Abstract
Background
A total of 30 000 people are treated with pelvic radiotherapy annually in the UK. Rectal bleeding is common following pelvic radiotherapy and one of the main causes is radiation proctopathy (RP). Six per cent develop severe bleeding from RP, leading to anaemia requiring iron +/− blood transfusion. There are very few safe, effective, evidence-based treatments. Purastat is a haemostatic agent licensed for gastrointestinal bleeding. It is a self-assembling peptide that forms a molecular mesh in contact with blood, thereby sealing blood vessels. There are numerous studies showing its efficacy and safety in various surgical/endoscopic settings. This service evaluation reports the first experience of the use of Purastat in RP.
Methods
Consecutive patients attending pelvic radiation disease clinic with severe refractory RP were offered treatment with Purastat. This was defined as rectal bleeding into the pan±anaemia with no response to rectal sucralfate. Purastat was applied endoscopically at four weekly intervals up to three times, with more as required. Bleeding severity, endoscopic grade and haemoglobin were recorded.
Results
Twenty-one patients were treated (18 men, median age 76 years) with a median of three treatments. Ten were on antithrombotics, 1 had thrombocytopenia and 13 had anaemia at baseline. Median episodes of bleeding reduced from 4.5 (0–27) to 2 (0–16) in the 7 days prior to the first and third treatment, respectively. Endoscopic grade was improved. Mean haemoglobin increased from 116.0 to 122.7. There were no complications.
Conclusion
Even in this cohort of severe refractory RP, there was an improvement in bleeding and endoscopic grade with Purastat. A randomised controlled trial is planned.
Keywords: radiation enteritis, anorectal disorders, angiodysplasia, gastrointestinal bleeding, endoscopy
Summary.
What is already known on the subject?
Six per cent of patients who have had pelvic radiotherapy will develop severe, debilitating bleeding from radiation proctopathy (RP), often leading to anaemia requiring iron replacement or blood transfusion.
There are very few safe, effective, evidence-based treatments available for RP based on well-designed prospective or randomised controlled trials.
Purastat is an endoscopically delivered haemostatic agent licensed as a medical device to treat bleeding in the gut, including RP. It is a hydrogel containing four peptides, which forms a molecular mesh in contact with blood. This closes damaged blood vessels, thereby stopping the bleeding.
Purastat is safe with no reported side effects.
What are the new findings?
Endoscopically delivered Purastat appears to be a promising treatment for RP and is licensed for this indication.
Even in this service evaluation of the most severe cases referred to a tertiary pelvic radiation disease clinic, there was an improvement in rectal bleeding, endoscopic grade and haemoglobin concentration, with five out of six patients no longer transfusion dependent after treatment.
Summary.
How might this impact on clinical practice in the foreseeable future?
This has the potential to revolutionise treatment for RP. Endoscopic delivery of Purastat is a simple technique that could be used widely and this medical device is already licensed for RP. A randomised controlled trial is planned to establish the role of Purastat in this patient group to address this ongoing significant area of unmet clinical need and to reduce associated morbidity and healthcare costs. This could lead to the widespread adoption of this treatment in clinical care within the next 5 years.
Introduction
In Europe, an estimated 500 000 people are treated with pelvic radiotherapy annually,1 30 000 of which are treated in the UK.2 Half of the patients develop gastrointestinal (GI) symptoms that adversely affect quality of life.3 Rectal bleeding affects up to 56% of patients following pelvic radiotherapy.4–6 One of the main causes of this is radiation proctopathy (RP).
RP is caused directly by the effect of radiation on the rectum. The rectum is fixed in the pelvis and so receives a high dose of radiation during pelvic radiotherapy. Radiation causes rectal ischaemia, which leads to fibrosis and the development of telangiectasia,7 which are the sources of the bleeding.
Six per cent of patients will develop severe bleeding from RP,8 often leading to anaemia requiring iron replacement or blood transfusion. There are very few safe, effective, evidence-based treatments available for RP based on well-designed prospective or randomised controlled trials. These comprise rectal sucralfate,9–11 radiofrequency ablation12 and hyperbaric oxygen.13–15 There are significant safety concerns regarding argon plasma coagulation, which has a 3%–40% complication rate including ulceration, perforation, strictures and fistulae.16–18 Rectal formalin can reduce bleeding19 but there are no long-term safety data. As such RP remains a significant area of unmet clinical need.
Purastat (3D Matrix, Tokyo, Japan) is an endoscopically delivered haemostatic agent licensed as a medical device to treat bleeding in the gut, including RP. It is a hydrogel containing four peptides that form a molecular mesh in contact with blood. This closes damaged blood vessels, thereby stopping the bleeding.20 21 It breaks down into amino acids, which can be used to repair the site of injury.22 Purastat is safe with no reported side effects.
Animal and human studies have shown the safety and efficacy of Purastat in haemostasis and healing during various surgical and endoscopic scenarios.21 23–25 We present the first data for the use of endoscopically delivered Purastat for haemorrhagic RP.
Patients/materials and methods
This was a service evaluation of 21 patients with severe refractory RP treated with endoscopically delivered Purastat from June 2018 to September 2019. Severe refractory RP was defined as rectal bleeding into the toilet bowl±anaemia with no response to rectal sucralfate (ongoing bleeding, recurrence of bleeding and/or unable to use due to mobility issues), our first-line treatment. All had full colonic evaluation (colonoscopy or CT colonogram) to exclude other causes of GI bleeding. Purastat was applied at four weekly intervals up to three times, with further treatments as determined by symptoms.
Bowel preparation was with phosphate enema or Moviprep (one sachet). A limited sigmoidoscopy was performed. A thin layer of Purastat was applied via a through-channel catheter, targeted to any active bleeding points preferentially, then to other areas of telangiectasia.
Severity of bleeding was recorded using patient-reported diaries completed during the 7 days prior to each treatment and physician-reported rectal bleeding score (online supplemental figure 1) at each treatment. Severity of RP was graded using the Zinicola score26 (online supplemental figure 2). Data were also collected on haemoglobin concentration and transfusion requirement.
flgastro-2020-101735supp001.pdf (68.5KB, pdf)
As a service evaluation of an intervention being used within its licence, no ethical approval was required. Purastat is licensed for treatment of bleeding from small vessels in the GI tract, including bleeding from the telangectasia of RP (3D Matrix regulatory department, the Medicines and Healthcare products Regulatory Agency devices team).
Patients were consented to sigmoidoscopy and Purastat delivery during the endoscopy admission as per standard practice, with no additional risk above the standard risks for sigmoidoscopy.
Results
Twenty-one patients were treated with endoscopically delivered Purastat (18 men; 17 prostate, 2 vaginal, 2 rectal; median age 76 years (range 47–84)). Baseline characteristics, including coexistent GI symptoms, are shown in table 1. All coexisting GI symptoms were investigated as per British Society of Gastroenterology guidelines27 and treated with either targeting treatments or symptomatic treatments (eg, loperamide, fybogel) to optimise bowel function.
Table 1.
Baseline patient characteristics
| Age (years) | 76 (47–84) |
| Gender | Male: 18 Female: 3 |
| Primary tumour | Prostate: 17 Vaginal: 2 Rectal: 2 |
| Previous treatments for RP | Rectal sucralfate: 16 Hyperbaric oxygen: 2 Argon plasma coagulation: 4 |
| Exacerbating medications | Warfarin: 2 Aspirin: 4 Aspirin and clopidogrel: 1 Rivaroxiban: 2 Apixaban: 1 |
| Exacerbating conditions | Cirrhosis with thrombocytopaenia: 1 |
| Coexistent GI symptoms | Nil: 8 Bowel urgency: 4 Faecal incontinence: 6 Diarrhoea: 7 Constipation: 4 Abdominal pain: 3 Bloating: 4 Nausea: 1 Weight loss: 1 |
| Anaemia at baseline | 13 |
|
Treatment for anaemia
(baseline) |
Oral iron: 12 IV iron: 5 Blood transfusion: 6
|
GI, gastrointestinal; RP, radiation proctopathy.
Primary cancer and cancer treatment details are shown in table 2. Median number of Purastat treatments was 3 (range 2–7). Median Purastat amount used was 5 mL (range 3–5 mL). There were no complications.
Table 2.
Cancer treatment details
| Patient | Stage | Grade | Cancer | Radiotherapy | Other treatments |
| 1 | T3A N0 M0 | No biopsy | Prostate | 60 Gy/20# | 6 months bicalutamide |
| 2 | T2c N0 M0 | Gleason 3+3 | Prostate | 60 Gy/20# | Nil |
| 3 | T2 N0 M0 | Gleason 3+4 | Prostate | 60 Gy/20# | 6 months LHRH antagonist |
| 4 | T2b N0 M0 | Gleason 4+3 | Prostate | 60 Gy/20# | 7 months LHRH antagonist |
| 5 | T2 N0 M0 | Grade 2 | Rectal | NK | Perianal resection of rectal lesion |
| 6 | T1c N0 M0 | Gleason 3+3 | Prostate | 57 Gy/19# | 3 months LHRH antagonist |
| 7 | Stage 1B with positive margins and lymphovascular invasion | Grade 3 | Vaginal | 45 Gy/25# | 2 x HDR brachytherapy |
| 8 | T1c N0 M0 | Gleason 3+4 | Prostate | 60 Gy/20# | 6 months LHRH antagonist |
| 9 | TXN0 | Grade 2 | Rectal | 25 Gy/5# | Papillon topical radiotherapy |
| 10 | T2b N0 M0 | Gleason 3+4 | Prostate | 60 Gy/20# | 7 months LHRH antagonist |
| 11 | Stage 2 | Grade 3 | Vaginal | 60 Gy/30# | Cisplatin x2 Early cessation due to toxicity |
| 12 | NK | NK | NK | NK | NK |
| 13 | T2a N0 M0 | Gleason 4+5 | Prostate | 60 Gy/20# | 25 months LHRH antagonist |
| 14 | T2c N0 M0 | Gleason 3+3 | Prostate | 72 Gy/32# | 6 months LHRH antagonist |
| 15 | T1c N0 M0 | Gleason 3+4 | Prostate | 60 Gy/20# | Nil |
| 16 | T4 N0 M0 | Gleason 3+3 | Prostate | 50 Gy/16# | Six cycles Docetaxel chemo pre-XRT in STAMPEDE trial 15 months LHRH antagonist |
| 17 | T2 N0 Mx | Gleason 3+4 | Prostate | NK | LHRH antagonist |
| 18 | T3b N0 M0 | Gleason 4+3 +5 | Prostate | 52.5 Gy/20# Prostate bed |
Radical prostatectomy |
| 19 | T3a N0 M0 | Gleason 4+3 | Prostate | 60 Gy/20# | 3 months LHRH antagonist |
| 20 | T2c N0 M0 | Gleason 3+4 | Prostate | 60 Gy/20# | Nil |
| 21 | T2 N0 M0 | Gleason 4+5 | Prostate | 60 Gy/20# | 6 months LHRH antagonist |
Data not obtainable from treating sites.
HDR, High dose-rate brachytherapy; LHRH, Luteinising hormone-releasing hormone; NK, not known; STAMPEDE, Systemic therapy in advancing or metastatic prostate cancer: evaluation of drug efficacy; XRT, Radiotherapy.
Anticoagulation
Ten patients were on anticoagulation or antiplatelets during the service evaluation, 9 of which were on treatment at the time of first pelvic radiation disease clinic review. Of these nine patients, five continued on their anticoagulation or antiplatelets following risk assessment (two of which were on warfarin for prosthetic valves); two stopped their treatment completely and two switched treatment from an anticoagulant to aspirin. One patient started rivaroxaban after referral to pelvic radiation disease clinic for the indication of acute deep vein thrombosis, but this was stopped due to worsening rectal haemorrhage.
Rectal bleeding
There was an improvement in rectal bleeding as determined by 7-day patient-reported bleeding diaries and physician-reported rectal bleeding score (table 3). Number of episodes of rectal bleeding into the toilet bowl reduced from a median of 4.5 (range 0–27) to 2 (range 0–16) from the 7 days prior to the first treatment to the 7 days prior to the third treatment. Eight patients had no rectal bleeding following treatment (38%) and 14 patients had a reduction in rectal bleeding episodes (67%) as determined by 7-day patient-reported bleeding diary.
Table 3.
Rectal bleeding outcomes
| First Treatment | Second Treatment | Third Treatment | |
| Patient-reported episodes of rectal bleeding into the toilet bowl* | |||
| Median rectal bleeding episodes (range) | 4.5 (0–27) | 1.5 (0–35) | 2 (0–16) |
| Physician-reported rectal bleeding score | |||
| Median total rectal bleeding score | 9 | 6 | 6.5 |
| Median rectal bleeding subset score | 3 | 2 | 2 |
| Median endoscopic subset score | 6 | 3 | 3 |
| Mean Hb concentration | |||
| Hb concentration (g/L) | 116.0 | 119.7 | 122.7 |
*Median number of episodes of fresh rectal bleeding into the toilet bowl reported in a diary for the 7 days preceding each Purastat treatment.
Hb, haemoglobin.
Endoscopic grade
Endoscopic grade, as determined by Zinicola score,26 improved in 12 patients, with no change in 9, 4 of whom had the lowest grade possible throughout treatment (grade A). Figure 1 shows the endoscopic appearance prior to and 4 weeks after the first Purastat treatment for one of the service evaluation patients. Five of those whose endoscopic grade remained stable had a reduction in rectal bleeding episodes as determined by 7-day patient-reported bleeding diary, with the rest with an unchanged number of rectal bleeding episodes. No patients within this service evaluation had macroscopic ulceration at endoscopy. Table 4 shows the change in Zinicola endoscopic score from baseline to final treatment.
Figure 1.
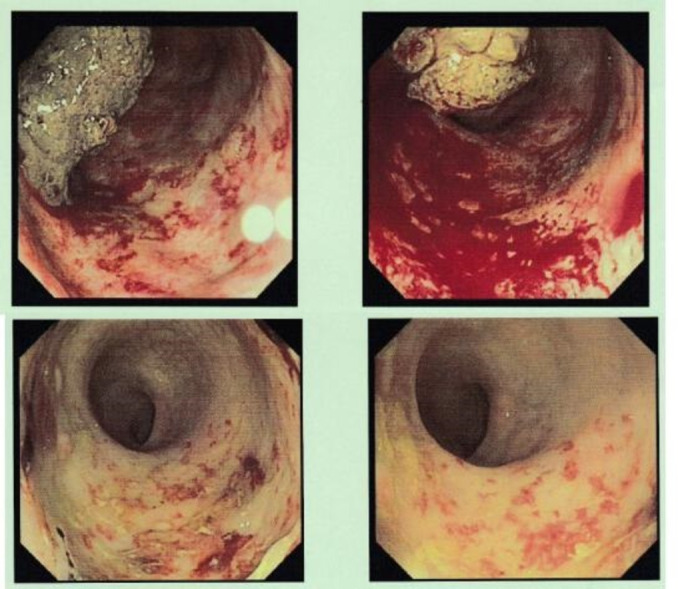
Effect of Purastat on radiation proctopathy. Top: endoscopic image prior to first Purastat treatment; bottom: endoscopic image 4 weeks postfirst Purastat treatment.
Table 4.
Change in Zinicola endoscopic score
| Number of patients | |||
| Grade A (least severe) |
Grade B | Grade C (most severe) |
|
| Baseline | 4 | 8 | 9 |
| Final Purastat treatment | 9 | 9 | 3 |
Haemoglobin concentration and transfusion requirements
Mean haemoglobin increased by 3.7 g/L between baseline to postthird treatment values and by 6.7 g/L between baseline and follow-up.
Prior to Purastat treatment, the range of units of blood transfusion required was 1–8 units (table 1). Of the six patients who had previously required blood transfusion, four required no further blood transfusions and two have required blood transfusions after starting Purastat treatment. Of these two, one has thrombocytopenia secondary to cirrhosis and one had a recurrence of severe bleeding due to recommencement of aspirin monotherapy for significant vascular disease. This has subsequently been stopped.
Long-term follow-up
The median time from first Purastat treatment to final follow-up information is 12 months (range 3–18). For those patients >12 months beyond their first Purastat treatment, only one has had recurrence of significant bleeding. Of the 16 patients who have been seen in clinic following Purastat treatment, 14 have had a marked improvement in their bleeding in terms of volume and frequency, both subjectively (patient-reported in clinic) and according to 7-day patient-reported bleeding diaries.
Discussion
RP remains a significant area of unmet clinical need in which patients often suffer debilitating symptoms for prolonged periods of time due to a paucity of safe, effective, evidence-based treatments. This service evaluation shows that endoscopically delivered Purastat reduced rectal bleeding in this group of patients with severe RP, along with improved endoscopic severity and haemoglobin concentration, and reduced blood transfusions.
This service evaluation has highlighted the lack of reliable tools with which to measure improvements and outcomes in RP. There are limitations in endoscopic grading tools. We report the Zinicola score25 in this service evaluation as we found it the best at picking up changes. The authors used other endoscopic severity scores, that is, Vienna proctoscopy score28 and the rectal telangiectasia score.29 We found that these two scores did not pick up changes well in this severe symptomatic group, that is, the highest score on the scales was very broad and not responsive enough to change. The highest scores encompassed this cohort of patients’ endoscopic findings even when there was symptomatic and endoscopic improvement. We found that the Vienna proctoscopy score was also not helpful as the endoscopic findings in this cohort were mucosal pallor and telangiectasia so the domains relating to oedema, ulceration, necrosis were not relevant.
The ideal would be to have a more objective measure of severity of RP. Unfortunately at present, there are no established methods with which to do this. Biopsies are high risk in the irradiated rectum due to concern regarding fistulation. Newer technologies exist to perform ‘optical’ biopsies, including Cellvizio. This technology is still a research tool and has not been studied in RP yet. This does offer potential solutions to objective assessments of severity and healing in future intervention studies.
The other issue is how to measure the severity of rectal bleeding objectively. We gathered data on episodes of bleeding on wiping, into the pan and passage of clots. On discussion with the patients and review, we presented the data on number of episodes of rectal bleeding into the pan over a 7-day period as being reflective of the most severe bleeding and the type of bleeding most likely to reduce quality-of-life. This was not, however, sensitive to changes in volume or amount of rectal bleeding, which is not easily objectively measured. Many of the patients did report that their rectal bleeding was less ‘heavy’ than before, but this is challenging to quantify.
The final issue is what the best primary outcome measure would be for intervention studies for RP. We feel that patient-reported rectal bleeding is the best primary outcome measure as it is the one cited by patients as having the greatest impact on quality-of-life. Endoscopic grade, regardless of the specific grading system being used, is a coarse tool at best and haemoglobin concentration is often confounded by treatment for anaemia.
While these initial data appear promising, this is a small service evaluation, which, by definition, has no control group and the results cannot be extrapolated. Questions remain that would be best addressed in a robust randomised controlled trial. While it appears that endoscopically delivered Purastat is effective at treating RP, the duration of response is neither known, nor the ideal treatment regime. The timings of treatments used in this service evaluation were chosen pragmatically. There are limited long-term data to determine whether the treatment effect is sustained.
There are also cost considerations. A sigmoidoscopy costs £488, with the additional cost of Purastat (3 mL at £250, 5 mL at £430, designated catheter £18). These costs, however, need to be balanced against the healthcare costs of uncontrolled haemorrhagic RP, for example, transfusion, iron therapy, repeated endoscopic evaluation and healthcare visits (GP, hospital appointments, Emergency Department attendances and inpatient stays). Formal health economic analysis is planned in future randomised trials.
In conclusion, endoscopically delivered Purastat appears to be a promising treatment for RP and is licensed for this indication. Even in this group of the most severe cases, there was an improvement in rectal bleeding, endoscopic grade and haemoglobin concentration, with five out of six transfusion-dependent patients becoming no longer transfusion dependent after treatment. A randomised controlled trial is planned to determine the safety and efficacy of Purastat in this patient group to address this ongoing significant area of unmet clinical need and reduce associated morbidity and healthcare costs.
Acknowledgments
Thanks to the Gastroenterology and Endoscopy Department at Wythenshawe Hospital.
Footnotes
Correction notice: This article has been corrected since it published online first. The provenance and peer review statement has been included.
Contributors: CCH planned the service evaluation, delivered the Purastat treatments, obtained the information and contributed to the writing of this paper. KW collated the data and summarised it and contributed to the writing of this paper.
Funding: This is a service evaluation of treatments provided via standard NHS care.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data are included in the paper.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Borras JM, Lievens Y, Barton M, et al. How many new cancer patients in Europe will require radiotherapy by 2025? an ESTRO-HERO analysis. Radiother Oncol 2016;119:5–11. 10.1016/j.radonc.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 2. Davidson SE. Number of people receiving pelvic radiotherapy in th UK estimated using the Christie NHS Foundation trust data, 2013. [Google Scholar]
- 3. Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. Lancet Oncol 2007;8:1007–17. 10.1016/S1470-2045(07)70341-8 [DOI] [PubMed] [Google Scholar]
- 4. Crook J, Esche B, Futter N. Effect of pelvic radiotherapy for prostate cancer on bowel, bladder, and sexual function: the patient's perspective. Urology 1996;47:387–94. 10.1016/S0090-4295(99)80458-0 [DOI] [PubMed] [Google Scholar]
- 5. Widmark A, Fransson P, Tavelin B. Self-Assessment questionnaire for evaluating urinary and intestinal late side effects after pelvic radiotherapy in patients with prostate cancer compared with an age-matched control population. Cancer 1994;74:2520–32. [DOI] [PubMed] [Google Scholar]
- 6. Dearnaley DP, Hall E, Lawrence D, et al. Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer 2005;92:488–98. 10.1038/sj.bjc.6602301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 2006;6:702–13. 10.1038/nrc1950 [DOI] [PubMed] [Google Scholar]
- 8. Gami B, Harrington K, Blake P, et al. How patients manage gastrointestinal symptoms after pelvic radiotherapy. Aliment Pharmacol Ther 2003;18:987–94. 10.1046/j.1365-2036.2003.01760.x [DOI] [PubMed] [Google Scholar]
- 9. Kochhar R, Patel F, Dhar A, et al. Radiation-Induced proctosigmoiditis. prospective, randomized, double-blind controlled trial of oral sulfasalazine plus rectal steroids versus rectal sucralfate. Dig Dis Sci 1991;36:103–7. 10.1007/BF01300096 [DOI] [PubMed] [Google Scholar]
- 10. Grigsby PW, Pilepich MV, Parsons CL. Preliminary results of a phase I/II study of sodium pentosanpolysulfate in the treatment of chronic radiation-induced proctitis. Am J Clin Oncol 1990;13:28–31. 10.1097/00000421-199002000-00008 [DOI] [PubMed] [Google Scholar]
- 11. Kochhar R, Sriram PV, Sharma SC, et al. Natural history of late radiation proctosigmoiditis treated with topical sucralfate suspension. Dig Dis Sci 1999;44:973–8. 10.1023/A:1026612731210 [DOI] [PubMed] [Google Scholar]
- 12. McCarty TR, Garg R, Rustagi T. Efficacy and safety of radiofrequency ablation for treatment of chronic radiation proctitis: a systematic review and meta-analysis. J Gastroenterol Hepatol 2019;34:1479–85. 10.1111/jgh.14729 [DOI] [PubMed] [Google Scholar]
- 13. Clarke RE, Tenorio LMC, Hussey JR, et al. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys 2008;72:134–43. 10.1016/j.ijrobp.2007.12.048 [DOI] [PubMed] [Google Scholar]
- 14. Sidik S, Hardjodisastro D, Setiabudy R, et al. Does hyperbaric oxygen administration decrease side effect and improve quality of life after pelvic radiation? Acta Med Indones 2007;39:169–73. [PubMed] [Google Scholar]
- 15. Glover M, Smerdon GR, Andreyev HJ, et al. Hyperbaric oxygen for patients with chronic bowel dysfunction after pelvic radiotherapy (HOT2): a randomised, double-blind, sham-controlled phase 3 trial. Lancet Oncol 2016;17:224–33. 10.1016/S1470-2045(15)00461-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Canard J-M, Védrenne B, Bors G, et al. [Long term results of treatment of hemorrhagic radiation proctitis by argon plasma coagulation]. Gastroenterol Clin Biol 2003;27:455–9. [PubMed] [Google Scholar]
- 17. Villavicencio RT, Rex DK, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc 2002;55:70–4. 10.1067/mge.2002.119877 [DOI] [PubMed] [Google Scholar]
- 18. Ravizza D, Fiori G, Trovato C, et al. Frequency and outcomes of rectal ulcers during argon plasma coagulation for chronic radiation-induced proctopathy. Gastrointest Endosc 2003;57:519–25. 10.1067/mge.2003.144 [DOI] [PubMed] [Google Scholar]
- 19. van de Wetering FT, Verleye L, Andreyev HJN, et al. Non‐surgical interventions for late rectal problems (proctopathy) of radiotherapy in people who have received radiotherapy to the pelvis. Cochrane Database of Systematic Reviews 2016;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhao X, Pan F, Lu JR. Recent development of peptide self-assembly. Progress in Natural Science 2008;18:653–60. 10.1016/j.pnsc.2008.01.012 [DOI] [Google Scholar]
- 21. Uraoka T, Ochiai Y, Fujimoto A, et al. A novel fully synthetic and self-assembled peptide solution for endoscopic submucosal dissection-induced ulcer in the stomach. Gastrointest Endosc 2016;83:1259–64. 10.1016/j.gie.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 22. Ellis-Behnke RG, Liang Y-X, Tay DKC, et al. Nano hemostat solution: immediate hemostasis at the nanoscale. Nanomedicine 2006;2:207–15. 10.1016/j.nano.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 23. Masuhara H, Fujii T, Watanabe Y, et al. Novel infectious agent-free hemostatic material (TDM-621) in cardiovascular surgery. Ann Thorac Cardiovasc Surg 2012;18:444–51. 10.5761/atcs.oa.12.01977 [DOI] [PubMed] [Google Scholar]
- 24. Heller M, Wei C. Self-Assembly peptide prevents blood loss. Nanomedicine 2006;2:216. 10.1016/j.nano.2006.10.158 [DOI] [PubMed] [Google Scholar]
- 25. Ellis-Behnke R, Rutledge E-B. At the nanoscale: nanohemostat, a new class of hemostatic agent. Wiley Interdiscip Rev Nanomed Nanobiotechnol 2011;3:70–8. 10.1002/wnan.110 [DOI] [PubMed] [Google Scholar]
- 26. Zinicola R, Rutter MD, Falasco G, et al. Haemorrhagic radiation proctitis: endoscopic severity may be useful to guide therapy. Int J Colorectal Dis 2003;18:439–44. 10.1007/s00384-003-0487-y [DOI] [PubMed] [Google Scholar]
- 27. Andreyev HJN, Davidson SE, Gillespie C, et al. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut 2012;61:179–92. 10.1136/gutjnl-2011-300563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wachter S, Gerstner N, Goldner G, et al. Endoscopic scoring of late rectal mucosal damage after conformal radiotherapy for prostatic carcinoma. Radiother Oncol 2000;54:11–19. 10.1016/S0167-8140(99)00173-5 [DOI] [PubMed] [Google Scholar]
- 29. Chi KD, Ehrenpreis ED, Jani AB. Accuracy and reliability of the endoscopic classification of chronic radiation-induced proctopathy using a novel grading method. J Clin Gastroenterol 2005;39:42–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2020-101735supp001.pdf (68.5KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. All relevant data are included in the paper.


