Abstract
Objective:
Our aim was to analyze the association between sugar-sweetened beverage consumption and depressive symptoms, as well as the extent to which TV viewing and physical activity moderate this association.
Methods:
We used cross-sectional data from the 2013 Brazilian National Survey (Pesquisa Nacional de Saúde) of 59,402 adults (33,482 women, mean age = 42.9 years, 95%CI 42.7-43.2 years). Depressive symptoms (Patient Health Questionnaire-9), physical activity, TV viewing, and sugar-sweetened beverage consumption, as well as potential confounders (chronological age, ethnicity, consumption of candy/sweets and fruit, multimorbidity, education, and employment status) were self-reported. Poisson regression models were used for association analyses.
Results:
The consumption of 16 or more glasses/week of sugar-sweetened beverages was associated with higher levels of severe depressive symptoms among women compared to no consumption (prevalence ratio [PR] 1.71 [95%CI 1.38-2.11]). Consistent interactions were observed between 1-5 glasses and TV viewing (PR 2.09 [95%CI 1.06-4.12]) and between 11-15 glasses and TV viewing (PR 2.90 [95%CI 1.29-6.50]) among men compared to no consumption, given that the co-occurrence of sugar-sweetened beverage consumption and elevated TV viewing was associated with higher odds of severe depressive symptoms. Sugar-sweetened beverage consumption did not interact with physical activity, only presenting an independent association.
Conclusion:
Sugar-sweetened beverage consumption was independently associated with severe depressive symptoms among women and interacted with TV viewing, but not with physical activity among men.
Keywords: Sedentary behavior, sitting, exercise, depression, mood
Introduction
Depression is a common mental disorder, with a prevalence of approximately 4% worldwide.1 People with depression have considerably lower life expectancy.2 Beyond the elevated burden of suicide, people with depression also have an elevated risk of cardiovascular disease.3 Therefore, depression is one of the leading causes of disability.4 Lifestyle changes, such as diet and exercise, have been suggested for depression treatment and prevention.5,6
Lifestyle behaviors can play an important role in the control of depressive symptoms in people without depression,7 and unhealthy dietary patterns are associated with increased depressive symptoms.8 Preliminary findings showed that increased consumption of ultra-processed food,9,10 specifically added sugar,11 is longitudinally associated with increased depressive symptoms. However, the association with sugar-sweetened beverages is less clear.11-13 When consumed in high quantities,14 sugar-sweetened beverages are associated with high glycemic load,15,16 increased inflammation, and oxidative stress,17 which in turn can be associated with elevated depressive symptoms.18 Regarding prospective studies, while Guo et al.12 found that sugar-sweetened beverages were associated with higher odds of depression, Sanchez-Villegas et al.11 and Knüppel et al.13 found inconclusive evidence of an association between sugar-sweetened beverages and depression.
Behaviors related to human movement, such as physical inactivity19 (< 150 min/week of moderate or vigorous physical activity [PA]20) and sedentary behavior (much time spent in a sitting, reclining, or lying posture with an energy expenditure ≤ 1.5 metabolic equivalents)21 have been related to an elevated risk of depression. Specifically, some types of movement behaviors, such as mentally passive sedentary behaviors (e.g., TV viewing), are recognized as independent risk factors for depression.5,22
Given the association between unhealthy dietary patterns and movement behaviors,23 ultra-processed food consumption, PA, and sedentary behavior can share mechanisms in relation to depression symptoms. For example, sugar-sweetened beverages,8,24 physical inactivity,25 and high sedentary behaviour26 can be associated with inflammation, which is associated with depression.27 However, it is not clear whether there is an independent association between the consumption of sugar-sweetened beverages and depressive symptoms or whether the co-occurrence with movement behaviors is involved. We highlight that the co-occurrence of TV viewing and the consumption of sugar-sweetened beverages may involve different mechanisms than the association between PA and consumption of sugar-sweetened beverages. For example, the consumption of unhealthy foods frequently occurs while viewing TV.28 In addition, many TV commercials advertise ultra-processed foods.29 On the other hand, the association between PA and sugar-sweetened beverages may be principally due to healthy or unhealthy lifestyles choices.30
Investigating the interactions between sugar-sweetened beverage consumption and high levels of TV viewing or physical inactivity in the association with depressive symptoms could help guide interventions that focus on individual behaviors or integrate different behaviors. Therefore, our primary aim was to analyze the association between sugar-sweetened beverage consumption and severe depressive symptoms. Our secondary aim was to assess the interaction between TV viewing and PA in this association.
Methods
Sample
Cross-sectional data from the 2013 Brazilian National Health Survey (Pesquisa Nacional de Saúde) were used. The sampling and weighting processes have been described in detail elsewhere.31 Briefly, the Survey was a cross-sectional epidemiological study conducted with a nationally representative sample of Brazilian adults (+ 18 years old). The sampling process involved multiple stages. First, census tracts were randomly selected; next, households were randomly selected; and finally, one adult was randomly selected from the selected households. The minimum sample size per state (n=27) was 1,800 households, and interviews were conducted in 64,348 households. The sample consisted of 59,402 individuals with complete data for all variables. The estimates were weighted, adjusting for nonresponse by sex, total population by sex and age, and the number of individuals per household.
Depressive symptoms
The outcome of this study was positive screening for severe depressive symptoms according to the Patient Health Questionnaire-9.32 In its nine questions, this tool evaluates the frequency of depressive symptoms (depressed mood, anhedonia, trouble sleeping, tiredness or lack of energy, change in appetite, feelings of guilt or uselessness, trouble concentrating, feeling slow or agitated, and having recurrent thoughts about death or suicidal ideation) in the two weeks prior to data collection. Each question has four possible answers on a Likert scale: not at all (value = 0); some days (value = 1); more than half the days (value = 2); and nearly every day (value = 3). This instrument has already been validated for Brazilian adults.33 The test’s algorithm was used to identify individuals at a higher risk of major depressive episode through the most common cut-point (sum of the values ≥ 10). The classification indicates which group is most likely to experience depressive episodes,33,34 which indicates severe depressive symptoms. Moreover, the questionnaire presented a good Cronbach’s alpha value (0.836) in the present sample.
Sugar-sweetened beverage consumption
Sugar-sweetened beverage consumption was evaluated through three questions. First, participants reported how many days they consumed soft drinks during a normal week. Second, participants who reported at least 1 day of consumption were asked how many glasses of soft drinks they consumed on those days (answers ranging from 1 to 3 or more). Third, participants who reported at least 1 day of soft drink consumption also reported whether they consumed sugar-sweetened beverages or artificially-sweetened soft drinks. For the analyses, we only considered sugar-sweetened beverages as soft drinks. We created a general score of total glasses consumed per week, which we classified into five categories to investigate linear increases: 0 glasses/week; 1-5 glasses/week; 6-10 glasses/week; 11-15 glasses/week; and 16 or more glasses/week. We used the highest category (16 or more glasses/week) for the main analysis, considering that this was the risk group. In addition, we used sensitivity analysis only among soft drink consumers, analyzing the frequency (7 days/week as a cut-off point) and number of glasses per day (two glasses/day as a reference).
Physical activity
PA was assessed with the Brazilian Ministry of Health’s Surveillance System for Risk and Protective Factors for Chronic Diseases by Telephone Survey (Pesquisa de Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico [Vigitel]). This survey assesses the frequency and duration of different domains of PA.31,35 The cut-off point for classification as physically active was 150 min of physical activity per week.
TV viewing
TV viewing was evaluated as a proxy of sedentary behavior with the question: How many hours a day do you usually spend watching TV? Five hours per day was considered the cut-off point for excessive time spent watching TV, given that this amount of time has been previously demonstrated to be associated with the presence of severe depressive symptoms.36
Covariates
Age was included as a continuous variable. Ethnicity was self-reported as white, black, mixed, or other. Educational status was determined through the question: What is your highest level of academic achievement? Based on the responses, three categories were created (1 = incomplete high school; 2 = complete high school; and 3 = at least some university level). Employment status was assessed by asking whether the respondent had a paying job in the last month: a yes or no response was used as the covariate. Smoking status was evaluated through the question: Do you use any tobacco product? Possible responses included yes (daily), yes (not daily), and no. We considered either positive response as exposure, and the dichotomous response was used as the covariate. The participants were also asked: How many days per week do you usually consume alcohol? The responses were classified as: 1 = non-consumers; 2 = weekly consumers (one to three days per week); 3 = almost daily consumers (4 or more days per week). The participants reported the number of days per week they consumed fruit and sweet foodstuffs (e.g., cake, sweets, chocolate, candy, or cookies). Seven days per week was considered the cutoff point for both indicators. The participants were asked whether a physician had ever diagnosed them with dyslipidemia, type 2 diabetes, hypertension, heart disease, stroke, or pulmonary disease. Response options were binary (no/yes). In line with previous research, participants with two or more chronic conditions were classified as having physical multimorbidity.37 Body mass and height were measured with a portable digital scale and stadiometer, respectively. Body mass index was subsequently calculated and categorized as: normal weight (≤ 24.99 kg/m2), overweight (25 kg/m2 to 29.9 kg/m2), or obese (≥ 30 kg/m2).
Statistical analysis
Sample characteristics regarding sugar-sweetened beverage consumption were reported using weighted frequency values and 95% confidence interval (95%CI). The association between different patterns of sugar-sweetened beverage consumption and severe depressive symptoms was assessed using crude and adjusted Poisson regression models with robust variance. The data were expressed as prevalence ratio (PR). Since sex interacted with sugar-sweetened beverage consumption in the association with severe depressive symptoms, we separated the analyses according to sex.
In addition, we analyzed the independent and combined association between patterns of sugar-sweetened beverage consumption and TV viewing, as well as patterns of sugar-sweetened beverage consumption and PA. The association with severe depressive symptoms was estimated through Poisson regression models with robust variance, inserting interaction terms. The interaction terms included the number of glasses/week, weekly frequency, number of glasses/day, no consumption, 1-6 days/week (with one glass as a reference), respectively, interacting with TV viewing (< 5 h/day as reference) and PA (physically active as a reference). All models were adjusted for potential confounders, considering the theoretical association with both sugar-sweetened beverage consumption and severe depressive symptoms: chronological age, ethnicity, educational status, employment status, smoking, alcohol consumption, fruit consumption, consumption of sweets/candies, multimorbidity, weight status, TV viewing (for analyses including PA), and PA (for analyses including TV viewing). The analyses were performed in Stata 15.1.
Ethics statement
All procedures performed in original studies involving human participants were approved by the National Council of Ethics in Research (CONEP 10853812.7.0000.0008) and were performed in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants.
All data have been published as microdata at https://www.ibge.gov.br/estatisticas/sociais/saude/9160-pesquisa-nacional-de-saude.html?=&t=microdados.
Results
Sample characteristics
The final sample consisted of 59,402 adults (33,482 females), with a mean age of 42.9 years (95%CI 42.7-43.2y). The sample characteristics are presented in Table 1. Among participants who drank 16 or more glasses of sugar-sweetened beverages per week, the majority were young adult men with higher TV viewing and lower PA. Depressive symptom scores according to sugar-sweetened beverage consumption groups are presented in Table 2. For both sexes, participants who consumed 16 or more glasses/week also had higher depressive symptom scores (men: 2.33 [2.06-2.61]; women: 4.92 [4.35-5.49]). Similarly, participants with daily sugar-sweetened beverage consumption reported higher depressive symptoms (men: 2.14 [1.93-2.35]; women: 3.98 [3.71-4.26]) and women who consumed two or more glasses/day also had higher depressive symptoms (3.53 [3.36-3.71]).
Table 1. Sample characteristics according to number of glasses of sugar-sweetened beverages consumed per week.
| Glasses of sugar-sweetened beverages consumed per week | |||||
|---|---|---|---|---|---|
| 0 (n=20,747) | 1-5 (n=21,832) | 6-10 (n=8,974) | 11-15 (n=4,734) | 16 or more (n=3,113) | |
| Sex (men) | 40.8 (39.6-42.0) | 45.8 (44.6-47.0) | 54.5 (52.6-56.3) | 59.4 (56.9-61.9) | 63.6 (60.6-66.5) |
| Age group | |||||
| 18-39 | 31.2 (30.0-32.2) | 47.9 (46.7-49.0) | 60.2 (58.4-62.0) | 68.5 (66.2-70.7) | 72.4 (69.5-75.0) |
| 40-59 | 39.7 (38.5-40.9) | 35.6 (34.5-36.8) | 29.5 (27.8-31.2) | 25.5 (23.5-27.7) | 23.8 (21.3-26.5) |
| 60+ | 29.1 (28.1-30.2) | 16.5 (15.6-17.4) | 10.3 (9.3-11.5) | 6.0 (5.1-7.1) | 3.8 (2.9-5.0) |
| Ethnicity | |||||
| White | 50.3 (49.1-51.5) | 45.5 (44.3-46.7) | 46.5 (44.6-48.3) | 46.1 (43.5-48.7) | 47.7 (44.5-50.8) |
| Black | 8.2 (7.6-8.9) | 9.3 (8.6-10.0) | 10.3 (9.1-11.7) | 10.6 (9.1-12.2) | 9.7 (8.0-11.7) |
| Mixed | 39.9 (38.7-41.0) | 43.8 (42.7-45.0) | 42.0 (40.2-43.8) | 42.3 (39.8-44.9) | 41.7 (38.7-44.8) |
| Other | 1.6 (1.3-1.9) | 1.4 (1.2-1.7) | 1.2 (1.0-1.6) | 1.0 (0.7-1.5) | 0.9 (0.6-1.4) |
| Educational status | |||||
| Incomplete high school | 15.9 (15.1-16.8) | 15.9 (15.1-16.8) | 15.6 (14.3-16.9) | 15.9 (14.1-17.8) | 13.1 (11.3-15.3) |
| High school | 66.7 (65.5-67.8) | 71.2 (70.2-72.2) | 71.5 (69.7-73.1) | 73.2 (70.8-75.4) | 77.9 (75.3-80.3) |
| At least some university | 17.4 (16.5-18.3) | 12.8 (12.1-13.6) | 13.0 (11.8-14.3) | 11.0 (9.5-12.7) | 9.0 (7.4-10.8) |
| Employment status (yes) | 50.4 (49.2-51.6) | 41.1 (40.0-42.3) | 35.9 (34.2-37.7) | 31.9 (29.5-34.4) | 29.7 (27.0-32.7) |
| TV viewing (≥ 5 h/day) | 8.0 (7.4-8.7) | 6.7 (6.4-7.6) | 9.6 (8.5-10.7) | 11.1 (9.7-12.7) | 15.5 (13.4-17.8) |
| Depressive symptoms (severe) | 9.0 (8.3-9.6) | 7.0 (6.4-7.6) | 7.5 (6.6-8.4) | 6.4 (5.5-7.5) | 10.3 (8.6-12.2) |
| Smoking (yes) | 13.6 (12.8-14.5) | 13.2 (12.5-14.0) | 16.5 (15.1-18.0) | 19.1 (17.1-21.4) | 21.0 (18.5-23.7) |
| Alcohol consumption (times/week) | |||||
| 0 | 79.9 (78.9-80.9) | 78.1 (77.1-79.0) | 71.1 (69.3-72.7) | 66.3 (63.8-68.8) | 63.7 (60.6-66.7) |
| 1-3 | 16.0 (15.1-16.9) | 19.3 (18.4-20.2) | 24.6 (23.0-26.2) | 29.3 (26.9-31.8) | 30.2 (27.4-33.1) |
| ≥ 4 | 4.1 (3.6-4.6) | 2.7 (2.3-3.1) | 4.3 (3.6-5.3) | 4.4 (3.5-5.4) | 6.1 (4.7-7.9) |
| Physical activity (active) | 23.0 (21.9-24.0) | 17.7 (16.8-18.6) | 16.8 (15.5-18.2) | 18.5 (16.3-20.8) | 18.0 (15.7-20.7) |
| Consumption of candies/sweets (7 days/week) | 12.0 (11.3-12.9) | 13.3 (12.5-14.1) | 18.6 (17.1-20.2) | 23.9 (21.6-26.2) | 28.3 (25.6-31.2) |
| Fruits consumption (7 days/week) | 40.6 (39.4-41.7) | 27.9 (26.9-29.0) | 21.9 (20.5-23.5) | 20.7 (18.8-22.7) | 20.5 (18.1-23.2) |
| Weight status | |||||
| Normal | 39.6 (38.5-40.8) | 43.5 (42.3-44.7) | 45.7 (43.9-47.6) | 49.0 (46.5-51.6) | 45.0 (41.9-48.2) |
| Overweight | 38.0 (36.8-39.2) | 36.4 (35.3-37.5) | 34.6 (32.9-36.3) | 31.6 (29.3-34.0) | 33.8 (30.9-36.8) |
| Obese | 22.4 (21.4-23.4) | 20.1 (19.2-21.0) | 19.7 (18.2-21.2) | 19.3 (17.4-21.4) | 21.2 (18.7-24.0) |
| Multimorbidity (yes) | 19.8 (18.8-20.7) | 10.6 (9.8-11.4) | 7.8 (6.7-9.0) | 6.3 (5.3-7.6) | 6.3 (4.9-7.9) |
Data presented as percentages (estimated 95% confidence intervals).
Table 2. Depressive symptom scores according to sugar-sweetened beverage consumption groups.
| Men | Women | |||
|---|---|---|---|---|
| n | Mean (95%CI) | n | Mean (95%CI) | |
| Number of glasses/week | ||||
| 0 | 7,634 | 1.24 (2.08-2.39) | 13,379 | 3.43 (3.30-3.57) |
| 1-5 | 9,299 | 1.72 (1.61-1.84) | 12,857 | 3.10 (2.95-3.24) |
| 6-10 | 4,612 | 1.93 (1.73-2.13) | 4,478 | 3.34 (3.10-3.57) |
| 11-15 | 2,567 | 1.84 (1.64-2.03) | 2,230 | 3.47 (3.16-3.77) |
| 16 or more | 1,808 | 2.33 (2.06-2.61) | 1,338 | 4.92 (4.35-5.49) |
| Weekly frequency (days/week) | ||||
| 1-6 | 22,205 | 1.81 (1.72-1.90) | 30,268 | 3.13 (3.02-2.25) |
| 7 | 3,715 | 2.14 (1.93-2.35) | 4,014 | 3.98 (3.71-4.26) |
| Number of glasses/day | ||||
| 1 | 7,496 | 1.80 (1.66-1.94) | 12,496 | 3.12 (2.99-3.25) |
| 2 or more | 11,647 | 1.94 (1.84-2.05) | 9,823 | 3.53 (3.36-3.71) |
95%CI = 95% confidence interval.
Association between sugar-sweetened beverage consumption and severe depressive symptoms
The associations between different patterns of sugar-sweetened beverage consumption and severe depressive symptoms are presented in Table 3. Considering the adjusted models, among women, consumption of 16 or more glasses/week was associated with a 71% higher prevalence of severe depressive symptoms (PR 1.71 [95%CI 1.38-2.11]), compared to those who reported drinking no sugar-sweetened beverages. Similarly, drinking sugar-sweetened beverages 7 days/week (PR 1.34 [95%CI 1.16-1.56]; 1-6 days/week as reference) and two or more glasses of sugar-sweetened beverages on each day of consumption (PR 1.21 [95%CI 1.06-1.37]; one glass as a reference) were also associated with a higher likelihood of severe depressive symptoms. No associations were found among men.
Table 3. The association between different indicators of sugar-sweetened beverage consumption and severe depressive symptoms according to sex.
| Men | Women | |||
|---|---|---|---|---|
| n | PR (95%CI) | n | PR (95%CI) | |
| Crude models | ||||
| Number of glasses/week | ||||
| 0 | 7,634 | Ref | 13,379 | Ref |
| 1-5 | 9,299 | 0.68 (0.54-0.85)* | 12,857 | 0.87 (0.77-0.98)* |
| 6-10 | 4,612 | 0.74 (0.55-0.98)* | 4,478 | 1.01 (0.86-1.18) |
| 11-15 | 2,567 | 0.63 (0.46-0.86)* | 2,230 | 0.94 (0.77-1.15) |
| 16 or more | 1,808 | 0.85 (0.61-1.19) | 1,338 | 1.80 (1.45-2.22)* |
| Weekly frequency (days/week) | ||||
| 1-6 | 22,205 | Ref | 30,268 | Ref |
| 7 | 3,715 | 1.20 (0.93-1.55) | 4,014 | 1.46 (1.26-1.69)* |
| Number of glasses/day | ||||
| 1 | 7,496 | Ref | 12,496 | Ref |
| 2 or more | 11,647 | 0.95 (0.76-1.20) | 9,823 | 1.19 (1.05-1.36)* |
| Adjusted models | ||||
| Number of glasses/week | ||||
| 0 | 7,634 | Ref | 13,379 | Ref |
| 1-5 | 9,299 | 0.80 (0.63-1.01) | 12,857 | 0.92 (0.81-1.04) |
| 6-10 | 4,612 | 0.88 (0.65-1.20) | 4,478 | 1.07 (0.91-1.25) |
| 11-15 | 2,567 | 0.76 (0.55-1.06) | 2,230 | 0.98 (0.79-1.21) |
| 16 or more | 1,808 | 1.05 (0.74-1.49) | 1,338 | 1.71 (1.38-2.11)* |
| Weekly frequency (days/week) | ||||
| 1-6 | 22,205 | Ref | 30,268 | Ref |
| 7 | 3,715 | 1.22 (0.93-1.60) | 4,014 | 1.34 (1.16-1.56)* |
| Number of glasses/day | ||||
| 1 | 7,496 | Ref | 12,496 | Ref |
| 2 or more | 11,647 | 1.06 (0.85-1.33) | 9,823 | 1.21 (1.06-1.37)* |
95%CI = 95% confidence interval; PR = prevalence ratio; Ref = reference category.
Adjusted models were adjusted for chronological age, ethnicity, educational status, employment status, smoking, consumption of alcohol, fruit, and sweets/candy, multimorbidity, and weight status.
p < 0.05.
Independent associations of sugar-sweetened beverage consumption, TV viewing, and physical activity with severe depressive symptoms
Regarding independent associations of sugar-sweetened beverage consumption, TV viewing, and physical inactivity with severe depressive symptoms (see Tables S1 and S2 (102.1KB, pdf) , available as online-only supplementary material), sugar-sweetened beverage consumption was associated with higher odds of severe depressive symptoms only among women, while higher TV viewing and physical inactivity were associated with higher odds of severe depressive symptoms in both sexes.
Combined associations of sugar-sweetened beverage consumption, TV viewing and physical activity with severe depressive symptoms
Considering only participants who reported consuming sugar-sweetened beverages, the combined association of consumption with TV viewing and PA in the association with severe depressive symptoms are presented in Table 4. In general, individuals with combined unhealthy behaviors (≥ 5 h TV viewing or physical inactivity and sugar-sweetened beverage consumption 7 days/week or two or more glasses/day) had a higher likelihood of severe depressive symptoms; drinking two or more glasses/day interacted with TV viewing among men (PR 2.11 [95%CI 1.19-3.74]).
Table 4. Combined associations of sugar-sweetened beverage consumption with PA and TV viewing in the association with severe depressive symptoms.
| Men (n=19,143) | Women (n=21,776) | |||
|---|---|---|---|---|
| Sugar-sweetened beverage consumption | n | PR (95%CI) | n | PR (95%CI) |
| 7 days/week | ||||
| No + < 5 h/day TV viewing | 20,754 | Ref | 27,400 | Ref |
| No + ≥ 5 h/day TV viewing | 1,451 | 1.86 (1.29-2.68)* | 2,868 | 1.27 (1.01-1.58)* |
| Yes + < 5 h/day TV viewing | 3,302 | 1.16 (0.86-1.57) | 3,394 | 1.32 (1.12-1.56)* |
| Yes + ≥ 5 h/day TV viewing | 413 | 2.08 (1.30-3.31)* | 620 | 1.70 (1.29-2.25)* |
| No + active | 4,714 | Ref | 5,223 | Ref |
| No + inactive | 17,491 | 1.91 (1.34-2.71)* | 25,045 | 1.17 (0.92-1.48) |
| Yes + active | 765 | 1.46 (0.79-2.68) | 510 | 1.09 (0.66-1.78) |
| Yes + inactive | 2,950 | 2.13 (1.41-3.20)* | 3,504 | 1.59 (1.23-2.05)* |
| 2 or more glasses/day | ||||
| No + < 5 h/day TV viewing | 7,027 | Ref | 11,403 | Ref |
| No + ≥ 5 h/day TV viewing | 469 | 1.11 (0.70-1.76) | 1,093 | 1.14 (0.87-1.49) |
| Yes + < 5 h/day TV viewing | 10,700 | 0.94 (0.74-1.20) | 8,567 | 1.16 (1.01-1.33)* |
| Yes + ≥ 5 h/day TV viewing | 947 | 2.21 (1.53-3.19)* | 1,256 | 1.66 (1.31-2.10)* |
| No + active | 1,546 | Ref | 2,024 | Ref |
| No + inactive | 5,950 | 2.09 (1.22-3.56)* | 10,472 | 1.13 (0.84-1.51) |
| Yes + active | 2,454 | 1.29 (0.69-2.39) | 1,372 | 1.01 (0.69-1.48) |
| Yes + inactive | 9,193 | 2.11 (1.24-3.60)* | 8,451 | 1.38 (1.04-1.85)* |
95%CI = 95% confidence interval; PA = physical activity; PR = prevalence ratio; Ref = reference category.
Adjusted for chronological age, ethnicity, educational status, employment status, smoking, consumption of alcohol, fruit, and sweets/candy, multimorbidity, weight status, TV viewing (for PA analysis), and PA (for TV viewing analysis). Interactions: a) more than 7 days/week × TV viewing (PR 0.97 [95%CI 0.52-1.78]) and women: PR 1.02 (95%CI 0.70-1.48); b) more than 7 days/week × PA (men: PR 0.76 [95%CI 0.39-1.49] and women: PR 1.25 [95%CI 0.75-2.09]); c) two or more glasses × TV viewing (men: PR 2.11 [95%CI 1.19-3.74] and women: PR 1.26 [95%CI 0.88-1.80]); and d) two or more glasses × PA (men: PR 0.78 [95%CI 0.41-1.50] and women: PR 1.22 [95%CI 0.81-1.83]).
p < 0.05.
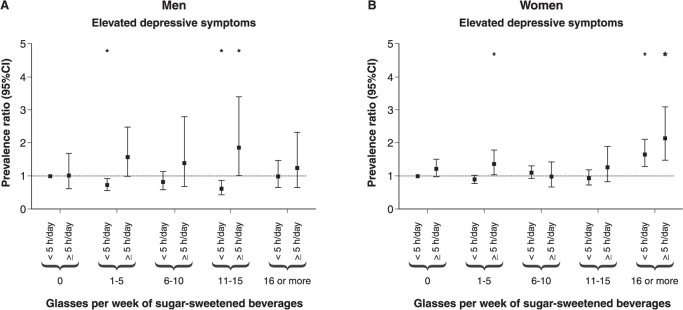
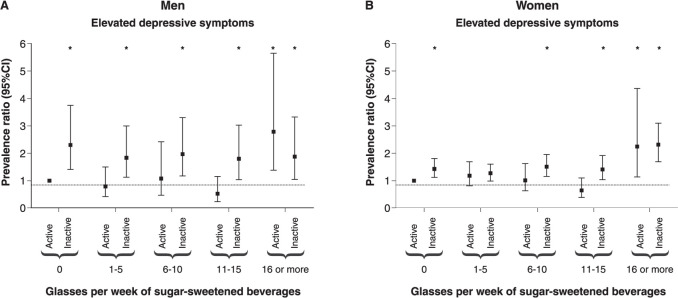
The combined associations between TV viewing and sugar-sweetened beverage consumption are presented in Figure 1. Lower consumption reduced the effects of higher TV viewing, and lower TV viewing reduced the harmful association between high sugar-sweetened beverage ingestion and severe depressive symptoms. Among men, there were consistent interactions between 1-5 glasses and TV viewing (PR 2.09 [95%CI 1.06-4.12]) and between 11-15 glasses and TV viewing (PR 2.90 [95%CI 1.29-6.50]). The combined associations between PA and sugar-sweetened beverage consumption are shown in Figure 2. There was an association between 16 glasses of sugar-sweetened beverages per week and severe depressive symptoms regardless of PA level, with no consistent interactions.
Figure 1. Combined associations of sugar-sweetened beverage consumption with TV viewing in the association with severe depressive symptoms, adjusted for age, ethnicity, educational status, employment status, smoking, consumption of alcohol, fruit, and sweets/candy, multimorbidity, TV viewing, weight status, and physical activity. Interactions: A) men: 1-5 glasses × TV viewing (prevalence ratio [PR] 2.09 [95% confidence interval {95%CI} 1.06-4.12]); 6-10 glasses × TV viewing (PR 1.64 [95%CI 0.68-3.97]), 11-15 glasses × TV viewing (PR 2.90 [95%CI 1.29-6.50]), 16 or more glasses × TV viewing (PR 1.22 [95%CI 0.53-2.79]); B) women: 1-5 glasses × TV viewing (PR 1.25 [95%CI 0.88-1.76]), 6-10 glasses × TV viewing (PR 0.73 [95%CI 0.47-1.13]), 11-15 glasses × TV viewing (PR 1.10 [95%CI 0.67-1.79]), 16 or more glasses × TV viewing (PR 1.06 [95%CI 0.67-1.68]). * p < 0.05.
Figure 2. Combined associations of sugar-sweetened beverage consumption with physical activity in the association with severe depressive symptoms, adjusted for chronological age, ethnicity, educational status, employment status, smoking, consumption of alcohol, fruit, and sweets/candy, multimorbidity, weight status, and TV viewing. Interactions: A) men: 1-5 glasses × TV viewing (prevalence ratio [PR] 1.00 [95% confidence interval {95%C} 0.51-1.96]), 6-10 glasses × physical activity (PA) (PR 0.79 [95%CI 0.33-1.86]), 11-15 glasses × PA (PR 1.45 [95%CI 0.63-3.37]), 16 or more glasses × PA (PR 0.29 [95%CI 0.13-0.64]); B) women: 1-5 glasses × PA (PR 0.75 [95%CI 0.51-1.10]), 6-10 glasses × PA (PR 1.03 [95%CI 0.63-1.70]), 11-15 glasses × PA (PR 1.50 [95%CI 0.85-2.62]), 16 or more glasses × PA (PR 0.71 [95%CI 0.35-1.43]). * p < 0.05.
Discussion
We aimed to investigate the association between sugar-sweetened beverage consumption and severe depressive symptoms, as well as whether TV viewing and PA can change the association between sugar-sweetened beverage consumption and severe depressive symptoms. We found that different indicators of sugar-sweetened beverage consumption and severe depressive symptoms were associated only among women. In addition, TV viewing interacted with sugar-sweetened beverage consumption among men, given that lower TV viewing reduced the association between sugar-sweetened beverage consumption and severe depressive symptoms. Sugar-sweetened beverage consumption did not interact with TV viewing among women. The associations of sugar-sweetened beverage consumption and TV viewing with severe depressive symptoms were independent in both sexes.
Our study showed that whether independently or dependently associated with TV viewing, higher ingestion of sugar-sweetened beverages was a risk factor for severe depressive symptoms. However, only high consumption was associated with severe depressive symptoms (16 glasses or more/week). The association between sugar-sweetened beverage consumption and severe depressive symptoms can be explained due to the high glycemic load of the beverages.15,16 A higher glycemic load is associated with inflammatory markers and oxidative stress,17 which are associated with severe depressive symptoms.18
Nevertheless, very small portions of sugar-sweetened beverages should not necessarily imply an immediate increase in glycemic load, which would explain why only high intake of sugar-sweetened beverages was associated with higher severe depressive symptoms. These beverages also include other industrialized components, such as artificial flavors and colors, as well as other additives that may also be related to depressive symptoms, although this is still under investigation.38 In addition, higher consumption of sugar-sweetened beverages may lead to lower consumption of fruits and vegetables and other foods that protect against depressive symptoms.8 Higher consumption of sugar-sweetened beverages is also associated with obesity,39 which is associated with depressive symptoms.40 However, we observed that, even when controlling for fruit consumption and weight status, high intake of sugar-sweetened beverages was still associated with depressive symptoms.
We also found that the association between sugar-sweetened beverage consumption and severe depressive symptoms was influenced by TV viewing, but not PA, among men. This finding can be explained through different paths, especially since TV viewing may share more social and behavioral mechanisms with sugar-sweetened beverage consumption than PA. First, watching television may increase food intake, such as well as sugar-sweetened beverage consumption due to their co-occurrence (i.e. eating and drinking while watching television).28 Second, many TV commercials are about ultra-processed foods, such as sugar-sweetened beverages, which consequently influences higher consumption.29,41 Third, high sitting time and sugar-sweetened beverage consumption share similar mechanisms that underlie the association with depressive symptoms, such as higher inflammatory levels42 and obesity,43 which are associated with higher depressive symptoms.18
We also found a sex difference in the association between sugar-sweetened beverage consumption and severe depressive symptoms. Obesity is more prevalent among women44 and women have a higher mean energy intake.45 It is plausible to infer that sugar-sweetened beverages have a greater impact on the glycemic index in women than men. However, we found higher consumption of sugar-sweetened beverages among men, which suggests that the same amount of sugar-sweetened beverages can have a greater effect on women than men with respect to severe depressive symptoms.
Our study has some limitations. First, due to the cross-sectional design, causality cannot be inferred. Thus, severe depressive symptoms can only be associated with risk behaviors, such as the consumption of sugar-sweetened beverages and physical inactivity.6 Second, we used self-report measures of lifestyle behaviors, which could produce bias. In addition, we only assessed one sedentary behavior activity (TV viewing) as a proxy, which should be considered when interpreting the findings, despite the fact that TV viewing is a mentally passive activity and has been associated with depressive symptoms.36,46 Finally, although recent evidence suggests that there is an association between artificially sweetened soft drinks and brain health,47 we did not include this in our analysis given the lack of a direct relationship with mental health, especially through shared mechanisms with PA and sedentary behaviors.12,48 Nevertheless, we could not adjust for potential confounders such as other nutrients, total energy intake, loneliness, and family history of mental disorders. On the other hand, we found combined associations of sugar-sweetened beverage consumption with PA and TV viewing in the association with depressive symptoms using a large representative cohort of Brazilian adults, which we consider a strength. We also controlled for important confounders, especially eating habits (e.g., consumption of alcohol, fruit, sweets/candy), specifying the role of sugar-sweetened beverages.
Thus, we conclude that sugar-sweetened beverage consumption is independently associated with severe depressive symptoms in women, while it interacted with TV viewing among men. In addition, sugar-sweetened beverage consumption did not interact with PA since it only presented independent associations. Future interventions should reduce the combination of TV viewing and sugar-sweetened beverage consumption, since they can interact in the association with severe depressive symptoms. Longitudinal studies are needed to test these associations and confirm these findings, especially considering the potential interactions between lifestyle behaviors in the prediction of severe depressive symptoms.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
We gratefully acknowledge the contributions of all participants of the present research, as well as the Instituto Brasileiro de Geografia e Estatística (IBGE) staff for the data collection. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. AOW is supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) with a PhD scholarship (FAPESP process: 2019/24124-7). This paper presents independent research. The views expressed in this publication are those of the authors and not necessarily those of the acknowledged institution.
Footnotes
How to cite this article: Werneck AO, Schuch FB, Stubbs B, Oyeyemi AL, Szwarcwald CL, Vancampfort D, et al. Independent and combined associations of sugar-sweetened beverage consumption, TV viewing, and physical activity with severe depressive symptoms among 59,402 adults. Braz J Psychiatry. 2021;43:574-583. http://dx.doi.org/10.1590/1516-4446-2020-1073
References
- 1.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang CK, Hayes RD, Perera G, Broadbent MT, Fernandes AC, Lee WE, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One. 2011;6:e19590. doi: 10.1371/journal.pone.0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Correll CU, Solmi M, Veronese N, Bortolato B, Rosson S, Santonastaso P, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16:163–80. doi: 10.1002/wps.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. 2018;175:631–48. doi: 10.1176/appi.ajp.2018.17111194. [DOI] [PubMed] [Google Scholar]
- 6.Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6:675–712. doi: 10.1016/S2215-0366(19)30132-4. [DOI] [PubMed] [Google Scholar]
- 7.Ashdown-Franks G, Sabiston CM, Stubbs B. The evidence for physical activity in the management of major mental illnesses: a concise overview to inform busy clinicians’ practice and guide policy. Curr Opin Psychiatry. 2019;32:375–80. doi: 10.1097/YCO.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 8.Firth J, Veronese N, Cotter J, Shivappa N, Hebert JR, Ee C, et al. What is the role of dietary inflammation in severe mental illness? A review of observational and experimental findings. Front Psychiatry. 2019;10:350. doi: 10.3389/fpsyt.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adjibade M, Julia C, Allès B, Touvier M, Lemogne C, Srour B, et al. Prospective association between ultra-processed food consumption and incident depressive symptoms in the French NutriNet-Santé cohort. BMC Med. 2019;17:78. doi: 10.1186/s12916-019-1312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gómez-Donoso C, Sánchez-Villegas A, Martínez-González MA, Gea A, Mendonça RD, Lahortiga-Ramos F, et al. Ultra-processed food consumption and the incidence of depression in a Mediterranean cohort: the SUN Project. Eur J Nutr. 2020;59:1093–103. doi: 10.1007/s00394-019-01970-1. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Villegas A, Zazpe I, Santiago S, Perez-Cornago A, Martinez-Gonzalez MA, Lahortiga-Ramos F. Added sugars and sugar-sweetened beverage consumption, dietary carbohydrate index and depression risk in the Seguimiento Universidad de Navarra (SUN) Project. Br J Nutr. 2018;119:211–21. doi: 10.1017/S0007114517003361. [DOI] [PubMed] [Google Scholar]
- 12.Guo X, Park Y, Freedman ND, Sinha R, Hollenbeck AR, Blair A, et al. Sweetened beverages, coffee, and tea and depression risk among older US adults. PLoS One. 2014;9:e94715. doi: 10.1371/journal.pone.0094715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knüppel A, Shipley MJ, Llewellyn CH, Brunner EJ. Sugar intake from sweet food and beverages, common mental disorder and depression: prospective findings from the Whitehall II study. Sci Rep. 2017;7:6287. doi: 10.1038/s41598-017-05649-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh GM, Micha R, Khatibzadeh S, Shi P, Lim S, Andrews KG, et al. Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: a systematic assessment of beverage intake in 187 countries. PLoS One. 2015;10:e0124845. doi: 10.1371/journal.pone.0124845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breymeyer KL, Lampe JW, McGregor BA, Neuhouser ML. Subjective mood and energy levels of healthy weight and overweight/obese healthy adults on high-and low-glycemic load experimental diets. Appetite. 2016;107:253–9. doi: 10.1016/j.appet.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantantzis K, Schlaghecken F, Sünram-Lea SI, Maylor EA. Sugar rush or sugar crash? A meta-analysis of carbohydrate effects on mood. Neurosci Biobehav Rev. 2019;101:45–67. doi: 10.1016/j.neubiorev.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Milajerdi A, Saneei P, Larijani B, Esmaillzadeh A. The effect of dietary glycemic index and glycemic load on inflammatory biomarkers: a systematic review and meta-analysis of randomized clinical trials. Am J Clin Nutr. 2018;107:593–606. doi: 10.1093/ajcn/nqx042. [DOI] [PubMed] [Google Scholar]
- 18.Osimo EF, Pillinger T, Rodriguez IM, Khandaker GM, Pariante CM, Howes OD. Inflammatory markers in depression: a meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav Immun. 2020;87:9019. doi: 10.1016/j.bbi.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization (WHO) Global recommendations on physical activity for health. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 21.Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, et al. Sedentary Behavior Research Network (SBRN) – Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallgren M, Owen N, Stubbs B, Zeebari Z, Vancampfort D, Schuch F, et al. Passive and mentally-active sedentary behaviors and incident major depressive disorder: a 13-year cohort study. J Affect Disord. 2018;241:579–85. doi: 10.1016/j.jad.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Mittal D, Stevenson RJ, Oaten MJ, Miller LA. Snacking while watching TV impairs food recall and promotes food intake on a later TV free test meal. Appl Cognit Psychol. 2011;25:871–7. [Google Scholar]
- 24.Lopes AE, Araújo LF, Levy RB, Barreto SM, Giatti L. Association between consumption of ultra-processed foods and serum C-reactive protein levels: cross-sectional results from the ELSA-Brasil study. Sao Paulo Med J. 2019;137:169–76. doi: 10.1590/1516-3180.2018.0363070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandes RA, Ritti-Dias RM, Balagopal PB, Conceição RD, Santos RD, Cucato GG, et al. Self-initiated physical activity is associated with high sensitivity C-reactive protein: a longitudinal study in 5,030 adults. Atherosclerosis. 2018;273:131–5. doi: 10.1016/j.atherosclerosis.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Allison MA, Jensky NE, Marshall SJ, Bertoni AG, Cushman M. Sedentary behavior and adiposity-associated inflammation: the multi-ethnic study of atherosclerosis. Am J Prev Med. 2012;42:8–13. doi: 10.1016/j.amepre.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Köhler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. 2017;135:373–87. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 28.Chapman CD, Nilsson VC, Thune HÅ, Cedernaes J, Le Grevès M, Hogenkamp PS, et al. Watching TV and food intake: the role of content. PLoS One. 2014;9:e100602. doi: 10.1371/journal.pone.0100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soares Guimarães J, Mais LA, Leite FH, Horta PM, Santana MO, Martins AP, et al. Ultra-processed food and beverage advertising on Brazilian television by International Network for Food and Obesity/Non-Communicable Diseases Research, Monitoring and Action Support benchmark. Public Health Nutr. 2020;23:2657–62. doi: 10.1017/S1368980020000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Žeželj SP, Jovanović GK, Krešić G. The association between the Mediterranean diet and high physical activity among the working population in Croatia. Med Pr. 2019;70:169–76. doi: 10.13075/mp.5893.00773. [DOI] [PubMed] [Google Scholar]
- 31.Instituto Brasileiro de Geografia e Estatística (IBGE) Pesquisa nacional de saúde 2013 [Internet] 2014. [cited 2020 Oct 22] http://biblioteca.ibge.gov.br/visualizacao/livros/liv94074.pdf.
- 32.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos IS, Tavares BF, Munhoz TN, Pio de Almeida LS, Barreto da Silva NT, Tams BD, et al. [Sensitivity and specificity of the Patient Health Questionnaire-9 (PHQ-9) among adults from the general population] Cad Saude Publica. 2013;29:1533–43. doi: 10.1590/0102-311x00144612. [DOI] [PubMed] [Google Scholar]
- 34.He C, Levis B, Riehm KE, Saadat N, Levis AW, Azar M, et al. The accuracy of the Patient Health Questionnaire-9 Algorithm for screening to detect major depression: an individual participant data meta-analysis. Psychother Psychosom. 2020;89:25–37. doi: 10.1159/000502294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malta DC, Stopa SR, Szwarcwald CL, Gomes NL, Silva JB, Júnior, Reis AA. A vigilância e o monitoramento das principais doenças crônicas não transmissíveis no Brasil – Pesquisa Nacional de Saúde, 2013. Rev Bras Epidemiol. 2015;18(Suppl 2):3–16. [Google Scholar]
- 36.Werneck AO, Oyeyemi AL, Szwarcwald CL, Vancampfort D, Silva DR. Associations between TV viewing and depressive symptoms among 60,202 Brazilian adults: the Brazilian national health survey. J Affect Disord. 2018;236:23–30. doi: 10.1016/j.jad.2018.04.083. [DOI] [PubMed] [Google Scholar]
- 37.Stubbs B, Vancampfort D, Veronese N, Kahl KG, Mitchell AJ, Lin PY, et al. Depression and physical health multimorbidity: primary data and country-wide meta-analysis of population data from 190 593 people across 43 low- and middle-income countries. Psychol Med. 2017;47:2107–17. doi: 10.1017/S0033291717000551. [DOI] [PubMed] [Google Scholar]
- 38.Kang D, Kim Y, Je Y. Non-alcoholic beverage consumption and risk of depression: epidemiological evidence from observational studies. Eur J Clin Nutr. 2018;72:1506–16. doi: 10.1038/s41430-018-0121-2. [DOI] [PubMed] [Google Scholar]
- 39.Garduño‐Alanís A, Malyutina S, Pajak A, Stepaniak U, Kubinova R, Denisova D, et al. Association between soft drink, fruit juice consumption and obesity in Eastern Europe: cross‐sectional and longitudinal analysis of the HAPIEE study. J Hum Nutr Diet. 2020;33:66–77. doi: 10.1111/jhn.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romain AJ, Marleau J, Baillot A. Association between physical multimorbidity, body mass index and mental health/disorders in a representative sample of people with obesity. J Epidemiol Community Health. 2019;73:874–80. doi: 10.1136/jech-2018-211497. [DOI] [PubMed] [Google Scholar]
- 41.Harris JL, Bargh JA, Brownell KD. Priming effects of television food advertising on eating behavior. Health Psychol. 2009;28:404–13. doi: 10.1037/a0014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips CM, Dillon CB, Perry IJ. Does replacing sedentary behaviour with light or moderate to vigorous physical activity modulate inflammatory status in adults? Int J Behav Nutr Phys Act. 2017;14:138. doi: 10.1186/s12966-017-0594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinonen I, Helajärvi H, Pahkala K, Heinonen OJ, Hirvensalo M, Pälve K, et al. Sedentary behaviours and obesity in adults: the Cardiovascular Risk in Young Finns Study. BMJ Open. 2013;3:e002901. doi: 10.1136/bmjopen-2013-002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NCD Risk Factor Collaboration (NCD-RisC) Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260–4. doi: 10.1038/s41586-019-1171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett E, Peters SA, Woodward M. Sex differences in macronutrient intake and adherence to dietary recommendations: findings from the UK Biobank. BMJ Open. 2018;8:e020017. doi: 10.1136/bmjopen-2017-020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallgren M, Dunstan DW, Owen N. Passive versus mentally active sedentary behaviors and depression. Exerc Sport Sci Rev. 2020;48:20–7. doi: 10.1249/JES.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 47.Anjum I, Jaffery SS, Fayyaz M, Wajid A, Ans AH. Sugar beverages and dietary sodas impact on brain health: a mini literature review. Cureus. 2018;10:e2756. doi: 10.7759/cureus.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pase MP, Himali JJ, Beiser AS, Aparicio HJ, Satizabal CL, Vasan RS, et al. Sugar- and artificially sweetened beverages and the risks of incident stroke and dementia: a prospective cohort study. Stroke. 2017;48:1139–46. doi: 10.1161/STROKEAHA.116.016027. [DOI] [PMC free article] [PubMed] [Google Scholar]