Abstract
Aim
Hyperleptinemia potentiates the effects of many atherogenic factors, such as inflammation, platelet aggregation, migration, hypertrophy, proliferation of vascular smooth muscle cells, and endothelial cell dysfunction. The present study analysed the effects of long-term hyperleptinemia in an in vivo myocardial ischemia-reperfusion model to demonstrate whether the in vivo deleterious effect also affects cardiac structure and function.
Main methods
Rats were subcutaneously administered leptin for 8 days to estimate the involvement of the JAK/STAT pathway. Data from 58 male Wistar rats were included in the final analysis. Myocardial infarction (MI) was modelled by the 30-minute ligation of the main left coronary artery followed by 120-minute reperfusion. Hemodynamic measurements, electrocardiography monitoring, echocardiography, myocardial infarct size and area at risk, blood biochemical parameters, leptin, IL-6, TNF-alpha, FGF-21, and cardiomyocyte morphology were measured. The expression of JAK2, p-JAK2, STAT3, p-STAT3 was assessed by Western Blot analysis. Statistical analyses were performed using IBM SPSS Statistics v.26.
Key findings
Eight-day hyperleptinemia in rats leads to an increase in blood pressure and heart rate, myocardial hypertrophy, impaired LV function, the frequency of ischemic arrhythmias, dyslipidemia, systemic inflammation, and the size of induced myocardial infarction. Significance: The blockade of the JAK/STAT signalling pathway effectively reverses the negative effects of leptin, including increased blood pressure and total cholesterol.
Keywords: Leptin, JAK/STAT pathway, Myocardial infarction, Hemodynamics, Arrhythmias, Dyslipidemia, Inflammation, Cardiac remodelling
Leptin; JAK/STAT pathway; Myocardial infarction; Hemodynamics; Arrhythmias; Dyslipidemia; Inflammation; Cardiac remodelling.
1. Introduction
Hyperleptinemia plays an important role in obesity-associated cardiovascular diseases, including atherosclerosis [1, 2]. is considered an important risk factor for coronary heart disease, particularly myocardial infarction (MI). Increases in the weight of white adipose tissue lead to leptin hypersecretion, as leptin is mainly produced by adipocytes [3]. The effects of hyperleptinemia are multidirectional, and evidence indicates that it may exaggerate ischemic injury to the myocardium [1, 3]. Therefore, the translation potential of experimental studies aimed at the modification of myocardial infarct size can be improved by using hyperleptinemia models. Theoretically, such an approach might be more relevant to clinical practice since many patients with MI have an increased plasma leptin level and negative leptin effect demonstrated in an experimental study [1]. Hyperleptinemia leads to an unfavourable course of a heart attack is observed, since increased cardiac hypertrophy, apoptosis, deterioration of cardiac structure and function, and impairment of energy, glucose, and fatty acid metabolism, which further accelerated heart damage due to myocardial infarction [1].
There is a direct correlation between the amount of body fat and serum leptin concentration, reaching ≥200 ng/mL in morbidly obese individuals compared with 10 ng/mL in non-obese individuals [4]. The main physiological functions of leptin include inhibition of food intake, a reduction of body weight, and the stimulation of energy expenditure [5]. Hyperleptinemia potentiates the effect of many atherogenic factors, including inflammation, platelet aggregation, migration, hypertrophy, proliferation of vascular smooth muscle cells, endothelial cell dysfunction, reactive oxygen species formation, and decreased paraoxonase activity. In addition, hyperleptinemia modulates the expression of several vascular genes associated with atherosclerosis and abnormal angiogenesis, including cytokines, growth factors, and extracellular matrix proteins [6, 7].
The expression of leptin receptors on the cardiomyocyte membrane has also been described, suggesting a direct effect of adipokines on myocardial function [2]. Several signalling pathways have been shown to be involved in cardiovascular regulation by leptin, including Janus activated kinase (JAK), signal transducer and activator of transcription 3 (STAT3), mitogen-activated protein (MAP) kinase, and nitric oxide [5], which are associated with a direct hypertrophic effect and can induce cardiac remodelling [2, 8]. Continuous short-term leptin infusion following MI in mice causes eccentric left ventricular dilation with increased systolic function [9]. In addition, the chronic blockade of leptin receptors (ObR) by the systemic administration of specific antibodies limits the development of post-infarct cardiac dysfunction in rats [10]. Interestingly, FGF21 expression is controlled by STAT3, a stress-responsive transcription factor that is also known to mediate leptin effects [11].
Cardiac ischemia-reperfusion injury (IRI) activates the renin-angiotensin-aldosterone system, resulting in the release of angiotensin II and endothelin-1, both of which drive myocardial remodelling via leptin induction and mediation [12]. Experimentally induced hyperleptinemia in rodents has been reported to result in hypertension and affect post-ischemic cardiac remodelling and myocardial dysfunction [8]. What was not observed with short-term administration of leptin [13, 14, 15, 16]. In contrast, clinical data suggest that the blood leptin levels are correlated with cardiovascular morbidity, obesity, MI, and heart failure [2, 17, 18, 19]. Moreover, hyperleptinemia is often considered a surrogate marker of cardiovascular diseases [20]. Rats with acute MI demonstrate preservation of myocardial function when cardiac leptin activity is counteracted [21].
Therefore, the cardiac effects of leptin are complex, and the simultaneous influence of other factors can modify the experimental results. In this study, we addressed the hypothesis that long-term hyperleptinemia might lead to more extensive myocardial IRI in a rat model and signaling relies on the JAK/STAT pathways. This study is different from other previous work that we have developed an experimental protocol based on the well-known model of myocardial ischemia-reperfusion and supplemented it with modelling long-term hyperleptinemia with blockade of one of the key signalling pathways of leptin with assessing of myocardial damage, cardiac remodelling, arrhythmias, hemodynamics, inflammation and metabolism.
Thus, the aim of this study was to analyse the effects of long-term hyperleptinemia in an in vivo myocardial ischemia-reperfusion model to demonstrate whether the in vivo deleterious effect also affects cardiac structure and function by subcutaneously administering rats with leptin for a period of 8 days. As the heart is a high-energy-demand organ, we propose that a possible link between obesity and the development of cardiac diseases may be related to the effect of circulating leptin on inflammation, cholesterol, and glucose metabolism. The JAK/STAT signalling pathway has been implicated in the physiological and pathological effects of leptin [1], and we examined the JAK/STAT signalling pathway as a potential mechanism by which leptin influences IRI. For that purpose, we first analysed the effects of prolonged leptin administration on myocardial IRI, and then investigated whether the effects of leptin could be reversed by a specific inhibitor of the JAK/STAT pathway.
2. Materials and methods
2.1. Animals
This study was conducted in conformance with the policies and procedures detailed by the “Institutional Animal Care and Use Committee” (IACUC). All animal experimental protocols and procedures were reviewed and approved by the Committee for the Control of the Maintenance and Use of Laboratory Animals of the V. A. Almazov National Medical Research Center (date: 06-FEB-2020, application number: 20-01).
All experiments involved male Wistar rats aged 11–12 weeks and weighing 250–300 g (Pushchino, Moscow region, Russian Federation). Body weight was assessed prior to all procedures and on day 8 of the experiment, before myocardial infarction modelling. The rats were housed under standard conditions in a humidity-controlled (50–60%) and temperature-controlled (22–24 °C) facility on a 12-h light-dark cycle (lights on at 8:00 AM). All rats were acclimatised for 2 weeks before the experiments with ad libitum access to food and water. All surgeries were performed under anaesthesia, and all efforts were made to minimise suffering.
Sixty-two male Wistar rats were used in the experiment. Four rats were removed from the study early: two died from anaesthetic failures and two died after myocardial infarction initiation. Thus, data from 58 rats were included in the final analysis.
2.2. Drugs
Recombinant rat leptin (endotoxin level <0.10 EU per 1 μg of protein by the Limulus amoebocyte lysate method) (R&D Systems, Minneapolis, MN, USA) was reconstituted at 1 mg/mL in a sterile vehicle: 20 mM Tris-HCI, pH 8.0. Chronic leptin treatment was delivered by an osmotic mini-pump (model 2ML1; ALZET, Cupertino, CA, USA) with an initial leptin concentration of 0.33 μg/μl and an infusion rate of 10 μl/h for 8 days, according to a previously described protocol [22].
JSI-124 (cucurbitacin I), a specific inhibitor of JAK/STAT, was purchased from Sigma-Aldrich (St. Louis, MO, USA). A 1 mg/ml JSI-124 stock solution was prepared in dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA), stored at ‒20 °C, and diluted in 0.9% NaCl to a concentration of 150 mM. JSI-124 was administered intraperitoneally at a daily dose of 1 mg/kg for 7 days, as described in an earlier study [23].
2.3. Osmotic pump implantation
An osmotic mini-pump was implanted subcutaneously in the midscapular region under isoflurane anaesthesia (Butler Schein Animal Health, Dublin, OH, USA) via an aesthetic vaporizer (Ohmeda; BOC Health Care, Steeton, UK). Continuous infusion of leptin was initiated for 8 days. After implantation, the animals were housed individually in normal cages under standard conditions.
2.4. Hemodynamic measurements and electrocardiography (ECG) monitoring
Heart rate, arterial blood pressure measurements, and ECG registration were performed during the experiment on day 8 after pump implantation, summarized in the following stages: initiation of anaesthesia, 1 h before ischemia, and coronary artery ligation up until the end of the experiment. After a midline cervical incision, the right carotid artery was cannulated for continuous blood pressure measurements. Heart rate and arterial blood pressure signals were recorded using a computer-based PhysExp Mini system (Cardioprotect Ltd., St. Petersburg, Russia).
A 3-lead surface ECG was recorded from subcutaneous needle electrodes attached to each limb in the prone position using the INCART acquisition and analysis system (LLC Ltd “INCART”, Saint-Petersburg, Russia). Arrhythmias were quantified according to Lambeth Convention guidelines [24]. Differences in the number of ischemic arrhythmia episodes per animal were analysed.
2.5. Echocardiography
Echocardiography (ECHO) was performed on day 8 of the experiment, before myocardial IRI modelling, under light isoflurane anaesthesia using 1.5% isoflurane (Isoflurane Baxter, Baxter A/S Allerød, Denmark) applied through a face mask. Cardiac morphology and function were analysed with ECG-gating at a heart rate of 400–450 bpm using a commercially available system (Vevo 2100; Fujifilm VisualSonics, Toronto, Canada).
ECG was monitored to verify that ECHO examinations were performed at a heart rate of 400–450 bpm. The ECHO parameters were measured using M-mode tracings for parasternal long- and short-axis views. All ECHO examinations were performed by an experienced technician who was blinded to the treatment groups and assessed by a blinded investigator. Each measurement was performed on at least three cardiac cycles, and the average values were calculated. The study parameters included end-diastolic left ventricular posterior wall thickness, end-diastolic interventricular septal thickness, left ventricular end-diastolic diameter, and left ventricular fractional shortening.
2.6. Surgical preparation and myocardial infarction (MI) modeling
The rats were initially anaesthetized in a transparent acrylic box flushed with 2.5–3.0% isoflurane. After the induction of anaesthesia, which lasted from 5 to 10 min, the rats were removed from the box. To manage 1.5% isoflurane anaesthesia and minimise respiratory complications, the rats were carefully intubated orotracheally and mechanically ventilated with oxygen-enriched air (FiO2 35%) after tracheal intubation. Anaesthesia was maintained until the end of the experiment and the animals were euthanized. The respiratory rate was adjusted to maintain the arterial pCO2 within physiological limits. A thermistor probe (8.0 mm) was inserted into the rectum to monitor the core body temperature that was maintained at normothermia between 37.0 °C and 38.0 °C. Body temperature was maintained using a feedback-controlled heating pad (TCAT2LV controller; Physitemp Instruments Inc., Clifton, NJ, USA).
After a 30-minute stabilisation period, left lateral thoracotomy and pericardiotomy were performed, and a 6-0 silk thread was passed below the main left coronary artery. The ends of the thread were passed through a propylene tube to form a snare. Successful coronary occlusion and MI initiation were verified visually by epicardial cyanosis and ST-segment elevation on ECG. Myocardial IRI was induced by 30-min occlusion of the left coronary artery followed by 120-min of reperfusion. Reperfusion was verified by ST-segment depression on ECG and the appearance of local hyperaemia in the left ventricular area, which was previously ischemic and pale. At the end of the reperfusion, Evans blue was injected intra-aortically, and the rats were euthanized by isoflurane overdose.
2.7. Terminal sampling
At the end of the protocol (on day 8), blood samples from the aorta (4 mL) were collected in tubes containing EDTA and centrifuged at 3,000 × g for 15 min at ambient temperature. The resulting supernatants were collected and stored at −80 °C until use. Following euthanasia, the base part of the heart was frozen rapidly in liquid nitrogen and stored at −80 °C. The heart apex was removed and fixed in 10% buffered neutral formaldehyde (pH 7.4) for 1 d and processed using the routine histological examination. The hearts were then processed for the AR and IS measurements.
2.8. Infarct size measurement and area at risk
Excised hearts were cut into 2-mm thick transverse slices. The slices were incubated at 37 °C for 20 min in 1% 2,3,5-triphenyl tetrazolium chloride buffer. The slices were then placed in 10% formaldehyde for 10 min to increase the contrast between stained tissue with a deep red colour and non-stained tissue. Slices were then photographed, and the percentages of the area at risk (AR) and infarct size (IS) were calculated using an image analysis program (ImageJ bundled with 64-bit Java 1.8.0_172). AR was expressed as a percentage of the whole slice, and IS was expressed as a percentage of AR. The values of AR and IS for each heart were obtained by summarising the data of the slices and calculating the mean values.
2.9. The experimental protocol and design
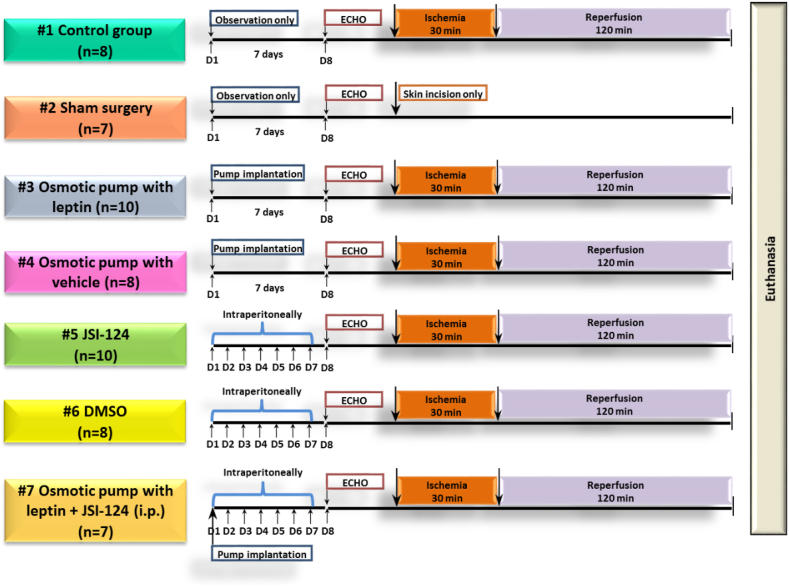
The animals were randomly (random number generation) assigned to one of seven study groups shown in Figure 1:
-
(1)
Control group (CON), n = 8: control MI modelling according to the protocol described above.
-
(2)
Sham surgery operated animals group (SH), n = 7: Thoracotomy was performed without coronary ligation.
-
(3)
Group with chronic administration of leptin using an osmotic mini-pump (LEP), n = 10.
-
(4)
Leptin-control group with chronic administration of vehicle (20 mM Tris-HCI, pH 8.0) using an osmotic mini-pump (PUMP + VEH), n = 8.
-
(5)
Group with chronic intraperitoneal administration of JAK/STAT inhibitor JSI-124 (Cucurbitacin-I) (JSI-124), n = 10.
-
(6)
JSI-124-control group with chronic intraperitoneal administration of vehicle (DMSO + NaCl) (DMSO), n = 8.
-
(7)
Group with chronic administration of leptin using the osmotic mini-pump and JSI-124 intraperitoneal administration (LEP + JSI-124), n = 7.
Figure 1.
Experimental protocol aimed at comparison of the cardiospecific effects of hyperleptinemia and JAK/STAT inhibition in the context of post-ischemic and reperfusion myocardial injury. D, day; ECHO, echocardiography; i.p., intraperitoneal injection; n, number of rats.
2.9.1. Leptin effects
The effects of chronic leptin administration on hemodynamic and metabolic parameters, inflammatory markers, and myocardial IRI were evaluated in four groups: rats received an infusion of either leptin (LEP group) or vehicle (PUMP + VEH group), and the results were compared to the control (CON group) and sham surgery (SH group) groups.
Three successive experimental series were designed: 1) Leptin effects (section 2.9.1), 2) JAK/STAT inhibitor effects (section 2.9.2), and 3) Leptin + JAK/STAT inhibitor effects (section 2.9.3).
2.9.2. JAK/STAT inhibitor effects
The impact of long-term treatment with JSI-124 (JAK/STAT inhibitor) on hemodynamic and metabolic parameters, inflammatory markers, and myocardial IRI was evaluated in four groups: rats were randomly allocated to groups that received an intraperitoneal infusion of JSI-124 (JSI-124 group) or vehicle (DMSO group), and the results were compared to the control (CON group) and sham surgery (SH group) groups.
2.9.3. Leptin + JAK/STAT inhibitor effects
The effects of JSI-124 treatment on leptin-induced changes in hemodynamic and metabolic parameters, inflammatory markers, and myocardial IRI were evaluated in three groups. Rats were randomly allocated to three different groups: (1) Leptin group: leptin administration without JSI-124 administration (LEP group); (2) JSI-124 group: JSI-124 injected intraperitoneally without leptin administration (JSI-124 group); (3) Leptin + JSI-124 group: JSI-124 was injected intraperitoneally combined with chronic leptin administration (LEP + JSI-124 group).
2.10. Evaluation of biochemical parameters
The plasma levels of leptin (Leptin Rat ELISA Kit; Thermo Fisher Scientific, Inc., Waltham, MA, USA), troponin-I (Rat Cardiac Troponin I ELISA Kit (ab246529); Cambridge, MA, USA), FGF-21 (RayBio® Mouse FGF-21 ELISA Kit; Peachtree Corners, GA, USA), IL-6 (RayBio® Rat IL-6 ELISA Kit; Peachtree Corners, GA, USA), and TNF-alpha (RayBio® Rat TNF-alpha ELISA Kit; Peachtree Corners, GA, USA) were measured using commercial ELISA kits, using specific ELISA kits, according to the manufacturer's instructions. Plasma glucose, total cholesterol, HDL, LDL, and triglyceride concentrations were determined using commercially available kits (Olvex Diagnosticum; Saint-Petersburg, Russia).
2.11. Morphological analysis
Heart apex samples were fixed in 10% buffered formalin, embedded in paraffin, and cut into 4-μm thick sections. The tissue sections were stained with haematoxylin-eosin (H&E) and examined under a light microscope (Eclipse E400; Nikon, Tokyo, Japan). The size of the cells in the field of view was studied using light microscopy. A morphometric study using the NIS Elements 4.3 Br software. The average cell size was calculated. The results are expressed as mean ± standard deviation, as there was a normal distribution. All analyses of histological data were performed by two experienced investigators blinded to the treatment groups.
2.12. Western blotting
Frozen fragments of myocardial tissue samples were homogenized in 2 ml centrifuge tubes with metal beads using the TissueLyser LT (QIAGEN, USA). Protein isolation was performed using the fresh lysis buffer (50 mM Tris-HCl, pH-7,6; 150 mM NaCl; 5 mM EDTA, pH-7,5; 100 mM NaF; 1% Na-deoxycholate; 1% Triton X-100; 10% Glycerol; PMSF (1 mM); NaF (100 mM), Na4P2O7 • 10H2O (10 mM); Na3OV4 (1 mM); and the ROCHE cOmplete™ Protease Inhibitor Cocktail (Roche, USA)), then centrifugation at 14000g/+4 °C for 30 min was carried out.
The supernatants were collected, and protein concentration was determined using a bicinchoninic acid assay kit. Proteins were separated by the SDS-PAGE method, transferred to a nitrocellulose membrane, and probed overnight at +4 °C with the following antibodies: anti-STAT3 (SAB2104912, Sigma-Aldrich); anti-phospho-STAT3 (pTyr705, SAB4300033, Sigma-Aldrich); anti-JAK2 (SAB4501600, Sigma-Aldrich) or anti-phospho-JAK2 (Tyr1007/1008, 07-606, Sigma-Aldrich). Then the membranes were washed three times in Tris-buffered saline and polysorbate 20 and incubated in the horseradish peroxidase-conjugated secondary antibody. An enhanced chemiluminescence solution was used to visualize the immunoreactive protein bands. The bands were quantified using the ImageJ software (USA). Protein concentration differences were assessed after correction for differences in protein loading, as determined by β-actin probes (mouse monoclonal Anti-β-Actin antibody, Sigma-Aldrich).
2.13. Statistical analysis
The materials used in this study were statistically processed using parametric and non-parametric methods. The collection, correction, systematisation of the initial information, and visualisation of the obtained results were carried out in Microsoft Office Excel (2016). Statistical analysis was performed using IBM SPSS Statistics v.26 (IBM Corporation).
Quantitative indicators were assessed against the normal distribution using the Shapiro-Wilk criterion. Combinations of quantitative indicators whose distribution differed from normal were described through the median (Me) and the bottom and top quartiles (Q1-Q3). When comparing several samples of quantitative data with a different distribution from the normal, the Kruskal-Wallis criterion was used as a non-parametric alternative to one-factor dispersion analysis. If the calculated value of the Kruskal-Wallis criterion exceeded the critical value, the differences in the indicators were considered to be statistically significant. Otherwise, the null hypothesis was accepted. Depended groups were analysed using Friedman's ANOVA with the Bonferroni post-hoc test. The Kruskal-Wallis test was used to show significant differences between the independent groups. This was followed by the post-hoc Bonferroni test, where significant differences between groups were also identified. All tests were two-tailed, and the significance was set at p < 0.05.
A predictive model describing the dependence of the quantitative variable on factors, also represented by quantitative indicators, was developed using the pair or multiple linear regression method to construct the following equation:
where y is the result quantitative characteristic, x1...xn are the values of the factors measured in the nominal, ordinal, or numerical scale, a1...an are the regression coefficients, and a0 is a constant.
The resulting regression models make it possible to find the theoretical values of the result characteristic y from the given values of factor x.
The linear correlation coefficient, rxy, was used as an indicator of connectivity. To evaluate the quality of the selection of a linear function, the square of the linear correlation coefficient R2, known as the determinism coefficient, was calculated. The determinant coefficient corresponds to the percentage of the factors considered in the model.
3. Results
3.1. The effects of chronic leptin administration on hemodynamic and metabolic parameters, inflammatory markers, and myocardial ischemia-reperfusion injury
Chronic subcutaneous leptin administration (at a concentration of 0.33 μg/μl and an infusion rate of 10 μl/h for 8 days, subcutaneously) altered hemodynamic and metabolic parameters, inflammatory markers, and myocardial IRI in a rat model of myocardial infarction.
In this experimental series, the following four animal groups were analysed: the LEP group with chronic leptin administration was compared with the CON, SH, and PUMP + VEH groups.
On day eight, the body weight increased significantly in all experimental groups compared with the baseline (Table 1).
Table 1.
Body weight dynamics.
| Group | n | Initial body weight (g) | Day 8 body weight (g) | p | |
|---|---|---|---|---|---|
| 1 | CON | 8 | 293.0 (263.0; 295.0) | 302.0 (270.5; 302.0) | 0.010 |
| 2 | SH | 7 | 273.0 (262.0; 279.0) | 281.0 (268.5; 288.5) | 0.018 |
| 3 | LEP | 10 | 279.0 (270.0; 296.0) | 267.0 (260.0; 281.0) | 0.005 |
| 4 | PUMP + VEH | 8 | 291.0 (266.0; 295.5) | 295.5 (274.5; 303.0) | 0.017 |
| 5 | JSI-124 | 10 | 289.0 (258.0; 297.0) | 301.0 (267.0; 308.0) | 0.017 |
| 6 | DMSO | 8 | 279.0 (270.5; 295.0) | 288.0 (276.5; 303.5) | 0.005 |
| 7 | LEP + JSI-124 | 7 | 286.0 (278.0; 289.0) | 275.0 (266.5; 281.5) | 0.011 |
| p | 0.787 | 0.046 | |||
CON, control group; SH, sham surgery group; LEP, leptin administration group; PUMP + VEH, group with osmotic pump with vehicle; JSI-124, group with JSI-124 administration intraperitoneally; DMSO, group with vehicle administration intraperitoneally; LEP + JSI-124, simultaneous leptin and JSI-124 administration group. Values are expressed as medians and interquartile ranges. n = 7–10 for each group.
There were no intergroup differences in body weight at the baseline or at the end of the experiment (Table 1).
Moreover, there were no differences between the CON, SH, LEP, and PUMP + VEH groups in terms of mean blood pressure and heart rate at the baseline, and between the groups CON, LEP and PUMP + VEH during ischemia and reperfusion (Table 2). Mean BP increased at the baseline in the LEP group compared with that in the CON group (p = 0.003). In addition, the heart rate was higher in the LEP group than in the CON group at the end of ischemia (p = 0.006).
Table 2.
Systemic hemodynamics in all study groups.
| Group | n | Baseline |
Ischemia 5 min |
Ischemia 30 min |
Reperfusion 60 min |
Reperfusion 120 min |
p | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||||
| BP, mean (mmHg) | |||||||||
| 1 | CON | 8 | 114.5 (108.5; 119.5) | 69.0 (65.5; 71.0) | 81.5 (78.0; 87.0) | 58.0 (54.0; 64.0) | 66.0 (60.5; 70.5) | <0.001 p1-2 = 0.012 р1-3 = 1.000 p1-4 < 0.001 р1-5 = 0.002 |
р2-3 = 0.481 р2-4 = 1.000 р2-5 = 1.000 р3-4 = 0.016 р3-5 = 0.143 р4-5 = 1.000 |
| 2 | SH | 7 | 119.0 (115.0; 122.0) | - | - | - | - | - | |
| 3 | LEP | 10 | 129.0 (127.0; 132.0) | 69.5 (64.0; 76.5) | 76.5 (71.0; 78.0) | 60.0 (54.0; 62.0) | 67.0 (61.0; 71.0) | <0.001 p1-2 = 0.030 р1-3 = 0.196 p1-4 < 0.001 р1-5 = 0.004 |
р2-3 = 1.000 р2-4 = 0.196 р2-5 = 1.000 р3-4 = 0.030 р3-5 = 1.000 р4-5 = 0.771 |
| 4 | PUMP + VEH | 8 | 120.5 (117.5; 121.5) | 71.0 (64.0; 76.5) | 79.5 (75.5; 84.0) | 54.0 (50.5; 60.5) | 69.0 (64.5; 71.0) | <0.001 p1-2 = 0.034 р1-3 = 1.000 p1-4 < 0.001 р1-5 = 0.009 |
р2-3 = 1.000 р2-4 = 0.481 р2-5 = 1.000 р3-4 = 0.007 р3-5 = 0.690 р4-5 = 1.000 |
| 5 | JSI-124 | 10 | 107.0 (99.0; 125.0) | 69.0 (67.0; 78.0) | 72.5 (69.0; 81.0) | 56.0 (54.0; 58.0) | 67.0 (64.0; 71.0) | <0.001 p1-2 = 0.109 р1-3 = 0.237 p1-4 < 0.001 р1-5 = 0.001 |
р2-3 = 1.000 р2-4 = 0.030 р2-5 = 1.000 р3-4 = 0.011 р3-5 = 1.000 р4-5 = 0.897 |
| 6 | DMSO | 8 | 118.5 (113.5; 122.0) | 72.5 (65.0; 76.5) | 76.0 (72.0; 80.5) | 55.5 (50.5; 61.5) | 68.5 (61.0; 72.5) | 0.001 p1-2 = 0.057 р1-3 = 0.820 p1-4 < 0.001 р1-5 = 0.007 р2-3 = 1.000 |
р2-4 = 0.481 р2-5 = 1.000 р3-4 = 0.027 р3-5 = 0.969 р4-5 = 1.000 |
| 7 |
LEP + JSI-124 | 7 | 106.0 (103.0; 120.0) | 79.0 (73.5; 79.0) | 75.0 (72.5; 77.5) | 58.0 (53.5; 60.0) | 65.0 (60.5; 69.5) | <0.001 p1-2 = 1.000 р1-3 = 0.225 p1-4 < 0.001 р1-5 = 0.003 |
р2-3 = 1.000 р2-4 = 0.041 р2-5 = 0.346 р3-4 = 0.346 р3-5 = 1.000 р4-5 = 1.000 |
| p |
0.001 р1-3 = 0.003 р3-5 = 0.001 р3-7 = 0.004 |
0.2 |
0.021 |
0.816 |
0.993 |
||||
| HR (beats/min) | |||||||||
| 1 | CON | 8 | 379.2 (346.8; 388.3) | 348.0 (342.0; 352.0) | 310.0 (306.0; 324.0) | 318.0 (196.5; 338.0) | 307.0 (303.0; 314.5) | <0.001 p1-2 = 1.000 р1-3 = 0.114 p1-4 = 0.016 р1-5 = 0.005 |
р2-3 = 0.269 р2-4 = 0.044 р2-5 = 0.016 р3-4 = 1.000 р3-5 = 1.000 р4-5 = 1.000 |
| 2 | SH | 7 | 366.7 (364.5; 376.3) | - | - | - | - | - | |
| 3 | LEP | 10 | 371.5 (365.0; 389.7) | 372.0 (355.0; 384.0) | 360.0 (342.0; 384.0) | 334.5 (324.0; 348.0) | 302.0 (290.0; 308.0) | <0.001 p1-2 = 1.000 р1-3 = 1.000 p1-4 = 0.058 р1-5 < 0.001 |
р2-3 = 1.000 р2-4 = 0.771 р2-5 = 0.003 р3-4 = 1.000 р3-5 = 0.024 р4-5 = 0.660 |
| 4 | PUMP + VEH | 8 | 376.3 (364.3; 387.3) | 348.0 (344.0; 360.0) | 326.0 (306.0; 344.0) | 349.0 (309.0; 357.0) | 305.0 (290.54 313.0) | <0.001 p1-2 = 1.000 р1-3 = 0.020 p1-4 = 0.269 р1-5 = 0.001 |
р2-3 = 0.177 р2-4 = 1.000 р2-5 = 0.016 р3-4 = 1.000 р3-5 = 1.000 р4-5 = 0.969 |
| 5 | JSI-124 | 10 | 384.5 (384.0; 385.7) | 378.0 (366.0; 402.0) | 357.0 (340.0; 366.0) | 311.5 (304.0; 322.0) | 304.5 (290.0; 308.0) | <0.001 p1-2 = 1.000 р1-3 = 0.477 p1-4 = 0.001 р1-5 < 0.001 |
р2-3 = 1.000 р2-4 = 0.007 р2-5 < 0.001 р3-4 = 0.477 р3-5 = 0.047 р4-5 = 1.000 |
| 6 | DMSO | 8 | 376.0 (371.3; 392.5) | 344.0 (324.5; 361.0) | 330.0 (322.0; 342.0) | 318.0 (303.5; 320.0) | 304.5 (289.0; 311.0) | <0.001 p1-2 = 0.690 р1-3 = 0.177 p1-4 = 0.001 р1-5 < 0.001 |
р2-3 = 1.000 р2-4 = 0.481 р2-5 = 0.044 р3-4 = 1.000 р3-5 = 0.219 р4-5 = 1.000 |
| 7 | LEP + JSI-124 | 7 | 389.7 (379.2; 396.5) | 372.0 (366.0; 390.0) | 378.0 (363.5; 390.0) | 320.0 (312.0; 325.0) | 297.0 (293.0; 306.0) | <0.001 p1-2 = 1.000 р1-3 = 1.000 p1-4 = 0.041 р1-5 = 0.002 |
р2-3 = 1.000 р2-4 = 0.112 р2-5 = 0.007 р3-4 = 0.280 р3-5 = 0.023 р45 = 1.000 |
| p | 0.18 | 0.005 | <0.001 р1-3 = 0.006 р1-5 = 0.008 р1-7 < 0.001 р4-7 = 0.008 |
0.069 | 0.702 | ||||
CON, control group; SH, sham surgery group; LEP, leptin administration group; PUMP + VEH, group with osmotic pump with vehicle; JSI-124, group with JSI-124 administration intraperitoneally; DMSO, group with vehicle administration intraperitoneally; LEP + JSI-124, simultaneous leptin and JSI-124 administration group. Values are expressed as medians and interquartile ranges. n = 7–10 for each group.
Myocardial ischemia led to a significant decrease in mean BP in all groups compared to baseline (p < 0.05). At the 60th minute and at the end of reperfusion, both the heart rate and mean BP decreased significantly in all seven experimental groups compared with the baseline. This reduction can be explained by cardiac dysfunction following acute myocardial infarction.
Cardiac morphology and function assessments were based on multiple echocardiographic parameters (Table 3).
Table 3.
LV function in all groups on day 8 (echocardiographic data).
| Group | n | ED LVPW (mm) |
ED IVS (mm) |
LV EDD (mm) |
LV ES (%) |
|
|---|---|---|---|---|---|---|
| 1 | CON | 8 | 1.3 (1.2; 1.5) | 1.0 (1.0; 1.1) | 6.1 (6.1; 6.4) | 67.3 (65.9; 68.9) |
| 2 | SH | 7 | 1.5 (1.4; 1.6) | 1.4 (1.3; 1.5) | 6.4 (6.3; 6.5) | 68.3 (66.4; 69.0) |
| 3 | LEP | 10 | 2.1 (2.0; 2.3) | 1.9 (1.9; 2.0) | 7.2 (7.0; 7.6) | 69.5 (66.1; 71.7) |
| 4 | PUMP + VEH | 8 | 1.5 (1.3; 1.6) | 1.4 (1.3; 1.5) | 6.5 (6.2; 6.6) | 70.9 (67.8; 72.0) |
| 5 | JSI-124 | 10 | 1.3 (1.1; .4) | 0.9 (0.9; 0.9) | 6.2 (6.0; 6.3) | 70.5 (66.4; 71.4) |
| 6 | DMSO | 8 | 1.3 (1.2; 1.4) | 0.9 (0.9; 1.2) | 6.4 (6.2; 6.5) | 68.6 (66.3; 71.3) |
| 7 | LEP + JSI-124 | 7 | 2.1 (2.0; 2.2) | 2.0 (1.9; 2.0) | 7.2 (7.0; 7.3) | 71.3 (69.7; 72.5) |
| p | <0.001 р1-3 = 0.001 р3-5 = 0.001 р3-6 = 0.001 р4-7 = 0.004 р5-7 = 0.002 р6-7 = 0.008 |
<0.001 р1-3 = 0.001 р3-5 < 0.001 р3-6 < 0.001 р4-7 = 0.004 р5-7 < 0.001 р6-7 = 0.001 |
<0.001 р1-3 < 0.001 р3-5 < 0.001 р3-6 = 0.014 р4-7 = 0.003 р5-7 < 0.001 |
0.020 р1-3 = 0.041 |
||
CON, control group; SH, sham surgery group; LEP, leptin administration group; PUMP + VEH, group with osmotic pump with vehicle; JSI-124, group with JSI-124 administration intraperitoneally; DMSO, group with vehicle administration intraperitoneally; LEP + JSI -124, simultaneous leptin and JSI-124 administration group; ED LVPW, end-diastolic left ventricular posterior wall thickness; ED IVS, end-diastolic interventricular septal thickness; LV EDD, left ventricular end-diastolic dimension; LV FS, left ventricular fractional shortening. Values are expressed as medians and interquartile ranges. n = 7–10 for each group.
Echocardiography analyses revealed the development of hypertrophy, including dilation and a significant reduction in LV function, in leptin-treated animals versus CON, SH, and PUMP + VEH groups of rats (Table 3). Compared to the controls, hyperleptinemic rats had an increased end-diastolic left ventricular posterior wall thickness (p = 0.001), end-diastolic interventricular septal thickness (p = 0.001), and LV end-diastolic dimension (LV EDD) (p < 0.001). These findings indicated significant LV enlargement, which markedly affected the LV systolic function. In particular, left ventricular fractional shortening (LV FS) was significantly higher (p = 0.041) in leptin-treated animals compared to CON. There were no differences in echocardiography data between groups SH and PUMP + VEH versus CON (Table 3). Thus, cardiac dysfunction, as measured by echocardiography on day 8, was exacerbated by leptin administration.
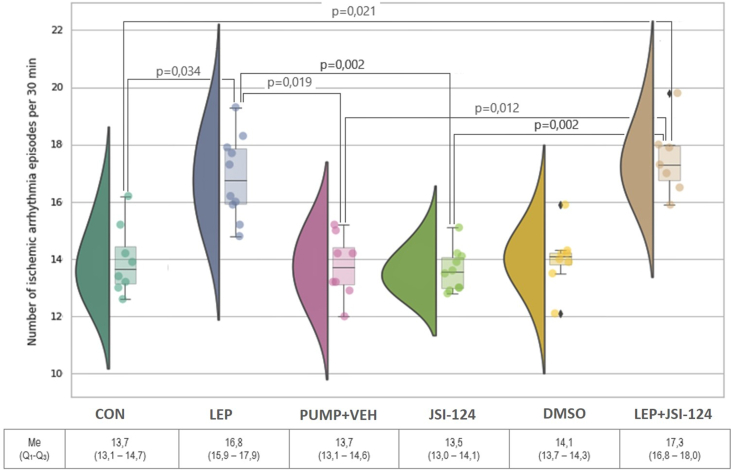
In addition, the number of ischemic arrhythmia episodes was analysed (Figure 2). In leptin-treated rats, there was a significantly higher number of ischemic arrhythmias than the CON and PUMP + VEH groups (Figure 2; p = 0.034 and p = 0.019, respectively).
Figure 2.
Number of arrhythmia episodes per 30 min registered on day 8 during ischemia in all groups.
The validity of the model of chronic hyperleptinemia has been confirmed by significantly elevated plasma leptin levels in the LEP group compared to the CON group. Compared with CON, leptin-treated rats developed higher plasma levels of total cholesterol and LDL (Table 4), as well as troponin-I, IL-6, TNF-alpha, and lower plasma levels of FGF-21 (Table 5). However, the levels of blood glucose, HDL, and plasma triglycerides were not significantly different between the LEP and CON groups. There were no differences in blood plasma parameters between the SH and PUMP + VEH groups compared with the CON group, except for the SH group with respect to troponin-I (Tables 4 and 5).
Table 4.
Biochemical parameters of blood plasma in all study groups.
| Group | n | Glucose (mmol/l) |
Total cholesterol (mmol/l) |
HDL (mmol/l) |
LDL (mmol/l) |
Triglyceride (mmol/l) |
|
|---|---|---|---|---|---|---|---|
| 1 | CON | 8 | 4.7 (4.5; 5.0) | 4.3 (4.1; 4.5) | 1.3 (1.2; 1.4) | 2.5 (2.3; 2.9) | 1.2 (1.1; 1.3) |
| 2 | SH | 7 | 5.0 (4.6; 5.3) | 4.4 (4.0; 4.6) | 1.4 (1.3; 1.7) | 3.0 (2.3; 3.1) | 1.2 (1.1; 1.2) |
| 3 | LEP | 10 | 4.9 (4.7; 5.1) | 5.6 (5.1; 5.9) | 1.4 (1.1; 1.8) | 3.5 (3.3; 3.6) | 1.1 (1.1; 1.3) |
| 4 | PUMP + VEH | 8 | 4.7 (4.4; 5.2) | 4.5 (4.2; 4.8) | 1.7 (1.5; 1.9) | 2.6 (2.3; 2.9) | 1.2 (1.2; 1.3) |
| 5 | JSI-124 | 10 | 4.9 (4.8; 5.2) | 4.3 (4.1; 4.7) | 1.5 (1.4; 1.7) | 2.5 (2.1; 2.9) | 0.3 (0.3; 0.4) |
| 6 | DMSO | 8 | 4.6 (4.4; 5.2) | 4.5 (4.0; 4.8) | 1.7 (1.3; 1.8) | 2.6 (2.3; 3.0) | 1.3 (1.2; 1.3) |
| 7 | LEP + JSI-124 | 7 | 5.1 (5.0; 5.5) | 4.7 (4.3; 4.7) | 1.4 (1.4; 1.5) | 3.2 (2.9; 3.0) | 0.9 (0.4; 1.0) |
| p | 0.406 | <0.001 p1-3 = 0.003 р2-3 = 0.005 р3-4 = 0.043 р3-5 = 0.001 р3-6 = 0.008 р3-7 = 0.028 |
0.140 | <0.001 p1-3 = 0.008 р3-4 = 0.012 р3-5 < 0.001 р3-6 = 0.027 |
<0.001 p1-5 = 0.002 р1-7 = 0.040 р2-5 = 0.035 р3-5 = 0.007 р4-5 < 0.001 р4-7 = 0.014 р5-6 < 0.001 р6-7 = 0.010 |
||
CON, control group; SH, sham surgery group; LEP, leptin administration group; PUMP + VEH, group with osmotic pump with vehicle; JSI-124 – group with JSI-124 administration intraperitoneally; DMSO – group with vehicle administration intraperitoneally; LEP + JSI-124 – simultaneous leptin and JSI-124 administration group. Values are expressed as medians and interquartile ranges. n = 7–10 for each group.
Table 5.
Plasma troponin-I, adipokines and cytokines in all study groups.
| Group | n | Troponin-I (ng/mL) |
Leptin (ng/mL) |
FGF-21 (pg/mL) |
IL-6 (pg/mL) |
TNF-alpha (pg/mL) |
|
|---|---|---|---|---|---|---|---|
| 1 | CON | 8 | 42.0 (39.0; 47.0) | 3.2 (3.0; 3.8) | 20.1 (15.0; 31.5) | 9.8 (9.5; 11.8) | 68.8 (61.2; 73.6) |
| 2 | SH | 7 | 2.1 (1.5; 3.1) | 3.5 (3.2; 3.7) | 32.4 (24.6; 35.7) | 13.9 (9.6; 15.9) | 64.8 (54.9; 73.1) |
| 3 | LEP | 10 | 84.5 (79.0; 87.0) | 70.2 (65.0; 71.6) | 6.6 (3.3; 7.8) | 24.9 (20.8; 29.9) | 182.0 (164.7; 234.9) |
| 4 | PUMP + VEH | 8 | 43.0 (40.0; 47.5) | 3.5 (3.1; 3.9) | 26.3 (16.3; 28.9) | 10.2 (8.6; 12.0) | 94.0 (53.6; 102.1) |
| 5 | JSI-124 | 10 | 45.0 (38.0; 47.0) | 2.8 (2.6; 3.2) | 33.7 (22.0; 40.5) | 11.1 (10.6; 14.5) | 153.0 (75.7; 168.7) |
| 6 | DMSO | 8 | 41.0 (38.5; 43.5) | 2.9 (2.7; 3.2) | 27.8 (25.5; 30.1) | 11.8 (10.4; 13.7) | 80.6 (71.2; 86.7) |
| 7 | LEP + JSI-124 | 7 | 77.0 (72.5; 81.0) | 73.1 (69.1; 76.1) | 3.3 (2.1; 5.7) | 16.7 (15.1; 17.5) | 80.6 (71.2; 86.7) |
| p | <0.001 p1-3 = 0.002 р3-4 = 0.004 р3-5 = 0.003 р3-6 < 0.001 р6-7 = 0.015 р1-2 < 0.001 р2-3 < 0.001 р2-4 < 0.001 р2-5 < 0.001 р2-6 < 0.001 р2-7 < 0.001 |
<0.001 p1-3 = 0.032 р1-7 = 0.021 р3-5 < 0.001 р3-6 = 0.001 р5-7 < 0.001 р6-7 = 0.001 |
<0.001 p2-3 = 0.003 р2-7 = 0.003 р3-4 = 0.049 р3-5 < 0.001 р3-6 = 0.011 р4-7 = 0.048 р5-7 = 0.001 р6-7 = 0.012 |
<0.001 p1-3 < 0.001 р2-3 = 0.023 р3-4 < 0.001 р3-5 = 0.003 р3-6 = 0.011 |
<0.001 p1-3 = 0.001 p1-7 = 0.004 р2-3 = 0.001 р2-7 = 0.004 р3-6 = 0.037 |
||
CON, control group; SH, sham surgery group; LEP, leptin administration group; PUMP + VEH, group with osmotic pump with vehicle; JSI-124 – group with JSI-124 administration intraperitoneally; DMSO – group with vehicle administration intraperitoneally; LEP + JSI-124 – simultaneous leptin and JSI-124 administration group. Values are expressed as medians and interquartile ranges. n = 7–10 for each group.
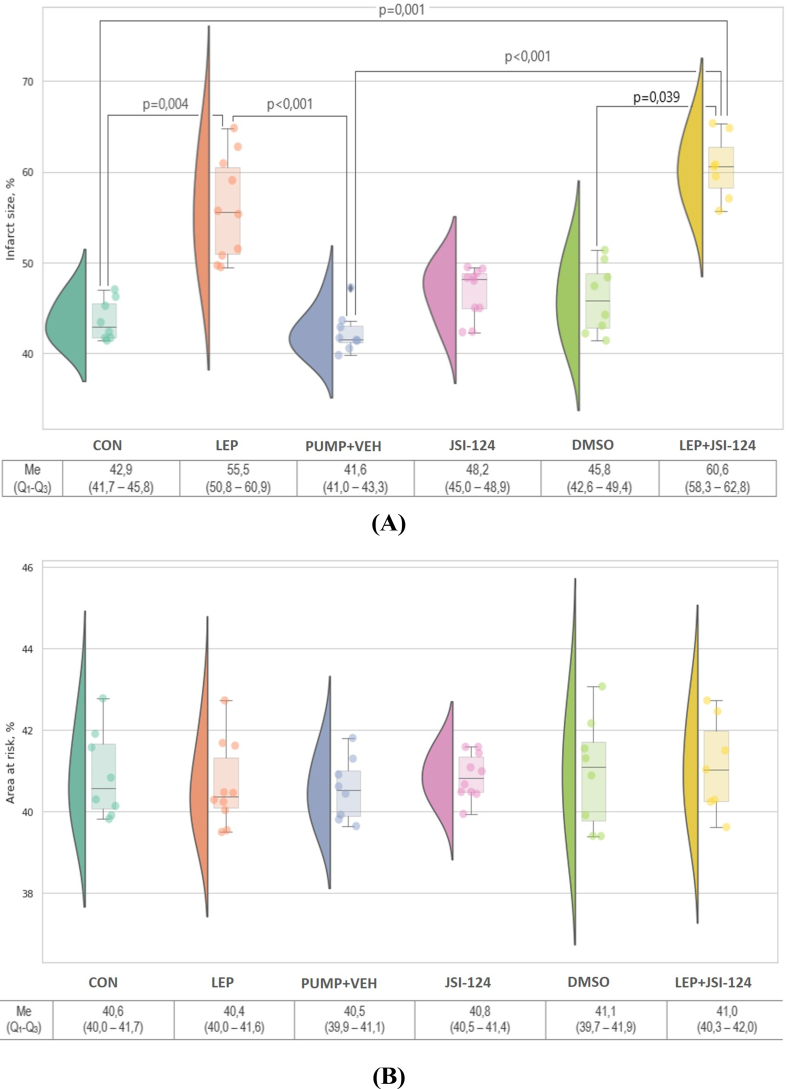
The myocardial infarct size and the area at risk are shown in Figure 3. The infarct size in rats with hyperleptinemia was larger than that in rats from the CON group (p = 0.004) and LEP group (p < 0.001) (Figure 3A). There were no intergroup differences in the area at risk (р>0.05) (Figure 3B).
Figure 3.
Infarct size (A) is expressed as a percentage of the area at risk and area at risk (B) expressed as a percentage of the left ventricle after 30 min of ischemia followed by 120 min of reperfusion in each group. Values are median and interquartile range. n = 7–10 for each group.
3.2. The impact of long-term treatment with JAK/STAT inhibitor on hemodynamic and metabolic parameters, inflammatory markers, and myocardial ischemia-reperfusion injury
Long-term JAK/STAT inhibition (at a dose of 1 mg/kg/d for 7 days intraperitoneally) had no effect on hemodynamic and metabolic parameters, inflammatory markers, or myocardial IRI in rats with myocardial infarction.
In this experimental series, the following four animal groups were analysed: JSI-124 group with the long-term intraperitoneal infusion of JSI-124 (JAK/STAT inhibitor) was compared to the CON, SH, and DMSO groups.
There were no differences between the CON, SH, JSI-124, and DMSO groups in terms of the mean blood pressure and heart rate at the baseline, and during ischemia and reperfusion in the groups CON, JSI-124 and DMSO (Table 2). However, in the JSI-124 treated group, the heart rate was higher than in the CON group at the end of ischemia (p = 0.008).
JAK/STAT inhibition did not influence the echocardiography data of rats. The parameters did not differ between the study groups (Table 3).
In addition, it was found that the administration of JSI-124 did not significantly change the frequency of ischemic arrhythmias when compared to the DMSO (p > 0.05, Figure 2).
JSI-124 administration was not associated with changes in metabolic parameters (Tables 4 and 5); the only exception was a significant decrease in plasma triglyceride levels compared to the CON group (p = 0.002) (Table 4).
The infarct size (Figure 3A) and area at risk (Figure 3B) in rats administered JSI-124 were not significantly different from those in the other groups (p > 0.05).
3.3. The effects of JAK/STAT inhibition on leptin-induced changes in hemodynamic and metabolic parameters, inflammatory markers, and myocardial ischemia-reperfusion injury
Inhibition of the JAK/STAT pathway partially reverses the changes induced by leptin administration in rats. In this experimental series, the following three animal groups were analysed: LEP, JSI-124, and LEP + JSI-124.
Our results showed that the hemodynamic, metabolic, inflammation, and ischemic myocardial conditioning were impaired after chronic subcutaneous leptin administration. Next, we examined whether the administration of a JAK/STAT inhibitor, JSI-124, could rescue the impairments induced by leptin treatment. Because the JAK/STAT signalling pathway has been reported to participate in a number of metabolic and cardiovascular functions [25], JSI-124 without leptin application also influences heart conditioning as evidenced by the results of the previous experimental series. Based on these results, we conducted an experiment with the application of leptin and JSI-124 (Figure 1) to determine whether blocking the JAK/STAT signalling pathway can rescue the leptin-induced impairments of metabolic and heart conditioning. This possibility was evaluated by conducting and comparing the experiments after subcutaneous leptin administration, after intraperitoneal infusion of JSI-124, and after both leptin and JSI-124 administration.
No differences were observed between LEP, JSI-124, and LEP + JSI-124 in terms of body weight at both, the baseline and the end of the experiment (Table 1).
Similarly, there were no differences in the heart rate during ischemia and reperfusion between the groups (Table 2). However, JAK/STAT inhibition led to a decrease in the baseline mean blood pressure in the group treated only with JSI-124 compared with leptin administration (p = 0.001) and in the group with simultaneous leptin and JSI-124 administration compared with the LEP group (p = 0.004).
There were no intergroup LEP and LEP + JSI-124 differences in the incidence of ischemic arrhythmia (Figure 2). However, JAK/STAT blockade without leptin administration led to a decrease in the incidence of ischemic arrhythmias compared with leptin and JSI-124 rats treated with leptin alone (Figure 2).
In the LEP and LEP + JSI-124 groups, ED LVPW, ED IVS, and LV EDD were more deteriorated than in animals receiving JSI-124 alone, according to ECHO data (Table 3).
Blood plasma analysis revealed several significant differences. JSI-124 administration compared with rats treated with leptin and leptin and JSI-124 was accompanied by a significant reduction in total cholesterol (Table 4) and leptin (Table 5). Differences were found between the LEP and JSI-124 groups. Smaller concentrations of LDL and triglycerides (Table 4) and troponin-I and IL-6 (Table 5) were observed in the JSI-124 group. Animals in which the JAK/STAT signalling pathway was blockaded had an increased FGF-21 plasma concentration compared with the LEP and LEP + JSI-124 groups (Table 5).
The infarct size and area at risk among rats from the LEP, JSI-124, and LEP + JSI-124 groups did not differ (p > 0.05) (Figure 3A, B).
Together, these results suggest that blocking the JAK/STAT pathway compared with leptin effects is sufficient to restoring hemodynamic, metabolic, and inflammation, but not myocardial impairment.
3.4. Relationship between plasma leptin concentration and myocardial infarction size (post-regression analysis)
A post-regression analysis of the role of the studied indicators on myocardial infarction size and the area at risk was performed. As a result, the correlation between infarct size and the level of leptin was found to be high (r = 0.830, Chaddock scale) and statistically significant (p < 0.001). In the resulting model, the leptin level determined the size of the infarction by 68.9% (R2 = 0.689).
The observed dependence is described by the following equation:
| Yinfarct size = 44.281 + 0.193∗Xleptin |
where Yinfarct size is the infarction size (%) and Xleptin is the blood plasma leptin level (ng/ml). According to this equation, if the plasma leptin level increased by 1 ng/ml, an increase of 0.193% in infarct size would be expected.
The Darbina-Watson criterion was 1.94. As this was in the range of 1–3, the independence of observations was respected. Estimation of the normal distribution of residues using the Shapiro-Wilk criterion (p < 0.001) indicated a normal distribution of residues, confirming the application of the regression model.
This pattern was found only for plasma leptin concentrations in relation to myocardial infarction size.
3.5. Effects of JAK/STAT inhibition on attenuation of cardiomyocytes hypertrophy in animals receiving leptin
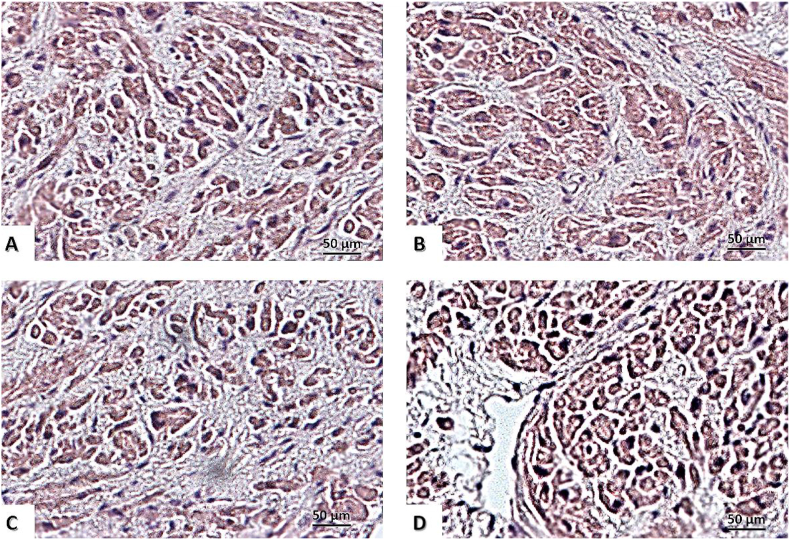
The size of the heart apex cardiomyocytes was assessed using light microscopy in the field of view (Figure 4). Among the four different groups (CON, LEP, JSI-124, and LEP + JSI-124), no significant intergroup difference was observed for cell diameter: 12.3 ± 0.1 μm, 13.7 ± 0.2 μm, 12.7 ± 0.2 μm, and 13.1 ± 0.2 μm in 1, 3, 5, and 7 groups (p > 0.05). However, the cell sectional area of cardiomyocytes in rats from the LEP group (324.7 ± 36.7 μm2) and the LEP + JSI-124 group (336.2 ± 38.3 μm2) was significantly larger than that in the CON group (262 ± 33.8 μm2) (p < 0.01) and significantly larger than that in the JSI-124 group (277 ± 38.1 μm2) (p < 0.01). The cross-sectional area of cardiomyocytes was not different between the JSI-124 (277 ± 38.1 μm2) and CON groups (p > 0.05).
Figure 4.
Myocardium staining with haematoxylin-eosin. Scale bar, 50 μm (×400). (A) Control group; (B) group with chronic leptin administration; (C) group with chronic administration of JAK/STAT inhibitor – JSI-124 (Cucurbitacin-I); (D) group with chronic administration of leptin and JSI-124.
3.6. Effects of leptin and JSI-124 on JAK2 and STAT3 phosphorylation
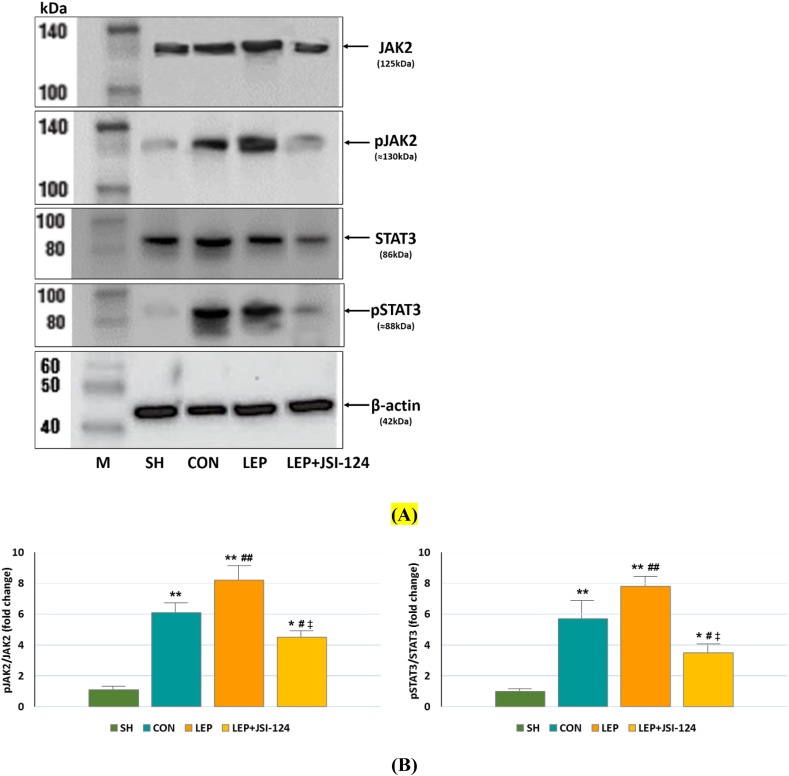
Verification of potential leptin influences on JAK/STAT signalling was performed with the analysis of JAK2 and STAT3 phosphorylation in the SH, CON, LEP and LEP + JSI-124 groups by Western blotting.
In the SH group, the expressions of phosphorylated JAK2 (pJAK2) and STAT3 (pSTAT3) were weak, while their expressions were increased in the CON and LEP groups (Figure 5A). In the leptin-treated rats, pJAK2 and pSTAT3 expressions were significantly suppressed following JSI-124 administration (Figure 5A).
Figure 5.
Western blotting of total and phosphorylated JAK2 and STAT3 in hearts of IRI-treated rats from the SH, CON, LEP, LEP + JSI-124 groups and marker (M). (A) Representative Western blot bands demonstrating JAK2, p-JAK2, STAT3, p-STAT3 and β-actin expressions. (B) The bar graph shows the expression of p-JAK2/JAK2 and p-STAT3/STAT3 after normalization to the SH data. Values are expressed as means and standard errors of means (SEM), n = 7–10 for each group. ∗p < 0.01, ∗∗p < 0.001 vs. SH; #p < 0.05, ##p < 0.01 vs. CON; ‡p < 0.01 LEP vs. LEP + JSI-124.
After standardization of pJAK2 and pSTAT3 levels to SH group values, the expression of pJAK2 protein was found higher than total JAK2 in the CON and LEP groups (Figure 5B). However, the pJAK2 level was significantly reduced in the JSI-124 group when compared with the LEP group (p < 0.01, Figure 5B), suggesting a significant suppressive effect of JSI-124. Furthermore, the ratio of pSTAT3/STAT3, the concentration of JAK2 cascade downstream molecules were also higher in the CON and LEP groups than in the SH group (Figure 5B). The level of pSTAT3 was reduced by JSI-124 treatment in rats receiving leptin (p < 0.01, Figure 5B). These results suggest that eight-day hyperleptinemia induces JAK2/STAT3 activation and daily intraperitoneal administration of JSI-124 (1 mg/kg/day) from days 1–8 in leptin-treated rats reduces JAK2 and STAT3 phosphorylation.
4. Discussion
This study provides evidence that the eight-day hyperleptinemia in rats leads to an increase in blood pressure and heart rate, myocardial hypertrophy, impaired LV function, the frequency of ischemic arrhythmias, dyslipidemia, systemic inflammation, and the increased size of induced myocardial infarction. In addition, the pharmacological inhibition of the JAK/STAT pathway by the intraperitoneal injection of JSI-124 reduces leptin-induced JAK2 and STAT3 phosphorylation and reverses changes in certain hemodynamic and metabolic parameters. The data suggest that JAK/STAT signalling could be involved in mediating mechanisms of both metabolic dysfunction and ischemic arrhythmogenesis in rats. Previous studies have provided insights regarding the mechanisms by which leptin-STAT signalling is implicated in the development of myocardial injury [1, 2, 8]. In addition to the leptin-STAT signalling pathway, other pathways, such as the MAPK and PI3K/AKT signalling pathways, are involved in the effector mechanisms of inflammatory responses [1].
Previous reports have suggested that leptin plays a complex and variable role in the regulation of hemodynamic, metabolic, and inflammatory conditions and ischemic/reperfusion myocardial damage, and can produce opposite metabolic effects under different conditions or contexts [10, 15, 26]. On the one hand, leptin may exert cardioprotective effects [27] when administered acutely. The short-term leptin administration reduces infarct size in isolated perfused rat hearts [13] and attenuates cardiomyocyte apoptosis after ischemia by increasing bcl-2 and survivin gene expression and by reducing caspase-3 activity [14]. Leptin has also been shown to possess cardioprotective properties in several ex vivo and in vitro studies performed in mice [15, 16]. On the other hand, evidence suggests that continuously elevated levels of leptin may be associated with maladaptive effects through hemodynamic factors, such as increased heart rate and blood pressure [28, 29], metabolic changes including augmented fatty acid or impairment of glucose utilisation [30, 31], induced cardiac apoptosis [32], or structural cardiac changes, such as cardiac lipid accumulation [31, 33], and increased myocardial hypertrophy [34]. Obesity-related hyperleptinemia is accompanied by a low-grade inflammatory profile, characterised by increased circulating levels of IL-6, IL-12, and TNF-alpha, and reduced concentrations of IL-10. Whether hyperleptinemia is a cause or consequence of the systemic inflammatory milieu in humans is worthy of further consideration in clinical studies and research [30]. Furthermore, our rat model may also simulate clinical scenarios of patients who experience acute myocardial infarction and reperfusion coinciding with leptin overexpression associated with a pre-existing inflammatory state. Adverse consequences attributed to this unfavourable association have been observed in patients suffering from inflammatory bowel disease [35] or rheumatoid arthritis [36], who exhibit a disproportionally higher degree of post-MI heart failure. Therefore, severe infection may render patients vulnerable to serious cardiovascular complications and progression of heart failure [37]. Our results imply that a worse outcome of post-MI cardiac dysfunction may be attributed to excessive blood leptin concentration.
In the present study, rats treated with leptin for 8 days developed structural and metabolic alterations, including cardiac hypertrophy, dyslipidemia, a more pronounced inflammatory response with IL-6 and TNF-α elevation, and FGF-21 plasma level depression. Although leptin administration in the current study increased the serum leptin levels, there was no significant difference in body weight, which is consistent with an earlier study [38]. The characteristics and metabolic parameters at the end of the study are consistent with a previous report, indicating that this animal model mimics the classical insulin resistance and leptin resistance features of human obesity [39]. This may explain the increase in mean BP and heart rate in the leptin-treated group.
Furthermore, there is evidence for the induction of both murine and human cardiomyocyte hypertrophy in response to leptin treatment, which appears to be mediated by the activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol-3 kinase [40]. Although some in vitro results suggest that leptin contributes to adverse cardiac remodelling and hypertrophy, the results from in vivo animal and human studies are inconclusive with regards to the direct role of leptin in cardiac hypertrophy [41].
In our study, we found myocardial hypertrophy and an increase in the cell cross-sectional area of cardiomyocytes in rats that received leptin. This pro-hypertrophic effect of leptin was not abolished by JSI-124, indicating that it is not dependent on the JAK/STAT pathway. Additionally, we found that elevated blood leptin levels drove excessive myocardial remodelling, which may lead to the progression of heart failure. As a consequence of structural changes in the myocardium and pro-arrhythmogenic effects, the administration of leptin was also accompanied by LV end-diastolic dimension elevation and a reduction of markers, such as LV systolic function and left ventricular fractional shortening.
Autocrine leptin signalling plays an important role in cardiomyocyte metabolism [2]. This may explain the more pronounced ischemic myocardial damage in rats receiving leptin. Hyperleptinemic rats had a larger infarct size and higher troponin-I concentration compared to the controls. A previous study showed that hearts from obese Zucker rats exhibited larger infarct size following ischemia–reperfusion than the control rats [42]. Our results confirm the findings of an earlier study demonstrating that high leptin levels, which were observed in fat-fed hypertensive rats compared to hypertensive-glucose intolerant rats (with lower leptin levels), and hyperleptinemia resulted in larger myocardial infarct size [43]. More importantly, we have found that JSI-124 preconditioning does not reduce myocardial damage in leptin-treated rats, possibly due to the small dose of JSI-124 or regulation of these processes through other signalling pathways. Similarly, the long-term inhibition of the JAK/STAT pathway (at a JSI-124 dose of 1 mg/kg/d) did not affect hemodynamics (except mean blood pressure), metabolism (except total cholesterol), inflammation, or ischemic myocardial injury in rats with myocardial infarction model compared with the controls.
The upregulation of leptin and the associated elevation of TNF-alpha, IL-6, and atherogenic lipoproteins are known to be involved in the pathological mechanisms of many human diseases, including cardiac, inflammatory, and autoimmune diseases, as well as inflammation-associated cancers [33, 44]. Blocking leptin signalling is effective in treating experimental models of these diseases [45, 46, 47]. Recently, the involvement of leptin in the mechanisms of ischemic heart disease has also been demonstrated [48, 49]. For example, a multicentre retrospective study suggested that blood leptin concentration is a significant risk factor for coronary heart disease in obese subjects [50]. In our study, we artificially elevated leptin levels to reproduce the pathological states caused by inflammatory and dysmetabolic conditions (Figure 6).
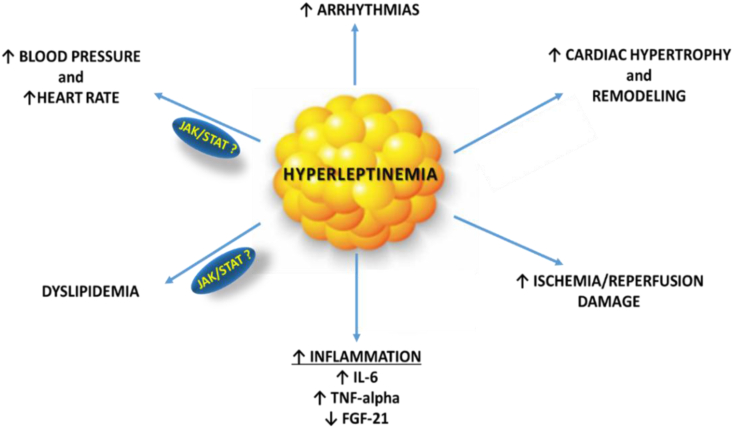
Figure 6.
Potential mechanisms by which hyperleptinemia influences cardiac function, and potential association with the JAK/STAT pathway according to the results of our study. Hyperleptinemia may exert maladaptive effects through hemodynamic factors, such as increased heart rate and blood pressure, metabolic and inflammatory changes, including dyslipidemia, reduced cardiac output, or structural cardiac changes, such as myocardial hypertrophy.
5. Conclusions
In conclusion, our results confirm the role of leptin in mediating leptin-associated mechanisms of metabolic damage and arrhythmogenesis in rats experiencing IRI. Eight-day hyperleptinemia in rats leads to an increase in blood pressure and heart rate, myocardial hypertrophy, impaired LV function, an increase in the frequency of ischemic arrhythmias, dyslipidemia, systemic inflammation, and an increase in the size of induced myocardial infarction. The plasma leptin level enhanced the size of the infarction by 68.9% in the experimental rat model, and an increase in the level of leptin in blood plasma for every 1 ng/ml was found to result in an increase of the infarct size by 0.193%.
Eight-day hyperleptinemia induces JAK/STAT activation and JSI-124 administration reduces JAK2 and STAT3 phosphorylation as confirmed by the protein expression analysis. The direct blockade of the JAK/STAT signalling pathway was effective in reversing the negative effects of leptin, including increased blood pressure and total cholesterol, but did not attenuate ischemic myocardial impairment and inflammation. Based on these findings, we speculate that leptin-JAK/STAT signalling may be a candidate for investigating the aetiology of obesity-related cardiac dysfunctions. Further study will be needed to determine the involvement of different signalling pathways in leptin-induced alterations and the interactions between them. In addition, these findings also imply that drugs targeting the leptin pathways could provide new treatments for cardiac disorders, especially in obese subjects, in the future.
We showed that hyperleptinemia, coinciding with myocardial ischemia and reperfusion, potentiates myocardial remodelling. This occurs via cardiomyocyte hypertrophy and heart remodelling, and may lead to augmented post-myocardial infarction heart failure. Our rat model simulates a clinical scenario of acute MI with delayed reperfusion, or myocardial ischemia and reperfusion in patients who suffer from obesity and/or inflammation, which is associated with endogenous leptin induction, for example, in obese patients.
Declarations
Author contribution statement
Ekaterina A. Polyakova: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Evgeny N. Mikhaylov: Performed the experiments.
Michael M. Galagudza: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Evgeny V. Shlyakhto: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (agreement 075-15-2020-800).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Western blot protein ladders of total and phosphorylated JAK2 and STAT3 and β-actin in cardiomyocytes of rats.
References
- 1.Kang K.W., Ok M., Lee S.K. Leptin as a key between obesity and cardiovascular disease. J. Obes. Metab. Syndr. 2020;29(4):248–259. doi: 10.7570/jomes20120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segers V.F.M., De Keulenaer G.W. Autocrine signaling in cardiac remodeling: a rich source of therapeutic targets. J. Am. Heart Assoc. 2021;10(3) doi: 10.1161/JAHA.120.019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima Júnior J.C., Moura-Assis A., Cintra R.M., Quinaglia T., Velloso L.A., Sposito A.C. Central role of obesity in endothelial cell dysfunction and cardiovascular risk. Rev. Assoc. Med. Bras. 1992;65(1):87–97. doi: 10.1590/1806-9282.65.1.87. [DOI] [PubMed] [Google Scholar]
- 4.Berzabá-Evoli E., Zazueta C., Cruz Hernández J.H., Gómez-Crisóstomo N.P., Juárez-Rojop I.E., De la Cruz-Hernández E.N., Martínez-Abundis E. Leptin modifies the rat heart performance associated with mitochondrial dysfunction independently of its prohypertrophic effects. Internet J. Endocrinol. 2018:6081415. doi: 10.1155/2018/6081415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obradovic M., Sudar-Milovanovic E., Soskic S., Essack M., Arya S., Stewart A.J., Gojobori T., Isenovic E.R. Leptin and obesity: role and clinical implication. Front. Endocrinol. (Lausanne) 2021;12:585887. doi: 10.3389/fendo.2021.585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montserrat-de la Paz S., Pérez-Pérez A., Vilariño-García T., Jiménez-Cortegana C., Muriana F.J.G., Millán-Linares M.C., Sánchez-Margalet V. Nutritional modulation of leptin expression and leptin action in obesity and obesity-associated complications. J. Nutr. Biochem. 2021;89:108561. doi: 10.1016/j.jnutbio.2020.108561. [DOI] [PubMed] [Google Scholar]
- 7.Ganguly R., Khanal S., Mathias A., Gupta S., Lallo J., Sahu S., Ohanyan V., Patel A., Storm K., Datta S., Raman P. TSP-1 (Thrombospondin-1) deficiency protects ApoE-/- mice against leptin-induced atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020;17 doi: 10.1161/ATVBAHA.120.314962. ATVBAHA120314962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H., Li M., Liu L., Zhu D., Tian G. Telmisartan improves myocardial remodeling by inhibiting leptin autocrine activity and activating PPARγ. Exp. Biol. Med. (Maywood) 2020;245(7):654–666. doi: 10.1177/1535370220908215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abe Y., Ono K., Kawamura T., Wada H., Kita T., Shimatsu A., Hasegawa K. Leptin induces elongation of cardiac myocytes and causes eccentric left ventricular dilatation with compensation. Am. J. Physiol. Heart Circ. Physiol. 2007;292(5):H2387–H2396. doi: 10.1152/ajpheart.00579.2006. [DOI] [PubMed] [Google Scholar]
- 10.Gan X.T., Zhao G., Huang C.X., Rowe A.C., Purdham D.M., Karmazyn M. Identification of fat mass and obesity associated (FTO) protein expression in cardiomyocytes: regulation by leptin and its contribution to leptin-induced hypertrophy. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asrih M., Veyrat-Durebex C., Poher A.L., Lyautey J., Rohner-Jeanrenaud F., Jornayvaz F.R. Leptin as a potential regulator of FGF21. Cell. Physiol. Biochem. 2016;38(3):1218–1225. doi: 10.1159/000443070. [DOI] [PubMed] [Google Scholar]
- 12.Sapouckey S.A., Morselli L.L., Deng G., Patil C.N., Balapattabi K., Oliveira V., Claflin K.E., Gomez J., Pearson N.A., Potthoff M.J., Gibson-Corley K.N., Sigmund C.D., Grobe J.L. Exploration of cardiometabolic and developmental significance of angiotensinogen expression by cells expressing the leptin receptor or agouti-related peptide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020;318(5):R855–R869. doi: 10.1152/ajpregu.00297.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moro C., Grauzam S., Ormezzano O., Toufektsian M.C., Tanguy S., Calabrese P., Coll J.L., Bak I., Juhasz B., Tosaki A., de Leiris J., Boucher F. Inhibition of cardiac leptin expression after infarction reduces subsequent dysfunction. J. Cell Mol. Med. 2011;15(8):1688–1694. doi: 10.1111/j.1582-4934.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGaffin K.R., Zou B., McTiernan C.F., O'Donnell C.P. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc. Res. 2009;83:313–324. doi: 10.1093/cvr/cvp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall M.E., Harmancey R., Stec D.E. Lean heart: role of leptin in cardiac hypertrophy and metabolism. World J. Cardiol. 2015;26:511–524. doi: 10.4330/wjc.v7.i9.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGaffin K.R., Witham W.G., Yester K.A., Romano L.C., O’Doherty R.M., McTiernan C.F., O'Donnell C.P. Cardiac-specific leptin receptor deletion exacerbates ischemic heart failure in mice. Cardiovasc. Res. 2011;89:60–71. doi: 10.1093/cvr/cvq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khafaji H.A., Bener A.B., Rizk N.M., Al Suwaidi J. Elevated serum leptin levels in patients with acute myocardial infarction; correlation with coronary angiographic and echocardiographic findings. BMC Res. Notes. 2012;5:262. doi: 10.1186/1756-0500-5-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Syed A.H., Lohana S., Aung N.H., Memon M.K., Shaikh A., Memon S., Hassan S.M., Kumar B. Correlation of leptin with acute myocardial infarction: a case control study. Cureus. 2020;12(12) doi: 10.7759/cureus.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall M.E., Smith G., Hall J.E., Stec D.E. Cardiomyocyte-specific deletion of leptin receptors causes lethal heart failure in Cre-recombinase-mediated cardiotoxicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R1241–R1250. doi: 10.1152/ajpregu.00292.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiérrez-Cuevas J., Sandoval-Rodriguez A., Meza-Rios A., Monroy-Ramírez H.C., Galicia-Moreno M., García-Bañuelos J., Santos A., Armendariz-Borunda J. Molecular mechanisms of obesity-linked cardiac dysfunction: an up-date on current knowledge. Cells. 2021;10(3):629. doi: 10.3390/cells10030629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kain D., Simon A.J., Greenberg A., Ben Zvi D., Gilburd B., Schneiderman J. Cardiac leptin overexpression in the context of acute MI and reperfusion potentiates myocardial remodeling and left ventricular dysfunction. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0203902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamy E., Neves S., Ferreira J., Rodrigues L., da Costa G., Cordeiro C., Fialho L., Lima M., Costa A.R., Antunes C.M., Lopes O., Amado F., Capela E., Silva F. Effects of hyperleptinemia in rat saliva composition, histology and ultrastructure of the major salivary glands. Arch. Oral Biol. 2018;96:1–12. doi: 10.1016/j.archoralbio.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Yuan G., Yan S., Xue H., Zhang P., Sun J., Li G. JSI-124 suppresses invasion and angiogenesis of glioblastoma cells in vitro. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0118894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis M.J., Hancox J.C., Farkas A., Wainwright C.L., Stables C.L., Saint D.A., Clements-Jewery H., Lambiase P.D., Billman G.E., Janse M.J., Pugsley M.K., Ng G.A., Roden D.M., Camm A.J., Walker M.J. The Lambeth Conventions (II): guidelines for the study of animal and human ventricular and supraventricular arrhythmias. Pharmacol. Ther. 2013;139:213-248. doi: 10.1016/j.pharmthera.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Kishore R., Verma S.K. Roles of STATs signaling in cardiovascular diseases. JAK-STAT. 2012;1(2):118–124. doi: 10.4161/jkst.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poetsch M.S., Strano A., Guan K. Role of leptin in cardiovascular diseases. Front. Endocrinol. (Lausanne) 2020;11:354. doi: 10.3389/fendo.2020.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith C.C., Mocanu M.M., Davidson S.M., Wynne A.M., Simpkin J.C., Yellon D.M. Leptin, the obesity-associated hormone, exhibits direct cardioprotective effects. Br. J. Pharmacol. 2006;149(1):5–13. doi: 10.1038/sj.bjp.0706834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruber T., Pan C., Contreras R.E., Wiedemann T., Morgan D.A., Skowronski A.A., Lefort S., De Bernardis Murat C., Le Thuc O., Legutko B., Ruiz-Ojeda F.J., Fuente-Fernández M., García-Villalón A.L., González-Hedström D., Huber M., Szigeti-Buck K., Müller T.D., Ussar S., Pfluger P., Woods S.C., Ertürk A., LeDuc C.A., Rahmouni K., Granado M., Horvath T.L., Tschöp M.H., García-Cáceres C. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metabol. 2021;33(6):1155–1170. doi: 10.1016/j.cmet.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlyle M., Jones O.B., Kuo J.J., Hall J.E. Chronic cardiovascular and renal actions of leptin: role of adrenergic activity. Hypertension. 2002;39(2 Pt 2):496–501. doi: 10.1161/hy0202.104398. [DOI] [PubMed] [Google Scholar]
- 30.Leon-Cabrera S., Solís-Lozano L., Suárez-Álvarez K., González-Chávez A., Béjar Y.L., Robles-Díaz G., Escobedo G. Hyperleptinemia is associated with parameters of low-grade systemic inflammation and metabolic dysfunction in obese human beings. Front. Integr. Neurosci. 2013:7. doi: 10.3389/fnint.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Calvo R., Samino S., Guaita-Esteruelas S., Martínez-Micaelo N., Heras M., Girona J., Yanes O., Correig X., Masana L. Plasma glucose, triglycerides, VLDL, leptin and resistin levels as potential biomarkers for myocardial fat in mice. Clín. Invest. Arterioscler. 2020;32(1):8–14. doi: 10.1016/j.arteri.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Barouch L.A., Gao D., Chen L., Miller K.L., Xu W., Phan A.C., Kittleson M.M., Minhas K.M., Berkowitz D.E., Wei C., Hare J.M. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ. Res. 2006;98(1):119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 33.Polyakova E.A., Mikhaylov E.N., Sonin D.L., Cheburkin Y.V., Galagudza M.M. Neurohumoral, cardiac and inflammatory markers in the evaluation of heart failure severity and progression. J. Geriatr. Cardiol. 2021;18(1):E1–E20. doi: 10.11909/j.issn.1671-5411.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubio B., Mora C., Pintado C., Mazuecos L., Fernández A., López V., Andrés A., Gallardo N. The nutrient sensing pathways FoxO1/3 and mTOR in the heart are coordinately regulated by central leptin through PPARβ/δ. Implications in cardiac remodeling. Metabolism. 2021;115:154453. doi: 10.1016/j.metabol.2020.154453. [DOI] [PubMed] [Google Scholar]
- 35.Biondi R.B., Salmazo P.S., Bazan S.G.Z., Hueb J.C., de Paiva S.A.R., Sassaki L.Y. Cardiovascular risk in individuals with inflammatory bowel disease. Clin. Exp. Gastroenterol. 2020;13:107–113. doi: 10.2147/CEG.S243478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rawla P. Cardiac and vascular complications in rheumatoid arthritis. Reumatologia. 2019;57(1):27–36. doi: 10.5114/reum.2019.83236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang S., Frangogiannis N.G. Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. Br. J. Pharmacol. 2018;175(9):1377–1400. doi: 10.1111/bph.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murata T., Asanuma K., Ara N., Iijima K., Hatta W., Hamada S., Asano N., Koike T., Imatani A., Masamune A., Shimosegawa T. Leptin aggravates reflux esophagitis by increasing tissue levels of macrophage migration inhibitory factor in rats. Tohoku J. Exp. Med. 2018;245(1):45–53. doi: 10.1620/tjem.245.45. [DOI] [PubMed] [Google Scholar]
- 39.Pretz D., Le Foll C., Rizwan M.Z., Lutz T.A., Tups A. Hyperleptinemia as a contributing factor for the impairment of glucose intolerance in obesity. The FASEB J. Feb. 2021;35(2) doi: 10.1096/fj.202001147R. [DOI] [PubMed] [Google Scholar]
- 40.Tajmir P., Ceddia R.B., Li R.K., Coe I.R., Sweeney G. Leptin increases cardiomyocyte hyperplasia via extracellular signal-regulated kinase- and phosphatidylinositol 3-kinase-dependent signaling pathways. Endocrinology. 2004;145(4):1550–1555. doi: 10.1210/en.2003-1128. [DOI] [PubMed] [Google Scholar]
- 41.Piñieiro R., Iglesias M.J., Eiras S., Viñuela J., Lago F., González-Juanatey J.R. Leptin does not induce hypertrophy, cell cycle alterations, or production of MCP-1 in cultured rat and mouse cardiomyocytes. Endocr. Res. 2005;31:375–386. doi: 10.1080/07435800500456937. [DOI] [PubMed] [Google Scholar]
- 42.Katakam P.V., Jordan J.E., Snipes J.A., Tulbert C.D., Miller A.W., Busija D.W. Myocardial preconditioning against ischemia–reperfusion injury is abolished in Zucker obese rats with insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R920–R926. doi: 10.1152/ajpregu.00520.2006. [DOI] [PubMed] [Google Scholar]
- 43.Mozaffari M.S., Schaffer S.W. Myocardial ischemic-reperfusion injury in a rat model of metabolic syndrome. Obesity. 2008;16(10):2253–2258. doi: 10.1038/oby.2008.356. [DOI] [PubMed] [Google Scholar]
- 44.Battineni G., Sagaro G.G., Chintalapudi N., Amenta F., Tomassoni D., Tayebati S.K. Impact of obesity-induced inflammation on cardiovascular diseases (CVD) Int. J. Mol. Sci. 2021;22(9):4798. doi: 10.3390/ijms22094798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang K.P., Goodson M.L., Vang W., Li H., Page A.J., Raybould H.E. Leptin signaling in vagal afferent neurons supports the absorption and storage of nutrients from high-fat diet. Int. J. Obes. (Lond) 2021;45(2):348–357. doi: 10.1038/s41366-020-00678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murayama S., Yamamoto K., Fujita S., Takei H., Inui T., Ogiso B., Kobayashi M. Extracellular glucose-dependent IPSC enhancement by leptin in fast-spiking to pyramidal neuron connections via JAK2-PI3K pathway in the rat insular cortex. Neuropharmacology. 2019;149:133–148. doi: 10.1016/j.neuropharm.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 47.Yang R., Barouch L.A. Leptin signaling and obesity: cardiovascular consequences. Circ. Res. 2007;101(6):545–559. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 48.Mohamadshahi M., Haybar H., Mousavi-Borazjani A., Haghighizadeh M., Abiri B. The association between dietary patterns with severity of coronary artery stenosis, serum leptin-to-adiponectin ratio, and some related risk factors in patients with coronary artery disease. J. Diabetes Metab. Disord. 2021;20(1):697–708. doi: 10.1007/s40200-021-00801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao P., Shi J., Liu X. Associations of leptin and leptin receptor genetic variants with coronary artery disease: a meta-analysis. Biosci. Rep. 2019;39(6) doi: 10.1042/BSR20190466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chai S.B., Sun F., Nie X.L., Wang J. Leptin and coronary heart disease: a systematic review and meta-analysis. Atherosclerosis. 2014;233(1):3–10. doi: 10.1016/j.atherosclerosis.2013.11.069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Western blot protein ladders of total and phosphorylated JAK2 and STAT3 and β-actin in cardiomyocytes of rats.
Data Availability Statement
Data will be made available on request.