Abstract
Background
Immune checkpoint inhibitor (ICI)-related myocarditis is an uncommon but potentially fatal immune-related adverse event. Corticoid-resistant myocarditis induced by ICI is an important therapeutic challenge.
Case summary
Here, we present a case of steroid-refractory ICI-related myocarditis and myositis treated with abatacept and mycophenolate mofetil (MMF). A 57-year-old male with metastatic renal cell carcinoma was diagnosed with immune-related myocarditis and myasthenia gravis-like myositis after first dose of combination ICIs with nivolumab (anti-programmed cell death-1) plus ipilimumab (anti-cytotoxic T-lymphocyte-associated antigen-4). Twelve days after ICI he was admitted to the hospital due to palpitations, headache, and pain in the extremities. Laboratory findings revealed elevated inflammatory markers and cardiac enzymes. Electrocardiogram showed first-degree atrioventricular (AV) block and right bundle branch block which developed into complete heart block within 48 h. Because of clinical and paraclinical deterioration despite immediate initiation of methylprednisolone abatacept and MMF was added. Following, gradual subjective improvement and termination of arrhythmia led to discharge of the patient from the hospital 6 weeks after the introduction of ICI.
Discussion
The key treatment of ICI-related myocarditis is glucocorticoid. For steroid-refractory myocarditis supplementary immune suppressive agents are recommended. Yet, data still relies on case reports and case series, due to lack of prospective studies. In this case, the use of abatacept and MMF led to resolution of steroid-resistant ICI-related myocarditis and myositis.
Keywords: Renal cell carcinoma, Immune checkpoint inhibitors, Immune-related adverse events, Management of immune-related myocarditis, Troponin I, Case report
Learning points
Immune checkpoint inhibitor (ICI)-related myocarditis is an infrequent adverse event with high mortality rate (50%), often occurring simultaneously with myositis.
Essential treatment of ICI-related myocarditis is glucocorticoid. Steroid-refractory cases are clinically challenging with high risk of fatal outcome. Treatment with abatacept and mycophenolate mofetil is an opportunity. In the future, clinical trials are needed to determine the optimal treatment of steroid-refractory ICI-related myocarditis.
Introduction
Immune checkpoint inhibitors (ICI) are approved as treatment of an increasing number of tumours. Immune-related adverse events (irAE) are generally manageable, however, serious, and potentially life-threatening irAEs can occur. Myocarditis and myositis are rare but severe immune-related side effects, appearing more frequently and with higher mortality rates with combined ICI than with monotherapy.1
Mycophenolate mofetil (MMF) is a prodrug of mycophenolic acid, which causes immune suppression by impeding synthesis of DNA and RNA especially in T and B cells. In several cases, MMF is presented to resolve irAEs such as colitis and hepatitis,2,3 while the effect on ICI-related myocarditis is sparsely described.4 Abatacept, a cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) agonist approved for use in rheumatic diseases, binds to CD80/CD86 on antigen-presenting cells, thereby inhibiting activation of cytotoxic T cells by blocking co-stimulatory ligand binding to CD28. Abatacept in combination with plasmapheresis has been reported to resolve steroid-refractory ICI-related myocarditis.5
Here, we present a case in which the use of abatacept concomitant with MMF presumably led to resolution of ICI-related steroid-refractory myocarditis and myositis with myasthenia gravis-like symptoms.
Timeline
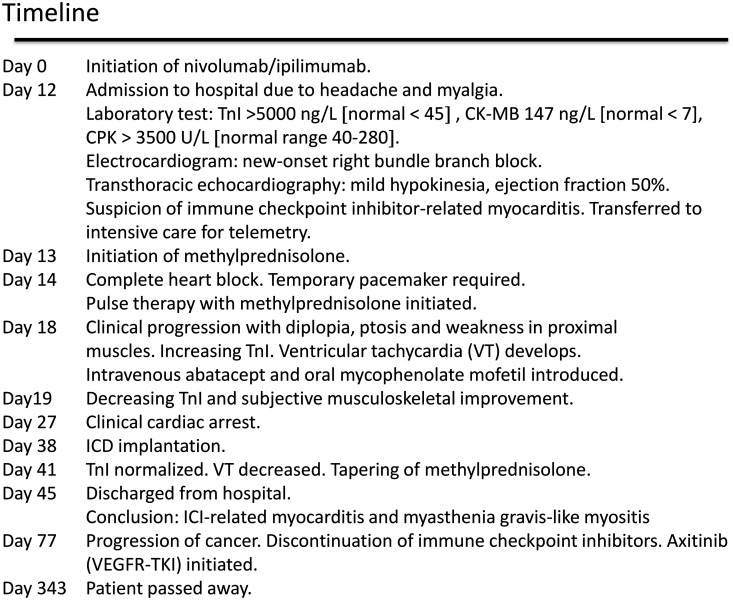
|
Case presentation
A 57-year-old male was diagnosed with renal cell carcinoma (clear cell adenocarcinoma with sarcomatoid component) metastasized to lungs, adrenal gland, and sacrum. Treatment with combination immunotherapy nivolumab, an anti-programmed cell death-1 (3 mg/kg), and ipilimumab, an anti-CTLA-4, (1 mg/kg), was initiated. Twelve days after receiving first dose of ICI, the patient was admitted to the hospital with persisting headache and myalgia in the lower extremities. He reported palpitations without other cardiac symptoms. The physical examination represented normal findings with vital parameters SpO2 96%, respiratory rate 20 breaths per minute, blood pressure 109/75 mmHg, pulse 119 beats per minute, and normal temperature. Laboratory test revealed elevated troponin I (TnI) and creatine phosphokinase (CPK) (both normal at baseline): TnI 5760 ng/L (normal < 45), creatine kinase-myocardial band 147 ng/L (normal < 7), myoglobin 5230 µg/L (normal range 22–77), CPK 3510 U/L (normal range 40–280) along with transaminitis [alanine aminotransferase 158 U/L (normal range 10–70) and aspartate aminotransferase 287 U/L (normal range 15–45)]. Electrocardiogram (ECG) demonstrated new-onset first-degree AV block and right bundle branch block (Figures 1 and 2). Transthoracic echocardiography (TTE) showed mild apical hypokinesia with slightly reduced left ventricular systolic function (ejection fraction 50%) (Videos 1 and 2), whereas the values were normal at the baseline multigated acquisition scan. Pituitary magnetic resonance imaging (MRI) could not exclude hypophysitis.
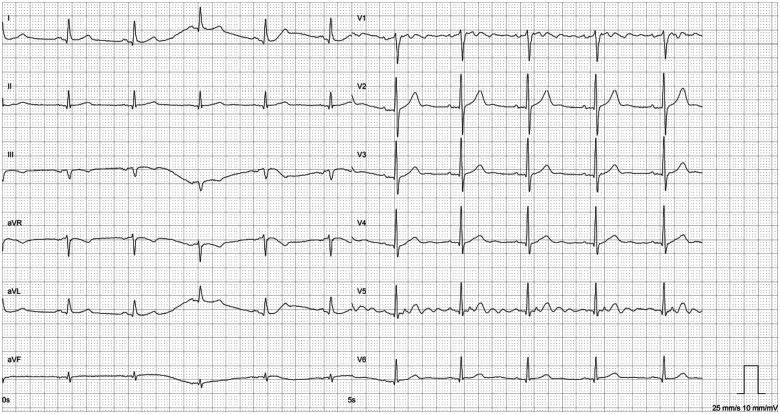
Figure 1.
Baseline electrocardiogram before initiation of immune checkpoint inhibitor showing sinus rhythm.
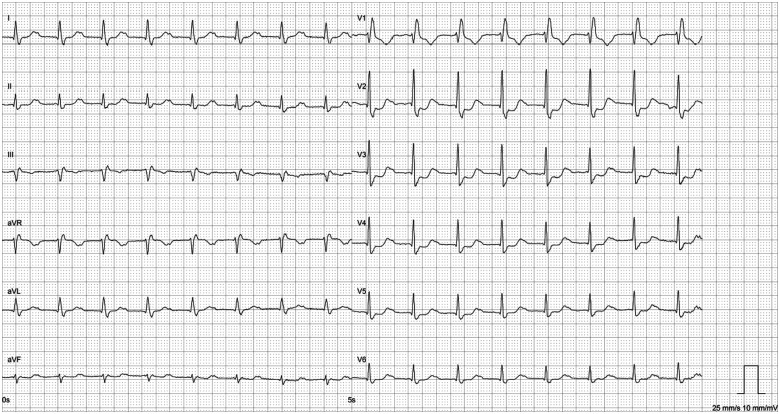
Figure 2.
Electrocardiogram at admission showing first-degree AV block and right bundle branch block.
As the patient had no cardiovascular risk factors, the initial work-up gave rise to suspicion of ICI-related myocarditis and myositis, assuming the elevated liver enzymes derived from affected inflammatory and cardiac markers. The patient was transferred to the intensive care unit for continuous cardiac monitoring (telemetry) and daily TTE. Cardiac MRI (CMR) failed due to arrhythmia (ventricular extra systoles). Subsequently, computed tomography (CT) coronary angiogram, or coronary angiography, was not performed to exclude acute coronary syndrome due to strong clinical suspicion of immune-related myocarditis. Considering the invasive character, endomyocardial biopsy was not carried out.
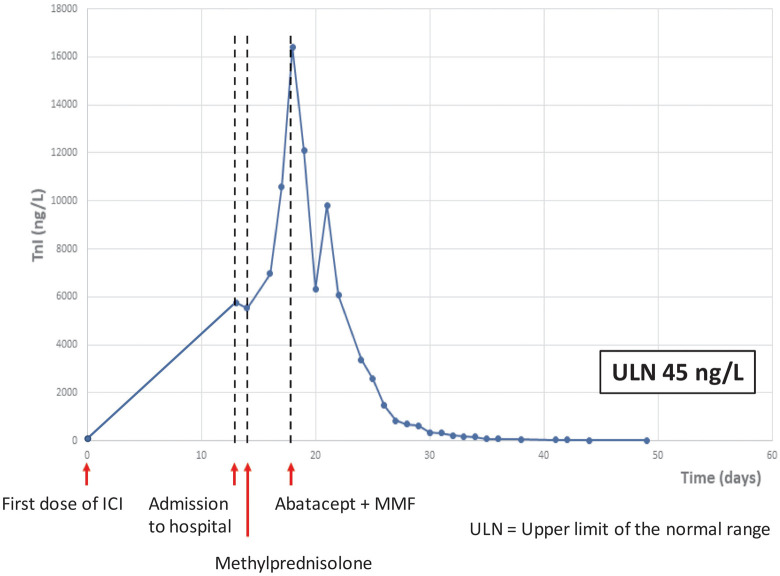
Within 48 h, the patient developed complete heart block requiring a temporary pacemaker. Despite immediate administration of high-dose glucocorticoids (initially intravenous methylprednisolone 2 mg/kg/day, increased to pulse therapy, i.e. 1000 mg intravenous methylprednisolone per day for 3 days), the TnI level increased to 16 400 ng/L (Figure 3), and ventricular tachycardia (VT) arose. Additionally, intermittent binocular diplopia, ptosis, and weakness in proximal muscles developed, and myalgia progressed. Supplementary diagnostic assessment demonstrated elevated myasthenia gravis-associated antibodies [anti-acetylcholine receptor (IgG) 2.7 nmol/L (normal < 0.4), anti-titin (IgG) 2.6 AU (normal < 1.0)]. Electromyography showed evidence of a myogenic disorder without dysfunction of the neuromuscular junction.
Figure 3.
Troponin I (baseline troponin I < upper limit of normal range). ICI: immune checkpoint inhibitor; MMF: mycophenolate mofetil; TnI, troponin I; ULN: upper limit of normal range.
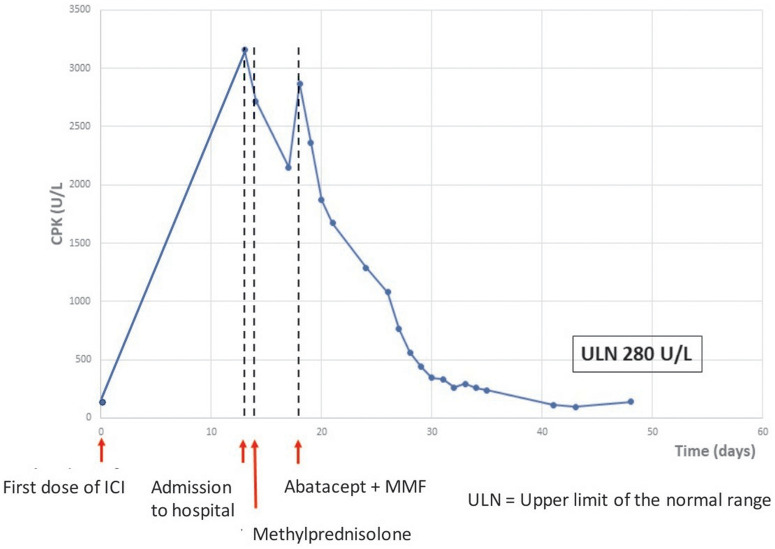
Clinical symptoms and paraclinical data were interpreted as ICI-related myocarditis and myositis with myasthenia gravis-like symptoms. Due to lack of improvement, intravenous abatacept (500 mg every 2 weeks, for a total of 5 doses) and oral MMF (1 g twice a day for 3 months) was introduced 18 days after ICI therapy. Intravenous methylprednisolone (2 mg/kg/day) was continued. Rapidly the TnI and CPK level decreased (Figures 3 and 4), and the patient reported instant musculoskeletal improvement. However, intermittent VT persisted, and clinical cardiac arrest caused by VT developed 21 days after ICI. External defibrillation was applied once, and paced rhythm was resumed. An implantable cardioverter-defibrillator was inserted.
Figure 4.
Creatine phosphokinase (baseline creatine phosphokinase < upper limit of normal range). CPK, creatine phosphokinase; ICI: immune checkpoint inhibitor; MMF: mycophenolate mofetil; ULN: upper limit of normal range.
Troponin I was normalized 6 weeks after initiation of ICI, VT decreased and tapering of steroid was initiated. Four days later the patient was discharged. Afterwards, TnI remained normal, while intermittent non-sustained VT (1–10 beats/min, heart rate 180 beats/min) continued for the next 2 months. Transthoracic echocardiography abnormalities persisted throughout life. Symptoms of myositis (myalgia, muscular weakness, ptosis, and diplopia) gradually decreased during the hospital stay, but gait support was still required at discharge. Ultimately, myositis almost remitted with marginal weakness of the upper limbs remaining.
Additional treatment with ICI was permanently discontinued. Positron emission tomography/CT executed 3 months after the introduction of ICI, showed progressive disease in lungs, adrenal gland, and bones. Targeted treatment with axitinib (a vascular endothelial growth factor receptor tyrosine kinase inhibitor) was introduced.
Eleven months after initiation of ICI the patient passed away due to cancer.
Discussion
Immune checkpoint inhibitors have remarkably improved clinical outcome for multiple malignancies but can cause high-grade irAEs.1 Immune checkpoint inhibitor-related myocarditis is an infrequent (<1.5%), but potentially life-threatening adverse event (50% fatal).1,6 The condition is often associated with other irAEs, particularly myositis (25%) and myasthenia gravis-like conditions (10%).1
Myocarditis caused by ICI can be challenging to identify due to lack of specific cardiac symptoms. Characteristically, TnI is elevated (94%), and ECG is abnormal (89%). The degree of TnI elevation seems to be a predictor of severe cardiac events, such as cardiac arrest. Normal TTE with preserved left ventricular ejection fraction is observed in 50% of myocarditis associated with ICI.7
When suspecting myocarditis, CMR is recommended for diagnostic assessment, while endomyocardial biopsy is considered the most accurate diagnostic test. Without CMR or biopsy, clinical symptoms and paraclinical results must be evaluated to determine the most probable diagnosis.8 In our case, there was a high clinical suspicion of ICI-related myocarditis, legalized by relevant changes in ECG and TTE, elevated biomarkers (with continuously increasing TnI), and presence of cardiac symptoms concurrent with signs of myositis in a patient, that had just received ICI and had no cardiovascular risks.9
Glucocorticoid is the treatment of choice for myocarditis caused by ICI, while steroids or other immune suppressive agents are less frequently required for non-ICI-related myocarditis. Steroid-refractory immune-related myocarditis represents an important clinical challenge with high mortality. Alternative immune suppressive therapy, e.g. MMF, anti-thymocyte globulin, intravenous immunoglobulin, infliximab, abatacept, and plasmapheresis can be considered for steroid-resistant cases.10,11 Yet, due to lack of prospective studies, the efficacy of the agents is uncertain.
Here, we report that abatacept combined with MMF resolved steroid-refractory myocarditis and myositis. Troponin I decreased rapidly after the introduction of abatacept and MMF, whereas arrhythmia (VT) persisted. Cardiac arrest was interpreted as secondary to myocarditis caused by ICI, as opposed to a side effect to the immune suppressive therapy.
To determine the optimal treatment of steroid-refractory immune-related myocarditis clinical trials are required.
Lead author biography

Dr Mette Syberg Jespersen is a clinical oncologist at Herlev Hospital, Denmark. She is undertaking a PhD with the University of Copenhagen, exploring the effects and side effects of immune checkpoint inhibitors in real-life populations.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the assistance of Inge Marie Svane, Kasper Karmark Iversen, and Finn Gustafsson in the review and editing process.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
References
- 1.Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A. et al. Spectrum of cardiovascular toxicities of immune checkpoint inhibitors: a pharmacovigilance study. Lancet Oncol 2018;19:1579–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mir R, Shaw HM, Nathan PD.. Mycophenolate mofetil alongside high-dose corticosteroids: optimizing the management of combination immune checkpoint inhibitor-induced colitis. Melanoma Res 2019;29:102–106. [DOI] [PubMed] [Google Scholar]
- 3.Cheung V, Gupta T, Payne M, Middleton MR, Collier JD, Simmons A. et al. Immunotherapy-related hepatitis: real-world experience from a tertiary centre. Frontline Gastroenterol 2019;10:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmood SS, Chen CL, Shapnik N, Krishnan U, Singh HS, Makker V.. Myocarditis with tremelimumab plus durvalumab combination therapy for endometrial cancer: a case report. Gynecol Oncol Rep 2018;25:74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salem J-E, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ. et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med 2019;380:2377–2379. [DOI] [PubMed] [Google Scholar]
- 6.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F. et al. Fatal toxic effects associated with immune checkpoint inhibitors a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM. et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A. et al. Myocarditis in the setting of cancer therapeutics. Proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation 2019;140:80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spallarossa P, Giacomo T, Sarocchi M, Arboscello E, Grossi F, Queirolo P. et al. Identification and management of immune checkpoint inhibitor-related myocarditis: use troponin wisely. J Clin Oncol 2019;37:2201–2205. [DOI] [PubMed] [Google Scholar]
- 10.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM. et al. ; in oration with the National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy. American Society of Clinical Oncology Clinical Practical Guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mir H, Alhussein M, Alrashidi S, Alzayer H, Alshatti A, Valettas N. et al. Cardiac complications associated with checkpoint inhibition: a systematic review of the literature in an important emerging area. Can J Cardiol 2018;34:1059–1068. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.