Abstract
Experimental preclinical models have been a cornerstone of lung cancer translational research. Work in these model systems has provided insights into the biology of lung cancer subtypes and their origins, contributed to our understanding of the mechanisms that underlie tumor progression, and revealed new therapeutic vulnerabilities. Initially patient-derived lung cancer cell lines were the main preclinical models available. The landscape is very different now with numerous preclinical models for research each with unique characteristics. These include genetically engineered mouse models (GEMMs), patient-derived xenografts (PDXs) and three-dimensional culture systems (“organoid” cultures). Here we review the development and applications of these models and describe their contributions to lung cancer research.
GENETICALLY ENGINEERED MOUSE MODELS (GEMMs) OF LUNG CANCER
Why Is There a Need for GEMMs?
Over the past 20 years, there has been a large increase in the number and complexity of genetically engineered mouse models (GEMMs) of lung cancer owing to advances in technology, as well as an increased appreciation for the ways in which GEMMs can be used to model the disease in an in vivo, immune-competent environment more closely resembling that of the human tumor being studied (Fig. 1). These mouse models, indeed, are valuable for modeling the tumor microenvironment, allowing for the study of interactions between the tumor cells, endothelial cells, immune cells and other stromal components in its specific tissue of origin. Therefore, through the use of GEMMs of lung cancer, we can model disease development and progression and its interaction with the lung tissue microenvironment as it progresses. Xenografts and syngeneic models (including orthotopic syngeneic implants) do not have this unique advantage of modeling tumor initiation and natural progression and, thus, may have different evolutionary trajectories that could, in part, be a result of different tumor microenvironments. Further, GEMMs enable the in vivo modeling of cancers driven by specific oncogenic drivers of lung cancer and engineered co-occurring alterations in a uniform germline genetic background compared to studies in patient-derived models. Additionally, these models are also critical for studying response to therapies at the organismal and physiological level, incorporating drug exposure and bioavailability at different organ sites. Indeed, studies using GEMMs have provided insights into lung cancer treatment with targeted therapies, given that they are an efficient system to evaluate therapeutic response and model acquired resistance (Politi et al. 2006; Pirazzoli et al. 2014; Starrett et al. 2020; Foggetti et al. 2021).
Figure 1.
Schema of the different models for the study of lung cancer. Compilation of several types of models developed for the study of lung cancer including genetically engineered mouse models (GEMMs) and patient-derived models and cell lines and organoids derived from them. The patient-derived models can be developed from specimens from primary tumors (lung) as well as from metastatic sites (brain, lymph nodes, liver, bone, etc). Specific injection techniques allow modeling of multiple steps of the tumorigenic process of lung cancer. (PDX) Patient-derived xenograft.
GEM Models of Lung Cancer
Over the last two decades, GEMMs of lung cancer that incorporate mutations identified in patient tumors have enabled studies of this disease in vivo, and have led to important discoveries in lung tumor biology, tumor progression, as well as in the development of therapeutic strategies for lung cancer (Fig. 2; Van Dyke and Jacks 2002; Meuwissen and Berns 2005; Politi and Pao 2011; DuPage and Jacks 2013; Politi and Herbst 2015; Rudin et al. 2019). Two main types of models have been used: those leveraging the inducible Tet-operator system in which doxycycline administration (given in the mouse drinking water or food) leads to expression of an oncogene (from a transgene) under the control of a tissue-specific promoter and conditional models in which Cre recombinase (often given intranasally or intratracheally to mice) targets LoxP sites that flank a gene region for deletion or activation (Gu et al. 1993; Kuhn et al. 1995; de Alboran et al. 2001; Jackson et al. 2001). Using these approaches, GEMMs have been used to model the different histological subtypes of lung cancer: non-small-cell lung cancer (NSCLC), which accounts for 85% of cases, and small-cell lung cancer (SCLC), which accounts for 15% of cases. NSCLC can be further subdivided into lung adenocarcinoma (LUAD, 40%), lung squamous cell carcinoma (LUSC, 30%), and large-cell carcinoma (15%). LUAD, the most prevalent histological subtype, usually arises in the cells lining the alveoli, typically from type II pneumocytes (Chen et al. 2014b).
Figure 2.
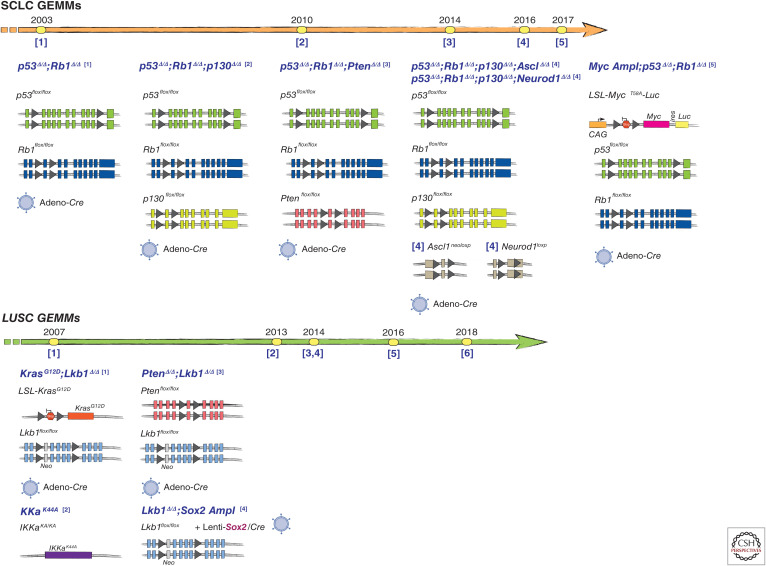
Timelines of lung cancer genetically engineered mouse model (GEMM) development by histological subtype (lung adenocarcinoma [LUAD], small-cell lung cancer [SCLC], and lung squamous cell carcinoma [LUSC] models). Highlights of noteworthy events and advances in the development of GEMMs for each histological subtype over the years are shown. Numbers under the timeline arrow indicate references where the models were described (see list below), while the year of publication is reported above the arrow. Schematics of the alleles used to develop the relevant lung cancer models are shown. Gray triangles represent loxP sites. Small vertical rectangles represent exons. Timeline reference numbers in lung adenocarcinoma models correspond as follows: [1] Fisher et al. 2001, [2] Jackson et al. 2001, [3] Politi et al. 2006, [4] Ji et al. 2006b, [5] Dankort et al. 2007, [6] Tran et al. 2008, [7] Soda et al. 2008, [8] DuPage et al. 2009, [9] Chen et al. 2010, [10] Trejo et al. 2013, [11] Yin et al. 2013, [12] Arai et al. 2013, [13] Platt et al. 2014, [14] Sánchez-Rivera et al. 2014, [15] Tchaicha et al. 2014, [16] Inoue et al. 2016, [17] Rogers et al. 2017, [18] Pyo et al. 2017, [19] Rogers et al. 2018, [20] Walter et al. 2019, and [21] Foggetti et al. 2021. Reference numbers in SCLC models correspond as follows: [1] Meuwissen et al. 2003, [2] Schaffer et al. 2010, [3] McFadden et al. 2014, [4] Borromeo et al. 2016, and [5] Mollaoglu et al. 2017. Reference numbers in LUSC models correspond as follows: [1] Ji et al. 2007a, [2] Xiao et al. 2013, [3] Xu et al. 2014a, [4] Mukhopadhyay et al. 2014, [5] Ferone et al. 2016, and [6] Mollaoglu et al. 2018. (Image of the virus from BioRender.com.)
Lung Adenocarcinoma
Approximately 70% of LUADs are characterized by the presence of mutations in known driver oncogenes. Mutations in KRAS are the most frequently occurring oncogenic driver mutations in LUAD, followed by EGFR and ALK alterations (Pao and Hutchinson 2012; Cancer Genome Atlas Research Network 2014; Skoulidis and Heymach 2019). Mutations in these oncogenes are largely mutually exclusive with one another (if one oncogene mutation occurs in a tumor, e.g., in KRAS, a mutually exclusive oncogene, e.g., EGFR, does not and vice versa). GEMMs for each of these major subsets of LUAD and other less commonly observed alterations have been developed. The first mouse models of Kras-mutant lung cancer demonstrated that oncogenic activation of Kras was sufficient to give rise to the disease, specifically causing LUADs in vivo (Jackson et al. 2001; Johnson et al. 2001). Many models have since been developed to specifically study Kras-mutant LUAD, using both inducible and conditional systems. The majority of studies have used a conditional lox-stop-lox allele that leads to expression of the KrasG12D mutation following Cre-mediated recombination. The mutation is engineered in the endogenous Kras locus, and tumors are induced by exposure to adeno-Cre or lenti-Cre viruses (Jackson et al. 2001; Meuwissen et al. 2001; DuPage et al. 2009). A doxycycline-inducible model was also developed in which oncogene expression was induced in type II pneumocytes (Fisher et al. 2001). Additional Kras alleles have been modeled using similar approaches, including a mouse model of KrasG12C-induced lung cancer, which is especially valuable for studies of the new KRASG12C inhibitors (Guerra et al. 2003; Li et al. 2018). When activating mutations in EGFR that confer sensitivity to EGFR tyrosine kinase inhibitors (TKIs) were discovered (Lynch et al. 2004; Paez et al. 2004; Pao et al. 2004), GEMMs of EGFR-mutant lung cancer were soon developed to model the specific mutations found in patient tumors. Inducible bitransgenic models were first developed that express activating EGFR exon 19 deletions or the L858R mutant in type II pneumocytes in the lung when the mice are administered doxycycline (Ji et al. 2006b; Politi et al. 2006). In both studies, expression of either EGFR mutant led to the development of LUADs, confirming the oncogenic potential of these two mutant alleles. Interestingly, while the EGFRL858R mutation resulted in diffuse lung cancer, the exon 19 deletion EGFR mutation resulted in multifocal adenocarcinomas, whereas the KrasG12D-mutant models developed focal tumors. In addition to demonstrating a role for the oncogenic variants in tumor initiation, the studies in the doxycycline-inducible systems also revealed the requirement for continued expression of the mutant oncogene for the survival of tumor cells in vivo. Of therapeutic importance, these preclinical model-based studies were also the first to demonstrate that lung cancer GEMMs could be used to accurately model responses to targeted therapies (Ji et al. 2006b; Politi et al. 2006).
ALK rearrangements are also responsible for driving a subset of NSCLCs and occur predominantly in LUAD (Takeuchi et al. 2008). Two studies using novel GEMMs of EML4-ALK-rearranged lung cancer first demonstrated the oncogenic effects of this gene fusion (Soda et al. 2008; Chen et al. 2010). More recently, a Cre-inducible model of EML4-ALK was developed, driven by the surfactant protein C (SFTPC) promoter, specific to alveolar type II pneumocytes, and has been used to evaluate response and resistance to ALK inhibitors (Pyo et al. 2017). Moreover, the ALK chromosomal rearrangement was modeled in one of the first studies to leverage CRISPR-Cas9 genome editing for in vivo cancer modeling (Maddalo et al. 2014). Other GEMMs have additionally been developed to study several of the less common driver mutations in LUAD. A Cre-inducible model of BrafV600E-mutant lung cancer, for example, develops benign lung tumors that only rarely progress to adenocarcinoma (Dankort et al. 2007). A Pik3caH1047R model did not develop tumors on its own, but when combined with the BrafV600E model promoted quicker tumor progression than in the BrafV600E model alone, with similar features to the KrasG12D model (Trejo et al. 2013). ROS1 fusion models (EZR-ROS1, CD74-ROS1, and SDC4-ROS1) have been developed using the SFTPC promoter to drive expression of the fusions and these have been used to study response to ROS1-targeted therapies (Arai et al. 2013; Inoue et al. 2016). Additionally, several models of MYC-driven LUAD exist. A model driven by c-Myc expression under the SFTPC promoter develops multifocal bronchioalveolar adenomas and eventually adenocarcinomas, yet tumor penetrance is incomplete (Ehrhardt et al. 2001). In one study, when c-Myc was placed under control of the CC10 promoter, only bronchioalveolar hyperplasias were observed (Geick et al. 2001), while in another study, overexpression of Myc under the CC10 promoter resulted in full-fledged LUADs (Tran et al. 2008). Importantly, another transgenic model with more physiologically relevant levels of Myc demonstrated that there is a threshold level of Myc expression that determines its biological consequences (Murphy et al. 2008), which could help explain differences among models.
Lung Squamous Cell Carcinoma
Until recently, LUSC has been the most challenging subtype of lung cancer to model in GEMMs. One of the reasons for this is that oncogenic alterations that drive LUSC have been harder to pinpoint even though a TCGA study identified targetable mutations and genomic differences in LUSC compared with LUAD (Cancer Genome Atlas Research Network 2012). One of the first models of LUSC emerged when expression of oncogenic KrasG12D was combined with inactivation of Lkb1, which resulted in a mixed spectrum of lung tumor types, including LUSCs (Ji et al. 2007a). A second model was developed by expression of kinase-dead IKKα leading exclusively to LUSCs, which had similarities to the molecular and inflammatory features of LUSC in patients (Xiao et al. 2013). A critical step forward occurred in 2014, with the development of two key models in which combinations of genetic alterations were introduced, namely through inactivation of Pten and Lkb1 (Ptenfl/fl;Lkb1fl/fl) or overexpression of Sox2 and inactivation of Lkb1 (Sox2;Lkb1fl/fl) (Mukhopadhyay et al. 2014; Xu et al. 2014a). The Sox2;Lkb1fl/fl model was generated using an advanced engineering approach through intranasal inhalation of a bicistronic lentivirus carrying both Sox2 and Cre recombinase under the control of two separate promoters. This study defined Sox2 as a driver of LUSC, and both studies together helped identify therapeutically targetable pathways altered in LUSC as well as changes in the immune and inflammatory response. Additional studies using the Sox2;Lkb1fl/fl model revealed an enrichment for tumor-associated neutrophils, as well as a role for reduced expression of Nkx2-1 in contributing to the squamous histological phenotype (Mollaoglu et al. 2018). Another study confirmed a role for Sox2 overexpression in the development of LUSC when combined with Cdkn2a/b and Pten loss (Ferone et al. 2016). Additional studies will be important to further define the cell-of-origin and molecular drivers of LUSC, which could enable the development of additional GEMMs of this hard-to-treat subtype of lung cancer (Sánchez-Danés and Blanpain 2018).
Small-Cell Lung Cancer
Several GEMMs of SCLC exist that recapitulate the histologic and molecular features described in patient tumors (Semenova et al. 2015). One of the first models of SCLC was developed by conditionally inactivating Rb1 and Trp53 (the hallmark tumor-suppressor gene changes found in patients’ SCLCs) in lung epithelial cells (Meuwissen et al. 2003). The resulting lung tumors displayed morphologic and immunophenotypic similarities to human SCLC and metastasized outside the lung. This study also demonstrated that inactivation of both Rb1 and Trp53 were necessary for SCLC pathogenesis. Several additional genetic alterations can accelerate tumorigenesis in this model including inactivation of Pten or of the Rb family member p130 (Schaffer et al. 2010; McFadden et al. 2014). More recently, SCLC subtypes were defined by differential expression of four critical transcription regulators: ASCL1, NEUROD1, YAP1, and POU2F3 (Rudin et al. 2019). Models now exist that recapitulate some, but not all, of these human molecular subtypes. Concomitant inactivation of Ascl1 with Rb1, Trp53, and p130 revealed that Ascl1 is required for SCLC tumorigenesis in this model, yet NeuroD1 was dispensable (Borromeo et al. 2016). Conversely, modeling Myc amplification, which commonly occurs in SCLC along with loss of Rb1 and Trp53 promotes highly aggressive, metastatic tumors, which are consistent with the NeuroD1-expressing subgroup of SCLC (Mollaoglu et al. 2017). The development of GEMMs that recapitulate the YAP1- and POU2F3-driven subtypes of SCLC will be important for further understanding the biology of these tumors and evaluating therapeutic strategies.
Overall, these models have recapitulated the histopathological features of human lung cancer. There is now a GEM model for each of the major oncogenic drivers of LUAD, and several reliable models of SCLC and LUSC. However, it has also become clear that some models with single-oncogene drivers may not be sufficient to consistently recapitulate the more complex features of tumor progression seen in patient tumors. Thus, models, driven by alterations in multiple oncogenes and/or tumor suppressors, that recapitulate the complexity of genetic alterations in human lung cancer are increasingly becoming of interest to the community to more accurately model the different subtypes of lung cancer.
Applications of GEM Models to Study Tumor Initiation and Progression
The generation of inducible and conditional models, as described above, represented an important advance in modeling lung cancer. Indeed, these approaches have the advantage of restricting tumor initiation to a particular subset of cells, which are maintained in their physiological microenvironment (Fisher et al. 2001; Jackson et al. 2001; Meuwissen et al. 2001; Kumar et al. 2008). An additional advantage of viral delivery systems is that they allow for extended tumor kinetics and thus improved survival of these models by mimicking tumor initiation from a single transduced cell, and by modulating temporal or spatial expression of the oncogenic driver (DuPage et al. 2009). Collectively, these advances in tumor modeling have allowed them to be used extensively for studies of lung tumor initiation and progression and therapeutic studies (see the section on Clinical Translation).
Lung Tumor Initiation and Cell of Origin
In this regard, GEMM studies have also helped define the cell-of-origin and biology of tumor initiation in lung cancer. Bronchioalveolar stem cells, positive for both club and type II alveolar cell markers, were identified using the Kras adenoviral Cre-induced GEMM, and are thought to be precursors to the bronchiolar club cells and alveolar cells that can give rise to adenocarcinoma (Kim et al. 2005). An additional study using CC10 (club cells) and Sftpc (type II alveolar) cell-specific promoters confirmed the role of bronchioalveolar stem cells as the cells of origin for Kras-induced hyperplasia, and further determined that only type II cells progressed to form LUADs (Xu et al. 2012). Notch signaling and Sox2 expression levels were shown to modulate the subtype of tumors that develop, such that overexpression of Sox2 leading to a reduction in Notch signaling results in widespread papillary adenocarcinomas throughout the bronchioles, yet Notch inhibition promotes squamous hyperplasia in alveoli (Xu et al. 2014b). Additional studies in GEMMs have revealed the mechanisms that underlie tumor initiation in specific cells of origin. Clonal 3D co-cultures of endothelial cells and bronchioalveolar stem cells revealed that the BMP4-NFATc1-TSP1 signaling pathway drives alveolar lineage-specific differentiation and regulates regeneration in vivo (Lee et al. 2014). Lgr5+ and Lgr6+ mesenchymal stem cells in the lung also influence alveolar differentiation of epithelial cell progenitors, which can occur through Wnt activity (Lee et al. 2017). More recently, the role of H3K9 methyltransferases and demethylases as regulators of tumor-propagating cells and therapeutic targets in GEMMs of lung cancer have been evaluated (Rowbotham et al. 2018). Further insights into the cells-of-orgin of different lung cancer subtypes come from studies using the Trp53- and Rb1-deficient model of SCLC. In this model, cell-type-specific adeno-Cre viruses identified neuroendocrine cells, and to a lesser extent alveolar type 2 (SFTPC-positive) cells, but not club cells, as the cell of origin in SCLC (Sutherland et al. 2011).
Another important application of GEMMs is to investigate tumor evolution and progression. In particular, single-cell sequencing of Kras-driven tumor models has revealed a major role for epigenomic regulation in cell-state transitions from early to advanced tumor stages (Drapkin and Minna 2020). Studies in the Kras model have described how phenotypic intratumoral heterogeneity increases along with tumor progression and have identified five main transcriptional programs to categorize cancer cells that appear during various stages of tumor initiation and progression (Marjanovic et al. 2020). These include cell states with an AT2 cell phenotype, an embryonic liver-like program, an EMT program that emerges at advanced tumor stages, and two previously undescribed programs: an early program characterized by both AT1 and AT2 cell features and a “highly mixed” one in which signatures of dramatically diverse cell lineages are found (Marjanovic et al. 2020). Interestingly, this “highly mixed” program defines a high-plasticity cell state (HPCS) profile characterized by cells with transcriptional profiles that overlap with multiple other cell types in the tumors and throughout tumor progression and the capacity to give rise to other cell states. These cells also have a distinct chromatin accessibility profile and express low and high levels of the Nkx2-1 and Runx transcription factors, respectively, and exhibit a pre-metastatic aggressive tumor cell state (LaFave et al. 2020; Marjanovic et al. 2020). Importantly, cells with HPCS profiles are found in human tumors opening up avenues for the investigation of possible novel therapeutic targets for this subset of tumor cells to prevent or delay tumor progression and drug resistance.
Lung Tumor Progression and Metastasis
Many GEMM studies, including some mentioned above, have also identified critical drivers of and factors that facilitate tumor progression. More than 50% of human tumors have alterations in TP53, and the same frequency is observed for lung cancer (Jordan et al. 2017; Skoulidis and Heymach 2019). In mice with oncogenic Kras, Trp53-deficient lung tumors, p53 restoration led to the regression of LUADs but not lung adenomas pointing to different functions of the tumor-suppressor gene at different stages of tumor progression (Feldser et al. 2010). Further, inactivation of Trp53 in the Kras-driven LUAD model promotes local and distal metastasis accompanied with more rapid tumor progression, highlighting the importance of this tumor-suppressor pathway to constrain metastasis formation in vivo (DuPage et al. 2009). Similarly, the addition of mutant Pik3ca with the BrafV600E mutation accelerated the latency for tumor development and the formation of LUADs (Trejo et al. 2013). In addition to the more rapid development of more aggressive tumors, some models also develop metastatic disease. For example, both local and distant metastases are observed in KrasG12D;p53fl/fl (DuPage et al. 2009), KrasG12D;Ptenfl/fl, and KrasG12D;Lkb1fl/fl models (Ji et al. 2007a; Iwanaga et al. 2008). However, distant metastases do not develop very readily in GEMMs and certainly not in all sites observed in humans. Moreover, many of the mouse models of lung cancer initiated with other oncogenic drivers do not develop metastatic disease. This may in part be due to the nature of models that develop tumors that are not always conducive to the time frame necessary for an advanced tumor to develop, with animals often succumbing to high tumor burden in the lungs. Alternatively, it could also be the result of different biological consequences of the different driver mutations, some of which yield more aggressive disease than others. Therefore, there is a significant need for the development of additional approaches to model metastatic disease in GEMMs especially for LUAD.
In SCLC models, metastatic disease develops more readily and this can be enhanced by the addition of other oncogenic alterations. For example, in the Rb1;Trp53-deficient model of SCLC, overexpression of Nfib, which typically occurs in human SCLC tumors, promotes tumor progression and metastasis (Denny et al. 2016; Semenova et al. 2016; Wu et al. 2016). Interestingly, the cell-of-origin can also influence the metastatic trajectory (Yang et al. 2018). One drawback is that current GEMMs of SCLC do not metastasize to the central nervous system (CNS), despite the fact that humans with SCLCs have the highest incidence of brain metastasis out of all lung cancer subtypes (Whitsett et al. 2013).
GEMMs, to date, have been excellent models in which to study the effects of oncogenic alterations individually or in combination with one to two additional cooperating events, and these studies have provided fruitful insights into the biology of the disease. Indeed, the lack of genetic complexity of GEMM models compared with human cancers (McFadden et al. 2016) is advantageous when studying specific genetic alterations on the biology of the tumor and on drug sensitivity. However, the lack of genomic complexity in many of the “classic” GEMMs is also a disadvantage, potentially precluding insights into the consequences of mutational heterogeneity on tumor progression and therapeutic response, including responses to immunotherapies that often rely on tumor immunogenicity for their efficacy (see Clinical Translation). Moreover, many lung cancer GEMMs do not develop metastases, and the metastatic models that do exist suggest that additional genetic alterations may be required to promote progression and metastasis.
Next-Generation GEMMs
Next-generation sequencing of human specimens through direct analysis of tumors or of circulating tumor DNA from liquid biopsies, has shown that multiple alterations affecting crucial pathways in tumorigenesis frequently co-occur in lung cancers (Cancer Genome Atlas Research Network 2012, 2014; Rudin et al. 2012; Campbell et al. 2016; Jordan et al. 2017; Leighl et al. 2019). How the presence of co-occurring alterations with the oncogenic driver affects tumor progression is still largely unknown. There is now increasing evidence that co-occurring alterations further define subsets of oncogene-driven cancers (Blakely et al. 2017; Skoulidis and Heymach 2019). For example, for KRAS-driven LUADs, co-occurring alterations in STK11/LKB1 or TP53 define subsets that differ with regard to their sensitivity to immunotherapies and features of the tumor immune microenvironment (Skoulidis et al. 2015, 2018). The genomic complexity of human tumors is less likely to be achieved with traditional GEMMs, since tumor initiation in vivo is often driven by activation of a single oncogenic driver, and the tumor kinetics—which are usually faster in GEMMs compared to humans—could limit the acquisition of certain advanced features that are present in the human disease. Nonetheless, in contrast to their human counterparts, LUADs in GEMMs harbor fewer somatic mutations (McFadden et al. 2016). Despite Trp53 inactivation leading to the acquisition of a more aggressive phenotype in the Kras-driven model, no difference in the mutation rates was observed between Trp53-deficient and wild-type Kras-mutant tumors (McFadden et al. 2016). Therefore, there is a need for the next generation of GEMMs to incorporate these important co-occurring alterations to more accurately recapitulate the disease observed in humans.
Over the course of the past decade, many studies have aimed to evaluate the role of co-occurring tumor-suppressor gene alterations in a Kras-driven model of LUAD—mainly conditional alleles to inactivate specific tumor-suppressor genes of interest (Efeyan et al. 2009; Liang et al. 2010; Gao et al. 2011; Curry et al. 2013; Schuster et al. 2014; Baumgart et al. 2015). More recently, these efforts have greatly increased with the advent of CRISPR-Cas9 genome editing. Several years ago, Sánchez-Rivera and colleagues (2014), for the first time, used CRISPR-Cas9-mediated genome editing in vivo in the Kras-driven LUAD model. They demonstrated that inactivating Pten had a selective advantage over retention of wild-type Pten due to increased PI3K/Akt signaling, and that Pten, Apc, and Nkx2-1 all constrain tumor growth, to varying degrees (Sánchez-Rivera et al. 2014). More recently, other studies leveraging in vivo somatic genome editing have elucidated the role of other additional tumor-suppressive pathways in lung cancer (Romero et al. 2017; Walter et al. 2017; Wang et al. 2019a). Loss of Setd2 showed robust effects on tumor growth providing evidence of a role for chromatin-modifying genes in lung cancer in vivo (Walter et al. 2017). Keap1 or Nf1 inactivation were shown to lead to metabolic reprogramming establishing a tumor dependency on glutaminolysis thus nominating potential therapeutic strategies for two major subsets of lung cancer patients with tumors harboring oncogenic KRAS mutations and KEAP1 or NF1 pathway alterations (Romero et al. 2017; Wang et al. 2019a). A major limitation of these in vivo studies is that the number of possible combinations to be investigated is very high and requires complex crosses and large numbers of experimental animals. To circumvent this problem, multiplexed systems have been developed to simultaneously interrogate multiple genomic interactions (Rogers et al. 2017, 2018; Winters et al. 2017; Li et al. 2021). By performing an in vivo screen to simultaneously interrogate the effect of inactivating 11 different tumor-suppressor genes frequently mutated in LUAD, Rogers and colleagues identified tumor-suppressor genes that significantly constrain the growth of Kras-driven tumors and others that do not (Rogers et al. 2017). For example, Setd2 and Lkb1 inactivation had the most profound effects on the growth of Kras-mutant LUADs, while Smad4 or Atm inactivation did not have any effect. Furthermore, they found that the functional effects on tumor growth further depend on the genomic interactions with co-occurring alterations in the p53 or Lkb1 pathways, which define two important subsets of KRAS-driven tumors (Skoulidis et al. 2015; Rogers et al. 2018). Indeed, recently, this CRISPR-Cas9 multiplexed screening approach was applied to an EGFR-driven Trp53-deficient model of LUAD. This study identified Rb1, Rbm10, and Apc inactivation as the strongest drivers of tumor growth in this model and studies are underway to understand mechanistically how these tumor-suppressor genes modulate the biology of EGFR-mutant tumors (Foggetti et al. 2021).
As shown above, most of the studies of the consequences of co-occurring alterations on LUADs have been limited to Kras-driven tumors. Therefore, we anticipate that additional in vivo studies in other oncogenic driver contexts (i.e., EGFR- and ALK-driven models) and other lung cancer subtypes will be important to further shed light on the biology of specific combinations of genomic alterations that are commonly found in human tumors. Overall, these innovative multiplexed models could lead to a more rapid identification of potential pathways that inform lung cancer biology, help predict responses to novel therapeutics, and shed light on mechanisms of resistance to therapies.
Preclinical Models to Study Cancer Immunology (from GEMMs to Syngeneic Models)
With increased recognition of the role played by the tumor microenvironment (TME) in tumorigenesis and drug sensitivity, interactions between tumor cells and the immune system are being studied (Chen et al. 2014b). An important advantage of GEMMs is that tumors develop within the tissue of interest where interactions between cancer cells and other cells in the TME can be investigated. For example, GEMMs of lung cancer have been used to establish the influence of oncogenic RAS and EGFR signaling on the tumor immune microenvironment, including stimulation of PD-1 pathway activity and the production of proinflammatory cytokines (Ji et al. 2006a; Wislez et al. 2006; Akbay et al. 2013). In a study that profiled the tumor immune microenvironment of three common models of NSCLC—KrasG12D, KrasG12D;Trp53fl/fl, and EGFRL858R—as well as the Rb1fl/fl; Trp53fl/fl model of SCLC, each was found to have a different immune cell composition (Busch et al. 2016). While both the EGFR- and Kras-mutant models had significant infiltration of myeloid cells, only the Kras-mutant tumors displayed T-cell-driven immune responses. Additionally, the SCLC model had fewer leukocytes than any of the LUAD models. Importantly, these findings in the mouse models were validated using patient samples. Further, additional studies have investigated the differences in immune cell composition in subsets of Kras-driven LUAD with specific co-occurring alterations in other genes. These studies demonstrated, for example, that the Kras;Lkb1-deficient subset displays an accumulation of proinflammatory cytokines and neutrophils with T-cell-suppressive effects (Koyama et al. 2016) while the Kras;Keap1-deficient subset of tumors, found to originate in bronchiolar cells, lack the pro-tumorigenic macrophages observed in tumors originating from alveolar cells (Best et al. 2019).
Immune checkpoint inhibitors (ICIs) have shown remarkable efficacy for the treatment of many cancer types including lung cancer, although response rates remain limited (Topalian et al. 2012; Rizvi et al. 2015; Doroshow et al. 2019). In LUAD, the sensitivity to ICIs depends in part on the tumor mutation burden (TMB) (Rizvi et al. 2015). This partially explains the higher response to ICIs observed in KRAS-driven tumors compared to EGFR-driven tumors, in which the TMB is lower (Gainor et al. 2016; Dong et al. 2017; Hastings et al. 2019). How a tumor grows and evolves to no longer be recognized by the immune system and how it can evade immunotherapy are critical questions currently under investigation. Therefore, there is an urgent need to generate improved preclinical models to understand the mechanisms that regulate the immune system during tumorigenesis and identify further vulnerabilities to optimize immunotherapy strategies.
GEMMs have a low tumor burden and seem to be less suitable to address these questions (McFadden et al. 2016). For this reason, mice that are exposed to carcinogens to accumulate more somatic single-nucleotide variants (SNVs) and develop tumors, can be used for understanding the influence of a complex mutational spectrum on the tumor microenvironment. However, studies with these carcinogen-induced tumor models in mice, which are mostly initiated by carcinogen-induced Kras alterations, limit these studies to this subset of LUADs (Westcott et al. 2015). Another challenge to studying immune responses in GEMMs is the lack of known tumor-associated antigens. To facilitate studies of tumor-specific T-cell responses, GEMMs have now been developed that express model tumor antigens, which elicit an antitumor T-cell response in a Kras-driven model of LUAD (DuPage et al. 2011; Joshi et al. 2015; Damo et al. 2021). These GEM models have helped inform our understanding of tumor cell interactions with the immune microenvironment, yet many challenges still exist to modeling immune responses in mice. There is still a need for improved models to better study response and resistance to immunotherapies, which may include models with an increased TMB and lung cancer–specific neoantigens.
An alternative in vivo approach to study the immune system in the response to ICIs involves the transplantation of murine tumor cell lines into syngeneic recipient mice. A successful example of this approach is described by Ambrogio et al. (2014), in which murine lung cancer cell lines derived from the KrasG12V model were transplanted orthotopically into the lungs of mice. Other groups have also established murine tumor cell lines from GEMMs (Azpilikueta et al. 2016; McFadden et al. 2016; Meeth et al. 2016; Wang et al. 2017). These more flexible models allow the manipulation of tumor cells in vitro by introducing additional alterations and transplanting them for in vivo studies, without losing their immunogenicity, when engrafted into syngeneic mice. Several studies with this approach have led to improved understanding of the molecular mechanisms that orchestrate the modulation of the immune system together with the tumor in response to ICIs (Azpilikueta et al. 2016; Meeth et al. 2016; Gettinger et al. 2017). Because targeted therapies or ICIs are most frequently used in advanced metastatic disease, studies in metastatic models where tumor cells are introduced systemically or transplanted at metastatic sites are very relevant (Gibbons et al. 2009; Padhye et al. 2019). Finally, syngeneic CRISPR-Cas9-mediated genome-editing screens are now being used in these models (Wang et al. 2019b).
Next-generation transplantation systems can be rapidly established and are, despite their simplicity, good models of the human counterparts (as elaborated in the following section). A potential disadvantage of transplantation of cell lines, however, is that the immune response to a transplanted tumor cell line may differ from that when a tumor develops autochthonously over time as would occur in GEMMs. As with the GEMMs, these systems have been mainly adopted in Kras-driven tumors; thus, the development of such models for oncogenic EGFR and ALK drivers would help uncover interactions between the immune system and other LUAD genotypes that can be exploited toward precision medicine.
PATIENT-DERIVED HUMAN TUMOR CELL LINES AND XENOGRAFT MODELS
Based on pioneering work in the 1970s, several collections of well-annotated human lung cancer cell lines were established (Gazdar et al. 2010b). Accordingly, between 300 and 400 of these cell lines continue to be widely used for research today. The molecular diversity of human cell lines is perhaps their greatest advantage and these lines encompass most of the recurrent genetic alterations found in human lung cancers, including gene mutations, deletions, amplifications, translocations, and methylation-induced gene silencing (Gazdar et al. 2010a). Cell lines have historically proven to be instrumental in identifying oncogenic drivers and molecular mediators of drug resistance and many cell line–based findings have been validated in human studies. Several notable examples include studies in lung cancer cell lines harboring KRAS mutations (Singh et al. 2009) and EGFR mutations (Paez et al. 2004; Pao et al. 2005) or ALK fusions (Soda et al. 2007). Below, we describe several major collections of well-annotated human cell lines that have been used for large-scale molecular and functional studies and describe their advantages and disadvantages. Moreover, we also review recent advances in the use of cell lines and patient-derived models for the establishment of 3D cultures and for studies in vivo.
Vast Collection of Cell Lines for In Vitro Studies
The National Cancer Institute (NCI) collection of a panel of 60 cell lines includes 10 NSCLC lines. The NCI-60 has been previously subjected to extensive molecular characterization, validation by analysis of SNPs (single-nucleotide polymorphisms), and served as a platform for screening and repurposing of drugs (Roschke et al. 2003; Adams et al. 2005; Lorenzi et al. 2009). A broader collection of 187 lung cell lines (including 53 SCLC, 28 LUSC, 54 LUAD, and other rarer subtypes) has been recently subjected to molecular and functional characterization as part of the Cancer Cell Line Encyclopedia (CCLE). This includes next-generation characterization by RNA sequencing, whole exome sequencing (WES), reverse-phase protein array (RPPA), reduced representation bisulfite sequencing (RRBS), microRNA expression profiling, and global histone modification profiling (Ghandi et al. 2019). In addition, functional studies using loss-of-function techniques including short hairpin RNA (shRNA) (Cowley et al. 2014; Tsherniak et al. 2017) and CRISPR screens (depmap.org/portal, Meyers et al. 2017; depmap.sanger.ac.uk, Behan et al. 2019) have been performed using a wide representation of lung cancer cell lines. For example, various studies have used up to 110 cells lines (Tsherniak et al. 2017) for shRNA screens, and up to 43 lines (Sanger, Behan et al. 2019) and 64 cell lines (Broad, Meyers et al. 2017; Nature Cancer, Corsello 2020) in the Dependency Map (DepMap) effort (Table 1). These rich data sets can be integrated and mined to reveal potential oncogenic dependencies. Another advantage is that cell line collections can be subjected to large-scale genomic high-throughput drug screening in culture. This approach is particularly amenable to repurpose drugs, which may be effective in cancer cells independent of their oncogenic dependencies (Nature Cancer, Corsello 2020). Finally, as described below, many lung cancer cell lines can be transplanted into animals for specific types of in vivo preclinical testing and validation.
Table 1.
Representative examples of collections of models of lung cancer
| Name | Model types | Lung cancer models | SCLC | LUAD | LUSC | Resources | Websites | References |
|---|---|---|---|---|---|---|---|---|
| Broad DepMap | Cell lines | 242 | x | x | x | CRISPR screen, shRNA screen, drug screen, RNA-seq, WES, chromatin profilinga | depmap.org/portal | Cowley et al. 2014; Meyers et al. 2017; Tsherniak et al. 2017; Ghandi et al. 2019; Corsello et al. 2020 |
| SANGER DepMap | Cell lines | 231 | x | x | x | CRISPR screen, shRNA screen, drug screen, RNA-seq, WES, chromatin profilinga | depmap.sanger.ac.uk | Behan et al. 2019 |
| Human Cancer Models Initiative (HCMI) NCI | Cell lines/PDO | 3 | n/a | x | n/a | RNA-seq, WGS | ocg.cancer.gov/programs/HCMI | Joshi et al. 2020 |
| Princess Margaret Cancer Center | PDX/PDO | 145/8 | n/a | n/a | n/a | www.livingbiobank.ca | ||
| EurOPDX European consortium | PDX | 71 | x | x | n/a | Ongoing characterization | www.europdx.eu/pdx-collection | |
| PDXnet | PDX | Coming soon | n/a | n/a | n/a | WES/RNA-seq | www.pdxnetwork.org | |
| ChampionPDX | PDX | 160 | n/a | n/a | n/a | WES/RNAseq (130)a | www.championsoncology.com | |
| Jackson laboratories | PDX | 79 | x | x | x | RNAseq, Biobank (4)a | www.jax.org, tumor .informatics.jax.org/mtbwi/pdxSearch.do |
aNot all resources available for all the lung cancer models listed.
(PDX) Patient-derived xenograft, (PDO) patient-derived organoid, (CDX) cell-line-derived xenograft, (SCLC) small cell lung cancer, (LUAD) lung adenocarcinoma, (LUSC) lung squamous carcinoma, (WGS) whole genome sequencing, (WES) whole exome sequencing, (n/a) not annotated.
Next-Generation In Vitro Models: 3D Cultures and Organoids
Despite the vast amount of knowledge we have collected from using lung cancer cell lines, these models have important limitations. These include genetic drift after extensive passages in culture, lack of differentiation and multistage pathology in vitro, and restricted representation of all histological and molecular subtypes of lung cancer. Notably, cell culture conditions used to date may bias toward the generation of cell lines of the adenocarcinoma subtype (Oie et al. 1996). Other general considerations include the fact that culture conditions select for cancer cell lines under nonphysiological conditions. First, the polystyrene surface where cells are plated is mechanically stiff (Young's modulus, E = 3-3.5 × 109 Pa) particularly when compared to the mechanosensing conditions in the lung microenvironment (∼2000 Pa) or metastatic sites (brain ∼100 Pa, liver ∼500–600 Pa) (Swift et al. 2013; Wells 2013; Barnes et al. 2017; Smith et al. 2018). Second, the rich nutrients and in particular amino-acid content of most tissue culture media might prime cell lines toward particular metabolic dependencies that diverge from their in vivo anabolic and catabolic activity (Muir et al. 2018). For example, lung cancer cell lines cultured in vitro use glutamine as a primary carbon substrate for fueling the TCA cycle, whereas lung tumors in vivo favor glucose consumption with a minor contribution of glutamine (Sellers et al. 2015; Davidson et al. 2016; Muir et al. 2017). Third, standard oxygen levels in culture do not match the same abundance as tumors in vivo and their tissue of origin (Vaupel et al. 1989; Walsh et al. 2014; Place et al. 2017). This could have significant consequences when interpreting results from functional studies using cell lines. For example, a loss-of-function screen comparing in vitro model systems and in vivo tumors identified a requirement for the enzyme (NSF1) depending on environmental oxygen levels (Alvarez et al. 2017). Selection of the NSF1 pathway by lung adenocarcinoma in vivo confers resistance to high oxygen tension and protects cells from oxidative damage and ferroptosis. Differences in oxygen levels and hypoxic conditions are also relevant at different metastatic sites in vivo (see below in the section Cell Line- and Patient-Derived Xenografts).
Various cell culture conditions have been developed to recapitulate particular physiologically relevant characteristics of airway epithelial differentiation and regeneration ex vivo. Some of these initial protocols include air–liquid interface (ALI) cultures (Barkauskas et al. 2017), growth with extracellular matrix components such as matrigel, co-culture with stromal cells (i.e., fibroblasts or endothelial cells), and mesenchymal factors (i.e., Noggin, BMPs, EGF, etc.). In particular, 3D and organoid models for alveolar and bronchial tissue have been developed from adult stem cells, and recapitulate their epithelial organization (McQualter et al. 2010; Chen et al. 2012a; Barkauskas et al. 2013; Tata et al. 2013; Lee et al. 2014). Moreover, advances in the growth of airway structures from human pluripotent stem cells (hPSCs) have been made (Chen et al. 2017; Miller et al. 2019). These protocols have been applied successfully to model complex lung diseases such as idiopathic lung fibrosis (IPF) (Strikoudis et al. 2019), cystic fibrosis (Mou et al. 2012a; Wong et al. 2012), or asthma (Danahay et al. 2015). Similar approaches have been most successfully adapted for the generation of epithelial organoids (Sato et al. 2009) and patient-derived tumor organoids (Boj et al. 2015; van de Wetering et al. 2015; Sachs et al. 2018) from the intestine. The same methodology may be applied to grow lung tumor organoids (Sachs et al. 2019; Shi et al. 2020). An important technical challenge is that this protocol also enriches for nonmalignant cells (Dijkstra et al. 2020). One way to minimize the coexpansion of nontumorigenic cells in organoid cultures is through selective killing of TP53 wild-type cells using Nutlin-3a, manual separation based on organoid morphology, or removing from the culture media airway epithelial cell growth factors (i.e., Wnt3a and Noggin) (Kim et al. 2019).
Organoids ultimately may more closely recapitulate the polarized epithelial–mesenchymal cellular interactions, maturation of airway structures, and retention of functional features including beating cilia and mucus secretion (Dye et al. 2015, 2016; Chen et al. 2017). Despite the overall challenges of genetically transducing primary cell cultures, organoids may also be readily genetically modified (Broutier et al. 2016). Finally, perhaps the greatest potential for using organoid cultures is the possibility to co-culture tumor cells with stromal cells to mimic specific tumor microenvironment interactions including with immune cells (Neal et al. 2018). One recent example is a protocol to establish patient-derived organotypic tumor spheroids (PDOTS), which are co-cultures of patient-derived organoids with autologous peripheral blood lymphocytes (Cattaneo et al. 2020). PDOTS provide an essential platform to enrich for tumor-reactive T cells (Dijkstra et al. 2018) and to perform preclinical testing of immunotherapies (Jenkins et al. 2018) or combination therapies (Ivanova et al. 2020).
In summary, based on experience with other cancer types, organoid culture conditions may be more suitable for the propagation of patient-derived lung cancer cells in vitro, thus facilitating the generation of new clinically relevant models to complement available cell line collections and mouse models.
Cell Line– and Patient-Derived Xenografts
The advantages and disadvantages of xenograft models, particularly when compared to syngeneic models of tumor progression have been extensively reviewed elsewhere (Gould et al. 2015; Byrne et al. 2017). Here, we consider how lung cancer cell–derived xenografts and patient-derived xenografts (PDXs) can specifically inform us about the biology of lung cancers and their response to relevant therapies in vivo.
As described above, diligently annotated collections of human lung cancer cell lines are widely available for research. Many of these human cell lines were isolated from patient tumor biospecimens and they can also be transplanted into immunocompromised mice to study lung tumorigenesis in vivo. An important advantage of cell lines in general is that they can be transplanted using various injection techniques, which are amenable to the quantification of different biological properties of lung cancer cells in vivo and the study of metastasis (Fig. 1). Examples include measuring the tumor-reinitiating capacity of lung cancer cells, survival of circulating tumor cells (CTCs), seeding rates of disseminating tumor cells (DTCs) in distant organs, monitoring micrometastatic to macrometastatic outgrowth, and measuring residual disease following treatment. Some xenograft models also recapitulate patterns of metastatic relapse observed in lung cancer patients. These include metastasis to the bone, adrenal gland, liver, and the CNS (Fig. 1; Riihimäki et al. 2014; Li et al. 2019a). In GEMMs of lung cancer, the presence of DTCs in circulation can also be documented during the later stages of progression (Caswell et al. 2014). However, as mentioned above, the latency of metastasis in GEMMs is generally long and to date, these models rarely, if ever, form metastases in the CNS, despite the fact that brain relapses are frequent in lung cancer patients.
During the past decade, we have seen a significant increase in the generation and use of PDXs in preclinical studies across various types of cancers and several well-described PDX collections are accessible for use (Table 1). It has been suggested that PDXs obviate the idiosyncrasies of cell lines established in vitro. PDXs in vivo can be generated from tumor tissue as well as from CTCs (referred to as CTC explants [CDXs]), which may be more readily collected from the clinic (Hodgkinson et al. 2014). In addition, an important opportunity that PDX models provide is the ability to propagate and study molecular subtypes of human lung cancers that cannot be readily maintained as cell lines. This is particularly relevant in the context of human NSCLCs driven by ROS1 and ALK rearrangements, which are detected in 2% and 5% of NSCLCs, respectively, and yet are not well represented in historical cell line collections. PDXs can also recapitulate the major histological characteristics of their original tumor tissue. Accordingly, several new collections of PDXs are being initiated by patients and patient advocates to expand the repertoire of human lung cancer subtypes that can be modeled in animals (see, for example, clinical trial NCT03497624).
Because of the increased genetic heterogeneity inherent to patient biospecimens, PDXs can model drug response and acquired drug resistance, and in turn help identify predictive and prognostic biomarkers (DeRose et al. 2011; Bertotti et al. 2015; Kavuri et al. 2015; Gupta et al. 2016). For instance, a study of PDXs from metastatic colorectal carcinomas identified vulnerabilities in patients with tumors resistant to cetuximab and identified HER2 as a predictor of resistance to anti-EGFR antibodies (Bertotti et al. 2011). In NSCLC, preclinical studies in PDXs have been able to identify a range of responses to the EGFR inhibitor gefitinib and oncogenic aberrations that render them insensitive to the drug (Zhang et al. 2013) or underlie erlotinib resistance (Fichtner et al. 2008), and TKI-resistant PDXs are increasingly being generated that will be useful for these purposes (Zahalka et al. 2017; Kita et al. 2019). Moreover, xenograft models have proven to be invaluable for ascertaining the potential toxicities and pharmacokinetic properties of drugs. The differences between murine and human genomes can also be leveraged to distinguish the molecular profiles of tumor cells from their surrounding stroma in situ (Sevenich et al. 2014; Wingrove et al. 2019), thus obviating the need to isolate a priori defined cell types that may also introduce technical artifacts.
Some limitations of xenograft models are particularly worthy of consideration when designing in vivo experiments. First, a deficient adaptive immune response in most immunodeficient recipient mice limits studies on the immune tumor microenvironment and efficacy of immunotherapies. Transplantation rates and tumor growth can also be influenced by the recipient mouse strain (Hidalgo et al. 2014; Jung et al. 2018). For instance, in colorectal cancer, engraftment is higher in non-obese-diabetic (NOD)/SCID compared to Nude mice, whereas in breast or lung cancer, both strains yield similar engraftment rates (24%–34%) (Perez-Soler et al. 2000; Fichtner et al. 2008). Moreover, some paracrine signaling molecules have poorly conserved activities across human and mice. One notable example is the HGF/Met signaling axis, as murine Hgf is a poor activator of human MET receptor tyrosine kinase (RTK) (Wilson et al. 2012). Furthermore, it is estimated that a significant number of paracrine cytokine receptor pairings in humans are incompatible or do not exist in mice (Manz 2007). Injection and surgical procedures can trigger inflammation, which conceivably may confound studies on tumor cell plasticity in response to the microenvironment. The passaging of cell lines or PDXs in mice also results in clonal selection or genetic drift in a manner that may differ from normal human tumor evolution (Ben-David et al. 2017). When compared to other tumor types, the initial transplantation rate of lung cancer biospecimens into mice is relatively low. The reasons for this remain unclear but may involve the dependency of lung cancer cells on the microenvironment. In this regard, significant consideration should be made on the site of tumor cell injection and growth. Tumors grown subcutaneously into the flank of mice are poorly perfused and more hypoxic when directly compared to tumors in the lungs (Vilalta et al. 2017). Lung cancer PDXs implanted in the renal capsule engrafted at a higher rate than in the subcutaneous flank in NOD SCID mice (90% vs. 24%) (Cutz et al. 2006; Fichtner et al. 2008). Injection of tumor cells into an orthotopic site resembling their tissue site of origin, despite being technically challenging, is probably the most relevant way to recapitulate some of the physiological and stromal conditions to which tumor cells are adapted (Wang et al. 1992; Hidalgo et al. 2014). This approach is especially relevant when using PDXs to study metastasis and may explain the higher take rate of metastatic biopsies when they are engrafted into their orthotopic site (Lee et al. 2015).
Next-Generation PDX Models: Incorporating the Human Immune System
A new generation of PDX models is being developed to overcome some of the limitations noted above. For instance, humanized mouse models can recapitulate aspects of the human immune system. These models are generated by engrafting human peripheral blood mononuclear cells (PBMCs), hematopoietic stem cells (HSCs), or fetal tissues (liver and thymus) into an immunodeficient mouse, preferentially the NSG and MISTRG (Rongvaux et al. 2014) mice. NSG mice, also known as NOD-Cg-PrkdcscidIl2rgtm1Wjll, have three key features: (1) they are on a NOD background characterized by hemolytic complement deficiency and a loss-of-function allele of Sirpa thus reducing phagocytosis; (2) severe combined immunodeficiency due to mutations in the Prkdc gene causing lack of T and B lymphocytes; and (3) a mutation in the IL-2 receptor common γ-chain (Il2rg) further exacerbating the immunodeficiency and causing natural killer (NK) cell defects (Ishikawa et al. 2005). NSG mice can be modified to express HLA-A2 (MHC class I haplotype) to facilitate the evaluation of antigen-specific T-cell responses (Shultz et al. 2010). MISTRG mice are immunodeficient Rag2−/−γc−/− mice that have been further manipulated to knock-in human genes that support myeloid cell function into the mouse loci (Rongvaux et al. 2014). These genes encoding for human macrophage colony-stimulating factor (M-CSF), interleukin-3 (IL3), thrombopoietin, granulocyte macrophage CSF (GM-CSF) and a transgene encoding human SIRPA. These models allow us to understand the biology of acquired immune response in diseases such as autoimmune disease, infectious diseases (i.e., HIV) and allograft rejection (Walsh et al. 2017; Allen et al. 2019). One potential pitfall of this approach is the difficulty to obtain sufficient autologous HSCs to conduct properly powered preclinical studies. Moreover, repopulation of all the hematopoietic lineages is incomplete: T-cell function is elicited only for a limited period of time and in some instances robust graft versus host disease (GvHD) develops (reviewed in Walsh et al. 2017). Nevertheless, humanized mice have contributed substantially to improvement of anticancer therapies (Norelli et al. 2018), CAR-T cell testing (Forsberg et al. 2019) and preclinical testing of human immunosuppressive agents (De La Rochere et al. 2018). In the era of immuno-oncology (IO), the generation of humanized PDX models is a requirement for preclinical testing (Morton et al. 2016) and modeling of resistance to these therapies. A few examples have been applied to lung cancer biology, specifically to test responses of PDXs to immune checkpoint inhibitors. Initial studies back in 2008 reported that implantation of nondisrupted pieces of primary NSCLC into NSG mice allowed expansion of tumor memory T cells (Simpson-Abelson et al. 2008). A similar approach was used more recently to study the effect of anti-PD1 therapy on T cells in implanted NSCLC tumor fragments (Gettinger et al. 2018). In other studies, NSG mice transplanted with fresh CD34+ HSCs derived from umbilical cord developed functional T and B lymphocytes, and NK cells and mice elicited a strong antitumor response to PD-1 checkpoint inhibitors pembrolizumab and nivolumab (Meraz et al. 2019).
Reprogrammed to Study Lung Differentiation and Lung Tumor Pathogenesis
Lung tissues derived from human embryonic (hESCs) and induced (iPSCs) pluripotent stem cells have emerged as an alternative to understand and study lung biology and pathogenesis. The first report describing a protocol to derive functional proximal airway epithelial cells from hESCs (Wong et al. 2012) was a proof of concept to model cystic fibrosis. Since then, methods have been improved by incorporating signaling molecules responsible for embryonic lung development (Huang et al. 2014; Dye et al. 2015; Konishi et al. 2016; Chen et al. 2017; McCauley et al. 2017). To date, lung epithelial cultures can be maintained and propagated for several months to generate distal and proximal progenitors and develop functional human airways. In the case of lung cancer, reprogramming of human stem cells has been implemented to generate rare pulmonary epithelial cell populations that are likely to be a target of transformation. This is the case of neuroendocrine cells, putative progenitors of SCLC (Sutherland et al. 2011; Song et al. 2012). Lung progenitors derived from hESCs have also been subjected to inhibition of NOTCH signaling, loss of RB1 and interference with TP53, to generate early-stage SCLC-like tumors when engrafted into mice (Chen et al. 2019). Another example is the reprogramming of lung and prostate normal epithelial cells by a defined set of oncogenic factors that include BCL2, RB1, and TP53 to generate neuroendocrine small-cell cancers (Park et al. 2018). These approaches, therefore, allow us to model rare cancer types, and to understand the common underlying oncogenic cues that enable transformation of normal cells into different lung cancer subtypes.
CLINICAL TRANSLATION
Therapeutic Studies in Preclinical Models of Lung Cancer
During the past decade there has been increased interest in developing models and applying them to investigate therapeutic approaches to treat cancer, including lung cancer (Table 2). These models have been valuable to provide insight into the mechanisms and efficacy of therapies, to uncover new drug targets, and to study drug resistance.
Table 2.
Representative examples of therapeutic studies in preclinical models of lung cancer
| Name | Chemotherapy | Targeted therapy | Immunotherapy | |||
|---|---|---|---|---|---|---|
| EGFR-mutant GEMM | Paclitaxel and pemetrexed | Regales et al. 2009 | Cetuximab | Ji et al. 2006b; Regales et al. 2007 | Anti-PD-1 | Akbay et al. 2013; Ayeni et al. 2019 |
| 1st Gen. EGFR TKI |
Ji et al. 2006a; Politi et al. 2006 | |||||
| 2nd Gen. EGFR TKI |
Regales et al. 2009; Pirazzoli et al. 2014 | |||||
| 3rd Gen. EGFR TKI |
Zhou et al. 2009; Starrett et al. 2020 | |||||
| EGFR TKI + MEK inhibitor |
Tricker et al. 2015 | |||||
| Kras-mutant GEMM | Cisplatin | Oliver et al. 2010 | MEK inhibitor | Ji et al. 2007b; Engelman et al. 2008; Faber et al. 2009 | Anti-PD-1 | DuPage et al. 2011; Li et al. 2020 |
| Docetaxel + MEK inhibitor |
Chen et al. 2012b | |||||
| Cisplatin + pemetrexed + MEK inhibitor | Li et al. 2018 | |||||
| Other LUAD GEMM | Docetaxel and pemetrexed | Chen et al. 2014a | ALK inhibitor (ALK GEMM) |
Soda et al. 2008; Chen et al. 2010; Chen et al. 2014a; Pyo et al. 2017 | ALK cancer vaccine | Voena et al. 2015 |
| ALK/ROS inhibitor (ROS1 GEMM) |
Inoue et al. 2016 | |||||
| SCLC GEMM | Cisplatin | Böttger et al. 2019 | Aurora kinase inhibitor | Mollaoglu et al. 2017 | Anti-CD47 | Weiskopf et al. 2016 |
| Xenograft | Cisplatin, docetaxel, and gemcitabine | Steiner et al. 2007 | Cetuximab | Steiner et al. 2007 | Anti-PD-1 (humanized model) |
Meraz et al. 2019 |
| Paclitaxel | Onn et al. 2003 | ALK inhibitor | Friboulet et al. 2014 | |||
| KRAS inhibitor | Janes et al. 2018; Canon et al. 2019; Hallin et al. 2020 | |||||
| EGFR TKI + MEK inhibitor |
Tricker et al. 2015 | |||||
| Syngeneic | Oxaliplatin + cyclophosphamide | Pfirschke et al. 2016 | ALK inhibitor | Ambrogio et al. 2014 | Anti-PD-1 | Gettinger et al. 2017 |
| KRAS inhibitor | Canon et al. 2019; Hallin et al. 2020 | ALK cancer vaccine | Voena et al. 2015 | |||
| PARP inhibitor + anti-PD-L1 | Sen et al. 2019 | |||||
| PDX | Etoposide and platinum | Drapkin et al. 2018 | Cetuximab | Fichtner et al. 2008; Merk et al. 2009 | CAR T-cell therapy | Norelli et al. 2018 |
| EGFR TKI | Fichtner et al. 2008; Merk et al. 2009; Zhang et al. 2013; Stewart et al. 2015 | Anti-PD-1 (humanized models) | Meraz et al. 2019 | |||
| ALK inhibitor | Gainor et al. 2016 | |||||
| KRAS inhibitor | Janes et al. 2018 | |||||
| PARP inhibitor | Lok et al. 2017; Farago et al. 2019 | |||||
(TKI) Tyrosine kinase inhibitor, (SCLC) small-cell lung cancer, (PDX) patient-derived xenografts.
Mechanism of Action and Efficacy
One of the most common uses of preclinical models has been to test the efficacy of therapeutic approaches to treat tumors. This has been achieved through the use of GEMMs, established cell lines, and more recently patient-derived models. Models of LUAD, in particular, have been valuable to evaluate targeted therapies and provide preclinical rationale for investigating specific agents alone or in combination in the clinic (Politi and Pao 2011). For example, GEMMs of EGFR and Kras-mutant lung cancer were used early on to evaluate the efficacy of therapies targeting these oncogenes or pathways that they control. The early studies in EGFR models demonstrated that the models could be used to recapitulate responses observed in patients and also to evaluate new TKIs and TKI combinations as they were developed (Ji et al. 2006b; Politi et al. 2006; Li et al. 2007). Many of these studies have served as the impetus for clinical trials. Similarly, for Kras-mutant lung cancer, proof-of-principle studies of agents targeting MEK have been tested in preclinical models. These studies uncovered, for example, that MEK blockade alone is insufficient to yield responses in GEMMs but that, when combined with PI3K inhibition tumors regress highlighting the importance of interfering with multiple signaling nodes to achieve tumor cell death (Faber et al. 2009). GEMMs have also been used to take these studies a step further to study how specific co-occurring alterations can affect the therapeutic response to specific therapies. In a coclinical trial (performed in mice and patients) of docetaxel and the MEK inhibitor selumetinib Kras;Lkb1-mutant lung tumors were found to be more resistant to combination therapy than Kras- or Kras;Trp53-mutant tumors, highlighting Lkb1 as a potential determinant of resistance to these agents (Chen et al. 2012b). Preclinical studies in GEMMs have also shed light on novel mechanisms of action of therapies. Thus, the combination of MAPK and CDK4/6 inhibitors (but neither agent alone) leads to NK cell activation and tumor regression in a Kras-driven model of LUAD (Ruscetti et al. 2018). Over the course of the past decade or so, patient-derived models of lung cancer have also increasingly been used to evaluate therapies and gain insights into mechanisms of sensitivity and resistance. Thus, lung cancer PDXs have been profiled for sensitivity to chemotherapeutic agents and EGFR-targeted therapies and analyzed to identify molecular patterns that underlie the observed sensitivity (Fichtner et al. 2008; Merk et al. 2009, 2011). Similarly, SCLC PDXs have been shown to recapitulate sensitivity to platinum etoposide (Drapkin et al. 2018). When used in a coclinical trial of olaparib plus temozolomide, SCLC PDXs were useful to identify biomarkers of resistance to the drug combination therapy (Farago et al. 2019). Moreover, primary cell-line-derived xenografts can be readily established from circulating SCLC cells and have been used extensively for therapeutic studies (Lallo et al. 2017).
Uncovering New Targets
Models can serve as excellent systems to discover and validate new potential therapeutic targets. Some prime examples of the success of using preclinical models as engines for the discovery of new targets come from studies in SCLC models. In GEMMs of an aggressive form of MYC-driven SCLC, aurora kinase was identified as a target and inhibition of this kinase in combination with chemotherapy prolonged mouse survival (Mollaoglu et al. 2017). Another target in this case revealing a potential immunotherapeutic strategy for SCLC was uncovered by integrating data from studies in human SCLC cell lines, PDXs, and GEM-derived models. Indeed, targeting CD47, which transduces a signal (termed the “don't eat me signal”) to macrophages to block their phagocytic function, was identified through studies using multiple types of models (Weiskopf et al. 2016). Functional genomic screens are emerging as a major approach to identify new therapeutic vulnerabilities and targets in lung cancer, including SCLC. Thus, in a CRISPR-Cas9 screen of druggable targets, loss or inhibition of an enzyme (dihydroorotate dehydrogenase [DHODH]) in the pyrimidine biosynthesis pathway was found to impair growth of SCLC in several different model systems highlighting its potential as a therapeutic target in this disease (Li et al. 2019b). LUAD models have also been shown to reveal novel targets for this disease. As an example, through transcriptional profiling of Kras-mutant GEMM tumors, a subset of tumors were found to resemble advanced human lung adenocarcinomas and characterized by elevated expression of the DDR kinase and Notch pathway activation. Inhibition of both of these pathways in both Kras-mutant GEMMs and orthotopic PDXs had an antitumor effect revealing this as a potential combination strategy (Ambrogio et al. 2016). Cell lines derived from GEMM models can also be used to identify new targets. As such, in a loss-of-function screen of epigenetic genes in cells derived from the oncogenic Kras;Trp53-deficient model, inactivation of the histone chaperone Asf1a was shown to sensitize tumors to anti-PD1 therapy (Jenkins et al. 2018).
Drug Resistance
Several preclinical model systems have been used to successfully model and identify opportunities to overcome drug resistance. Within the context of targeted therapies, generating resistant cell lines by culturing them over time in the presence of increasing doses of drug has revealed resistance mechanisms that were also found in patient tumors (Engelman et al. 2007; Godin-Heymann et al. 2008; Isozaki et al. 2016). For example, multiple studies that generated TKI-resistant PC9 lung cancer cells, which harbor an oncogenic EGFR mutation, found that the cells acquired the secondary T790M mutation in EGFR, mirroring what was seen in patient tumors (Pao et al. 2005; Ogino et al. 2007; Chmielecki et al. 2011). These models have enabled the evaluation of second-line strategies to overcome resistance, and specifically contributed to the approval of osimertinib for T790M-positive tumors (Cross et al. 2014; Mok et al. 2017). Cell lines, including those established directly from patient specimens, are beneficial for the high-throughput screening of potential genetic targets involved in resistance or drugs that could broadly overcome resistance (Crystal et al. 2014; de Bruin et al. 2014; Wilson et al. 2015; Dardaei et al. 2018). Other factors that influence the biology of resistance, such as growth dynamics or biochemical interaction partners, can readily be studied in a cell line system (Chmielecki et al. 2011; Takezawa et al. 2012). Cell lines have also revealed several mechanisms of resistance to the newest generation of TKIs in EGFR-mutant lung cancer (e.g., osimertinib), although, interestingly, acquired mutations in EGFR have not yet been readily observed in these cell line models (Eberlein et al. 2015; Galvani et al. 2015; Ichihara et al. 2017) highlighting the need for complementary approaches to study drug resistance.
Resistance to chemotherapy, targeted therapies, and immunotherapies can also be studied using mouse models of lung cancer. GEMMs of lung cancer have been critically important for defining mechanisms of resistance to EGFR-targeted therapies and enabling the in vivo evaluation of therapeutic strategies to overcome resistance (Regales et al. 2009; Zhou et al. 2009; Politi et al. 2010; Pirazzoli et al. 2014, 2016; Starrett et al. 2020), and several of these studies have informed clinical trials in the setting of resistance. Resistance to ALK inhibitors and therapies to overcome resistance have been studied using xenografts (Friboulet et al. 2014). Additionally, PDX models have a role in studying resistance and have been used for both EGFR- and ALK-targeted therapies (Stewart et al. 2015; Gainor et al. 2016). Interestingly, despite its broad clinical use, chemotherapy resistance is relatively understudied in lung cancer models, but this research has revealed a role for increased DNA repair pathways in resistant tumors (Oliver et al. 2010). While resistance to chemotherapy and targeted therapies can be modeled using cell lines or mouse models, resistance to immunotherapies must be studied in models with functional components of the immune systems (see below). Despite this limitation, PDXs of lung tumors resistant to ICIs have been useful to gain insights into tumor cell–intrinsic mechanisms of therapeutic resistance to these agents like defects in HLA class I antigen presentation (Gettinger et al. 2017).
Challenges with Clinical Translation
Although preclinical models of lung cancer have contributed significantly to the translation of findings from the bench to the bedside, several challenges remain. Given the advances in preclinical modeling there are a number of model systems to choose from and establishing which model system is the most appropriate to address the question at hand is not always immediately apparent. An example of this comes from studies of resistance to EGFR inhibitors in lung cancer. GEMM models are very good at recapitulating on target EGFR mutations that emerge at resistance to TKIs but current models do not readily recapitulate the spectrum of resistance mechanisms seen in patient tumors potentially, in part, due to the reduced genetic complexity of the tumors that develop in these animals. Conversely, patient-derived models are less likely to develop resistance to TKIs through the acquisition of additional EGFR mutations. Therefore, the type of model used for studies of drug resistance should account for these observations, and the use of multiple different models and complementary approaches may be warranted. Another important consideration is that even within specific categories of models there is variability. Thus, patient-derived models can be established as 2D cell lines, 3D cultures/organoids, or as subcutaneous or orthotopic xenografts in mice. Which of these models are most likely to provide information that can be translated to the clinic is unknown, and several efforts like the NCI's patient-derived models of cancer consortium and the mammalian models for translational oncology forum are attempting to address this question. Furthermore, individual patient specimens used to generate the patient-derived models will be very diverse and establishing criteria for selecting models for specific preclinical studies is essential. Analogous considerations apply to GEMMs where different combinations of oncogenic and tumor-suppressor gene alterations can be modeled and give different phenotypes: understanding the models and matching the most appropriate one(s) for the therapeutic study is important to maximize the relevance and impact of the study for patients.
Another major challenge for the clinical translation of studies in lung cancer models to the clinic comes from the fact that cancer immunotherapies are now used to treat many patients with lung cancer. However, most of the lung cancer preclinical models are not ideal for studies of cancer immunotherapy. Tumors that develop in GEMMs have a low mutational burden and, as a result, are poorly immunogenic and do not respond effectively to T-cell-directed therapies like immune checkpoint inhibitors (Westcott et al. 2015; McFadden et al. 2016; Pfirschke et al. 2016; Ayeni et al. 2019). Therefore, there is a critical need to develop GEMMs, which in addition to recapitulating specific oncogenic driver and tumor-suppressor alterations found in lung cancer, also have a similar mutational landscape to their human counterparts. Potential approaches to achieve this are through the use of carcinogens and through modulation of genes involved in DNA repair processes. As discussed above, standard patient-derived models are also generally devoid of immune cells from the patient's tumor and approaches to incorporate elements of the human immune system into these models are important for translational therapeutic studies.
The significant cost and logistical effort required to conduct statistically rigorous therapeutic studies in vivo either in GEMMs or PDXs represents an additional challenge to the use of these models and restricts the number of models that can be interrogated at given time. Potential remedies include the use of 3D culture systems that would allow experiments to be conducted on a larger scale. Additionally, the possibility of interrogating therapeutic responses of multiple different genotypes in an individual animal using multiplex approaches would also contribute to containing costs of statistically animal purchases and husbandry.
Finally, therapeutic studies are often conducted in an experimental context that is different from the clinical setting under investigation. Thus, in most GEMMs, therapies are tested for activity on the primary tumor that arises in the lung epithelium that models early-stage lung cancer. However, in the clinic, these agents are frequently used or being investigated in the metastatic setting. Therefore, how findings from the GEMMs are reflective of patients with advanced lung cancer is a matter of concern. While patient-derived models can be established from sites of metastatic disease, they are frequently implanted subcutaneously rather than at orthotopic sites that could affect how lung tumors respond to treatment.
CONCLUSIONS
In summary, the landscape of preclinical models of lung cancer has expanded significantly in recent years, with more accurate genetic modeling of specific individual and combinatorial alterations found in the different histological subtypes of the human disease and the increasing generation of new patient-derived models. This provides the field with an unprecedented wealth of models for mechanistic studies of lung cancer biology and therapeutics that are and will continue to contribute significant insights for the treatment of lung cancer.
ACKNOWLEDGMENTS
The authors would like to acknowledge support from the following funding sources: U01CA235747 (K.P. and D.X.N.), and P50CA196530 (K.P. and D.X.N.).
Footnotes
Editors: Christine M. Lovly, David P. Carbone, and John D. Minna
Additional Perspectives on Lung Cancer: Disease Biology and Its Potential for Clinical Translation available at www.perspectivesinmedicine.org
REFERENCES
- Adams S, Robbins FM, Chen D, Wagage D, Holbeck SL, Morse HC III, Stroncek D, Marincola FM. 2005. HLA class I and II genotype of the NCI-60 cell lines. J Transl Med 3: 11. 10.1186/1479-5876-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, et al. 2013. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3: 1355–1363. 10.1158/2159-8290.CD-13-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TM, Brehm MA, Bridges S, Ferguson S, Kumar P, Mirochnitchenko O, Palucka K, Pelanda R, Sanders-Beer B, Shultz LD, et al. 2019. Humanized immune system mouse models: progress, challenges and opportunities. Nat Immunol 20: 770–774. 10.1038/s41590-019-0416-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K, Possemato R. 2017. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature 551: 639–643. 10.1038/nature24637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogio C, Carmona FJ, Vidal A, Falcone M, Nieto P, Romero OA, Puertas S, Vizoso M, Nadal E, Poggio T, et al. 2014. Modeling lung cancer evolution and preclinical response by orthotopic mouse allografts. Cancer Res 74: 5978–5988. 10.1158/0008-5472.CAN-14-1606 [DOI] [PubMed] [Google Scholar]
- Ambrogio C, Gómez-López G, Falcone M, Vidal A, Nadal E, Crosetto N, Blasco RB, Fernández-Marcos PJ, Sánchez-Céspedes M, Ren X, et al. 2016. Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med 22: 270–277. 10.1038/nm.4041 [DOI] [PubMed] [Google Scholar]
- Arai Y, Totoki Y, Takahashi H, Nakamura H, Hama N, Kohno T, Tsuta K, Yoshida A, Asamura H, Mutoh M, et al. 2013. Mouse model for ROS1-rearranged lung cancer. PLoS ONE 8: e56010. 10.1371/journal.pone.0056010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayeni D, Miller B, Kuhlmann A, Ho PC, Robles-Oteiza C, Gaefele M, Levy S, de Miguel FJ, Perry C, Guan T, et al. 2019. Tumor regression mediated by oncogene withdrawal or erlotinib stimulates infiltration of inflammatory immune cells in EGFR mutant lung tumors. J Immunother Cancer 7: 172. 10.1186/s40425-019-0643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpilikueta A, Agorreta J, Labiano S, Pérez-Gracia JL, Sánchez-Paulete AR, Aznar MA, Ajona D, Gil-Bazo I, Larrayoz M, Teijeira A, et al. 2016. Successful immunotherapy against a transplantable mouse squamous lung carcinoma with anti-PD-1 and anti-CD137 monoclonal antibodies. J Thorac Oncol 11: 524–536. 10.1016/j.jtho.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. 2013. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 123: 3025–3036. 10.1172/JCI68782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas CE, Chung MI, Fioret B, Gao X, Katsura H, Hogan BL. 2017. Lung organoids: current uses and future promise. Development 144: 986–997. 10.1242/dev.140103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JM, Przybyla L, Weaver VM. 2017. Tissue mechanics regulate brain development, homeostasis and disease. J Cell Sci 130: 71–82. 10.1242/jcs.191742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart A, Mazur PK, Anton M, Rudelius M, Schwamborn K, Feuchtinger A, Behnke K, Walch A, Braren R, et al. 2015. Opposing role of Notch1 and Notch2 in a KrasG12D-driven murine non-small cell lung cancer model. Oncogene 34: 578–588. [DOI] [PubMed] [Google Scholar]
- Behan FM, Iorio F, Picco G, Gonçalves E, Beaver CM, Migliardi G, Santos R, Rao Y, Sassi F, Pinnelli M, et al. 2019. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature 568: 511–516. 10.1038/s41586-019-1103-9 [DOI] [PubMed] [Google Scholar]
- Ben-David U, Ha G, Tseng YY, Greenwald NF, Oh C, Shih J, McFarland JM, Wong B, Boehm JS, Beroukhim R, et al. 2017. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat Genet 49: 1567–1575. 10.1038/ng.3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Corà D, Di Nicolantonio F, Buscarino M, Petti C, et al. 2011. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 1: 508–523. 10.1158/2159-8290.CD-11-0109 [DOI] [PubMed] [Google Scholar]
- Bertotti A, Papp E, Jones S, Adleff V, Anagnostou V, Lupo B, Sausen M, Phallen J, Hruban CA, Tokheim C, et al. 2015. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature 526: 263–267. 10.1038/nature14969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best SA, Ding S, Kersbergen A, Dong X, Song JY, Xie Y, Reljic B, Li K, Vince JE, Rathi V, et al. 2019. Distinct initiating events underpin the immune and metabolic heterogeneity of KRAS-mutant lung adenocarcinoma. Nat Commun 10: 4190. 10.1038/s41467-019-12164-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely CM, Watkins TBK, Wu W, Gini B, Chabon JJ, McCoach CE, McGranahan N, Wilson GA, Birkbak NJ, Olivas VR, et al. 2017. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet 49: 1693–1704. 10.1038/ng.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, Jager M, Ponz-Sarvise M, Tiriac H, Spector MS, et al. 2015. Organoid models of human and mouse ductal pancreatic cancer. Cell 160: 324–338. 10.1016/j.cell.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borromeo MD, Savage TK, Kollipara RK, He M, Augustyn A, Osborne JK, Girard L, Minna JD, Gazdar AF, Cobb MH, et al. 2016. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep 16: 1259–1272. 10.1016/j.celrep.2016.06.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttger F, Semenova EA, Song JY, Ferone G, van der Vliet J, Cozijnsen M, Bhaskaran R, Bombardelli L, Piersma SR, Pham TV, et al. 2019. Tumor heterogeneity underlies differential cisplatin sensitivity in mouse models of small-cell lung cancer. Cell Rep 27: 3345–3358.e4. 10.1016/j.celrep.2019.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broutier L, Andersson-Rolf A, Hindley CJ, Boj SF, Clevers H, Koo BK, Huch M. 2016. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc 11: 1724–1743. 10.1038/nprot.2016.097 [DOI] [PubMed] [Google Scholar]
- Busch SE, Hanke ML, Kargl J, Metz HE, MacPherson D, Houghton AM. 2016. Lung cancer subtypes generate unique immune responses. J Immunol 197: 4493–4503. 10.4049/jimmunol.1600576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, Bruna A, Budinská E, Caldas C, Chang DK, et al. 2017. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer 17: 254–268. 10.1038/nrc.2016.140 [DOI] [PubMed] [Google Scholar]
- Campbell JD, Alexandrov A, Kim J, Wala J, Berger AH, Pedamallu CS, Shukla SA, Guo G, Brooks AN, Murray BA, et al. 2016. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet 48: 607–616. 10.1038/ng.3564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. 2012. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489: 519–525. 10.1038/nature11404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network. 2014. Comprehensive molecular profiling of lung adenocarcinoma. Nature 511: 543–550. 10.1038/nature13385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canon J, Rex K, Saiki AY, Mohr C, Cooke K, Bagal D, Gaida K, Holt T, Knutson CG, Koppada N, et al. 2019. The clinical KRASG12C inhibitor AMG 510 drives anti-tumour immunity. Nature 575: 217–223. 10.1038/s41586-019-1694-1 [DOI] [PubMed] [Google Scholar]
- Caswell DR, Chuang CH, Yang D, Chiou SH, Cheemalavagu S, Kim-Kiselak C, Connolly A, Winslow M. 2014. Obligate progression precedes lung adenocarcinoma dissemination. Cancer Discov 4: 781–789. 10.1158/2159-8290.CD-13-0862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo CM, Dijkstra KK, Fanchi LF, Kelderman S, Kaing S, van Rooij N, van den Brink S, Schumacher TN, Voest EE. 2020. Tumor organoid-T-cell coculture systems. Nat Protoc 15: 15–39. 10.1038/s41596-019-0232-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Sasaki T, Tan X, Carretero J, Shimamura T, Li D, Xu C, Wang Y, Adelmant GO, Capelletti M, et al. 2010. Inhibition of ALK, PI3K/MEK, and HSP90 in murine lung adenocarcinoma induced by EML4-ALK fusion oncogene. Cancer Res 70: 9827–9836. 10.1158/0008-5472.CAN-10-1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, et al. 2012a. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells 30: 1948–1960. 10.1002/stem.1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, Liu Y, Tupper T, Ouyang J, Li J, et al. 2012b. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 483: 613–617. 10.1038/nature10937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Akbay E, Mikse O, Tupper T, Cheng K, Wang Y, Tan X, Altabef A, Woo SA, Chen L, et al. 2014a. Co-clinical trials demonstrate superiority of crizotinib to chemotherapy in ALK-rearranged non-small cell lung cancer and predict strategies to overcome resistance. Clin Cancer Res 20: 1204–1211. 10.1158/1078-0432.CCR-13-1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. 2014b. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 14: 535–546. 10.1038/nrc3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Huang SX, de Carvalho ALRT, Ho SH, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova JL, Bhattacharya J, et al. 2017. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19: 542–549. 10.1038/ncb3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HJ, Poran A, Unni AM, Huang SX, Elemento O, Snoeck HW, Varmus H. 2019. Generation of pulmonary neuroendocrine cells and SCLC-like tumors from human embryonic stem cells. J Exp Med 216: 674–687. 10.1084/jem.20181155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, Wang L, Amato KR, Arcila M, Sos ML, et al. 2011. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 3: 90ra59. 10.1126/scitranslmed.3002356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsello SM, Nagari RT, Spangler RD, Rossen J, Kocak M, Bryan JG, Humeidi R, Peck D, Wu X, Tang AA, et al. 2020. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat Cancer 1: 235–248. 10.1038/s43018-019-0018-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley GS, Weir BA, Vazquez F, Tamayo P, Scott JA, Rusin S, East-Seletsky A, Ali LD, Gerath WF, Pantel SE, et al. 2014. Parallel genome-scale loss of function screens in 216 cancer cell lines for the identification of context-specific genetic dependencies. Sci Data 1: 140035. 10.1038/sdata.2014.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, et al. 2014. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 4: 1046–1061. 10.1158/2159-8290.CD-14-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal AS, Shaw AT, Sequist LV, Friboulet L, Niederst MJ, Lockerman EL, Frias RL, Gainor JF, Amzallag A, Greninger P, et al. 2014. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 346: 1480–1486. 10.1126/science.1254721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry NL, Mino-Kenudson M, Oliver TG, Yilmaz OH, Yilmaz VO, Moon JY, Jacks T, Sabatini DM, Kalaany NY. 2013. Pten-null tumors cohabiting the same lung display differential AKT activation and sensitivity to dietary restriction. Cancer Discov 3: 908–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutz JC, Guan J, Bayani J, Yoshimoto M, Xue H, Sutcliffe M, English J, Flint J, LeRiche J, Yee J, et al. 2006. Establishment in severe combined immunodeficiency mice of subrenal capsule xenografts and transplantable tumor lines from a variety of primary human lung cancers: potential models for studying tumor progression-related changes. Clin Cancer Res 12: 4043–4054. 10.1158/1078-0432.CCR-06-0252 [DOI] [PubMed] [Google Scholar]
- Damo M, Fitzgerald B, Lu Y, Nader M, William I, Cheung JF, Connolly KA, Foster GG, Akama-Garren E, Lee DY, et al. 2021. Inducible de novo expression of neoantigens in tumor cells and mice. Nat Biotechnol 39: 64–73. 10.1038/s41587-020-0613-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danahay H, Pessotti AD, Coote J, Montgomery BE, Xia D, Wilson A, Yang H, Wang Z, Bevan L, Thomas C, et al. 2015. Notch2 is required for inflammatory cytokine-driven goblet cell metaplasia in the lung. Cell Rep 10: 239–252. 10.1016/j.celrep.2014.12.017 [DOI] [PubMed] [Google Scholar]
- Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. 2007. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev 21: 379–384. 10.1101/gad.1516407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardaei L, Wang HQ, Singh M, Fordjour P, Shaw KX, Yoda S, Kerr G, Yu K, Liang J, Cao Y, et al. 2018. SHP2 inhibition restores sensitivity in ALK-rearranged non-small-cell lung cancer resistant to ALK inhibitors. Nat Med 24: 512–517. 10.1038/nm.4497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SM, Papagiannakopoulos T, Olenchock BA, Heyman JE, Keibler MA, Luengo A, Bauer MR, Jha AK, O'Brien JP, Pierce KA, et al. 2016. Environment impacts the metabolic dependencies of Ras-driven non-small cell lung cancer. Cell Metab 23: 517–528. 10.1016/j.cmet.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alboran IM, O'Hagan RC, Gärtner F, Malynn B, Davidson L, Rickert R, Rajewsky K, DePinho RA, Alt FW. 2001. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity 14: 45–55. 10.1016/S1074-7613(01)00088-7 [DOI] [PubMed] [Google Scholar]
- de Bruin EC, Cowell C, Warne PH, Jiang M, Saunders RE, Melnick MA, Gettinger S, Walther Z, Wurtz A, Heynen GJ, et al. 2014. Reduced NF1 expression confers resistance to EGFR inhibition in lung cancer. Cancer Discov 4: 606–619. 10.1158/2159-8290.CD-13-0741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Rochere P, Guil-Luna S, Decaudin D, Azar G, Sidhu SS, Piaggio E. 2018. Humanized mice for the study of immuno-oncology. Trends Immunol 39: 748–763. 10.1016/j.it.2018.07.001 [DOI] [PubMed] [Google Scholar]
- Denny SK, Yang D, Chuang CH, Brady JJ, Lim JS, Grüner BM, Chiou SH, Schep AN, Baral J, Hamard C, et al. 2016. Nfib promotes metastasis through a widespread increase in chromatin accessibility. Cell 166: 328–342. 10.1016/j.cell.2016.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, Factor R, Matsen C, Milash BA, Nelson E, et al. 2011. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med 17: 1514–1520. 10.1038/nm.2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, Slagter M, van der Velden DL, Kaing S, Kelderman S, et al. 2018. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174: 1586–1598.e12. 10.1016/j.cell.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra KK, Monkhorst K, Schipper LJ, Hartemink KJ, Smit EF, Kaing S, de Groot R, Wolkers MC, Clevers H, Cuppen E, et al. 2020. Challenges in establishing pure lung cancer organoids limit their utility for personalized medicine. Cell Rep 31: 107588. 10.1016/j.celrep.2020.107588 [DOI] [PubMed] [Google Scholar]
- Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, Zhou Q, Tu HY, Xu CR, Yan LX, et al. 2017. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology 6: e1356145. 10.1080/2162402X.2017.1356145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, Melero I, Schalper KA, Herbst RS. 2019. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res 25: 4592–4602. 10.1158/1078-0432.CCR-18-1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin BJ, Minna JD. 2020. Studying lineage plasticity one cell at a time. Cancer Cell 38: 150–152. 10.1016/j.ccell.2020.07.001 [DOI] [PubMed] [Google Scholar]
- Drapkin BJ, George J, Christensen CL, Mino-Kenudson M, Dries R, Sundaresan T, Phat S, Myers DT, Zhong J, Igo P, et al. 2018. Genomic and functional fidelity of small cell lung cancer patient-derived xenografts. Cancer Discov 8: 600–615. 10.1158/2159-8290.CD-17-0935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Jacks T. 2013. Genetically engineered mouse models of cancer reveal new insights about the antitumor immune response. Curr Opin Immunol 25: 192–199. 10.1016/j.coi.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Dooley AL, Jacks T. 2009. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc 4: 1064–1072. 10.1038/nprot.2009.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Cheung AF, Mazumdar C, Winslow MM, Bronson R, Schmidt LM, Crowley D, Chen J, Jacks T. 2011. Endogenous T cell responses to antigens expressed in lung adenocarcinomas delay malignant tumor progression. Cancer Cell 19: 72–85. 10.1016/j.ccr.2010.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, et al. 2015. In vitro generation of human pluripotent stem cell derived lung organoids. eLife 4: e05098. 10.7554/eLife.05098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BR, Dedhia PH, Miller AJ, Nagy MS, White ES, Shea LD, Spence JR. 2016. A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. eLife 5: e19732. 10.7554/eLife.19732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberlein CA, Stetson D, Markovets AA, Al-Kadhimi KJ, Lai Z, Fisher PR, Meador CB, Spitzler P, Ichihara E, Ross SJ, et al. 2015. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res 75: 2489–2500. 10.1158/0008-5472.CAN-14-3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Murga M, Martinez-Pastor B, Ortega-Molina A, Soria R, Collado M, Fernandez-Capetillo O, Serrano M. 2009. Limited role of murine ATM in oncogene-induced senescence and p53-dependent tumor suppression. PLoS ONE 4: e5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt A, Bartels T, Geick A, Klocke R, Paul D, Halter R. 2001. Development of pulmonary bronchiolo-alveolar adenocarcinomas in transgenic mice overexpressing murine c-myc and epidermal growth factor in alveolar type II pneumocytes. Br J Cancer 84: 813–818. 10.1054/bjoc.2000.1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. 2007. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316: 1039–1043. 10.1126/science.1141478 [DOI] [PubMed] [Google Scholar]
- Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, et al. 2008. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14: 1351–1356. 10.1038/nm.1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, Lifshits E, Chen Z, Maira SM, Garcia-Echeverria C, et al. 2009. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci 106: 19503–19508. 10.1073/pnas.0905056106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago AF, Yeap BY, Stanzione M, Hung YP, Heist RS, Marcoux JP, Zhong J, Rangachari D, Barbie DA, Phat S, et al. 2019. Combination olaparib and temozolomide in relapsed small-cell lung cancer. Cancer Discov 9: 1372–1387. 10.1158/2159-8290.CD-19-0582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldser DM, Kostova KK, Winslow MM, Taylor SE, Cashman C, Whittaker CA, Sanchez-Rivera FJ, Resnick R, Bronson R, Hemann MT, et al. 2010. Stage-specific sensitivity to p53 restoration during lung cancer progression. Nature 468: 572–575. 10.1038/nature09535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferone G, Song JY, Sutherland KD, Bhaskaran R, Monkhorst K, Lambooij JP, Proost N, Gargiulo G, Berns A. 2016. SOX2 is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell 30: 519–532. 10.1016/j.ccell.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner I, Rolff J, Soong R, Hoffmann J, Hammer S, Sommer A, Becker M, Merk J. 2008. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res 14: 6456–6468. 10.1158/1078-0432.CCR-08-0138 [DOI] [PubMed] [Google Scholar]
- Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, Whitsett JA, Koretsky A, Varmus HE. 2001. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev 15: 3249–3262. 10.1101/gad.947701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foggetti G, Li C, Cai H, Hellyer JA, Lin WY, Ayeni D, Hastings K, Choi J, Wurtz A, Andrejka L, et al. 2021. Genetic determinants of EGFR-driven lung cancer growth and therapeutic response in vivo. Cancer Discov 10.1158/2159-8290.CD-20-1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg EMV, Lindberg MF, Jespersen H, Alsén S, Bagge RO, Donia M, Svane IM, Nilsson O, Ny L, Nilsson LM, et al. 2019. HER2 CAR-T cells eradicate uveal melanoma and T-cell therapy-resistant human melanoma in IL2 transgenic NOD/SCID IL2 receptor knockout mice. Cancer Res 79: 899–904. 10.1158/0008-5472.CAN-18-3158 [DOI] [PubMed] [Google Scholar]
- Friboulet L, Li N, Katayama R, Lee CC, Gainor JF, Crystal AS, Michellys PY, Awad MM, Yanagitani N, Kim S, et al. 2014. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 4: 662–673. 10.1158/2159-8290.CD-13-0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, Dagogo-Jack I, Gadgeel S, Schultz K, Singh M, et al. 2016. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov 6: 1118–1133. 10.1158/2159-8290.CD-16-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvani E, Sun J, Leon LG, Sciarrillo R, Narayan RS, Sjin RT, Lee K, Ohashi K, Heideman DA, Alfieri RR, et al. 2015. NF-κB drives acquired resistance to a novel mutant-selective EGFR inhibitor. Oncotarget 6: 42717–42732. 10.18632/oncotarget.3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Steine EJ, Barrasa MI, Hockemeyer D, Pawlak M, Fu D, Reddy S, Bell GW, Jaenisch R. 2011. Deletion of the de novo DNA methyltransferase Dnmt3a promotes lung tumor progression. Proc Natl Acad Sci 108: 18061–18066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar AF, Gao B, Minna JD. 2010a. Lung cancer cell lines: useless artifacts or invaluable tools for medical science? Lung Cancer 68: 309–318. 10.1016/j.lungcan.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar AF, Girard L, Lockwood WW, Lam WL, Minna JD. 2010b. Lung cancer cell lines as tools for biomedical discovery and research. J Natl Cancer Inst 102: 1310–1321. 10.1093/jnci/djq279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geick A, Redecker P, Ehrhardt A, Klocke R, Paul D, Halter R. 2001. Uteroglobin promoter-targeted c-MYC expression in transgenic mice cause hyperplasia of Clara cells and malignant transformation of T-lymphoblasts and tubular epithelial cells. Transgenic Res 10: 501–511. 10.1023/A:1013085228119 [DOI] [PubMed] [Google Scholar]
- Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, et al. 2017. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov 7: 1420–1435. 10.1158/2159-8290.CD-17-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettinger SN, Choi J, Mani N, Sanmamed MF, Datar I, Sowell R, Du VY, Kaftan E, Goldberg S, Dong W, et al. 2018. A dormant TIL phenotype defines non-small cell lung carcinomas sensitive to immune checkpoint blockers. Nat Commun 9: 3196. 10.1038/s41467-018-05032-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghandi M, Huang FW, Jané-Valbuena J, Kryukov GV, Lo CC, McDonald ER III, Barretina J, Gelfand ET, Bielski CM, Li H, et al. 2019. Next-generation characterization of the cancer cell line encyclopedia. Nature 569: 503–508. 10.1038/s41586-019-1186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y, Pertsemlidis A, et al. 2009. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev 23: 2140–2151. 10.1101/gad.1820209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin-Heymann N, Ulkus L, Brannigan BW, McDermott U, Lamb J, Maheswaran S, Settleman J, Haber DA. 2008. The T790M “gatekeeper” mutation in EGFR mediates resistance to low concentrations of an irreversible EGFR inhibitor. Mol Cancer Ther 7: 874–879. 10.1158/1535-7163.MCT-07-2387 [DOI] [PubMed] [Google Scholar]
- Gould SE, Junttila MR, de Sauvage FJ. 2015. Translational value of mouse models in oncology drug development. Nat Med 21: 431–439. 10.1038/nm.3853 [DOI] [PubMed] [Google Scholar]
- Gu H, Zou YR, Rajewsky K. 1993. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell 73: 1155–1164. 10.1016/0092-8674(93)90644-6 [DOI] [PubMed] [Google Scholar]
- Guerra C, Mijimolle N, Dhawahir A, Dubus P, Barradas M, Serrano M, Campuzano V, Barbacid M. 2003. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell 4: 111–120. 10.1016/S1535-6108(03)00191-0 [DOI] [PubMed] [Google Scholar]
- Gupta SK, Kizilbash SH, Carlson BL, Mladek AC, Boakye-Agyeman F, Bakken KK, Pokorny JL, Schroeder MA, Decker PA, Cen L, et al. 2016. Delineation of MGMT hypermethylation as a biomarker for veliparib-mediated temozolomide-sensitizing therapy of glioblastoma. J Natl Cancer Inst 108: djv369. 10.1093/jnci/djv369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallin J, Engstrom LD, Hargis L, Calinisan A, Aranda R, Briere DM, Sudhakar N, Bowcut V, Baer BR, Ballard JA, et al. 2020. The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov 10: 54–71. 10.1158/2159-8290.CD-19-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings K, Yu HA, Wei W, Sanchez-Vega F, DeVeaux M, Choi J, Rizvi H, Lisberg A, Truini A, Lydon CA, et al. 2019. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann Oncol 30: 1311–1320. 10.1093/annonc/mdz141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Maelandsmo GM, et al. 2014. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 4: 998–1013. 10.1158/2159-8290.CD-14-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, Trapani F, Polanski R, Burt DJ, Simpson KL, Morris K, et al. 2014. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 20: 897–903. 10.1038/nm.3600 [DOI] [PubMed] [Google Scholar]
- Huang SX, Islam MN, O'Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, et al. 2014. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32: 84–91. 10.1038/nbt.2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara E, Westover D, Meador CB, Yan Y, Bauer JA, Lu P, Ye F, Kulick A, de Stanchina E, McEwen R, et al. 2017. SFK/FAK signaling attenuates osimertinib efficacy in both drug-sensitive and drug-resistant models of EGFR-mutant lung cancer. Cancer Res 77: 2990–3000. 10.1158/0008-5472.CAN-16-2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Toki H, Matsui J, Togashi Y, Dobashi A, Fukumura R, Gondo Y, Minowa O, Tanaka N, Mori S, et al. 2016. Mouse models for ROS1-fusion-positive lung cancers and their application to the analysis of multikinase inhibitor efficiency. Carcinogenesis 37: 452–460. 10.1093/carcin/bgw028 [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. 2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γ chainnull mice. Blood 106: 1565–1573. 10.1182/blood-2005-02-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isozaki H, Ichihara E, Takigawa N, Ohashi K, Ochi N, Yasugi M, Ninomiya T, Yamane H, Hotta K, Sakai K, et al. 2016. Non-small cell lung cancer cells acquire resistance to the ALK inhibitor alectinib by activating alternative receptor tyrosine kinases. Cancer Res 76: 1506–1516. 10.1158/0008-5472.CAN-15-1010 [DOI] [PubMed] [Google Scholar]
- Ivanova E, Kuraguchi M, Xu M, Portell AJ, Taus L, Diala I, Lalani AS, Choi J, Chambers ES, Li S, et al. 2020. Use of ex vivo patient-derived tumor organotypic spheroids to identify combination therapies for HER2 mutant non-small cell lung cancer. Clin Cancer Res 26: 2393–2403. 10.1158/1078-0432.CCR-19-1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga K, Yang Y, Raso MG, Ma L, Hanna AE, Thilaganathan N, Moghaddam S, Evans CM, Li H, Cai WW, et al. 2008. Pten inactivation accelerates oncogenic K-ras-initiated tumorigenesis in a mouse model of lung cancer. Cancer Res 68: 1119–1127. 10.1158/0008-5472.CAN-07-3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoy R, Jacks T, Tuveson DA. 2001. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 15: 3243–3248. 10.1101/gad.943001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, Chen Y, Babbar A, Firdaus SJ, Darjania L, et al. 2018. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell 172: 578–589.e17. 10.1016/j.cell.2018.01.006 [DOI] [PubMed] [Google Scholar]
- Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, Bowden M, Deng J, Liu H, Miao D, et al. 2018. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov 8: 196–215. 10.1158/2159-8290.CD-17-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, Tonon G, McNamara K, Marconcini LA, Hezel A, et al. 2006a. K-ras activation generates an inflammatory response in lung tumors. Oncogene 25: 2105–2112. 10.1038/sj.onc.1209237 [DOI] [PubMed] [Google Scholar]
- Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, Mahmood U, Mitchell A, Sun Y, Al-Hashem R, et al. 2006b. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell 9: 485–495. 10.1016/j.ccr.2006.04.022 [DOI] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, et al. 2007a. LKB1 modulates lung cancer differentiation and metastasis. Nature 448: 807–810. 10.1038/nature06030 [DOI] [PubMed] [Google Scholar]
- Ji H, Wang Z, Perera SA, Li D, Liang MC, Zaghlul S, McNamara K, Chen L, Albert M, Sun Y, et al. 2007b. Mutations in BRAF and KRAS converge on activation of the mitogen-activated protein kinase pathway in lung cancer mouse models. Cancer Res 67: 4933–4939. 10.1158/0008-5472.CAN-06-4592 [DOI] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, Jacks T. 2001. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 410: 1111–1116. 10.1038/35074129 [DOI] [PubMed] [Google Scholar]
- Jordan EJ, Kim HR, Arcila ME, Barron D, Chakravarty D, Gao J, Chang MT, Ni A, Kundra R, Jonsson P, et al. 2017. Prospective comprehensive molecular characterization of lung adenocarcinomas for efficient patient matching to approved and emerging therapies. Cancer Discov 7: 596–609. 10.1158/2159-8290.CD-16-1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF, et al. 2015. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 43: 579–590. 10.1016/j.immuni.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi R, Castro De Moura M, Pineyro D, Alvarez-Errico D, Arribas C, Esteller M. 2020. The DNA methylation landscape of human cancer organoids available at the American type culture collection. Epigenetics 15: 1167–1177. 10.1080/15592294.2020.1762398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Seol HS, Chang S. 2018. The generation and application of patient-derived xenograft model for cancer research. Cancer Res Treat 50: 1–10. 10.4143/crt.2017.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavuri SM, Jain N, Galimi F, Cottino F, Leto SM, Migliardi G, Searleman AC, Shen W, Monsey J, Trusolino L, et al. 2015. HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov 5: 832–841. 10.1158/2159-8290.CD-14-1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. 2005. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835. 10.1016/j.cell.2005.03.032 [DOI] [PubMed] [Google Scholar]
- Kim M, Mun H, Sung CO, Cho EJ, Jeon HJ, Chun SM, Jung DJ, Shin TH, Jeong GS, Kim DK, et al. 2019. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun 10: 3991. 10.1038/s41467-019-11867-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K, Fukuda K, Takahashi H, Tanimoto A, Nishiyama A, Arai S, Takeuchi S, Yamashita K, Ohtsubo K, Otani S, et al. 2019. Patient-derived xenograft models of non-small cell lung cancer for evaluating targeted drug sensitivity and resistance. Cancer Sci 110: 3215–3224. 10.1111/cas.14171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Gotoh S, Tateishi K, Yamamoto Y, Korogi Y, Nagasaki T, Matsumoto H, Muro S, Hirai T, Ito I, et al. 2016. Directed induction of functional multi-ciliated cells in proximal airway epithelial spheroids from human pluripotent stem cells. Stem Cell Reports 6: 18–25. 10.1016/j.stemcr.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, Buczkowski KA, Liu Y, Awad MM, Denning WL, et al. 2016. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res 76: 999–1008. 10.1158/0008-5472.CAN-15-1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. 1995. Inducible gene targeting in mice. Science 269: 1427–1429. 10.1126/science.7660125 [DOI] [PubMed] [Google Scholar]
- Kumar MS, Erkeland SJ, Pester RE, Chen CY, Ebert MS, Sharp PA, Jacks T. 2008. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci 105: 3903–3908. 10.1073/pnas.0712321105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFave LM, Kartha VK, Ma S, Meli K, Del Priore I, Lareau C, Naranjo S, Westcott PMK, Duarte FM, Sankar V, et al. 2020. Epigenomic state transitions characterize tumor progression in mouse lung adenocarcinoma. Cancer Cell 38: 212–228.e13. 10.1016/j.ccell.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallo A, Schenk MW, Frese KK, Blackhall F, Dive C. 2017. Circulating tumor cells and CDX models as a tool for preclinical drug development. Transl Lung Cancer Res 6: 397–408. 10.21037/tlcr.2017.08.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. 2014. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 156: 440–455. 10.1016/j.cell.2013.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Lee JI, Lee SJ, Cho HJ, Song HJ, Jeong DE, Seo YJ, Shin S, Joung JG, Kwon YJ, et al. 2015. Patient-derived xenografts from non-small cell lung cancer brain metastases are valuable translational platforms for the development of personalized targeted therapy. Clin Cancer Res 21: 1172–1182. 10.1158/1078-0432.CCR-14-1589 [DOI] [PubMed] [Google Scholar]
- Lee JH, Tammela T, Hofree M, Choi J, Marjanovic ND, Han S, Canner D, Wu K, Paschini M, Bhang DH, et al. 2017. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell 170: 1149–1163.e12. 10.1016/j.cell.2017.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighl NB, Page RD, Raymond VM, Daniel DB, Divers SG, Reckamp KL, Villalona-Calero MD, Dix D, Odegaard JI, Lanman RD, et al. 2019. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res 25: 4691–4700. 10.1158/1078-0432.CCR-19-0624 [DOI] [PubMed] [Google Scholar]
- Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, Liang MC, Perera SA, Zaghlul S, Borgman CL, et al. 2007. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell 12: 81–93. 10.1016/j.ccr.2007.06.005 [DOI] [PubMed] [Google Scholar]
- Li S, Liu S, Deng J, Akbay EA, Hai J, Ambrogio C, Zhang L, Zhou F, Jenkins RW, Adeegbe DO, et al. 2018. Assessing therapeutic efficacy of MEK inhibition in a KRASG12C-driven mouse model of lung cancer. Clin Cancer Res 24: 4854–4864. 10.1158/1078-0432.CCR-17-3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhu H, Sun L, Xu W, Wang X. 2019a. Prognostic value of site-specific metastases in lung cancer: a population based study. J Cancer 10: 3079–3086. 10.7150/jca.30463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ng SR, Colon CI, Drapkin BJ, Hsu PP, Li Z, Nabel CS, Lewis CA, Romero R, Mercer KL, et al. 2019b. Identification of DHODH as a therapeutic target in small cell lung cancer. Sci Transl Med 11: eaaw7852. 10.1126/scitranslmed.aaw7852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Huang Q, Luster TA, Hu H, Zhang H, Ng WL, Khodadadi-Jamayran A, Wang W, Chen T, Deng J, et al. 2020. In vivo epigenetic CRISPR screen identifies Asf1a as an immunotherapeutic target in Kras-mutant lung adenocarcinoma. Cancer Discov 10: 270–287. 10.1158/2159-8290.CD-19-0780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Lin WY, Rizvi H, Cai H, McFarland CD, Rogers ZN, Yousefi M, Winters IP, Rudin CM, Petrov DA, Winslow MM. 2021. Quantitative in vivo analyses reveal a complex pharmacogenomic landscape in lung adenocarcinoma. Cancer Res 10.1158/0008-5472.can-21-0716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang MC, Ma J, Chen L, Kozlowski P, Qin W, Li D, Goto J, Shimamura T, Hayes DN, Meyerson M, et al. 2010. TSC1 loss synergizes with KRAS activation in lung cancer development in the mouse and confers rapamycin sensitivity. Oncogene 29: 1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok BH, Gardner EE, Schneeberger VE, Ni A, Desmeules P, Rekhtman N, de Stanchina E, Teicher BA, Riaz N, Powell SN, et al. 2017. PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin Cancer Res 23: 523–535. 10.1158/1078-0432.CCR-16-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi PL, Reinhold WC, Varma S, Hutchinson AA, Pommier Y, Chanock SJ, Weinstein JN. 2009. DNA fingerprinting of the NCI-60 cell line panel. Mol Cancer Ther 8: 713–724. 10.1158/1535-7163.MCT-08-0921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. 2004. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129–2139. 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- Maddalo D, Manchado E, Concepcion CP, Bonetti C, Vidigal JA, Han YC, Ogrodowski P, Crippa A, Rekhtman N, de Stanchina E, et al. 2014. In vivo engineering of oncogenic chromosomal rearrangements with the CRISPR/Cas9 system. Nature 516: 423–427. 10.1038/nature13902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz MG. 2007. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity 26: 537–541. 10.1016/j.immuni.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Marjanovic ND, Hofree M, Chan JE, Canner D, Wu K, Trakala M, Hartmann GG, Smith OC, Kim JY, Evans KV, et al. 2020. Emergence of a high-plasticity cell state during lung cancer evolution. Cancer Cell 38: 229–246.e13. 10.1016/j.ccell.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN. 2017. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of Wnt signaling. Cell Stem Cell 20: 844–857.e6. 10.1016/j.stem.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, Stewart C, Carter SL, Cibulskis K, Bhutkar A, McKenna A, Dooley A, Vernon A, et al. 2014. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell 156: 1298–1311. 10.1016/j.cell.2014.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, Politi K, Bhutkar A, Chen FK, Song X, Pirun M, Santiago PM, Kim-Kiselak C, Platt JT, Lee E, et al. 2016. Mutational landscape of EGFR-, MYC-, and Kras-driven genetically engineered mouse models of lung adenocarcinoma. Proc Natl Acad Sci 113: E6409–E6417. 10.1073/pnas.1613601113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQualter JL, Yuen K, Williams B, Bertoncello I. 2010. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci 107: 1414–1419. 10.1073/pnas.0909207107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeth K, Wang JX, Micevic G, Damsky W, Bosenberg MW. 2016. The YUMM lines: a series of congenic mouse melanoma cell lines with defined genetic alterations. Pigment Cell Melanoma Res 29: 590–597. 10.1111/pcmr.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraz IM, Majidi M, Meng F, Shao R, Ha MJ, Neri S, Fang B, Lin SH, Tinkey PT, Shpall EJ, et al. 2019. An improved patient-derived xenograft humanized mouse model for evaluation of lung cancer immune responses. Cancer Immunol Res 7: 1267–1279. 10.1158/2326-6066.CIR-18-0874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk J, Rolff J, Becker M, Leschber G, Fichtner I. 2009. Patient-derived xenografts of non-small-cell lung cancer: a pre-clinical model to evaluate adjuvant chemotherapy? Eur J Cardiothorac Surg 36: 454–459. 10.1016/j.ejcts.2009.03.054 [DOI] [PubMed] [Google Scholar]
- Merk J, Rolff J, Dorn C, Leschber G, Fichtner I. 2011. Chemoresistance in non-small-cell lung cancer: can multidrug resistance markers predict the response of xenograft lung cancer models to chemotherapy? Eur J Cardiothorac Surg 40: e29–e33. 10.1016/j.ejcts.2011.02.010 [DOI] [PubMed] [Google Scholar]
- Meuwissen R, Berns A. 2005. Mouse models for human lung cancer. Genes Dev 19: 643–664. 10.1101/gad.1284505 [DOI] [PubMed] [Google Scholar]
- Meuwissen R, Linn SC, van der Valk M, Mooi WJ, Berns A. 2001. Mouse model for lung tumorigenesis through Cre/lox controlled sporadic activation of the K-Ras oncogene. Oncogene 20: 6551–6558. 10.1038/sj.onc.1204837 [DOI] [PubMed] [Google Scholar]
- Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. 2003. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4: 181–189. 10.1016/S1535-6108(03)00220-4 [DOI] [PubMed] [Google Scholar]
- Meyers RM, Bryan JG, McFarland JM, Weir BA, Sizemore AE, Xu H, Dharia NV, Montgomery PG, Cowley GS, Pantel S, et al. 2017. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat Genet 49: 1779–1784. 10.1038/ng.3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AJ, Dye BR, Ferrer-Torres D, Hill DR, Overeem AW, Shea LD, Spence JR. 2019. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc 14: 518–540. 10.1038/s41596-018-0104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok TS, Wu YL, Papadimitrakopoulou VA. 2017. Osimertinib in EGFR T790M-positive lung cancer. N Engl J Med 376: 1993–1994. [DOI] [PubMed] [Google Scholar]
- Mollaoglu G, Guthrie MR, Böhm S, Brägelmann J, Can I, Ballieu PM, Marx A, George J, Heinen C, Chalishazar MD, et al. 2017. MYC drives progression of small cell lung cancer to a variant neuroendocrine subtype with vulnerability to aurora kinase inhibition. Cancer Cell 31: 270–285. 10.1016/j.ccell.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollaoglu G, Jones A, Wait SJ, Mukhopadhyay A, Jeong S, Arya R, Camolotto SA, Mosbruger TL, Stubben CJ, Conley CJ, et al. 2018. The lineage-defining transcription factors SOX2 and NKX2-1 determine lung cancer cell fate and shape the tumor immune microenvironment. Immunity 49: 764–779.e9. 10.1016/j.immuni.2018.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JJ, Bird G, Keysar SB, Astling DP, Lyons TR, Anderson RT, Glogowska MJ, Estes P, Eagles JR, Le PN, et al. 2016. Xactmice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene 35: 290–300. 10.1038/onc.2015.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, et al. 2012. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 10: 385–397. 10.1016/j.stem.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A, Danai LV, Gui DY, Waingarten CY, Lewis CA, Vander Heiden MG. 2017. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. eLife 6: e27713. 10.7554/eLife.27713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A, Danai LV, Vander Heiden MG. 2018. Microenvironmental regulation of cancer cell metabolism: implications for experimental design and translational studies. Dis Model Mech 11. 10.1242/dmm.035758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay A, Berrett KC, Kc U, Clair PM, Pop SM, Carr SR, Witt BL, Oliver TG. 2014. Sox2 cooperates with Lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep 8: 40–49. 10.1016/j.celrep.2014.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ, Junttila MR, Pouyet L, Karnezis A, Shchors K, Bui DA, Brown-Swigart L, Johnson L, Evan GI. 2008. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell 14: 447–457. 10.1016/j.ccr.2008.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, et al. 2018. Organoid modeling of the tumor immune microenvironment. Cell 175: 1972–1988.e16. 10.1016/j.cell.2018.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, et al. 2018. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 24: 739–748. 10.1038/s41591-018-0036-4 [DOI] [PubMed] [Google Scholar]
- Ogino A, Kitao H, Hirano S, Uchida A, Ishiai M, Kozuki T, Takigawa N, Takata M, Kiura K, Tanimoto M. 2007. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non small cell lung cancer cell line. Cancer Res 67: 7807–7814. 10.1158/0008-5472.CAN-07-0681 [DOI] [PubMed] [Google Scholar]
- Oie HK, Russell EK, Carney DN, Gazdar AF. 1996. Cell culture methods for the establishment of the NCI series of lung cancer cell lines. J Cell Biochem Suppl 24: 24–31. [DOI] [PubMed] [Google Scholar]
- Oliver TG, Mercer KL, Sayles LC, Burke JR, Mendus D, Lovejoy KS, Cheng MH, Subramanian A, Mu D, Powers S, et al. 2010. Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer. Genes Dev 24: 837–852. 10.1101/gad.1897010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn A, Isobe T, Itasaka S, Wu W, O'Reilly MS, Ki Hong W, Fidler IJ, Herbst RS. 2003. Development of an orthotopic model to study the biology and therapy of primary human lung cancer in nude mice. Clin Cancer Res 9: 5532–5539. [PubMed] [Google Scholar]
- Padhye A, Ungewiss C, Fradette JJ, Rodriguez BL, Albritton JL, Miller JS, Gibbons DL. 2019. A novel ex vivo tumor system identifies Src-mediated invasion and metastasis in mesenchymal tumor cells in non-small cell lung cancer. Sci Rep 9: 4819. 10.1038/s41598-019-41301-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. 2004. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304: 1497–1500. 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- Pao W, Hutchinson KE. 2012. Chipping away at the lung cancer genome. Nat Med 18: 349–351. 10.1038/nm.2697 [DOI] [PubMed] [Google Scholar]
- Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. 2004. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci 101: 13306–13311. 10.1073/pnas.0405220101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. 2005. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2: e73. 10.1371/journal.pmed.0020073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Lee JK, Sheu KM, Wang L, Balanis NG, Nguyen K, Smith BA, Cheng C, Tsai BL, Cheng D, et al. 2018. Reprogramming normal human epithelial tissues to a common, lethal neuroendocrine cancer lineage. Science 362: 91–95. 10.1126/science.aat5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Soler R, Kemp B, Wu QP, Mao L, Gomez J, Zeleniuch-Jacquotte A, Yee H, Lee JS, Jagirdar J, Ling YH. 2000. Response and determinants of sensitivity to paclitaxel in human non-small cell lung cancer tumors heterotransplanted in nude mice. Clin Cancer Res 6: 4932–4938. [PubMed] [Google Scholar]
- Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, Yamazaki T, Poirier-Colame V, Newton A, Redouane Y, et al. 2016. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 44: 343–354. 10.1016/j.immuni.2015.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirazzoli V, Nebhan C, Song X, Wurtz A, Walther Z, Cai G, Zhao Z, Jia P, de Stanchina E, Shapiro EM, et al. 2014. Acquired resistance of EGFR-mutant lung adenocarcinomas to Afatinib plus cetuximab is associated with activation of mTORC1. Cell Rep 7: 999–1008. 10.1016/j.celrep.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirazzoli V, Ayeni D, Meador CB, Sanganahalli BG, Hyder F, de Stanchina E, Goldberg SB, Pao W, Politi K. 2016. Afatinib plus cetuximab delays resistance compared to single-agent erlotinib or Afatinib in mouse models of TKI-naive EGFR L858R-induced lung adenocarcinoma. Clin Cancer Res 22: 426–435. 10.1158/1078-0432.CCR-15-0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place TL, Domann FE, Case AJ. 2017. Limitations of oxygen delivery to cells in culture: an underappreciated problem in basic and translational research. Free Radic Biol Med 113: 311–322. 10.1016/j.freeradbiomed.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, et al. 2014. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159: 440–455. 10.1016/j.cell.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi K, Herbst RS. 2015. Lung cancer in the era of precision medicine. Clin Cancer Res 21: 2213–2220. 10.1158/1078-0432.CCR-14-2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi K, Pao W. 2011. How genetically engineered mouse tumor models provide insights into human cancers. J Clin Oncol 29: 2273–2281. 10.1200/JCO.2010.30.8304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. 2006. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev 20: 1496–1510. 10.1101/gad.1417406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi K, Fan PD, Shen R, Zakowski M, Varmus H. 2010. Erlotinib resistance in mouse models of epidermal growth factor receptor-induced lung adenocarcinoma. Dis Model Mech 3: 111–119. 10.1242/dmm.003681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo KH, Lim SM, Kim HR, Sung YH, Yun MR, Kim SM, Kim H, Kang HN, Lee JM, Kim SG, et al. 2017. Establishment of a conditional transgenic mouse model recapitulating EML4-ALK-positive human non-small cell lung cancer. J Thorac Oncol 12: 491–500. 10.1016/j.jtho.2016.10.022 [DOI] [PubMed] [Google Scholar]
- Regales L, Balak MN, Gong Y, Politi K, Sawai A, Le C, Koutcher JA, Solit DB, Rosen N, Zakowski MF, et al. 2007. Development of new mouse lung tumor models expressing EGFR T790M mutants associated with clinical resistance to kinase inhibitors. PLoS ONE 2: e810. 10.1371/journal.pone.0000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, Koutcher JA, Spassova M, Ouerfelli O, Mellinghoff IK, et al. 2009. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest 119: 3000–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riihimaki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. 2014. Metastatic sites and survival in lung cancer. Lung Cancer 86: 78–84. 10.1016/j.lungcan.2014.07.020 [DOI] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. 2015. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348: 124–128. 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ZN, McFarland CD, Winters IP, Naranjo S, Chuang CH, Petrov D, Winslow MM. 2017. A quantitative and multiplexed approach to uncover the fitness landscape of tumor suppression in vivo. Nat Methods 14: 737–742. 10.1038/nmeth.4297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ZN, McFarland CD, Winters IP, Seoane JA, Brady JJ, Yoon S, Curtis C, Petrov DA, Winslow MM. 2018. Mapping the in vivo fitness landscape of lung adenocarcinoma tumor suppression in mice. Nat Genet 50: 483–486. 10.1038/s41588-018-0083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Sayin VI, Davidson SM, Bauer MR, Singh SX, LeBoeuf SE, Karakousi TR, Ellis DC, Bhutkar A, Sánchez-Rivera FJ, et al. 2017. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med 23: 1362–1368. 10.1038/nm.4407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, et al. 2014. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol 32: 364–372. 10.1038/nbt.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschke AV, Tonon G, Gehlhaus KS, McTyre N, Bussey KJ, Lababidi S, Scudiero DA, Weinstein JN, Kirsch IR. 2003. Karyotypic complexity of the NCI-60 drug-screening panel. Cancer Res 63: 8634–8647. [PubMed] [Google Scholar]
- Rowbotham SP, Li F, Dost AFM, Louie SM, Marsh BP, Pessina P, Anbarasu CR, Brainson CF, Tuminello SJ, Lieberman A, et al. 2018. H3k9 methyltransferases and demethylases control lung tumor-propagating cells and lung cancer progression. Nat Commun 9: 4559. 10.1038/s41467-018-07077-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, Bergbower EA, Guan Y, Shin J, Guillory J, et al. 2012. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 44: 1111–1116. 10.1038/ng.2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, Heymach JV, Johnson JE, Lehman JM, MacPherson D, et al. 2019. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 19: 289–297. 10.1038/s41568-019-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti M, Leibold J, Bott MJ, Fennell M, Kulick A, Salgado NR, Chen CC, Ho YJ, Sanchez-Rivera FJ, Feucht J, et al. 2018. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 362: 1416–1422. 10.1126/science.aas9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, Balgobind AV, Wind K, Gracanin A, Begthel H, et al. 2018. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172: 373–386.e10. 10.1016/j.cell.2017.11.010 [DOI] [PubMed] [Google Scholar]
- Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Böttinger L, Klay D, Weeber F, Huelsz-Prince G, Iakobachvili N, Amatngalim GD, et al. 2019. Long-term expanding human airway organoids for disease modeling. EMBO J 38. 10.15252/embj.2018100300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Danés A, Blanpain C. 2018. Deciphering the cells of origin of squamous cell carcinomas. Nat Rev Cancer 18: 549–561. 10.1038/s41568-018-0024-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rivera FJ, Papagiannakopoulos T, Romero R, Tammela T, Bauer MR, Bhutkar A, Joshi NS, Subbaraj L, Bronson RT, Xue W, et al. 2014. Rapid modelling of cooperating genetic events in cancer through somatic genome editing. Nature 516: 428–431. 10.1038/nature13906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459: 262–265. 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- Schaffer BE, Park KS, Yiu G, Conklin JF, Lin C, Burkhart DL, Karnezis AN, Sweet-Cordero EA, Sage J. 2010. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res 70: 3877–3883. 10.1158/0008-5472.CAN-09-4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster K, Venkateswaran N, Rabellino A, Girard L, Pena-Llopis S, Scaglioni PP. 2014. Nullifying the CDKN2AB locus promotes mutant K-ras lung tumorigenesis. Mol Cancer Res 12: 912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers K, Fox MP, Bousamra M, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, et al. 2015. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest 125: 687–698. 10.1172/JCI72873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova EA, Nagel R, Berns A. 2015. Origins, genetic landscape, and emerging therapies of small cell lung cancer. Genes Dev 29: 1447–1462. 10.1101/gad.263145.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova EA, Kwon MC, Monkhorst K, Song JY, Bhaskaran R, Krijgsman O, Kuilman T, Peters D, Buikhuisen WA, Smit EF, et al. 2016. Transcription factor NFIB is a driver of small cell lung cancer progression in mice and marks metastatic disease in patients. Cell Rep 16: 631–643. 10.1016/j.celrep.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen T, Rodriguez BL, Chen L, Corte CMD, Morikawa N, Fujimoto J, Cristea S, Nguyen T, Diao L, Li L, et al. 2019. Targeting DNA damage response promotes antitumor immunity through STING-mediated t-cell activation in small cell lung cancer. Cancer Discov 9: 646–661. 10.1158/2159-8290.CD-18-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ, et al. 2014. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol 16: 876–888. 10.1038/ncb3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Radulovich N, Ng C, Liu N, Notsuda H, Cabanero M, Martins-Filho SN, Raghavan V, Li Q, Mer AS, et al. 2020. Organoid cultures as preclinical models of non-small cell lung cancer. Clin Cancer Res 26: 1162–1174. 10.1158/1078-0432.CCR-19-1376 [DOI] [PubMed] [Google Scholar]
- Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi Y, Tomizawa M, Doi T, Sone A, Suzuki N, Fujiwara H, et al. 2010. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r γnull humanized mice. Proc Natl Acad Sci 107: 13022–13027. 10.1073/pnas.1000475107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Abelson MR, Sonnenberg GF, Takita H, Yokota SJ, Conway TF Jr, Kelleher RJ Jr, Shultz LD, Barcos M, Bankert RB. 2008. Long-term engraftment and expansion of tumor-derived memory T cells following the implantation of non-disrupted pieces of human lung tumor into NOD-scid IL2Rγnull mice. J Immunol 180: 7009–7018. 10.4049/jimmunol.180.10.7009 [DOI] [PubMed] [Google Scholar]
- Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. 2009. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell 15: 489–500. 10.1016/j.ccr.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulidis F, Heymach JV. 2019. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat Rev Cancer 19: 495–509. 10.1038/s41568-019-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulidis F, Byers LA, Diao L, Papadimitrakopoulou VA, Tong P, Izzo J, Behrens C, Kadara H, Parra ER, Canales JR, et al. 2015. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 5: 860–877. 10.1158/2159-8290.CD-14-1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, Schrock AB, Hartmaier RJ, Trabucco SE, Gay L, et al. 2018. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov 8: 822–835. 10.1158/2159-8290.CD-18-0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LR, Cho S, Discher DE. 2018. Stem cell differentiation is regulated by extracellular matrix mechanics. Physiology (Bethesda) 33: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, et al. 2007. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448: 561–566. 10.1038/nature05945 [DOI] [PubMed] [Google Scholar]
- Soda M, Takada S, Takeuchi K, Choi YL, Enomoto M, Ueno T, Haruta H, Hamada T, Yamashita Y, Ishikawa Y, et al. 2008. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci 105: 19893–19897. 10.1073/pnas.0805381105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Yao E, Lin C, Gacayan R, Chen MH, Chuang PT. 2012. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci 109: 17531–17536. 10.1073/pnas.1207238109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrett JH, Guernet AA, Cuomo ME, Poels KE, van Alderwerelt van Rosenburgh IK, Nagelberg A, Farnsworth D, Price KS, Khan H, Ashtekar KD, et al. 2020. Drug sensitivity and allele-specificity of first-line osimertinib resistance EGFR mutations. Cancer Res 80: 2017–2030. 10.1158/0008-5472.CAN-19-3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P, Joynes C, Bassi R, Wang S, Tonra JR, Hadari YR, Hicklin DJ. 2007. Tumor growth inhibition with cetuximab and chemotherapy in non-small cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor. Clin Cancer Res 13: 1540–1551. 10.1158/1078-0432.CCR-06-1887 [DOI] [PubMed] [Google Scholar]
- Stewart EL, Mascaux C, Pham NA, Sakashita S, Sykes J, Kim L, Yanagawa N, Allo G, Ishizawa K, Wang D, et al. 2015. Clinical utility of patient-derived xenografts to determine biomarkers of prognosis and map resistance pathways in EGFR-mutant lung adenocarcinoma. J Clin Oncol 33: 2472–2480. 10.1200/JCO.2014.60.1492 [DOI] [PubMed] [Google Scholar]
- Strikoudis A, Cieślak A, Loffredo L, Chen YW, Patel N, Saqi A, Lederer DJ, Snoeck HW. 2019. Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep 27: 3709–3723.e5. 10.1016/j.celrep.2019.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. 2011. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 19: 754–764. 10.1016/j.ccr.2011.04.019 [DOI] [PubMed] [Google Scholar]
- Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al. 2013. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341: 1240104. 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Choi YL, Soda M, Inamura K, Togashi Y, Hatano S, Enomoto M, Takada S, Yamashita Y, Satoh Y, et al. 2008. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 14: 6618–6624. 10.1158/1078-0432.CCR-08-1018 [DOI] [PubMed] [Google Scholar]
- Takezawa K, Pirazzoli V, Arcila ME, Nebhan CA, Song X, de Stanchina E, Ohashi K, Janjigian YY, Spitzler PJ, Melnick MA, et al. 2012. HER2 amplification: a potential mechanism of acquired resistance to EGFR inhibition in EGFR-mutant lung cancers that lack the second-site EGFRT790M mutation. Cancer Discov 2: 922–933. 10.1158/2159-8290.CD-12-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. 2013. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503: 218–223. 10.1038/nature12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchaicha JH, Akbay EA, Altabef A, Mikse OR, Kikuchi E, Rhee K, Liao RG, Bronson RT, Sholl LM, Meyerson M, et al. 2014. Kinase domain activation of FGFR2 yields high-grade lung adenocarcinoma sensitive to a Pan-FGFR inhibitor in a mouse model of NSCLC. Cancer Res 74: 4676–4684. 10.1158/0008-5472.CAN-13-3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PT, Fan AC, Bendapudi PK, Koh S, Komatsubara K, Chen J, Horng G, Bellovin DI, Giuriato S, Wang CS, et al. 2008. Combined inactivation of MYC and K-Ras oncogenes reverses tumorigenesis in lung adenocarcinomas and lymphomas. PLoS One 3: e2125. 10.1371/journal.pone.0002125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo CL, Green S, Marsh V, Collisson EA, Iezza G, Phillips WA, McMahon M. 2013. Mutationally activated PIK3CAH1047R cooperates with BRAFV600E to promote lung cancer progression. Cancer Res 73: 6448–6461. 10.1158/0008-5472.CAN-13-0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricker EM, Xu C, Uddin S, Capelletti M, Ercan D, Ogino A, Pratilas CA, Rosen N, Gray NS, Wong KK, et al. 2015. Combined EGFR/MEK inhibition prevents the emergence of resistance in EGFR-mutant lung cancer. Cancer Discov 5: 960–971. 10.1158/2159-8290.CD-15-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, Gill S, Harrington WF, Pantel S, Krill-Burger JM, et al. 2017. Defining a cancer dependency map. Cell 170: 564–576.e16. 10.1016/j.cell.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J, Taylor-Weiner A, Kester L, et al. 2015. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161: 933–945. 10.1016/j.cell.2015.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T, Jacks T. 2002. Cancer modeling in the modern era: progress and challenges. Cell 108: 135–144. 10.1016/S0092-8674(02)00621-9 [DOI] [PubMed] [Google Scholar]
- Vaupel P, Kallinowski F, Okunieff P. 1989. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49: 6449–6465. [PubMed] [Google Scholar]
- Vilalta M, Hughes NP, Von Eyben R, Giaccia AJ, Graves EE. 2017. Patterns of vasculature in mouse models of lung cancer are dependent on location. Mol Imaging Biol 19: 215–224. 10.1007/s11307-016-1010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voena C, Menotti M, Mastini C, Di Giacomo F, Longo DL, Castella B, Merlo MEB, Ambrogio C, Wang Q, Minero VG, et al. 2015. Efficacy of a cancer vaccine against ALK-rearranged lung tumors. Cancer Immunol Res 3: 1333–1343. 10.1158/2326-6066.CIR-15-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. 2014. The clinical importance of assessing tumor hypoxia: relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal 21: 1516–1554. 10.1089/ars.2013.5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NC, Kenney LL, Jangalwe S, Aryee KE, Greiner DL, Brehm MA, Shultz LD. 2017. Humanized mouse models of clinical disease. Annu Rev Pathol 12: 187–215. 10.1146/annurev-pathol-052016-100332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter DM, Venancio OS, Buza EL, Tobias JW, Deshpande C, Gudiel AA, Kim-Kiselak C, Cicchini M, Yates TJ, Feldser DM. 2017. Systematic in vivo inactivation of chromatin-regulating enzymes identifies Setd2 as a potent tumor suppressor in lung adenocarcinoma. Cancer Res 77: 1719–1729. 10.1158/0008-5472.CAN-16-2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter DM, Yates TJ, Ruiz-Torres M, Kim-Kiselak C, Gudiel AA, Deshpande C, Wang WZ, Cicchini M, Stokes KL, Tobias JW, et al. 2019b. RB constrains lineage fidelity and multiple stages of tumour progression and metastasis. Nature 569: 423–427. 10.1038/s41586-019-1172-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fu X, Hoffman RM. 1992. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer 51: 992–995. 10.1002/ijc.2910510626 [DOI] [PubMed] [Google Scholar]
- Wang J, Perry CJ, Meeth K, Thakral D, Damsky W, Micevic G, Kaech S, Blenman K, Bosenberg M. 2017. UV-induced somatic mutations elicit a functional T cell response in the YUMMER1.7 mouse melanoma model. Pigment Cell Melanoma Res 30: 428–435. 10.1111/pcmr.12591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Min S, Liu H, Wu N, Liu X, Wang T, Li W, Shen Y, Wang H, Qian Z, et al. 2019a. Nf1 loss promotes Kras-driven lung adenocarcinoma and results in Psat1-mediated glutamate dependence. EMBO Mol Med 11: e9856. 10.15252/emmm.201809856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Chow RD, Bai Z, Zhu L, Errami Y, Dai X, Dong MB, Ye L, Zhang X, Renauer PA, et al. 2019b. Multiplexed activation of endogenous genes by CRISPRa elicits potent antitumor immunity. Nat Immunol 20: 1494–1505. 10.1038/s41590-019-0500-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer JP, Liu J, Lim JS, et al. 2016. CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest 126: 2610–2620. 10.1172/JCI81603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RG. 2013. Tissue mechanics and fibrosis. Biochim Biophys Acta 1832: 884–890. 10.1016/j.bbadis.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott PM, Halliwill KD, To MD, Rashid M, Rust AG, Keane TM, Delrosario R, Jen KY, Gurley KE, Kemp CJ, et al. 2015. The mutational landscapes of genetic and chemical models of Kras-driven lung cancer. Nature 517: 489–492. 10.1038/nature13898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett TG, Inge LJ, Dhruv HD, Cheung PY, Weiss GJ, Bremner RM, Winkles JA, Tran NL. 2013. Molecular determinants of lung cancer metastasis to the central nervous system. Transl Lung Cancer Res 2: 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, et al. 2012. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 487: 505–509. 10.1038/nature11249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FH, Johannessen CM, Piccioni F, Tamayo P, Kim JW, Van Allen EM, Corsello SM, Capelletti M, Calles A, Butaney M, et al. 2015. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell 27: 397–408. 10.1016/j.ccell.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove E, Liu ZZ, Patel KD, Arnal-Estapé A, Cai WL, Melnick MA, Politi K, Monteiro C, Zhu L, Valiente M, et al. 2019. Transcriptomic hallmarks of tumor plasticity and stromal interactions in brain metastasis. Cell Rep 27: 1277–1292.e7. 10.1016/j.celrep.2019.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters IP, Chiou SH, Paulk NK, McFarland CD, Lalgudi PV, Ma RK, Lisowski L, Connolly AJ, Petrov DA, Kay MA, et al. 2017. Multiplexed in vivo homology-directed repair and tumor barcoding enables parallel quantification of Kras variant oncogenicity. Nat Commun 8: 2053. 10.1038/s41467-017-01519-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wislez M, Fujimoto N, Izzo JG, Hanna AE, Cody DD, Langley RR, Tang H, Burdick MD, Sato M, Minna JD, et al. 2006. High expression of ligands for chemokine receptor CXCR2 in alveolar epithelial neoplasia induced by oncogenic kras. Cancer Res 66: 4198–4207. 10.1158/0008-5472.CAN-05-3842 [DOI] [PubMed] [Google Scholar]
- Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. 2012. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30: 876–882. 10.1038/nbt.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N, Jia D, Ibrahim AH, Bachurski CJ, Gronostajski RM, MacPherson D. 2016. NFIB overexpression cooperates with Rb/p53 deletion to promote small cell lung cancer. Oncotarget 7: 57514–57524. 10.18632/oncotarget.11583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Jiang Q, Willette-Brown J, Xi S, Zhu F, Burkett S, Back T, Song NY, Datla M, Sun Z, et al. 2013. The pivotal role of IKKα in the development of spontaneous lung squamous cell carcinomas. Cancer Cell 23: 527–540. 10.1016/j.ccr.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, Hogan BL, Onaitis MW. 2012. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proc Natl Acad Sci 109: 4910–4915. 10.1073/pnas.1112499109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Fillmore CM, Koyama S, Wu H, Zhao Y, Chen Z, Herter-Sprie GS, Akbay EA, Tchaicha JH, Altabef A, et al. 2014a. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 25: 590–604. 10.1016/j.ccr.2014.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Huang L, Futtner C, Schwab B, Rampersad RR, Lu Y, Sporn TA, Hogan BL, Onaitis MW. 2014b. The cell of origin and subtype of K-Ras-induced lung tumors are modified by Notch and Sox2. Genes Dev 28: 1929–1939. 10.1101/gad.243717.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Denny SK, Greenside PG, Chaikovsky AC, Brady JJ, Ouadah Y, Granja JM, Jahchan NS, Lim JS, Kwok S, et al. 2018. Intertumoral heterogeneity in SCLC is influenced by the cell type of origin. Cancer Discov 8: 1316–1331. 10.1158/2159-8290.CD-17-0987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Betsuyaku T, Garbow GR, Miao J, Govindan R, Ornitz DM. 2013. Rapid induction of lung adenocarcinoma by fibroblast growth factor 9 signaling through FGF receptor 3. Cancer Res 73: 5730–5741. 10.1158/0008-5472.CAN-13-0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahalka AH, Arnal-Estapé A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, Frenette PS. 2017. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358: 321–326. 10.1126/science.aah5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Zhang J, Li M, Huang XS, Yang XN, Zhong WZ, Xie L, Zhang L, Zhou M, Gavine P, et al. 2013. Establishment of patient-derived non-small cell lung cancer xenograft models with genetic aberrations within EGFR, KRAS and FGFR1: useful tools for preclinical studies of targeted therapies. J Transl Med 11: 168. 10.1186/1479-5876-11-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, Cortot AB, Chirieac L, Iacob RE, Padera R, et al. 2009. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 462: 1070–1074. 10.1038/nature08622 [DOI] [PMC free article] [PubMed] [Google Scholar]