Abstract
Heterotopic ossification (HO) occurs as a common complication after injury or in genetic disorders. The mechanisms underlying HO remain incompletely understood and there are no approved prophylactic or secondary treatments available. Here, we identify a self-amplifying, self-propagating loop of Yes-associated protein (YAP)-Sonic hedgehog (SHH) as a core molecular mechanism underlying diverse forms of HO. In mouse models of progressive osseous heteroplasia (POH), a disease caused by null mutations in GNAS, we found that Gnas−/− mesenchymal cells secreted SHH, which induced osteoblast differentiation of the surrounding wild-type cells. We further show that loss of Gnas led to activation of YAP transcription activity, which directly drove Shh expression. Secreted SHH further induced YAP activation, Shh expression, and osteoblast differentiation in surrounding wild-type cells. This self-propagated positive feedback loop is both necessary and sufficient for HO expansion and can act independently of Gnas in fibrodysplasia ossificans progressiva (FOP), another genetic HO, and nonhereditary HO mouse models. Genetic or pharmacological inhibition of YAP or SHH abolished HO in POH, FOP, and acquired HO mouse models without affecting normal bone homeostasis, providing a previously unrecognized therapeutic rationale to prevent, reduce and shrink HO.
One-sentence summary:
A self-amplifying, self-propagating loop of YAP-SHH is a core molecular mechanism underlying diverse forms of heterotopic ossification.
Introduction
Heterotopic ossification (HO) is a pathological condition in which bone forms in non-skeletal tissues. HO is a frequent complication of soft-tissue injuries and complicates recovery after surgery or trauma (1). It is also a manifestation of several congenital syndromic diseases (2). HO causes pain and restricts range-of-motion. Genetic forms can be severe, progressive, and life threatening, yet treatments are largely ineffective. As in normal skeletal morphogenesis, HO can form through an intramembranous and/or an endochondral process, suggesting multiple cellular mechanisms may be involved (2). Nevertheless, the principal cellular defect appears to be differentiation of the skeletal progenitor cells in soft tissues into chondrocytes, osteoblasts, or both cell types. The effects of HO are illustrated by two rare genetic disorders that are characterized by extensive and progressive replacement of soft tissues with bone: fibrodysplasia ossificans progressiva [(FOP), Online Mendelian Inheritance in Man (OMIM) #135100] (3) and progressive osseous heteroplasia [(POH), OMIM#166350] (4). HO in FOP is governed by endochondral bone formation, in which cartilage formation precedes bone development. Classic forms of FOP are caused by a highly conserved heterozygous mutation of arginine 206 to histidine (R206H) in the bone morphogenetic protein (BMP) type I receptor known as activin receptor-like kinase 2 (ALK2) encoded by ACVR1 (5-7). POH, on the other hand, results from heterozygous loss-of-function mutations in the human GNAS gene, which encodes the G-protein signaling subunit, Gαs. HO in POH is predominantly governed by intramembranous ossification, in which osteoblasts differentiate directly from mesenchymal progenitors, independently of chondrocytes (2, 8, 9).
Clinically, POH is characterized by dermal ossification during infancy, with mosaic distribution and progressive HO of subcutaneous and deep connective tissue such as muscle and fascia during childhood (10). Over time, HO expansion leads to ankylosis of affected joints and growth retardation of affected limbs. Non-hereditary forms of HO appear to be regulated by both endochondral and intramembranous bone formation, and occur frequently as complications of trauma, fractures, total hip arthroplasty, deep burns, and central nervous system injury (11-15). HO has an incidence of more than 50% in blast-related amputations, more than 10% after a fracture, multiple injuries or total hip arthroplasty (16-18). At present, preventative therapy for HO in high-risk clinical situations with nonsteroidal anti-inflammatory drugs (NSAIDs) and/or local irradiation may be marginally helpful, while surgical therapy to excise existing HO is limited by a high frequency of recurrence and complications, despite prophylactic therapy (19). Thus, HO remains a major unresolved medical challenge requiring more mechanistic studies to identify effective therapeutic targets to prevent, reduce and shrink HO.
Because ectopic osteoblast differentiation underlies all forms of HO, important molecular determinants for such cell-fate induction are likely to be identified in hereditary HO. Previously, we and others showed that, in both mesenchymal and neuronal cells, Gαs signaling suppresses hedgehog (HH) signaling by regulating protein kinase A (PKA)-induced GLI3 cleavage (20, 21). In POH mouse models, loss of Gαs signaling in the embryonic limb bud mesenchyme or adult subcutaneous mesenchymal progenitor cells (SMPs) activates HH signaling, which is both necessary and sufficient to induce osteoblast differentiation (20). As Gαs regulation of GLI activities are cell autonomous, key questions remain: What are the cellular and molecular mechanisms underlying extensive and progressive expansion of HO in POH? Could blocking bone growth in POH effectively alleviate HO symptoms? Does the molecular mechanism identified in POH studies also govern other forms of HO?
Whereas regulation of Gli transcriptional activities by Gαs is cell autonomous (20), HH signaling is controlled non-cell autonomously by HH ligands through the same pathway (22)—in mammals, these are Sonic hedgehog (SHH), Indian hedgehog (IHH), and Desert hedgehog (DHH). Determined by their expression patterns in the embryonic limb bud, Shh acts at early stages to regulate patterning and growth (23-25), while Ihh acts later, during endochondral bone formation, to not only control chondrocyte proliferation and hypertrophy, but also to induce osteoblast differentiation of mesenchymal progenitor cells in the perichondrium (26). Because HH signaling activation is both necessary and sufficient to drive HO (20, 21), to what extent HO expansion in POH is driven by a cell-autonomous HH signaling activation versus by secreted factors is important to determine.
In this study, we investigated the cellular and molecular mechanisms underlying progressive HO expansion in POH and found that HO expansion is driven by SHH secretion. We demonstrate that HH signaling activates Yes-associated protein (YAP), a Hippo-signaling pathway transcription factor that plays key regulatory roles in developmental and oncogenic processes (27, 28), and YAP directly drives Shh expression. Both YAP and SHH activities were upregulated in human nonhereditary HO. We identified a YAP-SHH positive feedback loop that is both necessary and sufficient to drive self-propagated osteoblast differentiation of cells with initially lower bone inductive activities in POH, FOP, and non-hereditary HO mouse models. Our studies demonstrate that inhibiting YAP and SHH activities could be promising therapeutic approaches for diverse HO forms.
Results
Recruitment of wild-type cells contributes to progressive expansion of ectopic bone in POH in mice
Because most HO occurs in adults, we established a conditional POH mouse model by subcutaneously injecting Adenovirus Cre (Ad-Cre) into the hind limbs of 4-week-old Gnasf/f mice, which allowed Cre-mediated Gαs inactivation by deleting exon 1 of the Gnas gene (20, 29). HO develops in young children with POH, who have heterozygous inactivating GNAS mutations. However, HO was only detected in old (~1 year of age) Gnas+/− mice (30, 31). In our study, the GnaSf/+ mice did not develop HO after Ad-Cre injection in the experimental time window (1.5-9.5 months of age), and therefore served as controls. A hallmark of POH is progressive heterotopic bone formation with associated pain and restricted range of motion (4), so the Ad-Cre-injected Gnasf/f mice were examined for ectopic bone formation by micro computed tomography (μCT) scanning and von Kossa staining of tissue sections (Fig. 1, A and B). From 6 weeks to 3 and 8 months after injection, HO expanded progressively from mostly subcutaneous regions (where the Gnas gene deletion was induced by Ad-Cre) to deeper muscle areas (Fig. 1, A and B). Quantitative-real-time reverse transcription polymerase chain reaction (qRT-PCR) analysis of osteoblast marker expression of osterix (Osx) and collagen 1a1 (Col1a1) also showed progressive increase from 6 weeks to 8 months (Fig. 1, C). We performed lineage tracing experiments to distinguish whether HO expansion was caused by proliferation of Gnas−/− cells or recruitment of wild-type (WT) cells by the Gnas−/− cells to the progressively forming bone. Gnas−/− cells were labeled with tdTomato (tdTMT) using the Ai9 R26LSL-tdTMT mouse strain (32) in the Gnasf/f; R26LSL-tdTMT mice. Subcutaneous Ad-Cre injection into the Gnasf/f; R26LSL-tdTMT mice allows simultaneous Gnas deletion and tdTMT expression. We showed previously that loss of Gnas led to ectopic osteoblast differentiation, as detected by OSX immunohistochemistry (20). Although many OSX+ cells were also tdTMT+ at 6 weeks, 3 or 8 months post injection, some of the OSX+ cells were tdTMT−. By 3 months post injection, OSX+tdTMT− cells were found in deeper muscle areas (fig. S1A). By 8 months post injection, the fraction of OSX+tdTMT− cells increased further, suggesting ectopic osteoblast differentiation could be controlled by Gnas−/− cells non-cell autonomously.
Fig. 1. HO progresses by recruitment of increasing numbers of wild-type cells to the ectopic bone in POH mouse models.
(A) Representative μCT images of the Gnasf/+; R26LSL-tdTMT (Ctrl) and Gnasf/f; R26LSL-tdTMT (KO) mouse tibia at indicated time points after subcutaneous Ad-Cre injections at 4-weeks-old. Red arrows indicate ectopic bone. White lines indicate section plane. N = 8 biological replicates. Scale bar: 1 mm. (B) Representative von Kossa staining of HO and control tissue sections from the Ad-Cre injected hindlimbs. Dashed lines indicate the interface between epidermis and dermis. Scale bar: 100 μm. (c) Upper panel: Analyses of gene expression in the HO and control tissue by qRT-PCR. (mean±SD; N = 3 biological replicates); Lower panel: quantification of ectopic bone volume from (A) **P < 0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (D) Representative immunofluorescent images of Col1a1-GFP and tdTMT in the ectopic bone sections of the indicated mice. Top panel: merged images with lower magnification. Dashed lines indicate the interface between epidermis and dermis. Lower panel: higher magnification images of the boxed regions. Arrows: GFP+; tdTMT− cells. DAPI stained the nucleus. N = 5 biological replicates. Scale bar: 100 μm. (E) Quantification of the GFP+ cell in (D). Total GFP+ cells/field of view (FOV) (left) and GFP+; tdTMT− cells vs. total GFP+/FOV (right). FOV: 800 × 600 μm **P < 0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests.
To further explore the non-cell-autonomous function of Gnas−/− cells, the Gnas−/− cell lineage was traced by tdTMT expression while all osteoblast cells were identified by GFP expression using the Col1a12.3-GFP reporter allele (33) (Fig. 1d). GFP+ osteoblast cells were detected in the endogenous bone (tibia) (fig. S1B). In the soft tissue, GFP+ osteoblast cells were only detected after Ad-Cre injection in the Gnasf/f mice and their numbers increased between 6 weeks and 8 months post injection (Fig. 1, D and E). Although the number of tdTMT+ cells was similar in Ad-Cre injected Gnadf/+ and Gnasf/f mice, the number of GFP+ cells increased progressively between 6 weeks and 3 to 8 months in the Gnasf/f mice (Fig. 1, D and E). Many GFP+ cells were not tdTMT+, particularly in the Gnasf/f mice at 3 or 8 months post-injection (Fig. 1d). Therefore, progressively more tdTMT− WT cells were induced by Gnas−/− cells to become osteoblast cells, contributing to progressive HO expansion. In addition, we found that Gnas loss also stimulated cell proliferation, as shown by Ki67 staining. At an early stage of HO induction (6 weeks after Ad-Cre injection), some of the Ki67+ cells expressed an osteoblast marker osteopontin (OPN) and most of the Ki67+ cells were also tdTMT+ (Fig. S1c). Since platelet-derived growth factor receptor α (PDGFRα) marks fibro/adipogenic progenitor (FAP) cells which contribute to ectopic bone formation (34), we examined whether the Ki67+ cells were PDGFRα+: indeed, most Ki67+ cells were PDGFRα+, and loss of Gnas increased PDGFRα+ cell numbers (fig. S1D).
Gnas loss induces bone formation non-cell autonomously by inducing Shh expression
To identify the molecular mechanism underlying the cell non-autonomous function of Gnas−/− cells during osteoblast differentiation, we cultured WT SMP cells in conditioned medium (CM) collected from Ad-GFP- or Ad-Cre-infected Gnasf/f SMP cells. Alkaline phosphatase (ALP) and von Kossa staining determined osteogenic differentiation and matrix mineralization 7 and 21 days later, respectively, and both were enhanced by CM from the Gnas−/− cells (Fig. 2, A). Expression of osteoblast differentiation markers, such as Osx and Col1a1, was also upregulated (Fig. 2, B). Thus, the Gnas−/− osteoblasts secreted factors that induced osteoblast differentiation of WT cells.
Fig. 2. Gnas loss induces bone formation non-cell autonomously by inducing SHH expression and secretion.
(A) Representative images of ALP and von Kossa staining of wild-type SMPs, cultured with conditioned medium (CM) from indicated adenovirus-infected SMPs for 7 days and 21 days, respectively. N=3 biological replicates. Two sets were shown. (B) Left panel: quantification of ALP activity from (a). Right panel: qRT-PCR analysis of gene expression in WT SMPs cultured with indicated CM for 7 days (mean±SD; N=3 biological replicates). **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (C) qRT-PCR analysis of Hh gene expression in Gnasf/f SMPs, 4 days after adenovirus infection. SHH protein, phosphorylated Creb (pCreb), and total Creb protein, were detected by Western blotting (right panel). β-ACTIN: loading control. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. NS: not significant. (D) Secreted SHH protein detected by Western blotting of CM from two independent sets of Gnasf/f SMPs, 4 days following adenovirus infection. (E) Left panel: ALP staining of wild-type SMPs, cultured with indicated CM and SHH monoclonal blocker (5E1; 200 ng/ml) for 7 days. Middle panel: quantification of ALP activiy. Right panel: qRT-PCR analysis of gene expression. N=3 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (F) Left panel: ALP staining of the indicated SMPs 7 days after adenovirus infection. Right panel: quantification of ALP activity. N=3 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (G) Representative μCT images of the indicated tibia bone 6 weeks or 3 months after subcutaneous Ad-Cre injection at 4 weeks of age. Red arrows indicate ectopic bone. White lines indicate section position. Lower panel: quantification of ectopic bone volume. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. N=5 biological replicates. Scale bar, 1 mm. (H) Representative immunofluorescent images of OSX and tdTMT expression in ectopic bone sections of indicated genotypes, 3 months after Ad-Cre injection of 4-week-old mice. Arrows: OSX+; tdTMT− cells. S: Skin; M: Muscle. Dashed lines indicate the interface between epidermis and dermis. N=5 biological replicates. Scale bar: 100 μm.
Previously, we showed that activated HH signaling was both necessary and sufficient to induce HO in POH (20). We reasoned that WT soft tissue cells could have been induced to become osteoblasts via activated HH signaling. Expression of Shh, Ihh and Dhh analyzed by qRT-PCR showed that Shh expression was the most upregulated, whereas Dhh expression did not change (Fig. 2, C). Furthermore, SHH accumulated in the Gnas−/− CM (Fig. 2, D), and a neutralizing monoclonal antibody against SHH (35) blocked the activity of the Gnas−/− CM in promoting osteoblast differentiation (Fig. 2, E). We then confirmed upregulation of Shh mRNA and protein by Gnas loss in HO tissues in vivo (fig. S2A). To determine whether increased SHH plays an essential role in HO, we generated Shhf/f; Gnasf/f mice (36) and both Shh and Gnas were removed by Ad-Cre infection of SMPs in vitro (Fig. 2, F). Shh loss reduced osteoblast differentiation and HH signaling (Fig. 2, F and fig. S2B). Furthermore, removing one copy of Shh in vivo markedly reduced Gnas−/−-induced HO, whereas removing both Shh copies further reduced HO with almost complete phenotypic and molecular rescue (Fig. 2, G and fig. S2C). Efficient Shh removal was confirmed by genomic PCR of the Shh floxed allele as we have done previously with other gene deletion (37) and Western blotting of SHH protein (fig. S2D). The residual HO suggests that Gnas−/− cells could differentiate into osteoblast cells even when SHH expression was largely reduced. In tissue sections, OSX+tdTMT− cells readily identified in the Gnasf/f; R26LSL-tdTMT mice were far fewer in the Shhf/f; Gnasf/f;R26LSL-tdTMT mice, which still contained a few OSX+tdTMT+ cells (Fig. 2, H). Taken together, these results show that SHH secretion by the Gnas−/− SMP cells promoted osteoblast differentiation of surrounding WT cells.
Gnas loss upregulates SHH by activating Yap transcription activities
To determine the mechanism driving SHH ectopic expression, gene expression in WT and Gnas-deficient SMP cells was analyzed by RNA seq (fig. S3A and B). YAP transcriptional activity was increased, as confirmed by qRT-PCR analyses of YAP target gene expression (Ctgf and Cyr61) as well as Western blotting of YAP and GLI1 protein, respectively, in WT and Gnas-deficient SMP cells (Fig. 3, A). Cyclic adenosine 3’, 5’-monophosphate (cAMP) signaling was paradoxically upregulated, possibly due to upregulation of some of the negative regulators of cAMP signaling such as phosphodiesterase 3a (Pde3a). PKA activity, shown by phosphor-CREB, was reduced in the Gnas-deficient SMP cells (Fig. 2, C). We focused our analyses on the Hippo/YAP pathway because, similar to HH signaling, it critically regulates cell proliferation, differentiation and survival in development and tumorigenesis (38-40). In Ad-Cre-induced HO tissues, YAP transcriptional activity and protein amounts were also increased as compared to the control (fig. S3C). Immunofluorescent staining further showed that YAP was upregulated in most tdTMT+ cells in Ad-Cre injected Gnasf/f;R26LSL-tdTMT mice (fig. S3D). These data show that loss of Gnas activated YAP activities both in vitro and in vivo in subcutaneous mesenchymal cells, consistent with previous studies in skin stem cells where loss of Gnas activated both GLI and YAP activities in the context of basal-cell carcinoma (41).
Fig. 3. Gnas loss upregulates SHH by activating Yap activity.
(A) Quantified gene expression in Gnasf/f SMPs infected by indicated adenovirus. N=3 biological replicates. Western blotting analyses (right panel) of indicated proteins in Gnasf/f SMPs infected by indicated adenovirus. (mean±SD). **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (B) Representative μCT images of indicated tibia bones 6 weeks or 3 months after Ad-Cre injection. Red arrows indicate ectopic bone. White lines indicate section position. Lower panel: quantification of ectopic bone volume. N=5 biological replicates. Scale bar, 1 mm. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (C) Representative immunofluorescent images of OSX and tdTMT in ectopic bone sections of indicated mice, 6 weeks after Ad-Cre injection. Dashed lines indicate the interface between epidermis and dermis. Arrows: OSX+; tdTMT− cells. S: Skin; M: Muscle. Scale bar: 100 μm. (D) Top: HO induction and treatment schedules. Representative μCT images of tibia bones from indicated mice treated with the Yap inhibitor verteporfin (VP) five days per week starting from the day of Ad-Cre injection. Upper panel: Topical application of VP (0.2 mg/ml) around the injection site. Lower panel: intraperitoneal VP injection (2.5 mg/kg). Red arrows indicate ectopic bone. Bottom panel: quantification of ectopic bone volume. N=5 biological replicates. Scale bar: 1 mm. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (E) Representative μCT images of the indicated tibia bones. Doxycycline water treatment started right after Ad-Cre injection. Red arrows indicate ectopic bone. Right panel: quantification of ectopic bone volume. N=5 biological replicates. Scale bar: 100 μm. (F) qRT-PCR analysis of gene expression in the ectopic bone region from mice in (E). (mean±SD; N=3 biological replicates). NS: not significant. *p<0.05 **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. Ad-Cre injection was always performed in 4-week-old mice.
Some YAP+ cells were tdTMT−, and the number of YAP+tdTMT− cells increased from 6 weeks to 3 months after Ad-Cre injection (fig. S3D), suggesting YAP activation can also be non-cell autonomous. To test whether YAP is required for HO and its expansion, we removed Yap and Gnas by generating Gnasf/f;Yapf/+ and Gnasf/f;Yapf/f mice (42), into which Ad-Cre was injected subcutaneously. Partial loss of Yap drastically reduced the HO caused by Gnas loss, while complete Yap loss more efficiently inhibited HO at 6 weeks or 3 months after Ad-Cre injection (Fig. 3, B). In mice with complete Yap loss, HO was not detected. In contrast, in mice with Shh loss, some HO was always present (compare Fig. 3, B with Fig. 2, G). Furthermore, in the Gnasf/f;R26LSL-tdTMT mice, both tdTMT+ and tdTMT− fractions of HO tissue contained many OSX+ cells; in contrast, in the Gnasf/f; Yapf/f; R26LSL-tdTMT mice, no OSX+ cells were detected (Fig. 3, C). In all of the Gnas, Shh or Yap single or double conditional mutant mice, HO was induced locally by Ad-Cre injection and no adverse effects were detected systemically (fig. S4A).
Since Yap loss abolished HO, we then tested whether HO can be inhibited by the YAP inhibitor verteporfin (VP), which interferes with YAP-TEAD binding that is required for YAP-mediated gene expression (43). VP was applied topically or via intraperitoneal (IP) injection to Gnasf/f mice immediately after Ad-Cre injection (Fig. 3d). Both delivery methods ameliorated HO, with IP injection being more effective (Fig. 3, D). Recently, we found Yap activity is enhanced in the nucleus by Cyclin Dependent Kinase 7 (CDK7) (44). Indeed, two distinct CDK7 inhibitors, THZ1 and CT7001 (ICEC0942) (45, 46), drastically reduced HO in Ad-Cre injected Gnasf/f mice (fig. S4B). Osteoblast differentiation was reduced when Gnas−/− SMP cells were treated in vitro with VP, THZ1, and CT7001, but not palbociclib (PD-0332991), an inhibitor of CDK4/6 that does not regulate YAP (fig. S4C). Furthermore, we found that starting VP injection 6 weeks after Ad-Cre injection, when HO was already evident (Fig. 1, A), also reduced HO (fig. S4D). These results indicate that YAP inhibitors can both prevent HO and reduce already formed HO. To determine whether YAP inhibition by VP treatment could reduce endogenous bone mass and therefore cause osteoporosis, we analyzed the tibia bones and found no significant difference between VP-injected and uninjected groups (fig. S5A), suggesting that YAP inhibition reduced HO, but not the normal bone. To test whether short inhibition could have long-term protective effects, we stopped the VP treatment after 6 weeks and harvested the samples 3 months after initial Ad-Cre injection (fig. S5B). Indeed, early and short VP treatment for 6 weeks reduced HO even after VP withdrawal. Furthermore, we found that endogenous bone mass was not reduced after 3 months of continuous VP treatment (fig. S5B), suggesting that the bone reduction effects of Yap inhibition are largely confined to HO.
To identify the cellular and molecular mechanism whereby YAP promotes HO, SMP cells were cultured under osteogenic conditions in vitro. Loss of both Yap and Gnas reduced osteoblast differentiation compared to Gnas loss alone (fig. S6A). HH signaling, Shh expression and SHH protein production were markedly reduced by Yap loss (fig. S6A and B). qRT-PCR analysis also showed reduced expression of osteoblast markers, HH-signaling target genes, and Shh in ectopic bone from Ad-Cre-injected Gnasf/f;Yapf/f mice compared to similarly treated Gnasf/f mice (fig. S6C). Therefore, YAP promotes Shh expression and HH signaling and is required for HO. To investigate whether YAP activation alone is sufficient to induce HO in vivo and in vitro, expression of a constitutively active, phospho-deficient YAP was induced by subcutaneous Ad-Cre injection and doxycycline (Dox) water treatment of the Cre- and Tet-inducible Yaptg/+; rtTAtg/+ mice (47) (Fig. 3, E). In these mice, YAP activation was sufficient to induce HO, which was more severe after longer duration of Dox induction (Fig. 3, E and F). Expression of osteoblast markers, Shh, Ihh, and HH signaling targets in ectopic bone tissues and SMP cells were increased by YAP activation (Fig. 3, F and fig. S6D). These results show that YAP activation is sufficient to induce ectopic osteoblast differentiation regardless of Gnas status. However, compared to Gnas loss, YAP activation induced less ectopic osteoblast differentiation and HO was preferentially found in the Achilles tendon, suggesting that YAP may require other transcription factors that are also activated by Gnas loss to efficiently induce osteoblast differentiation. Taken together, these results demonstrate that loss of Gnas requires YAP activation to induce Shh expression and cause HO. Our results also identify YAP or SHH inhibition as potential therapeutic strategies for HO reduction in POH.
YAP and SHH form a positive feedback loop
In SMPs, both YAP activation and SHH expression are downstream events of Gnas loss. To determine whether they regulate each other, we treated SMPs with the YAP inhibitor VP or a SHH monoclonal blocker for 7 days after Ad-Cre infection. Both treatments decreased osteoblast differentiation and SHH expression (Fig. 4, A and B and fig. S7A), suggesting SHH may activate Shh transcription via YAP activation, as Yap is both necessary and sufficient to activate Shh expression. We further tested whether YAP and SHH form a positive feedback loop by removing Yap or Shh in the Gnas−/− SMPs. SMPs from Gnasf/f, Gnasf/f;Yapf/f and Gnasf/f;Shhf/f mice were induced by Ad-Cre and CM was applied to WT SMPs. Gnas−/− CM promoted osteogenesis, whereas similar activity was abolished in CM from cells lacking either Yap or Shh (Fig. 4, C and fig. S7B). As expected, Yap deletion in SMPs reduced SHH amount in the CM (Fig. 4, D). These data indicate that YAP is required for Shh expression and the resulting SHH protein secretion, which induces osteoblast differentiation of WT cells. SHH also activates YAP in SMP cells. In Gnas−/− SMPs, Shh loss reduced YAP expression (fig. S7C and D). Activation of YAP by HH signaling has also been shown in the skin in the context of basal-cell carcinoma (48). In SMPs, recombinant SHH induced expression of YAP target genes, osteoblast markers and HH signaling targets (fig. S7E), indicating that SHH and YAP form a positive feedback loop that promotes osteoblast differentiation.
Fig. 4. Yap and SHH form a positive feedback loop.
(A) ALP staining of Gnasf/f SMPs, 7 days after indicated Ad-GFP or Ad-Cre infection and treatment with VP (200 ng/ml) or a SHH monoclonal blocker (200 ng/ml). Right panel: quantification of ALP activity. N=3 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (B) SHH protein detected by Western blotting of Gnasf/f SMPs, treated as in (a). GAPDH: loading control. (C) ALP staining of WT SMPs cultured for 7 days in indicated conditioned medium. Right panel: quantification of ALP activity. N=3 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (D) Western blotting analysis of secreted SHH protein in the indicated CM. (E Representative immunofluorescent images of ectopic bone sections of the indicated genotypes, 3 months (left panel) or 8 months (right panel) after Ad-Cre injection. Dashed lines indicate the interface between epidermis and dermis in (E-G). Arrows: Yap+; tdTMT − or SHH+; tdTMT− cells. S: Skin; M: Muscle. Scale bar: 100 μm. (F, G) Representative immunofluorescent images of Yap (F), SHH (G), and tdTMT in the ectopic bone section from the indicated mice 6 weeks after Ad-Cre injection. Arrows: Yap+; tdTMT− or SHH+; tdTMT− cells. S: Skin; M: Muscle. Scale bar: 100 μm. Ad-Cre injection was always performed in 4-week-old mice.
To determine the relationship between YAP and SHH in vivo, YAP and SHH immunohistochemistry was performed (Fig. 4, E). Upregulation of YAP and SHH expression was detected in both Gnas−/− cells marked by tdTMT + and the surrounding tdTMT− WT cells (Fig. 4, E). In the Gnasf/f; Shhf/f mice (Fig. 4, F) or Gnasf/f; Yapf/f mice (Fig. 4, G), ectopic YAP or SHH expression, respectively, was diminished, demonstrating that YAP and SHH regulate each other in vivo. These data suggest that both YAP and SHH were activated non-cell autonomously in WT cells, which were induced to become osteoblasts to drive HO expansion. In this process, SHH secreted by the Gnas−/− cells signals plays a key role to induce YAP activation, osteoblast differentiation as well as Shh expression in the surrounding WT cells. Repetition of this process may propagate the osteogenic activities to more WT cells. To further test whether WT cells with YAP activation will express and secrete SHH to induce osteoblast differentiation of more wild type cells, thereby progressively expanding the ectopically formed bone, we collected the CM from WT SMPs that had been induced by the CM from Gnas−/− cells, applied it to WT SMPs, and found that it also showed elevated osteoblast induction ability (fig. S8A). Therefore, the positive feedback loop between YAP and SHH triggers a self-amplifying and self-propagating loop in WT cells that results in recruitment of increasing numbers of cells into HO (fig. S8B).
YAP directly activates Shh expression to promote HO
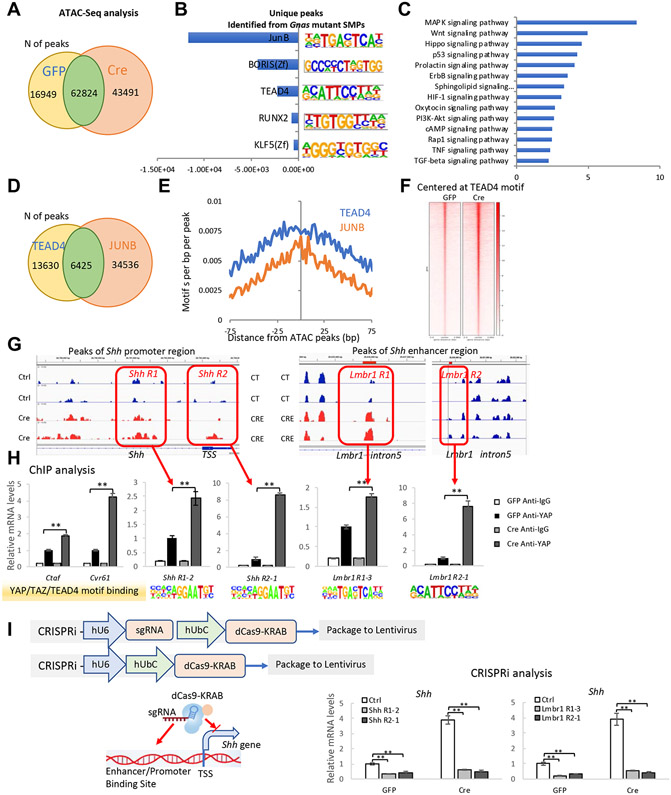
The ectopic Shh expression regulated by YAP prompted us to look for genome-wide gene-expression changes in Gnas−/− mutant SMP cells. Since chromatin remodeling occurs during cell-fate changes, we sought to understand the molecular mechanism underlying HO induction using a genome-wide approach. Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) was used to determine chromatin accessibility in WT and Gnas−/− SMP cells. Consistent with activated HH and Yap activities in the Gnas−/− SMP cells, the number of ATAC-seq peaks increased in the Gnas−/− SMP cells (Fig. 5, A), indicating that gene transcription is likely more active in the absence of Gnas. To understand changes of transcriptional regulation in the Gnas−/− SMP cells, unbiased de novo motif discovery in the 43491 unique peaks in the Gnas−/− cells was performed by the HOMER software (49) and the top 5 enriched transcription factor (TF) binding motifs were found to be binding sites of JUNB, BRIS or CCCTC-Binding Factor Like (CTCFL), TEAD4, Runt-related transcription factor 2 (RUNX2), and Krueppel-like factor 5 (KLF5) (Fig. 5, B). Enriched signal from the CTCFL binding motif reflected chromatin remodeling in the Gnas−/− SMP cells, consistent with the observed cell-fate changes; and increased signal at RUNX2 binding motifs confirmed osteoblastic differentiation. Since YAP/TEAD and AP1 (JUNB) coordinate gene regulation in various tumor cells (42, 48, 50), enriched signal at JUNB and TEAD4 binding motifs suggest Hippo and Mitogen-activated protein kinase (MAPK) signaling may play important roles in osteoblastic differentiation. Indeed, KEGG-pathway mapping of genes with a transcriptional start site (TSS) at the unique ATAC peaks of the Gnas−/− SMP cells showed both Hippo and MAPK signaling pathways were highly up-regulated (Fig. 5, C).
Fig. 5. Shh is a direct target of the YAP transcription factor.
(A) The number of ATAC-seq peaks detected in Ad-GFP- or Ad-Cre-infected Gnasf/f SMPs. (B) HOMER software for unbiased de novo motif discovery was applied to the 43491 unique peaks identified in the Ad-Cre infected cells and the top 5 enriched transcription factor (TF) binding motifs were identified. (C) KEGG-pathway mapping of genes with transcriptional start sites present in the unique ATAC peaks of the Ad-Cre infected Gnasf/f SMPs. (D) The number of JUNB and TEAD4 binding peaks in Ad-Cre infected Gnasf/f SMP cells. (E) The co-occurrence of JunB and TEAD4 binding peaks in the unique ATAC peaks of Ad-Cre-infected Gnasf/f SMPs. (F) ATAC seq signal intensities near TEAD4 motifs. (G) ATAC peaks at TSS and enhancer regions of the SHH locus. (H) ChIP-qPCR analysis of TEAD4 binding sites in each indicated region (boxed). (mean±SD; N=3 biological replicates.). **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (I) Shh expression in Ad-GFP or Ad-Cre-infected Gnasf/f SMP cells. TEAD4 sites in the Shh promotor/enhancer regions were occupied by dCas9-KRAB. Schematic is shown in the left panel. QRT-PCR results are shown as mean±SD; N=3 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests.
To test whether TEAD4 and JUNB coordinate in gene regulation, all identified putative TEAD4- and JUNB-binding peaks were separated into unique and overlapping sets; about 30% of TEAD4 binding peaks also contain JUNB binding motifs (Fig. 5, D). In addition, enrichment of co-occurrence of TEAD4 and JUNB binding motifs was observed in the unique ATAC peaks of the Gnas−/− SMP cells (Fig. 5, E). However, although 15% of the TEAD4 binding peaks contain RUNX2 binding sites, RUNX2 and TEAD4 binding sites were rarely found together (fig. S9). These data suggest that, in Gnas−/− SMP cells, YAP/TEAD also coordinate with AP1 transcription regulation to promote osteoblastic differentiation. To identify YAP/TEAD4-mediated gene regulation, we tested whether chromatin was more accessible around the TEAD4 binding sites in Gnas−/− SMP cells. TEAD4 motifs were assessed in all ATAC peaks, showing that ATAC-seq signal intensities were higher around TEAD4 motifs in Gnas−/− SMP cells (Fig. 5, H). Taken together, our ATAC seq data analyses support the notion that YAP transcription activities in Gnas−/− SMP cells was activated.
In agreement with Shh expression increase, we observed that during osteoblast differentiation of the SMPs, ATAC-seq signal intensities were strongly enhanced at the Shh promoter and enhancer [Lmbr1 intron 5 region (51-53)], suggesting that the chromatin comprising Shh regulatory regions was more accessible in Gnas−/− SMP cells compared to control cells (Fig. 5, G). To test whether Shh is a direct transcriptional target of YAP, the ATAC-seq results were analyzed further in the Shh promoter and enhancer, where several TEAD4 binding sites were identified and confirmed by Chromatin Immunoprecipitation-quantitative PCR (ChIP-qPCR) analysis (Fig. 5, H). To determine whether the identified TEAD4 binding sites are required for Shh expression, the TEAD4 sites in the Shh promoter/enhancer regions were functionally inhibited by placing a nuclease-deficient(d)Cas9-KRAB fusion protein (dCas9-KRAB) to the TEAD4 binding site with respective guide RNAs targeting each TEAD4 binding site via lentivirus (54, 55). Targeting dCas9-KRAB to putative enhancers or promoters recruits the H3K9 methyltransferase SETDB1, which increases local H3K9me3 binding and represses target-gene expression (55). As expected, dCas9-KRAB guided by RNAs targeting TEAD4 binding site in the Shh promoter/enhancer greatly reduced Shh expression in the Gnas−/− SMPs (Fig. 5, I). Taken together, these data demonstrate that Shh is a direct transcriptional target of YAP.
YAP and SHH are activated in and required for HO in FOP mouse models
HO is governed by distinct cellular mechanisms in POH vs. FOP (intramembranous vs. endochondral ossification, respectively), but both require HH signaling for osteoblast differentiation during normal bone development (56, 57). We therefore asked whether the Yap-SHH positive feedback loop also controls HO in FOP. FOP is caused by activating mutations of ACVR1 that render the type-1 receptor inappropriately or promiscuously active (7, 58, 59). Two animal models carrying floxed ACVR1 mutant alleles have been used to study FOP in vivo. The ACVR1Q207D mouse is a transgenic line that allows Cre-inducible expression of an artificial, constitutively active variant of the type I BMP receptor (ACVR1Q207D) (60), which leads to a phenotype similar to FOP with injury- and inflammation-dependent HO (61). Cre-mediated ACVR1Q207D expression driven by the tendon-specific ScxCre causes spontaneous HO (62-64). The advantage of the ACVR1Q207D FOP model is its robust HO. It was reported that heterotopic cartilage, but not bone, was derived from Scx+ lineage cells (63), suggesting HO in this model is formed cell non-autonomously. We re-established the ScxCre; ACVR1Q207D/+ HO model (Fig. 6, A) and found that expression of Shh, Ihh, as well as YAP and HH target genes, transforming growth factor β 1-3 (Tgfβ1-3) were upregulated in the mutant Achilles tendon (Fig. 6, B). Ectopic YAP, SHH, OSX, and OPN expression was found by immunofluorescent staining in the mutant Achilles tendon (Fig. 6, C). Although many cells with high YAP or SHH expression also expressed osteoblast markers OPN and OSX, we also detected YAP+OPN− or SHH+OSX− cells, suggesting that these cells were primed for differentiation into osteoblasts. To test the effects of ACVR1Q207D expression in vitro, we isolated ACVR1Q207D/+ SMPs, because SMPs also express PDGFRα and PDGFRα+ fibro/adipogenic cells contribute to HO in FOP models (65). Indeed, osteoblast differentiation was enhanced and expression of Shh, Ihh, YAP and HH target genes was increased by induced ACVR1Q207D expression in SMPs, and these effects were reduced by VP inhibition of Yap (Fig. 6, D and E). Ad-Cre infection also increased the number of YAP+ and SHH+ cells in the ACVR1Q207D/+ SMPs (Fig. 6, F). These results suggest that YAP and SHH/IHH are also required for HO in FOP models and that HO in distinct genetic models can be regulated by similar mechanisms.
Fig. 6. YAP and SHH are upregulated and required for HO, in an FOP mouse model.
(A) Representative μCT images of tibia bones from 4-week-old ACVR1Q207D/+ and ACVR1Q207D/+; ScxCre+ mice. Arrows indicate ectopic bone around tendon region. White lines indicate section position. Right panel: quantification of ectopic bone volume. N=5 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. Scale bar: 100 μm. (B) Gene expression analysis by qRT-PCR of ectopic bone regions in 4-week-old mice. Mean±SD; N=3 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (C) Representative immunofluorescent images of ectopic bone sections from 4-week-old ACVR1Q207D/+ and ACVR1Q207D/+; ScxCre+ mice. Lower panel: higher magnification of the boxed regions. Dashed lines indicate the tendon boundary. Arrows: YAP+; OPN− cells (left panel); SHH+; OSX− cells (right panel). Scale bar: 50 μm. T: Tendon. (D) ALP staining of ACVR1Q207D/+SMPs with Ad-GFP or Ad-Cre infection and VP (200 ng/ml) treatment for 7 days. Right panel: quantification of ALP activity. N=3 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (E) qRT-PCR analysis of gene expression in SMPs in (D). Mean±SD; N=3 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (F) Representative immunofluorescent images of YAP (left panel) or SHH (right panel) in ACVR1Q207D/+ SMPs, with Ad-GFP or Ad-Cre infection. N=3 biological replicates. Scale bar: 20 μm. (G, H) Representative μCT images of tibia bones from the indicated mice 6 weeks after intramuscular injection of indicated virus with cobra venom at 4 weeks of age. Red arrows indicate ectopic bone. Right panel: quantification of ectopic bone volume. N=6 biological replicates. Scale bar: 100 μm. Lower panel: qRT-PCR analysis of the osteoblast markers Col1a1, Osx, Shh/Ihh, YAP target genes (Ctgf and Cyr61); and the HH signaling targets, Ptch1, Gli1 and Hip. Mean±SD; N=3 biological replicates. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests.
To determine whether Yap and SHH are also required for HO in FOP, we generated Yapf/f; ACVR1Q207D/+ mice that allow Yap removal and ACVR1Q207D expression upon injection of Ad-Cre and cobra venom factor (66). Six weeks after injection, Yap deletion abolished the heterotopic bone (Fig. 6, G). Consistently, expression of osteoblast markers, YAP, and HH-signaling target genes, as well as Shh/Ihh, were markedly reduced (Fig. 6, G). Similarly, when Shh was removed in the Shhf/f; ACVR1Q207D/+ mice using the same approach, HO as well as gene expression associated with osteoblast differentiation, Shh/Ihh expression, HH signaling and YAP activity was decreased (Fig. 6, H). Partial reduction of Yap in ScxCre; ACVR1Q207D/+; Yapf/+ mice drastically reduced HO and Shh/Ihh expression (fig. S10A). These results show that YAP and SHH upregulation are also required for HO in the ACVR1Q207D/+ transgenic mouse models of HO. To ascertain if HO due to ACVR1R206H— the classic mutation found in 97% of human patients with FOP (7)—also exhibits the same requirement for YAP signal transduction, we tested the effect of YAP inhibition in the ScxCre; ACVR1R206H FOP model, in which Cre-induced ACVR1R206H expression leads to spontaneous and progressive HO in tendons, which is slightly milder than that in the ACVR1Q207D model (58, 63). Both ACVR1R206H mutant and wild-type cells have been found to participate in the progression of HO lesion formation (67), suggesting that HO in the ACVR1R206H FOP model may also involve a cell non-autonomous mechanism. Both Q207D and R206H mutations occur in the glycine-serine (GS) rich domain of ACVR1 and HO was detected in the tendon in 3-month-old ACVR1R206H; ScxCre mice, in which expression of YAP and HH target genes, Shh, and Tgfβ1-3 were increased (fig. S10B). Treatment of the ACVR1R206H; ScxCre mice with VP (4 mg/kg/d, 5 days per week) starting at 6 weeks of age for 8 weeks reduced tendon, ligament, and intra-articular HO (fig. S10C). These findings further demonstrate the importance of YAP and SHH for HO in FOP mouse models.
YAP and SHH are upregulated in and required for HO in an injury-induced HO mouse model
Genetic models provide critical insights into more common, non-genetic conditions. Since Yap and Shh are required for HO in both POH and FOP mouse models, we asked whether they are required for acquired HO. We adopted a trauma-induced HO mouse model that uses percutaneous Achilles tendon puncture (ATP) (66) to induce heterotopic bone formation. In this model, HO formed 6 weeks after puncture and continued to enlarge for up to 12 weeks (Fig. 7, A). The sham group showed weaker mineralization in the subcutaneous area as only the skin was punctured and injured. Expression of Col1a1, Runx2, Shh, YAP and HH-signaling target genes, as well as Tgfβ1-3, were increased in the injured Achilles tendon (Fig. 7, B). HH signaling and YAP activities were further examined in vivo in the injured tendon in Ptch1lacZ and Ctgf-GFP mice, respectively. LacZ expression from the Ptch1locus serves as a reporter for HH signaling activity and GFP expression is controlled by YAP (47, 68). Ten days after puncture, strong ectopic β-gal staining and GFP expression was found (Fig. 7, C and D), and immunostaining showed increased SHH and OSX expression (Fig. 7, E).
Fig. 7. Yap and SHH are upregulated in and required for HO in an injury-induced HO mouse model.
The Achilles tendon puncture (ATP) surgery was performed in 4-week-old WT mice. (A) Representative μCT images and the quantified ectopic bone volume of tibia bone and the Achilles tendon region, 6 or 12 weeks after Achilles tendon puncture (ATP) surgery. Arrows indicate ectopic bone around tendon region. N=5 biological replicates. Sub: Subcutaneous region. Scale bar: 100 μm. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. Right panel: Representative images of von Kossa staining of tendon sections, 12 weeks post ATP surgery. The boxed region is shown at higher magnification in the lower panel. Scale bar: 100 μm. Arrows indicate mineralization in the injured subcutaneous region in the sham sample. (B) QRT-PCR analysis of Achilles tendon samples, 6 weeks post ATP surgery. Expression of the YAP target gene Ctgf, Cyr61, osteoblast marker Col1a1, and Runx2, Shh/Ihh, and HH-signaling targets, Ptch1, Gli1 Hip, and Tgfβ1-3 are shown. Mean±SD; N=3 biological replicates.). **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (C) Top panel: X-ray images of the mouse hindlimbs. Low panel: β-Gal staining of tibia-tendon of 6-week-old Ptch1LacZ+ mice, 10 days after ATP surgery. Arrows indicate ectopic bone around the tendon. Scale bars: 100 μm. (D) Representative immunofluorescent images of tendon marker TNMD, GFP, and YAP in tendon sections from the Ctgf-GFP mice 6 weeks post ATP surgery in 4-week-old mice. Lower panel: higher magnification of boxed region. Scale bar: 50 μm. (E) Representative immunofluorescent staining for SHH and OSX in ectopic bone sections from WT mice, 6 weeks after ATP surgery. Arrows: SHH+ OSX− cells. Scale bar: 20 μm. (F, G) μCT images of the tibia/tendon region 6 weeks after ATP surgery with topical treatment of VP (20 μg/ml or 200 μg/ml) and THZ1 (1 mM) (F) or IP injection of VP (2.5 mg/kg) (G) 5 days per week for 6 weeks. Arrows indicate ectopic bone around tendon region. Lower panel: quantification of ectopic bone volume. N=5 biological replicates. Scale bar: 100 μm. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (H) μCT images of tibia/tendon region from the indicated mice 6 weeks after Ad-Cre tendon injection in 4-week-old mice. ATP was performed 1 day after Ad-Cre injection. Lower panel: quantification of ectopic bone volume. N=5 biological replicates. Scale bar: 100 μm. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests. (I) μCT images of the tibia/tendon region of WT mice 6 weeks after ATP surgery followed by IP injection of SHH monoclonal antibody (0.25 mg/kg, 5 days per week). Arrows: ectopic bone around tendon region. Lower panel: quantification of ectopic bone volume. N=5 biological replicates. Scale bar: 100 μm. **p<0.01 one-way ANOVA followed by Tukey’s multiple comparisons tests.
To test whether pharmacological inhibition of YAP attenuates HO in the ATP model, the YAP inhibitor VP and the CDK7 inhibitor THZ1 were topically applied to the skin of the punctured area 3 times a week from the day of ATP for 6 weeks. HO formation was reduced by VP (20 μg/ml) treatment, and completely halted by a higher dose of VP (200 μg/ml) and THZ1(1 mM) (Fig. 7, F). Similar HO blockage was observed in mice intraperitoneally injected with VP five times a week (2.5 mg/kg) from the day of ATP for 6 weeks (Fig. 7, G). To determine whether YAP inhibition by VP treatment reduces endogenous bone mass in ATP mouse model, we analyzed the tibia bones: there was no significant difference between VP-treated and control groups (fig. S11A). To confirm the essential role of Yap and Shh in HO, we removed Yap or Shh by injecting Ad-Cre to the Achilles tendon of the Yapf/f and Shhf/f mice, respectively. Both showed less HO after injury (Fig. 7, H). Likewise, injection of SHH monoclonal antibodies to WT mice greatly reduced HO caused by ATP (Fig. 7, I), suggesting a SHH monoclonal antibody-based therapeutic treatment strategy for HO.
To determine whether upregulation of YAP activity and SHH signaling also contribute to HO formation in humans, we collected 28 human HO samples from the Ossification of the Posterior Longitudinal Ligament (OPLL) patients and performed qRT-PCR analysis and immunostaining of HO and neighboring normal tissues (fig. S11B and C). OPLL is a multi-factorial disease involving HO in spinal ligaments, which causes serious neurological problems and affects 0.8-3.0% of aging Asian and 0.1-1.7% of aging European Caucasian individuals (69). In agreement with the data from the mouse models, expression of YAP target genes CTGF and CYR61, SHH, GLI1 and osteoblast markers SP7 (OSX) and OPN were upregulated in ossified tissues as compared to the surrounding soft tissues (fig. S11B). In addition, immunohistochemistry confirmed that YAP and OPN expression was upregulated in ossified tissues of patients with OPLL (fig. S11C). Although the precise cause for OPLL is unclear, our data suggest that increased YAP and SHH activities may be critical molecular mechanisms underlying HO in patients with OPLL. Taken together, we show that YAP and SHH activation is a common molecular mechanism whereby HO is induced and expanded in both genetic and acquired HO regardless of ossification mechanisms. Inhibition of HO by a YAP inhibitor or SHH antibody may be a therapeutic strategy for HO.
Discussion
Here we demonstrate that a self-amplifying and self-propagating loop for YAP activation and SHH ectopic expression is a core mechanism underlying bone formation and expansion in HO. This positive feedback loop and the resulting continuous osteoblast differentiation of wild-type mesenchymal progenitor cells is a shared mechanism driving HO progression in mouse models of two distinct genetic HOs and injury-induced, common and non-hereditary HO. YAP and HH signaling are also upregulated in human HO, therefore our findings hold promise for strategies to effectively treat human HO, which remains an unmet medical challenge. Our findings indicate that a small number of abnormally differentiating osteoblast cells can lead to extensive HO in normal soft tissues due to genetic mutations or injury. Based on this discovery, we have identified therapeutic approaches that not only prevented HO but also blocked or reduced expansion of established HO in mouse models.
Human patients with POH, which is caused by paternally inherited heterozygous inactivating GNAS mutations (8, 70), develop subcutaneous HO with a mosaic pattern in their childhood. Heterozygous inactivating GNAS mutations also cause mild HO in Albright's hereditary osteodystrophy (AHO); the two disorders share a common genetic basis (8, 70). In the more extreme cases, non-penetrance (the complete failure of disease expression in some carriers of a gene mutation) in two families with POH have been reported (70). Phenotypic expression of genetic disorders caused by single-gene mutations can be highly variable, particularly in autosomal dominant conditions. Such variability can be attributed to a complex set of genetic, epigenetic and environmental regulators and it is well known that GNAS expression is regulated by genomic imprinting. Our findings further suggest that difference in YAP and SHH signaling in human individuals may also contribute to phenotypic variation of POH and other forms of HO. The mosaic distribution of POH lesions could also be a form of phenotypic variance within the same individual, such that weak HH signaling activation in POH patient cells provides a foundation for modifications that either promote YAP/HH signaling above-threshold activity or maintain normal tissue homeostasis. The “seed” cells with higher YAP/HH activities may employ the mechanism we have identified to recruit surrounding phenotypically wild type cells with initially weaker HH signaling to expand the HO lesion.
Unlike human patients, mouse disease models can be made in the same genetic background and raised in the same environment, reducing phenotypic variance. In Gnas+/− mutant mice (30, 31), subcutaneous ossification is found by 1 year of age and resembles that seen in patients with AHO. We found that only homozygous Gnas deletion caused HO in young mice (20). The discrepancy between early HO phenotypes in POH patients and lack of early HO onset in Gnas+/− mice is likely due to the difference between mouse and human life spans, as slightly upregulated HH signaling in the Gnas+/− mutant cells must persist for a certain amount of time to reach threshold strength required for osteoblast differentiation. This time (12 months after birth) is short relative to human development, but much longer relative to mouse lifespan.
YAP is a critical transcription factor in the Hippo signaling pathway that regulates a diverse array of fundamental cellular activities in development and disease. Gαs can control YAP activities cell autonomously in basal cell carcinomas (BCC), Schwann cell proliferation and myelination, and intraductal papillary mucinous neoplasms (IPMNs) (41, 71). In a BCC mouse model, loss of Gnas in the epidermal stem cell compartment activated YAP and GLI transcriptional activities cell autonomously (41). However, while HH signaling is sufficient to activate YAP, YAP loss does not dampen HH or WNT signaling activities, indicating that YAP acts strictly downstream of HH and WNT signaling in BCC (48). Importantly, our results show that YAP does not act independently of HH signaling in the HO models. Instead, it acts both downstream and upstream of HH signaling activation in SMPs. Furthermore, abnormal bone expansion and frequent recurrence after surgical removal of HO bear certain similarities to rapid tumor growth and recurrence. Given the pro-tumor-growth roles of YAP and HH signaling activation, it will be interesting to determine whether a similar regulatory positive feedback loop regulates the rapid growth of certain solid tumors.
YAP serves as a co-regulator for phosphor-smad1/5/8, RUNX2, and β-catenin (72-74), all of which promote osteoblast differentiation. However, HO in soft tissues, but not normal bone formation, was inhibited by YAP genetic removal or pharmacological inhibition, indicating that regulation of osteoblast differentiation in adult soft tissues is not entirely the same as that in normal bone development or homeostasis. This notion is further supported by the surprisingly critical function of Shh in HO. Previously, Shh was only found to be required for early embryonic growth and patterning, but not bone formation. Ihh was also upregulated by Gαs loss, but not as robustly as Shh. In the FOP models, however, Ihh expression was stronger, similar to Shh expression, possibly due to the presence of cartilage and Ihh is normally expressed in the growth plate chondrocytes to induce osteoblast differentiation (75). The self-amplifying loop of secreted HH production and YAP activation suggest that loss of Shh or Ihh will reduce the expression of each other by reducing YAP activation. This explains why Shh loss blocked HO in distinct models. In addition, WNT signaling was upregulated in Gnas−/− cells. However, YAP and SHH are preferable therapeutic targets as they are not required for orthotopic skeletal development.
In comparing YAP and SHH, we found that SHH inhibition was less potent in blocking HO, likely due to the redundant functions of IHH and the cell autonomous role of Gαs in suppressing GLI transcription activities independently of HH ligands (20). The important roles YAP and SHH play in homeostatic processes suggest that preventing or reducing HO pharmacologically by inhibiting YAP or SHH may potentially cause undesired side effects such as delayed injury repair because HO is often associated with trauma. In addition, although we did not observe gross negative effects in maintaining normal bone, body weight and hair with the doses given to mice for as long as three months of treatment, it is possible that in humans similar inhibition may be less tolerated. In addition, treatment with YAP inhibitor VP led to reduction of formed HO and a short duration of treatment appeared to offer long-term protection against HO expansion in this study; However, the beneficial effects may not apply to mature HO, which has been considered difficult to reverse.
The YAP-SHH self-amplifying loop first found in POH mouse models also operates in FOP and ATP mouse models. In addition, TAZ promotes HO in trauma-induced HO (76). Thus, YAP/TAZ, transcription effectors of mechanical stress, appear to be common drivers for osteoblast differentiation in different forms of HO. Tendon is generally subject to stronger mechanical forces and this may explain why models of FOP and trauma-induced HO are mostly focused on tendon. It is notable that human non-hereditary HO samples from OPLL patients exhibited increased YAP expression. OPLL may result from aging, chronic trauma, overload and systemic conditions (77, 78). Our findings suggest that activation of YAP and SHH may be core events in HO pathogenesis and that inhibition of YAP and SHH may also inhibit human HO.
Materials and Methods
Study design.
The objective of this study was to investigate the underlying cellular and molecular mechanism whereby HO lesions expand progressively in genetic diseases or injury in mouse models and to develop optimal therapies to treat diverse forms of HO. The involvement of wild type mesenchymal cell recruitment into HO lesions was demonstrated by genetic lineage tracing in vivo and the roles of secreted SHH and YAP activation in HO were investigated both in genetic mutants and by treatment with pharmacological reagents that inhibit SHH or YAP. For in vivo studies, HO detected by μCT scanning in Gnas or Acvr1 mutant mice or mice that underwent ATP procedure was considered a primary end point, with motion impact by expanded HO at a later time point considered secondary end points. All mice were randomly grouped into mutant or treatment and control groups, and all μCT, histologic and molecular measurements were performed in a blinded manner with respect to genotypes and treatment. Multiple separate operators at two separate sites (Harvard School of Dental Medicine and Brigham Women’s Hospital) performed drug treatment and μCT measurements with different μCT machines for independent replication. Cohort sizes for in vivo experiments testing interventions (for example, HO measured volumetrically by μCT) were determined based on an average variability of about 25% observed in previous animal cohorts or experimental groups, and a hypothesized change of ½- or 2-fold due to the intervention. With these assumptions, sample sizes for sample group or cohorts of 4 would be 95% powered to detect this difference with an α of 0.05, while sample sizes with sample group or cohorts of 3 would be 90% powered to detect these differences with an α of 0.05. Thus, for each of the animal studies measuring HO volume as an endpoint, experimental arms were designed with an n of 4 or 5 in critical experiments, and a minimum n of 3 in replication studies. Because the genetic or pharmacological intervention in the experiments did not cause detectible general health or survival issue, we included all animals without exclusion. Histologic data were obtained by cryo-sectioning 4% paraformaldehyde-fixed HO or control tissues after Ad-Cre injection. A minimum of 20 sections from each animal subject were stained and at least 4-6 stained sections were photographed and scored in a blinded fashion by two independent observers. Histologic measurements and qRT-PCR-based gene expression analyses were presented as the mean and SD of all animals in each group and reported without exclusion. For in vitro studies, we hypothesized that loss of Gnas or other genetic alterations or drug treatment would affect a given readout by a minimum of 50% or 2-fold, with a percent of variation of 15 to 30%. Experimental groups consisting of three biological replicates would be 95% powered to this difference. Thus, in vitro studies were designed to include groups of 3 to 4 biological replicates at the outset of each experiment. In experiments that measured data from cells cultured in multiwell plates, we did not observe obvious variability between samples and all measurements were included in the analyses. Raw data are provided in data files S1 and S2. All mouse experiments were approved by the Institutional Animal Care and Use Committee at the Harvard Medical School or Brigham and Women’s Hospital. All human samples and clinical data were obtained with informed consent under an approved protocol with oversight from the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University.
Mice.
All mouse experiments were approved by the Institutional Animal Care and Use Committee at the Harvard Medical School or Brigham and Women’s Hospital. Both male (M) and female (F) mice were included in our studies and we did not see gender difference in HO. Control and experimental groups of mice, of equal male and female gender, were housed four per cage, fed a diet of normal chow and water, and exposed to light for 12 hours daily. The weights of the control and experimental mice are comparable. All mice are described in published literature: Gnasf/f (20), tdTMTtg/+ (Rosa26-TdTomato, Jackson stock# 007909) (32), Yapf/f (42), Yaptg/+; rtTAtg/+(47), Shhf/f (36), ScxCre (63), Ptch1LacZ (68), Ctgf-GFP (47), Col1a12.3-GFP(33), ACVR1Q207D/+(60), and ACVR1R206H/+(ACVR1FlExR206H/+)(63). For the ATP mouse model, 4-week-old male and female mice were anesthetized, and a 27-gauge needle punctured the Achilles tendon body from the lateral aspect, percutaneously. This process was repeated 6 times at different parts of Achilles tendon body for each mouse. For sham operations, the needle was punctured through the skin without touching the Achilles tendon. The floxed constitutively active ALK2 (caALK2) mice ACVR1Q207D/+ were provided by Dr. Yuji Mishina at University of Michigan. To induce expression of caALK2, adenoviral-Cre (from Berk; ~0.5x109 pfu/mouse) and cobra venom factor (EMD/Millipore, 233552; 0.03 μg per mouse) were injected into the limbs of male and female mice at 4 weeks of age (66). Six weeks later, limbs were collected and analyzed. The ACVR1FlExR206H mice conditionally express ACVR1R206H knock-in alleles, were kindly provided by Dr. Aris Economides (Regeneron Pharmaceuticals, Inc.), mated with ScxCre mice, to yield ScxCre; ACVR1FlExR206H/+ mice, which represent a FOP mouse model that develops spontaneous HO of tendons, ligaments, and joints (63). These mice were treated with verteporfin (VP) (4 mg/kg/d intraperitoneal (IP) injection, 5 days per week) or an equal volume of PBS for 8 weeks, starting at 6 weeks of age. In the POH model, VP (200μg/ml) or DMSO (vehicle control) were applied topically 5 days per week for 6 weeks starting at 4 weeks of age to the surface of the legs (hair has been shaved) of the Gnas mutant groups immediately after Ad-Cre injection. In another set of experiments, IP injection of the Gnas mutant mice 5 days a week for 6 weeks after Ad-Cre injection with VP (2.5 mg/kg) and THZ1 (10 mg/kg) starting from 4 weeks of age. CT7001 (10 mg/kg) was gavaged every other day immediately after Ad-Cre injection at 4 weeks of age. Equal volume of PBS was IP injected or gavaged as control.
SMP isolation and culture in osteogenic media.
Subcutaneous mesenchymal progenitor (SMP) cells are potential osteoprogenitor cells within the subcutaneous connective tissue which can be induced to become osteoblast cells and adipocytes (79-81). SMPs have been used to performed in vitro experiments in our previous studies (20). Mouse adipose deposits were removed under sterile conditions and washed in PBS supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin on ice. Tissue was minced and digested with 1 mg/ml collagenase type I and 0.5%Trypsin in 0.1% BSA, for 70 minutes at 37 °C. Digested tissue was centrifuged at 500 g for 10 min and the pellet was carefully collected after aspirating off the floating fat depots. After a second centrifugation at 500 g for 10 min, the cellular pellet was filtered through a 100-μm mesh filter to remove debris.
Immunohistochemistry.
Tissues were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) and processed for cryostat section according to standard protocol. The sections were blocked in 10% donkey serum and 0.1% Triton X-100 in PBS and immunohistochemistry was performed with primary antibodies and corresponding secondary antibodies. The sections were mounted in mounting medium with DAPI (Sigma, F6057). All the antibodies are listed in table S1.
Human subjects.
The study was approved by Ethics Committee of First Affiliated Hospital of Sun Yat-sen University. Written informed consent was obtained from all subjects. HO was identified radiographically from 28 patients (19 male and 9 female, previously healthy, nonsmoking individuals; age ranging from 39 to 79 years) who were diagnosed with ossification in the posterior longitudinal ligament, detailed information were shown in table S2. A region 5 mm away from the edge of the ossified area was defined as the para-ossified area and was used for RNA extraction. Sequences of qRT-PCR primers, ChIP-qPCR primers, and sgRNAs are included in table S3.
Statistical analysis.
Quantifications were done from at least three independent experimental groups. Statistical analysis between groups was performed by two-tailed Student’s t test to determine significance when only two groups were compared. One-way ANOVA with Tukey’s post-hoc tests were used to compare differences between multiple groups. P < 0.05 was considered statistically significant. Error bars on all graphs are presented as the SD of the mean unless otherwise indicated.
Supplementary Material
Acknowledgement
We thank the Yang lab members for stimulating discussions. We are grateful to Dr. Lee Weinstein (National Institute of Diabetes and Digestive and Kidney Diseases) for providing the Gnasf/f mice; Dr. Yuji Mishina (University of Michigan) for the floxed constitutively active ALK2 (caALK2) mice ACVR1Q207D/+ and Dr. Aris Economides (Regeneron Pharmaceuticals, Inc.) for the ACVR1FlExR206H mice that conditionally express a “knock in” allele of ACVR1R206H.
Funding:
The work in the Yang lab is supported by National Institutes of Health grants from National Institute of Dental and Craniofacial Research (DE025866), National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR070877) and National Cancer Institute (CA222571) and a pilot grant from Million Dollar Bike Ride grant program of the Orphan disease center at the University of Pennsylvania (MDBR-18-115-FD/MAS) to Y.Y. The work in the Yu lab is supported by NIH grants is supported by NIH grants from NIAMS (AR057374, R01-AR057374S1, and UG3-TR002617) and an International Fibrodysplasia Ossificans Progressiva Research Award to P.B.Y. T.Z. and R.X. were supported by visiting graduate student fellowships from the China Scholarship Council of the Chinese Ministry of Education. B.G. was supported by a visiting graduate student fellowships from Sun Yat-sen University. Z.L. was supported by the National Natural Science Foundation of China (NO.81772293).
Footnotes
Competing interests: Y.Y. has filed a U.S. Patent Application No.: 62/949,534 entitled “Methods and compositions for treating heterotopic ossification”. P.B.Y. is a co-founder and holds stock in Keros Therapeutics, which develops therapies for hematologic and musculoskeletal diseases targeting BMP and TGFβ signaling pathways. P.B.Y.’s interests are reviewed and managed by Brigham and Women’s Hospital in accordance with their conflict-of-interest policies. The remaining authors declare no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials. The raw and processed RNA seq and ATAC seq data have been uploaded to GEO database with the number GSE161889 and GSE161962, respectively.
References
- 1.Rigaux P, Benabid N, Darriet D, Delecourt C, Chieux V, Dudermel AF, Sutter B, Anselme K, Hardouin P, Study of serum factors potentially involved in the pathogenesis of heterotopic bone formation after severe brain injury. Joint Bone Spine 72, 146–149 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Shore EM, Kaplan FS, Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol 6, 518–527 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS, Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med 335, 555–561 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Kaplan FS, Hahn GV, Zasloff MA, Heterotopic Ossification: Two Rare Forms and What They Can Teach Us. J Am Acad Orthop Surg 2, 288–296 (1994). [DOI] [PubMed] [Google Scholar]
- 5.Xu R, Hu J, Zhou X, Yang Y, Heterotopic ossification: Mechanistic insights and clinical challenges. Bone 109, 134–142 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Chakkalakal SA, Shore EM, Heterotopic Ossification in Mouse Models of Fibrodysplasia Ossificans Progressiva. Methods Mol Biol 1891, 247–255 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS, A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet 38, 525–527 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Eddy MC, Jan De Beur SM, Yandow SM, McAlister WH, Shore EM, Kaplan FS, Whyte MP, Levine MA, Deficiency of the alpha-subunit of the stimulatory G protein and severe extraskeletal ossification. J Bone Miner Res 15, 2074–2083 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Adegbite NS, Xu M, Kaplan FS, Shore EM, Pignolo RJ, Diagnostic and mutational spectrum of progressive osseous heteroplasia (POH) and other forms of GNAS-based heterotopic ossification. Am J Med Genet A 146A, 1788–1796 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shore EM, Ahn J, Jan de Beur S, Li M, Xu M, Gardner RJ, Zasloff MA, Whyte MP, Levine MA, Kaplan FS, Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. N Engl J Med 346, 99–106 (2002). [DOI] [PubMed] [Google Scholar]
- 11.McCarthy EF, Sundaram M, Heterotopic ossification: a review. Skeletal Radiol 34, 609–619 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA, Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am 91, 1084–1091 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Neal B, Gray H, MacMahon S, Dunn L, Incidence of heterotopic bone formation after major hip surgery. ANZ J Surg 72, 808–821 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski D, Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg 14, S191–197 (2006). [DOI] [PubMed] [Google Scholar]
- 15.van Kuijk AA, Geurts AC, van Kuppevelt HJ, Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord 40, 313–326 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Forsberg JA, Potter BK, Heterotopic ossification in wartime wounds. J Surg Orthop Adv 19, 54–61 (2010). [PubMed] [Google Scholar]
- 17.Potter BK, Forsberg JA, Davis TA, Evans KN, Hawksworth JS, Tadaki D, Brown TS, Crane NJ, Burns TC, O'Brien FP, Elster EA, Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am 92 Suppl 2, 74–89 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Ahrengart L, Periarticular heterotopic ossification after total hip arthroplasty. Risk factors and consequences. Clin Orthop Relat Res, 49–58 (1991). [PubMed] [Google Scholar]
- 19.Mavrogenis AF, Soucacos PN, Papagelopoulos PJ, Heterotopic ossification revisited. Orthopedics 34, 177 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Regard JB, Malhotra D, Gvozdenovic-Jeremic J, Josey M, Chen M, Weinstein LS, Lu J, Shore EM, Kaplan FS, Yang Y, Activation of Hedgehog signaling by loss of GNAS causes heterotopic ossification. Nat Med 19, 1505–1512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He X, Zhang L, Chen Y, Remke M, Shih D, Lu F, Wang H, Deng Y, Yu Y, Xia Y, Wu X, Ramaswamy V, Hu T, Wang F, Zhou W, Burns DK, Kim SH, Kool M, Pfister SM, Weinstein LS, Pomeroy SL, Gilbertson RJ, Rubin JB, Hou Y, Wechsler-Reya R, Taylor MD, Lu QR, The G protein alpha subunit Galphas is a tumor suppressor in Sonic hedgehog-driven medulloblastoma. Nat Med 20, 1035–1042 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingham PW, McMahon AP, Hedgehog signaling in animal development: paradigms and principles. Genes Dev 15, 3059–3087 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP, Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75, 1417–1430 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Riddle RD, Johnson RL, Laufer E, Tabin C, Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75, 1401–1416 (1993). [DOI] [PubMed] [Google Scholar]
- 25.Zhu J, Nakamura E, Nguyen MT, Bao X, Akiyama H, Mackem S, Uncoupling Sonic hedgehog control of pattern and expansion of the developing limb bud. Dev Cell 14, 624–632 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kronenberg HM, Developmental regulation of the growth plate. Nature 423, 332–336 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Zheng Y, Pan D, The Hippo Signaling Pathway in Development and Disease. Dev Cell 50, 264–282 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng Z, Moroishi T, Guan KL, Mechanisms of Hippo pathway regulation. Genes Dev 30, 1–17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M, Gavrilova O, Zhao WQ, Nguyen A, Lorenzo J, Shen L, Nackers L, Pack S, Jou W, Weinstein LS, Increased glucose tolerance and reduced adiposity in the absence of fasting hypoglycemia in mice with liver-specific Gs alpha deficiency. J Clin Invest 115, 3217–3227 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huso DL, Edie S, Levine MA, Schwindinger W, Wang Y, Juppner H, Germain-Lee EL, Heterotopic ossifications in a mouse model of albright hereditary osteodystrophy. PloS one 6, e21755 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheeseman MT, Vowell K, Hough TA, Jones L, Pathak P, Tyrer HE, Kelly M, Cox R, Warren MV, Peters J, A mouse model for osseous heteroplasia. PLoS One 7, e51835 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H, A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D, Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. J Bone Miner Res 17, 15–25 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Lees-Shepard JB, Nicholas SE, Stoessel SJ, Devarakonda PM, Schneider MJ, Yamamoto M, Goldhamer DJ, Palovarotene reduces heterotopic ossification in juvenile FOP mice but exhibits pronounced skeletal toxicity. Elife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A, Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci U S A 101, 12561–12566 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP, Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell 105, 599–612 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y, Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev 18, 2404–2417 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan D, The hippo signaling pathway in development and cancer. Dev Cell 19, 491–505 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barron DA, Kagey JD, The role of the Hippo pathway in human disease and tumorigenesis. Clin Transl Med 3, 25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo JS, Park HW, Guan KL, The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 15, 642–656 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, Chen M, Weinstein LS, Taylor SS, Molinolo AA, Gutkind JS, Inactivation of a Galpha(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol 17, 793–803 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S, Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol 17, 1218–1227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giraud J, Molina-Castro S, Seeneevassen L, Sifre E, Izotte J, Tiffon C, Staedel C, Boeuf H, Fernandez S, Barthelemy P, Megraud F, Lehours P, Dubus P, Varon C, Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int J Cancer, (2019). [DOI] [PubMed] [Google Scholar]
- 44.Cho YS, Li S, Wang X, Zhu J, Zhuo S, Han Y, Yue T, Yang Y, Jiang J, CDK7 regulates organ size and tumor growth by safeguarding the Hippo pathway effector Yki/Yap/Taz in the nucleus. Genes Dev, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwiatkowski N, Zhang T, Rahl PB, Abraham BJ, Reddy J, Ficarro SB, Dastur A, Amzallag A, Ramaswamy S, Tesar B, Jenkins CE, Hannett NM, McMillin D, Sanda T, Sim T, Kim ND, Look T, Mitsiades CS, Weng AP, Brown JR, Benes CH, Marto JA, Young RA, Gray NS, Targeting transcription regulation in cancer with a covalent CDK7 inhibitor. Nature 511, 616–620 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel H, Periyasamy M, Sava GP, Bondke A, Slafer BW, Kroll SHB, Barbazanges M, Starkey R, Ottaviani S, Harrod A, Aboagye EO, Buluwela L, Fuchter MJ, Barrett AGM, Coombes RC, Ali S, ICEC0942, an Orally Bioavailable Selective Inhibitor of CDK7 for Cancer Treatment. Mol Cancer Ther 17, 1156–1166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD, Hippo pathway activity influences liver cell fate. Cell 157, 1324–1338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maglic D, Schlegelmilch K, Dost AF, Panero R, Dill MT, Calogero RA, Camargo FD, YAP-TEAD signaling promotes basal cell carcinoma development via a c-JUN/AP1 axis. EMBO J 37, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK, Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38, 576–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X, Li H, Rajurkar M, Li Q, Cotton JL, Ou J, Zhu LJ, Goel HL, Mercurio AM, Park JS, Davis RJ, Mao J, Tead and AP1 Coordinate Transcription and Motility. Cell Rep 14, 1169–1180 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, Goode D, Elgar G, Hill RE, de Graaff E, A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet 12, 1725–1735 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Sagai T, Hosoya M, Mizushina Y, Tamura M, Shiroishi T, Elimination of a long-range cis-regulatory module causes complete loss of limb-specific Shh expression and truncation of the mouse limb. Development 132, 797–803 (2005). [DOI] [PubMed] [Google Scholar]
- 53.Potuijt JWP, Baas M, Sukenik-Halevy R, Douben H, Nguyen P, Venter DJ, Gallagher R, Swagemakers SM, Hovius SER, van Nieuwenhoven CA, Galjaard RH, van der Spek PJ, Ahituv N, de Klein A, A point mutation in the pre-ZRS disrupts sonic hedgehog expression in the limb bud and results in triphalangeal thumb-polysyndactyly syndrome. Genet Med 20, 1405–1413 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Yeo NC, Chavez A, Lance-Byrne A, Chan Y, Menn D, Milanova D, Kuo CC, Guo X, Sharma S, Tung A, Cecchi RJ, Tuttle M, Pradhan S, Lim ET, Davidsohn N, Ebrahimkhani MR, Collins JJ, Lewis NE, Kiani S, Church GM, An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat Methods 15, 611–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thakore PI, D'Ippolito AM, Song L, Safi A, Shivakumar NK, Kabadi AM, Reddy TE, Crawford GE, Gersbach CA, Highly specific epigenome editing by CRISPR-Cas9 repressors for silencing of distal regulatory elements. Nat Methods 12, 1143–1149 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodda SJ, McMahon AP, Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133, 3231–3244 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Xu R, Khan SK, Zhou T, Gao B, Zhou Y, Zhou X, Yang Y, Galphas signaling controls intramembranous ossification during cranial bone development by regulating both Hedgehog and Wnt/beta-catenin signaling. Bone Res 6, 33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, Das N, Makhoul G, Chernomorsky R, D'Ambrosio D, Corpina RA, Schoenherr CJ, Feeley K, Yu PB, Yancopoulos GD, Murphy AJ, Economides AN, ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med 7, 303ra137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hino K, Ikeya M, Horigome K, Matsumoto Y, Ebise H, Nishio M, Sekiguchi K, Shibata M, Nagata S, Matsuda S, Toguchida J, Neofunction of ACVR1 in fibrodysplasia ossificans progressiva. Proc Natl Acad Sci U S A 112, 15438–15443 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, Yamada M, Mishina Y, Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis 44, 159–167 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD, BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med 14, 1363–1369 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bagarova J, Vonner AJ, Armstrong KA, Borgermann J, Lai CS, Deng DY, Beppu H, Alfano I, Filippakopoulos P, Morrell NW, Bullock AN, Knaus P, Mishina Y, Yu PB, Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol 33, 2413–2424 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dey D, Bagarova J, Hatsell SJ, Armstrong KA, Huang L, Ermann J, Vonner AJ, Shen Y, Mohedas AH, Lee A, Eekhoff EM, van Schie A, Demay MB, Keller C, Wagers AJ, Economides AN, Yu PB, Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci Transl Med 8, 366ra163 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agarwal S, Loder SJ, Cholok D, Peterson J, Li J, Breuler C, Cameron Brownley R, Hsin Sung H, Chung MT, Kamiya N, Li S, Zhao B, Kaartinen V, Davis TA, Qureshi AT, Schipani E, Mishina Y, Levi B, Scleraxis-Lineage Cells Contribute to Ectopic Bone Formation in Muscle and Tendon. Stem Cells 35, 705–710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lees-Shepard JB, Yamamoto M, Biswas AA, Stoessel SJ, Nicholas SE, Cogswell CA, Devarakonda PM, Schneider MJ Jr., Cummins SM, Legendre NP, Yamamoto S, Kaartinen V, Hunter JW, Goldhamer DJ, Activin-dependent signaling in fibro/adipogenic progenitors causes fibrodysplasia ossificans progressiva. Nature communications 9, 471 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, Li F, Xie L, Crane J, Zhen G, Mishina Y, Deng R, Gao B, Chen H, Liu S, Yang P, Gao M, Tu M, Wang Y, Wan M, Fan C, Cao X, Inhibition of overactive TGF-beta attenuates progression of heterotopic ossification in mice. Nature communications 9, 551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chakkalakal SA, Zhang D, Culbert AL, Convente MR, Caron RJ, Wright AC, Maidment AD, Kaplan FS, Shore EM, An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res 27, 1746–1756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodrich LV, Milenkovic L, Higgins KM, Scott MP, Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997). [DOI] [PubMed] [Google Scholar]
- 69.Yan L, Gao R, Liu Y, He B, Lv S, Hao D, The Pathogenesis of Ossification of the Posterior Longitudinal Ligament. Aging Dis 8, 570–582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shore EM, Ahn J, Jan de Beur S, Li M, Xu M, Gardner RJ, Zasloff MA, Whyte MP, Levine MA, Kaplan FS, Paternally inherited inactivating mutations of the GNAS1 gene in progressive osseous heteroplasia. The New England journal of medicine 346, 99–106 (2002). [DOI] [PubMed] [Google Scholar]
- 71.Ideno N, Yamaguchi H, Ghosh B, Gupta S, Okumura T, Steffen DJ, Fisher CG, Wood LD, Singhi AD, Nakamura M, Gutkind JS, Maitra A, GNAS(R201C) Induces Pancreatic Cystic Neoplasms in Mice That Express Activated KRAS by Inhibiting YAP1 Signaling. Gastroenterology 155, 1593–1607 e1512 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang Z, Hu J, Pan J, Wang Y, Hu G, Zhou J, Mei L, Xiong WC, YAP stabilizes SMAD1 and promotes BMP2-induced neocortical astrocytic differentiation. Development 143, 2398–2409 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaidi SK, Sullivan AJ, Medina R, Ito Y, van Wijnen AJ, Stein JL, Lian JB, Stein GS, Tyrosine phosphorylation controls Runx2-mediated subnuclear targeting of YAP to repress transcription. EMBO J 23, 790–799 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, Fassina A, Cordenonsi M, Piccolo S, YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell 158, 157–170 (2014). [DOI] [PubMed] [Google Scholar]
- 75.St-Jacques B, Hammerschmidt M, McMahon AP, Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13, 2072–2086. (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huber AK, Patel N, Pagani CA, Marini S, Padmanabhan KR, Matera DL, Said M, Hwang C, Hsu GC, Poli AA, Strong AL, Visser ND, Greenstein JA, Nelson R, Li S, Longaker MT, Tang Y, Weiss SJ, Baker BM, James AW, Levi B, Immobilization after injury alters extracellular matrix and stem cell fate. J Clin Invest 130, 5444–5460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chazal J, Tanguy A, Bourges M, Gaurel G, Escande G, Guillot M, Vanneuville G, Biomechanical properties of spinal ligaments and a histological study of the supraspinal ligament in traction. J Biomech 18, 167–176 (1985). [DOI] [PubMed] [Google Scholar]
- 78.Luo J, Wei X, Li JJ, [Clinical significance of nuchal ligament calcification and the discussion on biomechanics]. Zhongguo Gu Shang 23, 305–307 (2010). [PubMed] [Google Scholar]
- 79.Ritter A, Friemel A, Roth S, Kreis NN, Hoock SC, Safdar BK, Fischer K, Mollmann C, Solbach C, Louwen F, Yuan J, Subcutaneous and Visceral Adipose-Derived Mesenchymal Stem Cells: Commonality and Diversity. Cells 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frazier TP, Gimble JM, Devay JW, Tucker HA, Chiu ES, Rowan BG, Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue-derived stem cells. BMC Cell Biol 14, 34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Russo V, Yu C, Belliveau P, Hamilton A, Flynn LE, Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl Med 3, 206–217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.