Abstract
Objectives
The aim of this study was to determine the safety and tolerability of escalating doses of orally delivered cannabis oils predominant in cannabidiol (CBD), tetrahydrocannabinol (THC), or both CBD and THC in healthy cats.
Methods
In this placebo-controlled, blinded study, 20 healthy adult cats were randomized to one of five treatment groups (n = 4 per group): two placebo groups (sunflower oil [SF] or medium-chain triglyceride oil [MCT]), or three plant-derived cannabinoid oil groups (CBD in MCT, THC in MCT or CBD/THC [1.5:1] in SF). Up to 11 escalating doses of each formulation were delivered orally via syringe to fasted subjects, with at least 3 days separating doses. Safety and tolerability were determined from clinical observations, complete blood counts (CBCs) and clinical chemistry. Plasma cannabinoids (CBD, THC) and metabolites (7-COOH-CBD, 11-OH-THC) were assessed.
Results
Titration to maximum doses of 30.5 mg/kg CBD (CBD oil), 41.5 mg/kg THC (THC oil) or 13.0:8.4 mg/kg CBD:THC (CBD/THC oil) was safely achieved in all subjects. All observed adverse events (AEs) were mild, transient and resolved without medical intervention. Gastrointestinal AEs were more common with formulations containing MCT. Constitutional (lethargy, hypothermia), neurologic (ataxia) and ocular (protrusion membrana nictitans) AEs were more common with oils containing THC (CBD/THC and THC oils). There were no clinically significant changes in CBC or clinical chemistry across treatment groups. Higher plasma levels of the cannabinoids and their metabolites following administration of the CBD/THC combination product are suggestive of a pharmacokinetic interaction.
Conclusions and relevance
This is the first feline study to explore the safety and tolerability of CBD and THC, alone and in combination, in a controlled research setting. These findings will inform veterinarians of the safety profile of cannabinoids, particularly when considering the potential therapeutic use of CBD in cats or recognizing clinical signs associated with accidental exposure to THC-containing products.
Keywords: Cannabidiol, tetrahydrocannabinol, safety, cannabinoid adverse effects, cannabinoid administration and dosage
Introduction
There is growing interest in the potential therapeutic uses of cannabinoids (particularly cannabidiol [CBD]) in cats. In an online survey, 97.8% of respondents (n = 448) reported at least one behavioral problem in their cat, and many were open to considering cannabinoids as a treatment option. 1 To date, only one publication supports the safety of CBD (CBD/cannabidiolic acid [CBDA] in fish oil [4 mg/kg/day; q12h PO] for 12 weeks) in cats, 2 but no study has explored the tolerability of different doses of CBD and/or tetrahydrocannabinol (THC) in cats.
There are no published studies on the efficacy of cannabinoids in cats. In dogs with osteoarthritis, CBD has been shown to reduce pain and increase mobility when administered at 1.2 mg/kg daily for 4 weeks, 3 and to reduce pain and improve quality of life when administered at 2 mg/kg q24h for 12 weeks 4 or q12h (1:1 CBD:CBDA) for 4 weeks. 5 CBD (2.5 mg/kg q12h for 12 weeks) has also been shown to decrease seizure frequency in dogs with intractable idiopathic epilepsy. 6 Importantly, canine studies have concluded that efficacy is dependent on the dose administered and/or on plasma CBD concentration;3,6 higher dose and plasma concentration was associated with improved efficacy. Interestingly, dosing recommendations for the Food and Drug Administration-approved CBD isolate (Epidiolex), used in human patients aged >2 years for the treatment of seizures associated with two rare forms of epilepsy, range from 5 to 20 mg/kg/day. 7 Both canine and human data therefore suggest that higher doses may be required for CBD efficacy, depending on disease state. To date, no feline studies have explored the safety and tolerability of CBD doses >4 mg/kg/day.
The potential for accidental exposure to widely available human cannabinoid products is another clinically relevant research gap. While data exist to understand how dogs manifest exposure to THC (alone or with CBD) in a controlled research setting or via accidental exposure to human recreational or medical cannabis,8,9 data on cats are sparse, with only two published case reports on accidental cannabis intoxication.10,11
The primary objective of this placebo-controlled study was to determine the safety and tolerability of escalating doses of well-defined cannabis formulations predominant in CBD, THC or CBD plus THC (1.5:1) in healthy cats in a controlled research setting. As a secondary objective, this study also explored the plasma concentrations of CBD, THC and their major metabolites following serial dosing with CBD, THC or CBD plus THC.
Materials and methods
Study design
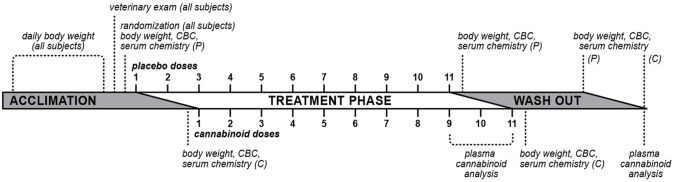
This was a randomized, placebo-controlled, blinded, parallel study. Following a ⩾10-day acclimation, 20 healthy cats (10 males, 10 females; age 1–1.2 years; weight 3–6 kg) were randomized to one of five treatment groups (four cats per group [two males, two females]): (1) CBD oil (18.3 mg/ml CBD in medium-chain triglyceride [MCT] oil); (2) THC oil (25.1 mg/ml THC in MCT oil); (3) CBD/THC oil (8.0:5.2 mg/ml CBD:THC, 1.5:1, in sunflower [SF] oil); (4) MCT oil placebo; or (5) SF oil placebo. The study investigated 11 escalating volumes/doses of placebo or test formulations with at least 3 days separating doses. Two placebo oils were tested as the cannabinoid oils included either MCT or SF oil as carriers. To ascertain tolerability to increasing volumes of carrier oil at each escalating dose, the treatment groups were on a staggered schedule whereby the first two doses were delivered to placebo group animals prior to the commencement of cannabinoid oil dosing in the remaining three treatment groups. The study timeline is shown in Figure 1. The total study duration, from the first day of acclimation to the last day of washout, was 71 days.
Figure 1.
Diagram of study timeline. Cats were acclimated to study housing conditions for ⩾10 days, during which time they underwent daily body weight measurement and one veterinary examination. Following randomization to treatment groups, cats were dosed with 11 escalating doses of placebo oils (P; medium-chain triglyceride or sunflower oils) or cannabinoid oils (C; cannabidiol [CBD], tetrahydrocannabinol [THC] or CBD/THC) with at least 3 days separating doses. Treatment groups were on a staggered schedule whereby the first two doses were delivered to placebo group animals prior to the commencement of cannabinoid oil dosing. Blood was collected (complete blood count [CBC] and serum chemistry) and body weight was measured 1 day before treatment initiation, twice weekly during the treatment phase, and 1 and 7 days after completion of the 11-dose treatment phase. Plasma cannabinoids were measured after the ninth, 10th and 11th (final) doses
Test article description and administration
Plant-derived cannabinoid distillate and placebo oils were acquired from Canopy Growth Corporation. The cannabis plants used to prepare the cannabinoid oils were grown indoors under tightly controlled environmental conditions. Within one lot, all plants were genetically identical. Upon harvest, plant material was trimmed, dried and extracted via supercritical carbon dioxide. The extracted resin was diluted with a food-grade oil (MCT or SF oil) to the target concentration.
An independent laboratory (RPC, Fredericton, NB, Canada) analyzed the composition of the cannabinoid oil formulations using validated methods. Solvent extraction and high performance liquid chromatography with diode array detection were used for cannabinoid analyses (accuracy: 90–113%; precision: 5.6–12.8%). Solvent extraction and gas chromatography/mass selective detector were used for terpene analyses (accuracy: 74–106%; relative standard deviation: 3.2–9.4%). Table 1 summarizes the levels of cannabinoids and terpenes in the formulations.
Table 1.
Select cannabinoid and terpene composition of cannabis oil formulations
| Constituent | CBD (in MCT oil) | THC (in MCT oil) | CBD/THC (in SF oil) |
|---|---|---|---|
| Cannabinoids (mg/ml)* | |||
| CBD | 18.3 | ND | 8.0 |
| Delta-9-THC | 0.7 | 25.1 | 5.2 |
| CBDA | 0.6 | ND | ND |
| Delta-9-THCA | ND | ND | ND |
| CBG | <RL † | 1.3 | ND |
| CBGA | ND | ND | ND |
| CBN | ND | 0.9 | <RL † |
| CBC | 0.8 | 0.6 | <RL † |
| Terpenes (%) | |||
| Caryophyllene | <RL ‡ | 0.05 | <RL ‡ |
| Humulene | <RL ‡ | 0.02 | <RL ‡ |
| Myrcene | <RL ‡ | <RL ‡ | 0.03 |
| Remaining terpenes § | <RL ‡ | ⩽RL ‡ | ⩽RL ‡ |
Solvent extraction and high performance liquid chromatography with diode array detection was used to measure cannabinoids
Cannabinoid reporting limit (RL) 0.5 mg/ml
Terpene RL 0.01%
Twenty-one terpenes were measured in cannabidiol (CBD) and tetrahydrocannabinol (THC) oils: alpha-pinene, beta-pinene, myrcene, limonene, terpinolene, linalool, terpineol, caryophyllene, humulene, 3-carene, cis-ocimene, eucalyptol, trans-ocimene, fenchol, borneol, valencene, cis-nerolidol, trans-nerolidol, guaiol, alpha-bisabolol, sabinene. Fifteen terpenes were measured in CBD/THC oil: alpha-pinene, beta-pinene, myrcene, limonene, terpinolene, linalool, terpineol, caryophyllene, humulene, 3-carene, cis-ocimene, eucalyptol, trans-ocimene, fenchol, borneol. All terpenes were measured by solvent extraction and gas chromatography/mass selective detector
MCT = medium-chain triglyceride; SF = sunflower; CBDA = cannabidiolic acid; THCA = tetrahydrocannabinolic acid; ND = not detectable; CBG = cannabigerol; CBGA = cannabigerolic acid; CBN = cannabinol; CBC = cannabichromene
At the study site (InterVivo Solutions, ON, Canada), products were stored in a locked controlled drug cabinet (protected from light at 15.7–24.7°C). Treatments were administered orally (syringe) to fasted subjects (⩾12 h). The volume was increased at each escalating dose. The range of volumes investigated was dependent on body weight and ranged from 0.5–0.8 ml (0.15 ml/kg; first dose) to 5.0–9.8 ml (1.67 ml/kg; last dose). CBD and THC doses (mg/kg) are reported in Table 2. The test articles were administered with attention to complete delivery and retention. Doses >5 ml in volume were divided into two administrations, separated by 30 (± 10) mins. For divided doses, dosing time was considered the completion time of the second administration. As such, timed blood collection or observations were based on the time of dosing completion for a given cat. If an animal was observed vomiting ⩽30 mins after administration, the dose was re-administered. To disrupt negative association with test article administration and study procedures, positive reinforcement (treats/handling) was performed on non-dosing days.
Table 2.
Cannabidiol (CBD) and tetrahydrocannabinol (THC) quantities per kilogram body weight delivered to cats across the cannabinoid treatment groups (n = 4/group)
| CBD oil | THC oil | CBD/THC oil | ||||
|---|---|---|---|---|---|---|
| Carrier oil | MCT | MCT | SF | |||
| BSN weight range (kg) | 3.5–5.5 | 3.2–5.0 | 3.8–5.2 | |||
| Dose number | CBD (mg/kg) | THC (mg/kg) | CBD (mg/kg)* | THC (mg/kg) | CBD (mg/kg) | THC (mg/kg) |
| 1 | 2.8 | 0.1 | – | 3.8 | 1.2 | 0.8 |
| 2 | 5.5 | 0.21 | – | 7.6 | 2.4 | 1.5 |
| 3 | 8.3 | 0.31 | – | 11.3 | 3.5 | 2.3 |
| 4 | 11.1 | 0.42 | – | 15.1 | 4.7 | 3.0 |
| 5 | 13.9 | 0.52 | – | 18.9 | 5.9 | 3.8 |
| 6 | 16.6 | 0.63 | – | 22.6 | 7.1 | 4.6 |
| 7 | 19.4 | 0.73 | – | 26.4 | 8.3 | 5.3 |
| 8 | 22.2 | 0.84 | – | 30.2 | 9.4 | 6.1 |
| 9 | 24.9 | 0.94 | – | 34.0 | 10.6 | 6.9 |
| 10 | 27.7 | 1.0 | – | 37.7 | 11.8 | 7.6 |
| 11 | 30.5 | 1.1 | – | 41.5 | 13.0 | 8.4 |
CBD was not detected in the cannabinoid analysis (mg/ml) of the THC oil formulation; CBD quantities at higher volumes (>1 ml) of the formulation are unknown
MCT = medium-chain triglyceride; SF = sunflower; BSN = baseline
Subject selection
The inclusion criteria for the study were good general health upon veterinary examination; stable weight (fluctuations ⩽0.3 kg) over 10 days during acclimation; weight of 2–6 kg; and age >1 year. Exclusion criteria included uncooperative disposition and/or receipt of cannabinoids within 2 months of the start of the study. Twenty-four cats were acclimated to study housing conditions for 10 days, and 20 cats were randomized to a treatment group.
Randomization and treatment allocation
Animals were blocked by body weight and stratified by sex. Randomization was conducted using a random number generator in Microsoft Excel 2016. Formulations were randomly assigned a code name using a random number generator in Microsoft Excel 2016. Personnel collecting data and/or administering the investigational products were blinded to the test groups and treatment conditions. Bottles containing test or placebo formulations were over-labeled with opaque white labels. Information about group allocation and treatment conditions were securely stored in the research facility’s archive room for the duration of the study.
Animal care
Animal care and experimental procedures were conducted under protocols approved by the research facility’s (VivoCore) Institutional Animal Care and Use Committee (protocol ID: VRI150-17219-FS; approval date: 17 January 2018) and the Veterinary Drug Directorate (Health Canada), in accordance with the principles of the Animals for Research Act and guidelines of the Canadian Council on Animal Care.12,13
Animals were individually housed in stainless steel metabolic cages (height × width × depth = 60 × 67 × 74 cm) and cats in the same treatment group were exercised together daily for at least 1 h, except on dosing days or when an adverse event (AE) was being monitored. Environmental controls for the animal housing area maintained a temperature of 19.5–21.2°C and a 12-h light/dark cycle. Animals were provided a standard commercial diet in stainless steel bowls (Purina ProPlan Savor Adult) once daily; food quantity offered was based on body weight and condition maintenance. On dosing days, food was offered 5 h following dose administration. Water was available ad libitum in stainless steel bowls.
Measurable outcomes
Animals were monitored 0.5, 1, 2, 3, 4, 6, 9, 12 and 24 h (±15 mins) following dosing by experienced veterinary technicians for clinical signs not expected in healthy cats and for the occurrence of AEs. Pre-dose and 1, 3, 6 and 24 h post-dose, heart rate (stethoscope), respiratory rate (via observation) and rectal temperature were measured. If rectal temperature was normal 3 h post-dose, subsequent measurements were not taken. Body weight was measured daily (calibrated and verified scale) over the acclimation period, twice weekly during the treatment phase, and 1 and 7 days after completion of dosing.
Adverse events
The Veterinary Cooperative Oncology Group – Common Terminology Criteria for Adverse Events was used to define AEs and rate their severity as mild (asymptomatic or mild clinical signs; intervention not indicated), moderate (minimal, non-invasive intervention indicated; moderate limitation of activities of daily living) or severe (medically significant but not immediately life-threatening). 14 Occurrence of a severe AE was a pre-established justification for the withdrawal of an animal from further dosing.
Blood collections and analyses
Blood was collected by direct venipuncture from a cephalic or jugular vein. The volume of blood drawn over the course of the study ranged from 3.4 ml/kg per month (largest cat) to 6.4 ml/kg per month (smallest cat).Blood samples for hematological analysis (complete blood count [CBC] and clinical chemistry) were collected during acclimation (7 days before treatment start) and 24 h and 7 days following the final dose. At each time point, 4 ml blood was drawn (2 ml placed in evacuated serum separator tube [SST] and another 2 ml placed in an evacuated potassium oxalate sodium fluoride tube). Blood in evacuated SSTs was allowed to clot for a minimum of 10 mins and centrifuged (1525 g) at 20°C for 10 mins within 1 h of collection. The evacuated SSTs and potassium oxalate sodium fluoride tubes were stored at 2–8°C and sent for analysis the same day as blood collection (Antech Diagnostics).
For cannabinoid analysis, blood was drawn immediately prior to and 1, 2, 3, 4, 6 and 24 h (±15 mins) following the ninth dose, and 4 and 24 h (±15 mins) following the 10th and final (11th) doses, as well as 7 days following the final dose. At each time point, 2 ml blood was placed into an evacuated K2EDTA tube and inverted gently. Within 30 mins of collection, tubes were centrifuged (1525 g) at 2–8°C for 10 mins. Plasma was separated into two equal aliquots and stored at –80°C until shipment on dry ice to the analytical laboratory (InterVivo Solutions). CBD, THC, 7-COOH-CBD and 11-OH-THC were analyzed by liquid chromatography tandem mass spectrometry (QTRAP 6500 with an Exion LC system; AB Sciex). Analytical methods have been described previously. 8 Calibration standards were prepared in blank pooled feline plasma (with K2EDTA as anticoagulant).
Data analysis
Poisson regression models using a logarithmic link function were fit to evaluate group differences in the number of AEs, with linear contrasts specified to evaluate differences across the following groups: MCT vs CBD, MCT vs THC, MCT vs SF, SF vs CBD/THC, CBD vs THC, CBD vs CBD/THC and THC vs CBD/THC. Evaluation of model fit revealed significant over-dispersion for hypersalivation AEs. Therefore, a negative binomial model was used for this outcome instead. Groups with zero conditional means for specific AEs were excluded from analyses owing to the instability of standard error estimation.
Pharmacokinetic outcomes, liver enzymes and body weights were evaluated using generalized estimating equations (GEEs) clustered within subject that assumed a Gaussian probability distribution and an unstructured working correlation structure. For plasma levels of cannabinoids and metabolites, linear contrasts evaluated differences between treatment groups (CBD, THC and CBD/THC) at each time point where data were available. Linear contrasts compared changes in liver enzyme levels across time points within each group (ie, baseline vs post-study, baseline vs washout and post-study vs washout). Two separate models for change in body weight were fit: linear slope change in body weight from baseline to dose 11, and change from dose 11 to the washout period. Linear contrasts compared the linear body weight change across the following groups: MCT vs CBD; MCT vs THC; MCT vs SF; SF vs CBD/THC; CBD vs THC; CBD vs CBD/THC; THC vs CBD/THC. Reported Z-scores indicate number of standard deviations a result is above (positive) or below (negative) the mean. All reported P values in all models were adjusted using Benjamini and Hochberg’s procedure to control for false discovery rate inflation due to multiple comparisons between groups for each outcome variable. 15
Inferential analyses were calculated using R version 4.0.0 (www.R-project.org). The R Stats Package was used for Poisson regression modeling, the MASS Package for negative binomial regression modeling and the geepack package for GEE analyses.16,17 Measures of central tendency and all figures were generated using GraphPad Prism version 8.4.2 for macOS (GraphPad Software).
Results
Of 24 subjects included in the acclimation period, four were excluded from study enrollment owing to incompatibility with metabolic cage housing. The 20 randomized subjects, aged 1.0–1.2 years, had baseline mean ± SD body weights of 4.6 ± 1.0 kg (MCT oil placebo), 4.3 ± 0.7 kg (SF oil placebo), 4.8 ± 0.9 kg (CBD oil), 4.4 ± 0.8 kg (THC oil) and 4.5 ± 0.7 kg (CBD/THC oil). Baseline body weight did not significantly differ between treatment groups (P >0.05).
All randomized subjects completed the study. The placebo and cannabinoid oils were tested in up to 11 escalating doses and all cats received all doses of the oil to which they were assigned. Across the cannabinoid oil groups, titration to 30.5 mg/kg CBD (CBD oil), 41.5 mg/kg THC (THC oil) or 13.0/8.4 mg/kg CBD/THC (CBD/THC oil) was achieved in individual subjects. Following dose escalation over a 6- to 7-week period, mean body weights at the end of the treatment period were 4.6 ± 1.0 kg (MCT oil placebo), 4.5 ± 0.7 kg (SF oil placebo), 5.0 ± 1.0 kg (CBD oil), 4.5 ± 0.9 kg (THC oil) and 4.5 ± 0.7 kg (CBD/THC oil), representing changes from baseline of +1.7%, +5.0%, +5.1%, +0.7% and –0.1%, respectively. Body weight increase over the treatment phase was significant for the CBD group compared with MCT oil placebo (Z = 3.20, P = 0.005). The CBD/THC oil group showed a small but significant decrease in body weight over the treatment phase relative to the SF oil placebo group (Z = −2.62, P = 0.015), as well as the THC oil group (Z = –2.72, P = 0.015) and the CBD oil group (Z = 4.46, P <0.001), whereas the CBD group exhibited a small increase in body weight relative to the THC oil group (Z = 2.36, P = 0.025). Following the completion of the last dose, the CBD/THC oil group exhibited a gain in weight relative to the SF placebo oil group (Z = 5.21, P <0.001). All other body weight differences were not statistically significant.
AEs
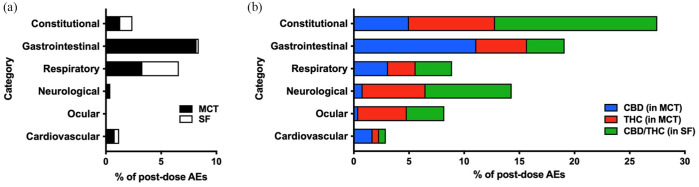
All AEs observed in the present study were rated as mild, and there were no moderate, severe or medically significant AEs. Overall, the total number of AEs differed between cannabinoid groups and their respective placebo, and between MCT and SF oils (all P <0.01), as well as between CBD and CBD/THC groups (Z = –3.45, P = 0.001) and THC and CBD/THC groups (Z = –2.33, P = 0.022). AEs that occurred within 24 h of dose administration across 11 escalating doses were classified as gastrointestinal (27.6%), respiratory (15.4%), neurologic (14.6%) or ocular (8.3%), or as constitutional signs (29.9%; non-specific clinical signs that can affect multiple body systems and have several potential causes) (Figure 2). Cardiovascular AEs (pale mucous membranes, bradycardia, tachycardia) and respiratory AEs (primarily tachypnoea), occurred with uniform frequency across all treatment groups (P >0.05 across all comparisons; Figure 2).
Figure 2.
Proportion of post-dose adverse events (AEs) following dose escalation of cannabis or placebo oils (n = 4 per treatment). AEs were observed within 24 h of oil administration across 11 doses. (a) Medium-chain triglyceride (MCT) oil placebo and sunflower (SF) oil placebo; (b) cannabidiol (CBD) (in MCT oil), tetrahydrocannabinol (THC) (in MCT oil) or CBD/THC (in SF oil). A single occurrence of a musculoskeletal AE (muscle stiffness) in the CBD/THC group was not plotted
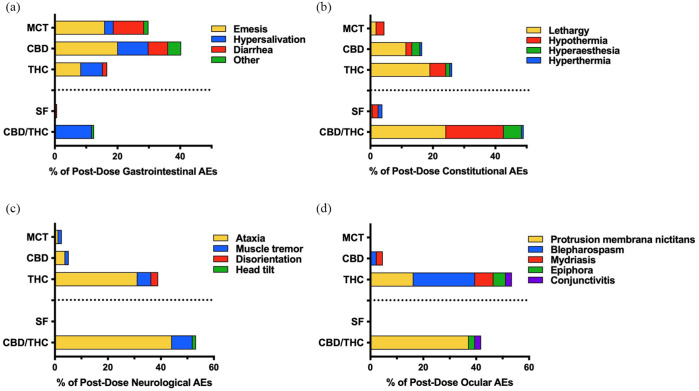
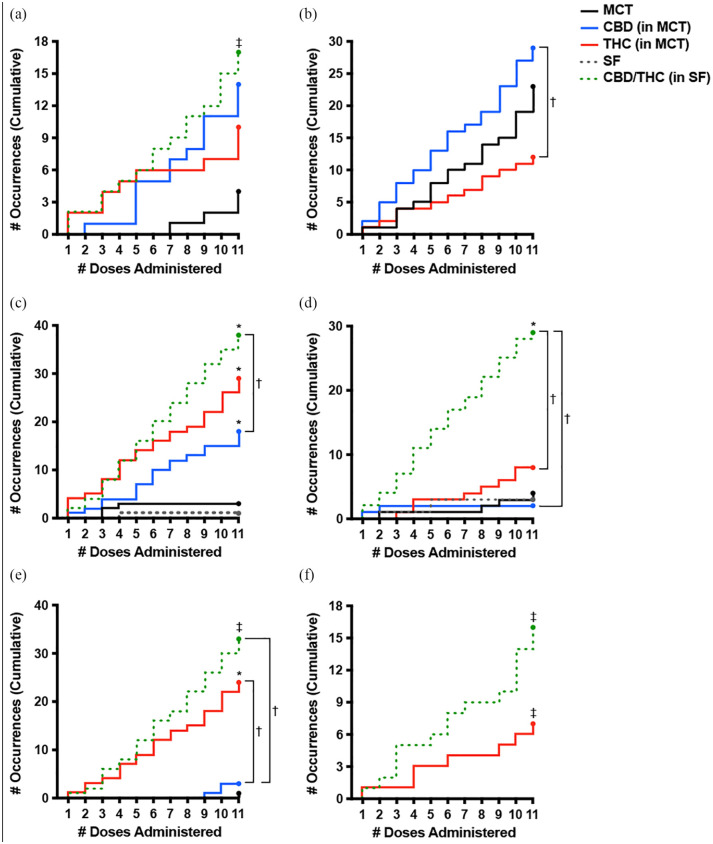
Occurrence of gastrointestinal AEs significantly differed (Z = 3.71, P <0.001) between the two placebo groups – their occurrence was greater with MCT oil (n = 43) than SF oil (n = 1; Figure 2). Moreover, 99% of emesis and diarrhea occurrences (n = 89/90) were with MCT oil formulations (placebo, CBD oil, THC oil) (Figure 3). The occurrence of the two most frequently observed gastrointestinal AEs – hypersalivation and emesis – did not significantly differ between CBD or THC vs MCT oil (P >0.05). Emesis was not observed in the CBD/THC or SF oil treatment groups, but was more frequently observed with CBD than THC oil (P = 0.030). Hypersalivation did not significantly differ between CBD, THC or CBD/THC treatment groups (all P >0.05) (Figure 4).
Figure 3.
Proportion and profile of post-dose adverse events (AEs) attributed to dose escalation of each oil in up to 11 doses (n = 4 per treatment). (a) Gastrointestinal, (b) constitutional, (c) neurologic and (d) ocular AEs were observed within 24 h of oil administration. ‘Other’ gastrointestinal AEs include abnormal excreta, bloody stool, retching and dehydration. MCT = medium-chain triglyceride oil; CBD = cannabidiol; THC = tetrahydrocannabinol; SF = sunflower oil
Figure 4.
Cumulative occurrence of frequently observed adverse events (AEs) across 11 escalating doses of the oil formulations (n = 4 per treatment). (a) Hypersalivation, (b) emesis, (c) lethargy, (d) hypothermia, (e) ataxia and (f) protrusion membrana nictitans were observed within 24 h of dose administration. *Significant difference (P <0.05) between the indicated cannabinoid oil group and its placebo (cannabidiol [CBD] vs medium-chain triglyceride [MCT] oil, tetrahydrocannabinol [THC] vs MCT or CBD/THC vs sunflower [SF] oil). †Significant difference (P <0.05) between the indicated cannabinoid oil groups (generalized estimating equation with alpha level set at P <0.05, two-tailed and false discovery rate adjustments made for multiple comparisons). ‡Statistical analysis between the indicated cannabinoid oil and its placebo was not performed owing to insufficient variability (zero occurrences) in the placebo group
While the occurrence of constitutional signs did not significantly differ between the two placebo groups (P = 0.782), constitutional signs occurred more frequently with CBD oil (Z = 3.08, P = 0.003) and THC oil (Z = 4.25, P <0.001) than MCT oil, and more frequently with CBD/THC oil (Z = 6.02, P <0.001) than SF oil. Moreover, constitutional signs were more frequently observed with the CBD/THC combination product than with either CBD (Z = 4.79, P <0.001) or THC (Z = 3.36, P = 0.001) (Figure 2). The most prominent constitutional signs were lethargy and hypothermia (Figure 3). While the occurrence of lethargy did not significantly differ between the two placebo groups (P = 0.341), lethargy occurred more frequently with CBD oil (Z = 2.87, P = 0.010) and THC oil (Z = 3.74, P = 0.001) than MCT oil, and more frequently with CBD/THC oil (Z = 3.59, P = 0.001) than SF oil. Moreover, lethargy was observed more frequently with THC-containing oils than CBD, but this relationship was only significant when comparing CBD/THC and CBD (Z = 2.61, P = 0.016) (Figure 4). On average, across all doses, lethargy began sooner after dose administration of CBD (~2.8 h) than administration of THC (~4.2 h) or CBD/THC (~4.3 h), and was of shorter duration with CBD (~1.2 h) than with THC (~8.0 h) or CBD/THC (~5.7 h) (Figure 5). Hypothermia did not differ between CBD and MCT oil (P = 0.706) or THC and MCT oil (P = 0.361) but was observed more frequently in the CBD/THC group vs the SF oil placebo group (Z = 3.741, P <0.001), CBD oil (Z = 3.741, P <0.001) and THC oil group (Z = 3.225, P = 0.003) (Figure 4).
Figure 5.
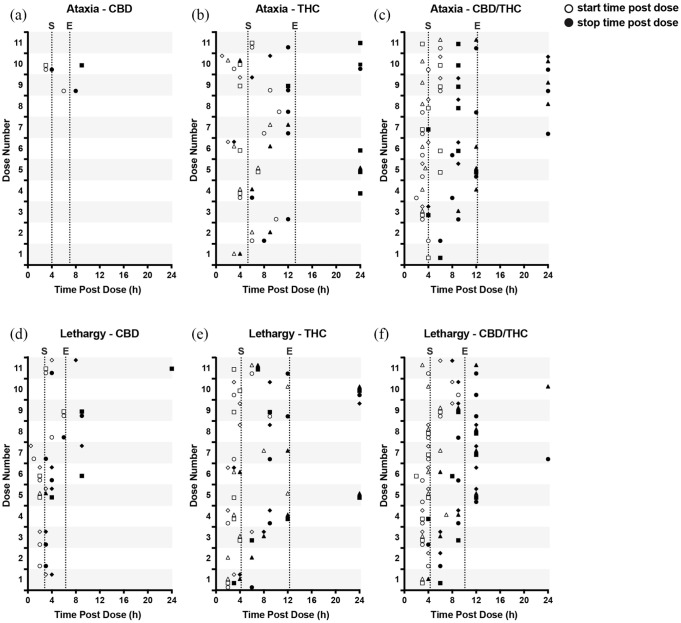
Start and end times (open and closed symbols, respectively) of (a–c) ataxia and (d–f) lethargy observed over 24 h following the administration of cannabinoid oil formulations. Within each plot, symbols (circle, square, triangle or diamond) are used to represent an individual cat across each dose when ataxia or lethargy were observed. Vertical dotted lines represent overall average start (S) and end (E) times across all observations during the study. CBD = cannabidiol; THC = tetrahydrocannabinol
Neurologic AEs, which were infrequently observed in the placebo groups, occurred more frequently with THC oil and CBD/THC oil than with CBD oil (both P <0.001) but did not differ between THC and CBD/THC groups (P = 0.292) (Figure 2). Ataxia occurred predominantly following the administration of THC-containing oils (Figure 3) and its frequency was eight-fold (n = 24; P = 0.002; THC oil) and 11-fold (n = 33; P <0.001; CBD/THC oil) greater than with CBD oil (n = 3) but did not differ between THC and CBD/THC treatment groups (P = 0.294) (Figure 4). Ataxia was observed in the CBD/THC and THC oil groups at each of the 11 doses (Figure 4), with every animal in these groups experiencing ataxia at least once (see Table 1 in the supplementary material). On the contrary, in the CBD oil group, ataxia did not occur until the ninth (24.9 mg/kg CBD + 0.94 mg/kg THC, one cat) and 10th (27.7 mg/kg CBD + 1 mg/kg THC, two cats) administered doses, and ataxia did not significantly differ between CBD and MCT oil placebo groups (P = 0.341) (Figure 4). Assessment of ataxia duration at cannabinoid doses comparable for THC quantity (eg, first dose of THC oil vs fifth dose of CBD/THC oil [3.8 mg/kg THC] or second dose of THC oil vs 10th dose of CBD/THC oil [7.6 mg/kg THC]) revealed an approximately seven-fold increased duration of this AE with the combination product (Figure 5).
Ocular AEs, which were not observed following the administration of placebo oils, occurred more frequently with THC oil (Z = 3.31, P = 0.003) and CBD/THC oil (Z = 2.95, P = 0.004) than CBD oil but did not significantly differ between THC and CBD/THC groups (P = 0.436) (Figure 2). The most frequently observed ocular AE was protrusion membrana nictitans (n = 23) (Figure 3), which occurred exclusively in the THC (n = 7 across three cats) and CBD/THC (n = 16 across four cats) treatment groups but did not significantly differ between these two groups (P = 0.068) (Figure 4).
Regarding behavioral observations, the greatest occurrence of vocalization was in the CBD/THC oil group (Figure 6) wherein all four cats vocalized at least once throughout dose escalation, beginning, on average, 4.2 h after dose administration (range 1.5–9 h post-dose) and lasting on average 5.6 h (range 0.5–21 h) (see Table 1 in the supplementary material).
Figure 6.
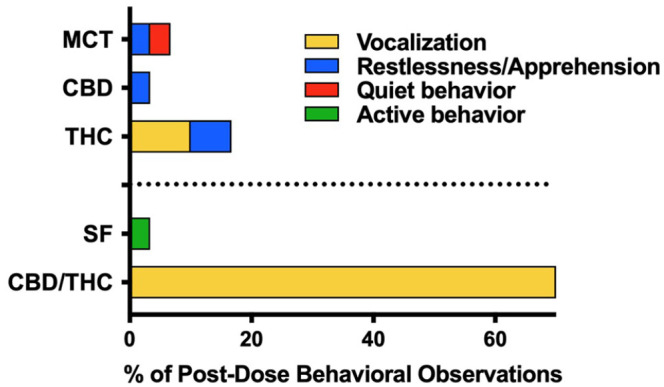
Proportion and profile of behavioral observations attributed to dose escalation of cannabis or placebo oils (n = 4 per treatment) that were observed within 24 h of oil administration across 11 escalating doses. Restlessness/apprehension includes observations of fractious behavior, pacing, agitation and/or nervousness. MCT = medium-chain triglyceride oil; CBD = cannabidiol; THC = tetrahydrocannabinol; SF = sunflower oil
Clinical chemistry and CBC
Liver markers (alkaline phosphatase [ALP], alanine aminotransferase [ALT], aspartate aminotransferase [AST], total bilirubin and gamma-glutamyl transpeptidase [GGTP]) remained within the normal reference intervals (RIs) at all measured time points (Table 3), with the exception of one cat receiving MCT placebo with elevated ALT (above the RI) 24 h following the final dose that normalized within 1 week.
Table 3.
Clinical chemistry plasma parameters indicative of liver function as measured in healthy cats administered cannabinoid or placebo oils (n = 4 per treatment group)
| Parameter | RI | Time point | MCT oil placebo | CBD (in MCT oil) |
THC (in MCT oil) |
SF oil placebo | CBD/THC (in SF oil) |
|---|---|---|---|---|---|---|---|
| ALP (U/l) | 5–131 | BSN | 44 ± 6.8 | 30.3 ± 6.8 | 41 ± 18.4 | 40.3 ± 11.1 | 42.8 ± 19.4 |
| 24 h post-FD | 35 ± 5.3* | 28.3 ± 6.0 | 37.8 ± 13.0 | 44.3 ± 11.5* | 37.8 ± 15.8 | ||
| 7 days post-FD | 41.5 ± 4.5 | 29 ± 2.9 | 34.8 ± 12.3 | 46.8 ± 13.4* | 36.3 ± 14.0 | ||
| ALT (U/l) |
12–118 | BSN | 43.8 ± 8.5 | 42 ± 4.3 | 45.5 ± 5.8 | 46.3 ± 9.2 | 41.8 ± 10.1 |
| 24 h post-FD | 78 ± 50.0 † | 39.8 ± 1.5 | 43.3 ± 6.7 | 51.8 ± 12.0 | 44.3 ± 9.5* | ||
| 7 days post-FD | 46.5 ± 7.7 | 44.3 ± 7.4 | 42.8 ± 8.3 | 50.8 ± 13.1 | 41.3 ± 12 | ||
| AST (U/l) |
15–66 | BSN | 17.8 ± 3.3 | 19.8 ± 3.5 | 24 ± 12.1 | 28.3 ± 17.3 | 20.8 ± 4.7 |
| 24 h post-FD | 28.5 ± 6.8* | 17.5 ± 1.3 | 22 ± 1.4 | 23.3 ± 8.1 | 19.8 ± 3.0 | ||
| 7 days post-FD | 20.8 ± 5.1 | 23.3 ± 4.6 | 21.8 ± 4.1 | 30.3 ± 22.0 | 18.8 ± 6.3 | ||
| Bilirubin (µmol/l) | 0–5.1 | BSN | 1.7 ± 0 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.3 ± 0.7 | 1.8 ± 0.1 |
| 24 h post-FD | 1.9 ± 0.1* | 1.8 ± 0.1 | 1.7 ± 0 | 1.9 ± 0.2 | 1.8 ± 0.2 | ||
| 7 days post-FD | 1.7 ± 0.1 | 2.1 ± 0.2* | 1.6 ± 0.5 | 1.5 ± 0.4 | 2 ± 0.9 | ||
| GGTP (U/l) ‡ | 1–12 | BSN | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| 24 h post-FD | 1.3 ± 0.5 | 1.8 ± 0.5 | 1.5 ± 0.6 | 1.8 ± 1 | 1 ± 0 | ||
| 7 days post-FD | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
Data are mean ± SD
Significant difference within a group for a specific parameter between 24 h post-final dose (FD) or 7 days post-FD and baseline (BSN; generalized estimating equation with alpha level set at P <0.05 [two-tailed] and false discovery rate adjustments made for multiple comparisons)
One subject receiving medium-chain triglyceride (MCT) oil placebo had elevated alanine aminotransferase (ALT) (153 U/l; above the reference interval [RI]) 24 h following the final dose. ALT normalized within 1 week of dosing cessation (54 U/l at 7 days post-FD)
Statistics were not conducted on gamma-glutamyl transpeptidase (GGTP) owing to insufficient variability
CBD = cannabidiol; THC = tetrahydrocannabinol; SF = sunflower; ALP = alkaline phosphatase; AST = aspartate aminotransferase
Relative to baseline values, concentrations at 24 h following the final dose showed increased AST (Z = 3.82, P = 0.002), decreased ALP (Z = 7.35, P <0.001) and increased bilirubin (Z = 3.21, P = 0.005) in the MCT oil group, increased ALT (Z = 5.77, P <0.001) in the CBD/THC group, and increased ALP (Z = 2.67, P = 0.016) in the SF group. Following a 7-day washout, liver enzyme changes returned to baseline level with the exception of increased bilirubin (Z = 2.69, P = 0.022) in the CBD oil group and increased ALP (Z = 4.06, P <0.001) in the SF group, which were significantly different from baseline in the washout period. Other observed fluctuations in CBC or clinical chemistry parameters following the last administered dose were not considered to be clinically significant by the attending veterinarian, and there were no observable trends relating to treatment group allocation (data not shown).
Plasma cannabinoids and metabolites
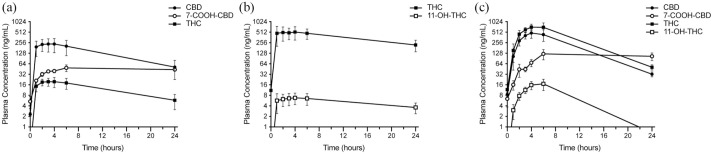
CBD, THC and their metabolites (7-COOH-CBD, 11-OH-THC) were measured in the plasma prior to and 1, 2, 3, 4, 6 and 24 h after the ninth administered dose of CBD oil (24.9/0.94 mg/kg CBD/THC), THC oil (34.0 mg/kg THC) or CBD/THC oil (10.6/6.9 mg/kg CBD/THC) (Figure 7). Following CBD oil administration, CBD and 7-COOH-CBD reached peak mean ± SD plasma levels of 236.0 ± 193.0 ng/ml (3 h) and 49.0 ± 21.1 ng/ml (6 h), respectively. At all measured time points following the ninth dose of CBD oil, both CBD and 7-COOH-CBD plasma levels were higher than pre-dose levels (P <0.05). Following THC oil administration, THC and 11-OH-THC reached peak mean plasma levels of 518.0 ± 428.0 ng/ml (4 h) and 6.8 ± 5.0 ng/ml (4 h), respectively. Plasma levels of THC and 11-OH-THC were significantly higher than those pre-dose (P <0.05) at all time points, with the exception of 1 h (THC and 11-OH-THC) and 24 h (11-OH-THC). Following CBD/THC oil administration, CBD reached peak mean plasma levels of 483.0 ± 281.0 ng/ml (4 h), and 7-COOH-CBD reached peak mean plasma levels of 123.0 ± 79.9 ng/ml (6 h). While plasma CBD did not differ significantly from pre-dose levels at any of the measured time points, plasma 7-COOH-CBD was significantly higher 4, 6 and 24 h following dosing (P <0.05). THC reached peak mean plasma levels of 715.0 ± 301.0 ng/ml (4 h), and 11-OH-THC reached peak mean plasma levels of 17.0 ± 12.1 ng/ml (6 h). Plasma levels of THC and 11-OH-THC were significantly higher than those pre-dose (P <0.05) at all time points with the exception of 1 h (THC) and 24 h (11-OH-THC)
Figure 7.
Mean ± SEM (n = 4/treatment) plasma levels of cannabidiol (CBD), tetrahydrocannabinol (THC) and their primary metabolites (7-COOH-CBD, 11-OH-THC) immediately prior to and 1, 2, 3, 4, 6 and 24 h following the ninth dose of (a) CBD oil (24.9/0.94 mg/kg CBD/THC), (b) THC oil (34.0 mg/kg THC) or (c) CBD/THC oil (10.6/6.9 mg/kg CBD/THC). The lower level of quantitation was 1 ng/ml. Data were not plotted for 11-OH-THC (following CBD) or CBD and 7-COOH-CBD (following THC), as their levels were not detected
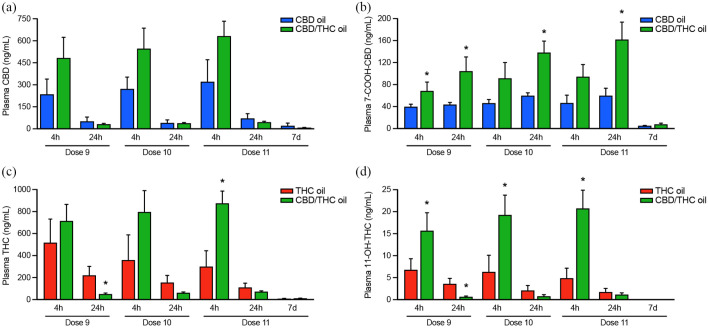
Additionally, CBD, THC, 7-COOH-CBD and 11-OH-THC were measured 4 and 24 h following the ninth, 10th and 11th (final) doses, and 7 days following the final dose, to compare cannabinoid/metabolite levels through consecutive increasing doses and after discontinuation of each cannabis oil (Figure 8). While the amount of CBD administered with CBD/THC oil was ~57% lower at each of doses 9, 10 and 11 than CBD oil, plasma levels of CBD trended to be higher 4 h following the administration of CBD and CBD/THC oils and plasma 7-COOH-CBD was significantly higher (dose 9 at 4 h; doses 9, 10, 11 at 24 h [all P <0.05]) with the combination product. Similarly, while ~80% less THC was administered at each dose with the CBD/THC combination product than with THC oil, plasma levels of THC (dose 11 at 4 h) and 11-OH-THC (doses 9, 10 and 11 at 4 h) were significantly higher with the combination product (P <0.05). Twenty-four hours after the ninth dose, both THC and 11-OH-THC were lower with the combination product (P = 0.015 and P = 0.032, respectively). While plasma levels of CBD, 7-COOH-CBD and THC were still detectable 7 days following completion of the dosing phase, their levels did not differ between CBD oil or THC oil and the CBD/THC combination product.
Figure 8.
Mean ± SEM (n = 4/treatment) plasma levels of cannabinoids and primary metabolites 4 and 24 h after the ninth, 10th and 11th doses, and 7 days after the final dose of cannabinoid oils. (a) Cannabidiol (CBD) and (b) its metabolite 7-COOH-CBD were measured following treatment with CBD or CBD/tetrahydrocannabinol (THC). (c) THC and (d) its metabolite 11-OH-THC were measured following treatments with THC oil or CBD/THC oil. The lower level of quantitation was 1 ng/ml. * Significant difference between CBD oil and CBD/THC oil or THC oil and CBD/THC oil for a given analyte at the specified timepoint (generalized estimating equation with alpha level set at P <0.05 [two-tailed] and adjustments made for multiple comparisons)
Discussion
In this study, dose escalation of cannabis oils, including a CBD-predominant oil (2.8–30.5 mg/kg CBD + 0.1–1.1 mg/kg THC), a THC-predominant oil (3.8–41.5 mg/kg THC) and a CBD/THC-predominant oil (1.2–13 mg/kg CBD + 0.8–8.4 mg/kg THC) was generally well tolerated by healthy cats, leading only to mild AEs. Importantly, over the 6- to 7-week dose escalation period, no clinically significant changes were observed in CBC or clinical chemistry parameters, including liver enzymes (ALP, ALT, AST, bilirubin and GGTP).
Comparison of gastrointestinal AE occurrence between the two placebo oils suggests that SF oil is better tolerated by healthy cats than MCT oil. Emesis and diarrhea secondary to CBD or THC oil administration may therefore be explained by intolerability to the MCT carrier oil rather than the cannabinoids themselves. To this end, emesis and diarrhea were not observed with CBD/THC oil administration (SF oil carrier). Notably, all three cannabinoid formulations led to a greater incidence of hypersalivation than either placebo – a trend also observed by our group in a similar canine dose escalation study. 8 Others have reported excessive licking and head shaking with cannabinoid administration (CBD:CBDA in fish oil), 2 suggesting an immediate aversion to the taste and/or smell of cannabinoid oils or gastrointestinal aversion following dosing (nausea, manifested as hypersalivation) in cats.
The similar incidence of respiratory AEs (tachypnoea) across all groups, irrespective of treatment, likely resulted from stress secondary to handling and dosing procedures. In cats, physiologic parameters (eg, respiratory rate) can be affected by stress,18,19 particularly in less familiar environments. 18 While they had undergone an acclimation period to adjust to housing conditions in the present study, the cats were naive to experimental procedures, including oral dosing, which may explain observations of tachypnoea across all groups at or around the time of dose administration.
Occurrence of constitutional (lethargy, hypothermia), neurologic (ataxia) and ocular (protrusion membrana nictitans) AEs was a clear differentiator of dose escalation of THC-containing oils (CBD/THC oil, THC oil) vs CBD oil. The CBD/THC combination oil (1.5:1) yielded the highest number of these AEs and behavioral observations (vocalization) despite delivering lower THC and CBD levels at each dose than THC oil or CBD oil, respectively. In a previous canine study from our group, 8 a combination CBD/THC (1.5:1) oil also resulted in a higher number and more severe AEs than THC oil or CBD oil, despite lower levels of THC or CBD in the combination oil. It thus appears that both cats and dogs experience a potentiation of the effect of THC when it is co-administered with CBD. The ability of CBD to act as a functional antagonist of THC has been widely popularized, but evidence from rodent and human studies indicates that CBD can also potentiate the psychoactive and physiologic effects of THC (eg, locomotor activity suppression, hypothermia and hypoactivity).20–25 Whether this interaction is pharmacokinetic (changes in cannabinoid absorption, distribution or elimination) or pharmacodynamic (changes to receptor interaction) may depend on whether the cannabinoids are administered simultaneously or in series,21,26,27 and on the dose ratio of CBD:THC.27,28 In published reports, when CBD/THC was simultaneously co-administered at a mean ± SD dose ratio of 8.1 ± 11.1, antagonistic effects of CBD on THC were seen (via a pharmacodynamic interaction), whereas at a ratio of 1.8 ± 1.4, CBD potentiated the effects of THC (via a pharmacokinetic interaction).27,28 In the present study, the CBD/THC dose ratio in the combination product was 1.5. It therefore follows that the effects of THC may have been potentiated by CBD via a pharmacokinetic interaction.
Plasma cannabinoid data following the ninth, 10th and 11th doses of each formulation support a pharmacokinetic interaction between CBD and THC in this study. Despite delivery at an 80% lower THC and 57% lower CBD concentration at each dose, the plasma levels of both CBD and THC 4 h after treatment with the CBD/THC combination product were approximately twofold (CBD) and 1.3–2.9-fold (THC) higher. Similarly, the plasma levels of metabolites were elevated by approximately 1.7–2.0-fold (7-COOH-CBD) and approximately 2.3–4.2-fold (11-OH-THC) with the combination product. While minor cannabinoid and most terpene levels in the CBD/THC oil fell below the reporting limit, their interaction with CBD and THC cannot be precluded. Similarly, the effect of carrier oil (MCT vs SF) on cannabinoid absorption and bioavailability warrants further exploration.
Fasted cats receiving the ninth dose of CBD (25 mg/kg) or THC (34 mg/kg) had a Tmax between 2 and 4 h (mean 3.3 h) but had achieved near-maximal cannabinoid plasma levels by 1 h post-dose and maintained this level for at least 6 h, trending downwards by 24 h post-dose. Others have noted a Tmax for CBD at 2 h in single-dose pharmacokinetics, with individual cat Tmax ranging from 1 to 4 h. 2 While there are no existing feline oral THC pharmacokinetic studies, in fasted dogs the median THC Tmax has been reported at 1.25 h following the administration of 1.5 mg/kg THC, 29 and at 1 h after administration of approximately 37 mg/kg THC. 8 The higher CBD Cmax achieved in the present study (250 ± 97 ng/ml) than what has been previously reported in cats following a 2 mg/kg total CBD + CBDA dose (43 ± 9 ng/ml) 2 is likely a reflection of higher CBD levels administered prior to pharmacokinetic analysis, as well as accumulation in plasma with dose escalation over time, but may also be related to test article matrix (MCT vs fish oil) and to the presence of THC in the CBD oil used in the present study.
Research conducted in the 1970s and 1980s in cats and rodents identified differentiating effects of THC (or cannabis extracts) vs CBD on intraocular pressure and ocular toxicity.30–32 While the adverse ocular effects of THC and a cannabis extract were similar (conjunctival erythema, chemosis, corneal opacification), CBD was devoid of ocular toxicity.30,31 An interesting hypothesis for THC-induced ocular effects is the involvement of the sympathetic nervous system, specifically the action of catecholamines (norepinephrine, epinephrine) transported to the eye via systemic circulation. 32
Unlike the THC oils (particularly CBD/THC oil), which led to vocalization, there were no occurrences of vocalization in any of the cats receiving the CBD oil over the dose escalation period. Cats have been described as having a complex vocal repertoire. 33 As the vocalizations in our study were not qualified, whether they were an expression of positive or negative emotional states is unknown.
This preliminary study used a small homogenous group of healthy cats, which may not be representative of client-owned animals, or of cats with pre-existing disease. In addition to the small sample size, alpha rate adjustments to control false discovery rate inflation due to multiple comparisons were required. Consequently, statistical tests were likely underpowered, and, as such, lack of statistical significance should not be interpreted as evidence for no difference between groups. Replication in a larger sample is therefore recommended. As the cannabinoid oils used in this study are proprietary formulations, with relatively high concentrations of CBD and/or THC and low concentrations of other cannabinoids and terpenes, the findings described here may not be applicable to the safety of other marketed formulations with different cannabinoid profiles and non-cannabinoid (eg, terpenes) constituents, and those delivered in a different matrix (different carrier oil or formulated as a solid chew).
To date, the safety and tolerability of CBD has been reviewed in rodents and humans,26,34,35 with a handful of recent studies addressing CBD safety in companion animal species, including dogs and cats.2,3,5,6,8,36 Overall, the findings support the safety and tolerability of CBD in these species. The present placebo-controlled escalating dose study, which delivered up to 30.5 mg/kg CBD in an MCT oil matrix, showed oral administration of CBD to be generally well tolerated by cats with no detrimental changes in CBC and clinical chemistry. Importantly, as compared with formulations containing THC, CBD oil was associated with the fewest number of constitutional, neurologic and ocular AEs and the fewest number of behavioral changes.
Conclusions
This study separates the relative safety and tolerability profiles of cannabis oil formulations predominant in CBD, THC or both CBD and THC (1.5:1) in cats. THC-containing oils (CBD/THC oil, THC oil) more frequently led to lethargy, ataxia, hypothermia, protrusion membrana nictitans and vocalization. Compared with THC oil or CBD/THC oil, CBD (⩽30.5 mg/kg) in MCT oil was associated with fewer constitutional, neurologic and ocular AEs, and fewer behavioral changes. Higher plasma cannabinoid and metabolite levels following administration of a CBD/THC combination product vs oils predominant in either CBD or THC are suggestive of a pharmacokinetic interaction between CBD and THC. Our findings support continuing research on the potential therapeutic uses of orally delivered CBD in cats, and for its consideration as a safe treatment option in veterinary medicine.
Supplemental Material
Adverse events and behavioral observations following administration of CBD, THC or CBD/THC across 11 escalating doses.
Acknowledgments
The authors would like to thank the staff at VivoCore for overseeing the study procedures, and Robert Menardi DVM (Canopy Animal Health) and Vivienne Marshall PhD for reviewing and providing feedback on the manuscript.
Footnotes
Accepted: 10 February 2021
Supplementary material: The following file is available online:
Table 1: Adverse events and behavioral observations following administration of CBD, THC or CBD/THC across 11 escalating doses.
JK, GE and DV are employed by Canopy Growth Corporation. LP was previously employed by Canopy Growth Corporation. Staff at VivoCore, and not the authors, were responsible for study conduct and data collection.
Funding: Canopy Animal Health, a division of Canopy Growth Corporation, financially supported this research. There were no conditions attached to the allocation of funds for this study.
Ethical approval: This work involved the use of experimental animals and the study therefore had ethical approval from an established committee as stated in the manuscript.
Informed consent: Informed consent (either verbal or written) was obtained from the owner or legal custodian of all animal(s) described in this work (either experimental or non-experimental animals) for the procedure(s) undertaken (either prospective or retrospective studies). No animals or humans are identifiable within this publication, and therefore additional informed consent for publication was not required.
ORCID iD: Justyna E Kulpa  https://orcid.org/0000-0001-9823-235X
https://orcid.org/0000-0001-9823-235X
References
- 1. Grigg EK, Kogan LR, van Haaften K, et al. Cat owners’ perceptions of psychoactive medications, supplements and pheromones for the treatment of feline behavior problems. J Feline Med Surg 2019; 21: 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Deabold KA, Schwark WS, Wolf L, et al. Single-dose pharmacokinetics and preliminary safety assessment with use of CBD-rich hemp nutraceutical in healthy dogs and cats. Animals (Basel) 2019; 9: 832. DOI: 10.3390/ani9100832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verrico CD, Wesson S, Konduri V, et al. A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain. Pain 2020; 161: 2191–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brioschi FA, Di Cesare F, Gioeni D, et al. Oral transmucosal cannabidiol oil formulation as part of a multimodal analgesic regimen: effects on pain relief and quality of life improvement in dogs affected by spontaneous osteoarthritis. Animals (Basel) 2020; 10: 1505. DOI: 10.3390/ani10091505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gamble LJ, Boesch JM, Frye CW, et al. Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. Front Vet Sci 2018; 5: 165. DOI: 10.3389/fvets.2018.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGrath S, Bartner LR, Rao S, et al. Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. J Am Vet Med Assoc 2019; 254: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 7. Greenwich Biosciences. Epidiolex (package insert). Carlsbad, CA: Greenwich Biosciences, 2018. [Google Scholar]
- 8. Vaughn D, Kulpa J, Paulionis L. Preliminary investigation of the safety of escalating cannabinoid doses in healthy dogs. Front Vet Sci 2020; 7: 51. DOI: 10.3389/fvets.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brutlag A, Hommerding H. Toxicology of marijuana, synthetic cannabinoids, and cannabidiol in dogs and cats. Vet Clin North Am Small Anim Pract 2018; 48: 1087–1102. [DOI] [PubMed] [Google Scholar]
- 10. Janeczek A, Zawadzki M, Szpot P, et al. Marijuana intoxication in a cat. Acta Vet Scand 2018; 60: 44. DOI: 10.1186/s13028-018-0398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amanollahi R, Soltaninejad H, Ghasemkhani N. Cannabis poisoning in a cat. Onl J Vet Res 2020; 24: 434–437. [Google Scholar]
- 12. Animals for Research Act, RSO, c.A.22. Available at: https://www.ontario.ca/laws/statute/90a22 (accessed September 8, 2020).
- 13. Canadian Council on Animal Care. Guidelines. Available at: https://www.ccac.ca/en/standards/guidelines/ (accessed September 8, 2020).
- 14. VCOG. Veterinary Cooperative Oncology Group – common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol 2016; 14: 417–446. [DOI] [PubMed] [Google Scholar]
- 15. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57: 289–300. [Google Scholar]
- 16. Venables WN, Ripley BD. Modern applied statistics with S. 4th ed. New York: Springer, 2002. [Google Scholar]
- 17. Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw 2006; 15: 1–11. [Google Scholar]
- 18. Quimby JM, Smith ML, Lunn KF. Evaluation of the effects of hospital visit stress on physiologic parameters in the cat. J Feline Med Surg 2011; 13: 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nibblett BM, Ketzis JK, Grigg EK. Comparison of stress exhibited by cats examined in a clinic versus a home setting. Appl Anim Behav Sci 2015; 173: 68–75. [Google Scholar]
- 20. Hayakawa K, Mishima K, Hazekawa M, et al. Cannabidiol potentiates pharmacological effects of delta-9-tetrahydrocannabinol via CB1 receptor-dependent mechanism. Brain Res 2008; 1188: 157–164. [DOI] [PubMed] [Google Scholar]
- 21. Klein C, Karanges E, Spiro A, et al. Cannabidiol potentiates delta-9-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats. Psychopharmacology (Berl) 2011; 218: 443–457. [DOI] [PubMed] [Google Scholar]
- 22. Fernandes M, Schabarek A, Cooper H, et al. Modification of delta-9-THC actions by cannabinol and cannabidiol in the rat. Psychopharmacologia 1974; 38: 329–338. [DOI] [PubMed] [Google Scholar]
- 23. Reid MJ, Bornheim LM. Cannabinoid-induced alterations in brain disposition of drugs of abuse. Biochem Pharmacol 2001; 61: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 24. Taffe MA, Creehan KM, Vandewater SA. Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Δ(9)-tetrahydrocannabinol in Sprague-Dawley rats. Br J Pharmacol 2015; 172: 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hollister LE, Gillespie H. Interactions in man of delta-9-tetrahydrocannabinol and cannabidiol. Clin Pharmacol Ther 1975; 18: 329–338. [DOI] [PubMed] [Google Scholar]
- 26. Bergamaschi MM, Queiroz RHC, Crippa JAS, et al. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr Drug Saf 2011; 6: 237–249. [DOI] [PubMed] [Google Scholar]
- 27. Zuardi AW, Hallak JEC, Crippa JAS. Interaction between cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC): influence of administration interval and dose ratio between the cannabinoids. Psychopharmacology (Berl) 2012; 219: 247–249. [DOI] [PubMed] [Google Scholar]
- 28. Zuardi AW, Karniol IG. Effects on variable-interval performance in rats of delta 9-tetrahydrocannabinol and cannabidiol, separately and in combination. Braz J Med Biol Res 1983; 16: 141–146. [PubMed] [Google Scholar]
- 29. Lebkowska-Wieruszewska B, Stefanelli F, Chericoni S, et al. Pharmacokinetics of Bedrocan®, a cannabis oil extract, in fasting and fed dogs: an explorative study. Res Vet Sci 2019; 123: 26–28. [DOI] [PubMed] [Google Scholar]
- 30. Colsanti BK, Brown RE, Craig CR. Ocular hypotension, ocular toxicity, and neurotoxicity in response to marihuana extract and cannabidiol. Gen Pharmacol 1984; 15: 479–484. [DOI] [PubMed] [Google Scholar]
- 31. Colsanti BK, Powell SR, Craig CR. Intraocular pressure, ocular toxicity, and neurotoxicity after administration of delta-9-tetrahydrocannabinol or cannabichromene. Exp Eye Res 1984; 38: 63–71. [DOI] [PubMed] [Google Scholar]
- 32. Korczyn AD. The ocular effects of cannabinoids. Gen Pharmacol 1980; 11: 419–423. [DOI] [PubMed] [Google Scholar]
- 33. Tavernier C, Ahmed S, Houpt KA, et al. Feline vocal communication. J Vet Sci 2020; 21: e18. DOI: 10.4142/jvs.2020.21.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant studies. Cannabis Cannabinoid Res 2017; 2: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization (WHO). Expert Committee on Drug Dependence Fortieth Meeting. Cannabidiol (CBD) critical review report 2018. Available at: https://www.who.int/medicines/access/controlled-substances/CannabidiolCriticalReview.pdf (accessed September 14, 2020).
- 36. McGrath S, Bartner LR, Rao S, et al. A report of adverse effects associated with the administration of cannabidiol in healthy dogs. Amer Holistic Vet Med Assoc 2018; 52: 34–38. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adverse events and behavioral observations following administration of CBD, THC or CBD/THC across 11 escalating doses.