Abstract
Study Objectives:
Lower therapeutic positive airway pressure (PAP) levels are associated with improved response to non-PAP therapies in the treatment of obstructive sleep apnea. The aim of this study was to evaluate the prevailing notion that patients with apnea-predominant obstructive sleep apnea require higher therapeutic PAP levels compared to patients with hypopnea-predominant obstructive sleep apnea.
Methods:
An institutional review board–approved retrospective review was performed using strict inclusion criteria: presence of type I or III sleep study, apnea-hypopnea index > 10 events/h, and adherence to auto-adjusting continuous positive airway pressure. Patients were stratified by apnea (> 50% apneas) or hypopnea (≤ 50% apneas) predominance, and PAP data were compared. Statistical analyses were performed using Student’s t test and linear regression modeling.
Results:
Between January 1, 2018 and January 1, 2020, 500 patients met inclusion criteria. Two hundred twenty-one (44.1%) patients were apnea-predominant and 279 (55.8%) were hypopnea-predominant. Apnea-predominant patients had a slightly greater mean PAP (9.01 vs 8.36, P = .002) than hypopnea-predominant patients. Univariable and multivariable linear regression of 7 variables (obstructive apnea percentage, age, sex, body mass index, apnea-hypopnea index, O2 nadir, mask type) showed obstructive apnea percentage was the weakest predictor of therapeutic PAP levels.
Conclusions:
Apnea-predominant individuals demonstrated a clinically insignificant difference in PAP level compared to hypopnea-predominant individuals; moreover, obstructive apnea percentage was not a strong predictor of therapeutic PAP levels. Of the modeled variables, the strongest predictor of PAP level was apnea-hypopnea index. Further studies are needed to explore these relationships as well as additional variables that may contribute to predicting therapeutic PAP levels.
Citation:
Yu JL, Liu Y, Tangutur A, et al. Influence of apnea vs hypopnea predominance in predicting mean therapeutic positive airway pressures among patients with obstructive sleep apnea. J Clin Sleep Med. 2021;17(11):2171–2178.
Keywords: apnea, hypopnea, positive airway pressure, auto-CPAP, OSA
BRIEF SUMMARY
Current Knowledge/Study Rationale: Differences in therapeutic positive airway pressure (PAP) levels are associated with response to PAP-alternative therapies for the treatment of obstructive sleep apnea. The aim of this study was to evaluate whether apnea/hypopnea predominance is a predictor of therapeutic PAP levels.
Study Impact: Our findings demonstrated that apnea-predominant individuals bear a clinically insignificant difference in PAP level compared to hypopnea-predominant individuals, with regression modeling showing obstructive apnea percentage to be a very weak contributor to predicting therapeutic PAP. These findings challenge the conceptual paradigm of apneas as a more severe physiologic form of obstruction and warrant further exploration into the driving factors of therapeutic PAP levels. An understanding of these variables will allow optimal patient selection for alternatives to PAP therapy.
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder that affects over 25 million Americans.1 Positive airway pressure (PAP) devices are considered first-line therapy for treatment of OSA; however, adherence to therapy ranges from 23% to 80%.2–4 Patients who do not tolerate PAP have a variety of second-line therapies, including positional sleep devices, mandibular advancement devices (MAD), and surgical interventions.4 Unfortunately, second-line therapies are not effective in all OSA patients, with success rates ranging between 30% and 82%.5–10 There is a continuing need to determine patient-specific factors that may be predictive of therapeutic success among the different PAP-alternative therapies in order to better guide treatment decisions.
One area of interest is the use of evidence from PAP therapy itself to guide PAP-alternative therapies. Lower therapeutic PAP levels have been associated with improved response rates among PAP-alternative therapies, including MAD and upper airway stimulation surgery.11,12 The mechanism for the ability of therapeutic PAP to predict such responses likely relates to its direct relationship with the severity of upper airway collapsibility.13 In other words, patients with higher therapeutic PAP levels have more collapsible upper airways, making treatment with PAP alternatives less effective.
However, not all patients considering PAP-alternative therapies will be able to have PAP data, given that they, by definition, have difficulty using PAP. Measurements of upper airway collapsibility are also difficult and cumbersome to perform. In this way, determining patient-derived factors as a correlate to upper airway collapsibility may be helpful in guiding PAP-alternative treatments when accurate PAP data cannot be obtained.
OSA is defined by the frequency of obstructive respiratory events that occur during sleep reported as a combination of complete obstructive events called apneas and partial obstructive events called hypopneas. Both events are included in the apnea-hypopnea index (AHI), the major OSA disease-defining metric.14 However, because apneas are caused by complete cessation of airflow, they are commonly described as a more severe form of upper airway collapse than hypopneas. If a greater proportion of apneas is associated with more significant upper airway collapsibility, then apnea-predominant OSA should be associated with greater therapeutic PAP requirements.
Currently, there are no studies in the literature comparing apnea or hypopnea predominance to therapeutic PAP levels. The aim of this study is to evaluate apnea vs hypopnea predominance in a large sample population of OSA patients receiving PAP therapy to determine if there are differences in therapeutic PAP levels between these groups. We hypothesize that among OSA patients being treated with an auto-adjusting continuous positive airway pressure (aPAP) device, those who are apnea-predominant will require a greater mean PAP level than those who are hypopnea-predominant. The second aim of this study was to determine other variables that may be predictive of PAP levels in univariable and multivariable linear regression models. We hypothesized that obstructive apnea (OA) percentage as well as 6 additional variables (age, sex, body mass index [BMI], AHI, O2 nadir, and mask type) will contribute significantly to predicting therapeutic PAP levels in linear regression modeling.
METHODS
Participants
The study is a retrospective cohort study performed at a single institution. Patients evaluated between January 1, 2018 and January 1, 2020 at the University of Pennsylvania Sleep Center who were diagnosed with OSA and prescribed an aPAP were included. Inclusion criteria were as follows: all patients 18 years or older who were diagnosed with OSA by a sleep study performed at the University of Pennsylvania and treated with an aPAP with initial 90-day follow-up adherence data. To ensure consistent treatment data, only 1 type of aPAP machine was studied, the DreamStation Auto-CPAP Machine (Philips Respironics, Murrysville, PA). Exclusion criteria were as follows: baseline AHI <10 events/h, greater than 25% central apneic events per hour, no follow up aPAP adherence data, or nonadherence on follow-up with adherence being defined as greater than 4 hours of nightly use, 70% of nights. The central sleep apnea index was calculated as the total number of central events divided by sleep time and the central sleep apnea percent was calculated by dividing this by the total AHI.
Procedures and data collection
Patient demographics, physical exam information (age, sex, BMI, neck circumference), and baseline sleep study reports were obtained from the electronic medical record (Epic; Epic Systems Corporation in Verona, WI). Type I (including split night studies) and type III polysomnography studies were included in the analysis. The following data variables were extracted from the sleep studies: study type, AHI, oxygen desaturation index, and percent oxygen desaturation nadir. All sleep studies were performed at the same institution and scored based on the American Academy of Sleep Medicine 2007 guidelines.14 Hypopneas were defined as a reduction of flow > 30% associated with a 4% oxygen desaturation. Patients whose AHI consisted of greater than 50% obstructive apneas were considered apnea-predominant and those with less than or equal to 50% apneas were considered hypopnea-predominant. Adherence monitoring was performed through a secured adherence monitoring software (EncoreAnywhere) provided by Philips Respironics. The following data variables were obtained from EncoreAnywhere: days aPAP used, average daily aPAP use, mean PAP, PAP > 90% of the time, peak pressure, and mask type. Mean PAP was our primary outcome variable. Adherence was based on the best 30 days of adherence in the initial 90 days of receiving the device (see manual of procedures in the supplemental material (3.8MB, pdf) ).
Statistical analysis
Continuous variables are reported as means and standard deviations or medians and ranges, as appropriate, and categorical variables using frequencies and percentages. Unadjusted comparisons between groups used parametric t tests or nonparametric Wilcoxon tests for continuous data and chi-square or Fisher’s exact tests for categorical data. Statistical significance of the data is based on a P value of < .05.
Univariate linear regression modeling was performed comparing mean PAP level to obstructive apnea (OA) percentage, a continuous variable calculated by dividing the obstructive apnea index by the total AHI, as well as 6 other variables: age, sex, BMI, total AHI, oxygen nadir, and mask type. These variables were chosen based on completeness of data as well as prior literature showing association with PAP therapy.13,15,16 Multivariable linear regression was used comparing OA percentage along with these 6 additional metrics to generate a model for predicting mean PAP levels. Beta coefficients with 95% confidence intervals are reported along with Pearson’s R correlation coefficient. The multivariable model also reports a standardized beta that is calculated from standard deviation to allow comparisons among the individual variables. Statistical significance of the model variables is based on a P value of < .05. To evaluate for multicollinearity in the multivariable linear regression, tolerance and variance inflation factors (VIF) were calculated for each variable with VIF values < 5 considered to be minimally collinear.
Two post-hoc analyses were additionally performed. A multivariable regression analysis was performed adding sleep study type to the model to determine if it contributed to changes in mean PAP level. Type I studies included both full overnight in-lab PSG as well as in-lab split-night studies that were then compared to type III studies. Mean PAP levels in nonadherent patients were also calculated and compared to adherent patients to determine if PAP data would be similar among nonadherent participants.
A sample-size calculation was performed for the primary aim, testing the difference in therapeutic PAP levels between apnea- and hypopnea-predominant participants. Previous studies have reported mean therapeutic PAP pressures among OSA patients to be approximately 9 cm H2O (standard deviation = 1.9).17 An a priori determination was made that a 2 cm H2O difference in effect size between the two groups would be considered significant based on clinical experience. In order to test a 2 cm H2O difference between groups, based on the significance level (α = 0.05) and power (1 – β = 0.80), a total sample of 76 patients was needed. Statistical analysis was performed using RStudio v.1.3.1073 (RStudio, PBC, Boston, MA).
RESULTS
Participant inclusion and demographics
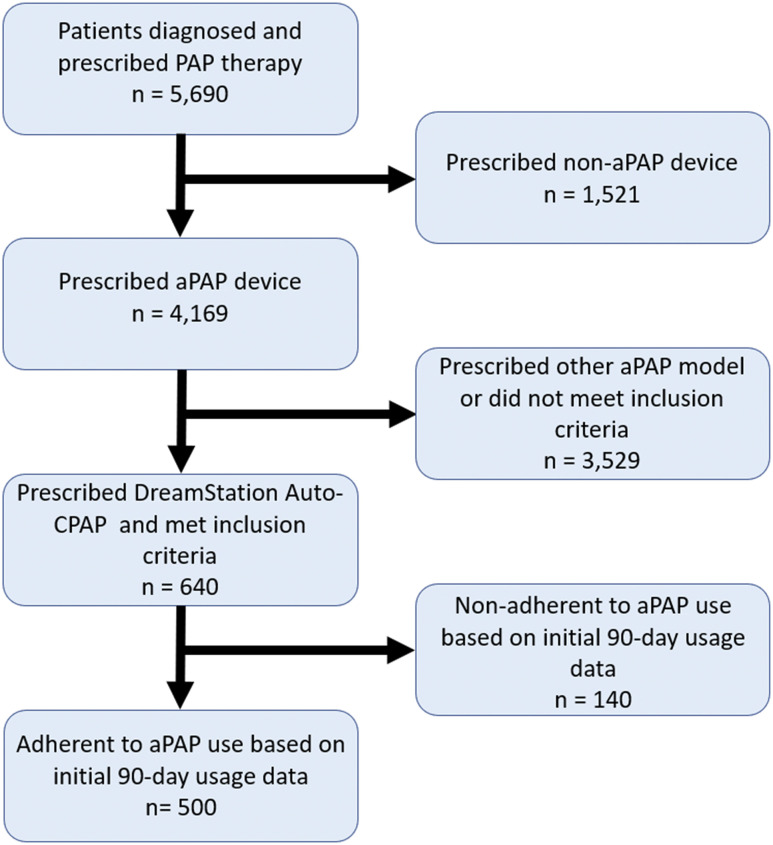
Between 1/1/2018 and 1/1/2020, 5,690 patients were diagnosed with OSA and prescribed a therapeutic PAP device at the University of Pennsylvania Sleep Center. Of these patients, 640 patients met our inclusion criteria and received a DreamStation Auto-CPAP Machine. One hundred forty patients were excluded because they did not meet adherence criteria (Figure 1). Table 1 shows the average baseline demographics and aPAP download data for the 500 participants included in our study sample. Of note, the sample demonstrated mean PAP levels of 8.2 cm H2O and a balance of nasal and full-face mask interfaces (48% vs 52%, respectively).
Figure 1. Flow diagram for patient inclusion in the study.
aPAP = auto-adjusting positive airway pressure, CPAP = continuous positive airway pressure, PAP = positive airway pressure.
Table 1.
Baseline demographic, sleep study, and aPAP data among all participants (n = 500).
| Characteristic | Value |
|---|---|
| Age, y | 53 (42–63) |
| Sex | |
| Female | 204 (41%) |
| Male | 292 (59%) |
| BMI, kg/m2 | 35 (30–41) |
| Neck, cm* | 41.9 (39.4–45.7) |
| AHI, events/h | 28 (17–49) |
| ODI, events/h | 25 (15–45) |
| O2 nadir, % | 78 (72–83) |
| Apnea, % | 45 (23–68) |
| Mean pressure, cm H2O | 8.20 (6.90–9.90) |
| Pressure > 90% time, cm H2O | 10.70 (9.00–12.60) |
| Peak pressure, cm H2O | 12.10 (9.90–14.12) |
| Mask type | |
| Full-face mask | 262 (53%) |
| Nasal mask | 237 (47%) |
| Unknown | 1 |
| Study type | |
| HSAT | 248 (50%) |
| PSG | 155 (31%) |
| Split-night study | 97 (19%) |
Statistics presented: median (IQR); n (%). *Neck circumference data were not available in all participants. AHI = apnea-hypopnea index, aPAP = auto-adjusting positive airway pressure, BMI = body mass index, HSAT = home sleep apnea test, IQR = interquartile range, ODI = oxygen desaturation index, PSG = polysomnography.
Primary outcome analysis
Table 2 compares apnea-predominant to hypopnea-predominant patients. Two hundred and twenty-one patients (44.2%) were considered apnea-predominant while 279 (55.8%) patients were hypopnea-predominant. The 2 groups were similar in age, neck circumference, and mask type. No differences were seen on positional apnea rates as well as rapid eye movement sleep AHI among participants in each group. The hypopnea-predominant group had higher BMI and had a greater proportion of women, while the apnea-predominant group had greater total AHI and lower oxygen nadir values. The apnea-predominant group had a mean PAP level of 9.01 cm H2O, while the hypopnea-predominant group had a mean PAP level of 8.36 cm H2O (P = .002).
Table 2.
Comparison of participants based on apnea or hypopnea predominance.
| Apnea-Predominant (n = 221) | Hypopnea-Predominant (n = 279) | P | |
|---|---|---|---|
| Age, y | 52.2 (14.4) | 52.5 (13.6) | .794 |
| Sex | .001 | ||
| Female | 72 (32.6%) | 132 (47.3%) | |
| Male | 149 (67.4%) | 147 (52.7%) | |
| BMI, kg/m2 | 34.4 (7.92) | 37.7 (9.25) | < .001 |
| Neck, cm* | 42.5 (5.16) | 41.7 (4.14) | .151 |
| AHI, events/h | 45.0 (27.8) | 30.6 (22.8) | < .001 |
| REM sleep AHI, events/h** | 52.6 (30.9) | 47.1 (27.8) | .193 |
| Positional apnea*** | 1.000 | ||
| Nonpositional | 62 (45.3%) | 68 (45.3%) | |
| Positional | 75 (54.7%) | 82 (54.7%) | |
| ODI, events/h | 39.1 (28.7) | 28.9 (22.0) | < .001 |
| O2 nadir, % | 75.2 (9.28) | 77.6 (7.88) | .002 |
| Mean pressure, cm H2O | 9.01 (2.54) | 8.36 (2.14) | .002 |
| Pressure > 90% time, cm H2O | 11.4 (2.89) | 10.7 (2.60) | .004 |
| Peak pressure, cm H2O | 12.5 (3.13) | 12.0 (2.78) | .039 |
| Mask type**** | .794 | ||
| Full-face mask | 118 (53.4%) | 143 (51.8%) | |
| Nasal mask | 103 (46.6%) | 133 (48.2%) | |
| Study type | .001 | ||
| 1 | 127 (57.5%) | 121 (43.4%) | |
| 2 | 49 (22.2%) | 106 (38.0%) | |
| 3 | 45 (20.4%) | 52 (18.6%) |
Categorical data are reported as counts with percent of the total sample in parenthesis, numerical data are reported as mean values with standard deviation in parenthesis. *Neck circumference data were not available in all participants (n = 129, n = 168 for apnea-predominant and hypopnea-predominant participants, respectively). **REM sleep data only present among full polysomnograms (n = 80, n = 137 for apnea-predominant and hypopnea-predominant participants, respectively). ***Sleep position data not available in all participants (n = 137, n = 150 for apnea-predominant and hypopnea-predominant participants, respectively). ****One hypopnea-predominant participant had an unknown mask type. AHI = apnea-hypopnea index, BMI = body mass index, ODI = oxygen desaturation index, REM = rapid eye movement.
Secondary outcome analysis
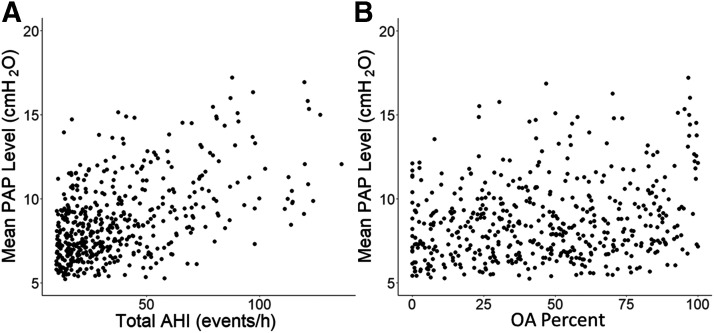
The unadjusted univariate linear regression analysis is shown in Table 3. All 7 variables were significantly correlated with mean aPAP levels with AHI having the greatest correlation (r = .51, P < .001) (Figure 2). The multivariable regression modeling results are shown in Table 4. The overall model showed a strong correlation to mean PAP level (r = .62, P < .001). Similar to the univariate comparisons, the strongest contributor to the multivariable model was the patient’s AHI with a standardized beta of 0.32. BMI, oxygen nadir, and mask type were moderate contributors to the model (0.16, –0.20, –0.18). Age, sex, and OA percentage weakly contributed to the model (–0.10, 0.08, 0.08). Tolerance and variable inflation factor calculations revealed no evidence of significant multicollinearity among the 7 variables in the model (Table S1 (3.8MB, pdf) in the supplemental material).
Table 3.
Univariate linear regression comparing mean pressure to other variables.
| Beta | 95% CI | R | R 2 | P | |
|---|---|---|---|---|---|
| Age, y | –0.04 | –0.06 to –0.03 | –0.27 | 0.07 | < .001 |
| Sex, male | 0.62 | 0.20 to 1.0 | 0.13 | 0.02 | .004 |
| BMI, kg/m2 | 0.08 | 0.06 to 0.10 | 0.30 | 0.09 | < .001 |
| AHI, events/h | 0.05 | 0.04 to 0.05 | 0.51 | 0.26 | < .001 |
| O2 nadir, % | –0.10 | –0.12 to –0.08 | –0.37 | 0.14 | < .001 |
| OA% | 0.02 | 0.01 to 0.03 | 0.22 | 0.05 | < .001 |
| Mask type, nasal | –1.2 | –1.6 to –0.81 | –0.26 | 0.07 | < .001 |
AHI = apnea-hypopnea index, BMI = body mass index, CI = confidence interval, OA% = obstructive apnea percent, ODI = oxygen desaturation index.
Figure 2. Scatterplot diagram of mean PAP compared to AHI and OA percentage.
Comparison of total AHI (A) and OA percent (B) to mean PAP level. AHI showed the strongest correlation (r = .51, P < .001) while OA percent was weakly correlated (r = .22, P < .001). AHI = apnea-hypopnea index, OA = obstructive apnea, PAP = positive airway pressure.
Table 4.
Adjusted multivariable linear regression analysis of mean pressure to other variables.
| Beta | 95% CI | Standardized Beta* | P | |
|---|---|---|---|---|
| Age, y | –0.02 | –0.03 to 0.00 | –0.10 | .011 |
| Sex, male | 0.39 | 0.04 to 0.75 | 0.08 | .031 |
| BMI, kg/m2 | 0.04 | 0.02 to 0.06 | 0.16 | < .001 |
| AHI, events/h | 0.03 | 0.02 to 0.04 | 0.32 | < .001 |
| O2 nadir, % | –0.05 | –0.07 to –0.03 | –0.20 | < .001 |
| OA% | 0.01 | 0.00 to 0.01 | 0.08 | .061 |
| Mask type, nasal | –0.84 | –1.2 to –0.50 | –0.18 | < .001 |
*Standardized beta values represent the regression based on z scores and allow for comparison of the relative contributions of each variable to the overall model. AHI = apnea-hypopnea index, BMI = body mass index, CI = confidence interval, OA% = obstructive apnea percent, ODI = oxygen desaturation index.
Post-hoc analysis
Post-hoc multivariable linear regression analysis with the addition of study type into the model showed no significant influence of sleep study type in predicting mean PAP level (Table S2 (3.8MB, pdf) ). Mean PAP levels were also found to be similar (8.65 cm H2O vs 8.68 cm H2O, P = .90) between adherent and nonadherent groups (Table S3 (3.8MB, pdf) ).
DISCUSSION
This study is the first to characterize OSA severity using therapeutic PAP levels obtained from an aPAP device and to compare apnea vs hypopnea predominance in a therapeutic context. The results show that apnea-predominant patients have minimally higher PAP levels than hypopnea-predominant patients. Other notable differences between groups include: greater male percentage, greater BMI, and greater overall AHI in the apnea-predominant group. Possible interactions explaining these differences include sex differences in OSA, with women having lower AHI and greater BMI compared to men, which could contribute to sex, AHI, and BMI differences observed between apnea and hypopnea-predominant groups.18 These findings highlight the complex nature of OSA physiology with multiple factors influencing disease characteristics and emphasize the need for a multivariable approach to modeling these relationships.
Univariable and multivariable linear regression modeling showed significant relationships between all variables and mean PAP level. AHI was the strongest contributor to predicting therapeutic PAP while OA percentage was the weakest contributor among all variables in our model. The small difference in mean PAP level between apnea- and hypopnea-predominant participants along with the weak contribution of OA percentage in our multivariable model imply that apnea-hypopnea predominance does not significantly influence therapeutic PAP levels.
Our results are consistent with prior studies examining the relationship between apnea/hypopnea predominance to treatment modalities. Studies have reported little difference in therapeutic outcomes between apnea- or hypopnea-predominant participants with respect to therapies, including tonsillectomy, mandibular advancement devices, and upper airway stimulation.19–21 The significance of AHI in predicting therapeutic PAP also agrees with prior studies that have shown a positive relationship of AHI to both therapeutic PAP and upper airway collapsibility.13,15,22 AHI is a complex trait that integrates several upper airway, ventilatory control, and sleep/wake mechanisms, making it a difficult metric to interpret in terms of the individual factors that characterize OSA.23,24 Prior to the advent of aPAP devices, several studies demonstrated variable contributions of a variety of demographic, anthropometric, and polysomnographic parameters to determining minimally effective therapeutic PAP levels.25–28 The present study extends these observations by demonstrating that apnea/hypopnea predominance adds minimally to the prediction of therapeutic PAP levels, suggesting that instead apnea/hypopnea predominance must reflect another aspect of upper airway collapse distinct from therapeutic PAP.
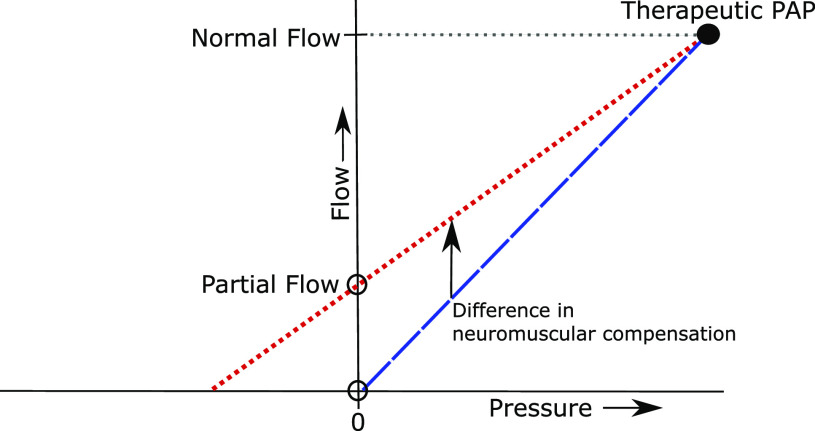
Investigators have demonstrated that the severity of upper airway obstruction during sleep is largely determined by pharyngeal mechanical loads and neuromotor tone.29–31 When OSA patients breathe at atmospheric pressure, airflow obstruction can elicit reflex neuromuscular responses that mitigate the obstruction. We propose that apnea/hypopnea predominance reflects the impact of airway structures and neuromuscular response mechanisms. At atmospheric pressure, compensatory responses can relieve airflow limitation to varying degrees, resulting in the partial (hypopnea) vs complete (apnea) obstruction that is observed in OSA (see Figure 3). When therapeutic PAP is applied, upper airway obstruction resolves completely, at which point compensatory neuromuscular responses wane and the pharynx becomes relatively hypotonic.30,32 Therapeutic PAP effectively minimizes the influence of neuromuscular activity on the otherwise passive mechanical loads obstructing the airway. Therapeutic PAP levels were similar in both of our patient groups, suggesting that their upper airways, in the hypotonic state, had similar levels of collapsibility (see Figure 3 depicting comparable levels of flow, a surrogate for pharyngeal collapsibility, at therapeutic PAP in both apnea- and hypopnea-predominant groups). The diminished slope of the pressure-flow relationship in hypopneic- vs apneic-predominant patients can therefore be attributed to the impact of compensatory neuromuscular responses that mitigate obstruction at atmospheric pressure (see Figure 3 showing partial flow vs zero flow at atmospheric pressure between hypopneic- and apneic-predominant participants). Observed differences in apnea/hypopnea predominance and/or therapeutic PAP can fuel inferences about the relative contribution of neuromuscular response and anatomic loads, respectively, to airflow obstruction in OSA. This parameter may have therapeutic implications in guiding future PAP-alternative therapies that could target the neuromuscular response mechanisms.
Figure 3. A pressure-flow schematic describing the role that apnea/hypopnea predominance and therapeutic PAP may play in determining the severity of upper airway obstruction during sleep.
Illustrative pressure-flow plots for a typical patient with predominant hypopneas (dotted red) or apneas (dashed blue) during sleep. These curves intersect the flow axis at a reduced level of flow during obstructive hypopnea or at zero flow during complete obstructive apneas (open dots). The curves converge at the minimally effective PAP level (the large black dot) when the flow is normal, ventilation is not obstructed, and PAP is therapeutic. Our findings suggest that therapeutic PAP is similar in both groups. At lower levels of PAP, progressive degrees of airflow obstruction can elicit ever-increasing neuromuscular responses that mitigate the obstruction. The slopes of the 2 lines are inversely related to the strength of neuromuscular compensation in hypopneic vs apneic patients for a given therapeutic PAP level (see arrow between the two lines). PAP = positive airway pressure.
The study methods exhibit several strengths. The study included a large sample (n = 500) of patients, all using the same aPAP device. This ensured the proprietary algorithm for calculating PAP therapy was consistent among all patients. All patients were treated at the same single institution and had consistent follow-up with appropriate adherence data recorded from a standardized online platform. All sleep studies were flow-based, were performed at the same institution, and used the same definition for obstructive hypopnea. Use of aPAP provides machine algorithm-based, real-world device usage as opposed to single-night, technologist-dependent in-laboratory titration studies. Finally, the average mean pressure reported by the aPAP device represents therapy that was averaged over 30 nights, thereby making it less susceptible to night-to-night variability of treatment.
Conversely, the study has ostensible limitations. The determination of OSA was based on single night type I and type III studies, which can be subject to night-to-night variability, and the distribution of apneas and hypopneas may have been influenced also by night-to-night variability.33 In addition, we included both type I and type III studies, but type III studies may underestimate the frequency of respiratory events because they do not record sleep time. Hypopneas may also be underestimated in home sleep apnea tests (HSATs) if arousals are included in the definition of hypopneas, which cannot be identified in an HSAT. However, our study design used standardized hypopnea definitions across both study types that did not include arousals in the hypopnea definition. From a clinical standpoint, many patients will present for evaluation having a history of a type III study, and therefore it is relevant to include these patients in the study sample. Furthermore, when study type was added to the regression model, it was shown not to be a significant contributor to predicting mean PAP levels (Table S2 (3.8MB, pdf) ).
The aPAP device used a proprietary algorithm for determining therapeutic PAP levels. It is therefore possible that the results of this study are related to an unknown calculation that might be artificially biasing our results. However, Philips Respironics provided detailed descriptions of their DreamStation Auto-CPAP algorithm and it was determined that bias from the algorithm was unlikely (a description of the algorithm provided by Philips Respironics is available in the supplemental material (3.8MB, pdf) ).
PAP data were also gathered based on averages of the best 30 days of adherence within the initial 90-day window, because all patients receiving PAP therapy at our institution are required to have a follow-up at 90 days for a review of adherence, which allowed for a standardized method to search the EncoreAnywhere database. This search ultimately excluded patients who did not initially meet adherence criteria but could have achieved adherence long-term, though initial adherence period is a strong predictor of long-term adherence and this excluded population is likely to have been small.34 We were also unable to identify if patients received additional treatment on initial prescription of PAP, such as the use of positional therapy, nor could we determine if any treatment changes occurred within the 90-day compliance period. It is therefore possible that certain patients could have changed mask types or had additional adjunct therapies within this 90-day window that could have influenced pressure measurements.
OSA physiology is complex with identifiable subtypes, including positional OSA or rapid eye movement sleep–related OSA, that could lead to differences in PAP therapy. These subgroup analyses are beyond the scope of this study but can be analyzed in future studies. We also excluded nonadherent patients from the initial study design out of concern that poor PAP adherence may have led to unreliable pressure data. However, the nonadherent group is clinically important because they are more likely to have been recommended for PAP-alternative therapies. A post-hoc analysis of PAP levels between adherent and nonadherent participants in our data showed comparable pressure measurements (Table S3 (3.8MB, pdf) ), suggesting the data may be helpful even in nonadherent patients and can be explored in future studies. In addition, no anatomic measurements of the upper airway or of upper airway collapsibility were obtained from patients in this retrospective study, although they may be important factors for determining relationships of anatomic load on therapeutic PAP levels and should also be explored in future studies.
CONCLUSIONS
The results of our study show that apnea/hypopnea predominance does not significantly influence therapeutic PAP levels, while AHI was the strongest predictor of therapeutic PAP levels in both univariable and multivariable modeling. These findings challenge the conceptual paradigm of apneas as a more severe physiologic form of obstruction, highlighting the complex interplay between anatomic load and neuromuscular control. Future studies into patient-derived factors for therapeutic PAP levels remain critical to personalizing patient selection for alternatives to PAP therapy.
DISCLOSURE STATEMENT
All authors have seen and approved the final manuscript. Dr. Yu is supported by T32 Training Grant in Sleep and Sleep Disorders (5-T32-HL–007953-18) at the Division of Sleep Medicine, University of Pennsylvania, and by a Sleep Research Society CDA Award (023-JP-19). Dr. Schwartz receives support relevant for this project from the National Institutes of Health (1R01HL144859-01). Dr. Dedhia receives support relevant for this project from the National Institutes of Health (1R01HL144859-01). The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Brendan Keenan for his assistance with the statistical analysis.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- aPAP
auto-adjusting positive airway pressure
- BMI
body mass index
- OA
obstructive apnea
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
REFERENCES
- 1. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM . Increased prevalence of sleep-disordered breathing in adults . Am J Epidemiol. 2013. ; 177 ( 9 ): 1006 – 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kohler M, Smith D, Tippett V, Stradling JR . Predictors of long-term compliance with continuous positive airway pressure . Thorax. 2010. ; 65 ( 9 ): 829 – 832. [DOI] [PubMed] [Google Scholar]
- 3. Bakker JP, Weaver TE, Parthasarathy S, Aloia MS . Adherence to CPAP: what should we be aiming for, and how can we get there? Chest. 2019. ; 155 ( 6 ): 1272 – 1287. [DOI] [PubMed] [Google Scholar]
- 4. Gottlieb DJ, Punjabi NM . Diagnosis and management of obstructive sleep apnea: a review . JAMA. 2020. ; 323 ( 14 ): 1389 – 1400. [DOI] [PubMed] [Google Scholar]
- 5. Verse T, Kroker BA, Pirsig W, Brosch S . Tonsillectomy as a treatment of obstructive sleep apnea in adults with tonsillar hypertrophy . Laryngoscope. 2000. ; 110 ( 9 ): 1556 – 1559. [DOI] [PubMed] [Google Scholar]
- 6. Sutherland K, Vanderveken OM, Tsuda H, et al . Oral appliance treatment for obstructive sleep apnea: an update . J Clin Sleep Med. 2014. ; 10 ( 2 ): 215 – 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramar K, Dort LC, Katz SG, et al . Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015 . J Clin Sleep Med. 2015. ; 11 ( 7 ): 773 – 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zaghi S, Holty J-EC, Certal V, et al . Maxillomandibular advancement for treatment of obstructive sleep apnea: a meta-analysis . JAMA Otolaryngol Head Neck Surg. 2016. ; 142 ( 1 ): 58 – 66. [DOI] [PubMed] [Google Scholar]
- 9. Friedman M, Salapatas AM, Bonzelaar LB . Updated Friedman staging system for obstructive sleep apnea . Adv Otorhinolaryngol. 2017. ; 80 : 41 – 48. [DOI] [PubMed] [Google Scholar]
- 10. Thaler E, Schwab R, Maurer J, et al . Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy . Laryngoscope. 2020. ; 130 ( 5 ): 1333 – 1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sutherland K, Phillips CL, Davies A, et al . CPAP pressure for prediction of oral appliance treatment response in obstructive sleep apnea . J Clin Sleep Med. 2014. ; 10 ( 9 ): 943 – 949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee CH, Seay EG, Walters BK, Scalzitti NJ, Dedhia RC . Therapeutic positive airway pressure level predicts response to hypoglossal nerve stimulation for obstructive sleep apnea . J Clin Sleep Med. 2019. ; 15 ( 8 ): 1165 – 1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Landry SA, Joosten SA, Eckert DJ, et al . Therapeutic CPAP level predicts upper airway collapsibility in patients with obstructive sleep apnea . Sleep. 2017. ; 40 ( 6 ): zsx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF ; for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed . Westchester, IL: : American Academy of Sleep Medicine; ; 2007. . [Google Scholar]
- 15. Sforza E, Petiau C, Weiss T, Thibault A, Krieger J . Pharyngeal critical pressure in patients with obstructive sleep apnea syndrome. Clinical implications . Am J Respir Crit Care Med. 1999. ; 159 ( 1 ): 149 – 157. [DOI] [PubMed] [Google Scholar]
- 16. Deshpande S, Joosten S, Turton A, et al . Oronasal masks require a higher pressure than nasal and nasal pillow masks for the treatment of obstructive sleep apnea . J Clin Sleep Med. 2016. ; 12 ( 9 ): 1263 – 1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Masa JF, Jiménez A, Durán J, et al . Alternative methods of titrating continuous positive airway pressure: a large multicenter study . Am J Respir Crit Care Med. 2004. ; 170 ( 11 ): 1218 – 1224. [DOI] [PubMed] [Google Scholar]
- 18. O’Connor C, Thornley KS, Hanly PJ . Gender differences in the polysomnographic features of obstructive sleep apnea . Am J Respir Crit Care Med. 2000. ; 161 ( 5 ): 1465 – 1472. [DOI] [PubMed] [Google Scholar]
- 19. Tang AL, Cohen AP, Benke JR, Stierer KD, Stanley J, Ishman SL . Obstructive sleep apnea resolution in hypopnea- vs apnea-predominant children after adenotonsillectomy . Otolaryngol Head Neck Surg. 2016. ; 155 ( 4 ): 670 – 675. [DOI] [PubMed] [Google Scholar]
- 20. Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA . Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea . J Clin Sleep Med. 2015. ; 11 ( 8 ): 861 – 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Op de Beeck S, Wellman A, Dieltjens M, et al . Endotypic mechanisms of successful hypoglossal nerve stimulation for obstructive sleep apnea . Am J Respir Crit Care Med. 2020. ;203(6):674–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oksenberg A, Arons E, Froom P . Does the severity of obstructive sleep apnea predict patients requiring high continuous positive airway pressure? Laryngoscope. 2006. ; 116 ( 6 ): 951 – 955. [DOI] [PubMed] [Google Scholar]
- 23. Younes M . Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea . Am J Respir Crit Care Med. 2003. ; 168 ( 6 ): 645 – 658. [DOI] [PubMed] [Google Scholar]
- 24. Wellman A, Jordan AS, Malhotra A, et al . Ventilatory control and airway anatomy in obstructive sleep apnea . Am J Respir Crit Care Med. 2004. ; 170 ( 11 ): 1225 – 1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miljeteig H, Hoffstein V . Determinants of continuous positive airway pressure level for treatment of obstructive sleep apnea . Am Rev Respir Dis. 1993. ; 147 ( 6 Pt 1 ): 1526 – 1530. [DOI] [PubMed] [Google Scholar]
- 26. Hoffstein V, Mateika S . Predicting nasal continuous positive airway pressure . Am J Respir Crit Care Med. 1994. ; 150 ( 2 ): 486 – 488. [DOI] [PubMed] [Google Scholar]
- 27. Oliver Z, Hoffstein V . Predicting effective continuous positive airway pressure . Chest. 2000. ; 117 ( 4 ): 1061 – 1064. [DOI] [PubMed] [Google Scholar]
- 28. Rowley JA, Tarbichi AGS, Badr MS . The use of a predicted CPAP equation improves CPAP titration success . Sleep Breath. 2005. ; 9 ( 1 ): 26 – 32. [DOI] [PubMed] [Google Scholar]
- 29. Isono S, Morrison DL, Launois SH, Feroah TR, Whitelaw WA, Remmers JE . Static mechanics of the velopharynx of patients with obstructive sleep apnea . J Appl Physiol. 1993. ; 75 ( 1 ): 148 – 154. [DOI] [PubMed] [Google Scholar]
- 30. Schwartz AR, O’Donnell CP, Baron J, et al . The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity . Am J Respir Crit Care Med. 1998. ; 157 ( 4 ): 1051 – 1057. [DOI] [PubMed] [Google Scholar]
- 31. Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL . Neuromechanical control of upper airway patency during sleep . J Appl Physiol 1985. 2007. ; 102 ( 2 ): 547 – 556. [DOI] [PubMed] [Google Scholar]
- 32. Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S . Upper airway pressure-flow relationships in obstructive sleep apnea . J Appl Physiol. 1988. ; 64 ( 2 ): 789 – 795. [DOI] [PubMed] [Google Scholar]
- 33. Weaver TE, Kribbs NB, Pack AI, et al . Night-to-night variability in CPAP use over the first three months of treatment . Sleep. 1997. ; 20 ( 4 ): 278 – 283. [DOI] [PubMed] [Google Scholar]
- 34. Weaver TE, Grunstein RR . Adherence to continuous positive airway pressure therapy: the challenge to effective treatment . Proc Am Thorac Soc. 2008. ; 5 ( 2 ): 173 – 178. [DOI] [PMC free article] [PubMed] [Google Scholar]