Summary
Primary somatosensory neurons convey salient information about our external environment and internal state to the central nervous system, allowing us to detect, perceive, and react to a wide range of innocuous and noxious stimuli. Pseudo-unipolar in shape, and among the largest (longest) cells of most mammals, dorsal root ganglia (DRG) somatosensory neurons have peripheral axons that extend into skin, muscle, viscera, or bone, and central axons that innervate the spinal cord and brainstem where they synaptically engage the central somatosensory circuitry. Here, we review the diversity of mammalian DRG neuron subtypes and the intrinsic and extrinsic mechanisms that control their development. We describe classical and contemporary advances that frame our understanding of DRG neurogenesis, transcriptional specification of DRG neurons, and the establishment of morphological, physiological, and synaptic diversification across somatosensory neuron subtypes.
In Brief
Meltzer et al. describe classical and contemporary advances that frame modern thinking of somatosensory neuron development, from neurogenesis and transcriptional specification of the somatosensory neuron subtypes to the acquisition of their mature morphological, physiological, and synaptic properties.
An overview of DRG somatosensory neuron subtypes
Primary somatosensory neurons are a highly diverse group of neurons that report features of the outside world as well as the internal state of our body, and thus these neurons are principal mediators of exteroception, interoception, and proprioception. The cell bodies of somatosensory neurons reside in sensory ganglia, which include the dorsal root ganglia (DRG) as well as the trigeminal ganglia (TG) and other cranial nerve ganglia. Decades of research has revealed a remarkably large cohort of physiologically distinct somatosensory neuron subtypes that populate these ganglia. Here, we review the cellular and molecular basis of DRG somatosensory neuron development.
Before considering the developmental steps leading to the DRG neuron subtypes and their integration into central somatosensory circuits, one must ask: how many mature DRG neuron subtypes exist and what are their distinguishing features? Answers to these questions naturally depend on how somatosensory neurons are classified. DRG sensory neurons can be distinguished based on known or presumed functions, cell body size, physiological response property, axon caliber and degree of myelination, transcriptional profile, morphology of their peripheral axonal endings, and the laminar location, morphological, and synaptic properties of their central axonal terminals within the spinal cord and brainstem. Functionally, DRG neurons are broadly classified as proprioceptors, mechanoreceptors, nociceptors, pruriceptors, and thermoreceptors (Figure 1B), although current functional classification schemes are oversimplified. Indeed, confounds of functional classification schemes include findings that many DRG neurons are polymodal or, in the case of some mechanoreceptors, exhibit responses to mechanical stimuli that scale with indentation forces from low-threshold to high-threshold and into the noxious range. These observations as well as new insights into molecular, physiological, and morphological properties of DRG neurons highlight a need for revising current DRG neuron subtype nomenclature and classification schemes. Moreover, despite a wealth of knowledge, our understanding of the physiological and morphological properties of some DRG neuron types remains far from complete. For example, properties of DRG neurons that innervate non-cutaneous targets such as vasculature, joints, gastrointestinal tract, spleen, and other internal organs remain largely uncharacterized. Thus, with these challenges in mind, below we describe known DRG neuron functional subtypes and some of their defining features.
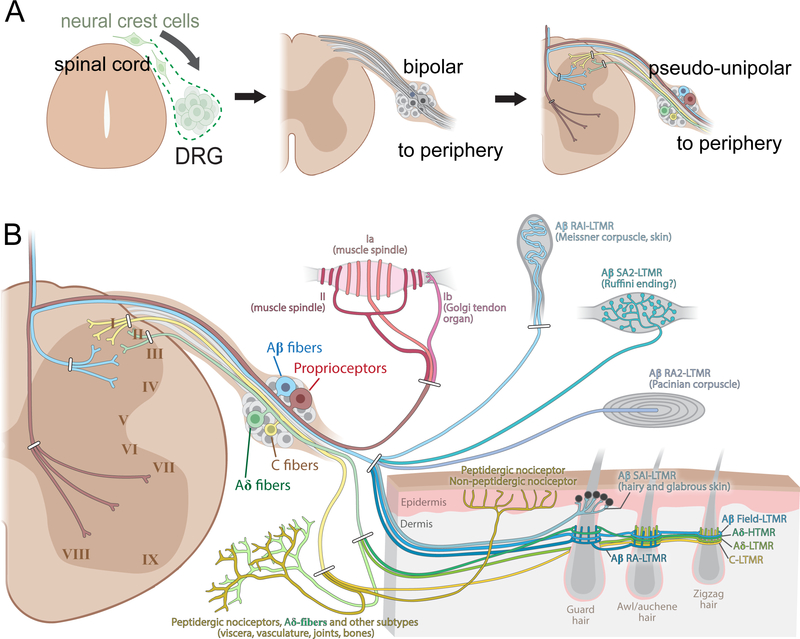
Figure 1. Overview of early development, anatomy, and the major DRG somatosensory neuron subtypes.
(A) Dorsal root ganglia (DRG) neurons derive from neural crest cells that delaminate from the dorsal neural tube and coalesce to form DRGs. Rodents typically have 30 or 31 pairs of DRGs; 8 pairs of cervical, 13 pairs of thoracic, 5 or 6 pairs of lumbar, and 4 pairs of sacral DRGs. Nascent DRG neurons assume a spindle-shaped, bipolar morphology, with axons emanating from opposite sides of the cell body. Then, a stem axonal protrusion containing the two axonal branches forms and refines to assume the mature T shape pseudo-unipolar morphology by birth.
(B) Somatosensory neuron cell bodies reside in DRG, adjacent to the spinal cord. DRG neurons have a pseudo-unipolar morphology with axonal branches extending into both the periphery and the spinal cord. The major peripheral end organs formed by DRG neuron subtypes are illustrated on the right, and the general, albeit oversimplified spinal cord lamination patterns of their central projections are also shown. Note that Aβ LTMR fibers and proprioceptors also have central branches with multiple collaterals extending along the rostral-caudal axis of the spinal cord and an additional branch that ascends via the dorsal column, often reaching the dorsal column nuclei of the brainstem.
Large-diameter DRG neurons with the largest caliber axons are classically defined as Aα fibers (referring to their fast conduction velocities) and consist of proprioceptors, which are further subdivided into three main functional classes. Group Ia and group II proprioceptors innervate muscle spindles and respond to stretch of intrafusal muscle fibers, whereas group Ib proprioceptors innervate Golgi tendon organs and respond to changes in muscle tension (Zampieri and de Nooij, 2021; Tuthill and Azim, 2018). Neurons with large diameter cell bodies and large caliber, myelinated Aβ fibers include cutaneous low-threshold mechanoreceptors (LTMRs). Depending on their different action potential firing patterns to sustained innocuous mechanical stimuli, LTMRs are further classified as rapidly adapting (RA), intermediate adapting (IA), or slowly adapting (SA) LTMRs. Main Aβ-LTMR subtypes include Aβ RA1-LTMRs, Aβ RA2-LTMRs, Aβ SA1-LTMRs, Aβ SA2-LTMRs, and Aβ field-LTMRs, which form morphologically distinct endings in the skin and extend central axons to the LTMR-recipient zone (LTMR-RZ) of the spinal cord dorsal horn and to the brainstem (Abraira and Ginty, 2013; Zimmerman et al., 2014; Bai et al., 2015). The Aβ-LTMR subtypes are differentially responsive to mechanical stimuli including skin indentation, vibration, hair deflection, skin stretch, and gentle stroking across the skin. Some myelinated nociceptors or high threshold mechanoreceptors (HTMRs), which encode mechanical forces into the noxious range, also exhibit an Aβ conduction velocity (Koerber et al., 1988; Djouhri and Lawson, 2004; Burgess and Perl, 1967; Woodbury and Koerber, 2003).
DRG neuron subtypes with medium diameter cell bodies and Aδ fibers with intermediate conduction velocities are also heterogeneous, with some responding to thermal stimuli, mechanical forces, and chemical irritants acting on the skin or internal organs. One major cutaneous subtype, the Aδ-LTMRs, originally called down (D-) hair cells, form lanceolate endings associated with hair follicles (Li et al., 2011; Rutlin et al., 2014) and respond to low force skin indentation and hair deflection (Brown and Iggo, 1967; Burgess et al., 1968; Iggo and Kornhuber, 1968; Rutlin et al., 2014; Walcher et al., 2018). Other Aδ subtypes, Aδ-high threshold mechanoreceptors (HTMRs), express the neuropeptide calcitonin-gene related peptide (CGRP), form either circumferential endings around hair follicles or free nerve endings, and respond to high force mechanical stimuli (Bai et al., 2015; Ghitani et al., 2017; Arcourt et al., 2017; Sharma et al., 2020).
Small diameter somatosensory neurons have small caliber, C fiber axons that are unmyelinated and thus exhibit the slowest conduction velocities. DRG neurons with C fibers include subtypes of nociceptors, thermoceptors, mechanoreceptors, pruriceptors and which collectively report tissue damage, chemical milieu, thermal stimuli, light mechanical forces acting on hairy skin, and the presence of pruritogens across the skin and certain internal organs (Cranfill and Luo, 2021). The peripheral axons of C-fiber neurons that innervate the skin, viscera, and bone have central axons that project mainly to distinct but overlapping superficial laminae of the dorsal horn. Historically, C-fiber nociceptors are classified as either peptidergic or non-peptidergic nociceptors (Basbaum et al., 2009), although this classification has begun to phase out as the field has progressed to using more specific molecular markers to define subtypes. Peptidergic nociceptors are heterogeneous and release the neuropeptides CGRP and substance P, while nonpeptidergic nociceptor subtypes do not express these peptides but do express the neurotrophic factor receptor Ret. Small diameter nociceptor subtypes are also distinguished based on expression of ion channels that confer sensitivity to heat (TRPV1), cold (TRPM8 and TRPA1), acidic milieu (ASICs), and chemical irritants (TRPA1). C-LTMRs, in contrast, are highly responsive to gentle forces acting on hairy skin (Zotterman, 1939) and in humans are implicated in affective touch (Olausson et al., 2010). In the mouse, C-LTMRs form lanceolate endings around hair follicles (Li et al., 2011) and have central axons that terminate in the superficial layer of the LTMR-recipient zone (Abraira et al., 2017; Seal et al., 2009; Li et al., 2011). Other C fiber subtypes are classified as HTMRs, and at least some of these neurons express the G protein-coupled receptor MrgprD and form “free nerve endings” that penetrate the epidermis (Cavanaugh et al., 2009; Zylka et al., 2005; Warwick et al., 2021).
Collectively, a remarkable diversity of somatosensory neuron subtypes underlies our ability to perceive and respond to a wide range of stimuli. Thus, a central question is how this diversity arises during development. Like development of cells across all organ systems, intrinsic cues and environmentally generated signals collaborate in the genesis of somatosensory neuron diversity. In the following sections, we describe classical and contemporary advances that frame our thinking along the trajectory of somatosensory neuron development (Figure 2), from neurogenesis and transcriptional specification of DRG neuron subtypes to the acquisition of their mature morphological, physiological, and synaptic properties.
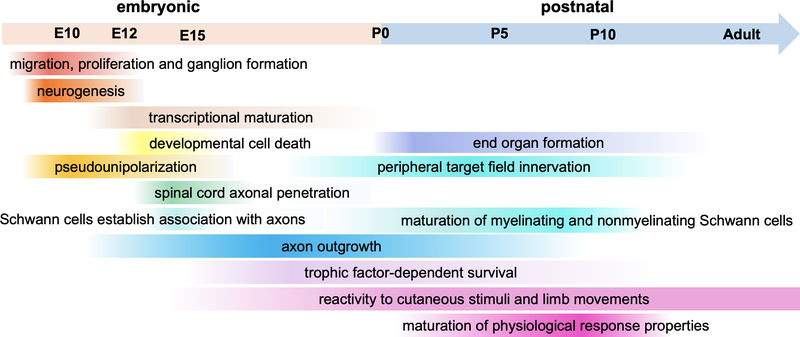
Figure 2. Overview of the timing of major stages of rodent somatosensory neuron development.
Developmental timing of milestone events during mouse somatosensory neuron development. Approximate embryonic (E) and postnatal (P) time points are indicated on the timeline above, and some of the times are estimations based on those measured in experiments using rats. Color gradients reflect ranges of times for the various developmental processes and the observation that different DRG neuron subtypes and neurons across different axial levels may have slightly different start or end time points for these processes.
Somatosensory neurogenesis and transcriptional specification of DRG neuron subtypes
Following their birth, immature DRG neurons progress through a series of specification steps governed by dedicated gene expression programs that, together with local environmental cues, orchestrate the emergence of the distinct subtypes. DRG neurons derive from neural crest cells which, upon exposure to specific inductive signals, delaminate from the dorsal neural tube and migrate along a ventral pathway in a chain-like fashion, coalescing into ganglia at regular intervals in the anterior half of each somite, adjacent to the neural tube (Figure 1A). Classical work of Nicole Le Douarin (Le Douarin et al., 1999) delineated migratory streams that neural crest progenitors take as they coalesce to form somatosensory ganglia. A subset of neural crest cells is fated to directly form the majority of DRG neurons. Other neural crest-derived progenitors, emerging from cells known as boundary cap cells and located at the dorsal root entry and ventral root exit zones, contribute an additional, albeit more minor stream of progenitors fated to become DRG neurons (Maro et al., 2004; Gresset et al., 2015). During the migratory process, guidance cues as well as adhesion between cell surface receptors, such as integrins, and extracellular matrix proteins, either promote or inhibit crest cell migration, resulting in the accumulation of cells in the form of nascent ganglia (Davies, 1990; Szabó and Mayor, 2018).
Early transcriptional specification of somatosensory neurons.
Prominently described in the literature as one of the earliest events in DRG neuron subtype specification is the onset of expression of the transcription factors Neurog1 and Neurog2 (Ma et al., 1999; Marmigère and Ernfors, 2007; Jenkins et al., 2019). This transcriptional event coincides with the exit from a neural crest progenitor status, as indicated by the loss of expression of Sox10, a marker of neural crest progenitors. As such, the onset of expression of Neurogenins and the POU domain-containing transcription factor Pou4f1/Brn3a is considered to establish commitment to the somatosensory neuron lineage. BrdU and radiolabeling based birth dating experiments imply that there are two main waves of progenitors committing to become DRG sensory neurons (Lawson and Biscoe, 1979), and in some reports a third wave (reviewed in Marmigère and Ernfors, 2007). The expression patterns of Neurog1 and Neurog2 have been superimposed onto the two principle waves of neurogenesis (Ma et al., 1999). Indeed, based on in situ hybridization analysis, it was suggested that the first main wave (E9.5–11) exclusively expresses Neurog2 and gives rise to neuronal subtypes with large- and medium-diameter cell bodies and A-fiber caliber axons, whereas the second main wave (E11–13) is marked by Neurog1 and gives rise to neurons with small-diameter cell bodies and unmyelinated C-fibers (Figure 3A).
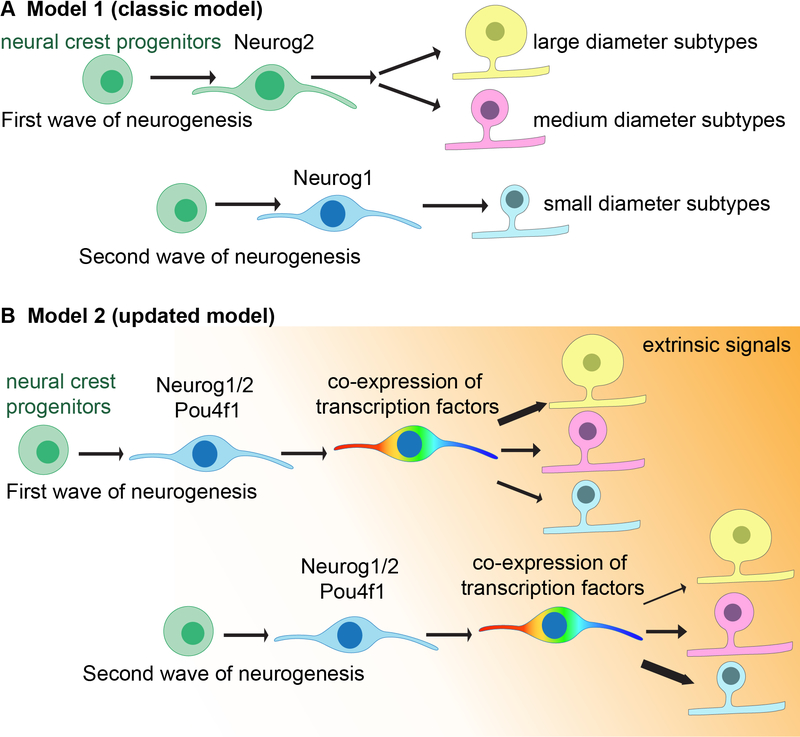
Figure 3. Models of neurogenesis and transcriptional maturation of somatosensory neurons.
(A) One longstanding model of neurogenesis and maturation of DRGs proposes that neurons born during the first wave of neurogenesis (E9.5–11) exclusively express Neurog2 and give rise to neurons with large-(yellow) and medium-(pink) diameter cell bodies. Neurons born during the second wave of neurogenesis (E11–13) express Neurog1 and give rise to unmyelinated C-fiber neurons with small-diameter cell bodies (cyan).
(B) An updated model incorporating insights from scRNASeq transcriptional analyses, genetic labeling experiments, gene knockout findings, birth-dating experiments, and iPSC experiments posits that following neurogenesis, nascent DRG neurons express Neurg1/2 and Pou4f1 and are transcriptionally poised to become any DRG neuron subtype. These newborn neurons begin to extend axons and they co-express a wide range of transcription factors (rainbow) that at later times become subtype-restricted. Temporally and spatially restricted environmental cues may bias early-born DRG neurons towards a large- or intermediate-diameter subtype fate, whereas other extrinsic cues instruct most later-born neurons towards a small-diameter subtype fate. The relative percentages of the subtype fates are indicated by the thickness of the arrows.
While the classic view has held that large-diameter, myelinated neuron subtypes are born days prior to small-diameter, unmyelinated subtypes, with Neurog2 and Neurog1 respectively instructing early stages of their specification, the classical literature may in fact be interpreted to support different models, especially considering newer findings. Indeed, experiments using tritiated thymidine or BrdU/EdU labeling have evaluated cell diameter size postnatally and do not strictly necessitate that large and small diameter cells arise from distinct lineages represented at E11.5, as the classic model would suggest. In fact, BrdU/EdU administration at E11.5, during the putative second wave, leads to labeling of a substantial number of neurons that go on to assume a large diameter identity (Kitao et al., 2002). Consistent with these findings, reports of nociceptor subtype birth dating with EdU confirmed that these subtypes are continuously born between E9.5-E13.5 (Landy et al., 2021). In addition, recent studies using mouse genetic based tracing strategies, which involve labeling sensory neuron progenitor cells expressing either Neurog1 or Neurog2, have suggested that both large and small diameter neurons, and indeed all DRG neuron subtypes, arise from newborn neurons expressing both of these transcription factors and may in fact arise from the same or a largely overlapping temporal compartment (Faure et al., 2020; Sharma et al., 2020). It is also noteworthy that the evidence suggesting that Neurog2 and Neurog1 promote distinct subtypes within the somatosensory lineage is based on knockout mutants followed by in situ expression analysis for TrkA (mostly, but not exclusively, a small diameter C-fiber neuron marker), TrkB (Aδ/Aβ marker) or TrkC (mostly an Aα/Aβ marker) (Ma et al., 1999), which represent a robust set of marker genes with a storied history of developmental studies linking them to specific subpopulations of somatosensory neurons (discussed below). However, the Neurog1 knockouts show a nuanced phenotype, with a complete loss of TrkA in the first and second cervical level DRGs but preservation of TrkA in a subset of neurons in more caudal DRGs (Marmigère and Ernfors, 2007; Bachy et al., 2011; Ventéo et al., 2019). More surprisingly, Neurog2 knockouts showed no change in expression of any of these marker genes. Only in the context of Neurog1/Neurog2 double knockouts were the expression of TrkA, TrkB, and TrkC lost (Ma et al., 1999). Also conflicting with the classical view are in vitro findings using iPSCs that show that virtually all of the main transcriptionally distinct DRG neuron subtypes can be generated by expression of Pou4f1 along with either Neurog1 or Neurog2 and exposure to a cocktail of neurotrophic growth factors (Blanchard et al., 2014).
A limitation of classic molecular analyses of the somatosensory neuron lineage was the reliance on a small set of marker genes to identify the diverse DRG neuron subtypes derived from this lineage. As such, understanding trajectories that cells must take to arrive at their terminally differentiated identity has required updating the molecular taxonomy of somatosensory neurons. This renaissance has been primarily fueled by the advent of next generation sequencing and single-cell genomic methodologies. Several studies have used modern clustering algorithms to categorize the expression profiles of hundreds to tens of thousands on transcriptomes, each of which represents a single somatosensory neuron (Oliver et al., 2021; Sharma et al., 2020; Usoskin et al., 2015; Zeisel et al., 2018; Wu et al., 2021), or genetically labeled and purified somatosensory neuron populations (Zheng et al., 2019). It is now clear that there are at least 15 transcriptionally distinct subtypes of DRG neurons with divergent gene expression patterns. However, considerable debate remains as to where to draw the boundaries between individual cell types and what criteria should be used, beyond transcriptomes, to define a sensory neuron subtype. A unified nomenclature has yet to emerge and will likely require a more comprehensive characterization of cellular function to help taxonomize transcriptomic clusters. Nevertheless, the revelation of full transcriptomes of DRG neurons provides an escape from the reliance on single marker genes when defining cell types.
Given these near full transcriptomes of DRG neurons during different stages of development, what can be learned about the steps leading to the highly divergent somatosensory neuron subtypes? Perhaps not surprisingly, the large scale scRNA-seq transcriptomic datasets of adult DRGs have shown that terminally differentiated DRG neuron subtypes express unique sets of transcription factors (Sharma et al., 2020; Usoskin et al., 2015; Zeisel et al., 2018). In some cases, DRG neuron subtype restricted transcription factors (TFs) have been previously described in the literature, as is the case for Etv1, which is expressed in proprioceptors and required for their development (Arber et al., 2000). Notably, Etv1 is also expressed in other subtypes in which it may also be required for proper maturation. In another example, the transcription factor Runx1 is expressed in non-peptidergic nociceptor subtypes and C-LTMRs, and deletion of Runx1 leads to major alterations in several C fiber subtypes (Chen et al., 2006b), indicating that subtype-restricted factors are indeed critical for the emergence of somatosensory neuron subtype identities. Consistent with this, analyses from germline mutants of Pou4f family members, Runx3, Prdm12, Shox2, and c-Maf knockouts, each of which are subtype-restricted TFs or, more accurately, expressed in combinations of subtypes during development, show a reduction in subtype-specific gene expression and defects in somatosensory neuron development (Chen et al., 2006a; Abdo et al., 2011; Zou et al., 2012; Badea et al., 2012; Desiderio et al., 2019; Scott et al., 2011; Sharma et al., 2020; Wende et al., 2012; Bartesaghi et al., 2019). It is worth noting that the subtype specification function of these TFs may be distinct when comparing embryogenesis and adulthood. For example, while developmental loss of the TF Prdm12 leads to dramatic alterations of genes selectively expressed in small diameter subtypes, distinct deficits are found when this TF is deleted from DRGs of adult mice (Landy et al., 2021; Bartesaghi et al., 2019).
Interestingly, transcriptomes collected during different stages of development reveal that a large array of DRG neuron subtype-restricted TFs, including those mentioned above, are broadly co-expressed early, beginning at times roughly coincident with the loss of Neurog1/2 expression (Sharma et al., 2020). While it is possible that this transient state of co-expression of TFs that become subtype restricted at later stages is an epiphenomenon of development, a contrasting interpretation is that early co-expression of this large TF cohort endows newborn somatosensory neurons with the ability to terminally differentiate into any of the array of possible somatosensory neuron subtypes. Thus, we favor an updated model that combines observations of BrdU/EdU birthdating, which formed the basis of the classical models, with insights gleaned from transcriptomic and genetic manipulation analyses to describe the early transcriptional control of somatosensory neuron specification (Figure 3B). In this new model, following neurogenesis, nascent somatosensory neurons co-express Neurogenins and Pou4f1, and upon extinction of the Neurogenins (but maintenance of Pou4f1) a plethora of subtype-defining TFs emerge. Then, progressive interactions of newborn neurons with a series of extrinsic cues as well as intrinsic TF-TF interactions uniquely combine to instruct the cohorts of TFs that will remain expressed in each somatosensory neuron. It is these remaining, unique combinations of subtype-defining TFs and their enhanced expression at subsequent stages that promote gene expression programs specific to each of the large number of transcriptionally distinct DRG neuron subtypes. In this model, environmental signals within and surrounding nascent ganglia nudge the majority of early born DRG neurons towards transcriptional states that promote large- or intermediate-diameter subtype fates, whereas different extrinsic cues acting on later born neurons instruct many, but not all, to adopt small-diameter subtype fates. Additional studies will be required to delineate the identities of the intrinsic and extrinsic mechanisms that allow for transition from an ambiguous TF expression state to the stable transcriptional states of subtypes observed in mature animals. It is worthwhile to note that an early, transient state of co-expression of TFs that become subtype restricted at later times, described here for DRG neurons, has been observed across a range of biological systems, with early reports coming from the hematopoietic system (Hu et al., 1997). Indeed, whole embryo scRNA-seq has suggested that early, broad co-expression of TFs that at later stages become subtype restricted may be a general feature of the cell type specification process (Wagner et al., 2018).
Interplay of intrinsic and extrinsic cues during transcriptional specification.
A key question is how intrinsic and extrinsic cues act in concert as nascent DRG neurons select certain TFs to be maintained and increased in their expression, while other TFs are extinguished, thus guiding specification and maturation of the different subtypes. We now appreciate that intermediate and peripheral target tissues provide growth and survival factors that not only instruct morphogenesis and survival of somatosensory neurons (see below), but also transcriptional maturation of individual subtypes. Indeed, the prototypic target-derived neurotrophic factor NGF and the transcription factor Runx1 coordinate postmitotic differentiation of nonpeptidergic C-fiber neurons (Huang et al., 2015; Chen et al., 2006b). Moreover, genome-wide analysis of mice lacking NGF has revealed dramatic alterations to the subtype restricted TF expression profiles (Sharma et al., 2020), consistent with earlier work suggesting a role for NGF in subtype maturation by showing the loss of individual known marker genes (Patel et al., 2000, Luo et al., 2007). Similarly, recent studies have lent support to the longstanding view that cues from muscle spindles play a key role in establishing the molecular identities of proprioceptor subtypes (Wu et al., 2019). In another example, the neurotrophic factor receptor Ret is required in a subset of Aβ-LTMRs, presumably the Aβ RA2-LTMRs of Pacinian corpuscles, for maintenance, but not initiation, of Etv1 expression (Fleming et al., 2016). Additionally, a role for intrinsic interactions between TFs is likely at play, analogous to motor neuron subtype differentiation, which is controlled by mutually antagonistic interactions of the Nkx/Hox family of transcription factors (Briscoe et al., 2000; Dasen et al., 2003; 2005). It is noteworthy that, in contrast to the host of broadly expressed TFs that go on to become subtype restricted, the key neurotrophic factor receptors TrkA, TrkB, TrkC, and Ret are expressed in highly distinct patterns during early stages of somatosensory neuron maturation (reviewed in Marmigère and Ernfors, 2007). This suggests that the initial expression patterns of neurotrophic growth factor receptors may be controlled independently of the subtype-restricted TFs, which are initially broadly co-expressed, potentially though ligand-receptor mediated positive feedback loops as observed in other PNS neuron types (Deppmann et al., 2008). This early differential expression of neurotrophic factor receptors is likely to endow subsets of newborn somatosensory neurons with differential sensitivity to several of the key extrinsic cues that shape transcriptional character of DRG neurons. Consistent with this thinking, neurotrophic growth factor receptor expression, TF expression, and subtype specification are intimately interlinked, as inserting the TrkC coding sequence into the TrkA locus results in a subset of neurons switching from a small diameter to a proprioceptor fate (Moqrich et al., 2004). Collectively, these findings underscore the need for understanding the mechanisms that establish the different neurotrophic growth factor receptor expression patterns, how and when these growth factor receptors begin to signal, and how their signals may influence distinct patterns of TF expression initiation or maintenance or TF-TF interactions. Our knowledge of the interplay between extrinsic and intrinsic determinants of early stages of DRG neuron specification is still in its infancy, and there is a clear need for more research in this area.
Axonogenesis, axon growth and guidance, and target-dependent neuronal survival
DRG neuron axonogenesis and acquisition of pseudo-unipolar morphology.
As first described in 1886 (His, 1886), mature DRG neurons display a T-shaped, pseudo-unipolar morphology, which may function as a low-pass filter and limit the frequency of action potentials that are relayed from the periphery to the spinal cord (Stoney, 1990). Just after their birth, however, nascent DRG neurons assume a spindle-shaped, bipolar morphology, with neurites emanating from opposite sides of the cell body (Figure 1A). The molecular and cellular mechanisms of initiation of axonal outgrowth on opposite poles of the nascent cell body remain unknown. Following axonogenesis, all DRG neurons undergo the remarkable transformation from the bipolar morphology to the mature, pseudo-unipolar axonal appearance. During this process, the two axonal branches on either end of the spindle-shaped cell body bend towards each other to form a bell-shaped bipolar morphology. Then, a stem axonal protrusion containing the two axonal branches forms, extends away from the cell body, and refines to assume the characteristic T shape, completing the pseudo-unipolarization process (Matsuda et al., 1998; Nascimento et al., 2018). The exact timing of pseudo-unipolarization varies between vertebrate species, and this morphological transformation is mostly finished by birth (Barber and Vaughn, 1986; Matsuda et al., 1996). In mice, retrograde HRP injection into the spinal cord at E12 labeled only bipolar and early transitional bipolar DRG neurons, and not pseudo-unipolar neurons, indicating that axonal projections to the spinal cord precede pseudo-unipolarization (Barber and Vaughn, 1986).
Although the cellular and molecular basis of pseudo-unipolarization is unknown, a few clues have been gleaned from in vitro experiments. Adding Schwann cells to cultured DRG neurons induces a mature pseudo-unipolar morphology (Mudge, 1984); whether this is triggered by the physical wrapping of immature DRG neurons by Schwann cells, imparting some physical constraints on them, or by Schwann-cell derived molecular cues is not known. On the other hand, in an in vitro study, co-expression of the transcription factor Pou4f1 and either Neurog1 or Neurog2 was sufficient to reprogram fibroblasts into electrically active somatosensory neuron subtypes exhibiting a pseudo-unipolar morphology. This finding argues that physical wrapping of glia cells may not be required for this morphological transformation (Blanchard et al., 2014). Defining the intercellular and intracellular signals that instruct somatosensory neuron pseudo-unipolarization represents an interesting future challenge.
Axonal extension along intermediate targets and into peripheral target fields.
Current models implicate a range of trophic and tropic cues in promoting somatosensory neuron axonal extension over long distances, towards final peripheral target fields. As defined, neurotrophic cues promote axonal extension, neuronal growth and survival, whereas neurotropic cues function as either attractants or repellents, providing directional valence to migrating cells or extending processes. The distinctions between trophic and tropic cues are not clear cut, however, and at least some, and perhaps all neurotrophic cues are also neurotropic. Indeed, the prototypical neurotrophic factor, NGF, may have been the first neurotropic cue described, attracting extending DRG sensory axons towards a source of the factor (Gundersen, 1985; Gundersen and Barrett, 1979; Letourneau, 1978; Tucker et al., 2001; Reichardt, 2006).
The peripheral axons of DRG neurons extend outward from the vertebral column, ultimately reaching a range of target tissues beginning ~E13.5 in the mouse (Hasegawa et al., 2007). Early embryological experiments in the chick suggest that factors produced in ectoderm act over long distances to attract somatosensory axons. When regions of ectoderm were removed, DRG neurons failed to extend axons toward the skin (Honig et al., 2004; Martin et al., 1989). Moreover, limb-bud removal in the chick embryo led to aberrant sensory neuron innervation of the tail, suggesting that some sensory axons find a secondary target when their primary target is absent (Calderó et al., 1998).
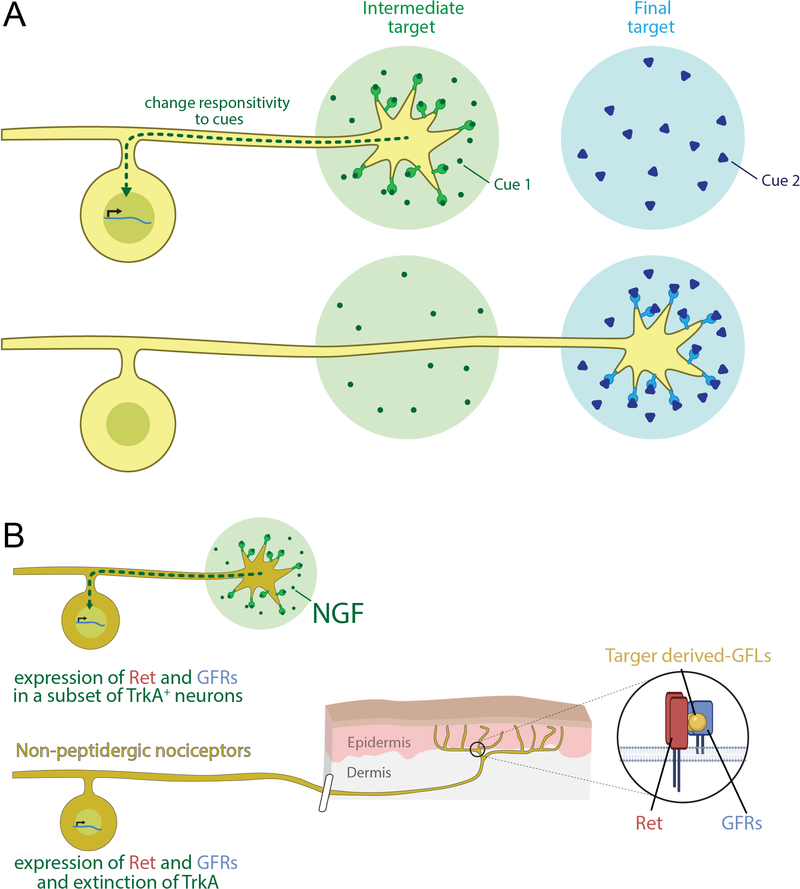
At this point it is instructive to step back and ask: Are newborn DRG neurons pre-specified to project to a particular target? Or, at the other end of a range of possibilities, perhaps nascent, unspecified DRG neurons first extend axons in a stochastic manner into different intermediate target regions, where distinct factors instruct neurons to acquire responsivity to new sets of factors for extension into new territories, and so on (Figure 4A). One reason to suspect something closer to the latter is that the number of neurotrophic and tropic cues may be too small to guide axons of myriad “pre-specified” DRG neuron subtypes through the intermediaries of their trajectories. Moreover, evidence for hierarchical actions of tropic cues has been found for other populations of neurons, including commissural neuron axons crossing the midline of the spinal cord (reviewed in Gorla and Bashaw, 2020). As mentioned, Trk receptors and Ret are expressed in distinct patterns in newborn DRG neurons, and the neurotrophins (NGF, BDNF, NT3 and NT4) and glial cell line-derived neurotrophic factors (GFLs) are thus candidates for exerting hierarchical control over sensory neuron subtypes. NGF, as a case in point, is required for developing C-fiber neurons to extend axons into peripheral target regions, but not into the spinal cord (Patel et al., 2000), and it also instructs transcriptional maturation of TrkA+ DRG neuron subtypes (Patel et al., 2000; Luo et al., 2007; Sharma et al., 2020) and late stages of responsivity of some C-fiber neurons to other extrinsic cues (Luo et al., 2007). Here, NGF acts on a subset of embryonic TrkA+ neurons to promote expression of GDNF family ligand receptors (GFRs) and Ret, which together form holoreceptors for the GFLs (Figure 4B). GFR and Ret receptor complexes are, in turn, required for this TrkA+ subset to adopt the mature nonpeptidergic C-fiber neuron fate and form their relevant skin innervation patterns (Luo et al., 2007). Not only does this example provide support for hierarchical, stepwise regulation of axonal extension and target field innervation patterns (Figure 4A), it also underscores the entwinement of extrinsic cue control over transcriptional specification of DRG neuron subtypes and subtype-specific axonal projection patterns.
Figure 4. Model of hierarchical axonal growth and guidance cues acting sequentially to control axonal extension towards target fields.
(A) Hierarchical actions of trophic cues act on extending axons of DRG neuron subtypes (yellow), at sequential steps along their trajectories. Cues present at intermediate targets (green) alter the neuron’s responses to new cues presented along subsequent intermediate targets or within final target fields (blue). This hypothetical model may explain how temporal differences in axonal extension of early and late born sensory neurons allows them to respond to distinct extrinsic cues that instruct the emergence of different subtypes. This model could also explain how subsets of developing neurons may encounter distinct sets of cues during axonal outgrowth, resulting in a diversity of axonal trajectories and target innervation patterns.
(B) An example summarizing hierarchical actions of trophic cues instructing non-peptidergic C-fiber neuron development (Luo et al., 2007). In this case, NGF acts on a subset of TrkA+ neurons to promote expression of GDNF family ligand receptors (GFRs) and Ret. These receptors form holoreceptors for the glial cell line-derived neurotrophic factors (GFLs), which are required for proper formation of skin innervation patterns of non-peptidergic C-fiber neurons and their molecular maturation.
An important consideration is that nascent somatosensory neurons exhibit dynamic interactions with other cell types, and they extend axons through intermediate and into final target tissues that are themselves developing and maturing. Schwann cell precursors, for example, migrate along extending axons and differentiate into myelinating Schwann cells that ensheathe large and medium diameter axons and nonmyelinating Schwann cells that form Remak bundles (Jessen and Mirsky, 2005; Zuchero and Barres, 2015). Another example is provided by the vasculature. Indeed, along their trajectory towards final target fields, bundles of sensory axons are often intimately associated with large blood vessels, a phenomenon known as neurovascular congruency (Miller, 1939). In developing limb skin of the mouse, for example, sensory axons and arteries are closely aligned by ~E14.5. Interestingly, cutaneous sensory axons promote maturation and branching patterns of arterial vessels in the skin through VEGF signaling (Mukouyama et al., 2002). Here, sensory neurons are a source of VEGF that promotes angiogenesis and acts through the VEGF coreceptor neuropilin-1 expressed in proliferating and migrating vascular endothelial cells (Mukouyama et al., 2005). On the other hand, although a close association between trigeminal sensory axon terminals and blood vessels in the whisker pad is established during development, these neural and vascular structures are patterned independently of one another by a common repulsive cue Sema3E (Oh and Gu, 2013). These examples highlight the general principle that somatosensory neuron axons, Schwann cells, and intermediate and final axonal target cells co-develop, in complex ways and in some instances relying on common cues.
Thus, there exists a highly dynamic interplay between DRG subtype transcriptional maturation, axonal growth and guidance, and target field innervation. We favor a general model in which environmental cues expressed along the trajectories of extending axons play key, instructive roles in directing sensory neuron subtype maturation, and in doing so shaping axonal responsivity to tropic and trophic cues as they further extend towards target fields (Figure 4A). This model points to early and intermediate target regions as principal influencers of both subtype maturation and axonal responsivity, as opposed to models reliant solely upon intrinsically defined and precisely timed genetic programs that are pre-specified within distinct newborn sensory neuron populations. Thus, as a variation or extension of the neurotrophic factor hypothesis (see below), we suggest that early and intermediate targets and the cues they express are central in promoting transcriptional and functional maturation of DRG neuron subtypes, in proper proportions, and for priming responsivity of their axons to enable final target innervation in proper amounts, to meet the physiological demands of different target fields. Tests of this and other models will require identification of the instructive early and intermediate target cells for DRG neuron axons and the neurotrophic cues they express. Also needed is a better understanding of the transcriptional or posttranscriptional events set in motion by these cues to instruct DRG subtype maturation events, growth factor responsivity, and acquisition of final target field innervation patterns. A related goal is to identify the cues that orchestrate DRG neuron central axon projection patterns, including the initial extension of axons to form dorsal roots and axonal penetration of the spinal cord.
The control of somatosensory neuron survival.
As developing somatosensory axons extend into the periphery and make their way to their final targets, neurotrophic factors present in intermediate and final target tissues act to support neuronal survival. The seminal discoveries of naturally-occurring neuronal death, target field dependence of neuronal survival, and the first neurotrophic factor NGF (Hamburger and Levi-Montalcini, 1949; Levi-Montalcini and Booker, 1960) led to the neurotrophic factor hypothesis, which remains a central tenet of developmental neuroscience (reviewed in Yuen et al., 1996). The neurotrophic factor hypothesis posits that neurons are initially over-produced, and only those reaching peripheral target fields and successfully competing for limited amounts of target-derived neurotrophic factors will survive; this process may ensure that an appropriately sized neuronal population exists to meet the demands of the target (Barde, 1989; Oppenheim, 1989). The technological revolution afforded by reverse genetic approaches in mice has made it possible to further test this model through targeted deletion of genes encoding neurotrophic growth factors and their receptors. Collectively, this body of work has demonstrated that specific neurotrophic factor signals are essential for supporting survival of select DRG neuron populations during development. Indeed, mice lacking either NGF or Ntrk1/TrkA display a loss of virtually all small and some medium/large diameter neurons of the DRG (as well as trigeminal sensory neurons and postganglionic sympathetic neurons) (Crowley et al., 1994; Smeyne et al., 1994). Correspondingly, NGF and TrkA knockout mice display a near complete absence of responses to painful stimuli, such as tail pinch, and human patients with mutations in NGF have congenital insensitivity to pain (Indo et al., 1996). A different complement of neurotrophic factors and their cognate receptors ensures survival of select large diameter DRG populations. Mice lacking NT3 or TrkC lack virtually all proprioceptive afferents and also display dramatic reductions in muscle spindles and Golgi tendon organs (Ernfors et al., 1994; Klein et al., 1994). Behaviorally, these mutants have severe ataxia and often die before three weeks of age. Mice lacking BDNF or TrkB also display a loss of a subset of DRG and trigeminal ganglia neurons (Jones et al., 1994; Klein et al., 1993). TrkB global or conditional knockouts exhibit a significant loss of intermediate-sized neurons of the DRG, as well as loss of Meissner corpuscles in glabrous skin and a reduction in hair-follicle associated endings (González-Martínez et al., 2004; Pérez-Piñera et al., 2008). BDNF but not NT4 knockouts have a similar reduction in Meissner corpuscles (González-Martínez et al., 2005).
As a recurring theme of this review, neurotrophic factors are multifunctional, playing roles not only in neuronal survival, but also in transcriptional maturation of DRG neuron subtypes and their peripheral and central target innervation patterns (Gundersen, 1985; Gundersen and Barrett, 1979; Letourneau, 1978; Tucker et al., 2001; Patel et al., 2000; 2003; Segal, 2003; Sharma et al., 2020). Consistent with this, neurotrophic factor signals can act locally in distal axons, to control peripheral axon arborization and target innervation, as well as retrogradely in the soma, to control gene expression, maturation, and survival (Cosker et al., 2008; Harrington and Ginty, 2013). Mechanistically, considerable evidence supports a model in which retrograde neurotrophic factor receptor signaling is mediated by signaling endosomes, which are cellular compartments whose membranes contain internalized neurotrophic receptor bound to ligand and which form intracellular scaffolds for the assembly and activation of downstream growth and survival signaling pathways (Ginty and Segal, 2002; Cosker et al., 2008; Scott-Solomon and Kuruvilla, 2018). Taken together, a host of neurotrophic factors derived from intermediate and final targets during embryonic stages are critical for somatosensory neuron survival, and these same survival-promoting cues also act, to varying degrees and from multiple sources, to instruct sensory neuron subtype-specific patterns of gene expression as well as axon extension, branching, and ending maturation within target tissues.
The development of target field innervation patterns and sensory ending morphologies
On the acquisition of form and function.
A striking feature of the somatosensory system is the large diversity of peripheral target field innervation patterns and axon terminal morphologies across the sensory neuron subtypes. Indeed, it is this diversity of axon terminals, together with distinct intrinsic molecular and biophysical properties, that underlie the wide range of physiological response properties, receptive field properties, and thus functions of somatosensory neuron subtypes. The exactness of form that underlies function is nicely illustrated by proprioceptor subtypes (Tuthill and Azim, 2018; Zampieri and de Nooij, 2021). Group Ia and group II proprioceptors form endings with muscle spindles allowing them to report the rate and magnitude of change in muscle length, respectively. On the other hand, axonal endings of Group Ib proprioceptors elaborate upon Golgi tendon organs enabling them to report changes in muscle tension. The LTMRs also nicely illustrate form underlying function in somatosensation, and indeed LTMR subtypes innervate different skin regions and exhibit a range of morphologies enabling them to report distinct features of vibrotactile stimuli acting on the skin (Jenkins and Lumpkin, 2017; Handler and Ginty, 2021). As examples, Aβ RA1-LTMRs terminate in Meissner corpuscles nestled within dermal papillae of glabrous skin, whereas Aβ RA2-LTMRs form the central axon of Pacinian corpuscles located within the deeper dermis, around the periosteum of certain bones, or within the mesentery (Figure 1B). These two axonal ending types in association with their distinct corpuscle structures and unique patterns of lamellar cell wrappings underlie differences in vibration tuning, with the Meissner’s Aβ RA1-LTMR optimally sensitive to 40–80 Hz vibratory stimuli and the Pacinian’s Aβ RA2-LTMRs responsive in the 100–300 Hz range. In hairy skin, on the other hand, Aβ RA-LTMRs form longitudinal lanceolate endings around hair follicles, endowing them with high sensitivity to hair deflection as well as indentation of the surrounding skin. Thus, a central question is: how do proprioceptors, LTMRs, and other DRG sensory neuron subtypes acquire their unique ending structures and intimate associations with end organ cell types? At a basic level, and related to considerations of subtype specification and maturation raised above, one may ask: Are newborn DRG neurons pre-specified to form unique axon terminal morphologies and assemble with particular end organs, or do local cues within intermediate or final target fields instruct unspecified neurons to assume characteristic morphological properties that reflect the needs of the target? The answer may lie somewhere in between.
Some observations pertaining to the morphological properties of LTMR endings in hairy skin suggest that newborn DRG neurons are destined to form a particular type of terminal morphology, to at least some degree, prior to skin innervation. For example, while the axonal terminals of Aβ RA-LTMRs and Aβ field-LTMRs both intimately associate with the upper bulge region of hair follicles, Aβ RA-LTMRs form lanceolate endings around hair follicles whereas Aβ field-LTMRs form circumferential endings around the same follicles. Importantly, these two LTMR populations can be indelibly labeled with different genetic tools at E12.5, which is well in advance of when they innervate the skin and form the relevant ending types (Luo et al., 2009; Bai et al., 2015; Li et al., 2011). This observation points to at least some degree of early specification of gene expression patterns in neurons that go on to exhibit distinct axon terminal morphologies. It would be informative to know if a developing sensory neuron that forms a particular terminal morphology within a particular target region can exhibit flexibility and form a different ending structure if forced to project to a different target region. For example, would an Aβ RA1-LTMR that forms endings within a Meissner corpuscle in glabrous skin instead form lanceolate endings around hair follicles if its axonal trajectory was diverted into hairy skin? It is interesting to note, in this regard, that genetically altering the dorsoventral character of limb mesenchyme in mice changes the molecular expression profiles of proprioceptors, suggesting that proprioceptors acquire certain features of their muscle-type identity from peripheral targets (Poliak et al., 2016).
The spatial arrangements of somatosensory neuron endings within target regions.
Beyond sensory neuron ending morphology, the patterns of axonal branches and distributed end organs across the skin and other target fields underlie sensory neuron subtype receptive field properties and thus are critical for sensory information processing. Some somatosensory neuron types form simple branching patterns within their target regions whereas others form elaborate arbors that branch extensively. For example, Aβ SA1-LTMRs that innervate trunk hairy skin typically have one major cutaneous axon that forms several short branches in close proximity to touch domes (clusters of Merkel cells), with individual terminals each associating with a single Merkel cell (Jenkins et al., 2019; Kuehn et al., 2019). Axons of Aβ field-LTMRs, on the other hand, branch extensively across a large area of hairy skin and form circumferential endings around as many as 180 hair follicles (Bai et al., 2015). Also, generally speaking, branches of neurons of the same subtype often exhibit little or no spatial overlap with one another (tiling) as do axonal arbors of individual neurons of the same neuron (self-avoidance). This phenomenon is particularly well described in invertebrate systems where visualization of processes of multiple neurons of a single class is feasible (Grueber and Sagasti, 2010a; Zipursky and Grueber, 2013). It is proposed that tiling and self-avoidance ensure that sensory neurons cover the entirety of sensory space while at the same time avoid overlap with neighboring neurons of the same class to minimize redundancy and establish accurate somatotopic representations of the periphery within the CNS. For mammalian somatosensory neurons, tiling of axon terminals has been observed for the principal cutaneous LTMR subtypes. Indeed, while heterotypically overlapping in hairy skin, axons of Aβ SA1-LTMRs, Aβ field-LTMRs, Aδ-LTMRs, and C-LTMRs exhibit minimal overlap with neighboring neurons of the same class, thus homotypically tiling across the skin (Kuehn et al., 2019). Similarly, axonal endings of the two Aβ-LTMR subtypes innervating Meissner corpuscles in glabrous skin are homotypically tiled but heterotypically offset (Neubarth et al., 2020). The identity of molecular cues and mechanisms underlying DRG somatosensory neuron subtype branching patterns, tiling, and self-avoidance are largely unknown. For tiling and self-avoidance, it is likely that repulsion between sensory axons restricts territorial overlap, similar to what has been observed in zebrafish trigeminal neurons, invertebrate sensory neurons, neurons of the mammalian retina, and elsewhere (Grueber and Sagasti, 2010b; Sagasti et al., 2005).
Maturation of peripheral axonal endings and their associated end-organs.
What can be said about the developmental steps, and the identity and source of instructional cues, for the varied and magnificent morphologies of somatosensory neuron subtype endings? We know that different target tissues provide unique signaling niches to support peripheral ending maturation. Studies of proprioceptor ending maturation, for example, point to a dynamic interplay between developing target cells and the sensory neuron ending types that form upon them. Moreover, proper muscle development is critical for proprioceptor maturation and their acquisition of muscle type identity (Zampieri and de Nooij, 2021). In Egr3 mutants, for example, where muscle spindle development is perturbed, group Ia/II proprioceptor specification and ending morphology are also severely disrupted (Oliveira Fernandes and Tourtellotte, 2015; Wu et al., 2019). Likewise, neuregulin 1 expressed in proprioceptors is an early induction signal of muscle spindle differentiation (Hippenmeyer, et al., 2002). In the developing Pacinian corpuscle, a RET-ER81-NRG1 signaling pathway coordinates interactions between Aβ RA2-LTMRs axons and the numerous terminal Schwann cells comprising the nascent corpuscle (Fleming et al., 2016; Luo et al., 2009; Sedý et al., 2006). Other cell types, such as epidermal stem cells associated with hair follicles (Cheng et al., 2018) and keratinocytes (Jenkins et al., 2019) also provide essential support for the formation of select sensory ending morphologies and LTMR end organs in the skin.
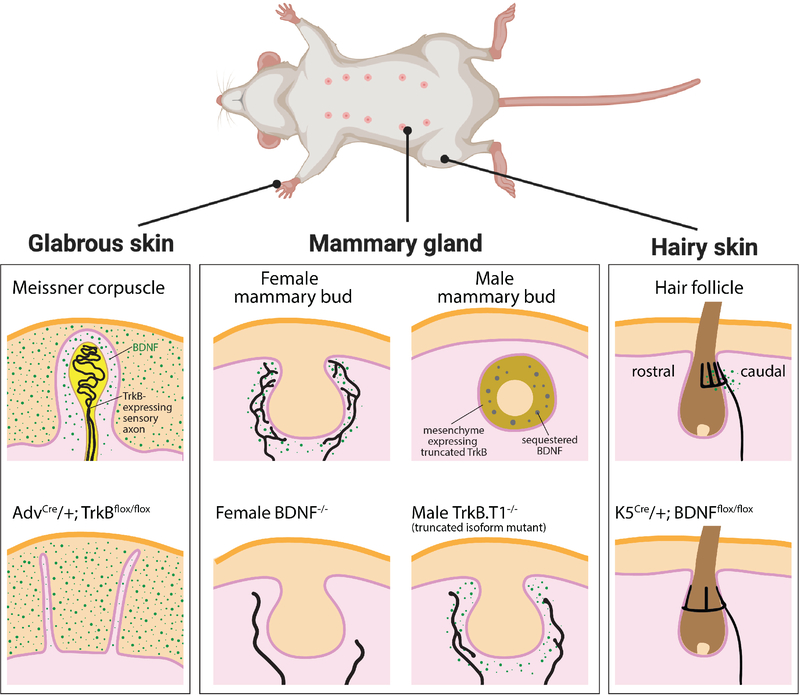
While relatively little is known about the identity of instructive cues that govern morphological maturation of the diverse DRG neuron ending types, we do know that at least some of the cues are repurposed in different ways and within different locales to instruct formation of distinct end organ structures. This point is nicely illustrated by the neurotrophic factor BDNF, which is expressed in several end organ niches and is required for maturation of at least four distinct somatosensory neuron peripheral ending morphologies (Figure 5). In one example, BDNF produced by mesenchymal cells plays an essential role in survival of the TrkB-expressing Aδ-LTMRs, whereas BDNF produced by epithelial cells on the caudal side of the hair follicles is dispensable for survival of these same neurons but instead instructs patterning of their nascent axonal endings (Rutlin et al., 2014). In this case, while epithelial-derived BDNF is not required for formation of lanceolate endings, its polarized spatial distribution pattern directs the biased localization of Aδ-LTMR lanceolate endings to the caudal side of the follicle, which underlies Aδ-LTMRs’ direction-selective responses to hair deflection. In a second example, in non-hairy (glabrous) skin, DRG sensory axons that form Meissner corpuscles reach the dermis by birth and form mature corpuscles during postnatal development (Idé, 1977) in a BDNF–TrkB-dependent manner. In this case, BDNF expressed in epithelial cells surrounding dermal papillae acts on TrkB expressed on sensory axons as they invade this dermal region during corpuscle formation, and corpuscles fail to develop in the absence of this signal (González-Martínez et al., 2005; Neubarth et al., 2020). In a third example, BDNF–TrkB signaling functions in a sexually dimorphic manner to direct DRG sensory neurons that innervate the mammary gland (Liu et al., 2012). In this case, BDNF produced by primordial mammary gland mesenchymal cells signals through TrkB receptors expressed on embryonic DRG neuron axons to establish mammary gland sensory innervation of both males and females during early development. Interestingly, BDNF–TrkB signaling becomes inhibited in sensory axons in males, which leads to an early, male-specific loss of mammary gland innervation. In a fourth and final example, morphological maturation of periodontal Ruffini-like endings is delayed in the absence of BDNF and Neurotrophin-4/5, although the source of these factors remains to be determined (Hoshino et al., 2003; Maruyama et al., 2005). Thus, an emerging view is that common sets of cues are used in different combinations and at different times within different niches to shape the varied peripheral sensory ending structures and thus the variations of physiological properties and functions of somatosensory neuron subtypes. Clearly, more work is needed to appreciate the dynamics of intercellular interactions and the identity of molecular cues that instruct somatosensory end organ morphogenesis and the form underlying function in somatosensation.
Figure 5. BDNF signaling is deployed at different times and in different locales to instruct a range of somatosensory neuron subtype axonal morphologies and functions.
The neurotrophic factor BDNF (green dots) is expressed in several end organ niches in the periphery, including cells of glabrous skin, mammary gland, and hairy skin. The BDNF receptor TrkB receptor is expressed in subsets of developing axons (black). Mutants lacking either BDNF in the periphery or the TrkB receptor in axons exhibit deficits in ending morphology and function, in each of these cases.
The formation of DRG neuron central projections and synapses.
All DRG neurons have a central axon that innervates the spinal cord. As with their peripheral counterparts, central axons exhibit a range of subtype specific termination patterns. Some, the proprioceptors and Aβ-LTMR subtypes, also have secondary branches that form off the main central axon and extend both rostrally and caudally within the spinal cord, typically for many segments, and many but not all of these neurons also have a branch that extends via the dorsal column to the dorsal column nuclei (DCN) of the brainstem (Figure 1B; Abraira and Ginty, 2013; Niu et al., 2013). As such, by our estimation, Aβ LTMRs and proprioceptors hold the distinction of being the largest (longest) cells of the body, with peripheral axons extending as far as distal hindlimbs and central projections that may extend all the way to the brainstem. Interestingly, spinal cord roof-plate derived radial glial cells are essential for growth of the ascending, dorsal column projecting axons of Aβ-LTMRs (Kridsada et al., 2018); identifying glial-produced molecules involved in this process represents an exciting future direction. In contrast to proprioceptors and Aβ-LTMRs, the Aδ-LTMRs, C-LTMRs, Aδ-HTMRs, C-HTMRs and nociceptor subtypes, and other C-fiber subtypes, form simpler, less expansive central branching patterns, and indeed most and possibly all of these subtypes lack an ascending branch within the dorsal column (Abraira and Ginty, 2013; Basbaum et al., 2009). Additional organizing features of the central somatosensory system include topographic organization of sensory neuron inputs to the spinal cord and DCN and a remarkable precision of the laminar location of sensory neuron subtype endings along the dorsal-ventral axis of the spinal cord (Figure 1B). With respect to the latter, axons of peptidergic nociceptor subtypes mainly terminate within lamina I and outer lamina II (IIo) of the dorsal horn, while axons belonging to non-peptidergic nociceptor or C-fiber subtypes and pruriceptors mainly terminate within inner lamina II (IIi) (Braz et al., 2014; Dong and Dong, 2018). Within the deeper dorsal horn (lamina IIiv-V), LTMR subtypes exhibit distinct but overlapping laminar termination patterns in a region termed the LTMR-RZ (Abraira and Ginty, 2013; Abraira et al., 2017). Proprioceptors that innervate different groups of muscles have spatially segregated trajectories and terminate more ventrally, within distinct regions of the intermediate zone and ventral spinal cord. Although little is known about the identity of cell surface molecules that specify the precise termination zones of somatosensory neuron subtypes, lamina-specific innervation patterns of nociceptive axons in the dorsal spinal cord are disrupted in runx1 mutants (Chen et al., 2006b), and the level of Runx3 expression informs proprioceptor afferent targeting within the developing spinal cord (Chen et al., 2006a). As such, identifying cell adhesion and central guidance cue recognition molecules regulated by Runx1 and Runx3 is likely to offer insights into how dorsal-ventral patterning of cutaneous afferents within the spinal cord is achieved. The mechanisms underlying the establishment of somatotopic alignment of sensory terminals, which underlies the topographic representation of the body in the CNS (Hodge et al., 2007; Kuehn et al., 2019; Li et al., 2011; Mirnics and Koerber, 1995a; Olson and Luo, 2018) are also largely unknown.
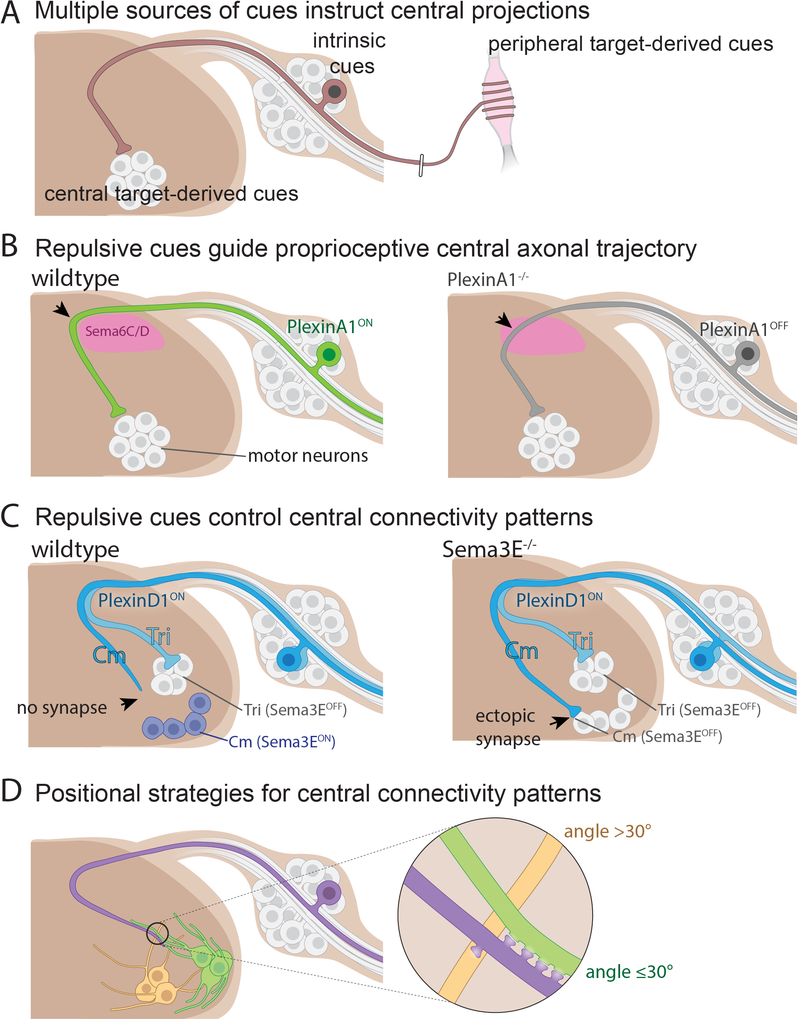
Similar to peripheral axon extension and targeting, central projections may respond to a series of instructive cues from intermediate targets en route to final target regions. Netrin-1 produced in the floor plate of the spinal cord repels sensory axons that express the receptor Unc5c to ensure that sensory axons project towards the dorsal root entry zone (Masuda et al., 2008). Upon their arrival to the dorsal spinal cord, sensory neuron axons exhibit a stereotyped “waiting period” before penetrating the spinal cord grey matter (Davis et al., 1989). After this waiting period and beginning ~E14 in the mouse, which roughly coincides with the beginning of peripheral target field innervation (Mirnics and Koerber, 1995b), sensory fibers enter the spinal cord in sequence, with ventrally projecting proprioceptive axons invading the spinal cord earlier than dorsally terminating axons (Mirnics and Koerber, 1995b; Ozaki and Snider, 1997). Sema5B expressed in the spinal cord may contribute to the waiting period and prevent premature extension of cutaneous C-fiber axons into the cord (Liu et al., 2014; Mirnics and Koerber, 1995b; Ozaki and Snider, 1997). With respect to dorsal-ventral laminar positioning, the observation that sensory axons strictly avoid incorrect laminae during development suggests that repulsive guidance cues play a key role in instructing axonal trajectories and the specificity of connectivity. Indeed, certain secreted semaphorins produced in the ventral spinal cord can selectively repel NGF responsive axons that normally terminate in dorsal lamina, whereas proprioceptors that normally innervate more ventral regions are unresponsive to these cues (Messersmith et al., 1995). Conversely, distinct semaphorin-PlexinA1 repulsive signals direct targeting of proprioceptive axons to the intermediate and ventral spinal cord by preventing these axons from terminating within more dorsal positions (Figure 6B) (Yoshida et al., 2006).
Figure 6. Repulsion and positional mechanisms for proprioceptor axon targeting in the spinal cord.
(A) Cues emanating from multiple sources instruct the central projections of DRG neurons.
(B) In wild-type mice, the presence of Sema6C and Sema6D in the dorsal horn (pink) repels proprioceptors that express PlexinA1. In the absence of PlexinA1, proprioceptive axons are disorganized in the superficial dorsal horn (indicated by the arrow).
(C) In wild-type mice, triceps (Tri) motor neurons (white) receive direct proprioceptor input (light blue), while cutaneous maximus (Cm) motor neurons do not receive input from Cm proprioceptors (blue). In the absence of the repulsive cue Sema3E, Cm proprioceptors directly form synaptic connections with Cm motor neurons (indicated by the arrow).
(D) Two patterning principles underlie the specificity of connectivity in sensory-motor circuits. First, the degree of axo-dendritic overlap between proprioceptive axon (purple) and motor neuron dendrites (yellow and green) influences the number of synaptic connections formed. Second, the angle of axo-dendritic approach influences the formation of synaptic clusters when sensory axons (purple) intersect dendrites from different motor neuron pools (yellow and green).
Beyond controlling laminar position of sensory axon terminals, repulsive cues play crucial roles in orchestrating sensory-motor connectivity and, in particular, the precision of synaptic connections between proprioceptor axons and motor neuron dendrites. Different subtypes of proprioceptors synapse upon distinct sets of target neurons in the ventral horn (Sürmeli et al., 2011). The overall central trajectory of proprioceptor subtypes does not appear to require positional cues emanating from motor neurons (Sürmeli et al., 2011). However, when sensory neuron axons intersect motor neuron dendritic arbors, the position of motor neuron subtypes, the angle of axo-dendritic approach, and the extent of axo-dendritic overlap together can account for selectivity in the formation of synaptic clusters between proprioceptor subtypes and their motor neuron partners (Figure 6D) (Balaskas et al., 2019). Moreover, Sema3E produced by motor neurons that innervate the cutaneous maximus muscle repels proprioceptors that express the Sema3E receptor Plexin D1. This repulsion signal thus instructs the precision of monosynaptic connections between proprioceptors and motor neurons (Figure 6C) (Fukuhara et al., 2013; Pecho-Vrieseling et al., 2009).
While the identity of some key spinal cord derived cues are becoming realized, an interesting, emerging view is that cues emanating from the periphery as well as those derived from sensory neurons themselves regulate the specificity of axonal targeting and the precision of synaptic connectivity within the spinal cord (Figure 6A). Insights into this have come from experiments using chick embryos showing that proprioceptors innervating duplicate muscles make similar types of central connections onto select motoneurons as those innervating correspondingly normal (single) muscles. This suggests that target muscles provide cues that instruct the central connection patterns of sensory neurons (Wenner and Frank, 1995). Along these lines, muscle-derived NT3 promotes expression of the ETS transcription factor Etv1 in proprioceptors to regulate the extension of proprioceptor central axons towards the ventral horn (Patel et al., 2003). Moreover, the loss of muscle spindle-derived NT3 in Egr3−/− mice causes a decrease in the amplitude of monosynaptic sensory-evoked responses in motor neurons (Chen et al., 2002), while overexpression of NT3 in muscles disrupts the selectivity of connections between group Ia proprioceptive afferents and motor neurons (Wang et al., 2007). Related to this, in the trigeminal system, BMP4 signals expressed in the craniofacial region of trigeminal sensory neurons direct central projections of sensory neurons to form proper whisker maps in the brain (Hodge et al., 2007).
In addition to peripherally derived cues, sensory neuron derived cues can instruct proper formation of sensorimotor circuits. Secretion of BDNF from proprioceptors and activation of TrkB signaling in spinal GABAergic interneurons is required for the accumulation of GABA synthetic enzyme GAD65 in presynaptic terminals of these interneurons, which mediate presynaptic inhibition of sensory inputs to the spinal cord (Betley et al., 2009). Furthermore, proprioceptor-derived NB2 and Caspr4a, together with spinal interneuron expression of NrCAM and CHL1, control formation of these GABAergic terminals onto proprioceptive terminals (Ashrafi et al., 2014). These findings, taken together, thus illustrate a general principle of the developing somatosensory system (Figure 6): peripheral target-derived cues and DRG neuron-derived cues collaborate with spinal cord-derived cues and positional information to promote formation and selectivity of somatosensory neuron connectivity patterns in the spinal cord.
The acquisition of DRG neuron functional response properties
A fundamental feature of somatosensation is the early acquisition of functional and behavioral responses to a range of stimuli. Indeed, reactivity to cutaneous tactile stimuli in fetuses in utero has been reported across mammalian species and may emerge earlier than responses to visual, auditory, gustatory and olfactory stimuli (Bradley and Mistretta, 1975; Narayanan et al., 1971), suggesting an early developmental dependence on somatosensation. This view is aligned with a range of observations indicating that genetic disruptions that alter mechanosensation and proprioception are generally found to be incompatible with postnatal viability (Nonomura et al., 2016; Ernfors et al., 1994; Klein et al., 1994; Ranade et al., 2014). Responses to somatosensory stimuli other than tactile stimuli across development have been less well characterized, although studies with neonatal rats suggest that flexor-withdrawal reflex responses to noxious mechanical and some thermal stimuli are present at birth, whereas behavioral responses to chemical irritants may develop later, in the second week of postnatal life (Fitzgerald and Gibson, 1984). Both in vitro and in vivo electrophysiological recordings of DRG neurons across development have yielded insights into the functional maturation of somatosensory neuron subtypes and the developmental changes in sensory neuron physiology that may underlie the maturation of behavioral responses to naturalistic stimuli (Figure 7).
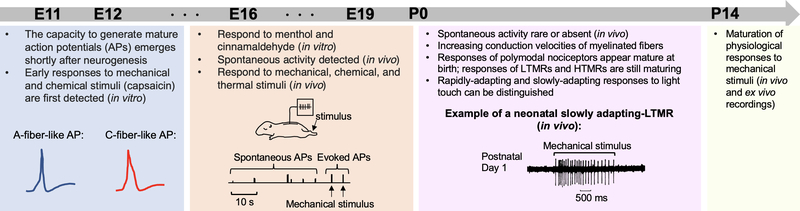
Figure 7. Timeline illustrating the emergence of functional responses in rodent DRG neurons.
Mature action potentials (APs) characteristic of A-fibers or C-fibers can first be detected in response to current injection at E11.5 for A-fiber-like responses and at E12.5 for C-fiber like responses, based on in vitro recordings of dissociated mouse embryonic neurons. Mechanosensitive currents emerge at E13.5, whereas functional responses to capsaicin and menthol are evident at E12.5 and E16.5, respectively. In vivo extracellular recordings of DRG neurons in rat fetuses detect spontaneous firing in the absence of applied stimuli; by postnatal Day 0, spontaneous activity disappears. The earliest evoked responses to physiological stimuli in vivo are detected at late embryonic stages (E17 onwards). Responses of polymodal nociceptors to noxious mechanical, thermal, or chemical stimuli appear mature at birth. In contrast, the physiological response properties of mechanosensitive DRG neurons continue to mature between P0-P14, displaying near adult-like features by Day 14. Schematics are adapted from Lechner et al., 2009, Fitzgerald, 1987, and Fitzgerald, 1987b.
Insights from in vitro studies.
The functional maturation of DRG neurons occurs concomitant with their molecular and morphological maturation. Electrophysiological recordings of acutely dissociated embryonic mouse DRG neurons revealed that as early as E11.5 a subset can generate action potentials in response to current injection (Lechner et al., 2009). These embryonic neurons display a narrow spike profile typical of proprioceptors and Aβ-LTMRs. Slightly later, at E12.5, cells are detected that can fire action potentials characteristic of nociceptors, with a larger width and a hump on the falling phase. At E13.5, rapidly adapting (RA) mechanosensitive currents can be detected in a small population of neurons expressing TrkB and TrkC transcripts. Beginning at E14.5, small diameter neurons are detected with RA-type mechanosensitive currents, and after birth a third population of small diameter neurons acquires an SA-type (slowly adapting) mechanosensitive current. Experiments using calcium imaging of acutely dissociated DRG neurons suggest that functional responses to chemical and thermal stimuli emerge slightly later, during late embryonic and early postnatal development (Hjerling-Leffler et al., 2007). In general, responses to select chemical stimuli coincide with the appearance of transcripts encoding particular receptors. For example, the first responses to capsaicin are detected at E12.5, which corresponds to the earliest time point at which transcripts for the capsaicin receptor TrpV1 are detected in DRG neurons. In contrast, the earliest responses to the TrpM8 agonist menthol or the TrpA1 agonist cinnamaldehyde are seen at E16.5 or P7, respectively. The emergence of these responses is consistent with when the transcripts of their respective receptors (TrpM8 or TrpA1) are detected. This is also consistent with the late emergence of behavioral responses to the TrpA1 agonist mustard oil observed in rat pups (Fitzgerald and Gibson, 1984). Taken together, findings from in vitro experiments suggest that DRG neurons acquire the ability to respond to mechanical, thermal, and chemical stimuli relatively early in fetal development, when they are beginning to innervate their targets but long before their peripheral arbors are morphologically mature. The extent to which this process results from the execution of an intrinsic developmental program or is dependent on cues that neurons encounter as they navigate towards their final targets is unknown.
Insights from in vivo and ex vivo studies.
Tour de force experiments done in the 1980s provided the first glimpse into the acquisition of DRG neuron functional responses in vivo during late embryonic and early postnatal development. Extracellular recordings of single unit activity in DRGs of rat fetuses demonstrated that at E16, DRG neurons display spontaneous activity but no detectable responses to thermal, mechanical, or chemical stimulation of the skin, although some units responded to electrical stimulation (Fitzgerald, 1987a). This stands in contrast to the detection of mechanosensitive responses to somal stimulation of acutely dissociated E13.5 mouse embryonic neurons (Lechner et al., 2009) and could reflect species-specific differences, incomplete innervation of peripheral targets at E16, or delayed maturation of mechanoreceptive endings or molecular machinery in peripheral axons. By E17, clear responses to mechanical stimulation of the limb were detected in rat fetal DRG neurons, and these could be classified as pressure responses, low threshold mechanoreceptor responses, or responses to limb and joint movement. By E19, responses to heating of the skin were also present (Fitzgerald, 1987a). As such, rudimentary responses to touch, temperature, and body and limb position develop at late embryonic stages.
In vivo extracellular recordings of DRG neurons in perinatal rats from P0-P14 revealed that DRG neurons lose their spontaneous activity postnatally, as their responses to external stimuli mature and increasingly resemble those of adult animals (Fitzgerald, 1987b). Conduction velocities are slower in perinatal animals compared to adults, and gradually increase for a subset of fibers, presumably reflecting the increase in myelination during postnatal development. Strikingly, as early as P0, cutaneous afferents could be classified as slowly adapting (SA) mechanoreceptors, rapidly adapting (RA) mechanoreceptors, pressure receptors, HTMRs, or polymodal nociceptors that responded to heating or pinching of the skin and in some cases to chemical irritants. For most of these classes, response thresholds and functional response profiles were largely stable during the first two weeks of postnatal life, with some exceptions. The firing frequencies and total number of spikes per stimulus of SA-LTMRs, RA-LTMRs, and some HTMRs increased with age. In contrast, responses of polymodal nociceptors did not significantly change over time (Fitzgerald, 1987b). Pressure receptors were more commonly observed from P0-P7 than at P14 and may correspond to an immature population, but future studies will be needed to resolve this. Interestingly, single unit recordings performed in cats suggest that the precise functional tuning properties of vibration-sensitive mechanoreceptors that innervate glabrous skin undergo considerable maturation during postnatal development (Ferrington and Rowe, 1980). In adult recordings, two classes of vibration sensitive fibers can be detected that are tuned to largely non-overlapping frequencies and that likely correspond to Aβ RA1-LTMRs and Aβ RA2-LTMRs innervating Meissner and Pacinian corpuscles, respectively. In contrast, in neonatal recordings there is no clear segregation of frequency tuning among vibration sensitive units, the range of frequencies that neurons respond to is narrower, and their capacity to phase-lock responses, or precisely entrain, to vibratory stimuli is lower compared to what is observed in adults. As the morphological properties of both Meissner and Pacinian corpuscles mature over the first few weeks to months of postnatal life in cats, these findings suggest that physiological and morphological maturation of LTMRs and their respective end organs are intimately coupled in time.
Ex vivo recordings from skin-nerve-DRG preparations built upon the foundation of the in vivo studies and provided considerable insight by correlating functional responses with specific neuronal morphologies (Koerber and Woodbury, 2002; Mirnics and Koerber, 1997; Woodbury et al., 2001; Woodbury and Koerber, 2003; Woodbury and Koerber, 2007). Neurobiotin fills following recordings at late embryonic and early postnatal stages revealed that cutaneous sensory neurons displaying narrow somal action potentials consistently had central projections confined to the deep dorsal horn, similar to the adult projection patterns of Aβ-LTMRs. The labeled sensory neurons with broad action potentials exhibited central projections restricted to superficial lamina, consistent with the pattern of C-fiber neurons in adults (Mirnics and Koerber, 1997). Rapidly adapting responses to low force skin indentation and to hair deflection were seen in afferents innervating hair follicles as early as P0 and P2 in mice, respectively (Woodbury et al., 2001). Similarly, slowly adapting responses to low forces were observed at P2 in afferents innervating touch domes in hairy skin; thresholds for these responses were indistinguishable from those seen in Aβ SA1-LTMRs afferents recorded in adult mice (Woodbury and Koerber, 2007). Responses of myelinated HTMRs to tactile stimulation in perinatal animals were also similar to those recorded from these populations of neurons in adults; interestingly, a subset of myelinated HTMRs formed central projections that span superficial and deep lamina of the dorsal horn at both perinatal and adult ages (Woodbury and Koerber, 2003). The exact identity of these neurons remains unclear, though they may correspond to the “pressure” units detected in perinatal DRG neurons in vivo (Fitzgerald et al., 1987b). In ex vivo recordings from P14 mice, the major mechanosensitive classes were detected (Aβ RA-LTMR, Aβ SA-LTMR, Aδ-LTMR, Aδ-HTMR, polymodal nociceptors), though there were signs that the functional maturation of LTMRs was still incomplete (Koltzenburg et al., 1997). In particular, many LTMR units could not be clearly classified as RA or SA, showing RA-like firing at low stimulus forces and SA-like discharges at higher forces, which may reflect the existence of an immature population.
Taken together, these classic studies indicate: 1) DRG neurons become electrically excitable within days following their birth; 2) at least some DRG neurons exhibit spontaneous activity during late embryonic but not postnatal periods; 3) DRG neurons are able to respond to some naturalistic stimuli during late embryonic development, long before their peripheral endings are morphologically mature; and 4) late embryonic and early postnatal physiological response properties are distinct from those of adult neurons. In principle, changes in either the morphologies of somatosensory neuron peripheral arbors and their associated end organs or in the intrinsic properties of somatosensory neurons, or both, could underlie the acquisition of precisely-tuned, subtype-specific physiological response properties and action potential firing patterns during neonatal and postnatal periods. Understanding whether and how changes in the expression patterns of ion channels contribute to the loss of spontaneous activity and the maturation of functional responses presents an exciting direction for future work. Similarly, experiments using modern genetic tools to perform targeted recordings of genetically and functionally defined somatosensory neurons over development, combined with detailed analyses of the sensory ending structural features, will help delineate the relationship between the morphological, ultrastructural, and functional maturation of these exquisitely tuned neurons.
The role of neural activity in development of somatosensory neurons
One fascinating and relatively under-explored question is the role of neural activity in the development of DRG somatosensory neurons and their engagement with the spinal cord and brainstem circuitry. In the visual and auditory systems, both spontaneous and stimulus-evoked activity play essential roles in the maturation of connectivity, the regulation of gene expression patterns, and topographic map formation (Cang and Feldheim, 2013; Kandler et al., 2009; Kirkby et al., 2013; Sitko and Goodrich, 2021). The role of neural activity in the development of the somatosensory system has primarily been studied in the context of the rodent whisker system and the cortical maps that represent the whiskers (Harding-Forrester and Feldman, 2018; Erzurumlu and Gaspar, 2020; Iwasato and Erzurumlu, 2018; Kania, 2021). Less is known about the role of activity in the maturation of DRG neurons and the downstream circuits that process tactile inputs from the majority of the mammalian body.
A role for neural activity in the development of DRG neurons has been investigated in the context of sensorimotor circuitry of the ventral spinal cord. Experiments in which chick embryos were treated with a neuromuscular blocking agent suggested that sensory-evoked activity is dispensable for establishing muscle-specific connectivity between proprioceptors and motor neurons (Mendelson and Frank, 1991). In treated embryos, all motor neurons received strong monosynaptic input from proprioceptors innervating the same muscle (homonymous), similar to what was detected in controls. The basic patterns of other types of connections (heteronymous or antagonistic) were also largely unchanged, except for some increases in heteronymous connections that were not detected in control animals. A study re-visited this question using mouse genetic tools to block synaptic transmission from proprioceptors throughout development. In this work, expression of the tetanus toxin light chain subunit in proprioceptors to block synaptic transmission during development led to an increase in the number of heteronymous connections between proprioceptors and motor neurons innervating muscles that are functionally related, but no change in homonymous or antagonistic connections (Mendelsohn et al., 2015). In addition, an examination of the development of pre-synaptic inhibition in the spinal cord revealed a key role for somatosensory neuron-derived glutamate in establishing inhibitory synapses onto proprioceptor afferent terminals (Mende et al., 2016). In vGlut1 knock-out mice, these spinal cord “GABA-pre” interneurons form abnormal inhibitory synapses onto proprioceptor axons, with reduced GAD67 and GAD65 localized to these synapses. Here, the metabotropic glutamate receptor mGluR1-beta is expressed on axons of GABA-pre interneurons that contact proprioceptors, and mGluR1 KO mice also have reduced GAD67 at inhibitory axoaxonic synapses and display reduced presynaptic inhibition (Mende et al., 2016). Together with the classic literature, these studies suggest that evoked activity in proprioceptors, while not critical for proprioceptor maturation or survival, plays an essential role in the establishment of their specific patterns of synaptic connectivity in the spinal cord.
The role of neural activity in development of primary somatosensory neuron populations other than proprioceptors remains largely unexplored. One study that used Wnt1-Cre to conditionally delete the vesicular glutamate transporter vGlut2 reported defects in the innervation of hair follicles by NFH+ endings and in the organization of terminal Schwann cells surrounding hair follicles during embryonic development, but the early lethality associated with this manipulation precluded further characterization of end organ development (Woo et al., 2012). Similarly, the role of activity or neurotransmitter release by LTMR or nociceptor afferents in the spinal cord or brainstem during central targeting and synapse formation remains unclear. While several early reports pointed to developmental refinement of LTMR central projections within the spinal cord, the extent of this maturation is controversial, and the identity of the populations undergoing refinement remains unknown. Bulk retrograde labeling to visualize A fibers during rat postnatal development suggested that these axons initially target the superficial dorsal horn but withdraw from it and become restricted to the deep dorsal horn by P21 (Beggs et al., 2002). Chronic exposure of the dorsal spinal cord to NMDA receptor antagonists prevented these structural rearrangements, but had no effect on C fibers whose central projections did not undergo similar refinement. More somatotopically specific retrograde labeling of cutaneous afferents in rat pups from P1 to adulthood revealed that spinal cord termination zones in young pups were much broader than in adults, and that chronic delivery of an NMDA receptor antagonist to the spinal cord from P6-P21 resulted in the central projections being broader compared to controls, effectively resembling the projections of young animals (Granmo et al., 2008).
These findings implicating activity in structural refinement of central projections have been challenged by studies in which neurobiotin fills were used to visualize central axonal morphologies of individual cutaneous neurons following their physiological characterization in a skin-nerve preparation (Koerber and Woodbury, 2002; Woodbury et al., 2001; Woodbury and Koerber, 2003; Woodbury and Koerber, 2007). In this work, the authors did not detect obvious structural maturation in the laminar termination zones of A-fiber inputs during postnatal development. The basis of this apparent discrepancy may reflect the specific identities of the populations undergoing refinement and could be resolved by using genetic tools to perform single cell labeling of distinct DRG subclasses and to assess the morphology of cells throughout late embryonic and early postnatal development.
The role of neural activity in the development of nociceptor populations also remains largely unknown, though work in humans and mice suggests a possible role for sodium channels in controlling development, maintenance, or maturation of C-fibers. A study of human patients with compound heterozygous loss of function mutations in Nav1.7 (Scn9A) and resultant congenital insensitivity to pain revealed dramatic reductions in intra-epidermal nerve fiber density (IENFD) in biopsies from the distal leg and proximal thigh of all three patients (McDermott et al., 2019). Other studies have also reported reduced IENFD in patients with Scn9A mutations (Marchi et al., 2018), though another reported no change in C-fiber nerve densities in Scn9A patients (Cox et al., 2006). Mice lacking Scn9A have normal IENFD (Gingras et al., 2014); however, another study reported that somatosensory neuron specific Scn9A KO mice (unlike Nav1.8 cKO or Nav1.9 cKO mice) display profound changes in gene expression in DRG neurons (Minett et al., 2015). Mis-regulated genes included the developmentally important transcription factors Runx1 and Pou4f2 as well as Penk, the precursor of the opioid receptor ligand Met-enkephalin. Additional experiments manipulating intracellular sodium levels suggest a possible role for sodium in regulating gene expression in sensory neurons. Interestingly, one study showed that Scn9A mutant mice show very little change in peripheral sensory neuron functional responses to noxious stimuli, but a severe deficit in glutamate release by their central terminals; this defect is reversed upon blocking opioid receptor signaling (MacDonald et al., 2021). Together, these findings suggest that sodium channels and sodium-based action potentials may play diverse roles in regulating small diameter DRG neuron properties and that some of these functions could be important during development, though further work will be needed to test this idea. Studies in the CNS have increasingly demonstrated that certain ion channels play critical roles early in development to control progenitor specification, migration, or differentiation (Smith and Walsh, 2020). Exploring the roles of select ion channels, action potential firing patterns, and both spontaneous and evoked neurotransmitter release during transcriptional, morphological, and functional maturation of somatosensory neurons represents an exciting future direction for the field.
A renewed emphasis on development and challenges ahead
An appreciation of how the cells and circuitry of somatosensation are assembled during development informs our understanding of how this sensory system works, why it may be dysfunctional in developmental disorders or following disease or injury, and steps that may be taken to repair it. In addition, because of its accessibility and historical focus, insights gleaned from studies of somatosensory system development have long advanced many of the principles of development of the nervous system at large. Advances in our understanding of the properties and functions of mature somatosensory neurons, and their synaptic organization within the CNS, render the field poised for renewed efforts towards understanding the steps leading to their development. Indeed, somatosensory neuron development, as a discipline, may now be more compelling than ever.
Studies over the next decade will make use of a wide range of new tools and techniques to tackle many outstanding questions of somatosensory neuron development. These questions include: What are the intrinsic and extrinsic cues that instruct sensory neuron subtype transcriptional and functional maturation, and where, when, and how do these cues collaborate to establish the proper numbers and range of somatosensory neuron subtypes, across axial levels, with respect to different peripheral tissues, and among species? What are the cellular and molecular mechanisms that instruct formation of the different somatosensory neuron end organs, in the skin and elsewhere, and which end organ features underlie the acquisition of mature physiological properties of somatosensory neuron subtypes? And, how are central lamination patterns, somatotopic organization, and the specificity of central synaptic connectivity achieved? Answers to these and a host of related questions will require better resolution on gene expression patterns and proteomics, not only for somatosensory neurons but also for glial cells and cells of co-developing tissues and end organs and in CNS synaptic partners, across all stages of development. Defining the basis of spontaneous neural activity and the roles of both spontaneous and evoked patterns of activity for the establishment of central circuitry represent additional exciting goals that may soon be realized with newly available tools.
As our knowledge of orchestration of somatosensory system development grows, so too does our appreciation for how somatosensory neuron populations and their central circuitry can go awry in certain developmental disorders and in chronic pain states. As such, additionally compelling are future studies that ask how dysfunction of developing somatosensory neurons may affect spinal cord and brain development, CNS function, and behavior. We anticipate that advanced genetic tools and fine morphological, transcriptional, biochemical, ultrastructural, and physiological approaches used in years ahead hold great promise for unraveling mysteries of somatosensory system development, in health and disease.
Acknowledgments
We thank Annie Handler, Rick Koerber, Charalampia Koutsioumpa, Brendan Lehnert, Qiufu Ma, Joriene De Nooij, Rosalind Segal, and Elizabeth Silagi for comments on this review. We thank Janet Iwasa for illustrations shown in Figures 1, 3, 4, 5, and 6. We thank Ginty laboratory members for valuable discussions on the topic of this review. The authors’ research addressing development of somatosensory neurons is supported by a Hanna Gray Fellowship (S.M.), NIH NRSA 5F32NS106807-03 (C. S.), NIH 1R01 AT011447-01 (D.D.G) and NIH R35 5R35NS097344-05 (D.D.G.). D.D.G. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Declaration of Interests
DDG is an associate editor of Neuron. DDG is a member of the Decibel Therapeutics Scientific Advisory Board and a consultant for Lab1636, LLC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdo H, Li L, Lallemend F, Bachy I, Xu X-J, Rice FL, and Ernfors P (2011). Dependence on the transcription factor Shox2 for specification of sensory neurons conveying discriminative touch. Eur J Neurosci 34, 1529–1541. [DOI] [PubMed] [Google Scholar]
- Abraira VE, and Ginty DD (2013). The Sensory Neurons of Touch. Neuron 79, 618–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraira VE, Kuehn ED, Chirila AM, Springel MW, Toliver AA, Zimmerman AL, Orefice LL, Boyle KA, Bai L, Song BJ, et al. (2017). The Cellular and Synaptic Architecture of the Mechanosensory Dorsal Horn. Cell 168, 295–310.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, and Jessell TM (2000). ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell 101, 485–498. [DOI] [PubMed] [Google Scholar]
- Arber S (2012). Motor Circuits in Action: Specification, Connectivity, and Function. Neuron 74, 975–989. [DOI] [PubMed] [Google Scholar]
- Arcourt A, Gorham L, Dhandapani R, Prato V, Taberner FJ, Wende H, Gangadharan V, Birchmeier C, Heppenstall PA, Lechner SG (2017). Touch Receptor-Derived Sensory Information Alleviates Acute Pain Signaling and Fine-Tunes Nociceptive Reflex Coordination. Neuron 93, 179–193. [DOI] [PubMed] [Google Scholar]
- Ashrafi S, Betley JN, Comer JD, Brenner-Morton S, Bar V, Shimoda Y, Watanabe K, Peles E, Jessell TM, and Kaltschmidt JA (2014). Neuronal Ig/Caspr recognition promotes the formation of axoaxonic synapses in mouse spinal cord. Neuron 81, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachy I, Franck MCM, Li L, Abdo H, Pattyn A, Ernfors P, (2011). The transcription factor Cux2 marks development of an A-delta sublineage of TrkA sensory neurons. Developmental Biology 360, 77–86. [DOI] [PubMed] [Google Scholar]
- Badea TC, Williams J, Smallwood P, Shi M, Motajo O, Nathans J, (2012). Combinatorial expression of Brn3 transcription factors in somatosensory neurons: genetic and morphologic analysis. J. Neurosci. 32, 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai L, Lehnert BP, Liu J, Neubarth NL, Dickendesher TL, Nwe PH, Cassidy C, Woodbury CJ, and Ginty DD (2015). Genetic Identification of an Expansive Mechanoreceptor Sensitive to Skin Stroking. Cell 163, 1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaskas N, Abbott LF, Jessell TM, and Ng D (2019). Positional Strategies for Connection Specificity and Synaptic Organization in Spinal Sensory-Motor Circuits. Neuron 102, 1143–1156.e1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RP, and Vaughn JE (1986). Differentiation of dorsal root ganglion cells with processes in their synaptic target zone of embryonic mouse spinal cord: a retrograde tracer study. J. Neurocytol. 15, 207–218. [DOI] [PubMed] [Google Scholar]
- Barde YA (1989). Trophic factors and neuronal survival. Neuron 2, 1525–1534. [DOI] [PubMed] [Google Scholar]
- Bartesaghi L, Wang Y, Fontanet P, Wanderoy S, Berger F, Wu H, Akkuratova N, Bouçanova F, Médard J-J, Petitpré C, Landy MA, Zhang M-D, Harrer P, Stendel C, Stucka R, Dusl M, Kastriti ME, Croci L, Lai HC, Consalez GG, Pattyn A, Ernfors P, Senderek J, Adameyko I, Lallemend F, Hadjab S, Chrast R, 2019. PRDM12 Is Required for Initiation of the Nociceptive Neuron Lineage during Neurogenesis. CellReports 26, 3484–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, and Julius D (2009). Cellular and molecular mechanisms of pain. Cell 139, 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs S, Torsney C, Drew LJ, and Fitzgerald M (2002). The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci 16, 1249–1258. [DOI] [PubMed] [Google Scholar]
- Betley JN, Wright CVE, Kawaguchi Y, ErdElyi F, SzabO G, Jessell TM, and Kaltschmidt JA (2009). Stringent Specificity in the Construction of a GABAergic Presynaptic Inhibitory Circuit. Cell 139, 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard JW, Eade KT, Szűcs A, Sardo Lo, V., Tsunemoto RK, Williams D, Pietro Paolo Sanna, and Baldwin KK (2014). Selective conversion of fibroblasts into peripheral sensory neurons. Nat Neurosci 18, 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RM, and Mistretta CM (1975). Fetal sensory receptors. Physiol Rev 55, 352–382. [DOI] [PubMed] [Google Scholar]
- Braz J, Solorzano C, Wang X, and Basbaum AI (2014). Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 82, 522–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, and Ericson J (2000). A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101, 435–445. [DOI] [PubMed] [Google Scholar]
- Brown AG, Iggo A (1967). A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J. Physiol. (Lond.) 193, 707–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PR, Petit D, Warren RM (1968). Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol 31, 833–848. [DOI] [PubMed] [Google Scholar]
- Burgess PR, Perl ER (1967). Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J. Physiol. (Lond.) 190, 541–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderó J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, and Oppenheim RW (1998). Peripheral target regulation of the development and survival of spinal sensory and motor neurons in the chick embryo. Journal of Neuroscience 18, 356–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang J, and Feldheim DA (2013). Developmental mechanisms of topographic map formation and alignment. Annu. Rev. Neurosci. 36, 51–77. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, and Anderson DJ (2009). Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl. Acad. Sci. U.S.a. 106, 9075–9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AI, de Nooij JC, and Jessell TM (2006a). Graded activity of transcription factor Runx3 specifies the laminar termination pattern of sensory axons in the developing spinal cord. Neuron 49, 395–408. [DOI] [PubMed] [Google Scholar]
- Chen C-L, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, and Ma Q (2006b). Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron 49, 365–377. [DOI] [PubMed] [Google Scholar]
- Chen H-H, Tourtellotte WG, and Frank E (2002). Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons. J. Neurosci. 22, 3512–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C-C, Tsutsui K, Taguchi T, Sanzen N, Nakagawa A, Kakiguchi K, Yonemura S, Tanegashima C, Keeley SD, Kiyonari H, et al. (2018). Hair follicle epidermal stem cells define a niche for tactile sensation. eLife 7, 1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Courchesne SL, Segal RA, (2008). Action in the axon: generation and transport of signaling endosomes. Current Opinion in Neurobiology 18, 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, Thornton G, Roberts E, Springell K, Karbani G, Jafri H, Mannan J, Raashid Y, et al. (2006). An SCN9A channelopathy causes congenital inability to experience pain. Nature 444, 894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranfill SL, and Luo W (2021). The development of somatosensory neurons: Insights into pain and itch (Elsevier Inc.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, and Levinson AD (1994). Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76, 1001–1011. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu J-P, and Jessell TM (2003). Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature 425, 926–933. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, and Jessell TM (2005). A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell 123, 477–491. [DOI] [PubMed] [Google Scholar]
- Davis BM, Frank E, Johnson FA, and Scott SA (1989). Development of central projections of lumbosacral sensory neurons in the chick. J. Comp. Neurol. 279, 556–566. [DOI] [PubMed] [Google Scholar]
- Davies A (1990). Ontogeny of the Somatosensory System: Origins and Early Development of Primary Sensory Neurons. Annu. Rev. Neurosci. 61–73. [DOI] [PubMed] [Google Scholar]
- Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, Ginty DD, (2008). A model for neuronal competition during development. Science 320, 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desiderio S, Vermeiren S, Van Campenhout C, Kricha S, Malki E, Richts S, Fletcher EV, Vanwelden T, Schmidt BZ, Henningfeld KA, et al. (2019). Prdm12 Directs Nociceptive Sensory Neuron Development by Regulating the Expression of the NGF Receptor TrkA. CellReports 26, 3522–3536.e3525. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN, (2004). Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev 46, 131–145. [DOI] [PubMed] [Google Scholar]
- Dong X, and Dong X (2018). Peripheral and Central Mechanisms of Itch. Neuron 98, 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Kucera J, and Jaenisch R (1994). Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell 77, 503–512. [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, and Gaspar P (2020). How the Barrel Cortex Became a Working Model for Developmental Plasticity: A Historical Perspective. J. Neurosci. 40, 6460–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure L, Wang Y, Kastriti ME, Fontanet P, Cheung KKY, Petitpré C, Wu H, Sun LL, Runge K, Croci L, et al. (2020). Single cell RNA sequencing identifies early diversity of sensory neurons forming via bi-potential intermediates. Nature Communications 11, 4175–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DG, and Rowe MJ (1980). Functional capacities of tactile afferent fibres in neonatal kittens. J. Physiol. (Lond.) 307, 335–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M (1987a). Spontaneous and evoked activity of fetal primary afferents in vivo. Nature 326, 603–605. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M (1987b). Cutaneous primary afferent properties in the hind limb of the neonatal rat. J. Physiol. (Lond.) 383, 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M, and Gibson S (1984). The postnatal physiological and neurochemical development of peripheral sensory C fibres. Neuroscience 13, 933–944. [DOI] [PubMed] [Google Scholar]
- Fleming MS, Li JJ, Ramos D, Li T, Talmage DA, Abe S-I, Arber S, and Luo W (2016). A RET-ER81-NRG1 Signaling Pathway Drives the Development of Pacinian Corpuscles. J. Neurosci. 36, 10337–10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara K, Imai F, Ladle DR, Katayama K-I, Leslie JR, Arber S, Jessell TM, and Yoshida Y (2013). Specificity of monosynaptic sensory-motor connections imposed by repellent Sema3E-PlexinD1 signaling. CellReports 5, 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras J, Smith S, Matson DJ, Johnson D, Nye K, Couture L, Feric E, Yin R, Moyer BD, Peterson ML, et al. (2014). Global Nav1.7 knockout mice recapitulate the phenotype of human congenital indifference to pain. PLoS ONE 9, e105895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty DD, Segal RA (2002). Retrograde neurotrophin signaling: Trk-ing along the axon. Current Opinion in Neurobiology 12, 268–274. [DOI] [PubMed] [Google Scholar]
- González-Martínez T, Fariñas I, Del Valle ME, Feito J, Germanà G, Cobo J, and Vega JA (2005). BDNF, but not NT-4, is necessary for normal development of Meissner corpuscles. Neurosci. Lett. 377, 12–15. [DOI] [PubMed] [Google Scholar]
- González-Martínez T, Germanà GP, Monjil DF, Silos-Santiago I, de Carlos F, Germanà G, Cobo J, and Vega JA (2004). Absence of Meissner corpuscles in the digital pads of mice lacking functional TrkB. Brain Res. 1002, 120–128. [DOI] [PubMed] [Google Scholar]
- Gorla M, Bashaw GJ (2020). Molecular mechanisms regulating axon responsiveness at the midline. Developmental Biology 466, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granmo M, Petersson P, and Schouenborg J (2008). Action-based body maps in the spinal cord emerge from a transitory floating organization. J. Neurosci. 28, 5494–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresset A, Coulpier F, Gerschenfeld G, Jourdon A, Matesic G, Richard L, Vallat J-M, Charnay P, Topilko P (2015). Boundary Caps Give Rise to Neurogenic Stem Cells and Terminal Glia in the Skin. Stem Cell Reports 5, 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, and Sagasti A (2010a). Self-avoidance and tiling: Mechanisms of dendrite and axon spacing. Cold Spring Harbor Perspectives in Biology 2, a001750–a001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, and Sagasti A (2010b). Self-avoidance and tiling: Mechanisms of dendrite and axon spacing. Cold Spring Harbor Perspectives in Biology 2, a001750–a001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen RW (1985). Sensory neurite growth cone guidance by substrate adsorbed nerve growth factor. J Neurosci Res 13, 199–212. [DOI] [PubMed] [Google Scholar]
- Gundersen RW, and Barrett JN (1979). Neuronal chemotaxis: chick dorsal-root axons turn toward high concentrations of nerve growth factor. Science 206, 1079–1080. [DOI] [PubMed] [Google Scholar]
- Handler A, Ginty DD (2021). The mechanosensory neurons of touch and their mechanisms of activation. Nat Rev Neurosci 22, 521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, and Levi-Montalcini R (1949). Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J Exp Zool 111, 457–501. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Abbott S, Han B-X, Qi Y, Wang F, (2007). Analyzing somatosensory axon projections with the sensory neuron-specific Advillin gene. J. Neurosci. 27, 14404–14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding-Forrester S, Feldman DE, (2018). Somatosensory maps. Handb Clin Neurol 151, 73–102. [DOI] [PubMed] [Google Scholar]
- Harrington AW, and Ginty DD (2013). Long-distance retrograde neurotrophic factor signalling in neurons. Nat Rev Neurosci 14, 177–187. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Shneider NA, Birchmeier C, Burden SJ, Jessell TM, Arber S, (2002). A role for neuregulin1 signaling in muscle spindle differentiation. Neuron 36, 1035–1049. [DOI] [PubMed] [Google Scholar]
- His W (1886). Zur Geschichte des menschlichen Rückenmarkes und der Nervenwurzeln. S. Hirzel, Leipzig. [Google Scholar]
- Hjerling-Leffer J, AlQatari M, Ernfors P, Koltzenburg M (2007). Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J. Neurosci. 27(10):2435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge LK, Klassen MP, Han B-X, Yiu G, Hurrell J, Howell A, Rousseau G, Lemaigre F, Tessier-Lavigne M, and Wang F (2007). Retrograde BMP signaling regulates trigeminal sensory neuron identities and the formation of precise face maps. Neuron 55, 572–586. [DOI] [PubMed] [Google Scholar]
- Honig MG, Camilli SJ, and Xue Q-S (2004). Ectoderm removal prevents cutaneous nerve formation and perturbs sensory axon growth in the chick hindlimb. Developmental Biology 266, 27–42. [DOI] [PubMed] [Google Scholar]
- Hoshino N, Harada F, Alkhamrah BA, Aita M, Kawano Y, Hanada K, and Maeda T (2003). Involvement of brain-derived neurotrophic factor (BDNF) in the development of periodontal Ruffini endings. Anat Rec a Discov Mol Cell Evol Biol 274, 807–816. [DOI] [PubMed] [Google Scholar]
- Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, and Enver T (1997). Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 11, 774–785. [DOI] [PubMed] [Google Scholar]
- Huang S, O’Donovan KJ, Turner EE, Zhong J, and Ginty DD (2015). Extrinsic and intrinsic signals converge on the Runx1/CBFβ transcription factor for nonpeptidergic nociceptor maturation. eLife 4, e10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idé C (1977). Development of Meissner corpuscle of mouse toe pad. Anat. Rec. 188, 49–67. [DOI] [PubMed] [Google Scholar]
- Iggo A, Kornhuber HH (1968). A quantitative analysis of non-myelinated cutaneous mechano-receptors. J. Physiol. (Lond.) 198, 113. [PubMed] [Google Scholar]
- Iggo A, and Ogawa H (1977). Correlative physiological and morphological studies of rapidly adapting mechanoreceptors in cat’s glabrous skin. J. Physiol. (Lond.) 266, 275–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, Mitsubuchi H, Tonoki H, Awaya Y, and Matsuda I (1996). Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet 13, 485–488. [DOI] [PubMed] [Google Scholar]
- Iwasato T, and Erzurumlu RS (2018). Development of tactile sensory circuits in the CNS. Current Opinion in Neurobiology 53, 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W (1971). Morphology of rapidly and slowly adapting mechano-receptors in the hairless skin of the cat’s hind foot. Brain Res. 217–231. [DOI] [PubMed] [Google Scholar]
- Jenkins BA, Lumpkin EA (2017). Developing a sense of touch. Development 144, 4078–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins BA, Fontecilla NM, Lu CP, Fuchs E, and Lumpkin EA (2019). The cellular basis of mechanosensory Merkel-cell innervation during development. eLife 8, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R, (2005). The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci 6, 671–682. [DOI] [PubMed] [Google Scholar]
- Jones KR, Fariñas I, Backus C, and Reichardt LF (1994). Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell 76, 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Clause A, and Noh J (2009). Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci 12, 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania A (2021). Sensational developments in somatosensory development? Current Opinion in Neurobiology 66, 212–223. [DOI] [PubMed] [Google Scholar]
- Kirkby LA, Sack GS, Firl A, and Feller MB (2013). A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, 1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao Y, Robertson B, Kudo M, and Grant G (2002). Proliferation patterns of dorsal root ganglion neurons of cutaneous, muscle and visceral nerves in the rat. J. Neurocytol. 31, 765–776. [DOI] [PubMed] [Google Scholar]
- Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, and Barbacid M (1994). Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature 368, 249–251. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, and Barbacid M (1993). Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 75, 113–122. [PubMed] [Google Scholar]
- Koerber HR, Druzinsky RE, Mendell LM (1988). Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol 60, 1584–1596. [DOI] [PubMed] [Google Scholar]
- Koerber HR, and Woodbury CJ (2002). Comprehensive phenotyping of sensory neurons using an ex vivo somatosensory system. Physiol Behav 77, 589–594. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, and Lewin GR (1997). Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol 78, 1841–1850. [DOI] [PubMed] [Google Scholar]
- Kridsada K, Niu J, Haldipur P, Wang Z, Ding L, Li JJ, Lindgren AG, Herrera E, Thomas GM, Chizhikov VV, et al. (2018). Roof Plate-Derived Radial Glial-like Cells Support Developmental Growth of Rapidly Adapting Mechanoreceptor Ascending Axons. CellReports 23, 2928–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn ED, Meltzer S, Abraira VE, Ho C-Y, and Ginty DD (2019). Tiling and somatotopic alignment of mammalian low-threshold mechanoreceptors. Proc. Natl. Acad. Sci. U.S.a. 116, 9168–9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy MA, Goyal M, Lai HC (2021). Nociceptor subtypes are born continuously over DRG development. Developmental Biology 479, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, and Biscoe TJ (1979). Development of mouse dorsal root ganglia: an autoradiographic and quantitative study. J. Neurocytol. 8, 265–274. [DOI] [PubMed] [Google Scholar]
- Le Douarin N, LeDouarin NM, and Kalcheim C (1999). The Neural Crest (Cambridge University Press; ). [Google Scholar]
- Lechner SG, Frenzel H, Wang R, and Lewin GR (2009). Developmental waves of mechanosensitivity acquisition in sensory neuron subtypes during embryonic development. The EMBO Journal 28, 1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC (1978). Chemotactic response of nerve fiber elongation to nerve growth factor. Developmental Biology 66, 183–196. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Booker B, 1960. Destruction of the sympathetic ganglia in mammals by an antiserum to a nerve-growth protein. Proc Natl Acad Sci USA 46, 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-L, Li K-C, Wu D, Chen Y, Luo H, Zhao J-R, Wang S-S, Sun M-M, Lu Y-J, Zhong Y-Q, et al. (2016). Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res 26, 967–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, et al. (2011). The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147, 1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RQ, Wang W, Legg A, Abramyan J, and O’Connor TP (2014). Semaphorin 5B is a repellent cue for sensory afferents projecting into the developing spinal cord. Development 141, 1940–1949. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rutlin M, Huang S, Barrick CA, Wang F, Jones KR, Tessarollo L, and Ginty DD (2012). Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science 338, 1357–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Enomoto H, Rice FL, Milbrandt J, and Ginty DD (2009). Molecular identification of rapidly adapting mechanoreceptors and their developmental dependence on ret signaling. Neuron 64, 841–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, and Ginty DD (2007). A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron 54, 739–754. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, and Anderson DJ (1999). Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 13, 1717–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald DI, Sikandar S, Weiss J, Pyrski M, Luiz AP, Millet Q, Emery EC, Mancini F, Iannetti GD, Alles SRA, Arcangeletti M, Zhao J, Cox JJ, Brownstone RM, Zufall F, Wood JN. (2021). A central mechanism of analgesia in mice and humans lacking the sodium channel Na V 1.7. Neuron 109(9):1497–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi M, Provitera V, Nolano M, Romano M, Maccora S, D’Amato I, Salvi E, Gerrits M, Santoro L, and Lauria G (2018). A novel SCN9A splicing mutation in a compound heterozygous girl with congenital insensitivity to pain, hyposmia and hypogeusia. J Peripher Nerv Syst 23, 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigère F, Ernfors P, 2007. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci 8, 114–127. [DOI] [PubMed] [Google Scholar]
- Martin P, Khan A, and Lewis J (1989). Cutaneous nerves of the embryonic chick wing do not develop in regions denuded of ectoderm. Development 106, 335–346. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Harada F, Jabbar S, Saito I, Aita M, Kawano Y, Suzuki A, Nozawa-Inoue K, and Maeda T (2005). Neurotrophin-4/5-depletion induces a delay in maturation of the periodontal Ruffini endings in mice. Arch. Histol. Cytol. 68, 267–288. [DOI] [PubMed] [Google Scholar]
- Maro GS, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P, Topilko P, (2004). Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci 7, 930–938. [DOI] [PubMed] [Google Scholar]
- Masuda T, Watanabe K, Sakuma C, Ikenaka K, Ono K, and Yaginuma H (2008). Netrin-1 acts as a repulsive guidance cue for sensory axonal projections toward the spinal cord. J. Neurosci. 28, 10380–10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Baluk P, Shimizu D, and Fujiwara T (1996). Dorsal root ganglion neuron development in chick and rat. Anat. Embryol. 193, 475–480. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Kobayashi N, Mominoki K, Wakisaka H, Mori M, and Murakami S (1998). [Morphological transformation of sensory ganglion neurons and satellite cells]. Kaibogaku Zasshi 73, 603–613. [PubMed] [Google Scholar]
- McDermott LA, Weir GA, Themistocleous AC, Segerdahl AR, Blesneac I, Baskozos G, Clark AJ, Millar V, Peck LJ, Ebner D, et al. (2019). Defining the Functional Role of NaV1.7 in Human Nociception. Neuron 101, 905–919.e908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mende M, Fletcher EV, Belluardo JL, Pierce JP, Bommareddy PK, Weinrich JA, Kabir ZD, Schierberl KC, Pagiazitis JG, Mendelsohn AI, et al. (2016). Sensory-Derived Glutamate Regulates Presynaptic Inhibitory Terminals in Mouse Spinal Cord. Neuron 90, 1189–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn AI, Simon CM, Abbott LF, Mentis GZ, and Jessell TM (2015). Activity Regulates the Incidence of Heteronymous Sensory-Motor Connections. Neuron 87, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson B, and Frank E (1991). Specific monosynaptic sensory-motor connections form in the absence of patterned neural activity and motoneuronal cell death. Journal of Neuroscience 11, 1390–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, and Kolodkin AL (1995). Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron 14, 949–959. [DOI] [PubMed] [Google Scholar]
- Miller R (1939). Observations upon the arrangement of the axillary artery and brachial plexus. American Journal of Anatomy 143–163. [Google Scholar]
- Minett MS, Pereira V, Sikandar S, Matsuyama A, Lolignier S, Kanellopoulos AH, Mancini F, Iannetti GD, Bogdanov YD, Santana-Varela S, et al. (2015). Endogenous opioids contribute to insensitivity to pain in humans and mice lacking sodium channel Nav1.7. Nature Communications 6, 8967–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirnics K, Koerber HR (1995a). Prenatal development of rat primary afferent fibers: I. Peripheral projection. J. Comp. Neurol. 355, 589–600. [DOI] [PubMed] [Google Scholar]
- Mirnics K, and Koerber HR (1995b). Prenatal development of rat primary afferent fibers: II. Central projections. J. Comp. Neurol. 355, 601–614. [DOI] [PubMed] [Google Scholar]
- Mirnics K, and Koerber HR (1997). Properties of individual embryonic primary afferents and their spinal projections in the rat. J Neurophysiol 78, 1590–1600. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Earley TJ, Watson J, Andahazy M, Backus C, Martin-Zanca D, Wright DE, Reichardt LF, and Patapoutian A (2004). Expressing TrkC from the TrkA locus causes a subset of dorsal root ganglia neurons to switch fate. Nat Neurosci 7, 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge A (1984). Schwann cells induce morphological transformation of sensory neurones. Nature 309, 367–369. [DOI] [PubMed] [Google Scholar]
- Mukouyama Y-S, Gerber H-P, Ferrara N, Gu C, and Anderson DJ (2005). Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development 132, 941–952. [DOI] [PubMed] [Google Scholar]
- Mukouyama Y-S, Shin D, Britsch S, Taniguchi M, and Anderson DJ (2002). Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 109, 693–705. [DOI] [PubMed] [Google Scholar]
- Narayanan CH, Fox MW, Hamburger V (1971). Prenatal development of spontaneous and evoked activity in the rat (Rattus norvegicus albinus). Behaviour 40, 100–134. [DOI] [PubMed] [Google Scholar]
- Nascimento AI, Mar FM, and Sousa MM (2018). The intriguing nature of dorsal root ganglion neurons: Linking structure with polarity and function. Prog. Neurobiol. 168, 86–103. [DOI] [PubMed] [Google Scholar]
- Neubarth NL, Emanuel AJ, Liu Y, Springel MW, Handler A, Zhang Q, Lehnert BP, Guo C, Orefice LL, Abdelaziz A, et al. (2020). Meissner corpuscles and their spatially intermingled afferents underlie gentle touch perception. Science 368, eabb2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Ding L, Li JJ, Kim H, Liu J, Li H, Moberly A, Badea TC, Duncan ID, Son Y-J, Scherer SS, Luo W, 2013. Modality-based organization of ascending somatosensory axons in the direct dorsal column pathway. J. Neurosci. 33, 17691–17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, Patapoutian A (2017). Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh W-J, and Gu C (2013). Establishment of neurovascular congruency in the mouse whisker system by an independent patterning mechanism. Neuron 80, 458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, Morrison I, McGlone F, and Vallbo A (2010). The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev 34, 185–191. [DOI] [PubMed] [Google Scholar]
- Oliveira Fernandes M, and Tourtellotte WG (2015). Egr3-dependent muscle spindle stretch receptor intrafusal muscle fiber differentiation and fusimotor innervation homeostasis. J. Neurosci. 35, 5566–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KM, Florez-Paz DM, Badea TC, Mentis GZ, Menon V, de Nooij JC, (2021). Molecular correlates of muscle spindle and Golgi tendon organ afferents. Nature Communications 12, 1451–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W, and Luo W (2018). Somatotopic organization of central arbors from nociceptive afferents develops independently of their intact peripheral target innervation. J. Comp. Neurol. 526, 3058–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim RW (1989). The neurotrophic theory and naturally occurring motoneuron death. Trends in Neurosciences 12, 252–255. [DOI] [PubMed] [Google Scholar]
- Ozaki S, and Snider WD (1997). Initial trajectories of sensory axons toward laminar targets in the developing mouse spinal cord. J. Comp. Neurol. 380, 215–229. [PubMed] [Google Scholar]
- Paré M, Smith AM, and Rice FL (2002). Distribution and terminal arborizations of cutaneous mechanoreceptors in the glabrous finger pads of the monkey. J. Comp. Neurol. 445, 347–359. [DOI] [PubMed] [Google Scholar]
- Patel TD, Jackman A, Rice FL, Kucera J, and Snider WD (2000). Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron 25, 345–357. [DOI] [PubMed] [Google Scholar]
- Patel TD, Kramer I, Kucera J, Niederkofler V, Jessell TM, Arber S, and Snider WD (2003). Peripheral NT3 signaling is required for ETS protein expression and central patterning of proprioceptive sensory afferents. Neuron 38, 403–416. [DOI] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, and Arber S (2009). Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature 459, 842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Piñera P, García-Suarez O, Germanà A, Díaz-Esnal B, de Carlos F, Silos-Santiago I, del Valle ME, Cobo J, and Vega JA (2008). Characterization of sensory deficits in TrkB knockout mice. Neurosci. Lett. 433, 43–47. [DOI] [PubMed] [Google Scholar]
- Poliak S, Norovich AL, Yamagata M, Sanes JR, and Jessell TM (2016). Muscle-type Identity of Proprioceptors Specified by Spatially Restricted Signals from Limb Mesenchyme. Cell 164, 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF (2006). Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 361, 1545–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Woo S-H, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Bégay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A (2014). Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutlin M, Ho C-Y, Abraira VE, Cassidy C, Bai L, Woodbury CJ, and Ginty DD (2014). The Cellular and Molecular Basis of Direction Selectivity of Aδ-LTMRs. Cell 159, 1640–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagasti A, Guido MR, Raible DW, and Schier AF (2005). Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Current Biology 15, 804–814. [DOI] [PubMed] [Google Scholar]
- Scott A, Hasegawa H, Sakurai K, Yaron A, Cobb J, and Wang F (2011). Transcription factor short stature homeobox 2 is required for proper development of tropomyosin-related kinase B-expressing mechanosensory neurons. J. Neurosci. 31, 6741–6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Solomon E, and Kuruvilla R (2018). Mechanisms of neurotrophin trafficking via Trk receptors. Mol Cell Neurosci 91, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH, (2009). Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462, 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedý J, Tseng S, Walro JM, Grim M, and Kucera J (2006). ETS transcription factor ER81 is required for the Pacinian corpuscle development. Dev. Dyn. 235, 1081–1089. [DOI] [PubMed] [Google Scholar]
- Segal RA (2003). Selectivity in neurotrophin signaling: theme and variations. Annu. Rev. Neurosci. 26, 299–330. [DOI] [PubMed] [Google Scholar]
- Sharma N, Flaherty K, Lezgiyeva K, Wagner DE, Klein AM, and Ginty DD (2020). The emergence of transcriptional identity in somatosensory neurons. Nature 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitko AA, and Goodrich LV (2021). Making sense of neural development by comparing wiring strategies for seeing and hearing. Science 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, and Barbacid M (1994). Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368, 246–249. [DOI] [PubMed] [Google Scholar]
- Smith RS, and Walsh CA (2020). Ion Channel Functions in Early Brain Development. Trends in Neurosciences 43, 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoney SD (1990). Limitations on impulse conduction at the branch point of afferent axons in frog dorsal root ganglion. Exp Brain Res 80, 512–524. [DOI] [PubMed] [Google Scholar]
- Sürmeli G, Akay T, Ippolito GC, Tucker PW, and Jessell TM (2011). Patterns of spinal sensory-motor connectivity prescribed by a dorsoventral positional template. Cell 147, 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó A, and Mayor M (2018). Mechanisms of Neural Crest Migration. 52, 43–63. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA, (2001). Neurotrophins are required for nerve growth during development. Nat Neurosci 4, 29–37. [DOI] [PubMed] [Google Scholar]
- Tuthill JC, and Azim E (2018). Proprioception. Current Biology 28, R194–R203. [DOI] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Ventéo S, Desiderio S, Cabochette P, Deslys A, Carroll P, Pattyn A, (2019). Neurog2 Deficiency Uncovers a Critical Period of Cell Fate Plasticity and Vulnerability among Neural-Crest-Derived Somatosensory Progenitors. CellReports 29, 2953–2960. [DOI] [PubMed] [Google Scholar]
- Wagner DE, Weinreb C, Collins ZM, Briggs JA, Megason SG, and Klein AM (2018). Single-cell mapping of gene expression landscapes and lineage in the zebrafish embryo. Science 360, 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcher J, Ojeda-Alonso J, Haseleu J, Oosthuizen MK, Rowe AH, Bennett NC, Lewin GR, 2018. Specialized mechanoreceptor systems in rodent glabrous skin. J. Physiol. (Lond.) 596, 4995–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li LY, Taylor MD, Wright DE, and Frank E (2007). Prenatal exposure to elevated NT3 disrupts synaptic selectivity in the spinal cord. J. Neurosci. 27, 3686–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende H, Lechner SG, Cheret C, Bourane S, Kolanczyk ME, Pattyn A, Reuter K, Munier FL, Carroll P, Lewin GR, et al. (2012). The transcription factor c-Maf controls touch receptor development and function. Science 335, 1373–1376. [DOI] [PubMed] [Google Scholar]
- Wenner P, and Frank E (1995). Peripheral target specification of synaptic connectivity of muscle spindle sensory neurons with spinal motoneurons. Journal of Neuroscience 15, 8191–8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S-H, Baba Y, Franco AM, Lumpkin EA, and Owens DM (2012). Excitatory glutamate is essential for development and maintenance of the piloneural mechanoreceptor. Development 139, 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ, Ritter AM, and Koerber HR (2001). Central anatomy of individual rapidly adapting low-threshold mechanoreceptors innervating the “hairy” skin of newborn mice: early maturation of hair follicle afferents. J. Comp. Neurol. 436, 304–323. [PubMed] [Google Scholar]
- Woodbury CJ and Koerber HR (2003). Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. J. Neurosci. 23(2): 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CJ and Koerber HR (2007). Central and peripheral anatomy of slowly adapting type I low-threshold mechanoreceptors innervating trunk skin of neonatal mice. The Journal of Comparative Neurology 505: 547–561. [DOI] [PubMed] [Google Scholar]
- Wu D, Schieren I, Qian Y, Zhang C, Jessell TM, and de Nooij JC (2019). A Role for Sensory end Organ-Derived Signals in Regulating Muscle Spindle Proprioceptor Phenotype. J. Neurosci. 39, 4252–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Petitpré C, Fontanet P, Sharma A, Bellardita C, Quadros RM, Jannig PR, Wang Y, Heimel JA, Cheung KKY, Wanderoy S, Xuan Y, Meletis K, Ruas J, Gurumurthy CB, Kiehn O, Hadjab S, Lallemend F (2021). Distinct subtypes of proprioceptive dorsal root ganglion neurons regulate adaptive proprioception in mice. Nature Communications 12, 1026–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Han B, Mendelsohn M, and Jessell TM (2006). PlexinA1 Signaling Directs the Segregation of Proprioceptive Sensory Axons in the Developing Spinal Cord. Neuron 52, 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EC, Howe CL, Li Y, Holtzman DM, and Mobley WC (1996). Nerve growth factor and the neurotrophic factor hypothesis. Brain Dev 18, 362–368. [DOI] [PubMed] [Google Scholar]
- Zampieri N, and de Nooij JC (2021). Regulating muscle spindle and Golgi tendon organ proprioceptor phenotypes. Current Opinion in Psychology 19, 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Liu P, Bai L, Trimmer JS, Bean BP, Ginty DD (2019). Deep Sequencing of Somatosensory Neurons Reveals Molecular Determinants of Intrinsic Physiological Properties. Neuron 103, 598–616.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman A, Bai L, and Ginty DD (2014). The gentle touch receptors of mammalian skin. Science 346, 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky SL, and Grueber WB (2013). The molecular basis of self-avoidance. Annu. Rev. Neurosci. 36, 547–568. [DOI] [PubMed] [Google Scholar]
- Zotterman Y (1939). Touch, pain and tickling: an electro-physiological investigation on cutaneous sensory nerves. 95, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou M, Li S, Klein WH, Xiang M, (2012). Brn3a/Pou4f1 regulates dorsal root ganglion sensory neuron specification and axonal projection into the spinal cord. Developmental Biology 364, 114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchero JB, Barres BA, (2015). Glia in mammalian development and disease. Development 142, 3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, and Anderson DJ (2005). Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 45, 17–25. [DOI] [PubMed] [Google Scholar]