ho CYX, et al. A meta-analysis of the use of probiotics to alleviate depressive symptoms. J Affect Disord 2018;228:13–19. [DOI] [PubMed] [Google Scholar]
- 144.Endo Y, Shoji T, Fukudo S. Epidemiology of irritable bowel syndrome. Ann Gastroenterol 2015;28:158–159. [PMC free article] [PubMed] [Google Scholar]
- 145.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016;150:1257–61. [DOI] [PubMed] [Google Scholar]
- 146.Zamani M, Alizadeh-Tabari S, Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2019;50:132–143. [DOI] [PubMed] [Google Scholar]
- 147.Halland M, Almazar A, Lee R, et al. A case-control study of childhood trauma in the development of irritable bowel syndrome. Neurogastroenterol Motil 2014;26:990–8. [DOI] [PubMed] [Google Scholar]
- 148.Sykes MA, Blanchard EB, Lackner J, et al. Psychopathology in irritable bowel syndrome: support for a psychophysiological model. J Behav Med 2003;26:361–72. [DOI] [PubMed] [Google Scholar]
- 149.Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther 2016;44:592–600. [DOI] [PubMed] [Google Scholar]
- 150.Mayer EA, Labus J, Aziz Q, et al. Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut 2019;68:1701–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011;140:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004;53:1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kano M, Dupont P, Aziz Q, et al. Understanding Neurogastroenterology From Neuroimaging Perspective: A Comprehensive Review of Functional and Structural Brain Imaging in Functional Gastrointestinal Disorders. J Neurogastroenterol Motil 2018;24:512–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest 2007;117:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Simren M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013;62:159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jeffery IB, O’Toole PW, Ohman L, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012;61:997–1006. [DOI] [PubMed] [Google Scholar]
- 157.Labus JS, Hollister EB, Jacobs J, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome 2017;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tap J, Derrien M, Tornblom H, et al. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology 2017;152:111–123 e8. [DOI] [PubMed] [Google Scholar]
- 159.Gargari G, Taverniti V, Gardana C, et al. Fecal Clostridiales distribution and short-chain fatty acids reflect bowel habits in irritable bowel syndrome. Environ Microbiol 2018;20:3201–3213. [DOI] [PubMed] [Google Scholar]
- 160.Osadchiy V, Martin CR, Mayer EA. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol 2019;17:322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kang DW, Park JG, Ilhan ZE, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 2013;8:e68322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thijssen AY, Mujagic Z, Jonkers DM, et al. Alterations in serotonin metabolism in the irritable bowel syndrome. Aliment Pharmacol Ther 2016;43:272–82. [DOI] [PubMed] [Google Scholar]
- 163.Tack J, Janssen P, Wouters M, et al. Targeting serotonin synthesis to treat irritable bowel syndrome. Gastroenterology 2011;141:420–2. [DOI] [PubMed] [Google Scholar]
- 164.Fakhfouri G, Rahimian R, Dyhrfjeld-Johnsen J, et al. 5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: The Iceberg Still Lies beneath the Surface. Pharmacol Rev 2019;71:383–412. [DOI] [PubMed] [Google Scholar]
- 165.Rebholz H, Friedman E, Castello J. Alterations of Expression of the Serotonin 5-HT4 Receptor in Brain Disorders. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Manabe N, Wong BS, Camilleri M. New-generation 5-HT4 receptor agonists: potential for treatment of gastrointestinal motility disorders. Expert Opin Investig Drugs 2010;19:765–75. [DOI] [PubMed] [Google Scholar]
- 167.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 2013;20:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kelly JR, Borre Y, C OB, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 2016;82:109–18. [DOI] [PubMed] [Google Scholar]
- 169.Zheng P, Zeng B, Zhou C, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 2016;21:786–96. [DOI] [PubMed] [Google Scholar]
- 170.Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol 2019;4:623–632. [DOI] [PubMed] [Google Scholar]
- 171.Algera J, Colomier E, Simren M. The Dietary Management of Patients with Irritable Bowel Syndrome: A Narrative Review of the Existing and Emerging Evidence. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Adan RAH, van der Beek EM, Buitelaar JK, et al. Nutritional psychiatry: Towards improving mental health by what you eat. Eur Neuropsychopharmacol 2019;29:1321–1332. [DOI] [PubMed] [Google Scholar]
- 173.Dinan TG, Stanton C, Long-Smith C, et al. Feeding melancholic microbes: MyNewGut recommendations on diet and mood. Clin Nutr 2019;38:1995–2001. [DOI] [PubMed] [Google Scholar]
- 174.Huang R, Wang K, Hu J. Effect of Probiotics on Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther 2011;33:1302–10. [DOI] [PubMed] [Google Scholar]
- 176.Preidis GA, Weizman AV, Kashyap PC, et al. AGA Technical Review on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Su GL, Ko CW, Bercik P, et al. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020. [DOI] [PubMed] [Google Scholar]
- 178.Xu D, Chen VL, Steiner CA, et al. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Am J Gastroenterol 2019;114:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Enck P. Primum non nocere: is faecal microbiota transplantation doing harm to patients with IBS? Gut 2019;68:1722–1723. [DOI] [PubMed] [Google Scholar]
- 180.El-Salhy M, Hatlebakk JG, Gilja OH, et al. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020;69:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Audet MC. Stress-induced disturbances along the gut microbiota-immune-brain axis and implications for mental health: Does sex matter? Front Neuroendocrinol 2019;54:100772. [DOI] [PubMed] [Google Scholar]
- 182.Vemuri R, Sylvia KE, Klein SL, et al. The microgenderome revealed: sex differences in bidirectional interactions between the microbiota, hormones, immunity and disease susceptibility. Semin Immunopathol 2019;41:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jaggar M, Rea K, Spichak S, et al. You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan. Front Neuroendocrinol 2020;56:100815. [DOI] [PubMed] [Google Scholar]
- 184.Mulak A, Tache Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol 2014;20:2433–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Royston KJ, Adedokun B, Olopade OI. Race, the microbiome and colorectal cancer. World J Gastrointest Oncol 2019;11:773–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Simren M, Tack J. New treatments and therapeutic targets for IBS and other functional bowel disorders. Nat Rev Gastroenterol Hepatol 2018;15:589–605. [DOI] [PubMed] [Google Scholar]