Notes
Editorial note
There is a more recent Cochrane review on this topic: https://doi.org/10.1002/14651858.CD013669.pub2
Abstract
Background
Self‐harm (SH; intentional self‐poisoning or self‐injury) is common, often repeated, and strongly associated with suicide. This is an update of a broader Cochrane review on psychosocial and pharmacological treatments for deliberate SH, first published in 1998 and previously updated in 1999. We have now divided the review into three separate reviews. This review is focused on pharmacological interventions in adults who self harm.
Objectives
To identify all randomised controlled trials of pharmacological agents or natural products for SH in adults, and to conduct meta‐analyses (where possible) to compare the effects of specific treatments with comparison types of treatment (e.g., placebo/alternative pharmacological treatment) for SH patients.
Search methods
For this update the Cochrane Depression, Anxiety and Neurosis Review Group (CCDAN) Trials Search Co‐ordinator searched the CCDAN Specialised Register (September 2014). Additional searches of MEDLINE, EMBASE, PsycINFO, and CENTRAL were conducted to October 2013.
Selection criteria
We included randomised controlled trials comparing pharmacological treatments or natural products with placebo/alternative pharmacological treatment in individuals with a recent (within six months) episode of SH resulting in presentation to clinical services.
Data collection and analysis
We independently selected trials, extracted data, and appraised trial quality. For binary outcomes, we calculated odds ratios (ORs) and their 95% confidence intervals (CIs). For continuous outcomes we calculated the mean difference (MD) and 95% CI. Meta‐analysis was only possible for one intervention (i.e. newer generation antidepressants) on repetition of SH at last follow‐up. For this analysis, we pooled data using a random‐effects model. The overall quality of evidence for the primary outcome was appraised for each intervention using the GRADE approach.
Main results
We included seven trials with a total of 546 patients. The largest trial included 167 participants. We found no significant treatment effect on repetition of SH for newer generation antidepressants (n = 243; k = 3; OR 0.76, 95% CI 0.42 to 1.36; GRADE: low quality of evidence), low‐dose fluphenazine (n = 53; k = 1; OR 1.51, 95% CI 0.50 to 4.58; GRADE: very low quality of evidence), mood stabilisers (n = 167; k = 1; OR 0.99, 95% CI 0.33 to 2.95; GRADE: low quality of evidence), or natural products (n = 49; k = 1; OR 1.33, 95% CI 0.38 to 4.62; GRADE: low quality of evidence). A significant reduction in SH repetition was found in a single trial of the antipsychotic flupenthixol (n = 30; k = 1; OR 0.09, 95% CI 0.02 to 0.50), although the quality of evidence for this trial, according to the GRADE criteria, was very low. No data on adverse effects, other than the planned outcomes relating to suicidal behaviour, were reported.
Authors' conclusions
Given the low or very low quality of the available evidence, and the small number of trials identified, it is not possible to make firm conclusions regarding pharmacological interventions in SH patients. More and larger trials of pharmacotherapy are required. In view of an indication of positive benefit for flupenthixol in an early small trial of low quality, these might include evaluation of newer atypical antipsychotics. Further work should include evaluation of adverse effects of pharmacological agents. Other research could include evaluation of combined pharmacotherapy and psychological treatment.
Keywords: Adult, Female, Humans, Male, Anticonvulsants, Anticonvulsants/therapeutic use, Antidepressive Agents, Antidepressive Agents/therapeutic use, Antipsychotic Agents, Antipsychotic Agents/therapeutic use, Fluphenazine, Fluphenazine/therapeutic use, Lithium Compounds, Lithium Compounds/therapeutic use, Randomized Controlled Trials as Topic, Recurrence, Self-Injurious Behavior, Self-Injurious Behavior/drug therapy
Plain language summary
Drugs and natural products for self‐harm in adults
We have reviewed the international literature regarding pharmacological (drug) and natural product (dietary supplementation) treatment trials in this field. A total of seven trials meeting our inclusion criteria were identified. There is little evidence of beneficial effects of either pharmacological or natural product treatments. However, few trials have been conducted and those that have are small, meaning that possible beneficial effects of some therapies cannot be ruled out.
Why is this review important?
Self‐harm (SH), which includes intentional self‐poisoning/overdose and self‐injury, is a major problem in many countries and is strongly linked to suicide. It is therefore important that effective treatments for SH patients are developed. Whilst there has been an increase in the use of psychosocial interventions for SH in adults (which is the focus of a separate review), drug treatments are frequently used in clinical practice. It is therefore important to assess the evidence for their effectiveness.
Who will be interested in this review?
Clinicians working with patients who engage in SH, patients themselves, and their relatives.
What questions does this review aim to answer?
This review is an update of a previous Cochrane review from 1999 which found little evidence of beneficial effects of drug treatments on repetition of SH apart from for flupenthixol. This update aims to further evaluate the evidence for effectiveness of drugs and natural products for patients with SH with a broader range of outcomes.
Which studies were included in the review?
To be included in the review, studies had to be randomised controlled trials of drug treatments for adults who had recently engaged in SH.
What does the evidence from the review tell us?
There is currently no clear evidence for the effectiveness of antidepressants, antipsychotics, mood stabilisers, or natural products in preventing repetition of SH.
What should happen next?
We recommend further trials of drugs for SH patients, possibly in combination with psychological treatment.
Summary of findings
Summary of findings 1. Newer generation antidepressants versus placebo.
| Newer generation antidepressants (nomifensine, mianserin, paroxetine) compared to placebo for adults who engage in SH. | ||||||
| Patient or population: adults who engage in SH Settings: outpatient Intervention: NGAs (nomifensine, mianserin, paroxetine) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | NGAs | |||||
| Repetition of SH at last follow‐up | Study population | OR 0.76 (0.42 to 1.36) | 243 (3 studies) | ⊕⊕⊝⊝ low1,2 | Quality was downgraded owing to serious risk of bias. Quality was further downgraded owing to serious imprecision. | |
| 375 per 1000 | 313 per 1000 (201 to 449) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NGA: newer generation antidepressant; OR: odds ratio; SH: self‐harm. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias was rated as SERIOUS as blinding of participants and blinding of outcome assessors was unclear leading to possible performance bias and detection bias. 2 Imprecision was rated as SERIOUS as the overall sample size for each trial is small.
Summary of findings 2. Antipsychotics versus placebo or another comparator drug/dose.
| Antipsychotics (flupenthixol, fluphenazine) compared to placebo or another comparator drug/dose for adults who engage in SH. | ||||||
| Patient or population: adults who engage in SH Settings: outpatient Intervention: antipsychotics (flupenthixol, fluphenazine) Comparison: placebo or another comparator drug/dose | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or another comparator drug/dose | Antipsychotics | |||||
|
Repetition of SH at six months (flupenthixol vs. placebo) |
Study population | OR 0.09 (0.02 to 0.50) | 30 (1 study) | ⊕⊝⊝⊝ very low1,2 | Quality of evidence was downgraded as this was a single, small trial (N = 37) in which random sequence generation, allocation concealment, and personnel blinding were rated as at unclear risk of bias. Quality was further downgraded owing to serious imprecision. | |
| 750 per 1000 | 213 per 1000 (57 to 600) | |||||
|
Repetition of SH at six months (low dose fluphenazine vs. ultra low dose fluphenazine) |
Study population | OR 1.51 (0.50 to 4.58) | 53 (1 study) | ⊕⊝⊝⊝ very low1,2 | Quality of evidence was downgraded as this was a single, small trial (N = 53). Quality was further downgraded owing to serious imprecision. | |
| 346 per 1000 | 444 per 1000 (209 to 708) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SH: self‐harm. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias was rated as VERY SERIOUS as no details of allocation concealment or personnel blinding were provided leading to possible selection, performance, and detection bias. 2 Imprecision was rated as SERIOUS as the forest plot indicates the trial was associated with a wide confidence interval.
Summary of findings 3. Mood stabilisers versus placebo.
| Mood stabilisers (lithium) compared to placebo for adults who engage in SH. | ||||||
| Patient or population: adults who engage in SH Settings: outpatients Intervention: mood stabilisers (lithium) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Mood stabilisers | |||||
| Repetition of SH at 12 months | Study population | OR 0.99 (0.33 to 2.95) | 167 (1 study) | ⊕⊕⊝⊝ low1,2 | Quality of evidence was downgraded as this was a single trial and there was evidence of a significant imbalance between the treatment and control groups at baseline. | |
| 84 per 1000 | 84 per 1000 (29 to 214) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SH: self‐harm. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias was rated as VERY SERIOUS as no details of allocation concealment or personnel blinding were provided leading to possible selection, performance, and detection bias. 2 The trial was further downgraded as there was substantial imbalance between the intervention and control groups in terms of the proportion of participants with a history of multiple suicide attempts, scores on the Suicide Intent Scale for the index attempt, and the proportion of participants diagnosed with any personality disorder suggesting the presence of possible confounding.
Summary of findings 4. Natural products versus placebo.
| Natural products (omega‐3 fatty acid supplementation) compared to placebo for adults who engage in SH. | ||||||
| Patient or population: adults who engage in SH Settings: outpatients Intervention: natural products (omega‐3 fatty acid supplementation) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Natural products | |||||
| Repetition of SH during 12 week treatment phase | Study population | OR 1.33 (0.38 to 4.62) | 49 (1 study) | ⊕⊕⊝⊝ low1,2 | Quality of evidence was downgraded owing to possible performance and detection bias and serious imprecision. | |
| 259 per 1000 | 318 per 1000 (117 to 618) | |||||
| *The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; SH: self‐harm. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Risk of bias was rated as SERIOUS as no details on outcome assessor blinding were provided leading to possible performance and detection bias. 2 Imprecision was also rated as SERIOUS owing to the wide confidence interval associated with the estimate of treatment effect.
Background
Description of the condition
The term ‘self‐harm’ is used to describe all intentional acts of self‐poisoning (e.g., overdoses) or self‐injury (e.g., self‐cutting), irrespective of degree of suicidal intent or other types of motivation (Hawton 2003a). Thus it includes acts intended to result in death (‘attempted suicide’), those without suicidal intent (e.g., to communicate distress, to temporarily reduce unpleasant feelings), and those with mixed motivation (Hjelmeland 2002; Scoliers 2009). The term ‘parasuicide’ was introduced by Kreitman 1969 to include the same range of behaviour. However, ‘parasuicide’ has been used in the United States of America (USA) to refer specifically to acts of self‐harm without suicidal intent (Linehan 1991), and the term has largely fallen into disuse in the United Kingdom (UK) and other countries. In the fifth version of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5; American Psychiatric Association 2013), two types of self‐harming behaviour are included as conditions for further study, namely "Non‐Suicidal Self Injury" (NSSI) and "Suicidal Behavior Disorder" (SBD). Many researchers and clinicians, however, believe this to be an artificial and somewhat misleading categorisation (Kapur 2013). We have therefore used the approach favoured in the UK and some other countries where all intentional self‐harm is conceptualised in a single category, namely self‐harm (SH). Within this category, suicidal intent is regarded as a dimensional rather than a categorical concept. For readers more familiar with the NSSI and SBD distinction, SH can be regarded as an umbrella term for these two behaviours (although it should be noted that neither NSSI nor SBD include non‐fatal self‐poisoning).
SH has been a growing problem in most countries over the past 40 years. In the UK it is estimated that there are now more than 200, 000 related presentations to general hospitals per year (Hawton 2007). In addition, SH often occurs in the community and does not result in presentation to hospital or other clinical services (Borges 2011). SH consumes considerable hospital resources in both high income countries (Gibbs 2004; Claassen 2006; Schmidtke 1996; Schmidtke 2004) and low to middle income countries (Fleischmann 2005; Kinyanda 2005; Parkar 2006). Methods of SH vary, however, between high income and low to middle income countries. In high income countries, self‐poisoning frequently involves overdoses of analgesics and psychotropic drugs (Hawton 2003a; Värnik 2004; Gjelsvik 2012). In low to middle income countries, pesticides are often consumed, particularly in rural areas (Eddleston 2000; Gunnell 2003). Self‐cutting and other forms of self‐mutilation are probably the most common forms of non‐fatal self‐injury in both high and low to middle income countries. However, fatal self‐injury in high income countries most commonly involves hanging and firearms (World Health Organization 2014), whereas in some low to middle income countries, self‐immolation is not uncommon (Ahmadi 2007).
In most countries, SH (unlike suicide) occurs more commonly in females than males. However, the gender difference decreases over the life cycle (Hawton 2008), and in some countries the difference between the genders may have decreased in recent years (Perry 2012). SH predominantly occurs in young people, with 60‐70% of individuals in many studies being aged under 35 years. In females, rates tend to be particularly high among 15‐24 year olds, whereas in males the highest rates are usually among those in their late 20s and early 30s. SH is less common in older people, but then tends to be associated with high suicidal intent (Hawton 2008), with consequent greater risk of suicide (Murphy 2012).
Many people who engage in SH are facing acute life problems, often in the context of longer‐term difficulties (Hawton 2003b). Common problems include disrupted relationships, employment issues, financial and housing trouble, and social isolation. In older people, physical health problems, bereavement, and threatened loss of independence become increasingly important. Alcohol abuse and, to a lesser extent, drug misuse are often present. There may be a history of physical and/or sexual abuse and other adverse experiences.
Both psychological and biological factors appear to increase vulnerability to SH. Many patients who present to hospital following SH have psychiatric disorders, especially depression, anxiety and substance misuse (Hawton 2013); these disorders frequently occur in combination with a personality disorder (Haw 2001). Biological factors include disturbances in the serotonergic and stress‐response systems (van Heeringen 2014).
SH is often repeated, with 15‐25% of individuals who present to hospital with SH returning to the same hospital following a repeat episode within a year (Owens 2002; Carroll 2014). Studies from Asia suggest a lower risk of repetition (Carroll 2014), although there may be other repeat episodes that result in presentation to another hospital, or do not result in hospital presentation at all. Repetition is more common in individuals who have a history of previous SH, personality disorder, psychiatric treatment, and alcohol or drug misuse (Larkin 2014).
The risk of death by suicide within one year amongst people who present to hospital with SH varies across studies from nearly 1% to over 3% (Owens 2002; Carroll 2014). This variation reflects the characteristics of the SH population and the background national suicide rate. In the UK, during the first year after an SH episode the risk is 50–100 times that of the general population (Hawton 1988; Hawton 2003b; Cooper 2005). Of people who die by suicide, over half will have a history of SH (Foster 1997) and at least 15% will have presented to hospital with SH in the preceding year (Gairin 2003). A history of SH is the strongest risk factor for suicide across a range of psychiatric disorders (Sakinofsky 2000), and repetition of SH further increases the risk of suicide (Zahl 2004).
Description of the intervention
Given the high prevalence of depression in patients who engage in SH, antidepressants are often used in treatment in the same dose range as is generally used to treat depression. However, owing to the increased risk of overdose in this population, including the likelihood that patients who engage in self‐poisoning may use their own medication, antidepressants associated with lower case fatality indices (e.g., selective serotonin reuptake inhibitors (SSRIs); Hawton 2010) will generally be preferred.
In patients with a history of repetition of SH, especially those with a diagnosis of borderline personality disorder (BPD), treatment with antipsychotics may be used, although there is little evidence for their efficacy in reducing suicidal behaviour (Stoffers 2010).
Attention has also focused on the potential efficacy of mood stabilisers for this population, including both antiepileptic medications and lithium, given the high prevalence of recurrent mood disorders in people who engage in SH. There is currently little evidence that antiepileptics reduce risk of suicidal behaviour; however, there is accumulating evidence that lithium has specific antisuicidal effects, including reducing both the risk of SH and suicide in patients with affective disorders (Cipriani 2013a).
The high prevalence of anxiety disorders in this population (Hawton 2013) also suggests that other pharmacological agents, such as benzodiazepines and other sedatives, might be expected to have an important role in treatment. However, benzodiazepines may increase the risk of repetition of SH (Verkes 2000). Therefore it is usually recommended that benzodiazepines are used very cautiously, if at all, in people at risk of SH (Verkes 2000).
There is also interest in the use of natural products (e.g., dietary supplementation of omega‐3 fatty acids) to treat a variety of mental disorders, including suicidal behaviour, but there is little convincing evidence of their efficacy at present (Ross 2007).
How the intervention might work
Antidepressants
Antidepressants in general would be expected to improve mood in individuals with depression and, hence, decrease thoughts and acts of SH. Antidepressant medications can be divided into tricyclics and newer generation antidepressants (e.g., SSRIs). Tricyclic antidepressants primarily inhibit both serotonin and norepinephrine reuptake, whereas SSRIs specifically target synaptic serotonergic reuptake (Feighner 1999). Given the link between serotonin activity, impulsivity, and suicidal behaviour (van Heeringen 2014), both tricyclic and SSRI antidepressants may be associated with a serotonin‐mediated reduction in impulsivity and enhanced emotion regulation which might possibly reduce the likelihood that an individual will engage in SH.
Antipsychotics
One risk factor for SH, including repetition of the behaviour, may be heightened arousal, especially in relation to stressful life events. Rationale for the use of antipsychotics is that by reducing this arousal, the urge to engage in SH may be reduced. Lower doses might be used to obtain this effect than are used in the treatment of psychotic disorders (Battaglia 1999).
Mood stabilisers (including antiepileptics)
Mood stabilisers have specific benefits for patients with bipolar disorder or unipolar depression, especially in terms of preventing recurrence of episodes of mood disorder (Geddes 2004; Cipriani 2013b). It might therefore be anticipated that these drugs would have benefits in terms of reducing the risk of suicidal behaviour, although currently such an effect has only been shown for lithium (Cipriani 2013a). Lithium may reduce the risk of suicidal behaviour via a serotonin‐mediated reduction in impulsivity and aggression. It is also possible that the long‐term clinical monitoring that all patients prescribed lithium treatment must undergo might contribute to a reduction in SH (Cipriani 2013a).
Other pharmacological agents
Benzodiazepines might be anticipated to reduce suicidal behaviour through their specific effects on anxiety (Tyrer 2012). However, because of their GABAminergic effects, benzodiazepines may also increase aggression and disinhibition (Albrecht 2014), which may increase the risk of suicidal behaviour. Other pharmacological agents, particularly the N‐Methyl‐D‐aspartate receptor antagonist ketamine, may also have beneficial effects on suicidal ideation in patients with major depression. However, it is presently unclear whether ketamine has a specific antisuicidal effect, or rather, whether its effectiveness is due to a reduction in general depressive symptomatology (Fond 2014).
Natural products
The main focus with regard to natural products and suicidal behaviour has been on dietary supplementation of omega‐3 fatty acids (fish oils; Tanskanen 2001). Omega‐3 fatty acids have been implicated in the neural network shown to correlate with the lethality of recent suicidal behaviour (Mann 2013). Blood plasma polyunsaturated fatty acid levels have also been implicated in the serotonin‐mediated link between low cholesterol and suicidal behaviour, suggesting that low omega‐3 fatty acid levels may have a negative impact on serotonin function (Sublette 2006). Omega‐3 supplementation, in contrast, might stimulate serotonin activity, thereby reducing the likelihood that an individual will engage in impulsive behaviours, such as SH (Brunner 2002).
Why it is important to do this review
SH is a major social and healthcare problem. It represents significant morbidity, is often repeated, and has strong links to suicide. It also leads to substantial healthcare costs (Sinclair 2011). Many countries now have suicide prevention strategies (World Health Organization 2014), all of which include a focus on improved management of patients presenting with SH because of their greatly elevated suicide risk, and also because of their high levels of psychopathology and distress. The National Suicide Prevention Strategy for England (Department of Health 2012) and the USA's National Strategy for Suicide Prevention (Office of the Surgeon General (US) 2012), for example, highlight SH patients as a key high risk group to be given special attention. In recent years there has also been considerable focus on improving the standards of general hospital care for SH patients. In 1994 the Royal College of Psychiatrists published consensus guidelines for such services (Royal College of Psychiatrists 1994), and in 2004 produced revised guidelines (Royal College of Psychiatrists 2004). While these guidelines focus particularly on organisation of services and assessment of patients, there is clearly a need for effective treatments for SH patients; these may include pharmacological as well as psychosocial interventions. In 2004 the National Institute for Clinical Excellence (NICE; NCCMH 2004) produced a guideline on SH which focused on its short‐term physical and psychological management. More recently, NICE produced a second guide with an aim towards longer‐term management (NICE 2011), using some interim data from the present review as the evidence base on therapeutic interventions. A similar guideline was produced in Australia and New Zealand (Boyce 2003). We had previously conducted a systematic review of treatment interventions for SH patients, in terms of reducing repetition of SH, which highlighted the paucity of evidence for effective treatments, at least in terms of this outcome (Hawton 1998; Hawton 1999); the first NICE guideline essentially reinforced this conclusion (NCCMH 2004). Using interim data from the present review, the second NICE guideline concluded that there was evidence showing clinical benefit of brief psychological interventions in reducing repetition of SH, compared with routine care (NICE 2011). However, there was no evidence of similar beneficial effects for pharmacological treatments.
We have now fully updated our original review in order to provide contemporary evidence to guide clinical policy and practice. We have also divided the review into three reviews: the present review which focuses on pharmacological interventions for adults, a second review on psychosocial interventions for adults, and the third on interventions for children and adolescents. In the earlier review we focused on repetition of SH and suicide as the main outcomes. In this update, we have now also included data on treatment adherence, depression, hopelessness, suicidal ideation, and problem‐solving.
Objectives
To identify all randomised controlled trials (RCTs) on pharmacological agents or natural products for SH in adults, and to conduct meta‐analyses (where possible) to compare the effects of specific treatments with comparison types of treatment (e.g., placebo or alternative pharmacological treatment) for SH patients.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials, including cluster randomised and cross‐over trials, of specific pharmacological agents or natural products versus placebo or any other pharmacological comparisons in the treatment of adult SH patients.
Types of participants
Participant characteristics
Participants were adult males and females (age 18 and over) of all ethnicities. We also included trials where there were a small minority (<15%) of adolescent participants. However, we undertook sensitivity analyses to assess the effect of inclusion of such trials.
Diagnosis
Participants who had engaged in any type of non‐fatal intentional self‐poisoning or self‐injury in the six months prior to trial entry resulting in presentation to clinical services were included. There were no restrictions on the frequency with which patients engaged in SH; thus, for example, we included trials where participants had frequently repeated SH (e.g., those with self‐harming behaviour as part of borderline personality disorder).
We defined SH as any non‐fatal intentional act of self‐poisoning or self‐injury, irrespective of degree of suicidal intent or other types of motivation. Thus it includes acts intended to result in death ("attempted suicide"), those without suicidal intent (e.g., to communicate distress, to temporarily reduce unpleasant feelings), and those with mixed motivation. Self‐poisoning includes both overdoses of medicinal drugs and ingestion of substances not intended for consumption (e.g., pesticides). Self‐injury includes acts such as self‐cutting, self‐mutilation, attempted hanging, and jumping in front of moving vehicles. We only included trials where participants presented to clinical services as a result of SH.
Co‐morbidities
There were no restrictions in terms of whether or not patients had psychiatric disorders, or the nature of those disorders, with the exception of intellectual disability, where any SH behaviour is likely to be repetitive (e.g., head banging) as the purpose of this behaviour is usually different from that involved in SH. The reader is instead referred to a recent Cochrane review of pharmacological interventions for self‐injury in this population (Rana 2013).
Setting
Interventions delivered in inpatient or outpatient settings were eligible for inclusion, as were trials from any country.
Subset data
We did not include trials in which only some participants had engaged in SH or trials of people with psychiatric disorders in which SH was an outcome variable but was not an inclusion criterion for entry into the trial.
Types of interventions
Experimental interventions
These included:
Tricyclic antidepressants (TADs; e.g., amitriptyline);
Newer generation antidepressants (NGAs), including SSRIs (e.g., fluoxetine), serotonin and noradrenaline reuptake inhibitors (SNRIs; e.g., venlafaxine), norepinephrine reuptake inhibitors (NRIs; e.g., reboxetine), tetracyclic antidepressants (TAs; e.g., maprotiline), noradrenergic specific serotonergic antidepressants (NaSSAs; e.g., mirtazapine), serotonin antagonist or reuptake inhibitors (SARIs; e.g., trazodone), or reversible inhibitors of monoamine oxidase type A (RIMAs; e.g., moclobemide);
Any other antidepressants such as irreversible mono‐amine oxidase inhibitors (MAOIs; e.g., bupropion);
Antipsychotics (e.g., olanzapine);
Mood stabilisers, including antiepileptics (e.g., sodium valporate) and lithium;
Other pharmacological agents (e.g., benzodiazepines, ketamine);
Natural products (e.g., omega‐3 essential fatty acid supplementation).
Comparator interventions
While treatment as usual (TAU), which usually refers to routine clinical service provision, is often used as a comparator in trials of psychosocial interventions, it is not generally used in pharmacological trials, where comparison with the specific effects of an active drug is being made. For the purposes of the current review, then, the comparator was placebo, which consisted of any pharmacologically inactive treatment such as sugar pills or injections with saline, or another comparator pharmacological intervention (e.g., another standard pharmacological agent, or reduced dose of the intervention agent).
Combination interventions
We also planned to include combination interventions where any pharmacological agent of any class as outlined above is combined with psychological therapy. However, as the focus of this review is the effectiveness of pharmacological agents for SH patients, we only included such trials if both the intervention and control groups received the same psychological therapy to ensure that any potential effect of the psychosocial therapy was balanced across both groups. The effectiveness of psychosocial therapy in adults is the subject of a separate review.
Types of outcome measures
Primary outcomes
The primary outcome measure in this review was the occurrence of repeated SH (defined above) over a maximum follow‐up period of two years. Repetition was identified through self‐report, collateral report, clinical records, or research monitoring systems. As we wished to incorporate the maximal amount of data from each trial, we included both self‐reported and hospital records of SH where available. We also assessed frequency of repetition of SH at final follow‐up.
Secondary outcomes
1. Treatment adherence
This was assessed using a range of measures of adherence, including pill counts, changes in blood measures, and the proportion of participants that both started and completed treatment.
2. Depression
This was assessed either continuously, as scores on psychometric measures of depression symptoms (for example total scores on Beck Depression Inventory (BDI; Beck 1961) or scores on the depression sub‐scale of the Hospital Anxiety and Depression Scale (HADS; Zigmond 1983)), or dichotomously, as the proportion of patients reaching defined diagnostic criteria for depression.
3. Hopelessness
This was assessed by scores on psychometric measures of hopelessness, for example, total scores on the Beck Hopelessness Scale (BHS; Beck 1974).
4. Suicidal ideation
This was assessed either continuously, as scores on psychometric measures of suicidal ideation (for example total scores on the Beck Scale for Suicidal Ideation (BSS; Beck 1988)), or dichotomously, as the proportion of patients reaching a defined cut‐off for ideation.
5. Problem‐solving
This was assessed either continuously, as scores on a psychiatric measure of problem‐solving ability (for example total scores on the Problem Solving Inventory (PSI; Heppner 1988)), or dichotomously, as the proportion of patients with improved problems.
6. Suicide
This included both register‐recorded deaths and reports from collateral informants such as family members or neighbours.
Timing of outcome assessment
We reported outcomes for the following time periods:
During treatment.
At the conclusion of the treatment period.
Between zero and six months after the conclusion of the treatment period.
Between six and 12 months after the conclusion of the treatment period.
Between 12 and 24 months after the conclusion of the treatment period.
Hierarchy of outcome assessment
Where a trial measured the same outcome (e.g., depression) in two or more ways, we used the most common measure across trials in any meta‐analysis, but we also reported scores from the other measure in the text of the review.
Search methods for identification of studies
Electronic searches
1. The Cochrane Depression, Anxiety and Neurosis Review Group's Specialised Register (CCDANCTR) The Cochrane Depression, Anxiety and Neurosis Group (CCDAN) maintains two clinical trials registers at their editorial base in Bristol, UK: a references register and a studies‐based register. The CCDANCTR‐References register contains over 37,500 reports of randomised controlled trials on depression, anxiety and neurosis. Approximately 60% of these references have been tagged to individual, coded trials. The coded trials are held in the CCDANCTR‐Studies register and records are linked between the two registers through the use of unique study ID tags. Coding of trials is based on the EU‐Psi coding manual. Please contact the CCDAN Trials Search Coordinator for further details.
Reports of trials for inclusion in the group's registers are collated from weekly generic searches of MEDLINE (1950 to date), EMBASE (1974 to date), and PsycINFO (1967 to date), as well as quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL).
The CCDANCTR (Studies and References) was searched on 2 September 2014 using terms for self‐harm (condition only), as outlined in Appendix 1.
No restrictions on date, language, or publication status were applied to the search.
2. Additional electronic database searches
Complementary searches of MEDLINE (1998 to 2013), EMBASE (1998 to 2013), PsycINFO (1998 to 2013), and CENTRAL (The Cochrane Library, 1998 to 2013) were conducted by Sarah Stockton, librarian at the University of Oxford, following the search strategy outlined in Appendix 2. Additionally, KW searched the Australian Suicide Prevention RCT Database (Christensen 2014). KW also conducted electronic searches of ClinicalTrials.gov and the ISRCTN registry using the keywords random* AND suicide attempt* OR self$harm* to identify relevant ongoing trials.
Both the original version of this review and an unpublished version also incorporated searches of SIGLE (1980 to March 2005) and SocioFile (1963‐July 2006).
Searching other resources
Hand searching
For the original version of this review, the authors hand searched ten specialty journals within the fields of psychology and psychiatry, including all English language suicidology journals, as outlined in Appendix 3. As these journals are now indexed in major electronic databases, hand searching was not repeated for this update.
Reference lists
The reference lists of all relevant papers known to the investigators were checked, as were the reference lists of major reviews which include a focus on interventions for SH patients (Baldessarini 2003; Baldessarini 2006; Beasley 1991; Brausch 2012; Burns 2000; Cipriani 2005; Cipriani 2013a; Comtois 2006; Crawford 2007a; Crawford 2007b; Daigle 2011; Daniel 2009; Dew 1987; Gould 2003; Gray 2001; Gunnell 1994; Hawton 1998; Hawton 1999; Hawton 2012; Hennen 2005; Hepp 2004; Hirsch 1982; Kapur 2010; Kliem 2010; Lester 1994; Links 2003; Lorillard 2011a; Lorillard 2011b; Luxton 2013; Mann 2005; McMain 2007; Milner 2015; Möller 1989; Möller, 1992; Montgomery 1995; Muehlenkamp 2006; Müller‐Oerlinghausen 2005; Nock 2007; Ougrin 2011; Ougrin 2015; Smith 2005; Stoffers 2010; Tarrier 2008; Tondo 1997; Tondo 2000; Tondo 2001; Townsend 2001; van der Sande 1997).
Correspondence
The authors of trials and other experts in the field of suicidal behaviour were consulted to find out if they were aware of any ongoing or unpublished RCTs concerning the use of pharmacological interventions for adult SH patients.
Data collection and analysis
For details of the data collection and analysis methods used in the original version of this review see Appendix 4.
Selection of studies
For this update of the review, all authors independently assessed the titles of trials identified by the systematic search for eligibility. A distinction was made between:
Eligible trials, in which any psychopharmacological treatment was compared with a control (e.g., placebo medication or comparator drug/dose).
General treatment trials (without any control treatment).
All trials identified as potentially eligible for inclusion then underwent a second screening. Pairs of review authors, working independently from one another, screened the full text of relevant trials to identify whether the trial met our inclusion criteria.
Disagreements were resolved following consultation with KH. Where disagreements could not be resolved from the information reported within the trial, or where it was unclear whether the trial satisfied our inclusion criteria, study authors were contacted to provide additional clarification.
Data extraction and management
In the current update, data from included trials was extracted by KW and one of either TTS, EA, DG, PH, ET, or KvH using a standardised extraction form. Review authors extracted data independently of one another. Where there were any disagreements, these were resolved through consensus discussions with KH.
Data extracted from each eligible trial concerned participant demographics, details of the treatment and control interventions, and information on the outcome measures used to evaluate the efficacy of the intervention. Study authors were contacted to provide raw data for outcomes that were not reported in the full text of included trials.
Both dichotomous and continuous outcome data were extracted from eligible trials. As the use of non‐validated psychometric scales is associated with bias, we extracted continuous data only if the psychometric scale used to measure the outcome of interest had been previously published in a peer‐reviewed journal (Marshall 2000), and was not subjected to item, scoring, or other modification by the trial authors.
We planned the following main comparisons.
Tricyclic antidepressants versus placebo.
Tricyclic antidepressants versus another comparator drug/dose.
Newer generation antidepressants versus placebo.
Newer generation antidepressants versus another comparator drug/dose.
Any other antidepressants versus placebo.
Any other antidepressants versus another comparator drug/dose.
Antipsychotics versus placebo.
Antipsychotics versus another comparator drug/dose.
Mood stabilisers versus placebo.
Mood stabilisers versus another comparator drug/dose.
Other pharmacological agents versus placebo.
Other pharmacological agents versus another comparator drug/dose.
Natural products versus placebo.
Natural products versus another comparator drug/dose.
Assessment of risk of bias in included studies
Given that highly biased trials are more likely to overestimate treatment effectiveness (Moher 1998), the quality of included trials was evaluated independently by KW and one of either TTS, EA, DG, PH, ET, or KvH using the criteria described in Higgins 2008a. This tool encourages consideration of the following domains:
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
Each study was judged as being at "low", "high", or "unclear" risk of each potential bias, and a supporting quotation from the trial report was incorporated to justify this judgment. Where inadequate details of the randomisation, blinding, or outcome assessment procedures were provided in the original report, we contacted authors to provide clarification. Disagreements were resolved following discussion with KH. Risk of bias for each included trial is reported in the text of the review.
Measures of treatment effect
Dichotomous outcomes
We summarised dichotomous outcomes, such as the number of participants engaging in a repeat SH episode and the number of deaths by suicide, using summary odds ratios (OR) and the accompanying 95% confidence interval (CI), as OR is the most appropriate effect size statistic for summarising associations between two dichotomous groups (Fleiss 1994).
Continuous outcomes
For outcomes measured on a continuous scale, we used mean differences (MD) and accompanying 95% CI where the same outcome measure was employed. In future updates of this review, if different scales are used to assess a given outcome, we will use the standardised mean difference (SMD) and its accompanying 95% CI.
Trials were aggregated for the purposes of meta‐analysis only if treatments were sufficiently similar. For trials that could not be included in a meta‐analysis, we have instead provided narrative descriptions of the results.
Unit of analysis issues
Zelen design trials
Trials in this area are increasingly employing Zelen's method in which consent is obtained subsequent to randomisation and treatment allocation. This design may lead to bias if, for example, participants allocated to one particular arm of the trial disproportionally refuse to provide consent for participation or, alternatively, if participants only provide consent if they are allowed to cross‐over to the other treatment arm (Torgerson 2004). No trial included in this review used Zelen's design. Given the uncertainty of whether to use data for the primary outcome based on all those randomised to the trial, or only those who consent to participation, should a trial using Zelen's method be identified in future updates of this review we plan to extract data using both sources where possible. We also plan to conduct sensitivity analyses to investigate what impact, if any, the inclusion of these trials may have on the pooled estimate of treatment effectiveness.
Cluster randomised trials
Cluster randomisation, for example by clinician or general practice, can lead to overestimation of the significance of a treatment effect, resulting in an inflation of the nominal type I error rate, unless appropriate adjustment is made for the effects of clustering (Donner 2002; Kerry 1998). Although no trials identified by this review used cluster randomisation methods, should any future trials in this area use this design we will follow the guidance outlined in Higgins 2008b, section 16.3.4.
Cross‐over trials
A primary concern with cross‐over trials is the "carry‐over" effect in which the effect of the intervention treatment (e.g., pharmacological, physiological, or psychological) influences the participant's response to the subsequent control condition (Elbourne 2002). As a consequence, on entry to the second phase of the trial, participants may differ systematically from their initial state despite a wash‐out phase. This, in turn, may result in a concomitant underestimation of the effectiveness of the treatment intervention (Curtin 2002a; Curtin 2002b). Once again, no trials included in the current review used cross‐over methodology. However, should we identify any such trials in future updates, only data from the first phase of the trial, prior to cross‐over, will be extracted to protect against the carry‐over effect.
Studies with multiple treatment groups
One trial in the current review included multiple treatment groups (Hirsch 1982). As both intervention arms in this trial investigated the effectiveness of a newer generation antidepressant, we combined dichotomous data from these two arms and compared the combined data with data from the placebo arm. For outcomes reported on a continuous scale, we combined data using the formula given in Higgins 2008c, section 7.7.3.8.
Studies with adjusted effect sizes
None of the trials included in the current update provided adjusted effect sizes. In future updates of this review, however, where trials report both unadjusted and adjusted effect sizes, we will include only unadjusted effect sizes.
Dealing with missing data
We as review authors did not impute missing data as we considered that the bias that would be introduced by doing this would have outweighed any benefit (in terms of increased statistical power) that may have been gained by the inclusion of imputed data. However, where authors omitted standard deviations (SD) for continuous measures, these were estimated using the method described in Townsend 2001.
Dichotomous data
Although many authors conducted their own intention‐to‐treat analyses, none presented intention‐to‐treat analyses as defined by Higgins 2008b. Therefore, outcome analyses for both dichotomous and continuous data were based on all information available on trial participants. For dichotomous outcomes, we included data on only those participants whose results were known, using as the denominator the total number of participants with data for the particular outcome of interest at follow‐up, as recommended (Higgins 2008b).
Continuous data
For continuous outcomes, we have included data only on observed cases.
Missing data
Where data on outcomes of interest were incomplete or were excluded from the text of the trial, study authors were contacted in order to try to obtain further information.
Assessment of heterogeneity
Between‐study heterogeneity can be assessed using either the Chi2 or I2 statistics. In this review, however, we used only the I2 statistic to determine heterogeneity as this is considered to be more reliable (Higgins 2003). The I2 statistic indicates the percentage of between‐study variation due to chance (Higgins 2003), and can take any value from 0% to 100%. We used the following values to denote unimportant, moderate, substantial, and considerable heterogeneity, respectively: 0% to 40%, 30% to 60%, 50% to 90%, and 75% to 100% as per the guidance in the Cochrane Handbook (Deeks 2008). Where we found substantial levels of heterogeneity (i.e., ≥ 75%), reasons for this heterogeneity were explored. We also planned to investigate heterogeneity when the I2 statistic was lower than 75% where either the direction or magnitude of a trial effect size was clearly discrepant from that of other trials included in the meta‐analysis (see Subgroup analysis and investigation of heterogeneity section for further information on these analyses).
Assessment of reporting biases
Reporting bias occurs when the decision to publish a particular trial is influenced by the direction and significance of its results (Egger 1997). Research suggests, for example, that trials with statistically significant findings are more likely to be submitted for publication and to subsequently be accepted for publication (Hopewell 2009), leading to possible overestimation of the true treatment effect. To assess whether trials included in any meta‐analysis were affected by reporting bias, we entered their data into a funnel plot but only, as recommended, when a meta‐analysis included results from at least ten trials. Where evidence of any small‐study effects were identified, reasons for funnel plot asymmetry, including the presence of publication bias, were explored (Egger 1997).
Data synthesis
For the purposes of meta‐analysis, we calculated the pooled OR and accompanying 95% CI using the random‐effects model as this is the most appropriate model for incorporating heterogeneity between trials (Deeks 2008, section 9.5.4). Specifically, for dichotomous data, the Mantel‐Haenszel method was used whilst the inverted variance method was used for continuous data. However, a fixed‐effect analysis was also undertaken to investigate the potential effect of method choice on the estimates of treatment effect. Any material differences in ORs between these two methods are reported descriptively in the text of the review. All analyses were undertaken in RevMan, version 5.3.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses
In the original version of this review, we had planned to undertake subgroup analyses by repeater status and gender but found there were insufficient data. Consequently, in this update we only undertook a priori subgroup analyses by gender or repeater status where there were sufficient data to do so.
Investigation of heterogeneity
Although no meta‐analysis was associated with substantial levels of between‐study heterogeneity (i.e., I2 ≥75%) in this review, in future updates, should this be the case KH and KW would independently triple‐check the data to ensure it had been correctly entered. Assuming data have been entered correctly, we would then investigate the source of this heterogeneity by visually inspecting the forest plot and removing each trial which had a very different result to the general pattern of the others until homogeneity was restored as indicted by an I2 statistic <75%. We would report the results of this sensitivity analysis in the text of the review alongside hypotheses regarding the likely causes of the heterogeneity.
Sensitivity analysis
We undertook sensitivity analyses, where appropriate, as outlined below:
Where a trial or trials made use of Zelen's method of randomisation (see Unit of analysis issues section).
Where a trial or trials contributed substantial levels of between‐study heterogeneity (see Subgroup analysis and investigation of heterogeneity section).
Where a trial or trials included a mixture of both adolescent and adult participants.
Where a trial or trials specifically recruited individuals diagnosed with borderline personality disorder.
'Summary of findings' tables
A 'Summary of findings' table was prepared for the primary outcome measure, repetition of SH, following recommendations outlined in Schünemann 2008a, section 11.5. This table provides information concerning the overall quality of evidence from each included trial. The 'Summary of findings' table was prepared using GRADEpro software (GRADEpro). Quality of the evidence was assessed following recommendations in Schünemann 2008b, section 12.2.
Results
Description of studies
Results of the search
For this update, a total of 23,725 citations were found using the search strategy outlined in Appendix 1 and Appendix 2. A further 10 were identified through correspondence and discussion with researchers in the field; these trials were ongoing at the time of the systematic search. All but one have subsequently been published and a report on the remaining trial is currently in preparation. We were able to include data for this trial, however, by correspondence with and permission from study authors. In consultation with CCDAN, we have since divided the original review into three separate reviews: the present review which focuses on pharmacological interventions for adults, a second review on psychosocial interventions for adults, and the third on interventions for children and adolescents. As these 10 trials evaluated psychosocial rather than pharmacological interventions, they are included in the related two reviews.
After deduplication, the initial number was reduced to 16,700. Of these, 16,459 were excluded after screening, whilst a further 217 were excluded after reviewing the full texts (Figure 1).
1.
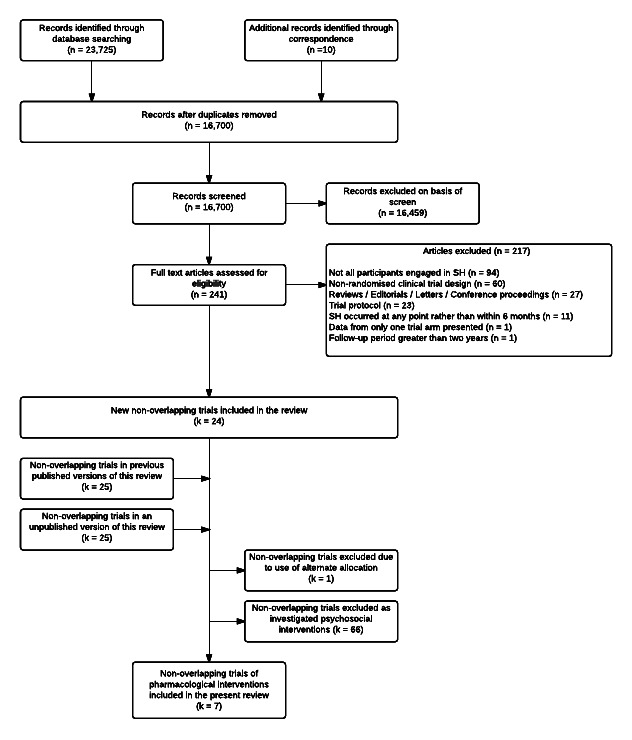
Search flow diagram of included and excluded trials.
Included studies
In the previous versions of this review (Hawton 1998; Hawton 1999; NICE 2011), seven trials of pharmacological interventions for SH patients were included. The present update failed to locate any additional trials of pharmacological agents. The present review therefore includes seven non‐overlapping trials (Battaglia 1999; Hallahan 2007; Hirsch 1982; Lauterbach 2008; Montgomery 1979; Montgomery 1983; Verkes 1998). No further reports provided additional information on these trials.
Two of the trials have not been published (Montgomery 1979Hirsch 1982). Unpublished data were obtained from study authors for three of the trials (Battaglia 1999; Hallahan 2007; Verkes 1998.
Two ongoing trials of pharmacological interventions were also identified (see Characteristics of ongoing studies section for further information on these trials).
Design
Of the seven trials, all were described as randomised controlled trials. All employed a simple randomisation procedure based on individual allocation to the intervention and control groups.
Participants
The included trials comprised a total of 546 participants. All had engaged in at least one episode of SH in the six months prior to randomisation.
Participant characteristics
Of the five trials that recorded information on age, the average age of participants at randomisation was 35.3 years (SD 3.1). Two trials included a small number of adolescent participants (i.e., under 18 years of age) but the precise number was not recorded in either trial (Hallahan 2007; Hirsch 1982). Of the six trials that reported information on gender, the majority of participants were female (63.5%), reflecting the typical pattern for SH (Hawton 2008).
Diagnosis
A history of SH prior to the index episode (i.e., multiple episodes of SH) was a requirement for participation in five trials (Battaglia 1999; Hallahan 2007; Lauterbach 2008; Montgomery 1979; Montgomery 1983). In one trial, almost one‐third (30.0%) of participants had a history of multiple episodes (Verkes 1998) whilst in the remaining trial the proportion was not reported (Hirsch 1982). Only one trial included participants who had made a "suicide attempt" (i.e., with evidence of suicidal intent; Lauterbach 2008) whilst in two others, although only those who made a "suicide attempt" were eligible to participate, it is unclear whether all participants intended to die as a result (Battaglia 1999; Verkes 1998). The remaining trials did not provide any information on intent.
Information on the methods of SH for the index episode was not reported in five trials (Battaglia 1999; Hallahan 2007; Montgomery 1979; Montgomery 1983; Verkes 1998). In one trial, only those participants who had engaged in self‐poisoning with either over‐the‐counter or prescription drugs (i.e., not illicit substances or poison) were eligible to participate (Hirsch 1982), whilst in the remaining trial (Lauterbach 2008) a variety of methods were used, including: self‐poisoning (73.2%), self‐injury (14.4%), jumping from a height (2.5%), and attempted hanging, attempted shooting, or attempted drowning (5.0%). The methods used by the remaining 4.9% of participants in this trial were not reported.
Co‐morbidities
Information on current psychiatric diagnoses were reported in five trials (see Table 5). In these trials, the most common psychiatric diagnoses were for borderline personality disorder (k = 3, mean 78.4%) and other personality disorder (k = 3, mean 52.0%). One trial included a high proportion of participants diagnosed with major depression (76.0%; Lauterbach 2008).
1. Major categories of psychiatric diagnoses in included studies.
| Reference | Psychiatric Diagnosis1 | |||||||||
| MDD n (%) |
Any Mood Disorder n (%) |
AD n (%) |
PTSD n (%) |
AUD n (%) |
DUD n (%) |
SUD n (%) |
Adjustment Disorder n (%) |
BPD n (%) |
PD n (%) |
|
| Battaglia 1999 | 33 (57.9) | 11 (19.0) | 33 (58.0) | 16 (28.0) | 8 (14.0) | 49 (85.0) | 2 | |||
| Hallahan 2007 | 35 (71.4) | 40 (81.6) | ||||||||
| Hirsch 19823 | ||||||||||
| Lauterbach 2008 | 127 (76.0) | 8 (4.8) | 12 (7.2) | 14 (8.4) | 32 (19.2) | 131 (42.7) | ||||
| Montgomery 19793 | ||||||||||
| Montgomery 1983 | 30 (78.9) | 12 (31.6) | ||||||||
| Verkes 1998 | 29 (31.9) | 4 (4.4) | 40 (43.9) | 19 (20.9) | ||||||
MDD: major depression disorder; AD: anxiety disorder; PTSD: post‐traumatic stress disorder; AUD: alcohol use disorder; DUD: drug use disorder; SUD: substance use disorder; BPD: borderline personality disorder; PD: any other personality disorder (not including borderline personality disorder).
1 All diagnoses represent current rather than lifetime diagnoses. Percentages for any one trial may be greater than 100% due to comorbidity.
2 As participants could be diagnosed with more than one psychiatric diagnosis, the absolute number of participants diagnosed with any other personality disorder in this trial is unclear.
3 No information on psychiatric diagnoses reported.
Details on comorbid diagnoses were reported in one trial (Lauterbach 2008). For this trial, the most common comorbidity was for any personality disorder (33.5%), followed by substance use disorder (8.4%), and any anxiety disorder (7.2%). In a second trial, 25.3% of the sample were diagnosed with more than one psychiatric disorder from the following: dysthymia, any anxiety disorder, any dissociative disorder, alcohol abuse, any adjustment disorder, and any depressive disorder (Verkes 1998). However, the proportion diagnosed with each comorbid condition was not provided.
Setting
Of the seven independent RCTs included in this review, three were from the UK (Hirsch 1982; Montgomery 1979; Montgomery 1983), and one was from each of the USA (Battaglia 1999), Germany (Lauterbach 2008), the Netherlands (Verkes 1998) and the Republic of Ireland (Hallahan 2007). Although all participants were identified following a hospital admission for SH, five trials did not clearly specify if treatment was delivered on an inpatient or outpatient basis. Two trials were described as being conducted in an outpatient setting (Lauterbach 2008; Verkes 1998).
Interventions
The trials included in this review investigated the effectiveness of various pharmacological agents:
Newer generation antidepressants (mianserin, nomifensine, paroxetine) versus placebo (Hirsch 1982; Montgomery 1983; Verkes 1998).
Antipsychotics vs. placebo (Montgomery 1979).
Antipsychotics vs. comparator drug/dose (Battaglia 1999).
Mood stabilisers (lithium) vs. placebo (Lauterbach 2008).
Natural products (omega‐3 essential fatty acid; n‐3EFA) vs. placebo (Hallahan 2007).
Outcomes
All trials reported information on the primary outcome, repetition of SH. In two trials this was based on self‐reported information (Battaglia 1999; Lauterbach 2008), and in two further trials on re‐presentation to hospital (Hallahan 2007; Hirsch 1982). For the remaining three trials the source of information for this outcome was unclear (Montgomery 1979; Montgomery 1983; Verkes 1998).
Treatment adherence was assessed using pill counts (Hallahan 2007; Verkes 1998). Depression was assessed using the BDI in two trials (Hallahan 2007; Verkes 1998) or the Hamilton Depression Rating Scale (HDRS; Hamilton 1960) in two further trials (Hallahan 2007; Lauterbach 2008). Hopelessness was assessed using the BHS (Lauterbach 2008; Verkes 1998). Suicidal ideation was assessed using either the sub‐scale of the Overt Aggression Scale (Hallahan 2007) or the BSS (Lauterbach 2008). It was unclear how suicide was assessed in any of the included trials. No trial reported information on problem‐solving.
Excluded studies
A total of 217 articles were excluded from this update: 94 were excluded because not all patients engaged in SH; 60 used a non‐randomised clinical trial design; 27 were reviews, editorials, letters to the editor, or conference proceedings; 23 were trial protocols; 11 were excluded as SH could have occurred at any point rather than within six months of randomisation; and one each were excluded either because only data from one trial arm were presented (however, a related publication in which data for both the intervention and control arms were presented was eligible for inclusion), or because the data reported in the article were for a period beyond two years (however, articles reporting data for earlier follow‐up periods for this trial were eligible for inclusion).
Details on the reasons for exclusion of 12 trials clearly related to pharmacological interventions for suicidality can be found in the Characteristics of excluded studies section.
Ongoing studies
Two ongoing trials of pharmacological interventions, one of oral lithium (Liang 2014) and one of oral ketamine (Sharon 2014), were identified. Full details of these trials are provided in the Characteristics of ongoing studies section.
Studies awaiting classification
There were no potentially eligible trials which have not been incorporated into the review.
Risk of bias in included studies
Summaries of the overall risk of bias for the included trials are presented in Figure 2 and Figure 3. Risk of bias for each included trial is also considered within the text of the review.
2.

Risk of bias graph: Review authors' judgements for each risk of bias item presented as percentages across all included trials.
3.

Risk of bias summary graph: review authors' judgements about each risk of bias item for each included trial.
Sequence generation
Of the seven independent trials, all used random allocation. The majority (k = 4; 57.1%) were rated as having an unclear risk of bias as no information on the method used to generate the random sequence was provided. Three trials were rated as being at low risk of bias: two used a computerised program to generate the random sequence (Hallahan 2007; Lauterbach 2008) whilst the third used alternate allocation of identical blister packs to randomly assign participants to the active or placebo groups (Verkes 1998).
Allocation concealment (selection bias)
Information on allocation concealment was not provided in the majority of trials, earning them a rating of unclear risk (k = 5; 71.4%). Two trials were rated as being at low risk of bias as the random sequence was generated by an offsite researcher (Verkes 1998) or a third party researcher working independently from the trial team (Hallahan 2007). For the latter trial, moreover, additional attempts were made to prevent participants from guessing to which treatment group they had been allocated by adding the taste of the experimental medication to the placebo (Hallahan 2007).
Blinding (performance bias and detection bias)
Blinding was assessed separately for participants, clinical personnel, and outcome assessors.
Blinding of participants
Blinding of participants was classified as resulting in a low risk of bias for all trials included in this review as participant blinding was maintained through the use of identical capsules or blister packs for both the active agent and placebo arms.
Blinding of personnel
The majority of trials in this review were rated as being at low risk (k = 4; 57.1%) as personnel blinding was maintained through the use of identical capsules or blister packs for both the active agent and placebo arms (Battaglia 1999; Hallahan 2007; Montgomery 1983; Verkes 1998). The remaining three trials were rated as at being at unclear risk of bias. For two, no information on personnel blinding was provided (Hirsch 1982; Montgomery 1979). For one trial, blinding could not be maintained in all cases due to the occurrence of serious suicidal behaviour or insufficient treatment adherence (Lauterbach 2008).
Blinding of outcome assessors
Four trials were rated as being at low risk of bias (Battaglia 1999; Lauterbach 2008; Montgomery 1979; Montgomery 1983) as outcome assessors were blind to treatment allocation. For the remaining three trials (Hallahan 2007; Hirsch 1982; Verkes 1998) no information on blinding of outcome assessors was provided. These three trials were therefore rated as being at unclear risk of bias for this item.
Incomplete outcome data (attrition bias)
Three trials reported conducting analyses on an intention‐to‐treat basis. Two did not report any further details on the method used to conduct intention‐to‐treat analyses (Lauterbach 2008; Verkes 1998) but as all participants were included in the analyses, these trials were nonetheless classified as being at low risk. One used the last observation carried forward method (Hallahan 2007), which we understand may introduce bias (Engles 2003), and so was classified as being at unclear risk of bias for this item. A further trial was rated as being at unclear risk of bias for this item as no details on incomplete outcome reporting were provided (Hirsch 1982). Three trials used per protocol analyses (Battaglia 1999; Montgomery 1979; Montgomery 1983). As there was a greater than 0% drop‐out rate for these three trials, all were rated as having high risk of bias for this item.
Selective reporting (reporting bias)
As the review authors did not have access to trial protocols for the trials included in this review, it is difficult to assess the degree to which selective outcome reporting could have occurred. Consequently the majority of trials were classified as being at unclear risk for this item (k = 5; 71.4%). For two trials, numerical data for non‐significant outcomes were not reported (Hirsch 1982; Verkes 1998).These trials were therefore rated as at being at high risk of bias for this item.
Other potential sources of bias
For one trial, significant imbalances between the active and placebo arms in terms of history of multiple "suicide attempts", personality disorder diagnosis, and scores on the Suicide Intent Scale for the index attempt were apparent (Lauterbach 2008). As in all cases this imbalance was suggestive of worse prognosis for the intervention group, this trial was rated as being at high risk of bias for this item. The remaining six trials were classified as having low risk of bias for this item as no additional sources of bias were apparent.
Few trials used systematic means to investigate whether participants were able to guess if they had been allocated to the active or placebo arm. Only one (Hallahan 2007) questioned participants at the conclusion of the trial, however, this information was not presented in the trial report.
The source of funding was not indicated in three trials (Hirsch 1982; Montgomery 1979; Montgomery 1983). In two, funding was jointly received from a pharmaceutical company and a government department (Lauterbach 2008; Verkes 1998), in a further trial funding was received from both government and university sources (Battaglia 1999), and for the remaining trial, funding was exclusively received from a university source (Hallahan 2007).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
As only newer generation antidepressants were evaluated against placebo in more than one non‐overlapping trial, meta‐analyses were only undertaken for this intervention.
Although antipsychotics were also evaluated in more than one independent trial, one used placebo as the comparator (Montgomery 1979) whilst the second used the intervention antipsychotic in an ultra‐low dosage as the comparator (Battaglia 1999). We therefore think it is inappropriate to pool these trials and have therefore analysed them separately. All other drug classes, which were evaluated in only single trials, are reported in the text.
There were no trials of tricyclic antidepressants, other antidepressants (e.g., MAOIs), antiepileptics, nor of other pharmacological agents (e.g., benzodiazepines, ketamine).
Comparison 1: newer generation antidepressants versus placebo
Three trials evaluated the effectiveness of different newer generation antidepressants (NGAs) in patients admitted to general hospital facilities following either self‐poisoning or attempted suicide. The first compared 30‐60 mg mianserin or 75‐150 mg nomifensine against placebo (N = 114; Hirsch 1982), the second compared 30 mg mianserin against placebo (N = 58; Montgomery 1983), whilst the third compared 40 mg paroxetine per day plus weekly/fortnightly supportive psychotherapy to placebo and supportive psychotherapy (N = 91; Verkes 1998). We acknowledge that these antidepressants are from different drug classes (heterocyclic, SSRI, and NDRI respectively); however, we have combined results for these agents into one comparison in order to address the question of whether antidepressant treatment using NGAs might be of general benefit in this patient population. We have also sub‐grouped the individual drugs in a post hoc analysis.
Primary outcome
1.1 Repetition of SH
There was no evidence of a significant treatment effect for mianserin or nomifensine at 12 weeks (15/76 versus 6/38; OR 1.31, 95% CI 0.46 to 3.71; k = 1; N = 114; Hirsch 1982) or for mianserin at six months (8/17 versus 12/21; OR 0.67, 95% CI 0.18 to 2.41; k = 1; N = 38; Montgomery 1983). In the trial involving paroxetine with adjunct psychotherapy, furthermore, there was also no evidence of a significant treatment effect at 12 months (15/46 versus 21/45; 95% OR 0.55, 0.24 to 1.29; k = 1; N = 91; Verkes 1998).
A post hoc analysis was performed to assess repetition for all three trials at the last follow‐up (i.e., 12 weeks (Hirsch 1982), six months (Montgomery 1983), and 12 months ( Verkes 1998). Again, there was no evidence of a significant treatment effect between groups (Analysis 1.1; OR 0.76, 95% CI 0.42 to 1.36; k = 3; N = 243). The quality of evidence, assessed using the GRADE criteria, was low (see Table 1).
1.1. Analysis.

Comparison 1: Newer generation antidepressants versus placebo, Outcome 1: Repetition of SH at last follow‐up
To assess the efficacy of each NGA drug, the intervention arms in Hirsch 1982 were separated into nomifensine vs. placebo (n = 76) and mianserin vs. placebo (n = 76). There was no evidence of a significant difference between drugs (Analysis 1.2; test for subgroup differences: Chi² = 1.25; df = 2; p = 0.53; I² = 0%).
1.2. Analysis.

Comparison 1: Newer generation antidepressants versus placebo, Outcome 2: Repetition of SH at last follow‐up (by NGA drug)
Secondary outcomes
1.2 Treatment adherence
Data on treatment adherence was reported in one trial (Verkes 1998), however no numerical data for treatment adherence were reported. Instead the trial authors state that "...analysis of capsule counts at each visit revealed no statistically significant differences between treatments" (p.545), and that "...levels of platelet serotonin were not substantially decreased at week two in five patients, indicating doubtful adherence, and showed a manifest increase following a previous definite decrease in four other patients—at week 8 (n = 2) and week 52 (n = 2)" (p.545).
1.3 Depression
Two trials reported outcome data for depression (Verkes 1998; Hirsch 1982). In Verkes 1998, however, no numerical data were reported. Instead the trial authors state there was "...no significant treatment effect..." for this outcome (p.545). Also, although mean scores on the HDRS were reported by Hirsch 1982, insufficient information was provided to enable calculation of accompanying SDs via imputation using the formula outlined by Townsend 2001.
1.4 Hopelessness
Information on hopelessness was reported in one trial (Verkes 1998). Once again, however, no numerical data were reported. Instead the trial authors state there was also "...no significant treatment effect..." for this outcome (p.545).
1.5 Suicidal ideation
No data available.
1.6 Problem‐solving
No data available.
1.7 Suicide
Numbers of suicides during follow‐up were available for two trials (Hirsch 1982; Verkes 1998). In the first, no participant died by suicide during the six month follow‐up period (Hirsch 1982). In the second, one suicide occurred in the control group by the 12 month follow‐up period; however, there was no evidence of a significant treatment effect for NGAs on suicide in this trial (0/46 versus 1/45; OR 0.32, 95% CI 0.01 to 8.04; k = 1; N = 91).
Comparison 2: antipsychotics versus placebo or other comparator drug/dose
The effectiveness of 'prophylactic' injections of the antipsychotic flupenthixol compared to placebo was investigated in one small trial of patients admitted to a general hospital following a "suicidal act" (N = 37; Montgomery 1979). A second trial investigated the effectiveness of low dose (12 mg) fluphenazine compared to ultra‐low dose (1.5 mg) fluphenazine in individuals admitted to an emergency psychiatric unit following a suicide attempt (N = 58; Battaglia 1999). The authors of this trial state that "[t]he 'ultra‐low' (1.5 mg) was chosen to represent the extreme low end of possible pharmacologic effect for fluphenazine treatment" (p.363). However, because the comparator in these two trials was different (i.e., placebo in Montgomery 1979 and ultra‐low dose fluphenazine in Battaglia 1999) we have not combined the results of these two trials in a meta‐analysis.
Primary outcome
2.1 Repetition of SH
A significant treatment effect for flupenthixol was found for repetition of SH in the six months following trial entry (3/14 vs. 12/16; OR 0.09, 95% CI 0.02 to 0.50; k = 1; N = 30), although the overall quality of evidence was very low (see Table 2).
There was no significant treatment effect for low‐dose fluphenazine, however, on repetition of SH during the six month follow‐up period (12/27 versus 9/26; OR 1.51, 95% CI 0.50 to 4.58; k = 1; N = 53). The quality of evidence for this outcome was very low (see Table 2).
Secondary outcomes
2.2 Treatment adherence
There was no evidence of a significant treatment effect for the number of participants who completed the full course of treatment in Montgomery 1979 (14/18 versus 16/19; OR 0.66, 95% CI 0.12 to 3.45; k = 1; N = 37).
No data on treatment adherence were available for Battaglia 1999.
2.3 Depression
No data available.
2.4 Hopelessness
No data available.
2.5 Suicidal ideation
No data available.
2.6 Problem‐solving
No data available.
2.7 Suicide
No data on suicides were available for Montgomery 1979. In Battaglia 1999, no participant died by suicide in either group during the six month follow‐up period.
Comparison 3: mood stabilisers versus placebo
In a single trial the effectiveness of lithium was compared to placebo in individuals who had made suicide attempts, defined as SH acts with explicit or implicit evidence that the individual intended to die, in the context of an depressive spectrum disorder (N = 167; Lauterbach 2008).
Primary outcome
3.1 Repetition of SH
There was no evidence of a significant treatment effect for lithium on repetition of SH at the 12 month follow‐up period (7/84 versus 7/83; OR 0.99, 95% CI 0.33 to 2.95; k = 1; N = 167). There was evidence of low quality for this intervention (see Table 3). Please note that these ORs differ modestly from those reported by the authors in correspondence; however, there is no material difference in either the overall direction or significance of these results.
Secondary outcomes
3.2 Treatment adherence
No data available.
3.3 Depression
There was also no evidence of a significant treatment effect for lithium on depression at either the three month (mean 9.11, SD 7.0, n = 59 versus mean 9.45, SD 6.8, n = 51; MD ‐0.34, 95% CI ‐2.92 to 2.24; k = 1; N = 110), six month (mean 9.48, SD 7.2, n = 47 versus mean 8.94, SD 8.7, n = 38; MD 0.54, 95% CI ‐2.91 to 3.99; k = 1; N = 85), or 12 month (mean 8.48, SD 7.5, n = 31 versus mean 8.87, SD 8.1, n = 33; MD ‐0.39, 95% CI ‐4.21 to 3.43; k = 1; N = 64) follow‐ups. It should be noted that these MDs differ modestly from those reported by the authors; however, there is no material difference in either the overall direction or significance of these results.
3.4 Hopelessness
There was no evidence of a significant treatment effect for lithium on hopelessness at either the three month (mean 8.91, SD 5.4, n = 45 versus mean 9.47, SD 6.2, n = 53; MD ‐0.56, 95% CI ‐2.86 to 1.74; k = 1; N = 98), six month (mean 8.32, SD 6.4, n = 28 versus mean 9.16, SD 7.0, n = 24; MD ‐0.84, 95% CI ‐4.51 to 2.83; k = 1; N = 52), or 12 month (mean 8.88, SD 5.4, n = 26 vs. mean 9.04, SD 6.1, n = 25; MD ‐0.16, 95% CI ‐3.33 to 3.01; k = 1; N = 51) follow‐ups. These MDs differ modestly from those reported by the authors; however, there is no material difference in either the overall direction or significance of these results.
3.5 Suicidal Ideation
There was no evidence of a significant treatment effect for lithium on the number of patients reporting suicidal ideation, defined as a score of greater than zero on the Scale for Suicidal Ideation, at either the three month (20/58 versus 16/51; OR 1.15, 95% CI 0.52 to 2.57; k = 1; N = 109), six month (15/45 versus 11/37; OR 1.18, 95% CI 0.46 to 3.02; k = 1; N = 82), or 12 month (8/31 versus 11/32; OR 0.66, 95% CI 0.22 to 1.97; k = 1; N = 63) follow‐ups. These ORs differ modestly from those reported by the authors in correspondence; however, there is no material difference in either the overall direction or significance of these results.
3.6 Problem‐solving
No data available.
3.7 Suicide
There was no evidence of a significant treatment effect for lithium on suicides (0/84 versus 3/83; OR 0.14, CI 0.01 to 2.68; k = 1; N = 167). The authors, however, state that: "[a]ssuming the number of rare events to be Poisson‐distributed and taking the event proportion in the placebo group as a comparison standard (3/83 = 3.6%), the probability of no event was lower than 5%...Taking into account the available person‐years that are somewhat larger in the lithium group compared with the placebo group, it can be shown that the 95% CI of the placebo incidence rate of completed suicides of IR = 0.065 (range 0.013–0.190) did not cover the zero IR of the lithium group (p = 0.049)" (p.475).
Comparison 4: natural products versus placebo
One trial investigated the effectiveness of dietary supplementation with omega‐3 essential fatty acid (n‐3EFA) in patients admitted to accident and emergency facilities following an episode of SH (N = 49; Hallahan 2007).
Primary outcome
4.1 Repetition of SH
There was no evidence of a significant treatment effect for natural products during the 12 week treatment phase (7/22 versus 7/27; OR 1.33, 95% CI 0.38 to 4.62; k = 1; N = 49). This was associated with a low quality of evidence, however (see Table 4).
Additionally, there was no difference between groups in the mean number of SH episodes per participant between those receiving the supplement and those receiving placebo (mean 0.41 versus mean 0.41). However, SDs were not reported and insufficient information was available to enable imputation of SDs using the formula outlined by Townsend 2001 for this outcome.
Secondary outcomes
4.2 Treatment adherence
There was no evidence of a significant treatment effect for natural products on treatment adherence as measured by pill counts (19/22 versus 20/27; OR 2.22, 95% CI 0.50 to 9.85; k = 1; N = 49).
4.3 Depression
Mean and SD scores on the BDI and HRSD were reported as adjusted improvement scores. The authors report that there were "significant improvements in BDI scores at 12 weeks (p = 0.004) in the [omega‐3] group. Moreover, more patients in the [omega‐3] group attained more than 50% (p = 0.001) and 70% (p = 0.001) reduction (response and remission respectively) in symptoms...Similar data were observed for the HRSD" (p.119).
4.4 Hopelessness
No data available.
4.5 Suicidal ideation
There was a significant treatment effect for natural products on the proportion of participants reporting suicidal ideation at the 12 week follow up (8/22 versus 19/27; OR 0.24, 95% CI 0.07 to 0.80; k = 1; N = 49).
4.6 Problem‐solving
No data available.
4.7 Suicide
No participant died by suicide in either group during the 12 week treatment period.
Discussion
This systematic review is an update of previous versions of the Cochrane review (Hawton 1998; Hawton 1999); it also adds to raw data we provided to the UK’s National Institute for Health and Care Excellence (NICE) in 2010 to contribute to its guideline on longer‐term management of self‐harm NICE 2011). Whilst the previous review included psychosocial interventions, this update is solely focused on psychopharmacological treatments. Previously we commented on the paucity of evidence on which to make firm conclusions about the most effective form of treatment for patients who have recently engaged in SH. In the previous versions of this review we only focused on a limited number of clinical outcomes; namely repetition of SH and suicide. In this update we have considerably expanded the range of clinically relevant outcomes that have been examined to include treatment adherence, depression, hopelessness, problem‐solving, and suicidal ideation where available.
In our previous review we commented on the fact that the majority of trials included either patients who had all taken overdoses, or samples where the majority had. This appears still to be the case, reflecting the types of patients who present to general hospitals following SH (Hawton 2007). However, there are other important patient subgroups, such as those who cut themselves. None of the pharmacological trials included in this review specifically focused on these patients. It should be noted that people who repeat SH may change the methods they use (Lilley 2008).
None of the trials included information on adverse effects of pharmacotherapy, other than further suicidal behaviour.
We have used the intention‐to‐treat method where data allowed. This was usually possible when examining the outcomes of repetition of SH and suicide. Where outcomes relied on patient interview, this was generally not possible and we have instead used all available case data.
Summary of main results
Newer generation antidepressants
Three trials in which newer generation antidepressants were evaluated in SH patients indicated no overall benefit or negative effect in terms of repetition of SH compared with placebo. However, it should be noted that the trials were relatively small and the confidence intervals around the point estimate of the treatment effects was relatively wide. As only one death by suicide was recorded it was not possible to determine whether there is an effect of newer generation antidepressants on suicide. In this review we combined three newer generation antidepressants from different drug classes (i.e., mianserin, nomifensine and paroxetine). Although we acknowledge that these drugs have different mechanisms of action, we chose to combine them for the purposes of meta‐analysis on the basis that their potential impacts on risk of SH, for example through a serotonin‐mediated reduction in impulsivity and enhanced emotion regulation, are likely to be similar. A post hoc analysis, furthermore, suggested that no one antidepressant was superior to the others in reducing repetition of SH.
Antipsychotics
In a single trial in patients with a history of multiple episodes of SH, the use of the depot antipsychotic medication flupenthixol appeared to have considerable benefits compared with placebo in terms of reduced repetition of SH (Montgomery 1979). This trial has not been replicated and similar trials have not been conducted with newer oral antipsychotics, although they have been conducted in patients with borderline personality disorder with or without a history of SH (Stoffers 2010). In another single trial, two different doses of fluphenazine did not appear to differ in their impact on repetition of SH in patients with a history of multiple episodes of SH (Battaglia 1999).
Mood Stabilisers
In a comparison of lithium versus placebo in suicide attempters with depression, no beneficial impact of lithium was found for repetition of SH, depression, hopelessness, or suicidal ideation (Lauterbach 2008). It should also be noted that despite randomisation the lithium‐treated group differed significantly from the placebo‐treated group in terms of more patients having a history of multiple suicide attempts and personality disorder. However, this group had lower suicidal intent scores associated with the index attempt.
Our analysis also did not show a beneficial effect for lithium on suicide, although the authors claimed that there was such an effect. This was based on there being no suicides in the lithium group and three in the placebo group, and taking account of exposure time in each group. While we must conclude that our result showed no significant effect on suicide, the trend revealed evidence of a beneficial effect of lithium on suicide and SH in affective disorders (Cipriani 2013a), suggesting that this is an area for future research.
Natural products
In a single trial comparing provision of omega‐3 essential fatty acids with placebo (in addition to routine therapy) for patients with a history of repeated episodes of SH, there appeared to be no impact on further repetition of SH. However, fewer patients who received the supplement reported suicidal ideation at follow‐up (Hallahan 2007).
Overall completeness and applicability of evidence
Completeness of evidence
There have been few trials of pharmacological interventions for SH patients (we identified just seven), especially compared with the number of trials of psychosocial treatments. Therefore our conclusions are limited to a small range of drugs and outcomes.
Three trials focused on newer generation antidepressants. This is in keeping with the high prevalence of depression in SH patients in general presenting to clinical services (Hawton 2013) and with evidence that antidepressants are commonly prescribed in SH patients (Haw 2001). However, these trials included relatively old drugs (i.e., mianserin, nomifensine and paroxetine). The antidepressants now most commonly used for treatment of depression are SSRIs, but only one older drug from this group (i.e., paroxetine) has been evaluated in SH patients. Increasingly, SNRIs are also being used for treatment of depression (Hollingworth 2010; Ilyas 2012; Olfson 2009), but none of this group of antidepressants has so far been evaluated in this clinical population. Because of the small number of trials of antidepressants, and the lack of clarity over the mechanism of action in relation to SH behaviour for these agents, we have combined results for the different antidepressants in order to establish whether there is evidence of a generalised effect of antidepressants in this patient population.
Limited data were available on secondary outcomes. Only three trials included information on depression and two on hopelessness and suicidal ideation. Information on suicide was only published in one trial (Lauterbach 2008) and had to be requested from authors for the remaining trials.
Applicability of evidence
The gender representation of participants in these trials appears to have been in accord with SH patients more generally (Hawton 2007). Five of the seven trials focused on individuals with a history of repeated SH, which is a particular issue in this clinical population (Owens 2002; Zahl 2004; Carroll 2014). Only one trial recorded information on intent of participants (Lauterbach 2008), which is surprising given the association of SH with future risk of suicide (Owens 2002; Carroll 2014).
It should be noted that this review focused exclusively on patients who have engaged in SH. As a result, we have excluded patients with conditions such as borderline personality disorder who have not engaged in SH and mixed trials of patients with either SH or suicidal ideation in the absence of suicidal behaviour. For further information on the treatment of patients with borderline personality disorder the reader is referred to Stoffers 2010.
Quality of the evidence
The trials included in this review were likely too small to detect significant differences in proportions of patients experiencing the primary outcome, namely repetition of SH. Additionally, quality of evidence, as assessed using the GRADE approach, was generally low to very low suggesting that further research is likely to have an important impact on our confidence in the estimate of treatment effectiveness, and may in fact change the estimate.
Limitations in design and implementation
Five trials possessed high risk of bias in relation to at least one aspect of trial design, with weaknesses most commonly observed with respect to incomplete outcome data. Attrition bias therefore cannot be ruled out. Details on personnel blinding were not reported in three trials whist a further two reported no information on outcome assessor blinding. Performance bias and detection bias also cannot be ruled out.
Participants prescribed psychotropic medication other than the intervention drugs were typically excluded from the majority of the included trials (Battaglia 1999; Hallahan 2007; Hirsch 1982; Lauterbach 2008; Verkes 1998). Information on medication use was not reported in one trial of antipsychotics (Montgomery 1979); consequently the effect of this intervention may have been confounded.
Indirectness of evidence
Repetition of SH was measured using either self‐reported information or hospital re‐presentation in all seven trials included in this review. Information on secondary outcomes was measured using widely validated psychometric measures (e.g., BDI, BHS) that were not subjected to modifications in scoring.
Unexplained heterogeneity or inconsistency of results
Meta‐analysis was only undertaken for newer generation antidepressants, for which results between trials were reasonably consistent as indicated by the I2 statistic values.
Imprecision of results
Results of the individual trials included in this review were associated with a high level of imprecision as indicated by the wide confidence intervals around the effect size estimates.
Probability of publication bias
Presence of publication bias could not be evaluated as no meta‐analysis in the present review included 10 or more trials. However, it is notable that one trial (Hirsch 1982) was never published in full whilst a second (Montgomery 1979) was not published in a peer‐reviewed journal.
Potential biases in the review process
We have no reason to believe we have not identified all relevant trials of pharmacological interventions. Nevertheless, by using the random‐effects model in all analyses, our results possess greater generalisability than if we had used the fixed‐effect model (Erez 1996). However, because our review criteria included only trials of patients who had all engaged in SH and presented to hospital in the preceding six months, we excluded trials where only some of the patients had engaged in SH and also trials where SH was an outcome measured in general pharmacological interventions for patients with psychiatric disorders. Data on repetition of SH were available for all the included trials and information on suicides was available for most (although the number of events was small).
We chose to combine three different newer generation antidepressants from three different drug classes in order to investigate whether there was an overall impact of antidepressant therapy, even though the pharmacological mechanisms of these drugs are likely to have differed. Also, the phenothiazine drugs flupenthixol and fluphenazine (investigated in two trials) are less often used in clinical practice than used to be the case.
We also combined data from different time points in our analysis of newer generation antidepressants (i.e., 12 weeks, six months, and 12 months) to make maximal use of the limited data on the efficacy of these drugs. This might however limit the applicability of the results.
Agreements and disagreements with other studies or reviews
We have identified one review of pharmacotherapy in "self‐mutilation", which included a wide range of evidence, not just from RCTs (Smith 2005). It also included trials in which not all participants had a history of SH. While there were encouraging findings, especially with regard to mood stabilisers and antipsychotics, effects were less strong in randomised than non‐randomised trials.
Another Cochrane review considered pharmacotherapy for borderline personality disorder and concluded that both antipsychotics and mood stabilisers could be beneficial for the core symptoms of the disorder but results were inconclusive for suicidal behaviour (Stoffers 2010).
Given the positive effects of lithium with regard to suicidal behaviour in patients with affective disorders found in a second Cochrane review (Cipriani 2013a), there may also be a role for lithium for some SH patients. Given that the review by Cipriani 2013a focused specifically on patients with either depression or bipolar disorder, rather than those with a history of SH, it may well be that the primary indication for lithium in the prevention of further SH is in patients with either of these disorders. While the negative results of the Lauterbach 2008 trial, which included patients with "an affective spectrum disorder", would appear contrary to this suggestion, it should be noted that there were no suicides in the lithium‐treated group.
A further Cochrane review examined pharmacological interventions for self‐injurious behaviour in adults with intellectual disabilities (Rana 2013). The authors reported that there was weak evidence that an opioid receptor antagonist (naltrexone) and an antidepressant (clomipramine) were more beneficial than placebo in reducing self‐injurious behaviour in this population. However, bias in the included trials precluded a definitive conclusion.
Authors' conclusions
Implications for practice.
Given the paucity of trials, which were generally of low quality, it is not possible to reach firm conclusions regarding pharmacological interventions in SH patients. While depression is common in these patients, we found no evidence that newer generation antidepressants prevent repetition of SH although it should be noted that one of these drugs, nomifensine, is no longer used in the UK. While in a single early trial of flupenthixol in those with multiple episodes of SH there was evidence of benefit for repetition of SH, this requires replication ‐ preferably involving more modern antipsychotics ‐ before such treatment can be considered for use in routine clinical practice. While our analysis of the impact of lithium on suicide following SH showed no effect, this differed from the conclusion of the authors (Lauterbach 2008). Clinicians treating SH patients with pharmacological agents must also be aware of the extra risks of overdose in this population, and especially the relative toxicity of different drugs that might be used.
Implications for research.
While the results of this review did not indicate any benefit of newer generation antidepressants, the high prevalence of depression in SH patients (Hawton 2013), the strong association between both depression and SH and suicide, and the frequent use of antidepressants in treatment following SH (Haw 2001) suggest that there should be further evaluation of antidepressants in this patient population. This is particularly true for patients with a diagnosis of depression, and would preferably involve the use of more modern and less toxic antidepressants, which could be combined with psychosocial interventions. The apparent benefit of flupenthixol in an old trial of its use in individuals with a history of multiple episodes of SH suggests there might be value in conducting future trials on the use of antipsychotic drugs in subgroups of patients with a history of multiple episodes of SH. The encouraging results of trials of lithium in patients with affective disorders in terms of suicidal behaviours (Cipriani 2013a), and the uncertainty around the impact of lithium on suicide in the one trial included in this review, suggest that there should be further evaluation of lithium in this patient population.
Evidence that self‐injury may be associated with endorphin release (Nock 2010), suggests that investigations of drugs that can block opioid receptors might be of benefit to those patients that engage in this form of SH.
In view of the paucity of RCTs of pharmacological agents in SH patients, perhaps reflecting difficulties in conducting these trials due to safety considerations, valuable information about the potential impacts of drug treatments on suicidal behaviour might be gained from population‐wide registry data in which information about drug prescriptions, hospital presentations for SH, and suicide are routinely recorded.
In trials of interventions in SH patients it is important that the characteristics of the participants are described in detail, including in addition to gender and age, details of history of SH, methods used, degree of suicidal intent, and presence of comorbid psychiatric disorders. Such information could help to provide evidence on prediction of response to medication according to clinically relevant sub‐groups.
Additionally, any pharmacological interventions in SH patients should include a range of outcome measures, not just SH and suicide, but also adherence, mood, and attitudes to treatment as these may help to identify contributors to any apparent benefits or lack of impact. It is also important that adverse effects of treatment medication, both short‐ and long‐term, are carefully evaluated (including possible use of the medication for self‐poisoning).
What's new
| Date | Event | Description |
|---|---|---|
| 14 September 2021 | Amended | Hyperlink added to editorial note. |
History
Review first published: Issue 7, 2015
| Date | Event | Description |
|---|---|---|
| 13 September 2021 | Amended | There is a more recent Cochrane review on this topic: https://doi.org/10.1002/14651858.CD013669.pub2 An editorial note has been added to redirect readers. |
| 25 June 2015 | New search has been performed | Original review CD001764 was split into three and updated; no new studies were identified for this review, but methodology was updated. |
| 25 June 2015 | New citation required but conclusions have not changed | Review updated |
Acknowledgements
We thank Brian Hallahan and John Rush for providing us with unpublished data.
We also wish to thank Andrea Cipriani, Jane Dennis, Jessica Sharp, and Catroina Shatford for advice on data extraction and management issues.
This project has previously had support from the National Co‐ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). KH is funded by Oxford Health NHS Foundation Trust. He is a National Institute for Health Research (NIHR) Senior Investigator and personal funding from NIHR helped support this update. The opinions expressed are solely those of the authors.
Appendices
Appendix 1. CCDANCTR search strategy
Date range searched: 01.01.56 to 02.09.14. #1. ((deliberat* or self*) NEXT (destruct* or harm* or injur* or mutilat* or poison*)):ab,ti,kw,ky,emt,mh,mc #2. DSH:ab #3. (parasuicid* or “para suicid*”) #4. (suicid* NEAR2 (attempt* or episod* or frequen* or future or histor* or multiple or previous* or recur* or repeat* or repetition)):ab,ti,kw,ky,emt,mh,mc #5. “post suicid*” #6. (suicid* and (BPD or “borderline personality disorder”)) #7. (overdos* or “over dos*”) #8. ((crisis or suicid*) NEAR (emergenc* or hospital or outpatient or “repeat* attend*” or “frequent* attend*” )):ab,ti,kw,ky,emt,mh,mc #9. (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) [ab:abstract; ti:title; kw:keywords; ky:additional keywords; emt:EMTREE headings; mh:MeSH headings; mc:MeSH checkwords]
Appendix 2. EMBASE, MEDLINE, PreMEDLINE, PsycINFO and CENTRAL Search Strategies
Search Strategy 2012 to 2013:
EMBASE, MEDLINE, PreMEDLINE, PsycINFO (OVID SP interface)
Date range searched: 01.01.1998 to 13.10.2013.
1. automutilation/ or drug overdose/ or exp suicidal behavior/ 2. 1 use emez 3. overdose/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ 4. 3 use mesz, prem 5. drug overdoses/ or self destructive behavior/ or exp self injurious behavior/ or attempted suicide/ or suicidal ideation/ or suicide/ or suicide prevention/ or suicide prevention centers/ or suicidology/ 6. 5 use psyh 7. (auto mutilat$ or automutilat$ or cutt$ or head bang$ or head bang$ or overdos$ or (self adj2 cut$) or self destruct$ or selfdestruct$ or self harm$ or selfharm$ or self immolat$ or selfimmolat$ or self inflict$ or selfinflict$ or self injur$ or selfinjur$ or selfmutilat$ or self mutilat$ or self poison$ or selfpoison$ or suicid$).ti,ab. 8. or/2,4,6‐7 9. exp "clinical trial (topic)"/ or exp clinical trial/ or crossover procedure/ or double blind procedure/ or placebo/ or randomization/ or random sample/ or single blind procedure/ 10. 9 use emez 11. exp clinical trial/ or exp "clinical trials as topic"/ or cross‐over studies/ or double‐blind method/ or placebos/ or random allocation/ or single‐blind method/ 12. 11 use mesz, prem 13. (clinical trials or placebo or random sampling).sh,id. 14. 13 use psyh 15. (clinical adj2 trial$).ti,ab. 16. (crossover or cross over).ti,ab. 17. (((single$ or doubl$ or trebl$ or tripl$) adj2 blind$) or mask$ or dummy or doubleblind$ or singleblind$ or trebleblind$ or tripleblind$).ti,ab. 18. (placebo$ or random$).ti,ab. 19. treatment outcome$.md. use psyh 20. animals/ not human$.mp. use emez 21. animal$/ not human$/ use mesz 22. (animal not human).po. use psyh 23. (or/10,12,14‐19) not (or/20‐22) 24. 8 and 23
CENTRAL (Wiley interface)
Date range searched: 01.01.1998 to 13.10.2013.
#1. MeSH descriptor: [Drug Overdose], this term only #2. MeSH descriptor: [Self‐Injurious Behavior], this term only #3. MeSH descriptor: [Self Mutilation], this term only #4. MeSH descriptor: [Suicide], this term only #5. MeSH descriptor: [Suicide, Attempted], this term only #6. MeSH descriptor: [Suicidal Ideation], this term only #7. auto mutilat*" or automutilat* or cutt* or "head bang*" or headbang* or overdos* or "self destruct*" or selfdestruct* or "self harm*" or selfharm* or "self immolat*" or selfimmolat* or "self inflict*" or selfinflict* or "self injur*" or selfinjur* or selfmutilat* or "self mutilat*" or "self poison*" or selfpoison* or suicid*:ti #8. "auto mutilat*" or automutilat* or cutt* or "head bang*" or "head bang*" or overdos* or "self destruct*" or selfdestruct* or "self harm*" or selfharm* or "self immolat*" or selfimmolat* or "self inflict*" or selfinflict* or "self injur*" or selfinjur* or selfmutilat* or "self mutilat*" or "self poison*" or selfpoison* or suicid*:ab #9. #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
Appendix 3. Journals hand‐searched for relevant literature in the original version of this review
1. Archives of Suicide Research (1995‐1998).
2. Crisis (1980‐1998).
3. Suicide and Life‐Threatening Behavior (1971‐1998).
4. Der Nervenarzt (1950‐1979).
5. Journal of Adolescence (1978‐1996).
6. Journal of Affective Disorders (1994‐1996).
7. Journal of the American Academy of Child and Adolescent Psychiatry (1978‐1996).
8. Journal of Clinical Psychiatry (1978‐1996).
9. Journal of Psychiatric Research (1961‐1972) and (1985‐1996).
10. Social Psychiatry (1966‐1987).
11. Social Psychiatry & Psychiatric Epidemiology (1988‐1996).
Appendix 4. Data collection and analysis methods used for the original review
Selection of studies
In the original version of this review, Sarah Stockton, Librarian at the University of Oxford, conducted the systematic search for trials. Two out of TTS, EA, ET, and KH then independently screened the titles of identified trials for relevancy. A distinction was made between:
1) eligible studies, in which any psychological and/or psychopharmacological treatment was compared with a control (e.g., standard or less intensive types of aftercare or medication), and;
2) general treatment studies, without any control treatment.
A second screening was then undertaken in which two of TTS, EA, ET, and KH independently screened the full text of relevant studies with reference to the following inclusion criteria:
1. All participants must have engaged in SH (i.e., self‐poisoning or self‐injury) shortly prior to randomisation;
2. Studies must have reported the number of participants engaging in a repeat episode of SH as an outcome measure;
3. Study participants must have been randomised to the treatment and control groups.
Data extraction and management
Data extraction was carried out by EA and a second member of the review group (TTS, ET, or KH) using a standardised data extraction form. Members of the review team extracted data independently from one another. Disputes were resolved through consensus discussions with a third member of the review group with assistance from the CCDAN editorial base.
We extracted data from each eligible trial concerning the characteristics of patients, the details of the interventions used, and information on the number of participants engaging in a repeat episode of SH during the follow‐up period. Where these details were unclear, corresponding authors were contacted to provide additional clarification.
Assessment of risk of bias
For the original version of this review, the quality of the studies was rated by three independent review authors (EA and ET plus another member of the review group). Review authors were blind to authorship according to the recommended Cochrane criteria for quality assessment (Sackett 1997).
Given that the quality of concealment of allocation can affect the results of trials (Schultz 1995), studies were assigned a quality of concealment rating ranging from C (poor quality) to A (high quality). Trials rated as inadequately concealed, for example via reference to an open random number table, were given a rating of C. Trials that did not provide adequate details about how the randomisation procedure was carried out were given a rating of B, and trials that were deemed to have taken adequate measures to conceal allocation, for example through the use of serially numbered, opaque, sealed envelopes or numbered or coded bottles or containers, were rated as A quality. Where the concealment of allocation was not clearly reported (i.e., where trials were initially in category B), we contacted corresponding authors for more information. Where raters disagreed as to the category to which a trial had been allocated, the final rating was made by consensus discussion in consultation with TTS, KH, and a third member of the review group.
Measures of treatment effect
RevMan version 3.0, was used to calculate summary odds ratios and accompanying 95% CIs for the number of participants engaging in a repeat episode of SH during the follow‐up period.
Unit of analysis issues
1. Cluster trials
Clustering was an issue in one included study, however, as the authors reported adjusting for the effects of clustering in their primary analyses, we reproduced the data from this study as if it came from a non‐cluster randomised study.
2. Studies with multiple treatment groups
One included study presented data for multiple treatment groups (Hirsch 1982). As both treatment groups were prescribed antidepressants in this study, we combined the data from these two treatment arms.
Dealing with missing data
Where data on the primary outcome measure were incomplete or excluded from the study, corresponding author(s) were contacted to obtain further information. Some authors used intention to treat analyses to account for missing data using a variety of different methods which was discussed within the 'Risk of bias' tables. We as review authors did not attempt to impute data for those studies in which intention‐to‐treat analyses had not been conducted, however. Instead, the effects of missing data were discussed in the text of the review.
Assessment of heterogeneity
Clinical heterogeneity was examined using the Chi2 statistic. Where this statistic was significant, potential causes of heterogeneity were investigated as outlined in the "Subgroup analysis and investigation of heterogeneity" section.
Assessment of reporting biases
To assess whether any meta‐analysis reported in the review were affected by reporting bias, we planned to construct funnel plots to investigate the likelihood that the results of our meta‐analysis were affected by reporting bias. We were unable to undertake these analyses, however, due to the very small number of trials included in our meta‐analyses.
Data synthesis
The Mantel‐Haenszel fixed‐effect method was used to calculate pooled summary ORs and accompanying 95% CIs.
Subgroup analysis and investigation of heterogeneity
In analyses resulting in significant heterogeneity, as indicated by the Chi2 statistic, an investigation into the source of this heterogeneity was conducted. We had planned to conduct subgroup analyses by repeater status and gender, however there were insufficient studies with appropriate data to enable these analyses to be undertaken.
Sensitivity analysis
Sensitivity analyses were undertaken where appropriate (e.g., in relation to risk of bias of included trials in the relative intensity of treatment).
Data and analyses
Comparison 1. Newer generation antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Repetition of SH at last follow‐up | 3 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.42, 1.36] |
| 1.2 Repetition of SH at last follow‐up (by NGA drug) | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 Mianserin vs. Placebo | 2 | 114 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.42, 3.29] |
| 1.2.2 Nomifensine vs. Placebo | 1 | 76 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.22, 2.91] |
| 1.2.3 Paroxetine vs. Placebo | 1 | 91 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.24, 1.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Battaglia 1999.
| Study characteristics | ||
| Methods |
Allocation: double‐blind randomisation. Follow‐up period: six months. N lost to follow up: 5/58 (8.6%) for repetition data. |
|
| Participants |
Inclusion criteria: i) aged 18‐65 years; ii) receiving treatment for suicide attempt that occurred within 30 days prior to trial entry; iii) at least 2 prior suicide attempts; iv) able to read English. Exclusion criteria: i) allergic/hypersensitive to fluphenazine; ii) tardive dyskinesia; iii) history of neuroleptic malignant syndrome; iv) narrow angle glaucoma; v) diagnosed with schizophrenia; vi) diagnosed with any terminal illness with less than 1 year life expectancy; vii) pregnant or of childbearing age and not using effective birth control; viii) current/expected to continue with treatment using medications with psychotropic effects. Numbers: of the 58 participants, 30 were allocated to the experimental arm, and 28 to the control arm. Profile: 44% (n = 28) were female, 100% (n = 58) had multiple episodes of SH prior to the index attempt, 79% (n = 45) were diagnosed with substance abuse, 35% (n = 20) with mood disorder, and 29% (n = 17) with anxiety disorder. Source of participants: patients presenting to a psychiatric hospital, screened for history of suicide attempts. Location: Dallas, Texas, USA. |
|
| Interventions |
Experimental: low dose (12 mg) fluphenazine decanoate. Control: ultra low dose (1.5 mg) fluphenazine decanoate. Therapist: none. Type of therapy offered: drug therapy. Length of treatment: six months. |
|
| Outcomes |
Included: i) repetition of SH according to self‐report; ii) suicide. Excluded: i) adverse effects; ii) drug and alcohol use. |
|
| Notes |
Source of funding: "This research was supported in part by a grant from The National Institute of Mental Health (MH‐53799) and by Mental Health Connections, a partnership between Dallas County Mental Health Mental Retardation and the Department of Psychiatry at the University of Texas South‐Western Medical Centre. Funding was from the Texas State Legislature and Dallas Country Mental Health and Mental Retardation" (p.370). Declaration of author interests: no details on author interests were provided. Other: data on suicides were obtained following correspondence with authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: “Participants...were randomized” (p.363) Comment: although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation sequence were provided. |
| Blinding (performance bias and detection bias) Of participants | Low risk |
Quote: treatment described as “double‐blinded” (p.364) Comment: given this was a drug trial, treatment could have been adequately blinded through, for example, the use of identical capsules for both the active agent and placebo. |
| Blinding (performance bias and detection bias) Of personnel | Low risk | Quote: “The research nurse and research psychiatrist...[were] both blinded to dose group” (p.364) |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk |
Quote: “The research nurse and research psychiatrist...[were] both blinded to dose group” (p.364). However, the authors also note that “measurement of side‐effects may have potentially unblinded the clinician raters to dose group..." (p.364), nevertheless, the authors suggest that "...these low rates [of side‐effects] suggest the impact was minimal” (p.364). Comment: given the low rate of adverse side‐effects observed, it is unlikely the measurement of side‐effects would have led to complete unblinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: of the 58 participants, 53 completed one month and 23 completed all six months of treatment. Intention‐to‐treat analyses were not provided, however. |
| Selective reporting (reporting bias) | Unclear risk | Comment: data on suicides were obtained from authors, suggesting that selective reporting bias may have been present. In the absence of the trial protocol, however, the degree of selective reporting cannot be ascertained. |
| Other bias | Low risk | Comment: no apparent other sources of bias. |
Hallahan 2007.
| Study characteristics | ||
| Methods |
Allocation: computer generated list. Randomisation code was revealed to researchers only after data collection was complete. Follow‐up period: 12 weeks. N lost to follow up: 0/49 (0%) for repetition of SH. |
|
| Participants |
Inclusion criteria: i) presenting to hospital following an episode of SH; ii) at least one previous episode of SH; iii) living inside the greater Dublin area. Exclusion criteria: i) current history of addiction, substance abuse, psychosis, or an eating disorder; ii) currently receiving psychotherapy; iii) history of dyslipidaemia; iv) involved with any treatment, diet, or illness known to interfere with lipid or n‐3 EFA metabolism; v) weight loss greater than 10% over the previous 3 months; vi) taking supplements containing n‐3 EFAs or have consumed fish more than once per week; vii) changes to, or introduction of, psychotropic medication during the previous 6 weeks. Numbers: of the 49 participants, 22 were allocated to the experimental arm, and 27 to the control arm. Profile: 65% (n = 32) were female, 100% (n = 49) had multiple episodes of SH prior to the index episode, 41% (n = 20) were diagnosed with alcohol misuse, 81.6% (n = 40) were diagnosed with any personality disorder, and 71.4% (n = 35) were diagnosed with borderline personality disorder. Additionally, 53% (n = 26) were currently taking psychotropic medication. Source of participants: patients presenting to hospital following an episode of SH. Location: Dublin, Republic of Ireland. |
|
| Interventions |
Experimental: EPAX 5500 in addition to usual psychiatric care. Participants received four capsules containing 305 mg EPA and 227 mg DHA daily. Total dose equalled 2128 mg per day of EPA plus DHA. Control: placebo in addition to usual psychiatric care. Participants received four capsules per day consisting of 99% corn oil and a 1% EPA/DHA mixture. Therapists: not applicable. Type of therapy offered: dietary supplement. Length of treatment: 12 weeks. |
|
| Outcomes |
Included: i) repetition of SH according to hospital records; ii) treatment adherence; iii) depression; iv) suicidal ideation; v) suicide. Excluded: i) aggression; ii) adverse effects; iii) impulsivity; iv) stress. |
|
| Notes |
Source of funding: "B.H. received salary support from the Department of Psychiatry, University of Illonois at Chicago, USA" (p.122). Declaration of author interests: "Pronova (now Epax) AS, Lysaker, Norway, provided the active preparation and placebo but were not otherwise involved in the study" (p.118). No other conflicts of interest were stated. Other: adherence was encouraged by weekly telephone calls and was determined by pill counts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: “An independent colleague dispensed either active or placebo capsules according to a computer‐generated list” (p.119) Comment: use of a computer‐generated list is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Comment: no details about allocation concealment were provided, however, the colleague responsible for allocation was referred to as "independent" suggesting that allocation may have been adequately concealed. |
| Blinding (performance bias and detection bias) Of participants | Low risk |
Quote: “Participants were prescribed four identical capsules of either active agent or placebo” (p.119) Comment: use of identical capsules for both the active agent and placebo arms means that participant blinding could have been achieved. |
| Blinding (performance bias and detection bias) Of personnel | Low risk |
Quote: "An independent colleague dispensed either active or placebo capsules according to a computer‐generated list. This code was only revealed to the researchers once data collection was complete" (p.119). Comment: use of identical capsules for both the active agent and placebo arms means that personnel blinding could have been achieved. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on outcome assessor blinding were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Analyses were performed using...the last observation carried forward...method" (p. 119). Comment: thirteen participants dropped out of the trial. Reasons given, for those allocated to the treatment arm, included: i) leaving the district (n = 2); ii) lost to follow‐up (n = 2); iii) admitted to a psychiatric hospital at time 1 (n = 2); iv) 'late' discontinuation (n = 1). Reasons for drop‐out for those randomised to the control arm included: i) 'late' discontinuation (n = 3); ii) lost to follow‐up (n = 2); iii) left district (n = 1). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured, however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no apparent other sources of bias. |
Hirsch 1982.
| Study characteristics | ||
| Methods |
Allocation: randomised placebo‐controlled trial. Follow‐up period: 12 weeks. N lost to follow‐up: 0/114 (0%) for repetition of SH data. |
|
| Participants |
Inclusion criteria: i) General Health Questionnaire score of over 20. Exclusion criteria: i) currently receiving antidepressant or antipsychotic medication. Numbers: of the 114 participants, 76 were allocated to the experimental arm and 38 to the control arm. Profile: not stated. Source of participants: patients admitted to hospital following an episode of deliberate self‐poisoning. Location: London, UK. |
|
| Interventions |
Experimental: oral administration of either 30‐60 mg/day of mianserin or 75‐150 mg/day of nomifensine. Control: placebo. Therapist: none. Type of therapy offered: drug therapy. Length of treatment: six weeks. |
|
| Outcomes |
Included: i) repetition of SH according to an unknown source; ii) suicide. Excluded: i) depression; ii) GHQ scores; iii) life events; iv) treatment adherence. |
|
| Notes |
Source of funding: no details on funding were provided. Declaration of author interests: no details on author interests were provided. Other: data on depression and treatment adherence were excluded due to inability to collect unpublished data relating to these outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "...randomised..." (p.307). Comment: although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment were provided. |
| Blinding (performance bias and detection bias) Of participants | Low risk | Comment: the nature of this trial means that participants are likely to have been blinded to allocation, possibly through the use of identical capsules for both the active agent and placebo arms. |
| Blinding (performance bias and detection bias) Of personnel | Unclear risk | Comment: no details on personnel blinding were provided. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on outcome assessor blinding were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no details on incomplete outcome data analyses were provided. |
| Selective reporting (reporting bias) | High risk | Comment: study authors claim that various outcomes are not significant, e.g., changes in GHQ and depression scores post‐treatment, however, no numerical data are reported to support these conclusions, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no apparent other sources of bias. |
Lauterbach 2008.
| Study characteristics | ||
| Methods |
Allocation: random assignment using a computerised randomisation sequence. Follow‐up period: 14 months. N lost to follow up: 22/169 (13.0%) at 1 month; 50/169 (29.6%) at 3 months; 62/169 (36.7%) at 6 months; 97/169 (57.4%) at 12 months. |
|
| Participants |
Inclusion criteria: i) at least 18 years of age; ii) suicide attempt within 3 months prior to first drug administration; iii) suicide attempt occurred within the context of a depressive spectrum disorder; iv) able to complete the screening and baseline assessment protocols; v) able to provide written informed consent. Exclusion criteria: i) diagnosed with a disorder associated with frequent suicidal behaviour (e.g., schizophrenia, borderline personality disorder, severe SH and/or substance‐related disorders including current substance addictions); ii) diagnosed with any disorder indicated for long‐term lithium treatment; iii) diagnosed with any disorder for which lithium treatment is contraindicated; iv) any other contraindications (e.g., pregnant, breast‐feeding, etc.) Numbers: of the 167 participants, 84 were allocated to the experimental arm and 83 to the control arm. Profile: 96 (57.5%) were female, 74 (44.3%) had multiple episodes of SH prior to the index attempt, 123 (76%) were diagnosed with major depression. Source of participants: patients admitted to one of five participating emergency departments following a suicide attempt. Location: various locations around Germany. |
|
| Interventions |
Experimental: oral administration of lithium carbonate according to a fixed schedule of dose augmentation (i.e., 200 mg/week) until an effective blood level of between 0.6‐0.8 mmol/L had been achieved. For most participants, this level was achieved in 3‐4 weeks. Pariticipants received, in addition, treatment as usual involving consultations with physicians in the community and referral to psychiatric treatment as necessary. Control: oral administration of a placebo capsule in addition to treatment as usual involving consultations with physicians in the community and referral to psychiatric treatment as necessary. Ingredients for the placebo capsule are not provided. Therapist: none. Type of therapy offered: drug therapy. Length of treatment: 12 months. |
|
| Outcomes |
Included: i) repetition of SH according to self‐report; ii) depression (through correspondence); iii) hopelessness (through correspondence); iv) suicidal ideation (through correspondence); v) suicide according to an unknown source. Excluded: none. |
|
| Notes |
Source of funding: "This research was supported by grants 01GI 9920 and 01GI 0220 from the German Ministry for Education and Research within the promotional emphasis 'German Research Network on Depression ' (subproject 1.2), and German Research Foundation grant LA 1975/2‐1 to Erik Lauterback. Additional funding was granted by Sanofi‐Aventis" (p.477). Declaration of author interests: "Dr. Ahrens has received a research grant from Sanofi‐Aventis" (p.477). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: "Participants were randomly assigned...using a computerised randomisation sequence" (p.471). Comment: use of a computerised randomisation sequence is likely to have minimised the role of bias in the generation of the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment were provided. |
| Blinding (performance bias and detection bias) Of participants | Low risk |
Quote: "Double‐blind assessment was conducted...although in some cases this procedure could not be maintained because of emergencies in relation to suicidal acts or insufficient drug compliance" (p.471). Comment: the nature of this trial means that participants are likely to have been blinded to allocation, possibly through the use of identical capsules for both the active agent and placebo arms. |
| Blinding (performance bias and detection bias) Of personnel | Unclear risk |
Quote: "...monitoring of lithium blood levels occurred in an independent laboratory...to ensure the double‐blind design, fake values on a randomised basis were provided for blood samples from individuals belonging to the placebo group" (p.472). Comment: as no specific details on blinding of personnel were provided, it is unclear if all personnel were blinded to allocation. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Double‐blind assessment was conducted...although in some cases this procedure could not be maintained because of emergencies in relation to suicidal acts or insufficient drug compliance" (p.471). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The evaluation of efficacy was based on an intention‐to‐treat analysis, including all participants randomised in the trial" (p.472). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured, although in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | High risk | Comment: significant imbalances between the intervention and control groups were apparent for the following characteristics: i) proportion with a history of multiple suicide attempts; ii) proportion diagnosed with any personality disorder; iii) scores on the Suicide Intent Scale for the index attempt. |
Montgomery 1979.
| Study characteristics | ||
| Methods |
Allocation: random allocation. Follow‐up period: six months. N lost to follow up: 7/37 (19%) for repetition of SH. |
|
| Participants |
Inclusion criteria: i) documented history of two or more episodes of SH. Exclusion criteria: i) diagnosis of depression or schizophrenia; ii) diagnosis of an organic illness. Numbers: of the 37 participants, 18 were allocated to the experimental arm and 19 to the control arm. Profile: 70.3% (n = 26) were female, 100% (n = 37) had multiple episodes of SH prior to the index episode. Source of participants: patients admitted to a general hospital following a suicidal act. Setting: Maidstone, UK. |
|
| Interventions |
Experimental: intramuscular administration of 20 mg flupenthixol decanoate. Control: placebo. Therapist: none. Type of therapy offered: drug therapy. Length of treatment: six months. |
|
| Outcomes |
Included: i) repetition of SH according to an unknown source; ii) treatment adherence. Excluded: i) adverse effects. |
|
| Notes |
Source of funding: no details on funding were provided. Declaration of author interests: no details on author interests were provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "Patients were randomly allocated" (p.227). Comment: although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment were provided. |
| Blinding (performance bias and detection bias) Of participants | Low risk |
Quote: “Patients...remained blind to actual treatment” (p.227). Comment: the nature of this trial means that participants are likely to have been blinded to allocation, possibly through the use of identical capsules for both the active agent and placebo arms. |
| Blinding (performance bias and detection bias) Of personnel | Unclear risk | Comment: no details on personnel blinding were provided. |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk |
Quote: “...and raters remained blind to actual treatment” (p.227) Comment: assuming ‘raters’ are outcome assessors, then outcome assessors were blinded as to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: there were seven dropouts. Reasons for drop‐out included: i) development of Parkinsonian side‐effects necessitating the removal of these patients to preserve blinding (n = 2); ii) unspecified reasons (n = 5; 2 in the experimental arm and 3 in the control arm). No attempt was made to use intention‐to‐treat analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured, however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no apparent other sources of bias. |
Montgomery 1983.
| Study characteristics | ||
| Methods |
Allocation: random allocation. Follow‐up period: six months. N lost to follow up: 0/58 (0%) for repetition of SH. |
|
| Participants |
Inclusion criteria: i) multiple previous episodes of SH; ii) diagnosis of personality disorder. Exclusion criteria: ii) diagnosis of depression or schizophrenia. Numbers: of the 58 participants, 17 were allocated to the experimental arm and 21 to the control arm. Profile: 38 (66%) were female, 58 (100%) had multiple episodes of SH prior to the index episode, and 58 (100%) were diagnosed with personality disorder (borderline or histrionic). Source of participants: patients admitted to a medical ward following SH. Location: London, UK. |
|
| Interventions |
Experimental: mianserin 30 mg daily. Control: placebo. Therapist: none. Type of therapy offered: drug therapy. Length of treatment: six months. |
|
| Outcomes |
Included: i) repetition of SH according to an unknown source. Excluded: i) depression; ii) compliance. |
|
| Notes |
Source of funding: no details on funding were provided. Declaration of author interests: no details on author interests were provided. Other: means and SDs were missing for the Montomery‐Åsberg Depression Rating Scale necessitating the omission of this outcome. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote: "[Participants were r]andomly allocated..." (p.184S). Comment: although it is likely the random sequence was adequately generated, without further information on the method used, this cannot be ascertained. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details on allocation concealment were provided. |
| Blinding (performance bias and detection bias) Of participants | Low risk | Quote: "Randomly allocated to treatment under double‐blind conditions..." (p.184S) Comment: the nature of this trial means that participants are likely to have been blinded to allocation, possibly through the use of identical capsules for both the active agent and placebo arms. |
| Blinding (performance bias and detection bias) Of personnel | Low risk | Quote: "Randomly allocated to treatment under double‐blind conditions..." (p.184S) |
| Blinding (performance bias and detection bias) Of outcome assessors | Low risk | Quote: "Randomly allocated to treatment under double‐blind conditions..." (p.184S) |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Quote: "Drop out rate of 34%" (p.185S). Comments: subsequent analyses include only those in the experimental and control groups who completed treatment, suggesting per protocol analyses were undertaken. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no reason to suspect that all outcomes were not measured, however, in the absence of the trial protocol, this cannot be ascertained. |
| Other bias | Low risk | Comment: no apparent other sources of bias. |
Verkes 1998.
| Study characteristics | ||
| Methods |
Allocation: randomised assignment. Follow‐up period: 12 months. N lost to follow up: 0/91 (0%) for repetition of SH data. |
|
| Participants |
Inclusion criteria: i) aged 18 years and over; ii) previous history of SH. Exclusion criteria: i) current diagnosis of major depression. Numbers: of the 91 participants, 46 were allocated to the experimental arm and 45 were allocated to the control arm. Profile: 100% (n = 91) had multiple episodes of SH prior to the index episode, 92% (n = 84) were diagnosed with a personality disorder, 6.5% (n = 6) were diagnosed with dysthymia, 4.4% (n = 4) were diagnosed with an anxiety disorder, 8.8% (n = 8) were diagnosed with dissociative disorder, 44% (n = 40) were diagnosed with alcohol abuse, 20.9% (n = 19) were diagnosed with adjustment disorder, 25.3% (n = 23) were diagnosed with depressive disorder, and 16.5% (n = 15) had no psychiatric diagnosis. Source of participants: patients were recruited from outpatient departments in accident and emergency wards of university hospitals in Leiden and Rotterdam. Location: Leiden and Rotterdam, the Netherlands. |
|
| Interventions |
Experimental: paroxetine 40 mg/day, plus weekly or fortnightly psychotherapy. Control: placebo, plus weekly or fortnightly psychotherapy. Therapist: none. Type of therapy offered: drug therapy. Length of treatment: 12 months. |
|
| Outcomes |
Included: i) repetition of SH according to an unknown source; ii) treatment adherence; iii) depression; iv) hopelessness; v) suicide. Excluded: i) adverse effects; ii) anger; iii) side‐effects. |
|
| Notes |
Source of funding: "Supported by grant 89‐110 CRO 012859 from the Dutch Ministry of Welfare, Health, and Cultural Affairs and a grant from SmithKline Beecham Pharmaceuticals" (p.543). Declaration of author interests: no details on author interests are provided. Other: authors note that "the number of previous suicide attempts...was manifestly associated with the risk of another suicide attempt.... With adjustment for this important predictive characteristic, paroxetine proved to reduce the recurrence of suicidal behavior significantly" (p.544‐555). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote: “Patients were randomly assigned” (p.544) Comments: correspondence with authors clarified that "all the medication...was packed in a series of blisters with consecutive numbers. This was done before the start of the study...A patient who entered the study got a consecutive number and subsequently received the medication blisters with the corresponding number." |
| Allocation concealment (selection bias) | Low risk | Comments: correspondence with authors clarified that randomisation was done at the pharmacy of SmithKline Beecham. Use of offsite allocation means that allocation was probably adequately concealed. |
| Blinding (performance bias and detection bias) Of participants | Low risk |
Quote: “Patients in the placebo group received matching placebo” (p.544) Comment: the nature of this trial means that participants are likely to have been blinded to allocation, possibly through the use of identical capsules for both the active agent and placebo arms. |
| Blinding (performance bias and detection bias) Of personnel | Low risk | Comment: use of identical capsules for both the active agent and placebo arms means that personnel blinding could have been achieved. |
| Blinding (performance bias and detection bias) Of outcome assessors | Unclear risk | Comment: no details on outcome assessor blinding were provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Efficacy was analyzed on an intention‐to‐treat basis; all available observations were used without the last point carried forward or estimating missing values. We also analyzed efficacy excluding the non‐compliant visits” (p.544) |
| Selective reporting (reporting bias) | High risk | Comment: numerical data were not provided for outcomes that were not significant, for example: i) treatment adherence; ii) depression; iii) hopelessness, suggesting that selective reporting bias may have been present. |
| Other bias | Low risk | Comment: no apparent other sources of bias. |
SH: self‐harm
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ciprani 2013 | Participants were not required to have engaged in SH prior to trial entry. |
| Gibbons 2012 | Participants were not required to have engaged in SH prior to trial entry. |
| Kelip 2010 | Participants were not required to have engaged in SH prior to trial entry. |
| Meltzer 2003 | Participants were not required to have engaged in SH prior to trial entry. |
| Nickel 2006 | Participants were not required to have engaged in SH prior to trial entry. |
| Oquendo 2011 | Participants could have engaged in SH at any point, rather than within six months. |
| Pompili 2012 | Participants were not required to have engaged in SH prior to trial entry. |
| Price 2014 | Participants were not required to have engaged in SH prior to trial entry. |
| Reeves 2008 | Participants were not required to have engaged in SH prior to trial entry. |
| Roth 2012 | Participants were not required to have engaged in SH prior to trial entry. |
| Rucci 2011 | Participants were not required to have engaged in SH prior to trial entry. |
| Sandman 2008 | Control participants were not required to have engaged in SH prior to trial entry. |
Characteristics of ongoing studies [ordered by study ID]
Liang 2014.
| Study name | Lithium for suicidal behavior in mood disorders (Li+) Trial Registration Number: NCT01928446. |
| Methods |
Allocation: double‐blind randomisation. Design: multi‐centre. Setting: Department of Veterans Affairs hospitals. Location: various locations throughout the USA. Follow‐up period: 12 months. |
| Participants |
Inclusion criteria: i) males and females; ii) any age; iii) attempted suicide within three months of randomisation, or were admitted to a mental health inpatient unit within three months of randomisation specifically for the prevention of suicide; iv) diagnosed, according to DSM‐IV‐TR criteria, with bipolar I disorder, bipolar II disorder, or current or recurrent major depression. Exclusion criteria: i) diagnosis of schizophrenia or schizoaffective disorder; ii) scoring below 10 on the Brief Orientation Memory and Concentration Test indicating presence of a major cognitive impairment; iii) lacking decision‐making capacity according to scores on Jeste's Brief Instrument for Assessing Decisional Capacity; iv) adjudicated as incompetent and have been appointed a guardian or conservator; v) six or more prior lifetime suicide attempts; vi) current or recent (within six months) use of lithium; vii) a history of adverse reactions to lithium; viii) diagnosed with congestive heart failure according to Framingham criteria; ix) diagnosed with chronic renal failure as defined by the Kidney Foundation Outcome Quality Initiative criteria; x) pregnant, may become pregnant, or using an unreliable method of birth control; xi) lactating or breastfeeding; xii) concurrently prescribed any diuretic medications, angiotensin converting enzyme inhibitors, angiotensin II receptor antagonists, haloperidol or clozapine; xiii) currently abusing alcohol, cocaine, methamphetamines, other stimulants, hallucinogens, or cannabis; xiv) enrolled in another randomised controlled trial. |
| Interventions | Individuals randomised to the intervention arm will receive oral administration of extended release lithium carbonate. Participants will be started on 600 mg/day until a steady blood plasma concentration of between 0.6 to 0.8 meg/litre is reached. Lithium will be prescribed for the duration of the 12 month follow‐up period. |
| Outcomes | Primary outcome measure: time until first repeated episode of either a suicide attempt or re‐hospitalisation for the prevention of suicide. |
| Starting date |
Start date: August, 2014. Proposed end date: August, 2018. |
| Contact information |
Name: Dr. Matthew Liang and Ms. Natalie Morgenstern. Affiliations: Department of Veterans Affairs, Boston, USA. emails: mailto:Matthew.Liang%40va.gov?subject=NCT01928446, 590, Lithium for Suicidal Behavior in Mood Disorders or mailto:Natalie.Morgenstern%40va.gov?subject=NCT01928446, 590, Lithium for Suicidal Behavior in Mood Disorders |
| Notes | Ms. Natalie Morgenstern very kindly confirmed the information provided in this record is correct. |
Sharon 2014.
| Study name | Oral ketamine for suicidal ideation. Trial registration number: NCT02037503. |
| Methods |
Allocation: double‐blind randomisation. Design: single‐centre (hospital). Setting: ambulatory patients living in the community. Follow‐up period: participants will be assessed each week during treatment for a total of three weeks, followed by a one week post‐treatment follow‐up period. Location: Tel Aviv, Israel. |
| Participants |
Inclusion criteria: i) males and females; ii) between 18 and 65 years of age; iii) admitted to the emergency department following a suicide attempt severe enough to necessitate medical intervention. Exclusion criteria: not stated. |
| Interventions | Participants randomised to the intervention arm will receive oral administration of a sub‐anaesthetic dose of ketamine. |
| Outcomes |
Primary outcome: scores on the Beck Scale for Suicidal ideation during treatment and for one week post‐treatment. Secondary outcomes: scores on Montgomery‐Åsberg Depression Rating Scale during treatment and for one week post‐treatment, treatment adherence measured as the proportion of patients who withdraw from the trial owing to the development of intolerable side‐effects, and the proportion of patients who engage in SH and/or make a suicide attempt during treatment and for one week post‐treatment. |
| Starting date |
Start date: January, 2014. Proposed end date: January, 2016. |
| Contact information |
Name: Dr. Haggai Sharon. Affiliation: Functional Brain Centre, Tel Aviv Sourasky Medical Centre, Tel Aviv, Israel. email:haggais@tlvmc.gov.il |
| Notes | Prof. Haggai Sharon very kindly provided unpublished information relating to this trial. |
Differences between protocol and review
In the original protocol for this review, we planned to assess dichotomous outcome data (i.e., repetition of self‐harm and suicide) using the Peto odds ratio. Following revisions to iterations of the Cochrane Handbook (Higgins 2003) and new statistical advice, however, we have instead used the Mantel Haenzel method in this update. For this version of the review we have also expanded the range of outcomes assessed to include depression, hopelessness, problem‐solving, and suicidal ideation. We have also used the I2 statistic, rather than the Chi2 test, to summarise between‐study heterogeneity in this version in light of revisions to the Cochrane Handbook (Higgins 2003).
We also planned to assess methodological quality of included trials by the means recommended by the contemporary version of the Cochrane Handbook (Higgins 2003). For this version of the review, we have therefore created 'Risk of bias' tables as per current recommendations. We have also refined the unit of analysis section, as per current recommendations, to include Zelen designed trials and trials that report adjusted effect sizes.
For the first comparison, a post hoc analysis was performed to assess repetition for all three trials at the last follow‐up (i.e., 12 weeks, six months, and 12 months) and by drug type (i.e., mianserin, nomifensine, and paroxetine).
We have also added four sensitivity analyses: one for trials which employed Zelen’s method of randomisation; one for trials that contributed to substantial (> 75%) levels of heterogeneity; one for trials that specifically recruited individuals diagnosed with borderline personality disorder; and a fourth for trials that included a small minority (< 15% ) of adolescent participants.
Contributions of authors
KH had the idea for the review. All authors extracted data and assessed risk of bias for included trials. Both TTS and KW conducted the statistical analyses. KH, TTS, and KW wrote the initial version of the report and all authors contributed to the writing of drafts. All authors also approved the final version of the review for publication.
Sources of support
Internal sources
University Department of Psychiatry, Warneford Hospital, Oxford, UK
Oxford Health NHS Foundation Trust, UK
External sources
NHS Executive Anglia and Oxford Research and Development Program, UK
NIHR Service Delivery and Organisation programme, UK
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Battaglia 1999 {published data only}
- Battaglia J, Wolff TK, Wagner-Johnson DS, Rush AJ, Carmody TJ, Basco MR. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. International Clinical Psychopharmacology 1999;14:361-72. [DOI] [PubMed] [Google Scholar]
Hallahan 2007 {published data only}
- Hallahan B, Hibbeln RJ, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm: Single-centre double-blind randomised controlled trial. The British Journal of Psychiatry 2007;190:118-22. [DOI] [PubMed] [Google Scholar]
Hirsch 1982 {published and unpublished data}
- Draper R, Hirsch SR. Treatment of parasuicide patients with mianserin, nomifensine and placebo: A double blind placebo controlled trial. Unpublished manuscript 1982.
- Hirsch S, Walsh C, Draper R. Parasuicide: A review of treatment interventions. Journal of Affective Disorders 1982;4:299-311. [DOI] [PubMed] [Google Scholar]
Lauterbach 2008 {published and unpublished data}
- Lauterbach E, Ahrens B, Felber W, Muller-Oerlinghausen B, Kilb B, Bischof G, et al. Suicide prevention by lithium (SUPLI): Challenges of a multi-center prospective study. Archives of Suicide Research 2005;9:27-34. [DOI] [PubMed] [Google Scholar]
- Lauterbach E, Felber W, Müller-Oerlinghausen B, Ahrens B, Bronisch T, Meyer T, et al. Adjunctive lithium treatment in the prevention of suicidal behaviour in depressive disorders: A randomised, placebo-controlled, 1-year trial. Acta Psychiatrica Scandinavica 2008;118:469-79. [DOI] [PubMed] [Google Scholar]
Montgomery 1979 {published data only}
- Montgomery S, Montgomery D, Jayanthi-Rani S, Roy D, Shaw P, McAuley R. Maintenance therapy in repeat suicidal behaviour: A placebo controlled trial. In: Proceedings of the 10th International Congress for Suicide Prevention and Crisis Intervention. Ottawa, Canada, 1979:227-9.
Montgomery 1983 {published data only}
- Montgomery S, Roy D, Montgomery D. The prevention of recurrent suicidal acts. British Journal of Clinical Pharmacology 1983;15:183s-8s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verkes 1998 {published data only}
- Verkes RJ, Mast RC, Hengeveld MW, Tuyl JP, Zwinderman AH, Kempen GMJ. Reduction by paroxetine of suicidal behavior in patients with repeated suicide attempts but not major depression. American Journal of Psychiatry 1998;155:543-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ciprani 2013 {published data only}
- Cipriani A, Girlanda F, Agrimi E, Barichello A, Beneduce R, Bighelli I, et al. Effectiveness of lithium in subjects with treatment-resistant depression and suicide risk: A protocol for a randomised, independent, pragmatic, multicentre, parallel-group, superiority clinical trial. BMC Psychiatry 2013;13:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbons 2012 {published data only}
- Gibbons RD, Brown H, Hur K, Davis JM, Mann JJ. Suicidal thoughts and behavior with antidepressant treatment: Reanalysis of the randomized placebo-controlled studies of fluoxetine and venlafaxine. Archives of General Psychiatry 2012;69:580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelip 2010 {published data only}
- Kelip JG, Oquendo MA, Stanley BH, Burke AK, Cooper TB, Malone KM, et al. Future suicide attempt and responses to serotonergic challenge. Neuropsychopharmacology 2010;35:1063-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meltzer 2003 {published data only}
- Meltzer HY, Alphs L, Green AI, Altamura C, Anard R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Archives of General Psychiatry 2003;60:82-91. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Reducing risk of suicide in schizophrenia. European Neuropsychopharmacology 2003;13:s163. [Google Scholar]
- Potkin SG, Alphs L, Hsu C, Krishnan RR, Anand R, Young FK, et al. Predicting suicidal risk in schizophrenic and schizoaffective patients in a prospective two-year trial. Biological Psychiatry 2003;54:444-52. [DOI] [PubMed] [Google Scholar]
Nickel 2006 {published data only}
- Nickel MK, Muehlbacher M, Nickel C, Kettler C, Gil FP, Bachler E, et al. Aripiprazole in the treatment of patients with borderline personality disorder: A double-blind, placebo-controlled study. The American Journal of Psychiatry 2006;163:833-8. [DOI] [PubMed] [Google Scholar]
Oquendo 2011 {published data only}
- Oquendo MA, Galfalvy HC, Currier D, Grunebaum MF, Sher L, Sullivan GM, et al. Treatment of suicide attempters with bipolar disorder: A randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. American Journal of Psychiatry 2011;168:1050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pompili 2012 {published data only}
- Pompili M, Rihmer Z, Gonda X, Serafini G, Sher L, Girardi P. Early onset of action and sleep-improving effect are crucial in decreasing suicide risk: The role of quetiapine XR in the treatment of unipolar and bipolar depression. Rivista di Psichiatria 2012;47:489-97. [DOI] [PubMed] [Google Scholar]
Price 2014 {published data only}
- Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. Effects of ketamine on explicit and implicit suicidal cognition: A randomized controlled trial in treatment-resistant depression. Depression and Anxiety 2014;31:335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2008 {published data only}
- Reeves H, Batra S, May RS, Zhang R, Dahl DC, Li X. Efficacy of risperidone augmentation to antidepressants in the management of suicidality in major depressive disorder: A randomized, double-blind, placebo-controlled pilot study. Journal of Clinical Psychiatry 2008;69:1228-36. [DOI] [PubMed] [Google Scholar]
Roth 2012 {published data only}
- Roth TL, Mahoney EM, Dundon WD, Pettinati HM. Combination pharmacotherapy sertraline and naltrexone decreases suicidal ideation scores in co-morbid depressed alcoholics. Alcoholism: Clinical and Experimental Research 2012;36:245A. [Google Scholar]
Rucci 2011 {published data only}
- Rucci P, Frank E, Scocco P, Calugi S, Miniati M, Fagiolini A, et al. Treatment-emergent suicidal ideation during 4 months of acute management of unipolar major depression with SSRI pharmacotherapy or interpersonal psychotherapy in a randomized clinical trial. Depression and Anxiety 2011;28:303-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandman 2008 {published data only}
- Sandman CA, Touchette PE, Marion SD, Chicz-DeMet A. The role of proopiomelanocortin (POMC) in sequentially dependent self-injurious behavior. Developmental Psychobiology 2008;7:680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Liang 2014 {published and unpublished data}
- NCT01928446. CSP #590 - Lithium for Suicidal Behavior in Mood Disorders. https://clinicaltrials.gov/ct2/show/NCT01928446 2013 (accessed 26-June-2015). [Google Scholar]
Sharon 2014 {published and unpublished data}
- NCT02037503. Effect of Oral Ketamine Treatment on Suicidal Ideation and Drug Resistant Major Depression, a Clinical and fMRI Study. https://clinicaltrials.gov/ct2/show/NCT02037503 2014 (accessed 26-June-2015). [Google Scholar]
Additional references
Ahmadi 2007
- Ahmadi A. Suicide by self-immolation: Comprehensive overview, experiences and suggestions. Journal of Burns Care Research 2007;28:30-41. [DOI] [PubMed] [Google Scholar]
Albrecht 2014
- Albrecht B, Staiger PK, Hall K, Miller P, Best D, Lubman DI. Benzodiazepine use and aggressive behaviour: A systematic review. Australian and New Zealand Jounral of Psychiatry 2014;48:1096-114. [DOI] [PubMed] [Google Scholar]
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
Baldessarini 2003
- Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: Update and new findings. Journal of Clinical Psychiatry 2003;64:s44-52. [PubMed] [Google Scholar]
Baldessarini 2006
- Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. Decreased risk of suicides and attempts during long-term lithium treatment: A meta-analytic review. Bipolar Disorders 2006;8:625-39. [DOI] [PubMed] [Google Scholar]
Beasley 1991
- Beasley CM, Dornseif BE, Bosomworth JC, Sayler ME, Rampey Jr AH, Heiligenstein JH, et al. Fluoxetine and suicide: A meta-analysis of controlled trials of treatment for depression. British Medical Journal 1991;303:685-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561. [DOI] [PubMed] [Google Scholar]
Beck 1974
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the Hopelessness Scale. Journal of Consulting and Clinical Psychology 1974;42:861-5. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Steer RA, Ranieri WF. Scale for Suicide Ideation: Psychometric properties of a self-report version. Journal of Clinical Psychology 1988;44:499-505. [DOI] [PubMed] [Google Scholar]
Borges 2011
- Borges G, Nock MK, Abad JMH, Hwang I, Sampson NA, Alonso J, et al. Twelve month prevalence of and risk factors for suicide attempts in the WHO World Mental Health Surveys. Journal of Clinical Psychiatry 2010;71(12):1617-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyce 2003
- Boyce P, Carter G, Penrose-Wall J, Wilhelm K, Goldney R. Summary Australian and New Zealand clinical practice guideline for the management of adult deliberate self-harm (2003). Australasian Psychiatry 2003;11:150-5. [Google Scholar]
Brausch 2012
- Brausch AM, Girresch SK. A review of empirical treatment studies for adolescent nonsuicidal self-injury. Journal of Cognitive Psychotherapy: An International Quarterly 2012;26:3-18. [Google Scholar]
Brunner 2002
- Brunner J, Parhofer KG, Schwandt P, Bronisch T. Cholesterol, essential fatty acids, and suicide. Pharmacopsychiatry 2002;35:1-5. [DOI] [PubMed] [Google Scholar]
Burns 2000
- Burns JM, Patton GC. Preventive interventions for youth suicide: A risk factor-based approach. Australian and New Zealand Journal of Psychiatry 2000;34:388-407. [DOI] [PubMed] [Google Scholar]
Carroll 2014
- Carroll R, Metcalfe C, Gunnell D. Hospital presenting self-harm and risk of fatal and non-fatal repetition: Systematic review and meta-analysis. PLoS One 2014;9:e89944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 2014
- Christensen H, Calear AL, Spijker B, Gosling J, Petrie K, Donker T, et al. Psychosocial interventions for suicidal ideation, plans, and attempts: A database of randomised controlled trials. BMC Psychiatry 2014;14:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2005
- Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicide behavior and all-cause mortality in patients with mood disorders: A systematic review of randomized trials. American Journal of Psychiatry 2005;162:1805-19. [DOI] [PubMed] [Google Scholar]
Cipriani 2013a
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta-analysis. British Medical Journal 2013;346:f3646. [DOI] [PubMed] [Google Scholar]
Cipriani 2013b
- Cipriani A, Reid K, Young AH, Macritchie K, Geddes J. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database of Systematic Reviews 2013;10:CD003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Claassen 2006
- Claassen CA, Trivedi MH, Shimizu I, Stewart S, Larkin GL, Litovitz T. Epidemiology of nonfatal deliberate self-harm in the United States as described in three medical databases. Suicide and Life-Threatening Behavior 2006;36:192-212. [DOI] [PubMed] [Google Scholar]
Comtois 2006
- Comtois KA, Linehan MM. Psychosocial treatments of suicidal behaviors: A practice-friendly review. Journal of Clinical Psychology in Session 2006;62:161-70. [DOI] [PubMed] [Google Scholar]
Cooper 2005
- Cooper J, Kapur N, Webb R, Lawlor M, Guthrie E, Mackway-Jones K, et al. Suicide after deliberate self-harm: A 4-year cohort study. American Journal of Psychiatry 2005;162:297-303. [DOI] [PubMed] [Google Scholar]
Crawford 2007a
- Crawford MJ, Thomas O, Khan N, Kulinskaya E. Psychosocial interventions following self-harm: Systematic review of their efficacy in preventing suicide. British Journal of Psychiatry 2007;190:11-7. [DOI] [PubMed] [Google Scholar]
Crawford 2007b
- Crawford MJ, Kumar P. Intervention following deliberate self-harm: Enough evidence to act? Evidence-Based Mental Health 2007;10:37-9. [DOI] [PubMed] [Google Scholar]
Curtin 2002a
- Curtin F, Elbourne D, Altman DG. Meta-analysis combining parallel and cross-over clinical trials. III: The issue of carry-over. Statistics in Medicine 2002;21:2161-73. [DOI] [PubMed] [Google Scholar]
Curtin 2002b
- Curtin F, Altman DG, Elbourne D. Meta-analysis combining parallel and cross-over clinical trials. I: Continuous outcomes. Statistics in Medicine 2002;21:2131-44. [DOI] [PubMed] [Google Scholar]
Daigle 2011
- Daigle MS, Pouliot L, Chagnon F, Greenfield B, Mishara B. Suicide attempts: Prevention of repetition. Canadian Journal of Psychiatry 2011;56:621-9. [DOI] [PubMed] [Google Scholar]
Daniel 2009
- Daniel SS, Goldston DB. Interventions for suicidal youth: A review of the literature and developmental considerations. Suicide and Life-Threatening Behavior 2009;39:252-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group. Analysing data and undertaking analyses. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Department of Health 2012
- Department of Health. Preventing suicide in England: A cross-government outcomes strategy to save lives. London: Her Majesty's Government/Department of Health, 2012. [Google Scholar]
Dew 1987
- Dew MA, Bromet EJ, Brent D. A quantitative literature review of the effectiveness of suicide prevention centers. Journal of Consulting and Clinical Psychology 1987;55:239-44. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971-80. [DOI] [PubMed] [Google Scholar]
Eddleston 2000
- Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. Quarterly Journal of Medicine: An International Journal of Medicine 2000;93:715-31. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: Methodological issues. International Journal of Epidemiology 2002;31:140-9. [DOI] [PubMed] [Google Scholar]
Engles 2003
- Engles JM, Diehr P. Imputation of missing longitudinal data: A comparison of methods. Journal of Clinical Epidemiology 2003;56:968-76. [DOI] [PubMed] [Google Scholar]
Erez 1996
- Erez A, Bloom MC, Wells MT. Using random rather than fixed effects models in meta-analysis: Implications for situation specificity and validity generalization. Personnel Psychology 1996;49:275-306. [Google Scholar]
Feighner 1999
- Feighner JP. Mechanism of action of antidepressant medications. Journal of Clinical Psychiatry 1999;60:s4-11. [PubMed] [Google Scholar]
Fleischmann 2005
- Fleischmann A, Bertolote JM, Leo D, Botega N, Phillips M, Sisask M, et al. Characteristics of attempted suicides seen in emergency-care settings of general hospitals in eight low- and middle-income countries. Psychological Medicine 2005;35:1467-74. [DOI] [PubMed] [Google Scholar]
Fleiss 1994
- Fleiss JL. Measures of effect size for categorical data. In: Cooper H & Hedges LV, editors(s). The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation, 1994:245-60. [Google Scholar]
Fond 2014
- Fond G, Loundou A, Rabu C, MacGregor A, Lançon C, Brittner M, et al. Ketamine administration in depressive disorders: A systematic review and meta-analysis. Psychopharmacology 2014;231:3663-76. [DOI] [PubMed] [Google Scholar]
Foster 1997
- Foster T, Gillespie K, McClelland R. Mental disorders and suicide in Northern Ireland. British Journal of Psychiatry 1997;170:447-52. [DOI] [PubMed] [Google Scholar]
Gairin 2003
- Gairin I, House A, Owens D. Attendance at the accident and emergency department in the year before suicide: Retrospective study. British Journal of Psychiatry 2003;183:28-33. [DOI] [PubMed] [Google Scholar]
Geddes 2004
- Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM. Long-term lithium therapy for bipolar disorder: Systematic review and meta-analysis of randomized controlled trials. American Journal of Psychiatry 2003;161:217-22. [DOI] [PubMed] [Google Scholar]
Gibbs 2004
- Gibbs S, Beautrais A. Epidemiology of attempted suicide in Canterbury Province, New Zealand (1993-2002). New Zealand Medical Journal 2004;117:U1141. [PubMed] [Google Scholar]
Gjelsvik 2012
- Gjelsvik B, Heyerdahl F, Hawton K. Prescribed medication availability and deliberate self-poisoning: A longitudinal study. Journal of Clinical Psychiatry 2012;73:e548-54. [DOI] [PubMed] [Google Scholar]
Gould 2003
- Gould MS, Greenberg T, Velting DM, Shaffer D. Youth suicide risk and preventive interventions: A review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42:386-405. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADEpro. Cochrane Informatics and Knowledge Management Department, Accessed 24 September, 2014.
Gray 2001
- Gray SM, Otto MW. Psychosocial approaches to suicide prevention: Applications to patients with bipolar disorder. Journal of Clinical Psychiatry 2001;62:s56-64. [PubMed] [Google Scholar]
Gunnell 1994
- Gunnell D, Frankel S. Prevention of suicide: Aspirations and evidence. British Medical Journal 1994;308:1227-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunnell 2003
- Gunnell D, Eddleston M. Suicide by intentional ingestion of pesticides:a continuing tragedy in developing countries. International Journal of Epidemiology 2003;32:902-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haw 2001
- Haw C, Hawton K, Houston K, Townsend E. Psychiatric and personality disorders in deliberate self-harm patients. British Journal of Psychiatry 2001;178:48-54. [DOI] [PubMed] [Google Scholar]
Hawton 1988
- Hawton K, Fagg J. Suicide, and other causes of death, following attempted suicide. British Journal of Psychiatry 1988;152:359-66. [DOI] [PubMed] [Google Scholar]
Hawton 2003a
- Hawton K, Harriss L, Hall S, Simkin S, Bale E, Bond A. Deliberate self-harm in Oxford, 1990-2000: A time of change in patient characteristics. Psychological Medicine 2003;33:987-96. [DOI] [PubMed] [Google Scholar]
Hawton 2003b
- Hawton K, Zahl D, Weatherall R. Suicide following deliberate self-harm: Long-term follow-up of patients who presented to a general hospital. British Journal of Psychiatry 2003;182:537-42. [DOI] [PubMed] [Google Scholar]
Hawton 2007
- Hawton K, Bergen H, Casey D, Simkin S, Palmer B, Cooper J, et al. Self-harm in England: A tale of three cities. Multicentre study of self-harm. Social Psychiatry and Psychiatric Epidemiology 2007;42:513-21. [DOI] [PubMed] [Google Scholar]
Hawton 2008
- Hawton K, Harriss L. The changing gender ratio in occurrence of deliberate self-harm across the life-cycle. Crisis 2008;29:4-10. [DOI] [PubMed] [Google Scholar]
Hawton 2010
- Hawton K, Bergen H, Simkin H, Cooper K, Waters K, Gunnell D, et al. Toxicity of antidepressants: Rates of suicide relative to prescribing and non-fatal overdose. British Journal of Psychiatry 2010;196:354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawton 2012
- Hawton K, Saunders KEA, O'Connor R. Self-harm and suicide in adolescents. Lancet 2012;379:2373-82. [DOI] [PubMed] [Google Scholar]
Hawton 2013
- Hawton K, Saunders KEA, Topiwala A, Haw C. Psychiatric disorders in patients presenting to hospital following self-harm: A systematic review. Journal of Affective Disorders 2013;151:821-30. [DOI] [PubMed] [Google Scholar]
Hennen 2005
- Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: A meta-analysis. Schizophrenia Research 2005;73:139-45. [DOI] [PubMed] [Google Scholar]
Hepp 2004
- Hepp U, Wittman L, Schnyder U, Michel K. Psychological and psychosocial interventions after attempted suicide: An overview of treatment studies. Crisis 2004;25:108-17. [DOI] [PubMed] [Google Scholar]
Heppner 1988
- Heppner P. The Problem-Solving Inventory. Palo Alto, CA: Consulting Psychologist Press, 1988. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008a
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Higgins 2008b
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group. Special topics in statistics. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Higgins 2008c
- Higgins JPT, Deeks JJ. Selecting studies and collecting data. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Hjelmeland 2002
- Hjelmeland H, Hawton K, Nordvik H, Bille-Brahe U, Leo D, Fekete S, et al. Why people engage in parasuicide: A cross-cultural study of intentions. Suicide and Life-Threatening Behavior 2002;32:380-93. [DOI] [PubMed] [Google Scholar]
Hollingworth 2010
- Hollingworth SA, Burgess PM, Whiteford HA. Affective and anxiety disorders: Prevalence, treatment and antidepressant medication use. Australian and New Zealand Journal of Psychiatry 2010;44:513-9. [DOI] [PubMed] [Google Scholar]
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: MR000006. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ilyas 2012
- Ilyas S, Moncrieff J. Trends in prescriptions and costs of drugs for mental disorders in England, 1998-2010. British Journal of Psychiatry 2012;200:393-8. [DOI] [PubMed] [Google Scholar]
Kapur 2010
- Kapur N, Cooper J, Bennewith O, Gunnell D, Hawton K. Postcards, green cards and telephone calls: Therapeutic contact with individuals following self-harm. British Journal of Psychiatry 2010;197:5-7. [DOI] [PubMed] [Google Scholar]
Kapur 2013
- Kapur N, Cooper J, O'Connor RC, Hawton K. Non-suicidal self-injury v. attempted suicide: New diagnosis or false dichotomy? British Journal of Psychiatry 2013;202:326-8. [DOI] [PubMed] [Google Scholar]
Kerry 1998
- Kerry SM, Bland JM. Analysis of a trial randomised in clusters. British Medical Journal 1998;316:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinyanda 2005
- Kinyanda E, Hjelmeland H, Musisi S. Psychological factors in deliberate self-harm as seen in an urban African population in Uganda: A case-control study. Suicide and Life-Threatening Behavior 2005;35:468-77. [DOI] [PubMed] [Google Scholar]
Kliem 2010
- Kliem S, Kröger C, Kosfelder J. Dialectical behavior therapy for borderline personality disorder: A meta-analysis using mixed-effects modeling. Journal of Consulting and Clinical Psychology 2010;78:936-51. [DOI] [PubMed] [Google Scholar]
Kreitman 1969
- Kreitman N, Philip AE, Greer S, Bagley CR. Parasuicide. British Journal of Psychiatry 1969;115:746-77. [DOI] [PubMed] [Google Scholar]
Larkin 2014
- Larkin C, di Blasi Z, Arensman E. Risk factors for repetition of self-harm: A systematic review of prospective hospital-based studies. PLoS One 2014;9:e84282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lester 1994
- Lester D. The effectiveness of centres for the prevention of suicide [L'efficacité des centres de prévention du suicide]. Santé Mentale au Québec 1994;19:15-24. [PubMed] [Google Scholar]
Lilley 2008
- Lilley R, Owens D, Horrocks J, House A, Noble R, Bergen H, et al. Hospital care and repetition following self-harm: Multicentre comparison of self-poisoning and self-injury. British Journal of Psychiatry 2008;192:440-5. [DOI] [PubMed] [Google Scholar]
Linehan 1991
- Linehan MM, Armstrong HE, Suarez A, Allmon D, Heard HL. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Archives of General Psychiatry 1991;48:1060-4. [DOI] [PubMed] [Google Scholar]
Links 2003
- Links PS, Bergmans Y, Cook M. Psychotherapeutic interventions to prevent repeated suicidal behavior. Brief Treatment and Crisis Intervention 2003;3:445-64. [Google Scholar]
Lorillard 2011a
- Lorillard S, Schmitt L, Andreoli A. How to treat deliberate self-harm from clinical research to effective treatment choice? Part 1: An update on treatment efficacy among unselected patients referred to emergency room with deliberate self-harm [Comment traiter la tentative de suicide? 1 re partie: Efficacité des interventions psychosociales chez des patients suicidants à la sortie des urgences]. Annales Médico-Psychologiques 2011;169:211-28. [Google Scholar]
Lorillard 2011b
- Lorillard S, Schmitt L, Andreoli A. How to treat suicide attempt? Part 2: A review of treatments and the efficiency among borderline personality disorder patients [Comment traiter la tentative de suicide? Seconde partie: Une revue des traitements et de leur efficacité chez de patients borderline]. Annales Médico-Psychologiques 2011;169:229-36. [Google Scholar]
Luxton 2013
- Luxton DD, June JD, Comtois KA. Can postdischarge follow-up contacts prevent suicide and suicidal behavior? A review of the evidence. Crisis 2013;34:32-41. [DOI] [PubMed] [Google Scholar]
Mann 2005
- Mann JJ, Apter A, Bertolote J, Beautrais A, Currier D, Haas A, et al. Suicide prevention strategies: A systematic review. Journal of the American Medical Association 2005;294:2064-74. [DOI] [PubMed] [Google Scholar]
Mann 2013
- Mann JJ. The serotonergic system in mood disorders and suicidal behaviour. Philosophical Transactions of The Royal Society (B Biological Sciences) 2013;368:20120537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton, M. Unpublished rating scales: A major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249-52. [DOI] [PubMed] [Google Scholar]
McMain 2007
- McMain S. Effectiveness of psychosocial treatments on suicidality in personality disorders. Canadian Journal of Psychiatry 2007;52:s103-14. [PubMed] [Google Scholar]
Milner 2015
- Milner AJ, Carter G, Pirkis J, Robinson J, Spittal MJ. Letters, green cards, telephone calls and postcards: Systematic and meta-analytic review of brief contact interventions for reducing self-harm, suicide attempts and suicide. British Journal of Psychiatry 2015;206:184-90. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609-13. [DOI] [PubMed] [Google Scholar]
Möller, 1992
- Möller H-J. Attempted suicide: Efficacy of different aftercare strategies. International Clinical Psychopharmacology 1992;6:s58-69. [PubMed] [Google Scholar]
Möller 1989
- Möller HJ. Efficacy of different strategies of aftercare for patients who have attempted suicide. Journal of the Royal Society of Medicine 1989;82:643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 1995
- Montgomery SA, Dunner DL, Dunbar GC. Reduction of suicidal thoughts with paroxetine in comparison with reference antidepressants and placebo. European Neuropsychopharmacology 1995;5:5-13. [DOI] [PubMed] [Google Scholar]
Muehlenkamp 2006
- Muehlenkamp JJ. Empirically supported treatments and general therapy guidelines for non-suicidal self-injury. Journal of Mental Health Counseling 2006;28:166-85. [Google Scholar]
Müller‐Oerlinghausen 2005
- Müller-Oerlinghausen B, Felber W, Berghöfer A, Lauterbach E, Ahrens B. The impact of lithium long-term medication of suicidal behavior and mortality of bipolar patients. Archives of Suicide Research 2005;9:307-19. [DOI] [PubMed] [Google Scholar]
Murphy 2012
- Murphy E, Kapur N, Webb R, Purandare N, Hawton K, Bergen H, et al. Risk factors for repetition and suicide following self-harm in older adults: Multicentre cohort study. British Journal of Psychiatry 2012;200:399-404. [DOI] [PubMed] [Google Scholar]
NCCMH 2004
- National Collaborating Centre for Mental Health. Clinical Guideline 16. Self-harm: the short-term physical and psychological management and secondary prevention of self-harm in primary and secondary care. London: National Institute for Clinical Excellence, 2004. [Google Scholar]
Nock 2007
- Nock MK, Teper R, Hollander M. Psychological treatment of self-injury among adolescents. Journal of Clinical Psychology 2007;63:1081-9. [DOI] [PubMed] [Google Scholar]
Nock 2010
- Nock MK. Self-injury. Annual Review of Psychology 2010;6:339-63. [DOI] [PubMed] [Google Scholar]
Office of the Surgeon General (US) 2012
- Office of the Surgeon General (US) / National Action Alliance for Suicide Prevention (US). 2012 National Strategy for Suicide Prevention: Goals and Objects for Action. A report of the U.S. Surgeon General and of the National Action Alliance for Suicide Prevention. Washington, DC: US Department of Health and Human Services, 2012. [PubMed] [Google Scholar]
Olfson 2009
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Archives of General Psychiatry 2009;66:848-56. [DOI] [PubMed] [Google Scholar]
Ougrin 2011
- Ougrin D, Latif S. Specific psychological treatment versus treatment as usual in adolescents with self-harm: Systematic review and meta-analysis. Crisis 2011;32:74-80. [DOI] [PubMed] [Google Scholar]
Ougrin 2015
- Ougrin D, Tranah T, Stahl D, Moran P, Asarnow JR. Therapeutic interventions for suicide attempts and self-harm in adolescents: Systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry 2015;54:97-107. [DOI] [PubMed] [Google Scholar]
Owens 2002
- Owens D, Horrocks J, House A. Fatal and non-fatal repetition of self-harm. Systematic review. British Journal of Psychiatry 2002;181:193-9. [DOI] [PubMed] [Google Scholar]
Parkar 2006
- Parkar SR, Dawani V, Weiss MG. Clinical diagnostic and sociocultural dimensions of deliberate self-harm in Mumbai, India. Suicide and Life-Threatening Behavior 2006;36:223-38. [DOI] [PubMed] [Google Scholar]
Perry 2012
- Perry IJ, Corcoran P, Fitzgerald AP, Keeley HS, Reulbach U, Arensman E. The incidence and repetition of hospital-treated deliberate self harm: Findings from the world's first national registry. PLoS One 2012;7:e31663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rana 2013
- Rana F, Gormez A, Varghese S. Pharmacological interventions for self-injurious behaviour in adults with intellectual disabilities. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD009084. [DOI: 10.1002/14651858.CD009084.pub2] [DOI] [PubMed] [Google Scholar]
Ross 2007
- Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: Which disorder and which fatty acid? Lipids in Health and Disease 2007;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Royal College of Psychiatrists 1994
- Royal College of Psychiatrists. The General Hospital Management of Adult Deliberate Self-Harm. Council Report CR32. London: Royal College of Psychiatrists, 1994. [Google Scholar]
Royal College of Psychiatrists 2004
- Royal College of Psychiatrists. Assessment Following Self-Harm in Adults. Council Report CR122. London: Royal College of Psychiatrists, 2004. [Google Scholar]
Sackett 1997
- Sackett D. The Cochrane Colloboration Handbook. Oxford: Oxford Univeersity Press, 1997. [Google Scholar]
Sakinofsky 2000
- Sakinofsky I. Repetition of suicidal behaviour. In: Hawton K, Heeringen K, editors(s). The International Handbook of Suicide and Attempted Suicide. Chichester: Wiley, 2000:385-404. [Google Scholar]
Schmidtke 1996
- Schmidtke A, Bille Brahe U, Leo D, Kerkhof A, Bjerke T, Crepet P, et al. Attempted suicide in Europe: Rates, trends and sociodemographic characteristics of suicide attempters during the period 1989-1992. Results of the WHO/EURO Multicentre Study on Parasuicide. Acta Psychiatrica Scandinavica 1996;93:327-38. [DOI] [PubMed] [Google Scholar]
Schmidtke 2004
- Schmidtke A, Weinacker B, Lähr C, Bille-Brahe U, Leo D, Kerkhof A, et al. Suicide and suicide attempts in Europe - An overview. In: Schmidtke A, Bille-Brahe U, Leo D, Kerkhof A, editors(s). Suicidal Behaviour in Europe: Results from the WHO/EURO Multicentre Study on Suicidal Behaviour. Göttingen, Germany: Hogrefe & Huber Publishers, 2004:15-28. [Google Scholar]
Schultz 1995
- Schultz KF. Subverting randomization in controlled trials. Journal of the American Medical Association 1995;274:1456-8. [PubMed] [Google Scholar]
Schünemann 2008a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Presenting results and 'summary of findings’ tables. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Schünemann 2008b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, 2008. [Google Scholar]
Scoliers 2009
- Scoliers G, Portzky G, Madge N, Hewitt A, Hawton K, Wilde EJ, et al. Reasons for adolescent deliberate self-harm: A cry of pain and/or a cry for help? Findings from the child and adolescent self-harm in Europe (CASE) study. Social Psychiatry and Psychiatric Epidemiology 2009;44:601-7. [DOI] [PubMed] [Google Scholar]
Sinclair 2011
- Sinclair JMA, Gray A, Rivero-Arias O, Saunders KEA, Hawton K. Healthcare and social services resource use and costs of self-harm patients. Social Psychiatry and Psychiatric Epidemiology 2011;46:263-71. [DOI] [PubMed] [Google Scholar]
Smith 2005
- Smith BD. Self-mutilation and pharmacotherapy. Psychiatry 2005;2:28-37. [PMC free article] [PubMed] [Google Scholar]
Stoffers 2010
- Stoffers J, Völlm BA, Rücker G, Timmer A, Huband N, Lieb K. Pharmacological interventions for borderline personality disorder. Cochrane Database of Systematic Reviews 2010, Issue 6. Art. No: CD005653. [DOI: 10.1002/14651858.CD005653.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sublette 2006
- Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. American Journal of Psychiatry 2006;163(6):1100-2. [DOI] [PubMed] [Google Scholar]
Tanskanen 2001
- Tanskanen A, Hibbeln JR, Hintikka J, Haatainen K, Honkalampi K, Viinamäki H. Fish consumption, depression, and suicidality in a general population. Archives of General Psychiatry 2001;58:512-13. [DOI] [PubMed] [Google Scholar]
Tarrier 2008
- Tarrier N, Taylor K, Gooding P. Cognitive-behavioral interventions to reduce suicide behavior: A systematic review and meta-analysis. Behavior Modification 2008;32:77-108. [DOI] [PubMed] [Google Scholar]
Tondo 1997
- Tondo L, Jamison KR, Baldessarini RJ. Effect of lithium maintenance on suicidal behavior in major mood disorders. Annals of the New York Academy of Science 1997;836:339-51. [DOI] [PubMed] [Google Scholar]
Tondo 2000
- Tondo L, Baldessarini RJ. Reduced suicide risk during lithium maintenance treatment. Journal of Clinical Psychiatry 2000;61:s97-104. [PubMed] [Google Scholar]
Tondo 2001
- Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: A meta-analysis. Acta Psychiatrica Scandinavica 2001;104:163-72. [DOI] [PubMed] [Google Scholar]
Torgerson 2004
- Torgerson D. The use of Zelen's design in randomised trials. British Journal of Obstetrics and Gynaecology: An International Journal of Obstetrics and Gynaecology 2004;111:2. [DOI] [PubMed] [Google Scholar]
Townsend 2001
- Townsend E, Hawton K, Altman D, Arensman E, Gunnell D, Hazell P, et al. The efficacy of problem-solving treatments after deliberate self-harm: Meta-analysis of randomized controlled trials with respect to depression, hopelessness and improvement in problems. Psychological Medicine 2001;31:979-88. [DOI] [PubMed] [Google Scholar]
Tyrer 2012
- Tyrer P. Why benzodiazepines are not going away. Commentary on benzodiazepines for anxiety disorders. Advances in Psychiatic Treatment 2012;18:259-62. [Google Scholar]
van der Sande 1997
- Sande R, Buskens E, Allart E, Graaf Y, Engeland H. Psychosocial intervention following suicide attempt: A systematic review of treatment interventions. Acta Psychiatrica Scandinavica 1997;96:43-50. [DOI] [PubMed] [Google Scholar]
van Heeringen 2014
- Heeringen K, Mann JJ. The neurobiology of suicide. The Lancet Psychiatry 2014;1:63-72. [DOI] [PubMed] [Google Scholar]
Värnik 2004
- Värnik A, Küpersepp O, Marandi T, Palo E. Suicidal behaviour in Estonia. In: Schmidtke A, Bille-Brahe U, Leo D, Kerkhof A, editors(s). Suicidal behaviour in Europe: Results from the WHO/EURO Multicentre Study on Suicidal Behaviour. Göttingen, Germany: Hogrefe & Huber Publishers, 2004:195-200. [Google Scholar]
Verkes 2000
- Verkes RJ, Cowen PJ. Pharmacotherapy of suicidal ideation and behaviour. In: Hawton K, Heeringen K, editors(s). The International Handbook of Suicide and Attempted Suicide. Chichester, UK: John Wiley and Sons, 2000. [Google Scholar]
World Health Organization 2014
- World Health Organization. Preventing suicide: A global imperative. Geneva, Switzerland: World Health Organization, 2014. [Google Scholar]
Zahl 2004
- Zahl D, Hawton K. Repetition of deliberate self-harm and subsequent suicide risk: long-term follow-up study in 11,583 patients. British Journal of Psychiatry 2004;185:70-5. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67:361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hawton 1998
- Hawton K, Arensman E, Townsend E, Bremner S, Feldman E, Goldney R, et al. Deliberate self harm: Systematic review of efficacy of psychosocial and pharmacological treatments in preventing repetition. British Medical Journal 1998;317:441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawton 1999
- Hawton K, Townsend E, Arensman E, Gunnell D, Hazell P, House A, et al. Psychosocial and pharmacological treatments for deliberate self harm. Cochrane Database of Systematic Reviews 1999, Issue 4. Art. No: CD001764. [DOI: 10.1002/14651858.CD001764] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. Self-harm: Longer-term Management. NICE clinical guideline 133. London: National Institute for Health and Clinical Excellence, 2011. [PubMed] [Google Scholar]


