Abstract
Accumulating evidence indicates that gut microbiota may regulate sex-hormone levels in the host, with effects on reproductive health. Very little is known about the development of intestinal microbiota during puberty in humans. To assess the connection between pubertal timing and fecal microbiota, and to assess how fecal microbiota develop during puberty in comparison with adult microbiota, we utilized a Finnish allergy-prevention-trial cohort (Flora). Data collected at 13-year follow-up were compared with adult data from a different Finnish cohort. Among the 13-year-old participants we collected questionnaire information, growth data from school-health-system records and fecal samples from 148 participants. Reference adult fecal samples were received from the Health and Early Life Microbiota (HELMi) cohort (n = 840). Fecal microbiota were analyzed using 16S rRNA gene amplicon sequencing; the data were correlated with pubertal timing and compared with data on adult microbiota. Probiotic intervention in the allergy-prevention-trial cohort was considered as a confounding factor only. The main outcome was composition of the microbiota in relation to pubertal timing (time to/from peak growth velocity) in both sexes separately, and similarity to adult microbiota. In girls, fecal microbiota became more adult-like with pubertal progression (p = 0.009). No such development was observed in boys (p = 0.9). Both sexes showed a trend towards increasing relative abundance of estrogen-metabolizing Clostridia and decreasing Bacteroidia with pubertal development, but this was statistically significant in girls only (p = 0.03). In girls, pubertal timing was associated positively with exposure to cephalosporins prior to the age of 10. Our data support the hypothesis that gut microbiota, particularly members of Ruminococcaceae, may affect pubertal timing, possibly via regulating host sex-hormone levels.
Trial registration The registration number for the allergy-prevention-trial cohort: ClinicalTrials.gov, NCT00298337, registered 1 March 2006—Retrospectively registered, https://clinicaltrials.gov/show/NCT00298337. The adult-comparison cohort (HELMi) is NCT03996304.
Subject terms: Endocrinology, Applied microbiology
Introduction
The onset of puberty is affected by genetic and environmental factors. Currently, variation in several hundred chromosomal loci are known to be associated with variation in the onset of puberty1, and external and internal cues such as diet2, exercise3, the amount of fat tissue4, intrauterine conditions5, psychosocial stress6, and chronic diseases7 are known to play a role.
There is rapidly growing evidence pointing to the role of gut microbiota in the regulation of growth via hormonal effects on bone growth8, widespread metabolic effects9 and effects on nutritional status10. Infant gut microbiota predict body mass index (BMI) at preschool age, and more specifically, Streptococcus and Bacteroides spp. are positively and bifidobacteria negatively connected to later BMI11. Modulation of gut microbiota in order to improve growth is routine practice in animal husbandry12. Gut microbiota may thus influence pubertal development via metabolic effects.
Gut microbiota are important regulators of circulating estrogens. Conjugated estrogen is secreted to bile, and certain gut microbes, particularly Ruminococcus and Faecalibacterium spp. secrete beta-glucuronidase, which deconjugates estrogen back to an active form13. Through the enterohepatic circulation, the deconjugated estrogens return to the systemic circulation. Several human studies have shown associations between the composition of gut microbiota and urinary and fecal estrogen levels14,15. In monkeys, a decrease of estrogen-metabolizing bacteria leads to a decrease in circulating estrogens16. In addition, dietary fiber consumption, which regulates the composition of the gut microbiota, affects the levels of serum estrogens17–19, suggesting an effect of gut microbiota on sex-hormone levels.
Sex hormones in connection with the composition of gut microbiota have been studied most thoroughly in mice, where gonadectomy changes the composition of the microbiota20, indicating an effect of sex hormones. Furthermore, it has been shown that the gut microbiota of mice influence sex-hormone levels, which in turn affects the hosts’ immune development21,22. In mice, gut microbiota undergo sex-specific changes during puberty that influence hormone levels22. Furthermore, fecal microbiota transplants (FMTs) from healthy female rats to those with polycystic ovary syndrome (PCOS) have been shown to ameliorate the condition23, indicating that the gut microbiota have a decisive impact on gonadal functioning in the host.
Accumulating evidence thus suggests that gut microbiota may have an important role in determining the timing of puberty via metabolic and hormonal effects. It is also possible that gut microbiota composition is dependent on hormonal signals. It has been reported that girls with idiopathic central precocious puberty (ICPP) have different gut microbiota compared with controls, especially as regards Ruminococcus and Gemmiger spp., which were found to be enriched in the ICPP group. The microbiota of ICPP patients were also more generally diverse and showed features that have been previously associated with the microbiota of obese individuals24.
Limited data exist on human adolescent gut microbiota. In a recent cross-sectional investigation it was discovered that the distinction in gut microbiota between sexes becomes more evident in puberty25. Differences have been found between adolescent and adult microbiota, and the amounts of bifidobacteria in particular have been found to diminish with age in several studies26–29, and age-related associations with Bacteroidetes and Firmicutes have also been reported27–29. However, significant individual variation exists in pubertal timing, and previous studies have not addressed the associations between pubertal stage and gut microbiota. Hence, we here investigated the association between intestinal microbiota and pubertal timing in humans in a well-characterized and longitudinally monitored cohort.
Methods
Participants and data sources
The study was implemented among an allergy-prevention-trial cohort including 1018 mother–child pairs where the child had a high risk of allergy30 (trial registration number NCT 00298337). The subjects randomly received a mixture of four probiotics in capsules: Lactobacillus rhamnosus GG(ATCC 53103), 5 × 109 colony-forming units (cfu); L. rhamnosus LC705(DSM 7061), 5 × 10^9 cfu; Bifidobacterium breve Bb99(DSM 13692), 2 × 108 cfu; and Propionibacterium freudenreichii ssp. shermanii JS(DSM 7076), 2 × 109 cfu, or placebo. Starting 2–4 weeks before the end of pregnancy, mothers took capsules twice daily and children from day 1–2, one daily capsule and a prebiotic oligosaccharide, or placebo for the first six months of life. The treatment had no effect on growth31.
At the age of 13 years, 960 participants were invited for a follow-up visit. For the visit, 642 participants filled in questionnaires with information on gastrointestinal symptoms and 422 participants provided a fecal sample. Growth data on 306 participants was obtained from school health-service records32. Data on lifetime antibiotic use were obtained from the drug-purchase register of the Finnish National Health Insurance scheme (Kela) for all participants (Suppl. Figure 1). Written informed consent was obtained from all subjects’ parents and/or legal guardians. The study was approved by the Helsinki University Hospital Ethics Committee. The ethical statement number is 78/13/03/03/2013.
Both growth data and fecal samples were available for 65 boys and 83 girls (total 148). This sub-set was used for the majority of the analyses. Gastrointestinal symptom data were missing for 4 boys and 2 girls and antibiotic purchase data were missing for 3 girls, so the complete data was available for 61 boys and 78 girls (total 139).
Fecal samples from adults of reproductive age were obtained from Finnish Health and Early Life Microbiota (HELMi) cohort33, and used as reference material. A total of 396 samples from women and 444 samples from men were included. The adult reference cohort consisted of generally healthy parents of newborn infants.
Determination of the timing of puberty from growth data
Reference growth-velocity data on prepubertal growth was obtained from previous work34. In total, 1127 pubertal (over eight years for girls and over 9 years for boys) measurements were available and the mean number of measurements per person was 7.6. Age at take-off of pubertal growth acceleration was defined as the age when growth velocity exceeded the age- and sex-specific mean plus 2 SD for the first time, followed by a period of accelerated growth typical for puberty. Age at peak-height velocity (APHV) was defined as the age when a participant’s growth velocity was highest during a phase of accelerated growth after eight and nine years of age in girls and boys, respectively. Only measurements that were at least six months apart were used for growth-velocity calculation.
For 11 boys and 23 girls, it was only possible to determine APHV according to the aforementioned criteria. In these cases, the age at take-off was imputed to be six months prior to APHV.
To assess the correlation between gut microbiota and the timing of puberty, two new variables were derived. First, the time between growth take-off and sample collection, and second, the time between APHV and sample collection.
Fecal sample analysis
Fecal samples were collected by the participants at home and frozen immediately. They were transported to the laboratory frozen and stored at − 80 °C until processing.
Bacterial DNA was extracted using a previously described repeated bead-beating method35 with the following modifications for automated DNA purification: ca. 125 mg of fecal material were suspended in 1 ml of sterile ice-cold PBS, and 175 μl of fecal suspension was combined with 235 μl of RBB lysis buffer (500 mM NaCl, 50 mM Tris–HCl (pH 8.0), 50 mM EDTA, 4% SDS) in a bead-beating tube from the Ambion Magmax™ Total Nucleic Acid Isolation Kit (Life Technologies, Carlsbad, CA, USA). After repeated bead-beating, 200 μl of the supernatant was used for DNA extraction with a KingFisher™ Flex automated purification system (ThermoFisher Scientific, Waltham, MA, USA) using a MagMAX™ Pathogen High Vol. Duo program. DNA was quantified using a Quanti-iT™ Pico Green dsDNA assay (Invitrogen, San Diego, CA, USA) and 1 ng was used for V3–V4-region amplicon PCR of the 16S rRNA gene as previously described36. Sequencing was carried out with Illumina HiSeq 2500 equipment in Rapid Run mode.
Statistical analysis and visualization
The sequencing reads were processed using the ProcessReads and TaxonomicTables functions in the R package mare37. The forward reads were quality and chimera filtered using USEARCH38 v 8.1 and mapped to the Silva39 reference database. After mapping the reads to the reference database, the reads were summarized at different taxonomic levels.
Statistical analysis was conducted in R40, partly using the R package mare, which relies on the R packages vegan41, nlme42, and MASS43. Plots and other figures were made with R40 using the R package ggplot244 and Microsoft Powerpoint for Mac45.
Similarity to microbiota in adults was assessed as the correlation between the log-transformed relative abundances of the bacterial taxa at species level between each participant and an adult of the same sex. The average similarity to adults for each participant was taken as a measure of microbiota maturity.
Associations between bacterial taxa and pubertal timing were analyzed using regression as described below. The number of reads per taxon was used as the response variable and the model was adjusted for probiotic use, time since the last antibiotic course, BMI and whole-grain intake as a proxy of dietary-fiber intake. The total number of reads per sample was used as an offset. For each taxon, a suitable model was identified based on standard statistical diagnostics. Initially, a generalized linear model (glm) with negative binomial distribution was fitted. In cases in which the model did not converge, a glm with Poisson distribution was fitted, and if that did not converge, finally a linear model (lm) using relative abundances instead of raw read counts was fitted (log-transformed if necessary depending on the data distribution). In cases in which there were patterns in the residuals, a generalized least squares (gls) model was fitted, also modelling the residual variation. If no model fulfilled standard statistical diagnostic criteria (normality and stochasticity of residuals), no p-value was obtained. Using this iterative approach, a suitable statistical model was identified for each taxon.
Results
Comparison of the whole allergy-prevention-trial-cohort at 13-years and the analysed cohort is presented in Suppl Table 1. The groups were reasonably similar, except for the prevalence of asthma (16.8% in whole cohort vs 7.2% in the analysed cohort). The difference was statistically significant (p = 0.004). The birth weight in whole cohort (average 3588 g) was higher compared to analyzed cohort (average 3452 g) and the difference was statistically significant (p = 0.002).
At 13 years of age, the boys, as expected, were less mature in terms of pubertal development than the girls (Suppl. Figure 2). Girls were mostly (81%) past their peak-growth velocity, while most boys (80%) had not yet reached it. Self-reported Tanner staging reflected a similar picture (Suppl. Table 2).
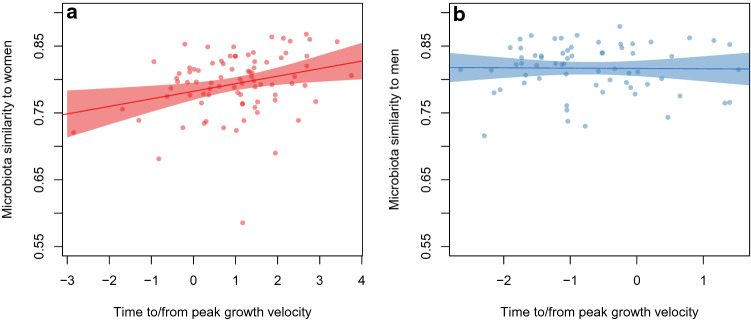
Gut microbiota were analyzed in fecal samples by extracting DNA, amplifying the 16S rRNA gene and sequencing the amplicons. To compare the compositions of gut microbiota in children and adults of the same sex, a microbiota maturity index was created. In girls, similarity to adult-female microbiota was positively correlated with pubertal progress: the more progressed the girl was in terms of puberty, the more similar were the microbiota (Fig. 1a, p = 0.009). The same was not evident as regards boys versus adult males (Fig. 1b, p = 0.9). The association between the time from growth take-off and microbiota maturity was similar to that of the time from peak growth velocity to maturity of the microbiota (girls, p = 0.01; boys, p = 0.85; Suppl. Figure 3). We tested whether antibiotic exposure, BMI, intake of probiotics, and intake of whole-grain foods (based on dietary questionnaires) could explain the association between pubertal timing and the overall similarity to microbiota in adults. However, after adjustment for each of these variables, the association remained significant in girls (p < 0.05).
Figure 1.
(a) Microbiota similarity to that in adults in relation to pubertal timing (time to/from peak growth velocity) in girls. (b) Microbiota similarity to that in adults in relation to pubertal timing (time to/from peak growth velocity) in boys.
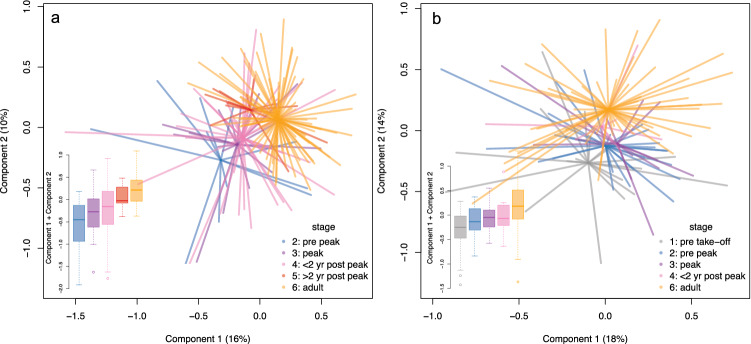
To assess the information on growth take-off and peak growth velocity, the children were divided into groups: prior to growth take-off (N = 18/0 boys/girls), prior to peak growth (post take-off, up to three months before peak, N = 27/11 boys/girls), peak growth (three months before to six months after peak, N = 13/17 boys/girls), immediately after peak growth (six months to two years after peak, N = 8/40 boys/girls) and more than two years after peak growth (N = 0/15 boys/girls). Principal coordinates analysis revealed that these groups differed in microbiota composition in both sexes, with the composition becoming more adult-like as puberty progressed, especially in girls (Fig. 2a). In girls, the summed score of the first two principal coordinates in the two post-peak groups was significantly higher than the score in the pre-peak group (< 2 years post-peak, p = 0.02; > 2 years post-peak, p = 0.0004). In boys, the same trend was evident (Fig. 2b), although nonsignificant (compared with pre-take-off: pre-peak, p = 0.09; peak, p = 0.06; < 2 years post-peak, p = 0.07).
Figure 2.
(a) Principal coordinates analysis of gut microbiota composition in girls compared with adults of the same sex. (b) Principal coordinates analysis of gut microbiota composition in boys compared with adults of the same sex.
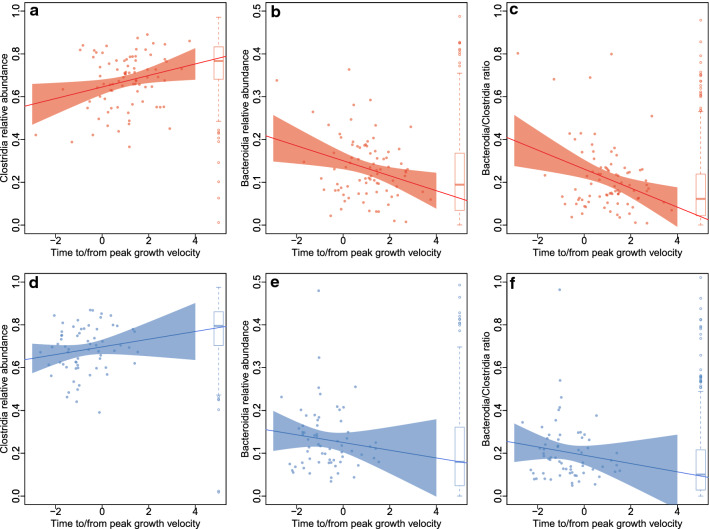
The main driver behind maturation of the microbiota in girls was a change in the relative abundances of the two most abundant classes of bacteria, Clostridiales and Bacteroidales (Fig. 3a–c). During puberty, the relative abundance of Clostridiales increased (p = 0.03, after adjusting for BMI, whole-grain intake, probiotic use and antibiotic exposure) and the relative abundance of Bacteroidales decreased (p = 0.03) towards adult-like levels. The same trends, although nonsignificant (Clostridiales, p = 0.14; Bacteroidales, p = 0.48), were evident in boys (Fig. 3d–f). At phylum level, the abundance of the Firmicutes increased with puberty progression in both sexes, significantly in girls (p = 0.01) and nonsignificantly in boys (p = 0.23), and the abundance of Bacteroidetes decreased (girls, p = 0.03; boys, p = 0.48).
Figure 3.
(a) Abundance of Clostridiales in relation to pubertal timing (time to/from peak growth velocity) in girls. (b) Abundance of Bacteroidales in relation to pubertal timing (time to/from peak growth velocity) in girls. (c) Bacteroidales/Clostridiales ratio in relation to pubertal timing (time to/from peak growth velocity) in girls. (d) Abundance of Clostridiales in relation to pubertal timing (time to/from peak growth velocity) in boys. (e) Abundance of Bacteroidales in relation to pubertal timing (time to/from peak growth velocity) in boys. (f) Bacteroidales/Clostridiales ratio in relation to pubertal timing (time to/from peak growth velocity) in boys.
At family and genus levels, the puberty-associated changes in the microbiota of girls were mainly attributable to an increase in members of Ruminococcaceae (Anaerofilum, p = 0.02; Anaerotruncus, p < 0.0001; Subdoligranulum, p = 0.02; uncultured, p = 0.002) and Lachnospiraceae (Dorea, p = 0.03; Syntrophococcus, p = 0.005) and a decrease of the order Bacteroidales (p = 0.03), especially Paludibacter (p = 0.04), Macellibacteroides (p = 0.005) and Barnesiella (p < 0.001). The same patterns were evident in boys, with only Barnesiella (p < 0.001), Macellibacteroides (p < 0.001) and uncultured Ruminococcaceae (p = 0.02) reaching statistical significance. In addition to the abundant taxa, there was a decline in low-abundance taxa Streptococcus (girls, p = 0.02; boys, p = 0.6), Lactobacillus (girls, p = 0.5; boys, p = 0.009), Coriobacteriaceae (girls, p = 0.1; boys, p = 0.04), and Escherichia (girls, p = 0.3; boys, p < 0.001).
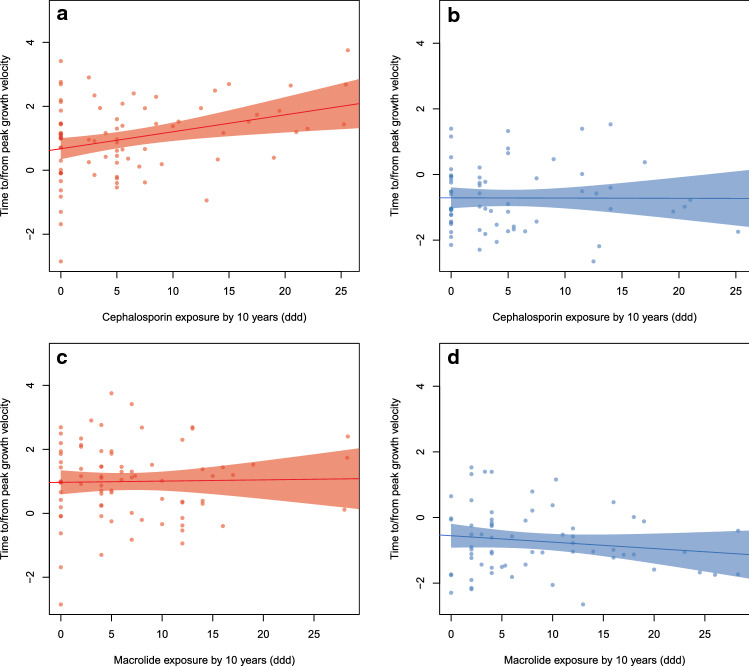
We considered the possibility that antibiotic exposure might influence the timing of puberty. For each sex, we identified the antibiotic type and timing of exposure that was most strongly associated with pubertal timing, using AIC-based model selection. For girls, the most strongly associated antibiotic variable was (first- and second-generation) cephalosporin exposure (cumulative defined daily doses, ddd) up to the age of 10 years, which was positively associated with pubertal progress (p = 0.004, Fig. 4a). In boys, cephalosporin exposure was not associated with the timing of puberty (p = 0.97, Fig. 4b). For boys, the most strongly associated variable was macrolide exposure by the age of 10 years, which showed a non-significant negative trend (p = 0.12, Fig. 4d). In girls, macrolide use was not associated with puberty (p = 0.66, Fig. 4c). We also tested for an association between BMI and pubertal timing. In both sexes, there was a modest but nonsignificant, positive trend (Suppl. Figure 4).
Figure 4.
(a) Association between exposure to cephalosporin and pubertal timing (time to/from peak growth velocity) in girls. (b) Association between exposure to cephalosporin and pubertal timing (time to/from peak growth velocity) in boys. (c) Association between exposure to macrolide, and pubertal timing (time to/from peak growth velocity) in girls. (d) Association between exposure to macrolide, and pubertal timing (time to/from peak growth velocity) in boys.
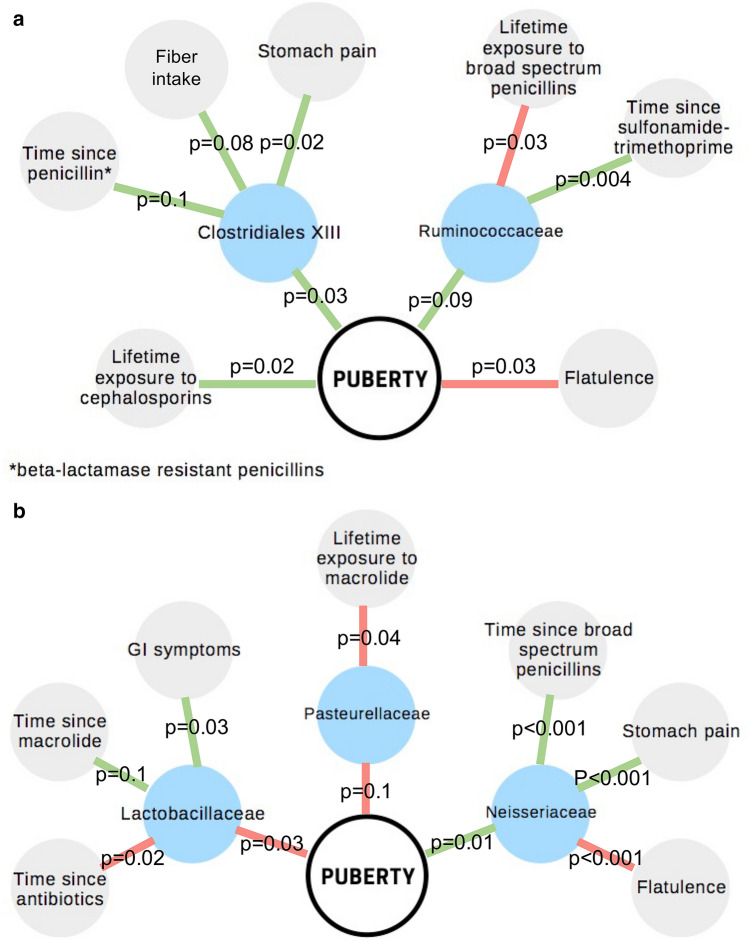
Finally, we created multivariate models for the timing of puberty, including gut microbiota at family level, detailed information on antibiotic exposure (time since last antibiotic course per antibiotic type, cumulative lifetime number of courses and ddd per antibiotic type), probiotic use, BMI, and gastrointestinal (GI) symptoms as potential confounders and explanatory variables for both the timing of puberty and for the relative abundances of the bacterial taxa. We used AIC-based model selection to arrive at the final model. Among girls, pubertal timing was associated positively with the abundance of Clostridiales family XIII bacteria and Ruminococcaceae, both members of the Clostridia class (Fig. 5a). In addition, in a multivariate model, timing of puberty was associated positively with lifetime exposure to cephalosporins and negatively with self-reported flatulence. In boys, a very different picture emerged. Timing of puberty was associated negatively with Lactobacillaceae and Pasteurellaceae and positively with Neisseriaceae (Fig. 5b). The latter taxa were associated with antibiotic exposure and with GI symptoms. In boys, the negative association between puberty and flatulence appeared to be mediated by Neisseriaceae.
Figure 5.
(a) Multivariate model for pubertal timing in girls. (b) Multivariate model for pubertal timing in boys.
Discussion
In this work we aimed to investigate the relationship between the timing of puberty and gut microbiota. Our results show, for the first time, that gut microbiota shifts towards adult-like composition as the puberty progress. These findings suggest an association between sex hormones and gut microbiota development.
Pubertal timing in girls was associated with antibiotic use, especially cephalosporin. It is already known that antibiotic exposure, very likely via changes in the gut microbiota, is connected to higher BMI46,47, and higher BMI is connected to early puberty48. However, in our dataset BMI was not significantly associated with pubertal timing and the association with cephalosporin use was also observable when adjusting for BMI as a confounding factor. This suggests that antibiotic-driven changes in gut microbiota may be causally related to pubertal timing, independently of the metabolic effects. However, the connection was not visible in boys in our cohort.
Comparison of pubertal and adult microbiota revealed that during puberty, girls’ gut microbiota develops in the direction of adult women’s microbiota, and the level of similarity is related to the degree of pubertal development. Importantly, the trend towards adult-like microbiota was visible in the first two principal coordinates, indicating that the main dimensions of variation in the gut microbiota are affected by pubertal progression in this age group. Among boys a similar trend was observed with respect to the first two principal coordinates (together explaining 32% of total microbiota variation), but not when considering the whole microbiota composition. It is possible that the route of microbiota development is different in men compared with women, but the most probable explanation for the difference is the lack of data from boys past the period of peak growth velocity, as a result of the generally later onset of puberty in boys, resulting in too few cases in this time window.
For both sexes, the dominant taxa Clostridia, including the abundant families Ruminococcaceae and Lachnospiraceae, and Bacteroidia, including the abundant genus Bacteroides, and their ratio, approached adult levels in parallel with pubertal development. The result shows that development of the gut microbiota that begins at the period of weaning49, with increasing abundance of Clostridia, still continues through puberty. Our data show that children do not achieve an adult-like gut microbiota composition before the later stages of puberty.
The increasing relative abundance of Clostridia with pubertal development is supported by earlier findings. An Italian study revealed a lower abundance of Bacteroidetes and a higher abundance of Firmicutes in normal-weight adults compared with normal-weight adolescents, but the pattern was opposite in obese individuals 29. In contrast, American study reported a higher abundance of Bacteroidetes in adults compared with 7- to 12-year-olds 28, corresponding to the pattern seen in obese Italians. The weight status of the participants in the American study was not reported. A Dutch study in which 6- to 9-year-old children were compared with adults showed a lower abundance of Bacteroides and higher abundances of Ruminococcus, Eubacterium and Clostridium species in adults50. We had mostly normal-weight individuals, with only four obese girls and five obese boys. Owing to small numbers it was not possible to reliably address whether or not gut microbiota development was different between obese and normal-weight adolescents, but adjusting for BMI in the models did not alter the results. It is possible that obesity or, for example, a high-fat diet could alter microbiota progression during puberty. In contrast to our findings, a recent Chinese study showed a higher abundance of Clostridia in 5-year-olds compared with 15-year-olds51. In the 15-year-olds, several genera were found to be associated with the level of serum testosterone, but estradiol concentrations were not associated with microbiota composition51.
Previous studies on the association between gut microbiota and reproductive stage in humans have shown that at the end of female reproductive life, the pattern that we observed reverses again, with increasing abundance of Bacteroidetes and decreasing abundances of Firmicutes during menopause52. Together, these results strongly indicate that sex hormones are a major driver of gut microbiota composition, particularly affecting the balance between the two major phyla, Firmicutes and Bacteroidetes. Furthermore, Bacteroides spp. were positively and Ruminococcus spp. negatively correlated with testosterone levels in a cohort of women with and without polycystic ovary syndrome53. Several Ruminococcaceae OTUs (operational taxonomic units) were at a lower abundance in women with polycystic ovary syndrome53, suggesting that their abundance is dependent on normal gonadal function of the host.
Several types of Clostridia, especially species of the Ruminococcaceae genera Faecalibacterium and Ruminococcus, are known to reactivate inactive conjugated forms of estrogen via their beta-glucuronidase activity13. The beta-glucuronidase enzymes of Ruminococcus and Faecalibacterium spp. have the ability to cleave both estrone and estradiol, while the enzyme of Bacteroides fragilis shows catalytic activity only to estrone—orders of magnitude weaker than that of the Clostridial enzymes13. The relative abundance levels of members of the Ruminococcaceae family have been positively correlated with levels of circulating estrogens14. In accordance with the higher estrogen-metabolizing activity of Ruminococcus spp., the ratio of estrogen metabolites to estrogen parent compounds in urine has been shown to correlate positively with the relative abundance of Ruminococcus and negatively with that of Bacteroides spp.15. Based on these data and our current results, it is plausible that gut microbiota may partly regulate the onset of puberty and menopause via their estrogen metabolism. This hypothesis suggests that microbiota-targeting treatments may be effective in regulating estrogen levels. Indeed, increased consumption of dietary fiber reduces the levels of circulating estrogens18. The effect has been shown to be caused specifically by wheat bran, but not by oat or corn bran19. Notably, wheat-bran consumption decreases the relative abundance of Ruminococcaceae, which are specialized in resistant starch fermentation17.
In addition to their estrogen metabolism, Clostridia and Bacteroidia, the two dominant bacterial classes in the adult human gut, have widespread and varying effects on host physiology9. The families Lachnospiraceae and Ruminococcaceae contain the most important butyrate producers, which are adapted to fermenting complex plant-based carbohydrates and are generally considered important for a healthy gut9. Members of the Ruminococcaceae family have been associated with healthy growth in a cohort of undernourished young children10.
While the capacity of specific gut microbes to metabolize estrogen suggests that gut microbiota may regulate puberty, the reverse is also possible. Sex hormones might affect maturation of the microbiota by directly affecting the growth of specific taxa, by influencing immune reactions to gut microbes, by affecting bile-acid composition20, or in some as yet uncharacterized manner. More investigation is warranted to better understand the underlining mechanisms.
There are some limitations in our study. Our investigation did not include hormone level measurements, and so the pubertal timing is based on the changes in growth velocity only. For boys 13 years-of-age was probably not the optimal time point to investigate puberty-related changes in gut microbiota, likely resulting in weaker results than in the girls.
The sample size in this study was substantially smaller than the whole 13-year follow-up sample. This was due to lack of growth and microbial data. The main reason for the limited availability of the growth data is, that some of the participants have moved away from the capital-city region of Finland, and therefore we couldn’t attain the growth information from the school health-service records of the local municipalities. In addition, many participants were unwilling to provide faecal samples, which contributed to the reduced sample size. However, it is unlikely that willingness to provide a faecal sample or continued residence in the capital region would confound the association between puberty and gut microbiota.
Our study population was selected on the basis of its high risk of allergic disease—they have at least one parent with atopic disease. The population was collected from a relatively limited geographic area in the capital-city region of Finland (population 1.5 million inhabitants and population density 800–3000 inhabitants/km2), 50% living in urban, 40% suburban and 10% in rural areas. These factors may affect the generalization of our findings.
Conclusion
Previously very little was known about the development of human gut microbiota in puberty. Our investigation opens interesting insights to the connection of the gut microbiota and pubertal development. Our data shows, that the development is sex-specific. Gut microbiota were connected to pubertal timing on girls, but not on boys, possible due to later development pattern on boys. The timing of puberty may be affected by the gut microbiota, particularly members of Ruminococcaceae, which may affect the timing via regulating host sex-hormone levels. Although we didn’t have hormonal data, growth curve analysis is a valid method to estimate the timing of puberty, even in the absence of serial measurements of gonadotropin and sex steroid levels. Pubertal timing on girls correlates with prepubertal antibiotic usage. The effect of lifestyle factors on pubertal timing may be mediated via changes in gut microbiota. More investigation is warranted to evaluate the generalizability of the findings and to clarify the cause-and-effect relations.
Ethics approval and consent to participate
All experiments were performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from all subjects’ parents and/or legal guardians. The study was approved by the Helsinki University Hospital Ethics Committee. The ethical statement number is 78/13/03/03/2013.
Supplementary Information
Acknowledgements
We thank the study participants and research nurse Rhea Paajanen, and Nick Bolton for language editing.
Author contributions
K.K. and S.K. analyzed the data and were the major contributors in writing of the manuscript. A.S. supervised and participated in the initial part of the data analysis. E.S., M.K., A.K. and S.K. collected and processed the Flora follow-up data. T.R., M.H. and P.M. provided endocrinological expertize for the analysis and manuscript text. E.K., L.K., M.S. and A.T. analyzed the growth data. T.R. and M.K. did the initial formulation of the investigation. W.M.dV., T.R. and M.K. had a major role in funding and supervision of the study. All authors contributed on the writing of the manuscript and read and approved the final manuscript.
Funding
This study was partially supported by the Biocodex Microbiota Foundation (AS), grant 1308255 (FINMIC) from the Academy of Finland (WMdV & MK), Foundation for Pediatric Research (SK, TR), Sigrid Juselius Foundation (TR) and the Spinoza Award 2008 of the Netherlands Organization for Scientific Research (WMdV).
Data availability
The raw dataset analysed during the current study is available in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB44673 (https://www.ebi.ac.uk/ena/browser/view/PRJEB44673).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Katri Korpela and Sampo Kallio.
These authors jointly supervised this work: Taneli Raivio and Mikael Kuitunen.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-02375-z.
References
- 1.Day FR, et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 2017;49(6):6. doi: 10.1038/ng.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ullah R, Raza A, Rauf N, Shen Y, Zhou Y-D, Fu J. Postnatal feeding with a fat rich diet induces precocious puberty independent of body weight, body fat, and leptin levels in female mice. Front. Endocrinol. 2019;10:758. doi: 10.3389/fendo.2019.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapczuk K. Elite athletes and pubertal delay. Minerva Pediatr. 2017;69(5):415–426. doi: 10.23736/S0026-4946.17.05044-7. [DOI] [PubMed] [Google Scholar]
- 4.Aksglaede L, Juul A, Olsen LW, Sørensen TIA. Age at puberty and the emerging obesity epidemic. PLoS ONE. 2009;4(12):e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng X, Li W, Luo Y, Liu S, Wen Y, Liu Q. Association between small fetuses and puberty timing: a systematic review and meta-analysis. Int. J. Environ. Res. Public Health. 2017;14(11):1. doi: 10.3390/ijerph14111377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XF, et al. Role of the posterodorsal medial amygdala in predator odour stress-induced puberty delay in female rats. J. Neuroendocrinol. 2019;31(6):1. doi: 10.1111/jne.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao KT, Denker M, Zacharin M, Wong SC. Pubertal abnormalities in adolescents with chronic disease. Best Pract. Res. Clin. Endocrinol. Metab. 2019;33(3):101275. doi: 10.1016/j.beem.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Yan J, et al. Gut microbiota induce IGF-1 and promote bone formation and growth. PNAS. 2016;113(47):E7554–E7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korpela K. Diet, microbiota, and metabolic health: trade-off between saccharolytic and proteolytic fermentation. Annu. Rev. Food Sci. Technol. 2018;9:65–84. doi: 10.1146/annurev-food-030117-012830. [DOI] [PubMed] [Google Scholar]
- 10.Blanton LV, et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351(6275):1. doi: 10.1126/science.aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korpela K, et al. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome. 2017;5(1):26. doi: 10.1186/s40168-017-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017;106:162–170. doi: 10.1016/j.micpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Ervin SM, et al. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019;294(49):18586–18599. doi: 10.1074/jbc.RA119.010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores R, et al. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: a cross-sectional study. J. Transl. Med. 2012;10:253. doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuhrman BJ, et al. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J. Clin. Endocrinol. Metab. 2014;99(12):4632–4640. doi: 10.1210/jc.2014-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker JM, Al-Nakkash L, Herbst-Kralovetz MM. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas. 2017;103:45–53. doi: 10.1016/j.maturitas.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Salonen A, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8(11):2218–2230. doi: 10.1038/ismej.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldin BR, et al. The effect of dietary fat and fiber on serum estrogen concentrations in premenopausal women under controlled dietary conditions. Cancer. 1994;74(3 Suppl):1125–1131. doi: 10.1002/1097-0142(19940801)74:3+<1125::aid-cncr2820741521>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Rose DP, Goldman M, Connolly JM, Strong LE. High-fiber diet reduces serum estrogen concentrations in premenopausal women. Am. J. Clin. Nutr. 1991;54(3):520–525. doi: 10.1093/ajcn/54.3.520. [DOI] [PubMed] [Google Scholar]
- 20.Org E, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7(4):313–322. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yurkovetskiy L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39(2):400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markle JGM, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 23.Guo Y, et al. Association between polycystic ovary syndrome and gut microbiota. PLoS ONE. 2016;11(4):e0153196. doi: 10.1371/journal.pone.0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong G, et al. The association of gut microbiota with idiopathic central precocious puberty in girls. Front. Endocrinol. 2019;10:1. doi: 10.3389/fendo.2019.00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan X, Chen R, Zhang Y, Lin X, Yang X. Sexual dimorphism of gut microbiota at different pubertal status. Microb. Cell Fact. 2020;19(1):152. doi: 10.1186/s12934-020-01412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, Paliy O. Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiol. Ecol. 2011;77(2):404–412. doi: 10.1111/j.1574-6941.2011.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringel-Kulka T, et al. Intestinal microbiota in healthy US young children and adults: a high throughput microarray analysis. PLoS ONE. 2013;8(5):64315. doi: 10.1371/journal.pone.0064315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollister EB, et al. Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome. 2015;3(1):36. doi: 10.1186/s40168-015-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Chierico F, et al. Gut microbiota markers in obese adolescent and adult patients: age-dependent differential patterns. Front. Microbiol. 2018;9:1210. doi: 10.3389/fmicb.2018.01210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kukkonen K, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 2007;119(1):192–198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Kuitunen M, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J. Allergy Clin. Immunol. 2009;123(2):335–341. doi: 10.1016/j.jaci.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Kallio S, Kukkonen AK, Savilahti E, Kuitunen M. Perinatal probiotic intervention prevented allergic disease in a Caesarean-delivered subgroup at 13-year follow-up. Clin. Exp. Allergy. 2019;49(4):506–515. doi: 10.1111/cea.13321. [DOI] [PubMed] [Google Scholar]
- 33.Korpela K, Dikareva E, Hanski E, Kolho K-L, de Vos WM, Salonen A. Cohort profile: Finnish Health and Early Life Microbiota (HELMi) longitudinal birth cohort. BMJ Open. 2019;9(6):e028500. doi: 10.1136/bmjopen-2018-028500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rikken B, Wit JM. Prepubertal height velocity references over a wide age range. Arch. Dis. Child. 1992;67(10):1277–1280. doi: 10.1136/adc.67.10.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salonen A, et al. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: effective recovery of bacterial and archaeal DNA using mechanical cell lysis. J. Microbiol. Methods. 2010;81(2):127–134. doi: 10.1016/j.mimet.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Korpela K, et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome. 2018;6:1. doi: 10.1186/s40168-018-0567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korpela, Katri, mare: Microbiota Analysis in R Easily. R package version 1.0. 2016. [Online]. Available: https://github.com/katrikorpela/mare
- 38.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 39.Quast C, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(D1):D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. [Online]. Available: https://www.R-project.org/
- 41.Jari Oksanen et al., vegan: Community Ecology Package. R package version 2.5-4. 2019. [Online]. Available: https://CRAN.R-project.org/package=vegan
- 42.Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D. & R Core Team, _nlme: Linear and Nonlinear Mixed Effects Models_. R package version 3.1-137. 2018. [Online]. Available: https://CRAN.R-project.org/package=nlme
- 43.Venables, W. N., & Ripley, B. D., Modern Applied Statistics with S. Fourth Edition. Springer, New York. ISBN 0-387-95457-0 (2002).
- 44.Wickham, H., ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016. [Online]. Available: https://ggplot2.tidyverse.org
- 45.Microsoft Powerpoint for Mac. 2020. [Online]. Available: https://www.microsoft.com
- 46.Stark CM, Susi A, Emerick J, Nylund CM. Antibiotic and acid-suppression medications during early childhood are associated with obesity. Gut. 2019;68(1):62–69. doi: 10.1136/gutjnl-2017-314971. [DOI] [PubMed] [Google Scholar]
- 47.Block JP, et al. Early antibiotic exposure and weight outcomes in young children. Pediatrics. 2018;142(6):1. doi: 10.1542/peds.2018-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L-K, et al. Trajectory of body mass index from ages 2 to 7 years and age at peak height velocity in boys and girls. J. Pediatr. 2020 doi: 10.1016/j.jpeds.2020.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korpela K, de Vos WM. Early life colonization of the human gut: microbes matter everywhere. Curr. Opin. Microbiol. 2018;44:70–78. doi: 10.1016/j.mib.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Zhong H, et al. Impact of early events and lifestyle on the gut microbiota and metabolic phenotypes in young school-age children. Microbiome. 2019;7(1):2. doi: 10.1186/s40168-018-0608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan X, Chen R, Zhang Y, Lin X, Yang X. Gut microbiota: effect of pubertal status. BMC Microbiol. 2020;20:1. doi: 10.1186/s12866-020-02021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos-Marcos JA, et al. Influence of gender and menopausal status on gut microbiota. Maturitas. 2018;116:43–53. doi: 10.1016/j.maturitas.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Liu R, et al. Dysbiosis of Gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front. Microbiol. 2017;8:1. doi: 10.3389/fmicb.2017.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw dataset analysed during the current study is available in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB44673 (https://www.ebi.ac.uk/ena/browser/view/PRJEB44673).