Key Points
Question
Are plasma metabolites that are associated with incident coronary heart disease (CHD) among non-Hispanic White individuals also associated with incident CHD among African American individuals?
Findings
In this cohort study of 2346 African American participants from the Jackson Heart Study and 1588 multiethnic participants from the Women’s Health Initiative with and without incident CHD, metabolomic profiles of African American individuals that had not been previously reported in this population were found to be associated with incident CHD. Many novel metabolites associated with incident CHD, including N-acylamides, leucine, and lipid species, were replicated in a multiethnic cohort.
Meaning
This study found that African American individuals shared certain incident CHD-associated metabolites with individuals of other racial groups, although specific associations may be distinct among African American individuals; these findings warrant follow-up studies of large diverse populations.
Abstract
Importance
African American individuals have disproportionate rates of coronary heart disease (CHD) but lower levels of coronary artery calcium (CAC), a marker of subclinical CHD, than non-Hispanic White individuals. African American individuals may have distinct metabolite profiles associated with incident CHD risk compared with non-Hispanic White individuals, and examination of these differences could highlight important processes that differ between them.
Objectives
To identify novel biomarkers of incident CHD and CAC among African American individuals and to replicate incident CHD findings in a multiethnic cohort.
Design, Setting, and Participants
This analysis targeted plasma metabolomic profiling of 2346 participants in the Jackson Heart Study (JHS), a prospective population-based cohort study that included 5306 African American participants who were examined at baseline (2000-2004) and 2 follow-up visits. Replication of CHD-associated metabolites was sought among 1588 multiethnic participants from the Women’s Health Initiative (WHI), a prospective population-based multiethnic cohort study of 161 808 postmenopausal women who were examined at baseline (1991-1995) and ongoing follow-up visits. Regression analyses were performed for each metabolite to examine the associations with incident CHD and CAC scores. Data were collected from the WHI between 1994 and 2009 and from the JHS between 2000 and 2015. All data were analyzed from November 2020 to August 2021.
Exposures
Plasma metabolites.
Main Outcomes and Measures
Incident CHD was defined as definite or probable myocardial infarction or definite fatal CHD in both the JHS and WHI cohorts. In the JHS cohort, silent myocardial infarction between examinations (as determined by electrocardiography) and coronary revascularization were included in the incident CHD analysis. Coronary artery calcium was measured using a 16-channel computed tomographic system and reported as an Agatston score.
Results
Among 2346 African American individuals in the JHS cohort, the mean (SD) age was 56 (13) years, and 1468 individuals (62.6%) were female. Among 1588 postmenopausal women in the WHI cohort, the mean (SD) age was 67 (7) years; 217 individuals (13.7%) self-identified as African American, 1219 (76.8%) as non-Hispanic White, and 152 (9.6%) as other races or ethnicities. In the fully adjusted model including 1876 individuals, 46 of 303 targeted metabolites were associated with incident CHD (false discovery rate q <0.100). Data for 32 of the 46 metabolites were available in the WHI cohort, and 13 incident CHD–associated metabolites from the JHS cohort were replicated in the WHI cohort. A total of 1439 participants from the JHS cohort with available CAC scores received metabolomic profiling. Nine metabolites were associated with CAC scores. Minimal overlap was found between the results from the incident CHD and CAC analyses, with only 3 metabolites shared between the 2 analyses.
Conclusions and Relevance
This cohort study identified metabolites that were associated with incident CHD among African American individuals, including 13 incident CHD–associated metabolites that were replicated in a multiethnic population and 9 novel metabolites that included N-acylamides, leucine, and lipid species. These findings may help to elucidate common and distinct metabolic processes that may be associated with CHD among individuals with different self-identified race.
This cohort study examines metabolites associated with incident coronary heart disease and coronary artery calcium among African American participants in the Jackson Heart Study and seeks to replicate coronary heart disease findings among multiethnic participants in the Women’s Health Initiative.
Introduction
African American individuals are at greater risk of coronary heart disease (CHD) than non-Hispanic White individuals; however, established risk prediction models, such as the Framingham risk score, do not fully capture their risk.1,2,3,4,5 The AHA/ACC (American College of Cardiology/American Heart Association) Guideline on the Primary Prevention of Cardiovascular Disease6 recommends using coronary artery calcium (CAC) scores to guide statin use among patients with an intermediate risk of atherosclerotic cardiovascular disease. Despite having higher rates of CHD, African American individuals have been found to have lower CAC scores than non-Hispanic White individuals,7,8,9 suggesting that CAC among African American individuals may not have the same implications for risk stratification as CAC among non-Hispanic White individuals.10
The reasons for the differences between African American and non-Hispanic White individuals with regard to CHD and intermediate phenotypes are not well understood. High throughput metabolite profiling of population-based cohorts has identified metabolites that are associated with the risk of CHD,11,12,13,14,15,16,17,18,19,20,21,22 but African American individuals have been underrepresented in these studies. The present cohort study examined whether metabolite markers of cardiometabolic risk identified in cohorts consisting primarily of non-Hispanic White individuals would also extend to African American individuals. We hypothesized that African American individuals would have distinct metabolite profiles associated with incident CHD risk compared with non-Hispanic White individuals and that examination of these differences could highlight important processes that differ between them. Furthermore, given the attenuated association between CAC and CHD outcomes among African American individuals, we hypothesized that metabolite profiles associated with incident CHD might not overlap with those associated with CAC. We used targeted metabolite profiling of baseline plasma samples from participants in the Jackson Heart Study (JHS) to define the metabolic mechanisms of CAC and CHD among African American individuals. We sought replication of our findings among a cohort from the Women’s Health Initiative (WHI), a population-based multiethnic cohort study of postmenopausal women across the US.
Methods
This cohort study of participants in the JHS and WHI cohorts was approved by the institutional review board of Beth Israel Deaconess Medical Center. All participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Study Populations
Jackson Heart Study
The JHS was a single-site prospective population-based cohort study of cardiovascular disease and its risk factors among African American individuals. The study included 5306 African American participants aged 21 to 84 years residing in 3 counties surrounding Jackson, Mississippi. Participants were examined according to standardized protocols at baseline (2000-2004) and 2 follow-up visits. The JHS was approved by the institutional review boards of Jackson State University, Tougaloo College, and the University of Mississippi Medical Center. All participants provided written informed consent. Additional characteristics are available in eMethods 1 and eTables 1 to 3 in the Supplement. The present study included 2346 participants from the JHS who received plasma metabolomic profiling after fasting for more than 8 hours and had no history of CHD. Exclusions are summarized in eFigure 1 in the Supplement.
Women’s Health Initiative
The replication cohort included participants from the WHI, a long-term national health study focused on heart disease, breast and colorectal cancer, and osteoporosis among postmenopausal women recruited from 40 sites across the US. The WHI included 161 808 women who were examined at baseline (1991-1995) and ongoing follow-up visits. The WHI was approved by the institutional review boards of each participating clinical center, and all participants provided written informed consent. Additional characteristics are available in eMethods 1 and eTable 4 in the Supplement. The present study comprised 1588 participants from the WHI who were included in a previous study of metabolomic profiles among women with CHD.22
Assessment of Incident CHD and Covariates
Incident CHD was defined as definite fatal CHD, definite or probable myocardial infarction (MI), silent MI between examinations (as determined by electrocardiography), or coronary revascularization. Prevalent CHD status was assessed by questionnaire to obtain participants’ history of MI, history of coronary revascularization, or evidence of MI determined by electrocardiography at visit 1. For the JHS cohort, covariates used in the analyses were measured at the baseline examination. For the WHI cohort, CHD was defined as incident fatal or nonfatal CHD; silent MI and coronary revascularization were not included in the analysis. A description of incident CHD assessments and covariates is available in eMethods 2 in the Supplement.
Assessment of Coronary Artery Calcium Score
Coronary artery calcium was measured using a 16-channel computed tomographic system and was reported as an Agatston score. The computed tomographic protocol for the JHS cohort has been published previously.23 To analyze CAC in a continuous manner and account for CAC scores of 0, we assessed metabolite associations using log (CAC score + 1). Additional details about the computed tomographic protocol and analysis of CAC scores are available in eMethods 3 in the Supplement.
Plasma Samples and Metabolomic Profiling
Plasma metabolite profiling was performed using liquid chromatography with tandem mass spectrometry.24,25,26 In brief, to measure water-soluble metabolites in the positive ionization mode, hydrophilic interaction liquid chromatography was performed using an ultra-high performance liquid chromatograph (Nexera X2 U-HPLC; Shimadzu) coupled to a mass spectrometer (Q Exactive; ThermoFisher Scientific). To measure organic acids and other intermediary metabolites in negative ionization or amide mode, chromatography was performed using an ultra-high performance liquid chromatograph (1290 Infinity LC system; Agilent Technologies) equipped with an amide column (XBridge; Waters) coupled to a triple quadrupole mass spectrometer (6490 Triple Quad LC/MS; Agilent Technologies). Liquid chromatography with tandem mass spectrometry was performed using Masshunter QQQ quantitative analysis software (Agilent Technologies). Additional details are available in eMethods 4 in the Supplement.
Prediction Models
To investigate whether identified metabolites were associated with improvements in incident CHD risk prediction, we constructed receiver operating characteristic (ROC) curves and calculated C statistics using 46 metabolites associated with incident CHD in the JHS cohort, and we aimed to replicate these findings in the WHI cohort using 32 metabolites available for replication. The 3 JHS models comprised (1) covariates alone, (2) covariates plus C-reactive protein (CRP) and N-terminal fragment of the prohormone brain natriuretic peptide (NT-proBNP), and (3) covariates, CRP, NT-proBNP, and 46 incident CHD–associated metabolites from the fully adjusted model. The 3 WHI models comprised (1) covariates alone, (2) covariates plus CRP (data on NT-proBNP were not available in the WHI cohort), and (3) covariates, CRP, and 32 incident CHD–associated metabolites from the fully adjusted JHS model that were available for replication. We corrected for optimism in the JHS cohort to account for overfitting due to testing in the derivation cohort by using the validation function of the rms package in R software, version 6.2-0 (R Foundation for Statistical Computing).
To further investigate whether metabolites of interest were associated with improvement in risk prediction, we constructed a metabolite risk score. A Cox model regularized by Ridge penalties was used to predict incident CHD–free survival. Analyses were conducted using R software, version 4.0.2. Ridge regression analyses were performed using the glmnet package in R software version 4.1.1. Additional details about the prediction models are available in eMethods 6 in the Supplement.
Statistical Analysis
A Cox regression analysis was performed to examine the association between metabolite levels (independent variable) and incident CHD (dependent variable), and linear regression analysis was used to assess the association between metabolite levels (independent variable) and log (CAC score + 1) (dependent variable). Metabolite levels were standardized to multiples of 1 SD. Two sets of covariates were used in the incident CHD and CAC analyses. The first set included age, sex, and batch as covariates; the second set included age, sex, batch, and traditional clinical risk factors as covariates.
Categorical variables were reported as frequencies with percentages, and continuous variables were reported as means with SDs. Continuous variables were compared using a paired t test or a Wilcoxon rank sum test, as appropriate, with a significance threshold of 2-tailed P < .05.
Correction for multiple hypothesis testing was performed using the Benjamin-Hochberg false discovery rate (FDR) method, with a significance threshold of q <0.100. In the cross-cohort analysis, which was conducted to replicate our findings in the WHI cohort, the significance threshold was P < .05 with consistent direction of effect. All statistical analyses were performed using R software, version 3.3.1.
To assess the significance of differences in β coefficient estimates between the JHS and WHI cohorts, a z score was created for each metabolite comparison by dividing the difference in β estimates in the 2 cohorts by the square root of the sum of the variances. Because the β estimates were normally distributed in each cohort, the P value for this difference was calculated using the probnorm function in SAS software, version 9.4 (SAS Institute Inc), with the significance level set at P < .01 owing to the exploratory nature of the analysis.
Data were collected from the WHI between 1994 and 2009 and from the JHS between 2000 and 2015. All data were analyzed from November 2020 to August 2021. Additional information is provided in eMethods 5 in the Supplement.
Results
Participant Characteristics
Jackson Heart Study
Among 2346 African American participants (1468 women [62.6%]; mean [SD] age, 56 [13] years) in the JHS cohort who were included in the incident CHD analysis, 190 individuals had incident CHD, and 2156 individuals did not (Table 1). In general, individuals with vs without incident CHD were older (mean [SD] age, 63 [10] years vs 55 [13] years), with lower kidney function (mean [SD] estimated glomerular filtration rate, 82 [22] mL/min/1.73 m2 vs 86 [17] mL/min/1.73 m2) and a higher prevalence of diabetes (75 individuals [39.5%] vs 390 individuals [18.1%]). The median time to an incident CHD event was 11.7 years (range, 10.8-12.7 years).
Table 1. Clinical Characteristics of Participants With Metabolomic Profiles in the Jackson Heart Study.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Incident CHD (n = 2346) | CAC (n = 1439) | |||||
| Yes | No | P value | <100 | ≥100 | P value | |
| Total participants, No. | 190 | 2156 | NA | 1089 | 350 | NA |
| Age, mean (SD), y | 63 (10) | 55 (13) | <.001 | 54 (10) | 63 (8) | <.001 |
| Sex | ||||||
| Female | 107 (56.3) | 1361 (63.1) | .06 | 732 (67.2) | 194 (55.4) | <.001 |
| Male | 83 (43.7) | 795 (36.9) | 357 (32.8) | 156 (44.6) | ||
| BMI, mean (SD) | 32 (7) | 31 (7) | .30 | 31 (7) | 31 (6) | .70 |
| eGFR, mean (SD), mL/min/1.73 m2 | 82 (22) | 86 (17) | .002 | 87 (16) | 83 (18) | <.001 |
| Diabetes | 75 (39.5) | 390 (18.1) | <.001 | 142 (13.0) | 111 (31.7) | <.001 |
| Hypertension | 156 (82.1) | 1261 (58.5) | <.001 | 599 (55.0) | 274 (78.3) | <.001 |
| Systolic blood pressure, mean (SD), mm Hg | 133 (17) | 126 (18) | <.001 | 127 (16) | 132 (18) | <.001 |
| Total cholesterol, mean (SD), mg/dL | 204 (43) | 200 (40) | .11 | 199 (40) | 207 (44) | .70 |
| HDL-C, mean (SD), mg/dL | 51 (15) | 52 (14) | .30 | 53 (15) | 50 (13) | .009 |
| Current smoker | 29 (15.3) | 245 (11.4) | .11 | 105 (9.6) | 39 (11.1) | .40 |
| Statin medication receipt | 41 (21.6) | 185 (8.6) | <.001 | 72 (6.6) | 78 (22.3) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAC, coronary artery calcium; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; NA, not applicable.
SI conversion factor: To convert total cholesterol and HDL-C to millimoles per liter, multiply by 0.0259.
Of 1439 participants with CAC data available, 350 individuals had CAC scores of 100 or greater, and 1089 individuals had CAC scores less than 100 (Table 1). Participants with CAC scores less than 100 were younger (mean [SD] age, 54 [10] years vs 63 [8] years, respectively) and more likely to be female (732 individuals [67.2%] vs 194 individuals [55.4%]), with a higher estimated glomerular filtration rate (mean [SD], 87 [16] mL/min/1.73 m2 vs 83 [18] mL/min/1.73 m2), a lower prevalence of diabetes (142 individuals [13.0%] vs 111 individuals [31.7%]), lower total cholesterol levels (mean [SD], 199 [40] mg/dL vs 207 [44] mg/dL; to convert to millimoles per liter, multiply by 0.0259), and lower receipt of statin medications (72 individuals [6.6%] vs 78 individuals [22.3%]) compared with participants with CAC scores of 100 or greater. The baseline characteristics of the 2750 participants who received metabolite profiling were representative of the overall JHS cohort (eTables 1-3 in the Supplement).
Women’s Health Initiative
We sought to replicate our findings among 1588 participants (mean [SD] age, 67 [7] years) in the WHI cohort (eTable 12 in the Supplement). Of those, 793 individuals had incident CHD, and 795 individuals did not. A total of 217 participants (13.7%) self-identified as African American, 1219 (76.8%) as non-Hispanic White, and 152 (9.6%) as other races or ethnicities, including American Indian or Alaska Native (14 individuals [0.9%]), Asian or Pacific Islander (34 individuals [2.1%]), Hispanic or Latino (52 individuals [3.3%]), and unknown or unreported race and/or ethnicity (52 individuals [3.3%]). Women with vs without incident CHD had a higher prevalence of diabetes (143 individuals [18.0%] vs 55 individuals [6.9%], respectively), hypertension (506 individuals [63.8%] vs 374 individuals [47.0%]), statin medication receipt (103 individuals [13.0%] vs 74 individuals [9.3%]), and current smoking (104 individuals [13.1%] vs 61 individuals [7.7%]). Individuals with vs without CHD also had a higher body mass index (mean [SD], 29 [6] vs 28 [6]; calculated as weight in kilograms divided by height in meters squared), higher systolic blood pressure (mean [SD], 136 [19] mm Hg vs 130 [18] mm Hg), and lower high-density lipoprotein cholesterol levels (mean [SD], 49 [15] mg/dL vs 55 [16] mg/dL) (eTable 4 in the Supplement).
Incident CHD Analysis
We first analyzed 58 metabolites previously associated with incident CHD among cohorts consisting primarily of non-Hispanic White individuals from 8 previous studies11,12,13,14,15,16,17,22; a full list of metabolites is available in eTable 5 in the Supplement. We performed age- and sex-adjusted analyses, then further adjusted for age, sex, total cholesterol level, high-density lipoprotein cholesterol level, presence of hypertension, systolic blood pressure, body mass index, presence of diabetes, estimated glomerular filtration rate, smoking status, and statin medication receipt. In the fully adjusted model, 10 of the 58 metabolites replicated previous findings (both directional consistency of effect and statistical significance). For example, the hazard ratio (HR) for phosphatidylethanolamine 34:2 (with numerical ratio denoting the number of total carbon atoms to the number of double bonds) was 1.30 (95% CI, 1.10-1.52; FDR q = 0.020), and the HR for uridine was 0.82 (95% CI, 0.70-0.95; FDR q = 0.080); these results were consistent with previous findings among primarily non-Hispanic White cohorts.11,12
In the JHS cohort, leucine, a branched-chain amino acid, was associated with a reduced risk of incident CHD (HR, 0.79; 95% CI, 0.65-0.94; FDR q = 0.070). This finding is notable given that branched-chain amino acids are well-established biomarkers of increased cardiometabolic risk.16,17,18,21 The association of isoleucine (HR, 0.84; 95% CI, 0.69-1.01; FDR q = 0.240) and valine (HR, 0.88; 95% CI, 0.75-1.04; FDR q = 0.450) with the risk of incident CHD was also inconsistent with previous findings.14,15,16 Other associations between metabolites and incident CHD that were not replicated among African American individuals in the JHS cohort included glutamate (HR, 0.86; 95% CI, 0.73-1.01; FDR q = 0.230) and glutamine (HR, 1.06; 95% CI, 0.89-1.27; FDR q = 0.790); this association has been previously described in populations consisting primarily of non-Hispanic White individuals.12,15,17,22
We performed analyses of the full panel of 303 metabolites from 1876 individuals using the same models. In the first model, 61 metabolites were significantly associated with incident CHD (FDR q <0.100) (eTable 6 in the Supplement). In the fully adjusted model, 46 of 303 metabolites were associated with incident CHD (Table 2), representing 15 distinct metabolite classes (eFigure 2 in the Supplement). Imidazole propronate, which has been associated with diabetes,27 was among the 15 metabolites that were no longer statistically significant in the fully adjusted model. Correlations among the 46 metabolites revealed several cassettes of associated metabolites (eg, lysophosphatidylcholines and deoxyuridine or pseudouridine) but substantial orthogonality (median R2 = 0.05; range, −0.44 to 0.91), suggesting broad coverage of plasma components (eFigure 3 in the Supplement). Twenty-six metabolites were associated with an increased risk of incident CHD, and 20 metabolites were associated with a reduced risk of CHD. Pseudouridine (HR, 1.48; 95% CI, 1.20-1.82; FDR q = 0.005) and 1-methylnicotinamide (HR, 0.69; 95% CI, 0.59-0.81; FDR q = 0.001) had the highest and lowest HRs for incident CHD, respectively (Table 2). N-acetylaspartic acid (HR, 1.44; 95% CI, 1.22-1.69; FDR q = 0.001) and linoleoyl ethanolamide (HR, 1.38; 95% CI, 1.19-1.60; FDR q = 0.001), both of which are N-acylamides, were among the metabolites with significant associations with CHD. Given that incident CHD in the JHS cohort was defined as MI as well as revascularization, we sought to identify differences in metabolite associations by type of outcome. An MI-only analysis revealed a loss of FDR significance in only 7 of the 46 metabolites (eTable 7 in the Supplement). To explore the potential association of dysmetabolism, we further adjusted for triglyceride levels and waist circumference. Five of the 46 metabolites lost FDR significance but remained statistically significant and retained consistent direction of effect (eTable 8 in the Supplement).
Table 2. Fully Adjusted Model of Metabolites Associated With Incident Coronary Heart Disease in the Jackson Heart Studya.
| Metabolite | HR (95% CI) | P value | FDR q value |
|---|---|---|---|
| 1-Methylnicotinamide | 0.69 (0.59-0.81) | 3.62 × 10−6 | 0.001 |
| Linoleoyl ethanolamide | 1.38 (1.19-1.60) | .00001 | 0.001 |
| N-acetylaspartic acid | 1.44 (1.22-1.69) | 9.72 × 10−6 | 0.001 |
| 4-Acetamidobutanoate | 1.46 (1.22-1.73) | .00002 | 0.001 |
| Homoarginine | 0.71 (0.60-0.83) | .00002 | 0.001 |
| Niacinamide | 0.75 (0.65-0.85) | .00001 | 0.001 |
| Trimethylbenzene | 0.73 (0.62-0.85) | .00004 | 0.002 |
| PE 36:2 | 1.41 (1.18-1.66) | .00006 | 0.002 |
| 2-Hydroxyglutaric acid | 1.36 (1.16-1.58) | .00008 | 0.003 |
| NMMA | 0.75 (0.64-0.86) | .00010 | 0.003 |
| Oxaloacetic acid | 1.36 (1.16-1.59) | .0001 | 0.003 |
| Pseudouridine | 1.48 (1.20-1.82) | .0002 | 0.005 |
| DMGV | 1.47 (1.19-1.81) | .0003 | 0.006 |
| SM 16:0 | 1.46 (1.17-1.81) | .0006 | 0.010 |
| Biliverdin | 0.76 (0.65-0.89) | .0007 | 0.010 |
| Lysine | 0.78 (0.67-0.90) | .0008 | 0.010 |
| Myristoleate | 0.77 (0.66-0.90) | .0008 | 0.010 |
| LPE B 18:0 | 0.77 (0.65-0.90) | .0009 | 0.020 |
| 4-Hydroxy-3-methylacetophenone | 0.79 (0.67-0.91) | .001 | 0.020 |
| Indole-3-lactic acid | 1.31 (1.11-1.55) | .001 | 0.020 |
| Histidine | 0.78 (0.66-0.91) | .001 | 0.020 |
| Cystine | 1.39 (1.13-1.71) | .002 | 0.020 |
| N2,N2-dimethylguanosine | 1.32 (1.10-1.57) | .002 | 0.020 |
| PE 34:2 | 1.30 (1.10-1.52) | .002 | 0.020 |
| 1-Methyladenosine | 1.33 (1.10-1.59) | .002 | 0.030 |
| PE plasmalogen 36:3 | 1.31 (1.10-1.56) | .002 | 0.030 |
| Arginine | 0.80 (0.68-0.93) | .004 | 0.040 |
| 1,5 Anhydroglucitol | 0.79 (0.67-0.93) | .005 | 0.050 |
| Homogentisic acid | 1.29 (1.08-1.53) | .004 | 0.050 |
| 2-Deoxyuridine | 1.31 (1.08-1.59) | .005 | 0.050 |
| LPC 16:1 | 0.79 (0.67-0.93) | .005 | 0.050 |
| Plasmalogen 16:1 | 0.80 (0.68-0.93) | .005 | 0.050 |
| N-acetyltryptophan | 1.26 (1.07-1.47) | .005 | 0.050 |
| Malic acid | 1.26 (1.06-1.48) | .006 | 0.060 |
| Methionine | 0.79 (0.66-0.94) | .008 | 0.070 |
| Glycochenodeoxycholic acid | 1.23 (1.05-1.42) | .008 | 0.070 |
| Leucine | 0.79 (0.65-0.94) | .009 | 0.070 |
| PC 36:2 | 1.26 (1.05-1.51) | .009 | 0.080 |
| Uridine | 0.82 (0.70-0.95) | .01 | 0.080 |
| N4-acetylcytidine | 1.25 (1.05-1.49) | .01 | 0.080 |
| D-gluconic acid | 1.30 (1.06-1.58) | .01 | 0.080 |
| LPC 20:1 | 0.82 (0.70-0.96) | .01 | 0.080 |
| LPC 18:3 | 0.82 (0.69-0.96) | .01 | 0.080 |
| Aconitic acid | 1.38 (1.07-1.79) | .01 | 0.080 |
| Fumaric acid | 1.21 (1.03-1.41) | .01 | 0.090 |
| SM 18:0 | 1.25 (1.04-1.50) | .02 | 0.090 |
Abbreviations: DMGV, dimethylguanidino valerate; FDR, false discovery rate; HR, hazard ratio; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; NMMA, N-methylmalonamic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin.
Covariates included age, sex, batch, total cholesterol level, high-density lipoprotein level, presence of hypertension, systolic blood pressure, body mass index (calculated as weight in kilograms divided by height in meters squared), presence of diabetes, estimated glomerular filtration rate, smoking status, and statin medication receipt.
CAC Analysis
The association between individual metabolites and log (CAC score + 1) was assessed among 1439 participants using linear regression analysis in 2 models. In the first model, which was adjusted for age and sex, 65 metabolites were significantly associated (FDR q <0.100) with log (CAC score + 1) (eTable 9 in the Supplement). In the second model, which was adjusted for age, sex, CHD risk factors, time between blood sampling, and results of computed tomographic scans, 9 metabolites (xanthosine, N-acetylputrescine, lysophosphatidylethanolamine-B 18:0, phosphatidylethanolamine 34:2, phosphatidylethanolamine 36:4, 2-hydroxyglutaric acid, lysophosphatidylethanolamine 18:1, lysophosphatidylethanolamine 20:0, and lysophosphatidylethanolamine 20:4) were associated with log (CAC score + 1) (eTable 10 in the Supplement); 3 of those metabolites (2-hydroxyglutaric acid [β = 0.18; 95% CI, 0.06-0.30; FDR q = 0.090], phosphatidylethanolamine 34:2 [β = 0.20; 95% CI, 0.08-0.33; FDR q = 0.070], and lysophosphatidylethanolamine B 18:0 [β = −0.19; 95% CI, −0.30 to −0.08; FDR q = 0.070]) were also associated with incident CHD and had the same direction of association. Overall, 43 of 46 metabolites associated with incident CHD were not associated with CAC (Box). Because statin therapy has been associated with accelerated calcification of atherosclerotic plaques,28,29 we also adjusted for statin medication receipt, which slightly attenuated our findings (eTable 10 in the Supplement).
Box. Metabolites Associated With Incident Coronary Heart Disease and CAC Score in an African American Cohort.
Incident coronary heart disease
1-Methylnicotinamide
Linoleoyl ethanolamide
N-acetylaspartic acid
4-Acetamidobutanoate
Homoarginine; niacinamide
Trimethylbenzene; PE 36:2; NMMA
Oxaloacetic acid; pseudouridine; DMGV; SM 16:0; biliverdin; lysine; myristoleate
4-Hydroxy-3-methylacetophenone
Indole-3-lactic acid; histidine; cystine
N2,N2-dimethylguanosine
1-Methyladenosine; PE 36:3; PE plasmalogen; arginine; 1,5-anhydroglucitol; homogentisic acid; 2-deoxyuridine
LPC 16:1; LPC 16:1 plasmalogen
N-acetyltryptophan; malate; methionine; glycochenodeoxycholic acid
Leucine; PC 36:2; uridine
N4-acetylcytidine; D-gluconic acid
LPC 20:1; LPC 18:3; aconitic acid
Fumaric acid; SM 18:0
CAC score
Xanthosine; N-acetylputrescine; PE 36:4; LPE 18:1; LPE 20:0; LPE 20:4
Both incident coronary heart disease and CAC score
PE 34:2
2-Hydroxyglutaric acid
LPE-B 18:0
Adjustment for Socioeconomic Factors
To address the potential role of socioeconomic factors associated with incident CHD among African American individuals, we further adjusted the incident CHD analysis in the JHS cohort for income level, educational level, and health insurance status (eTable 11 in the Supplement). Thirty-three metabolites remained significantly associated with incident CHD at the FDR significance threshold of q < 0.100. Among the 13 metabolites that did not meet FDR significance, 12 retained statistical significance.
Cross-cohort Analyses
Of the 61 metabolites significantly associated with incident CHD in the age-, sex-, and batch-adjusted model of the JHS cohort, 43 were available for replication in the WHI cohort. Of those, 24 metabolites (55.8%) replicated with a statistically significant (P < .05) association with CHD and a consistent direction of effect (eTable 13 in the Supplement). Among the 46 metabolites that were associated with incident CHD in the JHS cohort after multivariable adjustment, 32 were available for replication. Of those, 13 metabolites (40.6%; linoleoyl ethanolamide; 4-acetamidobutanoate; homoarginine; pseudouridine; sphingomyelin 16:0; lysine; histidine; N2,N2-dimethylguanosine; phosphatidylethanolamine plasmalogen 36:3, methionine, leucine, uridine, and N4-acetylcytidine) replicated with a statistically significant association with CHD and the same direction of effect (Table 3). Four of those 13 metabolites (homoarginine, histidine, methionine, and uridine) have been associated with incident CHD.11,13,14 The 9 novel metabolites associated with incident CHD included 2 amino acids (lysine and leucine [previously associated with increased risk14,16,17 but associated with decreased risk in the JHS cohort]), 2 lipids (sphingomyelin 16:0 and phosphatidylethanolamine plasmalogen 36:3), 3 nucleosides (pseudouridine, N4-acetylcytidine, and N2,N2-dimethylguanosine), 1 endocannabinoid (linoleoyl ethanolamide), and 1 monocarboxylic acid anion (4-acetamidobutanoate).
Table 3. Cross-cohort Analyses of Metabolites Available in Both Cohorts.
| Metabolitea | JHS model 2b | WHI model 2c | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | FDR q value | HR (95% CI) | P value | |
| 1-Methylnicotinamide | 0.69 (0.59-0.81) | 3.62 × 10−6 | 0.001 | 0.91 (0.81-1.02) | .10 |
| Linoleoyl ethanolamided | 1.38 (1.19-1.60) | .00001 | 0.001 | 2.15 (1.09-4.22) | .03 |
| N-acetylaspartic acid | 1.44 (1.22-1.69) | 9.72 × 10−6 | 0.001 | 0.97 (0.85-1.11) | .64 |
| 4-Acetamidobutanoated | 1.46 (1.22-1.73) | .00002 | 0.001 | 1.28 (1.12-1.46) | .0003 |
| Homoarginined | 0.71 (0.60-0.83) | .00002 | 0.001 | 0.85 (0.76-0.96) | .01 |
| Niacinamide | 0.75 (0.65-0.85) | .00001 | 0.001 | 0.97 (0.87-1.08) | .57 |
| Trimethylbenzene | 0.73 (0.62-0.85) | .00004 | 0.002 | 1.20 (1.05-1.36) | .006 |
| PE 36:2 | 1.41 (1.18-1.66) | .00006 | 0.002 | 0.99 (0.88-1.12) | .92 |
| NMMA | 0.75 (0.64-0.86) | .00010 | 0.003 | 0.89 (0.79-1.01) | .07 |
| Pseudouridined | 1.48 (1.20-1.82) | .0002 | 0.005 | 1.20 (1.05-1.36) | .005 |
| SM 16:0d | 1.46 (1.17-1.81) | .0006 | 0.010 | 1.17 (1.00-1.37) | .047 |
| Biliverdin | 0.76 (0.65-0.89) | .0007 | 0.010 | 0.98 (0.86-1.12) | .78 |
| Lysined | 0.78 (0.67-0.90) | .0008 | 0.010 | 0.85 (0.76-0.96) | .007 |
| Myristoleate | 0.77 (0.66-0.90) | .0008 | 0.010 | 1.04 (0.92-1.17) | .54 |
| Histidined | 0.78 (0.66-0.91) | .001 | 0.020 | 0.85 (0.75-0.96) | .01 |
| N2,N2-dimethylguanosined | 1.32 (1.10-1.57) | .002 | 0.020 | 1.18 (1.00-1.37) | .04 |
| PE 34:2 | 1.30 (1.10-1.52) | .002 | 0.020 | 1.05 (0.93-1.17) | .44 |
| 1-Methyladenosine | 1.33 (1.10-1.59) | .002 | 0.030 | 0.93 (0.83-1.04) | .22 |
| PE plasmalogen 36:3d | 1.31 (1.10-1.56) | .002 | 0.030 | 1.20 (1.07-1.36) | .003 |
| Arginine | 0.80 (0.68-0.93) | .004 | 0.040 | 0.97 (0.87-1.10) | .67 |
| LPC 16:1 | 0.79 (0.67-0.93) | .005 | 0.050 | 1.03 (0.91-1.16) | .68 |
| LPC plasmalogen 16:1 | 0.80 (0.68-0.93) | .005 | 0.050 | 1.08 (0.96-1.21) | .20 |
| Malic acid | 1.26 (1.06-1.48) | .006 | 0.060 | 1.11 (0.98-1.26) | .11 |
| Methionined | 0.79 (0.66-0.94) | .008 | 0.070 | 0.88 (0.78-0.98) | .02 |
| Glycochenodeoxycholic acid | 1.23 (1.05-1.42) | .008 | 0.070 | 1.02 (0.91-1.14) | .72 |
| Leucined | 0.79 (0.65-0.94) | .009 | 0.070 | 0.86 (0.76-0.97) | .01 |
| PC 36:2 | 1.26 (1.05-1.51) | .009 | 0.080 | 1.04 (0.91-1.19) | .57 |
| Uridined | 0.82 (0.70-0.95) | .01 | 0.080 | 0.80 (0.71-0.90) | .0003 |
| N4-acetylcytidined | 1.25 (1.05-1.49) | .01 | 0.080 | 1.20 (1.06-1.36) | .005 |
| LPC 20:1 | 0.82 (0.70-0.96) | .01 | 0.080 | 1.07 (0.94-1.21) | .31 |
| Aconitic acid | 1.38 (1.07-1.79) | .01 | 0.080 | 1.10 (0.96-1.25) | .18 |
| SM 18:0 | 1.25 (1.04-1.50) | .02 | 0.100 | 1.09 (0.94-1.25) | .25 |
Abbreviations: CHD, coronary heart disease; FDR, false discovery rate; HR, hazard ratio; JHS, Jackson Heart Study; LPC, lysophosphatidylcholine; NMMA, N-methylmalonamic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin; WHI, Women's Health Initiative.
The metabolites listed were those associated with incident CHD in the fully adjusted model of the JHS cohort and available for replication in the WHI cohort.
Covariates used for adjustment in the JHS cohort included age, sex, batch, total cholesterol level, high-density lipoprotein level, presence of hypertension, systolic blood pressure, body mass index (calculated as weight in kilograms divided by height in meters squared), presence of diabetes, estimated glomerular filtration rate, smoking status, and statin medication receipt.
Covariates used for adjustment in the WHI cohort included age, total cholesterol level, high-density lipoprotein level, presence of hypertension, systolic blood pressure, body mass index, presence of diabetes, smoking status, and statin medication receipt.
Metabolites with significant P values and concordant direction of effect in the WHI cohort.
Analyses of Risk Prediction
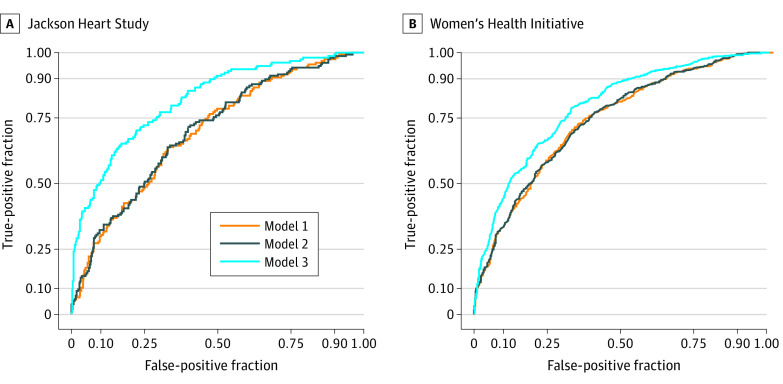
To assess the utility of the metabolite associations for the prediction of incident CHD beyond clinical risk factors, we compared the ROC curves for clinical risk factors with and without inclusion of the metabolites of interest. Using optimism correction to address overfitting, we found results suggesting significant improvement when metabolites were added. In the JHS cohort, the use of clinical risk factors to predict incident CHD produced an ROC curve with an optimism-corrected C statistic of 0.655. The addition of NT-proBNP and CRP increased the C statistic to 0.658 (change in optimism-corrected C statistic, 0.003). Further inclusion of the 46 incident CHD–associated metabolites increased the C statistic to 0.721 (change in optimism-corrected C statistic, 0.063) (Figure).
Figure. Receiver Operating Characteristic Curves for the Jackson Heart Study and the Women’s Health Initiative Cohorts.
A, Model 1 measured the predictive risk value of traditional clinical risk factors, including age, sex, total cholesterol level, high-density lipoprotein cholesterol level, presence of hypertension, systolic blood pressure, body mass index (calculated as weight in kilograms divided by height in meters squared), presence of diabetes, estimated glomerular filtration rate, smoking status, and statin medication receipt (uncorrected C statistic, 0.680; 95% CI, 0.653-0.732). Model 2 included all risk factors in model 1 plus the added value of C-reactive protein and N-terminal fragment of the prohormone brain natriuretic peptide (uncorrected C statistic, 0.685; 95% CI, 0.671-0.763; change in C statistic from model 1, 0.005). Model 3 included all risk factors in model 2 plus the added value of 46 metabolites associated with incident coronary heart disease (CHD) in the Jackson Heart Study cohort (uncorrected C statistic, 0.791; 95% CI, 0.772-0.841; change in C statistic from model 2, 0.106). Given overfitting in the derivation cohort, optimism correction was performed for C statistics in model 1 (overoptimism, 0.025; optimism-corrected C statistic, 0.655), model 2 (overoptimism, 0.027; optimism-corrected C statistic, 0.658; change in optimism-corrected C statistic from model 1, 0.003), and model 3 (overoptimism, 0.070; optimism-corrected C statistic, 0.721; change in optimism-corrected C statistic from model 2, 0.063). B, Model 1 measured the predictive risk value of the same traditional clinical risk factors used in model 1 of the Jackson Heart Study cohort (C statistic, 0.689; 95% CI, 0.642-0.735). Model 2 included all risk factors in model 1 plus the added value of C-reactive protein (C statistic, 0.684; 95% CI, 0.637-0.730; change in C statistic from model 1, 0.005). Model 3 included all risk factors in model 2 plus the added value of 32 metabolites associated with incident CHD in the JHS cohort and available for replication in the Women’s Health Initiative cohort (C statistic, 0.724; 95% CI, 0.679-0.768; change in C statistic from model 2, 0.040).
We next used the 32 metabolites available for replication in the WHI cohort to construct an ROC curve in an independent cohort. The use of clinical risk factors to predict incident CHD yielded a C statistic of 0.689 (95% CI, 0.642-0.735). The addition of CRP yielded a C statistic of 0.684 (95% CI, 0.637-0.730; change in C statistic, 0.005). Inclusion of the 32 incident CHD–associated metabolites available for replication increased the C statistic to 0.724 (95% CI, 0.679-0.768; change in C statistic, 0.040) (Figure).
We created a metabolite risk score in the JHS cohort and tested it in the WHI cohort. Using the 46 metabolites found to be significantly associated with CHD in the JHS cohort, the metabolite risk score had an HR of 4.50 (95% CI, 3.30-6.20; P = 6.5 × 10−21) (eTable 14 in the Supplement). We used the 32 metabolites available for replication in the WHI cohort to test our metabolite risk score. Across the WHI cohort, the metabolite risk score had an HR of 1.67 (95% CI, 1.23-2.28; P = .001).
We also constructed an ROC curve for the metabolite risk score applied to the WHI cohort. The C statistic for the metabolite risk score as an added predictive variable to clinical risk factors was 0.706 (95% CI, 0.660-0.752) compared with a C statistic of 0.684 (95% CI, 0.637-0.731; change in C statistic, 0.022) for clinical risk factors alone (eFigure 4 in the Supplement).
Analyses of Self-identified Race
To assess potential differences based on self-identified race, we compared β coefficients between African American and non-Hispanic White women in the WHI cohort. Given the exploratory nature of this analysis, we used a significance level of P < .10. A total of 6 metabolites met the significance threshold in this comparison (eTable 15 in the Supplement). Of the 6 metabolites, 3 metabolites (leucine [β = −0.50 for African American women vs −0.10 for non-Hispanic White women; P for change in β = 0.08], methionine [β = −0.58 for African American women vs −0.08 for non-Hispanic White women; P for change in β = 0.03], and sphingomyelin 16:0 [β = 0.76 for African American women vs 0.13 for non-Hispanic White women; P for change in β = 0.08]) were associated with incident CHD in the JHS cohort and replicated in the WHI cohort.
We also compared β coefficients between the African American participants in the JHS cohort and non-Hispanic White participants in the WHI cohort. Of the 32 metabolites that replicated in the WHI cohort, 13 had a significant difference based on race (these metabolites are denoted by an asterisk in eTable 16 in the Supplement). Notably, trimethylbenzene had a significant association with an increased risk of incident CHD among non-Hispanic White individuals in the WHI cohort (β = 0.19; P = .01) but a decreased risk of incident CHD among African American individuals in the JHS cohort (β = −0.32; P = 4.0 × 10−5).
Discussion
Given the high risk of CHD among African American individuals despite relatively low levels of CAC and the relative paucity of metabolomic data for African American individuals, this cohort study compared the metabolite profiles associated with incident CHD and CAC among African American participants in the JHS cohort. Forty-six metabolites were associated with incident CHD up to 16 years before the incident event. The disease-associated metabolites were minimally correlated, suggesting they may have been associated with nonoverlapping biological processes. Many of our metabolite findings were novel. More than one-half of our age- and sex-adjusted findings from the JHS cohort were able to be replicated in the WHI cohort, which comprised both African American and non-Hispanic White women. Approximately 40% of the fully adjusted findings could be replicated, including findings for 9 metabolites that were novel in their associations with incident CHD among all participants. In addition, we used CAC scores from the JHS cohort to compare metabolic processes between CHD events and CAC, a well-described intermediate phenotype, albeit one of less clinical utility among African American individuals.
Our metabolite findings added to the predictive value of traditional risk factors and established markers of disease, as observed in the improvement in the C statistic even after optimism correction. We also constructed a metabolite risk score in the JHS cohort that we applied to the WHI cohort. Although findings in the WHI cohort were attenuated, they continued to have a significant association with incident CHD. Although our analyses can be considered preliminary, the results may motivate the performance of analyses of large heterogeneous cohorts to further assess disease prediction. It is also notable that we found several metabolites that may be specifically associated with incident CHD among African American individuals.
Minimally adjusted models may highlight metabolites that are biological mediators of CHD, the primary focus of our analyses. For example, imidazole propionate was associated with incident CHD in our age- and sex-adjusted model, but the association was lost in our fully adjusted model. Imidazole propionate levels are higher in individuals with diabetes,27 and this association with diabetes may represent a process through which imidazole propionate is associated with incident CHD that merits additional investigation.
N-Acylamides
We identified a novel association between linoloeyl ethanolamide, an N-acylamide, and incident CHD in the JHS cohort and replicated this finding in the WHI cohort. Linoleoyl ethanolamide is an endocannabinoid and a lipid-signaling molecule that has anti-inflammatory effects on macrophages.30,31 In mice exposed to lipopolysaccharide, linoleoyl ethanolamide suppressed the expression of messenger RNA for tumor necrosis factor α, interleukin 1β, and interleukin 6 in activated macrophages.32 These effects on macrophages may be of particular interest given their central role in atherosclerosis and CHD.33
Branched-Chain Amino Acid Levels
In contrast to previous findings among populations of non-Hispanic White or Asian individuals,16,21,34,35 none of the branched-chain amino acids (leucine, isoleucine, or valine) were directly associated with incident CHD. Leucine was inversely associated with incident CHD (HR, 0.79; FDR q = 0.070). One previous study reported a potential association between leucine and protection from incident CHD.22 Within the WHI cohort, we found a difference in the association between leucine and the risk of incident CHD based on race, with a β of −0.50 among African American women vs −0.10 among non-Hispanic White women (P for change in β = 0.08) (eTable 15 in the Supplement). The observation that leucine may be associated with a reduced risk of CHD among postmenopausal non-Hispanic White women is noteworthy given previous findings.14,16,17 These observations highlight the need for further investigation of the association between branched-chain amino acids and cardiometabolic disease among African American individuals and women.
Differences Among Self-identified Racial Groups
This study found various metabolites that appeared to differ in their associations with incident CHD based on self-identified race. Many potential mechanisms exist to explain the differences between African American and non-Hispanic White individuals with regard to the associations between circulating metabolites and incident CHD, highlighting the importance of investigating the circulating metabolome as a possible intermediary of these mechanisms. African American individuals are subject to a distinct set of socioeconomic factors that may be associated with diseases as wide ranging as cancer, anxiety, and cardiovascular disease.1,36 Limitations of the tools available to capture these important factors have been noted. Studies have found that the specific choice of socioeconomic factors used to represent differences between social groups may be associated with the extent of the inequalities being examined.37,38 Given these factors, there remains no standardized approach to assess these factors across groups of individuals. Even less is known about how these factors may change as individuals age. Although socioeconomic factors are likely to have substantial consequences for the differences in baseline metabolic risk factors, genetic factors may also be relevant. Our investigation was not intended to be a definitive description of all factors associated with disparities between African American and non-Hispanic White individuals with regard to CHD but rather an effort to understand how the circulating metabolome is associated with incident CHD and CAC. Although beyond the scope of the current study, we are pursuing analyses investigating the association of genetic and socioeconomic factors with the levels of circulating metabolites in African American individuals.
Overlap Between CHD and CAC
Across our analyses of incident CHD and CAC, we found 52 distinct associations between metabolites and traits. However, only 3 metabolites were associated with both CAC scores and incident CHD. Differences in the metabolites associated with incident CHD vs CAC may offer clues regarding distinctive features of the interaction between this intermediate phenotype and CHD events. Because African American individuals are likely to have lower levels of CAC but higher rates of incident CHD,7,8,9 it is important to understand the extent to which CAC predicts risk among African American individuals.
Strengths and Limitations
This study has several strengths. First, the study includes a validated metabolomics platform, detailed covariate and phenotypic information, replication in a multiethnic cohort, and a large number of carefully adjudicated end points. Second, the study provides a detailed investigation of the metabolomics of CHD and CAC in an exclusively African American cohort, an understudied population with disproportionate rates of CHD. Our findings suggest that shared metabolite associations exist among African American and non-Hispanic White individuals, some of which are suggestive of differences between those populations. Third, we assayed many metabolites not previously studied in humans and focused on an African American cohort.
The study also has limitations. First, we lacked an exclusively African American population-based study in which to replicate our findings, reflecting the paucity of available data for African American individuals. Second, we were limited to analyzing metabolite levels 4 years before the measurement of CAC. Third, approximately 30% of our findings were not available for replication in the WHI cohort. These findings provide motivation to seek further validation among cohorts consisting primarily of African American individuals. To address the limitation of overfitting in our derivation ROC curve, we corrected for optimism. The resulting C statistics (and changes in C statistics) were similar to the results from the predictive models in the WHI cohort.
Conclusions
This cohort study found 46 metabolites that were associated with incident CHD in an African American cohort. Of 32 metabolites available for replication, 13 metabolites were replicated in a multiethnic population, 9 of which were novel in their association with incident CHD and included N-acylamides, leucine, and lipid species. Given minimal metabolite overlap, these findings suggested that distinct mechanisms may underlie CAC and CHD events. Furthermore, the analysis suggested that metabolites may be better markers of incident disease than traditional risk factors. Race-specific analyses identified several metabolites that may differ in their associations with incident CHD based on self-identified race. Further work is warranted to assess whether these findings are specific to African American individuals and to identify potential mechanistic differences in the development of CHD among African American individuals compared with individuals from other racial groups.
eMethods 1. Study Populations
eMethods 2. Assessment of Incident Coronary Heart Disease and Covariates
eMethods 3. Assessment of Coronary Artery Calcium Score
eMethods 4. Plasma Sample Selection and Metabolomic Profiling
eMethods 5. Statistics
eMethods 6. Prediction Models
eTable 1. Clinical Characteristics of the Entire Jackson Heart Study and the Population of Profiled Samples
eTable 2. Participants Without Prevalent Coronary Heart Disease in the Jackson Heart Study Cohort
eTable 3. Participants With Computed Tomographic Scans at Visit 2 in the Jackson Heart Study Cohort
eTable 4. Baseline Participant Characteristics by Self-Identified Race in the Women’s Health Initiative Cohort
eTable 5. Comparison of Metabolites Across Published Studies
eTable 6. Metabolites Associated With Incident Coronary Heart Disease in the Jackson Heart Study Cohort: Adjusted for Age, Sex, and Batch
eTable 7. Metabolites Associated With Incident Coronary Heart Disease: Fully-Adjusted Model With Sensitivity Analysis for Myocardial Infarction
eTable 8. Metabolites Associated With Incident Coronary Heart Disease: Fully-Adjusted Model With Additional Adjustment for Triglyceride Levels and Waist Circumference
eTable 9. Metabolites Associated With Coronary Artery Calcium in the Jackson Heart Study Cohort: Adjusted for Age, Sex, and Batch
eTable10. Metabolites Associated With Coronary Artery Calcium in a Fully-Adjusted Model of the Jackson Heart Study Cohort With Additional Adjustment for Statin Medication Receipt
eTable 11. Adjustment for Socioeconomic Status Factors
eTable 12. Participant Characteristics of the Women’s Health Initiative Cohort
eTable 13. Replication of Metabolite Findings in the Age-, Sex-, and Batch-Adjusted Analysis
eTable 14. Metabolite Risk Score
eTable 15. β Comparisons Between Self-Identified Race in the Women’s Health Initiative Cohort
eTable 16. β Comparisons Between Self-Identified Race Among African American Individuals in the Jackson Heart Study Cohort vs Non-Hispanic White Individuals in the Women’s Health Initiative Cohort
eFigure 1. Exclusions From the Jackson Heart Study Analysis
eFigure 2. Metabolite Classes Associated With Incident Coronary Heart Disease
eFigure 3. Metabolite Correlations
eFigure 4. Receiver Operating Characteristic Curves for Metabolite Risk Score in the Women’s Health Initiative Cohort
eReferences
Footnotes
Abbreviations: CAC, coronary artery calcification; DMGV, dimethylguanidino valerate; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; NMMA, N-methylmalonamic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; and SM, sphingomyelin.
References
- 1.Frieden TR; Centers for Disease Control and Prevention (CDC) . CDC Health Disparities and Inequalities Report—United States, 2013: foreword. MMWR Suppl. 2013;62(3):1-2. doi: 10.15585/mmwr.su6503a1 [DOI] [PubMed] [Google Scholar]
- 2.Murray CJL, Kulkarni SC, Michaud C, et al. Eight Americas: investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med. 2006;3(9):e260. doi: 10.1371/journal.pmed.0030260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurley LP, Dickinson LM, Estacio RO, Steiner JF, Havranek EP. Prediction of cardiovascular death in racial/ethnic minorities using Framingham risk factors. Circ Cardiovasc Qual Outcomes. 2010;3(2):181-187. doi: 10.1161/CIRCOUTCOMES.108.831073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosamond WD, Chambless LE, Heiss G, et al. Twenty-two–year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987-2008. Circulation. 2012;125(15):1848-1857. doi: 10.1161/CIRCULATIONAHA.111.047480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safford MM, Brown TM, Muntner PM, et al. ; REGARDS Investigators . Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768-1774. doi: 10.1001/jama.2012.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376-1414. doi: 10.1016/j.jacc.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman AB, Naydeck BL, Whittle J, Sutton-Tyrrell K, Edmundowicz D, Kuller LH. Racial differences in coronary artery calcification in older adults. Arterioscler Thromb Vasc Biol. 2002;22(3):424-430. doi: 10.1161/hq0302.105357 [DOI] [PubMed] [Google Scholar]
- 8.Lee TC, O’Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41(1):39-44. doi: 10.1016/S0735-1097(02)02618-9 [DOI] [PubMed] [Google Scholar]
- 9.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111(10):1313-1320. doi: 10.1161/01.CIR.0000157730.94423.4B [DOI] [PubMed] [Google Scholar]
- 10.Sung JH, Yeboah J, Lee JE, et al. Diagnostic value of coronary artery calcium score for cardiovascular disease in African Americans: the Jackson Heart Study. Br J Med Med Res. 2016;11(2):1-9. doi: 10.9734/BJMMR/2016/21449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Zhu C, Nambi V, et al. Metabolomic pattern predicts incident coronary heart disease. Arterioscler Thromb Vasc Biol. 2019;39(7):1475-1482. doi: 10.1161/ATVBAHA.118.312236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stegemann C, Pechlaner R, Willeit P, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129(18):1821-1831. doi: 10.1161/CIRCULATIONAHA.113.002500 [DOI] [PubMed] [Google Scholar]
- 13.Cavus E, Karakas M, Ojeda FM, et al. ; BiomarCare Consortium . Association of circulating metabolites with risk of coronary heart disease in a European population: results from the Biomarkers for Cardiovascular Risk Assessment in Europe (BiomarCare) Consortium. JAMA Cardiol. 2019;4(12):1270-1279. doi: 10.1001/jamacardio.2019.4130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy VL, Reis JP, Pico AR, et al. Comprehensive metabolic phenotyping refines cardiovascular risk in young adults. Circulation. 2020;142(22):2110-2127. doi: 10.1161/CIRCULATIONAHA.120.047689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magnusson M, Lewis GD, Ericson U, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34(26):1982-1989. doi: 10.1093/eurheartj/ehs424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wurtz P, Havulinna AS, Soininen P, et al. Metabolite profiling and cardiovascular event risk: a prospective study of 3 population-based cohorts. Circulation. 2015;131(9):774-785. doi: 10.1161/CIRCULATIONAHA.114.013116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah SH, Sun JL, Stevens RD, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. 2012;163(5):844-850. doi: 10.1016/j.ahj.2012.02.005 [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Semiz S, van der Lee SJ, et al. Metabolomics based markers predict type 2 diabetes in a 14-year follow-up study. Metabolomics. 2017;13(9):104. doi: 10.1007/s11306-017-1239-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez-Gallego E, Guirro M, Riera-Borrull M, et al. Mapping of the circulating metabolome reveals α-ketoglutarate as a predictor of morbid obesity–associated non-alcoholic fatty liver disease. Int J Obes (Lond). 2015;39(2):279-287. doi: 10.1038/ijo.2014.53 [DOI] [PubMed] [Google Scholar]
- 20.Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222-2231. doi: 10.1161/CIRCULATIONAHA.111.067827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimberly WT, Wang Y, Pham L, Furie KL, Gerszten RE. Metabolite profiling identifies a branched chain amino acid signature in acute cardioembolic stroke. Stroke. 2013;44(5):1389-1395. doi: 10.1161/STROKEAHA.111.000397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paynter NP, Balasubramanian R, Giulianini F, et al. Metabolic predictors of incident coronary heart disease in women. Circulation. 2018;137(8):841-853. doi: 10.1161/CIRCULATIONAHA.117.029468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson Heart Study Investigators. Jackson Heart Study (JHS) Manual 8: CT Scan Site Manual of Operations: Visit 2. Version 1.0. Jackson Heart Study Coordinating Center. January 12, 2007. Accessed December 2020. https://www.jacksonheartstudy.org/Portals/0/pdf/manuals2/Manual%208_CT_01-30-2007%20(1).pdf
- 24.Tahir UA, Katz DH, Zhao T, et al. Metabolomic profiles and heart failure risk in Black adults: insights from the Jackson Heart Study. Circ Heart Fail. 2021;14(1):e007275. doi: 10.1161/CIRCHEARTFAILURE.120.007275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngo D, Benson MD, Long JZ, et al. Proteomic profiling reveals biomarkers and pathways in type 2 diabetes risk. JCI Insight. 2021;6(5):e144392. doi: 10.1172/jci.insight.144392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rebholz CM, Gao Y, Talegawkar S, et al. Metabolomic markers of southern dietary patterns in the Jackson Heart Study. Mol Nutr Food Res. 2021;65(8):e2000796. doi: 10.1002/mnfr.202000796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molinaro A, Bel Lassen P, Henricsson M, et al. ; MetaCardis Consortium . Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat Commun. 2020;11(1):5881. doi: 10.1038/s41467-020-19589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saremi A, Bahn G, Reaven PD; VADT Investigators. Progression of vascular calcification is increased with statin use in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care. 2012;35(11):2390-2392. doi: 10.2337/dc12-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henein M, Granasen G, Wiklund U, et al. High dose and long-term statin therapy accelerate coronary artery calcification. Int J Cardiol. 2015;184:581-586. doi: 10.1016/j.ijcard.2015.02.072 [DOI] [PubMed] [Google Scholar]
- 30.Ishida T, Nishiumi S, Tanahashi T, et al. Linoleoyl ethanolamide reduces lipopolysaccharide-induced inflammation in macrophages and ameliorates 2,4-dinitrofluorobenzene–induced contact dermatitis in mice. Eur J Pharmacol. 2013;699(1-3):6-13. doi: 10.1016/j.ejphar.2012.11.030 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe K, Matsunaga T, Nakamura S, et al. Pharmacological effects in mice of anandamide and its related fatty acid ethanolamides, and enhancement of cataleptogenic effect of anandamide by phenylmethylsulfonyl fluoride. Biol Pharm Bull. 1999;22(4):366-370. doi: 10.1248/bpb.22.366 [DOI] [PubMed] [Google Scholar]
- 32.Hansen HS, Diep TA. N-acylethanolamines, anandamide and food intake. Biochem Pharmacol. 2009;78(6):553-560. doi: 10.1016/j.bcp.2009.04.024 [DOI] [PubMed] [Google Scholar]
- 33.Cao DJ. Macrophages in cardiovascular homeostasis and disease. Circulation. 2018;138(22):2452-2455. doi: 10.1161/CIRCULATIONAHA.118.035736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tobias DK, Lawler PR, Harada PH, et al. Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women. Circ Genom Precis Med. 2018;11(4):e002157. doi: 10.1161/CIRCGEN.118.002157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhattacharya S, Granger CB, Craig D, et al. Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis. 2014;232(1):191-196. doi: 10.1016/j.atherosclerosis.2013.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perneger TV, Whelton PK, Klag MJ. Race and end-stage renal disease. socioeconomic status and access to health care as mediating factors. Arch Intern Med. 1995;155(11):1201-1208. doi: 10.1001/archinte.1995.00430110121013 [DOI] [PubMed] [Google Scholar]
- 37.Darin-Mattsson A, Fors S, Kareholt I. Different indicators of socioeconomic status and their relative importance as determinants of health in old age. Int J Equity Health. 2017;16(1):173. doi: 10.1186/s12939-017-0670-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gornick ME. Measuring the effects of socioeconomic status on health care. In: Swift EK, ed; Institute of Medicine (US) Committee on Guidance for Designing a National Healthcare Disparities Report. Guidance for the National Healthcare Disparities Report. National Academies Press; 2002:chap 2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Study Populations
eMethods 2. Assessment of Incident Coronary Heart Disease and Covariates
eMethods 3. Assessment of Coronary Artery Calcium Score
eMethods 4. Plasma Sample Selection and Metabolomic Profiling
eMethods 5. Statistics
eMethods 6. Prediction Models
eTable 1. Clinical Characteristics of the Entire Jackson Heart Study and the Population of Profiled Samples
eTable 2. Participants Without Prevalent Coronary Heart Disease in the Jackson Heart Study Cohort
eTable 3. Participants With Computed Tomographic Scans at Visit 2 in the Jackson Heart Study Cohort
eTable 4. Baseline Participant Characteristics by Self-Identified Race in the Women’s Health Initiative Cohort
eTable 5. Comparison of Metabolites Across Published Studies
eTable 6. Metabolites Associated With Incident Coronary Heart Disease in the Jackson Heart Study Cohort: Adjusted for Age, Sex, and Batch
eTable 7. Metabolites Associated With Incident Coronary Heart Disease: Fully-Adjusted Model With Sensitivity Analysis for Myocardial Infarction
eTable 8. Metabolites Associated With Incident Coronary Heart Disease: Fully-Adjusted Model With Additional Adjustment for Triglyceride Levels and Waist Circumference
eTable 9. Metabolites Associated With Coronary Artery Calcium in the Jackson Heart Study Cohort: Adjusted for Age, Sex, and Batch
eTable10. Metabolites Associated With Coronary Artery Calcium in a Fully-Adjusted Model of the Jackson Heart Study Cohort With Additional Adjustment for Statin Medication Receipt
eTable 11. Adjustment for Socioeconomic Status Factors
eTable 12. Participant Characteristics of the Women’s Health Initiative Cohort
eTable 13. Replication of Metabolite Findings in the Age-, Sex-, and Batch-Adjusted Analysis
eTable 14. Metabolite Risk Score
eTable 15. β Comparisons Between Self-Identified Race in the Women’s Health Initiative Cohort
eTable 16. β Comparisons Between Self-Identified Race Among African American Individuals in the Jackson Heart Study Cohort vs Non-Hispanic White Individuals in the Women’s Health Initiative Cohort
eFigure 1. Exclusions From the Jackson Heart Study Analysis
eFigure 2. Metabolite Classes Associated With Incident Coronary Heart Disease
eFigure 3. Metabolite Correlations
eFigure 4. Receiver Operating Characteristic Curves for Metabolite Risk Score in the Women’s Health Initiative Cohort
eReferences