Abstract
A 74-year-old man was diagnosed with hepatocellular carcinoma. The tumor in the liver showed a complete response after transcatheter arterial chemoembolization, but lung, bone, and lymph node metastases were observed, so treatment with atezolizumab plus bevacizumab was initiated. After administration, the scans showed tumor growth, but after continuous administration of atezolizumab plus bevacizumab, the tumors finally reduced in size and showed a partial response. The transient growth of the tumors was considered to be pseudoprogression. Herein, we report a case of pseudoprogression in hepatocellular carcinoma treated with atezolizumab plus bevacizumab.
Keywords: Hepatocellular carcinoma, immune checkpoint inhibitor, atezolizumab, bevacizumab, pseudoprogression
Introduction
Immune checkpoint inhibitors (ICIs) have been increasingly used in several types of cancer treatment and have also been confirmed to be effective against hepatocellular carcinoma (HCC). The IMbrave150 trial showed that atezolizumab plus bevacizumab achieved notably favorable progression-free and overall survival compared with sorafenib in advanced metastatic or unresectable HCC. 1 According to the results of this clinical study, the first-line systemic therapy for advanced HCC has shifted from tyrosine kinase inhibitors such as sorafenib and lenvatinib to atezolizumab plus bevacizumab.2-4
Unlike conventional anticancer drugs, ICIs can also cause atypical response patterns. After ICI treatment, pseudoprogression may be observed, which is a phenomenon in which the tumor shrinks after transient tumor growth or the appearance of a new lesion.5,6 We report a case of pseudoprogression after atezolizumab plus bevacizumab treatment for HCC.
Case Report
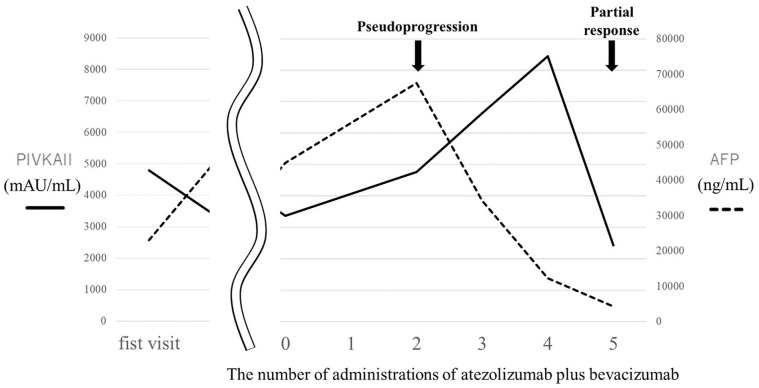
A 74-year-old man was referred to our hospital after abdominal ultrasonography revealed a liver S8 tumor. The patient’s medical history included alcoholic liver disease, type-2 diabetes mellitus, and hypertension. The Child–Pugh grade was class A, and laboratory evaluation showed elevated tumor markers: α-fetoprotein, 22,882 ng/mL; and protein induced by vitamin K absence/antagonist II, 4793.3 mAU/mL. A dynamic computed tomography (CT) scan of the abdomen revealed a round-shaped and hypodense S8 tumor measuring 54 mm in diameter, which was enhanced in the arterial phase and washed out in the portal venous and delayed phases (Figure 1). The patient was diagnosed with HCC arising from a history of alcoholic liver disease. Transcatheter arterial chemoembolization (TACE) was performed because the patient requested nonsurgical treatment. Right iliac metastasis was confirmed after the first TACE, and an additional TACE was performed for the primary tumor of the liver. Radiation therapy was used to treat the left iliac metastasis. The tumor in the liver showed a complete response on the CT images after performing TACE twice; however, the metastatic lesions of the left iliac increased, and bilateral lung and pelvic lymph node metastases were newly observed (Figure 2A). Therefore, the administration of atezolizumab plus bevacizumab was initiated 5 months after the initial treatment. After the administration of the second course of atezolizumab plus bevacizumab, the CT scan showed growth in the lung tumor, left iliac tumor, and pelvic lymph node tumor (Figure 2B). New lesions were also found in the lungs which indicated progressive disease (PD). Elevated levels of tumor markers were also observed (Figure 3). Although possible changes in the treatment method were considered, the treatment was continued at the discretion of the doctor. After administration of the third course of atezolizumab plus bevacizumab, the CT scan showed a decrease in both lung and iliac tumors (Figure 2C), and after administration of the fourth course of atezolizumab plus bevacizumab, the CT scan showed disappearance of the lung tumor (Figure 2D). After administration of the fifth course of atezolizumab plus bevacizumab, the CT scan showed no recurrence of the lung tumor and further reduction of iliac and pelvic lymph node metastases (Figure 2E), indicating a partial response. Along with the reduction of the tumor, a decrease in tumor marker levels was also observed (Figure 3). The patient is continuously receiving atezolizumab plus bevacizumab therapy without any major adverse events.
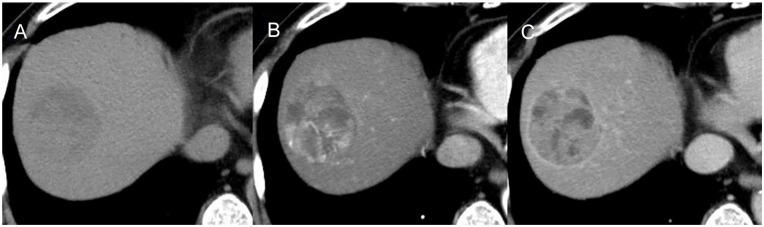
Figure 1.
CT images of the tumor.
Plain computed tomography (A) demonstrated a low-density tumor (54 mm in diameter) in the liver S8. The tumor was (B) enhanced in the arterial phase and (C) washed out in the equilibrium phase.
Abbreviation: CT, computed tomography.
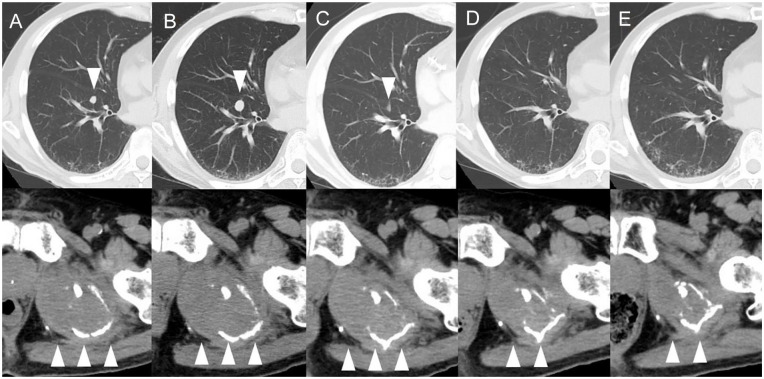
Figure 2.
CT scans over the course of treatment with atezolizumab plus bevacizumab.
The upper panels show the right lung and the lower panels show the left ilium. (A) CT images before the initiation of treatment showing metastatic tumors (arrows). (B) CT image after the second course showing an increase in the size of the tumors (arrows). (C) CT image after the third course showing a decrease in size of the tumor (arrows). (D) CT images after the fourth course showing disappearance of the right lung tumor and a decrease in the left iliac tumor (arrows). (E) No recurrence of metastatic lung tumors, and further reduction in the left iliac tumor after the fifth course (arrows).
Abbreviation: CT, computed tomography.
Figure 3.
Clinical course of tumor markers.
Discussion
For the last decade, sorafenib has been considered the standard of care for first-line systemic treatment of HCC.7,8 Although lenvatinib was approved as an alternative to sorafenib as first-line therapy based on the REFLECT trial results, 9 there were no systemic treatments that were superior to sorafenib for more than 10 years. The combination therapy of atezolizumab and bevacizumab significantly prolonged progression-free and overall survival in unresectable HCC patients compared to sorafenib in the IMbrave 150 Phase-3 clinical trial. 1 Based on this result, atezolizumab plus bevacizumab is now the first-line systemic treatment for liver cancer, and its use is expected to increase in the future.
Atezolizumab, an ICI, exerts antitumor effects by selectively targeting PD-L1, preventing interactions with receptors PD-1 and B7-1, and reversing T-cell repression. 10 Immune checkpoint inhibitors are anticancer agents that exert antitumor effects through the immune function of the host and have unique advantages in terms of efficacy and side effects. Conventional anticancer drugs are judged to be ineffective when the tumor shows a certain growth, but ICIs may exhibit a phenomenon called “pseudoprogression” in which the tumor grows before it shrinks. This phenomenon has been confirmed in various cancers, with an incidence rate of 10–15% in malignant melanomas, 11 5–7% in lung cancers,12,13 and 2–3% in head and neck cancers. 14 A previous systematic review and meta-analysis reported that the overall incidence of pseudoprogression was 6.0% in clinical trial reports of patients with cancer undergoing ICI treatment. 15
In our case, administration of atezolizumab for HCC showed transient tumor growth, which was thought to be pseudoprogression. To our knowledge, there are no reports of pseudoprogression after the use of atezolizumab plus bevacizumab for the treatment of HCC. The clinical features of pseudoprogression have been reported to be: no decline in the general condition and performance status despite the growth of the tumor as seen on the image, no deterioration in test values such as tumor markers, and the sustained infiltration of immune cells observed in the tissue biopsy. 16 However, there is no consensus on this information as yet. In our case, pseudoprogression was observed relatively early (6 weeks after the initiation of treatment) and tumor markers increased when pseudoprogression was confirmed. Further accumulation of cases is needed to determine the characteristics of pseudoprogression after the use of atezolizumab plus bevacizumab for the treatment of HCC.
In clinical practice, it is difficult to distinguish between true progression and pseudoprogression after only one diagnostic imaging, and if a false judgment is made, the administration of effective ICI treatment may be discontinued. As a result, the patient’s prognosis may worsen. As ICIs are well tolerated and not highly toxic, they have been previously recommended for continuous immunotherapy in locally progressive, asymptomatic, or slow-growing cases. 17 Immune-related response criteria (IrRC), immune-related response evaluation criteria in solid tumors (irRECIST), and immune RECIST (iRECIST) have been proposed for the judgment of therapeutic effect and can be used for ICIs.5,18,19 Each evaluation method requires multiple image evaluations at various intervals. In this case, the initial evaluation was PD according to RECIST; however, the new evaluation criteria did not result in PD, and the treatment evaluation based on the new evaluation criteria was appropriate. To avoid missing treatment opportunities in patients who may greatly benefit from ICI, it is necessary to carefully evaluate the therapeutic effect by making use of the new evaluation criteria.
Conclusion
In conclusion, we encountered a case of pseudoprogression in HCC treated with atezolizumab plus bevacizumab. It is expected that the use of ICIs for HCC will continue to increase in the future, and it will be necessary to improve the understanding and diagnosis of pseudoprogression.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patient for their anonymized information to be published in this article.
References
- 1. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med. 2020;382(20):1894-1905. [DOI] [PubMed] [Google Scholar]
- 2. Merle P. The new immuno-oncology-based therapies and their perspectives in hepatocellular carcinoma. Cancers. 2021;13238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu X, Lu Y, Qin S. Atezolizumab and bevacizumab for hepatocellular carcinoma: mechanism, pharmacokinetics and future treatment strategies. Future Oncol. 2021;17(17):2243-2256. [DOI] [PubMed] [Google Scholar]
- 4. Iwamoto H, Shimose S, Noda Y, et al. Initial experience of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in real-world clinical practice. Cancers (Basel). 2021;13(11):2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412-7420. [DOI] [PubMed] [Google Scholar]
- 6. Nishino M. Immune-related response evaluations during immune-checkpoint inhibitor therapy: establishing a “common language” for the new arena of cancer treatment. J Immunother Cancer. 2016;4:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34. [DOI] [PubMed] [Google Scholar]
- 8. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [DOI] [PubMed] [Google Scholar]
- 9. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [DOI] [PubMed] [Google Scholar]
- 10. Herbst RS, Soria J-C, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hodi FS, Hwu WJ, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. 2016;34:1510-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park HJ, Kim KW, Pyo J, et al. Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology. 2020;297(1):87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanizaki J, Hayashi H, Kimura M, et al. Report of two cases of pseudo-progression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer. 2016;102:44-48. [DOI] [PubMed] [Google Scholar]
- 17. Oxnard GR, Morris MJ, Hodi FS, et al. When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Inst. 2012;104:1534-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19:393643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143-e152. [DOI] [PMC free article] [PubMed] [Google Scholar]