Abstract
Background:
A healthy gut microbiome is critical for glucose metabolism during pregnancy. In vivo studies indicate that trace element affects the composition and function of the gut microbiome and potentially leads to metabolic disorders but their relationship are largely unknown. We aimed to investigate whether the gut microbiome plays a role in the relationship between trace element exposure and Gestational Diabetes Mellitus (GDM).
Methods:
In a prospective cohort study, blood levels of 22 trace elements and the fecal gut microbiome composition were assessed in 837 pregnant women in the second trimester between 22 and 24 weeks of pregnancy prior to GDM diagnosis. Regression and mediation analysis were used to explore the link between element exposure, the gut microbiome and GDM.
Results:
128 pregnant women (15.3%) were diagnosed with GDM. No individual trace elements were found significantly associated with GDM. In contrast, the composition of the gut microbiome was dramatically altered in women later diagnosed with GDM and characterized by lower alpha diversity and lower abundance of co-abundance groups (CAGs) composed of genera belonging to Ruminococcaceae and Lachnospiraceae. Rubidium (Rb) was positively associated with alpha diversity indices while mercury (Hg) and vanadium (V) showed negative associations. Elements including rubidium (Rb), thallium (Tl), arsenic (As) and antimony (Sb) significantly correlated with GDM-related CAGs and mediation analysis revealed that Rb and Sb are inversely related to GDM risk by altering abundance levels of CAGs enriched for Lachnospiraceae and Ruminococcaceae.
Conclusion:
Our study indicates that the influence of trace element exposure on certain features of gut microbiome increases risk of GDM, which could provide a new avenue for intervening in environmental exposure-related GDM.
Keywords: Gestational Diabetes Mellitus, trace element, gut microbiome, mediation effect
1. Introduction
The human gut microbiome plays a non-substitutable role in host nutrient, host defense against invading pathogens, and regulating xenobiotic metabolism (Koppel and Maini Rekdal 2017; Oliphant and Allen-Vercoe 2019). Gestational Diabetes Mellitus (GDM) is a common pregnancy complication which manifests as a transient glucose metabolite disorder with long lasting effects on both mothers and offspring (Ferrara 2007). The gut microbiome plays a critical role in carbohydrate/lipid metabolism, insulin homeostasis, and inflammation (Nielsenet al. 2003; Oliphant and Allen-Vercoe 2019; Vriezeet al. 2012) and accumulating evidence suggests a relationship between the gut microbiome and GDM (Cortezet al. 2019; Crusellet al. 2018; Wanget al. 2018). While previous cross-sectional studies have explored the gut microbiome composition in patients diagnosed with GDM (Cortezet al. 2019; Crusellet al. 2018; Wanget al. 2018), few studies characterized the gut microbiome before GDM diagnosis.
The composition of the gut microbiome is influenced by internal factors including genetic background, immune status, the endocrine system, and external environmental factors (Iszattet al. 2019; Liuet al. 2019a; Xinget al. 2018). Compared to host genetics, environmental factors including diet, drugs and anthropometric parameters are dominant in shaping gut microbiome (Rothschildet al. 2018). Besides the environmental factors mentioned above, trace element are commonly exposed during pregnancy and is causing widespread concern but a comprehensive view of whether these elements affect gut microbiome is lacking. Although in vivo studies show that trace element exposure can have profound effects on the gut microbiome and intestinal barrier integrity (Duanet al. 2020; Wanget al. 2015; Zhaiet al. 2016), these studies focused on a limited number of elements and used high dose exposures (Cattoet al. 2019; Chiet al. 2017; Dinget al. 2019; Gielda and DiRita 2012; Liet al. 2019; Srutet al. 2019). The impact of trace element exposure on gut microbiome and their linkage to human health outcomes remain inadequately addressed.
In this study, we conducted a prospective cohort study to determine a link between trace elements exposure, the gut microbiome and GDM. Our study provides new insights into the relationship between exposure to trace elements, the gut microbiome composition and the evidence for their interaction role in GDM development.
2. Methods
2.1. Study design and population
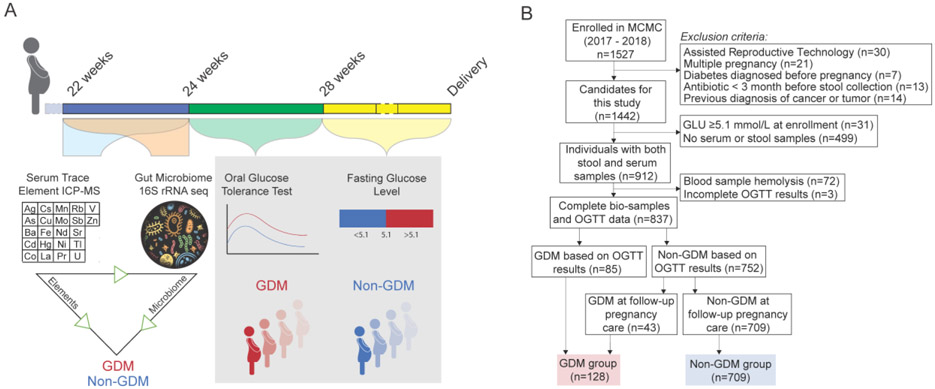
All participants were drawn from the Mother and Child Microbiome Cohort (MCMC) Study initiated and conducted at the affiliated hospital of Nanjing Medical University from the year 2017 to 2018. The medicine ethics committee of Nanjing Maternity and Child Health Care Hospital approved the study. Written informed consent was obtained from each participant at enrollment. In total, 1527 pregnant women agreed to participate and they provided informed consent for themselves. Serum and fecal samples were obtained from pregnant women when they registered for antenatal care during 22-24 weeks of pregnancy (averaged at 23.8 weeks of pregnancy). Due to the design of this study, we excluded participants according to the following criteria: (i) pregnant by artificial reproductive technology (ART), (ii) multiple pregnancy, (iii) with pregestational diabetes mellitus, (iv) treatment with antibiotics within 3 months before sample collection, and (v) previously diagnosed of tumor or cancer. We also excluded those diagnosed of GDM at enrollment. 64.6% of the participants provided both blood and fecal samples. We excluded the blood samples with hemolysis (n=72). A standardized 75-g oral glucose tolerance test (OGTT) between 24-28 weeks of pregnancy (averaged at 25.2 weeks of pregnancy) was taken and participants without complete OGTT results were also excluded. At the following pregnant care visits, fasting glucose levels were detected as part of biochemistry detection. Finally, a total of 837 pregnant women were included in this study (Fig. 1). According to GDM testing and diagnosis guidelines recommended by Ministry of Health (MOH) of China (Zhu and Yang 2013), the women with GDM were diagnosed by qualified doctors if one or more of the following glucose levels were elevated: fasting ⩾ 5.1 mmol/L, 1 hour ⩾ 10.0 mmol/L, or 2 hour ⩾ 8.5 mmol/L.
Fig. 1. Study design overview.
(A) Pregnant women were recruited at the first administration between 22 and 24 weeks of gestation (averaged at 23.8 weeks of gestation), at which time blood and stool samples were collected. Oral glucose tolerance test was carried out between 24 and 28 weeks of gestation (averaged at 25.2 weeks of gestation). Fasting glucose levels were also measured in the following pregnant care visit. The link between trace elements exposure, gut microbiome, and GDM was determined. (B) Flowchart of the population included in our final analysis.
Demographic information and clinical records of the study population were extracted from the structured questionnaires and the Hospital Information Systems, respectively. Feces and serum were frozen in −20 °C freezers immediately and stored at −80 °C until DNA extraction and trace element measurement. Impaired Glucose Tolerance (IGT) was diagnosed when both of the following conditions were satisfied: 1) fasting blood glucose level < 7.0 mmol/L; and 2) 2h blood glucose level ⩾7.8 and <11.10 mmol/L.
2.2. Serum elements measurement
Serum samples were digested with diluent (0.05% [v/v] Triton X-100, 0.1% [v/v] nitric acid plus 10 mg/L internal standards including Sc, Y, In, Tb, Bi) and were analyzed using an iCAP Q inductively coupled plasma mass spectrometry (ICP-MS) instrument (Thermo Scientific, Bremen, Germany) at Nanjing Medical University. Standard quality control samples (Seronorm Trace Elements Serum L-1 (ref. 203105, Sero, Billingstad, Norway) (one in every 20 samples) and mixed quality samples (mixed by 10% of target samples) (one in every 10 samples) were analyzed in parallel with study samples within 17 days’ examination. The target metals included silver (Ag), arsenic (As), barium (Ba), cadmium (Cd), cobalt (Co), cesium (Cs), copper (Cu), iron (Fe), mercury (Hg), lanthanum (La), manganese (Mn), molybdenum (Mo), neodymium (Nd), nickel (Ni), praseodymium (Pr), rubidium (Rb), antimony (Sb), strontium (Sr), thallium (Tl), uranium (U), vanadium (V), and zinc (Zn). The relative standard deviations (RSD) of quality control samples and mixed quality samples were under reference RSD (2002/657/EC) with vanadium in mixed quality samples slightly higher than the reference. The trace element analysis protocol was adapted from a previous report (Balcaenet al. 2014). Limits of detection (LODs) were calculated as 3 times the standard deviation of 10 consecutive measurements of the blank diluent. Values < LOD were replaced with LOD/√2 for individual trace element with detection rates of > 80% for later analysis as continuous variables. Trace elements with detection rates <80% (including Ba, Cd, La, Mn, Nd, Ni, Pr, and U, and their detection rates range from 5.7% to 44.9%) were not retained in this study as recommended (Johnsonet al. 2013).
2.3. DNA extraction and 16s rRNA gene sequencing
Total bacterial DNA was obtained from 180~220 mg of fecal in a sterile environment using QIAamp Fast DNA Stool Mini Kit (QIAGEN, Germany). The variable regions 3 and 4 (V3-V4) of the 16S rRNA gene was amplified and then sequenced using a MiSeq platform (Illumina Inc., San Diego, USA). Amplification of the V3-V4 region used the primers 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R (5’-GGACTACHVGGGTWTCTAAT-3’). The reaction was performed under the following conditions: 94 °C for 2 minutes, 30 cycles of 20 seconds at 94 °C, 20 seconds at 60 °C, 30 seconds at 72 °C, and a final step of 10 minutes at 72 °C. Nanodrop 2000 was used to measure DNA concentration and quantify the purity. 1% agarose gel electrophoresis was used to determine DNA integrity. Amplications were sequenced on an Illumina Miseq PE250 platform.
2.4. Sequencing data analysis
Sequence quality control was conducted with QIIME2-2019.1. The dada2 plugin was used to denoise sequences and amplicon sequence variants (ASVs) were obtained at 100% sequence homology, which method improves distinctive taxonomic classification at single-nucleotide accuracy. All representative reads were classified to the lowest taxonomic rank by the Silva database. Alpha diversity (Shannon index, Simpson and Observed OTUs) were calculated with QIIME2 with all the samples rarefied to the lowest sequence depth 28288. High-dimensional microbiome biomarkers at the family level were identified by LDA Effect Size (LEfSE) (logarithmic LDA scores >3.0).
2.5. Microbiome co-abundance groups (CAGs) network
The top 100 most abundant genera (with relative abundance of 98.7%) were used to construct co-abundance groups. Their Kendall correlation was calculated by the function “cor”. Their correlation was visualized by hierarchical Ward clustering with a Spearman correlation distance metric to define co-abundant group with the “Made4” package. The appropriate number of clustering was selected based on significance testing among each group of the original Kendall correlation matrix using “adonis” function in “Vegan” package. The number of significant differences with pairwise adonis test was chosen as the best clustering number. The sum of relative abundance belongs to the same CAG was calculated as the expression levels of this CAG. Spearman correlation of the genera in CAGs was visualized by Cytoscape 3.7.1 software.
2.6. Statistical analysis
We examine associations between elements exposure and GDM-related outcomes by general linear regression with adjusting for previous reported GDM-related covariates including maternal age, parity, pre-pregnant BMI and educational levels.
To explore trace elements that have effects on gut microbiome, we used linear regression adjusted for maternal age, parity, pre-pregnant BMI and educational levels. To explore the indirect effect of elements on GDM by gut microbiome, we conducted mediation analysis using Hayes PROCESS macro in SPSS 22.0. Alpha diversity indices, log-transformed relative expression levels of GDM-related CAGs were potential mediators. A Bootstrap process for 5000 times was conducted in each analysis. We used PICRUSt2 plugin (version 2.0.3-b) in QIIME2 to predict metagenome functions (Douglas GM 2019). MetaCyc pathways differently abundant between GDM and Non-GDM groups were identified by Welch’s test with FDR correction.
All statistical analysis was conducted in R software and SPSS 22.0. A two-tailed P value <0.05 was defined as statistically significant.
3. Results
3.1. Characteristics of the study population
To explore the possibility of the contribution of element exposure to GDM through modulating the gut microbiome, we conducted a population-based prospective cohort study in 1527 pregnant women. Between 22 and 24 weeks of gestation, the serum levels of 22 trace elements were measured by ICP-MS and the gut microbiome composition was measured by 16s rRNA sequencing of fecal samples (Fig. 1A). Screening for GDM was performed between 24 and 28 weeks of gestation using a 75-g 2-hour OGTT test and between 28 weeks of gestation and delivery using fasting glucose levels. After exclusion of women with multiple pregnancies, those who conceived using assisted reproductive technologies or with certain pre-existing conditions, 837 pregnant women with information of both serum trace elements and gut microbiome composition were enrolled in this study and were followed up (Fig. 1B). A total of 15.3% of the study population was diagnosed with GDM (N=128; Fig. 1B). Maternal age was significantly different between Non-GDM and GDM groups. Pregnant women 30 years and older were at significantly higher risk of GDM (p<0.001; Supplementary material Table S1). Women with GDM also had higher triglycerides (TG; p=0.009), hemoglobin A1C (p<0.001) and fasting blood glucose levels (p<0.001) (Supplementary material Table S1). Parity, education level, maternal pre-pregnant BMI and BMI at enrollment were not different between GDM and Non-GDM groups (Supplementary material Table S1).
3.2. Distribution of trace elements in the cohort
We measured 22 trace elements in serum between 22 and 24 weeks of gestation and their distribution is summarized in Supplementary material Table S2. Eleven of the 22 trace elements were also measured in pregnant Chinese women enrolled in the China Nutrition and Health Survey 2010–2012 (Liuet al. 2017). Compared to the reference levels, we observed similar levels in this survey. We then filtered the trace elements to only include those that were detected in at least 80% of samples (N=14; Supplementary material Table S2) and treated each element as a continuous variable for downstream association analysis. Correlation analysis among these 14 trace elements showed correlations ranging from −0.33 to 0.57. Significant positive correlations were found between rubidium and cesium (Spearman’s rho 0.57), zinc and cobalt (Spearman’s rho 0.45) and rubidium and iron (Spearman’s rho 0.44) while a negative correlation was observed between vanadium and molybdenum (Spearman’s rho −0.33) (Supplementary material Figure S1).
3.3. Associations between trace elements and GDM
The generalized linear regression model was used to test the association of elements exposure with GDM, Impaired Glucose Tolerance (IGT) and blood glucose levels. None of the 14 elements were significantly associated with GDM and IGT (Supplementary material Table S3). Ag was found to be significantly positively associated with OGTT at 0h glucose levels (Supplementary material Table S3), while Co, Cs, and V were found negatively associated with blood glucose levels (Supplementary material Table S3).
3.4. Links between the gut microbiome composition and GDM
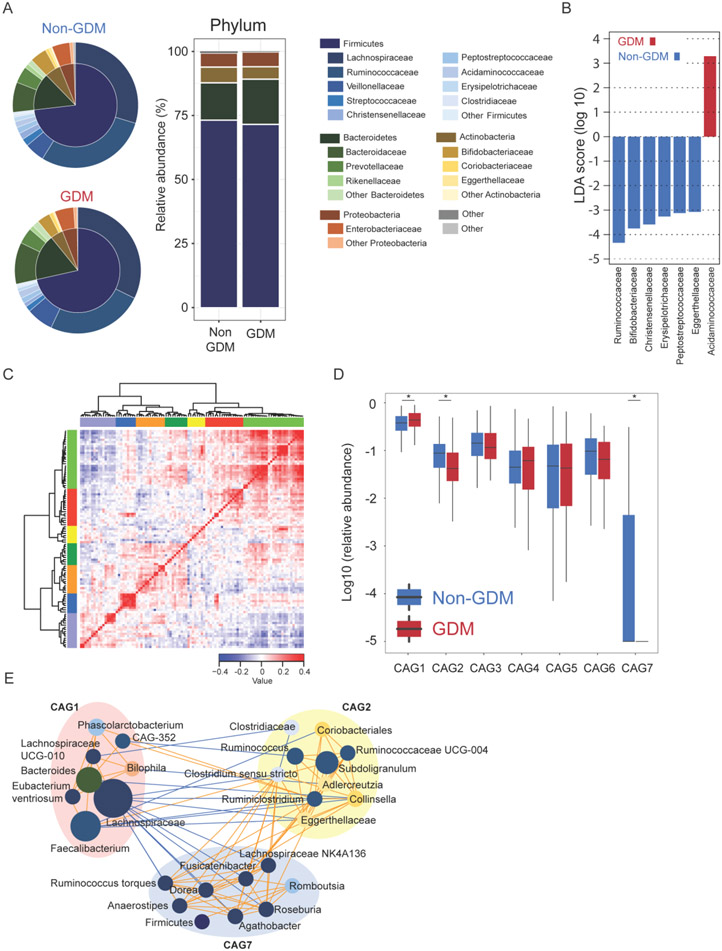
The gut microbiome plays an important role in human health and imbalance of the gut microbiome is involved in development of disease. We next determined the association of the gut microbiome composition at 22-24 weeks of gestation with GDM diagnosed after 24 weeks of gestation. Regression analysis, after adjusting for maternal age, parity, pre-pregnant BMI and educational levels, showed that the alpha-diversity indices of Shannon and Observed OTUs were significantly associated with GDM (Supplementary material Table S4). The gut microbiome of both the GDM and Non-GDM groups were dominated by taxa belongs to Firmicutes (mean±SD relative abundance, GDM: 71.5%±15.2%; Non-GDM: 73.2%±13.9%), Bacteroidetes (GDM: 17.7%±15.6%; Non-GDM: 14.6%±13.0%), Actinobacteria (GDM: 4.9%±7.0%; Non-GDM: 6.1%±7.4%), and Proteobacteria (GDM: 5.7%±8.0%; Non-GDM: 5.5%±8.3%). GDM patients had a relative lower abundance of Actinobacteria compared with Non-GDMs (Mann-Whitney U test, P value<0.05). At the family level, we found the abundances of Acidaminococcaceae was significantly higher while the abundances of Ruminococcaceae, Bifidobacteriaceae, Christensenellaceae, Erysipelotrichaceae, Peptostreptococcaceae, and Eggerthellaceae were significantly lower in pregnant women later diagnosed with GDM compared to Non-GDM (Fig. 2B).
Fig. 2. Gut microbiota structure was dramatically altered before GDM diagnosis.
(A) Mean relative abundance of the most abundant phyla and families in the cohort population gut microbiome. (B) LEfSE analysis to find gut microbiome biomarkers between groups at the family level. (C) Heatmap showing Kendall correlations between top 100 most abundant genera clustered by the Spearman correlation coefficient and hierarchical Ward clustering. (D) CAGs abundance between Non-GDM and GDM groups by Mann-Whitney U test. (E) Genera level network diagram showing the enrichments of the genera in the different groups based on significantly changed CAGs. Node size indicates the mean relative abundance of each genus. Lines indicate significant Spearman association between nodes with FDR corrected P-value less than 0.01. Orange indicates positive association and blue indicates negative association. * indicates P-value <0.05.
To identify differences in microbiota structure between groups, we clustered the top 100 most abundant genera according to their co-abundance correlations (Fig. 2C). Seven co-abundance groups (CAGs) were obtained and CAG1, CAG2 and CAG7, were differently expressed between GDM and Non-GDM groups (Fig. 2D; Supplementary material Table S4). CAGs depleted in GDM group (CAG2 and CAG7) were mainly composed of genera from Ruminococcaceae and Lachnospiraceae (Fig. 2E), which were also found in participants with impaired glucose tolerance (IGT) where glucose levels are in between normal and diabetic (Supplementary material Fig. S2).
3.5. Influence of elements exposure on gut microbiome composition
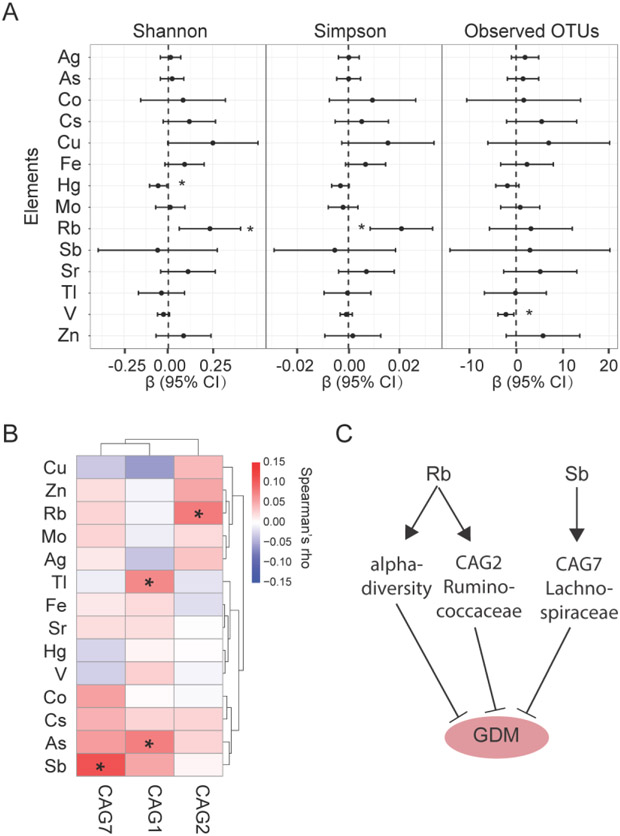
Although no significant association was found between element exposure and GDM, the microbiota structure was dramatically different in GDM group compared to the Non-GDM group. It has been reported that environmental chemical and element exposure can disrupt the gut microbiome and are linked to diseases (Liuet al. 2019a; Zhanget al. 2017). We next investigated the relationship between element exposure and gut microbiome alteration in GDM group. We found that the serum levels of Rb was significantly positively correlated with alpha diversity, whereas Hg and V exposure were inversely correlated with diversity indices (Fig. 3A). The three GDM-associated CAGs were positively correlated with rubidium (Rb), thallium (Tl), arsenic (As), and antimony (Sb) (Fig. 3B). Serum rubidium levels were positively correlated with CAG2, arsenic and thallium with CAG1 and antimony with CAG7. These findings suggest that element exposure significantly disturbed the composition of gut microbiome, and this alteration might induce the development of GDM. The strong correlation between elements exposure and gut microbiome and strong association between gut microbiome and GDM led us to hypothesize that there were indirect effects of elements on GDM by modulating gut microbiome. To test this hypothesis, we conducted mediation analysis. We found that rubidium exposure inversely affected GDM by altering CAG2. Similarly, antimony inversely affected GDM by altering CAG7 (Fig. 3C). Both CAG2 and CAG7 are largely enriched for bacterial families Ruminococcaceae and Lachnospiraceae. These results suggest that the contribution of trace elements exposure to GDM through influencing gut microbiome.
Fig. 3. Correlations between serum trace element exposure and gut microbiome composition.
(A) Linear regression of per unit increase of trace elements and alpha diversity indices. Models were adjusted for maternal age, parity, pre-pregnant BMI and educational levels. (B) Spearman correlation of trace elements exposure and relative abundance of GDM-related CAGs. (C) Mediating effects of alpha-diversity, CAG2 and CAG7 on GDM by rubidium (Rb) and antimony (Sb). * indicates P-value <0.05.
3.6. Functional alterations in the GDM gut microbiome
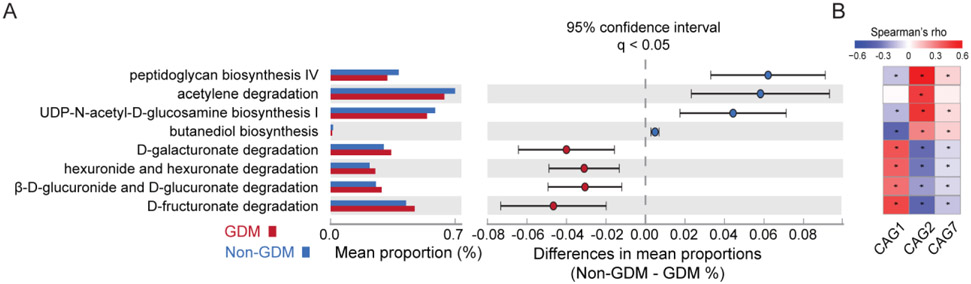
Finally, we explored the biological functions of the CAGs that were significantly associated with GDM using PICRUSt2. In this analysis, the amplicon sequence variants are used to predict the abundance of gene families and MetaCyc pathways. We found that complex and simple carbohydrate utilization pathways were enrich in the GDM group (Fig. 4A). D-galacturonate degradation was enriched in GDM vs Non-GDM groups. D-galacturonate is found in cell walls of plants and is the main monomeric constituent of pectin and is degraded into D-glyceraldehyde-3-phosphate and pyruvate. All these indicate an increased utilization of glucose as energy source in participants later diagnosed of GDM (Hasainet al. 2020). These pathways were significantly correlated with CAG7 abundance (Fig. 4B). Cell wall and envelope biosynthesis pathways including peptidoglycan biosynthesis and phospholipid biosynthesis were enriched in the Non-GDM group (Fig. 4A) and strongly correlated with CAG2 and CAG7 abundance (Fig. 4B), which suggests that the GDM protective effects of Rb and Sb are mediated by increased activity of these metabolic pathways.
Fig. 4. Functional analysis of CAGs associated with GDM.
(A) PICRUSt2 analysis results of predicted functional pathways of the CAGs that were significantly associated with GDM. Two-sided Welch’s t-test and FDR correction were used to identify the differentially abundant MetaCyc pathways (P < 0.05). (B) Spearman correlations of the differentially abundant MetaCyc pathways and CAGs between GDM and Non-GDM groups. * indicates P-value <0.05.
4. Discussion
In this study, we conducted a comprehensive analysis of the relationship between elements, gut microbiome and GDM, and provided strong evidence that gut microbiome plays an important role in the environmental exposure-related health. Our study revealed a strong link between element exposure and the composition of the gut microbiome and between the gut microbiota composition and GDM, and further showed that the influence of trace element exposure on certain features of gut microbiome can modulate GDM risk. Our findings suggest gut microbiome intervention could be an effective method to reduce the risk of elements exposure induced GDM.
The relationship between elements exposure and GDM has been explored in many epidemiological studies (Ettingeret al. 2009; Rawalet al. 2017; Shapiroet al. 2015; Wuet al. 2018; Xinget al. 2018) and some associations have been indicated. However, due to differences in study design, population and exposure biomarkers, our study did not find any individual trace element significantly associated with GDM.
We found that the composition of the gut microbiome was an important factor for GDM development. After adjusting for maternal age, parity, pre-pregnant BMI and educational levels, alpha diversity indices were significantly negatively associated with GDM and blood glucose levels. Lower alpha diversity levels were found in participants later diagnosed of GDM, which was also observed in previous cross-sectional studies (Crusellet al. 2018; Kuanget al. 2017). We analyzed microbiome biomarkers of GDM at the family level. Ruminococcaceae, the most dominant family in the gut of the Hadza hunter-gatherers with a foraging lifestyle (Schnorret al. 2014), was found the most depleted in the GDM group and this bacterial family was reportedly associated with the onset of obesity and metabolic syndrome (Lippertet al. 2017). However, in a small size study of 15 GDM and 60 Non-GDM with fecal samples collected at the first trimester, Ruminococcaceae was found to increase the risk of GDM (Mokkalaet al. 2017). As all the participants in the previous study are overweight and obese women, the conflict result might come from lifestyle change after pregnant. More researches regarding gut microbiota preceding GDM diagnosis are warranted to clarity the contradiction. We also investigated the gut microbiome by co-abundance groups which are highly correlated and work as functional groups. Three CAGs were found associated with GDM development. Among them, CAGs of low abundance in GDM participants were mainly composed of genera from Ruminococcaceae and Lachnospiraceae. Their members are previously found play a key role in degrading complex polysaccharides into short-chain fatty acids and their depletion is correlated with metabolic diseases (Biddle A. 2013; Chávez-Carbajalet al. 2019).
Although no association was found between element exposure and GDM, the alteration of gut microbiome composition before GDM diagnosis and the potential interaction between elements and microbiome inspired us to explore if the altered gut microbiome was influenced by elemental exposure. Until now, the effects of elemental exposure on the gut microbiome are rarely analyzed in prospective studies and there is still sparse information about the association of environmental exposure and the structure of the gut microbiome. We measured maternal elements exposure and gut microbiome composition during the second trimester. V and Hg were found to be negatively associated with alpha diversity indices. No study reported association of Hg or V exposure and alpha diversity during pregnancy, but some in vivo studies found decreased alpha diversity after Hg and V treatment (Liuet al. 2019b; Wanget al. 2012), which could result from inducing oxidative stress (Denget al. 2012) and damaging gut lining (Bernhoft 2012; Riceet al. 2014; Summerset al. 1993). Rb was found positively associated with alpha diversity indices in our study. This element is a possible essential trace element for human and its metabolism is closely related to that of potassium (Perez-Vizcainoet al. 1993). Although the effect of rubidium on the gut microbiome is unknown, food additives like potassium sorbate and acesulfame potassium are found to alter the structure of the gut microbiome (Frankenfeldet al. 2015; Penget al. 2019). In this study, we considered the gut microbiome as co-functional groups and found that microbial families Ruminococcaceae, and Lachnospiraceae play a role in mediating the effect of rubinium and antimony element exposure on GDM development. Exposure to Rb and Sb were positively correlated with Ruminococcaceae, and Lachnospiraceae, which in turn were lower in the GDM group. A previous cohort study showed also that antimony was negatively associated with incident diabetes (Yuanet al. 2018). In contrast, a cross-sectional NHANES study showed that higher urine antimony was associated with elevated diabetes prevalence in US adults (Menkeet al. 2016). The mediation effect of Ruminococcaceae, and Lachnospiraceae on Rb and Sb related GDM is not reported before.
This study focused on the complex relationship between environmental trace element exposure and the gut microbiome and their effect on GDM. On the one hand, trace elements might directly affect the gut microbiome composition and their metabolic function. On the other hand, the gut microbiome could be involved in the absorption and metabolic transformation of trace elements (Van de Wieleet al. 2010). Either path could lead to a change in disease risk. In our study, elements were found to affect gut microbes associated with GDM, which could be one of the mechanisms by which trace element exposure contributes to GDM development. However, the detailed mechanisms remain unknown and need to be further explored.
5. Conclusions
Our prospective cohort study determined a link between elemental exposure, the gut microbiome composition and the development of GDM. We identified microbiome components that could serve as mediators of element exposure effects on GDM indicating that the influence of trace element exposure on certain features of gut microbiome increases risk of GDM. The gut microbiome could be a new avenue for intervening in environmental exposure-related GDM. Prospective studies of larger sample sizes are warranted to further explore the causal relationship and mechanism between them.
Supplementary Material
Highlights.
The link between element exposure, the gut microbiome and GDM were assessed.
Gut microbiome is dramatically altered before GDM diagnosis.
Trace elements are closely related to gut microbiome composition.
Rubidium (Rb) and antimony (Sb) are inversely related to GDM risk by altering abundance levels of GDM-related co-abundance groups (CAGs).
Acknowledgements
The authors thank all the participants of the Mother and Child Microbiome Cohort (MCMC) Study. This work was supported by grants from NSFC-NIH Biomedical collaborative research program (grant no. 81961128022), U.S.-China Program for Biomedical Collaborative Research NIEHS: R01ES031322 (A.M.S.), Nanjing Science and technology development plan project (grant no. 201911040) and "333 High-level Talent Training Project" of the Jiangsu Province.
Abbreviations
- CAG
co-abundance group
- GDM
Gestational Diabetes Mellitus
- OGTT
Oral Glucose Tolerance Test
- IGT
Impaired Glucose Tolerance
- BMI
body mass index
- ICP-MS
inductively coupled plasma mass spectrometry
- OTU
operational taxonomic unit
Footnotes
Declaration of Competing Interest
The authors declare they have no actual or potential competing financial interests.
REFERENCES
- Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Official Journal of the European Communities; 2002/657/EC [Google Scholar]
- Balcaen L; Bolea-Fernandez E; Resano M; Vanhaecke F Accurate determination of ultra-trace levels of Ti in blood serum using ICP-MS/MS. Anal Chim Acta 2014;809:1–8 [DOI] [PubMed] [Google Scholar]
- Bernhoft RA Mercury toxicity and treatment: a review of the literature. J Environ Public Health 2012;2012:460508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle A, S. L, Blanchard J, Leschine S Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 2013;5:627–640 [Google Scholar]
- Catto C; Garuglieri E; Borruso L; Erba D; Casiraghi MC; Cappitelli F; Villa F; Zecchin S; Zanchi R Impacts of dietary silver nanoparticles and probiotic administration on the microbiota of an in-vitro gut model. Environ Pollut 2019;245:754–763 [DOI] [PubMed] [Google Scholar]
- Chávez-Carbajal A; Nirmalkar K; Pérez-Lizaur A; Hernández-Quiroz F; Ramírez-Del-Alto S; García-Mena J; Hernández-Guerrero C Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int J Mol Sci 2019;20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L; Gao B; Bian X; Tu P; Ru H; Lu K Manganese-induced sex-specific gut microbiome perturbations in C57BL/6 mice. Toxicol Appl Pharmacol 2017;331:142–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez RV; Taddei CR; Sparvoli LG; Angelo AGS; Padilha M; Mattar R; Daher S Microbiome and its relation to gestational diabetes. Endocrine 2019;64:254–264 [DOI] [PubMed] [Google Scholar]
- Crusell MKW; Hansen TH; Nielsen T; Allin KH; Ruhlemann MC; Damm P; Vestergaard H; Rorbye C; Jorgensen NR; Christiansen OB; Heinsen FA; Franke A; Hansen T; Lauenborg J; Pedersen O Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 2018;6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y; Cui H; Peng X; Fang J; Wang K; Cui W; Liu X Dietary vanadium induces oxidative stress in the intestine of broilers. Biol Trace Elem Res 2012;145:52–58 [DOI] [PubMed] [Google Scholar]
- Ding J; An XL; Lassen SB; Wang HT; Zhu D; Ke X Heavy metal-induced co-selection of antibiotic resistance genes in the gut microbiota of collembolans. Sci Total Environ 2019;683:210–215 [DOI] [PubMed] [Google Scholar]
- Douglas GM, M. V, Zaneveld J, Yurgel SN, Brown JR, Taylor CM, Huttenhower C, Langille MG. PICRUSt2: an improved and extensible approach for metagenome inference. BioRxiv 2019;672295 [Google Scholar]
- Duan H; Yu L; Tian F; Zhai Q; Fan L; Chen W Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Sci Total Environ 2020;742:140429. [DOI] [PubMed] [Google Scholar]
- Ettinger AS; Zota AR; Amarasiriwardena CJ; Hopkins MR; Schwartz J; Hu H; Wright RO Maternal arsenic exposure and impaired glucose tolerance during pregnancy. Environ Health Perspect 2009;117:1059–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara A Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30 Suppl 2:S141–146 [DOI] [PubMed] [Google Scholar]
- Frankenfeld CL; Sikaroodi M; Lamb E; Shoemaker S; Gillevet PM High-intensity sweetener consumption and gut microbiome content and predicted gene function in a cross-sectional study of adults in the United States. Ann Epidemiol 2015;25 [DOI] [PubMed] [Google Scholar]
- Gielda LM; DiRita VJ Zinc competition among the intestinal microbiota. MBio 2012;3:e00171–00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasain Z; Mokhtar NM; Kamaruddin NA; Mohamed Ismail NA; Razalli NH; Gnanou JV; Raja Ali RA Gut Microbiota and Gestational Diabetes Mellitus: A Review of Host-Gut Microbiota Interactions and Their Therapeutic Potential. Front Cell Infect Microbiol 2020;10:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iszatt N; Janssen S; Lenters V; Dahl C; Stigum H; Knight R; Mandal S; Peddada S; Gonzalez A; Midtvedt T; Eggesbo M Environmental toxicants in breast milk of Norwegian mothers and gut bacteria composition and metabolites in their infants at 1 month. Microbiome 2019;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL; Paulose-Ram R; Ogden CL; Carroll MD; Kruszon-Moran D; Dohrmann SM; Curtin LR National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital and health statistics Series 2, Data evaluation and methods research 2013; [PubMed] [Google Scholar]
- Koppel N; Maini Rekdal V Chemical transformation of xenobiotics by the human gut microbiota. Science 2017;356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang YS; Lu JH; Li SH; Li JH; Yuan MY; He JR; Chen NN; Xiao WQ; Shen SY; Qiu L; Wu YF; Hu CY; Wu YY; Li WD; Chen QZ; Deng HW; Papasian CJ; Xia HM; Qiu X Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 2017;6:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X; Brejnrod AD; Ernst M; Rykaer M; Herschend J; Olsen NMC; Dorrestein PC; Rensing C; Sorensen SJ Heavy metal exposure causes changes in the metabolic health-associated gut microbiome and metabolites. Environ Int 2019;126:454–467 [DOI] [PubMed] [Google Scholar]
- Lippert K; Kedenko L; Antonielli L; Kedenko I; Gemeier C; Leitner M; Kautzky-Willer A; Paulweber B; Hackl E Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes 2017;8:545–556 [DOI] [PubMed] [Google Scholar]
- Liu T; Chen X; Xu Y; Wu W; Tang W; Chen Z; Ji G; Peng J; Jiang Q; Xiao J; Li X; Zeng W; Xu X; Hu J; Guo Y; Zou F; Du Q; Zhou H; He Y; Ma W Gut microbiota partially mediates the effects of fine particulate matter on type 2 diabetes: Evidence from a population-based epidemiological study. Environ Int 2019a;130:104882. [DOI] [PubMed] [Google Scholar]
- Liu X; Zhang Y; Piao J; Mao D; Li Y; Li W; Yang L; Yang X Reference Values of 14 Serum Trace Elements for Pregnant Chinese Women: A Cross-Sectional Study in the China Nutrition and Health Survey 2010-2012. Nutrients 2017;9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y; Ji J; Zhang W; Suo Y; Zhao J; Lin X; Cui L; Li B; Hu H; Chen C; Li YF Selenium modulated gut flora and promoted decomposition of methylmercury in methylmercury-poisoned rats. Ecotoxicol Environ Saf 2019b;185:109720. [DOI] [PubMed] [Google Scholar]
- Menke A; Guallar E; Cowie CC Metals in Urine and Diabetes in U.S. Adults. Diabetes 2016;65:164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokkala K; Houttu N; Vahlberg T; Munukka E; Rönnemaa T; Laitinen K Gut microbiota aberrations precede diagnosis of gestational diabetes mellitus. Acta Diabetol 2017;54:1147–1149 [DOI] [PubMed] [Google Scholar]
- Nielsen DS; Møller PL; Rosenfeldt V; Paerregaard A; Michaelsen KF; Jakobsen M Case study of the distribution of mucosa-associated Bifidobacterium species, Lactobacillus species, and other lactic acid bacteria in the human colon. Appl Environ Microbiol 2003;69:7545–7548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant K; Allen-Vercoe E Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 2019;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q; Chang H; Wang R; You Z; Jiang S; Ma C; Huo D; Zhu X; Zhang J Potassium sorbate suppresses intestinal microbial activity and triggers immune regulation in zebrafish (Danio rerio). Food Funct 2019;10:7164–7173 [DOI] [PubMed] [Google Scholar]
- Perez-Vizcaino F; Casis O; Rodriguez R; Gomez LA; Garcia Rafanell J; Tamargo J Effects of the novel potassium channel opener, UR-8225, on contractile responses in rat isolated smooth muscle. Br J Pharmacol 1993;110:1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal S; Hinkle SN; Bao W; Zhu Y; Grewal J; Albert PS; Weir NL; Tsai MY; Zhang C A longitudinal study of iron status during pregnancy and the risk of gestational diabetes: findings from a prospective, multiracial cohort. Diabetologia 2017;60:249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KM; Walker EM Jr.; Wu M; Gillette C; Blough ER Environmental mercury and its toxic effects. J Prev Med Public Health 2014;47:74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild D; Weissbrod O; Barkan E; Kurilshikov A; Korem T; Zeevi D; Costea PI; Godneva A; Kalka IN; Bar N; Shilo S; Lador D; Vila AV; Zmora N; Pevsner-Fischer M; Israeli D; Kosower N; Malka G; Wolf BC; Avnit-Sagi T; Lotan-Pompan M; Weinberger A; Halpern Z; Carmi S; Fu J; Wijmenga C; Zhernakova A; Elinav E; Segal E Environment dominates over host genetics in shaping human gut microbiota. Nature 2018;555:210–215 [DOI] [PubMed] [Google Scholar]
- Schnorr SL; Candela M; Rampelli S; Centanni M; Consolandi C; Basaglia G; Turroni S; Biagi E; Peano C; Severgnini M; Fiori J; Gotti R; De Bellis G; Luiselli D; Brigidi P; Mabulla A; Marlowe F; Henry AG; Crittenden AN Gut microbiome of the Hadza hunter-gatherers. Nat Commun 2014;5:3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GD; Dodds L; Arbuckle TE; Ashley-Martin J; Fraser W; Fisher M; Taback S; Keely E; Bouchard MF; Monnier P; Dallaire R; Morisset A; Ettinger AS Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ Int 2015;83:63–71 [DOI] [PubMed] [Google Scholar]
- Srut M; Menke S; Hockner M; Sommer S Earthworms and cadmium - Heavy metal resistant gut bacteria as indicators for heavy metal pollution in soils? Ecotoxicol Environ Saf 2019;171:843–853 [DOI] [PubMed] [Google Scholar]
- Summers AO; Wireman J; Vimy MJ; Lorscheider FL; Marshall B; Levy SB; Bennett S; Billard L Mercury released from dental "silver" fillings provokes an increase in mercury- and antibiotic-resistant bacteria in oral and intestinal floras of primates. Antimicrob Agents Chemother 1993;37:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Wiele T; Gallawa CM; Kubachka KM; Creed JT; Basta N; Dayton EA; Whitacre S; Du Laing G; Bradham K Arsenic metabolism by human gut microbiota upon in vitro digestion of contaminated soils. Environ Health Perspect 2010;118:1004–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze A; Van Nood E; Holleman F; Salojärvi J; Kootte RS; Bartelsman JFWM; Dallinga-Thie GM; Ackermans MT; Serlie MJ; Oozeer R; Derrien M; Druesne A; Van Hylckama Vlieg JET; Bloks VW; Groen AK; Heilig HGHJ; Zoetendal EG; Stroes ES; de Vos WM; Hoekstra JBL; Nieuwdorp M Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012;143 [DOI] [PubMed] [Google Scholar]
- Wang J; Zheng J; Shi W; Du N; Xu X; Zhang Y; Ji P; Zhang F; Jia Z; Wang Y; Zheng Z; Zhang H; Zhao F Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018;67:1614–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K; Cui H; Deng Y; Peng X; Zuo Z; Fang J; Deng J; Cui W; Wu B Effect of dietary vanadium on intestinal microbiota in broiler. Biol Trace Elem Res 2012;149:212–218 [DOI] [PubMed] [Google Scholar]
- Wang Q; Qin D; Zhang S; Wang L; Li J; Rensing C; McDermott TR; Wang G Fate of arsenate following arsenite oxidation in Agrobacterium tumefaciens GW4. Environ Microbiol 2015;17:1926–1940 [DOI] [PubMed] [Google Scholar]
- Wu Y; Zhang J; Peng S; Wang X; Luo L; Liu L; Huang Q; Tian M; Zhang X; Shen H Multiple elements related to metabolic markers in the context of gestational diabetes mellitus in meconium. Environ Int 2018;121:1227–1234 [DOI] [PubMed] [Google Scholar]
- Xing Y; Xia W; Zhang B; Zhou A; Huang Z; Zhang H; Liu H; Jiang Y; Hu C; Chen X; Xu S; Li Y Relation between cadmium exposure and gestational diabetes mellitus. Environ Int 2018;113:300–305 [DOI] [PubMed] [Google Scholar]
- Yuan Y; Xiao Y; Yu Y; Liu Y; Feng W; Qiu G; Wang H; Liu B; Wang J; Zhou L; Liu K; Xu X; Yang H; Li X; Qi L; Zhang X; He M; Hu FB; Pan A; Wu T Associations of multiple plasma metals with incident type 2 diabetes in Chinese adults: The Dongfeng-Tongji Cohort. Environ Pollut 2018;237:917–925 [DOI] [PubMed] [Google Scholar]
- Zhai Q; Tian F; Zhao J; Zhang H; Narbad A; Chen W Oral Administration of Probiotics Inhibits Absorption of the Heavy Metal Cadmium by Protecting the Intestinal Barrier. Appl Environ Microbiol 2016;82:4429–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F; Zheng W; Guo R; Yao W Effect of dietary copper level on the gut microbiota and its correlation with serum inflammatory cytokines in Sprague-Dawley rats. J Microbiol 2017;55:694–702 [DOI] [PubMed] [Google Scholar]
- Zhu WW; Yang HX Diagnosis of gestational diabetes mellitus in China. Diabetes Care 2013;36:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.