Abstract
Currently approved anti-COVID-19 vaccines have been found to be safe and effective and almost 60% of Israeli residents are already vaccinated with BNT162b2 vaccine.
This observational study was designed to evaluate the adverse events of vaccine reported by 61 healthcare workers at least 7 days after the 2nd vaccination, and to investigate the correlation of adverse events and anti-SARS-CoV-2 IgG antibody levels.
The median participant's age was 51.25 years, 16 men and 45 women; 77% (44% of male and 84.5% of female participants) reported adverse events. Injection site pain, fatigue and fever were the most common symptoms, and significantly higher antibody levels (average 19,387 AU/mL) were found in participants who had fever compared to those who did not experience fever (average antibody levels of 9,977 AU/mL, p < 0.001). This finding corresponds to previous observations of higher anti-SARS-CoV-2 IgG antibody levels in COVID-19 patients presented with fever.
Keywords: Anti-SARS-CoV-2 S1-specific IgG antibody levels, Vaccination with BNT162b2 mRNA COVID-19 Vaccine, Individuals presented with fever after vaccination had higher antibody levels
1. Introduction
During the last century, vaccines have changed the modern world, and became a main strategy in reducing morbidity and mortality from infections worldwide. The spread of Coronavirus Disease (COVID-19) urged medical communities and pharmaceutical companies to seek effective vaccines with acceptable side effects. The rapid development of COVID-19 vaccines was an unprecedented challenge of balancing an urgent claim for effective vaccine, without compromising the safety of the product, based on strict quality assurance requirements. Currently there is a number of approved COVID-19 vaccines, which are safe and have relatively few clinically remarkable adverse effects. At the time of this writing (July 10, 2021), 3.42 billion COVID-19 vaccines doses have been administered globally.
In Israel, more than 5.5 million people (59.97% of the 9.3 million population) - have already been vaccinated with two doses of BNT162b2 mRNA vaccine [1], and the efficacy and safety were lately reported [2]. Israel's experience in responding to large-scale national emergencies played an important role in rapid planning and implementation of the vaccination process.
Some concerns were raised regarding the vaccines, including the frequency and severity of adverse events.
We have conducted an analysis of adverse events after vaccination with BNT162b2 mRNA Covid-19 Vaccine of Magen David Adom (MDA) healthcare workers and the correlation between adverse events and level of the anti-Spike protein (anti-S1) IgG antibodies. None of the participants were diagnosed with COVID-19 prior to vaccination.
2. Materials and methods
Clinical symptoms and anti-S1 anti-SARS-CoV-2 IgG antibodies were investigated in sixty-one MDA healthcare workers: laboratory technicians, phlebotomists and administration personnel, vaccinated between December 21st, 2020 and February 28th, 2021. All clinical symptoms, appeared from the day of injection of the first dose of spike messenger RNA (mRNA) BNT162b2 Covid-19 Vaccine (Pfizer-BioNTech) till day 21 after the second dose were considered adverse events after vaccination. The work was conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki). Participants younger than 18 years old and those who diagnosed as COVID-19 prior to vaccination or post vaccination were excluded from the study. All participants gave consent to report adverse events and to have blood drawn for investigation of anti-SARS-CoV-2 antibody levels after being vaccinated with 2 doses of BNT162b2. Clinical information was collected from participants by questionnaires (phone conversation), 7–27 days after second dose of BNT162b2 COVID-19 vaccine. Antibodies were measured in the MDA National Blood Services laboratory using SARS-CoV-2 IgG II Quant assay - a chemiluminescent microparticle immunoassay (CMIA, Abbott, USA), for the quantitative determination of immunoglobulin class G (IgG) antibodies to the receptor binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 on the ARCHITECT i2000SR (Abbott, USA) instrument [3]. The results were reported in arbitrary units per milliliter (AU/mL). Results equal or greater than 50 AU/ml were considered positive [3]. Based on the WHO International Standard study, a strong correlation between the Abbott AU/mL unit and WHO unit was found (binding antibody unit per mL [BAU/mL]) and the correlation could be calculated by the equation [4]:
BAU/mL = 0.142*AU/mL
Descriptive statistics (mean, median, standard deviation) were calculated. Participants were divided to groups by age (“younger” individuals, aged 30–49 and “older” aged 50–71) and by sex. Antibody levels were calculated in asymptomatic participants and in those who had adverse events after vaccination. Symptomatic participants divided to subgroups by symptoms. Antibody levels between groups of participants were compared using Chi- square test.
3. Results
Sixty-one vaccinated individuals, who got two doses of BNT162b2 mRNA Covid-1 vaccine at least 21 days apart, participated in the study. Sixty received the second dose within 28 days from first dose and one −46 days after the first dose. Venous blood samples were drawn once, at least 7 days after the second vaccine dose (average 15 days, 7–27 days). The median age of participants was 51.25 years, 16 men and 45 women. Seventy seven percent of participants (47/61) reported at least one symptom that was attributed to the vaccination; 14/61 (23%) did not have any symptoms. Adverse events presented in Table 1 .
Table 1.
Adverse events in vaccinated individuals after two doses of BNT162b2 vaccine and average Anti-SARS-CoV-2 Anti-S IgG levels (AU/mL) per group of participants with certain adverse event.
| Symptoms | Men,% N = 16 |
Women,% N = 45 |
All, (%) N = 61 |
Average Anti-SARS-CoV-2 Anti-S IgG levels, AU/mL |
|---|---|---|---|---|
| Any | 9 (44%) | 45 (84.5%) | 47 (77%) | 13,177 |
| Pain in the injection site | 6 (37.5%) | 16 (35.6%) | 22 (36.1%) | 12,727 |
| Fatigue | 3 (18.8%) | 16 (35.6%) | 19 (31.1%) | 12,023 |
| Fever (≥37.8 °C) | 4 (25%) | 13 (28.9%) | 17 (27.9%) | 19,387 |
| Myalgia | 1 (6.3%) | 14 (31.1%) | 15 (24.6%) | 13,317 |
| Nausea | 0 | 8 (17.8%) | 8 (13.1%) | 13,268 |
| Chills | 0 | 6 (13.3%) | 6 (9.8%) | 13,211 |
| Tiredness/sleepiness | 1 (6.3%) | 5 (11.1%) | 6 (9.8%) | 12,990 |
| Abdominal pain | 0 | 4 (8.9%) | 4 (6.6%) | 13,587 |
| Chest pain* | 0 | 3 (6.7%) | 3 (4.9%) | N/A |
| Back pain* | 0 | 2 (4.4%) | 2 (3.3%) | N/A |
| Arm numbness* | 0 | 1 (2.2%) | 1 (1.6%) | N/A |
| Dizziness* | 0 | 1 (2.2%) | 1 (1.6%) | N/A |
| Nasal congestion* | 0 | 1 (2.2%) | 1 (1.6%) | N/A |
AU/mL - arbitrary units per milliliter;
N/A – not applicable
chest pain, back pain, numbness, dizziness and nasal congestion were rare symptoms in our cohort, therefore the average antibody level was not calculated.
In general, male participants reported less symptoms than female, and 9/16 (56%) did not have any adverse events. Among the 45 women who participated in the study, 38/45 (84.5%) experienced at least 1 adverse event (1–6, an average 3) and only 7/45 (15.5%) were asymptomatic. The most common reported complain was pain in the injection site (36.1%) with equal distribution among men and women, followed by fatigue (31.1%) and fever (27.9%) - more common symptoms in women, and myalgia (24.6%) with equal distribution among both sexes. In contrast to finding of Polack et al [1] younger individuals reported less adverse events: 69.7% of 26 “younger” individuals, aged 30–49 (15 women and 11 men) reported at least one symptom compared to 82.9% of thirty-five “older” participants aged 50–71. Frequently reported adverse event in “younger” group was fever ≥ 37.8 °C (38.5% of participants), in “older” group this symptom was less frequent (20%). Nobody reported fever > 39 °C.
No severe adverse events (Grade 3 or 4) were reported [5].
IgG anti-S antibodies were detected in blood samples of all participants. Average level of IgG was 12,599 AU/mL (range 229–37,840 AU/mL), no significant difference was found between both sexes: men had an average level of 11,854 AU/mL and women −12,865 AU/mL Twenty six “younger” individuals had similar average antibody level than 35 “older”: 12,903 AU/mL versus 12,374 AU/mL, respectively. The average antibody level among individuals who reported adverse events after vaccination was not significantly different from the average level of antibodies in asymptomatic persons −13,177 AU/mL versus 10,663 AU/mL, respectively (p = 0.39).
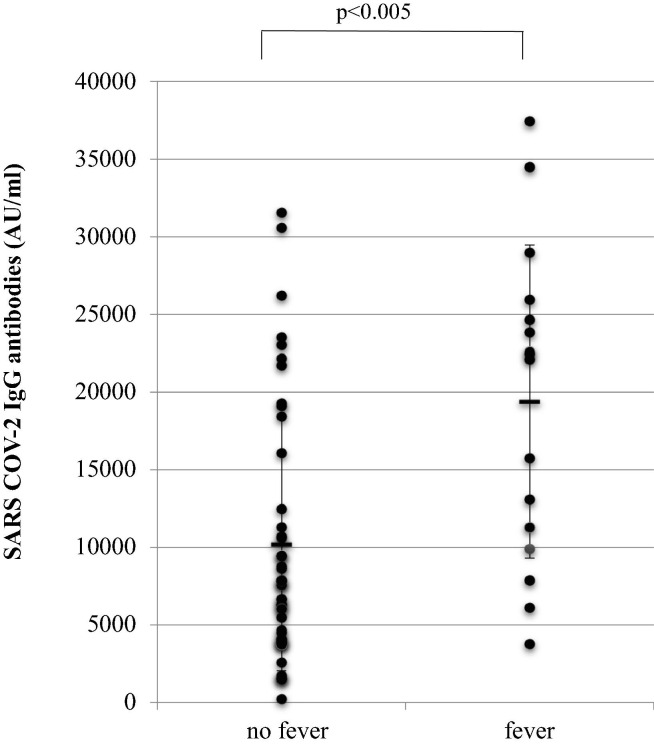
Participants who had fever as an adverse event had higher levels of antibodies (average 19,387 AU/mL) compared to those who did not have fever (average 9977 AU/mL), p < 0.005 (Fig. 1 ).
Fig. 1.
Average Anti-SARS-CoV-2 Anti-S IgG antibody levels (AU/mL) in individuals who had no fever and who had fever (≥37.8 °C) as an adverse event after injection of 2 doses of BNT162b2 vaccine. U/mL - arbitrary units per milliliter; p < 0.005.
Participants reporting fatigue, pain at injection site, headache, muscle aches, nausea, chills and sleepiness had similar levels of antibodies compared to those who did not complain on these symptoms.
4. Discussion
The potency of two doses of BNT162b2 mRNA vaccine were recently reported [1]. In Israel, with the successful campaign of vaccination of population and highest rate of vaccine doses per capita, first lessons concerning efficacy and safety are already learned. In this observational study we evaluated antibody responses and adverse events after injection of two doses of BNT162b2 mRNA vaccine in sixty one healthcare workers, vaccinated between December 21st, 2020 and February 28th, 2021. The symptoms were evaluated by phone questionnaires, at least 7 days after completion of vaccination schedule. The period of observation of post-vaccination symptoms and collection of blood samples was 7–27 days after the second dose of BNT162b2. The collection of data was completed on March 17, 2021. We found that two doses of mRNA vaccine drawn immune responses in all participants, with post-vaccination antibody titers ranged from 229 to 37,840 AU/mL. Most participants (77%) experienced some symptoms after vaccination, and the most common symptoms were pain in injection site, fatigue and fever. We found that female participants experienced more subjective symptoms than men. Similar rate of objective symptoms (e.g., fever) was found in both sexes. Antibody levels were similar between “younger” and “older” age groups.
No serious adverse events were observed. In general, vaccinated individuals who reported adverse events had similar antibody levels, comparing to those who did not have any symptoms. The only exclusion was fever (≥37.8 °C): persons experienced fever as an adverse event after vaccination had significantly higher antibody levels. The same phenomenon was described in recipients of other vaccines, e.g., young children, received measles-containing vaccines [6]. In COVID-19 patients who suffered from fever during the course of the disease, the antibody levels in plasma were higher than in those patients who did not have fever [7], [8]. The suggestion that a febrile temperature can boost the innate and adaptive immune and drive more prominent immune reactions was made years ago and the evidence from previous studies reviewed by Evans et al [9]. There is a controversy among medical professionals about the role of antipyretic drugs in prevention and treatment of post-vaccination febrile reactions. According to the observation of Prymula et al, antibody responses to some vaccines decreased due to use of prophylactic paracetamol in children, however, conclusions of another studies did not support this finding [10]. This conclusion is controversial, as in another trials it was shown that paracetamol given to children after influenza vaccine did not decrease an immune response [11].
It could be concluded that release of endogenous pyrogens, and, as a result post-vaccination febrile reaction, is associated with enhanced antibody production.
Our study had several limitations. We evaluated adverse events and antibody levels in small homogenous group of participants, aged 30–71 years old, most of them were women. There was no information concerning underlying health conditions of individuals participated in the study. There was no follow-up of antibody levels, and all results and adverse events were detected 7–27 days after the completion of vaccination schedule. Neutralizing antibodies were not tested and objective changes post-vaccination (e. g., changes in blood tests, as elevated C-Reactive Protein and Erythrocyte Sedimentation Rate) were not examined. Larger scale trials are needed to understand better the immune responses to COVID-19 vaccines and to investigate further the adverse events, especially delayed ones. We hope that ongoing follow-up studies will show whether immune responses to mRNA vaccines are maintained over time and what are the hallmarks of more prominent and prolonged immune processes in vaccinated persons.
5. Conclusions
This small observation study examined the possible correlation of anti-SARS-CoV-2 S1-specific IgG antibody levels after vaccination with BNT162b2 mRNA COVID-19 vaccine with adverse events following vaccination in sixty-one healthcare workers. Recently, antibody responses to single dose of BNT162b2 vaccine were investigated [12] and IgG antibodies were found in most participants of the study. In our study, all recipients of two doses of BNT162b2 vaccine produced IgG anti-S antibodies, with average level of 12,599 AU/mL (range 229–37,840 AU/mL). Adverse events after BNT162b2 vaccine [1] and after first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccines were reported recently [13] and were similar to adverse events experienced by healthcare workers participated in our study: injection-site pain, myalgia, fatigue, headache and fever. Younger individuals experienced fewer side effects in contrast to findings of Kim et al [13]; women reported more adverse events. Most of sixty-one participants reported mild adverse events, and fever ≥ 37.8 °C was an only significant predictor of higher IgG levels.
The current experience with reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2 was recently summarized in systematic review and meta-analysis [14] but there are still many questions and uncertainties, as a longevity of immune responses and protection of vaccinated persons against new mutated forms of COVID-19.
Up to date, this a first report of possible connection of febrile reaction after BNT162b2 vaccination and more explicit immune response with higher IgG anti-S antibody levels. Larger studies are needed for investigating the correlation of immune response to symptoms experienced after vaccination with BNT162b2. As an observation period after the completion of vaccination schedule in our study was short, the follow-up of vaccinated persons in terms of long-term safety of vaccine and stability of immune responses was not possible. Follow-up analysis of long-term safety profile of BNT162b2 vaccine and repeated antibody tests are planned for the study participants.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
M. Izak contributed to the data acquisition and wrote the manuscript, E. Stoyanova performed experiments and revised the manuscript, K. Dezuraev collected the data, E.Shinar devised the project, and revised the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott Alinity i SARS-CoV-2 IgG II Quant Reagent Instructions for Use. 2020.https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2.
- 4.World Health Organization Expert Committee on Biological Standardization Geneva, 9 - 10 December 2020. Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. https://www.who.int/docs/default-source/biologicals/ecbs/bs-2020-2403-sars-cov-2-ab-ik-17-nov-2020.
- 5.Dueck A.C., Mendoza T.R., Mitchell S.A., Reeve B.B., Castro K.M., Rogak L.J., et al. National Cancer Institute PRO-CTCAE Study Group (2015). Validity and Reliability of the US National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) JAMA Oncol. 2015;1(8):1051–1059. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carazo Perez S., Bureau A., De Serres G. Post-immunisation fever and the antibody response to measles containing vaccines. Epidemiol Infect. 2018;146(12):1584–1592. doi: 10.1017/S0950268818001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L., Tong X., Chen H., He R., Lv Q., Yang R., et al. Characteristics and serological patterns of COVID-19 convalescent plasma donors: optimal donors and timing of donation. Transfusion. 2020;60(8):1765–1772. doi: 10.1111/trf.15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 9.Evans S.S., Repasky E.A., Fisher D.T. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15(6):335–349. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prymula R., Siegrist C.-A., Chlibek R., Zemlickova H., Vackova M., Smetana J., et al. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet. 2009;374(9698):1339–1350. doi: 10.1016/S0140-6736(09)61208-3. [DOI] [PubMed] [Google Scholar]
- 11.Walter E.B., Hornik C.P., Grohskopf L., McGee C.E., Todd C.A., Museru O.I., et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671. doi: 10.1016/j.vaccine.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S.-H., Wi Y.M., Yun S.Y., Ryu J.S., Shin J.M., Lee E.H., et al. Adverse Events in Healthcare Workers after the First Dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 Vaccination: a Single Center Experience. J Korean Med Sci. 2021;36(14) doi: 10.3346/jkms.2021.36.e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald I., Murray S.M., Reynolds C.J., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. npj Vaccines. 2021;6(1):74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]