Abstract
Toxoplasma gondii is an apicomplexan parasite that affects both humans and livestock. Transmitted to humans through ingestion, it is the second-leading cause of foodborne illness-related death. Currently, there exists no approved vaccine for humans or most livestock against the parasite. DNA vaccines, a type of subunit vaccine which uses segments of the pathogen's DNA to generate immunity, have shown varying degrees of experimental efficacy against infection caused by the parasite. This review compiles DNA vaccine efforts against Toxoplasma gondii, segmenting the analysis by parasite antigen, as well as a review of concomitant adjuvant usage. No single antigenic group was consistently more effective within in vivo trials relative to others.
Keywords: Toxoplasma gondii, DNA vaccines, Adjuvants, Antigens
Toxoplasma gondii is an obligate intracellular parasite of the Apicomplexan phylum that can infect any nucleated mammalian cell and is carried by at least one-quarter of the world's human population. While chronic infection of healthy individuals is generally benign, transient cervical lymphadenopathy may occur during the initial acute phase of infection. However, infection of immunocompromised patients can cause pneumonia, encephalitis, and even death (Demar et al., 2007). Acute infection of pregnant women during or shortly before gestation may also cause neonatal death or fetal abnormalities due to transmission to the fetus (Assir et al., 2014). Once establishing a chronic infection, the parasite can be found in various solid organs, particularly muscle tissue and the brain (Dunay et al., 2018). Overall in the United States, it is estimated that T. gondii is the second leading cause of death due to foodborne illness and has an economic impact of over $3 billion each year (Scallan et al., 2011; Hoffmann et al., 2012).
Toxoplasma gondii has 3 stages to its life cycle: oocysts, tachyzoites, and bradyzoites. The oocyst stage is the only portion of the life cycle that is produced by sexual reproduction and originates in the feline intestine. Toxoplasma can be acquired by humans in several ways. Exposure to cat feces in food or water allows for the ingestion of oocysts, and eating undercooked mammalian flesh allows for the acquisition of Toxoplasma tissue cysts. Once inside the host, ingested parasites differentiate into tachyzoites, which define the acute stage of infection, leading to rapid replication in host tissue. Pressure from the host's immune system ultimately forces the parasites into the latent bradyzoite form, which results in intracellular cyst formation and characterizes chronic infection (Black and Boothroyd, 2000). The bradyzoite stage can be reactivated (usually through immune suppression) and will redifferentiate into tachyzoites, thus restarting the cycle of intracellular spread (McConkey et al., 2013).
Control of Toxoplasma in humans is limited due to several factors. Toxoplasma gondii can cross the blood-brain barrier to establish persistent infection in the central nervous system, making it a challenge for drug therapy capable of clearing infection due to the general chemical inaccessibility of this compartment (Alday and Doggett, 2017). Treatment for symptomatic disease (often in the form of reactivated toxoplasmosis) usually consists of a combination of 2 antimicrobials. Currently, the most common regimens include a combination of pyrimethamine and sulfadiazine, or pyrimethamine coadministered with other drugs such as clindamycin, atovaquone, clarithromycin, or azithromycin (Neville et al., 2015). Currently, all drugs used for clinical applications are only effective against the acute tachyzoite stage of infection and are associated with side effects, which has driven impressive efforts in search of additional antiparasitic compounds (McFarland et al., 2016).
Due to the limited applicability of these treatments, control may be more fully obtained via preventative measures such as that offered by vaccination. Ideally, a Toxoplasma vaccine would be able to offer protection for humans and agricultural animals, the latter leading to reduced transmission to humans (Innes et al., 2019). Currently, there is 1 vaccine on the market against Toxoplasma, called Toxovax, which is a live attenuated vaccine specifically designed to reduce fetal abortion in sheep. It is not approved for use in humans, or in the United States, and offers limited protection and duration of efficacy (Liu et al., 2012). More recently, Toxovax has been shown to reduce tissue cysts in pigs (Burrells et al., 2015).
There are several different methods for creating protective vaccines including live attenuated vaccines, inactivated or killed vaccines, toxoid vaccines, and subunit vaccines. Live attenuated vaccines are created by weakening or altering the pathogen in a way that it is non-disease causing. The immunogenicity of these vaccines varies based on attenuation, and thus their efficacy is variable. Additionally, fears of the parasite reverting to a pathogenic strain make this type of vaccine approach less appealing. Killed or inactivated vaccines function by altering the pathogen by heating or using chemicals such as formalin to destroy the pathogen's ability to replicate, and thus the pathogen cannot revert to its more virulent state, making them safer than live attenuated vaccines. Due to the intracellular nature of infection of T. gondii, a toxoid vaccine, a vaccine made with an altered form of pathogen-essential toxin, would be less viable. Finally, subunit vaccines aim to expose the host immune system to a limited, often defined, portion of antigens derived from the pathogen. Subunit vaccines are effective against some diseases but often suffer from relatively low immunogenicity (Liu et al., 2012).
Attempts to develop experimental T. gondii vaccines for the last 30 yr have met limited success (Wang et al., 2019). Live-attenuated vaccines have been based on mutant strains such as strain S48 used in Toxovax. Other strains such as T-263 are used in live vaccine attempts in felines to suppress oocyst shedding, but live T-263 is difficult to produce and deliver large-scale (Verma and Khanna, 2013; Innes et al., 2019). Recent developments in genetic technology allow for new live vaccines based on gene editing, or the deletion of certain genes, which may produce a higher immune response in hosts. For example, there has been 1 live vaccine made using a CRISPR/Cas9 approach to delete a gene in an attempt to stop the fertilization of oocysts in felines that has proved to be completely effective (Ramakrishnan et al., 2019). However, a broader safety profile and multi-species efficacy still need to be established. Inactivated or killed vaccines have been attempted but with less efficacy than live vaccines, though they are considered safer because they cannot revert to a pathogenic strain (Innes et al., 2019). Subunit vaccines have been investigated in over 200 studies for a Toxoplasma vaccine with emphasis on antigens that affect T. gondii attachment to host cells and evasion of host immune response, mostly recombinant protein antigens. The main method of delivery in the past has usually involved live, nonpathogenic bacteria, viruses, and other microbes (Wang et al., 2019). Notably, using a major histocompatibility complex (MHC)-matched parasite peptide antigen (HF10), Blanchard et al. (2008) achieved complete protection from lethal infection by using antigen-pulsed bone marrow-derived dendritic cells. Recently, other delivery methods involving nanoparticles and exosomes have been explored for their ability to protect the antigen and thus produce a higher immune response and lower number of cysts (Beauvillain et al., 2009; Assolini et al., 2017). Major challenges to all vaccination attempts include the genetic diversity amongst different parasite strains and hosts, the multistage life cycle, the lack of knowledge about the transition from the acute stage to latent stage, and the absence of standard vaccination procedures across attempts (Wang et al., 2019).
DNA vaccines are an emerging type of subunit vaccines in which a portion of the pathogen's DNA is inserted into a bacterial vehicle vector and delivered to the host. Once the DNA has been injected into tissue, some cells will uptake the vector, which is then translocated to the nucleus. Injection of DNA vaccines may be done intramuscularly, intradermally, or subcutaneously. Next, the pathogen's DNA is transcribed and translated as if it were from the host. Following translation, the protein is presented to the immune system where it is recognized as foreign (Hobernik and Bros, 2018).
The use of DNA vaccines provides several advantages (Smith et al., 2020; Yu et al., 2020). DNA vaccines are easily adaptable to new pathogens, as there is little work required to change the specific antigens of the vaccine. Additionally, plasmid vectors are extremely malleable and can be readily modified. Because of the ability to select the specific antigens present in the vaccine, it can be specifically engineered to produce a tailored immune response, especially those suited for efficacy against intracellular pathogens such as T. gondii. The multiple stages in a parasite's complex life cycle have different proteins that evoke an immune response, thus the ability to tailor the immune response with specific antigens allows for more efficacy across stages (Ghaffarifar, 2015). Another distinct advantage is the ease of production and distribution. Propagation of the vaccine is very rapid and the cost to do so is low, even on a large scale. DNA vaccines also do not require cold-chain storage for stability, which allows for easy distribution. Finally, DNA vaccines have been considered safer than other vaccine options, as they usually require low dosage and are unable to revert to their virulent state (Shedlock and Weiner, 2000).
The efficacy of DNA vaccines is continuing to be explored in other parasites besides T. gondii. In Trypanosoma cruzi, which causes Chagas disease in humans, clinical trials that explored administration of a DNA vaccine to mice during an ongoing infection have shown that after just 2 doses there was a limitation of disease progression (Dumonteil, 2007). A DNA vaccine is also currently being developed for Leishmania major, the parasite associated with cutaneous leishmaniasis. The vaccine, named LEISHDNAVAX, contains several Leishmania protein antigens that have been shown to elicit T-cell responses in people of different genetic backgrounds and may move forward into clinical trials (Sacks et al., 2016). With these promising results demonstrated in other parasites, a DNA vaccine against T. gondii could also be efficacious.
Our aim in this review is to summarize recent Toxoplasma DNA vaccine attempts and provide a concise collection of outcomes aligned by antigen or adjuvant which could promote future vaccine investigations that lead to increased potency and efficacy against Toxoplasma gondii.
MATERIALS AND METHODS
Manuscripts were selected from PubMed and Toxoblog.com using the search term “Toxoplasma gondii DNA vaccine,” with papers selected that were dated 2010 and after. In total 95 unique papers were reviewed to create a matrix that summarized methods used in T. gondii DNA vaccine experiments (Tables I–III). Papers were searched for genes used (and if they were used in conjunction with other genes), and the sequence of the genes and which parasite strain they were from, as well as the type of plasmid backbone and the inclusion of adjuvants. Papers excluded in this review matrix included papers that did not perform a parasite challenge in a mouse model, papers that did not use a plasmid delivery system (such an adenovirus) (Li et al., 2015a, 2016; Yin et al., 2015), papers that used a DNA prime/boost method to enhance response (Rashid et al., 2011, 2017; Min et al., 2012; Meng et al., 2013), and papers that were missing too many of the key variables such as challenge method and dosage. Further, publications before 2010 are only briefly summarized and not included in the tables. ToxoDB.org datasets were used to evaluate the levels of transcriptional expression of antigens listed in Table I.
Table I.
Summary of challenge results from single antigens DNA vaccines against Toxoplasma gondii. A summary of results from publications since 2010 employing a single parasite antigen (generally full-length) delivered via DNA vaccination followed by challenge, organized by antigen class. Tz = tachyzoites.
| Gene |
Dosage (per injection) |
Acute challenge |
Acute result* |
Chronic challenge |
Chronic result† |
Prominent stage expression |
Reference |
| Microneme Proteins (MICs) | |||||||
| MIC1 | 10 μg | 80 ME49 cysts | 40–50% survival | 40 ME49 cysts | 52% reduced | Bz§, Tz, Sp | Pinzan et al., 2015 |
| MIC3 | 100 μg | 10,000 RH Tz | +8 days | 100 RH Tz | unknown | Bz, Tz§, Sp | Gong et al., 2016 |
| MIC3‡ | 100 μg | 10,000 RH Tz | 20% survival | None | N.D. | Bz, Tz§, Sp | Ghaffarifar et al., 2019 |
| MIC3 | 100 μg | 1000 RH Tz | +7 days | None | N.D. | Bz, Tz§, Sp | Qu et al., 2013 |
| MIC4 | 10 μg | 80 ME49 cysts | 40–50% survival | 40 ME49 cysts | 47% reduced | Bz, Tz§, Sp | Pinzan et al., 2015 |
| MIC6 | 10 μg | 80 ME49 cysts | 40–50% survival | 40 ME49 cysts | 27% reduced | Bz§, Tz, Sp | Pinzan et al., 2015 |
| MIC6 | 100 μg | 80 Pru cysts | +20 days | 20 Pru cysts | 40% reduced | Bz§, Tz, Sp | Yan et al., 2012 |
| MIC6 | 100 μg | 1,000 RH Tz | +10 days | 20 Pru cysts | 38% reduced | Bz§, Tz, Sp | Xu et al., 2019 |
| MIC8‡ | 100 μg | 1,000 RH Tz | +6 days | 20 Pru cysts | 25% reduced | Bz§, Tz, Sp | Li et al., 2014 |
| MIC8‡ | 100 μg | 80 Pru cysts | +11 days | None | N.D. | Bz§, Tz, Sp | Li et al., 2014 |
| MIC8 | 100 μg | 1,000 RH Tz | +6 days | None | N.D. | Bz§, Tz, Sp | Liu et al., 2010a |
| MIC11 | 100 μg | 1,000 RH Tz | 17% survival | None | N.D. | Bz, Tz§, Sp | Tao et al., 2013 |
| MIC13 | 100 μg | 1,000 RH Tz | +21 days | 10 Pru cysts | 57% reduced | Bz, Tz, Sp§ | Yuan et al., 2013 |
| Surface Antigens (SAGs) | |||||||
| SAG1‡ | 50 μg | 50 RH Tz | 40% survival | None | N.D. | Bz, Tz§, Sp | Liu et al., 2010b |
| SAG1 | 100 μg | 1,000 RH Tz | +7 days | None | N.D. | Bz, Tz§, Sp | Mavi et al., 2019 |
| SAG1‡ | 100 μg | 1,000 RH Tz | +2 days | None | N.D. | Bz, Tz§, Sp | Maraghi et al., 2019 |
| SAG1 | 50 μg per plasmid (total of 100 μg) | 10,000 RH Tz | +4 days | None | N.D. | Bz, Tz§, Sp | Hoseinian Khosroshahi et al., 2011 |
| SAG1 | 100 μg | 10,000 RH Tz | +6 days | None | N.D. | Bz, Tz§, Sp | Meng et al., 2012 |
| SAG1 | 100 μg | 10,000 RH Tz | +7 days | 20 Pru cysts | 37% reduced | Bz, Tz§, Sp | Han et al., 2017b |
| SAG1 | 100 μg | 10,000 RH Tz | +6 days | None | N.D. | Bz, Tz§, Sp | Zhou et al., 2012 |
| SAG1 | 100 μg | 100,000 RH Tz | +3 days | None | N.D. | Bz, Tz§, Sp | Wu et al., 2012 |
| SAG2C | 100 μg | None | N.D. | 20 Pru cysts | 72% reduced | Bz | Zhang et al., 2013a |
| SAG2D | 100 μg | None | N.D. | 20 Pru cysts | 23% reduced | Bz | Zhang et al., 2013a |
| SAG2X | 100 μg | None | N.D. | 20 Pru cysts | 70% reduced | Bz | Zhang et al., 2013a |
| SAG4 | 100 μg | 100,000 RH Tz | +7 days | 20 Pru cysts | 66% reduction | Bz§, Tz | Zhou and Wang, 2017 |
| SAG5a | 100 μg | None | N.D. | 20 Pru cysts | 35% reduced | Low expression | Lu et al., 2015 |
| SAG5b | 100 μg | 10,000 RH Tz | +7 days | 20 Pru cysts | 44% reduced | Low expression | Lu et al., 2017 |
| SAG5c | 100 μg | 10,000 RH Tz | +6 days | 20 Pru cysts | 41% reduced | Tz | Lu et al., 2017 |
| SAG5d‡ | 100 μg | 10,000 RH Tz | +7 days | None | N.D. | Tz | Lu et al., 2014 |
| Dense Granule Proteins (GRAs) | |||||||
| GRA1 | 100 μg | 10,000 RH Tz | +6 days | 100 RH Tz | unknown | Bz, Tz§, Sp | Gong et al., 2016 |
| GRA1 | 100 μg | 100,000 RH Tz | Not significant | None | N.D. | Bz, Tz§, Sp | Wu et al., 2012 |
| GRA2 | 100 μg | 1,000 RH Tz | +3 days | None | N.D. | Bz§, Tz, Sp | Ching et al., 2017 |
| GRA2 | 100 μg | 10,000 RH Tz | +4 days | None | N.D. | Bz§, Tz, Sp | Zhou et al., 2012 |
| GRA5 | 100 μg | 1,000 RH Tz | +3 days | None | N.D. | Bz, Tz§, Sp | Ching et al., 2017 |
| GRA6‡ | 100 μg | 1,000 RH Tz | 40% survival | None | N.D. | Bz, Tz§, Sp | Sun et al., 2011 |
| GRA7‡ | 50 μg per plasmid (total of 100 μg) | 1,500 ME49 cysts | +12 days | 20 ME49 cysts | 55% reduced | Bz§, Tz, Sp | Quan et al., 2012 |
| GRA7 | 100 μg | 1,000 RH Tz | +5 days | None | N.D. | Bz§, Tz, Sp | Mavi et al., 2019 |
| GRA7‡ | 100 μg | 1,000 RH Tz | +12 days | None | N.D. | Bz§, Tz, Sp | Liu et al., 2014 |
| GRA8 | 100 μg | 1,000 RH Tz | +4 days | None | N.D. | Bz, Tz§, Sp | Chu et al., 2018 |
| GRA14‡ | 100 μg | 100,000 RH Tz | +5 days | None | N.D. | Bz§, Tz, Sp | Ahmadpour et al., 2017b |
| GRA14‡ | 100 μg | 100,000 RH Tz | +5 days | None | N.D. | Bz§, Tz, Sp | Ahmadpour et al., 2017a |
| GRA16 | 100 μg | 1,000 RH Tz | +2 days | 10 Pru cysts | 44% reduced | Bz, Tz§ | Hu et al., 2017 |
| GRA17 | 100 μg | 1,000 RH Tz | +2 days | None | N.D. | Bz, Tz§ | Zhu et al., 2017 |
| GRA23 | 100 μg | 1,000 RH Tz | +4 days | None | N.D. | Bz, Tz§ | Zhu et al., 2017 |
| GRA24 | 100 μg | 100 RH Tz | +25 days | None | N.D. | Bz§, Tz, Sp | Zheng et al., 2019b |
| GRA24 | 100 μg | 1,000 RH Tz | +5 days | 20 Pru cysts | 29% reduced | Bz§, Tz, Sp | Xu et al., 2019 |
| GRA25 | 100 μg | 1,000 RH Tz | +7 days | 20 Pru cysts | 41% reduced | Bz, Tz§, Sp | Xu et al., 2019 |
| Rhoptry Proteins (ROPs) | |||||||
| ROP1‡ | 50 μg per plasmid (total of 100 μg) | 1,500 ME49 cysts | 20% survival | 20 ME49 cysts | 55% reduced | Bz, Tz§, Sp | Quan et al., 2012 |
| ROP1 | 100 μg | 1,000 RH Tz | +12 days | None | N.D. | Bz, Tz§, Sp | Sonaimuthu et al., 2016 |
| ROP2 | 50 μg per plasmid (total of 100 μg) | 10,000 RH Tz | +2 days | None | N.D. | Low expression | Hoseinian Khosroshahi et al., 2011 |
| ROP5 | 100 μg | 10,000 RH Tz | +4 days | 20 Pru cysts | 58% reduced | Bz, Tz§, Sp | Wang et al., 2016b |
| ROP5 | 100 μg | 1,000 RH Tz | +14 days | 6 Pru cysts | 52% reduced | Bz, Tz§, Sp | Zhu et al., 2020 |
| ROP7 | 100 μg | 10,000 RH Tz | +5 days | 20 Pru cysts | 58% reduced | Bz, Tz§, Sp | Wang et al., 2016b |
| ROP8 | 100 μg | 1,000 RH Tz | 50% survival | None | N.D. | Bz, Tz§, Sp | Parthasarathy et al., 2013 |
| ROP8 | 100 μg | 2,000 RH Tz | +2 days | None | N.D. | Bz, Tz§, Sp | Foroutan et al., 2020b |
| ROP8‡ | 100 μg | 2,000 RH Tz | +2 days | None | N.D. | Bz, Tz§, Sp | Foroutan et al., 2020b |
| ROP9 | 100 μg | 1,000 RH Tz | +12 days | None | N.D. | Bz§, Tz, Sp | Chen et al., 2014b |
| ROP13‡ | 100 μg | 1,000 RH Tz | +49 days | 10 Pru cysts | 40% reduced | Bz§, Tz, Sp | Wang et al., 2012 |
| ROP13 | 100 μg | 10,000 RH Tz | N.D. | N.D. | N.D. | Bz§, Tz, Sp | Alizadeh et al., 2019 |
| ROP16‡ | 100 μg | 1,000 RH Tz | +13 days | None | N.D. | Bz§, Tz, Sp | Liu et al., 2014a |
| ROP16 | 100 μg | 1,000 RH Tz | +24 days | None | N.D. | Bz§, Tz, Sp | Yuan et al., 2011a |
| ROP17 | 100 μg | 1,000 RH Tz | +14 days | None | N.D. | Bz§, Tz, Sp | Wang et al., 2016a |
| ROP18 | 100 μg | 80 Pru cysts | +12 days | 20 Pru cysts | 30% reduced | Bz, Tz§, Sp | Chen et al., 2018 |
| ROP18 | 100 μg | 1,000 RH Tz | +17 days | 6 Pru cysts | 47% reduced | Bz, Tz§, Sp | Zhu et al., 2020 |
| ROP18 | 100 μg | 1,000 RH Tz | +9 days | None | N.D. | Bz, Tz§, Sp | Qu et al., 2013 |
| ROP18 | 100 μg | 1,000 RH Tz | +48 days | None | N.D. | Bz, Tz§, Sp | Yuan et al., 2011b |
| ROP19 | 100 μg | None | N.D. | 20 Pru cysts | 57% reduced | Bz, Tz§ | Zhou et al., 2016 |
| ROP21 | 100 μg | 1,000 RH Tz | +7 days | 10 Pru cysts | 37% reduced | Bz§, Tz | Zhang et al., 2018d |
| ROP22 | 100 μg | 1,000 RH Tz | +8 days | 10 Pru cysts | 40% reduced | Low expression | Zhang et al., 2019 |
| ROP29‡ | 100 μg | None | N.D. | 20 Pru cysts | 53% reduced | Low expression | Lu et al., 2018 |
| ROP35 | 100 μg | 1,000 RH Tz | +8 days | 10 Pru cysts | 38% reduced | Bz, Tz§, Sp | Zhang et al., 2018c |
| ROP38 | unknown | 1,000 RH Tz | +2 days | 10 Pru cysts | 77% reduced | Tz§ | Xu et al., 2014 |
| ROP54 | 100 μg | 1,000 RH Tz | +8 days | 10 Pru cysts | 36% reduced | Bz, Tz§, Sp | Yang et al., 2017 |
| Other Antigens | |||||||
| PLP1 | 100 μg | 80 Pru cysts | +20 days | 20 Pru cysts | 44% reduced | Bz§, Tz, Sp | Yan et al., 2012 |
| PLP1 | 100 μg | 80 Pru cysts | +12 days | 20 Pru cysts | 30% reduced | Bz§, Tz, Sp | Chen et al., 2018 |
| PLP1‡ | 100 μg | 1,000 RH Tz | +8 days | None | N.D. | Bz§, Tz, Sp | Yan et al., 2011 |
| ADF | 100 μg | 1,000 RH Tz | +2 days | None | N.D. | Bz, Tz§, Sp | Li et al., 2011 |
| ROM4‡ | 100 μg | 1,000 RH Tz | +6 days | None | N.D. | Bz, Tz§, Sp | Rahimi et al., 2017 |
| ROM4 | 100 μg | 10,000 RH Tz | +6 days | 20 Pru cysts | 41% reduced | Bz, Tz§, Sp | Han et al., 2017b |
| ROM4 (peptide) | 100 μg | 10,000 RH Tz | +5 days | 20 Pru cysts | 36% reduced | Bz, Tz§, Sp | Han et al., 2017b |
| ROM4 (full gene + peptide) | 100 μg | 10,000 RH Tz | +12 days | 20 Pru cysts | 57% reduced | Bz, Tz§, Sp | Han et al., 2017b |
| ROM4 | unknown | 1,000 RH Tz | 13.3% survival | 10 Pru cysts | 44% reduced | Bz, Tz§, Sp | Zhang et al., 2015 |
| ROM5 | unknown | 1,000 RH Tz | 20% survival | 10 Pru cysts | 72% reduced | Bz§, Tz, Sp | Zhang et al., 2015 |
| RON5 | 100 μg | 1,000 RH Tz | +8 days | 10 Pru cysts | 26% reduced | Bz§, Tz | Zhao et al., 2016 |
| ESA10 | 100 μg | 10,000 RH Tz | +10 days | None | N.D. | Bz§, Tz, Sp | Wang et al., 2015c |
| MDH | 100 μg | 20,000 RH Tz | +9 days | None | N.D. | Bz, Tz§ | Hassan et al., 2014c |
| DPA | 100 μg | 20,000 RH Tz | +12 days | None | N.D. | Bz | Hassan et al., 2014a |
| CDPK1‡ | 100 μg | 1,000 RH Tz | +17 days | 20 Pru cysts | 46% reduced | Bz§, Tz, Sp | Chen et al., 2014a |
| CDPK1‡ | 100 μg | 1,000 RH Tz | +14 days | 10 Pru cysts | 46% reduced | Bz§, Tz, Sp | Chen et al., 2016 |
| CDPK1 | 100 μg | 1,000 RH Tz | +10 days | None | N.D. | Bz§, Tz, Sp | Huang et al., 2019 |
| CPDK2 | 100 μg | 1,000 RH Tz | +11 days | None | N.D. | Bz§, Tz, Sp | Chen et al., 2017 |
| CDPK3 | 100 μg | 1,000 RH Tz | +17 days | 10 Pru cysts | 54% reduced | Bz, Tz, Sp§ | Zhang et al., 2013b |
| CDPK5 | 100 μg | 1,000 RH Tz | +4 days | 10 Pru cysts | 39% reduced | Bz§, Tz | Zhang et al., 2014 |
| IF-2a | 100 μg | 1,000 RH Tz | +16 days | 20 Pru cysts | 44% reduced | Bz, Tz§, Sp | Chen et al., 2013b |
| IF-4a | 100 μg | 1,000 RH Tz | +29 days | None | N.D. | Bz, Tz§, Sp | Chen et al., 2013a |
| EF-1a | 100 μg | 10,000 RH Tz | +9 days | None | N.D. | Bz, Tz§ | Wang et al., 2015a |
| CP-B | 100 μg | 10,000 RH Tz | +5 days | None | N.D. | Bz, Tz, Sp§ | Zhao et al., 2013a |
| CP-C1‡ | 100 μg | 10,000 RH Tz | +6 days | None | N.D. | Bz, Tz§, Sp | Han et al., 2017a |
| CP-L | 100 μg | 10,000 RH Tz | +4 days | None | N.D. | Bz, Tz, Sp§ | Zhao et al., 2013a |
| IMP1 | 100 μg | 500 RH Tz | +22 days | None | N.D. | Bz, Tz§, Sp | Cui et al., 2012 |
| ADF | 100 μg | 1,000 RH Tz | +2 days | None | N.D. | Bz, Tz§, Sp | Li et al., 2011 |
| DOC2C | 100 μg | 1,000 RH Tz | +10 days | 10 Pru cysts | 48% reduced | Bz§, Tz, Sp | Zhang et al., 2018b |
| HSP40 | 100 μg | 1,000 RH Tz | +3 days | 10 Pru cysts | 62% reduced | Bz§, Tz, Sp | Li et al., 2018b |
| HSP60 | 100 μg | 1,000 RH Tz | +19 days | 10 Pru cysts | 48% reduced | Bz, Tz§ | Li et al., 2018a |
| HSP70‡ | 10 μg | 10 ME49 cysts | N.D. | N.D. | N.D. | Bz§, Tz, Sp | Czarnewski et al., 2017 |
| ASP1 | 100 μg | 10,000 RH Tz | +9 days | None | N.D. | Bz, Tz, Sp§ | Zhao et al., 2013b |
| Profilin‡ | 100 μg | None | N.D. | 10 Pru cysts | 39% reduced | Bz, Tz, Sp§ | Gao et al., 2018 |
| Profilin | 100 μg | None | N.D. | 10 Pru Cysts | 49% reduced | Bz, Tz, Sp§ | Zhang et al., 2018a |
| MYR1 | 100 μg | 100 RH Tz | +29 days | None | N.D. | Bz, Tz§, Sp | Zheng et al., 2019a |
| SOD | 100 μg | 1,000 ME49 Tz | +10 days | None | N.D. | Bz, Tz§ | Liu et al., 2017 |
| NTPase | 5 μg | 2,000 RH(Δku80) | +10 days | 20 Pru cysts | 78% reduced | Bz, Tz§ | Zheng et al., 2017b |
| p14-3-3 | 100 μg | 10,000 RH Tz | +5 days | None | N.D. | Bz, Tz§, Sp | Meng et al., 2012 |
| Toxofilin‡ | 50 μg | 10,000 RH Tz | +9 days | 20 Pru cysts | 33% reduced | Bz§, Tz, Sp | Song et al., 2017 |
| CyP | 100 μg | 500 RH Tz | 38% survival | None | N.D. | Bz, Tz§, Sp | Gong et al., 2013 |
| PP2C | 100 μg | 10,000 RH Tz | +11 days | None | N.D. | Sp | Song et al., 2020 |
| GST | 100 μg | 10,000 RH Tz | +6 days | None | N.D. | Wang et al., 2015b | |
| SPATR | 100 μg | 100 RH Tz | +10 days | None | N.D. | Bz§, Tz, Sp | Zheng et al., 2017a |
| GR | 100 μg | 20,000 RH Tz | +7 days | None | N.D. | Bz, Tz§ | Hassan et al., 2014b |
| ERK7 | 100 μg | 1,000 GT1 Tz | +1 day | None | N.D. | Bz§, Tz, Sp | Guo et al., 2019 |
| ERK7 | 100 μg | 20 Pru Cysts | +2 days | None | N.D. | Bz§, Tz, Sp | Guo et al., 2019 |
Acute result: n % survival indicates the percentage of vaccinated mice surviving parasite challenge, typically via administration of parasite tachyzoites (Tz); or.+n days indicates the number of days vaccination permitted survival beyond the control group.
Chronic result: indicates reduction in brain cyst numbers compared to the unvaccinated group. N.D. indicates not determined.
Indicates antigen + adjuvant was also tested and included in Table III.
Indicates the parasite stage with the highest transcriptional expression according to ToxoDB.org. Bz: bradyzoite, Sp: sporozoite.
RESULTS
As of 2018, there were more than 500 clinical trials that focused on DNA vaccines, with most targeting viral infections and cancer; however, due to the safety, stability, and cost-effectiveness of DNA vaccines, they are a valuable focus for other areas including bacterial infection, autoimmune diseases, and parasites. Some DNA vaccines have been approved by the Food and Drug Administration (FDA) and U.S. Department of Agriculture (USDA) for veterinary use, including vaccines against West Nile Virus in horses and canine melanoma. To create a potent DNA vaccine, the design should include 2 well thought out elements: antigens (to target the immune response) and adjuvants (to enhance the immune response) (Hobernik and Bros, 2018).
Before the year 2000, there was minimal research into DNA vaccines against T. gondii. However, between 2000 and 2010, the investigation of DNA vaccines as possible protection against T. gondii began to take off, almost exclusively with work being conducted in mice. Early DNA vaccine work on T. gondii focused on antigens such as GRA1, GRA7, and ROP2 to determine whether DNA vaccines incorporating these antigens could elicit an immune response and whether they proved effective against T. gondii infection (Leyva et al., 2001; Bivas-Benita et al., 2003; Scorza et al., 2003; Martin et al., 2004; Roque-Reséndiz et al., 2004; Wei et al., 2006; Hiszczyńska-Sawicka et al., 2010). Several of these studies noted the efficacy of ROP2-base DNA vaccines to delay the death of mice given a lethal parasite challenge. GRA7 was also used in combination with other antigens, leading to increased survival rates (Jongert et al., 2007, 2008a; Rosenberg et al., 2009). Further studies investigated SAG1, MIC3, and GRA4 as antigens (Chen et al., 2002, 2009; Couper et al., 2003; Ismael et al., 2003, 2009; Martin et al., 2004; Yang et al., 2005; Zhang et al., 2007a; Liu et al., 2008; Fang et al., 2009, 2010; Shang et al., 2009). Many of these studies showed promising results at high doses or against non-fetal acquired parasites (Couper et al., 2003; Fachado et al., 2003). Additionally, some studies investigated the efficacy of bradyzoite antigens such as BAG1 and MAG1 (Nielsen et al., 2006; Dautu et al., 2007; Zimmermann et al., 2008). Studies also used different combinations of these antigens (Fachado et al., 2003; Martin et al., 2004; Cong et al., 2005; Mévélec et al., 2005; Yang et al., 2005; Jongert et al., 2007, 2008b; Zhang et al., 2007b; Cui et al., 2008; Xue et al., 2008a, 2008b; Liu et al., 2009; Qu et al., 2009; Rosenberg et al., 2009; Wang et al., 2009). Common adjuvants tested in these early DNA vaccines included CTxA2/b, GMCSF, CPG-ODN, IL-2, and IL-12 (Chen et al., 2002; Mévélec et al., 2005; Cong et al., 2008; Cui et al., 2008; Xue et al., 2008b; Zimmermann et al., 2008; Ismael et al., 2009).
Antigens
The biological function of the parasite antigen produced by a DNA vaccine, as well as its cellular localization, can play an important role in antigenicity. Figure 1 outlines several subcellular locations from which antigens are selected for vaccine experiments. Surface or secreted proteins allow for easier access by the immune system as opposed to intracellular proteins. As T. gondii is a complex organism with three different infectious stages, stage specificity and the variety of invasion pathways must be taken into consideration, as the acute and latent forms of toxoplasmosis are induced by very distinct forms of the pathogen. In addition, there are different strains of Toxoplasma, so the use of an antigen (portion) that is heavily conserved among strains makes the vaccine more versatile (Liu et al., 2012). Since immunity to Toxoplasma has been correlated to a Th1 immune response, most vaccines attempt to achieve a higher Th1 response (Ghaffarifar, 2015). Size is also an important consideration when choosing an antigen: a large plasmid will have a lower transfection rate when entering the cell, and also a large protein may have more difficulty in translation. While peptide-encoding DNA vaccines are less immunogenic than their full-length gene counterparts, they benefit from smaller size—however, fewer attempts with antigen protein regions have been conducted. It is also vital that the chosen antigen is not similar to those produced by humans, as these may be identified by the adaptive immune response as self-antigens and thereby ignored by the immune system (Dumonteil, 2007).
Figure 1.
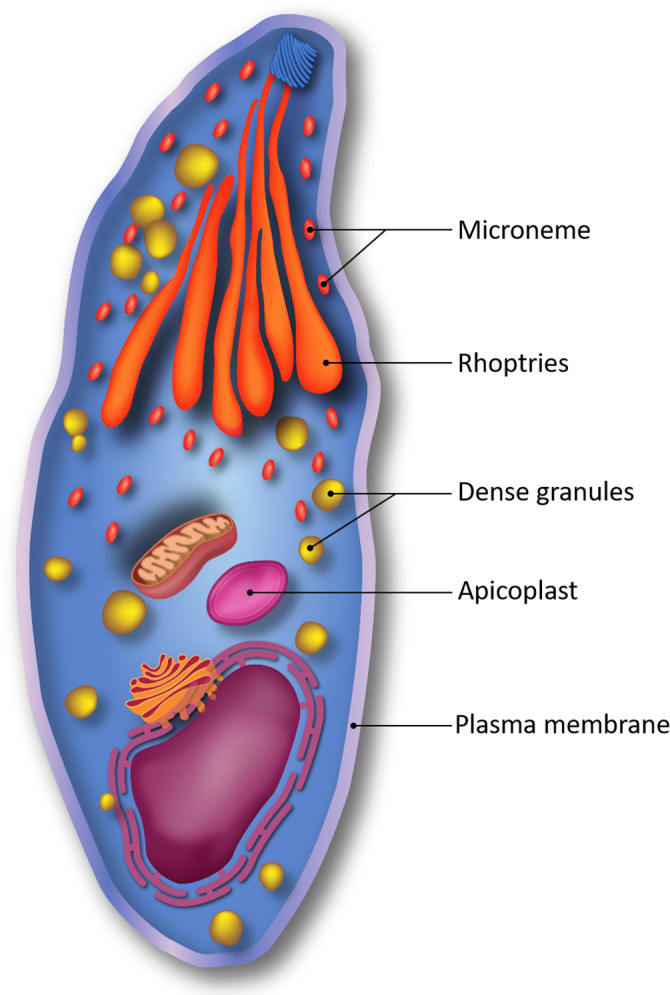
A graphical illustration of common subcellular sources of antigens from Toxoplasma gondii for the development of vaccines. Color version available online.
Codon optimization, or the accommodation of codon bias of the host organism, is also valuable when creating a DNA vaccine, as it can strongly enhance antigen expression in the host, thus providing greater amounts of antigen to be recognized by the immune response (Dumonteil, 2007). If foreign antigen production is sufficiently high, DNA vaccines have been shown to elicit antibody responses in vaccination trials. Notably, this is not always expected, as many DNA vaccine antigens are produced and remain in the cytoplasm where humoral immunity is considered inferior to cell-mediated immune responses (Shedlock and Weiner, 2000).
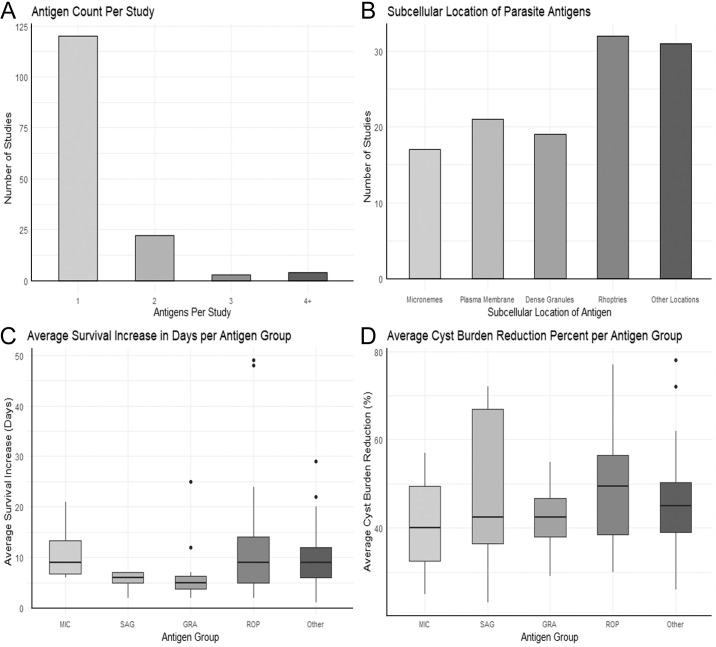
Given the nature of T. gondii virulence, antigens used in vaccines are often surface antigens (SAGs), and apically situated rhoptry antigens (ROPs), microneme antigens (MICs), and dense granule antigens (GRAs), as these are the main genes encoding surface proteins or secreted proteins necessary for successful host cell invasion, respectively (Fig. 2B). Another functional class of genes explored more recently as vaccine antigens are the essential parasite genes, those which encode proteins that are necessary for the parasite to replicate and survive. The majority of DNA vaccines also encode for most or all of the coding regions of antigen genes to ensure providing the highest number of epitopes.
Figure 2.
Metrics of published experimental Toxoplasma gondii DNA vaccine designs by antigen number and subcellular location. (A) Reviewed studies evaluated varying numbers of investigated DNA antigens, with few investigating multiple antigens (i.e., antigen cocktails). (B) Reviewed studies evaluated antigens found in various parasite subcellular locations. (C, D) Antigens found in various subcellular locations demonstrate overlapping levels of efficacy in vivo. MIC = microneme-associated proteins; SAG = surface antigen-associated proteins; GRA = dense granule-associated proteins; ROP = rhoptry-associated proteins.
Microneme proteins (MICs):
Microneme proteins (MICs) are released onto the parasite's surface and interact with host cell receptors to aid in gliding motility machinery and successful host cell invasion (Yuan et al., 2013). This critical role makes MIC proteins attractive targets for DNA vaccine studies, and 7 different MIC genes were observed in T. gondii DNA vaccine studies reviewed herein.
The T. gondii MIC1/MIC4/MIC6 complex is involved in host cell attachment. Thus, each of these genes has been tested as possible antigens against both acute and chronically infected mice. Parasite MIC1 and MIC4 both act as adhesins and will bind to host cell receptors (Reiss et al., 2001). DNA vaccination incorporating MIC1 administered to mice showed a 40–50% survival rate against the acute challenge of the parasite, and chronically infected mice showed a 52% cyst burden reduction. Similarly, MIC4 showed a 40–50% acute infection survival rate and 47% cyst burden reduction in chronically infected mice. DNA vaccination with MIC6, which acts as an escort for both MIC1 and MIC4, permitted a 40–50% survival rate amongst acutely infected mice and in all other studies showed an increase in survival ranging from 10 to 20 days post-treatment (Meissner et al., 2002). In preventing the establishment of the chronic stage of the parasite, MIC6-based DNA vaccines reduced cyst burden by 27–40%.
MIC3 is expressed in all 3 infectious stages of T. gondii and is a potent adhesion protein of the parasite. It has been shown to elicit an early and powerful immune response in vivo (Fang et al., 2009; Yuan et al., 2013). Incorporating MIC3 into a DNA vaccination led to 20% overall survival or an increase in survival by 7–8 days in acutely infected mice.
MIC8 and MIC13 have been described as involved in Toxoplasma host cell adhesion (Friedrich et al., 2010; Li et al., 2014). Treatment with a MIC8-based DNA vaccine led to an increased survival of acutely infected mice by 6–11 days and a 25% cyst burden reduction in chronically infected mice. MIC13-based DNA vaccination led to an increase in survival of 21 days against acutely infected mice and led to a 57% cyst burden reduction in chronically infected mice. MIC 11 has been described as involved in the organization of other MICs during adhesion (Tao et al., 2013). MIC11-based DNA vaccination led to a 17% survival of acutely infected mice.
A full summary of the use of micronemes in Toxoplasma DNA vaccine papers and their results can be found in Table I. In summary, DNA vaccines encoding MIC antigens reduced chronic parasite loads from 30–60%, while extending survival from acute infection by approximately 10 days (median).
Surface antigens (SAGs):
The surface of T. gondii is covered in often heavily modified DNA-encoded protein surface antigens (SAGs) that play various roles in the pathogenesis of the parasite, as they are the first portion of T. gondii to make contact with the host cell and are thus an attractive target for vaccine development. There are 5 proteins in the superfamily of surface antigens, SAG1–SAG5. SAG1 is the most abundant of the surface antigens, making up about 3–5% of all T. gondii tachyzoite proteins, and it plays a major role in tachyzoite adhesion and invasion of the host cell (Roozbehani et al., 2018). SAG1-based DNA vaccines led to an increase in survival ranging from 2 to 7 days against an acute challenge and a 37% chronic cyst burden reduction.
Unlike SAG1, the SAG2CDXY cluster, made of SAG2C, SAG2D, SAG2X, and SAGY, as well as SAG4, are detectable on the surface of bradyzoites, which contribute to chronic T. gondii infection (Zhang et al., 2013a; Zhou and Wang, 2017). SAG2C-based, SAG2D-based, and SAG2X-based vaccines led to 72, 23, and 70% reduction of chronic cyst burden, respectively. Studies that investigated SAG4 found increased survival of 7 days from the acute infection. Additionally, a 66% reduction in cyst burden was observed when tested against the chronic infection in mice. SAG5 is broken up into 5 subtypes (SAG5a–SAG5e). SAG5a, SAG5b, and SAG5c are all found on both tachyzoites and bradyzoites and specifically play a role in the persistence of cysts in the host (Lu et al., 2015). Administration of a SAG5a-based DNA vaccine in mice showed a 35% cyst burden reduction. DNA vaccines incorporating SAG5b and SAG5c showed an increased survival of 7 and 6 days, respectively. Additionally, chronically infected mice showed a 44% cyst burden reduction and 41% cyst burden reduction for SAG5b-based and SAG5c-based DNA vaccines, respectively. SAG5d, which is only expressed in tachyzoites, has been shown to have immunogenicity similar to SAG1 (Lu et al., 2014). Mice that were acutely infected with T. gondii, when administered with a SAG5d-based DNA vaccine, had an increased survival of 7 days in comparison to untreated mice.
An additional summary of the SAG proteins found in T. gondii DNA vaccine papers and their effectiveness against the parasite can be found in Table I. The non-SAG1 surface antigens, as a group, generally outperformed MIC DNA-encoded proteins in reducing cyst loads (37–72% reduction) associated with the chronic infection, while acutely challenged mice often experienced a reduced duration of survival (2–7 days beyond control death) among the SAG-vaccinated groups.
Dense granule proteins (GRAs):
Dense granule proteins (GRAs) play a vital role in host cell invasion by forming a parasitophorous vacuole (PV) from the membrane of the host cell. The PV is a wrapping that contains invading parasites and is derived from both host membrane and parasite secretions. The PV permits the parasites to grow within a self-contained vacuole and resists the leakage of antigens into the host cell, thereby limiting the activation of the immune response. Their secretory nature, as well as their role in host cell invasion function, makes GRAs an appealing target for DNA vaccines. Because of their role in host invasion, GRA proteins showed little efficacy against chronic infection, and many studies do not evaluate DNA vaccine constructs incorporating GRA antigens against chronic infection.
GRA1 is a calcium-binding protein that is secreted by both tachyzoites and bradyzoites and has been shown to illicit heavy T-cell proliferation (Vercammen et al., 2000). Against the acute stage of infection, DNA vaccines containing GRA1 as an antigen showed an increase in survival of 6 days. GRA2 is involved in the formation of the intravacuolar network of the PV (Ching et al., 2017). DNA vaccines incorporating GRA2 showed an increase in survival of 3 days against acute infection. GRA5 protects the parasite during cell invasion by inhibiting apoptosis of infected cells (Min et al., 2012). Against acute infection, a GRA5-based DNA vaccine showed an increased survival of 3 days.
Mice vaccinated using GRA6 as an antigen showed a 40% survival of the acute infection. A specific carboxyl-terminal portion of GRA6, known as HF10, has been known to have MHC-restricted immunodominance and protective capability against lethal infection (Sun et al., 2011). GRA7 plays an integral role in host cell invasion and, once inside the host cell, works in conjunction with other T. gondii proteins. GRA7-treated mice showed a 5–12 day increase against acute infection and a 55% chronic cyst burden reduction. GRA8 is commonly used to identify and diagnose acute infection (Ybañez et al., 2020). When tested as a DNA vaccine antigen, it led to a 4-day survival increase against the acute infection. GRA14, expressed in all stages of the Toxoplasma life cycle and during infection, is secreted into the PV where it can be transferred between the PV membrane and the intravacuolar network (Pagheh et al., 2019). Vaccination with a GRA14-based DNA vaccine showed increased survival of 5 days against an acute challenge.
GRA16 and GRA 24 are exported from the PV and mediate host gene expression. GRA16 regulates the production of p53, a protein involved in inflammatory host response and the cell cycle (Hu et al., 2017). DNA vaccines incorporating GRA16 as an antigen led to a 2-day survival increase of the acute stage and a 44% cyst burden reduction against the chronic stage. GRA24 binds with a mitogen-activated protein kinase known as p38α and causes transcriptional changes across the cell (Bougdour et al., 2014). GRA24-based DNA vaccines showed an increase in survival against the acute infection of 3–26 days. When chronically challenged, these vaccines led to a 29% cyst burden reduction. Similar to GRA16 and GRA24, GRA25 may act as an inhibitor of chemokines CCL2 and CXCL1, which are involved in host immune response. However, the mechanism by which GRA25 works is still unknown (Xu et al., 2019). When tested as a DNA vaccine antigen, GRA25 led to an increase in acute infection survival of 7 days and a 41% cyst burden reduction in chronic infection.
GRA17 and GRA23 are involved in PV membrane permeability and nutrient transportation (Gold et al., 2015). DNA vaccines containing these antigens were tested against acute infection and showed an increased survival of 2 and 4 days, respectively.
In comparison to SAG and MIC antigens, vaccines using GRA antigens showed variable success against both the acute and chronic stages of the parasite. GRA-based vaccines led to a 5-day (median) acute survival increase, which is similar to that of SAG-based DNA vaccines. Against the chronic stage, these vaccines showed a 29–55% cyst burden reduction, which is less than that of both SAG and MIC-based DNA vaccines. DNA vaccines that used GRA6 antigens showed the highest efficacy against the acute stage of the parasite, leading to 40% overall survival. This is comparable to DNA vaccines incorporating MIC4, MIC6, and SAG1. A full summary of GRA antigens explored in T. gondii DNA vaccine papers can be found in Table I.
Rhoptry proteins (ROPs):
Rhoptry proteins (ROPs) are apical secretory organelles that are known to be involved in parasites' penetration of the host cell, specifically in the formation of the PV. ROP proteins have a variety of different functions and contribute heavily to the virulence of the parasite. Some play a role in early, acute virulence, such as ROP1 and ROP9 (Chen et al., 2014b; Sonaimuthu et al., 2016). DNA vaccines incorporating ROP1 as an antigen led to 20% survival or a 12-day survival increase against acute infection and a 55% cyst burden reduction against chronic infection. ROP9-based DNA vaccines led to increased mouse survival of 12 days against acute infection.
Another family of ROP proteins exists that contributes to the virulence of the parasite and is involved in the membrane of the PV. Included in this family are ROP2, ROP7, ROP8 (Hajj et al., 2007; Labesse et al., 2009; Li et al., 2015b). Several studies have used these ROP proteins as antigens in DNA vaccine candidates. Against the acute stage of the parasite in mice, DNA vaccines using ROP2 as an antigen led to increased survival of 2 days. ROP7-based DNA vaccine-treated mice had an increased acute infection survival of 5 days and a 58% cyst burden reduction during the chronic stage. Against an acute challenge, DNA vaccines incorporating ROP8 showed 50% survival and increased survival of 2 days.
ROP5 is an essential virulence factor of T. gondii and leads to the inactivation of IFN-γ-inducible immunity-related GTPases (IRGs) (Wang et al., 2016b). These IRG proteins lead to an immunity from the parasite within mice by accumulating on the PV and killing the parasite (Khaminets et al., 2010). Studies that investigated ROP5 as a DNA vaccine antigen found that it led to a 4–14 day survival increase against the acute stage of the parasite and a 52–58% reduction in chronic infection cyst burden. Additionally, ROP5 complexes with ROP17 and ROP18, which are both ROP kinases, to aid in the mediation of the IRG pathway (Etheridge et al., 2014). ROP17 is also a member of the ROP2 family, and a ROP17-based DNA vaccine led to a 14-day survival increase against acute infection. ROP18, a polymorphic serine-threonine kinase that is secreted during the invasion process and can inhibit the host's innate and adaptive immune responses, has been studied more frequently against both acute and chronic infection. In acutely infected mice, those that had been administered a ROP18-based DNA vaccine led to 9–48-day survival increases in others. Against the chronic infection, 30–47% cyst burden reduction was found in mice administered this DNA vaccine.
Another category of these proteins is ROP kinases (ROPK), which include ROP17 and ROP 18 as mentioned previously, as well as ROP16, ROP19, ROP21, ROP22, ROP29, and ROP38 (Liu et al., 2014; Xu et al., 2014; Zhou et al., 2016; Lu et al., 2018; Zhang et al., 2019). Additionally, ROP54 is a pseudokinase (Yang et al., 2017). ROP16 is considered a key virulence factor in the pathogenesis of Toxoplasma, as it can subvert STAT3/6 signaling and target the host nucleus directly (Liu et al., 2014). Against acute infection, ROP16-based DNA vaccines led to an increase in survival of 13–27 days. ROP19 is another ROP kinase present in the PV membrane and is considered to be a target for less virulent strains of T. gondii (Zhou et al., 2016). An ROP19-based DNA vaccine was tested against the chronic stage of the parasite and led to a 57% cyst burden reduction. A DNA vaccine using ROP21 as an antigen showed a 7-day survival increase against acute infection and a 37% chronic cyst burden reduction. A ROP22-based vaccine led to an 8-day survival increase against acute infection and a 40% cyst burden reduction against the chronic stage. ROP29 was tested as a DNA vaccine antigen in mice against the chronic infection and showed a 53% cyst reduction. Mice administered a ROP38-based DNA vaccine showed a 2-day survival increase against the acute infection and a 77% cyst burden reduction against the chronic infection. A study that investigated ROP54 as a potential DNA vaccine antigen observed an 8-day survival increase against the acute infection and a 36% cyst reduction in chronically infected mice.
ROP13 has an effector function that is involved in the host cell response to T. gondii and is not similar to any other known protein. Studies have found it present in evacuoles, or rhoptry-derived secretory vesicles, and suggest that it might be toxic to the host (Håkansson et al., 2001; Turetzky et al., 2010). Studies testing a DNA vaccine incorporating ROP13 found a 49-day increased survival against the acute stage of the parasite and a 40% cyst burden reduction when chronically challenged.
ROP35 is a protein found within the PV membrane, though its direct function is unclear (Zhang et al., 2018c). Against acute infection, a ROP35-based DNA vaccine led to an 8-day survival increase. This DNA vaccine was tested against the chronic infection as well and a 38% cyst burden reduction was observed in mice that had the vaccine administered.
A full summary of ROP antigens that were examined in DNA vaccine papers against T. gondii can be found in Table I. Noticeably, ROP38 DNA vaccination stands out as producing an impressive 77% reduction in cyst load, compared to the other evaluated ROP proteins which averaged 44% reduction. ROP13-based and ROP18-based DNA vaccines also show great efficacy, leading to a 49- and 48-day survival increase against acute infection, respectively.
Other antigens:
Many other genes have been employed as DNA vaccine antigens against T. gondii, although with less regularity. Perforin-like proteins (PLPs) are pore-forming proteins, and the PLP1 protein has been shown to affect the permeability of the PV membrane in T. gondii (Kafsack et al., 2009). When explored as a potential DNA vaccine antigen, it showed an increase in acute infection survival by 8–20 days and a 30–44% cyst burden reduction against the chronic infection. Rhomboid proteases (ROMs) are used in T. gondii to cleave adhesive proteins to facilitate the entry into the host cells and the separation of parasites from receptors (Foroutan et al., 2019). Two ROMs have been explored as DNA vaccine candidates within the last decade, ROM4 and ROM5. DNA Vaccines incorporating ROM4 showed a 13% survival, or a 5–12-day survival, increase and generated a 36–57% cyst burden reduction against the chronic infection. ROM5 provided a 20% survival in acutely infected mice and produced a 72% cyst reduction. Toxoplasma gondii secreted protein with an altered thrombospondin repeat (SPATR) has been described as part of the microneme family and involved in virulence and invasion of the parasite (Zheng et al., 2017a). SPATR-based DNA vaccination led to a 10-day survival increase against an acute challenge.
Rhoptry neck (RON) proteins combine with ROP proteins mentioned earlier to create a larger moving junction complex and have only recently been discovered. RON5 is present in the moving junction as well and acts as an escort for other RON proteins (Zhao et al., 2016). Acutely infected mice that had been administered a RON5-based DNA vaccine survived 8 days longer than untreated control and produced a 26% cyst reduction in chronically infected mice.
Some studies have taken interest in molecules that function in the parasite's metabolism as possible DNA vaccine antigens. Toxoplasma gondii requires a lot of energy during the invasion, and the production of malate dehydrogenase (MDH) has been shown to contribute to virulence (Hassan et al., 2014c). MDH treatment led to 9 days of increased survival against the acute infection. Deoxyribose phosphate aldolase (DPA) is functional in the parasite's glycolysis cycle and functions to connect the MIC2 protein to the host cell surface (Hassan et al., 2014a). When mice that had been administered a DPA-based DNA vaccine were challenged with the acute parasite infection, they survived 12 days longer than untreated mice. Superoxide dismutase (SOD) is an enzyme that protects cells from oxidative stress and is involved in the process of metabolizing oxygen. In T. gondii, it is involved in the growth of both the acute and chronic stages of the parasite (Liu et al., 2017). Treatment with a DNA vaccine incorporating SOD as an antigen showed a 10-day survival increase against an acute challenge. Nucleoside triphosphate hydrolase (NTPase) promotes host invasion in T. gondii by breaking down ATP and ADP to AMP, forcing the parasite to acquire purines from host cells (Zheng et al., 2017b). DNA vaccines incorporating NTPase showed a 10-day survival increase against the acute infection and led to a 78% cyst burden reduction against a chronic challenge.
Calcium-dependent protein kinases (CDPKs) are kinases present in many apicomplexans and play a role in biological function. In T. gondii, CDPK1 is involved in the parasite's life during infection and in the secretion of microneme proteins (Chen et al., 2014a). In DNA vaccines using CDPK1 as an antigen, a 10–17 day survival increase against the acute form of the parasite and a 46% chronic stage cyst burden reduction were observed. CPDK2, which plays a role in amylopectin metabolism, was also tested as a DNA vaccine antigen and led to a survival increase of 11 days against the acute infection (Chen et al., 2017). Another kinase, CDPK3, has a similar function to other CDPKs and is located at the apical end of the parasite (Zhang et al., 2013b). A CDPK3-based DNA vaccine led to a 17-day survival increase in acutely infected mice and a 54% cyst burden reduction against the chronic infection. CPDK5 was also tested as a DNA vaccine antigen but showed less efficacy. The DOC2C protein acts downstream of CDPK proteins in T. gondii and facilitates secretory vesicle and plasma membrane fusion (Zhang et al., 2018b). A DNA vaccine incorporating DOC2C showed a 10-day survival increase in acutely infected mice.
Heat shock proteins (HSPs) assist the cell with post-translational processes such as stabilization, folding, and translocation (Li et al., 2018b). HSPs tested as DNA vaccine candidates include HSP40 and HSP60. HSP40 showed promise against the chronic stage of the parasite, which led to a 62% chronic cyst burden reduction. When tested against the acute infection, an HSP60-based DNA vaccine showed a 19-day survival increase and a 48% cyst burden reduction against the chronic stage.
Other molecules that displayed positive results as a DNA vaccine antigen include Initiation factor 2a, Initiation factor 4a, Immune mapped protein 1, Myc regulation 1, Phosphatase 2C, and Cyclophilin. Toxoplasma gondii eukaryotic initiation-factor 2a (IF-2a) is vital to the viability of the parasite when phosphorylated (Chen et al., 2013b). IF-2a antigen treatment led to a 16-day survival increase against the acute infection and a 44% reduction in cyst burden against a chronic challenge. Toxoplasma gondii eukaryotic translation initiation factor 4a (IF-4a) aids in beginning translation and unwrapping inhibiting mRNA (Chen et al., 2013a). Against an acute challenge, treatment with IF-4a led to a 29-day survival increase. Immune mapped protein 1 (IMP1) has shown immunogenicity and protection against Eimeria maxima. This protein is also present in the RH strain of T. gondii and, thus, shows significant interest as a vaccine candidate (Cui et al., 2012). IMP1 treatment showed increased survival against acute infection by 22 days. Myc regulation 1 (MYR1) is a recently discovered virulence factor in T. gondii present in the PV to help alter host cell transcription to promote host–parasite interactions. Additionally, it also serves to regulate several tachyzoite pathways (Zheng et al., 2019a). Against an acute challenge, treatment with the MYR1 antigen led to a 29-day survival increase. Phosphatase 2C (PP2C) is a protein described as a rhoptry secretory protein and interferes with host gene expression during infection (Song et al., 2020). Against an acute challenge, a PP2C-based DNA vaccine led to a survival increase of 11 days. Cyclophilin (CyP) has been described as a protein family responsible for protein folding in many prokaryotes and eukaryotes (Gong et al., 2013). CyP-based DNA vaccine treatment led to a 38% survival against an acute challenge.
Several other antigens were tested but did not display results as positive as those mentioned above. These include actin-depolymerizing factor (ADF), cathepsin proteases B, C1, and L (CP-B, CP-C1, CP-L), ESA10, Aspartic protease 1 (ASP1), elongation factor 1-alpha (EF-1α), Profilin (PF), p14-3-3, Toxofilin, Glutathione reductase (GR), Glutathione-S-Transferase (GST), and ERK7.
Many of these genes chosen as candidate vaccine antigens play a role in parasite invasion and metabolism, which is, perhaps, why they showed promising results as a DNA vaccine candidate. These other genes showed a survival increase of 9 days (median) and an average cyst burden reduction range of 26–78%. A full list of other genes that were tested in T. gondii DNA vaccines and their results can be found in Table I, and summarized in Figure 2.
Antigen considerations:
Two final important factors when designing the antigen contents of a DNA vaccine include the decision between using a full-length gene or a peptide sequence, as well as using one or multiple genes.
DNA-encoded peptide sequences have some advantages, such as their relative ease of production and storage, but they are less stable in vivo than the full-length gene (Roozbehani et al., 2018). In this T. gondii DNA vaccine review, 3 of 95 papers used peptide sequences in their vaccines. Two papers used various short amino acid sequences from multiple antigens, which led to protection for 7 days more than the control in the acute challenge in both cases: one tested SAG1, AMA1, ROP2, GRA4 while the other used SAG1, GRA2, GRA7, and ROP16 (Cao et al., 2015; Roozbehani et al., 2018).
It has also been shown that using multiple genes in a vaccine can offer a more wide-range immune response, especially for a pathogen such as Toxoplasma that has multiple stages in its life cycle during which not all genes are present. Of the papers reviewed, 29 of the 149 DNA vaccine candidates incorporated at least 2 separate antigens in their vaccine design (Figure 2A). A full summary of multiple genes and multiple peptide sequences explored in T. gondii DNA vaccine papers can be found in Table II.
Table II.
Summary of challenge results from DNA vaccines containing multiple Toxoplasma gondii antigens. A summary of results from publications since 2010 employing two or more generally full-length (or peptide portions, where indicated) parasite antigens delivered via DNA vaccination followed by challenge, organized by the number of antigens co-administered. Bz = bradyzoite, Tz = tachyzoites, Sp = sporozoite.
| Gene |
Acute challenge |
Acute result* |
Chronic challenge |
Chronic result† |
Reference |
| Dual Genes | |||||
| MIC1 + MIC4 | 80 ME49 cysts | 70% survival | 40 ME49 cysts | 59% reduced | Pinzan et al., 2015 |
| MIC3 + GRA1 | 10,000 RH Tz | 10% survival | 1,000 RH Tz | unknown | Gong et al., 2016 |
| MIC3 + ROP18 | 1,000 RH Tz | +12 days | None | N.D. | Qu et al., 2013 |
| MIC6 + PLP1‡ | 80 Pru cysts | +25 days | 20 Pru cysts | 62% reduced | Yan et al., 2012 |
| SAG1 + GRA1 | 100,000 RH Tz | 30% survival | None | N.D. | Wu et al., 2012 |
| SAG1 + GRA2‡ | 10,000 RH Tz | +9 days | None | N.D. | Zhou et al., 2012 |
| SAG1 + GRA5 | 10,000 RH Tz | +4 days | None | N.D. | Naserifar et al., 2015 |
| SAG1 + GRA7‡ | 1,000RH Tz | +8 days | None | N.D. | Mavi et al., 2019 |
| SAG1 + ROP2 | 1,000 RH Tz | +3 days | None | N.D. | Sun et al., 2020 |
| SAG1 + ROP2 | 10,000 RH Tz | +5 days | None | N.D. | Naserifar et al., 2015 |
| SAG1 + ROP2 | 10,000 RH Tz | +5 days | None | N.D. | Hoseinian Khosroshahi et al., 2011 |
| SAG1 + p14-3-3 | 10,000 RH Tz | +11 days | None | N.D. | Meng et al., 2012 |
| SAG5B + SAG5C | 10,000 RH Tz | +12 days | 20 Pru cysts | 70% reduced | Lu et al., 2017 |
| GRA5 + ROP2 | 10,000 RH Tz | +6 days | None | N.D. | Naserifar et al., 2015 |
| GRA7 + ROP1‡ | 1,500 ME49 cysts | 33% survival | 20 ME49 cysts | 62% reduced | Quan et al., 2012 |
| GRA7 + ROP16‡ | 1,000 RH Tz | +16 days | None | N.D. | Liu et al., 2014 |
| GRA14 + ROM4‡ | 1,000 RH Tz | +8 days | None | N.D. | Rahimi et al., 2017 |
| GRA17 + GRA23 | 1,000 RH Tz | +10 days | None | N.D. | Zhu et al., 2017 |
| ROP5 + ROP18‡ | 1,000 RH Tz | +23 days | 6 Pru cysts | 64% reduced | Zhu et al., 2020 |
| ROP5 + ROP7 | 1,000 RH Tz | +9 days | 20 Pru cysts | 75% reduced | Wang et al., 2016b |
| ROP18 + PLP1‡ | 80 Pru cysts | +28 days | 20 Pru cysts | 66% reduced | Chen et al., 2018 |
| CPB + CPL | 10,000 RH Tz | +10 days | None | N.D. | Zhao et al., 2013a |
| Tri-Gene | |||||
| MIC1 + MIC4 + MIC6 | 80 ME49 cysts | 80% survival | 40 ME49 cysts | 68% reduced | Pinzan et al., 2015 |
| SAG1 + GRA5 + ROP2 | 10,000 RH Tz | +6 days | None | N.D. | Naserifar et al., 2015 |
| SAG2C + SAG2D + SAG2X | None | N.D. | 20 Pru cysts | 77% reduced | Zhang et al., 2013a |
| Multiple DNA-encoded Peptides | |||||
| SAG1/AMA1/ROP2/GRA4‡ | 10,000 RH Tz | +7 days | None | N.D. | Roozbehani et al., 2018 |
| SAG1/GRA2/GRA7/ROP16‡ | 1,000 RH Tz | +7 days | None | N.D. | Cao et al., 2015 |
| ROP16/ROP18/MIC6/CDPK3 | None | N.D. | 10 Pru cysts | 38% reduced | Zhang et al., 2018a |
| PF/ROP16/ROP18/MIC6/CDPK3 | None | N.D. | 10 Pru cysts | 80% reduced | Zhang et al., 2018a |
| SAG1/ROP2/GRA1/GRA4/SAG2C/SAG2X | 1,000 RH Tz | 20% survival | None | N.D. | Cong et al., 2014 |
Acute result: n % survival indicates the percentage of vaccinated mice surviving parasite challenge, typically via administration of parasite tachyzoites (TzTz); or.+n days indicates the number of days vaccination permitted survival beyond the control group.
Chronic result: indicates reduction in brain cyst numbers compared to the unvaccinated group. N.D. indicates not determined.
Indicates antigen + adjuvant was also tested and included in Table III.
Dual antigens with the longest increase in days of protection included MIC6 + PLP1 and ROP18 + PLP1, which led to 25 and 28 days of increased survival, respectively (Yan et al., 2012; Chen et al., 2018). However, a vaccine using MIC1 + MIC4 resulted in 70–80% survival against the acute challenge (Lourenço et al., 2006; Pinzan et al., 2015). The most effective dual antigens vaccines against the chronic challenge were the combination of SAG5b + SAG5c with a 70% cyst reduction (Lu et al., 2017) and ROP5 + ROP7 with a 75% cyst reduction (Wang et al., 2016b). Multiple sources used vaccines with SAG1 in union with various other antigens that demonstrated between 4–8 days of increased survival for the acute challenge (Hoseinian Khosroshahi et al., 2011; Naserifar et al., 2015; Mavi et al., 2019). A DNA vaccine incorporating SAG1 with GRA1 led to 30% survival against the acute challenge (Wu et al., 2012). Three papers used a tri-gene attempt in creating a vaccine—the most effective being the combination of 3 MIC genes (MIC1, MIC4, MIC6), which led to an 80% survival against the acute challenge (Pinzan et al., 2015). Another of the 3 studies use a combination of 3 SAG antigens (SAG2C, SAG2D, and SAG2X), which resulted in a 77% reduction in cysts after a chronic challenge (Zhang et al., 2013a).
In summary, a vaccine containing multiple antigens had the potential to produce higher levels of protection depending on the antigens used. Against the acute challenge, results vary from 4 days of increased survival to 80% survival. Against the chronic challenges, results vary between 32–80% cyst reduction.
Ruminant models of vaccine efficacy
Few T. gondii DNA vaccine studies have been conducted in ruminant animals, despite human Toxoplasma infection often originating from consumption of cysts found in meat and the large economic losses that result from congenital abnormalities caused by toxoplasmosis in livestock. In sheep, it was found that the use of ROP1 in combination with CpG elicited a higher interferon-gamma response than did other vaccine candidates; however, no humoral response was observed against tachyzoite lysate (Li et al., 2010). Another study reported that the fusion of ROP1 with ovine CD154 could induce a mixed Th1/Th2 response, while ROP1 alone only produced a Th1 response (Hiszczyńska-Sawicka et al., 2011a). Another study in sheep found that a significant humoral and cellular response could be elicited in response to MIC3 (Hiszczyńska-Sawicka et al., 2012). A more antigen-diverse study in sheep found that of GRA1, GRA4, GRA6, or GRA7 DNA vaccine antigens, all constructs evoked some amount of humoral and cellular response, with GRA7 producing the most robust response. (Hiszczyńska-Sawicka et al., 2011b). DNA vaccine efficacy has been evaluated in pigs, including GRA1 and GRA7 in a DNA cocktail vaccine that produced strong interferon-gamma and antibody response to recombinant protein (Jongert et al., 2008a). Overall, the understanding of the efficacy of T. gondii DNA vaccines in rudiment animals is still in its infancy. In addition to exploring the humoral and cellular responses to DNA vaccines in these animals, it would be prudent to focus on effects against cyst formation and congenital defects.
Adjuvants
DNA vaccines evoke T-cells and potentially humoral responses; however, the efficacy of vaccination is related to dosage, antigenicity, and potentially the size of the recipient (Babiuk et al., 2003). Therefore, adjuvants have been considered for use in DNA vaccines to enhance immune responsiveness. Despite the potential of an adjuvant to enhance the immunogenicity, and therefore protective effect, of the vaccine, only 26 reviewed papers included an adjuvant. The adjuvants used generally fall into 2 categories: host-derived biomolecules or chemical elements. A summary of adjuvants explored in T. gondii DNA vaccine papers can be found in Table III.
Table III.
Summary of adjuvant effect on DNA vaccinations against Toxoplasma gondii. A summary of results from publications since 2010 employing adjuvants co-administered with or by DNA vaccination followed by challenge, organized by adjuvant class. Tz = tachyzoites.
| Adjuvant |
Antigen gene(s) |
Acute challenge |
Acute result* |
Chronic challenge |
Chronic result† |
Reference |
| Host Proteins | ||||||
| IL-12 | MIC3 | 10,000 RH Tz | +1 day | None | N.D. | Ghaffarifar et al., 2019 |
| IL-12 | GRA7 | 1,500 ME49 cysts | +17% survival | 20 ME49 cysts | +5% reduction | Quan et al., 2012 |
| IL-12 | GRA14 | 100,000 RH Tz | −3 days | None | N.D. | Ahmadpour et al., 2017a |
| IL-12 | ROP1 | 1,500 ME49 cysts | Not significant | 20 ME49 cysts | +5% reduction | Quan et al., 2012 |
| IL-12 | ROP8 | 2,000 RH Tz | +1 day | None | N.D. | Foroutan et al., 2020a |
| IL-12 | GRA7 + ROP1 | 1,500 ME49 cysts | +17% survival | 20 ME49 cysts | +5% reduction | Quan et al., 2012 |
| IL-15 | Profilin | None | N.D. | 10 Pru cysts | +2% reduction | Gao et al., 2018 |
| IL-18 | SAG1 | 50 RH Tz | +20% survival | None | N.D. | Liu et al., 2010b |
| IL-18 | ROP13 | 1,000 RH Tz | Not significant | 10 Pru cysts | +26% reduction | Wang et al., 2012 |
| IL-18 | PLP1 | 1,000 RH Tz | +1 day | None | N.D. | Yan et al., 2011 |
| IL-18 | MIC6 + PLP1 | 80 Pru cysts | +4 days | 20 Pru cysts | +3% reduction | Yan et al., 2012 |
| IL-18 | ROP18 + PLP1 | 80 Pru cysts | +3 days | 20 Pru cysts | +5% reduction | Chen et al., 2018 |
| IL-33 | ROP5 + ROP18 | 1,000 RH Tz | +7 days | 6 Pru cysts | +16% reduction | Zhu et al., 2020 |
| IL-7 + IL-15 | CDPK1 | 1,000 RH Tz | +5 days | 10 Pru cysts | +28% reduction | Chen et al., 2016 |
| IL-15 + IL-21 | MIC8 | 1,000 RH Tz | +7 days | 20 Pru cysts | +39% reduction | Li et al., 2014 |
| IL-15 + IL-21 | MIC8 | 80 Pru cysts | +16 days | None | N.D. | Li et al., 2014 |
| IL-15 + IL-21 | CDPK1 | 1,000 RH Tz | +3 days | 20 Pru cysts | +27% reduction | Chen et al., 2014a |
| RANTES | SAG1/GRA2/ GRA7/ROP16 | 1,000 RH Tz | +4 days | None | N.D. | Cao et al., 2015 |
| B7-2 | GRA7 | 1,000 RH Tz | +5 days | None | N.D. | Liu et al., 2014 |
| B7-2 | ROP16 | 1,000 RH Tz | +6 days | None | N.D. | Liu et al., 2014 |
| B7-2 | GRA7 + ROP16 | 1,000 RH Tz | +6 days | None | N.D. | Liu et al., 2014 |
| Other | ||||||
| FliC gene | SAG1 | 1,000 RH Tz | +3 days | None | N.D. | Maraghi et al., 2019 |
| CpG-ODN | SAG1 + GRA7 | 1,000 RH Tz | +11 days | None | N.D. | Mavi et al., 2019 |
| a-GalCer | SAG5d | 10,000 RH Tz | +4 days | None | N.D. | Lu et al., 2014 |
| a-GalCer | CP-C1 | 10,000 RH Tz | +6 days | None | N.D. | Han et al., 2017b |
| R848 | ROP29 | None | N.D. | 20 Pru cysts | +27% reduction | Lu et al., 2018 |
| Aluminum Hydroxide | HSP70 | 10 ME49 cysts | N.D. | N.D. | N.D. | Czarnewski et al., 2017 |
| Aluminum Hydroxide | SAG1/AMA1/ ROP2/GRA4 | 10,000 RH Tz | −5 days | None | N.D. | Roozbehani et al., 2018 |
| Aluminum Hydroxide | SAG1 | 1,000 RH Tz | +0 days | None | N.D. | Maraghi et al., 2019 |
| Aluminum Hydroxide | Toxofilin | 10,000 RH Tz | +13 days | 20 Pru Cysts | +9% reduction | Song et al., 2017 |
| MPLA | Toxofilin | 10,000 RH Tz | +14 days | 20 Pru Cysts | +16% reduction | Song et al., 2017 |
| Alum + MPLA | Toxofilin | 10,000 RH Tz | +23 days | 20 Pru cysts | +33% reduction | Song et al., 2017 |
| Calcium Phosphate Nanoparticles | GRA14 | 100,000 RH Tz | +0 days | None | N.D. | Ahmadpour et al., 2017b |
| Calcium Phosphate Nanoparticles | ROM4 | 1,000 RH Tz | +1 day | None | N.D. | Rahimi et al., 2017 |
| Calcium Phosphate Nanoparticles | ROM4 + GRA14 | 1,000 RH Tz | +1 day | None | N.D. | Rahimi et al., 2017 |
| Levamisole | GRA6 | 1,000 RH Tz | +13% survival | None | N.D. | Sun et al., 2011 |
| pSPreS2 | SAG1 + GRA2 | 10,000 RH Tz | +1 day | None | N.D. | Zhou et al., 2012 |
| Saponin | SAG1 | 1,000 RH Tz | +0 days | None | N.D. | Maraghi et al., 2019 |
| HbsAg | SAG1 + ROP2 | 1,000 RH Tz | 20% survival | None | N.D. | Sun et al., 2020 |
Acute result: n % survival indicates the percentage of vaccinated mice surviving parasite challenge; or +n days indicates the number of days vaccination permitted survival beyond antigen alone group.
Chronic result: indicates reduction in brain cyst numbers beyond antigen alone group. N.D. indicates not determined.
Host proteins as immunostimulants:
Using host immune molecules to produce a stronger immune response, often Th1-directed, is a potent option for DNA vaccination (Ghaffarifar, 2015; Ghaffarifar et al., 2019). The most common host genes used as adjuvants were interleukins capable of directing the host to a Th1 response, considered most desirable for an intracellular infection caused by T. gondii (Quan et al., 2012; Ahmadpour et al., 2017a; Rashid et al., 2017; Ghaffarifar et al., 2019). IL-12 has been the most extensively studied adjuvant due to its role in activating Th1 responses and natural killer cells and has shown varying efficacy (Ghaffarifar, 2015). In some experiments, it led to a decreased survival, such as when used with GRA14, but it has also led to increased survival by 17% when co-administered with both GRA7 and GRA7 + ROP1 (Quan et al., 2012; Ahmadpour et al., 2017a; Ghaffarifar et al., 2019). In chronic challenges, the addition of IL-12 increased the cyst reduction by an additional 5% with GRA7 + ROP1 (Quan et al., 2012). Another interleukin, IL-15, provided an additional 2% cyst reduction beyond profilin antigen alone (Gao et al., 2018). IL-18 enhanced survival by 20% when used with SAG1 (Liu et al., 2010b) and reduced cyst burden by a further 26% compared to ROP13 alone (Wang et al., 2012). Lastly, recently IL-33 was co-administered with ROP5 + ROP18, leading to a 7-day increase in survival for the acute challenge and a further 16% reduction in cysts for the chronic challenge (Zhu et al., 2020). Combinations of interleukins have also been attempted, leading to similar enhancements (Table III).
Other host molecules serving as adjuvants included RANTES, which is secreted from many cell types and is associated with chronic inflammation and wound repair (Cao et al., 2015). As an adjuvant, it functions by increasing the immune response of CD4+ and CD8+ T-cells. RANTES was used with the DNA vaccine encoding peptides from SAG1/GRA2/GRA7/ROP16 and contributed to increased survival by 4 days (Cao et al., 2015).
Other adjuvants:
Non-host derived adjuvants have also been explored to enhance T. gondii-focused DNA vaccines. For example, the flagellin FliC gene from Salmonella, which acts as a TLR5 agonist, increased acute survival by 3 days (Maraghi et al., 2019). CPG-ODN, a synthetic oligodeoxynucleotide containing unmethylated CpG motifs that can activate TLR9, coadministered with SAG1+GRA7 resulted in an additional 11 days of survival from an acute challenge (Mavi et al., 2019). The adjuvant α-GalCer, which stimulates natural killer T-cells, increased acute survival by 4–6 days (Han et al., 2017b).
Non-nucleic acid-based molecules have also been used as coadministered adjuvants for DNA vaccines against T. gondii. The R848 molecule that activates both TLR7 and TLR8 modestly reduced cyst loads (Lu et al., 2018) (Table III).
A protein from the HBV genome, HbsAg, encodes for a B- and T-cell epitope and has also been tested as a possible DNA vaccine adjuvant against T. gondii (Sun et al., 2020). When coadministered with a multiple-antigen DNA vaccine encoding SAG1 + ROP2, HbsAg allowed for a 20% survival against acute challenge.
Aluminum hydroxide gel (alum), when used with the DNA vaccine encoding Toxofilin, led to an increase in survival by 13 days (Song et al., 2017). However, when used with a DNA vaccine encoding peptides from SAG1/AMA1/ROP2/GRA4, the survival against an acute challenge was decreased by 5 days (Roozbehani et al., 2018). However, levamisole, a chemical used to treat parasitic worm infections and thought to foster a Th2 immune response, modestly increased survival by 13% when co-administered with GRA6 (Sun et al., 2011).
In summary, interleukins demonstrated varying degrees of efficacy in acute and chronic conditions: between a decrease in survival by 3 days to an increase in survival rates by 20% when added to antigens for acute challenges, and between 0–39% additional cyst reduction when added to antigens for chronic challenges. Other adjuvants also showed increases in the immune response: an increase in survival of 4–23 days when added to antigens for an acute challenge, and 0–27% additional cyst reduction when added to antigens for chronic challenges. Efficacy is likely a product of the combination of both antigen efficacy and adjuvant activity. A full summary of additional adjuvants can be found in Table III.
CONCLUSION
DNA vaccines are an attractive approach for controlling morbidity and mortality associated with T. gondii infection in humans as well as in susceptible animal species. While many studies have been undertaken, no single vaccine antigen or adjuvants stands out as the single most promising lead for a DNA vaccine candidate, e.g., one capable of 100% cyst reduction and complete protection against death from acute challenge in model outbred species. However, the comparison of antigen efficacy between studies is complicated by the fact that there is no standardization regarding immunization protocol and evaluation criteria, including dose and route of inoculation, or parasite strain and infection number (Liu et al., 2012). Chronic infection efficacy studies are somewhat more standardized in measuring reduction in cyst loads, but variation in mouse and parasite strain is common. Further, it is known to be difficult to measure a significant change in cyst load due to wide variations between individual mice (Watts et al., 2015).
This review attempts to join together varying approaches to provide a basis for comparison, particularly by antigens and adjuvants. In almost all cases, the use of an adjuvant increased the survival time or rate as well as decreased the cyst burden in mice, although the increase is rarely impressive (no more than 20%) (Liu et al., 2010b).
The DNA vaccines with the greatest efficacy against acute infection measured by percent survival were the vaccines using microneme proteins MIC1 + MIC4 + MIC6 and MIC1 + MIC4, which led to 80% and 70% survival over 30 days against an acute challenge of 80 ME49 cysts, respectively (Pinzan et al., 2015; Foroutan et al., 2018).
However, the two most effective DNA vaccines against acute infection, as measured by the number of days surviving beyond the untreated group, were ROP18 + PLP1 with the adjuvant IL-18 and ROP5 + ROP18 with the adjuvant IL-33, which led to an increase in survival by 31 and 30 days, respectively (Chen et al., 2018; Zhu et al., 2020).
The most impressive DNA vaccine attempt against the chronic challenge was the antigen ROP29 with adjuvant R848. Using 20 PRU cysts, the ROP29 and R848 vaccine demonstrated an 80% reduction in cysts (Lu et al., 2018). Another DNA vaccine that showed of similar efficacy was a multi-antigen vaccine encoding Profilin, ROP16, ROP18, MIC6, and CDPK3, which led to an 80% reduction in cyst burden (Zhang et al., 2018a). Two other antigens with impressive cyst reduction include NTPase with a 78% cyst reduction and ROP38 with a 77% cyst reduction (Xu et al., 2014; Zheng et al., 2017b). The adjuvants which contributed to the greatest reduction in cysts compared to an antigen-only vaccine was the combination of IL-15 + IL-21, which led to a 39% further cyst reduction than the antigen MIC8 when challenged with 20 PRU cysts (Li et al., 2014).
The combination of multiple genes, especially the microneme proteins and rhoptry genes, demonstrated more efficacy as antigens in DNA vaccines and may warrant further investigation. For adjuvants, the use of interleukins, especially the combination of IL-15 + IL-21, produced relatively effective results and thus may be of interest to be tested in conjunction with other antigens.
Taken together, the field of T. gondii DNA vaccines has demonstrated that the more potent DNA vaccine candidates have the following features: (1) multiple antigens, (2) antigens either found on the surface of, or is secreted by, the parasite, and (3) inclusion of an adjuvant. Perhaps in the future, some combination of these factors can be combined to produce a more potent outcome than is currently available.
ACKNOWLEDGMENTS
This work is supported by National Institute of Health NIH GM103427 and the University of Nebraska at Omaha Fund for Undergraduate Scholarly Experience (FUSE) program.
LITERATURE CITED
- Ahmadpour E, Sarvi S, Hashemi Soteh M. B, Sharif M, Rahimi M. T, Valadan R, Tehrani M, Khalilian A, Montazeri M, Daryani A. Evaluation of the immune response in BALB/c mice induced by a novel DNA vaccine expressing GRA14 against Toxoplasma gondii. Parasite Immunology. 2017;39:e12419. doi: 10.1111/pim.12419. a. [DOI] [PubMed] [Google Scholar]
- Ahmadpour E, Sarvi S, Hashemi Soteh M. B, Sharif M, Rahimi M. T, Valadan R, Tehrani M, Khalilian A, Montazeri M, Fashi-Ramandi M, et al. Enhancing immune responses to a DNA vaccine encoding Toxoplasma gondii GRA14 by calcium phosphate nanoparticles as an adjuvant. Immunology Letters. 2017;185:40–47. doi: 10.1016/j.imlet.2017.03.006. b. [DOI] [PubMed] [Google Scholar]
- Alday P. H, Doggett J. S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Design Development and Therapy. 2017;11:273–293. doi: 10.2147/DDDT.S60973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh P, Ahmadpour E, Daryani A, Kazemi T, Spotin A, Mahami-Oskouei M, Flynn R. J, Azadi Y, Rajabi S, Sandoghchian S. IL-17 and IL-22 elicited by a DNA vaccine encoding ROP13 associated with protection against Toxoplasma gondii in BALB/c mice. Journal of Cellular Physiology. 2019;234:10782–10788. doi: 10.1002/jcp.27747. [DOI] [PubMed] [Google Scholar]
- Assir M. Z. K, Ahmad H. I, Akram J, Yusuf N. W, Kamran U. An outbreak of pyrimethamine toxicity in patients with ischaemic heart disease in Pakistan. Basic & Clinical Pharmacology & Toxicology. 2014;115:291–296. doi: 10.1111/bcpt.12206. [DOI] [PubMed] [Google Scholar]
- Assolini J. P, Concato V. M, Gonçalves M. D, Carloto A. C. M, Conchon-Costa I, Pavanelli W. R, Melanda F. N, Costa I. N. Nanomedicine advances in toxoplasmosis: Diagnostic, treatment, and vaccine applications. Parasitology Research. 2017;116:1603–1615. doi: 10.1007/s00436-017-5458-2. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A, Pontarollo R, Babiuk S, Loehr B, van Drunen Littel-van den Hurk S. Induction of immune responses by DNA vaccines in large animals. Vaccine. 2003;21:649–658. doi: 10.1016/s0264-410x(02)00574-1. [DOI] [PubMed] [Google Scholar]
- Beauvillain C, Juste M. O, Dion S, Pierre J, Dimier-Poisson I. Exosomes are an effective vaccine against congenital toxoplasmosis in mice. Vaccine. 2009;27:1750–1757. doi: 10.1016/j.vaccine.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Bivas-Benita M, Laloup M, Versteyhe S, Dewit J, De Braekeleer J, Jongert E, Borchard G. Generation of Toxoplasma gondii GRA1 protein and DNA vaccine loaded chitosan particles: Preparation, characterization, and preliminary in vivo studies. International Journal of Pharmaceutics. 2003;266:17–27. doi: 10.1016/s0378-5173(03)00377-6. [DOI] [PubMed] [Google Scholar]
- Black M. W, Boothroyd J. C. Lytic cycle of Toxoplasma gondii. Microbiology and Molecular Biology Reviews. 2000;64:607–623. doi: 10.1128/mmbr.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard N, Gonzalez F, Schaeffer M, Joncker N. T, Cheng T, Shastri A. J, Robey E. A, Shastri N. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nature Immunology. 2008;9:937–944. doi: 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougdour A, Tardieux I, Hakimi M.-A. Toxoplasma exports dense granule proteins beyond the vacuole to the host cell nucleus and rewires the host genome expression. Cellular Microbiology. 2014;16:334–343. doi: 10.1111/cmi.12255. [DOI] [PubMed] [Google Scholar]
- Burrells A, Benavides J, Cantón G, Garcia J. L, Bartley P. M, Nath M, Thomson J, Chianini F, Innes E. A, Katzer F. Vaccination of pigs with the S48 strain of Toxoplasma gondii—Safer meat for human consumption. Veterinary Research. 2015;46:47. doi: 10.1186/s13567-015-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao A, Liu Y, Wang J, Li X, Wang S, Zhao Q, Cong H, He S, Zhou H. Toxoplasma gondii Vaccination with a DNA vaccine encoding T- and B-cell epitopes of SAG1, GRA2, GRA7 and ROP16 elicits protection against acute toxoplasmosis in mice. Vaccine. 2015;33:6757–6762. doi: 10.1016/j.vaccine.2015.10.077. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen H, Guo H, Zheng H. Protective effect of DNA-mediated immunization with a combination of SAG1 and IL-2 gene adjuvant against infection of Toxoplasma gondii in mice. Chinese Medical Journal. 2002;115:1448–1452. [PubMed] [Google Scholar]
- Chen J, Huang S.-Y, Li Z.-Y, Yuan Z.-G, Zhou D.-H, Petersen E, Zhang N.-Z, Zhu X.-Q. Protective immunity induced by a DNA vaccine expressing eIF4A of Toxoplasma gondii against acute toxoplasmosis in mice. Vaccine. 2013;31:1734–1739. doi: 10.1016/j.vaccine.2013.01.027. a. [DOI] [PubMed] [Google Scholar]
- Chen J, Huang S.-Y, Zhou D.-H, Li Z.-Y, Petersen E, Song H.-Q, Zhu X.-Q. DNA immunization with eukaryotic initiation factor-2α of Toxoplasma gondii induces protective immunity against acute and chronic toxoplasmosis in mice. Vaccine. 2013;31:6225–6231. doi: 10.1016/j.vaccine.2013.10.034. b. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Z.-Y, Huang S.-Y, Petersen E, Song H.-Q, Zhou D.-H, Zhu X.-Q. Protective efficacy of Toxoplasma gondii calcium-dependent protein kinase 1 (TgCDPK1) adjuvated with recombinant IL-15 and IL-21 against experimental toxoplasmosis in mice. BMC Infectious Diseases. 2014;14:487. doi: 10.1186/1471-2334-14-487. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Z.-Y, Petersen E, Liu W.-G, Zhu X.-Q. Co-administration of interleukins 7 and 15 with DNA vaccine improves protective immunity against Toxoplasma gondii. Experimental Parasitology. 2016;162:18–23. doi: 10.1016/j.exppara.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou D.-H, Li Z.-Y, Petersen E, Huang S.-Y, Song H.-Q, Zhu X.-Q. Toxoplasma gondii Protective immunity induced by rhoptry protein 9 (TgROP9) against acute toxoplasmosis. Experimental Parasitology. 2014;139:42–48. doi: 10.1016/j.exppara.2014.02.016. b. [DOI] [PubMed] [Google Scholar]
- Chen K, Wang J.-L, Huang S.-Y, Yang W.-B, Zhu W.-N, Zhu X.-Q. Immune responses and protection after DNA vaccination against Toxoplasma gondii calcium-dependent protein kinase 2 (TgCDPK2) Parasite. 2017;24:41. doi: 10.1051/parasite/2017045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Lu S.-H, Tong Q.-B, Lou D, Shi D.-Y, Jia B.-B, Huang G.-P, Wang J.-F. Protective effect of DNA-mediated immunization with liposome-encapsulated GRA4 against infection of Toxoplasma gondii Journal of Zhejiang University. Science. B. 2009;10:512–521. doi: 10.1631/jzus.B0820300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yu M, Hemandez J. A, Li J, Yuan Z.-G, Yan H. Immuno-efficacy of DNA vaccines encoding PLP1 and ROP18 against experimental Toxoplasma gondii infection in mice. Experimental Parasitology. 2018;188:73–78. doi: 10.1016/j.exppara.2018.04.003. [DOI] [PubMed] [Google Scholar]
- Ching X. T, Fong M. Y, Lau Y. L. Evaluation of the protective effect of deoxyribonucleic acid vaccines encoding granule antigen 2 and 5 against acute toxoplasmosis in BALB/c Mice. American Journal of Tropical Medicine and Hygiene. 2017;96:1441–1447. doi: 10.4269/ajtmh.16-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J.-Q, Huang S, Ye W, Fan X.-Y, Huang R, Ye S.-C, Yu C.-Y, Wu W.-Y, Zhou Y, Zhou W, et al. Evaluation of protective immune response induced by a DNA vaccine encoding GRA8 against acute toxoplasmosis in a murine model. Korean Journal of Parasitology. 2018;56:325–334. doi: 10.3347/kjp.2018.56.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong H, Gu Q.-M, Yin H. E, Wang J. W, Zhao Q. L, Zhou H. Y, Li Y, Zhang J. Q. Multi-epitope DNA vaccine linked to the A2/B subunit of cholera toxin protect mice against Toxoplasma gondii. Vaccine. 2008;26:3913–3921. doi: 10.1016/j.vaccine.2008.04.046. [DOI] [PubMed] [Google Scholar]
- Cong H, Gu Q.-M, Zhou H.-Y, Guo L, Yang T.-T, He S.-Y, Li Y, Zhao Q.-L. [Oral mixed DNA vaccine protects mice from infection of Toxoplasma gondii. Chinese Journal of Parasitology & Parasitic Diseases. 2005;23:159–162. [PubMed] [Google Scholar]
- Cong H, Yuan Q, Zhao Q, Zhao L, Yin H, Zhou H, He S, Wang Z. Comparative efficacy of a multi-epitope DNA vaccine via intranasal, peroral, and intramuscular delivery against lethal Toxoplasma gondii infection in mice. Parasites & Vectors. 2014;7:145. doi: 10.1186/1756-3305-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper K. N, Nielsen H. V, Petersen E, Roberts F, Roberts C. W, Alexander J. DNA vaccination with the immunodominant tachyzoite surface antigen (SAG-1) protects against adult acquired Toxoplasma gondii infection but does not prevent maternofoetal transmission. Vaccine. 2003;21:2813–2820. doi: 10.1016/s0264-410x(03)00163-4. [DOI] [PubMed] [Google Scholar]
- Cui X, Lei T, Yang D, Hao P, Li B, Liu Q. Toxoplasma gondii immune mapped protein-1 (TgIMP1) is a novel vaccine candidate against toxoplasmosis. Vaccine. 2012;30:2282–2287. doi: 10.1016/j.vaccine.2012.01.073. [DOI] [PubMed] [Google Scholar]
- Cui Y.-L, He S.-Y, Xue M.-F, Zhang J, Wang H.-X, Yao Y. Protective effect of a multiantigenic DNA vaccine against Toxoplasma gondii with co-delivery of IL-12 in mice. Parasite Immunology. 2008;30:309–313. doi: 10.1111/j.1365-3024.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- Czarnewski P, Araújo E. C. B, Oliveira M. C, Mineo T. W. P, Silva N. M. Recombinant TgHSP70 immunization protects against Toxoplasma gondii brain cyst formation by enhancing inducible nitric oxide expression. Frontiers in Cellular and Infection Microbiology. 2017;7:142. doi: 10.3389/fcimb.2017.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautu G, Munyaka B, Carmen G, Zhang G, Omata Y, Xuenan X, Igarashi M. Toxoplasma gondii DNA vaccination with genes encoding antigens MIC2, M2AP, AMA1 and BAG1 and evaluation of their immunogenic potential. Experimental Parasitology. 2007;116:273–282. doi: 10.1016/j.exppara.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Demar M, Ajzenberg D, Maubon D, Djossou F, Panchoe D, Punwasi W, Valery N, Peneau C, Daigre J.-L, Aznar C, et al. Fatal outbreak of human toxoplasmosis along the Maroni River: Epidemiological, clinical, and parasitological aspects. Clinical Infectious Diseases. 2007;45:e88–e95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- Dumonteil E. DNA Vaccines against protozoan parasites: Advances and challenges. Journal of Biomedicine and Biotechnology. 2007;2007:e90520. doi: 10.1155/2007/90520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunay I. R, Gajurel K, Dhakal R, Liesenfeld O, Montoya J. G. Treatment of toxoplasmosis: Historical perspective, animal models, and current clinical practice. Clinical Microbiology Reviews. 2018;31:e00057–17. doi: 10.1128/CMR.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge R. D, Alaganan A, Tang K, Lou H. J, Turk B. E, Sibley L. D. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host & Microbe. 2014;15:537–550. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachado A, Rodriguez A, Angel S. O, Pinto D. C, Vila I, Acosta A, Amendoeira R. R, Lannes-Vieira J. Protective effect of a naked DNA vaccine cocktail against lethal toxoplasmosis in mice. Vaccine. 2003;21:1327–1335. doi: 10.1016/s0264-410x(02)00692-8. [DOI] [PubMed] [Google Scholar]
- Fang R, Feng H, Nie H, Wang L, Tu P, Song Q, Zhou Y, Zhao J. Construction and immunogenicity of pseudotype baculovirus expressing Toxoplasma gondii SAG1 protein in BALB/c mice model. Vaccine. 2010;28:1803–1807. doi: 10.1016/j.vaccine.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Fang R, Nie H, Wang Z, Tu P, Zhou D, Wang L, He L, Zhou Y, Zhao J. Protective immune response in BALB/c mice induced by a suicidal DNA vaccine of the MIC3 gene of Toxoplasma gondii. Veterinary Parasitology. 2009;164:134–140. doi: 10.1016/j.vetpar.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Foroutan M, Barati M, Ghaffarifar F. Enhancing immune responses by a novel multi-epitope ROP8 DNA vaccine plus interleukin-12 plasmid as a genetic adjuvant against acute Toxoplasma gondii infection in BALB/c mice. Microbial Pathogenesis. 2020;147:104435. doi: 10.1016/j.micpath.2020.104435. a. [DOI] [PubMed] [Google Scholar]
- Foroutan M, Ghaffarifar F, Sharifi Z, Dalimi A. Vaccination with a novel multi-epitope ROP8 DNA vaccine against acute Toxoplasma gondii infection induces strong B and T cell responses in mice. Comparative Immunology Microbiology and Infectious Diseases. 2020;69:101413. doi: 10.1016/j.cimid.2020.101413. b. [DOI] [PubMed] [Google Scholar]
- Foroutan M, Zaki L, Ghaffarifar F. Recent progress in microneme-based vaccines development against Toxoplasma gondii. Clinical and Experimental Vaccine Research. 2018;7:93–103. doi: 10.7774/cevr.2018.7.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroutan M, Zaki L, Tavakoli S, Soltani S, Taghipour A, Ghaffarifar E. Rhomboid antigens are promising targets in the vaccine development against Toxoplasma gondii. EXCLI Journal. 2019;18:259–272. doi: 10.17179/excli2018-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich N, Santos J. M, Liu Y, Palma A. S, Leon E, Saouros S, Kiso M, Blackman M. J, Matthews S, Feizi T, et al. Members of a novel protein family containing microneme adhesive repeat domains act as sialic acid-binding lectins during host cell invasion by apicomplexan parasites. Journal of Biological Chemistry. 2010;285:2064–2076. doi: 10.1074/jbc.M109.060988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Zhang N.-Z, Zhang F.-K, Wang M, Hu L.-Y, Zhu X.-Q. Immune response and protective effect against chronic Toxoplasma gondii infection induced by vaccination with a DNA vaccine encoding profilin. BMC Infectious Diseases. 2018;18:117. doi: 10.1186/s12879-018-3022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffarifar F. Strategies of DNA vaccines against toxoplasmosis. Reviews in Medical Microbiology. 2015;26:88–90. doi: 10.1097/MRM.0000000000000037. [DOI] [Google Scholar]
- Ghaffarifar F, Jafarimodrek M, Vazini H, Sharifi Z, Dalimi A, Dayer M. S. Assessment of DNA vaccine encoding Toxoplasma gondii microneme complete gene and IL-12 as adjuvant in BALB/c mice. Iranian Journal of Basic Medical Sciences. 2019;22:901–907. doi: 10.22038/ijbms.2019.34872.8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold D. A, Kaplan A. D, Lis A, Bett G. C. L, Rosowski E. E, Cirelli K. M, Bongdour A, Sidik S. M, Cirelli K. M, Lourido S, et al. The Toxoplasma dense granule proteins GRA17 and GRA23 mediate the movement of small molecules between the host and the parasitophorous vacuole. Cell Host & Microbe. 2015;17:642–652. doi: 10.1016/j.chom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P, Cao L, Guo Y, Dong H, Yuan S, Yao X, Ren W, Yao L, Xu Z, Sun Q, et al. Toxoplasma gondii Protective immunity induced by a DNA vaccine expressing GRA1 and MIC3 against toxoplasmosis in BALB/c mice. Experimental Parasitology. 2016;166:131–136. doi: 10.1016/j.exppara.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Gong P, Huang X, Yu Q, Li Y, Huang J, Li J, Yang J, Li H, Zhang G, Ren W, et al. The protective effect of a DNA vaccine encoding the Toxoplasma gondii cyclophilin gene in BALB/c mice. Parasite Immunology. 2013;35:140–146. doi: 10.1111/pim.12024. [DOI] [PubMed] [Google Scholar]
- Guo H.-T, Li Z.-Y, Wang J.-L, Geng Z.-Y, Zhu X.-Q. Immune responses induced by pVAX/TgERK7 against Toxoplasma gondii infection in BALB/c mice. Iranian Journal of Parasitology. 2019;14:552–562. [PMC free article] [PubMed] [Google Scholar]
- Hajj H. E, Lebrun M, Fourmaux M. N, Vial H, Dubremetz J. F. Inverted topology of the Toxoplasma gondii ROP5 rhoptry protein provides new insights into the association of the ROP2 protein family with the parasitophorous vacuole membrane. Cellular Microbiology. 2007;9:54–64. doi: 10.1111/j.1462-5822.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- Håkansson S, Charron A. J, Sibley L. D. Toxoplasma evacuoles: A two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO Journal. 2001;20:3132–3144. doi: 10.1093/emboj/20.12.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhou A, Lu G, Zhao G, Sha W, Wang L, Guo J, Zhou J, Zhou H, Cong H, et al. DNA vaccines encoding Toxoplasma gondii cathepsin C 1 induce protection against toxoplasmosis in mice. Korean Journal of Parasitology. 2017;55:505–512. doi: 10.3347/kjp.2017.55.5.505. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Zhou A, Lu G, Zhao G, Wang L, Guo J, Song P, Zhou J, Zhou H, Cong H, et al. Protection via a ROM4 DNA vaccine and peptide against Toxoplasma gondii in BALB/c mice. BMC Infectious Diseases. 2017;17:59. doi: 10.1186/s12879-016-2104-z. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan I. A, Wang S, Xu L, Yan R, Song X, Li X. DNA vaccination with a gene encoding Toxoplasma gondii deoxyribose phosphate aldolase (TgDPA) induces partial protective immunity against lethal challenge in mice. Parasites & Vectors. 2014;7:431. doi: 10.1186/1756-3305-7-431. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan I. A, Wang S, Xu L, Yan R, Song X, Li X. Immunoglobulin and cytokine changes induced following immunization with a DNA vaccine encoding Toxoplasma gondii selenium-dependent glutathione reductase protein. Experimental Parasitology. 2014;146:1–10. doi: 10.1016/j.exppara.2014.08.011. b. [DOI] [PubMed] [Google Scholar]
- Hassan I. A, Wang S, Xu L, Yan R, Song X, XiangRui L. Immunological response and protection of mice immunized with plasmid encoding Toxoplasma gondii glycolytic enzyme malate dehydrogenase. Parasite Immunology. 2014;36:674–683. doi: 10.1111/pim.12146. c. [DOI] [PubMed] [Google Scholar]
- Hiszczyńska-Sawicka E, Li H, Boyu Xu J, Akhtar M, Holec-Gasior L, Kur J, Bickerstaffe R, Stankiewicz M. Induction of immune responses in sheep by vaccination with liposome-entrapped DNA complexes encoding Toxoplasma gondii MIC3 gene. Polish Journal of Veterinary Sciences. 2012;15:3–9. doi: 10.2478/v10181-011-0107-7. [DOI] [PubMed] [Google Scholar]
- Hiszczyńska-Sawicka E, Li H, Boyu Xu J, Holec-Gąsior L, Kur J, Sedcole R, Bickerstaffe R, Stankiewicz M. Modulation of immune response to Toxoplasma gondii in sheep by immunization with a DNA vaccine encoding ROP1 antigen as a fusion protein with ovine CD154. Veterinary Parasitology. 2011;183:72–78. doi: 10.1016/j.vetpar.2011.06.010. a. [DOI] [PubMed] [Google Scholar]
- Hiszczyńska-Sawicka E, Li H, Boyu Xu J, Olędzka G, Kur J, Bickerstaffe R, Stankiewicz M. Comparison of immune response in sheep immunized with DNA vaccine encoding Toxoplasma gondii GRA7 antigen in different adjuvant formulations. Experimental Parasitology. 2010;124:365–372. doi: 10.1016/j.exppara.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Hiszczyńska-Sawicka E, Olędzka G, Holec-Gąsior L, Li H, Xu J. B, Sedcole R, Kur J, Bickerstaffe R, Stankiewicz M. Evaluation of immune responses in sheep induced by DNA immunization with genes encoding GRA1, GRA4, GRA6 and GRA7 antigens of Toxoplasma gondii. Veterinary Parasitology. 2011;177:281–289. doi: 10.1016/j.vetpar.2010.11.047. b. [DOI] [PubMed] [Google Scholar]
- Hobernik D, Bros M. DNA Vaccines—How far from clinical use? International Journal of Molecular Sciences. 2018;19:3605. doi: 10.3390/ijms19113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Batz M. B, Morris J. G. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. Journal of Food Protection. 2012;75:1292–1302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- Hoseinian Khosroshahi K, Ghaffarifar F, D'Souza S, Sharifi Z, Dalimi A. Evaluation of the immune response induced by DNA vaccine cocktail expressing complete SAG1 and ROP2 genes against toxoplasmosis. Vaccine. 2011;29:778–783. doi: 10.1016/j.vaccine.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Hu L.-Y, Zhang N.-Z, Zhang F.-K, Wang M, Gao Q, Wang J.-L, Zhu X.-Q. Resistance to chronic Toxoplasma gondii infection induced by a DNA vaccine expressing GRA16. BioMed Research International. 2017;2017:1295038. doi: 10.1155/2017/1295038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.-Y, Chen K, Wang J.-L, Yang B, Zhu X.-Q. Evaluation of protective immunity induced by recombinant calcium-dependent protein kinase 1 (TgCDPK1) protein against acute toxoplasmosis in mice. Microbial Pathogenesis. 2019;133:103560. doi: 10.1016/j.micpath.2019.103560. [DOI] [PubMed] [Google Scholar]
- Innes E. A, Hamilton C, Garcia J. L, Chryssafidis A, Smith D. A one health approach to vaccines against Toxoplasma gondii. Food and Waterborne Parasitology. 2019;15:e00053. doi: 10.1016/j.fawpar.2019.e00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismael A. B, Hedhli D, Cérède O, Lebrun M, Dimier-Poisson I, Mévélec M.-N. Further analysis of protection induced by the MIC3 DNA vaccine against T. gondii CD4 and CD8 T cells are the major effectors of the MIC3 DNA vaccine-induced protection, both Lectin-like and EGF-like domains of MIC3 conferred protection. Vaccine. 2009;27:2959–2966. doi: 10.1016/j.vaccine.2009.02.107. [DOI] [PubMed] [Google Scholar]
- Ismael A. B, Sekkai D, Collin C, Bout D, Mévélec M.-N. The MIC3 gene of Toxoplasma gondii is a novel potent vaccine candidate against toxoplasmosis. Infection and Immunity. 2003;71:6222–6228. doi: 10.1128/IAI.71.11.6222-6228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongert E, Craeye S. D, Dewit J, Huygen K. GRA7 provides protective immunity in cocktail DNA vaccines against Toxoplasma gondii. Parasite Immunology. 2007;29:445–453. doi: 10.1111/j.1365-3024.2007.00961.x. [DOI] [PubMed] [Google Scholar]
- Jongert E, Melkebeek V, De Craeye S, Dewit J, Verhelst D, Cox E. An enhanced GRA1-GRA7 cocktail DNA vaccine primes anti-Toxoplasma immune responses in pigs. Vaccine. 2008;26:1025–1031. doi: 10.1016/j.vaccine.2007.11.058. a. [DOI] [PubMed] [Google Scholar]
- Jongert E, Verhelst D, Abady M, Petersen E, Gargano N. Protective Th1 immune responses against chronic toxoplasmosis induced by a protein-protein vaccine combination but not by its DNA-protein counterpart. Vaccine. 2008;26:5289–5295. doi: 10.1016/j.vaccine.2008.07.032. b. [DOI] [PubMed] [Google Scholar]
- Kafsack B. F. C, Pena J. D. O, Coppens I, Ravindran S, Boothroyd J. C, Carruthers V. B. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science. 2009;323:530–533. doi: 10.1126/science.1165740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaminets A, Hunn J. P, Könen-Waisman S, Zhao Y. O, Preukschat D, Coers J, Boyle J. P, Ong Y.-C, Boothroyd J. C, Reichmann G, et al. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cellular Microbiology. 2010;12:939–961. doi: 10.1111/j.1462-5822.2010.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labesse G, Gelin M, Bessin Y, Lebrun M, Papoin J, Cerdan R, Arold S. T, Dubremetz J.-F. ROP2 from Toxoplasma gondii A virulence factor with a protein-kinase fold and no enzymatic activity. Structure. 2009;17:139–146. doi: 10.1016/j.str.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Leyva R, Hérion P, Saavedra R. Genetic immunization with plasmid DNA coding for the ROP2 protein of Toxoplasma gondii. Parasitology Research. 2001;87:70–79. doi: 10.1007/s004360000296. [DOI] [PubMed] [Google Scholar]
- Li B, Oledzka G, McFarlane R. G, Spellerberg M. B, Smith S. M, Gelder F. B, Kur J, Stankiewicz M M. Immunological response of sheep to injections of plasmids encoding Toxoplasma gondii SAG1 and ROP1 genes. Parasite Immunology. 2010;32(9–10):671–683. doi: 10.1111/j.1365-3024.2010.01228.x. [DOI] [PubMed] [Google Scholar]
- Li J, Huang X, Zhang G, Gong P, Zhang X, Wu L. Immune response and protective efficacy against homologous challenge in BALB/c mice vaccinated with DNA vaccine encoding Toxoplasma gondii actin depolymerizing factor gene. Veterinary Parasitology. 2011;179:1–6. doi: 10.1016/j.vetpar.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Li X-Z, Lv L, Zhang X, Anchang K. Y, Abdullahi A. Y, Tu L, Wang X, Xia L, Zhang X.-X, Feng W, et al. Recombinant canine adenovirus type-2 expressing TgROP16 provides partial protection against acute Toxoplasma gondii infection in mice. Infection Genetics and Evolution. 2016;45:447–453. doi: 10.1016/j.meegid.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Li X.-Z, Wang X.-H, Xia L.-J, Weng Y.-B, Hernandez J. A, Tu L.-Q, Li L.-T, Li S.-J, Yuan Z.-G. Protective efficacy of recombinant canine adenovirus type-2 expressing TgROP18 (CAV-2-ROP18) against acute and chronic Toxoplasma gondii infection in mice. BMC Infectious Diseases. 2015;15:114. doi: 10.1186/s12879-015-0815-1. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.-Y, Chen J, Lu J, Wang C. R, Zhu X.-Q. Sequence variation in ROP8 gene among Toxoplasma gondii isolates from different hosts and geographical localities. Genetics and molecular research GMR. 2015;14:11403–11409. doi: 10.4238/2015.September.25.8. b. [DOI] [PubMed] [Google Scholar]
- Li Z.-Y, Chen J, Petersen E, Zhou D.-H, Huang S.-Y, Song H.-Q, Zhu X.-Q. Synergy of mIL-21 and mIL-15 in enhancing DNA vaccine efficacy against acute and chronic Toxoplasma gondii infection in mice. Vaccine. 2014;32:3058–3065. doi: 10.1016/j.vaccine.2014.03.042. [DOI] [PubMed] [Google Scholar]
- Li Z.-Y, Lu J, Zhang N.-Z, Chen J, Zhu X.-Q. Immune responses induced by HSP60 DNA vaccine against Toxoplasma gondii infection in Kunming mice. Korean Journal of Parasitology. 2018;56:237–245. doi: 10.3347/kjp.2018.56.3.237. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.-Y, Lu J, Zhang N.-Z, Elsheikha H. M, Hou J.-L, Guo H.-T, Zhu X.-Q. Immunization with plasmid DNA expressing Heat Shock Protein 40 confers prophylactic protection against chronic Toxoplasma gondii infection in Kunming mice. Parasite. 2018;25:37. doi: 10.1051/parasite/2018040. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. M, Yuan Z. G, Peng G. H, Zhou D. H, He X. H, Yan C, Yin C.C, He Y, Lin R. Q, Song H. Q, et al. Toxoplasma gondii microneme protein 8 (MIC8) is a potential vaccine candidate against toxoplasmosis. Parasitology Research. 2010;106:1079–1084. doi: 10.1007/s00436-010-1742-0. a. [DOI] [PubMed] [Google Scholar]
- Liu Q, Gao S, Jiang L, Shang L, Men J, Wang Z, Zhai Y, Xia Z, Hu R, Zhang X, et al. A recombinant pseudorabies virus expressing TgSAG1 protects against challenge with the virulent Toxoplasma gondii RH strain and pseudorabies in BALB/c mice. Microbes and Infection. 2008;10:1355–1362. doi: 10.1016/j.micinf.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Liu Q, Shang L, Jin H, Wei F, Zhu X.-Q, Gao H. The protective effect of a Toxoplasma gondii SAG1 plasmid DNA vaccine in mice is enhanced with IL-18. Research in Veterinary Science. 2010;89:93–97. doi: 10.1016/j.rvsc.2010.01.007. b. [DOI] [PubMed] [Google Scholar]
- Liu Q, Singla L. D, Zhou H. Vaccines against Toxoplasma gondii Status, challenges and future directions. Human Vaccines & Immunotherapeutics. 2012;8:1305–1308. doi: 10.4161/hv.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wang F, Wang G, Zhao Q, Min J, Wang S, Cong H, Li Y, He S, Zhou H. Toxoplasma gondii Immune response and protective efficacy induced by ROP16/GRA7 multicomponent DNA vaccine with a genetic adjuvant B7-2. Human Vaccines & Immunotherapeutics. 2014;10:184–191. doi: 10.4161/hv.26703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shi L, Cheng Y, Fan G, Ren H, Yuan Y. Evaluation of protective effect of multi-epitope DNA vaccine encoding six antigen segments of Toxoplasma gondii in mice. Parasitology Research. 2009;105:267–274. doi: 10.1007/s00436-009-1393-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cao A, Li Y, Li X, Cong H, He S, Zhou H. Immunization with a DNA vaccine encoding Toxoplasma gondii Superoxide dismutase (TgSOD) induces partial immune protection against acute toxoplasmosis in BALB/c mice. BMC Infectious Diseases. 2017;17:403. doi: 10.1186/s12879-017-2507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço E. V, Bernardes E. S, Silva N. M, Mineo J. R, Panunto-Castelo A, Roque-Barreira M. C. Immunization with MIC1 and MIC4 induces protective immunity against Toxoplasma gondii. Microbes and Infection. 2006;8:1244–1251. doi: 10.1016/j.micinf.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Lu G, Wang L, Zhou A, Han Y, Guo J, Song P, Zhou H, Cong H, Zhao Q, He S. Epitope analysis, expression and protection of SAG5A vaccine against Toxoplasma gondii. Acta Tropica. 2015;146:66–72. doi: 10.1016/j.actatropica.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Lu G, Zhou A, Meng M, Wang L, Han Y, Guo J, Zhou H, Cong H, Zhao Q, Zhu X.-Q, et al. Alpha-galactosylceramide enhances protective immunity induced by DNA vaccine of the SAG5D gene of Toxoplasma gondii. BMC Infectious Diseases. 2014;14:3862. doi: 10.1186/s12879-014-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Zhou J, Zhao Y. H, Wang L. DNA vaccine ROP29 from Toxoplasma gondii containing R848 enhances protective immunity in mice. Parasite Immunology. 2018;40:e12578. doi: 10.1111/pim.12578. [DOI] [PubMed] [Google Scholar]
- Lu G, Zhou J, Zhou A, Han Y, Guo J, Song P, Zhou H, Cong H, Hou M, Wang L, et al. SAG5B and SAG5C combined vaccine protects mice against Toxoplasma gondii infection. Parasitology International. 2017;66:596–602. doi: 10.1016/j.parint.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Maraghi S, Ghadiri A. A, Tavalla M, Shojaee S, Abdizadeh R. Evaluation of immunogenicity and protective effect of DNA vaccine encoding surface antigen1 (SAG1) of Toxoplasma gondii and TLR-5 ligand as a genetic adjuvant against acute toxoplasmosis in BALB/c mice. Biologicals. 2019;62:39–49. doi: 10.1016/j.biologicals.2019.10.002. [DOI] [PubMed] [Google Scholar]
- Martin V, Supanitsky A, Echeverria P. C, Litwin S, Tanos T, Roodt A. R. D, Guarnera E. A, Angel S. O. Recombinant GRA4 or ROP2 protein combined with alum or the gra4 gene provides partial protection in chronic murine models of toxoplasmosis. Clinical and Diagnostic Laboratory Immunology. 2004;11:704–710. doi: 10.1128/CDLI.11.4.704-710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavi S. A, Modarressi M. H, Mohebali M, Shojaee S, Zeraati H, Teimouri A, Keshavarz H. Assessment of the immunogenicity and protective efficiency of a novel dual-promoter DNA vaccine, harboring SAG1 and GRA7 genes, from RH strain of Toxoplasma gondii in BALB/c mice. Infection and Drug Resistance. 2019;12:2519–2530. doi: 10.2147/IDR.S209270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey G. A, Martin H. L, Bristow G. C, Webster J. P. Toxoplasma gondii infection and behaviour—Location, location, location? Journal of Experimental Biology. 2013;216:113–119. doi: 10.1242/jeb.074153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland M. M, Zach S. J, Wang X, Potluri L.-P, Neville A. J, Vennerstrom J. L, Davis P. H. Review of experimental compounds demonstrating anti-Toxoplasma activity. Antimicrobial Agents and Chemotherapy. 2016;60:7017–7034. doi: 10.1128/AAC.01176-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner M, Reiss M, Viebig N, Carruthers V. B, Toursel C, Tomavo S, Ajioka J. W, Soldati D. A family of transmembrane microneme proteins of Toxoplasma gondii contain EGF-like domains and function as escorters. Journal of Cell Science. 2002;115:563–574. doi: 10.1242/jcs.115.3.563. [DOI] [PubMed] [Google Scholar]
- Meng M, He S, Zhao G, Bai Y, Zhou H, Cong H, Lu G, Zhao Q, Zhu X.-Q. Evaluation of protective immune responses induced by DNA vaccines encoding Toxoplasma gondii surface antigen 1 (SAG1) and 14-3-3 protein in BALB/c mice. Parasites & Vectors. 2012;5:273. doi: 10.1186/1756-3305-5-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng M, Zhou A, Lu G, Wang L, Zhao G, Han Y, Zhou H, Cong H, Zhao Q, Zhu X.-Q, et al. DNA prime and peptide boost immunization protocol encoding the Toxoplasma gondii GRA4 induces strong protective immunity in BALB/c mice. BMC Infectious Diseases. 2013;13:494. doi: 10.1186/1471-2334-13-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mévélec M.-N, Bout D, Desolme B, Marchand H, Magné R, Bruneel O, Buzoni-Gatel D. Evaluation of protective effect of DNA vaccination with genes encoding antigens GRA4 and SAG1 associated with GM-CSF plasmid, against acute, chronical and congenital toxoplasmosis in mice. Vaccine. 2005;23:4489–4499. doi: 10.1016/j.vaccine.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Min J, Qu D, Li C, Song X, Zhao Q, Li X, Yang Y, Liu Q, He S, Zhou H. Enhancement of protective immune responses induced by Toxoplasma gondii dense granule antigen 7 (GRA7) against toxoplasmosis in mice using a prime-boost vaccination strategy. Vaccine. 2012;30:5631–5636. doi: 10.1016/j.vaccine.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Naserifar R, Ghaffarifar F, Dalimi A, Sharifi Z, Solhjoo K, Hosseinian Khosroshahi K. Evaluation of immunogenicity of cocktail DNA vaccine containing plasmids encoding complete GRA5, SAG1, and ROP2 antigens of Toxoplasma gondii in BALB/C mice. Iranian Journal of Parasitology 590–598. 2015. [PMC free article] [PubMed]
- Neville A. J, Zach S. J, Wang X, Larson J. J, Judge A. K, Davis L. A, Vennerstrom J. L, Davis P. H. Clinically available medicines demonstrating anti-Toxoplasma activity. Antimicrobial Agents and Chemotherapy. 2015;59:7161–7169. doi: 10.1128/AAC.02009-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H. V, Di Cristina M, Beghetto E, Spadoni A, Petersen E, Gargano N. Toxoplasma gondii DNA vaccination with bradyzoite antigens induces protective immunity in mice against oral infection with parasite cysts. Experimental Parasitology. 2006;112:274–279. doi: 10.1016/j.exppara.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Pagheh A. S, Sarvi S, Gholami S, Asgarian-Omran H, Valadan R, Hassannia H, Ahmadpour E, Fasihi-Ramadie M, Dodangeh S, Hosseni-Khah Z, et al. Protective efficacy induced by DNA prime and recombinant protein boost vaccination with Toxoplasma gondii GRA14 in mice. Microbial Pathogenesis. 2019;134:103601. doi: 10.1016/j.micpath.2019.103601. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S, Fong M. Y, Ramaswamy K, Lau Y. L. Protective immune response in BALB/c mice induced by DNA vaccine of the ROP8 gene of Toxoplasma gondii. American Journal of Tropical Medicine and Hygiene. 2013;88:883–887. doi: 10.4269/ajtmh.12-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzan C. F, Sardinha-Silva A, Almeida F, Lai L, Lopes C. D, Lourenço E. V, Panunto-Castelo A, Matthews S, Roque-Barreira M. C. Vaccination with recombinant microneme proteins confers protection against experimental toxoplasmosis in mice. Public Library of Science. PLOS ONE. 2015;10:e0143087. doi: 10.1371/journal.pone.0143087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D, Han J, Du A. Evaluation of protective effect of multiantigenic DNA vaccine encoding MIC3 and ROP18 antigen segments of Toxoplasma gondii in mice. Parasitology Research. 2013;112:2593–2599. doi: 10.1007/s00436-013-3425-0. [DOI] [PubMed] [Google Scholar]
- Qu D, Yu H, Wang S, Cai W, Du A. Induction of protective immunity by multiantigenic DNA vaccine delivered in attenuated Salmonella typhimurium against Toxoplasma gondii infection in mice. Veterinary Parasitology. 2009;166:220–227. doi: 10.1016/j.vetpar.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Quan J.-H, Chu J.-Q, Ismail H. A. H. A, Zhou W, Jo E.-K, Cha G.-H, Lee Y.-H. Induction of protective immune responses by a multiantigenic DNA vaccine encoding GRA7 and ROP1 of Toxoplasma gondii. Clinical and Vaccine Immunology. 2012;19:666–674. doi: 10.1128/CVI.05385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi M. T, Sarvi S, Sharif M, Abediankenari S, Ahmadpour E, Valadan R, Ramandie M. F, Hosseini S.-A, Daryani A. Immunological evaluation of a DNA cocktail vaccine with co-delivery of calcium phosphate nanoparticles (CaPNs) against the Toxoplasma gondii RH strain in BALB/c mice. Parasitology Research. 2017;116:609–616. doi: 10.1007/s00436-016-5325-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan C, Maier S, Walker R. A, Rehrauer H, Joekel D. E, Winiger R. R, Basso W. U, Grigg M. E, Hehl A. B, Deplazes P, et al. An experimental genetically attenuated live vaccine to prevent transmission of Toxoplasma gondii by cats. Nature Publishing Group. Scientific Reports. 2019;9:1474. doi: 10.1038/s41598-018-37671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid I, Hedhli D, Moiré N, Pierre J, Debierre-Grockiego F, Dimier-Poisson I, Mévélec M.-N. Immunological responses induced by a DNA vaccine expressing RON4 and by immunogenic recombinant protein RON4 failed to protect mice against chronic toxoplasmosis. Vaccine. 2011;29:8838–8846. doi: 10.1016/j.vaccine.2011.09.099. [DOI] [PubMed] [Google Scholar]
- Rashid I, Moiré N, Héraut B, Dimier-Poisson I, Mévélec M.-N. Enhancement of the protective efficacy of a ROP18 vaccine against chronic toxoplasmosis by nasal route. Medical Microbiology and Immunology. 2017;206:53–62. doi: 10.1007/s00430-016-0483-9. [DOI] [PubMed] [Google Scholar]
- Reiss M, Viebig N, Brecht S, Fourmaux M.-N, Soete M, Di Cristina M, Dubremetz J. F, Soldati D. Identification and characterization of an escorter for two secretory adhesins in Toxoplasma gondii. Journal of Cell Biology. 2001;152:563–578. doi: 10.1083/jcb.152.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozbehani M, Falak R, Mohammadi M, Hemphill A, Razmjou E, Meamar A. R, Masoori L, Khoshmirsafa M, Moradi M, Gharavi M.-J. Characterization of a multi-epitope peptide with selective MHC-binding capabilities encapsulated in PLGA nanoparticles as a novel vaccine candidate against Toxoplasma gondii infection. Vaccine. 2018;36:6124–6132. doi: 10.1016/j.vaccine.2018.08.068. [DOI] [PubMed] [Google Scholar]
- Roque-Reséndiz J. L, Rosales R, Herion P. MVA ROP2 vaccinia virus recombinant as a vaccine candidate for toxoplasmosis. Parasitology. 2004;128:397–405. doi: 10.1017/s0031182003004761. [DOI] [PubMed] [Google Scholar]
- Rosenberg C, De Craeye S, Jongert E, Gargano N, Beghetto E, Del Porto P, Vorup-Jensen T, Petersen E. Induction of partial protection against infection with Toxoplasma gondii genotype II by DNA vaccination with recombinant chimeric tachyzoite antigens. Vaccine. 2009;27:2489–2498. doi: 10.1016/j.vaccine.2009.02.058. [DOI] [PubMed] [Google Scholar]
- Sacks D. L, Peters N. C, Bethony J. M. Bloom B. R, Lambert P.-H, editors. Vaccines against parasites. The Vaccine Book 2nd ed. 2016. pp. 331–360. In . (eds.) Academic Press, London, U.K. p. [DOI]
- Scallan E, Hoekstra R. M, Angulo F. J, Tauxe R. V, Widdowson M. A, Roy S. L, Jones J. L, Griffin P. M. Foodborne illness acquired in the United States—Major pathogens. Emerging Infectious Diseases. 2011;17:7–15. doi: 10.3201/eid1701.p11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza T, D'Souza S, Laloup M, Dewit J, De Braekeleer J, Verschueren H, Vercammen M, Huygen K, Jongert E. A GRA1 DNA vaccine primes cytolytic CD8+ T cells to control acute Toxoplasma gondii infection. Infection and Immunity. 2003;71:309–316. doi: 10.1128/IAI.71.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L, Liu Q, Liu W, Men J, Gao S, Jiang L, Wang Z, Zhai Y, Jin H, Lian H, et al. Protection in mice immunized with a heterologous prime-boost regime using DNA and recombinant pseudorabies expressing TgSAG1 against Toxoplasma gondii challenge. Vaccine. 2009;27:2741–2745. doi: 10.1016/j.vaccine.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Shedlock D. J, Weiner D. B. DNA vaccination: Antigen presentation and the induction of immunity. Journal of Leukocyte Biology. 2000;68:793–806. [PubMed] [Google Scholar]
- Smith T. R. F, Patel A, Ramos S, Elwood D, Zhu X, Yan J, Gary E. N, Walker S. N, Schultheis K, Purwar M, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nature Communications. 2020;11:2601. doi: 10.1038/s41467-020-16505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonaimuthu P, Ching X. T, Fong M. Y, Kalyanasundaram R, Lau Y. L. Induction of protective immunity against toxoplasmosis in BALB/c mice vaccinated with Toxoplasma gondii rhoptry-1. Frontiers in Microbiology. 2016;7:808. doi: 10.3389/fmicb.2016.00808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, He S, Zhou A, Lv G, Guo J, Zhou J, Han Y, Zhou H, Hao Z, Cong H. Vaccination with toxofilin DNA in combination with an alum-monophosphoryl lipid A mixed adjuvant induces significant protective immunity against Toxoplasma gondii. BMC Infectious Diseases. 2017;17:19. doi: 10.1186/s12879-016-2147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P. X, Yao S. H, Yao Y, Zhou J, Li Q. F, Cao Y. H, He S. Y. Epitope analysis and efficacy evaluation of phosphatase 2C (PP2C) DNA vaccine against Toxoplasma gondii infection. Journal of Parasitology. 2020;106:513–521. doi: 10.1645/18-210. [DOI] [PubMed] [Google Scholar]
- Sun H, Li J, Xiao T, Huang X.-D, Wang L, Huang B, Yin K, Liu G, Xu C, Wei Q. Protective immunity induced by a DNA vaccine cocktail expressing TgSAG1, TgROP2, and the genetic adjuvant HBsAg against Toxoplasma gondii infection. Microbial Pathogenesis. 2020;147:104441. doi: 10.1016/j.micpath.2020.104441. [DOI] [PubMed] [Google Scholar]
- Sun X.-M, Zou J, Saeed AA E, Yan W.-C, Liu X.-Y, Suo X, Wang H, Chen Q.-J. DNA vaccination with a gene encoding Toxoplasma gondii GRA6 induces partial protection against toxoplasmosis in BALB/c mice. Parasites & Vectors. 2011;4:213. doi: 10.1186/1756-3305-4-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Q, Fang R, Zhang W, Wang Y, Cheng J, Li Y, Fang K, Khan M. K, Hu M, Zhou Y, et al. Protective immunity induced by a DNA vaccine-encoding Toxoplasma gondii microneme protein 11 against acute toxoplasmosis in BALB/c mice. Parasitology Research. 2013;112:2871–2877. doi: 10.1007/s00436-013-3458-4. [DOI] [PubMed] [Google Scholar]
- Turetzky J. M, Chu D. K, Hajagos B. E, Bradley P. J. Processing and secretion of ROP13: A unique Toxoplasma effector protein. International Journal for Parasitology. 2010;40:1037–1044. doi: 10.1016/j.ijpara.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen M, Scorza T, Huygen K, De Braekeleer J, Diet R, Jacobs D, Saman E, Verschueren H. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infection and Immunity. 2000;68:38–45. doi: 10.1128/IAI.68.1.38-45.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Khanna P. Development of Toxoplasma gondii vaccine. Human Vaccines & Immunotherapeutics. 2013;9:291–293. doi: 10.4161/hv.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, He S, Yao Y, Cong H, Zhao H, Li T, Zhu X.-Q. Toxoplasma gondii Protective effect of an intranasal SAG1 and MIC4 DNA vaccine in mice. Experimental Parasitology. 2009;122:226–232. doi: 10.1016/j.exppara.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Wang H.-L, Wang Y.-J, Pei Y.-J, Bai J.-Z, Yin L.-T, Guo R, Yin G.-R. DNA vaccination with a gene encoding Toxoplasma gondii rhoptry protein 17 induces partial protective immunity against lethal challenge in mice. Parasite. 2016;23:4. doi: 10.1051/parasite/2016004. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-L, Zhang N.-Z, Li T.-T, He J.-J, Elsheikha H. M, Zhu X.-Q. Advances in the development of anti-Toxoplasma gondii vaccines: Challenges, opportunities, and perspectives. Trends in Parasitology. 2019;35:239–253. doi: 10.1016/j.pt.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Wang L, Lu G, Zhou A, Han Y, Guo J, Zhou H, Cong H, He S. Evaluation of immune responses induced by rhoptry protein 5 and rhoptry protein 7 DNA vaccines against Toxoplasma gondii. Parasite Immunology. 2016;38:209–217. doi: 10.1111/pim.12306. b. [DOI] [PubMed] [Google Scholar]
- Wang P.-Y, Yuan Z.-G, Peterson E, Li J, Zhang X.-X, Li X.-Z, Li H.-Z, Lv Z.-C, Cheng T, Ren D, et al. Protective efficacy of a Toxoplasma gondii rhoptry protein 13 plasmid DNA vaccine in mice. Clinical and Vaccine Immunology. 2012;19:1916–1920. doi: 10.1128/CVI.00397-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Hassan I. A, Liu X, Xu L, Yan R, Song X, Li X. Immunological changes induced by Toxoplasma gondii glutathione-s-transferase (TgGST) delivered as a DNA vaccine. Research in Veterinary Science. 2015;99:157–164. doi: 10.1016/j.rvsc.2014.12.006. a. [DOI] [PubMed] [Google Scholar]
- Wang S, Wang Y, Sun X, Zhang Z, Liu T, Gadahi J. A, Hassan I. A, Xu L, Yan R, Song X, et al. Protective immunity against acute toxoplasmosis in BALB/c mice induced by a DNA vaccine encoding Toxoplasma gondii elongation factor 1-alpha. BMC Infectious Diseases. 2015;15:448. doi: 10.1186/s12879-015-1220-5. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Wang Y, Sun X, Zhang Z, Liu T, Gadahi J. A, Xu L, Yan R, Song X, Li X. Protective immunity against acute toxoplasmosis in BALB/c mice induced by a DNA vaccine encoding Toxoplasma gondii 10kDa excretory–secretory antigen (TgESA10) Veterinary Parasitology. 2015;214:40–48. doi: 10.1016/j.vetpar.2015.09.016. c. [DOI] [PubMed] [Google Scholar]
- Watts E, Zhao Y, Dhara A, Eller B, Patwardhan A, Sinai A. P. Novel approaches reveal that Toxoplasma gondii bradyzoites within tissue cysts are dynamic and replicating entities in vivo. mBio. 2015;6:e01155–15. doi: 10.1128/mBio.01155-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q, Li J, Fu T.-X, Bai X.-L, Cui Y, Zhang D.-B, Wang H.-F, Liu Y.-B, Fu B, Zai D.-F, et al. [Studies on the immuno-protection of ROP2 nuclei acid vaccine in Toxoplasma gondii infection] Chinese Journal of Parasitology & Parasitic Diseases. 2006;24:337–341. [PubMed] [Google Scholar]
- Wu X.-N, Lin J, Lin X, Chen J, Chen Z.-L, Lin J.-Y. Multicomponent DNA vaccine-encoding Toxoplasma gondii GRA1 and SAG1 primes: Anti-Toxoplasma immune response in mice. Parasitology Research. 2012;111:2001–2009. doi: 10.1007/s00436-012-3047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.-P, Liu W.-G, Xu Q.-M, Zhu X.-Q, Chen J. Evaluation of immune protection against Toxoplasma gondii infection in mice induced by a multi-antigenic DNA vaccine containing TgGRA24, TgGRA25 and TgMIC6. Parasite. 2019;26:58. doi: 10.1051/parasite/2019050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang N.-Z, Tan Q.-D, Chen J, Lu J, Xu Q.-M, Zhu X.-Q. Evaluation of immuno-efficacy of a novel DNA vaccine encoding Toxoplasma gondii rhoptry protein 38 (TgROP38) against chronic toxoplasmosis in a murine model. BMC Infectious Diseases. 2014;14:525. doi: 10.1186/1471-2334-14-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, He S, Cui Y, Yao Y, Wang H. Evaluation of the immune response elicited by multi-antigenic DNA vaccine expressing SAG1, ROP2 and GRA2 against Toxoplasma gondii. Parasitology International. 2008;57:424–429. doi: 10.1016/j.parint.2008.05.001. a. [DOI] [PubMed] [Google Scholar]
- Xue M, He S, Zhang J, Cui Y, Yao Y, Wang H. Comparison of cholera toxin A2/B and murine interleukin-12 as adjuvants of Toxoplasma multi-antigenic SAG1-ROP2 DNA vaccine. Experimental Parasitology. 2008;119:352–357. doi: 10.1016/j.exppara.2008.03.005. b. [DOI] [PubMed] [Google Scholar]
- Yan H.-K, Yuan Z.-G, Petersen E, Zhang X.-X, Zhou D.-H, Liu Q, He Y, Lin R.-Q, Xu M.-J, Chen X.-L, et al. Toxoplasma gondii Protective immunity against experimental toxoplasmosis induced by a DNA vaccine encoding the perforin-like protein 1. Experimental Parasitology. 2011;128:38–43. doi: 10.1016/j.exppara.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Yan H.-K, Yuan Z.-G, Song H.-Q, Petersen E, Zhou Y, Ren D, Zhou D.-H, Li H.-X, Lin R.-Q, Yang G.-L, et al. Vaccination with a DNA vaccine coding for perforin-like protein 1 and MIC6 induces significant protective immunity against Toxoplasma gondii. Clinical and Vaccine Immunology. 2012;19:684–689. doi: 10.1128/CVI.05578-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, He S, Jiang H, Gu Q, Cong H, Zhou H, Zhang J, Li Y, Zhao Q. [Construction of monovalent and compound nucleic acid vaccines against Toxoplasma gondii with gene encoding p30] Chinese Journal of Parasitology & Parasitic Diseases. 2005;23:14–17. [PubMed] [Google Scholar]
- Yang W.-B, Zhou D.-H, Zou Y, Chen K, Liu Q, Wang J.-L, Zhu X.-Q, Zhao G.-H. Vaccination with a DNA vaccine encoding Toxoplasma gondii ROP54 induces protective immunity against toxoplasmosis in mice. Acta Tropica. 2017;176:427–432. doi: 10.1016/j.actatropica.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Ybañez R. H. D, Ybañez A. P, Nishikawa Y. Review on the current trends of toxoplasmosis serodiagnosis in humans. Frontiers in Cellular and Infection Microbiology. 2020;10:204. doi: 10.3389/fcimb.2020.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Zhao L, Wang T, Zhou H, He S, Cong H. A Toxoplasma gondii vaccine encoding multistage antigens in conjunction with ubiquitin confers protective immunity to BALB/c mice against parasite infection. Parasites & Vectors. 2015;8:498. doi: 10.1186/s13071-015-1108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Tostanoski L. H, Peter L, Mercado N. B, McMahan K, Mahrokhian S. H, Nkolola J. P, Liu J, Li Z, Chandrashekar A, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z.-G, Ren D, Zhou D.-H, Zhang X.-X, Petersen E, Li X.-Z, Zhou Y, Yang G.-L, Zhu X.-Q. Evaluation of protective effect of pVAX-TgMIC13 plasmid against acute and chronic Toxoplasma gondii infection in a murine model. Vaccine. 2013;31:3135–3139. doi: 10.1016/j.vaccine.2013.05.040. [DOI] [PubMed] [Google Scholar]
- Yuan Z.-G, Zhang X.-X, He X.-H, Petersen E, Zhou D.-H, He Y, Lin R.-Q, Li X.-Z, Chen X.-L, Shi X.-R, et al. Protective immunity induced by Toxoplasma gondii rhoptry protein 16 against toxoplasmosis in mice. Clinical and Vaccine Immunology. 2011;18:119–124. doi: 10.1128/CVI.00312-10. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z.-G, Zhang X.-X, Lin R.-Q, Petersen E, He S, Yu M, He X.-H, Zhou D.-H, He Y, Li H.-X, et al. Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine. 2011;29:6614–6619. doi: 10.1016/j.vaccine.2011.06.110. b. [DOI] [PubMed] [Google Scholar]
- Zhang G, Huong V. T. T, Battur B, Zhou J, Zhang H, Liao M, Kawase O, Lee E. G, Dautu G, Igarashi M, et al. A heterologous prime-boost vaccination regime using DNA and a vaccinia virus, both expressing GRA4, induced protective immunity against Toxoplasma gondii infection in mice. Parasitology. 2007;134:1339–1346. doi: 10.1017/S0031182007002892. a. [DOI] [PubMed] [Google Scholar]
- Zhang J, He S, Jiang H, Yang T, Cong H, Zhou H, Zhang J, Gu Q, Li Y, Zhao Q. Evaluation of the immune response induced by multiantigenic DNA vaccine encoding SAG1 and ROP2 of Toxoplasma gondii and the adjuvant properties of murine interleukin-12 plasmid in BALB/c mice. Parasitology Research. 2007;101:331–338. doi: 10.1007/s00436-007-0465-3. b. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhao L, Song J, Li Y, Zhao Q, He S, Cong H. DNA vaccine encoding the Toxoplasma gondii bradyzoite-specific surface antigens SAG2CDX protect BALB/c mice against type II parasite infection. Vaccine. 2013;31:4536–4540. doi: 10.1016/j.vaccine.2013.07.065. a. [DOI] [PubMed] [Google Scholar]
- Zhang N.-Z, Gao Q, Wang M, Elsheikha H. M, Wang B, Wang J.-L, Zhang F.-K, Hu L.-Y, Zhu X.-Q. Immunization with a DNA vaccine cocktail encoding TgPF, TgROP16, TgROP18, TgMIC6, and TgCDPK3 genes protects mice against chronic toxoplasmosis. Frontiers in Immunology. 2018;9:1505. doi: 10.3389/fimmu.2018.01505. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.-Z, Gao Q, Wang M, Hou J.-L, Zhang F.-K, Hu L.-Y, Zhu X.-Q. Protective efficacy against acute and chronic Toxoplasma gondii infection induced by immunization with the DNA vaccine TgDOC2C. Frontiers in Microbiology. 2018;9:2965. doi: 10.3389/fmicb.2018.02965. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.-Z, Huang S.-Y, Xu Y, Chen J, Wang J.-L, Tian W.-P, Zhu X.-Q. Evaluation of immune responses in mice after DNA immunization with putative Toxoplasma gondii calcium-dependent protein kinase 5. Clinical and Vaccine Immunology. 2014;21:924–929. doi: 10.1128/CVI.00059-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N.-Z, Huang S.-Y, Zhou D.-H, Chen J, Xu Y, Tian W.-P, Lu J, Zhu X.-Q. Protective immunity against Toxoplasma gondii induced by DNA immunization with the gene encoding a novel vaccine candidate: Calcium-dependent protein kinase 3. BMC Infectious Diseases. 2013;13:512. doi: 10.1186/1471-2334-13-512. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li Y, Liang Y, Wang S, Xie Q, Nan X, Li P, Hong G, Liu Q, Li X. Molecular characterization and protective immunity of rhoptry protein 35 (ROP35) of Toxoplasma gondii as a DNA vaccine. Veterinary Parasitology. 2018;260:12–21. doi: 10.1016/j.vetpar.2018.06.016. c. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li Y, Wang M, Xie Q, Li P, Zuo S, Kong L, Wang C, Wang S. Immune protection of rhoptry protein 21 (ROP21) of Toxoplasma gondii as a DNA vaccine against toxoplasmosis. Frontiers in Microbiology. 2018;9:909. doi: 10.3389/fmicb.2018.00909. d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li Y, Xie Q, Li P, Nan X, Kong L, Zeng D, Ding Z, Wang S. The molecular characterization and immunity identification of rhoptry protein 22 of Toxoplasma gondii as a DNA vaccine candidate against toxoplasmosis. Journal of Eukaryotic Microbiology. 2019;66:147–157. doi: 10.1111/jeu.12639. [DOI] [PubMed] [Google Scholar]
- Zhang N.-Z, Xu Y, Wang M, Petersen E, Chen J, Huang S.-Y, Zhu X.-Q. Protective efficacy of two novel DNA vaccines expressing Toxoplasma gondii rhomboid 4 and rhomboid 5 proteins against acute and chronic toxoplasmosis in mice. Expert Review of Vaccines. 2015;14:1289–1297. doi: 10.1586/14760584.2015.1061938. [DOI] [PubMed] [Google Scholar]
- Zhao G, Zhou A, Lu G, Meng M, Sun M, Bai Y, Han Y, Wang L, Zhou H, Cong H, et al. Identification and characterization of Toxoplasma gondii aspartic protease 1 as a novel vaccine candidate against toxoplasmosis. Parasites & Vectors. 2013;6:175. doi: 10.1186/1756-3305-6-175. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Zhou A, Lu G, Meng M, Sun M, Bai Y, Han Y, Wang L, Zhou H, Cong H, et al. Toxoplasma gondii cathepsin proteases are undeveloped prominent vaccine antigens against toxoplasmosis. BMC Infectious Diseases. 2013;13:207. doi: 10.1186/1471-2334-13-207. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Li Z.-Y, Chen J, Sun X.-L, Liu S.-S, Zhu X.-Q, Zhou D.-H. Protective efficacy of pVAX-RON5p against acute and chronic infections of Toxoplasma gondii in BALB/c mice. Experimental Parasitology. 2016;163:24–30. doi: 10.1016/j.exppara.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Zheng B, Ding J, Chen X, Yu H, Lou D, Tong Q, Kong Q, Lu S. Immuno-efficacy of a T. gondii secreted protein with an altered thrombospondin repeat (TgSPATR) as a novel DNA vaccine candidate against acute toxoplasmosis in BALB/c mice. Frontiers in Microbiology. 2017;8:216. doi: 10.3389/fmicb.2017.00216. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Ding J, Lou D, Tong Q, Zhuo X, Ding H, Kong Q, Lu S. The virulence-related MYR1 protein of Toxoplasma gondii as a novel DNA vaccine against toxoplasmosis in mice. Frontiers in Microbiology. 2019;10:734. doi: 10.3389/fmicb.2019.00734. a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Lou D, Ding J, Zhuo X, Ding H, Kong Q, Lu S. GRA24-based DNA vaccine prolongs survival in mice challenged with a virulent Toxoplasma gondii strain. Frontiers in Immunology. 2019;10:418. doi: 10.3389/fimmu.2019.00418. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Hu Y, Hua Q, Luo F, Xie G, Li X, Lin J, Wan Y, Ren S, Pan C, et al. Protective immune response in mice induced by a suicidal DNA vaccine encoding NTPase-II gene of Toxoplasma gondii. Acta Tropica. 2017;166:336–342. doi: 10.1016/j.actatropica.2016.12.004. b. [DOI] [PubMed] [Google Scholar]
- Zhou H, Min J, Zhao Q, Gu Q, Cong H, Li Y, He S. Protective immune response against Toxoplasma gondii elicited by a recombinant DNA vaccine with a novel genetic adjuvant. Vaccine. 2012;30:1800–1806. doi: 10.1016/j.vaccine.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang L. SAG4 DNA and peptide vaccination provides partial protection against T. gondii infection in BALB/c mice. Frontiers in Microbiology. 2017;8:1733. doi: 10.3389/fmicb.2017.01733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang L, Lu G, Zhou A, Zhu M, Li Q, Wang Z, Arken M, Wang A, He S. Epitope analysis and protection by a ROP19 DNA vaccine against Toxoplasma gondii. Parasite. 2016;23:17. doi: 10.1051/parasite/2016017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.-N, Wang J.-L, Chen K, Yue D.-M, Zhang X.-X, Huang S.-Y, Zhu X.-Q. Evaluation of protective immunity induced by DNA vaccination with genes encoding Toxoplasma gondii GRA17 and GRA23 against acute toxoplasmosis in mice. Experimental Parasitology. 2017;179:20–27. doi: 10.1016/j.exppara.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Zhu Y.-C, He Y, Liu J.-F, Chen J. Adjuvantic cytokine IL-33 improves the protective immunity of cocktailed DNA vaccine of ROP5 and ROP18 against Toxoplasma gondii infection in mice. Parasite. 2020;27:26. doi: 10.1051/parasite/2020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Dalpke A, Heeg K. CpG oligonucleotides as adjuvant in therapeutic vaccines against parasitic infections. International Journal of Medical Microbiology. 2008;298:39–44. doi: 10.1016/j.ijmm.2007.07.011. [DOI] [PubMed] [Google Scholar]