Abstract
The disruption of glucose homeostasis associated with the use of nicotine delivery systems may be due to a shift to lipid metabolism. Indirect calorimetry was used to measure the respiratory exchange ratio (RER) in female (N = 21) and male (N = 21) C57BL/6J mice exposed to room air (control) or e-cigarette vapor in a 1L chamber to test the hypothesis that lipid metabolism predominates in vaped mice. Metabolism was quantified via RER using a GA-200 gas analyzer (iWorx, Inc) and LabScribe v.4 (iWorx, Inc.) software. Blood glucose levels were assessed from a subset of the population using an Accu-Check glucometer (Roche Diagnostics, Inc.). Statistical analyses were conducted using R v.4.0.3. Median RER for controls was lower in females. Older females showed a reduction in RER when exposure occurred in the afternoon (p < 0.001), and in males when exposure occurred in the morning (p = 0.007). Glucose concentrations (mg/dL) were higher after e-cigarette inhalation compared with controls, but this difference was not significant (p = 0.464). The reduction in the respiratory exchange ratio supports the hypothesis that e-cigarette inhalation promotes lipid metabolism, and the magnitude of the effect is influenced by gender, age and time of day.
Keywords: Electronic cigarette, Metabolism, Respiratory exchange ratio, Indirect calorimetry, C57BL/6J mice, Gender
Highlights
-
•
A reduction in RER followed inhalation of secondary e-cigarette vapor by female and male C57BL/6J mice.
-
•
Metabolic changes following e-cigarette inhalation were influenced by the time of day that exposure occurred.
-
•
Reductions in the respiratory exchange ratio after e-cigarette vapor inhalation were more evident in older animals.
1. Introduction
The use of electronic cigarettes (ENDS) in the U.S. is widespread across several age groups (e.g. Ref. [1]. Though gaps in our understanding exist, there is a building consensus regarding the influence of e-cigarette components on the body. Chronic inhalation of e-cigarette vapor can exert a myriad of effects, including on the cardiovascular, immune, and pulmonary systems [[2], [3], [4]] and cancer risk [5]. Physiological effects from e-cigarette use are evident after 3 consecutive days of exposure [6].
Despite their growing popularity, the possible impacts of e-cigarette use on metabolism are not fully understood [3] but hypotheses can be informed by studies with tobacco. Tobacco is a suspected agent of metabolic dysfunction [7,8]. Chronic tobacco users have an increased risk of developing Type II diabetes [8] and use is associated with dyslipidemia [7] and hyperglycemia [9]. Metabolic dysfunction following tobacco use may be because of nicotine, which promotes lipolysis, leading to insulin resistance and imbalances in blood glucose homeostasis [8,10].
Both tobacco and electronic cigarettes have chemical profiles that include nicotine at varying concentrations suspended in other compounds [11,12]. Most volatile compounds are likely disseminated via secondary vapor and not inhaled [13] but the concentration of nicotine inhaled via secondary vapor is often significant [13], so that the effect of nicotine on metabolism would be similar for both electronic and tobacco cigarettes [13]. Nicotine suppresses the appetite center in the hypothalamus, which activates the sympathetic nervous system to increase energy expenditure [3] by increasing the rate of lipolysis. The predominance of lipids decreases the cellular use of carbohydrates, promoting dysfunction in glucose homeostasis [7,8,14]. If chronic e-cigarette use promotes lipolysis, a shift to lipid metabolism should be detectable over a period of inhalation. In a 12-week study of e-cigarette on C57BL/6J mice [15], found no association between inhalation and insulin resistance, though a response may not be evident in this time frame, and metabolic parameters (i.e., RER) were not measured. A focused, longer-term investigation may provide a more definitive understanding of the metabolic consequences of e-cigarette use.
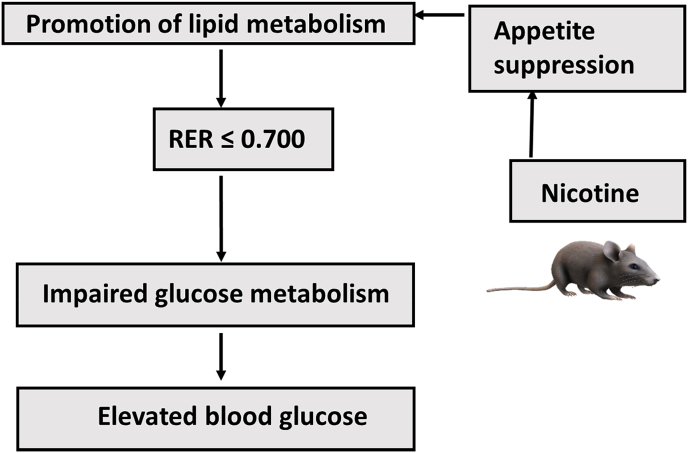
We investigated whether a shift in metabolism following e-cigarette use is observable in C57BL/6J mice using indirect calorimetry [16,17], in which the respiratory exchange ratio (RER) parameter represents the volumes of CO2 produced to O2 consumed per minute to elucidate the predominant metabolic pathway. Lipid metabolism will yield an RER ≤0.70 and carbohydrate metabolism should yield an RER >> 0.70 [17]. This physiological association allows us to test the hypothesis: if inhalation of e-cigarette vapor containing nicotine shifts the body cells to lipid metabolism, then inhalation should produce an RER ≤0.7 in test subjects (Fig. 1). The possible effect of e-cigarette inhalation on glucose metabolism was also assessed in a subset of the study population.
Fig. 1.
Hypothesized connection between nicotine use and metabolism. Nicotine use may shift body cells to lipid – based metabolism, resulting in a respiratory exchange ratio (RER) ≤ 0.700 and elevated blood glucose concentration (mg/dL).
2. Subjects and methods
2.1. Animal subjects
All animal research was conducted under approved IACUC protocols (#DC-2018-1, #DC-2019-1, #DC-2020-1). We examined the metabolic effects of secondary e-cigarette inhalation using the C57BL/6J rodent model. The average life expectancy for C57BL/6J ranges from 2 to 3.5 years [18,19]. The strains' unique longevity and susceptibility to metabolic dysfunction make it ideal for protracted examinations of metabolic dynamics [20]. The C57BL/6J also exhibits a phenotypic response similar to humans [21].
Care of C57BL/6J mice was performed using recommended guidelines [22]. All mice were housed in boxes 11-1/2″ long x 7-1/2″ wide x 5″ deep and covered with stainless steel wire bar lid that accepts a water bottle and rodent chow. Exercise wheels were not provided. The photoperiod is set with an automatic timer to 12 hours of daylight followed by 12 hours of darkness. The room temperature was set at 72 o F or 22 o C (65–75 o F or 18–24 o C), as recommended by Humane Society of America. Animals are maintained on Laboratory Diet 5001 (LabDiet, Inc.) provided ad libitum. Lab Diet 5001 maintains body weight and health with a lower protein (23%) and fat content (4.5%). Water was provided ad libitum.
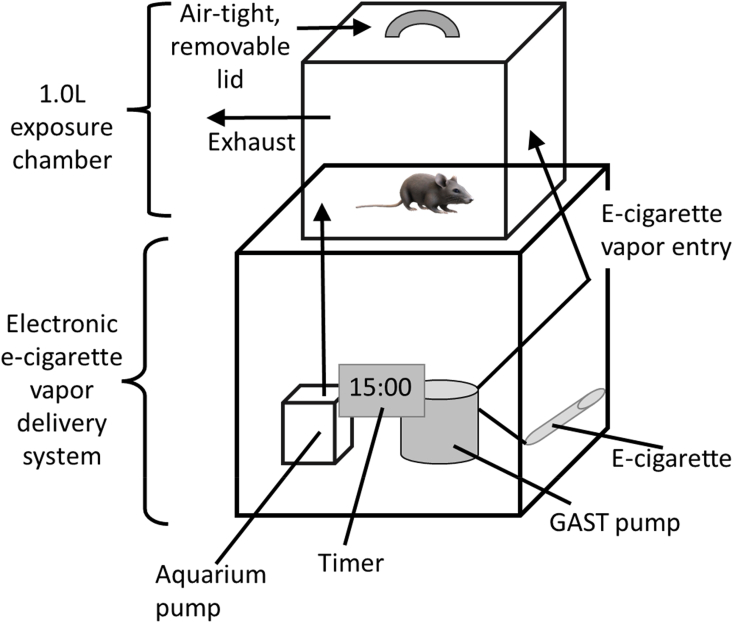
A random sample of C57BL/6J mice were selected from a total population of 75 animals (36 ♀, 37♂) to produce a study population of 42 animals divided into two study groups of equivalent size, gender and age distribution. Selection produced two groups of 21 females and 21 males. Mice were aged between 33 and 855 days at the start of the research. Each animal was exposed to room air (control) or e-cigarette treatments while housed in a 1L exposure chamber (Fig. 2) for 15 minutes. Three sets of control data were collected to optimize detection of any physiological effect [6]. Inhalation of e-cigarette vapor by each C57BL/6J mouse occurred over four consecutive days to emulate patterns of human use [6]. Outlier detection was completed using boxplot functions in the ggplot2 package and the interquartile (IQR) range criterion. Values that fell below the minimum Q1 (i.e. Q1 – 1.5*IQR) or above Q3 (i.e. Q3 + 1.5*IQR) were removed. This process was continued for 16- months until experimental data was obtained for the entire study population.
Fig. 2.
A basic schematic of the 1.0L exposure chamber depicting the mechanism used to pump e-cigarette vapor from the e-cigarette into the 1L chamber. Details in text.
2.2. Experimental approach
Control and e-cigarette treatment data were obtained from each test subject. Animals were initially placed in the exposure chamber on three separate occasions without data collection to facilitate acclimation to the experimental settings. Following, three sets of control data were collected per animal, the minimum number recommended to detect physiological effects [6].
Electronic cigarette inhalation data was obtained by exposing animals to secondary e-cigarette vapor over a consecutive 4-day period. A 15-s “puff” of 18 mg/mL nicotine reference cigarette (VaporFi) was pumped into the chamber and allowed to circulate in the exposure chamber for an additional 15-min. Given the average human puff is 4 seconds [[23], [24], [25]], this exposure represents about 4 puffs. The VaporFi Classic electronic cigarette containing 1.8% nicotine by volume (but see Ref. [26] was connected to a Gast 10D DC Series Miniature vacuum pump with a flow rate capacity of 4.3 L/min at 21oC (70oF) and zero pressure (https://gastmfg.com/). When operating at 21oC (70oF), the flow rate averages 2.2 L/min. Under these conditions, the pump vaporizes about 18 mg of e-liquid per puff; each puff containing about 2 μg/mg nicotine, 8 mg vegetable glycerol, and 11 mg propylene glycol [27]. E-cigarette vapor was exhausted from the chamber following exposure using a 120-V aquarium pump (Fig. 2). Animals were shifted into a metabolic chamber (iWorx, Inc.) and metabolic (RER) data were measured using a GA-200 analyzer (iWorx Systems, 2013). Gas sensors were calibrated prior to experiments with gas standards containing known concentrations of O2, CO2, and N2 (iWorx, Inc.). The ratio of VCO2/VO2 (RER) was reported using LabScribe v.4.0 (iWorx, Inc.). The average RER for control and e-cigarette treatments for each animal was used in subsequent statistical analyses.
Blood glucose levels were assessed for control and vaped mice across a subset of the study population. After a 6-h period of fasting, each animal was placed in a restraining tube to obtain ∼2 mL volume of blood from the caudal vein and blood glucose levels (mg/dL) were measured using the Accu-Check glucometer (Roche Diagnostics, Inc.).
2.3. Statistical analyses
Statistical analyses examined the effect of independent variables on each dependent variable using R v.4.0.3 (R Foundation for Statistical Computing, 2017). The Shapiro-Wilks test was used to evaluate data normality and density plots were calculated with the Rcpp and ggplot2 packages. Correlation tests were conducted using Spearman's Rank correlation ρ statistic. Variables included the respiratory exchange quotient (RER), a binary variable TX that represented either control or e-cigarette treatment groups. The variable EXP_TURN represented the number of consecutive days of e-cigarette exposure, to emulate the multi-day use patterns of humans [6]. Other variables included animal weight, relative time of day (AM_PM), the number of days since the last exposure (DAYS_SINCE) and age (days). The relationship of TX on RER was evaluated with the Wilcoxon test and the relationship of EXP_TURN on RER was evaluated using the Kruskal-Wallis test. Both tests were conducted on the full dataset and on subsets of data partitioned by gender and age with the dplyr package.
The relationship of independent variables on the factor TX was evaluated using two techniques. A model to predict the probability of correctly classifying TX from the explanatory variables across all gender and age groups was constructed with binary logistic regression using the ISLR2 package. Data were first randomly divided into training (70%) and testing (30%) datasets. The number of explanatory variables in the initial regression model was reduced in subsequent models to reduce overfitting. The recursive classification partitioning algorithm in Random Forests [28] was used to evaluate the effect of independent variables on TX, first using the complete dataset and again with subsets of data defined by gender and age. Data were randomly partitioned into training (70%) and testing (30%) samples. Training data were bootstrapped with replacement to build an ensemble of 500 trees to predict the classification of the testing data. Three metrics were used to evaluate the accuracy of each model: the mean decrease in the Gini Index, the Out-of-Bag (OOB) error and the area under the ROC curve (AUC) statistic and an AUC ≥0.7 indicates robust model discrimination [29].
3. Results
3.1. Effect of e-cigarette inhalation on study sample
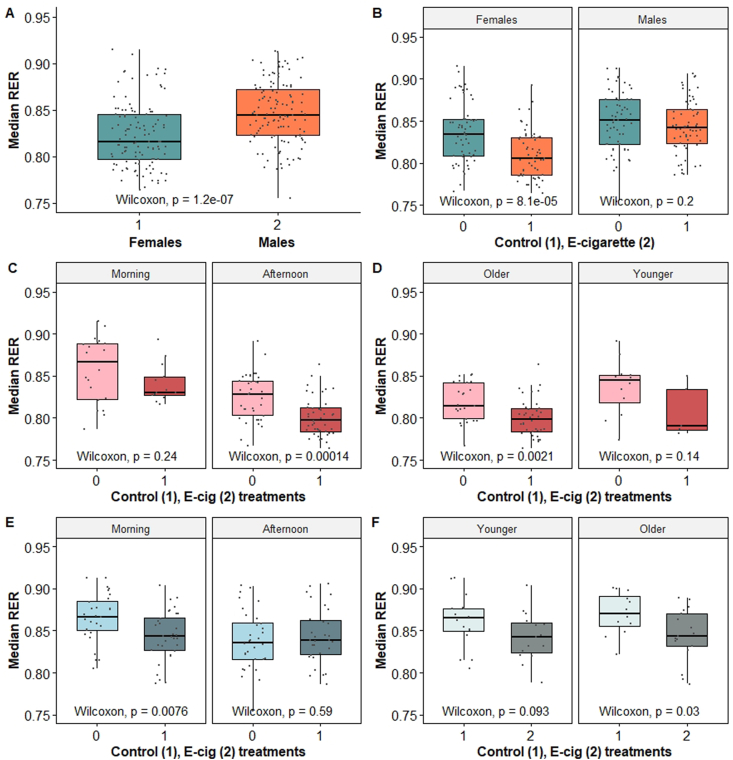
Metabolic data was collected from 42 individuals (21 ♀, 21 ♂) to yield 254 records. Data not representative of a minimum of three control trials and at least four consecutive experimental trials per animal were excluded to minimize error. This procedure resulted in a final dataset of 235 records, consisting of control (N = 117) and e-cigarette exposure (N = 118) treatments from 21 females (N = 113) and 21 males (N = 122) that ranged in age from 33 to 855 days (equivalent to roughly 10–70 years in humans [30]; Table 1). A significant departure from normality was reported for RER (W = 0.977, p = 0.006), CO2 (W = 0.941, p = 0.001) and O2 (W = 0.972, p = 0.002). Median RER across genders was 0.834 ± 0.037, was significantly lower for females (Fig. 3A) and was significantly different between control and e-cigarette treatments for females (Fig. 3B). Results of the multiple binary logistic regression indicated that animals with a lower median RER had higher odds of being in the e-cigarette treatment group (OR = 0.001, 95% CI: 0.00 to 0.003; p = 0.006). For each unit of increase in the value of RER, the log odds of an animal being in the e-cigarette treatment group decreased by 16.18. A classification analysis of independent variables on TX showed that age, RER, time of day and gender were important predictors (OOB = 19.39%, AUC = 0.777; Table 2). There was a slight but non-significant trend for median RER to increase with the number of days elapsed since the last e-cigarette exposure. The effect of e-cigarette exposure on metabolism was next examined in each gender separately to control for the effect of gender.
Table 1.
Sample sizes for data examined in study.
| Treatment | Females (Total, AM/PM) | Males (Total, AM/PM) |
|---|---|---|
| None (controls) | 57, 20/37 | 60, 28/32 |
| E-cigarette exposure | ||
| Total | 56, 13/43 | 62, 32/30 |
| 1 | 15, 5/10 | 17, 10/7 |
| 2 | 15, 3/12 | 13, 7/6 |
| 3 | 14, 3/11 | 14, 6/8 |
| 4 | 12, 2/10 | 10, 4/6 |
| 5 | – | 8, 5/3 |
| Total sample size | 113 | 122 |
Fig. 3.
(A) Median RER across all treatments was significantly lower for females, and (B) median RER was significantly lower following e-cigarette inhalation only for females. (C) Variation in median RER as a function of treatment in females during morning and afternoon hours, and in PM females (D). (E) Variation in median RER in males during morning and afternoon hours and (F) in AM males as a function of relative age. Mouse age categories were determined by classification analysis of independent variables on treatment (TX). Treatment groups are defined as control (TX = 1) and e-cigarette exposure (TX = 2).
Table 2.
Predictors based on variable importance in classification models of treatment (TX; control and e-cigarette treatments) as a function of RER, gender, age and time of day. Variables as defined in text.
| Model | Model parameter | Mean decrease in Gini Index |
|---|---|---|
| All | Age | 25.54 |
| RER | 23.98 | |
| Time of Day | 12.53 | |
| Gender | 11.04 | |
| Females | RER | 21.27 |
| Age | 18.74 | |
| Time of day | 9.41 | |
| AM Females | RER | 18.25 |
| Age | 17.44 | |
| PM Females | RER | 20.61 |
| Age | 10.16 | |
| Males | Age | 44.99 |
| Time of day | 11.99 | |
| RER | 8.99 | |
| AM Males | RER | 9.56 |
| Age | 19.95 | |
| PM Males | Age | 27.56 |
| RER | -3.20 |
3.2. Effect of e-cigarette inhalation on females
The effects of e-cigarette exposure on respiratory parameters and metabolism were evaluated in 21 females that ranged in age from 33 to 855 days and represented both control (N = 57) and experimental (N = 56) data (Table 1). Normality was violated for respiratory exchange quotient (RER; W = 0.976, p = 0.018), O2 (W = 0.942, p = 0.015) and CO2 (W = 0.892, p < 0.001; Fig. A1). Age and weight were significantly correlated (rho = 0.407, p = 0.006) and weight was excluded in subsequent analyses. Classification analysis of independent variables on TX showed that RER and age were the top two variables ranked for importance, followed by time of day (OOB = 26.86%, AUC = 0.735; Table 2). Median RER was significantly greater (p < 0.001) in controls (0.835 ± 0.035) compared to e-cigarette (0.805 ± 0.028) treatments across age groups (Fig. 3B) and ranged from 0.828 in “older” (≥509 days) females to 0.848 in “younger” females (>509 days). Median RER following e-cigarette treatment was significantly lower (p = 0.001) in older females (N = 77) compared to younger females (N = 36). Median RER collected during the morning hours was greater for both control (0.867 ± 0.038) and e-cigarette (0.830 ± 0.023) treatments compared with the same treatments in the afternoon (0.828 ± 0.027 and 0.797 ± 0.023, respectively) and these differences were significant (p = 0.003, controls; p < 0.001, e-cigarette exposure). Data for females was divided into “AM” (N = 33) and “PM” (N = 80) groups for subsequent analyses.
The AM female data was composed of 33 records collected from 11 individual animals and separated into control (N = 20) and e-cigarette exposure (N = 13) treatment data. Animals ranged in age from 46 to 854 days. Values of RER ranged from 0.787 to 0.915, with a median of 0.845 (±0.034). The classification tree with the best support (OOB error = 12.12%; AUC = 0.954) for predicting TX included RER and age (Table 2). A mouse age of ≥509 days or ˂ 509 days divided control and e-cigarette treatment groups into “older” and “younger” females with similar sample sizes for older (N = 14) and younger (N = 19) females. Most females (60%) in the control group demonstrated a median RER ≥0.875 while 92% of the vaped females had a median RER <0.875. Control females with a median RER ≥0.875 were aged >509 days (“Older”). Median RER across age groups was lower in the experimental group (0.830 ± 0.023) compared with controls (0.866 ± 0.038) but this difference was not significant (W = 162.5, p = 0.238; Fig. 3C). No significant difference for median RER between controls and one or more consecutive e-cigarette exposures was observed (H (4) = 4.06, p = 0.700).
The PM female data was composed of 80 records across control (N = 37) and e-cigarette treatment (N = 43) groups from 19 animals that ranged in age from 33 days to 855 days. Values of RER ranged from 0.764 to 0.891, with a median of 0.808 ± 0.027. Median RER was lower in animals exposed to e-cigarette vapors (0.797 ± 0.023) compared with controls (0.828 ± 0.027) and this difference was significant (W = 1191, p < 0.001; Fig. 3C). The tree with the best support for predicting TX included RER and age (OOB error = 22.86%, AUC = 0.888; Table 2). Classification analysis divided the sample population into older females aged ≥534.5 days and younger females ˂ 534.5 days, and the sample size was greater for older (N = 63) than for younger (N = 17) females. Most of the females (92%) in the control group demonstrated a median RER ≥0.808. A majority (66%) of the vaped females demonstrated a median RER <0.808, including a significantly reduced median RER in older females (W = 1191.0, p = 0.002). The median RER for older females in the e-cigarette treatment group (0.801 ± 0.022) was significantly lower compared to older females in the control group (0.829 ± 0.036; Fig. 3D). Median RER across all ages declined significantly with the number of consecutive e-cigarette exposures compared with control data (H (4) = 16.2, p = 0.003).
3.3. Effect of e-cigarette inhalation on males
The effects of e-cigarette inhalation on metabolism were evaluated in 21 male C57BL/6J mice that ranged in age from 33 to 796 days and represented both control (N = 60) and experimental (N = 62) data (Table 1). Tests for normality were violated for O2 (W = 0.979, p = 0.051) and CO2 (W = 0.971, p < 0.001; Fig. A1). Age was correlated with weight (rho = 0.717, p < 0.001) and weight was removed from subsequent analyses. Values for RER ranged from 0.755 to 0.914 and a median of 0.845 ± 0.037. Classification analysis of independent variables on TX showed that age and time of day were the top two variables ranked for importance, followed by RER (OOB = 17.21%, AUC = 0.812; Table 2). About 28% of control males were <60.5 days old and about 28% of experimental males were >780 days. Median RER was not significantly different between control and experimental groups (W = 2030, p = 0.385), or for animals repeatedly exposed to e-cigarette vapor compared to controls (H (5) = 7.22, p = 0.204). Median RER was significantly greater for controls for morning hours (0.866 ± 0.028) compared to the afternoon (0.836 ± 0.035; W = 646.5, p = 0.003). Data was divided into “AM” (N = 60) and “PM” (N = 62) groups for subsequent analyses.
The “AM” male group consisted of 60 records for control (N = 28) and e-cigarette exposure (N = 32) data from sixteen males that ranged in age from 33 to 796 days. Median RER was significantly lower in the experimental group (0.843 ± 0.029) compared with controls (0.867 ± 0.028; W = 578.5, p = 0.007; Fig. 3E). The median RER value was significantly lower in AM males that were vaped two or four times consecutively compared to controls (H (5) = 11.18, p = 0.025). The tree with the best support for predicting TX included RER and age (OOB error = 21.67%) with an AUC value of 0.713 (Table 2). Classification analysis divided data into “older” (≥737.5 days old) and “younger” (<737.5 days) groups. The median RER for older males in the e-cigarette treatment group (0.837 ± 0.031) was significantly lower compared to older males in the control group (0.868 ± 0.029; Fig. 3F). The median RER, though lower for younger males in the e-cigarette treatment group, was marginally significantly different to similarly aged males in the control group (p = 0.09; Fig. 3F).
The “PM” group consisted of 62 records composed of control (N = 32) and experimental N = 30) data from twenty males that ranged in age from 34 to 795 days. Median RER values for control (0.836 ± 0.035) and experimental groups (0.839 ± 0.034) were not significantly different (W = 441, p = 0.587). The tree with the best support for predicting TX included animal age and RER (OOB error = 32.14%; AUC = 0.838; Table 2). Classification analysis of independent variables on TX divided PM males into two groups composed of older (≥720 days old) and “younger” (<720 days old) males. Most of the control males (69%) demonstrated a median RER ≥0.817 and 34% of these animals were young animals with an RER >0.835. Most (83%) of the vaped males demonstrated an RER <0.817 and 56% of these males were older than 712 days.
3.4. Effect of e-cigarette inhalation on blood glucose
Blood glucose (mg/dL) was obtained for control and e-cigarette treatments from five animals (3 females; 2 males) still alive after indirect calorimetry data collection ended. Glucose concentration data (mg/dL) were distributed normally (Controls, W = 0.928, p = 0.583; E-cigarette, W = 0.924, p = 0.561) and were greater, on average, after e-cigarette inhalation (165.8) compared with controls (156.1), but this difference was not significant (t = −0.793, df = 4.89, p = 0.464).
4. Discussion
Conclusions regarding the effect of e-cigarette inhalation on metabolism in C57BL/6J mice are tentative due to several limitations. Nicotine, a major constituent of secondary vapor [13], may be the causal agent behind the shift to lipid metabolism, but quantification of chemical compounds in the exposure chamber is needed. The focus of the current work was to assess the effect of e-cigarette inhalation on the respiratory exchange ratio (RER), but the collection of additional endpoints such as lipid profiles and metabolic tolerance tests are planned for future work to refine the physiological response.
Secondary e-cigarette vapor inhalation by female and male C57BL/6J mice led to a reduction of the respiratory exchange ratio (RER). Though a median RER ≤0.700, indicative of lipid metabolism, was not observed in this study, this result appears broadly consistent with a shift to lipid metabolism that is predicted with cigarette use [3,7,8,31]. An increasing but non-significant trend in blood glucose concentrations after e-cigarette inhalation was observed, consistent with a shift to lipid metabolism, but conclusions are tentative until additional data can be examined. The reduction in median RER cannot be attributed to food because all animals in the study received the same diet. Instead, RER reductions were uneven between treatments because of the interaction with gender, age, and time of day.
Gender differences in RER were observed in the control data. Median RER was greater for controls across genders and was greater overall for males (Fig. 3A and B). Males preferentially use carbohydrates to meet the significant oxygen demands of muscular tissue of a greater body mass [[32], [33], [34]]. Lipids are the primary substrate for energy generation in females [[32], [33], [34], [35], [36]].
The lower RER in older C57BL/6J female controls (Fig. 3) may be due to several factors. The reduction in RER is often attributed to physical exercise that favors lipid mobilization for fuel [37,38]. Though older control animals tend to be more sedentary [34,35], neurological changes associated with dementia may increase restlessness and lead to lower RER values, especially at night [39]. The reduced RER in control females could also be due to a shift to lipid metabolism, possibly linked to declining estrogen [32,34,36,40]. It is also possible that older females are more active during a time of day that was not monitored in the study. Activity in older males often peaks late at night [41]. Distinguishing among the several alternative hypotheses will require modifications of the study design including 24-h observations of animal activity.
Median RER for males in the control group was greater during the morning (0.865 ± 0.028) than for afternoon (0.836 ± 0.035). Males prefer carbohydrates, are more physically active in the morning [42], so a greater median RER at that time is expected. The greater levels of physical activity expected in younger animals [41] is also reflected by the greater median RER observed in younger control males.
Female C57BL/6J demonstrated a significantly lower median RER after e-cigarette inhalation (Fig. 3). These results are attributed to the effect of nicotine, which was the only compound found at high concentration in the exposure chamber (Curtis and Corbin, unpublished data) and the concentration of nicotine in secondary vapor is often significant [13]. Nicotine is known to exert gender-specific effects on metabolism (e.g. Refs. [7,43,44]. By suppressing the appetite, nicotine favors lipolysis [3] and a decrease in RER is expected because more O2 is required to catabolize lipid compounds in body cells [17].
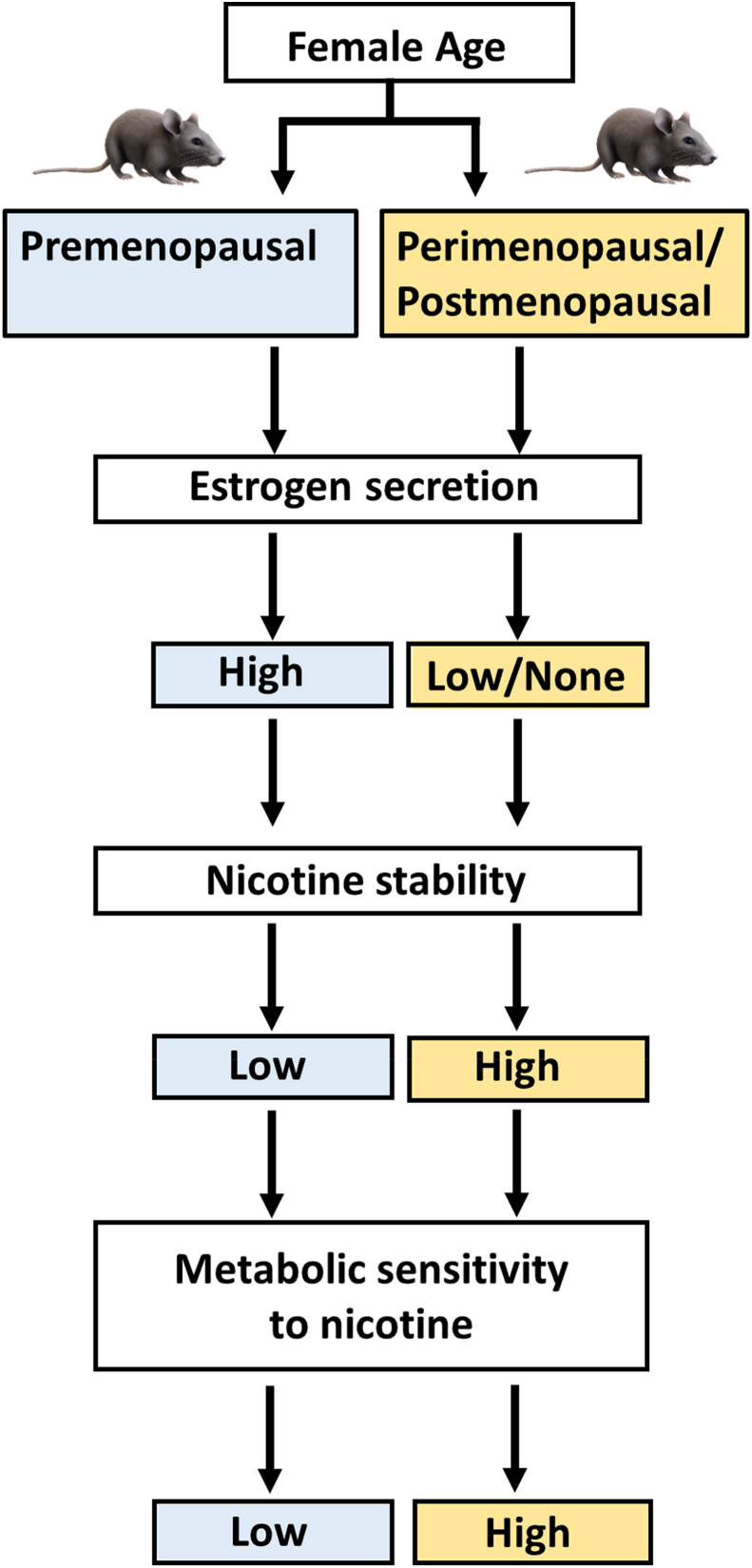
The further reduction in RER following e-cigarette inhalation was observed in older females may be due to hormonal changes associated with the aging process. One of the main physiological actions of nicotine is to stimulate lipolysis and lipid metabolism [3,7,8,31]. Female sex hormones may regulate the action of nicotine and nicotine, which in turn can influence metabolism (Fig. 1; [[32], [33], [34]]. In females, nicotine is metabolized at a faster rate, likely due to the influence of estrogen [44,45]. At the same time, nicotine reduces plasma estrogen in premenopausal women [40] so that the overall effect of nicotine on lipid metabolism depends on the balance of nicotine and estrogen. Nicotine metabolism is expected to slow as estrogen secretion declines in post-menopausal mice beginning at about age 480 days (∼1.3 years; [34]. The lower rate of nicotine metabolism in post-menopausal females translates to a lower rate of degradation of nicotine into cotinine so that a smaller amount of nicotine can exert observable pharmacological [45], and possibly metabolic effects (Fig. 4). The faster rate of nicotine degradation expected in younger females means that a greater concentration of nicotine may be necessary to produce a significant metabolic shift. Future studies could include assignment of treatment groups defined by their exposure to different concentrations of e-cigarette vapor. This modification would help evaluate the effect of a varied e-cigarette “burden” on metabolism as a function of gender and age. The measurement of plasma cotinine levels could also help quantify the clearance rate of nicotine as a function of age.
Fig. 4.
Hypothesized connections between age, nicotine metabolism and RER in female C57BL/6J examined in the current study.
Analyses of current data also support a “time of day effect” for females. Females exposed to e-cigarette vapor in the afternoon demonstrated a greater reduction in median RER (0.797) compared to females tested during the morning hours (0.830; Fig. 3C), though the difference in median RER between controls and exposed animals was only significant in the afternoon (Fig. 3C). Diurnal variability in metabolism is a widely reported phenomenon, including variation in basal metabolic rate and the exchange of respiratory gases [16,42]. Higher O2 consumption and increased physical activity during the morning hours is reported in female C57BL/6J [42] and female rats [46,47]. Conclusions about how the time of day of e-cigarette inhalation may influence metabolism are tentative until a greater sample size can be evaluated, especially for AM comparisons (Table 1) where there were uneven sample sizes between young (Y) and old (O) females for AM data (Y = 14, O = 19) and PM data (Y = 17, O = 63). The greater proportion of older females in the PM data may explain the lower RER for afternoon exposure (Fig. 3D). Future work will require greater sample sizes distributed more equally across experimental treatments.
The effect of e-cigarette inhalation on RER in males was influenced by time of day and age. A significant decrease in RER for males was documented when inhalation occurred in the morning, despite similar sample sizes for AM/PM and older/younger age comparisons. Males may have a greater nicotine sensitivity in the morning due to an abstinence interval as found in studies with human subjects [48]. Older males (aged ≥737.5 days) demonstrated the greatest RER reduction during the morning, like the pattern observed in older females (Fig. 3E and F). The reduced RER in older males during the morning may be due to a combination of nicotine sensitivity and the effect of nicotine on physiology. Nicotine has a dose-dependent effect on reducing plasma testosterone [49]. Normal testosterone production decreases with age, leading to changes in body composition that can precipitate the development of metabolic syndrome [50]. Nicotine inhalation in older males further reduces plasma testosterone, making the shift to lipid metabolism more likely.
Study findings advance the understanding of e-cigarette effects on metabolism in several ways. Inhalation of e-cigarette vapor by C57BL/6J produced effects that are expected to mirror those in humans [3,9]. The study adds to the handful of studies [15,51] that reveal metabolic impacts from e-cigarette use. Our results show that metabolic impacts of e-cigarette inhalation are not equal across gender or age groups. Comparatively few studies have explicitly examined gender differences in the physiological response of e-cigarette inhalation [[52], [53], [54]]. Concern about the physiological effects of e-cigarette use on adolescents has focused much on pulmonary [4,6] and neurological [55] effects. However, our study shows that metabolic effects from e-cigarette inhalation can also be considerable and be more pronounced in older animals. These findings have potentially significant ramifications for long-term e-cigarette use in human subjects and demonstrate the need for more long-term studies. Additional data in this area can help inform future policies regarding the potential health risks from e-cigarette use and how changes manifest over time.
Data statement
Data may be available upon reasonable request.
Author contributions
Dr. Dolly Crawford: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing - original draft; Writing - review & editing. Ms. Alexis Phillips: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing - original draft; Writing - review & editing. Ms. Taylor Williams: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing - original draft; Writing - review & editing.
CRediT authorship contribution statement
Dolly L. Crawford: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Alexis R. Phillips: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Taylor R. Williams: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgements
We express our gratitude to Mr. Cameron Middis for the design and construction of the e-cigarette exposure chamber. We also appreciate the funding and logistical support provided by members of the Ashland University community: specifically, the help provided by Dr. Amiel Jarstfer, Provost for the College of Arts and Sciences, and Dr. Paul Hyman, Chair of the Department of Biology and Toxicology.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2021.100150.
Contributor Information
Dolly L. Crawford, Email: dcrawfo9@ashland.edu.
Alexis R. Phillips, Email: alexisryannephillips@gmail.com.
Taylor R. Williams, Email: taylorwilliams102199@gmail.com.
Appendix A.
Fig. A1.
QQ plots of the dependent variables respiratory exchange ratio (RER), CO2 production (CO2) and O2 consumption in females (A - C), and males (D - F).
Appendix B. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Schoenborn C.A., Gindi R.M. NCHS Data Brief No; 2015. Electronic cigarette use among adults: United States, 2014; p. 217. [PubMed] [Google Scholar]
- 2.Sussan T.E., Gajghate S., Thimmulappa R.K., Ma J., Kim J.H., Sudini K., Consolini N., Cormier S.A., Lomnicki S., Hasan F., Pekosz A., Biswani S. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116861. 10.1371/journal. pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verhaegen A., Van Gaal L. Do e-cigarette induce weight changes and increase cardiovascular risk? A signal for the future. Obes Rev. 2017;18:1136–1146. doi: 10.1111/obr.12568. [DOI] [PubMed] [Google Scholar]
- 4.McAlinden K.D., Eapen M.S., Wenying L., Sharma P., Sohal S.S. The rise of electronic nicotine delivery systems and the emergence of electronic-cigarette-driven disease. Am J Physiol Lung Cell Mol Physiol. 2020;319(4):585–595. doi: 10.1152/ajplung.00160.2020. [DOI] [PubMed] [Google Scholar]
- 5.Lee H.W., Park S.H., Weng M., Wang H.T., Huang W.C., Lepor H., Wu X.R., Chen L.C., Tang M. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proc Natl Acad Sci USA. 2018;115(7):1560–1569. doi: 10.1073/pnas.1718185115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McConnell R., Barrington-Trimis J.L., Wang K., Urman R., Hong H., Unger J. Electronic cigarette use and respiratory symptoms in adolescents. Am J Respir Crit Care Med. 2017;195(8):1043–1049. doi: 10.1164/rccm.201604-0804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facchini F.S., Hollenbeck C.B., Jeppesen J., Chen Y.D., Reaven G.M. Insulin resistance and cigarette smoking. Lancet. 1992;339:1128–1130. doi: 10.1016/0140-6736(92)90730-q. [DOI] [PubMed] [Google Scholar]
- 8.Willi C., Bodenmann P., Ghali W.A., Faris P.D., Cornuz J. Active smoking and the risk of Type 2 diabetes. J Am Med Assoc. 2007;298:2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 9.Chen H., Hansen M.J., Jones J.E., Vlahos R., Anderson G.P., Morris M.J. Detrimental metabolic effects of combining long-term cigarette smoke exposure and high-fat diet in mice. Am J Physiol Endocrinol Metab. 2007;293:1564–1571. doi: 10.1152/ajpendo.00442.2007. [DOI] [PubMed] [Google Scholar]
- 10.Saponaro C., Gaggini M., Carli F., Gastaldelli A. The subtle balance between lipolysis and lipogenesis: a critical point in metabolic homeostasis. Nutrients. 2015;7(11):9453–9474. doi: 10.3390/nu7115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng T. Chemical evaluation of electronic cigarettes. Tobac Control. 2014;23:11–17. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips B., Titz B., Kogel U., Sharma D., Leroy P., Xiang Y., Vuillaume G., Lebrun S., Sciuscio D., Ho J., Nury C., Guedj E., Elamin A., Esposito M., Krishman S., Schlage W.K., Veljikovic E., Ivanov N.V., Martin F., Peitsch M.C., Hoeng J., Vanscheeuwijick P. Toxicity of the main electronic cigarette components, propylene glycol, glycerin, and nicotine, in Sprague-Dawley rats in a 90-day OECD inhalation study complemented by molecular endpoints. Food Chem Toxicol. 2017;109:315–332. doi: 10.1016/j.fct.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Czogala J., Goniewicz M.L., Fidelus B.F., Zielinska-Danch W., Travers M.J., Sobczak A. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res. 2014;16(6):655–662. doi: 10.1093/ntr/ntt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parhofer K.G. Interaction between glucose and lipid metabolism: more than diabetic dyslipidemia. Diabetes Metab J. 2015;39:353–362. doi: 10.4093/dmj.2015.39.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orimoloye O.A., Uddin S.M.I., Chen L.C., Osei A.D., Mirbolouk M., Malovichko M.V., Sithu I.D., Dzaye O., Conklin D.J., Srivastava S., Blaha M.J. Electronic cigarettes and insulin resistance in animals and humans: results of a controlled animal study and the National Health and Nutrition Examination Survey (NHANES 2013-2016) PLoS One. 2019;14(12) doi: 10.1371/journal.pone.0226744. 0.1371/journal.pone.0226744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speakman J.R. Measuring energy metabolism in the mouse – theoretical, practical, and analytical considerations. Front Physiol. 2013;4(34):1–23. doi: 10.3389/fphys.2013.00034. doi.org/10.3389/fphys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta R.D., Ramachandran R., Venkatesan P., Anoop S., Joseph M., Thomas N. Indirect calorimetry: from bench to bedside. Indian J Endocrinol Metabol. 2017;21:594–599. doi: 10.4103/ijem.IJEM_484_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mewissen D.J. Natural tumor incidence in a population of mice as a reference index. Fed Proc. 1971;30:311. [Google Scholar]
- 19.Goodrick C.L. Lifespan and the inheritance of longevity in inbred mice. J Gerontol. 1975;30:257–263. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- 20.Benedé-Ubieto R., Estévez-Vazquez O., Ramadori P., Cubero F.J., Nevzorova Y.A. Guidelines and considerations for metabolic tolerance tests in mice. Diabetes, Metab Syndrome Obes Targets Ther. 2020;13:439–450. doi: 10.2147/DMSO.S234665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pacini G., Omar B., Ahren B. 2013. Methods and models for metabolic assessment in mice; pp. 1–8. J Diabetes Res 2013. 10.1155/2013/986906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Research Council . eighth ed. The National Academies Press; Washington, DC: 2011. Guide for the care and use of laboratory animals. [DOI] [Google Scholar]
- 23.Farsalinos K.E., Romagna G., Tsiapras D., Kyrzopoulos S., Voudris V. Evaluation of electronic cigarette use (vaping) topography and estimation of liquid consumption: implications for research protocol standards definition and for public health authorities' regulation. Int J Environ Res Publ Health. 2013;10:2500–2514. doi: 10.3390/ijerph10062500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hua M., Alfi M., Talbot P. Health-related effects reported by electronic cigarette users in online forums. J Med Internet Res. 2013;15(4):e59. doi: 10.2196/jmir.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinson R.J., Hensel E.C., Morabito P.N., Roundtree K.A. Electronic cigarette topography in the natural environment. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogunwale M.A., Chen Y.C., Theis W.S., Nantz M.H., Conklin D.J., Fu X.A. A novel method of nicotine quantification in electronic cigarette liquids and aerosols. Anal Methods. 2017;9(29):4261–4266. doi: 10.1039/C7AY00501F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Havel C.M., Benowitz N.L., Jacob P., St Helen G. An electronic cigarette vaping machine for the characterization of aerosol delivery and composition. Nicotine Tob Res. 2017;19:1224–1231. doi: 10.1093/ntr/ntw147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Breiman L. Random Forests. Mach Learn. 2001;45:5–32. [Google Scholar]
- 29.Swets J.A. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- 30.Flurkey K., Currer J.M., Harrison D.E. In: The mouse in biomedical research: volume 3 Normative biology, husbandry, and models. Fox J.G., Barthold S.W., Davisson M.T., Newcomber C.E., Quimby F.W., Smith A.L., editors. Academic Press; Burlington, MA: 2007. Mouse models in aging research; pp. 637–669. [Google Scholar]
- 31.Heck J.D., Gaworski C.L., Rajendran N., Morrissey R.L. Toxicologic evaluation of humectants added to cigarette tobacco: 13-week smoke inhalation study of glycerin and propylene glycol in Fischer 344 rats. Inhal Toxicol. 2002;14:135–1152. doi: 10.1080/08958370290084827. [DOI] [PubMed] [Google Scholar]
- 32.Lamont L. Gender differences in amino acid use during endurance exercise. Nutr Rev. 2005;63(12):419–422. doi: 10.1301/nr.2005.dec.419-422. [DOI] [PubMed] [Google Scholar]
- 33.Lundsgaard A., Kiens B. Gender differences in skeletal muscle substrate metabolism- molecular mechanisms and insulin sensitivity. Front Endocrinol. 2014;5(195):1–16. doi: 10.3389/fendo.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMullan R.C., Kelly S.A., Hua K., Buckley B.K., Faber J.E., Pardo-Manuel de Villena F., Pomp D. Long-term exercise in mice has sex-dependent benefits on body composition and metabolism during aging. Phys Rep. 2016;4(21):1–16. doi: 10.14814/phy2.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houtkooper R.H., Argmann C., Houten S.M., Canto C., Jeninga E.H., Andreux P.A., Thomas C., Doenlen R., Schoonjans K., Auwerx J. The metabolic footprint of aging in mice. Sci Rep. 2011;1:134. doi: 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi H.M., Kim H.R., Kim E.K., Byun Y.S., Won Y.S., Yoon W.K., Kim H.C., Kang J.G., Nam K.H. An age-dependent alteration of the respiratory exchange ratio in the db/db mouse. Lab Anim Res. 2015;31(1):1–6. doi: 10.5625/lar.2015.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kane S.L., Garland T., Carter P.A. Basal metabolic rate of aged mice is affected by random genetic drift not by selective breeding for high early-age locomotor activity or chronic wheel access. Physiol Biochem Zool. 2008;81(3):288–300. doi: 10.1086/587093. [DOI] [PubMed] [Google Scholar]
- 38.O'Neal T.J., Friend D.M., Guo J., Hall K.D., Kravitz A.V. Increases in physical activity result in diminishing increments in daily energy expenditure in mice. Curr Biol. 2017;27:423–430. doi: 10.1016/j.cub.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joly-Amado A., Serraneau K.S., Brownlow M., de Evsikova C.M., Speakman J.R., Gordon M.N., Morgan D. Metabolic changes over the course of aging in a mouse model of tau deposition. Neurobiol Aging. 2016;44:62–73. doi: 10.1016/j.neurobiolaging.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szkup M., Jurczak A., Karakiewicz B., Kotwas A., Kopeć J., Grochans E. Influence of cigarette smoking on hormone and lipid metabolism in women in late reproductive stage. Clin Interv Aging. 2018;13:109–115. doi: 10.2147/CIA.S140487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentinuzzi V.S., Scarbrough K., Takahashi J.S., Turek F.W. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6J mice. Regul Integr Physiol. 1997;273(6):1957–1964. doi: 10.1152/ajpregu.1997.273.6.r1957. [DOI] [PubMed] [Google Scholar]
- 42.Fuhrman G.J., McLin E.D., Turner M.L. The effect of time of day on the metabolic rate of albino mice: a manometric method. Am J Physiol. 1946;147:284–288. doi: 10.1152/ajplegacy.1946.147.2.284. /abs/10.1152/ajplegacy.1946.147.2.284. [DOI] [PubMed] [Google Scholar]
- 43.Rubinstein M.L., Shiffman S., Rait M.A., Benowitz N.L. Race, gender, and nicotine metabolism in adolescent smokers. Nicotine Tob Res. 2013;15(7):1311–1315. doi: 10.1093/ntr/nts272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong G., Kuguru K., Krishnan-Sarin S. Gender differences in U.S. adolescent e-cigarette use. Curr Addict Rep. 2017;4(4):422–430. doi: 10.1007/s40429-017-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benowitz N.L., Lessov-Schlaggar C.N., Swan G.E., Jacob P. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79(5):480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Horst K., Mendel L.B., Benedict F.G. The influence of previous exercise upon the metabolism, the rectal temperature, and the body composition of the rat. J Nutr. 1934;7(3):251–275. doi: 10.1093/jn/7.3.251. [DOI] [Google Scholar]
- 47.Horst K., Mendel L.B., Benedict F.G. The effects of some external factors upon the metabolism of the rat: one figure. J Nutr. 1934;7(3):277–303. doi: 10.1093/jn/7.3.277. [DOI] [Google Scholar]
- 48.St Helen G., Ross K.C., Dempsey D.A., Havel C.M., Jacob P., Benowitz N.L. Nicotine delivery and vaping during ad libitum e-cigarette access. Tob Regul Sci. 2016;2(4):363–376. doi: 10.18001/TRS.2.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oyeyipo I.P., Raji Y., Bolarinwa A.F. Nicotine alters male reproductive hormones in male albino rats: the role of cessation. J Hum Reprod Sci. 2013;6(1):40–44. doi: 10.4103/0974-1208.112380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawano H. The relationship between testosterone and metabolic syndrome. Hypertens Res. 2010;33(6):537–538. doi: 10.1038/hr.2010.52. [DOI] [PubMed] [Google Scholar]
- 51.Golli N.El., Dkhili H., Dallagi Y., Rahali D., Lasram M., Bini-Dhouib I., Lebret M., Rosa J.P., El Fazaa S., Asmi M. Comparison between electronic cigarette refill liquid and nicotine on metabolic parameters in rats. Life Sci. 2016;146:131–136. doi: 10.1016/j.lfs.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 52.Lefever T.W., Lee Y., Kovach A.L., Silinski M.A.R., Marusich J.A., Thomas B.F., Wiley J. Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug Alcohol Depend. 2017;172:80–87. doi: 10.1016/j.drugalcdep.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.March T.H., Wilder J.A., Esparaza D.C., Cossey P.Y., Blair L.F., Herrera L.K., McDonald J.D., Campen M.J., Mauderly J.L., Seagrave J. Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol Sci. 2006;92(2):545–559. doi: 10.1093/toxsci/kfl016. [DOI] [PubMed] [Google Scholar]
- 54.Van Winkle L.S., Gunderson A.D., Shimizu J.A., Baker G.L., Brown C.D. Gender differences in naphthalene – induced acute lung injury. Am J Physiol Lung Cell Mol. 2002;282:1122–1134. doi: 10.1152/ajplung.00309.2001. [DOI] [PubMed] [Google Scholar]
- 55.Lydon D.M., Wilson S.J., Child A., Geier C.F. Adolescent brain maturation and smoking: what we know and where we’re headed. Neurosci Biobehav Rev. 2014;45:323–342. doi: 10.1016/j.neubiorev.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.