Abstract
Hemodialysis-central venous catheter (HD-CVC) insertion is a most often performed procedure, with approximately 80% of patients with end-stage kidney disease in the United States initiating kidney replacement therapy through a HD-CVC. Certain adverse events arising from HD-CVC placement, including catheter-related bloodstream infections (CR-BSIs), thrombosis, and central vein stenosis, can complicate the clinical course of patients and lead to considerable financial impact on the health care system. Medical professionals with different training backgrounds are responsible for performing this procedure, and therefore, comprehensive operator guidelines are crucial to improve the success rate of HD-CVC insertion and prevent complications. In this review article, we not only discuss the basic principles behind the use of HD-CVCs but also address frequently asked questions and myths regarding catheter asepsis, length selection, tip positioning, and flow rate assessment.
Keywords: central venous catheter, cuffed catheter, dialysis vascular access, hemodialysis, tunneled catheter
HD-CVC is a type of central venous access device specifically designed to facilitate kidney replacement therapy in patients without a functioning long-term HD vascular access. The lumen of a HD-CVC has a larger diameter compared with that of a typical CVC used for infusion to provide a desired extracorporeal blood flow of 300 to 400 ml/min. Common indications for HD-CVC placement include acute kidney injury requiring HD support, therapeutic apheresis, end-stage kidney disease without previous creation of a permanent vascular access (arteriovenous fistula or graft), or dysfunction of a preexisting vascular access.1
Complications arising from HD-CVC placement include CR-BSIs, thrombosis, and venous stenosis, which contribute significantly to morbidity and mortality of a patient at a considerable economic burden on the society.2,3 Although there have been concerted efforts to increase early establishment of arteriovenous fistula and graft access, approximately 80% of patients with end-stage kidney disease in the United States continue to require a HD-CVC to initiate kidney replacement therapy,4 possibly owing to late referrals to nephrologists,5 socioeconomic status,6 and lack of patient awareness regarding different HD modalities.
Consequently, a range of medical professionals with varying levels of skills and competency are entrusted with the placement of HD-CVCs. Therefore, rigorous guidelines for operator training are imperative to mitigate the risk of complications and improve success rates of HD-CVC insertion, such as those provided by medical organizations, including the American Society of Diagnostic and Interventional Nephrology.7
In this review article, we provide a comprehensive overview of different types of HD-CVCs and the approach to device and access site selection to address common myths circulating among trainees and nonproceduralists. Furthermore, we provide a comprehensive overview regarding prevention of CR-BSIs, benefit of ultrasound guidance, and appropriate catheter site, length selection, and tip positioning.
Types of HD-CVCs and Access Site Considerations
Nontunneled Versus Tunneled HD-CVCs
A nontunneled HD-CVC is intended for short-term access (up to 1 week in duration) when kidney replacement therapy is indicated for acute and emergent situations.1 A tunneled HD-CVC is preferred when intermediate- or long-term HD vascular access is required.8
A tunneled HD-CVC is different in several aspects compared with a nontunneled HD-CVC. First, a tunneled HD-CVC has a Dacron cuff that wraps around the tubing, allowing tissue integration to anchor the catheter inside the tunnel in 4 to 6 weeks and protect against pericatheter bacterial entry into the bloodstream.9 A systematic review by Maki et al.10 reported that tunneled HD-CVCs are associated with lower rates of CR-BSIs compared with nontunneled HD-CVCs (1.6 vs. 4.8 per 1000 catheter days; 95% CI 1.5–1.7 and 4.2–5.3, respectively). Second, tunneled HD-CVCs are made with soft polymers with a soft flexible tip and are less prone to mechanical complications, such as vessel perforation, as compared with the more rigid nontunneled catheters (relative risk [RR] 13.6; P = 0.001).11 Third, tunneled HD-CVCs provide greater average blood flow rates than nontunneled HD-CVCs, not only because they are available in larger sizes, such as 15.5 or 16 Fr as opposed to 13.5 Fr, but also owing to the positioning of the catheter tip in the upper to mid-right atrium instead of the superior vena cava.12
Consequently, nontunneled HD-CVCs are primarily used in situations when kidney recovery is anticipated within 1 week or if patients cannot be transported to the procedure room owing to cardiorespiratory instability or if there are contraindications to tunneled HD-CVC insertion, such as severe, uncorrectable coagulopathies, uncontrolled sepsis, or chronic infections. Although the safety of conversion of nontunneled to tunneled HD-CVCs is similar to de novo placement of tunneled HD-CVCs with no difference in the rates of catheter dysfunction or CR-BSI,13, 14, 15 the de novo placement of tunneled HD-CVCs may still be preferred in light of their aforementioned advantages, even in the intensive care unit, especially because there are no known predictors of recovery of kidney function in <1 week.16
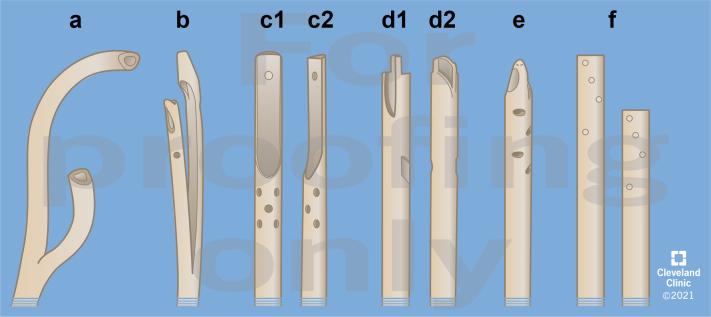
Tunneled HD-CVCs are manufactured in multiple unique designs. Many distinguishable features involve catheter tip design and shape and location of the side holes of the catheter (Fig. 1 for tunneled HD-CVC tip designs). These include 2 separate single lumen catheters or double lumen catheters with standard split tip, preformed curved split tip, step tip, and symmetrical tip with side slots or holes. Although the designs seem to improve blood flow, reduce recirculation, and mitigate the risk of catheter tip occlusion with in vivo tests, 1 meta-analysis observed no difference when comparing these different designs in terms of long-term functional outcomes, albeit the preformed curved split tip design was not evaluated in this comparison.17
Figure 1.
Tunneled hemodialysis catheter tips. (a) Split tip with preformed curved tips. (b) Split tip standard. (c) c1/c2—step tip. (d) d1/d2—symmetric tip with side slots. (e) Symmetric tip with side holes. (f) Dual catheter (e.g., Tesio twin catheter).
Coated Versus Uncoated HD-CVCs
Antimicrobial- and antithrombogenic-coated catheters have been found to reduce CR-BSIs and thrombosis in conventional CVCs used for infusion. Two meta-analyses of chlorhexidine-silver sulfadiazine–coated catheters revealed significant reductions in CR-BSI (odds ratio 0.56; 95% CI 0.37–0.84; P = 0.005 and odds ratio 0.68; 95% CI 0.47–0.98, respectively) and significantly lower rates of catheter colonization (odds ratio 0.44; 95% CI 0.36–0.54; P < 0.001 and odds ratio 0.51; 95% CI 0.42–0.61, respectively).18,19 Similarly, heparin-bonded CVCs for infusion are associated with a significantly lower rate of catheter thrombosis (8% vs. 44%; P = 0.004).20 Consequently, antimicrobial-coated catheters are preferred in situations where adherence to maximal antisepsis measures have not adequately reduced rates of CR-BSI.21,22 Nevertheless, it must be acknowledged that antimicrobial coatings may only help to prevent CR-BSIs that occur secondary to bacterial pericatheter migration along the subcutaneous course of the catheter, with little influence on the risk of catheter hub contamination and hematogenous spread.23
In contrast, according to a systematic review, antimicrobial-coated HD-CVCs failed to significantly reduce rates of CR-BSIs compared with noncoated HD-CVCs,24 and observational studies revealed similar rates of catheter dysfunction between heparin-coated and uncoated HD-CVCs.25,26 Therefore, even though surface-coated CVCs for infusion are routinely used in many centers in the United States, randomized controlled trials are required to reveal the long-term efficacy of a coating in the dialysis setting. Given the inadequate clinical data, the potential additional cost of using surface-coated HD-CVCs is yet to be justified,2 because an antimicrobial-coated nontunneled HD-CVC is estimated to cost at approximately $20 more than an uncoated catheter, whereas a tunneled HD-CVC with surface heparinization costs approximately $100 more than a standard catheter.27
Internal Jugular Versus Subclavian Versus Femoral HD-CVCs
The right internal jugular vein is the preferred site for insertion of both nontunneled and tunneled HD-CVCs as it provides a direct path into the superior vena cava, minimizing difficulties during catheter placement, as opposed to the left internal jugular vein, which requires the HD-CVC to make 2 right angle bends and an anteroposterior bend over the pulmonary arch before reaching the superior vena cava.28,29 Left-sided internal jugular HD-CVCs have also been found to be associated with higher rates of infection (0.50 vs. 0.27 per 100 catheter days; P = 0.005) and catheter dysfunction (0.25 vs. 0.11 per 100 catheter days; P = 0.036) compared with right-sided jugular catheters.30
The common femoral vein may be accessed for HD in patients with occluded jugular veins or when the jugular veins are occupied by other central lines. Femoral HD access circumvents complications associated with central thoracic vein access, including pneumothorax and air embolism. Furthermore, a randomized controlled trial comparing jugular and femoral vein catheterization revealed that the rates of catheter colonization (35.7 vs. 40.8 per 1000 catheter days, respectively; P = 0.031) and CR-BSI (2.3 vs. 1.5 per 1000 catheter days, respectively; P = 0.42) were similar in both groups.31 Nevertheless, femoral access should be avoided in patients with higher body mass indices as femoral catheterization can significantly increase the incidence of catheter colonization (50.9 vs. 24.5 per 1000 catheter days; P < 0.001) in patients with body mass index > 28.4.31 Femoral vein catheterization should especially be avoided in patients with abdominal wall obesity or panniculus morbidus, because the panniculus, consisting of excess subcutaneous fat, can extend to cover the femoral catheter site, predisposing the patient to recurrent skin exit-site infections and CR-BSIs.32 Furthermore, in the absence of clinical factors, such as emergency situations, respiratory distress, and uncooperative patients, nonfemoral access points are generally preferred owing to ease of care and ability to permit ambulation.33
Lastly, subclavian vein catheterization for HD should be avoided because the risk of developing vein stenosis with subclavian catheters is 4-fold compared with that with internal jugular catheters, compromising the potential placement of permanent arteriovenous HD access.34
Appropriate Length Selection for HD-CVCs
Myth—Internal Jugular and Femoral HD-CVCs Are Always 15 Cm and 20 Cm in Length, Respectively
The length of a HD-CVC is determined by the type of central vein being accessed and the desired catheter tip location. Nevertheless, ideal catheter length is also dependent on laterality of the catheter location and the patient’s body habitus (see Table 1 for available lengths of tunneled HD-CVCs).
Table 1.
Available lengths for tunneled HD-CVCs
| Location of tunneled HD-CVCs | Length of tunneled HD-CVCs (cm) |
|---|---|
| Right internal jugular | 19– 31 |
| Left internal jugular | 23–36 |
| Right femoral | 36–55 |
| Left femoral | 55 |
HD-CVC, hemodialysis-central venous catheters.
The optimal tip location for nontunneled internal jugular HD-CVCs is considered to be the caudal superior vena cava or pericavoatrial junction,1 and catheters approximately 15 cm in length are deemed appropriate for right-sided catheters. In contrast, the tip of tunneled internal jugular HD-CVCs should be positioned within the upper to mid-right atrium,35 with corresponding catheters ranging from 19 to 31 cm in length (tip to cuff).
The length of left-sided internal jugular HD-CVCs tends to be greater because catheters inserted from the left negotiate the angulation of the brachiocephalic vein to enter the superior vena cava. The likelihood of complications, such as central vein erosion and perforation, is associated with the angle of catheter impingement on the superior vena cava.36 Consequently, with left-sided catheters having to make 2 necessary right-angled bends and an anteroposterior curve over the pulmonary arch along their route, they can erode the weak lateral wall of the superior vena cava and potentially lead to perforation.37,38 Therefore, left-sided internal jugular HD-CVCs are typically placed after choosing an appropriate length, such that the catheter tip resides in the upper right atrium so that they lie parallel within the long axis of the superior vena cava.35,39
Adequate flow rates for nontunneled femoral HD-CVCs are best observed with the catheter tip residing centrally in the inferior vena cava.40 A total of 15 to 20 cm nontunneled femoral HD-CVCs will typically only extend to the level of the common iliac vein, resulting in higher recirculation and catheter dysfunction.41 Therefore, when placing tunneled femoral HD-CVCs for long-term use, longer catheters (33–45 cm) are inserted for extension into the inferior vena cava.42
There have been multiple studies that have looked at the pediatric population for optimal length of insertion of right- and left-sided CVCs with their analyses revealing age, height, and weight had significant correlations with optimal insertion lengths.43, 44, 45 In contrast, formulas for CVC length based on height have been investigated for adults, but there is no substantial evidence supporting their use.46,47 Nevertheless, 1 study did reveal that obesity significantly influences HD-CVC migration with median catheter tip migration between inspiration and expiration measured at 15 mm (interquartile range 5–23 mm) in obese patients versus 9 mm (interquartile range 6–18 mm) in the nonobese group (P < 0.001).48 Nonetheless, validated formulae based on height and weight are still required in the adult population to select appropriate length of HD-CVCs.
Although HD-CVC length is influenced by the factors mentioned previously (desired catheter tip location, type and laterality of central vein, patient’s body habitus), fluoroscopic guidance continues to be integral in the determination of the appropriate length of HD-CVCs because it can help accurately position the tip of the catheter.48
Overview of Complications of HD-CVCs
Complications associated with HD-CVCs can broadly be divided into the following 2 categories: (i) immediate mechanical complications associated with catheter insertion and access-related issues and (ii) delayed (>1 week) complications, such as catheter-related infection, central vein stenosis or thrombosis, and catheter dysfunction.
Rates of mechanical access-related complications, such as pneumothorax, venous air embolism, arterial injury, arrhythmias, and catheter malposition, have precipitously decreased with the routine use of ultrasound and fluoroscopic guidance for HD-CVC placement.49, 50, 51
CR-BSIs occur in patients with HD-CVCs at a rate ranging from 0.6 to 6.5 episodes per 1000 catheter days,52,53 and the Hemodialysis (HEMO) study revealed that although only 7.6% of all patients had HD-CVCs for vascular access, this group accounted for 32% of all patients hospitalized for access-related infections.54 Consequently, arteriovenous fistulas and grafts are preferred over HD-CVCs for vascular access.
Central venous obstruction, including stenosis and thrombosis, is strongly associated with central vein cannulation because catheters can directly cause vessel injury at the point of cannulation and within the vein, leading to areas of fibrotic stenosis on healing.55 Furthermore, HD-CVCs are associated with higher blood flows when compared with conventional CVCs used for infusion, creating areas of turbulent flow beyond the tip of the catheter, stimulating endothelial proliferation and subsequent fibrosis and stenosis/thrombosis.56 The incidence of central vein stenosis in patients with HD-CVCs is approximately 20% to 40%,57 with the risk amplified by longer catheter dwell time and the choice of vascular access site (increased with placement in the left internal jugular or subclavian vein vs. the right internal jugular or femoral vein).58
Catheter dysfunction leading to inadequate dialysis has been defined as the inability to maintain an extracorporeal blood flow sufficient to perform HD without significantly lengthening treatment.1 It can be categorized as early or late catheter dysfunction, with early dysfunction defined as a catheter that never performed adequately after insertion, typically owing to improper positioning of the tip, kinking, or constriction by exit-site sutures.59 In contrast, late catheter dysfunction occurs mostly secondary to thrombotic occlusion or fibrin sheath formation after a catheter has previously functioned adequately.60
Infection Control Measures for HD-CVCs
CR-BSIs with HD-CVCs can be prevented by adhering to established protocols for sterile technique, including hand hygiene before insertion, ensuring maximal barrier precautions and chlorhexidine skin antisepsis, strict maintenance of asepsis while handling needles, guidewires and catheters, and suture-less securement and maintenance of HD-CVCs.61 Proper maintenance of HD-CVCs entails catheter hub disinfection and application of antimicrobial ointments during dressing changes.62
Maintaining adequate hand hygiene, preferably with chlorhexidine-based surgical scrubs before donning sterile gloves, remains one of the most important measures for the prevention of catheter-associated infections. One study investigating the effectiveness of hand-cleansing methods by plate culturing the fingers of subjects for 24 hours revealed that alcohol-based cleansers were significantly less effective than the chlorhexidine gluconate-based surgical scrubs (P < 0.001).63 Maximal barrier precautions dictate that all operators should wear a nonsterile mask and cap, sterile gown, and gloves along with a sterile full-body drape placed on the patient and a long sterile cover over the ultrasound probe.22 Although a randomized controlled trial64 which compared maximal barrier precautions with a control group involving only gloves and a small drape revealed indeterminate findings for decreased catheter colonization and CR-BSI, several observational studies have found that hand hygiene in combination with maximal barrier precautions reduces the frequency of CR-BSI.65,66
Furthermore, the use of a chlorhexidine-based antiseptic solution for skin disinfection at the catheter insertion site reduces the risk of infection and has been found to be superior to povidone-iodine in minimizing catheter colonization and CR-BSI. A 49% reduction in CR-BSI (RR 0.51; 95% CI 0.27–0.97) was revealed in a meta-analysis of 8 randomized controlled trials comparing chlorhexidine with aqueous povidone-iodine for disinfection of the site of catheter insertion.67 Similarly, alcohol-based povidone-iodine was associated with a higher incidence of CR-BSI than chlorhexidine-alcohol in a randomized trial (1.77 vs. 0.28 per 1000 catheter days, respectively; hazard ratio 0.15; 95% CI 0.05–0.41; P = 0.0002).68
The hub of a HD-CVC refers to the end that connects to the blood lines or cap. Catheter hub contamination is a risk factor for CR-BSI.69 The Centers for Disease Control and Prevention/Healthcare Infection Control Practices Advisory Committee guidelines for the prevention of intravascular catheter-related infections have described the “scrub the hub” protocol,70 which delineates a recommended approach to preparing catheter hubs before accessing the HD-CVC. This involves using a scrubbing device with chlorhexidine and alcohol to disinfect the catheter hub and stopcocks. One observational study that was conducted to evaluate the efficacy of the “scrub the hub” protocol before accessing central line hubs depicted a 65% reduction in CR-BSI events in the inpatient dialysis population in the postimplementation period (P = 0.0493).71
Moreover, the application of exit-site antimicrobial agents at the catheter site during dressing changes may prevent HD-CVC–related infections. Nevertheless, it should be kept in mind that resistance to topical antimicrobial agents is a potential risk of such therapies, and mupirocin resistance, for instance, is an emergent problem in the United States.72 One case-control study also reported increased risk of developing yeast-positive exit-site cultures with the use of polysporin double ointment (bacitracin and polymyxin B) in the treatment of exit-site infections with central HD-CVCs.73 Similarly, a randomized controlled trial involving peritoneal dialysis catheters revealed increased fungal exit-site infections in patients using polysporin triple ointment (bacitracin/gramicidin/polymyxin B) compared with those using mupirocin (0.07 vs. 0.01; P = 0.02).74 Nonetheless, topical antimicrobial ointments have been recommended by the Society of Critical Care Medicine and the Infectious Diseases Society of America70 specifically for HD-CVCs after insertion and at the end of each HD session owing to a quality improvement project that revealed a 20% reduction in bloodstream infections (P < 0.001) and a decrease in sepsis-related hospitalizations (0.069 per catheter-year vs. 0.095 per catheter-year in controls; P < 0.05).75 Similarly, a meta-analysis also reported that the use of topical antibiotics compared with no antibiotic therapy reduced rates of catheter-related bacteremia (RR 0.22; 95% CI 0.12–0.40) and exit-site infections (RR 0.17; 95% CI 0.08–0.38).76
Prophylactic antimicrobial catheter locking solutions and caps may be considered in patients at increased risk for recurrent CR-BSI, especially in facilities with uncontrolled rates of infection.77 Although a systematic review24 involving 3005 catheters revealed decreased risk of CR-BSI with antimicrobial lock solutions (RR 0.33; 95% CI 0.24–0.45) and a multicenter randomized controlled trial78 revealed a significant 69% reduction in the rate of CR-BSIs when using an antibacterial barrier cap device containing a chlorhexidine-coated rod compared with standard practices (0.22 vs. 0.72 per 1000 catheter days; P = 0.01), there are still concerns regarding the development of antibiotic resistant organisms with prolonged exposure to antimicrobial agents.79 One potential strategy to address these concerns would be to use antibiotics, such as minocycline, which are not typically administered to combat serious infections. As such, a randomized open-label trial80 was able to reveal a lower rate of catheter-related bacteremia with a lock solution containing minocycline/ethylenediamine tetraacetic acid compared with heparin (1.1 vs. 4.3 per 1000 catheter days; P = 0.005). Nonetheless, in contrast to the recommendations regarding exit-site antimicrobial agents and catheter hub disinfection, guidelines still do not advocate for the routine use of prophylactic antimicrobial catheter locking solutions or caps.1
Lastly, in addition to their antithrombotic properties, prophylactic nonantimicrobial catheter locking solutions, including heparin and citrate, have also been investigated with regard to their ability to inhibit or promote biofilm formation, which can contribute to the development of bacteremia. An in vitro study revealed that heparin promotes biofilm, whereas citrate inhibits its development at levels > 0.5%.81 Accordingly, a randomized controlled trial revealed that rates of CR-BSI in tunneled and nontunneled HD-CVCs were lower with trisodium citrate (1.1 per 1000 catheter days) than with heparin locking solution (4.1 per 1000 catheter days) (P < 0.001).82
Utility of Ultrasound Guidance in HD-CVC Placement
Ultrasonography is integral to various aspects of establishing central vascular access for HD. Ultrasound is used to assess vein size and patency to determine the minimum luminal diameter required to place a large bore HD-CVC (see Table 2 for available diameters of HD-CVCs).83 It is also used for monitoring the progression of the catheter after insertion84 and identifying early puncture-related complications, such as pneumothorax, nerve injury, or local hematoma, and late complications, such as catheter malposition and venous thrombosis.85
Table 2.
Utility of ultrasound in vein size assessment: determination of the minimum luminal diameter required to insert central venous access devices
| Type of CVC | CVC size (Fr) | Outer diameter of CVC (mm) |
|---|---|---|
| Nontunneled HD-CVC | 11.5–13.5 | 3.8–4.5 |
| Tunneled HD-CVC | 14.5–16 | 4.8–5.3 |
| Single lumen CVC for infusion | 5–8 | 1.7–2.7 |
Note. 1 Fr = 0.33 mm.
HD-CVC, hemodialysis-central venous catheters; Fr, French.
Furthermore, the use of ultrasound also increases the chances of initial successful insertion of HD-CVCs, mitigating the risk of developing the aforementioned early and late complications, reducing patient stress and pain, and consequently improving patient satisfaction.86 A meta-analysis87 reported that ultrasound-guided HD-CVC placement not only decreases the risk of early puncture-related complications, such as access site hematoma (RR 0.27; 95% CI 0.08–0.88) and arterial puncture (RR 0.22; 95% CI 0.06–0.81), but also increases the likelihood of the catheter being placed successfully on first attempt (RR 0.40; 95% CI 0.29–0.56).
For internal jugular vein HD access in particular, an ultrasound-guided technique provides an important advantage because it can allow the operator to puncture the vein at a site in close proximity to the clavicle, which may potentially decrease the likelihood of kinking and developing catheter dysfunction. Similarly, for femoral HD-CVCs, ultrasonography was found to reduce the rate of complications (5.5% vs. 18.2%; P = 0.039) and improve the first attempt success rate (85.5% vs. 54.5%; P = 0.000) when compared with an anatomical landmark-guided technique.88
Optimal HD-CVC Tip Positioning
Myth—Optimal Catheter Tip Position for Internal Jugular HD-CVCs Is Always at the Junction of Superior Vena Cava and Right Atrium
Atrial placement has been traditionally avoided with noncompliant, stiff-tipped nontunneled catheters because they can increase the likelihood of cardiac complications.89 Therefore, the distal tip of nontunneled jugular HD-CVCs is typically positioned in the lower superior vena cava or pericavoatrial junction.1 In contrast, tunneled HD-CVCs have been found to malfunction if they end up being positioned in the superior vena cava or brachiocephalic vein.35 A retrospective analysis of internal jugular HD-CVCs reported that left-sided catheters terminating in the right atrium had significantly fewer episodes of catheter malfunction when compared with catheters that ended up in the superior vena cava or pericavoatrial junction (0.35 vs. 0.84, respectively; P = 0.006).30 A systematic review revealed that cardiac tamponade is an extremely rare complication owing to vessel/cardiac perforation caused by the tip of a central line positioned inside the right atrium.90 Furthermore, catheter placement within the right atrium does not seem to increase the risk of arrhythmias significantly.35 Therefore, the optimal position for the tip of tunneled jugular HD-CVCs is within the upper right atrium with the patient placed supine during the procedure.1
Confirmation of HD-CVC Tip Position
Myth—Confirmation of Catheter Tip Positioning Is Only Needed for Internal Jugular HD-CVCs
Confirmation of HD-CVC tip location is typically performed by radiography, fluoroscopy, or ultrasound. Although fluoroscopy still represents the most accurate method for confirming catheter tip positioning, in nonlife-threatening scenarios when fluoroscopy has not been used for placement, a chest radiograph is usually obtained after placement of internal jugular catheters to confirm the course of the catheter and tip position.91 In contrast, in emergency situations, one study suggests that when immediate use of a nontunneled right internal jugular catheter may be required, routine radiography for confirmation of tip positioning may be bypassed in favor of early initiation of therapy, albeit the study did not evaluate nontunneled HD-CVCs.92 Ultrasound of heart and central vessels using a phased array probe is another potential modality that may be used in emergency scenarios to confirm catheter positioning and detect early puncture-related complications, such as pneumothorax or hemothorax.93
Traditionally, femoral venous catheters, unlike internal jugular venous catheters, have been used immediately after placement without confirmation of positioning. An abdominal film is performed to confirm the course of the catheter and position of the tip only if the femoral catheter malfunctions. Nevertheless, it may be necessary in certain situations to ensure proper femoral catheter positioning before clinical use. For instance, the correct placement of femoral HD-CVCs is crucial to their function because acceptable flow rates can often only be maintained with a femoral catheter tip that is present in the common iliac vein or caudal inferior vena cava. Furthermore, when placing tunneled femoral HD-CVCs for long-term use, flow rates are often better achieved with catheters extending centrally into the inferior vena cava.42
Flow Rate Assessment—Right- Versus Left-Sided HD-CVCs
Myth—There Is No Difference in Flow Rate Between a Right-Sided and a Left-Sided Internal Jugular HD-CVC
According to Poiseuille’s Law, the resistance to flow in a tube is directly proportional to the length of the tube. Blood flow rates are consistently higher with right-sided jugular HD-CVCs than with left-sided catheters because placement of catheters into the left internal jugular vein requires that the catheter make 2 right angle bends and an anteroposterior bend over the pulmonary arch before reaching the superior vena cava.28,29 There is additional resistance to flow not only from the multiple bends but also from the longer length of the catheter for left-sided venous access.
Consequently, the right internal jugular vein, which traverses a relatively straighter path to the superior vena cava is preferred for HD access because there are fewer challenges faced during catheter insertion and there is a lower incidence of catheter dysfunction. As mentioned previously, HD-CVC dysfunction has been defined as the inability to maintain an extracorporeal blood flow sufficient to perform HD without significantly lengthening treatment.1 A retrospective analysis of jugular HD-CVCs depicted that left-sided internal jugular HD-CVCs had higher rates of catheter dysfunction (0.25 vs. 0.11 per 100-catheter days; P = 0.036) compared with those inserted from the right.30 Therefore, the longer path that a left-sided catheter has to traverse predisposes it to developing catheter dysfunction as opposed to a right-sided catheter, which has a relatively shorter, less meandering course to the superior vena cava.
Concomitant Insertion of a HD-CVC With a CVC for Infusion
Myth—a HD-CVC and a CVC for Infusion Cannot Be Simultaneously Placed in the Same Central Vein
The prospect of concomitant placement of multiple catheters in a single central vein may ignite concerns regarding increased risk of catheter-related complications, including infections, thrombosis, and puncture-related complications, such as pneumothorax. Furthermore, with concomitant placement of a HD-CVC with a CVC for infusion, there may even be concerns regarding HD-CVC dysfunction. A retrospective analysis94 compared patients who had undergone concomitant placement of a tunneled HD-CVC and a CVC for infusion with patients who only had a HD-CVC placed in their right internal jugular vein. No significant differences were found between the aforementioned 2 groups of patients in the incidence of thrombosis (1.0% vs. 0.0%; P > 0.999), line infection (2.1% vs. 0.0%; P = 0.519), or line dysfunction (2.1% vs. 0.0%; P = 0.516). No puncture-related complications, such as pneumothorax, were reported for either group. Therefore, even though multiple catheters are typically not routinely placed in the same central vein, the simultaneous placement of multiple catheters may be considered if necessary, without increasing the risk of complications.
In addition, multiple techniques for placing guidewires and catheters were used throughout the duration of the study. In some instances, both guidewires were placed first, followed by insertion of both catheters. At other times, the infusion catheter guidewire and infusion catheter were inserted first, followed by the dialysis catheter guidewire and dialysis catheter being placed. Although catheter damage was not observed with either technique in the study, placing both guidewires first might be preferred to err on the side of caution and prevent complications.
Conclusions
Although arteriovenous access is still preferred for establishing permanent HD access, tunneled HD-CVCs continue to be a reasonable long-term option for a select patient population, especially those with limited life expectancy and who have anatomical vascular issues with multiple failed attempts at establishing fistulas/grafts. In contrast, nontunneled HD-CVCs continue to be important for providing short-term vascular access for emergent kidney replacement therapy in the inpatient setting. Nevertheless, it must be acknowledged that HD-CVCs lead to a myriad of complications ranging from immediate placement-related complications to delayed adverse events, including central vein stenosis/thrombosis and CR-BSIs, not only associated with significant morbidity and mortality resulting in increasing health care cost. This review article is intended for physicians looking for a comprehensive resource for reviewing the principles governing the use of HD-CVCs and addressing common misconceptions, with the ultimate goal to enhance awareness and implementation of strategies, which have been found to improve safety of HD-CVC insertion and reduce the incidence of catheter-related complications.
Disclosure
All the authors declared no competing interests.
References
- 1.Lok C.E., Huber T.S., Lee T., et al. KDOQI Clinical Practice Guideline for vascular access: 2019 update [published correction appears in. Am J Kidney Dis. 2021;77:551] Am J Kidney Dis. 2020;75(suppl 2):S1–S164. doi: 10.1053/j.ajkd.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Al-Balas A., Lee T., Young C.J., Kepes J.A., Barker-Finkel J., Allon M. The clinical and economic effect of vascular access selection in patients initiating hemodialysis with a catheter. J Am Soc Nephrol. 2017;28:3679–3687. doi: 10.1681/ASN.2016060707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Balas A., Shariff S., Lee T., Young C., Allon M. Clinical outcomes and economic impact of starting hemodialysis with a catheter after predialysis arteriovenous fistula creation. Am J Nephrol. 2019;50:221–227. doi: 10.1159/000502050. [DOI] [PubMed] [Google Scholar]
- 4.Saran R., Robinson B., Abbott K.C., et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1 suppl 1):A6–A7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Astor B.C., Eustace J.A., Powe N.R., et al. Timing of nephrologist referral and arteriovenous access use: the CHOICE study. Am J Kidney Dis. 2001;38:494–501. doi: 10.1053/ajkd.2001.26833. [DOI] [PubMed] [Google Scholar]
- 6.Zarkowsky D.S., Arhuidese I.J., Hicks C.W., et al. Racial/ethnic disparities associated with initial hemodialysis access. JAMA Surg. 2015;150:529–536. doi: 10.1001/jamasurg.2015.0287. [DOI] [PubMed] [Google Scholar]
- 7.Hoggard J., Saad T., Schon D., et al. Guidelines for venous access in patients with chronic kidney disease. A position statement from the American Society of Diagnostic and Interventional Nephrology, Clinical Practice Committee and the Association for Vascular Access [published correction appears in Semin Dial. 2009;22:221-222] Semin Dial. 2008;21:186–191. doi: 10.1111/j.1525-139X.2008.00421.x. [DOI] [PubMed] [Google Scholar]
- 8.Schwab S.J., Beathard G. The hemodialysis catheter conundrum: hate living with them, but can’t live without them. Kidney Int. 1999;56:1–17. doi: 10.1046/j.1523-1755.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- 9.Mandolfo S., Acconcia P., Bucci R., et al. Hemodialysis tunneled central venous catheters: five-year outcome analysis. J Vasc Access. 2014;15:461–465. doi: 10.5301/jva.5000236. [DOI] [PubMed] [Google Scholar]
- 10.Maki D.G., Kluger D.M., Crnich C.J. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 11.Mendu M.L., May M.F., Kaze A.D., et al. Non-tunneled versus tunneled dialysis catheters for acute kidney injury requiring renal replacement therapy: a prospective cohort study. BMC Nephrol. 2017;18:351. doi: 10.1186/s12882-017-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asif A., Cherla G., Merrill D., Cipleu C.D., Briones P., Pennell P. Conversion of tunneled hemodialysis catheter-consigned patients to arteriovenous fistula. Kidney Int. 2005;67:2399–2406. doi: 10.1111/j.1523-1755.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 13.Falk A., Prabhuram N., Parthasarathy S. Conversion of temporary hemodialysis catheters to permanent hemodialysis catheters: a retrospective study of catheter exchange versus classic de novo placement. Semin Dial. 2005;18:425–430. doi: 10.1111/j.1525-139X.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj S.K., Ciacci J., Kirsch M., Ebersole J.D. A single institutional experience of conversion of non-tunneled to tunneled hemodialysis catheters: a comparison to de novo placement. Int Urol Nephrol. 2013;45:1753–1759. doi: 10.1007/s11255-013-0508-x. [DOI] [PubMed] [Google Scholar]
- 15.Criddle J.M., Hieb R.A., White S.B., et al. Evaluation of catheter infection rates in converted dialysis catheters versus de novo placement in the setting of chlorhexidine use. J Vasc Access. 2016;17:162–166. doi: 10.5301/jva.5000490. [DOI] [PubMed] [Google Scholar]
- 16.Coryell L., Lott J.P., Stavropoulos S.W., et al. The case for primary placement of tunneled hemodialysis catheters in acute kidney injury. J Vasc Interv Radiol. 2009;20:1578–1582. doi: 10.1016/j.jvir.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Ling X.C., Lu H.P., Loh E.W., et al. A systematic review and meta-analysis of the comparison of performance among step-tip, split-tip, and symmetrical-tip hemodialysis catheters. J Vasc Surg. 2019;69:1282–1292. doi: 10.1016/j.jvs.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Veenstra D.L., Saint S., Saha S., Lumley T., Sullivan S.D. Efficacy of antiseptic-impregnated central venous catheters in preventing catheter-related bloodstream infection: a meta-analysis. JAMA. 1999;281:261–267. doi: 10.1001/jama.281.3.261. [DOI] [PubMed] [Google Scholar]
- 19.Casey A.L., Mermel L.A., Nightingale P., Elliott T.S. Antimicrobial central venous catheters in adults: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8:763–776. doi: 10.1016/S1473-3099(08)70280-9. [DOI] [PubMed] [Google Scholar]
- 20.Krafte-Jacobs B., Sivit C.J., Mejia R., Pollack M.M. Catheter-related thrombosis in critically ill children: comparison of catheters with and without heparin bonding. J Pediatr. 1995;126:50–54. doi: 10.1016/s0022-3476(95)70499-x. [DOI] [PubMed] [Google Scholar]
- 21.O’Grady N.P., Alexander M., Burns L.A., et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–e193. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marschall J., Mermel L.A., Fakih M., et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:753–771. doi: 10.1086/676533. [DOI] [PubMed] [Google Scholar]
- 23.Trautner B.W., Darouiche R.O. Catheter-associated infections: pathogenesis affects prevention. Arch Intern Med. 2004;164:842–850. doi: 10.1001/archinte.164.8.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabindranath K.S., Bansal T., Adams J., et al. Systematic review of antimicrobials for the prevention of haemodialysis catheter-related infections. Nephrol Dial Transplant. 2009;24:3763–3774. doi: 10.1093/ndt/gfp327. [DOI] [PubMed] [Google Scholar]
- 25.Jain G., Allon M., Saddekni S., Barker J.F., Maya I.D. Does heparin coating improve patency or reduce infection of tunneled dialysis catheters? Clin J Am Soc Nephrol. 2009;4:1787–1790. doi: 10.2215/CJN.03920609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark T.W., Jacobs D., Charles H.W., et al. Comparison of heparin-coated and conventional split-tip hemodialysis catheters. Cardiovasc Interv Radiol. 2009;32:703–706. doi: 10.1007/s00270-009-9608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dwyer A. Surface-treated catheters—a review. Semin Dial. 2008;21:542–546. doi: 10.1111/j.1525-139X.2008.00499.x. [DOI] [PubMed] [Google Scholar]
- 28.Oliver M.J., Edwards L.J., Treleaven D.J., Lambert K., Margetts P.J. Randomized study of temporary hemodialysis catheters. Int J Artif Organs. 2002;25:40–44. doi: 10.1177/039139880202500107. [DOI] [PubMed] [Google Scholar]
- 29.Salik E., Daftary A., Tal M.G. Three-dimensional anatomy of the left central veins: implications for dialysis catheter placement. J Vasc Interv Radiol. 2007;18:361–364. doi: 10.1016/j.jvir.2006.12.721. [DOI] [PubMed] [Google Scholar]
- 30.Engstrom B.I., Horvath J.J., Stewart J.K., et al. Tunneled internal jugular hemodialysis catheters: impact of laterality and tip position on catheter dysfunction and infection rates. J Vasc Interv Radiol. 2013;24:1295–1302. doi: 10.1016/j.jvir.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 31.Parienti J.J., Thirion M., Mégarbane B., et al. Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: a randomized controlled trial. JAMA. 2008;299:2413–2422. doi: 10.1001/jama.299.20.2413. [DOI] [PubMed] [Google Scholar]
- 32.Hillenbrand A., Henne-Bruns D., Wolf A.M. Panniculus, giant hernias and surgical problems in patients with morbid obesity. GMS Interdiscip Plast Reconstr Surg DGPW. 2012;1:Doc05. doi: 10.3205/iprs000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Society of Anesthesiologists Task Force on Central Venous Access. Rupp S.M., Apfelbaum J.L., et al. Practice guidelines for central venous access: a report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology. 2012;116:539–573. doi: 10.1097/ALN.0b013e31823c9569. [DOI] [PubMed] [Google Scholar]
- 34.Schillinger F., Schillinger D., Montagnac R., Milcent T. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant. 1991;6:722–724. doi: 10.1093/ndt/6.10.722. [DOI] [PubMed] [Google Scholar]
- 35.Vesely T.M. Central venous catheter tip position: a continuing controversy. J Vasc Interv Radiol. 2003;14:527–534. doi: 10.1097/01.rvi.0000071097.76348.72. [DOI] [PubMed] [Google Scholar]
- 36.Polderman K.H., Girbes A.J. Central venous catheter use. Part 1: mechanical complications. Intensive Care Med. 2002;28:1–17. doi: 10.1007/s00134-001-1154-9. [DOI] [PubMed] [Google Scholar]
- 37.Duntley P., Siever J., Korwes M.L., Harpel K., Heffner J.E. Vascular erosion by central venous catheters. Clinical features and outcome. Chest. 1992;101:1633–1638. doi: 10.1378/chest.101.6.1633. [DOI] [PubMed] [Google Scholar]
- 38.Mukau L., Talamini M.A., Sitzmann J.V. Risk factors for central venous catheter-related vascular erosions. JPEN J Parenter Enter Nutr. 1991;15:513–516. doi: 10.1177/0148607191015005513. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher S.J., Bodenham A.R. Safe placement of central venous catheters: where should the tip of the catheter lie? Br J Anaesth. 2000;85:188–191. doi: 10.1093/bja/85.2.188. [DOI] [PubMed] [Google Scholar]
- 40.Huriaux L., Costille P., Quintard H., Journois D., Kellum J.A., Rimmelé T. Haemodialysis catheters in the intensive care unit. Anaesth Crit Care Pain Med. 2017;36:313–319. doi: 10.1016/j.accpm.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Falk A. Use of the femoral vein as insertion site for tunneled hemodialysis catheters. J Vasc Interv Radiol. 2007;18:217–225. doi: 10.1016/j.jvir.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Chalkiadis G.A., Goucke C.R. Depth of central venous catheter insertion in adults: an audit and assessment of a technique to improve tip position. Anaesthesiol Intensive Care. 1998;26:61–66. doi: 10.1177/0310057X9802600109. [DOI] [PubMed] [Google Scholar]
- 43.Andropoulos D.B., Bent S.T., Skjonsby B., Stayer S.A. The optimal length of insertion of central venous catheters for pediatric patients. Anesth Analg. 2001;93:883–886. doi: 10.1097/00000539-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Choi Y.H., Cheon J.E., Shin S.H., et al. Optimal insertion lengths of right and left internal jugular central venous catheters in children. Pediatr Radiol. 2015;45:1206–1211. doi: 10.1007/s00247-015-3289-9. [DOI] [PubMed] [Google Scholar]
- 45.Kim H., Jeong C.H., Byon H.J., et al. Predicting the optimal depth of left-sided central venous catheters in children. Anaesthesia. 2013;68:1033–1037. doi: 10.1111/anae.12371. [DOI] [PubMed] [Google Scholar]
- 46.Czepizak C.A., O’Callaghan J.M., Venus B. Evaluation of formulas for optimal positioning of central venous catheters. Chest. 1995;107:1662–1664. doi: 10.1378/chest.107.6.1662. [DOI] [PubMed] [Google Scholar]
- 47.Peres P.W. Positioning central venous catheters—a prospective survey. Anaesth Intensive Care. 1990;18:536–539. doi: 10.1177/0310057X9001800422. [DOI] [PubMed] [Google Scholar]
- 48.Planert M., Stahlberg E., Anton S., Jacob F., Nitschke M. Determination of the correct length of percutaneously implanted LongTerm hemodialysis catheters and the influence of obesity on post-interventional catheter migration. Ann Vasc Med Res. 2017;4:1077. [Google Scholar]
- 49.Oner B., Karam A.R., Surapaneni P., Phillips D.A. Pneumothorax following ultrasound-guided jugular vein puncture for central venous access in interventional radiology: 4 years of experience. J Intensive Care Med. 2012;27:370–372. doi: 10.1177/0885066611415494. [DOI] [PubMed] [Google Scholar]
- 50.Wong S.S., Kwaan H.C., Ing T.S. Venous air embolism related to the use of central catheters revisited: with emphasis on dialysis catheters. Clin Kidney J. 2017;10:797–803. doi: 10.1093/ckj/sfx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beathard G.A., Litchfield T. Physician Operators Forum of RMS Lifeline, Inc. Effectiveness and safety of dialysis vascular access procedures performed by interventional nephrologists. Kidney Int. 2004;66:1622–1632. doi: 10.1111/j.1523-1755.2004.00928.x. [DOI] [PubMed] [Google Scholar]
- 52.Inrig J.K., Reed S.D., Szczech L.A., et al. Relationship between clinical outcomes and vascular access type among hemodialysis patients with Staphylococcus aureus bacteremia. Clin J Am Soc Nephrol. 2006;1:518–524. doi: 10.2215/CJN.01301005. [DOI] [PubMed] [Google Scholar]
- 53.Lok C.E., Mokrzycki M.H. Prevention and management of catheter-related infection in hemodialysis patients. Kidney Int. 2011;79:587–598. doi: 10.1038/ki.2010.471. [DOI] [PubMed] [Google Scholar]
- 54.Allon M., Depner T.A., Radeva M., et al. Impact of dialysis dose and membrane on infection-related hospitalization and death: results of the HEMO Study. J Am Soc Nephrol. 2003;14:1863–1870. doi: 10.1097/01.asn.0000074237.78764.d1. [DOI] [PubMed] [Google Scholar]
- 55.Dolmatch B.L., Gurley J.C., Baskin K.M., et al. Society of Interventional Radiology Reporting Standards for Thoracic Central Vein Obstruction: Endorsed by the American Society of Diagnostic and Interventional Nephrology (ASDIN), British Society of Interventional Radiology (BSIR), Canadian Interventional Radiology Association (CIRA), Heart Rhythm Society (HRS), Indian Society of Vascular and Interventional Radiology (ISVIR), Vascular Access Society of the Americas (VASA), and Vascular Access Society of Britain and Ireland (VASBI) J Vasc Access. 2019;20(2):114–122. doi: 10.1177/1129729818791409. [DOI] [PubMed] [Google Scholar]
- 56.Schwab S.J., Quarles L.D., Middleton J.P., Cohan R.H., Saeed M., Dennis V.W. Hemodialysis-associated subclavian vein stenosis. Kidney Int. 1988;33:1156–1159. doi: 10.1038/ki.1988.124. [DOI] [PubMed] [Google Scholar]
- 57.McFall R.G., Lu T. Application of intravascular ultrasound in end-stage renal patients with central venous occlusive disease. Methodist Debakey CardioVasc J. 2018;14:196–199. doi: 10.14797/mdcj-14-3-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agarwal A.K. Central vein stenosis: current concepts. Adv Chronic Kidney Dis. 2009;16:360–370. doi: 10.1053/j.ackd.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 59.Liangos O., Gul A., Madias N.E., Jaber B.L. Long-term management of the tunneled venous catheter. Semin Dial. 2006;19:158–164. doi: 10.1111/j.1525-139X.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 60.Sehgal A.R., Snow R.J., Singer M.E., et al. Barriers to adequate delivery of hemodialysis. Am J Kidney Dis. 1998;31:593–601. doi: 10.1053/ajkd.1998.v31.pm9531174. [DOI] [PubMed] [Google Scholar]
- 61.Boyce J.M. Prevention of central line-associated bloodstream infections in hemodialysis patients. Infect Control Hosp Epidemiol. 2012;33:936–944. doi: 10.1086/667369. [DOI] [PubMed] [Google Scholar]
- 62.Golestaneh L., Mokrzycki M.H. Prevention of hemodialysis catheter infections: ointments, dressings, locks, and catheter hub devices. Hemodial Int. 2018;22(suppl 2):S75–S82. doi: 10.1111/hdi.12703. [DOI] [PubMed] [Google Scholar]
- 63.Burch T.M., Stanger B., Mizuguchi K.A., Zurakowski D., Reid S.D. Is alcohol-based hand disinfection equivalent to surgical scrub before placing a central venous catheter? Anesth Analg. 2012;114:622–625. doi: 10.1213/ANE.0b013e31824083b8. [DOI] [PubMed] [Google Scholar]
- 64.Raad I.I., Hohn D.C., Gilbreath B.J., et al. Prevention of central venous catheter-related infections by using maximal sterile barrier precautions during insertion. Infect Control Hosp Epidemiol. 1994;15:231–238. [PubMed] [Google Scholar]
- 65.Pronovost P., Needham D., Berenholtz S., et al. An intervention to decrease catheter-related bloodstream infections in the ICU [published correction appears in N Engl J Med. 2007;356:2660] N Engl J Med. 2006;355:2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 66.Munoz-Price L.S., Dezfulian C., Wyckoff M., et al. Effectiveness of stepwise interventions targeted to decrease central catheter-associated bloodstream infections. Crit Care Med. 2012;40:1464–1469. doi: 10.1097/CCM.0b013e31823e9f5b. [DOI] [PubMed] [Google Scholar]
- 67.Chaiyakunapruk N., Veenstra D.L., Lipsky B.A., Saint S. Chlorhexidine compared with povidone-iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med. 2002;136:792–801. doi: 10.7326/0003-4819-136-11-200206040-00007. [DOI] [PubMed] [Google Scholar]
- 68.Mimoz O., Lucet J.C., Kerforne T., et al. Skin antisepsis with chlorhexidine-alcohol versus povidone iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet. 2015;386:2069–2077. doi: 10.1016/S0140-6736(15)00244-5. [DOI] [PubMed] [Google Scholar]
- 69.Safdar N., Kluger D.M., Maki D.G. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters: implications for preventive strategies. Med (Baltim) 2002;81:466–479. doi: 10.1097/00005792-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 70.O’Grady N.P., Alexander M., Burns L.A., et al. Summary of recommendations: guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:1087–1099. doi: 10.1093/cid/cir138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marty Cooney R., Manickam N., Becherer P., et al. The use of 3.15% chlorhexidine gluconate/70% alcohol hub disinfection to prevent central line-associated bloodstream infections in dialysis patients. Br J Nurs. 2020;29:S24–S26. doi: 10.12968/bjon.2020.29.2.S24. [DOI] [PubMed] [Google Scholar]
- 72.Farr B.M. Mupirocin to prevent S. aureus infections. N Engl J Med. 2002;346:1905–1906. doi: 10.1056/NEJMed020048. [DOI] [PubMed] [Google Scholar]
- 73.Oliveira L., Graham J., Lok C., MacFarlane S., Zimmerman D. Risk factors for yeast superinfection in the treatment of suspected exit site infections: a case-control study. J Vasc Access. 2008;9:35–38. [PubMed] [Google Scholar]
- 74.McQuillan R.F., Chiu E., Nessim S., et al. A randomized controlled trial comparing mupirocin and polysporin triple ointments in peritoneal dialysis patients: the MP3 Study. Clin J Am Soc Nephrol. 2012;7:297–303. doi: 10.2215/CJN.07970811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenblum A., Wang W., Ball L.K., Latham C., Maddux F.W., Lacson E., Jr. Hemodialysis catheter care strategies: a cluster-randomized quality improvement initiative. Am J Kidney Dis. 2014;63:259–267. doi: 10.1053/j.ajkd.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 76.James M.T., Conley J., Tonelli M., et al. Meta-analysis: antibiotics for prophylaxis against hemodialysis catheter-related infections. Ann Intern Med. 2008;148:596–605. doi: 10.7326/0003-4819-148-8-200804150-00004. [DOI] [PubMed] [Google Scholar]
- 77.Fisher M., Golestaneh L., Allon M., Abreo K., Mokrzycki M.H. Prevention of bloodstream infections in patients undergoing hemodialysis. Clin J Am Soc Nephrol. 2020;15:132–151. doi: 10.2215/CJN.06820619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hymes J.L., Mooney A., Van Zandt C., Lynch L., Ziebol R., Killion D. Dialysis catheter-related bloodstream infections: a cluster-randomized trial of the ClearGuard HD antimicrobial barrier cap. Am J Kidney Dis. 2017;69:220–227. doi: 10.1053/j.ajkd.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 79.Jaffer Y., Selby N.M., Taal M.W., Fluck R.J., McIntyre C.W. A meta-analysis of hemodialysis catheter locking solutions in the prevention of catheter-related infection. Am J Kidney Dis. 2008;51:233–241. doi: 10.1053/j.ajkd.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 80.Campos R.P., do Nascimento M.M., Chula D.C., Riella M.C. Minocycline-EDTA lock solution prevents catheter-related bacteremia in hemodialysis. J Am Soc Nephrol. 2011;22:1939–1945. doi: 10.1681/ASN.2010121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shanks R.M., Sargent J.L., Martinez R.M., Graber M.L., O'Toole G.A. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol Dial Transplant. 2006;21:2247–2255. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 82.Weijmer M.C., van den Dorpel M.A., Van de Ven P.J., et al. Randomized, clinical trial comparison of trisodium citrate 30% and heparin as catheter-locking solution in hemodialysis patients. J Am Soc Nephrol. 2005;16:2769–2777. doi: 10.1681/ASN.2004100870. [DOI] [PubMed] [Google Scholar]
- 83.Wilkin T.D., Kraus M.A., Lane K.A., Trerotola S.O. Internal jugular vein thrombosis associated with hemodialysis catheters. Radiology. 2003;228:697–700. doi: 10.1148/radiol.2283020681. [DOI] [PubMed] [Google Scholar]
- 84.La Greca A., Biasucci D.G., Emoli A., Pittiruti M. Improving the “global use” of ultrasound for central venous access: a new supraclavicular scan by microconvex probe. Crit Ultrasound J. 2014;6(suppl 2):A11. doi: 10.1186/2036-7902-6-S2-A11. [DOI] [Google Scholar]
- 85.Lin B.S., Huang T.P., Tang G.J., Tarng D.C., Kong C.W. Ultrasound-guided cannulation of the internal jugular vein for dialysis vascular access in uremic patients. Nephron. 1998;78:423–428. doi: 10.1159/000044971. [DOI] [PubMed] [Google Scholar]
- 86.Geddes C.C., Walbaum D., Fox J.G., Mactier R.A. Insertion of internal jugular temporary hemodialysis cannulae by direct ultrasound guidance—a prospective comparison of experienced and inexperienced operators. Clin Nephrol. 1998;50:320–325. [PubMed] [Google Scholar]
- 87.Rabindranath K.S., Kumar E., Shail R., Vaux E.C. Ultrasound use for the placement of haemodialysis catheters. Cochrane Database Syst Rev. 2011;11:CD005279. doi: 10.1002/14651858.CD005279.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prabhu M.V., Juneja D., Gopal P.B., et al. Ultrasound-guided femoral dialysis access placement: a single-center randomized trial. Clin J Am Soc Nephrol. 2010;5:235–239. doi: 10.2215/CJN.04920709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shamir M.Y., Bruce L.J. Central venous catheter-induced cardiac tamponade: a preventable complication. Anesth Analg. 2011;112:1280–1282. doi: 10.1213/ANE.0b013e318214b544. [DOI] [PubMed] [Google Scholar]
- 90.Pittiruti M., Lamperti M. Late cardiac tamponade in adults secondary to tip position in the right atrium: an urban legend? A systematic review of the literature. J Cardiothorac Vasc Anesth. 2015;29:491–495. doi: 10.1053/j.jvca.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 91.Abood G.J., Davis K.A., Esposito T.J., Luchette F.A., Gamelli R.L. Comparison of routine chest radiograph versus clinician judgment to determine adequate central line placement in critically ill patients. J Trauma. 2007;63:50–56. doi: 10.1097/TA.0b013e31806bf1a3. [DOI] [PubMed] [Google Scholar]
- 92.Lessnau K.D. Is chest radiography necessary after uncomplicated insertion of a triple-lumen catheter in the right internal jugular vein, using the anterior approach? Chest. 2005;127:220–223. doi: 10.1378/chest.127.1.220. [DOI] [PubMed] [Google Scholar]
- 93.Vezzani A., Brusasco C., Palermo S., Launo C., Mergoni M., Corradi F. Ultrasound localization of central vein catheter and detection of postprocedural pneumothorax: an alternative to chest radiography. Crit Care Med. 2010;38:533–538. doi: 10.1097/CCM.0b013e3181c0328f. [DOI] [PubMed] [Google Scholar]
- 94.Spitzer B., Kirkland K., Reyes J., Helmer S.D., Ammar C. Subbarao C Concomitant placement of dialysis and infusion catheters in the right internal jugular vein in the intensive care setting: is it safe? J Vasc Access. 2021;22:359–363. doi: 10.1177/1129729820938209. [DOI] [PubMed] [Google Scholar]